User login
Rhythm control may be best for atrial fib in HFpEF
CHICAGO – Atrial fibrillation with good heart rate control in patients who have heart failure with preserved ejection fraction is independently associated with exercise intolerance, impaired contractile reserve, and a sharply higher mortality rate than in matched HFpEF patients without the arrhythmia, a retrospective analysis showed.
“Our study, the largest of its kind, provides mechanistic evidence from cardiopulmonary testing that a rhythm control strategy may potentially improve peak exercise capacity and survival in this patient population, a finding that of course requires future prospective appraisal in randomized trials comparing rate and rhythm control of atrial fibrillation in HFpEF,” Dr. Mohamed Badreldin Elshazly reported at the annual meeting of the American College of Cardiology.
In the meantime, his study also shows the useful role cardiopulmonary stress testing can play in the setting of atrial fibrillation (AF) in HFpEF, he added.
“Cardiopulmonary stress tests are cheap and easy to do. They’re a big asset for personalized medicine. Using an objective measure like cardiopulmonary stress testing to define the physiologic and hemodynamic consequences of atrial fibrillation in individual patients may help identify those in whom rhythm control may improve exercise tolerance and quality of life, and those who may be okay with rate control,” according to Dr. Elshazly of the Cleveland Clinic.
He noted that while it’s well established that atrial fibrillation is associated with exercise intolerance in patients with heart failure with reduced ejection fraction (HFrEF) and that restoration of sinus rhythm in such patients has a positive impact on exercise hemodynamics, symptom severity, and quality of life, the situation is murkier regarding AF in patients with HFpEF. Prior studies were generally small and unable to establish whether AF was independently associated with exercise intolerance or if HFpEF patients who developed AF were sicker and higher risk.
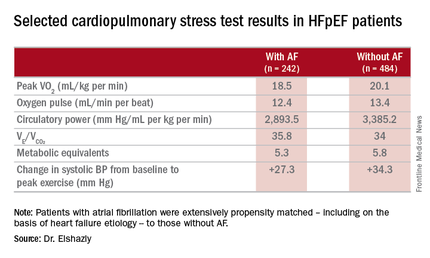
He presented a retrospective, case-control study in a cohort of 1,825 patients with HFpEF referred for maximal, symptom-limited cardiopulmonary stress testing at the Cleveland Clinic. Among these were 242 patients with AF. They were extensively propensity matched – including on the basis of heart failure etiology – to 484 HFpEF patients without AF.
“That’s what makes our study strong. We were the first to be able to do propensity matching and therefore account for other risk factors in our analysis,” Dr. Elshazly explained.
The investigators measured peak oxygen uptake (VO2), the minute ventilation–carbon dioxide production relationship (VE/VCO2) as an indicator of ventilatory efficiency, metabolic equivalents (METS), ventilatory anaerobic threshold, circulatory power as a proxy for cardiac power, peak oxygen pulse as a surrogate for stroke volume, and resting and peak heart rate and systolic blood pressure. The patients with AF were in fibrillation at the time of their cardiopulmonary stress testing.
The HFpEF patients with AF had a mean resting heart rate of 70 beats per minute and a peak rate of 130 bpm. This group showed evidence of impaired peak exercise tolerance as reflected in lower peak VO2, oxygen pulse, and circulatory power at peak exercise. Their VE/VCO2 was higher, indicating impaired ventilatory efficiency. Notably, however, their submaximal exercise capacity was similar to the non-AF controls.
“Atrial fibrillation in these patients is really more of a disease that shows itself in patients when you take them to their peak exercise capacity,” he observed.
All-cause mortality was significantly higher in the AF as compared with no-AF patients with HFpEF. The mortality curves separated early and the divergence grew larger over the course of 8 years of follow-up.
One audience member pointed out that the large mortality difference between the two groups seems disproportionate to the rather modest differences in exercise capacity.
“It brings up an interesting point,” Dr. Elshazly replied. “Maybe the increase in total mortality that we see is being driven by other things besides cardiovascular mortality. Our data doesn’t capture the specific cause of death, be it cancer, for example, but it does raise the idea that this mortality difference is not all driven by cardiovascular mortality, but by atrial fibrillation.”
Dr. Elshazly reported having no financial conflicts of interest regarding his institutionally supported study.
CHICAGO – Atrial fibrillation with good heart rate control in patients who have heart failure with preserved ejection fraction is independently associated with exercise intolerance, impaired contractile reserve, and a sharply higher mortality rate than in matched HFpEF patients without the arrhythmia, a retrospective analysis showed.
“Our study, the largest of its kind, provides mechanistic evidence from cardiopulmonary testing that a rhythm control strategy may potentially improve peak exercise capacity and survival in this patient population, a finding that of course requires future prospective appraisal in randomized trials comparing rate and rhythm control of atrial fibrillation in HFpEF,” Dr. Mohamed Badreldin Elshazly reported at the annual meeting of the American College of Cardiology.
In the meantime, his study also shows the useful role cardiopulmonary stress testing can play in the setting of atrial fibrillation (AF) in HFpEF, he added.
“Cardiopulmonary stress tests are cheap and easy to do. They’re a big asset for personalized medicine. Using an objective measure like cardiopulmonary stress testing to define the physiologic and hemodynamic consequences of atrial fibrillation in individual patients may help identify those in whom rhythm control may improve exercise tolerance and quality of life, and those who may be okay with rate control,” according to Dr. Elshazly of the Cleveland Clinic.
He noted that while it’s well established that atrial fibrillation is associated with exercise intolerance in patients with heart failure with reduced ejection fraction (HFrEF) and that restoration of sinus rhythm in such patients has a positive impact on exercise hemodynamics, symptom severity, and quality of life, the situation is murkier regarding AF in patients with HFpEF. Prior studies were generally small and unable to establish whether AF was independently associated with exercise intolerance or if HFpEF patients who developed AF were sicker and higher risk.
He presented a retrospective, case-control study in a cohort of 1,825 patients with HFpEF referred for maximal, symptom-limited cardiopulmonary stress testing at the Cleveland Clinic. Among these were 242 patients with AF. They were extensively propensity matched – including on the basis of heart failure etiology – to 484 HFpEF patients without AF.
“That’s what makes our study strong. We were the first to be able to do propensity matching and therefore account for other risk factors in our analysis,” Dr. Elshazly explained.
The investigators measured peak oxygen uptake (VO2), the minute ventilation–carbon dioxide production relationship (VE/VCO2) as an indicator of ventilatory efficiency, metabolic equivalents (METS), ventilatory anaerobic threshold, circulatory power as a proxy for cardiac power, peak oxygen pulse as a surrogate for stroke volume, and resting and peak heart rate and systolic blood pressure. The patients with AF were in fibrillation at the time of their cardiopulmonary stress testing.
The HFpEF patients with AF had a mean resting heart rate of 70 beats per minute and a peak rate of 130 bpm. This group showed evidence of impaired peak exercise tolerance as reflected in lower peak VO2, oxygen pulse, and circulatory power at peak exercise. Their VE/VCO2 was higher, indicating impaired ventilatory efficiency. Notably, however, their submaximal exercise capacity was similar to the non-AF controls.

“Atrial fibrillation in these patients is really more of a disease that shows itself in patients when you take them to their peak exercise capacity,” he observed.
All-cause mortality was significantly higher in the AF as compared with no-AF patients with HFpEF. The mortality curves separated early and the divergence grew larger over the course of 8 years of follow-up.
One audience member pointed out that the large mortality difference between the two groups seems disproportionate to the rather modest differences in exercise capacity.
“It brings up an interesting point,” Dr. Elshazly replied. “Maybe the increase in total mortality that we see is being driven by other things besides cardiovascular mortality. Our data doesn’t capture the specific cause of death, be it cancer, for example, but it does raise the idea that this mortality difference is not all driven by cardiovascular mortality, but by atrial fibrillation.”
Dr. Elshazly reported having no financial conflicts of interest regarding his institutionally supported study.
CHICAGO – Atrial fibrillation with good heart rate control in patients who have heart failure with preserved ejection fraction is independently associated with exercise intolerance, impaired contractile reserve, and a sharply higher mortality rate than in matched HFpEF patients without the arrhythmia, a retrospective analysis showed.
“Our study, the largest of its kind, provides mechanistic evidence from cardiopulmonary testing that a rhythm control strategy may potentially improve peak exercise capacity and survival in this patient population, a finding that of course requires future prospective appraisal in randomized trials comparing rate and rhythm control of atrial fibrillation in HFpEF,” Dr. Mohamed Badreldin Elshazly reported at the annual meeting of the American College of Cardiology.
In the meantime, his study also shows the useful role cardiopulmonary stress testing can play in the setting of atrial fibrillation (AF) in HFpEF, he added.
“Cardiopulmonary stress tests are cheap and easy to do. They’re a big asset for personalized medicine. Using an objective measure like cardiopulmonary stress testing to define the physiologic and hemodynamic consequences of atrial fibrillation in individual patients may help identify those in whom rhythm control may improve exercise tolerance and quality of life, and those who may be okay with rate control,” according to Dr. Elshazly of the Cleveland Clinic.
He noted that while it’s well established that atrial fibrillation is associated with exercise intolerance in patients with heart failure with reduced ejection fraction (HFrEF) and that restoration of sinus rhythm in such patients has a positive impact on exercise hemodynamics, symptom severity, and quality of life, the situation is murkier regarding AF in patients with HFpEF. Prior studies were generally small and unable to establish whether AF was independently associated with exercise intolerance or if HFpEF patients who developed AF were sicker and higher risk.
He presented a retrospective, case-control study in a cohort of 1,825 patients with HFpEF referred for maximal, symptom-limited cardiopulmonary stress testing at the Cleveland Clinic. Among these were 242 patients with AF. They were extensively propensity matched – including on the basis of heart failure etiology – to 484 HFpEF patients without AF.
“That’s what makes our study strong. We were the first to be able to do propensity matching and therefore account for other risk factors in our analysis,” Dr. Elshazly explained.
The investigators measured peak oxygen uptake (VO2), the minute ventilation–carbon dioxide production relationship (VE/VCO2) as an indicator of ventilatory efficiency, metabolic equivalents (METS), ventilatory anaerobic threshold, circulatory power as a proxy for cardiac power, peak oxygen pulse as a surrogate for stroke volume, and resting and peak heart rate and systolic blood pressure. The patients with AF were in fibrillation at the time of their cardiopulmonary stress testing.
The HFpEF patients with AF had a mean resting heart rate of 70 beats per minute and a peak rate of 130 bpm. This group showed evidence of impaired peak exercise tolerance as reflected in lower peak VO2, oxygen pulse, and circulatory power at peak exercise. Their VE/VCO2 was higher, indicating impaired ventilatory efficiency. Notably, however, their submaximal exercise capacity was similar to the non-AF controls.

“Atrial fibrillation in these patients is really more of a disease that shows itself in patients when you take them to their peak exercise capacity,” he observed.
All-cause mortality was significantly higher in the AF as compared with no-AF patients with HFpEF. The mortality curves separated early and the divergence grew larger over the course of 8 years of follow-up.
One audience member pointed out that the large mortality difference between the two groups seems disproportionate to the rather modest differences in exercise capacity.
“It brings up an interesting point,” Dr. Elshazly replied. “Maybe the increase in total mortality that we see is being driven by other things besides cardiovascular mortality. Our data doesn’t capture the specific cause of death, be it cancer, for example, but it does raise the idea that this mortality difference is not all driven by cardiovascular mortality, but by atrial fibrillation.”
Dr. Elshazly reported having no financial conflicts of interest regarding his institutionally supported study.
AT ACC 16
Key clinical point: Atrial fibrillation in patients with heart failure with preserved ejection fraction is associated with exercise intolerance and increased mortality.
Major finding: Mean peak VO2 was 18.5 mL/kg per minute in patients with HFpEF and atrial fibrillation, significantly less than the 20.1 mL/kg per minute in controls.
Data source: A retrospective, single-institution study of cardiopulmonary stress test findings and 8-year mortality in 242 patients with HFpEF and atrial fibrillation and 484 propensity-matched controls with HFpEF and no arrhythmia.
Disclosures: The presenter reported having no financial conflicts of interest regarding his institutionally supported study.
Prompt antidepressant treatment swiftly chops cardiovascular risk
CHICAGO – Prompt, effective treatment for depression in the primary care setting appears to swiftly reduce the elevated cardiovascular risk known to be tied to the mood disorder, Heidi Thomas May, Ph.D., reported at the annual meeting of the American College of Cardiology.
“We know that depression is a risk factor for long-term adverse cardiovascular outcomes. Our study shows that it can also have immediate effects on someone’s cardiovascular health. I think our study highlights the importance of screening for depression in the primary care setting – and if someone’s depressed, they need to be treated,” said Dr. May, a cardiovascular and genetic epidemiologist at Intermountain Medical Center in Murray, Utah.
She presented an observational study of the electronic medical records of 7,559 Intermountain Healthcare patients over age 40 years who completed the Patient Health Questionnaire-9 (PHQ-9) depression screening tool during a visit to an Intermountain primary care clinic for any reason. They completed another PHQ-9 a median of 2.7 years later. Under the Intermountain system, a PHQ-9 score of 10 or more triggers implementation of a depression treatment pathway, the specifics of which vary depending upon the severity of symptoms.
On the basis of their two PHQ-9 scores, all patients were classified into one of four groups: The “nondepressed” group of 3,286 patients had a score of 9 or less on both occasions; the “remained depressed” cohort of 1,987 patients scored 10 or more on both PHQ-9s; the “no longer depressed” group of 1,542 patients scored at least 10 but subsequently improved by at least 5 points to a score of 9 or less; and the 735 patients in the “became depressed” group first scored 9 or less on the PHQ-9 but subsequently had at least a 5-point increase to a score of 10 or more.
