User login
Pharmacotherapy for alcohol use disorder in patients with hepatic impairment
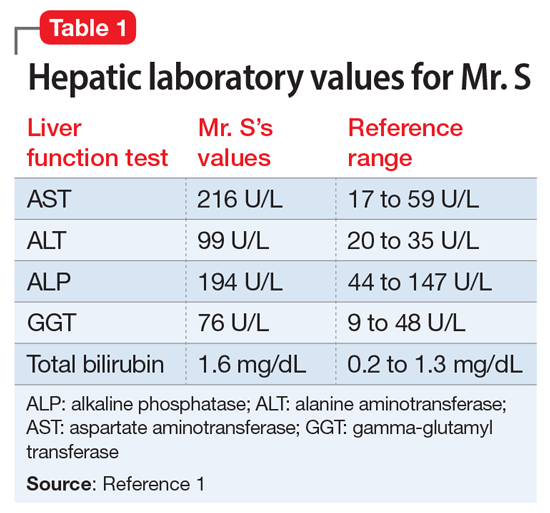
Mr. S, age 64, presents for an outpatient follow-up after a recent hospital discharge for alcohol detoxification. He reports a long history of alcohol use, which has resulted in numerous hospital admissions. He has recently been receiving care from a gastroenterologist because the results of laboratory testing suggested hepatic impairment (Table 1). Mr. S says that a friend of his was able to stop drinking by taking a medication, and he wonders if he can be prescribed a medication to help him as well.
A chart review shows that Mr. S recently underwent paracentesis, during which 6 liters of fluid were removed. Additionally, an abdominal ultrasound confirmed hepatic cirrhosis.
According to the World Health Organization, alcohol consumption contributes to 3 million deaths annually.2 The highest proportion of these deaths (21.3%) is due to alcohol-associated gastrointestinal complications, including alcoholic and infectious hepatitis, pancreatitis, and cirrhosis. Because the liver is the primary site of ethanol metabolism, it sustains the greatest degree of tissue injury with heavy alcohol consumption. Additionally, the association of harmful use of alcohol with risky sexual behavior may partially explain the higher prevalence of viral hepatitis among persons with alcohol use disorder (AUD) compared with the general population. Alcoholic liver disease (ALD) progresses through several stages, beginning with hepatic steatosis and progressing through alcohol-related hepatitis, fibrosis, cirrhosis, and potentially hepatocellular carcinoma.3
Liver markers of alcohol use
Although biological markers can be used in clinical practice to screen and monitor for alcohol abuse, making a diagnosis of ALD can be challenging. Typically, a history of heavy alcohol consumption in addition to certain physical signs and laboratory tests for liver disease are the best indicators of ALD. However, the clinical assessment can be confounded by patients who deny or minimize how much alcohol they have consumed. Furthermore, physical and laboratory findings may not be specific to ALD.
Liver enzymes, including aspartate aminotransferase (AST), alanine aminotransferase (ALT), and gamma-glutamyltransferase (GGT), have historically been used as the basis of diagnosing ALD. In addition to elevated bilirubin and evidence of macrocytic anemia, elevations in these enzymes may suggest heavy alcohol use, but these values alone are inadequate to establish ALD. Gamma-glutamyltransferase is found in cell membranes of several body tissues, including the liver and spleen, and therefore is not specific to liver damage. However, elevated GGT is the best indicator of excessive alcohol consumption because it has greater sensitivity than AST and ALT.1,3,4
Although these biomarkers are helpful in diagnosing ALD, they lose some of their utility in patients with advanced liver disease. Patients with severe liver dysfunction may not have elevated serum aminotransferase levels because the degree of liver enzyme elevation does not correlate well with the severity of ALD. For example, patients with advanced cirrhosis may have liver enzyme levels that appear normal. However, the pattern of elevation in transaminases can be helpful in making a diagnosis of liver dysfunction; using the ratio of AST to ALT may aid in diagnosing ALD, because AST is elevated more than twice that of ALT in >80% of patients with ALD.1,3,4
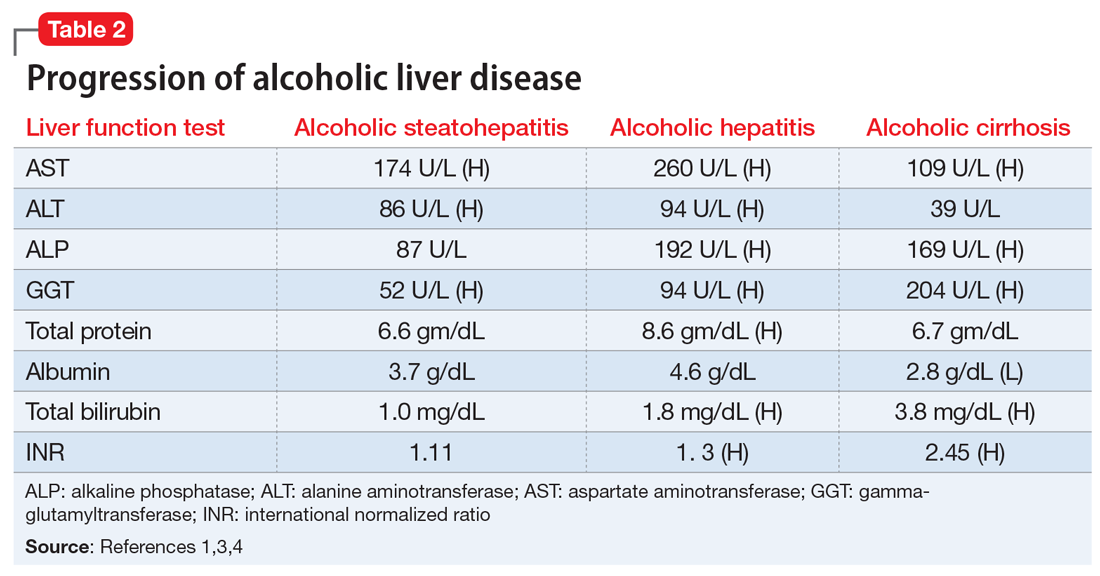
Table 21,3,4 shows the progression of ALD from steatohepatitis to alcoholic hepatitis to cirrhosis. In steatohepatitis, transaminitis is present but all other biomarkers normal. In alcoholic hepatitis, transaminitis is present along with elevated alkaline phosphatase, elevated bilirubin, and elevated international normalized ratio (INR). In alcoholic cirrhosis, the AST-to-ALT ratio is >2, and hypoalbuminemia, hyperbilirubinemia, and coagulopathy (evidenced by elevated INR) are present, consistent with long-term liver damage.1,3,4
Continue to: FDA-approved medications
FDA-approved medications
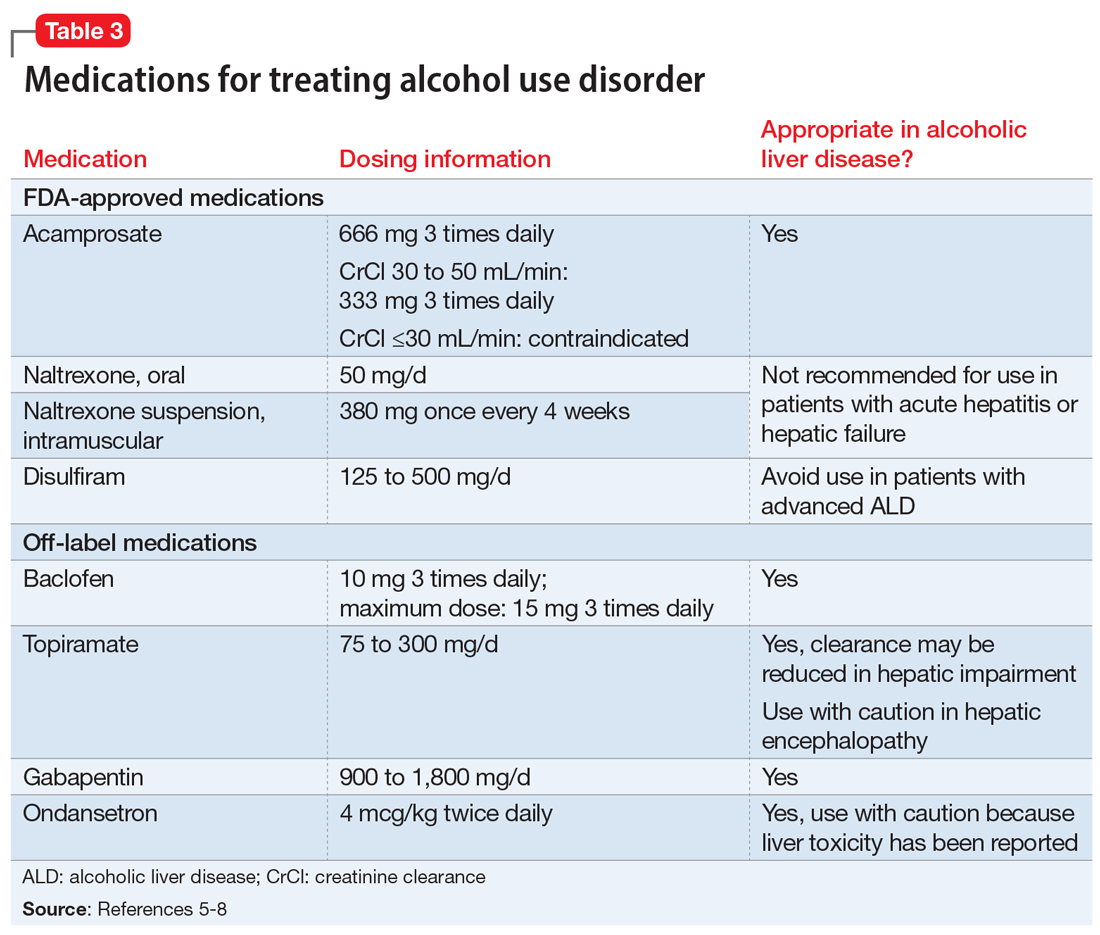
Three medications—acamprosate, naltrexone, and disulfiram—currently are FDA-approved for treating AUD.5,6 Additionally, several other medications have shown varying levels of efficacy in treating patients with AUD but are not FDA-approved for this indication (Table 3).5-8
Acamprosate is thought to create a balance of inhibitor and excitatory neurotransmitters by functioning as a glutamate antagonist and gamma-aminobutyric acid (GABA) agonist. This is speculated to aid in abstinence from alcohol. Data suggests that acamprosate may be more effective for maintaining abstinence than for inducing remission in individuals who have not yet detoxified from alcohol. Because of its renal excretion, acamprosate is the only FDA-approved medication for AUD that is not associated with liver toxicity. The most commonly reported adverse effect with acamprosate use is diarrhea.
Naltrexone, a mu-opioid receptor antagonist, is available in both tablet and long-acting IM injection formulations. Naltrexone blocks the binding of endorphins created by alcohol consumption to opioid receptors. This results in diminished dopamine release and is speculated to decrease reward and positive reinforcement with alcohol consumption, leading to fewer heavy drinking days. Due to hepatic metabolism, naltrexone use carries a risk of liver injury. Cases of hepatitis and clinically significant liver dysfunction as well as transient, asymptomatic, hepatic transaminase elevations have been observed in patients who receive naltrexone. Because of the absence of first-pass metabolism, long-acting IM naltrexone may produce less hepatotoxicity than the oral formulation. When the FDA approved both formulations of naltrexone, a “black-box” warning was issued concerning the risk of liver damage; however, these warnings have since been removed from their respective prescribing information.
Disulfiram inhibits acetaldehyde dehydrogenase, resulting in elevated acetaldehyde concentrations after consuming alcohol. In theory, this medication reduces a person’s desire to drink due to the negative physiological and physical effects associated with increased acetaldehyde, including hypotension, flushing, nausea, and vomiting. Although most of these reactions are short-lived, disulfiram can induce hepatotoxicity and liver failure that may prove fatal. Disulfiram should be avoided in patients with advanced ALD.
Off-label medications for AUD
Additional pharmacotherapeutic agents have been evaluated in patients with AUD. Baclofen, topiramate, gabapentin, and ondansetron have shown varying levels of efficacy and pose minimal concern in patients with ALD.
Continue to: Baclofen
Baclofen. Although findings are conflicting, baclofen is the only agent that has been specifically studied for treating AUD in patients with ALD. A GABA B receptor antagonist, baclofen is currently FDA-approved for treating spasticity. In a series of open-label and double-blind studies, baclofen has been shown to effectively reduce alcohol intake, promote abstinence, and prevent relapse.5,6 Further studies identified a possible dose-related response, noting that 20 mg taken 3 times daily may confer additional response over 10 mg taken 3 times daily.5,6 Conversely, the ALPADIR study failed to demonstrate superiority of baclofen vs placebo in the maintenance of abstinence from alcohol despite dosing at 180 mg/d.9 This study did, however, find a significant reduction in alcohol craving in favor of baclofen.9 Further, in a randomized controlled trial (RCT) conducted in veterans with chronic hepatitis C, baclofen 30 mg/d failed to show superiority over placebo with regard to increasing abstinence or reducing alcohol use
Topiramate. A recent meta-analysis found that topiramate use may result in fewer drinking days, heavy drinking days, and number of drinks per drinking day.7 Additionally, topiramate has demonstrated a statistically significant reduction in alcohol craving as well as the ability to decrease all liver function test values.5 This agent should be used with caution in patients with hepatic encephalopathy because the adverse cognitive effects associated with topiramate may confound the clinical course and treatment of such.
Gabapentin. The use of gabapentin to treat patients with AUD is supported by multiple RCTs. In studies that evaluated dose-related response, higher doses of gabapentin (up to 1,800 mg/d) showed greater efficacy than lower doses (ie, 900 mg/d).8 Because gabapentin does not undergo hepatic metabolism, its use in patients with ALD is considered safe. Although the abuse potential of gabapentin is less defined in patients with AUD, there have been reports of abuse in other high-risk populations (ie, those with opioid use disorder, incarcerated persons, and those who misuse prescriptions recreationally).8
Ondansetron is speculated to decrease the reward from alcohol via the down-regulation of dopaminergic neurons. Studies examining ondansetron for patients with AUD have found that it decreases alcohol cravings in those with early-onset alcoholism (initial onset at age ≤25), but not in late-onset alcoholism (initial onset at age >25).5 However, the ondansetron doses used in these trials were very low (4 mcg/kg), and those doses are not available commercially.5
CASE CONTINUED
Following a discussion of available pharmacotherapeutic options for AUD, Mr. S is started on baclofen, 10 mg 3 times daily, with plans for dose titration. At a 2-week follow-up appointment, Mr. S reports that he had not been taking baclofen as often as instructed; however, he denies further alcohol consumption and re-commits to baclofen treatment. Unfortunately, Mr. S is soon admitted to hospice care due to continued decompensation and is unable to attend any additional outpatient follow-up appointments. Three months after his initial outpatient contact, Mr. S dies due to alcoholic cirrhosis.
Related Resources
• Crabb DW, Im GY, Szabo G, et al. Diagnosis and treatment of alcohol-related liver diseases: 2019 practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2020;71(1):306-333.
• Murail AR, Carey WD. Disease management. Liver test interpretation - approach to the patient with liver disease: a guide to commonly used liver tests. Cleveland Clinic Center for Continuing Education. Updated August 2017. www.clevelandclinicmeded. com/medicalpubs/diseasemanagement/hepatology/ guide-to-common-liver-tests/
Drug Brand Names
Acamprosate • Campral
Baclofen • Lioresal
Disulfiram • Antabuse
Gabapentin • Neurontin
Naltrexone • Revia, Vivitrol
Ondansetron • Zofran
Topiramate • Topamax
1. Agrawal S, Dhiman RK, Limdi JK. Evaluation of abnormal liver function tests. Postgrad Med J. 2016;92(1086):223-234.
2. World Health Organization. Global status report on alcohol and health 2018. Published 2018. Accessed November 5, 2020. https://www.who.int/substance_abuse/publications/global_alcohol_report/gsr_2018/en/
3. Osna NA, Donohue TM, Kharbanda KK. Alcoholic liver disease: pathogenesis and current management. Alcohol Res. 2017;38(2):147-161.
4. Leggio L, Lee MR. Treatment of alcohol use disorder in patients with alcoholic liver disease. Am J Med. 2017;130(2):124-134.
5. Addolorato G, Mirijello A, Leggio L, et al. Management of alcohol dependence in patients with liver disease. CNS Drugs. 2013;27(4):287-299.
6. Vuittonet CL, Halse M, Leggio L, et al. Pharmacotherapy for alcoholic patients with alcoholic liver disease. Am J Health Syst Pharm. 2014;71(15):1265-1276.
7. Jonas DE, Amick HR, Feltner C, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings. JAMA. 2014;311(18):1889-1900.
8. Mason BJ, Quello S, Shadan F. Gabapentin for the treatment of alcohol use disorder. Expert Opin Investig Drugs. 2018;27(1):113-124.
9. Reynaud M, Aubin HJ, Trinquet F, et al. A randomized, placebo-controlled study of high-dose baclofen in alcohol-dependent patients-the ALPADIR study. Alcohol Alcohol. 2017;52(4):439-446.
10. Hauser P, Fuller B, Ho S, et al. The safety and efficacy of baclofen to reduce alcohol use in veterans with chronic hepatitis C: a randomized controlled trial. Addiction. 2017;112(7):1173-1183.
Mr. S, age 64, presents for an outpatient follow-up after a recent hospital discharge for alcohol detoxification. He reports a long history of alcohol use, which has resulted in numerous hospital admissions. He has recently been receiving care from a gastroenterologist because the results of laboratory testing suggested hepatic impairment (Table 1). Mr. S says that a friend of his was able to stop drinking by taking a medication, and he wonders if he can be prescribed a medication to help him as well.
A chart review shows that Mr. S recently underwent paracentesis, during which 6 liters of fluid were removed. Additionally, an abdominal ultrasound confirmed hepatic cirrhosis.
According to the World Health Organization, alcohol consumption contributes to 3 million deaths annually.2 The highest proportion of these deaths (21.3%) is due to alcohol-associated gastrointestinal complications, including alcoholic and infectious hepatitis, pancreatitis, and cirrhosis. Because the liver is the primary site of ethanol metabolism, it sustains the greatest degree of tissue injury with heavy alcohol consumption. Additionally, the association of harmful use of alcohol with risky sexual behavior may partially explain the higher prevalence of viral hepatitis among persons with alcohol use disorder (AUD) compared with the general population. Alcoholic liver disease (ALD) progresses through several stages, beginning with hepatic steatosis and progressing through alcohol-related hepatitis, fibrosis, cirrhosis, and potentially hepatocellular carcinoma.3
Liver markers of alcohol use
Although biological markers can be used in clinical practice to screen and monitor for alcohol abuse, making a diagnosis of ALD can be challenging. Typically, a history of heavy alcohol consumption in addition to certain physical signs and laboratory tests for liver disease are the best indicators of ALD. However, the clinical assessment can be confounded by patients who deny or minimize how much alcohol they have consumed. Furthermore, physical and laboratory findings may not be specific to ALD.
Liver enzymes, including aspartate aminotransferase (AST), alanine aminotransferase (ALT), and gamma-glutamyltransferase (GGT), have historically been used as the basis of diagnosing ALD. In addition to elevated bilirubin and evidence of macrocytic anemia, elevations in these enzymes may suggest heavy alcohol use, but these values alone are inadequate to establish ALD. Gamma-glutamyltransferase is found in cell membranes of several body tissues, including the liver and spleen, and therefore is not specific to liver damage. However, elevated GGT is the best indicator of excessive alcohol consumption because it has greater sensitivity than AST and ALT.1,3,4
Although these biomarkers are helpful in diagnosing ALD, they lose some of their utility in patients with advanced liver disease. Patients with severe liver dysfunction may not have elevated serum aminotransferase levels because the degree of liver enzyme elevation does not correlate well with the severity of ALD. For example, patients with advanced cirrhosis may have liver enzyme levels that appear normal. However, the pattern of elevation in transaminases can be helpful in making a diagnosis of liver dysfunction; using the ratio of AST to ALT may aid in diagnosing ALD, because AST is elevated more than twice that of ALT in >80% of patients with ALD.1,3,4
Table 21,3,4 shows the progression of ALD from steatohepatitis to alcoholic hepatitis to cirrhosis. In steatohepatitis, transaminitis is present but all other biomarkers normal. In alcoholic hepatitis, transaminitis is present along with elevated alkaline phosphatase, elevated bilirubin, and elevated international normalized ratio (INR). In alcoholic cirrhosis, the AST-to-ALT ratio is >2, and hypoalbuminemia, hyperbilirubinemia, and coagulopathy (evidenced by elevated INR) are present, consistent with long-term liver damage.1,3,4
Continue to: FDA-approved medications
FDA-approved medications
Three medications—acamprosate, naltrexone, and disulfiram—currently are FDA-approved for treating AUD.5,6 Additionally, several other medications have shown varying levels of efficacy in treating patients with AUD but are not FDA-approved for this indication (Table 3).5-8
Acamprosate is thought to create a balance of inhibitor and excitatory neurotransmitters by functioning as a glutamate antagonist and gamma-aminobutyric acid (GABA) agonist. This is speculated to aid in abstinence from alcohol. Data suggests that acamprosate may be more effective for maintaining abstinence than for inducing remission in individuals who have not yet detoxified from alcohol. Because of its renal excretion, acamprosate is the only FDA-approved medication for AUD that is not associated with liver toxicity. The most commonly reported adverse effect with acamprosate use is diarrhea.
Naltrexone, a mu-opioid receptor antagonist, is available in both tablet and long-acting IM injection formulations. Naltrexone blocks the binding of endorphins created by alcohol consumption to opioid receptors. This results in diminished dopamine release and is speculated to decrease reward and positive reinforcement with alcohol consumption, leading to fewer heavy drinking days. Due to hepatic metabolism, naltrexone use carries a risk of liver injury. Cases of hepatitis and clinically significant liver dysfunction as well as transient, asymptomatic, hepatic transaminase elevations have been observed in patients who receive naltrexone. Because of the absence of first-pass metabolism, long-acting IM naltrexone may produce less hepatotoxicity than the oral formulation. When the FDA approved both formulations of naltrexone, a “black-box” warning was issued concerning the risk of liver damage; however, these warnings have since been removed from their respective prescribing information.
Disulfiram inhibits acetaldehyde dehydrogenase, resulting in elevated acetaldehyde concentrations after consuming alcohol. In theory, this medication reduces a person’s desire to drink due to the negative physiological and physical effects associated with increased acetaldehyde, including hypotension, flushing, nausea, and vomiting. Although most of these reactions are short-lived, disulfiram can induce hepatotoxicity and liver failure that may prove fatal. Disulfiram should be avoided in patients with advanced ALD.
Off-label medications for AUD
Additional pharmacotherapeutic agents have been evaluated in patients with AUD. Baclofen, topiramate, gabapentin, and ondansetron have shown varying levels of efficacy and pose minimal concern in patients with ALD.
Continue to: Baclofen
Baclofen. Although findings are conflicting, baclofen is the only agent that has been specifically studied for treating AUD in patients with ALD. A GABA B receptor antagonist, baclofen is currently FDA-approved for treating spasticity. In a series of open-label and double-blind studies, baclofen has been shown to effectively reduce alcohol intake, promote abstinence, and prevent relapse.5,6 Further studies identified a possible dose-related response, noting that 20 mg taken 3 times daily may confer additional response over 10 mg taken 3 times daily.5,6 Conversely, the ALPADIR study failed to demonstrate superiority of baclofen vs placebo in the maintenance of abstinence from alcohol despite dosing at 180 mg/d.9 This study did, however, find a significant reduction in alcohol craving in favor of baclofen.9 Further, in a randomized controlled trial (RCT) conducted in veterans with chronic hepatitis C, baclofen 30 mg/d failed to show superiority over placebo with regard to increasing abstinence or reducing alcohol use
Topiramate. A recent meta-analysis found that topiramate use may result in fewer drinking days, heavy drinking days, and number of drinks per drinking day.7 Additionally, topiramate has demonstrated a statistically significant reduction in alcohol craving as well as the ability to decrease all liver function test values.5 This agent should be used with caution in patients with hepatic encephalopathy because the adverse cognitive effects associated with topiramate may confound the clinical course and treatment of such.
