User login
Progression in Huntington’s linked to CAG repeat number
The progression, not just age of onset, of Huntington’s disease can be predicted by a measurable genetic factor, researchers have learned.
Huntington’s, an inherited neurodegenerative disease that affects motor function and cognition, is caused by an expansion of the CAG trinucleotide sequence on the huntingtin gene. Scientists have previously linked younger age at onset to a higher number of CAG repeats on the gene, but the association between these and the rate of progression after onset was poorly understood.
In research published online August 12 in JAMA Neurology, investigators linked the rate of progression – which, like age at onset, is highly variable in Huntington’s – to CAG repeat length. CAG repeat length was strongly associated with distinct patterns of brain damage, as well as clinical measures of cognitive and motor decline.
For their research, Douglas R. Langbehn, MD, PhD, of the University of Iowa, Iowa City, and colleagues used data from two longitudinal observational studies in gene carriers for Huntington’s and nonrelated controls. The researchers looked at data from 443 participants (56% female; mean age, 44.4 years) who were followed for a mean of 4 years, with more than 2,000 study visits across the multisite cohort. Neuropsychiatric testing and brain imaging were conducted annually, using composite scoring systems of the investigators’ design. These composite scores sought to be more sensitive by combining results from several validated clinical and imaging tests.
Age and speed of decline in total functional capacity tracked with more CAG repeats, the researchers found. For example, in people with 40 CAG repeats, the estimated mean age of initial motor-cognitive score change was 42.46 years; for those with 45 repeats, 26.65 years, and for people with 50 CAG repeats, 18.49 years. Higher repeats were seen significantly associated with accelerated, nonlinear decline on both clinical and brain-volume measures, except gray matter volume, according to principal component analyses conducted on the data.
“We derived a single summary measure capturing the motor-cognitive phenotype and showed that the accelerating progression of the phenotype with aging is highly CAG repeat length dependent (i.e., those with higher CAG decline earlier and faster). Contrary to some previous assertions, this CAG dependence continues well past the onset of clinical illness,” Dr. Langbehn and colleagues wrote in their analysis. “By characterizing these CAG repeat length–dependent disease trajectories, we provide insights into disease progression that may guide future therapeutic approaches and identify the most appropriate intervention ages to prevent clinical decline.”
Dr. Langbehn and colleagues acknowledged as a limitation of their study its likely exclusion of the sickest subjects because of the cohorts’ design. The CHDI Foundation funded the study. Of the 16 coauthors, 13 reported receiving funding from CHDI and/or from pharmaceutical manufacturers.
SOURCE: Langbehn et al. JAMA Neurol. 2019 Aug 12. doi: 10.1001/jamaneurol.2019.2328
The progression, not just age of onset, of Huntington’s disease can be predicted by a measurable genetic factor, researchers have learned.
Huntington’s, an inherited neurodegenerative disease that affects motor function and cognition, is caused by an expansion of the CAG trinucleotide sequence on the huntingtin gene. Scientists have previously linked younger age at onset to a higher number of CAG repeats on the gene, but the association between these and the rate of progression after onset was poorly understood.
In research published online August 12 in JAMA Neurology, investigators linked the rate of progression – which, like age at onset, is highly variable in Huntington’s – to CAG repeat length. CAG repeat length was strongly associated with distinct patterns of brain damage, as well as clinical measures of cognitive and motor decline.
For their research, Douglas R. Langbehn, MD, PhD, of the University of Iowa, Iowa City, and colleagues used data from two longitudinal observational studies in gene carriers for Huntington’s and nonrelated controls. The researchers looked at data from 443 participants (56% female; mean age, 44.4 years) who were followed for a mean of 4 years, with more than 2,000 study visits across the multisite cohort. Neuropsychiatric testing and brain imaging were conducted annually, using composite scoring systems of the investigators’ design. These composite scores sought to be more sensitive by combining results from several validated clinical and imaging tests.
Age and speed of decline in total functional capacity tracked with more CAG repeats, the researchers found. For example, in people with 40 CAG repeats, the estimated mean age of initial motor-cognitive score change was 42.46 years; for those with 45 repeats, 26.65 years, and for people with 50 CAG repeats, 18.49 years. Higher repeats were seen significantly associated with accelerated, nonlinear decline on both clinical and brain-volume measures, except gray matter volume, according to principal component analyses conducted on the data.
“We derived a single summary measure capturing the motor-cognitive phenotype and showed that the accelerating progression of the phenotype with aging is highly CAG repeat length dependent (i.e., those with higher CAG decline earlier and faster). Contrary to some previous assertions, this CAG dependence continues well past the onset of clinical illness,” Dr. Langbehn and colleagues wrote in their analysis. “By characterizing these CAG repeat length–dependent disease trajectories, we provide insights into disease progression that may guide future therapeutic approaches and identify the most appropriate intervention ages to prevent clinical decline.”
Dr. Langbehn and colleagues acknowledged as a limitation of their study its likely exclusion of the sickest subjects because of the cohorts’ design. The CHDI Foundation funded the study. Of the 16 coauthors, 13 reported receiving funding from CHDI and/or from pharmaceutical manufacturers.
SOURCE: Langbehn et al. JAMA Neurol. 2019 Aug 12. doi: 10.1001/jamaneurol.2019.2328
The progression, not just age of onset, of Huntington’s disease can be predicted by a measurable genetic factor, researchers have learned.
Huntington’s, an inherited neurodegenerative disease that affects motor function and cognition, is caused by an expansion of the CAG trinucleotide sequence on the huntingtin gene. Scientists have previously linked younger age at onset to a higher number of CAG repeats on the gene, but the association between these and the rate of progression after onset was poorly understood.
In research published online August 12 in JAMA Neurology, investigators linked the rate of progression – which, like age at onset, is highly variable in Huntington’s – to CAG repeat length. CAG repeat length was strongly associated with distinct patterns of brain damage, as well as clinical measures of cognitive and motor decline.
For their research, Douglas R. Langbehn, MD, PhD, of the University of Iowa, Iowa City, and colleagues used data from two longitudinal observational studies in gene carriers for Huntington’s and nonrelated controls. The researchers looked at data from 443 participants (56% female; mean age, 44.4 years) who were followed for a mean of 4 years, with more than 2,000 study visits across the multisite cohort. Neuropsychiatric testing and brain imaging were conducted annually, using composite scoring systems of the investigators’ design. These composite scores sought to be more sensitive by combining results from several validated clinical and imaging tests.
Age and speed of decline in total functional capacity tracked with more CAG repeats, the researchers found. For example, in people with 40 CAG repeats, the estimated mean age of initial motor-cognitive score change was 42.46 years; for those with 45 repeats, 26.65 years, and for people with 50 CAG repeats, 18.49 years. Higher repeats were seen significantly associated with accelerated, nonlinear decline on both clinical and brain-volume measures, except gray matter volume, according to principal component analyses conducted on the data.
“We derived a single summary measure capturing the motor-cognitive phenotype and showed that the accelerating progression of the phenotype with aging is highly CAG repeat length dependent (i.e., those with higher CAG decline earlier and faster). Contrary to some previous assertions, this CAG dependence continues well past the onset of clinical illness,” Dr. Langbehn and colleagues wrote in their analysis. “By characterizing these CAG repeat length–dependent disease trajectories, we provide insights into disease progression that may guide future therapeutic approaches and identify the most appropriate intervention ages to prevent clinical decline.”
Dr. Langbehn and colleagues acknowledged as a limitation of their study its likely exclusion of the sickest subjects because of the cohorts’ design. The CHDI Foundation funded the study. Of the 16 coauthors, 13 reported receiving funding from CHDI and/or from pharmaceutical manufacturers.
SOURCE: Langbehn et al. JAMA Neurol. 2019 Aug 12. doi: 10.1001/jamaneurol.2019.2328
FROM JAMA NEUROLOGY
Key clinical point:
Major finding: CAG closely tracked the rate of cognitive and motor decline among patients with HD.
Study details: Brain imaging and neuropsychiatric testing data from 443 patients enrolled in cohort studies in people with HD-causing mutations
Disclosures: CHDI sponsored the study, and most coauthors disclosed financial relationships with the sponsor and/or pharmaceutical firms.
Source: Langbehn et al. JAMA Neurol. 2019 Aug 12. doi: 10.1001/jamaneurol.2019.2328.
Huntington’s symptom domains correlate with structural differences
Differences in the prominence of motor, cognitive, and psychiatric symptoms of Huntington’s disease among individuals can be attributed to differences in gray and white matter structural alterations, according to a neuroimaging study of 43 Huntington’s disease gene carriers conducted by Clara Garcia-Gorro, PhD, of the Bellvitge Institute for Biomedical Research and Bellvitge Hospital, Barcelona, and colleagues.
Their work detected a common neurobiological basis for the carriers’ cognitive and motor symptoms in patterns of reductions in gray matter, cortical thickness, and white matter integrity in cognitive and motor networks. They also found that depressive symptoms were associated with imaging findings primarily characterized by reduced cortical thickness in limbic and paralimbic regions.
“These results are relevant in the context of clinical trials, since they could be used to define specific biomarkers for each symptom profile, even before clinical signs appear. Having more homogeneous groups would potentially increase the likelihood of detecting successful interventions and help to find individualized treatments that target specific cognitive, motor, and psychiatric disturbances,” the authors concluded.
SOURCE: Garcia-Gorro C et al. Neuroimage Clin. 2019 Jun 15. doi: 10.1016/j.nicl.2019.101900.
Differences in the prominence of motor, cognitive, and psychiatric symptoms of Huntington’s disease among individuals can be attributed to differences in gray and white matter structural alterations, according to a neuroimaging study of 43 Huntington’s disease gene carriers conducted by Clara Garcia-Gorro, PhD, of the Bellvitge Institute for Biomedical Research and Bellvitge Hospital, Barcelona, and colleagues.
Their work detected a common neurobiological basis for the carriers’ cognitive and motor symptoms in patterns of reductions in gray matter, cortical thickness, and white matter integrity in cognitive and motor networks. They also found that depressive symptoms were associated with imaging findings primarily characterized by reduced cortical thickness in limbic and paralimbic regions.
“These results are relevant in the context of clinical trials, since they could be used to define specific biomarkers for each symptom profile, even before clinical signs appear. Having more homogeneous groups would potentially increase the likelihood of detecting successful interventions and help to find individualized treatments that target specific cognitive, motor, and psychiatric disturbances,” the authors concluded.
SOURCE: Garcia-Gorro C et al. Neuroimage Clin. 2019 Jun 15. doi: 10.1016/j.nicl.2019.101900.
Differences in the prominence of motor, cognitive, and psychiatric symptoms of Huntington’s disease among individuals can be attributed to differences in gray and white matter structural alterations, according to a neuroimaging study of 43 Huntington’s disease gene carriers conducted by Clara Garcia-Gorro, PhD, of the Bellvitge Institute for Biomedical Research and Bellvitge Hospital, Barcelona, and colleagues.
Their work detected a common neurobiological basis for the carriers’ cognitive and motor symptoms in patterns of reductions in gray matter, cortical thickness, and white matter integrity in cognitive and motor networks. They also found that depressive symptoms were associated with imaging findings primarily characterized by reduced cortical thickness in limbic and paralimbic regions.
“These results are relevant in the context of clinical trials, since they could be used to define specific biomarkers for each symptom profile, even before clinical signs appear. Having more homogeneous groups would potentially increase the likelihood of detecting successful interventions and help to find individualized treatments that target specific cognitive, motor, and psychiatric disturbances,” the authors concluded.
SOURCE: Garcia-Gorro C et al. Neuroimage Clin. 2019 Jun 15. doi: 10.1016/j.nicl.2019.101900.
FROM NEUROIMAGE: CLINICAL
Novel genetic therapy reduces key protein in Huntington’s disease
according to a study published in the New England Journal of Medicine.
Huntington’s disease is an autosomal-dominant neurodegenerative disease caused by CAG trinucleotide repeat expansion in HTT, resulting in a mutant huntingtin protein. No disease-modifying treatment currently exists. The experimental therapy tested in this trial, developed by Ionis Pharmaceuticals and licensed to Roche as HTTRx, is an antisense oligonucleotide that inhibits HTT messenger RNA signaling specific to the production of the mutant huntingtin protein implicated in Huntington’s disease. Whether HTTRx, which is delivered intrathecally, can produce functional or cognitive improvement is yet unclear, as this randomized, double-blinded, multiple-ascending-dose, placebo-controlled trial, which enrolled 46 patients in Canada, Germany, and the United Kingdom, was primarily a safety study.
For the phase 1-2a trial, lead author Sarah J. Tabrizi, MB, ChB, PhD, of University College London and colleagues assigned patients with early Huntington’s disease to monthly intrathecal injections of one of five different doses of HTTRx (10, 30, 60, 90 or 120 mg), or placebo. Most patients (n = 34) received active drug. After the 85-day treatment period, in which four doses were delivered, patients were followed for 4 months.
The treatment groups saw a mean dose-dependent reduction from baseline in the concentration of CSF mutant huntingtin of between –20% and –42% at 28 days post dosing, while the placebo arm saw an increase of a mean 10%. The most common adverse events seen in the trial were procedure-related pain and headache following spinal puncture.
Other endpoints in the study included concentrations of mutant huntingtin in plasma, the effect of treatment on other neurodegenerative biomarkers, and cognitive scores.
The median peak plasma concentrations of HTTRx were reached within 4 hours after the bolus intrathecal administration and declined to less than 30% of the peak concentration by 24 hours after administration. There was no evidence of accumulation of concentration in plasma 24 hours after dose administration.
Functional, cognitive, psychiatric, and neurologic clinical outcomes were generally unchanged at the dose-group level during the trial, and no meaningful differences were observed between patients who received placebo and patients who received active treatment, regardless of the dose level.
An open-label, follow-up study in the same group of patients, all of whom have been assigned to the 120-mg dose monthly or every other month, is expected to end in October 2019. While the extension study is also mainly a safety study, it will also look at biomarkers and cognitive scores over a longer treatment period.
The study was funded by Ionis Pharmaceuticals and F. Hoffmann–La Roche, and most of the authors, including Dr. Tabrizi, reported financial relationships with one or both entities.
SOURCE: Tabrizi SJ et al. N Eng J Med. 2019:380;2307-16.
according to a study published in the New England Journal of Medicine.
Huntington’s disease is an autosomal-dominant neurodegenerative disease caused by CAG trinucleotide repeat expansion in HTT, resulting in a mutant huntingtin protein. No disease-modifying treatment currently exists. The experimental therapy tested in this trial, developed by Ionis Pharmaceuticals and licensed to Roche as HTTRx, is an antisense oligonucleotide that inhibits HTT messenger RNA signaling specific to the production of the mutant huntingtin protein implicated in Huntington’s disease. Whether HTTRx, which is delivered intrathecally, can produce functional or cognitive improvement is yet unclear, as this randomized, double-blinded, multiple-ascending-dose, placebo-controlled trial, which enrolled 46 patients in Canada, Germany, and the United Kingdom, was primarily a safety study.
For the phase 1-2a trial, lead author Sarah J. Tabrizi, MB, ChB, PhD, of University College London and colleagues assigned patients with early Huntington’s disease to monthly intrathecal injections of one of five different doses of HTTRx (10, 30, 60, 90 or 120 mg), or placebo. Most patients (n = 34) received active drug. After the 85-day treatment period, in which four doses were delivered, patients were followed for 4 months.
The treatment groups saw a mean dose-dependent reduction from baseline in the concentration of CSF mutant huntingtin of between –20% and –42% at 28 days post dosing, while the placebo arm saw an increase of a mean 10%. The most common adverse events seen in the trial were procedure-related pain and headache following spinal puncture.
Other endpoints in the study included concentrations of mutant huntingtin in plasma, the effect of treatment on other neurodegenerative biomarkers, and cognitive scores.
The median peak plasma concentrations of HTTRx were reached within 4 hours after the bolus intrathecal administration and declined to less than 30% of the peak concentration by 24 hours after administration. There was no evidence of accumulation of concentration in plasma 24 hours after dose administration.
Functional, cognitive, psychiatric, and neurologic clinical outcomes were generally unchanged at the dose-group level during the trial, and no meaningful differences were observed between patients who received placebo and patients who received active treatment, regardless of the dose level.
An open-label, follow-up study in the same group of patients, all of whom have been assigned to the 120-mg dose monthly or every other month, is expected to end in October 2019. While the extension study is also mainly a safety study, it will also look at biomarkers and cognitive scores over a longer treatment period.
The study was funded by Ionis Pharmaceuticals and F. Hoffmann–La Roche, and most of the authors, including Dr. Tabrizi, reported financial relationships with one or both entities.
SOURCE: Tabrizi SJ et al. N Eng J Med. 2019:380;2307-16.
according to a study published in the New England Journal of Medicine.
Huntington’s disease is an autosomal-dominant neurodegenerative disease caused by CAG trinucleotide repeat expansion in HTT, resulting in a mutant huntingtin protein. No disease-modifying treatment currently exists. The experimental therapy tested in this trial, developed by Ionis Pharmaceuticals and licensed to Roche as HTTRx, is an antisense oligonucleotide that inhibits HTT messenger RNA signaling specific to the production of the mutant huntingtin protein implicated in Huntington’s disease. Whether HTTRx, which is delivered intrathecally, can produce functional or cognitive improvement is yet unclear, as this randomized, double-blinded, multiple-ascending-dose, placebo-controlled trial, which enrolled 46 patients in Canada, Germany, and the United Kingdom, was primarily a safety study.
For the phase 1-2a trial, lead author Sarah J. Tabrizi, MB, ChB, PhD, of University College London and colleagues assigned patients with early Huntington’s disease to monthly intrathecal injections of one of five different doses of HTTRx (10, 30, 60, 90 or 120 mg), or placebo. Most patients (n = 34) received active drug. After the 85-day treatment period, in which four doses were delivered, patients were followed for 4 months.
The treatment groups saw a mean dose-dependent reduction from baseline in the concentration of CSF mutant huntingtin of between –20% and –42% at 28 days post dosing, while the placebo arm saw an increase of a mean 10%. The most common adverse events seen in the trial were procedure-related pain and headache following spinal puncture.
