User login
Unrecognized placenta accreta spectrum: Intraoperative management
CASE Concerning finding on repeat CD
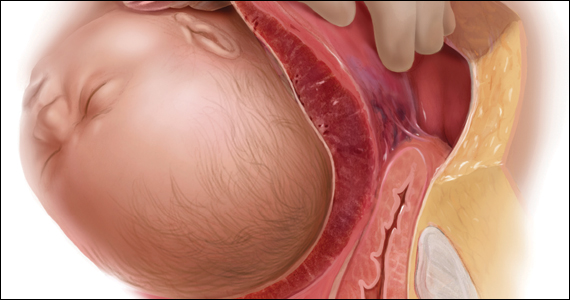
A 30-year-old woman with a history of 1 prior cesarean delivery (CD) presents to labor and delivery at 38 weeks of gestation with symptoms of mild cramping. Her prenatal care was uncomplicated. The covering team made a decision to proceed with a repeat CD. A Pfannenstiel incision is made to enter the abdomen, and inspection of the lower uterine segment is concerning for a placenta accreta spectrum (PAS) (FIGURE).
What would be your next steps?

Placenta accreta spectrum describes the range of disorders of placental implantation, including placenta accreta, increta, and percreta. PAS is a significant cause of severe maternal morbidity and mortality, primarily due to massive hemorrhage at the time of delivery. The incidence of PAS continues to rise along with the CD rate. The authors of a recent meta-analysis reported a pooled prevalence rate of 1 in 588 women.1 Notably, in women with PAS, the rate of hysterectomy is 52.2%, and the transfusion-dependent hemorrhage rate is 46.9%.1
Ideally, PAS should be diagnosed or at least suspected antenatally during prenatal ultrasonography, leading to delivery planning by a multidisciplinary team.2 The presence of a multidisciplinary team—in addition to the primary obstetric and surgical teams—composed of experienced anesthesiologists, a blood bank able to respond to massive transfusion needs, critical care specialists, and interventional radiologists is associated with improved outcomes.3-5
Occasionally, a patient is found to have an advanced PAS (increta or percreta) at the time of delivery. In these situations, it is paramount that the appropriate resources be assembled as expeditiously as possible to optimize maternal outcomes. Surgical management can be challenging even for experienced pelvic surgeons, and appropriate resuscitation cannot be provided by a single anesthesiologist working alone. A cavalier attitude of proceeding with the delivery “as usual” in the face of an unexpected PAS situation can lead to disastrous consequences, including maternal death.
In this article, we review the important steps to take when faced with the unexpected situation of a PAS at the time of CD.
Continue to: Stop and collect your multidisciplinary team...
Stop and collect your multidisciplinary team
Once the diagnosis of an advanced PAS is suspected, the first step is to stop and request the presence of your institution’s multidisciplinary surgical team. This team typically includes a maternal-fetal specialist or, if not available, an experienced obstetrician, and an expert pelvic surgeon, which varies by institution (gynecologic oncologist, trauma surgeon, urologist, urogynecologist, vascular surgeon). An interventional radiology team is an additional useful resource that can assist with the control of pelvic hemorrhage using embolization techniques.
In our opinion, it is not appropriate to have a surgical backup team available only as needed at a certain distance from the hospital or even in the building. Because of the acuity and magnitude of bleeding that can occur in a short time, the most appropriate approach is to have your surgical team scrubbed and ready to assist or take over the procedure immediately if indicated.
Additional support staff also may be required. A single circulating nurse may not be sufficient, and available nursing staff may need to be called. The surgical technician scrubbed on the case may be familiar only with uncomplicated CDs and can be overwhelmed during a PAS case. Having a more experienced surgical technologist can optimize the availability of the appropriate instruments for the surgical team.
If a multidisciplinary surgical team with PAS management expertise is not available at your institution and the patient is stable, it is appropriate to consider transferring her to the nearest center that can meet the high-risk needs of this situation.6
Prepare for resuscitation
While you are calling your multidisciplinary team members, implement plans for resuscitation by notifying the anesthesiologist about the PAS findings. This will allow the gathering of needed resources that may include calling on additional anesthesiologists with experience in high-risk obstetrics, trauma, or critical care.
Placing large-bore intravenous lines or a central line to allow rapid transfusion is essential. Strongly consider inserting an arterial line for hemodynamic monitoring and intraoperative blood draws to monitor blood loss, blood gases, electrolytes, and coagulation parameters, which can guide resuscitative efforts and replacement therapies.
Simultaneously, inform the blood bank to prepare blood and blood products for possible activation of a massive transfusion protocol. It is imperative to have the products available in the operating room (OR) prior to proceeding with the surgery. Our current practice is to have 10 units of packed red blood cells and fresh frozen plasma available in the OR for all our prenatally diagnosed electively planned PAS cases.
Optimize exposure of the surgical field
Appropriate exposure of the surgical field is essential and should include exposure of the uterine fundus and the pelvic sidewalls. The uterine incision should avoid the placenta; typically it is placed at the level of the uterine fundus. Exposure of the pelvic sidewalls is needed to open the retroperitoneum and identify the ureter and the iliac vessels.
Vertical extension of the fascial incision probably will be needed to achieve appropriate exposure. Although at times this can be done without a concomitant vertical skin incision, often an inverted T incision is required. Be mindful that PAS is a life-threatening condition and that aesthetics are not a priority. After extending the fascial incision, adequate exposure can be achieved with any of the commonly used retractors or wound protectors (depending on institutional availability and surgeon preference) or by the surgical assistants using body wall retractors.
We routinely place the patient in lithotomy position. This allows us to monitor for vaginal bleeding (often a site of unrecognized massive hemorrhage) during the surgery, facilitate retrograde bladder filling, and provide a vaginal access to the pelvis. In addition, the lithotomy position allows for cystoscopy and placement of ureteral stents, which can be performed before starting the surgery to help prevent urinary tract injuries or at the end of the procedure in case one is suspected.7
Continue to: Performing the hysterectomy...
Performing the hysterectomy
A complete review of all surgical techniques for managing PAS is beyond the scope of this article. However, we briefly cover important procedural steps and offer suggestions on how to minimize the risk of bleeding.
In our experience. The areas with the highest risk of massive bleeding that can be difficult to control include the pelvic sidewall when there is lateral extension of the PAS, the vesicouterine space, and placenta previa vaginally. Be mindful of these areas at risk and have a plan in place in case of bleeding.
Uterine incision
Avoid the placenta when making the uterine incision, which is typically done in the fundal part of the uterus. Cut and tie the cord and return it to the uterine cavity. Close the incision in a single layer. Use of a surgical stapler can be used for the hysterotomy and can decrease the amount of blood loss.8
Superior attachments of the uterus
The superior attachments of the uterus include the round ligament, the utero-ovarian ligament, and the fallopian tubes. With meticulous dissection, develop an avascular space underneath these structures and, in turn, individually divide and suture ligate; this is typically achieved with minimal blood loss.
In addition, isolate the engorged veins of the broad ligament and divide them in a similar fashion.
In our experience. Use of a vessel-sealing device can facilitate division of all the former structures. Simply excise the fallopian tubes with the vessel-sealing device either at this time or after the uterus is removed.
Pelvic sidewall
Once the superior attachments of the uterus have been divided, the next step involves exposing the pelvic sidewall structures, that is, the ureter and the pelvic vessels. Expose the ureter from the pelvic brim to the level of the uterine artery. The hypogastric artery is exposed as well in this process and the pararectal space developed.
When the PAS has extended laterally, perform stepwise division of the lateral attachments of the placenta to the pelvic sidewall using a combination of electrocautery, hemoclips, and the vessel-sealing device. In laterally extended PAS cases, it often is necessary to divide the uterine artery either at its origin or at the level of the ureter to allow for the completion of the separation of the placenta from the pelvic sidewall.
In our experience. During this lateral dissection, significant bleeding may be encountered from the neovascular network that has developed in the pelvic sidewall. The bleeding may be diffuse and difficult to control with the methods described above. In this situation, we have found that placing hemostatic agents in this area and packing the sidewall with laparotomy pads can achieve hemostasis in most cases, thus allowing the surgery to proceed.
1. Stop and collect your multidisciplinary team. If required resources are not available at your institution and the patient is stable, consider transferring her to the nearest center of expertise
2. Prepare for resuscitation: Have blood products available in the operating room and optimize IV access and arterial line
3. Optimize exposure of the surgical field: place in lithotomy position, extend fascial incision, perform hysterotomy to avoid the placenta, and expose pelvic sidewall and ureters
4. Be mindful of likely sources of massive bleeding: pelvic sidewall, bladder/vesicouterine space, and/or placenta previa vaginally
5. Proceed with meticulous dissection to minimize the risk of hemorrhage, retrograde fill the bladder, be mindful of the utility of packing
6. Be prepared to move to an expeditious hysterectomy in case of massive bleeding
Continue to: Bladder dissection...
Bladder dissection
The next critical part of the surgery involves developing the vesicovaginal space to mobilize the bladder. Prior to initiating the bladder dissection, we routinely retrograde fill the bladder with 180 to 240 mL of saline mixed with methylene blue. This delineates the superior edge of the bladder and indicates the appropriate level to start the dissection. Then slowly develop the vesicouterine space using a combination of electrocautery and a vessel-sealing device until the bladder is mobilized to the level of the anterior vaginal wall. Many vascular connections are encountered at that level, and meticulous dissection and patience is required to systematically divide them all.
In our experience. This part of the surgery presents several challenges. The bladder wall may be invaded by the placenta, which will lead to an increased risk of bleeding and cystotomy during the dissection. In these cases, it might be preferable to create an intentional cystotomy to guide the dissection; at times, a limited excision of the involved bladder wall may be required. In other cases, even in the absence of bladder wall invasion, the bladder may be densely adherent to the uterine wall (usually due to a history of prior CDs), and bladder mobilization may be complicated by bleeding from the neovascular network that has developed between the placenta and bladder.
Uterine arteries and cervix
Once the placenta is separated from its lateral attachments and the bladder is mobilized, the next steps are similar to those in a standard abdominal hysterectomy. If the uterine arteries were not yet divided during the pelvic sidewall dissection, they are clamped, divided, and suture ligated at the level of the uterine isthmus. The decision whether to perform a supracervical or total hysterectomy depends on the level of cervical involvement by the placenta, surgeon preference, anatomic distortion, and bleeding from the cervix and anterior vaginal wall.
