Placement of a left ventricular assist device (LVAD) after a heart attack has been found to suppress certain cellular signaling pathways that protect coronary tissue, but at the same time LVAD placement seemed to help normalize other protective properties in areas of the heart closest to the infarcted region, investigators reported in a recent study.
The findings could have implications in determining the best method for unloading and other medical therapies in the aftermath of a heart attack, Dr. Keshava Rajagopal of the University of Texas, Houston, and associates reported in the November issue of the Journal of Thoracic and Cardiovascular Surgery (2015;150:1332-41).
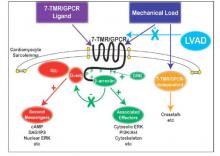
To study the effect of LVAD on cardiac tissue, the investigators induced myocardial infarction in sheep and then placed the animals on LVAD support for 2 weeks. After 10 more weeks of observation, the investigators harvested and analyzed the myocardial specimens. The principal goal of the study was to investigate how heart attack and subsequent short-term mechanical support of the left ventricle can influence signaling controlled by the protein beta-arrestin.
They found that an infarction of myocardial tissue caused activation of the beta-arrestin protein that regulates cellular pathways that can benefit cardiac cells. At the same time, LVAD support inhibited beta-arrestin activation, specifically in regulating pathways of two cardioprotective proteins: Akt, also called protein kinase B (PKB), and, to a lesser extent, ERK-1 and -2.
They also found that MI resulted in regional activation of load-induced signaling of cardiac G protein-coupled receptor (GPCR) via G proteins.
“These studies demonstrate that small platform catheter-based LVAD support exerts suppressive effects on cardioprotective beta-arrestin–mediated signal transduction, while normalizing the signaling networks of G-alpha-q–coupled cardiac GPCRS in the MI-adjacent zone,” Dr. Rajagopal and colleagues said.
They acknowledged that further studies are needed to better understand the roles that specific GPCRs in beta-arrestin–regulated signaling play in left ventricle dysfunction after a heart attack and to help define the optimal timing for LVAD based on signaling and genetic markers along with standard LV functional endpoints.
The authors had no disclosures.