User login
Deprescribing in older adults: An overview
Mr. J, age 73, has a 25-year history of generalized anxiety disorder and major depressive disorder. His medical history includes hypertension, hyperlipidemia, type 2 diabetes mellitus, hypothyroidism, osteoarthritis, insomnia, and allergic rhinitis. His last laboratory test results indicate his hemoglobin A1c, thyroid-stimulating hormone, low-density lipoprotein, and blood pressure measurements are at goal. He believes his conditions are well controlled but cites concerns about taking multiple medications each day and being able to afford his medications.
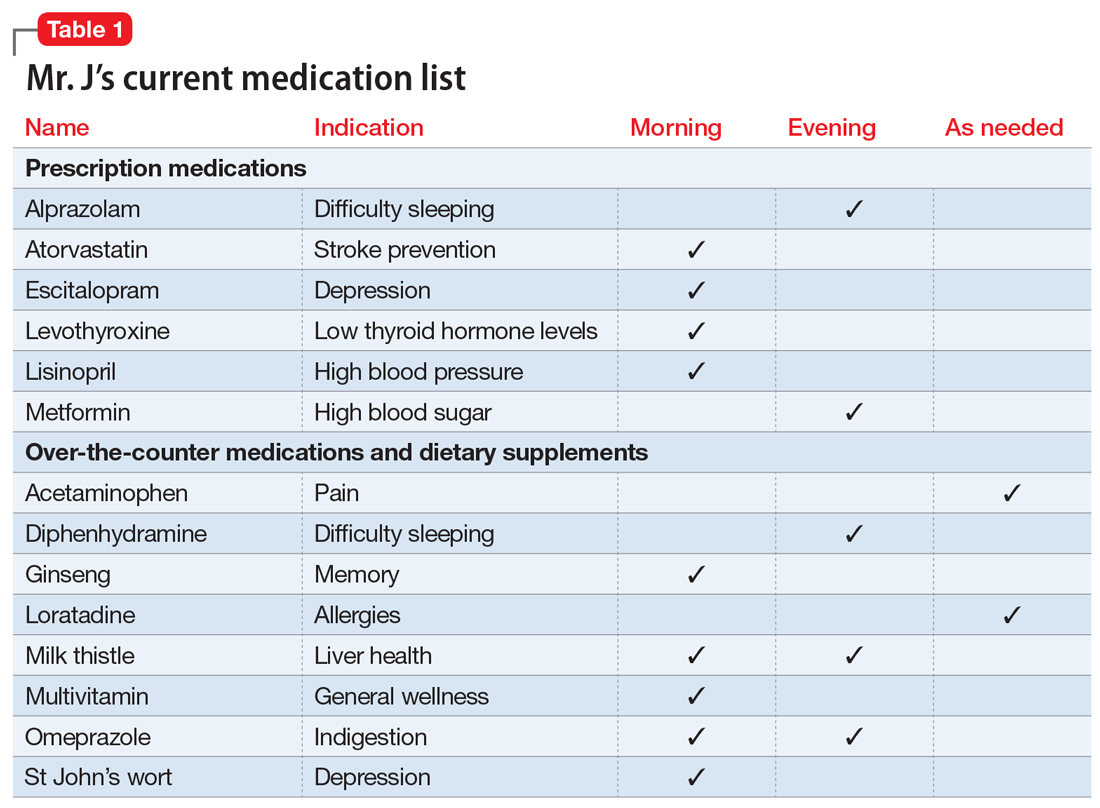
You review the list of Mr. J’s current prescription medications, which include alprazolam 0.5 mg/d, atorvastatin 40 mg/d, escitalopram 10 mg/d, levothyroxine 0.125 mg/d, lisinopril 20 mg/d, and metformin XR 1,000 mg/d. Mr. J reports taking over-the-counter (OTC) acetaminophen as needed for pain, diphenhydramine for insomnia, loratadine as needed for allergic rhinitis, and omeprazole for 2 years for indigestion. After further questioning, he also reports taking ginseng, milk thistle, a multivitamin, and, based on a friend’s recommendation, St John’s Wort (Table 1).
Similar to Mr. J, many older adults take multiple medications to manage chronic health conditions and promote their overall health. On average, 30% of older adults take ≥5 medications.1 Among commonly prescribed medications for these patients, an estimated 1 in 5 of may be inappropriate.1 Older adults have high rates of polypharmacy (often defined as taking ≥5 medications1), age-related physiological changes, increased number of comorbidities, and frailty, all of which can increase the risk of medication-related adverse events.2 As a result, older patients’ medications should be regularly evaluated to determine if each medication is appropriate to continue or should be tapered or stopped.
Deprescribing, in which medications are tapered or discontinued using a patient-centered approach, should be considered when a patient is no longer receiving benefit from a medication, or when the harm may exceed the benefit.1,3
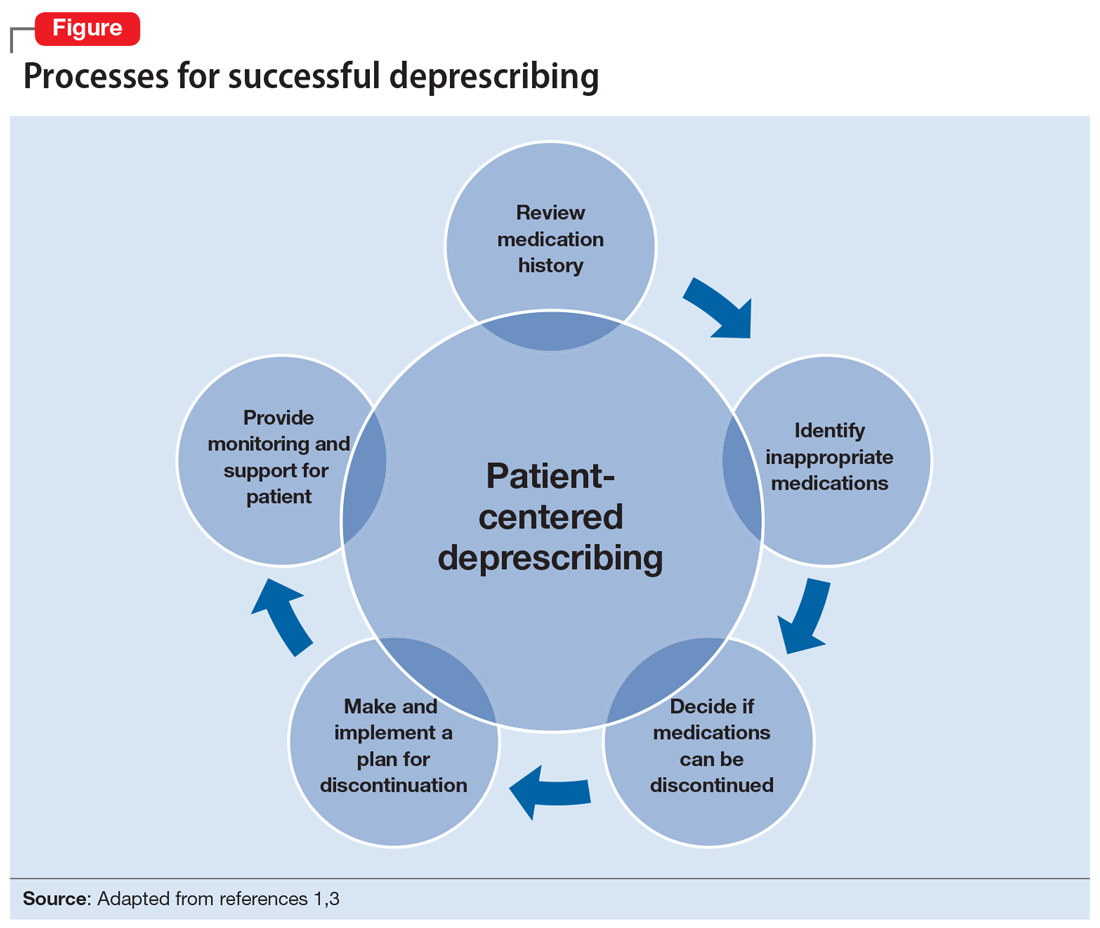
Several researchers1,3 and organizations have published detailed descriptions of and guidelines for the process of deprescribing (see Related Resources). Here we provide a brief overview of this process (Figure1,3). The first step is to assemble a list of all prescription and OTC medications, herbal products, vitamins, or nutritional supplements the patient is taking. It is important to specifically ask patients about their use of nonprescription products, because these products are infrequently documented in medical records.
The second step is to evaluate the indication, effectiveness, safety, and patient’s adherence to each medication while beginning to consider opportunities to limit treatment burden and the risk of harm from medications. Ideally, this assessment should involve a patient-centered conversation that considers the patient’s goals, preferences, and treatment values. Many resources can be used to evaluate which medications might be inappropriate for an older adult. Two examples are the American Geriatrics Society Beers Criteria5 and STOPP/START criteria.6 By looking at these resources, you could identify that (for example) anticholinergic medications should be avoided in older patients due to an increased risk of adverse effects, change in cognitive status, and falls.5,6 These resources can aid in identifying, prioritizing, and deprescribing potentially harmful and/or inappropriate medications.
The next step is to decide whether any medications should be discontinued. Whenever possible, include the patient in this conversation, as they may have strong feelings about their current medication regimen. When there are multiple medications that can be discontinued, consider which medication to stop first based on potential harm, patient resistance, and other factors.
Continue to: Subsequently, work with...
Subsequently, work with the patient to create a plan for stopping or lowering the dose or frequency of the medication. These changes should be individualized based on the patient’s preferences as well as the properties of the medication. For example, some medications can be immediately discontinued, while others (eg, benzodiazepines) may need to be slowly tapered. It is important to consider if the patient will need to switch to a safer medication, change their behaviors (eg, lifestyle changes), or engage in alternative treatments (such as cognitive-behavioral therapy for insomnia) when they stop their current medication. Take an active role in monitoring your patient during this process, and encourage them to reach out to you or to their primary clinician if they have concerns.
CASE CONTINUED
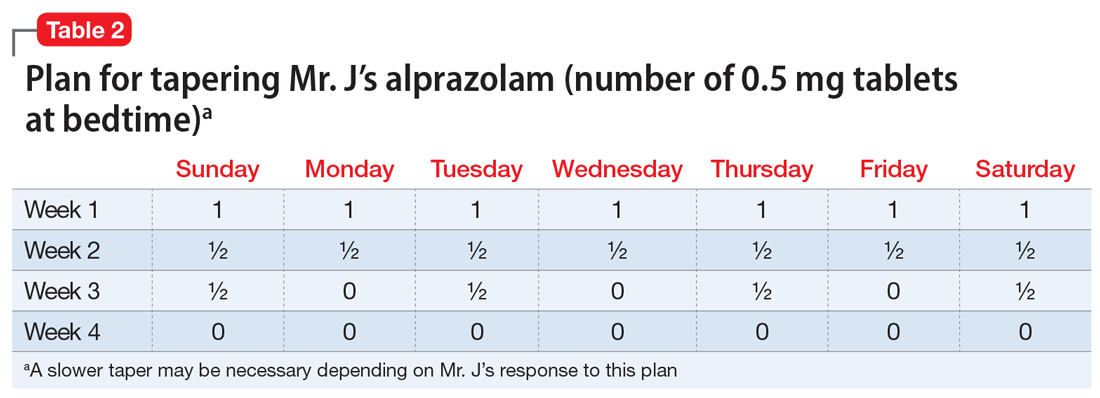
Mr. J is a candidate for deprescribing because he has expressed concerns about his current regimen, and because he is taking potentially unsafe medications. The 2 medications he’s taking that may cause the most harm are diphenhydramine and alprazolam, due to the risk of cognitive impairment and falls. Through a patient-centered conversation, Mr. J says he is willing to stop diphenhydramine immediately and taper off the alprazolam over the next month, with the support of a tapering chart (Table 2). You explain to him that a long tapering of alprazolam may be necessary. He is willing to try good sleep hygiene practices and will put off starting trazodone as an alternative to diphenhydramine until he sees if it will be necessary. You make a note to follow up with him in 1 week to assess his insomnia and adherence to the new treatment plan. You also teach Mr. J that some of his supplements may interact with his prescription medications, such as St John’s Wort with escitalopram (ie, risk of serotonin syndrome) and ginseng with metformin (ie, risk for hypoglycemia). He says he doesn’t take ginseng, milk thistle, or St John’s Wort regularly, and because he feels they do not offer any benefit, he will stop taking them. He says that at his next visit with his primary care physician, he will bring up the idea of stopping omeprazole.
Related Resources
- Deprescribing.org. Deprescribing guidelines and algorithms. https://deprescribing.org/resources/deprescribing-guidelines-algorithms/
- US Deprescribing Research Network. Resources for Clinicians. https://deprescribingresearch.org/resources-2/resources-for-clinicians/
Drug Brand Names
Alprazolam • Xanax
Atorvastatin • Lipitor
Escitalopram • Lexapro
Levothyroxine • Synthroid
Lisinopril • Zestril
Metformin XR • Glucophage XR
Trazodone • Desyrel
1. Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175(5):827-834.
2. Gibson G, Kennedy LH, Barlow G. Polypharmacy in older adults. Current Psychiatry. 2020;19(4):40-46.
3. Reeve E, Shakib S, Hendrix I, et al. Review of deprescribing processes and development of an evidence-based, patient-centred deprescribing process. Br J Clin Pharmcol. 2014;78(4):738-747.
4. Iyer S, Naganathan V, McLachlan AJ, et al. Medication withdrawal trials in people aged 65 years and older: a systematic review. Drugs Aging. 2008;25(12):1021-1031.
5. 2019 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694.
6. O’Mahony D, O’Sullivan D, Byrne S, et al. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2015;44(2):213-218.
Mr. J, age 73, has a 25-year history of generalized anxiety disorder and major depressive disorder. His medical history includes hypertension, hyperlipidemia, type 2 diabetes mellitus, hypothyroidism, osteoarthritis, insomnia, and allergic rhinitis. His last laboratory test results indicate his hemoglobin A1c, thyroid-stimulating hormone, low-density lipoprotein, and blood pressure measurements are at goal. He believes his conditions are well controlled but cites concerns about taking multiple medications each day and being able to afford his medications.
You review the list of Mr. J’s current prescription medications, which include alprazolam 0.5 mg/d, atorvastatin 40 mg/d, escitalopram 10 mg/d, levothyroxine 0.125 mg/d, lisinopril 20 mg/d, and metformin XR 1,000 mg/d. Mr. J reports taking over-the-counter (OTC) acetaminophen as needed for pain, diphenhydramine for insomnia, loratadine as needed for allergic rhinitis, and omeprazole for 2 years for indigestion. After further questioning, he also reports taking ginseng, milk thistle, a multivitamin, and, based on a friend’s recommendation, St John’s Wort (Table 1).
Similar to Mr. J, many older adults take multiple medications to manage chronic health conditions and promote their overall health. On average, 30% of older adults take ≥5 medications.1 Among commonly prescribed medications for these patients, an estimated 1 in 5 of may be inappropriate.1 Older adults have high rates of polypharmacy (often defined as taking ≥5 medications1), age-related physiological changes, increased number of comorbidities, and frailty, all of which can increase the risk of medication-related adverse events.2 As a result, older patients’ medications should be regularly evaluated to determine if each medication is appropriate to continue or should be tapered or stopped.
Deprescribing, in which medications are tapered or discontinued using a patient-centered approach, should be considered when a patient is no longer receiving benefit from a medication, or when the harm may exceed the benefit.1,3
Several researchers1,3 and organizations have published detailed descriptions of and guidelines for the process of deprescribing (see Related Resources). Here we provide a brief overview of this process (Figure1,3). The first step is to assemble a list of all prescription and OTC medications, herbal products, vitamins, or nutritional supplements the patient is taking. It is important to specifically ask patients about their use of nonprescription products, because these products are infrequently documented in medical records.
The second step is to evaluate the indication, effectiveness, safety, and patient’s adherence to each medication while beginning to consider opportunities to limit treatment burden and the risk of harm from medications. Ideally, this assessment should involve a patient-centered conversation that considers the patient’s goals, preferences, and treatment values. Many resources can be used to evaluate which medications might be inappropriate for an older adult. Two examples are the American Geriatrics Society Beers Criteria5 and STOPP/START criteria.6 By looking at these resources, you could identify that (for example) anticholinergic medications should be avoided in older patients due to an increased risk of adverse effects, change in cognitive status, and falls.5,6 These resources can aid in identifying, prioritizing, and deprescribing potentially harmful and/or inappropriate medications.
The next step is to decide whether any medications should be discontinued. Whenever possible, include the patient in this conversation, as they may have strong feelings about their current medication regimen. When there are multiple medications that can be discontinued, consider which medication to stop first based on potential harm, patient resistance, and other factors.
Continue to: Subsequently, work with...
Subsequently, work with the patient to create a plan for stopping or lowering the dose or frequency of the medication. These changes should be individualized based on the patient’s preferences as well as the properties of the medication. For example, some medications can be immediately discontinued, while others (eg, benzodiazepines) may need to be slowly tapered. It is important to consider if the patient will need to switch to a safer medication, change their behaviors (eg, lifestyle changes), or engage in alternative treatments (such as cognitive-behavioral therapy for insomnia) when they stop their current medication. Take an active role in monitoring your patient during this process, and encourage them to reach out to you or to their primary clinician if they have concerns.
CASE CONTINUED
Mr. J is a candidate for deprescribing because he has expressed concerns about his current regimen, and because he is taking potentially unsafe medications. The 2 medications he’s taking that may cause the most harm are diphenhydramine and alprazolam, due to the risk of cognitive impairment and falls. Through a patient-centered conversation, Mr. J says he is willing to stop diphenhydramine immediately and taper off the alprazolam over the next month, with the support of a tapering chart (Table 2). You explain to him that a long tapering of alprazolam may be necessary. He is willing to try good sleep hygiene practices and will put off starting trazodone as an alternative to diphenhydramine until he sees if it will be necessary. You make a note to follow up with him in 1 week to assess his insomnia and adherence to the new treatment plan. You also teach Mr. J that some of his supplements may interact with his prescription medications, such as St John’s Wort with escitalopram (ie, risk of serotonin syndrome) and ginseng with metformin (ie, risk for hypoglycemia). He says he doesn’t take ginseng, milk thistle, or St John’s Wort regularly, and because he feels they do not offer any benefit, he will stop taking them. He says that at his next visit with his primary care physician, he will bring up the idea of stopping omeprazole.
Related Resources
- Deprescribing.org. Deprescribing guidelines and algorithms. https://deprescribing.org/resources/deprescribing-guidelines-algorithms/
- US Deprescribing Research Network. Resources for Clinicians. https://deprescribingresearch.org/resources-2/resources-for-clinicians/
Drug Brand Names
Alprazolam • Xanax
Atorvastatin • Lipitor
Escitalopram • Lexapro
Levothyroxine • Synthroid
Lisinopril • Zestril
Metformin XR • Glucophage XR
Trazodone • Desyrel
Mr. J, age 73, has a 25-year history of generalized anxiety disorder and major depressive disorder. His medical history includes hypertension, hyperlipidemia, type 2 diabetes mellitus, hypothyroidism, osteoarthritis, insomnia, and allergic rhinitis. His last laboratory test results indicate his hemoglobin A1c, thyroid-stimulating hormone, low-density lipoprotein, and blood pressure measurements are at goal. He believes his conditions are well controlled but cites concerns about taking multiple medications each day and being able to afford his medications.
You review the list of Mr. J’s current prescription medications, which include alprazolam 0.5 mg/d, atorvastatin 40 mg/d, escitalopram 10 mg/d, levothyroxine 0.125 mg/d, lisinopril 20 mg/d, and metformin XR 1,000 mg/d. Mr. J reports taking over-the-counter (OTC) acetaminophen as needed for pain, diphenhydramine for insomnia, loratadine as needed for allergic rhinitis, and omeprazole for 2 years for indigestion. After further questioning, he also reports taking ginseng, milk thistle, a multivitamin, and, based on a friend’s recommendation, St John’s Wort (Table 1).
Similar to Mr. J, many older adults take multiple medications to manage chronic health conditions and promote their overall health. On average, 30% of older adults take ≥5 medications.1 Among commonly prescribed medications for these patients, an estimated 1 in 5 of may be inappropriate.1 Older adults have high rates of polypharmacy (often defined as taking ≥5 medications1), age-related physiological changes, increased number of comorbidities, and frailty, all of which can increase the risk of medication-related adverse events.2 As a result, older patients’ medications should be regularly evaluated to determine if each medication is appropriate to continue or should be tapered or stopped.
Deprescribing, in which medications are tapered or discontinued using a patient-centered approach, should be considered when a patient is no longer receiving benefit from a medication, or when the harm may exceed the benefit.1,3
Several researchers1,3 and organizations have published detailed descriptions of and guidelines for the process of deprescribing (see Related Resources). Here we provide a brief overview of this process (Figure1,3). The first step is to assemble a list of all prescription and OTC medications, herbal products, vitamins, or nutritional supplements the patient is taking. It is important to specifically ask patients about their use of nonprescription products, because these products are infrequently documented in medical records.
The second step is to evaluate the indication, effectiveness, safety, and patient’s adherence to each medication while beginning to consider opportunities to limit treatment burden and the risk of harm from medications. Ideally, this assessment should involve a patient-centered conversation that considers the patient’s goals, preferences, and treatment values. Many resources can be used to evaluate which medications might be inappropriate for an older adult. Two examples are the American Geriatrics Society Beers Criteria5 and STOPP/START criteria.6 By looking at these resources, you could identify that (for example) anticholinergic medications should be avoided in older patients due to an increased risk of adverse effects, change in cognitive status, and falls.5,6 These resources can aid in identifying, prioritizing, and deprescribing potentially harmful and/or inappropriate medications.
The next step is to decide whether any medications should be discontinued. Whenever possible, include the patient in this conversation, as they may have strong feelings about their current medication regimen. When there are multiple medications that can be discontinued, consider which medication to stop first based on potential harm, patient resistance, and other factors.
Continue to: Subsequently, work with...
Subsequently, work with the patient to create a plan for stopping or lowering the dose or frequency of the medication. These changes should be individualized based on the patient’s preferences as well as the properties of the medication. For example, some medications can be immediately discontinued, while others (eg, benzodiazepines) may need to be slowly tapered. It is important to consider if the patient will need to switch to a safer medication, change their behaviors (eg, lifestyle changes), or engage in alternative treatments (such as cognitive-behavioral therapy for insomnia) when they stop their current medication. Take an active role in monitoring your patient during this process, and encourage them to reach out to you or to their primary clinician if they have concerns.
CASE CONTINUED
Mr. J is a candidate for deprescribing because he has expressed concerns about his current regimen, and because he is taking potentially unsafe medications. The 2 medications he’s taking that may cause the most harm are diphenhydramine and alprazolam, due to the risk of cognitive impairment and falls. Through a patient-centered conversation, Mr. J says he is willing to stop diphenhydramine immediately and taper off the alprazolam over the next month, with the support of a tapering chart (Table 2). You explain to him that a long tapering of alprazolam may be necessary. He is willing to try good sleep hygiene practices and will put off starting trazodone as an alternative to diphenhydramine until he sees if it will be necessary. You make a note to follow up with him in 1 week to assess his insomnia and adherence to the new treatment plan. You also teach Mr. J that some of his supplements may interact with his prescription medications, such as St John’s Wort with escitalopram (ie, risk of serotonin syndrome) and ginseng with metformin (ie, risk for hypoglycemia). He says he doesn’t take ginseng, milk thistle, or St John’s Wort regularly, and because he feels they do not offer any benefit, he will stop taking them. He says that at his next visit with his primary care physician, he will bring up the idea of stopping omeprazole.
Related Resources
- Deprescribing.org. Deprescribing guidelines and algorithms. https://deprescribing.org/resources/deprescribing-guidelines-algorithms/
- US Deprescribing Research Network. Resources for Clinicians. https://deprescribingresearch.org/resources-2/resources-for-clinicians/
Drug Brand Names
Alprazolam • Xanax
Atorvastatin • Lipitor
Escitalopram • Lexapro
Levothyroxine • Synthroid
Lisinopril • Zestril
Metformin XR • Glucophage XR
Trazodone • Desyrel
1. Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175(5):827-834.
2. Gibson G, Kennedy LH, Barlow G. Polypharmacy in older adults. Current Psychiatry. 2020;19(4):40-46.
3. Reeve E, Shakib S, Hendrix I, et al. Review of deprescribing processes and development of an evidence-based, patient-centred deprescribing process. Br J Clin Pharmcol. 2014;78(4):738-747.
4. Iyer S, Naganathan V, McLachlan AJ, et al. Medication withdrawal trials in people aged 65 years and older: a systematic review. Drugs Aging. 2008;25(12):1021-1031.
5. 2019 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694.
6. O’Mahony D, O’Sullivan D, Byrne S, et al. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2015;44(2):213-218.
1. Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175(5):827-834.
2. Gibson G, Kennedy LH, Barlow G. Polypharmacy in older adults. Current Psychiatry. 2020;19(4):40-46.
3. Reeve E, Shakib S, Hendrix I, et al. Review of deprescribing processes and development of an evidence-based, patient-centred deprescribing process. Br J Clin Pharmcol. 2014;78(4):738-747.
4. Iyer S, Naganathan V, McLachlan AJ, et al. Medication withdrawal trials in people aged 65 years and older: a systematic review. Drugs Aging. 2008;25(12):1021-1031.
5. 2019 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694.
6. O’Mahony D, O’Sullivan D, Byrne S, et al. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2015;44(2):213-218.
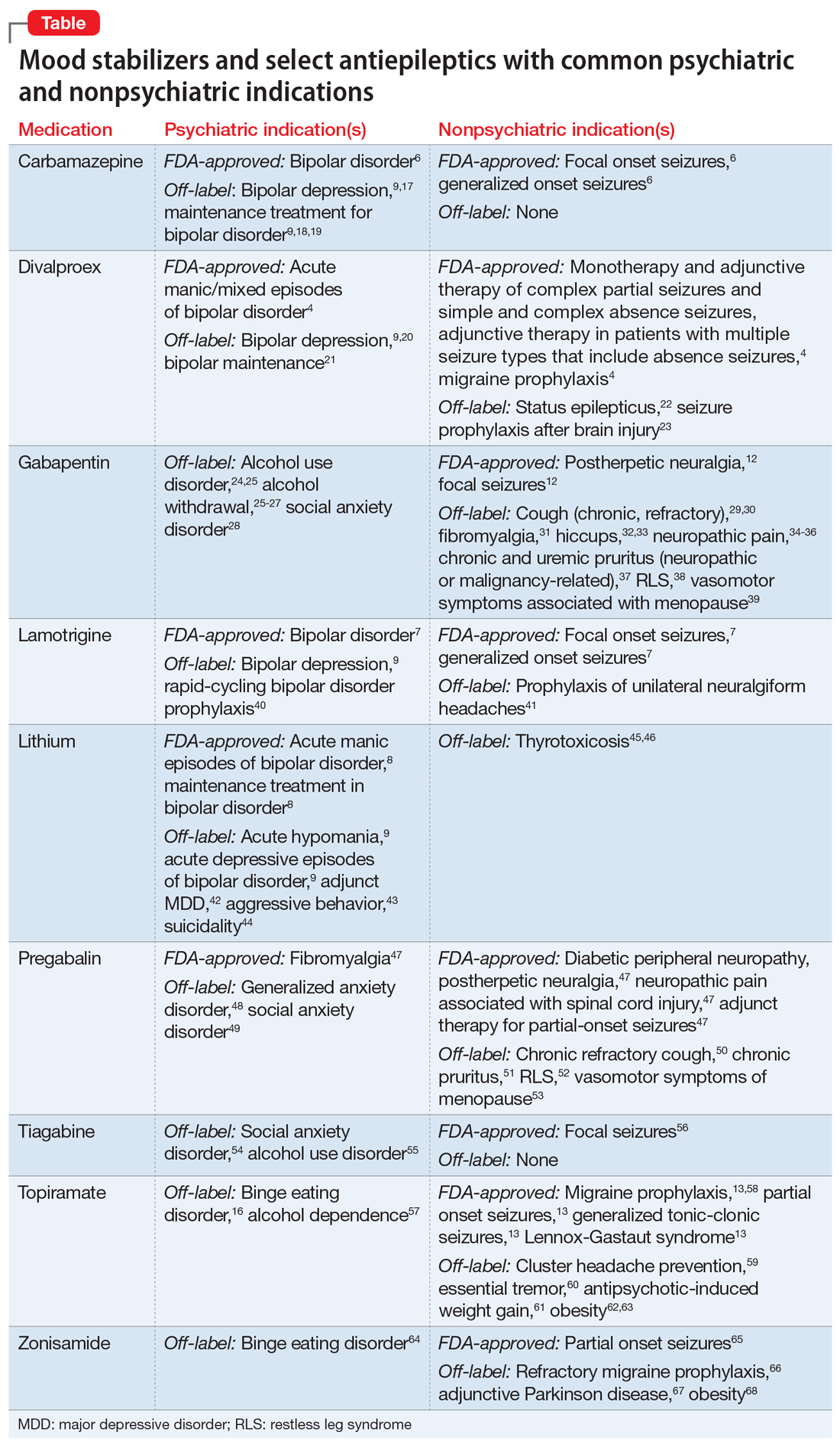
Psychiatric and nonpsychiatric indications for mood stabilizers and select antiepileptics
Mr. B, age 64, is being treated in the psychiatric clinic for generalized anxiety disorder. He also has a history of type 2 diabetes mellitus and osteoarthritis. His present medications include metformin 500 mg twice daily, escitalopram 20 mg/d, and a multivitamin.
Three months after a shingles outbreak on his left trunk, Mr. B develops a sharp, burning pain and hypersensitivity to light in the same area as the shingles flare-up. He is diagnosed with postherpetic neuralgia. Despite a 12-week trial of cognitive-behavioral therapy, Mr. B continues to report excessive worry, irritability, poor concentration, psychomotor restlessness, and poor sleep.
Contrasting with the serendipitous discovery of iproniazid and chlorpromazine leading to the development of the current spectrum of antidepressant and antipsychotic agents, discovery of the benefits various antiepileptic agents have in bipolar disorder has not led to a similar proliferation of medication development for bipolar mania or depression.1-3 Divalproex, one of the most commonly used antiepileptic drugs (AEDs) in psychiatry, was thought to be an inactive organic solvent until it was used in 1962 to test the anticonvulsant activity of other compounds. This led to the discovery and subsequent use of divalproex in patients with epilepsy, followed by FDA approval in bipolar disorder.4,5 Off-label use of many AEDs as mood-stabilizing agents in bipolar disorder led to the emergence of carbamazepine, divalproex, and lamotrigine, which joined lithium as classic mood-stabilizing agents.4,6-8 Amid varying definitions of “mood stabilizer,” many AEDs have failed to demonstrate mood-stabilizing effects in bipolar disorder and therefore should not all be considered mood stabilizers.9 Nonetheless, the dual use of a single AED for both psychiatric and nonpsychiatric indications can decrease polypharmacy and increase acceptability of medications in patients who have low insight into their illness.10,11
Because AEDs were originally purposed to treat neurologic disease, psychiatric indications must first be established before considering other indications. AEDs as a class have broad pharmacologic actions, but are generally CNS depressants, decreasing brain signaling through mechanisms such as ion channel antagonism (carbamazepine, gabapentin) or alterations to gamma-aminobutyric acid/glutamate signaling (divalproex, topiramate).4,6,12,13 Compared to antidepressants and antipsychotics, whose primary use for psychiatric conditions is firmly rooted in evidence, rational use of AEDs for psychiatric conditions and symptoms depends on the agent-specific efficacy. Patients with comorbid psychiatric and neurologic disorders are ideal candidates for dually indicated AEDs due to these agents’ class effects rooted in epilepsy. Due to the history of positive psychiatric benefits with AEDs, newer agents may be psychiatrically beneficial but will likely follow the discovery of these benefits in patients for whom epilepsy is the primary diagnosis.
Consider the limitations
Using AEDs to reduce polypharmacy should be done judiciously from a drug-drug interaction perspective, because certain AEDs (eg, carbamazepine, divalproex) can greatly influence the metabolism of other medications, which may defeat the best intentions of the original intervention.4,6
Several other limitations should be considered. This article does not include all AEDs, only those commonly used for psychiatric indications with known nonpsychiatric benefits. Some may worsen psychiatric conditions (such as rage and irritability in the case of levetiracetam), and all AEDs have an FDA warning regarding suicidal behaviors and ideation.14,15 Another important limitation is the potential for differential dosing across indications; tolerability concerns may limit adequate dosing across multiple uses. For example, topiramate’s migraine prophylaxis effect can be achieved at much lower doses than the patient-specific efficacy dosing seen in binge eating disorder, with higher doses increasing the propensity for adverse effects.13,16Dual-use AEDs should be considered wherever possible, but judicious review of evidence is necessary to appropriately adjudicate a specific patient’s risk vs benefit. The Table4,6-9,12,13,16-68 provides information on select AEDs with both psychiatric and nonpsychiatric indications, including both FDA-approved and common off-label uses. These indications are limited to adult use only.
CASE CONTINUED
After reviewing Mr. B’s medical history, the treating medical team decides to cross-taper escitalopram to duloxetine 30 mg twice daily. Though his pain lessens after several weeks, it persists enough to interfere with Mr. B’s daily life. In addition to duloxetine, he is started on pregabalin 50 mg 3 times a day. Mr. B’s pain decreases to a tolerable level, and he reports decreased worrying and restlessness, and improvements in concentration and sleep.
1. Meyer JM. A concise guide to monoamine oxidase inhibitors. Current Psychiatry. 2017;16(12):14-16,18-23,47,A.
2. Ban TA. Fifty years chlorpromazine: a historical perspective. Neuropsychiatr Dis Treat. 2007;3(4):495-500.
3. López-Mun
4. Depakote [package insert]. North Chicago, IL: AbbVie, Inc; 2021.
5. Henry TR. The history of valproate in clinical neuroscience. Psychopharmacol Bull. 2003;37 Suppl 2:5-16.
6. Tegretol and Tegretol-XR [package insert]. East Hanover, NJ: Pharmaceuticals Co.; 2020.
7. Lamictal [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2009.
8. Lithobid [package insert]. Baudette, MN: ANI Pharmaceuticals, Inc; 2009.
9. Yatham LN, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018;20(2):97-170.
10. National Alliance on Mental Illness. Anosognosia. Common with mental illness. Accessed March 3, 2022. https://www.nami.org/About-Mental-Illness/Common-with-Mental-Illness/Anosognosia
11. Hales CM, Servais J, Martin CB, et al. Prescription drug use among adults aged 40-79 in the United States and Canada. NCHS Data Brief. 2019(347):1-8.
12. Neurontin [package insert]. New York, NY: Pfizer; 2017.
13. Topamax [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc; 2009.
14. Molokwu OA, Ezeala-Adikaibe BA, Onwuekwe IO. Levetiracetam-induced rage and suicidality: two case reports and review of literature. Epilepsy Behav Case Rep. 2015;4:79-81.
15. U.S. Food & Drug Administration. FDA Statistical Review and Evaluation. Antiepileptic Drugs and Suicidality. 2008. Accessed March 3, 2022. https://www.fda.gov/files/drugs/published/Statistical-Review-and-Evaluation--Antiepileptic-Drugs-and-Suicidality.pdf
16. McElroy SL, Hudson JI, Capece JA, et al. Topiramate for the treatment of binge eating disorder associated with obesity: a placebo-controlled study. Biol Psychiatry. 2007;61(9):1039-1048.
17. Zhang ZJ, Kang WH, Tan QR, et al. Adjunctive herbal medicine with carbamazepine for bipolar disorders: a double-blind, randomized, placebo-controlled study. J Psychiatr Res. 2007;41(3-4):360-369.
18. Kleindienst N, Greil W. Differential efficacy of lithium and carbamazepine in the prophylaxis of bipolar disorder: results of the MAP study. Neuropsychobiology. 2000;42 Suppl 1:2-10.
19. Goodwin GM, Haddad PM, Ferrier IN, et al. Evidence-based guidelines for treating bipolar disorder: revised third edition recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2016;30(6):495-553.
20. Davis LL, Bartolucci A, Petty F. Divalproex in the treatment of bipolar depression: a placebo-controlled study. J Affect Disord. 2005;85(3):259-266.
21. Gyulai L, Bowden CL, McElroy SL, et al. Maintenance efficacy of divalproex in the prevention of bipolar depression. Neuropsychopharmacology. 2003;28(7):1374-1382.
22. Limdi NA, Shimpi AV, Faught E, et al. Efficacy of rapid IV administration of valproic acid for status epilepticus. Neurology. 2005;64(2):353-355.
23. Temkin NR, Dikmen SS, Anderson GD, et al. Valproate therapy for prevention of posttraumatic seizures: a randomized trial. J Neurosurg. 1999; 91(4):593-600.
24. Reus VI, Fochtmann LJ, Bukstein O, et al. The American Psychiatric Association practice guideline for the pharmacological treatment of patients with alcohol use disorder. Am J Psychiatry. 2018;175(1):86-90.
25. US Dept of Veterans Affairs, US Dept of Defense, The Management of Substance Use Disorders Work Group. VA/DoD clinical practice guideline for the management of substance use disorders. US Dept of Veterans Affairs/Dept of Defense; 2015. Accessed March 3, 2022. http://www.healthquality.va.gov/guidelines/MH/sud/VADoDSUDCPGRevised22216.pdf
26. Myrick H, Malcolm R, Randall PK, et al. A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res. 2009;33(9):1582-1588.
27. Ahmed S, Stanciu CN, Kotapati PV, et al. Effectiveness of gabapentin in reducing cravings and withdrawal in alcohol use disorder: a meta-analytic review. Prim Care Companion CNS Disord. 2019;21(4):19r02465.
28. Pande AC, Davidson JR, Jefferson JW, et al. Treatment of social phobia with gabapentin: a placebo-controlled study. J Clin Psychopharmacol. 1999;19(4):341-348.
29. Ryan NM, Birring SS, Gibson PG. Gabapentin for refractory chronic cough: a randomized, double-blind, placebo-controlled trial. Lancet. 2012;380(9853):1583-1589.
30. Gibson P, Wang G, McGarvey L, et al. Treatment of unexplained chronic cough: CHEST guideline and expert panel report. Chest. 2016;149(1):27-44.
31. Arnold LM, Goldenberg DL, Stanford SB, et al. Gabapentin in the treatment of fibromyalgia: a randomized, double-blind, placebo-controlled, multicenter trial. Arthritis Rheum. 2007;56(4):1336-1344.
32. Alonso-Navarro H, Rubio L, Jiménez-Jiménez FJ. Refractory hiccup: successful treatment with gabapentin. Clin Neuropharmacol. 2007;30(3):186-187.
33. Jatzko A, Stegmeier-Petroianu A, Petroianu GA. Alpha-2-delta ligands for singultus (hiccup) treatment: three case reports. J Pain Symptom Manage. 2007;33(6):756-760.
34. Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14(2):162-173.
35. Moore RA, Wiffen PJ, Derry S, et al. Gabapentin for chronic neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2014;2014(4):CD007938.
36. Yuan M, Zhou HY, Xiao ZL, et al. Efficacy and safety of gabapentin vs. carbamazepine in the treatment of trigeminal neuralgia: a meta-analysis. Pain Pract. 2016;16(8):1083-1091.
37. Weisshaar E, Szepietowski JC, Darsow U, et al. European guideline on chronic pruritus. Acta Derm Venereol. 2012;92(5):563-581.
38. Garcia-Borreguero D, Silber MH, Winkelman JW, et al. Guidelines for the first-line treatment of restless legs syndrome/Willis-Ekbom disease, prevention and treatment of dopaminergic augmentation: a combined task force of the IRLSSG, EURLSSG, and the RLS-Foundation. Sleep Med. 2016;21:1-11.
39. Cobin RH, Goodman NF; AACE Reproductive Endocrinology Scientific Committee. American Association of Clinical Endocrinologists and American College of Endocrinology position statement on menopause—2017 update [published correction appears in Endocr Pract. 2017;23 (12):1488]. Endocr Pract. 2017;23(7):869-880.
40. Calabrese JR, Suppes T, Bowden CL, et al. A double-blind, placebo-controlled, prophylaxis study of lamotrigine in rapid-cycling bipolar disorder: Lamictal 614 Study Group. J Clin Psychiatry. 2000;60(11):841-850.
41. May A, Leone M, Afra J, et al. EFNS guidelines on the treatment of cluster headache and other trigeminal-autonomic cephalalgias. Eur J Neurol. 2006;13(10):1066-1077.
42. Stein G, Bernadt M. Lithium augmentation therapy in tricyclic-resistant depression. A controlled trial using lithium in low and normal doses. Br J Psychiatry. 1993;162:634-640.
43. Craft M, Ismail IA, Krishnamurti D, et al. Lithium in the treatment of aggression in mentally handicapped patients: a double-blind trial. Br J Psychiatry. 1987;150:685-689.
44. Cipriani A, Pretty H, Hawton K, et al. Lithium in the prevention of suicidal behavior and all-cause mortality in patients with mood disorders: a systematic review of randomized trials. Am J Psychiatry. 2005;162(10):1805-1819.
45. Dickstein G, Shechner C, Adawi F, et al. Lithium treatment in amiodarone-induced thyrotoxicosis. Am J Med. 1997;102(5):454-458.
46. Bogazzi F, Bartalena L, Brogioni S, et al. Comparison of radioiodine with radioiodine plus lithium in the treatment of Graves’ hyperthyroidism. J Clin Endocrinol Metab. 1999;84(2):499-503.
47. Lyrica [package insert]. New York, NY: Parke-Davis, Division of Pfizer Inc; 2020.
48. Lydiard RB, Rickels K, Herman B, et al. Comparative efficacy of pregabalin and benzodiazepines in treating the psychic and somatic symptoms of generalized anxiety disorder. Int J Neuropsychopharmacol. 2010;13(2):229-241.
49. Pande AC, Feltner DE, Jefferson JW, et al. Efficacy of the novel anxiolytic pregabalin in social anxiety disorder: a placebo-controlled, multicenter study. J Clin Psychopharmacol. 2004;24(2):141-149.
50. Vertigan AE, Kapela SL, Ryan NM, et al. Pregabalin and speech pathology combination therapy for refractory chronic cough: a randomized controlled trial. Chest. 2016;149(3):639-648.
51. Matsuda KM, Sharma D, Schonfeld AR, et al. Gabapentin and pregabalin for the treatment of chronic pruritus. J Am Acad Dermatol. 2016;75(3):619-625.e6.
52. Allen R, Chen C, Soaita A, et al. A randomized, double-blind, 6-week, dose-ranging study of pregabalin in patients with restless legs syndrome. Sleep Med. 2010;11(6):512-519.
53. Loprinzi CL, Qin R, Balcueva EP, et al. Phase III, randomized, double-blind, placebo-controlled evaluation of pregabalin for alleviating hot flashes, N07C1 [published correction appears in J Clin Oncol. 2010;28(10):1808]. J Clin Oncol. 2010;28(4):641-647.
54. Dunlop BW, Papp L, Garlow SJ, et al. Tiagabine for social anxiety disorder. Hum Psychopharmacol. 2007;22(4):241-244.
55. Paparrigopoulos T, Tzavellas E, Karaiskos D, et al. An open pilot study of tiagabine in alcohol dependence: tolerability and clinical effects. J Psychopharmacol. 2010;24(9):1375-1380.
56. Gabitril [package insert]. North Wales, PA: Teva Pharmaceuticals USA, Inc; 2015.
57. Johnson BA, Ait-Daoud N, Bowden C, et al. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003;361(9370):1677-1685.
58. Linde M, Mulleners WM, Chronicle EP, et al. Topiramate for the prophylaxis of episodic migraine in adults. Cochrane Database Syst Rev. 2013;2013(6):CD010610.
59. Pascual J, Láinez MJ, Dodick D, et al. Antiepileptic drugs for the treatment of chronic and episodic cluster headache: a review. Headache. 2007;47(1):81-89.
60. Ondo WG, Jankovic J, Connor GS, et al. Topiramate in essential tremor: a double-blind, placebo-controlled trial. Neurology. 2006;66(5):672-677.
61. Ko YH, Joe SH, Jung IK, et al. Topiramate as an adjuvant treatment with atypical antipsychotics in schizophrenic patients experiencing weight gain. Clin Neuropharmacol. 2005;28(4):169-175.
62. Wilding J, Van Gaal L, Rissanen A, et al. A randomized double-blind placebo-controlled study of the long-term efficacy and safety of topiramate in the treatment of obese subjects. Int J Obes Relat Metab Disord. 2004;28(11):1399-1410.
63. Rosenstock J, Hollander P, Gadde KM, et al. A randomized, double-blind, placebo-controlled, multicenter study to assess the efficacy and safety of topiramate controlled release in the treatment of obese type 2 diabetic patients. Diabetes Care. 2007; 30(6):1480-1486.
64. McElroy SL, Kotwal R, Guerdjikova AI, et al. Zonisamide in the treatment of binge eating disorder with obesity: a randomized controlled trial. J Clin Psychiatry. 2006;67(12):1897-1906.
65. Zonegran [package insert]. Teaneck, NJ: Eisai Inc; 2006.
66. Drake ME Jr, Greathouse NI, Renner JB, et al. Open-label zonisamide for refractory migraine. Clin Neuropharmacol. 2004;27(6):278-280.
67. Matsunaga S, Kishi T, Iwata N. Combination therapy with zonisamide and antiparkinson drugs for Parkinson’s disease: a meta-analysis. J Alzheimers Dis. 2017;56(4):1229-1239.
68. Gadde KM, Kopping MF, Wagner HR 2nd, et al. Zonisamide for weight reduction in obese adults: a 1-year randomized controlled trial. Arch Intern Med. 2012;172(20):1557-1564.
Mr. B, age 64, is being treated in the psychiatric clinic for generalized anxiety disorder. He also has a history of type 2 diabetes mellitus and osteoarthritis. His present medications include metformin 500 mg twice daily, escitalopram 20 mg/d, and a multivitamin.
Three months after a shingles outbreak on his left trunk, Mr. B develops a sharp, burning pain and hypersensitivity to light in the same area as the shingles flare-up. He is diagnosed with postherpetic neuralgia. Despite a 12-week trial of cognitive-behavioral therapy, Mr. B continues to report excessive worry, irritability, poor concentration, psychomotor restlessness, and poor sleep.
Contrasting with the serendipitous discovery of iproniazid and chlorpromazine leading to the development of the current spectrum of antidepressant and antipsychotic agents, discovery of the benefits various antiepileptic agents have in bipolar disorder has not led to a similar proliferation of medication development for bipolar mania or depression.1-3 Divalproex, one of the most commonly used antiepileptic drugs (AEDs) in psychiatry, was thought to be an inactive organic solvent until it was used in 1962 to test the anticonvulsant activity of other compounds. This led to the discovery and subsequent use of divalproex in patients with epilepsy, followed by FDA approval in bipolar disorder.4,5 Off-label use of many AEDs as mood-stabilizing agents in bipolar disorder led to the emergence of carbamazepine, divalproex, and lamotrigine, which joined lithium as classic mood-stabilizing agents.4,6-8 Amid varying definitions of “mood stabilizer,” many AEDs have failed to demonstrate mood-stabilizing effects in bipolar disorder and therefore should not all be considered mood stabilizers.9 Nonetheless, the dual use of a single AED for both psychiatric and nonpsychiatric indications can decrease polypharmacy and increase acceptability of medications in patients who have low insight into their illness.10,11
Because AEDs were originally purposed to treat neurologic disease, psychiatric indications must first be established before considering other indications. AEDs as a class have broad pharmacologic actions, but are generally CNS depressants, decreasing brain signaling through mechanisms such as ion channel antagonism (carbamazepine, gabapentin) or alterations to gamma-aminobutyric acid/glutamate signaling (divalproex, topiramate).4,6,12,13 Compared to antidepressants and antipsychotics, whose primary use for psychiatric conditions is firmly rooted in evidence, rational use of AEDs for psychiatric conditions and symptoms depends on the agent-specific efficacy. Patients with comorbid psychiatric and neurologic disorders are ideal candidates for dually indicated AEDs due to these agents’ class effects rooted in epilepsy. Due to the history of positive psychiatric benefits with AEDs, newer agents may be psychiatrically beneficial but will likely follow the discovery of these benefits in patients for whom epilepsy is the primary diagnosis.
Consider the limitations
Using AEDs to reduce polypharmacy should be done judiciously from a drug-drug interaction perspective, because certain AEDs (eg, carbamazepine, divalproex) can greatly influence the metabolism of other medications, which may defeat the best intentions of the original intervention.4,6
Several other limitations should be considered. This article does not include all AEDs, only those commonly used for psychiatric indications with known nonpsychiatric benefits. Some may worsen psychiatric conditions (such as rage and irritability in the case of levetiracetam), and all AEDs have an FDA warning regarding suicidal behaviors and ideation.14,15 Another important limitation is the potential for differential dosing across indications; tolerability concerns may limit adequate dosing across multiple uses. For example, topiramate’s migraine prophylaxis effect can be achieved at much lower doses than the patient-specific efficacy dosing seen in binge eating disorder, with higher doses increasing the propensity for adverse effects.13,16Dual-use AEDs should be considered wherever possible, but judicious review of evidence is necessary to appropriately adjudicate a specific patient’s risk vs benefit. The Table4,6-9,12,13,16-68 provides information on select AEDs with both psychiatric and nonpsychiatric indications, including both FDA-approved and common off-label uses. These indications are limited to adult use only.
CASE CONTINUED
After reviewing Mr. B’s medical history, the treating medical team decides to cross-taper escitalopram to duloxetine 30 mg twice daily. Though his pain lessens after several weeks, it persists enough to interfere with Mr. B’s daily life. In addition to duloxetine, he is started on pregabalin 50 mg 3 times a day. Mr. B’s pain decreases to a tolerable level, and he reports decreased worrying and restlessness, and improvements in concentration and sleep.
Mr. B, age 64, is being treated in the psychiatric clinic for generalized anxiety disorder. He also has a history of type 2 diabetes mellitus and osteoarthritis. His present medications include metformin 500 mg twice daily, escitalopram 20 mg/d, and a multivitamin.
Three months after a shingles outbreak on his left trunk, Mr. B develops a sharp, burning pain and hypersensitivity to light in the same area as the shingles flare-up. He is diagnosed with postherpetic neuralgia. Despite a 12-week trial of cognitive-behavioral therapy, Mr. B continues to report excessive worry, irritability, poor concentration, psychomotor restlessness, and poor sleep.
Contrasting with the serendipitous discovery of iproniazid and chlorpromazine leading to the development of the current spectrum of antidepressant and antipsychotic agents, discovery of the benefits various antiepileptic agents have in bipolar disorder has not led to a similar proliferation of medication development for bipolar mania or depression.1-3 Divalproex, one of the most commonly used antiepileptic drugs (AEDs) in psychiatry, was thought to be an inactive organic solvent until it was used in 1962 to test the anticonvulsant activity of other compounds. This led to the discovery and subsequent use of divalproex in patients with epilepsy, followed by FDA approval in bipolar disorder.4,5 Off-label use of many AEDs as mood-stabilizing agents in bipolar disorder led to the emergence of carbamazepine, divalproex, and lamotrigine, which joined lithium as classic mood-stabilizing agents.4,6-8 Amid varying definitions of “mood stabilizer,” many AEDs have failed to demonstrate mood-stabilizing effects in bipolar disorder and therefore should not all be considered mood stabilizers.9 Nonetheless, the dual use of a single AED for both psychiatric and nonpsychiatric indications can decrease polypharmacy and increase acceptability of medications in patients who have low insight into their illness.10,11
Because AEDs were originally purposed to treat neurologic disease, psychiatric indications must first be established before considering other indications. AEDs as a class have broad pharmacologic actions, but are generally CNS depressants, decreasing brain signaling through mechanisms such as ion channel antagonism (carbamazepine, gabapentin) or alterations to gamma-aminobutyric acid/glutamate signaling (divalproex, topiramate).4,6,12,13 Compared to antidepressants and antipsychotics, whose primary use for psychiatric conditions is firmly rooted in evidence, rational use of AEDs for psychiatric conditions and symptoms depends on the agent-specific efficacy. Patients with comorbid psychiatric and neurologic disorders are ideal candidates for dually indicated AEDs due to these agents’ class effects rooted in epilepsy. Due to the history of positive psychiatric benefits with AEDs, newer agents may be psychiatrically beneficial but will likely follow the discovery of these benefits in patients for whom epilepsy is the primary diagnosis.
Consider the limitations
Using AEDs to reduce polypharmacy should be done judiciously from a drug-drug interaction perspective, because certain AEDs (eg, carbamazepine, divalproex) can greatly influence the metabolism of other medications, which may defeat the best intentions of the original intervention.4,6
Several other limitations should be considered. This article does not include all AEDs, only those commonly used for psychiatric indications with known nonpsychiatric benefits. Some may worsen psychiatric conditions (such as rage and irritability in the case of levetiracetam), and all AEDs have an FDA warning regarding suicidal behaviors and ideation.14,15 Another important limitation is the potential for differential dosing across indications; tolerability concerns may limit adequate dosing across multiple uses. For example, topiramate’s migraine prophylaxis effect can be achieved at much lower doses than the patient-specific efficacy dosing seen in binge eating disorder, with higher doses increasing the propensity for adverse effects.13,16Dual-use AEDs should be considered wherever possible, but judicious review of evidence is necessary to appropriately adjudicate a specific patient’s risk vs benefit. The Table4,6-9,12,13,16-68 provides information on select AEDs with both psychiatric and nonpsychiatric indications, including both FDA-approved and common off-label uses. These indications are limited to adult use only.
CASE CONTINUED
After reviewing Mr. B’s medical history, the treating medical team decides to cross-taper escitalopram to duloxetine 30 mg twice daily. Though his pain lessens after several weeks, it persists enough to interfere with Mr. B’s daily life. In addition to duloxetine, he is started on pregabalin 50 mg 3 times a day. Mr. B’s pain decreases to a tolerable level, and he reports decreased worrying and restlessness, and improvements in concentration and sleep.
1. Meyer JM. A concise guide to monoamine oxidase inhibitors. Current Psychiatry. 2017;16(12):14-16,18-23,47,A.
2. Ban TA. Fifty years chlorpromazine: a historical perspective. Neuropsychiatr Dis Treat. 2007;3(4):495-500.
3. López-Mun
4. Depakote [package insert]. North Chicago, IL: AbbVie, Inc; 2021.
5. Henry TR. The history of valproate in clinical neuroscience. Psychopharmacol Bull. 2003;37 Suppl 2:5-16.
6. Tegretol and Tegretol-XR [package insert]. East Hanover, NJ: Pharmaceuticals Co.; 2020.
7. Lamictal [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2009.
8. Lithobid [package insert]. Baudette, MN: ANI Pharmaceuticals, Inc; 2009.
9. Yatham LN, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018;20(2):97-170.
10. National Alliance on Mental Illness. Anosognosia. Common with mental illness. Accessed March 3, 2022. https://www.nami.org/About-Mental-Illness/Common-with-Mental-Illness/Anosognosia
11. Hales CM, Servais J, Martin CB, et al. Prescription drug use among adults aged 40-79 in the United States and Canada. NCHS Data Brief. 2019(347):1-8.
12. Neurontin [package insert]. New York, NY: Pfizer; 2017.
13. Topamax [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc; 2009.
14. Molokwu OA, Ezeala-Adikaibe BA, Onwuekwe IO. Levetiracetam-induced rage and suicidality: two case reports and review of literature. Epilepsy Behav Case Rep. 2015;4:79-81.
15. U.S. Food & Drug Administration. FDA Statistical Review and Evaluation. Antiepileptic Drugs and Suicidality. 2008. Accessed March 3, 2022. https://www.fda.gov/files/drugs/published/Statistical-Review-and-Evaluation--Antiepileptic-Drugs-and-Suicidality.pdf
16. McElroy SL, Hudson JI, Capece JA, et al. Topiramate for the treatment of binge eating disorder associated with obesity: a placebo-controlled study. Biol Psychiatry. 2007;61(9):1039-1048.
17. Zhang ZJ, Kang WH, Tan QR, et al. Adjunctive herbal medicine with carbamazepine for bipolar disorders: a double-blind, randomized, placebo-controlled study. J Psychiatr Res. 2007;41(3-4):360-369.
18. Kleindienst N, Greil W. Differential efficacy of lithium and carbamazepine in the prophylaxis of bipolar disorder: results of the MAP study. Neuropsychobiology. 2000;42 Suppl 1:2-10.
19. Goodwin GM, Haddad PM, Ferrier IN, et al. Evidence-based guidelines for treating bipolar disorder: revised third edition recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2016;30(6):495-553.
20. Davis LL, Bartolucci A, Petty F. Divalproex in the treatment of bipolar depression: a placebo-controlled study. J Affect Disord. 2005;85(3):259-266.
21. Gyulai L, Bowden CL, McElroy SL, et al. Maintenance efficacy of divalproex in the prevention of bipolar depression. Neuropsychopharmacology. 2003;28(7):1374-1382.
22. Limdi NA, Shimpi AV, Faught E, et al. Efficacy of rapid IV administration of valproic acid for status epilepticus. Neurology. 2005;64(2):353-355.
23. Temkin NR, Dikmen SS, Anderson GD, et al. Valproate therapy for prevention of posttraumatic seizures: a randomized trial. J Neurosurg. 1999; 91(4):593-600.
24. Reus VI, Fochtmann LJ, Bukstein O, et al. The American Psychiatric Association practice guideline for the pharmacological treatment of patients with alcohol use disorder. Am J Psychiatry. 2018;175(1):86-90.
25. US Dept of Veterans Affairs, US Dept of Defense, The Management of Substance Use Disorders Work Group. VA/DoD clinical practice guideline for the management of substance use disorders. US Dept of Veterans Affairs/Dept of Defense; 2015. Accessed March 3, 2022. http://www.healthquality.va.gov/guidelines/MH/sud/VADoDSUDCPGRevised22216.pdf
26. Myrick H, Malcolm R, Randall PK, et al. A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res. 2009;33(9):1582-1588.
27. Ahmed S, Stanciu CN, Kotapati PV, et al. Effectiveness of gabapentin in reducing cravings and withdrawal in alcohol use disorder: a meta-analytic review. Prim Care Companion CNS Disord. 2019;21(4):19r02465.
28. Pande AC, Davidson JR, Jefferson JW, et al. Treatment of social phobia with gabapentin: a placebo-controlled study. J Clin Psychopharmacol. 1999;19(4):341-348.
29. Ryan NM, Birring SS, Gibson PG. Gabapentin for refractory chronic cough: a randomized, double-blind, placebo-controlled trial. Lancet. 2012;380(9853):1583-1589.
30. Gibson P, Wang G, McGarvey L, et al. Treatment of unexplained chronic cough: CHEST guideline and expert panel report. Chest. 2016;149(1):27-44.
31. Arnold LM, Goldenberg DL, Stanford SB, et al. Gabapentin in the treatment of fibromyalgia: a randomized, double-blind, placebo-controlled, multicenter trial. Arthritis Rheum. 2007;56(4):1336-1344.
32. Alonso-Navarro H, Rubio L, Jiménez-Jiménez FJ. Refractory hiccup: successful treatment with gabapentin. Clin Neuropharmacol. 2007;30(3):186-187.
33. Jatzko A, Stegmeier-Petroianu A, Petroianu GA. Alpha-2-delta ligands for singultus (hiccup) treatment: three case reports. J Pain Symptom Manage. 2007;33(6):756-760.
34. Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14(2):162-173.
35. Moore RA, Wiffen PJ, Derry S, et al. Gabapentin for chronic neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2014;2014(4):CD007938.
36. Yuan M, Zhou HY, Xiao ZL, et al. Efficacy and safety of gabapentin vs. carbamazepine in the treatment of trigeminal neuralgia: a meta-analysis. Pain Pract. 2016;16(8):1083-1091.
37. Weisshaar E, Szepietowski JC, Darsow U, et al. European guideline on chronic pruritus. Acta Derm Venereol. 2012;92(5):563-581.
38. Garcia-Borreguero D, Silber MH, Winkelman JW, et al. Guidelines for the first-line treatment of restless legs syndrome/Willis-Ekbom disease, prevention and treatment of dopaminergic augmentation: a combined task force of the IRLSSG, EURLSSG, and the RLS-Foundation. Sleep Med. 2016;21:1-11.
39. Cobin RH, Goodman NF; AACE Reproductive Endocrinology Scientific Committee. American Association of Clinical Endocrinologists and American College of Endocrinology position statement on menopause—2017 update [published correction appears in Endocr Pract. 2017;23 (12):1488]. Endocr Pract. 2017;23(7):869-880.
40. Calabrese JR, Suppes T, Bowden CL, et al. A double-blind, placebo-controlled, prophylaxis study of lamotrigine in rapid-cycling bipolar disorder: Lamictal 614 Study Group. J Clin Psychiatry. 2000;60(11):841-850.
41. May A, Leone M, Afra J, et al. EFNS guidelines on the treatment of cluster headache and other trigeminal-autonomic cephalalgias. Eur J Neurol. 2006;13(10):1066-1077.
42. Stein G, Bernadt M. Lithium augmentation therapy in tricyclic-resistant depression. A controlled trial using lithium in low and normal doses. Br J Psychiatry. 1993;162:634-640.
43. Craft M, Ismail IA, Krishnamurti D, et al. Lithium in the treatment of aggression in mentally handicapped patients: a double-blind trial. Br J Psychiatry. 1987;150:685-689.
44. Cipriani A, Pretty H, Hawton K, et al. Lithium in the prevention of suicidal behavior and all-cause mortality in patients with mood disorders: a systematic review of randomized trials. Am J Psychiatry. 2005;162(10):1805-1819.
45. Dickstein G, Shechner C, Adawi F, et al. Lithium treatment in amiodarone-induced thyrotoxicosis. Am J Med. 1997;102(5):454-458.
46. Bogazzi F, Bartalena L, Brogioni S, et al. Comparison of radioiodine with radioiodine plus lithium in the treatment of Graves’ hyperthyroidism. J Clin Endocrinol Metab. 1999;84(2):499-503.
47. Lyrica [package insert]. New York, NY: Parke-Davis, Division of Pfizer Inc; 2020.
48. Lydiard RB, Rickels K, Herman B, et al. Comparative efficacy of pregabalin and benzodiazepines in treating the psychic and somatic symptoms of generalized anxiety disorder. Int J Neuropsychopharmacol. 2010;13(2):229-241.
49. Pande AC, Feltner DE, Jefferson JW, et al. Efficacy of the novel anxiolytic pregabalin in social anxiety disorder: a placebo-controlled, multicenter study. J Clin Psychopharmacol. 2004;24(2):141-149.
50. Vertigan AE, Kapela SL, Ryan NM, et al. Pregabalin and speech pathology combination therapy for refractory chronic cough: a randomized controlled trial. Chest. 2016;149(3):639-648.
51. Matsuda KM, Sharma D, Schonfeld AR, et al. Gabapentin and pregabalin for the treatment of chronic pruritus. J Am Acad Dermatol. 2016;75(3):619-625.e6.
52. Allen R, Chen C, Soaita A, et al. A randomized, double-blind, 6-week, dose-ranging study of pregabalin in patients with restless legs syndrome. Sleep Med. 2010;11(6):512-519.
53. Loprinzi CL, Qin R, Balcueva EP, et al. Phase III, randomized, double-blind, placebo-controlled evaluation of pregabalin for alleviating hot flashes, N07C1 [published correction appears in J Clin Oncol. 2010;28(10):1808]. J Clin Oncol. 2010;28(4):641-647.
54. Dunlop BW, Papp L, Garlow SJ, et al. Tiagabine for social anxiety disorder. Hum Psychopharmacol. 2007;22(4):241-244.
55. Paparrigopoulos T, Tzavellas E, Karaiskos D, et al. An open pilot study of tiagabine in alcohol dependence: tolerability and clinical effects. J Psychopharmacol. 2010;24(9):1375-1380.
56. Gabitril [package insert]. North Wales, PA: Teva Pharmaceuticals USA, Inc; 2015.
57. Johnson BA, Ait-Daoud N, Bowden C, et al. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003;361(9370):1677-1685.
58. Linde M, Mulleners WM, Chronicle EP, et al. Topiramate for the prophylaxis of episodic migraine in adults. Cochrane Database Syst Rev. 2013;2013(6):CD010610.
59. Pascual J, Láinez MJ, Dodick D, et al. Antiepileptic drugs for the treatment of chronic and episodic cluster headache: a review. Headache. 2007;47(1):81-89.
60. Ondo WG, Jankovic J, Connor GS, et al. Topiramate in essential tremor: a double-blind, placebo-controlled trial. Neurology. 2006;66(5):672-677.
61. Ko YH, Joe SH, Jung IK, et al. Topiramate as an adjuvant treatment with atypical antipsychotics in schizophrenic patients experiencing weight gain. Clin Neuropharmacol. 2005;28(4):169-175.
62. Wilding J, Van Gaal L, Rissanen A, et al. A randomized double-blind placebo-controlled study of the long-term efficacy and safety of topiramate in the treatment of obese subjects. Int J Obes Relat Metab Disord. 2004;28(11):1399-1410.
63. Rosenstock J, Hollander P, Gadde KM, et al. A randomized, double-blind, placebo-controlled, multicenter study to assess the efficacy and safety of topiramate controlled release in the treatment of obese type 2 diabetic patients. Diabetes Care. 2007; 30(6):1480-1486.
64. McElroy SL, Kotwal R, Guerdjikova AI, et al. Zonisamide in the treatment of binge eating disorder with obesity: a randomized controlled trial. J Clin Psychiatry. 2006;67(12):1897-1906.
65. Zonegran [package insert]. Teaneck, NJ: Eisai Inc; 2006.
66. Drake ME Jr, Greathouse NI, Renner JB, et al. Open-label zonisamide for refractory migraine. Clin Neuropharmacol. 2004;27(6):278-280.
67. Matsunaga S, Kishi T, Iwata N. Combination therapy with zonisamide and antiparkinson drugs for Parkinson’s disease: a meta-analysis. J Alzheimers Dis. 2017;56(4):1229-1239.
68. Gadde KM, Kopping MF, Wagner HR 2nd, et al. Zonisamide for weight reduction in obese adults: a 1-year randomized controlled trial. Arch Intern Med. 2012;172(20):1557-1564.
1. Meyer JM. A concise guide to monoamine oxidase inhibitors. Current Psychiatry. 2017;16(12):14-16,18-23,47,A.
2. Ban TA. Fifty years chlorpromazine: a historical perspective. Neuropsychiatr Dis Treat. 2007;3(4):495-500.
3. López-Mun
4. Depakote [package insert]. North Chicago, IL: AbbVie, Inc; 2021.
5. Henry TR. The history of valproate in clinical neuroscience. Psychopharmacol Bull. 2003;37 Suppl 2:5-16.
6. Tegretol and Tegretol-XR [package insert]. East Hanover, NJ: Pharmaceuticals Co.; 2020.
7. Lamictal [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2009.
8. Lithobid [package insert]. Baudette, MN: ANI Pharmaceuticals, Inc; 2009.
9. Yatham LN, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018;20(2):97-170.
10. National Alliance on Mental Illness. Anosognosia. Common with mental illness. Accessed March 3, 2022. https://www.nami.org/About-Mental-Illness/Common-with-Mental-Illness/Anosognosia
11. Hales CM, Servais J, Martin CB, et al. Prescription drug use among adults aged 40-79 in the United States and Canada. NCHS Data Brief. 2019(347):1-8.
12. Neurontin [package insert]. New York, NY: Pfizer; 2017.
13. Topamax [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc; 2009.
14. Molokwu OA, Ezeala-Adikaibe BA, Onwuekwe IO. Levetiracetam-induced rage and suicidality: two case reports and review of literature. Epilepsy Behav Case Rep. 2015;4:79-81.
15. U.S. Food & Drug Administration. FDA Statistical Review and Evaluation. Antiepileptic Drugs and Suicidality. 2008. Accessed March 3, 2022. https://www.fda.gov/files/drugs/published/Statistical-Review-and-Evaluation--Antiepileptic-Drugs-and-Suicidality.pdf
16. McElroy SL, Hudson JI, Capece JA, et al. Topiramate for the treatment of binge eating disorder associated with obesity: a placebo-controlled study. Biol Psychiatry. 2007;61(9):1039-1048.
17. Zhang ZJ, Kang WH, Tan QR, et al. Adjunctive herbal medicine with carbamazepine for bipolar disorders: a double-blind, randomized, placebo-controlled study. J Psychiatr Res. 2007;41(3-4):360-369.
18. Kleindienst N, Greil W. Differential efficacy of lithium and carbamazepine in the prophylaxis of bipolar disorder: results of the MAP study. Neuropsychobiology. 2000;42 Suppl 1:2-10.
19. Goodwin GM, Haddad PM, Ferrier IN, et al. Evidence-based guidelines for treating bipolar disorder: revised third edition recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2016;30(6):495-553.
20. Davis LL, Bartolucci A, Petty F. Divalproex in the treatment of bipolar depression: a placebo-controlled study. J Affect Disord. 2005;85(3):259-266.
21. Gyulai L, Bowden CL, McElroy SL, et al. Maintenance efficacy of divalproex in the prevention of bipolar depression. Neuropsychopharmacology. 2003;28(7):1374-1382.
22. Limdi NA, Shimpi AV, Faught E, et al. Efficacy of rapid IV administration of valproic acid for status epilepticus. Neurology. 2005;64(2):353-355.
23. Temkin NR, Dikmen SS, Anderson GD, et al. Valproate therapy for prevention of posttraumatic seizures: a randomized trial. J Neurosurg. 1999; 91(4):593-600.
24. Reus VI, Fochtmann LJ, Bukstein O, et al. The American Psychiatric Association practice guideline for the pharmacological treatment of patients with alcohol use disorder. Am J Psychiatry. 2018;175(1):86-90.
25. US Dept of Veterans Affairs, US Dept of Defense, The Management of Substance Use Disorders Work Group. VA/DoD clinical practice guideline for the management of substance use disorders. US Dept of Veterans Affairs/Dept of Defense; 2015. Accessed March 3, 2022. http://www.healthquality.va.gov/guidelines/MH/sud/VADoDSUDCPGRevised22216.pdf
26. Myrick H, Malcolm R, Randall PK, et al. A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res. 2009;33(9):1582-1588.
27. Ahmed S, Stanciu CN, Kotapati PV, et al. Effectiveness of gabapentin in reducing cravings and withdrawal in alcohol use disorder: a meta-analytic review. Prim Care Companion CNS Disord. 2019;21(4):19r02465.
28. Pande AC, Davidson JR, Jefferson JW, et al. Treatment of social phobia with gabapentin: a placebo-controlled study. J Clin Psychopharmacol. 1999;19(4):341-348.
29. Ryan NM, Birring SS, Gibson PG. Gabapentin for refractory chronic cough: a randomized, double-blind, placebo-controlled trial. Lancet. 2012;380(9853):1583-1589.
30. Gibson P, Wang G, McGarvey L, et al. Treatment of unexplained chronic cough: CHEST guideline and expert panel report. Chest. 2016;149(1):27-44.
31. Arnold LM, Goldenberg DL, Stanford SB, et al. Gabapentin in the treatment of fibromyalgia: a randomized, double-blind, placebo-controlled, multicenter trial. Arthritis Rheum. 2007;56(4):1336-1344.
32. Alonso-Navarro H, Rubio L, Jiménez-Jiménez FJ. Refractory hiccup: successful treatment with gabapentin. Clin Neuropharmacol. 2007;30(3):186-187.
33. Jatzko A, Stegmeier-Petroianu A, Petroianu GA. Alpha-2-delta ligands for singultus (hiccup) treatment: three case reports. J Pain Symptom Manage. 2007;33(6):756-760.
34. Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14(2):162-173.
35. Moore RA, Wiffen PJ, Derry S, et al. Gabapentin for chronic neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2014;2014(4):CD007938.
36. Yuan M, Zhou HY, Xiao ZL, et al. Efficacy and safety of gabapentin vs. carbamazepine in the treatment of trigeminal neuralgia: a meta-analysis. Pain Pract. 2016;16(8):1083-1091.
37. Weisshaar E, Szepietowski JC, Darsow U, et al. European guideline on chronic pruritus. Acta Derm Venereol. 2012;92(5):563-581.
38. Garcia-Borreguero D, Silber MH, Winkelman JW, et al. Guidelines for the first-line treatment of restless legs syndrome/Willis-Ekbom disease, prevention and treatment of dopaminergic augmentation: a combined task force of the IRLSSG, EURLSSG, and the RLS-Foundation. Sleep Med. 2016;21:1-11.
39. Cobin RH, Goodman NF; AACE Reproductive Endocrinology Scientific Committee. American Association of Clinical Endocrinologists and American College of Endocrinology position statement on menopause—2017 update [published correction appears in Endocr Pract. 2017;23 (12):1488]. Endocr Pract. 2017;23(7):869-880.
40. Calabrese JR, Suppes T, Bowden CL, et al. A double-blind, placebo-controlled, prophylaxis study of lamotrigine in rapid-cycling bipolar disorder: Lamictal 614 Study Group. J Clin Psychiatry. 2000;60(11):841-850.
41. May A, Leone M, Afra J, et al. EFNS guidelines on the treatment of cluster headache and other trigeminal-autonomic cephalalgias. Eur J Neurol. 2006;13(10):1066-1077.
42. Stein G, Bernadt M. Lithium augmentation therapy in tricyclic-resistant depression. A controlled trial using lithium in low and normal doses. Br J Psychiatry. 1993;162:634-640.
43. Craft M, Ismail IA, Krishnamurti D, et al. Lithium in the treatment of aggression in mentally handicapped patients: a double-blind trial. Br J Psychiatry. 1987;150:685-689.
44. Cipriani A, Pretty H, Hawton K, et al. Lithium in the prevention of suicidal behavior and all-cause mortality in patients with mood disorders: a systematic review of randomized trials. Am J Psychiatry. 2005;162(10):1805-1819.
45. Dickstein G, Shechner C, Adawi F, et al. Lithium treatment in amiodarone-induced thyrotoxicosis. Am J Med. 1997;102(5):454-458.
46. Bogazzi F, Bartalena L, Brogioni S, et al. Comparison of radioiodine with radioiodine plus lithium in the treatment of Graves’ hyperthyroidism. J Clin Endocrinol Metab. 1999;84(2):499-503.
47. Lyrica [package insert]. New York, NY: Parke-Davis, Division of Pfizer Inc; 2020.
48. Lydiard RB, Rickels K, Herman B, et al. Comparative efficacy of pregabalin and benzodiazepines in treating the psychic and somatic symptoms of generalized anxiety disorder. Int J Neuropsychopharmacol. 2010;13(2):229-241.
49. Pande AC, Feltner DE, Jefferson JW, et al. Efficacy of the novel anxiolytic pregabalin in social anxiety disorder: a placebo-controlled, multicenter study. J Clin Psychopharmacol. 2004;24(2):141-149.
50. Vertigan AE, Kapela SL, Ryan NM, et al. Pregabalin and speech pathology combination therapy for refractory chronic cough: a randomized controlled trial. Chest. 2016;149(3):639-648.
51. Matsuda KM, Sharma D, Schonfeld AR, et al. Gabapentin and pregabalin for the treatment of chronic pruritus. J Am Acad Dermatol. 2016;75(3):619-625.e6.
52. Allen R, Chen C, Soaita A, et al. A randomized, double-blind, 6-week, dose-ranging study of pregabalin in patients with restless legs syndrome. Sleep Med. 2010;11(6):512-519.
53. Loprinzi CL, Qin R, Balcueva EP, et al. Phase III, randomized, double-blind, placebo-controlled evaluation of pregabalin for alleviating hot flashes, N07C1 [published correction appears in J Clin Oncol. 2010;28(10):1808]. J Clin Oncol. 2010;28(4):641-647.
54. Dunlop BW, Papp L, Garlow SJ, et al. Tiagabine for social anxiety disorder. Hum Psychopharmacol. 2007;22(4):241-244.
55. Paparrigopoulos T, Tzavellas E, Karaiskos D, et al. An open pilot study of tiagabine in alcohol dependence: tolerability and clinical effects. J Psychopharmacol. 2010;24(9):1375-1380.
56. Gabitril [package insert]. North Wales, PA: Teva Pharmaceuticals USA, Inc; 2015.
57. Johnson BA, Ait-Daoud N, Bowden C, et al. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003;361(9370):1677-1685.
58. Linde M, Mulleners WM, Chronicle EP, et al. Topiramate for the prophylaxis of episodic migraine in adults. Cochrane Database Syst Rev. 2013;2013(6):CD010610.
59. Pascual J, Láinez MJ, Dodick D, et al. Antiepileptic drugs for the treatment of chronic and episodic cluster headache: a review. Headache. 2007;47(1):81-89.
60. Ondo WG, Jankovic J, Connor GS, et al. Topiramate in essential tremor: a double-blind, placebo-controlled trial. Neurology. 2006;66(5):672-677.
61. Ko YH, Joe SH, Jung IK, et al. Topiramate as an adjuvant treatment with atypical antipsychotics in schizophrenic patients experiencing weight gain. Clin Neuropharmacol. 2005;28(4):169-175.
62. Wilding J, Van Gaal L, Rissanen A, et al. A randomized double-blind placebo-controlled study of the long-term efficacy and safety of topiramate in the treatment of obese subjects. Int J Obes Relat Metab Disord. 2004;28(11):1399-1410.
63. Rosenstock J, Hollander P, Gadde KM, et al. A randomized, double-blind, placebo-controlled, multicenter study to assess the efficacy and safety of topiramate controlled release in the treatment of obese type 2 diabetic patients. Diabetes Care. 2007; 30(6):1480-1486.