Responding to massive bleeding
Not uncommonly, and despite the best efforts to avoid it, massive bleeding may develop from the areas at risk as noted above. If the bleeding is significant and originates from multiple areas (including vaginal bleeding from placenta previa), we recommend proceeding with an expeditious hysterectomy to remove the specimen, and then reassess the pelvic field for hemostatic control and any organ damage that may have occurred.
The challenge of PAS
Surgical management of PAS is one the most challenging procedures in pelvic surgery. Successful outcomes require a multidisciplinary team approach and an experienced team dedicated to the management of this condition.9 By contrast, proceeding “as usual” in the face of an unexpected PAS situation can lead to disastrous consequences in terms of maternal morbidity and mortality. ●
- Jauniaux E, Bunce C, Gronbeck L, et al. Prevalence and main outcomes of placenta accreta spectrum: a systematic review and meta-analysis. Am J Obstet Gynecol. 2019;221:208-218.
- Society of Gynecologic Oncology, American College of Obstetricians and Gynecologists, Society for Maternal-Fetal Medicine, et al. Placenta accreta spectrum. Am J Obstet Gynecol. 2018;219:B2-B16.
- Eller AG, Bennett MA, Sharshiner M, et al. Maternal morbidity in cases of placenta accreta managed by a multidisciplinary care team compared with standard obstetric care. Obstet Gynecol. 2011;117(2 pt 1):331-337.
- Shamshirsaz AA, Fox KA, Salmanian B, et al. Maternal morbidity in patients with morbidly adherent placenta treated with and without a standardized multidisciplinary approach. Am J Obstet Gynecol. 2015;212:218.e1-9.
- Collins SL, Alemdar B, van Beekhuizen HJ, et al; International Society for Abnormally Invasive Placenta. Evidence-based guidelines for the management of abnormally invasive placenta: recommendations from the International Society for Abnormally Invasive Placenta. Am J Obstet Gynecol. 2019;220:511-526.
- Silver RM, Fox KA, Barton JR, et al. Center of excellence for placenta accreta. Am J Obstet Gynecol. 2015;212:561-568.
- Tam Tam KB, Dozier J, Martin JN Jr. Approaches to reduce urinary tract injury during management of placenta accreta, increta, and percreta: a systematic review. J Matern Fetal Neonatal Med. 2012;25:329-334.
- Belfort MA, Shamshiraz AA, Fox K. Minimizing blood loss at cesarean-hysterectomy for placenta previa percreta. Am J Obstet Gynecol. 2017;216:78.e1-78.e2.
- Shamshirsaz AA, Fox KA, Erfani H, et al. Multidisciplinary team learning in the management of the morbidly adherent placenta: outcome improvements over time. Am J Obstet Gynecol. 2017;216:612.e1-612.e5.
CASE Concerning finding on repeat CD
A 30-year-old woman with a history of 1 prior cesarean delivery (CD) presents to labor and delivery at 38 weeks of gestation with symptoms of mild cramping. Her prenatal care was uncomplicated. The covering team made a decision to proceed with a repeat CD. A Pfannenstiel incision is made to enter the abdomen, and inspection of the lower uterine segment is concerning for a placenta accreta spectrum (PAS) (FIGURE).
What would be your next steps?

Placenta accreta spectrum describes the range of disorders of placental implantation, including placenta accreta, increta, and percreta. PAS is a significant cause of severe maternal morbidity and mortality, primarily due to massive hemorrhage at the time of delivery. The incidence of PAS continues to rise along with the CD rate. The authors of a recent meta-analysis reported a pooled prevalence rate of 1 in 588 women.1 Notably, in women with PAS, the rate of hysterectomy is 52.2%, and the transfusion-dependent hemorrhage rate is 46.9%.1
Ideally, PAS should be diagnosed or at least suspected antenatally during prenatal ultrasonography, leading to delivery planning by a multidisciplinary team.2 The presence of a multidisciplinary team—in addition to the primary obstetric and surgical teams—composed of experienced anesthesiologists, a blood bank able to respond to massive transfusion needs, critical care specialists, and interventional radiologists is associated with improved outcomes.3-5
Occasionally, a patient is found to have an advanced PAS (increta or percreta) at the time of delivery. In these situations, it is paramount that the appropriate resources be assembled as expeditiously as possible to optimize maternal outcomes. Surgical management can be challenging even for experienced pelvic surgeons, and appropriate resuscitation cannot be provided by a single anesthesiologist working alone. A cavalier attitude of proceeding with the delivery “as usual” in the face of an unexpected PAS situation can lead to disastrous consequences, including maternal death.
In this article, we review the important steps to take when faced with the unexpected situation of a PAS at the time of CD.
Continue to: Stop and collect your multidisciplinary team...
Stop and collect your multidisciplinary team
Once the diagnosis of an advanced PAS is suspected, the first step is to stop and request the presence of your institution’s multidisciplinary surgical team. This team typically includes a maternal-fetal specialist or, if not available, an experienced obstetrician, and an expert pelvic surgeon, which varies by institution (gynecologic oncologist, trauma surgeon, urologist, urogynecologist, vascular surgeon). An interventional radiology team is an additional useful resource that can assist with the control of pelvic hemorrhage using embolization techniques.
In our opinion, it is not appropriate to have a surgical backup team available only as needed at a certain distance from the hospital or even in the building. Because of the acuity and magnitude of bleeding that can occur in a short time, the most appropriate approach is to have your surgical team scrubbed and ready to assist or take over the procedure immediately if indicated.
Additional support staff also may be required. A single circulating nurse may not be sufficient, and available nursing staff may need to be called. The surgical technician scrubbed on the case may be familiar only with uncomplicated CDs and can be overwhelmed during a PAS case. Having a more experienced surgical technologist can optimize the availability of the appropriate instruments for the surgical team.
If a multidisciplinary surgical team with PAS management expertise is not available at your institution and the patient is stable, it is appropriate to consider transferring her to the nearest center that can meet the high-risk needs of this situation.6

Prepare for resuscitation
While you are calling your multidisciplinary team members, implement plans for resuscitation by notifying the anesthesiologist about the PAS findings. This will allow the gathering of needed resources that may include calling on additional anesthesiologists with experience in high-risk obstetrics, trauma, or critical care.
Placing large-bore intravenous lines or a central line to allow rapid transfusion is essential. Strongly consider inserting an arterial line for hemodynamic monitoring and intraoperative blood draws to monitor blood loss, blood gases, electrolytes, and coagulation parameters, which can guide resuscitative efforts and replacement therapies.
Simultaneously, inform the blood bank to prepare blood and blood products for possible activation of a massive transfusion protocol. It is imperative to have the products available in the operating room (OR) prior to proceeding with the surgery. Our current practice is to have 10 units of packed red blood cells and fresh frozen plasma available in the OR for all our prenatally diagnosed electively planned PAS cases.
Optimize exposure of the surgical field
Appropriate exposure of the surgical field is essential and should include exposure of the uterine fundus and the pelvic sidewalls. The uterine incision should avoid the placenta; typically it is placed at the level of the uterine fundus. Exposure of the pelvic sidewalls is needed to open the retroperitoneum and identify the ureter and the iliac vessels.
Vertical extension of the fascial incision probably will be needed to achieve appropriate exposure. Although at times this can be done without a concomitant vertical skin incision, often an inverted T incision is required. Be mindful that PAS is a life-threatening condition and that aesthetics are not a priority. After extending the fascial incision, adequate exposure can be achieved with any of the commonly used retractors or wound protectors (depending on institutional availability and surgeon preference) or by the surgical assistants using body wall retractors.
We routinely place the patient in lithotomy position. This allows us to monitor for vaginal bleeding (often a site of unrecognized massive hemorrhage) during the surgery, facilitate retrograde bladder filling, and provide a vaginal access to the pelvis. In addition, the lithotomy position allows for cystoscopy and placement of ureteral stents, which can be performed before starting the surgery to help prevent urinary tract injuries or at the end of the procedure in case one is suspected.7
Continue to: Performing the hysterectomy...
Performing the hysterectomy
A complete review of all surgical techniques for managing PAS is beyond the scope of this article. However, we briefly cover important procedural steps and offer suggestions on how to minimize the risk of bleeding.
In our experience. The areas with the highest risk of massive bleeding that can be difficult to control include the pelvic sidewall when there is lateral extension of the PAS, the vesicouterine space, and placenta previa vaginally. Be mindful of these areas at risk and have a plan in place in case of bleeding.
Uterine incision
Avoid the placenta when making the uterine incision, which is typically done in the fundal part of the uterus. Cut and tie the cord and return it to the uterine cavity. Close the incision in a single layer. Use of a surgical stapler can be used for the hysterotomy and can decrease the amount of blood loss.8
Superior attachments of the uterus
The superior attachments of the uterus include the round ligament, the utero-ovarian ligament, and the fallopian tubes. With meticulous dissection, develop an avascular space underneath these structures and, in turn, individually divide and suture ligate; this is typically achieved with minimal blood loss.
In addition, isolate the engorged veins of the broad ligament and divide them in a similar fashion.
In our experience. Use of a vessel-sealing device can facilitate division of all the former structures. Simply excise the fallopian tubes with the vessel-sealing device either at this time or after the uterus is removed.
Pelvic sidewall
Once the superior attachments of the uterus have been divided, the next step involves exposing the pelvic sidewall structures, that is, the ureter and the pelvic vessels. Expose the ureter from the pelvic brim to the level of the uterine artery. The hypogastric artery is exposed as well in this process and the pararectal space developed.
When the PAS has extended laterally, perform stepwise division of the lateral attachments of the placenta to the pelvic sidewall using a combination of electrocautery, hemoclips, and the vessel-sealing device. In laterally extended PAS cases, it often is necessary to divide the uterine artery either at its origin or at the level of the ureter to allow for the completion of the separation of the placenta from the pelvic sidewall.
In our experience. During this lateral dissection, significant bleeding may be encountered from the neovascular network that has developed in the pelvic sidewall. The bleeding may be diffuse and difficult to control with the methods described above. In this situation, we have found that placing hemostatic agents in this area and packing the sidewall with laparotomy pads can achieve hemostasis in most cases, thus allowing the surgery to proceed.