Other endpoints in the study included concentrations of mutant huntingtin in plasma, the effect of treatment on other neurodegenerative biomarkers, and cognitive scores.
The median peak plasma concentrations of HTTRx were reached within 4 hours after the bolus intrathecal administration and declined to less than 30% of the peak concentration by 24 hours after administration. There was no evidence of accumulation of concentration in plasma 24 hours after dose administration.
Functional, cognitive, psychiatric, and neurologic clinical outcomes were generally unchanged at the dose-group level during the trial, and no meaningful differences were observed between patients who received placebo and patients who received active treatment, regardless of the dose level.
An open-label, follow-up study in the same group of patients, all of whom have been assigned to the 120-mg dose monthly or every other month, is expected to end in October 2019. While the extension study is also mainly a safety study, it will also look at biomarkers and cognitive scores over a longer treatment period.
The study was funded by Ionis Pharmaceuticals and F. Hoffmann–La Roche, and most of the authors, including Dr. Tabrizi, reported financial relationships with one or both entities.
SOURCE: Tabrizi SJ et al. N Eng J Med. 2019:380;2307-16.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Mutant huntingtin and neurofilament light are potential biomarkers in Huntington’s disease
PHILADELPHIA – according to an investigation presented at the annual meeting of the American Academy of Neurology. These biomarkers appear to reflect the earliest detectable changes in the natural history of Huntington’s disease, but the longitudinal prognostic value of changes in these biomarkers requires further investigation, the researchers said.
Huntington’s disease has a long prodromal phase and is associated with long survival. Investigators still need well-validated biomarkers of disease progression, prognosis, and pharmacodynamics to aid drug development, said Filipe B. Rodrigues, MD, clinical research fellow at University College London. After several years of study, Dr. Rodrigues and colleagues found mutant huntingtin and neurofilament light (NfL) to be the most promising potential biomarkers in Huntington’s disease. They sought to understand how these two biomarkers compare with each other, what their predictive ability is, and how they change longitudinally.
To this end, Dr. Rodrigues and colleagues designed the HD-CSF study, a prospective, observational, longitudinal cohort study with a 2-year follow-up. They recruited 20 healthy controls, 20 patients with premanifest Huntington’s disease, and 40 patients with manifest Huntington’s disease. All participants underwent regular clinical assessments and standardized collections of cerebrospinal fluid (CSF) and blood. They also had the option of undergoing brain MRI scans.
The investigators analyzed their data using multiple linear regression models, Pearson’s correlations, receiver operating characteristic curves, and sample size calculations. They used an event-based model to evaluate the temporal sequence of changes in Huntington’s disease-related biomarkers.
Dr. Rodrigues and colleagues first observed that all three biomarkers successfully distinguished between healthy controls, patients with premanifest Huntington’s disease, and patients with Huntington’s disease. Mutant huntingtin, the pathogenic agent in Huntington’s disease, discriminated perfectly between healthy controls and mutation carriers, as the researchers had expected. CSF and plasma levels of NfL also discriminated well between healthy controls and mutation carriers. These biomarkers had areas under the ROC curve greater than 0.9. NfL in plasma and CSF also distinguished well between patients with premanifest Huntington’s disease and those with manifest Huntington’s disease, with areas under the curve greater than 0.9. Their discriminative ability in this regard was significantly better than that of mutant huntingtin.
When the researchers examined the relationship between the three biomarkers, they found that CSF levels of NfL were strongly correlated in a linear fashion with plasma levels of NfL. CSF levels of mutant huntingtin were moderately associated with CSF levels of NfL.
Levels of all three biomarkers increased significantly as the disease progressed and were associated with all clinical scales and imaging measures. CSF and plasma levels of NfL had superior predictive ability for clinical and imaging measures, compared with mutant huntingtin. CSF and plasma NfL were associated with brain volume, but mutant huntingtin was not.
All three biomarkers were stable during a 6-week period. Dr. Rodrigues and colleagues calculated sample sizes for a two-arm interventional trial involving various hypothetical therapeutic effects. They found that the required sample sizes were small enough to be incorporated easily into ongoing and future clinical trials.
In silico modeling suggested among the markers measured in the HD-CSF study, the three biofluid biomarkers were the first factors to be altered in the course of Huntington’s disease. Alterations in the biomarkers were followed by changes in imaging markers, and then by changes in clinical markers (for example, motor and cognitive function).
Finally, Dr. Rodrigues and colleagues found preliminary evidence that levels of NfL in CSF and plasma increase over time at different rates in patients with Huntington’s disease, compared with healthy controls. NfL appears to be more useful than mutant huntingtin for evaluating the rate of disease progression than for gauging response to treatment, said Dr. Rodrigues. “If [we] can prove that we can assess response to treatment by measuring NfL, I think that would be great.”
The investigators are currently analyzing the longitudinal predictive value of changes in these biomarkers. They also have begun analyzing other markers such as tau and brain-derived neurotrophic factor.
This study was funded by the Medical Research Council UK, the CHDI Foundation, and F. Hoffmann-La Roche.
This article was updated 6/18/19.
SOURCE: Rodrigues FB et al. AAN 2019, Abstract S16.003.
PHILADELPHIA – according to an investigation presented at the annual meeting of the American Academy of Neurology. These biomarkers appear to reflect the earliest detectable changes in the natural history of Huntington’s disease, but the longitudinal prognostic value of changes in these biomarkers requires further investigation, the researchers said.
Huntington’s disease has a long prodromal phase and is associated with long survival. Investigators still need well-validated biomarkers of disease progression, prognosis, and pharmacodynamics to aid drug development, said Filipe B. Rodrigues, MD, clinical research fellow at University College London. After several years of study, Dr. Rodrigues and colleagues found mutant huntingtin and neurofilament light (NfL) to be the most promising potential biomarkers in Huntington’s disease. They sought to understand how these two biomarkers compare with each other, what their predictive ability is, and how they change longitudinally.
To this end, Dr. Rodrigues and colleagues designed the HD-CSF study, a prospective, observational, longitudinal cohort study with a 2-year follow-up. They recruited 20 healthy controls, 20 patients with premanifest Huntington’s disease, and 40 patients with manifest Huntington’s disease. All participants underwent regular clinical assessments and standardized collections of cerebrospinal fluid (CSF) and blood. They also had the option of undergoing brain MRI scans.
The investigators analyzed their data using multiple linear regression models, Pearson’s correlations, receiver operating characteristic curves, and sample size calculations. They used an event-based model to evaluate the temporal sequence of changes in Huntington’s disease-related biomarkers.
Dr. Rodrigues and colleagues first observed that all three biomarkers successfully distinguished between healthy controls, patients with premanifest Huntington’s disease, and patients with Huntington’s disease. Mutant huntingtin, the pathogenic agent in Huntington’s disease, discriminated perfectly between healthy controls and mutation carriers, as the researchers had expected. CSF and plasma levels of NfL also discriminated well between healthy controls and mutation carriers. These biomarkers had areas under the ROC curve greater than 0.9. NfL in plasma and CSF also distinguished well between patients with premanifest Huntington’s disease and those with manifest Huntington’s disease, with areas under the curve greater than 0.9. Their discriminative ability in this regard was significantly better than that of mutant huntingtin.
When the researchers examined the relationship between the three biomarkers, they found that CSF levels of NfL were strongly correlated in a linear fashion with plasma levels of NfL. CSF levels of mutant huntingtin were moderately associated with CSF levels of NfL.
Levels of all three biomarkers increased significantly as the disease progressed and were associated with all clinical scales and imaging measures. CSF and plasma levels of NfL had superior predictive ability for clinical and imaging measures, compared with mutant huntingtin. CSF and plasma NfL were associated with brain volume, but mutant huntingtin was not.
All three biomarkers were stable during a 6-week period. Dr. Rodrigues and colleagues calculated sample sizes for a two-arm interventional trial involving various hypothetical therapeutic effects. They found that the required sample sizes were small enough to be incorporated easily into ongoing and future clinical trials.
In silico modeling suggested among the markers measured in the HD-CSF study, the three biofluid biomarkers were the first factors to be altered in the course of Huntington’s disease. Alterations in the biomarkers were followed by changes in imaging markers, and then by changes in clinical markers (for example, motor and cognitive function).
Finally, Dr. Rodrigues and colleagues found preliminary evidence that levels of NfL in CSF and plasma increase over time at different rates in patients with Huntington’s disease, compared with healthy controls. NfL appears to be more useful than mutant huntingtin for evaluating the rate of disease progression than for gauging response to treatment, said Dr. Rodrigues. “If [we] can prove that we can assess response to treatment by measuring NfL, I think that would be great.”
The investigators are currently analyzing the longitudinal predictive value of changes in these biomarkers. They also have begun analyzing other markers such as tau and brain-derived neurotrophic factor.
This study was funded by the Medical Research Council UK, the CHDI Foundation, and F. Hoffmann-La Roche.
This article was updated 6/18/19.
SOURCE: Rodrigues FB et al. AAN 2019, Abstract S16.003.
PHILADELPHIA – according to an investigation presented at the annual meeting of the American Academy of Neurology. These biomarkers appear to reflect the earliest detectable changes in the natural history of Huntington’s disease, but the longitudinal prognostic value of changes in these biomarkers requires further investigation, the researchers said.
Huntington’s disease has a long prodromal phase and is associated with long survival. Investigators still need well-validated biomarkers of disease progression, prognosis, and pharmacodynamics to aid drug development, said Filipe B. Rodrigues, MD, clinical research fellow at University College London. After several years of study, Dr. Rodrigues and colleagues found mutant huntingtin and neurofilament light (NfL) to be the most promising potential biomarkers in Huntington’s disease. They sought to understand how these two biomarkers compare with each other, what their predictive ability is, and how they change longitudinally.
To this end, Dr. Rodrigues and colleagues designed the HD-CSF study, a prospective, observational, longitudinal cohort study with a 2-year follow-up. They recruited 20 healthy controls, 20 patients with premanifest Huntington’s disease, and 40 patients with manifest Huntington’s disease. All participants underwent regular clinical assessments and standardized collections of cerebrospinal fluid (CSF) and blood. They also had the option of undergoing brain MRI scans.
The investigators analyzed their data using multiple linear regression models, Pearson’s correlations, receiver operating characteristic curves, and sample size calculations. They used an event-based model to evaluate the temporal sequence of changes in Huntington’s disease-related biomarkers.
Dr. Rodrigues and colleagues first observed that all three biomarkers successfully distinguished between healthy controls, patients with premanifest Huntington’s disease, and patients with Huntington’s disease. Mutant huntingtin, the pathogenic agent in Huntington’s disease, discriminated perfectly between healthy controls and mutation carriers, as the researchers had expected. CSF and plasma levels of NfL also discriminated well between healthy controls and mutation carriers. These biomarkers had areas under the ROC curve greater than 0.9. NfL in plasma and CSF also distinguished well between patients with premanifest Huntington’s disease and those with manifest Huntington’s disease, with areas under the curve greater than 0.9. Their discriminative ability in this regard was significantly better than that of mutant huntingtin.
When the researchers examined the relationship between the three biomarkers, they found that CSF levels of NfL were strongly correlated in a linear fashion with plasma levels of NfL. CSF levels of mutant huntingtin were moderately associated with CSF levels of NfL.
Levels of all three biomarkers increased significantly as the disease progressed and were associated with all clinical scales and imaging measures. CSF and plasma levels of NfL had superior predictive ability for clinical and imaging measures, compared with mutant huntingtin. CSF and plasma NfL were associated with brain volume, but mutant huntingtin was not.
All three biomarkers were stable during a 6-week period. Dr. Rodrigues and colleagues calculated sample sizes for a two-arm interventional trial involving various hypothetical therapeutic effects. They found that the required sample sizes were small enough to be incorporated easily into ongoing and future clinical trials.
In silico modeling suggested among the markers measured in the HD-CSF study, the three biofluid biomarkers were the first factors to be altered in the course of Huntington’s disease. Alterations in the biomarkers were followed by changes in imaging markers, and then by changes in clinical markers (for example, motor and cognitive function).
Finally, Dr. Rodrigues and colleagues found preliminary evidence that levels of NfL in CSF and plasma increase over time at different rates in patients with Huntington’s disease, compared with healthy controls. NfL appears to be more useful than mutant huntingtin for evaluating the rate of disease progression than for gauging response to treatment, said Dr. Rodrigues. “If [we] can prove that we can assess response to treatment by measuring NfL, I think that would be great.”
The investigators are currently analyzing the longitudinal predictive value of changes in these biomarkers. They also have begun analyzing other markers such as tau and brain-derived neurotrophic factor.
This study was funded by the Medical Research Council UK, the CHDI Foundation, and F. Hoffmann-La Roche.
This article was updated 6/18/19.
SOURCE: Rodrigues FB et al. AAN 2019, Abstract S16.003.
REPORTING FROM AAN 2019
Key clinical point: Mutant huntingtin and plasma and cerebrospinal fluid levels of neurofilament light are useful potential biomarkers in Huntington’s disease.
Major finding: Levels of neurofilament light in plasma are correlated with those in cerebrospinal fluid.
Study details: A prospective, observational, longitudinal cohort study including 80 participants with and without Huntington’s disease.
Disclosures: This study was funded by the Medical Research Council UK, the CHDI Foundation, and F. Hoffmann-La Roche.
Source: Rodrigues FB et al. AAN 2019, Abstract S16.003.
Laquinimod may not improve motor function in Huntington’s disease
PHILADELPHIA – Laquinimod appears not to improve motor function or clinical outcomes in patients with Huntington’s disease, according to a study presented at the annual meeting of the American Academy of Neurology. However, the drug reduced brain volume loss in the caudate and other regions.
Laquinimod is an investigational immunomodulatory drug that prevents inflammation and neurodegeneration in the CNS. The treatment was studied as a therapy for multiple sclerosis (MS), but development for this indication has been stopped. Researchers have observed that laquinimod modulates inflammatory pathways that are involved in Huntington’s disease pathology.
Ralf Reilmann, MD, PhD, founder of the George Huntington Institute and chair of the Huntington unit at the University of Münster (Germany), and colleagues conducted the phase 2 LEGATO-HD study at 48 sites in 10 countries to examine the efficacy and safety of laquinimod in patients with early Huntington’s disease. Participants were randomized in double-blind fashion to daily placebo or 0.5-mg, 1.0-mg, or 1.5-mg doses of laquinimod for 52 weeks. After the initiation of this trial, studies of the drug in MS indicated that the 1.5-mg dose was associated with cardiovascular risks, and Dr. Reilmann and his colleagues discontinued the 1.5-mg arm of their trial as a precaution.
The primary endpoint of LEGATO-HD was the change from baseline in the Unified Huntington’s Disease Rating Scale (UHDRS)–Total Motor Score (TMS). The secondary endpoint was the percent change in caudate volume at week 52 for the 1.0-mg dose group, compared with controls. The investigators also examined exploratory endpoints such as changes in MRI volume measures and Quantitative Motor, Clinician Interview-Based Impression of Change plus caregiver input, UHDRS–Total Functional Capacity and UHDRS–Functional Assessment scores. Adverse event reporting and clinical and laboratory examinations constituted the safety measures.
Dr. Reilmann and colleagues found no difference between the treated patients and controls in UHDRS-TMS. However, they did observe less caudate volume loss in the laquinimod group, compared with controls. All MRI exploratory measures also favored laquinimod. The researchers found no treatment effects of laquinimod in rater-dependent clinical outcome measures. Laquinimod was well tolerated, and the study yielded no new safety findings.
Dr. Reilmann has received research support from Teva, which supported the LEGATO-HD trial and in 2018 sold development and commercial rights for laquinimod to Active Biotech. He has received research support from a variety of other pharmaceutical companies and organizations, including the CHDI Foundation and the European Huntington Disease Network.
SOURCE: Reilmann R et al. AAN 2019, Abstract S16.007.
PHILADELPHIA – Laquinimod appears not to improve motor function or clinical outcomes in patients with Huntington’s disease, according to a study presented at the annual meeting of the American Academy of Neurology. However, the drug reduced brain volume loss in the caudate and other regions.
Laquinimod is an investigational immunomodulatory drug that prevents inflammation and neurodegeneration in the CNS. The treatment was studied as a therapy for multiple sclerosis (MS), but development for this indication has been stopped. Researchers have observed that laquinimod modulates inflammatory pathways that are involved in Huntington’s disease pathology.
Ralf Reilmann, MD, PhD, founder of the George Huntington Institute and chair of the Huntington unit at the University of Münster (Germany), and colleagues conducted the phase 2 LEGATO-HD study at 48 sites in 10 countries to examine the efficacy and safety of laquinimod in patients with early Huntington’s disease. Participants were randomized in double-blind fashion to daily placebo or 0.5-mg, 1.0-mg, or 1.5-mg doses of laquinimod for 52 weeks. After the initiation of this trial, studies of the drug in MS indicated that the 1.5-mg dose was associated with cardiovascular risks, and Dr. Reilmann and his colleagues discontinued the 1.5-mg arm of their trial as a precaution.
The primary endpoint of LEGATO-HD was the change from baseline in the Unified Huntington’s Disease Rating Scale (UHDRS)–Total Motor Score (TMS). The secondary endpoint was the percent change in caudate volume at week 52 for the 1.0-mg dose group, compared with controls. The investigators also examined exploratory endpoints such as changes in MRI volume measures and Quantitative Motor, Clinician Interview-Based Impression of Change plus caregiver input, UHDRS–Total Functional Capacity and UHDRS–Functional Assessment scores. Adverse event reporting and clinical and laboratory examinations constituted the safety measures.
Dr. Reilmann and colleagues found no difference between the treated patients and controls in UHDRS-TMS. However, they did observe less caudate volume loss in the laquinimod group, compared with controls. All MRI exploratory measures also favored laquinimod. The researchers found no treatment effects of laquinimod in rater-dependent clinical outcome measures. Laquinimod was well tolerated, and the study yielded no new safety findings.
Dr. Reilmann has received research support from Teva, which supported the LEGATO-HD trial and in 2018 sold development and commercial rights for laquinimod to Active Biotech. He has received research support from a variety of other pharmaceutical companies and organizations, including the CHDI Foundation and the European Huntington Disease Network.
