User login
Robot pill wins AGA Shark Tank competition
SAN FRANCISCO – No one yet has figured out how to shrink doctors so they can make house calls inside the human blood stream as they did in the science fiction movie “Fantastic Voyage.” But the founders of a gastroenterology startup think they have the next best thing – a remote-controlled robot so small it can be swallowed like a pill.
The concept captured the imagination of a panel of judges earlier this month at the 2023 American Gastroenterological Association Tech Summit where it was named the winner of the annual Shark Tank innovation competition. The AGA Tech Summit and Shark Tank are the flagship events of the AGA Center for GI Innovation and Technology.
“This could be a game-changing investment down the line,” one of the judges, Amrita Sethi, MD, from Columbia University Medical Center in New York, said in an interview.

COURTESY AMERICAN GASTROENTEROLOGICAL ASSOCIATION
Hawyard, Calif.–based Endiatx is early in its voyage. , but CEO Torrey Smith, an aerospace engineer, sees future generations of the device operating on any diseased tissues that can be treated with surgery. “We believe teeny robots can go anywhere in the body,” he said.
The company executives envision that one day, robots small enough to enter the human brain will be able to eat away at tumors. “Imagine having your brain surgery while you’re on a ride at Disneyland,” said Endiatx cofounder and chair Alex Luebke. If that sounds fanciful, Mr. Smith cites a case report of a botfly larva that wormed its way into a human skull and ate a golf-ball sized chunk of brain.
Endiatx has raised $3 million and sent 24 of its robots swimming into the stomachs of its founding team. Mr. Smith himself has swallowed 15. Operators can use an external device with a joystick. Engineers have experimented with an Xbox video game controller to navigate around the stomach. The procedure requires no anesthesia.
The company expects to apply for Food and Drug Administration approval in 2025 or 2026. Mr. Smith is hoping the agency will approve it quickly because the robot pills are similar enough to passive camera pills that have been on the market for years.
But he also sees it as a crucial step forward because controlling the robot with three electric motors squirting water in six directions will allow physicians to point it at what they really need to see, not just hope to get a lucky shot of a problem area as the device floats by.
The most immediate technical challenge is improving the quality of the pill’s video. “We’re evaluating different cameras but we know we can’t be inferior on the imaging side,” Mr. Smith said.
Attention from the AGA is crucial because the team of engineers wants physicians to help it improve the robot pill, Mr. Luebeke said. “We can build anything, but we need guidance about what the market needs. Doctors have to say, ‘We need you to tweak it this way or that way.’ ”
The business opportunity is large, Mr. Smith said, with 7.5 million upper endoscopies out of 223 million endoscopic procedures done per year in the United States.
Endiatx figures the gross margin on procedures with the robot pills is 90%-95% because the manufacturing cost is about $50 per pill, but physicians can bill $500 for them using existing CPT codes for passive pill cameras.
Dr. Sethi said the robot pill stood out among other contenders because of the dire need for improved endoscopy technology.
Endiatx will represent AGA at the 2023 Digestive Disease Week® (DDW) Shark Tank pitch competition.
Four other finalists
The choice that received the most votes from the audience was Ezalife’s Button Huggie, a device for securing gastrostomy and cecostomy buttons. It includes a reusable, child-proof lid with a disposable, biodegradable, gauze sponge and a base layer held in place with a long-wearing adhesive. This prevents button movement in the tract, which can delay wound healing and lead to complications. In addition, the Button Huggie is much easier to put in place. “Our device is novel, with no direct competitors,” said CTO/COO Tyler Mironuck.
Currently patients are advised to fasten gastrostomy and cecostomy buttons with tape, but the buttons are dislodged 7% of the time, he said. The company estimates that patients spend an average of $100 a month on tape and gauze. The Button Huggie can be manufactured for $56, and the company envisions selling them for $300.
The device is exempt from needing a 510K FDA approval, so it can get to the market quickly. Nevertheless, the company is conducting a clinical trial with 200 patients at five children’s hospitals, Mr. Mironuck said.
NovaScan was a finalist for nsCanary, a device that uses electrical impedance to detect cancer. The device hinges on the company’s discovery that the Cole relaxation frequency is orders of magnitude different for cancerous and benign tissue, yet not affected by mass. By measuring this frequency, the nsCanary can find cancer in tissue acquired through biopsy forceps, snare polypectomy, mucosal resection, and endoscopic ultrasound-guided fine needle biopsy. It works in seconds without the need to interpret images.
Atlas Endoscopy was recognized for REN, a robotic colonoscopy system. The operator uses an external actuating magnet above the patient to guide a disposable ultracompliant endoscope through the colon. The company says this form of navigation prevents looping, reduces pain, and minimizes tissue stress.
Limaca Medical was recognized for Precision, a motorized, automated, rotational cutting and coring needle for endoscopic ultrasound biopsy. Manual biopsy needles now on the market require repeat passes in and out of the endoscope to obtain fragments of tissue, but Precision obtains larger intact samples of tumor tissue in a single pass.
Dr. Sethi has served as a consultant for Boston Scientific, Medtronic and Olympus; as a board member for EndoSound and has received grant support from FUJIFILM.
SAN FRANCISCO – No one yet has figured out how to shrink doctors so they can make house calls inside the human blood stream as they did in the science fiction movie “Fantastic Voyage.” But the founders of a gastroenterology startup think they have the next best thing – a remote-controlled robot so small it can be swallowed like a pill.
The concept captured the imagination of a panel of judges earlier this month at the 2023 American Gastroenterological Association Tech Summit where it was named the winner of the annual Shark Tank innovation competition. The AGA Tech Summit and Shark Tank are the flagship events of the AGA Center for GI Innovation and Technology.
“This could be a game-changing investment down the line,” one of the judges, Amrita Sethi, MD, from Columbia University Medical Center in New York, said in an interview.

COURTESY AMERICAN GASTROENTEROLOGICAL ASSOCIATION
Hawyard, Calif.–based Endiatx is early in its voyage. , but CEO Torrey Smith, an aerospace engineer, sees future generations of the device operating on any diseased tissues that can be treated with surgery. “We believe teeny robots can go anywhere in the body,” he said.
The company executives envision that one day, robots small enough to enter the human brain will be able to eat away at tumors. “Imagine having your brain surgery while you’re on a ride at Disneyland,” said Endiatx cofounder and chair Alex Luebke. If that sounds fanciful, Mr. Smith cites a case report of a botfly larva that wormed its way into a human skull and ate a golf-ball sized chunk of brain.
Endiatx has raised $3 million and sent 24 of its robots swimming into the stomachs of its founding team. Mr. Smith himself has swallowed 15. Operators can use an external device with a joystick. Engineers have experimented with an Xbox video game controller to navigate around the stomach. The procedure requires no anesthesia.
The company expects to apply for Food and Drug Administration approval in 2025 or 2026. Mr. Smith is hoping the agency will approve it quickly because the robot pills are similar enough to passive camera pills that have been on the market for years.
But he also sees it as a crucial step forward because controlling the robot with three electric motors squirting water in six directions will allow physicians to point it at what they really need to see, not just hope to get a lucky shot of a problem area as the device floats by.
The most immediate technical challenge is improving the quality of the pill’s video. “We’re evaluating different cameras but we know we can’t be inferior on the imaging side,” Mr. Smith said.
Attention from the AGA is crucial because the team of engineers wants physicians to help it improve the robot pill, Mr. Luebeke said. “We can build anything, but we need guidance about what the market needs. Doctors have to say, ‘We need you to tweak it this way or that way.’ ”
The business opportunity is large, Mr. Smith said, with 7.5 million upper endoscopies out of 223 million endoscopic procedures done per year in the United States.
Endiatx figures the gross margin on procedures with the robot pills is 90%-95% because the manufacturing cost is about $50 per pill, but physicians can bill $500 for them using existing CPT codes for passive pill cameras.
Dr. Sethi said the robot pill stood out among other contenders because of the dire need for improved endoscopy technology.
Endiatx will represent AGA at the 2023 Digestive Disease Week® (DDW) Shark Tank pitch competition.
Four other finalists
The choice that received the most votes from the audience was Ezalife’s Button Huggie, a device for securing gastrostomy and cecostomy buttons. It includes a reusable, child-proof lid with a disposable, biodegradable, gauze sponge and a base layer held in place with a long-wearing adhesive. This prevents button movement in the tract, which can delay wound healing and lead to complications. In addition, the Button Huggie is much easier to put in place. “Our device is novel, with no direct competitors,” said CTO/COO Tyler Mironuck.
Currently patients are advised to fasten gastrostomy and cecostomy buttons with tape, but the buttons are dislodged 7% of the time, he said. The company estimates that patients spend an average of $100 a month on tape and gauze. The Button Huggie can be manufactured for $56, and the company envisions selling them for $300.
The device is exempt from needing a 510K FDA approval, so it can get to the market quickly. Nevertheless, the company is conducting a clinical trial with 200 patients at five children’s hospitals, Mr. Mironuck said.
NovaScan was a finalist for nsCanary, a device that uses electrical impedance to detect cancer. The device hinges on the company’s discovery that the Cole relaxation frequency is orders of magnitude different for cancerous and benign tissue, yet not affected by mass. By measuring this frequency, the nsCanary can find cancer in tissue acquired through biopsy forceps, snare polypectomy, mucosal resection, and endoscopic ultrasound-guided fine needle biopsy. It works in seconds without the need to interpret images.
Atlas Endoscopy was recognized for REN, a robotic colonoscopy system. The operator uses an external actuating magnet above the patient to guide a disposable ultracompliant endoscope through the colon. The company says this form of navigation prevents looping, reduces pain, and minimizes tissue stress.
Limaca Medical was recognized for Precision, a motorized, automated, rotational cutting and coring needle for endoscopic ultrasound biopsy. Manual biopsy needles now on the market require repeat passes in and out of the endoscope to obtain fragments of tissue, but Precision obtains larger intact samples of tumor tissue in a single pass.
Dr. Sethi has served as a consultant for Boston Scientific, Medtronic and Olympus; as a board member for EndoSound and has received grant support from FUJIFILM.
SAN FRANCISCO – No one yet has figured out how to shrink doctors so they can make house calls inside the human blood stream as they did in the science fiction movie “Fantastic Voyage.” But the founders of a gastroenterology startup think they have the next best thing – a remote-controlled robot so small it can be swallowed like a pill.
The concept captured the imagination of a panel of judges earlier this month at the 2023 American Gastroenterological Association Tech Summit where it was named the winner of the annual Shark Tank innovation competition. The AGA Tech Summit and Shark Tank are the flagship events of the AGA Center for GI Innovation and Technology.
“This could be a game-changing investment down the line,” one of the judges, Amrita Sethi, MD, from Columbia University Medical Center in New York, said in an interview.

COURTESY AMERICAN GASTROENTEROLOGICAL ASSOCIATION
Hawyard, Calif.–based Endiatx is early in its voyage. , but CEO Torrey Smith, an aerospace engineer, sees future generations of the device operating on any diseased tissues that can be treated with surgery. “We believe teeny robots can go anywhere in the body,” he said.
The company executives envision that one day, robots small enough to enter the human brain will be able to eat away at tumors. “Imagine having your brain surgery while you’re on a ride at Disneyland,” said Endiatx cofounder and chair Alex Luebke. If that sounds fanciful, Mr. Smith cites a case report of a botfly larva that wormed its way into a human skull and ate a golf-ball sized chunk of brain.
Endiatx has raised $3 million and sent 24 of its robots swimming into the stomachs of its founding team. Mr. Smith himself has swallowed 15. Operators can use an external device with a joystick. Engineers have experimented with an Xbox video game controller to navigate around the stomach. The procedure requires no anesthesia.
The company expects to apply for Food and Drug Administration approval in 2025 or 2026. Mr. Smith is hoping the agency will approve it quickly because the robot pills are similar enough to passive camera pills that have been on the market for years.
But he also sees it as a crucial step forward because controlling the robot with three electric motors squirting water in six directions will allow physicians to point it at what they really need to see, not just hope to get a lucky shot of a problem area as the device floats by.
The most immediate technical challenge is improving the quality of the pill’s video. “We’re evaluating different cameras but we know we can’t be inferior on the imaging side,” Mr. Smith said.
Attention from the AGA is crucial because the team of engineers wants physicians to help it improve the robot pill, Mr. Luebeke said. “We can build anything, but we need guidance about what the market needs. Doctors have to say, ‘We need you to tweak it this way or that way.’ ”
The business opportunity is large, Mr. Smith said, with 7.5 million upper endoscopies out of 223 million endoscopic procedures done per year in the United States.
Endiatx figures the gross margin on procedures with the robot pills is 90%-95% because the manufacturing cost is about $50 per pill, but physicians can bill $500 for them using existing CPT codes for passive pill cameras.
Dr. Sethi said the robot pill stood out among other contenders because of the dire need for improved endoscopy technology.
Endiatx will represent AGA at the 2023 Digestive Disease Week® (DDW) Shark Tank pitch competition.
Four other finalists
The choice that received the most votes from the audience was Ezalife’s Button Huggie, a device for securing gastrostomy and cecostomy buttons. It includes a reusable, child-proof lid with a disposable, biodegradable, gauze sponge and a base layer held in place with a long-wearing adhesive. This prevents button movement in the tract, which can delay wound healing and lead to complications. In addition, the Button Huggie is much easier to put in place. “Our device is novel, with no direct competitors,” said CTO/COO Tyler Mironuck.
Currently patients are advised to fasten gastrostomy and cecostomy buttons with tape, but the buttons are dislodged 7% of the time, he said. The company estimates that patients spend an average of $100 a month on tape and gauze. The Button Huggie can be manufactured for $56, and the company envisions selling them for $300.
The device is exempt from needing a 510K FDA approval, so it can get to the market quickly. Nevertheless, the company is conducting a clinical trial with 200 patients at five children’s hospitals, Mr. Mironuck said.
NovaScan was a finalist for nsCanary, a device that uses electrical impedance to detect cancer. The device hinges on the company’s discovery that the Cole relaxation frequency is orders of magnitude different for cancerous and benign tissue, yet not affected by mass. By measuring this frequency, the nsCanary can find cancer in tissue acquired through biopsy forceps, snare polypectomy, mucosal resection, and endoscopic ultrasound-guided fine needle biopsy. It works in seconds without the need to interpret images.
Atlas Endoscopy was recognized for REN, a robotic colonoscopy system. The operator uses an external actuating magnet above the patient to guide a disposable ultracompliant endoscope through the colon. The company says this form of navigation prevents looping, reduces pain, and minimizes tissue stress.
Limaca Medical was recognized for Precision, a motorized, automated, rotational cutting and coring needle for endoscopic ultrasound biopsy. Manual biopsy needles now on the market require repeat passes in and out of the endoscope to obtain fragments of tissue, but Precision obtains larger intact samples of tumor tissue in a single pass.
Dr. Sethi has served as a consultant for Boston Scientific, Medtronic and Olympus; as a board member for EndoSound and has received grant support from FUJIFILM.
AT THE 2023 AGA TECH SUMMIT
Should GI own the obesity field?
SAN FRANCISCO –
“We see this as a field that GI should own,” said Naresh T. Gunaratnam, MD, a gastroenterologist at Huron Gastro in Ypsilanti, Mich., who has made obesity treatment an important part of his practice.
Gastroenterologists are uniquely qualified in endoscopic sleeve gastroplasty and in the placement of intragastric balloons and can also bring their internal medicine training to bear in patient education and medical prescription, Dr. Gunaratnam said in an interview.
He and three colleagues spoke about innovation in obesity and metabolism at the 2023 AGA Tech Summit sponsored by the AGA Center for GI Innovation and Technology.
Significant hurdles remain to launching new endoscopic devices for treating obesity, but evidence shows that the existing treatments are effective when combined with medication and other treatments, said panelist Reem Z. Sharaiha, MD, MSc, a gastroenterologist with expertise in obesity at Weill Cornell Medicine in New York. “You can never find just one cure for obesity, you should always think about a combination.”
Obesity rates continue to spiral worldwide, with over 100 million adults in the United States weighing in at over 30 kg/m2, said Dr. Sharaiha, but less than 5% per year receive adequate treatment. The condition is driving upticks in diabetes and nonalcoholic fatty liver disease and contributing to cancer, heart disease, stroke, and COVID-19 infections.