64. McElroy SL, Kotwal R, Guerdjikova AI, et al. Zonisamide in the treatment of binge eating disorder with obesity: a randomized controlled trial. J Clin Psychiatry. 2006;67(12):1897-1906.
65. Zonegran [package insert]. Teaneck, NJ: Eisai Inc; 2006.
66. Drake ME Jr, Greathouse NI, Renner JB, et al. Open-label zonisamide for refractory migraine. Clin Neuropharmacol. 2004;27(6):278-280.
67. Matsunaga S, Kishi T, Iwata N. Combination therapy with zonisamide and antiparkinson drugs for Parkinson’s disease: a meta-analysis. J Alzheimers Dis. 2017;56(4):1229-1239.
68. Gadde KM, Kopping MF, Wagner HR 2nd, et al. Zonisamide for weight reduction in obese adults: a 1-year randomized controlled trial. Arch Intern Med. 2012;172(20):1557-1564.
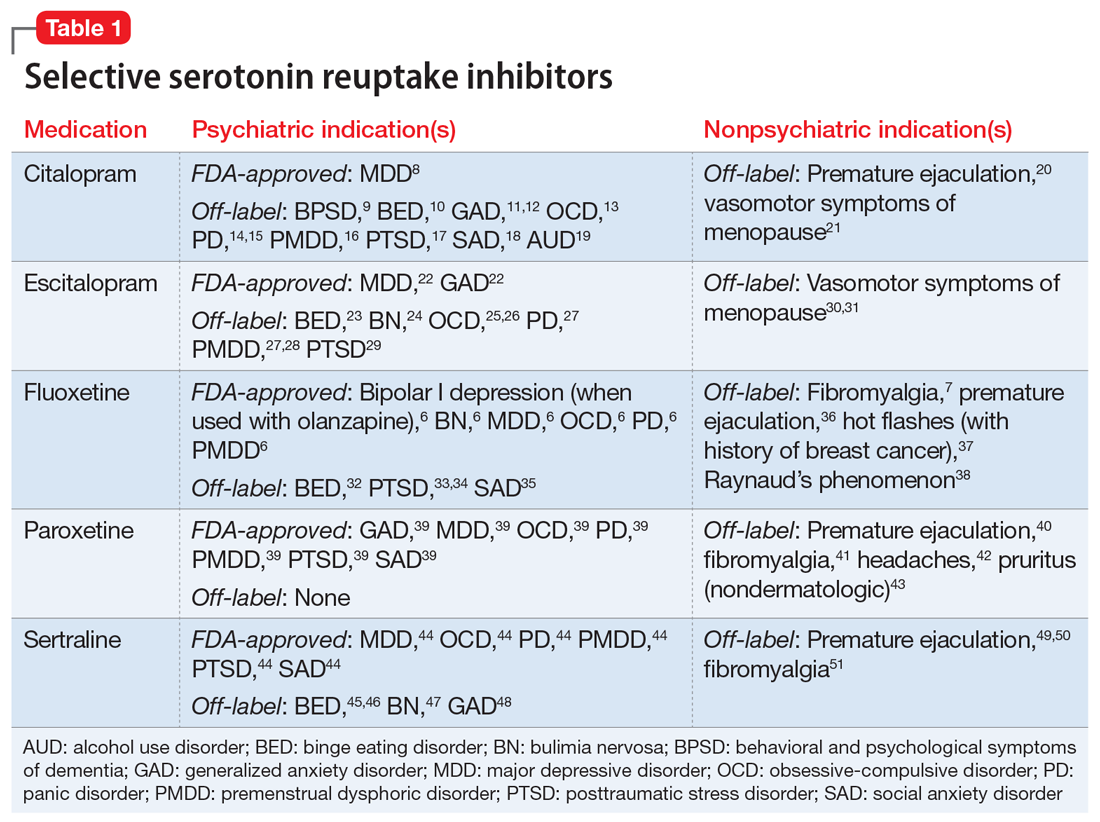
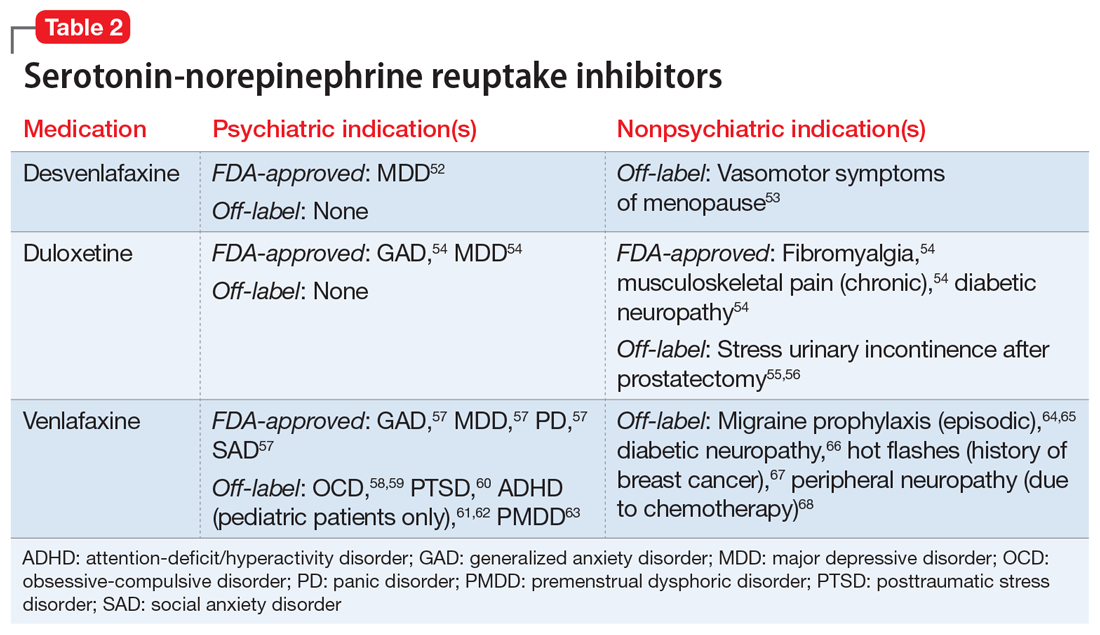
Nonpsychiatric indications for antidepressants and antipsychotics
Ms. A, age 45, is hospitalized for abdominal pain. She is noted to have hiccups, the onset of which she reports was >1 month ago and did not have a clear precipitant. Abdominal and head imaging return no acute findings, and data from a serum electrolyte test, hepatic function test, and thyroid function test are within normal limits. The medical team notices that Ms. A’s speech is pressured, she hardly sleeps, and she appears animated, full of ideas and energy.
Ms. A has a history of bipolar I disorder, hypertension, hyperlipidemia, gastroesophageal reflux disease, and hypothyroidism. Her present medications include hydrochlorothiazide 25 mg/d; levothyroxine 25 mcg/d; omeprazole 20 mg/d; and lovastatin 20 mg/d. She states that she was remotely treated for bipolar disorder, but she was cured by a shamanic healer, and therefore no longer needs treatment.
Approximately 35% of adults in the United States age 60 to 79 reported taking ≥5 prescription medications in 2016, compared to 15% of adults age 40 to 59.1 In a study of 372 patients with advanced, life-limiting illness, Schenker et al2 found that those who took multiple medications (mean: 11.6 medications) had a lower quality of life and worse symptoms. Optimizing medications to patients’ specific needs and diagnoses in order to reduce pill burden can be a favorable intervention. In addition, some patients—approximately 30% of those with schizophrenia and 20% of those with bipolar disorder—may not have insight into their mental illness as they do with their medical conditions, and may be more accepting of treatment for the latter.3 Dual-indication prescribing may be a useful way to decrease polypharmacy, reduce potential drug-drug interactions (DDIs), increase patient acceptance and adherence, and improve a patient’s overall health.
Continue on for: Multiple uses for antidepressants and antipsychotics...
Multiple uses for antidepressants and antipsychotics
One of the first medications discovered to have antidepressant effects was iproniazid, a monoamine oxidase inhibitor (MAOI) initially used to treat tuberculosis.4 Since then, numerous classes of antidepressant medications have been developed that capitalize on monoamine reuptake through several different mechanisms of action. These drugs can be grouped into subclasses that include selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, tricyclic antidepressants, MAOIs, and others. True to their roots in iproniazid, these medications can have a myriad of effects not limited to mental health and can therefore be beneficial for a variety of comorbid conditions.
As was the case with antidepressants, the first medication approved in the antipsychotic class, chlorpromazine, was serendipitously discovered to treat psychosis and agitation after being approved and used to treat presurgical apprehension.5 The term “antipsychotic” is almost a misnomer given these agents’ broad pharmacology profiles and impact on various mental illnesses, including bipolar disorder, depressive disorders, anxiety disorders, and many other mental conditions. First-generation antipsychotics (FGAs) were the first to enter the market; they work primarily by blocking dopamine-2 (D2) receptors. Second-generation antipsychotics have less movement-based adverse effects than FGAs by having higher affinity for serotonin 5-HT2A receptors than for D2 receptors. However, they tend to carry a higher risk for weight gain and metabolic syndrome.
Antidepressants and antipsychotics are widely utilized in psychiatry. Many have been found to have additional uses beyond their original FDA-approved indication and can therefore be beneficial for a variety of comorbid conditions.
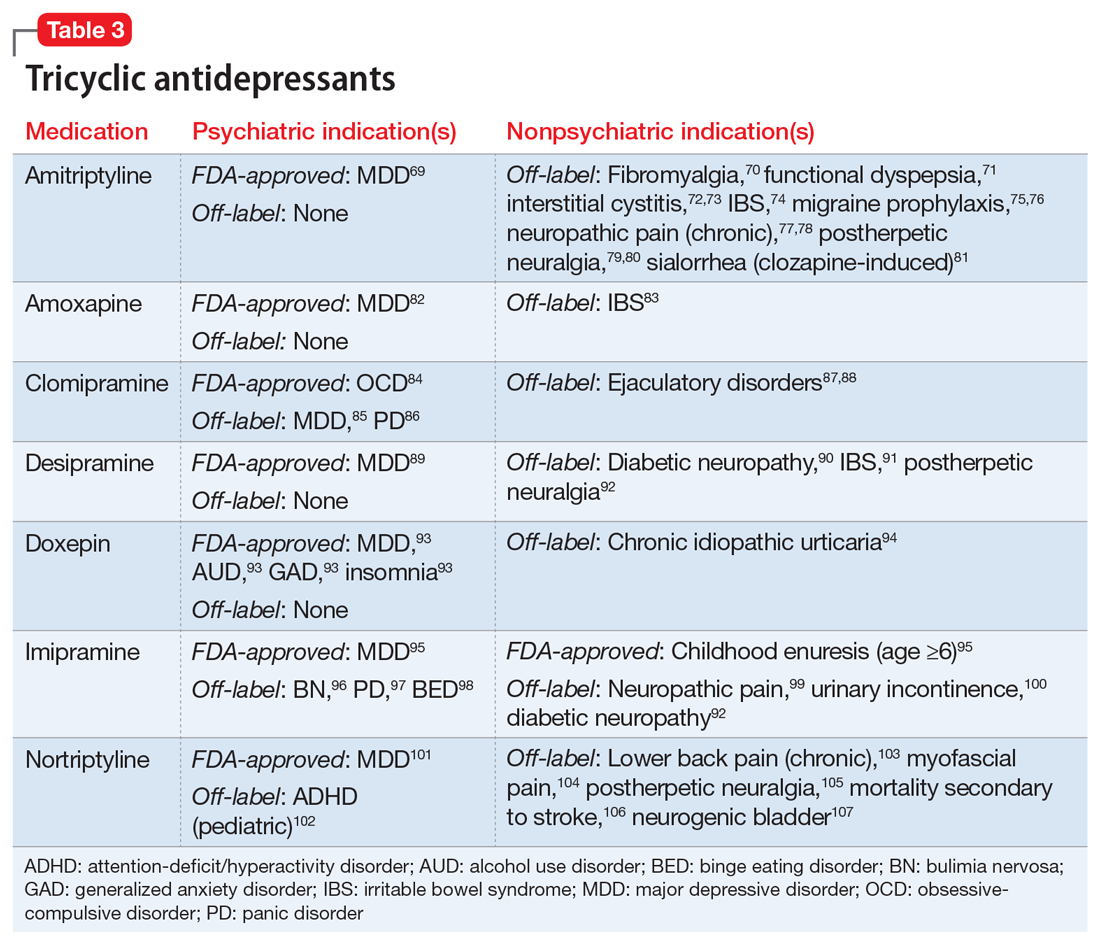
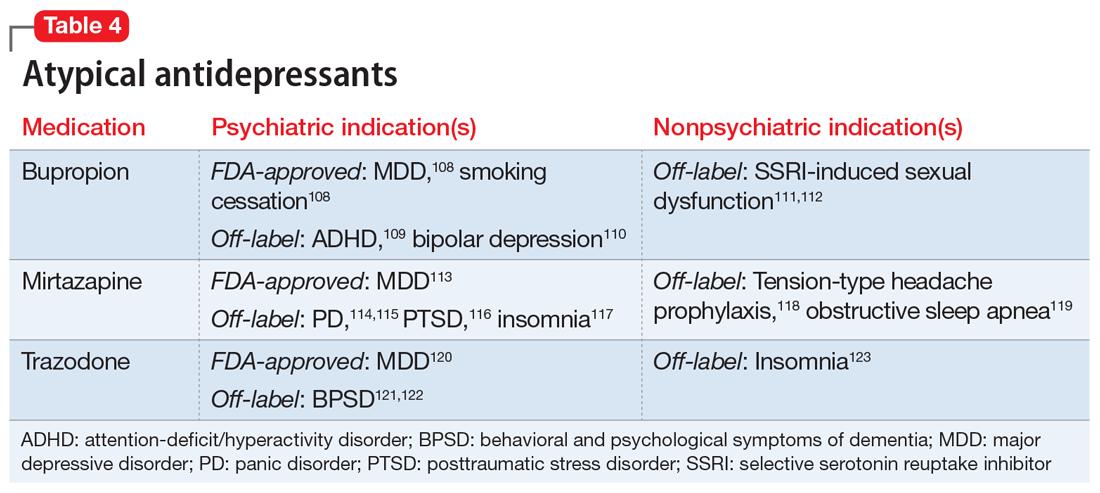
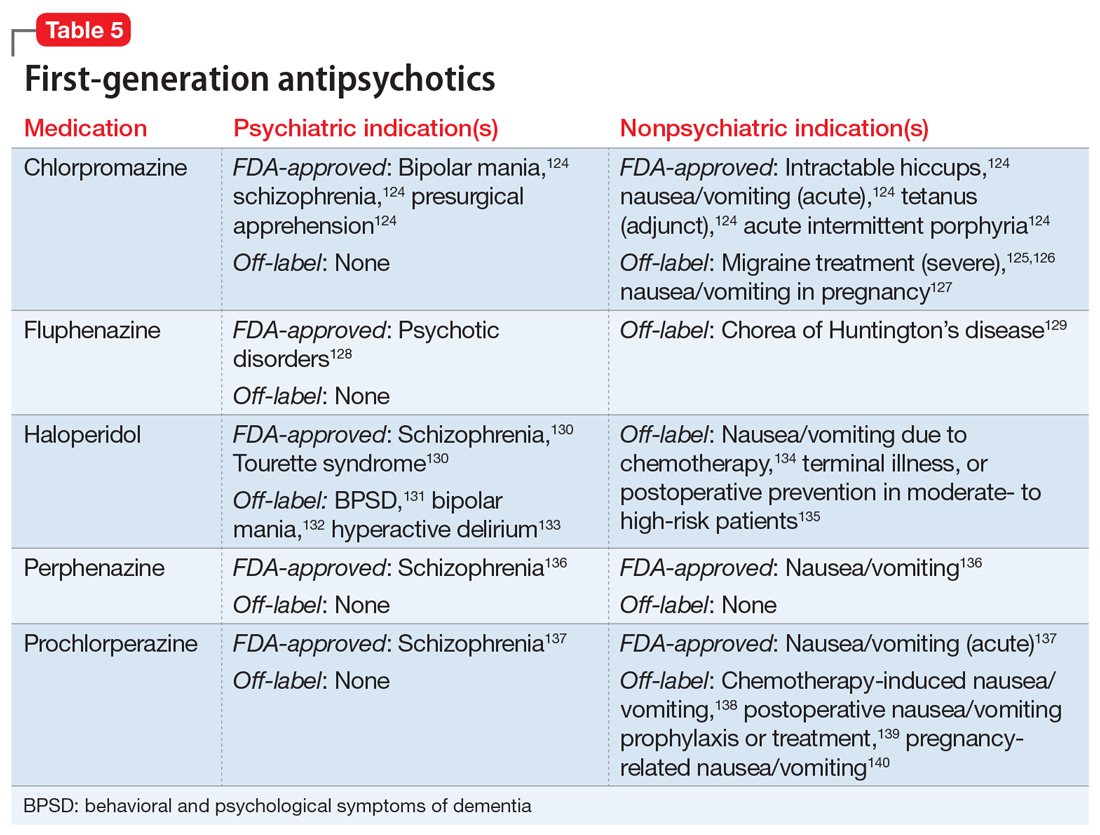
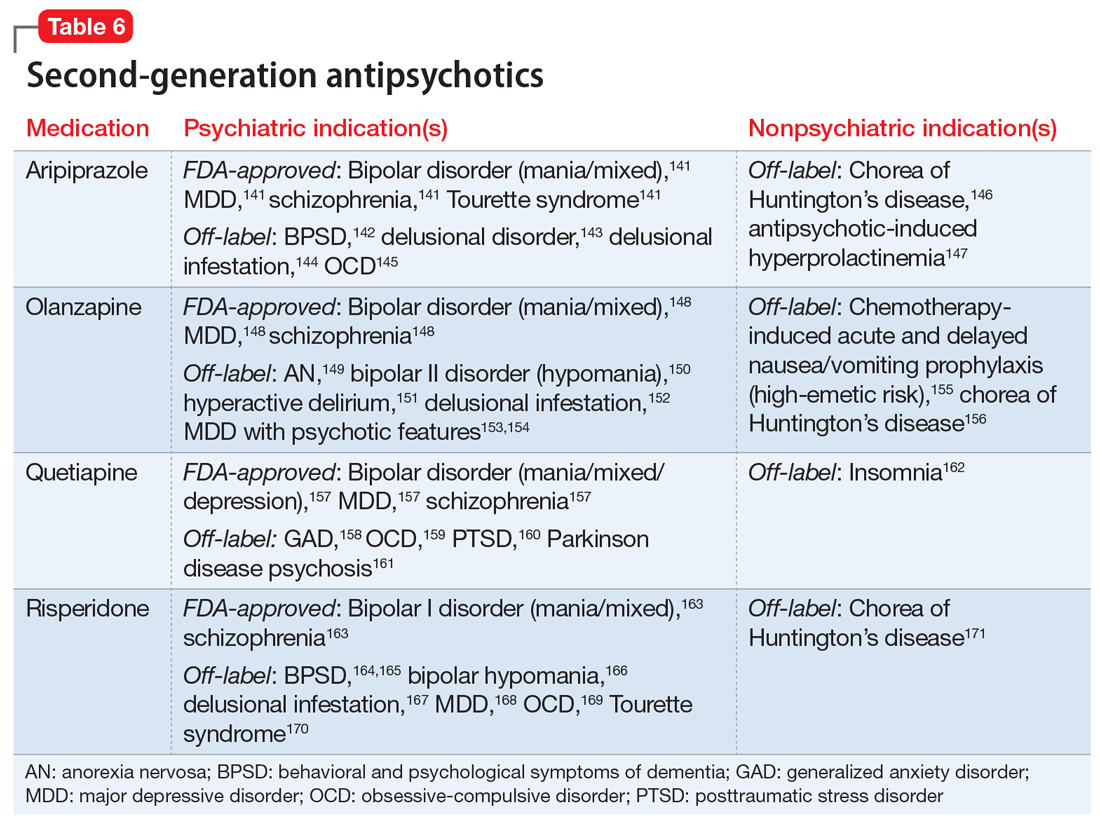
One limitation of using psychiatric medications for nonpsychiatric indications is that different doses of antidepressants and antipsychotics are typically targeted for different indications based on receptor binding affinity. A common example of this is trazodone, where doses below 100 mg are used as needed for insomnia, but higher doses ranging from 200 to 600 mg/d are used for depression. Another important consideration is DDIs. For example, the possibility of adding an agent such as fluoxetine to a complex pain regimen for fibromyalgia could impact the clearance of other agents that are cytochrome P450 (CYP) 2D6 substrates due to fluoxetine’s potent inhibition of the enzyme.6,7 Table 16-51, Table 252-68, Table 369-107, and Table 4108-123 provide information on select antidepressants, while Table 5124-140 and Table 6141-171 provide information on select antipsychotics. Each table lists psychiatric and nonpsychiatric indications for the respective medications, including both FDA-approved (where applicable) and common off-label uses. Most of the indications listed are for adult use only, unless otherwise noted.
Continue on to: Case Continued...
CASE CONTINUED
After reviewing Ms. A’s medical history, the treatment team initiates chlorpromazine, 25 mg 3 times a day, for intractable hiccups, and increases the dosage to 50 mg 3 times a day after 3 days. Chlorpromazine is FDA-approved for treating bipolar mania, and also for treating intractable hiccups. Shortly thereafter, Ms. A’s hiccups subside, she sleeps for longer periods, and her manic symptoms resolve.
1. Hales CM, Servais J, Martin CB, et al. Prescription drug use among adults aged 40-79 in the United States and Canada. National Center for Health Statistics (Centers for Disease Control and Prevention). 2019. NCHS Data Brief No. 347. https://www.cdc.gov/nchs/products/databriefs/db347.htm
2. Schenker Y, Park SY, Jeong K, et al. Associations between polypharmacy, symptom burden, and quality of life in patients with advanced, life-limiting illness. J Gen Intern Med. 2019;34(4):559-566.
3. National Alliance on Mental Illness. Anosognosia. 2021. https://www.nami.org/About-Mental-Illness/Common-with-Mental-Illness/Anosognosia
4. Meyer JM. A concise guide to monoamine oxidase inhibitors. Current Psychiatry. 2017;16(12):14-16,18-23,47,A.
5. Ban TA. Fifty years chlorpromazine: a historical perspective. Neuropsychiatr Dis Treat. 2007;3(4):495-500.
6. Prozac [package insert]. Indianapolis, IN: Eli Lilly and Company; 2009.
7. Arnold LM, Hess EV, Hudson JI, et al. A randomized, placebo-controlled, double-blind, flexible-dose study of fluoxetine in the treatment of women with fibromyalgia. Am J Med. 2002;112(3):191-197.
8. Celexa [package insert]. St. Louis, MO: Forest Pharmaceuticals, Inc; 2009.
9. Porsteinsson AP, Drye LT, Pollock BG, et al. Effect of citalopram on agitation in Alzheimer disease: the CitAD randomized clinical trial. JAMA. 2014;311(7):682-691.
10. McElroy SL, Hudson JI, Malhotra S, et al. Citalopram in the treatment of binge-eating disorder: a placebo-controlled trial. J Clin Psychiatry. 2003;64(7):807-813.
11. Blank S, Lenze EJ, Mulsant BH, et al. Outcomes of late-life anxiety disorders during 32 weeks of citalopram treatment. J Clin Psychiatry. 2006;67(3):468-472.
12. Lenze EJ, Mulsant BH, Shear MK, et al. Efficacy and tolerability of citalopram in the treatment of late-life anxiety disorders: results from an 8-week randomized, placebo-controlled trial. Am J Psychiatry. 2005;162(1):146-150.
13. Montgomery SA, Kasper S, Stein DJ, et al. Citalopram 20 mg, 40 mg and 60 mg are all effective and well tolerated compared with placebo in obsessive-compulsive disorder. Int Clin Psychopharmacol. 2001;16(2):75-86.
14. Leinonen E, Lepola U, Koponen H, et al. Citalopram controls phobic symptoms in patients with panic disorder: randomized controlled trial. J Psychiatry Neurosci. 2000;25(1):24-32.
15. Perna G, Bertani A, Caldirola D, et al. A comparison of citalopram and paroxetine in the treatment of panic disorder: a randomized, single-blind study. Pharmacopsychiatry. 2001;34(3):85-90.
16. Wikander I, Sundblad C, Andersch B, et al. Citalopram in premenstrual dysphoria: is intermittent treatment during luteal phases more effective than continuous medication throughout the menstrual cycle? J Clin Psychopharmacol. 1998;18(5):390-398.
17. English BA, Jewell M, Jewell G, et al. Treatment of chronic posttraumatic stress disorder in combat veterans with citalopram: an open trial. J Clin Psychopharmacol. 2006;26(1):84-88.
18. Furmark T, Appel L, Michelgård A, et al. Cerebral blood flow changes after treatment of social phobia with neurokinin-1 antagonist GR205171, citalopram, or placebo. Biol Psychiatry. 2005;58(2):132-142.
19. Naranjo CA, Poulos CX, Bremner KE, et al. Citalopram decreases desirability, liking, and consumption of alcohol in alcohol-dependent drinkers. Clin Pharmacol Ther. 1992;51(6):729-739.
20. Safarinejad MR, Hosseini SY. Safety and efficacy of citalopram in the treatment of premature ejaculation: a double-blind placebo-controlled, fixed dose, randomized study. Int J Impot Res. 2006;18(2):164-169.
21. Shams T, Firwana B, Habib F, et al. SSRIs for hot flashes: a systematic review and meta-analysis of randomized trials. J Gen Intern Med. 2014;29(1):204-213.
22. Lexapro [package insert]. Irvine, CA: Allergan USA, Inc; 2016.
23. Guerdjikova AI, McElroy SL, Kotwal R, et al. High-dose escitalopram in the treatment of binge-eating disorder with obesity: a placebo-controlled monotherapy trial. Hum Psychopharmacol. 2008;23(1):1-11.
24. Aigner M, Treasure J, Kaye W, et al. World federation of societies of biological psychiatry (WFSBP) guidelines for pharmacological treatment of eating disorders. World J Biol Psychiatry. 2011;12:400-443.
25. Fineberg NA, Tonnoir B, Lemming O, et al. Escitalopram prevents relapse of obsessive-compulsive disorder. Eur Neuropsychopharmacol. 2007;17(6-7):430-439.
26. Stein DJ, Andersen EW, Tonnoir B, et al. Escitalopram in obsessive-compulsive disorder: a randomized, placebo-controlled, paroxetine-referenced, fixed-dose, 24-week study. Curr Med Res Opin. 2007;23(4):701-711.
27. Stahl SM, Gergel I, Li D. Escitalopram in the treatment of panic disorder: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2003;64(11):1322-1327.
28. Freeman EW, Sondheimer SJ, Sammel MD, et al. A preliminary study of luteal phase versus symptom-onset dosing with escitalopram for premenstrual dysphoric disorder. J Clin Psychiatry. 2005;66(6):769-773.
29. Qi W, Gevonden M, Shalev A. Efficacy and tolerability of high-dose escitalopram in posttraumatic stress disorder. J Clin Psychopharmacol. 2017;37(1):89-93.
30. Carpenter JS, Guthrie KA, Larson JC, et al. Effect of escitalopram on hot flash interference: a randomized, controlled trial. Fertil Steril. 2012;97(6):1399-1404.
31. Freeman EW, Guthrie KA, Caan B, et al. Efficacy of escitalopram for hot flashes in healthy menopausal women: a randomized controlled trial. JAMA. 2011;305(3):267-274.
32. Arnold LM, McElroy SL, Hudson JI, et al. A placebo-controlled, randomized trial of fluoxetine in the treatment of binge-eating disorder. J Clin Psychiatry. 2002;63(11):1028-1033.
33. Connor KM, Sutherland SM, Tupler LA, et al. Fluoxetine in posttraumatic stress disorder. Randomized, double-blind study. Br J Psychiatry. 1999;175:17-22.
34. Martenyi F, Brown EB, Zhang H, et al. Fluoxetine versus placebo in posttraumatic stress disorder. J Clin Psychiatry. 2002;63(3):199-206.
35. Davidson JR, Foa EB, Huppert JD, et al. Fluoxetine, comprehensive cognitive behavioral therapy, and placebo in generalized social phobia. Arch Gen Psychiatry. 2004;61(10):1005-1013.
36. Kara H, Aydin S, Yücel M, et al. The efficacy of fluoxetine in the treatment of premature ejaculation: a double-blind placebo-controlled study. J Urol. 1996;156(5):1631-1632.
37. Loprinzi CL, Sloan JA, Perez EA, et al. Phase III evaluation of fluoxetine for treatment of hot flashes. J Clin Oncol. 2002;20(6):1578-1583.
38. Coleiro B, Marshall SE, Denton CP, et al. Treatment of Raynaud’s phenomenon with the selective serotonin reuptake inhibitor fluoxetine. Rheumatology (Oxford). 2001;40(9):1038-1043.
39. Paxil [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2019.
40. Zhang D, Cheng Y, Wu K, et al. Paroxetine in the treatment of premature ejaculation: a systematic review and meta-analysis. BMC Urol. 2019;19(1):2.
41. Walitt B, Urrútia G, Nishishinya MB. Selective serotonin reuptake inhibitors for fibromyalgia syndrome. Cochrane Database Syst Rev. 2015;(6):CD011735.
42. Foster CA, Bafaloukos J. Paroxetine in the treatment of chronic daily headache. Headache. 1994;34:587-589.
43. Zylicz Z, Krajnik M, Sorge A, et al. Paroxetine in the treatment of severe non-dermatological pruritus: a randomized, controlled trial. J Pain Symptom Manage. 2003;26(3):1105-1112.
44. Zoloft [package insert]. New York, NY: Pfizer; 2016.
45. Leombruni P, Pierò A, Lavagnino L, et al. A randomized, double-blind trial comparing sertraline and fluoxetine 6-month treatment in obese patients with binge eating disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(6):1599-1605.
46. McElroy SL, Casuto LS, Nelson EB, et al. Placebo-controlled trial of sertraline in the treatment of binge eating disorder. Am J Psychiatry. 2000;157(6):1004-1006.
47. Milano W, Petrella C, Sabatino C, et al. Treatment of bulimia nervosa with sertraline: a randomized controlled trial. Adv Ther. 2004;21(4):232-237.
48. Brawman-Mintzer O, Knapp RG, Rynn M, et al. Sertraline treatment for generalized anxiety disorder: a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2006;67(6):874-881.
49. McMahon CG. Treatment of premature ejaculation with sertraline hydrochloride: a single-blind placebo-controlled crossover study. J Urol. 1998;159(6):1935-1938.
50. Yi ZM, Chen SD, Tang QY, et al. Efficacy and safety of sertraline for the treatment of premature ejaculation: systematic review and meta-analysis. Medicine (Baltimore). 2019;98(23):e15989.
51. Uçeyler N, Häuser W, Sommer C. A systematic review on the effectiveness of treatment with antidepressants in fibromyalgia syndrome. Arthritis Rheum. 2008;59(9):1279-1298.
52. Pristiq [package insert]. Philadelphia, PA: Wyeth Pharmaceuticals, Inc; 2011.
53. Sun Z, Hao Y, Zhang M. Efficacy and safety of desvenlafaxine treatment for hot flashes associated with menopause: a meta-analysis of randomized controlled trials. Gynecol Obstet Invest. 2013;75(4):255-262.
54. Cymbalta [package insert]. Indianapolis, IN: Eli Lilly and Company; 2008.
55. Li J, Yang L, Pu C, et al. The role of duloxetine in stress urinary incontinence: a systemic review and meta-analysis. Int Urol Nephrol. 2013;45(3):679-686.
56. Filocamo MT, Li Marzi V, Del Popolo G, et al. Pharmacologic treatment in postprostatectomy stress urinary incontinence. Eur Urol. 2007;51(6):1559-1564.
57. Effexor XR [package insert]. Philadelphia, PA: Wyeth Pharmaceuticals, Inc; 2017.
58. Denys D, Van der Wee N, Van Megen HJ, et al. A double-blind comparison of venlafaxine and paroxetine in obsessive-compulsive disorder. J Clin Psychopharmacol. 2003;23(6):568-575.
59. Albert U, Aguglia E, Maina G, et al. Venlafaxine versus clomipramine in the treatment of obsessive-compulsive disorder: a preliminary single-blind, 12-week, controlled study. J Clin Psychiatry. 2002;63(11):1004-1009.
60. Davidson J, Baldwin D, Stein DJ, et al. Treatment of posttraumatic stress disorder with venlafaxine extended release: a 6-month randomized controlled trial. Arch Gen Psychiatry. 2006;63(10):1158-1165.
61. Zarinara AR, Mohammad MR, Hazrati N, et al. Venlafaxine versus methylphenidate in pediatric outpatients with attention deficit hyperactivity disorder: a randomized, double-blind comparison trial. Hum Psychopharmacol. 2010;25(7-8):530-535.
62. Mukaddes NM, Abali O. Venlafaxine in children and adolescents with attention deficit hyperactivity disorder. Psychiatry Clin Neurosci. 2004;58(1):92-95.
63. Cohen LS, Soares CN, Lyster A, et al. Efficacy and tolerability of premenstrual use of venlafaxine (flexible dose) in the treatment of premenstrual dysphoric disorder. J Clin Psychopharmacol. 2004;24(5):540-543.
64. Ozyalcin SN, Talu GK, Kiziltan E, et al. The efficacy and safety of venlafaxine in the prophylaxis of migraine. Headache. 2005;45(2):144-152.
65. Tarlaci S. Escitalopram and venlafaxine for the prophylaxis of migraine headache without mood disorders. Clin Neuropharmacol. 2009;32(5):254-258.
66. Kadiroglu AK, Sit D, Kayabasi H, et al. The effect of venlafaxine HCl on painful peripheral diabetic neuropathy in patients with type 2 diabetes mellitus. J Diabetes Complications. 2008;22(4):241-245.
67. Evans ML, Pritts E, Vittinghoff E, et al. Management of postmenopausal hot flushes with venlafaxine hydrochloride: a randomized, controlled trial. Obstet Gynecol. 2005;105(1):161-166.
68. Farshchian N, Alavi A, Heydarheydari S, et al. Comparative study of the effects of venlafaxine and duloxetine on chemotherapy-induced peripheral neuropathy. Cancer Chemother Pharmacol. 2018;82(5):787-793.
69. Amitriptyline Hydrochloride [package insert]. Princeton, NJ: Sandoz Inc; 2014.
70. Hauser W, Wolfe F, Tolle T, et al. The role of antidepressants in the management of fibromyalgia syndrome: a systemic review and meta-analysis. CNS Drugs. 2012;26(4):297-307.
71. Braak B, Klooker T, Lei A, et al. Randomised clinical trial: the effects of amitriptyline on drinking capacity and symptoms in patients with functional dyspepsia, a double-blind placebo-controlled study. Aliment Pharmacol Ther. 2011;34(6):638-648.
72. Van Ophoven A, Pokupic S, Heinecke A, et al. A prospective, randomized, placebo controlled, double-blind study of amitriptyline for the treatment of interstitial cystitis. J Urol. 2004;172(2):533-536.
73. Foster HE Jr, Hanno P, Nickel JC, et al; Interstitial Cystitis Collaborative Research Network. Effect of amitriptyline on symptoms in treatment naïve patients with interstitial cystitis/painful bladder syndrome. J Urol. 2010;183(5):1853-1858.
74. Vahedi H, Merat S, Momtahen S, et al. Clinical trial: the effect of amitriptyline in patients with diarrhoea-predominent irritable bowel syndrome. Aliment Pharmacol Ther. 2008;27(8):678-684.
75. Bulut S, Berilgen MS, Baran A, et al. Venlafaxine versus amitriptyline in the prophylactic treatment of migraine: a randomized, double-blind, crossover study. Clin Neurol Neurosurg. 2004;107(1):44-48.
76. Keskinbora K, Aydinli I. A double-blind randomized controlled trial of topiramate and amitriptyline either alone or in combination for the prevention of migraine. Clin Neurol Neurosurg. 2008;110(10):979-984.
77. Max MB, Lynch SA, Muir J, et al. Effects of desipramine, amitriptyline, and fluoxetine on pain in diabetic neuropathy. N Engl J Med. 1992;326(19):1250-1256.
78. Boyle J, Eriksson M, Gribble L, et al. Randomized, placebo-controlled comparison of amitriptyline, duloxetine, and pregabalin in patients with chronic diabetic peripheral neuropathic pain: impact on pain, polysomnographic sleep, daytime functioning, and quality of life. Diabetes Care. 2012;35(12):2451-2458.
79. Graff-Radford SB, Shaw LR, Naliboff BN. Amitriptyline and fluphenazine in the treatment of postherpetic neuralgia. Clin J Pain. 2000;16(3):188-192.
80. Watson CP, Evans RJ, Reed K, et al. Amitriptyline versus placebo in postherpetic neuralgia. Neurology. 1982;32(6):671-673.
81. Sinha S, Simlai J, Praharaj SK. Very low dose amitriptyline for clozapine-associated sialorrhea. Curr Drug Saf. 2016;11(3):262-263.
82. Amoxapine [package insert]. Parsippany, NJ: Watson Pharma, Inc; 2014.
83. Weinberg DS, Smalley W, Heidelbaugh JJ, et al. American Gastroenterological Association institute guideline on the pharmacological management of irritable bowel syndrome. Gastroenterology. 2014;147(5):1146-1148.
84. Anafranil (clomipramine hydrochloride) [package insert]. Whitby, Ontario: Patheon Inc; 2012.
85. Clomipramine dose-effect study in patients with depression: clinical end points and pharmacokinetics. Danish University Antidepressant Group (DUAG). Clin Pharmacol Ther. 1999;66(2):152-165.
86. Caillard V, Rouillon F, Viel J, et al. Comparative effects of low and high doses of clomipramine and placebo in panic disorder: a double-blind controlled study. Acta Psychiatr Scand. 1999;99(1):51-58.
87. Segraves RT, Saran A, Segraves K, et al. Clomipramine versus placebo in the treatment of premature ejaculation: a pilot study. J Sex Marital Therap. 1993;19(3):198-200.
88. Rowland DL, de Gouveia Brazao CA, Koos Slob A. Effective daily treatment with clomipramine in men with premature ejaculation when 25 mg (as required) is ineffective. BJU Int. 2001;87(4):357-360.
89. Norpramin (desipramine hydrochloride) [package insert]. Bridgewater, NJ: sanofi-aventis U.S. LLC; 2014.
90. Max MB, Kishore-Kumar R, Schafer SC, et al. Efficacy of desipramine in painful diabetic neuropathy: a placebo-controlled trial. Pain. 1991;45(1):3-9.
91. Drossman DA, Toner BB, Whitehead WE, et al. Cognitive-behavioral therapy versus education and desipramine versus placebo for moderate to severe functional bowel disorders. Gastroenterology. 2003;125(1):19-31.
92. Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systemic review and meta-analysis. Lancet Neurol. 2015;14(2):162-173.
93. Doxepin hydrochloride [package insert]. Morgantown, WV: Mylan Pharmaceuticals, Inc; 2014.
94. Goldsobel AB, Rohr AS, Siegel SC, et al. Efficacy of doxepin in the treatment of chronic idiopathic urticaria. J Allergy Clin Immunol. 1986;78(5 Pt 1):867-873.
95. Imipramine hydrochloride [package insert]. Fairfield, NJ: Excellium Pharmaceutical, Inc; 2012.
96. Pope HG Jr, Hudson JI, Jonas JM, et al. Bulimia treated with imipramine: a placebo-controlled, double-blind study. Am J Psychiatry. 1983;140(5):554-558.
97. Barlow DH, Gorman JM, Shear MK, et al. Cognitive-behavioral therapy, imipramine, or their combination for panic disorder: a randomized controlled trial. JAMA. 2000;283(19):2529-2536.
98. Laederach-Hofmann K, Graf C, Horber F, et al. Imipramine and diet counseling with psychological support in the treatment of obese binge eaters: a randomized, placebo-controlled double-blind study. Int J Eat Disord. 1999;26(3):231-244.
99. Sindrup SH, Bach FW, Madsen C, et al. Venlafaxine versus imipramine in painful polyneuropathy: a randomized, controlled trial. Neurology. 2003;60(8):1284-1289.
100. Lin HH, Sheu BC, Lo MC, et al. Comparison of treatment outcomes of imipramine for female genuine stress incontinence. Br J Obstet Gynaecol. 1999;106(10):1089-1092.
101. Pamelor (nortriptyline) [package insert]. Hazelwood, MO: Mallinckrodt Inc; 2007.
102. Spencer T, Biederman J, Wilens T, et al. Nortriptyline treatment of children with attention-deficit hyperactivity disorder and tic disorder or Tourette’s syndrome. J Am Acad Child Adolesc Psychiatry. 1993;32(1):205-210.
103. Atkinson JH, Slater MA, Williams RA, et al. A placebo-controlled randomized clinical trial of nortriptyline for chronic low back pain. Pain. 1998;76(3):287-296.
104. Desai MJ, Saini V, Saini S. Myofacial pain syndrome: a treatment review. Pain Ther. 2013;2(1):21-36.
105. Chandra K, Shafiq N, Pandhi P, et al. Gabapentin versus nortriptyline in post-herpetic neuralgia patients: a randomized, double-blind clinical trial – the GONIP trial. Int J Clin Pharmacol Ther. 2006;44(8):358-363.
106. Jorge RE, Robinson RG, Arndt S, et al. Mortality and poststroke depression: a placebo-controlled trial of antidepressants. Am J Psychiatry. 2003;160(10):1823-1829.
107. Martin MR, Schiff AA. Fluphenazine/nortriptyline in the irritable bladder syndrome. A double-blind placebo-controlled study. Br J Urol. 1984;56(2):178-179.
108. Wellbutrin (bupropion hydrochloride) [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2017.
109. Maneeton N, Maneeton B, Srisurapanont M, et al. Bupropion for adults with attention-deficit hyperactivity disorder: meta-analysis of randomized, placebo-controlled trials. Psychiatry Clin Neurosci. 2011;65(7):611-617.
110. Li DJ, Tseng PT, Chen YW, et al. Significant treatment effect of bupropion in patients with bipolar disorder but similar phase-shifting rate as other antidepressants: a meta-analysis following the PRISMA guidelines. Medicine (Baltimore). 2016;95(13):e3165.
111. Clayton AH, Warnock JK, Kornstein SG, et al. A placebo-controlled trial of bupropion SR as an antidote for selective serotonin reuptake inhibitor-induced sexual dysfunction. J Clin Psychiatry. 2004;65(1):62-67.
112. Safarinejad MR. Reversal of SSRI-induced female sexual dysfunction by adjunctive bupropion in menstruating women: a double-blind, placebo-controlled and randomized study. J Psychopharmacol. 2011;25(3):370-378.
113. Remeron (mirtazapine) [package insert]. Whitehouse Station, NJ: Merck & Co, Inc; 2020.
114. Boshuisen ML, Slaap BR, Vester-Blokland ED, et al. The effect of mirtazapine in panic disorder: an open label pilot study with a single-blind placebo run-in period. Int Clin Psychopharmacol. 2001;16(6):363-368.
115. Sarchiapone M, Amore M, De Risio S, et al. Mirtazapine in the treatment of panic disorder: an open-label trial. Int Clin Psychopharmacol. 2003;18(1):35-38.
116. Connor KM, Davidson JR, Weisler RH, et al. A pilot study of mirtazapine in post-traumatic stress disorder. Int Clin Psychopharmacol. 1999;14(1):29-31.
117. Wichniak A, Wierzbicka A, Walecka M, et al. Effects of antidepressants on sleep. Curr Psychiatry Rep. 2017;19(9):63.
118. Bedtsen L, Jensen R. Mirtazapine is effective in the prophylactic treatment of chronic tension-type headache. Neurology. 2004;62(10):1706-1711.
119. AbdelFattah MR, Jung SW, Greenspan MA, et al. Efficacy of antidepressants in the treatment of obstructive sleep apnea compared to placebo. A systemic review with meta-analysis. Sleep Breath. 2020;24(2):443-453.
120. Desyrel [package insert]. Locust Valley, NY: Pragma Pharmaceuticals, LLC; 2017.
121. Lebert F, Stekke W, Hasenbroekx C, et al. Frontotemporal dementia: a randomized, controlled trial with trazodone. Dement Geriatr Cogn Disord. 2004;17(4):355-359.
122. Sultzer DL, Gray KF, Gunay I, et al. A double-blind comparison of trazodone and haloperidol for treatment of agitation in patients with dementia. Am J Geriatr Psychiatry. 1997;5(1):60-69.
123. Yi XY, Ni SF, Ghadami MR, et al. Trazodone for the treatment of insomnia: a meta-analysis of randomized placebo-controlled trials. Sleep Med. 2018;45:25-32.
124. Chlorpromazine hydrochloride [package insert]. Minneapolis, MN: Upsher-Smith Laboratories, Inc; 2010.
125. Bigal ME, Bordini CA, Speciali JG. Intravenous chlorpromazine in the emergency department treatment of migraines: a randomized controlled trial. J Emerg Med. 2002;23(2):141-148.
126. Bell R, Montoya D, Shuaib A, et al. A comparative trial of three agents in the treatment of acute migraine headache. Ann Emerg Med. 1990;19(10):1079-1082.
127. Committee on Practice Bulletins-Obstetrics. ACOG Practice Bulletin No. 189: Nausea and vomiting of pregnancy. Obstet Gynecol. 2018;131(1):e15-e30.
128. Fluphenazine hydrochloride [package insert]. Philadelphia, PA: Lannett Company, Inc; 2019.
129. Bonelli RM, Wenning GK. Pharmacological management of Huntington’s disease: an evidence-based review. Curr Pharm Des. 2006;12(21):2701-2720.
130. Haldol [package insert]. Columbus, OH: American Health Packaging; 2020.
131. MacDonald K, Wilson M, Minassian A, et al. A naturalistic study for intramuscular haloperidol versus intramuscular olanzapine for the management of acute agitation. J Clin Psychopharmacol. 2012;32(3):317-322.
132. Goikolea JM, Colom F, Capapey J, et al. Faster onset of antimanic action with haloperidol compared to second-generation antipsychotics. A meta-analysis of randomized clinical trials in acute mania. Eur Neuropsychopharmacol. 2013;23(4):305-316.
133. Girard TD, Exline MC, Carson SS, et al. Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med. 2018;379(26):2506-2516.
134. Lohr L. Chemotherapy-induced nausea and vomiting. Cancer J. 2008;14(2):85-93.
135. Büttner M, Walder B, von Elm E, et al. Is low-dose haloperidol a useful antiemetic?: A meta-analysis of published and unpublished randomized trials. Anesthesiology. 2004;101(6):1454-1463.
136. Perphenazine [package insert]. Princeton, NJ: Sandoz Inc; 2010.
137. Compazine [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2004.
138. Hesketh PJ. Chemotherapy-induced nausea and vomiting. N Engl J Med. 2008;358(23):2482-2494.
139. Chen JJ, Frame DG, White TJ. Efficacy of ondansetron and prochlorperazine for the prevention of postoperative nausea and vomiting after total hip replacement or total knee replacement procedures: a randomized, double-blind, comparative trial. Arch Intern Med. 1998;158(19):2124-2128.
140. Campbell K, Rowe H, Azzam H, et al. The management of nausea and vomiting of pregnancy. J Obstet Gynaecol Can. 2016;38(12):1127-1137.
141. Abilify [package insert]. Rockville, MD: Otsuka America Pharmaceutical, Inc; 2014.
142. Kinon BJ, Stauffer VL, Kollack-Walker S, et al. Olanzapine versus aripiprazole for the treatment of agitation in acutely ill patients with schizophrenia. J Clin Psychopharmacol. 2008;28(6):601-607.
143. Iannuzzi GL, Patel AA, Stewart JT. Aripiprazole and delusional disorder. J Psychiatr Pract. 2019;25(2):132-134.
144. Campbell EH, Elston DM, Hawthorne JD, et al. Diagnosis and management of delusional parasitosis. J Am Acad Dermatol. 2019;80(5):1428-1434.
145. Sayyah M, Sayyah M, Boostani H, et al. Effects of aripiprazole augmentation in treatment-resistant obsessive-compulsive disorder (a double-blind clinical trial). Depress Anxiety. 2012;29(10):850-854.
146. Lin WC, Chou YH. Aripiprazole effects on psychosis and chorea in a patient with Huntington’s disease. Am J Psychiatry. 2008;165(9):1207-1208.
147. Li X, Tang Y, Wang C. Adjunctive aripiprazole versus placebo for antipsychotic-induced hyperprolactinemia: meta-analysis of randomized controlled trials. PLoS One. 2013;8(8):e70179.
148. Zyprexa [package insert]. Indianapolis, IN: Eli Lilly and Company; 1997.
149. Attia E, Steinglass JE, Walsh BT, et al. Olanzapine versus placebo in adult outpatients with anorexia nervosa: a randomized clinical trial. Am J Psychiatry. 2019;176(6):449-456.
150. Dennehy EB, Doyle K, Suppes T. The efficacy of olanzapine monotherapy for acute hypomania or mania in an outpatient setting. Int Clin Psychopharmacol. 2003;18(3):143-145.
151. Grover S, Kumar V, Chakrabarti S. Comparative efficacy study of haloperidol, olanzapine and risperidone in delirium. J Psychosom Res. 2011;71(4):277-281.
152. Bosmans A, Verbanck P. Successful treatment of delusional disorder of the somatic type or “delusional parasitosis” with olanzapine. Pharmacopsychiatry. 2008;41(3):121-122.
153. Meyers BS, Flint AJ, Rothschild AJ, et al; STOP-PD Group. A double-blind randomized controlled trial of olanzapine plus sertraline vs olanzapine plus placebo for psychotic depression: the study of pharmacotherapy of psychotic depression (STOP-PD). Arch Gen Psychiatry. 2009;66(8):838-847.
154. Rothschild AJ, Williamson DJ, Tohen MF, et al. A double-blind, randomized study of olanzapine and olanzapine/fluoxetine combination for major depression with psychotic features. J Clin Psychopharmacol. 2004;24(4):365-373.
155. Navari RM, Gray SE, Kerr AC. Olanzapine versus aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a randomized phase III trial. J Support Oncol. 2011;9(5):188-195.
156. Bonelli RM, Mahnert FA, Niederwieser G. Olanzapine for Huntington’s disease: an open label study. Clin Neuropharmacol. 2002;25(5):263-265.
157. Seroquel [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2013.
158. Khan A, Atkinson S, Mezhebovsky I, et al. Extended-release quetiapine fumarate (quetiapine XR) as adjunctive therapy in patients with generalized anxiety disorder and a history of inadequate treatment response: a randomized, double-blind study. Ann Clin Psychiatry. 2014;26(1):3-18.
159. Dold M, Aigner M, Lanzenberger R, et al. Antipsychotic augmentation of serotonin reuptake inhibitors in treatment-resistant obsessive-compulsive disorder: a meta-analysis of double-blind, randomized, placebo-controlled trials. Int J Neuropsychopharmacol. 2013;16(3):557-574.
160. Villarreal G, Hamner MB, Cañive JM, et al. Efficacy of quetiapine monotherapy in posttraumatic stress disorder: a randomized, placebo-controlled trial. Am J Psychiatry. 2016;173(12):1205-1212.
161. Fernandez HH, Friedman JH, Jacques C, et al. Quetiapine for the treatment of drug-induced psychosis in Parkinson’s disease. Mov Disord. 1999;14(3):484-487.
162. Doroudgar S, Chou T, Yu J, et al. Evaluation of trazodone and quetiapine for insomnia: an observational study in psychiatric inpatients. Prim Care Companion CNS Disord. 2013;15(6):PCC.13m01558. doi: 10.4088/PCC.13m01558
163. Risperdal [package insert]. Titusville, NJ: Janssen Pharamceuticals, Inc; 2007.
164. Lim HK, Kim JJ, Pae CU, et al. Comparison of risperidone orodispersible tablet and intramuscular haloperidol in the treatment of acute psychotic agitation: a randomized open, prospective study. Neuropsychobiology. 2010;62(2):81-86.
165. Currier GW, Chou J, Feifel D, et al. Acute treatment of psychotic agitation: a randomized comparison of oral treatment with risperidone and lorazepam versus intramuscular treatment with haloperidol and lorazepam. J Clin Psychiatry. 2004;65(3):386-394.
166. Bahk WM, Yoon JS, Kim YH, et al. Risperidone in combination with mood stabilizers for acute mania: a multicentre, open study. Int Clin Psychopharmacol. 2004;19(5):299-303.
167. Freudenmann RW, Lepping P. Second-generation antipsychotics in primary and secondary delusional parasitosis: outcome and efficacy. J Clin Psychopharmacol. 2008;28(5):500-508.
168. Nelson JC, Papakostas GI. Atypical antipsychotic augmentation in major depressive disorder: a meta-analysis of placebo-controlled randomized trials. Am J Psychiatry. 2009;166(9): 980-991.
169. McDougle CJ, Epperson CN, Pelton GH, et al. A double-blind, placebo-controlled study of risperidone addition in serotonin reuptake inhibitor-refractory obsessive-compulsive disorder. Arch Gen Psychiatry. 2000;57(8):794-801.
170. Scahill L, Leckman JF, Schulz RT, et al. A placebo-controlled trial of risperidone in Tourette syndrome. Neurology. 2003;60(7):1130-1135.
171. Dallocchio C, Buffa C, Tinelli C, et al. Effectiveness of risperidone in Huntington Chorea patients. J Clin Psychopharmacol. 1999;19(1):101-103.
Ms. A, age 45, is hospitalized for abdominal pain. She is noted to have hiccups, the onset of which she reports was >1 month ago and did not have a clear precipitant. Abdominal and head imaging return no acute findings, and data from a serum electrolyte test, hepatic function test, and thyroid function test are within normal limits. The medical team notices that Ms. A’s speech is pressured, she hardly sleeps, and she appears animated, full of ideas and energy.
Ms. A has a history of bipolar I disorder, hypertension, hyperlipidemia, gastroesophageal reflux disease, and hypothyroidism. Her present medications include hydrochlorothiazide 25 mg/d; levothyroxine 25 mcg/d; omeprazole 20 mg/d; and lovastatin 20 mg/d. She states that she was remotely treated for bipolar disorder, but she was cured by a shamanic healer, and therefore no longer needs treatment.
Approximately 35% of adults in the United States age 60 to 79 reported taking ≥5 prescription medications in 2016, compared to 15% of adults age 40 to 59.1 In a study of 372 patients with advanced, life-limiting illness, Schenker et al2 found that those who took multiple medications (mean: 11.6 medications) had a lower quality of life and worse symptoms. Optimizing medications to patients’ specific needs and diagnoses in order to reduce pill burden can be a favorable intervention. In addition, some patients—approximately 30% of those with schizophrenia and 20% of those with bipolar disorder—may not have insight into their mental illness as they do with their medical conditions, and may be more accepting of treatment for the latter.3 Dual-indication prescribing may be a useful way to decrease polypharmacy, reduce potential drug-drug interactions (DDIs), increase patient acceptance and adherence, and improve a patient’s overall health.
Continue on for: Multiple uses for antidepressants and antipsychotics...
Multiple uses for antidepressants and antipsychotics
One of the first medications discovered to have antidepressant effects was iproniazid, a monoamine oxidase inhibitor (MAOI) initially used to treat tuberculosis.4 Since then, numerous classes of antidepressant medications have been developed that capitalize on monoamine reuptake through several different mechanisms of action. These drugs can be grouped into subclasses that include selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, tricyclic antidepressants, MAOIs, and others. True to their roots in iproniazid, these medications can have a myriad of effects not limited to mental health and can therefore be beneficial for a variety of comorbid conditions.
As was the case with antidepressants, the first medication approved in the antipsychotic class, chlorpromazine, was serendipitously discovered to treat psychosis and agitation after being approved and used to treat presurgical apprehension.5 The term “antipsychotic” is almost a misnomer given these agents’ broad pharmacology profiles and impact on various mental illnesses, including bipolar disorder, depressive disorders, anxiety disorders, and many other mental conditions. First-generation antipsychotics (FGAs) were the first to enter the market; they work primarily by blocking dopamine-2 (D2) receptors. Second-generation antipsychotics have less movement-based adverse effects than FGAs by having higher affinity for serotonin 5-HT2A receptors than for D2 receptors. However, they tend to carry a higher risk for weight gain and metabolic syndrome.
Antidepressants and antipsychotics are widely utilized in psychiatry. Many have been found to have additional uses beyond their original FDA-approved indication and can therefore be beneficial for a variety of comorbid conditions.
One limitation of using psychiatric medications for nonpsychiatric indications is that different doses of antidepressants and antipsychotics are typically targeted for different indications based on receptor binding affinity. A common example of this is trazodone, where doses below 100 mg are used as needed for insomnia, but higher doses ranging from 200 to 600 mg/d are used for depression. Another important consideration is DDIs. For example, the possibility of adding an agent such as fluoxetine to a complex pain regimen for fibromyalgia could impact the clearance of other agents that are cytochrome P450 (CYP) 2D6 substrates due to fluoxetine’s potent inhibition of the enzyme.6,7 Table 16-51, Table 252-68, Table 369-107, and Table 4108-123 provide information on select antidepressants, while Table 5124-140 and Table 6141-171 provide information on select antipsychotics. Each table lists psychiatric and nonpsychiatric indications for the respective medications, including both FDA-approved (where applicable) and common off-label uses. Most of the indications listed are for adult use only, unless otherwise noted.
Continue on to: Case Continued...
CASE CONTINUED
After reviewing Ms. A’s medical history, the treatment team initiates chlorpromazine, 25 mg 3 times a day, for intractable hiccups, and increases the dosage to 50 mg 3 times a day after 3 days. Chlorpromazine is FDA-approved for treating bipolar mania, and also for treating intractable hiccups. Shortly thereafter, Ms. A’s hiccups subside, she sleeps for longer periods, and her manic symptoms resolve.
Ms. A, age 45, is hospitalized for abdominal pain. She is noted to have hiccups, the onset of which she reports was >1 month ago and did not have a clear precipitant. Abdominal and head imaging return no acute findings, and data from a serum electrolyte test, hepatic function test, and thyroid function test are within normal limits. The medical team notices that Ms. A’s speech is pressured, she hardly sleeps, and she appears animated, full of ideas and energy.
Ms. A has a history of bipolar I disorder, hypertension, hyperlipidemia, gastroesophageal reflux disease, and hypothyroidism. Her present medications include hydrochlorothiazide 25 mg/d; levothyroxine 25 mcg/d; omeprazole 20 mg/d; and lovastatin 20 mg/d. She states that she was remotely treated for bipolar disorder, but she was cured by a shamanic healer, and therefore no longer needs treatment.
Approximately 35% of adults in the United States age 60 to 79 reported taking ≥5 prescription medications in 2016, compared to 15% of adults age 40 to 59.1 In a study of 372 patients with advanced, life-limiting illness, Schenker et al2 found that those who took multiple medications (mean: 11.6 medications) had a lower quality of life and worse symptoms. Optimizing medications to patients’ specific needs and diagnoses in order to reduce pill burden can be a favorable intervention. In addition, some patients—approximately 30% of those with schizophrenia and 20% of those with bipolar disorder—may not have insight into their mental illness as they do with their medical conditions, and may be more accepting of treatment for the latter.3 Dual-indication prescribing may be a useful way to decrease polypharmacy, reduce potential drug-drug interactions (DDIs), increase patient acceptance and adherence, and improve a patient’s overall health.
Continue on for: Multiple uses for antidepressants and antipsychotics...
Multiple uses for antidepressants and antipsychotics
One of the first medications discovered to have antidepressant effects was iproniazid, a monoamine oxidase inhibitor (MAOI) initially used to treat tuberculosis.4 Since then, numerous classes of antidepressant medications have been developed that capitalize on monoamine reuptake through several different mechanisms of action. These drugs can be grouped into subclasses that include selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, tricyclic antidepressants, MAOIs, and others. True to their roots in iproniazid, these medications can have a myriad of effects not limited to mental health and can therefore be beneficial for a variety of comorbid conditions.
As was the case with antidepressants, the first medication approved in the antipsychotic class, chlorpromazine, was serendipitously discovered to treat psychosis and agitation after being approved and used to treat presurgical apprehension.5 The term “antipsychotic” is almost a misnomer given these agents’ broad pharmacology profiles and impact on various mental illnesses, including bipolar disorder, depressive disorders, anxiety disorders, and many other mental conditions. First-generation antipsychotics (FGAs) were the first to enter the market; they work primarily by blocking dopamine-2 (D2) receptors. Second-generation antipsychotics have less movement-based adverse effects than FGAs by having higher affinity for serotonin 5-HT2A receptors than for D2 receptors. However, they tend to carry a higher risk for weight gain and metabolic syndrome.
Antidepressants and antipsychotics are widely utilized in psychiatry. Many have been found to have additional uses beyond their original FDA-approved indication and can therefore be beneficial for a variety of comorbid conditions.
One limitation of using psychiatric medications for nonpsychiatric indications is that different doses of antidepressants and antipsychotics are typically targeted for different indications based on receptor binding affinity. A common example of this is trazodone, where doses below 100 mg are used as needed for insomnia, but higher doses ranging from 200 to 600 mg/d are used for depression. Another important consideration is DDIs. For example, the possibility of adding an agent such as fluoxetine to a complex pain regimen for fibromyalgia could impact the clearance of other agents that are cytochrome P450 (CYP) 2D6 substrates due to fluoxetine’s potent inhibition of the enzyme.6,7 Table 16-51, Table 252-68, Table 369-107, and Table 4108-123 provide information on select antidepressants, while Table 5124-140 and Table 6141-171 provide information on select antipsychotics. Each table lists psychiatric and nonpsychiatric indications for the respective medications, including both FDA-approved (where applicable) and common off-label uses. Most of the indications listed are for adult use only, unless otherwise noted.
Continue on to: Case Continued...
CASE CONTINUED
After reviewing Ms. A’s medical history, the treatment team initiates chlorpromazine, 25 mg 3 times a day, for intractable hiccups, and increases the dosage to 50 mg 3 times a day after 3 days. Chlorpromazine is FDA-approved for treating bipolar mania, and also for treating intractable hiccups. Shortly thereafter, Ms. A’s hiccups subside, she sleeps for longer periods, and her manic symptoms resolve.
1. Hales CM, Servais J, Martin CB, et al. Prescription drug use among adults aged 40-79 in the United States and Canada. National Center for Health Statistics (Centers for Disease Control and Prevention). 2019. NCHS Data Brief No. 347. https://www.cdc.gov/nchs/products/databriefs/db347.htm
2. Schenker Y, Park SY, Jeong K, et al. Associations between polypharmacy, symptom burden, and quality of life in patients with advanced, life-limiting illness. J Gen Intern Med. 2019;34(4):559-566.
3. National Alliance on Mental Illness. Anosognosia. 2021. https://www.nami.org/About-Mental-Illness/Common-with-Mental-Illness/Anosognosia
4. Meyer JM. A concise guide to monoamine oxidase inhibitors. Current Psychiatry. 2017;16(12):14-16,18-23,47,A.
5. Ban TA. Fifty years chlorpromazine: a historical perspective. Neuropsychiatr Dis Treat. 2007;3(4):495-500.
6. Prozac [package insert]. Indianapolis, IN: Eli Lilly and Company; 2009.
7. Arnold LM, Hess EV, Hudson JI, et al. A randomized, placebo-controlled, double-blind, flexible-dose study of fluoxetine in the treatment of women with fibromyalgia. Am J Med. 2002;112(3):191-197.
8. Celexa [package insert]. St. Louis, MO: Forest Pharmaceuticals, Inc; 2009.
9. Porsteinsson AP, Drye LT, Pollock BG, et al. Effect of citalopram on agitation in Alzheimer disease: the CitAD randomized clinical trial. JAMA. 2014;311(7):682-691.
10. McElroy SL, Hudson JI, Malhotra S, et al. Citalopram in the treatment of binge-eating disorder: a placebo-controlled trial. J Clin Psychiatry. 2003;64(7):807-813.
11. Blank S, Lenze EJ, Mulsant BH, et al. Outcomes of late-life anxiety disorders during 32 weeks of citalopram treatment. J Clin Psychiatry. 2006;67(3):468-472.
12. Lenze EJ, Mulsant BH, Shear MK, et al. Efficacy and tolerability of citalopram in the treatment of late-life anxiety disorders: results from an 8-week randomized, placebo-controlled trial. Am J Psychiatry. 2005;162(1):146-150.
13. Montgomery SA, Kasper S, Stein DJ, et al. Citalopram 20 mg, 40 mg and 60 mg are all effective and well tolerated compared with placebo in obsessive-compulsive disorder. Int Clin Psychopharmacol. 2001;16(2):75-86.
14. Leinonen E, Lepola U, Koponen H, et al. Citalopram controls phobic symptoms in patients with panic disorder: randomized controlled trial. J Psychiatry Neurosci. 2000;25(1):24-32.
15. Perna G, Bertani A, Caldirola D, et al. A comparison of citalopram and paroxetine in the treatment of panic disorder: a randomized, single-blind study. Pharmacopsychiatry. 2001;34(3):85-90.
16. Wikander I, Sundblad C, Andersch B, et al. Citalopram in premenstrual dysphoria: is intermittent treatment during luteal phases more effective than continuous medication throughout the menstrual cycle? J Clin Psychopharmacol. 1998;18(5):390-398.
17. English BA, Jewell M, Jewell G, et al. Treatment of chronic posttraumatic stress disorder in combat veterans with citalopram: an open trial. J Clin Psychopharmacol. 2006;26(1):84-88.
18. Furmark T, Appel L, Michelgård A, et al. Cerebral blood flow changes after treatment of social phobia with neurokinin-1 antagonist GR205171, citalopram, or placebo. Biol Psychiatry. 2005;58(2):132-142.
19. Naranjo CA, Poulos CX, Bremner KE, et al. Citalopram decreases desirability, liking, and consumption of alcohol in alcohol-dependent drinkers. Clin Pharmacol Ther. 1992;51(6):729-739.
20. Safarinejad MR, Hosseini SY. Safety and efficacy of citalopram in the treatment of premature ejaculation: a double-blind placebo-controlled, fixed dose, randomized study. Int J Impot Res. 2006;18(2):164-169.
21. Shams T, Firwana B, Habib F, et al. SSRIs for hot flashes: a systematic review and meta-analysis of randomized trials. J Gen Intern Med. 2014;29(1):204-213.
22. Lexapro [package insert]. Irvine, CA: Allergan USA, Inc; 2016.
23. Guerdjikova AI, McElroy SL, Kotwal R, et al. High-dose escitalopram in the treatment of binge-eating disorder with obesity: a placebo-controlled monotherapy trial. Hum Psychopharmacol. 2008;23(1):1-11.
24. Aigner M, Treasure J, Kaye W, et al. World federation of societies of biological psychiatry (WFSBP) guidelines for pharmacological treatment of eating disorders. World J Biol Psychiatry. 2011;12:400-443.
25. Fineberg NA, Tonnoir B, Lemming O, et al. Escitalopram prevents relapse of obsessive-compulsive disorder. Eur Neuropsychopharmacol. 2007;17(6-7):430-439.
26. Stein DJ, Andersen EW, Tonnoir B, et al. Escitalopram in obsessive-compulsive disorder: a randomized, placebo-controlled, paroxetine-referenced, fixed-dose, 24-week study. Curr Med Res Opin. 2007;23(4):701-711.
27. Stahl SM, Gergel I, Li D. Escitalopram in the treatment of panic disorder: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2003;64(11):1322-1327.
28. Freeman EW, Sondheimer SJ, Sammel MD, et al. A preliminary study of luteal phase versus symptom-onset dosing with escitalopram for premenstrual dysphoric disorder. J Clin Psychiatry. 2005;66(6):769-773.
29. Qi W, Gevonden M, Shalev A. Efficacy and tolerability of high-dose escitalopram in posttraumatic stress disorder. J Clin Psychopharmacol. 2017;37(1):89-93.
30. Carpenter JS, Guthrie KA, Larson JC, et al. Effect of escitalopram on hot flash interference: a randomized, controlled trial. Fertil Steril. 2012;97(6):1399-1404.
31. Freeman EW, Guthrie KA, Caan B, et al. Efficacy of escitalopram for hot flashes in healthy menopausal women: a randomized controlled trial. JAMA. 2011;305(3):267-274.
32. Arnold LM, McElroy SL, Hudson JI, et al. A placebo-controlled, randomized trial of fluoxetine in the treatment of binge-eating disorder. J Clin Psychiatry. 2002;63(11):1028-1033.
33. Connor KM, Sutherland SM, Tupler LA, et al. Fluoxetine in posttraumatic stress disorder. Randomized, double-blind study. Br J Psychiatry. 1999;175:17-22.
34. Martenyi F, Brown EB, Zhang H, et al. Fluoxetine versus placebo in posttraumatic stress disorder. J Clin Psychiatry. 2002;63(3):199-206.
35. Davidson JR, Foa EB, Huppert JD, et al. Fluoxetine, comprehensive cognitive behavioral therapy, and placebo in generalized social phobia. Arch Gen Psychiatry. 2004;61(10):1005-1013.
36. Kara H, Aydin S, Yücel M, et al. The efficacy of fluoxetine in the treatment of premature ejaculation: a double-blind placebo-controlled study. J Urol. 1996;156(5):1631-1632.
37. Loprinzi CL, Sloan JA, Perez EA, et al. Phase III evaluation of fluoxetine for treatment of hot flashes. J Clin Oncol. 2002;20(6):1578-1583.
38. Coleiro B, Marshall SE, Denton CP, et al. Treatment of Raynaud’s phenomenon with the selective serotonin reuptake inhibitor fluoxetine. Rheumatology (Oxford). 2001;40(9):1038-1043.
39. Paxil [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2019.
40. Zhang D, Cheng Y, Wu K, et al. Paroxetine in the treatment of premature ejaculation: a systematic review and meta-analysis. BMC Urol. 2019;19(1):2.
41. Walitt B, Urrútia G, Nishishinya MB. Selective serotonin reuptake inhibitors for fibromyalgia syndrome. Cochrane Database Syst Rev. 2015;(6):CD011735.
42. Foster CA, Bafaloukos J. Paroxetine in the treatment of chronic daily headache. Headache. 1994;34:587-589.
43. Zylicz Z, Krajnik M, Sorge A, et al. Paroxetine in the treatment of severe non-dermatological pruritus: a randomized, controlled trial. J Pain Symptom Manage. 2003;26(3):1105-1112.
44. Zoloft [package insert]. New York, NY: Pfizer; 2016.
45. Leombruni P, Pierò A, Lavagnino L, et al. A randomized, double-blind trial comparing sertraline and fluoxetine 6-month treatment in obese patients with binge eating disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(6):1599-1605.
46. McElroy SL, Casuto LS, Nelson EB, et al. Placebo-controlled trial of sertraline in the treatment of binge eating disorder. Am J Psychiatry. 2000;157(6):1004-1006.
47. Milano W, Petrella C, Sabatino C, et al. Treatment of bulimia nervosa with sertraline: a randomized controlled trial. Adv Ther. 2004;21(4):232-237.
48. Brawman-Mintzer O, Knapp RG, Rynn M, et al. Sertraline treatment for generalized anxiety disorder: a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2006;67(6):874-881.
49. McMahon CG. Treatment of premature ejaculation with sertraline hydrochloride: a single-blind placebo-controlled crossover study. J Urol. 1998;159(6):1935-1938.
50. Yi ZM, Chen SD, Tang QY, et al. Efficacy and safety of sertraline for the treatment of premature ejaculation: systematic review and meta-analysis. Medicine (Baltimore). 2019;98(23):e15989.
51. Uçeyler N, Häuser W, Sommer C. A systematic review on the effectiveness of treatment with antidepressants in fibromyalgia syndrome. Arthritis Rheum. 2008;59(9):1279-1298.
52. Pristiq [package insert]. Philadelphia, PA: Wyeth Pharmaceuticals, Inc; 2011.
53. Sun Z, Hao Y, Zhang M. Efficacy and safety of desvenlafaxine treatment for hot flashes associated with menopause: a meta-analysis of randomized controlled trials. Gynecol Obstet Invest. 2013;75(4):255-262.
54. Cymbalta [package insert]. Indianapolis, IN: Eli Lilly and Company; 2008.
55. Li J, Yang L, Pu C, et al. The role of duloxetine in stress urinary incontinence: a systemic review and meta-analysis. Int Urol Nephrol. 2013;45(3):679-686.
56. Filocamo MT, Li Marzi V, Del Popolo G, et al. Pharmacologic treatment in postprostatectomy stress urinary incontinence. Eur Urol. 2007;51(6):1559-1564.
57. Effexor XR [package insert]. Philadelphia, PA: Wyeth Pharmaceuticals, Inc; 2017.
58. Denys D, Van der Wee N, Van Megen HJ, et al. A double-blind comparison of venlafaxine and paroxetine in obsessive-compulsive disorder. J Clin Psychopharmacol. 2003;23(6):568-575.
59. Albert U, Aguglia E, Maina G, et al. Venlafaxine versus clomipramine in the treatment of obsessive-compulsive disorder: a preliminary single-blind, 12-week, controlled study. J Clin Psychiatry. 2002;63(11):1004-1009.
60. Davidson J, Baldwin D, Stein DJ, et al. Treatment of posttraumatic stress disorder with venlafaxine extended release: a 6-month randomized controlled trial. Arch Gen Psychiatry. 2006;63(10):1158-1165.
61. Zarinara AR, Mohammad MR, Hazrati N, et al. Venlafaxine versus methylphenidate in pediatric outpatients with attention deficit hyperactivity disorder: a randomized, double-blind comparison trial. Hum Psychopharmacol. 2010;25(7-8):530-535.
62. Mukaddes NM, Abali O. Venlafaxine in children and adolescents with attention deficit hyperactivity disorder. Psychiatry Clin Neurosci. 2004;58(1):92-95.
63. Cohen LS, Soares CN, Lyster A, et al. Efficacy and tolerability of premenstrual use of venlafaxine (flexible dose) in the treatment of premenstrual dysphoric disorder. J Clin Psychopharmacol. 2004;24(5):540-543.
64. Ozyalcin SN, Talu GK, Kiziltan E, et al. The efficacy and safety of venlafaxine in the prophylaxis of migraine. Headache. 2005;45(2):144-152.
65. Tarlaci S. Escitalopram and venlafaxine for the prophylaxis of migraine headache without mood disorders. Clin Neuropharmacol. 2009;32(5):254-258.
66. Kadiroglu AK, Sit D, Kayabasi H, et al. The effect of venlafaxine HCl on painful peripheral diabetic neuropathy in patients with type 2 diabetes mellitus. J Diabetes Complications. 2008;22(4):241-245.
67. Evans ML, Pritts E, Vittinghoff E, et al. Management of postmenopausal hot flushes with venlafaxine hydrochloride: a randomized, controlled trial. Obstet Gynecol. 2005;105(1):161-166.
68. Farshchian N, Alavi A, Heydarheydari S, et al. Comparative study of the effects of venlafaxine and duloxetine on chemotherapy-induced peripheral neuropathy. Cancer Chemother Pharmacol. 2018;82(5):787-793.
69. Amitriptyline Hydrochloride [package insert]. Princeton, NJ: Sandoz Inc; 2014.
70. Hauser W, Wolfe F, Tolle T, et al. The role of antidepressants in the management of fibromyalgia syndrome: a systemic review and meta-analysis. CNS Drugs. 2012;26(4):297-307.
71. Braak B, Klooker T, Lei A, et al. Randomised clinical trial: the effects of amitriptyline on drinking capacity and symptoms in patients with functional dyspepsia, a double-blind placebo-controlled study. Aliment Pharmacol Ther. 2011;34(6):638-648.
72. Van Ophoven A, Pokupic S, Heinecke A, et al. A prospective, randomized, placebo controlled, double-blind study of amitriptyline for the treatment of interstitial cystitis. J Urol. 2004;172(2):533-536.
73. Foster HE Jr, Hanno P, Nickel JC, et al; Interstitial Cystitis Collaborative Research Network. Effect of amitriptyline on symptoms in treatment naïve patients with interstitial cystitis/painful bladder syndrome. J Urol. 2010;183(5):1853-1858.
74. Vahedi H, Merat S, Momtahen S, et al. Clinical trial: the effect of amitriptyline in patients with diarrhoea-predominent irritable bowel syndrome. Aliment Pharmacol Ther. 2008;27(8):678-684.
75. Bulut S, Berilgen MS, Baran A, et al. Venlafaxine versus amitriptyline in the prophylactic treatment of migraine: a randomized, double-blind, crossover study. Clin Neurol Neurosurg. 2004;107(1):44-48.
76. Keskinbora K, Aydinli I. A double-blind randomized controlled trial of topiramate and amitriptyline either alone or in combination for the prevention of migraine. Clin Neurol Neurosurg. 2008;110(10):979-984.
77. Max MB, Lynch SA, Muir J, et al. Effects of desipramine, amitriptyline, and fluoxetine on pain in diabetic neuropathy. N Engl J Med. 1992;326(19):1250-1256.
78. Boyle J, Eriksson M, Gribble L, et al. Randomized, placebo-controlled comparison of amitriptyline, duloxetine, and pregabalin in patients with chronic diabetic peripheral neuropathic pain: impact on pain, polysomnographic sleep, daytime functioning, and quality of life. Diabetes Care. 2012;35(12):2451-2458.
79. Graff-Radford SB, Shaw LR, Naliboff BN. Amitriptyline and fluphenazine in the treatment of postherpetic neuralgia. Clin J Pain. 2000;16(3):188-192.
