User login
Using SNRIs to prevent migraines in patients with depression
Ms. D, age 45, has major depressive disorder (MDD), generalized anxiety disorder (GAD), migraines, and hypertension. At a follow-up visit, she says she has been under a lot of stress at work in the past several months and feels her antidepressant is not working well for her depression or anxiety. Ms. D notes that lately she has had more frequent migraines, occurring approximately 4 times per month during the past 3 months. She describes a severe throbbing frontal pain that occurs primarily on the left side of her head, but sometimes on the right side. Ms. D says she experiences nausea, vomiting, and photophobia during these migraine episodes. The migraines last up to 12 hours, but often resolve with sumatriptan 50 mg as needed.
Ms. D takes fluoxetine 60 mg/d for depression and anxiety, lisinopril 20 mg/d for hypertension, as well as a women’s multivitamin and vitamin D3 daily. She has not tried other antidepressants and misses doses of her medications about once every other week. Her blood pressure is 125/80 mm Hg; heart rate is 80 beats per minute; and temperature is 37° C. Ms. D’s treatment team is considering switching her to a medication that can act as preventative therapy for migraines while also treating her depression and anxiety.
Migraine is a chronic, disabling neurovascular disorder that affects approximately 15% of the United States population.1 It is the second-leading disabling condition worldwide and may negatively affect social, family, personal, academic, and occupational domains.2 Migraine is often characterized by throbbing pain, is frequently unilateral, and may last 24 to 72 hours.3 It may occur with or without aura and can be associated with nausea, vomiting, or sensitivity to light.3 Episodic migraines occur <15 days a month, while chronic migraines occur ≥15 days a month.4
Many psychiatric, neurologic, vascular, and cardiac comorbidities are more prevalent in individuals who experience migraine headaches compared to the general population. Common psychiatric comorbidities found in patients with migraines are depression, bipolar disorder, GAD, panic disorder, and posttraumatic stress disorder5; MDD is the most common.4 A person who experiences migraine headaches is 2 to 4 times more likely to develop MDD than one who does not experience migraine headaches.4
First-line treatments for preventing migraine including divalproex, topiramate, metoprolol, propranolol, and timolol.6 However, for some patients with migraines and comorbid depression or anxiety, an antidepressant may be an option. This article briefly reviews the evidence for using antidepressants that have been studied for their ability to decrease migraine frequency.
Antidepressants that can prevent migraine
Tricyclic antidepressants (TCAs) are second- or third-line options for migraine prevention.6 While TCAs have proven to be effective for preventing migraines, many patients are unable to tolerate their adverse effects (ie, anticholinergic effects, sedation).7 TCAs may be more appealing for younger patients, who may be less bothered by anticholinergic burden, or those who have difficulty sleeping.
Serotonin-norepinephrine reuptake inhibitors (SNRIs). There has been growing interest in understanding the potential utility of SNRIs as a preventative treatment for migraines. Research has found that SNRIs are as effective as TCAs for preventing migraines and also more tolerable in terms of adverse effects.7 SNRIs such as venlafaxine and duloxetine are currently prescribed off-label to prevent migraines despite a lack of FDA approval for this indication.8
Continue to: Understanding the safety and efficacy...
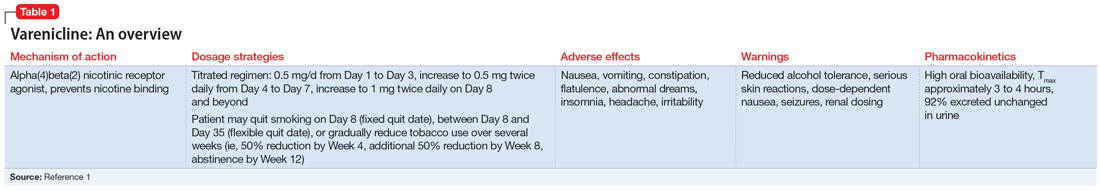
Understanding the safety and efficacy of SNRIs as preventative treatment for episodic migraines is useful, particularly for patients with comorbid depression. The Table8-17 details clinical information related to SNRI use.
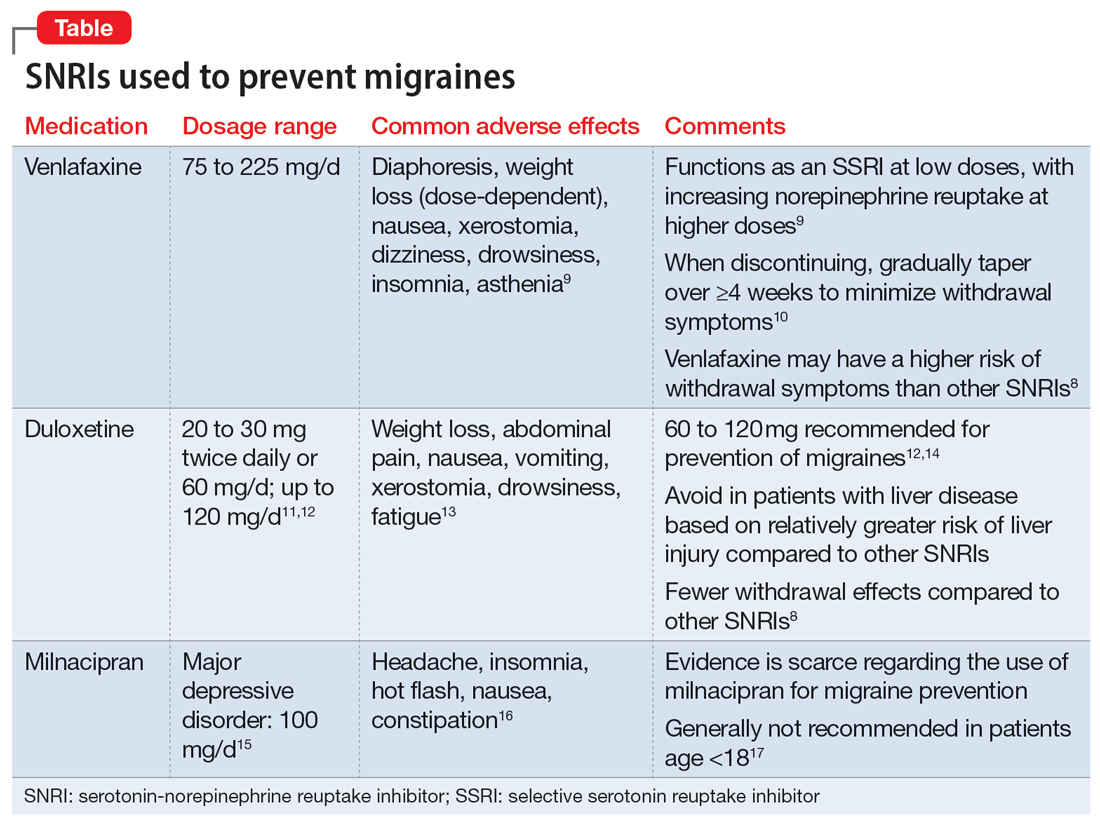
Duloxetine has demonstrated efficacy in preventing migraines in patients with comorbid depression.8 In a 2019 study, Kisler et al14 found that duloxetine 60 mg/d for 7 weeks was more effective for migraine prophylaxis than placebo as measured by the percentage of self-estimated migraine improvement by each patient compared to pretreatment levels (duloxetine: 52.3% ± 30.4%; placebo: 26.0% ± 27.3%; P = .001).
Venlafaxine has also demonstrated efficacy for preventing migraines in patients with comorbid depression.8 One study demonstrated a significant decrease in headaches per month with the use of venlafaxine 150 mg/d compared to placebo.18 Adelman et al19 found a reduction in migraine headaches per month (16.1 to 11.1, P < .0001) in patients who took venlafaxine for an average of 6 months with a mean dose of 150 mg/d. In a study of patients who did not have a mood disorder, Tarlaci20 found that venlafaxine reduced migraine headache independent of its antidepressant action.
Though milnacipran has not been studied as extensively as other SNRIs, evidence suggests it reduces the incidence of headaches and migraines, especially among episodic migraine patients. Although it has an equipotent effect on both serotonin and norepinephrine (NE) reuptake, milnacipran has a greater NE effect compared to other SNRIs approved for treating mood disorders. A prospective, single-arm study by Engel et al21 found a significant (P < .005) reduction from baseline in all headache and migraine days per month with the use of milnacipran 100 mg/d over the course of 3 months. The number of headache days per month was reduced by 4.2 compared to baseline. This same study reported improved functionality and reduced use of acute and symptomatic medications overall due to the decrease in headaches and migraines.21
In addition to demonstrating that certain SNRIs can effectively prevent migraine, some evidence suggests certain patients may benefit from the opportunity to decrease pill burden by using a single medication to treat both depression and migraine.22 Duloxetine may be preferred for patients who struggle with adherence (such as Ms. D) due to its relatively lower incidence of withdrawal symptoms compared to venlafaxine.8
CASE CONTINUED
Ms. D’s psychiatrist concludes she would be an appropriate candidate for treatment with an SNRI due to her history of MDD and chronic migraines. Because Ms. D expresses some difficulty remembering to take her medications, the psychiatrist recommends duloxetine because it is less likely to produce withdrawal symptoms compared to venlafaxine. To decrease pill burden, fluoxetine 60 mg is stopped with no taper due to its long half-life, and duloxetine is started at 30 mg/d, with a planned increase to 60 mg/d after 1 to 2 weeks as tolerated to target both mood and migraine prophylaxis. Duloxetine will not interact with Ms. D’s current medication regimen, including lisinopril, women’s multivitamin, or vitamin D3. The psychiatrist discusses the importance of medication adherence to improve her conditions effectively and safely. Ms. D’s heart rate and blood pressure will continue to be monitored.
Related Resources
- Leo RJ, Khalid K. Antidepressants for chronic pain. Current Psychiatry. 2019;18(2):8-16,21-22.
- Williams AM, Knox ED. When to prescribe antidepressants to treat comorbid depression and pain disorders. Current Psychiatry. 2017;16(1):55-58.
Drug Brand Names
Divalproex • Depakote
Duloxetine • Cymbalta
Fluoxetine • Prozac
Lisinopril • Zestril, Prinivil
Milnacipran • Savella
Sumatriptan • Imitrex
Topiramate • Topamax
Venlafaxine • Effexor
1. Burch R, Rizzoli P, Loder E. The prevalence and impact of migraine and severe headache in the United States: figures and trends from government health studies. Headache. 2018;58(4):496-505. doi:10.1111/head.13281
2. GBD 2016 Headache Collaborators. Global, regional, and national burden of migraine and tension-type headache, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17(11):954-976. doi:10.1016/S1474-4422(18)30322-3
3. Goadsby PJ, Lipton RB, Ferrari MD. Migraine--current understanding and treatment. N Engl J Med. 2002;346(4):257-270. doi:10.1056/NEJMra010917
4. Amoozegar F. Depression comorbidity in migraine. Int Rev Psychiatry. 2017;29(5):504-515. doi:10.1080/09540261.2017.1326882
5. Burch RC, Buse DC, Lipton RB. Migraine: epidemiology, burden, and comorbidity. Neurol Clin. 2019;37(4):631-649. doi:10.1016/j.ncl.2019.06.001
6. Ha H, Gonzalez A. Migraine headache prophylaxis. Am Fam Physician. 2019;99(1):17-24.
7. Xu XM, Liu Y, Dong MX, et al. Tricyclic antidepressants for preventing migraine in adults. Medicine (Baltimore). 2017;96(22):e6989. doi:10.1097/MD.0000000000006989
8. Burch R. Antidepressants for preventive treatment of migraine. Curr Treat Options Neurol. 2019;21(4):18. doi:10.1007/s11940-019-0557-2
9. Venlafaxine. Lexicomp. 2021. http://online.lexi.com/
10. Ogle NR, Akkerman SR. Guidance for the discontinuation or switching of antidepressant therapies in adults. J Pharm Pract. 2013;26(4):389-396. doi:10.1177/0897190012467210
11. Duloxetine [package insert]. Indianapolis, IN: Eli Lilly and Company; 2004.
12. Young WB, Bradley KC, Anjum MW, et al. Duloxetine prophylaxis for episodic migraine in persons without depression: a prospective study. Headache. 2013;53(9):1430-1437.
13. Duloxetine. Lexicomp. 2021. http://online.lexi.com/
14. Kisler LB, Weissman-Fogel I, Coghill RC, et al. Individualization of migraine prevention: a randomized controlled trial of psychophysical-based prediction of duloxetine efficacy. Clin J Pain. 2019;35(9):753-765.
15. Mansuy L. Antidepressant therapy with milnacipran and venlafaxine. Neuropsychiatr Dis Treat. 2010;6 (Suppl I):17-22.
16. Milnacipran. Lexicomp. 2021. http://online.lexi.com/
17. Milnacipran. MedlinePlus. Updated January 22, 2022. Accessed August 19, 2022. https://medlineplus.gov/druginfo/meds/a609016.html
18. Ozyalcin SN, Talu GK, Kiziltan E, et al. The efficacy and safety of venlafaxine in the prophylaxis of migraine. Headache. 2005;45(2):144-152. doi:10.1111/j.1526-4610.2005.05029.x
19. Adelman LC, Adelman JU, Von Seggern R, et al. Venlafaxine extended release (XR) for the prophylaxis of migraine and tension-type headache: a retrospective study in a clinical setting. Headache. 2000;40(7):572-580. doi:10.1046/j.1526-4610.2000.00089.x
20. Tarlaci S. Escitalopram and venlafaxine for the prophylaxis of migraine headache without mood disorders. Clin Neuropharmacol. 2009;32(5):254-258. doi:10.1097/WNF.0b013e3181a8c84f
21. Engel ER, Kudrow D, Rapoport AM. A prospective, open-label study of milnacipran in the prevention of headache in patients with episodic or chronic migraine. Neurol Sci. 2014;35(3):429-435. doi:10.1007/s10072-013-1536-0
22. Baumgartner A, Drame K, Geutjens S, et al. Does the polypill improve patient adherence compared to its individual formulations? A systematic review. Pharmaceutics. 2020;12(2):190.
Ms. D, age 45, has major depressive disorder (MDD), generalized anxiety disorder (GAD), migraines, and hypertension. At a follow-up visit, she says she has been under a lot of stress at work in the past several months and feels her antidepressant is not working well for her depression or anxiety. Ms. D notes that lately she has had more frequent migraines, occurring approximately 4 times per month during the past 3 months. She describes a severe throbbing frontal pain that occurs primarily on the left side of her head, but sometimes on the right side. Ms. D says she experiences nausea, vomiting, and photophobia during these migraine episodes. The migraines last up to 12 hours, but often resolve with sumatriptan 50 mg as needed.
Ms. D takes fluoxetine 60 mg/d for depression and anxiety, lisinopril 20 mg/d for hypertension, as well as a women’s multivitamin and vitamin D3 daily. She has not tried other antidepressants and misses doses of her medications about once every other week. Her blood pressure is 125/80 mm Hg; heart rate is 80 beats per minute; and temperature is 37° C. Ms. D’s treatment team is considering switching her to a medication that can act as preventative therapy for migraines while also treating her depression and anxiety.
Migraine is a chronic, disabling neurovascular disorder that affects approximately 15% of the United States population.1 It is the second-leading disabling condition worldwide and may negatively affect social, family, personal, academic, and occupational domains.2 Migraine is often characterized by throbbing pain, is frequently unilateral, and may last 24 to 72 hours.3 It may occur with or without aura and can be associated with nausea, vomiting, or sensitivity to light.3 Episodic migraines occur <15 days a month, while chronic migraines occur ≥15 days a month.4
Many psychiatric, neurologic, vascular, and cardiac comorbidities are more prevalent in individuals who experience migraine headaches compared to the general population. Common psychiatric comorbidities found in patients with migraines are depression, bipolar disorder, GAD, panic disorder, and posttraumatic stress disorder5; MDD is the most common.4 A person who experiences migraine headaches is 2 to 4 times more likely to develop MDD than one who does not experience migraine headaches.4
First-line treatments for preventing migraine including divalproex, topiramate, metoprolol, propranolol, and timolol.6 However, for some patients with migraines and comorbid depression or anxiety, an antidepressant may be an option. This article briefly reviews the evidence for using antidepressants that have been studied for their ability to decrease migraine frequency.
Antidepressants that can prevent migraine
Tricyclic antidepressants (TCAs) are second- or third-line options for migraine prevention.6 While TCAs have proven to be effective for preventing migraines, many patients are unable to tolerate their adverse effects (ie, anticholinergic effects, sedation).7 TCAs may be more appealing for younger patients, who may be less bothered by anticholinergic burden, or those who have difficulty sleeping.
Serotonin-norepinephrine reuptake inhibitors (SNRIs). There has been growing interest in understanding the potential utility of SNRIs as a preventative treatment for migraines. Research has found that SNRIs are as effective as TCAs for preventing migraines and also more tolerable in terms of adverse effects.7 SNRIs such as venlafaxine and duloxetine are currently prescribed off-label to prevent migraines despite a lack of FDA approval for this indication.8
Continue to: Understanding the safety and efficacy...
Understanding the safety and efficacy of SNRIs as preventative treatment for episodic migraines is useful, particularly for patients with comorbid depression. The Table8-17 details clinical information related to SNRI use.

Duloxetine has demonstrated efficacy in preventing migraines in patients with comorbid depression.8 In a 2019 study, Kisler et al14 found that duloxetine 60 mg/d for 7 weeks was more effective for migraine prophylaxis than placebo as measured by the percentage of self-estimated migraine improvement by each patient compared to pretreatment levels (duloxetine: 52.3% ± 30.4%; placebo: 26.0% ± 27.3%; P = .001).
Venlafaxine has also demonstrated efficacy for preventing migraines in patients with comorbid depression.8 One study demonstrated a significant decrease in headaches per month with the use of venlafaxine 150 mg/d compared to placebo.18 Adelman et al19 found a reduction in migraine headaches per month (16.1 to 11.1, P < .0001) in patients who took venlafaxine for an average of 6 months with a mean dose of 150 mg/d. In a study of patients who did not have a mood disorder, Tarlaci20 found that venlafaxine reduced migraine headache independent of its antidepressant action.
Though milnacipran has not been studied as extensively as other SNRIs, evidence suggests it reduces the incidence of headaches and migraines, especially among episodic migraine patients. Although it has an equipotent effect on both serotonin and norepinephrine (NE) reuptake, milnacipran has a greater NE effect compared to other SNRIs approved for treating mood disorders. A prospective, single-arm study by Engel et al21 found a significant (P < .005) reduction from baseline in all headache and migraine days per month with the use of milnacipran 100 mg/d over the course of 3 months. The number of headache days per month was reduced by 4.2 compared to baseline. This same study reported improved functionality and reduced use of acute and symptomatic medications overall due to the decrease in headaches and migraines.21
In addition to demonstrating that certain SNRIs can effectively prevent migraine, some evidence suggests certain patients may benefit from the opportunity to decrease pill burden by using a single medication to treat both depression and migraine.22 Duloxetine may be preferred for patients who struggle with adherence (such as Ms. D) due to its relatively lower incidence of withdrawal symptoms compared to venlafaxine.8
CASE CONTINUED
Ms. D’s psychiatrist concludes she would be an appropriate candidate for treatment with an SNRI due to her history of MDD and chronic migraines. Because Ms. D expresses some difficulty remembering to take her medications, the psychiatrist recommends duloxetine because it is less likely to produce withdrawal symptoms compared to venlafaxine. To decrease pill burden, fluoxetine 60 mg is stopped with no taper due to its long half-life, and duloxetine is started at 30 mg/d, with a planned increase to 60 mg/d after 1 to 2 weeks as tolerated to target both mood and migraine prophylaxis. Duloxetine will not interact with Ms. D’s current medication regimen, including lisinopril, women’s multivitamin, or vitamin D3. The psychiatrist discusses the importance of medication adherence to improve her conditions effectively and safely. Ms. D’s heart rate and blood pressure will continue to be monitored.
Related Resources
- Leo RJ, Khalid K. Antidepressants for chronic pain. Current Psychiatry. 2019;18(2):8-16,21-22.
