User login
Vesicovaginal and rectovaginal fistulas from obstetric-related causes: Diagnosis and management
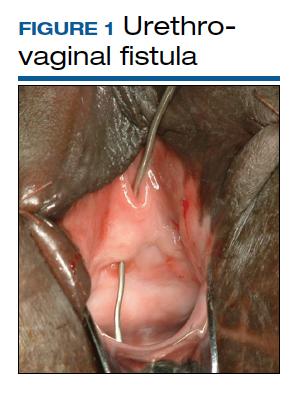
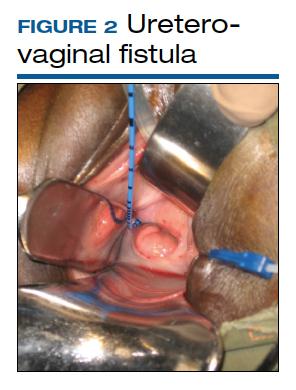
Although rare in the United States and more common in low-resource countries, fistulas due to obstructed labor do occur. In developed countries, other obstetric causes for fistula are usually surgery, trauma, or infection related. An abnormal communication between organs—be it the urethra, bladder, ureter, uterus, cervix, or rectum—can develop1 and lead to vesicovaginal fistula (VVF), urethrovaginal fistula (FIGURE 1), vesicocervical fistula, vesicouterine fistula, ureterovaginal fistula (FIGURE 2), and rectovaginal fistula (RVF). Other nonobstetric causes include gynecologic surgery, radiation, malignancy, and congenital malformations.
During labor, hypoxia, subsequent ischemia, and pressure necrosis contribute to fistula formation. Injury sustained during a cesarean delivery (CD) or cesarean hysterectomy can lead to fistula formation; at times, however, complications are unavoidable given the nature of the pathologic condition that the patient presents with.
VVF and RVF have a devastating impact on a woman’s quality of life as they lead to significant morbidity and short- and long-term psychological distress. The fistula may not be recognized at the time of injury. The presenting signs and symptoms may be intermittent and confusing. Immediate surgical intervention may not be possible due to ongoing inflammation or infection. Recovery often is prolonged. As there is significant concomitant postpartum anxiety and depression, patients with fistula often require psychosocial support and counseling. After repair, there is still a risk for recurrence and voiding dysfunction.
Fistula signs and symptoms and evaluation
In cases of VVF, patients present with continuing large or small volume urinary incontinence. Depending on the time to diagnosis, patients may have calculi formation, prolapse, scarring, external perineal dermatitis, perineal nerve injury, and even motor weakness. Cyclic hematuria may be seen in vesicouterine fistulas.2
Multiple classification systems for diagnosis and staging of VVF have been suggested.3,4 A classification system for RVF was published by Tsang and colleagues.5 All these classification systems have attempted to characterize fistulas in terms of level of surgical complexity for repair, providing a guideline for preoperative assessment. These classification systems do not translate into prediction regarding outcomes.
Evaluation of pelvic fistula from the urinary tract starts with a thorough history that includes onset, duration, and description of leakage (continuous, intermittent, or positional) and whether there is concomitant stress and urge incontinence. A detailed obstetric history, including circumstances around the mode of delivery, underlying risk factors, and psychosocial history, should be obtained.
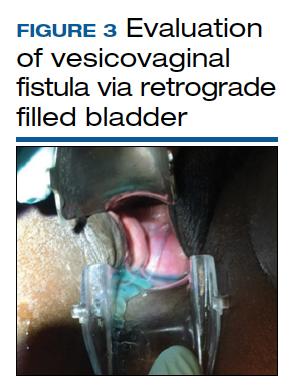
The pelvic examination with a plastic speculum and adequate lighting should assess the external perineum for dermatitis; bulbocavernosus and anal reflexes; and the vagina for length, caliber, level of scarring, and any prolapse. For VVFs, the location, size, and number of the fistula tracts can be visualized and confirmed with a retrograde fill of the bladder via a Foley catheter with saline or water mixed with methylene blue or any other blue dye (FIGURE 3). If a ureterovaginal fistula is suspected, the patient can simultaneously be given oral phenazopyridine and a tampon inserted within the vagina; the patient can then ambulate, and re-examination of the end of the tampon can reveal orange staining. The bladder meanwhile is retrograde filled with blue dye, with no blue staining of the tampon.
For RVF, history taking should include the onset, duration, and description of leakage, and the external anal sphincter should be assessed, with careful examination of the distal vagina at the vestibule as this is the most common location for RVF (fistula in ano). Patients may describe vaginal flatus and sometimes only brownish discharge, which can be intermittent, leading to an incorrect diagnosis of vaginitis that is treated repeatedly without success.
There is no consensus regarding optimal imaging for the assessment of VVF. Imaging used for diagnosis of VVF includes a voiding cystogram with opacification of the vagina after filling the bladder with contrast if there is a fistula. A cystoscopy can evaluate for calculi, retained suture, level of inflammation, and location of the ureters in relation to the fistula. Renal ultrasonography is of limited use. Intravenous pyelography can miss lesions by the trigone. In general, a computed tomography (CT) urogram and magnetic resonance imaging (MRI) with bladder contrast are more sensitive.
In the diagnosis of RVF, contrast vaginoscopy, double contrast barium enema, CT scan with contrast, and MRI can be used. MRI is more sensitive.6 A high index of suspicion is required based on the patient’s history as these imaging modalities do not always confirm RVF despite patient’s clear history of leakage. When the history is convincing, a thorough rectovaginal exam under anesthesia may be imperative. If rectal trauma is present, endoanal ultrasonography can delineate external and internal anal sphincter defects.
Prolonged Foley catheter placement after obstetric injury can lead to successful closure of a VVF. Prior to surgical intervention, assessing if there is possible ureteral involvement and use of intraoperative ureteral stents is a consideration. The route of surgery can be vaginal, abdominal, combined abdominal-vaginal, laparoscopic, or robotic.7 The robotic approach is increasingly utilized.8,9 However, the general consensus among fistula surgeons is that the vaginal approach should be considered first.
Continue to: Surgical repair...
Surgical repair
VVF repair. Factors that influence successful repair of VVF include the size and number of fistula, location, degree of scarring, bladder capacity, and urethral length.
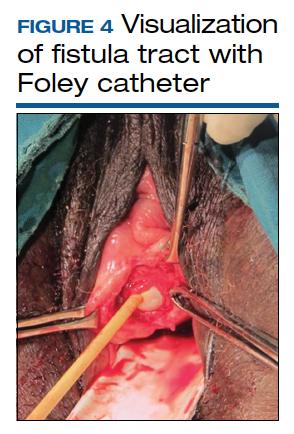
Surgical technique requires wide mobilization and adequate exposure. The fistula tract can be delineated and manipulated with a pediatric Foley catheter, ureteral stent, or even a ureteral guidewire to aid in dissection (FIGURE 4). Intraoperative visualization of the ureters, including stenting, often is needed. The fistulous track is excised depending on the level of scarring. Closure of the bladder uroepithelium for the first layer is with absorbable interrupted 3-0 or 2-0 sutures in a tension-free closure. The bladder is then evaluated with a retrograde fill with saline and methylene blue to ensure a watertight closure for the first layer. If the first layer is not watertight, the second layer closure will not compensate and the fistula will persist. Particular attention is paid to the angles of the fistula at the first layer closure to prevent recurrence of the fistula at the angles. A running second layer with absorbable 2-0 suture is done. At times, a Martius flap or an omental J flap can be used to provide an additional layer for support and to increase vascularity.10 The patient is sent home with a Foley catheter for drainage for 10 to 14 days.11 Antibiotics are not needed postoperatively for VVF surgery.12
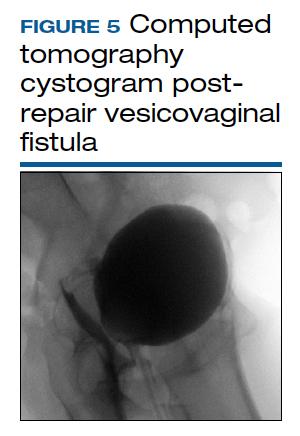
CT cystogram or retrograde cystogram is usually done to evaluate closure of the fistula prior to removal of the Foley catheter; retrograde fill with contrast directly into the bladder with 300 mL is sufficient (FIGURE 5). Patients are advised to refrain from sexual activity for a minimum of 6 weeks, but depending on the level of complexity and scarring, this can be up to 12 weeks.
The success rate in general is in the 95% range. Patients with successful closure of VVF are at risk for urge incontinence due to decreased bladder capacity, stress incontinence especially if the continence mechanism or urethra is involved, vaginal scarring, dyspareunia, and infertility.13 In general, sexual function improves after surgical repair.
RVF repair. Prior to surgical repair of RVF, the integrity of the external anal sphincter must be determined. If it is not involved, a vertical incision is made in the posterior vaginal wall, the vaginal epithelium is separated from the vaginal muscularis, and the fistula tract is identified. After complete wide mobilization of the tissue surrounding the tract, it is excised. The rectal wall is repaired with 3-0 or 4-0 absorbable interrupted sutures; a second layer and if possible even a third layer and finally the vaginal epithelium are all closed with 2-0 absorbable interrupted sutures.
If the sphincter complex is involved, the dissection involves an inverted U incision separating the vaginal wall from the rectum. The fistula tract is excised, the rectal wall is closed, and the internal anal sphincter is identified and reapproximated with interrupted absorbable 2-0 or 0 sutures. The disrupted external sphincter is then reapproximated with 2-0 or 0 sutures, and finally the transverse perineal and bulbocavernosus muscles are brought together with Lembert 0 sutures prior to closure of the external skin. Perioperative antibiotics have been shown to improve success rates in the correction of RVF.5 In patients with sphincter trauma and known RVF, outcomes with a sphincteroplasty are better, compared with endorectal advancement flaps. The patient is discharged with a bowel regimen and dietary precautions that aim for daily soft bowel movements.
After surgical treatment of fistulas, patients benefit from pelvic floor physical therapy that focuses on pelvic floor strengthening. Incorporating the habit of Kegel exercises after every void, timed (scheduled) bladder voiding, and avoidance of straining with urination or defecation should be emphasized.
Continue to: CASE 1 Pregnant woman with rectal bleeding...
CASE 1 Pregnant woman with rectal bleeding
A 37-year-old woman at 36 3/7 weeks’ gestation presented with acute rectal bleeding and pain. This was found to result from a catastrophic rupture of a pelvic arteriovenous malformation that caused an 11 x 7 x 9.5 cm size inferior pelvic hematoma and a full-thickness rectal tear at the dentate line. During examination under anesthesia, the baby was delivered by a stat CD due to breech presentation and a prolonged fetal heart rate deceleration. The patient underwent embolization of the right middle rectal artery and right internal iliac artery by a radiologic intervention. Further bleeding required surgical intervention for evacuation of about 1,000 mL of hematoma, repair of the rectal tear, and laparoscopic diverting loop ileostomy. In total, the patient received 8 U of packed red blood cells, 6 U of fresh frozen plasma, 5 L of crystalloid solution, and 2 g of tranexamic acid. The patient reported increased foul-smelling vaginal discharge, bedside exam suggested possible fistulous tract, and on postoperative day 16, an exam under anesthesia by Urogynecology confirmed a rectovaginal fistula in the right mid vagina. After 2 months of observation to allow resolution of inflammation, successful excision of the fistula tract and repair of RVF using the above-mentioned technique was accomplished.
CASE 2 Patient with VVF after cesarean hysterectomy
A 40-year-old (G6P2222) patient underwent cesarean hysterectomy for placenta percreta and uterine rupture at 24 weeks’ gestation. Intraoperatively, there were right ureteral ligation and posterior bladder wall cystotomies. The right ureter was reimplanted in the right upper posterior wall and the cystostomies were closed. As the patient had continuous urinary leakage postoperatively, a CT urogram was obtained, which showed left ureteral obstruction and VVF. Urinary incontinence persisted despite bilateral robotic ureteral reimplantation with omental flap by the urology team. Percutaneous nephrostomy tubes were placed bilaterally. The patient underwent additional imaging studies, including MRI, with findings of VVF and possible ureterovaginal fistula.
On referral to Urogynecology, the patient underwent cystoscopy with antegrade pyelogram, and the bilateral ureteroneocystostomy orifices had 5 French open-ended ureteral stents placed. A 10 French pediatric Foley catheter was inserted intravaginally into the bladder through the VVF. Via the vaginal approach, cervical remnant and skin bridges overlying the VVF were excised. The scarred fistula tract was excised with a circumferential incision. Horizontal interrupted Lembert sutures with 3-0 absorbable suture were used to reapproximate the first layer, which was confirmed to be watertight on testing with retrograde fill. Second-layer closure was completed with horizontal mattress 2-0 absorbable sutures, followed by a third-layer closure done in similar fashion. Fibrin glue was then placed. The vaginal epithelium was closed with 2-0 absorbable suture. Percutaneous nephrostomy tubes were removed. Postoperatively, the patient had a CT cystogram with no leak and no incontinence, but she developed urgency, which was controlled with timed voids and oxybutynin.
- Adler AJ, Ronsmans C, Calvert C, et al. Estimating the presence of obstetric fistula: a systematic review and meta-analysis BMC Pregnancy Childbirth. 2013;13:246.
- Battacharjee S, Kohli UA, Sood A, et al. Vesicouterine fistula: Youssef’s syndrome. Med J Armed Forces India. 2015;71(suppl 1):S175-S177. doi: 10.1016/j.mjafi.2013.11.006.
- Waaldijk K. Step-by-Step Surgery of Vesicovaginal Fistulas. Campion Press; 1994.
- Goh, JTW. A new classification for female genital tract fistula. Aust N Z J Ob Gynecol. 2004:44:502-504.
- Tsang CB, Rothenberger DA. Rectovaginal fistulas: therapeutic options. Surg Clin North Am. 1997;77:95-114.
- Champagne BJ, McGee MF. Rectovaginal fistula. Surg Clin North Am. 2010;90:69-82.
- Bodner-Adler B, Hanzal E, Pablik E, et al. Management of vesicovaginal fistulas in women following benign gynecologic surgery: a systematic review and meta-analysis. PLoS One. 2017;12:e0171554.
- Randazzo M, Lengauer L, Rochat CH, et al. Best practices in robotic-assisted repair of vesicovaginal fistula: a consensus report from the European Association of Urology Robotic Urology Section Scientific Working Group for Reconstructive Urology. Eur Urol. 2020;78: 432-442.
- Miklos JR, Moore RD, Chinthakanan O. Laparoscopic and robotic assisted vesicovaginal fistula repair: a systematic review of the literature. J Minim Invasive Gynecol. 2015:22:727-736.
- Hancock B. Practical Obstetric Fistula Surgery. Royal Society of Medicine Press; 2009.
- Nardos R, Menber B, Browning A. Outcome of obstetric fistula repair after 10-day versus 14-day Foley catheterization. Int J Gynaecol 0bstet. 2012;118:21-23.
- Tomlinson AJ, Thornton JG. A randomized controlled trial of antibiotic prophylaxis for vesico-vaginal fistula repair. Br J Obstet Gynaecol. 2005;105:397-399.
- Bengtson AM, Kopp D, Tang JH, et al. Identifying patients with vesicovaginal fistula at high risk of urinary incontinence after surgery. Obstet Gynecol. 2016;128:945-953.
Although rare in the United States and more common in low-resource countries, fistulas due to obstructed labor do occur. In developed countries, other obstetric causes for fistula are usually surgery, trauma, or infection related. An abnormal communication between organs—be it the urethra, bladder, ureter, uterus, cervix, or rectum—can develop1 and lead to vesicovaginal fistula (VVF), urethrovaginal fistula (FIGURE 1), vesicocervical fistula, vesicouterine fistula, ureterovaginal fistula (FIGURE 2), and rectovaginal fistula (RVF). Other nonobstetric causes include gynecologic surgery, radiation, malignancy, and congenital malformations.
During labor, hypoxia, subsequent ischemia, and pressure necrosis contribute to fistula formation. Injury sustained during a cesarean delivery (CD) or cesarean hysterectomy can lead to fistula formation; at times, however, complications are unavoidable given the nature of the pathologic condition that the patient presents with.
VVF and RVF have a devastating impact on a woman’s quality of life as they lead to significant morbidity and short- and long-term psychological distress. The fistula may not be recognized at the time of injury. The presenting signs and symptoms may be intermittent and confusing. Immediate surgical intervention may not be possible due to ongoing inflammation or infection. Recovery often is prolonged. As there is significant concomitant postpartum anxiety and depression, patients with fistula often require psychosocial support and counseling. After repair, there is still a risk for recurrence and voiding dysfunction.
Fistula signs and symptoms and evaluation
In cases of VVF, patients present with continuing large or small volume urinary incontinence. Depending on the time to diagnosis, patients may have calculi formation, prolapse, scarring, external perineal dermatitis, perineal nerve injury, and even motor weakness. Cyclic hematuria may be seen in vesicouterine fistulas.2
Multiple classification systems for diagnosis and staging of VVF have been suggested.3,4 A classification system for RVF was published by Tsang and colleagues.5 All these classification systems have attempted to characterize fistulas in terms of level of surgical complexity for repair, providing a guideline for preoperative assessment. These classification systems do not translate into prediction regarding outcomes.
Evaluation of pelvic fistula from the urinary tract starts with a thorough history that includes onset, duration, and description of leakage (continuous, intermittent, or positional) and whether there is concomitant stress and urge incontinence. A detailed obstetric history, including circumstances around the mode of delivery, underlying risk factors, and psychosocial history, should be obtained.
The pelvic examination with a plastic speculum and adequate lighting should assess the external perineum for dermatitis; bulbocavernosus and anal reflexes; and the vagina for length, caliber, level of scarring, and any prolapse. For VVFs, the location, size, and number of the fistula tracts can be visualized and confirmed with a retrograde fill of the bladder via a Foley catheter with saline or water mixed with methylene blue or any other blue dye (FIGURE 3). If a ureterovaginal fistula is suspected, the patient can simultaneously be given oral phenazopyridine and a tampon inserted within the vagina; the patient can then ambulate, and re-examination of the end of the tampon can reveal orange staining. The bladder meanwhile is retrograde filled with blue dye, with no blue staining of the tampon.
For RVF, history taking should include the onset, duration, and description of leakage, and the external anal sphincter should be assessed, with careful examination of the distal vagina at the vestibule as this is the most common location for RVF (fistula in ano). Patients may describe vaginal flatus and sometimes only brownish discharge, which can be intermittent, leading to an incorrect diagnosis of vaginitis that is treated repeatedly without success.
There is no consensus regarding optimal imaging for the assessment of VVF. Imaging used for diagnosis of VVF includes a voiding cystogram with opacification of the vagina after filling the bladder with contrast if there is a fistula. A cystoscopy can evaluate for calculi, retained suture, level of inflammation, and location of the ureters in relation to the fistula. Renal ultrasonography is of limited use. Intravenous pyelography can miss lesions by the trigone. In general, a computed tomography (CT) urogram and magnetic resonance imaging (MRI) with bladder contrast are more sensitive.
In the diagnosis of RVF, contrast vaginoscopy, double contrast barium enema, CT scan with contrast, and MRI can be used. MRI is more sensitive.6 A high index of suspicion is required based on the patient’s history as these imaging modalities do not always confirm RVF despite patient’s clear history of leakage. When the history is convincing, a thorough rectovaginal exam under anesthesia may be imperative. If rectal trauma is present, endoanal ultrasonography can delineate external and internal anal sphincter defects.
Prolonged Foley catheter placement after obstetric injury can lead to successful closure of a VVF. Prior to surgical intervention, assessing if there is possible ureteral involvement and use of intraoperative ureteral stents is a consideration. The route of surgery can be vaginal, abdominal, combined abdominal-vaginal, laparoscopic, or robotic.7 The robotic approach is increasingly utilized.8,9 However, the general consensus among fistula surgeons is that the vaginal approach should be considered first.
Continue to: Surgical repair...
Surgical repair
VVF repair. Factors that influence successful repair of VVF include the size and number of fistula, location, degree of scarring, bladder capacity, and urethral length.
Surgical technique requires wide mobilization and adequate exposure. The fistula tract can be delineated and manipulated with a pediatric Foley catheter, ureteral stent, or even a ureteral guidewire to aid in dissection (FIGURE 4). Intraoperative visualization of the ureters, including stenting, often is needed. The fistulous track is excised depending on the level of scarring. Closure of the bladder uroepithelium for the first layer is with absorbable interrupted 3-0 or 2-0 sutures in a tension-free closure. The bladder is then evaluated with a retrograde fill with saline and methylene blue to ensure a watertight closure for the first layer. If the first layer is not watertight, the second layer closure will not compensate and the fistula will persist. Particular attention is paid to the angles of the fistula at the first layer closure to prevent recurrence of the fistula at the angles. A running second layer with absorbable 2-0 suture is done. At times, a Martius flap or an omental J flap can be used to provide an additional layer for support and to increase vascularity.10 The patient is sent home with a Foley catheter for drainage for 10 to 14 days.11 Antibiotics are not needed postoperatively for VVF surgery.12
CT cystogram or retrograde cystogram is usually done to evaluate closure of the fistula prior to removal of the Foley catheter; retrograde fill with contrast directly into the bladder with 300 mL is sufficient (FIGURE 5). Patients are advised to refrain from sexual activity for a minimum of 6 weeks, but depending on the level of complexity and scarring, this can be up to 12 weeks.
The success rate in general is in the 95% range. Patients with successful closure of VVF are at risk for urge incontinence due to decreased bladder capacity, stress incontinence especially if the continence mechanism or urethra is involved, vaginal scarring, dyspareunia, and infertility.13 In general, sexual function improves after surgical repair.
