User login
FDA adds safety warnings to certain type 2 diabetes medications
Type 2 diabetes medicines that contain saxagliptin and alogliptin may increase the risk of heart failure, especially in patients who already have heart or kidney disease, according to results from an Food and Drug Administration safety review.
The development, which was announced by MedWatch on April 5, 2016, means that the FDA will add new warnings to the drug labels about this safety issue. “Health care professionals should consider discontinuing medications containing saxagliptin and alogliptin in patients who develop heart failure and monitor their diabetes control,” the communication states. “If a patient’s blood sugar level is not well-controlled with their current treatment, other diabetes medicines may be required.”
The medications of concern include Onglyza (saxagliptin); Kombiglyze XR (saxagliptin and metformin extended release); Nesina (alogliptin); Kazano (alogliptin and metformin), and Oseni (alogliptin and pioglitazone). The move comes after two clinical trials showed that more patients who received saxagliptin- or alogliptin-containing medicines were hospitalized for heart failure, compared with patients who received placebo (for specifics, see the data summary section in the FDA Drug Safety Communication).
The communication noted that patients taking these medicines should contact their health care clinician if they develop signs and symptoms of heart failure such as: unusual shortness of breath during daily activities; trouble breathing when lying down; tiredness, weakness, or fatigue; and weight gain with swelling in the ankles, feet, legs, or stomach.
Clinicians and patients can report adverse events or side effects related to the use of these products at www.accessdata.fda.gov/scripts/medwatch/index.cfm?action=reporting.home.
Type 2 diabetes medicines that contain saxagliptin and alogliptin may increase the risk of heart failure, especially in patients who already have heart or kidney disease, according to results from an Food and Drug Administration safety review.
The development, which was announced by MedWatch on April 5, 2016, means that the FDA will add new warnings to the drug labels about this safety issue. “Health care professionals should consider discontinuing medications containing saxagliptin and alogliptin in patients who develop heart failure and monitor their diabetes control,” the communication states. “If a patient’s blood sugar level is not well-controlled with their current treatment, other diabetes medicines may be required.”
The medications of concern include Onglyza (saxagliptin); Kombiglyze XR (saxagliptin and metformin extended release); Nesina (alogliptin); Kazano (alogliptin and metformin), and Oseni (alogliptin and pioglitazone). The move comes after two clinical trials showed that more patients who received saxagliptin- or alogliptin-containing medicines were hospitalized for heart failure, compared with patients who received placebo (for specifics, see the data summary section in the FDA Drug Safety Communication).
The communication noted that patients taking these medicines should contact their health care clinician if they develop signs and symptoms of heart failure such as: unusual shortness of breath during daily activities; trouble breathing when lying down; tiredness, weakness, or fatigue; and weight gain with swelling in the ankles, feet, legs, or stomach.
Clinicians and patients can report adverse events or side effects related to the use of these products at www.accessdata.fda.gov/scripts/medwatch/index.cfm?action=reporting.home.
Type 2 diabetes medicines that contain saxagliptin and alogliptin may increase the risk of heart failure, especially in patients who already have heart or kidney disease, according to results from an Food and Drug Administration safety review.
The development, which was announced by MedWatch on April 5, 2016, means that the FDA will add new warnings to the drug labels about this safety issue. “Health care professionals should consider discontinuing medications containing saxagliptin and alogliptin in patients who develop heart failure and monitor their diabetes control,” the communication states. “If a patient’s blood sugar level is not well-controlled with their current treatment, other diabetes medicines may be required.”
The medications of concern include Onglyza (saxagliptin); Kombiglyze XR (saxagliptin and metformin extended release); Nesina (alogliptin); Kazano (alogliptin and metformin), and Oseni (alogliptin and pioglitazone). The move comes after two clinical trials showed that more patients who received saxagliptin- or alogliptin-containing medicines were hospitalized for heart failure, compared with patients who received placebo (for specifics, see the data summary section in the FDA Drug Safety Communication).
The communication noted that patients taking these medicines should contact their health care clinician if they develop signs and symptoms of heart failure such as: unusual shortness of breath during daily activities; trouble breathing when lying down; tiredness, weakness, or fatigue; and weight gain with swelling in the ankles, feet, legs, or stomach.
Clinicians and patients can report adverse events or side effects related to the use of these products at www.accessdata.fda.gov/scripts/medwatch/index.cfm?action=reporting.home.
High-dose vitamin D improves heart structure, function in chronic heart failure
High-dose oral vitamin D supplements taken for 1 year significantly improved cardiac structure and function in patients with chronic heart failure secondary to left ventricular systolic dysfunction, according to results from a new study.
However, the same study. led by Dr. Klaus Witte of the University of Leeds (England), found that 6-minute walk distance – the study’s primary outcome measure – was not improved after a year’s supplementation with vitamin D.
It is unclear why vitamin D deficiency co-occurs in a majority of people with chronic heart failure (CHF) due to left ventricular systolic dysfunction (LVSD) or to what degree reversing it can improve outcomes. However, vitamin D deficiency is thought to interfere with calcium transport in cardiac cells, and may contribute to cardiac fibrosis and inflammation, leading to faster progression to heart failure following damage to cardiac muscle.
The new VINDICATE study randomized 223 patients with CHF due to LVSD and vitamin D deficiency to 1 year’s treatment with 4,000 IU of 25(OH) vitamin D3 daily, or placebo, Dr. Witte and associates concluded at the annual meeting of the American College of Cardiology. The results were published online April 4 in JACC (doi: 10.1016/j.jacc.2016.03.508).
Of these patients, 163 completed follow-up at 12 months, and 6-minute walk distance (MWT) and echocardiography findings were recorded at baseline and follow-up.
Dr. Witte and colleagues found significant evidence of improved function in the vitamin D–treated patients as measured by left ventricular ejection fraction +6.07% (95% confidence interval 3.20, 8.95; P less than .0001); and a reversal of left ventricular remodeling (left ventricular end diastolic diameter –2.49 mm (95% CI –4.09, –0.90; P equal to .002) and left ventricular end systolic diameter –2.09 mm (95% CI –4.11; –0.06; P equal to .043).
The researchers also drew blood at 3-month intervals to check for serum calcium concentration, renal function, and vitamin D levels. Treatment was well tolerated, and no patients suffered hypervitaminosis or required a dose adjustment.
“There was no effect of vitamin D supplementation on the primary endpoint of 6 MWT distance but there were statistically significant, and prognostically and clinically relevant improvements in the secondary outcomes of left ventricular ejection fraction, dimensions, and volumes, suggesting that vitamin D is leading to beneficial reverse remodeling,” the investigators wrote in their analysis.
The study’s failure to meet its primary endpoint despite significant results from its secondary endpoints led Dr. Witte and colleagues to say that its design led to underpowering.
“Variability in the walk distance measure at baseline was much greater than predicted from our pilot study such that our sample size only had 7% post hoc power to detect a difference between the groups,” meaning it was underpowered to detect a clinically relevant change in walk distance. The findings “have implications for future studies using 6-minute walk distance as an outcome measure,” they wrote.
The investigators championed the addition of vitamin D3 to CHF treatment regimens.
As new therapies for CHF are “often expensive, increasingly technical, and frequently fail to meet the rigorous demands of large phase III clinical trials,” Dr. Witte and colleagues wrote, vitamin D “might be a cheap and safe additional option for CHF patients and may have beneficial effects on multiple features of the syndrome.”
The U.K.’s National Institute for Health Research supported the study, and none of its authors declared conflicts of interest.
High-dose oral vitamin D supplements taken for 1 year significantly improved cardiac structure and function in patients with chronic heart failure secondary to left ventricular systolic dysfunction, according to results from a new study.
However, the same study. led by Dr. Klaus Witte of the University of Leeds (England), found that 6-minute walk distance – the study’s primary outcome measure – was not improved after a year’s supplementation with vitamin D.
It is unclear why vitamin D deficiency co-occurs in a majority of people with chronic heart failure (CHF) due to left ventricular systolic dysfunction (LVSD) or to what degree reversing it can improve outcomes. However, vitamin D deficiency is thought to interfere with calcium transport in cardiac cells, and may contribute to cardiac fibrosis and inflammation, leading to faster progression to heart failure following damage to cardiac muscle.
The new VINDICATE study randomized 223 patients with CHF due to LVSD and vitamin D deficiency to 1 year’s treatment with 4,000 IU of 25(OH) vitamin D3 daily, or placebo, Dr. Witte and associates concluded at the annual meeting of the American College of Cardiology. The results were published online April 4 in JACC (doi: 10.1016/j.jacc.2016.03.508).
Of these patients, 163 completed follow-up at 12 months, and 6-minute walk distance (MWT) and echocardiography findings were recorded at baseline and follow-up.
Dr. Witte and colleagues found significant evidence of improved function in the vitamin D–treated patients as measured by left ventricular ejection fraction +6.07% (95% confidence interval 3.20, 8.95; P less than .0001); and a reversal of left ventricular remodeling (left ventricular end diastolic diameter –2.49 mm (95% CI –4.09, –0.90; P equal to .002) and left ventricular end systolic diameter –2.09 mm (95% CI –4.11; –0.06; P equal to .043).
The researchers also drew blood at 3-month intervals to check for serum calcium concentration, renal function, and vitamin D levels. Treatment was well tolerated, and no patients suffered hypervitaminosis or required a dose adjustment.
“There was no effect of vitamin D supplementation on the primary endpoint of 6 MWT distance but there were statistically significant, and prognostically and clinically relevant improvements in the secondary outcomes of left ventricular ejection fraction, dimensions, and volumes, suggesting that vitamin D is leading to beneficial reverse remodeling,” the investigators wrote in their analysis.
The study’s failure to meet its primary endpoint despite significant results from its secondary endpoints led Dr. Witte and colleagues to say that its design led to underpowering.
“Variability in the walk distance measure at baseline was much greater than predicted from our pilot study such that our sample size only had 7% post hoc power to detect a difference between the groups,” meaning it was underpowered to detect a clinically relevant change in walk distance. The findings “have implications for future studies using 6-minute walk distance as an outcome measure,” they wrote.
The investigators championed the addition of vitamin D3 to CHF treatment regimens.
As new therapies for CHF are “often expensive, increasingly technical, and frequently fail to meet the rigorous demands of large phase III clinical trials,” Dr. Witte and colleagues wrote, vitamin D “might be a cheap and safe additional option for CHF patients and may have beneficial effects on multiple features of the syndrome.”
The U.K.’s National Institute for Health Research supported the study, and none of its authors declared conflicts of interest.
High-dose oral vitamin D supplements taken for 1 year significantly improved cardiac structure and function in patients with chronic heart failure secondary to left ventricular systolic dysfunction, according to results from a new study.
However, the same study. led by Dr. Klaus Witte of the University of Leeds (England), found that 6-minute walk distance – the study’s primary outcome measure – was not improved after a year’s supplementation with vitamin D.
It is unclear why vitamin D deficiency co-occurs in a majority of people with chronic heart failure (CHF) due to left ventricular systolic dysfunction (LVSD) or to what degree reversing it can improve outcomes. However, vitamin D deficiency is thought to interfere with calcium transport in cardiac cells, and may contribute to cardiac fibrosis and inflammation, leading to faster progression to heart failure following damage to cardiac muscle.