SOURCE: Reilmann R et al. AAN 2019, Abstract S16.007.
PHILADELPHIA – Laquinimod appears not to improve motor function or clinical outcomes in patients with Huntington’s disease, according to a study presented at the annual meeting of the American Academy of Neurology. However, the drug reduced brain volume loss in the caudate and other regions.
Laquinimod is an investigational immunomodulatory drug that prevents inflammation and neurodegeneration in the CNS. The treatment was studied as a therapy for multiple sclerosis (MS), but development for this indication has been stopped. Researchers have observed that laquinimod modulates inflammatory pathways that are involved in Huntington’s disease pathology.
Ralf Reilmann, MD, PhD, founder of the George Huntington Institute and chair of the Huntington unit at the University of Münster (Germany), and colleagues conducted the phase 2 LEGATO-HD study at 48 sites in 10 countries to examine the efficacy and safety of laquinimod in patients with early Huntington’s disease. Participants were randomized in double-blind fashion to daily placebo or 0.5-mg, 1.0-mg, or 1.5-mg doses of laquinimod for 52 weeks. After the initiation of this trial, studies of the drug in MS indicated that the 1.5-mg dose was associated with cardiovascular risks, and Dr. Reilmann and his colleagues discontinued the 1.5-mg arm of their trial as a precaution.
The primary endpoint of LEGATO-HD was the change from baseline in the Unified Huntington’s Disease Rating Scale (UHDRS)–Total Motor Score (TMS). The secondary endpoint was the percent change in caudate volume at week 52 for the 1.0-mg dose group, compared with controls. The investigators also examined exploratory endpoints such as changes in MRI volume measures and Quantitative Motor, Clinician Interview-Based Impression of Change plus caregiver input, UHDRS–Total Functional Capacity and UHDRS–Functional Assessment scores. Adverse event reporting and clinical and laboratory examinations constituted the safety measures.
Dr. Reilmann and colleagues found no difference between the treated patients and controls in UHDRS-TMS. However, they did observe less caudate volume loss in the laquinimod group, compared with controls. All MRI exploratory measures also favored laquinimod. The researchers found no treatment effects of laquinimod in rater-dependent clinical outcome measures. Laquinimod was well tolerated, and the study yielded no new safety findings.
Dr. Reilmann has received research support from Teva, which supported the LEGATO-HD trial and in 2018 sold development and commercial rights for laquinimod to Active Biotech. He has received research support from a variety of other pharmaceutical companies and organizations, including the CHDI Foundation and the European Huntington Disease Network.
SOURCE: Reilmann R et al. AAN 2019, Abstract S16.007.
REPORTING FROM AAN 2019
Key clinical point: Investigators found no difference between laquinimod and placebo on motor function in Huntington’s disease.
Major finding: The study examined 0.5-mg, 1.0-mg, and 1.5-mg doses of laquinimod.
Study details: The phase 2 LEGATO-HD trial included 352 patients with Huntington’s disease who underwent a 52-week treatment period.
Disclosures: Dr. Reilmann has received research support from Teva, which supported the LEGATO-HD trial and in 2018, sold development and commercial rights for laquinimod to Active Biotech. He has received research support from a variety of other pharmaceutical companies and organizations, including the CHDI Foundation and the European Huntington Disease Network.
Source: Reilmann R et al. AAN 2019, Abstract S16.007.
Polyglutamine diseases are rare, but not the mutations that cause them
Polyglutamine diseases are a group of hereditary neurodegenerative disorders caused by mutations in which a trinucleotide repeat expands pathologically on a disease-associated gene. The diseases are rare, with the most common among them – Huntington disease – affecting between 10 and 14 per 100,000 people in Western countries, where prevalence is highest.
In polyglutamine diseases, which include the spinocerebellar ataxias, dentatorubral-pallidoluysian atrophy, and spinal bulbar muscular atrophy, higher CAG (cytosine-adenine-guanine) repeat numbers are associated with greater disease severity, faster progression, or earlier age at onset.
In research published April 1 in JAMA Neurology, investigators report that more than one-tenth of the European population carries CAG expansions that fall short of the repeats needed to cause any of 9 polyglutamine diseases – but are enough to put them at risk of having children who develop one. A smaller number of people – about 1% – carry enough CAG repeats to cause one of the diseases late in life.
For their research, Sarah L. Gardiner, MD, of Leiden (the Netherlands) University, and her colleagues looked at polyglutamine expansion variants for nine diseases in samples from 14,196 adults (56% of whom were women) from the Netherlands, Scotland, and Ireland. The samples were taken from five population-based cohort studies conducted between 1997 and 2012, and all subjects were without a history of polyglutamine disease or major depression.
Of these, 10.7% had a CAG repeat number on a disease-associated gene that was in the intermediate range, defined as a number of repeats that cannot cause disease but for which “expansion into the fully pathological range has been observed on intergenerational transmission,” Dr. Gardiner and her colleagues wrote. And some 1.3% of subjects were found to have CAG repeats within the disease-causing range, “mostly in the lower range associated with elderly onset.”
The investigators found no differences in sex, age, or body mass index between individuals with CAG repeat numbers within the pathological range and individuals with CAG repeat numbers within the normal or intermediate range.
Whether carriers of immediate or lower-range pathological CAG repeats went on to develop disease could not be measured, as follow-up data were not available. Another limitation of the study, the investigators acknowledged, was that the genotyping method used “did not allow us to determine the presence of trinucleotide interruptions,” which can affect disease penetrance.
“A late age at onset, a reduced penetrance, or the presence of interruptions could all explain the asymptomatic status of our carriers of intermediate and pathological polyglutamine disease–associated alleles at the time of assessment,” Dr. Gardiner and her colleagues wrote.
This study was funded by the European Union and Dutch government agencies; one of the population-based cohort studies from which the study sample was taken received some support from Bristol-Myers-Squibb. One of Dr. Gardiner’s coauthors, Raymund A. C. Roos, MD, PhD, disclosed being an adviser for UniQure, a gene-therapy firm, and no other conflicts of interest were reported.
SOURCE: Gardiner et al. JAMA Neurol. 2019 Apr 1. doi: 10.001/jamaneurol.2019.042.
Gardiner et al. describe the results of an appraisal of polyglutamine expansion variants in more than 14,000 individuals from the Netherlands, Scotland, and Ireland. Given the relative rarity of polyglutamine repeat disease, the first question that comes to mind is why were so many individuals identified with repeats in the pathogenic range? Based on our understanding of disease prevalence, it is unlikely that each of these individuals will become affected; therefore, this work suggests a reduced penetrance of these mutations. The findings are illustrative of a growing theme in human disease genetics: There are a very large number of apparently healthy individuals in the general population who carry mutations associated with various diseases. The phenomenon of reduced penetrance, where mutations cause disease in some but not all carriers, overlaps and arguably may be the same as that of variable expressivity, where the same mutation can lead to very different disease outcomes in different individuals. It is extremely likely that second-generation sequencing and population-scale screening will continue to reveal similar themes. We continue to appreciate the increasing complexity of the human genome and its relationship to disease, even those diseases we thought of previously as simple “single-gene” disorders.
Monia B. Hammer, PhD, and Andrew B. Singleton, PhD, are with the National Institute on Aging, National Institutes of Health, Bethesda, Md. Dr. Hammer and Dr. Singleton report no financial conflicts of interest related to their editorial.
Gardiner et al. describe the results of an appraisal of polyglutamine expansion variants in more than 14,000 individuals from the Netherlands, Scotland, and Ireland. Given the relative rarity of polyglutamine repeat disease, the first question that comes to mind is why were so many individuals identified with repeats in the pathogenic range? Based on our understanding of disease prevalence, it is unlikely that each of these individuals will become affected; therefore, this work suggests a reduced penetrance of these mutations. The findings are illustrative of a growing theme in human disease genetics: There are a very large number of apparently healthy individuals in the general population who carry mutations associated with various diseases. The phenomenon of reduced penetrance, where mutations cause disease in some but not all carriers, overlaps and arguably may be the same as that of variable expressivity, where the same mutation can lead to very different disease outcomes in different individuals. It is extremely likely that second-generation sequencing and population-scale screening will continue to reveal similar themes. We continue to appreciate the increasing complexity of the human genome and its relationship to disease, even those diseases we thought of previously as simple “single-gene” disorders.
Monia B. Hammer, PhD, and Andrew B. Singleton, PhD, are with the National Institute on Aging, National Institutes of Health, Bethesda, Md. Dr. Hammer and Dr. Singleton report no financial conflicts of interest related to their editorial.
Gardiner et al. describe the results of an appraisal of polyglutamine expansion variants in more than 14,000 individuals from the Netherlands, Scotland, and Ireland. Given the relative rarity of polyglutamine repeat disease, the first question that comes to mind is why were so many individuals identified with repeats in the pathogenic range? Based on our understanding of disease prevalence, it is unlikely that each of these individuals will become affected; therefore, this work suggests a reduced penetrance of these mutations. The findings are illustrative of a growing theme in human disease genetics: There are a very large number of apparently healthy individuals in the general population who carry mutations associated with various diseases. The phenomenon of reduced penetrance, where mutations cause disease in some but not all carriers, overlaps and arguably may be the same as that of variable expressivity, where the same mutation can lead to very different disease outcomes in different individuals. It is extremely likely that second-generation sequencing and population-scale screening will continue to reveal similar themes. We continue to appreciate the increasing complexity of the human genome and its relationship to disease, even those diseases we thought of previously as simple “single-gene” disorders.
Monia B. Hammer, PhD, and Andrew B. Singleton, PhD, are with the National Institute on Aging, National Institutes of Health, Bethesda, Md. Dr. Hammer and Dr. Singleton report no financial conflicts of interest related to their editorial.
Polyglutamine diseases are a group of hereditary neurodegenerative disorders caused by mutations in which a trinucleotide repeat expands pathologically on a disease-associated gene. The diseases are rare, with the most common among them – Huntington disease – affecting between 10 and 14 per 100,000 people in Western countries, where prevalence is highest.
In polyglutamine diseases, which include the spinocerebellar ataxias, dentatorubral-pallidoluysian atrophy, and spinal bulbar muscular atrophy, higher CAG (cytosine-adenine-guanine) repeat numbers are associated with greater disease severity, faster progression, or earlier age at onset.
In research published April 1 in JAMA Neurology, investigators report that more than one-tenth of the European population carries CAG expansions that fall short of the repeats needed to cause any of 9 polyglutamine diseases – but are enough to put them at risk of having children who develop one. A smaller number of people – about 1% – carry enough CAG repeats to cause one of the diseases late in life.
For their research, Sarah L. Gardiner, MD, of Leiden (the Netherlands) University, and her colleagues looked at polyglutamine expansion variants for nine diseases in samples from 14,196 adults (56% of whom were women) from the Netherlands, Scotland, and Ireland. The samples were taken from five population-based cohort studies conducted between 1997 and 2012, and all subjects were without a history of polyglutamine disease or major depression.
Of these, 10.7% had a CAG repeat number on a disease-associated gene that was in the intermediate range, defined as a number of repeats that cannot cause disease but for which “expansion into the fully pathological range has been observed on intergenerational transmission,” Dr. Gardiner and her colleagues wrote. And some 1.3% of subjects were found to have CAG repeats within the disease-causing range, “mostly in the lower range associated with elderly onset.”
The investigators found no differences in sex, age, or body mass index between individuals with CAG repeat numbers within the pathological range and individuals with CAG repeat numbers within the normal or intermediate range.
Whether carriers of immediate or lower-range pathological CAG repeats went on to develop disease could not be measured, as follow-up data were not available. Another limitation of the study, the investigators acknowledged, was that the genotyping method used “did not allow us to determine the presence of trinucleotide interruptions,” which can affect disease penetrance.
“A late age at onset, a reduced penetrance, or the presence of interruptions could all explain the asymptomatic status of our carriers of intermediate and pathological polyglutamine disease–associated alleles at the time of assessment,” Dr. Gardiner and her colleagues wrote.
This study was funded by the European Union and Dutch government agencies; one of the population-based cohort studies from which the study sample was taken received some support from Bristol-Myers-Squibb. One of Dr. Gardiner’s coauthors, Raymund A. C. Roos, MD, PhD, disclosed being an adviser for UniQure, a gene-therapy firm, and no other conflicts of interest were reported.
SOURCE: Gardiner et al. JAMA Neurol. 2019 Apr 1. doi: 10.001/jamaneurol.2019.042.
Polyglutamine diseases are a group of hereditary neurodegenerative disorders caused by mutations in which a trinucleotide repeat expands pathologically on a disease-associated gene. The diseases are rare, with the most common among them – Huntington disease – affecting between 10 and 14 per 100,000 people in Western countries, where prevalence is highest.
In polyglutamine diseases, which include the spinocerebellar ataxias, dentatorubral-pallidoluysian atrophy, and spinal bulbar muscular atrophy, higher CAG (cytosine-adenine-guanine) repeat numbers are associated with greater disease severity, faster progression, or earlier age at onset.
In research published April 1 in JAMA Neurology, investigators report that more than one-tenth of the European population carries CAG expansions that fall short of the repeats needed to cause any of 9 polyglutamine diseases – but are enough to put them at risk of having children who develop one. A smaller number of people – about 1% – carry enough CAG repeats to cause one of the diseases late in life.
For their research, Sarah L. Gardiner, MD, of Leiden (the Netherlands) University, and her colleagues looked at polyglutamine expansion variants for nine diseases in samples from 14,196 adults (56% of whom were women) from the Netherlands, Scotland, and Ireland. The samples were taken from five population-based cohort studies conducted between 1997 and 2012, and all subjects were without a history of polyglutamine disease or major depression.
Of these, 10.7% had a CAG repeat number on a disease-associated gene that was in the intermediate range, defined as a number of repeats that cannot cause disease but for which “expansion into the fully pathological range has been observed on intergenerational transmission,” Dr. Gardiner and her colleagues wrote. And some 1.3% of subjects were found to have CAG repeats within the disease-causing range, “mostly in the lower range associated with elderly onset.”
The investigators found no differences in sex, age, or body mass index between individuals with CAG repeat numbers within the pathological range and individuals with CAG repeat numbers within the normal or intermediate range.
Whether carriers of immediate or lower-range pathological CAG repeats went on to develop disease could not be measured, as follow-up data were not available. Another limitation of the study, the investigators acknowledged, was that the genotyping method used “did not allow us to determine the presence of trinucleotide interruptions,” which can affect disease penetrance.
“A late age at onset, a reduced penetrance, or the presence of interruptions could all explain the asymptomatic status of our carriers of intermediate and pathological polyglutamine disease–associated alleles at the time of assessment,” Dr. Gardiner and her colleagues wrote.
This study was funded by the European Union and Dutch government agencies; one of the population-based cohort studies from which the study sample was taken received some support from Bristol-Myers-Squibb. One of Dr. Gardiner’s coauthors, Raymund A. C. Roos, MD, PhD, disclosed being an adviser for UniQure, a gene-therapy firm, and no other conflicts of interest were reported.
SOURCE: Gardiner et al. JAMA Neurol. 2019 Apr 1. doi: 10.001/jamaneurol.2019.042.
FROM JAMA NEUROLOGY
Huntington’s research returns to Latin America, as scientists tread with care
BARRANQUILLA, COLOMBIA – “We don’t like to call them brigades. That sounds militant,” said neuropsychologist Johan Acosta-López, PhD.
Dr. Acosta-López, the head of cognitive neurosciences at Simón Bolivar University in this city on Colombia’s Atlantic coast, was among five Colombian clinicians – neurologists, psychiatrists, and neuropsychologists – stuffed into a car on their way to a conference hotel in July 2018.

The following day they would be joined by clinicians and researchers from North America, other Latin American countries, and Europe for a first-of-its kind meeting on Huntington’s disease (HD) in the region, sponsored by Factor-H, an HD charity working in Latin America.
Once the talks wrapped up, the researchers – clinicians and basic scientists – were invited to see patients at a hospital in a town an hour inland with a large concentration of HD families, most of them extremely poor. For some, the Factor-H–sponsored “brigade” would be their first hands-on experience with HD patients in a developing country.
There was some debate in the car about what to call such events: brigades, “integrated health days,” or clinics. Around here – where HD abounded but patients were weary of researchers – terminology mattered.
“We’ve had so many investigators arrive in this area – foreigners and Colombians – telling people ‘we’ve got this huge, great project that you’ll benefit from.’ And they take blood samples and never return,” Dr. Acosta-López said.
Even as a local investigator, Dr. Acosta-López has faced challenges getting a new study off the ground. Dr. Acosta-López and his colleagues are working under a grant from the Colombian government to recruit 241 presymptomatic subjects with confirmed genetic markers for HD, and evaluate them for cognitive and neurologic changes preceding disease onset.
It’s a cross-sectional study, and such studies are usually funded for a year. But the investigators knew it would take much more than a year to recruit patients here, and planned their study for 3 years. As of July, the team had been engaging with the community for 6 months but still didn’t have a single blood sample.
“We’ve had to convince everyone that this time is different,” he said, “and that means focusing on the social aspect” – setting up a legal-assistance program through the university to help families claim health benefits and a job-training program sponsored by local businesses.
It’s unusual for researchers to find themselves playing such extensive roles in coordinating social and economic support for their subjects. But with HD, it’s happening across Latin America, where researchers speak frequently of a “debt” owed to HD families in this region.
Huntington’s disease is a neurodegenerative disease caused by a genetic mutation in the huntingtin (HTT) gene, changing the normal protein it expresses in the body to a toxic form that damages cortical and basal ganglia neurons. It affects between 0.5 to 1 in 10,000 people worldwide, with higher prevalence in the United States, Europe, and Australia.
HD is inherited in an autosomal dominant pattern; a child of a parent with the mutation has a 50% chance of developing the disease. Patients develop cognitive symptoms that progress to dementia, along with the debilitating involuntary, dancelike movements that gave the disease the name by which it was formerly known: Huntington’s chorea.