Even small reductions in body weight can significantly improve these conditions, she said. Less than a 5% in total body weight on average results in significant reductions in HbA1c, triglycerides, blood pressure and steatosis.
In recent years, the Food and Drug Administration has several devices that gastroenterologists can use to treat obesity, Dr. Sharaiha said, including three brands of intragastric balloon used to reduce appetite by filling the stomach. The AGA now recommends such an intragastric balloon for people with obesity who have “failed a trial of conventional weight-loss strategies.”
But many devices have been withdrawn from the market, including two of the balloon systems. Why do so many devices fail? Sometimes the FDA demands trials that are too expensive, Dr. Sharaiha said. The COVID-19 pandemic put financial pressure on some companies that have already secured FDA approval. Some insurance companies are not willing to pay for the devices, even after the FDA has approved them. Some are not cost effective.
And sometimes patients don’t accept them. That may have been one challenge with Aspire’s AspireAssist, which allowed patients to empty their stomachs into the toilet using a surgically implanted tube, though the company cited “the financial impact of the COVID-19 pandemic” when it withdrew the device from the market last year.
More devices are in the pipeline, but they face an uncertain path forward, Dr. Sharaiha said. “Device companies are usually startups that need funding. With the economic downturn, venture capital funding is hard to get.”
In the meantime, patients with class 3 obesity in particular may benefit from surgery, she said.
For others, medications are playing a more important role in the obesity epidemic, with an average 10%-15% body weight loss, Dr. Sharaiha said. Injections with semaglutide (Ozempic), a glucagon-like peptide 1 (GLP-1) receptor agonist that is approved to improve glycemic control in adults with type 2 diabetes mellitus, is leading the charge.
Tirzepatide (Mounjaro) may be even more effective, Dr. Sharaiha said. The FDA approved the drug last year to improve blood sugar control in adults with type 2 diabetes and was fast-tracked in October for the treatment of adults with obesity, or who are overweight with weight-related comorbidities.
Medications provide add-on benefits to many patients who have been treated with intragastric balloons or endoscopic sleeve gastroplasty, Dr. Sharaiha said.
Also lifestyle and education should not be neglected, said Dr. Gunaratnam, who lost 50 pounds by changing his diet. He urged gastroenterologists to take on the challenge of treating obesity. It’s not the part of his practice with the best reimbursement, but it is the most satisfying. “I get more hugs, cards, and tears by doing this because when you change weight, you’re impacting every part of their lives,” he said.
Dr. Gunaratnam is the founder of Lean Medical LLC and Satya Health Sciences. Dr. Sharaiha has served as a consultant for Boston Scientific Corporation and Cook Medical Inc.
SAN FRANCISCO –
“We see this as a field that GI should own,” said Naresh T. Gunaratnam, MD, a gastroenterologist at Huron Gastro in Ypsilanti, Mich., who has made obesity treatment an important part of his practice.
Gastroenterologists are uniquely qualified in endoscopic sleeve gastroplasty and in the placement of intragastric balloons and can also bring their internal medicine training to bear in patient education and medical prescription, Dr. Gunaratnam said in an interview.
He and three colleagues spoke about innovation in obesity and metabolism at the 2023 AGA Tech Summit sponsored by the AGA Center for GI Innovation and Technology.
Significant hurdles remain to launching new endoscopic devices for treating obesity, but evidence shows that the existing treatments are effective when combined with medication and other treatments, said panelist Reem Z. Sharaiha, MD, MSc, a gastroenterologist with expertise in obesity at Weill Cornell Medicine in New York. “You can never find just one cure for obesity, you should always think about a combination.”
Obesity rates continue to spiral worldwide, with over 100 million adults in the United States weighing in at over 30 kg/m2, said Dr. Sharaiha, but less than 5% per year receive adequate treatment. The condition is driving upticks in diabetes and nonalcoholic fatty liver disease and contributing to cancer, heart disease, stroke, and COVID-19 infections.
Even small reductions in body weight can significantly improve these conditions, she said. Less than a 5% in total body weight on average results in significant reductions in HbA1c, triglycerides, blood pressure and steatosis.
In recent years, the Food and Drug Administration has several devices that gastroenterologists can use to treat obesity, Dr. Sharaiha said, including three brands of intragastric balloon used to reduce appetite by filling the stomach. The AGA now recommends such an intragastric balloon for people with obesity who have “failed a trial of conventional weight-loss strategies.”
But many devices have been withdrawn from the market, including two of the balloon systems. Why do so many devices fail? Sometimes the FDA demands trials that are too expensive, Dr. Sharaiha said. The COVID-19 pandemic put financial pressure on some companies that have already secured FDA approval. Some insurance companies are not willing to pay for the devices, even after the FDA has approved them. Some are not cost effective.
And sometimes patients don’t accept them. That may have been one challenge with Aspire’s AspireAssist, which allowed patients to empty their stomachs into the toilet using a surgically implanted tube, though the company cited “the financial impact of the COVID-19 pandemic” when it withdrew the device from the market last year.
More devices are in the pipeline, but they face an uncertain path forward, Dr. Sharaiha said. “Device companies are usually startups that need funding. With the economic downturn, venture capital funding is hard to get.”
In the meantime, patients with class 3 obesity in particular may benefit from surgery, she said.
For others, medications are playing a more important role in the obesity epidemic, with an average 10%-15% body weight loss, Dr. Sharaiha said. Injections with semaglutide (Ozempic), a glucagon-like peptide 1 (GLP-1) receptor agonist that is approved to improve glycemic control in adults with type 2 diabetes mellitus, is leading the charge.
Tirzepatide (Mounjaro) may be even more effective, Dr. Sharaiha said. The FDA approved the drug last year to improve blood sugar control in adults with type 2 diabetes and was fast-tracked in October for the treatment of adults with obesity, or who are overweight with weight-related comorbidities.
Medications provide add-on benefits to many patients who have been treated with intragastric balloons or endoscopic sleeve gastroplasty, Dr. Sharaiha said.
Also lifestyle and education should not be neglected, said Dr. Gunaratnam, who lost 50 pounds by changing his diet. He urged gastroenterologists to take on the challenge of treating obesity. It’s not the part of his practice with the best reimbursement, but it is the most satisfying. “I get more hugs, cards, and tears by doing this because when you change weight, you’re impacting every part of their lives,” he said.
Dr. Gunaratnam is the founder of Lean Medical LLC and Satya Health Sciences. Dr. Sharaiha has served as a consultant for Boston Scientific Corporation and Cook Medical Inc.
SAN FRANCISCO –
“We see this as a field that GI should own,” said Naresh T. Gunaratnam, MD, a gastroenterologist at Huron Gastro in Ypsilanti, Mich., who has made obesity treatment an important part of his practice.
Gastroenterologists are uniquely qualified in endoscopic sleeve gastroplasty and in the placement of intragastric balloons and can also bring their internal medicine training to bear in patient education and medical prescription, Dr. Gunaratnam said in an interview.
He and three colleagues spoke about innovation in obesity and metabolism at the 2023 AGA Tech Summit sponsored by the AGA Center for GI Innovation and Technology.
Significant hurdles remain to launching new endoscopic devices for treating obesity, but evidence shows that the existing treatments are effective when combined with medication and other treatments, said panelist Reem Z. Sharaiha, MD, MSc, a gastroenterologist with expertise in obesity at Weill Cornell Medicine in New York. “You can never find just one cure for obesity, you should always think about a combination.”
Obesity rates continue to spiral worldwide, with over 100 million adults in the United States weighing in at over 30 kg/m2, said Dr. Sharaiha, but less than 5% per year receive adequate treatment. The condition is driving upticks in diabetes and nonalcoholic fatty liver disease and contributing to cancer, heart disease, stroke, and COVID-19 infections.
Even small reductions in body weight can significantly improve these conditions, she said. Less than a 5% in total body weight on average results in significant reductions in HbA1c, triglycerides, blood pressure and steatosis.
In recent years, the Food and Drug Administration has several devices that gastroenterologists can use to treat obesity, Dr. Sharaiha said, including three brands of intragastric balloon used to reduce appetite by filling the stomach. The AGA now recommends such an intragastric balloon for people with obesity who have “failed a trial of conventional weight-loss strategies.”
But many devices have been withdrawn from the market, including two of the balloon systems. Why do so many devices fail? Sometimes the FDA demands trials that are too expensive, Dr. Sharaiha said. The COVID-19 pandemic put financial pressure on some companies that have already secured FDA approval. Some insurance companies are not willing to pay for the devices, even after the FDA has approved them. Some are not cost effective.
And sometimes patients don’t accept them. That may have been one challenge with Aspire’s AspireAssist, which allowed patients to empty their stomachs into the toilet using a surgically implanted tube, though the company cited “the financial impact of the COVID-19 pandemic” when it withdrew the device from the market last year.
More devices are in the pipeline, but they face an uncertain path forward, Dr. Sharaiha said. “Device companies are usually startups that need funding. With the economic downturn, venture capital funding is hard to get.”
In the meantime, patients with class 3 obesity in particular may benefit from surgery, she said.
For others, medications are playing a more important role in the obesity epidemic, with an average 10%-15% body weight loss, Dr. Sharaiha said. Injections with semaglutide (Ozempic), a glucagon-like peptide 1 (GLP-1) receptor agonist that is approved to improve glycemic control in adults with type 2 diabetes mellitus, is leading the charge.
Tirzepatide (Mounjaro) may be even more effective, Dr. Sharaiha said. The FDA approved the drug last year to improve blood sugar control in adults with type 2 diabetes and was fast-tracked in October for the treatment of adults with obesity, or who are overweight with weight-related comorbidities.
Medications provide add-on benefits to many patients who have been treated with intragastric balloons or endoscopic sleeve gastroplasty, Dr. Sharaiha said.
Also lifestyle and education should not be neglected, said Dr. Gunaratnam, who lost 50 pounds by changing his diet. He urged gastroenterologists to take on the challenge of treating obesity. It’s not the part of his practice with the best reimbursement, but it is the most satisfying. “I get more hugs, cards, and tears by doing this because when you change weight, you’re impacting every part of their lives,” he said.
Dr. Gunaratnam is the founder of Lean Medical LLC and Satya Health Sciences. Dr. Sharaiha has served as a consultant for Boston Scientific Corporation and Cook Medical Inc.
AT THE 2023 AGA TECH SUMMIT
Scant evidence for proton pump inhibitor role in gastric cancer
The available evidence suggests that proton pump inhibitors (PPIs) do not cause gastric cancer, researchers say.
A new study could help resolve a controversy over one of the most serious side effects attributed to the widely used medications.
“Our findings are reassuring, especially to all those patients who have an indication for long-term PPI use and need persistent and effective gastric acid suppression to prevent serious health consequences,” said Daniele Piovani, MSc, PhD, an assistant professor of medical statistics at Humanitas University, Milan, in an email to this news organization.
Previous studies did not take into account the probability that the diseases for which the medications were prescribed might have caused the cancer, Dr. Piovani and colleagues write in Alimentary Pharmacology and Therapeutics.
Researchers have worried about the potential of PPIs to cause cancer after finding that they are associated with enterochromaffin-like cells, gastric atrophy, and changes in gut microbiota and gastric mucosal immunology.
Observational studies and meta-analyses showed a link between PPIs and an increased risk for gastric cancer.
“However, the underlying conditions for which PPIs are prescribed are associated with gastric cancer,” said Dr. Piovani. “This may result in an apparent association between PPIs and gastric cancer.”
Another potential confounding factor is that as-yet undiagnosed cancer might also cause symptoms that are treated with PPIs. Patient behavior also may play a role, she noted.
“Let’s imagine a patient with peptic ulcer who takes PPIs,” said Dr. Piovani. “He may not only have peptic ulcer but also be a heavy smoker. He may drink much more alcohol, have a different dietary pattern, be more likely to be exposed to high levels of stress, etc. in respect to a control [patient] who does not have peptic ulcer and does not take PPIs.”
Comparing two drug classes
More recent studies have compared people taking PPIs to people taking histamine-2 receptor antagonists (H2RAs). H2RAs are often used to treat the same conditions as PPIs, but they are not as strongly linked to hypergastrinemia and are not associated with gastric atrophy, so they might serve as good comparators.
Since results of these studies have been conflicting, Dr. Piovani and colleagues attempted to weigh them together in a systematic review and meta-analysis. They identified two randomized clinical trials and 12 observational studies with a total of over 6 million patients.
One randomized controlled trial involved Helicobacter pylori–negative patients with bleeding ulcers. Researchers assigned 138 to 20 mg daily rabeprazole (a PPI) and 132 to 40 mg famotidine (an H2RA). After a year, no cancer occurred.
The other randomized controlled trial involved H. pylori–negative patients with idiopathic peptic ulcers. Investigators assigned 114 to 30 mg lansoprazole (another PPI) and 114 to 40 mg famotidine. In 2 years, one patient receiving famotidine developed cancer.
The researchers found several methodological problems with these trials. One flaw is that the study periods were not long enough to accurately measure what effects the medications might have on gastric cancers, which are a rare outcome, they note. The evidence from these studies was so weak they could not draw conclusions from the results, the investigators conclude.
Pooling data from the 11 observational trials they were able to combine, the researchers found that PPI users had a one-third higher random relative risk of cancer than H2RA users (95% confidence interval, 1.11-1.59). However, these studies were heterogenous, and five of them did not adjust for age and sex, as well as other potentially confounding covariates.
The remaining six observational studies adjusted for age, sex, and at least two other covariates that could affect the risk for gastric cancer. These studies had a total of 2.5 million patients and 7,372 gastric cancers. Combined, these studies showed an RR of gastric cancer in PPI users, compared with H2RA users of 1.07, which was not statistically significant (95% CI, 0.97-1.19).
The researchers found no clear evidence of a dose-response or of an increased risk with longer-term use of PPIs.
Findings support practice guidance
“I found this relatively reassuring,” Mark Lewis, MD, director of gastrointestinal oncology at Intermountain Healthcare, Murray, Utah, told this news organization.
PPIs do dramatically increase the pH of the stomach, stimulating the stomach to try to compensate in a process that can sometimes give rise to tumors, Dr. Lewis said. But these tumors appear to be benign.
Other concerns about PPI use, such as reduction in bone density, remain under investigation, he said.
Some H2RA blockers might actually pose a greater cancer risk than PPIs, said Dr. Lewis, and many clinicians seem to favor PPIs. “I have seen a huge sea change where most patients are on PPIs. And I would say that H2RA blockers are older and increasingly the exception in terms of usage, not the rule.”
The investigators note that observational studies by their nature cannot prove cause and effect, but because gastric cancer is so rare, a randomized controlled trial of PPIs versus H2RAs that is large enough to be definitive may not be feasible.
They conclude that their findings support the American Gastroenterological Association recommendation that “the decision to discontinue PPIs should be based solely on the lack of an indication for use and not because of concern for PPI-associated adverse effects.”
Dr. Piovani and Dr. Lewis report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The available evidence suggests that proton pump inhibitors (PPIs) do not cause gastric cancer, researchers say.
A new study could help resolve a controversy over one of the most serious side effects attributed to the widely used medications.
“Our findings are reassuring, especially to all those patients who have an indication for long-term PPI use and need persistent and effective gastric acid suppression to prevent serious health consequences,” said Daniele Piovani, MSc, PhD, an assistant professor of medical statistics at Humanitas University, Milan, in an email to this news organization.
Previous studies did not take into account the probability that the diseases for which the medications were prescribed might have caused the cancer, Dr. Piovani and colleagues write in Alimentary Pharmacology and Therapeutics.
Researchers have worried about the potential of PPIs to cause cancer after finding that they are associated with enterochromaffin-like cells, gastric atrophy, and changes in gut microbiota and gastric mucosal immunology.
Observational studies and meta-analyses showed a link between PPIs and an increased risk for gastric cancer.
“However, the underlying conditions for which PPIs are prescribed are associated with gastric cancer,” said Dr. Piovani. “This may result in an apparent association between PPIs and gastric cancer.”