The subjects were then followed for major adverse cardiovascular events, or MACE – defined as a composite of death, diagnosis of coronary artery disease, acute MI, stroke, and heart failure hospitalization – for a median of 208 days after completing their second PHQ-9.
The MACE rate was 4.8% in the nondepressed group and similar at 4.6% in the “no longer depressed” group, Dr. May reported. Both groups fared significantly better than the “remained depressed” and “became depressed” groups, which had MACE rates of 6% and 6.4%, respectively.
In a multivariate regression analysis adjusted for demographics, cardiovascular risk factors, prior disease diagnoses, medications, and other potential confounders, the “remained depressed” group was 33% more likely to experience a cardiovascular event than was the nondepressed group, she said. The “became depressed” group had a 44% increase in risk, compared with the nondepressed individuals. In contrast, the MACE risk in patients in the “no longer depressed” group was not significantly different from that of patients who weren’t depressed at either time point. And the MACE risk of patients who became depressed during the course of the study was no different from that of patients who remained depressed at both time points.
This is the first study of its kind, Dr. May said. Hence, the results require confirmation, ideally in a randomized clinical trial.
She reported having no financial conflicts regarding the study, which was supported by Intermountain Healthcare.
CHICAGO – Prompt, effective treatment for depression in the primary care setting appears to swiftly reduce the elevated cardiovascular risk known to be tied to the mood disorder, Heidi Thomas May, Ph.D., reported at the annual meeting of the American College of Cardiology.
“We know that depression is a risk factor for long-term adverse cardiovascular outcomes. Our study shows that it can also have immediate effects on someone’s cardiovascular health. I think our study highlights the importance of screening for depression in the primary care setting – and if someone’s depressed, they need to be treated,” said Dr. May, a cardiovascular and genetic epidemiologist at Intermountain Medical Center in Murray, Utah.
She presented an observational study of the electronic medical records of 7,559 Intermountain Healthcare patients over age 40 years who completed the Patient Health Questionnaire-9 (PHQ-9) depression screening tool during a visit to an Intermountain primary care clinic for any reason. They completed another PHQ-9 a median of 2.7 years later. Under the Intermountain system, a PHQ-9 score of 10 or more triggers implementation of a depression treatment pathway, the specifics of which vary depending upon the severity of symptoms.
On the basis of their two PHQ-9 scores, all patients were classified into one of four groups: The “nondepressed” group of 3,286 patients had a score of 9 or less on both occasions; the “remained depressed” cohort of 1,987 patients scored 10 or more on both PHQ-9s; the “no longer depressed” group of 1,542 patients scored at least 10 but subsequently improved by at least 5 points to a score of 9 or less; and the 735 patients in the “became depressed” group first scored 9 or less on the PHQ-9 but subsequently had at least a 5-point increase to a score of 10 or more.
The subjects were then followed for major adverse cardiovascular events, or MACE – defined as a composite of death, diagnosis of coronary artery disease, acute MI, stroke, and heart failure hospitalization – for a median of 208 days after completing their second PHQ-9.
The MACE rate was 4.8% in the nondepressed group and similar at 4.6% in the “no longer depressed” group, Dr. May reported. Both groups fared significantly better than the “remained depressed” and “became depressed” groups, which had MACE rates of 6% and 6.4%, respectively.
In a multivariate regression analysis adjusted for demographics, cardiovascular risk factors, prior disease diagnoses, medications, and other potential confounders, the “remained depressed” group was 33% more likely to experience a cardiovascular event than was the nondepressed group, she said. The “became depressed” group had a 44% increase in risk, compared with the nondepressed individuals. In contrast, the MACE risk in patients in the “no longer depressed” group was not significantly different from that of patients who weren’t depressed at either time point. And the MACE risk of patients who became depressed during the course of the study was no different from that of patients who remained depressed at both time points.
This is the first study of its kind, Dr. May said. Hence, the results require confirmation, ideally in a randomized clinical trial.
She reported having no financial conflicts regarding the study, which was supported by Intermountain Healthcare.
CHICAGO – Prompt, effective treatment for depression in the primary care setting appears to swiftly reduce the elevated cardiovascular risk known to be tied to the mood disorder, Heidi Thomas May, Ph.D., reported at the annual meeting of the American College of Cardiology.
“We know that depression is a risk factor for long-term adverse cardiovascular outcomes. Our study shows that it can also have immediate effects on someone’s cardiovascular health. I think our study highlights the importance of screening for depression in the primary care setting – and if someone’s depressed, they need to be treated,” said Dr. May, a cardiovascular and genetic epidemiologist at Intermountain Medical Center in Murray, Utah.
She presented an observational study of the electronic medical records of 7,559 Intermountain Healthcare patients over age 40 years who completed the Patient Health Questionnaire-9 (PHQ-9) depression screening tool during a visit to an Intermountain primary care clinic for any reason. They completed another PHQ-9 a median of 2.7 years later. Under the Intermountain system, a PHQ-9 score of 10 or more triggers implementation of a depression treatment pathway, the specifics of which vary depending upon the severity of symptoms.
On the basis of their two PHQ-9 scores, all patients were classified into one of four groups: The “nondepressed” group of 3,286 patients had a score of 9 or less on both occasions; the “remained depressed” cohort of 1,987 patients scored 10 or more on both PHQ-9s; the “no longer depressed” group of 1,542 patients scored at least 10 but subsequently improved by at least 5 points to a score of 9 or less; and the 735 patients in the “became depressed” group first scored 9 or less on the PHQ-9 but subsequently had at least a 5-point increase to a score of 10 or more.
The subjects were then followed for major adverse cardiovascular events, or MACE – defined as a composite of death, diagnosis of coronary artery disease, acute MI, stroke, and heart failure hospitalization – for a median of 208 days after completing their second PHQ-9.
The MACE rate was 4.8% in the nondepressed group and similar at 4.6% in the “no longer depressed” group, Dr. May reported. Both groups fared significantly better than the “remained depressed” and “became depressed” groups, which had MACE rates of 6% and 6.4%, respectively.
In a multivariate regression analysis adjusted for demographics, cardiovascular risk factors, prior disease diagnoses, medications, and other potential confounders, the “remained depressed” group was 33% more likely to experience a cardiovascular event than was the nondepressed group, she said. The “became depressed” group had a 44% increase in risk, compared with the nondepressed individuals. In contrast, the MACE risk in patients in the “no longer depressed” group was not significantly different from that of patients who weren’t depressed at either time point. And the MACE risk of patients who became depressed during the course of the study was no different from that of patients who remained depressed at both time points.
This is the first study of its kind, Dr. May said. Hence, the results require confirmation, ideally in a randomized clinical trial.
She reported having no financial conflicts regarding the study, which was supported by Intermountain Healthcare.
AT ACC 16
Key clinical point: Event rate was no different in “no longer depressed” group than in “never depressed.”
Major finding: Major adverse cardiovascular events were 44% more likely in primary care patients who became depressed during a median 2.7-year period, compared with those who weren’t depressed at either time point.
Data source: An observational study of 7,550 patients screened for depression in primary care clinics.
Disclosures: The study was supported by Intermountain Healthcare. Dr. May reported having no financial conflicts of interest.
Exercise is protective but underutilized in atrial fib patients
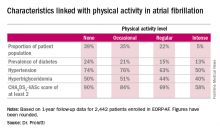
CHICAGO – Efforts to encourage even modest amounts of physical activity in sedentary patients with atrial fibrillation are likely to pay off in reduced risks of cardiovascular and all-cause mortality, according to a report from the EurObservational Research Program Pilot Survey on Atrial Fibrillation General Registry.
“Clearly we would recommend regular physical activity for patients with atrial fibrillation on the basis of the mortality benefit we see in the registry. If we give patients with atrial fibrillation oral anticoagulation, they are protected against stroke risk, but clearly they are still dying a lot,” Dr. Marco Proietti said at the annual meeting of the American College of Cardiology.
He presented 1-year follow-up data on 2,442 “real world” patients enrolled in the nine-country, observational, prospective registry, known as EORP-AF, shortly after being diagnosed with AF. One of the goals of EORP-AF is to learn whether physical exercise protects against cardiovascular events and all-cause mortality in AF patients, as has been well established in the general population and in patients at high cardiovascular risk.
One striking finding was that nearly 40% of patients in EORP-AF reported engaging in no physical activity, defined for study purposes as zero to less than 3 hours of physical activity per week for less than 2 years.
The other three categories employed by investigators were “occasional,” meaning less than 3 hours per week but for 2 years or more; “regular,” defined as at least 3 hours weekly for at least 2 years; and “intense,” which required more than 7 hours of physical activity per week for at least 2 years. Levels of cardiovascular and stroke risk factors decreased progressively with increasing levels of physical activity. Only 5% of the AF patients met the ‘intense’ standard, noted Dr. Proietti of the University of Birmingham (England).
The 1-year cardiovascular mortality rate approached 6% in the no physical activity group and hovered around 1% in the other three groups. The 1-year all-cause mortality rate exceeded 12% in the no-exercise group, was 4%% in the occasional exercisers, and 1%-2% in the groups reporting regular or intense physical activity.
The 1-year composite endpoint of cardiovascular death, any thromboembolism, or a bleeding event occurred in 12% of the sedentary patients, a rate two-to-three times higher than in the others.
Updated outcomes are to be reported from the EORP-AF pilot registry after 2 and 3 years of follow-up. Meanwhile, on the basis of the success of the pilot registry, more than 10,000 patients with AF have been enrolled in the EORP-AF main registry, according to Dr. Proietti.
A study limitation, he conceded, is that the registry includes no objective measure of physical capacity, such as METS.
Session co-chair Dr. Brian Olshansky, emeritus professor of internal medicine at the University of Iowa, Iowa City, observed that the registry data raise a classic chicken-versus-egg issue: Do the sedentary patients do worse because they’re inactive, or are they inactive because they are sicker and hence have worse outcomes?
Dr. Proietti said the registry data provide some support for the latter idea, since the no-physical-activity group had higher prevalences of coronary artery disease and heart failure.
Dr. Olshansky raised another point: “It’s interesting to me that there’s a whole bunch of literature showing that elite endurance athletes – bike racers, cross country skiers – have a very high incidence of atrial fibrillation. It seems to be either an inflammatory or an autonomic issue.”
Dr. Proietti replied that he’s familiar with that extensive literature, but the EORP-AF data through 1 year don’t provide validation. While the intense physical activity group tended to have more symptomatic AF than the other groups, they were no more likely to show progression from paroxysmal to permanent AF. The much larger main registry now underway may be able to better clarify the relationship between physical activity and incidence and progression of AF, including the possibility of a U-shaped dose-response curve.
The EORP-AF registry is supported by the European Society of Cardiology. Dr. Proietti reported having no financial conflicts of interest.
CHICAGO – Efforts to encourage even modest amounts of physical activity in sedentary patients with atrial fibrillation are likely to pay off in reduced risks of cardiovascular and all-cause mortality, according to a report from the EurObservational Research Program Pilot Survey on Atrial Fibrillation General Registry.
“Clearly we would recommend regular physical activity for patients with atrial fibrillation on the basis of the mortality benefit we see in the registry. If we give patients with atrial fibrillation oral anticoagulation, they are protected against stroke risk, but clearly they are still dying a lot,” Dr. Marco Proietti said at the annual meeting of the American College of Cardiology.
He presented 1-year follow-up data on 2,442 “real world” patients enrolled in the nine-country, observational, prospective registry, known as EORP-AF, shortly after being diagnosed with AF. One of the goals of EORP-AF is to learn whether physical exercise protects against cardiovascular events and all-cause mortality in AF patients, as has been well established in the general population and in patients at high cardiovascular risk.
One striking finding was that nearly 40% of patients in EORP-AF reported engaging in no physical activity, defined for study purposes as zero to less than 3 hours of physical activity per week for less than 2 years.
The other three categories employed by investigators were “occasional,” meaning less than 3 hours per week but for 2 years or more; “regular,” defined as at least 3 hours weekly for at least 2 years; and “intense,” which required more than 7 hours of physical activity per week for at least 2 years. Levels of cardiovascular and stroke risk factors decreased progressively with increasing levels of physical activity. Only 5% of the AF patients met the ‘intense’ standard, noted Dr. Proietti of the University of Birmingham (England).
The 1-year cardiovascular mortality rate approached 6% in the no physical activity group and hovered around 1% in the other three groups. The 1-year all-cause mortality rate exceeded 12% in the no-exercise group, was 4%% in the occasional exercisers, and 1%-2% in the groups reporting regular or intense physical activity.
The 1-year composite endpoint of cardiovascular death, any thromboembolism, or a bleeding event occurred in 12% of the sedentary patients, a rate two-to-three times higher than in the others.
Updated outcomes are to be reported from the EORP-AF pilot registry after 2 and 3 years of follow-up. Meanwhile, on the basis of the success of the pilot registry, more than 10,000 patients with AF have been enrolled in the EORP-AF main registry, according to Dr. Proietti.
A study limitation, he conceded, is that the registry includes no objective measure of physical capacity, such as METS.
Session co-chair Dr. Brian Olshansky, emeritus professor of internal medicine at the University of Iowa, Iowa City, observed that the registry data raise a classic chicken-versus-egg issue: Do the sedentary patients do worse because they’re inactive, or are they inactive because they are sicker and hence have worse outcomes?
Dr. Proietti said the registry data provide some support for the latter idea, since the no-physical-activity group had higher prevalences of coronary artery disease and heart failure.
Dr. Olshansky raised another point: “It’s interesting to me that there’s a whole bunch of literature showing that elite endurance athletes – bike racers, cross country skiers – have a very high incidence of atrial fibrillation. It seems to be either an inflammatory or an autonomic issue.”
Dr. Proietti replied that he’s familiar with that extensive literature, but the EORP-AF data through 1 year don’t provide validation. While the intense physical activity group tended to have more symptomatic AF than the other groups, they were no more likely to show progression from paroxysmal to permanent AF. The much larger main registry now underway may be able to better clarify the relationship between physical activity and incidence and progression of AF, including the possibility of a U-shaped dose-response curve.