The new VINDICATE study randomized 223 patients with CHF due to LVSD and vitamin D deficiency to 1 year’s treatment with 4,000 IU of 25(OH) vitamin D3 daily, or placebo, Dr. Witte and associates concluded at the annual meeting of the American College of Cardiology. The results were published online April 4 in JACC (doi: 10.1016/j.jacc.2016.03.508).
Of these patients, 163 completed follow-up at 12 months, and 6-minute walk distance (MWT) and echocardiography findings were recorded at baseline and follow-up.
Dr. Witte and colleagues found significant evidence of improved function in the vitamin D–treated patients as measured by left ventricular ejection fraction +6.07% (95% confidence interval 3.20, 8.95; P less than .0001); and a reversal of left ventricular remodeling (left ventricular end diastolic diameter –2.49 mm (95% CI –4.09, –0.90; P equal to .002) and left ventricular end systolic diameter –2.09 mm (95% CI –4.11; –0.06; P equal to .043).
The researchers also drew blood at 3-month intervals to check for serum calcium concentration, renal function, and vitamin D levels. Treatment was well tolerated, and no patients suffered hypervitaminosis or required a dose adjustment.
“There was no effect of vitamin D supplementation on the primary endpoint of 6 MWT distance but there were statistically significant, and prognostically and clinically relevant improvements in the secondary outcomes of left ventricular ejection fraction, dimensions, and volumes, suggesting that vitamin D is leading to beneficial reverse remodeling,” the investigators wrote in their analysis.
The study’s failure to meet its primary endpoint despite significant results from its secondary endpoints led Dr. Witte and colleagues to say that its design led to underpowering.
“Variability in the walk distance measure at baseline was much greater than predicted from our pilot study such that our sample size only had 7% post hoc power to detect a difference between the groups,” meaning it was underpowered to detect a clinically relevant change in walk distance. The findings “have implications for future studies using 6-minute walk distance as an outcome measure,” they wrote.
The investigators championed the addition of vitamin D3 to CHF treatment regimens.
As new therapies for CHF are “often expensive, increasingly technical, and frequently fail to meet the rigorous demands of large phase III clinical trials,” Dr. Witte and colleagues wrote, vitamin D “might be a cheap and safe additional option for CHF patients and may have beneficial effects on multiple features of the syndrome.”
The U.K.’s National Institute for Health Research supported the study, and none of its authors declared conflicts of interest.
FROM ACC16
Key clinical point: Oral supplementation of high-dose vitamin D3 led to significantly improved left ventricular function and structure in a cohort of vitamin-deficient patients.
Major finding: Treated patients had significantly improved left ventricular ejection fraction of +6.07% vs. nontreated patients at 1 year, and significant reversal of left ventricular remodeling (left ventricular end diastolic diameter –2.49 mm and left ventricular end systolic diameter –2.09 mm).
Data source: A single-site randomized trial in which 229 patients with LV CHF received high-dose vitamin D or placebo for 12 months.
Disclosures: The U.K.’s National Institute for Health Research supported the study, and none of its authors declared conflicts of interest.
VIDEO: STICHES trial update boosts CABG in ischemic cardiomyopathy
CHICAGO – The results of the Surgical Treatment for Ischemic Heart Failure Extension Study (STICHES) presented at the annual meeting of the American College of Cardiology ought to change the clinical management of patients with coronary artery disease and heart failure with severe left ventricular dysfunction, according to Dr. Robert O. Bonow.
STICHES is the 10-year follow-up of 1,212 such patients who were randomized to coronary artery bypass graft surgery plus optimal guideline-directed medical therapy or to the medical therapy alone. At 10 years, the CABG group showed a significant survival advantage: an all-cause mortality rate of 58.9%, a significant 16% relative risk reduction compared with the 66.1% rate in the medically managed group. Secondary endpoints were also strongly in favor of the CABG group.
These findings indicate CABG is beneficial in patients with ischemic cardiomyopathy, and patients deserve to be so informed, according to Dr. Bonow, a member of the STICHES publication committee and professor of cardiology and director of the Center for Cardiovascular Innovation at Northwestern University in Chicago, who discussed the findings in this video interview.
He reported having no financial conflicts regarding this National Institutes of Health–funded study.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – The results of the Surgical Treatment for Ischemic Heart Failure Extension Study (STICHES) presented at the annual meeting of the American College of Cardiology ought to change the clinical management of patients with coronary artery disease and heart failure with severe left ventricular dysfunction, according to Dr. Robert O. Bonow.
STICHES is the 10-year follow-up of 1,212 such patients who were randomized to coronary artery bypass graft surgery plus optimal guideline-directed medical therapy or to the medical therapy alone. At 10 years, the CABG group showed a significant survival advantage: an all-cause mortality rate of 58.9%, a significant 16% relative risk reduction compared with the 66.1% rate in the medically managed group. Secondary endpoints were also strongly in favor of the CABG group.
These findings indicate CABG is beneficial in patients with ischemic cardiomyopathy, and patients deserve to be so informed, according to Dr. Bonow, a member of the STICHES publication committee and professor of cardiology and director of the Center for Cardiovascular Innovation at Northwestern University in Chicago, who discussed the findings in this video interview.
He reported having no financial conflicts regarding this National Institutes of Health–funded study.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – The results of the Surgical Treatment for Ischemic Heart Failure Extension Study (STICHES) presented at the annual meeting of the American College of Cardiology ought to change the clinical management of patients with coronary artery disease and heart failure with severe left ventricular dysfunction, according to Dr. Robert O. Bonow.
STICHES is the 10-year follow-up of 1,212 such patients who were randomized to coronary artery bypass graft surgery plus optimal guideline-directed medical therapy or to the medical therapy alone. At 10 years, the CABG group showed a significant survival advantage: an all-cause mortality rate of 58.9%, a significant 16% relative risk reduction compared with the 66.1% rate in the medically managed group. Secondary endpoints were also strongly in favor of the CABG group.
These findings indicate CABG is beneficial in patients with ischemic cardiomyopathy, and patients deserve to be so informed, according to Dr. Bonow, a member of the STICHES publication committee and professor of cardiology and director of the Center for Cardiovascular Innovation at Northwestern University in Chicago, who discussed the findings in this video interview.
He reported having no financial conflicts regarding this National Institutes of Health–funded study.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT ACC 16
VIDEO: STICHES trial update boosts CABG in ischemic cardiomyopathy
CHICAGO – The results of the Surgical Treatment for Ischemic Heart Failure Extension Study (STICHES) presented at the annual meeting of the American College of Cardiology ought to change the clinical management of patients with coronary artery disease and heart failure with severe left ventricular dysfunction, according to Dr. Robert O. Bonow.
STICHES is the 10-year follow-up of 1,212 such patients who were randomized to coronary artery bypass graft surgery plus optimal guideline-directed medical therapy or to the medical therapy alone. At 10 years, the CABG group showed a significant survival advantage: an all-cause mortality rate of 58.9%, a significant 16% relative risk reduction compared with the 66.1% rate in the medically managed group. Secondary endpoints were also strongly in favor of the CABG group.
These findings indicate CABG is beneficial in patients with ischemic cardiomyopathy, and patients deserve to be so informed, according to Dr. Bonow, a member of the STICHES publication committee and professor of cardiology and director of the Center for Cardiovascular Innovation at Northwestern University in Chicago, who discussed the findings in this video interview.
He reported having no financial conflicts regarding this National Institutes of Health–funded study.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – The results of the Surgical Treatment for Ischemic Heart Failure Extension Study (STICHES) presented at the annual meeting of the American College of Cardiology ought to change the clinical management of patients with coronary artery disease and heart failure with severe left ventricular dysfunction, according to Dr. Robert O. Bonow.
STICHES is the 10-year follow-up of 1,212 such patients who were randomized to coronary artery bypass graft surgery plus optimal guideline-directed medical therapy or to the medical therapy alone. At 10 years, the CABG group showed a significant survival advantage: an all-cause mortality rate of 58.9%, a significant 16% relative risk reduction compared with the 66.1% rate in the medically managed group. Secondary endpoints were also strongly in favor of the CABG group.
These findings indicate CABG is beneficial in patients with ischemic cardiomyopathy, and patients deserve to be so informed, according to Dr. Bonow, a member of the STICHES publication committee and professor of cardiology and director of the Center for Cardiovascular Innovation at Northwestern University in Chicago, who discussed the findings in this video interview.
He reported having no financial conflicts regarding this National Institutes of Health–funded study.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – The results of the Surgical Treatment for Ischemic Heart Failure Extension Study (STICHES) presented at the annual meeting of the American College of Cardiology ought to change the clinical management of patients with coronary artery disease and heart failure with severe left ventricular dysfunction, according to Dr. Robert O. Bonow.
STICHES is the 10-year follow-up of 1,212 such patients who were randomized to coronary artery bypass graft surgery plus optimal guideline-directed medical therapy or to the medical therapy alone. At 10 years, the CABG group showed a significant survival advantage: an all-cause mortality rate of 58.9%, a significant 16% relative risk reduction compared with the 66.1% rate in the medically managed group. Secondary endpoints were also strongly in favor of the CABG group.
These findings indicate CABG is beneficial in patients with ischemic cardiomyopathy, and patients deserve to be so informed, according to Dr. Bonow, a member of the STICHES publication committee and professor of cardiology and director of the Center for Cardiovascular Innovation at Northwestern University in Chicago, who discussed the findings in this video interview.
He reported having no financial conflicts regarding this National Institutes of Health–funded study.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT ACC 16
Heart failure severity at AMI predicts long-term CV death risk
CHICAGO – The severity of heart failure in the setting of acute myocardial infarction predicts long-term cardiovascular death risk, according to a post hoc analysis of data from the IMPROVE IT Trial.
Among 11,185 individuals with MI and known Killip Classification who were part of that randomized, double-blind trial, those with Killip Class II or greater had more than double the risk of long-term cardiovascular death, compared with those with Killip Class I heart failure, Dr Michael G. Silverman, a cardiovascular medicine fellow at Brigham and Women’s Hospital, Boston and a research fellow at the Thrombolysis in MI (TIMI) Study Group reported in a poster at the annual meeting of the American College of Cardiology.
After adjusting for a number of factors, including age, gender, diabetes, hypertension, left ventricular ejection fraction (LVEF), beta blocker and ACE inhibitor/angiotensin receptor blocker use at randomization, and percutaneous coronary intervention at the index event, the 7-year event rate was 14.5% among those with Killip Class II or higher vs. 5.7% those with Killip Class I heart failure (adjusted hazard ratio, 1.9), Dr. Silverman reported on behalf of the TIMI Study Group.
The event rates from 30 days to 6 months were 4.85% and 1.25% in the groups, respectively (adjusted hazard ratio, 1.96), and from 6 months to 7 years they were 1.52% and 0.61%, in the groups, respectively, (adjusted hazard ratio, 1.85).
Further, the increased risk of cardiovascular death associated with Killip Class II or higher was also apparent among important subgroups, including those with ST Segment Elevation MI, those with non-STEMI, those with LVEF of 50 or greater, those with LVEF less than 50, those with diabetes, those without diabetes, men, and women (adjusted hazard ratios ranging from 1.6 to 2.1), Dr Silverman explained in an interview.
