User login
5 years out, sleeve safer than gastric bypass
Five years out, sleeve gastrectomy had a lower risk of mortality, complications, and reinterventions than gastric bypass, but there was a higher risk of surgical revision, including conversion to another bariatric surgery, gastrectomy, or anastomotic revision, according to a new analysis.
Sleeve gastrectomy has gained rapid popularity, and now represents 60% of all bariatric procedures. It has demonstrated good efficacy and short-term safety, it is easier to perform than laparoscopic Roux-en-Y gastric bypass, and it is a safe option for high-risk patients, authors led by Ryan Howard, MD, of University of Michigan, Ann Arbor, wrote in JAMA Surgery.
Still, there are few comparative data on the long-term efficacy of the two procedures. Randomized, controlled trials have conducted long-term follow-up, but their small size has made it difficult to detect differences in rare outcomes. Observational studies are limited by the potential for bias. A novel approach to limiting bias is instrumental variables analysis, which controls for possible confounding using a factor that impacts treatment choice, but not patient outcome, to control for possible confounders. Studies using this approach confirmed the superior safety profile of sleeve gastrectomy in the short term.
The current study’s authors, used that method to examined 5-year outcomes in a Medicare population, in which obesity and its complications are especially frequent. Partly because of that lack of data, the Medical Evidence Development and Coverage Committee has called for more data in older patients and in patients with disabilities.
The researchers analyzed data from 95,405 Medicare claims between 2012 and 2018, using state-level variation in sleeve gastrectomy as the instrumental variable.
At 5 years, sleeve gastrectomy was associated with a lower cumulative frequency of mortality (4.27%; 95% confidence interval, 4.25%-4.30% vs. 5.67%; 95% CI, 5.63%-5.69%]), complications (22.10%; 95% CI, 22.06%-22.13% vs. 29.03%; 95% CI, 28.99%-29.08%), and reintervention (25.23%; 95% CI, 25.19%-25.27% vs. 33.57%; 95% CI, 33.52%-33.63%). At 5 years, surgical revision was more common in the sleeve gastrectomy group (2.91%; 95% CI, 2.90%-2.93% vs. 1.46%; 95% CI, 1.45%-1.47%).
The sleeve gastrectomy group had lower odds of all-cause hospitalization at 1 year (adjusted hazard ratio, 0.83; 95% CI, 0.80-0.86) and 3 years (aHR, 0.94; 95% CI, 0.90-0.98), as well as emergency department use at 1 year (aHR, 0.87; 95% CI, 0.84-0.90) and 3 years (aHR, 0.93; 95% CI, 0.90-0.97). There was no significant difference between the two groups at 5 years with respect to either outcome.
The effort to understand long-term outcomes of these two procedures has been challenging because follow-up is often incomplete, and because reporting isn’t always standardized, according Anita P. Courcoulas, MD, MPH, and Bestoun Ahmed, MD, in an accompanying editorial in JAMA Surgery. They noted that the differences in mortality is a new finding and the difference in surgical revisions confirmed something often seen in clinical practice. “Overall, these novel methods, which creatively balance unmeasured factors, have succeeded in providing important incremental findings about the long-term comparative safety outcomes between bariatric procedures that will be helpful in clinical practice,” the editorial authors wrote.
The complications discussed in the study are also difficult to interpret, according to Ali Aminian, MD, who is a professor of surgery and director of Bariatric and Metabolic Institute at Cleveland Clinic. They may be related to the surgery, or they may be complications that accrue as patients age. “So that doesn’t mean those were surgical complications, but [the findings are] in line with the other literature that [gastric sleeve] may be safer than gastric bypass, but in a different cohort of patients,” said Dr. Aminian, who was asked to comment.
“I thought it validated that which many of us in clinical practice see on a day to day basis,” said Shanu Kothari, MD, chair of surgery at Prisma Health, and the current president of American Society for Bariatric and Metabolic Surgery. He pointed out that the study was limited by its reliance on administrative claims, which makes it impossible to know the reduction in weight and obesity-related comorbid conditions following the procedures, as well as factors driving individual decisions: A surgeon might offer sleeve to a patient at higher risk of complications, but a gastric bypass to someone with more comorbidities. “What we don’t know is how to interpret this 35,000-foot view of Medicare data to that conversation with the patient sitting right in front of you,” said Dr. Kothari.
The authors similarly cited the “lack of clinical granularity in administrative claims data” among study limitations, as well as how the use of instrumental variables may leave the findings less applicable to patients more strongly indicated for one procedure over the other.
“Longer-term randomized clinical trials and observational studies are warranted to confirm these findings,” the study authors concluded. “Understanding the risk profile of various bariatric operations may further help patients and surgeons make the most appropriate decisions regarding plans of care.”
The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases. Some study authors and editorialists reported funding from various groups and institutions, such as the National Institutes of Health and the VA Ann Arbor Health System. Dr. Kothari and Dr. Aminian have no relevant financial disclosures.
Five years out, sleeve gastrectomy had a lower risk of mortality, complications, and reinterventions than gastric bypass, but there was a higher risk of surgical revision, including conversion to another bariatric surgery, gastrectomy, or anastomotic revision, according to a new analysis.
Sleeve gastrectomy has gained rapid popularity, and now represents 60% of all bariatric procedures. It has demonstrated good efficacy and short-term safety, it is easier to perform than laparoscopic Roux-en-Y gastric bypass, and it is a safe option for high-risk patients, authors led by Ryan Howard, MD, of University of Michigan, Ann Arbor, wrote in JAMA Surgery.
Still, there are few comparative data on the long-term efficacy of the two procedures. Randomized, controlled trials have conducted long-term follow-up, but their small size has made it difficult to detect differences in rare outcomes. Observational studies are limited by the potential for bias. A novel approach to limiting bias is instrumental variables analysis, which controls for possible confounding using a factor that impacts treatment choice, but not patient outcome, to control for possible confounders. Studies using this approach confirmed the superior safety profile of sleeve gastrectomy in the short term.
The current study’s authors, used that method to examined 5-year outcomes in a Medicare population, in which obesity and its complications are especially frequent. Partly because of that lack of data, the Medical Evidence Development and Coverage Committee has called for more data in older patients and in patients with disabilities.
The researchers analyzed data from 95,405 Medicare claims between 2012 and 2018, using state-level variation in sleeve gastrectomy as the instrumental variable.
At 5 years, sleeve gastrectomy was associated with a lower cumulative frequency of mortality (4.27%; 95% confidence interval, 4.25%-4.30% vs. 5.67%; 95% CI, 5.63%-5.69%]), complications (22.10%; 95% CI, 22.06%-22.13% vs. 29.03%; 95% CI, 28.99%-29.08%), and reintervention (25.23%; 95% CI, 25.19%-25.27% vs. 33.57%; 95% CI, 33.52%-33.63%). At 5 years, surgical revision was more common in the sleeve gastrectomy group (2.91%; 95% CI, 2.90%-2.93% vs. 1.46%; 95% CI, 1.45%-1.47%).
The sleeve gastrectomy group had lower odds of all-cause hospitalization at 1 year (adjusted hazard ratio, 0.83; 95% CI, 0.80-0.86) and 3 years (aHR, 0.94; 95% CI, 0.90-0.98), as well as emergency department use at 1 year (aHR, 0.87; 95% CI, 0.84-0.90) and 3 years (aHR, 0.93; 95% CI, 0.90-0.97). There was no significant difference between the two groups at 5 years with respect to either outcome.
The effort to understand long-term outcomes of these two procedures has been challenging because follow-up is often incomplete, and because reporting isn’t always standardized, according Anita P. Courcoulas, MD, MPH, and Bestoun Ahmed, MD, in an accompanying editorial in JAMA Surgery. They noted that the differences in mortality is a new finding and the difference in surgical revisions confirmed something often seen in clinical practice. “Overall, these novel methods, which creatively balance unmeasured factors, have succeeded in providing important incremental findings about the long-term comparative safety outcomes between bariatric procedures that will be helpful in clinical practice,” the editorial authors wrote.
The complications discussed in the study are also difficult to interpret, according to Ali Aminian, MD, who is a professor of surgery and director of Bariatric and Metabolic Institute at Cleveland Clinic. They may be related to the surgery, or they may be complications that accrue as patients age. “So that doesn’t mean those were surgical complications, but [the findings are] in line with the other literature that [gastric sleeve] may be safer than gastric bypass, but in a different cohort of patients,” said Dr. Aminian, who was asked to comment.
“I thought it validated that which many of us in clinical practice see on a day to day basis,” said Shanu Kothari, MD, chair of surgery at Prisma Health, and the current president of American Society for Bariatric and Metabolic Surgery. He pointed out that the study was limited by its reliance on administrative claims, which makes it impossible to know the reduction in weight and obesity-related comorbid conditions following the procedures, as well as factors driving individual decisions: A surgeon might offer sleeve to a patient at higher risk of complications, but a gastric bypass to someone with more comorbidities. “What we don’t know is how to interpret this 35,000-foot view of Medicare data to that conversation with the patient sitting right in front of you,” said Dr. Kothari.
The authors similarly cited the “lack of clinical granularity in administrative claims data” among study limitations, as well as how the use of instrumental variables may leave the findings less applicable to patients more strongly indicated for one procedure over the other.
“Longer-term randomized clinical trials and observational studies are warranted to confirm these findings,” the study authors concluded. “Understanding the risk profile of various bariatric operations may further help patients and surgeons make the most appropriate decisions regarding plans of care.”
The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases. Some study authors and editorialists reported funding from various groups and institutions, such as the National Institutes of Health and the VA Ann Arbor Health System. Dr. Kothari and Dr. Aminian have no relevant financial disclosures.
Five years out, sleeve gastrectomy had a lower risk of mortality, complications, and reinterventions than gastric bypass, but there was a higher risk of surgical revision, including conversion to another bariatric surgery, gastrectomy, or anastomotic revision, according to a new analysis.
Sleeve gastrectomy has gained rapid popularity, and now represents 60% of all bariatric procedures. It has demonstrated good efficacy and short-term safety, it is easier to perform than laparoscopic Roux-en-Y gastric bypass, and it is a safe option for high-risk patients, authors led by Ryan Howard, MD, of University of Michigan, Ann Arbor, wrote in JAMA Surgery.
Still, there are few comparative data on the long-term efficacy of the two procedures. Randomized, controlled trials have conducted long-term follow-up, but their small size has made it difficult to detect differences in rare outcomes. Observational studies are limited by the potential for bias. A novel approach to limiting bias is instrumental variables analysis, which controls for possible confounding using a factor that impacts treatment choice, but not patient outcome, to control for possible confounders. Studies using this approach confirmed the superior safety profile of sleeve gastrectomy in the short term.
The current study’s authors, used that method to examined 5-year outcomes in a Medicare population, in which obesity and its complications are especially frequent. Partly because of that lack of data, the Medical Evidence Development and Coverage Committee has called for more data in older patients and in patients with disabilities.
The researchers analyzed data from 95,405 Medicare claims between 2012 and 2018, using state-level variation in sleeve gastrectomy as the instrumental variable.
At 5 years, sleeve gastrectomy was associated with a lower cumulative frequency of mortality (4.27%; 95% confidence interval, 4.25%-4.30% vs. 5.67%; 95% CI, 5.63%-5.69%]), complications (22.10%; 95% CI, 22.06%-22.13% vs. 29.03%; 95% CI, 28.99%-29.08%), and reintervention (25.23%; 95% CI, 25.19%-25.27% vs. 33.57%; 95% CI, 33.52%-33.63%). At 5 years, surgical revision was more common in the sleeve gastrectomy group (2.91%; 95% CI, 2.90%-2.93% vs. 1.46%; 95% CI, 1.45%-1.47%).
The sleeve gastrectomy group had lower odds of all-cause hospitalization at 1 year (adjusted hazard ratio, 0.83; 95% CI, 0.80-0.86) and 3 years (aHR, 0.94; 95% CI, 0.90-0.98), as well as emergency department use at 1 year (aHR, 0.87; 95% CI, 0.84-0.90) and 3 years (aHR, 0.93; 95% CI, 0.90-0.97). There was no significant difference between the two groups at 5 years with respect to either outcome.
The effort to understand long-term outcomes of these two procedures has been challenging because follow-up is often incomplete, and because reporting isn’t always standardized, according Anita P. Courcoulas, MD, MPH, and Bestoun Ahmed, MD, in an accompanying editorial in JAMA Surgery. They noted that the differences in mortality is a new finding and the difference in surgical revisions confirmed something often seen in clinical practice. “Overall, these novel methods, which creatively balance unmeasured factors, have succeeded in providing important incremental findings about the long-term comparative safety outcomes between bariatric procedures that will be helpful in clinical practice,” the editorial authors wrote.
The complications discussed in the study are also difficult to interpret, according to Ali Aminian, MD, who is a professor of surgery and director of Bariatric and Metabolic Institute at Cleveland Clinic. They may be related to the surgery, or they may be complications that accrue as patients age. “So that doesn’t mean those were surgical complications, but [the findings are] in line with the other literature that [gastric sleeve] may be safer than gastric bypass, but in a different cohort of patients,” said Dr. Aminian, who was asked to comment.
“I thought it validated that which many of us in clinical practice see on a day to day basis,” said Shanu Kothari, MD, chair of surgery at Prisma Health, and the current president of American Society for Bariatric and Metabolic Surgery. He pointed out that the study was limited by its reliance on administrative claims, which makes it impossible to know the reduction in weight and obesity-related comorbid conditions following the procedures, as well as factors driving individual decisions: A surgeon might offer sleeve to a patient at higher risk of complications, but a gastric bypass to someone with more comorbidities. “What we don’t know is how to interpret this 35,000-foot view of Medicare data to that conversation with the patient sitting right in front of you,” said Dr. Kothari.
The authors similarly cited the “lack of clinical granularity in administrative claims data” among study limitations, as well as how the use of instrumental variables may leave the findings less applicable to patients more strongly indicated for one procedure over the other.
“Longer-term randomized clinical trials and observational studies are warranted to confirm these findings,” the study authors concluded. “Understanding the risk profile of various bariatric operations may further help patients and surgeons make the most appropriate decisions regarding plans of care.”
The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases. Some study authors and editorialists reported funding from various groups and institutions, such as the National Institutes of Health and the VA Ann Arbor Health System. Dr. Kothari and Dr. Aminian have no relevant financial disclosures.
FROM JAMA SURGERY
Weight-loss surgery linked to fewer cardiovascular events, more so with RYGB
Those are the key findings of a retrospective analysis of a large group of patients who received care at the Cleveland Clinic between 1998 and 2017. MACE is defined as first occurrence of coronary artery events, cerebrovascular events, heart failure, nephropathy, atrial fibrillation, and all-cause mortality.
“I think what it tells us is that, in making these choices and in counseling patients about the potential advantages of undergoing bariatric surgery for their obesity and diabetes, that they should know that they’re more likely to be protected by a Roux-en-Y gastric bypass, although certainly sleeve gastrectomy is effective,” said study coauthor Steven E. Nissen, MD, who is the chief academic officer of the Heart and Vascular Institute at the Cleveland Clinic.
Previous studies have shown a benefit to metabolic surgery in patients with type 2 diabetes and obesity, improving diabetes control and altering cardiometabolic risk factors. Others have shown a link between surgery and reduced mortality. Most studies examined the impact of RYGB. SG is a newer procedure, but its relative simplicity and lower complication rate have helped it become the most commonly performed metabolic surgery in the world.
“There was no study to compare gastric bypass and sleeve gastrectomy head to head in terms of reduction in risk of cardiovascular disease. There are studies comparing these two procedures for diabetes control and weight loss, but not specifically in terms of effects on their risk of developing cardiovascular disease. That’s the unique feature of this study,” said lead author Ali Aminian, MD, who is director of the Bariatric and Metabolic Institute at the Cleveland Clinic.
The researchers included 2,287 adults with type 2 diabetes and a body mass index of at least 30 kg/m2, with no history of solid organ transplant, severe heart failure, or active cancer. 1,362 underwent RYGB, and 693 SG. Outcomes were compared with 11,435 matched nonsurgical patients.
At 5 years, 13.7% of the RYGB group experienced a MACE (95% confidence interval, 11.4-15.9), compared with 24.7% of the SG group for a relative reduction of 33% (95% CI, 19.0-30.0; adjusted hazard ratio, 0.77; P = .035). The nonsurgical group had a 5-year MACE incidence of 30.4% (95% CI, 29.4-31.5). Compared with usual care, the risk of MACE was lower in both the RYGB group (HR, 0.53; P < .001) and the SG group (HR, 0.69; P < .001). The researchers also analyzed the cumulative incidence of all-cause mortality, myocardial infarction, and ischemic stroke (three-component MACE) at 5 years. The cumulative incidence of three-component MACE at 5 years was 15.5% in the usual care group, 6.4% in the RYGB group (HR, 0.53 versus usual care; P < .001) and 11.8% in the SG group (HR vs. usual care, 0.65; P = .006).