80. Watson CP, Evans RJ, Reed K, et al. Amitriptyline versus placebo in postherpetic neuralgia. Neurology. 1982;32(6):671-673.
81. Sinha S, Simlai J, Praharaj SK. Very low dose amitriptyline for clozapine-associated sialorrhea. Curr Drug Saf. 2016;11(3):262-263.
82. Amoxapine [package insert]. Parsippany, NJ: Watson Pharma, Inc; 2014.
83. Weinberg DS, Smalley W, Heidelbaugh JJ, et al. American Gastroenterological Association institute guideline on the pharmacological management of irritable bowel syndrome. Gastroenterology. 2014;147(5):1146-1148.
84. Anafranil (clomipramine hydrochloride) [package insert]. Whitby, Ontario: Patheon Inc; 2012.
85. Clomipramine dose-effect study in patients with depression: clinical end points and pharmacokinetics. Danish University Antidepressant Group (DUAG). Clin Pharmacol Ther. 1999;66(2):152-165.
86. Caillard V, Rouillon F, Viel J, et al. Comparative effects of low and high doses of clomipramine and placebo in panic disorder: a double-blind controlled study. Acta Psychiatr Scand. 1999;99(1):51-58.
87. Segraves RT, Saran A, Segraves K, et al. Clomipramine versus placebo in the treatment of premature ejaculation: a pilot study. J Sex Marital Therap. 1993;19(3):198-200.
88. Rowland DL, de Gouveia Brazao CA, Koos Slob A. Effective daily treatment with clomipramine in men with premature ejaculation when 25 mg (as required) is ineffective. BJU Int. 2001;87(4):357-360.
89. Norpramin (desipramine hydrochloride) [package insert]. Bridgewater, NJ: sanofi-aventis U.S. LLC; 2014.
90. Max MB, Kishore-Kumar R, Schafer SC, et al. Efficacy of desipramine in painful diabetic neuropathy: a placebo-controlled trial. Pain. 1991;45(1):3-9.
91. Drossman DA, Toner BB, Whitehead WE, et al. Cognitive-behavioral therapy versus education and desipramine versus placebo for moderate to severe functional bowel disorders. Gastroenterology. 2003;125(1):19-31.
92. Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systemic review and meta-analysis. Lancet Neurol. 2015;14(2):162-173.
93. Doxepin hydrochloride [package insert]. Morgantown, WV: Mylan Pharmaceuticals, Inc; 2014.
94. Goldsobel AB, Rohr AS, Siegel SC, et al. Efficacy of doxepin in the treatment of chronic idiopathic urticaria. J Allergy Clin Immunol. 1986;78(5 Pt 1):867-873.
95. Imipramine hydrochloride [package insert]. Fairfield, NJ: Excellium Pharmaceutical, Inc; 2012.
96. Pope HG Jr, Hudson JI, Jonas JM, et al. Bulimia treated with imipramine: a placebo-controlled, double-blind study. Am J Psychiatry. 1983;140(5):554-558.
97. Barlow DH, Gorman JM, Shear MK, et al. Cognitive-behavioral therapy, imipramine, or their combination for panic disorder: a randomized controlled trial. JAMA. 2000;283(19):2529-2536.
98. Laederach-Hofmann K, Graf C, Horber F, et al. Imipramine and diet counseling with psychological support in the treatment of obese binge eaters: a randomized, placebo-controlled double-blind study. Int J Eat Disord. 1999;26(3):231-244.
99. Sindrup SH, Bach FW, Madsen C, et al. Venlafaxine versus imipramine in painful polyneuropathy: a randomized, controlled trial. Neurology. 2003;60(8):1284-1289.
100. Lin HH, Sheu BC, Lo MC, et al. Comparison of treatment outcomes of imipramine for female genuine stress incontinence. Br J Obstet Gynaecol. 1999;106(10):1089-1092.
101. Pamelor (nortriptyline) [package insert]. Hazelwood, MO: Mallinckrodt Inc; 2007.
102. Spencer T, Biederman J, Wilens T, et al. Nortriptyline treatment of children with attention-deficit hyperactivity disorder and tic disorder or Tourette’s syndrome. J Am Acad Child Adolesc Psychiatry. 1993;32(1):205-210.
103. Atkinson JH, Slater MA, Williams RA, et al. A placebo-controlled randomized clinical trial of nortriptyline for chronic low back pain. Pain. 1998;76(3):287-296.
104. Desai MJ, Saini V, Saini S. Myofacial pain syndrome: a treatment review. Pain Ther. 2013;2(1):21-36.
105. Chandra K, Shafiq N, Pandhi P, et al. Gabapentin versus nortriptyline in post-herpetic neuralgia patients: a randomized, double-blind clinical trial – the GONIP trial. Int J Clin Pharmacol Ther. 2006;44(8):358-363.
106. Jorge RE, Robinson RG, Arndt S, et al. Mortality and poststroke depression: a placebo-controlled trial of antidepressants. Am J Psychiatry. 2003;160(10):1823-1829.
107. Martin MR, Schiff AA. Fluphenazine/nortriptyline in the irritable bladder syndrome. A double-blind placebo-controlled study. Br J Urol. 1984;56(2):178-179.
108. Wellbutrin (bupropion hydrochloride) [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2017.
109. Maneeton N, Maneeton B, Srisurapanont M, et al. Bupropion for adults with attention-deficit hyperactivity disorder: meta-analysis of randomized, placebo-controlled trials. Psychiatry Clin Neurosci. 2011;65(7):611-617.
110. Li DJ, Tseng PT, Chen YW, et al. Significant treatment effect of bupropion in patients with bipolar disorder but similar phase-shifting rate as other antidepressants: a meta-analysis following the PRISMA guidelines. Medicine (Baltimore). 2016;95(13):e3165.
111. Clayton AH, Warnock JK, Kornstein SG, et al. A placebo-controlled trial of bupropion SR as an antidote for selective serotonin reuptake inhibitor-induced sexual dysfunction. J Clin Psychiatry. 2004;65(1):62-67.
112. Safarinejad MR. Reversal of SSRI-induced female sexual dysfunction by adjunctive bupropion in menstruating women: a double-blind, placebo-controlled and randomized study. J Psychopharmacol. 2011;25(3):370-378.
113. Remeron (mirtazapine) [package insert]. Whitehouse Station, NJ: Merck & Co, Inc; 2020.
114. Boshuisen ML, Slaap BR, Vester-Blokland ED, et al. The effect of mirtazapine in panic disorder: an open label pilot study with a single-blind placebo run-in period. Int Clin Psychopharmacol. 2001;16(6):363-368.
115. Sarchiapone M, Amore M, De Risio S, et al. Mirtazapine in the treatment of panic disorder: an open-label trial. Int Clin Psychopharmacol. 2003;18(1):35-38.
116. Connor KM, Davidson JR, Weisler RH, et al. A pilot study of mirtazapine in post-traumatic stress disorder. Int Clin Psychopharmacol. 1999;14(1):29-31.
117. Wichniak A, Wierzbicka A, Walecka M, et al. Effects of antidepressants on sleep. Curr Psychiatry Rep. 2017;19(9):63.
118. Bedtsen L, Jensen R. Mirtazapine is effective in the prophylactic treatment of chronic tension-type headache. Neurology. 2004;62(10):1706-1711.
119. AbdelFattah MR, Jung SW, Greenspan MA, et al. Efficacy of antidepressants in the treatment of obstructive sleep apnea compared to placebo. A systemic review with meta-analysis. Sleep Breath. 2020;24(2):443-453.
120. Desyrel [package insert]. Locust Valley, NY: Pragma Pharmaceuticals, LLC; 2017.
121. Lebert F, Stekke W, Hasenbroekx C, et al. Frontotemporal dementia: a randomized, controlled trial with trazodone. Dement Geriatr Cogn Disord. 2004;17(4):355-359.
122. Sultzer DL, Gray KF, Gunay I, et al. A double-blind comparison of trazodone and haloperidol for treatment of agitation in patients with dementia. Am J Geriatr Psychiatry. 1997;5(1):60-69.
123. Yi XY, Ni SF, Ghadami MR, et al. Trazodone for the treatment of insomnia: a meta-analysis of randomized placebo-controlled trials. Sleep Med. 2018;45:25-32.
124. Chlorpromazine hydrochloride [package insert]. Minneapolis, MN: Upsher-Smith Laboratories, Inc; 2010.
125. Bigal ME, Bordini CA, Speciali JG. Intravenous chlorpromazine in the emergency department treatment of migraines: a randomized controlled trial. J Emerg Med. 2002;23(2):141-148.
126. Bell R, Montoya D, Shuaib A, et al. A comparative trial of three agents in the treatment of acute migraine headache. Ann Emerg Med. 1990;19(10):1079-1082.
127. Committee on Practice Bulletins-Obstetrics. ACOG Practice Bulletin No. 189: Nausea and vomiting of pregnancy. Obstet Gynecol. 2018;131(1):e15-e30.
128. Fluphenazine hydrochloride [package insert]. Philadelphia, PA: Lannett Company, Inc; 2019.
129. Bonelli RM, Wenning GK. Pharmacological management of Huntington’s disease: an evidence-based review. Curr Pharm Des. 2006;12(21):2701-2720.
130. Haldol [package insert]. Columbus, OH: American Health Packaging; 2020.
131. MacDonald K, Wilson M, Minassian A, et al. A naturalistic study for intramuscular haloperidol versus intramuscular olanzapine for the management of acute agitation. J Clin Psychopharmacol. 2012;32(3):317-322.
132. Goikolea JM, Colom F, Capapey J, et al. Faster onset of antimanic action with haloperidol compared to second-generation antipsychotics. A meta-analysis of randomized clinical trials in acute mania. Eur Neuropsychopharmacol. 2013;23(4):305-316.
133. Girard TD, Exline MC, Carson SS, et al. Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med. 2018;379(26):2506-2516.
134. Lohr L. Chemotherapy-induced nausea and vomiting. Cancer J. 2008;14(2):85-93.
135. Büttner M, Walder B, von Elm E, et al. Is low-dose haloperidol a useful antiemetic?: A meta-analysis of published and unpublished randomized trials. Anesthesiology. 2004;101(6):1454-1463.
136. Perphenazine [package insert]. Princeton, NJ: Sandoz Inc; 2010.
137. Compazine [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2004.
138. Hesketh PJ. Chemotherapy-induced nausea and vomiting. N Engl J Med. 2008;358(23):2482-2494.
139. Chen JJ, Frame DG, White TJ. Efficacy of ondansetron and prochlorperazine for the prevention of postoperative nausea and vomiting after total hip replacement or total knee replacement procedures: a randomized, double-blind, comparative trial. Arch Intern Med. 1998;158(19):2124-2128.
140. Campbell K, Rowe H, Azzam H, et al. The management of nausea and vomiting of pregnancy. J Obstet Gynaecol Can. 2016;38(12):1127-1137.
141. Abilify [package insert]. Rockville, MD: Otsuka America Pharmaceutical, Inc; 2014.
142. Kinon BJ, Stauffer VL, Kollack-Walker S, et al. Olanzapine versus aripiprazole for the treatment of agitation in acutely ill patients with schizophrenia. J Clin Psychopharmacol. 2008;28(6):601-607.
143. Iannuzzi GL, Patel AA, Stewart JT. Aripiprazole and delusional disorder. J Psychiatr Pract. 2019;25(2):132-134.
144. Campbell EH, Elston DM, Hawthorne JD, et al. Diagnosis and management of delusional parasitosis. J Am Acad Dermatol. 2019;80(5):1428-1434.
145. Sayyah M, Sayyah M, Boostani H, et al. Effects of aripiprazole augmentation in treatment-resistant obsessive-compulsive disorder (a double-blind clinical trial). Depress Anxiety. 2012;29(10):850-854.
146. Lin WC, Chou YH. Aripiprazole effects on psychosis and chorea in a patient with Huntington’s disease. Am J Psychiatry. 2008;165(9):1207-1208.
147. Li X, Tang Y, Wang C. Adjunctive aripiprazole versus placebo for antipsychotic-induced hyperprolactinemia: meta-analysis of randomized controlled trials. PLoS One. 2013;8(8):e70179.
148. Zyprexa [package insert]. Indianapolis, IN: Eli Lilly and Company; 1997.
149. Attia E, Steinglass JE, Walsh BT, et al. Olanzapine versus placebo in adult outpatients with anorexia nervosa: a randomized clinical trial. Am J Psychiatry. 2019;176(6):449-456.
150. Dennehy EB, Doyle K, Suppes T. The efficacy of olanzapine monotherapy for acute hypomania or mania in an outpatient setting. Int Clin Psychopharmacol. 2003;18(3):143-145.
151. Grover S, Kumar V, Chakrabarti S. Comparative efficacy study of haloperidol, olanzapine and risperidone in delirium. J Psychosom Res. 2011;71(4):277-281.
152. Bosmans A, Verbanck P. Successful treatment of delusional disorder of the somatic type or “delusional parasitosis” with olanzapine. Pharmacopsychiatry. 2008;41(3):121-122.
153. Meyers BS, Flint AJ, Rothschild AJ, et al; STOP-PD Group. A double-blind randomized controlled trial of olanzapine plus sertraline vs olanzapine plus placebo for psychotic depression: the study of pharmacotherapy of psychotic depression (STOP-PD). Arch Gen Psychiatry. 2009;66(8):838-847.
154. Rothschild AJ, Williamson DJ, Tohen MF, et al. A double-blind, randomized study of olanzapine and olanzapine/fluoxetine combination for major depression with psychotic features. J Clin Psychopharmacol. 2004;24(4):365-373.
155. Navari RM, Gray SE, Kerr AC. Olanzapine versus aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a randomized phase III trial. J Support Oncol. 2011;9(5):188-195.
156. Bonelli RM, Mahnert FA, Niederwieser G. Olanzapine for Huntington’s disease: an open label study. Clin Neuropharmacol. 2002;25(5):263-265.
157. Seroquel [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2013.
158. Khan A, Atkinson S, Mezhebovsky I, et al. Extended-release quetiapine fumarate (quetiapine XR) as adjunctive therapy in patients with generalized anxiety disorder and a history of inadequate treatment response: a randomized, double-blind study. Ann Clin Psychiatry. 2014;26(1):3-18.
159. Dold M, Aigner M, Lanzenberger R, et al. Antipsychotic augmentation of serotonin reuptake inhibitors in treatment-resistant obsessive-compulsive disorder: a meta-analysis of double-blind, randomized, placebo-controlled trials. Int J Neuropsychopharmacol. 2013;16(3):557-574.
160. Villarreal G, Hamner MB, Cañive JM, et al. Efficacy of quetiapine monotherapy in posttraumatic stress disorder: a randomized, placebo-controlled trial. Am J Psychiatry. 2016;173(12):1205-1212.
161. Fernandez HH, Friedman JH, Jacques C, et al. Quetiapine for the treatment of drug-induced psychosis in Parkinson’s disease. Mov Disord. 1999;14(3):484-487.
162. Doroudgar S, Chou T, Yu J, et al. Evaluation of trazodone and quetiapine for insomnia: an observational study in psychiatric inpatients. Prim Care Companion CNS Disord. 2013;15(6):PCC.13m01558. doi: 10.4088/PCC.13m01558
163. Risperdal [package insert]. Titusville, NJ: Janssen Pharamceuticals, Inc; 2007.
164. Lim HK, Kim JJ, Pae CU, et al. Comparison of risperidone orodispersible tablet and intramuscular haloperidol in the treatment of acute psychotic agitation: a randomized open, prospective study. Neuropsychobiology. 2010;62(2):81-86.
165. Currier GW, Chou J, Feifel D, et al. Acute treatment of psychotic agitation: a randomized comparison of oral treatment with risperidone and lorazepam versus intramuscular treatment with haloperidol and lorazepam. J Clin Psychiatry. 2004;65(3):386-394.
166. Bahk WM, Yoon JS, Kim YH, et al. Risperidone in combination with mood stabilizers for acute mania: a multicentre, open study. Int Clin Psychopharmacol. 2004;19(5):299-303.
167. Freudenmann RW, Lepping P. Second-generation antipsychotics in primary and secondary delusional parasitosis: outcome and efficacy. J Clin Psychopharmacol. 2008;28(5):500-508.
168. Nelson JC, Papakostas GI. Atypical antipsychotic augmentation in major depressive disorder: a meta-analysis of placebo-controlled randomized trials. Am J Psychiatry. 2009;166(9): 980-991.
169. McDougle CJ, Epperson CN, Pelton GH, et al. A double-blind, placebo-controlled study of risperidone addition in serotonin reuptake inhibitor-refractory obsessive-compulsive disorder. Arch Gen Psychiatry. 2000;57(8):794-801.
170. Scahill L, Leckman JF, Schulz RT, et al. A placebo-controlled trial of risperidone in Tourette syndrome. Neurology. 2003;60(7):1130-1135.
171. Dallocchio C, Buffa C, Tinelli C, et al. Effectiveness of risperidone in Huntington Chorea patients. J Clin Psychopharmacol. 1999;19(1):101-103.
1. Hales CM, Servais J, Martin CB, et al. Prescription drug use among adults aged 40-79 in the United States and Canada. National Center for Health Statistics (Centers for Disease Control and Prevention). 2019. NCHS Data Brief No. 347. https://www.cdc.gov/nchs/products/databriefs/db347.htm
2. Schenker Y, Park SY, Jeong K, et al. Associations between polypharmacy, symptom burden, and quality of life in patients with advanced, life-limiting illness. J Gen Intern Med. 2019;34(4):559-566.
3. National Alliance on Mental Illness. Anosognosia. 2021. https://www.nami.org/About-Mental-Illness/Common-with-Mental-Illness/Anosognosia
4. Meyer JM. A concise guide to monoamine oxidase inhibitors. Current Psychiatry. 2017;16(12):14-16,18-23,47,A.
5. Ban TA. Fifty years chlorpromazine: a historical perspective. Neuropsychiatr Dis Treat. 2007;3(4):495-500.
6. Prozac [package insert]. Indianapolis, IN: Eli Lilly and Company; 2009.
7. Arnold LM, Hess EV, Hudson JI, et al. A randomized, placebo-controlled, double-blind, flexible-dose study of fluoxetine in the treatment of women with fibromyalgia. Am J Med. 2002;112(3):191-197.
8. Celexa [package insert]. St. Louis, MO: Forest Pharmaceuticals, Inc; 2009.
9. Porsteinsson AP, Drye LT, Pollock BG, et al. Effect of citalopram on agitation in Alzheimer disease: the CitAD randomized clinical trial. JAMA. 2014;311(7):682-691.
10. McElroy SL, Hudson JI, Malhotra S, et al. Citalopram in the treatment of binge-eating disorder: a placebo-controlled trial. J Clin Psychiatry. 2003;64(7):807-813.
11. Blank S, Lenze EJ, Mulsant BH, et al. Outcomes of late-life anxiety disorders during 32 weeks of citalopram treatment. J Clin Psychiatry. 2006;67(3):468-472.
12. Lenze EJ, Mulsant BH, Shear MK, et al. Efficacy and tolerability of citalopram in the treatment of late-life anxiety disorders: results from an 8-week randomized, placebo-controlled trial. Am J Psychiatry. 2005;162(1):146-150.
13. Montgomery SA, Kasper S, Stein DJ, et al. Citalopram 20 mg, 40 mg and 60 mg are all effective and well tolerated compared with placebo in obsessive-compulsive disorder. Int Clin Psychopharmacol. 2001;16(2):75-86.
14. Leinonen E, Lepola U, Koponen H, et al. Citalopram controls phobic symptoms in patients with panic disorder: randomized controlled trial. J Psychiatry Neurosci. 2000;25(1):24-32.
15. Perna G, Bertani A, Caldirola D, et al. A comparison of citalopram and paroxetine in the treatment of panic disorder: a randomized, single-blind study. Pharmacopsychiatry. 2001;34(3):85-90.
16. Wikander I, Sundblad C, Andersch B, et al. Citalopram in premenstrual dysphoria: is intermittent treatment during luteal phases more effective than continuous medication throughout the menstrual cycle? J Clin Psychopharmacol. 1998;18(5):390-398.
17. English BA, Jewell M, Jewell G, et al. Treatment of chronic posttraumatic stress disorder in combat veterans with citalopram: an open trial. J Clin Psychopharmacol. 2006;26(1):84-88.
18. Furmark T, Appel L, Michelgård A, et al. Cerebral blood flow changes after treatment of social phobia with neurokinin-1 antagonist GR205171, citalopram, or placebo. Biol Psychiatry. 2005;58(2):132-142.
19. Naranjo CA, Poulos CX, Bremner KE, et al. Citalopram decreases desirability, liking, and consumption of alcohol in alcohol-dependent drinkers. Clin Pharmacol Ther. 1992;51(6):729-739.
20. Safarinejad MR, Hosseini SY. Safety and efficacy of citalopram in the treatment of premature ejaculation: a double-blind placebo-controlled, fixed dose, randomized study. Int J Impot Res. 2006;18(2):164-169.
21. Shams T, Firwana B, Habib F, et al. SSRIs for hot flashes: a systematic review and meta-analysis of randomized trials. J Gen Intern Med. 2014;29(1):204-213.
22. Lexapro [package insert]. Irvine, CA: Allergan USA, Inc; 2016.
23. Guerdjikova AI, McElroy SL, Kotwal R, et al. High-dose escitalopram in the treatment of binge-eating disorder with obesity: a placebo-controlled monotherapy trial. Hum Psychopharmacol. 2008;23(1):1-11.
24. Aigner M, Treasure J, Kaye W, et al. World federation of societies of biological psychiatry (WFSBP) guidelines for pharmacological treatment of eating disorders. World J Biol Psychiatry. 2011;12:400-443.
25. Fineberg NA, Tonnoir B, Lemming O, et al. Escitalopram prevents relapse of obsessive-compulsive disorder. Eur Neuropsychopharmacol. 2007;17(6-7):430-439.
26. Stein DJ, Andersen EW, Tonnoir B, et al. Escitalopram in obsessive-compulsive disorder: a randomized, placebo-controlled, paroxetine-referenced, fixed-dose, 24-week study. Curr Med Res Opin. 2007;23(4):701-711.
27. Stahl SM, Gergel I, Li D. Escitalopram in the treatment of panic disorder: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2003;64(11):1322-1327.
28. Freeman EW, Sondheimer SJ, Sammel MD, et al. A preliminary study of luteal phase versus symptom-onset dosing with escitalopram for premenstrual dysphoric disorder. J Clin Psychiatry. 2005;66(6):769-773.
29. Qi W, Gevonden M, Shalev A. Efficacy and tolerability of high-dose escitalopram in posttraumatic stress disorder. J Clin Psychopharmacol. 2017;37(1):89-93.
30. Carpenter JS, Guthrie KA, Larson JC, et al. Effect of escitalopram on hot flash interference: a randomized, controlled trial. Fertil Steril. 2012;97(6):1399-1404.
31. Freeman EW, Guthrie KA, Caan B, et al. Efficacy of escitalopram for hot flashes in healthy menopausal women: a randomized controlled trial. JAMA. 2011;305(3):267-274.
32. Arnold LM, McElroy SL, Hudson JI, et al. A placebo-controlled, randomized trial of fluoxetine in the treatment of binge-eating disorder. J Clin Psychiatry. 2002;63(11):1028-1033.
33. Connor KM, Sutherland SM, Tupler LA, et al. Fluoxetine in posttraumatic stress disorder. Randomized, double-blind study. Br J Psychiatry. 1999;175:17-22.
34. Martenyi F, Brown EB, Zhang H, et al. Fluoxetine versus placebo in posttraumatic stress disorder. J Clin Psychiatry. 2002;63(3):199-206.
35. Davidson JR, Foa EB, Huppert JD, et al. Fluoxetine, comprehensive cognitive behavioral therapy, and placebo in generalized social phobia. Arch Gen Psychiatry. 2004;61(10):1005-1013.
36. Kara H, Aydin S, Yücel M, et al. The efficacy of fluoxetine in the treatment of premature ejaculation: a double-blind placebo-controlled study. J Urol. 1996;156(5):1631-1632.
37. Loprinzi CL, Sloan JA, Perez EA, et al. Phase III evaluation of fluoxetine for treatment of hot flashes. J Clin Oncol. 2002;20(6):1578-1583.
38. Coleiro B, Marshall SE, Denton CP, et al. Treatment of Raynaud’s phenomenon with the selective serotonin reuptake inhibitor fluoxetine. Rheumatology (Oxford). 2001;40(9):1038-1043.
39. Paxil [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2019.
40. Zhang D, Cheng Y, Wu K, et al. Paroxetine in the treatment of premature ejaculation: a systematic review and meta-analysis. BMC Urol. 2019;19(1):2.
41. Walitt B, Urrútia G, Nishishinya MB. Selective serotonin reuptake inhibitors for fibromyalgia syndrome. Cochrane Database Syst Rev. 2015;(6):CD011735.
42. Foster CA, Bafaloukos J. Paroxetine in the treatment of chronic daily headache. Headache. 1994;34:587-589.
43. Zylicz Z, Krajnik M, Sorge A, et al. Paroxetine in the treatment of severe non-dermatological pruritus: a randomized, controlled trial. J Pain Symptom Manage. 2003;26(3):1105-1112.
44. Zoloft [package insert]. New York, NY: Pfizer; 2016.
45. Leombruni P, Pierò A, Lavagnino L, et al. A randomized, double-blind trial comparing sertraline and fluoxetine 6-month treatment in obese patients with binge eating disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(6):1599-1605.
46. McElroy SL, Casuto LS, Nelson EB, et al. Placebo-controlled trial of sertraline in the treatment of binge eating disorder. Am J Psychiatry. 2000;157(6):1004-1006.
47. Milano W, Petrella C, Sabatino C, et al. Treatment of bulimia nervosa with sertraline: a randomized controlled trial. Adv Ther. 2004;21(4):232-237.
48. Brawman-Mintzer O, Knapp RG, Rynn M, et al. Sertraline treatment for generalized anxiety disorder: a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2006;67(6):874-881.
49. McMahon CG. Treatment of premature ejaculation with sertraline hydrochloride: a single-blind placebo-controlled crossover study. J Urol. 1998;159(6):1935-1938.
50. Yi ZM, Chen SD, Tang QY, et al. Efficacy and safety of sertraline for the treatment of premature ejaculation: systematic review and meta-analysis. Medicine (Baltimore). 2019;98(23):e15989.
51. Uçeyler N, Häuser W, Sommer C. A systematic review on the effectiveness of treatment with antidepressants in fibromyalgia syndrome. Arthritis Rheum. 2008;59(9):1279-1298.
52. Pristiq [package insert]. Philadelphia, PA: Wyeth Pharmaceuticals, Inc; 2011.
53. Sun Z, Hao Y, Zhang M. Efficacy and safety of desvenlafaxine treatment for hot flashes associated with menopause: a meta-analysis of randomized controlled trials. Gynecol Obstet Invest. 2013;75(4):255-262.
54. Cymbalta [package insert]. Indianapolis, IN: Eli Lilly and Company; 2008.
55. Li J, Yang L, Pu C, et al. The role of duloxetine in stress urinary incontinence: a systemic review and meta-analysis. Int Urol Nephrol. 2013;45(3):679-686.
56. Filocamo MT, Li Marzi V, Del Popolo G, et al. Pharmacologic treatment in postprostatectomy stress urinary incontinence. Eur Urol. 2007;51(6):1559-1564.
57. Effexor XR [package insert]. Philadelphia, PA: Wyeth Pharmaceuticals, Inc; 2017.
58. Denys D, Van der Wee N, Van Megen HJ, et al. A double-blind comparison of venlafaxine and paroxetine in obsessive-compulsive disorder. J Clin Psychopharmacol. 2003;23(6):568-575.
59. Albert U, Aguglia E, Maina G, et al. Venlafaxine versus clomipramine in the treatment of obsessive-compulsive disorder: a preliminary single-blind, 12-week, controlled study. J Clin Psychiatry. 2002;63(11):1004-1009.
60. Davidson J, Baldwin D, Stein DJ, et al. Treatment of posttraumatic stress disorder with venlafaxine extended release: a 6-month randomized controlled trial. Arch Gen Psychiatry. 2006;63(10):1158-1165.
61. Zarinara AR, Mohammad MR, Hazrati N, et al. Venlafaxine versus methylphenidate in pediatric outpatients with attention deficit hyperactivity disorder: a randomized, double-blind comparison trial. Hum Psychopharmacol. 2010;25(7-8):530-535.
62. Mukaddes NM, Abali O. Venlafaxine in children and adolescents with attention deficit hyperactivity disorder. Psychiatry Clin Neurosci. 2004;58(1):92-95.
63. Cohen LS, Soares CN, Lyster A, et al. Efficacy and tolerability of premenstrual use of venlafaxine (flexible dose) in the treatment of premenstrual dysphoric disorder. J Clin Psychopharmacol. 2004;24(5):540-543.
64. Ozyalcin SN, Talu GK, Kiziltan E, et al. The efficacy and safety of venlafaxine in the prophylaxis of migraine. Headache. 2005;45(2):144-152.
65. Tarlaci S. Escitalopram and venlafaxine for the prophylaxis of migraine headache without mood disorders. Clin Neuropharmacol. 2009;32(5):254-258.
66. Kadiroglu AK, Sit D, Kayabasi H, et al. The effect of venlafaxine HCl on painful peripheral diabetic neuropathy in patients with type 2 diabetes mellitus. J Diabetes Complications. 2008;22(4):241-245.
67. Evans ML, Pritts E, Vittinghoff E, et al. Management of postmenopausal hot flushes with venlafaxine hydrochloride: a randomized, controlled trial. Obstet Gynecol. 2005;105(1):161-166.
68. Farshchian N, Alavi A, Heydarheydari S, et al. Comparative study of the effects of venlafaxine and duloxetine on chemotherapy-induced peripheral neuropathy. Cancer Chemother Pharmacol. 2018;82(5):787-793.
69. Amitriptyline Hydrochloride [package insert]. Princeton, NJ: Sandoz Inc; 2014.
70. Hauser W, Wolfe F, Tolle T, et al. The role of antidepressants in the management of fibromyalgia syndrome: a systemic review and meta-analysis. CNS Drugs. 2012;26(4):297-307.
71. Braak B, Klooker T, Lei A, et al. Randomised clinical trial: the effects of amitriptyline on drinking capacity and symptoms in patients with functional dyspepsia, a double-blind placebo-controlled study. Aliment Pharmacol Ther. 2011;34(6):638-648.
72. Van Ophoven A, Pokupic S, Heinecke A, et al. A prospective, randomized, placebo controlled, double-blind study of amitriptyline for the treatment of interstitial cystitis. J Urol. 2004;172(2):533-536.
73. Foster HE Jr, Hanno P, Nickel JC, et al; Interstitial Cystitis Collaborative Research Network. Effect of amitriptyline on symptoms in treatment naïve patients with interstitial cystitis/painful bladder syndrome. J Urol. 2010;183(5):1853-1858.
74. Vahedi H, Merat S, Momtahen S, et al. Clinical trial: the effect of amitriptyline in patients with diarrhoea-predominent irritable bowel syndrome. Aliment Pharmacol Ther. 2008;27(8):678-684.
75. Bulut S, Berilgen MS, Baran A, et al. Venlafaxine versus amitriptyline in the prophylactic treatment of migraine: a randomized, double-blind, crossover study. Clin Neurol Neurosurg. 2004;107(1):44-48.
76. Keskinbora K, Aydinli I. A double-blind randomized controlled trial of topiramate and amitriptyline either alone or in combination for the prevention of migraine. Clin Neurol Neurosurg. 2008;110(10):979-984.
77. Max MB, Lynch SA, Muir J, et al. Effects of desipramine, amitriptyline, and fluoxetine on pain in diabetic neuropathy. N Engl J Med. 1992;326(19):1250-1256.
78. Boyle J, Eriksson M, Gribble L, et al. Randomized, placebo-controlled comparison of amitriptyline, duloxetine, and pregabalin in patients with chronic diabetic peripheral neuropathic pain: impact on pain, polysomnographic sleep, daytime functioning, and quality of life. Diabetes Care. 2012;35(12):2451-2458.
79. Graff-Radford SB, Shaw LR, Naliboff BN. Amitriptyline and fluphenazine in the treatment of postherpetic neuralgia. Clin J Pain. 2000;16(3):188-192.
80. Watson CP, Evans RJ, Reed K, et al. Amitriptyline versus placebo in postherpetic neuralgia. Neurology. 1982;32(6):671-673.
81. Sinha S, Simlai J, Praharaj SK. Very low dose amitriptyline for clozapine-associated sialorrhea. Curr Drug Saf. 2016;11(3):262-263.
82. Amoxapine [package insert]. Parsippany, NJ: Watson Pharma, Inc; 2014.
83. Weinberg DS, Smalley W, Heidelbaugh JJ, et al. American Gastroenterological Association institute guideline on the pharmacological management of irritable bowel syndrome. Gastroenterology. 2014;147(5):1146-1148.
84. Anafranil (clomipramine hydrochloride) [package insert]. Whitby, Ontario: Patheon Inc; 2012.
85. Clomipramine dose-effect study in patients with depression: clinical end points and pharmacokinetics. Danish University Antidepressant Group (DUAG). Clin Pharmacol Ther. 1999;66(2):152-165.
86. Caillard V, Rouillon F, Viel J, et al. Comparative effects of low and high doses of clomipramine and placebo in panic disorder: a double-blind controlled study. Acta Psychiatr Scand. 1999;99(1):51-58.
87. Segraves RT, Saran A, Segraves K, et al. Clomipramine versus placebo in the treatment of premature ejaculation: a pilot study. J Sex Marital Therap. 1993;19(3):198-200.
88. Rowland DL, de Gouveia Brazao CA, Koos Slob A. Effective daily treatment with clomipramine in men with premature ejaculation when 25 mg (as required) is ineffective. BJU Int. 2001;87(4):357-360.
89. Norpramin (desipramine hydrochloride) [package insert]. Bridgewater, NJ: sanofi-aventis U.S. LLC; 2014.
90. Max MB, Kishore-Kumar R, Schafer SC, et al. Efficacy of desipramine in painful diabetic neuropathy: a placebo-controlled trial. Pain. 1991;45(1):3-9.
91. Drossman DA, Toner BB, Whitehead WE, et al. Cognitive-behavioral therapy versus education and desipramine versus placebo for moderate to severe functional bowel disorders. Gastroenterology. 2003;125(1):19-31.
92. Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systemic review and meta-analysis. Lancet Neurol. 2015;14(2):162-173.
93. Doxepin hydrochloride [package insert]. Morgantown, WV: Mylan Pharmaceuticals, Inc; 2014.
94. Goldsobel AB, Rohr AS, Siegel SC, et al. Efficacy of doxepin in the treatment of chronic idiopathic urticaria. J Allergy Clin Immunol. 1986;78(5 Pt 1):867-873.
95. Imipramine hydrochloride [package insert]. Fairfield, NJ: Excellium Pharmaceutical, Inc; 2012.
96. Pope HG Jr, Hudson JI, Jonas JM, et al. Bulimia treated with imipramine: a placebo-controlled, double-blind study. Am J Psychiatry. 1983;140(5):554-558.
97. Barlow DH, Gorman JM, Shear MK, et al. Cognitive-behavioral therapy, imipramine, or their combination for panic disorder: a randomized controlled trial. JAMA. 2000;283(19):2529-2536.
98. Laederach-Hofmann K, Graf C, Horber F, et al. Imipramine and diet counseling with psychological support in the treatment of obese binge eaters: a randomized, placebo-controlled double-blind study. Int J Eat Disord. 1999;26(3):231-244.
99. Sindrup SH, Bach FW, Madsen C, et al. Venlafaxine versus imipramine in painful polyneuropathy: a randomized, controlled trial. Neurology. 2003;60(8):1284-1289.
100. Lin HH, Sheu BC, Lo MC, et al. Comparison of treatment outcomes of imipramine for female genuine stress incontinence. Br J Obstet Gynaecol. 1999;106(10):1089-1092.
101. Pamelor (nortriptyline) [package insert]. Hazelwood, MO: Mallinckrodt Inc; 2007.
102. Spencer T, Biederman J, Wilens T, et al. Nortriptyline treatment of children with attention-deficit hyperactivity disorder and tic disorder or Tourette’s syndrome. J Am Acad Child Adolesc Psychiatry. 1993;32(1):205-210.
103. Atkinson JH, Slater MA, Williams RA, et al. A placebo-controlled randomized clinical trial of nortriptyline for chronic low back pain. Pain. 1998;76(3):287-296.
104. Desai MJ, Saini V, Saini S. Myofacial pain syndrome: a treatment review. Pain Ther. 2013;2(1):21-36.
105. Chandra K, Shafiq N, Pandhi P, et al. Gabapentin versus nortriptyline in post-herpetic neuralgia patients: a randomized, double-blind clinical trial – the GONIP trial. Int J Clin Pharmacol Ther. 2006;44(8):358-363.
106. Jorge RE, Robinson RG, Arndt S, et al. Mortality and poststroke depression: a placebo-controlled trial of antidepressants. Am J Psychiatry. 2003;160(10):1823-1829.
107. Martin MR, Schiff AA. Fluphenazine/nortriptyline in the irritable bladder syndrome. A double-blind placebo-controlled study. Br J Urol. 1984;56(2):178-179.
108. Wellbutrin (bupropion hydrochloride) [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2017.
109. Maneeton N, Maneeton B, Srisurapanont M, et al. Bupropion for adults with attention-deficit hyperactivity disorder: meta-analysis of randomized, placebo-controlled trials. Psychiatry Clin Neurosci. 2011;65(7):611-617.
110. Li DJ, Tseng PT, Chen YW, et al. Significant treatment effect of bupropion in patients with bipolar disorder but similar phase-shifting rate as other antidepressants: a meta-analysis following the PRISMA guidelines. Medicine (Baltimore). 2016;95(13):e3165.
111. Clayton AH, Warnock JK, Kornstein SG, et al. A placebo-controlled trial of bupropion SR as an antidote for selective serotonin reuptake inhibitor-induced sexual dysfunction. J Clin Psychiatry. 2004;65(1):62-67.
112. Safarinejad MR. Reversal of SSRI-induced female sexual dysfunction by adjunctive bupropion in menstruating women: a double-blind, placebo-controlled and randomized study. J Psychopharmacol. 2011;25(3):370-378.
113. Remeron (mirtazapine) [package insert]. Whitehouse Station, NJ: Merck & Co, Inc; 2020.
114. Boshuisen ML, Slaap BR, Vester-Blokland ED, et al. The effect of mirtazapine in panic disorder: an open label pilot study with a single-blind placebo run-in period. Int Clin Psychopharmacol. 2001;16(6):363-368.
115. Sarchiapone M, Amore M, De Risio S, et al. Mirtazapine in the treatment of panic disorder: an open-label trial. Int Clin Psychopharmacol. 2003;18(1):35-38.
116. Connor KM, Davidson JR, Weisler RH, et al. A pilot study of mirtazapine in post-traumatic stress disorder. Int Clin Psychopharmacol. 1999;14(1):29-31.