In the 1980s and 1990s, several generations of Latin American HD families provided data that allowed for some of the greatest research advances in the disease – and they may represent a large share of the world’s HD cases. Yet, they continue to live in extreme poverty and have benefited little from the findings of the past 3 decades.
Without recognizing this and working to improve the families’ well-being, the researchers at the conference said it’s unlikely that promising therapies in the pipeline will ever reach the populations that need them the most.
Discovery in Venezuela
Some 8 hours by car from Barranquilla sits Lake Maracaibo, Venezuela, home to the largest known clusters of HD patients worldwide. The disease is believed to have come to the shores of Lake Maracaibo with a lone European immigrant – a Spanish sailor, many claim – at the end of the 18th century. Cases were first described in the 1950s by a young Venezuelan physician named Américo Negrette, MD.
Dr. Negrette’s findings were ignored by health officials in Venezuela and went unnoticed in the international research community until 1972, when a student of Dr. Negrette’s presented at an HD conference in Ohio. There he drew the attention of the American neuropsychologist Nancy S. Wexler, PhD. Dr. Wexler’s own mother had died of HD, and her father Milton, a noted psychoanalyst, founded the first research foundation dedicated to the disease.
While prevalence of HD in North America, Australia, and Europe is about 1 in 10,000, the region around Lake Maracaibo saw 70 times that rate at the time, thanks to high birth rates, geographic isolation, and extensive intermarriage within a handful of families. The families comprised mostly poor fishermen who lived in makeshift homes in towns ringing the lake.
In 1979, Dr. Wexler, with funding from the U.S. National Institutes of Health, began making annual research visits to Lake Maracaibo, and in 1983, the research group she coordinated, using data from blood and tissue samples donated by affected families, identified the location of the huntingtin gene on chromosome 4 (Nature. 1983 Nov 17;306[5940]:234-8). A decade later, the researchers isolated the mutant version of the gene and found it to be a triplet (CAG) expansion mutation, with more CAG repetitions associated with earlier age at disease onset (Cell. 1993 Mar 26;72[6]:971-83). Dr. Wexler and her colleagues’ findings led to the first genetic tests for HD.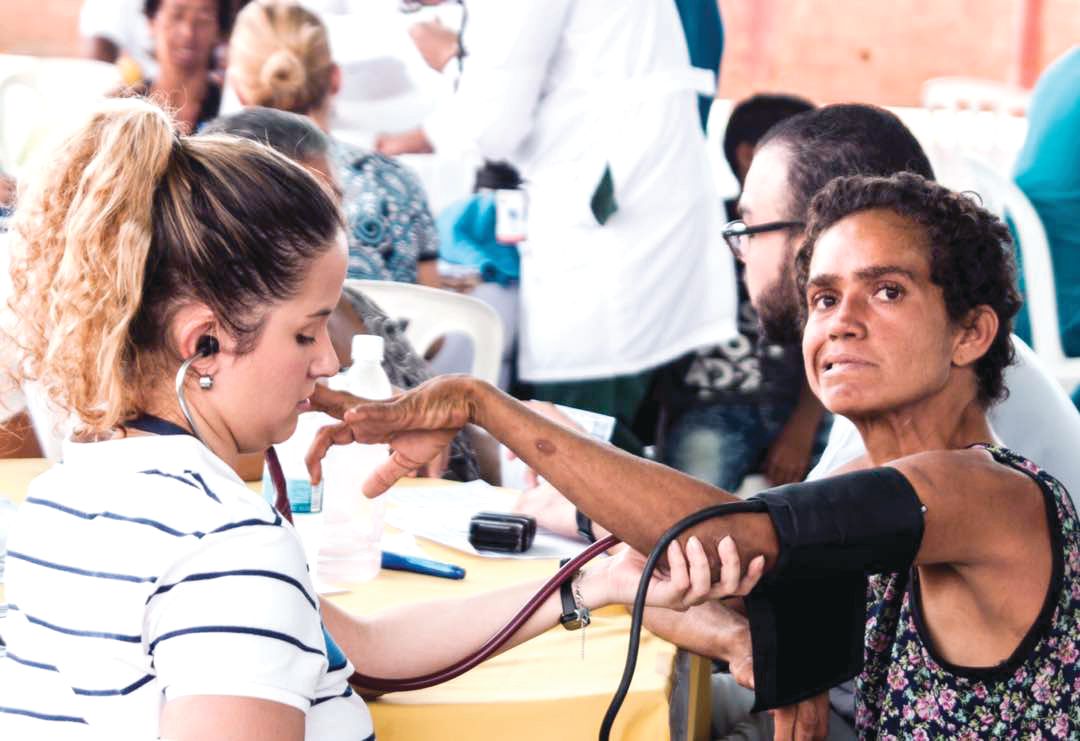
Nationalist policies in Venezuela ended Dr. Wexler and her colleagues’ annual visits to Lake Maracaibo in 2002, along with the food, clothing, and medicines that the group routinely distributed to the families when they came.
Over 23 years, the researchers obtained data from some 18,000 individuals, but the families did not benefit in any durable way from the research. Local investigators with whom Dr. Wexler’s group collaborated lacked the resources and training to continue independently.
Access to medications is limited in Venezuela, and there is no institutional support for hundreds of HD patients living in extreme poverty, many of them descendants of the patients who contributed to the research and generation of these samples. The families’ biological material was sent to labs abroad, where investigators continue to derive findings from it today. Though genetic testing was performed on thousands at risk for the disease, few received access to their results through genetic counseling. A hospice established by Dr. Wexler’s foundation limped along until 2014, when it was finally shuttered.
Rebuilding bridges
A handful of families from the Lake Maracaibo towns attended the conference in Barranquilla. Their travel costs were picked up by Factor-H, which sponsored the event.
Ignacio Muñoz-Sanjuán, PhD, Factor-H’s founder and president, knew the families personally. He’s visited them regularly for years. In 2017, Dr. Muñoz-Sanjuán, a molecular biologist known affectionately in the HD community as “Nacho,” invited several to Rome for a meeting with Pope Francis, as part of an effort to raise awareness of HD and to request support from the Catholic Church for the Latin American families.
Humanitarian work is relatively new to Dr. Muñoz-Sanjuán, who’s spent his career in drug development. In addition to his unpaid work with Factor-H, he is vice president of biology with the CHDI Foundation, a Los Angeles–based nonprofit that funds drug research in HD. CHDI is reported to have about $100 million in annual funding – about triple the NIH budget in recent years for HD research. Its major donors are a group of investors who for years have remained anonymous and do not publicly discuss their philanthropy.
The Spanish-born Dr. Muñoz-Sanjuán had little direct experience with HD populations in Latin America until a few years ago, he said.
At a CHDI meeting in Brazil, he said, “I was talking with physicians and patient advocates from Latin America, telling them they had to be willing to be involved, that these communities with high prevalence had a lot to offer science,” Dr. Muñoz-Sanjuán said in an interview. “I was told that it was me who needed to understand the conditions in which HD patients lived. It completely put me on the spot.”
HD tends to strike during the most productive years of a person’s life, from the late 30s onward, keeping them from working and obliging family members to stop working to care for them. In a poor community, it can condemn a family to a state of extreme poverty for generations. Tetrabenazine (Xenazine), a medication to quiet chorea symptoms, is costly enough that many patients must do without it. Ensuring adequate calorie intake is difficult in HD patients, whose constant movements cause them to lose weight.
Dr. Muñoz-Sanjuán traveled to Colombia, Venezuela, and Brazil, meeting HD families and doctors like neurologist Gustavo Barrios, MD, of Hospital Occidente de Kennedy in Bogotá, Colombia. In a talk at the Barranquilla conference, Dr. Barrios related the experience of his first visit to El Dificil, a community in northern Colombia where some large HD families are forced to survive on the equivalent of $5 a day. “I had to confront not only the fact that these families were living with a terrible disease but in conditions of extreme deprivation,” he said. “My life as a doctor changed that day.”
Dr. Muñoz-Sanjuán helped form a Latin American HD network to involve clinicians like Dr. Barrios who worked with HD clusters, most of them poorly studied. “These are all neglected communities that share similar features,” Dr. Muñoz-Sanjuán said.
On Colombia’s Caribbean coast, for example, HD had been documented since the early 1990s, but genotyping was not performed until recently. Prevalence data are “virtually nonexistent” in Colombia, said Sonia Moreno, PhD, a neuropsychologist at the University of Antioquia in Medellin. In a pilot study presented this year at the CHDI Foundation’s Enroll-HD Congress, Dr. Moreno and her colleagues mined Colombian public health data for likely HD cases, and argued for the creation of a national registry.
In 2012, Dr. Muñoz-Sanjuán founded Factor-H with the aim of improving living conditions for Latin American communities with HD.
Factor-H does not receive funds from CHDI Foundation and instead relies on donations from individuals and companies; its annual budget is less than $200,000. But through contracts with local nongovernmental organizations, it has sponsored health clinics and ongoing food assistance, delivered shipments of medicines and clothing, and started a sponsorship program for young people in HD families, whose studies often are interrupted caring for sick parents. It hopes to build permanent support centers in Colombia and Venezuela where HD families can get their food and medical needs met.
“The traditional thinking in the HD research community is that we’re helping people by doing the legwork to make medicine – and that’s not necessarily enough. You need a more holistic approach,” Dr. Muñoz-Sanjuán said.
Lennie Pineda, MSc, who recently retired as a geneticist with the University of Zulia in Maracaibo, Venezuela, said that Dr. Muñoz-Sanjuán was viewed skeptically when he first visited, in part because of his biomedical research background.
Ms. Pineda, who worked with the region’s HD families her whole career, has been wary of past research efforts in Venezuela. In 2010, she published a paper critical of Dr. Wexler’s and his colleagues’ approach (Revista Redbioética/UNESCO. 2010;1[2]:50-61), particularly regarding issues of informed consent.
“I was very cold to Nacho,” she laughed. “We all looked at him suspiciously.”
Ms. Pineda said Dr. Muñoz-Sanjuán won her over with his interest and creativity in finding concrete ways improve the lives of families in the Lake Maracaibo towns.
In a talk at the conference, Edison Soto, a young man from San Luis, a town on Lake Maracaibo that is a key cluster of HD, said Dr. Muñoz-Sanjuán’s visits had reawakened hope among the families there. “For years, no one thought about us, and because of the situation in the country it’s been hard, really hard,” he said.
“Nacho’s smart,” Ms. Pineda said. “He’s not coming to build a research cohort, he’s coming with genuine intention to help. But if one day conditions are adequate to support investigation, and the people here are well informed and volunteer for a study with full consent, well, all the better,” she said.
Dr. Muñoz-Sanjuán acknowledged that his humanitarian work could be perceived as preparing the ground for future clinical trials.
“I’m not doing anything research oriented with Latin America,” he said. “I would never approach these communities and recommend they take part in a study or give samples, unless their conditions change significantly. But the idea of cross-contamination is a problem I might need to fix. There may come a day where I need to depersonalize Factor-H from me.”
A research platform, a novel agent
Though HD research in Latin America remains rife with challenges, a number of investigators at the conference talked optimistically about planned and ongoing HD studies in Latin America.
The biggest of these is ENROLL-HD, a long-term global observational study of families with HD that uses a standardized approach to data collection. The platform, launched in 2013, aims to enroll 20,000 participants for yearly (or more frequent) assessment. Data from ENROLL-HD will support a diverse range of studies on everything from biomarkers to genetic modifiers to quality of life measures in HD.
ENROLL-HD has opened study sites in Argentina, Chile, and Colombia, and plans to launch a site near Lima, Peru, that is home to an HD cluster. Venezuela is considered out of reach, at least for now.
In Barranquilla, Claudia Perandones, MD, PhD, a genetics researcher in Argentina who manages ENROLL-HD for Latin America and is a cofounder of Factor-H, explained why the kind of clusters seen in Latin America are so valuable scientifically.
The extended family groups share a disease haplotype, eat the same foods, and live in similar environments, Dr. Perandones noted. Because not all the variation in HD can be explained by the number of CAG repeats a patient has, having a large sample with a common haplotype would help researchers pinpoint other environmental and genetic factors that can modify the onset or progress of the disease.
Another key goal of ENROLL-HD, investigators say, is to speed recruitment into clinical trials as they arise. And for the first time in history, potentially game-changing therapies are being developed specifically for HD.
For the past 5 years the Swiss pharmaceutical giant Roche has worked with a smaller biotech firm, Ionis Pharmaceuticals, on an agent called RG6042, which was known until recently as IONIS-HTTRx. CHDI was extensively involved in the agent’s preclinical development, contributing some $10 million to get it off the ground.
RG6042 is an antisense oligonucleotide, delivered by spinal injection, which works by interrupting an mRNA signaling pathway to suppress production of mutant HTT (mHTT) protein in the brain. Antisense oligonucleotides, sometimes called gene silencing therapies, are a new and promising approach in neurodegenerative diseases. Two have received FDA approval to treat spinal muscular atrophy and Duchenne muscular dystrophy.
In April 2018, Roche announced positive results from phase 1/2a study in 46 HD patients in Europe and North America. Patients in that 13-week study saw significant (up to 60%) dose-dependent reductions of the mHTT in their cerebrospinal fluid; a post hoc analysis also found some evidence of functional improvement (Neurology. 2018;90[15 Supplement]:CT.002).
These encouraging findings led to Roche’s announcement of a global phase 3 randomized, controlled trial that is scheduled to begin enrolling in 2019. Roche hopes to randomize 660 patients with mild HD across 15 countries for the 2-year trial, called GENERATION-HD1.
Sites in Latin America are expected to include Argentina, Chile, and Colombia.
At the Barranquilla meeting, Daniel Ciriano, MD, Roche’s Argentina-based medical director for Latin America, extolled the company’s commitment to ethics and social welfare in the region. In recent years, Roche has increased its humanitarian commitments across Latin America, including helping rebuild a Chilean village after an earthquake and offering free breast cancer and kidney disease treatments.
RG6042 is only one of a number of promising approaches to HD. Other therapies in the pipeline include gene silencing delivered by viral vectors instead of repeated spinal injections, an oral drug that interrupts mHTT production, immunotherapies, and even CRISPR gene–editing techniques.
Little was said at the conference, however, about how Latin American HD communities might be able to afford RG6042 or any other therapy that emerges from the pipeline.
Dr. Muñoz-Sanjuán called the issue “a theme for future discussion.”
“This is an area that has to be handled carefully and not one we are heavily invested in yet, although it’s very important,” he said.
On the ground
Several of the European and North American scientists who presented in Barranquilla took pains to express their concern with the well-being of HD patients in Latin America and to demonstrate goodwill toward the local researchers and clinicians.
Hilal A. Lashuel, PhD, a molecular biologist working on the structure and behavior of the HTT protein, said his participation in the Factor-H event at the Vatican the year before had awakened him to “the real human part of HD,” and changed the way he does science.
Normally, Dr. Lashuel said, “we do research disconnected from the realities of the diseases we work with.”
“We need to not just to do research but [to ensure] that research is done right,” he said, which means also focusing on improving patients’ standard of living.
The room broke out in applause when Dr. Lashuel announced new internships for investigators from developing countries. He also presented a parting video from his research team at the École Polytechnique Fédérale de Lausanne (Switzerland), complete with music and affectionate messages in Spanish.
Pharmacologist Elena Cattaneo, PhD, a stem cell researcher long active in the HD community, and also a senator in Italy’s parliament, delivered a similarly warm, carefully choreographed video message from her laboratory at the University of Milan.
Just days later in the town of Juan de Acosta, an hour inland of Barranquilla, the same researchers sat down with patients and families who crowded the waiting room of the town’s only hospital, as the sun beat in through the windows and as mule carts, stray dogs, and buses passed by on the main drag outside.
The event had been titled a “brigade” after all, but the HD families did not seem to mind – and indeed so many showed up that a sign had to be placed on the door saying that no one who arrived after noon could be seen. Consults were not limited to HD-related matters, so families could be seen for any complaint.
HD was first documented in this town in the early 1990s, but much remains to be understood about the size of the cluster, the haplotype, and its relation to other clusters in Colombia or Venezuela. The families here share a handful of last names and likely share a common ancestor. In the early 19th century, the Barranquilla region was flooded with European migrants who reached the city by ship. (HD clusters in Latin America tend to be concentrated in coastal regions, possibly because of migration patterns.)
The waiting room of the hospital was loud with chatter. Small children played as their relatives waited for consults. Some showed the characteristic restless movements and emaciated bodies of people with advanced HD.
The foreign scientists were barred from taking any patient data out of the hospital or asking for samples. Even picture taking was prohibited. Instead they performed genetic counseling and neuropsychological tests; they sorted out differential diagnoses and advised on medications. Visiting Colombian and Venezuelan physicians did the same, while their assistants met with families in the waiting room, taking medical histories and sketching out basic genealogies.
Some of the foreign researchers reported fruitful interactions with patients, while others seemed perplexed by what they’d experienced. Alba di Pardo, PhD, a genetic epidemiologist at the Istituto Neurologico Mediterraneo Pozzilli (Italy), said she’d spent the morning doing genetic counseling with families and going over genealogies to assess risk. Yet, despite the fact that anyone with an HD parent has a 50% chance of developing the disease, some family members acted uninterested, she said.
Dr. di Pardo’s colleague at the Istituto, biologist Vittorio Maglione, PhD, reported having a similar experience. As he was counseling a young woman about her risk for HD, she scrolled indifferently through Facebook posts on her phone, he said.
On some level, Dr. Maglione said, he could understand patients’ reluctance to engage noting that, while there were many potential HD therapies to try, any new treatment paradigm for HD was many years away from a place like this – and potentially very costly. Dr. Maglione – along with Dr. di Pardo – is researching the SP1 axis, a sphingolipid pathway implicated in neurodegenerative such diseases as HD and which has potential as a drug target (Trends Pharmacol Sci. 2018;39[5]:468-80).
Psychologist Pedro Puentes Rozo of Simón Bolivar University, who is working with Dr. Acosta-López on the local cohort study of presymptomatic HD patients, said that, for most of the families in the clinic that day, any seeming indifference probably masked deeper fears. People already were well aware of their risk. “They’ve known about it forever, said Dr. Puentes Rozo, who has been working with this HD population for a decade. “But this is a catastrophic illness and can generate a lot of anxiety.”
