User login
Mood stabilizers: Balancing tolerability, serum levels, and dosage
Mr. B, age 32, was diagnosed with bipolar disorder 10 years ago after experiencing a manic episode that resulted in his first psychiatric hospitalization. He was prescribed quetiapine, 400 mg/d, and remained stable for the next several years. Unfortunately, Mr. B developed significant metabolic adverse effects, including diabetes and a 30-pound weight gain, so he was switched from quetiapine to lithium. Mr. B was unable to tolerate the sedation and cognitive effects of lithium, and the dose could not be titrated to within the therapeutic window. As a result, Mr. B experienced a moderate depressive episode. His current clinician would like to initiate lamotrigine at a starting dose of 25 mg/d. Mr. B has not had a manic episode since the index hospitalization, and this is his first depressive episode.
The term “mood stabilizer” has come to refer to medications that treat a depressive and/or manic episode without inducing the other. In conventional terms, it refers to non-antipsychotic medications such as lithium, divalproex, and lamotrigine. Except for lithium, mood stabilizers are also antiepileptic drugs (AEDs). The role of AEDs for treating psychiatric conditions was discovered after they were originally FDA-approved for treating seizures. Following this discovery, the recommended doses and therapeutic ranges for these agents when applied to psychiatric treatment fell into a gray area.
Every patient is different and requires an individualized treatment plan, but this often leaves the clinician wondering, “How high is too high for this mood stabilizer?” or “My patient is responding well, but could a higher dose be even more effective?” In the case of Mr. B, who has trialed 2 medications with poor tolerability, how high can the lamotrigine dose be titrated to achieve a therapeutic response without adverse effects? The literature on this topic does not provide an exact answer, but does shed some light on key considerations for such decisions.
Which mood stabilizers are recommended?
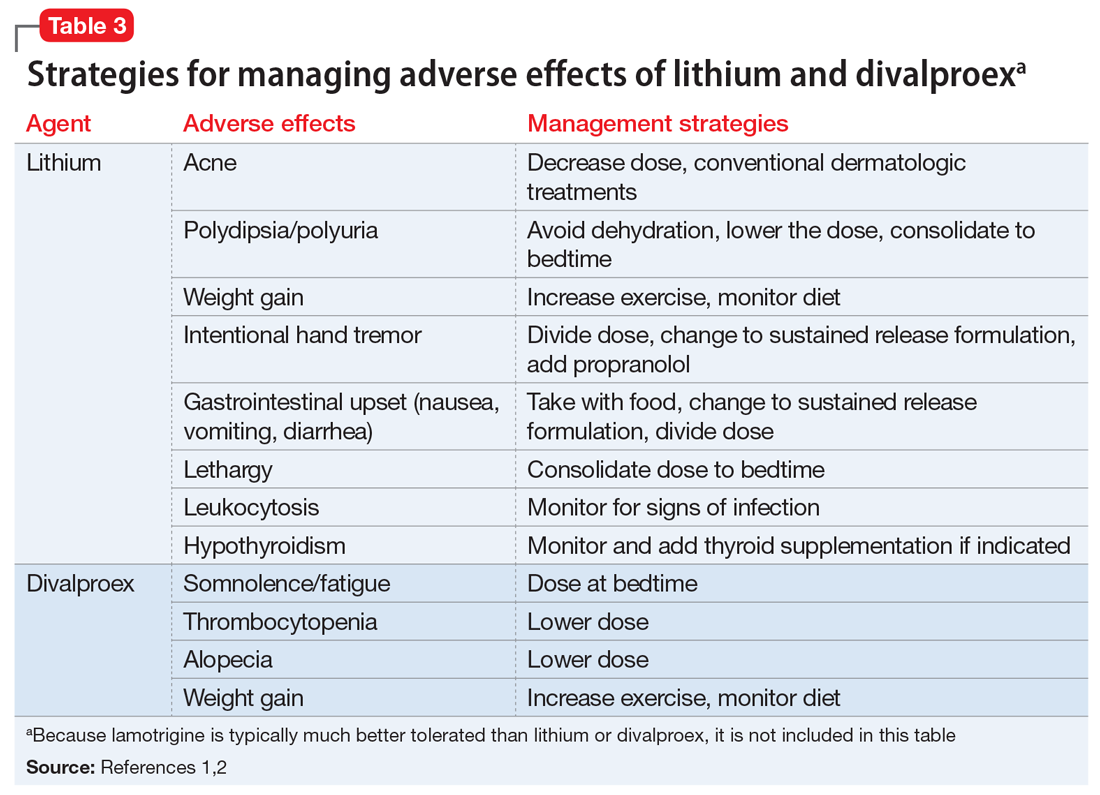
One of the most recently updated guidelines for the treatment of bipolar disorder was released in 2018 by the Canadian Network for Mood and Anxiety Treatments (CANMAT).1 Lithium, divalproex, and lamotrigine were each recommended as a first-line option for treating bipolar disorder. For lithium and divalproex, the CANMAT guidelines recommend serum level monitoring for efficacy and tolerability; however, they do not recommend serum level monitoring for lamotrigine. Lithium and divalproex each have safety and tolerability concerns, particularly when selected for maintenance therapy, whereas lamotrigine is typically much better tolerated.1 Divalproex and lithium can cause weight gain, gastrointestinal adverse effects (nausea, vomiting, diarrhea), and tremor. Additional tolerability concerns with lithium include renal toxicity, electrocardiogram abnormalities, hypothyroidism, cognitive impairment, and dermatologic reactions. Divalproex can produce greater levels of sedation and may impact reproductive function (oligomenorrhea or hyperandrogenism). One of the most common adverse effects of lamotrigine is a non-serious rash; however, slow dose titration is necessary to decrease the risk of a serious, life-threatening rash such as Stevens-Johnson syndrome.
Lithium
Lithium continues to be regarded as a gold-standard therapy for bipolar disorder. The exact serum levels corresponding to efficacy and tolerability vary. The Lithiumeter: Version 2.0 is a schematic that incorporates the various levels recommended by different clinical guidelines.2 The recommended serum levels range from 0.6 to 1.0 mEq/L for mania and 0.4 to 0.8 mEq/L for depression.2 One of the main issues with lithium dosing is balancing a therapeutic level with tolerability and toxicity. Toxicity may begin when lithium levels exceed 1.2 mEq/L, and levels >2.0 mEq/L can be lethal. Signs of acute toxicity include tremor, headache, arrhythmia, nausea, vomiting, diarrhea, polyuria, and polydipsia. Conversely, chronic lithium use may lead to chronic toxicity as patients age and their physical health changes. Signs of chronic toxicity include ataxia, confusion, renal dysfunction, and tremor. There is no “one size fits all” when it comes to lithium dosing. Individualized dosing is necessary to balance efficacy and tolerability.
Divalproex
Divalproex was initially studied for use as an AED, and its therapeutic levels as an AED are not the same as those indicated for bipolar disorder. Generally, patients with bipolar disorder require a divalproex serum level >50 µg/mL. Ranges closer to 100 µg/mL have been found to be most effective for treating acute mania.3 A loading dose of 20 to 30 mg/kg/d can be administered to help achieve mood stabilization. Again, efficacy must be balanced against toxicity. The maximum dose of divalproex is 60 mg/kg/d, which is rarely seen in psychiatric practice. Early studies of divalproex found adverse effects greatest in individuals with plasma levels >100 µg/mL. Reported adverse effects included alopecia, weight gain, tremor, and mental status changes.4
Lamotrigine
Unlike lithium and divalproex, lamotrigine therapeutic drug monitoring is not common. The accepted therapeutic reference range (TRR) for lamotrigine as an AED is 3,000 to 14,000 ng/mL. Unholzer et al5 evaluated the dose and TRR for individuals with bipolar disorder treated with lamotrigine. No statistically significant difference in lamotrigine serum levels was found in responders vs nonresponders.5 Most patients were prescribed ≤200 mg/d; however, some were prescribed higher doses. The maximum dose recommended when lamotrigine is used as an AED is 400 mg/d; however, this study furthered the evidence that lower doses tend to be effective in bipolar disorder.
Continue to: CASE
CASE CONTINUED
It has been 3 months since Mr. B was initiated on lamotrigine, and he has since been titrated to his current, stable dose of 100 mg/d. Mr. B is no longer experiencing the sedation he had with lithium and has the energy to commit to an exercise routine. This has allowed him to lose 15 pounds so far and greatly improve control of his diabetes.
Dosage summary
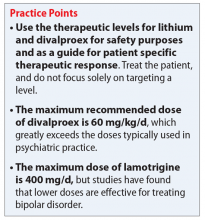
Most available evidence supports dosing lithium and divalproex to effect, typically seen between 0.6 to 1.0 mEq/L and 50 to 125 µg/mL, respectively. Higher plasma levels tend to correspond to more adverse effects and toxicity. Lamotrigine does not have such a narrow therapeutic window. Lamotrigine for psychiatric treatment yields greatest efficacy at approximately 200 mg/d, but doses can be increased if warranted, which could be the case in Mr. B.
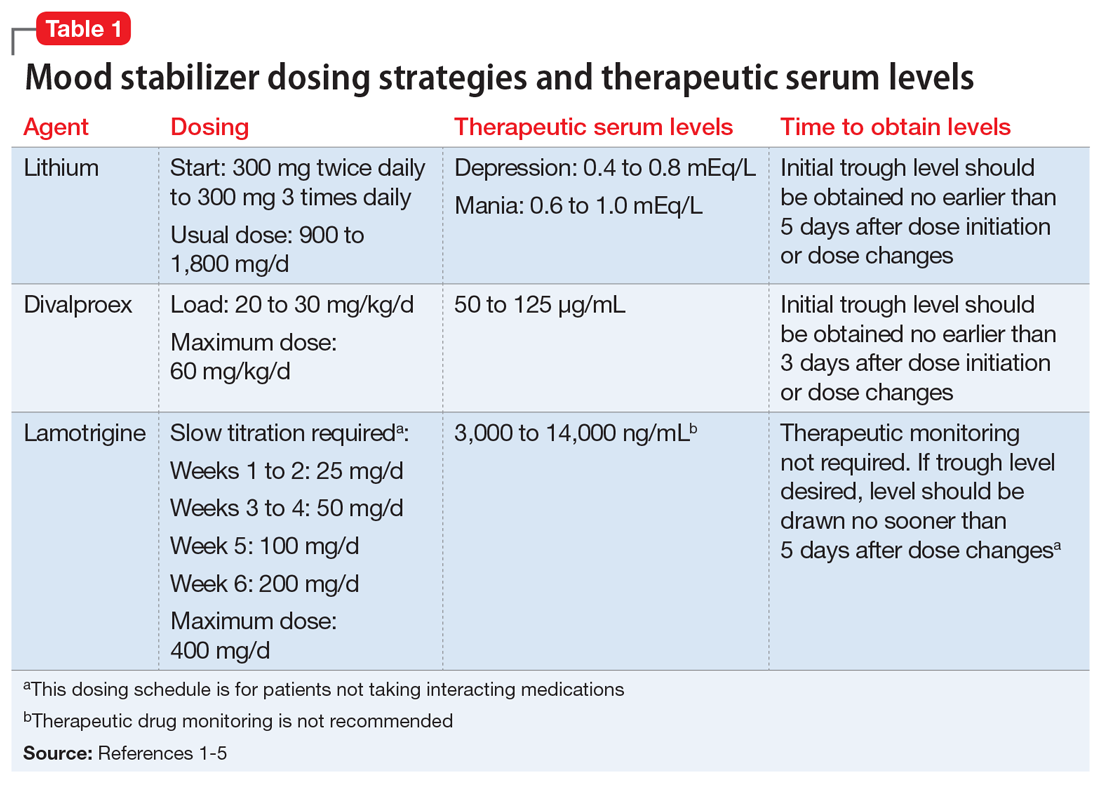
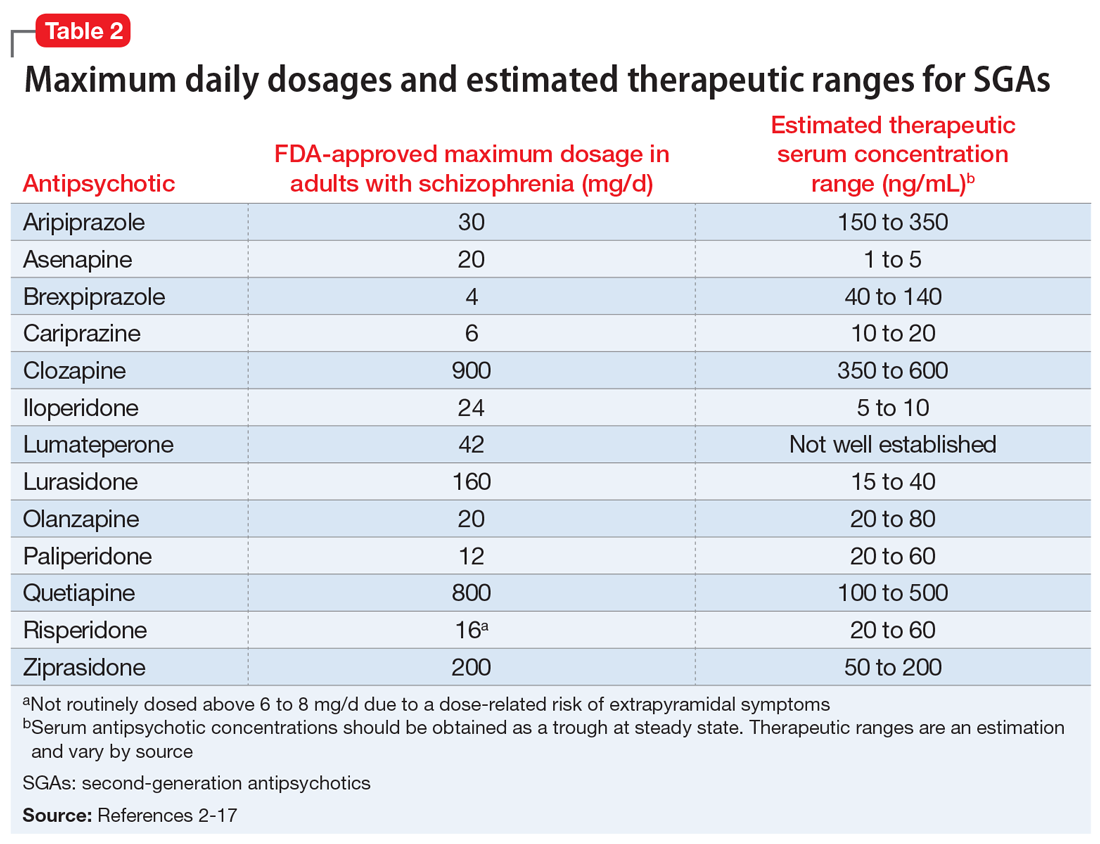
Table 11-5 outlines dosing strategies and therapeutic serum levels for lithium, divalproex, and lamotrigine. Table 22 lists signs and symptoms of lithium toxicity, and Table 31,2 describes strategies for managing adverse effects of lithium and divalproex.
1. Yatham LN, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018;20(2):97-170.
2. Malhi GS, Gershon S, Outhred T. Lithiumeter: version 2.0. Bipolar Disord. 2016;18(8):631-641.
3. Allen MH, Hirschfeld RM, Wozniak PJ, et al. Linear relationship of valproate serum concentration to response and optimal serum levels for acute mania. Am J Psychiatry. 2006;163(2):272-275.
4. Turnbull DM, Rawlins MD, Weightman D, et al. Plasma concentrations of sodium valproate: their clinical value. Ann Neurol. 1983;14(1):38-42.
5. Unholzer S, Haen E. Retrospective analysis of therapeutic drug monitoring data for treatment of bipolar disorder with lamotrigine. Pharmacopsychiatry. 2015;48(7):296.
Mr. B, age 32, was diagnosed with bipolar disorder 10 years ago after experiencing a manic episode that resulted in his first psychiatric hospitalization. He was prescribed quetiapine, 400 mg/d, and remained stable for the next several years. Unfortunately, Mr. B developed significant metabolic adverse effects, including diabetes and a 30-pound weight gain, so he was switched from quetiapine to lithium. Mr. B was unable to tolerate the sedation and cognitive effects of lithium, and the dose could not be titrated to within the therapeutic window. As a result, Mr. B experienced a moderate depressive episode. His current clinician would like to initiate lamotrigine at a starting dose of 25 mg/d. Mr. B has not had a manic episode since the index hospitalization, and this is his first depressive episode.
The term “mood stabilizer” has come to refer to medications that treat a depressive and/or manic episode without inducing the other. In conventional terms, it refers to non-antipsychotic medications such as lithium, divalproex, and lamotrigine. Except for lithium, mood stabilizers are also antiepileptic drugs (AEDs). The role of AEDs for treating psychiatric conditions was discovered after they were originally FDA-approved for treating seizures. Following this discovery, the recommended doses and therapeutic ranges for these agents when applied to psychiatric treatment fell into a gray area.
Every patient is different and requires an individualized treatment plan, but this often leaves the clinician wondering, “How high is too high for this mood stabilizer?” or “My patient is responding well, but could a higher dose be even more effective?” In the case of Mr. B, who has trialed 2 medications with poor tolerability, how high can the lamotrigine dose be titrated to achieve a therapeutic response without adverse effects? The literature on this topic does not provide an exact answer, but does shed some light on key considerations for such decisions.
Which mood stabilizers are recommended?
One of the most recently updated guidelines for the treatment of bipolar disorder was released in 2018 by the Canadian Network for Mood and Anxiety Treatments (CANMAT).1 Lithium, divalproex, and lamotrigine were each recommended as a first-line option for treating bipolar disorder. For lithium and divalproex, the CANMAT guidelines recommend serum level monitoring for efficacy and tolerability; however, they do not recommend serum level monitoring for lamotrigine. Lithium and divalproex each have safety and tolerability concerns, particularly when selected for maintenance therapy, whereas lamotrigine is typically much better tolerated.1 Divalproex and lithium can cause weight gain, gastrointestinal adverse effects (nausea, vomiting, diarrhea), and tremor. Additional tolerability concerns with lithium include renal toxicity, electrocardiogram abnormalities, hypothyroidism, cognitive impairment, and dermatologic reactions. Divalproex can produce greater levels of sedation and may impact reproductive function (oligomenorrhea or hyperandrogenism). One of the most common adverse effects of lamotrigine is a non-serious rash; however, slow dose titration is necessary to decrease the risk of a serious, life-threatening rash such as Stevens-Johnson syndrome.
Lithium
Lithium continues to be regarded as a gold-standard therapy for bipolar disorder. The exact serum levels corresponding to efficacy and tolerability vary. The Lithiumeter: Version 2.0 is a schematic that incorporates the various levels recommended by different clinical guidelines.2 The recommended serum levels range from 0.6 to 1.0 mEq/L for mania and 0.4 to 0.8 mEq/L for depression.2 One of the main issues with lithium dosing is balancing a therapeutic level with tolerability and toxicity. Toxicity may begin when lithium levels exceed 1.2 mEq/L, and levels >2.0 mEq/L can be lethal. Signs of acute toxicity include tremor, headache, arrhythmia, nausea, vomiting, diarrhea, polyuria, and polydipsia. Conversely, chronic lithium use may lead to chronic toxicity as patients age and their physical health changes. Signs of chronic toxicity include ataxia, confusion, renal dysfunction, and tremor. There is no “one size fits all” when it comes to lithium dosing. Individualized dosing is necessary to balance efficacy and tolerability.