The RYGB group had less nephropathy at 5 years (2.8% vs. 8.3%; HR, 0.47; P = .005), and experienced a greater reduction in weight, glycated hemoglobin, and diabetes and cardiovascular medication use. At 5 years, RYGB was associated with a higher frequency of upper endoscopy (45.8% vs. 35.6%, P < .001) and abdominal surgical procedures (10.8% vs. 5.4%, P = .001), compared with SG.
“Both procedures are extremely safe and extremely effective,” said Dr. Aminian. He pointed out the need to consider multiple factors when choosing between the procedures, including overall health, weight, comorbidities, and the patient’s values and goals.
A few factors may be contraindicated for one procedure or another. The sleeve may worsen severe reflux disease, while the gastric bypass may interfere more with absorption of psychiatric medications. Some patients may have multiple comorbidities that could point to a less risky procedure. “Decision-making should not be solely based on findings of this study. All these conditions need to be considered when patients and surgeons make a final decision about the most appropriate procedure,” said Dr. Aminian.
Dr. Nissen noted that the associations were wide ranging, including classic outcomes like death, stroke, and heart failure, but also extending to heart failure, coronary events, cerebral vascular events, nephropathy, and atrial fibrillation. “I found the nephropathy results to be amongst the most striking, that Roux-en-Y really dramatically reduced the risk of neuropathy,” he added. That’s a particularly important point because end-stage renal disease is a common cause of diabetes mortality.
Dr. Nissen acknowledged the limitations of the retrospective nature of the study, though he feels confident that the relationships are causal. “Bariatric surgery desperately needs a randomized, controlled trial, where both groups get intensive dietary and lifestyle counseling, but one group gets metabolic surgery and the other doesn’t. Given the dramatic effects in diabetic patients of reducing their hemoglobin A1c in a sustained way, reducing their body weight. We think these are very strong data to suggest that we have a major reduction in all the endpoints. If we’re right about this, the randomized controlled trial will show that dramatic effect, and will convince even the skeptics that metabolic surgery is the best way to go.”
Those are the key findings of a retrospective analysis of a large group of patients who received care at the Cleveland Clinic between 1998 and 2017. MACE is defined as first occurrence of coronary artery events, cerebrovascular events, heart failure, nephropathy, atrial fibrillation, and all-cause mortality.
“I think what it tells us is that, in making these choices and in counseling patients about the potential advantages of undergoing bariatric surgery for their obesity and diabetes, that they should know that they’re more likely to be protected by a Roux-en-Y gastric bypass, although certainly sleeve gastrectomy is effective,” said study coauthor Steven E. Nissen, MD, who is the chief academic officer of the Heart and Vascular Institute at the Cleveland Clinic.
Previous studies have shown a benefit to metabolic surgery in patients with type 2 diabetes and obesity, improving diabetes control and altering cardiometabolic risk factors. Others have shown a link between surgery and reduced mortality. Most studies examined the impact of RYGB. SG is a newer procedure, but its relative simplicity and lower complication rate have helped it become the most commonly performed metabolic surgery in the world.
“There was no study to compare gastric bypass and sleeve gastrectomy head to head in terms of reduction in risk of cardiovascular disease. There are studies comparing these two procedures for diabetes control and weight loss, but not specifically in terms of effects on their risk of developing cardiovascular disease. That’s the unique feature of this study,” said lead author Ali Aminian, MD, who is director of the Bariatric and Metabolic Institute at the Cleveland Clinic.
The researchers included 2,287 adults with type 2 diabetes and a body mass index of at least 30 kg/m2, with no history of solid organ transplant, severe heart failure, or active cancer. 1,362 underwent RYGB, and 693 SG. Outcomes were compared with 11,435 matched nonsurgical patients.
At 5 years, 13.7% of the RYGB group experienced a MACE (95% confidence interval, 11.4-15.9), compared with 24.7% of the SG group for a relative reduction of 33% (95% CI, 19.0-30.0; adjusted hazard ratio, 0.77; P = .035). The nonsurgical group had a 5-year MACE incidence of 30.4% (95% CI, 29.4-31.5). Compared with usual care, the risk of MACE was lower in both the RYGB group (HR, 0.53; P < .001) and the SG group (HR, 0.69; P < .001). The researchers also analyzed the cumulative incidence of all-cause mortality, myocardial infarction, and ischemic stroke (three-component MACE) at 5 years. The cumulative incidence of three-component MACE at 5 years was 15.5% in the usual care group, 6.4% in the RYGB group (HR, 0.53 versus usual care; P < .001) and 11.8% in the SG group (HR vs. usual care, 0.65; P = .006).
The RYGB group had less nephropathy at 5 years (2.8% vs. 8.3%; HR, 0.47; P = .005), and experienced a greater reduction in weight, glycated hemoglobin, and diabetes and cardiovascular medication use. At 5 years, RYGB was associated with a higher frequency of upper endoscopy (45.8% vs. 35.6%, P < .001) and abdominal surgical procedures (10.8% vs. 5.4%, P = .001), compared with SG.
“Both procedures are extremely safe and extremely effective,” said Dr. Aminian. He pointed out the need to consider multiple factors when choosing between the procedures, including overall health, weight, comorbidities, and the patient’s values and goals.
A few factors may be contraindicated for one procedure or another. The sleeve may worsen severe reflux disease, while the gastric bypass may interfere more with absorption of psychiatric medications. Some patients may have multiple comorbidities that could point to a less risky procedure. “Decision-making should not be solely based on findings of this study. All these conditions need to be considered when patients and surgeons make a final decision about the most appropriate procedure,” said Dr. Aminian.
Dr. Nissen noted that the associations were wide ranging, including classic outcomes like death, stroke, and heart failure, but also extending to heart failure, coronary events, cerebral vascular events, nephropathy, and atrial fibrillation. “I found the nephropathy results to be amongst the most striking, that Roux-en-Y really dramatically reduced the risk of neuropathy,” he added. That’s a particularly important point because end-stage renal disease is a common cause of diabetes mortality.
Dr. Nissen acknowledged the limitations of the retrospective nature of the study, though he feels confident that the relationships are causal. “Bariatric surgery desperately needs a randomized, controlled trial, where both groups get intensive dietary and lifestyle counseling, but one group gets metabolic surgery and the other doesn’t. Given the dramatic effects in diabetic patients of reducing their hemoglobin A1c in a sustained way, reducing their body weight. We think these are very strong data to suggest that we have a major reduction in all the endpoints. If we’re right about this, the randomized controlled trial will show that dramatic effect, and will convince even the skeptics that metabolic surgery is the best way to go.”
Those are the key findings of a retrospective analysis of a large group of patients who received care at the Cleveland Clinic between 1998 and 2017. MACE is defined as first occurrence of coronary artery events, cerebrovascular events, heart failure, nephropathy, atrial fibrillation, and all-cause mortality.
“I think what it tells us is that, in making these choices and in counseling patients about the potential advantages of undergoing bariatric surgery for their obesity and diabetes, that they should know that they’re more likely to be protected by a Roux-en-Y gastric bypass, although certainly sleeve gastrectomy is effective,” said study coauthor Steven E. Nissen, MD, who is the chief academic officer of the Heart and Vascular Institute at the Cleveland Clinic.
Previous studies have shown a benefit to metabolic surgery in patients with type 2 diabetes and obesity, improving diabetes control and altering cardiometabolic risk factors. Others have shown a link between surgery and reduced mortality. Most studies examined the impact of RYGB. SG is a newer procedure, but its relative simplicity and lower complication rate have helped it become the most commonly performed metabolic surgery in the world.
“There was no study to compare gastric bypass and sleeve gastrectomy head to head in terms of reduction in risk of cardiovascular disease. There are studies comparing these two procedures for diabetes control and weight loss, but not specifically in terms of effects on their risk of developing cardiovascular disease. That’s the unique feature of this study,” said lead author Ali Aminian, MD, who is director of the Bariatric and Metabolic Institute at the Cleveland Clinic.
The researchers included 2,287 adults with type 2 diabetes and a body mass index of at least 30 kg/m2, with no history of solid organ transplant, severe heart failure, or active cancer. 1,362 underwent RYGB, and 693 SG. Outcomes were compared with 11,435 matched nonsurgical patients.
At 5 years, 13.7% of the RYGB group experienced a MACE (95% confidence interval, 11.4-15.9), compared with 24.7% of the SG group for a relative reduction of 33% (95% CI, 19.0-30.0; adjusted hazard ratio, 0.77; P = .035). The nonsurgical group had a 5-year MACE incidence of 30.4% (95% CI, 29.4-31.5). Compared with usual care, the risk of MACE was lower in both the RYGB group (HR, 0.53; P < .001) and the SG group (HR, 0.69; P < .001). The researchers also analyzed the cumulative incidence of all-cause mortality, myocardial infarction, and ischemic stroke (three-component MACE) at 5 years. The cumulative incidence of three-component MACE at 5 years was 15.5% in the usual care group, 6.4% in the RYGB group (HR, 0.53 versus usual care; P < .001) and 11.8% in the SG group (HR vs. usual care, 0.65; P = .006).
The RYGB group had less nephropathy at 5 years (2.8% vs. 8.3%; HR, 0.47; P = .005), and experienced a greater reduction in weight, glycated hemoglobin, and diabetes and cardiovascular medication use. At 5 years, RYGB was associated with a higher frequency of upper endoscopy (45.8% vs. 35.6%, P < .001) and abdominal surgical procedures (10.8% vs. 5.4%, P = .001), compared with SG.
“Both procedures are extremely safe and extremely effective,” said Dr. Aminian. He pointed out the need to consider multiple factors when choosing between the procedures, including overall health, weight, comorbidities, and the patient’s values and goals.
A few factors may be contraindicated for one procedure or another. The sleeve may worsen severe reflux disease, while the gastric bypass may interfere more with absorption of psychiatric medications. Some patients may have multiple comorbidities that could point to a less risky procedure. “Decision-making should not be solely based on findings of this study. All these conditions need to be considered when patients and surgeons make a final decision about the most appropriate procedure,” said Dr. Aminian.
Dr. Nissen noted that the associations were wide ranging, including classic outcomes like death, stroke, and heart failure, but also extending to heart failure, coronary events, cerebral vascular events, nephropathy, and atrial fibrillation. “I found the nephropathy results to be amongst the most striking, that Roux-en-Y really dramatically reduced the risk of neuropathy,” he added. That’s a particularly important point because end-stage renal disease is a common cause of diabetes mortality.
Dr. Nissen acknowledged the limitations of the retrospective nature of the study, though he feels confident that the relationships are causal. “Bariatric surgery desperately needs a randomized, controlled trial, where both groups get intensive dietary and lifestyle counseling, but one group gets metabolic surgery and the other doesn’t. Given the dramatic effects in diabetic patients of reducing their hemoglobin A1c in a sustained way, reducing their body weight. We think these are very strong data to suggest that we have a major reduction in all the endpoints. If we’re right about this, the randomized controlled trial will show that dramatic effect, and will convince even the skeptics that metabolic surgery is the best way to go.”
FROM DIABETES CARE
Bariatric surgery leads to better cardiovascular function in pregnancy
Pregnant women with a history of bariatric surgery have better cardiovascular adaptation to pregnancy compared with women who have similar early-pregnancy body mass index (BMI) but no history of weight loss surgery, new data suggest.
“Pregnant women who have had bariatric surgery demonstrate better cardiovascular adaptation through lower blood pressure, heart rate, and cardiac output, more favorable diastolic indices, and better systolic function,” reported Deesha Patel, MBBS MRCOG, specialist registrar, Chelsea and Westminster Hospital, London.
“Because the groups were matched for early pregnancy BMI, it’s unlikely that the results are due to weight loss alone but indicate that the metabolic alterations as a result of the surgery, via the enterocardiac axis, play an important role,” Dr. Patel continued.
The findings were presented at the Royal College of Obstetricians and Gynecologists 2021 Virtual World Congress.
Although obesity is known for its inflammatory and toxic effects on the cardiovascular system, it is not clear to what extent the various treatment options for obesity modify these risks in the long term, said Hutan Ashrafian, MD, clinical lecturer in surgery, Imperial College London.
“It is even less clear how anti-obesity interventions affect the cardiovascular system in pregnancy,” Dr. Ashrafian told this news organization.
“This very novel study in pregnant mothers having undergone the most successful and consistent intervention for severe obesity – bariatric or metabolic surgery – gives new clues as to the extent that bariatric procedures can alter cardiovascular risk in pregnant mothers,” continued Dr. Ashrafian, who was not involved in the study.
The results show how bariatric surgery has favorable effects on cardiac adaptation in pregnancy and in turn “might offer protection from pregnancy-related cardiovascular pathology such as preeclampsia,” explained Dr. Ashrafian. “This adds to the known effects of cardiovascular protection of bariatric surgery through the enterocardiac axis, which may explain a wider range of effects that can be translated within pregnancy and possibly following pregnancy in the postpartum era and beyond.”
A history of bariatric surgery versus no surgery
The prospective, longitudinal study compared 41 women who had a history of bariatric surgery with 41 women who had not undergone surgery. Patients’ characteristics were closely matched for age, BMI (34.5 kg/m2 and 34.3 kg/m2 in the surgery and bariatric surgery groups, respectively) and race. Hypertensive disorders in the post-surgery group were significantly less common compared with the no-surgery group (0% vs. 9.8%).
During the study, participants underwent cardiovascular assessment at 12-14 weeks, 20-24 weeks, and 30-32 weeks of gestation. The assessment included measurement of blood pressure and heart rate, transthoracic echocardiography, and 2D speckle tracking, performed offline to assess global longitudinal and circumferential strain.
Blood pressure readings across the three trimesters were consistently lower in the women who had undergone bariatric surgery compared with those in the no-surgery group, and all differences were statistically significant. Likewise, heart rate and cardiac output across the three trimesters were lower in the post-surgery cohort. However, there was no difference in stroke volume between the two groups.
As for diastolic function, there were more favorable indices in the post-surgery group with a higher E/A ratio, a marker of left ventricle filling (P < .001), and lower left atrial volume (P < .05), Dr. Patel reported.
With respect to systolic function, there was no difference in ejection fraction, but there was lower global longitudinal strain (P < .01) and global circumferential strain in the post-bariatric group (P = .02), suggesting better systolic function.
“Strain is a measure of differences in motion and velocity between regions of the myocardium through the cardiac cycle and can detect subclinical changes when ejection fraction is normal,” she added.
“This is a fascinating piece of work. The author should be congratulated on gathering so many [pregnant] women who had had bariatric surgery. The work gives a unique glimpse into metabolic syndrome,” said Philip Toozs-Hobson, MD, who moderated the session.
“We are increasingly recognizing the impact [of bariatric surgery] on metabolic syndrome, and the fact that this study demonstrates that there is more to it than just weight is important,” continued Dr. Toosz-Hobson, who is a consultant gynecologist at Birmingham Women’s Hospital NHS Foundation Trust, United Kingdom.
Cardiovascular benefits of bariatric surgery
Bariatric surgery has been associated with loss of excess body weight of up to 55% and with approximately 40% reduction in all-cause mortality in the general population. The procedure also reduces the risk for heart disease, diabetes, and cancer.
The cardiovascular benefits of bariatric surgery include reduced hypertension, remodeling of the heart with a reduction in left ventricular mass, and an improvement in diastolic and systolic function.
“Traditionally, the cardiac changes were thought to be due to weight loss and blood pressure reduction, but it is now conceivable that the metabolic components contribute to the reverse modeling via changes to the enterocardiac axis involving changes to gut hormones,” said Dr. Patel. These hormones include secretin, glucagon, and vasoactive intestinal peptide, which are known to have inotropic effects, as well as adiponectin and leptin, which are known to have cardiac effects, she added.
“Pregnancy following bariatric surgery is associated with a reduced risk of hypertensive disorders, as well as a reduced risk of gestational diabetes, large-for-gestational-age neonates, and a small increased risk of small-for-gestational-age neonates,” said Dr. Patel.
Dr. Patel and Dr. Toosz-Hobson have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pregnant women with a history of bariatric surgery have better cardiovascular adaptation to pregnancy compared with women who have similar early-pregnancy body mass index (BMI) but no history of weight loss surgery, new data suggest.
“Pregnant women who have had bariatric surgery demonstrate better cardiovascular adaptation through lower blood pressure, heart rate, and cardiac output, more favorable diastolic indices, and better systolic function,” reported Deesha Patel, MBBS MRCOG, specialist registrar, Chelsea and Westminster Hospital, London.
“Because the groups were matched for early pregnancy BMI, it’s unlikely that the results are due to weight loss alone but indicate that the metabolic alterations as a result of the surgery, via the enterocardiac axis, play an important role,” Dr. Patel continued.
The findings were presented at the Royal College of Obstetricians and Gynecologists 2021 Virtual World Congress.
Although obesity is known for its inflammatory and toxic effects on the cardiovascular system, it is not clear to what extent the various treatment options for obesity modify these risks in the long term, said Hutan Ashrafian, MD, clinical lecturer in surgery, Imperial College London.
“It is even less clear how anti-obesity interventions affect the cardiovascular system in pregnancy,” Dr. Ashrafian told this news organization.