Dr. Puentes Rozo said the group’s planned study, unlike studies in the past, would be conducted under strict “international ethical norms and standards.” Subjects would receive ongoing psychological support, and the researchers were working to establish a genetic counseling center so that people who want to know their status “can be prepared,” he said, and plan for their lives and families.
By fall 2018, the cohort study was underway. The group had sponsored several more hospital brigades – or “integrated health days” as they preferred to call them, at the hospital in Juan de Acosta, giving them a chance to work face to face with families.
They drew no blood during the clinics, as investigators in the past had done. Instead, they explained the study to patients, performed the initial screenings, and invited them to designated study appointments at the university. Legal assistance was up and running, and the jobs program would start in 2019.
Enrollment was climbing. And the group was steadily accumulating data.
BARRANQUILLA, COLOMBIA – “We don’t like to call them brigades. That sounds militant,” said neuropsychologist Johan Acosta-López, PhD.
Dr. Acosta-López, the head of cognitive neurosciences at Simón Bolivar University in this city on Colombia’s Atlantic coast, was among five Colombian clinicians – neurologists, psychiatrists, and neuropsychologists – stuffed into a car on their way to a conference hotel in July 2018.

The following day they would be joined by clinicians and researchers from North America, other Latin American countries, and Europe for a first-of-its kind meeting on Huntington’s disease (HD) in the region, sponsored by Factor-H, an HD charity working in Latin America.
Once the talks wrapped up, the researchers – clinicians and basic scientists – were invited to see patients at a hospital in a town an hour inland with a large concentration of HD families, most of them extremely poor. For some, the Factor-H–sponsored “brigade” would be their first hands-on experience with HD patients in a developing country.
There was some debate in the car about what to call such events: brigades, “integrated health days,” or clinics. Around here – where HD abounded but patients were weary of researchers – terminology mattered.
“We’ve had so many investigators arrive in this area – foreigners and Colombians – telling people ‘we’ve got this huge, great project that you’ll benefit from.’ And they take blood samples and never return,” Dr. Acosta-López said.
Even as a local investigator, Dr. Acosta-López has faced challenges getting a new study off the ground. Dr. Acosta-López and his colleagues are working under a grant from the Colombian government to recruit 241 presymptomatic subjects with confirmed genetic markers for HD, and evaluate them for cognitive and neurologic changes preceding disease onset.
It’s a cross-sectional study, and such studies are usually funded for a year. But the investigators knew it would take much more than a year to recruit patients here, and planned their study for 3 years. As of July, the team had been engaging with the community for 6 months but still didn’t have a single blood sample.
“We’ve had to convince everyone that this time is different,” he said, “and that means focusing on the social aspect” – setting up a legal-assistance program through the university to help families claim health benefits and a job-training program sponsored by local businesses.
It’s unusual for researchers to find themselves playing such extensive roles in coordinating social and economic support for their subjects. But with HD, it’s happening across Latin America, where researchers speak frequently of a “debt” owed to HD families in this region.
Huntington’s disease is a neurodegenerative disease caused by a genetic mutation in the huntingtin (HTT) gene, changing the normal protein it expresses in the body to a toxic form that damages cortical and basal ganglia neurons. It affects between 0.5 to 1 in 10,000 people worldwide, with higher prevalence in the United States, Europe, and Australia.
HD is inherited in an autosomal dominant pattern; a child of a parent with the mutation has a 50% chance of developing the disease. Patients develop cognitive symptoms that progress to dementia, along with the debilitating involuntary, dancelike movements that gave the disease the name by which it was formerly known: Huntington’s chorea.
In the 1980s and 1990s, several generations of Latin American HD families provided data that allowed for some of the greatest research advances in the disease – and they may represent a large share of the world’s HD cases. Yet, they continue to live in extreme poverty and have benefited little from the findings of the past 3 decades.
Without recognizing this and working to improve the families’ well-being, the researchers at the conference said it’s unlikely that promising therapies in the pipeline will ever reach the populations that need them the most.
Discovery in Venezuela
Some 8 hours by car from Barranquilla sits Lake Maracaibo, Venezuela, home to the largest known clusters of HD patients worldwide. The disease is believed to have come to the shores of Lake Maracaibo with a lone European immigrant – a Spanish sailor, many claim – at the end of the 18th century. Cases were first described in the 1950s by a young Venezuelan physician named Américo Negrette, MD.
Dr. Negrette’s findings were ignored by health officials in Venezuela and went unnoticed in the international research community until 1972, when a student of Dr. Negrette’s presented at an HD conference in Ohio. There he drew the attention of the American neuropsychologist Nancy S. Wexler, PhD. Dr. Wexler’s own mother had died of HD, and her father Milton, a noted psychoanalyst, founded the first research foundation dedicated to the disease.
While prevalence of HD in North America, Australia, and Europe is about 1 in 10,000, the region around Lake Maracaibo saw 70 times that rate at the time, thanks to high birth rates, geographic isolation, and extensive intermarriage within a handful of families. The families comprised mostly poor fishermen who lived in makeshift homes in towns ringing the lake.
In 1979, Dr. Wexler, with funding from the U.S. National Institutes of Health, began making annual research visits to Lake Maracaibo, and in 1983, the research group she coordinated, using data from blood and tissue samples donated by affected families, identified the location of the huntingtin gene on chromosome 4 (Nature. 1983 Nov 17;306[5940]:234-8). A decade later, the researchers isolated the mutant version of the gene and found it to be a triplet (CAG) expansion mutation, with more CAG repetitions associated with earlier age at disease onset (Cell. 1993 Mar 26;72[6]:971-83). Dr. Wexler and her colleagues’ findings led to the first genetic tests for HD.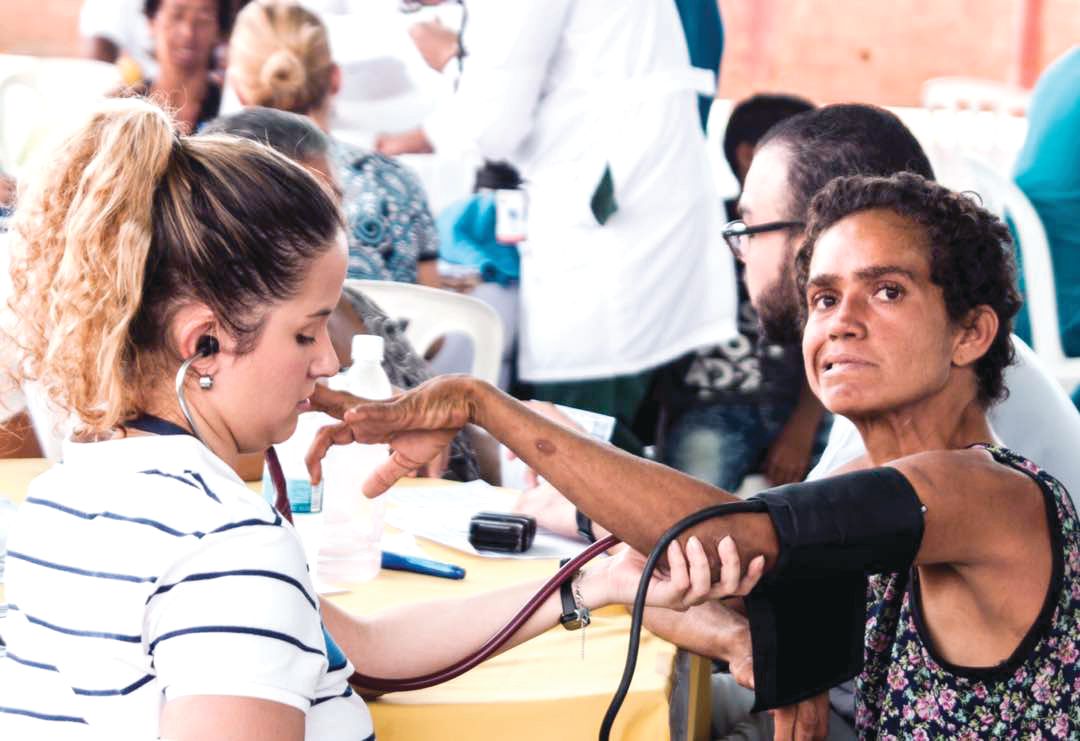
Nationalist policies in Venezuela ended Dr. Wexler and her colleagues’ annual visits to Lake Maracaibo in 2002, along with the food, clothing, and medicines that the group routinely distributed to the families when they came.
Over 23 years, the researchers obtained data from some 18,000 individuals, but the families did not benefit in any durable way from the research. Local investigators with whom Dr. Wexler’s group collaborated lacked the resources and training to continue independently.
Access to medications is limited in Venezuela, and there is no institutional support for hundreds of HD patients living in extreme poverty, many of them descendants of the patients who contributed to the research and generation of these samples. The families’ biological material was sent to labs abroad, where investigators continue to derive findings from it today. Though genetic testing was performed on thousands at risk for the disease, few received access to their results through genetic counseling. A hospice established by Dr. Wexler’s foundation limped along until 2014, when it was finally shuttered.
Rebuilding bridges
A handful of families from the Lake Maracaibo towns attended the conference in Barranquilla. Their travel costs were picked up by Factor-H, which sponsored the event.
Ignacio Muñoz-Sanjuán, PhD, Factor-H’s founder and president, knew the families personally. He’s visited them regularly for years. In 2017, Dr. Muñoz-Sanjuán, a molecular biologist known affectionately in the HD community as “Nacho,” invited several to Rome for a meeting with Pope Francis, as part of an effort to raise awareness of HD and to request support from the Catholic Church for the Latin American families.
Humanitarian work is relatively new to Dr. Muñoz-Sanjuán, who’s spent his career in drug development. In addition to his unpaid work with Factor-H, he is vice president of biology with the CHDI Foundation, a Los Angeles–based nonprofit that funds drug research in HD. CHDI is reported to have about $100 million in annual funding – about triple the NIH budget in recent years for HD research. Its major donors are a group of investors who for years have remained anonymous and do not publicly discuss their philanthropy.
The Spanish-born Dr. Muñoz-Sanjuán had little direct experience with HD populations in Latin America until a few years ago, he said.
At a CHDI meeting in Brazil, he said, “I was talking with physicians and patient advocates from Latin America, telling them they had to be willing to be involved, that these communities with high prevalence had a lot to offer science,” Dr. Muñoz-Sanjuán said in an interview. “I was told that it was me who needed to understand the conditions in which HD patients lived. It completely put me on the spot.”
HD tends to strike during the most productive years of a person’s life, from the late 30s onward, keeping them from working and obliging family members to stop working to care for them. In a poor community, it can condemn a family to a state of extreme poverty for generations. Tetrabenazine (Xenazine), a medication to quiet chorea symptoms, is costly enough that many patients must do without it. Ensuring adequate calorie intake is difficult in HD patients, whose constant movements cause them to lose weight.
Dr. Muñoz-Sanjuán traveled to Colombia, Venezuela, and Brazil, meeting HD families and doctors like neurologist Gustavo Barrios, MD, of Hospital Occidente de Kennedy in Bogotá, Colombia. In a talk at the Barranquilla conference, Dr. Barrios related the experience of his first visit to El Dificil, a community in northern Colombia where some large HD families are forced to survive on the equivalent of $5 a day. “I had to confront not only the fact that these families were living with a terrible disease but in conditions of extreme deprivation,” he said. “My life as a doctor changed that day.”
Dr. Muñoz-Sanjuán helped form a Latin American HD network to involve clinicians like Dr. Barrios who worked with HD clusters, most of them poorly studied. “These are all neglected communities that share similar features,” Dr. Muñoz-Sanjuán said.
On Colombia’s Caribbean coast, for example, HD had been documented since the early 1990s, but genotyping was not performed until recently. Prevalence data are “virtually nonexistent” in Colombia, said Sonia Moreno, PhD, a neuropsychologist at the University of Antioquia in Medellin. In a pilot study presented this year at the CHDI Foundation’s Enroll-HD Congress, Dr. Moreno and her colleagues mined Colombian public health data for likely HD cases, and argued for the creation of a national registry.
In 2012, Dr. Muñoz-Sanjuán founded Factor-H with the aim of improving living conditions for Latin American communities with HD.
Factor-H does not receive funds from CHDI Foundation and instead relies on donations from individuals and companies; its annual budget is less than $200,000. But through contracts with local nongovernmental organizations, it has sponsored health clinics and ongoing food assistance, delivered shipments of medicines and clothing, and started a sponsorship program for young people in HD families, whose studies often are interrupted caring for sick parents. It hopes to build permanent support centers in Colombia and Venezuela where HD families can get their food and medical needs met.
“The traditional thinking in the HD research community is that we’re helping people by doing the legwork to make medicine – and that’s not necessarily enough. You need a more holistic approach,” Dr. Muñoz-Sanjuán said.
Lennie Pineda, MSc, who recently retired as a geneticist with the University of Zulia in Maracaibo, Venezuela, said that Dr. Muñoz-Sanjuán was viewed skeptically when he first visited, in part because of his biomedical research background.
Ms. Pineda, who worked with the region’s HD families her whole career, has been wary of past research efforts in Venezuela. In 2010, she published a paper critical of Dr. Wexler’s and his colleagues’ approach (Revista Redbioética/UNESCO. 2010;1[2]:50-61), particularly regarding issues of informed consent.
“I was very cold to Nacho,” she laughed. “We all looked at him suspiciously.”
Ms. Pineda said Dr. Muñoz-Sanjuán won her over with his interest and creativity in finding concrete ways improve the lives of families in the Lake Maracaibo towns.
In a talk at the conference, Edison Soto, a young man from San Luis, a town on Lake Maracaibo that is a key cluster of HD, said Dr. Muñoz-Sanjuán’s visits had reawakened hope among the families there. “For years, no one thought about us, and because of the situation in the country it’s been hard, really hard,” he said.
“Nacho’s smart,” Ms. Pineda said. “He’s not coming to build a research cohort, he’s coming with genuine intention to help. But if one day conditions are adequate to support investigation, and the people here are well informed and volunteer for a study with full consent, well, all the better,” she said.
Dr. Muñoz-Sanjuán acknowledged that his humanitarian work could be perceived as preparing the ground for future clinical trials.
“I’m not doing anything research oriented with Latin America,” he said. “I would never approach these communities and recommend they take part in a study or give samples, unless their conditions change significantly. But the idea of cross-contamination is a problem I might need to fix. There may come a day where I need to depersonalize Factor-H from me.”
A research platform, a novel agent
Though HD research in Latin America remains rife with challenges, a number of investigators at the conference talked optimistically about planned and ongoing HD studies in Latin America.
The biggest of these is ENROLL-HD, a long-term global observational study of families with HD that uses a standardized approach to data collection. The platform, launched in 2013, aims to enroll 20,000 participants for yearly (or more frequent) assessment. Data from ENROLL-HD will support a diverse range of studies on everything from biomarkers to genetic modifiers to quality of life measures in HD.
ENROLL-HD has opened study sites in Argentina, Chile, and Colombia, and plans to launch a site near Lima, Peru, that is home to an HD cluster. Venezuela is considered out of reach, at least for now.
In Barranquilla, Claudia Perandones, MD, PhD, a genetics researcher in Argentina who manages ENROLL-HD for Latin America and is a cofounder of Factor-H, explained why the kind of clusters seen in Latin America are so valuable scientifically.
The extended family groups share a disease haplotype, eat the same foods, and live in similar environments, Dr. Perandones noted. Because not all the variation in HD can be explained by the number of CAG repeats a patient has, having a large sample with a common haplotype would help researchers pinpoint other environmental and genetic factors that can modify the onset or progress of the disease.
Another key goal of ENROLL-HD, investigators say, is to speed recruitment into clinical trials as they arise. And for the first time in history, potentially game-changing therapies are being developed specifically for HD.
For the past 5 years the Swiss pharmaceutical giant Roche has worked with a smaller biotech firm, Ionis Pharmaceuticals, on an agent called RG6042, which was known until recently as IONIS-HTTRx. CHDI was extensively involved in the agent’s preclinical development, contributing some $10 million to get it off the ground.
RG6042 is an antisense oligonucleotide, delivered by spinal injection, which works by interrupting an mRNA signaling pathway to suppress production of mutant HTT (mHTT) protein in the brain. Antisense oligonucleotides, sometimes called gene silencing therapies, are a new and promising approach in neurodegenerative diseases. Two have received FDA approval to treat spinal muscular atrophy and Duchenne muscular dystrophy.
In April 2018, Roche announced positive results from phase 1/2a study in 46 HD patients in Europe and North America. Patients in that 13-week study saw significant (up to 60%) dose-dependent reductions of the mHTT in their cerebrospinal fluid; a post hoc analysis also found some evidence of functional improvement (Neurology. 2018;90[15 Supplement]:CT.002).
These encouraging findings led to Roche’s announcement of a global phase 3 randomized, controlled trial that is scheduled to begin enrolling in 2019. Roche hopes to randomize 660 patients with mild HD across 15 countries for the 2-year trial, called GENERATION-HD1.
Sites in Latin America are expected to include Argentina, Chile, and Colombia.
At the Barranquilla meeting, Daniel Ciriano, MD, Roche’s Argentina-based medical director for Latin America, extolled the company’s commitment to ethics and social welfare in the region. In recent years, Roche has increased its humanitarian commitments across Latin America, including helping rebuild a Chilean village after an earthquake and offering free breast cancer and kidney disease treatments.
RG6042 is only one of a number of promising approaches to HD. Other therapies in the pipeline include gene silencing delivered by viral vectors instead of repeated spinal injections, an oral drug that interrupts mHTT production, immunotherapies, and even CRISPR gene–editing techniques.
Little was said at the conference, however, about how Latin American HD communities might be able to afford RG6042 or any other therapy that emerges from the pipeline.
Dr. Muñoz-Sanjuán called the issue “a theme for future discussion.”
“This is an area that has to be handled carefully and not one we are heavily invested in yet, although it’s very important,” he said.
On the ground
Several of the European and North American scientists who presented in Barranquilla took pains to express their concern with the well-being of HD patients in Latin America and to demonstrate goodwill toward the local researchers and clinicians.