The EORP-AF registry is supported by the European Society of Cardiology. Dr. Proietti reported having no financial conflicts of interest.
CHICAGO – Efforts to encourage even modest amounts of physical activity in sedentary patients with atrial fibrillation are likely to pay off in reduced risks of cardiovascular and all-cause mortality, according to a report from the EurObservational Research Program Pilot Survey on Atrial Fibrillation General Registry.
“Clearly we would recommend regular physical activity for patients with atrial fibrillation on the basis of the mortality benefit we see in the registry. If we give patients with atrial fibrillation oral anticoagulation, they are protected against stroke risk, but clearly they are still dying a lot,” Dr. Marco Proietti said at the annual meeting of the American College of Cardiology.
He presented 1-year follow-up data on 2,442 “real world” patients enrolled in the nine-country, observational, prospective registry, known as EORP-AF, shortly after being diagnosed with AF. One of the goals of EORP-AF is to learn whether physical exercise protects against cardiovascular events and all-cause mortality in AF patients, as has been well established in the general population and in patients at high cardiovascular risk.
One striking finding was that nearly 40% of patients in EORP-AF reported engaging in no physical activity, defined for study purposes as zero to less than 3 hours of physical activity per week for less than 2 years.
The other three categories employed by investigators were “occasional,” meaning less than 3 hours per week but for 2 years or more; “regular,” defined as at least 3 hours weekly for at least 2 years; and “intense,” which required more than 7 hours of physical activity per week for at least 2 years. Levels of cardiovascular and stroke risk factors decreased progressively with increasing levels of physical activity. Only 5% of the AF patients met the ‘intense’ standard, noted Dr. Proietti of the University of Birmingham (England).
The 1-year cardiovascular mortality rate approached 6% in the no physical activity group and hovered around 1% in the other three groups. The 1-year all-cause mortality rate exceeded 12% in the no-exercise group, was 4%% in the occasional exercisers, and 1%-2% in the groups reporting regular or intense physical activity.
The 1-year composite endpoint of cardiovascular death, any thromboembolism, or a bleeding event occurred in 12% of the sedentary patients, a rate two-to-three times higher than in the others.
Updated outcomes are to be reported from the EORP-AF pilot registry after 2 and 3 years of follow-up. Meanwhile, on the basis of the success of the pilot registry, more than 10,000 patients with AF have been enrolled in the EORP-AF main registry, according to Dr. Proietti.
A study limitation, he conceded, is that the registry includes no objective measure of physical capacity, such as METS.
Session co-chair Dr. Brian Olshansky, emeritus professor of internal medicine at the University of Iowa, Iowa City, observed that the registry data raise a classic chicken-versus-egg issue: Do the sedentary patients do worse because they’re inactive, or are they inactive because they are sicker and hence have worse outcomes?
Dr. Proietti said the registry data provide some support for the latter idea, since the no-physical-activity group had higher prevalences of coronary artery disease and heart failure.
Dr. Olshansky raised another point: “It’s interesting to me that there’s a whole bunch of literature showing that elite endurance athletes – bike racers, cross country skiers – have a very high incidence of atrial fibrillation. It seems to be either an inflammatory or an autonomic issue.”
Dr. Proietti replied that he’s familiar with that extensive literature, but the EORP-AF data through 1 year don’t provide validation. While the intense physical activity group tended to have more symptomatic AF than the other groups, they were no more likely to show progression from paroxysmal to permanent AF. The much larger main registry now underway may be able to better clarify the relationship between physical activity and incidence and progression of AF, including the possibility of a U-shaped dose-response curve.
The EORP-AF registry is supported by the European Society of Cardiology. Dr. Proietti reported having no financial conflicts of interest.
AT ACC 16
Key clinical point: Atrial fibrillation patients who report engaging in even occasional physical activity have a markedly lower risk of all-cause mortality than those who are sedentary.
Major finding: The 1-year composite outcome of cardiovascular death, any thromboembolism, or a bleeding event occurred in 12% in patients with atrial fibrillation who were sedentary, a rate two to three times greater than in those who engaged in various amounts of physical activity.
Data source: An analysis of 1-year outcomes in 2,442 patients with AF enrolled in the prospective, observational EORP-AF pilot registry.
Disclosures: The EORP-AF registry is supported by the European Society of Cardiology. The presenter reported having no financial conflicts of interest.
Drilling down on end-of-life health care costs in heart failure
CHICAGO – Health care costs for heart failure patients spike dramatically in the last 6 months of life, with lack of adherence to guideline-directed outpatient medical therapy being the major modifiable factor driving the costs, Jason P. Swindle, Ph.D., reported at the annual meeting of the American College of Cardiology.
He presented a retrospective study of heart failure–related and total health care costs during the final 24 months of life for 48,026 Medicare Advantage managed care plan members with heart failure.
The researchers were interested in exploring possible racial/ethnic differences in costs, particularly in light of evidence that African Americans have a higher risk of heart failure and higher all-cause mortality. And while a first look at the data indicated racial differences in the size of end-of-life cost spikes, those differences lost their significance in multivariate analysis.
“Lack of guideline-directed outpatient heart failure therapy was a key. Also older age and the presence of coronary heart disease – those were really the big three items that were driving the spike in costs,” said Dr. Swindle of the Chicago office of Optum, a health care consulting group.
He was quick to add that, since the study was based upon administrative data, the lack of adherence to guideline-directed therapy may be unrelated to physician prescribing.
“We see the prescriptions that patients are actually filling. Patients may very well be seeing their cardiologist and being prescribed a medication, but they simply don’t fill the prescription,” he explained in an interview.
Over patients’ final 2 years of life, semiannual all-cause health care costs climbed from a baseline of roughly $10,000 during months 24-19 before death by about 4-fold during months 6-1 before death. Heart failure–related medical costs jumped 10-fold in Asians, 7.8-fold in Hispanics, 6.6-fold in African Americans, and 6.7-fold in whites. Most of the increases occurred in the final 6 months.
Zeroing in on the final 6 months of life, the mean cumulative total medical costs were $44,599, with heart failure–related medical costs accounting for $24,818 of that figure. Total medical costs averaged just under $5,000 during month 6 prior to death and rose roughly 3.5-fold over the remaining months.
This study was supported by Novartis Pharmaceuticals.
CHICAGO – Health care costs for heart failure patients spike dramatically in the last 6 months of life, with lack of adherence to guideline-directed outpatient medical therapy being the major modifiable factor driving the costs, Jason P. Swindle, Ph.D., reported at the annual meeting of the American College of Cardiology.
He presented a retrospective study of heart failure–related and total health care costs during the final 24 months of life for 48,026 Medicare Advantage managed care plan members with heart failure.
The researchers were interested in exploring possible racial/ethnic differences in costs, particularly in light of evidence that African Americans have a higher risk of heart failure and higher all-cause mortality. And while a first look at the data indicated racial differences in the size of end-of-life cost spikes, those differences lost their significance in multivariate analysis.
“Lack of guideline-directed outpatient heart failure therapy was a key. Also older age and the presence of coronary heart disease – those were really the big three items that were driving the spike in costs,” said Dr. Swindle of the Chicago office of Optum, a health care consulting group.
He was quick to add that, since the study was based upon administrative data, the lack of adherence to guideline-directed therapy may be unrelated to physician prescribing.
“We see the prescriptions that patients are actually filling. Patients may very well be seeing their cardiologist and being prescribed a medication, but they simply don’t fill the prescription,” he explained in an interview.
Over patients’ final 2 years of life, semiannual all-cause health care costs climbed from a baseline of roughly $10,000 during months 24-19 before death by about 4-fold during months 6-1 before death. Heart failure–related medical costs jumped 10-fold in Asians, 7.8-fold in Hispanics, 6.6-fold in African Americans, and 6.7-fold in whites. Most of the increases occurred in the final 6 months.
Zeroing in on the final 6 months of life, the mean cumulative total medical costs were $44,599, with heart failure–related medical costs accounting for $24,818 of that figure. Total medical costs averaged just under $5,000 during month 6 prior to death and rose roughly 3.5-fold over the remaining months.
This study was supported by Novartis Pharmaceuticals.
CHICAGO – Health care costs for heart failure patients spike dramatically in the last 6 months of life, with lack of adherence to guideline-directed outpatient medical therapy being the major modifiable factor driving the costs, Jason P. Swindle, Ph.D., reported at the annual meeting of the American College of Cardiology.
He presented a retrospective study of heart failure–related and total health care costs during the final 24 months of life for 48,026 Medicare Advantage managed care plan members with heart failure.
The researchers were interested in exploring possible racial/ethnic differences in costs, particularly in light of evidence that African Americans have a higher risk of heart failure and higher all-cause mortality. And while a first look at the data indicated racial differences in the size of end-of-life cost spikes, those differences lost their significance in multivariate analysis.
“Lack of guideline-directed outpatient heart failure therapy was a key. Also older age and the presence of coronary heart disease – those were really the big three items that were driving the spike in costs,” said Dr. Swindle of the Chicago office of Optum, a health care consulting group.
He was quick to add that, since the study was based upon administrative data, the lack of adherence to guideline-directed therapy may be unrelated to physician prescribing.
“We see the prescriptions that patients are actually filling. Patients may very well be seeing their cardiologist and being prescribed a medication, but they simply don’t fill the prescription,” he explained in an interview.
Over patients’ final 2 years of life, semiannual all-cause health care costs climbed from a baseline of roughly $10,000 during months 24-19 before death by about 4-fold during months 6-1 before death. Heart failure–related medical costs jumped 10-fold in Asians, 7.8-fold in Hispanics, 6.6-fold in African Americans, and 6.7-fold in whites. Most of the increases occurred in the final 6 months.
Zeroing in on the final 6 months of life, the mean cumulative total medical costs were $44,599, with heart failure–related medical costs accounting for $24,818 of that figure. Total medical costs averaged just under $5,000 during month 6 prior to death and rose roughly 3.5-fold over the remaining months.
This study was supported by Novartis Pharmaceuticals.
AT ACC 16
Key clinical point: Addressing lack of adherence to guideline-directed medical therapy could curb end-of-life health care costs in heart failure.
Major finding: Total monthly medical costs in heart failure patients during their final 6 months of life climbed roughly 3.5-fold.
Data source: This was a retrospective study of total and heart failure–related health care costs during the final 24 months of life for more than 48,000 patients with heart failure.
Disclosures: This study was supported by Novartis Pharmaceuticals. Dr. Swindle is an employee of Optum, which conducted the research.
Acute heart failure mortality climbs with severity of peripheral edema
CHICAGO – Breathlessness typically results in hospital admission for patients with heart failure, but peripheral edema is what prolongs their stay, according to Dr. John G.F. Cleland.
Moreover, it’s not only hospital length of stay that climbs with increasing severity of peripheral edema on admission. So does mortality, both during the index admission and long term, Dr. Cleland reported at the annual meeting of the American College of Cardiology.
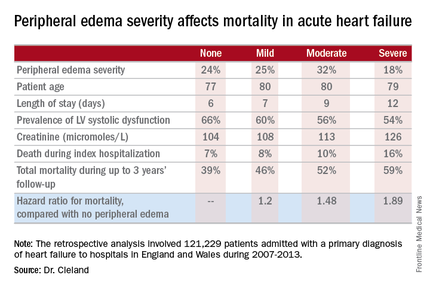
He presented a retrospective analysis of 121,229 patients admitted with a primary diagnosis of heart failure to more than 90% of the hospitals in England and Wales during 2007-2013.
“It turns out that the majority of patients we’re seeing admitted with heart failure at U.K. hospitals are not admitted because of severe breathlessness at rest; they’re admitted for increasing fluid retention. They have lots of peripheral edema, which is actually associated with bad outcome. The patients who are very breathless tend to have the better outcome because they have left heart failure. The ones who are full of peripheral edema have more renal dysfunction and anemia, and they’re more likely to have right heart failure,” said Dr. Cleland, professor of cardiology at Imperial College London.
“I’m not saying the breathless group isn’t a target, but this group with peripheral edema is a bigger target – and we’re not designing trials to address their problems,” he added.
Compared with patients with no peripheral edema on hospital admission, the risk of mortality during that hospitalization and up to 3 years of subsequent follow-up was increased 1.2-fold in those with mild peripheral edema, 1.48-fold with moderate peripheral edema, and 1.89-fold in those with severe peripheral edema.
“We’re designing all the big clinical trials in acute heart failure to capture what I regard as neither fish nor fowl. They’re recruiting patients 6-12 hours after admission for acute heart failure. But by that point they’ve pretty well responded to their intravenous diuretics, and we’re just catching the tail end of their breathlessness. They’re not an emergency. The problem is really their peripheral edema, and that’s a day 2/day 3 problem. The first 6 hours of care really isn’t relevant to this group,” according to the internationally renowned heart failure researcher.
Indeed, he said he’d like to see the term “acute heart failure” laid to rest.
“If you think of acute decompensated heart failure, you think of patients wearing an oxygen mask coming in by an ambulance with blue lights flashing, and it’s an emergency. We need to move the mind-set. We shouldn’t call it acute heart failure at all, we should start talking about hospitalized heart failure, of which some is acute but much of it is subacute in people who have been deteriorating over several weeks and have just gotten to the point where they’re not coping at home anymore. They call a taxi or a friend who takes them to the hospital. Then they take a wheelchair from the taxi to the ER, but they don’t really need an ER at all,” Dr. Cleland said.
Many of these patients could be redirected to a different sort of facility at great cost savings, he added.
“In the United Kingdom and I think in the States, we’re now talking about furosemide lounges where, rather than admit the patient, you can bring them up as a day case, give them intravenous therapy, then [have them] go home at night, perhaps coming back for 3 or 4 days if needed. People are also now looking at home infusion services, and there’s a nice device for giving subcutaneous doses of furosemide as well,” the cardiologist said.
