User login
PACT-HF: Transitional care derives no overall benefit
Women respond more to intervention
PHILADELPHIA – A clinical trial of a program that transitions heart failure patients after they’re discharged from the hospital didn’t result in any appreciable improvement in all-cause death, readmissions or emergency department visits after 6 months overall, but it did show that women responded more favorably than men.
Harriette G.C. Van Spall, MD, MPH, reported 6-month results of the Patient-Centered Transitional Care Services in Heart Failure (PACT-HF) trial of 2,494 HF patients at 10 hospitals in Ontario during February 2015 to March 2016. They were randomized to the care-transition program or usual care. The findings, she said at the American Heart Association scientific sessions, “highlight the gap between efficacy that’s often demonstrated in mechanistic clinical trials and effectiveness when we aim to implement these results in real-world settings.” Three-month PACT-HF results were reported previously (JAMA. 2019 Feb 26;321:753-61).
The transitional-care model consisted of a comprehensive needs assessment by a nurse who also provided self-care education, a patient-centered discharge summary, and follow-up with a family physician within 7 days of discharge, which Dr. Van Spall noted “is not current practice in our health care system.”
Patients deemed high risk for readmission or death also received nurse home visits and scheduled visits to a multidisciplinary heart function clinic within 2-4 weeks of discharge and continuing as long as clinically suitable, said Dr. Van Spall, a principal investigator at the Population Health Research Institute, Hamilton, Ont., and assistant professor in cardiology at McMaster University in Hamilton.
The trial found no difference between the intervention and usual-care groups in the two composite endpoints at 6 months, Dr. Van Spall said: all-cause death, readmissions, or ED visits (63.1% and 64.5%, respectively; P = .50); or all-cause readmissions or ED visits (60.8% and 62.4%; P = .36).
“Despite the mutual overall clinical outcomes, we noted specific differences in response to treatment,” she said. With regard to the composite endpoint that included all-cause death, “Men had an attenuated response to the treatment with a hazard ratio of 1.05 (95% confidence interval, 0.87-1.26), whereas women had a hazard ratio of 0.85 (95% CI, 0.71-1.03), demonstrating that women have more of a treatment response to this health care service,” she said.
In men, rates for the first primary composite outcome were 66.3% and 64.1% in the intervention and usual-care groups, whereas in women those rates were 59.9% and 64.8% (P = .04 for sex interaction).
In the second composite endpoint, all-cause readmission or ED visit, “again, men had an attenuated response” with a HR of 1.03, whereas women had a HR of 0.83. Results were similar to those for the first primary composite outcome: 63.4% and 61.7% for intervention and usual care in men and 57.7% and 63% in women (P = .03 for sex interaction).
In putting the findings into context, Dr. Van Spall said tailoring services to risk in HF patients may be fraught with pitfalls. “We delivered intensive services to those patients at high risk of readmission or death, but it is quite possible they are the least likely to derive benefit by virtue of their advanced heart failure,” she said. “It may be that more benefit would have been derived had we chosen low- or moderate-risk patients to receive the intervention.”
She also said the sex-specific outcomes must be interpreted with caution. “But they do give us pause to consider that services could be titrated more effectively if delivered to patients who are more likely to derive benefit,” Dr. Van Spall said. The finding that women derived more of a benefit is in line with other prospective and observational studies that have found that women have a higher sense of self-care, self-efficacy, and confidence in managing their own health care needs than men.
Dr. Van Spall has no financial relationships to disclose.
Women respond more to intervention
Women respond more to intervention
PHILADELPHIA – A clinical trial of a program that transitions heart failure patients after they’re discharged from the hospital didn’t result in any appreciable improvement in all-cause death, readmissions or emergency department visits after 6 months overall, but it did show that women responded more favorably than men.
Harriette G.C. Van Spall, MD, MPH, reported 6-month results of the Patient-Centered Transitional Care Services in Heart Failure (PACT-HF) trial of 2,494 HF patients at 10 hospitals in Ontario during February 2015 to March 2016. They were randomized to the care-transition program or usual care. The findings, she said at the American Heart Association scientific sessions, “highlight the gap between efficacy that’s often demonstrated in mechanistic clinical trials and effectiveness when we aim to implement these results in real-world settings.” Three-month PACT-HF results were reported previously (JAMA. 2019 Feb 26;321:753-61).
The transitional-care model consisted of a comprehensive needs assessment by a nurse who also provided self-care education, a patient-centered discharge summary, and follow-up with a family physician within 7 days of discharge, which Dr. Van Spall noted “is not current practice in our health care system.”
Patients deemed high risk for readmission or death also received nurse home visits and scheduled visits to a multidisciplinary heart function clinic within 2-4 weeks of discharge and continuing as long as clinically suitable, said Dr. Van Spall, a principal investigator at the Population Health Research Institute, Hamilton, Ont., and assistant professor in cardiology at McMaster University in Hamilton.
The trial found no difference between the intervention and usual-care groups in the two composite endpoints at 6 months, Dr. Van Spall said: all-cause death, readmissions, or ED visits (63.1% and 64.5%, respectively; P = .50); or all-cause readmissions or ED visits (60.8% and 62.4%; P = .36).
“Despite the mutual overall clinical outcomes, we noted specific differences in response to treatment,” she said. With regard to the composite endpoint that included all-cause death, “Men had an attenuated response to the treatment with a hazard ratio of 1.05 (95% confidence interval, 0.87-1.26), whereas women had a hazard ratio of 0.85 (95% CI, 0.71-1.03), demonstrating that women have more of a treatment response to this health care service,” she said.
In men, rates for the first primary composite outcome were 66.3% and 64.1% in the intervention and usual-care groups, whereas in women those rates were 59.9% and 64.8% (P = .04 for sex interaction).
In the second composite endpoint, all-cause readmission or ED visit, “again, men had an attenuated response” with a HR of 1.03, whereas women had a HR of 0.83. Results were similar to those for the first primary composite outcome: 63.4% and 61.7% for intervention and usual care in men and 57.7% and 63% in women (P = .03 for sex interaction).
In putting the findings into context, Dr. Van Spall said tailoring services to risk in HF patients may be fraught with pitfalls. “We delivered intensive services to those patients at high risk of readmission or death, but it is quite possible they are the least likely to derive benefit by virtue of their advanced heart failure,” she said. “It may be that more benefit would have been derived had we chosen low- or moderate-risk patients to receive the intervention.”
She also said the sex-specific outcomes must be interpreted with caution. “But they do give us pause to consider that services could be titrated more effectively if delivered to patients who are more likely to derive benefit,” Dr. Van Spall said. The finding that women derived more of a benefit is in line with other prospective and observational studies that have found that women have a higher sense of self-care, self-efficacy, and confidence in managing their own health care needs than men.
Dr. Van Spall has no financial relationships to disclose.
PHILADELPHIA – A clinical trial of a program that transitions heart failure patients after they’re discharged from the hospital didn’t result in any appreciable improvement in all-cause death, readmissions or emergency department visits after 6 months overall, but it did show that women responded more favorably than men.
Harriette G.C. Van Spall, MD, MPH, reported 6-month results of the Patient-Centered Transitional Care Services in Heart Failure (PACT-HF) trial of 2,494 HF patients at 10 hospitals in Ontario during February 2015 to March 2016. They were randomized to the care-transition program or usual care. The findings, she said at the American Heart Association scientific sessions, “highlight the gap between efficacy that’s often demonstrated in mechanistic clinical trials and effectiveness when we aim to implement these results in real-world settings.” Three-month PACT-HF results were reported previously (JAMA. 2019 Feb 26;321:753-61).
The transitional-care model consisted of a comprehensive needs assessment by a nurse who also provided self-care education, a patient-centered discharge summary, and follow-up with a family physician within 7 days of discharge, which Dr. Van Spall noted “is not current practice in our health care system.”
Patients deemed high risk for readmission or death also received nurse home visits and scheduled visits to a multidisciplinary heart function clinic within 2-4 weeks of discharge and continuing as long as clinically suitable, said Dr. Van Spall, a principal investigator at the Population Health Research Institute, Hamilton, Ont., and assistant professor in cardiology at McMaster University in Hamilton.
The trial found no difference between the intervention and usual-care groups in the two composite endpoints at 6 months, Dr. Van Spall said: all-cause death, readmissions, or ED visits (63.1% and 64.5%, respectively; P = .50); or all-cause readmissions or ED visits (60.8% and 62.4%; P = .36).
“Despite the mutual overall clinical outcomes, we noted specific differences in response to treatment,” she said. With regard to the composite endpoint that included all-cause death, “Men had an attenuated response to the treatment with a hazard ratio of 1.05 (95% confidence interval, 0.87-1.26), whereas women had a hazard ratio of 0.85 (95% CI, 0.71-1.03), demonstrating that women have more of a treatment response to this health care service,” she said.
In men, rates for the first primary composite outcome were 66.3% and 64.1% in the intervention and usual-care groups, whereas in women those rates were 59.9% and 64.8% (P = .04 for sex interaction).
In the second composite endpoint, all-cause readmission or ED visit, “again, men had an attenuated response” with a HR of 1.03, whereas women had a HR of 0.83. Results were similar to those for the first primary composite outcome: 63.4% and 61.7% for intervention and usual care in men and 57.7% and 63% in women (P = .03 for sex interaction).
In putting the findings into context, Dr. Van Spall said tailoring services to risk in HF patients may be fraught with pitfalls. “We delivered intensive services to those patients at high risk of readmission or death, but it is quite possible they are the least likely to derive benefit by virtue of their advanced heart failure,” she said. “It may be that more benefit would have been derived had we chosen low- or moderate-risk patients to receive the intervention.”
She also said the sex-specific outcomes must be interpreted with caution. “But they do give us pause to consider that services could be titrated more effectively if delivered to patients who are more likely to derive benefit,” Dr. Van Spall said. The finding that women derived more of a benefit is in line with other prospective and observational studies that have found that women have a higher sense of self-care, self-efficacy, and confidence in managing their own health care needs than men.
Dr. Van Spall has no financial relationships to disclose.
REPORTING FROM AHA 2019
Analyses clarify who benefits from ARNI-ARB combination
PHILADELPHIA – Two clinical trials of the combination therapy of the neprilysin inhibitor sacubitril and the angiotensin II receptor blocker valsartan in patients with heart failure and reduced ejection fraction found that it lowered rates of all-cause death, compared to a renin-angiotensin-system inhibitor alone.
Furthermore, the treatment produced a more beneficial effect in women, who are more prone to heart failure and preserved ejection fraction (HFpEF), lead investigators reported at the American Heart Association scientific sessions.
A prespecified subgroup analysis of 4,796 patients in the PARAGON-HF trial found that the sacubitril/valsartan, or sac/val, combination had a significantly more beneficial risk reduction of first and recurrent hospitalizations for heart failure, as well as cardiovascular death, in women than men. A prespecified pooled analysis of 13,195 patients in the PARAGON-HF and the PARADIGM-HF trials also found women derived a greater benefit from the combination therapy than men, but also concluded that patients with heart failure and even mildly reduced ejection fraction had better outcomes. The results of both studies were published simultaneously with the presentations on Nov. 17 in Circulation (doi: 10.1161/circulationaha.119.044491; doi: 10.1161/circulationaha.119.044586).
The findings underscore the effectiveness of sac/val combination in patients with HF and EF in the lower ranges, defined as 40% or less, commented discussant Lynne Warner Stevenson, MD, of Vanderbilt Heart and Vascular Institute in Nashville, Tenn. “We all agree now that the use of sacubitril/valsartan is very appropriate to improve outcomes in those patients, even if they’ve never been hospitalized,” she said in an interview.
PARAGON-HF subanalysis
John J.V. McMurray, MD, of the University of Glasgow presented the PARAGON-HF subgroup analysis. He said it initially focused on 12 subgroups, but that only two baseline variables showed a modified effect of sac/val: sex and left-ventricle ejection fraction (LVEF). The findings, he said, “stood up in a very robust, multivariable analysis.”
The women in the subgroup analysis were older, had higher baseline New York Heart Association class status, and worse quality of life as measured by Kansas City Cardiomyopathy Questionnaire clinical summary score. At baseline, women also had higher average LVEF (59% vs. 56%), lower N-terminal prohormone brain natriuretic peptide levels, and higher rates of renal dysfunction and chronic kidney disease, but lower incidence of a previous MI and coronary artery disease. Prestudy treatments were similar between the sexes.
In terms of the primary outcome – a composite of total hospitalizations for heart failure and cardiovascular death – “there was an apparent 27% relative risk reduction in women and no overall effect in men,” Dr. McMurray said of the treatment group. “The difference was driven completely by hospitalizations.” Rates of CV death were similar between the valsartan-only and sac/val groups in both men and women, he said.
In the analysis of LVEF, women in the treatment group seemed to cross over to a heightened risk of hospitalization and CV death at an LVEF in the 60%-65% range, Dr. McMurray said, whereas men made that cross over in the 50%-55% range. “It looks as though women might be getting more benefit from this treatment up to a higher EF than in men,” he said.
However, the differences between men and women did not hold up in the analysis of secondary outcomes. At 8 months, women in the sac/val group had a 0.6-point greater decline than did the valsartan-only patients in KCCQ-CSS score, whereas men on sac/val had a 2.8-point lesser decline than did those on valsartan only. Similar differences were seen between the treatment and valsartan-only groups within the sexes, with women showing a noticeable improvement surpassing the men.
Posttreatment hypotension rates in both sexes were higher in the sac/val groups, and the risk of renal dysfunction was a bit less in both treatment groups. Women in the treatment group had significantly higher rates of angioedema than did the valsartan-only group and men in either group.
“Compared to valsartan, it’s important to say that sacubitril/valsartan seemed to reduce the risk of heart failure and hospitalization more in women than men, but we didn’t find a similar differential for other endpoints,” Dr. McMurray said. “Therefore, we’re not sure this is a real effect or a chance finding. It’s very statistically robust, but it could still be a chance finding.”
A possible explanation could be than men may not be responding to sac/val, or that valsartan alone may be more effective in men than women, he said. “This possible effect modification of sac/val vs. valsartan by sex deserves further investigation,” he said.
PARAGON-HF and PARADIGM-HF pooled analysis
Likewise, the prespecified pooled analysis of the PARADIGM-HF and PARAGON-HF trials found a greater benefit of sac/val in women, according to results presented by Scott D. Solomon, MD, of Brigham and Women’s Hospital in Boston. Where PARAGON-HF compared combination therapy with valsartan 160 mg twice daily alone, PARADIGM-HF used enalapril 10 mg twice daily alone as the comparator renin-angiotensin-system (RAS) inhibitor.
“These data suggest that the therapeutic effect of sacubitril/valsartan vs. RAS inhibition alone appear to extend to patients with heart failure and mildly reduced EF, with therapeutic benefits that extend to a higher left-ventricle EF range in women compared to men,” Dr. Solomon said.
The pooled analysis divided patients into six different EF groups: up to 22.5%, then in 10-point increments from 22.5% to 62.5%, and 62.5% or greater. PARADIGM-HF enrolled patients age 18 years and older, whereas PARAGON-HF involved those aged 50 years and older.
The analysis showed that, as LVEF rates increased across the EF groups, the rates of the primary composite outcome – HF hospitalizations, CV death, and all-cause mortality – decreased, but the decline was greatest for CV death and less so for HF hospitalization. And while rates of all-cause mortality decreased as EF increased, rates of non-CV death increased substantially with increasing LVEF.
“For each of these endpoints, there are significant benefits to sacubitril/valsartan in the pooled analysis, and this includes HF hospitalization, CV death, either total or first events, and all-cause mortality, which was reduced overall by 12% in the combination group,” Dr. Solomon said. That benefit was seen in the first five categories of EF, but all but disappeared in the highest category (at least 62.5%), he said.
At the lower end of the EF spectrum, the effect of sac/val is more pronounced and similar for men and women, Dr. Solomon said. “But as EF goes up, we see an attenuation of that effect in both men and women, but it occurs at a different point,” he said. “Women seem to derive a benefit to a higher ejection fraction than men.” As in Dr. McMurray’s research, the benefit seems to extend to LVEF of 55%-60% in men and 65%-70% in women.
“These findings were driven by an observed benefit in patients with chronic heart failure and LVEF below the normal range,” he said. “The benefit in the EF range above the ranking ‘reduced’ but below normal was driven primarily by reduction in HF hospitalization.”
Dr. Stevenson said that these findings indicate that a previous hospitalization for HF with preserved EF may be a telling marker for the effectiveness of sac/val. “As opposed to the patient who has exertional dyspnea but has never decompensated to the level needing hospitalization, if they have pEF, our current analyses would suggest sac/val may not offer them much benefit,” she said.
In real-world practice, cost would be an issue, Dr. Stevenson said. “This drug is very expensive; the majority of patients pay more than $100 a month in out-of-pocket costs, and we have to recognize this is not a therapy that everyone can afford,” she said in an interview. “In many areas, and particularly in the disadvantaged populations, this is not going to be a therapy that we’re going to be able to offer everyone, and that gives me great concern as we move toward trying to treat the whole disease that we’re developing therapies that will be limited by finance rather than by physiology. That’s a major call to action for all of us.”
Novartis sponsored the studies. Dr. McMurray has no disclosures. Dr. Solomon disclosed financial relationships with trial sponsor Novartis along with numerous pharmaceutical companies and the National Heart, Lung, and Blood Institute.
SOURCE: McMurray JJ and Solomon SD. AHA 2019, Late Breaking Science Session 5.
PHILADELPHIA – Two clinical trials of the combination therapy of the neprilysin inhibitor sacubitril and the angiotensin II receptor blocker valsartan in patients with heart failure and reduced ejection fraction found that it lowered rates of all-cause death, compared to a renin-angiotensin-system inhibitor alone.