117. Wichniak A, Wierzbicka A, Walecka M, et al. Effects of antidepressants on sleep. Curr Psychiatry Rep. 2017;19(9):63.
118. Bedtsen L, Jensen R. Mirtazapine is effective in the prophylactic treatment of chronic tension-type headache. Neurology. 2004;62(10):1706-1711.
119. AbdelFattah MR, Jung SW, Greenspan MA, et al. Efficacy of antidepressants in the treatment of obstructive sleep apnea compared to placebo. A systemic review with meta-analysis. Sleep Breath. 2020;24(2):443-453.
120. Desyrel [package insert]. Locust Valley, NY: Pragma Pharmaceuticals, LLC; 2017.
121. Lebert F, Stekke W, Hasenbroekx C, et al. Frontotemporal dementia: a randomized, controlled trial with trazodone. Dement Geriatr Cogn Disord. 2004;17(4):355-359.
122. Sultzer DL, Gray KF, Gunay I, et al. A double-blind comparison of trazodone and haloperidol for treatment of agitation in patients with dementia. Am J Geriatr Psychiatry. 1997;5(1):60-69.
123. Yi XY, Ni SF, Ghadami MR, et al. Trazodone for the treatment of insomnia: a meta-analysis of randomized placebo-controlled trials. Sleep Med. 2018;45:25-32.
124. Chlorpromazine hydrochloride [package insert]. Minneapolis, MN: Upsher-Smith Laboratories, Inc; 2010.
125. Bigal ME, Bordini CA, Speciali JG. Intravenous chlorpromazine in the emergency department treatment of migraines: a randomized controlled trial. J Emerg Med. 2002;23(2):141-148.
126. Bell R, Montoya D, Shuaib A, et al. A comparative trial of three agents in the treatment of acute migraine headache. Ann Emerg Med. 1990;19(10):1079-1082.
127. Committee on Practice Bulletins-Obstetrics. ACOG Practice Bulletin No. 189: Nausea and vomiting of pregnancy. Obstet Gynecol. 2018;131(1):e15-e30.
128. Fluphenazine hydrochloride [package insert]. Philadelphia, PA: Lannett Company, Inc; 2019.
129. Bonelli RM, Wenning GK. Pharmacological management of Huntington’s disease: an evidence-based review. Curr Pharm Des. 2006;12(21):2701-2720.
130. Haldol [package insert]. Columbus, OH: American Health Packaging; 2020.
131. MacDonald K, Wilson M, Minassian A, et al. A naturalistic study for intramuscular haloperidol versus intramuscular olanzapine for the management of acute agitation. J Clin Psychopharmacol. 2012;32(3):317-322.
132. Goikolea JM, Colom F, Capapey J, et al. Faster onset of antimanic action with haloperidol compared to second-generation antipsychotics. A meta-analysis of randomized clinical trials in acute mania. Eur Neuropsychopharmacol. 2013;23(4):305-316.
133. Girard TD, Exline MC, Carson SS, et al. Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med. 2018;379(26):2506-2516.
134. Lohr L. Chemotherapy-induced nausea and vomiting. Cancer J. 2008;14(2):85-93.
135. Büttner M, Walder B, von Elm E, et al. Is low-dose haloperidol a useful antiemetic?: A meta-analysis of published and unpublished randomized trials. Anesthesiology. 2004;101(6):1454-1463.
136. Perphenazine [package insert]. Princeton, NJ: Sandoz Inc; 2010.
137. Compazine [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2004.
138. Hesketh PJ. Chemotherapy-induced nausea and vomiting. N Engl J Med. 2008;358(23):2482-2494.
139. Chen JJ, Frame DG, White TJ. Efficacy of ondansetron and prochlorperazine for the prevention of postoperative nausea and vomiting after total hip replacement or total knee replacement procedures: a randomized, double-blind, comparative trial. Arch Intern Med. 1998;158(19):2124-2128.
140. Campbell K, Rowe H, Azzam H, et al. The management of nausea and vomiting of pregnancy. J Obstet Gynaecol Can. 2016;38(12):1127-1137.
141. Abilify [package insert]. Rockville, MD: Otsuka America Pharmaceutical, Inc; 2014.
142. Kinon BJ, Stauffer VL, Kollack-Walker S, et al. Olanzapine versus aripiprazole for the treatment of agitation in acutely ill patients with schizophrenia. J Clin Psychopharmacol. 2008;28(6):601-607.
143. Iannuzzi GL, Patel AA, Stewart JT. Aripiprazole and delusional disorder. J Psychiatr Pract. 2019;25(2):132-134.
144. Campbell EH, Elston DM, Hawthorne JD, et al. Diagnosis and management of delusional parasitosis. J Am Acad Dermatol. 2019;80(5):1428-1434.
145. Sayyah M, Sayyah M, Boostani H, et al. Effects of aripiprazole augmentation in treatment-resistant obsessive-compulsive disorder (a double-blind clinical trial). Depress Anxiety. 2012;29(10):850-854.
146. Lin WC, Chou YH. Aripiprazole effects on psychosis and chorea in a patient with Huntington’s disease. Am J Psychiatry. 2008;165(9):1207-1208.
147. Li X, Tang Y, Wang C. Adjunctive aripiprazole versus placebo for antipsychotic-induced hyperprolactinemia: meta-analysis of randomized controlled trials. PLoS One. 2013;8(8):e70179.
148. Zyprexa [package insert]. Indianapolis, IN: Eli Lilly and Company; 1997.
149. Attia E, Steinglass JE, Walsh BT, et al. Olanzapine versus placebo in adult outpatients with anorexia nervosa: a randomized clinical trial. Am J Psychiatry. 2019;176(6):449-456.
150. Dennehy EB, Doyle K, Suppes T. The efficacy of olanzapine monotherapy for acute hypomania or mania in an outpatient setting. Int Clin Psychopharmacol. 2003;18(3):143-145.
151. Grover S, Kumar V, Chakrabarti S. Comparative efficacy study of haloperidol, olanzapine and risperidone in delirium. J Psychosom Res. 2011;71(4):277-281.
152. Bosmans A, Verbanck P. Successful treatment of delusional disorder of the somatic type or “delusional parasitosis” with olanzapine. Pharmacopsychiatry. 2008;41(3):121-122.
153. Meyers BS, Flint AJ, Rothschild AJ, et al; STOP-PD Group. A double-blind randomized controlled trial of olanzapine plus sertraline vs olanzapine plus placebo for psychotic depression: the study of pharmacotherapy of psychotic depression (STOP-PD). Arch Gen Psychiatry. 2009;66(8):838-847.
154. Rothschild AJ, Williamson DJ, Tohen MF, et al. A double-blind, randomized study of olanzapine and olanzapine/fluoxetine combination for major depression with psychotic features. J Clin Psychopharmacol. 2004;24(4):365-373.
155. Navari RM, Gray SE, Kerr AC. Olanzapine versus aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a randomized phase III trial. J Support Oncol. 2011;9(5):188-195.
156. Bonelli RM, Mahnert FA, Niederwieser G. Olanzapine for Huntington’s disease: an open label study. Clin Neuropharmacol. 2002;25(5):263-265.
157. Seroquel [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2013.
158. Khan A, Atkinson S, Mezhebovsky I, et al. Extended-release quetiapine fumarate (quetiapine XR) as adjunctive therapy in patients with generalized anxiety disorder and a history of inadequate treatment response: a randomized, double-blind study. Ann Clin Psychiatry. 2014;26(1):3-18.
159. Dold M, Aigner M, Lanzenberger R, et al. Antipsychotic augmentation of serotonin reuptake inhibitors in treatment-resistant obsessive-compulsive disorder: a meta-analysis of double-blind, randomized, placebo-controlled trials. Int J Neuropsychopharmacol. 2013;16(3):557-574.
160. Villarreal G, Hamner MB, Cañive JM, et al. Efficacy of quetiapine monotherapy in posttraumatic stress disorder: a randomized, placebo-controlled trial. Am J Psychiatry. 2016;173(12):1205-1212.
161. Fernandez HH, Friedman JH, Jacques C, et al. Quetiapine for the treatment of drug-induced psychosis in Parkinson’s disease. Mov Disord. 1999;14(3):484-487.
162. Doroudgar S, Chou T, Yu J, et al. Evaluation of trazodone and quetiapine for insomnia: an observational study in psychiatric inpatients. Prim Care Companion CNS Disord. 2013;15(6):PCC.13m01558. doi: 10.4088/PCC.13m01558
163. Risperdal [package insert]. Titusville, NJ: Janssen Pharamceuticals, Inc; 2007.
164. Lim HK, Kim JJ, Pae CU, et al. Comparison of risperidone orodispersible tablet and intramuscular haloperidol in the treatment of acute psychotic agitation: a randomized open, prospective study. Neuropsychobiology. 2010;62(2):81-86.
165. Currier GW, Chou J, Feifel D, et al. Acute treatment of psychotic agitation: a randomized comparison of oral treatment with risperidone and lorazepam versus intramuscular treatment with haloperidol and lorazepam. J Clin Psychiatry. 2004;65(3):386-394.
166. Bahk WM, Yoon JS, Kim YH, et al. Risperidone in combination with mood stabilizers for acute mania: a multicentre, open study. Int Clin Psychopharmacol. 2004;19(5):299-303.
167. Freudenmann RW, Lepping P. Second-generation antipsychotics in primary and secondary delusional parasitosis: outcome and efficacy. J Clin Psychopharmacol. 2008;28(5):500-508.
168. Nelson JC, Papakostas GI. Atypical antipsychotic augmentation in major depressive disorder: a meta-analysis of placebo-controlled randomized trials. Am J Psychiatry. 2009;166(9): 980-991.
169. McDougle CJ, Epperson CN, Pelton GH, et al. A double-blind, placebo-controlled study of risperidone addition in serotonin reuptake inhibitor-refractory obsessive-compulsive disorder. Arch Gen Psychiatry. 2000;57(8):794-801.
170. Scahill L, Leckman JF, Schulz RT, et al. A placebo-controlled trial of risperidone in Tourette syndrome. Neurology. 2003;60(7):1130-1135.
171. Dallocchio C, Buffa C, Tinelli C, et al. Effectiveness of risperidone in Huntington Chorea patients. J Clin Psychopharmacol. 1999;19(1):101-103.
Antipsychotic-induced priapism: Mitigating the risk
Mr. J, age 35, is brought to the hospital from prison due to priapism that does not improve with treatment. He says he has had priapism 5 times previously, with the first incidence occurring “years ago” due to trazodone.
Recently, he has been receiving risperidone, which the treatment team believes is the cause of his current priapism. His medical history includes asthma, schizophrenia, hypertension, seizures, and sickle cell trait. Mr. J is experiencing auditory hallucinations, which he describes as “continuous, neutral voices that are annoying.” He would like relief from his auditory hallucinations and is willing to change his antipsychotic, but does not want additional treatment for his priapism. His present medications include risperidone, 1 mg twice a day, escitalopram, 10 mg/d, benztropine, 1 mg twice a day, and phenytoin, 500 mg/d at bedtime.
Priapism is a prolonged, persistent, and often painful erection that occurs without sexual stimulation. Although relatively rare, it can result in potentially serious long-term complications, including impotence and gangrene, and requires immediate evaluation and management.
There are 2 types of priapism: nonischemic, or “high-flow,” priapism, and ischemic, or “low-flow,” priapism (Table 1). While nonischemic priapism is typically caused by penile or perineal trauma, ischemic priapism can occur as a result of medications, including antipsychotics, antidepressants, anxiolytics, and antihypertensives, or hematological conditions such as sickle cell disease.1 Other risk factors associated with priapism include substance abuse, hyperprolactinemia, diabetes, and liver disease.4
Antipsychotic-induced priapism
Medication-induced priapism is a rare adverse drug reaction (ADR). Of the medication classes associated with priapism, antipsychotics have the highest incidence and account for approximately 20% of all cases.1
The mechanism of priapism associated with antipsychotics is thought to be related to alpha-1 blockade in the corpora cavernosa of the penis. Although antipsychotics within each class share common characteristics, each agent has a unique profile of receptor affinities. As such, antipsychotics have varying affinities for the alpha-adrenergic receptor (Table 2). Agents such as ziprasidone, chlorpromazine, and risperidone—which have the highest affinity for the alpha-1 adrenoceptors—may be more likely to cause priapism compared with agents with lower affinity, such as olanzapine. Priapism may occur at any time during antipsychotic treatment, and does not appear to be dose-related.
Continue to: Antipsychotic drug interactions and priapism...
Antipsychotic drug interactions and priapism
Patients who are receiving multiple medications as treatment for chronic medical or psychiatric conditions have an increased likelihood of experiencing drug-drug interactions (DDIs) that lead to adverse effects.
Various case reports have described priapism as a result of DDIs related to antipsychotic agents combined with other psychotropic or nonpsychotropic medications.3 Most of these DDIs have been attributed to the cytochrome P450 (CYP) family of enzymes, including CYP2D6, CYP1A2, and CYP3A4/5, which are major enzymes implicated in the metabolism of antipsychotics (Table 3).
It is imperative to be vigilant during the concomitant administration of antipsychotics with other medications that may be substrates, inducers, or inhibitors of CYP enzymes, as this could alter the metabolism and kinetics of the antipsychotic and result in ADRs such as priapism. For example, drug interactions exist between strong CYP2D6 inhibitors—such as the antidepressants paroxetine, fluoxetine, and bupropion—and antipsychotics that are substrates of CYP2D6, such as risperidone, aripiprazole, haloperidol, and perphenazine. This interaction can lead to higher levels of the antipsychotic, which would increase the patient’s risk of experiencing ADRs. Because psychotic illnesses and depression/anxiety often coexist, it is not uncommon for individuals with these conditions to be receiving both an antipsychotic and an antidepressant.
Because there is a high incidence of comorbidities such as HIV and cardiovascular disease among individuals with mental illnesses, clinicians must also be cognizant of any nonpsychotropic medications the patient may be taking. For instance, clinically relevant DDIs exist between protease inhibitors, such as ritonavir, a strong CYP3A4 inhibitor, and antipsychotics that are substrates of CYP3A4, such as pimozide, aripiprazole, and quetiapine.
Mitigating the risk of priapism
Although there are associated risk factors for priapism, there are no concrete indicators to predict the onset or development of the condition. The best predictor may be a history of prolonged and painless erections.3
As such, when choosing an antipsychotic, it is critical to screen the patient for the previously mentioned risk factors, including the presence of medications with strong alpha-1 receptor affinity and CYP interactions, especially to minimize the risk of recurrence of priapism in those with prior or similar episodes. Management of patients with priapism due to antipsychotics has involved reducing the dose of the offending agent and/or changing the medication to one with a lower alpha-adrenergic affinity (Table 22).
Similar to most situations, management is patient-specific and depends on several factors, including the severity of the patient’s psychiatric disease, history/severity of priapism and treatment, concurrent medication list, etc. For example, although clozapine is considered to have relatively high affinity for the alpha-1 receptor, it is also the agent of choice for treatment-refractory schizophrenia. Risks and benefits must be weighed on a individualized basis. Case reports have described symptom improvement via lowering the dose of clozapine and adding on or switching to an antipsychotic agent with minimal alpha-1 receptor affinity.4
After considering Mr. J’s history, risk factors, and preferences, the treatment team discontinues risperidone and initiates haloperidol, 5 mg twice a day. Soon after, Mr. J no longer experiences priapism.
1. Weiner DM, Lowe FC. Psychotropic drug-induced priapism. Mol Diag Ther 9. 1998;371-379. doi:10.2165/00023210-199809050-00004
2. Andersohn F, Schmedt N, Weinmann S, et al. Priapism associated with antipsychotics: role of alpha1 adrenoceptor affinity. J Clin Psychopharmacol. 2010;30(1):68-71. doi:10.1097/JCP.0b013e3181c8273d
3. Sood S, James W, Bailon MJ. Priapism associated with atypical antipsychotic medications: a review. Int Clin Psychopharmacol. 2008;23(1):9-17.
4. Sinkeviciute I, Kroken RA, Johnsen E. Priapism in antipsychotic drug use: a rare but important side effect. Case Rep Psychiatry. 2012;2012:496364. doi:10.1155/2012/496364
5. Mora F, Martín JDD, Zubillaga E, et al. CYP450 and its implications in the clinical use of antipsychotic drugs. Clin Exp Pharmacol. 2015;5(176):1-10. doi:10.4172/2161-1459.1000176
6. Puangpetch A, Vanwong N, Nuntamool N, et al. CYP2D6 polymorphisms and their influence on risperidone treatment. Pharmgenomics Pers Med. 2016;9:131-147. doi:10.2147/PGPM.S107772
Mr. J, age 35, is brought to the hospital from prison due to priapism that does not improve with treatment. He says he has had priapism 5 times previously, with the first incidence occurring “years ago” due to trazodone.
Recently, he has been receiving risperidone, which the treatment team believes is the cause of his current priapism. His medical history includes asthma, schizophrenia, hypertension, seizures, and sickle cell trait. Mr. J is experiencing auditory hallucinations, which he describes as “continuous, neutral voices that are annoying.” He would like relief from his auditory hallucinations and is willing to change his antipsychotic, but does not want additional treatment for his priapism. His present medications include risperidone, 1 mg twice a day, escitalopram, 10 mg/d, benztropine, 1 mg twice a day, and phenytoin, 500 mg/d at bedtime.
Priapism is a prolonged, persistent, and often painful erection that occurs without sexual stimulation. Although relatively rare, it can result in potentially serious long-term complications, including impotence and gangrene, and requires immediate evaluation and management.
There are 2 types of priapism: nonischemic, or “high-flow,” priapism, and ischemic, or “low-flow,” priapism (Table 1). While nonischemic priapism is typically caused by penile or perineal trauma, ischemic priapism can occur as a result of medications, including antipsychotics, antidepressants, anxiolytics, and antihypertensives, or hematological conditions such as sickle cell disease.1 Other risk factors associated with priapism include substance abuse, hyperprolactinemia, diabetes, and liver disease.4
Antipsychotic-induced priapism
Medication-induced priapism is a rare adverse drug reaction (ADR). Of the medication classes associated with priapism, antipsychotics have the highest incidence and account for approximately 20% of all cases.1
The mechanism of priapism associated with antipsychotics is thought to be related to alpha-1 blockade in the corpora cavernosa of the penis. Although antipsychotics within each class share common characteristics, each agent has a unique profile of receptor affinities. As such, antipsychotics have varying affinities for the alpha-adrenergic receptor (Table 2). Agents such as ziprasidone, chlorpromazine, and risperidone—which have the highest affinity for the alpha-1 adrenoceptors—may be more likely to cause priapism compared with agents with lower affinity, such as olanzapine. Priapism may occur at any time during antipsychotic treatment, and does not appear to be dose-related.
Continue to: Antipsychotic drug interactions and priapism...
Antipsychotic drug interactions and priapism
Patients who are receiving multiple medications as treatment for chronic medical or psychiatric conditions have an increased likelihood of experiencing drug-drug interactions (DDIs) that lead to adverse effects.
Various case reports have described priapism as a result of DDIs related to antipsychotic agents combined with other psychotropic or nonpsychotropic medications.3 Most of these DDIs have been attributed to the cytochrome P450 (CYP) family of enzymes, including CYP2D6, CYP1A2, and CYP3A4/5, which are major enzymes implicated in the metabolism of antipsychotics (Table 3).
It is imperative to be vigilant during the concomitant administration of antipsychotics with other medications that may be substrates, inducers, or inhibitors of CYP enzymes, as this could alter the metabolism and kinetics of the antipsychotic and result in ADRs such as priapism. For example, drug interactions exist between strong CYP2D6 inhibitors—such as the antidepressants paroxetine, fluoxetine, and bupropion—and antipsychotics that are substrates of CYP2D6, such as risperidone, aripiprazole, haloperidol, and perphenazine. This interaction can lead to higher levels of the antipsychotic, which would increase the patient’s risk of experiencing ADRs. Because psychotic illnesses and depression/anxiety often coexist, it is not uncommon for individuals with these conditions to be receiving both an antipsychotic and an antidepressant.
Because there is a high incidence of comorbidities such as HIV and cardiovascular disease among individuals with mental illnesses, clinicians must also be cognizant of any nonpsychotropic medications the patient may be taking. For instance, clinically relevant DDIs exist between protease inhibitors, such as ritonavir, a strong CYP3A4 inhibitor, and antipsychotics that are substrates of CYP3A4, such as pimozide, aripiprazole, and quetiapine.
Mitigating the risk of priapism
Although there are associated risk factors for priapism, there are no concrete indicators to predict the onset or development of the condition. The best predictor may be a history of prolonged and painless erections.3
As such, when choosing an antipsychotic, it is critical to screen the patient for the previously mentioned risk factors, including the presence of medications with strong alpha-1 receptor affinity and CYP interactions, especially to minimize the risk of recurrence of priapism in those with prior or similar episodes. Management of patients with priapism due to antipsychotics has involved reducing the dose of the offending agent and/or changing the medication to one with a lower alpha-adrenergic affinity (Table 22).
Similar to most situations, management is patient-specific and depends on several factors, including the severity of the patient’s psychiatric disease, history/severity of priapism and treatment, concurrent medication list, etc. For example, although clozapine is considered to have relatively high affinity for the alpha-1 receptor, it is also the agent of choice for treatment-refractory schizophrenia. Risks and benefits must be weighed on a individualized basis. Case reports have described symptom improvement via lowering the dose of clozapine and adding on or switching to an antipsychotic agent with minimal alpha-1 receptor affinity.4
After considering Mr. J’s history, risk factors, and preferences, the treatment team discontinues risperidone and initiates haloperidol, 5 mg twice a day. Soon after, Mr. J no longer experiences priapism.
Mr. J, age 35, is brought to the hospital from prison due to priapism that does not improve with treatment. He says he has had priapism 5 times previously, with the first incidence occurring “years ago” due to trazodone.
Recently, he has been receiving risperidone, which the treatment team believes is the cause of his current priapism. His medical history includes asthma, schizophrenia, hypertension, seizures, and sickle cell trait. Mr. J is experiencing auditory hallucinations, which he describes as “continuous, neutral voices that are annoying.” He would like relief from his auditory hallucinations and is willing to change his antipsychotic, but does not want additional treatment for his priapism. His present medications include risperidone, 1 mg twice a day, escitalopram, 10 mg/d, benztropine, 1 mg twice a day, and phenytoin, 500 mg/d at bedtime.
Priapism is a prolonged, persistent, and often painful erection that occurs without sexual stimulation. Although relatively rare, it can result in potentially serious long-term complications, including impotence and gangrene, and requires immediate evaluation and management.
There are 2 types of priapism: nonischemic, or “high-flow,” priapism, and ischemic, or “low-flow,” priapism (Table 1). While nonischemic priapism is typically caused by penile or perineal trauma, ischemic priapism can occur as a result of medications, including antipsychotics, antidepressants, anxiolytics, and antihypertensives, or hematological conditions such as sickle cell disease.1 Other risk factors associated with priapism include substance abuse, hyperprolactinemia, diabetes, and liver disease.4
Antipsychotic-induced priapism
Medication-induced priapism is a rare adverse drug reaction (ADR). Of the medication classes associated with priapism, antipsychotics have the highest incidence and account for approximately 20% of all cases.1
The mechanism of priapism associated with antipsychotics is thought to be related to alpha-1 blockade in the corpora cavernosa of the penis. Although antipsychotics within each class share common characteristics, each agent has a unique profile of receptor affinities. As such, antipsychotics have varying affinities for the alpha-adrenergic receptor (Table 2). Agents such as ziprasidone, chlorpromazine, and risperidone—which have the highest affinity for the alpha-1 adrenoceptors—may be more likely to cause priapism compared with agents with lower affinity, such as olanzapine. Priapism may occur at any time during antipsychotic treatment, and does not appear to be dose-related.
Continue to: Antipsychotic drug interactions and priapism...
Antipsychotic drug interactions and priapism
Patients who are receiving multiple medications as treatment for chronic medical or psychiatric conditions have an increased likelihood of experiencing drug-drug interactions (DDIs) that lead to adverse effects.
Various case reports have described priapism as a result of DDIs related to antipsychotic agents combined with other psychotropic or nonpsychotropic medications.3 Most of these DDIs have been attributed to the cytochrome P450 (CYP) family of enzymes, including CYP2D6, CYP1A2, and CYP3A4/5, which are major enzymes implicated in the metabolism of antipsychotics (Table 3).
It is imperative to be vigilant during the concomitant administration of antipsychotics with other medications that may be substrates, inducers, or inhibitors of CYP enzymes, as this could alter the metabolism and kinetics of the antipsychotic and result in ADRs such as priapism. For example, drug interactions exist between strong CYP2D6 inhibitors—such as the antidepressants paroxetine, fluoxetine, and bupropion—and antipsychotics that are substrates of CYP2D6, such as risperidone, aripiprazole, haloperidol, and perphenazine. This interaction can lead to higher levels of the antipsychotic, which would increase the patient’s risk of experiencing ADRs. Because psychotic illnesses and depression/anxiety often coexist, it is not uncommon for individuals with these conditions to be receiving both an antipsychotic and an antidepressant.
Because there is a high incidence of comorbidities such as HIV and cardiovascular disease among individuals with mental illnesses, clinicians must also be cognizant of any nonpsychotropic medications the patient may be taking. For instance, clinically relevant DDIs exist between protease inhibitors, such as ritonavir, a strong CYP3A4 inhibitor, and antipsychotics that are substrates of CYP3A4, such as pimozide, aripiprazole, and quetiapine.
Mitigating the risk of priapism
Although there are associated risk factors for priapism, there are no concrete indicators to predict the onset or development of the condition. The best predictor may be a history of prolonged and painless erections.3
As such, when choosing an antipsychotic, it is critical to screen the patient for the previously mentioned risk factors, including the presence of medications with strong alpha-1 receptor affinity and CYP interactions, especially to minimize the risk of recurrence of priapism in those with prior or similar episodes. Management of patients with priapism due to antipsychotics has involved reducing the dose of the offending agent and/or changing the medication to one with a lower alpha-adrenergic affinity (Table 22).
Similar to most situations, management is patient-specific and depends on several factors, including the severity of the patient’s psychiatric disease, history/severity of priapism and treatment, concurrent medication list, etc. For example, although clozapine is considered to have relatively high affinity for the alpha-1 receptor, it is also the agent of choice for treatment-refractory schizophrenia. Risks and benefits must be weighed on a individualized basis. Case reports have described symptom improvement via lowering the dose of clozapine and adding on or switching to an antipsychotic agent with minimal alpha-1 receptor affinity.4
After considering Mr. J’s history, risk factors, and preferences, the treatment team discontinues risperidone and initiates haloperidol, 5 mg twice a day. Soon after, Mr. J no longer experiences priapism.
1. Weiner DM, Lowe FC. Psychotropic drug-induced priapism. Mol Diag Ther 9. 1998;371-379. doi:10.2165/00023210-199809050-00004
2. Andersohn F, Schmedt N, Weinmann S, et al. Priapism associated with antipsychotics: role of alpha1 adrenoceptor affinity. J Clin Psychopharmacol. 2010;30(1):68-71. doi:10.1097/JCP.0b013e3181c8273d
3. Sood S, James W, Bailon MJ. Priapism associated with atypical antipsychotic medications: a review. Int Clin Psychopharmacol. 2008;23(1):9-17.
4. Sinkeviciute I, Kroken RA, Johnsen E. Priapism in antipsychotic drug use: a rare but important side effect. Case Rep Psychiatry. 2012;2012:496364. doi:10.1155/2012/496364
5. Mora F, Martín JDD, Zubillaga E, et al. CYP450 and its implications in the clinical use of antipsychotic drugs. Clin Exp Pharmacol. 2015;5(176):1-10. doi:10.4172/2161-1459.1000176
6. Puangpetch A, Vanwong N, Nuntamool N, et al. CYP2D6 polymorphisms and their influence on risperidone treatment. Pharmgenomics Pers Med. 2016;9:131-147. doi:10.2147/PGPM.S107772
1. Weiner DM, Lowe FC. Psychotropic drug-induced priapism. Mol Diag Ther 9. 1998;371-379. doi:10.2165/00023210-199809050-00004
2. Andersohn F, Schmedt N, Weinmann S, et al. Priapism associated with antipsychotics: role of alpha1 adrenoceptor affinity. J Clin Psychopharmacol. 2010;30(1):68-71. doi:10.1097/JCP.0b013e3181c8273d
3. Sood S, James W, Bailon MJ. Priapism associated with atypical antipsychotic medications: a review. Int Clin Psychopharmacol. 2008;23(1):9-17.
4. Sinkeviciute I, Kroken RA, Johnsen E. Priapism in antipsychotic drug use: a rare but important side effect. Case Rep Psychiatry. 2012;2012:496364. doi:10.1155/2012/496364
5. Mora F, Martín JDD, Zubillaga E, et al. CYP450 and its implications in the clinical use of antipsychotic drugs. Clin Exp Pharmacol. 2015;5(176):1-10. doi:10.4172/2161-1459.1000176
6. Puangpetch A, Vanwong N, Nuntamool N, et al. CYP2D6 polymorphisms and their influence on risperidone treatment. Pharmgenomics Pers Med. 2016;9:131-147. doi:10.2147/PGPM.S107772
Using measurement-based care to improve outcomes for patients with depression
Ms. H, age 42, is being treated by her family physician for her second episode of major depressive disorder (MDD). When she was 35, Ms. H experienced her first episode of MDD, which was successfully treated with
At the 8-week follow-up appointment, the physician notes how much better Ms. H seems to be doing. He says that because she has had such a good response, she should continue the fluoxetine and come back in 3 months. Later that evening, Ms. H reflects on her visit. Although she feels better, she still does not feel normal. In fact, she is not sure that she has really felt normal since before her first depressive episode. Ms. H decides to see a psychiatrist.
At her first appointment, the psychiatrist asks Ms. H to complete the Quick Inventory of Depressive Symptoms–Self Rated (QIDS-SR) scale. Her QIDS-SR score is 6, which is consistent with mild residual symptoms of depression.1 The psychiatrist increases the fluoxetine dosage to 40 mg/d and recommends that she complete a course of cognitive-behavioral therapy (CBT).
Although psychiatry currently does not have tests that provide continuous data such as blood pressure or HbA1c, well-validated rating scales can help clinicians in getting their patients to achieve symptom remission. Measurement-based care is the “systematic use of measurement tools to monitor progress and guide treatment choices.”1 Originally, psychometric rating scales were designed for research; typically, they were administered by the clinician, and were too long to be used in routine outpatient clinical practice. Subsequently, it was determined that patients without psychotic symptoms or cognitive deficits can accurately assess their own symptoms, and this led to the development of short self-assessment scales that have a high level of reliability when compared with longer, clinician-administered instruments. Despite the availability of several validated, brief rating scales, it is estimated that only approximately 18% of psychiatrists use them in clinical practice.2
Self-rated scales for depression have been shown to be as valid as clinician-rated scales. For depression, the Patient Health Questionaire-9 (PHQ-9), based on the 9 symptom criteria associated with a diagnosis of MDD, is likely the most commonly used self-assessment scale.1 However, the QIDS-SR and the Beck Depression Inventory are both well-validated.1 In particular, QIDS-SR scores and score changes have been shown to be comparable with those on the QIDS-Clinician Rating (QIDS-C) scale.3 A 50% decrease in score typically is defined as a clinical response. Remission of symptoms is often defined as a score ≤4 on the PHQ-9 or ≤5 on the QIDS-SR (Table1). Similar to laboratory tests, rating scales are not diagnostic, but are a piece of information for the clinician to use in making diagnostic and treatment decisions.
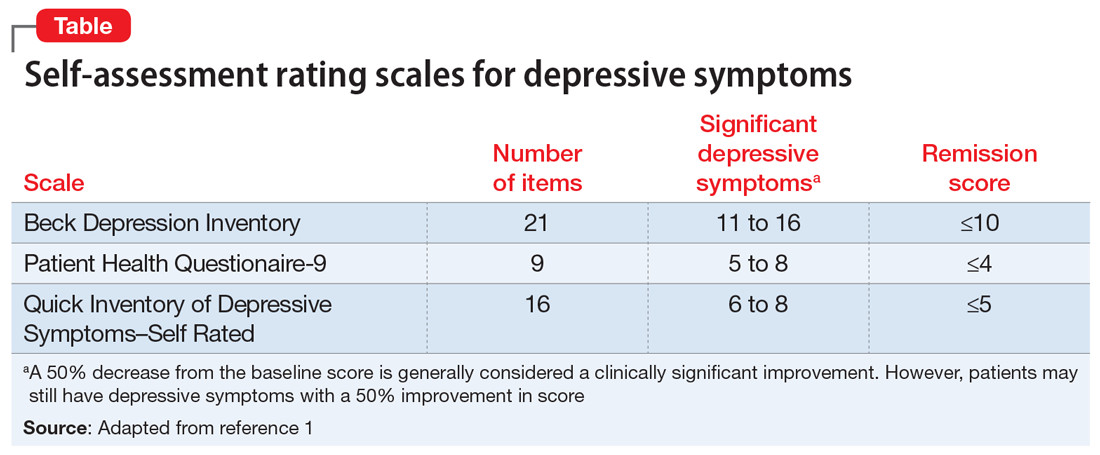
The use of brief rating scales can help identify symptoms that may not come up in discussion with the patient, and it provides a systematic method of reviewing symptoms. Patients may be encouraged when they see a decrease in their scores after beginning treatment.2 Patients with depression need to complete rating scales frequently, just as a patient with hypertension would need their blood pressure frequently monitored.2 Frequent measurement with rating scales may help identify residual depressive symptoms that indicate the need for additional intervention. Residual depressive symptoms are the best predictor of the recurrence of depression, and treatment to remission is essential in preventing recurrence. In fact, recurrence is 2 to 3 times more likely in patients who do not achieve remission.1
Continue to: Optimizing the use of self-rating scales...
Optimizing the use of self-rating scales
To save time, patients can complete a rating scale before seeing the clinician, and the use of computerized applications can automatically sum scores and plot response graphs.4 Some researchers have suggested that some patients may be more honest in completing a self-assessment than in their verbal responses to the clinician.4 It is important to discuss the rating scale results with the patient.2 With a newly diagnosed patient, goals for treatment and the treatment plan can be outlined. During follow-up visits, clinicians should note areas of improvement and provide encouragement. If the patient’s symptoms are not improving appropriately, the clinician should discuss treatment options and offer the patient hope. This may improve the patient’s engagement in care and their understanding of how symptoms are associated with their illness.2 Studies have suggested that the use of validated rating tools (along with other interventions) can result in faster improvement in symptoms and higher response rates, and can assist in achieving remission.1,2,5
CASE CONTINUED
After 6 weeks of CBT and the increased fluoxetine dose, Ms. H returns to her psychiatrist for a follow-up visit. Her QIDS-SR score is 4, which is down from her initial score of 6. Ms. H is elated when she sees that her symptoms score has decreased since the previous visit. To confirm this finding, the psychiatrist completes the QIDS-C, and records a score of 3. The psychiatrist discusses the appropriate continuation of fluoxetine and CBT.
In this case, the use of a brief clinical rating scale helped Ms. H’s psychiatrist identify residual depressive symptoms and modify treatment so that she achieved remission. Using patient-reported outcomes also helps facilitate meaningful conversations between the patient and clinician and helps identify symptoms suggestive of relapse.2 Although this case focused on the use of measurement-based care in depression, brief symptom rating scales for most major psychiatric disorders—many of them self-assessments—also are available, as are brief rating scales to assess medication adverse effects and adherence.5
Just as clinicians in other areas of medicine use assessments such as laboratory tests and blood pressure monitoring for initial assessment and in following response to treatment, measurement-based care allows for a quasi-objective evaluation of patients with psychiatric disorders. Improved response rates, time to response, and patient engagement are all positive results of measurement-based care
Related Resources
- Martin-Cook K, Palmer L, Thornton L, et al. Setting measurement-based care in motion: practical lessons in the implementation and integration of measurement-based care in psychiatry clinical practice. Neuropsychiatric Disease & Treatment. 2021;17:1621-1631.
- Aboraya A, Nasrallah HA, Elswick DE, et al. Measurementbased care in psychiatry-past, present, and future. Innov Clin Neurosci. 2018;15(11-12):13-26.
Drug Brand Names
Fluoxetine • Prozac
- Self-rated scales are believed to be as reliable as clinician-rated scales in assessing symptoms in patients who are not cognitively impaired.
- The use of rating scales can enhance engagement of the patient with the clinician.
- Utilizing computer- or smartphone appbased rating scales allows for automatic scoring and graphing.
- The use of rating scales in the pharmacotherapy of depression has been associated with more rapid symptoms improvement, greater response rates, and a greater likelihood of achieving remission.
- Trivedi MH. Tools and strategies for ongoing assessment of depression: a measurement-based approach to remission. J Clin Psychiatry 2009;70(suppl 6):26-31. doi:10.4088/ JCP.8133su1c.04
- Lewis CC, Boyd M, Puspitasari A, et al. Implementing measurement-based care in behavioral health: a review. JAMA Psychiatry. 2019;76(3):324-335.
- Trivedi MH, Rush AJ, Ibrahim HM, et al. The Inventory of Depressive Symptomatology, Clinician Rating (IDS-C) and Self-Report (IDS-SR), and the Quick Inventory of Depressive Symptomatology, Clinician Rating (QIDS-C) and Self-Report (QIDS-SR) in public sector patients with mood disorders: a psychometric evaluation. Psychol Med. 2004;34(1):73-82.
- Trivedi MH, Papakostas GI, Jackson WC, et al. Implementing measurement-based care to determine and treat inadequate response. J Clin Psychiatry 2020;81(3):OT19037BR1. doi: 10.4088/JCP.OT19037BR1
- Morris DW, Trivedi MH. Measurement-based care for unipolar depression. Curr Psychiatry Rep. 2011;13(6):446-458.
Ms. H, age 42, is being treated by her family physician for her second episode of major depressive disorder (MDD). When she was 35, Ms. H experienced her first episode of MDD, which was successfully treated with
At the 8-week follow-up appointment, the physician notes how much better Ms. H seems to be doing. He says that because she has had such a good response, she should continue the fluoxetine and come back in 3 months. Later that evening, Ms. H reflects on her visit. Although she feels better, she still does not feel normal. In fact, she is not sure that she has really felt normal since before her first depressive episode. Ms. H decides to see a psychiatrist.
At her first appointment, the psychiatrist asks Ms. H to complete the Quick Inventory of Depressive Symptoms–Self Rated (QIDS-SR) scale. Her QIDS-SR score is 6, which is consistent with mild residual symptoms of depression.1 The psychiatrist increases the fluoxetine dosage to 40 mg/d and recommends that she complete a course of cognitive-behavioral therapy (CBT).
