User login
Then and now: Liver disease
In the late 2000s, we witnessed revolutionary discoveries and advances in our understanding and management of chronic hepatitis C. Who knew that when IL-28B was first described in 2009, providing a genetic basis for patients’ response to interferon-based therapies, its impact would also be so swiftly supplanted by the introduction of direct acting antivirals a few years later? The pipeline for HCV treatment was feverish for several years, which resulted in a complete transformation of HCV treatment from a long, exhausting, side-effect filled course to a simple 8-to-12-week regimen. Furthermore, we now have established protocols for organ transplantation for patients without active HCV infection to receive HCV-positive organs due to the effectiveness of treatments for HCV. This kind of progress in our field demonstrates how awe-inspiring medical advances can be and how fortunate we are to have witnessed and lived this progress in such a short period of time.
In recent years, non-alcoholic fatty liver disease (NAFLD) has supplanted HCV as the most prevalent chronic liver disease seen in GI and hepatology practices across the country.
The sheer number of these patients can be overwhelming for any practice, whether a GI practice or primary care. It has become clear that we have an urgent need for improved and easily accessible non-invasive methods to risk stratify NAFLD to identify patients at most risk for developing advanced fibrosis, decompensated cirrhosis, and hepatocellular carcinoma. Furthermore, effective strategies for prevention of these adverse outcomes in the general population still need to be further characterized. For treatment of non-alcoholic steatohepatitis, therapeutic agents being studied for their efficacy are wide ranging with particular interest in weight loss medications, diabetic medications, and anti-inflammatory medications. Yet, we can all see that there are sizeable gaps in our understanding and management of patients with NAFLD. However, rather than being intimidated, we should look forward to the progress that will surely come in the next 15 years.
Dr. Jou is associate professor of medicine, division of gastroenterology and hepatology, School of Medicine Fellowship program director, Medicine, Division of Gastroenterology and Hepatology, School of Medicine, Oregon Health and Science University, Portland. She reported no relevant financial conflicts of interest.
In the late 2000s, we witnessed revolutionary discoveries and advances in our understanding and management of chronic hepatitis C. Who knew that when IL-28B was first described in 2009, providing a genetic basis for patients’ response to interferon-based therapies, its impact would also be so swiftly supplanted by the introduction of direct acting antivirals a few years later? The pipeline for HCV treatment was feverish for several years, which resulted in a complete transformation of HCV treatment from a long, exhausting, side-effect filled course to a simple 8-to-12-week regimen. Furthermore, we now have established protocols for organ transplantation for patients without active HCV infection to receive HCV-positive organs due to the effectiveness of treatments for HCV. This kind of progress in our field demonstrates how awe-inspiring medical advances can be and how fortunate we are to have witnessed and lived this progress in such a short period of time.
In recent years, non-alcoholic fatty liver disease (NAFLD) has supplanted HCV as the most prevalent chronic liver disease seen in GI and hepatology practices across the country.
The sheer number of these patients can be overwhelming for any practice, whether a GI practice or primary care. It has become clear that we have an urgent need for improved and easily accessible non-invasive methods to risk stratify NAFLD to identify patients at most risk for developing advanced fibrosis, decompensated cirrhosis, and hepatocellular carcinoma. Furthermore, effective strategies for prevention of these adverse outcomes in the general population still need to be further characterized. For treatment of non-alcoholic steatohepatitis, therapeutic agents being studied for their efficacy are wide ranging with particular interest in weight loss medications, diabetic medications, and anti-inflammatory medications. Yet, we can all see that there are sizeable gaps in our understanding and management of patients with NAFLD. However, rather than being intimidated, we should look forward to the progress that will surely come in the next 15 years.
Dr. Jou is associate professor of medicine, division of gastroenterology and hepatology, School of Medicine Fellowship program director, Medicine, Division of Gastroenterology and Hepatology, School of Medicine, Oregon Health and Science University, Portland. She reported no relevant financial conflicts of interest.