Apart from diuretics, there really aren’t any effective medications at present for peripheral edema in heart failure. But there are novel investigational agents worthy of evaluation, according to Dr. Cleland, including drugs aimed at improving mitochondrial function, agents that inhibit channels that allow edema to gather, and iron therapy.
The study was supported by the British Society for Heart Failure and the National Institute for Cardiovascular Outcomes Research. Dr. Cleland reported having no relevant financial conflicts.
CHICAGO – Breathlessness typically results in hospital admission for patients with heart failure, but peripheral edema is what prolongs their stay, according to Dr. John G.F. Cleland.
Moreover, it’s not only hospital length of stay that climbs with increasing severity of peripheral edema on admission. So does mortality, both during the index admission and long term, Dr. Cleland reported at the annual meeting of the American College of Cardiology.
He presented a retrospective analysis of 121,229 patients admitted with a primary diagnosis of heart failure to more than 90% of the hospitals in England and Wales during 2007-2013.
“It turns out that the majority of patients we’re seeing admitted with heart failure at U.K. hospitals are not admitted because of severe breathlessness at rest; they’re admitted for increasing fluid retention. They have lots of peripheral edema, which is actually associated with bad outcome. The patients who are very breathless tend to have the better outcome because they have left heart failure. The ones who are full of peripheral edema have more renal dysfunction and anemia, and they’re more likely to have right heart failure,” said Dr. Cleland, professor of cardiology at Imperial College London.
“I’m not saying the breathless group isn’t a target, but this group with peripheral edema is a bigger target – and we’re not designing trials to address their problems,” he added.
Compared with patients with no peripheral edema on hospital admission, the risk of mortality during that hospitalization and up to 3 years of subsequent follow-up was increased 1.2-fold in those with mild peripheral edema, 1.48-fold with moderate peripheral edema, and 1.89-fold in those with severe peripheral edema.

“We’re designing all the big clinical trials in acute heart failure to capture what I regard as neither fish nor fowl. They’re recruiting patients 6-12 hours after admission for acute heart failure. But by that point they’ve pretty well responded to their intravenous diuretics, and we’re just catching the tail end of their breathlessness. They’re not an emergency. The problem is really their peripheral edema, and that’s a day 2/day 3 problem. The first 6 hours of care really isn’t relevant to this group,” according to the internationally renowned heart failure researcher.
Indeed, he said he’d like to see the term “acute heart failure” laid to rest.
“If you think of acute decompensated heart failure, you think of patients wearing an oxygen mask coming in by an ambulance with blue lights flashing, and it’s an emergency. We need to move the mind-set. We shouldn’t call it acute heart failure at all, we should start talking about hospitalized heart failure, of which some is acute but much of it is subacute in people who have been deteriorating over several weeks and have just gotten to the point where they’re not coping at home anymore. They call a taxi or a friend who takes them to the hospital. Then they take a wheelchair from the taxi to the ER, but they don’t really need an ER at all,” Dr. Cleland said.
Many of these patients could be redirected to a different sort of facility at great cost savings, he added.
“In the United Kingdom and I think in the States, we’re now talking about furosemide lounges where, rather than admit the patient, you can bring them up as a day case, give them intravenous therapy, then [have them] go home at night, perhaps coming back for 3 or 4 days if needed. People are also now looking at home infusion services, and there’s a nice device for giving subcutaneous doses of furosemide as well,” the cardiologist said.
Apart from diuretics, there really aren’t any effective medications at present for peripheral edema in heart failure. But there are novel investigational agents worthy of evaluation, according to Dr. Cleland, including drugs aimed at improving mitochondrial function, agents that inhibit channels that allow edema to gather, and iron therapy.
The study was supported by the British Society for Heart Failure and the National Institute for Cardiovascular Outcomes Research. Dr. Cleland reported having no relevant financial conflicts.
CHICAGO – Breathlessness typically results in hospital admission for patients with heart failure, but peripheral edema is what prolongs their stay, according to Dr. John G.F. Cleland.
Moreover, it’s not only hospital length of stay that climbs with increasing severity of peripheral edema on admission. So does mortality, both during the index admission and long term, Dr. Cleland reported at the annual meeting of the American College of Cardiology.
He presented a retrospective analysis of 121,229 patients admitted with a primary diagnosis of heart failure to more than 90% of the hospitals in England and Wales during 2007-2013.
“It turns out that the majority of patients we’re seeing admitted with heart failure at U.K. hospitals are not admitted because of severe breathlessness at rest; they’re admitted for increasing fluid retention. They have lots of peripheral edema, which is actually associated with bad outcome. The patients who are very breathless tend to have the better outcome because they have left heart failure. The ones who are full of peripheral edema have more renal dysfunction and anemia, and they’re more likely to have right heart failure,” said Dr. Cleland, professor of cardiology at Imperial College London.
“I’m not saying the breathless group isn’t a target, but this group with peripheral edema is a bigger target – and we’re not designing trials to address their problems,” he added.
Compared with patients with no peripheral edema on hospital admission, the risk of mortality during that hospitalization and up to 3 years of subsequent follow-up was increased 1.2-fold in those with mild peripheral edema, 1.48-fold with moderate peripheral edema, and 1.89-fold in those with severe peripheral edema.

“We’re designing all the big clinical trials in acute heart failure to capture what I regard as neither fish nor fowl. They’re recruiting patients 6-12 hours after admission for acute heart failure. But by that point they’ve pretty well responded to their intravenous diuretics, and we’re just catching the tail end of their breathlessness. They’re not an emergency. The problem is really their peripheral edema, and that’s a day 2/day 3 problem. The first 6 hours of care really isn’t relevant to this group,” according to the internationally renowned heart failure researcher.
Indeed, he said he’d like to see the term “acute heart failure” laid to rest.
“If you think of acute decompensated heart failure, you think of patients wearing an oxygen mask coming in by an ambulance with blue lights flashing, and it’s an emergency. We need to move the mind-set. We shouldn’t call it acute heart failure at all, we should start talking about hospitalized heart failure, of which some is acute but much of it is subacute in people who have been deteriorating over several weeks and have just gotten to the point where they’re not coping at home anymore. They call a taxi or a friend who takes them to the hospital. Then they take a wheelchair from the taxi to the ER, but they don’t really need an ER at all,” Dr. Cleland said.
Many of these patients could be redirected to a different sort of facility at great cost savings, he added.
“In the United Kingdom and I think in the States, we’re now talking about furosemide lounges where, rather than admit the patient, you can bring them up as a day case, give them intravenous therapy, then [have them] go home at night, perhaps coming back for 3 or 4 days if needed. People are also now looking at home infusion services, and there’s a nice device for giving subcutaneous doses of furosemide as well,” the cardiologist said.
Apart from diuretics, there really aren’t any effective medications at present for peripheral edema in heart failure. But there are novel investigational agents worthy of evaluation, according to Dr. Cleland, including drugs aimed at improving mitochondrial function, agents that inhibit channels that allow edema to gather, and iron therapy.
The study was supported by the British Society for Heart Failure and the National Institute for Cardiovascular Outcomes Research. Dr. Cleland reported having no relevant financial conflicts.
AT ACC 16
Key clinical point: Leg swelling warrants greater attention in patients hospitalized for acute heart failure.
Major finding: In-hospital mortality was more than twice as great in patients admitted for acute heart failure with severe peripheral edema, compared with no leg swelling.
Data source: A retrospective study of more than 121,000 patients hospitalized for acute heart failure in England and Wales.
Disclosures: The study was supported by the British Society for Heart Failure and the National Institute for Cardiovascular Outcomes Research. The presenter reported having no relevant financial conflicts.
Valve hemodynamic deterioration 2.5% at 1 year
CHICAGO – The incidence of valve hemodynamic deterioration in the first year after transcatheter aortic valve replacement is about 2.5%, but this event wasn’t clearly associated with adverse clinical outcomes out to 18 months of follow-up in an analysis of the large U.S. registry collaboratively maintained by the Society of Thoracic Surgeons and the American College of Cardiology.
“These findings, especially the patient and procedural predictors of valve hemodynamic deterioration we identified, may help to inform TAVR care, including patient selection, surveillance, and preventive strategies,” Dr. Sreekanth Vemulapalli reported at the annual meeting of the American College of Cardiology.
Recent reports have linked TAVR to subsequent development of leaflet abnormalities and valve thrombosis, with widely ranging estimates of incidence. Definitive answers as to the true rate of these adverse events and the underlying mechanisms will come from ongoing prospective studies using advanced imaging via four-dimensional CT or transesophageal echocardiography, but those studies will take years to complete, noted Dr. Vemulapalli of the Duke Clinical Research Institute in Durham, N.C.
In the meantime, he continued, the STS/ACC Transcatheter Valve Therapy Registry provides a unique opportunity to shed light on the incidence and consequences of valve hemodynamic deterioration (VHD) in real-world clinical practice. The registry includes all commercial TAVR procedures performed in the United States, with transthoracic echocardiograms obtained pre- and post-TAVR, at 30 days, and at 1 year after the procedure.
To examine the short- and longer-term rates of VHD, which Dr. Vemulapalli and his coinvestigators defined as an increase in the mean aortic valve gradient of 10 mm or more, the researchers assembled two separate patient cohorts. They comprised a short-term–risk group of 10,095 patients who underwent TAVR at 334 sites, with an incidence of VHD of 2.1% during the first 30 days after the procedure, and 3,175 patients at 254 sites, whose incidence of VHD from day 30 through 1 year post TAVR was 2.5%.
The combined rate of VHD and all-cause mortality during the first 30 days was 7.1%. For the long-term cohort, the combined endpoint rate from day 30 to 1 year was 23.5%.
Importantly, the occurrence of VHD was not associated with an excess of the composite endpoint of mortality, stroke, heart failure hospitalization, or aortic valve reintervention at 1 year in either the short- or long-term cohort. The same held true in an analysis covering the period of 12-18 months post TAVR, according to Dr. Vemulapalli.
In a multivariate analysis, the significant predictors of VHD in the short-term cohort were male sex; increased body mass index, with the risk rising stepwise with every additional 5 kg/m above normal weight; baseline severe chronic lung disease; a valve-in-valve procedure; a larger baseline aortic valve gradient; a TAVR valve size of 23 mm or less; and severe patient/prosthesis mismatch.
In the long-term cohort, the risk factors for VHD were hospital discharge on a factor Xa inhibitor and a larger predischarge aortic valve gradient.
Change in left ventricular ejection fraction over the course of the study bore no relation to VHD risk. Neither did which of the two commercially available TAVR valves a patient received.
This study was funded by the American College of Cardiology’s National Cardiovascular Data Registry. Dr. Vemulapalli reported serving as a consultant to Novella and Premiere and receiving research grants from the Agency for Healthcare Research and Quality, Boston Scientific, Abbott Vascular, and the ACC.
CHICAGO – The incidence of valve hemodynamic deterioration in the first year after transcatheter aortic valve replacement is about 2.5%, but this event wasn’t clearly associated with adverse clinical outcomes out to 18 months of follow-up in an analysis of the large U.S. registry collaboratively maintained by the Society of Thoracic Surgeons and the American College of Cardiology.
“These findings, especially the patient and procedural predictors of valve hemodynamic deterioration we identified, may help to inform TAVR care, including patient selection, surveillance, and preventive strategies,” Dr. Sreekanth Vemulapalli reported at the annual meeting of the American College of Cardiology.
Recent reports have linked TAVR to subsequent development of leaflet abnormalities and valve thrombosis, with widely ranging estimates of incidence. Definitive answers as to the true rate of these adverse events and the underlying mechanisms will come from ongoing prospective studies using advanced imaging via four-dimensional CT or transesophageal echocardiography, but those studies will take years to complete, noted Dr. Vemulapalli of the Duke Clinical Research Institute in Durham, N.C.
In the meantime, he continued, the STS/ACC Transcatheter Valve Therapy Registry provides a unique opportunity to shed light on the incidence and consequences of valve hemodynamic deterioration (VHD) in real-world clinical practice. The registry includes all commercial TAVR procedures performed in the United States, with transthoracic echocardiograms obtained pre- and post-TAVR, at 30 days, and at 1 year after the procedure.
To examine the short- and longer-term rates of VHD, which Dr. Vemulapalli and his coinvestigators defined as an increase in the mean aortic valve gradient of 10 mm or more, the researchers assembled two separate patient cohorts. They comprised a short-term–risk group of 10,095 patients who underwent TAVR at 334 sites, with an incidence of VHD of 2.1% during the first 30 days after the procedure, and 3,175 patients at 254 sites, whose incidence of VHD from day 30 through 1 year post TAVR was 2.5%.
The combined rate of VHD and all-cause mortality during the first 30 days was 7.1%. For the long-term cohort, the combined endpoint rate from day 30 to 1 year was 23.5%.
Importantly, the occurrence of VHD was not associated with an excess of the composite endpoint of mortality, stroke, heart failure hospitalization, or aortic valve reintervention at 1 year in either the short- or long-term cohort. The same held true in an analysis covering the period of 12-18 months post TAVR, according to Dr. Vemulapalli.
In a multivariate analysis, the significant predictors of VHD in the short-term cohort were male sex; increased body mass index, with the risk rising stepwise with every additional 5 kg/m above normal weight; baseline severe chronic lung disease; a valve-in-valve procedure; a larger baseline aortic valve gradient; a TAVR valve size of 23 mm or less; and severe patient/prosthesis mismatch.
In the long-term cohort, the risk factors for VHD were hospital discharge on a factor Xa inhibitor and a larger predischarge aortic valve gradient.
Change in left ventricular ejection fraction over the course of the study bore no relation to VHD risk. Neither did which of the two commercially available TAVR valves a patient received.
This study was funded by the American College of Cardiology’s National Cardiovascular Data Registry. Dr. Vemulapalli reported serving as a consultant to Novella and Premiere and receiving research grants from the Agency for Healthcare Research and Quality, Boston Scientific, Abbott Vascular, and the ACC.