Divalproex
Divalproex was initially studied for use as an AED, and its therapeutic levels as an AED are not the same as those indicated for bipolar disorder. Generally, patients with bipolar disorder require a divalproex serum level >50 µg/mL. Ranges closer to 100 µg/mL have been found to be most effective for treating acute mania.3 A loading dose of 20 to 30 mg/kg/d can be administered to help achieve mood stabilization. Again, efficacy must be balanced against toxicity. The maximum dose of divalproex is 60 mg/kg/d, which is rarely seen in psychiatric practice. Early studies of divalproex found adverse effects greatest in individuals with plasma levels >100 µg/mL. Reported adverse effects included alopecia, weight gain, tremor, and mental status changes.4
Lamotrigine
Unlike lithium and divalproex, lamotrigine therapeutic drug monitoring is not common. The accepted therapeutic reference range (TRR) for lamotrigine as an AED is 3,000 to 14,000 ng/mL. Unholzer et al5 evaluated the dose and TRR for individuals with bipolar disorder treated with lamotrigine. No statistically significant difference in lamotrigine serum levels was found in responders vs nonresponders.5 Most patients were prescribed ≤200 mg/d; however, some were prescribed higher doses. The maximum dose recommended when lamotrigine is used as an AED is 400 mg/d; however, this study furthered the evidence that lower doses tend to be effective in bipolar disorder.
Continue to: CASE
CASE CONTINUED
It has been 3 months since Mr. B was initiated on lamotrigine, and he has since been titrated to his current, stable dose of 100 mg/d. Mr. B is no longer experiencing the sedation he had with lithium and has the energy to commit to an exercise routine. This has allowed him to lose 15 pounds so far and greatly improve control of his diabetes.
Dosage summary
Most available evidence supports dosing lithium and divalproex to effect, typically seen between 0.6 to 1.0 mEq/L and 50 to 125 µg/mL, respectively. Higher plasma levels tend to correspond to more adverse effects and toxicity. Lamotrigine does not have such a narrow therapeutic window. Lamotrigine for psychiatric treatment yields greatest efficacy at approximately 200 mg/d, but doses can be increased if warranted, which could be the case in Mr. B.
Table 11-5 outlines dosing strategies and therapeutic serum levels for lithium, divalproex, and lamotrigine. Table 22 lists signs and symptoms of lithium toxicity, and Table 31,2 describes strategies for managing adverse effects of lithium and divalproex.
Mr. B, age 32, was diagnosed with bipolar disorder 10 years ago after experiencing a manic episode that resulted in his first psychiatric hospitalization. He was prescribed quetiapine, 400 mg/d, and remained stable for the next several years. Unfortunately, Mr. B developed significant metabolic adverse effects, including diabetes and a 30-pound weight gain, so he was switched from quetiapine to lithium. Mr. B was unable to tolerate the sedation and cognitive effects of lithium, and the dose could not be titrated to within the therapeutic window. As a result, Mr. B experienced a moderate depressive episode. His current clinician would like to initiate lamotrigine at a starting dose of 25 mg/d. Mr. B has not had a manic episode since the index hospitalization, and this is his first depressive episode.
The term “mood stabilizer” has come to refer to medications that treat a depressive and/or manic episode without inducing the other. In conventional terms, it refers to non-antipsychotic medications such as lithium, divalproex, and lamotrigine. Except for lithium, mood stabilizers are also antiepileptic drugs (AEDs). The role of AEDs for treating psychiatric conditions was discovered after they were originally FDA-approved for treating seizures. Following this discovery, the recommended doses and therapeutic ranges for these agents when applied to psychiatric treatment fell into a gray area.
Every patient is different and requires an individualized treatment plan, but this often leaves the clinician wondering, “How high is too high for this mood stabilizer?” or “My patient is responding well, but could a higher dose be even more effective?” In the case of Mr. B, who has trialed 2 medications with poor tolerability, how high can the lamotrigine dose be titrated to achieve a therapeutic response without adverse effects? The literature on this topic does not provide an exact answer, but does shed some light on key considerations for such decisions.
Which mood stabilizers are recommended?
One of the most recently updated guidelines for the treatment of bipolar disorder was released in 2018 by the Canadian Network for Mood and Anxiety Treatments (CANMAT).1 Lithium, divalproex, and lamotrigine were each recommended as a first-line option for treating bipolar disorder. For lithium and divalproex, the CANMAT guidelines recommend serum level monitoring for efficacy and tolerability; however, they do not recommend serum level monitoring for lamotrigine. Lithium and divalproex each have safety and tolerability concerns, particularly when selected for maintenance therapy, whereas lamotrigine is typically much better tolerated.1 Divalproex and lithium can cause weight gain, gastrointestinal adverse effects (nausea, vomiting, diarrhea), and tremor. Additional tolerability concerns with lithium include renal toxicity, electrocardiogram abnormalities, hypothyroidism, cognitive impairment, and dermatologic reactions. Divalproex can produce greater levels of sedation and may impact reproductive function (oligomenorrhea or hyperandrogenism). One of the most common adverse effects of lamotrigine is a non-serious rash; however, slow dose titration is necessary to decrease the risk of a serious, life-threatening rash such as Stevens-Johnson syndrome.
Lithium
Lithium continues to be regarded as a gold-standard therapy for bipolar disorder. The exact serum levels corresponding to efficacy and tolerability vary. The Lithiumeter: Version 2.0 is a schematic that incorporates the various levels recommended by different clinical guidelines.2 The recommended serum levels range from 0.6 to 1.0 mEq/L for mania and 0.4 to 0.8 mEq/L for depression.2 One of the main issues with lithium dosing is balancing a therapeutic level with tolerability and toxicity. Toxicity may begin when lithium levels exceed 1.2 mEq/L, and levels >2.0 mEq/L can be lethal. Signs of acute toxicity include tremor, headache, arrhythmia, nausea, vomiting, diarrhea, polyuria, and polydipsia. Conversely, chronic lithium use may lead to chronic toxicity as patients age and their physical health changes. Signs of chronic toxicity include ataxia, confusion, renal dysfunction, and tremor. There is no “one size fits all” when it comes to lithium dosing. Individualized dosing is necessary to balance efficacy and tolerability.
Divalproex
Divalproex was initially studied for use as an AED, and its therapeutic levels as an AED are not the same as those indicated for bipolar disorder. Generally, patients with bipolar disorder require a divalproex serum level >50 µg/mL. Ranges closer to 100 µg/mL have been found to be most effective for treating acute mania.3 A loading dose of 20 to 30 mg/kg/d can be administered to help achieve mood stabilization. Again, efficacy must be balanced against toxicity. The maximum dose of divalproex is 60 mg/kg/d, which is rarely seen in psychiatric practice. Early studies of divalproex found adverse effects greatest in individuals with plasma levels >100 µg/mL. Reported adverse effects included alopecia, weight gain, tremor, and mental status changes.4
Lamotrigine
Unlike lithium and divalproex, lamotrigine therapeutic drug monitoring is not common. The accepted therapeutic reference range (TRR) for lamotrigine as an AED is 3,000 to 14,000 ng/mL. Unholzer et al5 evaluated the dose and TRR for individuals with bipolar disorder treated with lamotrigine. No statistically significant difference in lamotrigine serum levels was found in responders vs nonresponders.5 Most patients were prescribed ≤200 mg/d; however, some were prescribed higher doses. The maximum dose recommended when lamotrigine is used as an AED is 400 mg/d; however, this study furthered the evidence that lower doses tend to be effective in bipolar disorder.
Continue to: CASE
CASE CONTINUED
It has been 3 months since Mr. B was initiated on lamotrigine, and he has since been titrated to his current, stable dose of 100 mg/d. Mr. B is no longer experiencing the sedation he had with lithium and has the energy to commit to an exercise routine. This has allowed him to lose 15 pounds so far and greatly improve control of his diabetes.
Dosage summary
Most available evidence supports dosing lithium and divalproex to effect, typically seen between 0.6 to 1.0 mEq/L and 50 to 125 µg/mL, respectively. Higher plasma levels tend to correspond to more adverse effects and toxicity. Lamotrigine does not have such a narrow therapeutic window. Lamotrigine for psychiatric treatment yields greatest efficacy at approximately 200 mg/d, but doses can be increased if warranted, which could be the case in Mr. B.
Table 11-5 outlines dosing strategies and therapeutic serum levels for lithium, divalproex, and lamotrigine. Table 22 lists signs and symptoms of lithium toxicity, and Table 31,2 describes strategies for managing adverse effects of lithium and divalproex.
1. Yatham LN, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018;20(2):97-170.
2. Malhi GS, Gershon S, Outhred T. Lithiumeter: version 2.0. Bipolar Disord. 2016;18(8):631-641.
3. Allen MH, Hirschfeld RM, Wozniak PJ, et al. Linear relationship of valproate serum concentration to response and optimal serum levels for acute mania. Am J Psychiatry. 2006;163(2):272-275.
4. Turnbull DM, Rawlins MD, Weightman D, et al. Plasma concentrations of sodium valproate: their clinical value. Ann Neurol. 1983;14(1):38-42.
5. Unholzer S, Haen E. Retrospective analysis of therapeutic drug monitoring data for treatment of bipolar disorder with lamotrigine. Pharmacopsychiatry. 2015;48(7):296.
1. Yatham LN, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018;20(2):97-170.
2. Malhi GS, Gershon S, Outhred T. Lithiumeter: version 2.0. Bipolar Disord. 2016;18(8):631-641.
3. Allen MH, Hirschfeld RM, Wozniak PJ, et al. Linear relationship of valproate serum concentration to response and optimal serum levels for acute mania. Am J Psychiatry. 2006;163(2):272-275.
4. Turnbull DM, Rawlins MD, Weightman D, et al. Plasma concentrations of sodium valproate: their clinical value. Ann Neurol. 1983;14(1):38-42.
5. Unholzer S, Haen E. Retrospective analysis of therapeutic drug monitoring data for treatment of bipolar disorder with lamotrigine. Pharmacopsychiatry. 2015;48(7):296.
Efficacy and safety of high-dose antipsychotic therapy
Mr. K, age 21, is admitted to the psychiatry unit with agitation, disorganized behavior, and paranoia. Upon presentation, he has no known medical history or current medications. He is diagnosed with schizophrenia and subsequently tolerates but does not respond to adequate durations of treatment with fluphenazine, 20 mg/d; aripiprazole, 30 mg/d; and risperidone, 6 mg/d. Medication adherence is verified, but Mr. K is reluctant to try a fourth antipsychotic. The treatment team suspects that Mr. K may be a cytochrome P450 (CYP) 2D6 ultra-rapid metabolizer, so they obtain a serum risperidone level. The serum risperidone concentration is subtherapeutic (10 ng/mL). What should be considered next?
Several factors must be considered when a patient with psychosis does not experience significant symptomatic improvement with an adequate antipsychotic trial. This article focuses on high-dose second-generation antipsychotic (SGA) therapy in adults with psychosis. “High-dose” antipsychotic therapy is dosing that exceeds the standard maximum dosage for a given antipsychotic. Existing evidence on the use of high-dose SGAs consists of open-label studies and case reports, as well as a handful of randomized controlled trials (RCTs) with small sample sizes and high dropout rates. In some studies, the use of concomitant interventions (eg, duplicate antipsychotic therapy) limit the interpretation of data. High-dose first-generation antipsychotic therapy is discouraged because of a heightened risk of extrapyramidal symptoms (EPS).
Steps to take before increasing the dose
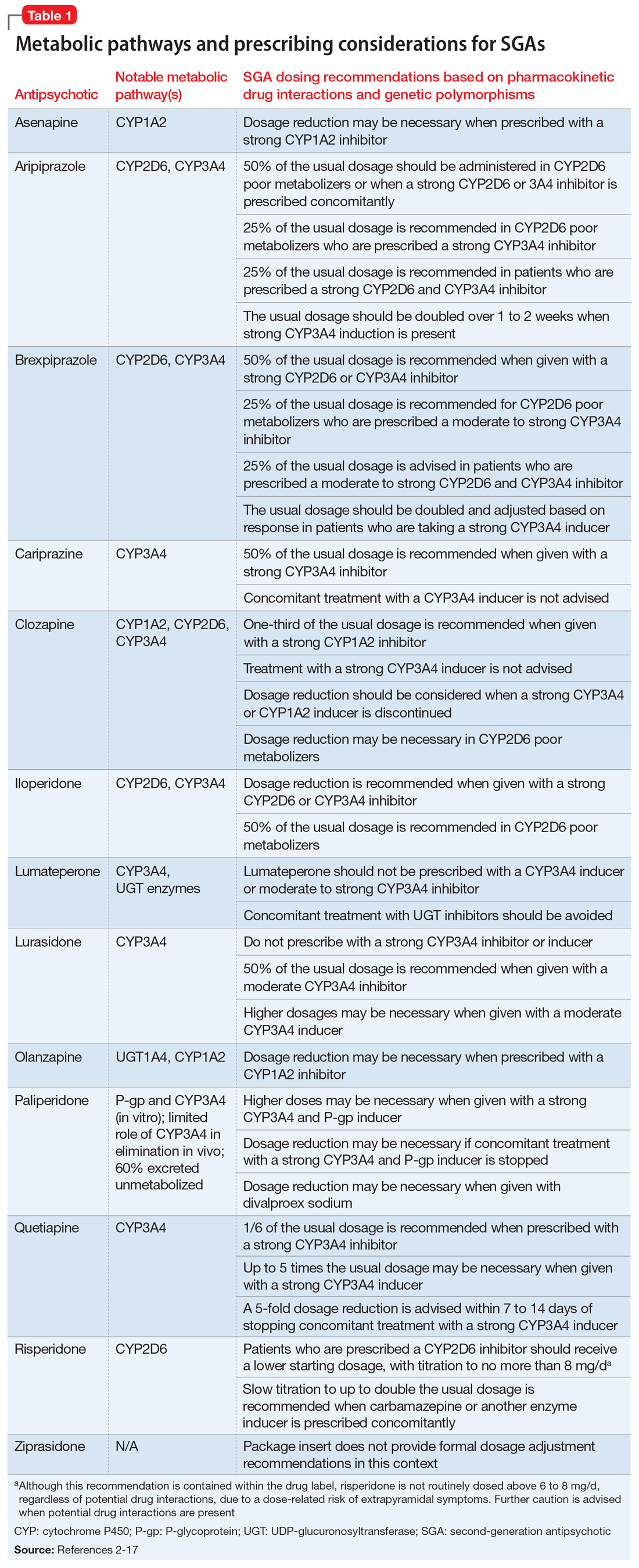
When considering prescribing high-dose antipsychotic therapy, first confirm that the patient has been adherent to the current medication regimen. Also, screen for factors that might impair drug absorption, such as bariatric surgery or noncompliance with administration precautions.1 For example, administration of lurasidone with less than 350 calories may considerably decrease absorption.2 Dosage requirements may vary based on ethnicity, gender, CYP polymorphisms, and pharmacokinetic drug interactions (Table 12-17).1,18,19 Causes of inadequate efficacy should be addressed before considering the use of high-dose antipsychotic therapy.1 Under certain circumstances, serum drug concentrations may be used to guide antipsychotic dosing (Table 22-17). Inadequate response despite a therapeutic serum concentration may indicate pharmacodynamic failure.1 Inadequate response in the context of subtherapeutic serum concentrations, good medication adherence, and compliance to administration precautions may be indicative of a genetic polymorphism or drug interaction.1 Changes in antipsychotic dosing or selection may be warranted, depending on associated risks and benefits.
SGAs and high-dose administration
The SGA with the greatest evidence for high-dose administration is olanzapine, which is similar in structure and receptor pharmacology to clozapine.20,21 The use of high-dose olanzapine is controversial. High-dose olanzapine has been compared to clozapine in patients with treatment-resistant schizophrenia (TRS) and schizoaffective disorder. Meltzer et al22 reported similar efficacy with clozapine, 300 to 900 mg/d, and olanzapine, 25 to 45 mg/d. In this study, high-dose olanzapine caused more weight gain when compared to clozapine. Olanzapine dosages of up to 100 mg/d have been prescribed for TRS; however, this is not common practice.23 A study comparing 10, 20, and 40 mg/d in patients with non-TRS or schizoaffective disorder showed no advantage with higher dosages.24
There is limited data on high-dose treatment with other SGAs.17 Orthostasis may limit iloperidone’s safety at high doses, and single doses of asenapine should not exceed 10 mg.25 Limited sublingual surface area and saliva saturation result in decreased bioavailability with higher asenapine doses.25,26 In a small RCT of patients with stable schizophrenia or schizoaffective disorder, aripiprazole was relatively well-tolerated up to 75 mg/d, whereas akathisia and tachycardia occurred with 90 mg/d.27 Case reports have documented successful treatment with aripiprazole, 60 to 75 mg/d; however, dizziness and worsening psychosis, agitation, and confusion have been observed.28-31
There is a paucity of data on high-dose risperidone and paliperidone, possibly due to their potent dopamine-2 (D2) receptor antagonism and dose-related risk of EPS.1 At risperidone dosages >6 mg/d, the balance between D2 and serotonin-2A (5-HT2A) receptor potency is lost, which increases the potential for EPS.32 In one RCT, long-acting injectable (LAI) risperidone, up to 100 mg biweekly, was well-tolerated but no more effective for TRS than 50 mg biweekly.33 A case report suggested improvement of TRS in a patient administered risperidone LAI, 75 mg vs 37.5 mg biweekly, but it is unclear if a 50-mg dosage was tried.34 Another case report documented improvement in schizophrenia symptoms with risperidone LAI, 125 mg biweekly; however, anticholinergic therapy was required for EPS.35
Dose-dependent adverse effects, including EPS, sedation, anticholinergic effects, orthostasis, hyperprolactinemia, and QTc prolongation, may limit the safety of high-dose antipsychotic therapy.1,20,36 Two studies showed no correlation between QTc prolongation and ziprasidone dosages of up to 320 mg/d for psychosis.37,38 QTc prolongation was more likely at higher ziprasidone concentrations.37 Higher concentrations, but not higher dosages, also trended toward improvement in positive symptoms, and concentrations >100 ng/mL were associated with more negative symptoms.37 A case report described improvement in positive symptoms of schizoaffective disorder with ziprasidone, 320 mg/d, but activation, hostility, and depression worsened.39
Continue to: Compared with other antipsychotics...
Compared with other antipsychotics, high-dose clozapine and quetiapine may be less likely to cause EPS due to lower D2 receptor occupancies.40 Nevertheless, increased activity at other postsynaptic receptors may lead to constipation, metabolic effects, and sedation.1,41,42 Case reports suggest efficacy with quetiapine, 1,200 to 2,400 mg/d, vs lower dosages for patients with TRS.43,44 However, RCTs of quetiapine, 600 and 800 mg/d vs 1,200 mg/d, have not demonstrated an efficacy advantage with high-dose treatment in patients with schizophrenia or schizoaffective disorder.41,45 High-dose quetiapine has also resulted in photopsia, cardiotoxicity, orthostasis, dysphagia, and sedation.43,46,47
Proceed with caution
In light of safety concerns and a lack of high-quality evidence for high-dose antipsychotic therapy, alternative solutions for inadequate response to treatment should be considered. Underlying causes of poor response should be addressed, and alternative antipsychotics should be utilized, when appropriate. A clozapine trial remains first-line for TRS. Olanzapine may be the best-supported high-dose antipsychotic alternative when clozapine is not an option. High antipsychotic dosages are not well-studied in patients with genetic polymorphisms or unavoidable drug interactions. Serum antipsychotic concentrations may facilitate dosing in these patients.