Another potential confounding factor is that as-yet undiagnosed cancer might also cause symptoms that are treated with PPIs. Patient behavior also may play a role, she noted.
“Let’s imagine a patient with peptic ulcer who takes PPIs,” said Dr. Piovani. “He may not only have peptic ulcer but also be a heavy smoker. He may drink much more alcohol, have a different dietary pattern, be more likely to be exposed to high levels of stress, etc. in respect to a control [patient] who does not have peptic ulcer and does not take PPIs.”
Comparing two drug classes
More recent studies have compared people taking PPIs to people taking histamine-2 receptor antagonists (H2RAs). H2RAs are often used to treat the same conditions as PPIs, but they are not as strongly linked to hypergastrinemia and are not associated with gastric atrophy, so they might serve as good comparators.
Since results of these studies have been conflicting, Dr. Piovani and colleagues attempted to weigh them together in a systematic review and meta-analysis. They identified two randomized clinical trials and 12 observational studies with a total of over 6 million patients.
One randomized controlled trial involved Helicobacter pylori–negative patients with bleeding ulcers. Researchers assigned 138 to 20 mg daily rabeprazole (a PPI) and 132 to 40 mg famotidine (an H2RA). After a year, no cancer occurred.
The other randomized controlled trial involved H. pylori–negative patients with idiopathic peptic ulcers. Investigators assigned 114 to 30 mg lansoprazole (another PPI) and 114 to 40 mg famotidine. In 2 years, one patient receiving famotidine developed cancer.
The researchers found several methodological problems with these trials. One flaw is that the study periods were not long enough to accurately measure what effects the medications might have on gastric cancers, which are a rare outcome, they note. The evidence from these studies was so weak they could not draw conclusions from the results, the investigators conclude.
Pooling data from the 11 observational trials they were able to combine, the researchers found that PPI users had a one-third higher random relative risk of cancer than H2RA users (95% confidence interval, 1.11-1.59). However, these studies were heterogenous, and five of them did not adjust for age and sex, as well as other potentially confounding covariates.
The remaining six observational studies adjusted for age, sex, and at least two other covariates that could affect the risk for gastric cancer. These studies had a total of 2.5 million patients and 7,372 gastric cancers. Combined, these studies showed an RR of gastric cancer in PPI users, compared with H2RA users of 1.07, which was not statistically significant (95% CI, 0.97-1.19).
The researchers found no clear evidence of a dose-response or of an increased risk with longer-term use of PPIs.
Findings support practice guidance
“I found this relatively reassuring,” Mark Lewis, MD, director of gastrointestinal oncology at Intermountain Healthcare, Murray, Utah, told this news organization.
PPIs do dramatically increase the pH of the stomach, stimulating the stomach to try to compensate in a process that can sometimes give rise to tumors, Dr. Lewis said. But these tumors appear to be benign.
Other concerns about PPI use, such as reduction in bone density, remain under investigation, he said.
Some H2RA blockers might actually pose a greater cancer risk than PPIs, said Dr. Lewis, and many clinicians seem to favor PPIs. “I have seen a huge sea change where most patients are on PPIs. And I would say that H2RA blockers are older and increasingly the exception in terms of usage, not the rule.”
The investigators note that observational studies by their nature cannot prove cause and effect, but because gastric cancer is so rare, a randomized controlled trial of PPIs versus H2RAs that is large enough to be definitive may not be feasible.
They conclude that their findings support the American Gastroenterological Association recommendation that “the decision to discontinue PPIs should be based solely on the lack of an indication for use and not because of concern for PPI-associated adverse effects.”
Dr. Piovani and Dr. Lewis report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The available evidence suggests that proton pump inhibitors (PPIs) do not cause gastric cancer, researchers say.
A new study could help resolve a controversy over one of the most serious side effects attributed to the widely used medications.
“Our findings are reassuring, especially to all those patients who have an indication for long-term PPI use and need persistent and effective gastric acid suppression to prevent serious health consequences,” said Daniele Piovani, MSc, PhD, an assistant professor of medical statistics at Humanitas University, Milan, in an email to this news organization.
Previous studies did not take into account the probability that the diseases for which the medications were prescribed might have caused the cancer, Dr. Piovani and colleagues write in Alimentary Pharmacology and Therapeutics.
Researchers have worried about the potential of PPIs to cause cancer after finding that they are associated with enterochromaffin-like cells, gastric atrophy, and changes in gut microbiota and gastric mucosal immunology.
Observational studies and meta-analyses showed a link between PPIs and an increased risk for gastric cancer.
“However, the underlying conditions for which PPIs are prescribed are associated with gastric cancer,” said Dr. Piovani. “This may result in an apparent association between PPIs and gastric cancer.”
Another potential confounding factor is that as-yet undiagnosed cancer might also cause symptoms that are treated with PPIs. Patient behavior also may play a role, she noted.
“Let’s imagine a patient with peptic ulcer who takes PPIs,” said Dr. Piovani. “He may not only have peptic ulcer but also be a heavy smoker. He may drink much more alcohol, have a different dietary pattern, be more likely to be exposed to high levels of stress, etc. in respect to a control [patient] who does not have peptic ulcer and does not take PPIs.”
Comparing two drug classes
More recent studies have compared people taking PPIs to people taking histamine-2 receptor antagonists (H2RAs). H2RAs are often used to treat the same conditions as PPIs, but they are not as strongly linked to hypergastrinemia and are not associated with gastric atrophy, so they might serve as good comparators.
Since results of these studies have been conflicting, Dr. Piovani and colleagues attempted to weigh them together in a systematic review and meta-analysis. They identified two randomized clinical trials and 12 observational studies with a total of over 6 million patients.
One randomized controlled trial involved Helicobacter pylori–negative patients with bleeding ulcers. Researchers assigned 138 to 20 mg daily rabeprazole (a PPI) and 132 to 40 mg famotidine (an H2RA). After a year, no cancer occurred.
The other randomized controlled trial involved H. pylori–negative patients with idiopathic peptic ulcers. Investigators assigned 114 to 30 mg lansoprazole (another PPI) and 114 to 40 mg famotidine. In 2 years, one patient receiving famotidine developed cancer.
The researchers found several methodological problems with these trials. One flaw is that the study periods were not long enough to accurately measure what effects the medications might have on gastric cancers, which are a rare outcome, they note. The evidence from these studies was so weak they could not draw conclusions from the results, the investigators conclude.
Pooling data from the 11 observational trials they were able to combine, the researchers found that PPI users had a one-third higher random relative risk of cancer than H2RA users (95% confidence interval, 1.11-1.59). However, these studies were heterogenous, and five of them did not adjust for age and sex, as well as other potentially confounding covariates.
The remaining six observational studies adjusted for age, sex, and at least two other covariates that could affect the risk for gastric cancer. These studies had a total of 2.5 million patients and 7,372 gastric cancers. Combined, these studies showed an RR of gastric cancer in PPI users, compared with H2RA users of 1.07, which was not statistically significant (95% CI, 0.97-1.19).
The researchers found no clear evidence of a dose-response or of an increased risk with longer-term use of PPIs.
Findings support practice guidance
“I found this relatively reassuring,” Mark Lewis, MD, director of gastrointestinal oncology at Intermountain Healthcare, Murray, Utah, told this news organization.
PPIs do dramatically increase the pH of the stomach, stimulating the stomach to try to compensate in a process that can sometimes give rise to tumors, Dr. Lewis said. But these tumors appear to be benign.
Other concerns about PPI use, such as reduction in bone density, remain under investigation, he said.
Some H2RA blockers might actually pose a greater cancer risk than PPIs, said Dr. Lewis, and many clinicians seem to favor PPIs. “I have seen a huge sea change where most patients are on PPIs. And I would say that H2RA blockers are older and increasingly the exception in terms of usage, not the rule.”
The investigators note that observational studies by their nature cannot prove cause and effect, but because gastric cancer is so rare, a randomized controlled trial of PPIs versus H2RAs that is large enough to be definitive may not be feasible.
They conclude that their findings support the American Gastroenterological Association recommendation that “the decision to discontinue PPIs should be based solely on the lack of an indication for use and not because of concern for PPI-associated adverse effects.”
Dr. Piovani and Dr. Lewis report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ALIMENTARY PHARMACOLOGY AND THERAPEUTICS
Expert panel forms strategy for eosinophilic esophagitis monitoring
“Follow-up should comprise symptom assessment and periodic or repeated endoscopy with histological assessment in specific EoE settings,” wrote Ulrike von Arnim, MD, from University Hospital Magdeburg (Germany), and an international team of colleagues in Clinical Gastroenterology and Hepatology.
Although medicine and diet can control EoE, there is presently no cure, and long-term management is needed to prevent recurrence and long-term effects such as esophageal remodeling, fibrosis, and stricture, the researchers said. Yet they could find no evidence-based recommendations for clinical monitoring of the condition.
With the participation of The International Gastrointestinal Eosinophil Researchers (TIGER) and the European Consortium for Eosinophilic Diseases of the GI Tract (EUREOS), they assembled a team of 18 gastroenterologists, pathologists, and allergists from the United States and Western Europe with expertise in the condition.
Almost all panelists had more than 10 years of subspecialty EoE care and more than five relevant research publications. All were members of TIGER or EUREOS. The panel met by video conferencing and responded to surveys to develop a consensus about why, by what means, and when to monitor patients with EoE.
The group reached 75% or greater agreement on 11 statements on these subjects.
Regular follow-ups are needed because they enable clinicians to detect whether treatments have stopped working, improve therapy adherence, and introduce patients to any new treatments that become available, while preventing gaps in care that can worsen outcomes, the group wrote.
Symptoms don’t give a precise indication of esophageal healing and shouldn’t be the sole measure for disease activity, the experts wrote. They recommended other approaches to monitoring, including biopsies. They also endorse the Endoscopic Reference Score as an outcome measure.
The panel recommended noninvasive tissue sampling, mentioning the esophageal string test and the Cytosponge as examples, but called for more research on these two techniques.
Blood markers, oral swabs, breath condensates, and stool and urine samples are not recommended as approaches for monitoring EoE, they wrote.
The optimal interval to measure the efficacy of a therapy is more difficult to decide, the panel noted.
“The clinician’s decision should take into account the clinical severity of the disease, estimated risk of imminent subsequent food impaction, presence of stenosis, as well as mode of action and reported outcome of the chosen medical, dietary, or mechanical treatment,” they wrote. Intervals from 6 to 24 weeks may be appropriate.
For diets and topical corticosteroids, they agreed on an interval of 8-12 weeks to confirm remission but say a longer time might be preferred for slower-acting therapies, such as monoclonal antibodies.
The panel had the most trouble reaching a consensus on how often to follow up on patients whose disease is in remission or is stable. They settled on 12 to 24 months after the last endoscopy. Any longer than 2 years risks missing increased disease activity, they wrote.
This follow-up should include assessment of symptoms and a gastrointestinal endoscopy in cases of relapse or suspected stricture, as well as when treatment modification is being considered or when assessment of histological activity is desired, the panel recommended.
Almost all the panelists disclosed financial relationships with pharmaceutical or medical device companies.
A version of this article first appeared on Medscape.com.
“Follow-up should comprise symptom assessment and periodic or repeated endoscopy with histological assessment in specific EoE settings,” wrote Ulrike von Arnim, MD, from University Hospital Magdeburg (Germany), and an international team of colleagues in Clinical Gastroenterology and Hepatology.
Although medicine and diet can control EoE, there is presently no cure, and long-term management is needed to prevent recurrence and long-term effects such as esophageal remodeling, fibrosis, and stricture, the researchers said. Yet they could find no evidence-based recommendations for clinical monitoring of the condition.
With the participation of The International Gastrointestinal Eosinophil Researchers (TIGER) and the European Consortium for Eosinophilic Diseases of the GI Tract (EUREOS), they assembled a team of 18 gastroenterologists, pathologists, and allergists from the United States and Western Europe with expertise in the condition.
Almost all panelists had more than 10 years of subspecialty EoE care and more than five relevant research publications. All were members of TIGER or EUREOS. The panel met by video conferencing and responded to surveys to develop a consensus about why, by what means, and when to monitor patients with EoE.
The group reached 75% or greater agreement on 11 statements on these subjects.
Regular follow-ups are needed because they enable clinicians to detect whether treatments have stopped working, improve therapy adherence, and introduce patients to any new treatments that become available, while preventing gaps in care that can worsen outcomes, the group wrote.
Symptoms don’t give a precise indication of esophageal healing and shouldn’t be the sole measure for disease activity, the experts wrote. They recommended other approaches to monitoring, including biopsies. They also endorse the Endoscopic Reference Score as an outcome measure.
The panel recommended noninvasive tissue sampling, mentioning the esophageal string test and the Cytosponge as examples, but called for more research on these two techniques.
Blood markers, oral swabs, breath condensates, and stool and urine samples are not recommended as approaches for monitoring EoE, they wrote.
The optimal interval to measure the efficacy of a therapy is more difficult to decide, the panel noted.
“The clinician’s decision should take into account the clinical severity of the disease, estimated risk of imminent subsequent food impaction, presence of stenosis, as well as mode of action and reported outcome of the chosen medical, dietary, or mechanical treatment,” they wrote. Intervals from 6 to 24 weeks may be appropriate.
For diets and topical corticosteroids, they agreed on an interval of 8-12 weeks to confirm remission but say a longer time might be preferred for slower-acting therapies, such as monoclonal antibodies.
The panel had the most trouble reaching a consensus on how often to follow up on patients whose disease is in remission or is stable. They settled on 12 to 24 months after the last endoscopy. Any longer than 2 years risks missing increased disease activity, they wrote.
This follow-up should include assessment of symptoms and a gastrointestinal endoscopy in cases of relapse or suspected stricture, as well as when treatment modification is being considered or when assessment of histological activity is desired, the panel recommended.
Almost all the panelists disclosed financial relationships with pharmaceutical or medical device companies.
A version of this article first appeared on Medscape.com.
“Follow-up should comprise symptom assessment and periodic or repeated endoscopy with histological assessment in specific EoE settings,” wrote Ulrike von Arnim, MD, from University Hospital Magdeburg (Germany), and an international team of colleagues in Clinical Gastroenterology and Hepatology.
Although medicine and diet can control EoE, there is presently no cure, and long-term management is needed to prevent recurrence and long-term effects such as esophageal remodeling, fibrosis, and stricture, the researchers said. Yet they could find no evidence-based recommendations for clinical monitoring of the condition.
With the participation of The International Gastrointestinal Eosinophil Researchers (TIGER) and the European Consortium for Eosinophilic Diseases of the GI Tract (EUREOS), they assembled a team of 18 gastroenterologists, pathologists, and allergists from the United States and Western Europe with expertise in the condition.
Almost all panelists had more than 10 years of subspecialty EoE care and more than five relevant research publications. All were members of TIGER or EUREOS. The panel met by video conferencing and responded to surveys to develop a consensus about why, by what means, and when to monitor patients with EoE.
The group reached 75% or greater agreement on 11 statements on these subjects.
Regular follow-ups are needed because they enable clinicians to detect whether treatments have stopped working, improve therapy adherence, and introduce patients to any new treatments that become available, while preventing gaps in care that can worsen outcomes, the group wrote.
Symptoms don’t give a precise indication of esophageal healing and shouldn’t be the sole measure for disease activity, the experts wrote. They recommended other approaches to monitoring, including biopsies. They also endorse the Endoscopic Reference Score as an outcome measure.
The panel recommended noninvasive tissue sampling, mentioning the esophageal string test and the Cytosponge as examples, but called for more research on these two techniques.
Blood markers, oral swabs, breath condensates, and stool and urine samples are not recommended as approaches for monitoring EoE, they wrote.
The optimal interval to measure the efficacy of a therapy is more difficult to decide, the panel noted.
“The clinician’s decision should take into account the clinical severity of the disease, estimated risk of imminent subsequent food impaction, presence of stenosis, as well as mode of action and reported outcome of the chosen medical, dietary, or mechanical treatment,” they wrote. Intervals from 6 to 24 weeks may be appropriate.
For diets and topical corticosteroids, they agreed on an interval of 8-12 weeks to confirm remission but say a longer time might be preferred for slower-acting therapies, such as monoclonal antibodies.