CHICAGO – The incidence of valve hemodynamic deterioration in the first year after transcatheter aortic valve replacement is about 2.5%, but this event wasn’t clearly associated with adverse clinical outcomes out to 18 months of follow-up in an analysis of the large U.S. registry collaboratively maintained by the Society of Thoracic Surgeons and the American College of Cardiology.
“These findings, especially the patient and procedural predictors of valve hemodynamic deterioration we identified, may help to inform TAVR care, including patient selection, surveillance, and preventive strategies,” Dr. Sreekanth Vemulapalli reported at the annual meeting of the American College of Cardiology.
Recent reports have linked TAVR to subsequent development of leaflet abnormalities and valve thrombosis, with widely ranging estimates of incidence. Definitive answers as to the true rate of these adverse events and the underlying mechanisms will come from ongoing prospective studies using advanced imaging via four-dimensional CT or transesophageal echocardiography, but those studies will take years to complete, noted Dr. Vemulapalli of the Duke Clinical Research Institute in Durham, N.C.
In the meantime, he continued, the STS/ACC Transcatheter Valve Therapy Registry provides a unique opportunity to shed light on the incidence and consequences of valve hemodynamic deterioration (VHD) in real-world clinical practice. The registry includes all commercial TAVR procedures performed in the United States, with transthoracic echocardiograms obtained pre- and post-TAVR, at 30 days, and at 1 year after the procedure.
To examine the short- and longer-term rates of VHD, which Dr. Vemulapalli and his coinvestigators defined as an increase in the mean aortic valve gradient of 10 mm or more, the researchers assembled two separate patient cohorts. They comprised a short-term–risk group of 10,095 patients who underwent TAVR at 334 sites, with an incidence of VHD of 2.1% during the first 30 days after the procedure, and 3,175 patients at 254 sites, whose incidence of VHD from day 30 through 1 year post TAVR was 2.5%.
The combined rate of VHD and all-cause mortality during the first 30 days was 7.1%. For the long-term cohort, the combined endpoint rate from day 30 to 1 year was 23.5%.
Importantly, the occurrence of VHD was not associated with an excess of the composite endpoint of mortality, stroke, heart failure hospitalization, or aortic valve reintervention at 1 year in either the short- or long-term cohort. The same held true in an analysis covering the period of 12-18 months post TAVR, according to Dr. Vemulapalli.
In a multivariate analysis, the significant predictors of VHD in the short-term cohort were male sex; increased body mass index, with the risk rising stepwise with every additional 5 kg/m above normal weight; baseline severe chronic lung disease; a valve-in-valve procedure; a larger baseline aortic valve gradient; a TAVR valve size of 23 mm or less; and severe patient/prosthesis mismatch.
In the long-term cohort, the risk factors for VHD were hospital discharge on a factor Xa inhibitor and a larger predischarge aortic valve gradient.
Change in left ventricular ejection fraction over the course of the study bore no relation to VHD risk. Neither did which of the two commercially available TAVR valves a patient received.
This study was funded by the American College of Cardiology’s National Cardiovascular Data Registry. Dr. Vemulapalli reported serving as a consultant to Novella and Premiere and receiving research grants from the Agency for Healthcare Research and Quality, Boston Scientific, Abbott Vascular, and the ACC.
AT ACC 16
Key clinical point: The incidence of valve hemodynamic deterioration after transaortic valve replacement is 2.5% from day 30 through 12 months post procedure.
Major finding: Patients who experienced valve hemodynamic deterioration had a rate of adverse clinical outcomes similar to those without valve deterioration.
Data source: This was a retrospective study of 18-month outcomes in the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry, which covers all commercial transcatheter valve replacements done in the United States.
Disclosures: This study was funded by the American College of Cardiology’s National Cardiovascular Data Registry. The presenter reported serving as a consultant to Novella and Premiere and receiving research grants from the Agency for Healthcare Research and Quality, Boston Scientific, Abbott Vascular, and the ACC.
No rise in serious HF seen in patients taking saxagliptin or sitagliptin
Neither saxagliptin nor sitagliptin, the two oral DPP-4 inhibitors most commonly used as antihyperglycemic medications, raised the risk of hospitalization for heart failure in a large population-based cohort study that analyzed data from a Food and Drug Administration surveillance program.
The report was published online April 25 in Annals of Internal Medicine.
The cardiovascular safety of DPP-4 inhibitors is controversial: Several postmarketing studies have produced conflicting results, particularly with regard to HF risk. “Patients with diabetes have a higher HF risk than those without, so any antihyperglycemic agent that modifies the risk warrants further examination,” said Sengwee Toh, Sc.D., a pharmacoepidemiologist in the department of population medicine, Harvard Medical School and Harvard Pilgrim Health Institute, Boston, and his associates.
They compared rates of HF among demographically and geographically diverse patients who initiated antidiabetic medications during a 7-year period in routine clinical settings. The study population included 78,553 adults who initiated saxagliptin and 298,124 who initiated sitagliptin, who were compared with patients who initiated pioglitazone, second-generation sulfonylureas, or long-acting insulins. Mean follow-up was 7-9 months.
There was no evidence of an increased risk of hospitalization for HF among new users of saxagliptin or sitagliptin. The hazard ratios for developing HF were 0.83 for saxagliptin vs. sitagliptin, 0.63 for saxagliptin vs. pioglitazone, 0.69 for saxagliptin vs. sulfonylureas, and 0.61 for saxagliptin vs. insulin. Similarly, the hazard ratios for developing HF were 0.74 for sitagliptin vs. pioglitazone, 0.86 for sitagliptin vs. sulfonylureas, and 0.71 for sitagliptin vs. insulin.
These results were consistent across sensitivity analyses and subgroup analyses that categorized patients by whether or not they had preexisting cardiovascular disease and whether or not they had a history of prior HF, the investigators said (Ann Intern Med. 2016 April 25. doi:10.7326/M15-2568).
However, this was an observational study with a relatively short follow-up. “Well-designed randomized trials with hospitalization for HF as the main endpoint or observational studies that address the limitations of our study will help provide more definitive evidence on the topic,” Dr. Toh and his associates said.
This study was supported by the FDA. Dr. Toh reported having no relevant financial disclosures; one of his associates reported receiving personal fees from Novartis unrelated to this work.
The findings of Toh et al. allay concerns about a saxagliptin- or sitagliptin-associated risk for heart failure. This risk was similar between the two agents and either comparable to or lower than that in all other comparator groups.
Beyond reassuring clinicians, this study illustrates the value of large, longitudinal databases built from clinical and administrative data, to complement the findings of clinical trials. These investigators were able to draw their conclusions from rich demographic, diagnostic, prescription, and utilization data based in routine real-world practice.
Joseph V. Selby, M.D., is at the Patient-Centered Outcomes Research Institute, Washington. He reported having no relevant financial disclosures. Dr. Selby made these remarks in an editorial accompanying Dr. Toh’s report (Ann. Intern. Med. 2016 April 25. doi:10.7326/M16-0869).
The findings of Toh et al. allay concerns about a saxagliptin- or sitagliptin-associated risk for heart failure. This risk was similar between the two agents and either comparable to or lower than that in all other comparator groups.
Beyond reassuring clinicians, this study illustrates the value of large, longitudinal databases built from clinical and administrative data, to complement the findings of clinical trials. These investigators were able to draw their conclusions from rich demographic, diagnostic, prescription, and utilization data based in routine real-world practice.
Joseph V. Selby, M.D., is at the Patient-Centered Outcomes Research Institute, Washington. He reported having no relevant financial disclosures. Dr. Selby made these remarks in an editorial accompanying Dr. Toh’s report (Ann. Intern. Med. 2016 April 25. doi:10.7326/M16-0869).
The findings of Toh et al. allay concerns about a saxagliptin- or sitagliptin-associated risk for heart failure. This risk was similar between the two agents and either comparable to or lower than that in all other comparator groups.
Beyond reassuring clinicians, this study illustrates the value of large, longitudinal databases built from clinical and administrative data, to complement the findings of clinical trials. These investigators were able to draw their conclusions from rich demographic, diagnostic, prescription, and utilization data based in routine real-world practice.
Joseph V. Selby, M.D., is at the Patient-Centered Outcomes Research Institute, Washington. He reported having no relevant financial disclosures. Dr. Selby made these remarks in an editorial accompanying Dr. Toh’s report (Ann. Intern. Med. 2016 April 25. doi:10.7326/M16-0869).
Neither saxagliptin nor sitagliptin, the two oral DPP-4 inhibitors most commonly used as antihyperglycemic medications, raised the risk of hospitalization for heart failure in a large population-based cohort study that analyzed data from a Food and Drug Administration surveillance program.
The report was published online April 25 in Annals of Internal Medicine.
The cardiovascular safety of DPP-4 inhibitors is controversial: Several postmarketing studies have produced conflicting results, particularly with regard to HF risk. “Patients with diabetes have a higher HF risk than those without, so any antihyperglycemic agent that modifies the risk warrants further examination,” said Sengwee Toh, Sc.D., a pharmacoepidemiologist in the department of population medicine, Harvard Medical School and Harvard Pilgrim Health Institute, Boston, and his associates.
They compared rates of HF among demographically and geographically diverse patients who initiated antidiabetic medications during a 7-year period in routine clinical settings. The study population included 78,553 adults who initiated saxagliptin and 298,124 who initiated sitagliptin, who were compared with patients who initiated pioglitazone, second-generation sulfonylureas, or long-acting insulins. Mean follow-up was 7-9 months.
There was no evidence of an increased risk of hospitalization for HF among new users of saxagliptin or sitagliptin. The hazard ratios for developing HF were 0.83 for saxagliptin vs. sitagliptin, 0.63 for saxagliptin vs. pioglitazone, 0.69 for saxagliptin vs. sulfonylureas, and 0.61 for saxagliptin vs. insulin. Similarly, the hazard ratios for developing HF were 0.74 for sitagliptin vs. pioglitazone, 0.86 for sitagliptin vs. sulfonylureas, and 0.71 for sitagliptin vs. insulin.
These results were consistent across sensitivity analyses and subgroup analyses that categorized patients by whether or not they had preexisting cardiovascular disease and whether or not they had a history of prior HF, the investigators said (Ann Intern Med. 2016 April 25. doi:10.7326/M15-2568).
However, this was an observational study with a relatively short follow-up. “Well-designed randomized trials with hospitalization for HF as the main endpoint or observational studies that address the limitations of our study will help provide more definitive evidence on the topic,” Dr. Toh and his associates said.
This study was supported by the FDA. Dr. Toh reported having no relevant financial disclosures; one of his associates reported receiving personal fees from Novartis unrelated to this work.
Neither saxagliptin nor sitagliptin, the two oral DPP-4 inhibitors most commonly used as antihyperglycemic medications, raised the risk of hospitalization for heart failure in a large population-based cohort study that analyzed data from a Food and Drug Administration surveillance program.
The report was published online April 25 in Annals of Internal Medicine.
The cardiovascular safety of DPP-4 inhibitors is controversial: Several postmarketing studies have produced conflicting results, particularly with regard to HF risk. “Patients with diabetes have a higher HF risk than those without, so any antihyperglycemic agent that modifies the risk warrants further examination,” said Sengwee Toh, Sc.D., a pharmacoepidemiologist in the department of population medicine, Harvard Medical School and Harvard Pilgrim Health Institute, Boston, and his associates.
They compared rates of HF among demographically and geographically diverse patients who initiated antidiabetic medications during a 7-year period in routine clinical settings. The study population included 78,553 adults who initiated saxagliptin and 298,124 who initiated sitagliptin, who were compared with patients who initiated pioglitazone, second-generation sulfonylureas, or long-acting insulins. Mean follow-up was 7-9 months.
There was no evidence of an increased risk of hospitalization for HF among new users of saxagliptin or sitagliptin. The hazard ratios for developing HF were 0.83 for saxagliptin vs. sitagliptin, 0.63 for saxagliptin vs. pioglitazone, 0.69 for saxagliptin vs. sulfonylureas, and 0.61 for saxagliptin vs. insulin. Similarly, the hazard ratios for developing HF were 0.74 for sitagliptin vs. pioglitazone, 0.86 for sitagliptin vs. sulfonylureas, and 0.71 for sitagliptin vs. insulin.
These results were consistent across sensitivity analyses and subgroup analyses that categorized patients by whether or not they had preexisting cardiovascular disease and whether or not they had a history of prior HF, the investigators said (Ann Intern Med. 2016 April 25. doi:10.7326/M15-2568).
However, this was an observational study with a relatively short follow-up. “Well-designed randomized trials with hospitalization for HF as the main endpoint or observational studies that address the limitations of our study will help provide more definitive evidence on the topic,” Dr. Toh and his associates said.
This study was supported by the FDA. Dr. Toh reported having no relevant financial disclosures; one of his associates reported receiving personal fees from Novartis unrelated to this work.
FROM ANNALS OF INTERNAL MEDICINE
Key clinical point: No increase in the risk of hospitalization for heart failure was found in a large cohort study that used an FDA surveillance program.
Major finding: The hazard ratios for developing HF were 0.83 for saxagliptin vs. sitagliptin, 0.63 for saxagliptin vs. pioglitazone, 0.69 for saxagliptin vs. sulfonylureas, and 0.61 for saxagliptin vs. insulin.
Data source: A population-based retrospective cohort study involving 78,553 new users of saxagliptin and 298,124 of sitagliptin during a 7-year period.
Disclosures: This study was supported by the FDA. Dr. Toh reported having no relevant financial disclosures; one of his associates reported receiving personal fees from Novartis unrelated to this work.
VIDEO: Serial lung fluid measurement improved heart failure outcomes
CHICAGO – Regular assessment of a heart failure patient’s lung fluid volume using a device that measures electrical conduction through the chest – lung impedance – helped guide clinicians to make timely adjustments in a patient’s medications and thereby significantly reduce mortality and hospitalizations during an average 4 years of follow-up in a randomized, controlled study with 256 patients.