If high-dose antipsychotic therapy is deemed necessary, its ongoing appropriateness should be continually re-evaluated. Higher antipsychotic dosages and D2 receptor occupancies may be required to manage acute psychosis, but efficacy may be maintained and adverse effects limited with the use of lower dosages during maintenance treatment.48,49 Long-term treatment with high-dose antipsychotic therapy should be avoided, when possible. If high-dose antipsychotic therapy is prescribed, the rationale should be well-documented. Dosage adjustments should not be made until steady state is reached on a given dosage. Electrocardiograms should be obtained at baseline, steady state, and routinely thereafter.3,20 Tolerability should be assessed regularly, and screening for drug interactions should be conducted when new medications are initiated.
Case CONTINUED
Because Mr. K’s serum risperidone level is subtherapeutic (10 ng/mL), his risperidone dosage is cautiously titrated to 10 mg/d, divided (serum concentration: 22 ng/mL). Mr. K develops mild orthostasis but denies other adverse effects. His psychotic symptoms resolve, and he is discharged with education on nonpharmacologic management of orthostasis. The rationale for high-dose risperidone is relayed to his outpatient psychiatrist, as well as a recommendation to monitor Mr. K closely for continued efficacy and tolerability.
Related Resource
- Barnes TRE, Drake R, Paton C, et al. Evidence-based guidelines for the pharmacological treatment of schizophrenia: updated recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2020;34(1):3-78.
Drug Brand Names
Aripiprazole • Abilify
Asenapine • Saphris
Brexpiprazole • Rexulti
Cariprazine • Vraylar
Clozapine • Clozaril
Iloperidone • Fanapt
Lumateperone • Caplyta
Lurasidone • Latuda
Olanzapine • Zyprexa
Paliperidone • Invega
Quetiapine • Seroquel
Risperidone • Risperdal
Risperidone long-acting injection • Risperdal Consta
Ziprasidone • Geodon
1. Morrissette DA, Stahl SM. Treating the violence patient with psychosis or impulsivity utilizing antipsychotic polypharmacy and high-dose monotherapy. CNS Spectrums. 2014;19(5):439-448.
2. Latuda [package insert]. Marlborough, MA: Sunovion Pharmaceuticals Inc.; 2019.
3. Taylor D, Paton C, Kapur S. The Maudsley prescribing guidelines in psychiatry. 12th ed. Wiley Blackwell; 2015.
4. Vyas P, Hwang BJ, Brasic JR. An evaluation of lumateperone tosylate for the treatment of schizophrenia. Expert Opin Pharmacother. 2020;21(2):139-145.
5. Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1-02):9-62.
6. Saphris [package insert]. Irvine, CA: Allergan USA, Inc; 2017.
7. Abilify [package insert]. Tokyo, Japan: Otsuka America Pharmaceutical, Inc.; 2014.
8. Rexulti [package insert]. Rockville, MD: Otsuka America Pharmaceutical, Inc.; 2020.
9. Vraylar [package insert]. Madison, NJ: Allergan USA, Inc.; 2019.
10. Clozaril [package insert]. Rosemont, PA: Novartis Pharmaceuticals Corporation; 2017.
11. Fanapt [package insert]. Washington, DC: Vanda Pharmaceuticals Inc.; 2016.
12. Caplyta [package insert]. New York, NY: Intra-Cellular Therapies, Inc.; 2019.
13. Zyprexa [package insert]. Indianapolis, IN: Lilly USA, LLC.; 2020.
14. Invega [package insert]. Titusville, NJ: Janssen Pharmaceutical Companies; 2019.
15. Seroquel [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2016.
16. Risperdal [package insert]. Titusville, NJ: Janssen Pharmaceutical Companies; 2020.
17. Geodon [package insert]. New York, NY: Pfizer Inc.; 2020.
18. Chaudhry IB, Neelam K, Duddu V, et al. Ethnicity and psychopharmacology. J Psychopharmacol. 2008;22(6):673-680.
19. Seeman MV. Men and women respond differently to antipsychotic drugs. Neuropharmacology. 2020;163:107631. doi: 10.1016/j.neuropharm.2019.05.008
20. Barnes TRE, Drake R, Paton C, et al. Evidence-based guidelines for the pharmacological treatment of schizophrenia: updated recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2020;34(1):3-78.
21. Citrome L, McEvoy JP, Todtenkopf MS, et al. A commentary on the efficacy of olanzapine for the treatment of schizophrenia: the past, present, and future. Neuropsych Dis Treat. 2019;15:2559-2569.
22. Meltzer HY, Bobo WV, Ajanta R, et al. A randomized, double-blind comparison of clozapine and high-dose olanzapine in treatment-resistant patients with schizophrenia. J Clin Psychiatry. 2008;69(2):274-285.
23. Batail JM, Langree B, Robert G, et al. Use of very-high-dose olanzapine in treatment-resistant schizophrenia. Schizophr Res. 2014;159(2-3):411-414.
24. Kinon BJ, Volavka J, Stauffer V, et al. Standard and higher dose of olanzapine in patients with schizophrenia or schizoaffective disorder. J Clin Psychopharmacol. 2008;28(4):392-400.
25. Stahl SM. Stahl’s essential psychopharmacology prescriber’s guide. 6th ed. Cambridge University Press; 2017.
26. Bartlett JA, van der Voort Maarschalk K. Understanding the oral mucosal absorption and resulting clinical pharmacokinetics of asenapine. AAPS PharmSciTech. 2012;13(4):1110-1115.
27. Auby P, Saha A, Ali M, et al. Safety and tolerability of aripiprazole at doses higher than 30 mg. Eur Neuropsychopharm. 2002;12(3):288.
28. Chavez B, Poveda R. Efficacy with high-dose aripiprazole after olanzapine-related metabolic disturbances. Ann Pharmacother. 2006;40(12):2265-2268.
29. Duggal HS, Mendhekar DN. High-dose aripiprazole in treatment-resistant schizophrenia. J Clin Psychiatry. 2006;67(4):674-675.
30. Thone J. Worsened agitation and confusion in schizophrenia subsequent to high-dose aripiprazole. J Neuropsychiatry Clin Neurosci. 2007;19(4):481-482.
31. Saatcioglu O, Gumus S, Kamberyan K, et al. Efficacy of high-dose aripiprazole for treatment-resistant schizoaffective disorder: a case report. Psychopharmacol Bull. 2010;43(4):70-72.
32. Thomson SR, Chogtu B, Bhattacharjee D, et al. Extrapyramidal symptoms probably related to risperidone treatment: a case series. Ann Neurosci. 2017;24(3):155-163.
33. Meltzer HY, Lindenmayer JP, Kwentus J, et al. A six month randomized controlled trial of long acting injectable risperidone 50 and 100 mg in treatment resistant schizophrenia. Schizophr Res. 2014;154(1-3):14-22.
34. Hou Y, Lai C. The response of psychotic symptoms in a patient with resistant schizophrenia under treatment of high-dose risperidone long-acting injection. J Neuropsychiatry Clin Neurosci. 2014;26(3):E16-E17. doi: 10.1176/appi.neuropsych.13070150
35. Albrecht A, Morena PG, Baumann P, et al. High dose of depot risperidone in a nonresponder schizophrenic patient. J Clin Psychopharmacol. 2004;24(6):673-674.
36. Mace S, Taylor D. Reducing the rates of prescribing high-dose antipsychotics and polypharmacy on psychiatric inpatient and intensive care units: results of a 6-year quality improvement programme. Ther Adv Psychopharmacol. 2015;5(1):4-12.
37. Goff DC, McEvoy JP, Citrome L, et al. High-dose oral ziprasidone versus conventional dosing in schizophrenia patients with residual symptoms. J Clin Psychopharmacol. 2013;33:485-490.
38. Levy WO, Robichaux-Keene NR, Nunez C. No significant QTc interval changes with high-dose ziprasidone: a case series. J Psychiatr Pract. 2004;10(4):227-232.
39. Kaushik S, Maccabee N, Kaushik S, et al. Activation induced by high-dose ziprasidone: a case report. J Clin Psychiatry. 2009;70(9):1326-1327.
40. Seeman P. Targeting the dopamine D2 receptor in schizophrenia. Expert Opin Ther Targets. 2006;10(4):515-531.
41. Honer WG, MacEwan W, Gendron A, et al. A randomized, double-blind, placebo-controlled study of safety and tolerability of high-dose quetiapine in patients with persistent symptoms of schizophrenia or schizoaffective disorder. J Clin Psychiatry. 2012;73(1):13-20.
42. Sokolski KN, Brown BJ, Meldon M. Urinary retention following repeated high-dose quetiapine. Ann Pharmacother. 2004;38(5):899-890.
43. Chandrappa P, Ho L. Case reports of patients with treatment-resistant schizophrenia and related psychotic disorders intolerant to clozapine responding to high doses of quetiapine. Ther Adv Psychopharmacol. 2012;2(5):207-209.
44. Pierre JM, Wirshing DA, Wirshing WC, et al. High-dose quetiapine in treatment refractory schizophrenia. Schizophr Res. 2005;73:373-375.
45. Lindenmyer JP, Citrome L, Khan A, et al. A randomized, double-blind parallel-group, fixed-dose, clinical trial of quetiapine at 600 vs. 1200 mg/d for patients with treatment-resistant schizophrenia or schizoaffective disorder. J Clin Psychopharmacol. 2011;31(2):160-168.
46. Hazra M, Culo S, Mamo D. High-dose quetiapine and photopsia. J Clin Psychopharmacol. 2006;26(5):546-547.
47. Smolders DME, Smolders WAP. Case report and review of the literature: cardiomyopathy in a young woman on high-dose quetiapine. Cardiovasc Toxicol. 2017;17(4):478-481.
48. Takeuchi H, Suzuki T, Bies RR, et al. Dose reduction of risperidone and olanzapine and estimated D2 receptor occupancy in stable patients with schizophrenia: findings from an open-label, randomized, controlled study. J Clin Psychiatry. 2014;75(11):1209-1214.
49. Kumar V, Rao NP, Narasimha V, et al. Antipsychotic dose in maintenance treatment of schizophrenia: a retrospective study. Psychiatry Res. 2016;245:311-316.
Mr. K, age 21, is admitted to the psychiatry unit with agitation, disorganized behavior, and paranoia. Upon presentation, he has no known medical history or current medications. He is diagnosed with schizophrenia and subsequently tolerates but does not respond to adequate durations of treatment with fluphenazine, 20 mg/d; aripiprazole, 30 mg/d; and risperidone, 6 mg/d. Medication adherence is verified, but Mr. K is reluctant to try a fourth antipsychotic. The treatment team suspects that Mr. K may be a cytochrome P450 (CYP) 2D6 ultra-rapid metabolizer, so they obtain a serum risperidone level. The serum risperidone concentration is subtherapeutic (10 ng/mL). What should be considered next?
Several factors must be considered when a patient with psychosis does not experience significant symptomatic improvement with an adequate antipsychotic trial. This article focuses on high-dose second-generation antipsychotic (SGA) therapy in adults with psychosis. “High-dose” antipsychotic therapy is dosing that exceeds the standard maximum dosage for a given antipsychotic. Existing evidence on the use of high-dose SGAs consists of open-label studies and case reports, as well as a handful of randomized controlled trials (RCTs) with small sample sizes and high dropout rates. In some studies, the use of concomitant interventions (eg, duplicate antipsychotic therapy) limit the interpretation of data. High-dose first-generation antipsychotic therapy is discouraged because of a heightened risk of extrapyramidal symptoms (EPS).
Steps to take before increasing the dose
When considering prescribing high-dose antipsychotic therapy, first confirm that the patient has been adherent to the current medication regimen. Also, screen for factors that might impair drug absorption, such as bariatric surgery or noncompliance with administration precautions.1 For example, administration of lurasidone with less than 350 calories may considerably decrease absorption.2 Dosage requirements may vary based on ethnicity, gender, CYP polymorphisms, and pharmacokinetic drug interactions (Table 12-17).1,18,19 Causes of inadequate efficacy should be addressed before considering the use of high-dose antipsychotic therapy.1 Under certain circumstances, serum drug concentrations may be used to guide antipsychotic dosing (Table 22-17). Inadequate response despite a therapeutic serum concentration may indicate pharmacodynamic failure.1 Inadequate response in the context of subtherapeutic serum concentrations, good medication adherence, and compliance to administration precautions may be indicative of a genetic polymorphism or drug interaction.1 Changes in antipsychotic dosing or selection may be warranted, depending on associated risks and benefits.
SGAs and high-dose administration
The SGA with the greatest evidence for high-dose administration is olanzapine, which is similar in structure and receptor pharmacology to clozapine.20,21 The use of high-dose olanzapine is controversial. High-dose olanzapine has been compared to clozapine in patients with treatment-resistant schizophrenia (TRS) and schizoaffective disorder. Meltzer et al22 reported similar efficacy with clozapine, 300 to 900 mg/d, and olanzapine, 25 to 45 mg/d. In this study, high-dose olanzapine caused more weight gain when compared to clozapine. Olanzapine dosages of up to 100 mg/d have been prescribed for TRS; however, this is not common practice.23 A study comparing 10, 20, and 40 mg/d in patients with non-TRS or schizoaffective disorder showed no advantage with higher dosages.24
There is limited data on high-dose treatment with other SGAs.17 Orthostasis may limit iloperidone’s safety at high doses, and single doses of asenapine should not exceed 10 mg.25 Limited sublingual surface area and saliva saturation result in decreased bioavailability with higher asenapine doses.25,26 In a small RCT of patients with stable schizophrenia or schizoaffective disorder, aripiprazole was relatively well-tolerated up to 75 mg/d, whereas akathisia and tachycardia occurred with 90 mg/d.27 Case reports have documented successful treatment with aripiprazole, 60 to 75 mg/d; however, dizziness and worsening psychosis, agitation, and confusion have been observed.28-31
There is a paucity of data on high-dose risperidone and paliperidone, possibly due to their potent dopamine-2 (D2) receptor antagonism and dose-related risk of EPS.1 At risperidone dosages >6 mg/d, the balance between D2 and serotonin-2A (5-HT2A) receptor potency is lost, which increases the potential for EPS.32 In one RCT, long-acting injectable (LAI) risperidone, up to 100 mg biweekly, was well-tolerated but no more effective for TRS than 50 mg biweekly.33 A case report suggested improvement of TRS in a patient administered risperidone LAI, 75 mg vs 37.5 mg biweekly, but it is unclear if a 50-mg dosage was tried.34 Another case report documented improvement in schizophrenia symptoms with risperidone LAI, 125 mg biweekly; however, anticholinergic therapy was required for EPS.35
Dose-dependent adverse effects, including EPS, sedation, anticholinergic effects, orthostasis, hyperprolactinemia, and QTc prolongation, may limit the safety of high-dose antipsychotic therapy.1,20,36 Two studies showed no correlation between QTc prolongation and ziprasidone dosages of up to 320 mg/d for psychosis.37,38 QTc prolongation was more likely at higher ziprasidone concentrations.37 Higher concentrations, but not higher dosages, also trended toward improvement in positive symptoms, and concentrations >100 ng/mL were associated with more negative symptoms.37 A case report described improvement in positive symptoms of schizoaffective disorder with ziprasidone, 320 mg/d, but activation, hostility, and depression worsened.39
Continue to: Compared with other antipsychotics...
Compared with other antipsychotics, high-dose clozapine and quetiapine may be less likely to cause EPS due to lower D2 receptor occupancies.40 Nevertheless, increased activity at other postsynaptic receptors may lead to constipation, metabolic effects, and sedation.1,41,42 Case reports suggest efficacy with quetiapine, 1,200 to 2,400 mg/d, vs lower dosages for patients with TRS.43,44 However, RCTs of quetiapine, 600 and 800 mg/d vs 1,200 mg/d, have not demonstrated an efficacy advantage with high-dose treatment in patients with schizophrenia or schizoaffective disorder.41,45 High-dose quetiapine has also resulted in photopsia, cardiotoxicity, orthostasis, dysphagia, and sedation.43,46,47
Proceed with caution
In light of safety concerns and a lack of high-quality evidence for high-dose antipsychotic therapy, alternative solutions for inadequate response to treatment should be considered. Underlying causes of poor response should be addressed, and alternative antipsychotics should be utilized, when appropriate. A clozapine trial remains first-line for TRS. Olanzapine may be the best-supported high-dose antipsychotic alternative when clozapine is not an option. High antipsychotic dosages are not well-studied in patients with genetic polymorphisms or unavoidable drug interactions. Serum antipsychotic concentrations may facilitate dosing in these patients.
If high-dose antipsychotic therapy is deemed necessary, its ongoing appropriateness should be continually re-evaluated. Higher antipsychotic dosages and D2 receptor occupancies may be required to manage acute psychosis, but efficacy may be maintained and adverse effects limited with the use of lower dosages during maintenance treatment.48,49 Long-term treatment with high-dose antipsychotic therapy should be avoided, when possible. If high-dose antipsychotic therapy is prescribed, the rationale should be well-documented. Dosage adjustments should not be made until steady state is reached on a given dosage. Electrocardiograms should be obtained at baseline, steady state, and routinely thereafter.3,20 Tolerability should be assessed regularly, and screening for drug interactions should be conducted when new medications are initiated.
Case CONTINUED
Because Mr. K’s serum risperidone level is subtherapeutic (10 ng/mL), his risperidone dosage is cautiously titrated to 10 mg/d, divided (serum concentration: 22 ng/mL). Mr. K develops mild orthostasis but denies other adverse effects. His psychotic symptoms resolve, and he is discharged with education on nonpharmacologic management of orthostasis. The rationale for high-dose risperidone is relayed to his outpatient psychiatrist, as well as a recommendation to monitor Mr. K closely for continued efficacy and tolerability.
Related Resource
- Barnes TRE, Drake R, Paton C, et al. Evidence-based guidelines for the pharmacological treatment of schizophrenia: updated recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2020;34(1):3-78.
Drug Brand Names
Aripiprazole • Abilify
Asenapine • Saphris
Brexpiprazole • Rexulti
Cariprazine • Vraylar
Clozapine • Clozaril
Iloperidone • Fanapt
Lumateperone • Caplyta
Lurasidone • Latuda
Olanzapine • Zyprexa
Paliperidone • Invega
Quetiapine • Seroquel
Risperidone • Risperdal
Risperidone long-acting injection • Risperdal Consta
Ziprasidone • Geodon
Mr. K, age 21, is admitted to the psychiatry unit with agitation, disorganized behavior, and paranoia. Upon presentation, he has no known medical history or current medications. He is diagnosed with schizophrenia and subsequently tolerates but does not respond to adequate durations of treatment with fluphenazine, 20 mg/d; aripiprazole, 30 mg/d; and risperidone, 6 mg/d. Medication adherence is verified, but Mr. K is reluctant to try a fourth antipsychotic. The treatment team suspects that Mr. K may be a cytochrome P450 (CYP) 2D6 ultra-rapid metabolizer, so they obtain a serum risperidone level. The serum risperidone concentration is subtherapeutic (10 ng/mL). What should be considered next?
Several factors must be considered when a patient with psychosis does not experience significant symptomatic improvement with an adequate antipsychotic trial. This article focuses on high-dose second-generation antipsychotic (SGA) therapy in adults with psychosis. “High-dose” antipsychotic therapy is dosing that exceeds the standard maximum dosage for a given antipsychotic. Existing evidence on the use of high-dose SGAs consists of open-label studies and case reports, as well as a handful of randomized controlled trials (RCTs) with small sample sizes and high dropout rates. In some studies, the use of concomitant interventions (eg, duplicate antipsychotic therapy) limit the interpretation of data. High-dose first-generation antipsychotic therapy is discouraged because of a heightened risk of extrapyramidal symptoms (EPS).