Gabapentin. The use of gabapentin to treat patients with AUD is supported by multiple RCTs. In studies that evaluated dose-related response, higher doses of gabapentin (up to 1,800 mg/d) showed greater efficacy than lower doses (ie, 900 mg/d).8 Because gabapentin does not undergo hepatic metabolism, its use in patients with ALD is considered safe. Although the abuse potential of gabapentin is less defined in patients with AUD, there have been reports of abuse in other high-risk populations (ie, those with opioid use disorder, incarcerated persons, and those who misuse prescriptions recreationally).8
Ondansetron is speculated to decrease the reward from alcohol via the down-regulation of dopaminergic neurons. Studies examining ondansetron for patients with AUD have found that it decreases alcohol cravings in those with early-onset alcoholism (initial onset at age ≤25), but not in late-onset alcoholism (initial onset at age >25).5 However, the ondansetron doses used in these trials were very low (4 mcg/kg), and those doses are not available commercially.5
CASE CONTINUED
Following a discussion of available pharmacotherapeutic options for AUD, Mr. S is started on baclofen, 10 mg 3 times daily, with plans for dose titration. At a 2-week follow-up appointment, Mr. S reports that he had not been taking baclofen as often as instructed; however, he denies further alcohol consumption and re-commits to baclofen treatment. Unfortunately, Mr. S is soon admitted to hospice care due to continued decompensation and is unable to attend any additional outpatient follow-up appointments. Three months after his initial outpatient contact, Mr. S dies due to alcoholic cirrhosis.
Related Resources
• Crabb DW, Im GY, Szabo G, et al. Diagnosis and treatment of alcohol-related liver diseases: 2019 practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2020;71(1):306-333.
• Murail AR, Carey WD. Disease management. Liver test interpretation - approach to the patient with liver disease: a guide to commonly used liver tests. Cleveland Clinic Center for Continuing Education. Updated August 2017. www.clevelandclinicmeded. com/medicalpubs/diseasemanagement/hepatology/ guide-to-common-liver-tests/
Drug Brand Names
Acamprosate • Campral
Baclofen • Lioresal
Disulfiram • Antabuse
Gabapentin • Neurontin
Naltrexone • Revia, Vivitrol
Ondansetron • Zofran
Topiramate • Topamax
Mr. S, age 64, presents for an outpatient follow-up after a recent hospital discharge for alcohol detoxification. He reports a long history of alcohol use, which has resulted in numerous hospital admissions. He has recently been receiving care from a gastroenterologist because the results of laboratory testing suggested hepatic impairment (Table 1). Mr. S says that a friend of his was able to stop drinking by taking a medication, and he wonders if he can be prescribed a medication to help him as well.
A chart review shows that Mr. S recently underwent paracentesis, during which 6 liters of fluid were removed. Additionally, an abdominal ultrasound confirmed hepatic cirrhosis.
According to the World Health Organization, alcohol consumption contributes to 3 million deaths annually.2 The highest proportion of these deaths (21.3%) is due to alcohol-associated gastrointestinal complications, including alcoholic and infectious hepatitis, pancreatitis, and cirrhosis. Because the liver is the primary site of ethanol metabolism, it sustains the greatest degree of tissue injury with heavy alcohol consumption. Additionally, the association of harmful use of alcohol with risky sexual behavior may partially explain the higher prevalence of viral hepatitis among persons with alcohol use disorder (AUD) compared with the general population. Alcoholic liver disease (ALD) progresses through several stages, beginning with hepatic steatosis and progressing through alcohol-related hepatitis, fibrosis, cirrhosis, and potentially hepatocellular carcinoma.3
Liver markers of alcohol use
Although biological markers can be used in clinical practice to screen and monitor for alcohol abuse, making a diagnosis of ALD can be challenging. Typically, a history of heavy alcohol consumption in addition to certain physical signs and laboratory tests for liver disease are the best indicators of ALD. However, the clinical assessment can be confounded by patients who deny or minimize how much alcohol they have consumed. Furthermore, physical and laboratory findings may not be specific to ALD.
Liver enzymes, including aspartate aminotransferase (AST), alanine aminotransferase (ALT), and gamma-glutamyltransferase (GGT), have historically been used as the basis of diagnosing ALD. In addition to elevated bilirubin and evidence of macrocytic anemia, elevations in these enzymes may suggest heavy alcohol use, but these values alone are inadequate to establish ALD. Gamma-glutamyltransferase is found in cell membranes of several body tissues, including the liver and spleen, and therefore is not specific to liver damage. However, elevated GGT is the best indicator of excessive alcohol consumption because it has greater sensitivity than AST and ALT.1,3,4
Although these biomarkers are helpful in diagnosing ALD, they lose some of their utility in patients with advanced liver disease. Patients with severe liver dysfunction may not have elevated serum aminotransferase levels because the degree of liver enzyme elevation does not correlate well with the severity of ALD. For example, patients with advanced cirrhosis may have liver enzyme levels that appear normal. However, the pattern of elevation in transaminases can be helpful in making a diagnosis of liver dysfunction; using the ratio of AST to ALT may aid in diagnosing ALD, because AST is elevated more than twice that of ALT in >80% of patients with ALD.1,3,4
Table 21,3,4 shows the progression of ALD from steatohepatitis to alcoholic hepatitis to cirrhosis. In steatohepatitis, transaminitis is present but all other biomarkers normal. In alcoholic hepatitis, transaminitis is present along with elevated alkaline phosphatase, elevated bilirubin, and elevated international normalized ratio (INR). In alcoholic cirrhosis, the AST-to-ALT ratio is >2, and hypoalbuminemia, hyperbilirubinemia, and coagulopathy (evidenced by elevated INR) are present, consistent with long-term liver damage.1,3,4
Continue to: FDA-approved medications
FDA-approved medications
Three medications—acamprosate, naltrexone, and disulfiram—currently are FDA-approved for treating AUD.5,6 Additionally, several other medications have shown varying levels of efficacy in treating patients with AUD but are not FDA-approved for this indication (Table 3).5-8
Acamprosate is thought to create a balance of inhibitor and excitatory neurotransmitters by functioning as a glutamate antagonist and gamma-aminobutyric acid (GABA) agonist. This is speculated to aid in abstinence from alcohol. Data suggests that acamprosate may be more effective for maintaining abstinence than for inducing remission in individuals who have not yet detoxified from alcohol. Because of its renal excretion, acamprosate is the only FDA-approved medication for AUD that is not associated with liver toxicity. The most commonly reported adverse effect with acamprosate use is diarrhea.
Naltrexone, a mu-opioid receptor antagonist, is available in both tablet and long-acting IM injection formulations. Naltrexone blocks the binding of endorphins created by alcohol consumption to opioid receptors. This results in diminished dopamine release and is speculated to decrease reward and positive reinforcement with alcohol consumption, leading to fewer heavy drinking days. Due to hepatic metabolism, naltrexone use carries a risk of liver injury. Cases of hepatitis and clinically significant liver dysfunction as well as transient, asymptomatic, hepatic transaminase elevations have been observed in patients who receive naltrexone. Because of the absence of first-pass metabolism, long-acting IM naltrexone may produce less hepatotoxicity than the oral formulation. When the FDA approved both formulations of naltrexone, a “black-box” warning was issued concerning the risk of liver damage; however, these warnings have since been removed from their respective prescribing information.
Disulfiram inhibits acetaldehyde dehydrogenase, resulting in elevated acetaldehyde concentrations after consuming alcohol. In theory, this medication reduces a person’s desire to drink due to the negative physiological and physical effects associated with increased acetaldehyde, including hypotension, flushing, nausea, and vomiting. Although most of these reactions are short-lived, disulfiram can induce hepatotoxicity and liver failure that may prove fatal. Disulfiram should be avoided in patients with advanced ALD.
Off-label medications for AUD
Additional pharmacotherapeutic agents have been evaluated in patients with AUD. Baclofen, topiramate, gabapentin, and ondansetron have shown varying levels of efficacy and pose minimal concern in patients with ALD.
Continue to: Baclofen
Baclofen. Although findings are conflicting, baclofen is the only agent that has been specifically studied for treating AUD in patients with ALD. A GABA B receptor antagonist, baclofen is currently FDA-approved for treating spasticity. In a series of open-label and double-blind studies, baclofen has been shown to effectively reduce alcohol intake, promote abstinence, and prevent relapse.5,6 Further studies identified a possible dose-related response, noting that 20 mg taken 3 times daily may confer additional response over 10 mg taken 3 times daily.5,6 Conversely, the ALPADIR study failed to demonstrate superiority of baclofen vs placebo in the maintenance of abstinence from alcohol despite dosing at 180 mg/d.9 This study did, however, find a significant reduction in alcohol craving in favor of baclofen.9 Further, in a randomized controlled trial (RCT) conducted in veterans with chronic hepatitis C, baclofen 30 mg/d failed to show superiority over placebo with regard to increasing abstinence or reducing alcohol use
Topiramate. A recent meta-analysis found that topiramate use may result in fewer drinking days, heavy drinking days, and number of drinks per drinking day.7 Additionally, topiramate has demonstrated a statistically significant reduction in alcohol craving as well as the ability to decrease all liver function test values.5 This agent should be used with caution in patients with hepatic encephalopathy because the adverse cognitive effects associated with topiramate may confound the clinical course and treatment of such.
Gabapentin. The use of gabapentin to treat patients with AUD is supported by multiple RCTs. In studies that evaluated dose-related response, higher doses of gabapentin (up to 1,800 mg/d) showed greater efficacy than lower doses (ie, 900 mg/d).8 Because gabapentin does not undergo hepatic metabolism, its use in patients with ALD is considered safe. Although the abuse potential of gabapentin is less defined in patients with AUD, there have been reports of abuse in other high-risk populations (ie, those with opioid use disorder, incarcerated persons, and those who misuse prescriptions recreationally).8
Ondansetron is speculated to decrease the reward from alcohol via the down-regulation of dopaminergic neurons. Studies examining ondansetron for patients with AUD have found that it decreases alcohol cravings in those with early-onset alcoholism (initial onset at age ≤25), but not in late-onset alcoholism (initial onset at age >25).5 However, the ondansetron doses used in these trials were very low (4 mcg/kg), and those doses are not available commercially.5
CASE CONTINUED
Following a discussion of available pharmacotherapeutic options for AUD, Mr. S is started on baclofen, 10 mg 3 times daily, with plans for dose titration. At a 2-week follow-up appointment, Mr. S reports that he had not been taking baclofen as often as instructed; however, he denies further alcohol consumption and re-commits to baclofen treatment. Unfortunately, Mr. S is soon admitted to hospice care due to continued decompensation and is unable to attend any additional outpatient follow-up appointments. Three months after his initial outpatient contact, Mr. S dies due to alcoholic cirrhosis.
Related Resources
• Crabb DW, Im GY, Szabo G, et al. Diagnosis and treatment of alcohol-related liver diseases: 2019 practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2020;71(1):306-333.
• Murail AR, Carey WD. Disease management. Liver test interpretation - approach to the patient with liver disease: a guide to commonly used liver tests. Cleveland Clinic Center for Continuing Education. Updated August 2017. www.clevelandclinicmeded. com/medicalpubs/diseasemanagement/hepatology/ guide-to-common-liver-tests/
Drug Brand Names
Acamprosate • Campral
Baclofen • Lioresal
Disulfiram • Antabuse
Gabapentin • Neurontin
Naltrexone • Revia, Vivitrol
Ondansetron • Zofran
Topiramate • Topamax
1. Agrawal S, Dhiman RK, Limdi JK. Evaluation of abnormal liver function tests. Postgrad Med J. 2016;92(1086):223-234.
2. World Health Organization. Global status report on alcohol and health 2018. Published 2018. Accessed November 5, 2020. https://www.who.int/substance_abuse/publications/global_alcohol_report/gsr_2018/en/
3. Osna NA, Donohue TM, Kharbanda KK. Alcoholic liver disease: pathogenesis and current management. Alcohol Res. 2017;38(2):147-161.
4. Leggio L, Lee MR. Treatment of alcohol use disorder in patients with alcoholic liver disease. Am J Med. 2017;130(2):124-134.
5. Addolorato G, Mirijello A, Leggio L, et al. Management of alcohol dependence in patients with liver disease. CNS Drugs. 2013;27(4):287-299.
6. Vuittonet CL, Halse M, Leggio L, et al. Pharmacotherapy for alcoholic patients with alcoholic liver disease. Am J Health Syst Pharm. 2014;71(15):1265-1276.
7. Jonas DE, Amick HR, Feltner C, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings. JAMA. 2014;311(18):1889-1900.
8. Mason BJ, Quello S, Shadan F. Gabapentin for the treatment of alcohol use disorder. Expert Opin Investig Drugs. 2018;27(1):113-124.
9. Reynaud M, Aubin HJ, Trinquet F, et al. A randomized, placebo-controlled study of high-dose baclofen in alcohol-dependent patients-the ALPADIR study. Alcohol Alcohol. 2017;52(4):439-446.
10. Hauser P, Fuller B, Ho S, et al. The safety and efficacy of baclofen to reduce alcohol use in veterans with chronic hepatitis C: a randomized controlled trial. Addiction. 2017;112(7):1173-1183.
1. Agrawal S, Dhiman RK, Limdi JK. Evaluation of abnormal liver function tests. Postgrad Med J. 2016;92(1086):223-234.
2. World Health Organization. Global status report on alcohol and health 2018. Published 2018. Accessed November 5, 2020. https://www.who.int/substance_abuse/publications/global_alcohol_report/gsr_2018/en/
3. Osna NA, Donohue TM, Kharbanda KK. Alcoholic liver disease: pathogenesis and current management. Alcohol Res. 2017;38(2):147-161.
4. Leggio L, Lee MR. Treatment of alcohol use disorder in patients with alcoholic liver disease. Am J Med. 2017;130(2):124-134.
5. Addolorato G, Mirijello A, Leggio L, et al. Management of alcohol dependence in patients with liver disease. CNS Drugs. 2013;27(4):287-299.
6. Vuittonet CL, Halse M, Leggio L, et al. Pharmacotherapy for alcoholic patients with alcoholic liver disease. Am J Health Syst Pharm. 2014;71(15):1265-1276.
7. Jonas DE, Amick HR, Feltner C, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings. JAMA. 2014;311(18):1889-1900.
8. Mason BJ, Quello S, Shadan F. Gabapentin for the treatment of alcohol use disorder. Expert Opin Investig Drugs. 2018;27(1):113-124.
9. Reynaud M, Aubin HJ, Trinquet F, et al. A randomized, placebo-controlled study of high-dose baclofen in alcohol-dependent patients-the ALPADIR study. Alcohol Alcohol. 2017;52(4):439-446.
10. Hauser P, Fuller B, Ho S, et al. The safety and efficacy of baclofen to reduce alcohol use in veterans with chronic hepatitis C: a randomized controlled trial. Addiction. 2017;112(7):1173-1183.
Impact of the MTHFR C677T genetic variant on depression
Ms. T, age 55, presents to her psychiatrist’s clinic with a chief complaint of ongoing symptoms of anhedonia and lethargy related to her diagnosis of major depressive disorder (MDD). She also has a history of peripheral arterial disease, hypothyroidism, and generalized anxiety disorder. Her current antidepressant regimen is duloxetine, 60 mg/d, and mirtazapine, 15 mg at night. She recently elected to undergo pharmacogenetic testing, which showed that she is heterozygous for the methylenetetrahydrofolate reductase (MTHFR) C677T mutation (MTHFR C677T CT carrier). Her test report states that she may have impaired folate metabolism. Her psychiatrist adds L-methylfolate, 15 mg/d, to her current antidepressant regimen.
What is the relationship between folic acid and MTHFR?
Methylenetetrahydrofolate reductase is an intracellular enzyme responsible for one of several steps involved in converting dietary folic acid to its physiologically active form, L-methylfolate.1 Once active, L-methylfolate can be transported into the CNS, where it participates in one-carbon transfer reactions.2,3 Mutations in the MTHFR gene have been associated with decreased activity of the enzyme, which has been shown to result in accumulation of homocysteine and may lead to decreased synthesis of neurotransmitters.2,4Commercial pharmacogenetic testing panels may offer MTHFR genetic testing to assist with prescribing decisions for patients with mental illness. The most well-characterized mutation currently is C677T (rsID1801133), which is a single amino acid base pair change (cytosine [C] to thymine [T]) that leads to increased thermolability and instability of the enzyme.5 Carrying 1 or 2 T alleles can lead to a 35% or 70% reduction in enzyme activity, respectively. The T variant allele is most frequent in Hispanics (20% to 25%), Asians (up to 63%), and Caucasians (8% to 20%); however, it is relatively uncommon in African Americans (<2%).5,6 Another variant, A1289C (rs1801131), has also been associated with decreased enzyme function, particularly when analyzed in combination with C677T. However, carrying the 1289C variant allele does not appear to result in as large of a reduction of enzyme function as the 677T variant.7
What is the relationship between MTHFR C677T and depression?
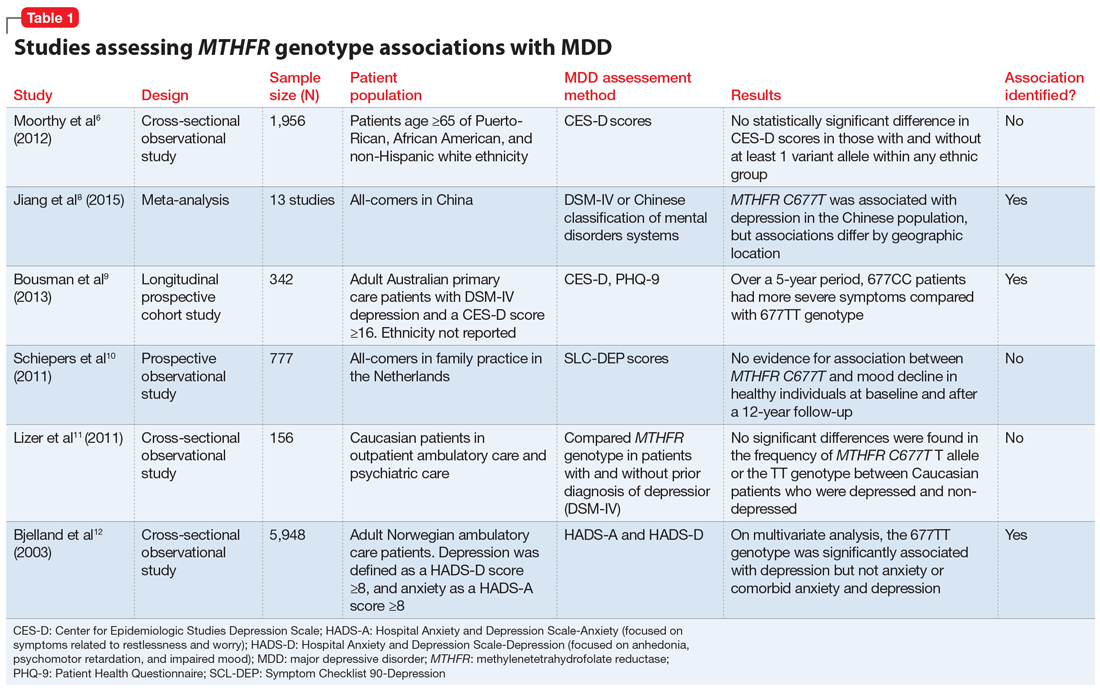
Some researchers have proposed that the C677T mutation in MTHFR may be associated with depression as a result of decreased neurotransmitter synthesis, but studies have not consistently supported this hypothesis. Several studies suggest an association between MTHFR mutations and MDD8-10:
Jiang et al8 performed a meta-analysis of 13 studies including 1,295 Chinese patients and found that having at least 1 C677T variant allele was significantly associated with an increased risk of depression (for T vs C odds ratio 1.52, 95% confidence interval 1.24 to 1.85). The authors noted a stronger association identified in the Northern Chinese population compared with the Southern Chinese population.8
Bousman et al9 found that American patients with MDD and the 677CC genotype had greater Patient Health Questionnaire-9 (PHQ-9) scores at assessments at 24, 36, and 48 months post-baseline compared with those with the 677TT genotype (P = .024), which was unexpected based on previously reported associations.9
Schiepers et al10 also assessed the association between the MTHFR genotype in a Dutch ambulatory care population over 12 years. There was no association identified between scores on the depression subscale of the Symptom Checklist 90 and C677T diplotype.10
Table 16,8-12 provides summaries of these and other selected studies on MTHFR and MDD. Overall, although a pathophysiological basis for depression and decreased MTHFR function has been proposed, the current body of literature does not indicate a consistent link between MTHFR C677T genetic variants alone and depression.
Continue to: Medication changes based on MTHFR: What is the evidence?
Medication changes based on MTHFR: What is the evidence?
Some evidence supports the use of active folate supplementation to improve symptoms of MDD.
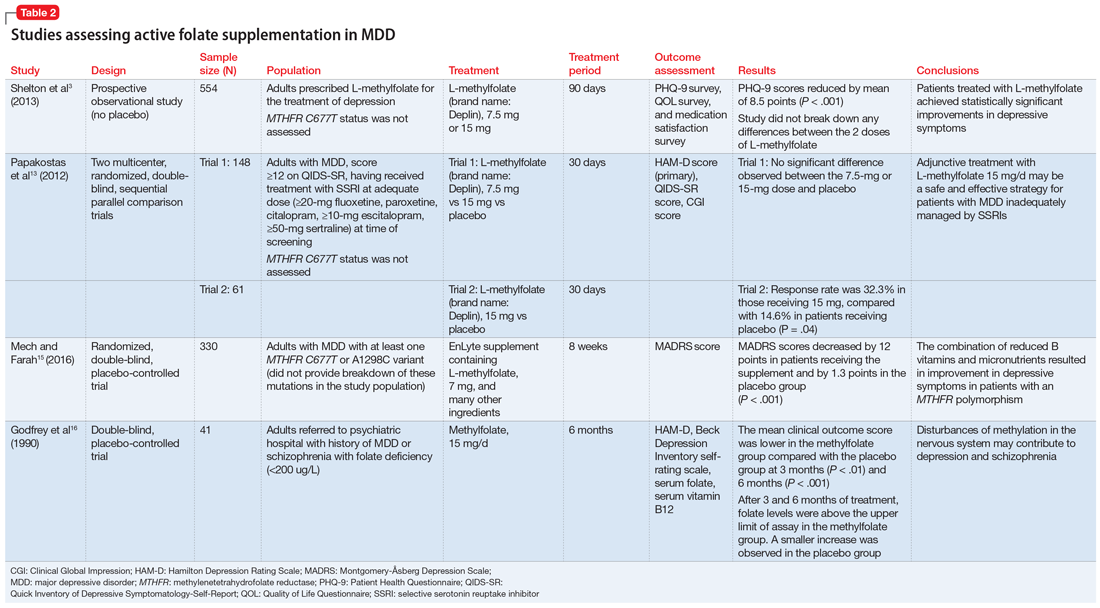
Shelton et al3 conducted an observational study that assessed the effects of adding L-methylfolate (brand name: Deplin), 7.5 or 15 mg, to existing antidepressant therapy in 502 patients with MDD who had baseline PHQ-9 scores of at least 5. After an average 95 days of therapy, PHQ-9 scores were reduced by a mean of 8.5 points, with 67.9% of patients achieving at least a 50% reduction in PHQ-9 scores. The study did not take into account patients’ MTHFR genotype or differentiate results between the 2 doses of L-methylfolate.3
Papakostas et al13 performed 2 randomized, double-blind, parallel-sequential, placebo-controlled trials of L-methylfolate for patients with MDD. The first compared L-methylfolate, 7.5 and 15 mg, to placebo, without regard to MTHFR genotype.13 There was no significant difference between the 7.5-mg dose and placebo, or the 15-mg dose and placebo. However, among the group receiving the 15-mg dose, the response rate was 24%, vs 9% in the placebo group, which approached significance (P = .1). Papakostas et al13 followed up with a smaller trial comparing the 15-mg dose alone to placebo, and found the response rate was 32.3% in patients treated with L-methylfolate compared with 14.6% in the placebo group (P = .04).13
Although the Shelton et al3 and Papakostas et al13 studies showed some improvement in depressive symptom scores among patients who received L-methylfolate supplementation, an important consideration is if MTHFR genotype may predict patient response to this therapy.
Papakostas et al14 performed a post hoc analysis of their earlier study to assess potential associations amongst multiple other biomarkers of inflammation and metabolic disturbances hypothesized by the authors to be associated with MDD, as well as body mass index (BMI), with treatment outcome.14 When change in the Hamilton Depression Rating Scale-28 (HDRS-28) was analyzed by C677T and A1298C variant groups (677 CT vs TT and 1298 AC vs CC), no statistically significant improvements were identified (C677T mean change from baseline −3.8 points, P = .087; A1298C mean change from baseline −0.5 points, P = .807).14 However, statistically significant improvements in HDRS-28 scores were observed compared with baseline when the C677T genotype was pooled with other biomarkers, including methionine synthase (MTR 2756 AG/GG, −23.3 points vs baseline, P < .001) and a voltage-dependent calcium channel (CACNAIC AG/AA, −9 points vs baseline, P < .001), as well as with BMI ≥ 30 kg/m2 (−9.9 points vs baseline, P = .001).14
Continue to: Mech and Farah...