Furthermore, the treatment produced a more beneficial effect in women, who are more prone to heart failure and preserved ejection fraction (HFpEF), lead investigators reported at the American Heart Association scientific sessions.
A prespecified subgroup analysis of 4,796 patients in the PARAGON-HF trial found that the sacubitril/valsartan, or sac/val, combination had a significantly more beneficial risk reduction of first and recurrent hospitalizations for heart failure, as well as cardiovascular death, in women than men. A prespecified pooled analysis of 13,195 patients in the PARAGON-HF and the PARADIGM-HF trials also found women derived a greater benefit from the combination therapy than men, but also concluded that patients with heart failure and even mildly reduced ejection fraction had better outcomes. The results of both studies were published simultaneously with the presentations on Nov. 17 in Circulation (doi: 10.1161/circulationaha.119.044491; doi: 10.1161/circulationaha.119.044586).
The findings underscore the effectiveness of sac/val combination in patients with HF and EF in the lower ranges, defined as 40% or less, commented discussant Lynne Warner Stevenson, MD, of Vanderbilt Heart and Vascular Institute in Nashville, Tenn. “We all agree now that the use of sacubitril/valsartan is very appropriate to improve outcomes in those patients, even if they’ve never been hospitalized,” she said in an interview.
PARAGON-HF subanalysis
John J.V. McMurray, MD, of the University of Glasgow presented the PARAGON-HF subgroup analysis. He said it initially focused on 12 subgroups, but that only two baseline variables showed a modified effect of sac/val: sex and left-ventricle ejection fraction (LVEF). The findings, he said, “stood up in a very robust, multivariable analysis.”
The women in the subgroup analysis were older, had higher baseline New York Heart Association class status, and worse quality of life as measured by Kansas City Cardiomyopathy Questionnaire clinical summary score. At baseline, women also had higher average LVEF (59% vs. 56%), lower N-terminal prohormone brain natriuretic peptide levels, and higher rates of renal dysfunction and chronic kidney disease, but lower incidence of a previous MI and coronary artery disease. Prestudy treatments were similar between the sexes.
In terms of the primary outcome – a composite of total hospitalizations for heart failure and cardiovascular death – “there was an apparent 27% relative risk reduction in women and no overall effect in men,” Dr. McMurray said of the treatment group. “The difference was driven completely by hospitalizations.” Rates of CV death were similar between the valsartan-only and sac/val groups in both men and women, he said.
In the analysis of LVEF, women in the treatment group seemed to cross over to a heightened risk of hospitalization and CV death at an LVEF in the 60%-65% range, Dr. McMurray said, whereas men made that cross over in the 50%-55% range. “It looks as though women might be getting more benefit from this treatment up to a higher EF than in men,” he said.
However, the differences between men and women did not hold up in the analysis of secondary outcomes. At 8 months, women in the sac/val group had a 0.6-point greater decline than did the valsartan-only patients in KCCQ-CSS score, whereas men on sac/val had a 2.8-point lesser decline than did those on valsartan only. Similar differences were seen between the treatment and valsartan-only groups within the sexes, with women showing a noticeable improvement surpassing the men.
Posttreatment hypotension rates in both sexes were higher in the sac/val groups, and the risk of renal dysfunction was a bit less in both treatment groups. Women in the treatment group had significantly higher rates of angioedema than did the valsartan-only group and men in either group.
“Compared to valsartan, it’s important to say that sacubitril/valsartan seemed to reduce the risk of heart failure and hospitalization more in women than men, but we didn’t find a similar differential for other endpoints,” Dr. McMurray said. “Therefore, we’re not sure this is a real effect or a chance finding. It’s very statistically robust, but it could still be a chance finding.”
A possible explanation could be than men may not be responding to sac/val, or that valsartan alone may be more effective in men than women, he said. “This possible effect modification of sac/val vs. valsartan by sex deserves further investigation,” he said.
PARAGON-HF and PARADIGM-HF pooled analysis
Likewise, the prespecified pooled analysis of the PARADIGM-HF and PARAGON-HF trials found a greater benefit of sac/val in women, according to results presented by Scott D. Solomon, MD, of Brigham and Women’s Hospital in Boston. Where PARAGON-HF compared combination therapy with valsartan 160 mg twice daily alone, PARADIGM-HF used enalapril 10 mg twice daily alone as the comparator renin-angiotensin-system (RAS) inhibitor.
“These data suggest that the therapeutic effect of sacubitril/valsartan vs. RAS inhibition alone appear to extend to patients with heart failure and mildly reduced EF, with therapeutic benefits that extend to a higher left-ventricle EF range in women compared to men,” Dr. Solomon said.
The pooled analysis divided patients into six different EF groups: up to 22.5%, then in 10-point increments from 22.5% to 62.5%, and 62.5% or greater. PARADIGM-HF enrolled patients age 18 years and older, whereas PARAGON-HF involved those aged 50 years and older.
The analysis showed that, as LVEF rates increased across the EF groups, the rates of the primary composite outcome – HF hospitalizations, CV death, and all-cause mortality – decreased, but the decline was greatest for CV death and less so for HF hospitalization. And while rates of all-cause mortality decreased as EF increased, rates of non-CV death increased substantially with increasing LVEF.
“For each of these endpoints, there are significant benefits to sacubitril/valsartan in the pooled analysis, and this includes HF hospitalization, CV death, either total or first events, and all-cause mortality, which was reduced overall by 12% in the combination group,” Dr. Solomon said. That benefit was seen in the first five categories of EF, but all but disappeared in the highest category (at least 62.5%), he said.
At the lower end of the EF spectrum, the effect of sac/val is more pronounced and similar for men and women, Dr. Solomon said. “But as EF goes up, we see an attenuation of that effect in both men and women, but it occurs at a different point,” he said. “Women seem to derive a benefit to a higher ejection fraction than men.” As in Dr. McMurray’s research, the benefit seems to extend to LVEF of 55%-60% in men and 65%-70% in women.
“These findings were driven by an observed benefit in patients with chronic heart failure and LVEF below the normal range,” he said. “The benefit in the EF range above the ranking ‘reduced’ but below normal was driven primarily by reduction in HF hospitalization.”
Dr. Stevenson said that these findings indicate that a previous hospitalization for HF with preserved EF may be a telling marker for the effectiveness of sac/val. “As opposed to the patient who has exertional dyspnea but has never decompensated to the level needing hospitalization, if they have pEF, our current analyses would suggest sac/val may not offer them much benefit,” she said.
In real-world practice, cost would be an issue, Dr. Stevenson said. “This drug is very expensive; the majority of patients pay more than $100 a month in out-of-pocket costs, and we have to recognize this is not a therapy that everyone can afford,” she said in an interview. “In many areas, and particularly in the disadvantaged populations, this is not going to be a therapy that we’re going to be able to offer everyone, and that gives me great concern as we move toward trying to treat the whole disease that we’re developing therapies that will be limited by finance rather than by physiology. That’s a major call to action for all of us.”
Novartis sponsored the studies. Dr. McMurray has no disclosures. Dr. Solomon disclosed financial relationships with trial sponsor Novartis along with numerous pharmaceutical companies and the National Heart, Lung, and Blood Institute.
SOURCE: McMurray JJ and Solomon SD. AHA 2019, Late Breaking Science Session 5.
PHILADELPHIA – Two clinical trials of the combination therapy of the neprilysin inhibitor sacubitril and the angiotensin II receptor blocker valsartan in patients with heart failure and reduced ejection fraction found that it lowered rates of all-cause death, compared to a renin-angiotensin-system inhibitor alone.
Furthermore, the treatment produced a more beneficial effect in women, who are more prone to heart failure and preserved ejection fraction (HFpEF), lead investigators reported at the American Heart Association scientific sessions.
A prespecified subgroup analysis of 4,796 patients in the PARAGON-HF trial found that the sacubitril/valsartan, or sac/val, combination had a significantly more beneficial risk reduction of first and recurrent hospitalizations for heart failure, as well as cardiovascular death, in women than men. A prespecified pooled analysis of 13,195 patients in the PARAGON-HF and the PARADIGM-HF trials also found women derived a greater benefit from the combination therapy than men, but also concluded that patients with heart failure and even mildly reduced ejection fraction had better outcomes. The results of both studies were published simultaneously with the presentations on Nov. 17 in Circulation (doi: 10.1161/circulationaha.119.044491; doi: 10.1161/circulationaha.119.044586).
The findings underscore the effectiveness of sac/val combination in patients with HF and EF in the lower ranges, defined as 40% or less, commented discussant Lynne Warner Stevenson, MD, of Vanderbilt Heart and Vascular Institute in Nashville, Tenn. “We all agree now that the use of sacubitril/valsartan is very appropriate to improve outcomes in those patients, even if they’ve never been hospitalized,” she said in an interview.
PARAGON-HF subanalysis
John J.V. McMurray, MD, of the University of Glasgow presented the PARAGON-HF subgroup analysis. He said it initially focused on 12 subgroups, but that only two baseline variables showed a modified effect of sac/val: sex and left-ventricle ejection fraction (LVEF). The findings, he said, “stood up in a very robust, multivariable analysis.”
The women in the subgroup analysis were older, had higher baseline New York Heart Association class status, and worse quality of life as measured by Kansas City Cardiomyopathy Questionnaire clinical summary score. At baseline, women also had higher average LVEF (59% vs. 56%), lower N-terminal prohormone brain natriuretic peptide levels, and higher rates of renal dysfunction and chronic kidney disease, but lower incidence of a previous MI and coronary artery disease. Prestudy treatments were similar between the sexes.
In terms of the primary outcome – a composite of total hospitalizations for heart failure and cardiovascular death – “there was an apparent 27% relative risk reduction in women and no overall effect in men,” Dr. McMurray said of the treatment group. “The difference was driven completely by hospitalizations.” Rates of CV death were similar between the valsartan-only and sac/val groups in both men and women, he said.
In the analysis of LVEF, women in the treatment group seemed to cross over to a heightened risk of hospitalization and CV death at an LVEF in the 60%-65% range, Dr. McMurray said, whereas men made that cross over in the 50%-55% range. “It looks as though women might be getting more benefit from this treatment up to a higher EF than in men,” he said.
However, the differences between men and women did not hold up in the analysis of secondary outcomes. At 8 months, women in the sac/val group had a 0.6-point greater decline than did the valsartan-only patients in KCCQ-CSS score, whereas men on sac/val had a 2.8-point lesser decline than did those on valsartan only. Similar differences were seen between the treatment and valsartan-only groups within the sexes, with women showing a noticeable improvement surpassing the men.
Posttreatment hypotension rates in both sexes were higher in the sac/val groups, and the risk of renal dysfunction was a bit less in both treatment groups. Women in the treatment group had significantly higher rates of angioedema than did the valsartan-only group and men in either group.
“Compared to valsartan, it’s important to say that sacubitril/valsartan seemed to reduce the risk of heart failure and hospitalization more in women than men, but we didn’t find a similar differential for other endpoints,” Dr. McMurray said. “Therefore, we’re not sure this is a real effect or a chance finding. It’s very statistically robust, but it could still be a chance finding.”
A possible explanation could be than men may not be responding to sac/val, or that valsartan alone may be more effective in men than women, he said. “This possible effect modification of sac/val vs. valsartan by sex deserves further investigation,” he said.
PARAGON-HF and PARADIGM-HF pooled analysis
Likewise, the prespecified pooled analysis of the PARADIGM-HF and PARAGON-HF trials found a greater benefit of sac/val in women, according to results presented by Scott D. Solomon, MD, of Brigham and Women’s Hospital in Boston. Where PARAGON-HF compared combination therapy with valsartan 160 mg twice daily alone, PARADIGM-HF used enalapril 10 mg twice daily alone as the comparator renin-angiotensin-system (RAS) inhibitor.
“These data suggest that the therapeutic effect of sacubitril/valsartan vs. RAS inhibition alone appear to extend to patients with heart failure and mildly reduced EF, with therapeutic benefits that extend to a higher left-ventricle EF range in women compared to men,” Dr. Solomon said.
The pooled analysis divided patients into six different EF groups: up to 22.5%, then in 10-point increments from 22.5% to 62.5%, and 62.5% or greater. PARADIGM-HF enrolled patients age 18 years and older, whereas PARAGON-HF involved those aged 50 years and older.
The analysis showed that, as LVEF rates increased across the EF groups, the rates of the primary composite outcome – HF hospitalizations, CV death, and all-cause mortality – decreased, but the decline was greatest for CV death and less so for HF hospitalization. And while rates of all-cause mortality decreased as EF increased, rates of non-CV death increased substantially with increasing LVEF.
“For each of these endpoints, there are significant benefits to sacubitril/valsartan in the pooled analysis, and this includes HF hospitalization, CV death, either total or first events, and all-cause mortality, which was reduced overall by 12% in the combination group,” Dr. Solomon said. That benefit was seen in the first five categories of EF, but all but disappeared in the highest category (at least 62.5%), he said.
At the lower end of the EF spectrum, the effect of sac/val is more pronounced and similar for men and women, Dr. Solomon said. “But as EF goes up, we see an attenuation of that effect in both men and women, but it occurs at a different point,” he said. “Women seem to derive a benefit to a higher ejection fraction than men.” As in Dr. McMurray’s research, the benefit seems to extend to LVEF of 55%-60% in men and 65%-70% in women.
“These findings were driven by an observed benefit in patients with chronic heart failure and LVEF below the normal range,” he said. “The benefit in the EF range above the ranking ‘reduced’ but below normal was driven primarily by reduction in HF hospitalization.”
Dr. Stevenson said that these findings indicate that a previous hospitalization for HF with preserved EF may be a telling marker for the effectiveness of sac/val. “As opposed to the patient who has exertional dyspnea but has never decompensated to the level needing hospitalization, if they have pEF, our current analyses would suggest sac/val may not offer them much benefit,” she said.
In real-world practice, cost would be an issue, Dr. Stevenson said. “This drug is very expensive; the majority of patients pay more than $100 a month in out-of-pocket costs, and we have to recognize this is not a therapy that everyone can afford,” she said in an interview. “In many areas, and particularly in the disadvantaged populations, this is not going to be a therapy that we’re going to be able to offer everyone, and that gives me great concern as we move toward trying to treat the whole disease that we’re developing therapies that will be limited by finance rather than by physiology. That’s a major call to action for all of us.”
Novartis sponsored the studies. Dr. McMurray has no disclosures. Dr. Solomon disclosed financial relationships with trial sponsor Novartis along with numerous pharmaceutical companies and the National Heart, Lung, and Blood Institute.
SOURCE: McMurray JJ and Solomon SD. AHA 2019, Late Breaking Science Session 5.
REPORTING FROM THE AHA SCIENTIFIC SESSIONS
Farxiga granted Priority Review for treatment of adults with HFrEF
The Food and Drug Administration has accepted a supplemental New Drug Application and granted Priority Review for dapagliflozin (Farxiga) for the reduction of risk of cardiovascular death or worsening of heart failure in adult patients with heart failure with reduced ejection fraction (HFrEF).
The application was based on results from the landmark, phase 3 DAPA-HF trial, published in September 2019 in the New England Journal of Medicine. The study showed that dapagliflozin plus standard care reduced the incidence of cardiovascular death and worsening of heart failure versus placebo in patients with HFrEF.
Dapagliflozin was granted Fast Track designation for heart failure by the FDA in September 2019. In August 2019, the FDA also granted Fast Track designation to dapagliflozin for the delayed progression of renal failure and prevention of cardiovascular and renal death in patients with chronic kidney disease.
The drug is currently indicated for the improvement of glycemic control in adults with type 2 diabetes as either monotherapy or in combination. The FDA approved dapagliflozin in October 2019 for the reduction of heart failure hospitalization risk in patients with type 2 diabetes and cardiovascular risk factors.
“Farxiga is well established in the treatment of type 2 diabetes and this Priority Review shows its potential to also impact millions of patients with heart failure. If approved, Farxiga will be the first and only medicine of its kind indicated to treat patients with heart failure,” said Mene Pangalos, executive vice president of biopharmaceutical research and development at AstraZeneca.
Find the full press release on the AstraZeneca website.
The Food and Drug Administration has accepted a supplemental New Drug Application and granted Priority Review for dapagliflozin (Farxiga) for the reduction of risk of cardiovascular death or worsening of heart failure in adult patients with heart failure with reduced ejection fraction (HFrEF).
The application was based on results from the landmark, phase 3 DAPA-HF trial, published in September 2019 in the New England Journal of Medicine. The study showed that dapagliflozin plus standard care reduced the incidence of cardiovascular death and worsening of heart failure versus placebo in patients with HFrEF.
Dapagliflozin was granted Fast Track designation for heart failure by the FDA in September 2019. In August 2019, the FDA also granted Fast Track designation to dapagliflozin for the delayed progression of renal failure and prevention of cardiovascular and renal death in patients with chronic kidney disease.
The drug is currently indicated for the improvement of glycemic control in adults with type 2 diabetes as either monotherapy or in combination. The FDA approved dapagliflozin in October 2019 for the reduction of heart failure hospitalization risk in patients with type 2 diabetes and cardiovascular risk factors.
“Farxiga is well established in the treatment of type 2 diabetes and this Priority Review shows its potential to also impact millions of patients with heart failure. If approved, Farxiga will be the first and only medicine of its kind indicated to treat patients with heart failure,” said Mene Pangalos, executive vice president of biopharmaceutical research and development at AstraZeneca.
Find the full press release on the AstraZeneca website.
The Food and Drug Administration has accepted a supplemental New Drug Application and granted Priority Review for dapagliflozin (Farxiga) for the reduction of risk of cardiovascular death or worsening of heart failure in adult patients with heart failure with reduced ejection fraction (HFrEF).