RVF repair. Prior to surgical repair of RVF, the integrity of the external anal sphincter must be determined. If it is not involved, a vertical incision is made in the posterior vaginal wall, the vaginal epithelium is separated from the vaginal muscularis, and the fistula tract is identified. After complete wide mobilization of the tissue surrounding the tract, it is excised. The rectal wall is repaired with 3-0 or 4-0 absorbable interrupted sutures; a second layer and if possible even a third layer and finally the vaginal epithelium are all closed with 2-0 absorbable interrupted sutures.
If the sphincter complex is involved, the dissection involves an inverted U incision separating the vaginal wall from the rectum. The fistula tract is excised, the rectal wall is closed, and the internal anal sphincter is identified and reapproximated with interrupted absorbable 2-0 or 0 sutures. The disrupted external sphincter is then reapproximated with 2-0 or 0 sutures, and finally the transverse perineal and bulbocavernosus muscles are brought together with Lembert 0 sutures prior to closure of the external skin. Perioperative antibiotics have been shown to improve success rates in the correction of RVF.5 In patients with sphincter trauma and known RVF, outcomes with a sphincteroplasty are better, compared with endorectal advancement flaps. The patient is discharged with a bowel regimen and dietary precautions that aim for daily soft bowel movements.
After surgical treatment of fistulas, patients benefit from pelvic floor physical therapy that focuses on pelvic floor strengthening. Incorporating the habit of Kegel exercises after every void, timed (scheduled) bladder voiding, and avoidance of straining with urination or defecation should be emphasized.
Continue to: CASE 1 Pregnant woman with rectal bleeding...
CASE 1 Pregnant woman with rectal bleeding
A 37-year-old woman at 36 3/7 weeks’ gestation presented with acute rectal bleeding and pain. This was found to result from a catastrophic rupture of a pelvic arteriovenous malformation that caused an 11 x 7 x 9.5 cm size inferior pelvic hematoma and a full-thickness rectal tear at the dentate line. During examination under anesthesia, the baby was delivered by a stat CD due to breech presentation and a prolonged fetal heart rate deceleration. The patient underwent embolization of the right middle rectal artery and right internal iliac artery by a radiologic intervention. Further bleeding required surgical intervention for evacuation of about 1,000 mL of hematoma, repair of the rectal tear, and laparoscopic diverting loop ileostomy. In total, the patient received 8 U of packed red blood cells, 6 U of fresh frozen plasma, 5 L of crystalloid solution, and 2 g of tranexamic acid. The patient reported increased foul-smelling vaginal discharge, bedside exam suggested possible fistulous tract, and on postoperative day 16, an exam under anesthesia by Urogynecology confirmed a rectovaginal fistula in the right mid vagina. After 2 months of observation to allow resolution of inflammation, successful excision of the fistula tract and repair of RVF using the above-mentioned technique was accomplished.
CASE 2 Patient with VVF after cesarean hysterectomy
A 40-year-old (G6P2222) patient underwent cesarean hysterectomy for placenta percreta and uterine rupture at 24 weeks’ gestation. Intraoperatively, there were right ureteral ligation and posterior bladder wall cystotomies. The right ureter was reimplanted in the right upper posterior wall and the cystostomies were closed. As the patient had continuous urinary leakage postoperatively, a CT urogram was obtained, which showed left ureteral obstruction and VVF. Urinary incontinence persisted despite bilateral robotic ureteral reimplantation with omental flap by the urology team. Percutaneous nephrostomy tubes were placed bilaterally. The patient underwent additional imaging studies, including MRI, with findings of VVF and possible ureterovaginal fistula.
On referral to Urogynecology, the patient underwent cystoscopy with antegrade pyelogram, and the bilateral ureteroneocystostomy orifices had 5 French open-ended ureteral stents placed. A 10 French pediatric Foley catheter was inserted intravaginally into the bladder through the VVF. Via the vaginal approach, cervical remnant and skin bridges overlying the VVF were excised. The scarred fistula tract was excised with a circumferential incision. Horizontal interrupted Lembert sutures with 3-0 absorbable suture were used to reapproximate the first layer, which was confirmed to be watertight on testing with retrograde fill. Second-layer closure was completed with horizontal mattress 2-0 absorbable sutures, followed by a third-layer closure done in similar fashion. Fibrin glue was then placed. The vaginal epithelium was closed with 2-0 absorbable suture. Percutaneous nephrostomy tubes were removed. Postoperatively, the patient had a CT cystogram with no leak and no incontinence, but she developed urgency, which was controlled with timed voids and oxybutynin.
Although rare in the United States and more common in low-resource countries, fistulas due to obstructed labor do occur. In developed countries, other obstetric causes for fistula are usually surgery, trauma, or infection related. An abnormal communication between organs—be it the urethra, bladder, ureter, uterus, cervix, or rectum—can develop1 and lead to vesicovaginal fistula (VVF), urethrovaginal fistula (FIGURE 1), vesicocervical fistula, vesicouterine fistula, ureterovaginal fistula (FIGURE 2), and rectovaginal fistula (RVF). Other nonobstetric causes include gynecologic surgery, radiation, malignancy, and congenital malformations.
During labor, hypoxia, subsequent ischemia, and pressure necrosis contribute to fistula formation. Injury sustained during a cesarean delivery (CD) or cesarean hysterectomy can lead to fistula formation; at times, however, complications are unavoidable given the nature of the pathologic condition that the patient presents with.
VVF and RVF have a devastating impact on a woman’s quality of life as they lead to significant morbidity and short- and long-term psychological distress. The fistula may not be recognized at the time of injury. The presenting signs and symptoms may be intermittent and confusing. Immediate surgical intervention may not be possible due to ongoing inflammation or infection. Recovery often is prolonged. As there is significant concomitant postpartum anxiety and depression, patients with fistula often require psychosocial support and counseling. After repair, there is still a risk for recurrence and voiding dysfunction.
Fistula signs and symptoms and evaluation
In cases of VVF, patients present with continuing large or small volume urinary incontinence. Depending on the time to diagnosis, patients may have calculi formation, prolapse, scarring, external perineal dermatitis, perineal nerve injury, and even motor weakness. Cyclic hematuria may be seen in vesicouterine fistulas.2
Multiple classification systems for diagnosis and staging of VVF have been suggested.3,4 A classification system for RVF was published by Tsang and colleagues.5 All these classification systems have attempted to characterize fistulas in terms of level of surgical complexity for repair, providing a guideline for preoperative assessment. These classification systems do not translate into prediction regarding outcomes.
Evaluation of pelvic fistula from the urinary tract starts with a thorough history that includes onset, duration, and description of leakage (continuous, intermittent, or positional) and whether there is concomitant stress and urge incontinence. A detailed obstetric history, including circumstances around the mode of delivery, underlying risk factors, and psychosocial history, should be obtained.
The pelvic examination with a plastic speculum and adequate lighting should assess the external perineum for dermatitis; bulbocavernosus and anal reflexes; and the vagina for length, caliber, level of scarring, and any prolapse. For VVFs, the location, size, and number of the fistula tracts can be visualized and confirmed with a retrograde fill of the bladder via a Foley catheter with saline or water mixed with methylene blue or any other blue dye (FIGURE 3). If a ureterovaginal fistula is suspected, the patient can simultaneously be given oral phenazopyridine and a tampon inserted within the vagina; the patient can then ambulate, and re-examination of the end of the tampon can reveal orange staining. The bladder meanwhile is retrograde filled with blue dye, with no blue staining of the tampon.
For RVF, history taking should include the onset, duration, and description of leakage, and the external anal sphincter should be assessed, with careful examination of the distal vagina at the vestibule as this is the most common location for RVF (fistula in ano). Patients may describe vaginal flatus and sometimes only brownish discharge, which can be intermittent, leading to an incorrect diagnosis of vaginitis that is treated repeatedly without success.
There is no consensus regarding optimal imaging for the assessment of VVF. Imaging used for diagnosis of VVF includes a voiding cystogram with opacification of the vagina after filling the bladder with contrast if there is a fistula. A cystoscopy can evaluate for calculi, retained suture, level of inflammation, and location of the ureters in relation to the fistula. Renal ultrasonography is of limited use. Intravenous pyelography can miss lesions by the trigone. In general, a computed tomography (CT) urogram and magnetic resonance imaging (MRI) with bladder contrast are more sensitive.
In the diagnosis of RVF, contrast vaginoscopy, double contrast barium enema, CT scan with contrast, and MRI can be used. MRI is more sensitive.6 A high index of suspicion is required based on the patient’s history as these imaging modalities do not always confirm RVF despite patient’s clear history of leakage. When the history is convincing, a thorough rectovaginal exam under anesthesia may be imperative. If rectal trauma is present, endoanal ultrasonography can delineate external and internal anal sphincter defects.
Prolonged Foley catheter placement after obstetric injury can lead to successful closure of a VVF. Prior to surgical intervention, assessing if there is possible ureteral involvement and use of intraoperative ureteral stents is a consideration. The route of surgery can be vaginal, abdominal, combined abdominal-vaginal, laparoscopic, or robotic.7 The robotic approach is increasingly utilized.8,9 However, the general consensus among fistula surgeons is that the vaginal approach should be considered first.
Continue to: Surgical repair...
Surgical repair
VVF repair. Factors that influence successful repair of VVF include the size and number of fistula, location, degree of scarring, bladder capacity, and urethral length.
Surgical technique requires wide mobilization and adequate exposure. The fistula tract can be delineated and manipulated with a pediatric Foley catheter, ureteral stent, or even a ureteral guidewire to aid in dissection (FIGURE 4). Intraoperative visualization of the ureters, including stenting, often is needed. The fistulous track is excised depending on the level of scarring. Closure of the bladder uroepithelium for the first layer is with absorbable interrupted 3-0 or 2-0 sutures in a tension-free closure. The bladder is then evaluated with a retrograde fill with saline and methylene blue to ensure a watertight closure for the first layer. If the first layer is not watertight, the second layer closure will not compensate and the fistula will persist. Particular attention is paid to the angles of the fistula at the first layer closure to prevent recurrence of the fistula at the angles. A running second layer with absorbable 2-0 suture is done. At times, a Martius flap or an omental J flap can be used to provide an additional layer for support and to increase vascularity.10 The patient is sent home with a Foley catheter for drainage for 10 to 14 days.11 Antibiotics are not needed postoperatively for VVF surgery.12
CT cystogram or retrograde cystogram is usually done to evaluate closure of the fistula prior to removal of the Foley catheter; retrograde fill with contrast directly into the bladder with 300 mL is sufficient (FIGURE 5). Patients are advised to refrain from sexual activity for a minimum of 6 weeks, but depending on the level of complexity and scarring, this can be up to 12 weeks.
The success rate in general is in the 95% range. Patients with successful closure of VVF are at risk for urge incontinence due to decreased bladder capacity, stress incontinence especially if the continence mechanism or urethra is involved, vaginal scarring, dyspareunia, and infertility.13 In general, sexual function improves after surgical repair.
RVF repair. Prior to surgical repair of RVF, the integrity of the external anal sphincter must be determined. If it is not involved, a vertical incision is made in the posterior vaginal wall, the vaginal epithelium is separated from the vaginal muscularis, and the fistula tract is identified. After complete wide mobilization of the tissue surrounding the tract, it is excised. The rectal wall is repaired with 3-0 or 4-0 absorbable interrupted sutures; a second layer and if possible even a third layer and finally the vaginal epithelium are all closed with 2-0 absorbable interrupted sutures.
If the sphincter complex is involved, the dissection involves an inverted U incision separating the vaginal wall from the rectum. The fistula tract is excised, the rectal wall is closed, and the internal anal sphincter is identified and reapproximated with interrupted absorbable 2-0 or 0 sutures. The disrupted external sphincter is then reapproximated with 2-0 or 0 sutures, and finally the transverse perineal and bulbocavernosus muscles are brought together with Lembert 0 sutures prior to closure of the external skin. Perioperative antibiotics have been shown to improve success rates in the correction of RVF.5 In patients with sphincter trauma and known RVF, outcomes with a sphincteroplasty are better, compared with endorectal advancement flaps. The patient is discharged with a bowel regimen and dietary precautions that aim for daily soft bowel movements.
After surgical treatment of fistulas, patients benefit from pelvic floor physical therapy that focuses on pelvic floor strengthening. Incorporating the habit of Kegel exercises after every void, timed (scheduled) bladder voiding, and avoidance of straining with urination or defecation should be emphasized.
Continue to: CASE 1 Pregnant woman with rectal bleeding...
CASE 1 Pregnant woman with rectal bleeding
A 37-year-old woman at 36 3/7 weeks’ gestation presented with acute rectal bleeding and pain. This was found to result from a catastrophic rupture of a pelvic arteriovenous malformation that caused an 11 x 7 x 9.5 cm size inferior pelvic hematoma and a full-thickness rectal tear at the dentate line. During examination under anesthesia, the baby was delivered by a stat CD due to breech presentation and a prolonged fetal heart rate deceleration. The patient underwent embolization of the right middle rectal artery and right internal iliac artery by a radiologic intervention. Further bleeding required surgical intervention for evacuation of about 1,000 mL of hematoma, repair of the rectal tear, and laparoscopic diverting loop ileostomy. In total, the patient received 8 U of packed red blood cells, 6 U of fresh frozen plasma, 5 L of crystalloid solution, and 2 g of tranexamic acid. The patient reported increased foul-smelling vaginal discharge, bedside exam suggested possible fistulous tract, and on postoperative day 16, an exam under anesthesia by Urogynecology confirmed a rectovaginal fistula in the right mid vagina. After 2 months of observation to allow resolution of inflammation, successful excision of the fistula tract and repair of RVF using the above-mentioned technique was accomplished.
CASE 2 Patient with VVF after cesarean hysterectomy
A 40-year-old (G6P2222) patient underwent cesarean hysterectomy for placenta percreta and uterine rupture at 24 weeks’ gestation. Intraoperatively, there were right ureteral ligation and posterior bladder wall cystotomies. The right ureter was reimplanted in the right upper posterior wall and the cystostomies were closed. As the patient had continuous urinary leakage postoperatively, a CT urogram was obtained, which showed left ureteral obstruction and VVF. Urinary incontinence persisted despite bilateral robotic ureteral reimplantation with omental flap by the urology team. Percutaneous nephrostomy tubes were placed bilaterally. The patient underwent additional imaging studies, including MRI, with findings of VVF and possible ureterovaginal fistula.
On referral to Urogynecology, the patient underwent cystoscopy with antegrade pyelogram, and the bilateral ureteroneocystostomy orifices had 5 French open-ended ureteral stents placed. A 10 French pediatric Foley catheter was inserted intravaginally into the bladder through the VVF. Via the vaginal approach, cervical remnant and skin bridges overlying the VVF were excised. The scarred fistula tract was excised with a circumferential incision. Horizontal interrupted Lembert sutures with 3-0 absorbable suture were used to reapproximate the first layer, which was confirmed to be watertight on testing with retrograde fill. Second-layer closure was completed with horizontal mattress 2-0 absorbable sutures, followed by a third-layer closure done in similar fashion. Fibrin glue was then placed. The vaginal epithelium was closed with 2-0 absorbable suture. Percutaneous nephrostomy tubes were removed. Postoperatively, the patient had a CT cystogram with no leak and no incontinence, but she developed urgency, which was controlled with timed voids and oxybutynin.
- Adler AJ, Ronsmans C, Calvert C, et al. Estimating the presence of obstetric fistula: a systematic review and meta-analysis BMC Pregnancy Childbirth. 2013;13:246.
- Battacharjee S, Kohli UA, Sood A, et al. Vesicouterine fistula: Youssef’s syndrome. Med J Armed Forces India. 2015;71(suppl 1):S175-S177. doi: 10.1016/j.mjafi.2013.11.006.
- Waaldijk K. Step-by-Step Surgery of Vesicovaginal Fistulas. Campion Press; 1994.
- Goh, JTW. A new classification for female genital tract fistula. Aust N Z J Ob Gynecol. 2004:44:502-504.
- Tsang CB, Rothenberger DA. Rectovaginal fistulas: therapeutic options. Surg Clin North Am. 1997;77:95-114.
- Champagne BJ, McGee MF. Rectovaginal fistula. Surg Clin North Am. 2010;90:69-82.
- Bodner-Adler B, Hanzal E, Pablik E, et al. Management of vesicovaginal fistulas in women following benign gynecologic surgery: a systematic review and meta-analysis. PLoS One. 2017;12:e0171554.
- Randazzo M, Lengauer L, Rochat CH, et al. Best practices in robotic-assisted repair of vesicovaginal fistula: a consensus report from the European Association of Urology Robotic Urology Section Scientific Working Group for Reconstructive Urology. Eur Urol. 2020;78: 432-442.
- Miklos JR, Moore RD, Chinthakanan O. Laparoscopic and robotic assisted vesicovaginal fistula repair: a systematic review of the literature. J Minim Invasive Gynecol. 2015:22:727-736.
- Hancock B. Practical Obstetric Fistula Surgery. Royal Society of Medicine Press; 2009.
- Nardos R, Menber B, Browning A. Outcome of obstetric fistula repair after 10-day versus 14-day Foley catheterization. Int J Gynaecol 0bstet. 2012;118:21-23.
- Tomlinson AJ, Thornton JG. A randomized controlled trial of antibiotic prophylaxis for vesico-vaginal fistula repair. Br J Obstet Gynaecol. 2005;105:397-399.
- Bengtson AM, Kopp D, Tang JH, et al. Identifying patients with vesicovaginal fistula at high risk of urinary incontinence after surgery. Obstet Gynecol. 2016;128:945-953.
- Adler AJ, Ronsmans C, Calvert C, et al. Estimating the presence of obstetric fistula: a systematic review and meta-analysis BMC Pregnancy Childbirth. 2013;13:246.
- Battacharjee S, Kohli UA, Sood A, et al. Vesicouterine fistula: Youssef’s syndrome. Med J Armed Forces India. 2015;71(suppl 1):S175-S177. doi: 10.1016/j.mjafi.2013.11.006.
- Waaldijk K. Step-by-Step Surgery of Vesicovaginal Fistulas. Campion Press; 1994.
- Goh, JTW. A new classification for female genital tract fistula. Aust N Z J Ob Gynecol. 2004:44:502-504.
- Tsang CB, Rothenberger DA. Rectovaginal fistulas: therapeutic options. Surg Clin North Am. 1997;77:95-114.
- Champagne BJ, McGee MF. Rectovaginal fistula. Surg Clin North Am. 2010;90:69-82.
- Bodner-Adler B, Hanzal E, Pablik E, et al. Management of vesicovaginal fistulas in women following benign gynecologic surgery: a systematic review and meta-analysis. PLoS One. 2017;12:e0171554.
- Randazzo M, Lengauer L, Rochat CH, et al. Best practices in robotic-assisted repair of vesicovaginal fistula: a consensus report from the European Association of Urology Robotic Urology Section Scientific Working Group for Reconstructive Urology. Eur Urol. 2020;78: 432-442.
- Miklos JR, Moore RD, Chinthakanan O. Laparoscopic and robotic assisted vesicovaginal fistula repair: a systematic review of the literature. J Minim Invasive Gynecol. 2015:22:727-736.
- Hancock B. Practical Obstetric Fistula Surgery. Royal Society of Medicine Press; 2009.
- Nardos R, Menber B, Browning A. Outcome of obstetric fistula repair after 10-day versus 14-day Foley catheterization. Int J Gynaecol 0bstet. 2012;118:21-23.
- Tomlinson AJ, Thornton JG. A randomized controlled trial of antibiotic prophylaxis for vesico-vaginal fistula repair. Br J Obstet Gynaecol. 2005;105:397-399.
- Bengtson AM, Kopp D, Tang JH, et al. Identifying patients with vesicovaginal fistula at high risk of urinary incontinence after surgery. Obstet Gynecol. 2016;128:945-953.
OR safety and efficiency: Measuring and monitoring all factors—including surgical volume
The operating room (OR) is a key contributor to a hospital’s profitability. It is a complex environment with ever-advancing technology. A successful surgery completed without complications within an optimal time depends not only on the surgeon’s experience, skills, and knowledge but also on numerous other structural, human, and nontechnical factors over which the surgeon has limited control.
As in any setting that deals with human life, in the OR, team dynamics, communication, and environment play a major role. Research has indicated the benefits of dedicated teams, reduced handoffs, and innovative modalities that continuously and systematically monitor potential breakdowns and propose solutions for the detected problems.
Finally, who should perform your loved one’s hysterectomy? This article also attempts to address the impact of surgeons’ and hospitals’ volume on operative outcomes with a diminishing number of hysterectomies but an increasing number of approaches.