Although psychiatry currently does not have tests that provide continuous data such as blood pressure or HbA1c, well-validated rating scales can help clinicians in getting their patients to achieve symptom remission. Measurement-based care is the “systematic use of measurement tools to monitor progress and guide treatment choices.”1 Originally, psychometric rating scales were designed for research; typically, they were administered by the clinician, and were too long to be used in routine outpatient clinical practice. Subsequently, it was determined that patients without psychotic symptoms or cognitive deficits can accurately assess their own symptoms, and this led to the development of short self-assessment scales that have a high level of reliability when compared with longer, clinician-administered instruments. Despite the availability of several validated, brief rating scales, it is estimated that only approximately 18% of psychiatrists use them in clinical practice.2
Self-rated scales for depression have been shown to be as valid as clinician-rated scales. For depression, the Patient Health Questionaire-9 (PHQ-9), based on the 9 symptom criteria associated with a diagnosis of MDD, is likely the most commonly used self-assessment scale.1 However, the QIDS-SR and the Beck Depression Inventory are both well-validated.1 In particular, QIDS-SR scores and score changes have been shown to be comparable with those on the QIDS-Clinician Rating (QIDS-C) scale.3 A 50% decrease in score typically is defined as a clinical response. Remission of symptoms is often defined as a score ≤4 on the PHQ-9 or ≤5 on the QIDS-SR (Table1). Similar to laboratory tests, rating scales are not diagnostic, but are a piece of information for the clinician to use in making diagnostic and treatment decisions.

The use of brief rating scales can help identify symptoms that may not come up in discussion with the patient, and it provides a systematic method of reviewing symptoms. Patients may be encouraged when they see a decrease in their scores after beginning treatment.2 Patients with depression need to complete rating scales frequently, just as a patient with hypertension would need their blood pressure frequently monitored.2 Frequent measurement with rating scales may help identify residual depressive symptoms that indicate the need for additional intervention. Residual depressive symptoms are the best predictor of the recurrence of depression, and treatment to remission is essential in preventing recurrence. In fact, recurrence is 2 to 3 times more likely in patients who do not achieve remission.1
Continue to: Optimizing the use of self-rating scales...
Optimizing the use of self-rating scales
To save time, patients can complete a rating scale before seeing the clinician, and the use of computerized applications can automatically sum scores and plot response graphs.4 Some researchers have suggested that some patients may be more honest in completing a self-assessment than in their verbal responses to the clinician.4 It is important to discuss the rating scale results with the patient.2 With a newly diagnosed patient, goals for treatment and the treatment plan can be outlined. During follow-up visits, clinicians should note areas of improvement and provide encouragement. If the patient’s symptoms are not improving appropriately, the clinician should discuss treatment options and offer the patient hope. This may improve the patient’s engagement in care and their understanding of how symptoms are associated with their illness.2 Studies have suggested that the use of validated rating tools (along with other interventions) can result in faster improvement in symptoms and higher response rates, and can assist in achieving remission.1,2,5
CASE CONTINUED
After 6 weeks of CBT and the increased fluoxetine dose, Ms. H returns to her psychiatrist for a follow-up visit. Her QIDS-SR score is 4, which is down from her initial score of 6. Ms. H is elated when she sees that her symptoms score has decreased since the previous visit. To confirm this finding, the psychiatrist completes the QIDS-C, and records a score of 3. The psychiatrist discusses the appropriate continuation of fluoxetine and CBT.
In this case, the use of a brief clinical rating scale helped Ms. H’s psychiatrist identify residual depressive symptoms and modify treatment so that she achieved remission. Using patient-reported outcomes also helps facilitate meaningful conversations between the patient and clinician and helps identify symptoms suggestive of relapse.2 Although this case focused on the use of measurement-based care in depression, brief symptom rating scales for most major psychiatric disorders—many of them self-assessments—also are available, as are brief rating scales to assess medication adverse effects and adherence.5
Just as clinicians in other areas of medicine use assessments such as laboratory tests and blood pressure monitoring for initial assessment and in following response to treatment, measurement-based care allows for a quasi-objective evaluation of patients with psychiatric disorders. Improved response rates, time to response, and patient engagement are all positive results of measurement-based care
Related Resources
- Martin-Cook K, Palmer L, Thornton L, et al. Setting measurement-based care in motion: practical lessons in the implementation and integration of measurement-based care in psychiatry clinical practice. Neuropsychiatric Disease & Treatment. 2021;17:1621-1631.
- Aboraya A, Nasrallah HA, Elswick DE, et al. Measurementbased care in psychiatry-past, present, and future. Innov Clin Neurosci. 2018;15(11-12):13-26.
Drug Brand Names
Fluoxetine • Prozac
- Self-rated scales are believed to be as reliable as clinician-rated scales in assessing symptoms in patients who are not cognitively impaired.
- The use of rating scales can enhance engagement of the patient with the clinician.
- Utilizing computer- or smartphone appbased rating scales allows for automatic scoring and graphing.
- The use of rating scales in the pharmacotherapy of depression has been associated with more rapid symptoms improvement, greater response rates, and a greater likelihood of achieving remission.
Ms. H, age 42, is being treated by her family physician for her second episode of major depressive disorder (MDD). When she was 35, Ms. H experienced her first episode of MDD, which was successfully treated with
At the 8-week follow-up appointment, the physician notes how much better Ms. H seems to be doing. He says that because she has had such a good response, she should continue the fluoxetine and come back in 3 months. Later that evening, Ms. H reflects on her visit. Although she feels better, she still does not feel normal. In fact, she is not sure that she has really felt normal since before her first depressive episode. Ms. H decides to see a psychiatrist.
At her first appointment, the psychiatrist asks Ms. H to complete the Quick Inventory of Depressive Symptoms–Self Rated (QIDS-SR) scale. Her QIDS-SR score is 6, which is consistent with mild residual symptoms of depression.1 The psychiatrist increases the fluoxetine dosage to 40 mg/d and recommends that she complete a course of cognitive-behavioral therapy (CBT).
Although psychiatry currently does not have tests that provide continuous data such as blood pressure or HbA1c, well-validated rating scales can help clinicians in getting their patients to achieve symptom remission. Measurement-based care is the “systematic use of measurement tools to monitor progress and guide treatment choices.”1 Originally, psychometric rating scales were designed for research; typically, they were administered by the clinician, and were too long to be used in routine outpatient clinical practice. Subsequently, it was determined that patients without psychotic symptoms or cognitive deficits can accurately assess their own symptoms, and this led to the development of short self-assessment scales that have a high level of reliability when compared with longer, clinician-administered instruments. Despite the availability of several validated, brief rating scales, it is estimated that only approximately 18% of psychiatrists use them in clinical practice.2
Self-rated scales for depression have been shown to be as valid as clinician-rated scales. For depression, the Patient Health Questionaire-9 (PHQ-9), based on the 9 symptom criteria associated with a diagnosis of MDD, is likely the most commonly used self-assessment scale.1 However, the QIDS-SR and the Beck Depression Inventory are both well-validated.1 In particular, QIDS-SR scores and score changes have been shown to be comparable with those on the QIDS-Clinician Rating (QIDS-C) scale.3 A 50% decrease in score typically is defined as a clinical response. Remission of symptoms is often defined as a score ≤4 on the PHQ-9 or ≤5 on the QIDS-SR (Table1). Similar to laboratory tests, rating scales are not diagnostic, but are a piece of information for the clinician to use in making diagnostic and treatment decisions.

The use of brief rating scales can help identify symptoms that may not come up in discussion with the patient, and it provides a systematic method of reviewing symptoms. Patients may be encouraged when they see a decrease in their scores after beginning treatment.2 Patients with depression need to complete rating scales frequently, just as a patient with hypertension would need their blood pressure frequently monitored.2 Frequent measurement with rating scales may help identify residual depressive symptoms that indicate the need for additional intervention. Residual depressive symptoms are the best predictor of the recurrence of depression, and treatment to remission is essential in preventing recurrence. In fact, recurrence is 2 to 3 times more likely in patients who do not achieve remission.1
Continue to: Optimizing the use of self-rating scales...
Optimizing the use of self-rating scales
To save time, patients can complete a rating scale before seeing the clinician, and the use of computerized applications can automatically sum scores and plot response graphs.4 Some researchers have suggested that some patients may be more honest in completing a self-assessment than in their verbal responses to the clinician.4 It is important to discuss the rating scale results with the patient.2 With a newly diagnosed patient, goals for treatment and the treatment plan can be outlined. During follow-up visits, clinicians should note areas of improvement and provide encouragement. If the patient’s symptoms are not improving appropriately, the clinician should discuss treatment options and offer the patient hope. This may improve the patient’s engagement in care and their understanding of how symptoms are associated with their illness.2 Studies have suggested that the use of validated rating tools (along with other interventions) can result in faster improvement in symptoms and higher response rates, and can assist in achieving remission.1,2,5
CASE CONTINUED
After 6 weeks of CBT and the increased fluoxetine dose, Ms. H returns to her psychiatrist for a follow-up visit. Her QIDS-SR score is 4, which is down from her initial score of 6. Ms. H is elated when she sees that her symptoms score has decreased since the previous visit. To confirm this finding, the psychiatrist completes the QIDS-C, and records a score of 3. The psychiatrist discusses the appropriate continuation of fluoxetine and CBT.
In this case, the use of a brief clinical rating scale helped Ms. H’s psychiatrist identify residual depressive symptoms and modify treatment so that she achieved remission. Using patient-reported outcomes also helps facilitate meaningful conversations between the patient and clinician and helps identify symptoms suggestive of relapse.2 Although this case focused on the use of measurement-based care in depression, brief symptom rating scales for most major psychiatric disorders—many of them self-assessments—also are available, as are brief rating scales to assess medication adverse effects and adherence.5
Just as clinicians in other areas of medicine use assessments such as laboratory tests and blood pressure monitoring for initial assessment and in following response to treatment, measurement-based care allows for a quasi-objective evaluation of patients with psychiatric disorders. Improved response rates, time to response, and patient engagement are all positive results of measurement-based care
Related Resources
- Martin-Cook K, Palmer L, Thornton L, et al. Setting measurement-based care in motion: practical lessons in the implementation and integration of measurement-based care in psychiatry clinical practice. Neuropsychiatric Disease & Treatment. 2021;17:1621-1631.
- Aboraya A, Nasrallah HA, Elswick DE, et al. Measurementbased care in psychiatry-past, present, and future. Innov Clin Neurosci. 2018;15(11-12):13-26.
Drug Brand Names
Fluoxetine • Prozac
- Self-rated scales are believed to be as reliable as clinician-rated scales in assessing symptoms in patients who are not cognitively impaired.
- The use of rating scales can enhance engagement of the patient with the clinician.
- Utilizing computer- or smartphone appbased rating scales allows for automatic scoring and graphing.
- The use of rating scales in the pharmacotherapy of depression has been associated with more rapid symptoms improvement, greater response rates, and a greater likelihood of achieving remission.
- Trivedi MH. Tools and strategies for ongoing assessment of depression: a measurement-based approach to remission. J Clin Psychiatry 2009;70(suppl 6):26-31. doi:10.4088/ JCP.8133su1c.04
- Lewis CC, Boyd M, Puspitasari A, et al. Implementing measurement-based care in behavioral health: a review. JAMA Psychiatry. 2019;76(3):324-335.
- Trivedi MH, Rush AJ, Ibrahim HM, et al. The Inventory of Depressive Symptomatology, Clinician Rating (IDS-C) and Self-Report (IDS-SR), and the Quick Inventory of Depressive Symptomatology, Clinician Rating (QIDS-C) and Self-Report (QIDS-SR) in public sector patients with mood disorders: a psychometric evaluation. Psychol Med. 2004;34(1):73-82.
- Trivedi MH, Papakostas GI, Jackson WC, et al. Implementing measurement-based care to determine and treat inadequate response. J Clin Psychiatry 2020;81(3):OT19037BR1. doi: 10.4088/JCP.OT19037BR1
- Morris DW, Trivedi MH. Measurement-based care for unipolar depression. Curr Psychiatry Rep. 2011;13(6):446-458.
- Trivedi MH. Tools and strategies for ongoing assessment of depression: a measurement-based approach to remission. J Clin Psychiatry 2009;70(suppl 6):26-31. doi:10.4088/ JCP.8133su1c.04
- Lewis CC, Boyd M, Puspitasari A, et al. Implementing measurement-based care in behavioral health: a review. JAMA Psychiatry. 2019;76(3):324-335.
- Trivedi MH, Rush AJ, Ibrahim HM, et al. The Inventory of Depressive Symptomatology, Clinician Rating (IDS-C) and Self-Report (IDS-SR), and the Quick Inventory of Depressive Symptomatology, Clinician Rating (QIDS-C) and Self-Report (QIDS-SR) in public sector patients with mood disorders: a psychometric evaluation. Psychol Med. 2004;34(1):73-82.
- Trivedi MH, Papakostas GI, Jackson WC, et al. Implementing measurement-based care to determine and treat inadequate response. J Clin Psychiatry 2020;81(3):OT19037BR1. doi: 10.4088/JCP.OT19037BR1
- Morris DW, Trivedi MH. Measurement-based care for unipolar depression. Curr Psychiatry Rep. 2011;13(6):446-458.
Calcineurin inhibitor–induced psychosis
Mrs. C, age 68, is experiencing worsening paranoid delusions. She believes that because she lied about her income when she was younger, the FBI is tracking her and wants to poison her food and spray dangerous fumes in her house. Her paranoia has made it hard for her to sleep, eat, or feel safe in her home.
Mrs. C’s daughter reports that her mother’s delusions started 3 years ago and have worsened in recent months. Mrs. C has no psychiatric history. Her medical history is significant for renal transplant in 2015, type 2 diabetes, hyperlipidemia, hypertension, and hypothyroidism. She is currently normotensive. Mrs. C’s home medications include cyclosporine, which is a calcineurin inhibitor, 125 mg twice daily (trough level = 138 mcg/L); mycophenolate mofetil, 500 mg twice daily; cinacalcet, 30 mg 3 times a week; metformin, 500 mg twice daily; amlodipine, 5 mg twice daily; levothyroxine, 112 mcg/d; and atorvastatin, 40 mg at bedtime.
As you review her medications, you wonder if the cyclosporine she began receiving after her kidney transplant could be causing or contributing to her worsening paranoid delusions.
Kidney transplantation has become an ideal treatment for patients with end-stage renal disease. In 2020, 22,817 kidney transplants were performed in the United States.1 Compared with dialysis, kidney transplant allows patients the chance to return to a satisfactory quality of life.2 However, to ensure a successful and long-lasting transplant, patients must be started and maintained on lifelong immunosuppressant agents that have potential adverse effects, including nephrotoxicity and hypertension. Further, immunosuppressant agents—particularly calcineurin inhibitors—are associated with potential adverse effects on mental health. The most commonly documented mental health-related adverse effects include insomnia, anxiety, depression, and confusion.3 In this article, we discuss the risk of severe psychosis associated with the use of calcineurin inhibitors.
Calcineurin inhibitors and psychiatric symptoms
Cyclosporine and tacrolimus are calcineurin inhibitors that are commonly used as immunosuppressant agents after kidney transplantation. They primarily work by specifically and competitively binding to and inhibiting the calcineurin protein to reduce the transcriptional activation of cytokine genes for interleukin-2, tumor necrosis factor-alpha, interleukin-3, interleukin-4, CD40L (CD40 ligand), granulocyte-macrophage colony-stimulating factor, and interferon-gamma.4,5 The ultimate downstream effect is reduced proliferation of T lymphocytes and thereby an immunosuppressed state that will protect against organ rejection. However, this is not the only downstream effect that can occur from inhibiting calcineurin. Cyclosporine and tacrolimus may modulate the activity of dopamine and N-methyl-
An increased effect of dopamine in the mesocortical dopaminergic pathway has long been a suspected cause for psychotic symptoms. A study conducted in rodents suggested that tacrolimus selectively modifies the responsivity and sensitivity of postsynaptic dopamine-2 (D2) and dopamine-3 (D3) receptors.9 These receptors are important when discussing psychosis because antipsychotic medications work primarily by blocking dopamine D2, while many also block the D3 receptor. We hypothesize that modifying the responsivity and sensitivity of these 2 receptors could increase the risk of a person developing psychosis. It may also provide insight into how to best treat a psychotic episode.
Tacrolimus has been shown to elicit inhibition of NMDA-induced neurotransmitter release and augmentation of depolarization-induced neurotransmitter release.10 In theory, this potential inhibition at the NMDA receptors may lead to a compensatory and excessive release of glutamate. Elevated glutamate levels in the brain could lead to psychiatric symptoms, including psychosis. This is supported by the psychosis caused by many NMDA receptor antagonists, such as phencyclidine (PCP) and ketamine. Furthermore, a study examining calcineurin in knockout mice showed that the spectrum of behavioral abnormalities was strikingly similar to those in schizophrenia models.11 These mice displayed impaired working memory, impaired attentional function, social withdrawal, and psychomotor agitation. This further supports the idea that calcineurin inhibition can play a significant role in causing psychiatric symptoms by affecting both dopamine and NMDA receptors.
Continue to: How to address calcineurin inhibitor–induced psychosis...
How to address calcineurin inhibitor–induced psychosis
Here we outline a potential treatment strategy to combat psychosis secondary to calcineurin inhibitors. First, evaluate the patient’s calcineurin inhibitor level (either cyclosporine or tacrolimus). Levels should be drawn as a true trough and doses adjusted if necessary via appropriate consultation with a transplant specialist. Because many of the adverse effects associated with these agents are dose-dependent, we suspect that the risk of psychosis and other mental health–related adverse effects may also follow this trend.
Assuming that the calcineurin inhibitor cannot be stopped, changed to a different agent, or subject to a dose decrease, we recommend initiating an antipsychotic medication to control psychotic symptoms. Given the potential effect of calcineurin inhibitors on dopamine, we suggest trialing a second-generation antipsychotic with relatively high affinity for dopamine D2 receptors, such as risperidone or paliperidone. However, compared with patients with schizophrenia, patients receiving a calcineurin inhibitor may be more likely to develop extrapyramidal symptoms (EPS). Therefore, to avoid potential adverse effects, consider using a lower starting dose or an antipsychotic medication with less dopamine D2 affinity, such as quetiapine, olanzapine, or aripiprazole. Furthermore, because post-transplant patients may be at a higher risk for depression, which may be secondary to medication adverse effects, we suggest avoiding first-generation antipsychotics (FGAs) such as haloperidol because FGAs may worsen depressive symptoms.
We recommend initiating risperidone, 1 mg twice a day, for patients with psychosis secondary to a calcineurin inhibitor. If the patient develops EPS, consider switching to an antipsychotic medication with a less potent dopamine D2 blockade, such as quetiapine, olanzapine, or aripiprazole. We recommend an antipsychotic switch rather than adding benztropine or diphenhydramine to the regimen because many transplant recipients may be older patients, and adding anticholinergic medications can be problematic for this population. However, if the patient is younger or has responded particularly well to risperidone, the benefit of adding an anticholinergic medication may outweigh the risks. This decision should be made on an individual basis and may include other options, such as a switch to quetiapine, olanzapine, or aripiprazole. While these agents may not block the D2 receptor as strongly as risperidone, they all are effective and approved for adjunct therapy in major depressive disorder. We recommend quetiapine and olanzapine as second-line agents because of their potential for sedation and significant weight gain. While aripiprazole has a great metabolic adverse effect profile, its mechanism of action as a partial D2 agonist may make it difficult to control psychotic symptoms in this patient population compared with true D2 antagonists.
Continue to: CASE CONTINUED...
CASE CONTINUED
Mrs. C is admitted to the inpatient psychiatric unit and started on risperidone, 1 mg twice daily. Initially, she complains of lightheadedness at night due to the risperidone, so her dose is changed to 2 mg at bedtime. Gradually, she begins to show mild improvement. Previously, she reported feeling frightened of staff members, but after a few days she reports that she feels safe at the hospital. However, her delusions of being monitored by the FBI persist.
After 9 days of hospitalization, Mrs. C is discharged home to the care of her daughter. At first, she does well, but unfortunately she begins to refuse to take her medication and returns to her baseline.
Related Resources
- Gok F, Eroglu MZ. Acute psychotic disorder associated with immunosuppressive agent use after renal transplantation: a case report. Psychiatry and Clinical Psychopharmacology. 2017;3:314-316.
- Bersani G, Marino P, Valerani G, et al. Manic-like psychosis associated with elevated trough tacrolimus blood concentrations 17 years after kidney transplant. Case Rep Psychiatry. 2013;2013:926395. doi: 10.1155/2013/926395
Drug Brand Names
Amlodipine • Norvasc
Aripiprazole • Abilify
Atorvastatin • Lipitor
Benztropine • Cogentin
Cinacalcet • Sensipar
Cyclosporine • Gengraf
Haloperidol • Haldol
Ketamine • Ketalar
Levothyroxine • Synthroid
Metformin • Glucophage
Mycophenolate mofetil • CellCept
Olanzapine • Zyprexa
Quetiapine • Seroquel
Paliperidone • Invega
Risperidone • Risperdal
Tacrolimus • Prograf
1. Health Resources & Services Administration. US Government Information on Organ Donor Transplantation. Organ Donation Statistics. Updated October 1, 2020. Accessed October 8, 2021. https://www.organdonor.gov/learn/organ-donation-statistics/detailed-description#fig1
2. De Pasquale C, Veroux M, Indelicato L, et al. Psychopathological aspects of kidney transplantation: efficacy of a multidisciplinary team. World J Transplant. 2014;4(4):267-275.
3. Gengraf capsules [package insert]. North Chicago, IL: AbbVie Inc; 2017.
4. Wiederrecht G, Lam E, Hung S, et al. The mechanism of action of FK-506 and cyclosporin A. Ann N Y Acad Sci. 1993;696:9-19.
5. Schreiber SL, Crabtree GR. The mechanism of action of cyclosporin A and FK506. Immunol Today. 1992;13(4):136-142.
6. Scherrer U, Vissing SF, Morgan BJ, et al. Cyclosporine-induced sympathetic activation and hypertension after heart transplantation. N Engl J Med. 1990;323(11):693-699.
7. Fulya G, Meliha ZE. Acute psychotic disorder associated with immunosuppressive agent use after renal transplantation: a case report. Psychiatry and Clinical Psychopharmacology. 2017;27(3):314-316.
8. Tan TC, Robinson PJ. Mechanisms of calcineurin inhibitor-induced neurotoxicity. Transplant Rev. 2006;20(1):49-60.
9. Masatsuna S, Norio M, Nori Takei, et al. Tacrolimus, a specific inhibitor of calcineurin, modifies the locomotor activity of quinpirole, but not that of SKF82958, in male rats. Eur J Pharmacol. 2002;438(1-2):93-97.
10. Gold BG. FK506 and the role of immunophilins in nerve regeneration. Mol Neurobiol. 1997;15(3):285-306.
11. Miyakawa T, Leiter LM, Gerber DJ. Conditional calcineurin knockout mice exhibit multiple abnormal behaviors related to schizophrenia. Proc Natl Acad Sci U S A. 2003;100(15): 8987-8992.
Mrs. C, age 68, is experiencing worsening paranoid delusions. She believes that because she lied about her income when she was younger, the FBI is tracking her and wants to poison her food and spray dangerous fumes in her house. Her paranoia has made it hard for her to sleep, eat, or feel safe in her home.
Mrs. C’s daughter reports that her mother’s delusions started 3 years ago and have worsened in recent months. Mrs. C has no psychiatric history. Her medical history is significant for renal transplant in 2015, type 2 diabetes, hyperlipidemia, hypertension, and hypothyroidism. She is currently normotensive. Mrs. C’s home medications include cyclosporine, which is a calcineurin inhibitor, 125 mg twice daily (trough level = 138 mcg/L); mycophenolate mofetil, 500 mg twice daily; cinacalcet, 30 mg 3 times a week; metformin, 500 mg twice daily; amlodipine, 5 mg twice daily; levothyroxine, 112 mcg/d; and atorvastatin, 40 mg at bedtime.
As you review her medications, you wonder if the cyclosporine she began receiving after her kidney transplant could be causing or contributing to her worsening paranoid delusions.
Kidney transplantation has become an ideal treatment for patients with end-stage renal disease. In 2020, 22,817 kidney transplants were performed in the United States.1 Compared with dialysis, kidney transplant allows patients the chance to return to a satisfactory quality of life.2 However, to ensure a successful and long-lasting transplant, patients must be started and maintained on lifelong immunosuppressant agents that have potential adverse effects, including nephrotoxicity and hypertension. Further, immunosuppressant agents—particularly calcineurin inhibitors—are associated with potential adverse effects on mental health. The most commonly documented mental health-related adverse effects include insomnia, anxiety, depression, and confusion.3 In this article, we discuss the risk of severe psychosis associated with the use of calcineurin inhibitors.

Calcineurin inhibitors and psychiatric symptoms
Cyclosporine and tacrolimus are calcineurin inhibitors that are commonly used as immunosuppressant agents after kidney transplantation. They primarily work by specifically and competitively binding to and inhibiting the calcineurin protein to reduce the transcriptional activation of cytokine genes for interleukin-2, tumor necrosis factor-alpha, interleukin-3, interleukin-4, CD40L (CD40 ligand), granulocyte-macrophage colony-stimulating factor, and interferon-gamma.4,5 The ultimate downstream effect is reduced proliferation of T lymphocytes and thereby an immunosuppressed state that will protect against organ rejection. However, this is not the only downstream effect that can occur from inhibiting calcineurin. Cyclosporine and tacrolimus may modulate the activity of dopamine and N-methyl-
An increased effect of dopamine in the mesocortical dopaminergic pathway has long been a suspected cause for psychotic symptoms. A study conducted in rodents suggested that tacrolimus selectively modifies the responsivity and sensitivity of postsynaptic dopamine-2 (D2) and dopamine-3 (D3) receptors.9 These receptors are important when discussing psychosis because antipsychotic medications work primarily by blocking dopamine D2, while many also block the D3 receptor. We hypothesize that modifying the responsivity and sensitivity of these 2 receptors could increase the risk of a person developing psychosis. It may also provide insight into how to best treat a psychotic episode.
Tacrolimus has been shown to elicit inhibition of NMDA-induced neurotransmitter release and augmentation of depolarization-induced neurotransmitter release.10 In theory, this potential inhibition at the NMDA receptors may lead to a compensatory and excessive release of glutamate. Elevated glutamate levels in the brain could lead to psychiatric symptoms, including psychosis. This is supported by the psychosis caused by many NMDA receptor antagonists, such as phencyclidine (PCP) and ketamine. Furthermore, a study examining calcineurin in knockout mice showed that the spectrum of behavioral abnormalities was strikingly similar to those in schizophrenia models.11 These mice displayed impaired working memory, impaired attentional function, social withdrawal, and psychomotor agitation. This further supports the idea that calcineurin inhibition can play a significant role in causing psychiatric symptoms by affecting both dopamine and NMDA receptors.
Continue to: How to address calcineurin inhibitor–induced psychosis...
How to address calcineurin inhibitor–induced psychosis
Here we outline a potential treatment strategy to combat psychosis secondary to calcineurin inhibitors. First, evaluate the patient’s calcineurin inhibitor level (either cyclosporine or tacrolimus). Levels should be drawn as a true trough and doses adjusted if necessary via appropriate consultation with a transplant specialist. Because many of the adverse effects associated with these agents are dose-dependent, we suspect that the risk of psychosis and other mental health–related adverse effects may also follow this trend.
Assuming that the calcineurin inhibitor cannot be stopped, changed to a different agent, or subject to a dose decrease, we recommend initiating an antipsychotic medication to control psychotic symptoms. Given the potential effect of calcineurin inhibitors on dopamine, we suggest trialing a second-generation antipsychotic with relatively high affinity for dopamine D2 receptors, such as risperidone or paliperidone. However, compared with patients with schizophrenia, patients receiving a calcineurin inhibitor may be more likely to develop extrapyramidal symptoms (EPS). Therefore, to avoid potential adverse effects, consider using a lower starting dose or an antipsychotic medication with less dopamine D2 affinity, such as quetiapine, olanzapine, or aripiprazole. Furthermore, because post-transplant patients may be at a higher risk for depression, which may be secondary to medication adverse effects, we suggest avoiding first-generation antipsychotics (FGAs) such as haloperidol because FGAs may worsen depressive symptoms.
We recommend initiating risperidone, 1 mg twice a day, for patients with psychosis secondary to a calcineurin inhibitor. If the patient develops EPS, consider switching to an antipsychotic medication with a less potent dopamine D2 blockade, such as quetiapine, olanzapine, or aripiprazole. We recommend an antipsychotic switch rather than adding benztropine or diphenhydramine to the regimen because many transplant recipients may be older patients, and adding anticholinergic medications can be problematic for this population. However, if the patient is younger or has responded particularly well to risperidone, the benefit of adding an anticholinergic medication may outweigh the risks. This decision should be made on an individual basis and may include other options, such as a switch to quetiapine, olanzapine, or aripiprazole. While these agents may not block the D2 receptor as strongly as risperidone, they all are effective and approved for adjunct therapy in major depressive disorder. We recommend quetiapine and olanzapine as second-line agents because of their potential for sedation and significant weight gain. While aripiprazole has a great metabolic adverse effect profile, its mechanism of action as a partial D2 agonist may make it difficult to control psychotic symptoms in this patient population compared with true D2 antagonists.
Continue to: CASE CONTINUED...
CASE CONTINUED
Mrs. C is admitted to the inpatient psychiatric unit and started on risperidone, 1 mg twice daily. Initially, she complains of lightheadedness at night due to the risperidone, so her dose is changed to 2 mg at bedtime. Gradually, she begins to show mild improvement. Previously, she reported feeling frightened of staff members, but after a few days she reports that she feels safe at the hospital. However, her delusions of being monitored by the FBI persist.
After 9 days of hospitalization, Mrs. C is discharged home to the care of her daughter. At first, she does well, but unfortunately she begins to refuse to take her medication and returns to her baseline.
Related Resources
- Gok F, Eroglu MZ. Acute psychotic disorder associated with immunosuppressive agent use after renal transplantation: a case report. Psychiatry and Clinical Psychopharmacology. 2017;3:314-316.
- Bersani G, Marino P, Valerani G, et al. Manic-like psychosis associated with elevated trough tacrolimus blood concentrations 17 years after kidney transplant. Case Rep Psychiatry. 2013;2013:926395. doi: 10.1155/2013/926395
Drug Brand Names
Amlodipine • Norvasc
Aripiprazole • Abilify
Atorvastatin • Lipitor
Benztropine • Cogentin
Cinacalcet • Sensipar
Cyclosporine • Gengraf
Haloperidol • Haldol
Ketamine • Ketalar
Levothyroxine • Synthroid
Metformin • Glucophage
Mycophenolate mofetil • CellCept
Olanzapine • Zyprexa
Quetiapine • Seroquel
Paliperidone • Invega
Risperidone • Risperdal
Tacrolimus • Prograf
Mrs. C, age 68, is experiencing worsening paranoid delusions. She believes that because she lied about her income when she was younger, the FBI is tracking her and wants to poison her food and spray dangerous fumes in her house. Her paranoia has made it hard for her to sleep, eat, or feel safe in her home.
Mrs. C’s daughter reports that her mother’s delusions started 3 years ago and have worsened in recent months. Mrs. C has no psychiatric history. Her medical history is significant for renal transplant in 2015, type 2 diabetes, hyperlipidemia, hypertension, and hypothyroidism. She is currently normotensive. Mrs. C’s home medications include cyclosporine, which is a calcineurin inhibitor, 125 mg twice daily (trough level = 138 mcg/L); mycophenolate mofetil, 500 mg twice daily; cinacalcet, 30 mg 3 times a week; metformin, 500 mg twice daily; amlodipine, 5 mg twice daily; levothyroxine, 112 mcg/d; and atorvastatin, 40 mg at bedtime.
As you review her medications, you wonder if the cyclosporine she began receiving after her kidney transplant could be causing or contributing to her worsening paranoid delusions.
Kidney transplantation has become an ideal treatment for patients with end-stage renal disease. In 2020, 22,817 kidney transplants were performed in the United States.1 Compared with dialysis, kidney transplant allows patients the chance to return to a satisfactory quality of life.2 However, to ensure a successful and long-lasting transplant, patients must be started and maintained on lifelong immunosuppressant agents that have potential adverse effects, including nephrotoxicity and hypertension. Further, immunosuppressant agents—particularly calcineurin inhibitors—are associated with potential adverse effects on mental health. The most commonly documented mental health-related adverse effects include insomnia, anxiety, depression, and confusion.3 In this article, we discuss the risk of severe psychosis associated with the use of calcineurin inhibitors.

Calcineurin inhibitors and psychiatric symptoms
Cyclosporine and tacrolimus are calcineurin inhibitors that are commonly used as immunosuppressant agents after kidney transplantation. They primarily work by specifically and competitively binding to and inhibiting the calcineurin protein to reduce the transcriptional activation of cytokine genes for interleukin-2, tumor necrosis factor-alpha, interleukin-3, interleukin-4, CD40L (CD40 ligand), granulocyte-macrophage colony-stimulating factor, and interferon-gamma.4,5 The ultimate downstream effect is reduced proliferation of T lymphocytes and thereby an immunosuppressed state that will protect against organ rejection. However, this is not the only downstream effect that can occur from inhibiting calcineurin. Cyclosporine and tacrolimus may modulate the activity of dopamine and N-methyl-
An increased effect of dopamine in the mesocortical dopaminergic pathway has long been a suspected cause for psychotic symptoms. A study conducted in rodents suggested that tacrolimus selectively modifies the responsivity and sensitivity of postsynaptic dopamine-2 (D2) and dopamine-3 (D3) receptors.9 These receptors are important when discussing psychosis because antipsychotic medications work primarily by blocking dopamine D2, while many also block the D3 receptor. We hypothesize that modifying the responsivity and sensitivity of these 2 receptors could increase the risk of a person developing psychosis. It may also provide insight into how to best treat a psychotic episode.
Tacrolimus has been shown to elicit inhibition of NMDA-induced neurotransmitter release and augmentation of depolarization-induced neurotransmitter release.10 In theory, this potential inhibition at the NMDA receptors may lead to a compensatory and excessive release of glutamate. Elevated glutamate levels in the brain could lead to psychiatric symptoms, including psychosis. This is supported by the psychosis caused by many NMDA receptor antagonists, such as phencyclidine (PCP) and ketamine. Furthermore, a study examining calcineurin in knockout mice showed that the spectrum of behavioral abnormalities was strikingly similar to those in schizophrenia models.11 These mice displayed impaired working memory, impaired attentional function, social withdrawal, and psychomotor agitation. This further supports the idea that calcineurin inhibition can play a significant role in causing psychiatric symptoms by affecting both dopamine and NMDA receptors.
Continue to: How to address calcineurin inhibitor–induced psychosis...
How to address calcineurin inhibitor–induced psychosis
Here we outline a potential treatment strategy to combat psychosis secondary to calcineurin inhibitors. First, evaluate the patient’s calcineurin inhibitor level (either cyclosporine or tacrolimus). Levels should be drawn as a true trough and doses adjusted if necessary via appropriate consultation with a transplant specialist. Because many of the adverse effects associated with these agents are dose-dependent, we suspect that the risk of psychosis and other mental health–related adverse effects may also follow this trend.
Assuming that the calcineurin inhibitor cannot be stopped, changed to a different agent, or subject to a dose decrease, we recommend initiating an antipsychotic medication to control psychotic symptoms. Given the potential effect of calcineurin inhibitors on dopamine, we suggest trialing a second-generation antipsychotic with relatively high affinity for dopamine D2 receptors, such as risperidone or paliperidone. However, compared with patients with schizophrenia, patients receiving a calcineurin inhibitor may be more likely to develop extrapyramidal symptoms (EPS). Therefore, to avoid potential adverse effects, consider using a lower starting dose or an antipsychotic medication with less dopamine D2 affinity, such as quetiapine, olanzapine, or aripiprazole. Furthermore, because post-transplant patients may be at a higher risk for depression, which may be secondary to medication adverse effects, we suggest avoiding first-generation antipsychotics (FGAs) such as haloperidol because FGAs may worsen depressive symptoms.
We recommend initiating risperidone, 1 mg twice a day, for patients with psychosis secondary to a calcineurin inhibitor. If the patient develops EPS, consider switching to an antipsychotic medication with a less potent dopamine D2 blockade, such as quetiapine, olanzapine, or aripiprazole. We recommend an antipsychotic switch rather than adding benztropine or diphenhydramine to the regimen because many transplant recipients may be older patients, and adding anticholinergic medications can be problematic for this population. However, if the patient is younger or has responded particularly well to risperidone, the benefit of adding an anticholinergic medication may outweigh the risks. This decision should be made on an individual basis and may include other options, such as a switch to quetiapine, olanzapine, or aripiprazole. While these agents may not block the D2 receptor as strongly as risperidone, they all are effective and approved for adjunct therapy in major depressive disorder. We recommend quetiapine and olanzapine as second-line agents because of their potential for sedation and significant weight gain. While aripiprazole has a great metabolic adverse effect profile, its mechanism of action as a partial D2 agonist may make it difficult to control psychotic symptoms in this patient population compared with true D2 antagonists.
Continue to: CASE CONTINUED...
CASE CONTINUED
Mrs. C is admitted to the inpatient psychiatric unit and started on risperidone, 1 mg twice daily. Initially, she complains of lightheadedness at night due to the risperidone, so her dose is changed to 2 mg at bedtime. Gradually, she begins to show mild improvement. Previously, she reported feeling frightened of staff members, but after a few days she reports that she feels safe at the hospital. However, her delusions of being monitored by the FBI persist.
After 9 days of hospitalization, Mrs. C is discharged home to the care of her daughter. At first, she does well, but unfortunately she begins to refuse to take her medication and returns to her baseline.
Related Resources
- Gok F, Eroglu MZ. Acute psychotic disorder associated with immunosuppressive agent use after renal transplantation: a case report. Psychiatry and Clinical Psychopharmacology. 2017;3:314-316.
- Bersani G, Marino P, Valerani G, et al. Manic-like psychosis associated with elevated trough tacrolimus blood concentrations 17 years after kidney transplant. Case Rep Psychiatry. 2013;2013:926395. doi: 10.1155/2013/926395
Drug Brand Names
Amlodipine • Norvasc
Aripiprazole • Abilify
Atorvastatin • Lipitor
Benztropine • Cogentin
Cinacalcet • Sensipar
Cyclosporine • Gengraf
Haloperidol • Haldol
Ketamine • Ketalar
Levothyroxine • Synthroid
Metformin • Glucophage
Mycophenolate mofetil • CellCept
Olanzapine • Zyprexa
Quetiapine • Seroquel
Paliperidone • Invega
Risperidone • Risperdal
Tacrolimus • Prograf
1. Health Resources & Services Administration. US Government Information on Organ Donor Transplantation. Organ Donation Statistics. Updated October 1, 2020. Accessed October 8, 2021. https://www.organdonor.gov/learn/organ-donation-statistics/detailed-description#fig1
2. De Pasquale C, Veroux M, Indelicato L, et al. Psychopathological aspects of kidney transplantation: efficacy of a multidisciplinary team. World J Transplant. 2014;4(4):267-275.