The application was based on results from the landmark, phase 3 DAPA-HF trial, published in September 2019 in the New England Journal of Medicine. The study showed that dapagliflozin plus standard care reduced the incidence of cardiovascular death and worsening of heart failure versus placebo in patients with HFrEF.
Dapagliflozin was granted Fast Track designation for heart failure by the FDA in September 2019. In August 2019, the FDA also granted Fast Track designation to dapagliflozin for the delayed progression of renal failure and prevention of cardiovascular and renal death in patients with chronic kidney disease.
The drug is currently indicated for the improvement of glycemic control in adults with type 2 diabetes as either monotherapy or in combination. The FDA approved dapagliflozin in October 2019 for the reduction of heart failure hospitalization risk in patients with type 2 diabetes and cardiovascular risk factors.
“Farxiga is well established in the treatment of type 2 diabetes and this Priority Review shows its potential to also impact millions of patients with heart failure. If approved, Farxiga will be the first and only medicine of its kind indicated to treat patients with heart failure,” said Mene Pangalos, executive vice president of biopharmaceutical research and development at AstraZeneca.
Find the full press release on the AstraZeneca website.
Navigators improve medication adherence in HFrEF
PHILADELPHIA – Treatment guidelines are clear about optimal treatment of heart failure in patients with reduced ejection fraction (HFrEF), but adherence breakdowns often occur.
So, Brigham and Women’s Hospital in Boston implemented a navigator-administered patient outreach program that led to improved medication adherence over usual care, according to study results reported at the American Heart Association scientific sessions.
Although the study was done at a major academic center, the findings have implications for community practitioners, lead study author Akshay S. Desai, MD, MPH, said in an interview. “The impact of the intervention is clearly greater in those practitioners who manage heart failure and have the least support around them,” he said.
“Our sense is that the kind of population where this intervention would have the greater impact would be a community-dwelling heart failure population managed by community cardiologists, where the infrastructure to provide longitudinal heart failure care is less robust than may be in an academic center,” Dr. Desai said.
The study evaluated adherence in guideline-directed medical therapy (GDMT) at 3 months. “The navigator-led remote medication optimization strategy improved utilization and dosing of all categories of GDMP and was associated with a lower rate of adverse events,” Dr. Desai said. “The impact was more pronounced in patients followed by general practitioners than by a HF specialist.” In the outreach, health navigators contacted patients by phone and managed medications based on remote surveillance of labs, blood pressure, and symptoms under supervision of a pharmacist, nurse practitioner, and heart failure specialist.
The study included 1,028 patients with chronic HFrEF who’d visited a cardiologist at Brigham and Women’s in the year prior to the study: 197 patients and their providers consented to participate in the program with the remainder serving as the reference usual-care group. Most HF specialists at Brigham and Women’s declined to participate in the navigator-led program, Dr. Desai said.
Treating providers were approached for consent to adjust medical therapy according to a sequential, stepped titration algorithm modeled on the current American College of Cardiology/American Heart Association HF Guidelines. The study population did not include patients with end-stage HF, those with a severe noncardiac illness with a life expectancy of less than a year, and patients with a pattern of nonadherence. Baseline characteristics of the two groups were well balanced, Dr. Desai said.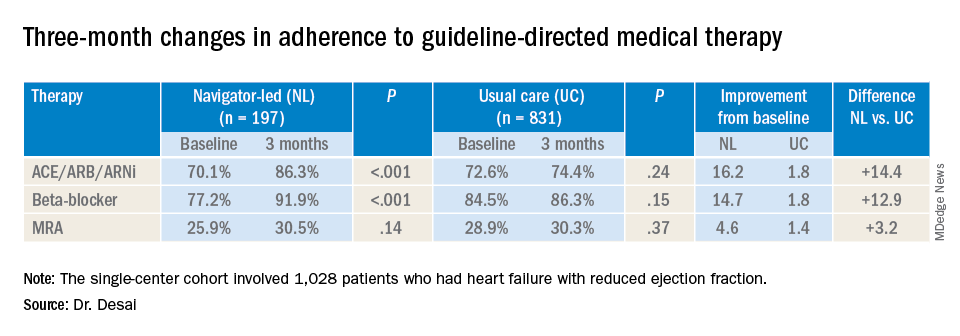
At baseline, 74% (759) participants were treated with ACE inhibitors/angiotensin receptor blockers/angiotensin-receptor neprilysin inhibitors (ACE/ARB/ARNi), 73% (746) with guideline-directed beta-blockers, and 29% (303) with mineralocorticoid receptor antagonists (MRAs), with 10% (107) and 11% (117) treated with target doses of ACE/ARB/ARNi and beta-blockers, respectively.
In the navigator-led group, beta-blocker adherence improved from 77.2% at baseline to 91.9% at 3 months (P less than 0.001) compared with an increase from 84.5% to 86.3% in the usual-care patients (P = 0.15), Dr. Desai said. ACE/ARB/ARNi adherence increased 16.2 percentage points to 86.3% (P less than 0.001) in the navigator-group versus 1.8 percentage points to 74.4% (P = 0.24) for usual care. In the MRA subgroup, 3-month adherence to GDMT was almost identical: 30.5% (P = 0.14) and 30.3% (P = 0.37) for the two treatment groups, respectively, although the navigator-led patients averaged a larger increase of 4.6 versus 1.4 percentage points from baseline.
Adverse event rates were similar in both groups, although the navigator group had “slightly higher rates” of hypotension and hyperkalemia but no serious events, Dr. Desai said. This group also had similarly higher rates of worsening renal function, but most were asymptomatic change in creatinine that was addressed with medication changes, he said. There were no hospitalizations for adverse events.
He said the navigator-led optimization has potential in a community setting because the referral nature of Brigham and Women’s HF population “reflects potentially a worst-case scenario for such a program.” The greatest impact was seen in patients managed by general cardiologists, he said. “If we were to move this forward, which we hope to do with scale, the impact might be greater in a community population where there are fewer specialists and less severe illnesses present.”
This study represents a proof of concept, Dr. Desai said in an interview. “What we would like to do is demonstrate that this can be done on a larger scale,” he said. “That might involve partnership with a payer or health care system to see if we can replicate these findings across a broader range of providers.”
Dr. Desai disclosed financial relationships with Novartis, AstraZeneca, Abbott, Boehringer-Ingelheim, Coston Scientific, Biofourmis, DalCor, Relypsa, Regeneron, and Alnylam. Novartis provided an unrestricted grant for the investigator-initiated trial.
SOURCE: Desai AS. AHA 2019 Featured Science session AOS.07.
PHILADELPHIA – Treatment guidelines are clear about optimal treatment of heart failure in patients with reduced ejection fraction (HFrEF), but adherence breakdowns often occur.
So, Brigham and Women’s Hospital in Boston implemented a navigator-administered patient outreach program that led to improved medication adherence over usual care, according to study results reported at the American Heart Association scientific sessions.
Although the study was done at a major academic center, the findings have implications for community practitioners, lead study author Akshay S. Desai, MD, MPH, said in an interview. “The impact of the intervention is clearly greater in those practitioners who manage heart failure and have the least support around them,” he said.
“Our sense is that the kind of population where this intervention would have the greater impact would be a community-dwelling heart failure population managed by community cardiologists, where the infrastructure to provide longitudinal heart failure care is less robust than may be in an academic center,” Dr. Desai said.
The study evaluated adherence in guideline-directed medical therapy (GDMT) at 3 months. “The navigator-led remote medication optimization strategy improved utilization and dosing of all categories of GDMP and was associated with a lower rate of adverse events,” Dr. Desai said. “The impact was more pronounced in patients followed by general practitioners than by a HF specialist.” In the outreach, health navigators contacted patients by phone and managed medications based on remote surveillance of labs, blood pressure, and symptoms under supervision of a pharmacist, nurse practitioner, and heart failure specialist.
The study included 1,028 patients with chronic HFrEF who’d visited a cardiologist at Brigham and Women’s in the year prior to the study: 197 patients and their providers consented to participate in the program with the remainder serving as the reference usual-care group. Most HF specialists at Brigham and Women’s declined to participate in the navigator-led program, Dr. Desai said.
Treating providers were approached for consent to adjust medical therapy according to a sequential, stepped titration algorithm modeled on the current American College of Cardiology/American Heart Association HF Guidelines. The study population did not include patients with end-stage HF, those with a severe noncardiac illness with a life expectancy of less than a year, and patients with a pattern of nonadherence. Baseline characteristics of the two groups were well balanced, Dr. Desai said.
At baseline, 74% (759) participants were treated with ACE inhibitors/angiotensin receptor blockers/angiotensin-receptor neprilysin inhibitors (ACE/ARB/ARNi), 73% (746) with guideline-directed beta-blockers, and 29% (303) with mineralocorticoid receptor antagonists (MRAs), with 10% (107) and 11% (117) treated with target doses of ACE/ARB/ARNi and beta-blockers, respectively.
In the navigator-led group, beta-blocker adherence improved from 77.2% at baseline to 91.9% at 3 months (P less than 0.001) compared with an increase from 84.5% to 86.3% in the usual-care patients (P = 0.15), Dr. Desai said. ACE/ARB/ARNi adherence increased 16.2 percentage points to 86.3% (P less than 0.001) in the navigator-group versus 1.8 percentage points to 74.4% (P = 0.24) for usual care. In the MRA subgroup, 3-month adherence to GDMT was almost identical: 30.5% (P = 0.14) and 30.3% (P = 0.37) for the two treatment groups, respectively, although the navigator-led patients averaged a larger increase of 4.6 versus 1.4 percentage points from baseline.
Adverse event rates were similar in both groups, although the navigator group had “slightly higher rates” of hypotension and hyperkalemia but no serious events, Dr. Desai said. This group also had similarly higher rates of worsening renal function, but most were asymptomatic change in creatinine that was addressed with medication changes, he said. There were no hospitalizations for adverse events.
He said the navigator-led optimization has potential in a community setting because the referral nature of Brigham and Women’s HF population “reflects potentially a worst-case scenario for such a program.” The greatest impact was seen in patients managed by general cardiologists, he said. “If we were to move this forward, which we hope to do with scale, the impact might be greater in a community population where there are fewer specialists and less severe illnesses present.”
This study represents a proof of concept, Dr. Desai said in an interview. “What we would like to do is demonstrate that this can be done on a larger scale,” he said. “That might involve partnership with a payer or health care system to see if we can replicate these findings across a broader range of providers.”
Dr. Desai disclosed financial relationships with Novartis, AstraZeneca, Abbott, Boehringer-Ingelheim, Coston Scientific, Biofourmis, DalCor, Relypsa, Regeneron, and Alnylam. Novartis provided an unrestricted grant for the investigator-initiated trial.
SOURCE: Desai AS. AHA 2019 Featured Science session AOS.07.
PHILADELPHIA – Treatment guidelines are clear about optimal treatment of heart failure in patients with reduced ejection fraction (HFrEF), but adherence breakdowns often occur.
So, Brigham and Women’s Hospital in Boston implemented a navigator-administered patient outreach program that led to improved medication adherence over usual care, according to study results reported at the American Heart Association scientific sessions.
Although the study was done at a major academic center, the findings have implications for community practitioners, lead study author Akshay S. Desai, MD, MPH, said in an interview. “The impact of the intervention is clearly greater in those practitioners who manage heart failure and have the least support around them,” he said.
“Our sense is that the kind of population where this intervention would have the greater impact would be a community-dwelling heart failure population managed by community cardiologists, where the infrastructure to provide longitudinal heart failure care is less robust than may be in an academic center,” Dr. Desai said.
The study evaluated adherence in guideline-directed medical therapy (GDMT) at 3 months. “The navigator-led remote medication optimization strategy improved utilization and dosing of all categories of GDMP and was associated with a lower rate of adverse events,” Dr. Desai said. “The impact was more pronounced in patients followed by general practitioners than by a HF specialist.” In the outreach, health navigators contacted patients by phone and managed medications based on remote surveillance of labs, blood pressure, and symptoms under supervision of a pharmacist, nurse practitioner, and heart failure specialist.
The study included 1,028 patients with chronic HFrEF who’d visited a cardiologist at Brigham and Women’s in the year prior to the study: 197 patients and their providers consented to participate in the program with the remainder serving as the reference usual-care group. Most HF specialists at Brigham and Women’s declined to participate in the navigator-led program, Dr. Desai said.
Treating providers were approached for consent to adjust medical therapy according to a sequential, stepped titration algorithm modeled on the current American College of Cardiology/American Heart Association HF Guidelines. The study population did not include patients with end-stage HF, those with a severe noncardiac illness with a life expectancy of less than a year, and patients with a pattern of nonadherence. Baseline characteristics of the two groups were well balanced, Dr. Desai said.
At baseline, 74% (759) participants were treated with ACE inhibitors/angiotensin receptor blockers/angiotensin-receptor neprilysin inhibitors (ACE/ARB/ARNi), 73% (746) with guideline-directed beta-blockers, and 29% (303) with mineralocorticoid receptor antagonists (MRAs), with 10% (107) and 11% (117) treated with target doses of ACE/ARB/ARNi and beta-blockers, respectively.
In the navigator-led group, beta-blocker adherence improved from 77.2% at baseline to 91.9% at 3 months (P less than 0.001) compared with an increase from 84.5% to 86.3% in the usual-care patients (P = 0.15), Dr. Desai said. ACE/ARB/ARNi adherence increased 16.2 percentage points to 86.3% (P less than 0.001) in the navigator-group versus 1.8 percentage points to 74.4% (P = 0.24) for usual care. In the MRA subgroup, 3-month adherence to GDMT was almost identical: 30.5% (P = 0.14) and 30.3% (P = 0.37) for the two treatment groups, respectively, although the navigator-led patients averaged a larger increase of 4.6 versus 1.4 percentage points from baseline.
Adverse event rates were similar in both groups, although the navigator group had “slightly higher rates” of hypotension and hyperkalemia but no serious events, Dr. Desai said. This group also had similarly higher rates of worsening renal function, but most were asymptomatic change in creatinine that was addressed with medication changes, he said. There were no hospitalizations for adverse events.
He said the navigator-led optimization has potential in a community setting because the referral nature of Brigham and Women’s HF population “reflects potentially a worst-case scenario for such a program.” The greatest impact was seen in patients managed by general cardiologists, he said. “If we were to move this forward, which we hope to do with scale, the impact might be greater in a community population where there are fewer specialists and less severe illnesses present.”
This study represents a proof of concept, Dr. Desai said in an interview. “What we would like to do is demonstrate that this can be done on a larger scale,” he said. “That might involve partnership with a payer or health care system to see if we can replicate these findings across a broader range of providers.”
Dr. Desai disclosed financial relationships with Novartis, AstraZeneca, Abbott, Boehringer-Ingelheim, Coston Scientific, Biofourmis, DalCor, Relypsa, Regeneron, and Alnylam. Novartis provided an unrestricted grant for the investigator-initiated trial.
SOURCE: Desai AS. AHA 2019 Featured Science session AOS.07.
REPORTING FROM AHA 2019
Low RAAS inhibitor dosing linked to MACE risk
Suboptimal dosing of renin-angiotensin-aldosterone system (RAAS) inhibitors to reduce the risk of hyperkalemia could increase the risk of major adverse cardiac events (MACE) and all-cause mortality in patients with chronic kidney disease (CKD) or heart failure.
Researchers reported the outcomes of an observational study that explored the real-world associations between RAAS inhibitor dose, hyperkalemia, and clinical outcomes.
RAAS inhibitors – such as ACE inhibitors, angiotensin receptor blockers, and mineralocorticoid receptor antagonists – are known to reduce potassium excretion and therefore increase the risk of high potassium levels.
Dr. Cecilia Linde, from the Karolinska University Hospital and Karolinska Institutet in Stockholm, and coauthors wrote that management of serum potassium levels often requires reducing the dosage of RAAS inhibitors or stopping them altogether. However, this is also associated with risks in patients with heart failure or CKD.
In this study, researchers looked at data from 100,572 people with nondialysis CKD and 13,113 with new-onset heart failure who were prescribed RAAS inhibitors during 2006-2015.
Overall, 58% of patients with CKD and 63% of patients with heart failure spent the majority of follow-up on prescribed optimal doses of RAAS inhibitors – defined as at least 50% of the guidelines-recommended dose.
Patients with hyperkalemia were more likely to have down-titrations or discontinue their RAAS inhibitors, and this increased with increasing hyperkalemia severity.
The study found consistently lower mortality rates among patients who spent most of their follow-up time on at least 50% of the guideline-recommended dose of RAAS inhibitors.
In patients with CKD, mortality rates were 7.2 deaths per 1,000 patient-years in those taking at least 50% of the recommended dose, compared with 57.7 deaths per 1,000 patient-years for those on suboptimal doses. The rates of MACE were 73 and 130 per 1,000 patient-years, respectively.
The differences were even more pronounced in patients with heart failure. Those taking at least 50% of the recommended dose had mortality rates of 12.5 per 1000 patient-years, compared with 141.7 among those on suboptimal doses. The rates of MACE were 148.5 and 290.4, respectively.
“The results highlight the potential negative impact of suboptimal RAASi dosing, indicate the generalizability of [European Society of Cardiology–recommended] RAASi doses in HF to CKD patients, and emphasize the need for strategies that allow patients to be maintained on appropriate therapy, avoiding RAASi dose modification or discontinuation,” the authors wrote.
The study was funded by AstraZeneca. One author was an employee and stockholder of AstraZeneca, and five authors declared funding and support from the pharmaceutical sector, including AstraZeneca.
SOURCE: Linde C et al. J Am Heart Assoc. 2019 Nov 12. doi: 10.1161/JAHA.119.012655.