The panel had the most trouble reaching a consensus on how often to follow up on patients whose disease is in remission or is stable. They settled on 12 to 24 months after the last endoscopy. Any longer than 2 years risks missing increased disease activity, they wrote.
This follow-up should include assessment of symptoms and a gastrointestinal endoscopy in cases of relapse or suspected stricture, as well as when treatment modification is being considered or when assessment of histological activity is desired, the panel recommended.
Almost all the panelists disclosed financial relationships with pharmaceutical or medical device companies.
A version of this article first appeared on Medscape.com.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Lifestyle changes may reduce colorectal cancer risk
Changes regarding smoking, drinking, body weight, and physical activity may alter the risk for colorectal cancer (CRC), the results of a study on a large European cohort suggest.
“This is a clear message that practicing clinicians and gastroenterologists could give to their patients and to CRC screening participants to improve CRC prevention,” write Edoardo Botteri, PhD, Cancer Registry of Norway, Oslo, and colleagues in an article published in the American Journal of Gastroenterology.
Previous studies have shown a correlation between cancer in general and unhealthy lifestyle factors. They have also shown an association between weight gain and an increased risk for CRC and a reduced risk with smoking cessation. But Dr. Botteri and colleagues could not find any published research on the association of other lifestyle factors and the risk for CRC specifically, they write.
To help fill this gap, they followed 295,865 people who participated in the European Prospective Investigation into Cancer (EPIC) for a median of 7.8 years. The participants were mostly aged from 35 to 70 years and lived in Denmark, France, Germany, Greece, Italy, the Netherlands, Norway, Spain, Sweden, and the United Kingdom.
The researchers calculated a healthy lifestyle index (HLI) score on the basis of smoking status, alcohol consumption, body mass index, and physical activity. The median time between baseline and the follow-up questionnaire was 5.7 years.
They awarded points as indicated in the following table.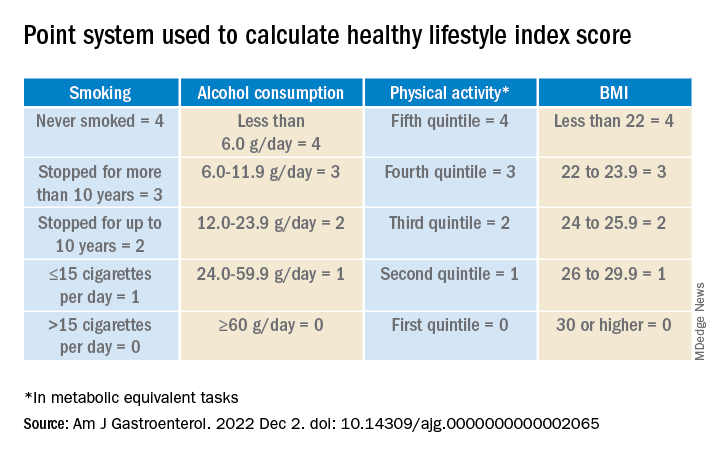
Participants’ scores ranged from 0 to 16. At baseline, the mean HLI score was 10.04. It dipped slightly to 9.95 at follow-up.
Men had more favorable changes than women, and the associations between the HLI score and CRC risk were only statistically significant among men.
Overall, a 1-unit increase in the HLI score was associated with a 3% lower risk for CRC.
When the HLI scores were grouped into tertiles, improvements from an “unfavorable lifestyle” (0-9) to a “favorable lifestyle” (12-16) were associated with a 23% lower risk for CRC (compared with no change). Likewise, a decline from a “favorable lifestyle” to an “unfavorable lifestyle” was associated with a 34% higher risk.
Changes in the BMI score from baseline showed a trend toward an association with CRC risk.
Decreases in alcohol consumption were significantly associated with a reduction in CRC risk among participants aged 55 years or younger at baseline.
Increases in physical activity were significantly associated with a lower risk for proximal colon cancer, especially in younger participants.
On the other hand, reductions in smoking were associated with an increase in CRC risk. This correlation might be the result of “inverse causation,” the researchers note; that is, people may have quit smoking because they experienced early symptoms of CRC. Smoking had only a marginal influence on the HLI calculations in this study because only a small proportion of participants changed their smoking rates.
Information on diet was collected only at baseline, so changes in this factor could not be measured. The researchers adjusted their analysis for diet at baseline, but they acknowledge that their inability to incorporate diet into the HLI score was a limitation of the study.
Similarly, they used education as a marker of socioeconomic status but acknowledge that this is only a proxy.
“The HLI score may therefore not accurately capture the complex relationship between lifestyle habits and risk for CRC,” they write.
Still, if the results of this observational study are confirmed by other research, the findings could provide evidence to design intervention studies to prevent CRC, they conclude.
The study was supported by the grant LIBERTY from the French Institut National du Cancer. Financial supporters of the national cohorts and the coordination of EPIC are listed in the published study. The researchers reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Changes regarding smoking, drinking, body weight, and physical activity may alter the risk for colorectal cancer (CRC), the results of a study on a large European cohort suggest.
“This is a clear message that practicing clinicians and gastroenterologists could give to their patients and to CRC screening participants to improve CRC prevention,” write Edoardo Botteri, PhD, Cancer Registry of Norway, Oslo, and colleagues in an article published in the American Journal of Gastroenterology.
Previous studies have shown a correlation between cancer in general and unhealthy lifestyle factors. They have also shown an association between weight gain and an increased risk for CRC and a reduced risk with smoking cessation. But Dr. Botteri and colleagues could not find any published research on the association of other lifestyle factors and the risk for CRC specifically, they write.
To help fill this gap, they followed 295,865 people who participated in the European Prospective Investigation into Cancer (EPIC) for a median of 7.8 years. The participants were mostly aged from 35 to 70 years and lived in Denmark, France, Germany, Greece, Italy, the Netherlands, Norway, Spain, Sweden, and the United Kingdom.
The researchers calculated a healthy lifestyle index (HLI) score on the basis of smoking status, alcohol consumption, body mass index, and physical activity. The median time between baseline and the follow-up questionnaire was 5.7 years.
They awarded points as indicated in the following table.
Participants’ scores ranged from 0 to 16. At baseline, the mean HLI score was 10.04. It dipped slightly to 9.95 at follow-up.
Men had more favorable changes than women, and the associations between the HLI score and CRC risk were only statistically significant among men.
Overall, a 1-unit increase in the HLI score was associated with a 3% lower risk for CRC.
When the HLI scores were grouped into tertiles, improvements from an “unfavorable lifestyle” (0-9) to a “favorable lifestyle” (12-16) were associated with a 23% lower risk for CRC (compared with no change). Likewise, a decline from a “favorable lifestyle” to an “unfavorable lifestyle” was associated with a 34% higher risk.
Changes in the BMI score from baseline showed a trend toward an association with CRC risk.
Decreases in alcohol consumption were significantly associated with a reduction in CRC risk among participants aged 55 years or younger at baseline.
Increases in physical activity were significantly associated with a lower risk for proximal colon cancer, especially in younger participants.
On the other hand, reductions in smoking were associated with an increase in CRC risk. This correlation might be the result of “inverse causation,” the researchers note; that is, people may have quit smoking because they experienced early symptoms of CRC. Smoking had only a marginal influence on the HLI calculations in this study because only a small proportion of participants changed their smoking rates.
Information on diet was collected only at baseline, so changes in this factor could not be measured. The researchers adjusted their analysis for diet at baseline, but they acknowledge that their inability to incorporate diet into the HLI score was a limitation of the study.
Similarly, they used education as a marker of socioeconomic status but acknowledge that this is only a proxy.
“The HLI score may therefore not accurately capture the complex relationship between lifestyle habits and risk for CRC,” they write.
Still, if the results of this observational study are confirmed by other research, the findings could provide evidence to design intervention studies to prevent CRC, they conclude.
The study was supported by the grant LIBERTY from the French Institut National du Cancer. Financial supporters of the national cohorts and the coordination of EPIC are listed in the published study. The researchers reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Changes regarding smoking, drinking, body weight, and physical activity may alter the risk for colorectal cancer (CRC), the results of a study on a large European cohort suggest.
“This is a clear message that practicing clinicians and gastroenterologists could give to their patients and to CRC screening participants to improve CRC prevention,” write Edoardo Botteri, PhD, Cancer Registry of Norway, Oslo, and colleagues in an article published in the American Journal of Gastroenterology.
Previous studies have shown a correlation between cancer in general and unhealthy lifestyle factors. They have also shown an association between weight gain and an increased risk for CRC and a reduced risk with smoking cessation. But Dr. Botteri and colleagues could not find any published research on the association of other lifestyle factors and the risk for CRC specifically, they write.
To help fill this gap, they followed 295,865 people who participated in the European Prospective Investigation into Cancer (EPIC) for a median of 7.8 years. The participants were mostly aged from 35 to 70 years and lived in Denmark, France, Germany, Greece, Italy, the Netherlands, Norway, Spain, Sweden, and the United Kingdom.
The researchers calculated a healthy lifestyle index (HLI) score on the basis of smoking status, alcohol consumption, body mass index, and physical activity. The median time between baseline and the follow-up questionnaire was 5.7 years.
They awarded points as indicated in the following table.
Participants’ scores ranged from 0 to 16. At baseline, the mean HLI score was 10.04. It dipped slightly to 9.95 at follow-up.
Men had more favorable changes than women, and the associations between the HLI score and CRC risk were only statistically significant among men.
Overall, a 1-unit increase in the HLI score was associated with a 3% lower risk for CRC.
When the HLI scores were grouped into tertiles, improvements from an “unfavorable lifestyle” (0-9) to a “favorable lifestyle” (12-16) were associated with a 23% lower risk for CRC (compared with no change). Likewise, a decline from a “favorable lifestyle” to an “unfavorable lifestyle” was associated with a 34% higher risk.
Changes in the BMI score from baseline showed a trend toward an association with CRC risk.
Decreases in alcohol consumption were significantly associated with a reduction in CRC risk among participants aged 55 years or younger at baseline.
Increases in physical activity were significantly associated with a lower risk for proximal colon cancer, especially in younger participants.
On the other hand, reductions in smoking were associated with an increase in CRC risk. This correlation might be the result of “inverse causation,” the researchers note; that is, people may have quit smoking because they experienced early symptoms of CRC. Smoking had only a marginal influence on the HLI calculations in this study because only a small proportion of participants changed their smoking rates.
Information on diet was collected only at baseline, so changes in this factor could not be measured. The researchers adjusted their analysis for diet at baseline, but they acknowledge that their inability to incorporate diet into the HLI score was a limitation of the study.
Similarly, they used education as a marker of socioeconomic status but acknowledge that this is only a proxy.
“The HLI score may therefore not accurately capture the complex relationship between lifestyle habits and risk for CRC,” they write.
Still, if the results of this observational study are confirmed by other research, the findings could provide evidence to design intervention studies to prevent CRC, they conclude.
The study was supported by the grant LIBERTY from the French Institut National du Cancer. Financial supporters of the national cohorts and the coordination of EPIC are listed in the published study. The researchers reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE AMERICAN JOURNAL OF GASTROENTEROLOGY
Dupilumab effective for eosinophilic esophagitis up to 52 weeks
Few side effects, other than injection pain, emerged in the year-long phase 3 trial that convinced the Food and Drug Administration to approve the drug for EoE in May, said Evan S. Dellon, MD, a gastroenterologist at the University of North Carolina at Chapel Hill.
The FDA approved dupilumab for the treatment of EoE in people 12 years and older who weigh at least 40 kg (about 88 pounds). The study included patients who had failed to benefit from an 8-week course of high-dose proton pump inhibitor (PPI) therapy, and most also hadn’t had relief from topical steroids.
Because dupilumab is a systemic biologic, such patients are the most likely candidates for the medication, Dr. Dellon said.
Dr. Dellon and colleagues published the study results in the New England Journal of Medicine.
A chronic, progressive, type 2 inflammatory disease of the esophagus, EoE can make it difficult to swallow and cause abdominal and chest pain or vomiting. Specialized diets, topical steroids, and PPIs can help manage EoE for many patients, but these therapies don’t always work, diets are difficult to follow, and topical steroids and PPIs can be difficult to take or cause side effects.
A fully human monoclonal antibody developed by Regeneron Pharmaceuticals and Sanofi, dupilumab blocks the shared receptor component for interleukin-4 and interleukin-13, central drivers of type 2 inflammation in EoE. The FDA has approved dupilumab for atopic dermatitis, asthma, and chronic rhinosinusitis with nasal polyps.
Three-part trial
For the three-part study conducted in Australia, Canada, Europe, and the United States, participants received 300 mg dupilumab by subcutaneous injection.
In part A, investigators randomly assigned 42 patients to weekly doses of dupilumab and 39 to weekly placebo injections for 24 weeks.
In part B, they randomly divided patients into three groups: Eighty received dupilumab weekly; 81 received dupilumab every 2 weeks; and 79 received a placebo weekly, for 24 weeks.
In part C, the intervention and placebo groups from part A received dupilumab weekly for another 28 weeks. For the two intervention groups in part B, treatment remained the same, while the part B placebo group was evenly split to receive dupilumab weekly or every 2 weeks for 28 weeks.
Over half of patients in remission
In part A, 60% of patients receiving weekly dupilumab achieved histologic remission (defined as no more than six eosinophils per high-power field), compared with 5% of patients receiving placebo, a significant difference (P < .001).
In part B, 59% of patients receiving weekly dupilumab, 60% of those receiving biweekly dupilumab, and 6% of those receiving placebo achieved histologic remission. The difference between those receiving weekly dupilumab or placebo was significant (P < .001).
Symptoms improved with weekly dupilumab, as measured via the Dysphagia Symptom Questionnaire score, which can range from 0 to 84, with higher values indicating more frequent or more severe dysphagia. The mean score at baseline was 33.6 in part A and 36.7 in part B. Scores in patients receiving weekly dupilumab decreased by 12.3 in part A and 9.9 in part B (both P < .001). However, among part B patients receiving biweekly dupilumab, the mean score dropped by only 0.5, which was not significant.
Dupilumab reached a higher serum concentration with the weekly than the biweekly regimen, which may explain the improved symptoms with more frequent dosing, Dr. Dellon said.
Only nine patients experienced serious adverse events during the part A or B treatment period (seven who received weekly dupilumab, one who received biweekly dupilumab, and one who received placebo) and just one patient during the part C treatment period who received placebo in part A and weekly dupilumab in part C. None of these events, except one, were related to the regimen, according to investigators.
Patients who received weekly dupilumab in part A and continued to part C maintained similar treatment effects to week 52, Dr. Dellon said. Although the data on part B patients who continued into part C were not included in the published paper, they were similar, he said.
“The people who responded to the first 24 weeks maintain that response for up to 52 weeks, and there was even a gain of response for some measures,” he said.
Patients, on average, also experienced improvements as measured by endoscopic healing, histological severity scores, and even gene expression, Dr. Dellon said.
‘Welcome addition’
Dupilumab is “a welcome addition to what we do,” said Philip Katz, MD, a professor of medicine in the division of gastroenterology at Weill Cornell Medicine in New York. The publication of this “pivotal” trial provides reassurance about its safety and effectiveness for EoE, he added.
“Dupilumab, or Dupixent, is going to be used in my practice for people who are refractory to both PPIs and topical steroids,” Dr. Katz, who was not involved in the research, said in an interview.
However, it will take practice to know how best to use the drug, and its high cost may make some payers reluctant to cover it, Dr. Katz added.
The study was funded by Sanofi and Regeneron. Dr. Dellon has reported financial relationships with multiple pharmaceutical companies, including Regeneron and Sanofi US. Dr. Katz has reported financial relationships with Phantom Pharmaceuticals, AstraZeneca, and Braintree Laboratories.
A version of this article first appeared on Medscape.com.
Few side effects, other than injection pain, emerged in the year-long phase 3 trial that convinced the Food and Drug Administration to approve the drug for EoE in May, said Evan S. Dellon, MD, a gastroenterologist at the University of North Carolina at Chapel Hill.
The FDA approved dupilumab for the treatment of EoE in people 12 years and older who weigh at least 40 kg (about 88 pounds). The study included patients who had failed to benefit from an 8-week course of high-dose proton pump inhibitor (PPI) therapy, and most also hadn’t had relief from topical steroids.