- Williams AM, Knox ED. When to prescribe antidepressants to treat comorbid depression and pain disorders. Current Psychiatry. 2017;16(1):55-58.
Drug Brand Names
Divalproex • Depakote
Duloxetine • Cymbalta
Fluoxetine • Prozac
Lisinopril • Zestril, Prinivil
Milnacipran • Savella
Sumatriptan • Imitrex
Topiramate • Topamax
Venlafaxine • Effexor
Ms. D, age 45, has major depressive disorder (MDD), generalized anxiety disorder (GAD), migraines, and hypertension. At a follow-up visit, she says she has been under a lot of stress at work in the past several months and feels her antidepressant is not working well for her depression or anxiety. Ms. D notes that lately she has had more frequent migraines, occurring approximately 4 times per month during the past 3 months. She describes a severe throbbing frontal pain that occurs primarily on the left side of her head, but sometimes on the right side. Ms. D says she experiences nausea, vomiting, and photophobia during these migraine episodes. The migraines last up to 12 hours, but often resolve with sumatriptan 50 mg as needed.
Ms. D takes fluoxetine 60 mg/d for depression and anxiety, lisinopril 20 mg/d for hypertension, as well as a women’s multivitamin and vitamin D3 daily. She has not tried other antidepressants and misses doses of her medications about once every other week. Her blood pressure is 125/80 mm Hg; heart rate is 80 beats per minute; and temperature is 37° C. Ms. D’s treatment team is considering switching her to a medication that can act as preventative therapy for migraines while also treating her depression and anxiety.
Migraine is a chronic, disabling neurovascular disorder that affects approximately 15% of the United States population.1 It is the second-leading disabling condition worldwide and may negatively affect social, family, personal, academic, and occupational domains.2 Migraine is often characterized by throbbing pain, is frequently unilateral, and may last 24 to 72 hours.3 It may occur with or without aura and can be associated with nausea, vomiting, or sensitivity to light.3 Episodic migraines occur <15 days a month, while chronic migraines occur ≥15 days a month.4
Many psychiatric, neurologic, vascular, and cardiac comorbidities are more prevalent in individuals who experience migraine headaches compared to the general population. Common psychiatric comorbidities found in patients with migraines are depression, bipolar disorder, GAD, panic disorder, and posttraumatic stress disorder5; MDD is the most common.4 A person who experiences migraine headaches is 2 to 4 times more likely to develop MDD than one who does not experience migraine headaches.4
First-line treatments for preventing migraine including divalproex, topiramate, metoprolol, propranolol, and timolol.6 However, for some patients with migraines and comorbid depression or anxiety, an antidepressant may be an option. This article briefly reviews the evidence for using antidepressants that have been studied for their ability to decrease migraine frequency.
Antidepressants that can prevent migraine
Tricyclic antidepressants (TCAs) are second- or third-line options for migraine prevention.6 While TCAs have proven to be effective for preventing migraines, many patients are unable to tolerate their adverse effects (ie, anticholinergic effects, sedation).7 TCAs may be more appealing for younger patients, who may be less bothered by anticholinergic burden, or those who have difficulty sleeping.
Serotonin-norepinephrine reuptake inhibitors (SNRIs). There has been growing interest in understanding the potential utility of SNRIs as a preventative treatment for migraines. Research has found that SNRIs are as effective as TCAs for preventing migraines and also more tolerable in terms of adverse effects.7 SNRIs such as venlafaxine and duloxetine are currently prescribed off-label to prevent migraines despite a lack of FDA approval for this indication.8
Continue to: Understanding the safety and efficacy...
Understanding the safety and efficacy of SNRIs as preventative treatment for episodic migraines is useful, particularly for patients with comorbid depression. The Table8-17 details clinical information related to SNRI use.

Duloxetine has demonstrated efficacy in preventing migraines in patients with comorbid depression.8 In a 2019 study, Kisler et al14 found that duloxetine 60 mg/d for 7 weeks was more effective for migraine prophylaxis than placebo as measured by the percentage of self-estimated migraine improvement by each patient compared to pretreatment levels (duloxetine: 52.3% ± 30.4%; placebo: 26.0% ± 27.3%; P = .001).
Venlafaxine has also demonstrated efficacy for preventing migraines in patients with comorbid depression.8 One study demonstrated a significant decrease in headaches per month with the use of venlafaxine 150 mg/d compared to placebo.18 Adelman et al19 found a reduction in migraine headaches per month (16.1 to 11.1, P < .0001) in patients who took venlafaxine for an average of 6 months with a mean dose of 150 mg/d. In a study of patients who did not have a mood disorder, Tarlaci20 found that venlafaxine reduced migraine headache independent of its antidepressant action.
Though milnacipran has not been studied as extensively as other SNRIs, evidence suggests it reduces the incidence of headaches and migraines, especially among episodic migraine patients. Although it has an equipotent effect on both serotonin and norepinephrine (NE) reuptake, milnacipran has a greater NE effect compared to other SNRIs approved for treating mood disorders. A prospective, single-arm study by Engel et al21 found a significant (P < .005) reduction from baseline in all headache and migraine days per month with the use of milnacipran 100 mg/d over the course of 3 months. The number of headache days per month was reduced by 4.2 compared to baseline. This same study reported improved functionality and reduced use of acute and symptomatic medications overall due to the decrease in headaches and migraines.21
In addition to demonstrating that certain SNRIs can effectively prevent migraine, some evidence suggests certain patients may benefit from the opportunity to decrease pill burden by using a single medication to treat both depression and migraine.22 Duloxetine may be preferred for patients who struggle with adherence (such as Ms. D) due to its relatively lower incidence of withdrawal symptoms compared to venlafaxine.8
CASE CONTINUED
Ms. D’s psychiatrist concludes she would be an appropriate candidate for treatment with an SNRI due to her history of MDD and chronic migraines. Because Ms. D expresses some difficulty remembering to take her medications, the psychiatrist recommends duloxetine because it is less likely to produce withdrawal symptoms compared to venlafaxine. To decrease pill burden, fluoxetine 60 mg is stopped with no taper due to its long half-life, and duloxetine is started at 30 mg/d, with a planned increase to 60 mg/d after 1 to 2 weeks as tolerated to target both mood and migraine prophylaxis. Duloxetine will not interact with Ms. D’s current medication regimen, including lisinopril, women’s multivitamin, or vitamin D3. The psychiatrist discusses the importance of medication adherence to improve her conditions effectively and safely. Ms. D’s heart rate and blood pressure will continue to be monitored.
Related Resources
- Leo RJ, Khalid K. Antidepressants for chronic pain. Current Psychiatry. 2019;18(2):8-16,21-22.
- Williams AM, Knox ED. When to prescribe antidepressants to treat comorbid depression and pain disorders. Current Psychiatry. 2017;16(1):55-58.
Drug Brand Names
Divalproex • Depakote
Duloxetine • Cymbalta
Fluoxetine • Prozac
Lisinopril • Zestril, Prinivil
Milnacipran • Savella
Sumatriptan • Imitrex
Topiramate • Topamax
Venlafaxine • Effexor
1. Burch R, Rizzoli P, Loder E. The prevalence and impact of migraine and severe headache in the United States: figures and trends from government health studies. Headache. 2018;58(4):496-505. doi:10.1111/head.13281
2. GBD 2016 Headache Collaborators. Global, regional, and national burden of migraine and tension-type headache, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17(11):954-976. doi:10.1016/S1474-4422(18)30322-3
3. Goadsby PJ, Lipton RB, Ferrari MD. Migraine--current understanding and treatment. N Engl J Med. 2002;346(4):257-270. doi:10.1056/NEJMra010917
4. Amoozegar F. Depression comorbidity in migraine. Int Rev Psychiatry. 2017;29(5):504-515. doi:10.1080/09540261.2017.1326882
5. Burch RC, Buse DC, Lipton RB. Migraine: epidemiology, burden, and comorbidity. Neurol Clin. 2019;37(4):631-649. doi:10.1016/j.ncl.2019.06.001
6. Ha H, Gonzalez A. Migraine headache prophylaxis. Am Fam Physician. 2019;99(1):17-24.
7. Xu XM, Liu Y, Dong MX, et al. Tricyclic antidepressants for preventing migraine in adults. Medicine (Baltimore). 2017;96(22):e6989. doi:10.1097/MD.0000000000006989
8. Burch R. Antidepressants for preventive treatment of migraine. Curr Treat Options Neurol. 2019;21(4):18. doi:10.1007/s11940-019-0557-2
9. Venlafaxine. Lexicomp. 2021. http://online.lexi.com/
10. Ogle NR, Akkerman SR. Guidance for the discontinuation or switching of antidepressant therapies in adults. J Pharm Pract. 2013;26(4):389-396. doi:10.1177/0897190012467210
11. Duloxetine [package insert]. Indianapolis, IN: Eli Lilly and Company; 2004.
12. Young WB, Bradley KC, Anjum MW, et al. Duloxetine prophylaxis for episodic migraine in persons without depression: a prospective study. Headache. 2013;53(9):1430-1437.
13. Duloxetine. Lexicomp. 2021. http://online.lexi.com/
14. Kisler LB, Weissman-Fogel I, Coghill RC, et al. Individualization of migraine prevention: a randomized controlled trial of psychophysical-based prediction of duloxetine efficacy. Clin J Pain. 2019;35(9):753-765.
15. Mansuy L. Antidepressant therapy with milnacipran and venlafaxine. Neuropsychiatr Dis Treat. 2010;6 (Suppl I):17-22.
16. Milnacipran. Lexicomp. 2021. http://online.lexi.com/
17. Milnacipran. MedlinePlus. Updated January 22, 2022. Accessed August 19, 2022. https://medlineplus.gov/druginfo/meds/a609016.html
18. Ozyalcin SN, Talu GK, Kiziltan E, et al. The efficacy and safety of venlafaxine in the prophylaxis of migraine. Headache. 2005;45(2):144-152. doi:10.1111/j.1526-4610.2005.05029.x
19. Adelman LC, Adelman JU, Von Seggern R, et al. Venlafaxine extended release (XR) for the prophylaxis of migraine and tension-type headache: a retrospective study in a clinical setting. Headache. 2000;40(7):572-580. doi:10.1046/j.1526-4610.2000.00089.x
20. Tarlaci S. Escitalopram and venlafaxine for the prophylaxis of migraine headache without mood disorders. Clin Neuropharmacol. 2009;32(5):254-258. doi:10.1097/WNF.0b013e3181a8c84f
21. Engel ER, Kudrow D, Rapoport AM. A prospective, open-label study of milnacipran in the prevention of headache in patients with episodic or chronic migraine. Neurol Sci. 2014;35(3):429-435. doi:10.1007/s10072-013-1536-0
22. Baumgartner A, Drame K, Geutjens S, et al. Does the polypill improve patient adherence compared to its individual formulations? A systematic review. Pharmaceutics. 2020;12(2):190.
1. Burch R, Rizzoli P, Loder E. The prevalence and impact of migraine and severe headache in the United States: figures and trends from government health studies. Headache. 2018;58(4):496-505. doi:10.1111/head.13281
2. GBD 2016 Headache Collaborators. Global, regional, and national burden of migraine and tension-type headache, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17(11):954-976. doi:10.1016/S1474-4422(18)30322-3
3. Goadsby PJ, Lipton RB, Ferrari MD. Migraine--current understanding and treatment. N Engl J Med. 2002;346(4):257-270. doi:10.1056/NEJMra010917
4. Amoozegar F. Depression comorbidity in migraine. Int Rev Psychiatry. 2017;29(5):504-515. doi:10.1080/09540261.2017.1326882
5. Burch RC, Buse DC, Lipton RB. Migraine: epidemiology, burden, and comorbidity. Neurol Clin. 2019;37(4):631-649. doi:10.1016/j.ncl.2019.06.001
6. Ha H, Gonzalez A. Migraine headache prophylaxis. Am Fam Physician. 2019;99(1):17-24.
7. Xu XM, Liu Y, Dong MX, et al. Tricyclic antidepressants for preventing migraine in adults. Medicine (Baltimore). 2017;96(22):e6989. doi:10.1097/MD.0000000000006989
8. Burch R. Antidepressants for preventive treatment of migraine. Curr Treat Options Neurol. 2019;21(4):18. doi:10.1007/s11940-019-0557-2
9. Venlafaxine. Lexicomp. 2021. http://online.lexi.com/
10. Ogle NR, Akkerman SR. Guidance for the discontinuation or switching of antidepressant therapies in adults. J Pharm Pract. 2013;26(4):389-396. doi:10.1177/0897190012467210
11. Duloxetine [package insert]. Indianapolis, IN: Eli Lilly and Company; 2004.
12. Young WB, Bradley KC, Anjum MW, et al. Duloxetine prophylaxis for episodic migraine in persons without depression: a prospective study. Headache. 2013;53(9):1430-1437.
13. Duloxetine. Lexicomp. 2021. http://online.lexi.com/
14. Kisler LB, Weissman-Fogel I, Coghill RC, et al. Individualization of migraine prevention: a randomized controlled trial of psychophysical-based prediction of duloxetine efficacy. Clin J Pain. 2019;35(9):753-765.
15. Mansuy L. Antidepressant therapy with milnacipran and venlafaxine. Neuropsychiatr Dis Treat. 2010;6 (Suppl I):17-22.
16. Milnacipran. Lexicomp. 2021. http://online.lexi.com/
17. Milnacipran. MedlinePlus. Updated January 22, 2022. Accessed August 19, 2022. https://medlineplus.gov/druginfo/meds/a609016.html
18. Ozyalcin SN, Talu GK, Kiziltan E, et al. The efficacy and safety of venlafaxine in the prophylaxis of migraine. Headache. 2005;45(2):144-152. doi:10.1111/j.1526-4610.2005.05029.x
19. Adelman LC, Adelman JU, Von Seggern R, et al. Venlafaxine extended release (XR) for the prophylaxis of migraine and tension-type headache: a retrospective study in a clinical setting. Headache. 2000;40(7):572-580. doi:10.1046/j.1526-4610.2000.00089.x
20. Tarlaci S. Escitalopram and venlafaxine for the prophylaxis of migraine headache without mood disorders. Clin Neuropharmacol. 2009;32(5):254-258. doi:10.1097/WNF.0b013e3181a8c84f
21. Engel ER, Kudrow D, Rapoport AM. A prospective, open-label study of milnacipran in the prevention of headache in patients with episodic or chronic migraine. Neurol Sci. 2014;35(3):429-435. doi:10.1007/s10072-013-1536-0
22. Baumgartner A, Drame K, Geutjens S, et al. Does the polypill improve patient adherence compared to its individual formulations? A systematic review. Pharmaceutics. 2020;12(2):190.
Drug-induced progressive multifocal leukoencephalopathy: Rare but serious
Mr. P, age 67, presents to the clinic with vision changes and memory loss following a fall in his home due to limb weakness. Six years ago, his care team diagnosed him with rheumatoid arthritis (RA). Mr. P’s current medication regimen includes methotrexate 20 mg once weekly and etanercept 50 mg once weekly, and he has been stable on this plan for 3 years. Mr. P also was recently diagnosed with major depressive disorder (MDD), but has not yet started treatment. Following a complete workup, an MRI of Mr. P’s brain revealed white matter demyelination. Due to these findings, he is scheduled for a brain biopsy, which confirms a diagnosis of progressive multifocal leukoencephalopathy (PML).
PML is a demyelinating disease of the central nervous system caused by the John Cunningham virus (JCV), or JC polyomavirus, named for the first patient identified to have contracted the virus.1 Asymptomatic infection of JCV often occurs in childhood, and antibodies are found in ≤70% of healthy adults. In most individuals, JCV remains latent in the kidneys and lymphoid organs, but immunosuppression can cause it to reactivate.2
JCV infects oligodendrocytes, astrocytes, and neurons, which results in white matter demyelination. Due to this demyelination, individuals can experience visual field defects, speech disturbances, ataxia, paresthesia, and cognitive impairments.2 Limb weakness presents in 60% of patients with PML, visual disturbances in 20%, and gait disturbances in 65%.3 Progression of these symptoms can lead to a more severe clinical presentation, including focal seizures in ≤10% of patients, and the mortality rate is 30% to 50%.3 Patients with comorbid HIV have a mortality rate ≤90%.2
Currently, there are no biomarkers that can identify PML in its early stages. A PML diagnosis is typically based on the patient’s clinical presentation, radiological imaging, and detection of JCV DNA. A brain biopsy is the gold standard for PML diagnosis.1
Interestingly, data suggest that glial cells harboring JCV in the brain express receptors for serotonin and dopamine.4 Researchers pinpointed 5HT2A receptors as JCV entry points into cells, and theorized that medications competing for binding, such as certain psychotropic agents, might decrease JCV entry. Cells lacking the 5HT2A receptor have shown immunity to JCV infection and the ability of cells to be infected was restored through transfection of 5HT2A receptors.4
Immunosuppressant medications can cause PML
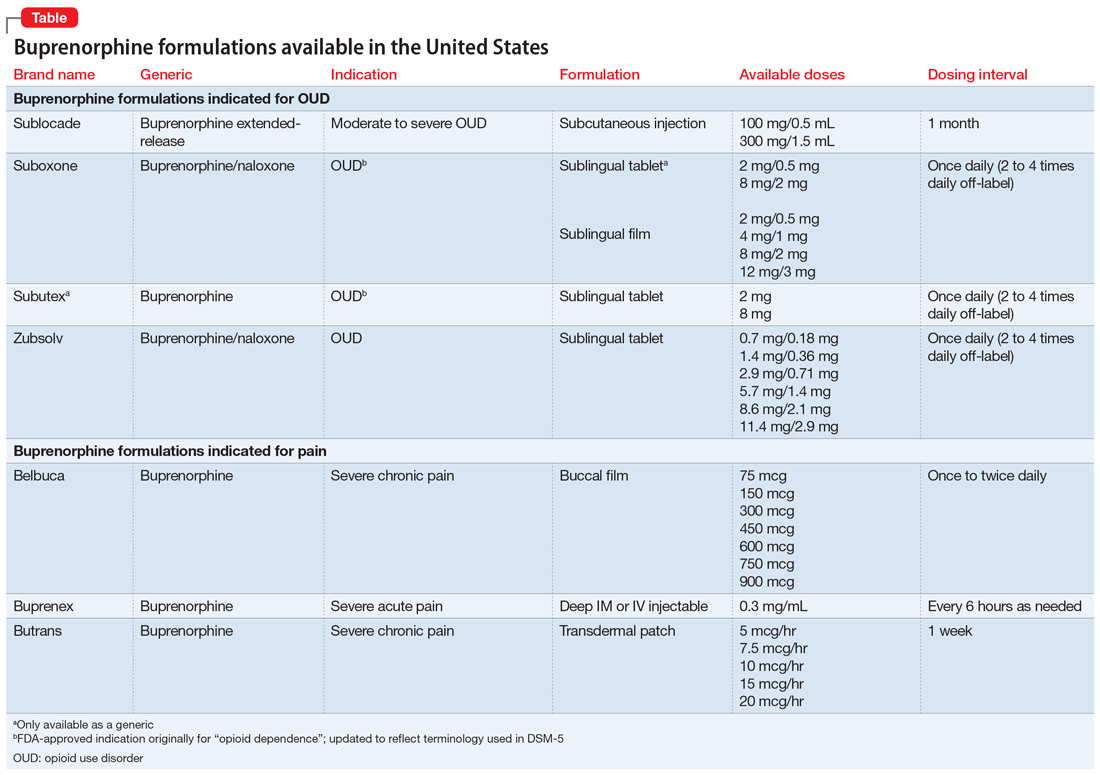
PML was initially seen in individuals with conditions that cause immunosuppression, such as malignancies and HIV. However, “drug-induced PML” refers to cases in which drug-induced immunosuppression creates an environment that allows JCV to reactivate and disseminate back into the CNS.4 It is important to emphasize that drug-induced PML is a very rare effect of certain immunosuppressant medications. Medications that can weaken the immune system include glucocorticoids, monoclonal antibodies, alkylating agents, purine analogues, antimetabolites, and immunosuppressants (Table).1
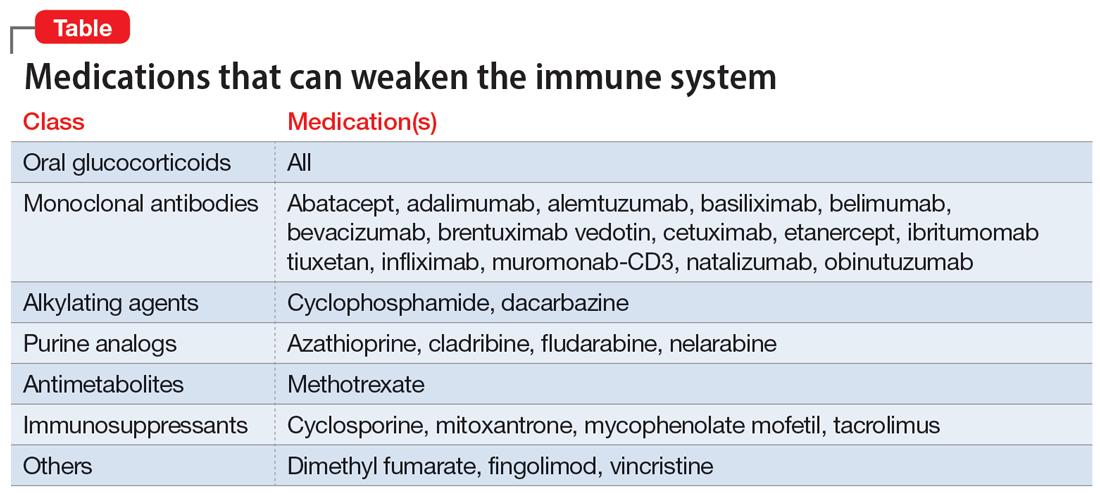
These medications are used to treat conditions such as multiple sclerosis, RA, psoriatic arthritis, and lupus. Although drug-induced PML can result from the use of any of these agents, the highest incidence (1%) is found with natalizumab. Rates of incidence with other agents are either unknown or as low as .002%.1 Evidence suggests that the risk for PML increases with the duration of therapy.5
Continue to: Management
Management: Stop the offending agent, restore immune function
Specific pharmacologic treatments for PML are lacking. Management of drug-induced PML starts with discontinuing the offending agent. Restoring immune function has been found to be the most effective approach to treat PML.3 Restoration is possible through interleukin-2 (IL-2), IL-7, and T-cell infusions. Other treatment options are theoretical and include the development of a JCV vaccine to stimulate host response, plasma exchange to remove the medication from the host, and antiviral therapy targeting JCV replication. Diclofenac, isotretinoin, and mefloquine can inhibit JCV replication.3
Based on the theory that JCV requires 5HT2A receptors for entry into cells, researchers have studied medications that block this receptor as a treatment for PML. The first-generation antipsychotic chlorpromazine did not show benefit when combined with cidofovir, a replication inhibitor.3 Antipsychotics agents such as ziprasidone and olanzapine have shown in vitro inhibition of JCV, while risperidone has mixed results, with 1 trial failing to find a difference on JCV in fetal glial cells.3 Second-generation antipsychotics may be the preferred option due to more potent antagonism of the 5HT2A receptors and fewer adverse effects compared to agents such as chlorpromazine.4 The antidepressant mirtazapine has shown to have promising results, with evidence indicating that earlier initiation is more beneficial.3 Overall, data involving the use of medications that act on the 5HT2A receptor are mixed. Recent data suggest that JCV might enter cells independent of 5HT2A receptors; however, more research in this area is needed.2
The best strategy for treating drug-induced PML has not yet been determined. While combination therapy is thought to be more successful than monotherapy, ultimately, it depends on the patient’s immune response. If a psychotropic medication is chosen as adjunct treatment for drug-induced PML, it is prudent to assess the patient’s entire clinical picture to determine the specific indication for therapy (ie, treating symptomatology or drug-induced PML).