In the late 2000s, we witnessed revolutionary discoveries and advances in our understanding and management of chronic hepatitis C. Who knew that when IL-28B was first described in 2009, providing a genetic basis for patients’ response to interferon-based therapies, its impact would also be so swiftly supplanted by the introduction of direct acting antivirals a few years later? The pipeline for HCV treatment was feverish for several years, which resulted in a complete transformation of HCV treatment from a long, exhausting, side-effect filled course to a simple 8-to-12-week regimen. Furthermore, we now have established protocols for organ transplantation for patients without active HCV infection to receive HCV-positive organs due to the effectiveness of treatments for HCV. This kind of progress in our field demonstrates how awe-inspiring medical advances can be and how fortunate we are to have witnessed and lived this progress in such a short period of time.
In recent years, non-alcoholic fatty liver disease (NAFLD) has supplanted HCV as the most prevalent chronic liver disease seen in GI and hepatology practices across the country.
The sheer number of these patients can be overwhelming for any practice, whether a GI practice or primary care. It has become clear that we have an urgent need for improved and easily accessible non-invasive methods to risk stratify NAFLD to identify patients at most risk for developing advanced fibrosis, decompensated cirrhosis, and hepatocellular carcinoma. Furthermore, effective strategies for prevention of these adverse outcomes in the general population still need to be further characterized. For treatment of non-alcoholic steatohepatitis, therapeutic agents being studied for their efficacy are wide ranging with particular interest in weight loss medications, diabetic medications, and anti-inflammatory medications. Yet, we can all see that there are sizeable gaps in our understanding and management of patients with NAFLD. However, rather than being intimidated, we should look forward to the progress that will surely come in the next 15 years.
Dr. Jou is associate professor of medicine, division of gastroenterology and hepatology, School of Medicine Fellowship program director, Medicine, Division of Gastroenterology and Hepatology, School of Medicine, Oregon Health and Science University, Portland. She reported no relevant financial conflicts of interest.
Fewer GI docs, more alcohol-associated liver disease deaths
People are more likely to die of alcohol-associated liver disease (ALD) when there are fewer gastroenterologists in their state, researchers say.
The finding raises questions about steps that policymakers could take to increase the number of gastroenterologists and spread them more evenly around the United States.
“We found that there’s a fivefold difference in density of gastroenterologists through different states,” said Brian P. Lee, MD, MAS, an assistant professor of clinical medicine at the University of Southern California, Los Angeles.
Dr. Lee and colleagues published their findings in Clinical Gastroenterology and Hepatology.
ALD is becoming more common, and it is killing more people. Research among veterans has linked visits to gastroenterologists to a lower risk for death from liver disease.
To see whether that correlation applies more broadly, Dr. Lee and colleagues compared multiple datasets. One from the U.S. Health Resources & Service Administration provided the number of gastroenterologists per 100,000 population. The other from the U.S. Centers for Disease Control and Prevention provided ALD-related deaths per 1,000,000 adults for each state and the District of Columbia.
The researchers adjusted for many variables that could affect the relationship between the availability of gastroenterologists and deaths related to ALD, including the age distribution of the population in each state, the gender balance, race and ethnicity, binge drinking, household income, obesity, and the proportion of rural residents.
They found that for every additional gastroenterologist, there is almost one fewer ALD-related death each year per 100,000 population (9.0 [95% confidence interval, 1.3-16.7] fewer ALD-related deaths per 1,000,000 population for each additional gastroenterologist per 100,000 population).
The strength of the association appeared to plateau when there were at least 7.5 gastroenterologists per 100,000 people.
From these findings, the researchers calculated that as many as 40% of deaths from ALD nationwide could be prevented by providing more gastroenterologists in the places where they are lacking.
The mean number of gastroenterologists per 100,000 people in the United States was 4.6, and the annual ALD-related death rate was 85.6 per 1,000,000 people.
The Atlantic states had the greatest concentration of gastroenterologists and the lowest ALD-related mortality, whereas the Mountain states had the lowest concentration of gastroenterologists and the highest ALD-related mortality.
The lowest mortality related to ALD was in New Jersey, Maryland, and Hawaii, with 52 per 1,000,000 people, and the highest was in Wyoming, with 289.
Study shines spotlight on general GI care
Access to liver transplants did not make a statistically significant difference in mortality from ALD.