The severity of heart failure according to Killip Class is a strong independent predictor of mortality in the setting of acute MI, and the current findings demonstrate that it also predicts cardiovascular death for at least 7 years, suggesting a need for careful attention to the findings of the physical exam in AMI, as it can serve as an important biomarker of long-term cardiovascular death risk, he said.
“AMI patients with Killip Class II or greater warrant continued close medical follow-up and adherence to guideline -directed medial therapy beyond the acute hospitalization to prevent this potentially modifiable outcome,” he concluded.
Dr. Silverman reported having no disclosures.
CHICAGO – The severity of heart failure in the setting of acute myocardial infarction predicts long-term cardiovascular death risk, according to a post hoc analysis of data from the IMPROVE IT Trial.
Among 11,185 individuals with MI and known Killip Classification who were part of that randomized, double-blind trial, those with Killip Class II or greater had more than double the risk of long-term cardiovascular death, compared with those with Killip Class I heart failure, Dr Michael G. Silverman, a cardiovascular medicine fellow at Brigham and Women’s Hospital, Boston and a research fellow at the Thrombolysis in MI (TIMI) Study Group reported in a poster at the annual meeting of the American College of Cardiology.
After adjusting for a number of factors, including age, gender, diabetes, hypertension, left ventricular ejection fraction (LVEF), beta blocker and ACE inhibitor/angiotensin receptor blocker use at randomization, and percutaneous coronary intervention at the index event, the 7-year event rate was 14.5% among those with Killip Class II or higher vs. 5.7% those with Killip Class I heart failure (adjusted hazard ratio, 1.9), Dr. Silverman reported on behalf of the TIMI Study Group.
The event rates from 30 days to 6 months were 4.85% and 1.25% in the groups, respectively (adjusted hazard ratio, 1.96), and from 6 months to 7 years they were 1.52% and 0.61%, in the groups, respectively, (adjusted hazard ratio, 1.85).
Further, the increased risk of cardiovascular death associated with Killip Class II or higher was also apparent among important subgroups, including those with ST Segment Elevation MI, those with non-STEMI, those with LVEF of 50 or greater, those with LVEF less than 50, those with diabetes, those without diabetes, men, and women (adjusted hazard ratios ranging from 1.6 to 2.1), Dr Silverman explained in an interview.
The severity of heart failure according to Killip Class is a strong independent predictor of mortality in the setting of acute MI, and the current findings demonstrate that it also predicts cardiovascular death for at least 7 years, suggesting a need for careful attention to the findings of the physical exam in AMI, as it can serve as an important biomarker of long-term cardiovascular death risk, he said.
“AMI patients with Killip Class II or greater warrant continued close medical follow-up and adherence to guideline -directed medial therapy beyond the acute hospitalization to prevent this potentially modifiable outcome,” he concluded.
Dr. Silverman reported having no disclosures.
CHICAGO – The severity of heart failure in the setting of acute myocardial infarction predicts long-term cardiovascular death risk, according to a post hoc analysis of data from the IMPROVE IT Trial.
Among 11,185 individuals with MI and known Killip Classification who were part of that randomized, double-blind trial, those with Killip Class II or greater had more than double the risk of long-term cardiovascular death, compared with those with Killip Class I heart failure, Dr Michael G. Silverman, a cardiovascular medicine fellow at Brigham and Women’s Hospital, Boston and a research fellow at the Thrombolysis in MI (TIMI) Study Group reported in a poster at the annual meeting of the American College of Cardiology.
After adjusting for a number of factors, including age, gender, diabetes, hypertension, left ventricular ejection fraction (LVEF), beta blocker and ACE inhibitor/angiotensin receptor blocker use at randomization, and percutaneous coronary intervention at the index event, the 7-year event rate was 14.5% among those with Killip Class II or higher vs. 5.7% those with Killip Class I heart failure (adjusted hazard ratio, 1.9), Dr. Silverman reported on behalf of the TIMI Study Group.
The event rates from 30 days to 6 months were 4.85% and 1.25% in the groups, respectively (adjusted hazard ratio, 1.96), and from 6 months to 7 years they were 1.52% and 0.61%, in the groups, respectively, (adjusted hazard ratio, 1.85).
Further, the increased risk of cardiovascular death associated with Killip Class II or higher was also apparent among important subgroups, including those with ST Segment Elevation MI, those with non-STEMI, those with LVEF of 50 or greater, those with LVEF less than 50, those with diabetes, those without diabetes, men, and women (adjusted hazard ratios ranging from 1.6 to 2.1), Dr Silverman explained in an interview.
The severity of heart failure according to Killip Class is a strong independent predictor of mortality in the setting of acute MI, and the current findings demonstrate that it also predicts cardiovascular death for at least 7 years, suggesting a need for careful attention to the findings of the physical exam in AMI, as it can serve as an important biomarker of long-term cardiovascular death risk, he said.
“AMI patients with Killip Class II or greater warrant continued close medical follow-up and adherence to guideline -directed medial therapy beyond the acute hospitalization to prevent this potentially modifiable outcome,” he concluded.
Dr. Silverman reported having no disclosures.
AT ACC 16
Key clinical point: The severity of heart failure in the setting of acute myocardial infarction predicts long-term cardiovascular death risk, according to a post hoc analysis of data from the IMPROVE IT Trial.
Major finding: The 7-year event rate was 14.5% among those with Killip Class II or higher vs. 5.7% among those with Killip Class I heart failure (adjusted hazard ratio, 1.9).
Data source: A post-hoc analysis of data from 11,185 subjects from the IMPROVE IT trial.
Disclosures: Dr. Silverman reported having no disclosures.
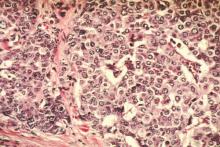
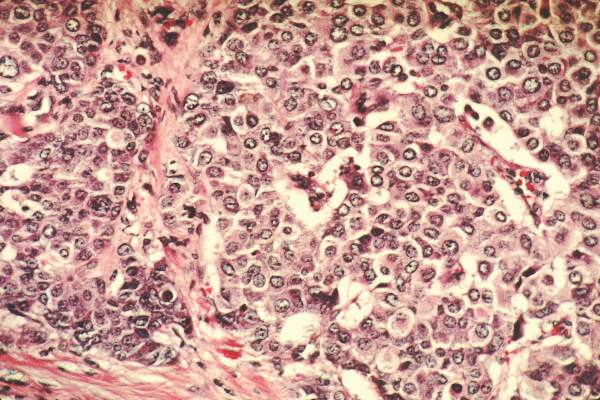
Breast cancer treatment linked to mild systolic dysfunction
AMSTERDAM – Breast cancer patients who underwent chemotherapy or radiotherapy had about a two-fold increased prevalence of mild systolic cardiac dysfunction a median of 10 years after treatment, compared with age-matched controls in a study that included a total of 700 people.
But even longer follow-up of treated breast cancer patients is needed to determine whether the excess mild cardiac dysfunction seen in this analysis eventually progresses to more severe cardiac impairment, Liselotte M. Boerman said at the European Breast Cancer Conference.
Data from the Breast Cancer Long-term Outcome of Cardiac Dysfunction (BLOC) study showed that 175 breast cancer patients who received chemotherapy (and may have also received radiotherapy) had a 2.5-fold higher prevalence of a left ventricular ejection fraction (LVEF) below 54% (95% confidence interval, 1.2-5.4) when measured by echocardiography a median of 10 years after treatment, compared with an equal number of age-matched individuals from the general population.
A separate group of 175 patients treated with radiotherapy only and evaluated by echocardiography a median of 10 years later had a 2.3-fold increased prevalence (1.1-4.7) of a LVEF below 54% when compared with an equal number of age-matched individuals, said Ms. Boerman, an epidemiology researcher at the University of Groningen (the Netherlands).
This degree of left-ventricular dysfunction was found in 15% of the chemotherapy patients and 6% of their controls, and in 16% of the radiotherapy patients and 8% of their controls.
However, the treated breast cancer patients had no long-term increase in their prevalence of more significant systolic cardiac dysfunction, defined as a LVEF of less than 45%, compared with the controls, and the overall rate of systolic dysfunction of this severity was low, affecting fewer than 1% of patients.
Also, the chemotherapy and radiotherapy patients showed no significant increase in the prevalence of diastolic cardiac dysfunction, defined as delayed cardiac relaxation beyond the age-appropriate range. Treated patients did show, after 10 years, a suggestion of an increased prevalence of diagnosed cardiovascular disease, which was 2.3-fold higher (1.0-4.9) in the chemotherapy-receiving patients, compared with their controls; and 70% higher (0.9-3.4) among the patients treated with radiotherapy, compared with their controls, Ms. Boerman said.
The study used data collected from breast cancer patients younger than 80 years old treated after 1980 and controls seen by general practice Dutch physicians. The chemotherapy patients were diagnosed at an average age of 49 years old (range 26-66 years old). About 78% had received treatment with an anthracycline agent and 7% had received trastuzumab (Herceptin). Radiotherapy had also been administered to 70%, while 62% had also received hormonal therapy, and 7% either had a recurrence or developed a tumor in their contralateral breast.
None of the radiotherapy-only patients had received chemotherapy, but 21% had also received hormonal therapy. Their average age at diagnosis was 54 years old (range 32-79 years old), and 10% had a recurrence or a contralateral tumor.
Follow-up echocardiography occurred 5-34 years after the index treatment, at a median age of 60 years old. Echocardiography follow-up occurred in 70% of the chemotherapy breast cancer patients contacted, and in 63% of those who received radiotherapy only. Among controls, about half of those selected by age matching, and contacted, agreed to participate.
Rates of cardiovascular-disease risk factors – dyslipidemia, hypertension, and diabetes – were at roughly similar levels in the cases and controls at the time of breast cancer diagnosis. But the rate of current smoking at the time of diagnosis appeared higher in the cases (30% among those who received chemotherapy and 33% among those on radiotherapy), compared with their respective control groups (22% and 30%). Ms. Boerman said that a multivariate analysis had not yet been run on the data but should occur soon.
“The prevalence of cardiac dysfunction was higher [in treated patients] than I would have expected, but there is potential bias as only 70% of invited patients actually participated,” commented Dr. Robert Mansel, a professor at the Institute of Cancer & Genetics at Cardiff University (Wales). He also noted the very low rate of patients who developed severe cardiac dysfunction.
Ms. Boerman and Dr. Mansel reported having no financial disclosures.
On Twitter @mitchelzoler
AMSTERDAM – Breast cancer patients who underwent chemotherapy or radiotherapy had about a two-fold increased prevalence of mild systolic cardiac dysfunction a median of 10 years after treatment, compared with age-matched controls in a study that included a total of 700 people.
But even longer follow-up of treated breast cancer patients is needed to determine whether the excess mild cardiac dysfunction seen in this analysis eventually progresses to more severe cardiac impairment, Liselotte M. Boerman said at the European Breast Cancer Conference.
Data from the Breast Cancer Long-term Outcome of Cardiac Dysfunction (BLOC) study showed that 175 breast cancer patients who received chemotherapy (and may have also received radiotherapy) had a 2.5-fold higher prevalence of a left ventricular ejection fraction (LVEF) below 54% (95% confidence interval, 1.2-5.4) when measured by echocardiography a median of 10 years after treatment, compared with an equal number of age-matched individuals from the general population.