Human factors in the OR
Human factors research was born as a product of the industrial revolution and mass production. It aims to optimize human experience and improve system performance by studying how humans interact with system. The aviation industry, for example, minimized errors significantly by using methods developed by human factors scientists. As another industry with no tolerance for mistakes, the health care sector followed suit. Ultimately, the goal of human factors research in health care is to improve patient safety, optimize work and environment, reduce costs, and enhance employees’ physical and mental health, engagement, comfort, and quality of life (FIGURE 1).1
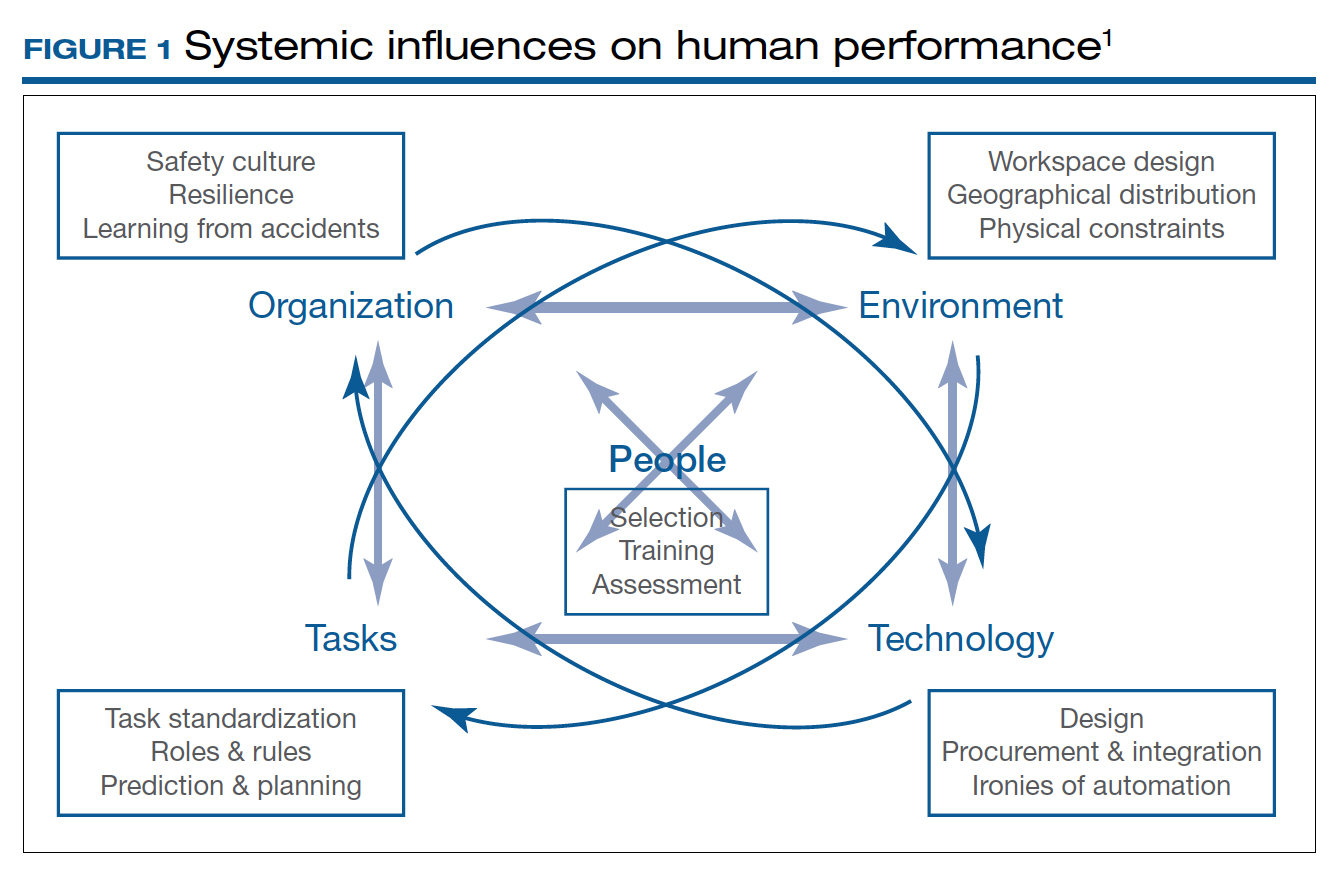
Today’s OR is so complex that it is hard to understand its dynamics without human factors research. Every new OR technology is first tested in controlled and simulated environments to determine “work as imagined.” However, it is necessary to study “work as done” in the real world via direct observation, video recording, questionnaires, and semistructured interviews by an on-site multidisciplinary team. This process not only focuses on surgical skills, process efficiency, and outcomes but also monitors the entire process according to Human Factors and Ergonomics Engineering principles to explore otherwise hidden complexities and latent safety concerns. The Systems Engineering Initiative for Patient Safety (SEIPS) framework is used to study the impact of interactions between people, tasks, technologies, environment, and organization.1
Robot-assisted surgery (RAS), an increasingly popular surgical approach among gynecologic surgeons, recently has been the focus of human factors science. A robotic OR poses unique challenges: the surgeon is not scrubbed, is removed from the operating table, and controls a complex highly technologic device in a crowded and darkened room. These are ideal conditions waiting to be optimized by human factor experts. To demonstrate the importance of human factors in the OR, we review the evidence for RAS.
Continue to: Impact of flow disruptions...
Impact of flow disruptions
Flow disruptions (FDs) were found to be more common in RAS. Catchpole and colleagues identified a mean of 9.62 FDs per hour in 89 robotic procedures, including hysterectomies and sacrocolpopexies, from a variety of fields; FDs occurred more often during the docking stage, followed by the console time, and they mostly were caused by communication breakdown and lack of team familiarity.2
Surgeon experience significantly reduced FDs. Surgeons who had done more than 700 RAS cases experienced 60% fewer FDs than those who had done less than 250 cases (13 vs 8 per hour).2 A study focusing on residents’ impact on RAS outcomes found that each FD increased the total operative time by an average 2.4 minutes, with the number significantly higher when a resident was involved.3 About one-quarter of the training-related FDs were procedure-specific instructions, while one-third were related to instrument and robotic instruction. However, pauses to teach residents did not appear to create significant intraoperative delays. Expectedly, experienced surgeons could anticipate and reduce these disruptions by supporting the whole team.
Human ergonomics, turnover time, and robot-specific skills
In a study of human ergonomics in RAS, Yu and colleagues noted that bedside assistants could experience neck posture problems. Surprisingly, the console could constrain the surgeon’s neck-shoulder region.4 Studies that reported on communication problems in a robotic OR suggest that innovative forms of verbal and nonverbal communication may support successful team communication.5
On the learning curve for RAS, OR turnover time, a key value metric, has been longer. However, turnover time was reduced almost by half from 99.2 to 53.2 minutes over 3 months after concepts from motor racing pit stops were employed, including briefings, leadership, role definition, task allocation, and task sequencing. Average room-ready time also was lowered from 42.2 to 27.2 minutes.6 RAS presents new challenges with sterile instrument processing as well. A successful RAS program, therefore, has organizational needs that include the training of OR and sterile processing staff and appropriate shift management.1
In a robotic OR, not only the surgeon but also the whole team requires robot-specific skills. New training approaches to teamwork, communication, and situation awareness skills are necessary. Robotic equipment, with its data and power cables, 2 consoles, and changing movement paths, necessitate larger rooms with a specific layout.7
In a review of recordings of RAS that used a multidimensional assessment tool to measure team effectiveness and cognitive load, Sexton and colleagues identified anticipation, active team engagement, and higher familiarity scores as the best predictors of team efficiency.8 Several studies emphasized the need for a stable team, especially in the learning phase of robotic surgery.5,9,10 A dedicated robotic team reduced the operative time by 18% during robot-assisted sacrocolpopexy (RASCP).10 RASCP procedures that extended into the afternoon took significantly longer time.9 A dedicated anesthesiologist improved the preoperative time.9 Surgical team handoffs also have reduced OR efficiency.11,12
Studying the impact of human factors is paramount for safe and efficient surgery. It is especially necessary in ORs that are equipped with high technologic instruments such as those used in RAS.
Surgical Black Box: Using data for OR safety and efficiency
Surgical procedures account for more than 50% of medical errors in a hospital setting, many of which are preventable. Postevent analysis with traditional methods, such as “Morbidity and Mortality” meetings held many days later, misses many adverse events in the OR.13 Another challenge with ever-changing and fast-multiplying surgical approaches is the development of effective surgical skill. Reviewing video recording of surgical procedures has been proposed as an instrument for recognizing adverse events and perfecting surgical skills.Recently, an innovative data-capture platform called the OR Black Box, developed by Teodor Grantcharov, MD, PhD, and colleagues, went beyond simple audiovisual recording.14 This high technologic platform not only video records the actual surgical procedure with laparoscopic camera capture (and wearable cameras for open cases) but also monitors the entire OR environment via wide-angle cameras, utilizes sensors, and records both the patient’s and the surgeon’s physiologic parameters.
The OR Black Box generates a holistic view of the OR after synchronization, encryption, and secure storage of all inputs for further analysis by experts and software-based algorithms (FIGURE 2). Computer vision algorithms can recognize improper dissection techniques and complications, such as bleeding. Adverse events are flagged with an automated software on a procedural timeline to facilitate review of procedural steps, disruptive environmental and organizational factors, OR team technical and nontechnical skills, surgeon physiologic stress, and intraoperative errors, events, and rectification processes using validated instruments.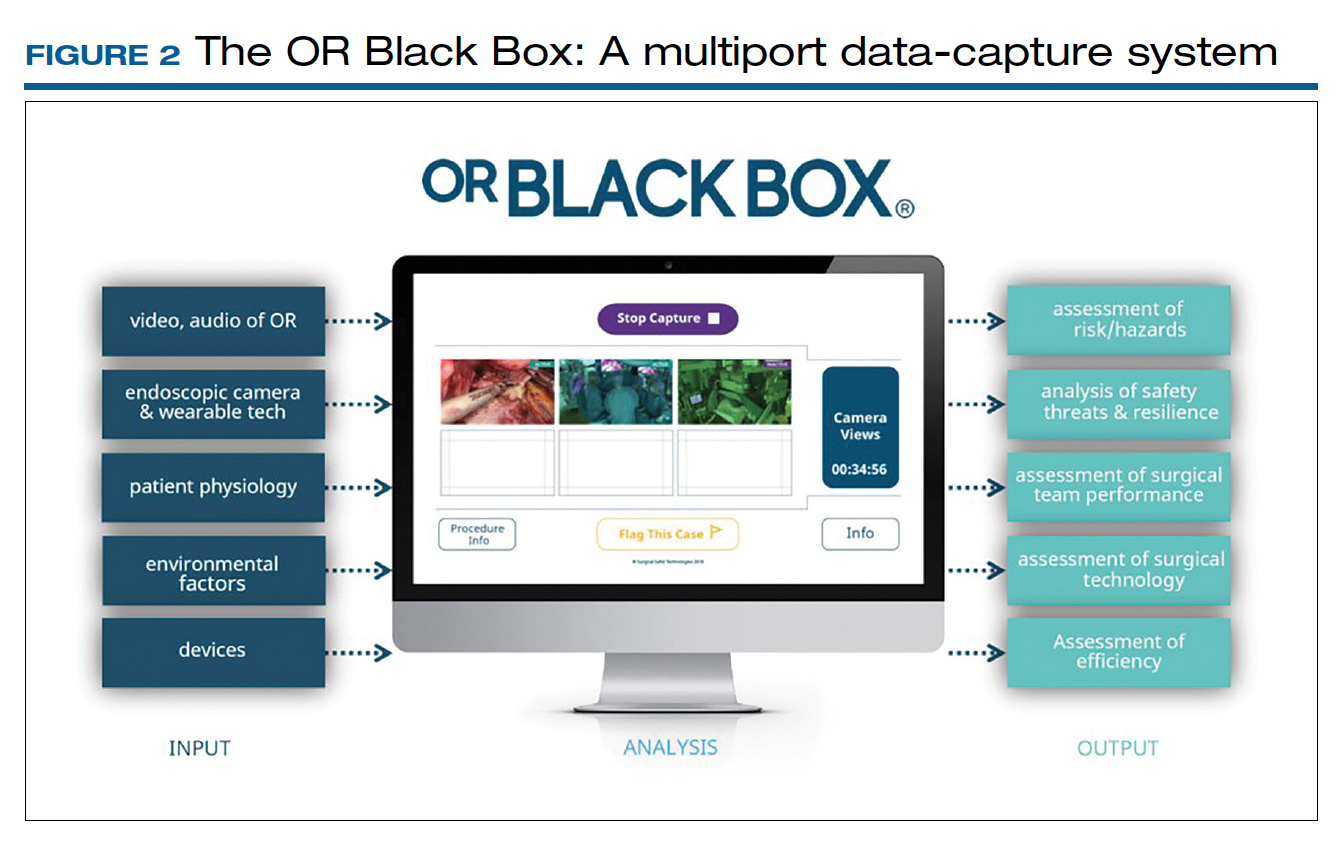
Artificial intelligence built into this platform can automatically extract objective, high-quality, and structured data to generate explainable insights by recognizing adverse events and procedural segments of interest for training and quality improvement and provide a foundation with objective measurements of technical and nontechnical performance for formative and summative assessment. This system, a major step up compared with retrospective review of likely biased medical records and labor-intensive multidisciplinary human observers, has the potential to increase efficiency and reduce costs by studying human factors that include clinical design, technology, and organization. OR efficiency, measured in real time objectively and thoroughly, may save time and resources.
OR Black Box platforms have already started to generate meaningful data. It is not surprising that auditory disruptions—OR doors opening, loud noises, pagers beeping, telephones ringing—were recorded almost every minute during laparoscopic procedures.15 Most technical errors occurred during dissection, resection, and reconstruction and most commonly were associated with improper estimations of force applied to tissue and distance to the target tissue during operative steps of a laparoscopic procedure.16 Another study based on this system showed that technical performance was an independent predictor of postoperative outcomes.17 The OR Black Box identified a device-related interruption in 30% of elective laparoscopic general surgery cases, most commonly in sleeve gastrectomy and oncologic gastrectomy procedures. This sophisticated surgical data recording system also demonstrated a significantly better ability to detect Veress needle injuries (12 vs 3) and near misses (47 vs 0) when compared with traditional chart review.18
Data from the OR Black Box also have been applied to better analyze nontechnical performance, including teamwork and interpersonal dynamics.19 Surgeons most commonly exhibited adept situational awareness and leadership, while the nurse team excelled at task management and situational awareness.19 Of the total care provider team studied, the surgeon and scrub nurse demonstrated the most favorable nontechnical behavior.19 Of note, continuous physiologic monitoring of the surgeon with this system revealed that surgeons under stress had 66% higher adverse events.
The OR Black Box is currently utilized at 20 institutions in North America and Europe. The data compiled from all these institutions revealed that there was a 10% decrease in intraoperative adverse events for each 10-point increase in technical skill score on a scale of 0 to 100 (unpublished data). This centralized data indicated that turnover time ranged widely between 7 and 91 minutes, with variation of cleanup time from 1 to 25 minutes and setup time from 22 to 43 minutes. Institutions can learn from each other using this platform. For example, the information about block time utilization (20%–99%) across institutions provides opportunities for system improvements.
With any revolutionary technology, it is imperative to study its effects on outcomes, training, costs, and privacy before it is widely implemented. We, obstetricians and gynecologists, are very familiar with the impact of electronic fetal monitoring, a great example of a technologic advance that did not improve perinatal outcomes but led to unintended consequences, such as higher rates of cesarean deliveries and lawsuits. Such a tool may lead to potential misrepresentation of intraoperative events unless legal aspects are clearly delineated. As exciting as it is, this disruptive technology requires further exploration with scientific vigor.
Continue to: Surgeon and hospital volume: Surgical outcomes paradigm...
Surgeon and hospital volume: Surgical outcomes paradigm
A landmark study in 1979 that showed decreased mortality in high-volume centers underscored the need for regionalization for certain surgical procedures.20 This association was further substantiated by 2 reports on 2.5 million Medicare beneficiaries that demonstrated significantly lower mortality for all 14 cardiovascular and oncologic procedures for hospitals with larger surgical volume (16% vs 4%) and high-volume surgeons for certain procedures, for example, 15% versus 5% for pancreatic resections for cancer.21,22
A similar association was found for all routes of hysterectomies performed for benign indications. Boyd and colleagues showed that gynecologists who performed fewer than 10 hysterectomies per year had a higher perioperative morbidity rate (16.5%) compared with those who did more (11.7%).23 Specific to vaginal hysterectomy, in a study of more than 6,000 women, surgeons who performed 13 procedures per year had 31% less risk of operative injury than those who did 5.5 procedures per year (2.5% vs 1.7%).24 Overall perioperative complications (5.0% vs 4.0%) and medical complications (5.7% vs 3.9%) were also reduced for higher-volume surgeons. In a cohort of approximately 8,000 women who underwent a laparoscopic hysterectomy, high-volume surgeons had a considerably lower complication rate (4.2% vs 6.2%).25
As expected, lower complication rates of high-volume surgeons led to lower resource utilization, including lower transfusion rates, less intensive care unit utilization, and shorter operative times and, in several studies, length of stay.24,25 Of note, low-volume surgeons were less likely to offer minimally invasive routes and were more likely to convert to laparotomy.26 In addition, significant cost savings have been associated with high surgical volume, which one study showed was 16% ($6,500 vs $5,600) for high-volume surgeons.26 With regard to mortality, a study of 7,800 women found that perioperative mortality increased more than 10-fold for surgeons who performed an average 1 case per year compared with all other surgeons (2.5% vs 0.2%).27
When gynecologic cancers are concerned, arguably, long-term survival outcomes may be more critical than perioperative morbidity and mortality. Higher surgeon and hospital volume are associated with improved perioperative outcomes for endometrial and cervical cancers.28 Importantly, minimally invasive hysterectomy was offered for endometrial cancer significantly more often by surgeons with high volume.28 Survival outcomes were not affected by surgeon or hospital volume, likely due to overall more favorable prognosis for endometrial cancer after treatment.
Although it is intuitive to assume that a surgeon’s skills and experience would make the most impact in procedures for ovarian cancer due to the complexity of ovarian cancer surgery, evidence on short-term outcomes has been mixed. Intriguingly, some studies reported that high-volume institutions had higher complication and readmission rates. However, evidence supports that the surgeon’s volume, and especially hospital volume, improves long-term survival for ovarian cancer, with a negative impact on immediate postoperative morbidity.29 This may suggest that a more aggressive surgical effort improves long-term survival but also can cause more perioperative complications. Further, longer survival may result not only from operative skills but also because of better care by a structured multidisciplinary team at more established high-volume cancer centers.
The association of improved outcomes with higher volume led to public reporting of hospital outcomes. Policy efforts toward regionalization have impacted surgical practice. Based on their analysis of 3.2 million Medicare patients who underwent 1 of 8 different cancer surgeries or cardiovascular operations from 1999 to 2008, Finks and colleagues demonstrated that care was concentrated to fewer hospitals over time for many of these procedures.29 This trend was noted for gynecologic cancer surgery but not for benign gynecologic surgery.
Regionalization of care limits access particularly for minority and underserved communities because of longer travel distances, logistic challenges, and financial strain. An alternative to regionalization of care is targeted quality improvement by rigorous adherence to quality guidelines at low-volume hospitals.
Is there a critical minimum volume that may be used as a requirement for surgeons to maintain their privileges and for hospitals to offer certain procedures? In 2015, minimum volume standards for a number of common procedures were proposed by Johns Hopkins Medicine and Dartmouth-Hitchcock Medical Center, such as 50 hip replacement surgeries per hospital and 25 per physician per year, and 20 pancreatectomies per hospital and 5 per surgeon per year.30 A modeling study for hysterectomy showed that a volume cut point of >1 procedure in the prior year would restrict privileges for a substantial number of surgeons performing abdominal (17.5%), robot-assisted (12.5%), laparoscopic (16.8%), and vaginal (27.6%) hysterectomies.27 This study concluded that minimum-volume standards for hysterectomy for even the lowest volume physicians would restrict a significant number of gynecologic surgeons, including many with outcomes that are better than predicted.
Therefore, while there is good evidence that favors better outcomes in the hands of high-volume surgeons in gynecology, the impact of such policies on gynecologic practice clearly warrants careful monitoring and further study.
- What factors besides the surgeon’s skills influence surgical safety and efficiency?
- Are you ready to have audio, video, and sensor-based recording of everything in the OR?
- Who should perform your loved one’s hysterectomy? Do the surgeon’s and hospital’s volume matter?
- Catchpole K, Bisantz A, Hallbeck MS, et al. Human factors in robotic assisted surgery: lessons from studies ‘in the wild’. Appl Ergon. 2019;78:270-276.
- Catchpole K, Perkins C, Bresee C, et al. Safety, efficiency and learning curves in robotic surgery: a human factors analysis. Surg Endosc. 2016;30:3749-3761.
- Jain M, Fry BT, Hess LW, et al. Barriers to efficiency in robotic surgery: the resident effect. J Surg. Res. 2016;205:296-304.
- Yu D, Dural C, Morrow MM, et al. Intraoperative workload in robotic surgery assessed by wearable motion tracking sensors and questionnaires. Surg Endosc. 2017;31:877-886.
- Randell R, Honey S, Alvarado N, et al. Embedding robotic surgery into routine practice and impacts on communication and decision making: a review of the experience of surgical teams. Cognit Technol Work. 2016;18:423-437.
- Souders CP, Catchpole KR, Wood LN, et al. Reducing operating room turnover time for robotic surgery using a motor racing pit stop model. World J Surg. 2017;4:1943–1949.
- Ahmad N, Hussein AA, Cavuoto L, et al. Ambulatory movements, team dynamics and interactions during robot-assisted surgery. BJU Int. 2016;118:132-139.
- Sexton K, Johnson A, Gotsch A, et al. Anticipation, teamwork, and cognitive load: chasing efficiency during robot-assisted surgery. BMJ Qual Saf. 2018;27:148-154.
- Harmanli O, Solak S, Bayram A, et al. Optimizing the robotic surgery team: an operations management perspective. Int Urogynecol J. 2021;32:1379-1385.
- Carter-Brooks CM, Du AL, Bonidie MJ, et al. The impact of a dedicated robotic team on robotic-assisted sacrocolpopexy outcomes. Female Pelvic Med Reconstr Surg. 2018;24:13-16.
- Giugale LE, Sears S, Lavelle ES, et al. Evaluating the impact of intraoperative surgical team handoffs on patient outcomes. Female Pelvic Med Reconstr Surg. 2017;23:288-292.
- Geynisman-Tan J, Brown O, Mueller M, et al. Operating room efficiency: examining the impact of personnel handoffs. Female Pelvic Med Reconstr Surg. 2018;24:87-89.
- Alsubaie H, Goldenberg M, Grantcharov T. Quantifying recall bias in surgical safety: a need for a modern approach to morbidity and mortality reviews. Can J Surg. 2019;62:39-43.
- Goldenberg MG, Jung J, Grantcharov TP. Using data to enhance performance and improve quality and safety in surgery. JAMA Surg. 2017;152:972-973.