1. Stop and collect your multidisciplinary team. If required resources are not available at your institution and the patient is stable, consider transferring her to the nearest center of expertise
2. Prepare for resuscitation: Have blood products available in the operating room and optimize IV access and arterial line
3. Optimize exposure of the surgical field: place in lithotomy position, extend fascial incision, perform hysterotomy to avoid the placenta, and expose pelvic sidewall and ureters
4. Be mindful of likely sources of massive bleeding: pelvic sidewall, bladder/vesicouterine space, and/or placenta previa vaginally
5. Proceed with meticulous dissection to minimize the risk of hemorrhage, retrograde fill the bladder, be mindful of the utility of packing
6. Be prepared to move to an expeditious hysterectomy in case of massive bleeding
Continue to: Bladder dissection...
Bladder dissection
The next critical part of the surgery involves developing the vesicovaginal space to mobilize the bladder. Prior to initiating the bladder dissection, we routinely retrograde fill the bladder with 180 to 240 mL of saline mixed with methylene blue. This delineates the superior edge of the bladder and indicates the appropriate level to start the dissection. Then slowly develop the vesicouterine space using a combination of electrocautery and a vessel-sealing device until the bladder is mobilized to the level of the anterior vaginal wall. Many vascular connections are encountered at that level, and meticulous dissection and patience is required to systematically divide them all.
In our experience. This part of the surgery presents several challenges. The bladder wall may be invaded by the placenta, which will lead to an increased risk of bleeding and cystotomy during the dissection. In these cases, it might be preferable to create an intentional cystotomy to guide the dissection; at times, a limited excision of the involved bladder wall may be required. In other cases, even in the absence of bladder wall invasion, the bladder may be densely adherent to the uterine wall (usually due to a history of prior CDs), and bladder mobilization may be complicated by bleeding from the neovascular network that has developed between the placenta and bladder.
Uterine arteries and cervix
Once the placenta is separated from its lateral attachments and the bladder is mobilized, the next steps are similar to those in a standard abdominal hysterectomy. If the uterine arteries were not yet divided during the pelvic sidewall dissection, they are clamped, divided, and suture ligated at the level of the uterine isthmus. The decision whether to perform a supracervical or total hysterectomy depends on the level of cervical involvement by the placenta, surgeon preference, anatomic distortion, and bleeding from the cervix and anterior vaginal wall.
Responding to massive bleeding
Not uncommonly, and despite the best efforts to avoid it, massive bleeding may develop from the areas at risk as noted above. If the bleeding is significant and originates from multiple areas (including vaginal bleeding from placenta previa), we recommend proceeding with an expeditious hysterectomy to remove the specimen, and then reassess the pelvic field for hemostatic control and any organ damage that may have occurred.
The challenge of PAS
Surgical management of PAS is one the most challenging procedures in pelvic surgery. Successful outcomes require a multidisciplinary team approach and an experienced team dedicated to the management of this condition.9 By contrast, proceeding “as usual” in the face of an unexpected PAS situation can lead to disastrous consequences in terms of maternal morbidity and mortality. ●
CASE Concerning finding on repeat CD
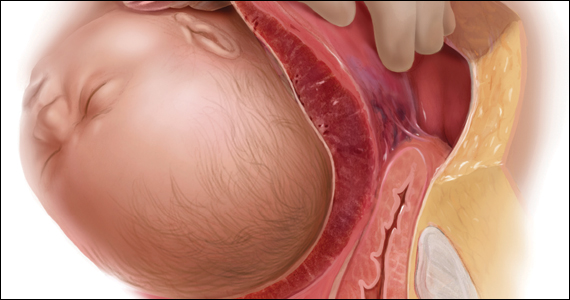
A 30-year-old woman with a history of 1 prior cesarean delivery (CD) presents to labor and delivery at 38 weeks of gestation with symptoms of mild cramping. Her prenatal care was uncomplicated. The covering team made a decision to proceed with a repeat CD. A Pfannenstiel incision is made to enter the abdomen, and inspection of the lower uterine segment is concerning for a placenta accreta spectrum (PAS) (FIGURE).
What would be your next steps?

Placenta accreta spectrum describes the range of disorders of placental implantation, including placenta accreta, increta, and percreta. PAS is a significant cause of severe maternal morbidity and mortality, primarily due to massive hemorrhage at the time of delivery. The incidence of PAS continues to rise along with the CD rate. The authors of a recent meta-analysis reported a pooled prevalence rate of 1 in 588 women.1 Notably, in women with PAS, the rate of hysterectomy is 52.2%, and the transfusion-dependent hemorrhage rate is 46.9%.1
Ideally, PAS should be diagnosed or at least suspected antenatally during prenatal ultrasonography, leading to delivery planning by a multidisciplinary team.2 The presence of a multidisciplinary team—in addition to the primary obstetric and surgical teams—composed of experienced anesthesiologists, a blood bank able to respond to massive transfusion needs, critical care specialists, and interventional radiologists is associated with improved outcomes.3-5
Occasionally, a patient is found to have an advanced PAS (increta or percreta) at the time of delivery. In these situations, it is paramount that the appropriate resources be assembled as expeditiously as possible to optimize maternal outcomes. Surgical management can be challenging even for experienced pelvic surgeons, and appropriate resuscitation cannot be provided by a single anesthesiologist working alone. A cavalier attitude of proceeding with the delivery “as usual” in the face of an unexpected PAS situation can lead to disastrous consequences, including maternal death.
In this article, we review the important steps to take when faced with the unexpected situation of a PAS at the time of CD.
Continue to: Stop and collect your multidisciplinary team...
Stop and collect your multidisciplinary team
Once the diagnosis of an advanced PAS is suspected, the first step is to stop and request the presence of your institution’s multidisciplinary surgical team. This team typically includes a maternal-fetal specialist or, if not available, an experienced obstetrician, and an expert pelvic surgeon, which varies by institution (gynecologic oncologist, trauma surgeon, urologist, urogynecologist, vascular surgeon). An interventional radiology team is an additional useful resource that can assist with the control of pelvic hemorrhage using embolization techniques.
In our opinion, it is not appropriate to have a surgical backup team available only as needed at a certain distance from the hospital or even in the building. Because of the acuity and magnitude of bleeding that can occur in a short time, the most appropriate approach is to have your surgical team scrubbed and ready to assist or take over the procedure immediately if indicated.
Additional support staff also may be required. A single circulating nurse may not be sufficient, and available nursing staff may need to be called. The surgical technician scrubbed on the case may be familiar only with uncomplicated CDs and can be overwhelmed during a PAS case. Having a more experienced surgical technologist can optimize the availability of the appropriate instruments for the surgical team.
If a multidisciplinary surgical team with PAS management expertise is not available at your institution and the patient is stable, it is appropriate to consider transferring her to the nearest center that can meet the high-risk needs of this situation.6

Prepare for resuscitation
While you are calling your multidisciplinary team members, implement plans for resuscitation by notifying the anesthesiologist about the PAS findings. This will allow the gathering of needed resources that may include calling on additional anesthesiologists with experience in high-risk obstetrics, trauma, or critical care.
Placing large-bore intravenous lines or a central line to allow rapid transfusion is essential. Strongly consider inserting an arterial line for hemodynamic monitoring and intraoperative blood draws to monitor blood loss, blood gases, electrolytes, and coagulation parameters, which can guide resuscitative efforts and replacement therapies.
Simultaneously, inform the blood bank to prepare blood and blood products for possible activation of a massive transfusion protocol. It is imperative to have the products available in the operating room (OR) prior to proceeding with the surgery. Our current practice is to have 10 units of packed red blood cells and fresh frozen plasma available in the OR for all our prenatally diagnosed electively planned PAS cases.
Optimize exposure of the surgical field
Appropriate exposure of the surgical field is essential and should include exposure of the uterine fundus and the pelvic sidewalls. The uterine incision should avoid the placenta; typically it is placed at the level of the uterine fundus. Exposure of the pelvic sidewalls is needed to open the retroperitoneum and identify the ureter and the iliac vessels.
Vertical extension of the fascial incision probably will be needed to achieve appropriate exposure. Although at times this can be done without a concomitant vertical skin incision, often an inverted T incision is required. Be mindful that PAS is a life-threatening condition and that aesthetics are not a priority. After extending the fascial incision, adequate exposure can be achieved with any of the commonly used retractors or wound protectors (depending on institutional availability and surgeon preference) or by the surgical assistants using body wall retractors.
We routinely place the patient in lithotomy position. This allows us to monitor for vaginal bleeding (often a site of unrecognized massive hemorrhage) during the surgery, facilitate retrograde bladder filling, and provide a vaginal access to the pelvis. In addition, the lithotomy position allows for cystoscopy and placement of ureteral stents, which can be performed before starting the surgery to help prevent urinary tract injuries or at the end of the procedure in case one is suspected.7
Continue to: Performing the hysterectomy...
Performing the hysterectomy
A complete review of all surgical techniques for managing PAS is beyond the scope of this article. However, we briefly cover important procedural steps and offer suggestions on how to minimize the risk of bleeding.
In our experience. The areas with the highest risk of massive bleeding that can be difficult to control include the pelvic sidewall when there is lateral extension of the PAS, the vesicouterine space, and placenta previa vaginally. Be mindful of these areas at risk and have a plan in place in case of bleeding.
Uterine incision
Avoid the placenta when making the uterine incision, which is typically done in the fundal part of the uterus. Cut and tie the cord and return it to the uterine cavity. Close the incision in a single layer. Use of a surgical stapler can be used for the hysterotomy and can decrease the amount of blood loss.8
Superior attachments of the uterus
The superior attachments of the uterus include the round ligament, the utero-ovarian ligament, and the fallopian tubes. With meticulous dissection, develop an avascular space underneath these structures and, in turn, individually divide and suture ligate; this is typically achieved with minimal blood loss.
In addition, isolate the engorged veins of the broad ligament and divide them in a similar fashion.
In our experience. Use of a vessel-sealing device can facilitate division of all the former structures. Simply excise the fallopian tubes with the vessel-sealing device either at this time or after the uterus is removed.
Pelvic sidewall
Once the superior attachments of the uterus have been divided, the next step involves exposing the pelvic sidewall structures, that is, the ureter and the pelvic vessels. Expose the ureter from the pelvic brim to the level of the uterine artery. The hypogastric artery is exposed as well in this process and the pararectal space developed.
When the PAS has extended laterally, perform stepwise division of the lateral attachments of the placenta to the pelvic sidewall using a combination of electrocautery, hemoclips, and the vessel-sealing device. In laterally extended PAS cases, it often is necessary to divide the uterine artery either at its origin or at the level of the ureter to allow for the completion of the separation of the placenta from the pelvic sidewall.