A separate group of 175 patients treated with radiotherapy only and evaluated by echocardiography a median of 10 years later had a 2.3-fold increased prevalence (1.1-4.7) of a LVEF below 54% when compared with an equal number of age-matched individuals, said Ms. Boerman, an epidemiology researcher at the University of Groningen (the Netherlands).
This degree of left-ventricular dysfunction was found in 15% of the chemotherapy patients and 6% of their controls, and in 16% of the radiotherapy patients and 8% of their controls.
However, the treated breast cancer patients had no long-term increase in their prevalence of more significant systolic cardiac dysfunction, defined as a LVEF of less than 45%, compared with the controls, and the overall rate of systolic dysfunction of this severity was low, affecting fewer than 1% of patients.
Also, the chemotherapy and radiotherapy patients showed no significant increase in the prevalence of diastolic cardiac dysfunction, defined as delayed cardiac relaxation beyond the age-appropriate range. Treated patients did show, after 10 years, a suggestion of an increased prevalence of diagnosed cardiovascular disease, which was 2.3-fold higher (1.0-4.9) in the chemotherapy-receiving patients, compared with their controls; and 70% higher (0.9-3.4) among the patients treated with radiotherapy, compared with their controls, Ms. Boerman said.
The study used data collected from breast cancer patients younger than 80 years old treated after 1980 and controls seen by general practice Dutch physicians. The chemotherapy patients were diagnosed at an average age of 49 years old (range 26-66 years old). About 78% had received treatment with an anthracycline agent and 7% had received trastuzumab (Herceptin). Radiotherapy had also been administered to 70%, while 62% had also received hormonal therapy, and 7% either had a recurrence or developed a tumor in their contralateral breast.
None of the radiotherapy-only patients had received chemotherapy, but 21% had also received hormonal therapy. Their average age at diagnosis was 54 years old (range 32-79 years old), and 10% had a recurrence or a contralateral tumor.
Follow-up echocardiography occurred 5-34 years after the index treatment, at a median age of 60 years old. Echocardiography follow-up occurred in 70% of the chemotherapy breast cancer patients contacted, and in 63% of those who received radiotherapy only. Among controls, about half of those selected by age matching, and contacted, agreed to participate.
Rates of cardiovascular-disease risk factors – dyslipidemia, hypertension, and diabetes – were at roughly similar levels in the cases and controls at the time of breast cancer diagnosis. But the rate of current smoking at the time of diagnosis appeared higher in the cases (30% among those who received chemotherapy and 33% among those on radiotherapy), compared with their respective control groups (22% and 30%). Ms. Boerman said that a multivariate analysis had not yet been run on the data but should occur soon.
“The prevalence of cardiac dysfunction was higher [in treated patients] than I would have expected, but there is potential bias as only 70% of invited patients actually participated,” commented Dr. Robert Mansel, a professor at the Institute of Cancer & Genetics at Cardiff University (Wales). He also noted the very low rate of patients who developed severe cardiac dysfunction.
Ms. Boerman and Dr. Mansel reported having no financial disclosures.
On Twitter @mitchelzoler
AMSTERDAM – Breast cancer patients who underwent chemotherapy or radiotherapy had about a two-fold increased prevalence of mild systolic cardiac dysfunction a median of 10 years after treatment, compared with age-matched controls in a study that included a total of 700 people.
But even longer follow-up of treated breast cancer patients is needed to determine whether the excess mild cardiac dysfunction seen in this analysis eventually progresses to more severe cardiac impairment, Liselotte M. Boerman said at the European Breast Cancer Conference.
Data from the Breast Cancer Long-term Outcome of Cardiac Dysfunction (BLOC) study showed that 175 breast cancer patients who received chemotherapy (and may have also received radiotherapy) had a 2.5-fold higher prevalence of a left ventricular ejection fraction (LVEF) below 54% (95% confidence interval, 1.2-5.4) when measured by echocardiography a median of 10 years after treatment, compared with an equal number of age-matched individuals from the general population.
A separate group of 175 patients treated with radiotherapy only and evaluated by echocardiography a median of 10 years later had a 2.3-fold increased prevalence (1.1-4.7) of a LVEF below 54% when compared with an equal number of age-matched individuals, said Ms. Boerman, an epidemiology researcher at the University of Groningen (the Netherlands).
This degree of left-ventricular dysfunction was found in 15% of the chemotherapy patients and 6% of their controls, and in 16% of the radiotherapy patients and 8% of their controls.
However, the treated breast cancer patients had no long-term increase in their prevalence of more significant systolic cardiac dysfunction, defined as a LVEF of less than 45%, compared with the controls, and the overall rate of systolic dysfunction of this severity was low, affecting fewer than 1% of patients.
Also, the chemotherapy and radiotherapy patients showed no significant increase in the prevalence of diastolic cardiac dysfunction, defined as delayed cardiac relaxation beyond the age-appropriate range. Treated patients did show, after 10 years, a suggestion of an increased prevalence of diagnosed cardiovascular disease, which was 2.3-fold higher (1.0-4.9) in the chemotherapy-receiving patients, compared with their controls; and 70% higher (0.9-3.4) among the patients treated with radiotherapy, compared with their controls, Ms. Boerman said.
The study used data collected from breast cancer patients younger than 80 years old treated after 1980 and controls seen by general practice Dutch physicians. The chemotherapy patients were diagnosed at an average age of 49 years old (range 26-66 years old). About 78% had received treatment with an anthracycline agent and 7% had received trastuzumab (Herceptin). Radiotherapy had also been administered to 70%, while 62% had also received hormonal therapy, and 7% either had a recurrence or developed a tumor in their contralateral breast.
None of the radiotherapy-only patients had received chemotherapy, but 21% had also received hormonal therapy. Their average age at diagnosis was 54 years old (range 32-79 years old), and 10% had a recurrence or a contralateral tumor.
Follow-up echocardiography occurred 5-34 years after the index treatment, at a median age of 60 years old. Echocardiography follow-up occurred in 70% of the chemotherapy breast cancer patients contacted, and in 63% of those who received radiotherapy only. Among controls, about half of those selected by age matching, and contacted, agreed to participate.
Rates of cardiovascular-disease risk factors – dyslipidemia, hypertension, and diabetes – were at roughly similar levels in the cases and controls at the time of breast cancer diagnosis. But the rate of current smoking at the time of diagnosis appeared higher in the cases (30% among those who received chemotherapy and 33% among those on radiotherapy), compared with their respective control groups (22% and 30%). Ms. Boerman said that a multivariate analysis had not yet been run on the data but should occur soon.
“The prevalence of cardiac dysfunction was higher [in treated patients] than I would have expected, but there is potential bias as only 70% of invited patients actually participated,” commented Dr. Robert Mansel, a professor at the Institute of Cancer & Genetics at Cardiff University (Wales). He also noted the very low rate of patients who developed severe cardiac dysfunction.
Ms. Boerman and Dr. Mansel reported having no financial disclosures.
On Twitter @mitchelzoler
AT EBCC10
Key clinical point: Breast cancer patients treated with chemotherapy or radiotherapy showed a doubled rate of mild left-ventricular dysfunction, compared with matched controls 10 years after treatment.
Major finding: Mildly reduced left-ventricular function occurred in 15% of post-chemotherapy patients, compared with 6% of controls.
Data source: Echocardiography examinations conducted on 350 Dutch breast cancer patients and an equal number of age-matched controls.
Disclosures: Ms. Boerman and Dr. Mansel reported having no financial disclosures.
Incretin-based diabetes drugs don’t raise heart failure risk
Incretin-based antidiabetic drugs didn’t raise the risk of hospitalization for heart failure in an international observational study involving 1.5 million patients reported online March 24 in the New England Journal of Medicine.
The safety of dipeptidyl peptidase 4 (DPP-4) inhibitors such as sitagliptin, saxagliptin, and linagliptin, and of glucagon-like peptide–1 (GLP-1) analogues such as exenatide and liraglutide is controversial. Some clinical trials have reported these agents raise the risk of heart failure (HF) while others have found no increase in risk, but all of the studies are underpowered to settle the question, said Kristian B. Filion, Ph.D., of McGill University and the Center for Clinical Epidemiology, Lady Davis Research Institute, Jewish General Hospital, and his associates.
They examined this issue by analyzing data from several large cohorts of diabetes patients treated in routine clinical practice in the United States, Canada, and England. Their study population comprised 1,499,650 adults who began taking noninsulin antidiabetic drugs at or after the date that incretin-based agents entered the market. “With 3.2 million person-years of observations, we had the statistical power to robustly assess this important drug safety issue,” the investigators said.
Patients taking DPP-4 inhibitors and GLP-1 analogues were compared with those taking non–incretin-based drugs such as biguanides, sulfonylureas, thiazolidinediones, alpha-glucosidase inhibitors, meglitinides, and sodium-glucose cotransporter-2 inhibitors. A total of 29,741 patients were hospitalized for HF, for an overall rate of 9.2 events per 1,000 person-years.
Incretin-based drugs were not associated with an increased rate of hospitalization for HF when compared with other antidiabetic drugs (hazard ratio, 0.82) among the roughly 1.4 million patients who had no history of HF at baseline. Individually, neither DPP-4 inhibitors (HR, 0.84) nor GLP-1 analogues (HR, 0.95) were associated with an increased risk of hospitalization for HF. These findings remained consistent through several subgroup and sensitivity analyses that categorized the data according to duration of exposure, presence or absence of a history of MI, and duration of diabetes, Dr. Filion and his associates said (N Engl J Med. 2016 Mar 24. doi: 10.1056/NEJMoa1506115).
Similarly, incretin-based drugs were not associated with an increased rate of hospitalization for HF when compared with other antidiabetic drugs among the approximately 80,000 patients who had a history of HF at baseline (HR, 0.86).
This study was supported by the Canadian Institutes of Health Research and the Quebec Foundation for Health Research. Dr. Filion reported having no relevant financial disclosures; some of his associates reported ties to numerous industry sources.
Incretin-based antidiabetic drugs didn’t raise the risk of hospitalization for heart failure in an international observational study involving 1.5 million patients reported online March 24 in the New England Journal of Medicine.
The safety of dipeptidyl peptidase 4 (DPP-4) inhibitors such as sitagliptin, saxagliptin, and linagliptin, and of glucagon-like peptide–1 (GLP-1) analogues such as exenatide and liraglutide is controversial. Some clinical trials have reported these agents raise the risk of heart failure (HF) while others have found no increase in risk, but all of the studies are underpowered to settle the question, said Kristian B. Filion, Ph.D., of McGill University and the Center for Clinical Epidemiology, Lady Davis Research Institute, Jewish General Hospital, and his associates.
They examined this issue by analyzing data from several large cohorts of diabetes patients treated in routine clinical practice in the United States, Canada, and England. Their study population comprised 1,499,650 adults who began taking noninsulin antidiabetic drugs at or after the date that incretin-based agents entered the market. “With 3.2 million person-years of observations, we had the statistical power to robustly assess this important drug safety issue,” the investigators said.
Patients taking DPP-4 inhibitors and GLP-1 analogues were compared with those taking non–incretin-based drugs such as biguanides, sulfonylureas, thiazolidinediones, alpha-glucosidase inhibitors, meglitinides, and sodium-glucose cotransporter-2 inhibitors. A total of 29,741 patients were hospitalized for HF, for an overall rate of 9.2 events per 1,000 person-years.