Because dupilumab is a systemic biologic, such patients are the most likely candidates for the medication, Dr. Dellon said.
Dr. Dellon and colleagues published the study results in the New England Journal of Medicine.
A chronic, progressive, type 2 inflammatory disease of the esophagus, EoE can make it difficult to swallow and cause abdominal and chest pain or vomiting. Specialized diets, topical steroids, and PPIs can help manage EoE for many patients, but these therapies don’t always work, diets are difficult to follow, and topical steroids and PPIs can be difficult to take or cause side effects.
A fully human monoclonal antibody developed by Regeneron Pharmaceuticals and Sanofi, dupilumab blocks the shared receptor component for interleukin-4 and interleukin-13, central drivers of type 2 inflammation in EoE. The FDA has approved dupilumab for atopic dermatitis, asthma, and chronic rhinosinusitis with nasal polyps.
Three-part trial
For the three-part study conducted in Australia, Canada, Europe, and the United States, participants received 300 mg dupilumab by subcutaneous injection.
In part A, investigators randomly assigned 42 patients to weekly doses of dupilumab and 39 to weekly placebo injections for 24 weeks.
In part B, they randomly divided patients into three groups: Eighty received dupilumab weekly; 81 received dupilumab every 2 weeks; and 79 received a placebo weekly, for 24 weeks.
In part C, the intervention and placebo groups from part A received dupilumab weekly for another 28 weeks. For the two intervention groups in part B, treatment remained the same, while the part B placebo group was evenly split to receive dupilumab weekly or every 2 weeks for 28 weeks.
Over half of patients in remission
In part A, 60% of patients receiving weekly dupilumab achieved histologic remission (defined as no more than six eosinophils per high-power field), compared with 5% of patients receiving placebo, a significant difference (P < .001).
In part B, 59% of patients receiving weekly dupilumab, 60% of those receiving biweekly dupilumab, and 6% of those receiving placebo achieved histologic remission. The difference between those receiving weekly dupilumab or placebo was significant (P < .001).
Symptoms improved with weekly dupilumab, as measured via the Dysphagia Symptom Questionnaire score, which can range from 0 to 84, with higher values indicating more frequent or more severe dysphagia. The mean score at baseline was 33.6 in part A and 36.7 in part B. Scores in patients receiving weekly dupilumab decreased by 12.3 in part A and 9.9 in part B (both P < .001). However, among part B patients receiving biweekly dupilumab, the mean score dropped by only 0.5, which was not significant.
Dupilumab reached a higher serum concentration with the weekly than the biweekly regimen, which may explain the improved symptoms with more frequent dosing, Dr. Dellon said.
Only nine patients experienced serious adverse events during the part A or B treatment period (seven who received weekly dupilumab, one who received biweekly dupilumab, and one who received placebo) and just one patient during the part C treatment period who received placebo in part A and weekly dupilumab in part C. None of these events, except one, were related to the regimen, according to investigators.
Patients who received weekly dupilumab in part A and continued to part C maintained similar treatment effects to week 52, Dr. Dellon said. Although the data on part B patients who continued into part C were not included in the published paper, they were similar, he said.
“The people who responded to the first 24 weeks maintain that response for up to 52 weeks, and there was even a gain of response for some measures,” he said.
Patients, on average, also experienced improvements as measured by endoscopic healing, histological severity scores, and even gene expression, Dr. Dellon said.
‘Welcome addition’
Dupilumab is “a welcome addition to what we do,” said Philip Katz, MD, a professor of medicine in the division of gastroenterology at Weill Cornell Medicine in New York. The publication of this “pivotal” trial provides reassurance about its safety and effectiveness for EoE, he added.
“Dupilumab, or Dupixent, is going to be used in my practice for people who are refractory to both PPIs and topical steroids,” Dr. Katz, who was not involved in the research, said in an interview.
However, it will take practice to know how best to use the drug, and its high cost may make some payers reluctant to cover it, Dr. Katz added.
The study was funded by Sanofi and Regeneron. Dr. Dellon has reported financial relationships with multiple pharmaceutical companies, including Regeneron and Sanofi US. Dr. Katz has reported financial relationships with Phantom Pharmaceuticals, AstraZeneca, and Braintree Laboratories.
A version of this article first appeared on Medscape.com.
Few side effects, other than injection pain, emerged in the year-long phase 3 trial that convinced the Food and Drug Administration to approve the drug for EoE in May, said Evan S. Dellon, MD, a gastroenterologist at the University of North Carolina at Chapel Hill.
The FDA approved dupilumab for the treatment of EoE in people 12 years and older who weigh at least 40 kg (about 88 pounds). The study included patients who had failed to benefit from an 8-week course of high-dose proton pump inhibitor (PPI) therapy, and most also hadn’t had relief from topical steroids.
Because dupilumab is a systemic biologic, such patients are the most likely candidates for the medication, Dr. Dellon said.
Dr. Dellon and colleagues published the study results in the New England Journal of Medicine.
A chronic, progressive, type 2 inflammatory disease of the esophagus, EoE can make it difficult to swallow and cause abdominal and chest pain or vomiting. Specialized diets, topical steroids, and PPIs can help manage EoE for many patients, but these therapies don’t always work, diets are difficult to follow, and topical steroids and PPIs can be difficult to take or cause side effects.
A fully human monoclonal antibody developed by Regeneron Pharmaceuticals and Sanofi, dupilumab blocks the shared receptor component for interleukin-4 and interleukin-13, central drivers of type 2 inflammation in EoE. The FDA has approved dupilumab for atopic dermatitis, asthma, and chronic rhinosinusitis with nasal polyps.
Three-part trial
For the three-part study conducted in Australia, Canada, Europe, and the United States, participants received 300 mg dupilumab by subcutaneous injection.
In part A, investigators randomly assigned 42 patients to weekly doses of dupilumab and 39 to weekly placebo injections for 24 weeks.
In part B, they randomly divided patients into three groups: Eighty received dupilumab weekly; 81 received dupilumab every 2 weeks; and 79 received a placebo weekly, for 24 weeks.
In part C, the intervention and placebo groups from part A received dupilumab weekly for another 28 weeks. For the two intervention groups in part B, treatment remained the same, while the part B placebo group was evenly split to receive dupilumab weekly or every 2 weeks for 28 weeks.
Over half of patients in remission
In part A, 60% of patients receiving weekly dupilumab achieved histologic remission (defined as no more than six eosinophils per high-power field), compared with 5% of patients receiving placebo, a significant difference (P < .001).
In part B, 59% of patients receiving weekly dupilumab, 60% of those receiving biweekly dupilumab, and 6% of those receiving placebo achieved histologic remission. The difference between those receiving weekly dupilumab or placebo was significant (P < .001).
Symptoms improved with weekly dupilumab, as measured via the Dysphagia Symptom Questionnaire score, which can range from 0 to 84, with higher values indicating more frequent or more severe dysphagia. The mean score at baseline was 33.6 in part A and 36.7 in part B. Scores in patients receiving weekly dupilumab decreased by 12.3 in part A and 9.9 in part B (both P < .001). However, among part B patients receiving biweekly dupilumab, the mean score dropped by only 0.5, which was not significant.
Dupilumab reached a higher serum concentration with the weekly than the biweekly regimen, which may explain the improved symptoms with more frequent dosing, Dr. Dellon said.
Only nine patients experienced serious adverse events during the part A or B treatment period (seven who received weekly dupilumab, one who received biweekly dupilumab, and one who received placebo) and just one patient during the part C treatment period who received placebo in part A and weekly dupilumab in part C. None of these events, except one, were related to the regimen, according to investigators.
Patients who received weekly dupilumab in part A and continued to part C maintained similar treatment effects to week 52, Dr. Dellon said. Although the data on part B patients who continued into part C were not included in the published paper, they were similar, he said.
“The people who responded to the first 24 weeks maintain that response for up to 52 weeks, and there was even a gain of response for some measures,” he said.
Patients, on average, also experienced improvements as measured by endoscopic healing, histological severity scores, and even gene expression, Dr. Dellon said.
‘Welcome addition’
Dupilumab is “a welcome addition to what we do,” said Philip Katz, MD, a professor of medicine in the division of gastroenterology at Weill Cornell Medicine in New York. The publication of this “pivotal” trial provides reassurance about its safety and effectiveness for EoE, he added.
“Dupilumab, or Dupixent, is going to be used in my practice for people who are refractory to both PPIs and topical steroids,” Dr. Katz, who was not involved in the research, said in an interview.
However, it will take practice to know how best to use the drug, and its high cost may make some payers reluctant to cover it, Dr. Katz added.
The study was funded by Sanofi and Regeneron. Dr. Dellon has reported financial relationships with multiple pharmaceutical companies, including Regeneron and Sanofi US. Dr. Katz has reported financial relationships with Phantom Pharmaceuticals, AstraZeneca, and Braintree Laboratories.
A version of this article first appeared on Medscape.com.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Kiwifruit found effective for constipation
Kiwifruit can increase the frequency of bowel movements for people with constipation, according to researchers in New Zealand.
“In addition to improved measures of constipation status, there was a significant improvement in stool consistency, reduction in constipation, indigestion/reflux, and abdominal pain resulting in an improved overall level of GI comfort,” write Richard Gearry, MD, PhD, of the University of Otago, New Zealand, and colleagues.
Dr. Gearry and colleagues describe their international multicenter controlled trial of kiwifruit as a constipation treatment in the American Journal of Gastroenterology.
Although constipation is common, previous researchers have reported a high rate of dissatisfaction with pharmaceutical treatments.
Other components of kiwifruit, such as raphides, may alter mucin production, leading to improved laxation.
Several previous studies have suggested that regular consumption of fresh, green kiwifruit may be effective as a treatment for constipation. However, these studies have typically been small with nonstandardized endpoints, Dr. Gearry and colleagues note. In their article, they did not comment on studies of other fresh fruits or vegetables as constipation treatments.
The researchers set out to test the benefits of the fruit in a more rigorous trial. They recruited adults in New Zealand, Italy, and Japan who had either functional constipation or constipation-predominant irritable bowel syndrome (IBS-C), as well as healthy people as controls. The primary difference between functional constipation and IBS-C is that people with IBS-C experience abdominal pain along with constipation, they write.
Between June 12, 2014, and June 17, 2017, 184 participants were enrolled. Participants included 136 women and 48 men, a proportion consistent with the gender prevalence of constipation. The mean age was 30.5 years in Japan, 36.9 years in Italy, and 44.8 years in New Zealand, and the mean body mass index was 20.6 in Japan, 23.0 in Italy, and 25.4 in New Zealand.
For 2 weeks following recruitment, the participants became accustomed to recording their bowel movements with respect to frequency, completeness, spontaneity, stool form, laxative use, and degree of straining.
Participants were then randomly assigned to consume either two ripe green kiwifruit (Actinidia chinensis var. deliciosa “Hayward”) without the skins, or 7.5 g of psyllium per day for 4 weeks. Psyllium is considered a first-line treatment for both the constipation conditions with which participants were diagnosed. The fiber content of psyllium is similar to that of kiwifruit.
After 4 weeks, treatments were stopped for 4 weeks as a washout period, after which patients were switched to the other treatment for another 4 weeks.
The researchers provided them with two bisacodyl (5-g suppositories) as a pharmacologic rescue therapy.
Overall, 169 participants completed the study, and compliance with diary completion was more than 80%.
At the end of the 4-week treatment periods, the people with functional constipation who ate kiwifruit demonstrated an average increase of 1.53 bowel movements per week, a statistically significant increase (P < .0001). Those with IBS-C demonstrated an increase of 1.73 (P = .0001). Both groups reported significantly improved gastrointestinal comfort on the gastrointestinal symptom rating scale.
The frequency of bowel movements in the healthy participants did not increase.
Among those participants who took psyllium, only those with IBS-C experienced a statistically significant increase in the frequency of their bowel movements or a decrease in their gastrointestinal symptoms. Their bowel movements increased by an average of 1.87 per week (P = .0051).
The participants who ate kiwifruit reported softening of stool consistency, reduction in straining, and improvement in quality of life compared with baseline, and these improvements in straining and stool consistency were better than was reported by those who took psyllium.
“Taken in conjunction with previous clinical trials of green kiwifruit and the emerging physiological data from functional studies, consumption of two green kiwifruit can be safely recommended as an effective treatment for constipation in those with functional gastrointestinal disorders that will also provide improvements in symptoms of GI comfort,” the researchers conclude.
Two of the researchers were employed by Zespri International, a cooperative of kiwifruit growers, which partially funded the study and approved its design. The New Zealand study center trial was jointly funded by a grant from the New Zealand government.
A version of this article first appeared on Medscape.com.
Kiwifruit can increase the frequency of bowel movements for people with constipation, according to researchers in New Zealand.
“In addition to improved measures of constipation status, there was a significant improvement in stool consistency, reduction in constipation, indigestion/reflux, and abdominal pain resulting in an improved overall level of GI comfort,” write Richard Gearry, MD, PhD, of the University of Otago, New Zealand, and colleagues.
Dr. Gearry and colleagues describe their international multicenter controlled trial of kiwifruit as a constipation treatment in the American Journal of Gastroenterology.
Although constipation is common, previous researchers have reported a high rate of dissatisfaction with pharmaceutical treatments.
Other components of kiwifruit, such as raphides, may alter mucin production, leading to improved laxation.
Several previous studies have suggested that regular consumption of fresh, green kiwifruit may be effective as a treatment for constipation. However, these studies have typically been small with nonstandardized endpoints, Dr. Gearry and colleagues note. In their article, they did not comment on studies of other fresh fruits or vegetables as constipation treatments.
The researchers set out to test the benefits of the fruit in a more rigorous trial. They recruited adults in New Zealand, Italy, and Japan who had either functional constipation or constipation-predominant irritable bowel syndrome (IBS-C), as well as healthy people as controls. The primary difference between functional constipation and IBS-C is that people with IBS-C experience abdominal pain along with constipation, they write.
Between June 12, 2014, and June 17, 2017, 184 participants were enrolled. Participants included 136 women and 48 men, a proportion consistent with the gender prevalence of constipation. The mean age was 30.5 years in Japan, 36.9 years in Italy, and 44.8 years in New Zealand, and the mean body mass index was 20.6 in Japan, 23.0 in Italy, and 25.4 in New Zealand.
For 2 weeks following recruitment, the participants became accustomed to recording their bowel movements with respect to frequency, completeness, spontaneity, stool form, laxative use, and degree of straining.
Participants were then randomly assigned to consume either two ripe green kiwifruit (Actinidia chinensis var. deliciosa “Hayward”) without the skins, or 7.5 g of psyllium per day for 4 weeks. Psyllium is considered a first-line treatment for both the constipation conditions with which participants were diagnosed. The fiber content of psyllium is similar to that of kiwifruit.
After 4 weeks, treatments were stopped for 4 weeks as a washout period, after which patients were switched to the other treatment for another 4 weeks.
The researchers provided them with two bisacodyl (5-g suppositories) as a pharmacologic rescue therapy.
Overall, 169 participants completed the study, and compliance with diary completion was more than 80%.
At the end of the 4-week treatment periods, the people with functional constipation who ate kiwifruit demonstrated an average increase of 1.53 bowel movements per week, a statistically significant increase (P < .0001). Those with IBS-C demonstrated an increase of 1.73 (P = .0001). Both groups reported significantly improved gastrointestinal comfort on the gastrointestinal symptom rating scale.
The frequency of bowel movements in the healthy participants did not increase.
Among those participants who took psyllium, only those with IBS-C experienced a statistically significant increase in the frequency of their bowel movements or a decrease in their gastrointestinal symptoms. Their bowel movements increased by an average of 1.87 per week (P = .0051).
The participants who ate kiwifruit reported softening of stool consistency, reduction in straining, and improvement in quality of life compared with baseline, and these improvements in straining and stool consistency were better than was reported by those who took psyllium.
“Taken in conjunction with previous clinical trials of green kiwifruit and the emerging physiological data from functional studies, consumption of two green kiwifruit can be safely recommended as an effective treatment for constipation in those with functional gastrointestinal disorders that will also provide improvements in symptoms of GI comfort,” the researchers conclude.