“This very novel study in pregnant mothers having undergone the most successful and consistent intervention for severe obesity – bariatric or metabolic surgery – gives new clues as to the extent that bariatric procedures can alter cardiovascular risk in pregnant mothers,” continued Dr. Ashrafian, who was not involved in the study.
The results show how bariatric surgery has favorable effects on cardiac adaptation in pregnancy and in turn “might offer protection from pregnancy-related cardiovascular pathology such as preeclampsia,” explained Dr. Ashrafian. “This adds to the known effects of cardiovascular protection of bariatric surgery through the enterocardiac axis, which may explain a wider range of effects that can be translated within pregnancy and possibly following pregnancy in the postpartum era and beyond.”
A history of bariatric surgery versus no surgery
The prospective, longitudinal study compared 41 women who had a history of bariatric surgery with 41 women who had not undergone surgery. Patients’ characteristics were closely matched for age, BMI (34.5 kg/m2 and 34.3 kg/m2 in the surgery and bariatric surgery groups, respectively) and race. Hypertensive disorders in the post-surgery group were significantly less common compared with the no-surgery group (0% vs. 9.8%).
During the study, participants underwent cardiovascular assessment at 12-14 weeks, 20-24 weeks, and 30-32 weeks of gestation. The assessment included measurement of blood pressure and heart rate, transthoracic echocardiography, and 2D speckle tracking, performed offline to assess global longitudinal and circumferential strain.
Blood pressure readings across the three trimesters were consistently lower in the women who had undergone bariatric surgery compared with those in the no-surgery group, and all differences were statistically significant. Likewise, heart rate and cardiac output across the three trimesters were lower in the post-surgery cohort. However, there was no difference in stroke volume between the two groups.
As for diastolic function, there were more favorable indices in the post-surgery group with a higher E/A ratio, a marker of left ventricle filling (P < .001), and lower left atrial volume (P < .05), Dr. Patel reported.
With respect to systolic function, there was no difference in ejection fraction, but there was lower global longitudinal strain (P < .01) and global circumferential strain in the post-bariatric group (P = .02), suggesting better systolic function.
“Strain is a measure of differences in motion and velocity between regions of the myocardium through the cardiac cycle and can detect subclinical changes when ejection fraction is normal,” she added.
“This is a fascinating piece of work. The author should be congratulated on gathering so many [pregnant] women who had had bariatric surgery. The work gives a unique glimpse into metabolic syndrome,” said Philip Toozs-Hobson, MD, who moderated the session.
“We are increasingly recognizing the impact [of bariatric surgery] on metabolic syndrome, and the fact that this study demonstrates that there is more to it than just weight is important,” continued Dr. Toosz-Hobson, who is a consultant gynecologist at Birmingham Women’s Hospital NHS Foundation Trust, United Kingdom.
Cardiovascular benefits of bariatric surgery
Bariatric surgery has been associated with loss of excess body weight of up to 55% and with approximately 40% reduction in all-cause mortality in the general population. The procedure also reduces the risk for heart disease, diabetes, and cancer.
The cardiovascular benefits of bariatric surgery include reduced hypertension, remodeling of the heart with a reduction in left ventricular mass, and an improvement in diastolic and systolic function.
“Traditionally, the cardiac changes were thought to be due to weight loss and blood pressure reduction, but it is now conceivable that the metabolic components contribute to the reverse modeling via changes to the enterocardiac axis involving changes to gut hormones,” said Dr. Patel. These hormones include secretin, glucagon, and vasoactive intestinal peptide, which are known to have inotropic effects, as well as adiponectin and leptin, which are known to have cardiac effects, she added.
“Pregnancy following bariatric surgery is associated with a reduced risk of hypertensive disorders, as well as a reduced risk of gestational diabetes, large-for-gestational-age neonates, and a small increased risk of small-for-gestational-age neonates,” said Dr. Patel.
Dr. Patel and Dr. Toosz-Hobson have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pregnant women with a history of bariatric surgery have better cardiovascular adaptation to pregnancy compared with women who have similar early-pregnancy body mass index (BMI) but no history of weight loss surgery, new data suggest.
“Pregnant women who have had bariatric surgery demonstrate better cardiovascular adaptation through lower blood pressure, heart rate, and cardiac output, more favorable diastolic indices, and better systolic function,” reported Deesha Patel, MBBS MRCOG, specialist registrar, Chelsea and Westminster Hospital, London.
“Because the groups were matched for early pregnancy BMI, it’s unlikely that the results are due to weight loss alone but indicate that the metabolic alterations as a result of the surgery, via the enterocardiac axis, play an important role,” Dr. Patel continued.
The findings were presented at the Royal College of Obstetricians and Gynecologists 2021 Virtual World Congress.
Although obesity is known for its inflammatory and toxic effects on the cardiovascular system, it is not clear to what extent the various treatment options for obesity modify these risks in the long term, said Hutan Ashrafian, MD, clinical lecturer in surgery, Imperial College London.
“It is even less clear how anti-obesity interventions affect the cardiovascular system in pregnancy,” Dr. Ashrafian told this news organization.
“This very novel study in pregnant mothers having undergone the most successful and consistent intervention for severe obesity – bariatric or metabolic surgery – gives new clues as to the extent that bariatric procedures can alter cardiovascular risk in pregnant mothers,” continued Dr. Ashrafian, who was not involved in the study.
The results show how bariatric surgery has favorable effects on cardiac adaptation in pregnancy and in turn “might offer protection from pregnancy-related cardiovascular pathology such as preeclampsia,” explained Dr. Ashrafian. “This adds to the known effects of cardiovascular protection of bariatric surgery through the enterocardiac axis, which may explain a wider range of effects that can be translated within pregnancy and possibly following pregnancy in the postpartum era and beyond.”
A history of bariatric surgery versus no surgery
The prospective, longitudinal study compared 41 women who had a history of bariatric surgery with 41 women who had not undergone surgery. Patients’ characteristics were closely matched for age, BMI (34.5 kg/m2 and 34.3 kg/m2 in the surgery and bariatric surgery groups, respectively) and race. Hypertensive disorders in the post-surgery group were significantly less common compared with the no-surgery group (0% vs. 9.8%).
During the study, participants underwent cardiovascular assessment at 12-14 weeks, 20-24 weeks, and 30-32 weeks of gestation. The assessment included measurement of blood pressure and heart rate, transthoracic echocardiography, and 2D speckle tracking, performed offline to assess global longitudinal and circumferential strain.
Blood pressure readings across the three trimesters were consistently lower in the women who had undergone bariatric surgery compared with those in the no-surgery group, and all differences were statistically significant. Likewise, heart rate and cardiac output across the three trimesters were lower in the post-surgery cohort. However, there was no difference in stroke volume between the two groups.
As for diastolic function, there were more favorable indices in the post-surgery group with a higher E/A ratio, a marker of left ventricle filling (P < .001), and lower left atrial volume (P < .05), Dr. Patel reported.
With respect to systolic function, there was no difference in ejection fraction, but there was lower global longitudinal strain (P < .01) and global circumferential strain in the post-bariatric group (P = .02), suggesting better systolic function.
“Strain is a measure of differences in motion and velocity between regions of the myocardium through the cardiac cycle and can detect subclinical changes when ejection fraction is normal,” she added.
“This is a fascinating piece of work. The author should be congratulated on gathering so many [pregnant] women who had had bariatric surgery. The work gives a unique glimpse into metabolic syndrome,” said Philip Toozs-Hobson, MD, who moderated the session.
“We are increasingly recognizing the impact [of bariatric surgery] on metabolic syndrome, and the fact that this study demonstrates that there is more to it than just weight is important,” continued Dr. Toosz-Hobson, who is a consultant gynecologist at Birmingham Women’s Hospital NHS Foundation Trust, United Kingdom.
Cardiovascular benefits of bariatric surgery
Bariatric surgery has been associated with loss of excess body weight of up to 55% and with approximately 40% reduction in all-cause mortality in the general population. The procedure also reduces the risk for heart disease, diabetes, and cancer.
The cardiovascular benefits of bariatric surgery include reduced hypertension, remodeling of the heart with a reduction in left ventricular mass, and an improvement in diastolic and systolic function.
“Traditionally, the cardiac changes were thought to be due to weight loss and blood pressure reduction, but it is now conceivable that the metabolic components contribute to the reverse modeling via changes to the enterocardiac axis involving changes to gut hormones,” said Dr. Patel. These hormones include secretin, glucagon, and vasoactive intestinal peptide, which are known to have inotropic effects, as well as adiponectin and leptin, which are known to have cardiac effects, she added.
“Pregnancy following bariatric surgery is associated with a reduced risk of hypertensive disorders, as well as a reduced risk of gestational diabetes, large-for-gestational-age neonates, and a small increased risk of small-for-gestational-age neonates,” said Dr. Patel.
Dr. Patel and Dr. Toosz-Hobson have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Bariatric surgery tied to 22% lower 5-year stroke risk
Patients with obesity who underwent bariatric surgery had 46% lower odds of stroke 1 year later, similar odds of stroke 3 years later, and 22% lower odds of stroke 5 years later, compared with matched control patients, in new research.
Michael D. Williams, MD, presented the study findings (abstract A002) at the annual meeting of the American Society for Metabolic & Bariatric Surgery.
The findings are “very good news,” even though the protection against stroke declined further out from the surgery, John D. Scott, MD, scientific program chair of the ASMBS meeting, told this news organization.
The investigators matched more than 56,000 patients with obesity who had bariatric surgery with an equal number of similar patients who did not have this surgery, from a large national insurance database, in what they believe is the largest study of this to date.
“Any intervention that decreases your risk of [cardiovascular] events is good news,” said Dr. Scott, a clinical professor of surgery at the University of South Carolina, Greenville, and metabolic and bariatric surgery director at Prisma Health in Greenville, S.C. “And having a 22%-45% chance of reduction in stroke risk is a very worthwhile intervention.”
Asked how this would change the way clinicians inform patients of what to expect from bariatric surgery, he said: “I would advise patients that studies like this show that surgery would not increase your risk of having a stroke.
“This is consistent with many studies that show that the risks of all macrovascular events decrease after the comorbidity reductions seen after surgery.”
According to Dr. Scott, “the next steps might include a prospective randomized trial of medical treatment versus surgery alone for [cardiovascular]/stroke outcomes, but this is unlikely.”
Similarly, Dr. Williams told this news organization that “I would tell [patients] that surgery is an effective and durable method for weight loss. It also can improve comorbid conditions, particularly diabetes and hypertension.”
Even with this study, “I’m not sure it’s appropriate to say that bariatric surgery will reduce the risk of stroke,” he cautioned.
“However, as we continue to investigate the effects of bariatric surgery, this study contributes to the greater body of knowledge that suggests that reduction in ischemic stroke risk is yet another benefit of bariatric surgery.”
The assigned discussant, Corrigan L. McBride, MD, MBA wanted to know if the lower odds ratio at 1 year might be because preoperative patient selection might eliminate patients at high risk of poor cardiovascular outcomes.
Dr. Williams, a resident at Rush Medical College, Chicago, replied that it is difficult to eliminate potential selection bias, despite best efforts, but this study shows that he can tell patients: “Having surgery is not going to increases your risk of stroke.”
“This is an important study,” Dr. McBride, professor and chief of minimally invasive surgery and bariatric surgery, University of Nebraska Medical Center, Omaha, told this news organization.
“It is the first large study to show a decreased [or no increased] risk of stroke 1, 3, and 5 years after bariatric surgery compared to matched patients, and it had enough data to look at stroke as a standalone endpoint,” Dr. McBride said. “It is important too, for patients and their physicians to understand that there is a lower chance of them having a stroke if they have surgery than if they do not.”
‘Important,’ ‘good news’ for stroke risk after bariatric surgery
The impact of bariatric surgery on remission of type 2 diabetes is well known, Dr. Williams noted, and other studies have reported how bariatric surgery affects the risk of major adverse cardiovascular events – a composite of stroke, myocardial infarction, coronary artery disease, and all-cause death – including a study presented in the same meeting session.
However, a very large sample size is needed to be able to demonstrate the effect of bariatric surgery on stroke, since stroke is a rare event.
The researchers analyzed data from the Mariner (PearlDiver.) all-payer insurance national claims database of patients in the United States.
They matched 56,514 patients with a body mass index over 35 kg/m2 and comorbidities or a BMI of more than 40 who underwent sleeve gastrectomy or Roux-en-Y gastric bypass during 2010-2019 with 56,514 control patients who did not undergo bariatric surgery.
A year after bariatric surgery, patients in that group had a lower stroke rate than patients in the control group (0.6% vs. 1.2%), and they had close to 50% lower odds of having a stroke (odds ratio, 0.54; 95% CI, 0.47-0.61).
Three years after bariatric surgery, there were 44,948 patients in each group; the rate of stroke was 2.1% in the surgery group and 2.2% in the control group, and there was no significant difference in the odds of having a stroke (OR, 0.96; 95% CI, 0.91-1.00).
Five years after bariatric surgery, there were 27,619 patients in each group; the stroke rate was lower in the bariatric surgery group than in the control group (2.8% vs 3.6%), but reduced odds of stroke was not as great as after 1 year (OR, 0.78; 95% CI, 0.65-0.90).
Dr. Williams has no relevant financial disclosures. Dr. McBride and Dr. Scott disclosed that they are speakers/trainers/faculty advisers for Gore. Dr. Scott is also a consultant for C-SATS (part of Johnson & Johnson).
Patients with obesity who underwent bariatric surgery had 46% lower odds of stroke 1 year later, similar odds of stroke 3 years later, and 22% lower odds of stroke 5 years later, compared with matched control patients, in new research.
Michael D. Williams, MD, presented the study findings (abstract A002) at the annual meeting of the American Society for Metabolic & Bariatric Surgery.
The findings are “very good news,” even though the protection against stroke declined further out from the surgery, John D. Scott, MD, scientific program chair of the ASMBS meeting, told this news organization.
The investigators matched more than 56,000 patients with obesity who had bariatric surgery with an equal number of similar patients who did not have this surgery, from a large national insurance database, in what they believe is the largest study of this to date.
“Any intervention that decreases your risk of [cardiovascular] events is good news,” said Dr. Scott, a clinical professor of surgery at the University of South Carolina, Greenville, and metabolic and bariatric surgery director at Prisma Health in Greenville, S.C. “And having a 22%-45% chance of reduction in stroke risk is a very worthwhile intervention.”
Asked how this would change the way clinicians inform patients of what to expect from bariatric surgery, he said: “I would advise patients that studies like this show that surgery would not increase your risk of having a stroke.
“This is consistent with many studies that show that the risks of all macrovascular events decrease after the comorbidity reductions seen after surgery.”
According to Dr. Scott, “the next steps might include a prospective randomized trial of medical treatment versus surgery alone for [cardiovascular]/stroke outcomes, but this is unlikely.”
Similarly, Dr. Williams told this news organization that “I would tell [patients] that surgery is an effective and durable method for weight loss. It also can improve comorbid conditions, particularly diabetes and hypertension.”
Even with this study, “I’m not sure it’s appropriate to say that bariatric surgery will reduce the risk of stroke,” he cautioned.
“However, as we continue to investigate the effects of bariatric surgery, this study contributes to the greater body of knowledge that suggests that reduction in ischemic stroke risk is yet another benefit of bariatric surgery.”
The assigned discussant, Corrigan L. McBride, MD, MBA wanted to know if the lower odds ratio at 1 year might be because preoperative patient selection might eliminate patients at high risk of poor cardiovascular outcomes.
Dr. Williams, a resident at Rush Medical College, Chicago, replied that it is difficult to eliminate potential selection bias, despite best efforts, but this study shows that he can tell patients: “Having surgery is not going to increases your risk of stroke.”
“This is an important study,” Dr. McBride, professor and chief of minimally invasive surgery and bariatric surgery, University of Nebraska Medical Center, Omaha, told this news organization.
“It is the first large study to show a decreased [or no increased] risk of stroke 1, 3, and 5 years after bariatric surgery compared to matched patients, and it had enough data to look at stroke as a standalone endpoint,” Dr. McBride said. “It is important too, for patients and their physicians to understand that there is a lower chance of them having a stroke if they have surgery than if they do not.”
‘Important,’ ‘good news’ for stroke risk after bariatric surgery
The impact of bariatric surgery on remission of type 2 diabetes is well known, Dr. Williams noted, and other studies have reported how bariatric surgery affects the risk of major adverse cardiovascular events – a composite of stroke, myocardial infarction, coronary artery disease, and all-cause death – including a study presented in the same meeting session.
However, a very large sample size is needed to be able to demonstrate the effect of bariatric surgery on stroke, since stroke is a rare event.
The researchers analyzed data from the Mariner (PearlDiver.) all-payer insurance national claims database of patients in the United States.
They matched 56,514 patients with a body mass index over 35 kg/m2 and comorbidities or a BMI of more than 40 who underwent sleeve gastrectomy or Roux-en-Y gastric bypass during 2010-2019 with 56,514 control patients who did not undergo bariatric surgery.