“It makes you realize that transplant will only be accessible for really just a small fraction of the population who needs it,” Dr. Lee told this news organization.
General gastroenterologic care appears to make a bigger difference in saving patients’ lives. “Are they getting endoscopy for bleeding from varices?” Dr. Lee asked. “Are they getting appropriate antibiotics prescribed to prevent bacterial infection of ascites?”
The concentration of primary care physicians did not reduce mortality from ALD, and neither did the concentration of substance use, behavioral disorder, and mental health counselors.
Previous research has shown that substance abuse therapy is effective. But many people do not want to undertake it, or they face barriers of transportation, language, or insurance, said Dr. Lee.
“I have many patients whose insurance will provide them access to medical visits to me but will not to substance-use rehab, for example,” he said.
To see whether the effect was more generally due to the concentration of medical specialists, the researchers examined the state-level density of ophthalmologists and dermatologists. They found no significant difference in ALD-related mortality.
The finding builds on reports by the American Gastroenterological Association and the American Association for the Study of Liver Diseases that the number of gastroenterologists has not kept up with the U.S. population nor the burden of digestive diseases, and that predicts a critical shortage in the future.
Overcoming barriers to care for liver disease
The overall supply of gastroenterologists could be increased by reducing the educational requirements and increasing the funding for fellowships, said Dr. Lee.
“We have to have a better understanding as to the barriers to gastroenterology practice in certain areas, then interventions to address those barriers and also incentives to attract gastroenterologists to those areas,” Dr. Lee said.
The study underscores the importance of access to gastroenterological care, said George Cholankeril, MD, assistant professor of medicine at Baylor College of Medicine, Houston, who was not involved in the study. That urgency has only grown as ALD has spiraled up with the COVID-19 pandemic, he said.
“Anyone in clinical practice right now will be able to say that there’s been a clear rising tide of patients with alcohol-related liver disease,” he told this news organization. “There’s an urgent need to address this and provide the necessary resources.”
Prevention remains essential, Dr. Cholankeril said.
Gastroenterologists and primary care physicians can help stem the tide of ALD by screening their patients for the disease through a tool like AUDIT (Alcohol Use Disorders Identification Test), he said. They can then refer patients to substance abuse treatment centers or to psychologists and psychiatrists.
Dr. Lee and Dr. Cholankeril report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
People are more likely to die of alcohol-associated liver disease (ALD) when there are fewer gastroenterologists in their state, researchers say.
The finding raises questions about steps that policymakers could take to increase the number of gastroenterologists and spread them more evenly around the United States.
“We found that there’s a fivefold difference in density of gastroenterologists through different states,” said Brian P. Lee, MD, MAS, an assistant professor of clinical medicine at the University of Southern California, Los Angeles.
Dr. Lee and colleagues published their findings in Clinical Gastroenterology and Hepatology.
ALD is becoming more common, and it is killing more people. Research among veterans has linked visits to gastroenterologists to a lower risk for death from liver disease.
To see whether that correlation applies more broadly, Dr. Lee and colleagues compared multiple datasets. One from the U.S. Health Resources & Service Administration provided the number of gastroenterologists per 100,000 population. The other from the U.S. Centers for Disease Control and Prevention provided ALD-related deaths per 1,000,000 adults for each state and the District of Columbia.
The researchers adjusted for many variables that could affect the relationship between the availability of gastroenterologists and deaths related to ALD, including the age distribution of the population in each state, the gender balance, race and ethnicity, binge drinking, household income, obesity, and the proportion of rural residents.
They found that for every additional gastroenterologist, there is almost one fewer ALD-related death each year per 100,000 population (9.0 [95% confidence interval, 1.3-16.7] fewer ALD-related deaths per 1,000,000 population for each additional gastroenterologist per 100,000 population).
The strength of the association appeared to plateau when there were at least 7.5 gastroenterologists per 100,000 people.
From these findings, the researchers calculated that as many as 40% of deaths from ALD nationwide could be prevented by providing more gastroenterologists in the places where they are lacking.