Two of the researchers were employed by Zespri International, a cooperative of kiwifruit growers, which partially funded the study and approved its design. The New Zealand study center trial was jointly funded by a grant from the New Zealand government.
A version of this article first appeared on Medscape.com.
Kiwifruit can increase the frequency of bowel movements for people with constipation, according to researchers in New Zealand.
“In addition to improved measures of constipation status, there was a significant improvement in stool consistency, reduction in constipation, indigestion/reflux, and abdominal pain resulting in an improved overall level of GI comfort,” write Richard Gearry, MD, PhD, of the University of Otago, New Zealand, and colleagues.
Dr. Gearry and colleagues describe their international multicenter controlled trial of kiwifruit as a constipation treatment in the American Journal of Gastroenterology.
Although constipation is common, previous researchers have reported a high rate of dissatisfaction with pharmaceutical treatments.
Other components of kiwifruit, such as raphides, may alter mucin production, leading to improved laxation.
Several previous studies have suggested that regular consumption of fresh, green kiwifruit may be effective as a treatment for constipation. However, these studies have typically been small with nonstandardized endpoints, Dr. Gearry and colleagues note. In their article, they did not comment on studies of other fresh fruits or vegetables as constipation treatments.
The researchers set out to test the benefits of the fruit in a more rigorous trial. They recruited adults in New Zealand, Italy, and Japan who had either functional constipation or constipation-predominant irritable bowel syndrome (IBS-C), as well as healthy people as controls. The primary difference between functional constipation and IBS-C is that people with IBS-C experience abdominal pain along with constipation, they write.
Between June 12, 2014, and June 17, 2017, 184 participants were enrolled. Participants included 136 women and 48 men, a proportion consistent with the gender prevalence of constipation. The mean age was 30.5 years in Japan, 36.9 years in Italy, and 44.8 years in New Zealand, and the mean body mass index was 20.6 in Japan, 23.0 in Italy, and 25.4 in New Zealand.
For 2 weeks following recruitment, the participants became accustomed to recording their bowel movements with respect to frequency, completeness, spontaneity, stool form, laxative use, and degree of straining.
Participants were then randomly assigned to consume either two ripe green kiwifruit (Actinidia chinensis var. deliciosa “Hayward”) without the skins, or 7.5 g of psyllium per day for 4 weeks. Psyllium is considered a first-line treatment for both the constipation conditions with which participants were diagnosed. The fiber content of psyllium is similar to that of kiwifruit.
After 4 weeks, treatments were stopped for 4 weeks as a washout period, after which patients were switched to the other treatment for another 4 weeks.
The researchers provided them with two bisacodyl (5-g suppositories) as a pharmacologic rescue therapy.
Overall, 169 participants completed the study, and compliance with diary completion was more than 80%.
At the end of the 4-week treatment periods, the people with functional constipation who ate kiwifruit demonstrated an average increase of 1.53 bowel movements per week, a statistically significant increase (P < .0001). Those with IBS-C demonstrated an increase of 1.73 (P = .0001). Both groups reported significantly improved gastrointestinal comfort on the gastrointestinal symptom rating scale.
The frequency of bowel movements in the healthy participants did not increase.
Among those participants who took psyllium, only those with IBS-C experienced a statistically significant increase in the frequency of their bowel movements or a decrease in their gastrointestinal symptoms. Their bowel movements increased by an average of 1.87 per week (P = .0051).
The participants who ate kiwifruit reported softening of stool consistency, reduction in straining, and improvement in quality of life compared with baseline, and these improvements in straining and stool consistency were better than was reported by those who took psyllium.
“Taken in conjunction with previous clinical trials of green kiwifruit and the emerging physiological data from functional studies, consumption of two green kiwifruit can be safely recommended as an effective treatment for constipation in those with functional gastrointestinal disorders that will also provide improvements in symptoms of GI comfort,” the researchers conclude.
Two of the researchers were employed by Zespri International, a cooperative of kiwifruit growers, which partially funded the study and approved its design. The New Zealand study center trial was jointly funded by a grant from the New Zealand government.
A version of this article first appeared on Medscape.com.
FROM THE AMERICAN JOURNAL OF GASTROENTEROLOGY
Lifestyle guidance app may be effective in NASH
After 48 weeks, nonalcoholic fatty liver disease activity scores (NAS) improved in 13 out of 19 patients who used the NASH app developed by CureApp, according to Masaya Sato of the University of Tokyo and colleagues.
If confirmed by a controlled trial, these preliminary results could show promise for digital therapeutics, the researchers stated in an article published in The American Journal of Gastroenterology.
“The widespread use of smartphones, which can process and communicate data in real time, makes them an ideal platform for therapeutic interventions,” they said.
Although lifestyle changes can reduce NASH activity, many patients have difficulty keeping up these changes. Not enough counselors are available to guide patients in healthy practices, and hiring the counselors is expensive, the researchers wrote.
Smartphone applications aimed at instilling healthy behavior have been tried in diabetes, smoking, hypertension, alcoholism, and even cancer, they noted. They wanted to see whether something similar could be done with NASH.
The researchers recruited 19 patients with biopsy-confirmed NASH who consumed no more than moderate amounts of alcohol and had a body mass index (BMI) of at least 25 kg/m2. Their mean age was 52 years, mean BMI was 32, and mean NAS was 5.0.
The patients downloaded the NASH app onto their phones and entered their baseline profile information, including age, gender, diet and exercise practices, and social characteristics. On the basis of this information and daily weight measurements, the system proposed lifestyle improvement programs tailored to each individual. Its chatbot presented them in the form of behavioral goals and lectures from virtual nurses.
While patients used the app for 48 weeks, they also received standard outpatient care for NASH from live physicians, who also promoted the use of the app and provided additional education related to NASH.
The patients underwent liver biopsies within 90 days prior to beginning the study and at the end of 48 weeks. The researchers compared the changes in these patients versus those in a hypothetical control group, which they based on the placebo group in a previous study.
In the patients who used the app, the mean NAS change from baseline to week 48, the main endpoint, was –2.05 (95% confidence interval, –3.00 to –1.11). This result was statistically significant compared with the hypothetical control group, in which the mean change in NAS was –0.7 (P < .001).
In 11 of the patients, NAS decreased by at least 2 points without worsening of liver fibrosis. In eight patients, the researchers observed resolution of steatohepatitis, which they defined as disappearance of hepatocyte ballooning.
In 12 patients with stage F2 or F3 fibrosis, the average stage went from 2.5 to 2.0 (P = .02). No patient with stage F1 fibrosis showed a reduction in fibrosis stage. The scores for steatosis decreased in 11 patients, for lobular inflammation in 9 patients, and for ballooning in 10 patients.
The patients lost an average of 8.3% of their body weight, which was significant, compared with their baseline (P < .001). The patients also notched significant reductions in average serum levels of AST, ALT, gamma-glutamyltransferase, alkaline phosphatase, and triglycerides.
The researchers noted that the lack of a real control group and the small size of the study population limited the importance of their findings. A larger randomized, controlled trial is needed to confirm their results.
During the study, physicians browsed the patients’ data and provided them with feedback about it, the researchers wrote. But the study did not measure the amount of time the physicians spent on this activity.
CureApp founded the study, and one of the authors is a consultant for the company.
A version of this article first appeared on Medscape.com.
After 48 weeks, nonalcoholic fatty liver disease activity scores (NAS) improved in 13 out of 19 patients who used the NASH app developed by CureApp, according to Masaya Sato of the University of Tokyo and colleagues.
If confirmed by a controlled trial, these preliminary results could show promise for digital therapeutics, the researchers stated in an article published in The American Journal of Gastroenterology.
“The widespread use of smartphones, which can process and communicate data in real time, makes them an ideal platform for therapeutic interventions,” they said.
Although lifestyle changes can reduce NASH activity, many patients have difficulty keeping up these changes. Not enough counselors are available to guide patients in healthy practices, and hiring the counselors is expensive, the researchers wrote.
Smartphone applications aimed at instilling healthy behavior have been tried in diabetes, smoking, hypertension, alcoholism, and even cancer, they noted. They wanted to see whether something similar could be done with NASH.
The researchers recruited 19 patients with biopsy-confirmed NASH who consumed no more than moderate amounts of alcohol and had a body mass index (BMI) of at least 25 kg/m2. Their mean age was 52 years, mean BMI was 32, and mean NAS was 5.0.
The patients downloaded the NASH app onto their phones and entered their baseline profile information, including age, gender, diet and exercise practices, and social characteristics. On the basis of this information and daily weight measurements, the system proposed lifestyle improvement programs tailored to each individual. Its chatbot presented them in the form of behavioral goals and lectures from virtual nurses.
While patients used the app for 48 weeks, they also received standard outpatient care for NASH from live physicians, who also promoted the use of the app and provided additional education related to NASH.
The patients underwent liver biopsies within 90 days prior to beginning the study and at the end of 48 weeks. The researchers compared the changes in these patients versus those in a hypothetical control group, which they based on the placebo group in a previous study.
In the patients who used the app, the mean NAS change from baseline to week 48, the main endpoint, was –2.05 (95% confidence interval, –3.00 to –1.11). This result was statistically significant compared with the hypothetical control group, in which the mean change in NAS was –0.7 (P < .001).
In 11 of the patients, NAS decreased by at least 2 points without worsening of liver fibrosis. In eight patients, the researchers observed resolution of steatohepatitis, which they defined as disappearance of hepatocyte ballooning.
In 12 patients with stage F2 or F3 fibrosis, the average stage went from 2.5 to 2.0 (P = .02). No patient with stage F1 fibrosis showed a reduction in fibrosis stage. The scores for steatosis decreased in 11 patients, for lobular inflammation in 9 patients, and for ballooning in 10 patients.
The patients lost an average of 8.3% of their body weight, which was significant, compared with their baseline (P < .001). The patients also notched significant reductions in average serum levels of AST, ALT, gamma-glutamyltransferase, alkaline phosphatase, and triglycerides.
The researchers noted that the lack of a real control group and the small size of the study population limited the importance of their findings. A larger randomized, controlled trial is needed to confirm their results.
During the study, physicians browsed the patients’ data and provided them with feedback about it, the researchers wrote. But the study did not measure the amount of time the physicians spent on this activity.
CureApp founded the study, and one of the authors is a consultant for the company.
A version of this article first appeared on Medscape.com.
After 48 weeks, nonalcoholic fatty liver disease activity scores (NAS) improved in 13 out of 19 patients who used the NASH app developed by CureApp, according to Masaya Sato of the University of Tokyo and colleagues.
If confirmed by a controlled trial, these preliminary results could show promise for digital therapeutics, the researchers stated in an article published in The American Journal of Gastroenterology.
“The widespread use of smartphones, which can process and communicate data in real time, makes them an ideal platform for therapeutic interventions,” they said.
Although lifestyle changes can reduce NASH activity, many patients have difficulty keeping up these changes. Not enough counselors are available to guide patients in healthy practices, and hiring the counselors is expensive, the researchers wrote.
Smartphone applications aimed at instilling healthy behavior have been tried in diabetes, smoking, hypertension, alcoholism, and even cancer, they noted. They wanted to see whether something similar could be done with NASH.
The researchers recruited 19 patients with biopsy-confirmed NASH who consumed no more than moderate amounts of alcohol and had a body mass index (BMI) of at least 25 kg/m2. Their mean age was 52 years, mean BMI was 32, and mean NAS was 5.0.
The patients downloaded the NASH app onto their phones and entered their baseline profile information, including age, gender, diet and exercise practices, and social characteristics. On the basis of this information and daily weight measurements, the system proposed lifestyle improvement programs tailored to each individual. Its chatbot presented them in the form of behavioral goals and lectures from virtual nurses.
While patients used the app for 48 weeks, they also received standard outpatient care for NASH from live physicians, who also promoted the use of the app and provided additional education related to NASH.
The patients underwent liver biopsies within 90 days prior to beginning the study and at the end of 48 weeks. The researchers compared the changes in these patients versus those in a hypothetical control group, which they based on the placebo group in a previous study.
In the patients who used the app, the mean NAS change from baseline to week 48, the main endpoint, was –2.05 (95% confidence interval, –3.00 to –1.11). This result was statistically significant compared with the hypothetical control group, in which the mean change in NAS was –0.7 (P < .001).
In 11 of the patients, NAS decreased by at least 2 points without worsening of liver fibrosis. In eight patients, the researchers observed resolution of steatohepatitis, which they defined as disappearance of hepatocyte ballooning.
In 12 patients with stage F2 or F3 fibrosis, the average stage went from 2.5 to 2.0 (P = .02). No patient with stage F1 fibrosis showed a reduction in fibrosis stage. The scores for steatosis decreased in 11 patients, for lobular inflammation in 9 patients, and for ballooning in 10 patients.
The patients lost an average of 8.3% of their body weight, which was significant, compared with their baseline (P < .001). The patients also notched significant reductions in average serum levels of AST, ALT, gamma-glutamyltransferase, alkaline phosphatase, and triglycerides.
The researchers noted that the lack of a real control group and the small size of the study population limited the importance of their findings. A larger randomized, controlled trial is needed to confirm their results.
During the study, physicians browsed the patients’ data and provided them with feedback about it, the researchers wrote. But the study did not measure the amount of time the physicians spent on this activity.
CureApp founded the study, and one of the authors is a consultant for the company.
A version of this article first appeared on Medscape.com.
FROM THE AMERICAN JOURNAL OF GASTROENTEROLOGY
Ustekinumab has comparatively low infection rate in IBD
Patients with inflammatory bowel disease (IBD) taking ustekinumab (Stelara) are less likely to have infections as a side effect than those taking tofacitinib (Xeljanz) or anti–tumor necrosis factor-alpha (anti-TNF), researchers say.
Although the risk of infections is not large for any of these drugs, clinicians should nonetheless take it into account, given their similar efficacy, said Ashwin Ananthakrishnan, MD, associate professor of medicine at Massachusetts General Hospital, Boston.
“These findings may not dramatically change prescribing patterns, but in those who may be particularly vulnerable for infection, they could point toward the safer biologic being Stelara,” he told this news organization.
The study by Dr. Ananthakrishnan and his colleagues was published in Clinical Gastroenterology and Hepatology.
Biologic and small-molecule immunosuppressive therapies have emerged as effective treatments for Crohn’s disease and ulcerative colitis, the two conditions that comprise IBD.
But the recent proliferation of drugs in these classes with different mechanisms of action has made prescribing decisions complicated. And because the drugs suppress the immune system, they can make patients vulnerable to infection.
Randomized controlled trials, even those comparing the drugs head to head, have not been large enough to compare safety outcomes with statistical accuracy, Dr. Ananthakrishnan and his colleagues found.
Accessing a large, real-world cohort
To help fill this gap, they analyzed data on 89,972,617 people enrolled in Aetna, the nationwide U.S. health insurance plan, from 2008-2019.
They identified 19,096 patients whose IBD was treated with anti-TNF agents, 2,420 with ustekinumab, and 305 with tofacitinib. The number of patients taking tofacitinib is small because it is a newer drug, Dr. Ananthakrishnan said.
They found a higher rate of infection and rate of infection-related hospitalization for the patients taking anti-TNF agents (44% and 7%, respectively), compared with those taking ustekinumab (32% and 4%, respectively) over the course of up to a year.
The researchers adjusted for age, sex, IBD duration, prior corticosteroid use, prior immunomodulator use, prior IBD hospitalization, and comorbidities, after which they then compared the risks of infection.
They found that patients taking ustekinumab were 7% less likely to have any infection than patients taking anti-TNF agents (hazard ratio, 0.93), a statistically significant difference (95% confidence interval, 0.86-0.99; P = .041). The reduction in the risk of infection-related hospitalizations was similar but not statistically significant.
The advantage with ustekinumab over anti-TNF agents was larger for patients with comorbidities. There was a 25% reduction in the risk of infections for patients taking ustekinumab who had a Charlson comorbidity index score of at least 2 and who were younger than 65 years (HRm 0.71; 95% CI, 0.58-0.87; P < .001).