Suboptimal dosing of renin-angiotensin-aldosterone system (RAAS) inhibitors to reduce the risk of hyperkalemia could increase the risk of major adverse cardiac events (MACE) and all-cause mortality in patients with chronic kidney disease (CKD) or heart failure.
Researchers reported the outcomes of an observational study that explored the real-world associations between RAAS inhibitor dose, hyperkalemia, and clinical outcomes.
RAAS inhibitors – such as ACE inhibitors, angiotensin receptor blockers, and mineralocorticoid receptor antagonists – are known to reduce potassium excretion and therefore increase the risk of high potassium levels.
Dr. Cecilia Linde, from the Karolinska University Hospital and Karolinska Institutet in Stockholm, and coauthors wrote that management of serum potassium levels often requires reducing the dosage of RAAS inhibitors or stopping them altogether. However, this is also associated with risks in patients with heart failure or CKD.
In this study, researchers looked at data from 100,572 people with nondialysis CKD and 13,113 with new-onset heart failure who were prescribed RAAS inhibitors during 2006-2015.
Overall, 58% of patients with CKD and 63% of patients with heart failure spent the majority of follow-up on prescribed optimal doses of RAAS inhibitors – defined as at least 50% of the guidelines-recommended dose.
Patients with hyperkalemia were more likely to have down-titrations or discontinue their RAAS inhibitors, and this increased with increasing hyperkalemia severity.
The study found consistently lower mortality rates among patients who spent most of their follow-up time on at least 50% of the guideline-recommended dose of RAAS inhibitors.
In patients with CKD, mortality rates were 7.2 deaths per 1,000 patient-years in those taking at least 50% of the recommended dose, compared with 57.7 deaths per 1,000 patient-years for those on suboptimal doses. The rates of MACE were 73 and 130 per 1,000 patient-years, respectively.
The differences were even more pronounced in patients with heart failure. Those taking at least 50% of the recommended dose had mortality rates of 12.5 per 1000 patient-years, compared with 141.7 among those on suboptimal doses. The rates of MACE were 148.5 and 290.4, respectively.
“The results highlight the potential negative impact of suboptimal RAASi dosing, indicate the generalizability of [European Society of Cardiology–recommended] RAASi doses in HF to CKD patients, and emphasize the need for strategies that allow patients to be maintained on appropriate therapy, avoiding RAASi dose modification or discontinuation,” the authors wrote.
The study was funded by AstraZeneca. One author was an employee and stockholder of AstraZeneca, and five authors declared funding and support from the pharmaceutical sector, including AstraZeneca.
SOURCE: Linde C et al. J Am Heart Assoc. 2019 Nov 12. doi: 10.1161/JAHA.119.012655.
Suboptimal dosing of renin-angiotensin-aldosterone system (RAAS) inhibitors to reduce the risk of hyperkalemia could increase the risk of major adverse cardiac events (MACE) and all-cause mortality in patients with chronic kidney disease (CKD) or heart failure.
Researchers reported the outcomes of an observational study that explored the real-world associations between RAAS inhibitor dose, hyperkalemia, and clinical outcomes.
RAAS inhibitors – such as ACE inhibitors, angiotensin receptor blockers, and mineralocorticoid receptor antagonists – are known to reduce potassium excretion and therefore increase the risk of high potassium levels.
Dr. Cecilia Linde, from the Karolinska University Hospital and Karolinska Institutet in Stockholm, and coauthors wrote that management of serum potassium levels often requires reducing the dosage of RAAS inhibitors or stopping them altogether. However, this is also associated with risks in patients with heart failure or CKD.
In this study, researchers looked at data from 100,572 people with nondialysis CKD and 13,113 with new-onset heart failure who were prescribed RAAS inhibitors during 2006-2015.
Overall, 58% of patients with CKD and 63% of patients with heart failure spent the majority of follow-up on prescribed optimal doses of RAAS inhibitors – defined as at least 50% of the guidelines-recommended dose.
Patients with hyperkalemia were more likely to have down-titrations or discontinue their RAAS inhibitors, and this increased with increasing hyperkalemia severity.
The study found consistently lower mortality rates among patients who spent most of their follow-up time on at least 50% of the guideline-recommended dose of RAAS inhibitors.
In patients with CKD, mortality rates were 7.2 deaths per 1,000 patient-years in those taking at least 50% of the recommended dose, compared with 57.7 deaths per 1,000 patient-years for those on suboptimal doses. The rates of MACE were 73 and 130 per 1,000 patient-years, respectively.
The differences were even more pronounced in patients with heart failure. Those taking at least 50% of the recommended dose had mortality rates of 12.5 per 1000 patient-years, compared with 141.7 among those on suboptimal doses. The rates of MACE were 148.5 and 290.4, respectively.
“The results highlight the potential negative impact of suboptimal RAASi dosing, indicate the generalizability of [European Society of Cardiology–recommended] RAASi doses in HF to CKD patients, and emphasize the need for strategies that allow patients to be maintained on appropriate therapy, avoiding RAASi dose modification or discontinuation,” the authors wrote.
The study was funded by AstraZeneca. One author was an employee and stockholder of AstraZeneca, and five authors declared funding and support from the pharmaceutical sector, including AstraZeneca.
SOURCE: Linde C et al. J Am Heart Assoc. 2019 Nov 12. doi: 10.1161/JAHA.119.012655.
FROM THE JOURNAL OF THE AMERICAN HEART ASSOCIATION
Mechanical circulatory support in PCI needs clearer guidance
PHILADELPHIA – Use of the Impella ventricular-assist device in patients with cardiogenic shock having percutaneous coronary interventions (PCI) has increased rapidly since its approval in 2008, but two studies comparing it with intra-aortic balloon pumps in PCI patients have raised questions about the safety, effectiveness, and cost of the ventricular-assist device, according to results of two studies presented at the American Heart Association scientific sessions.
The results of an observational analysis of 48,306 patients and a national real-world study of 28,304 patients may not be telling the complete story of the utility of ventricular assist in patients requiring mechanical circulatory support (MCS), one interventional cardiologist said in an interview. “It’s concerning; it’s sobering,” said Ranya N. Sweis, MD, of Northwestern University, Chicago. However, the data didn’t parse out patients who would have been routed to palliative care and otherwise wouldn’t have been candidates for PCI without MCS.
“What I take from it is that we need to get more randomized data,” she said. “Who are the patients that were doing worse? Who are the patients who really needed the Impella support for the PCI after cardiogenic shock?”
In the observational study, Amit P. Amin, MD, of Washington University, St. Louis, said that the use of MCS devices increased steadily to 32% of all PCI patients receiving MCS from 2008 to 2016 while use of intra-aortic balloon pump (IABP) declined, but that Impella was less likely to be used in critically ill patients. The study analyzed patients in the Premier Healthcare Database who had PCI with MCS at 432 hospitals from 2004 to 2016.
Outcomes in what Dr. Amin called “the Impella era,” showed significantly higher risks for death, acute kidney injury, and stroke, with odds ratios of 1.17, 1.91 and 3.34, respectively (P less than .001 for all). In the patient-level comparison of Impella versus IABP, Impella had a 24% higher risk of death (P less than .0001), 10% for bleeding (P = .0445), 8% for acute kidney injury (P = .0521) and 34% for stroke (P less than .0001). The findings were published simultaneously with the presentation (Circulation. 2019 Nov 17. doi: 10.1161/CIRCULATIONAHA.119.044007)
“The total length of stay, as well as the ICU length of stay, were actually lower with Impella use, by approximately a half day to 1 day,” Dr. Amin said. “Despite that, the total costs were approximately $15,000.”
Yet, the study found wide variation in the use of Impella among hospitals, some doing no cases with the device and others all of them, Dr. Amin said. The risk analysis also found wide variations in outcomes across hospitals using Impella. “We saw a 2.5-fold variation in bleeding across hospitals and a 1.5-fold variation in acute kidney injury, stroke and death,” he noted. The study found less variation in hospital stays and total cost of Impella, “perhaps related to the uniformly high device acquisition costs.”
“These data underscore the need for defining the appropriate use of mechanical circulatory support in patients undergoing PCI,” Dr. Amin said.
Dr. Sweis wasn’t surprised by the cost findings. “New technology is going to cost more,” she said in an interview. “I’m actually surprised that the cost wasn’t more significantly different just knowing the cost of some of these devices.
Patients who require MCS represent a small portion of PCI cases: 2%, according to Dr. Sweis. “It’s not like all PCI has increased because of MCS, and there’s a potential improvement in the length of stay so there are going to be cost savings that way.”
The national real-world study that Sanket S. Dhruva, MD, MHS, of the University of California San Francisco, reported on focused on Impella and IABP in PCI patients with acute MI complicated by cardiogenic shock (CS). The study used outcomes of patients with AMI-CS who had PCI from October 2015 to December 2017 in the National Cardiovascular Data Registry’s CathPCI and Chest Pain–MI registries. An estimated 4%-12% of AMIs present with CS.
Most patients in the study population had medical therapy only, but this study focused on the 1,768 who had Impella only and the 8,471 who had IABP only. The rates of in-hospital death and bleeding were 34.1% 16% in the IABP group, and 45% and 31.3% in the Impella group, Dr. Dhruva said. In this study population, the rate of Impella use increased from 3.5% in 2015 to 8.7% by the end of 2017 (P less than .001).
Dr. Dhruva acknowledged a number of limitations to the study findings, including residual confounding. However, the “robust propensity match” of 95% of the Impella-only patients and the results were consistent across multiple sensitivity analyses. “There may have been questions about the clinical severity of AMI-CS patients in the NCDR Registry,” he said. “However, the registry definition is similar to that used in the trials.”
The trial also failed to distinguish between the different types of Impella devices, but the results mostly pertain to the Impella 2.5 and CP because the 5.0 device requires a surgical cutdown, and the study excluded patients who received multiple devices.
“Better evidence and guidance are needed regarding the optimal management of patients with AMI-CS as well as the role of mechanical circulatory support devices in general and Impella in particular,” he said, adding that Impella has been on the U.S. market since 2008, but with limited randomized clinical trial evidence in cardiogenic shock.
The study population of patient’s with CS is “only a piece of the puzzle,” Dr. Sweis said. “We know that there are sick hearts that aren’t in shock right now, but you’re going to do triple-vessel intervention and use atherectomy. Those patients would not do very well during the procedure itself and it may not even be offered to them if there weren’t support.”
Impella is not going away, Dr. Sweis said. “It provides an option that a patient wouldn’t otherwise have. This is really stressing to me that we need to get rid of that variability in the safety related to these devices.”
Dr. Amin disclosed financial relationships with Terumo and GE Healthcare. Dr. Dhruva had no financial relationships to disclose. The study was supported in part by a Center of Excellence in Regulatory Science and Innovation grant from the Food and Drug Administration and the American College of Cardiology’s National Cardiovascular Data Registry.
PHILADELPHIA – Use of the Impella ventricular-assist device in patients with cardiogenic shock having percutaneous coronary interventions (PCI) has increased rapidly since its approval in 2008, but two studies comparing it with intra-aortic balloon pumps in PCI patients have raised questions about the safety, effectiveness, and cost of the ventricular-assist device, according to results of two studies presented at the American Heart Association scientific sessions.
The results of an observational analysis of 48,306 patients and a national real-world study of 28,304 patients may not be telling the complete story of the utility of ventricular assist in patients requiring mechanical circulatory support (MCS), one interventional cardiologist said in an interview. “It’s concerning; it’s sobering,” said Ranya N. Sweis, MD, of Northwestern University, Chicago. However, the data didn’t parse out patients who would have been routed to palliative care and otherwise wouldn’t have been candidates for PCI without MCS.
“What I take from it is that we need to get more randomized data,” she said. “Who are the patients that were doing worse? Who are the patients who really needed the Impella support for the PCI after cardiogenic shock?”
In the observational study, Amit P. Amin, MD, of Washington University, St. Louis, said that the use of MCS devices increased steadily to 32% of all PCI patients receiving MCS from 2008 to 2016 while use of intra-aortic balloon pump (IABP) declined, but that Impella was less likely to be used in critically ill patients. The study analyzed patients in the Premier Healthcare Database who had PCI with MCS at 432 hospitals from 2004 to 2016.
Outcomes in what Dr. Amin called “the Impella era,” showed significantly higher risks for death, acute kidney injury, and stroke, with odds ratios of 1.17, 1.91 and 3.34, respectively (P less than .001 for all). In the patient-level comparison of Impella versus IABP, Impella had a 24% higher risk of death (P less than .0001), 10% for bleeding (P = .0445), 8% for acute kidney injury (P = .0521) and 34% for stroke (P less than .0001). The findings were published simultaneously with the presentation (Circulation. 2019 Nov 17. doi: 10.1161/CIRCULATIONAHA.119.044007)
“The total length of stay, as well as the ICU length of stay, were actually lower with Impella use, by approximately a half day to 1 day,” Dr. Amin said. “Despite that, the total costs were approximately $15,000.”
Yet, the study found wide variation in the use of Impella among hospitals, some doing no cases with the device and others all of them, Dr. Amin said. The risk analysis also found wide variations in outcomes across hospitals using Impella. “We saw a 2.5-fold variation in bleeding across hospitals and a 1.5-fold variation in acute kidney injury, stroke and death,” he noted. The study found less variation in hospital stays and total cost of Impella, “perhaps related to the uniformly high device acquisition costs.”
“These data underscore the need for defining the appropriate use of mechanical circulatory support in patients undergoing PCI,” Dr. Amin said.
Dr. Sweis wasn’t surprised by the cost findings. “New technology is going to cost more,” she said in an interview. “I’m actually surprised that the cost wasn’t more significantly different just knowing the cost of some of these devices.
Patients who require MCS represent a small portion of PCI cases: 2%, according to Dr. Sweis. “It’s not like all PCI has increased because of MCS, and there’s a potential improvement in the length of stay so there are going to be cost savings that way.”
The national real-world study that Sanket S. Dhruva, MD, MHS, of the University of California San Francisco, reported on focused on Impella and IABP in PCI patients with acute MI complicated by cardiogenic shock (CS). The study used outcomes of patients with AMI-CS who had PCI from October 2015 to December 2017 in the National Cardiovascular Data Registry’s CathPCI and Chest Pain–MI registries. An estimated 4%-12% of AMIs present with CS.
Most patients in the study population had medical therapy only, but this study focused on the 1,768 who had Impella only and the 8,471 who had IABP only. The rates of in-hospital death and bleeding were 34.1% 16% in the IABP group, and 45% and 31.3% in the Impella group, Dr. Dhruva said. In this study population, the rate of Impella use increased from 3.5% in 2015 to 8.7% by the end of 2017 (P less than .001).
Dr. Dhruva acknowledged a number of limitations to the study findings, including residual confounding. However, the “robust propensity match” of 95% of the Impella-only patients and the results were consistent across multiple sensitivity analyses. “There may have been questions about the clinical severity of AMI-CS patients in the NCDR Registry,” he said. “However, the registry definition is similar to that used in the trials.”
The trial also failed to distinguish between the different types of Impella devices, but the results mostly pertain to the Impella 2.5 and CP because the 5.0 device requires a surgical cutdown, and the study excluded patients who received multiple devices.
“Better evidence and guidance are needed regarding the optimal management of patients with AMI-CS as well as the role of mechanical circulatory support devices in general and Impella in particular,” he said, adding that Impella has been on the U.S. market since 2008, but with limited randomized clinical trial evidence in cardiogenic shock.
The study population of patient’s with CS is “only a piece of the puzzle,” Dr. Sweis said. “We know that there are sick hearts that aren’t in shock right now, but you’re going to do triple-vessel intervention and use atherectomy. Those patients would not do very well during the procedure itself and it may not even be offered to them if there weren’t support.”
Impella is not going away, Dr. Sweis said. “It provides an option that a patient wouldn’t otherwise have. This is really stressing to me that we need to get rid of that variability in the safety related to these devices.”
Dr. Amin disclosed financial relationships with Terumo and GE Healthcare. Dr. Dhruva had no financial relationships to disclose. The study was supported in part by a Center of Excellence in Regulatory Science and Innovation grant from the Food and Drug Administration and the American College of Cardiology’s National Cardiovascular Data Registry.
PHILADELPHIA – Use of the Impella ventricular-assist device in patients with cardiogenic shock having percutaneous coronary interventions (PCI) has increased rapidly since its approval in 2008, but two studies comparing it with intra-aortic balloon pumps in PCI patients have raised questions about the safety, effectiveness, and cost of the ventricular-assist device, according to results of two studies presented at the American Heart Association scientific sessions.
The results of an observational analysis of 48,306 patients and a national real-world study of 28,304 patients may not be telling the complete story of the utility of ventricular assist in patients requiring mechanical circulatory support (MCS), one interventional cardiologist said in an interview. “It’s concerning; it’s sobering,” said Ranya N. Sweis, MD, of Northwestern University, Chicago. However, the data didn’t parse out patients who would have been routed to palliative care and otherwise wouldn’t have been candidates for PCI without MCS.
“What I take from it is that we need to get more randomized data,” she said. “Who are the patients that were doing worse? Who are the patients who really needed the Impella support for the PCI after cardiogenic shock?”
In the observational study, Amit P. Amin, MD, of Washington University, St. Louis, said that the use of MCS devices increased steadily to 32% of all PCI patients receiving MCS from 2008 to 2016 while use of intra-aortic balloon pump (IABP) declined, but that Impella was less likely to be used in critically ill patients. The study analyzed patients in the Premier Healthcare Database who had PCI with MCS at 432 hospitals from 2004 to 2016.