Monthly measurement of lung impedance and medication adjustments based on the information led to a 58% reduction in hospitalizations for acute heart failure during the first year of the study, compared with control patients, and a 56% reduction in heart failure hospitalizations, compared with controls, during the entire course of the study, the study’s two primary endpoints, Dr. Michael K. Shochat reported at the annual meeting of the American College of Cardiology.
The results also showed that performing regular lung impedance measurements and using the results to guide treatment led to a 43% reduction in all-cause mortality and a 62% drop in heart failure mortality during the average 4-year course of the study, said Dr. Shochat, a cardiologist at the Heart Institute of Hillel Yaffe Medical Center in Hadera, Israel. Concurrent with Dr. Shochat’s report at the meeting the results also appeared in an article published online (J Card Failure. 2016;doi:10.1016/j.cardfail.2016.03.015).
A key aspect of the study was that the clinicians who treated the enrolled patients who underwent lung impedance monitoring used this information to adjust medications the patients received. Overall, patients who underwent monitoring had more than twice the number of medication dose adjustments, compared with the control patients. These adjustments particularly focused on diuretic dosages, which changed three times as often in the monitored patients, compared with controls, Dr. Shochat reported. Changes in the dosages of beta-blockers and ACE inhibitors also showed marked increases in the monitored patients, compared with the controls.
The Non-Invasive Lung IMPEDANCE-Guided Preemptive Treatment in Chronic Heart Failure Patients (IMPEDANCE-HF) trial enrolled 256 patients at two centers in Israel during 2005-2014. Patients had New York Heart Association class II-IV heart failure and a left ventricular ejection fraction of 35% or less. The enrolled patients averaged 67 years of age, and 80% were men.
Clinicians measured lung impedance using a proprietary device that places external electrodes on opposite sides of the patient’s chest. Calculation of impedance used a formula that eliminated the noise from chest wall impedance and focused exclusively on lung impedance. Once the electrodes are placed collection of the impedance data takes about 1 minute, Dr. Shochat said. The study protocol called for impedance data to be collected monthly, and in practice it occurred about 11 times a year during the study.
The investigators calculated for each patient in the active arm of the study a “basal” lung impedance level that reflected their level of lung conductivity when their lungs were clear of excess fluid. Participating clinicians were instructed to intervene by altering medications when the impedance level dropped more than 18% below the basal level. Their goal was to prevent impedance from dropping to more than 24% below the basal level, which correlated with when heart failure patients usually required hospitalization for acute decompensation. The specifics of how to adjust medications to manage patients who showed these signs of fluid overload were left to the discretion of each attending physician.
MPEDANCE-HF was sponsored by the RSMM Company, which is developing the lung impedance measurement device used in the study. Dr. Shochat is a cofounder of RSMM and is a member of the company’s board of directors.
On Twitter @mitchelzoler
The very exciting results reported by Dr. Shochat came from a small, positive trial that showed impedance monitoring was an effective way to detect an increased amount of fluid in a heart failure patient’s lungs. This resulted in improved outcomes, compared with patients managed using usual care, including fewer hospitalizations and reduced mortality.
These results suggest that when physicians had lung impedance information, they identified episodes of acute heart failure decompensation sooner and that they used this alert to change treatment and prevent patient worsening. Heart failure exacerbations and decompensation events are a recurring problem for heart failure patients, and the earlier they are identified and addressed with altered treatment, the better it is for the patient’s well being. The next step is to see if these positive results can be confirmed by other research groups and in larger numbers of patients.
These results contrast with the findings from a German study reported in 2015 that used lung impedance information collected by implantable cardioverter defibrillators in heart failure patients to identify episodes of fluid buildup and decompensation. That study failed to show a statistically significant impact on patient outcomes. The researchers speculated that this may have been because patients often did not go online to allow their information to get transmitted to their physician, and physicians often did not act on the information because the patients reported no coincident change in symptoms.
This problem with the German study highlights that collecting lung impedance information will only improve outcomes if physicians then act on the information and modify a patient’s treatment. In the new study reported by Dr. Shochat, patients consistently underwent evaluation for their lung impedance status every month, and when the results suggested a growing problem of fluid overload the physicians consistently acted on the information by adjusting medication dosages.
Use of lung impedance measurement is similar to another approach for monitoring patients with heart failure that recently entered routine U.S. practice, an implanted device to monitor pulmonary artery pressure and identify episodes of fluid overload and acute decompensation. In the future, it will be interesting to compare the efficacy and ease of use of managing heart failure patients with pulmonary artery pressure monitoring with an implanted device and monitoring fluid build up in the lungs with lung impedance.
Dr. John A. Jarcho is a cardiologist at Brigham and Women’s Hospital, Boston. He had no disclosures. He made these comments as a discussant of Dr. Shochat’s report and in an interview.
The very exciting results reported by Dr. Shochat came from a small, positive trial that showed impedance monitoring was an effective way to detect an increased amount of fluid in a heart failure patient’s lungs. This resulted in improved outcomes, compared with patients managed using usual care, including fewer hospitalizations and reduced mortality.
These results suggest that when physicians had lung impedance information, they identified episodes of acute heart failure decompensation sooner and that they used this alert to change treatment and prevent patient worsening. Heart failure exacerbations and decompensation events are a recurring problem for heart failure patients, and the earlier they are identified and addressed with altered treatment, the better it is for the patient’s well being. The next step is to see if these positive results can be confirmed by other research groups and in larger numbers of patients.
These results contrast with the findings from a German study reported in 2015 that used lung impedance information collected by implantable cardioverter defibrillators in heart failure patients to identify episodes of fluid buildup and decompensation. That study failed to show a statistically significant impact on patient outcomes. The researchers speculated that this may have been because patients often did not go online to allow their information to get transmitted to their physician, and physicians often did not act on the information because the patients reported no coincident change in symptoms.
This problem with the German study highlights that collecting lung impedance information will only improve outcomes if physicians then act on the information and modify a patient’s treatment. In the new study reported by Dr. Shochat, patients consistently underwent evaluation for their lung impedance status every month, and when the results suggested a growing problem of fluid overload the physicians consistently acted on the information by adjusting medication dosages.
Use of lung impedance measurement is similar to another approach for monitoring patients with heart failure that recently entered routine U.S. practice, an implanted device to monitor pulmonary artery pressure and identify episodes of fluid overload and acute decompensation. In the future, it will be interesting to compare the efficacy and ease of use of managing heart failure patients with pulmonary artery pressure monitoring with an implanted device and monitoring fluid build up in the lungs with lung impedance.
Dr. John A. Jarcho is a cardiologist at Brigham and Women’s Hospital, Boston. He had no disclosures. He made these comments as a discussant of Dr. Shochat’s report and in an interview.
The very exciting results reported by Dr. Shochat came from a small, positive trial that showed impedance monitoring was an effective way to detect an increased amount of fluid in a heart failure patient’s lungs. This resulted in improved outcomes, compared with patients managed using usual care, including fewer hospitalizations and reduced mortality.
These results suggest that when physicians had lung impedance information, they identified episodes of acute heart failure decompensation sooner and that they used this alert to change treatment and prevent patient worsening. Heart failure exacerbations and decompensation events are a recurring problem for heart failure patients, and the earlier they are identified and addressed with altered treatment, the better it is for the patient’s well being. The next step is to see if these positive results can be confirmed by other research groups and in larger numbers of patients.
These results contrast with the findings from a German study reported in 2015 that used lung impedance information collected by implantable cardioverter defibrillators in heart failure patients to identify episodes of fluid buildup and decompensation. That study failed to show a statistically significant impact on patient outcomes. The researchers speculated that this may have been because patients often did not go online to allow their information to get transmitted to their physician, and physicians often did not act on the information because the patients reported no coincident change in symptoms.
This problem with the German study highlights that collecting lung impedance information will only improve outcomes if physicians then act on the information and modify a patient’s treatment. In the new study reported by Dr. Shochat, patients consistently underwent evaluation for their lung impedance status every month, and when the results suggested a growing problem of fluid overload the physicians consistently acted on the information by adjusting medication dosages.
Use of lung impedance measurement is similar to another approach for monitoring patients with heart failure that recently entered routine U.S. practice, an implanted device to monitor pulmonary artery pressure and identify episodes of fluid overload and acute decompensation. In the future, it will be interesting to compare the efficacy and ease of use of managing heart failure patients with pulmonary artery pressure monitoring with an implanted device and monitoring fluid build up in the lungs with lung impedance.
Dr. John A. Jarcho is a cardiologist at Brigham and Women’s Hospital, Boston. He had no disclosures. He made these comments as a discussant of Dr. Shochat’s report and in an interview.
CHICAGO – Regular assessment of a heart failure patient’s lung fluid volume using a device that measures electrical conduction through the chest – lung impedance – helped guide clinicians to make timely adjustments in a patient’s medications and thereby significantly reduce mortality and hospitalizations during an average 4 years of follow-up in a randomized, controlled study with 256 patients.
Monthly measurement of lung impedance and medication adjustments based on the information led to a 58% reduction in hospitalizations for acute heart failure during the first year of the study, compared with control patients, and a 56% reduction in heart failure hospitalizations, compared with controls, during the entire course of the study, the study’s two primary endpoints, Dr. Michael K. Shochat reported at the annual meeting of the American College of Cardiology.
The results also showed that performing regular lung impedance measurements and using the results to guide treatment led to a 43% reduction in all-cause mortality and a 62% drop in heart failure mortality during the average 4-year course of the study, said Dr. Shochat, a cardiologist at the Heart Institute of Hillel Yaffe Medical Center in Hadera, Israel. Concurrent with Dr. Shochat’s report at the meeting the results also appeared in an article published online (J Card Failure. 2016;doi:10.1016/j.cardfail.2016.03.015).
A key aspect of the study was that the clinicians who treated the enrolled patients who underwent lung impedance monitoring used this information to adjust medications the patients received. Overall, patients who underwent monitoring had more than twice the number of medication dose adjustments, compared with the control patients. These adjustments particularly focused on diuretic dosages, which changed three times as often in the monitored patients, compared with controls, Dr. Shochat reported. Changes in the dosages of beta-blockers and ACE inhibitors also showed marked increases in the monitored patients, compared with the controls.
The Non-Invasive Lung IMPEDANCE-Guided Preemptive Treatment in Chronic Heart Failure Patients (IMPEDANCE-HF) trial enrolled 256 patients at two centers in Israel during 2005-2014. Patients had New York Heart Association class II-IV heart failure and a left ventricular ejection fraction of 35% or less. The enrolled patients averaged 67 years of age, and 80% were men.
Clinicians measured lung impedance using a proprietary device that places external electrodes on opposite sides of the patient’s chest. Calculation of impedance used a formula that eliminated the noise from chest wall impedance and focused exclusively on lung impedance. Once the electrodes are placed collection of the impedance data takes about 1 minute, Dr. Shochat said. The study protocol called for impedance data to be collected monthly, and in practice it occurred about 11 times a year during the study.
The investigators calculated for each patient in the active arm of the study a “basal” lung impedance level that reflected their level of lung conductivity when their lungs were clear of excess fluid. Participating clinicians were instructed to intervene by altering medications when the impedance level dropped more than 18% below the basal level. Their goal was to prevent impedance from dropping to more than 24% below the basal level, which correlated with when heart failure patients usually required hospitalization for acute decompensation. The specifics of how to adjust medications to manage patients who showed these signs of fluid overload were left to the discretion of each attending physician.
MPEDANCE-HF was sponsored by the RSMM Company, which is developing the lung impedance measurement device used in the study. Dr. Shochat is a cofounder of RSMM and is a member of the company’s board of directors.
On Twitter @mitchelzoler
CHICAGO – Regular assessment of a heart failure patient’s lung fluid volume using a device that measures electrical conduction through the chest – lung impedance – helped guide clinicians to make timely adjustments in a patient’s medications and thereby significantly reduce mortality and hospitalizations during an average 4 years of follow-up in a randomized, controlled study with 256 patients.
Monthly measurement of lung impedance and medication adjustments based on the information led to a 58% reduction in hospitalizations for acute heart failure during the first year of the study, compared with control patients, and a 56% reduction in heart failure hospitalizations, compared with controls, during the entire course of the study, the study’s two primary endpoints, Dr. Michael K. Shochat reported at the annual meeting of the American College of Cardiology.
The results also showed that performing regular lung impedance measurements and using the results to guide treatment led to a 43% reduction in all-cause mortality and a 62% drop in heart failure mortality during the average 4-year course of the study, said Dr. Shochat, a cardiologist at the Heart Institute of Hillel Yaffe Medical Center in Hadera, Israel. Concurrent with Dr. Shochat’s report at the meeting the results also appeared in an article published online (J Card Failure. 2016;doi:10.1016/j.cardfail.2016.03.015).
A key aspect of the study was that the clinicians who treated the enrolled patients who underwent lung impedance monitoring used this information to adjust medications the patients received. Overall, patients who underwent monitoring had more than twice the number of medication dose adjustments, compared with the control patients. These adjustments particularly focused on diuretic dosages, which changed three times as often in the monitored patients, compared with controls, Dr. Shochat reported. Changes in the dosages of beta-blockers and ACE inhibitors also showed marked increases in the monitored patients, compared with the controls.
The Non-Invasive Lung IMPEDANCE-Guided Preemptive Treatment in Chronic Heart Failure Patients (IMPEDANCE-HF) trial enrolled 256 patients at two centers in Israel during 2005-2014. Patients had New York Heart Association class II-IV heart failure and a left ventricular ejection fraction of 35% or less. The enrolled patients averaged 67 years of age, and 80% were men.
Clinicians measured lung impedance using a proprietary device that places external electrodes on opposite sides of the patient’s chest. Calculation of impedance used a formula that eliminated the noise from chest wall impedance and focused exclusively on lung impedance. Once the electrodes are placed collection of the impedance data takes about 1 minute, Dr. Shochat said. The study protocol called for impedance data to be collected monthly, and in practice it occurred about 11 times a year during the study.