- Jung JJ, Grantcharov TP. The operating room black box: a prospective observational study of the operating room. J Am Coll Surg. 2017;225:S127-S128.
- Jung JJ, Jüni P, Lebovic G, et al. First-year analysis of the operating room black box study. Ann Surg. 2020;271:122-127.
- Jung JJ, Kashfi A, Sharma S, et al. Characterization of device-related interruptions in minimally invasive surgery: need for intraoperative data and effective mitigation strategies. Surg Endosc. 2019;33:717-723.
- Jung JJ, Adams-McGavin RC, Grantcharov TP. Underreporting of Veress needle injuries: comparing direct observation and chart review methods. J Surg Res. 2019;236:266-270.
- Fesco AB, Kuzulugil SS, Babaoglu C, et al. Relationship between intraoperative nontechnical performance and technical events in bariatric surgery. Br J Surg. 2018;105:1044-1050.
- Luft HS, Bunker JP, Enthoven AC. Should operations be regionalized? The empirical relation between surgical volume and mortality. N Engl J Med. 1979;301:1364-1369.
- Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128-1137.
- Birkmeyer JD, Stukel TA, Siewers AE, et al. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:21172127.
- Boyd LR, Novetsky AP, Curtin JP. Effect of surgical volume on route of hysterectomy and short-term morbidity. Obstet Gynecol. 2010;116:909-915.
- Rogo-Gupta LJ, Lewin SN, Kim JH, et al. The effect of surgeon volume on outcomes and resource use for vaginal hysterectomy. Obstet Gynecol. 2010;116:1341-1347.
- Wallenstein MR, Ananth CV, Kim JH, et al. Effect of surgical volume on outcomes for laparoscopic hysterectomy for benign indications. Obstet Gynecol. 2012;119:709-716.
- Bretschneider CE, Frazzini Padilla P, Das D, et al. The impact of surgeon volume on perioperative adverse events in women undergoing minimally invasive hysterectomy for the large uterus. Am J Obstet Gynecol. 2018;219:490.e1-490.e8.
- Ruiz MP, Chen L, Hou JY, et al. Outcomes of hysterectomy performed by very low-volume surgeons. Obstet Gynecol. 2018;131:981-990.
- Wright JD. The volume-outcome paradigm for gynecologic surgery: clinical and policy implications. Clin Obstet Gynecol. 2020;63:252-265.
- Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high risk surgery. N Engl J Med. 2011;364:21282137.
- Sternberg S. Hospitals move to limit low-volume surgeries. US News & World Report. May 19, 2015. www.usnews.com/news /articles/2015/05/19/hospitals-move-to-limit-low-volume-surgeries. Accessed April 19, 2022.
The operating room (OR) is a key contributor to a hospital’s profitability. It is a complex environment with ever-advancing technology. A successful surgery completed without complications within an optimal time depends not only on the surgeon’s experience, skills, and knowledge but also on numerous other structural, human, and nontechnical factors over which the surgeon has limited control.
As in any setting that deals with human life, in the OR, team dynamics, communication, and environment play a major role. Research has indicated the benefits of dedicated teams, reduced handoffs, and innovative modalities that continuously and systematically monitor potential breakdowns and propose solutions for the detected problems.
Finally, who should perform your loved one’s hysterectomy? This article also attempts to address the impact of surgeons’ and hospitals’ volume on operative outcomes with a diminishing number of hysterectomies but an increasing number of approaches.
Human factors in the OR
Human factors research was born as a product of the industrial revolution and mass production. It aims to optimize human experience and improve system performance by studying how humans interact with system. The aviation industry, for example, minimized errors significantly by using methods developed by human factors scientists. As another industry with no tolerance for mistakes, the health care sector followed suit. Ultimately, the goal of human factors research in health care is to improve patient safety, optimize work and environment, reduce costs, and enhance employees’ physical and mental health, engagement, comfort, and quality of life (FIGURE 1).1

Today’s OR is so complex that it is hard to understand its dynamics without human factors research. Every new OR technology is first tested in controlled and simulated environments to determine “work as imagined.” However, it is necessary to study “work as done” in the real world via direct observation, video recording, questionnaires, and semistructured interviews by an on-site multidisciplinary team. This process not only focuses on surgical skills, process efficiency, and outcomes but also monitors the entire process according to Human Factors and Ergonomics Engineering principles to explore otherwise hidden complexities and latent safety concerns. The Systems Engineering Initiative for Patient Safety (SEIPS) framework is used to study the impact of interactions between people, tasks, technologies, environment, and organization.1
Robot-assisted surgery (RAS), an increasingly popular surgical approach among gynecologic surgeons, recently has been the focus of human factors science. A robotic OR poses unique challenges: the surgeon is not scrubbed, is removed from the operating table, and controls a complex highly technologic device in a crowded and darkened room. These are ideal conditions waiting to be optimized by human factor experts. To demonstrate the importance of human factors in the OR, we review the evidence for RAS.
Continue to: Impact of flow disruptions...
Impact of flow disruptions
Flow disruptions (FDs) were found to be more common in RAS. Catchpole and colleagues identified a mean of 9.62 FDs per hour in 89 robotic procedures, including hysterectomies and sacrocolpopexies, from a variety of fields; FDs occurred more often during the docking stage, followed by the console time, and they mostly were caused by communication breakdown and lack of team familiarity.2
Surgeon experience significantly reduced FDs. Surgeons who had done more than 700 RAS cases experienced 60% fewer FDs than those who had done less than 250 cases (13 vs 8 per hour).2 A study focusing on residents’ impact on RAS outcomes found that each FD increased the total operative time by an average 2.4 minutes, with the number significantly higher when a resident was involved.3 About one-quarter of the training-related FDs were procedure-specific instructions, while one-third were related to instrument and robotic instruction. However, pauses to teach residents did not appear to create significant intraoperative delays. Expectedly, experienced surgeons could anticipate and reduce these disruptions by supporting the whole team.
Human ergonomics, turnover time, and robot-specific skills
In a study of human ergonomics in RAS, Yu and colleagues noted that bedside assistants could experience neck posture problems. Surprisingly, the console could constrain the surgeon’s neck-shoulder region.4 Studies that reported on communication problems in a robotic OR suggest that innovative forms of verbal and nonverbal communication may support successful team communication.5
On the learning curve for RAS, OR turnover time, a key value metric, has been longer. However, turnover time was reduced almost by half from 99.2 to 53.2 minutes over 3 months after concepts from motor racing pit stops were employed, including briefings, leadership, role definition, task allocation, and task sequencing. Average room-ready time also was lowered from 42.2 to 27.2 minutes.6 RAS presents new challenges with sterile instrument processing as well. A successful RAS program, therefore, has organizational needs that include the training of OR and sterile processing staff and appropriate shift management.1
In a robotic OR, not only the surgeon but also the whole team requires robot-specific skills. New training approaches to teamwork, communication, and situation awareness skills are necessary. Robotic equipment, with its data and power cables, 2 consoles, and changing movement paths, necessitate larger rooms with a specific layout.7
In a review of recordings of RAS that used a multidimensional assessment tool to measure team effectiveness and cognitive load, Sexton and colleagues identified anticipation, active team engagement, and higher familiarity scores as the best predictors of team efficiency.8 Several studies emphasized the need for a stable team, especially in the learning phase of robotic surgery.5,9,10 A dedicated robotic team reduced the operative time by 18% during robot-assisted sacrocolpopexy (RASCP).10 RASCP procedures that extended into the afternoon took significantly longer time.9 A dedicated anesthesiologist improved the preoperative time.9 Surgical team handoffs also have reduced OR efficiency.11,12
Studying the impact of human factors is paramount for safe and efficient surgery. It is especially necessary in ORs that are equipped with high technologic instruments such as those used in RAS.
Surgical Black Box: Using data for OR safety and efficiency
Surgical procedures account for more than 50% of medical errors in a hospital setting, many of which are preventable. Postevent analysis with traditional methods, such as “Morbidity and Mortality” meetings held many days later, misses many adverse events in the OR.13 Another challenge with ever-changing and fast-multiplying surgical approaches is the development of effective surgical skill. Reviewing video recording of surgical procedures has been proposed as an instrument for recognizing adverse events and perfecting surgical skills.Recently, an innovative data-capture platform called the OR Black Box, developed by Teodor Grantcharov, MD, PhD, and colleagues, went beyond simple audiovisual recording.14 This high technologic platform not only video records the actual surgical procedure with laparoscopic camera capture (and wearable cameras for open cases) but also monitors the entire OR environment via wide-angle cameras, utilizes sensors, and records both the patient’s and the surgeon’s physiologic parameters.
The OR Black Box generates a holistic view of the OR after synchronization, encryption, and secure storage of all inputs for further analysis by experts and software-based algorithms (FIGURE 2). Computer vision algorithms can recognize improper dissection techniques and complications, such as bleeding. Adverse events are flagged with an automated software on a procedural timeline to facilitate review of procedural steps, disruptive environmental and organizational factors, OR team technical and nontechnical skills, surgeon physiologic stress, and intraoperative errors, events, and rectification processes using validated instruments.
Artificial intelligence built into this platform can automatically extract objective, high-quality, and structured data to generate explainable insights by recognizing adverse events and procedural segments of interest for training and quality improvement and provide a foundation with objective measurements of technical and nontechnical performance for formative and summative assessment. This system, a major step up compared with retrospective review of likely biased medical records and labor-intensive multidisciplinary human observers, has the potential to increase efficiency and reduce costs by studying human factors that include clinical design, technology, and organization. OR efficiency, measured in real time objectively and thoroughly, may save time and resources.
OR Black Box platforms have already started to generate meaningful data. It is not surprising that auditory disruptions—OR doors opening, loud noises, pagers beeping, telephones ringing—were recorded almost every minute during laparoscopic procedures.15 Most technical errors occurred during dissection, resection, and reconstruction and most commonly were associated with improper estimations of force applied to tissue and distance to the target tissue during operative steps of a laparoscopic procedure.16 Another study based on this system showed that technical performance was an independent predictor of postoperative outcomes.17 The OR Black Box identified a device-related interruption in 30% of elective laparoscopic general surgery cases, most commonly in sleeve gastrectomy and oncologic gastrectomy procedures. This sophisticated surgical data recording system also demonstrated a significantly better ability to detect Veress needle injuries (12 vs 3) and near misses (47 vs 0) when compared with traditional chart review.18
Data from the OR Black Box also have been applied to better analyze nontechnical performance, including teamwork and interpersonal dynamics.19 Surgeons most commonly exhibited adept situational awareness and leadership, while the nurse team excelled at task management and situational awareness.19 Of the total care provider team studied, the surgeon and scrub nurse demonstrated the most favorable nontechnical behavior.19 Of note, continuous physiologic monitoring of the surgeon with this system revealed that surgeons under stress had 66% higher adverse events.
The OR Black Box is currently utilized at 20 institutions in North America and Europe. The data compiled from all these institutions revealed that there was a 10% decrease in intraoperative adverse events for each 10-point increase in technical skill score on a scale of 0 to 100 (unpublished data). This centralized data indicated that turnover time ranged widely between 7 and 91 minutes, with variation of cleanup time from 1 to 25 minutes and setup time from 22 to 43 minutes. Institutions can learn from each other using this platform. For example, the information about block time utilization (20%–99%) across institutions provides opportunities for system improvements.
With any revolutionary technology, it is imperative to study its effects on outcomes, training, costs, and privacy before it is widely implemented. We, obstetricians and gynecologists, are very familiar with the impact of electronic fetal monitoring, a great example of a technologic advance that did not improve perinatal outcomes but led to unintended consequences, such as higher rates of cesarean deliveries and lawsuits. Such a tool may lead to potential misrepresentation of intraoperative events unless legal aspects are clearly delineated. As exciting as it is, this disruptive technology requires further exploration with scientific vigor.
Continue to: Surgeon and hospital volume: Surgical outcomes paradigm...
Surgeon and hospital volume: Surgical outcomes paradigm
A landmark study in 1979 that showed decreased mortality in high-volume centers underscored the need for regionalization for certain surgical procedures.20 This association was further substantiated by 2 reports on 2.5 million Medicare beneficiaries that demonstrated significantly lower mortality for all 14 cardiovascular and oncologic procedures for hospitals with larger surgical volume (16% vs 4%) and high-volume surgeons for certain procedures, for example, 15% versus 5% for pancreatic resections for cancer.21,22
A similar association was found for all routes of hysterectomies performed for benign indications. Boyd and colleagues showed that gynecologists who performed fewer than 10 hysterectomies per year had a higher perioperative morbidity rate (16.5%) compared with those who did more (11.7%).23 Specific to vaginal hysterectomy, in a study of more than 6,000 women, surgeons who performed 13 procedures per year had 31% less risk of operative injury than those who did 5.5 procedures per year (2.5% vs 1.7%).24 Overall perioperative complications (5.0% vs 4.0%) and medical complications (5.7% vs 3.9%) were also reduced for higher-volume surgeons. In a cohort of approximately 8,000 women who underwent a laparoscopic hysterectomy, high-volume surgeons had a considerably lower complication rate (4.2% vs 6.2%).25
As expected, lower complication rates of high-volume surgeons led to lower resource utilization, including lower transfusion rates, less intensive care unit utilization, and shorter operative times and, in several studies, length of stay.24,25 Of note, low-volume surgeons were less likely to offer minimally invasive routes and were more likely to convert to laparotomy.26 In addition, significant cost savings have been associated with high surgical volume, which one study showed was 16% ($6,500 vs $5,600) for high-volume surgeons.26 With regard to mortality, a study of 7,800 women found that perioperative mortality increased more than 10-fold for surgeons who performed an average 1 case per year compared with all other surgeons (2.5% vs 0.2%).27
When gynecologic cancers are concerned, arguably, long-term survival outcomes may be more critical than perioperative morbidity and mortality. Higher surgeon and hospital volume are associated with improved perioperative outcomes for endometrial and cervical cancers.28 Importantly, minimally invasive hysterectomy was offered for endometrial cancer significantly more often by surgeons with high volume.28 Survival outcomes were not affected by surgeon or hospital volume, likely due to overall more favorable prognosis for endometrial cancer after treatment.
Although it is intuitive to assume that a surgeon’s skills and experience would make the most impact in procedures for ovarian cancer due to the complexity of ovarian cancer surgery, evidence on short-term outcomes has been mixed. Intriguingly, some studies reported that high-volume institutions had higher complication and readmission rates. However, evidence supports that the surgeon’s volume, and especially hospital volume, improves long-term survival for ovarian cancer, with a negative impact on immediate postoperative morbidity.29 This may suggest that a more aggressive surgical effort improves long-term survival but also can cause more perioperative complications. Further, longer survival may result not only from operative skills but also because of better care by a structured multidisciplinary team at more established high-volume cancer centers.
The association of improved outcomes with higher volume led to public reporting of hospital outcomes. Policy efforts toward regionalization have impacted surgical practice. Based on their analysis of 3.2 million Medicare patients who underwent 1 of 8 different cancer surgeries or cardiovascular operations from 1999 to 2008, Finks and colleagues demonstrated that care was concentrated to fewer hospitals over time for many of these procedures.29 This trend was noted for gynecologic cancer surgery but not for benign gynecologic surgery.
Regionalization of care limits access particularly for minority and underserved communities because of longer travel distances, logistic challenges, and financial strain. An alternative to regionalization of care is targeted quality improvement by rigorous adherence to quality guidelines at low-volume hospitals.
Is there a critical minimum volume that may be used as a requirement for surgeons to maintain their privileges and for hospitals to offer certain procedures? In 2015, minimum volume standards for a number of common procedures were proposed by Johns Hopkins Medicine and Dartmouth-Hitchcock Medical Center, such as 50 hip replacement surgeries per hospital and 25 per physician per year, and 20 pancreatectomies per hospital and 5 per surgeon per year.30 A modeling study for hysterectomy showed that a volume cut point of >1 procedure in the prior year would restrict privileges for a substantial number of surgeons performing abdominal (17.5%), robot-assisted (12.5%), laparoscopic (16.8%), and vaginal (27.6%) hysterectomies.27 This study concluded that minimum-volume standards for hysterectomy for even the lowest volume physicians would restrict a significant number of gynecologic surgeons, including many with outcomes that are better than predicted.
Therefore, while there is good evidence that favors better outcomes in the hands of high-volume surgeons in gynecology, the impact of such policies on gynecologic practice clearly warrants careful monitoring and further study.
- What factors besides the surgeon’s skills influence surgical safety and efficiency?
- Are you ready to have audio, video, and sensor-based recording of everything in the OR?
- Who should perform your loved one’s hysterectomy? Do the surgeon’s and hospital’s volume matter?
The operating room (OR) is a key contributor to a hospital’s profitability. It is a complex environment with ever-advancing technology. A successful surgery completed without complications within an optimal time depends not only on the surgeon’s experience, skills, and knowledge but also on numerous other structural, human, and nontechnical factors over which the surgeon has limited control.
As in any setting that deals with human life, in the OR, team dynamics, communication, and environment play a major role. Research has indicated the benefits of dedicated teams, reduced handoffs, and innovative modalities that continuously and systematically monitor potential breakdowns and propose solutions for the detected problems.
Finally, who should perform your loved one’s hysterectomy? This article also attempts to address the impact of surgeons’ and hospitals’ volume on operative outcomes with a diminishing number of hysterectomies but an increasing number of approaches.
Human factors in the OR
Human factors research was born as a product of the industrial revolution and mass production. It aims to optimize human experience and improve system performance by studying how humans interact with system. The aviation industry, for example, minimized errors significantly by using methods developed by human factors scientists. As another industry with no tolerance for mistakes, the health care sector followed suit. Ultimately, the goal of human factors research in health care is to improve patient safety, optimize work and environment, reduce costs, and enhance employees’ physical and mental health, engagement, comfort, and quality of life (FIGURE 1).1

Today’s OR is so complex that it is hard to understand its dynamics without human factors research. Every new OR technology is first tested in controlled and simulated environments to determine “work as imagined.” However, it is necessary to study “work as done” in the real world via direct observation, video recording, questionnaires, and semistructured interviews by an on-site multidisciplinary team. This process not only focuses on surgical skills, process efficiency, and outcomes but also monitors the entire process according to Human Factors and Ergonomics Engineering principles to explore otherwise hidden complexities and latent safety concerns. The Systems Engineering Initiative for Patient Safety (SEIPS) framework is used to study the impact of interactions between people, tasks, technologies, environment, and organization.1
Robot-assisted surgery (RAS), an increasingly popular surgical approach among gynecologic surgeons, recently has been the focus of human factors science. A robotic OR poses unique challenges: the surgeon is not scrubbed, is removed from the operating table, and controls a complex highly technologic device in a crowded and darkened room. These are ideal conditions waiting to be optimized by human factor experts. To demonstrate the importance of human factors in the OR, we review the evidence for RAS.
Continue to: Impact of flow disruptions...
Impact of flow disruptions
Flow disruptions (FDs) were found to be more common in RAS. Catchpole and colleagues identified a mean of 9.62 FDs per hour in 89 robotic procedures, including hysterectomies and sacrocolpopexies, from a variety of fields; FDs occurred more often during the docking stage, followed by the console time, and they mostly were caused by communication breakdown and lack of team familiarity.2
Surgeon experience significantly reduced FDs. Surgeons who had done more than 700 RAS cases experienced 60% fewer FDs than those who had done less than 250 cases (13 vs 8 per hour).2 A study focusing on residents’ impact on RAS outcomes found that each FD increased the total operative time by an average 2.4 minutes, with the number significantly higher when a resident was involved.3 About one-quarter of the training-related FDs were procedure-specific instructions, while one-third were related to instrument and robotic instruction. However, pauses to teach residents did not appear to create significant intraoperative delays. Expectedly, experienced surgeons could anticipate and reduce these disruptions by supporting the whole team.
Human ergonomics, turnover time, and robot-specific skills
In a study of human ergonomics in RAS, Yu and colleagues noted that bedside assistants could experience neck posture problems. Surprisingly, the console could constrain the surgeon’s neck-shoulder region.4 Studies that reported on communication problems in a robotic OR suggest that innovative forms of verbal and nonverbal communication may support successful team communication.5
On the learning curve for RAS, OR turnover time, a key value metric, has been longer. However, turnover time was reduced almost by half from 99.2 to 53.2 minutes over 3 months after concepts from motor racing pit stops were employed, including briefings, leadership, role definition, task allocation, and task sequencing. Average room-ready time also was lowered from 42.2 to 27.2 minutes.6 RAS presents new challenges with sterile instrument processing as well. A successful RAS program, therefore, has organizational needs that include the training of OR and sterile processing staff and appropriate shift management.1
In a robotic OR, not only the surgeon but also the whole team requires robot-specific skills. New training approaches to teamwork, communication, and situation awareness skills are necessary. Robotic equipment, with its data and power cables, 2 consoles, and changing movement paths, necessitate larger rooms with a specific layout.7
In a review of recordings of RAS that used a multidimensional assessment tool to measure team effectiveness and cognitive load, Sexton and colleagues identified anticipation, active team engagement, and higher familiarity scores as the best predictors of team efficiency.8 Several studies emphasized the need for a stable team, especially in the learning phase of robotic surgery.5,9,10 A dedicated robotic team reduced the operative time by 18% during robot-assisted sacrocolpopexy (RASCP).10 RASCP procedures that extended into the afternoon took significantly longer time.9 A dedicated anesthesiologist improved the preoperative time.9 Surgical team handoffs also have reduced OR efficiency.11,12
Studying the impact of human factors is paramount for safe and efficient surgery. It is especially necessary in ORs that are equipped with high technologic instruments such as those used in RAS.