CASE CONTINUED
Following diagnosis, Mr. P is provided supportive therapy, and his care team discontinues methotrexate and etanercept. Although data are mixed on the efficacy of medications that work on 5HT2A receptors, because Mr. P was recently diagnosed with MDD, he is started on mirtazapine 15 mg/d at night in an attempt to manage both MDD and PML. It is possible that his depressive symptoms developed as a result of drug-induced PML rather than major depressive disorder. Discontinuing methotrexate and etanercept stabilizes Mr. P’s PML symptoms but leads to an exacerbation of his RA symptoms. Mr. P is initiated on hyd
Related Resources
- Castle D, Robertson NP. Treatment of progressive multifocal leukoencephalopathy. J Neurol. 2019;266(10):2587-2589. doi:10.1007/s00415-019-09501-y
Drug Brand Names
Abatacept • Orencia
Adalimumab • Humira
Alemtuzumab • Campath
Azathioprine • Azasan, Imuran
Basiliximab • Simulect
Belimumab • Benlysta
Bevacizumab • Avastin
Brentuximab vedotin • Adcetris
Cetuximab • Erbitux
Chlorpromazine • Thorazine, Largactil
Cidofovir • Vistide
Cladribine • Mavenclad
Cyclophosphamide • Cytoxan
Cyclosporine • Gengraf, Neoral
Dacarbazine • DTIC-Dome
Diclofenac • Cambia, Zorvolex
Dimethyl fumarate • Tecfidera
Etanercept • Enbrel
Fingolimod • Gilenya
Fludarabine • Fludara
Hydroxychloroquine • Plaquenil
Ibritumomab tiuxetan • Zevalin
Infliximab • Avsola, Inflectra
Isotretinoin • Absorica, Claravis
Mefloquine • Lariam
Methotrexate • Rheumatrex, Trexall
Mirtazapine • Remeron
Mitoxantrone • Novantrone
Muromonab-CD3 • Orthoclone OKT3
Mycophenolate mofetil • CellCept
Natalizumab • Tysabri
Nelarabine • Arranon
Obinutuzumab • Gazyva
Olanzapine • Zyprexa
Risperidone • Risperdal
Tacrolimus • Prograf
Vincristine • Vincasar PFS
Ziprasidone • Geodon
1. Yukitake M. Drug-induced progressive multifocal leukoencephalopathy in multiple sclerosis: a comprehensive review. Clin Exp Neuroimmunol. 2018;9(1):37-47. doi:10.1111/cen3.12440
2. Alstadhaug KB, Myhr KM, Rinaldo CH. Progressive multifocal leukoencephalopathy. Tidsskr Nor Laegeforen. 2017;137(23-24):10.4045/tidsskr.16.1092. doi:10.4045/tidsskr.16.1092
3. Williamson EML, Berger JR. Diagnosis and treatment of progressive multifocal leukoencephalopathy associated with multiple sclerosis therapies. Neurotherapeutics. 2017;14(4):961-973. doi:10.1007/s13311-017-0570-7
4. Altschuler EL, Kast RE. The atypical antipsychotic agents ziprasidone, risperidone and olanzapine as treatment for and prophylaxis against progressive multifocal leukoencephalopathy. Med Hypotheses. 2005;65(3):585-586.
5. Vinhas de Souza M, Keller-Stanislawski B, Blake K, et al. Drug-induced PML: a global agenda for a global challenge. Clin Pharmacol Ther. 2012;91(4):747-750. doi:10.1038/clpt.2012.4
Mr. P, age 67, presents to the clinic with vision changes and memory loss following a fall in his home due to limb weakness. Six years ago, his care team diagnosed him with rheumatoid arthritis (RA). Mr. P’s current medication regimen includes methotrexate 20 mg once weekly and etanercept 50 mg once weekly, and he has been stable on this plan for 3 years. Mr. P also was recently diagnosed with major depressive disorder (MDD), but has not yet started treatment. Following a complete workup, an MRI of Mr. P’s brain revealed white matter demyelination. Due to these findings, he is scheduled for a brain biopsy, which confirms a diagnosis of progressive multifocal leukoencephalopathy (PML).
PML is a demyelinating disease of the central nervous system caused by the John Cunningham virus (JCV), or JC polyomavirus, named for the first patient identified to have contracted the virus.1 Asymptomatic infection of JCV often occurs in childhood, and antibodies are found in ≤70% of healthy adults. In most individuals, JCV remains latent in the kidneys and lymphoid organs, but immunosuppression can cause it to reactivate.2
JCV infects oligodendrocytes, astrocytes, and neurons, which results in white matter demyelination. Due to this demyelination, individuals can experience visual field defects, speech disturbances, ataxia, paresthesia, and cognitive impairments.2 Limb weakness presents in 60% of patients with PML, visual disturbances in 20%, and gait disturbances in 65%.3 Progression of these symptoms can lead to a more severe clinical presentation, including focal seizures in ≤10% of patients, and the mortality rate is 30% to 50%.3 Patients with comorbid HIV have a mortality rate ≤90%.2
Currently, there are no biomarkers that can identify PML in its early stages. A PML diagnosis is typically based on the patient’s clinical presentation, radiological imaging, and detection of JCV DNA. A brain biopsy is the gold standard for PML diagnosis.1
Interestingly, data suggest that glial cells harboring JCV in the brain express receptors for serotonin and dopamine.4 Researchers pinpointed 5HT2A receptors as JCV entry points into cells, and theorized that medications competing for binding, such as certain psychotropic agents, might decrease JCV entry. Cells lacking the 5HT2A receptor have shown immunity to JCV infection and the ability of cells to be infected was restored through transfection of 5HT2A receptors.4
Immunosuppressant medications can cause PML
PML was initially seen in individuals with conditions that cause immunosuppression, such as malignancies and HIV. However, “drug-induced PML” refers to cases in which drug-induced immunosuppression creates an environment that allows JCV to reactivate and disseminate back into the CNS.4 It is important to emphasize that drug-induced PML is a very rare effect of certain immunosuppressant medications. Medications that can weaken the immune system include glucocorticoids, monoclonal antibodies, alkylating agents, purine analogues, antimetabolites, and immunosuppressants (Table).1

These medications are used to treat conditions such as multiple sclerosis, RA, psoriatic arthritis, and lupus. Although drug-induced PML can result from the use of any of these agents, the highest incidence (1%) is found with natalizumab. Rates of incidence with other agents are either unknown or as low as .002%.1 Evidence suggests that the risk for PML increases with the duration of therapy.5
Continue to: Management
Management: Stop the offending agent, restore immune function
Specific pharmacologic treatments for PML are lacking. Management of drug-induced PML starts with discontinuing the offending agent. Restoring immune function has been found to be the most effective approach to treat PML.3 Restoration is possible through interleukin-2 (IL-2), IL-7, and T-cell infusions. Other treatment options are theoretical and include the development of a JCV vaccine to stimulate host response, plasma exchange to remove the medication from the host, and antiviral therapy targeting JCV replication. Diclofenac, isotretinoin, and mefloquine can inhibit JCV replication.3
Based on the theory that JCV requires 5HT2A receptors for entry into cells, researchers have studied medications that block this receptor as a treatment for PML. The first-generation antipsychotic chlorpromazine did not show benefit when combined with cidofovir, a replication inhibitor.3 Antipsychotics agents such as ziprasidone and olanzapine have shown in vitro inhibition of JCV, while risperidone has mixed results, with 1 trial failing to find a difference on JCV in fetal glial cells.3 Second-generation antipsychotics may be the preferred option due to more potent antagonism of the 5HT2A receptors and fewer adverse effects compared to agents such as chlorpromazine.4 The antidepressant mirtazapine has shown to have promising results, with evidence indicating that earlier initiation is more beneficial.3 Overall, data involving the use of medications that act on the 5HT2A receptor are mixed. Recent data suggest that JCV might enter cells independent of 5HT2A receptors; however, more research in this area is needed.2
The best strategy for treating drug-induced PML has not yet been determined. While combination therapy is thought to be more successful than monotherapy, ultimately, it depends on the patient’s immune response. If a psychotropic medication is chosen as adjunct treatment for drug-induced PML, it is prudent to assess the patient’s entire clinical picture to determine the specific indication for therapy (ie, treating symptomatology or drug-induced PML).
CASE CONTINUED
Following diagnosis, Mr. P is provided supportive therapy, and his care team discontinues methotrexate and etanercept. Although data are mixed on the efficacy of medications that work on 5HT2A receptors, because Mr. P was recently diagnosed with MDD, he is started on mirtazapine 15 mg/d at night in an attempt to manage both MDD and PML. It is possible that his depressive symptoms developed as a result of drug-induced PML rather than major depressive disorder. Discontinuing methotrexate and etanercept stabilizes Mr. P’s PML symptoms but leads to an exacerbation of his RA symptoms. Mr. P is initiated on hyd
Related Resources
- Castle D, Robertson NP. Treatment of progressive multifocal leukoencephalopathy. J Neurol. 2019;266(10):2587-2589. doi:10.1007/s00415-019-09501-y
Drug Brand Names
Abatacept • Orencia
Adalimumab • Humira
Alemtuzumab • Campath
Azathioprine • Azasan, Imuran
Basiliximab • Simulect
Belimumab • Benlysta
Bevacizumab • Avastin
Brentuximab vedotin • Adcetris
Cetuximab • Erbitux
Chlorpromazine • Thorazine, Largactil
Cidofovir • Vistide
Cladribine • Mavenclad
Cyclophosphamide • Cytoxan
Cyclosporine • Gengraf, Neoral
Dacarbazine • DTIC-Dome
Diclofenac • Cambia, Zorvolex
Dimethyl fumarate • Tecfidera
Etanercept • Enbrel
Fingolimod • Gilenya
Fludarabine • Fludara
Hydroxychloroquine • Plaquenil
Ibritumomab tiuxetan • Zevalin
Infliximab • Avsola, Inflectra
Isotretinoin • Absorica, Claravis
Mefloquine • Lariam
Methotrexate • Rheumatrex, Trexall
Mirtazapine • Remeron
Mitoxantrone • Novantrone
Muromonab-CD3 • Orthoclone OKT3
Mycophenolate mofetil • CellCept
Natalizumab • Tysabri
Nelarabine • Arranon
Obinutuzumab • Gazyva
Olanzapine • Zyprexa
Risperidone • Risperdal
Tacrolimus • Prograf
Vincristine • Vincasar PFS
Ziprasidone • Geodon
Mr. P, age 67, presents to the clinic with vision changes and memory loss following a fall in his home due to limb weakness. Six years ago, his care team diagnosed him with rheumatoid arthritis (RA). Mr. P’s current medication regimen includes methotrexate 20 mg once weekly and etanercept 50 mg once weekly, and he has been stable on this plan for 3 years. Mr. P also was recently diagnosed with major depressive disorder (MDD), but has not yet started treatment. Following a complete workup, an MRI of Mr. P’s brain revealed white matter demyelination. Due to these findings, he is scheduled for a brain biopsy, which confirms a diagnosis of progressive multifocal leukoencephalopathy (PML).
PML is a demyelinating disease of the central nervous system caused by the John Cunningham virus (JCV), or JC polyomavirus, named for the first patient identified to have contracted the virus.1 Asymptomatic infection of JCV often occurs in childhood, and antibodies are found in ≤70% of healthy adults. In most individuals, JCV remains latent in the kidneys and lymphoid organs, but immunosuppression can cause it to reactivate.2
JCV infects oligodendrocytes, astrocytes, and neurons, which results in white matter demyelination. Due to this demyelination, individuals can experience visual field defects, speech disturbances, ataxia, paresthesia, and cognitive impairments.2 Limb weakness presents in 60% of patients with PML, visual disturbances in 20%, and gait disturbances in 65%.3 Progression of these symptoms can lead to a more severe clinical presentation, including focal seizures in ≤10% of patients, and the mortality rate is 30% to 50%.3 Patients with comorbid HIV have a mortality rate ≤90%.2
Currently, there are no biomarkers that can identify PML in its early stages. A PML diagnosis is typically based on the patient’s clinical presentation, radiological imaging, and detection of JCV DNA. A brain biopsy is the gold standard for PML diagnosis.1
Interestingly, data suggest that glial cells harboring JCV in the brain express receptors for serotonin and dopamine.4 Researchers pinpointed 5HT2A receptors as JCV entry points into cells, and theorized that medications competing for binding, such as certain psychotropic agents, might decrease JCV entry. Cells lacking the 5HT2A receptor have shown immunity to JCV infection and the ability of cells to be infected was restored through transfection of 5HT2A receptors.4
Immunosuppressant medications can cause PML
PML was initially seen in individuals with conditions that cause immunosuppression, such as malignancies and HIV. However, “drug-induced PML” refers to cases in which drug-induced immunosuppression creates an environment that allows JCV to reactivate and disseminate back into the CNS.4 It is important to emphasize that drug-induced PML is a very rare effect of certain immunosuppressant medications. Medications that can weaken the immune system include glucocorticoids, monoclonal antibodies, alkylating agents, purine analogues, antimetabolites, and immunosuppressants (Table).1

These medications are used to treat conditions such as multiple sclerosis, RA, psoriatic arthritis, and lupus. Although drug-induced PML can result from the use of any of these agents, the highest incidence (1%) is found with natalizumab. Rates of incidence with other agents are either unknown or as low as .002%.1 Evidence suggests that the risk for PML increases with the duration of therapy.5
Continue to: Management
Management: Stop the offending agent, restore immune function
Specific pharmacologic treatments for PML are lacking. Management of drug-induced PML starts with discontinuing the offending agent. Restoring immune function has been found to be the most effective approach to treat PML.3 Restoration is possible through interleukin-2 (IL-2), IL-7, and T-cell infusions. Other treatment options are theoretical and include the development of a JCV vaccine to stimulate host response, plasma exchange to remove the medication from the host, and antiviral therapy targeting JCV replication. Diclofenac, isotretinoin, and mefloquine can inhibit JCV replication.3
Based on the theory that JCV requires 5HT2A receptors for entry into cells, researchers have studied medications that block this receptor as a treatment for PML. The first-generation antipsychotic chlorpromazine did not show benefit when combined with cidofovir, a replication inhibitor.3 Antipsychotics agents such as ziprasidone and olanzapine have shown in vitro inhibition of JCV, while risperidone has mixed results, with 1 trial failing to find a difference on JCV in fetal glial cells.3 Second-generation antipsychotics may be the preferred option due to more potent antagonism of the 5HT2A receptors and fewer adverse effects compared to agents such as chlorpromazine.4 The antidepressant mirtazapine has shown to have promising results, with evidence indicating that earlier initiation is more beneficial.3 Overall, data involving the use of medications that act on the 5HT2A receptor are mixed. Recent data suggest that JCV might enter cells independent of 5HT2A receptors; however, more research in this area is needed.2
The best strategy for treating drug-induced PML has not yet been determined. While combination therapy is thought to be more successful than monotherapy, ultimately, it depends on the patient’s immune response. If a psychotropic medication is chosen as adjunct treatment for drug-induced PML, it is prudent to assess the patient’s entire clinical picture to determine the specific indication for therapy (ie, treating symptomatology or drug-induced PML).