The investigators calculated for each patient in the active arm of the study a “basal” lung impedance level that reflected their level of lung conductivity when their lungs were clear of excess fluid. Participating clinicians were instructed to intervene by altering medications when the impedance level dropped more than 18% below the basal level. Their goal was to prevent impedance from dropping to more than 24% below the basal level, which correlated with when heart failure patients usually required hospitalization for acute decompensation. The specifics of how to adjust medications to manage patients who showed these signs of fluid overload were left to the discretion of each attending physician.
MPEDANCE-HF was sponsored by the RSMM Company, which is developing the lung impedance measurement device used in the study. Dr. Shochat is a cofounder of RSMM and is a member of the company’s board of directors.
On Twitter @mitchelzoler
AT ACC 2016
Key clinical point: Monthly, noninvasive measurement of lung fluid levels using lung impedance produced better fluid control in heart failure patients and significantly fewer deaths and heart failure hospitalizations.
Major finding: Lung impedance–based management produced a 56% cut in heart failure hospitalizations, compared with standard care.
Data source: IMPEDANCE-HF, a randomized study with 256 heart failure patients at two Israeli centers.
Disclosures: IMPEDANCE-HF was sponsored by the RSMM Company, which is developing the lung impedance measurement device used in the study. Dr. Shochat is a cofounder of RSMM and is a member of the company’s board of directors.
DANAMI 3-iPOST: No significant benefit with ischemic postconditioning after STEMI
CHICAGO – Ischemic postconditioning in patients with ST-segment elevation myocardial infarction failed to significantly reduce death from any cause or hospitalization for heart failure in the randomized, controlled DANAMI 3-iPOST trial.
At a mean follow-up of 37.5 months, the primary composite endpoint of death from any cause and hospitalization for heart failure occurred in 69 of 617 patients with STEMI who received standard angioplasty and in 65 of 617 patients who received ischemic postconditioning in DANAMI 3-iPOST (the Third Danish Study of Optimal Acute Treatment of Patients With ST-Segment Elevation Myocardial Infarction: iPOST conditioning during primary PCI). The 7% difference (hazard ratio, 0.93) was not statistically significant, Dr. Thomas Engstrøm reported at the annual meeting of the American College of Cardiology.
For the individual component of all-cause mortality, the reduction in the ischemic postconditioning group was 25%, occurring in 50 patients, compared with 38 in the conventionally treated group (hazard ratio, 0.75), but this difference also did not reach statistical significance, Dr. Engstrøm said.
Thirty patients in each group required hospitalization for heart failure.
However, an improvement in a secondary endpoint of left ventricular ejection fraction above 45% in patients with anterior infarcts was statistically significant, occurring in 72% of patients in the standard angioplasty group and 80% of those in the ischemic postconditioning group, said Dr. Engstrøm of Rigshospitalet University of Copenhagen.
This finding may translate into improved survival with longer follow-up, he noted.
Patients in the DANAMI-3 iPOST trial, who had a mean age of age 61 years, had acute STEMI (ST-segment elevation MI) symptoms of less than 12 hours’ duration at the time of randomization. They were followed for at least 2 years.
Ischemic postconditioning – a variation on angioplasty that involves using 30-second bursts of blood flow interspersed with 30-second pauses to restore blood flow to the heart – was shown in earlier studies to improve ST-segment resolution, reduce damage to heart muscle, and – in some patients – limit the extent of reperfusion injury.
Whether these factors would reduce hospitalizations or improve patient survival remained unclear, Dr. Engstrøm said.
Abrupt reperfusion by angioplasty may itself damage the heart muscle. In fact, up to 35% of patients may experience such injury during angioplasty.
“The thinking was that performing the reperfusion in a gentle, graded fashion would protect the heart against reperfusion injury,” Dr. Engstrøm explained.
The findings of DANAMI 3-iPOST – the first large clinical trial designed to evaluate clinical outcomes in STEMI patients (as opposed to surrogate endpoints such as ST-segment resolution) were disappointing, but larger trials may be required to definitively establish whether ischemic postconditioning improves clinical outcomes, Dr. Engstrøm said.
The DANAMI 3-iPOST trial was funded by the Danish Agency for Science, Technology, and Innovation and the Danish Council for Strategic Research. Dr. Engstrøm reported having no relevant financial disclosures.
CHICAGO – Ischemic postconditioning in patients with ST-segment elevation myocardial infarction failed to significantly reduce death from any cause or hospitalization for heart failure in the randomized, controlled DANAMI 3-iPOST trial.
At a mean follow-up of 37.5 months, the primary composite endpoint of death from any cause and hospitalization for heart failure occurred in 69 of 617 patients with STEMI who received standard angioplasty and in 65 of 617 patients who received ischemic postconditioning in DANAMI 3-iPOST (the Third Danish Study of Optimal Acute Treatment of Patients With ST-Segment Elevation Myocardial Infarction: iPOST conditioning during primary PCI). The 7% difference (hazard ratio, 0.93) was not statistically significant, Dr. Thomas Engstrøm reported at the annual meeting of the American College of Cardiology.
For the individual component of all-cause mortality, the reduction in the ischemic postconditioning group was 25%, occurring in 50 patients, compared with 38 in the conventionally treated group (hazard ratio, 0.75), but this difference also did not reach statistical significance, Dr. Engstrøm said.
Thirty patients in each group required hospitalization for heart failure.
However, an improvement in a secondary endpoint of left ventricular ejection fraction above 45% in patients with anterior infarcts was statistically significant, occurring in 72% of patients in the standard angioplasty group and 80% of those in the ischemic postconditioning group, said Dr. Engstrøm of Rigshospitalet University of Copenhagen.
This finding may translate into improved survival with longer follow-up, he noted.
Patients in the DANAMI-3 iPOST trial, who had a mean age of age 61 years, had acute STEMI (ST-segment elevation MI) symptoms of less than 12 hours’ duration at the time of randomization. They were followed for at least 2 years.
Ischemic postconditioning – a variation on angioplasty that involves using 30-second bursts of blood flow interspersed with 30-second pauses to restore blood flow to the heart – was shown in earlier studies to improve ST-segment resolution, reduce damage to heart muscle, and – in some patients – limit the extent of reperfusion injury.
Whether these factors would reduce hospitalizations or improve patient survival remained unclear, Dr. Engstrøm said.
Abrupt reperfusion by angioplasty may itself damage the heart muscle. In fact, up to 35% of patients may experience such injury during angioplasty.
“The thinking was that performing the reperfusion in a gentle, graded fashion would protect the heart against reperfusion injury,” Dr. Engstrøm explained.
The findings of DANAMI 3-iPOST – the first large clinical trial designed to evaluate clinical outcomes in STEMI patients (as opposed to surrogate endpoints such as ST-segment resolution) were disappointing, but larger trials may be required to definitively establish whether ischemic postconditioning improves clinical outcomes, Dr. Engstrøm said.
The DANAMI 3-iPOST trial was funded by the Danish Agency for Science, Technology, and Innovation and the Danish Council for Strategic Research. Dr. Engstrøm reported having no relevant financial disclosures.
CHICAGO – Ischemic postconditioning in patients with ST-segment elevation myocardial infarction failed to significantly reduce death from any cause or hospitalization for heart failure in the randomized, controlled DANAMI 3-iPOST trial.
At a mean follow-up of 37.5 months, the primary composite endpoint of death from any cause and hospitalization for heart failure occurred in 69 of 617 patients with STEMI who received standard angioplasty and in 65 of 617 patients who received ischemic postconditioning in DANAMI 3-iPOST (the Third Danish Study of Optimal Acute Treatment of Patients With ST-Segment Elevation Myocardial Infarction: iPOST conditioning during primary PCI). The 7% difference (hazard ratio, 0.93) was not statistically significant, Dr. Thomas Engstrøm reported at the annual meeting of the American College of Cardiology.
For the individual component of all-cause mortality, the reduction in the ischemic postconditioning group was 25%, occurring in 50 patients, compared with 38 in the conventionally treated group (hazard ratio, 0.75), but this difference also did not reach statistical significance, Dr. Engstrøm said.
Thirty patients in each group required hospitalization for heart failure.
However, an improvement in a secondary endpoint of left ventricular ejection fraction above 45% in patients with anterior infarcts was statistically significant, occurring in 72% of patients in the standard angioplasty group and 80% of those in the ischemic postconditioning group, said Dr. Engstrøm of Rigshospitalet University of Copenhagen.
This finding may translate into improved survival with longer follow-up, he noted.
Patients in the DANAMI-3 iPOST trial, who had a mean age of age 61 years, had acute STEMI (ST-segment elevation MI) symptoms of less than 12 hours’ duration at the time of randomization. They were followed for at least 2 years.
Ischemic postconditioning – a variation on angioplasty that involves using 30-second bursts of blood flow interspersed with 30-second pauses to restore blood flow to the heart – was shown in earlier studies to improve ST-segment resolution, reduce damage to heart muscle, and – in some patients – limit the extent of reperfusion injury.
Whether these factors would reduce hospitalizations or improve patient survival remained unclear, Dr. Engstrøm said.
Abrupt reperfusion by angioplasty may itself damage the heart muscle. In fact, up to 35% of patients may experience such injury during angioplasty.
“The thinking was that performing the reperfusion in a gentle, graded fashion would protect the heart against reperfusion injury,” Dr. Engstrøm explained.
The findings of DANAMI 3-iPOST – the first large clinical trial designed to evaluate clinical outcomes in STEMI patients (as opposed to surrogate endpoints such as ST-segment resolution) were disappointing, but larger trials may be required to definitively establish whether ischemic postconditioning improves clinical outcomes, Dr. Engstrøm said.
The DANAMI 3-iPOST trial was funded by the Danish Agency for Science, Technology, and Innovation and the Danish Council for Strategic Research. Dr. Engstrøm reported having no relevant financial disclosures.
AT ACC 16
Key clinical point: Ischemic postconditioning in patients with STEMI failed to significantly reduce death from any cause or hospitalization for heart failure in the randomized, controlled DANAMI 3-iPOST trial.
Major finding: No significant difference was seen in the primary composite endpoint of death from any cause and hospitalization for heart failure in standard angioplasty and ischemic postconditioning patients (HR, 0.93).
Data source: A randomized, controlled, open-label study of 1,234 patients from the DANAMI 3-iPOST trial.
Disclosures: The DANAMI 3-iPOST trial was funded by the Danish Agency for Science, Technology, and Innovation and the Danish Council for Strategic Research. Dr. Engstrøm reported having no relevant financial disclosures.
Stem cells show heart failure benefits in phase II trial
CHICAGO – After rattling around in early-stage clinical studies for more than a decade, stem cell therapy for heart failure may have finally gained the efficacy evidence to send it to the next level: large-scale, phase III trials.
Patients with ischemic cardiomyopathy and severe heart failure showed a statistically significant 37% relative reduction in their combined rate of death and cardiovascular hospitalization during 1 year of follow-up after autologous stem cell injections to their left ventricular myocardium in a multicenter, fully blinded control, phase II trial with 109 North American patients.
The treatment used a technique in commercial development by Vericel that selectively expands ex vivo bone marrow cells taken from the heart failure patient. Clinicians inject 0.4 mL aliquots of the expanded cells – enriched for mesenchymal stem cells and M2 macrophages – via a transcatheter approach into the left ventricular myocardium using 12-17 injections per patient. The bone marrow preparation during ex vivo expansion is called ixmyelocel-T.
This treatment now needs testing in more patients, Dr. Timothy D. Henry said at the annual meeting of the American College of Cardiology. “We need a new generation of cell trials in larger studies with completely double-blind, placebo controls using a more uniform preparation of cells,” said Dr. Henry.
“To the best of our knowledge, ixCELL-DCM is the largest randomized, double-blind clinical trial to date for cell therapy use in congestive heart failure,” said Dr. Henry and his associates in their report. The concept of stem cell therapy to replace damaged myocardium “has been very attractive, but most clinical trials to date have been small and unblinded, and used unselected bone marrow cells,” explained Dr. Henry, director of cardiology at the Cedars-Sinai Heart Institute in Los Angeles.
The ixCELL-DCM study ran at 31 sites in the United States and Canada. About 90% of patients had New York Heart Association class III disease, the average left ventricular ejection fraction was about 25%, patients on average would cover about 310 m during a 6-minute walk test, and the average serum level of NT-ProBNP was about 1,900 pg/L. Patients in the control arm all underwent the same bone marrow retrieval and transcatheter injection into the left ventricle, but the injections only contained carrier material without active cells.
The primary endpoint of death or a cardiovascular event, primarily hospitalization, occurred at a rate of 110 events per 100 patient years during 1-year follow-up of 51 patients in the sham-treatment group. In the active-treatment arm, the endpoint occurred at a rate of 70 events per 100 patient years among 58 patients. The difference was primarily driven by a 3% death rate with cell therapy, compared with a 14% rate in the controls, and a 38% hospitalization rate, compared with a 47% rate among controls.
The study results appeared online concurrent with Dr. Henry’s report (Lancet. 2016 Apr 5. doi: 10.1016/S0140-6736[16]30137-4).
The results showed no significant differences between the active and sham groups for changes in left ventricular size, ejection fraction, and 6-minute walk distance.
“This trial was designed to look at events. It is not a cause for concern that we did not see effects on heart function,” Dr. Henry said. The current results were also generally consistent with results from two earlier, controlled, phase II studies with a total of 61 patients (Circ Res. 2014 Sep 26;115[8]:730-7).
In the safety analysis, done in 114 patients, the rates of all adverse events and major adverse cardiovascular events were similar in the two arms. The rate of serious adverse events was significantly reduced in the patients treated with expanded bone marrow cells, compared with the controls.