The mean number of gastroenterologists per 100,000 people in the United States was 4.6, and the annual ALD-related death rate was 85.6 per 1,000,000 people.
The Atlantic states had the greatest concentration of gastroenterologists and the lowest ALD-related mortality, whereas the Mountain states had the lowest concentration of gastroenterologists and the highest ALD-related mortality.
The lowest mortality related to ALD was in New Jersey, Maryland, and Hawaii, with 52 per 1,000,000 people, and the highest was in Wyoming, with 289.
Study shines spotlight on general GI care
Access to liver transplants did not make a statistically significant difference in mortality from ALD.
“It makes you realize that transplant will only be accessible for really just a small fraction of the population who needs it,” Dr. Lee told this news organization.
General gastroenterologic care appears to make a bigger difference in saving patients’ lives. “Are they getting endoscopy for bleeding from varices?” Dr. Lee asked. “Are they getting appropriate antibiotics prescribed to prevent bacterial infection of ascites?”
The concentration of primary care physicians did not reduce mortality from ALD, and neither did the concentration of substance use, behavioral disorder, and mental health counselors.
Previous research has shown that substance abuse therapy is effective. But many people do not want to undertake it, or they face barriers of transportation, language, or insurance, said Dr. Lee.
“I have many patients whose insurance will provide them access to medical visits to me but will not to substance-use rehab, for example,” he said.
To see whether the effect was more generally due to the concentration of medical specialists, the researchers examined the state-level density of ophthalmologists and dermatologists. They found no significant difference in ALD-related mortality.
The finding builds on reports by the American Gastroenterological Association and the American Association for the Study of Liver Diseases that the number of gastroenterologists has not kept up with the U.S. population nor the burden of digestive diseases, and that predicts a critical shortage in the future.
Overcoming barriers to care for liver disease
The overall supply of gastroenterologists could be increased by reducing the educational requirements and increasing the funding for fellowships, said Dr. Lee.
“We have to have a better understanding as to the barriers to gastroenterology practice in certain areas, then interventions to address those barriers and also incentives to attract gastroenterologists to those areas,” Dr. Lee said.
The study underscores the importance of access to gastroenterological care, said George Cholankeril, MD, assistant professor of medicine at Baylor College of Medicine, Houston, who was not involved in the study. That urgency has only grown as ALD has spiraled up with the COVID-19 pandemic, he said.
“Anyone in clinical practice right now will be able to say that there’s been a clear rising tide of patients with alcohol-related liver disease,” he told this news organization. “There’s an urgent need to address this and provide the necessary resources.”
Prevention remains essential, Dr. Cholankeril said.
Gastroenterologists and primary care physicians can help stem the tide of ALD by screening their patients for the disease through a tool like AUDIT (Alcohol Use Disorders Identification Test), he said. They can then refer patients to substance abuse treatment centers or to psychologists and psychiatrists.
Dr. Lee and Dr. Cholankeril report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
People are more likely to die of alcohol-associated liver disease (ALD) when there are fewer gastroenterologists in their state, researchers say.
The finding raises questions about steps that policymakers could take to increase the number of gastroenterologists and spread them more evenly around the United States.
“We found that there’s a fivefold difference in density of gastroenterologists through different states,” said Brian P. Lee, MD, MAS, an assistant professor of clinical medicine at the University of Southern California, Los Angeles.
Dr. Lee and colleagues published their findings in Clinical Gastroenterology and Hepatology.
ALD is becoming more common, and it is killing more people. Research among veterans has linked visits to gastroenterologists to a lower risk for death from liver disease.
To see whether that correlation applies more broadly, Dr. Lee and colleagues compared multiple datasets. One from the U.S. Health Resources & Service Administration provided the number of gastroenterologists per 100,000 population. The other from the U.S. Centers for Disease Control and Prevention provided ALD-related deaths per 1,000,000 adults for each state and the District of Columbia.
The researchers adjusted for many variables that could affect the relationship between the availability of gastroenterologists and deaths related to ALD, including the age distribution of the population in each state, the gender balance, race and ethnicity, binge drinking, household income, obesity, and the proportion of rural residents.