Incretin-based drugs were not associated with an increased rate of hospitalization for HF when compared with other antidiabetic drugs (hazard ratio, 0.82) among the roughly 1.4 million patients who had no history of HF at baseline. Individually, neither DPP-4 inhibitors (HR, 0.84) nor GLP-1 analogues (HR, 0.95) were associated with an increased risk of hospitalization for HF. These findings remained consistent through several subgroup and sensitivity analyses that categorized the data according to duration of exposure, presence or absence of a history of MI, and duration of diabetes, Dr. Filion and his associates said (N Engl J Med. 2016 Mar 24. doi: 10.1056/NEJMoa1506115).
Similarly, incretin-based drugs were not associated with an increased rate of hospitalization for HF when compared with other antidiabetic drugs among the approximately 80,000 patients who had a history of HF at baseline (HR, 0.86).
This study was supported by the Canadian Institutes of Health Research and the Quebec Foundation for Health Research. Dr. Filion reported having no relevant financial disclosures; some of his associates reported ties to numerous industry sources.
Incretin-based antidiabetic drugs didn’t raise the risk of hospitalization for heart failure in an international observational study involving 1.5 million patients reported online March 24 in the New England Journal of Medicine.
The safety of dipeptidyl peptidase 4 (DPP-4) inhibitors such as sitagliptin, saxagliptin, and linagliptin, and of glucagon-like peptide–1 (GLP-1) analogues such as exenatide and liraglutide is controversial. Some clinical trials have reported these agents raise the risk of heart failure (HF) while others have found no increase in risk, but all of the studies are underpowered to settle the question, said Kristian B. Filion, Ph.D., of McGill University and the Center for Clinical Epidemiology, Lady Davis Research Institute, Jewish General Hospital, and his associates.
They examined this issue by analyzing data from several large cohorts of diabetes patients treated in routine clinical practice in the United States, Canada, and England. Their study population comprised 1,499,650 adults who began taking noninsulin antidiabetic drugs at or after the date that incretin-based agents entered the market. “With 3.2 million person-years of observations, we had the statistical power to robustly assess this important drug safety issue,” the investigators said.
Patients taking DPP-4 inhibitors and GLP-1 analogues were compared with those taking non–incretin-based drugs such as biguanides, sulfonylureas, thiazolidinediones, alpha-glucosidase inhibitors, meglitinides, and sodium-glucose cotransporter-2 inhibitors. A total of 29,741 patients were hospitalized for HF, for an overall rate of 9.2 events per 1,000 person-years.
Incretin-based drugs were not associated with an increased rate of hospitalization for HF when compared with other antidiabetic drugs (hazard ratio, 0.82) among the roughly 1.4 million patients who had no history of HF at baseline. Individually, neither DPP-4 inhibitors (HR, 0.84) nor GLP-1 analogues (HR, 0.95) were associated with an increased risk of hospitalization for HF. These findings remained consistent through several subgroup and sensitivity analyses that categorized the data according to duration of exposure, presence or absence of a history of MI, and duration of diabetes, Dr. Filion and his associates said (N Engl J Med. 2016 Mar 24. doi: 10.1056/NEJMoa1506115).
Similarly, incretin-based drugs were not associated with an increased rate of hospitalization for HF when compared with other antidiabetic drugs among the approximately 80,000 patients who had a history of HF at baseline (HR, 0.86).
This study was supported by the Canadian Institutes of Health Research and the Quebec Foundation for Health Research. Dr. Filion reported having no relevant financial disclosures; some of his associates reported ties to numerous industry sources.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Incretin-based antidiabetic drugs did not raise the risk of hospitalization for heart failure in a large international observational study.
Major finding: Neither DPP-4 inhibitors (HR, 0.84) nor GLP-1 analogues (HR, 0.95) were associated with an increased risk of hospitalization for HF, compared with non–incretin-based antidiabetic drugs.
Data source: A retrospective international observational cohort study involving roughly 1.5 million diabetes patients, of whom 29,741 were hospitalized for HF.
Disclosures: This study was supported by the Canadian Institutes of Health Research and the Quebec Foundation for Health Research. Dr. Filion reported having no relevant financial disclosures; some of his associates reported ties to numerous industry sources.
Gait speed hones risk stratification of elderly heart failure patients
Gait speed was independently associated with hospitalization and death and aided in risk stratification of elderly patients with heart failure in a study reported online March 9 in JACC Heart Failure.
Optimal clinical management of heart failure is dependent on accurate prognostic stratification, Dr. Giovanni Pulignano and his coinvestigators wrote. Geriatric conditions such as frailty, cognitive impairment, and disability impact prognosis along with comorbidities and cardiac disease. Further, gait speed is a marker of frailty and is predictive of adverse health events in older patients, including mortality. However, there is limited research on the impact of geriatric conditions in heart failure studies.
Dr. Pulignano of San Camillo Hospital in Rome and colleagues sought to examine the relationship between gait speed in older patients with heart failure and the risk of hospitalization and death.
The investigators calculated the predicted all-cause mortality using the Cardiac and Comorbid Conditions Heart Failure (3C-HF) score. Variables included in the 3C-HF score are left ventricular ejection fraction of less than 20%, New York Heart Association class III-IV heart failure, no renin-angiotensin inhibitor treatment, no beta-blocker treatment, severe valvular heart disease, diabetes with macro- or microangiopathy, atrial fibrillation, hypertension, anemia, renal dysfunction, and older age.
Participants were at least 70 years old, with clinically stable heart failure with normal or reduced left ventricular ejection fraction and a previous hospitalization necessitating intravenous inotropes, diuretics, and/or vasodilators for heart failure.
Exclusion criteria included a condition that would decrease their walking speed, valvular heart disease with surgery planned, long-term intravenous inotrope treatment, or living in a nursing home.
Gait speed was assessed over 4 meters and grouped into groups of slow walkers (up to 0.65 m/s), intermediate walkers (0.66-0.99 m/s), and fast walkers (at least 1.0 m/s). Participants were allowed to use a walker or cane as a walking aid.
Data were analyzed on 331 patients (mean age 78 years, 43% women) with clinically stable and optimized chronic heart failure (mean NYHA class 2.7, mean left ventricular ejection fraction 35%). The mean 3C-HF score was 19.7 points.
The mean gait speed was 0.74 m/s, with 35% (115 patients) demonstrating severely reduced (less than or equal to 0.65 m/s) gait speed.
After 1 year of follow-up, they found a significant association between the measured gait speed and 1-year mortality, with 9.1%, 21.9%, and 38.3% for the high, intermediate, and low tertiles, respectively (P less than .001). A similar relationship was found for gait speed and heart failure–related hospitalization (26.6%, 58.6%, and 71.3%, respectively; P = .002) and all-cause hospitalization (26.6%, 58.6%, and 71.3%, respectively; P = .002).
Multivariate analysis revealed an independent association between gait speed and lower risk of all-cause death (hazard ratio, 0.62; P = .008). Gait speed was also associated with a lower risk for all-cause hospitalizations (HR, 0.74; P = .002) and heart failure–related hospitalizations (HR, 0.69; P = .004).
Finally, when gait speed was included in the 3C-HF risk score, accuracy of risk stratification for all-cause death (net reclassification improvement, 0.49; P less than .001) and heart failure admissions (NRI, 0.37; P less than .001) was improved (JCHF. 2016 Mar 9. doi:10.1016/j.jchf.2015.12.017).
“Gait speed, in combination with a validated clinical risk score, improves prognosis prediction in older HF patients,” the investigators concluded. “Frailty assessment using gait speed is simple and inexpensive and suggests new strategies for intervention. Its measurement should be incorporated in the routine clinical evaluation of older patients with HF.”
The study was supported by the ADRIANO-Italian Association for Research on Cardiac Disease in Older Patients. The authors had no disclosures.
Gait speed was independently associated with hospitalization and death and aided in risk stratification of elderly patients with heart failure in a study reported online March 9 in JACC Heart Failure.
Optimal clinical management of heart failure is dependent on accurate prognostic stratification, Dr. Giovanni Pulignano and his coinvestigators wrote. Geriatric conditions such as frailty, cognitive impairment, and disability impact prognosis along with comorbidities and cardiac disease. Further, gait speed is a marker of frailty and is predictive of adverse health events in older patients, including mortality. However, there is limited research on the impact of geriatric conditions in heart failure studies.
Dr. Pulignano of San Camillo Hospital in Rome and colleagues sought to examine the relationship between gait speed in older patients with heart failure and the risk of hospitalization and death.
The investigators calculated the predicted all-cause mortality using the Cardiac and Comorbid Conditions Heart Failure (3C-HF) score. Variables included in the 3C-HF score are left ventricular ejection fraction of less than 20%, New York Heart Association class III-IV heart failure, no renin-angiotensin inhibitor treatment, no beta-blocker treatment, severe valvular heart disease, diabetes with macro- or microangiopathy, atrial fibrillation, hypertension, anemia, renal dysfunction, and older age.
Participants were at least 70 years old, with clinically stable heart failure with normal or reduced left ventricular ejection fraction and a previous hospitalization necessitating intravenous inotropes, diuretics, and/or vasodilators for heart failure.
Exclusion criteria included a condition that would decrease their walking speed, valvular heart disease with surgery planned, long-term intravenous inotrope treatment, or living in a nursing home.
Gait speed was assessed over 4 meters and grouped into groups of slow walkers (up to 0.65 m/s), intermediate walkers (0.66-0.99 m/s), and fast walkers (at least 1.0 m/s). Participants were allowed to use a walker or cane as a walking aid.
Data were analyzed on 331 patients (mean age 78 years, 43% women) with clinically stable and optimized chronic heart failure (mean NYHA class 2.7, mean left ventricular ejection fraction 35%). The mean 3C-HF score was 19.7 points.
The mean gait speed was 0.74 m/s, with 35% (115 patients) demonstrating severely reduced (less than or equal to 0.65 m/s) gait speed.
After 1 year of follow-up, they found a significant association between the measured gait speed and 1-year mortality, with 9.1%, 21.9%, and 38.3% for the high, intermediate, and low tertiles, respectively (P less than .001). A similar relationship was found for gait speed and heart failure–related hospitalization (26.6%, 58.6%, and 71.3%, respectively; P = .002) and all-cause hospitalization (26.6%, 58.6%, and 71.3%, respectively; P = .002).
Multivariate analysis revealed an independent association between gait speed and lower risk of all-cause death (hazard ratio, 0.62; P = .008). Gait speed was also associated with a lower risk for all-cause hospitalizations (HR, 0.74; P = .002) and heart failure–related hospitalizations (HR, 0.69; P = .004).
Finally, when gait speed was included in the 3C-HF risk score, accuracy of risk stratification for all-cause death (net reclassification improvement, 0.49; P less than .001) and heart failure admissions (NRI, 0.37; P less than .001) was improved (JCHF. 2016 Mar 9. doi:10.1016/j.jchf.2015.12.017).
“Gait speed, in combination with a validated clinical risk score, improves prognosis prediction in older HF patients,” the investigators concluded. “Frailty assessment using gait speed is simple and inexpensive and suggests new strategies for intervention. Its measurement should be incorporated in the routine clinical evaluation of older patients with HF.”