A year after bariatric surgery, patients in that group had a lower stroke rate than patients in the control group (0.6% vs. 1.2%), and they had close to 50% lower odds of having a stroke (odds ratio, 0.54; 95% CI, 0.47-0.61).
Three years after bariatric surgery, there were 44,948 patients in each group; the rate of stroke was 2.1% in the surgery group and 2.2% in the control group, and there was no significant difference in the odds of having a stroke (OR, 0.96; 95% CI, 0.91-1.00).
Five years after bariatric surgery, there were 27,619 patients in each group; the stroke rate was lower in the bariatric surgery group than in the control group (2.8% vs 3.6%), but reduced odds of stroke was not as great as after 1 year (OR, 0.78; 95% CI, 0.65-0.90).
Dr. Williams has no relevant financial disclosures. Dr. McBride and Dr. Scott disclosed that they are speakers/trainers/faculty advisers for Gore. Dr. Scott is also a consultant for C-SATS (part of Johnson & Johnson).
Patients with obesity who underwent bariatric surgery had 46% lower odds of stroke 1 year later, similar odds of stroke 3 years later, and 22% lower odds of stroke 5 years later, compared with matched control patients, in new research.
Michael D. Williams, MD, presented the study findings (abstract A002) at the annual meeting of the American Society for Metabolic & Bariatric Surgery.
The findings are “very good news,” even though the protection against stroke declined further out from the surgery, John D. Scott, MD, scientific program chair of the ASMBS meeting, told this news organization.
The investigators matched more than 56,000 patients with obesity who had bariatric surgery with an equal number of similar patients who did not have this surgery, from a large national insurance database, in what they believe is the largest study of this to date.
“Any intervention that decreases your risk of [cardiovascular] events is good news,” said Dr. Scott, a clinical professor of surgery at the University of South Carolina, Greenville, and metabolic and bariatric surgery director at Prisma Health in Greenville, S.C. “And having a 22%-45% chance of reduction in stroke risk is a very worthwhile intervention.”
Asked how this would change the way clinicians inform patients of what to expect from bariatric surgery, he said: “I would advise patients that studies like this show that surgery would not increase your risk of having a stroke.
“This is consistent with many studies that show that the risks of all macrovascular events decrease after the comorbidity reductions seen after surgery.”
According to Dr. Scott, “the next steps might include a prospective randomized trial of medical treatment versus surgery alone for [cardiovascular]/stroke outcomes, but this is unlikely.”
Similarly, Dr. Williams told this news organization that “I would tell [patients] that surgery is an effective and durable method for weight loss. It also can improve comorbid conditions, particularly diabetes and hypertension.”
Even with this study, “I’m not sure it’s appropriate to say that bariatric surgery will reduce the risk of stroke,” he cautioned.
“However, as we continue to investigate the effects of bariatric surgery, this study contributes to the greater body of knowledge that suggests that reduction in ischemic stroke risk is yet another benefit of bariatric surgery.”
The assigned discussant, Corrigan L. McBride, MD, MBA wanted to know if the lower odds ratio at 1 year might be because preoperative patient selection might eliminate patients at high risk of poor cardiovascular outcomes.
Dr. Williams, a resident at Rush Medical College, Chicago, replied that it is difficult to eliminate potential selection bias, despite best efforts, but this study shows that he can tell patients: “Having surgery is not going to increases your risk of stroke.”
“This is an important study,” Dr. McBride, professor and chief of minimally invasive surgery and bariatric surgery, University of Nebraska Medical Center, Omaha, told this news organization.
“It is the first large study to show a decreased [or no increased] risk of stroke 1, 3, and 5 years after bariatric surgery compared to matched patients, and it had enough data to look at stroke as a standalone endpoint,” Dr. McBride said. “It is important too, for patients and their physicians to understand that there is a lower chance of them having a stroke if they have surgery than if they do not.”
‘Important,’ ‘good news’ for stroke risk after bariatric surgery
The impact of bariatric surgery on remission of type 2 diabetes is well known, Dr. Williams noted, and other studies have reported how bariatric surgery affects the risk of major adverse cardiovascular events – a composite of stroke, myocardial infarction, coronary artery disease, and all-cause death – including a study presented in the same meeting session.
However, a very large sample size is needed to be able to demonstrate the effect of bariatric surgery on stroke, since stroke is a rare event.
The researchers analyzed data from the Mariner (PearlDiver.) all-payer insurance national claims database of patients in the United States.
They matched 56,514 patients with a body mass index over 35 kg/m2 and comorbidities or a BMI of more than 40 who underwent sleeve gastrectomy or Roux-en-Y gastric bypass during 2010-2019 with 56,514 control patients who did not undergo bariatric surgery.
A year after bariatric surgery, patients in that group had a lower stroke rate than patients in the control group (0.6% vs. 1.2%), and they had close to 50% lower odds of having a stroke (odds ratio, 0.54; 95% CI, 0.47-0.61).
Three years after bariatric surgery, there were 44,948 patients in each group; the rate of stroke was 2.1% in the surgery group and 2.2% in the control group, and there was no significant difference in the odds of having a stroke (OR, 0.96; 95% CI, 0.91-1.00).
Five years after bariatric surgery, there were 27,619 patients in each group; the stroke rate was lower in the bariatric surgery group than in the control group (2.8% vs 3.6%), but reduced odds of stroke was not as great as after 1 year (OR, 0.78; 95% CI, 0.65-0.90).
Dr. Williams has no relevant financial disclosures. Dr. McBride and Dr. Scott disclosed that they are speakers/trainers/faculty advisers for Gore. Dr. Scott is also a consultant for C-SATS (part of Johnson & Johnson).
FROM ASMBS 2021
Medically suspect criterion can determine bariatric surgery coverage
A delaying tactic used by some U.S. health insurers to limit coverage of bariatric surgery does not jibe with the clinical experience at one U.S. center with 461 patients who underwent primary or revisional bariatric surgery.
The tactic applies to patients with a baseline body mass index (BMI) of 35-39 kg/m2 who usually also need at least one comorbidity to qualify for insurance coverage for bariatric surgery, and specifically to the subgroup for whom hypertension is the qualifying comorbidity.
Some insurers limit surgery coverage to patients with hypertension who fail to reach their goal blood pressure on agents from three different drug classes, a policy that is “extremely frustrating and dangerous,” said Yannis Raftopoulos, MD, PhD, in his presentation at the annual meeting of the American Society for Metabolic and Bariatric Surgery.
Using number of antihypertensive drugs ‘is not correct’
“Using the number of antihypertensive medications to justify surgery is not correct because blood pressure control is not [always] better when patients take two or three medications, compared with when they are taking one. This harms patients because the more severe their hypertension, the worse their control,” said Dr. Raftopoulos, director of the weight management program at Holyoke (Mass.) Medical Center.
He presented findings from a retrospective study of 461 patients who underwent either sleeve gastrectomy or laparoscopic Roux-en-Y gastric bypass at his center, including 213 (46%) diagnosed with hypertension at the time of their surgery. Within this group were 68 patients with a BMI of 35-39, which meant that they could get insurance coverage for bariatric surgery only if they also had a relevant comorbidity such as hypertension, diabetes, or severe sleep apnea.
Among these patients, 36 (17% of those with hypertension) had only hypertension as their relevant comorbidity and would not have qualified for bariatric surgery under the strictest criteria applied by some insurers that require patients to remain hypertensive despite treatment with at least three different antihypertensive medications. (These 36 patients underwent bariatric surgery because their insurance coverage did not have this restriction.)
The analyses Dr. Raftopoulos presented also documented the rate of hypertension resolution among patients in the series who had hypertension at baseline and 1-year follow-up results. Among 65 patients on one antihypertensive drug at baseline, 43 (66%) had complete resolution of their hypertension after 1 year, defined as blood pressure of less than 130/90 mm Hg while completely off antihypertensive treatment. In contrast, among 55 patients on two antihypertensive medications at baseline, 28 (51%) had complete resolution after 1 year, and among 24 patients on three or more antihypertensive medications at baseline, 3 (13%) had complete resolution 1 year after bariatric surgery, he reported.
“Patients who were treated with one oral antihypertensive medication preoperatively had a higher likelihood of postoperative hypertension resolution,” concluded Dr. Raftopoulos.
Restricting access to bariatric surgery to patients with a BMI of less than 40 based on the preoperative intensity of their antihypertensive treatment “is not supported by our data, and can be potentially harmful,” he declared.
“This study was the result of discussions about this problem with multiple insurers in my area,” he added. “This affects a good number of patients.”
Waiting for hypertension to become less treatable
The results Dr. Raftopoulos presented “are not surprising, because they confirm the hypothesis that earlier intervention in the course of a disease like hypertension is more likely to be successful,” commented Bruce D. Schirmer, MD, a professor of surgery at the University of Virginia, Charlottesville, and designated discussant for the report.
The policy followed by some health insurers to delay coverage for bariatric surgery until patients fail three medications “forces patients with more treatable hypertension to wait until their disease worsens and becomes less treatable before they can receive appropriate treatment,” he said.
Dr. Schirmer attributed the motivation for this approach to a “despicable” and “reprehensible” reason: “Actuarial calculations that show paying for curative therapy is not cost effective in the short term. The duration of a patient’s policy may not be long enough to yield a positive financial outcome, so it becomes more appropriate to deny optimal care and have patients become sicker from their disease.”
“I applaud the authors for accumulating the data that point out this unfortunate rule of some insurance companies,” Dr. Schirmer added.
The practice is comparable with an insurer requiring that a patient’s cancer must be metastatic before allowing coverage for treatment, commented Ann M. Rogers, MD, professor and director of the Penn State University surgical weight loss program in Hershey, Penn., and a moderator of the session.
Dr. Raftopoulos, Dr. Schirmer, and Dr. Rogers had no disclosures.
A delaying tactic used by some U.S. health insurers to limit coverage of bariatric surgery does not jibe with the clinical experience at one U.S. center with 461 patients who underwent primary or revisional bariatric surgery.
The tactic applies to patients with a baseline body mass index (BMI) of 35-39 kg/m2 who usually also need at least one comorbidity to qualify for insurance coverage for bariatric surgery, and specifically to the subgroup for whom hypertension is the qualifying comorbidity.
Some insurers limit surgery coverage to patients with hypertension who fail to reach their goal blood pressure on agents from three different drug classes, a policy that is “extremely frustrating and dangerous,” said Yannis Raftopoulos, MD, PhD, in his presentation at the annual meeting of the American Society for Metabolic and Bariatric Surgery.
Using number of antihypertensive drugs ‘is not correct’
“Using the number of antihypertensive medications to justify surgery is not correct because blood pressure control is not [always] better when patients take two or three medications, compared with when they are taking one. This harms patients because the more severe their hypertension, the worse their control,” said Dr. Raftopoulos, director of the weight management program at Holyoke (Mass.) Medical Center.
He presented findings from a retrospective study of 461 patients who underwent either sleeve gastrectomy or laparoscopic Roux-en-Y gastric bypass at his center, including 213 (46%) diagnosed with hypertension at the time of their surgery. Within this group were 68 patients with a BMI of 35-39, which meant that they could get insurance coverage for bariatric surgery only if they also had a relevant comorbidity such as hypertension, diabetes, or severe sleep apnea.
Among these patients, 36 (17% of those with hypertension) had only hypertension as their relevant comorbidity and would not have qualified for bariatric surgery under the strictest criteria applied by some insurers that require patients to remain hypertensive despite treatment with at least three different antihypertensive medications. (These 36 patients underwent bariatric surgery because their insurance coverage did not have this restriction.)
The analyses Dr. Raftopoulos presented also documented the rate of hypertension resolution among patients in the series who had hypertension at baseline and 1-year follow-up results. Among 65 patients on one antihypertensive drug at baseline, 43 (66%) had complete resolution of their hypertension after 1 year, defined as blood pressure of less than 130/90 mm Hg while completely off antihypertensive treatment. In contrast, among 55 patients on two antihypertensive medications at baseline, 28 (51%) had complete resolution after 1 year, and among 24 patients on three or more antihypertensive medications at baseline, 3 (13%) had complete resolution 1 year after bariatric surgery, he reported.
“Patients who were treated with one oral antihypertensive medication preoperatively had a higher likelihood of postoperative hypertension resolution,” concluded Dr. Raftopoulos.
Restricting access to bariatric surgery to patients with a BMI of less than 40 based on the preoperative intensity of their antihypertensive treatment “is not supported by our data, and can be potentially harmful,” he declared.
“This study was the result of discussions about this problem with multiple insurers in my area,” he added. “This affects a good number of patients.”
Waiting for hypertension to become less treatable
The results Dr. Raftopoulos presented “are not surprising, because they confirm the hypothesis that earlier intervention in the course of a disease like hypertension is more likely to be successful,” commented Bruce D. Schirmer, MD, a professor of surgery at the University of Virginia, Charlottesville, and designated discussant for the report.
The policy followed by some health insurers to delay coverage for bariatric surgery until patients fail three medications “forces patients with more treatable hypertension to wait until their disease worsens and becomes less treatable before they can receive appropriate treatment,” he said.
Dr. Schirmer attributed the motivation for this approach to a “despicable” and “reprehensible” reason: “Actuarial calculations that show paying for curative therapy is not cost effective in the short term. The duration of a patient’s policy may not be long enough to yield a positive financial outcome, so it becomes more appropriate to deny optimal care and have patients become sicker from their disease.”
“I applaud the authors for accumulating the data that point out this unfortunate rule of some insurance companies,” Dr. Schirmer added.
The practice is comparable with an insurer requiring that a patient’s cancer must be metastatic before allowing coverage for treatment, commented Ann M. Rogers, MD, professor and director of the Penn State University surgical weight loss program in Hershey, Penn., and a moderator of the session.
Dr. Raftopoulos, Dr. Schirmer, and Dr. Rogers had no disclosures.
A delaying tactic used by some U.S. health insurers to limit coverage of bariatric surgery does not jibe with the clinical experience at one U.S. center with 461 patients who underwent primary or revisional bariatric surgery.
The tactic applies to patients with a baseline body mass index (BMI) of 35-39 kg/m2 who usually also need at least one comorbidity to qualify for insurance coverage for bariatric surgery, and specifically to the subgroup for whom hypertension is the qualifying comorbidity.
Some insurers limit surgery coverage to patients with hypertension who fail to reach their goal blood pressure on agents from three different drug classes, a policy that is “extremely frustrating and dangerous,” said Yannis Raftopoulos, MD, PhD, in his presentation at the annual meeting of the American Society for Metabolic and Bariatric Surgery.
Using number of antihypertensive drugs ‘is not correct’
“Using the number of antihypertensive medications to justify surgery is not correct because blood pressure control is not [always] better when patients take two or three medications, compared with when they are taking one. This harms patients because the more severe their hypertension, the worse their control,” said Dr. Raftopoulos, director of the weight management program at Holyoke (Mass.) Medical Center.
He presented findings from a retrospective study of 461 patients who underwent either sleeve gastrectomy or laparoscopic Roux-en-Y gastric bypass at his center, including 213 (46%) diagnosed with hypertension at the time of their surgery. Within this group were 68 patients with a BMI of 35-39, which meant that they could get insurance coverage for bariatric surgery only if they also had a relevant comorbidity such as hypertension, diabetes, or severe sleep apnea.
Among these patients, 36 (17% of those with hypertension) had only hypertension as their relevant comorbidity and would not have qualified for bariatric surgery under the strictest criteria applied by some insurers that require patients to remain hypertensive despite treatment with at least three different antihypertensive medications. (These 36 patients underwent bariatric surgery because their insurance coverage did not have this restriction.)
The analyses Dr. Raftopoulos presented also documented the rate of hypertension resolution among patients in the series who had hypertension at baseline and 1-year follow-up results. Among 65 patients on one antihypertensive drug at baseline, 43 (66%) had complete resolution of their hypertension after 1 year, defined as blood pressure of less than 130/90 mm Hg while completely off antihypertensive treatment. In contrast, among 55 patients on two antihypertensive medications at baseline, 28 (51%) had complete resolution after 1 year, and among 24 patients on three or more antihypertensive medications at baseline, 3 (13%) had complete resolution 1 year after bariatric surgery, he reported.
“Patients who were treated with one oral antihypertensive medication preoperatively had a higher likelihood of postoperative hypertension resolution,” concluded Dr. Raftopoulos.
Restricting access to bariatric surgery to patients with a BMI of less than 40 based on the preoperative intensity of their antihypertensive treatment “is not supported by our data, and can be potentially harmful,” he declared.
“This study was the result of discussions about this problem with multiple insurers in my area,” he added. “This affects a good number of patients.”
Waiting for hypertension to become less treatable
The results Dr. Raftopoulos presented “are not surprising, because they confirm the hypothesis that earlier intervention in the course of a disease like hypertension is more likely to be successful,” commented Bruce D. Schirmer, MD, a professor of surgery at the University of Virginia, Charlottesville, and designated discussant for the report.
The policy followed by some health insurers to delay coverage for bariatric surgery until patients fail three medications “forces patients with more treatable hypertension to wait until their disease worsens and becomes less treatable before they can receive appropriate treatment,” he said.