In our experience. During this lateral dissection, significant bleeding may be encountered from the neovascular network that has developed in the pelvic sidewall. The bleeding may be diffuse and difficult to control with the methods described above. In this situation, we have found that placing hemostatic agents in this area and packing the sidewall with laparotomy pads can achieve hemostasis in most cases, thus allowing the surgery to proceed.
1. Stop and collect your multidisciplinary team. If required resources are not available at your institution and the patient is stable, consider transferring her to the nearest center of expertise
2. Prepare for resuscitation: Have blood products available in the operating room and optimize IV access and arterial line
3. Optimize exposure of the surgical field: place in lithotomy position, extend fascial incision, perform hysterotomy to avoid the placenta, and expose pelvic sidewall and ureters
4. Be mindful of likely sources of massive bleeding: pelvic sidewall, bladder/vesicouterine space, and/or placenta previa vaginally
5. Proceed with meticulous dissection to minimize the risk of hemorrhage, retrograde fill the bladder, be mindful of the utility of packing
6. Be prepared to move to an expeditious hysterectomy in case of massive bleeding
Continue to: Bladder dissection...
Bladder dissection
The next critical part of the surgery involves developing the vesicovaginal space to mobilize the bladder. Prior to initiating the bladder dissection, we routinely retrograde fill the bladder with 180 to 240 mL of saline mixed with methylene blue. This delineates the superior edge of the bladder and indicates the appropriate level to start the dissection. Then slowly develop the vesicouterine space using a combination of electrocautery and a vessel-sealing device until the bladder is mobilized to the level of the anterior vaginal wall. Many vascular connections are encountered at that level, and meticulous dissection and patience is required to systematically divide them all.
In our experience. This part of the surgery presents several challenges. The bladder wall may be invaded by the placenta, which will lead to an increased risk of bleeding and cystotomy during the dissection. In these cases, it might be preferable to create an intentional cystotomy to guide the dissection; at times, a limited excision of the involved bladder wall may be required. In other cases, even in the absence of bladder wall invasion, the bladder may be densely adherent to the uterine wall (usually due to a history of prior CDs), and bladder mobilization may be complicated by bleeding from the neovascular network that has developed between the placenta and bladder.
Uterine arteries and cervix
Once the placenta is separated from its lateral attachments and the bladder is mobilized, the next steps are similar to those in a standard abdominal hysterectomy. If the uterine arteries were not yet divided during the pelvic sidewall dissection, they are clamped, divided, and suture ligated at the level of the uterine isthmus. The decision whether to perform a supracervical or total hysterectomy depends on the level of cervical involvement by the placenta, surgeon preference, anatomic distortion, and bleeding from the cervix and anterior vaginal wall.
Responding to massive bleeding
Not uncommonly, and despite the best efforts to avoid it, massive bleeding may develop from the areas at risk as noted above. If the bleeding is significant and originates from multiple areas (including vaginal bleeding from placenta previa), we recommend proceeding with an expeditious hysterectomy to remove the specimen, and then reassess the pelvic field for hemostatic control and any organ damage that may have occurred.
The challenge of PAS
Surgical management of PAS is one the most challenging procedures in pelvic surgery. Successful outcomes require a multidisciplinary team approach and an experienced team dedicated to the management of this condition.9 By contrast, proceeding “as usual” in the face of an unexpected PAS situation can lead to disastrous consequences in terms of maternal morbidity and mortality. ●
- Jauniaux E, Bunce C, Gronbeck L, et al. Prevalence and main outcomes of placenta accreta spectrum: a systematic review and meta-analysis. Am J Obstet Gynecol. 2019;221:208-218.
- Society of Gynecologic Oncology, American College of Obstetricians and Gynecologists, Society for Maternal-Fetal Medicine, et al. Placenta accreta spectrum. Am J Obstet Gynecol. 2018;219:B2-B16.
- Eller AG, Bennett MA, Sharshiner M, et al. Maternal morbidity in cases of placenta accreta managed by a multidisciplinary care team compared with standard obstetric care. Obstet Gynecol. 2011;117(2 pt 1):331-337.
- Shamshirsaz AA, Fox KA, Salmanian B, et al. Maternal morbidity in patients with morbidly adherent placenta treated with and without a standardized multidisciplinary approach. Am J Obstet Gynecol. 2015;212:218.e1-9.
- Collins SL, Alemdar B, van Beekhuizen HJ, et al; International Society for Abnormally Invasive Placenta. Evidence-based guidelines for the management of abnormally invasive placenta: recommendations from the International Society for Abnormally Invasive Placenta. Am J Obstet Gynecol. 2019;220:511-526.
- Silver RM, Fox KA, Barton JR, et al. Center of excellence for placenta accreta. Am J Obstet Gynecol. 2015;212:561-568.
- Tam Tam KB, Dozier J, Martin JN Jr. Approaches to reduce urinary tract injury during management of placenta accreta, increta, and percreta: a systematic review. J Matern Fetal Neonatal Med. 2012;25:329-334.
- Belfort MA, Shamshiraz AA, Fox K. Minimizing blood loss at cesarean-hysterectomy for placenta previa percreta. Am J Obstet Gynecol. 2017;216:78.e1-78.e2.
- Shamshirsaz AA, Fox KA, Erfani H, et al. Multidisciplinary team learning in the management of the morbidly adherent placenta: outcome improvements over time. Am J Obstet Gynecol. 2017;216:612.e1-612.e5.
- Jauniaux E, Bunce C, Gronbeck L, et al. Prevalence and main outcomes of placenta accreta spectrum: a systematic review and meta-analysis. Am J Obstet Gynecol. 2019;221:208-218.
- Society of Gynecologic Oncology, American College of Obstetricians and Gynecologists, Society for Maternal-Fetal Medicine, et al. Placenta accreta spectrum. Am J Obstet Gynecol. 2018;219:B2-B16.
- Eller AG, Bennett MA, Sharshiner M, et al. Maternal morbidity in cases of placenta accreta managed by a multidisciplinary care team compared with standard obstetric care. Obstet Gynecol. 2011;117(2 pt 1):331-337.
- Shamshirsaz AA, Fox KA, Salmanian B, et al. Maternal morbidity in patients with morbidly adherent placenta treated with and without a standardized multidisciplinary approach. Am J Obstet Gynecol. 2015;212:218.e1-9.
- Collins SL, Alemdar B, van Beekhuizen HJ, et al; International Society for Abnormally Invasive Placenta. Evidence-based guidelines for the management of abnormally invasive placenta: recommendations from the International Society for Abnormally Invasive Placenta. Am J Obstet Gynecol. 2019;220:511-526.
- Silver RM, Fox KA, Barton JR, et al. Center of excellence for placenta accreta. Am J Obstet Gynecol. 2015;212:561-568.
- Tam Tam KB, Dozier J, Martin JN Jr. Approaches to reduce urinary tract injury during management of placenta accreta, increta, and percreta: a systematic review. J Matern Fetal Neonatal Med. 2012;25:329-334.
- Belfort MA, Shamshiraz AA, Fox K. Minimizing blood loss at cesarean-hysterectomy for placenta previa percreta. Am J Obstet Gynecol. 2017;216:78.e1-78.e2.
- Shamshirsaz AA, Fox KA, Erfani H, et al. Multidisciplinary team learning in the management of the morbidly adherent placenta: outcome improvements over time. Am J Obstet Gynecol. 2017;216:612.e1-612.e5.
Transvaginal reconstructive surgery for POP: Innovative approach to graft augmentation in the post-mesh era
Pelvic organ prolapse (POP) is a common occurrence over the course of a woman’s lifetime, especially in parous women (up to 50% of women who have given birth).1 The anterior vaginal wall is the most common site of POP and has the highest recurrence rate of up to 70%.2 The risk of developing POP increases with age, obesity, White race, family history, and prior pelvic surgery, such as hysterectomy. It affects more than 3 million women in the United States alone, often negatively impacting sexual function and overall quality of life.3,4
Because women are living longer than ever before and are more active in their senior years, a long-lasting, durable surgical repair is desirable, if not necessary. To be cost-effective and to avoid general anesthesia, the surgical approach ideally should be vaginal.
Biologic and synthetic grafts to augment transvaginal repair traditionally are used to improve on the well-recognized high failure rate of native-tissue repair that is often seen at both short-term and medium-term follow-up.5 The failure rate is commonly referenced as 30% to 40% at 2-year follow-up and 61% to 70% at 5-year follow-up, well-established by the results of the OPTIMAL randomized clinical trial.6 The more recent Descent trial likewise demonstrates a higher failure rate of native-tissue repair versus transvaginal mesh repair at a shorter term of 30 to 42 months.7 Furthermore, the use of permanent versus absorbable suture in suspension of the vaginal apex is associated with lower short-term failure rates.8
Despite this Level I evidence that demonstrates a clear advantage for obtaining a longer or more durable repair with permanent materials, native-tissue repairs with absorbable suture are still performed routinely. Since the US Food and Drug Administration (FDA) ordered that the use of transvaginal surgical mesh augmentation for pelvic reconstructive surgery be discontinued, it is more important than ever to explore evolving alternative native-tissue augmentation repair techniques that hopefully can preserve the advantages and merits of vaginal surgery and achieve longer durability.9
Biologic graft augmentation use in transvaginal reconstruction
All biologic grafts, including allografts derived from human tissue and xenografts derived from animal tissue, are acellular constructs composed of extracellular matrix (ECM) that acts as scaffolding for the host tissue. The ECM is predominantly composed of collagen (types I and III) and noncollagenous fibronectin, laminin, and glycosaminoglycans in various amounts depending on the source tissue. The 3D presentation of ECM’s complex molecules allows for rapid repopulation of host cells and revascularization with eventual regeneration.
Once a biologic graft is placed surgically, the body’s response to the scaffold ECM mimics the normal wound-healing process, beginning with fibrin-rich matrix hemostasis and the subsequent innate immune response of neutrophil and M1 macrophage infiltration. M1 macrophages are proinflammatory and clear cellular debris and begin the process of graft scaffold degradation. The host tissue then begins the process of remodeling through pro-remodeling M2 macrophages and stem cell recruitment, proliferation, and differentiation.10 As the biologic graft provides initial structure and strength for pelvic repairs, the ideal ECM scaffold would not degrade before the host is able to fully undergo regeneration and maintain its structure and strength.