CASE CONTINUED
Following diagnosis, Mr. P is provided supportive therapy, and his care team discontinues methotrexate and etanercept. Although data are mixed on the efficacy of medications that work on 5HT2A receptors, because Mr. P was recently diagnosed with MDD, he is started on mirtazapine 15 mg/d at night in an attempt to manage both MDD and PML. It is possible that his depressive symptoms developed as a result of drug-induced PML rather than major depressive disorder. Discontinuing methotrexate and etanercept stabilizes Mr. P’s PML symptoms but leads to an exacerbation of his RA symptoms. Mr. P is initiated on hyd
Related Resources
- Castle D, Robertson NP. Treatment of progressive multifocal leukoencephalopathy. J Neurol. 2019;266(10):2587-2589. doi:10.1007/s00415-019-09501-y
Drug Brand Names
Abatacept • Orencia
Adalimumab • Humira
Alemtuzumab • Campath
Azathioprine • Azasan, Imuran
Basiliximab • Simulect
Belimumab • Benlysta
Bevacizumab • Avastin
Brentuximab vedotin • Adcetris
Cetuximab • Erbitux
Chlorpromazine • Thorazine, Largactil
Cidofovir • Vistide
Cladribine • Mavenclad
Cyclophosphamide • Cytoxan
Cyclosporine • Gengraf, Neoral
Dacarbazine • DTIC-Dome
Diclofenac • Cambia, Zorvolex
Dimethyl fumarate • Tecfidera
Etanercept • Enbrel
Fingolimod • Gilenya
Fludarabine • Fludara
Hydroxychloroquine • Plaquenil
Ibritumomab tiuxetan • Zevalin
Infliximab • Avsola, Inflectra
Isotretinoin • Absorica, Claravis
Mefloquine • Lariam
Methotrexate • Rheumatrex, Trexall
Mirtazapine • Remeron
Mitoxantrone • Novantrone
Muromonab-CD3 • Orthoclone OKT3
Mycophenolate mofetil • CellCept
Natalizumab • Tysabri
Nelarabine • Arranon
Obinutuzumab • Gazyva
Olanzapine • Zyprexa
Risperidone • Risperdal
Tacrolimus • Prograf
Vincristine • Vincasar PFS
Ziprasidone • Geodon
1. Yukitake M. Drug-induced progressive multifocal leukoencephalopathy in multiple sclerosis: a comprehensive review. Clin Exp Neuroimmunol. 2018;9(1):37-47. doi:10.1111/cen3.12440
2. Alstadhaug KB, Myhr KM, Rinaldo CH. Progressive multifocal leukoencephalopathy. Tidsskr Nor Laegeforen. 2017;137(23-24):10.4045/tidsskr.16.1092. doi:10.4045/tidsskr.16.1092
3. Williamson EML, Berger JR. Diagnosis and treatment of progressive multifocal leukoencephalopathy associated with multiple sclerosis therapies. Neurotherapeutics. 2017;14(4):961-973. doi:10.1007/s13311-017-0570-7
4. Altschuler EL, Kast RE. The atypical antipsychotic agents ziprasidone, risperidone and olanzapine as treatment for and prophylaxis against progressive multifocal leukoencephalopathy. Med Hypotheses. 2005;65(3):585-586.
5. Vinhas de Souza M, Keller-Stanislawski B, Blake K, et al. Drug-induced PML: a global agenda for a global challenge. Clin Pharmacol Ther. 2012;91(4):747-750. doi:10.1038/clpt.2012.4
1. Yukitake M. Drug-induced progressive multifocal leukoencephalopathy in multiple sclerosis: a comprehensive review. Clin Exp Neuroimmunol. 2018;9(1):37-47. doi:10.1111/cen3.12440
2. Alstadhaug KB, Myhr KM, Rinaldo CH. Progressive multifocal leukoencephalopathy. Tidsskr Nor Laegeforen. 2017;137(23-24):10.4045/tidsskr.16.1092. doi:10.4045/tidsskr.16.1092
3. Williamson EML, Berger JR. Diagnosis and treatment of progressive multifocal leukoencephalopathy associated with multiple sclerosis therapies. Neurotherapeutics. 2017;14(4):961-973. doi:10.1007/s13311-017-0570-7
4. Altschuler EL, Kast RE. The atypical antipsychotic agents ziprasidone, risperidone and olanzapine as treatment for and prophylaxis against progressive multifocal leukoencephalopathy. Med Hypotheses. 2005;65(3):585-586.
5. Vinhas de Souza M, Keller-Stanislawski B, Blake K, et al. Drug-induced PML: a global agenda for a global challenge. Clin Pharmacol Ther. 2012;91(4):747-750. doi:10.1038/clpt.2012.4
How bariatric surgery affects psychotropic drug absorption
Ms. B, age 60, presents to the clinic with high blood pressure, hyperlipidemia, type 2 diabetes mellitus, depression, and anxiety. Her blood pressure is 138/82 mm Hg and pulse is 70 beats per minute. Her body mass index (BMI) is 41, which indicates she is obese. She has always struggled with her weight and has tried diet and lifestyle modifications, as well as medications, for the past 5 years with no success. Her current medication regimen includes lisinopril 40 mg daily, amlodipine 5 mg daily, atorvastatin 40 mg daily, metformin 500 mg twice daily, dulaglutide 0.75 mg weekly, lithium 600 mg daily, venlafaxine extended-release (XR) 150 mg daily, and alprazolam 0.5 mg as needed up to twice daily. Due to Ms. B’s BMI and because she has ≥1 comorbid health condition, her primary care physician refers her to a gastroenterologist to discuss gastric bypass surgery options.
Ms. B is scheduled for Roux-en-Y gastric bypass surgery. You need to determine if any changes should be made to her psychotropic medications after she undergoes this surgery.
There are multiple types of bariatric surgeries, including Roux-en-Y gastric bypass, sleeve gastrectomy, laparoscopic adjustable gastric band, and biliopancreatic diversion with duodenal switch (BPD/DS) (Figure1-4). These procedures all restrict the stomach’s capacity to hold food. In most cases, they also bypass areas of absorption in the intestine and cause increased secretion of hormones in the gut, including (but not limited to) peptide-YY (PYY) and glucagon-like peptide 1 (GLP-1). These hormonal changes impact several factors, including satiety, hunger, and blood sugar levels.5
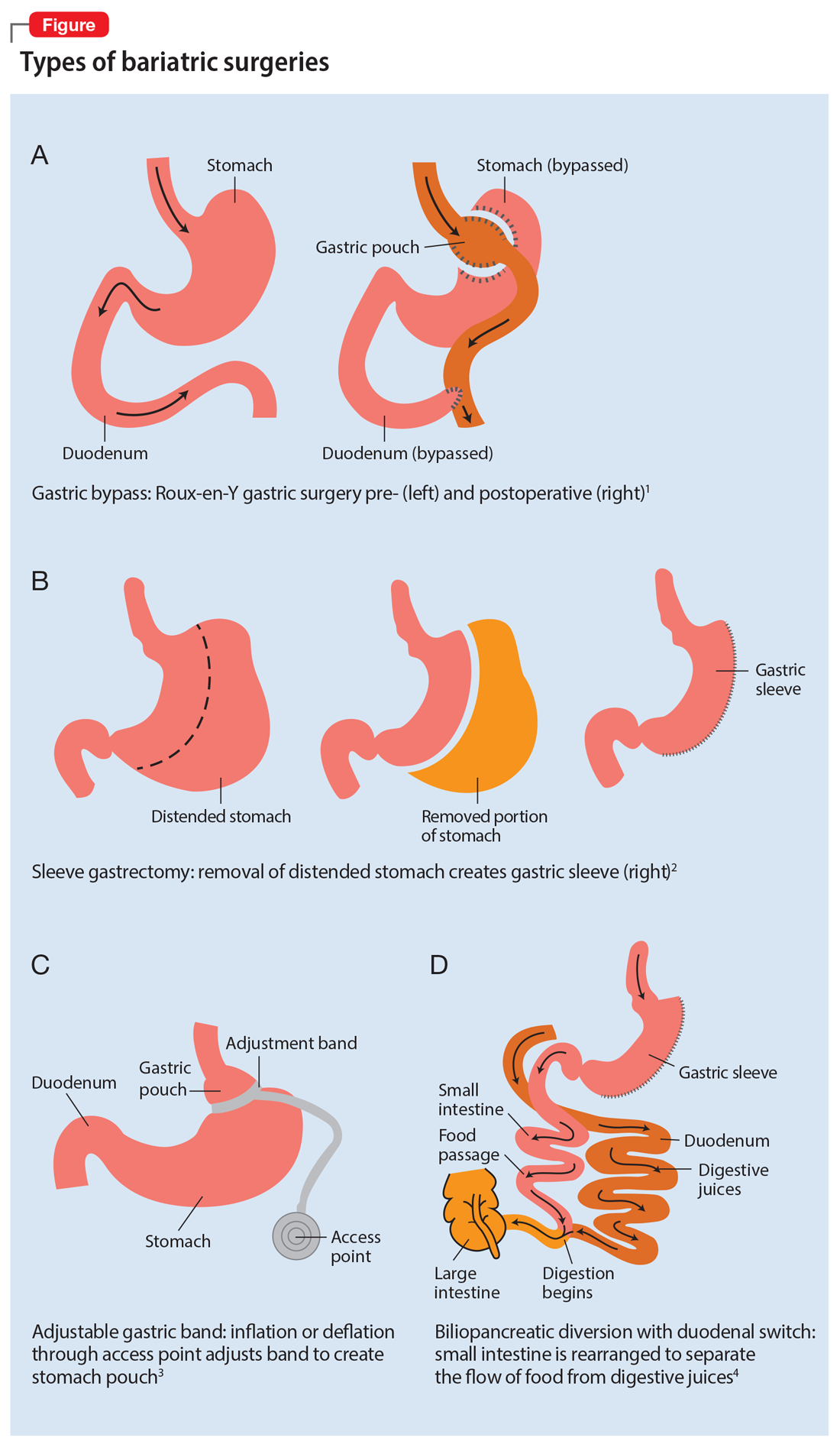
Roux-en-Y is commonly referred to as the gold standard of weight loss surgery. It divides the top of the stomach into a smaller stomach pouch that connects directly to the small intestine to facilitate smaller meals and alters the release of gut hormones. Additionally, a segment of the small intestine that normally absorbs nutrients and medications is completely bypassed. In contrast, the sleeve gastrectomy removes approximately 80% of the stomach, consequently reducing the amount of food that can be consumed. The greatest impact of the sleeve gastrectomy procedure appears to result from changes in gut hormones. The adjustable gastric band procedure works by placing a band around the upper portion of the stomach to create a small pouch above the band to satisfy hunger with a smaller amount of food. Lastly, BPD/DS is a procedure that creates a tubular stomach pouch and bypasses a large portion of the small intestine. Like the gastric bypass and sleeve gastrectomy, BPD/DS affects gut hormones impacting hunger, satiety, and blood sugar control.
How bariatric surgery can affect drug absorption
As illustrated in the Table,6-19 each type of bariatric surgery may impact drug absorption differently depending on the mechanism by which the stomach is restricted.
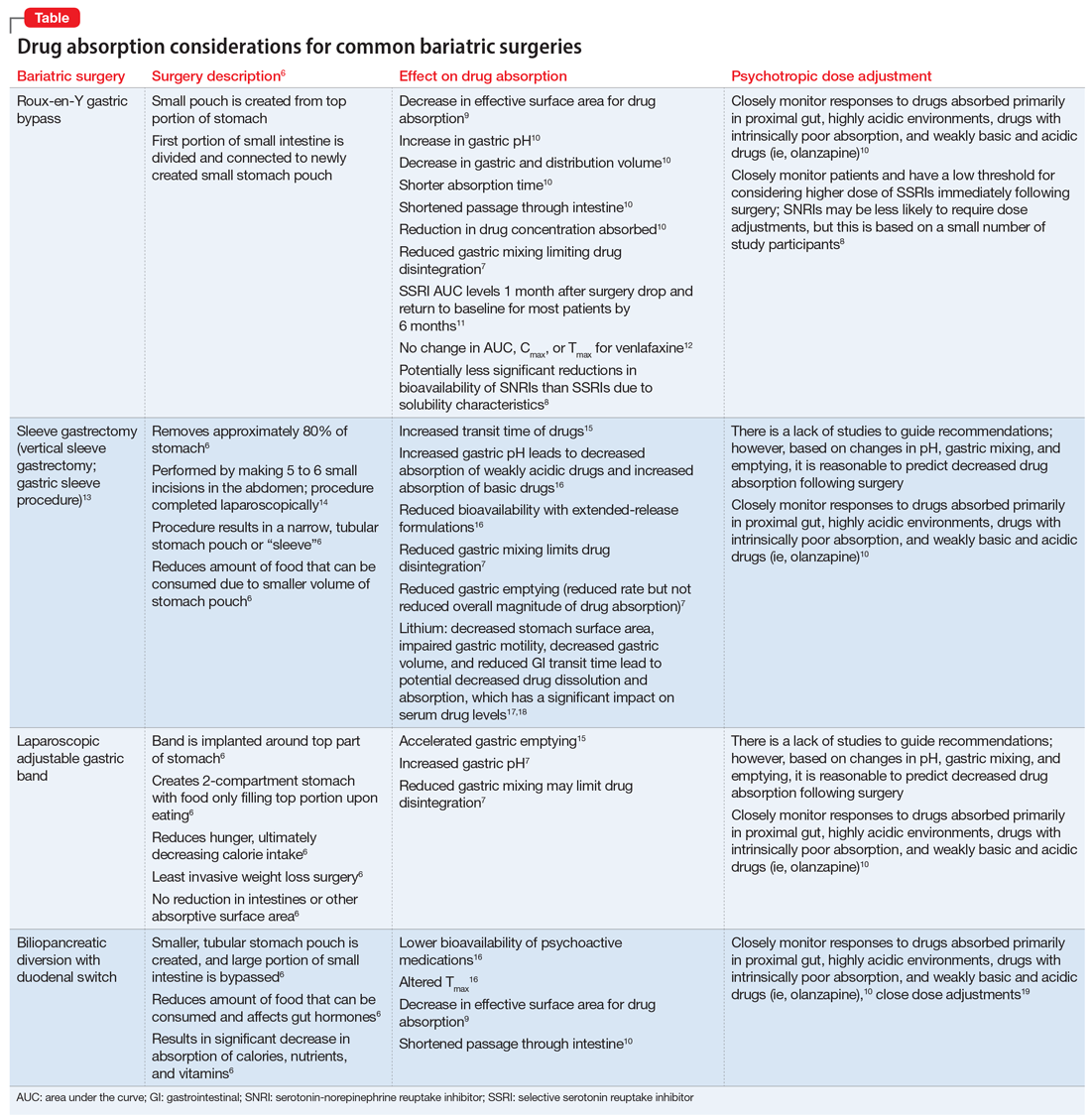
Drug malabsorption is a concern for clinicians with patients who have undergone bariatric surgery. There is limited research measuring changes in psychotropic exposure and outcomes following bariatric surgery. A 2009 literature review by Padwal et al7 found that one-third of the 26 studies evaluated provided evidence of decreased absorption following bariatric surgery in patients taking medications that had intrinsic poor absorption, high lipophilicity, and/or undergo enterohepatic recirculation. In a review that included a small study of patients taking selective serotonin reuptake inhibitors or venlafaxine, Godini et al8 demonstrated that although there was a notable decrease in drug absorption closely following the surgery, drug absorption recovered for some patients 1 month after Roux-en-Y surgery. These reviews suggest patients who have undergone any form of bariatric surgery must be observed closely because drug absorption may vary based on the individual, the medication administered, and the amount of time postprocedure.
Until more research becomes available, current evidence supports recommendations to assist patients who have a decreased ability to absorb medications after gastric bypass surgery by switching from an extended-release formulation to an immediate-release or solution formulation. This allows patients to rely less on gastric mixing and unpredictable changes in drug release from extended- or controlled-release formulations.
Continue to: Aside from altered...
Aside from altered pharmacokinetics after bariatric surgery, many patients experience an increased risk of self-harm and suicide.20 Therefore, a continued emphasis on and reinforcement of proper antidepressant use and adjustment in these patients is important. This can be facilitated through frequent follow-up visits, either in-person or via telehealth.
Understanding the effect of bariatric surgery on drug absorption is critical to identifying a potential need to adjust a medication dose or formulation after the surgery. Available evidence and data suggest it is reasonable to switch from an extended- or sustained-release formulation to an immediate-release formulation, and to monitor patients more frequently immediately following the surgery.
CASE CONTINUED
Related Resources
- Colvin C, Tsia W, Silverman AL, et al. Nothing up his sleeve: decompensation after bariatric surgery. Current Psychiatry. 2021;20(4):15-19. doi:10.12788/cp.010
Drug Brand Names
Alprazolam • Xanax
Amlodipine • Norvasc
Atorvastatin • Lipitor
Dulaglutide • Trulicity
Lisinopril • Zestril, Prinivil
Lithium • Eskalith, Lithobid
Metformin • Glucophage
Olanzapine • Zyprexa
Venlafaxine • Effexor
1. Obesity Treatments: Gastric Bypass Surgery. UCLA Health. Accessed April 4, 2021. http://surgery.ucla.edu/bariatrics-gastric-bypass
2. Thomas L. Gastric bypass more likely to require further treatment than gastric sleeve. News Medical. January 15, 2020. Accessed April 4, 2021. https://www.news-medical.net/news/20200115/Gastric-bypass-more-likely-to-require-further-treatment-than-gastric-sleeve.aspx
3. Lap Adjustable Gastric Banding. Laser Stone Surgery & Endoscopy Centre. September 5, 2016. Accessed April 4, 2021. http://www.laserstonesurgery.org/project/lap-adjustable-gastric-banding/
4. BPD/DS Weight-Loss Surgery. Johns Hopkins Medicine. Accessed April 4, 2021. https://www.hopkinsmedicine.org/health/treatment-tests-and-therapies/bpdds-weightloss-surgery
5. Holst JJ, Madsbad S, Bojsen-Møller KN, et al. Mechanisms in bariatric surgery: gut hormones, diabetes resolution, and weight loss. Surg Obes Relat Dis. 2018;14(5):708-714. doi:10.1016/j.soard.2018.03.003
6. Public Education Committee. Bariatric Surgery Procedures. American Society for Metabolic and Bariatric Surgery. Updated May 2021. Accessed September 4, 2021. https://asmbs.org/patients/bariatric-surgery-procedures
7. Padwal R, Brocks D, Sharma AM. A systematic review of drug absorption following bariatric surgery and its theoretical implications. Obes Rev. 2010;11(1):41-50. doi:10.1111/j.1467-789x.2009.00614.x
8. Godini L, Castellini G, Facchiano E, et al. Mood disorders and bariatric surgery patients: pre- and post- surgery clinical course- an overview. J Obes Weight Loss Medicat. 2016;2(1). doi:10.23937/2572-4010.1510012
9. Smith A, Henriksen B, Cohen A. Pharmacokinetic considerations in Roux-en-Y gastric bypass patients. Am J Health Syst Pharm. 2011;68(23):2241-2247. doi:10.2146/ajhp100630
10. Brocks DR, Ben-Eltriki M, Gabr RQ, et al. The effects of gastric bypass surgery on drug absorption and pharmacokinetics. Expert Opin Drug Metab Toxicol. 2012;8(12):1505-1519. doi:10.1517/17425255.2012.722757
11. Hamad GG, Helsel JC, Perel JM, et al. The effect of gastric bypass on the pharmacokinetics of serotonin reuptake inhibitors. Am J Psychiatry. 2012;169(3):256-263. doi:10.1176/appi.ajp.2011.11050719
12. Angeles PC, Robertsen I, Seeberg LT, et al. The influence of bariatric surgery on oral drug bioavailability in patients with obesity: a systematic review. Obes Rev. 2019;20(9):1299-1311. doi:10.1111/obr.12869
13. Laparoscopic Sleeve Gastrectomy. University of California San Francisco Department of Surgery. Accessed April 1, 2021. https://surgery.ucsf.edu/conditions--procedures/laparoscopic-sleeve-gastrectomy.aspx
14. Brethauer S, Schauer P. Laparoscopic Sleeve Gastrectomy: A Newcomer to Bariatric Surgery. Obesity Action Coalition. 2007. Accessed May 15, 2021. https://www.obesityaction.org/community/article-library/laparoscopic-sleeve-gastrectomy-a-newcomer-to-bariatric-surgery/
15. Roerig JL, Steffen K. Psychopharmacology and bariatric surgery. Eur Eat Disord Rev. 2015;23(6):463-469. doi:10.1002/erv.2396
16. Bland CM, Quidley AM, Love BL, et al. Long-term pharmacotherapy considerations in the bariatric surgery patient. A J Health Syst Pharm. 2016;73(16):1230-1242. doi:10.2146/ajhp151062
17. Lin YH, Liu SW, Wu HL, et al. Lithium toxicity with prolonged neurologic sequelae following sleeve gastrectomy: a case report and review of literature. Medicine (Baltimore). 2020;99(28):e21122. doi:10.1097/MD.0000000000021122
18. Lorico S, Colton B. Medication management and pharmacokinetic changes after bariatric surgery. Can Fam Physician. 2020;66(6):409-416.