Mech and Farah15 performed a randomized, double-blind, placebo-controlled study of the use of EnLyte, a supplement containing 7-mg L-methylfolate, in patients with at least 1 variant of MTHFR (either C677T or A1298C) over an 8-week period. In addition to L-methylfolate, this supplement contains other active ingredients, including leucovorin (or folinic acid), magnesium ascorbate, and ferrous glycine cysteinate. Montgomery-Åsberg Depression Scale (MADRS) scores improved by 12 points in patients who received the supplement and by 1.3 points in patients who received placebo. However, because the supplement contained many ingredients, the response observed in this study cannot be attributed to L-methylfolate alone.15
Table 23,13,15,16 contains summaries of these and other selected studies assessing active folate supplementation in MDD.
CASE CONTINUED
Over the next several weeks, Ms. T experiences some modest improvement in mood while taking L-methylfolate and her antidepressant regimen, and she experiences no notable adverse effects. Unfortunately, after 3 months, Ms. T discontinues the supplement due to the cost.
The value of MTHFR testing
Ms. T’s case is an example of how clinicians may respond to MTHFR pharmacogenetic testing. Although L-methylfolate has shown some benefit in several randomized clinical trials, available data do not confirm the relevance of MTHFR functional status to symptom response. Additionally, there is likely interplay among multiple factors affecting patients’ response to L-methylfolate. Larger randomized trials prospectively assessing other pharmacogenetic and lifestyle factors may shed more light on which patients would benefit.
Based on available data, the decision to prescribe L-methylfolate should not necessarily hinge on MTHFR genetics alone. Both patients and clinicians must be aware of the potentially prohibitive cost if L-methylfolate is recommended, as prescription insurance may not provide coverage (eg, a recent search on GoodRx.com showed that generic L-methylfolate was approximately $40 for 30 tablets; prices may vary). Additionally, clinicians should be aware that L-methylfolate is regulated as a medical food product and is not subject to strict quality standards required for prescription medications. Future prospective studies assessing the use of L-methylfolate specifically in patients with a MTHFR variants while investigating other relevant covariates may help identify which specific patient populations would benefit from supplementation.
Continue to: Related Resources
Related Resources
- Gilbody S, Lewis S, Lightfoot T. Methylenetetrahydrofolate reductase (MTHFR) genetic polymorphisms and psychiatric disorders: a HuGE review. Am J Epidemiol. 2007;165(1):1-13.
- Trimmer E. Methylenetetrahydrofolate reductase: biochemical characterization and medical significance. Current Pharmaceutical Design. 2013;19(4):2574-3595.
Drug Brand Names
Citalopram • Celexa
Duloxetine • Cymbalta
Escitalopram • Lexapro
Fluoxetine • Prozac
L-methylfolate • Deplin
Mirtazapine • Remeron
Paroxetine • Paxil
Sertraline • Zoloft
1. Scaglione F, Panzavolta G. Folate, folic acid and 5-methyltetrahydrofolate are not the same thing. Xenobiotica. 2014;44(5):480-488.
2. Jadavji N, Wieske F, Dirnagl U, et al. Methylenetetrahydrofolate reductase deficiency alters levels of glutamate and gamma-aminobutyric acid in brain tissue. Molecular Genetics and Metabolism Reports. 2015;3(Issue C):1-4.
3. Shelton R, Manning J, Barrentine L, et al. Assessing effects of L-methylfolate in depression management: results of a real-world patient experience trial. Prim Care Companion CNS Disord. 2013;15(4):pii:PCC.13m01520. doi: 10.4088/PCC.13m01520.
4. Brustolin S, Giugliani R, Felix T. Genetics of homocysteine metabolism and associated disorders. Braz J Med Biol Res. 2010;43(1):1-7.
5. Blom H, Smulders Y. Overview of homocysteine and folate metabolism. With special references to cardiovascular disease and neural tube defects. J Inherit Metab Dis. 2011;34:75-81.
6. Moorthy D, Peter I, Scott T, et al. Status of vitamins B-12 and B-6 but not of folate, homocysteine, and the methylenetetrahydrofolate reductase C677T polymorphism are associated with impaired cognition and depression in adults. J Nutr. 2012;142:1554-1560.
7. Lievers K, Boers G, Verhoef P, et al. A second common variant in the methylenetetrahydrofolate reductase (MTHFR) gene and its relationship to MTHFR enzyme activity, homocysteine, and cardiovascular disease risk. J Mol Med (Berl). 2001;79(9):522-528.
8. Jiang W, Xu J, Lu X, et al. Association between MTHFR C677T polymorphism and depression: a meta-analysis in the Chinese population. Psychol Health Med. 2015;21(6):675-685.
9. Bousman C, Potiriadis M, Everall I, et al. Methylenetetrahydrofolate reductase (MTHFR) genetic variation and major depressive disorder prognosis: a five-year prospective cohort study of primary care attendees. Am J Med Genet B Neuropsychiatr Genet. 2014;165B(1):68-76.
10. Schiepers O, Van Boxtel M, de Groot R, et al. Genetic variation in folate metabolism is not associated with cognitive functioning or mood in healthy adults. Prog Neuro-Psychopharmacol Biol Psychiatry. 2011;35(7):1682-1688.
11. Lizer M, Bogdan R, Kidd R. Comparison of the frequency of the methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism in depressed versus nondepressed patients. J Psychiatr Pract. 2011;17(6):404-409.
12. Bjelland I, Tell G, Vollset S, et al. Folate, vitamin B12, homocysteine, and the MTHFR 677C->T polymorphism in anxiety and depression: the Hordaland Homocysteine Study. Arch Gen Psychiatry. 2003;60(6):618-626.
13. Papakostas G, Shelton R, Zajecka J, et al. L-methylfolate as adjunctive therapy for SSRI-resistant major depression: results of two randomized, double-blind, parallel sequential trials. Am J Psychiatry. 2012;169(12):1267-1274.
14. Papakostas G, Shelton R, Zajecka J, et al. Effect of adjunctive L-methylfolate 15 mg among inadequate responders to SSRIs in depressed patients who were stratified by biomarker levels and genotype: results from a randomized clinical trial. J Clin Psychiatry. 2014;75(8):855-863.
15. Mech A, Farah A. Correlation of clinical response with homocysteine reduction during therapy with reduced B vitamins in patients with MDD who are positive for MTHFR C677T or A1298C polymorphism: a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2016;77(5):668-671.
16. Godfrey P, Toone B, Carney M, et al. Enhancement of recovery from psychiatric illness by methylfolate. Lancet. 1990;336(8712):392-395.
Ms. T, age 55, presents to her psychiatrist’s clinic with a chief complaint of ongoing symptoms of anhedonia and lethargy related to her diagnosis of major depressive disorder (MDD). She also has a history of peripheral arterial disease, hypothyroidism, and generalized anxiety disorder. Her current antidepressant regimen is duloxetine, 60 mg/d, and mirtazapine, 15 mg at night. She recently elected to undergo pharmacogenetic testing, which showed that she is heterozygous for the methylenetetrahydrofolate reductase (MTHFR) C677T mutation (MTHFR C677T CT carrier). Her test report states that she may have impaired folate metabolism. Her psychiatrist adds L-methylfolate, 15 mg/d, to her current antidepressant regimen.
What is the relationship between folic acid and MTHFR?
Methylenetetrahydrofolate reductase is an intracellular enzyme responsible for one of several steps involved in converting dietary folic acid to its physiologically active form, L-methylfolate.1 Once active, L-methylfolate can be transported into the CNS, where it participates in one-carbon transfer reactions.2,3 Mutations in the MTHFR gene have been associated with decreased activity of the enzyme, which has been shown to result in accumulation of homocysteine and may lead to decreased synthesis of neurotransmitters.2,4Commercial pharmacogenetic testing panels may offer MTHFR genetic testing to assist with prescribing decisions for patients with mental illness. The most well-characterized mutation currently is C677T (rsID1801133), which is a single amino acid base pair change (cytosine [C] to thymine [T]) that leads to increased thermolability and instability of the enzyme.5 Carrying 1 or 2 T alleles can lead to a 35% or 70% reduction in enzyme activity, respectively. The T variant allele is most frequent in Hispanics (20% to 25%), Asians (up to 63%), and Caucasians (8% to 20%); however, it is relatively uncommon in African Americans (<2%).5,6 Another variant, A1289C (rs1801131), has also been associated with decreased enzyme function, particularly when analyzed in combination with C677T. However, carrying the 1289C variant allele does not appear to result in as large of a reduction of enzyme function as the 677T variant.7
What is the relationship between MTHFR C677T and depression?
Some researchers have proposed that the C677T mutation in MTHFR may be associated with depression as a result of decreased neurotransmitter synthesis, but studies have not consistently supported this hypothesis. Several studies suggest an association between MTHFR mutations and MDD8-10:
Jiang et al8 performed a meta-analysis of 13 studies including 1,295 Chinese patients and found that having at least 1 C677T variant allele was significantly associated with an increased risk of depression (for T vs C odds ratio 1.52, 95% confidence interval 1.24 to 1.85). The authors noted a stronger association identified in the Northern Chinese population compared with the Southern Chinese population.8
Bousman et al9 found that American patients with MDD and the 677CC genotype had greater Patient Health Questionnaire-9 (PHQ-9) scores at assessments at 24, 36, and 48 months post-baseline compared with those with the 677TT genotype (P = .024), which was unexpected based on previously reported associations.9
Schiepers et al10 also assessed the association between the MTHFR genotype in a Dutch ambulatory care population over 12 years. There was no association identified between scores on the depression subscale of the Symptom Checklist 90 and C677T diplotype.10
Table 16,8-12 provides summaries of these and other selected studies on MTHFR and MDD. Overall, although a pathophysiological basis for depression and decreased MTHFR function has been proposed, the current body of literature does not indicate a consistent link between MTHFR C677T genetic variants alone and depression.

Continue to: Medication changes based on MTHFR: What is the evidence?
Medication changes based on MTHFR: What is the evidence?
Some evidence supports the use of active folate supplementation to improve symptoms of MDD.
Shelton et al3 conducted an observational study that assessed the effects of adding L-methylfolate (brand name: Deplin), 7.5 or 15 mg, to existing antidepressant therapy in 502 patients with MDD who had baseline PHQ-9 scores of at least 5. After an average 95 days of therapy, PHQ-9 scores were reduced by a mean of 8.5 points, with 67.9% of patients achieving at least a 50% reduction in PHQ-9 scores. The study did not take into account patients’ MTHFR genotype or differentiate results between the 2 doses of L-methylfolate.3
Papakostas et al13 performed 2 randomized, double-blind, parallel-sequential, placebo-controlled trials of L-methylfolate for patients with MDD. The first compared L-methylfolate, 7.5 and 15 mg, to placebo, without regard to MTHFR genotype.13 There was no significant difference between the 7.5-mg dose and placebo, or the 15-mg dose and placebo. However, among the group receiving the 15-mg dose, the response rate was 24%, vs 9% in the placebo group, which approached significance (P = .1). Papakostas et al13 followed up with a smaller trial comparing the 15-mg dose alone to placebo, and found the response rate was 32.3% in patients treated with L-methylfolate compared with 14.6% in the placebo group (P = .04).13
Although the Shelton et al3 and Papakostas et al13 studies showed some improvement in depressive symptom scores among patients who received L-methylfolate supplementation, an important consideration is if MTHFR genotype may predict patient response to this therapy.
Papakostas et al14 performed a post hoc analysis of their earlier study to assess potential associations amongst multiple other biomarkers of inflammation and metabolic disturbances hypothesized by the authors to be associated with MDD, as well as body mass index (BMI), with treatment outcome.14 When change in the Hamilton Depression Rating Scale-28 (HDRS-28) was analyzed by C677T and A1298C variant groups (677 CT vs TT and 1298 AC vs CC), no statistically significant improvements were identified (C677T mean change from baseline −3.8 points, P = .087; A1298C mean change from baseline −0.5 points, P = .807).14 However, statistically significant improvements in HDRS-28 scores were observed compared with baseline when the C677T genotype was pooled with other biomarkers, including methionine synthase (MTR 2756 AG/GG, −23.3 points vs baseline, P < .001) and a voltage-dependent calcium channel (CACNAIC AG/AA, −9 points vs baseline, P < .001), as well as with BMI ≥ 30 kg/m2 (−9.9 points vs baseline, P = .001).14
Continue to: Mech and Farah...
Mech and Farah15 performed a randomized, double-blind, placebo-controlled study of the use of EnLyte, a supplement containing 7-mg L-methylfolate, in patients with at least 1 variant of MTHFR (either C677T or A1298C) over an 8-week period. In addition to L-methylfolate, this supplement contains other active ingredients, including leucovorin (or folinic acid), magnesium ascorbate, and ferrous glycine cysteinate. Montgomery-Åsberg Depression Scale (MADRS) scores improved by 12 points in patients who received the supplement and by 1.3 points in patients who received placebo. However, because the supplement contained many ingredients, the response observed in this study cannot be attributed to L-methylfolate alone.15
Table 23,13,15,16 contains summaries of these and other selected studies assessing active folate supplementation in MDD.

CASE CONTINUED
Over the next several weeks, Ms. T experiences some modest improvement in mood while taking L-methylfolate and her antidepressant regimen, and she experiences no notable adverse effects. Unfortunately, after 3 months, Ms. T discontinues the supplement due to the cost.
The value of MTHFR testing
Ms. T’s case is an example of how clinicians may respond to MTHFR pharmacogenetic testing. Although L-methylfolate has shown some benefit in several randomized clinical trials, available data do not confirm the relevance of MTHFR functional status to symptom response. Additionally, there is likely interplay among multiple factors affecting patients’ response to L-methylfolate. Larger randomized trials prospectively assessing other pharmacogenetic and lifestyle factors may shed more light on which patients would benefit.
Based on available data, the decision to prescribe L-methylfolate should not necessarily hinge on MTHFR genetics alone. Both patients and clinicians must be aware of the potentially prohibitive cost if L-methylfolate is recommended, as prescription insurance may not provide coverage (eg, a recent search on GoodRx.com showed that generic L-methylfolate was approximately $40 for 30 tablets; prices may vary). Additionally, clinicians should be aware that L-methylfolate is regulated as a medical food product and is not subject to strict quality standards required for prescription medications. Future prospective studies assessing the use of L-methylfolate specifically in patients with a MTHFR variants while investigating other relevant covariates may help identify which specific patient populations would benefit from supplementation.
Continue to: Related Resources
Related Resources
- Gilbody S, Lewis S, Lightfoot T. Methylenetetrahydrofolate reductase (MTHFR) genetic polymorphisms and psychiatric disorders: a HuGE review. Am J Epidemiol. 2007;165(1):1-13.
- Trimmer E. Methylenetetrahydrofolate reductase: biochemical characterization and medical significance. Current Pharmaceutical Design. 2013;19(4):2574-3595.
Drug Brand Names
Citalopram • Celexa
Duloxetine • Cymbalta
Escitalopram • Lexapro
Fluoxetine • Prozac
L-methylfolate • Deplin
Mirtazapine • Remeron
Paroxetine • Paxil
Sertraline • Zoloft
Ms. T, age 55, presents to her psychiatrist’s clinic with a chief complaint of ongoing symptoms of anhedonia and lethargy related to her diagnosis of major depressive disorder (MDD). She also has a history of peripheral arterial disease, hypothyroidism, and generalized anxiety disorder. Her current antidepressant regimen is duloxetine, 60 mg/d, and mirtazapine, 15 mg at night. She recently elected to undergo pharmacogenetic testing, which showed that she is heterozygous for the methylenetetrahydrofolate reductase (MTHFR) C677T mutation (MTHFR C677T CT carrier). Her test report states that she may have impaired folate metabolism. Her psychiatrist adds L-methylfolate, 15 mg/d, to her current antidepressant regimen.
What is the relationship between folic acid and MTHFR?
Methylenetetrahydrofolate reductase is an intracellular enzyme responsible for one of several steps involved in converting dietary folic acid to its physiologically active form, L-methylfolate.1 Once active, L-methylfolate can be transported into the CNS, where it participates in one-carbon transfer reactions.2,3 Mutations in the MTHFR gene have been associated with decreased activity of the enzyme, which has been shown to result in accumulation of homocysteine and may lead to decreased synthesis of neurotransmitters.2,4Commercial pharmacogenetic testing panels may offer MTHFR genetic testing to assist with prescribing decisions for patients with mental illness. The most well-characterized mutation currently is C677T (rsID1801133), which is a single amino acid base pair change (cytosine [C] to thymine [T]) that leads to increased thermolability and instability of the enzyme.5 Carrying 1 or 2 T alleles can lead to a 35% or 70% reduction in enzyme activity, respectively. The T variant allele is most frequent in Hispanics (20% to 25%), Asians (up to 63%), and Caucasians (8% to 20%); however, it is relatively uncommon in African Americans (<2%).5,6 Another variant, A1289C (rs1801131), has also been associated with decreased enzyme function, particularly when analyzed in combination with C677T. However, carrying the 1289C variant allele does not appear to result in as large of a reduction of enzyme function as the 677T variant.7
What is the relationship between MTHFR C677T and depression?
Some researchers have proposed that the C677T mutation in MTHFR may be associated with depression as a result of decreased neurotransmitter synthesis, but studies have not consistently supported this hypothesis. Several studies suggest an association between MTHFR mutations and MDD8-10:
Jiang et al8 performed a meta-analysis of 13 studies including 1,295 Chinese patients and found that having at least 1 C677T variant allele was significantly associated with an increased risk of depression (for T vs C odds ratio 1.52, 95% confidence interval 1.24 to 1.85). The authors noted a stronger association identified in the Northern Chinese population compared with the Southern Chinese population.8
Bousman et al9 found that American patients with MDD and the 677CC genotype had greater Patient Health Questionnaire-9 (PHQ-9) scores at assessments at 24, 36, and 48 months post-baseline compared with those with the 677TT genotype (P = .024), which was unexpected based on previously reported associations.9
Schiepers et al10 also assessed the association between the MTHFR genotype in a Dutch ambulatory care population over 12 years. There was no association identified between scores on the depression subscale of the Symptom Checklist 90 and C677T diplotype.10
Table 16,8-12 provides summaries of these and other selected studies on MTHFR and MDD. Overall, although a pathophysiological basis for depression and decreased MTHFR function has been proposed, the current body of literature does not indicate a consistent link between MTHFR C677T genetic variants alone and depression.

Continue to: Medication changes based on MTHFR: What is the evidence?
Medication changes based on MTHFR: What is the evidence?
Some evidence supports the use of active folate supplementation to improve symptoms of MDD.
Shelton et al3 conducted an observational study that assessed the effects of adding L-methylfolate (brand name: Deplin), 7.5 or 15 mg, to existing antidepressant therapy in 502 patients with MDD who had baseline PHQ-9 scores of at least 5. After an average 95 days of therapy, PHQ-9 scores were reduced by a mean of 8.5 points, with 67.9% of patients achieving at least a 50% reduction in PHQ-9 scores. The study did not take into account patients’ MTHFR genotype or differentiate results between the 2 doses of L-methylfolate.3
Papakostas et al13 performed 2 randomized, double-blind, parallel-sequential, placebo-controlled trials of L-methylfolate for patients with MDD. The first compared L-methylfolate, 7.5 and 15 mg, to placebo, without regard to MTHFR genotype.13 There was no significant difference between the 7.5-mg dose and placebo, or the 15-mg dose and placebo. However, among the group receiving the 15-mg dose, the response rate was 24%, vs 9% in the placebo group, which approached significance (P = .1). Papakostas et al13 followed up with a smaller trial comparing the 15-mg dose alone to placebo, and found the response rate was 32.3% in patients treated with L-methylfolate compared with 14.6% in the placebo group (P = .04).13
Although the Shelton et al3 and Papakostas et al13 studies showed some improvement in depressive symptom scores among patients who received L-methylfolate supplementation, an important consideration is if MTHFR genotype may predict patient response to this therapy.
Papakostas et al14 performed a post hoc analysis of their earlier study to assess potential associations amongst multiple other biomarkers of inflammation and metabolic disturbances hypothesized by the authors to be associated with MDD, as well as body mass index (BMI), with treatment outcome.14 When change in the Hamilton Depression Rating Scale-28 (HDRS-28) was analyzed by C677T and A1298C variant groups (677 CT vs TT and 1298 AC vs CC), no statistically significant improvements were identified (C677T mean change from baseline −3.8 points, P = .087; A1298C mean change from baseline −0.5 points, P = .807).14 However, statistically significant improvements in HDRS-28 scores were observed compared with baseline when the C677T genotype was pooled with other biomarkers, including methionine synthase (MTR 2756 AG/GG, −23.3 points vs baseline, P < .001) and a voltage-dependent calcium channel (CACNAIC AG/AA, −9 points vs baseline, P < .001), as well as with BMI ≥ 30 kg/m2 (−9.9 points vs baseline, P = .001).14
Continue to: Mech and Farah...
Mech and Farah15 performed a randomized, double-blind, placebo-controlled study of the use of EnLyte, a supplement containing 7-mg L-methylfolate, in patients with at least 1 variant of MTHFR (either C677T or A1298C) over an 8-week period. In addition to L-methylfolate, this supplement contains other active ingredients, including leucovorin (or folinic acid), magnesium ascorbate, and ferrous glycine cysteinate. Montgomery-Åsberg Depression Scale (MADRS) scores improved by 12 points in patients who received the supplement and by 1.3 points in patients who received placebo. However, because the supplement contained many ingredients, the response observed in this study cannot be attributed to L-methylfolate alone.15
Table 23,13,15,16 contains summaries of these and other selected studies assessing active folate supplementation in MDD.

CASE CONTINUED
Over the next several weeks, Ms. T experiences some modest improvement in mood while taking L-methylfolate and her antidepressant regimen, and she experiences no notable adverse effects. Unfortunately, after 3 months, Ms. T discontinues the supplement due to the cost.
The value of MTHFR testing
Ms. T’s case is an example of how clinicians may respond to MTHFR pharmacogenetic testing. Although L-methylfolate has shown some benefit in several randomized clinical trials, available data do not confirm the relevance of MTHFR functional status to symptom response. Additionally, there is likely interplay among multiple factors affecting patients’ response to L-methylfolate. Larger randomized trials prospectively assessing other pharmacogenetic and lifestyle factors may shed more light on which patients would benefit.
Based on available data, the decision to prescribe L-methylfolate should not necessarily hinge on MTHFR genetics alone. Both patients and clinicians must be aware of the potentially prohibitive cost if L-methylfolate is recommended, as prescription insurance may not provide coverage (eg, a recent search on GoodRx.com showed that generic L-methylfolate was approximately $40 for 30 tablets; prices may vary). Additionally, clinicians should be aware that L-methylfolate is regulated as a medical food product and is not subject to strict quality standards required for prescription medications. Future prospective studies assessing the use of L-methylfolate specifically in patients with a MTHFR variants while investigating other relevant covariates may help identify which specific patient populations would benefit from supplementation.
Continue to: Related Resources
Related Resources
- Gilbody S, Lewis S, Lightfoot T. Methylenetetrahydrofolate reductase (MTHFR) genetic polymorphisms and psychiatric disorders: a HuGE review. Am J Epidemiol. 2007;165(1):1-13.
- Trimmer E. Methylenetetrahydrofolate reductase: biochemical characterization and medical significance. Current Pharmaceutical Design. 2013;19(4):2574-3595.
Drug Brand Names
Citalopram • Celexa
Duloxetine • Cymbalta
Escitalopram • Lexapro
Fluoxetine • Prozac
L-methylfolate • Deplin
Mirtazapine • Remeron
Paroxetine • Paxil
Sertraline • Zoloft
1. Scaglione F, Panzavolta G. Folate, folic acid and 5-methyltetrahydrofolate are not the same thing. Xenobiotica. 2014;44(5):480-488.
2. Jadavji N, Wieske F, Dirnagl U, et al. Methylenetetrahydrofolate reductase deficiency alters levels of glutamate and gamma-aminobutyric acid in brain tissue. Molecular Genetics and Metabolism Reports. 2015;3(Issue C):1-4.
3. Shelton R, Manning J, Barrentine L, et al. Assessing effects of L-methylfolate in depression management: results of a real-world patient experience trial. Prim Care Companion CNS Disord. 2013;15(4):pii:PCC.13m01520. doi: 10.4088/PCC.13m01520.
4. Brustolin S, Giugliani R, Felix T. Genetics of homocysteine metabolism and associated disorders. Braz J Med Biol Res. 2010;43(1):1-7.
5. Blom H, Smulders Y. Overview of homocysteine and folate metabolism. With special references to cardiovascular disease and neural tube defects. J Inherit Metab Dis. 2011;34:75-81.
6. Moorthy D, Peter I, Scott T, et al. Status of vitamins B-12 and B-6 but not of folate, homocysteine, and the methylenetetrahydrofolate reductase C677T polymorphism are associated with impaired cognition and depression in adults. J Nutr. 2012;142:1554-1560.