For patients taking tofacitinib, there were no significant differences in the rate of infection (HR, 0.97; 95% CI, 0.75-1.24) or infection-related hospitalizations (HR, 0.59; 95% CI, 0.27-1.05), compared with TNF-antagonists.
The respiratory system and the urinary tract were the most common sites of infections. Bacterial, viral, and fungal infections were similarly distributed in the three groups.
Remaining questions
Further research is needed, said Dr. Ananthakrishnan, as “to understand the comparative safety may be particularly important for vulnerable populations, like those who are older or have other underlying comorbidity, where safety is increasingly an important issue.”
The findings are similar to those of other studies comparing infections in association with ustekinumab to anti-TNF medications for related conditions, such as psoriatic arthritis and psoriasis, he said.
Clinicians have seen similar differences among the drugs in clinical practice, said Miguel Regueiro, MD, chair of the Digestive Disease and Surgery Institute at Cleveland Clinic, Ohio, who was not involved in the study.
“It aligns well with what we’ve thought, but it’s nice to see in a publication,” he said in an interview.
The implications of the study are limited, because it was not a prospective randomized trial and because the number of patients taking tofacitinib was so small, Dr. Regueiro added.
Another limitation is that the patients who were admitted to the hospital in this database were not necessarily admitted because of their infections, said Stephen Hanauer, MD, medical director of the Digestive Health Center at Northwestern University, Chicago, who also was not involved in the study.
Dr. Hanauer told this news organization that comparisons with other agents would be helpful.
“They didn’t look at vedolizumab (Entyvio) in this database,” he said. “Entyvio is generally considered to be safer than TNF inhibitors or tofacitinib with fairly comparable safety to ustekinumab.”
Dr. Ananthakrishnan reported financial relationships with Gilead, Ikena Therapeutics, and Sun Pharma. Dr. Regueiro reported financial relationships with AbbVie, BMS, Janssen, UCB, Pfizer, Takeda, Celgene, Genentech, Gilead, UCB, Miraca Labs, Amgen, Celgene, Seres, Allergan, Salix, Prometheus, Lilly, TARGET Pharma Solutions, Alfasigigma, and BMS. Dr. Hanauer reported financial relationships with Janssen Pharmaceuticals, AbbVie, Takeda Pharmaceutical, and Pfizer.
A version of this article first appeared on Medscape.com.
With our growing armamentarium of effective medical therapies for Crohn’s disease (CD) and ulcerative colitis (UC) come increasing decisions for patients and providers for treatments based on comparative effectiveness and safety. One of the most frequent concerns by both patients and providers is risk of infection. While we have partial data on safety from clinical trials, trials are underpowered to compare safety outcomes and also typically compare placebo rather than other active treatments.
This study by Cheng et al. provides further reassurance that ustekinumab and tofacitinib are at least as safe from an infection standpoint as TNF inhibitors, with UST having a small, but statistically significant lower risk of infection overall compared to TNF inhibitors. While there have been signals that ustekinumab may have lower infection risk compared to TNF inhibitors from clinical trials and real-world analyses of ustekinumab in other disease states, this study is remarkable in that it studies CD and UC specifically. Ustekinumab dosing for CD and UC are higher than that used for other indications, so it’s highly relevant to study ustekinumab in CD and UC, specifically for safety. This study is also notable in that no statistically significant difference in infection, particularly herpes zoster, was observed in tofacitinib vs. TNF inhibitors.
This study has limitations as a retrospective administrative dataset, including its inability to determine indication of prescription for CD or UC vs. rheumatologic or dermatologic condition, lack of adjustment for concomitant immunomodulator use, and inability to determine primary indication for hospitalization. However, this study should allow providers to discuss with patients with greater confidence that infection risks of ustekinumab and tofacitinib were overall low and that ustekinumab has lower risks of infections than TNF inhibitors.
Jason K. Hou, MD, MS, is an investigator in the clinical epidemiology and outcomes program in the Center for Innovations in Quality, Effectiveness and Safety at the Michael E. DeBakey VA Medical Center and an associate professor at the Baylor College of Medicine, both in Houston. He has no relevant conflicts of interest.
With our growing armamentarium of effective medical therapies for Crohn’s disease (CD) and ulcerative colitis (UC) come increasing decisions for patients and providers for treatments based on comparative effectiveness and safety. One of the most frequent concerns by both patients and providers is risk of infection. While we have partial data on safety from clinical trials, trials are underpowered to compare safety outcomes and also typically compare placebo rather than other active treatments.
This study by Cheng et al. provides further reassurance that ustekinumab and tofacitinib are at least as safe from an infection standpoint as TNF inhibitors, with UST having a small, but statistically significant lower risk of infection overall compared to TNF inhibitors. While there have been signals that ustekinumab may have lower infection risk compared to TNF inhibitors from clinical trials and real-world analyses of ustekinumab in other disease states, this study is remarkable in that it studies CD and UC specifically. Ustekinumab dosing for CD and UC are higher than that used for other indications, so it’s highly relevant to study ustekinumab in CD and UC, specifically for safety. This study is also notable in that no statistically significant difference in infection, particularly herpes zoster, was observed in tofacitinib vs. TNF inhibitors.
This study has limitations as a retrospective administrative dataset, including its inability to determine indication of prescription for CD or UC vs. rheumatologic or dermatologic condition, lack of adjustment for concomitant immunomodulator use, and inability to determine primary indication for hospitalization. However, this study should allow providers to discuss with patients with greater confidence that infection risks of ustekinumab and tofacitinib were overall low and that ustekinumab has lower risks of infections than TNF inhibitors.
Jason K. Hou, MD, MS, is an investigator in the clinical epidemiology and outcomes program in the Center for Innovations in Quality, Effectiveness and Safety at the Michael E. DeBakey VA Medical Center and an associate professor at the Baylor College of Medicine, both in Houston. He has no relevant conflicts of interest.
With our growing armamentarium of effective medical therapies for Crohn’s disease (CD) and ulcerative colitis (UC) come increasing decisions for patients and providers for treatments based on comparative effectiveness and safety. One of the most frequent concerns by both patients and providers is risk of infection. While we have partial data on safety from clinical trials, trials are underpowered to compare safety outcomes and also typically compare placebo rather than other active treatments.
This study by Cheng et al. provides further reassurance that ustekinumab and tofacitinib are at least as safe from an infection standpoint as TNF inhibitors, with UST having a small, but statistically significant lower risk of infection overall compared to TNF inhibitors. While there have been signals that ustekinumab may have lower infection risk compared to TNF inhibitors from clinical trials and real-world analyses of ustekinumab in other disease states, this study is remarkable in that it studies CD and UC specifically. Ustekinumab dosing for CD and UC are higher than that used for other indications, so it’s highly relevant to study ustekinumab in CD and UC, specifically for safety. This study is also notable in that no statistically significant difference in infection, particularly herpes zoster, was observed in tofacitinib vs. TNF inhibitors.
This study has limitations as a retrospective administrative dataset, including its inability to determine indication of prescription for CD or UC vs. rheumatologic or dermatologic condition, lack of adjustment for concomitant immunomodulator use, and inability to determine primary indication for hospitalization. However, this study should allow providers to discuss with patients with greater confidence that infection risks of ustekinumab and tofacitinib were overall low and that ustekinumab has lower risks of infections than TNF inhibitors.
Jason K. Hou, MD, MS, is an investigator in the clinical epidemiology and outcomes program in the Center for Innovations in Quality, Effectiveness and Safety at the Michael E. DeBakey VA Medical Center and an associate professor at the Baylor College of Medicine, both in Houston. He has no relevant conflicts of interest.
Patients with inflammatory bowel disease (IBD) taking ustekinumab (Stelara) are less likely to have infections as a side effect than those taking tofacitinib (Xeljanz) or anti–tumor necrosis factor-alpha (anti-TNF), researchers say.
Although the risk of infections is not large for any of these drugs, clinicians should nonetheless take it into account, given their similar efficacy, said Ashwin Ananthakrishnan, MD, associate professor of medicine at Massachusetts General Hospital, Boston.
“These findings may not dramatically change prescribing patterns, but in those who may be particularly vulnerable for infection, they could point toward the safer biologic being Stelara,” he told this news organization.
The study by Dr. Ananthakrishnan and his colleagues was published in Clinical Gastroenterology and Hepatology.
Biologic and small-molecule immunosuppressive therapies have emerged as effective treatments for Crohn’s disease and ulcerative colitis, the two conditions that comprise IBD.
But the recent proliferation of drugs in these classes with different mechanisms of action has made prescribing decisions complicated. And because the drugs suppress the immune system, they can make patients vulnerable to infection.
Randomized controlled trials, even those comparing the drugs head to head, have not been large enough to compare safety outcomes with statistical accuracy, Dr. Ananthakrishnan and his colleagues found.
Accessing a large, real-world cohort
To help fill this gap, they analyzed data on 89,972,617 people enrolled in Aetna, the nationwide U.S. health insurance plan, from 2008-2019.
They identified 19,096 patients whose IBD was treated with anti-TNF agents, 2,420 with ustekinumab, and 305 with tofacitinib. The number of patients taking tofacitinib is small because it is a newer drug, Dr. Ananthakrishnan said.
They found a higher rate of infection and rate of infection-related hospitalization for the patients taking anti-TNF agents (44% and 7%, respectively), compared with those taking ustekinumab (32% and 4%, respectively) over the course of up to a year.
The researchers adjusted for age, sex, IBD duration, prior corticosteroid use, prior immunomodulator use, prior IBD hospitalization, and comorbidities, after which they then compared the risks of infection.
They found that patients taking ustekinumab were 7% less likely to have any infection than patients taking anti-TNF agents (hazard ratio, 0.93), a statistically significant difference (95% confidence interval, 0.86-0.99; P = .041). The reduction in the risk of infection-related hospitalizations was similar but not statistically significant.
The advantage with ustekinumab over anti-TNF agents was larger for patients with comorbidities. There was a 25% reduction in the risk of infections for patients taking ustekinumab who had a Charlson comorbidity index score of at least 2 and who were younger than 65 years (HRm 0.71; 95% CI, 0.58-0.87; P < .001).
For patients taking tofacitinib, there were no significant differences in the rate of infection (HR, 0.97; 95% CI, 0.75-1.24) or infection-related hospitalizations (HR, 0.59; 95% CI, 0.27-1.05), compared with TNF-antagonists.
The respiratory system and the urinary tract were the most common sites of infections. Bacterial, viral, and fungal infections were similarly distributed in the three groups.
Remaining questions
Further research is needed, said Dr. Ananthakrishnan, as “to understand the comparative safety may be particularly important for vulnerable populations, like those who are older or have other underlying comorbidity, where safety is increasingly an important issue.”
The findings are similar to those of other studies comparing infections in association with ustekinumab to anti-TNF medications for related conditions, such as psoriatic arthritis and psoriasis, he said.
Clinicians have seen similar differences among the drugs in clinical practice, said Miguel Regueiro, MD, chair of the Digestive Disease and Surgery Institute at Cleveland Clinic, Ohio, who was not involved in the study.
“It aligns well with what we’ve thought, but it’s nice to see in a publication,” he said in an interview.
The implications of the study are limited, because it was not a prospective randomized trial and because the number of patients taking tofacitinib was so small, Dr. Regueiro added.
Another limitation is that the patients who were admitted to the hospital in this database were not necessarily admitted because of their infections, said Stephen Hanauer, MD, medical director of the Digestive Health Center at Northwestern University, Chicago, who also was not involved in the study.
Dr. Hanauer told this news organization that comparisons with other agents would be helpful.
“They didn’t look at vedolizumab (Entyvio) in this database,” he said. “Entyvio is generally considered to be safer than TNF inhibitors or tofacitinib with fairly comparable safety to ustekinumab.”
Dr. Ananthakrishnan reported financial relationships with Gilead, Ikena Therapeutics, and Sun Pharma. Dr. Regueiro reported financial relationships with AbbVie, BMS, Janssen, UCB, Pfizer, Takeda, Celgene, Genentech, Gilead, UCB, Miraca Labs, Amgen, Celgene, Seres, Allergan, Salix, Prometheus, Lilly, TARGET Pharma Solutions, Alfasigigma, and BMS. Dr. Hanauer reported financial relationships with Janssen Pharmaceuticals, AbbVie, Takeda Pharmaceutical, and Pfizer.
A version of this article first appeared on Medscape.com.
Patients with inflammatory bowel disease (IBD) taking ustekinumab (Stelara) are less likely to have infections as a side effect than those taking tofacitinib (Xeljanz) or anti–tumor necrosis factor-alpha (anti-TNF), researchers say.
Although the risk of infections is not large for any of these drugs, clinicians should nonetheless take it into account, given their similar efficacy, said Ashwin Ananthakrishnan, MD, associate professor of medicine at Massachusetts General Hospital, Boston.
“These findings may not dramatically change prescribing patterns, but in those who may be particularly vulnerable for infection, they could point toward the safer biologic being Stelara,” he told this news organization.
The study by Dr. Ananthakrishnan and his colleagues was published in Clinical Gastroenterology and Hepatology.
Biologic and small-molecule immunosuppressive therapies have emerged as effective treatments for Crohn’s disease and ulcerative colitis, the two conditions that comprise IBD.
But the recent proliferation of drugs in these classes with different mechanisms of action has made prescribing decisions complicated. And because the drugs suppress the immune system, they can make patients vulnerable to infection.
Randomized controlled trials, even those comparing the drugs head to head, have not been large enough to compare safety outcomes with statistical accuracy, Dr. Ananthakrishnan and his colleagues found.
Accessing a large, real-world cohort
To help fill this gap, they analyzed data on 89,972,617 people enrolled in Aetna, the nationwide U.S. health insurance plan, from 2008-2019.
They identified 19,096 patients whose IBD was treated with anti-TNF agents, 2,420 with ustekinumab, and 305 with tofacitinib. The number of patients taking tofacitinib is small because it is a newer drug, Dr. Ananthakrishnan said.
They found a higher rate of infection and rate of infection-related hospitalization for the patients taking anti-TNF agents (44% and 7%, respectively), compared with those taking ustekinumab (32% and 4%, respectively) over the course of up to a year.
The researchers adjusted for age, sex, IBD duration, prior corticosteroid use, prior immunomodulator use, prior IBD hospitalization, and comorbidities, after which they then compared the risks of infection.
They found that patients taking ustekinumab were 7% less likely to have any infection than patients taking anti-TNF agents (hazard ratio, 0.93), a statistically significant difference (95% confidence interval, 0.86-0.99; P = .041). The reduction in the risk of infection-related hospitalizations was similar but not statistically significant.
The advantage with ustekinumab over anti-TNF agents was larger for patients with comorbidities. There was a 25% reduction in the risk of infections for patients taking ustekinumab who had a Charlson comorbidity index score of at least 2 and who were younger than 65 years (HRm 0.71; 95% CI, 0.58-0.87; P < .001).
For patients taking tofacitinib, there were no significant differences in the rate of infection (HR, 0.97; 95% CI, 0.75-1.24) or infection-related hospitalizations (HR, 0.59; 95% CI, 0.27-1.05), compared with TNF-antagonists.
The respiratory system and the urinary tract were the most common sites of infections. Bacterial, viral, and fungal infections were similarly distributed in the three groups.
Remaining questions
Further research is needed, said Dr. Ananthakrishnan, as “to understand the comparative safety may be particularly important for vulnerable populations, like those who are older or have other underlying comorbidity, where safety is increasingly an important issue.”
The findings are similar to those of other studies comparing infections in association with ustekinumab to anti-TNF medications for related conditions, such as psoriatic arthritis and psoriasis, he said.
Clinicians have seen similar differences among the drugs in clinical practice, said Miguel Regueiro, MD, chair of the Digestive Disease and Surgery Institute at Cleveland Clinic, Ohio, who was not involved in the study.
“It aligns well with what we’ve thought, but it’s nice to see in a publication,” he said in an interview.
The implications of the study are limited, because it was not a prospective randomized trial and because the number of patients taking tofacitinib was so small, Dr. Regueiro added.
Another limitation is that the patients who were admitted to the hospital in this database were not necessarily admitted because of their infections, said Stephen Hanauer, MD, medical director of the Digestive Health Center at Northwestern University, Chicago, who also was not involved in the study.