Hilal A. Lashuel, PhD, a molecular biologist working on the structure and behavior of the HTT protein, said his participation in the Factor-H event at the Vatican the year before had awakened him to “the real human part of HD,” and changed the way he does science.
Normally, Dr. Lashuel said, “we do research disconnected from the realities of the diseases we work with.”
“We need to not just to do research but [to ensure] that research is done right,” he said, which means also focusing on improving patients’ standard of living.
The room broke out in applause when Dr. Lashuel announced new internships for investigators from developing countries. He also presented a parting video from his research team at the École Polytechnique Fédérale de Lausanne (Switzerland), complete with music and affectionate messages in Spanish.
Pharmacologist Elena Cattaneo, PhD, a stem cell researcher long active in the HD community, and also a senator in Italy’s parliament, delivered a similarly warm, carefully choreographed video message from her laboratory at the University of Milan.
Just days later in the town of Juan de Acosta, an hour inland of Barranquilla, the same researchers sat down with patients and families who crowded the waiting room of the town’s only hospital, as the sun beat in through the windows and as mule carts, stray dogs, and buses passed by on the main drag outside.
The event had been titled a “brigade” after all, but the HD families did not seem to mind – and indeed so many showed up that a sign had to be placed on the door saying that no one who arrived after noon could be seen. Consults were not limited to HD-related matters, so families could be seen for any complaint.
HD was first documented in this town in the early 1990s, but much remains to be understood about the size of the cluster, the haplotype, and its relation to other clusters in Colombia or Venezuela. The families here share a handful of last names and likely share a common ancestor. In the early 19th century, the Barranquilla region was flooded with European migrants who reached the city by ship. (HD clusters in Latin America tend to be concentrated in coastal regions, possibly because of migration patterns.)
The waiting room of the hospital was loud with chatter. Small children played as their relatives waited for consults. Some showed the characteristic restless movements and emaciated bodies of people with advanced HD.
The foreign scientists were barred from taking any patient data out of the hospital or asking for samples. Even picture taking was prohibited. Instead they performed genetic counseling and neuropsychological tests; they sorted out differential diagnoses and advised on medications. Visiting Colombian and Venezuelan physicians did the same, while their assistants met with families in the waiting room, taking medical histories and sketching out basic genealogies.
Some of the foreign researchers reported fruitful interactions with patients, while others seemed perplexed by what they’d experienced. Alba di Pardo, PhD, a genetic epidemiologist at the Istituto Neurologico Mediterraneo Pozzilli (Italy), said she’d spent the morning doing genetic counseling with families and going over genealogies to assess risk. Yet, despite the fact that anyone with an HD parent has a 50% chance of developing the disease, some family members acted uninterested, she said.
Dr. di Pardo’s colleague at the Istituto, biologist Vittorio Maglione, PhD, reported having a similar experience. As he was counseling a young woman about her risk for HD, she scrolled indifferently through Facebook posts on her phone, he said.
On some level, Dr. Maglione said, he could understand patients’ reluctance to engage noting that, while there were many potential HD therapies to try, any new treatment paradigm for HD was many years away from a place like this – and potentially very costly. Dr. Maglione – along with Dr. di Pardo – is researching the SP1 axis, a sphingolipid pathway implicated in neurodegenerative such diseases as HD and which has potential as a drug target (Trends Pharmacol Sci. 2018;39[5]:468-80).
Psychologist Pedro Puentes Rozo of Simón Bolivar University, who is working with Dr. Acosta-López on the local cohort study of presymptomatic HD patients, said that, for most of the families in the clinic that day, any seeming indifference probably masked deeper fears. People already were well aware of their risk. “They’ve known about it forever, said Dr. Puentes Rozo, who has been working with this HD population for a decade. “But this is a catastrophic illness and can generate a lot of anxiety.”
Dr. Puentes Rozo said the group’s planned study, unlike studies in the past, would be conducted under strict “international ethical norms and standards.” Subjects would receive ongoing psychological support, and the researchers were working to establish a genetic counseling center so that people who want to know their status “can be prepared,” he said, and plan for their lives and families.
By fall 2018, the cohort study was underway. The group had sponsored several more hospital brigades – or “integrated health days” as they preferred to call them, at the hospital in Juan de Acosta, giving them a chance to work face to face with families.
They drew no blood during the clinics, as investigators in the past had done. Instead, they explained the study to patients, performed the initial screenings, and invited them to designated study appointments at the university. Legal assistance was up and running, and the jobs program would start in 2019.
Enrollment was climbing. And the group was steadily accumulating data.
BARRANQUILLA, COLOMBIA – “We don’t like to call them brigades. That sounds militant,” said neuropsychologist Johan Acosta-López, PhD.
Dr. Acosta-López, the head of cognitive neurosciences at Simón Bolivar University in this city on Colombia’s Atlantic coast, was among five Colombian clinicians – neurologists, psychiatrists, and neuropsychologists – stuffed into a car on their way to a conference hotel in July 2018.

The following day they would be joined by clinicians and researchers from North America, other Latin American countries, and Europe for a first-of-its kind meeting on Huntington’s disease (HD) in the region, sponsored by Factor-H, an HD charity working in Latin America.
Once the talks wrapped up, the researchers – clinicians and basic scientists – were invited to see patients at a hospital in a town an hour inland with a large concentration of HD families, most of them extremely poor. For some, the Factor-H–sponsored “brigade” would be their first hands-on experience with HD patients in a developing country.
There was some debate in the car about what to call such events: brigades, “integrated health days,” or clinics. Around here – where HD abounded but patients were weary of researchers – terminology mattered.
“We’ve had so many investigators arrive in this area – foreigners and Colombians – telling people ‘we’ve got this huge, great project that you’ll benefit from.’ And they take blood samples and never return,” Dr. Acosta-López said.
Even as a local investigator, Dr. Acosta-López has faced challenges getting a new study off the ground. Dr. Acosta-López and his colleagues are working under a grant from the Colombian government to recruit 241 presymptomatic subjects with confirmed genetic markers for HD, and evaluate them for cognitive and neurologic changes preceding disease onset.
It’s a cross-sectional study, and such studies are usually funded for a year. But the investigators knew it would take much more than a year to recruit patients here, and planned their study for 3 years. As of July, the team had been engaging with the community for 6 months but still didn’t have a single blood sample.
“We’ve had to convince everyone that this time is different,” he said, “and that means focusing on the social aspect” – setting up a legal-assistance program through the university to help families claim health benefits and a job-training program sponsored by local businesses.
It’s unusual for researchers to find themselves playing such extensive roles in coordinating social and economic support for their subjects. But with HD, it’s happening across Latin America, where researchers speak frequently of a “debt” owed to HD families in this region.
Huntington’s disease is a neurodegenerative disease caused by a genetic mutation in the huntingtin (HTT) gene, changing the normal protein it expresses in the body to a toxic form that damages cortical and basal ganglia neurons. It affects between 0.5 to 1 in 10,000 people worldwide, with higher prevalence in the United States, Europe, and Australia.
HD is inherited in an autosomal dominant pattern; a child of a parent with the mutation has a 50% chance of developing the disease. Patients develop cognitive symptoms that progress to dementia, along with the debilitating involuntary, dancelike movements that gave the disease the name by which it was formerly known: Huntington’s chorea.
In the 1980s and 1990s, several generations of Latin American HD families provided data that allowed for some of the greatest research advances in the disease – and they may represent a large share of the world’s HD cases. Yet, they continue to live in extreme poverty and have benefited little from the findings of the past 3 decades.
Without recognizing this and working to improve the families’ well-being, the researchers at the conference said it’s unlikely that promising therapies in the pipeline will ever reach the populations that need them the most.
Discovery in Venezuela
Some 8 hours by car from Barranquilla sits Lake Maracaibo, Venezuela, home to the largest known clusters of HD patients worldwide. The disease is believed to have come to the shores of Lake Maracaibo with a lone European immigrant – a Spanish sailor, many claim – at the end of the 18th century. Cases were first described in the 1950s by a young Venezuelan physician named Américo Negrette, MD.
Dr. Negrette’s findings were ignored by health officials in Venezuela and went unnoticed in the international research community until 1972, when a student of Dr. Negrette’s presented at an HD conference in Ohio. There he drew the attention of the American neuropsychologist Nancy S. Wexler, PhD. Dr. Wexler’s own mother had died of HD, and her father Milton, a noted psychoanalyst, founded the first research foundation dedicated to the disease.
While prevalence of HD in North America, Australia, and Europe is about 1 in 10,000, the region around Lake Maracaibo saw 70 times that rate at the time, thanks to high birth rates, geographic isolation, and extensive intermarriage within a handful of families. The families comprised mostly poor fishermen who lived in makeshift homes in towns ringing the lake.
In 1979, Dr. Wexler, with funding from the U.S. National Institutes of Health, began making annual research visits to Lake Maracaibo, and in 1983, the research group she coordinated, using data from blood and tissue samples donated by affected families, identified the location of the huntingtin gene on chromosome 4 (Nature. 1983 Nov 17;306[5940]:234-8). A decade later, the researchers isolated the mutant version of the gene and found it to be a triplet (CAG) expansion mutation, with more CAG repetitions associated with earlier age at disease onset (Cell. 1993 Mar 26;72[6]:971-83). Dr. Wexler and her colleagues’ findings led to the first genetic tests for HD.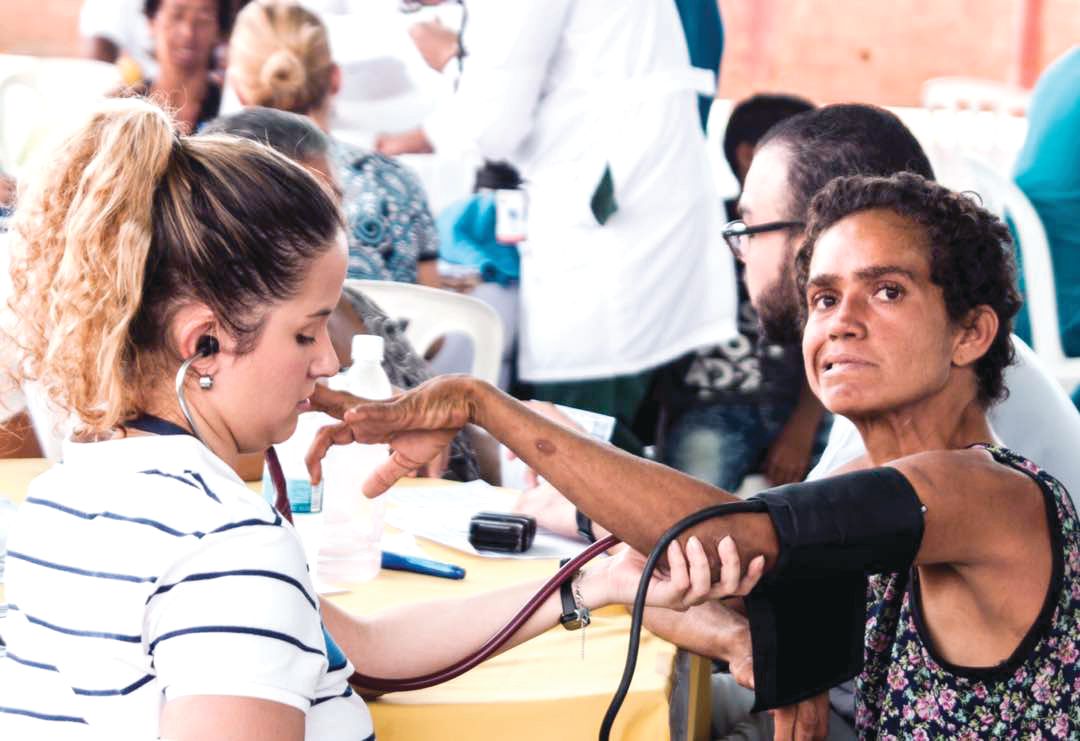
Nationalist policies in Venezuela ended Dr. Wexler and her colleagues’ annual visits to Lake Maracaibo in 2002, along with the food, clothing, and medicines that the group routinely distributed to the families when they came.
Over 23 years, the researchers obtained data from some 18,000 individuals, but the families did not benefit in any durable way from the research. Local investigators with whom Dr. Wexler’s group collaborated lacked the resources and training to continue independently.
Access to medications is limited in Venezuela, and there is no institutional support for hundreds of HD patients living in extreme poverty, many of them descendants of the patients who contributed to the research and generation of these samples. The families’ biological material was sent to labs abroad, where investigators continue to derive findings from it today. Though genetic testing was performed on thousands at risk for the disease, few received access to their results through genetic counseling. A hospice established by Dr. Wexler’s foundation limped along until 2014, when it was finally shuttered.
Rebuilding bridges
A handful of families from the Lake Maracaibo towns attended the conference in Barranquilla. Their travel costs were picked up by Factor-H, which sponsored the event.
Ignacio Muñoz-Sanjuán, PhD, Factor-H’s founder and president, knew the families personally. He’s visited them regularly for years. In 2017, Dr. Muñoz-Sanjuán, a molecular biologist known affectionately in the HD community as “Nacho,” invited several to Rome for a meeting with Pope Francis, as part of an effort to raise awareness of HD and to request support from the Catholic Church for the Latin American families.
Humanitarian work is relatively new to Dr. Muñoz-Sanjuán, who’s spent his career in drug development. In addition to his unpaid work with Factor-H, he is vice president of biology with the CHDI Foundation, a Los Angeles–based nonprofit that funds drug research in HD. CHDI is reported to have about $100 million in annual funding – about triple the NIH budget in recent years for HD research. Its major donors are a group of investors who for years have remained anonymous and do not publicly discuss their philanthropy.
The Spanish-born Dr. Muñoz-Sanjuán had little direct experience with HD populations in Latin America until a few years ago, he said.
At a CHDI meeting in Brazil, he said, “I was talking with physicians and patient advocates from Latin America, telling them they had to be willing to be involved, that these communities with high prevalence had a lot to offer science,” Dr. Muñoz-Sanjuán said in an interview. “I was told that it was me who needed to understand the conditions in which HD patients lived. It completely put me on the spot.”
HD tends to strike during the most productive years of a person’s life, from the late 30s onward, keeping them from working and obliging family members to stop working to care for them. In a poor community, it can condemn a family to a state of extreme poverty for generations. Tetrabenazine (Xenazine), a medication to quiet chorea symptoms, is costly enough that many patients must do without it. Ensuring adequate calorie intake is difficult in HD patients, whose constant movements cause them to lose weight.
Dr. Muñoz-Sanjuán traveled to Colombia, Venezuela, and Brazil, meeting HD families and doctors like neurologist Gustavo Barrios, MD, of Hospital Occidente de Kennedy in Bogotá, Colombia. In a talk at the Barranquilla conference, Dr. Barrios related the experience of his first visit to El Dificil, a community in northern Colombia where some large HD families are forced to survive on the equivalent of $5 a day. “I had to confront not only the fact that these families were living with a terrible disease but in conditions of extreme deprivation,” he said. “My life as a doctor changed that day.”
Dr. Muñoz-Sanjuán helped form a Latin American HD network to involve clinicians like Dr. Barrios who worked with HD clusters, most of them poorly studied. “These are all neglected communities that share similar features,” Dr. Muñoz-Sanjuán said.
On Colombia’s Caribbean coast, for example, HD had been documented since the early 1990s, but genotyping was not performed until recently. Prevalence data are “virtually nonexistent” in Colombia, said Sonia Moreno, PhD, a neuropsychologist at the University of Antioquia in Medellin. In a pilot study presented this year at the CHDI Foundation’s Enroll-HD Congress, Dr. Moreno and her colleagues mined Colombian public health data for likely HD cases, and argued for the creation of a national registry.
In 2012, Dr. Muñoz-Sanjuán founded Factor-H with the aim of improving living conditions for Latin American communities with HD.
Factor-H does not receive funds from CHDI Foundation and instead relies on donations from individuals and companies; its annual budget is less than $200,000. But through contracts with local nongovernmental organizations, it has sponsored health clinics and ongoing food assistance, delivered shipments of medicines and clothing, and started a sponsorship program for young people in HD families, whose studies often are interrupted caring for sick parents. It hopes to build permanent support centers in Colombia and Venezuela where HD families can get their food and medical needs met.
“The traditional thinking in the HD research community is that we’re helping people by doing the legwork to make medicine – and that’s not necessarily enough. You need a more holistic approach,” Dr. Muñoz-Sanjuán said.
Lennie Pineda, MSc, who recently retired as a geneticist with the University of Zulia in Maracaibo, Venezuela, said that Dr. Muñoz-Sanjuán was viewed skeptically when he first visited, in part because of his biomedical research background.
Ms. Pineda, who worked with the region’s HD families her whole career, has been wary of past research efforts in Venezuela. In 2010, she published a paper critical of Dr. Wexler’s and his colleagues’ approach (Revista Redbioética/UNESCO. 2010;1[2]:50-61), particularly regarding issues of informed consent.
“I was very cold to Nacho,” she laughed. “We all looked at him suspiciously.”
Ms. Pineda said Dr. Muñoz-Sanjuán won her over with his interest and creativity in finding concrete ways improve the lives of families in the Lake Maracaibo towns.
In a talk at the conference, Edison Soto, a young man from San Luis, a town on Lake Maracaibo that is a key cluster of HD, said Dr. Muñoz-Sanjuán’s visits had reawakened hope among the families there. “For years, no one thought about us, and because of the situation in the country it’s been hard, really hard,” he said.
“Nacho’s smart,” Ms. Pineda said. “He’s not coming to build a research cohort, he’s coming with genuine intention to help. But if one day conditions are adequate to support investigation, and the people here are well informed and volunteer for a study with full consent, well, all the better,” she said.
Dr. Muñoz-Sanjuán acknowledged that his humanitarian work could be perceived as preparing the ground for future clinical trials.
“I’m not doing anything research oriented with Latin America,” he said. “I would never approach these communities and recommend they take part in a study or give samples, unless their conditions change significantly. But the idea of cross-contamination is a problem I might need to fix. There may come a day where I need to depersonalize Factor-H from me.”