7. Lievers K, Boers G, Verhoef P, et al. A second common variant in the methylenetetrahydrofolate reductase (MTHFR) gene and its relationship to MTHFR enzyme activity, homocysteine, and cardiovascular disease risk. J Mol Med (Berl). 2001;79(9):522-528.
8. Jiang W, Xu J, Lu X, et al. Association between MTHFR C677T polymorphism and depression: a meta-analysis in the Chinese population. Psychol Health Med. 2015;21(6):675-685.
9. Bousman C, Potiriadis M, Everall I, et al. Methylenetetrahydrofolate reductase (MTHFR) genetic variation and major depressive disorder prognosis: a five-year prospective cohort study of primary care attendees. Am J Med Genet B Neuropsychiatr Genet. 2014;165B(1):68-76.
10. Schiepers O, Van Boxtel M, de Groot R, et al. Genetic variation in folate metabolism is not associated with cognitive functioning or mood in healthy adults. Prog Neuro-Psychopharmacol Biol Psychiatry. 2011;35(7):1682-1688.
11. Lizer M, Bogdan R, Kidd R. Comparison of the frequency of the methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism in depressed versus nondepressed patients. J Psychiatr Pract. 2011;17(6):404-409.
12. Bjelland I, Tell G, Vollset S, et al. Folate, vitamin B12, homocysteine, and the MTHFR 677C->T polymorphism in anxiety and depression: the Hordaland Homocysteine Study. Arch Gen Psychiatry. 2003;60(6):618-626.
13. Papakostas G, Shelton R, Zajecka J, et al. L-methylfolate as adjunctive therapy for SSRI-resistant major depression: results of two randomized, double-blind, parallel sequential trials. Am J Psychiatry. 2012;169(12):1267-1274.
14. Papakostas G, Shelton R, Zajecka J, et al. Effect of adjunctive L-methylfolate 15 mg among inadequate responders to SSRIs in depressed patients who were stratified by biomarker levels and genotype: results from a randomized clinical trial. J Clin Psychiatry. 2014;75(8):855-863.
15. Mech A, Farah A. Correlation of clinical response with homocysteine reduction during therapy with reduced B vitamins in patients with MDD who are positive for MTHFR C677T or A1298C polymorphism: a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2016;77(5):668-671.
16. Godfrey P, Toone B, Carney M, et al. Enhancement of recovery from psychiatric illness by methylfolate. Lancet. 1990;336(8712):392-395.
1. Scaglione F, Panzavolta G. Folate, folic acid and 5-methyltetrahydrofolate are not the same thing. Xenobiotica. 2014;44(5):480-488.
2. Jadavji N, Wieske F, Dirnagl U, et al. Methylenetetrahydrofolate reductase deficiency alters levels of glutamate and gamma-aminobutyric acid in brain tissue. Molecular Genetics and Metabolism Reports. 2015;3(Issue C):1-4.
3. Shelton R, Manning J, Barrentine L, et al. Assessing effects of L-methylfolate in depression management: results of a real-world patient experience trial. Prim Care Companion CNS Disord. 2013;15(4):pii:PCC.13m01520. doi: 10.4088/PCC.13m01520.
4. Brustolin S, Giugliani R, Felix T. Genetics of homocysteine metabolism and associated disorders. Braz J Med Biol Res. 2010;43(1):1-7.
5. Blom H, Smulders Y. Overview of homocysteine and folate metabolism. With special references to cardiovascular disease and neural tube defects. J Inherit Metab Dis. 2011;34:75-81.
6. Moorthy D, Peter I, Scott T, et al. Status of vitamins B-12 and B-6 but not of folate, homocysteine, and the methylenetetrahydrofolate reductase C677T polymorphism are associated with impaired cognition and depression in adults. J Nutr. 2012;142:1554-1560.
7. Lievers K, Boers G, Verhoef P, et al. A second common variant in the methylenetetrahydrofolate reductase (MTHFR) gene and its relationship to MTHFR enzyme activity, homocysteine, and cardiovascular disease risk. J Mol Med (Berl). 2001;79(9):522-528.
8. Jiang W, Xu J, Lu X, et al. Association between MTHFR C677T polymorphism and depression: a meta-analysis in the Chinese population. Psychol Health Med. 2015;21(6):675-685.
9. Bousman C, Potiriadis M, Everall I, et al. Methylenetetrahydrofolate reductase (MTHFR) genetic variation and major depressive disorder prognosis: a five-year prospective cohort study of primary care attendees. Am J Med Genet B Neuropsychiatr Genet. 2014;165B(1):68-76.
10. Schiepers O, Van Boxtel M, de Groot R, et al. Genetic variation in folate metabolism is not associated with cognitive functioning or mood in healthy adults. Prog Neuro-Psychopharmacol Biol Psychiatry. 2011;35(7):1682-1688.
11. Lizer M, Bogdan R, Kidd R. Comparison of the frequency of the methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism in depressed versus nondepressed patients. J Psychiatr Pract. 2011;17(6):404-409.
12. Bjelland I, Tell G, Vollset S, et al. Folate, vitamin B12, homocysteine, and the MTHFR 677C->T polymorphism in anxiety and depression: the Hordaland Homocysteine Study. Arch Gen Psychiatry. 2003;60(6):618-626.
13. Papakostas G, Shelton R, Zajecka J, et al. L-methylfolate as adjunctive therapy for SSRI-resistant major depression: results of two randomized, double-blind, parallel sequential trials. Am J Psychiatry. 2012;169(12):1267-1274.
14. Papakostas G, Shelton R, Zajecka J, et al. Effect of adjunctive L-methylfolate 15 mg among inadequate responders to SSRIs in depressed patients who were stratified by biomarker levels and genotype: results from a randomized clinical trial. J Clin Psychiatry. 2014;75(8):855-863.
15. Mech A, Farah A. Correlation of clinical response with homocysteine reduction during therapy with reduced B vitamins in patients with MDD who are positive for MTHFR C677T or A1298C polymorphism: a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2016;77(5):668-671.
16. Godfrey P, Toone B, Carney M, et al. Enhancement of recovery from psychiatric illness by methylfolate. Lancet. 1990;336(8712):392-395.
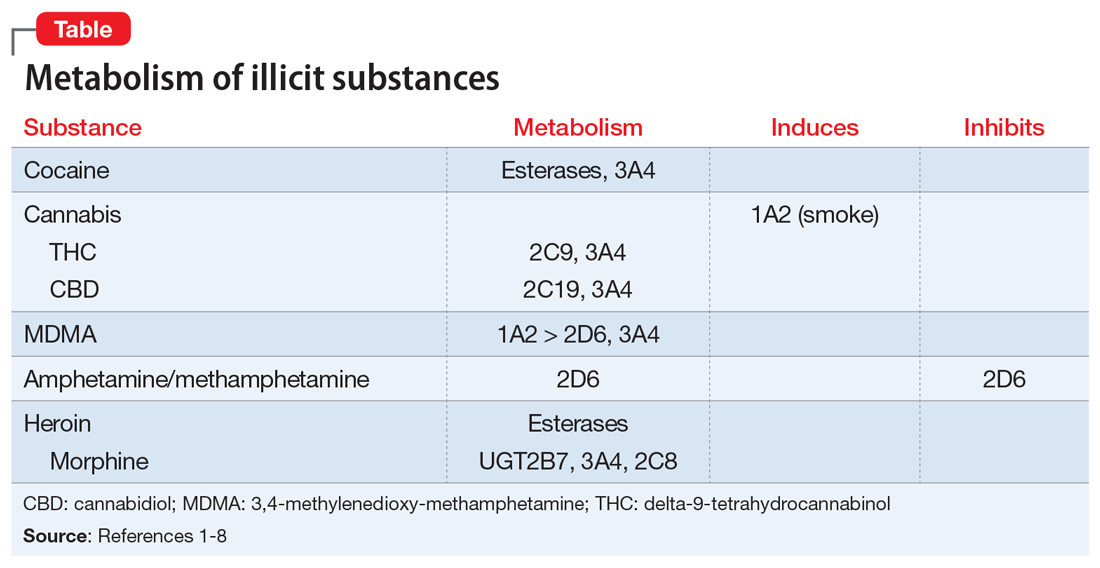
CYP450 interactions between illicit substances and prescription medications
Ms. L, age 37, presents to psychiatric emergency services with command auditory hallucinations, ideas of reference, and suicidal ideation.
Ms. L has a 22-year history of schizophrenia. Additionally, she has a history of cocaine use disorder (in remission for 12 years), cannabis use disorder (in remission for 6 months), type 2 diabetes mellitus, and hypertension. Her psychotic symptoms are well controlled on a regimen of
On interview, Ms. L reports smoking cannabis each day for the past month and using $400 worth of cocaine over 2 days. She is experiencing intense guilt over these relapses and is admitted to the inpatient adult psychiatry unit. On admission, Ms. L’s clozapine and norclozapine trough levels (drawn approximately 12 hours after last administration documented by the ACT member) are 300 and 275 ng/mL, respectively. Generally, clozapine levels >350 to 420 ng/mL are considered therapeutic, and a clozapine-to-norclozapine ratio of 2:1 is desirable for maximum efficacy and tolerability. Because Ms. L’s clozapine level is <350 and her ratio is approximately 1:1, her clozapine treatment is subtherapeutic.
Because Ms. L has a history of documented adherence to and benefit from her current medication regimen, no changes are made during her 3-week hospital stay. She notices a gradual reduction in auditory hallucinations, no longer wants to harm herself, and is motivated to regain sobriety.
At the time of discharge, Ms. L’s clozapine and norclozapine trough levels are 550 and 250 ng/mL, respectively, which indicates a more favorable clozapine-to-norclozapine ratio of approximately 2:1 and a clozapine level greater than the recommended minimum threshold of 350 ng/mL. While cocaine ingestion presumably played a role in her acute decompensation, the treatment team determined that Ms. L’s relapse to cannabis use likely contributed to low clozapine levels by induction of cytochrome P450 (CYP) 1A2, and subsequently led to the delayed recovery of symptom control.1
The use of illicit substances is a widespread, growing problem. According to the 2017 National Survey on Drug Use and Health, 11.5% of Americans age ≥12 had used an illicit substance (ie, use of marijuana, cocaine, heroin, hallucinogens, inhalants, or methamphetamine, or misuse of prescription psychotherapeutics) in the past month.2 While illicit substance use is of particular public health interest due to a known increase in mortality and health care spending, there has been little discussion of the impact of illicit drug use on concurrent pharmacologic therapy. Just as prescription medications have pharmacokinetic drug–drug interactions with each other, so do illicit substances, though far less is known about their impact on the treatment of medical conditions.
Pharmacokinetic interactions
Key pharmacokinetic interactions have been reported with cocaine, marijuana, amphetamines, and opioids. The Table1-8 summarizes the metabolism of illicit substances.
Continue to: Cocaine
Cocaine is largely metabolized by serum esterases such as pseudocholinesterase, human carboxylesterase-1 (hCE-1), and human carboxylesterase-2 (hCE-2), to inactive metabolites benzoylecgonine (35% to 45%) and ecgonine (32% to 49%).2 However, a smaller portion (2.6% to 6.2%) undergoes hepatic N-demethylation by CYP3A4 to norcocaine.3 Norcocaine is an active metabolite responsible for some of the toxic effects of cocaine (eg, hepatotoxicity).4,5 Several commonly prescribed medications are known inducers of CYP3A4 (eg, phenytoin, carbamazepine) and may lead to increased levels of the toxic metabolite when used concurrently with cocaine. Additionally, the use of cocaine with acetylcholinesterase inhibitors, such as donepezil, may lead to reduction of serum esterases and shunt cocaine metabolism toward the hepatic pathway, thus increasing norcocaine formation.3
Cannabis. The metabolism and drug–drug interactions of cannabis can be separated by its 2 main components: delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD). A review conducted in 2014 concluded that THC is primarily metabolized by CYP2C9 and 3A4, while CBD is metabolized by CYP2C19 and 3A4.6 Oral administration of ketoconazole, a CYP3A4 inhibitor, along with cannabis extract has been shown to increase the maximum concentration (Cmax) and area under the curve (AUC) of THC by 1.2- and 1.8-fold, respectively, while increasing both Cmaxand AUC of CBD by 2-fold.6 In addition, CYP2C9 poor metabolizers have been shown to experience significant increases in THC exposure and reductions in metabolite formation, further supporting the role of CYP enzymes in cannabis metabolism.6
There is also evidence of enzyme induction by cannabis. Individuals who reported smoking marijuana experienced greater clearance of theophylline, a substrate of CYP1A2, than did those who reported not smoking marijuana.1,6 As with cigarette smoking, this effect appears to be a direct result of the hydrocarbons found in marijuana smoke rather than the cannabis itself, as there is a lack of evidence for enzyme induction when the drug is orally ingested.6
Amphetamine and methamphetamine appear to be both substrates and competitive inhibitors of CYP2D6.7 Rats administered quinidine (a strong 2D6 inhibitor) had 2-fold elevations in AUC and decreased clearance of amphetamine and its metabolites.8 Amphetamine-related recreational drugs, such as 3,4-methylenedioxy-methamphetamine (MDMA) and 3,4-methylenedioxyamphetamine (MDA), are substrates of CYP2D6 and CYP3A4, while MDMA also undergoes substantial metabolism by CYP1A2.3,7,9
Opioids. Heroin is metabolized to 6‑monoacetylmorphine (6-MAM) and morphine by hCE-1, hCE-2, and pseudocholinesterase, and has minimal impact on CYP enzymes. However, while morphine is primarily metabolized to inactive metabolites by UGT2B7, it does undergo minor metabolism through CYP3A4 and 2C8 pathways, creating potential for drug interactions with medications that inhibit or induce CYP3A4.10
Continue to: An underappreciated risk of illicit substance use
An underappreciated risk of illicit substance use
There is a paucity of evidence regarding the metabolism and pharmacokinetic interactions with illicit substances, and further research is needed. Despite the absence of comprehensive data on the subject, the available information indicates the use of illicit substances may have a significant impact on medications used to treat comorbid conditions. Alternatively, those medications may affect the kinetics of recreationally used substances. The risk of adverse consequences of drug–drug interactions is yet another reason patients should be encouraged to avoid use of substances and seek treatment for substance use disorders. When determining the most appropriate therapy for comorbid conditions for patients who are using illicit substances and are likely to continue to do so, clinicians should take into consideration potential interactions among prescription medications and the specific illicit substances the patient uses.
Related Resources
- Lindsey W, Stewart D, Childress D. Drug interactions between common illicit drugs and prescription therapies. Am J Drug Alcohol Abuse. 2012;38(4):334-343.
- Maurer H, Sauer C, Theobald D. Toxicokinetics of drugs of abuse: current knowledge of the isoenzymes involved in the human metabolism of tetrahydrocannabinol, cocaine, heroin, morphine, and codeine. Ther Drug Monit. 2006;28(3):447-453.
- Dean A. Illicit drugs and drug interactions. Pharmacist. 2006;25(9):684-689.
Drug Brand Names
Carbamazepine • Carbatrol, Tegretol
Clozapine • Clozaril
Donepezil • Aricept
Ketoconazole • Nizoral
Paliperidone palmitate • Invega sustenna
Phenytoin • Dilantin, Phenytek
Quinidine • Cardioquin, Duraquin
Theophylline • Elixophylline, Theochron
1. Jusko WJ, Schentag JJ, Clark JH, et al. Enhanced biotransformation of theophylline in marihuana and tobacco smokers. Clin Pharmacol Ther. 1978;24(4):405-410.
2. Substance Abuse and Mental Health Services Administration. Results from the 2017 National Survey on Drug Use and Health: Detailed Tables. https://www.samhsa.gov/data/sites/default/files/cbhsq-reports/NSDUHDetailedTabs2017/NSDUHDetailedTabs2017.htm#tab1-1A. Published 2019. Accessed February 7, 2020.
3. Quinn D, Wodak A, Day R. Pharmacokinetic and pharmacodynamic principles of illicit drug use and treatment of illicit drug users. Clin Pharmacokinet. 1997;33(5):344-400.
4. Ndikum-Moffor FM, Schoeb TR, Roberts SM. Liver toxicity from norcocaine nitroxide, an N-oxidative metabolite of cocaine. J Pharmacol Exp Ther. 1998;284(1):413-419.
5. Pellinen P, Honkakoski P, Stenbäck F, et al. Cocaine N-demethylation and the metabolism-related hepatotoxicity can be prevented by cytochrome P450 3A inhibitors. Eur J Pharmacol. 1994;270(1):35-43.
6. Stout S, Cimino N. Exogenous cannabinoids as substrates, inhibitors, and inducers of human drug metabolizing enzymes: a systematic review. Drug Metab Rev. 2013;46(1):86-95.
7. Kraemer T, Maurer H. Toxicokinetics of amphetamines: metabolism and toxicokinetic data of designer drugs, amphetamine, methamphetamine, and their N-alkyl derivatives. Ther Drug Monit. 2002;24(2):277-289.
8. Markowitz J, Patrick K. Pharmacokinetic and pharmacodynamic drug interactions in the treatment of attention-deficit hyperactivity disorder. Clin Pharmacokinet. 2001;40(10):753-772.
9. Kreth K, Kovar K, Schwab M, et al. Identification of the human cytochromes P450 involved in the oxidative metabolism of “ecstasy”-related designer drugs. Biochem Pharmacol. 2000;59(12):1563-1571.
10. Meyer M, Maurer H. Absorption, distribution, metabolism and excretion pharmacogenomics of drugs of abuse. Pharmacogenomics. 2011;12(2):215-233.
Ms. L, age 37, presents to psychiatric emergency services with command auditory hallucinations, ideas of reference, and suicidal ideation.
Ms. L has a 22-year history of schizophrenia. Additionally, she has a history of cocaine use disorder (in remission for 12 years), cannabis use disorder (in remission for 6 months), type 2 diabetes mellitus, and hypertension. Her psychotic symptoms are well controlled on a regimen of
On interview, Ms. L reports smoking cannabis each day for the past month and using $400 worth of cocaine over 2 days. She is experiencing intense guilt over these relapses and is admitted to the inpatient adult psychiatry unit. On admission, Ms. L’s clozapine and norclozapine trough levels (drawn approximately 12 hours after last administration documented by the ACT member) are 300 and 275 ng/mL, respectively. Generally, clozapine levels >350 to 420 ng/mL are considered therapeutic, and a clozapine-to-norclozapine ratio of 2:1 is desirable for maximum efficacy and tolerability. Because Ms. L’s clozapine level is <350 and her ratio is approximately 1:1, her clozapine treatment is subtherapeutic.
Because Ms. L has a history of documented adherence to and benefit from her current medication regimen, no changes are made during her 3-week hospital stay. She notices a gradual reduction in auditory hallucinations, no longer wants to harm herself, and is motivated to regain sobriety.
At the time of discharge, Ms. L’s clozapine and norclozapine trough levels are 550 and 250 ng/mL, respectively, which indicates a more favorable clozapine-to-norclozapine ratio of approximately 2:1 and a clozapine level greater than the recommended minimum threshold of 350 ng/mL. While cocaine ingestion presumably played a role in her acute decompensation, the treatment team determined that Ms. L’s relapse to cannabis use likely contributed to low clozapine levels by induction of cytochrome P450 (CYP) 1A2, and subsequently led to the delayed recovery of symptom control.1
The use of illicit substances is a widespread, growing problem. According to the 2017 National Survey on Drug Use and Health, 11.5% of Americans age ≥12 had used an illicit substance (ie, use of marijuana, cocaine, heroin, hallucinogens, inhalants, or methamphetamine, or misuse of prescription psychotherapeutics) in the past month.2 While illicit substance use is of particular public health interest due to a known increase in mortality and health care spending, there has been little discussion of the impact of illicit drug use on concurrent pharmacologic therapy. Just as prescription medications have pharmacokinetic drug–drug interactions with each other, so do illicit substances, though far less is known about their impact on the treatment of medical conditions.
Pharmacokinetic interactions
Key pharmacokinetic interactions have been reported with cocaine, marijuana, amphetamines, and opioids. The Table1-8 summarizes the metabolism of illicit substances.
Continue to: Cocaine
Cocaine is largely metabolized by serum esterases such as pseudocholinesterase, human carboxylesterase-1 (hCE-1), and human carboxylesterase-2 (hCE-2), to inactive metabolites benzoylecgonine (35% to 45%) and ecgonine (32% to 49%).2 However, a smaller portion (2.6% to 6.2%) undergoes hepatic N-demethylation by CYP3A4 to norcocaine.3 Norcocaine is an active metabolite responsible for some of the toxic effects of cocaine (eg, hepatotoxicity).4,5 Several commonly prescribed medications are known inducers of CYP3A4 (eg, phenytoin, carbamazepine) and may lead to increased levels of the toxic metabolite when used concurrently with cocaine. Additionally, the use of cocaine with acetylcholinesterase inhibitors, such as donepezil, may lead to reduction of serum esterases and shunt cocaine metabolism toward the hepatic pathway, thus increasing norcocaine formation.3
Cannabis. The metabolism and drug–drug interactions of cannabis can be separated by its 2 main components: delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD). A review conducted in 2014 concluded that THC is primarily metabolized by CYP2C9 and 3A4, while CBD is metabolized by CYP2C19 and 3A4.6 Oral administration of ketoconazole, a CYP3A4 inhibitor, along with cannabis extract has been shown to increase the maximum concentration (Cmax) and area under the curve (AUC) of THC by 1.2- and 1.8-fold, respectively, while increasing both Cmaxand AUC of CBD by 2-fold.6 In addition, CYP2C9 poor metabolizers have been shown to experience significant increases in THC exposure and reductions in metabolite formation, further supporting the role of CYP enzymes in cannabis metabolism.6
There is also evidence of enzyme induction by cannabis. Individuals who reported smoking marijuana experienced greater clearance of theophylline, a substrate of CYP1A2, than did those who reported not smoking marijuana.1,6 As with cigarette smoking, this effect appears to be a direct result of the hydrocarbons found in marijuana smoke rather than the cannabis itself, as there is a lack of evidence for enzyme induction when the drug is orally ingested.6
Amphetamine and methamphetamine appear to be both substrates and competitive inhibitors of CYP2D6.7 Rats administered quinidine (a strong 2D6 inhibitor) had 2-fold elevations in AUC and decreased clearance of amphetamine and its metabolites.8 Amphetamine-related recreational drugs, such as 3,4-methylenedioxy-methamphetamine (MDMA) and 3,4-methylenedioxyamphetamine (MDA), are substrates of CYP2D6 and CYP3A4, while MDMA also undergoes substantial metabolism by CYP1A2.3,7,9
Opioids. Heroin is metabolized to 6‑monoacetylmorphine (6-MAM) and morphine by hCE-1, hCE-2, and pseudocholinesterase, and has minimal impact on CYP enzymes. However, while morphine is primarily metabolized to inactive metabolites by UGT2B7, it does undergo minor metabolism through CYP3A4 and 2C8 pathways, creating potential for drug interactions with medications that inhibit or induce CYP3A4.10
Continue to: An underappreciated risk of illicit substance use
An underappreciated risk of illicit substance use
There is a paucity of evidence regarding the metabolism and pharmacokinetic interactions with illicit substances, and further research is needed. Despite the absence of comprehensive data on the subject, the available information indicates the use of illicit substances may have a significant impact on medications used to treat comorbid conditions. Alternatively, those medications may affect the kinetics of recreationally used substances. The risk of adverse consequences of drug–drug interactions is yet another reason patients should be encouraged to avoid use of substances and seek treatment for substance use disorders. When determining the most appropriate therapy for comorbid conditions for patients who are using illicit substances and are likely to continue to do so, clinicians should take into consideration potential interactions among prescription medications and the specific illicit substances the patient uses.
Related Resources
- Lindsey W, Stewart D, Childress D. Drug interactions between common illicit drugs and prescription therapies. Am J Drug Alcohol Abuse. 2012;38(4):334-343.
- Maurer H, Sauer C, Theobald D. Toxicokinetics of drugs of abuse: current knowledge of the isoenzymes involved in the human metabolism of tetrahydrocannabinol, cocaine, heroin, morphine, and codeine. Ther Drug Monit. 2006;28(3):447-453.
- Dean A. Illicit drugs and drug interactions. Pharmacist. 2006;25(9):684-689.
Drug Brand Names
Carbamazepine • Carbatrol, Tegretol
Clozapine • Clozaril
Donepezil • Aricept
Ketoconazole • Nizoral
Paliperidone palmitate • Invega sustenna
Phenytoin • Dilantin, Phenytek
Quinidine • Cardioquin, Duraquin
Theophylline • Elixophylline, Theochron
Ms. L, age 37, presents to psychiatric emergency services with command auditory hallucinations, ideas of reference, and suicidal ideation.