Surgical Black Box: Using data for OR safety and efficiency
Surgical procedures account for more than 50% of medical errors in a hospital setting, many of which are preventable. Postevent analysis with traditional methods, such as “Morbidity and Mortality” meetings held many days later, misses many adverse events in the OR.13 Another challenge with ever-changing and fast-multiplying surgical approaches is the development of effective surgical skill. Reviewing video recording of surgical procedures has been proposed as an instrument for recognizing adverse events and perfecting surgical skills.Recently, an innovative data-capture platform called the OR Black Box, developed by Teodor Grantcharov, MD, PhD, and colleagues, went beyond simple audiovisual recording.14 This high technologic platform not only video records the actual surgical procedure with laparoscopic camera capture (and wearable cameras for open cases) but also monitors the entire OR environment via wide-angle cameras, utilizes sensors, and records both the patient’s and the surgeon’s physiologic parameters.
The OR Black Box generates a holistic view of the OR after synchronization, encryption, and secure storage of all inputs for further analysis by experts and software-based algorithms (FIGURE 2). Computer vision algorithms can recognize improper dissection techniques and complications, such as bleeding. Adverse events are flagged with an automated software on a procedural timeline to facilitate review of procedural steps, disruptive environmental and organizational factors, OR team technical and nontechnical skills, surgeon physiologic stress, and intraoperative errors, events, and rectification processes using validated instruments.
Artificial intelligence built into this platform can automatically extract objective, high-quality, and structured data to generate explainable insights by recognizing adverse events and procedural segments of interest for training and quality improvement and provide a foundation with objective measurements of technical and nontechnical performance for formative and summative assessment. This system, a major step up compared with retrospective review of likely biased medical records and labor-intensive multidisciplinary human observers, has the potential to increase efficiency and reduce costs by studying human factors that include clinical design, technology, and organization. OR efficiency, measured in real time objectively and thoroughly, may save time and resources.
OR Black Box platforms have already started to generate meaningful data. It is not surprising that auditory disruptions—OR doors opening, loud noises, pagers beeping, telephones ringing—were recorded almost every minute during laparoscopic procedures.15 Most technical errors occurred during dissection, resection, and reconstruction and most commonly were associated with improper estimations of force applied to tissue and distance to the target tissue during operative steps of a laparoscopic procedure.16 Another study based on this system showed that technical performance was an independent predictor of postoperative outcomes.17 The OR Black Box identified a device-related interruption in 30% of elective laparoscopic general surgery cases, most commonly in sleeve gastrectomy and oncologic gastrectomy procedures. This sophisticated surgical data recording system also demonstrated a significantly better ability to detect Veress needle injuries (12 vs 3) and near misses (47 vs 0) when compared with traditional chart review.18
Data from the OR Black Box also have been applied to better analyze nontechnical performance, including teamwork and interpersonal dynamics.19 Surgeons most commonly exhibited adept situational awareness and leadership, while the nurse team excelled at task management and situational awareness.19 Of the total care provider team studied, the surgeon and scrub nurse demonstrated the most favorable nontechnical behavior.19 Of note, continuous physiologic monitoring of the surgeon with this system revealed that surgeons under stress had 66% higher adverse events.
The OR Black Box is currently utilized at 20 institutions in North America and Europe. The data compiled from all these institutions revealed that there was a 10% decrease in intraoperative adverse events for each 10-point increase in technical skill score on a scale of 0 to 100 (unpublished data). This centralized data indicated that turnover time ranged widely between 7 and 91 minutes, with variation of cleanup time from 1 to 25 minutes and setup time from 22 to 43 minutes. Institutions can learn from each other using this platform. For example, the information about block time utilization (20%–99%) across institutions provides opportunities for system improvements.
With any revolutionary technology, it is imperative to study its effects on outcomes, training, costs, and privacy before it is widely implemented. We, obstetricians and gynecologists, are very familiar with the impact of electronic fetal monitoring, a great example of a technologic advance that did not improve perinatal outcomes but led to unintended consequences, such as higher rates of cesarean deliveries and lawsuits. Such a tool may lead to potential misrepresentation of intraoperative events unless legal aspects are clearly delineated. As exciting as it is, this disruptive technology requires further exploration with scientific vigor.
Continue to: Surgeon and hospital volume: Surgical outcomes paradigm...
Surgeon and hospital volume: Surgical outcomes paradigm
A landmark study in 1979 that showed decreased mortality in high-volume centers underscored the need for regionalization for certain surgical procedures.20 This association was further substantiated by 2 reports on 2.5 million Medicare beneficiaries that demonstrated significantly lower mortality for all 14 cardiovascular and oncologic procedures for hospitals with larger surgical volume (16% vs 4%) and high-volume surgeons for certain procedures, for example, 15% versus 5% for pancreatic resections for cancer.21,22
A similar association was found for all routes of hysterectomies performed for benign indications. Boyd and colleagues showed that gynecologists who performed fewer than 10 hysterectomies per year had a higher perioperative morbidity rate (16.5%) compared with those who did more (11.7%).23 Specific to vaginal hysterectomy, in a study of more than 6,000 women, surgeons who performed 13 procedures per year had 31% less risk of operative injury than those who did 5.5 procedures per year (2.5% vs 1.7%).24 Overall perioperative complications (5.0% vs 4.0%) and medical complications (5.7% vs 3.9%) were also reduced for higher-volume surgeons. In a cohort of approximately 8,000 women who underwent a laparoscopic hysterectomy, high-volume surgeons had a considerably lower complication rate (4.2% vs 6.2%).25
As expected, lower complication rates of high-volume surgeons led to lower resource utilization, including lower transfusion rates, less intensive care unit utilization, and shorter operative times and, in several studies, length of stay.24,25 Of note, low-volume surgeons were less likely to offer minimally invasive routes and were more likely to convert to laparotomy.26 In addition, significant cost savings have been associated with high surgical volume, which one study showed was 16% ($6,500 vs $5,600) for high-volume surgeons.26 With regard to mortality, a study of 7,800 women found that perioperative mortality increased more than 10-fold for surgeons who performed an average 1 case per year compared with all other surgeons (2.5% vs 0.2%).27
When gynecologic cancers are concerned, arguably, long-term survival outcomes may be more critical than perioperative morbidity and mortality. Higher surgeon and hospital volume are associated with improved perioperative outcomes for endometrial and cervical cancers.28 Importantly, minimally invasive hysterectomy was offered for endometrial cancer significantly more often by surgeons with high volume.28 Survival outcomes were not affected by surgeon or hospital volume, likely due to overall more favorable prognosis for endometrial cancer after treatment.
Although it is intuitive to assume that a surgeon’s skills and experience would make the most impact in procedures for ovarian cancer due to the complexity of ovarian cancer surgery, evidence on short-term outcomes has been mixed. Intriguingly, some studies reported that high-volume institutions had higher complication and readmission rates. However, evidence supports that the surgeon’s volume, and especially hospital volume, improves long-term survival for ovarian cancer, with a negative impact on immediate postoperative morbidity.29 This may suggest that a more aggressive surgical effort improves long-term survival but also can cause more perioperative complications. Further, longer survival may result not only from operative skills but also because of better care by a structured multidisciplinary team at more established high-volume cancer centers.
The association of improved outcomes with higher volume led to public reporting of hospital outcomes. Policy efforts toward regionalization have impacted surgical practice. Based on their analysis of 3.2 million Medicare patients who underwent 1 of 8 different cancer surgeries or cardiovascular operations from 1999 to 2008, Finks and colleagues demonstrated that care was concentrated to fewer hospitals over time for many of these procedures.29 This trend was noted for gynecologic cancer surgery but not for benign gynecologic surgery.
Regionalization of care limits access particularly for minority and underserved communities because of longer travel distances, logistic challenges, and financial strain. An alternative to regionalization of care is targeted quality improvement by rigorous adherence to quality guidelines at low-volume hospitals.
Is there a critical minimum volume that may be used as a requirement for surgeons to maintain their privileges and for hospitals to offer certain procedures? In 2015, minimum volume standards for a number of common procedures were proposed by Johns Hopkins Medicine and Dartmouth-Hitchcock Medical Center, such as 50 hip replacement surgeries per hospital and 25 per physician per year, and 20 pancreatectomies per hospital and 5 per surgeon per year.30 A modeling study for hysterectomy showed that a volume cut point of >1 procedure in the prior year would restrict privileges for a substantial number of surgeons performing abdominal (17.5%), robot-assisted (12.5%), laparoscopic (16.8%), and vaginal (27.6%) hysterectomies.27 This study concluded that minimum-volume standards for hysterectomy for even the lowest volume physicians would restrict a significant number of gynecologic surgeons, including many with outcomes that are better than predicted.
Therefore, while there is good evidence that favors better outcomes in the hands of high-volume surgeons in gynecology, the impact of such policies on gynecologic practice clearly warrants careful monitoring and further study.
- What factors besides the surgeon’s skills influence surgical safety and efficiency?
- Are you ready to have audio, video, and sensor-based recording of everything in the OR?
- Who should perform your loved one’s hysterectomy? Do the surgeon’s and hospital’s volume matter?
- Catchpole K, Bisantz A, Hallbeck MS, et al. Human factors in robotic assisted surgery: lessons from studies ‘in the wild’. Appl Ergon. 2019;78:270-276.
- Catchpole K, Perkins C, Bresee C, et al. Safety, efficiency and learning curves in robotic surgery: a human factors analysis. Surg Endosc. 2016;30:3749-3761.
- Jain M, Fry BT, Hess LW, et al. Barriers to efficiency in robotic surgery: the resident effect. J Surg. Res. 2016;205:296-304.
- Yu D, Dural C, Morrow MM, et al. Intraoperative workload in robotic surgery assessed by wearable motion tracking sensors and questionnaires. Surg Endosc. 2017;31:877-886.
- Randell R, Honey S, Alvarado N, et al. Embedding robotic surgery into routine practice and impacts on communication and decision making: a review of the experience of surgical teams. Cognit Technol Work. 2016;18:423-437.
- Souders CP, Catchpole KR, Wood LN, et al. Reducing operating room turnover time for robotic surgery using a motor racing pit stop model. World J Surg. 2017;4:1943–1949.
- Ahmad N, Hussein AA, Cavuoto L, et al. Ambulatory movements, team dynamics and interactions during robot-assisted surgery. BJU Int. 2016;118:132-139.
- Sexton K, Johnson A, Gotsch A, et al. Anticipation, teamwork, and cognitive load: chasing efficiency during robot-assisted surgery. BMJ Qual Saf. 2018;27:148-154.
- Harmanli O, Solak S, Bayram A, et al. Optimizing the robotic surgery team: an operations management perspective. Int Urogynecol J. 2021;32:1379-1385.
- Carter-Brooks CM, Du AL, Bonidie MJ, et al. The impact of a dedicated robotic team on robotic-assisted sacrocolpopexy outcomes. Female Pelvic Med Reconstr Surg. 2018;24:13-16.
- Giugale LE, Sears S, Lavelle ES, et al. Evaluating the impact of intraoperative surgical team handoffs on patient outcomes. Female Pelvic Med Reconstr Surg. 2017;23:288-292.
- Geynisman-Tan J, Brown O, Mueller M, et al. Operating room efficiency: examining the impact of personnel handoffs. Female Pelvic Med Reconstr Surg. 2018;24:87-89.
- Alsubaie H, Goldenberg M, Grantcharov T. Quantifying recall bias in surgical safety: a need for a modern approach to morbidity and mortality reviews. Can J Surg. 2019;62:39-43.
- Goldenberg MG, Jung J, Grantcharov TP. Using data to enhance performance and improve quality and safety in surgery. JAMA Surg. 2017;152:972-973.
- Jung JJ, Grantcharov TP. The operating room black box: a prospective observational study of the operating room. J Am Coll Surg. 2017;225:S127-S128.
- Jung JJ, Jüni P, Lebovic G, et al. First-year analysis of the operating room black box study. Ann Surg. 2020;271:122-127.
- Jung JJ, Kashfi A, Sharma S, et al. Characterization of device-related interruptions in minimally invasive surgery: need for intraoperative data and effective mitigation strategies. Surg Endosc. 2019;33:717-723.
- Jung JJ, Adams-McGavin RC, Grantcharov TP. Underreporting of Veress needle injuries: comparing direct observation and chart review methods. J Surg Res. 2019;236:266-270.
- Fesco AB, Kuzulugil SS, Babaoglu C, et al. Relationship between intraoperative nontechnical performance and technical events in bariatric surgery. Br J Surg. 2018;105:1044-1050.
- Luft HS, Bunker JP, Enthoven AC. Should operations be regionalized? The empirical relation between surgical volume and mortality. N Engl J Med. 1979;301:1364-1369.
- Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128-1137.
- Birkmeyer JD, Stukel TA, Siewers AE, et al. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:21172127.
- Boyd LR, Novetsky AP, Curtin JP. Effect of surgical volume on route of hysterectomy and short-term morbidity. Obstet Gynecol. 2010;116:909-915.
- Rogo-Gupta LJ, Lewin SN, Kim JH, et al. The effect of surgeon volume on outcomes and resource use for vaginal hysterectomy. Obstet Gynecol. 2010;116:1341-1347.
- Wallenstein MR, Ananth CV, Kim JH, et al. Effect of surgical volume on outcomes for laparoscopic hysterectomy for benign indications. Obstet Gynecol. 2012;119:709-716.
- Bretschneider CE, Frazzini Padilla P, Das D, et al. The impact of surgeon volume on perioperative adverse events in women undergoing minimally invasive hysterectomy for the large uterus. Am J Obstet Gynecol. 2018;219:490.e1-490.e8.
- Ruiz MP, Chen L, Hou JY, et al. Outcomes of hysterectomy performed by very low-volume surgeons. Obstet Gynecol. 2018;131:981-990.
- Wright JD. The volume-outcome paradigm for gynecologic surgery: clinical and policy implications. Clin Obstet Gynecol. 2020;63:252-265.
- Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high risk surgery. N Engl J Med. 2011;364:21282137.
- Sternberg S. Hospitals move to limit low-volume surgeries. US News & World Report. May 19, 2015. www.usnews.com/news /articles/2015/05/19/hospitals-move-to-limit-low-volume-surgeries. Accessed April 19, 2022.
- Catchpole K, Bisantz A, Hallbeck MS, et al. Human factors in robotic assisted surgery: lessons from studies ‘in the wild’. Appl Ergon. 2019;78:270-276.
- Catchpole K, Perkins C, Bresee C, et al. Safety, efficiency and learning curves in robotic surgery: a human factors analysis. Surg Endosc. 2016;30:3749-3761.
- Jain M, Fry BT, Hess LW, et al. Barriers to efficiency in robotic surgery: the resident effect. J Surg. Res. 2016;205:296-304.
- Yu D, Dural C, Morrow MM, et al. Intraoperative workload in robotic surgery assessed by wearable motion tracking sensors and questionnaires. Surg Endosc. 2017;31:877-886.
- Randell R, Honey S, Alvarado N, et al. Embedding robotic surgery into routine practice and impacts on communication and decision making: a review of the experience of surgical teams. Cognit Technol Work. 2016;18:423-437.
- Souders CP, Catchpole KR, Wood LN, et al. Reducing operating room turnover time for robotic surgery using a motor racing pit stop model. World J Surg. 2017;4:1943–1949.
- Ahmad N, Hussein AA, Cavuoto L, et al. Ambulatory movements, team dynamics and interactions during robot-assisted surgery. BJU Int. 2016;118:132-139.
- Sexton K, Johnson A, Gotsch A, et al. Anticipation, teamwork, and cognitive load: chasing efficiency during robot-assisted surgery. BMJ Qual Saf. 2018;27:148-154.
- Harmanli O, Solak S, Bayram A, et al. Optimizing the robotic surgery team: an operations management perspective. Int Urogynecol J. 2021;32:1379-1385.
- Carter-Brooks CM, Du AL, Bonidie MJ, et al. The impact of a dedicated robotic team on robotic-assisted sacrocolpopexy outcomes. Female Pelvic Med Reconstr Surg. 2018;24:13-16.
- Giugale LE, Sears S, Lavelle ES, et al. Evaluating the impact of intraoperative surgical team handoffs on patient outcomes. Female Pelvic Med Reconstr Surg. 2017;23:288-292.
- Geynisman-Tan J, Brown O, Mueller M, et al. Operating room efficiency: examining the impact of personnel handoffs. Female Pelvic Med Reconstr Surg. 2018;24:87-89.
- Alsubaie H, Goldenberg M, Grantcharov T. Quantifying recall bias in surgical safety: a need for a modern approach to morbidity and mortality reviews. Can J Surg. 2019;62:39-43.
- Goldenberg MG, Jung J, Grantcharov TP. Using data to enhance performance and improve quality and safety in surgery. JAMA Surg. 2017;152:972-973.
- Jung JJ, Grantcharov TP. The operating room black box: a prospective observational study of the operating room. J Am Coll Surg. 2017;225:S127-S128.
- Jung JJ, Jüni P, Lebovic G, et al. First-year analysis of the operating room black box study. Ann Surg. 2020;271:122-127.
- Jung JJ, Kashfi A, Sharma S, et al. Characterization of device-related interruptions in minimally invasive surgery: need for intraoperative data and effective mitigation strategies. Surg Endosc. 2019;33:717-723.
- Jung JJ, Adams-McGavin RC, Grantcharov TP. Underreporting of Veress needle injuries: comparing direct observation and chart review methods. J Surg Res. 2019;236:266-270.
- Fesco AB, Kuzulugil SS, Babaoglu C, et al. Relationship between intraoperative nontechnical performance and technical events in bariatric surgery. Br J Surg. 2018;105:1044-1050.
- Luft HS, Bunker JP, Enthoven AC. Should operations be regionalized? The empirical relation between surgical volume and mortality. N Engl J Med. 1979;301:1364-1369.
- Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128-1137.
- Birkmeyer JD, Stukel TA, Siewers AE, et al. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:21172127.
- Boyd LR, Novetsky AP, Curtin JP. Effect of surgical volume on route of hysterectomy and short-term morbidity. Obstet Gynecol. 2010;116:909-915.
- Rogo-Gupta LJ, Lewin SN, Kim JH, et al. The effect of surgeon volume on outcomes and resource use for vaginal hysterectomy. Obstet Gynecol. 2010;116:1341-1347.
- Wallenstein MR, Ananth CV, Kim JH, et al. Effect of surgical volume on outcomes for laparoscopic hysterectomy for benign indications. Obstet Gynecol. 2012;119:709-716.
- Bretschneider CE, Frazzini Padilla P, Das D, et al. The impact of surgeon volume on perioperative adverse events in women undergoing minimally invasive hysterectomy for the large uterus. Am J Obstet Gynecol. 2018;219:490.e1-490.e8.
- Ruiz MP, Chen L, Hou JY, et al. Outcomes of hysterectomy performed by very low-volume surgeons. Obstet Gynecol. 2018;131:981-990.
- Wright JD. The volume-outcome paradigm for gynecologic surgery: clinical and policy implications. Clin Obstet Gynecol. 2020;63:252-265.
- Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high risk surgery. N Engl J Med. 2011;364:21282137.
- Sternberg S. Hospitals move to limit low-volume surgeries. US News & World Report. May 19, 2015. www.usnews.com/news /articles/2015/05/19/hospitals-move-to-limit-low-volume-surgeries. Accessed April 19, 2022.
Society of Gynecologic Surgeons meeting champions training of future gynecologic surgeons
It was such a pleasure at the 48th Annual Meeting of the Society of Gynecologic Surgeons (SGS) to witness record meeting attendance and strong enthusiasm after 2 depressing years with the COVID-19 pandemic. Evidently, everyone was tired of virtual gatherings and presentations. As a dedicated surgical educator and a passionate vaginal surgeon, SGS President Carl Zimmerman, MD, chose “Gynecologic surgery training: Lessons from the past, looking to the future” as the theme for this year’s meeting. Our keynote speakers, Patricia Turner, MD, MBA, Executive Director of the American College of Surgeons, and Marta Crispens, MD, MBA, Professor and Division Director of Gynecologic Oncology at Vanderbilt, were spot on. They reviewed the current status of surgical training eloquently with convincing statistics. They mapped out the path forward by stressing collaboration and proposing strategies that might produce competent surgeons in all fields.
The meeting featured 2 panel discussions. The first, titled “Innovations in training gynecologic surgeons,” reviewed tracking in residency, use of simulation for surgical proficiency, and European perspective on training. The panelists emphasized the dwindling numbers of surgical procedures, especially vaginal hysterectomies. Cecile Ferrando, MD, suggested that tracking might be part of the answer, based on their experience, which provided a structure for residents to obtain concentrated training in their areas of interest. Douglas Miyazaki, MD, presented the prospects for his innovative, federally funded vaginal surgery simulation model. Oliver Preyer, MD, presented Austrian trainees’ low case volumes, showing that the grass was not actually greener on the other side. Finally, this panel reinvigorated ongoing debate about separating Obstetrics and Gynecology.
The second panel, “Operating room safety and efficiency,” shed light on human and nontechnical factors that might be as critical as surgeons’ skills and experience, and it highlighted an innovative technology that monitored and analyzed all operating room parameters to improve operational processes and surgical technique. Points by Jason Wright, MD, on the relationship between surgical volume and outcomes complemented the meeting theme and the first panel discussion. He underlined how much surgical volume of individual surgeons and hospitals mattered, but he also indicated that restrictive credentialing strategies might lead to unintended consequences.
Importantly, the SGS Women’s Council held a panel on the “Impact of Texas legislation on the physician/patient relationship” to provide a platform for members who had mixed feelings about attending this meeting in Texas.