Steps to take before increasing the dose
When considering prescribing high-dose antipsychotic therapy, first confirm that the patient has been adherent to the current medication regimen. Also, screen for factors that might impair drug absorption, such as bariatric surgery or noncompliance with administration precautions.1 For example, administration of lurasidone with less than 350 calories may considerably decrease absorption.2 Dosage requirements may vary based on ethnicity, gender, CYP polymorphisms, and pharmacokinetic drug interactions (Table 12-17).1,18,19 Causes of inadequate efficacy should be addressed before considering the use of high-dose antipsychotic therapy.1 Under certain circumstances, serum drug concentrations may be used to guide antipsychotic dosing (Table 22-17). Inadequate response despite a therapeutic serum concentration may indicate pharmacodynamic failure.1 Inadequate response in the context of subtherapeutic serum concentrations, good medication adherence, and compliance to administration precautions may be indicative of a genetic polymorphism or drug interaction.1 Changes in antipsychotic dosing or selection may be warranted, depending on associated risks and benefits.
SGAs and high-dose administration
The SGA with the greatest evidence for high-dose administration is olanzapine, which is similar in structure and receptor pharmacology to clozapine.20,21 The use of high-dose olanzapine is controversial. High-dose olanzapine has been compared to clozapine in patients with treatment-resistant schizophrenia (TRS) and schizoaffective disorder. Meltzer et al22 reported similar efficacy with clozapine, 300 to 900 mg/d, and olanzapine, 25 to 45 mg/d. In this study, high-dose olanzapine caused more weight gain when compared to clozapine. Olanzapine dosages of up to 100 mg/d have been prescribed for TRS; however, this is not common practice.23 A study comparing 10, 20, and 40 mg/d in patients with non-TRS or schizoaffective disorder showed no advantage with higher dosages.24
There is limited data on high-dose treatment with other SGAs.17 Orthostasis may limit iloperidone’s safety at high doses, and single doses of asenapine should not exceed 10 mg.25 Limited sublingual surface area and saliva saturation result in decreased bioavailability with higher asenapine doses.25,26 In a small RCT of patients with stable schizophrenia or schizoaffective disorder, aripiprazole was relatively well-tolerated up to 75 mg/d, whereas akathisia and tachycardia occurred with 90 mg/d.27 Case reports have documented successful treatment with aripiprazole, 60 to 75 mg/d; however, dizziness and worsening psychosis, agitation, and confusion have been observed.28-31
There is a paucity of data on high-dose risperidone and paliperidone, possibly due to their potent dopamine-2 (D2) receptor antagonism and dose-related risk of EPS.1 At risperidone dosages >6 mg/d, the balance between D2 and serotonin-2A (5-HT2A) receptor potency is lost, which increases the potential for EPS.32 In one RCT, long-acting injectable (LAI) risperidone, up to 100 mg biweekly, was well-tolerated but no more effective for TRS than 50 mg biweekly.33 A case report suggested improvement of TRS in a patient administered risperidone LAI, 75 mg vs 37.5 mg biweekly, but it is unclear if a 50-mg dosage was tried.34 Another case report documented improvement in schizophrenia symptoms with risperidone LAI, 125 mg biweekly; however, anticholinergic therapy was required for EPS.35
Dose-dependent adverse effects, including EPS, sedation, anticholinergic effects, orthostasis, hyperprolactinemia, and QTc prolongation, may limit the safety of high-dose antipsychotic therapy.1,20,36 Two studies showed no correlation between QTc prolongation and ziprasidone dosages of up to 320 mg/d for psychosis.37,38 QTc prolongation was more likely at higher ziprasidone concentrations.37 Higher concentrations, but not higher dosages, also trended toward improvement in positive symptoms, and concentrations >100 ng/mL were associated with more negative symptoms.37 A case report described improvement in positive symptoms of schizoaffective disorder with ziprasidone, 320 mg/d, but activation, hostility, and depression worsened.39
Continue to: Compared with other antipsychotics...
Compared with other antipsychotics, high-dose clozapine and quetiapine may be less likely to cause EPS due to lower D2 receptor occupancies.40 Nevertheless, increased activity at other postsynaptic receptors may lead to constipation, metabolic effects, and sedation.1,41,42 Case reports suggest efficacy with quetiapine, 1,200 to 2,400 mg/d, vs lower dosages for patients with TRS.43,44 However, RCTs of quetiapine, 600 and 800 mg/d vs 1,200 mg/d, have not demonstrated an efficacy advantage with high-dose treatment in patients with schizophrenia or schizoaffective disorder.41,45 High-dose quetiapine has also resulted in photopsia, cardiotoxicity, orthostasis, dysphagia, and sedation.43,46,47
Proceed with caution
In light of safety concerns and a lack of high-quality evidence for high-dose antipsychotic therapy, alternative solutions for inadequate response to treatment should be considered. Underlying causes of poor response should be addressed, and alternative antipsychotics should be utilized, when appropriate. A clozapine trial remains first-line for TRS. Olanzapine may be the best-supported high-dose antipsychotic alternative when clozapine is not an option. High antipsychotic dosages are not well-studied in patients with genetic polymorphisms or unavoidable drug interactions. Serum antipsychotic concentrations may facilitate dosing in these patients.
If high-dose antipsychotic therapy is deemed necessary, its ongoing appropriateness should be continually re-evaluated. Higher antipsychotic dosages and D2 receptor occupancies may be required to manage acute psychosis, but efficacy may be maintained and adverse effects limited with the use of lower dosages during maintenance treatment.48,49 Long-term treatment with high-dose antipsychotic therapy should be avoided, when possible. If high-dose antipsychotic therapy is prescribed, the rationale should be well-documented. Dosage adjustments should not be made until steady state is reached on a given dosage. Electrocardiograms should be obtained at baseline, steady state, and routinely thereafter.3,20 Tolerability should be assessed regularly, and screening for drug interactions should be conducted when new medications are initiated.
Case CONTINUED
Because Mr. K’s serum risperidone level is subtherapeutic (10 ng/mL), his risperidone dosage is cautiously titrated to 10 mg/d, divided (serum concentration: 22 ng/mL). Mr. K develops mild orthostasis but denies other adverse effects. His psychotic symptoms resolve, and he is discharged with education on nonpharmacologic management of orthostasis. The rationale for high-dose risperidone is relayed to his outpatient psychiatrist, as well as a recommendation to monitor Mr. K closely for continued efficacy and tolerability.
Related Resource
- Barnes TRE, Drake R, Paton C, et al. Evidence-based guidelines for the pharmacological treatment of schizophrenia: updated recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2020;34(1):3-78.
Drug Brand Names
Aripiprazole • Abilify
Asenapine • Saphris
Brexpiprazole • Rexulti
Cariprazine • Vraylar
Clozapine • Clozaril
Iloperidone • Fanapt
Lumateperone • Caplyta
Lurasidone • Latuda
Olanzapine • Zyprexa
Paliperidone • Invega
Quetiapine • Seroquel
Risperidone • Risperdal
Risperidone long-acting injection • Risperdal Consta
Ziprasidone • Geodon
1. Morrissette DA, Stahl SM. Treating the violence patient with psychosis or impulsivity utilizing antipsychotic polypharmacy and high-dose monotherapy. CNS Spectrums. 2014;19(5):439-448.
2. Latuda [package insert]. Marlborough, MA: Sunovion Pharmaceuticals Inc.; 2019.
3. Taylor D, Paton C, Kapur S. The Maudsley prescribing guidelines in psychiatry. 12th ed. Wiley Blackwell; 2015.
4. Vyas P, Hwang BJ, Brasic JR. An evaluation of lumateperone tosylate for the treatment of schizophrenia. Expert Opin Pharmacother. 2020;21(2):139-145.
5. Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1-02):9-62.
6. Saphris [package insert]. Irvine, CA: Allergan USA, Inc; 2017.
7. Abilify [package insert]. Tokyo, Japan: Otsuka America Pharmaceutical, Inc.; 2014.
8. Rexulti [package insert]. Rockville, MD: Otsuka America Pharmaceutical, Inc.; 2020.
9. Vraylar [package insert]. Madison, NJ: Allergan USA, Inc.; 2019.
10. Clozaril [package insert]. Rosemont, PA: Novartis Pharmaceuticals Corporation; 2017.
11. Fanapt [package insert]. Washington, DC: Vanda Pharmaceuticals Inc.; 2016.
12. Caplyta [package insert]. New York, NY: Intra-Cellular Therapies, Inc.; 2019.
13. Zyprexa [package insert]. Indianapolis, IN: Lilly USA, LLC.; 2020.
14. Invega [package insert]. Titusville, NJ: Janssen Pharmaceutical Companies; 2019.
15. Seroquel [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2016.
16. Risperdal [package insert]. Titusville, NJ: Janssen Pharmaceutical Companies; 2020.
17. Geodon [package insert]. New York, NY: Pfizer Inc.; 2020.
18. Chaudhry IB, Neelam K, Duddu V, et al. Ethnicity and psychopharmacology. J Psychopharmacol. 2008;22(6):673-680.
19. Seeman MV. Men and women respond differently to antipsychotic drugs. Neuropharmacology. 2020;163:107631. doi: 10.1016/j.neuropharm.2019.05.008
20. Barnes TRE, Drake R, Paton C, et al. Evidence-based guidelines for the pharmacological treatment of schizophrenia: updated recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2020;34(1):3-78.
21. Citrome L, McEvoy JP, Todtenkopf MS, et al. A commentary on the efficacy of olanzapine for the treatment of schizophrenia: the past, present, and future. Neuropsych Dis Treat. 2019;15:2559-2569.
22. Meltzer HY, Bobo WV, Ajanta R, et al. A randomized, double-blind comparison of clozapine and high-dose olanzapine in treatment-resistant patients with schizophrenia. J Clin Psychiatry. 2008;69(2):274-285.
23. Batail JM, Langree B, Robert G, et al. Use of very-high-dose olanzapine in treatment-resistant schizophrenia. Schizophr Res. 2014;159(2-3):411-414.
24. Kinon BJ, Volavka J, Stauffer V, et al. Standard and higher dose of olanzapine in patients with schizophrenia or schizoaffective disorder. J Clin Psychopharmacol. 2008;28(4):392-400.
25. Stahl SM. Stahl’s essential psychopharmacology prescriber’s guide. 6th ed. Cambridge University Press; 2017.
26. Bartlett JA, van der Voort Maarschalk K. Understanding the oral mucosal absorption and resulting clinical pharmacokinetics of asenapine. AAPS PharmSciTech. 2012;13(4):1110-1115.
27. Auby P, Saha A, Ali M, et al. Safety and tolerability of aripiprazole at doses higher than 30 mg. Eur Neuropsychopharm. 2002;12(3):288.
28. Chavez B, Poveda R. Efficacy with high-dose aripiprazole after olanzapine-related metabolic disturbances. Ann Pharmacother. 2006;40(12):2265-2268.
29. Duggal HS, Mendhekar DN. High-dose aripiprazole in treatment-resistant schizophrenia. J Clin Psychiatry. 2006;67(4):674-675.
30. Thone J. Worsened agitation and confusion in schizophrenia subsequent to high-dose aripiprazole. J Neuropsychiatry Clin Neurosci. 2007;19(4):481-482.
31. Saatcioglu O, Gumus S, Kamberyan K, et al. Efficacy of high-dose aripiprazole for treatment-resistant schizoaffective disorder: a case report. Psychopharmacol Bull. 2010;43(4):70-72.
32. Thomson SR, Chogtu B, Bhattacharjee D, et al. Extrapyramidal symptoms probably related to risperidone treatment: a case series. Ann Neurosci. 2017;24(3):155-163.
33. Meltzer HY, Lindenmayer JP, Kwentus J, et al. A six month randomized controlled trial of long acting injectable risperidone 50 and 100 mg in treatment resistant schizophrenia. Schizophr Res. 2014;154(1-3):14-22.
34. Hou Y, Lai C. The response of psychotic symptoms in a patient with resistant schizophrenia under treatment of high-dose risperidone long-acting injection. J Neuropsychiatry Clin Neurosci. 2014;26(3):E16-E17. doi: 10.1176/appi.neuropsych.13070150
35. Albrecht A, Morena PG, Baumann P, et al. High dose of depot risperidone in a nonresponder schizophrenic patient. J Clin Psychopharmacol. 2004;24(6):673-674.
36. Mace S, Taylor D. Reducing the rates of prescribing high-dose antipsychotics and polypharmacy on psychiatric inpatient and intensive care units: results of a 6-year quality improvement programme. Ther Adv Psychopharmacol. 2015;5(1):4-12.
37. Goff DC, McEvoy JP, Citrome L, et al. High-dose oral ziprasidone versus conventional dosing in schizophrenia patients with residual symptoms. J Clin Psychopharmacol. 2013;33:485-490.
38. Levy WO, Robichaux-Keene NR, Nunez C. No significant QTc interval changes with high-dose ziprasidone: a case series. J Psychiatr Pract. 2004;10(4):227-232.
39. Kaushik S, Maccabee N, Kaushik S, et al. Activation induced by high-dose ziprasidone: a case report. J Clin Psychiatry. 2009;70(9):1326-1327.
40. Seeman P. Targeting the dopamine D2 receptor in schizophrenia. Expert Opin Ther Targets. 2006;10(4):515-531.
41. Honer WG, MacEwan W, Gendron A, et al. A randomized, double-blind, placebo-controlled study of safety and tolerability of high-dose quetiapine in patients with persistent symptoms of schizophrenia or schizoaffective disorder. J Clin Psychiatry. 2012;73(1):13-20.
42. Sokolski KN, Brown BJ, Meldon M. Urinary retention following repeated high-dose quetiapine. Ann Pharmacother. 2004;38(5):899-890.
43. Chandrappa P, Ho L. Case reports of patients with treatment-resistant schizophrenia and related psychotic disorders intolerant to clozapine responding to high doses of quetiapine. Ther Adv Psychopharmacol. 2012;2(5):207-209.
44. Pierre JM, Wirshing DA, Wirshing WC, et al. High-dose quetiapine in treatment refractory schizophrenia. Schizophr Res. 2005;73:373-375.
45. Lindenmyer JP, Citrome L, Khan A, et al. A randomized, double-blind parallel-group, fixed-dose, clinical trial of quetiapine at 600 vs. 1200 mg/d for patients with treatment-resistant schizophrenia or schizoaffective disorder. J Clin Psychopharmacol. 2011;31(2):160-168.
46. Hazra M, Culo S, Mamo D. High-dose quetiapine and photopsia. J Clin Psychopharmacol. 2006;26(5):546-547.
47. Smolders DME, Smolders WAP. Case report and review of the literature: cardiomyopathy in a young woman on high-dose quetiapine. Cardiovasc Toxicol. 2017;17(4):478-481.
48. Takeuchi H, Suzuki T, Bies RR, et al. Dose reduction of risperidone and olanzapine and estimated D2 receptor occupancy in stable patients with schizophrenia: findings from an open-label, randomized, controlled study. J Clin Psychiatry. 2014;75(11):1209-1214.
49. Kumar V, Rao NP, Narasimha V, et al. Antipsychotic dose in maintenance treatment of schizophrenia: a retrospective study. Psychiatry Res. 2016;245:311-316.
1. Morrissette DA, Stahl SM. Treating the violence patient with psychosis or impulsivity utilizing antipsychotic polypharmacy and high-dose monotherapy. CNS Spectrums. 2014;19(5):439-448.
2. Latuda [package insert]. Marlborough, MA: Sunovion Pharmaceuticals Inc.; 2019.
3. Taylor D, Paton C, Kapur S. The Maudsley prescribing guidelines in psychiatry. 12th ed. Wiley Blackwell; 2015.
4. Vyas P, Hwang BJ, Brasic JR. An evaluation of lumateperone tosylate for the treatment of schizophrenia. Expert Opin Pharmacother. 2020;21(2):139-145.
5. Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1-02):9-62.
6. Saphris [package insert]. Irvine, CA: Allergan USA, Inc; 2017.
7. Abilify [package insert]. Tokyo, Japan: Otsuka America Pharmaceutical, Inc.; 2014.
8. Rexulti [package insert]. Rockville, MD: Otsuka America Pharmaceutical, Inc.; 2020.
9. Vraylar [package insert]. Madison, NJ: Allergan USA, Inc.; 2019.
10. Clozaril [package insert]. Rosemont, PA: Novartis Pharmaceuticals Corporation; 2017.
11. Fanapt [package insert]. Washington, DC: Vanda Pharmaceuticals Inc.; 2016.
12. Caplyta [package insert]. New York, NY: Intra-Cellular Therapies, Inc.; 2019.
13. Zyprexa [package insert]. Indianapolis, IN: Lilly USA, LLC.; 2020.
14. Invega [package insert]. Titusville, NJ: Janssen Pharmaceutical Companies; 2019.
15. Seroquel [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2016.
16. Risperdal [package insert]. Titusville, NJ: Janssen Pharmaceutical Companies; 2020.
17. Geodon [package insert]. New York, NY: Pfizer Inc.; 2020.
18. Chaudhry IB, Neelam K, Duddu V, et al. Ethnicity and psychopharmacology. J Psychopharmacol. 2008;22(6):673-680.
19. Seeman MV. Men and women respond differently to antipsychotic drugs. Neuropharmacology. 2020;163:107631. doi: 10.1016/j.neuropharm.2019.05.008
20. Barnes TRE, Drake R, Paton C, et al. Evidence-based guidelines for the pharmacological treatment of schizophrenia: updated recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2020;34(1):3-78.
21. Citrome L, McEvoy JP, Todtenkopf MS, et al. A commentary on the efficacy of olanzapine for the treatment of schizophrenia: the past, present, and future. Neuropsych Dis Treat. 2019;15:2559-2569.
22. Meltzer HY, Bobo WV, Ajanta R, et al. A randomized, double-blind comparison of clozapine and high-dose olanzapine in treatment-resistant patients with schizophrenia. J Clin Psychiatry. 2008;69(2):274-285.
23. Batail JM, Langree B, Robert G, et al. Use of very-high-dose olanzapine in treatment-resistant schizophrenia. Schizophr Res. 2014;159(2-3):411-414.
24. Kinon BJ, Volavka J, Stauffer V, et al. Standard and higher dose of olanzapine in patients with schizophrenia or schizoaffective disorder. J Clin Psychopharmacol. 2008;28(4):392-400.
25. Stahl SM. Stahl’s essential psychopharmacology prescriber’s guide. 6th ed. Cambridge University Press; 2017.
26. Bartlett JA, van der Voort Maarschalk K. Understanding the oral mucosal absorption and resulting clinical pharmacokinetics of asenapine. AAPS PharmSciTech. 2012;13(4):1110-1115.
27. Auby P, Saha A, Ali M, et al. Safety and tolerability of aripiprazole at doses higher than 30 mg. Eur Neuropsychopharm. 2002;12(3):288.
28. Chavez B, Poveda R. Efficacy with high-dose aripiprazole after olanzapine-related metabolic disturbances. Ann Pharmacother. 2006;40(12):2265-2268.
29. Duggal HS, Mendhekar DN. High-dose aripiprazole in treatment-resistant schizophrenia. J Clin Psychiatry. 2006;67(4):674-675.
30. Thone J. Worsened agitation and confusion in schizophrenia subsequent to high-dose aripiprazole. J Neuropsychiatry Clin Neurosci. 2007;19(4):481-482.
31. Saatcioglu O, Gumus S, Kamberyan K, et al. Efficacy of high-dose aripiprazole for treatment-resistant schizoaffective disorder: a case report. Psychopharmacol Bull. 2010;43(4):70-72.