Outcomes in what Dr. Amin called “the Impella era,” showed significantly higher risks for death, acute kidney injury, and stroke, with odds ratios of 1.17, 1.91 and 3.34, respectively (P less than .001 for all). In the patient-level comparison of Impella versus IABP, Impella had a 24% higher risk of death (P less than .0001), 10% for bleeding (P = .0445), 8% for acute kidney injury (P = .0521) and 34% for stroke (P less than .0001). The findings were published simultaneously with the presentation (Circulation. 2019 Nov 17. doi: 10.1161/CIRCULATIONAHA.119.044007)
“The total length of stay, as well as the ICU length of stay, were actually lower with Impella use, by approximately a half day to 1 day,” Dr. Amin said. “Despite that, the total costs were approximately $15,000.”
Yet, the study found wide variation in the use of Impella among hospitals, some doing no cases with the device and others all of them, Dr. Amin said. The risk analysis also found wide variations in outcomes across hospitals using Impella. “We saw a 2.5-fold variation in bleeding across hospitals and a 1.5-fold variation in acute kidney injury, stroke and death,” he noted. The study found less variation in hospital stays and total cost of Impella, “perhaps related to the uniformly high device acquisition costs.”
“These data underscore the need for defining the appropriate use of mechanical circulatory support in patients undergoing PCI,” Dr. Amin said.
Dr. Sweis wasn’t surprised by the cost findings. “New technology is going to cost more,” she said in an interview. “I’m actually surprised that the cost wasn’t more significantly different just knowing the cost of some of these devices.
Patients who require MCS represent a small portion of PCI cases: 2%, according to Dr. Sweis. “It’s not like all PCI has increased because of MCS, and there’s a potential improvement in the length of stay so there are going to be cost savings that way.”
The national real-world study that Sanket S. Dhruva, MD, MHS, of the University of California San Francisco, reported on focused on Impella and IABP in PCI patients with acute MI complicated by cardiogenic shock (CS). The study used outcomes of patients with AMI-CS who had PCI from October 2015 to December 2017 in the National Cardiovascular Data Registry’s CathPCI and Chest Pain–MI registries. An estimated 4%-12% of AMIs present with CS.
Most patients in the study population had medical therapy only, but this study focused on the 1,768 who had Impella only and the 8,471 who had IABP only. The rates of in-hospital death and bleeding were 34.1% 16% in the IABP group, and 45% and 31.3% in the Impella group, Dr. Dhruva said. In this study population, the rate of Impella use increased from 3.5% in 2015 to 8.7% by the end of 2017 (P less than .001).
Dr. Dhruva acknowledged a number of limitations to the study findings, including residual confounding. However, the “robust propensity match” of 95% of the Impella-only patients and the results were consistent across multiple sensitivity analyses. “There may have been questions about the clinical severity of AMI-CS patients in the NCDR Registry,” he said. “However, the registry definition is similar to that used in the trials.”
The trial also failed to distinguish between the different types of Impella devices, but the results mostly pertain to the Impella 2.5 and CP because the 5.0 device requires a surgical cutdown, and the study excluded patients who received multiple devices.
“Better evidence and guidance are needed regarding the optimal management of patients with AMI-CS as well as the role of mechanical circulatory support devices in general and Impella in particular,” he said, adding that Impella has been on the U.S. market since 2008, but with limited randomized clinical trial evidence in cardiogenic shock.
The study population of patient’s with CS is “only a piece of the puzzle,” Dr. Sweis said. “We know that there are sick hearts that aren’t in shock right now, but you’re going to do triple-vessel intervention and use atherectomy. Those patients would not do very well during the procedure itself and it may not even be offered to them if there weren’t support.”
Impella is not going away, Dr. Sweis said. “It provides an option that a patient wouldn’t otherwise have. This is really stressing to me that we need to get rid of that variability in the safety related to these devices.”
Dr. Amin disclosed financial relationships with Terumo and GE Healthcare. Dr. Dhruva had no financial relationships to disclose. The study was supported in part by a Center of Excellence in Regulatory Science and Innovation grant from the Food and Drug Administration and the American College of Cardiology’s National Cardiovascular Data Registry.
REPORTING FROM AHA 2019
Minimum transcatheter mitral valve intervention case load recommended
U.S. sites that perform transcatheter mitral valve interventions should treat a minimum of 20 such patients each year or at least 40 every 2 years, according to revised recommendations and requirements released on Dec. 16, 2019, by several U.S. cardiology and cardiac surgery societies.
The revised recommendations also for the first time address patient selection for using transcatheter mitral valve (MV) intervention in patients with mitral regurgitation (MR) secondary to heart failure and a left ventricular ejection fraction of 21%-49%, a patient target for transcatheter interventions approved by the Food and Drug Administration in March 2019. The FDA first approved the same transcatheter MV intervention system (MitraClip) in 2013 for patients with primary MR caused by an abnormality of the MV and a prohibitive risk for surgical MV repair or replacement, and the same societies had previously issued their recommendations based on that approval (J Am Coll Cardiol. 2014 Oct; 64[14]:1515-26).
The need for updated recommendations on the delivery of transcatheter MV interventions including the personnel and facilities required for a valid U.S. program was driven by “new transcatheter technology, new trial results, and the new FDA-approved indication” of secondary MR. “We did the update for patients with secondary MR,” said Robert O. Bonow, MD, professor of medicine at Northwestern University, Chicago, and chair of the committee that wrote the revised recommendations.
“Primary and secondary MR are like two different diseases. Primary MR is a disease of the MV leaflets. Secondary MR is a disease of heart failure, left ventricular dysfunction, and left ventricular enlargement.” That makes secondary MR an indication with a potentially huge upside, given how many patients have heart failure today, plus projections for steadily increasing numbers of patients as the geriatric population grows.
“Heart failure is a huge issue, and the numbers are growing, which is why we felt it was important to say which heart failure patients should be candidates. We did not endorse this for all patients, although MR is very common in heart failure,” with estimated prevalence rates as high as two-thirds of all heart failure patients, Dr. Bonow said.
The new recommendations spell out an approach to patient selection that aims to apply transcatheter MV intervention to patients who match those enrolled in the COAPT (Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients With Functional Mitral Regurgitation) trial, which randomized 614 patients with MR secondary to heart failure and found for that MV intervention cut the rate of heart failure hospitalization by about half during 2 years of follow-up, a statistically significant effect for the study’s primary endpoint (N Engl J Med. 2018 Dec 13;379[24]:2307-18). The new recommendations provide “a way to replicate the COAPT trial” in routine practice, Dr. Bonow said in an interview.”We thought it was important to try to replicate the COAPT results,” and a key step toward trying to accomplish that is to apply the multidisciplinary team concept to evaluating patients and determining their management strategy. Dr. Bonow coauthored a commentary that discussed how the COAPT results should influence routine practice (N Engl J Med. 2018 Dec 13;379[24]:2374-6).
“In this case, perhaps the most important member of the multidisciplinary team is the heart failure specialist, because secondary MR is a disease of heart failure and it’s important to also optimize medical therapy to reduce MR and prolong life,” he said. Treatment optimization before undertaking a transcatheter MV intervention involves not only drug treatment but also treatment with indicated devices, such as biventricular pacing, to optimize left ventricular size and function. The 2019 FDA approval of the transcatheter approach for treating MV disease specified that eligible patients had to continue to have significant MR despite receiving optimal drug and device treatment.
Treatment decisions for patients with MR must be highly individualized, and unlike transcatheter aortic valve replacement (TAVR), which occurs against a background of “really good results” for surgical aortic valve replacement, surgical treatment of MR “has never been shown to prolong life, although it can improve function,” Dr. Bonow said. In addition, open surgical repair or replacement of a faulty MV “is much better than the MitraClip for elimination a MV leak; usually with transcatheter intervention there is some residual leak. And other etiologies of MR, such as atrial fibrillation causing atrial enlargement and dilatation of the mitral annulus, generally have much better results with surgery than with a transcatheter intervention. For both primary and secondary MR, deciding when to use surgery and when to do a transcatheter intervention is very individualized,” concluded Dr. Bonow, and a fact that distinguishes transcatheter MV interventions from TAVR, which has been shown to have efficacy that’s comparable with surgical aortic valve replacement. The MitraClip system is currently the only device with U.S. marketing approval for transcatheter MV intervention, although other devices are in development.
Case volume requirements
The designation of a minimum transcatheter MV intervention case load of 20 procedures a year or 40 every 2 years reflected a “consensus among interventional cardiologists and cardiac surgeons of what the experience had to be for MV repair,” Dr. Bonow said. This number contrasts with a minimum case volume of 50 procedures/year or 100 every 2 years to maintain a TAVR program proposed in 2018 by a similar expert panel organized by U.S. cardiology and cardiac surgery societies (J Am Coll of Cardiol. 2019 Jan;73[3]:340-74). The new MV recommendations “follow a similar template [as the TAVR recommendations], but the numbers are what we thought would be best for optimal transcatheter MV expertise. MV interventions will likely increase, and we felt it would be best to define the transcatheter operators and are the right patients; the volume is unclear. There are a lot of heart failure patients, but we know from COAPT that not everyone is a candidate. The existing MV device does not fit all settings. We thought the numbers we selected were most appropriate, at least when we are starting.”
Dr. Bonow and coauthors who wrote the new recommendations will rely on payers, particularly the Centers for Medicare & Medicaid Services, to adopt the societal recommendations as part of their criteria for reimbursement and thereby give them teeth. In June 2019, CMS announced its Medicare coverage determination for TAVR, which included procedure minimums of 20 per year or 40 over 2 years for TAVR programs, a number that fell substantially below the 50 per year or 100 over 2 years that had been proposed by the societies. “We hope CMS will use our MV recommendations as a starting point,” but the final CMS coverage decision for transcatheter MV intervention could again differ from what the societies proposed, Dr. Bonow acknowledged.
In addition to strongly promoting a multidisciplinary team approach (and spelling out the members of the team) and shared decision making involvement of the patient with the team, the new recommendations also endorse participation of MV intervention programs in the Transcatheter Valve Therapy U.S. patient registry that’s maintained by two of the societies that helped organize the writing committee. The recommendations discuss the need to collect 30-day (and longer) outcomes data from transcatheter MV intervention programs through the registry as is now done for TAVR programs (N Engl J Med. 2019 Jun 27;380[26]:2541-50). Dr. Bonow declined to predict when 30-day outcomes data may start appearing for programs performing transcatheter MV interventions.
Dr. Bonow had no disclosures. The COAPT study was funded by Abbott, the company that markets the MitrClip clip delivery system.
SOURCE: Bonow RO et al. J Am Coll Cardiol. 2019 Dec 16. doi: 10.1016/j.jacc.2019.12.002.
U.S. sites that perform transcatheter mitral valve interventions should treat a minimum of 20 such patients each year or at least 40 every 2 years, according to revised recommendations and requirements released on Dec. 16, 2019, by several U.S. cardiology and cardiac surgery societies.
The revised recommendations also for the first time address patient selection for using transcatheter mitral valve (MV) intervention in patients with mitral regurgitation (MR) secondary to heart failure and a left ventricular ejection fraction of 21%-49%, a patient target for transcatheter interventions approved by the Food and Drug Administration in March 2019. The FDA first approved the same transcatheter MV intervention system (MitraClip) in 2013 for patients with primary MR caused by an abnormality of the MV and a prohibitive risk for surgical MV repair or replacement, and the same societies had previously issued their recommendations based on that approval (J Am Coll Cardiol. 2014 Oct; 64[14]:1515-26).
The need for updated recommendations on the delivery of transcatheter MV interventions including the personnel and facilities required for a valid U.S. program was driven by “new transcatheter technology, new trial results, and the new FDA-approved indication” of secondary MR. “We did the update for patients with secondary MR,” said Robert O. Bonow, MD, professor of medicine at Northwestern University, Chicago, and chair of the committee that wrote the revised recommendations.
“Primary and secondary MR are like two different diseases. Primary MR is a disease of the MV leaflets. Secondary MR is a disease of heart failure, left ventricular dysfunction, and left ventricular enlargement.” That makes secondary MR an indication with a potentially huge upside, given how many patients have heart failure today, plus projections for steadily increasing numbers of patients as the geriatric population grows.
“Heart failure is a huge issue, and the numbers are growing, which is why we felt it was important to say which heart failure patients should be candidates. We did not endorse this for all patients, although MR is very common in heart failure,” with estimated prevalence rates as high as two-thirds of all heart failure patients, Dr. Bonow said.
The new recommendations spell out an approach to patient selection that aims to apply transcatheter MV intervention to patients who match those enrolled in the COAPT (Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients With Functional Mitral Regurgitation) trial, which randomized 614 patients with MR secondary to heart failure and found for that MV intervention cut the rate of heart failure hospitalization by about half during 2 years of follow-up, a statistically significant effect for the study’s primary endpoint (N Engl J Med. 2018 Dec 13;379[24]:2307-18). The new recommendations provide “a way to replicate the COAPT trial” in routine practice, Dr. Bonow said in an interview.”We thought it was important to try to replicate the COAPT results,” and a key step toward trying to accomplish that is to apply the multidisciplinary team concept to evaluating patients and determining their management strategy. Dr. Bonow coauthored a commentary that discussed how the COAPT results should influence routine practice (N Engl J Med. 2018 Dec 13;379[24]:2374-6).
“In this case, perhaps the most important member of the multidisciplinary team is the heart failure specialist, because secondary MR is a disease of heart failure and it’s important to also optimize medical therapy to reduce MR and prolong life,” he said. Treatment optimization before undertaking a transcatheter MV intervention involves not only drug treatment but also treatment with indicated devices, such as biventricular pacing, to optimize left ventricular size and function. The 2019 FDA approval of the transcatheter approach for treating MV disease specified that eligible patients had to continue to have significant MR despite receiving optimal drug and device treatment.
Treatment decisions for patients with MR must be highly individualized, and unlike transcatheter aortic valve replacement (TAVR), which occurs against a background of “really good results” for surgical aortic valve replacement, surgical treatment of MR “has never been shown to prolong life, although it can improve function,” Dr. Bonow said. In addition, open surgical repair or replacement of a faulty MV “is much better than the MitraClip for elimination a MV leak; usually with transcatheter intervention there is some residual leak. And other etiologies of MR, such as atrial fibrillation causing atrial enlargement and dilatation of the mitral annulus, generally have much better results with surgery than with a transcatheter intervention. For both primary and secondary MR, deciding when to use surgery and when to do a transcatheter intervention is very individualized,” concluded Dr. Bonow, and a fact that distinguishes transcatheter MV interventions from TAVR, which has been shown to have efficacy that’s comparable with surgical aortic valve replacement. The MitraClip system is currently the only device with U.S. marketing approval for transcatheter MV intervention, although other devices are in development.
Case volume requirements
The designation of a minimum transcatheter MV intervention case load of 20 procedures a year or 40 every 2 years reflected a “consensus among interventional cardiologists and cardiac surgeons of what the experience had to be for MV repair,” Dr. Bonow said. This number contrasts with a minimum case volume of 50 procedures/year or 100 every 2 years to maintain a TAVR program proposed in 2018 by a similar expert panel organized by U.S. cardiology and cardiac surgery societies (J Am Coll of Cardiol. 2019 Jan;73[3]:340-74). The new MV recommendations “follow a similar template [as the TAVR recommendations], but the numbers are what we thought would be best for optimal transcatheter MV expertise. MV interventions will likely increase, and we felt it would be best to define the transcatheter operators and are the right patients; the volume is unclear. There are a lot of heart failure patients, but we know from COAPT that not everyone is a candidate. The existing MV device does not fit all settings. We thought the numbers we selected were most appropriate, at least when we are starting.”
Dr. Bonow and coauthors who wrote the new recommendations will rely on payers, particularly the Centers for Medicare & Medicaid Services, to adopt the societal recommendations as part of their criteria for reimbursement and thereby give them teeth. In June 2019, CMS announced its Medicare coverage determination for TAVR, which included procedure minimums of 20 per year or 40 over 2 years for TAVR programs, a number that fell substantially below the 50 per year or 100 over 2 years that had been proposed by the societies. “We hope CMS will use our MV recommendations as a starting point,” but the final CMS coverage decision for transcatheter MV intervention could again differ from what the societies proposed, Dr. Bonow acknowledged.
In addition to strongly promoting a multidisciplinary team approach (and spelling out the members of the team) and shared decision making involvement of the patient with the team, the new recommendations also endorse participation of MV intervention programs in the Transcatheter Valve Therapy U.S. patient registry that’s maintained by two of the societies that helped organize the writing committee. The recommendations discuss the need to collect 30-day (and longer) outcomes data from transcatheter MV intervention programs through the registry as is now done for TAVR programs (N Engl J Med. 2019 Jun 27;380[26]:2541-50). Dr. Bonow declined to predict when 30-day outcomes data may start appearing for programs performing transcatheter MV interventions.
Dr. Bonow had no disclosures. The COAPT study was funded by Abbott, the company that markets the MitrClip clip delivery system.
SOURCE: Bonow RO et al. J Am Coll Cardiol. 2019 Dec 16. doi: 10.1016/j.jacc.2019.12.002.
U.S. sites that perform transcatheter mitral valve interventions should treat a minimum of 20 such patients each year or at least 40 every 2 years, according to revised recommendations and requirements released on Dec. 16, 2019, by several U.S. cardiology and cardiac surgery societies.
The revised recommendations also for the first time address patient selection for using transcatheter mitral valve (MV) intervention in patients with mitral regurgitation (MR) secondary to heart failure and a left ventricular ejection fraction of 21%-49%, a patient target for transcatheter interventions approved by the Food and Drug Administration in March 2019. The FDA first approved the same transcatheter MV intervention system (MitraClip) in 2013 for patients with primary MR caused by an abnormality of the MV and a prohibitive risk for surgical MV repair or replacement, and the same societies had previously issued their recommendations based on that approval (J Am Coll Cardiol. 2014 Oct; 64[14]:1515-26).