Dr. Schirmer attributed the motivation for this approach to a “despicable” and “reprehensible” reason: “Actuarial calculations that show paying for curative therapy is not cost effective in the short term. The duration of a patient’s policy may not be long enough to yield a positive financial outcome, so it becomes more appropriate to deny optimal care and have patients become sicker from their disease.”
“I applaud the authors for accumulating the data that point out this unfortunate rule of some insurance companies,” Dr. Schirmer added.
The practice is comparable with an insurer requiring that a patient’s cancer must be metastatic before allowing coverage for treatment, commented Ann M. Rogers, MD, professor and director of the Penn State University surgical weight loss program in Hershey, Penn., and a moderator of the session.
Dr. Raftopoulos, Dr. Schirmer, and Dr. Rogers had no disclosures.
FROM ASMBS 2021
Consider risk for Barrett’s esophagus after bariatric surgery
Barrett’s esophagus occurred in nearly 12% of patients who underwent esophagogastroduodenoscopy after sleeve gastrectomy, but it was not associated with postoperative gastroesophageal reflux disease (GERD), based on data from 10 studies that totaled 680 adult patients.
Sleeve gastrectomy has become more popular in recent years as an effective strategy for patients with severe obesity, wrote Bashar J. Qumseya, MD, of the University of Florida, Gainesville, and colleagues. However, GERD is a common concern for patients undergoing sleeve gastrectomy and is the major risk factor for Barrett’s esophagus. However, the prevalence of Barrett’s esophagus in the sleeve gastrectomy population has not been examined.
In a meta-analysis published in Gastrointestinal Endoscopy, the researchers reviewed 10 studies that totaled 680 patients who underwent esophagogastroduodenoscopy 6 months to 10 years after a sleeve gastrectomy procedure. The primary outcome was Barrett’s esophagus prevalence in sleeve gastrectomy patients, with the prevalence of erosive esophagitis and GERD at follow-up as secondary outcomes.
Overall, 54 patients developed Barrett’s esophagus, for a pooled prevalence of 11.6%, and all cases were nondysplastic and de novo. There was no significant association between Barrett’s esophagus and the presence of postoperative GERD, the researchers said (odds ratio, 1.74; P = .37).
However, the rate of erosive esophagitis increased by 86% in five studies with long-term follow-up and by 35% in two studies with short-term follow-up, which suggests an increased risk of 13% each year after sleeve gastrectomy, the researchers noted.
Besides the risk of Barrett’s esophagus after sleeve gastrectomy, “the risk of [erosive esophagitis] is also of significant interest and shares the same pathophysiology with [Barrett’s esophagus] and GERD,” they emphasized.
The study findings were limited by several factors including the small sample size and the focus on Barrett’s esophagus rather than erosive esophagitis or GERD as the primary outcome, the researchers noted. However, the results indicate that sleeve gastrectomy patients are at increased risk for Barrett’s esophagus, and larger studies are needed to better understand the pathophysiology. Furthermore, although there is some debate regarding the risk of GERD and erosive esophagitis after sleeve gastrectomy, the authors wrote that the data from their study showed a “consistent and substantial trend” toward more erosive esophagitis after sleeve gastrectomy.
“Gastroenterologists, primary care providers, and bariatric surgeons should be aware” of the data and should discuss the risks of sleeve gastrectomy with patients before the procedure, including the risks and benefits of postprocedure screening for Barrett’s esophagus, they concluded.
Consider surveillance for Barrett’s
The study is important because of the increased rates of GERD and potentially Barrett’s esophagus that have been noted after sleeve gastrectomy, Gyanprakash A. Ketwaroo, MD, of Baylor College of Medicine, Houston, said in an interview.
“Many of these studies have been small, and the findings of meta-analyses have been limited by high heterogeneity,” he noted. “With the rise in popularity of sleeve gastrectomy, it is important to accurately assess potential long-term complications.”
Dr. Ketwaroo said he was not surprised by the study findings given several reports of increased GERD after sleeve gastrectomy. “Given the accepted pathophysiology of Barrett’s esophagus, I anticipated increased risk of Barrett’s esophagus after sleeve gastrectomy as well.
“Clinicians should consider surveillance for Barrett’s esophagus after sleeve gastrectomy, and possible early proton pump inhibitor use for both GERD/erosive esophagitis and Barrett’s esophagus chemoprophylaxis. Patients with longer-segment or dysplastic Barrett’s esophagus prior to sleeve gastrectomy may have to be monitored more closely after surgery,” he said.
Dr. Ketwaroo noted that the study was limited by the small sample size, “with only approximately 50 patients with Barrett’s esophagus after surgery among 680 overall.” He emphasized that “we will need a much larger prospective study to confirm this finding. Additionally, I would want to explore if sleeve gastrectomy increases rate of progression of dysplasia in those who develop Barrett’s esophagus.”
The study received no outside funding. Lead author Dr. Qumseya had no financial conflicts to disclose. Dr. Ketwaroo serves on the GI & Hepatology News editorial advisory board.
Barrett’s esophagus occurred in nearly 12% of patients who underwent esophagogastroduodenoscopy after sleeve gastrectomy, but it was not associated with postoperative gastroesophageal reflux disease (GERD), based on data from 10 studies that totaled 680 adult patients.
Sleeve gastrectomy has become more popular in recent years as an effective strategy for patients with severe obesity, wrote Bashar J. Qumseya, MD, of the University of Florida, Gainesville, and colleagues. However, GERD is a common concern for patients undergoing sleeve gastrectomy and is the major risk factor for Barrett’s esophagus. However, the prevalence of Barrett’s esophagus in the sleeve gastrectomy population has not been examined.
In a meta-analysis published in Gastrointestinal Endoscopy, the researchers reviewed 10 studies that totaled 680 patients who underwent esophagogastroduodenoscopy 6 months to 10 years after a sleeve gastrectomy procedure. The primary outcome was Barrett’s esophagus prevalence in sleeve gastrectomy patients, with the prevalence of erosive esophagitis and GERD at follow-up as secondary outcomes.
Overall, 54 patients developed Barrett’s esophagus, for a pooled prevalence of 11.6%, and all cases were nondysplastic and de novo. There was no significant association between Barrett’s esophagus and the presence of postoperative GERD, the researchers said (odds ratio, 1.74; P = .37).
However, the rate of erosive esophagitis increased by 86% in five studies with long-term follow-up and by 35% in two studies with short-term follow-up, which suggests an increased risk of 13% each year after sleeve gastrectomy, the researchers noted.
Besides the risk of Barrett’s esophagus after sleeve gastrectomy, “the risk of [erosive esophagitis] is also of significant interest and shares the same pathophysiology with [Barrett’s esophagus] and GERD,” they emphasized.
The study findings were limited by several factors including the small sample size and the focus on Barrett’s esophagus rather than erosive esophagitis or GERD as the primary outcome, the researchers noted. However, the results indicate that sleeve gastrectomy patients are at increased risk for Barrett’s esophagus, and larger studies are needed to better understand the pathophysiology. Furthermore, although there is some debate regarding the risk of GERD and erosive esophagitis after sleeve gastrectomy, the authors wrote that the data from their study showed a “consistent and substantial trend” toward more erosive esophagitis after sleeve gastrectomy.
“Gastroenterologists, primary care providers, and bariatric surgeons should be aware” of the data and should discuss the risks of sleeve gastrectomy with patients before the procedure, including the risks and benefits of postprocedure screening for Barrett’s esophagus, they concluded.
Consider surveillance for Barrett’s
The study is important because of the increased rates of GERD and potentially Barrett’s esophagus that have been noted after sleeve gastrectomy, Gyanprakash A. Ketwaroo, MD, of Baylor College of Medicine, Houston, said in an interview.
“Many of these studies have been small, and the findings of meta-analyses have been limited by high heterogeneity,” he noted. “With the rise in popularity of sleeve gastrectomy, it is important to accurately assess potential long-term complications.”
Dr. Ketwaroo said he was not surprised by the study findings given several reports of increased GERD after sleeve gastrectomy. “Given the accepted pathophysiology of Barrett’s esophagus, I anticipated increased risk of Barrett’s esophagus after sleeve gastrectomy as well.
“Clinicians should consider surveillance for Barrett’s esophagus after sleeve gastrectomy, and possible early proton pump inhibitor use for both GERD/erosive esophagitis and Barrett’s esophagus chemoprophylaxis. Patients with longer-segment or dysplastic Barrett’s esophagus prior to sleeve gastrectomy may have to be monitored more closely after surgery,” he said.
Dr. Ketwaroo noted that the study was limited by the small sample size, “with only approximately 50 patients with Barrett’s esophagus after surgery among 680 overall.” He emphasized that “we will need a much larger prospective study to confirm this finding. Additionally, I would want to explore if sleeve gastrectomy increases rate of progression of dysplasia in those who develop Barrett’s esophagus.”
The study received no outside funding. Lead author Dr. Qumseya had no financial conflicts to disclose. Dr. Ketwaroo serves on the GI & Hepatology News editorial advisory board.
Barrett’s esophagus occurred in nearly 12% of patients who underwent esophagogastroduodenoscopy after sleeve gastrectomy, but it was not associated with postoperative gastroesophageal reflux disease (GERD), based on data from 10 studies that totaled 680 adult patients.
Sleeve gastrectomy has become more popular in recent years as an effective strategy for patients with severe obesity, wrote Bashar J. Qumseya, MD, of the University of Florida, Gainesville, and colleagues. However, GERD is a common concern for patients undergoing sleeve gastrectomy and is the major risk factor for Barrett’s esophagus. However, the prevalence of Barrett’s esophagus in the sleeve gastrectomy population has not been examined.
In a meta-analysis published in Gastrointestinal Endoscopy, the researchers reviewed 10 studies that totaled 680 patients who underwent esophagogastroduodenoscopy 6 months to 10 years after a sleeve gastrectomy procedure. The primary outcome was Barrett’s esophagus prevalence in sleeve gastrectomy patients, with the prevalence of erosive esophagitis and GERD at follow-up as secondary outcomes.
Overall, 54 patients developed Barrett’s esophagus, for a pooled prevalence of 11.6%, and all cases were nondysplastic and de novo. There was no significant association between Barrett’s esophagus and the presence of postoperative GERD, the researchers said (odds ratio, 1.74; P = .37).
However, the rate of erosive esophagitis increased by 86% in five studies with long-term follow-up and by 35% in two studies with short-term follow-up, which suggests an increased risk of 13% each year after sleeve gastrectomy, the researchers noted.
Besides the risk of Barrett’s esophagus after sleeve gastrectomy, “the risk of [erosive esophagitis] is also of significant interest and shares the same pathophysiology with [Barrett’s esophagus] and GERD,” they emphasized.
The study findings were limited by several factors including the small sample size and the focus on Barrett’s esophagus rather than erosive esophagitis or GERD as the primary outcome, the researchers noted. However, the results indicate that sleeve gastrectomy patients are at increased risk for Barrett’s esophagus, and larger studies are needed to better understand the pathophysiology. Furthermore, although there is some debate regarding the risk of GERD and erosive esophagitis after sleeve gastrectomy, the authors wrote that the data from their study showed a “consistent and substantial trend” toward more erosive esophagitis after sleeve gastrectomy.
“Gastroenterologists, primary care providers, and bariatric surgeons should be aware” of the data and should discuss the risks of sleeve gastrectomy with patients before the procedure, including the risks and benefits of postprocedure screening for Barrett’s esophagus, they concluded.
Consider surveillance for Barrett’s
The study is important because of the increased rates of GERD and potentially Barrett’s esophagus that have been noted after sleeve gastrectomy, Gyanprakash A. Ketwaroo, MD, of Baylor College of Medicine, Houston, said in an interview.
“Many of these studies have been small, and the findings of meta-analyses have been limited by high heterogeneity,” he noted. “With the rise in popularity of sleeve gastrectomy, it is important to accurately assess potential long-term complications.”
Dr. Ketwaroo said he was not surprised by the study findings given several reports of increased GERD after sleeve gastrectomy. “Given the accepted pathophysiology of Barrett’s esophagus, I anticipated increased risk of Barrett’s esophagus after sleeve gastrectomy as well.
“Clinicians should consider surveillance for Barrett’s esophagus after sleeve gastrectomy, and possible early proton pump inhibitor use for both GERD/erosive esophagitis and Barrett’s esophagus chemoprophylaxis. Patients with longer-segment or dysplastic Barrett’s esophagus prior to sleeve gastrectomy may have to be monitored more closely after surgery,” he said.
Dr. Ketwaroo noted that the study was limited by the small sample size, “with only approximately 50 patients with Barrett’s esophagus after surgery among 680 overall.” He emphasized that “we will need a much larger prospective study to confirm this finding. Additionally, I would want to explore if sleeve gastrectomy increases rate of progression of dysplasia in those who develop Barrett’s esophagus.”
The study received no outside funding. Lead author Dr. Qumseya had no financial conflicts to disclose. Dr. Ketwaroo serves on the GI & Hepatology News editorial advisory board.
FROM GASTROINTESTINAL ENDOSCOPY
Bariatric surgery gives 10-year cure for some advanced diabetes
A small, single-center randomized trial of patients with obesity and advanced type 2 diabetes, defined as diabetes for ≥ 5 years and A1c ≥ 7%, found that a quarter to a half of patients who had metabolic surgery had diabetes remission (cure) that lasted 5-9 years.
That is, of the 60 randomized patients, 50% who had biliopancreatic diversion and 25% who had Roux-en-Y gastric bypass – but none who had received current medical therapy – still had diabetes remission a decade later.
Until now, there had only been 5-year follow-up data from this and similar trials, Geltrude Mingrone, MD, PhD, and colleagues noted in the study published online Jan. 23 in The Lancet.
These results provide “the most robust scientific evidence yet that full-blown type 2 diabetes is a curable disease, not inevitably progressive, and irreversible,” senior author Francesco Rubino, MD, chair of bariatric and metabolic surgery at King’s College London, said in a statement from his institution.
“The results of this trial will make a noticeable difference in the field and convince even the most skeptical of clinicians about the role of metabolic surgery as part of optimal care for their patients with difficult to control type 2 diabetes,” predicted two editorialists.
Alexander D. Miras, PhD, section of metabolism, digestion, and reproduction, Imperial College London, and Carel le Roux, MBChB, PhD, of the Diabetes Complications Research Centre, University College Dublin, penned the accompanying commentary.
Patients who had metabolic surgery also had greater weight loss, reduced medication use, lower cardiovascular risk, better quality of life, and a lower incidence of diabetes-related complications compared with those who received medical therapy.
“Clinicians and policymakers should ensure that metabolic surgery is appropriately considered in the management of patients with obesity and type 2 diabetes,” advised Dr. Mingrone of King’s College London and the Catholic University of Rome, and colleagues.
“Reassuring results, will make a difference in the field”
“It is reassuring that we now have 10-year data showing greater efficacy of metabolic surgery than conventional medical therapy,” Dr. Miras and Dr. le Roux wrote in their commentary.
There were no unexpected risks associated with surgery, they noted, and the findings are consistent with those of 12 other randomized controlled trials in the past 12 years.
“New generations of diabetologists are now more open to the use of metabolic surgery for patients with suboptimal responses to medical treatments,” they wrote, rather than endlessly intensifying insulin and blaming poor response on poor compliance.
And Dr. Miras and Dr. le Roux “eagerly await” 10-year data from the 150-patient STAMPEDE trial – which is examining sleeve gastrectomy, currently the most widely performed bariatric procedure, as well as Roux-en-Y gastric bypass and medical therapy – following the 5-year results published in 2017.
Diabetes for at least 5 years, mid 40s, half on insulin
Dr. Mingrone and colleagues previously reported 5-year findings from the 60 patients with obesity and advanced diabetes who were seen in a single center in Rome and randomized to three treatments (20 in each group) in 2009-2011.
Biliopancreatic diversion “remains infrequently performed but is still considered the best operation for glycemic control,” the researchers noted.
The primary endpoint was diabetes remission at 2 years (fasting plasma glucose < 100 mg/dL [5.6 mmol/L] and A1c < 6.5%) without the need for ongoing pharmacological treatment for at least 1 year.
Patients were a mean age of 44 years and had a mean body mass index of 44 kg/m2. About half were men. They had diabetes for a mean of 5.8 years and an average A1c of 8.6%. About half were taking insulin.
Patient retention rate was high (95%) and trial outcomes were assessed by nonsurgeons.
At 10 years, patients’ mean A1c had dropped to 6.4%, 6.7%, and 7.6%, in the biliopancreatic diversion, Roux-en-Y gastric bypass, and medical therapy groups, respectively; only 2.5% of patients in the surgery groups, versus 53% in the medical therapy group, required insulin.
At study end, patients in the surgery groups had lost about 29% of their initial weight versus a weight loss of 4.2% in the medical therapy group.
First 2 years after surgery is key
“We also learnt that patients who do not go into remission after 2 years are very unlikely to ever do so,” Dr. Miras and Dr. le Roux observed, which “might help us to intensify modern and potent glucose-lowering therapies like SGLT2 inhibitors and GLP-1 receptor agonists earlier after metabolic surgery.”