Biologic grafts differ in source (allograft or xenograft), type (pericardium, dermis, or bladder), developmental stage (fetal or adult), decellularization processing, and sterilization techniques. These 5 aspects determine the distinct 3D ECM scaffold structure, strength, and longevity. If the ECM scaffold is damaged or retains noncollagenous proteins during the preparation process, an inflammatory response is triggered in which the graft is degraded, resorbed, and replaced with scar tissue. Furthermore, certain processing techniques aimed at extending the ECM’s durability—that is, cross-linking collagen—results in the foreign body response in which there is no vascular infiltration or cellular penetration of the graft and a collagen capsule is created around the empty matrix.11 To avoid resorption or encapsulation of the graft, the ECM scaffolds of biologic grafts must be optimized to induce regeneration.
Continue to: Choosing surgical POP repair...
Choosing surgical POP repair
The decision to undergo surgical treatment for prolapse is a shared decision-making process between the patient and surgeon and always should be individualized. The type of procedure and the surgical approach will depend on the patient’s goals, the degree of prolapse, clinical history, risk tolerance, the surgeon’s skill set, and whether or not there is an indication or relative contraindication for uterine removal at the time of prolapse repair.
While the FDA’s order does not apply to transabdominally placed surgical mesh, such as sacrocolpopexy, not all patients are ideal candidates for an abdominal sacrocolpopexy. Most notable are women with a history of multiple prior abdominal surgeries with higher rates of intraperitoneal adhesions. Ideally, to be cost-effective and to avoid general anesthesia, the surgical approach should be vaginal whenever possible.
Biologic versus native-tissue grafts
Currently, only low-quality evidence exists that compares the outcomes of biologic grafts with traditional native-tissue repairs in POP. Studies have been limited by poor reporting of methods, inconsistency in technique and materials used, and imprecise definitions. One Cochrane Review on the surgical management of POP concluded that biologic graft augmentation was associated with a lower failure rate (18%) within 1 to 2 years when compared with a traditional anterior colporrhaphy (28%).12
Based on consideration of all Cochrane Database Reviews and recent large systematic reviews, there clearly is a paucity of information on which to draw well-defined conclusions regarding the advantage of biomaterials in prolapse surgery.12-14 This is due in part to the variation in graft material used and the surgical technique employed.
Similarly, evidence is lacking regarding the superiority of one type of biologic graft over another. Furthermore, some of the grafts previously studied are no longer on the market.15 With the FDA’s removal of all transvaginal mesh, including xenografts, only allografts are available for pelvic floor reconstruction. Currently, only 3 commercial manufacturers market allografts for pelvic floor reconstruction. Each allograft is available in various sizes and all can be trimmed at the time of the surgical procedure to customize both the size and shape to fit the individual patient.
A novel technique using Axis Dermis and polypropylene suture
One of the commercially available allografts, Axis Dermis (Coloplast), is non–cross-linked and is derived from human cadaveric dermal tissue from the back and dorsum of the upper leg. It is sterilized by a proprietary Tutoplast️ sterilization process that uses gamma irradiation to inactivate and prevent the transmission of pathogens. This unique technique involving solvent dehydration means the graft is never freeze dried; thus, the natural tissue matrix is preserved.
Additionally, the allograft is antigen-free, which decreases the risk of tissue reaction (scarring/fibrosis) and aids in the process of host tissue remodeling; invasion by growth factors, blood cells, collagen, elastin, and neovascularization. This natural tissue remodeling facilitates the anticipated “reabsorption” of the graft by the host tissue, leaving the patient with a tissue scaffold, that is, a stronger layer of “fascia” beneath the muscularis.16 As a result of this “biocompatible” graft, the host tissue remodeling has been shown in the rat model to involve early cellular infiltration and angiogenesis (in the first week after implantation), that leads to an organized cellular architecture with greater tensile strength by week 4, and ultimately inability to distinguish host collagen from the implant by 8 to 12 weeks.17,18
Continue to: Steps in performing the technique...
Steps in performing the technique
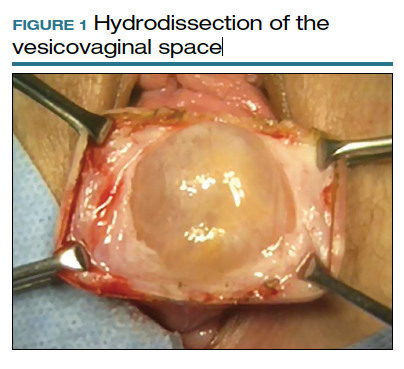
To ensure that the graft is placed adjacent to the vaginal serosa, a full-thickness dissection is carried out to enter the true vesicovaginal space, which lies below all 4 histologic layers of the vagina (nonkeratinized stratified squamous epithelium, lamina propria, muscularis, and serosa). For the anterior dissection, a Tuohy epidural needle is used to achieve an accurate and consistent depth when injecting fluid (hydrodissection) to enter this true pelvic space (FIGURE 1). Correct entry into the vesicovaginal space can be confirmed visually by the presence of adipose tissue.
Many pelvic surgeons use the sacrospinous ligament (SSL) as a strong and reliable point of attachment for vaginal prolapse repair. It can be approached either anteriorly or posteriorly with careful dissection. Permanent suture (0-Prolene) is used to “bridge” the attachment between the SSL, the Axis Dermis graft, and the cervix (or vaginal apex). The suture is placed in the middle third and lower half of the ligament to avoid injury to nearby neurovascular structures.
While the surgeon may use any suture-capturing device, we prefer the Anchosure System (Neomedic). This device delivers a small anchor securely into the ligament through a single point of entry, minimizing the risk of postoperative pain for the patient. A 6 cm x 8 cm size Axis Dermis graft is then trimmed to meet the specifications of the patient’s anatomy.
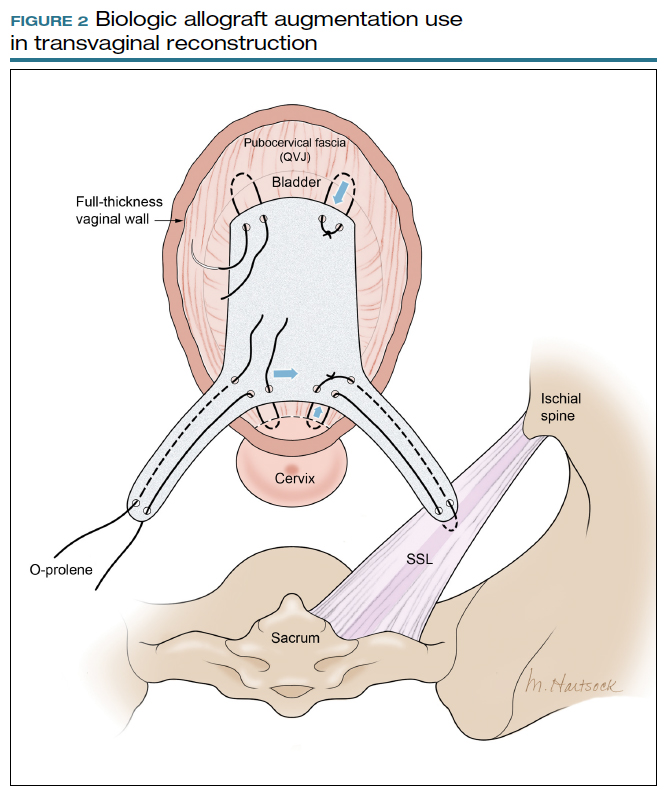
Most commonly, we measure, mark, and trim the body of the graft to 5.5 cm in length with a width of 3 cm. The bilateral arms are approximately 1 cm in width and comprise the remaining length of the 8 cm graft (FIGURE 2). As shown in Figure 2, pre-made holes are marked and punched out using a large hollow needle. These serve as the points of attachment for the permanent suture to be “weaved” into the graft arms and delayed absorbable “tacking suture” to be attached from the pubocervical fascia at the bladder neck to the distal end of the graft. This facilitates fixation of the graft in the midline of the anterior vaginal wall, overlying any central distention-type defect.
Finally, following attachment of the SSL permanent suture to the distal graft arm, this suture is then attached to the proximal U-shaped end of the graft body (in the midline), followed by a deep and secure bite through the cervix (or vaginal vault apex) and back through the proximal graft. These SSL suspension sutures are then tied such that the distal arms of the graft advance down to the ligament. Care is taken not to tie down to the SSL itself, rather until the cervix (or apex) is reduced to its normal anatomical location.
After the graft is secured in place, the full-thickness vaginal wall is closed with delayed absorbable suture. Sterile 1-inch ribbon packing is placed in the vagina immediately to close any dead space between the vagina and the graft to decrease the risk of seroma or hematoma formation.
This newly developed technique, like many surgeries for POP, requires extensive knowledge of pelvic anatomy and skill in vaginal surgery, and we recommend referral to a subspecialist in Female Pelvic Medicine and Reconstructive Surgery.

Continue to: Upcoming plans to share outcomes data...
Upcoming plans to share outcomes data
We are in the process of performing a retrospective review of all of the cases we have performed at our institution using this technique of permanent suture bridging to the SSL within the arm of the biograft. Given the relatively recent FDA announcement, we have yet to establish any long-term outcomes data. However, the preliminary results at 6-month follow-up are promising and demonstrate a low (2.6%) failure rate, without significant safety concerns. We hope to publish these data as well as more data on longitudinal outcomes in the future.
In summary
Many women are at risk for native-tissue repair failure or are not well suited for an abdominal procedure to correct their pelvic support defect and restore their quality of life. As expert pelvic surgeons, we play an important role in the search for innovative solutions for these women. There is ample opportunity for future research and clinical trials to determine the best biologic materials and their optimal use in pelvic reconstructive surgery.
Originally, polypropylene mesh was designed for use in augmenting abdominal hernia repairs and later was adapted by manufacturers for use in POP repair. The FDA removal from the market of existing transvaginal synthetic mesh kits was a unique catalyst that challenged our community to develop transvaginal repairs using biologic grafts that are genuinely tailored to the unique needs of the female pelvic anatomy. ●
- Maher C, Feiner B, Baessler K. Surgical management of pelvic organ prolapse in women. Cochrane Database Syst Rev. 2013:CD004014.
- Weber AM, Walters MD, Piedmonte MR, et al. Anterior colporrhaphy: a randomized trial of three surgical techniques. Am J Obstet Gynecol. 2001;185:1299-1304.
- Walters MD, Ridgeway BM. Surgical treatment of vaginal apex prolapse. Obstet Gynecol. 2013;121(2 pt 1):354-374.
- Meister MRL, Sutcliffe S, Lowder JL. Definitions of apical vaginal support loss: a systematic review. Am J Obstet Gynecol. 2017;216:232. e1-232.e14.