The need for updated recommendations on the delivery of transcatheter MV interventions including the personnel and facilities required for a valid U.S. program was driven by “new transcatheter technology, new trial results, and the new FDA-approved indication” of secondary MR. “We did the update for patients with secondary MR,” said Robert O. Bonow, MD, professor of medicine at Northwestern University, Chicago, and chair of the committee that wrote the revised recommendations.
“Primary and secondary MR are like two different diseases. Primary MR is a disease of the MV leaflets. Secondary MR is a disease of heart failure, left ventricular dysfunction, and left ventricular enlargement.” That makes secondary MR an indication with a potentially huge upside, given how many patients have heart failure today, plus projections for steadily increasing numbers of patients as the geriatric population grows.
“Heart failure is a huge issue, and the numbers are growing, which is why we felt it was important to say which heart failure patients should be candidates. We did not endorse this for all patients, although MR is very common in heart failure,” with estimated prevalence rates as high as two-thirds of all heart failure patients, Dr. Bonow said.
The new recommendations spell out an approach to patient selection that aims to apply transcatheter MV intervention to patients who match those enrolled in the COAPT (Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients With Functional Mitral Regurgitation) trial, which randomized 614 patients with MR secondary to heart failure and found for that MV intervention cut the rate of heart failure hospitalization by about half during 2 years of follow-up, a statistically significant effect for the study’s primary endpoint (N Engl J Med. 2018 Dec 13;379[24]:2307-18). The new recommendations provide “a way to replicate the COAPT trial” in routine practice, Dr. Bonow said in an interview.”We thought it was important to try to replicate the COAPT results,” and a key step toward trying to accomplish that is to apply the multidisciplinary team concept to evaluating patients and determining their management strategy. Dr. Bonow coauthored a commentary that discussed how the COAPT results should influence routine practice (N Engl J Med. 2018 Dec 13;379[24]:2374-6).
“In this case, perhaps the most important member of the multidisciplinary team is the heart failure specialist, because secondary MR is a disease of heart failure and it’s important to also optimize medical therapy to reduce MR and prolong life,” he said. Treatment optimization before undertaking a transcatheter MV intervention involves not only drug treatment but also treatment with indicated devices, such as biventricular pacing, to optimize left ventricular size and function. The 2019 FDA approval of the transcatheter approach for treating MV disease specified that eligible patients had to continue to have significant MR despite receiving optimal drug and device treatment.
Treatment decisions for patients with MR must be highly individualized, and unlike transcatheter aortic valve replacement (TAVR), which occurs against a background of “really good results” for surgical aortic valve replacement, surgical treatment of MR “has never been shown to prolong life, although it can improve function,” Dr. Bonow said. In addition, open surgical repair or replacement of a faulty MV “is much better than the MitraClip for elimination a MV leak; usually with transcatheter intervention there is some residual leak. And other etiologies of MR, such as atrial fibrillation causing atrial enlargement and dilatation of the mitral annulus, generally have much better results with surgery than with a transcatheter intervention. For both primary and secondary MR, deciding when to use surgery and when to do a transcatheter intervention is very individualized,” concluded Dr. Bonow, and a fact that distinguishes transcatheter MV interventions from TAVR, which has been shown to have efficacy that’s comparable with surgical aortic valve replacement. The MitraClip system is currently the only device with U.S. marketing approval for transcatheter MV intervention, although other devices are in development.
Case volume requirements
The designation of a minimum transcatheter MV intervention case load of 20 procedures a year or 40 every 2 years reflected a “consensus among interventional cardiologists and cardiac surgeons of what the experience had to be for MV repair,” Dr. Bonow said. This number contrasts with a minimum case volume of 50 procedures/year or 100 every 2 years to maintain a TAVR program proposed in 2018 by a similar expert panel organized by U.S. cardiology and cardiac surgery societies (J Am Coll of Cardiol. 2019 Jan;73[3]:340-74). The new MV recommendations “follow a similar template [as the TAVR recommendations], but the numbers are what we thought would be best for optimal transcatheter MV expertise. MV interventions will likely increase, and we felt it would be best to define the transcatheter operators and are the right patients; the volume is unclear. There are a lot of heart failure patients, but we know from COAPT that not everyone is a candidate. The existing MV device does not fit all settings. We thought the numbers we selected were most appropriate, at least when we are starting.”
Dr. Bonow and coauthors who wrote the new recommendations will rely on payers, particularly the Centers for Medicare & Medicaid Services, to adopt the societal recommendations as part of their criteria for reimbursement and thereby give them teeth. In June 2019, CMS announced its Medicare coverage determination for TAVR, which included procedure minimums of 20 per year or 40 over 2 years for TAVR programs, a number that fell substantially below the 50 per year or 100 over 2 years that had been proposed by the societies. “We hope CMS will use our MV recommendations as a starting point,” but the final CMS coverage decision for transcatheter MV intervention could again differ from what the societies proposed, Dr. Bonow acknowledged.
In addition to strongly promoting a multidisciplinary team approach (and spelling out the members of the team) and shared decision making involvement of the patient with the team, the new recommendations also endorse participation of MV intervention programs in the Transcatheter Valve Therapy U.S. patient registry that’s maintained by two of the societies that helped organize the writing committee. The recommendations discuss the need to collect 30-day (and longer) outcomes data from transcatheter MV intervention programs through the registry as is now done for TAVR programs (N Engl J Med. 2019 Jun 27;380[26]:2541-50). Dr. Bonow declined to predict when 30-day outcomes data may start appearing for programs performing transcatheter MV interventions.
Dr. Bonow had no disclosures. The COAPT study was funded by Abbott, the company that markets the MitrClip clip delivery system.
SOURCE: Bonow RO et al. J Am Coll Cardiol. 2019 Dec 16. doi: 10.1016/j.jacc.2019.12.002.
SZC passes extension test for hyperkalemia
Treatment with sodium zirconium cyclosilicate (SZC) led to lasting improvement of hyperkalemia, according to results from an 11-month open-label extension study of the HARMONIZE randomized clinical trial.
SZC selectively binds potassium ions in the colon, reducing absorption and promoting excretion. In the original study, 248 patients with mild hyperkalemia were randomized to SZC or placebo. Within 48 hours, the drug returned potassium to normal and maintained those levels out to 4 weeks.
In the extension study, 123 patients with measured potassium levels of 3.5-6.2 mmol/L, 48 of whom had previously been assigned to placebo, received a 5- to 10-g dose of SZC once per day for up to 337 days. Median daily dose was 10 g, with a dose range of 2.5-15 g (Am J Nephrol. 2019;50[6]:473-480).
Just under 65% of patients completed the 11 months of the open-label extension study, with 88.3% of those achieving the primary endpoint of mean serum potassium levels of 5.1 mmol/L or lower, according to Simon D. Roger, MD, a nephrologist based in Gosford, Australia, and colleagues.
Most patients (83) were taking renin–angiotensin–aldosterone system inhibitors at baseline of the extension study; 78.3% continued a stable dose throughout the open-label phase, 8.4% increased the dose, and 3.6% discontinued.
Two-thirds of patients reported adverse events, most commonly gastrointestinal disorders (18.7%). Constipation was the most frequent (5.7%), followed by nausea, vomiting, and diarrhea (3.3% each).
Adverse events that occurred in 5% or more of participants included hypertension (12.2%), urinary tract infection (8.9%), and peripheral edema (8.1%). Hypertension severity was either mild (46.7%) or moderate (53.3%), and only one case was believed to be associated with the study medication. Thirteen percent of participants reported a total of 17 SMQ edema events. Eleven of the 16 patients had baseline risk factors for edema, leading the authors to conclude that causality between SZC and edema could not be established.
Serious adverse events occurred in 19.5% of participants, and 4.9% of participants discontinued SZC as a result.
SZC is approved for the treatment of hyperkalemia in the United States and Europe. The study was funded by AstraZeneca.
SOURCE: Roger S et al. Am J Nephrol;2019:50(6):473-80.
Treatment with sodium zirconium cyclosilicate (SZC) led to lasting improvement of hyperkalemia, according to results from an 11-month open-label extension study of the HARMONIZE randomized clinical trial.
SZC selectively binds potassium ions in the colon, reducing absorption and promoting excretion. In the original study, 248 patients with mild hyperkalemia were randomized to SZC or placebo. Within 48 hours, the drug returned potassium to normal and maintained those levels out to 4 weeks.
In the extension study, 123 patients with measured potassium levels of 3.5-6.2 mmol/L, 48 of whom had previously been assigned to placebo, received a 5- to 10-g dose of SZC once per day for up to 337 days. Median daily dose was 10 g, with a dose range of 2.5-15 g (Am J Nephrol. 2019;50[6]:473-480).
Just under 65% of patients completed the 11 months of the open-label extension study, with 88.3% of those achieving the primary endpoint of mean serum potassium levels of 5.1 mmol/L or lower, according to Simon D. Roger, MD, a nephrologist based in Gosford, Australia, and colleagues.
Most patients (83) were taking renin–angiotensin–aldosterone system inhibitors at baseline of the extension study; 78.3% continued a stable dose throughout the open-label phase, 8.4% increased the dose, and 3.6% discontinued.
Two-thirds of patients reported adverse events, most commonly gastrointestinal disorders (18.7%). Constipation was the most frequent (5.7%), followed by nausea, vomiting, and diarrhea (3.3% each).
Adverse events that occurred in 5% or more of participants included hypertension (12.2%), urinary tract infection (8.9%), and peripheral edema (8.1%). Hypertension severity was either mild (46.7%) or moderate (53.3%), and only one case was believed to be associated with the study medication. Thirteen percent of participants reported a total of 17 SMQ edema events. Eleven of the 16 patients had baseline risk factors for edema, leading the authors to conclude that causality between SZC and edema could not be established.
Serious adverse events occurred in 19.5% of participants, and 4.9% of participants discontinued SZC as a result.
SZC is approved for the treatment of hyperkalemia in the United States and Europe. The study was funded by AstraZeneca.
SOURCE: Roger S et al. Am J Nephrol;2019:50(6):473-80.
Treatment with sodium zirconium cyclosilicate (SZC) led to lasting improvement of hyperkalemia, according to results from an 11-month open-label extension study of the HARMONIZE randomized clinical trial.
SZC selectively binds potassium ions in the colon, reducing absorption and promoting excretion. In the original study, 248 patients with mild hyperkalemia were randomized to SZC or placebo. Within 48 hours, the drug returned potassium to normal and maintained those levels out to 4 weeks.
In the extension study, 123 patients with measured potassium levels of 3.5-6.2 mmol/L, 48 of whom had previously been assigned to placebo, received a 5- to 10-g dose of SZC once per day for up to 337 days. Median daily dose was 10 g, with a dose range of 2.5-15 g (Am J Nephrol. 2019;50[6]:473-480).
Just under 65% of patients completed the 11 months of the open-label extension study, with 88.3% of those achieving the primary endpoint of mean serum potassium levels of 5.1 mmol/L or lower, according to Simon D. Roger, MD, a nephrologist based in Gosford, Australia, and colleagues.
Most patients (83) were taking renin–angiotensin–aldosterone system inhibitors at baseline of the extension study; 78.3% continued a stable dose throughout the open-label phase, 8.4% increased the dose, and 3.6% discontinued.
Two-thirds of patients reported adverse events, most commonly gastrointestinal disorders (18.7%). Constipation was the most frequent (5.7%), followed by nausea, vomiting, and diarrhea (3.3% each).
Adverse events that occurred in 5% or more of participants included hypertension (12.2%), urinary tract infection (8.9%), and peripheral edema (8.1%). Hypertension severity was either mild (46.7%) or moderate (53.3%), and only one case was believed to be associated with the study medication. Thirteen percent of participants reported a total of 17 SMQ edema events. Eleven of the 16 patients had baseline risk factors for edema, leading the authors to conclude that causality between SZC and edema could not be established.
Serious adverse events occurred in 19.5% of participants, and 4.9% of participants discontinued SZC as a result.
SZC is approved for the treatment of hyperkalemia in the United States and Europe. The study was funded by AstraZeneca.
SOURCE: Roger S et al. Am J Nephrol;2019:50(6):473-80.
FROM AMERICAN JOURNAL OF NEPHROLOGY
Uncertain generalizability limits AFib ablation in HFrEF
Despite several reports of dramatic efficacy and reasonable safety using catheter ablation of atrial fibrillation (AFib) in patients with heart failure, many clinicians, including many heart failure specialists, remain skeptical about whether existing evidence supports using ablation routinely in selected heart failure patients.
Though concerns vary, one core stumbling block is inadequate confidence that the ablation outcomes reported from studies represent the benefit that the average American heart failure patient might expect to receive from ablation done outside of a study. A related issue is whether atrial fibrillation ablation in patients with heart failure is cost effective, especially at sites that did not participate in the published studies.
The first part of this article discussed the building evidence that radiofrequency catheter ablation of atrial fibrillation (AFib) can produce striking reductions in all-cause mortality of nearly 50%, and a greater than one-third cut in cardiovascular hospitalizations in patients with heart failure with reduced ejection fraction (HFrEF), according to one recent meta-analysis (Circ Arrhythm Electrophysiol. 2019 Sep;12[9]:e007414). A key question about the implications of these findings is their generalizability.
“Experience is an issue, and I agree that not every operator should do it. A common perception is that ablation doesn’t work, but that mindset is changing,” said Luigi Di Biase, MD, director of arrhythmia services at Montefiore Medical Center and professor of medicine at Albert Einstein College of Medicine in New York. He noted that some apparent ablations failures happen because the treatment is used too late. “Ablation will fail if it is done too late. Think about using ablation earlier,” he advised. “The earlier you ablate, the earlier you reduce the AFib burden and the sooner the patient benefits. Ablation is a cost-effective, first-line strategy for younger patients with paroxysmal AFib. The unanswered question is whether it is cost effective for patients who have both AFib and heart failure. It may be, because in addition to the mortality benefit, there are likely savings from a lower rate of hospitalizations. A clearer picture should emerge from the cost-effectiveness analysis of CASTLE-AF.”
CASTLE-AF (Catheter Ablation Versus Standard Conventional Therapy in Patients With Left Ventricular Dysfunction and Atrial Fibrillation), which randomized patients with heart failure and AFib to ablation or medical management (N Engl J Med. 2018 Feb 1;378[5]:417-27), is one of the highest-profile studies reported so far showing AFib ablation’s efficacy in patients with heart failure. However, it has drawn skepticism over its generalizability because of its long enrollment period of 8 years despite running at 33 worldwide sites, and by its winnowing of 3,013 patients assessed down to the 398 actually enrolled and 363 randomized and included in the efficacy analysis.
“CASTLE-HF showed a remarkable benefit. The problem was that it took years and years to enroll the patients,” commented Mariell Jessup, MD, a heart failure specialist and chief science and medical officer of the American Heart Association in Dallas.
At the annual congress of the European Society of Cardiology in September 2019, French researchers reported data that supported the generalizability of the CASTLE-AF findings. The study used data collected from 252,395 patients in the French national hospital-discharge database during 2010-2018 who had diagnoses of both heart failure and AFib. Among these patients, 1,384 underwent catheter ablation and the remaining 251,011 were managed without ablation.
During a median follow-up of 537 days (about 1.5 years), the incidence of both all-cause death and heart failure hospitalization were both significantly lower in the ablated patients. The ablated patients were also much younger and were more often men, but both groups had several prevalent comorbidities at roughly similar rates. To better match the groups, the French researchers ran both a multivariate analysis, and then an even more intensively adjusting propensity-score analysis that compared the ablated patients with 1,384 closely matched patients from the nonablated group. Both analyses showed substantial incremental benefit from ablation. In the propensity score–matched analysis, ablation was linked with a relative 66% cut in all-cause death, and a relative 71% reduction in heart failure hospitalizations, compared with the patients who did not undergo ablation, reported Arnaud Bisson, MD, a cardiologist at the University of Tours (France).
Another recent assessment of the generalizability of the AFib ablation trial findings used data from nearly 184,000 U.S. patients treated for AFib during 2009-2016 in an administrative database, including more than 12,000 treated with ablation. This analysis did not take into account the coexistence of heart failure. After propensity-score matching of the ablated patients with a similar subgroup of those managed medically, the results showed a 25% relative cut in the combined primary endpoint used in the CABANA (Catheter Ablation vs. Anti-Arrhythmic Drug Therapy for Atrial Fibrillation Trial) study (JAMA. 2019 Mar 15;321[134]:1261-74). Among the 74% of ablated patients who met the enrollment criteria for CABANA, the primary endpoint reduction was even greater, a 30% drop relative to matched patients who did not undergo ablation (Eur Heart J. 2019 Apr 21;40[16]:1257-64).
“Professional societies are working to clarify best practices for procedural volume, outcomes, etc.,” said Jonathan P. Piccini, MD, a cardiac electrophysiologist at Duke University, Durham, N.C., and a CABANA coinvestigator. “There are some data on ablation cost effectiveness, and they generally favor” positive cost efficacy, with more analyses now in progress,” he noted in an interview.
Many unanswered questions remain about AFib in heart failure patients and how aggressively to use ablation to treat it. Most of the data so far have come from patients with HFrEF, and so most experts consider AFib ablation in patients with heart failure with preserved ejection fraction (HFpEF) a big unknown, although nearly 80% of the heart failure patients enrolled in CABANA (the largest randomized trial of AFib ablation with more than 2,200 patients) had left ventricular ejection fraction of 50% or greater, which translates into HFpEF. Another gray area is how to think about asymptomatic (also called subclinical) AFib and whether that warrants ablation in heart failure patients. The presence or absence of symptoms is a major consideration because the traditional indication for ablation has been to reduce or eliminate symptoms like palpitations, a step that can substantially improve patients’ quality of life as well as their left ventricular function. The indication to ablate asymptomatic AFib for the purpose of improving survival and reducing hospitalizations is the new and controversial concept. Yet it has been embraced by some heart failure physicians.