The study was supported by the ADRIANO-Italian Association for Research on Cardiac Disease in Older Patients. The authors had no disclosures.
Gait speed was independently associated with hospitalization and death and aided in risk stratification of elderly patients with heart failure in a study reported online March 9 in JACC Heart Failure.
Optimal clinical management of heart failure is dependent on accurate prognostic stratification, Dr. Giovanni Pulignano and his coinvestigators wrote. Geriatric conditions such as frailty, cognitive impairment, and disability impact prognosis along with comorbidities and cardiac disease. Further, gait speed is a marker of frailty and is predictive of adverse health events in older patients, including mortality. However, there is limited research on the impact of geriatric conditions in heart failure studies.
Dr. Pulignano of San Camillo Hospital in Rome and colleagues sought to examine the relationship between gait speed in older patients with heart failure and the risk of hospitalization and death.
The investigators calculated the predicted all-cause mortality using the Cardiac and Comorbid Conditions Heart Failure (3C-HF) score. Variables included in the 3C-HF score are left ventricular ejection fraction of less than 20%, New York Heart Association class III-IV heart failure, no renin-angiotensin inhibitor treatment, no beta-blocker treatment, severe valvular heart disease, diabetes with macro- or microangiopathy, atrial fibrillation, hypertension, anemia, renal dysfunction, and older age.
Participants were at least 70 years old, with clinically stable heart failure with normal or reduced left ventricular ejection fraction and a previous hospitalization necessitating intravenous inotropes, diuretics, and/or vasodilators for heart failure.
Exclusion criteria included a condition that would decrease their walking speed, valvular heart disease with surgery planned, long-term intravenous inotrope treatment, or living in a nursing home.
Gait speed was assessed over 4 meters and grouped into groups of slow walkers (up to 0.65 m/s), intermediate walkers (0.66-0.99 m/s), and fast walkers (at least 1.0 m/s). Participants were allowed to use a walker or cane as a walking aid.
Data were analyzed on 331 patients (mean age 78 years, 43% women) with clinically stable and optimized chronic heart failure (mean NYHA class 2.7, mean left ventricular ejection fraction 35%). The mean 3C-HF score was 19.7 points.
The mean gait speed was 0.74 m/s, with 35% (115 patients) demonstrating severely reduced (less than or equal to 0.65 m/s) gait speed.
After 1 year of follow-up, they found a significant association between the measured gait speed and 1-year mortality, with 9.1%, 21.9%, and 38.3% for the high, intermediate, and low tertiles, respectively (P less than .001). A similar relationship was found for gait speed and heart failure–related hospitalization (26.6%, 58.6%, and 71.3%, respectively; P = .002) and all-cause hospitalization (26.6%, 58.6%, and 71.3%, respectively; P = .002).
Multivariate analysis revealed an independent association between gait speed and lower risk of all-cause death (hazard ratio, 0.62; P = .008). Gait speed was also associated with a lower risk for all-cause hospitalizations (HR, 0.74; P = .002) and heart failure–related hospitalizations (HR, 0.69; P = .004).
Finally, when gait speed was included in the 3C-HF risk score, accuracy of risk stratification for all-cause death (net reclassification improvement, 0.49; P less than .001) and heart failure admissions (NRI, 0.37; P less than .001) was improved (JCHF. 2016 Mar 9. doi:10.1016/j.jchf.2015.12.017).
“Gait speed, in combination with a validated clinical risk score, improves prognosis prediction in older HF patients,” the investigators concluded. “Frailty assessment using gait speed is simple and inexpensive and suggests new strategies for intervention. Its measurement should be incorporated in the routine clinical evaluation of older patients with HF.”
The study was supported by the ADRIANO-Italian Association for Research on Cardiac Disease in Older Patients. The authors had no disclosures.
JACC HEART FAILURE
Key clinical point: Gait speed was independently associated with hospitalization and death, and aided in risk stratification of elderly patients with heart failure.
Major finding: When gait speed was included in the 3C-HF risk score, accuracy of risk stratification for all-cause death (net reclassification improvement, 0.49) and heart failure admissions (NRI, 0.37) was improved significantly.
Data source: Gait speed was tested in 331 elderly patients with clinically stable heart failure who were prospectively followed for 1 year to assess mortality and hospitalization rate.
Disclosures: The study was supported by the ADRIANO-Italian Association for Research on Cardiac Disease in Older Patients. The authors report no disclosures.
No LIGHT shed on CV safety of naltrexone-bupropion
The cardiovascular risks of the weight-loss combination drug naltrexone-bupropion are still uncertain, because the FDA-mandated safety trial, LIGHT, was terminated prematurely due to the sponsoring drug company’s breach of confidentiality. Other irregularities also were discovered, according to a report published online March 8 in JAMA.
Orexigen violated its agreement with FDA not to disclose the findings of an interim analysis performed after only 25% of expected CV events accrued. These findings appeared highly favorable for the drug, prompting the sponsor to publicly, and prematurely, claim naltrexone-bupropion reduced CV risk by 41% independently of its weight-loss effect. This favorable outcome was later found to be “driven almost exclusively by a 12-to-1 imbalance in death favoring naltrexone-bupropion among patients who were no longer taking the study drug,” said Dr. Steven E. Nissen of the Cleveland Clinic Center for Cardiovascular Research and his associates.
They now report the results of a second interim analysis of LIGHT (Cardiovascular Outcomes Study of Naltrexone SR/Bupropion SR in Overweight and Obese Subjects With Cardiovascular Risk Factors) performed after 50% of expected CV events accrued, as well as those of a final analysis performed when the trial was halted and 64% of expected CV events accrued. These findings are much less favorable, showing no significant difference between naltrexone-bupropion and placebo in the rate of major adverse cardiovascular events (MACE). In addition, extremely poor adherence to both active treatment and placebo over time, together with the complex statistical issues involved, call into question any interpretation of the results and preclude “any reliable conclusions about the long-term safety of naltrexone-bupropion,” the investigators said.
“The cardiovascular safety of this treatment remains uncertain and will require evaluation in a new, adequately powered outcome trial.” The results of such a study, should it be undertaken, wouldn’t be available for at least 3-4 years, they noted.
In their report, the investigators described in detail why the FDA required a CV safety study even though the agency had already approved naltrexone-bupropion as a weight-loss treatment.
The study was a phase IIIb randomized, double-blind, placebo-controlled trial involving 8,910 overweight/obese patients aged 45 or older (mean age, 61 years) who were at risk of adverse CV outcomes due to preexisting CV disease; exercise-induced angina; an unfavorable ankle/brachial index; stenosis of coronary, carotid, or lower extremity arteries; type 2 diabetes; hypertension; dyslipidemia; or smoking. These participants received daily tablets of either 32 mg naltrexone plus 360 mg bupropion or a matching placebo and were to be followed for 4 years at 266 U.S. medical centers.
The analysis performed after 50% of expected CV events accrued showed that MACE – the primary safety outcome – occurred in 2.0% of the naltrexone-bupropion group and 2.3% of the placebo group, a nonsignificant difference. Differences between the two study groups also were nonsignificant for the individual components of the primary outcome, including cardiovascular death (0.4% vs. 0.8%), nonfatal stroke (0.5% vs. 0.4%), and nonfatal MI (1.2% vs. 1.2%). These results were confirmed in a sensitivity analysis based on the final data collection, when 64% of expected CV events accrued: MACE occurred in 2.7% of the naltrexone-bupropion group and 2.8% of the placebo group, another nonsignificant difference.
The active treatment also was no better than placebo regarding all secondary outcomes, including all-cause mortality, CV hospitalization, and coronary revascularization, Dr. Nissen and his associates said (JAMA. 2016 Mar 8. doi: 10.1001/jama.2016.1558). Treatment adherence was low. At 16 weeks, only 64% of the naltrexone-bupropion group and 72% of the placebo group were still taking the study treatment. By 2 years, only 27% of the naltrexone-bupropion group and 17% of the placebo group were. The mean duration of treatment was only 18.4 weeks for naltrexone-bupropion and 16.3 weeks for placebo. Most treatment discontinuations were due to a failure to lose weight, but a substantial proportion occurred because of treatment-related increases in blood pressure or heart rate.
The active treatment’s ability to reduce body weight was deemed “modest.” When the trial was halted, the mean decrease in weight was 3.9 kg with naltrexone-bupropion, representing a 3.6% reduction in total weight, and was 1.2 kg with placebo, representing a 1.1% reduction.
Adverse effects developed in 28.1% of patients taking naltrexone-bupropion, which was a significantly greater proportion than the 8.7% rate in the placebo group. The most common adverse events leading to discontinuation of the study drug affected the gastrointestinal system (nausea, constipation, vomiting), the CNS (tremor, dizziness, headache), and mental/emotional problems (insomnia, anxiety, hallucinations, depression).
The sponsor of this trial, Orexigen Therapeutics, disregarded the multiple harms caused by breaches of confidentiality, treated the academic investigators unfairly, ignored the trial’s data monitoring committee, and defied the FDA. Their statements about the naltrexone-bupropion’s cardiovascular efficacy were highly misleading, and there were obvious signs that the supporting data were not reliable. For example, nearly all of the CV mortality in the first interim analysis occurred well after participants had stopped taking their medication.
Yet the drug company was not sanctioned, and the naltrexone-bupropion retains FDA approval. At a minimum, the FDA should withhold approval until a viable replacement study is conducted, or it should require Risk Evaluation and Mitigation Strategies to counter the misinformation disseminated by the study sponsor, or it should restrict use of the drug outright.
Clinicians should be aware of these research improprieties and should know that the purported weight-loss benefit of naltrexone-bupropion is only 2.7 kg more than that achieved with placebo. How does this modest benefit balance against an unknown cardiovascular risk? In addition, the drug’s lack of efficacy clearly contributed to the very poor adherence rate in this trial: At 1 year, only 37.5% were still taking naltrexone-bupropion and 26.3% were still taking placebo.
Dr. Joshua M. Sharfstein is in the department of health policy and management at Johns Hopkins Bloomberg School of Public Health, Baltimore. Dr. Bruce M. Psaty is in the Cardiovascular Health Research Unit and the departments of medicine, epidemiology, and health services at the University of Washington, Seattle. They reported that this work was supported by the National Heart, Lung, and Blood Institute. Dr. Sharfstein reported serving as principal deputy commissioner of the FDA in 2009-2011. Dr. Psaty reported serving on the data monitoring committee of a clinical trial funded by Zoll LifeCor, on the steering committee of the Yale Open Data Access Project funded by Johnson & Johnson, and on the FDA Science Board. Dr. Sharfstein and Dr. Psaty made these remarks in an editorial accompanying Dr. Nissen’s report (JAMA. 2016;315:984-6).
The sponsor of this trial, Orexigen Therapeutics, disregarded the multiple harms caused by breaches of confidentiality, treated the academic investigators unfairly, ignored the trial’s data monitoring committee, and defied the FDA. Their statements about the naltrexone-bupropion’s cardiovascular efficacy were highly misleading, and there were obvious signs that the supporting data were not reliable. For example, nearly all of the CV mortality in the first interim analysis occurred well after participants had stopped taking their medication.