- Cox A, Herschorn S. Evaluation of current biologic meshes in pelvic organ prolapse repair. Curr Urol Rep. 2012;13:247-255.
- Jelovsek JE, Barber M, Brubaker K, et al. Effect of uterosacral ligament suspension vs sacrospinous ligament fixation with or without perioperative behavioral therapy for pelvic organ vaginal prolapse on surgical outcomes and prolapse symptoms at 5 years in the OPTIMAL randomized clinical trial. JAMA. 2018:319:1554-1565.
- Bowen ST, Moalli P, Abramowitch S, et al. Outcomes of the defining mechanisms of anterior vaginal wall descent trial [abstract 15]. Am J Obstet Gynecol. 2020;222:S770-S771.
- Chung CP, Miskimins R, Kuehl TJ, et al. Permanent suture used in uterosacral ligament suspension offers better anatomical support than delayed absorbable suture. Int Urogynecol J. 2012;23:223-227.
- US Food and Drug Administration. FDA takes action to protect women’s health, orders manufacturers of surgical mesh intended for transvaginal repair of pelvic organ prolapse to stop selling all devices. https://www.fda.gov/news-events/press-announcements/fda-takes-action-protect-womens-health-orders-manufacturers-surgical -mesh-intended-transvaginal. April 16, 2019. Accessed September 1, 2020.
- Londono R, Badylak SF. Biologic scaffolds for regenerative medicine: mechanisms of in vivo remodeling. Ann Biomed Eng. 2015;43:577-592.
- Cornwell KG, Landsman A, James KS. Extracellular matrix biomaterials for soft tissue repair. Clin Podiatr Med Surg. 2009;26: 507-523.
- Maher CM, Feiner B, Baessler K, et al. Surgical management of pelvic organ prolapse in women: the updated summary version Cochrane review. Int Urogynecol J. 2011;22:1445-1447.
- Maher C, Feiner B, Baessler K, et al. Surgery for women with anterior compartment prolapse. Cochrane Database Syst Rev. 2016;11:CD004014.
- Maher C, Feiner B, Baessler K, et al. Transvaginal mesh or grafts compared with native tissue repair for vaginal prolapse. Cochrane Database Syst Rev. 2016;2:CD012179.
- Rosenblatt P, Von Bargen E. Use of biologic grafts in pelvic organ prolapse surgery. Contemporary OB/GYN. 2017;62:14-19.
- Greenspan DC, Hernandez R, Faleris J. Histology of surgically implanted Tutoplast processed dermis. http://www.zimmerbiomet .co.il/images/lib_artHistologyDermis%2010.pdf. Accessed September 2, 2020.
- Williams D. Revisiting the definition of biocompatibility. Med Device Technol. 2003;14:10-13.
- Nosti PA, Carter CM, Sokol AI, et al. Transvaginal versus transabdominal placement of synthetic mesh at time of sacrocolpopexy. Female Pelvic Med Reconstr Surg. 2016;22:151-155.
Pelvic organ prolapse (POP) is a common occurrence over the course of a woman’s lifetime, especially in parous women (up to 50% of women who have given birth).1 The anterior vaginal wall is the most common site of POP and has the highest recurrence rate of up to 70%.2 The risk of developing POP increases with age, obesity, White race, family history, and prior pelvic surgery, such as hysterectomy. It affects more than 3 million women in the United States alone, often negatively impacting sexual function and overall quality of life.3,4
Because women are living longer than ever before and are more active in their senior years, a long-lasting, durable surgical repair is desirable, if not necessary. To be cost-effective and to avoid general anesthesia, the surgical approach ideally should be vaginal.
Biologic and synthetic grafts to augment transvaginal repair traditionally are used to improve on the well-recognized high failure rate of native-tissue repair that is often seen at both short-term and medium-term follow-up.5 The failure rate is commonly referenced as 30% to 40% at 2-year follow-up and 61% to 70% at 5-year follow-up, well-established by the results of the OPTIMAL randomized clinical trial.6 The more recent Descent trial likewise demonstrates a higher failure rate of native-tissue repair versus transvaginal mesh repair at a shorter term of 30 to 42 months.7 Furthermore, the use of permanent versus absorbable suture in suspension of the vaginal apex is associated with lower short-term failure rates.8
Despite this Level I evidence that demonstrates a clear advantage for obtaining a longer or more durable repair with permanent materials, native-tissue repairs with absorbable suture are still performed routinely. Since the US Food and Drug Administration (FDA) ordered that the use of transvaginal surgical mesh augmentation for pelvic reconstructive surgery be discontinued, it is more important than ever to explore evolving alternative native-tissue augmentation repair techniques that hopefully can preserve the advantages and merits of vaginal surgery and achieve longer durability.9
Biologic graft augmentation use in transvaginal reconstruction
All biologic grafts, including allografts derived from human tissue and xenografts derived from animal tissue, are acellular constructs composed of extracellular matrix (ECM) that acts as scaffolding for the host tissue. The ECM is predominantly composed of collagen (types I and III) and noncollagenous fibronectin, laminin, and glycosaminoglycans in various amounts depending on the source tissue. The 3D presentation of ECM’s complex molecules allows for rapid repopulation of host cells and revascularization with eventual regeneration.
Once a biologic graft is placed surgically, the body’s response to the scaffold ECM mimics the normal wound-healing process, beginning with fibrin-rich matrix hemostasis and the subsequent innate immune response of neutrophil and M1 macrophage infiltration. M1 macrophages are proinflammatory and clear cellular debris and begin the process of graft scaffold degradation. The host tissue then begins the process of remodeling through pro-remodeling M2 macrophages and stem cell recruitment, proliferation, and differentiation.10 As the biologic graft provides initial structure and strength for pelvic repairs, the ideal ECM scaffold would not degrade before the host is able to fully undergo regeneration and maintain its structure and strength.
Biologic grafts differ in source (allograft or xenograft), type (pericardium, dermis, or bladder), developmental stage (fetal or adult), decellularization processing, and sterilization techniques. These 5 aspects determine the distinct 3D ECM scaffold structure, strength, and longevity. If the ECM scaffold is damaged or retains noncollagenous proteins during the preparation process, an inflammatory response is triggered in which the graft is degraded, resorbed, and replaced with scar tissue. Furthermore, certain processing techniques aimed at extending the ECM’s durability—that is, cross-linking collagen—results in the foreign body response in which there is no vascular infiltration or cellular penetration of the graft and a collagen capsule is created around the empty matrix.11 To avoid resorption or encapsulation of the graft, the ECM scaffolds of biologic grafts must be optimized to induce regeneration.
Continue to: Choosing surgical POP repair...
Choosing surgical POP repair
The decision to undergo surgical treatment for prolapse is a shared decision-making process between the patient and surgeon and always should be individualized. The type of procedure and the surgical approach will depend on the patient’s goals, the degree of prolapse, clinical history, risk tolerance, the surgeon’s skill set, and whether or not there is an indication or relative contraindication for uterine removal at the time of prolapse repair.
While the FDA’s order does not apply to transabdominally placed surgical mesh, such as sacrocolpopexy, not all patients are ideal candidates for an abdominal sacrocolpopexy. Most notable are women with a history of multiple prior abdominal surgeries with higher rates of intraperitoneal adhesions. Ideally, to be cost-effective and to avoid general anesthesia, the surgical approach should be vaginal whenever possible.
Biologic versus native-tissue grafts
Currently, only low-quality evidence exists that compares the outcomes of biologic grafts with traditional native-tissue repairs in POP. Studies have been limited by poor reporting of methods, inconsistency in technique and materials used, and imprecise definitions. One Cochrane Review on the surgical management of POP concluded that biologic graft augmentation was associated with a lower failure rate (18%) within 1 to 2 years when compared with a traditional anterior colporrhaphy (28%).12
Based on consideration of all Cochrane Database Reviews and recent large systematic reviews, there clearly is a paucity of information on which to draw well-defined conclusions regarding the advantage of biomaterials in prolapse surgery.12-14 This is due in part to the variation in graft material used and the surgical technique employed.
Similarly, evidence is lacking regarding the superiority of one type of biologic graft over another. Furthermore, some of the grafts previously studied are no longer on the market.15 With the FDA’s removal of all transvaginal mesh, including xenografts, only allografts are available for pelvic floor reconstruction. Currently, only 3 commercial manufacturers market allografts for pelvic floor reconstruction. Each allograft is available in various sizes and all can be trimmed at the time of the surgical procedure to customize both the size and shape to fit the individual patient.
A novel technique using Axis Dermis and polypropylene suture
One of the commercially available allografts, Axis Dermis (Coloplast), is non–cross-linked and is derived from human cadaveric dermal tissue from the back and dorsum of the upper leg. It is sterilized by a proprietary Tutoplast️ sterilization process that uses gamma irradiation to inactivate and prevent the transmission of pathogens. This unique technique involving solvent dehydration means the graft is never freeze dried; thus, the natural tissue matrix is preserved.
Additionally, the allograft is antigen-free, which decreases the risk of tissue reaction (scarring/fibrosis) and aids in the process of host tissue remodeling; invasion by growth factors, blood cells, collagen, elastin, and neovascularization. This natural tissue remodeling facilitates the anticipated “reabsorption” of the graft by the host tissue, leaving the patient with a tissue scaffold, that is, a stronger layer of “fascia” beneath the muscularis.16 As a result of this “biocompatible” graft, the host tissue remodeling has been shown in the rat model to involve early cellular infiltration and angiogenesis (in the first week after implantation), that leads to an organized cellular architecture with greater tensile strength by week 4, and ultimately inability to distinguish host collagen from the implant by 8 to 12 weeks.17,18
Continue to: Steps in performing the technique...
Steps in performing the technique
To ensure that the graft is placed adjacent to the vaginal serosa, a full-thickness dissection is carried out to enter the true vesicovaginal space, which lies below all 4 histologic layers of the vagina (nonkeratinized stratified squamous epithelium, lamina propria, muscularis, and serosa). For the anterior dissection, a Tuohy epidural needle is used to achieve an accurate and consistent depth when injecting fluid (hydrodissection) to enter this true pelvic space (FIGURE 1). Correct entry into the vesicovaginal space can be confirmed visually by the presence of adipose tissue.

Many pelvic surgeons use the sacrospinous ligament (SSL) as a strong and reliable point of attachment for vaginal prolapse repair. It can be approached either anteriorly or posteriorly with careful dissection. Permanent suture (0-Prolene) is used to “bridge” the attachment between the SSL, the Axis Dermis graft, and the cervix (or vaginal apex). The suture is placed in the middle third and lower half of the ligament to avoid injury to nearby neurovascular structures.