Ten of 19 patients (53%) in the biliopancreatic diversion group and 10 of 15 patients (67%) in the Roux-en-Y gastric bypass group who had diabetes remission at 2 years had a diabetes relapse, but at 10 years, they all had adequate glycemic control (mean A1c 6.7%), despite drastically reduced use of diabetes medications.
The two patients who crossed over to surgery from the medical therapy group had postoperative diabetes remission, which was maintained at 10 years in one patient.
Better risk-to-benefit ratio with Roux-en-y gastric bypass
No patient in the medical therapy group had a serious adverse event, but one patient in each surgery group had deep vein thrombosis or pulmonary embolism, and one patient in the biliopancreatic diversion group had an episode of atrial fibrillation. There were no late surgical complications.
Iron deficiency and mild osteopenia occurred in both surgical groups, but were more common in the biliopancreatic diversion group. And osteoporosis, transient nyctalopia (night blindness) due to vitamin A deficiency, and kidney stones were observed only with biliopancreatic diversion.
This suggests that despite the greater antidiabetic potential of biliopancreatic diversion, Roux-en-Y gastric bypass might have a more favorable risk-to-benefit profile as a standard surgical option for the treatment of type 2 diabetes, Dr. Mingrone and colleagues concluded.
The authors and Dr. Miras have reported no relevant financial relationships. Dr. le Roux has reported receiving funding from the Science Foundation Ireland, the Health Research Board, and the Irish Research Council for type 2 diabetes research, and serves on several advisory boards outside of the scope of the current study.
A version of this article first appeared on Medscape.com.
A small, single-center randomized trial of patients with obesity and advanced type 2 diabetes, defined as diabetes for ≥ 5 years and A1c ≥ 7%, found that a quarter to a half of patients who had metabolic surgery had diabetes remission (cure) that lasted 5-9 years.
That is, of the 60 randomized patients, 50% who had biliopancreatic diversion and 25% who had Roux-en-Y gastric bypass – but none who had received current medical therapy – still had diabetes remission a decade later.
Until now, there had only been 5-year follow-up data from this and similar trials, Geltrude Mingrone, MD, PhD, and colleagues noted in the study published online Jan. 23 in The Lancet.
These results provide “the most robust scientific evidence yet that full-blown type 2 diabetes is a curable disease, not inevitably progressive, and irreversible,” senior author Francesco Rubino, MD, chair of bariatric and metabolic surgery at King’s College London, said in a statement from his institution.
“The results of this trial will make a noticeable difference in the field and convince even the most skeptical of clinicians about the role of metabolic surgery as part of optimal care for their patients with difficult to control type 2 diabetes,” predicted two editorialists.
Alexander D. Miras, PhD, section of metabolism, digestion, and reproduction, Imperial College London, and Carel le Roux, MBChB, PhD, of the Diabetes Complications Research Centre, University College Dublin, penned the accompanying commentary.
Patients who had metabolic surgery also had greater weight loss, reduced medication use, lower cardiovascular risk, better quality of life, and a lower incidence of diabetes-related complications compared with those who received medical therapy.
“Clinicians and policymakers should ensure that metabolic surgery is appropriately considered in the management of patients with obesity and type 2 diabetes,” advised Dr. Mingrone of King’s College London and the Catholic University of Rome, and colleagues.
“Reassuring results, will make a difference in the field”
“It is reassuring that we now have 10-year data showing greater efficacy of metabolic surgery than conventional medical therapy,” Dr. Miras and Dr. le Roux wrote in their commentary.
There were no unexpected risks associated with surgery, they noted, and the findings are consistent with those of 12 other randomized controlled trials in the past 12 years.
“New generations of diabetologists are now more open to the use of metabolic surgery for patients with suboptimal responses to medical treatments,” they wrote, rather than endlessly intensifying insulin and blaming poor response on poor compliance.
And Dr. Miras and Dr. le Roux “eagerly await” 10-year data from the 150-patient STAMPEDE trial – which is examining sleeve gastrectomy, currently the most widely performed bariatric procedure, as well as Roux-en-Y gastric bypass and medical therapy – following the 5-year results published in 2017.
Diabetes for at least 5 years, mid 40s, half on insulin
Dr. Mingrone and colleagues previously reported 5-year findings from the 60 patients with obesity and advanced diabetes who were seen in a single center in Rome and randomized to three treatments (20 in each group) in 2009-2011.
Biliopancreatic diversion “remains infrequently performed but is still considered the best operation for glycemic control,” the researchers noted.
The primary endpoint was diabetes remission at 2 years (fasting plasma glucose < 100 mg/dL [5.6 mmol/L] and A1c < 6.5%) without the need for ongoing pharmacological treatment for at least 1 year.
Patients were a mean age of 44 years and had a mean body mass index of 44 kg/m2. About half were men. They had diabetes for a mean of 5.8 years and an average A1c of 8.6%. About half were taking insulin.
Patient retention rate was high (95%) and trial outcomes were assessed by nonsurgeons.
At 10 years, patients’ mean A1c had dropped to 6.4%, 6.7%, and 7.6%, in the biliopancreatic diversion, Roux-en-Y gastric bypass, and medical therapy groups, respectively; only 2.5% of patients in the surgery groups, versus 53% in the medical therapy group, required insulin.
At study end, patients in the surgery groups had lost about 29% of their initial weight versus a weight loss of 4.2% in the medical therapy group.
First 2 years after surgery is key
“We also learnt that patients who do not go into remission after 2 years are very unlikely to ever do so,” Dr. Miras and Dr. le Roux observed, which “might help us to intensify modern and potent glucose-lowering therapies like SGLT2 inhibitors and GLP-1 receptor agonists earlier after metabolic surgery.”
Ten of 19 patients (53%) in the biliopancreatic diversion group and 10 of 15 patients (67%) in the Roux-en-Y gastric bypass group who had diabetes remission at 2 years had a diabetes relapse, but at 10 years, they all had adequate glycemic control (mean A1c 6.7%), despite drastically reduced use of diabetes medications.
The two patients who crossed over to surgery from the medical therapy group had postoperative diabetes remission, which was maintained at 10 years in one patient.
Better risk-to-benefit ratio with Roux-en-y gastric bypass
No patient in the medical therapy group had a serious adverse event, but one patient in each surgery group had deep vein thrombosis or pulmonary embolism, and one patient in the biliopancreatic diversion group had an episode of atrial fibrillation. There were no late surgical complications.
Iron deficiency and mild osteopenia occurred in both surgical groups, but were more common in the biliopancreatic diversion group. And osteoporosis, transient nyctalopia (night blindness) due to vitamin A deficiency, and kidney stones were observed only with biliopancreatic diversion.
This suggests that despite the greater antidiabetic potential of biliopancreatic diversion, Roux-en-Y gastric bypass might have a more favorable risk-to-benefit profile as a standard surgical option for the treatment of type 2 diabetes, Dr. Mingrone and colleagues concluded.
The authors and Dr. Miras have reported no relevant financial relationships. Dr. le Roux has reported receiving funding from the Science Foundation Ireland, the Health Research Board, and the Irish Research Council for type 2 diabetes research, and serves on several advisory boards outside of the scope of the current study.
A version of this article first appeared on Medscape.com.
A small, single-center randomized trial of patients with obesity and advanced type 2 diabetes, defined as diabetes for ≥ 5 years and A1c ≥ 7%, found that a quarter to a half of patients who had metabolic surgery had diabetes remission (cure) that lasted 5-9 years.
That is, of the 60 randomized patients, 50% who had biliopancreatic diversion and 25% who had Roux-en-Y gastric bypass – but none who had received current medical therapy – still had diabetes remission a decade later.
Until now, there had only been 5-year follow-up data from this and similar trials, Geltrude Mingrone, MD, PhD, and colleagues noted in the study published online Jan. 23 in The Lancet.
These results provide “the most robust scientific evidence yet that full-blown type 2 diabetes is a curable disease, not inevitably progressive, and irreversible,” senior author Francesco Rubino, MD, chair of bariatric and metabolic surgery at King’s College London, said in a statement from his institution.
“The results of this trial will make a noticeable difference in the field and convince even the most skeptical of clinicians about the role of metabolic surgery as part of optimal care for their patients with difficult to control type 2 diabetes,” predicted two editorialists.
Alexander D. Miras, PhD, section of metabolism, digestion, and reproduction, Imperial College London, and Carel le Roux, MBChB, PhD, of the Diabetes Complications Research Centre, University College Dublin, penned the accompanying commentary.
Patients who had metabolic surgery also had greater weight loss, reduced medication use, lower cardiovascular risk, better quality of life, and a lower incidence of diabetes-related complications compared with those who received medical therapy.
“Clinicians and policymakers should ensure that metabolic surgery is appropriately considered in the management of patients with obesity and type 2 diabetes,” advised Dr. Mingrone of King’s College London and the Catholic University of Rome, and colleagues.
“Reassuring results, will make a difference in the field”
“It is reassuring that we now have 10-year data showing greater efficacy of metabolic surgery than conventional medical therapy,” Dr. Miras and Dr. le Roux wrote in their commentary.
There were no unexpected risks associated with surgery, they noted, and the findings are consistent with those of 12 other randomized controlled trials in the past 12 years.
“New generations of diabetologists are now more open to the use of metabolic surgery for patients with suboptimal responses to medical treatments,” they wrote, rather than endlessly intensifying insulin and blaming poor response on poor compliance.
And Dr. Miras and Dr. le Roux “eagerly await” 10-year data from the 150-patient STAMPEDE trial – which is examining sleeve gastrectomy, currently the most widely performed bariatric procedure, as well as Roux-en-Y gastric bypass and medical therapy – following the 5-year results published in 2017.
Diabetes for at least 5 years, mid 40s, half on insulin
Dr. Mingrone and colleagues previously reported 5-year findings from the 60 patients with obesity and advanced diabetes who were seen in a single center in Rome and randomized to three treatments (20 in each group) in 2009-2011.
Biliopancreatic diversion “remains infrequently performed but is still considered the best operation for glycemic control,” the researchers noted.
The primary endpoint was diabetes remission at 2 years (fasting plasma glucose < 100 mg/dL [5.6 mmol/L] and A1c < 6.5%) without the need for ongoing pharmacological treatment for at least 1 year.
Patients were a mean age of 44 years and had a mean body mass index of 44 kg/m2. About half were men. They had diabetes for a mean of 5.8 years and an average A1c of 8.6%. About half were taking insulin.
Patient retention rate was high (95%) and trial outcomes were assessed by nonsurgeons.
At 10 years, patients’ mean A1c had dropped to 6.4%, 6.7%, and 7.6%, in the biliopancreatic diversion, Roux-en-Y gastric bypass, and medical therapy groups, respectively; only 2.5% of patients in the surgery groups, versus 53% in the medical therapy group, required insulin.
At study end, patients in the surgery groups had lost about 29% of their initial weight versus a weight loss of 4.2% in the medical therapy group.
First 2 years after surgery is key
“We also learnt that patients who do not go into remission after 2 years are very unlikely to ever do so,” Dr. Miras and Dr. le Roux observed, which “might help us to intensify modern and potent glucose-lowering therapies like SGLT2 inhibitors and GLP-1 receptor agonists earlier after metabolic surgery.”
Ten of 19 patients (53%) in the biliopancreatic diversion group and 10 of 15 patients (67%) in the Roux-en-Y gastric bypass group who had diabetes remission at 2 years had a diabetes relapse, but at 10 years, they all had adequate glycemic control (mean A1c 6.7%), despite drastically reduced use of diabetes medications.
The two patients who crossed over to surgery from the medical therapy group had postoperative diabetes remission, which was maintained at 10 years in one patient.
Better risk-to-benefit ratio with Roux-en-y gastric bypass
No patient in the medical therapy group had a serious adverse event, but one patient in each surgery group had deep vein thrombosis or pulmonary embolism, and one patient in the biliopancreatic diversion group had an episode of atrial fibrillation. There were no late surgical complications.
Iron deficiency and mild osteopenia occurred in both surgical groups, but were more common in the biliopancreatic diversion group. And osteoporosis, transient nyctalopia (night blindness) due to vitamin A deficiency, and kidney stones were observed only with biliopancreatic diversion.
This suggests that despite the greater antidiabetic potential of biliopancreatic diversion, Roux-en-Y gastric bypass might have a more favorable risk-to-benefit profile as a standard surgical option for the treatment of type 2 diabetes, Dr. Mingrone and colleagues concluded.
The authors and Dr. Miras have reported no relevant financial relationships. Dr. le Roux has reported receiving funding from the Science Foundation Ireland, the Health Research Board, and the Irish Research Council for type 2 diabetes research, and serves on several advisory boards outside of the scope of the current study.
A version of this article first appeared on Medscape.com.
Bariatric surgery might reduce severity of COVID-19 infection
and the disease was less severe than among COVID patients with obesity who had not undergone the surgery, a new retrospective analysis shows.
The research was published in Surgery for Obesity and Related Diseases.
Because obesity is a well-known risk factor for poor COVID-19 outcomes, Ali Aminian, MD, Bariatric and Metabolic Institute, Cleveland Clinic, and colleagues decided to study whether weight-loss surgery had a bearing on outcomes of patients with COVID-19.
They matched 33 COVID-19 patients who had undergone metabolic surgery with 330 control patients with obesity who were infected with the virus during the first wave of the pandemic.
Surgery was associated with a 69% reduction in the risk of being hospitalized as a result of COVID-19. None of the surgery patients required intensive care, mechanical ventilation, or dialysis, and none died.
“Patients after bariatric surgery become significantly healthier and can fight the virus better,” said Dr. Aminian in a statement from his institution. “If confirmed by future studies, this can be added to the long list of health benefits of bariatric surgery.”
COVID-19 is a wake-up call for the consequences of obesity
Dr. Aminian said in an interview that COVID-19 is a “wake-up call to show the public and health care professionals that obesity is a major health problem and has multiple health consequences.”
More than 300 articles in the literature show that obesity is a major risk factor for poor outcomes following COVID-19 infection. Dr. Aminian said the pandemic has “improved public awareness about the consequences of obesity.”
Compared with last year at his institution, the intake of new patients “who would like to join a program to have surgery or have some tools to help them to lose weight is almost double,” he noted.
Furthermore, referrals to their unit from primary care physicians, as well as from endocrinologists and cardiologists, for bariatric surgery nearly doubled in recent months.
Although the unit had to stop all bariatric surgeries for around 6 weeks in April because of COVID-19, it has performed the same number of procedures this year as in 2019 and 2018.
Because of the recent surge in COVID cases in Ohio, bariatric procedures are once again on hold. “Elective operations that require hospital beds after surgery have been paused to provide beds for patients who have COVID-19,” he explained.
Small sample size, study should be repeated
For their study, Dr. Aminian and colleagues examined the records of 4,365 patients at the Cleveland Clinic Health System who tested positive for the virus between March 8 and July 22, 2020.
Of these, 1,003 had a body mass index of at least 35 mg/kg2; 482 had a BMI of at least 40. The team identified 33 patients who had previously undergone metabolic surgery, comprising 20 sleeve gastrectomies and 13 Roux-en-Y gastric bypasses.
The surgical patients were propensity matched in a 1:10 ratio with nonsurgical control patients with a BMI of at least 40. The patients were matched on the basis of age, sex, ethnicity, location, smoking status, and history of chronic obstructive pulmonary disease.
The mean BMI of surgical patients was 49.1 before their procedure. It fell to 37.2 by the time they tested positive for COVID-19. This compares with an average of 46.7 in the control group at the time they tested positive for the virus.
The team found that 18.2% of metabolic surgery patients were admitted to hospital versus 42.1% of control patients (P = .013).
Moreover, metabolic surgery patients did not require admission to the intensive care unit, nor did they require mechanical ventilation or dialysis, and none died. This compares with 13.0% (P = .021), 6.7% (P = .24), 1.5%, and 2.4%, respectively, of patients in the control group.
Multivariate analysis indicated that prior metabolic surgery was associated with lower hospital admission, at an odds ratio of 0.31 (P = .028), in comparison with control patients with obesity.
Acknowledging the limited sample size of their study, the team wrote: “As this study reflects findings early in the course of the pandemic, it will be of interest to repeat this study with larger data sets and later in the course of the pandemic.”
Continue as many aspects of obesity management as possible during pandemic
Dr. Aminian underlined that, for him, the take-home message from the study is that health care professionals should “ideally” continue all aspects of obesity management during the pandemic, including “medical management, behavioral therapy, lifestyle changes, and access to bariatric surgery.”
This is despite the fact that insurance coverage for bariatric surgery has “always been a challenge for many patients, since many insurance plans do not cover” bariatric procedures, he noted.
In July, the American Society for Metabolic and Bariatric Surgery issued a statement declaring that obesity surgery should not be considered an elective procedure and should be resumed as soon as it’s safe to do so during any resurgence of the COVID-19 pandemic.
The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
and the disease was less severe than among COVID patients with obesity who had not undergone the surgery, a new retrospective analysis shows.