32. Thomson SR, Chogtu B, Bhattacharjee D, et al. Extrapyramidal symptoms probably related to risperidone treatment: a case series. Ann Neurosci. 2017;24(3):155-163.
33. Meltzer HY, Lindenmayer JP, Kwentus J, et al. A six month randomized controlled trial of long acting injectable risperidone 50 and 100 mg in treatment resistant schizophrenia. Schizophr Res. 2014;154(1-3):14-22.
34. Hou Y, Lai C. The response of psychotic symptoms in a patient with resistant schizophrenia under treatment of high-dose risperidone long-acting injection. J Neuropsychiatry Clin Neurosci. 2014;26(3):E16-E17. doi: 10.1176/appi.neuropsych.13070150
35. Albrecht A, Morena PG, Baumann P, et al. High dose of depot risperidone in a nonresponder schizophrenic patient. J Clin Psychopharmacol. 2004;24(6):673-674.
36. Mace S, Taylor D. Reducing the rates of prescribing high-dose antipsychotics and polypharmacy on psychiatric inpatient and intensive care units: results of a 6-year quality improvement programme. Ther Adv Psychopharmacol. 2015;5(1):4-12.
37. Goff DC, McEvoy JP, Citrome L, et al. High-dose oral ziprasidone versus conventional dosing in schizophrenia patients with residual symptoms. J Clin Psychopharmacol. 2013;33:485-490.
38. Levy WO, Robichaux-Keene NR, Nunez C. No significant QTc interval changes with high-dose ziprasidone: a case series. J Psychiatr Pract. 2004;10(4):227-232.
39. Kaushik S, Maccabee N, Kaushik S, et al. Activation induced by high-dose ziprasidone: a case report. J Clin Psychiatry. 2009;70(9):1326-1327.
40. Seeman P. Targeting the dopamine D2 receptor in schizophrenia. Expert Opin Ther Targets. 2006;10(4):515-531.
41. Honer WG, MacEwan W, Gendron A, et al. A randomized, double-blind, placebo-controlled study of safety and tolerability of high-dose quetiapine in patients with persistent symptoms of schizophrenia or schizoaffective disorder. J Clin Psychiatry. 2012;73(1):13-20.
42. Sokolski KN, Brown BJ, Meldon M. Urinary retention following repeated high-dose quetiapine. Ann Pharmacother. 2004;38(5):899-890.
43. Chandrappa P, Ho L. Case reports of patients with treatment-resistant schizophrenia and related psychotic disorders intolerant to clozapine responding to high doses of quetiapine. Ther Adv Psychopharmacol. 2012;2(5):207-209.
44. Pierre JM, Wirshing DA, Wirshing WC, et al. High-dose quetiapine in treatment refractory schizophrenia. Schizophr Res. 2005;73:373-375.
45. Lindenmyer JP, Citrome L, Khan A, et al. A randomized, double-blind parallel-group, fixed-dose, clinical trial of quetiapine at 600 vs. 1200 mg/d for patients with treatment-resistant schizophrenia or schizoaffective disorder. J Clin Psychopharmacol. 2011;31(2):160-168.
46. Hazra M, Culo S, Mamo D. High-dose quetiapine and photopsia. J Clin Psychopharmacol. 2006;26(5):546-547.
47. Smolders DME, Smolders WAP. Case report and review of the literature: cardiomyopathy in a young woman on high-dose quetiapine. Cardiovasc Toxicol. 2017;17(4):478-481.
48. Takeuchi H, Suzuki T, Bies RR, et al. Dose reduction of risperidone and olanzapine and estimated D2 receptor occupancy in stable patients with schizophrenia: findings from an open-label, randomized, controlled study. J Clin Psychiatry. 2014;75(11):1209-1214.
49. Kumar V, Rao NP, Narasimha V, et al. Antipsychotic dose in maintenance treatment of schizophrenia: a retrospective study. Psychiatry Res. 2016;245:311-316.
Prazosin for PTSD: Sorting out the evidence
Mr. H, age 43, presents to your clinic for management of posttraumatic stress disorder (PTSD). At his last appointment 8 weeks ago, he was continued on fluoxetine, 60 mg/d; he had been stable on this medication for 6 months. Today, Mr. H reports an increase in the frequency and severity of nightmares. He states that he wakes at least 3 times every week with “disturbing dreams” about his time in the military and does not feel rested even when he sleeps through the night. His Clinician-Administered PTSD Scale (CAPS) score is 95 on this visit, suggesting extreme PTSD symptomatology. Mr. H asks if anything can be done to reduce the frequency and intensity of his nightmares.
PTSD is the development of characteristic symptoms following exposure to ≥1 traumatic events. According to DSM-5, PTSD symptoms include the presence of ≥1 intrusion symptoms (recurrent, intrusive memories of the traumatic event; recurrent distressing dreams; dissociative reactions), persistent avoidance of stimuli, negative alterations in cognition and mood, and marked alterations in arousal and reactivity associated with the traumatic event(s).1 The symptoms must be present for >1 month, cause clinically significant distress or impairment in functioning, and not be attributable to the psychologic effects of a substance or medical conditions.1 This article focuses specifically on the hyperarousal symptoms, and the clinical controversies surrounding the use of prazosin for PTSD.
Prazosin for PTSD treatment
Sleep disorders are extremely common in patients with PTSD. Up to 90% of patients report sleep disturbances, and up to 70% report nightmares.2 Prazosin has been widely used in the treatment of PTSD-related sleep disorders and nightmares.The American Psychiatric Association3 and the British Association of Psychopharmacology4 guidelines in-clude prazosin as a first-line recommendation for treatment of PTSD. However, updated 2017 guidelines from the Veterans Affairs/Department of Defense (VA/DoD)5 and data from the 2018 Prazosin and Combat Trauma PTSD (PACT) trial6 contradict these original recommendations. Previously, the 2010 VA/DoD guideline said prazosin had insufficient evidence for monotherapy, but recommended it as adjunctive treatment for sleep and nightmares.7 The updated 2017 VA/DoD guideline recommends “weak against” prazosin use for global symptoms of PTSD, and says there is insufficient evidence for its use in nightmares.5 Below we summarize the findings of studies that contributed to those original recommendations, along with results of the PACT trial.
Raskind et al8,9 conducted 2 studies of prazosin use in combat veterans with PTSD. In both studies, prazosin had significant positive effects on the Clinician-Administered PTSD Scale (CAPS) and Clinical Global Impression of Change (CGIC) scores.8,9 The 2007 study also found significant effects of prazosin on Pittsburgh Sleep Quality Index (PSQI) scores.9
Raskind et al10 conducted another study in 2013 of prazosin use for active-duty soldiers who had combat trauma PTSD with nightmares. Prazosin had positive effects for nightmares, sleep quality, and CAPS scores.10
Germain et al11 reviewed prazosin for treating sleep disturbances in US military veterans. Prazosin was associated with significant improvements in insomnia and daytime PTSD symptom severity as demonstrated by changes in PSQI and CAPS scores.11
Taylor et al12 examined the effects of prazosin on sleep measures and clinical symptoms in civilians with PTSD. Prazosin significantly increased total sleep time, rapid eye movement sleep time, and CGIC scores while significantly decreasing trauma-related nightmares.12
Continue to: Overall, these trials...
Overall, these trials found efficacy for the use of prazosin for patients diagnosed with PTSD; however, the population size in each of these studies was small.
Results of the PACT trial
The PACT trial was a 26-week, multicenter, double-blind, randomized, placebo-controlled trial conducted across 12 VA medical centers.6 During the first 5 weeks, participants were randomized to receive placebo or prazosin, which could be titrated up to 20 mg/d in men and 12 mg/d in women. Participants remained on that dose from the end of Week 5 through Week 10. At that time, other pharmacologic therapies and psychotherapy could be added, discontinued, or adjusted. The mean maintenance total daily dose of prazosin was 14.8 mg.
A total of 413 patients were screened, 304 were randomized (152 per group), and 271 completed the 10-week primary outcome assessment. The population was almost entirely male (96.1% in the prazosin group and 99.3% in the placebo group), and most participants were White (64.5% in the prazosin group and 69.1% in the placebo group), with an average age of approximately 50 years. Primary outcomes included change from baseline to Week 10 in both CAPS item B2 (“recurrent distressing dreams”) and PSQI scores. CGIC score was evaluated at Week 10.
At Week 10, none of the primary outcomes were found to be statistically significant. The mean difference in change from baseline to Week 10 in CAPS item B2 score and PSQI score were 0.2 (P = .38) and 0.1 (P = .80), respectively. There was no significant difference in mean CGIC scores (P = .96). Repeated measures of CAPS item B2, PSQI, and CGIC scores were conducted through Week 26 as secondary outcomes. No significant differences were found. This study concluded that prazosin did not alleviate distressing dreams, improve sleep quality, or improve overall clinical symptoms.6
The PACT trial: Strengths and weaknesses
The PACT trial is the largest placebo-controlled trial for prazosin use in PTSD to date. It failed to show efficacy of prazosin for PTSD-associated nightmares, which contradicts previous studies. Although the mean total daily dose of prazosin was adequate and primary outcomes were measured with appropriate scales, the study failed to enroll the desired number of patients, which increased the possibility of false-negative results. Furthermore, participant recruitment may have led to selection bias because all participants were clinically stable, which could explain the lack of efficacy. However, the average CAPS scores were 80.7 in the prazosin group and 81.9 in the placebo group, which indicates that these patients had significant symptomatology at baseline and before entering the study.
Continue to: A major theme...
A major theme of studies evaluating prazosin treatment for PTSD is a focus on a military population and military-related trauma. Other than Taylor et al12 (N=13), none of these trials included patients who were diagnosed with PTSD due to other traumas, such as sexual trauma, which limits the generalizability of the results. Furthermore, apart from the PACT trial, none of these studies had >100 participants, which further reduces external validity. Current guidelines have not been updated to include the results of the PACT trial, and it is unclear if the results of this trial are strong enough to change clinical practice.
CASE CONTINUED
To ensure patient-centered care, the treating clinicians conduct a risk/benefit discussion with the patient regarding starting prazosin. Mr. H opts to try prazosin, so the clinicians initiate a low dose (1 mg/d) to mitigate adverse effects, and plan to titrate to clinical effect or intolerability. Per evidence from the trials discussed, it is likely Mr. H will need to be titrated to at least 5 to 6 mg/d to see a clinical effect.
Related Resource
North CS, Hong BA, Downs DL. PTSD: A systematic approach to diagnosis and treatment. Current Psychiatry 2018;17(4):35-43.
Drug Brand Names
Fluoxetine • Prozac
Prazosin • Minipress
1. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
2. Maher MJ, Rego SA, Asnis, GM. Sleep disturbances in patients with post-traumatic stress disorder: epidemiology, impact and approaches to management. CNS Drugs. 2006;20(7):567-590.
3. Benedek DM, Friedman MJ, Zatzick D, et al. Guideline watch (March 2009): Practice guideline for the treatment of patients with acute stress disorder and posttraumatic stress disorder. APA Practice Guidelines. Published 2010. Accessed March 14, 2021. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/acutestressdisorderptsd-watch.pdf
4. Baldwin DS, Anderson IM, Nutt DJ, et al. Evidence-based pharmacological treatment of anxiety disorders, post-traumatic stress disorder and obsessive-compulsive disorder: a revision of the 2005 guidelines from the British Association for Psychopharmacology. J Psychopharmacol. 2014;28(5):403-439. doi: 10.1177/0269881114525674
5. Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline for the management of posttraumatic stress disorder and acute stress disorder. Version 3.0. Published 2017. Accessed February 5, 2021. https://www.healthquality.va.gov/guidelines/MH/ptsd/VADoDPTSDCPGFinal012418.pdf
6. Raskind MA, Peskind ER, Chow B, et al. Trial of prazosin for post-traumatic stress disorder in military veterans. N Engl J Med. 2018;378(6):507-517.
7. Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline: management of post-traumatic stress. Version 2.0. Published 2010. Accessed February 5, 2021. https://www.healthquality.va.gov/guidelines/MH/ptsd/cpg_PTSD-full-201011612.PDF
8. Raskind MA, Peskind ER, Katner ED, et al. Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: a placebo-controlled study. Am J Psychiatry. 2003;160(2):371-373.
9. Raskind MA, Peskind ER, Hoff DJ, et al. A parallel group placebo-controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol Psychiatry. 2007;61(8):928-934.
10. Raskind MA, Peterson K, Williams T, et al. A trial of prazosin for combat trauma PTSD with nightmares in active-duty soldiers returned from Iraq and Afghanistan. Am J Psychiatry. 2013;170(9):1003-1010.
11. Germain A, Richardson R, Moul DE, et al. Placebo-controlled comparison of prazosin and cognitive-behavioral treatments for sleep disturbances in US military veterans. J Psychosom Res. 2012;72(2):89-96.
12. Taylor FB, Martin P, Thompson C, et al. Prazosin effects on objective sleep measures and clinical symptoms in civilian trauma posttraumatic stress disorder: a placebo-controlled study. Biol Psychiatry. 2008;63(6):629-632.
Mr. H, age 43, presents to your clinic for management of posttraumatic stress disorder (PTSD). At his last appointment 8 weeks ago, he was continued on fluoxetine, 60 mg/d; he had been stable on this medication for 6 months. Today, Mr. H reports an increase in the frequency and severity of nightmares. He states that he wakes at least 3 times every week with “disturbing dreams” about his time in the military and does not feel rested even when he sleeps through the night. His Clinician-Administered PTSD Scale (CAPS) score is 95 on this visit, suggesting extreme PTSD symptomatology. Mr. H asks if anything can be done to reduce the frequency and intensity of his nightmares.
PTSD is the development of characteristic symptoms following exposure to ≥1 traumatic events. According to DSM-5, PTSD symptoms include the presence of ≥1 intrusion symptoms (recurrent, intrusive memories of the traumatic event; recurrent distressing dreams; dissociative reactions), persistent avoidance of stimuli, negative alterations in cognition and mood, and marked alterations in arousal and reactivity associated with the traumatic event(s).1 The symptoms must be present for >1 month, cause clinically significant distress or impairment in functioning, and not be attributable to the psychologic effects of a substance or medical conditions.1 This article focuses specifically on the hyperarousal symptoms, and the clinical controversies surrounding the use of prazosin for PTSD.
Prazosin for PTSD treatment
Sleep disorders are extremely common in patients with PTSD. Up to 90% of patients report sleep disturbances, and up to 70% report nightmares.2 Prazosin has been widely used in the treatment of PTSD-related sleep disorders and nightmares.The American Psychiatric Association3 and the British Association of Psychopharmacology4 guidelines in-clude prazosin as a first-line recommendation for treatment of PTSD. However, updated 2017 guidelines from the Veterans Affairs/Department of Defense (VA/DoD)5 and data from the 2018 Prazosin and Combat Trauma PTSD (PACT) trial6 contradict these original recommendations. Previously, the 2010 VA/DoD guideline said prazosin had insufficient evidence for monotherapy, but recommended it as adjunctive treatment for sleep and nightmares.7 The updated 2017 VA/DoD guideline recommends “weak against” prazosin use for global symptoms of PTSD, and says there is insufficient evidence for its use in nightmares.5 Below we summarize the findings of studies that contributed to those original recommendations, along with results of the PACT trial.
Raskind et al8,9 conducted 2 studies of prazosin use in combat veterans with PTSD. In both studies, prazosin had significant positive effects on the Clinician-Administered PTSD Scale (CAPS) and Clinical Global Impression of Change (CGIC) scores.8,9 The 2007 study also found significant effects of prazosin on Pittsburgh Sleep Quality Index (PSQI) scores.9
Raskind et al10 conducted another study in 2013 of prazosin use for active-duty soldiers who had combat trauma PTSD with nightmares. Prazosin had positive effects for nightmares, sleep quality, and CAPS scores.10
Germain et al11 reviewed prazosin for treating sleep disturbances in US military veterans. Prazosin was associated with significant improvements in insomnia and daytime PTSD symptom severity as demonstrated by changes in PSQI and CAPS scores.11
Taylor et al12 examined the effects of prazosin on sleep measures and clinical symptoms in civilians with PTSD. Prazosin significantly increased total sleep time, rapid eye movement sleep time, and CGIC scores while significantly decreasing trauma-related nightmares.12
Continue to: Overall, these trials...
Overall, these trials found efficacy for the use of prazosin for patients diagnosed with PTSD; however, the population size in each of these studies was small.
Results of the PACT trial
The PACT trial was a 26-week, multicenter, double-blind, randomized, placebo-controlled trial conducted across 12 VA medical centers.6 During the first 5 weeks, participants were randomized to receive placebo or prazosin, which could be titrated up to 20 mg/d in men and 12 mg/d in women. Participants remained on that dose from the end of Week 5 through Week 10. At that time, other pharmacologic therapies and psychotherapy could be added, discontinued, or adjusted. The mean maintenance total daily dose of prazosin was 14.8 mg.
A total of 413 patients were screened, 304 were randomized (152 per group), and 271 completed the 10-week primary outcome assessment. The population was almost entirely male (96.1% in the prazosin group and 99.3% in the placebo group), and most participants were White (64.5% in the prazosin group and 69.1% in the placebo group), with an average age of approximately 50 years. Primary outcomes included change from baseline to Week 10 in both CAPS item B2 (“recurrent distressing dreams”) and PSQI scores. CGIC score was evaluated at Week 10.
At Week 10, none of the primary outcomes were found to be statistically significant. The mean difference in change from baseline to Week 10 in CAPS item B2 score and PSQI score were 0.2 (P = .38) and 0.1 (P = .80), respectively. There was no significant difference in mean CGIC scores (P = .96). Repeated measures of CAPS item B2, PSQI, and CGIC scores were conducted through Week 26 as secondary outcomes. No significant differences were found. This study concluded that prazosin did not alleviate distressing dreams, improve sleep quality, or improve overall clinical symptoms.6
The PACT trial: Strengths and weaknesses
The PACT trial is the largest placebo-controlled trial for prazosin use in PTSD to date. It failed to show efficacy of prazosin for PTSD-associated nightmares, which contradicts previous studies. Although the mean total daily dose of prazosin was adequate and primary outcomes were measured with appropriate scales, the study failed to enroll the desired number of patients, which increased the possibility of false-negative results. Furthermore, participant recruitment may have led to selection bias because all participants were clinically stable, which could explain the lack of efficacy. However, the average CAPS scores were 80.7 in the prazosin group and 81.9 in the placebo group, which indicates that these patients had significant symptomatology at baseline and before entering the study.
Continue to: A major theme...
A major theme of studies evaluating prazosin treatment for PTSD is a focus on a military population and military-related trauma. Other than Taylor et al12 (N=13), none of these trials included patients who were diagnosed with PTSD due to other traumas, such as sexual trauma, which limits the generalizability of the results. Furthermore, apart from the PACT trial, none of these studies had >100 participants, which further reduces external validity. Current guidelines have not been updated to include the results of the PACT trial, and it is unclear if the results of this trial are strong enough to change clinical practice.
CASE CONTINUED
To ensure patient-centered care, the treating clinicians conduct a risk/benefit discussion with the patient regarding starting prazosin. Mr. H opts to try prazosin, so the clinicians initiate a low dose (1 mg/d) to mitigate adverse effects, and plan to titrate to clinical effect or intolerability. Per evidence from the trials discussed, it is likely Mr. H will need to be titrated to at least 5 to 6 mg/d to see a clinical effect.