19. Homan J, Schijns W, Aarts EO, et al. Treatment of vitamin and mineral deficiencies after biliopancreatic diversion with or without duodenal switch: a major challenge. Obes Surg. 2018;28(1):234-241. doi:10.1007/s11695-017-2841-0
20. Neovius M, Bruze G, Jacobson P, et al. Risk of suicide and non-fatal self-harm after bariatric surgery: results from two matched cohort studies. Lancet Diabetes Endocrinol. 2018;6(3):197-207. doi:10.1016/S2213-8587(17)30437-0
Ms. B, age 60, presents to the clinic with high blood pressure, hyperlipidemia, type 2 diabetes mellitus, depression, and anxiety. Her blood pressure is 138/82 mm Hg and pulse is 70 beats per minute. Her body mass index (BMI) is 41, which indicates she is obese. She has always struggled with her weight and has tried diet and lifestyle modifications, as well as medications, for the past 5 years with no success. Her current medication regimen includes lisinopril 40 mg daily, amlodipine 5 mg daily, atorvastatin 40 mg daily, metformin 500 mg twice daily, dulaglutide 0.75 mg weekly, lithium 600 mg daily, venlafaxine extended-release (XR) 150 mg daily, and alprazolam 0.5 mg as needed up to twice daily. Due to Ms. B’s BMI and because she has ≥1 comorbid health condition, her primary care physician refers her to a gastroenterologist to discuss gastric bypass surgery options.
Ms. B is scheduled for Roux-en-Y gastric bypass surgery. You need to determine if any changes should be made to her psychotropic medications after she undergoes this surgery.
There are multiple types of bariatric surgeries, including Roux-en-Y gastric bypass, sleeve gastrectomy, laparoscopic adjustable gastric band, and biliopancreatic diversion with duodenal switch (BPD/DS) (Figure1-4). These procedures all restrict the stomach’s capacity to hold food. In most cases, they also bypass areas of absorption in the intestine and cause increased secretion of hormones in the gut, including (but not limited to) peptide-YY (PYY) and glucagon-like peptide 1 (GLP-1). These hormonal changes impact several factors, including satiety, hunger, and blood sugar levels.5

Roux-en-Y is commonly referred to as the gold standard of weight loss surgery. It divides the top of the stomach into a smaller stomach pouch that connects directly to the small intestine to facilitate smaller meals and alters the release of gut hormones. Additionally, a segment of the small intestine that normally absorbs nutrients and medications is completely bypassed. In contrast, the sleeve gastrectomy removes approximately 80% of the stomach, consequently reducing the amount of food that can be consumed. The greatest impact of the sleeve gastrectomy procedure appears to result from changes in gut hormones. The adjustable gastric band procedure works by placing a band around the upper portion of the stomach to create a small pouch above the band to satisfy hunger with a smaller amount of food. Lastly, BPD/DS is a procedure that creates a tubular stomach pouch and bypasses a large portion of the small intestine. Like the gastric bypass and sleeve gastrectomy, BPD/DS affects gut hormones impacting hunger, satiety, and blood sugar control.
How bariatric surgery can affect drug absorption
As illustrated in the Table,6-19 each type of bariatric surgery may impact drug absorption differently depending on the mechanism by which the stomach is restricted.

Drug malabsorption is a concern for clinicians with patients who have undergone bariatric surgery. There is limited research measuring changes in psychotropic exposure and outcomes following bariatric surgery. A 2009 literature review by Padwal et al7 found that one-third of the 26 studies evaluated provided evidence of decreased absorption following bariatric surgery in patients taking medications that had intrinsic poor absorption, high lipophilicity, and/or undergo enterohepatic recirculation. In a review that included a small study of patients taking selective serotonin reuptake inhibitors or venlafaxine, Godini et al8 demonstrated that although there was a notable decrease in drug absorption closely following the surgery, drug absorption recovered for some patients 1 month after Roux-en-Y surgery. These reviews suggest patients who have undergone any form of bariatric surgery must be observed closely because drug absorption may vary based on the individual, the medication administered, and the amount of time postprocedure.
Until more research becomes available, current evidence supports recommendations to assist patients who have a decreased ability to absorb medications after gastric bypass surgery by switching from an extended-release formulation to an immediate-release or solution formulation. This allows patients to rely less on gastric mixing and unpredictable changes in drug release from extended- or controlled-release formulations.
Continue to: Aside from altered...
Aside from altered pharmacokinetics after bariatric surgery, many patients experience an increased risk of self-harm and suicide.20 Therefore, a continued emphasis on and reinforcement of proper antidepressant use and adjustment in these patients is important. This can be facilitated through frequent follow-up visits, either in-person or via telehealth.
Understanding the effect of bariatric surgery on drug absorption is critical to identifying a potential need to adjust a medication dose or formulation after the surgery. Available evidence and data suggest it is reasonable to switch from an extended- or sustained-release formulation to an immediate-release formulation, and to monitor patients more frequently immediately following the surgery.
CASE CONTINUED
Related Resources
- Colvin C, Tsia W, Silverman AL, et al. Nothing up his sleeve: decompensation after bariatric surgery. Current Psychiatry. 2021;20(4):15-19. doi:10.12788/cp.010
Drug Brand Names
Alprazolam • Xanax
Amlodipine • Norvasc
Atorvastatin • Lipitor
Dulaglutide • Trulicity
Lisinopril • Zestril, Prinivil
Lithium • Eskalith, Lithobid
Metformin • Glucophage
Olanzapine • Zyprexa
Venlafaxine • Effexor
Ms. B, age 60, presents to the clinic with high blood pressure, hyperlipidemia, type 2 diabetes mellitus, depression, and anxiety. Her blood pressure is 138/82 mm Hg and pulse is 70 beats per minute. Her body mass index (BMI) is 41, which indicates she is obese. She has always struggled with her weight and has tried diet and lifestyle modifications, as well as medications, for the past 5 years with no success. Her current medication regimen includes lisinopril 40 mg daily, amlodipine 5 mg daily, atorvastatin 40 mg daily, metformin 500 mg twice daily, dulaglutide 0.75 mg weekly, lithium 600 mg daily, venlafaxine extended-release (XR) 150 mg daily, and alprazolam 0.5 mg as needed up to twice daily. Due to Ms. B’s BMI and because she has ≥1 comorbid health condition, her primary care physician refers her to a gastroenterologist to discuss gastric bypass surgery options.
Ms. B is scheduled for Roux-en-Y gastric bypass surgery. You need to determine if any changes should be made to her psychotropic medications after she undergoes this surgery.
There are multiple types of bariatric surgeries, including Roux-en-Y gastric bypass, sleeve gastrectomy, laparoscopic adjustable gastric band, and biliopancreatic diversion with duodenal switch (BPD/DS) (Figure1-4). These procedures all restrict the stomach’s capacity to hold food. In most cases, they also bypass areas of absorption in the intestine and cause increased secretion of hormones in the gut, including (but not limited to) peptide-YY (PYY) and glucagon-like peptide 1 (GLP-1). These hormonal changes impact several factors, including satiety, hunger, and blood sugar levels.5

Roux-en-Y is commonly referred to as the gold standard of weight loss surgery. It divides the top of the stomach into a smaller stomach pouch that connects directly to the small intestine to facilitate smaller meals and alters the release of gut hormones. Additionally, a segment of the small intestine that normally absorbs nutrients and medications is completely bypassed. In contrast, the sleeve gastrectomy removes approximately 80% of the stomach, consequently reducing the amount of food that can be consumed. The greatest impact of the sleeve gastrectomy procedure appears to result from changes in gut hormones. The adjustable gastric band procedure works by placing a band around the upper portion of the stomach to create a small pouch above the band to satisfy hunger with a smaller amount of food. Lastly, BPD/DS is a procedure that creates a tubular stomach pouch and bypasses a large portion of the small intestine. Like the gastric bypass and sleeve gastrectomy, BPD/DS affects gut hormones impacting hunger, satiety, and blood sugar control.
How bariatric surgery can affect drug absorption
As illustrated in the Table,6-19 each type of bariatric surgery may impact drug absorption differently depending on the mechanism by which the stomach is restricted.

Drug malabsorption is a concern for clinicians with patients who have undergone bariatric surgery. There is limited research measuring changes in psychotropic exposure and outcomes following bariatric surgery. A 2009 literature review by Padwal et al7 found that one-third of the 26 studies evaluated provided evidence of decreased absorption following bariatric surgery in patients taking medications that had intrinsic poor absorption, high lipophilicity, and/or undergo enterohepatic recirculation. In a review that included a small study of patients taking selective serotonin reuptake inhibitors or venlafaxine, Godini et al8 demonstrated that although there was a notable decrease in drug absorption closely following the surgery, drug absorption recovered for some patients 1 month after Roux-en-Y surgery. These reviews suggest patients who have undergone any form of bariatric surgery must be observed closely because drug absorption may vary based on the individual, the medication administered, and the amount of time postprocedure.
Until more research becomes available, current evidence supports recommendations to assist patients who have a decreased ability to absorb medications after gastric bypass surgery by switching from an extended-release formulation to an immediate-release or solution formulation. This allows patients to rely less on gastric mixing and unpredictable changes in drug release from extended- or controlled-release formulations.
Continue to: Aside from altered...
Aside from altered pharmacokinetics after bariatric surgery, many patients experience an increased risk of self-harm and suicide.20 Therefore, a continued emphasis on and reinforcement of proper antidepressant use and adjustment in these patients is important. This can be facilitated through frequent follow-up visits, either in-person or via telehealth.
Understanding the effect of bariatric surgery on drug absorption is critical to identifying a potential need to adjust a medication dose or formulation after the surgery. Available evidence and data suggest it is reasonable to switch from an extended- or sustained-release formulation to an immediate-release formulation, and to monitor patients more frequently immediately following the surgery.
CASE CONTINUED
Related Resources
- Colvin C, Tsia W, Silverman AL, et al. Nothing up his sleeve: decompensation after bariatric surgery. Current Psychiatry. 2021;20(4):15-19. doi:10.12788/cp.010
Drug Brand Names
Alprazolam • Xanax
Amlodipine • Norvasc
Atorvastatin • Lipitor
Dulaglutide • Trulicity
Lisinopril • Zestril, Prinivil
Lithium • Eskalith, Lithobid
Metformin • Glucophage
Olanzapine • Zyprexa
Venlafaxine • Effexor
1. Obesity Treatments: Gastric Bypass Surgery. UCLA Health. Accessed April 4, 2021. http://surgery.ucla.edu/bariatrics-gastric-bypass
2. Thomas L. Gastric bypass more likely to require further treatment than gastric sleeve. News Medical. January 15, 2020. Accessed April 4, 2021. https://www.news-medical.net/news/20200115/Gastric-bypass-more-likely-to-require-further-treatment-than-gastric-sleeve.aspx
3. Lap Adjustable Gastric Banding. Laser Stone Surgery & Endoscopy Centre. September 5, 2016. Accessed April 4, 2021. http://www.laserstonesurgery.org/project/lap-adjustable-gastric-banding/
4. BPD/DS Weight-Loss Surgery. Johns Hopkins Medicine. Accessed April 4, 2021. https://www.hopkinsmedicine.org/health/treatment-tests-and-therapies/bpdds-weightloss-surgery
5. Holst JJ, Madsbad S, Bojsen-Møller KN, et al. Mechanisms in bariatric surgery: gut hormones, diabetes resolution, and weight loss. Surg Obes Relat Dis. 2018;14(5):708-714. doi:10.1016/j.soard.2018.03.003
6. Public Education Committee. Bariatric Surgery Procedures. American Society for Metabolic and Bariatric Surgery. Updated May 2021. Accessed September 4, 2021. https://asmbs.org/patients/bariatric-surgery-procedures
7. Padwal R, Brocks D, Sharma AM. A systematic review of drug absorption following bariatric surgery and its theoretical implications. Obes Rev. 2010;11(1):41-50. doi:10.1111/j.1467-789x.2009.00614.x
8. Godini L, Castellini G, Facchiano E, et al. Mood disorders and bariatric surgery patients: pre- and post- surgery clinical course- an overview. J Obes Weight Loss Medicat. 2016;2(1). doi:10.23937/2572-4010.1510012
9. Smith A, Henriksen B, Cohen A. Pharmacokinetic considerations in Roux-en-Y gastric bypass patients. Am J Health Syst Pharm. 2011;68(23):2241-2247. doi:10.2146/ajhp100630
10. Brocks DR, Ben-Eltriki M, Gabr RQ, et al. The effects of gastric bypass surgery on drug absorption and pharmacokinetics. Expert Opin Drug Metab Toxicol. 2012;8(12):1505-1519. doi:10.1517/17425255.2012.722757
11. Hamad GG, Helsel JC, Perel JM, et al. The effect of gastric bypass on the pharmacokinetics of serotonin reuptake inhibitors. Am J Psychiatry. 2012;169(3):256-263. doi:10.1176/appi.ajp.2011.11050719
12. Angeles PC, Robertsen I, Seeberg LT, et al. The influence of bariatric surgery on oral drug bioavailability in patients with obesity: a systematic review. Obes Rev. 2019;20(9):1299-1311. doi:10.1111/obr.12869
13. Laparoscopic Sleeve Gastrectomy. University of California San Francisco Department of Surgery. Accessed April 1, 2021. https://surgery.ucsf.edu/conditions--procedures/laparoscopic-sleeve-gastrectomy.aspx
14. Brethauer S, Schauer P. Laparoscopic Sleeve Gastrectomy: A Newcomer to Bariatric Surgery. Obesity Action Coalition. 2007. Accessed May 15, 2021. https://www.obesityaction.org/community/article-library/laparoscopic-sleeve-gastrectomy-a-newcomer-to-bariatric-surgery/
15. Roerig JL, Steffen K. Psychopharmacology and bariatric surgery. Eur Eat Disord Rev. 2015;23(6):463-469. doi:10.1002/erv.2396
16. Bland CM, Quidley AM, Love BL, et al. Long-term pharmacotherapy considerations in the bariatric surgery patient. A J Health Syst Pharm. 2016;73(16):1230-1242. doi:10.2146/ajhp151062
17. Lin YH, Liu SW, Wu HL, et al. Lithium toxicity with prolonged neurologic sequelae following sleeve gastrectomy: a case report and review of literature. Medicine (Baltimore). 2020;99(28):e21122. doi:10.1097/MD.0000000000021122
18. Lorico S, Colton B. Medication management and pharmacokinetic changes after bariatric surgery. Can Fam Physician. 2020;66(6):409-416.
19. Homan J, Schijns W, Aarts EO, et al. Treatment of vitamin and mineral deficiencies after biliopancreatic diversion with or without duodenal switch: a major challenge. Obes Surg. 2018;28(1):234-241. doi:10.1007/s11695-017-2841-0
20. Neovius M, Bruze G, Jacobson P, et al. Risk of suicide and non-fatal self-harm after bariatric surgery: results from two matched cohort studies. Lancet Diabetes Endocrinol. 2018;6(3):197-207. doi:10.1016/S2213-8587(17)30437-0
1. Obesity Treatments: Gastric Bypass Surgery. UCLA Health. Accessed April 4, 2021. http://surgery.ucla.edu/bariatrics-gastric-bypass
2. Thomas L. Gastric bypass more likely to require further treatment than gastric sleeve. News Medical. January 15, 2020. Accessed April 4, 2021. https://www.news-medical.net/news/20200115/Gastric-bypass-more-likely-to-require-further-treatment-than-gastric-sleeve.aspx
3. Lap Adjustable Gastric Banding. Laser Stone Surgery & Endoscopy Centre. September 5, 2016. Accessed April 4, 2021. http://www.laserstonesurgery.org/project/lap-adjustable-gastric-banding/
4. BPD/DS Weight-Loss Surgery. Johns Hopkins Medicine. Accessed April 4, 2021. https://www.hopkinsmedicine.org/health/treatment-tests-and-therapies/bpdds-weightloss-surgery
5. Holst JJ, Madsbad S, Bojsen-Møller KN, et al. Mechanisms in bariatric surgery: gut hormones, diabetes resolution, and weight loss. Surg Obes Relat Dis. 2018;14(5):708-714. doi:10.1016/j.soard.2018.03.003
6. Public Education Committee. Bariatric Surgery Procedures. American Society for Metabolic and Bariatric Surgery. Updated May 2021. Accessed September 4, 2021. https://asmbs.org/patients/bariatric-surgery-procedures
7. Padwal R, Brocks D, Sharma AM. A systematic review of drug absorption following bariatric surgery and its theoretical implications. Obes Rev. 2010;11(1):41-50. doi:10.1111/j.1467-789x.2009.00614.x
8. Godini L, Castellini G, Facchiano E, et al. Mood disorders and bariatric surgery patients: pre- and post- surgery clinical course- an overview. J Obes Weight Loss Medicat. 2016;2(1). doi:10.23937/2572-4010.1510012
9. Smith A, Henriksen B, Cohen A. Pharmacokinetic considerations in Roux-en-Y gastric bypass patients. Am J Health Syst Pharm. 2011;68(23):2241-2247. doi:10.2146/ajhp100630
10. Brocks DR, Ben-Eltriki M, Gabr RQ, et al. The effects of gastric bypass surgery on drug absorption and pharmacokinetics. Expert Opin Drug Metab Toxicol. 2012;8(12):1505-1519. doi:10.1517/17425255.2012.722757
11. Hamad GG, Helsel JC, Perel JM, et al. The effect of gastric bypass on the pharmacokinetics of serotonin reuptake inhibitors. Am J Psychiatry. 2012;169(3):256-263. doi:10.1176/appi.ajp.2011.11050719
12. Angeles PC, Robertsen I, Seeberg LT, et al. The influence of bariatric surgery on oral drug bioavailability in patients with obesity: a systematic review. Obes Rev. 2019;20(9):1299-1311. doi:10.1111/obr.12869
13. Laparoscopic Sleeve Gastrectomy. University of California San Francisco Department of Surgery. Accessed April 1, 2021. https://surgery.ucsf.edu/conditions--procedures/laparoscopic-sleeve-gastrectomy.aspx
14. Brethauer S, Schauer P. Laparoscopic Sleeve Gastrectomy: A Newcomer to Bariatric Surgery. Obesity Action Coalition. 2007. Accessed May 15, 2021. https://www.obesityaction.org/community/article-library/laparoscopic-sleeve-gastrectomy-a-newcomer-to-bariatric-surgery/
15. Roerig JL, Steffen K. Psychopharmacology and bariatric surgery. Eur Eat Disord Rev. 2015;23(6):463-469. doi:10.1002/erv.2396
16. Bland CM, Quidley AM, Love BL, et al. Long-term pharmacotherapy considerations in the bariatric surgery patient. A J Health Syst Pharm. 2016;73(16):1230-1242. doi:10.2146/ajhp151062
17. Lin YH, Liu SW, Wu HL, et al. Lithium toxicity with prolonged neurologic sequelae following sleeve gastrectomy: a case report and review of literature. Medicine (Baltimore). 2020;99(28):e21122. doi:10.1097/MD.0000000000021122
18. Lorico S, Colton B. Medication management and pharmacokinetic changes after bariatric surgery. Can Fam Physician. 2020;66(6):409-416.
19. Homan J, Schijns W, Aarts EO, et al. Treatment of vitamin and mineral deficiencies after biliopancreatic diversion with or without duodenal switch: a major challenge. Obes Surg. 2018;28(1):234-241. doi:10.1007/s11695-017-2841-0
20. Neovius M, Bruze G, Jacobson P, et al. Risk of suicide and non-fatal self-harm after bariatric surgery: results from two matched cohort studies. Lancet Diabetes Endocrinol. 2018;6(3):197-207. doi:10.1016/S2213-8587(17)30437-0
Smoking cessation: Varenicline and the risk of neuropsychiatric adverse events
Mr. T, age 34, is a veteran who recently returned to civilian life. He presents to his local Veteran Affairs facility for transition of care. During active duty, he had been diagnosed with obstructive sleep apnea, tobacco use disorder, posttraumatic stress disorder (PTSD) secondary to combat exposure, and insomnia. Mr. T says he wants to quit smoking; currently, he smokes 2 packs of cigarettes per day. The primary care clinician notes that Mr. T has uncontrolled PTSD symptoms and poor sleep, and refers him for an outpatient mental health appointment.