Ms. L has a 22-year history of schizophrenia. Additionally, she has a history of cocaine use disorder (in remission for 12 years), cannabis use disorder (in remission for 6 months), type 2 diabetes mellitus, and hypertension. Her psychotic symptoms are well controlled on a regimen of
On interview, Ms. L reports smoking cannabis each day for the past month and using $400 worth of cocaine over 2 days. She is experiencing intense guilt over these relapses and is admitted to the inpatient adult psychiatry unit. On admission, Ms. L’s clozapine and norclozapine trough levels (drawn approximately 12 hours after last administration documented by the ACT member) are 300 and 275 ng/mL, respectively. Generally, clozapine levels >350 to 420 ng/mL are considered therapeutic, and a clozapine-to-norclozapine ratio of 2:1 is desirable for maximum efficacy and tolerability. Because Ms. L’s clozapine level is <350 and her ratio is approximately 1:1, her clozapine treatment is subtherapeutic.
Because Ms. L has a history of documented adherence to and benefit from her current medication regimen, no changes are made during her 3-week hospital stay. She notices a gradual reduction in auditory hallucinations, no longer wants to harm herself, and is motivated to regain sobriety.
At the time of discharge, Ms. L’s clozapine and norclozapine trough levels are 550 and 250 ng/mL, respectively, which indicates a more favorable clozapine-to-norclozapine ratio of approximately 2:1 and a clozapine level greater than the recommended minimum threshold of 350 ng/mL. While cocaine ingestion presumably played a role in her acute decompensation, the treatment team determined that Ms. L’s relapse to cannabis use likely contributed to low clozapine levels by induction of cytochrome P450 (CYP) 1A2, and subsequently led to the delayed recovery of symptom control.1
The use of illicit substances is a widespread, growing problem. According to the 2017 National Survey on Drug Use and Health, 11.5% of Americans age ≥12 had used an illicit substance (ie, use of marijuana, cocaine, heroin, hallucinogens, inhalants, or methamphetamine, or misuse of prescription psychotherapeutics) in the past month.2 While illicit substance use is of particular public health interest due to a known increase in mortality and health care spending, there has been little discussion of the impact of illicit drug use on concurrent pharmacologic therapy. Just as prescription medications have pharmacokinetic drug–drug interactions with each other, so do illicit substances, though far less is known about their impact on the treatment of medical conditions.
Pharmacokinetic interactions
Key pharmacokinetic interactions have been reported with cocaine, marijuana, amphetamines, and opioids. The Table1-8 summarizes the metabolism of illicit substances.
Continue to: Cocaine
Cocaine is largely metabolized by serum esterases such as pseudocholinesterase, human carboxylesterase-1 (hCE-1), and human carboxylesterase-2 (hCE-2), to inactive metabolites benzoylecgonine (35% to 45%) and ecgonine (32% to 49%).2 However, a smaller portion (2.6% to 6.2%) undergoes hepatic N-demethylation by CYP3A4 to norcocaine.3 Norcocaine is an active metabolite responsible for some of the toxic effects of cocaine (eg, hepatotoxicity).4,5 Several commonly prescribed medications are known inducers of CYP3A4 (eg, phenytoin, carbamazepine) and may lead to increased levels of the toxic metabolite when used concurrently with cocaine. Additionally, the use of cocaine with acetylcholinesterase inhibitors, such as donepezil, may lead to reduction of serum esterases and shunt cocaine metabolism toward the hepatic pathway, thus increasing norcocaine formation.3
Cannabis. The metabolism and drug–drug interactions of cannabis can be separated by its 2 main components: delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD). A review conducted in 2014 concluded that THC is primarily metabolized by CYP2C9 and 3A4, while CBD is metabolized by CYP2C19 and 3A4.6 Oral administration of ketoconazole, a CYP3A4 inhibitor, along with cannabis extract has been shown to increase the maximum concentration (Cmax) and area under the curve (AUC) of THC by 1.2- and 1.8-fold, respectively, while increasing both Cmaxand AUC of CBD by 2-fold.6 In addition, CYP2C9 poor metabolizers have been shown to experience significant increases in THC exposure and reductions in metabolite formation, further supporting the role of CYP enzymes in cannabis metabolism.6
There is also evidence of enzyme induction by cannabis. Individuals who reported smoking marijuana experienced greater clearance of theophylline, a substrate of CYP1A2, than did those who reported not smoking marijuana.1,6 As with cigarette smoking, this effect appears to be a direct result of the hydrocarbons found in marijuana smoke rather than the cannabis itself, as there is a lack of evidence for enzyme induction when the drug is orally ingested.6
Amphetamine and methamphetamine appear to be both substrates and competitive inhibitors of CYP2D6.7 Rats administered quinidine (a strong 2D6 inhibitor) had 2-fold elevations in AUC and decreased clearance of amphetamine and its metabolites.8 Amphetamine-related recreational drugs, such as 3,4-methylenedioxy-methamphetamine (MDMA) and 3,4-methylenedioxyamphetamine (MDA), are substrates of CYP2D6 and CYP3A4, while MDMA also undergoes substantial metabolism by CYP1A2.3,7,9
Opioids. Heroin is metabolized to 6‑monoacetylmorphine (6-MAM) and morphine by hCE-1, hCE-2, and pseudocholinesterase, and has minimal impact on CYP enzymes. However, while morphine is primarily metabolized to inactive metabolites by UGT2B7, it does undergo minor metabolism through CYP3A4 and 2C8 pathways, creating potential for drug interactions with medications that inhibit or induce CYP3A4.10
Continue to: An underappreciated risk of illicit substance use
An underappreciated risk of illicit substance use
There is a paucity of evidence regarding the metabolism and pharmacokinetic interactions with illicit substances, and further research is needed. Despite the absence of comprehensive data on the subject, the available information indicates the use of illicit substances may have a significant impact on medications used to treat comorbid conditions. Alternatively, those medications may affect the kinetics of recreationally used substances. The risk of adverse consequences of drug–drug interactions is yet another reason patients should be encouraged to avoid use of substances and seek treatment for substance use disorders. When determining the most appropriate therapy for comorbid conditions for patients who are using illicit substances and are likely to continue to do so, clinicians should take into consideration potential interactions among prescription medications and the specific illicit substances the patient uses.
Related Resources
- Lindsey W, Stewart D, Childress D. Drug interactions between common illicit drugs and prescription therapies. Am J Drug Alcohol Abuse. 2012;38(4):334-343.
- Maurer H, Sauer C, Theobald D. Toxicokinetics of drugs of abuse: current knowledge of the isoenzymes involved in the human metabolism of tetrahydrocannabinol, cocaine, heroin, morphine, and codeine. Ther Drug Monit. 2006;28(3):447-453.
- Dean A. Illicit drugs and drug interactions. Pharmacist. 2006;25(9):684-689.
Drug Brand Names
Carbamazepine • Carbatrol, Tegretol
Clozapine • Clozaril
Donepezil • Aricept
Ketoconazole • Nizoral
Paliperidone palmitate • Invega sustenna
Phenytoin • Dilantin, Phenytek
Quinidine • Cardioquin, Duraquin
Theophylline • Elixophylline, Theochron
1. Jusko WJ, Schentag JJ, Clark JH, et al. Enhanced biotransformation of theophylline in marihuana and tobacco smokers. Clin Pharmacol Ther. 1978;24(4):405-410.
2. Substance Abuse and Mental Health Services Administration. Results from the 2017 National Survey on Drug Use and Health: Detailed Tables. https://www.samhsa.gov/data/sites/default/files/cbhsq-reports/NSDUHDetailedTabs2017/NSDUHDetailedTabs2017.htm#tab1-1A. Published 2019. Accessed February 7, 2020.
3. Quinn D, Wodak A, Day R. Pharmacokinetic and pharmacodynamic principles of illicit drug use and treatment of illicit drug users. Clin Pharmacokinet. 1997;33(5):344-400.
4. Ndikum-Moffor FM, Schoeb TR, Roberts SM. Liver toxicity from norcocaine nitroxide, an N-oxidative metabolite of cocaine. J Pharmacol Exp Ther. 1998;284(1):413-419.
5. Pellinen P, Honkakoski P, Stenbäck F, et al. Cocaine N-demethylation and the metabolism-related hepatotoxicity can be prevented by cytochrome P450 3A inhibitors. Eur J Pharmacol. 1994;270(1):35-43.
6. Stout S, Cimino N. Exogenous cannabinoids as substrates, inhibitors, and inducers of human drug metabolizing enzymes: a systematic review. Drug Metab Rev. 2013;46(1):86-95.
7. Kraemer T, Maurer H. Toxicokinetics of amphetamines: metabolism and toxicokinetic data of designer drugs, amphetamine, methamphetamine, and their N-alkyl derivatives. Ther Drug Monit. 2002;24(2):277-289.
8. Markowitz J, Patrick K. Pharmacokinetic and pharmacodynamic drug interactions in the treatment of attention-deficit hyperactivity disorder. Clin Pharmacokinet. 2001;40(10):753-772.
9. Kreth K, Kovar K, Schwab M, et al. Identification of the human cytochromes P450 involved in the oxidative metabolism of “ecstasy”-related designer drugs. Biochem Pharmacol. 2000;59(12):1563-1571.
10. Meyer M, Maurer H. Absorption, distribution, metabolism and excretion pharmacogenomics of drugs of abuse. Pharmacogenomics. 2011;12(2):215-233.
1. Jusko WJ, Schentag JJ, Clark JH, et al. Enhanced biotransformation of theophylline in marihuana and tobacco smokers. Clin Pharmacol Ther. 1978;24(4):405-410.
2. Substance Abuse and Mental Health Services Administration. Results from the 2017 National Survey on Drug Use and Health: Detailed Tables. https://www.samhsa.gov/data/sites/default/files/cbhsq-reports/NSDUHDetailedTabs2017/NSDUHDetailedTabs2017.htm#tab1-1A. Published 2019. Accessed February 7, 2020.
3. Quinn D, Wodak A, Day R. Pharmacokinetic and pharmacodynamic principles of illicit drug use and treatment of illicit drug users. Clin Pharmacokinet. 1997;33(5):344-400.
4. Ndikum-Moffor FM, Schoeb TR, Roberts SM. Liver toxicity from norcocaine nitroxide, an N-oxidative metabolite of cocaine. J Pharmacol Exp Ther. 1998;284(1):413-419.
5. Pellinen P, Honkakoski P, Stenbäck F, et al. Cocaine N-demethylation and the metabolism-related hepatotoxicity can be prevented by cytochrome P450 3A inhibitors. Eur J Pharmacol. 1994;270(1):35-43.
6. Stout S, Cimino N. Exogenous cannabinoids as substrates, inhibitors, and inducers of human drug metabolizing enzymes: a systematic review. Drug Metab Rev. 2013;46(1):86-95.
7. Kraemer T, Maurer H. Toxicokinetics of amphetamines: metabolism and toxicokinetic data of designer drugs, amphetamine, methamphetamine, and their N-alkyl derivatives. Ther Drug Monit. 2002;24(2):277-289.
8. Markowitz J, Patrick K. Pharmacokinetic and pharmacodynamic drug interactions in the treatment of attention-deficit hyperactivity disorder. Clin Pharmacokinet. 2001;40(10):753-772.
9. Kreth K, Kovar K, Schwab M, et al. Identification of the human cytochromes P450 involved in the oxidative metabolism of “ecstasy”-related designer drugs. Biochem Pharmacol. 2000;59(12):1563-1571.
10. Meyer M, Maurer H. Absorption, distribution, metabolism and excretion pharmacogenomics of drugs of abuse. Pharmacogenomics. 2011;12(2):215-233.
Polypharmacy in older adults
Mrs. B, age 66, presents to the emergency department with altered mental status, impaired gait, and tremors. Her son says she has had these symptoms for 3 days. He adds that she has been experiencing more knee pain than usual, and began taking naproxen, 220 mg twice daily, approximately 1 week ago.
Mrs. B’s medical history includes coronary artery disease (CAD), gastroesophageal reflux disease (GERD), hip fracture, osteoarthritis, and osteoporosis. She also has a history of insomnia and bipolar disorder.
Further, Mrs. B reports that 2 months ago, after watching a television program about mental health, she began taking ginkgo biloba, 60 mg/d by mouth for “memory,” and kava kava, 100 mg by mouth 3 times a day for “anxiety.” She did not tell her physician or pharmacist that she began using these supplements because she believes that “natural supplements wouldn’t affect her prescription medications.”
In addition to naproxen, gingko biloba, and kava kava, Mrs. B takes the following medications orally:
Mrs. B’s blood pressure is 132/74 mm Hg (at goal for her age) and her laboratory workup is unremarkable, except for the following results: serum creatinine level of 1.1 mg/dL, blood urea nitrogen/serum creatinine ratio of 40, and creatinine clearance rate of approximately 85 mL/min. An electrocardiogram shows normal sinus rhythm with a QTc of 489 ms. A lithium serum concentration level, drawn randomly, is 1.6 mEq/mL, suggesting lithium toxicity.
Although there is no consensus definition of polypharmacy, the most commonly referenced is concurrent use of ≥5 medications.1 During the last 2 decades, the percentage of adults who report receiving polypharmacy has markedly increased, from 8.2% to 15%.2 Geriatric patients, defined as those age >65, typically receive ≥5 prescription medications.2 Polypharmacy is associated with increased1:
- mortality
- adverse drug reactions
- falls
- length of hospital stay
- readmission rates.
Older adults are particularly vulnerable to the negative outcomes associated with polypharmacy because both increasing age and number of medications received are positively correlated with the risk of adverse events.3 However, the use of multiple medications may be clinically appropriate and necessary in patients with multiple chronic conditions. Recent research suggests that in addition to prescription medications, over-the-counter (OTC) medications and dietary supplements also pose polypharmacy concerns for geriatric patients.3 Here we discuss the risks of OTC medications and dietary supplements for older patients who may be receiving polypharmacy, and highlight specific agents and interactions to watch for in these individuals based on Mrs. B’s case.
Continue to: Factors that increase the risks of OTC medications
Factors that increase the risks of OTC medications
Although older adults account for only 15% of the present population, they purchase 40% of all OTC medications.4 These patients may inadvertently use OTC medications containing unnecessary or potentially harmful active ingredients because of unfamiliarity with the specific product, variability among products, or decreased health literacy. According to research presented at a 2010 Institute of Medicine Workshop on Safe Use Initiative and Health Literacy, many patients have a limited understanding of OTC medication indications and therapeutic duplication.5 For example, researchers found that almost 70% of patients thought they could take 2 products containing the same ingredient.5 Most patients were not able to determine the active ingredients or maximum daily dose of an OTC medication. Patients who were older, had lower literacy, or were African American were more likely to misunderstand medication labeling.5 Additional literature suggests that up to 20% of medical admissions can be attributed to adverse effects of OTC medications.6
Misconceptions regarding dietary supplements
The use of alternative and complementary medicine also is on the rise among geriatric patients.7-9 A recent study found that 70% of older adults in the United States consumed at least 1 dietary supplement in the past 30 days, with 29% consuming ≥4 natural products. Women consumed twice as many supplements as men.10
The perceived safety of natural medicines and dietary supplements is a common and potentially dangerous misconception.11 Because patients typically assume dietary supplements are safe, they often do not report their use to their clinicians, especially if clinicians do not explicitly ask them about supplement use.12 This is especially concerning because the FDA does not have the authority to review or regulate natural medicines or dietary supplements.13,14
With no requirements or regulations regarding quality control of these products, the obvious question is: “How do patients know what they’re ingesting?” The uncertainty regarding the true composition of dietary supplements is a cause for concern because federal regulations do not provide a standard way to verify the purity, quality, and safety. As a result, there is a dearth of information regarding drug–dietary supplement interactions and drug–dietary supplement–disease state interactions.8,15
What to watch for
Table 116-22 outlines OTC medication classes and potential medication and/or disease state interactions. Table 223-45 outlines potential interactions between select dietary supplements, medications, and disease states. Here we discuss several of these potential interactions based on the medications that Mrs. B was taking.
Continue to: Nonsteroidal anti-inflammatory drugs (NSAIDs)
Nonsteroidal anti-inflammatory drugs (NSAIDs). All OTC NSAIDs, except aspirin and salicylates, increase the risk for lithium toxicity by decreasing glomerular filtration rate and promoting lithium reabsorption in the kidneys.16 Additionally, NSAIDs increase the risk of developing gastric ulcers and may initiate or exacerbate GERD by suppressing gastric prostaglandin synthesis. Gastric prostaglandins facilitate the formation of a protective lipid-layer in the gastrointestinal (GI) tract.18,46-48 For Mrs. B, the naproxen she was taking resulted in lithium toxicity.
Ginkgo biloba is a plant used most commonly for its reported effect on memory. However, many drug–dietary supplement interactions have been associated with ginkgo biloba that may pose a problem for geriatric patients who receive polypharmacy.49 Mrs. B may have experienced decreased effectiveness of omeprazole and increased sedation or orthostatic hypotension with trazodone.
Kava kava is a natural sedative that can worsen cognition, increase the risk of falls, and potentially cause hepatotoxicity.50 The sedative effects of kava kava are thought to be a direct result of gamma-aminobutyric acid (GABA) modulation via the blockage of voltage-gated sodium ion channels.51 In Mrs. B’s case, when used in combination with diphenhydramine and trazodone, kava kava had the potential to further increase her risk of sedation and falls.
Gastroesophageal reflux disease medications. Older adults may be at an increased risk of GERD due to diseases that affect the esophagus and GI tract, such as diabetes, Parkinson’s disease, and Alzheimer’s disease. Medications may also contribute to gastric reflux by loosening the esophageal tone. Nitrates, benzodiazepines, anticholinergics, antidepressants, and lidocaine have been implicated in precipitating or exacerbating GERD.52
Numerous OTC products can be used to treat heartburn. Calcium carbonate supplements are typically recommended as first-line agents to treat occasional heartburn; histamine-2 receptor antagonists (H2RAs) and proton pump inhibitors (PPIs) generally are reserved for patients who experience heartburn more frequently.47 Per the American Geriatrics Society Beers Criteria for Potentially Inappropriate Medication Use in Older Adults, H2RAs were removed from the “avoid” list for patients with dementia or cognitive impairment due to a lack of strong evidence; however, H2RAs remain on the “avoid” list for patients with delirium.17 Low-dose H2RAs can be used safely in geriatric patients who have renal impairment. Although PPIs are not listedon the Beers Criteria, they have been associated with an increased risk of dementia, osteoporosis, and infections.53,54 There is robust evidence to support bone loss and fractures associated with chronic use of PPIs. However, the data linking PPI use and dementia is controversial due to multiple confounders identified in the studies, such as concomitant use of benzodiazepines.48 PPIs should be prescribed sparingly and judiciously in geriatric patients, and the need for continued PPI therapy should frequently be reassessed.48 Mrs. B’s use of omeprazole, a PPI, may put her at an increased risk for hip fracture compounded by an elevated fall risk associated with other medications she was taking.
Continue to: Trazodone
Trazodone causes sedative effects via anti-alpha 1 activity, which is thought to be responsible for orthostasis and may further increase the risk of falls.51 Mrs. B’s use of trazodone may have increased her risk of sedation and falls.
Antihistaminergic medications are associated with sedation, confusion, cognitive dysfunction, falls, and delirium in geriatric patients. Medications that act on histamine receptors can be particularly detrimental in the geriatric population because of their decreased clearance, smaller volume of distribution, and decreased tolerance.17,18
Anticholinergic medications. Although atropine and benztropine are widely recognized as anticholinergic agents, other medications, such as digoxin, paroxetine, and colchicine, also demonstrate anticholinergic activity that can cause problematic central and peripheral effects in geriatric patients.55 Central anticholinergic inhibition can lead to reduced cognitive function and impairments in attention and short-term memory. The peripheral effects of anticholinergic medications are similar to those of antihistamines and may include, but are not limited to, dry eyes and mouth via increased inhibition of acetylcholine-mediated muscle contraction of salivary glands.55 These effects can be compounded by the use of OTC medications that exhibit anticholinergic activity.
Diphenhydramine causes sedation through its activity on cholinergic and histaminergic receptors. Patients may not be aware that many OTC cough-and-cold combination products (such as NyQuil, Theraflu, etc.) and OTC nighttime analgesic products (such as Tylenol PM, Aleve PM, Motrin PM, etc.) contain diphenhydramine. For a geriatric patient, such as Mrs. B, diphenhydramine may increase the risk of falls and worsen cognition.
Teach patients to disclose everything they take
Polypharmacy can be detrimental to older patients’ health due to the increased risk of toxicity caused by therapeutic duplication, drug–drug interactions, and drug-disease interactions. Most patients are unable to navigate the nuances of medication indications, maximum dosages, and therapeutic duplications. Older adults frequently take OTC medications and have the greatest risk of developing adverse effects from these medications due to decreased renal and hepatic clearance, increased drug sensitivity, and decreased volume of distribution. Dietary supplements pose a unique risk because they are not FDA-regulated and their purity, quality, and content cannot be verified. Educating patients and family members about the importance of reporting all their prescription medications, OTC medications, and dietary supplements to their pharmacists and clinicians is critical in order to identify and mitigate the risks associated with polypharmacy in geriatric patients.
Continue to: CASE
CASE CONTINUED
Mrs. B is diagnosed with lithium toxicity due to a drug–drug interaction with naproxen. Her lithium is held, and IV fluids are administered. Her symptoms resolve over the next few days. Mrs. B and her son are taught about the interaction between lithium and NSAIDs, and she is counseled to avoid all OTC NSAIDs other than aspirin. Her clinician recommends taking acetaminophen because it will not interact with her medications and is the recommended OTC treatment for mild or moderate pain in geriatric patients.17,56
Next, the clinician addresses Mrs. B’s GERD. Although Mrs. B had been taking PPIs twice daily, her physician recommends decreasing the omeprazole frequency to once daily to minimize adverse effects and pill burden. She also decreases Mrs. B’s aspirin from 325 to 81 mg/d because evidence suggests that when used to prevent CAD, lower-dose aspirin is effective as high-dose aspirin and has fewer adverse effects.57 Finally, she advises Mrs. B to stop taking ginkgo biloba and kava kava and to always check with her primary care physician or pharmacist before beginning any new medication, dietary supplement, or vitamin.
Mrs. B agrees to first check with her clinicians before following advice from mass media. A follow-up appointment is scheduled for 2 weeks to assess renal function, a lithium serum concentration, and adherence to her simplified medication regimen.
Related Resources
- US Department of Health and Human Services. National Institutes of Health. MedlinePlus. Herbs and supplements. https://medlineplus.gov/druginfo/herb_All.html.
- US Department of Health and Human Services. National Center for Complementary and Integrative Health. https://nccih.nih.gov/.
Drug Brand Names
Atorvastatin • Lipitor
Atropine • Atropen
Benztropine • Cogentin
Clozapine • Clozaril
Clopidogrel • Plavix
Colchicine • Colcrys, Gloperba
Digoxin • Cardoxin, Digitek
Lidocaine • Lidoderm, Xylocaine Viscous
Lithium • Eskalith, Lithobid
Methadone • Methadose
Morphine • Kadian, Morphabond
Paroxetine • Paxil
Trazodone • Desyrel
Warfarin • Coumadin, Jantoven
1. Masnoon N, Shakib S, Kalisch-Ellett, et al. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17:230.
2. Kantor ED, Rehm CD, Haas JS, et al. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA. 2015;314(17):1818-1831.
3. Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13(1):57-65.
4. Maiese DR. Healthy People 2010-leading health indicators for women. Womens Health Issues. 2002;12(4):155-164.
5. National Academy of Sciences. Institute of Medicine (US) Roundtable on Health Literacy. The Safe Use Initiative and Health Literacy: workshop summary. https://www.ncbi.nlm.nih.gov/books/NBK209756/. Published 2010. Accessed January 22, 2020.
6. Caranasos GJ, Stewart RB, Cluff LE. Drug-induced illness leading to hospitalisation. JAMA. 1974;228(6):713-717.
7. Agbabiaka T. Prevalence of drug–herb and drug-supplement interactions in older adults: a cross-sectional survey. Br J Gen Pract. 2018;68(675):e711-e717. doi: 10.3399/bjgp18X699101.
8. Agbabiaka T, Wider B, Watson L, et al. Concurrent use of prescription drugs and herbal medicinal products in older adults: a systematic review. Drugs Aging. 2017;34(12):891-905.
9. de Souza Silva JE, Santos Souza CA, da Silva TB, et al. Use of herbal medicines by elderly patients: a systematic review. Arch Gerontol Geriatr. 2014;59(2):227-233.
10. Gahche J, Bailey RL, Potischman N, et al. Dietary supplement use was very high among older adults in the United States in 2011-2014. J Nutr. 2017;147(10):1968-1976.
11. Nisly NL, Gryzlak BM, Zimmerman MB et al. Dietary supplement polypharmacy: an unrecognized public health problem? Evid Based Complement Alternat Med. 2010;7(1):107-113.