Related Resource
North CS, Hong BA, Downs DL. PTSD: A systematic approach to diagnosis and treatment. Current Psychiatry 2018;17(4):35-43.
Drug Brand Names
Fluoxetine • Prozac
Prazosin • Minipress
Mr. H, age 43, presents to your clinic for management of posttraumatic stress disorder (PTSD). At his last appointment 8 weeks ago, he was continued on fluoxetine, 60 mg/d; he had been stable on this medication for 6 months. Today, Mr. H reports an increase in the frequency and severity of nightmares. He states that he wakes at least 3 times every week with “disturbing dreams” about his time in the military and does not feel rested even when he sleeps through the night. His Clinician-Administered PTSD Scale (CAPS) score is 95 on this visit, suggesting extreme PTSD symptomatology. Mr. H asks if anything can be done to reduce the frequency and intensity of his nightmares.
PTSD is the development of characteristic symptoms following exposure to ≥1 traumatic events. According to DSM-5, PTSD symptoms include the presence of ≥1 intrusion symptoms (recurrent, intrusive memories of the traumatic event; recurrent distressing dreams; dissociative reactions), persistent avoidance of stimuli, negative alterations in cognition and mood, and marked alterations in arousal and reactivity associated with the traumatic event(s).1 The symptoms must be present for >1 month, cause clinically significant distress or impairment in functioning, and not be attributable to the psychologic effects of a substance or medical conditions.1 This article focuses specifically on the hyperarousal symptoms, and the clinical controversies surrounding the use of prazosin for PTSD.
Prazosin for PTSD treatment
Sleep disorders are extremely common in patients with PTSD. Up to 90% of patients report sleep disturbances, and up to 70% report nightmares.2 Prazosin has been widely used in the treatment of PTSD-related sleep disorders and nightmares.The American Psychiatric Association3 and the British Association of Psychopharmacology4 guidelines in-clude prazosin as a first-line recommendation for treatment of PTSD. However, updated 2017 guidelines from the Veterans Affairs/Department of Defense (VA/DoD)5 and data from the 2018 Prazosin and Combat Trauma PTSD (PACT) trial6 contradict these original recommendations. Previously, the 2010 VA/DoD guideline said prazosin had insufficient evidence for monotherapy, but recommended it as adjunctive treatment for sleep and nightmares.7 The updated 2017 VA/DoD guideline recommends “weak against” prazosin use for global symptoms of PTSD, and says there is insufficient evidence for its use in nightmares.5 Below we summarize the findings of studies that contributed to those original recommendations, along with results of the PACT trial.
Raskind et al8,9 conducted 2 studies of prazosin use in combat veterans with PTSD. In both studies, prazosin had significant positive effects on the Clinician-Administered PTSD Scale (CAPS) and Clinical Global Impression of Change (CGIC) scores.8,9 The 2007 study also found significant effects of prazosin on Pittsburgh Sleep Quality Index (PSQI) scores.9
Raskind et al10 conducted another study in 2013 of prazosin use for active-duty soldiers who had combat trauma PTSD with nightmares. Prazosin had positive effects for nightmares, sleep quality, and CAPS scores.10
Germain et al11 reviewed prazosin for treating sleep disturbances in US military veterans. Prazosin was associated with significant improvements in insomnia and daytime PTSD symptom severity as demonstrated by changes in PSQI and CAPS scores.11
Taylor et al12 examined the effects of prazosin on sleep measures and clinical symptoms in civilians with PTSD. Prazosin significantly increased total sleep time, rapid eye movement sleep time, and CGIC scores while significantly decreasing trauma-related nightmares.12
Continue to: Overall, these trials...
Overall, these trials found efficacy for the use of prazosin for patients diagnosed with PTSD; however, the population size in each of these studies was small.
Results of the PACT trial
The PACT trial was a 26-week, multicenter, double-blind, randomized, placebo-controlled trial conducted across 12 VA medical centers.6 During the first 5 weeks, participants were randomized to receive placebo or prazosin, which could be titrated up to 20 mg/d in men and 12 mg/d in women. Participants remained on that dose from the end of Week 5 through Week 10. At that time, other pharmacologic therapies and psychotherapy could be added, discontinued, or adjusted. The mean maintenance total daily dose of prazosin was 14.8 mg.
A total of 413 patients were screened, 304 were randomized (152 per group), and 271 completed the 10-week primary outcome assessment. The population was almost entirely male (96.1% in the prazosin group and 99.3% in the placebo group), and most participants were White (64.5% in the prazosin group and 69.1% in the placebo group), with an average age of approximately 50 years. Primary outcomes included change from baseline to Week 10 in both CAPS item B2 (“recurrent distressing dreams”) and PSQI scores. CGIC score was evaluated at Week 10.
At Week 10, none of the primary outcomes were found to be statistically significant. The mean difference in change from baseline to Week 10 in CAPS item B2 score and PSQI score were 0.2 (P = .38) and 0.1 (P = .80), respectively. There was no significant difference in mean CGIC scores (P = .96). Repeated measures of CAPS item B2, PSQI, and CGIC scores were conducted through Week 26 as secondary outcomes. No significant differences were found. This study concluded that prazosin did not alleviate distressing dreams, improve sleep quality, or improve overall clinical symptoms.6
The PACT trial: Strengths and weaknesses
The PACT trial is the largest placebo-controlled trial for prazosin use in PTSD to date. It failed to show efficacy of prazosin for PTSD-associated nightmares, which contradicts previous studies. Although the mean total daily dose of prazosin was adequate and primary outcomes were measured with appropriate scales, the study failed to enroll the desired number of patients, which increased the possibility of false-negative results. Furthermore, participant recruitment may have led to selection bias because all participants were clinically stable, which could explain the lack of efficacy. However, the average CAPS scores were 80.7 in the prazosin group and 81.9 in the placebo group, which indicates that these patients had significant symptomatology at baseline and before entering the study.
Continue to: A major theme...
A major theme of studies evaluating prazosin treatment for PTSD is a focus on a military population and military-related trauma. Other than Taylor et al12 (N=13), none of these trials included patients who were diagnosed with PTSD due to other traumas, such as sexual trauma, which limits the generalizability of the results. Furthermore, apart from the PACT trial, none of these studies had >100 participants, which further reduces external validity. Current guidelines have not been updated to include the results of the PACT trial, and it is unclear if the results of this trial are strong enough to change clinical practice.
CASE CONTINUED
To ensure patient-centered care, the treating clinicians conduct a risk/benefit discussion with the patient regarding starting prazosin. Mr. H opts to try prazosin, so the clinicians initiate a low dose (1 mg/d) to mitigate adverse effects, and plan to titrate to clinical effect or intolerability. Per evidence from the trials discussed, it is likely Mr. H will need to be titrated to at least 5 to 6 mg/d to see a clinical effect.
Related Resource
North CS, Hong BA, Downs DL. PTSD: A systematic approach to diagnosis and treatment. Current Psychiatry 2018;17(4):35-43.
Drug Brand Names
Fluoxetine • Prozac
Prazosin • Minipress
1. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
2. Maher MJ, Rego SA, Asnis, GM. Sleep disturbances in patients with post-traumatic stress disorder: epidemiology, impact and approaches to management. CNS Drugs. 2006;20(7):567-590.
3. Benedek DM, Friedman MJ, Zatzick D, et al. Guideline watch (March 2009): Practice guideline for the treatment of patients with acute stress disorder and posttraumatic stress disorder. APA Practice Guidelines. Published 2010. Accessed March 14, 2021. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/acutestressdisorderptsd-watch.pdf
4. Baldwin DS, Anderson IM, Nutt DJ, et al. Evidence-based pharmacological treatment of anxiety disorders, post-traumatic stress disorder and obsessive-compulsive disorder: a revision of the 2005 guidelines from the British Association for Psychopharmacology. J Psychopharmacol. 2014;28(5):403-439. doi: 10.1177/0269881114525674
5. Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline for the management of posttraumatic stress disorder and acute stress disorder. Version 3.0. Published 2017. Accessed February 5, 2021. https://www.healthquality.va.gov/guidelines/MH/ptsd/VADoDPTSDCPGFinal012418.pdf
6. Raskind MA, Peskind ER, Chow B, et al. Trial of prazosin for post-traumatic stress disorder in military veterans. N Engl J Med. 2018;378(6):507-517.
7. Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline: management of post-traumatic stress. Version 2.0. Published 2010. Accessed February 5, 2021. https://www.healthquality.va.gov/guidelines/MH/ptsd/cpg_PTSD-full-201011612.PDF
8. Raskind MA, Peskind ER, Katner ED, et al. Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: a placebo-controlled study. Am J Psychiatry. 2003;160(2):371-373.
9. Raskind MA, Peskind ER, Hoff DJ, et al. A parallel group placebo-controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol Psychiatry. 2007;61(8):928-934.
10. Raskind MA, Peterson K, Williams T, et al. A trial of prazosin for combat trauma PTSD with nightmares in active-duty soldiers returned from Iraq and Afghanistan. Am J Psychiatry. 2013;170(9):1003-1010.
11. Germain A, Richardson R, Moul DE, et al. Placebo-controlled comparison of prazosin and cognitive-behavioral treatments for sleep disturbances in US military veterans. J Psychosom Res. 2012;72(2):89-96.
12. Taylor FB, Martin P, Thompson C, et al. Prazosin effects on objective sleep measures and clinical symptoms in civilian trauma posttraumatic stress disorder: a placebo-controlled study. Biol Psychiatry. 2008;63(6):629-632.
1. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
2. Maher MJ, Rego SA, Asnis, GM. Sleep disturbances in patients with post-traumatic stress disorder: epidemiology, impact and approaches to management. CNS Drugs. 2006;20(7):567-590.
3. Benedek DM, Friedman MJ, Zatzick D, et al. Guideline watch (March 2009): Practice guideline for the treatment of patients with acute stress disorder and posttraumatic stress disorder. APA Practice Guidelines. Published 2010. Accessed March 14, 2021. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/acutestressdisorderptsd-watch.pdf
4. Baldwin DS, Anderson IM, Nutt DJ, et al. Evidence-based pharmacological treatment of anxiety disorders, post-traumatic stress disorder and obsessive-compulsive disorder: a revision of the 2005 guidelines from the British Association for Psychopharmacology. J Psychopharmacol. 2014;28(5):403-439. doi: 10.1177/0269881114525674
5. Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline for the management of posttraumatic stress disorder and acute stress disorder. Version 3.0. Published 2017. Accessed February 5, 2021. https://www.healthquality.va.gov/guidelines/MH/ptsd/VADoDPTSDCPGFinal012418.pdf
6. Raskind MA, Peskind ER, Chow B, et al. Trial of prazosin for post-traumatic stress disorder in military veterans. N Engl J Med. 2018;378(6):507-517.
7. Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline: management of post-traumatic stress. Version 2.0. Published 2010. Accessed February 5, 2021. https://www.healthquality.va.gov/guidelines/MH/ptsd/cpg_PTSD-full-201011612.PDF
8. Raskind MA, Peskind ER, Katner ED, et al. Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: a placebo-controlled study. Am J Psychiatry. 2003;160(2):371-373.
9. Raskind MA, Peskind ER, Hoff DJ, et al. A parallel group placebo-controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol Psychiatry. 2007;61(8):928-934.
10. Raskind MA, Peterson K, Williams T, et al. A trial of prazosin for combat trauma PTSD with nightmares in active-duty soldiers returned from Iraq and Afghanistan. Am J Psychiatry. 2013;170(9):1003-1010.
11. Germain A, Richardson R, Moul DE, et al. Placebo-controlled comparison of prazosin and cognitive-behavioral treatments for sleep disturbances in US military veterans. J Psychosom Res. 2012;72(2):89-96.
12. Taylor FB, Martin P, Thompson C, et al. Prazosin effects on objective sleep measures and clinical symptoms in civilian trauma posttraumatic stress disorder: a placebo-controlled study. Biol Psychiatry. 2008;63(6):629-632.
Antidepressants: Is a higher dose always better?
Mr. E, age 39, presents to the mental health (MH) intake clinic, reporting he has had depressed mood almost every day, lack of interests, poor appetite, difficulty sleeping, inability to concentrate on daily activities, low energy and motivation, and feelings of guilt. He is diagnosed with major depressive disorder and agrees to a trial of sertraline, which is titrated up to 100 mg/d. He is also referred to the MH pharmacy clinic for interim visits.
Four weeks later during a follow-up visit, Mr. E reports tolerating sertraline, 100 mg/d, with a slight improvement in his mood. He reports that he has started working on his previous hobbies again and tries to consistently eat 2 meals a day. He feels that his sleep remains unchanged. He would like to enroll in school again, but is concerned about his poor concentration. He asks whether a further increase in his sertraline dose would improve his symptoms. What would you advise?
Escalating antidepressant doses up to, or even above, the FDA-approved maximum dose is a strategy for clinicians to consider for patients who are nonresponders or partial responders to treatment. This practice assumes that the effectiveness of an antidepressant is dependent on the dosage. However, based on our review of available literature, this recommendation is equivocally supported for general practice.
Selective serotonin reuptake inhibitors
The Table1-3 summarizes the results of 3 studies of high-dose selective serotonin reuptake inhibitors (SSRIs).
Adli et al1 evaluated 3 types of studies—studies of patients with treatment-resistant depression receiving high-dose treatment, comparative dose studies, and studies of therapeutic drug-monitoring (TDM) of antidepressants—to assess the effectiveness of high-dose antidepressants after a treatment failure with a medium dose. They concluded that SSRIs exhibit a flat dose-dependency pattern, where increasing a dose above the minimum effective dose (MED) does not increase efficacy but results in more adverse effects. Because treatment at the MED inhibits 70% of serotonin reuptake and is only marginally less effective than medium therapeutic doses, the authors recommended reserving treatment at higher doses for patients who have failed other standard treatment options, such as augmentation.
Ruhe et al2 evaluated 8 randomized controlled trials and 3 systematic analyses that investigated dose escalation of SSRIs, including paroxetine, fluoxetine, and sertraline. The authors noted that all included studies had methodological limitations and discussed 1 study that showed potential benefit from dose escalation when dropouts due to adverse effects were excluded from analysis. They determined that the evidence for increased efficacy with dose escalation was inconclusive; however, dose escalation un-doubtedly resulted in more adverse effects.
Hieronymus et al3 found a dose-dependency pattern with selected SSRIs—citalopram, paroxetine, and sertraline—in a mega-analysis of studies of adult patients with depression. All company-funded, acute-phase, placebo-controlled fixed-dose trials of these agents were included in this analysis. It included a total of 2,859 patients: 600 patients received citalopram (10 to 60 mg/d); 1,043 patients received paroxetine (10 to 40 mg/d); 481 patients received sertraline (50 to 400 mg/d); and 735 patients received placebo. They further divided the SSRIs into “low” vs “optimal” doses based on the dose curves of these agents. For citalopram, 10 to 20 mg/d was considered low vs 40 to 60 mg/d, which was considered optimal. For paroxetine, 10 mg/d was considered low vs other doses as optimal (20, 30, and 40 mg/d). For sertraline, 50 mg was considered low vs other doses as optimal (100, 200, and 400 mg/d). The authors concluded that at low doses, these antidepressants were superior to placebo but inferior to higher doses. Interestingly, they suggested that the dose-response relationship plateaued at 20 mg/d for paroxetine, 40 mg/d for citalopram, and 100 mg/d for sertraline. One of the limitations of the study was a lack of information on the tolerability of higher vs lower doses.
Continue to: Other antidepressants
Other antidepressants
Adli et al1 found a high-dose study and several comparative studies that supported a dose-response relationship with a reasonable degree of tolerability for venlafaxine, but there were no pertinent studies that evaluated mirtazapine. The only fixed-dose study found for bupropion did not support a dose-response relationship.1
The authors also concluded that there may be evidence supporting high-dose prescribing of tricyclic and tetracyclic antidepressants (TCAs and TeCAs, respectively). Despite the lack of clinical data that directly addressed the dose-dependency of TCAs and TeCAs, the authors supported dose escalation with amitriptyline, clomipramine, imipramine, desipramine, nortriptyline, and maprotiline, based on the data from comparative dose and TDM studies.1 The authors urged caution in interpreting and applying the results of TDM studies because the pharmacodynamic of each medication—such as being linear, curvilinear, or uncorrelated— may vary, which suggests there is a targeted therapeutic dose range.1
Important considerations
Differences in the pharmacokinetic and pharmacogenetic properties of individual medications may account for the mixed outcomes found when evaluating antidepressant dose-response relationships. Genetic polymorphisms of cytochrome (CYP) P450 enzymes, mainly CYP2D6 and CYP2D19, have been shown to directly affect antidepressants’ serum levels. Depending on the patient’s phenotype expression, such as poor, intermediate, extensive (ie, normal), or ultra-metabolizers, use of a specific antidepressant at a similar dose may result in therapeutic effectiveness, ineffectiveness, or toxicity. For antidepressants such as TCAs, which have a narrow therapeutic index compared with SSRIs, the differences in pharmacokinetic and pharmacogenetic properties becomes more impactful.1,4
Escalation within approved dose ranges
Few quality studies have conclusively found a relationship between antidepressant dose escalation within the FDA-approved dose ranges and efficacy, and there are few to no recommendations for prescribing doses above FDA-approved ranges. However, in clinical practice, clinicians may consider a dose escalation within the allowable dose ranges based on anecdotal evidence from previous patient cases. Consideration of relevant pharmacokinetic parameters and the patient’s individual pharmacogenetic factors may further guide clinicians and patients in making an informed decision on dose escalation to and beyond the FDA-approved doses.
CASE CONTINUED
After reviewing the evidence of antidepressant dose escalation and Mr. E’s progress, the MH pharmacist recommends that the psychiatrist increase Mr. E’s sertraline to 150 mg/d with close monitoring.
Related Resources
- Berney P. Dose-response relationship of recent antidepressants in the short-term treatment of depression. Dialogues Clin Neurosci. 2005;7:249.
- Jakubovski E, Varigonda AL, Freemantle N, et al. Systematic review and meta-analysis: dose-response relationship of selective serotonin reuptake inhibitors in major depressive disorder. Am J Psychiatry. 2016;173:174-183.
Drug Brand Names
Amitriptyline • Elavil
Bupropion • Wellbutrin
Citalopram • Celexa
Clomipramine • Anafranil
Desipramine • Norpramin
Fluoxetine • Prozac
Imipramine • Tofranil
Maprotiline • Ludiomil
Mirtazapine • Remeron
Nortriptyline • Pamelor
Paroxetine • Paxil
Sertraline • Zoloft
Venlafaxine • Effexor
1. Adli M, Baethge C, Heinz A, et al. Is dose escalation of antidepressants a rational strategy after a medium-dose treatment has failed? A systematic review. Eur Arch Psychiatry Clin Neurosci. 2005;255(6):387-400.
2. Ruhe HG, Huyser J, Swinkels JA, et al. Dose escalation for insufficient response to standard-dose selective serotonin reuptake inhibitors in major depressive disorder. Bri J Psychiatry. 2006;189:309-316.
3. Hieronymus F, Nilsson S, Eriksson E. A mega-analysis of fixed-dose trials reveals dose dependency and a rapid onset of action for the antidepressant effect of three selective serotonin reuptake inhibitors. Transl Psychiatry. 2016;6(6):e834. doi: 10.1038/tp.2016.104
4. Nassan M, Nicholson WY, Elliott MA, et al. Pharmacokinetic pharmacogenetic prescribing guidelines for antidepressants: a template for psychiatric precision medicine. Mayo Clin Proc. 2016;91(7):897-907.