They found that for every additional gastroenterologist, there is almost one fewer ALD-related death each year per 100,000 population (9.0 [95% confidence interval, 1.3-16.7] fewer ALD-related deaths per 1,000,000 population for each additional gastroenterologist per 100,000 population).
The strength of the association appeared to plateau when there were at least 7.5 gastroenterologists per 100,000 people.
From these findings, the researchers calculated that as many as 40% of deaths from ALD nationwide could be prevented by providing more gastroenterologists in the places where they are lacking.
The mean number of gastroenterologists per 100,000 people in the United States was 4.6, and the annual ALD-related death rate was 85.6 per 1,000,000 people.
The Atlantic states had the greatest concentration of gastroenterologists and the lowest ALD-related mortality, whereas the Mountain states had the lowest concentration of gastroenterologists and the highest ALD-related mortality.
The lowest mortality related to ALD was in New Jersey, Maryland, and Hawaii, with 52 per 1,000,000 people, and the highest was in Wyoming, with 289.
Study shines spotlight on general GI care
Access to liver transplants did not make a statistically significant difference in mortality from ALD.
“It makes you realize that transplant will only be accessible for really just a small fraction of the population who needs it,” Dr. Lee told this news organization.
General gastroenterologic care appears to make a bigger difference in saving patients’ lives. “Are they getting endoscopy for bleeding from varices?” Dr. Lee asked. “Are they getting appropriate antibiotics prescribed to prevent bacterial infection of ascites?”
The concentration of primary care physicians did not reduce mortality from ALD, and neither did the concentration of substance use, behavioral disorder, and mental health counselors.
Previous research has shown that substance abuse therapy is effective. But many people do not want to undertake it, or they face barriers of transportation, language, or insurance, said Dr. Lee.
“I have many patients whose insurance will provide them access to medical visits to me but will not to substance-use rehab, for example,” he said.
To see whether the effect was more generally due to the concentration of medical specialists, the researchers examined the state-level density of ophthalmologists and dermatologists. They found no significant difference in ALD-related mortality.
The finding builds on reports by the American Gastroenterological Association and the American Association for the Study of Liver Diseases that the number of gastroenterologists has not kept up with the U.S. population nor the burden of digestive diseases, and that predicts a critical shortage in the future.
Overcoming barriers to care for liver disease
The overall supply of gastroenterologists could be increased by reducing the educational requirements and increasing the funding for fellowships, said Dr. Lee.
“We have to have a better understanding as to the barriers to gastroenterology practice in certain areas, then interventions to address those barriers and also incentives to attract gastroenterologists to those areas,” Dr. Lee said.
The study underscores the importance of access to gastroenterological care, said George Cholankeril, MD, assistant professor of medicine at Baylor College of Medicine, Houston, who was not involved in the study. That urgency has only grown as ALD has spiraled up with the COVID-19 pandemic, he said.
“Anyone in clinical practice right now will be able to say that there’s been a clear rising tide of patients with alcohol-related liver disease,” he told this news organization. “There’s an urgent need to address this and provide the necessary resources.”
Prevention remains essential, Dr. Cholankeril said.
Gastroenterologists and primary care physicians can help stem the tide of ALD by screening their patients for the disease through a tool like AUDIT (Alcohol Use Disorders Identification Test), he said. They can then refer patients to substance abuse treatment centers or to psychologists and psychiatrists.
Dr. Lee and Dr. Cholankeril report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Gene variants found to protect against liver disease
Rare gene variants are associated with a reduced risk for multiple types of liver disease, including cirrhosis, researchers say.
People with certain variants in the gene CIDEB are one-third less likely to develop any sort of liver disease, according to Aris Baras, MD, a senior vice president at Regeneron, and colleagues.
“The unprecedented protective effect that these CIDEB genetic variants have against liver disease provides us with one of our most exciting targets and potential therapeutic approaches for a notoriously hard-to-treat disease where there are currently no approved treatments,” said Dr. Baras in a press release.
Dr. Baras and colleagues published the finding in The New England Journal of Medicine.
The finding follows on a similar discovery about a common variant in the gene HSD17B13. Treatments targeting this gene are being tested in clinical trials.