12. Kennedy J, Wang CC, Wu CH. Patient disclosure about herb and supplement use among adults in the US. Evid Based Complement Alternat Med. 2008;5(4):451-456.
13. Dickinson A. History and overview of DSHEA. Fitoterapia. 2011;82(1):5-10.
14. Dietary Supplement Health and Education Act of 1994. Public Law 103-417,103rd Congress. https://www.congress.gov/bill/103rd-congress/senate-bill/784. Accessed February 20, 2020.
15. US Department of Health & Human Services. National Institute on Aging. Dietary supplements. https://www.nia.nih.gov/health/dietary-supplements. Reviewed November 30, 2017. Accessed January 22, 2020.
16. Ragheb M. The clinical significance of lithium-nonsteroidal. J Clin Psychopharmacol. 1990;10(5):350-354.
17. 2019 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 Updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694.
18. Cho H, Myung J, Suh HS, et al. Antihistamine use and the risk of injurious falls or fracture in elderly patients: a systematic review and meta-analysis. Osteoporos Int. 2018;29(10):2163-2170.
19. Manlucu J, Tonelli M, Ray JG, et al. Dose-reducing H2 receptor antagonists in the presence of low glomerular filtration rate: a systematic review of the evidence. Nephrol Dial Transplant. 2005;20(11):2376-2384.
20. Sudafed [package insert]. Fort Washington, PA: McNeil Consumer Healthcare Division; 2018.
21. US National Library of Medicine. National Center for Biotechnology Information. PubChem Compound Summary: Dextromethorphan; CID=5360696. https://pubchem.ncbi.nlm.nih.gov/compound/5360696. Accessed January 22, 2020.
22. Hedya SA, Swoboda HD. Lithium toxicity. https://www.ncbi.nlm.nih.gov/books/NBK499992/. Updated August 14, 2019. Accessed January 22, 2020.
23. US Department of Health & Human Services. National Center for Complementary and Integrative Health. Herb-drug interactions: what the science says. https://www.nccih.nih.gov/health/providers/digest/herb-drug-interactions-science. Published September 2015. Accessed January 22, 2020.
24. Shader RI, Greenblatt DJ. Bees, ginseng and MAOIs revisited. J Clin Psychopharmacol. 1988;8(4):235.
25. Chua YT. Interaction between warfarin and Chinese herbal medicines. Singapore Med J. 2015;56(1):11-18.
26. Bonetto N, Santelli L, Battistin L, et al. Serotonin syndrome and rhabdomyolysis induced by concomitant use of triptans, fluoxetine and hypericum. Cephalalgia. 2007;27(12):1421-1423.
27. Henderson L, Yue QY, Bergquist C, et al. St John’s wort (Hypericum perforatum): drug interactions and clinical outcomes. Br J Clin Pharmacol. 2002;54(4):349-356.
28. Johne A, Schmider J, Brockmöller J, et al. Decreased plasma levels of amitriptyline and its metabolites on comedication with an extract from St John’s wort (Hypericum perforatum). J Clin Psychopharmacol. 2002;22(1):46-54.
29. Eich-Höchli D, Oppliger R, Golay KP, et al. Methadone maintenance treatment and St John’s wort: a case report. Pharmacopsychiatry. 2003;36(1):35-37.
30. Johne A, Brockmöller J, Bauer S, et al. Pharmacokinetic interaction of digoxin with an herbal extract from St John’s wort (Hypericum perforatum). Clin Pharmacol Ther. 1999;66(4):338-345.
31. Andrén L, Andreasson A, Eggertsen R. Interaction between a commercially available St John’s wort product (Movina) and atorvastatin in patients with hypercholesterolemia. Eur J Clin Pharmacol. 2007;63(10):913-916.
32. Van Strater AC. Interaction of St John’s wort (Hypericum perforatum) with clozapine. Int Clin Psychopharmacol. 2012;27(2):121-124.
33. Nöldner M, Chatterjee SS. Inhibition of haloperidol-induced catalepsy in rats by root extracts from Piper methysticum F. Phytomedicine. 1999;6(4):285-286.
34. Boerner RJ, Klement S. Attenuation of neuroleptic-induced extrapyramidal side effects by kava special extract WS 1490. Wien Med Wochenschr. 2004;154(21-22):508-510.
35. Schelosky L, Raffauf C, Jendroska K, et al. Kava and dopamine antagonism. J Neurol Neurosurg Psychiatry. 1995;58(5):639-640.
36. Singh YN. Potential for interaction of kava and St. John’s wort with drugs. J Ethnopharmacol. 2005;100(1-2):108-113.
37. Patel NM, Derkits R. Possible increase in liver enzymes secondary to atorvastatin and black cohosh administration. J Pharm Prac. 2007;20(4):341-346.
38. Rockwell S, Liu Y, Higgins SA. Alteration of the effects of cancer therapy agents on breast cancer cells by the herbal medicine black cohosh. Breast Cancer Res Treat. 2005;90(3):233-239.
39. Granger AS. Ginkgo biloba precipitating epileptic seizures. Age Ageing. 2001;30(6):523-525.
40. Mohutsky MA, Anderson GD, Miller JW, et al. Ginkgo biloba: evaluation of CYP2C9 drug interactions in vitro and in vivo. Am J Ther. 2006;13(1):24-31.
41. Zhang XY, Zhou DF, Zhang PY, et al. A double-blind, placebo controlled trial of extract of Ginkgo biloba added to haloperidol in treatment-resistant patients with schizophrenia. J Clin Psychiatry. 2001;62(11):878-883.
42. Atmaca M, Tezcan E, Kuloglu M, et al. The effect of extract of ginkgo biloba addition to olanzapine on therapeutic effect and antioxidant enzyme levels in patients with schizophrenia. Psychiatry Clin Neurosci. 2005;59(6):652-656.
43. Doruk A, Uzun O, Ozsahin A. A placebo-controlled study of extract of ginkgo biloba added to clozapine in patients with treatment-resistant schizophrenia. Int Clin Psychopharmacol. 2008;23(4):223-237.
44. Vaes LP. Interactions of warfarin with garlic, ginger, ginkgo, or ginseng: nature of the evidence. Ann Pharmacother. 2000;34(12):1478-1482.
45. Kanji S, Seely D, Yazdi F, et al. Interactions of commonly used dietary supplements with cardiovascular drugs: a systematic review. Syst Rev. 2012;1:26.
46. Wallace JL. Pathogenesis of NSAID-induced gastroduodenal mucosal injury. Best Pract Res Clin Gastroenterol. 2001;15(5):691-703.
47. Triadafilopoulos G, Sharma R. Features of symptomatic gastroesophageal reflux disease in elderly patients. Am J Gastroenterol. 1997;92(11):2007-2011.
48. Haastrup PF, Thompson W, Søndergaard J, et al. Side effects of long-term proton pump inhibitor use: a review. Basic Clin Pharmacol Toxicol. 2018;123(2):114-121.
49. Diamond BJ, Bailey MR. Ginkgo biloba: indications, mechanisms and safety. Psychiatr Clin N Am. 2013;36:73-83.
50. White CM. The pharmacology, pharmacokinetics, efficacy, and adverse events associated with kava. J Clin Pharmacol. 2018;58(11):1396-1405.
51. Gleitz J, Beile A, Peters T. (+/-)-Kavain inhibits veratridine-activated voltage-dependent Na(+)-channels in synaptosomes prepared from rat cerebral cortex. Neuropharmacology. 1995;34(9):1133-1138.
52. Kahrilas PJ. Gastroesophageal reflux disease and its complications. In: Feldman M, ed. Sleisenger & Fordtran’s Gastrointestinal and Liver Disease. 6th ed. Philadelphia, PA: WB Saunders Company; 1998:498-516.
53. Haenisch B, von Holt K, Wiese B, et al. Risk of dementia in elderly patients with the use of proton pump inhibitors. Eur Arch Psychiatry Clin Neurosci. 2015;265(5):419-428.
54. Sheen E, Triadafilopoulos G. Adverse effects of long-term proton pump inhibitor therapy. Dig Dis Sci. 2011;56(4):931-950.
55. Pitkälä KH, Suominen MH, Bell JS, et al. Herbal medications and other dietary supplements. A clinical review for physicians caring for older people. Ann Medicine. 2016;48(8):586-602.
56. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. Arthritis Rheum. 2000;43(9):1905-1915.
57. Vandvik PO, Lincoff AM, Core JM, et al. Primary and secondary prevention of cardiovascular disease: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):e637S-e668S. doi: 10.1378/chest.11-2306.
Mrs. B, age 66, presents to the emergency department with altered mental status, impaired gait, and tremors. Her son says she has had these symptoms for 3 days. He adds that she has been experiencing more knee pain than usual, and began taking naproxen, 220 mg twice daily, approximately 1 week ago.
Mrs. B’s medical history includes coronary artery disease (CAD), gastroesophageal reflux disease (GERD), hip fracture, osteoarthritis, and osteoporosis. She also has a history of insomnia and bipolar disorder.
Further, Mrs. B reports that 2 months ago, after watching a television program about mental health, she began taking ginkgo biloba, 60 mg/d by mouth for “memory,” and kava kava, 100 mg by mouth 3 times a day for “anxiety.” She did not tell her physician or pharmacist that she began using these supplements because she believes that “natural supplements wouldn’t affect her prescription medications.”
In addition to naproxen, gingko biloba, and kava kava, Mrs. B takes the following medications orally:
Mrs. B’s blood pressure is 132/74 mm Hg (at goal for her age) and her laboratory workup is unremarkable, except for the following results: serum creatinine level of 1.1 mg/dL, blood urea nitrogen/serum creatinine ratio of 40, and creatinine clearance rate of approximately 85 mL/min. An electrocardiogram shows normal sinus rhythm with a QTc of 489 ms. A lithium serum concentration level, drawn randomly, is 1.6 mEq/mL, suggesting lithium toxicity.
Although there is no consensus definition of polypharmacy, the most commonly referenced is concurrent use of ≥5 medications.1 During the last 2 decades, the percentage of adults who report receiving polypharmacy has markedly increased, from 8.2% to 15%.2 Geriatric patients, defined as those age >65, typically receive ≥5 prescription medications.2 Polypharmacy is associated with increased1:
- mortality
- adverse drug reactions
- falls
- length of hospital stay
- readmission rates.
Older adults are particularly vulnerable to the negative outcomes associated with polypharmacy because both increasing age and number of medications received are positively correlated with the risk of adverse events.3 However, the use of multiple medications may be clinically appropriate and necessary in patients with multiple chronic conditions. Recent research suggests that in addition to prescription medications, over-the-counter (OTC) medications and dietary supplements also pose polypharmacy concerns for geriatric patients.3 Here we discuss the risks of OTC medications and dietary supplements for older patients who may be receiving polypharmacy, and highlight specific agents and interactions to watch for in these individuals based on Mrs. B’s case.
Continue to: Factors that increase the risks of OTC medications
Factors that increase the risks of OTC medications
Although older adults account for only 15% of the present population, they purchase 40% of all OTC medications.4 These patients may inadvertently use OTC medications containing unnecessary or potentially harmful active ingredients because of unfamiliarity with the specific product, variability among products, or decreased health literacy. According to research presented at a 2010 Institute of Medicine Workshop on Safe Use Initiative and Health Literacy, many patients have a limited understanding of OTC medication indications and therapeutic duplication.5 For example, researchers found that almost 70% of patients thought they could take 2 products containing the same ingredient.5 Most patients were not able to determine the active ingredients or maximum daily dose of an OTC medication. Patients who were older, had lower literacy, or were African American were more likely to misunderstand medication labeling.5 Additional literature suggests that up to 20% of medical admissions can be attributed to adverse effects of OTC medications.6
Misconceptions regarding dietary supplements
The use of alternative and complementary medicine also is on the rise among geriatric patients.7-9 A recent study found that 70% of older adults in the United States consumed at least 1 dietary supplement in the past 30 days, with 29% consuming ≥4 natural products. Women consumed twice as many supplements as men.10
The perceived safety of natural medicines and dietary supplements is a common and potentially dangerous misconception.11 Because patients typically assume dietary supplements are safe, they often do not report their use to their clinicians, especially if clinicians do not explicitly ask them about supplement use.12 This is especially concerning because the FDA does not have the authority to review or regulate natural medicines or dietary supplements.13,14
With no requirements or regulations regarding quality control of these products, the obvious question is: “How do patients know what they’re ingesting?” The uncertainty regarding the true composition of dietary supplements is a cause for concern because federal regulations do not provide a standard way to verify the purity, quality, and safety. As a result, there is a dearth of information regarding drug–dietary supplement interactions and drug–dietary supplement–disease state interactions.8,15
What to watch for
Table 116-22 outlines OTC medication classes and potential medication and/or disease state interactions. Table 223-45 outlines potential interactions between select dietary supplements, medications, and disease states. Here we discuss several of these potential interactions based on the medications that Mrs. B was taking.
Continue to: Nonsteroidal anti-inflammatory drugs (NSAIDs)
Nonsteroidal anti-inflammatory drugs (NSAIDs). All OTC NSAIDs, except aspirin and salicylates, increase the risk for lithium toxicity by decreasing glomerular filtration rate and promoting lithium reabsorption in the kidneys.16 Additionally, NSAIDs increase the risk of developing gastric ulcers and may initiate or exacerbate GERD by suppressing gastric prostaglandin synthesis. Gastric prostaglandins facilitate the formation of a protective lipid-layer in the gastrointestinal (GI) tract.18,46-48 For Mrs. B, the naproxen she was taking resulted in lithium toxicity.
Ginkgo biloba is a plant used most commonly for its reported effect on memory. However, many drug–dietary supplement interactions have been associated with ginkgo biloba that may pose a problem for geriatric patients who receive polypharmacy.49 Mrs. B may have experienced decreased effectiveness of omeprazole and increased sedation or orthostatic hypotension with trazodone.
Kava kava is a natural sedative that can worsen cognition, increase the risk of falls, and potentially cause hepatotoxicity.50 The sedative effects of kava kava are thought to be a direct result of gamma-aminobutyric acid (GABA) modulation via the blockage of voltage-gated sodium ion channels.51 In Mrs. B’s case, when used in combination with diphenhydramine and trazodone, kava kava had the potential to further increase her risk of sedation and falls.
Gastroesophageal reflux disease medications. Older adults may be at an increased risk of GERD due to diseases that affect the esophagus and GI tract, such as diabetes, Parkinson’s disease, and Alzheimer’s disease. Medications may also contribute to gastric reflux by loosening the esophageal tone. Nitrates, benzodiazepines, anticholinergics, antidepressants, and lidocaine have been implicated in precipitating or exacerbating GERD.52
Numerous OTC products can be used to treat heartburn. Calcium carbonate supplements are typically recommended as first-line agents to treat occasional heartburn; histamine-2 receptor antagonists (H2RAs) and proton pump inhibitors (PPIs) generally are reserved for patients who experience heartburn more frequently.47 Per the American Geriatrics Society Beers Criteria for Potentially Inappropriate Medication Use in Older Adults, H2RAs were removed from the “avoid” list for patients with dementia or cognitive impairment due to a lack of strong evidence; however, H2RAs remain on the “avoid” list for patients with delirium.17 Low-dose H2RAs can be used safely in geriatric patients who have renal impairment. Although PPIs are not listedon the Beers Criteria, they have been associated with an increased risk of dementia, osteoporosis, and infections.53,54 There is robust evidence to support bone loss and fractures associated with chronic use of PPIs. However, the data linking PPI use and dementia is controversial due to multiple confounders identified in the studies, such as concomitant use of benzodiazepines.48 PPIs should be prescribed sparingly and judiciously in geriatric patients, and the need for continued PPI therapy should frequently be reassessed.48 Mrs. B’s use of omeprazole, a PPI, may put her at an increased risk for hip fracture compounded by an elevated fall risk associated with other medications she was taking.
Continue to: Trazodone
Trazodone causes sedative effects via anti-alpha 1 activity, which is thought to be responsible for orthostasis and may further increase the risk of falls.51 Mrs. B’s use of trazodone may have increased her risk of sedation and falls.
Antihistaminergic medications are associated with sedation, confusion, cognitive dysfunction, falls, and delirium in geriatric patients. Medications that act on histamine receptors can be particularly detrimental in the geriatric population because of their decreased clearance, smaller volume of distribution, and decreased tolerance.17,18
Anticholinergic medications. Although atropine and benztropine are widely recognized as anticholinergic agents, other medications, such as digoxin, paroxetine, and colchicine, also demonstrate anticholinergic activity that can cause problematic central and peripheral effects in geriatric patients.55 Central anticholinergic inhibition can lead to reduced cognitive function and impairments in attention and short-term memory. The peripheral effects of anticholinergic medications are similar to those of antihistamines and may include, but are not limited to, dry eyes and mouth via increased inhibition of acetylcholine-mediated muscle contraction of salivary glands.55 These effects can be compounded by the use of OTC medications that exhibit anticholinergic activity.
Diphenhydramine causes sedation through its activity on cholinergic and histaminergic receptors. Patients may not be aware that many OTC cough-and-cold combination products (such as NyQuil, Theraflu, etc.) and OTC nighttime analgesic products (such as Tylenol PM, Aleve PM, Motrin PM, etc.) contain diphenhydramine. For a geriatric patient, such as Mrs. B, diphenhydramine may increase the risk of falls and worsen cognition.
Teach patients to disclose everything they take
Polypharmacy can be detrimental to older patients’ health due to the increased risk of toxicity caused by therapeutic duplication, drug–drug interactions, and drug-disease interactions. Most patients are unable to navigate the nuances of medication indications, maximum dosages, and therapeutic duplications. Older adults frequently take OTC medications and have the greatest risk of developing adverse effects from these medications due to decreased renal and hepatic clearance, increased drug sensitivity, and decreased volume of distribution. Dietary supplements pose a unique risk because they are not FDA-regulated and their purity, quality, and content cannot be verified. Educating patients and family members about the importance of reporting all their prescription medications, OTC medications, and dietary supplements to their pharmacists and clinicians is critical in order to identify and mitigate the risks associated with polypharmacy in geriatric patients.
Continue to: CASE
CASE CONTINUED
Mrs. B is diagnosed with lithium toxicity due to a drug–drug interaction with naproxen. Her lithium is held, and IV fluids are administered. Her symptoms resolve over the next few days. Mrs. B and her son are taught about the interaction between lithium and NSAIDs, and she is counseled to avoid all OTC NSAIDs other than aspirin. Her clinician recommends taking acetaminophen because it will not interact with her medications and is the recommended OTC treatment for mild or moderate pain in geriatric patients.17,56
Next, the clinician addresses Mrs. B’s GERD. Although Mrs. B had been taking PPIs twice daily, her physician recommends decreasing the omeprazole frequency to once daily to minimize adverse effects and pill burden. She also decreases Mrs. B’s aspirin from 325 to 81 mg/d because evidence suggests that when used to prevent CAD, lower-dose aspirin is effective as high-dose aspirin and has fewer adverse effects.57 Finally, she advises Mrs. B to stop taking ginkgo biloba and kava kava and to always check with her primary care physician or pharmacist before beginning any new medication, dietary supplement, or vitamin.
Mrs. B agrees to first check with her clinicians before following advice from mass media. A follow-up appointment is scheduled for 2 weeks to assess renal function, a lithium serum concentration, and adherence to her simplified medication regimen.
Related Resources
- US Department of Health and Human Services. National Institutes of Health. MedlinePlus. Herbs and supplements. https://medlineplus.gov/druginfo/herb_All.html.
- US Department of Health and Human Services. National Center for Complementary and Integrative Health. https://nccih.nih.gov/.
Drug Brand Names
Atorvastatin • Lipitor
Atropine • Atropen
Benztropine • Cogentin
Clozapine • Clozaril
Clopidogrel • Plavix
Colchicine • Colcrys, Gloperba
Digoxin • Cardoxin, Digitek
Lidocaine • Lidoderm, Xylocaine Viscous
Lithium • Eskalith, Lithobid
Methadone • Methadose
Morphine • Kadian, Morphabond
Paroxetine • Paxil
Trazodone • Desyrel
Warfarin • Coumadin, Jantoven
Mrs. B, age 66, presents to the emergency department with altered mental status, impaired gait, and tremors. Her son says she has had these symptoms for 3 days. He adds that she has been experiencing more knee pain than usual, and began taking naproxen, 220 mg twice daily, approximately 1 week ago.
Mrs. B’s medical history includes coronary artery disease (CAD), gastroesophageal reflux disease (GERD), hip fracture, osteoarthritis, and osteoporosis. She also has a history of insomnia and bipolar disorder.
Further, Mrs. B reports that 2 months ago, after watching a television program about mental health, she began taking ginkgo biloba, 60 mg/d by mouth for “memory,” and kava kava, 100 mg by mouth 3 times a day for “anxiety.” She did not tell her physician or pharmacist that she began using these supplements because she believes that “natural supplements wouldn’t affect her prescription medications.”
In addition to naproxen, gingko biloba, and kava kava, Mrs. B takes the following medications orally:
Mrs. B’s blood pressure is 132/74 mm Hg (at goal for her age) and her laboratory workup is unremarkable, except for the following results: serum creatinine level of 1.1 mg/dL, blood urea nitrogen/serum creatinine ratio of 40, and creatinine clearance rate of approximately 85 mL/min. An electrocardiogram shows normal sinus rhythm with a QTc of 489 ms. A lithium serum concentration level, drawn randomly, is 1.6 mEq/mL, suggesting lithium toxicity.
Although there is no consensus definition of polypharmacy, the most commonly referenced is concurrent use of ≥5 medications.1 During the last 2 decades, the percentage of adults who report receiving polypharmacy has markedly increased, from 8.2% to 15%.2 Geriatric patients, defined as those age >65, typically receive ≥5 prescription medications.2 Polypharmacy is associated with increased1:
- mortality
- adverse drug reactions
- falls
- length of hospital stay
- readmission rates.
Older adults are particularly vulnerable to the negative outcomes associated with polypharmacy because both increasing age and number of medications received are positively correlated with the risk of adverse events.3 However, the use of multiple medications may be clinically appropriate and necessary in patients with multiple chronic conditions. Recent research suggests that in addition to prescription medications, over-the-counter (OTC) medications and dietary supplements also pose polypharmacy concerns for geriatric patients.3 Here we discuss the risks of OTC medications and dietary supplements for older patients who may be receiving polypharmacy, and highlight specific agents and interactions to watch for in these individuals based on Mrs. B’s case.
Continue to: Factors that increase the risks of OTC medications
Factors that increase the risks of OTC medications
Although older adults account for only 15% of the present population, they purchase 40% of all OTC medications.4 These patients may inadvertently use OTC medications containing unnecessary or potentially harmful active ingredients because of unfamiliarity with the specific product, variability among products, or decreased health literacy. According to research presented at a 2010 Institute of Medicine Workshop on Safe Use Initiative and Health Literacy, many patients have a limited understanding of OTC medication indications and therapeutic duplication.5 For example, researchers found that almost 70% of patients thought they could take 2 products containing the same ingredient.5 Most patients were not able to determine the active ingredients or maximum daily dose of an OTC medication. Patients who were older, had lower literacy, or were African American were more likely to misunderstand medication labeling.5 Additional literature suggests that up to 20% of medical admissions can be attributed to adverse effects of OTC medications.6
Misconceptions regarding dietary supplements
The use of alternative and complementary medicine also is on the rise among geriatric patients.7-9 A recent study found that 70% of older adults in the United States consumed at least 1 dietary supplement in the past 30 days, with 29% consuming ≥4 natural products. Women consumed twice as many supplements as men.10
The perceived safety of natural medicines and dietary supplements is a common and potentially dangerous misconception.11 Because patients typically assume dietary supplements are safe, they often do not report their use to their clinicians, especially if clinicians do not explicitly ask them about supplement use.12 This is especially concerning because the FDA does not have the authority to review or regulate natural medicines or dietary supplements.13,14
With no requirements or regulations regarding quality control of these products, the obvious question is: “How do patients know what they’re ingesting?” The uncertainty regarding the true composition of dietary supplements is a cause for concern because federal regulations do not provide a standard way to verify the purity, quality, and safety. As a result, there is a dearth of information regarding drug–dietary supplement interactions and drug–dietary supplement–disease state interactions.8,15
What to watch for
Table 116-22 outlines OTC medication classes and potential medication and/or disease state interactions. Table 223-45 outlines potential interactions between select dietary supplements, medications, and disease states. Here we discuss several of these potential interactions based on the medications that Mrs. B was taking.