Mr. E, age 39, presents to the mental health (MH) intake clinic, reporting he has had depressed mood almost every day, lack of interests, poor appetite, difficulty sleeping, inability to concentrate on daily activities, low energy and motivation, and feelings of guilt. He is diagnosed with major depressive disorder and agrees to a trial of sertraline, which is titrated up to 100 mg/d. He is also referred to the MH pharmacy clinic for interim visits.
Four weeks later during a follow-up visit, Mr. E reports tolerating sertraline, 100 mg/d, with a slight improvement in his mood. He reports that he has started working on his previous hobbies again and tries to consistently eat 2 meals a day. He feels that his sleep remains unchanged. He would like to enroll in school again, but is concerned about his poor concentration. He asks whether a further increase in his sertraline dose would improve his symptoms. What would you advise?
Escalating antidepressant doses up to, or even above, the FDA-approved maximum dose is a strategy for clinicians to consider for patients who are nonresponders or partial responders to treatment. This practice assumes that the effectiveness of an antidepressant is dependent on the dosage. However, based on our review of available literature, this recommendation is equivocally supported for general practice.
Selective serotonin reuptake inhibitors
The Table1-3 summarizes the results of 3 studies of high-dose selective serotonin reuptake inhibitors (SSRIs).
Adli et al1 evaluated 3 types of studies—studies of patients with treatment-resistant depression receiving high-dose treatment, comparative dose studies, and studies of therapeutic drug-monitoring (TDM) of antidepressants—to assess the effectiveness of high-dose antidepressants after a treatment failure with a medium dose. They concluded that SSRIs exhibit a flat dose-dependency pattern, where increasing a dose above the minimum effective dose (MED) does not increase efficacy but results in more adverse effects. Because treatment at the MED inhibits 70% of serotonin reuptake and is only marginally less effective than medium therapeutic doses, the authors recommended reserving treatment at higher doses for patients who have failed other standard treatment options, such as augmentation.
Ruhe et al2 evaluated 8 randomized controlled trials and 3 systematic analyses that investigated dose escalation of SSRIs, including paroxetine, fluoxetine, and sertraline. The authors noted that all included studies had methodological limitations and discussed 1 study that showed potential benefit from dose escalation when dropouts due to adverse effects were excluded from analysis. They determined that the evidence for increased efficacy with dose escalation was inconclusive; however, dose escalation un-doubtedly resulted in more adverse effects.
Hieronymus et al3 found a dose-dependency pattern with selected SSRIs—citalopram, paroxetine, and sertraline—in a mega-analysis of studies of adult patients with depression. All company-funded, acute-phase, placebo-controlled fixed-dose trials of these agents were included in this analysis. It included a total of 2,859 patients: 600 patients received citalopram (10 to 60 mg/d); 1,043 patients received paroxetine (10 to 40 mg/d); 481 patients received sertraline (50 to 400 mg/d); and 735 patients received placebo. They further divided the SSRIs into “low” vs “optimal” doses based on the dose curves of these agents. For citalopram, 10 to 20 mg/d was considered low vs 40 to 60 mg/d, which was considered optimal. For paroxetine, 10 mg/d was considered low vs other doses as optimal (20, 30, and 40 mg/d). For sertraline, 50 mg was considered low vs other doses as optimal (100, 200, and 400 mg/d). The authors concluded that at low doses, these antidepressants were superior to placebo but inferior to higher doses. Interestingly, they suggested that the dose-response relationship plateaued at 20 mg/d for paroxetine, 40 mg/d for citalopram, and 100 mg/d for sertraline. One of the limitations of the study was a lack of information on the tolerability of higher vs lower doses.
Continue to: Other antidepressants
Other antidepressants
Adli et al1 found a high-dose study and several comparative studies that supported a dose-response relationship with a reasonable degree of tolerability for venlafaxine, but there were no pertinent studies that evaluated mirtazapine. The only fixed-dose study found for bupropion did not support a dose-response relationship.1
The authors also concluded that there may be evidence supporting high-dose prescribing of tricyclic and tetracyclic antidepressants (TCAs and TeCAs, respectively). Despite the lack of clinical data that directly addressed the dose-dependency of TCAs and TeCAs, the authors supported dose escalation with amitriptyline, clomipramine, imipramine, desipramine, nortriptyline, and maprotiline, based on the data from comparative dose and TDM studies.1 The authors urged caution in interpreting and applying the results of TDM studies because the pharmacodynamic of each medication—such as being linear, curvilinear, or uncorrelated— may vary, which suggests there is a targeted therapeutic dose range.1
Important considerations
Differences in the pharmacokinetic and pharmacogenetic properties of individual medications may account for the mixed outcomes found when evaluating antidepressant dose-response relationships. Genetic polymorphisms of cytochrome (CYP) P450 enzymes, mainly CYP2D6 and CYP2D19, have been shown to directly affect antidepressants’ serum levels. Depending on the patient’s phenotype expression, such as poor, intermediate, extensive (ie, normal), or ultra-metabolizers, use of a specific antidepressant at a similar dose may result in therapeutic effectiveness, ineffectiveness, or toxicity. For antidepressants such as TCAs, which have a narrow therapeutic index compared with SSRIs, the differences in pharmacokinetic and pharmacogenetic properties becomes more impactful.1,4
Escalation within approved dose ranges
Few quality studies have conclusively found a relationship between antidepressant dose escalation within the FDA-approved dose ranges and efficacy, and there are few to no recommendations for prescribing doses above FDA-approved ranges. However, in clinical practice, clinicians may consider a dose escalation within the allowable dose ranges based on anecdotal evidence from previous patient cases. Consideration of relevant pharmacokinetic parameters and the patient’s individual pharmacogenetic factors may further guide clinicians and patients in making an informed decision on dose escalation to and beyond the FDA-approved doses.
CASE CONTINUED
After reviewing the evidence of antidepressant dose escalation and Mr. E’s progress, the MH pharmacist recommends that the psychiatrist increase Mr. E’s sertraline to 150 mg/d with close monitoring.
Related Resources
- Berney P. Dose-response relationship of recent antidepressants in the short-term treatment of depression. Dialogues Clin Neurosci. 2005;7:249.
- Jakubovski E, Varigonda AL, Freemantle N, et al. Systematic review and meta-analysis: dose-response relationship of selective serotonin reuptake inhibitors in major depressive disorder. Am J Psychiatry. 2016;173:174-183.
Drug Brand Names
Amitriptyline • Elavil
Bupropion • Wellbutrin
Citalopram • Celexa
Clomipramine • Anafranil
Desipramine • Norpramin
Fluoxetine • Prozac
Imipramine • Tofranil
Maprotiline • Ludiomil
Mirtazapine • Remeron
Nortriptyline • Pamelor
Paroxetine • Paxil
Sertraline • Zoloft
Venlafaxine • Effexor
Mr. E, age 39, presents to the mental health (MH) intake clinic, reporting he has had depressed mood almost every day, lack of interests, poor appetite, difficulty sleeping, inability to concentrate on daily activities, low energy and motivation, and feelings of guilt. He is diagnosed with major depressive disorder and agrees to a trial of sertraline, which is titrated up to 100 mg/d. He is also referred to the MH pharmacy clinic for interim visits.
Four weeks later during a follow-up visit, Mr. E reports tolerating sertraline, 100 mg/d, with a slight improvement in his mood. He reports that he has started working on his previous hobbies again and tries to consistently eat 2 meals a day. He feels that his sleep remains unchanged. He would like to enroll in school again, but is concerned about his poor concentration. He asks whether a further increase in his sertraline dose would improve his symptoms. What would you advise?
Escalating antidepressant doses up to, or even above, the FDA-approved maximum dose is a strategy for clinicians to consider for patients who are nonresponders or partial responders to treatment. This practice assumes that the effectiveness of an antidepressant is dependent on the dosage. However, based on our review of available literature, this recommendation is equivocally supported for general practice.
Selective serotonin reuptake inhibitors
The Table1-3 summarizes the results of 3 studies of high-dose selective serotonin reuptake inhibitors (SSRIs).
Adli et al1 evaluated 3 types of studies—studies of patients with treatment-resistant depression receiving high-dose treatment, comparative dose studies, and studies of therapeutic drug-monitoring (TDM) of antidepressants—to assess the effectiveness of high-dose antidepressants after a treatment failure with a medium dose. They concluded that SSRIs exhibit a flat dose-dependency pattern, where increasing a dose above the minimum effective dose (MED) does not increase efficacy but results in more adverse effects. Because treatment at the MED inhibits 70% of serotonin reuptake and is only marginally less effective than medium therapeutic doses, the authors recommended reserving treatment at higher doses for patients who have failed other standard treatment options, such as augmentation.
Ruhe et al2 evaluated 8 randomized controlled trials and 3 systematic analyses that investigated dose escalation of SSRIs, including paroxetine, fluoxetine, and sertraline. The authors noted that all included studies had methodological limitations and discussed 1 study that showed potential benefit from dose escalation when dropouts due to adverse effects were excluded from analysis. They determined that the evidence for increased efficacy with dose escalation was inconclusive; however, dose escalation un-doubtedly resulted in more adverse effects.
Hieronymus et al3 found a dose-dependency pattern with selected SSRIs—citalopram, paroxetine, and sertraline—in a mega-analysis of studies of adult patients with depression. All company-funded, acute-phase, placebo-controlled fixed-dose trials of these agents were included in this analysis. It included a total of 2,859 patients: 600 patients received citalopram (10 to 60 mg/d); 1,043 patients received paroxetine (10 to 40 mg/d); 481 patients received sertraline (50 to 400 mg/d); and 735 patients received placebo. They further divided the SSRIs into “low” vs “optimal” doses based on the dose curves of these agents. For citalopram, 10 to 20 mg/d was considered low vs 40 to 60 mg/d, which was considered optimal. For paroxetine, 10 mg/d was considered low vs other doses as optimal (20, 30, and 40 mg/d). For sertraline, 50 mg was considered low vs other doses as optimal (100, 200, and 400 mg/d). The authors concluded that at low doses, these antidepressants were superior to placebo but inferior to higher doses. Interestingly, they suggested that the dose-response relationship plateaued at 20 mg/d for paroxetine, 40 mg/d for citalopram, and 100 mg/d for sertraline. One of the limitations of the study was a lack of information on the tolerability of higher vs lower doses.
Continue to: Other antidepressants
Other antidepressants
Adli et al1 found a high-dose study and several comparative studies that supported a dose-response relationship with a reasonable degree of tolerability for venlafaxine, but there were no pertinent studies that evaluated mirtazapine. The only fixed-dose study found for bupropion did not support a dose-response relationship.1
The authors also concluded that there may be evidence supporting high-dose prescribing of tricyclic and tetracyclic antidepressants (TCAs and TeCAs, respectively). Despite the lack of clinical data that directly addressed the dose-dependency of TCAs and TeCAs, the authors supported dose escalation with amitriptyline, clomipramine, imipramine, desipramine, nortriptyline, and maprotiline, based on the data from comparative dose and TDM studies.1 The authors urged caution in interpreting and applying the results of TDM studies because the pharmacodynamic of each medication—such as being linear, curvilinear, or uncorrelated— may vary, which suggests there is a targeted therapeutic dose range.1
Important considerations
Differences in the pharmacokinetic and pharmacogenetic properties of individual medications may account for the mixed outcomes found when evaluating antidepressant dose-response relationships. Genetic polymorphisms of cytochrome (CYP) P450 enzymes, mainly CYP2D6 and CYP2D19, have been shown to directly affect antidepressants’ serum levels. Depending on the patient’s phenotype expression, such as poor, intermediate, extensive (ie, normal), or ultra-metabolizers, use of a specific antidepressant at a similar dose may result in therapeutic effectiveness, ineffectiveness, or toxicity. For antidepressants such as TCAs, which have a narrow therapeutic index compared with SSRIs, the differences in pharmacokinetic and pharmacogenetic properties becomes more impactful.1,4
Escalation within approved dose ranges
Few quality studies have conclusively found a relationship between antidepressant dose escalation within the FDA-approved dose ranges and efficacy, and there are few to no recommendations for prescribing doses above FDA-approved ranges. However, in clinical practice, clinicians may consider a dose escalation within the allowable dose ranges based on anecdotal evidence from previous patient cases. Consideration of relevant pharmacokinetic parameters and the patient’s individual pharmacogenetic factors may further guide clinicians and patients in making an informed decision on dose escalation to and beyond the FDA-approved doses.
CASE CONTINUED
After reviewing the evidence of antidepressant dose escalation and Mr. E’s progress, the MH pharmacist recommends that the psychiatrist increase Mr. E’s sertraline to 150 mg/d with close monitoring.
Related Resources
- Berney P. Dose-response relationship of recent antidepressants in the short-term treatment of depression. Dialogues Clin Neurosci. 2005;7:249.
- Jakubovski E, Varigonda AL, Freemantle N, et al. Systematic review and meta-analysis: dose-response relationship of selective serotonin reuptake inhibitors in major depressive disorder. Am J Psychiatry. 2016;173:174-183.
Drug Brand Names
Amitriptyline • Elavil
Bupropion • Wellbutrin
Citalopram • Celexa
Clomipramine • Anafranil
Desipramine • Norpramin
Fluoxetine • Prozac
Imipramine • Tofranil
Maprotiline • Ludiomil
Mirtazapine • Remeron
Nortriptyline • Pamelor
Paroxetine • Paxil
Sertraline • Zoloft
Venlafaxine • Effexor
1. Adli M, Baethge C, Heinz A, et al. Is dose escalation of antidepressants a rational strategy after a medium-dose treatment has failed? A systematic review. Eur Arch Psychiatry Clin Neurosci. 2005;255(6):387-400.
2. Ruhe HG, Huyser J, Swinkels JA, et al. Dose escalation for insufficient response to standard-dose selective serotonin reuptake inhibitors in major depressive disorder. Bri J Psychiatry. 2006;189:309-316.
3. Hieronymus F, Nilsson S, Eriksson E. A mega-analysis of fixed-dose trials reveals dose dependency and a rapid onset of action for the antidepressant effect of three selective serotonin reuptake inhibitors. Transl Psychiatry. 2016;6(6):e834. doi: 10.1038/tp.2016.104
4. Nassan M, Nicholson WY, Elliott MA, et al. Pharmacokinetic pharmacogenetic prescribing guidelines for antidepressants: a template for psychiatric precision medicine. Mayo Clin Proc. 2016;91(7):897-907.
1. Adli M, Baethge C, Heinz A, et al. Is dose escalation of antidepressants a rational strategy after a medium-dose treatment has failed? A systematic review. Eur Arch Psychiatry Clin Neurosci. 2005;255(6):387-400.
2. Ruhe HG, Huyser J, Swinkels JA, et al. Dose escalation for insufficient response to standard-dose selective serotonin reuptake inhibitors in major depressive disorder. Bri J Psychiatry. 2006;189:309-316.
3. Hieronymus F, Nilsson S, Eriksson E. A mega-analysis of fixed-dose trials reveals dose dependency and a rapid onset of action for the antidepressant effect of three selective serotonin reuptake inhibitors. Transl Psychiatry. 2016;6(6):e834. doi: 10.1038/tp.2016.104
4. Nassan M, Nicholson WY, Elliott MA, et al. Pharmacokinetic pharmacogenetic prescribing guidelines for antidepressants: a template for psychiatric precision medicine. Mayo Clin Proc. 2016;91(7):897-907.
Management of major depressive disorder with psychotic features
Mrs. C, age 56, has a history of major depressive disorder (MDD). She has been stable for 5 years without medication. Six months ago, she presented to you, along with her son, seeking help. She reported that she had been experiencing insomnia, fatigue, and was not engaging in hobbies. Her son told you that his mother had lost weight and had been avoiding family dinners. Mrs. C reported recurrent thoughts of dying and heard voices vividly telling her that she was a burden and that her family would be better off without her. However, there was no imminent danger of self-harm. At that appointment, you initiated
Since that time, Mrs. C has followed up with you monthly with good response to the medications. Currently, she states her depression is much improved, and she denies hearing voices for approximately 5 months.
Based on her presentation and response, what do the data suggest about her length of treatment, and when should you consider tapering the antipsychotic medication?
In DSM-5, MDD with psychotic features is a severe subtype of MDD that is defined as a major depressive episode characterized by delusions and/or hallucinations.1 In the general population, the lifetime prevalence of this disorder varies from 0.35% to 1%, and the rate is higher in older patients.2 Risk factors include female gender, family history, and concomitant bipolar disorder.2
Epidemiologic studies have shown that psychotic features can occur in 15% to 20% of patients with MDD. The psychotic features that occur during these episodes are delusions and hallucinations.1 These features can be either mood-congruent (related to the depressive themes of worthlessness or guilt) or mood-incongruent (ie, unrelated to depressive themes).1
Treatment options: ECT or pharmacotherapy
Guidelines from the American Psychiatric Association3 and the National Institute for Clinical Excellence4 recommend treating depression with psychosis with electroconvulsive therapy (ECT) or with combined antidepressant and antipsychotic medications as first-line options. The Texas Medication Algorithm Project (TMAP) Algorithm for MDD,5 which closely focuses on treatment of MDD with psychotic features, can be used for treatment decisions (see Related Resources).
Electroconvulsive therapy is known to be efficacious in treating patients with MDD with psychotic features and should be considered as a treatment option. However, medication therapy is often chosen as the initial treatment due to the limitations of ECT, including accessibility, cost, and patient preference. However, in certain cases, ECT is the preferred option because it can provide rapid and significant improvement in patients with severe psychosis, suicidality, or risk of imminent harm.
Continue to: Pharmacotherapy
Pharmacotherapy for the treatment of MDD with psychotic features should consist of a combination of an antidepressant and antipsychotic medication. This combination has been shown to be more effective than either agent alone. Some combinations have been studied specifically for MDD with psychosis. The Study of the Pharmacotherapy of Psychotic Depression (STOP-PD), a 12-week, double-blind, randomized controlled trial, found that the combination of sertraline and olanzapine was efficacious and superior to monotherapy with olanzapine in an acute setting.6 In another study, the combination of olanzapine and
How long should treatment last?
The optimal timeline for treating patients with MDD with psychotic features is unknown. According to the TMAP algorithm and expert opinion, the continuation phase of pharmacotherapy should include treatment for at least 4 months with an antipsychotic medication and at least 2 years to lifetime treatment with an antidepressant.5 The STOP-PD II study, which was a continuation of the 12-week STOP-PD study, examined antipsychotic duration to determine the effects of continuing olanzapine once an episode of psychotic depression had responded to olanzapine and sertraline.11 Patients who had achieved remission after receiving olanzapine and sertraline were randomized to continue to receive this combination or to receive sertraline plus placebo for 36 weeks. The primary outcome was relapse, which was broadly defined as 1 of the following11:
- a Structured Clinical Interview for the DSM (SCID)-rated assessment that revealed the patient had enough symptoms to meet criteria for a DSM-IV major depressive episode
- a 17-item HAM-D scoren of ≥18
- SCID-rated psychosis
- other significant clinical worsening, defined as having a suicide plan or attempting suicide, developing SCID-rated symptoms of mania or hypomania, or being hospitalized in a psychiatric unit.
Compared with sertraline plus placebo, continuing sertraline plus olanzapine reduced the risk of relapse over 36 weeks (hazard ratio, 0.25; 95% confidence interval, 0.13 to 0.48; P < .001).11 However, as expected, the incidence of adverse effects such as weight gain and parkinsonism was higher in the olanzapine group. Therefore, it is important to consider the potential long-term adverse effects of continuing antipsychotic medications.
Weighing the evidence
Electroconvulsive therapy is considered a first-line treatment option for MDD with psychotic features; however, because of limitations associated with this approach, antidepressants plus antipsychotics are often utilized as an initial treatment.