Yet the drug company was not sanctioned, and the naltrexone-bupropion retains FDA approval. At a minimum, the FDA should withhold approval until a viable replacement study is conducted, or it should require Risk Evaluation and Mitigation Strategies to counter the misinformation disseminated by the study sponsor, or it should restrict use of the drug outright.
Clinicians should be aware of these research improprieties and should know that the purported weight-loss benefit of naltrexone-bupropion is only 2.7 kg more than that achieved with placebo. How does this modest benefit balance against an unknown cardiovascular risk? In addition, the drug’s lack of efficacy clearly contributed to the very poor adherence rate in this trial: At 1 year, only 37.5% were still taking naltrexone-bupropion and 26.3% were still taking placebo.
Dr. Joshua M. Sharfstein is in the department of health policy and management at Johns Hopkins Bloomberg School of Public Health, Baltimore. Dr. Bruce M. Psaty is in the Cardiovascular Health Research Unit and the departments of medicine, epidemiology, and health services at the University of Washington, Seattle. They reported that this work was supported by the National Heart, Lung, and Blood Institute. Dr. Sharfstein reported serving as principal deputy commissioner of the FDA in 2009-2011. Dr. Psaty reported serving on the data monitoring committee of a clinical trial funded by Zoll LifeCor, on the steering committee of the Yale Open Data Access Project funded by Johnson & Johnson, and on the FDA Science Board. Dr. Sharfstein and Dr. Psaty made these remarks in an editorial accompanying Dr. Nissen’s report (JAMA. 2016;315:984-6).
The sponsor of this trial, Orexigen Therapeutics, disregarded the multiple harms caused by breaches of confidentiality, treated the academic investigators unfairly, ignored the trial’s data monitoring committee, and defied the FDA. Their statements about the naltrexone-bupropion’s cardiovascular efficacy were highly misleading, and there were obvious signs that the supporting data were not reliable. For example, nearly all of the CV mortality in the first interim analysis occurred well after participants had stopped taking their medication.
Yet the drug company was not sanctioned, and the naltrexone-bupropion retains FDA approval. At a minimum, the FDA should withhold approval until a viable replacement study is conducted, or it should require Risk Evaluation and Mitigation Strategies to counter the misinformation disseminated by the study sponsor, or it should restrict use of the drug outright.
Clinicians should be aware of these research improprieties and should know that the purported weight-loss benefit of naltrexone-bupropion is only 2.7 kg more than that achieved with placebo. How does this modest benefit balance against an unknown cardiovascular risk? In addition, the drug’s lack of efficacy clearly contributed to the very poor adherence rate in this trial: At 1 year, only 37.5% were still taking naltrexone-bupropion and 26.3% were still taking placebo.
Dr. Joshua M. Sharfstein is in the department of health policy and management at Johns Hopkins Bloomberg School of Public Health, Baltimore. Dr. Bruce M. Psaty is in the Cardiovascular Health Research Unit and the departments of medicine, epidemiology, and health services at the University of Washington, Seattle. They reported that this work was supported by the National Heart, Lung, and Blood Institute. Dr. Sharfstein reported serving as principal deputy commissioner of the FDA in 2009-2011. Dr. Psaty reported serving on the data monitoring committee of a clinical trial funded by Zoll LifeCor, on the steering committee of the Yale Open Data Access Project funded by Johnson & Johnson, and on the FDA Science Board. Dr. Sharfstein and Dr. Psaty made these remarks in an editorial accompanying Dr. Nissen’s report (JAMA. 2016;315:984-6).
The cardiovascular risks of the weight-loss combination drug naltrexone-bupropion are still uncertain, because the FDA-mandated safety trial, LIGHT, was terminated prematurely due to the sponsoring drug company’s breach of confidentiality. Other irregularities also were discovered, according to a report published online March 8 in JAMA.
Orexigen violated its agreement with FDA not to disclose the findings of an interim analysis performed after only 25% of expected CV events accrued. These findings appeared highly favorable for the drug, prompting the sponsor to publicly, and prematurely, claim naltrexone-bupropion reduced CV risk by 41% independently of its weight-loss effect. This favorable outcome was later found to be “driven almost exclusively by a 12-to-1 imbalance in death favoring naltrexone-bupropion among patients who were no longer taking the study drug,” said Dr. Steven E. Nissen of the Cleveland Clinic Center for Cardiovascular Research and his associates.
They now report the results of a second interim analysis of LIGHT (Cardiovascular Outcomes Study of Naltrexone SR/Bupropion SR in Overweight and Obese Subjects With Cardiovascular Risk Factors) performed after 50% of expected CV events accrued, as well as those of a final analysis performed when the trial was halted and 64% of expected CV events accrued. These findings are much less favorable, showing no significant difference between naltrexone-bupropion and placebo in the rate of major adverse cardiovascular events (MACE). In addition, extremely poor adherence to both active treatment and placebo over time, together with the complex statistical issues involved, call into question any interpretation of the results and preclude “any reliable conclusions about the long-term safety of naltrexone-bupropion,” the investigators said.
“The cardiovascular safety of this treatment remains uncertain and will require evaluation in a new, adequately powered outcome trial.” The results of such a study, should it be undertaken, wouldn’t be available for at least 3-4 years, they noted.
In their report, the investigators described in detail why the FDA required a CV safety study even though the agency had already approved naltrexone-bupropion as a weight-loss treatment.
The study was a phase IIIb randomized, double-blind, placebo-controlled trial involving 8,910 overweight/obese patients aged 45 or older (mean age, 61 years) who were at risk of adverse CV outcomes due to preexisting CV disease; exercise-induced angina; an unfavorable ankle/brachial index; stenosis of coronary, carotid, or lower extremity arteries; type 2 diabetes; hypertension; dyslipidemia; or smoking. These participants received daily tablets of either 32 mg naltrexone plus 360 mg bupropion or a matching placebo and were to be followed for 4 years at 266 U.S. medical centers.
The analysis performed after 50% of expected CV events accrued showed that MACE – the primary safety outcome – occurred in 2.0% of the naltrexone-bupropion group and 2.3% of the placebo group, a nonsignificant difference. Differences between the two study groups also were nonsignificant for the individual components of the primary outcome, including cardiovascular death (0.4% vs. 0.8%), nonfatal stroke (0.5% vs. 0.4%), and nonfatal MI (1.2% vs. 1.2%). These results were confirmed in a sensitivity analysis based on the final data collection, when 64% of expected CV events accrued: MACE occurred in 2.7% of the naltrexone-bupropion group and 2.8% of the placebo group, another nonsignificant difference.
The active treatment also was no better than placebo regarding all secondary outcomes, including all-cause mortality, CV hospitalization, and coronary revascularization, Dr. Nissen and his associates said (JAMA. 2016 Mar 8. doi: 10.1001/jama.2016.1558). Treatment adherence was low. At 16 weeks, only 64% of the naltrexone-bupropion group and 72% of the placebo group were still taking the study treatment. By 2 years, only 27% of the naltrexone-bupropion group and 17% of the placebo group were. The mean duration of treatment was only 18.4 weeks for naltrexone-bupropion and 16.3 weeks for placebo. Most treatment discontinuations were due to a failure to lose weight, but a substantial proportion occurred because of treatment-related increases in blood pressure or heart rate.
The active treatment’s ability to reduce body weight was deemed “modest.” When the trial was halted, the mean decrease in weight was 3.9 kg with naltrexone-bupropion, representing a 3.6% reduction in total weight, and was 1.2 kg with placebo, representing a 1.1% reduction.
Adverse effects developed in 28.1% of patients taking naltrexone-bupropion, which was a significantly greater proportion than the 8.7% rate in the placebo group. The most common adverse events leading to discontinuation of the study drug affected the gastrointestinal system (nausea, constipation, vomiting), the CNS (tremor, dizziness, headache), and mental/emotional problems (insomnia, anxiety, hallucinations, depression).
The cardiovascular risks of the weight-loss combination drug naltrexone-bupropion are still uncertain, because the FDA-mandated safety trial, LIGHT, was terminated prematurely due to the sponsoring drug company’s breach of confidentiality. Other irregularities also were discovered, according to a report published online March 8 in JAMA.
Orexigen violated its agreement with FDA not to disclose the findings of an interim analysis performed after only 25% of expected CV events accrued. These findings appeared highly favorable for the drug, prompting the sponsor to publicly, and prematurely, claim naltrexone-bupropion reduced CV risk by 41% independently of its weight-loss effect. This favorable outcome was later found to be “driven almost exclusively by a 12-to-1 imbalance in death favoring naltrexone-bupropion among patients who were no longer taking the study drug,” said Dr. Steven E. Nissen of the Cleveland Clinic Center for Cardiovascular Research and his associates.
They now report the results of a second interim analysis of LIGHT (Cardiovascular Outcomes Study of Naltrexone SR/Bupropion SR in Overweight and Obese Subjects With Cardiovascular Risk Factors) performed after 50% of expected CV events accrued, as well as those of a final analysis performed when the trial was halted and 64% of expected CV events accrued. These findings are much less favorable, showing no significant difference between naltrexone-bupropion and placebo in the rate of major adverse cardiovascular events (MACE). In addition, extremely poor adherence to both active treatment and placebo over time, together with the complex statistical issues involved, call into question any interpretation of the results and preclude “any reliable conclusions about the long-term safety of naltrexone-bupropion,” the investigators said.
“The cardiovascular safety of this treatment remains uncertain and will require evaluation in a new, adequately powered outcome trial.” The results of such a study, should it be undertaken, wouldn’t be available for at least 3-4 years, they noted.
In their report, the investigators described in detail why the FDA required a CV safety study even though the agency had already approved naltrexone-bupropion as a weight-loss treatment.
The study was a phase IIIb randomized, double-blind, placebo-controlled trial involving 8,910 overweight/obese patients aged 45 or older (mean age, 61 years) who were at risk of adverse CV outcomes due to preexisting CV disease; exercise-induced angina; an unfavorable ankle/brachial index; stenosis of coronary, carotid, or lower extremity arteries; type 2 diabetes; hypertension; dyslipidemia; or smoking. These participants received daily tablets of either 32 mg naltrexone plus 360 mg bupropion or a matching placebo and were to be followed for 4 years at 266 U.S. medical centers.
The analysis performed after 50% of expected CV events accrued showed that MACE – the primary safety outcome – occurred in 2.0% of the naltrexone-bupropion group and 2.3% of the placebo group, a nonsignificant difference. Differences between the two study groups also were nonsignificant for the individual components of the primary outcome, including cardiovascular death (0.4% vs. 0.8%), nonfatal stroke (0.5% vs. 0.4%), and nonfatal MI (1.2% vs. 1.2%). These results were confirmed in a sensitivity analysis based on the final data collection, when 64% of expected CV events accrued: MACE occurred in 2.7% of the naltrexone-bupropion group and 2.8% of the placebo group, another nonsignificant difference.
The active treatment also was no better than placebo regarding all secondary outcomes, including all-cause mortality, CV hospitalization, and coronary revascularization, Dr. Nissen and his associates said (JAMA. 2016 Mar 8. doi: 10.1001/jama.2016.1558). Treatment adherence was low. At 16 weeks, only 64% of the naltrexone-bupropion group and 72% of the placebo group were still taking the study treatment. By 2 years, only 27% of the naltrexone-bupropion group and 17% of the placebo group were. The mean duration of treatment was only 18.4 weeks for naltrexone-bupropion and 16.3 weeks for placebo. Most treatment discontinuations were due to a failure to lose weight, but a substantial proportion occurred because of treatment-related increases in blood pressure or heart rate.