At the mental health appointment 3 weeks later, Mr. T asks about medications to quit smoking, specifically varenicline (Table 11). Mr. T’s PTSD Checklist for DSM-5 score is 52, which indicates severe PTSD symptomatology. He says he sees shadowy figures in his periphery every day, and worries they are spying on him. His wife reports Mr. T has had these symptoms for most of their 10-year marriage but has never been treated for them. After a discussion with the outpatient team, Mr. T says he is willing to engage in exposure therapy for PTSD, but he does not want to take any medications other than varenicline for smoking cessation.
Cigarette smoke is a known carcinogen and risk factor for the development of cardiovascular and respiratory diseases and other comorbidities. People with severe mental illness (SMI) are 3 to 5 times more likely to smoke, and they often face multiple barriers to cessation, including low socioeconomic status and lack of support.2 Even when patients with SMI are provided appropriate behavioral and pharmacologic interventions, they often require more frequent monitoring and counseling, receive a longer duration of drug therapy, and experience lower smoking cessation rates than the general population.2
Current guidelines recommend nicotine replacement therapy (NRT), bupropion, varenicline, and behavioral support as first-line therapies for smoking cessation in patients with and without SMI.2 Evidence suggests that varenicline is more effective than other pharmacologic options; however, in 2009 a black-box warning was added to both varenicline and bupropion to highlight an increased risk of neuropsychiatric events in individuals with SMI.2 This led some clinicians to hesitate to prescribe varenicline or bupropion to patients with psychiatric illness. However, in 2016, the EAGLES trial evaluated the safety of varenicline, bupropion, and NRT in smokers with and without psychiatric disorders, and based on the findings, the black-box warning was removed.2
This article reviews the evidence regarding the use of varenicline and the risk of neuropsychiatric adverse events in patients with psychiatric illness. Table 23-6 provides a summary of each varenicline trial we discuss.
The EAGLES trial
EAGLES was a multicenter, multinational, randomized, double-blind, triple-dummy, placebo- and active-controlled trial of 8,144 individuals who received treatment for smoking cessation.3 The primary endpoint was the incidence of a composite measure of moderate to severe neuropsychiatric events (NPSAEs).3 Participants were split into psychiatric (N = 4,116) and nonpsychiatric (N = 4,028) cohorts and randomized into 4 treatment arms: varenicline 1 mg twice a day, bupropion 150 mg twice a day, nicotine patch 21 mg/d with taper, or placebo, all for 12 weeks with an additional 12 weeks of follow-up. All participants smoked ≥10 cigarettes per day. Individuals in the psychiatric cohort had to be psychiatrically stable (no exacerbations for 6 months and stable treatment for 3 months). Exclusionary diagnoses included psychotic disorders (except schizophrenia and schizoaffective disorder), dementia, substance use (except nicotine), and personality disorders (except borderline personality disorder).2
The rates of moderate to severe NPSAEs in the varenicline groups were 1.25% (95% CI, 0.60 to 1.90) in the nonpsychiatric cohort and 6.42% (95% CI, 4.91 to 7.93) in the psychiatric cohort.3 However, when comparing the varenicline group of the psychiatric cohort to the other arms of the psychiatric cohort, there were no differences (bupropion 6.62% [95% CI, 5.09 to 8.15], nicotine patch 5.20% [95% CI, 3.84 to 6.56], placebo 4.83% [95% CI, 3.51 to 6.16], respectively). The primary efficacy endpoint was continuous abstinence rates (CAR) for Week 9 through Week 12. In the psychiatric cohort, varenicline was superior compared to placebo (odds ratio [OR] 3.24; 95% CI, 2.56 to 4.11), bupropion (OR 1.74; 95% CI, 1.41 to 2.14), and nicotine patch (OR 1.62; 95% CI, 1.32 to 1.99).3
Continue to: Further analysis of EAGLES
Further analysis of EAGLES
Beard et al4 used Bayes factor testing for additional analysis of EAGLES data to determine whether the data were insensitive to neuropsychiatric effects secondary to a lack of statistical power. In the psychiatric cohort, the varenicline and bupropion groups exhibited suggestive but not conclusive data that there was no increase in NPSAEs compared to placebo (Bayes factor 0.52 and 0.71, respectively).4
Another EAGLES analysis by Ayers et al5 evaluated participants with anxiety disorders (N = 712), including PTSD (N = 192), generalized anxiety disorder (GAD) (N = 243), and panic disorder (N = 277).Of those with PTSD who received varenicline, there were no statistically significant differences in CAR from Week 9 to Week 12 vs placebo.5 However, there was a significant difference in individuals with GAD (OR 4.53; 95% CI, 1.20 to 17.10), and panic disorder (OR 8.49; 95% CI, 1.57 to 45.78).5 In contrast to CAR from Week 9 to Week 12, 7-day point prevalence abstinence at Week 12 for participants with PTSD was significant (OR 4.04; 95% CI, 1.39 to 11.74) when comparing varenicline to placebo. Within the anxiety disorder cohort, there were no significant differences in moderate to severe NPSAE rates based on treatment group. Calculated risk differences comparing varenicline to placebo were: PTSD group -7.73 (95% CI, -21.95 to 6.49), GAD group 2.80 (95% CI, -6.63 to 12.23), and panic disorder group -0.18 (95% CI, -9.57 to 9.21).5
Other studies
Evins et al6 conducted a randomized controlled trial to evaluate the safety of varenicline maintenance therapy in patients with schizophrenia or bipolar disorder. To be deemed clinically stable, participants in this study needed to be taking a stable dose of an antipsychotic or mood-stabilizing agent(s) for ≥30 days, compared to the 3-month requirement of the EAGLES trial.3,6 Participants received 12 weeks of open-label varenicline; those who achieved abstinence (N = 87) entered the relapse-prevention phase and were randomized to varenicline 1 mg twice a day or placebo for 40 weeks. Of those who entered relapse-prevention, 5 in the placebo group and 2 in the varenicline group were psychiatrically hospitalized (risk ratio 0.45; 95% CI, 0.04 to 2.9).6 These researchers concluded that varenicline maintenance therapy prolonged abstinence rates with no significant increase in neuropsychiatric events.6
Although treatment options for smoking cessation have advanced, individuals with SMI are still disproportionately affected by the negative outcomes of cigarette smoking. Current literature suggests that varenicline does not confer an appreciable risk of neuropsychiatric events in otherwise stable patients and is the preferred first-line treatment. However, there is a gap in understanding the impact of this medication on individuals with unstable psychiatric illness. Health care professionals should be encouraged to use varenicline with careful monitoring for appropriate patients with psychiatric disorders as a standard of care to help them quit smoking.
CASE CONTINUED
After consulting with the psychiatric pharmacist and discussing the risks and benefits of varenicline, Mr. T is started on the appropriate titration schedule (Table 11). A pharmacist provides varenicline education, including the possibility of psychiatric adverse effects, and tells Mr. T to report any worsening psychiatric symptoms. Mr. T is scheduled for frequent follow-up visits to monitor possible adverse effects and his tobacco use. He says he understands the potential adverse effects of varenicline and agrees to frequent follow-up appointments while taking it.
Related Resources
- Leone FT, Zhang Y, Evers-Casey S, et al. Initiating pharmacologic treatment in tobacco-dependent adults. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;202(2):e5-e31. doi:10.1164/rccm.202005.1982ST
- Cieslak K, Freudenreich O. 4 Ways to help your patients with schizophrenia quit smoking. Current Psychiatry. 2018; 17(2):28,33.
Drug Brand Names
Bupropion • Wellbutrin
Varenicline • Chantix
1. Chantix [package insert]. New York, NY: Pfizer Inc; 2019.
2. Sharma R, Alla K, Pfeffer D, et al. An appraisal of practice guidelines for smoking cessation in people with severe mental illness. Aust N Z J Psychiatry. 2017;51(11):1106-1120. doi:10.1177/0004867417726176
3. Anthenelli RM, Benowitz NL, West R, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet. 2016;387(10037):2507-2520. doi:10.1016/s0140-6736(16)30272-0
4. Beard E, Jackson SE, Anthenelli RM, et al. Estimation of risk of neuropsychiatric adverse events from varenicline, bupropion and nicotine patch versus placebo: secondary analysis of results from the EAGLES trial using Bayes factors. Addiction. 2021;116(10):2816-2824. doi:10.1111/add.15440
5. Ayers CR, Heffner JL, Russ C, et al. Efficacy and safety of pharmacotherapies for smoking cessation in anxiety disorders: subgroup analysis of the randomized, active- and placebo-controlled EAGLES trial. Depress Anxiety. 2020;37(3)247-260. doi:10.1002/da.22982
6. Evins AE, Cather C, Pratt SA, et al. Maintenance treatment with varenicline for smoking cessation in patients with schizophrenia and bipolar disorder: a randomized clinical trial. JAMA. 2014;311(2):145-154. doi:10.1001/jama.2013.285113
Mr. T, age 34, is a veteran who recently returned to civilian life. He presents to his local Veteran Affairs facility for transition of care. During active duty, he had been diagnosed with obstructive sleep apnea, tobacco use disorder, posttraumatic stress disorder (PTSD) secondary to combat exposure, and insomnia. Mr. T says he wants to quit smoking; currently, he smokes 2 packs of cigarettes per day. The primary care clinician notes that Mr. T has uncontrolled PTSD symptoms and poor sleep, and refers him for an outpatient mental health appointment.
At the mental health appointment 3 weeks later, Mr. T asks about medications to quit smoking, specifically varenicline (Table 11). Mr. T’s PTSD Checklist for DSM-5 score is 52, which indicates severe PTSD symptomatology. He says he sees shadowy figures in his periphery every day, and worries they are spying on him. His wife reports Mr. T has had these symptoms for most of their 10-year marriage but has never been treated for them. After a discussion with the outpatient team, Mr. T says he is willing to engage in exposure therapy for PTSD, but he does not want to take any medications other than varenicline for smoking cessation.
Cigarette smoke is a known carcinogen and risk factor for the development of cardiovascular and respiratory diseases and other comorbidities. People with severe mental illness (SMI) are 3 to 5 times more likely to smoke, and they often face multiple barriers to cessation, including low socioeconomic status and lack of support.2 Even when patients with SMI are provided appropriate behavioral and pharmacologic interventions, they often require more frequent monitoring and counseling, receive a longer duration of drug therapy, and experience lower smoking cessation rates than the general population.2
Current guidelines recommend nicotine replacement therapy (NRT), bupropion, varenicline, and behavioral support as first-line therapies for smoking cessation in patients with and without SMI.2 Evidence suggests that varenicline is more effective than other pharmacologic options; however, in 2009 a black-box warning was added to both varenicline and bupropion to highlight an increased risk of neuropsychiatric events in individuals with SMI.2 This led some clinicians to hesitate to prescribe varenicline or bupropion to patients with psychiatric illness. However, in 2016, the EAGLES trial evaluated the safety of varenicline, bupropion, and NRT in smokers with and without psychiatric disorders, and based on the findings, the black-box warning was removed.2
This article reviews the evidence regarding the use of varenicline and the risk of neuropsychiatric adverse events in patients with psychiatric illness. Table 23-6 provides a summary of each varenicline trial we discuss.
The EAGLES trial
EAGLES was a multicenter, multinational, randomized, double-blind, triple-dummy, placebo- and active-controlled trial of 8,144 individuals who received treatment for smoking cessation.3 The primary endpoint was the incidence of a composite measure of moderate to severe neuropsychiatric events (NPSAEs).3 Participants were split into psychiatric (N = 4,116) and nonpsychiatric (N = 4,028) cohorts and randomized into 4 treatment arms: varenicline 1 mg twice a day, bupropion 150 mg twice a day, nicotine patch 21 mg/d with taper, or placebo, all for 12 weeks with an additional 12 weeks of follow-up. All participants smoked ≥10 cigarettes per day. Individuals in the psychiatric cohort had to be psychiatrically stable (no exacerbations for 6 months and stable treatment for 3 months). Exclusionary diagnoses included psychotic disorders (except schizophrenia and schizoaffective disorder), dementia, substance use (except nicotine), and personality disorders (except borderline personality disorder).2
The rates of moderate to severe NPSAEs in the varenicline groups were 1.25% (95% CI, 0.60 to 1.90) in the nonpsychiatric cohort and 6.42% (95% CI, 4.91 to 7.93) in the psychiatric cohort.3 However, when comparing the varenicline group of the psychiatric cohort to the other arms of the psychiatric cohort, there were no differences (bupropion 6.62% [95% CI, 5.09 to 8.15], nicotine patch 5.20% [95% CI, 3.84 to 6.56], placebo 4.83% [95% CI, 3.51 to 6.16], respectively). The primary efficacy endpoint was continuous abstinence rates (CAR) for Week 9 through Week 12. In the psychiatric cohort, varenicline was superior compared to placebo (odds ratio [OR] 3.24; 95% CI, 2.56 to 4.11), bupropion (OR 1.74; 95% CI, 1.41 to 2.14), and nicotine patch (OR 1.62; 95% CI, 1.32 to 1.99).3
Continue to: Further analysis of EAGLES
Further analysis of EAGLES
Beard et al4 used Bayes factor testing for additional analysis of EAGLES data to determine whether the data were insensitive to neuropsychiatric effects secondary to a lack of statistical power. In the psychiatric cohort, the varenicline and bupropion groups exhibited suggestive but not conclusive data that there was no increase in NPSAEs compared to placebo (Bayes factor 0.52 and 0.71, respectively).4
Another EAGLES analysis by Ayers et al5 evaluated participants with anxiety disorders (N = 712), including PTSD (N = 192), generalized anxiety disorder (GAD) (N = 243), and panic disorder (N = 277).Of those with PTSD who received varenicline, there were no statistically significant differences in CAR from Week 9 to Week 12 vs placebo.5 However, there was a significant difference in individuals with GAD (OR 4.53; 95% CI, 1.20 to 17.10), and panic disorder (OR 8.49; 95% CI, 1.57 to 45.78).5 In contrast to CAR from Week 9 to Week 12, 7-day point prevalence abstinence at Week 12 for participants with PTSD was significant (OR 4.04; 95% CI, 1.39 to 11.74) when comparing varenicline to placebo. Within the anxiety disorder cohort, there were no significant differences in moderate to severe NPSAE rates based on treatment group. Calculated risk differences comparing varenicline to placebo were: PTSD group -7.73 (95% CI, -21.95 to 6.49), GAD group 2.80 (95% CI, -6.63 to 12.23), and panic disorder group -0.18 (95% CI, -9.57 to 9.21).5
Other studies
Evins et al6 conducted a randomized controlled trial to evaluate the safety of varenicline maintenance therapy in patients with schizophrenia or bipolar disorder. To be deemed clinically stable, participants in this study needed to be taking a stable dose of an antipsychotic or mood-stabilizing agent(s) for ≥30 days, compared to the 3-month requirement of the EAGLES trial.3,6 Participants received 12 weeks of open-label varenicline; those who achieved abstinence (N = 87) entered the relapse-prevention phase and were randomized to varenicline 1 mg twice a day or placebo for 40 weeks. Of those who entered relapse-prevention, 5 in the placebo group and 2 in the varenicline group were psychiatrically hospitalized (risk ratio 0.45; 95% CI, 0.04 to 2.9).6 These researchers concluded that varenicline maintenance therapy prolonged abstinence rates with no significant increase in neuropsychiatric events.6
Although treatment options for smoking cessation have advanced, individuals with SMI are still disproportionately affected by the negative outcomes of cigarette smoking. Current literature suggests that varenicline does not confer an appreciable risk of neuropsychiatric events in otherwise stable patients and is the preferred first-line treatment. However, there is a gap in understanding the impact of this medication on individuals with unstable psychiatric illness. Health care professionals should be encouraged to use varenicline with careful monitoring for appropriate patients with psychiatric disorders as a standard of care to help them quit smoking.
CASE CONTINUED
After consulting with the psychiatric pharmacist and discussing the risks and benefits of varenicline, Mr. T is started on the appropriate titration schedule (Table 11). A pharmacist provides varenicline education, including the possibility of psychiatric adverse effects, and tells Mr. T to report any worsening psychiatric symptoms. Mr. T is scheduled for frequent follow-up visits to monitor possible adverse effects and his tobacco use. He says he understands the potential adverse effects of varenicline and agrees to frequent follow-up appointments while taking it.
Related Resources
- Leone FT, Zhang Y, Evers-Casey S, et al. Initiating pharmacologic treatment in tobacco-dependent adults. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;202(2):e5-e31. doi:10.1164/rccm.202005.1982ST
- Cieslak K, Freudenreich O. 4 Ways to help your patients with schizophrenia quit smoking. Current Psychiatry. 2018; 17(2):28,33.
Drug Brand Names
Bupropion • Wellbutrin
Varenicline • Chantix
Mr. T, age 34, is a veteran who recently returned to civilian life. He presents to his local Veteran Affairs facility for transition of care. During active duty, he had been diagnosed with obstructive sleep apnea, tobacco use disorder, posttraumatic stress disorder (PTSD) secondary to combat exposure, and insomnia. Mr. T says he wants to quit smoking; currently, he smokes 2 packs of cigarettes per day. The primary care clinician notes that Mr. T has uncontrolled PTSD symptoms and poor sleep, and refers him for an outpatient mental health appointment.
At the mental health appointment 3 weeks later, Mr. T asks about medications to quit smoking, specifically varenicline (Table 11). Mr. T’s PTSD Checklist for DSM-5 score is 52, which indicates severe PTSD symptomatology. He says he sees shadowy figures in his periphery every day, and worries they are spying on him. His wife reports Mr. T has had these symptoms for most of their 10-year marriage but has never been treated for them. After a discussion with the outpatient team, Mr. T says he is willing to engage in exposure therapy for PTSD, but he does not want to take any medications other than varenicline for smoking cessation.
Cigarette smoke is a known carcinogen and risk factor for the development of cardiovascular and respiratory diseases and other comorbidities. People with severe mental illness (SMI) are 3 to 5 times more likely to smoke, and they often face multiple barriers to cessation, including low socioeconomic status and lack of support.2 Even when patients with SMI are provided appropriate behavioral and pharmacologic interventions, they often require more frequent monitoring and counseling, receive a longer duration of drug therapy, and experience lower smoking cessation rates than the general population.2
Current guidelines recommend nicotine replacement therapy (NRT), bupropion, varenicline, and behavioral support as first-line therapies for smoking cessation in patients with and without SMI.2 Evidence suggests that varenicline is more effective than other pharmacologic options; however, in 2009 a black-box warning was added to both varenicline and bupropion to highlight an increased risk of neuropsychiatric events in individuals with SMI.2 This led some clinicians to hesitate to prescribe varenicline or bupropion to patients with psychiatric illness. However, in 2016, the EAGLES trial evaluated the safety of varenicline, bupropion, and NRT in smokers with and without psychiatric disorders, and based on the findings, the black-box warning was removed.2
This article reviews the evidence regarding the use of varenicline and the risk of neuropsychiatric adverse events in patients with psychiatric illness. Table 23-6 provides a summary of each varenicline trial we discuss.