Continue to: Nonsteroidal anti-inflammatory drugs (NSAIDs)
Nonsteroidal anti-inflammatory drugs (NSAIDs). All OTC NSAIDs, except aspirin and salicylates, increase the risk for lithium toxicity by decreasing glomerular filtration rate and promoting lithium reabsorption in the kidneys.16 Additionally, NSAIDs increase the risk of developing gastric ulcers and may initiate or exacerbate GERD by suppressing gastric prostaglandin synthesis. Gastric prostaglandins facilitate the formation of a protective lipid-layer in the gastrointestinal (GI) tract.18,46-48 For Mrs. B, the naproxen she was taking resulted in lithium toxicity.
Ginkgo biloba is a plant used most commonly for its reported effect on memory. However, many drug–dietary supplement interactions have been associated with ginkgo biloba that may pose a problem for geriatric patients who receive polypharmacy.49 Mrs. B may have experienced decreased effectiveness of omeprazole and increased sedation or orthostatic hypotension with trazodone.
Kava kava is a natural sedative that can worsen cognition, increase the risk of falls, and potentially cause hepatotoxicity.50 The sedative effects of kava kava are thought to be a direct result of gamma-aminobutyric acid (GABA) modulation via the blockage of voltage-gated sodium ion channels.51 In Mrs. B’s case, when used in combination with diphenhydramine and trazodone, kava kava had the potential to further increase her risk of sedation and falls.
Gastroesophageal reflux disease medications. Older adults may be at an increased risk of GERD due to diseases that affect the esophagus and GI tract, such as diabetes, Parkinson’s disease, and Alzheimer’s disease. Medications may also contribute to gastric reflux by loosening the esophageal tone. Nitrates, benzodiazepines, anticholinergics, antidepressants, and lidocaine have been implicated in precipitating or exacerbating GERD.52
Numerous OTC products can be used to treat heartburn. Calcium carbonate supplements are typically recommended as first-line agents to treat occasional heartburn; histamine-2 receptor antagonists (H2RAs) and proton pump inhibitors (PPIs) generally are reserved for patients who experience heartburn more frequently.47 Per the American Geriatrics Society Beers Criteria for Potentially Inappropriate Medication Use in Older Adults, H2RAs were removed from the “avoid” list for patients with dementia or cognitive impairment due to a lack of strong evidence; however, H2RAs remain on the “avoid” list for patients with delirium.17 Low-dose H2RAs can be used safely in geriatric patients who have renal impairment. Although PPIs are not listedon the Beers Criteria, they have been associated with an increased risk of dementia, osteoporosis, and infections.53,54 There is robust evidence to support bone loss and fractures associated with chronic use of PPIs. However, the data linking PPI use and dementia is controversial due to multiple confounders identified in the studies, such as concomitant use of benzodiazepines.48 PPIs should be prescribed sparingly and judiciously in geriatric patients, and the need for continued PPI therapy should frequently be reassessed.48 Mrs. B’s use of omeprazole, a PPI, may put her at an increased risk for hip fracture compounded by an elevated fall risk associated with other medications she was taking.
Continue to: Trazodone
Trazodone causes sedative effects via anti-alpha 1 activity, which is thought to be responsible for orthostasis and may further increase the risk of falls.51 Mrs. B’s use of trazodone may have increased her risk of sedation and falls.
Antihistaminergic medications are associated with sedation, confusion, cognitive dysfunction, falls, and delirium in geriatric patients. Medications that act on histamine receptors can be particularly detrimental in the geriatric population because of their decreased clearance, smaller volume of distribution, and decreased tolerance.17,18
Anticholinergic medications. Although atropine and benztropine are widely recognized as anticholinergic agents, other medications, such as digoxin, paroxetine, and colchicine, also demonstrate anticholinergic activity that can cause problematic central and peripheral effects in geriatric patients.55 Central anticholinergic inhibition can lead to reduced cognitive function and impairments in attention and short-term memory. The peripheral effects of anticholinergic medications are similar to those of antihistamines and may include, but are not limited to, dry eyes and mouth via increased inhibition of acetylcholine-mediated muscle contraction of salivary glands.55 These effects can be compounded by the use of OTC medications that exhibit anticholinergic activity.
Diphenhydramine causes sedation through its activity on cholinergic and histaminergic receptors. Patients may not be aware that many OTC cough-and-cold combination products (such as NyQuil, Theraflu, etc.) and OTC nighttime analgesic products (such as Tylenol PM, Aleve PM, Motrin PM, etc.) contain diphenhydramine. For a geriatric patient, such as Mrs. B, diphenhydramine may increase the risk of falls and worsen cognition.
Teach patients to disclose everything they take
Polypharmacy can be detrimental to older patients’ health due to the increased risk of toxicity caused by therapeutic duplication, drug–drug interactions, and drug-disease interactions. Most patients are unable to navigate the nuances of medication indications, maximum dosages, and therapeutic duplications. Older adults frequently take OTC medications and have the greatest risk of developing adverse effects from these medications due to decreased renal and hepatic clearance, increased drug sensitivity, and decreased volume of distribution. Dietary supplements pose a unique risk because they are not FDA-regulated and their purity, quality, and content cannot be verified. Educating patients and family members about the importance of reporting all their prescription medications, OTC medications, and dietary supplements to their pharmacists and clinicians is critical in order to identify and mitigate the risks associated with polypharmacy in geriatric patients.
Continue to: CASE
CASE CONTINUED
Mrs. B is diagnosed with lithium toxicity due to a drug–drug interaction with naproxen. Her lithium is held, and IV fluids are administered. Her symptoms resolve over the next few days. Mrs. B and her son are taught about the interaction between lithium and NSAIDs, and she is counseled to avoid all OTC NSAIDs other than aspirin. Her clinician recommends taking acetaminophen because it will not interact with her medications and is the recommended OTC treatment for mild or moderate pain in geriatric patients.17,56
Next, the clinician addresses Mrs. B’s GERD. Although Mrs. B had been taking PPIs twice daily, her physician recommends decreasing the omeprazole frequency to once daily to minimize adverse effects and pill burden. She also decreases Mrs. B’s aspirin from 325 to 81 mg/d because evidence suggests that when used to prevent CAD, lower-dose aspirin is effective as high-dose aspirin and has fewer adverse effects.57 Finally, she advises Mrs. B to stop taking ginkgo biloba and kava kava and to always check with her primary care physician or pharmacist before beginning any new medication, dietary supplement, or vitamin.
Mrs. B agrees to first check with her clinicians before following advice from mass media. A follow-up appointment is scheduled for 2 weeks to assess renal function, a lithium serum concentration, and adherence to her simplified medication regimen.
Related Resources
- US Department of Health and Human Services. National Institutes of Health. MedlinePlus. Herbs and supplements. https://medlineplus.gov/druginfo/herb_All.html.
- US Department of Health and Human Services. National Center for Complementary and Integrative Health. https://nccih.nih.gov/.
Drug Brand Names
Atorvastatin • Lipitor
Atropine • Atropen
Benztropine • Cogentin
Clozapine • Clozaril
Clopidogrel • Plavix
Colchicine • Colcrys, Gloperba
Digoxin • Cardoxin, Digitek
Lidocaine • Lidoderm, Xylocaine Viscous
Lithium • Eskalith, Lithobid
Methadone • Methadose
Morphine • Kadian, Morphabond
Paroxetine • Paxil
Trazodone • Desyrel
Warfarin • Coumadin, Jantoven
1. Masnoon N, Shakib S, Kalisch-Ellett, et al. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17:230.
2. Kantor ED, Rehm CD, Haas JS, et al. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA. 2015;314(17):1818-1831.
3. Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13(1):57-65.
4. Maiese DR. Healthy People 2010-leading health indicators for women. Womens Health Issues. 2002;12(4):155-164.
5. National Academy of Sciences. Institute of Medicine (US) Roundtable on Health Literacy. The Safe Use Initiative and Health Literacy: workshop summary. https://www.ncbi.nlm.nih.gov/books/NBK209756/. Published 2010. Accessed January 22, 2020.
6. Caranasos GJ, Stewart RB, Cluff LE. Drug-induced illness leading to hospitalisation. JAMA. 1974;228(6):713-717.
7. Agbabiaka T. Prevalence of drug–herb and drug-supplement interactions in older adults: a cross-sectional survey. Br J Gen Pract. 2018;68(675):e711-e717. doi: 10.3399/bjgp18X699101.
8. Agbabiaka T, Wider B, Watson L, et al. Concurrent use of prescription drugs and herbal medicinal products in older adults: a systematic review. Drugs Aging. 2017;34(12):891-905.
9. de Souza Silva JE, Santos Souza CA, da Silva TB, et al. Use of herbal medicines by elderly patients: a systematic review. Arch Gerontol Geriatr. 2014;59(2):227-233.
10. Gahche J, Bailey RL, Potischman N, et al. Dietary supplement use was very high among older adults in the United States in 2011-2014. J Nutr. 2017;147(10):1968-1976.
11. Nisly NL, Gryzlak BM, Zimmerman MB et al. Dietary supplement polypharmacy: an unrecognized public health problem? Evid Based Complement Alternat Med. 2010;7(1):107-113.
12. Kennedy J, Wang CC, Wu CH. Patient disclosure about herb and supplement use among adults in the US. Evid Based Complement Alternat Med. 2008;5(4):451-456.
13. Dickinson A. History and overview of DSHEA. Fitoterapia. 2011;82(1):5-10.
14. Dietary Supplement Health and Education Act of 1994. Public Law 103-417,103rd Congress. https://www.congress.gov/bill/103rd-congress/senate-bill/784. Accessed February 20, 2020.
15. US Department of Health & Human Services. National Institute on Aging. Dietary supplements. https://www.nia.nih.gov/health/dietary-supplements. Reviewed November 30, 2017. Accessed January 22, 2020.
16. Ragheb M. The clinical significance of lithium-nonsteroidal. J Clin Psychopharmacol. 1990;10(5):350-354.
17. 2019 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 Updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694.
18. Cho H, Myung J, Suh HS, et al. Antihistamine use and the risk of injurious falls or fracture in elderly patients: a systematic review and meta-analysis. Osteoporos Int. 2018;29(10):2163-2170.
19. Manlucu J, Tonelli M, Ray JG, et al. Dose-reducing H2 receptor antagonists in the presence of low glomerular filtration rate: a systematic review of the evidence. Nephrol Dial Transplant. 2005;20(11):2376-2384.
20. Sudafed [package insert]. Fort Washington, PA: McNeil Consumer Healthcare Division; 2018.
21. US National Library of Medicine. National Center for Biotechnology Information. PubChem Compound Summary: Dextromethorphan; CID=5360696. https://pubchem.ncbi.nlm.nih.gov/compound/5360696. Accessed January 22, 2020.
22. Hedya SA, Swoboda HD. Lithium toxicity. https://www.ncbi.nlm.nih.gov/books/NBK499992/. Updated August 14, 2019. Accessed January 22, 2020.
23. US Department of Health & Human Services. National Center for Complementary and Integrative Health. Herb-drug interactions: what the science says. https://www.nccih.nih.gov/health/providers/digest/herb-drug-interactions-science. Published September 2015. Accessed January 22, 2020.
24. Shader RI, Greenblatt DJ. Bees, ginseng and MAOIs revisited. J Clin Psychopharmacol. 1988;8(4):235.
25. Chua YT. Interaction between warfarin and Chinese herbal medicines. Singapore Med J. 2015;56(1):11-18.
26. Bonetto N, Santelli L, Battistin L, et al. Serotonin syndrome and rhabdomyolysis induced by concomitant use of triptans, fluoxetine and hypericum. Cephalalgia. 2007;27(12):1421-1423.
27. Henderson L, Yue QY, Bergquist C, et al. St John’s wort (Hypericum perforatum): drug interactions and clinical outcomes. Br J Clin Pharmacol. 2002;54(4):349-356.
28. Johne A, Schmider J, Brockmöller J, et al. Decreased plasma levels of amitriptyline and its metabolites on comedication with an extract from St John’s wort (Hypericum perforatum). J Clin Psychopharmacol. 2002;22(1):46-54.
29. Eich-Höchli D, Oppliger R, Golay KP, et al. Methadone maintenance treatment and St John’s wort: a case report. Pharmacopsychiatry. 2003;36(1):35-37.
30. Johne A, Brockmöller J, Bauer S, et al. Pharmacokinetic interaction of digoxin with an herbal extract from St John’s wort (Hypericum perforatum). Clin Pharmacol Ther. 1999;66(4):338-345.
31. Andrén L, Andreasson A, Eggertsen R. Interaction between a commercially available St John’s wort product (Movina) and atorvastatin in patients with hypercholesterolemia. Eur J Clin Pharmacol. 2007;63(10):913-916.
32. Van Strater AC. Interaction of St John’s wort (Hypericum perforatum) with clozapine. Int Clin Psychopharmacol. 2012;27(2):121-124.
33. Nöldner M, Chatterjee SS. Inhibition of haloperidol-induced catalepsy in rats by root extracts from Piper methysticum F. Phytomedicine. 1999;6(4):285-286.
34. Boerner RJ, Klement S. Attenuation of neuroleptic-induced extrapyramidal side effects by kava special extract WS 1490. Wien Med Wochenschr. 2004;154(21-22):508-510.
35. Schelosky L, Raffauf C, Jendroska K, et al. Kava and dopamine antagonism. J Neurol Neurosurg Psychiatry. 1995;58(5):639-640.
36. Singh YN. Potential for interaction of kava and St. John’s wort with drugs. J Ethnopharmacol. 2005;100(1-2):108-113.
37. Patel NM, Derkits R. Possible increase in liver enzymes secondary to atorvastatin and black cohosh administration. J Pharm Prac. 2007;20(4):341-346.
38. Rockwell S, Liu Y, Higgins SA. Alteration of the effects of cancer therapy agents on breast cancer cells by the herbal medicine black cohosh. Breast Cancer Res Treat. 2005;90(3):233-239.
39. Granger AS. Ginkgo biloba precipitating epileptic seizures. Age Ageing. 2001;30(6):523-525.
40. Mohutsky MA, Anderson GD, Miller JW, et al. Ginkgo biloba: evaluation of CYP2C9 drug interactions in vitro and in vivo. Am J Ther. 2006;13(1):24-31.
41. Zhang XY, Zhou DF, Zhang PY, et al. A double-blind, placebo controlled trial of extract of Ginkgo biloba added to haloperidol in treatment-resistant patients with schizophrenia. J Clin Psychiatry. 2001;62(11):878-883.
42. Atmaca M, Tezcan E, Kuloglu M, et al. The effect of extract of ginkgo biloba addition to olanzapine on therapeutic effect and antioxidant enzyme levels in patients with schizophrenia. Psychiatry Clin Neurosci. 2005;59(6):652-656.
43. Doruk A, Uzun O, Ozsahin A. A placebo-controlled study of extract of ginkgo biloba added to clozapine in patients with treatment-resistant schizophrenia. Int Clin Psychopharmacol. 2008;23(4):223-237.
44. Vaes LP. Interactions of warfarin with garlic, ginger, ginkgo, or ginseng: nature of the evidence. Ann Pharmacother. 2000;34(12):1478-1482.
45. Kanji S, Seely D, Yazdi F, et al. Interactions of commonly used dietary supplements with cardiovascular drugs: a systematic review. Syst Rev. 2012;1:26.
46. Wallace JL. Pathogenesis of NSAID-induced gastroduodenal mucosal injury. Best Pract Res Clin Gastroenterol. 2001;15(5):691-703.
47. Triadafilopoulos G, Sharma R. Features of symptomatic gastroesophageal reflux disease in elderly patients. Am J Gastroenterol. 1997;92(11):2007-2011.
48. Haastrup PF, Thompson W, Søndergaard J, et al. Side effects of long-term proton pump inhibitor use: a review. Basic Clin Pharmacol Toxicol. 2018;123(2):114-121.
49. Diamond BJ, Bailey MR. Ginkgo biloba: indications, mechanisms and safety. Psychiatr Clin N Am. 2013;36:73-83.
50. White CM. The pharmacology, pharmacokinetics, efficacy, and adverse events associated with kava. J Clin Pharmacol. 2018;58(11):1396-1405.
51. Gleitz J, Beile A, Peters T. (+/-)-Kavain inhibits veratridine-activated voltage-dependent Na(+)-channels in synaptosomes prepared from rat cerebral cortex. Neuropharmacology. 1995;34(9):1133-1138.
52. Kahrilas PJ. Gastroesophageal reflux disease and its complications. In: Feldman M, ed. Sleisenger & Fordtran’s Gastrointestinal and Liver Disease. 6th ed. Philadelphia, PA: WB Saunders Company; 1998:498-516.
53. Haenisch B, von Holt K, Wiese B, et al. Risk of dementia in elderly patients with the use of proton pump inhibitors. Eur Arch Psychiatry Clin Neurosci. 2015;265(5):419-428.
54. Sheen E, Triadafilopoulos G. Adverse effects of long-term proton pump inhibitor therapy. Dig Dis Sci. 2011;56(4):931-950.
55. Pitkälä KH, Suominen MH, Bell JS, et al. Herbal medications and other dietary supplements. A clinical review for physicians caring for older people. Ann Medicine. 2016;48(8):586-602.
56. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. Arthritis Rheum. 2000;43(9):1905-1915.
57. Vandvik PO, Lincoff AM, Core JM, et al. Primary and secondary prevention of cardiovascular disease: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):e637S-e668S. doi: 10.1378/chest.11-2306.
1. Masnoon N, Shakib S, Kalisch-Ellett, et al. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17:230.
2. Kantor ED, Rehm CD, Haas JS, et al. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA. 2015;314(17):1818-1831.
3. Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13(1):57-65.
4. Maiese DR. Healthy People 2010-leading health indicators for women. Womens Health Issues. 2002;12(4):155-164.
5. National Academy of Sciences. Institute of Medicine (US) Roundtable on Health Literacy. The Safe Use Initiative and Health Literacy: workshop summary. https://www.ncbi.nlm.nih.gov/books/NBK209756/. Published 2010. Accessed January 22, 2020.
6. Caranasos GJ, Stewart RB, Cluff LE. Drug-induced illness leading to hospitalisation. JAMA. 1974;228(6):713-717.
7. Agbabiaka T. Prevalence of drug–herb and drug-supplement interactions in older adults: a cross-sectional survey. Br J Gen Pract. 2018;68(675):e711-e717. doi: 10.3399/bjgp18X699101.
8. Agbabiaka T, Wider B, Watson L, et al. Concurrent use of prescription drugs and herbal medicinal products in older adults: a systematic review. Drugs Aging. 2017;34(12):891-905.
9. de Souza Silva JE, Santos Souza CA, da Silva TB, et al. Use of herbal medicines by elderly patients: a systematic review. Arch Gerontol Geriatr. 2014;59(2):227-233.
10. Gahche J, Bailey RL, Potischman N, et al. Dietary supplement use was very high among older adults in the United States in 2011-2014. J Nutr. 2017;147(10):1968-1976.
11. Nisly NL, Gryzlak BM, Zimmerman MB et al. Dietary supplement polypharmacy: an unrecognized public health problem? Evid Based Complement Alternat Med. 2010;7(1):107-113.
12. Kennedy J, Wang CC, Wu CH. Patient disclosure about herb and supplement use among adults in the US. Evid Based Complement Alternat Med. 2008;5(4):451-456.
13. Dickinson A. History and overview of DSHEA. Fitoterapia. 2011;82(1):5-10.
14. Dietary Supplement Health and Education Act of 1994. Public Law 103-417,103rd Congress. https://www.congress.gov/bill/103rd-congress/senate-bill/784. Accessed February 20, 2020.
15. US Department of Health & Human Services. National Institute on Aging. Dietary supplements. https://www.nia.nih.gov/health/dietary-supplements. Reviewed November 30, 2017. Accessed January 22, 2020.
16. Ragheb M. The clinical significance of lithium-nonsteroidal. J Clin Psychopharmacol. 1990;10(5):350-354.
17. 2019 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 Updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694.
18. Cho H, Myung J, Suh HS, et al. Antihistamine use and the risk of injurious falls or fracture in elderly patients: a systematic review and meta-analysis. Osteoporos Int. 2018;29(10):2163-2170.
19. Manlucu J, Tonelli M, Ray JG, et al. Dose-reducing H2 receptor antagonists in the presence of low glomerular filtration rate: a systematic review of the evidence. Nephrol Dial Transplant. 2005;20(11):2376-2384.
20. Sudafed [package insert]. Fort Washington, PA: McNeil Consumer Healthcare Division; 2018.
21. US National Library of Medicine. National Center for Biotechnology Information. PubChem Compound Summary: Dextromethorphan; CID=5360696. https://pubchem.ncbi.nlm.nih.gov/compound/5360696. Accessed January 22, 2020.
22. Hedya SA, Swoboda HD. Lithium toxicity. https://www.ncbi.nlm.nih.gov/books/NBK499992/. Updated August 14, 2019. Accessed January 22, 2020.
23. US Department of Health & Human Services. National Center for Complementary and Integrative Health. Herb-drug interactions: what the science says. https://www.nccih.nih.gov/health/providers/digest/herb-drug-interactions-science. Published September 2015. Accessed January 22, 2020.
24. Shader RI, Greenblatt DJ. Bees, ginseng and MAOIs revisited. J Clin Psychopharmacol. 1988;8(4):235.
25. Chua YT. Interaction between warfarin and Chinese herbal medicines. Singapore Med J. 2015;56(1):11-18.
26. Bonetto N, Santelli L, Battistin L, et al. Serotonin syndrome and rhabdomyolysis induced by concomitant use of triptans, fluoxetine and hypericum. Cephalalgia. 2007;27(12):1421-1423.
27. Henderson L, Yue QY, Bergquist C, et al. St John’s wort (Hypericum perforatum): drug interactions and clinical outcomes. Br J Clin Pharmacol. 2002;54(4):349-356.
28. Johne A, Schmider J, Brockmöller J, et al. Decreased plasma levels of amitriptyline and its metabolites on comedication with an extract from St John’s wort (Hypericum perforatum). J Clin Psychopharmacol. 2002;22(1):46-54.
29. Eich-Höchli D, Oppliger R, Golay KP, et al. Methadone maintenance treatment and St John’s wort: a case report. Pharmacopsychiatry. 2003;36(1):35-37.
30. Johne A, Brockmöller J, Bauer S, et al. Pharmacokinetic interaction of digoxin with an herbal extract from St John’s wort (Hypericum perforatum). Clin Pharmacol Ther. 1999;66(4):338-345.
31. Andrén L, Andreasson A, Eggertsen R. Interaction between a commercially available St John’s wort product (Movina) and atorvastatin in patients with hypercholesterolemia. Eur J Clin Pharmacol. 2007;63(10):913-916.
32. Van Strater AC. Interaction of St John’s wort (Hypericum perforatum) with clozapine. Int Clin Psychopharmacol. 2012;27(2):121-124.
33. Nöldner M, Chatterjee SS. Inhibition of haloperidol-induced catalepsy in rats by root extracts from Piper methysticum F. Phytomedicine. 1999;6(4):285-286.
34. Boerner RJ, Klement S. Attenuation of neuroleptic-induced extrapyramidal side effects by kava special extract WS 1490. Wien Med Wochenschr. 2004;154(21-22):508-510.
35. Schelosky L, Raffauf C, Jendroska K, et al. Kava and dopamine antagonism. J Neurol Neurosurg Psychiatry. 1995;58(5):639-640.
36. Singh YN. Potential for interaction of kava and St. John’s wort with drugs. J Ethnopharmacol. 2005;100(1-2):108-113.
37. Patel NM, Derkits R. Possible increase in liver enzymes secondary to atorvastatin and black cohosh administration. J Pharm Prac. 2007;20(4):341-346.
38. Rockwell S, Liu Y, Higgins SA. Alteration of the effects of cancer therapy agents on breast cancer cells by the herbal medicine black cohosh. Breast Cancer Res Treat. 2005;90(3):233-239.
39. Granger AS. Ginkgo biloba precipitating epileptic seizures. Age Ageing. 2001;30(6):523-525.
40. Mohutsky MA, Anderson GD, Miller JW, et al. Ginkgo biloba: evaluation of CYP2C9 drug interactions in vitro and in vivo. Am J Ther. 2006;13(1):24-31.
41. Zhang XY, Zhou DF, Zhang PY, et al. A double-blind, placebo controlled trial of extract of Ginkgo biloba added to haloperidol in treatment-resistant patients with schizophrenia. J Clin Psychiatry. 2001;62(11):878-883.
42. Atmaca M, Tezcan E, Kuloglu M, et al. The effect of extract of ginkgo biloba addition to olanzapine on therapeutic effect and antioxidant enzyme levels in patients with schizophrenia. Psychiatry Clin Neurosci. 2005;59(6):652-656.
43. Doruk A, Uzun O, Ozsahin A. A placebo-controlled study of extract of ginkgo biloba added to clozapine in patients with treatment-resistant schizophrenia. Int Clin Psychopharmacol. 2008;23(4):223-237.
44. Vaes LP. Interactions of warfarin with garlic, ginger, ginkgo, or ginseng: nature of the evidence. Ann Pharmacother. 2000;34(12):1478-1482.