The SGS meeting also included several popular postgraduate courses on multidisciplinary management of Müllerian anomalies, pelvic fistula treatment, surgical simulation, management modalities for uterine fibroids, and medical innovation and entrepreneurship. In this special section and in the next issue of OBG M
It was such a pleasure at the 48th Annual Meeting of the Society of Gynecologic Surgeons (SGS) to witness record meeting attendance and strong enthusiasm after 2 depressing years with the COVID-19 pandemic. Evidently, everyone was tired of virtual gatherings and presentations. As a dedicated surgical educator and a passionate vaginal surgeon, SGS President Carl Zimmerman, MD, chose “Gynecologic surgery training: Lessons from the past, looking to the future” as the theme for this year’s meeting. Our keynote speakers, Patricia Turner, MD, MBA, Executive Director of the American College of Surgeons, and Marta Crispens, MD, MBA, Professor and Division Director of Gynecologic Oncology at Vanderbilt, were spot on. They reviewed the current status of surgical training eloquently with convincing statistics. They mapped out the path forward by stressing collaboration and proposing strategies that might produce competent surgeons in all fields.
The meeting featured 2 panel discussions. The first, titled “Innovations in training gynecologic surgeons,” reviewed tracking in residency, use of simulation for surgical proficiency, and European perspective on training. The panelists emphasized the dwindling numbers of surgical procedures, especially vaginal hysterectomies. Cecile Ferrando, MD, suggested that tracking might be part of the answer, based on their experience, which provided a structure for residents to obtain concentrated training in their areas of interest. Douglas Miyazaki, MD, presented the prospects for his innovative, federally funded vaginal surgery simulation model. Oliver Preyer, MD, presented Austrian trainees’ low case volumes, showing that the grass was not actually greener on the other side. Finally, this panel reinvigorated ongoing debate about separating Obstetrics and Gynecology.
The second panel, “Operating room safety and efficiency,” shed light on human and nontechnical factors that might be as critical as surgeons’ skills and experience, and it highlighted an innovative technology that monitored and analyzed all operating room parameters to improve operational processes and surgical technique. Points by Jason Wright, MD, on the relationship between surgical volume and outcomes complemented the meeting theme and the first panel discussion. He underlined how much surgical volume of individual surgeons and hospitals mattered, but he also indicated that restrictive credentialing strategies might lead to unintended consequences.
Importantly, the SGS Women’s Council held a panel on the “Impact of Texas legislation on the physician/patient relationship” to provide a platform for members who had mixed feelings about attending this meeting in Texas.
The SGS meeting also included several popular postgraduate courses on multidisciplinary management of Müllerian anomalies, pelvic fistula treatment, surgical simulation, management modalities for uterine fibroids, and medical innovation and entrepreneurship. In this special section and in the next issue of OBG M
It was such a pleasure at the 48th Annual Meeting of the Society of Gynecologic Surgeons (SGS) to witness record meeting attendance and strong enthusiasm after 2 depressing years with the COVID-19 pandemic. Evidently, everyone was tired of virtual gatherings and presentations. As a dedicated surgical educator and a passionate vaginal surgeon, SGS President Carl Zimmerman, MD, chose “Gynecologic surgery training: Lessons from the past, looking to the future” as the theme for this year’s meeting. Our keynote speakers, Patricia Turner, MD, MBA, Executive Director of the American College of Surgeons, and Marta Crispens, MD, MBA, Professor and Division Director of Gynecologic Oncology at Vanderbilt, were spot on. They reviewed the current status of surgical training eloquently with convincing statistics. They mapped out the path forward by stressing collaboration and proposing strategies that might produce competent surgeons in all fields.
The meeting featured 2 panel discussions. The first, titled “Innovations in training gynecologic surgeons,” reviewed tracking in residency, use of simulation for surgical proficiency, and European perspective on training. The panelists emphasized the dwindling numbers of surgical procedures, especially vaginal hysterectomies. Cecile Ferrando, MD, suggested that tracking might be part of the answer, based on their experience, which provided a structure for residents to obtain concentrated training in their areas of interest. Douglas Miyazaki, MD, presented the prospects for his innovative, federally funded vaginal surgery simulation model. Oliver Preyer, MD, presented Austrian trainees’ low case volumes, showing that the grass was not actually greener on the other side. Finally, this panel reinvigorated ongoing debate about separating Obstetrics and Gynecology.
The second panel, “Operating room safety and efficiency,” shed light on human and nontechnical factors that might be as critical as surgeons’ skills and experience, and it highlighted an innovative technology that monitored and analyzed all operating room parameters to improve operational processes and surgical technique. Points by Jason Wright, MD, on the relationship between surgical volume and outcomes complemented the meeting theme and the first panel discussion. He underlined how much surgical volume of individual surgeons and hospitals mattered, but he also indicated that restrictive credentialing strategies might lead to unintended consequences.
Importantly, the SGS Women’s Council held a panel on the “Impact of Texas legislation on the physician/patient relationship” to provide a platform for members who had mixed feelings about attending this meeting in Texas.
The SGS meeting also included several popular postgraduate courses on multidisciplinary management of Müllerian anomalies, pelvic fistula treatment, surgical simulation, management modalities for uterine fibroids, and medical innovation and entrepreneurship. In this special section and in the next issue of OBG M
How to teach vaginal surgery through simulation
Vaginal surgery, including vaginal hysterectomy, is slowly becoming a dying art. According to the National Inpatient Sample and the Nationwide Ambulatory Surgery Sample from 2018, only 11.8% of all hysterectomies were performed vaginally.1 The combination of uterine-sparing surgeries, advances in conservative therapies for benign uterine conditions, and the diversification of minimally invasive routes (laparoscopic and robotic) has resulted in a continued downtrend in vaginal surgical volumes. This shift has led to fewer operative learning opportunities and declining graduating resident surgical volume.2 According to the Accreditation Council for Graduate Medical Education (ACGME), the minimum number of vaginal hysterectomies is 15, which represents only the minimum accepted exposure and does not imply competency.
In response, surgical simulation has been used for skill acquisition and maintenance outside of the operating room in a learning environment that is safe for the learners and does not expose patients to additional risk. Educators are uniquely poised to use simulation to teach residents and to evaluate their procedural competency. Although vaginal surgery, specifically vaginal hysterectomy, continues to decline, it can be resuscitated with the assistance of surgical simulation.
In this article, we provide a broad overview of vaginal surgical simulation. We discuss the basic tenets of simulation, review how to teach and evaluate vaginal surgical skills, and present some of the commonly available vaginal surgery simulation models and their associated resources, cost, setup time, fidelity, and limitations.
Simulation principles relevant for vaginal hysterectomy simulation
Here, we review simulation-based learning principles that will help place specific simulation models into perspective.
One size does not fit all
Simulation, like many educational interventions, does not work via a “one-size-fits-all” approach. While the American College of Obstetricians and Gynecologists (ACOG) Simulations Working Group (SWG) has created a toolkit (available online at https://www.acog.org/education-and-events/simulations/about/curriculum) with many ready-to-use how-to simulation descriptions and lesson plans that cover common topics, what works in one setting may not work in another. The SWG created those modules to help educators save time and resources and to avoid reinventing the wheel for each simulation session. However, these simulations need to be adapted to the local needs of trainees and resources, such as faculty time, space, models, and funding.
Cost vs fidelity
It is important to distinguish between cost and fidelity. “Low cost” is often incorrectly used interchangeably with “low fidelity” when referring to models and simulations. The most basic principle of fidelity is that it is associated with situational realism that in turn, drives learning.3,4 For example, the term high fidelity does apply to a virtual reality robotic surgery simulator, which also is high cost. However, a low-cost beef tongue model of fourth-degree laceration5 is high fidelity, while more expensive commercial models are less realistic, which makes them high cost and low fidelity.6 When selecting simulation models, educators need to consider cost based on their available resources and the level of fidelity needed for their learners.
Continue to: Task breakdown...
Task breakdown
As surgeon-educators, we love to teach! And while educators are passionate about imparting vaginal hysterectomy skills to the next generation of surgeons, it is important to assess where the learners are technically. Vaginal hysterectomy is a high-complexity procedure, with each step involving a unique skill set that is new to residents as learners; this is where the science of learning can help us teach more effectively.7 Focusing on doing the entire procedure all at once is more likely to result in cognitive overload, while a better approach is to break the procedure down into several components and practice those parts until goal proficiency is reached.
Deliberate practice
The idea of deliberate practice was popularized by Malcolm Gladwell in his book titled Outliers, in which he gives examples of how 10,000 hours of practice leads to mastery of complex skills. This concept was deepened by the work of cognitive psychologist Anders Ericsson, who emphasized that not only the duration but also the quality of practice—which involves concentration, analysis, and problem-solving—leads to the most effective training.8
In surgical education, this concept translates into many domains. For example, an individualized learning plan includes frequent low-stakes assessments, video recording for later viewing and analysis, surgical coaching, and detailed planning of future training sessions to incorporate past performance. “Just doing” surgery on a simulator (or in the operating room) results in missed learning opportunities.
Logistics and implementation: Who, where, when
The simulation “formula” takes into account multiple factors but should start with learning objectives and then an assessment of what resources are available to address them. For example, if one surgeon-educator and one resident-learner are available for 30 minutes in between cases in the operating room, and the goal is to teach the resident clamp-and-tie technique on pedicles, the “milk carton” model9 and a few instruments from the vaginal hysterectomy tray are ideal for this training. On the other hand, if it is important to achieve competency for an entire procedure prior to operating room debut and a group of surgeon-educators is available to share the time commitment of 2-hour sessions per each resident, then the PROMPT (PRactical Obstetric Multi-Professional Training) shoulder dystocia model could be used (TABLE).10-14
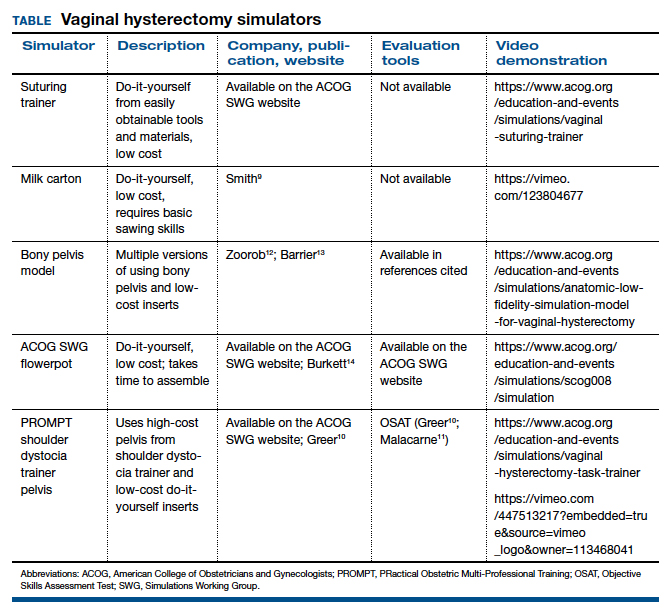
Learning curves
Ideally, educators would like to know how many simulated training sessions are needed for a learner to reach a proficiency level and become operating room ready. Such information about learning curves, unfortunately, is not available yet for vaginal hysterectomies. The first step in the process is to establish a baseline for performance to know a starting point, with assessment tools specific to each simulator; the next step is to study how many “takes” are needed for learners to move through their learning curve.15 The use of assessment tools can help assess each learner’s progression.
Continue to: Evaluation, assessment, and feedback...
Evaluation, assessment, and feedback
With more emphasis being placed on patient safety and transparency in every aspect of health care, including surgical training, graduate medical education leaders increasingly highlight the importance of objective assessment tools and outcome-based standards for certification of competency in surgery.16,17 Commonly used assessment tools that have reliability and validity evidence include surgical checklists and global rating scales. Checklists for common gynecologic procedures, including vaginal hysterectomy, as well as a global rating scale specifically developed for vaginal surgery (Vaginal Surgical Skills Index, VSSI)18 are accessible on the ACOG Simulations Working Group Surgical Curriculum in Obstetrics and Gynecology website.19
While checklists contain the main steps of each procedure, these lists do not assess for how well each step of the procedure is performed. By contrast, global rating scales, such as the VSSI, can discriminate between surgeons with different skill levels both in the simulation and operating room settings; each metric within the global rating scale (for example, time and motion) does not pertain to the performance of a procedure’s specific step but rather to the overall performance of the entire procedure.18,20 Hence, to provide detailed feedback, especially for formative assessment, both checklists and global rating scales often are used together.
Although standardized, checklists and global rating scales ultimately are still subjective (not objective) assessment tools. Recently, more attention has been to use surgical data science, particularly artificial intelligence methods, to objectively assess surgical performance by analyzing data generated during the performance of surgery, such as instrumental motion and video.21 These methods have been applied to a wide range of surgical techniques, including open, laparoscopic, robotic, endoscopic, and microsurgical approaches. Most of these types of studies have used assessment of surgical skill as the main outcome, with fewer studies correlating skill with clinically relevant metrics, such as patient outcomes.22-25 Although this is an area of active research, these methods are still being developed, and their validity and utility are not well established. For now, educators should continue to use validated checklists and global rating skills to help assess any type of surgical performance, particularly vaginal surgery.
Vaginal surgical simulation models
Vaginal surgery requires a surgeon to operate in a narrow, deep space. This requires ambidexterity, accurate depth perception, understanding of how to handle tissues, and use of movements that are efficient, fluid, and rhythmic. Multiple proposed simulation models are relevant to vaginal surgery, and these vary based on level of fidelity, cost, feasibility, ability to maintain standardization, ease of construction (if required), and generalizability to all of pelvic surgery (that is, procedure specific vs basic skills focused).10,11,13,26-31
Below, we describe various simulation models that are available for teaching vaginal surgical skills.
Vaginal hysterectomy simulation model
One commercially available simulation model for vaginal hysterectomy (as well as other vaginal surgical procedures, such as midurethral sling and anterior and posterior colporrhaphy) is the Miya Model (Miyazaki Enterprises) (FIGURE 1) and its accompanying MiyaMODEL App. In a multi-institutional study funded by the National Institutes of Health (NIH), the Miya Model, when used with the VSSI, was shown to be a valid assessment tool in terms of ability to differentiate a competent from a noncompetent surgeon.20 Currently, an ongoing NIH-sponsored multi-institutional study is assessing the Miya Model as a teaching tool and whether skills acquired on the Miya Model are transferable to the operating room.
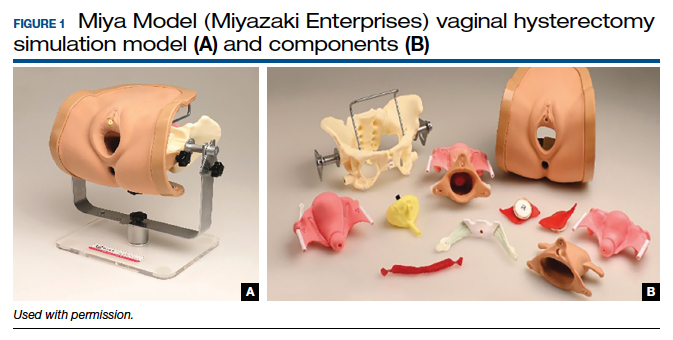
Continue to: Low-cost vaginal hysterectomy models...
Low-cost vaginal hysterectomy models
Multiple low-cost vaginal hysterectomy simulation models are described. Two models developed many years ago include the ACOG SWG flowerpot model14 and the PROMPT shoulder dystocia pelvic trainer model.10,11,14 The former model is low cost as it can be constructed from easily obtained household materials, but its downside is that it takes time and effort to obtain the materials and to assemble them. The latter model is faster to assemble but requires one to use a PROMPT pelvis for shoulder dystocia training, which has a considerable upfront cost. However, it is available in most hospitals with considerable obstetrical volume, and it allows for the most realistic perineum, which is helpful in recreating the feel of vaginal surgery, including retraction and exposure.
Many models created and described in the literature are variations of the models mentioned above, and many use commercially available low-cost bony pelvis models and polyvinyl chloride (PVC) pipes as a foundation for the soft tissue inserts to attach.12,13,31-33 Each model varies on what it “teaches best” regarding realism—for example, teaching anatomy, working in a tight space, dissection, or clamp placement and suture ligature.
Furthermore, since vaginal hysterectomy is a high-complexity procedure in terms of skills (working in confined space, limited view, “upside-down” anatomy, and need to direct assistants for retraction and exposure), task breakdown is important for simulation learning, as it is not efficient to repeat the entire procedure until proficiency is reached. Two trainers have been described to address that need: the milk carton and the vaginal suturing trainer. The latter allows learners to practice clamp placement and pedicle ligation multiple times, including in confined space (FIGURE 2), and the former allows them to do the same in a procedural matter as the clamp placement moves caudad to cephalad during the procedure (FIGURE 3).
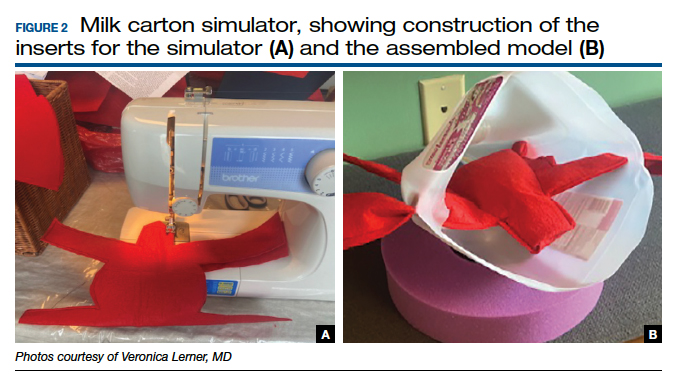

Native tissue pelvic floor surgery simulation
While there are few publications regarding surgical simulation models for native tissue pelvic floor surgeries, a low-cost anterior and posterior repair model was developed for the ACOG SWG Simulation Toolkit and published online in 2017, after their peer-review process. The fidelity is moderate for this low-cost model, which costs less than $5 per use. The simulation model requires a new vaginal insert for each learner, which is fast and easy to make and requires only a few components; however, the bony pelvis (for example, the flowerpot model) needs to be purchased or created. The stage of the anterior wall prolapse can be adjusted by the amount of fluid placed in the balloon, which is used to simulate the bladder. The more fluid that is placed in the “bladder,” the more severe the anterior wall prolapse appears. The vaginal caliber can be adjusted, if needed, by increasing or decreasing the size of the components to create the vagina, but the suggested sizes simulate a significantly widened vaginal caliber that would benefit from a posterior repair with perineorrhaphy. Although there is no validity evidence for this model, a skills assessment is available through the ACOG Simulation Surgical Curriculum. Of note, native tissue colpopexy repairs are also possible with this model (or another high-fidelity model, such as the Miya Model), if the sacrospinous ligaments and/or uterosacral ligaments are available on the pelvic model in use. This model’s limitations include the absence of a high-fidelity plane of dissection of the vaginal muscularis, and that no bleeding is encountered, which is the case for many low-cost models.19,34
Fundamentals of Vaginal Surgery (FVS) basic surgical skills simulation
The FVS simulation system, consisting of a task trainer paired with 6 selected surgical tasks, was developed to teach basic skills used in vaginal surgery.35 The FVS task trainer is 3D printed and has 3 main components: a base piece that allows for different surgical materials to be secured, a depth extender, and a width reducer. In addition, it has a mobile phone mount and a window into the system to enable video capture of skills exercises.
The FVS simulator is designed to enable 6 surgical tasks, including one-handed knot tying, two-handed knot tying, running suturing, plication suturing, Heaney transfixion pedicle ligation, and free pedicle ligation (FIGURE 4). In a pilot study, the FVS simulation system was deemed representative of the intended surgical field, useful for inclusion in a training program, and favored as a tool for both training and testing. Additionally, an initial proficiency score of 400 was set, which discriminated between novice and expert surgeons.35
An advantage of this simulation system is that it allows learners to focus on basic skills, rather than on an entire specific procedure. Further, the system is standardized, as it is commercially manufactured; this also allows for easy assembly. The disadvantage of this model is that it cannot be modified to teach specific vaginal procedures, and it must be purchased, rather than constructed on site. Further studies are needed to create generalizable proficiency scores and to assess its use in training and testing. For more information on the FVS simulation model, visit the Arbor Simulation website (http://arborsim.com).
Surgical simulation’s important role
Surgical skills can be learned and improved in the simulation setting in a low-stakes, low-pressure environment. Simulation can enable basic skills development and then higher-level learning of complex procedures. Skill assessment is important to aid in learning (via formative assessments) and for examination or certification (summative assessments).
With decreasing vaginal surgical volumes occurring nationally, it is becoming even more important to use surgical simulation to teach and maintain vaginal surgical skills. In this article, we reviewed various different simulation models that can be used for developing vaginal surgical skills and presented the advantages, limitations, and resources relevant for each simulation model. ●
- Wright JD, Huang Y, Li AH, et al. Nationwide estimates of annual inpatient and outpatient hysterectomies performed in the United States. Obstet Gynecol. 2022;139:446-448.
- Gressel GM, Potts JR 3rd, Cha S, et al. Hysterectomy route and numbers reported by graduating residents in obstetrics and gynecology training programs. Obstet Gynecol. 2020;135:268-273.
- Lioce L, ed. Healthcare Simulation Dictionary. 2nd ed. Rockville, MD; Agency for Healthcare Research and Quality: 2020. AHRQ Publication No. 20-0019.
- Norman G, Dore K, Grierson L. The minimal relationship between simulation fidelity and transfer of learning. Med Educ. 2012;46:636-647.
- Illston JD, Ballard AC, Ellington DR, et al. Modified beef tongue model for fourth-degree laceration repair simulation. Obstet Gynecol. 2017;129:491-496.
- WorldPoint website. 3B Scientific Episiotomy and Suturing Trainer. https://www.worldpoint.com/3b-episiotomy-and-suturing-sim. Accessed April 20, 2022.
- Balafoutas D, Joukhadar R, Kiesel M, et al. The role of deconstructive teaching in the training of laparoscopy. JSLS. 2019;23:e2019.00020.
- Ericsson KA, Harwell KW. Deliberate practice and proposed limits on the effects of practice on the acquisition of expert performance: why the original definition matters and recommendations for future research. Front Psychol. 2019;10:2396.
- Smith TM, Fenner DE. Vaginal hysterectomy teaching model—an educational video. Female Pelvic Med Reconstr Surg. 2012;18:S43. Abstract.