The EAGLES trial
EAGLES was a multicenter, multinational, randomized, double-blind, triple-dummy, placebo- and active-controlled trial of 8,144 individuals who received treatment for smoking cessation.3 The primary endpoint was the incidence of a composite measure of moderate to severe neuropsychiatric events (NPSAEs).3 Participants were split into psychiatric (N = 4,116) and nonpsychiatric (N = 4,028) cohorts and randomized into 4 treatment arms: varenicline 1 mg twice a day, bupropion 150 mg twice a day, nicotine patch 21 mg/d with taper, or placebo, all for 12 weeks with an additional 12 weeks of follow-up. All participants smoked ≥10 cigarettes per day. Individuals in the psychiatric cohort had to be psychiatrically stable (no exacerbations for 6 months and stable treatment for 3 months). Exclusionary diagnoses included psychotic disorders (except schizophrenia and schizoaffective disorder), dementia, substance use (except nicotine), and personality disorders (except borderline personality disorder).2
The rates of moderate to severe NPSAEs in the varenicline groups were 1.25% (95% CI, 0.60 to 1.90) in the nonpsychiatric cohort and 6.42% (95% CI, 4.91 to 7.93) in the psychiatric cohort.3 However, when comparing the varenicline group of the psychiatric cohort to the other arms of the psychiatric cohort, there were no differences (bupropion 6.62% [95% CI, 5.09 to 8.15], nicotine patch 5.20% [95% CI, 3.84 to 6.56], placebo 4.83% [95% CI, 3.51 to 6.16], respectively). The primary efficacy endpoint was continuous abstinence rates (CAR) for Week 9 through Week 12. In the psychiatric cohort, varenicline was superior compared to placebo (odds ratio [OR] 3.24; 95% CI, 2.56 to 4.11), bupropion (OR 1.74; 95% CI, 1.41 to 2.14), and nicotine patch (OR 1.62; 95% CI, 1.32 to 1.99).3
Continue to: Further analysis of EAGLES
Further analysis of EAGLES
Beard et al4 used Bayes factor testing for additional analysis of EAGLES data to determine whether the data were insensitive to neuropsychiatric effects secondary to a lack of statistical power. In the psychiatric cohort, the varenicline and bupropion groups exhibited suggestive but not conclusive data that there was no increase in NPSAEs compared to placebo (Bayes factor 0.52 and 0.71, respectively).4
Another EAGLES analysis by Ayers et al5 evaluated participants with anxiety disorders (N = 712), including PTSD (N = 192), generalized anxiety disorder (GAD) (N = 243), and panic disorder (N = 277).Of those with PTSD who received varenicline, there were no statistically significant differences in CAR from Week 9 to Week 12 vs placebo.5 However, there was a significant difference in individuals with GAD (OR 4.53; 95% CI, 1.20 to 17.10), and panic disorder (OR 8.49; 95% CI, 1.57 to 45.78).5 In contrast to CAR from Week 9 to Week 12, 7-day point prevalence abstinence at Week 12 for participants with PTSD was significant (OR 4.04; 95% CI, 1.39 to 11.74) when comparing varenicline to placebo. Within the anxiety disorder cohort, there were no significant differences in moderate to severe NPSAE rates based on treatment group. Calculated risk differences comparing varenicline to placebo were: PTSD group -7.73 (95% CI, -21.95 to 6.49), GAD group 2.80 (95% CI, -6.63 to 12.23), and panic disorder group -0.18 (95% CI, -9.57 to 9.21).5
Other studies
Evins et al6 conducted a randomized controlled trial to evaluate the safety of varenicline maintenance therapy in patients with schizophrenia or bipolar disorder. To be deemed clinically stable, participants in this study needed to be taking a stable dose of an antipsychotic or mood-stabilizing agent(s) for ≥30 days, compared to the 3-month requirement of the EAGLES trial.3,6 Participants received 12 weeks of open-label varenicline; those who achieved abstinence (N = 87) entered the relapse-prevention phase and were randomized to varenicline 1 mg twice a day or placebo for 40 weeks. Of those who entered relapse-prevention, 5 in the placebo group and 2 in the varenicline group were psychiatrically hospitalized (risk ratio 0.45; 95% CI, 0.04 to 2.9).6 These researchers concluded that varenicline maintenance therapy prolonged abstinence rates with no significant increase in neuropsychiatric events.6
Although treatment options for smoking cessation have advanced, individuals with SMI are still disproportionately affected by the negative outcomes of cigarette smoking. Current literature suggests that varenicline does not confer an appreciable risk of neuropsychiatric events in otherwise stable patients and is the preferred first-line treatment. However, there is a gap in understanding the impact of this medication on individuals with unstable psychiatric illness. Health care professionals should be encouraged to use varenicline with careful monitoring for appropriate patients with psychiatric disorders as a standard of care to help them quit smoking.
CASE CONTINUED
After consulting with the psychiatric pharmacist and discussing the risks and benefits of varenicline, Mr. T is started on the appropriate titration schedule (Table 11). A pharmacist provides varenicline education, including the possibility of psychiatric adverse effects, and tells Mr. T to report any worsening psychiatric symptoms. Mr. T is scheduled for frequent follow-up visits to monitor possible adverse effects and his tobacco use. He says he understands the potential adverse effects of varenicline and agrees to frequent follow-up appointments while taking it.
Related Resources
- Leone FT, Zhang Y, Evers-Casey S, et al. Initiating pharmacologic treatment in tobacco-dependent adults. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;202(2):e5-e31. doi:10.1164/rccm.202005.1982ST
- Cieslak K, Freudenreich O. 4 Ways to help your patients with schizophrenia quit smoking. Current Psychiatry. 2018; 17(2):28,33.
Drug Brand Names
Bupropion • Wellbutrin
Varenicline • Chantix
1. Chantix [package insert]. New York, NY: Pfizer Inc; 2019.
2. Sharma R, Alla K, Pfeffer D, et al. An appraisal of practice guidelines for smoking cessation in people with severe mental illness. Aust N Z J Psychiatry. 2017;51(11):1106-1120. doi:10.1177/0004867417726176
3. Anthenelli RM, Benowitz NL, West R, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet. 2016;387(10037):2507-2520. doi:10.1016/s0140-6736(16)30272-0
4. Beard E, Jackson SE, Anthenelli RM, et al. Estimation of risk of neuropsychiatric adverse events from varenicline, bupropion and nicotine patch versus placebo: secondary analysis of results from the EAGLES trial using Bayes factors. Addiction. 2021;116(10):2816-2824. doi:10.1111/add.15440
5. Ayers CR, Heffner JL, Russ C, et al. Efficacy and safety of pharmacotherapies for smoking cessation in anxiety disorders: subgroup analysis of the randomized, active- and placebo-controlled EAGLES trial. Depress Anxiety. 2020;37(3)247-260. doi:10.1002/da.22982
6. Evins AE, Cather C, Pratt SA, et al. Maintenance treatment with varenicline for smoking cessation in patients with schizophrenia and bipolar disorder: a randomized clinical trial. JAMA. 2014;311(2):145-154. doi:10.1001/jama.2013.285113
1. Chantix [package insert]. New York, NY: Pfizer Inc; 2019.
2. Sharma R, Alla K, Pfeffer D, et al. An appraisal of practice guidelines for smoking cessation in people with severe mental illness. Aust N Z J Psychiatry. 2017;51(11):1106-1120. doi:10.1177/0004867417726176
3. Anthenelli RM, Benowitz NL, West R, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet. 2016;387(10037):2507-2520. doi:10.1016/s0140-6736(16)30272-0
4. Beard E, Jackson SE, Anthenelli RM, et al. Estimation of risk of neuropsychiatric adverse events from varenicline, bupropion and nicotine patch versus placebo: secondary analysis of results from the EAGLES trial using Bayes factors. Addiction. 2021;116(10):2816-2824. doi:10.1111/add.15440
5. Ayers CR, Heffner JL, Russ C, et al. Efficacy and safety of pharmacotherapies for smoking cessation in anxiety disorders: subgroup analysis of the randomized, active- and placebo-controlled EAGLES trial. Depress Anxiety. 2020;37(3)247-260. doi:10.1002/da.22982
6. Evins AE, Cather C, Pratt SA, et al. Maintenance treatment with varenicline for smoking cessation in patients with schizophrenia and bipolar disorder: a randomized clinical trial. JAMA. 2014;311(2):145-154. doi:10.1001/jama.2013.285113
Sublingual buprenorphine plus buprenorphine XR for opioid use disorder
Mr. L, age 31, presents to the emergency department (ED) with somnolence after sustaining an arm laceration at work. While in the ED, Mr. L explains he has opioid use disorder (OUD) and last week received an initial 300 mg injection of extended-release buprenorphine (BUP-XR). Due to ongoing opioid cravings, he took nonprescribed fentanyl and alprazolam before work.
The ED clinicians address Mr. L’s arm injury and transfer him to the hospital’s low-threshold outpatient addiction clinic for further assessment and management. There, he is prescribed sublingual buprenorphine/naloxone (SL-BUP) 8 mg/2 mg daily as needed for 1 week to address ongoing opioid cravings, and is encouraged to return for another visit the following week.
The United States continues to struggle with the overdose crisis, largely fueled by illicitly manufactured opioids such as fentanyl.1 Opioid agonist and partial agonist treatments such as methadone and buprenorphine decrease the risk of death in individuals with OUD by up to 50%.2 While methadone has a history of proven effectiveness for OUD, accessibility is fraught with barriers (eg, patients must attend an opioid treatment program daily to receive a dose, pharmacies are unable to dispense methadone for OUD).
Buprenorphine has been shown to decrease opioid cravings while limiting euphoria due to its partial—as opposed to full—agonist activity.3 Several buprenorphine formulations are available (Table). Buprenorphine presents an opportunity to treat OUD like other chronic illnesses. In accordance with the US Department of Health and Human Services Practice Guideline (2021), any clinician can obtain a waiver to prescribe buprenorphine in any treatment setting, and patients can receive the medication at a pharmacy.4
However, many patients have barriers to consistent daily dosing of buprenorphine due to strict clinic/prescriber requirements, transportation difficulties, continued cravings, and other factors. BUP-XR, a buprenorphine injection administered once a month, may address several of these concerns, most notably the potential for better suppression of cravings by delivering a consistent level of buprenorphine over the course of 28 days.5 Since BUP-XR was FDA-approved in 2017, questions remain whether it can adequately quell opioid cravings in early treatment months prior to steady-state concentration.
This article addresses whether clinicians should consider supplemental SL-BUP in addition to BUP-XR during early treatment months and/or prior to steady-state.
Pharmacokinetics of BUP-XR
BUP-XR is administered by subcutaneous injection via an ATRIGEL delivery system (BUP-XR; Albany Molecular Research, Burlington, Massachusetts).6 Upon injection, approximately 7% of the buprenorphine dose dissipates with the solvent, leading to maximum concentration approximately 24 hours post-dose. The remaining dose hardens to create a depot that elutes buprenorphine gradually over 28 days.7
Continue to: Buprenorphine requires...
Buprenorphine requires ≥70% mu-opioid receptor (MOR) occupancy to effectively suppress symptoms of craving and withdrawal in patients with OUD. Buprenorphine serum concentration correlates significantly with MOR occupancy, such that concentrations of 2 to 3 ng/mL are acknowledged as baseline minimums for clinical efficacy.8
BUP-XR is administered in 1 of 2 dosing regimens. In both, 2 separate 300 mg doses are administered 28 days apart during Month 1 and Month 2, followed by maintenance doses of either 300 mg (300/300 mg dosing regimen) or 100 mg (300/100 mg dosing regimen) every 28 days thereafter. Combined Phase II and Phase III data analyzing serum concentrations of BUP-XR across both dosing regimens revealed that, for most patients, there is a noticeable period during Month 1 and Month 2 when serum concentrations fall below 2 ng/mL.7 Steady-state concentrations of both regimens develop after 4 to 6 appropriately timed injections, providing average steady-state serum concentrations in Phase II and Phase III trials of 6.54 ng/mL for the 300/300 mg dosing regimen and 3.00 ng/mL for 300/100 mg dosing regimen.7
Real-world experiences with BUP-XR
The theoretical need for supplementation has been voiced in practice. A case series by Peckham et al9 noted that 55% (n = 22) of patients required SL-BUP supplementation for up to 120 days after the first BUP-XR injection to quell cravings and reduce nonprescribed opioid use.
The RECOVER trial by Ling et al10 demonstrated the importance of the first 2 months of BUP-XR therapy in the overall treatment success for patients with OUD. In this analysis, patients maintained on BUP-XR for 12 months reported a 75% likelihood of abstinence, compared to 24% for patients receiving 0 to 2 months of BUP-XR treatment. Other benefits included improved employment status and reduced depression rates. This trial did not specifically discuss supplemental SL-BUP or subthreshold concentrations of buprenorphine during early months.10
Individualized treatment should be based on OUD symptoms
While BUP-XR was designed to continuously deliver at least 2 ng/mL of buprenorphine, serum concentrations are labile during the first 2 months of treatment. This may result in breakthrough OUD symptoms, particularly withdrawal or opioid cravings. Additionally, due to individual variability, some patients may still experience serum concentrations below 2 ng/mL after Month 2 and until steady-state is achieved between Month 4 and Month 6.7
Continue to: Beyond a theoretical...
Beyond a theoretical need for supplementation with SL-BUP, there is limited information regarding optimal dosing, dosage intervals, or length of supplementation. Therefore, clear guidance is not available at this time, and treatment should be individualized based on subjective and objective OUD symptoms.
What also remains unknown are potential barriers patients may face in receiving 2 concurrent buprenorphine prescriptions. BUP-XR, administered in a health care setting, can be obtained 2 ways. A clinician can directly order the medication from the distributor to be administered via buy-and-bill. An alternate option requires the clinician to send a prescription to an appropriately credentialed pharmacy that will ship patient-specific orders directly to the clinic. Despite this, most SL-BUP prescriptions are billed and dispensed from community pharmacies. At the insurance level, there is risk the prescription claim will be rejected for duplication of therapy, which may require additional collaboration between the prescribing clinician, pharmacist, and insurance representative to ensure patients have access to the medication.
Pending studies and approvals may also provide greater guidance and flexibility in decision-making for patients with OUD. The CoLAB study currently underway in Australia is examining the efficacy and outcomes of an intermediate dose (200 mg) of BUP-XR and will also allow for supplemental SL-BUP doses.11 Additionally, an alternative BUP-XR formulation, Brixadi, currently in use in the European Union as Buvidal, has submitted an application for FDA approval in the United States. The application indicates that Brixadi will be available with a wider range of doses and at both weekly and monthly intervals. Approval has been delayed due to deficiencies in the United States–based third-party production facilities. It is unclear how the FDA and manufacturer plan to proceed.12
Short-term supplementation with SL-BUP during early the months of treatment with BUP-XR should be considered to control OUD symptoms and assist with patient retention. Once steady-state is achieved, trough concentrations of buprenorphine are not expected to drop below 2 ng/mL with continued on-time maintenance doses and thus, supplementation can likely cease.
CASE CONTINUED
Mr. L is seen in the low-threshold outpatient clinic 1 week after his ED visit. His arm laceration is healing well, and he is noticeably more alert and engaged. Each morning this week, he awakes with cravings, sweating, and anxiety. These symptoms alleviate after he takes SL-BUP. Mr. L’s clinician gives him a copy of the Subjective Opioid Withdrawal Scale so he can assess his withdrawal symptoms each morning and provide this data at follow-up appointments. Mr. L and his clinician decide to meet weekly until his next injection to continue assessing his current supplemental dose, symptoms, and whether there should be additional adjustments to his treatment plan.
Related Resources
- Cho J, Bhimani J, Patel M, et al. Substance abuse among older adults: a growing problem. Current Psychiatry. 2018;17(3):14-20.
- Verma S. Opioid use disorder in adolescents: an overview. Current Psychiatry. 2020;19(2):12-14,16-21.
Drug Brand Names
Alprazolam • Xanax
Buprenorphine • Sublocade, Subutex
Buprenorphine/naloxone • Suboxone, Zubsolv
Methadone • Methadose
1. Mattson CL, Tanz LJ, Quinn K, et al. Trends and geographic patterns in drug and synthetic opioid overdose deaths - United States, 2013-2019. MMWR Morb Mortal Wkly Rep. 2021;70(6):202-207. doi:10.15585/mmwr.mm7006a4
2. Ma J, Bao YP, Wang RJ, et al. Effects of medication-assisted treatment on mortality among opioids users: a systematic review and meta-analysis. Mol Psychiatry. 2019;24(12):1868-1883. doi:10.1038/s41380-018-0094-5
3. Coe MA, Lofwall MR, Walsh SL. Buprenorphine pharmacology review: update on transmucosal and long-acting formulations. J Addict Med. 2019;13(2):93-103. doi:10.1097/ADM.0000000000000457
4. Becerra X. Practice Guidelines for the Administration of Buprenorphine for Treating Opioid Use Disorder. US Dept of Health and Human Services; 2021:22439-22440. FR Document 2021-08961. Accessed April 5, 2021. https://www.federalregister.gov/documents/2021/04/28/2021-08961/practice-guidelines-for-the-administration-of-buprenorphine-for-treating-opioid-use-disorder
5. Haight BR, Learned SM, Laffont CM, et al. Efficacy and safety of a monthly buprenorphine depot injection for opioid use disorder: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2019;393(10173):778-790. doi:10.1016/S0140-6736(18)32259-1
6. Sublocade [package insert]. North Chesterfield, VA: Indivior Inc; 2021.
7. Jones AK, Ngaimisi E, Gopalakrishnan M, et al. Population pharmacokinetics of a monthly buprenorphine depot injection for the treatment of opioid use disorder: a combined analysis of phase II and phase III trials. Clin Pharmacokinet. 2021;60(4):527-540. doi:10.1007/s40262-020-00957-0
8. Greenwald MK, Comer SD, Fiellin DA. Buprenorphine maintenance and mu-opioid receptor availability in the treatment of opioid use disorder: implications for clinical use and policy. Drug Alcohol Depend. 2014;144:1-11. doi:10.1016/j.drugalcdep.2014.07.035
9. Peckham AM, Kehoe LG, Gray JR, et al. Real-world outcomes with extended-release buprenorphine (XR-BUP) in a low threshold bridge clinic: a retrospective case series. J Subst Abuse Treat. 2021;126:108316. doi:10.1016/j.jsat.2021.108316
10. Ling W, Nadipelli VR, Aldridge AP, et al. Recovery from opioid use disorder (OUD) after monthly long-acting buprenorphine treatment: 12-month longitudinal outcomes from RECOVER, an observational study. J Addict Med. 2020;14(5):e233-e240. doi:10.1097/ADM.0000000000000647
11. Larance B, Byrne M, Lintzeris N, et al. Open-label, multicentre, single-arm trial of monthly injections of depot buprenorphine in people with opioid dependence: protocol for the CoLAB study. BMJ Open. 2020;10(7):e034389. doi:10.1136/bmjopen-2019-034389
12. Braeburn receives new Complete Response Letter for Brixadi in the US. News release. News Powered by Cision. December 15, 2021. Accessed April 13, 2022. https://news.cision.com/camurus-ab/r/braeburn-receives-new-complete-response-letter-for-brixadi-in-the-us,c3473281
Mr. L, age 31, presents to the emergency department (ED) with somnolence after sustaining an arm laceration at work. While in the ED, Mr. L explains he has opioid use disorder (OUD) and last week received an initial 300 mg injection of extended-release buprenorphine (BUP-XR). Due to ongoing opioid cravings, he took nonprescribed fentanyl and alprazolam before work.
The ED clinicians address Mr. L’s arm injury and transfer him to the hospital’s low-threshold outpatient addiction clinic for further assessment and management. There, he is prescribed sublingual buprenorphine/naloxone (SL-BUP) 8 mg/2 mg daily as needed for 1 week to address ongoing opioid cravings, and is encouraged to return for another visit the following week.
The United States continues to struggle with the overdose crisis, largely fueled by illicitly manufactured opioids such as fentanyl.1 Opioid agonist and partial agonist treatments such as methadone and buprenorphine decrease the risk of death in individuals with OUD by up to 50%.2 While methadone has a history of proven effectiveness for OUD, accessibility is fraught with barriers (eg, patients must attend an opioid treatment program daily to receive a dose, pharmacies are unable to dispense methadone for OUD).
Buprenorphine has been shown to decrease opioid cravings while limiting euphoria due to its partial—as opposed to full—agonist activity.3 Several buprenorphine formulations are available (Table). Buprenorphine presents an opportunity to treat OUD like other chronic illnesses. In accordance with the US Department of Health and Human Services Practice Guideline (2021), any clinician can obtain a waiver to prescribe buprenorphine in any treatment setting, and patients can receive the medication at a pharmacy.4
However, many patients have barriers to consistent daily dosing of buprenorphine due to strict clinic/prescriber requirements, transportation difficulties, continued cravings, and other factors. BUP-XR, a buprenorphine injection administered once a month, may address several of these concerns, most notably the potential for better suppression of cravings by delivering a consistent level of buprenorphine over the course of 28 days.5 Since BUP-XR was FDA-approved in 2017, questions remain whether it can adequately quell opioid cravings in early treatment months prior to steady-state concentration.