45. Kanji S, Seely D, Yazdi F, et al. Interactions of commonly used dietary supplements with cardiovascular drugs: a systematic review. Syst Rev. 2012;1:26.
46. Wallace JL. Pathogenesis of NSAID-induced gastroduodenal mucosal injury. Best Pract Res Clin Gastroenterol. 2001;15(5):691-703.
47. Triadafilopoulos G, Sharma R. Features of symptomatic gastroesophageal reflux disease in elderly patients. Am J Gastroenterol. 1997;92(11):2007-2011.
48. Haastrup PF, Thompson W, Søndergaard J, et al. Side effects of long-term proton pump inhibitor use: a review. Basic Clin Pharmacol Toxicol. 2018;123(2):114-121.
49. Diamond BJ, Bailey MR. Ginkgo biloba: indications, mechanisms and safety. Psychiatr Clin N Am. 2013;36:73-83.
50. White CM. The pharmacology, pharmacokinetics, efficacy, and adverse events associated with kava. J Clin Pharmacol. 2018;58(11):1396-1405.
51. Gleitz J, Beile A, Peters T. (+/-)-Kavain inhibits veratridine-activated voltage-dependent Na(+)-channels in synaptosomes prepared from rat cerebral cortex. Neuropharmacology. 1995;34(9):1133-1138.
52. Kahrilas PJ. Gastroesophageal reflux disease and its complications. In: Feldman M, ed. Sleisenger & Fordtran’s Gastrointestinal and Liver Disease. 6th ed. Philadelphia, PA: WB Saunders Company; 1998:498-516.
53. Haenisch B, von Holt K, Wiese B, et al. Risk of dementia in elderly patients with the use of proton pump inhibitors. Eur Arch Psychiatry Clin Neurosci. 2015;265(5):419-428.
54. Sheen E, Triadafilopoulos G. Adverse effects of long-term proton pump inhibitor therapy. Dig Dis Sci. 2011;56(4):931-950.
55. Pitkälä KH, Suominen MH, Bell JS, et al. Herbal medications and other dietary supplements. A clinical review for physicians caring for older people. Ann Medicine. 2016;48(8):586-602.
56. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. Arthritis Rheum. 2000;43(9):1905-1915.
57. Vandvik PO, Lincoff AM, Core JM, et al. Primary and secondary prevention of cardiovascular disease: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):e637S-e668S. doi: 10.1378/chest.11-2306.
Therapeutic drug monitoring of antipsychotics
Mr. Q, age 36, has a history of schizophrenia. He is brought to the hospital due to persistent auditory hallucinations and paranoid delusions. His history documents a trial and failure of risperidone, 4 mg twice daily, and aripiprazole, 20 mg/d. Based on this, the treatment team initiates haloperidol, 5 mg twice daily. Because he experiences persistent auditory hallucinations and paranoid delusions, Mr. Q is titrated to increasing doses of haloperidol over 2 weeks during the course of the hospitalization. Once Mr. Q is receiving a total haloperidol dose of 30 mg/d, the team decides to obtain a serum haloperidol level due to his persistent psychotic symptoms and the development of drug-induced parkinsonism. His serum haloperidol level is 24 ng/mL, which is within the expected range for his dose, but above the therapeutic window for efficacy. The team decides that the severity of Mr. Q’s illness and documented treatment resistance (failing at least 2 adequate trials of antipsychotics) warrant a trial of clozapine.
Despite a long history of therapeutic drug monitoring (TDM) within psychiatry, routine monitoring of antipsychotic serum levels has not been unanimously adopted as standard practice. Clinical practice typically results in mostly a subjective assessment of the safety and efficacy of antipsychotics. This practice is in contrast to agents such as valproic acid and lithium, which are routinely monitored for safety and efficacy using both subjective and laboratory measures. Clinicians may adhere to these monitoring practices for lithium and valproic acid because of these agents’ narrow “therapeutic window” between toxicity and efficacy. However, antipsychotics can be viewed in a similar fashion.
To help conceptualize the therapeutic window for antipsychotics, it is important to understand that in most cases, the pharmacologic target for antipsychotics is dopamine (D2)receptor antagonism between 60% to 80%.1 Total drug exposure would thus determine a patient’s likelihood of minimizing positive symptoms, or exposure to adverse effects related to total dopamine antagonism. Serum drug concentrations are a better metric than total daily dose for determining drug exposure and achieving the pharmacologic target.2 Evaluating serum antipsychotic levels also is a better method of determining true treatment failure than relying on the clinical judgment of the treating psychiatrist.3
Pros and cons of TDM
Benefits of using TDM for patients being treated with antipsychotics include4:
- ensuring adherence
- quantitatively adjusting dosages for medication interactions or genetic variations
- ensuring an adequate trial of a medication before considering it a treatment failure.
Potential drawbacks to TDM include:
- Delayed results. Access to expeditious testing may not be possible in certain laboratories, and this may require send-out testing, which could result in a delay in obtaining results. Continued advocacy and research on the value of TDM in antipsychotics may improve access to these resources in the future. Nonetheless, obtaining antipsychotic serum levels will still give clinicians insight into the antipsychotic exposure at a given dose. Further, obtaining antipsychotic serum levels may strengthen decisions about treatment resistance and the assessment of interactions, adherence, or the likelihood of adverse effects.
- Lack of guidance. Unfortunately, there is no established guidance outlining what to do once antipsychotic serum levels are obtained. The correlation of serum levels of commonly used second-generation antipsychotics with clinical efficacy needs to be more closely investigated; however, certain agents do have more data associated with appropriate ranges for efficacy/toxicity. While researchers should continue to study the precise relationship between antipsychotic serum levels and effect, clinicians still have resources available to help determine what the expected serum value for a given patient may be. Knowing an expected serum level may help clinicians determine whether there is an unknown interaction or genetic variation that is causing lower- or higher-than-expected levels. This may also help determine whether a patient is adhering to their medication regimen.
Growing evidence for TDM
In recent years, evidence supporting the use of TDM in patients receiving antipsychotics has been increasing, and recommendations from consensus groups have been strengthened. One of the most comprehensive assessments of these practices was published by the Arbeitsgemeinschaft für Neuropsychopharmakologie und Pharmakopsychiatrie (AGNP), a German-based psychopharmacology group consisting of researchers and clinicians. In 2018, the AGNP published consensus guidelines for TDM in various neuropsychiatric medication classes and recommendations for collecting, interpreting, and decision-making elements of the process.5 The Table5 lists the AGNP-recommended therapeutic serum range for several commonly used second-generation antipsychotics.
Researchers should be encouraged to contribute to the body of knowledge on the correlation of clinical response to serum level. However, there is compelling evidence for the use of TDM across many first- and second-generation antipsychotics. Of the most common, haloperidol and clozapine have evidence of a therapeutic range that is better correlated with serum level than daily dose. Specifically, haloperidol appears to lose benefit with dose increases beyond serum levels of approximately 10 ng/mL.6
Continue to: Clozapine levels may be...
Clozapine levels may be reported by measuring the metabolite norclozapine, which is not correlated with efficacy, or as a total level (combination of clozapine and norclozapine). While norclozapine is not associated with efficacy, the ratio of clozapine to norclozapine may indicate adherence to the medication, or any enzymatic modulation (genetic or drug–drug interaction) that may increase or decrease total exposure. A ratio of 1.5 to 2.0 (clozapine to norclozapine) is optimal; a ratio <0.5 may indicate nonadherence; and a ratio >2.0 may indicate inhibited drug clearance. A 12-hour serum clozapine level of ≥350 ng/mL is more likely to predict treatment response.7
CASE CONTINUED
Mr. Q is carefully tapered from haloperidol while initiating clozapine at 25 mg/d. As he is titrated on clozapine, Mr. Q’s serum levels are periodically checked and compared with expected levels and levels associated with efficacy. Eventually, Mr. Q is titrated to a clozapine dose of 400 mg/d at bedtime.
While receiving clozapine for 4 weeks, Mr. Q’s psychotic symptoms resolve, and he is scheduled for follow-up in the outpatient clozapine clinic.
Related Resources
- De Leon J. A critical commentary on the 2017 AGNP consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology. Pharmacopsychiatry. 2018; 51(1-02):63-68.
- Meyer JM. Is monitoring of plasma antipsychotic levels useful? Current Psychiatry. 2015;14(11):16,19-20.
Drug Brand Names
Aripiprazole • Abilify
Clozapine • Clozaril
Fluphenazine • Prolixin
Haloperidol • Haldol
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Olanzapine • Zyprexa
Perphenazine • Trilafon
Quetiapine • Seroquel
Risperidone • Risperdal
Valproic acid • Depakene, Depakote
Ziprasidone • Geodon
1. Stahl SM. Stahl’s essential psychopharmacology, neuroscientific basis and practical applications, 4th ed. Cambridge, United Kingdom: Cambridge University Press; 2013:129-236.
2. Potkin SG, Keator DB, Kesler-West ML, et al. D2 receptor occupancy following lurasidone treatment in patients with schizophrenia or schizoaffective disorder. CNS Spectr. 2014;19(2):176-181.
3. McCutcheon R, Beck K, D’Ambrosio E, et al. Antipsychotic plasma levels in the assessment of poor treatment response in schizophrenia. Acta Psychiatr Scand. 2018;137(1):39-46.
4. Horvitz-Lennon M, Mattke S, Predmore Z, et al. The role of antipsychotic plasma levels in the treatment of schizophrenia. Am J Psychiatry. 2017;174(5):421-426.
5. Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1-02):9-62.
6. Van Putten T, Marder SR, Mintz J, et al. Haloperidol plasma levels and clinical response: a therapeutic window relationship. Am J Psychiatry. 1992;149(4):500-505.
7. Couchman L, Morgan PE, Spencer EP, et al. Plasma clozapine, norclozapine, and the clozapine:norclozapine ratio in relation to prescribed dose and other factors: data from a therapeutic drug monitoring service, 1993-2007. Ther Drug Monit. 2010;32(4):438-447.
Mr. Q, age 36, has a history of schizophrenia. He is brought to the hospital due to persistent auditory hallucinations and paranoid delusions. His history documents a trial and failure of risperidone, 4 mg twice daily, and aripiprazole, 20 mg/d. Based on this, the treatment team initiates haloperidol, 5 mg twice daily. Because he experiences persistent auditory hallucinations and paranoid delusions, Mr. Q is titrated to increasing doses of haloperidol over 2 weeks during the course of the hospitalization. Once Mr. Q is receiving a total haloperidol dose of 30 mg/d, the team decides to obtain a serum haloperidol level due to his persistent psychotic symptoms and the development of drug-induced parkinsonism. His serum haloperidol level is 24 ng/mL, which is within the expected range for his dose, but above the therapeutic window for efficacy. The team decides that the severity of Mr. Q’s illness and documented treatment resistance (failing at least 2 adequate trials of antipsychotics) warrant a trial of clozapine.
Despite a long history of therapeutic drug monitoring (TDM) within psychiatry, routine monitoring of antipsychotic serum levels has not been unanimously adopted as standard practice. Clinical practice typically results in mostly a subjective assessment of the safety and efficacy of antipsychotics. This practice is in contrast to agents such as valproic acid and lithium, which are routinely monitored for safety and efficacy using both subjective and laboratory measures. Clinicians may adhere to these monitoring practices for lithium and valproic acid because of these agents’ narrow “therapeutic window” between toxicity and efficacy. However, antipsychotics can be viewed in a similar fashion.
To help conceptualize the therapeutic window for antipsychotics, it is important to understand that in most cases, the pharmacologic target for antipsychotics is dopamine (D2)receptor antagonism between 60% to 80%.1 Total drug exposure would thus determine a patient’s likelihood of minimizing positive symptoms, or exposure to adverse effects related to total dopamine antagonism. Serum drug concentrations are a better metric than total daily dose for determining drug exposure and achieving the pharmacologic target.2 Evaluating serum antipsychotic levels also is a better method of determining true treatment failure than relying on the clinical judgment of the treating psychiatrist.3
Pros and cons of TDM
Benefits of using TDM for patients being treated with antipsychotics include4:
- ensuring adherence
- quantitatively adjusting dosages for medication interactions or genetic variations
- ensuring an adequate trial of a medication before considering it a treatment failure.
Potential drawbacks to TDM include:
- Delayed results. Access to expeditious testing may not be possible in certain laboratories, and this may require send-out testing, which could result in a delay in obtaining results. Continued advocacy and research on the value of TDM in antipsychotics may improve access to these resources in the future. Nonetheless, obtaining antipsychotic serum levels will still give clinicians insight into the antipsychotic exposure at a given dose. Further, obtaining antipsychotic serum levels may strengthen decisions about treatment resistance and the assessment of interactions, adherence, or the likelihood of adverse effects.
- Lack of guidance. Unfortunately, there is no established guidance outlining what to do once antipsychotic serum levels are obtained. The correlation of serum levels of commonly used second-generation antipsychotics with clinical efficacy needs to be more closely investigated; however, certain agents do have more data associated with appropriate ranges for efficacy/toxicity. While researchers should continue to study the precise relationship between antipsychotic serum levels and effect, clinicians still have resources available to help determine what the expected serum value for a given patient may be. Knowing an expected serum level may help clinicians determine whether there is an unknown interaction or genetic variation that is causing lower- or higher-than-expected levels. This may also help determine whether a patient is adhering to their medication regimen.
Growing evidence for TDM
In recent years, evidence supporting the use of TDM in patients receiving antipsychotics has been increasing, and recommendations from consensus groups have been strengthened. One of the most comprehensive assessments of these practices was published by the Arbeitsgemeinschaft für Neuropsychopharmakologie und Pharmakopsychiatrie (AGNP), a German-based psychopharmacology group consisting of researchers and clinicians. In 2018, the AGNP published consensus guidelines for TDM in various neuropsychiatric medication classes and recommendations for collecting, interpreting, and decision-making elements of the process.5 The Table5 lists the AGNP-recommended therapeutic serum range for several commonly used second-generation antipsychotics.

Researchers should be encouraged to contribute to the body of knowledge on the correlation of clinical response to serum level. However, there is compelling evidence for the use of TDM across many first- and second-generation antipsychotics. Of the most common, haloperidol and clozapine have evidence of a therapeutic range that is better correlated with serum level than daily dose. Specifically, haloperidol appears to lose benefit with dose increases beyond serum levels of approximately 10 ng/mL.6
Continue to: Clozapine levels may be...
Clozapine levels may be reported by measuring the metabolite norclozapine, which is not correlated with efficacy, or as a total level (combination of clozapine and norclozapine). While norclozapine is not associated with efficacy, the ratio of clozapine to norclozapine may indicate adherence to the medication, or any enzymatic modulation (genetic or drug–drug interaction) that may increase or decrease total exposure. A ratio of 1.5 to 2.0 (clozapine to norclozapine) is optimal; a ratio <0.5 may indicate nonadherence; and a ratio >2.0 may indicate inhibited drug clearance. A 12-hour serum clozapine level of ≥350 ng/mL is more likely to predict treatment response.7
CASE CONTINUED
Mr. Q is carefully tapered from haloperidol while initiating clozapine at 25 mg/d. As he is titrated on clozapine, Mr. Q’s serum levels are periodically checked and compared with expected levels and levels associated with efficacy. Eventually, Mr. Q is titrated to a clozapine dose of 400 mg/d at bedtime.
While receiving clozapine for 4 weeks, Mr. Q’s psychotic symptoms resolve, and he is scheduled for follow-up in the outpatient clozapine clinic.
Related Resources
- De Leon J. A critical commentary on the 2017 AGNP consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology. Pharmacopsychiatry. 2018; 51(1-02):63-68.
- Meyer JM. Is monitoring of plasma antipsychotic levels useful? Current Psychiatry. 2015;14(11):16,19-20.
Drug Brand Names
Aripiprazole • Abilify
Clozapine • Clozaril
Fluphenazine • Prolixin
Haloperidol • Haldol
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Olanzapine • Zyprexa
Perphenazine • Trilafon
Quetiapine • Seroquel
Risperidone • Risperdal
Valproic acid • Depakene, Depakote
Ziprasidone • Geodon
Mr. Q, age 36, has a history of schizophrenia. He is brought to the hospital due to persistent auditory hallucinations and paranoid delusions. His history documents a trial and failure of risperidone, 4 mg twice daily, and aripiprazole, 20 mg/d. Based on this, the treatment team initiates haloperidol, 5 mg twice daily. Because he experiences persistent auditory hallucinations and paranoid delusions, Mr. Q is titrated to increasing doses of haloperidol over 2 weeks during the course of the hospitalization. Once Mr. Q is receiving a total haloperidol dose of 30 mg/d, the team decides to obtain a serum haloperidol level due to his persistent psychotic symptoms and the development of drug-induced parkinsonism. His serum haloperidol level is 24 ng/mL, which is within the expected range for his dose, but above the therapeutic window for efficacy. The team decides that the severity of Mr. Q’s illness and documented treatment resistance (failing at least 2 adequate trials of antipsychotics) warrant a trial of clozapine.
Despite a long history of therapeutic drug monitoring (TDM) within psychiatry, routine monitoring of antipsychotic serum levels has not been unanimously adopted as standard practice. Clinical practice typically results in mostly a subjective assessment of the safety and efficacy of antipsychotics. This practice is in contrast to agents such as valproic acid and lithium, which are routinely monitored for safety and efficacy using both subjective and laboratory measures. Clinicians may adhere to these monitoring practices for lithium and valproic acid because of these agents’ narrow “therapeutic window” between toxicity and efficacy. However, antipsychotics can be viewed in a similar fashion.
To help conceptualize the therapeutic window for antipsychotics, it is important to understand that in most cases, the pharmacologic target for antipsychotics is dopamine (D2)receptor antagonism between 60% to 80%.1 Total drug exposure would thus determine a patient’s likelihood of minimizing positive symptoms, or exposure to adverse effects related to total dopamine antagonism. Serum drug concentrations are a better metric than total daily dose for determining drug exposure and achieving the pharmacologic target.2 Evaluating serum antipsychotic levels also is a better method of determining true treatment failure than relying on the clinical judgment of the treating psychiatrist.3
Pros and cons of TDM
Benefits of using TDM for patients being treated with antipsychotics include4:
- ensuring adherence
- quantitatively adjusting dosages for medication interactions or genetic variations
- ensuring an adequate trial of a medication before considering it a treatment failure.
Potential drawbacks to TDM include:
- Delayed results. Access to expeditious testing may not be possible in certain laboratories, and this may require send-out testing, which could result in a delay in obtaining results. Continued advocacy and research on the value of TDM in antipsychotics may improve access to these resources in the future. Nonetheless, obtaining antipsychotic serum levels will still give clinicians insight into the antipsychotic exposure at a given dose. Further, obtaining antipsychotic serum levels may strengthen decisions about treatment resistance and the assessment of interactions, adherence, or the likelihood of adverse effects.
- Lack of guidance. Unfortunately, there is no established guidance outlining what to do once antipsychotic serum levels are obtained. The correlation of serum levels of commonly used second-generation antipsychotics with clinical efficacy needs to be more closely investigated; however, certain agents do have more data associated with appropriate ranges for efficacy/toxicity. While researchers should continue to study the precise relationship between antipsychotic serum levels and effect, clinicians still have resources available to help determine what the expected serum value for a given patient may be. Knowing an expected serum level may help clinicians determine whether there is an unknown interaction or genetic variation that is causing lower- or higher-than-expected levels. This may also help determine whether a patient is adhering to their medication regimen.
Growing evidence for TDM
In recent years, evidence supporting the use of TDM in patients receiving antipsychotics has been increasing, and recommendations from consensus groups have been strengthened. One of the most comprehensive assessments of these practices was published by the Arbeitsgemeinschaft für Neuropsychopharmakologie und Pharmakopsychiatrie (AGNP), a German-based psychopharmacology group consisting of researchers and clinicians. In 2018, the AGNP published consensus guidelines for TDM in various neuropsychiatric medication classes and recommendations for collecting, interpreting, and decision-making elements of the process.5 The Table5 lists the AGNP-recommended therapeutic serum range for several commonly used second-generation antipsychotics.

Researchers should be encouraged to contribute to the body of knowledge on the correlation of clinical response to serum level. However, there is compelling evidence for the use of TDM across many first- and second-generation antipsychotics. Of the most common, haloperidol and clozapine have evidence of a therapeutic range that is better correlated with serum level than daily dose. Specifically, haloperidol appears to lose benefit with dose increases beyond serum levels of approximately 10 ng/mL.6
Continue to: Clozapine levels may be...
Clozapine levels may be reported by measuring the metabolite norclozapine, which is not correlated with efficacy, or as a total level (combination of clozapine and norclozapine). While norclozapine is not associated with efficacy, the ratio of clozapine to norclozapine may indicate adherence to the medication, or any enzymatic modulation (genetic or drug–drug interaction) that may increase or decrease total exposure. A ratio of 1.5 to 2.0 (clozapine to norclozapine) is optimal; a ratio <0.5 may indicate nonadherence; and a ratio >2.0 may indicate inhibited drug clearance. A 12-hour serum clozapine level of ≥350 ng/mL is more likely to predict treatment response.7
CASE CONTINUED
Mr. Q is carefully tapered from haloperidol while initiating clozapine at 25 mg/d. As he is titrated on clozapine, Mr. Q’s serum levels are periodically checked and compared with expected levels and levels associated with efficacy. Eventually, Mr. Q is titrated to a clozapine dose of 400 mg/d at bedtime.
While receiving clozapine for 4 weeks, Mr. Q’s psychotic symptoms resolve, and he is scheduled for follow-up in the outpatient clozapine clinic.
Related Resources
- De Leon J. A critical commentary on the 2017 AGNP consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology. Pharmacopsychiatry. 2018; 51(1-02):63-68.
- Meyer JM. Is monitoring of plasma antipsychotic levels useful? Current Psychiatry. 2015;14(11):16,19-20.
Drug Brand Names
Aripiprazole • Abilify
Clozapine • Clozaril
Fluphenazine • Prolixin
Haloperidol • Haldol
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Olanzapine • Zyprexa
Perphenazine • Trilafon
Quetiapine • Seroquel
Risperidone • Risperdal
Valproic acid • Depakene, Depakote
Ziprasidone • Geodon
1. Stahl SM. Stahl’s essential psychopharmacology, neuroscientific basis and practical applications, 4th ed. Cambridge, United Kingdom: Cambridge University Press; 2013:129-236.
2. Potkin SG, Keator DB, Kesler-West ML, et al. D2 receptor occupancy following lurasidone treatment in patients with schizophrenia or schizoaffective disorder. CNS Spectr. 2014;19(2):176-181.
3. McCutcheon R, Beck K, D’Ambrosio E, et al. Antipsychotic plasma levels in the assessment of poor treatment response in schizophrenia. Acta Psychiatr Scand. 2018;137(1):39-46.
4. Horvitz-Lennon M, Mattke S, Predmore Z, et al. The role of antipsychotic plasma levels in the treatment of schizophrenia. Am J Psychiatry. 2017;174(5):421-426.
5. Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1-02):9-62.
6. Van Putten T, Marder SR, Mintz J, et al. Haloperidol plasma levels and clinical response: a therapeutic window relationship. Am J Psychiatry. 1992;149(4):500-505.
7. Couchman L, Morgan PE, Spencer EP, et al. Plasma clozapine, norclozapine, and the clozapine:norclozapine ratio in relation to prescribed dose and other factors: data from a therapeutic drug monitoring service, 1993-2007. Ther Drug Monit. 2010;32(4):438-447.
1. Stahl SM. Stahl’s essential psychopharmacology, neuroscientific basis and practical applications, 4th ed. Cambridge, United Kingdom: Cambridge University Press; 2013:129-236.
2. Potkin SG, Keator DB, Kesler-West ML, et al. D2 receptor occupancy following lurasidone treatment in patients with schizophrenia or schizoaffective disorder. CNS Spectr. 2014;19(2):176-181.
3. McCutcheon R, Beck K, D’Ambrosio E, et al. Antipsychotic plasma levels in the assessment of poor treatment response in schizophrenia. Acta Psychiatr Scand. 2018;137(1):39-46.
4. Horvitz-Lennon M, Mattke S, Predmore Z, et al. The role of antipsychotic plasma levels in the treatment of schizophrenia. Am J Psychiatry. 2017;174(5):421-426.
5. Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1-02):9-62.
6. Van Putten T, Marder SR, Mintz J, et al. Haloperidol plasma levels and clinical response: a therapeutic window relationship. Am J Psychiatry. 1992;149(4):500-505.
7. Couchman L, Morgan PE, Spencer EP, et al. Plasma clozapine, norclozapine, and the clozapine:norclozapine ratio in relation to prescribed dose and other factors: data from a therapeutic drug monitoring service, 1993-2007. Ther Drug Monit. 2010;32(4):438-447.