- Greer JA, Segal S, Salva CR, et al. Development and validation of simulation training for vaginal hysterectomy. J Minim Invasive Gynecol. 2014;21:74-82.
- Malacarne DR, Escobar CM, Lam CJ, et al. Teaching vaginal hysterectomy via simulation: creation and validation of the objective skills assessment tool for simulated vaginal hysterectomy on a task trainer and performance among different levels of trainees. Female Pelvic Med Reconstr Surg. 2019;25:298-304.
- Zoorob D, Frenn R, Moffitt M, et al. Multi-institutional validation of a vaginal hysterectomy simulation model for resident training. J Minim Invasive Gynecol. 2021;28:1490-1496.e1.
- Barrier BF, Thompson AB, McCullough MW, et al. A novel and inexpensive vaginal hysterectomy simulator. Simul Healthc. 2012;7:374-379.
- Burkett LS, Makin J, Ackenbom M, et al. Validation of transvaginal hysterectomy surgical model—modification of the flowerpot model to improve vesicovaginal plane simulation. J Minim Invasive Gynecol. 2021;28:1526-1530.
- Escobar C, Malacarne Pape D, Ferrante KL, et al. Who should be teaching vaginal hysterectomy on a task trainer? A multicenter randomized trial of peer versus expert coaching. J Surg Simul. 2020;7:63-72.
- The obstetrics and gynecology milestone project. J Grad Med Educ. 2014;6(1 suppl 1):129-143.
- Nasca TJ, Philibert I, Brigham T, et al. The next GME accreditation system—rationale and benefits. N Engl J Med. 2012;366:1051-1056.
- Chen CCG, Korn A, Klingele C, et al. Objective assessment of vaginal surgical skills. Am J Obstet Gynecol. 2010;203:79.e1-8.
- American College of Obstetricians and Gynecologists. Surgical curriculum in obstetrics and gynecology. https://www.acog.org /education-and-events/simulations/surgical-curriculum-in-ob-gyn.
- Chen CCG, Lockrow EG, DeStephano CC, et al. Establishing validity for a vaginal hysterectomy simulation model for surgical skills assessment. Obstet Gynecol. 2020;136:942-949.
- Vedula SS, Hager GD. Surgical data science: the new knowledge domain. Innov Surg Sci. 2017;2:109-121.
- Witthaus MW, Farooq S, Melnyk R, et al. Incorporation and validation of clinically relevant performance metrics of simulation (CRPMS) into a novel full-immersion simulation platform for nerve-sparing robot-assisted radical prostatectomy (NS-RARP) utilizing three-dimensional printing and hydrogel casting technology. BJU Int. 2020;125:322-332.
- Vedula SS, Malpani A, Ahmidi N, et al. Task-level vs segment-level quantitative metrics for surgical skill assessment. J Surg Educ. 2016;73:482-489.
- Maier-Hein L, Eisenmann M, Sarikaya D, et al. Surgical data science—from concepts toward clinical translation. Med Image Anal. 2022;76:102306.
- Hung AJ, Chen J, Gill IS. Automated performance metrics and machine learning algorithms to measure surgeon performance and anticipate clinical outcomes in robotic surgery. JAMA Surg. 2018;153:770-771.
- Altman K, Chen G, Chou B, et al. Surgical curriculum in obstetrics and gynecology: vaginal hysterectomy simulation. https://cfweb.acog. org/scog/scog008/Simulation.cfm.
- DeLancey JOL. Basic Exercises: Surgical Technique. Davis + Geck; Brooklyn, NY: 1987.
- Geoffrion R, Suen MW, Koenig NA, et al. Teaching vaginal surgery to junior residents: initial validation of 3 novel procedure-specific low-fidelity models. J Surg Educ. 2016;73:157-161.
- Pandey VA, Wolfe JHN, Lindhal AK, et al. Validity of an exam assessment in surgical skill: EBSQ-VASC pilot study. Eur J Vasc Endovasc Surg. 2004;27:341-348.
- Limbs&Things website. Knot Tying Trainer. https://limbsandthings. com/us/products/50050/50050-knot-tying-trainer. Accessed April 20, 2022.
- Vaughan MH, Kim-Fine S, Hullfish KL, et al. Validation of the simulated vaginal hysterectomy trainer. J Minim Invasive Gynecol. 2018;25:1101-1106.
- Braun K, Henley B, Ray C, et al. Teaching vaginal hysterectomy: low fidelity trainer provides effective simulation at low cost. Obstet Gynecol. 2017;130:44S.
- Anand M, Duffy CP, Vragovic O, et al. Surgical anatomy of vaginal hysterectomy—impact of a resident-constructed simulation model. Female Pelvic Med Reconstr Surg. 2018;24:176-182.
- Chen CC, Vaccaro CM. ACOG Simulation Consortium Surgical Curriculum: anterior and posterior repair. 2017. https://cfweb.acog. org/scog/.
- Schmidt PC, Fairchild PS, Fenner DE, et al. The Fundamentals of Vaginal Surgery pilot study: developing, validating, and setting proficiency scores for a vaginal surgical skills simulation system. Am J Obstet Gynecol. 2021;225:558.e1-558.e11.
Vaginal surgery, including vaginal hysterectomy, is slowly becoming a dying art. According to the National Inpatient Sample and the Nationwide Ambulatory Surgery Sample from 2018, only 11.8% of all hysterectomies were performed vaginally.1 The combination of uterine-sparing surgeries, advances in conservative therapies for benign uterine conditions, and the diversification of minimally invasive routes (laparoscopic and robotic) has resulted in a continued downtrend in vaginal surgical volumes. This shift has led to fewer operative learning opportunities and declining graduating resident surgical volume.2 According to the Accreditation Council for Graduate Medical Education (ACGME), the minimum number of vaginal hysterectomies is 15, which represents only the minimum accepted exposure and does not imply competency.
In response, surgical simulation has been used for skill acquisition and maintenance outside of the operating room in a learning environment that is safe for the learners and does not expose patients to additional risk. Educators are uniquely poised to use simulation to teach residents and to evaluate their procedural competency. Although vaginal surgery, specifically vaginal hysterectomy, continues to decline, it can be resuscitated with the assistance of surgical simulation.
In this article, we provide a broad overview of vaginal surgical simulation. We discuss the basic tenets of simulation, review how to teach and evaluate vaginal surgical skills, and present some of the commonly available vaginal surgery simulation models and their associated resources, cost, setup time, fidelity, and limitations.
Simulation principles relevant for vaginal hysterectomy simulation
Here, we review simulation-based learning principles that will help place specific simulation models into perspective.
One size does not fit all
Simulation, like many educational interventions, does not work via a “one-size-fits-all” approach. While the American College of Obstetricians and Gynecologists (ACOG) Simulations Working Group (SWG) has created a toolkit (available online at https://www.acog.org/education-and-events/simulations/about/curriculum) with many ready-to-use how-to simulation descriptions and lesson plans that cover common topics, what works in one setting may not work in another. The SWG created those modules to help educators save time and resources and to avoid reinventing the wheel for each simulation session. However, these simulations need to be adapted to the local needs of trainees and resources, such as faculty time, space, models, and funding.
Cost vs fidelity
It is important to distinguish between cost and fidelity. “Low cost” is often incorrectly used interchangeably with “low fidelity” when referring to models and simulations. The most basic principle of fidelity is that it is associated with situational realism that in turn, drives learning.3,4 For example, the term high fidelity does apply to a virtual reality robotic surgery simulator, which also is high cost. However, a low-cost beef tongue model of fourth-degree laceration5 is high fidelity, while more expensive commercial models are less realistic, which makes them high cost and low fidelity.6 When selecting simulation models, educators need to consider cost based on their available resources and the level of fidelity needed for their learners.
Continue to: Task breakdown...
Task breakdown
As surgeon-educators, we love to teach! And while educators are passionate about imparting vaginal hysterectomy skills to the next generation of surgeons, it is important to assess where the learners are technically. Vaginal hysterectomy is a high-complexity procedure, with each step involving a unique skill set that is new to residents as learners; this is where the science of learning can help us teach more effectively.7 Focusing on doing the entire procedure all at once is more likely to result in cognitive overload, while a better approach is to break the procedure down into several components and practice those parts until goal proficiency is reached.
Deliberate practice
The idea of deliberate practice was popularized by Malcolm Gladwell in his book titled Outliers, in which he gives examples of how 10,000 hours of practice leads to mastery of complex skills. This concept was deepened by the work of cognitive psychologist Anders Ericsson, who emphasized that not only the duration but also the quality of practice—which involves concentration, analysis, and problem-solving—leads to the most effective training.8
In surgical education, this concept translates into many domains. For example, an individualized learning plan includes frequent low-stakes assessments, video recording for later viewing and analysis, surgical coaching, and detailed planning of future training sessions to incorporate past performance. “Just doing” surgery on a simulator (or in the operating room) results in missed learning opportunities.
Logistics and implementation: Who, where, when
The simulation “formula” takes into account multiple factors but should start with learning objectives and then an assessment of what resources are available to address them. For example, if one surgeon-educator and one resident-learner are available for 30 minutes in between cases in the operating room, and the goal is to teach the resident clamp-and-tie technique on pedicles, the “milk carton” model9 and a few instruments from the vaginal hysterectomy tray are ideal for this training. On the other hand, if it is important to achieve competency for an entire procedure prior to operating room debut and a group of surgeon-educators is available to share the time commitment of 2-hour sessions per each resident, then the PROMPT (PRactical Obstetric Multi-Professional Training) shoulder dystocia model could be used (TABLE).10-14

Learning curves
Ideally, educators would like to know how many simulated training sessions are needed for a learner to reach a proficiency level and become operating room ready. Such information about learning curves, unfortunately, is not available yet for vaginal hysterectomies. The first step in the process is to establish a baseline for performance to know a starting point, with assessment tools specific to each simulator; the next step is to study how many “takes” are needed for learners to move through their learning curve.15 The use of assessment tools can help assess each learner’s progression.
Continue to: Evaluation, assessment, and feedback...
Evaluation, assessment, and feedback
With more emphasis being placed on patient safety and transparency in every aspect of health care, including surgical training, graduate medical education leaders increasingly highlight the importance of objective assessment tools and outcome-based standards for certification of competency in surgery.16,17 Commonly used assessment tools that have reliability and validity evidence include surgical checklists and global rating scales. Checklists for common gynecologic procedures, including vaginal hysterectomy, as well as a global rating scale specifically developed for vaginal surgery (Vaginal Surgical Skills Index, VSSI)18 are accessible on the ACOG Simulations Working Group Surgical Curriculum in Obstetrics and Gynecology website.19
While checklists contain the main steps of each procedure, these lists do not assess for how well each step of the procedure is performed. By contrast, global rating scales, such as the VSSI, can discriminate between surgeons with different skill levels both in the simulation and operating room settings; each metric within the global rating scale (for example, time and motion) does not pertain to the performance of a procedure’s specific step but rather to the overall performance of the entire procedure.18,20 Hence, to provide detailed feedback, especially for formative assessment, both checklists and global rating scales often are used together.
Although standardized, checklists and global rating scales ultimately are still subjective (not objective) assessment tools. Recently, more attention has been to use surgical data science, particularly artificial intelligence methods, to objectively assess surgical performance by analyzing data generated during the performance of surgery, such as instrumental motion and video.21 These methods have been applied to a wide range of surgical techniques, including open, laparoscopic, robotic, endoscopic, and microsurgical approaches. Most of these types of studies have used assessment of surgical skill as the main outcome, with fewer studies correlating skill with clinically relevant metrics, such as patient outcomes.22-25 Although this is an area of active research, these methods are still being developed, and their validity and utility are not well established. For now, educators should continue to use validated checklists and global rating skills to help assess any type of surgical performance, particularly vaginal surgery.
Vaginal surgical simulation models
Vaginal surgery requires a surgeon to operate in a narrow, deep space. This requires ambidexterity, accurate depth perception, understanding of how to handle tissues, and use of movements that are efficient, fluid, and rhythmic. Multiple proposed simulation models are relevant to vaginal surgery, and these vary based on level of fidelity, cost, feasibility, ability to maintain standardization, ease of construction (if required), and generalizability to all of pelvic surgery (that is, procedure specific vs basic skills focused).10,11,13,26-31
Below, we describe various simulation models that are available for teaching vaginal surgical skills.
Vaginal hysterectomy simulation model
One commercially available simulation model for vaginal hysterectomy (as well as other vaginal surgical procedures, such as midurethral sling and anterior and posterior colporrhaphy) is the Miya Model (Miyazaki Enterprises) (FIGURE 1) and its accompanying MiyaMODEL App. In a multi-institutional study funded by the National Institutes of Health (NIH), the Miya Model, when used with the VSSI, was shown to be a valid assessment tool in terms of ability to differentiate a competent from a noncompetent surgeon.20 Currently, an ongoing NIH-sponsored multi-institutional study is assessing the Miya Model as a teaching tool and whether skills acquired on the Miya Model are transferable to the operating room.

Continue to: Low-cost vaginal hysterectomy models...
Low-cost vaginal hysterectomy models
Multiple low-cost vaginal hysterectomy simulation models are described. Two models developed many years ago include the ACOG SWG flowerpot model14 and the PROMPT shoulder dystocia pelvic trainer model.10,11,14 The former model is low cost as it can be constructed from easily obtained household materials, but its downside is that it takes time and effort to obtain the materials and to assemble them. The latter model is faster to assemble but requires one to use a PROMPT pelvis for shoulder dystocia training, which has a considerable upfront cost. However, it is available in most hospitals with considerable obstetrical volume, and it allows for the most realistic perineum, which is helpful in recreating the feel of vaginal surgery, including retraction and exposure.
Many models created and described in the literature are variations of the models mentioned above, and many use commercially available low-cost bony pelvis models and polyvinyl chloride (PVC) pipes as a foundation for the soft tissue inserts to attach.12,13,31-33 Each model varies on what it “teaches best” regarding realism—for example, teaching anatomy, working in a tight space, dissection, or clamp placement and suture ligature.
Furthermore, since vaginal hysterectomy is a high-complexity procedure in terms of skills (working in confined space, limited view, “upside-down” anatomy, and need to direct assistants for retraction and exposure), task breakdown is important for simulation learning, as it is not efficient to repeat the entire procedure until proficiency is reached. Two trainers have been described to address that need: the milk carton and the vaginal suturing trainer. The latter allows learners to practice clamp placement and pedicle ligation multiple times, including in confined space (FIGURE 2), and the former allows them to do the same in a procedural matter as the clamp placement moves caudad to cephalad during the procedure (FIGURE 3).


Native tissue pelvic floor surgery simulation
While there are few publications regarding surgical simulation models for native tissue pelvic floor surgeries, a low-cost anterior and posterior repair model was developed for the ACOG SWG Simulation Toolkit and published online in 2017, after their peer-review process. The fidelity is moderate for this low-cost model, which costs less than $5 per use. The simulation model requires a new vaginal insert for each learner, which is fast and easy to make and requires only a few components; however, the bony pelvis (for example, the flowerpot model) needs to be purchased or created. The stage of the anterior wall prolapse can be adjusted by the amount of fluid placed in the balloon, which is used to simulate the bladder. The more fluid that is placed in the “bladder,” the more severe the anterior wall prolapse appears. The vaginal caliber can be adjusted, if needed, by increasing or decreasing the size of the components to create the vagina, but the suggested sizes simulate a significantly widened vaginal caliber that would benefit from a posterior repair with perineorrhaphy. Although there is no validity evidence for this model, a skills assessment is available through the ACOG Simulation Surgical Curriculum. Of note, native tissue colpopexy repairs are also possible with this model (or another high-fidelity model, such as the Miya Model), if the sacrospinous ligaments and/or uterosacral ligaments are available on the pelvic model in use. This model’s limitations include the absence of a high-fidelity plane of dissection of the vaginal muscularis, and that no bleeding is encountered, which is the case for many low-cost models.19,34
Fundamentals of Vaginal Surgery (FVS) basic surgical skills simulation
The FVS simulation system, consisting of a task trainer paired with 6 selected surgical tasks, was developed to teach basic skills used in vaginal surgery.35 The FVS task trainer is 3D printed and has 3 main components: a base piece that allows for different surgical materials to be secured, a depth extender, and a width reducer. In addition, it has a mobile phone mount and a window into the system to enable video capture of skills exercises.
The FVS simulator is designed to enable 6 surgical tasks, including one-handed knot tying, two-handed knot tying, running suturing, plication suturing, Heaney transfixion pedicle ligation, and free pedicle ligation (FIGURE 4). In a pilot study, the FVS simulation system was deemed representative of the intended surgical field, useful for inclusion in a training program, and favored as a tool for both training and testing. Additionally, an initial proficiency score of 400 was set, which discriminated between novice and expert surgeons.35
An advantage of this simulation system is that it allows learners to focus on basic skills, rather than on an entire specific procedure. Further, the system is standardized, as it is commercially manufactured; this also allows for easy assembly. The disadvantage of this model is that it cannot be modified to teach specific vaginal procedures, and it must be purchased, rather than constructed on site. Further studies are needed to create generalizable proficiency scores and to assess its use in training and testing. For more information on the FVS simulation model, visit the Arbor Simulation website (http://arborsim.com).
Surgical simulation’s important role
Surgical skills can be learned and improved in the simulation setting in a low-stakes, low-pressure environment. Simulation can enable basic skills development and then higher-level learning of complex procedures. Skill assessment is important to aid in learning (via formative assessments) and for examination or certification (summative assessments).
With decreasing vaginal surgical volumes occurring nationally, it is becoming even more important to use surgical simulation to teach and maintain vaginal surgical skills. In this article, we reviewed various different simulation models that can be used for developing vaginal surgical skills and presented the advantages, limitations, and resources relevant for each simulation model. ●
Vaginal surgery, including vaginal hysterectomy, is slowly becoming a dying art. According to the National Inpatient Sample and the Nationwide Ambulatory Surgery Sample from 2018, only 11.8% of all hysterectomies were performed vaginally.1 The combination of uterine-sparing surgeries, advances in conservative therapies for benign uterine conditions, and the diversification of minimally invasive routes (laparoscopic and robotic) has resulted in a continued downtrend in vaginal surgical volumes. This shift has led to fewer operative learning opportunities and declining graduating resident surgical volume.2 According to the Accreditation Council for Graduate Medical Education (ACGME), the minimum number of vaginal hysterectomies is 15, which represents only the minimum accepted exposure and does not imply competency.
In response, surgical simulation has been used for skill acquisition and maintenance outside of the operating room in a learning environment that is safe for the learners and does not expose patients to additional risk. Educators are uniquely poised to use simulation to teach residents and to evaluate their procedural competency. Although vaginal surgery, specifically vaginal hysterectomy, continues to decline, it can be resuscitated with the assistance of surgical simulation.
In this article, we provide a broad overview of vaginal surgical simulation. We discuss the basic tenets of simulation, review how to teach and evaluate vaginal surgical skills, and present some of the commonly available vaginal surgery simulation models and their associated resources, cost, setup time, fidelity, and limitations.
Simulation principles relevant for vaginal hysterectomy simulation
Here, we review simulation-based learning principles that will help place specific simulation models into perspective.
One size does not fit all
Simulation, like many educational interventions, does not work via a “one-size-fits-all” approach. While the American College of Obstetricians and Gynecologists (ACOG) Simulations Working Group (SWG) has created a toolkit (available online at https://www.acog.org/education-and-events/simulations/about/curriculum) with many ready-to-use how-to simulation descriptions and lesson plans that cover common topics, what works in one setting may not work in another. The SWG created those modules to help educators save time and resources and to avoid reinventing the wheel for each simulation session. However, these simulations need to be adapted to the local needs of trainees and resources, such as faculty time, space, models, and funding.
Cost vs fidelity
It is important to distinguish between cost and fidelity. “Low cost” is often incorrectly used interchangeably with “low fidelity” when referring to models and simulations. The most basic principle of fidelity is that it is associated with situational realism that in turn, drives learning.3,4 For example, the term high fidelity does apply to a virtual reality robotic surgery simulator, which also is high cost. However, a low-cost beef tongue model of fourth-degree laceration5 is high fidelity, while more expensive commercial models are less realistic, which makes them high cost and low fidelity.6 When selecting simulation models, educators need to consider cost based on their available resources and the level of fidelity needed for their learners.
Continue to: Task breakdown...
Task breakdown
As surgeon-educators, we love to teach! And while educators are passionate about imparting vaginal hysterectomy skills to the next generation of surgeons, it is important to assess where the learners are technically. Vaginal hysterectomy is a high-complexity procedure, with each step involving a unique skill set that is new to residents as learners; this is where the science of learning can help us teach more effectively.7 Focusing on doing the entire procedure all at once is more likely to result in cognitive overload, while a better approach is to break the procedure down into several components and practice those parts until goal proficiency is reached.
Deliberate practice
The idea of deliberate practice was popularized by Malcolm Gladwell in his book titled Outliers, in which he gives examples of how 10,000 hours of practice leads to mastery of complex skills. This concept was deepened by the work of cognitive psychologist Anders Ericsson, who emphasized that not only the duration but also the quality of practice—which involves concentration, analysis, and problem-solving—leads to the most effective training.8
In surgical education, this concept translates into many domains. For example, an individualized learning plan includes frequent low-stakes assessments, video recording for later viewing and analysis, surgical coaching, and detailed planning of future training sessions to incorporate past performance. “Just doing” surgery on a simulator (or in the operating room) results in missed learning opportunities.