Dr. Hanauer told this news organization that comparisons with other agents would be helpful.
“They didn’t look at vedolizumab (Entyvio) in this database,” he said. “Entyvio is generally considered to be safer than TNF inhibitors or tofacitinib with fairly comparable safety to ustekinumab.”
Dr. Ananthakrishnan reported financial relationships with Gilead, Ikena Therapeutics, and Sun Pharma. Dr. Regueiro reported financial relationships with AbbVie, BMS, Janssen, UCB, Pfizer, Takeda, Celgene, Genentech, Gilead, UCB, Miraca Labs, Amgen, Celgene, Seres, Allergan, Salix, Prometheus, Lilly, TARGET Pharma Solutions, Alfasigigma, and BMS. Dr. Hanauer reported financial relationships with Janssen Pharmaceuticals, AbbVie, Takeda Pharmaceutical, and Pfizer.
A version of this article first appeared on Medscape.com.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Pervasive ‘forever chemical’ linked to liver cancer
The correlation does not prove that PFOS causes this cancer, and more research is needed, but in the meantime, people should limit their exposure to it and others in its class, said Jesse Goodrich, PhD, a postdoctoral scholar in environmental medicine at the University of Southern California, Los Angeles.
“If you’re at risk for liver cancer because you have other risk factors, then these chemicals have the potential to kind of send you over the edge,” he told this news organization.
Dr. Goodrich and colleagues published their research online in JHEP Reports.
Dubbed “forever chemicals” because they can take thousands of years to break down, polyfluoroalkyl substances (PFAS) figure in makeup, food packaging, waterproof clothing, nonstick cookware, firefighting foams, and groundwater. They have spread through the atmosphere into rain and can be found in the blood of most Americans. PFOS is one of the most widely used PFAS.
“You can’t really escape them,” Dr. Goodrich said.
Previous research has linked PFAS to infertility, pregnancy complications, learning and behavioral problems in children, immune system issues, and higher cholesterol, as well as other cancers. Some experiments in animals suggested PFAS could cause liver cancer, and others showed a correlation between PFAS serum levels and biomarkers associated with liver cancer. But many of these health effects take a long time to develop.
“It wasn’t until we started to get really highly exposed groups of people that we started, as scientists, to be able to figure out what was going on,” said Dr. Goodrich.
High exposure, increased incidence
To measure the relationship between PFAS exposure and the incidence of hepatocellular carcinoma more definitively, Dr. Goodrich and colleagues analyzed data from the Multiethnic Cohort Study, a cohort of more than 200,000 people of African, Latin, Native Hawaiian, Japanese, and European ancestry tracked since the early 1990s in California and Hawaii. About 67,000 participants provided blood samples from 2001 to 2007.
From this cohort, the researchers found 50 people who later developed hepatocellular carcinoma. The researchers matched these patients with 50 controls of similar age at blood collection, sex, race, ethnicity, and study area who did not develop the cancer.
They found that people with more than 54.9 mcg/L of PFOS in their blood before any diagnosis of hepatocellular carcinoma were almost five times more likely to get the cancer (odds ratio 4.5; 95% confidence interval, 1.2-16.0), which was statistically significant (P = .02).
This level of PFOS corresponds to the 90th percentile found in the U.S. National Health and Nutrition Examination Survey (NHANES).
To get some idea of the mechanism by which PFOS might do its damage, the researchers also looked for linkage to levels of metabolites.
They found an overlap among high PFOS levels, hepatocellular carcinoma, and high levels of glucose, butyric acid (a short chain fatty acid), alpha-Ketoisovaleric acid (alpha branched-chain alpha-keto acid), and 7alpha-Hydroxy-3-oxo-4-cholestenoate (a bile acid). These metabolites have been associated in previous studies with metabolic disorders and liver disease.
Similarly, the researchers identified an association among the cancer, PFOS, and alterations in amino acid and glycan biosynthesis pathways.
Risk mitigation
The half-life of PFAS in the human body is about 3-7 years, said Dr. Goodrich.
“There’s not much you can do once they’re in there,” he said. “So, the focus needs to be on preventing the exposure in the first place.”
People can limit exposure by avoiding water contaminated with PFAS or filtering it out, Dr. Goodrich said. He recommended avoiding fish from contaminated waterways and nonstick cookware. The Environmental Protection Agency has more detailed recommendations.
But giving patients individualized recommendations is difficult, said Vincent Chen, MD, MS, a clinical instructor in gastroenterology at the University of Michigan, Ann Arbor, who was not involved in the study. Most clinicians don’t know their patients’ PFOS levels.
“It’s not that easy to get a test,” Dr. Chen told this news organization.
People can also mitigate their risk factors for hepatocellular carcinoma, such as a poor diet, a lack of exercise, and smoking, said Dr. Goodrich.
The researchers found that patients with hepatocellular carcinoma were more likely to be overweight and have diabetes, and PFOS was associated with higher fasting glucose levels. This raises the possibility that PFOS increases the risk for hepatocellular carcinoma by causing diabetes and obesity.
Dr. Goodrich and his colleagues tried to address this question by adjusting for baseline body mass index (BMI) and diabetes diagnosis in their statistical analysis.
After adjusting for BMI, they found that the association between PFOS and hepatocellular carcinoma diminished to a threefold risk (OR, 2.90; 95% CI, 0.78-10.00) and was no longer statistically significant (P = .11).
On the other hand, adjusting for diabetes did not change the significance of the relationship between PFOS and the cancer (OR, 5.7; 95% CI, 1.10-30.00; P = .04).
The sample size was probably too small to adequately tease out this relationship, Dr. Chen said. Still, he said, “I thought it was a very, very important study.”
The levels of PFOS found in the blood of Americans has been declining since the 1999-2000 NHANES, Dr. Chen pointed out. But that’s not as reassuring as it sounds.
“The problem is that if you put a regulation limiting the use of one PFAS, what people can do is just substitute with another PFAS or another molecule, which for all we know could be equally harmful,” Dr. Chen said.
Funding was provided by the Southern California Environmental Health Science Center supported by the National Institutes of Health. Dr. Goodrich and Dr. Chen report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The correlation does not prove that PFOS causes this cancer, and more research is needed, but in the meantime, people should limit their exposure to it and others in its class, said Jesse Goodrich, PhD, a postdoctoral scholar in environmental medicine at the University of Southern California, Los Angeles.
“If you’re at risk for liver cancer because you have other risk factors, then these chemicals have the potential to kind of send you over the edge,” he told this news organization.
Dr. Goodrich and colleagues published their research online in JHEP Reports.
Dubbed “forever chemicals” because they can take thousands of years to break down, polyfluoroalkyl substances (PFAS) figure in makeup, food packaging, waterproof clothing, nonstick cookware, firefighting foams, and groundwater. They have spread through the atmosphere into rain and can be found in the blood of most Americans. PFOS is one of the most widely used PFAS.
“You can’t really escape them,” Dr. Goodrich said.
Previous research has linked PFAS to infertility, pregnancy complications, learning and behavioral problems in children, immune system issues, and higher cholesterol, as well as other cancers. Some experiments in animals suggested PFAS could cause liver cancer, and others showed a correlation between PFAS serum levels and biomarkers associated with liver cancer. But many of these health effects take a long time to develop.
“It wasn’t until we started to get really highly exposed groups of people that we started, as scientists, to be able to figure out what was going on,” said Dr. Goodrich.
High exposure, increased incidence
To measure the relationship between PFAS exposure and the incidence of hepatocellular carcinoma more definitively, Dr. Goodrich and colleagues analyzed data from the Multiethnic Cohort Study, a cohort of more than 200,000 people of African, Latin, Native Hawaiian, Japanese, and European ancestry tracked since the early 1990s in California and Hawaii. About 67,000 participants provided blood samples from 2001 to 2007.
From this cohort, the researchers found 50 people who later developed hepatocellular carcinoma. The researchers matched these patients with 50 controls of similar age at blood collection, sex, race, ethnicity, and study area who did not develop the cancer.
They found that people with more than 54.9 mcg/L of PFOS in their blood before any diagnosis of hepatocellular carcinoma were almost five times more likely to get the cancer (odds ratio 4.5; 95% confidence interval, 1.2-16.0), which was statistically significant (P = .02).
This level of PFOS corresponds to the 90th percentile found in the U.S. National Health and Nutrition Examination Survey (NHANES).
To get some idea of the mechanism by which PFOS might do its damage, the researchers also looked for linkage to levels of metabolites.
They found an overlap among high PFOS levels, hepatocellular carcinoma, and high levels of glucose, butyric acid (a short chain fatty acid), alpha-Ketoisovaleric acid (alpha branched-chain alpha-keto acid), and 7alpha-Hydroxy-3-oxo-4-cholestenoate (a bile acid). These metabolites have been associated in previous studies with metabolic disorders and liver disease.
Similarly, the researchers identified an association among the cancer, PFOS, and alterations in amino acid and glycan biosynthesis pathways.
Risk mitigation
The half-life of PFAS in the human body is about 3-7 years, said Dr. Goodrich.
“There’s not much you can do once they’re in there,” he said. “So, the focus needs to be on preventing the exposure in the first place.”
People can limit exposure by avoiding water contaminated with PFAS or filtering it out, Dr. Goodrich said. He recommended avoiding fish from contaminated waterways and nonstick cookware. The Environmental Protection Agency has more detailed recommendations.
But giving patients individualized recommendations is difficult, said Vincent Chen, MD, MS, a clinical instructor in gastroenterology at the University of Michigan, Ann Arbor, who was not involved in the study. Most clinicians don’t know their patients’ PFOS levels.
“It’s not that easy to get a test,” Dr. Chen told this news organization.
People can also mitigate their risk factors for hepatocellular carcinoma, such as a poor diet, a lack of exercise, and smoking, said Dr. Goodrich.
The researchers found that patients with hepatocellular carcinoma were more likely to be overweight and have diabetes, and PFOS was associated with higher fasting glucose levels. This raises the possibility that PFOS increases the risk for hepatocellular carcinoma by causing diabetes and obesity.
Dr. Goodrich and his colleagues tried to address this question by adjusting for baseline body mass index (BMI) and diabetes diagnosis in their statistical analysis.
After adjusting for BMI, they found that the association between PFOS and hepatocellular carcinoma diminished to a threefold risk (OR, 2.90; 95% CI, 0.78-10.00) and was no longer statistically significant (P = .11).
On the other hand, adjusting for diabetes did not change the significance of the relationship between PFOS and the cancer (OR, 5.7; 95% CI, 1.10-30.00; P = .04).
The sample size was probably too small to adequately tease out this relationship, Dr. Chen said. Still, he said, “I thought it was a very, very important study.”
The levels of PFOS found in the blood of Americans has been declining since the 1999-2000 NHANES, Dr. Chen pointed out. But that’s not as reassuring as it sounds.
“The problem is that if you put a regulation limiting the use of one PFAS, what people can do is just substitute with another PFAS or another molecule, which for all we know could be equally harmful,” Dr. Chen said.
Funding was provided by the Southern California Environmental Health Science Center supported by the National Institutes of Health. Dr. Goodrich and Dr. Chen report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The correlation does not prove that PFOS causes this cancer, and more research is needed, but in the meantime, people should limit their exposure to it and others in its class, said Jesse Goodrich, PhD, a postdoctoral scholar in environmental medicine at the University of Southern California, Los Angeles.
“If you’re at risk for liver cancer because you have other risk factors, then these chemicals have the potential to kind of send you over the edge,” he told this news organization.
Dr. Goodrich and colleagues published their research online in JHEP Reports.
Dubbed “forever chemicals” because they can take thousands of years to break down, polyfluoroalkyl substances (PFAS) figure in makeup, food packaging, waterproof clothing, nonstick cookware, firefighting foams, and groundwater. They have spread through the atmosphere into rain and can be found in the blood of most Americans. PFOS is one of the most widely used PFAS.
“You can’t really escape them,” Dr. Goodrich said.
Previous research has linked PFAS to infertility, pregnancy complications, learning and behavioral problems in children, immune system issues, and higher cholesterol, as well as other cancers. Some experiments in animals suggested PFAS could cause liver cancer, and others showed a correlation between PFAS serum levels and biomarkers associated with liver cancer. But many of these health effects take a long time to develop.
“It wasn’t until we started to get really highly exposed groups of people that we started, as scientists, to be able to figure out what was going on,” said Dr. Goodrich.
High exposure, increased incidence
To measure the relationship between PFAS exposure and the incidence of hepatocellular carcinoma more definitively, Dr. Goodrich and colleagues analyzed data from the Multiethnic Cohort Study, a cohort of more than 200,000 people of African, Latin, Native Hawaiian, Japanese, and European ancestry tracked since the early 1990s in California and Hawaii. About 67,000 participants provided blood samples from 2001 to 2007.
From this cohort, the researchers found 50 people who later developed hepatocellular carcinoma. The researchers matched these patients with 50 controls of similar age at blood collection, sex, race, ethnicity, and study area who did not develop the cancer.
They found that people with more than 54.9 mcg/L of PFOS in their blood before any diagnosis of hepatocellular carcinoma were almost five times more likely to get the cancer (odds ratio 4.5; 95% confidence interval, 1.2-16.0), which was statistically significant (P = .02).
This level of PFOS corresponds to the 90th percentile found in the U.S. National Health and Nutrition Examination Survey (NHANES).
To get some idea of the mechanism by which PFOS might do its damage, the researchers also looked for linkage to levels of metabolites.
They found an overlap among high PFOS levels, hepatocellular carcinoma, and high levels of glucose, butyric acid (a short chain fatty acid), alpha-Ketoisovaleric acid (alpha branched-chain alpha-keto acid), and 7alpha-Hydroxy-3-oxo-4-cholestenoate (a bile acid). These metabolites have been associated in previous studies with metabolic disorders and liver disease.
Similarly, the researchers identified an association among the cancer, PFOS, and alterations in amino acid and glycan biosynthesis pathways.
Risk mitigation
The half-life of PFAS in the human body is about 3-7 years, said Dr. Goodrich.
“There’s not much you can do once they’re in there,” he said. “So, the focus needs to be on preventing the exposure in the first place.”
People can limit exposure by avoiding water contaminated with PFAS or filtering it out, Dr. Goodrich said. He recommended avoiding fish from contaminated waterways and nonstick cookware. The Environmental Protection Agency has more detailed recommendations.
But giving patients individualized recommendations is difficult, said Vincent Chen, MD, MS, a clinical instructor in gastroenterology at the University of Michigan, Ann Arbor, who was not involved in the study. Most clinicians don’t know their patients’ PFOS levels.
“It’s not that easy to get a test,” Dr. Chen told this news organization.
People can also mitigate their risk factors for hepatocellular carcinoma, such as a poor diet, a lack of exercise, and smoking, said Dr. Goodrich.
The researchers found that patients with hepatocellular carcinoma were more likely to be overweight and have diabetes, and PFOS was associated with higher fasting glucose levels. This raises the possibility that PFOS increases the risk for hepatocellular carcinoma by causing diabetes and obesity.
Dr. Goodrich and his colleagues tried to address this question by adjusting for baseline body mass index (BMI) and diabetes diagnosis in their statistical analysis.
After adjusting for BMI, they found that the association between PFOS and hepatocellular carcinoma diminished to a threefold risk (OR, 2.90; 95% CI, 0.78-10.00) and was no longer statistically significant (P = .11).
On the other hand, adjusting for diabetes did not change the significance of the relationship between PFOS and the cancer (OR, 5.7; 95% CI, 1.10-30.00; P = .04).
The sample size was probably too small to adequately tease out this relationship, Dr. Chen said. Still, he said, “I thought it was a very, very important study.”
The levels of PFOS found in the blood of Americans has been declining since the 1999-2000 NHANES, Dr. Chen pointed out. But that’s not as reassuring as it sounds.
“The problem is that if you put a regulation limiting the use of one PFAS, what people can do is just substitute with another PFAS or another molecule, which for all we know could be equally harmful,” Dr. Chen said.
Funding was provided by the Southern California Environmental Health Science Center supported by the National Institutes of Health. Dr. Goodrich and Dr. Chen report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JHEP REPORTS