3. Gengraf capsules [package insert]. North Chicago, IL: AbbVie Inc; 2017.
4. Wiederrecht G, Lam E, Hung S, et al. The mechanism of action of FK-506 and cyclosporin A. Ann N Y Acad Sci. 1993;696:9-19.
5. Schreiber SL, Crabtree GR. The mechanism of action of cyclosporin A and FK506. Immunol Today. 1992;13(4):136-142.
6. Scherrer U, Vissing SF, Morgan BJ, et al. Cyclosporine-induced sympathetic activation and hypertension after heart transplantation. N Engl J Med. 1990;323(11):693-699.
7. Fulya G, Meliha ZE. Acute psychotic disorder associated with immunosuppressive agent use after renal transplantation: a case report. Psychiatry and Clinical Psychopharmacology. 2017;27(3):314-316.
8. Tan TC, Robinson PJ. Mechanisms of calcineurin inhibitor-induced neurotoxicity. Transplant Rev. 2006;20(1):49-60.
9. Masatsuna S, Norio M, Nori Takei, et al. Tacrolimus, a specific inhibitor of calcineurin, modifies the locomotor activity of quinpirole, but not that of SKF82958, in male rats. Eur J Pharmacol. 2002;438(1-2):93-97.
10. Gold BG. FK506 and the role of immunophilins in nerve regeneration. Mol Neurobiol. 1997;15(3):285-306.
11. Miyakawa T, Leiter LM, Gerber DJ. Conditional calcineurin knockout mice exhibit multiple abnormal behaviors related to schizophrenia. Proc Natl Acad Sci U S A. 2003;100(15): 8987-8992.
1. Health Resources & Services Administration. US Government Information on Organ Donor Transplantation. Organ Donation Statistics. Updated October 1, 2020. Accessed October 8, 2021. https://www.organdonor.gov/learn/organ-donation-statistics/detailed-description#fig1
2. De Pasquale C, Veroux M, Indelicato L, et al. Psychopathological aspects of kidney transplantation: efficacy of a multidisciplinary team. World J Transplant. 2014;4(4):267-275.
3. Gengraf capsules [package insert]. North Chicago, IL: AbbVie Inc; 2017.
4. Wiederrecht G, Lam E, Hung S, et al. The mechanism of action of FK-506 and cyclosporin A. Ann N Y Acad Sci. 1993;696:9-19.
5. Schreiber SL, Crabtree GR. The mechanism of action of cyclosporin A and FK506. Immunol Today. 1992;13(4):136-142.
6. Scherrer U, Vissing SF, Morgan BJ, et al. Cyclosporine-induced sympathetic activation and hypertension after heart transplantation. N Engl J Med. 1990;323(11):693-699.
7. Fulya G, Meliha ZE. Acute psychotic disorder associated with immunosuppressive agent use after renal transplantation: a case report. Psychiatry and Clinical Psychopharmacology. 2017;27(3):314-316.
8. Tan TC, Robinson PJ. Mechanisms of calcineurin inhibitor-induced neurotoxicity. Transplant Rev. 2006;20(1):49-60.
9. Masatsuna S, Norio M, Nori Takei, et al. Tacrolimus, a specific inhibitor of calcineurin, modifies the locomotor activity of quinpirole, but not that of SKF82958, in male rats. Eur J Pharmacol. 2002;438(1-2):93-97.
10. Gold BG. FK506 and the role of immunophilins in nerve regeneration. Mol Neurobiol. 1997;15(3):285-306.
11. Miyakawa T, Leiter LM, Gerber DJ. Conditional calcineurin knockout mice exhibit multiple abnormal behaviors related to schizophrenia. Proc Natl Acad Sci U S A. 2003;100(15): 8987-8992.
Serotonergic antidepressants’ effects on bone health
Mrs. D, age 67, has a history of major depressive disorder. She has had adequate treatment trials with duloxetine, mirtazapine, and sertraline; each failed to produce remission. She is currently prescribed paroxetine, 40 mg/d, and aripiprazole, 10 mg/d, with good efficacy. She also has a history of hypertension and seasonal allergies, for which she receives amlodipine, 10 mg/d, and loratadine, 10 mg/d, respectively.
Mrs. D’s depressive symptoms were well controlled until 2 months ago, when she fell and fractured her hip. With encouragement from her prescriber, she enrolled in a partial hospitalization program for more intensive psychotherapy. During a medication education session, she is surprised to learn that antidepressants may affect bone health.
During a medication management meeting with her prescriber, Mrs. D asks about the risk of osteoporosis, and whether her antidepressant could have contributed to her hip fracture.
Bone is a dynamic tissue that undergoes a continuous process of remodeling. Osteoblasts are responsible for bone formation, whereas osteoclasts are responsible for bone resorption. Osteocytes—the predominant cell type in bone—along with cytokines, hormones, and growth factors help to orchestrate these actions.1 Serotonin is increasingly recognized as a factor in bone homeostasis. Bone synthesizes serotonin, expresses serotonin transporters, and contains a variety of serotonin receptors.2
Serotonin serves many physiologic functions outside of the CNS, and it appears to have opposing actions on bone metabolism (Table 11,3). Peripheral (gut-derived) serotonin inhibits bone formation through its effects on osteoblasts, whereas the actions of serotonin in the CNS promote bone growth through inhibitory effects on sympathetic output.2 Selective serotonin reuptake inhibitor (SSRI) enhancement of peripheral serotonin and its negative effect on bone may outweigh the benefits caused by SSRI enhancement of central serotonin neurotransmission.1 In vitro data suggest SSRIs inhibit osteoblast and osteoclast function, theoretically decreasing bone turnover and increasing fracture risk.4 Other data indicate SSRI treatment may decrease procollagen type 1 N-terminal propeptide, a peripheral marker of bone formation.5 Both SSRIs and serotonin-norepinephrine reuptake inhibitors (SNRIs) have been associated with lower cortical bone mineral density (BMD).6Table 27,8 details the relative affinity of select antidepressants for the serotonin transporter.
Both serotonergic antidepressants and depression have been associated with decreased BMD and increased fracture risk.1,9 Behavioral aspects of depression, such as inadequate nutrition or physical inactivity, overlap with risk factors for poor bone health. In addition, elevated levels of circulating cortisol and proinflammatory cytokines in patients with depressive symptoms may contribute to decreased bone mass.10,11 Modifiable risk factors for osteoporosis and fractures include low calcium and vitamin D intake, low body weight, and a sedentary lifestyle. Nonmodifiable risk factors include advancing age, female sex, Asian or White ethnicity, malabsorptive conditions, and chronic corticosteroid use.12
What the evidence says
Evidence for the correlation between fractures and serotonergic antidepressant use is mixed. One meta-analysis found a significant association between SSRIs and fractures, suggesting a 1.62-fold increased risk.13 Another meta-analysis investigated SSRIs and SNRIs and the risk of fracture.14 The SSRIs had a 1.67-fold increased risk; however, a lack of studies prohibited making conclusions about SNRIs. The number needed to harm was calculated at 85, 46, and 19 with 1, 2, and 5 years of SSRI exposure, respectively. A third meta-analysis found increased fracture risk related to depression and reported a hazard ratio of 1.26 after adjusting for confounders.9 This analysis suggests depression affects fracture risk and may limit the interpretation of causation from SSRI use. Studies included in these meta-analyses had significant heterogeneity.
Continue to: The effect of SSRIs...
The effect of SSRIs vs non-SSRIs on BMD also has been studied. The SSRIs were associated with significantly reduced BMD of the lumbar spine but not the total hip or femoral neck as compared to non-SSRIs; however, this BMD loss was not examined in relation to the presence of fractures. Older patients had more pronounced bone loss.15 Conversely, another meta-analysis examined BMD in women receiving SSRIs or tricyclic antidepressants.10 Neither medication class was associated with lower BMD at measured locations, including lumbar spine, femoral neck, and total hip. This analysis was limited by the lack of available trials; only 4 were included.
Other recent research has continued to explore the relationship between antidepressants and fracture in various patient populations. In a study of patients receiving maintenance dialysis treatment, short- and long-term SSRI use increased hip fracture risk. The authors speculated that short-term risk may be mediated by adverse effects that increase fall risk (eg, hyponatremia, orthostasis), whereas long-term risk may be influenced by changes in bone homeostasis.16 In two 6-month analyses of fluoxetine treatment in patients following an acute stroke, fluoxetine increased the risk of bone fractures.17,18 Finally, in women with osteoporosis receiving risedronate or teriparatide, in both groups a higher fracture risk was observed for patients who were also receiving an SSRI or SNRI.19
Monitor BMD and educate patients about bone health
Available literature has not identified any clear risk factors for fracture with SSRI use. Guidelines suggest monitoring BMD in patients with risk factors for osteoporosis, if clinically indicated, as well as monitoring BMD in those receiving long-term antidepressant treatment.20-22 Educate patients on strategies that promote optimal bone health, such as consuming a balanced diet that meets the recommended dietary allowance of calcium, vitamin D, and limits soda consumption. Teach patients to avoid tobacco and excessive alcohol use because both adversely impact BMD. Maintaining a healthy weight, physical activity, and adequate sleep also support bone health.11 Instruct patients receiving antidepressants to report unexplained bone pain, tenderness, swelling, or bruising because these symptoms may be indicative of fracture.
CASE CONTINUED
Mrs. D’s age, sex, and depression place her at higher risk of fracture. Paroxetine is the only SSRI that has bone fracture listed as a precaution in its labeling.23 In addition, it is the most anticholinergic SSRI and may have contributed to her fall. Switching to bupropion by cross titration may benefit Mrs. D because bupropion is not serotonergic. Little data exist regarding the effects of bupropion on bone. Her prescriber monitors Mrs. D’s BMD periodically, and educates her on dietary considerations. He also recommends calcium, 1,200 mg/d, and vitamin D, 800 IU/d, to help prevent fractures,24 and that she continue physical therapy exercises and increase physical activity as tolerated.
Related Resources
- Cosman F, de Beur SJ, LeBoff MS, et al. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359-2581.
- Dodd S, Mitchell PB, Bauer M, et al. Monitoring for antidepressant-associated adverse events in the treatment of patients with major depressive disorder: an international consensus statement. World J Biol Psychiatry. 2018;19(5):330-348.
- Fernandes BS, Hodge JM, Pasco JA, et al. Effects of depression and serotonergic antidepressants on bone: mechanisms and implications for the treatment of depression. Drugs Aging. 2016;33(1):21-25.
- US National Library of Medicine. DailyMed. https://dailymed.nlm.nih.gov/dailymed
Drug Brand Names
Amitriptyline • Elavil
Amlodipine • Norvasc
Aripiprazole • Abilify
Bupropion • Wellbutrin
Citalopram • Celexa
Clomipramine • Anafranil
Desipramine • Norpramin
Doxepin • Silenor, Sinequan
Duloxetine • Cymbalta
Escitalopram • Lexapro
Fluoxetine • Prozac
Fluvoxamine • Luvox
Imipramine • Tofranil
Levomilnacipran • Fetzima
Loratadine • Claritin
Mirtazapine • Remeron
Nortriptyline • Pamelor
Paroxetine • Paxil
Risedronate • Actonel
Sertraline • Zoloft
Teriparatide • Forteo
Trazodone • Desyrel
Venlafaxine • Effexor
Vortioxetine • Trintellix
1. Fernandes BS, Hodge JM, Pasco JA, et al. Effects of depression and serotonergic antidepressants on bone: mechanisms and implications for the treatment of depression. Drugs Aging. 2016;33(1):21-25.
2. Lavoie B, Lian JB, Mawe GM. Regulation of bone metabolism by serotonin. Adv Exp Med Biol. 2017;1033:35-46.
3. Berger M, Gray JA, Roth BL. The expanded biology of serotonin. Annu Rev Med. 2009;60:355-366.
4. Hodge JM, Wang Y, Berk M, et al. Selective serotonin reuptake inhibitors inhibit human osteoclast and osteoblast formation and function. Biol Psychiatry. 2013;74(1):32-39.
5. Kumar M, Jiloha RC, Kataria D, et al. Effect of selective serotonin reuptake inhibitors on markers of bone loss. Psychiatry Res. 2019;276:39-44.
6. Agarwal S, Germosen C, Kil N, et al. Current anti-depressant use is associated with cortical bone deficits and reduced physical function in elderly women. Bone. 2020;140:115552.
7. DeBattista C. Antidepressant agents. In: Katzung BG, ed. Basic and clinical pharmacology. 14th ed. McGraw-Hill; 2018.
8. Kasper S, Pail G. Milnacipran: a unique antidepressant? Neuropsychiatr Dis Treat. 2010;6(Suppl 1):23-31.
9. Wu Q, Liu B, Tonmoy S. Depression and risk of fracture and bone loss: an updated meta-analysis of prospective studies. Osteoporos Int. 2018;29(6):1303-1312.
10. Schweiger JU, Schweiger U, Hüppe M, et al. The use of antidepressant agents and bone mineral density in women: a meta-analysis. Int J Environ Res Public Health. 2018;15(7):1373.
11. Rizzoli R, Cooper C, Reginster JY, et al. Antidepressant medications and osteoporosis. Bone. 2012;51(3):606-613.
12. Rice JN, Gillett CB, Malas NM. The impact of psychotropic medications on bone health in youth. Curr Psychiatry Rep. 2018;20(11):104.
13. Kumar M, Bajpai R, Shaik AR, et al. Alliance between selective serotonin reuptake inhibitors and fracture risk: an updated systematic review and meta-analysis. Eur J Clin Pharmacol. 2020;76(10):1373-1392.
14. Khanassov V, Hu J, Reeves D, et al. Selective serotonin reuptake inhibitor and selective serotonin and norepinephrine reuptake inhibitor use and risk of fractures in adults: a systematic review and meta-analysis. Int J Geriatr Psychiatry. 2018;33(12):1688-1708.
15. Zhou C, Fang L, Chen Y, et al. Effect of selective serotonin reuptake inhibitors on bone mineral density: a systematic review and meta-analysis. Osteoporos Int. 2018;29(6):1243-1251.
16. Vangala C, Niu J, Montez-Rath ME, et al. Selective serotonin reuptake inhibitor use and hip fracture risk among patients on hemodialysis. Am J Kidney Dis. 2020;75(3):351-360.
17. Hankey GJ, Hackett ML, Almeida OP, et al. Safety and efficacy of fluoxetine on functional outcome after acute stroke (AFFINITY): a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2020;19(8):651-660.
18. Lundström E, Isaksson E, Näsman P, et al. Safety and efficacy of fluoxetine on functional recovery after acute stroke (EFFECTS): a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2020;19(8):661-669.
19. Kendler DL, Marin F, Geusens P, et al. Psychotropic medications and proton pump inhibitors and the risk of fractures in the teriparatide versus risedronate VERO clinical trial. Bone. 2020;130:115113.
20. Dodd S, Mitchell PB, Bauer M, et al. Monitoring for antidepressant-associated adverse events in the treatment of patients with major depressive disorder: an international consensus statement. World J Biol Psychiatry. 2018;19(5):330-348.
21. American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder. Published October 2010. Accessed February 8, 2021. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf
22. Agacayak KS, Guler R, Ilyasov B. Evaluation of the effect of long-term use of antidepressants in the SSRI group on bone density with dental volumetric tomography. Drug Des Devel Ther. 2019;13:3477-3484.
23. US National Library of Medicine. DailyMed. Accessed February 8, 2021. https://dailymed.nlm.nih.gov/dailymed
24. Cosman F, de Beur SJ, LeBoff MS, et al. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359-2581.
Mrs. D, age 67, has a history of major depressive disorder. She has had adequate treatment trials with duloxetine, mirtazapine, and sertraline; each failed to produce remission. She is currently prescribed paroxetine, 40 mg/d, and aripiprazole, 10 mg/d, with good efficacy. She also has a history of hypertension and seasonal allergies, for which she receives amlodipine, 10 mg/d, and loratadine, 10 mg/d, respectively.
Mrs. D’s depressive symptoms were well controlled until 2 months ago, when she fell and fractured her hip. With encouragement from her prescriber, she enrolled in a partial hospitalization program for more intensive psychotherapy. During a medication education session, she is surprised to learn that antidepressants may affect bone health.
During a medication management meeting with her prescriber, Mrs. D asks about the risk of osteoporosis, and whether her antidepressant could have contributed to her hip fracture.
Bone is a dynamic tissue that undergoes a continuous process of remodeling. Osteoblasts are responsible for bone formation, whereas osteoclasts are responsible for bone resorption. Osteocytes—the predominant cell type in bone—along with cytokines, hormones, and growth factors help to orchestrate these actions.1 Serotonin is increasingly recognized as a factor in bone homeostasis. Bone synthesizes serotonin, expresses serotonin transporters, and contains a variety of serotonin receptors.2
Serotonin serves many physiologic functions outside of the CNS, and it appears to have opposing actions on bone metabolism (Table 11,3). Peripheral (gut-derived) serotonin inhibits bone formation through its effects on osteoblasts, whereas the actions of serotonin in the CNS promote bone growth through inhibitory effects on sympathetic output.2 Selective serotonin reuptake inhibitor (SSRI) enhancement of peripheral serotonin and its negative effect on bone may outweigh the benefits caused by SSRI enhancement of central serotonin neurotransmission.1 In vitro data suggest SSRIs inhibit osteoblast and osteoclast function, theoretically decreasing bone turnover and increasing fracture risk.4 Other data indicate SSRI treatment may decrease procollagen type 1 N-terminal propeptide, a peripheral marker of bone formation.5 Both SSRIs and serotonin-norepinephrine reuptake inhibitors (SNRIs) have been associated with lower cortical bone mineral density (BMD).6Table 27,8 details the relative affinity of select antidepressants for the serotonin transporter.
Both serotonergic antidepressants and depression have been associated with decreased BMD and increased fracture risk.1,9 Behavioral aspects of depression, such as inadequate nutrition or physical inactivity, overlap with risk factors for poor bone health. In addition, elevated levels of circulating cortisol and proinflammatory cytokines in patients with depressive symptoms may contribute to decreased bone mass.10,11 Modifiable risk factors for osteoporosis and fractures include low calcium and vitamin D intake, low body weight, and a sedentary lifestyle. Nonmodifiable risk factors include advancing age, female sex, Asian or White ethnicity, malabsorptive conditions, and chronic corticosteroid use.12
What the evidence says
Evidence for the correlation between fractures and serotonergic antidepressant use is mixed. One meta-analysis found a significant association between SSRIs and fractures, suggesting a 1.62-fold increased risk.13 Another meta-analysis investigated SSRIs and SNRIs and the risk of fracture.14 The SSRIs had a 1.67-fold increased risk; however, a lack of studies prohibited making conclusions about SNRIs. The number needed to harm was calculated at 85, 46, and 19 with 1, 2, and 5 years of SSRI exposure, respectively. A third meta-analysis found increased fracture risk related to depression and reported a hazard ratio of 1.26 after adjusting for confounders.9 This analysis suggests depression affects fracture risk and may limit the interpretation of causation from SSRI use. Studies included in these meta-analyses had significant heterogeneity.
Continue to: The effect of SSRIs...
The effect of SSRIs vs non-SSRIs on BMD also has been studied. The SSRIs were associated with significantly reduced BMD of the lumbar spine but not the total hip or femoral neck as compared to non-SSRIs; however, this BMD loss was not examined in relation to the presence of fractures. Older patients had more pronounced bone loss.15 Conversely, another meta-analysis examined BMD in women receiving SSRIs or tricyclic antidepressants.10 Neither medication class was associated with lower BMD at measured locations, including lumbar spine, femoral neck, and total hip. This analysis was limited by the lack of available trials; only 4 were included.
Other recent research has continued to explore the relationship between antidepressants and fracture in various patient populations. In a study of patients receiving maintenance dialysis treatment, short- and long-term SSRI use increased hip fracture risk. The authors speculated that short-term risk may be mediated by adverse effects that increase fall risk (eg, hyponatremia, orthostasis), whereas long-term risk may be influenced by changes in bone homeostasis.16 In two 6-month analyses of fluoxetine treatment in patients following an acute stroke, fluoxetine increased the risk of bone fractures.17,18 Finally, in women with osteoporosis receiving risedronate or teriparatide, in both groups a higher fracture risk was observed for patients who were also receiving an SSRI or SNRI.19
Monitor BMD and educate patients about bone health
Available literature has not identified any clear risk factors for fracture with SSRI use. Guidelines suggest monitoring BMD in patients with risk factors for osteoporosis, if clinically indicated, as well as monitoring BMD in those receiving long-term antidepressant treatment.20-22 Educate patients on strategies that promote optimal bone health, such as consuming a balanced diet that meets the recommended dietary allowance of calcium, vitamin D, and limits soda consumption. Teach patients to avoid tobacco and excessive alcohol use because both adversely impact BMD. Maintaining a healthy weight, physical activity, and adequate sleep also support bone health.11 Instruct patients receiving antidepressants to report unexplained bone pain, tenderness, swelling, or bruising because these symptoms may be indicative of fracture.
CASE CONTINUED
Mrs. D’s age, sex, and depression place her at higher risk of fracture. Paroxetine is the only SSRI that has bone fracture listed as a precaution in its labeling.23 In addition, it is the most anticholinergic SSRI and may have contributed to her fall. Switching to bupropion by cross titration may benefit Mrs. D because bupropion is not serotonergic. Little data exist regarding the effects of bupropion on bone. Her prescriber monitors Mrs. D’s BMD periodically, and educates her on dietary considerations. He also recommends calcium, 1,200 mg/d, and vitamin D, 800 IU/d, to help prevent fractures,24 and that she continue physical therapy exercises and increase physical activity as tolerated.
Related Resources
- Cosman F, de Beur SJ, LeBoff MS, et al. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359-2581.
- Dodd S, Mitchell PB, Bauer M, et al. Monitoring for antidepressant-associated adverse events in the treatment of patients with major depressive disorder: an international consensus statement. World J Biol Psychiatry. 2018;19(5):330-348.
- Fernandes BS, Hodge JM, Pasco JA, et al. Effects of depression and serotonergic antidepressants on bone: mechanisms and implications for the treatment of depression. Drugs Aging. 2016;33(1):21-25.
- US National Library of Medicine. DailyMed. https://dailymed.nlm.nih.gov/dailymed
Drug Brand Names
Amitriptyline • Elavil
Amlodipine • Norvasc
Aripiprazole • Abilify
Bupropion • Wellbutrin
Citalopram • Celexa
Clomipramine • Anafranil
Desipramine • Norpramin
Doxepin • Silenor, Sinequan
Duloxetine • Cymbalta
Escitalopram • Lexapro
Fluoxetine • Prozac
Fluvoxamine • Luvox
Imipramine • Tofranil
Levomilnacipran • Fetzima
Loratadine • Claritin
Mirtazapine • Remeron
Nortriptyline • Pamelor
Paroxetine • Paxil
Risedronate • Actonel
Sertraline • Zoloft
Teriparatide • Forteo
Trazodone • Desyrel
Venlafaxine • Effexor
Vortioxetine • Trintellix
Mrs. D, age 67, has a history of major depressive disorder. She has had adequate treatment trials with duloxetine, mirtazapine, and sertraline; each failed to produce remission. She is currently prescribed paroxetine, 40 mg/d, and aripiprazole, 10 mg/d, with good efficacy. She also has a history of hypertension and seasonal allergies, for which she receives amlodipine, 10 mg/d, and loratadine, 10 mg/d, respectively.
Mrs. D’s depressive symptoms were well controlled until 2 months ago, when she fell and fractured her hip. With encouragement from her prescriber, she enrolled in a partial hospitalization program for more intensive psychotherapy. During a medication education session, she is surprised to learn that antidepressants may affect bone health.
During a medication management meeting with her prescriber, Mrs. D asks about the risk of osteoporosis, and whether her antidepressant could have contributed to her hip fracture.
Bone is a dynamic tissue that undergoes a continuous process of remodeling. Osteoblasts are responsible for bone formation, whereas osteoclasts are responsible for bone resorption. Osteocytes—the predominant cell type in bone—along with cytokines, hormones, and growth factors help to orchestrate these actions.1 Serotonin is increasingly recognized as a factor in bone homeostasis. Bone synthesizes serotonin, expresses serotonin transporters, and contains a variety of serotonin receptors.2
Serotonin serves many physiologic functions outside of the CNS, and it appears to have opposing actions on bone metabolism (Table 11,3). Peripheral (gut-derived) serotonin inhibits bone formation through its effects on osteoblasts, whereas the actions of serotonin in the CNS promote bone growth through inhibitory effects on sympathetic output.2 Selective serotonin reuptake inhibitor (SSRI) enhancement of peripheral serotonin and its negative effect on bone may outweigh the benefits caused by SSRI enhancement of central serotonin neurotransmission.1 In vitro data suggest SSRIs inhibit osteoblast and osteoclast function, theoretically decreasing bone turnover and increasing fracture risk.4 Other data indicate SSRI treatment may decrease procollagen type 1 N-terminal propeptide, a peripheral marker of bone formation.5 Both SSRIs and serotonin-norepinephrine reuptake inhibitors (SNRIs) have been associated with lower cortical bone mineral density (BMD).6Table 27,8 details the relative affinity of select antidepressants for the serotonin transporter.
Both serotonergic antidepressants and depression have been associated with decreased BMD and increased fracture risk.1,9 Behavioral aspects of depression, such as inadequate nutrition or physical inactivity, overlap with risk factors for poor bone health. In addition, elevated levels of circulating cortisol and proinflammatory cytokines in patients with depressive symptoms may contribute to decreased bone mass.10,11 Modifiable risk factors for osteoporosis and fractures include low calcium and vitamin D intake, low body weight, and a sedentary lifestyle. Nonmodifiable risk factors include advancing age, female sex, Asian or White ethnicity, malabsorptive conditions, and chronic corticosteroid use.12
What the evidence says
Evidence for the correlation between fractures and serotonergic antidepressant use is mixed. One meta-analysis found a significant association between SSRIs and fractures, suggesting a 1.62-fold increased risk.13 Another meta-analysis investigated SSRIs and SNRIs and the risk of fracture.14 The SSRIs had a 1.67-fold increased risk; however, a lack of studies prohibited making conclusions about SNRIs. The number needed to harm was calculated at 85, 46, and 19 with 1, 2, and 5 years of SSRI exposure, respectively. A third meta-analysis found increased fracture risk related to depression and reported a hazard ratio of 1.26 after adjusting for confounders.9 This analysis suggests depression affects fracture risk and may limit the interpretation of causation from SSRI use. Studies included in these meta-analyses had significant heterogeneity.
Continue to: The effect of SSRIs...
The effect of SSRIs vs non-SSRIs on BMD also has been studied. The SSRIs were associated with significantly reduced BMD of the lumbar spine but not the total hip or femoral neck as compared to non-SSRIs; however, this BMD loss was not examined in relation to the presence of fractures. Older patients had more pronounced bone loss.15 Conversely, another meta-analysis examined BMD in women receiving SSRIs or tricyclic antidepressants.10 Neither medication class was associated with lower BMD at measured locations, including lumbar spine, femoral neck, and total hip. This analysis was limited by the lack of available trials; only 4 were included.
Other recent research has continued to explore the relationship between antidepressants and fracture in various patient populations. In a study of patients receiving maintenance dialysis treatment, short- and long-term SSRI use increased hip fracture risk. The authors speculated that short-term risk may be mediated by adverse effects that increase fall risk (eg, hyponatremia, orthostasis), whereas long-term risk may be influenced by changes in bone homeostasis.16 In two 6-month analyses of fluoxetine treatment in patients following an acute stroke, fluoxetine increased the risk of bone fractures.17,18 Finally, in women with osteoporosis receiving risedronate or teriparatide, in both groups a higher fracture risk was observed for patients who were also receiving an SSRI or SNRI.19
Monitor BMD and educate patients about bone health
Available literature has not identified any clear risk factors for fracture with SSRI use. Guidelines suggest monitoring BMD in patients with risk factors for osteoporosis, if clinically indicated, as well as monitoring BMD in those receiving long-term antidepressant treatment.20-22 Educate patients on strategies that promote optimal bone health, such as consuming a balanced diet that meets the recommended dietary allowance of calcium, vitamin D, and limits soda consumption. Teach patients to avoid tobacco and excessive alcohol use because both adversely impact BMD. Maintaining a healthy weight, physical activity, and adequate sleep also support bone health.11 Instruct patients receiving antidepressants to report unexplained bone pain, tenderness, swelling, or bruising because these symptoms may be indicative of fracture.
CASE CONTINUED
Mrs. D’s age, sex, and depression place her at higher risk of fracture. Paroxetine is the only SSRI that has bone fracture listed as a precaution in its labeling.23 In addition, it is the most anticholinergic SSRI and may have contributed to her fall. Switching to bupropion by cross titration may benefit Mrs. D because bupropion is not serotonergic. Little data exist regarding the effects of bupropion on bone. Her prescriber monitors Mrs. D’s BMD periodically, and educates her on dietary considerations. He also recommends calcium, 1,200 mg/d, and vitamin D, 800 IU/d, to help prevent fractures,24 and that she continue physical therapy exercises and increase physical activity as tolerated.
Related Resources
- Cosman F, de Beur SJ, LeBoff MS, et al. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359-2581.
- Dodd S, Mitchell PB, Bauer M, et al. Monitoring for antidepressant-associated adverse events in the treatment of patients with major depressive disorder: an international consensus statement. World J Biol Psychiatry. 2018;19(5):330-348.
- Fernandes BS, Hodge JM, Pasco JA, et al. Effects of depression and serotonergic antidepressants on bone: mechanisms and implications for the treatment of depression. Drugs Aging. 2016;33(1):21-25.
- US National Library of Medicine. DailyMed. https://dailymed.nlm.nih.gov/dailymed
Drug Brand Names
Amitriptyline • Elavil
Amlodipine • Norvasc
Aripiprazole • Abilify
Bupropion • Wellbutrin
Citalopram • Celexa
Clomipramine • Anafranil
Desipramine • Norpramin
Doxepin • Silenor, Sinequan
Duloxetine • Cymbalta
Escitalopram • Lexapro
Fluoxetine • Prozac
Fluvoxamine • Luvox
Imipramine • Tofranil
Levomilnacipran • Fetzima
Loratadine • Claritin
Mirtazapine • Remeron
Nortriptyline • Pamelor
Paroxetine • Paxil
Risedronate • Actonel
Sertraline • Zoloft
Teriparatide • Forteo
Trazodone • Desyrel
Venlafaxine • Effexor
Vortioxetine • Trintellix
1. Fernandes BS, Hodge JM, Pasco JA, et al. Effects of depression and serotonergic antidepressants on bone: mechanisms and implications for the treatment of depression. Drugs Aging. 2016;33(1):21-25.
2. Lavoie B, Lian JB, Mawe GM. Regulation of bone metabolism by serotonin. Adv Exp Med Biol. 2017;1033:35-46.
3. Berger M, Gray JA, Roth BL. The expanded biology of serotonin. Annu Rev Med. 2009;60:355-366.
4. Hodge JM, Wang Y, Berk M, et al. Selective serotonin reuptake inhibitors inhibit human osteoclast and osteoblast formation and function. Biol Psychiatry. 2013;74(1):32-39.
5. Kumar M, Jiloha RC, Kataria D, et al. Effect of selective serotonin reuptake inhibitors on markers of bone loss. Psychiatry Res. 2019;276:39-44.
6. Agarwal S, Germosen C, Kil N, et al. Current anti-depressant use is associated with cortical bone deficits and reduced physical function in elderly women. Bone. 2020;140:115552.
7. DeBattista C. Antidepressant agents. In: Katzung BG, ed. Basic and clinical pharmacology. 14th ed. McGraw-Hill; 2018.
8. Kasper S, Pail G. Milnacipran: a unique antidepressant? Neuropsychiatr Dis Treat. 2010;6(Suppl 1):23-31.
9. Wu Q, Liu B, Tonmoy S. Depression and risk of fracture and bone loss: an updated meta-analysis of prospective studies. Osteoporos Int. 2018;29(6):1303-1312.
10. Schweiger JU, Schweiger U, Hüppe M, et al. The use of antidepressant agents and bone mineral density in women: a meta-analysis. Int J Environ Res Public Health. 2018;15(7):1373.
11. Rizzoli R, Cooper C, Reginster JY, et al. Antidepressant medications and osteoporosis. Bone. 2012;51(3):606-613.
12. Rice JN, Gillett CB, Malas NM. The impact of psychotropic medications on bone health in youth. Curr Psychiatry Rep. 2018;20(11):104.
13. Kumar M, Bajpai R, Shaik AR, et al. Alliance between selective serotonin reuptake inhibitors and fracture risk: an updated systematic review and meta-analysis. Eur J Clin Pharmacol. 2020;76(10):1373-1392.
14. Khanassov V, Hu J, Reeves D, et al. Selective serotonin reuptake inhibitor and selective serotonin and norepinephrine reuptake inhibitor use and risk of fractures in adults: a systematic review and meta-analysis. Int J Geriatr Psychiatry. 2018;33(12):1688-1708.
15. Zhou C, Fang L, Chen Y, et al. Effect of selective serotonin reuptake inhibitors on bone mineral density: a systematic review and meta-analysis. Osteoporos Int. 2018;29(6):1243-1251.
16. Vangala C, Niu J, Montez-Rath ME, et al. Selective serotonin reuptake inhibitor use and hip fracture risk among patients on hemodialysis. Am J Kidney Dis. 2020;75(3):351-360.
17. Hankey GJ, Hackett ML, Almeida OP, et al. Safety and efficacy of fluoxetine on functional outcome after acute stroke (AFFINITY): a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2020;19(8):651-660.
18. Lundström E, Isaksson E, Näsman P, et al. Safety and efficacy of fluoxetine on functional recovery after acute stroke (EFFECTS): a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2020;19(8):661-669.
19. Kendler DL, Marin F, Geusens P, et al. Psychotropic medications and proton pump inhibitors and the risk of fractures in the teriparatide versus risedronate VERO clinical trial. Bone. 2020;130:115113.
20. Dodd S, Mitchell PB, Bauer M, et al. Monitoring for antidepressant-associated adverse events in the treatment of patients with major depressive disorder: an international consensus statement. World J Biol Psychiatry. 2018;19(5):330-348.
21. American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder. Published October 2010. Accessed February 8, 2021. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf
22. Agacayak KS, Guler R, Ilyasov B. Evaluation of the effect of long-term use of antidepressants in the SSRI group on bone density with dental volumetric tomography. Drug Des Devel Ther. 2019;13:3477-3484.
23. US National Library of Medicine. DailyMed. Accessed February 8, 2021. https://dailymed.nlm.nih.gov/dailymed
24. Cosman F, de Beur SJ, LeBoff MS, et al. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359-2581.
1. Fernandes BS, Hodge JM, Pasco JA, et al. Effects of depression and serotonergic antidepressants on bone: mechanisms and implications for the treatment of depression. Drugs Aging. 2016;33(1):21-25.
2. Lavoie B, Lian JB, Mawe GM. Regulation of bone metabolism by serotonin. Adv Exp Med Biol. 2017;1033:35-46.
3. Berger M, Gray JA, Roth BL. The expanded biology of serotonin. Annu Rev Med. 2009;60:355-366.
4. Hodge JM, Wang Y, Berk M, et al. Selective serotonin reuptake inhibitors inhibit human osteoclast and osteoblast formation and function. Biol Psychiatry. 2013;74(1):32-39.
5. Kumar M, Jiloha RC, Kataria D, et al. Effect of selective serotonin reuptake inhibitors on markers of bone loss. Psychiatry Res. 2019;276:39-44.
6. Agarwal S, Germosen C, Kil N, et al. Current anti-depressant use is associated with cortical bone deficits and reduced physical function in elderly women. Bone. 2020;140:115552.
7. DeBattista C. Antidepressant agents. In: Katzung BG, ed. Basic and clinical pharmacology. 14th ed. McGraw-Hill; 2018.
8. Kasper S, Pail G. Milnacipran: a unique antidepressant? Neuropsychiatr Dis Treat. 2010;6(Suppl 1):23-31.
9. Wu Q, Liu B, Tonmoy S. Depression and risk of fracture and bone loss: an updated meta-analysis of prospective studies. Osteoporos Int. 2018;29(6):1303-1312.
10. Schweiger JU, Schweiger U, Hüppe M, et al. The use of antidepressant agents and bone mineral density in women: a meta-analysis. Int J Environ Res Public Health. 2018;15(7):1373.
11. Rizzoli R, Cooper C, Reginster JY, et al. Antidepressant medications and osteoporosis. Bone. 2012;51(3):606-613.
12. Rice JN, Gillett CB, Malas NM. The impact of psychotropic medications on bone health in youth. Curr Psychiatry Rep. 2018;20(11):104.
13. Kumar M, Bajpai R, Shaik AR, et al. Alliance between selective serotonin reuptake inhibitors and fracture risk: an updated systematic review and meta-analysis. Eur J Clin Pharmacol. 2020;76(10):1373-1392.
14. Khanassov V, Hu J, Reeves D, et al. Selective serotonin reuptake inhibitor and selective serotonin and norepinephrine reuptake inhibitor use and risk of fractures in adults: a systematic review and meta-analysis. Int J Geriatr Psychiatry. 2018;33(12):1688-1708.
15. Zhou C, Fang L, Chen Y, et al. Effect of selective serotonin reuptake inhibitors on bone mineral density: a systematic review and meta-analysis. Osteoporos Int. 2018;29(6):1243-1251.
16. Vangala C, Niu J, Montez-Rath ME, et al. Selective serotonin reuptake inhibitor use and hip fracture risk among patients on hemodialysis. Am J Kidney Dis. 2020;75(3):351-360.
17. Hankey GJ, Hackett ML, Almeida OP, et al. Safety and efficacy of fluoxetine on functional outcome after acute stroke (AFFINITY): a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2020;19(8):651-660.
18. Lundström E, Isaksson E, Näsman P, et al. Safety and efficacy of fluoxetine on functional recovery after acute stroke (EFFECTS): a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2020;19(8):661-669.
19. Kendler DL, Marin F, Geusens P, et al. Psychotropic medications and proton pump inhibitors and the risk of fractures in the teriparatide versus risedronate VERO clinical trial. Bone. 2020;130:115113.
20. Dodd S, Mitchell PB, Bauer M, et al. Monitoring for antidepressant-associated adverse events in the treatment of patients with major depressive disorder: an international consensus statement. World J Biol Psychiatry. 2018;19(5):330-348.
21. American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder. Published October 2010. Accessed February 8, 2021. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf
22. Agacayak KS, Guler R, Ilyasov B. Evaluation of the effect of long-term use of antidepressants in the SSRI group on bone density with dental volumetric tomography. Drug Des Devel Ther. 2019;13:3477-3484.
23. US National Library of Medicine. DailyMed. Accessed February 8, 2021. https://dailymed.nlm.nih.gov/dailymed
24. Cosman F, de Beur SJ, LeBoff MS, et al. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359-2581.