A research platform, a novel agent
Though HD research in Latin America remains rife with challenges, a number of investigators at the conference talked optimistically about planned and ongoing HD studies in Latin America.
The biggest of these is ENROLL-HD, a long-term global observational study of families with HD that uses a standardized approach to data collection. The platform, launched in 2013, aims to enroll 20,000 participants for yearly (or more frequent) assessment. Data from ENROLL-HD will support a diverse range of studies on everything from biomarkers to genetic modifiers to quality of life measures in HD.
ENROLL-HD has opened study sites in Argentina, Chile, and Colombia, and plans to launch a site near Lima, Peru, that is home to an HD cluster. Venezuela is considered out of reach, at least for now.
In Barranquilla, Claudia Perandones, MD, PhD, a genetics researcher in Argentina who manages ENROLL-HD for Latin America and is a cofounder of Factor-H, explained why the kind of clusters seen in Latin America are so valuable scientifically.
The extended family groups share a disease haplotype, eat the same foods, and live in similar environments, Dr. Perandones noted. Because not all the variation in HD can be explained by the number of CAG repeats a patient has, having a large sample with a common haplotype would help researchers pinpoint other environmental and genetic factors that can modify the onset or progress of the disease.
Another key goal of ENROLL-HD, investigators say, is to speed recruitment into clinical trials as they arise. And for the first time in history, potentially game-changing therapies are being developed specifically for HD.
For the past 5 years the Swiss pharmaceutical giant Roche has worked with a smaller biotech firm, Ionis Pharmaceuticals, on an agent called RG6042, which was known until recently as IONIS-HTTRx. CHDI was extensively involved in the agent’s preclinical development, contributing some $10 million to get it off the ground.
RG6042 is an antisense oligonucleotide, delivered by spinal injection, which works by interrupting an mRNA signaling pathway to suppress production of mutant HTT (mHTT) protein in the brain. Antisense oligonucleotides, sometimes called gene silencing therapies, are a new and promising approach in neurodegenerative diseases. Two have received FDA approval to treat spinal muscular atrophy and Duchenne muscular dystrophy.
In April 2018, Roche announced positive results from phase 1/2a study in 46 HD patients in Europe and North America. Patients in that 13-week study saw significant (up to 60%) dose-dependent reductions of the mHTT in their cerebrospinal fluid; a post hoc analysis also found some evidence of functional improvement (Neurology. 2018;90[15 Supplement]:CT.002).
These encouraging findings led to Roche’s announcement of a global phase 3 randomized, controlled trial that is scheduled to begin enrolling in 2019. Roche hopes to randomize 660 patients with mild HD across 15 countries for the 2-year trial, called GENERATION-HD1.
Sites in Latin America are expected to include Argentina, Chile, and Colombia.
At the Barranquilla meeting, Daniel Ciriano, MD, Roche’s Argentina-based medical director for Latin America, extolled the company’s commitment to ethics and social welfare in the region. In recent years, Roche has increased its humanitarian commitments across Latin America, including helping rebuild a Chilean village after an earthquake and offering free breast cancer and kidney disease treatments.
RG6042 is only one of a number of promising approaches to HD. Other therapies in the pipeline include gene silencing delivered by viral vectors instead of repeated spinal injections, an oral drug that interrupts mHTT production, immunotherapies, and even CRISPR gene–editing techniques.
Little was said at the conference, however, about how Latin American HD communities might be able to afford RG6042 or any other therapy that emerges from the pipeline.
Dr. Muñoz-Sanjuán called the issue “a theme for future discussion.”
“This is an area that has to be handled carefully and not one we are heavily invested in yet, although it’s very important,” he said.
On the ground
Several of the European and North American scientists who presented in Barranquilla took pains to express their concern with the well-being of HD patients in Latin America and to demonstrate goodwill toward the local researchers and clinicians.
Hilal A. Lashuel, PhD, a molecular biologist working on the structure and behavior of the HTT protein, said his participation in the Factor-H event at the Vatican the year before had awakened him to “the real human part of HD,” and changed the way he does science.
Normally, Dr. Lashuel said, “we do research disconnected from the realities of the diseases we work with.”
“We need to not just to do research but [to ensure] that research is done right,” he said, which means also focusing on improving patients’ standard of living.
The room broke out in applause when Dr. Lashuel announced new internships for investigators from developing countries. He also presented a parting video from his research team at the École Polytechnique Fédérale de Lausanne (Switzerland), complete with music and affectionate messages in Spanish.
Pharmacologist Elena Cattaneo, PhD, a stem cell researcher long active in the HD community, and also a senator in Italy’s parliament, delivered a similarly warm, carefully choreographed video message from her laboratory at the University of Milan.
Just days later in the town of Juan de Acosta, an hour inland of Barranquilla, the same researchers sat down with patients and families who crowded the waiting room of the town’s only hospital, as the sun beat in through the windows and as mule carts, stray dogs, and buses passed by on the main drag outside.
The event had been titled a “brigade” after all, but the HD families did not seem to mind – and indeed so many showed up that a sign had to be placed on the door saying that no one who arrived after noon could be seen. Consults were not limited to HD-related matters, so families could be seen for any complaint.
HD was first documented in this town in the early 1990s, but much remains to be understood about the size of the cluster, the haplotype, and its relation to other clusters in Colombia or Venezuela. The families here share a handful of last names and likely share a common ancestor. In the early 19th century, the Barranquilla region was flooded with European migrants who reached the city by ship. (HD clusters in Latin America tend to be concentrated in coastal regions, possibly because of migration patterns.)
The waiting room of the hospital was loud with chatter. Small children played as their relatives waited for consults. Some showed the characteristic restless movements and emaciated bodies of people with advanced HD.
The foreign scientists were barred from taking any patient data out of the hospital or asking for samples. Even picture taking was prohibited. Instead they performed genetic counseling and neuropsychological tests; they sorted out differential diagnoses and advised on medications. Visiting Colombian and Venezuelan physicians did the same, while their assistants met with families in the waiting room, taking medical histories and sketching out basic genealogies.
Some of the foreign researchers reported fruitful interactions with patients, while others seemed perplexed by what they’d experienced. Alba di Pardo, PhD, a genetic epidemiologist at the Istituto Neurologico Mediterraneo Pozzilli (Italy), said she’d spent the morning doing genetic counseling with families and going over genealogies to assess risk. Yet, despite the fact that anyone with an HD parent has a 50% chance of developing the disease, some family members acted uninterested, she said.
Dr. di Pardo’s colleague at the Istituto, biologist Vittorio Maglione, PhD, reported having a similar experience. As he was counseling a young woman about her risk for HD, she scrolled indifferently through Facebook posts on her phone, he said.
On some level, Dr. Maglione said, he could understand patients’ reluctance to engage noting that, while there were many potential HD therapies to try, any new treatment paradigm for HD was many years away from a place like this – and potentially very costly. Dr. Maglione – along with Dr. di Pardo – is researching the SP1 axis, a sphingolipid pathway implicated in neurodegenerative such diseases as HD and which has potential as a drug target (Trends Pharmacol Sci. 2018;39[5]:468-80).
Psychologist Pedro Puentes Rozo of Simón Bolivar University, who is working with Dr. Acosta-López on the local cohort study of presymptomatic HD patients, said that, for most of the families in the clinic that day, any seeming indifference probably masked deeper fears. People already were well aware of their risk. “They’ve known about it forever, said Dr. Puentes Rozo, who has been working with this HD population for a decade. “But this is a catastrophic illness and can generate a lot of anxiety.”
Dr. Puentes Rozo said the group’s planned study, unlike studies in the past, would be conducted under strict “international ethical norms and standards.” Subjects would receive ongoing psychological support, and the researchers were working to establish a genetic counseling center so that people who want to know their status “can be prepared,” he said, and plan for their lives and families.
By fall 2018, the cohort study was underway. The group had sponsored several more hospital brigades – or “integrated health days” as they preferred to call them, at the hospital in Juan de Acosta, giving them a chance to work face to face with families.
They drew no blood during the clinics, as investigators in the past had done. Instead, they explained the study to patients, performed the initial screenings, and invited them to designated study appointments at the university. Legal assistance was up and running, and the jobs program would start in 2019.
Enrollment was climbing. And the group was steadily accumulating data.
Huntington’s progression tracks with levels of mutant huntingtin, neurofilament light
Concentrations of mutant huntingtin protein and neurofilament light proteins in cerebrospinal fluid and blood may be the first signs of progression in Huntington’s disease, according to a paper published online Sept. 12 in Science Translational Medicine.
In a cohort of 40 Huntington’s mutation carriers with manifest disease, 20 carriers without clinical symptoms, and 20 healthy controls, researchers examined levels of mutant huntingtin (mHTT) and neurofilament light (NfL) protein in biofluids, in parallel with clinical evaluations and MRI imaging.
They found that concentrations of mHTT in the cerebrospinal fluid (CSF) and concentrations of NfL proteins in the CSF and plasma were significantly higher in participants with manifest Huntington’s disease (HD) than in those without manifest disease or in controls.
Researchers also saw that CSF concentrations of mHTT showed the earliest detectable change in progression of the disease, followed by plasma and CSF levels of NfL. After that came changes in caudate and global brain volume, motor score, word reading, and other clinical measures.
“These results suggest that as our understanding grows further, analysis of mHTT and NfL might be useful for developing HD therapeutics and for clinical management,” wrote Lauren M. Byrne of the Huntington’s Disease Centre at the University College London Institute of Neurology and her coauthors.
Plasma concentrations of NfL showed the strongest association with clinical severity, even after adjusting for the number of CAG (or cytosine, adenine, and guanine) repeats – a measure of disease severity – and age.
“Our previous work suggests that NfL is a dynamic marker of ongoing neuronal damage in HD that predicts subsequent progression,” the authors wrote. “This perhaps reflects that NfL, as a marker of axonal damage, has a more direct relationship with the development of clinical manifestations and brain atrophy.”
NfL concentrations in CSF more closely predicted brain volume than did plasma NfL or CSF concentrations of mHTT.
In participants who carried the Huntington’s mutation, CSF concentrations of mHTT and NfL were strongly correlated. Researchers also noted that mutation carriers had a significantly higher CSF-to-plasma ratio of NfL than did controls.
The study also showed that mHTT in the CSF and NfL in the cerebrospinal fluid and plasma, were very stable within individuals over 4-8 weeks.
“The very high intraclass correlation values of the three markers revealed them to be highly stable, suggesting that intraindividual variation in these analytes is likely to be a minimal source of noise in natural history and therapeutic studies,” the authors wrote.
This work was supported by the Medical Research Council U.K., the CHDI Foundation, the Wellcome Trust, the U.K. Department of Health’s National Institute for Health Research Biomedical Research Centres funding scheme, the U.K. Dementia Research Institute, F. Hoffmann-La Roche, the Horizon 2020 Framework Programme, and the Engineering and Physical Sciences Research Council. A number of authors disclosed consulting or serving on advisory boards for F. Hoffmann-La Roche and/or other companies. Three authors are full-time employees of F. Hoffmann-La Roche.
SOURCE: Byrne L et al. Sci Transl Med. 2018;10:eaat7108. doi: 10.1126/scitranslmed.aat7108.
Concentrations of mutant huntingtin protein and neurofilament light proteins in cerebrospinal fluid and blood may be the first signs of progression in Huntington’s disease, according to a paper published online Sept. 12 in Science Translational Medicine.
In a cohort of 40 Huntington’s mutation carriers with manifest disease, 20 carriers without clinical symptoms, and 20 healthy controls, researchers examined levels of mutant huntingtin (mHTT) and neurofilament light (NfL) protein in biofluids, in parallel with clinical evaluations and MRI imaging.
They found that concentrations of mHTT in the cerebrospinal fluid (CSF) and concentrations of NfL proteins in the CSF and plasma were significantly higher in participants with manifest Huntington’s disease (HD) than in those without manifest disease or in controls.
Researchers also saw that CSF concentrations of mHTT showed the earliest detectable change in progression of the disease, followed by plasma and CSF levels of NfL. After that came changes in caudate and global brain volume, motor score, word reading, and other clinical measures.
“These results suggest that as our understanding grows further, analysis of mHTT and NfL might be useful for developing HD therapeutics and for clinical management,” wrote Lauren M. Byrne of the Huntington’s Disease Centre at the University College London Institute of Neurology and her coauthors.
Plasma concentrations of NfL showed the strongest association with clinical severity, even after adjusting for the number of CAG (or cytosine, adenine, and guanine) repeats – a measure of disease severity – and age.
“Our previous work suggests that NfL is a dynamic marker of ongoing neuronal damage in HD that predicts subsequent progression,” the authors wrote. “This perhaps reflects that NfL, as a marker of axonal damage, has a more direct relationship with the development of clinical manifestations and brain atrophy.”
NfL concentrations in CSF more closely predicted brain volume than did plasma NfL or CSF concentrations of mHTT.
In participants who carried the Huntington’s mutation, CSF concentrations of mHTT and NfL were strongly correlated. Researchers also noted that mutation carriers had a significantly higher CSF-to-plasma ratio of NfL than did controls.
The study also showed that mHTT in the CSF and NfL in the cerebrospinal fluid and plasma, were very stable within individuals over 4-8 weeks.
“The very high intraclass correlation values of the three markers revealed them to be highly stable, suggesting that intraindividual variation in these analytes is likely to be a minimal source of noise in natural history and therapeutic studies,” the authors wrote.
This work was supported by the Medical Research Council U.K., the CHDI Foundation, the Wellcome Trust, the U.K. Department of Health’s National Institute for Health Research Biomedical Research Centres funding scheme, the U.K. Dementia Research Institute, F. Hoffmann-La Roche, the Horizon 2020 Framework Programme, and the Engineering and Physical Sciences Research Council. A number of authors disclosed consulting or serving on advisory boards for F. Hoffmann-La Roche and/or other companies. Three authors are full-time employees of F. Hoffmann-La Roche.
SOURCE: Byrne L et al. Sci Transl Med. 2018;10:eaat7108. doi: 10.1126/scitranslmed.aat7108.
Concentrations of mutant huntingtin protein and neurofilament light proteins in cerebrospinal fluid and blood may be the first signs of progression in Huntington’s disease, according to a paper published online Sept. 12 in Science Translational Medicine.
In a cohort of 40 Huntington’s mutation carriers with manifest disease, 20 carriers without clinical symptoms, and 20 healthy controls, researchers examined levels of mutant huntingtin (mHTT) and neurofilament light (NfL) protein in biofluids, in parallel with clinical evaluations and MRI imaging.
They found that concentrations of mHTT in the cerebrospinal fluid (CSF) and concentrations of NfL proteins in the CSF and plasma were significantly higher in participants with manifest Huntington’s disease (HD) than in those without manifest disease or in controls.
Researchers also saw that CSF concentrations of mHTT showed the earliest detectable change in progression of the disease, followed by plasma and CSF levels of NfL. After that came changes in caudate and global brain volume, motor score, word reading, and other clinical measures.
“These results suggest that as our understanding grows further, analysis of mHTT and NfL might be useful for developing HD therapeutics and for clinical management,” wrote Lauren M. Byrne of the Huntington’s Disease Centre at the University College London Institute of Neurology and her coauthors.
Plasma concentrations of NfL showed the strongest association with clinical severity, even after adjusting for the number of CAG (or cytosine, adenine, and guanine) repeats – a measure of disease severity – and age.
“Our previous work suggests that NfL is a dynamic marker of ongoing neuronal damage in HD that predicts subsequent progression,” the authors wrote. “This perhaps reflects that NfL, as a marker of axonal damage, has a more direct relationship with the development of clinical manifestations and brain atrophy.”
NfL concentrations in CSF more closely predicted brain volume than did plasma NfL or CSF concentrations of mHTT.
In participants who carried the Huntington’s mutation, CSF concentrations of mHTT and NfL were strongly correlated. Researchers also noted that mutation carriers had a significantly higher CSF-to-plasma ratio of NfL than did controls.
The study also showed that mHTT in the CSF and NfL in the cerebrospinal fluid and plasma, were very stable within individuals over 4-8 weeks.
“The very high intraclass correlation values of the three markers revealed them to be highly stable, suggesting that intraindividual variation in these analytes is likely to be a minimal source of noise in natural history and therapeutic studies,” the authors wrote.
This work was supported by the Medical Research Council U.K., the CHDI Foundation, the Wellcome Trust, the U.K. Department of Health’s National Institute for Health Research Biomedical Research Centres funding scheme, the U.K. Dementia Research Institute, F. Hoffmann-La Roche, the Horizon 2020 Framework Programme, and the Engineering and Physical Sciences Research Council. A number of authors disclosed consulting or serving on advisory boards for F. Hoffmann-La Roche and/or other companies. Three authors are full-time employees of F. Hoffmann-La Roche.
SOURCE: Byrne L et al. Sci Transl Med. 2018;10:eaat7108. doi: 10.1126/scitranslmed.aat7108.
FROM SCIENCE TRANSLATIONAL MEDICINE
Key clinical point: Cerebrospinal levels of mutant huntingtin could be earliest sign of Huntington’s disease progression.
Major finding: Changing levels of mutant huntingtin in the cerebrospinal fluid are the first sign of disease progression.
Study details: Cohort study in 60 Huntington’s disease mutation carriers and 20 controls.
Disclosures: This work was supported by the Medical Research Council U.K., the CHDI Foundation, the Wellcome Trust, the U.K. Department of Health’s National Institute for Health Research Biomedical Research Centres funding scheme, the U.K. Dementia Research Institute, F. Hoffmann-La Roche, the Horizon 2020 Framework Programme, and the Engineering and Physical Sciences Research Council. A number of authors disclosed consulting or serving on advisory boards for F. Hoffmann-La Roche and/or other companies. Three authors are full-time employees of F. Hoffmann-La Roche.
Source: Byrne L et al. Sci Transl Med. 2018;10:eaat7108. doi: 10.1126/scitranslmed.aat7108.
VIDEO: Encouraging results reported for novel Huntington’s disease therapy
LOS ANGELES – Results from the first-in-human trial of the investigational antisense oligonucleotide therapy IONIS-HTTRx showed strong dose-dependent reductions in the toxic huntingtin protein in Huntington’s disease patients’ cerebrospinal fluid when compared with placebo.
