Postoperative analgesia was provided based on a standard institutional animal care and use committee protocol. Rabbits were permitted full cage activity and nutrition ad libitum. Wound healing, body weight, and signs of distress were monitored daily.
Outcome Measures
Seven days after surgery, the rabbits were euthanized with administration of phenobarbital (100 mg/kg of body weight). Arterial blood samples were obtained from the auricular vein to ensure that the rabbits were not systemically infected. Using a sterile technique, the screw, polyethylene washer, lateral femoral condyle bone from the defect, and joint capsule were cultured. Harvested bone and soft tissues were weighed and immediately homogenized (PowerGen Model 35 Handheld Homogenizer; Thermo Fisher Scientific, Inc, Waltham, Massachusetts). Implants were sonicated (UBATH-Y; World Precision Instruments, Inc, Sarasota, Florida) in cold saline to obtain a sensitive culture.18
Bacterial quantification was determined by using trypticase soy agar plates after 24 hours of growth. Final CFU were calculated after serial dilutions and were standardized per gram of biopsied tissues.19 Members of the team were blinded to the treatment type.
Statistical Analysis
Statistical differences in mean bacterial burden were calculated independently for lateral condyle bone, joint capsule, polyethylene, and screws by conducting a Student t test.
Results
Treatment effect was higher than expected, and the study was terminated after 8 animals completed the protocol. All 8 rabbits tolerated the procedures well and were appropriately monitored during the postoperative period. No animals had signs of systemic infection or positive blood culture. All local cultures for screw, polyethylene washer, lateral femoral condyle defect, and joint capsule were positive.
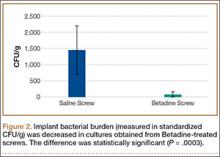
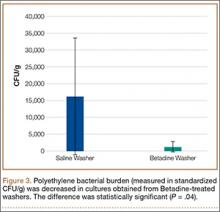
Statistically significant decreases were shown in the bacterial burden of the Betadine-irrigated screws and the Betadine-irrigated polyethylene washers compared with the saline-irrigated controls. Betadine-irrigated screws grew an average of 7.16 × 101 CFU of S aureus/g, whereas screws from control knees grew an average of 1.45 × 103 CFU/g (P = .0003) (Figure 2). Betadine-treated washers grew an average of 1.28 × 103 CFU/g compared with 1.62 × 104 CFU/g for control washers (P =. 04) (Figure 3).
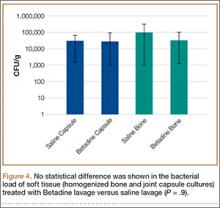
A trend toward decreased bacterial counts was shown in Betadine-treated soft tissues compared with saline-treated soft tissues, but the difference did not reach statistical significance (P = .9). Biopsied joint capsule from knees treated with Betadine grew an average of 2.84 × 104 CFU/g compared with an average of 3.16 × 104 CFU/g in control-rabbit knees (Figure 4). Cultured lateral condyle from Betadine-treated knees had an average bacterial load of 3.22 × 104 CFU/g compared with an average bacterial load of 1 × 105 CFU/g in control knees (Figure 4).
Discussion
Knees irrigated with Betadine showed a significant (P = .0003) decrease in metal implant–related S aureus bacterial counts by 20-fold and a significant (P < .05) decrease in polyethylene implant–related counts by more than 10-fold. This arthroplasty model used Betadine lavage as a treatment adjunct with intravenously administered antibiotics and polyethylene exchange. Our 1-week interval after the index procedure classifies the infection as an acute postoperative arthroplasty infection (occurring less than 4 weeks postoperatively).
The gold standard treatment for these infections is irrigation and débridement with component retention.18 The success rate has been reported to be as high as 71%20 but was closer to 44% in a study by Fridkin and colleagues,21 especially with more virulent bacteria. Staphylococcal species, higher American Society of Anesthesiologists scores, and frank pus around the prosthesis were markers of débridement failure in a recent study by Azzam and colleagues.18
The majority of postoperative joint arthroplasty infections are caused by S aureus, and the incidence of MRSA bacteria continues to rise.22 Community-acquired MRSA is increasing at an alarming rate and is now the predominant organism in skin and soft-tissue infections.23 Organism resistance also occurs at a cellular level by the formation of a glycocalyx layer, or biofilm. This layer assists in changing the phenotypic properties of the organism and decreases the efficacy of antibiotics.24 The self-produced layer of extracellular matrices, deoxyribonucleic acid, and polysaccharides attaches to inert material, preventing phagocytic action by neutrophils. In addition to antibacterial activity, povidone-iodine has antibiofilm activity against Staphylococcal species.25 The active ingredient targets the gene that produces biofilm. This correlates to our study in which the largest decrease in bacterial counts was noted on the implants.
The use of Betadine lavage has shown some promise in vivo as well. A prospective randomized controlled trial26 used 3.5% Betadine irrigation to prevent spine infection. No infections occurred in the Betadine group compared with a deep-infection rate of 2.9% in the control group. Brown and colleagues8 reviewed 1862 hip and knee arthroplasty cases before the use of Betadine lavage and 688 cases after the use of Betadine lavage and found a decrease in infection rate, from 0.97% to 0.15%. S aureus caused 13 of the 18 infections in the control group. These studies8,26 used Betadine lavage for prophylaxis and prevention of deep spine and arthroplasty infection. Betadine lavage as a treatment adjunct for acute arthroplasty infection has not been studied clinically. It has the potential to increase isolated incision and débridement success and to improve component survivorship.