Continue to: CASE
CASE CONTINUED
Based on the results of the STOP-PD and STOP-PD II trials, Mrs. C should be continued on sertraline plus olanzapine for at least another 3 to 6 months before an olanzapine taper should be considered. At that time, the risks and benefits of a taper vs continuing therapy should be considered. Given her history of MDD and the severity of this most recent episode, sertraline therapy should be continued for at least 2 years, and possibly indefinitely.
Related Resources
- Texas Medication Algorithm Project. Algorithm for the treatment of major depressive disorder with psychotic features. https://chsciowa.org/sites/chsciowa.org/files/resource/files/9_-_depression_med_algorithm_supplement.pdf
- Dold M, Bartova L, Kautzky A, et al. Psychotic features in patients with major depressive disorder: a report from the European Group for the Study of Resistant Depression. J Clin Psychiatry. 2019;80(1):17m12090. doi: 10.4088/ JCP.17m12090
- Flint AJ, Meyers BS, Rothschild AJ, et al. Effect of continuing olanzapine vs placebo on relapse among patients with psychotic depression in remission: the STOP-PD II randomized clinical trial. JAMA. 2019;322(7): 622-631.
Drug Brand Names
Amitriptyline • Elavil, Endep
Fluoxetine • Prozac
Haloperidol • Haldol
Olanzapine • Zyprexa
Quetiapine • Seroquel
Sertraline • Zoloft
Venlafaxine • Effexor
1. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
2. Jääskeläinen E, Juola T, Korpela H, et al. Epidemiology of psychotic depression - systematic review and meta-analysis. Psychol Med. 2018;48(6):905-918.
3. American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder (revision). Am J Psychiatry. 2000;157(4)(suppl):1-45.
4. National Institute for Clinical Excellence. Depression in adults: recognition and management: clinical guideline [CG90]. National Institute for Health and Clinical Excellence. Published October 28, 2009. Accessed January 12, 2021. https://www.nice.org.uk/guidance/cg90
5. Crimson ML, Trivedi M, Pigott TA, et al. The Texas Medication Algorithm Project: report of the Texas Consensus Conference Panel on medication treatment of major depressive disorder. J Clin Psychiatry. 1999;60(3):142-156.
6. Meyers BS, Flint AJ, Rothschild AJ, et al. A double-blind randomized controlled trial of olanzapine plus sertraline vs olanzapine plus placebo for psychotic depression: the study of pharmacotherapy for psychotic depression -- the STOP-PD study. Arch Gen Psychiatry. 2009;66(8):838-847.
7. Rothschild AJ, Williamson DJ, Tohen MF, et al. A double-blind, randomized study of olanzapine and olanzapine/fluoxetine combination for major depression with psychotic features. J Clin Psychopharmacol. 2004;24(4):365-373.
8. Wijkstra J, Burger H, van den Broek WW, et al. Treatment of unipolar psychotic depression: a randomized, doubleblind study comparing imipramine, venlafaxine, and venlafaxine plus quetiapine. Acta Psychiatr Scand. 2010;21(3):190-200.
9. Muller-Siecheneder F, Muller M, Hillert A, et al. Risperidone versus haloperidol and amitriptyline in the treatment of patients with a combined psychotic and depressive syndrome. J Clin Psychopharm. 1998;18(2):111-120.
10. Spiker DG, Weiss JC, Dealy RS, et al. The pharmacological treatment of delusional depression. Am J Psychiatry. 1985;142(4):430-436.
11. Flint AJ, Meyers BS, Rothschild AJ, et al. Effect of continuing olanzapine vs placebo on relapse among patients with psychotic depression in remission: the STOP-PD II randomized clinical trial. JAMA. 2019;322(7):622-631.
Mrs. C, age 56, has a history of major depressive disorder (MDD). She has been stable for 5 years without medication. Six months ago, she presented to you, along with her son, seeking help. She reported that she had been experiencing insomnia, fatigue, and was not engaging in hobbies. Her son told you that his mother had lost weight and had been avoiding family dinners. Mrs. C reported recurrent thoughts of dying and heard voices vividly telling her that she was a burden and that her family would be better off without her. However, there was no imminent danger of self-harm. At that appointment, you initiated
Since that time, Mrs. C has followed up with you monthly with good response to the medications. Currently, she states her depression is much improved, and she denies hearing voices for approximately 5 months.
Based on her presentation and response, what do the data suggest about her length of treatment, and when should you consider tapering the antipsychotic medication?
In DSM-5, MDD with psychotic features is a severe subtype of MDD that is defined as a major depressive episode characterized by delusions and/or hallucinations.1 In the general population, the lifetime prevalence of this disorder varies from 0.35% to 1%, and the rate is higher in older patients.2 Risk factors include female gender, family history, and concomitant bipolar disorder.2
Epidemiologic studies have shown that psychotic features can occur in 15% to 20% of patients with MDD. The psychotic features that occur during these episodes are delusions and hallucinations.1 These features can be either mood-congruent (related to the depressive themes of worthlessness or guilt) or mood-incongruent (ie, unrelated to depressive themes).1
Treatment options: ECT or pharmacotherapy
Guidelines from the American Psychiatric Association3 and the National Institute for Clinical Excellence4 recommend treating depression with psychosis with electroconvulsive therapy (ECT) or with combined antidepressant and antipsychotic medications as first-line options. The Texas Medication Algorithm Project (TMAP) Algorithm for MDD,5 which closely focuses on treatment of MDD with psychotic features, can be used for treatment decisions (see Related Resources).
Electroconvulsive therapy is known to be efficacious in treating patients with MDD with psychotic features and should be considered as a treatment option. However, medication therapy is often chosen as the initial treatment due to the limitations of ECT, including accessibility, cost, and patient preference. However, in certain cases, ECT is the preferred option because it can provide rapid and significant improvement in patients with severe psychosis, suicidality, or risk of imminent harm.
Continue to: Pharmacotherapy
Pharmacotherapy for the treatment of MDD with psychotic features should consist of a combination of an antidepressant and antipsychotic medication. This combination has been shown to be more effective than either agent alone. Some combinations have been studied specifically for MDD with psychosis. The Study of the Pharmacotherapy of Psychotic Depression (STOP-PD), a 12-week, double-blind, randomized controlled trial, found that the combination of sertraline and olanzapine was efficacious and superior to monotherapy with olanzapine in an acute setting.6 In another study, the combination of olanzapine and
How long should treatment last?
The optimal timeline for treating patients with MDD with psychotic features is unknown. According to the TMAP algorithm and expert opinion, the continuation phase of pharmacotherapy should include treatment for at least 4 months with an antipsychotic medication and at least 2 years to lifetime treatment with an antidepressant.5 The STOP-PD II study, which was a continuation of the 12-week STOP-PD study, examined antipsychotic duration to determine the effects of continuing olanzapine once an episode of psychotic depression had responded to olanzapine and sertraline.11 Patients who had achieved remission after receiving olanzapine and sertraline were randomized to continue to receive this combination or to receive sertraline plus placebo for 36 weeks. The primary outcome was relapse, which was broadly defined as 1 of the following11:
- a Structured Clinical Interview for the DSM (SCID)-rated assessment that revealed the patient had enough symptoms to meet criteria for a DSM-IV major depressive episode
- a 17-item HAM-D scoren of ≥18
- SCID-rated psychosis
- other significant clinical worsening, defined as having a suicide plan or attempting suicide, developing SCID-rated symptoms of mania or hypomania, or being hospitalized in a psychiatric unit.
Compared with sertraline plus placebo, continuing sertraline plus olanzapine reduced the risk of relapse over 36 weeks (hazard ratio, 0.25; 95% confidence interval, 0.13 to 0.48; P < .001).11 However, as expected, the incidence of adverse effects such as weight gain and parkinsonism was higher in the olanzapine group. Therefore, it is important to consider the potential long-term adverse effects of continuing antipsychotic medications.
Weighing the evidence
Electroconvulsive therapy is considered a first-line treatment option for MDD with psychotic features; however, because of limitations associated with this approach, antidepressants plus antipsychotics are often utilized as an initial treatment.
Continue to: CASE
CASE CONTINUED
Based on the results of the STOP-PD and STOP-PD II trials, Mrs. C should be continued on sertraline plus olanzapine for at least another 3 to 6 months before an olanzapine taper should be considered. At that time, the risks and benefits of a taper vs continuing therapy should be considered. Given her history of MDD and the severity of this most recent episode, sertraline therapy should be continued for at least 2 years, and possibly indefinitely.
Related Resources
- Texas Medication Algorithm Project. Algorithm for the treatment of major depressive disorder with psychotic features. https://chsciowa.org/sites/chsciowa.org/files/resource/files/9_-_depression_med_algorithm_supplement.pdf
- Dold M, Bartova L, Kautzky A, et al. Psychotic features in patients with major depressive disorder: a report from the European Group for the Study of Resistant Depression. J Clin Psychiatry. 2019;80(1):17m12090. doi: 10.4088/ JCP.17m12090
- Flint AJ, Meyers BS, Rothschild AJ, et al. Effect of continuing olanzapine vs placebo on relapse among patients with psychotic depression in remission: the STOP-PD II randomized clinical trial. JAMA. 2019;322(7): 622-631.
Drug Brand Names
Amitriptyline • Elavil, Endep
Fluoxetine • Prozac
Haloperidol • Haldol
Olanzapine • Zyprexa
Quetiapine • Seroquel
Sertraline • Zoloft
Venlafaxine • Effexor
Mrs. C, age 56, has a history of major depressive disorder (MDD). She has been stable for 5 years without medication. Six months ago, she presented to you, along with her son, seeking help. She reported that she had been experiencing insomnia, fatigue, and was not engaging in hobbies. Her son told you that his mother had lost weight and had been avoiding family dinners. Mrs. C reported recurrent thoughts of dying and heard voices vividly telling her that she was a burden and that her family would be better off without her. However, there was no imminent danger of self-harm. At that appointment, you initiated
Since that time, Mrs. C has followed up with you monthly with good response to the medications. Currently, she states her depression is much improved, and she denies hearing voices for approximately 5 months.
Based on her presentation and response, what do the data suggest about her length of treatment, and when should you consider tapering the antipsychotic medication?
In DSM-5, MDD with psychotic features is a severe subtype of MDD that is defined as a major depressive episode characterized by delusions and/or hallucinations.1 In the general population, the lifetime prevalence of this disorder varies from 0.35% to 1%, and the rate is higher in older patients.2 Risk factors include female gender, family history, and concomitant bipolar disorder.2
Epidemiologic studies have shown that psychotic features can occur in 15% to 20% of patients with MDD. The psychotic features that occur during these episodes are delusions and hallucinations.1 These features can be either mood-congruent (related to the depressive themes of worthlessness or guilt) or mood-incongruent (ie, unrelated to depressive themes).1
Treatment options: ECT or pharmacotherapy
Guidelines from the American Psychiatric Association3 and the National Institute for Clinical Excellence4 recommend treating depression with psychosis with electroconvulsive therapy (ECT) or with combined antidepressant and antipsychotic medications as first-line options. The Texas Medication Algorithm Project (TMAP) Algorithm for MDD,5 which closely focuses on treatment of MDD with psychotic features, can be used for treatment decisions (see Related Resources).
Electroconvulsive therapy is known to be efficacious in treating patients with MDD with psychotic features and should be considered as a treatment option. However, medication therapy is often chosen as the initial treatment due to the limitations of ECT, including accessibility, cost, and patient preference. However, in certain cases, ECT is the preferred option because it can provide rapid and significant improvement in patients with severe psychosis, suicidality, or risk of imminent harm.
Continue to: Pharmacotherapy
Pharmacotherapy for the treatment of MDD with psychotic features should consist of a combination of an antidepressant and antipsychotic medication. This combination has been shown to be more effective than either agent alone. Some combinations have been studied specifically for MDD with psychosis. The Study of the Pharmacotherapy of Psychotic Depression (STOP-PD), a 12-week, double-blind, randomized controlled trial, found that the combination of sertraline and olanzapine was efficacious and superior to monotherapy with olanzapine in an acute setting.6 In another study, the combination of olanzapine and
How long should treatment last?
The optimal timeline for treating patients with MDD with psychotic features is unknown. According to the TMAP algorithm and expert opinion, the continuation phase of pharmacotherapy should include treatment for at least 4 months with an antipsychotic medication and at least 2 years to lifetime treatment with an antidepressant.5 The STOP-PD II study, which was a continuation of the 12-week STOP-PD study, examined antipsychotic duration to determine the effects of continuing olanzapine once an episode of psychotic depression had responded to olanzapine and sertraline.11 Patients who had achieved remission after receiving olanzapine and sertraline were randomized to continue to receive this combination or to receive sertraline plus placebo for 36 weeks. The primary outcome was relapse, which was broadly defined as 1 of the following11:
- a Structured Clinical Interview for the DSM (SCID)-rated assessment that revealed the patient had enough symptoms to meet criteria for a DSM-IV major depressive episode
- a 17-item HAM-D scoren of ≥18
- SCID-rated psychosis
- other significant clinical worsening, defined as having a suicide plan or attempting suicide, developing SCID-rated symptoms of mania or hypomania, or being hospitalized in a psychiatric unit.
Compared with sertraline plus placebo, continuing sertraline plus olanzapine reduced the risk of relapse over 36 weeks (hazard ratio, 0.25; 95% confidence interval, 0.13 to 0.48; P < .001).11 However, as expected, the incidence of adverse effects such as weight gain and parkinsonism was higher in the olanzapine group. Therefore, it is important to consider the potential long-term adverse effects of continuing antipsychotic medications.
Weighing the evidence
Electroconvulsive therapy is considered a first-line treatment option for MDD with psychotic features; however, because of limitations associated with this approach, antidepressants plus antipsychotics are often utilized as an initial treatment.
Continue to: CASE
CASE CONTINUED
Based on the results of the STOP-PD and STOP-PD II trials, Mrs. C should be continued on sertraline plus olanzapine for at least another 3 to 6 months before an olanzapine taper should be considered. At that time, the risks and benefits of a taper vs continuing therapy should be considered. Given her history of MDD and the severity of this most recent episode, sertraline therapy should be continued for at least 2 years, and possibly indefinitely.
Related Resources
- Texas Medication Algorithm Project. Algorithm for the treatment of major depressive disorder with psychotic features. https://chsciowa.org/sites/chsciowa.org/files/resource/files/9_-_depression_med_algorithm_supplement.pdf
- Dold M, Bartova L, Kautzky A, et al. Psychotic features in patients with major depressive disorder: a report from the European Group for the Study of Resistant Depression. J Clin Psychiatry. 2019;80(1):17m12090. doi: 10.4088/ JCP.17m12090
- Flint AJ, Meyers BS, Rothschild AJ, et al. Effect of continuing olanzapine vs placebo on relapse among patients with psychotic depression in remission: the STOP-PD II randomized clinical trial. JAMA. 2019;322(7): 622-631.
Drug Brand Names
Amitriptyline • Elavil, Endep
Fluoxetine • Prozac
Haloperidol • Haldol
Olanzapine • Zyprexa
Quetiapine • Seroquel
Sertraline • Zoloft
Venlafaxine • Effexor
1. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
2. Jääskeläinen E, Juola T, Korpela H, et al. Epidemiology of psychotic depression - systematic review and meta-analysis. Psychol Med. 2018;48(6):905-918.
3. American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder (revision). Am J Psychiatry. 2000;157(4)(suppl):1-45.
4. National Institute for Clinical Excellence. Depression in adults: recognition and management: clinical guideline [CG90]. National Institute for Health and Clinical Excellence. Published October 28, 2009. Accessed January 12, 2021. https://www.nice.org.uk/guidance/cg90
5. Crimson ML, Trivedi M, Pigott TA, et al. The Texas Medication Algorithm Project: report of the Texas Consensus Conference Panel on medication treatment of major depressive disorder. J Clin Psychiatry. 1999;60(3):142-156.
6. Meyers BS, Flint AJ, Rothschild AJ, et al. A double-blind randomized controlled trial of olanzapine plus sertraline vs olanzapine plus placebo for psychotic depression: the study of pharmacotherapy for psychotic depression -- the STOP-PD study. Arch Gen Psychiatry. 2009;66(8):838-847.
7. Rothschild AJ, Williamson DJ, Tohen MF, et al. A double-blind, randomized study of olanzapine and olanzapine/fluoxetine combination for major depression with psychotic features. J Clin Psychopharmacol. 2004;24(4):365-373.
8. Wijkstra J, Burger H, van den Broek WW, et al. Treatment of unipolar psychotic depression: a randomized, doubleblind study comparing imipramine, venlafaxine, and venlafaxine plus quetiapine. Acta Psychiatr Scand. 2010;21(3):190-200.
9. Muller-Siecheneder F, Muller M, Hillert A, et al. Risperidone versus haloperidol and amitriptyline in the treatment of patients with a combined psychotic and depressive syndrome. J Clin Psychopharm. 1998;18(2):111-120.
10. Spiker DG, Weiss JC, Dealy RS, et al. The pharmacological treatment of delusional depression. Am J Psychiatry. 1985;142(4):430-436.
11. Flint AJ, Meyers BS, Rothschild AJ, et al. Effect of continuing olanzapine vs placebo on relapse among patients with psychotic depression in remission: the STOP-PD II randomized clinical trial. JAMA. 2019;322(7):622-631.
1. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
2. Jääskeläinen E, Juola T, Korpela H, et al. Epidemiology of psychotic depression - systematic review and meta-analysis. Psychol Med. 2018;48(6):905-918.
3. American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder (revision). Am J Psychiatry. 2000;157(4)(suppl):1-45.
4. National Institute for Clinical Excellence. Depression in adults: recognition and management: clinical guideline [CG90]. National Institute for Health and Clinical Excellence. Published October 28, 2009. Accessed January 12, 2021. https://www.nice.org.uk/guidance/cg90
5. Crimson ML, Trivedi M, Pigott TA, et al. The Texas Medication Algorithm Project: report of the Texas Consensus Conference Panel on medication treatment of major depressive disorder. J Clin Psychiatry. 1999;60(3):142-156.
6. Meyers BS, Flint AJ, Rothschild AJ, et al. A double-blind randomized controlled trial of olanzapine plus sertraline vs olanzapine plus placebo for psychotic depression: the study of pharmacotherapy for psychotic depression -- the STOP-PD study. Arch Gen Psychiatry. 2009;66(8):838-847.
7. Rothschild AJ, Williamson DJ, Tohen MF, et al. A double-blind, randomized study of olanzapine and olanzapine/fluoxetine combination for major depression with psychotic features. J Clin Psychopharmacol. 2004;24(4):365-373.
8. Wijkstra J, Burger H, van den Broek WW, et al. Treatment of unipolar psychotic depression: a randomized, doubleblind study comparing imipramine, venlafaxine, and venlafaxine plus quetiapine. Acta Psychiatr Scand. 2010;21(3):190-200.
9. Muller-Siecheneder F, Muller M, Hillert A, et al. Risperidone versus haloperidol and amitriptyline in the treatment of patients with a combined psychotic and depressive syndrome. J Clin Psychopharm. 1998;18(2):111-120.
10. Spiker DG, Weiss JC, Dealy RS, et al. The pharmacological treatment of delusional depression. Am J Psychiatry. 1985;142(4):430-436.
11. Flint AJ, Meyers BS, Rothschild AJ, et al. Effect of continuing olanzapine vs placebo on relapse among patients with psychotic depression in remission: the STOP-PD II randomized clinical trial. JAMA. 2019;322(7):622-631.