“Whether or not AFib is symptomatic doesn’t matter” in a heart failure patient, said Maria Rosa Costanzo, MD, a heart failure physician at Edward Heart Hospital in Naperville, Ill. “A patient with AFib doesn’t get the atrial contribution to cardiac output. When we look deeper, a patient with ‘asymptomatic’ AFib often has symptoms, such as new fatigue or obstructive sleep apnea, so when you see a patient with asymptomatic AFib look for sleep apnea, a trigger for AFib,” Dr. Costanzo advised. “Sleep apnea, AFib, and heart failure form a triad” that often clusters in patients, and the three conditions interact in a vicious circle of reinforcing comorbidities, she said in an interview.
The cardiac electrophysiology and arrhythmia community clearly realizes that catheter ablation of AFib, in patients with or without heart failure, has many unaddressed questions about who should administer it and who should undergo it. In March 2019, the National Heart, Lung, and Blood Institute held a workshop on AFib ablation. “Numerous knowledge gaps remain” about the best way to use ablation, said a summary of the workshop (Circulation. 2019 Nov 20;doi: 10.1161/CIRCULATIONAHA.119.042706). Among the research needs highlighted by the workshop was “more definitive studies ... to delineate the impact of AFib ablation on outcomes in patients with heart failure with preserved ejection fraction.” The workshop recommended establishing a national U.S. registry for AFib ablations with a reliable source of funding, as well as “establishing the cause-effect relationship between ventricular dysfunction and AFib, and the potential moderating role of atrial structure and function.” The workshop also raised the possibility of sham-controlled assessments of AFib ablation, while conceding that enrollment into such trials would probably be very challenging.
The upshot is that, even while ablation advocates agree on the need for more study, clinicians are using AFib ablation on a growing number of heart failure patients (as well as on growing numbers of patients with AFib but without heart failure), with a focus on treating those who “have refractory symptoms or evidence of tachycardia-induced cardiomyopathy,” said Dr. Piccini. Extending that to a first-line, class I indication for heart failure patients seems to need more data, and also needs clinicians to collectively raise their comfort level with the ablation concept. If results from additional studies now underway support the dramatic efficacy and reasonable safety that’s already been seen with ablation, then increased comfort should follow.
CABANA received funding from Biosense Webster, Boston Scientific, Medtronic, and St. Jude. CASTLE-AF was funded by Biotronik. Dr. Di Biase, Dr. Jessup, and Dr. Bisson had no disclosures. Dr. Piccini has been a consultant to Allergan, Biotronik, Medtronic, Phillips, and Sanofi Aventis; he has received research funding from Abbott, ARCA, Boston Scientific, Gilead, and Johnson & Johnson; and he had a financial relationship with GlaxoSmithKline. Dr. Costanzo has been a consultant to Abbott.
This is part 2 of a 2-part story. See part 1 here.
Despite several reports of dramatic efficacy and reasonable safety using catheter ablation of atrial fibrillation (AFib) in patients with heart failure, many clinicians, including many heart failure specialists, remain skeptical about whether existing evidence supports using ablation routinely in selected heart failure patients.
Though concerns vary, one core stumbling block is inadequate confidence that the ablation outcomes reported from studies represent the benefit that the average American heart failure patient might expect to receive from ablation done outside of a study. A related issue is whether atrial fibrillation ablation in patients with heart failure is cost effective, especially at sites that did not participate in the published studies.
The first part of this article discussed the building evidence that radiofrequency catheter ablation of atrial fibrillation (AFib) can produce striking reductions in all-cause mortality of nearly 50%, and a greater than one-third cut in cardiovascular hospitalizations in patients with heart failure with reduced ejection fraction (HFrEF), according to one recent meta-analysis (Circ Arrhythm Electrophysiol. 2019 Sep;12[9]:e007414). A key question about the implications of these findings is their generalizability.
“Experience is an issue, and I agree that not every operator should do it. A common perception is that ablation doesn’t work, but that mindset is changing,” said Luigi Di Biase, MD, director of arrhythmia services at Montefiore Medical Center and professor of medicine at Albert Einstein College of Medicine in New York. He noted that some apparent ablations failures happen because the treatment is used too late. “Ablation will fail if it is done too late. Think about using ablation earlier,” he advised. “The earlier you ablate, the earlier you reduce the AFib burden and the sooner the patient benefits. Ablation is a cost-effective, first-line strategy for younger patients with paroxysmal AFib. The unanswered question is whether it is cost effective for patients who have both AFib and heart failure. It may be, because in addition to the mortality benefit, there are likely savings from a lower rate of hospitalizations. A clearer picture should emerge from the cost-effectiveness analysis of CASTLE-AF.”
CASTLE-AF (Catheter Ablation Versus Standard Conventional Therapy in Patients With Left Ventricular Dysfunction and Atrial Fibrillation), which randomized patients with heart failure and AFib to ablation or medical management (N Engl J Med. 2018 Feb 1;378[5]:417-27), is one of the highest-profile studies reported so far showing AFib ablation’s efficacy in patients with heart failure. However, it has drawn skepticism over its generalizability because of its long enrollment period of 8 years despite running at 33 worldwide sites, and by its winnowing of 3,013 patients assessed down to the 398 actually enrolled and 363 randomized and included in the efficacy analysis.
“CASTLE-HF showed a remarkable benefit. The problem was that it took years and years to enroll the patients,” commented Mariell Jessup, MD, a heart failure specialist and chief science and medical officer of the American Heart Association in Dallas.
At the annual congress of the European Society of Cardiology in September 2019, French researchers reported data that supported the generalizability of the CASTLE-AF findings. The study used data collected from 252,395 patients in the French national hospital-discharge database during 2010-2018 who had diagnoses of both heart failure and AFib. Among these patients, 1,384 underwent catheter ablation and the remaining 251,011 were managed without ablation.
During a median follow-up of 537 days (about 1.5 years), the incidence of both all-cause death and heart failure hospitalization were both significantly lower in the ablated patients. The ablated patients were also much younger and were more often men, but both groups had several prevalent comorbidities at roughly similar rates. To better match the groups, the French researchers ran both a multivariate analysis, and then an even more intensively adjusting propensity-score analysis that compared the ablated patients with 1,384 closely matched patients from the nonablated group. Both analyses showed substantial incremental benefit from ablation. In the propensity score–matched analysis, ablation was linked with a relative 66% cut in all-cause death, and a relative 71% reduction in heart failure hospitalizations, compared with the patients who did not undergo ablation, reported Arnaud Bisson, MD, a cardiologist at the University of Tours (France).
Another recent assessment of the generalizability of the AFib ablation trial findings used data from nearly 184,000 U.S. patients treated for AFib during 2009-2016 in an administrative database, including more than 12,000 treated with ablation. This analysis did not take into account the coexistence of heart failure. After propensity-score matching of the ablated patients with a similar subgroup of those managed medically, the results showed a 25% relative cut in the combined primary endpoint used in the CABANA (Catheter Ablation vs. Anti-Arrhythmic Drug Therapy for Atrial Fibrillation Trial) study (JAMA. 2019 Mar 15;321[134]:1261-74). Among the 74% of ablated patients who met the enrollment criteria for CABANA, the primary endpoint reduction was even greater, a 30% drop relative to matched patients who did not undergo ablation (Eur Heart J. 2019 Apr 21;40[16]:1257-64).
“Professional societies are working to clarify best practices for procedural volume, outcomes, etc.,” said Jonathan P. Piccini, MD, a cardiac electrophysiologist at Duke University, Durham, N.C., and a CABANA coinvestigator. “There are some data on ablation cost effectiveness, and they generally favor” positive cost efficacy, with more analyses now in progress,” he noted in an interview.
Many unanswered questions remain about AFib in heart failure patients and how aggressively to use ablation to treat it. Most of the data so far have come from patients with HFrEF, and so most experts consider AFib ablation in patients with heart failure with preserved ejection fraction (HFpEF) a big unknown, although nearly 80% of the heart failure patients enrolled in CABANA (the largest randomized trial of AFib ablation with more than 2,200 patients) had left ventricular ejection fraction of 50% or greater, which translates into HFpEF. Another gray area is how to think about asymptomatic (also called subclinical) AFib and whether that warrants ablation in heart failure patients. The presence or absence of symptoms is a major consideration because the traditional indication for ablation has been to reduce or eliminate symptoms like palpitations, a step that can substantially improve patients’ quality of life as well as their left ventricular function. The indication to ablate asymptomatic AFib for the purpose of improving survival and reducing hospitalizations is the new and controversial concept. Yet it has been embraced by some heart failure physicians.
“Whether or not AFib is symptomatic doesn’t matter” in a heart failure patient, said Maria Rosa Costanzo, MD, a heart failure physician at Edward Heart Hospital in Naperville, Ill. “A patient with AFib doesn’t get the atrial contribution to cardiac output. When we look deeper, a patient with ‘asymptomatic’ AFib often has symptoms, such as new fatigue or obstructive sleep apnea, so when you see a patient with asymptomatic AFib look for sleep apnea, a trigger for AFib,” Dr. Costanzo advised. “Sleep apnea, AFib, and heart failure form a triad” that often clusters in patients, and the three conditions interact in a vicious circle of reinforcing comorbidities, she said in an interview.
The cardiac electrophysiology and arrhythmia community clearly realizes that catheter ablation of AFib, in patients with or without heart failure, has many unaddressed questions about who should administer it and who should undergo it. In March 2019, the National Heart, Lung, and Blood Institute held a workshop on AFib ablation. “Numerous knowledge gaps remain” about the best way to use ablation, said a summary of the workshop (Circulation. 2019 Nov 20;doi: 10.1161/CIRCULATIONAHA.119.042706). Among the research needs highlighted by the workshop was “more definitive studies ... to delineate the impact of AFib ablation on outcomes in patients with heart failure with preserved ejection fraction.” The workshop recommended establishing a national U.S. registry for AFib ablations with a reliable source of funding, as well as “establishing the cause-effect relationship between ventricular dysfunction and AFib, and the potential moderating role of atrial structure and function.” The workshop also raised the possibility of sham-controlled assessments of AFib ablation, while conceding that enrollment into such trials would probably be very challenging.
The upshot is that, even while ablation advocates agree on the need for more study, clinicians are using AFib ablation on a growing number of heart failure patients (as well as on growing numbers of patients with AFib but without heart failure), with a focus on treating those who “have refractory symptoms or evidence of tachycardia-induced cardiomyopathy,” said Dr. Piccini. Extending that to a first-line, class I indication for heart failure patients seems to need more data, and also needs clinicians to collectively raise their comfort level with the ablation concept. If results from additional studies now underway support the dramatic efficacy and reasonable safety that’s already been seen with ablation, then increased comfort should follow.
CABANA received funding from Biosense Webster, Boston Scientific, Medtronic, and St. Jude. CASTLE-AF was funded by Biotronik. Dr. Di Biase, Dr. Jessup, and Dr. Bisson had no disclosures. Dr. Piccini has been a consultant to Allergan, Biotronik, Medtronic, Phillips, and Sanofi Aventis; he has received research funding from Abbott, ARCA, Boston Scientific, Gilead, and Johnson & Johnson; and he had a financial relationship with GlaxoSmithKline. Dr. Costanzo has been a consultant to Abbott.
This is part 2 of a 2-part story. See part 1 here.
Despite several reports of dramatic efficacy and reasonable safety using catheter ablation of atrial fibrillation (AFib) in patients with heart failure, many clinicians, including many heart failure specialists, remain skeptical about whether existing evidence supports using ablation routinely in selected heart failure patients.
Though concerns vary, one core stumbling block is inadequate confidence that the ablation outcomes reported from studies represent the benefit that the average American heart failure patient might expect to receive from ablation done outside of a study. A related issue is whether atrial fibrillation ablation in patients with heart failure is cost effective, especially at sites that did not participate in the published studies.
The first part of this article discussed the building evidence that radiofrequency catheter ablation of atrial fibrillation (AFib) can produce striking reductions in all-cause mortality of nearly 50%, and a greater than one-third cut in cardiovascular hospitalizations in patients with heart failure with reduced ejection fraction (HFrEF), according to one recent meta-analysis (Circ Arrhythm Electrophysiol. 2019 Sep;12[9]:e007414). A key question about the implications of these findings is their generalizability.
“Experience is an issue, and I agree that not every operator should do it. A common perception is that ablation doesn’t work, but that mindset is changing,” said Luigi Di Biase, MD, director of arrhythmia services at Montefiore Medical Center and professor of medicine at Albert Einstein College of Medicine in New York. He noted that some apparent ablations failures happen because the treatment is used too late. “Ablation will fail if it is done too late. Think about using ablation earlier,” he advised. “The earlier you ablate, the earlier you reduce the AFib burden and the sooner the patient benefits. Ablation is a cost-effective, first-line strategy for younger patients with paroxysmal AFib. The unanswered question is whether it is cost effective for patients who have both AFib and heart failure. It may be, because in addition to the mortality benefit, there are likely savings from a lower rate of hospitalizations. A clearer picture should emerge from the cost-effectiveness analysis of CASTLE-AF.”
CASTLE-AF (Catheter Ablation Versus Standard Conventional Therapy in Patients With Left Ventricular Dysfunction and Atrial Fibrillation), which randomized patients with heart failure and AFib to ablation or medical management (N Engl J Med. 2018 Feb 1;378[5]:417-27), is one of the highest-profile studies reported so far showing AFib ablation’s efficacy in patients with heart failure. However, it has drawn skepticism over its generalizability because of its long enrollment period of 8 years despite running at 33 worldwide sites, and by its winnowing of 3,013 patients assessed down to the 398 actually enrolled and 363 randomized and included in the efficacy analysis.
“CASTLE-HF showed a remarkable benefit. The problem was that it took years and years to enroll the patients,” commented Mariell Jessup, MD, a heart failure specialist and chief science and medical officer of the American Heart Association in Dallas.
At the annual congress of the European Society of Cardiology in September 2019, French researchers reported data that supported the generalizability of the CASTLE-AF findings. The study used data collected from 252,395 patients in the French national hospital-discharge database during 2010-2018 who had diagnoses of both heart failure and AFib. Among these patients, 1,384 underwent catheter ablation and the remaining 251,011 were managed without ablation.
During a median follow-up of 537 days (about 1.5 years), the incidence of both all-cause death and heart failure hospitalization were both significantly lower in the ablated patients. The ablated patients were also much younger and were more often men, but both groups had several prevalent comorbidities at roughly similar rates. To better match the groups, the French researchers ran both a multivariate analysis, and then an even more intensively adjusting propensity-score analysis that compared the ablated patients with 1,384 closely matched patients from the nonablated group. Both analyses showed substantial incremental benefit from ablation. In the propensity score–matched analysis, ablation was linked with a relative 66% cut in all-cause death, and a relative 71% reduction in heart failure hospitalizations, compared with the patients who did not undergo ablation, reported Arnaud Bisson, MD, a cardiologist at the University of Tours (France).
Another recent assessment of the generalizability of the AFib ablation trial findings used data from nearly 184,000 U.S. patients treated for AFib during 2009-2016 in an administrative database, including more than 12,000 treated with ablation. This analysis did not take into account the coexistence of heart failure. After propensity-score matching of the ablated patients with a similar subgroup of those managed medically, the results showed a 25% relative cut in the combined primary endpoint used in the CABANA (Catheter Ablation vs. Anti-Arrhythmic Drug Therapy for Atrial Fibrillation Trial) study (JAMA. 2019 Mar 15;321[134]:1261-74). Among the 74% of ablated patients who met the enrollment criteria for CABANA, the primary endpoint reduction was even greater, a 30% drop relative to matched patients who did not undergo ablation (Eur Heart J. 2019 Apr 21;40[16]:1257-64).
“Professional societies are working to clarify best practices for procedural volume, outcomes, etc.,” said Jonathan P. Piccini, MD, a cardiac electrophysiologist at Duke University, Durham, N.C., and a CABANA coinvestigator. “There are some data on ablation cost effectiveness, and they generally favor” positive cost efficacy, with more analyses now in progress,” he noted in an interview.
Many unanswered questions remain about AFib in heart failure patients and how aggressively to use ablation to treat it. Most of the data so far have come from patients with HFrEF, and so most experts consider AFib ablation in patients with heart failure with preserved ejection fraction (HFpEF) a big unknown, although nearly 80% of the heart failure patients enrolled in CABANA (the largest randomized trial of AFib ablation with more than 2,200 patients) had left ventricular ejection fraction of 50% or greater, which translates into HFpEF. Another gray area is how to think about asymptomatic (also called subclinical) AFib and whether that warrants ablation in heart failure patients. The presence or absence of symptoms is a major consideration because the traditional indication for ablation has been to reduce or eliminate symptoms like palpitations, a step that can substantially improve patients’ quality of life as well as their left ventricular function. The indication to ablate asymptomatic AFib for the purpose of improving survival and reducing hospitalizations is the new and controversial concept. Yet it has been embraced by some heart failure physicians.
“Whether or not AFib is symptomatic doesn’t matter” in a heart failure patient, said Maria Rosa Costanzo, MD, a heart failure physician at Edward Heart Hospital in Naperville, Ill. “A patient with AFib doesn’t get the atrial contribution to cardiac output. When we look deeper, a patient with ‘asymptomatic’ AFib often has symptoms, such as new fatigue or obstructive sleep apnea, so when you see a patient with asymptomatic AFib look for sleep apnea, a trigger for AFib,” Dr. Costanzo advised. “Sleep apnea, AFib, and heart failure form a triad” that often clusters in patients, and the three conditions interact in a vicious circle of reinforcing comorbidities, she said in an interview.