The research was published in Surgery for Obesity and Related Diseases.
Because obesity is a well-known risk factor for poor COVID-19 outcomes, Ali Aminian, MD, Bariatric and Metabolic Institute, Cleveland Clinic, and colleagues decided to study whether weight-loss surgery had a bearing on outcomes of patients with COVID-19.
They matched 33 COVID-19 patients who had undergone metabolic surgery with 330 control patients with obesity who were infected with the virus during the first wave of the pandemic.
Surgery was associated with a 69% reduction in the risk of being hospitalized as a result of COVID-19. None of the surgery patients required intensive care, mechanical ventilation, or dialysis, and none died.
“Patients after bariatric surgery become significantly healthier and can fight the virus better,” said Dr. Aminian in a statement from his institution. “If confirmed by future studies, this can be added to the long list of health benefits of bariatric surgery.”
COVID-19 is a wake-up call for the consequences of obesity
Dr. Aminian said in an interview that COVID-19 is a “wake-up call to show the public and health care professionals that obesity is a major health problem and has multiple health consequences.”
More than 300 articles in the literature show that obesity is a major risk factor for poor outcomes following COVID-19 infection. Dr. Aminian said the pandemic has “improved public awareness about the consequences of obesity.”
Compared with last year at his institution, the intake of new patients “who would like to join a program to have surgery or have some tools to help them to lose weight is almost double,” he noted.
Furthermore, referrals to their unit from primary care physicians, as well as from endocrinologists and cardiologists, for bariatric surgery nearly doubled in recent months.
Although the unit had to stop all bariatric surgeries for around 6 weeks in April because of COVID-19, it has performed the same number of procedures this year as in 2019 and 2018.
Because of the recent surge in COVID cases in Ohio, bariatric procedures are once again on hold. “Elective operations that require hospital beds after surgery have been paused to provide beds for patients who have COVID-19,” he explained.
Small sample size, study should be repeated
For their study, Dr. Aminian and colleagues examined the records of 4,365 patients at the Cleveland Clinic Health System who tested positive for the virus between March 8 and July 22, 2020.
Of these, 1,003 had a body mass index of at least 35 mg/kg2; 482 had a BMI of at least 40. The team identified 33 patients who had previously undergone metabolic surgery, comprising 20 sleeve gastrectomies and 13 Roux-en-Y gastric bypasses.
The surgical patients were propensity matched in a 1:10 ratio with nonsurgical control patients with a BMI of at least 40. The patients were matched on the basis of age, sex, ethnicity, location, smoking status, and history of chronic obstructive pulmonary disease.
The mean BMI of surgical patients was 49.1 before their procedure. It fell to 37.2 by the time they tested positive for COVID-19. This compares with an average of 46.7 in the control group at the time they tested positive for the virus.
The team found that 18.2% of metabolic surgery patients were admitted to hospital versus 42.1% of control patients (P = .013).
Moreover, metabolic surgery patients did not require admission to the intensive care unit, nor did they require mechanical ventilation or dialysis, and none died. This compares with 13.0% (P = .021), 6.7% (P = .24), 1.5%, and 2.4%, respectively, of patients in the control group.
Multivariate analysis indicated that prior metabolic surgery was associated with lower hospital admission, at an odds ratio of 0.31 (P = .028), in comparison with control patients with obesity.
Acknowledging the limited sample size of their study, the team wrote: “As this study reflects findings early in the course of the pandemic, it will be of interest to repeat this study with larger data sets and later in the course of the pandemic.”
Continue as many aspects of obesity management as possible during pandemic
Dr. Aminian underlined that, for him, the take-home message from the study is that health care professionals should “ideally” continue all aspects of obesity management during the pandemic, including “medical management, behavioral therapy, lifestyle changes, and access to bariatric surgery.”
This is despite the fact that insurance coverage for bariatric surgery has “always been a challenge for many patients, since many insurance plans do not cover” bariatric procedures, he noted.
In July, the American Society for Metabolic and Bariatric Surgery issued a statement declaring that obesity surgery should not be considered an elective procedure and should be resumed as soon as it’s safe to do so during any resurgence of the COVID-19 pandemic.
The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
and the disease was less severe than among COVID patients with obesity who had not undergone the surgery, a new retrospective analysis shows.
The research was published in Surgery for Obesity and Related Diseases.
Because obesity is a well-known risk factor for poor COVID-19 outcomes, Ali Aminian, MD, Bariatric and Metabolic Institute, Cleveland Clinic, and colleagues decided to study whether weight-loss surgery had a bearing on outcomes of patients with COVID-19.
They matched 33 COVID-19 patients who had undergone metabolic surgery with 330 control patients with obesity who were infected with the virus during the first wave of the pandemic.
Surgery was associated with a 69% reduction in the risk of being hospitalized as a result of COVID-19. None of the surgery patients required intensive care, mechanical ventilation, or dialysis, and none died.
“Patients after bariatric surgery become significantly healthier and can fight the virus better,” said Dr. Aminian in a statement from his institution. “If confirmed by future studies, this can be added to the long list of health benefits of bariatric surgery.”
COVID-19 is a wake-up call for the consequences of obesity
Dr. Aminian said in an interview that COVID-19 is a “wake-up call to show the public and health care professionals that obesity is a major health problem and has multiple health consequences.”
More than 300 articles in the literature show that obesity is a major risk factor for poor outcomes following COVID-19 infection. Dr. Aminian said the pandemic has “improved public awareness about the consequences of obesity.”
Compared with last year at his institution, the intake of new patients “who would like to join a program to have surgery or have some tools to help them to lose weight is almost double,” he noted.
Furthermore, referrals to their unit from primary care physicians, as well as from endocrinologists and cardiologists, for bariatric surgery nearly doubled in recent months.
Although the unit had to stop all bariatric surgeries for around 6 weeks in April because of COVID-19, it has performed the same number of procedures this year as in 2019 and 2018.
Because of the recent surge in COVID cases in Ohio, bariatric procedures are once again on hold. “Elective operations that require hospital beds after surgery have been paused to provide beds for patients who have COVID-19,” he explained.
Small sample size, study should be repeated
For their study, Dr. Aminian and colleagues examined the records of 4,365 patients at the Cleveland Clinic Health System who tested positive for the virus between March 8 and July 22, 2020.
Of these, 1,003 had a body mass index of at least 35 mg/kg2; 482 had a BMI of at least 40. The team identified 33 patients who had previously undergone metabolic surgery, comprising 20 sleeve gastrectomies and 13 Roux-en-Y gastric bypasses.
The surgical patients were propensity matched in a 1:10 ratio with nonsurgical control patients with a BMI of at least 40. The patients were matched on the basis of age, sex, ethnicity, location, smoking status, and history of chronic obstructive pulmonary disease.
The mean BMI of surgical patients was 49.1 before their procedure. It fell to 37.2 by the time they tested positive for COVID-19. This compares with an average of 46.7 in the control group at the time they tested positive for the virus.
The team found that 18.2% of metabolic surgery patients were admitted to hospital versus 42.1% of control patients (P = .013).
Moreover, metabolic surgery patients did not require admission to the intensive care unit, nor did they require mechanical ventilation or dialysis, and none died. This compares with 13.0% (P = .021), 6.7% (P = .24), 1.5%, and 2.4%, respectively, of patients in the control group.
Multivariate analysis indicated that prior metabolic surgery was associated with lower hospital admission, at an odds ratio of 0.31 (P = .028), in comparison with control patients with obesity.
Acknowledging the limited sample size of their study, the team wrote: “As this study reflects findings early in the course of the pandemic, it will be of interest to repeat this study with larger data sets and later in the course of the pandemic.”
Continue as many aspects of obesity management as possible during pandemic
Dr. Aminian underlined that, for him, the take-home message from the study is that health care professionals should “ideally” continue all aspects of obesity management during the pandemic, including “medical management, behavioral therapy, lifestyle changes, and access to bariatric surgery.”
This is despite the fact that insurance coverage for bariatric surgery has “always been a challenge for many patients, since many insurance plans do not cover” bariatric procedures, he noted.
In July, the American Society for Metabolic and Bariatric Surgery issued a statement declaring that obesity surgery should not be considered an elective procedure and should be resumed as soon as it’s safe to do so during any resurgence of the COVID-19 pandemic.
The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
‘Disordered eating’ drops after teens undergo bariatric surgery
Kristina M. Decker, PhD, a postdoctoral fellow at Cincinnati Children’s Hospital Medical Center, presented these findings during the virtual ObesityWeek 2020.
Dr. Decker and associates examined rates of disordered eating in more than 200 adolescents (aged 13-18 years) who were severely obese, of whom 141 underwent bariatric surgery and the remainder did not.
At baseline (presurgery), the teens in both groups had rates of disordered eating ranging from 11% to 50%, with higher rates in those who went on to have bariatric surgery.
Six years later, rates of disordered eating were much lower in those who had bariatric surgery.
The data nevertheless “underscore that young adults with persistent severe obesity are at high risk for poor health and well-being,” Dr. Decker said in an interview.
“This means disordered eating behaviors should be closely monitored” in all such patients, both those who undergo surgery and those who don’t, she stressed.
Robust findings because of long follow-up and controls
The findings are not unexpected, based on adult bariatric literature, but are “novel because of the age of the patients,” senior author Margaret H. Zeller, PhD, Cincinnati Children’s Hospital Medical Center and professor at the University of Cincinnati, added.
In a comment comment, psychologist Kajsa Järvholm, PhD, of the Childhood Obesity Unit at Skåne University Hospital, Malmö̈, Sweden, who has published related work, said that this is “a needed study.”
Notably, it had “long-term follow-up and a control group,” and it “confirms that adolescents are in better control of their eating after surgery.”
However, an important additional takeaway for clinicians is that “disordered eating is associated with other mental health problems and self-worth. Clinicians treating obesity must address problems related to eating disorders to improve outcomes and well-being,” she stressed.
How does bariatric surgery impact overeating, binge eating, in teens?
“For teens with severe obesity, metabolic and bariatric surgery is the most effective treatment for improved cardiometabolic functioning, weight loss, and improved quality of life,” Dr. Decker stressed.
However, pre- and postsurgical disordered eating behaviors have been associated with a lower percentage change in body mass index (BMI), although this has not been well studied.
To investigate how disordered eating is affected by bariatric surgery in adolescents with severe obesity, researchers used data from Teen-LABS, which enrolled 242 participants aged 19 years and under who mainly underwent Roux-en-Y gastric bypass (67%) or sleeve gastrectomy (28%) from 2007 to 2012 at five adolescent bariatric surgery centers.
The current analysis examined data from 141 participants in Teen-LABS who underwent bariatric surgery at a mean age of 16.8 years. Mean BMI was 51.5, most were girls (80%), and they had diverse race/ethnicity (66% were White).
Researchers also identified a control group of 83 adolescents of a similar age and gender who had diverse race/ethnicity (54% White) and a mean BMI of 46.9.
At year 6, data were available for 123 young adults in the surgery group (who by then had a mean BMI of 39.7) and 63 young adults in the nonsurgery group (who had a mean BMI of 52.6).
At baseline and year 6, participants replied to questionnaires that identified three eating disorders: continuous eating (eating in an unplanned and repetitious way between meals and snacks), objective overeating (eating a “large” amount of food without loss of control), and objective binge eating (eating a “large” amount of food with loss of control).
At baseline, rates of continuous eating, overeating, and binge eating were higher in the surgical group (50%, 40%, and 30%, respectively) than the nonsurgical group (40%, 22%, and 11%, respectively).
Six years later, when participants were aged 19-24 years, rates of continuous eating, overeating, and binge eating had declined in the surgical group (to 17%, 5%, and 1%, respectively). In the nonsurgical group, only continuous eating and overeating declined (to 24% and 7%, respectively), and binge eating increased slightly (to 13%).
Disordered eating associated with low self-worth, anxiety, and depression
In young adulthood in both groups, disordered eating was associated with lower self-worth. In the surgical group, it was also associated with lower weight-related quality of life, and in the nonsurgical group, it was also associated with anxiety and/or depression.
“The current findings cannot tell us whether disordered eating is a direct result or caused by anxiety, depression, low self-worth, or poor quality of life,” Dr. Decker said.
“These findings do give us insight about what other areas of clinical concern might present together [in] young adults (e.g., disordered eating, low self-esteem).”
Bariatric surgery affects the amount of food people can eat at one time, she noted in reply to a question from the audience. If people eat too much at a time they can experience vomiting, dumping syndrome (where certain food is “dumped” into the small intestine without being digested, causing nausea and vomiting), and plugging (a sense of food becoming stuck).
The home environment and transition to adulthood might impact disordered eating in young adults, she said in reply to another question, but these issues were not examined in this study.
A version of this article originally appeared on Medscape.com.
Kristina M. Decker, PhD, a postdoctoral fellow at Cincinnati Children’s Hospital Medical Center, presented these findings during the virtual ObesityWeek 2020.
Dr. Decker and associates examined rates of disordered eating in more than 200 adolescents (aged 13-18 years) who were severely obese, of whom 141 underwent bariatric surgery and the remainder did not.
At baseline (presurgery), the teens in both groups had rates of disordered eating ranging from 11% to 50%, with higher rates in those who went on to have bariatric surgery.
Six years later, rates of disordered eating were much lower in those who had bariatric surgery.
The data nevertheless “underscore that young adults with persistent severe obesity are at high risk for poor health and well-being,” Dr. Decker said in an interview.
“This means disordered eating behaviors should be closely monitored” in all such patients, both those who undergo surgery and those who don’t, she stressed.
Robust findings because of long follow-up and controls
The findings are not unexpected, based on adult bariatric literature, but are “novel because of the age of the patients,” senior author Margaret H. Zeller, PhD, Cincinnati Children’s Hospital Medical Center and professor at the University of Cincinnati, added.
In a comment comment, psychologist Kajsa Järvholm, PhD, of the Childhood Obesity Unit at Skåne University Hospital, Malmö̈, Sweden, who has published related work, said that this is “a needed study.”
Notably, it had “long-term follow-up and a control group,” and it “confirms that adolescents are in better control of their eating after surgery.”
However, an important additional takeaway for clinicians is that “disordered eating is associated with other mental health problems and self-worth. Clinicians treating obesity must address problems related to eating disorders to improve outcomes and well-being,” she stressed.
How does bariatric surgery impact overeating, binge eating, in teens?
“For teens with severe obesity, metabolic and bariatric surgery is the most effective treatment for improved cardiometabolic functioning, weight loss, and improved quality of life,” Dr. Decker stressed.
However, pre- and postsurgical disordered eating behaviors have been associated with a lower percentage change in body mass index (BMI), although this has not been well studied.
To investigate how disordered eating is affected by bariatric surgery in adolescents with severe obesity, researchers used data from Teen-LABS, which enrolled 242 participants aged 19 years and under who mainly underwent Roux-en-Y gastric bypass (67%) or sleeve gastrectomy (28%) from 2007 to 2012 at five adolescent bariatric surgery centers.
The current analysis examined data from 141 participants in Teen-LABS who underwent bariatric surgery at a mean age of 16.8 years. Mean BMI was 51.5, most were girls (80%), and they had diverse race/ethnicity (66% were White).
Researchers also identified a control group of 83 adolescents of a similar age and gender who had diverse race/ethnicity (54% White) and a mean BMI of 46.9.
At year 6, data were available for 123 young adults in the surgery group (who by then had a mean BMI of 39.7) and 63 young adults in the nonsurgery group (who had a mean BMI of 52.6).
At baseline and year 6, participants replied to questionnaires that identified three eating disorders: continuous eating (eating in an unplanned and repetitious way between meals and snacks), objective overeating (eating a “large” amount of food without loss of control), and objective binge eating (eating a “large” amount of food with loss of control).
At baseline, rates of continuous eating, overeating, and binge eating were higher in the surgical group (50%, 40%, and 30%, respectively) than the nonsurgical group (40%, 22%, and 11%, respectively).
Six years later, when participants were aged 19-24 years, rates of continuous eating, overeating, and binge eating had declined in the surgical group (to 17%, 5%, and 1%, respectively). In the nonsurgical group, only continuous eating and overeating declined (to 24% and 7%, respectively), and binge eating increased slightly (to 13%).
Disordered eating associated with low self-worth, anxiety, and depression
In young adulthood in both groups, disordered eating was associated with lower self-worth. In the surgical group, it was also associated with lower weight-related quality of life, and in the nonsurgical group, it was also associated with anxiety and/or depression.
“The current findings cannot tell us whether disordered eating is a direct result or caused by anxiety, depression, low self-worth, or poor quality of life,” Dr. Decker said.
“These findings do give us insight about what other areas of clinical concern might present together [in] young adults (e.g., disordered eating, low self-esteem).”
Bariatric surgery affects the amount of food people can eat at one time, she noted in reply to a question from the audience. If people eat too much at a time they can experience vomiting, dumping syndrome (where certain food is “dumped” into the small intestine without being digested, causing nausea and vomiting), and plugging (a sense of food becoming stuck).