The high rate of death and hospitalization of patients with severe heart failure “is a very large, unmet need, so it’s a natural to go to a larger trial,” Dr. Henry said. “The cell preparation was very safe and easy to do.”
Another pressing research issue is to try to understand the mechanism by which the cell treatment improves clinical outcomes, with improved heart function or improved exercise capacity apparently excluded as mechanisms.
The trial was sponsored by Vericel, the company developing the ex vivo protocol for selective marrow cell expansion. Dr. Henry has been a consultant to or received honoraria from Abbott Vascular, Baxter, Capricor, Cytori, Eli Lilly, and the Medicines Company, and he has received research grants from Aastrom, Baxter International, Mesoblast, and Vericel.
On Twitter @mitchelzoler
The results reported by Dr. Henry come from one of the first trials of stem cell or bone marrow treatment of failing hearts that used clinical outcomes as the primary endpoint. In contrast, prior studies focused on changes in functional characteristics of patients, such as 6-minute walk distance or left ventricular ejection fraction or size. What makes Dr. Henry’s study distinctive is that it showed benefit for a clinical outcome: the rate of death or cardiovascular hospitalization.
Another distinct difference, compared with the vast majority of earlier trials, was the way the bone marrow was handled prior to placement in a heart. The bone marrow cells underwent a 12-day period of ex vivo treatment designed to expand the content of certain mesenchymal stem cells and macrophages.
The current study was also larger than most prior reported studies, with 114 randomized patients available for the safety analysis and 109 for the efficacy analysis. But by no means was this a large study; in fact, it is relatively small. Although it produced a statistically significant result for the primary endpoint, the efficacy needs expanded testing in larger numbers.
It’s currently unclear how the expanded bone marrow cell injections improve clinical status and lead to reduced deaths and hospitalization. The results show essentially no impact from the treatment on ejection fraction or 6-minute walk distance, raising the question of what alternative mechanisms link this treatment to improved clinical outcomes.
Until now, it has not been possible to move beyond early-stage trial designs for cell therapy of failing hearts. Now, for the first time, we have study results that suggest a phase III trial is indicated.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Dr. John A. Jarcho is a deputy editor of the New England Journal of Medicine and a cardiologist at Brigham and Women’s Hospital, both in Boston. He had no disclosures. He made these comments as a discussant of Dr. Henry’s report and in an interview.
The results reported by Dr. Henry come from one of the first trials of stem cell or bone marrow treatment of failing hearts that used clinical outcomes as the primary endpoint. In contrast, prior studies focused on changes in functional characteristics of patients, such as 6-minute walk distance or left ventricular ejection fraction or size. What makes Dr. Henry’s study distinctive is that it showed benefit for a clinical outcome: the rate of death or cardiovascular hospitalization.
Another distinct difference, compared with the vast majority of earlier trials, was the way the bone marrow was handled prior to placement in a heart. The bone marrow cells underwent a 12-day period of ex vivo treatment designed to expand the content of certain mesenchymal stem cells and macrophages.
The current study was also larger than most prior reported studies, with 114 randomized patients available for the safety analysis and 109 for the efficacy analysis. But by no means was this a large study; in fact, it is relatively small. Although it produced a statistically significant result for the primary endpoint, the efficacy needs expanded testing in larger numbers.
It’s currently unclear how the expanded bone marrow cell injections improve clinical status and lead to reduced deaths and hospitalization. The results show essentially no impact from the treatment on ejection fraction or 6-minute walk distance, raising the question of what alternative mechanisms link this treatment to improved clinical outcomes.
Until now, it has not been possible to move beyond early-stage trial designs for cell therapy of failing hearts. Now, for the first time, we have study results that suggest a phase III trial is indicated.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Dr. John A. Jarcho is a deputy editor of the New England Journal of Medicine and a cardiologist at Brigham and Women’s Hospital, both in Boston. He had no disclosures. He made these comments as a discussant of Dr. Henry’s report and in an interview.
The results reported by Dr. Henry come from one of the first trials of stem cell or bone marrow treatment of failing hearts that used clinical outcomes as the primary endpoint. In contrast, prior studies focused on changes in functional characteristics of patients, such as 6-minute walk distance or left ventricular ejection fraction or size. What makes Dr. Henry’s study distinctive is that it showed benefit for a clinical outcome: the rate of death or cardiovascular hospitalization.
Another distinct difference, compared with the vast majority of earlier trials, was the way the bone marrow was handled prior to placement in a heart. The bone marrow cells underwent a 12-day period of ex vivo treatment designed to expand the content of certain mesenchymal stem cells and macrophages.
The current study was also larger than most prior reported studies, with 114 randomized patients available for the safety analysis and 109 for the efficacy analysis. But by no means was this a large study; in fact, it is relatively small. Although it produced a statistically significant result for the primary endpoint, the efficacy needs expanded testing in larger numbers.
It’s currently unclear how the expanded bone marrow cell injections improve clinical status and lead to reduced deaths and hospitalization. The results show essentially no impact from the treatment on ejection fraction or 6-minute walk distance, raising the question of what alternative mechanisms link this treatment to improved clinical outcomes.
Until now, it has not been possible to move beyond early-stage trial designs for cell therapy of failing hearts. Now, for the first time, we have study results that suggest a phase III trial is indicated.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Dr. John A. Jarcho is a deputy editor of the New England Journal of Medicine and a cardiologist at Brigham and Women’s Hospital, both in Boston. He had no disclosures. He made these comments as a discussant of Dr. Henry’s report and in an interview.
CHICAGO – After rattling around in early-stage clinical studies for more than a decade, stem cell therapy for heart failure may have finally gained the efficacy evidence to send it to the next level: large-scale, phase III trials.
Patients with ischemic cardiomyopathy and severe heart failure showed a statistically significant 37% relative reduction in their combined rate of death and cardiovascular hospitalization during 1 year of follow-up after autologous stem cell injections to their left ventricular myocardium in a multicenter, fully blinded control, phase II trial with 109 North American patients.
The treatment used a technique in commercial development by Vericel that selectively expands ex vivo bone marrow cells taken from the heart failure patient. Clinicians inject 0.4 mL aliquots of the expanded cells – enriched for mesenchymal stem cells and M2 macrophages – via a transcatheter approach into the left ventricular myocardium using 12-17 injections per patient. The bone marrow preparation during ex vivo expansion is called ixmyelocel-T.
This treatment now needs testing in more patients, Dr. Timothy D. Henry said at the annual meeting of the American College of Cardiology. “We need a new generation of cell trials in larger studies with completely double-blind, placebo controls using a more uniform preparation of cells,” said Dr. Henry.
“To the best of our knowledge, ixCELL-DCM is the largest randomized, double-blind clinical trial to date for cell therapy use in congestive heart failure,” said Dr. Henry and his associates in their report. The concept of stem cell therapy to replace damaged myocardium “has been very attractive, but most clinical trials to date have been small and unblinded, and used unselected bone marrow cells,” explained Dr. Henry, director of cardiology at the Cedars-Sinai Heart Institute in Los Angeles.
The ixCELL-DCM study ran at 31 sites in the United States and Canada. About 90% of patients had New York Heart Association class III disease, the average left ventricular ejection fraction was about 25%, patients on average would cover about 310 m during a 6-minute walk test, and the average serum level of NT-ProBNP was about 1,900 pg/L. Patients in the control arm all underwent the same bone marrow retrieval and transcatheter injection into the left ventricle, but the injections only contained carrier material without active cells.
The primary endpoint of death or a cardiovascular event, primarily hospitalization, occurred at a rate of 110 events per 100 patient years during 1-year follow-up of 51 patients in the sham-treatment group. In the active-treatment arm, the endpoint occurred at a rate of 70 events per 100 patient years among 58 patients. The difference was primarily driven by a 3% death rate with cell therapy, compared with a 14% rate in the controls, and a 38% hospitalization rate, compared with a 47% rate among controls.
The study results appeared online concurrent with Dr. Henry’s report (Lancet. 2016 Apr 5. doi: 10.1016/S0140-6736[16]30137-4).
The results showed no significant differences between the active and sham groups for changes in left ventricular size, ejection fraction, and 6-minute walk distance.
“This trial was designed to look at events. It is not a cause for concern that we did not see effects on heart function,” Dr. Henry said. The current results were also generally consistent with results from two earlier, controlled, phase II studies with a total of 61 patients (Circ Res. 2014 Sep 26;115[8]:730-7).
In the safety analysis, done in 114 patients, the rates of all adverse events and major adverse cardiovascular events were similar in the two arms. The rate of serious adverse events was significantly reduced in the patients treated with expanded bone marrow cells, compared with the controls.
The high rate of death and hospitalization of patients with severe heart failure “is a very large, unmet need, so it’s a natural to go to a larger trial,” Dr. Henry said. “The cell preparation was very safe and easy to do.”
Another pressing research issue is to try to understand the mechanism by which the cell treatment improves clinical outcomes, with improved heart function or improved exercise capacity apparently excluded as mechanisms.
The trial was sponsored by Vericel, the company developing the ex vivo protocol for selective marrow cell expansion. Dr. Henry has been a consultant to or received honoraria from Abbott Vascular, Baxter, Capricor, Cytori, Eli Lilly, and the Medicines Company, and he has received research grants from Aastrom, Baxter International, Mesoblast, and Vericel.
On Twitter @mitchelzoler
CHICAGO – After rattling around in early-stage clinical studies for more than a decade, stem cell therapy for heart failure may have finally gained the efficacy evidence to send it to the next level: large-scale, phase III trials.
Patients with ischemic cardiomyopathy and severe heart failure showed a statistically significant 37% relative reduction in their combined rate of death and cardiovascular hospitalization during 1 year of follow-up after autologous stem cell injections to their left ventricular myocardium in a multicenter, fully blinded control, phase II trial with 109 North American patients.
The treatment used a technique in commercial development by Vericel that selectively expands ex vivo bone marrow cells taken from the heart failure patient. Clinicians inject 0.4 mL aliquots of the expanded cells – enriched for mesenchymal stem cells and M2 macrophages – via a transcatheter approach into the left ventricular myocardium using 12-17 injections per patient. The bone marrow preparation during ex vivo expansion is called ixmyelocel-T.
This treatment now needs testing in more patients, Dr. Timothy D. Henry said at the annual meeting of the American College of Cardiology. “We need a new generation of cell trials in larger studies with completely double-blind, placebo controls using a more uniform preparation of cells,” said Dr. Henry.
“To the best of our knowledge, ixCELL-DCM is the largest randomized, double-blind clinical trial to date for cell therapy use in congestive heart failure,” said Dr. Henry and his associates in their report. The concept of stem cell therapy to replace damaged myocardium “has been very attractive, but most clinical trials to date have been small and unblinded, and used unselected bone marrow cells,” explained Dr. Henry, director of cardiology at the Cedars-Sinai Heart Institute in Los Angeles.
The ixCELL-DCM study ran at 31 sites in the United States and Canada. About 90% of patients had New York Heart Association class III disease, the average left ventricular ejection fraction was about 25%, patients on average would cover about 310 m during a 6-minute walk test, and the average serum level of NT-ProBNP was about 1,900 pg/L. Patients in the control arm all underwent the same bone marrow retrieval and transcatheter injection into the left ventricle, but the injections only contained carrier material without active cells.
The primary endpoint of death or a cardiovascular event, primarily hospitalization, occurred at a rate of 110 events per 100 patient years during 1-year follow-up of 51 patients in the sham-treatment group. In the active-treatment arm, the endpoint occurred at a rate of 70 events per 100 patient years among 58 patients. The difference was primarily driven by a 3% death rate with cell therapy, compared with a 14% rate in the controls, and a 38% hospitalization rate, compared with a 47% rate among controls.
The study results appeared online concurrent with Dr. Henry’s report (Lancet. 2016 Apr 5. doi: 10.1016/S0140-6736[16]30137-4).
The results showed no significant differences between the active and sham groups for changes in left ventricular size, ejection fraction, and 6-minute walk distance.
“This trial was designed to look at events. It is not a cause for concern that we did not see effects on heart function,” Dr. Henry said. The current results were also generally consistent with results from two earlier, controlled, phase II studies with a total of 61 patients (Circ Res. 2014 Sep 26;115[8]:730-7).
In the safety analysis, done in 114 patients, the rates of all adverse events and major adverse cardiovascular events were similar in the two arms. The rate of serious adverse events was significantly reduced in the patients treated with expanded bone marrow cells, compared with the controls.
The high rate of death and hospitalization of patients with severe heart failure “is a very large, unmet need, so it’s a natural to go to a larger trial,” Dr. Henry said. “The cell preparation was very safe and easy to do.”
Another pressing research issue is to try to understand the mechanism by which the cell treatment improves clinical outcomes, with improved heart function or improved exercise capacity apparently excluded as mechanisms.
The trial was sponsored by Vericel, the company developing the ex vivo protocol for selective marrow cell expansion. Dr. Henry has been a consultant to or received honoraria from Abbott Vascular, Baxter, Capricor, Cytori, Eli Lilly, and the Medicines Company, and he has received research grants from Aastrom, Baxter International, Mesoblast, and Vericel.
On Twitter @mitchelzoler
AT ACC 16
Key clinical point: Severe, ischemic heart failure patients had a significant cut in death and cardiovascular hospitalizations 1 year after endovascular myocardial injection with selectively expanded autologous bone marrow cells in a fully blinded, placebo-controlled phase II study.
Major finding: Cell-treated patients had a 37% drop in death and cardiovascular hospitalization relative to controls in 1-year follow-up.
Data source: A multicenter, fully blinded study with 109 patients for the per protocol efficacy analysis, and 114 patients for the safety analysis.
Disclosures: The trial was sponsored by Vericel, the company developing the ex vivo protocol for selective marrow cell expansion. Dr. Henry has been a consultant to or received honoraria from Abbott Vascular, Baxter, Capricor, Cytori, Eli Lilly, and the Medicines Company, and he has received research grants from Aastrom, Baxter International, Mesoblast, and Vericel.