An exploratory analysis also showed signals of clinical improvement among patients receiving the therapy, the study’s lead author, Sarah Tabrizi, MD, PhD, of University College London, reported at the annual meeting of the American Academy of Neurology.
The findings represent a potential breakthrough in the way Huntington’s – and possibly other neurodegenerative diseases – are treated, said Dr. Tabrizi of University College London. The intrathecally-delivered drug binds to mutant huntingtin protein mRNA to reduce the level of the toxic protein being made.
In the phase 1/2a trial, Dr. Tabrizi and her colleagues enrolled 46 patients with early-stage Huntington’s disease and randomized them to four doses of IONIS-HTTRx or placebo. Patients received four monthly injections of the study drug into the cerebrospinal fluid followed by a 4-month untreated follow-up period. IONIS-HTTRx was delivered in five ascending-dose cohorts.
IONIS-HTTRx was safe and well tolerated and all patients completed the study, Dr. Tabrizi reported. “Our results show that we had significant lowering of the toxic mutant huntingtin protein in the spinal fluid of the patients,” she said, noting that patients receiving the highest doses saw 40%-60% lowering of the protein, which preclinical work suggests correlates to reductions of the protein in the brain.
Dr. Tabrizi stressed that the main outcome measures in the study were reduction of the mutant protein in the CSF and safety and tolerance of the study drug. The investigators did not expect to see clinical measures to change in such a short, small study, she said.
“But then when we looked more carefully at the data with exploratory analysis we found a link between lowering of CSF mutant huntingtin and improvement in total motor score, which is a measure of neurological function, and also, improvement in the Symbol Digit Modalities Test.”
The clinical results are exploratory and require confirmation in larger studies, Dr. Tabrizi stressed.
The study was supported by Ionis Pharmaceuticals. Roche will develop the drug in further clinical trials.
SOURCE: Tabrizi S et al. AAN 2018 abstract CT.002.
LOS ANGELES – Results from the first-in-human trial of the investigational antisense oligonucleotide therapy IONIS-HTTRx showed strong dose-dependent reductions in the toxic huntingtin protein in Huntington’s disease patients’ cerebrospinal fluid when compared with placebo.
An exploratory analysis also showed signals of clinical improvement among patients receiving the therapy, the study’s lead author, Sarah Tabrizi, MD, PhD, of University College London, reported at the annual meeting of the American Academy of Neurology.
The findings represent a potential breakthrough in the way Huntington’s – and possibly other neurodegenerative diseases – are treated, said Dr. Tabrizi of University College London. The intrathecally-delivered drug binds to mutant huntingtin protein mRNA to reduce the level of the toxic protein being made.
In the phase 1/2a trial, Dr. Tabrizi and her colleagues enrolled 46 patients with early-stage Huntington’s disease and randomized them to four doses of IONIS-HTTRx or placebo. Patients received four monthly injections of the study drug into the cerebrospinal fluid followed by a 4-month untreated follow-up period. IONIS-HTTRx was delivered in five ascending-dose cohorts.
IONIS-HTTRx was safe and well tolerated and all patients completed the study, Dr. Tabrizi reported. “Our results show that we had significant lowering of the toxic mutant huntingtin protein in the spinal fluid of the patients,” she said, noting that patients receiving the highest doses saw 40%-60% lowering of the protein, which preclinical work suggests correlates to reductions of the protein in the brain.
Dr. Tabrizi stressed that the main outcome measures in the study were reduction of the mutant protein in the CSF and safety and tolerance of the study drug. The investigators did not expect to see clinical measures to change in such a short, small study, she said.
“But then when we looked more carefully at the data with exploratory analysis we found a link between lowering of CSF mutant huntingtin and improvement in total motor score, which is a measure of neurological function, and also, improvement in the Symbol Digit Modalities Test.”
The clinical results are exploratory and require confirmation in larger studies, Dr. Tabrizi stressed.
The study was supported by Ionis Pharmaceuticals. Roche will develop the drug in further clinical trials.
SOURCE: Tabrizi S et al. AAN 2018 abstract CT.002.
LOS ANGELES – Results from the first-in-human trial of the investigational antisense oligonucleotide therapy IONIS-HTTRx showed strong dose-dependent reductions in the toxic huntingtin protein in Huntington’s disease patients’ cerebrospinal fluid when compared with placebo.
An exploratory analysis also showed signals of clinical improvement among patients receiving the therapy, the study’s lead author, Sarah Tabrizi, MD, PhD, of University College London, reported at the annual meeting of the American Academy of Neurology.
The findings represent a potential breakthrough in the way Huntington’s – and possibly other neurodegenerative diseases – are treated, said Dr. Tabrizi of University College London. The intrathecally-delivered drug binds to mutant huntingtin protein mRNA to reduce the level of the toxic protein being made.
In the phase 1/2a trial, Dr. Tabrizi and her colleagues enrolled 46 patients with early-stage Huntington’s disease and randomized them to four doses of IONIS-HTTRx or placebo. Patients received four monthly injections of the study drug into the cerebrospinal fluid followed by a 4-month untreated follow-up period. IONIS-HTTRx was delivered in five ascending-dose cohorts.
IONIS-HTTRx was safe and well tolerated and all patients completed the study, Dr. Tabrizi reported. “Our results show that we had significant lowering of the toxic mutant huntingtin protein in the spinal fluid of the patients,” she said, noting that patients receiving the highest doses saw 40%-60% lowering of the protein, which preclinical work suggests correlates to reductions of the protein in the brain.
Dr. Tabrizi stressed that the main outcome measures in the study were reduction of the mutant protein in the CSF and safety and tolerance of the study drug. The investigators did not expect to see clinical measures to change in such a short, small study, she said.
“But then when we looked more carefully at the data with exploratory analysis we found a link between lowering of CSF mutant huntingtin and improvement in total motor score, which is a measure of neurological function, and also, improvement in the Symbol Digit Modalities Test.”
The clinical results are exploratory and require confirmation in larger studies, Dr. Tabrizi stressed.
The study was supported by Ionis Pharmaceuticals. Roche will develop the drug in further clinical trials.
SOURCE: Tabrizi S et al. AAN 2018 abstract CT.002.
REPORTING FROM AAN 2018
Gene silencer reduces mutant huntingtin protein in early-stage Huntington’s patients
An investigational gene-silencing molecule safely and dose-dependently reduced production of the mutant huntingtin protein in people with early-stage Huntington’s disease in a small, early-phase study, according to an announcement from the drug’s developer, Ionis Pharmaceuticals.
The antisense oligonucleotide IONIS-HTTRx – the first potentially disease-modifying drug for Huntington’s – will now go forward in a larger study to determine whether lowering mutant huntingtin protein (mHTT) confers any clinical benefits upon patients with the fatal neurodegenerative disease.
IONIS-HTTRx reduced mHTT by fractions that “exceeded expectations” set for the 46-person trial, C. Frank Bennett, PhD, senior vice president of research at Ionis, said in a press statement.
“The results of this trial are of groundbreaking importance for Huntington’s disease patients and families,” said Dr. Tabrizi, director of the Huntington’s Disease Centre at University College London. “For the first time a drug has lowered the level of the toxic disease-causing protein in the nervous system, and the drug was safe and well tolerated. The key now is to move quickly to a larger trial to test whether the drug slows disease progression.”
Upon receiving the positive data, Roche Pharma exercised its $45 million option to license the molecule. Roche now takes all regulatory and clinical development responsibility for IONIS-HTTRx.
There are few publicly available data on the IONIS-HTTRx study. It enrolled 46 patients with early-stage Huntington’s who were recruited from nine sites in the United Kingdom, Germany, and Canada. They were randomized to placebo or to four ascending doses of IONIS-HTTRx. The primary outcomes were mHTT levels in spinal fluid, safety, and tolerability. It produced significant, dose-dependent reductions of mHTT without concerning or dose-limiting safety signals, the press statement noted.
A larger study with clinical endpoints is next up, according to a statement Ionis and Roche jointly issued to the Huntington’s Disease Society of America.
“The next step for this program will be to conduct a safety and efficacy study to investigate if decreasing mutant huntingtin protein with IONIS-HTTRx can benefit people with Huntington’s disease,” the statement noted. “Future studies for the program will be conducted globally, including in the U.S. Roche will announce details about future studies, including eligibility criteria and planned start dates, as this information becomes available. All relevant information on upcoming studies will also be posted on HDTrialFinder.org and ClinicalTrials.gov.”
Huntington’s disease is caused by an expansion of at least 36 repeats of the CAG trinucleotide sequence in the huntingtin gene. The resulting mHTT is toxic and gradually damages neurons. IONIS-HTTRx interrupts the messenger RNA that fuels this toxic protein buildup, and it is the only drug that has ever attacked the disease at this level. This development is “a historic moment in the fight against Huntington’s, as it represents the successful completion of the first trial to treat the underlying cause of Huntington’s disease, the genetic mutation itself,” according to a statement by Louise Vetter, president of the Huntington’s Disease Society of America.
“The fact that levels of mutant huntingtin were reduced in correlation to the dose of IONIS-HTTRx that was given is significant, and the fact that participants in this first Phase 1/2a study are able to continue on the drug through an open-label extension gives us optimism regarding its safety,” Ms. Vetter said.
In January 2016, the Food and Drug Administration granted orphan drug status to IONIS-HTTRX; the European Medicines Agency had previously granted it similar status.
The press release from Ionis Pharmaceuticals sounds very promising. There is reason to believe that lowering mHTT protein might prevent or delay Huntington’s disease, and this antisense molecule appears to be safe and to lower mHTT in cerebrospinal fluid. However, there are several caveats.
First, it is unclear whether lowering mHTT protein might help those who already have clinical Huntington’s disease.
Third, no information is given in the press release about the degree of reduction of mHTT observed and whether there is evidence that this lowering might be sufficient for a therapeutic effect.
Fourth, neurotoxicity may not only result from the mHTT protein but also directly from the mRNA itself. The contribution of mutant mRNA to pathogenesis is a key open question in the study of Huntington’s disease and other related “repeat disorders.”
Michael Wolfe, PhD , is the Mathias P. Mertes Professor of Medicinal Chemistry at the University of Kansas, Lawrence. He has no relevant disclosures.
The press release from Ionis Pharmaceuticals sounds very promising. There is reason to believe that lowering mHTT protein might prevent or delay Huntington’s disease, and this antisense molecule appears to be safe and to lower mHTT in cerebrospinal fluid. However, there are several caveats.
First, it is unclear whether lowering mHTT protein might help those who already have clinical Huntington’s disease.
Third, no information is given in the press release about the degree of reduction of mHTT observed and whether there is evidence that this lowering might be sufficient for a therapeutic effect.
Fourth, neurotoxicity may not only result from the mHTT protein but also directly from the mRNA itself. The contribution of mutant mRNA to pathogenesis is a key open question in the study of Huntington’s disease and other related “repeat disorders.”
Michael Wolfe, PhD , is the Mathias P. Mertes Professor of Medicinal Chemistry at the University of Kansas, Lawrence. He has no relevant disclosures.
The press release from Ionis Pharmaceuticals sounds very promising. There is reason to believe that lowering mHTT protein might prevent or delay Huntington’s disease, and this antisense molecule appears to be safe and to lower mHTT in cerebrospinal fluid. However, there are several caveats.
First, it is unclear whether lowering mHTT protein might help those who already have clinical Huntington’s disease.
Third, no information is given in the press release about the degree of reduction of mHTT observed and whether there is evidence that this lowering might be sufficient for a therapeutic effect.
Fourth, neurotoxicity may not only result from the mHTT protein but also directly from the mRNA itself. The contribution of mutant mRNA to pathogenesis is a key open question in the study of Huntington’s disease and other related “repeat disorders.”
Michael Wolfe, PhD , is the Mathias P. Mertes Professor of Medicinal Chemistry at the University of Kansas, Lawrence. He has no relevant disclosures.
An investigational gene-silencing molecule safely and dose-dependently reduced production of the mutant huntingtin protein in people with early-stage Huntington’s disease in a small, early-phase study, according to an announcement from the drug’s developer, Ionis Pharmaceuticals.
The antisense oligonucleotide IONIS-HTTRx – the first potentially disease-modifying drug for Huntington’s – will now go forward in a larger study to determine whether lowering mutant huntingtin protein (mHTT) confers any clinical benefits upon patients with the fatal neurodegenerative disease.
IONIS-HTTRx reduced mHTT by fractions that “exceeded expectations” set for the 46-person trial, C. Frank Bennett, PhD, senior vice president of research at Ionis, said in a press statement.
“The results of this trial are of groundbreaking importance for Huntington’s disease patients and families,” said Dr. Tabrizi, director of the Huntington’s Disease Centre at University College London. “For the first time a drug has lowered the level of the toxic disease-causing protein in the nervous system, and the drug was safe and well tolerated. The key now is to move quickly to a larger trial to test whether the drug slows disease progression.”
Upon receiving the positive data, Roche Pharma exercised its $45 million option to license the molecule. Roche now takes all regulatory and clinical development responsibility for IONIS-HTTRx.
There are few publicly available data on the IONIS-HTTRx study. It enrolled 46 patients with early-stage Huntington’s who were recruited from nine sites in the United Kingdom, Germany, and Canada. They were randomized to placebo or to four ascending doses of IONIS-HTTRx. The primary outcomes were mHTT levels in spinal fluid, safety, and tolerability. It produced significant, dose-dependent reductions of mHTT without concerning or dose-limiting safety signals, the press statement noted.
A larger study with clinical endpoints is next up, according to a statement Ionis and Roche jointly issued to the Huntington’s Disease Society of America.
“The next step for this program will be to conduct a safety and efficacy study to investigate if decreasing mutant huntingtin protein with IONIS-HTTRx can benefit people with Huntington’s disease,” the statement noted. “Future studies for the program will be conducted globally, including in the U.S. Roche will announce details about future studies, including eligibility criteria and planned start dates, as this information becomes available. All relevant information on upcoming studies will also be posted on HDTrialFinder.org and ClinicalTrials.gov.”
Huntington’s disease is caused by an expansion of at least 36 repeats of the CAG trinucleotide sequence in the huntingtin gene. The resulting mHTT is toxic and gradually damages neurons. IONIS-HTTRx interrupts the messenger RNA that fuels this toxic protein buildup, and it is the only drug that has ever attacked the disease at this level. This development is “a historic moment in the fight against Huntington’s, as it represents the successful completion of the first trial to treat the underlying cause of Huntington’s disease, the genetic mutation itself,” according to a statement by Louise Vetter, president of the Huntington’s Disease Society of America.
“The fact that levels of mutant huntingtin were reduced in correlation to the dose of IONIS-HTTRx that was given is significant, and the fact that participants in this first Phase 1/2a study are able to continue on the drug through an open-label extension gives us optimism regarding its safety,” Ms. Vetter said.
In January 2016, the Food and Drug Administration granted orphan drug status to IONIS-HTTRX; the European Medicines Agency had previously granted it similar status.
An investigational gene-silencing molecule safely and dose-dependently reduced production of the mutant huntingtin protein in people with early-stage Huntington’s disease in a small, early-phase study, according to an announcement from the drug’s developer, Ionis Pharmaceuticals.
The antisense oligonucleotide IONIS-HTTRx – the first potentially disease-modifying drug for Huntington’s – will now go forward in a larger study to determine whether lowering mutant huntingtin protein (mHTT) confers any clinical benefits upon patients with the fatal neurodegenerative disease.
IONIS-HTTRx reduced mHTT by fractions that “exceeded expectations” set for the 46-person trial, C. Frank Bennett, PhD, senior vice president of research at Ionis, said in a press statement.
“The results of this trial are of groundbreaking importance for Huntington’s disease patients and families,” said Dr. Tabrizi, director of the Huntington’s Disease Centre at University College London. “For the first time a drug has lowered the level of the toxic disease-causing protein in the nervous system, and the drug was safe and well tolerated. The key now is to move quickly to a larger trial to test whether the drug slows disease progression.”
Upon receiving the positive data, Roche Pharma exercised its $45 million option to license the molecule. Roche now takes all regulatory and clinical development responsibility for IONIS-HTTRx.
There are few publicly available data on the IONIS-HTTRx study. It enrolled 46 patients with early-stage Huntington’s who were recruited from nine sites in the United Kingdom, Germany, and Canada. They were randomized to placebo or to four ascending doses of IONIS-HTTRx. The primary outcomes were mHTT levels in spinal fluid, safety, and tolerability. It produced significant, dose-dependent reductions of mHTT without concerning or dose-limiting safety signals, the press statement noted.
A larger study with clinical endpoints is next up, according to a statement Ionis and Roche jointly issued to the Huntington’s Disease Society of America.
“The next step for this program will be to conduct a safety and efficacy study to investigate if decreasing mutant huntingtin protein with IONIS-HTTRx can benefit people with Huntington’s disease,” the statement noted. “Future studies for the program will be conducted globally, including in the U.S. Roche will announce details about future studies, including eligibility criteria and planned start dates, as this information becomes available. All relevant information on upcoming studies will also be posted on HDTrialFinder.org and ClinicalTrials.gov.”
Huntington’s disease is caused by an expansion of at least 36 repeats of the CAG trinucleotide sequence in the huntingtin gene. The resulting mHTT is toxic and gradually damages neurons. IONIS-HTTRx interrupts the messenger RNA that fuels this toxic protein buildup, and it is the only drug that has ever attacked the disease at this level. This development is “a historic moment in the fight against Huntington’s, as it represents the successful completion of the first trial to treat the underlying cause of Huntington’s disease, the genetic mutation itself,” according to a statement by Louise Vetter, president of the Huntington’s Disease Society of America.
“The fact that levels of mutant huntingtin were reduced in correlation to the dose of IONIS-HTTRx that was given is significant, and the fact that participants in this first Phase 1/2a study are able to continue on the drug through an open-label extension gives us optimism regarding its safety,” Ms. Vetter said.
In January 2016, the Food and Drug Administration granted orphan drug status to IONIS-HTTRX; the European Medicines Agency had previously granted it similar status.