The home environment and transition to adulthood might impact disordered eating in young adults, she said in reply to another question, but these issues were not examined in this study.
A version of this article originally appeared on Medscape.com.
Kristina M. Decker, PhD, a postdoctoral fellow at Cincinnati Children’s Hospital Medical Center, presented these findings during the virtual ObesityWeek 2020.
Dr. Decker and associates examined rates of disordered eating in more than 200 adolescents (aged 13-18 years) who were severely obese, of whom 141 underwent bariatric surgery and the remainder did not.
At baseline (presurgery), the teens in both groups had rates of disordered eating ranging from 11% to 50%, with higher rates in those who went on to have bariatric surgery.
Six years later, rates of disordered eating were much lower in those who had bariatric surgery.
The data nevertheless “underscore that young adults with persistent severe obesity are at high risk for poor health and well-being,” Dr. Decker said in an interview.
“This means disordered eating behaviors should be closely monitored” in all such patients, both those who undergo surgery and those who don’t, she stressed.
Robust findings because of long follow-up and controls
The findings are not unexpected, based on adult bariatric literature, but are “novel because of the age of the patients,” senior author Margaret H. Zeller, PhD, Cincinnati Children’s Hospital Medical Center and professor at the University of Cincinnati, added.
In a comment comment, psychologist Kajsa Järvholm, PhD, of the Childhood Obesity Unit at Skåne University Hospital, Malmö̈, Sweden, who has published related work, said that this is “a needed study.”
Notably, it had “long-term follow-up and a control group,” and it “confirms that adolescents are in better control of their eating after surgery.”
However, an important additional takeaway for clinicians is that “disordered eating is associated with other mental health problems and self-worth. Clinicians treating obesity must address problems related to eating disorders to improve outcomes and well-being,” she stressed.
How does bariatric surgery impact overeating, binge eating, in teens?
“For teens with severe obesity, metabolic and bariatric surgery is the most effective treatment for improved cardiometabolic functioning, weight loss, and improved quality of life,” Dr. Decker stressed.
However, pre- and postsurgical disordered eating behaviors have been associated with a lower percentage change in body mass index (BMI), although this has not been well studied.
To investigate how disordered eating is affected by bariatric surgery in adolescents with severe obesity, researchers used data from Teen-LABS, which enrolled 242 participants aged 19 years and under who mainly underwent Roux-en-Y gastric bypass (67%) or sleeve gastrectomy (28%) from 2007 to 2012 at five adolescent bariatric surgery centers.
The current analysis examined data from 141 participants in Teen-LABS who underwent bariatric surgery at a mean age of 16.8 years. Mean BMI was 51.5, most were girls (80%), and they had diverse race/ethnicity (66% were White).
Researchers also identified a control group of 83 adolescents of a similar age and gender who had diverse race/ethnicity (54% White) and a mean BMI of 46.9.
At year 6, data were available for 123 young adults in the surgery group (who by then had a mean BMI of 39.7) and 63 young adults in the nonsurgery group (who had a mean BMI of 52.6).
At baseline and year 6, participants replied to questionnaires that identified three eating disorders: continuous eating (eating in an unplanned and repetitious way between meals and snacks), objective overeating (eating a “large” amount of food without loss of control), and objective binge eating (eating a “large” amount of food with loss of control).
At baseline, rates of continuous eating, overeating, and binge eating were higher in the surgical group (50%, 40%, and 30%, respectively) than the nonsurgical group (40%, 22%, and 11%, respectively).
Six years later, when participants were aged 19-24 years, rates of continuous eating, overeating, and binge eating had declined in the surgical group (to 17%, 5%, and 1%, respectively). In the nonsurgical group, only continuous eating and overeating declined (to 24% and 7%, respectively), and binge eating increased slightly (to 13%).
Disordered eating associated with low self-worth, anxiety, and depression
In young adulthood in both groups, disordered eating was associated with lower self-worth. In the surgical group, it was also associated with lower weight-related quality of life, and in the nonsurgical group, it was also associated with anxiety and/or depression.
“The current findings cannot tell us whether disordered eating is a direct result or caused by anxiety, depression, low self-worth, or poor quality of life,” Dr. Decker said.
“These findings do give us insight about what other areas of clinical concern might present together [in] young adults (e.g., disordered eating, low self-esteem).”
Bariatric surgery affects the amount of food people can eat at one time, she noted in reply to a question from the audience. If people eat too much at a time they can experience vomiting, dumping syndrome (where certain food is “dumped” into the small intestine without being digested, causing nausea and vomiting), and plugging (a sense of food becoming stuck).
The home environment and transition to adulthood might impact disordered eating in young adults, she said in reply to another question, but these issues were not examined in this study.
A version of this article originally appeared on Medscape.com.
Bariatric surgery tied to lower aortic dissection risk
The finding is the latest in a series of benefits researchers have linked to the surgery, not all of which appear to directly result from weight loss.
“It has an incredible impact on hyperlipidemia and hypertension,” said Luis Felipe Okida, MD, from Cleveland Clinic Florida, Weston. “Those are the main risk factors for aortic dissection.”
He presented the finding at the virtual American Congress of Surgeons Clinical Congress 2020. The study was also published online in the Journal of the American College of Surgeons.
Although uncommon, acute aortic dissection proves fatal to half the people it strikes if patients do not receive treatment within 72 hours, Dr. Okida said in an interview.
To learn whether there is an association between bariatric surgery and risk for aortic dissection, Dr. Okida and colleagues analyzed data from the National Inpatient Sample (NIS) database from 2010 to 2015. The NIS comprises about 20% of hospital inpatient admissions in the United States.
Among the patients in the sample, 296,041 adults had undergone bariatric surgery, and 2,004,804 adults had obesity (body mass index ≥35 kg/m2) but had never undergone bariatric surgery. This latter group represented the control group.
Among the control group, 1,411 patients (.070%) experienced aortic dissection; among the bariatric surgery group, 94 patients (0.032%) experienced aortic dissection. This was a statistically significant difference (P < .0001).
The groups differed significantly in many ways. The mean age of the patients in the control group was 54.4 years, which was a mean of 2.5 years older than the bariatric surgery group. Additionally, the control group included a higher percentage of women and a lower percentage of White persons.
Those in the control group were also more likely to have a history of tobacco use, hypertension (64.2% vs. 48.9% in the surgery group), hyperlipidemia (32.7% vs. 18.3%), diabetes, aortic aneurysm (20.6% vs. 12.0%), and bicuspid aortic valves but were less likely to have Marfan/Ehlers-Danlos syndrome.
A multivariate analysis showed that gender, age, history of tobacco use, hypertension, hyperlipidemia, and Marfan/Ehlers-Danlos syndrome were associated with an increased risk for aortic dissection. Diabetes was associated with a lower risk. All of these findings had previously been reported in the literature, Dr. Okida said, but the reasons for the negative association with diabetes is not well understood.
The association between the surgery and aortic dissection applied to younger patients as well as older ones.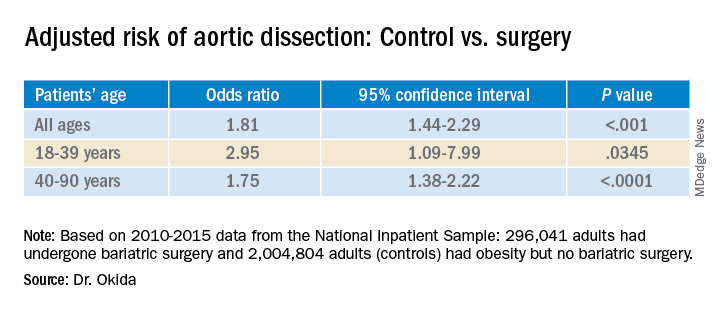
“In elderly patients, the main risk factor for aortic dissection is hypertension, and in younger patients, below 40 years old, the main risk factors are diseases of the collagen and diseases of the aorta,” said Dr. Okida during his presentation. “But these younger patients still have a high prevalence of hypertension, and that’s why bariatric surgery is beneficial.”
Although the finding regarding risk for aortic dissection supports the value of bariatric surgery, it does not in itself provide justification for undergoing the procedure. “It’s not even one of the comorbidities that insurance companies would recognize as key in approving this procedure,” said senior author Emanuele Lo Menzo, MD, PhD, also from the Cleveland Clinic Florida.
“I don’t think a physician would ever recommend this procedure specifically to avoid aortic dissection,” he said in an interview. “It’s sort of an extended benefit.”
The study raises interesting questions about the effects of the surgery, said Shanu Kothari, MD, president-elect of the American Society for Metabolic and Bariatric Surgery.
“We’ve known for a long time that patients with chronic obesity who undergo weight-loss surgery live longer than those who don’t,” he said in an interview. “They have less cardiovascular disease and cancer. Is this one more reason that they live longer?”
Bariatric surgery produces benefits for people with diabetes the day after the surgery, long before patients lose weight as a result of the procedure, Dr. Kothari said.
The effects on metabolism are complex, he added. Besides caloric restriction, they include changes in bile salt absorption and the gut microbiome, which in turn can affect hormones and inflammation.
A key question is how long after the surgery the risk for aortic dissection starts to decline, said Dr. Kothari.
The study could not answer such questions, and Dr. Okida could not find any previous studies that explored the association. He also couldn’t find any study that examined whether weight loss by other means might also reduce the risk for aortic dissection.
Dr. Okida, Dr. Lo Menzo, and Dr. Kothari disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The finding is the latest in a series of benefits researchers have linked to the surgery, not all of which appear to directly result from weight loss.
“It has an incredible impact on hyperlipidemia and hypertension,” said Luis Felipe Okida, MD, from Cleveland Clinic Florida, Weston. “Those are the main risk factors for aortic dissection.”
He presented the finding at the virtual American Congress of Surgeons Clinical Congress 2020. The study was also published online in the Journal of the American College of Surgeons.
Although uncommon, acute aortic dissection proves fatal to half the people it strikes if patients do not receive treatment within 72 hours, Dr. Okida said in an interview.
To learn whether there is an association between bariatric surgery and risk for aortic dissection, Dr. Okida and colleagues analyzed data from the National Inpatient Sample (NIS) database from 2010 to 2015. The NIS comprises about 20% of hospital inpatient admissions in the United States.
Among the patients in the sample, 296,041 adults had undergone bariatric surgery, and 2,004,804 adults had obesity (body mass index ≥35 kg/m2) but had never undergone bariatric surgery. This latter group represented the control group.
Among the control group, 1,411 patients (.070%) experienced aortic dissection; among the bariatric surgery group, 94 patients (0.032%) experienced aortic dissection. This was a statistically significant difference (P < .0001).
The groups differed significantly in many ways. The mean age of the patients in the control group was 54.4 years, which was a mean of 2.5 years older than the bariatric surgery group. Additionally, the control group included a higher percentage of women and a lower percentage of White persons.
Those in the control group were also more likely to have a history of tobacco use, hypertension (64.2% vs. 48.9% in the surgery group), hyperlipidemia (32.7% vs. 18.3%), diabetes, aortic aneurysm (20.6% vs. 12.0%), and bicuspid aortic valves but were less likely to have Marfan/Ehlers-Danlos syndrome.
A multivariate analysis showed that gender, age, history of tobacco use, hypertension, hyperlipidemia, and Marfan/Ehlers-Danlos syndrome were associated with an increased risk for aortic dissection. Diabetes was associated with a lower risk. All of these findings had previously been reported in the literature, Dr. Okida said, but the reasons for the negative association with diabetes is not well understood.
The association between the surgery and aortic dissection applied to younger patients as well as older ones.
“In elderly patients, the main risk factor for aortic dissection is hypertension, and in younger patients, below 40 years old, the main risk factors are diseases of the collagen and diseases of the aorta,” said Dr. Okida during his presentation. “But these younger patients still have a high prevalence of hypertension, and that’s why bariatric surgery is beneficial.”
Although the finding regarding risk for aortic dissection supports the value of bariatric surgery, it does not in itself provide justification for undergoing the procedure. “It’s not even one of the comorbidities that insurance companies would recognize as key in approving this procedure,” said senior author Emanuele Lo Menzo, MD, PhD, also from the Cleveland Clinic Florida.
“I don’t think a physician would ever recommend this procedure specifically to avoid aortic dissection,” he said in an interview. “It’s sort of an extended benefit.”
The study raises interesting questions about the effects of the surgery, said Shanu Kothari, MD, president-elect of the American Society for Metabolic and Bariatric Surgery.
“We’ve known for a long time that patients with chronic obesity who undergo weight-loss surgery live longer than those who don’t,” he said in an interview. “They have less cardiovascular disease and cancer. Is this one more reason that they live longer?”
Bariatric surgery produces benefits for people with diabetes the day after the surgery, long before patients lose weight as a result of the procedure, Dr. Kothari said.
The effects on metabolism are complex, he added. Besides caloric restriction, they include changes in bile salt absorption and the gut microbiome, which in turn can affect hormones and inflammation.
A key question is how long after the surgery the risk for aortic dissection starts to decline, said Dr. Kothari.
The study could not answer such questions, and Dr. Okida could not find any previous studies that explored the association. He also couldn’t find any study that examined whether weight loss by other means might also reduce the risk for aortic dissection.
Dr. Okida, Dr. Lo Menzo, and Dr. Kothari disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The finding is the latest in a series of benefits researchers have linked to the surgery, not all of which appear to directly result from weight loss.
“It has an incredible impact on hyperlipidemia and hypertension,” said Luis Felipe Okida, MD, from Cleveland Clinic Florida, Weston. “Those are the main risk factors for aortic dissection.”
He presented the finding at the virtual American Congress of Surgeons Clinical Congress 2020. The study was also published online in the Journal of the American College of Surgeons.
Although uncommon, acute aortic dissection proves fatal to half the people it strikes if patients do not receive treatment within 72 hours, Dr. Okida said in an interview.
To learn whether there is an association between bariatric surgery and risk for aortic dissection, Dr. Okida and colleagues analyzed data from the National Inpatient Sample (NIS) database from 2010 to 2015. The NIS comprises about 20% of hospital inpatient admissions in the United States.
Among the patients in the sample, 296,041 adults had undergone bariatric surgery, and 2,004,804 adults had obesity (body mass index ≥35 kg/m2) but had never undergone bariatric surgery. This latter group represented the control group.
Among the control group, 1,411 patients (.070%) experienced aortic dissection; among the bariatric surgery group, 94 patients (0.032%) experienced aortic dissection. This was a statistically significant difference (P < .0001).
The groups differed significantly in many ways. The mean age of the patients in the control group was 54.4 years, which was a mean of 2.5 years older than the bariatric surgery group. Additionally, the control group included a higher percentage of women and a lower percentage of White persons.
Those in the control group were also more likely to have a history of tobacco use, hypertension (64.2% vs. 48.9% in the surgery group), hyperlipidemia (32.7% vs. 18.3%), diabetes, aortic aneurysm (20.6% vs. 12.0%), and bicuspid aortic valves but were less likely to have Marfan/Ehlers-Danlos syndrome.
A multivariate analysis showed that gender, age, history of tobacco use, hypertension, hyperlipidemia, and Marfan/Ehlers-Danlos syndrome were associated with an increased risk for aortic dissection. Diabetes was associated with a lower risk. All of these findings had previously been reported in the literature, Dr. Okida said, but the reasons for the negative association with diabetes is not well understood.
The association between the surgery and aortic dissection applied to younger patients as well as older ones.
“In elderly patients, the main risk factor for aortic dissection is hypertension, and in younger patients, below 40 years old, the main risk factors are diseases of the collagen and diseases of the aorta,” said Dr. Okida during his presentation. “But these younger patients still have a high prevalence of hypertension, and that’s why bariatric surgery is beneficial.”
Although the finding regarding risk for aortic dissection supports the value of bariatric surgery, it does not in itself provide justification for undergoing the procedure. “It’s not even one of the comorbidities that insurance companies would recognize as key in approving this procedure,” said senior author Emanuele Lo Menzo, MD, PhD, also from the Cleveland Clinic Florida.
“I don’t think a physician would ever recommend this procedure specifically to avoid aortic dissection,” he said in an interview. “It’s sort of an extended benefit.”
The study raises interesting questions about the effects of the surgery, said Shanu Kothari, MD, president-elect of the American Society for Metabolic and Bariatric Surgery.
“We’ve known for a long time that patients with chronic obesity who undergo weight-loss surgery live longer than those who don’t,” he said in an interview. “They have less cardiovascular disease and cancer. Is this one more reason that they live longer?”
Bariatric surgery produces benefits for people with diabetes the day after the surgery, long before patients lose weight as a result of the procedure, Dr. Kothari said.
The effects on metabolism are complex, he added. Besides caloric restriction, they include changes in bile salt absorption and the gut microbiome, which in turn can affect hormones and inflammation.
A key question is how long after the surgery the risk for aortic dissection starts to decline, said Dr. Kothari.
The study could not answer such questions, and Dr. Okida could not find any previous studies that explored the association. He also couldn’t find any study that examined whether weight loss by other means might also reduce the risk for aortic dissection.
Dr. Okida, Dr. Lo Menzo, and Dr. Kothari disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.