Logistics and implementation: Who, where, when
The simulation “formula” takes into account multiple factors but should start with learning objectives and then an assessment of what resources are available to address them. For example, if one surgeon-educator and one resident-learner are available for 30 minutes in between cases in the operating room, and the goal is to teach the resident clamp-and-tie technique on pedicles, the “milk carton” model9 and a few instruments from the vaginal hysterectomy tray are ideal for this training. On the other hand, if it is important to achieve competency for an entire procedure prior to operating room debut and a group of surgeon-educators is available to share the time commitment of 2-hour sessions per each resident, then the PROMPT (PRactical Obstetric Multi-Professional Training) shoulder dystocia model could be used (TABLE).10-14

Learning curves
Ideally, educators would like to know how many simulated training sessions are needed for a learner to reach a proficiency level and become operating room ready. Such information about learning curves, unfortunately, is not available yet for vaginal hysterectomies. The first step in the process is to establish a baseline for performance to know a starting point, with assessment tools specific to each simulator; the next step is to study how many “takes” are needed for learners to move through their learning curve.15 The use of assessment tools can help assess each learner’s progression.
Continue to: Evaluation, assessment, and feedback...
Evaluation, assessment, and feedback
With more emphasis being placed on patient safety and transparency in every aspect of health care, including surgical training, graduate medical education leaders increasingly highlight the importance of objective assessment tools and outcome-based standards for certification of competency in surgery.16,17 Commonly used assessment tools that have reliability and validity evidence include surgical checklists and global rating scales. Checklists for common gynecologic procedures, including vaginal hysterectomy, as well as a global rating scale specifically developed for vaginal surgery (Vaginal Surgical Skills Index, VSSI)18 are accessible on the ACOG Simulations Working Group Surgical Curriculum in Obstetrics and Gynecology website.19
While checklists contain the main steps of each procedure, these lists do not assess for how well each step of the procedure is performed. By contrast, global rating scales, such as the VSSI, can discriminate between surgeons with different skill levels both in the simulation and operating room settings; each metric within the global rating scale (for example, time and motion) does not pertain to the performance of a procedure’s specific step but rather to the overall performance of the entire procedure.18,20 Hence, to provide detailed feedback, especially for formative assessment, both checklists and global rating scales often are used together.
Although standardized, checklists and global rating scales ultimately are still subjective (not objective) assessment tools. Recently, more attention has been to use surgical data science, particularly artificial intelligence methods, to objectively assess surgical performance by analyzing data generated during the performance of surgery, such as instrumental motion and video.21 These methods have been applied to a wide range of surgical techniques, including open, laparoscopic, robotic, endoscopic, and microsurgical approaches. Most of these types of studies have used assessment of surgical skill as the main outcome, with fewer studies correlating skill with clinically relevant metrics, such as patient outcomes.22-25 Although this is an area of active research, these methods are still being developed, and their validity and utility are not well established. For now, educators should continue to use validated checklists and global rating skills to help assess any type of surgical performance, particularly vaginal surgery.
Vaginal surgical simulation models
Vaginal surgery requires a surgeon to operate in a narrow, deep space. This requires ambidexterity, accurate depth perception, understanding of how to handle tissues, and use of movements that are efficient, fluid, and rhythmic. Multiple proposed simulation models are relevant to vaginal surgery, and these vary based on level of fidelity, cost, feasibility, ability to maintain standardization, ease of construction (if required), and generalizability to all of pelvic surgery (that is, procedure specific vs basic skills focused).10,11,13,26-31
Below, we describe various simulation models that are available for teaching vaginal surgical skills.
Vaginal hysterectomy simulation model
One commercially available simulation model for vaginal hysterectomy (as well as other vaginal surgical procedures, such as midurethral sling and anterior and posterior colporrhaphy) is the Miya Model (Miyazaki Enterprises) (FIGURE 1) and its accompanying MiyaMODEL App. In a multi-institutional study funded by the National Institutes of Health (NIH), the Miya Model, when used with the VSSI, was shown to be a valid assessment tool in terms of ability to differentiate a competent from a noncompetent surgeon.20 Currently, an ongoing NIH-sponsored multi-institutional study is assessing the Miya Model as a teaching tool and whether skills acquired on the Miya Model are transferable to the operating room.

Continue to: Low-cost vaginal hysterectomy models...
Low-cost vaginal hysterectomy models
Multiple low-cost vaginal hysterectomy simulation models are described. Two models developed many years ago include the ACOG SWG flowerpot model14 and the PROMPT shoulder dystocia pelvic trainer model.10,11,14 The former model is low cost as it can be constructed from easily obtained household materials, but its downside is that it takes time and effort to obtain the materials and to assemble them. The latter model is faster to assemble but requires one to use a PROMPT pelvis for shoulder dystocia training, which has a considerable upfront cost. However, it is available in most hospitals with considerable obstetrical volume, and it allows for the most realistic perineum, which is helpful in recreating the feel of vaginal surgery, including retraction and exposure.
Many models created and described in the literature are variations of the models mentioned above, and many use commercially available low-cost bony pelvis models and polyvinyl chloride (PVC) pipes as a foundation for the soft tissue inserts to attach.12,13,31-33 Each model varies on what it “teaches best” regarding realism—for example, teaching anatomy, working in a tight space, dissection, or clamp placement and suture ligature.
Furthermore, since vaginal hysterectomy is a high-complexity procedure in terms of skills (working in confined space, limited view, “upside-down” anatomy, and need to direct assistants for retraction and exposure), task breakdown is important for simulation learning, as it is not efficient to repeat the entire procedure until proficiency is reached. Two trainers have been described to address that need: the milk carton and the vaginal suturing trainer. The latter allows learners to practice clamp placement and pedicle ligation multiple times, including in confined space (FIGURE 2), and the former allows them to do the same in a procedural matter as the clamp placement moves caudad to cephalad during the procedure (FIGURE 3).


Native tissue pelvic floor surgery simulation
While there are few publications regarding surgical simulation models for native tissue pelvic floor surgeries, a low-cost anterior and posterior repair model was developed for the ACOG SWG Simulation Toolkit and published online in 2017, after their peer-review process. The fidelity is moderate for this low-cost model, which costs less than $5 per use. The simulation model requires a new vaginal insert for each learner, which is fast and easy to make and requires only a few components; however, the bony pelvis (for example, the flowerpot model) needs to be purchased or created. The stage of the anterior wall prolapse can be adjusted by the amount of fluid placed in the balloon, which is used to simulate the bladder. The more fluid that is placed in the “bladder,” the more severe the anterior wall prolapse appears. The vaginal caliber can be adjusted, if needed, by increasing or decreasing the size of the components to create the vagina, but the suggested sizes simulate a significantly widened vaginal caliber that would benefit from a posterior repair with perineorrhaphy. Although there is no validity evidence for this model, a skills assessment is available through the ACOG Simulation Surgical Curriculum. Of note, native tissue colpopexy repairs are also possible with this model (or another high-fidelity model, such as the Miya Model), if the sacrospinous ligaments and/or uterosacral ligaments are available on the pelvic model in use. This model’s limitations include the absence of a high-fidelity plane of dissection of the vaginal muscularis, and that no bleeding is encountered, which is the case for many low-cost models.19,34
Fundamentals of Vaginal Surgery (FVS) basic surgical skills simulation
The FVS simulation system, consisting of a task trainer paired with 6 selected surgical tasks, was developed to teach basic skills used in vaginal surgery.35 The FVS task trainer is 3D printed and has 3 main components: a base piece that allows for different surgical materials to be secured, a depth extender, and a width reducer. In addition, it has a mobile phone mount and a window into the system to enable video capture of skills exercises.
The FVS simulator is designed to enable 6 surgical tasks, including one-handed knot tying, two-handed knot tying, running suturing, plication suturing, Heaney transfixion pedicle ligation, and free pedicle ligation (FIGURE 4). In a pilot study, the FVS simulation system was deemed representative of the intended surgical field, useful for inclusion in a training program, and favored as a tool for both training and testing. Additionally, an initial proficiency score of 400 was set, which discriminated between novice and expert surgeons.35
An advantage of this simulation system is that it allows learners to focus on basic skills, rather than on an entire specific procedure. Further, the system is standardized, as it is commercially manufactured; this also allows for easy assembly. The disadvantage of this model is that it cannot be modified to teach specific vaginal procedures, and it must be purchased, rather than constructed on site. Further studies are needed to create generalizable proficiency scores and to assess its use in training and testing. For more information on the FVS simulation model, visit the Arbor Simulation website (http://arborsim.com).
Surgical simulation’s important role
Surgical skills can be learned and improved in the simulation setting in a low-stakes, low-pressure environment. Simulation can enable basic skills development and then higher-level learning of complex procedures. Skill assessment is important to aid in learning (via formative assessments) and for examination or certification (summative assessments).
With decreasing vaginal surgical volumes occurring nationally, it is becoming even more important to use surgical simulation to teach and maintain vaginal surgical skills. In this article, we reviewed various different simulation models that can be used for developing vaginal surgical skills and presented the advantages, limitations, and resources relevant for each simulation model. ●
- Wright JD, Huang Y, Li AH, et al. Nationwide estimates of annual inpatient and outpatient hysterectomies performed in the United States. Obstet Gynecol. 2022;139:446-448.
- Gressel GM, Potts JR 3rd, Cha S, et al. Hysterectomy route and numbers reported by graduating residents in obstetrics and gynecology training programs. Obstet Gynecol. 2020;135:268-273.
- Lioce L, ed. Healthcare Simulation Dictionary. 2nd ed. Rockville, MD; Agency for Healthcare Research and Quality: 2020. AHRQ Publication No. 20-0019.
- Norman G, Dore K, Grierson L. The minimal relationship between simulation fidelity and transfer of learning. Med Educ. 2012;46:636-647.
- Illston JD, Ballard AC, Ellington DR, et al. Modified beef tongue model for fourth-degree laceration repair simulation. Obstet Gynecol. 2017;129:491-496.
- WorldPoint website. 3B Scientific Episiotomy and Suturing Trainer. https://www.worldpoint.com/3b-episiotomy-and-suturing-sim. Accessed April 20, 2022.
- Balafoutas D, Joukhadar R, Kiesel M, et al. The role of deconstructive teaching in the training of laparoscopy. JSLS. 2019;23:e2019.00020.
- Ericsson KA, Harwell KW. Deliberate practice and proposed limits on the effects of practice on the acquisition of expert performance: why the original definition matters and recommendations for future research. Front Psychol. 2019;10:2396.
- Smith TM, Fenner DE. Vaginal hysterectomy teaching model—an educational video. Female Pelvic Med Reconstr Surg. 2012;18:S43. Abstract.
- Greer JA, Segal S, Salva CR, et al. Development and validation of simulation training for vaginal hysterectomy. J Minim Invasive Gynecol. 2014;21:74-82.
- Malacarne DR, Escobar CM, Lam CJ, et al. Teaching vaginal hysterectomy via simulation: creation and validation of the objective skills assessment tool for simulated vaginal hysterectomy on a task trainer and performance among different levels of trainees. Female Pelvic Med Reconstr Surg. 2019;25:298-304.
- Zoorob D, Frenn R, Moffitt M, et al. Multi-institutional validation of a vaginal hysterectomy simulation model for resident training. J Minim Invasive Gynecol. 2021;28:1490-1496.e1.
- Barrier BF, Thompson AB, McCullough MW, et al. A novel and inexpensive vaginal hysterectomy simulator. Simul Healthc. 2012;7:374-379.
- Burkett LS, Makin J, Ackenbom M, et al. Validation of transvaginal hysterectomy surgical model—modification of the flowerpot model to improve vesicovaginal plane simulation. J Minim Invasive Gynecol. 2021;28:1526-1530.
- Escobar C, Malacarne Pape D, Ferrante KL, et al. Who should be teaching vaginal hysterectomy on a task trainer? A multicenter randomized trial of peer versus expert coaching. J Surg Simul. 2020;7:63-72.
- The obstetrics and gynecology milestone project. J Grad Med Educ. 2014;6(1 suppl 1):129-143.
- Nasca TJ, Philibert I, Brigham T, et al. The next GME accreditation system—rationale and benefits. N Engl J Med. 2012;366:1051-1056.
- Chen CCG, Korn A, Klingele C, et al. Objective assessment of vaginal surgical skills. Am J Obstet Gynecol. 2010;203:79.e1-8.
- American College of Obstetricians and Gynecologists. Surgical curriculum in obstetrics and gynecology. https://www.acog.org /education-and-events/simulations/surgical-curriculum-in-ob-gyn.
- Chen CCG, Lockrow EG, DeStephano CC, et al. Establishing validity for a vaginal hysterectomy simulation model for surgical skills assessment. Obstet Gynecol. 2020;136:942-949.
- Vedula SS, Hager GD. Surgical data science: the new knowledge domain. Innov Surg Sci. 2017;2:109-121.
- Witthaus MW, Farooq S, Melnyk R, et al. Incorporation and validation of clinically relevant performance metrics of simulation (CRPMS) into a novel full-immersion simulation platform for nerve-sparing robot-assisted radical prostatectomy (NS-RARP) utilizing three-dimensional printing and hydrogel casting technology. BJU Int. 2020;125:322-332.
- Vedula SS, Malpani A, Ahmidi N, et al. Task-level vs segment-level quantitative metrics for surgical skill assessment. J Surg Educ. 2016;73:482-489.
- Maier-Hein L, Eisenmann M, Sarikaya D, et al. Surgical data science—from concepts toward clinical translation. Med Image Anal. 2022;76:102306.
- Hung AJ, Chen J, Gill IS. Automated performance metrics and machine learning algorithms to measure surgeon performance and anticipate clinical outcomes in robotic surgery. JAMA Surg. 2018;153:770-771.
- Altman K, Chen G, Chou B, et al. Surgical curriculum in obstetrics and gynecology: vaginal hysterectomy simulation. https://cfweb.acog. org/scog/scog008/Simulation.cfm.
- DeLancey JOL. Basic Exercises: Surgical Technique. Davis + Geck; Brooklyn, NY: 1987.
- Geoffrion R, Suen MW, Koenig NA, et al. Teaching vaginal surgery to junior residents: initial validation of 3 novel procedure-specific low-fidelity models. J Surg Educ. 2016;73:157-161.
- Pandey VA, Wolfe JHN, Lindhal AK, et al. Validity of an exam assessment in surgical skill: EBSQ-VASC pilot study. Eur J Vasc Endovasc Surg. 2004;27:341-348.
- Limbs&Things website. Knot Tying Trainer. https://limbsandthings. com/us/products/50050/50050-knot-tying-trainer. Accessed April 20, 2022.
- Vaughan MH, Kim-Fine S, Hullfish KL, et al. Validation of the simulated vaginal hysterectomy trainer. J Minim Invasive Gynecol. 2018;25:1101-1106.
- Braun K, Henley B, Ray C, et al. Teaching vaginal hysterectomy: low fidelity trainer provides effective simulation at low cost. Obstet Gynecol. 2017;130:44S.
- Anand M, Duffy CP, Vragovic O, et al. Surgical anatomy of vaginal hysterectomy—impact of a resident-constructed simulation model. Female Pelvic Med Reconstr Surg. 2018;24:176-182.
- Chen CC, Vaccaro CM. ACOG Simulation Consortium Surgical Curriculum: anterior and posterior repair. 2017. https://cfweb.acog. org/scog/.
- Schmidt PC, Fairchild PS, Fenner DE, et al. The Fundamentals of Vaginal Surgery pilot study: developing, validating, and setting proficiency scores for a vaginal surgical skills simulation system. Am J Obstet Gynecol. 2021;225:558.e1-558.e11.
- Wright JD, Huang Y, Li AH, et al. Nationwide estimates of annual inpatient and outpatient hysterectomies performed in the United States. Obstet Gynecol. 2022;139:446-448.
- Gressel GM, Potts JR 3rd, Cha S, et al. Hysterectomy route and numbers reported by graduating residents in obstetrics and gynecology training programs. Obstet Gynecol. 2020;135:268-273.
- Lioce L, ed. Healthcare Simulation Dictionary. 2nd ed. Rockville, MD; Agency for Healthcare Research and Quality: 2020. AHRQ Publication No. 20-0019.
- Norman G, Dore K, Grierson L. The minimal relationship between simulation fidelity and transfer of learning. Med Educ. 2012;46:636-647.
- Illston JD, Ballard AC, Ellington DR, et al. Modified beef tongue model for fourth-degree laceration repair simulation. Obstet Gynecol. 2017;129:491-496.
- WorldPoint website. 3B Scientific Episiotomy and Suturing Trainer. https://www.worldpoint.com/3b-episiotomy-and-suturing-sim. Accessed April 20, 2022.
- Balafoutas D, Joukhadar R, Kiesel M, et al. The role of deconstructive teaching in the training of laparoscopy. JSLS. 2019;23:e2019.00020.
- Ericsson KA, Harwell KW. Deliberate practice and proposed limits on the effects of practice on the acquisition of expert performance: why the original definition matters and recommendations for future research. Front Psychol. 2019;10:2396.
- Smith TM, Fenner DE. Vaginal hysterectomy teaching model—an educational video. Female Pelvic Med Reconstr Surg. 2012;18:S43. Abstract.
- Greer JA, Segal S, Salva CR, et al. Development and validation of simulation training for vaginal hysterectomy. J Minim Invasive Gynecol. 2014;21:74-82.
- Malacarne DR, Escobar CM, Lam CJ, et al. Teaching vaginal hysterectomy via simulation: creation and validation of the objective skills assessment tool for simulated vaginal hysterectomy on a task trainer and performance among different levels of trainees. Female Pelvic Med Reconstr Surg. 2019;25:298-304.
- Zoorob D, Frenn R, Moffitt M, et al. Multi-institutional validation of a vaginal hysterectomy simulation model for resident training. J Minim Invasive Gynecol. 2021;28:1490-1496.e1.
- Barrier BF, Thompson AB, McCullough MW, et al. A novel and inexpensive vaginal hysterectomy simulator. Simul Healthc. 2012;7:374-379.
- Burkett LS, Makin J, Ackenbom M, et al. Validation of transvaginal hysterectomy surgical model—modification of the flowerpot model to improve vesicovaginal plane simulation. J Minim Invasive Gynecol. 2021;28:1526-1530.
- Escobar C, Malacarne Pape D, Ferrante KL, et al. Who should be teaching vaginal hysterectomy on a task trainer? A multicenter randomized trial of peer versus expert coaching. J Surg Simul. 2020;7:63-72.
- The obstetrics and gynecology milestone project. J Grad Med Educ. 2014;6(1 suppl 1):129-143.
- Nasca TJ, Philibert I, Brigham T, et al. The next GME accreditation system—rationale and benefits. N Engl J Med. 2012;366:1051-1056.
- Chen CCG, Korn A, Klingele C, et al. Objective assessment of vaginal surgical skills. Am J Obstet Gynecol. 2010;203:79.e1-8.
- American College of Obstetricians and Gynecologists. Surgical curriculum in obstetrics and gynecology. https://www.acog.org /education-and-events/simulations/surgical-curriculum-in-ob-gyn.
- Chen CCG, Lockrow EG, DeStephano CC, et al. Establishing validity for a vaginal hysterectomy simulation model for surgical skills assessment. Obstet Gynecol. 2020;136:942-949.
- Vedula SS, Hager GD. Surgical data science: the new knowledge domain. Innov Surg Sci. 2017;2:109-121.
- Witthaus MW, Farooq S, Melnyk R, et al. Incorporation and validation of clinically relevant performance metrics of simulation (CRPMS) into a novel full-immersion simulation platform for nerve-sparing robot-assisted radical prostatectomy (NS-RARP) utilizing three-dimensional printing and hydrogel casting technology. BJU Int. 2020;125:322-332.
- Vedula SS, Malpani A, Ahmidi N, et al. Task-level vs segment-level quantitative metrics for surgical skill assessment. J Surg Educ. 2016;73:482-489.
- Maier-Hein L, Eisenmann M, Sarikaya D, et al. Surgical data science—from concepts toward clinical translation. Med Image Anal. 2022;76:102306.
- Hung AJ, Chen J, Gill IS. Automated performance metrics and machine learning algorithms to measure surgeon performance and anticipate clinical outcomes in robotic surgery. JAMA Surg. 2018;153:770-771.
- Altman K, Chen G, Chou B, et al. Surgical curriculum in obstetrics and gynecology: vaginal hysterectomy simulation. https://cfweb.acog. org/scog/scog008/Simulation.cfm.
- DeLancey JOL. Basic Exercises: Surgical Technique. Davis + Geck; Brooklyn, NY: 1987.
- Geoffrion R, Suen MW, Koenig NA, et al. Teaching vaginal surgery to junior residents: initial validation of 3 novel procedure-specific low-fidelity models. J Surg Educ. 2016;73:157-161.
- Pandey VA, Wolfe JHN, Lindhal AK, et al. Validity of an exam assessment in surgical skill: EBSQ-VASC pilot study. Eur J Vasc Endovasc Surg. 2004;27:341-348.
- Limbs&Things website. Knot Tying Trainer. https://limbsandthings. com/us/products/50050/50050-knot-tying-trainer. Accessed April 20, 2022.
- Vaughan MH, Kim-Fine S, Hullfish KL, et al. Validation of the simulated vaginal hysterectomy trainer. J Minim Invasive Gynecol. 2018;25:1101-1106.
- Braun K, Henley B, Ray C, et al. Teaching vaginal hysterectomy: low fidelity trainer provides effective simulation at low cost. Obstet Gynecol. 2017;130:44S.
- Anand M, Duffy CP, Vragovic O, et al. Surgical anatomy of vaginal hysterectomy—impact of a resident-constructed simulation model. Female Pelvic Med Reconstr Surg. 2018;24:176-182.
- Chen CC, Vaccaro CM. ACOG Simulation Consortium Surgical Curriculum: anterior and posterior repair. 2017. https://cfweb.acog. org/scog/.
- Schmidt PC, Fairchild PS, Fenner DE, et al. The Fundamentals of Vaginal Surgery pilot study: developing, validating, and setting proficiency scores for a vaginal surgical skills simulation system. Am J Obstet Gynecol. 2021;225:558.e1-558.e11.