The active treatment’s ability to reduce body weight was deemed “modest.” When the trial was halted, the mean decrease in weight was 3.9 kg with naltrexone-bupropion, representing a 3.6% reduction in total weight, and was 1.2 kg with placebo, representing a 1.1% reduction.
Adverse effects developed in 28.1% of patients taking naltrexone-bupropion, which was a significantly greater proportion than the 8.7% rate in the placebo group. The most common adverse events leading to discontinuation of the study drug affected the gastrointestinal system (nausea, constipation, vomiting), the CNS (tremor, dizziness, headache), and mental/emotional problems (insomnia, anxiety, hallucinations, depression).
FROM JAMA
Key clinical point: The cardiovascular risks of the weight-loss combination drug naltrexone-bupropion are still uncertain.
Major finding: The primary safety outcome, major adverse cardiovascular events, occurred in 2.0% of the naltrexone-bupropion group and 2.3% of the placebo group, a nonsignificant difference.
Data source: LIGHT, a multicenter randomized placebo-controlled double-blind noninferiority trial involving 8,910 overweight/obese patients.
Disclosures: This trial was sponsored by Orexigen Therapeutics and Takeda Pharmaceuticals. Dr. Nissen reported receiving grants from The Medicines Company, Amgen, Pfizer, AstraZeneca, Esperion Therapeutics, Eli Lilly, and Cerenis, and consulting for numerous drug companies that pay his fees directly to charities. His associates reported ties to numerous industry sources.
Two new drugs
Two new drugs have arrived to challenge our prescription pad or electronic record, depending on which you use. Empagliflozin (Jardiance), a drug used to modify glucose metabolism in type 2 diabetes in patients with preexisting cardiovascular disease, demonstrated a decrease in cardiovascular mortality. The other drug, Entresto, appears to provide an added benefit in heart failure therapy and was compared with a standard ACE inhibitor.
The results of EMPA-REG OUTCOME, reported at the European Association for the Study of Diabetes meeting in Stockholm, showed empagliflozin to be the first drug to decrease the mortality and morbidity of cardiovascular disease in diabetes. It is one of a group of new sodium-glucose cotransporter 2 (SGLT-2) blockers being tested in type 2 diabetes with established cardiovascular disease. Patients were randomized to placebo or empagliflozin while receiving standard medical and cardiovascular medications. After 3 years of follow-up, patients receiving the drug experienced a lower cardiovascular mortality rate, compared with placebo patients (3.7% vs. 5.9%, a 38% reduction) in addition to a decrease in hospitalization for heart failure and death from any cause. No effect was observed on the incidence of myocardial infarction or stroke. In addition, the drug also lowered blood glucose and blood pressure and led to some significant weight loss. The drug also was shown to decrease vascular resistance and albuminuria (N Engl J Med. 2015. 373:2117-2).
Furthermore, an analysis of EMPA-REG OUTCOME presented in November at the American Society of Nephrology meeting in San Diego, showed a profound benefit on the new onset and progression of chronic renal disease in diabetes patients. The importance of these results needs emphasis. Up until recently, the Food and Drug Administration has given a pass to diabetes drugs in regard to cardiovascular endpoints; approval has been based on their primary effect on lowering blood glucose. Some drugs in the past, such as rosiglitazone, actually have shown an increase in mortality in some diabetes patients. At long last, FDA approval for diabetes drugs hinges on acceptable outcomes in cardiovascular endpoints. The addition of a drug that can actually affect cardiovascular mortality and morbidity, the major risk factor of diabetes, provides an important addition to therapy.
The other drug that provides a choice of drugs for the treatment of heart failure is Entresto, a combination of sacubitril, a neprilysin inhibitor, and the angiotensin receptor inhibitor valsartan, approved in July 2015. In PARADIGM-HF, the compound was compared to enalapril in the treatment of patients with class II, III, and IV heart failure who were also receiving beta-blockers. Entresto-treated patients reported a 21.8% incidence of the primary outcome measure, cardiovascular death and hospitalization for heart failure, compared with the enalapril alone incidence of 26.5% (P less than .001) (N Engl J Med. 2014;371:993-1004). Investigators initially excluded 11.4% of the recruited patients from the study who could not tolerate Entresto or enalapril therapy. The drugs were well tolerated without any adverse reactions during therapy. Entresto was more effective than enalapril in regards to the occurrence of heart failure and death from any cause over a 27-month average follow-up.
The observations in this study emphasize how much the mortality of heart failure has decreased over the last decade. Cardiovascular deaths have decreased to roughly 7% per year and rehospitalization occurs in about 8% in the first year. Both drugs provide an important incremental benefit in heart failure patients.
Dr. Goldstein, medical editor of Cardiology News, is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.
Two new drugs have arrived to challenge our prescription pad or electronic record, depending on which you use. Empagliflozin (Jardiance), a drug used to modify glucose metabolism in type 2 diabetes in patients with preexisting cardiovascular disease, demonstrated a decrease in cardiovascular mortality. The other drug, Entresto, appears to provide an added benefit in heart failure therapy and was compared with a standard ACE inhibitor.
The results of EMPA-REG OUTCOME, reported at the European Association for the Study of Diabetes meeting in Stockholm, showed empagliflozin to be the first drug to decrease the mortality and morbidity of cardiovascular disease in diabetes. It is one of a group of new sodium-glucose cotransporter 2 (SGLT-2) blockers being tested in type 2 diabetes with established cardiovascular disease. Patients were randomized to placebo or empagliflozin while receiving standard medical and cardiovascular medications. After 3 years of follow-up, patients receiving the drug experienced a lower cardiovascular mortality rate, compared with placebo patients (3.7% vs. 5.9%, a 38% reduction) in addition to a decrease in hospitalization for heart failure and death from any cause. No effect was observed on the incidence of myocardial infarction or stroke. In addition, the drug also lowered blood glucose and blood pressure and led to some significant weight loss. The drug also was shown to decrease vascular resistance and albuminuria (N Engl J Med. 2015. 373:2117-2).
Furthermore, an analysis of EMPA-REG OUTCOME presented in November at the American Society of Nephrology meeting in San Diego, showed a profound benefit on the new onset and progression of chronic renal disease in diabetes patients. The importance of these results needs emphasis. Up until recently, the Food and Drug Administration has given a pass to diabetes drugs in regard to cardiovascular endpoints; approval has been based on their primary effect on lowering blood glucose. Some drugs in the past, such as rosiglitazone, actually have shown an increase in mortality in some diabetes patients. At long last, FDA approval for diabetes drugs hinges on acceptable outcomes in cardiovascular endpoints. The addition of a drug that can actually affect cardiovascular mortality and morbidity, the major risk factor of diabetes, provides an important addition to therapy.
The other drug that provides a choice of drugs for the treatment of heart failure is Entresto, a combination of sacubitril, a neprilysin inhibitor, and the angiotensin receptor inhibitor valsartan, approved in July 2015. In PARADIGM-HF, the compound was compared to enalapril in the treatment of patients with class II, III, and IV heart failure who were also receiving beta-blockers. Entresto-treated patients reported a 21.8% incidence of the primary outcome measure, cardiovascular death and hospitalization for heart failure, compared with the enalapril alone incidence of 26.5% (P less than .001) (N Engl J Med. 2014;371:993-1004). Investigators initially excluded 11.4% of the recruited patients from the study who could not tolerate Entresto or enalapril therapy. The drugs were well tolerated without any adverse reactions during therapy. Entresto was more effective than enalapril in regards to the occurrence of heart failure and death from any cause over a 27-month average follow-up.
The observations in this study emphasize how much the mortality of heart failure has decreased over the last decade. Cardiovascular deaths have decreased to roughly 7% per year and rehospitalization occurs in about 8% in the first year. Both drugs provide an important incremental benefit in heart failure patients.
Dr. Goldstein, medical editor of Cardiology News, is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.
Two new drugs have arrived to challenge our prescription pad or electronic record, depending on which you use. Empagliflozin (Jardiance), a drug used to modify glucose metabolism in type 2 diabetes in patients with preexisting cardiovascular disease, demonstrated a decrease in cardiovascular mortality. The other drug, Entresto, appears to provide an added benefit in heart failure therapy and was compared with a standard ACE inhibitor.
The results of EMPA-REG OUTCOME, reported at the European Association for the Study of Diabetes meeting in Stockholm, showed empagliflozin to be the first drug to decrease the mortality and morbidity of cardiovascular disease in diabetes. It is one of a group of new sodium-glucose cotransporter 2 (SGLT-2) blockers being tested in type 2 diabetes with established cardiovascular disease. Patients were randomized to placebo or empagliflozin while receiving standard medical and cardiovascular medications. After 3 years of follow-up, patients receiving the drug experienced a lower cardiovascular mortality rate, compared with placebo patients (3.7% vs. 5.9%, a 38% reduction) in addition to a decrease in hospitalization for heart failure and death from any cause. No effect was observed on the incidence of myocardial infarction or stroke. In addition, the drug also lowered blood glucose and blood pressure and led to some significant weight loss. The drug also was shown to decrease vascular resistance and albuminuria (N Engl J Med. 2015. 373:2117-2).
Furthermore, an analysis of EMPA-REG OUTCOME presented in November at the American Society of Nephrology meeting in San Diego, showed a profound benefit on the new onset and progression of chronic renal disease in diabetes patients. The importance of these results needs emphasis. Up until recently, the Food and Drug Administration has given a pass to diabetes drugs in regard to cardiovascular endpoints; approval has been based on their primary effect on lowering blood glucose. Some drugs in the past, such as rosiglitazone, actually have shown an increase in mortality in some diabetes patients. At long last, FDA approval for diabetes drugs hinges on acceptable outcomes in cardiovascular endpoints. The addition of a drug that can actually affect cardiovascular mortality and morbidity, the major risk factor of diabetes, provides an important addition to therapy.
The other drug that provides a choice of drugs for the treatment of heart failure is Entresto, a combination of sacubitril, a neprilysin inhibitor, and the angiotensin receptor inhibitor valsartan, approved in July 2015. In PARADIGM-HF, the compound was compared to enalapril in the treatment of patients with class II, III, and IV heart failure who were also receiving beta-blockers. Entresto-treated patients reported a 21.8% incidence of the primary outcome measure, cardiovascular death and hospitalization for heart failure, compared with the enalapril alone incidence of 26.5% (P less than .001) (N Engl J Med. 2014;371:993-1004). Investigators initially excluded 11.4% of the recruited patients from the study who could not tolerate Entresto or enalapril therapy. The drugs were well tolerated without any adverse reactions during therapy. Entresto was more effective than enalapril in regards to the occurrence of heart failure and death from any cause over a 27-month average follow-up.
The observations in this study emphasize how much the mortality of heart failure has decreased over the last decade. Cardiovascular deaths have decreased to roughly 7% per year and rehospitalization occurs in about 8% in the first year. Both drugs provide an important incremental benefit in heart failure patients.
Dr. Goldstein, medical editor of Cardiology News, is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.