This article addresses whether clinicians should consider supplemental SL-BUP in addition to BUP-XR during early treatment months and/or prior to steady-state.
Pharmacokinetics of BUP-XR
BUP-XR is administered by subcutaneous injection via an ATRIGEL delivery system (BUP-XR; Albany Molecular Research, Burlington, Massachusetts).6 Upon injection, approximately 7% of the buprenorphine dose dissipates with the solvent, leading to maximum concentration approximately 24 hours post-dose. The remaining dose hardens to create a depot that elutes buprenorphine gradually over 28 days.7
Continue to: Buprenorphine requires...
Buprenorphine requires ≥70% mu-opioid receptor (MOR) occupancy to effectively suppress symptoms of craving and withdrawal in patients with OUD. Buprenorphine serum concentration correlates significantly with MOR occupancy, such that concentrations of 2 to 3 ng/mL are acknowledged as baseline minimums for clinical efficacy.8
BUP-XR is administered in 1 of 2 dosing regimens. In both, 2 separate 300 mg doses are administered 28 days apart during Month 1 and Month 2, followed by maintenance doses of either 300 mg (300/300 mg dosing regimen) or 100 mg (300/100 mg dosing regimen) every 28 days thereafter. Combined Phase II and Phase III data analyzing serum concentrations of BUP-XR across both dosing regimens revealed that, for most patients, there is a noticeable period during Month 1 and Month 2 when serum concentrations fall below 2 ng/mL.7 Steady-state concentrations of both regimens develop after 4 to 6 appropriately timed injections, providing average steady-state serum concentrations in Phase II and Phase III trials of 6.54 ng/mL for the 300/300 mg dosing regimen and 3.00 ng/mL for 300/100 mg dosing regimen.7
Real-world experiences with BUP-XR
The theoretical need for supplementation has been voiced in practice. A case series by Peckham et al9 noted that 55% (n = 22) of patients required SL-BUP supplementation for up to 120 days after the first BUP-XR injection to quell cravings and reduce nonprescribed opioid use.
The RECOVER trial by Ling et al10 demonstrated the importance of the first 2 months of BUP-XR therapy in the overall treatment success for patients with OUD. In this analysis, patients maintained on BUP-XR for 12 months reported a 75% likelihood of abstinence, compared to 24% for patients receiving 0 to 2 months of BUP-XR treatment. Other benefits included improved employment status and reduced depression rates. This trial did not specifically discuss supplemental SL-BUP or subthreshold concentrations of buprenorphine during early months.10
Individualized treatment should be based on OUD symptoms
While BUP-XR was designed to continuously deliver at least 2 ng/mL of buprenorphine, serum concentrations are labile during the first 2 months of treatment. This may result in breakthrough OUD symptoms, particularly withdrawal or opioid cravings. Additionally, due to individual variability, some patients may still experience serum concentrations below 2 ng/mL after Month 2 and until steady-state is achieved between Month 4 and Month 6.7
Continue to: Beyond a theoretical...
Beyond a theoretical need for supplementation with SL-BUP, there is limited information regarding optimal dosing, dosage intervals, or length of supplementation. Therefore, clear guidance is not available at this time, and treatment should be individualized based on subjective and objective OUD symptoms.
What also remains unknown are potential barriers patients may face in receiving 2 concurrent buprenorphine prescriptions. BUP-XR, administered in a health care setting, can be obtained 2 ways. A clinician can directly order the medication from the distributor to be administered via buy-and-bill. An alternate option requires the clinician to send a prescription to an appropriately credentialed pharmacy that will ship patient-specific orders directly to the clinic. Despite this, most SL-BUP prescriptions are billed and dispensed from community pharmacies. At the insurance level, there is risk the prescription claim will be rejected for duplication of therapy, which may require additional collaboration between the prescribing clinician, pharmacist, and insurance representative to ensure patients have access to the medication.
Pending studies and approvals may also provide greater guidance and flexibility in decision-making for patients with OUD. The CoLAB study currently underway in Australia is examining the efficacy and outcomes of an intermediate dose (200 mg) of BUP-XR and will also allow for supplemental SL-BUP doses.11 Additionally, an alternative BUP-XR formulation, Brixadi, currently in use in the European Union as Buvidal, has submitted an application for FDA approval in the United States. The application indicates that Brixadi will be available with a wider range of doses and at both weekly and monthly intervals. Approval has been delayed due to deficiencies in the United States–based third-party production facilities. It is unclear how the FDA and manufacturer plan to proceed.12
Short-term supplementation with SL-BUP during early the months of treatment with BUP-XR should be considered to control OUD symptoms and assist with patient retention. Once steady-state is achieved, trough concentrations of buprenorphine are not expected to drop below 2 ng/mL with continued on-time maintenance doses and thus, supplementation can likely cease.
CASE CONTINUED
Mr. L is seen in the low-threshold outpatient clinic 1 week after his ED visit. His arm laceration is healing well, and he is noticeably more alert and engaged. Each morning this week, he awakes with cravings, sweating, and anxiety. These symptoms alleviate after he takes SL-BUP. Mr. L’s clinician gives him a copy of the Subjective Opioid Withdrawal Scale so he can assess his withdrawal symptoms each morning and provide this data at follow-up appointments. Mr. L and his clinician decide to meet weekly until his next injection to continue assessing his current supplemental dose, symptoms, and whether there should be additional adjustments to his treatment plan.
Related Resources
- Cho J, Bhimani J, Patel M, et al. Substance abuse among older adults: a growing problem. Current Psychiatry. 2018;17(3):14-20.
- Verma S. Opioid use disorder in adolescents: an overview. Current Psychiatry. 2020;19(2):12-14,16-21.
Drug Brand Names
Alprazolam • Xanax
Buprenorphine • Sublocade, Subutex
Buprenorphine/naloxone • Suboxone, Zubsolv
Methadone • Methadose
Mr. L, age 31, presents to the emergency department (ED) with somnolence after sustaining an arm laceration at work. While in the ED, Mr. L explains he has opioid use disorder (OUD) and last week received an initial 300 mg injection of extended-release buprenorphine (BUP-XR). Due to ongoing opioid cravings, he took nonprescribed fentanyl and alprazolam before work.
The ED clinicians address Mr. L’s arm injury and transfer him to the hospital’s low-threshold outpatient addiction clinic for further assessment and management. There, he is prescribed sublingual buprenorphine/naloxone (SL-BUP) 8 mg/2 mg daily as needed for 1 week to address ongoing opioid cravings, and is encouraged to return for another visit the following week.
The United States continues to struggle with the overdose crisis, largely fueled by illicitly manufactured opioids such as fentanyl.1 Opioid agonist and partial agonist treatments such as methadone and buprenorphine decrease the risk of death in individuals with OUD by up to 50%.2 While methadone has a history of proven effectiveness for OUD, accessibility is fraught with barriers (eg, patients must attend an opioid treatment program daily to receive a dose, pharmacies are unable to dispense methadone for OUD).
Buprenorphine has been shown to decrease opioid cravings while limiting euphoria due to its partial—as opposed to full—agonist activity.3 Several buprenorphine formulations are available (Table). Buprenorphine presents an opportunity to treat OUD like other chronic illnesses. In accordance with the US Department of Health and Human Services Practice Guideline (2021), any clinician can obtain a waiver to prescribe buprenorphine in any treatment setting, and patients can receive the medication at a pharmacy.4
However, many patients have barriers to consistent daily dosing of buprenorphine due to strict clinic/prescriber requirements, transportation difficulties, continued cravings, and other factors. BUP-XR, a buprenorphine injection administered once a month, may address several of these concerns, most notably the potential for better suppression of cravings by delivering a consistent level of buprenorphine over the course of 28 days.5 Since BUP-XR was FDA-approved in 2017, questions remain whether it can adequately quell opioid cravings in early treatment months prior to steady-state concentration.
This article addresses whether clinicians should consider supplemental SL-BUP in addition to BUP-XR during early treatment months and/or prior to steady-state.
Pharmacokinetics of BUP-XR
BUP-XR is administered by subcutaneous injection via an ATRIGEL delivery system (BUP-XR; Albany Molecular Research, Burlington, Massachusetts).6 Upon injection, approximately 7% of the buprenorphine dose dissipates with the solvent, leading to maximum concentration approximately 24 hours post-dose. The remaining dose hardens to create a depot that elutes buprenorphine gradually over 28 days.7
Continue to: Buprenorphine requires...
Buprenorphine requires ≥70% mu-opioid receptor (MOR) occupancy to effectively suppress symptoms of craving and withdrawal in patients with OUD. Buprenorphine serum concentration correlates significantly with MOR occupancy, such that concentrations of 2 to 3 ng/mL are acknowledged as baseline minimums for clinical efficacy.8
BUP-XR is administered in 1 of 2 dosing regimens. In both, 2 separate 300 mg doses are administered 28 days apart during Month 1 and Month 2, followed by maintenance doses of either 300 mg (300/300 mg dosing regimen) or 100 mg (300/100 mg dosing regimen) every 28 days thereafter. Combined Phase II and Phase III data analyzing serum concentrations of BUP-XR across both dosing regimens revealed that, for most patients, there is a noticeable period during Month 1 and Month 2 when serum concentrations fall below 2 ng/mL.7 Steady-state concentrations of both regimens develop after 4 to 6 appropriately timed injections, providing average steady-state serum concentrations in Phase II and Phase III trials of 6.54 ng/mL for the 300/300 mg dosing regimen and 3.00 ng/mL for 300/100 mg dosing regimen.7
Real-world experiences with BUP-XR
The theoretical need for supplementation has been voiced in practice. A case series by Peckham et al9 noted that 55% (n = 22) of patients required SL-BUP supplementation for up to 120 days after the first BUP-XR injection to quell cravings and reduce nonprescribed opioid use.
The RECOVER trial by Ling et al10 demonstrated the importance of the first 2 months of BUP-XR therapy in the overall treatment success for patients with OUD. In this analysis, patients maintained on BUP-XR for 12 months reported a 75% likelihood of abstinence, compared to 24% for patients receiving 0 to 2 months of BUP-XR treatment. Other benefits included improved employment status and reduced depression rates. This trial did not specifically discuss supplemental SL-BUP or subthreshold concentrations of buprenorphine during early months.10
Individualized treatment should be based on OUD symptoms
While BUP-XR was designed to continuously deliver at least 2 ng/mL of buprenorphine, serum concentrations are labile during the first 2 months of treatment. This may result in breakthrough OUD symptoms, particularly withdrawal or opioid cravings. Additionally, due to individual variability, some patients may still experience serum concentrations below 2 ng/mL after Month 2 and until steady-state is achieved between Month 4 and Month 6.7
Continue to: Beyond a theoretical...
Beyond a theoretical need for supplementation with SL-BUP, there is limited information regarding optimal dosing, dosage intervals, or length of supplementation. Therefore, clear guidance is not available at this time, and treatment should be individualized based on subjective and objective OUD symptoms.
What also remains unknown are potential barriers patients may face in receiving 2 concurrent buprenorphine prescriptions. BUP-XR, administered in a health care setting, can be obtained 2 ways. A clinician can directly order the medication from the distributor to be administered via buy-and-bill. An alternate option requires the clinician to send a prescription to an appropriately credentialed pharmacy that will ship patient-specific orders directly to the clinic. Despite this, most SL-BUP prescriptions are billed and dispensed from community pharmacies. At the insurance level, there is risk the prescription claim will be rejected for duplication of therapy, which may require additional collaboration between the prescribing clinician, pharmacist, and insurance representative to ensure patients have access to the medication.
Pending studies and approvals may also provide greater guidance and flexibility in decision-making for patients with OUD. The CoLAB study currently underway in Australia is examining the efficacy and outcomes of an intermediate dose (200 mg) of BUP-XR and will also allow for supplemental SL-BUP doses.11 Additionally, an alternative BUP-XR formulation, Brixadi, currently in use in the European Union as Buvidal, has submitted an application for FDA approval in the United States. The application indicates that Brixadi will be available with a wider range of doses and at both weekly and monthly intervals. Approval has been delayed due to deficiencies in the United States–based third-party production facilities. It is unclear how the FDA and manufacturer plan to proceed.12
Short-term supplementation with SL-BUP during early the months of treatment with BUP-XR should be considered to control OUD symptoms and assist with patient retention. Once steady-state is achieved, trough concentrations of buprenorphine are not expected to drop below 2 ng/mL with continued on-time maintenance doses and thus, supplementation can likely cease.
CASE CONTINUED
Mr. L is seen in the low-threshold outpatient clinic 1 week after his ED visit. His arm laceration is healing well, and he is noticeably more alert and engaged. Each morning this week, he awakes with cravings, sweating, and anxiety. These symptoms alleviate after he takes SL-BUP. Mr. L’s clinician gives him a copy of the Subjective Opioid Withdrawal Scale so he can assess his withdrawal symptoms each morning and provide this data at follow-up appointments. Mr. L and his clinician decide to meet weekly until his next injection to continue assessing his current supplemental dose, symptoms, and whether there should be additional adjustments to his treatment plan.
Related Resources
- Cho J, Bhimani J, Patel M, et al. Substance abuse among older adults: a growing problem. Current Psychiatry. 2018;17(3):14-20.
- Verma S. Opioid use disorder in adolescents: an overview. Current Psychiatry. 2020;19(2):12-14,16-21.
Drug Brand Names
Alprazolam • Xanax
Buprenorphine • Sublocade, Subutex
Buprenorphine/naloxone • Suboxone, Zubsolv
Methadone • Methadose
1. Mattson CL, Tanz LJ, Quinn K, et al. Trends and geographic patterns in drug and synthetic opioid overdose deaths - United States, 2013-2019. MMWR Morb Mortal Wkly Rep. 2021;70(6):202-207. doi:10.15585/mmwr.mm7006a4
2. Ma J, Bao YP, Wang RJ, et al. Effects of medication-assisted treatment on mortality among opioids users: a systematic review and meta-analysis. Mol Psychiatry. 2019;24(12):1868-1883. doi:10.1038/s41380-018-0094-5
3. Coe MA, Lofwall MR, Walsh SL. Buprenorphine pharmacology review: update on transmucosal and long-acting formulations. J Addict Med. 2019;13(2):93-103. doi:10.1097/ADM.0000000000000457
4. Becerra X. Practice Guidelines for the Administration of Buprenorphine for Treating Opioid Use Disorder. US Dept of Health and Human Services; 2021:22439-22440. FR Document 2021-08961. Accessed April 5, 2021. https://www.federalregister.gov/documents/2021/04/28/2021-08961/practice-guidelines-for-the-administration-of-buprenorphine-for-treating-opioid-use-disorder
5. Haight BR, Learned SM, Laffont CM, et al. Efficacy and safety of a monthly buprenorphine depot injection for opioid use disorder: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2019;393(10173):778-790. doi:10.1016/S0140-6736(18)32259-1
6. Sublocade [package insert]. North Chesterfield, VA: Indivior Inc; 2021.
7. Jones AK, Ngaimisi E, Gopalakrishnan M, et al. Population pharmacokinetics of a monthly buprenorphine depot injection for the treatment of opioid use disorder: a combined analysis of phase II and phase III trials. Clin Pharmacokinet. 2021;60(4):527-540. doi:10.1007/s40262-020-00957-0
8. Greenwald MK, Comer SD, Fiellin DA. Buprenorphine maintenance and mu-opioid receptor availability in the treatment of opioid use disorder: implications for clinical use and policy. Drug Alcohol Depend. 2014;144:1-11. doi:10.1016/j.drugalcdep.2014.07.035
9. Peckham AM, Kehoe LG, Gray JR, et al. Real-world outcomes with extended-release buprenorphine (XR-BUP) in a low threshold bridge clinic: a retrospective case series. J Subst Abuse Treat. 2021;126:108316. doi:10.1016/j.jsat.2021.108316
10. Ling W, Nadipelli VR, Aldridge AP, et al. Recovery from opioid use disorder (OUD) after monthly long-acting buprenorphine treatment: 12-month longitudinal outcomes from RECOVER, an observational study. J Addict Med. 2020;14(5):e233-e240. doi:10.1097/ADM.0000000000000647
11. Larance B, Byrne M, Lintzeris N, et al. Open-label, multicentre, single-arm trial of monthly injections of depot buprenorphine in people with opioid dependence: protocol for the CoLAB study. BMJ Open. 2020;10(7):e034389. doi:10.1136/bmjopen-2019-034389
12. Braeburn receives new Complete Response Letter for Brixadi in the US. News release. News Powered by Cision. December 15, 2021. Accessed April 13, 2022. https://news.cision.com/camurus-ab/r/braeburn-receives-new-complete-response-letter-for-brixadi-in-the-us,c3473281
1. Mattson CL, Tanz LJ, Quinn K, et al. Trends and geographic patterns in drug and synthetic opioid overdose deaths - United States, 2013-2019. MMWR Morb Mortal Wkly Rep. 2021;70(6):202-207. doi:10.15585/mmwr.mm7006a4
2. Ma J, Bao YP, Wang RJ, et al. Effects of medication-assisted treatment on mortality among opioids users: a systematic review and meta-analysis. Mol Psychiatry. 2019;24(12):1868-1883. doi:10.1038/s41380-018-0094-5
3. Coe MA, Lofwall MR, Walsh SL. Buprenorphine pharmacology review: update on transmucosal and long-acting formulations. J Addict Med. 2019;13(2):93-103. doi:10.1097/ADM.0000000000000457
4. Becerra X. Practice Guidelines for the Administration of Buprenorphine for Treating Opioid Use Disorder. US Dept of Health and Human Services; 2021:22439-22440. FR Document 2021-08961. Accessed April 5, 2021. https://www.federalregister.gov/documents/2021/04/28/2021-08961/practice-guidelines-for-the-administration-of-buprenorphine-for-treating-opioid-use-disorder
5. Haight BR, Learned SM, Laffont CM, et al. Efficacy and safety of a monthly buprenorphine depot injection for opioid use disorder: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2019;393(10173):778-790. doi:10.1016/S0140-6736(18)32259-1
6. Sublocade [package insert]. North Chesterfield, VA: Indivior Inc; 2021.
7. Jones AK, Ngaimisi E, Gopalakrishnan M, et al. Population pharmacokinetics of a monthly buprenorphine depot injection for the treatment of opioid use disorder: a combined analysis of phase II and phase III trials. Clin Pharmacokinet. 2021;60(4):527-540. doi:10.1007/s40262-020-00957-0
8. Greenwald MK, Comer SD, Fiellin DA. Buprenorphine maintenance and mu-opioid receptor availability in the treatment of opioid use disorder: implications for clinical use and policy. Drug Alcohol Depend. 2014;144:1-11. doi:10.1016/j.drugalcdep.2014.07.035
9. Peckham AM, Kehoe LG, Gray JR, et al. Real-world outcomes with extended-release buprenorphine (XR-BUP) in a low threshold bridge clinic: a retrospective case series. J Subst Abuse Treat. 2021;126:108316. doi:10.1016/j.jsat.2021.108316
10. Ling W, Nadipelli VR, Aldridge AP, et al. Recovery from opioid use disorder (OUD) after monthly long-acting buprenorphine treatment: 12-month longitudinal outcomes from RECOVER, an observational study. J Addict Med. 2020;14(5):e233-e240. doi:10.1097/ADM.0000000000000647
11. Larance B, Byrne M, Lintzeris N, et al. Open-label, multicentre, single-arm trial of monthly injections of depot buprenorphine in people with opioid dependence: protocol for the CoLAB study. BMJ Open. 2020;10(7):e034389. doi:10.1136/bmjopen-2019-034389
12. Braeburn receives new Complete Response Letter for Brixadi in the US. News release. News Powered by Cision. December 15, 2021. Accessed April 13, 2022. https://news.cision.com/camurus-ab/r/braeburn-receives-new-complete-response-letter-for-brixadi-in-the-us,c3473281