The cardiac electrophysiology and arrhythmia community clearly realizes that catheter ablation of AFib, in patients with or without heart failure, has many unaddressed questions about who should administer it and who should undergo it. In March 2019, the National Heart, Lung, and Blood Institute held a workshop on AFib ablation. “Numerous knowledge gaps remain” about the best way to use ablation, said a summary of the workshop (Circulation. 2019 Nov 20;doi: 10.1161/CIRCULATIONAHA.119.042706). Among the research needs highlighted by the workshop was “more definitive studies ... to delineate the impact of AFib ablation on outcomes in patients with heart failure with preserved ejection fraction.” The workshop recommended establishing a national U.S. registry for AFib ablations with a reliable source of funding, as well as “establishing the cause-effect relationship between ventricular dysfunction and AFib, and the potential moderating role of atrial structure and function.” The workshop also raised the possibility of sham-controlled assessments of AFib ablation, while conceding that enrollment into such trials would probably be very challenging.
The upshot is that, even while ablation advocates agree on the need for more study, clinicians are using AFib ablation on a growing number of heart failure patients (as well as on growing numbers of patients with AFib but without heart failure), with a focus on treating those who “have refractory symptoms or evidence of tachycardia-induced cardiomyopathy,” said Dr. Piccini. Extending that to a first-line, class I indication for heart failure patients seems to need more data, and also needs clinicians to collectively raise their comfort level with the ablation concept. If results from additional studies now underway support the dramatic efficacy and reasonable safety that’s already been seen with ablation, then increased comfort should follow.
CABANA received funding from Biosense Webster, Boston Scientific, Medtronic, and St. Jude. CASTLE-AF was funded by Biotronik. Dr. Di Biase, Dr. Jessup, and Dr. Bisson had no disclosures. Dr. Piccini has been a consultant to Allergan, Biotronik, Medtronic, Phillips, and Sanofi Aventis; he has received research funding from Abbott, ARCA, Boston Scientific, Gilead, and Johnson & Johnson; and he had a financial relationship with GlaxoSmithKline. Dr. Costanzo has been a consultant to Abbott.
This is part 2 of a 2-part story. See part 1 here.
New heart failure trial data presage guideline revisions
PHILADELPHIA – The definition and treatment of heart failure with reduced ejection fraction should change based on recent findings and analyses from major trials, said a key heart failure leader at the American Heart Association scientific sessions.
The people charged with writing U.S. guidelines for heart failure management already have enough evidence to change the recommended way of using sacubitril/valsartan (Entresto) in patients with heart failure with reduced ejection fraction (HFrEF), said Clyde W. Yancy, MD, professor of medicine and chief of cardiology at Northwestern University, Chicago. Accumulated evidence from studies and more than 5 years of experience in routine practice with the angiotensin receptor neprilysin inhibitor (ARNI) combination sacubitril/valsartan for treating HFrEF patients justifies striking the existing recommendation to first start patients on an ACE inhibitor or angiotensin receptor blocker and only after that switching to sacubitril/valsartan, a sequence that has rankled some clinicians as an unnecessary delay and barrier to starting patients on the ARNI regimen.
U.S. guidelines should now suggest that ARNI treatment start immediately, suggested Dr. Yancy, who chaired the AHA/American College of Cardiology panel that updated U.S. guidelines for heart failure management in 2013 (Circulation. 2013 Oct 15;128[16]:e240-327), 2016 (J Am Coll Cardiol. 2016 Sep;68[13]:1476-88), and 2017 (Circulation. 2017 Aug 8; 136[6]:e137-61).
Expanding the heart failure group for sacubitril/valsartan
Dr. Yancy also proposed a second major and immediate change to the existing heart failure guideline based on a new appreciation of a heart failure population that could benefit from ARNI treatment: patients with “mid-range” heart failure, defined by a left ventricular ejection fraction (LVEF) of 41%-49% that places them between patients with HFrEF with an ejection fraction of 40% or less, and those with heart failure with preserved ejection fraction (HFpEF) of 50% or more. As yet unchanged in the 2013 AHA/ACC heart failure guideline is the proposition that patients with heart failure and an ejection fraction of 41%-49% have “borderline” heart failure with characteristics, treatment patterns, and outcomes “similar to patients with HFpEF.”
That premise should now go out the window, urged Dr. Yancy, based on a new analysis of data collected from both the recent PARAGON-HF trial of sacubitril/valsartan in patients with HFpEF and ejection fractions of 45% or higher (N Engl J Med. 2019 Oct 24;381[17]:1609-20) and the landmark PARADIGM-HF trial that established sacubitril/valsartan as a treatment for patients with HFrEF (N Engl J Med. 2014 Sep 11;371[11]:993-1004). A combined analysis of the more than 13,000 total patients in both studies suggested that “patients with ejection fraction lower than normal, which includes those with so-called heart failure with mid-range ejection fraction or borderline ejection fraction, would likely benefit from sacubitril/valsartan, compared with RAS inhibition,” concluded the authors of the new analysis (Circulation. 2019 Nov 17. doi: 10.1161/CIRCULATIONAHA.119.044586).
Dr. Yancy argued that, based on this new analysis, a further revision to the 2013 guideline should say that patients with heart failure with a LVEF of 41%-49% have characteristics, treatment responses, and outcomes that “appear similar to those of patient with HFrEF,” a sharp departure from the existing text that lumps these patients with the HFpEF subgroup. “There appears to be a signal that extends the benefit of ARNI to patients with ejection fractions above the current threshold for HFrEF but below what is typically HFpEF,” he said.
Bringing SGLT2 inhibitors into heart failure management
Dr. Yancy also cited recently reported data from another landmark trial, DAPA-HF (Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure), as an impetus for both another immediate change to the guideline and for a potential second change pending a report of confirmatory evidence that may arrive in 2020.
The DAPA-HF results showed that the sodium-glucose cotransporter 2 (SGLT2) inhibitor dapagliflozin (Farxiga) was just as effective for preventing all-cause death and heart failure hospitalizations and urgent visits in patients without type 2 diabetes as it is in patients with type 2 diabetes (N Engl J Med. 2019 Nov 21;381[21]:1995-2008), a remarkable finding for an agent that came onto the U.S. market as a diabetes drug specifically aimed at reducing levels of glycosylated hemoglobin.
Dr. Yancy proposed an immediate guideline change to acknowledge the proven protection against incident heart failure that treatment with a SGLT2 inhibitor gives patients with type 2 diabetes. There is now “a strong opportunity to use an SGLT2 inhibitor in patients with type 2 diabetes to reduce the incidence of heart failure,” he said.
And he added that, if results from EMPEROR REDUCED (Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Reduced Ejection Fraction), studying the SGLT2 inhibitor empagliflozin (Jardiance) in HFrEF patients with and without type 2 diabetes, can confirm the efficacy of a second drug from this class in preventing heart failure events in patients with HFrEF but without diabetes, then the time will have arrived for another guideline change to establish the SGLT2 inhibitors as a new “foundational” drug for the management of all HFrEF patients, regardless of their level of glycemic control. The SGLT2 inhibitors are a particularly attractive additional drug because they are taken once daily orally with no need for dosage adjustment, so far they have shown excellent safety in patients without diabetes with no episodes of hypoglycemia or ketoacidosis, and they have even shown evidence for heart failure benefit in patients older than 75 years, Dr. Yancy noted.
Dr. Yancy had no relevant disclosures.
PHILADELPHIA – The definition and treatment of heart failure with reduced ejection fraction should change based on recent findings and analyses from major trials, said a key heart failure leader at the American Heart Association scientific sessions.
The people charged with writing U.S. guidelines for heart failure management already have enough evidence to change the recommended way of using sacubitril/valsartan (Entresto) in patients with heart failure with reduced ejection fraction (HFrEF), said Clyde W. Yancy, MD, professor of medicine and chief of cardiology at Northwestern University, Chicago. Accumulated evidence from studies and more than 5 years of experience in routine practice with the angiotensin receptor neprilysin inhibitor (ARNI) combination sacubitril/valsartan for treating HFrEF patients justifies striking the existing recommendation to first start patients on an ACE inhibitor or angiotensin receptor blocker and only after that switching to sacubitril/valsartan, a sequence that has rankled some clinicians as an unnecessary delay and barrier to starting patients on the ARNI regimen.
U.S. guidelines should now suggest that ARNI treatment start immediately, suggested Dr. Yancy, who chaired the AHA/American College of Cardiology panel that updated U.S. guidelines for heart failure management in 2013 (Circulation. 2013 Oct 15;128[16]:e240-327), 2016 (J Am Coll Cardiol. 2016 Sep;68[13]:1476-88), and 2017 (Circulation. 2017 Aug 8; 136[6]:e137-61).
Expanding the heart failure group for sacubitril/valsartan
Dr. Yancy also proposed a second major and immediate change to the existing heart failure guideline based on a new appreciation of a heart failure population that could benefit from ARNI treatment: patients with “mid-range” heart failure, defined by a left ventricular ejection fraction (LVEF) of 41%-49% that places them between patients with HFrEF with an ejection fraction of 40% or less, and those with heart failure with preserved ejection fraction (HFpEF) of 50% or more. As yet unchanged in the 2013 AHA/ACC heart failure guideline is the proposition that patients with heart failure and an ejection fraction of 41%-49% have “borderline” heart failure with characteristics, treatment patterns, and outcomes “similar to patients with HFpEF.”
That premise should now go out the window, urged Dr. Yancy, based on a new analysis of data collected from both the recent PARAGON-HF trial of sacubitril/valsartan in patients with HFpEF and ejection fractions of 45% or higher (N Engl J Med. 2019 Oct 24;381[17]:1609-20) and the landmark PARADIGM-HF trial that established sacubitril/valsartan as a treatment for patients with HFrEF (N Engl J Med. 2014 Sep 11;371[11]:993-1004). A combined analysis of the more than 13,000 total patients in both studies suggested that “patients with ejection fraction lower than normal, which includes those with so-called heart failure with mid-range ejection fraction or borderline ejection fraction, would likely benefit from sacubitril/valsartan, compared with RAS inhibition,” concluded the authors of the new analysis (Circulation. 2019 Nov 17. doi: 10.1161/CIRCULATIONAHA.119.044586).
Dr. Yancy argued that, based on this new analysis, a further revision to the 2013 guideline should say that patients with heart failure with a LVEF of 41%-49% have characteristics, treatment responses, and outcomes that “appear similar to those of patient with HFrEF,” a sharp departure from the existing text that lumps these patients with the HFpEF subgroup. “There appears to be a signal that extends the benefit of ARNI to patients with ejection fractions above the current threshold for HFrEF but below what is typically HFpEF,” he said.
Bringing SGLT2 inhibitors into heart failure management
Dr. Yancy also cited recently reported data from another landmark trial, DAPA-HF (Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure), as an impetus for both another immediate change to the guideline and for a potential second change pending a report of confirmatory evidence that may arrive in 2020.
The DAPA-HF results showed that the sodium-glucose cotransporter 2 (SGLT2) inhibitor dapagliflozin (Farxiga) was just as effective for preventing all-cause death and heart failure hospitalizations and urgent visits in patients without type 2 diabetes as it is in patients with type 2 diabetes (N Engl J Med. 2019 Nov 21;381[21]:1995-2008), a remarkable finding for an agent that came onto the U.S. market as a diabetes drug specifically aimed at reducing levels of glycosylated hemoglobin.
Dr. Yancy proposed an immediate guideline change to acknowledge the proven protection against incident heart failure that treatment with a SGLT2 inhibitor gives patients with type 2 diabetes. There is now “a strong opportunity to use an SGLT2 inhibitor in patients with type 2 diabetes to reduce the incidence of heart failure,” he said.
And he added that, if results from EMPEROR REDUCED (Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Reduced Ejection Fraction), studying the SGLT2 inhibitor empagliflozin (Jardiance) in HFrEF patients with and without type 2 diabetes, can confirm the efficacy of a second drug from this class in preventing heart failure events in patients with HFrEF but without diabetes, then the time will have arrived for another guideline change to establish the SGLT2 inhibitors as a new “foundational” drug for the management of all HFrEF patients, regardless of their level of glycemic control. The SGLT2 inhibitors are a particularly attractive additional drug because they are taken once daily orally with no need for dosage adjustment, so far they have shown excellent safety in patients without diabetes with no episodes of hypoglycemia or ketoacidosis, and they have even shown evidence for heart failure benefit in patients older than 75 years, Dr. Yancy noted.
Dr. Yancy had no relevant disclosures.
PHILADELPHIA – The definition and treatment of heart failure with reduced ejection fraction should change based on recent findings and analyses from major trials, said a key heart failure leader at the American Heart Association scientific sessions.
The people charged with writing U.S. guidelines for heart failure management already have enough evidence to change the recommended way of using sacubitril/valsartan (Entresto) in patients with heart failure with reduced ejection fraction (HFrEF), said Clyde W. Yancy, MD, professor of medicine and chief of cardiology at Northwestern University, Chicago. Accumulated evidence from studies and more than 5 years of experience in routine practice with the angiotensin receptor neprilysin inhibitor (ARNI) combination sacubitril/valsartan for treating HFrEF patients justifies striking the existing recommendation to first start patients on an ACE inhibitor or angiotensin receptor blocker and only after that switching to sacubitril/valsartan, a sequence that has rankled some clinicians as an unnecessary delay and barrier to starting patients on the ARNI regimen.
U.S. guidelines should now suggest that ARNI treatment start immediately, suggested Dr. Yancy, who chaired the AHA/American College of Cardiology panel that updated U.S. guidelines for heart failure management in 2013 (Circulation. 2013 Oct 15;128[16]:e240-327), 2016 (J Am Coll Cardiol. 2016 Sep;68[13]:1476-88), and 2017 (Circulation. 2017 Aug 8; 136[6]:e137-61).
Expanding the heart failure group for sacubitril/valsartan
Dr. Yancy also proposed a second major and immediate change to the existing heart failure guideline based on a new appreciation of a heart failure population that could benefit from ARNI treatment: patients with “mid-range” heart failure, defined by a left ventricular ejection fraction (LVEF) of 41%-49% that places them between patients with HFrEF with an ejection fraction of 40% or less, and those with heart failure with preserved ejection fraction (HFpEF) of 50% or more. As yet unchanged in the 2013 AHA/ACC heart failure guideline is the proposition that patients with heart failure and an ejection fraction of 41%-49% have “borderline” heart failure with characteristics, treatment patterns, and outcomes “similar to patients with HFpEF.”
That premise should now go out the window, urged Dr. Yancy, based on a new analysis of data collected from both the recent PARAGON-HF trial of sacubitril/valsartan in patients with HFpEF and ejection fractions of 45% or higher (N Engl J Med. 2019 Oct 24;381[17]:1609-20) and the landmark PARADIGM-HF trial that established sacubitril/valsartan as a treatment for patients with HFrEF (N Engl J Med. 2014 Sep 11;371[11]:993-1004). A combined analysis of the more than 13,000 total patients in both studies suggested that “patients with ejection fraction lower than normal, which includes those with so-called heart failure with mid-range ejection fraction or borderline ejection fraction, would likely benefit from sacubitril/valsartan, compared with RAS inhibition,” concluded the authors of the new analysis (Circulation. 2019 Nov 17. doi: 10.1161/CIRCULATIONAHA.119.044586).
Dr. Yancy argued that, based on this new analysis, a further revision to the 2013 guideline should say that patients with heart failure with a LVEF of 41%-49% have characteristics, treatment responses, and outcomes that “appear similar to those of patient with HFrEF,” a sharp departure from the existing text that lumps these patients with the HFpEF subgroup. “There appears to be a signal that extends the benefit of ARNI to patients with ejection fractions above the current threshold for HFrEF but below what is typically HFpEF,” he said.
Bringing SGLT2 inhibitors into heart failure management
Dr. Yancy also cited recently reported data from another landmark trial, DAPA-HF (Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure), as an impetus for both another immediate change to the guideline and for a potential second change pending a report of confirmatory evidence that may arrive in 2020.
The DAPA-HF results showed that the sodium-glucose cotransporter 2 (SGLT2) inhibitor dapagliflozin (Farxiga) was just as effective for preventing all-cause death and heart failure hospitalizations and urgent visits in patients without type 2 diabetes as it is in patients with type 2 diabetes (N Engl J Med. 2019 Nov 21;381[21]:1995-2008), a remarkable finding for an agent that came onto the U.S. market as a diabetes drug specifically aimed at reducing levels of glycosylated hemoglobin.
Dr. Yancy proposed an immediate guideline change to acknowledge the proven protection against incident heart failure that treatment with a SGLT2 inhibitor gives patients with type 2 diabetes. There is now “a strong opportunity to use an SGLT2 inhibitor in patients with type 2 diabetes to reduce the incidence of heart failure,” he said.
And he added that, if results from EMPEROR REDUCED (Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Reduced Ejection Fraction), studying the SGLT2 inhibitor empagliflozin (Jardiance) in HFrEF patients with and without type 2 diabetes, can confirm the efficacy of a second drug from this class in preventing heart failure events in patients with HFrEF but without diabetes, then the time will have arrived for another guideline change to establish the SGLT2 inhibitors as a new “foundational” drug for the management of all HFrEF patients, regardless of their level of glycemic control. The SGLT2 inhibitors are a particularly attractive additional drug because they are taken once daily orally with no need for dosage adjustment, so far they have shown excellent safety in patients without diabetes with no episodes of hypoglycemia or ketoacidosis, and they have even shown evidence for heart failure benefit in patients older than 75 years, Dr. Yancy noted.
Dr. Yancy had no relevant disclosures.
EXPERT ANALYSIS FROM AHA 2019