While the surgeon may use any suture-capturing device, we prefer the Anchosure System (Neomedic). This device delivers a small anchor securely into the ligament through a single point of entry, minimizing the risk of postoperative pain for the patient. A 6 cm x 8 cm size Axis Dermis graft is then trimmed to meet the specifications of the patient’s anatomy.
Most commonly, we measure, mark, and trim the body of the graft to 5.5 cm in length with a width of 3 cm. The bilateral arms are approximately 1 cm in width and comprise the remaining length of the 8 cm graft (FIGURE 2). As shown in Figure 2, pre-made holes are marked and punched out using a large hollow needle. These serve as the points of attachment for the permanent suture to be “weaved” into the graft arms and delayed absorbable “tacking suture” to be attached from the pubocervical fascia at the bladder neck to the distal end of the graft. This facilitates fixation of the graft in the midline of the anterior vaginal wall, overlying any central distention-type defect.

Finally, following attachment of the SSL permanent suture to the distal graft arm, this suture is then attached to the proximal U-shaped end of the graft body (in the midline), followed by a deep and secure bite through the cervix (or vaginal vault apex) and back through the proximal graft. These SSL suspension sutures are then tied such that the distal arms of the graft advance down to the ligament. Care is taken not to tie down to the SSL itself, rather until the cervix (or apex) is reduced to its normal anatomical location.
After the graft is secured in place, the full-thickness vaginal wall is closed with delayed absorbable suture. Sterile 1-inch ribbon packing is placed in the vagina immediately to close any dead space between the vagina and the graft to decrease the risk of seroma or hematoma formation.
This newly developed technique, like many surgeries for POP, requires extensive knowledge of pelvic anatomy and skill in vaginal surgery, and we recommend referral to a subspecialist in Female Pelvic Medicine and Reconstructive Surgery.

Continue to: Upcoming plans to share outcomes data...
Upcoming plans to share outcomes data
We are in the process of performing a retrospective review of all of the cases we have performed at our institution using this technique of permanent suture bridging to the SSL within the arm of the biograft. Given the relatively recent FDA announcement, we have yet to establish any long-term outcomes data. However, the preliminary results at 6-month follow-up are promising and demonstrate a low (2.6%) failure rate, without significant safety concerns. We hope to publish these data as well as more data on longitudinal outcomes in the future.
In summary
Many women are at risk for native-tissue repair failure or are not well suited for an abdominal procedure to correct their pelvic support defect and restore their quality of life. As expert pelvic surgeons, we play an important role in the search for innovative solutions for these women. There is ample opportunity for future research and clinical trials to determine the best biologic materials and their optimal use in pelvic reconstructive surgery.
Originally, polypropylene mesh was designed for use in augmenting abdominal hernia repairs and later was adapted by manufacturers for use in POP repair. The FDA removal from the market of existing transvaginal synthetic mesh kits was a unique catalyst that challenged our community to develop transvaginal repairs using biologic grafts that are genuinely tailored to the unique needs of the female pelvic anatomy. ●
Pelvic organ prolapse (POP) is a common occurrence over the course of a woman’s lifetime, especially in parous women (up to 50% of women who have given birth).1 The anterior vaginal wall is the most common site of POP and has the highest recurrence rate of up to 70%.2 The risk of developing POP increases with age, obesity, White race, family history, and prior pelvic surgery, such as hysterectomy. It affects more than 3 million women in the United States alone, often negatively impacting sexual function and overall quality of life.3,4
Because women are living longer than ever before and are more active in their senior years, a long-lasting, durable surgical repair is desirable, if not necessary. To be cost-effective and to avoid general anesthesia, the surgical approach ideally should be vaginal.
Biologic and synthetic grafts to augment transvaginal repair traditionally are used to improve on the well-recognized high failure rate of native-tissue repair that is often seen at both short-term and medium-term follow-up.5 The failure rate is commonly referenced as 30% to 40% at 2-year follow-up and 61% to 70% at 5-year follow-up, well-established by the results of the OPTIMAL randomized clinical trial.6 The more recent Descent trial likewise demonstrates a higher failure rate of native-tissue repair versus transvaginal mesh repair at a shorter term of 30 to 42 months.7 Furthermore, the use of permanent versus absorbable suture in suspension of the vaginal apex is associated with lower short-term failure rates.8
Despite this Level I evidence that demonstrates a clear advantage for obtaining a longer or more durable repair with permanent materials, native-tissue repairs with absorbable suture are still performed routinely. Since the US Food and Drug Administration (FDA) ordered that the use of transvaginal surgical mesh augmentation for pelvic reconstructive surgery be discontinued, it is more important than ever to explore evolving alternative native-tissue augmentation repair techniques that hopefully can preserve the advantages and merits of vaginal surgery and achieve longer durability.9
Biologic graft augmentation use in transvaginal reconstruction
All biologic grafts, including allografts derived from human tissue and xenografts derived from animal tissue, are acellular constructs composed of extracellular matrix (ECM) that acts as scaffolding for the host tissue. The ECM is predominantly composed of collagen (types I and III) and noncollagenous fibronectin, laminin, and glycosaminoglycans in various amounts depending on the source tissue. The 3D presentation of ECM’s complex molecules allows for rapid repopulation of host cells and revascularization with eventual regeneration.
Once a biologic graft is placed surgically, the body’s response to the scaffold ECM mimics the normal wound-healing process, beginning with fibrin-rich matrix hemostasis and the subsequent innate immune response of neutrophil and M1 macrophage infiltration. M1 macrophages are proinflammatory and clear cellular debris and begin the process of graft scaffold degradation. The host tissue then begins the process of remodeling through pro-remodeling M2 macrophages and stem cell recruitment, proliferation, and differentiation.10 As the biologic graft provides initial structure and strength for pelvic repairs, the ideal ECM scaffold would not degrade before the host is able to fully undergo regeneration and maintain its structure and strength.
Biologic grafts differ in source (allograft or xenograft), type (pericardium, dermis, or bladder), developmental stage (fetal or adult), decellularization processing, and sterilization techniques. These 5 aspects determine the distinct 3D ECM scaffold structure, strength, and longevity. If the ECM scaffold is damaged or retains noncollagenous proteins during the preparation process, an inflammatory response is triggered in which the graft is degraded, resorbed, and replaced with scar tissue. Furthermore, certain processing techniques aimed at extending the ECM’s durability—that is, cross-linking collagen—results in the foreign body response in which there is no vascular infiltration or cellular penetration of the graft and a collagen capsule is created around the empty matrix.11 To avoid resorption or encapsulation of the graft, the ECM scaffolds of biologic grafts must be optimized to induce regeneration.
Continue to: Choosing surgical POP repair...
Choosing surgical POP repair
The decision to undergo surgical treatment for prolapse is a shared decision-making process between the patient and surgeon and always should be individualized. The type of procedure and the surgical approach will depend on the patient’s goals, the degree of prolapse, clinical history, risk tolerance, the surgeon’s skill set, and whether or not there is an indication or relative contraindication for uterine removal at the time of prolapse repair.
While the FDA’s order does not apply to transabdominally placed surgical mesh, such as sacrocolpopexy, not all patients are ideal candidates for an abdominal sacrocolpopexy. Most notable are women with a history of multiple prior abdominal surgeries with higher rates of intraperitoneal adhesions. Ideally, to be cost-effective and to avoid general anesthesia, the surgical approach should be vaginal whenever possible.
Biologic versus native-tissue grafts
Currently, only low-quality evidence exists that compares the outcomes of biologic grafts with traditional native-tissue repairs in POP. Studies have been limited by poor reporting of methods, inconsistency in technique and materials used, and imprecise definitions. One Cochrane Review on the surgical management of POP concluded that biologic graft augmentation was associated with a lower failure rate (18%) within 1 to 2 years when compared with a traditional anterior colporrhaphy (28%).12
Based on consideration of all Cochrane Database Reviews and recent large systematic reviews, there clearly is a paucity of information on which to draw well-defined conclusions regarding the advantage of biomaterials in prolapse surgery.12-14 This is due in part to the variation in graft material used and the surgical technique employed.
Similarly, evidence is lacking regarding the superiority of one type of biologic graft over another. Furthermore, some of the grafts previously studied are no longer on the market.15 With the FDA’s removal of all transvaginal mesh, including xenografts, only allografts are available for pelvic floor reconstruction. Currently, only 3 commercial manufacturers market allografts for pelvic floor reconstruction. Each allograft is available in various sizes and all can be trimmed at the time of the surgical procedure to customize both the size and shape to fit the individual patient.
A novel technique using Axis Dermis and polypropylene suture
One of the commercially available allografts, Axis Dermis (Coloplast), is non–cross-linked and is derived from human cadaveric dermal tissue from the back and dorsum of the upper leg. It is sterilized by a proprietary Tutoplast️ sterilization process that uses gamma irradiation to inactivate and prevent the transmission of pathogens. This unique technique involving solvent dehydration means the graft is never freeze dried; thus, the natural tissue matrix is preserved.
Additionally, the allograft is antigen-free, which decreases the risk of tissue reaction (scarring/fibrosis) and aids in the process of host tissue remodeling; invasion by growth factors, blood cells, collagen, elastin, and neovascularization. This natural tissue remodeling facilitates the anticipated “reabsorption” of the graft by the host tissue, leaving the patient with a tissue scaffold, that is, a stronger layer of “fascia” beneath the muscularis.16 As a result of this “biocompatible” graft, the host tissue remodeling has been shown in the rat model to involve early cellular infiltration and angiogenesis (in the first week after implantation), that leads to an organized cellular architecture with greater tensile strength by week 4, and ultimately inability to distinguish host collagen from the implant by 8 to 12 weeks.17,18
Continue to: Steps in performing the technique...
Steps in performing the technique
To ensure that the graft is placed adjacent to the vaginal serosa, a full-thickness dissection is carried out to enter the true vesicovaginal space, which lies below all 4 histologic layers of the vagina (nonkeratinized stratified squamous epithelium, lamina propria, muscularis, and serosa). For the anterior dissection, a Tuohy epidural needle is used to achieve an accurate and consistent depth when injecting fluid (hydrodissection) to enter this true pelvic space (FIGURE 1). Correct entry into the vesicovaginal space can be confirmed visually by the presence of adipose tissue.

