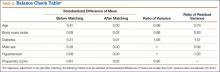
The balance of matching is checked using criteria suggested by Rubin21: (1) standardized difference of means of propensity score, (2) ratio of variances in propensity score in treated and control groups, and (3) for each covariate, ratio of variance in residuals orthogonal to propensity score in treated and control groups.
Table 1 lists the means of the background covariates for each group before and after matching. Table 2 lists the balance check results suggested by Rubin.21 After matching, all standardized differences of means are smaller than 0.25, and the variance ratios are between 0.5 and 2, which are the standards suggested21 for regression adjustment to be valid after matching.
After genetic matching, 31 bridged-therapy patients and 57 warfarin-only patients remained. After optimal matching, there were 31 patients in each group. Poisson regressions of datasets before and after matching adjustment were fitted.
Results
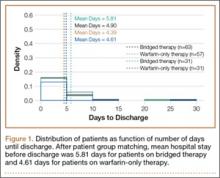
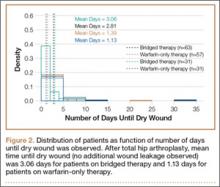
Wounds of bridged-therapy patients took longer to heal than wounds of warfarin-only patients both before (odds ratio, 2.16; P < .05) and after matching data (odds ratio, 2.39; P < .05) with respect to confounding factors. In addition, bridged-therapy patients had longer hospital stays both before (odds ratio 1.20; P < .05) and after matching data (odds ratio, 1.27; P < .05) with respect to confounding factors. Figures 1 and 2 are histograms displaying the 2 groups and their outcomes.
Discussion
For patients undergoing THA procedures, several important considerations should be taken into account. Colwell and colleagues2 showed that, compared with warfarin, enoxaparin offered a 0.1% higher rate of protection against venous thromboembolic disease after THA. However, patients given enoxaparin may face increased risks.25 Hallevi and colleagues26 demonstrated that, compared with warfarin, enoxaparin bridging increased the risk for serious bleeding in patients with cardioembolic stroke. In our review of the literature, we learned that the benefits of bridge therapy in thromboembolic disease have yet to be investigated in THA.
At our academic hospital, the extra costs associated with bridge therapy can be as much as about $200027 per day per patient. These costs can go much higher, depending on type of patient and types of resources used. Over the 2-year period covered by our study, the costs of using enoxaparin amounted to about $151,200 ($2000 × 1.2 days per patient). If bridging offers no significant protection against thromboembolic disease, then it would be more cost-effective to use a single anticoagulant, particularly enoxaparin, for high-risk patients.
There are significant risk factors associated with prolonged healing of surgical wounds. Protocols outlining these factors may help reduce costs. In addition, when deciding on the use of aggressive anticoagulation therapy, surgeons must consider the risks for prolonged leakage and infection in addition to the risk for thromboembolic disease. Protocols may aid in this process as well. Our study results showed that, compared with warfarin-only therapy, bridged therapy (enoxaparin and warfarin) was associated with longer hospital stays. Further research should examine whether there are advantages that justify the higher risks of delayed wound healing and subsequent infection. Improving our understanding of risk factors associated with anticoagulation therapy will make orthopedic surgery safer for patients.