Many pelvic surgeons use the sacrospinous ligament (SSL) as a strong and reliable point of attachment for vaginal prolapse repair. It can be approached either anteriorly or posteriorly with careful dissection. Permanent suture (0-Prolene) is used to “bridge” the attachment between the SSL, the Axis Dermis graft, and the cervix (or vaginal apex). The suture is placed in the middle third and lower half of the ligament to avoid injury to nearby neurovascular structures.
While the surgeon may use any suture-capturing device, we prefer the Anchosure System (Neomedic). This device delivers a small anchor securely into the ligament through a single point of entry, minimizing the risk of postoperative pain for the patient. A 6 cm x 8 cm size Axis Dermis graft is then trimmed to meet the specifications of the patient’s anatomy.
Most commonly, we measure, mark, and trim the body of the graft to 5.5 cm in length with a width of 3 cm. The bilateral arms are approximately 1 cm in width and comprise the remaining length of the 8 cm graft (FIGURE 2). As shown in Figure 2, pre-made holes are marked and punched out using a large hollow needle. These serve as the points of attachment for the permanent suture to be “weaved” into the graft arms and delayed absorbable “tacking suture” to be attached from the pubocervical fascia at the bladder neck to the distal end of the graft. This facilitates fixation of the graft in the midline of the anterior vaginal wall, overlying any central distention-type defect.

Finally, following attachment of the SSL permanent suture to the distal graft arm, this suture is then attached to the proximal U-shaped end of the graft body (in the midline), followed by a deep and secure bite through the cervix (or vaginal vault apex) and back through the proximal graft. These SSL suspension sutures are then tied such that the distal arms of the graft advance down to the ligament. Care is taken not to tie down to the SSL itself, rather until the cervix (or apex) is reduced to its normal anatomical location.
After the graft is secured in place, the full-thickness vaginal wall is closed with delayed absorbable suture. Sterile 1-inch ribbon packing is placed in the vagina immediately to close any dead space between the vagina and the graft to decrease the risk of seroma or hematoma formation.
This newly developed technique, like many surgeries for POP, requires extensive knowledge of pelvic anatomy and skill in vaginal surgery, and we recommend referral to a subspecialist in Female Pelvic Medicine and Reconstructive Surgery.

Continue to: Upcoming plans to share outcomes data...
Upcoming plans to share outcomes data
We are in the process of performing a retrospective review of all of the cases we have performed at our institution using this technique of permanent suture bridging to the SSL within the arm of the biograft. Given the relatively recent FDA announcement, we have yet to establish any long-term outcomes data. However, the preliminary results at 6-month follow-up are promising and demonstrate a low (2.6%) failure rate, without significant safety concerns. We hope to publish these data as well as more data on longitudinal outcomes in the future.
In summary
Many women are at risk for native-tissue repair failure or are not well suited for an abdominal procedure to correct their pelvic support defect and restore their quality of life. As expert pelvic surgeons, we play an important role in the search for innovative solutions for these women. There is ample opportunity for future research and clinical trials to determine the best biologic materials and their optimal use in pelvic reconstructive surgery.
Originally, polypropylene mesh was designed for use in augmenting abdominal hernia repairs and later was adapted by manufacturers for use in POP repair. The FDA removal from the market of existing transvaginal synthetic mesh kits was a unique catalyst that challenged our community to develop transvaginal repairs using biologic grafts that are genuinely tailored to the unique needs of the female pelvic anatomy. ●
- Maher C, Feiner B, Baessler K. Surgical management of pelvic organ prolapse in women. Cochrane Database Syst Rev. 2013:CD004014.
- Weber AM, Walters MD, Piedmonte MR, et al. Anterior colporrhaphy: a randomized trial of three surgical techniques. Am J Obstet Gynecol. 2001;185:1299-1304.
- Walters MD, Ridgeway BM. Surgical treatment of vaginal apex prolapse. Obstet Gynecol. 2013;121(2 pt 1):354-374.
- Meister MRL, Sutcliffe S, Lowder JL. Definitions of apical vaginal support loss: a systematic review. Am J Obstet Gynecol. 2017;216:232. e1-232.e14.
- Cox A, Herschorn S. Evaluation of current biologic meshes in pelvic organ prolapse repair. Curr Urol Rep. 2012;13:247-255.
- Jelovsek JE, Barber M, Brubaker K, et al. Effect of uterosacral ligament suspension vs sacrospinous ligament fixation with or without perioperative behavioral therapy for pelvic organ vaginal prolapse on surgical outcomes and prolapse symptoms at 5 years in the OPTIMAL randomized clinical trial. JAMA. 2018:319:1554-1565.
- Bowen ST, Moalli P, Abramowitch S, et al. Outcomes of the defining mechanisms of anterior vaginal wall descent trial [abstract 15]. Am J Obstet Gynecol. 2020;222:S770-S771.
- Chung CP, Miskimins R, Kuehl TJ, et al. Permanent suture used in uterosacral ligament suspension offers better anatomical support than delayed absorbable suture. Int Urogynecol J. 2012;23:223-227.
- US Food and Drug Administration. FDA takes action to protect women’s health, orders manufacturers of surgical mesh intended for transvaginal repair of pelvic organ prolapse to stop selling all devices. https://www.fda.gov/news-events/press-announcements/fda-takes-action-protect-womens-health-orders-manufacturers-surgical -mesh-intended-transvaginal. April 16, 2019. Accessed September 1, 2020.
- Londono R, Badylak SF. Biologic scaffolds for regenerative medicine: mechanisms of in vivo remodeling. Ann Biomed Eng. 2015;43:577-592.
- Cornwell KG, Landsman A, James KS. Extracellular matrix biomaterials for soft tissue repair. Clin Podiatr Med Surg. 2009;26: 507-523.
- Maher CM, Feiner B, Baessler K, et al. Surgical management of pelvic organ prolapse in women: the updated summary version Cochrane review. Int Urogynecol J. 2011;22:1445-1447.
- Maher C, Feiner B, Baessler K, et al. Surgery for women with anterior compartment prolapse. Cochrane Database Syst Rev. 2016;11:CD004014.
- Maher C, Feiner B, Baessler K, et al. Transvaginal mesh or grafts compared with native tissue repair for vaginal prolapse. Cochrane Database Syst Rev. 2016;2:CD012179.
- Rosenblatt P, Von Bargen E. Use of biologic grafts in pelvic organ prolapse surgery. Contemporary OB/GYN. 2017;62:14-19.
- Greenspan DC, Hernandez R, Faleris J. Histology of surgically implanted Tutoplast processed dermis. http://www.zimmerbiomet .co.il/images/lib_artHistologyDermis%2010.pdf. Accessed September 2, 2020.
- Williams D. Revisiting the definition of biocompatibility. Med Device Technol. 2003;14:10-13.
- Nosti PA, Carter CM, Sokol AI, et al. Transvaginal versus transabdominal placement of synthetic mesh at time of sacrocolpopexy. Female Pelvic Med Reconstr Surg. 2016;22:151-155.
- Maher C, Feiner B, Baessler K. Surgical management of pelvic organ prolapse in women. Cochrane Database Syst Rev. 2013:CD004014.
- Weber AM, Walters MD, Piedmonte MR, et al. Anterior colporrhaphy: a randomized trial of three surgical techniques. Am J Obstet Gynecol. 2001;185:1299-1304.
- Walters MD, Ridgeway BM. Surgical treatment of vaginal apex prolapse. Obstet Gynecol. 2013;121(2 pt 1):354-374.
- Meister MRL, Sutcliffe S, Lowder JL. Definitions of apical vaginal support loss: a systematic review. Am J Obstet Gynecol. 2017;216:232. e1-232.e14.
- Cox A, Herschorn S. Evaluation of current biologic meshes in pelvic organ prolapse repair. Curr Urol Rep. 2012;13:247-255.
- Jelovsek JE, Barber M, Brubaker K, et al. Effect of uterosacral ligament suspension vs sacrospinous ligament fixation with or without perioperative behavioral therapy for pelvic organ vaginal prolapse on surgical outcomes and prolapse symptoms at 5 years in the OPTIMAL randomized clinical trial. JAMA. 2018:319:1554-1565.
- Bowen ST, Moalli P, Abramowitch S, et al. Outcomes of the defining mechanisms of anterior vaginal wall descent trial [abstract 15]. Am J Obstet Gynecol. 2020;222:S770-S771.
- Chung CP, Miskimins R, Kuehl TJ, et al. Permanent suture used in uterosacral ligament suspension offers better anatomical support than delayed absorbable suture. Int Urogynecol J. 2012;23:223-227.
- US Food and Drug Administration. FDA takes action to protect women’s health, orders manufacturers of surgical mesh intended for transvaginal repair of pelvic organ prolapse to stop selling all devices. https://www.fda.gov/news-events/press-announcements/fda-takes-action-protect-womens-health-orders-manufacturers-surgical -mesh-intended-transvaginal. April 16, 2019. Accessed September 1, 2020.
- Londono R, Badylak SF. Biologic scaffolds for regenerative medicine: mechanisms of in vivo remodeling. Ann Biomed Eng. 2015;43:577-592.
- Cornwell KG, Landsman A, James KS. Extracellular matrix biomaterials for soft tissue repair. Clin Podiatr Med Surg. 2009;26: 507-523.
- Maher CM, Feiner B, Baessler K, et al. Surgical management of pelvic organ prolapse in women: the updated summary version Cochrane review. Int Urogynecol J. 2011;22:1445-1447.
- Maher C, Feiner B, Baessler K, et al. Surgery for women with anterior compartment prolapse. Cochrane Database Syst Rev. 2016;11:CD004014.
- Maher C, Feiner B, Baessler K, et al. Transvaginal mesh or grafts compared with native tissue repair for vaginal prolapse. Cochrane Database Syst Rev. 2016;2:CD012179.
- Rosenblatt P, Von Bargen E. Use of biologic grafts in pelvic organ prolapse surgery. Contemporary OB/GYN. 2017;62:14-19.
- Greenspan DC, Hernandez R, Faleris J. Histology of surgically implanted Tutoplast processed dermis. http://www.zimmerbiomet .co.il/images/lib_artHistologyDermis%2010.pdf. Accessed September 2, 2020.
- Williams D. Revisiting the definition of biocompatibility. Med Device Technol. 2003;14:10-13.
- Nosti PA, Carter CM, Sokol AI, et al. Transvaginal versus transabdominal placement of synthetic mesh at time of sacrocolpopexy. Female Pelvic Med Reconstr Surg. 2016;22:151-155.