To search for more such genes, the researchers analyzed human exomes – the part of the genome that codes for proteins – to look for associations between gene variants and liver function.
The researchers used exome sequencing on 542,904 people from the UK Biobank, the Geisinger Health System MyCode cohort, and other datasets.
They found that coding variants in APOB, ABCB4, SLC30A10, and TM6SF2 were associated with increased aminotransferase levels and an increased risk for liver disease.
But variants in CIDEB were associated with decreased levels of alanine aminotransferase, a biomarker of hepatocellular injury. And they were associated with a decreased risk for liver disease of any cause (odds ratio per allele, 0.67; 95% confidence interval, 0.57-0.79).
The CIDEB variants were present in only 0.7% of the persons in the study.
Zeroing in on various kinds of liver disease, the researchers found that the CIDEB variants were associated with a reduced risk for alcoholic liver disease, nonalcoholic liver disease, any liver cirrhosis, alcoholic liver cirrhosis, nonalcoholic liver cirrhosis, and viral hepatitis.
In 3,599 patients who had undergone bariatric surgery, variants in CIDEB were associated with a reduced nonalcoholic fatty liver disease activity score of –0.98 beta per allele in score units, where scores range from 0-8, with a higher score indicating more severe disease.
In patients for whom MRI data were available, those with rare coding variants in CIDEB had lower proportions of liver fat. However, percentage of liver fat did not fully explain the reduced risk for liver disease.
Pursuing another line of investigation, the researchers found that they could prevent the buildup of large lipid droplets in oleic acid-treated human hepatoma cell lines by silencing the CIDEB gene using small interfering RNA.
The association was particularly strong among people with higher body mass indices and type 2 diabetes.
The associations with the rare protective CIDEB variants were consistent across ancestries, but people of non-European ancestry, who might be disproportionately affected by liver disease, were underrepresented in the database, the researchers note.
The study was supported by Regeneron Pharmaceuticals, which also employed several of the researchers.
A version of this article first appeared on Medscape.com.
Rare gene variants are associated with a reduced risk for multiple types of liver disease, including cirrhosis, researchers say.
People with certain variants in the gene CIDEB are one-third less likely to develop any sort of liver disease, according to Aris Baras, MD, a senior vice president at Regeneron, and colleagues.
“The unprecedented protective effect that these CIDEB genetic variants have against liver disease provides us with one of our most exciting targets and potential therapeutic approaches for a notoriously hard-to-treat disease where there are currently no approved treatments,” said Dr. Baras in a press release.
Dr. Baras and colleagues published the finding in The New England Journal of Medicine.
The finding follows on a similar discovery about a common variant in the gene HSD17B13. Treatments targeting this gene are being tested in clinical trials.
To search for more such genes, the researchers analyzed human exomes – the part of the genome that codes for proteins – to look for associations between gene variants and liver function.
The researchers used exome sequencing on 542,904 people from the UK Biobank, the Geisinger Health System MyCode cohort, and other datasets.
They found that coding variants in APOB, ABCB4, SLC30A10, and TM6SF2 were associated with increased aminotransferase levels and an increased risk for liver disease.
But variants in CIDEB were associated with decreased levels of alanine aminotransferase, a biomarker of hepatocellular injury. And they were associated with a decreased risk for liver disease of any cause (odds ratio per allele, 0.67; 95% confidence interval, 0.57-0.79).
The CIDEB variants were present in only 0.7% of the persons in the study.
Zeroing in on various kinds of liver disease, the researchers found that the CIDEB variants were associated with a reduced risk for alcoholic liver disease, nonalcoholic liver disease, any liver cirrhosis, alcoholic liver cirrhosis, nonalcoholic liver cirrhosis, and viral hepatitis.
In 3,599 patients who had undergone bariatric surgery, variants in CIDEB were associated with a reduced nonalcoholic fatty liver disease activity score of –0.98 beta per allele in score units, where scores range from 0-8, with a higher score indicating more severe disease.
In patients for whom MRI data were available, those with rare coding variants in CIDEB had lower proportions of liver fat. However, percentage of liver fat did not fully explain the reduced risk for liver disease.
Pursuing another line of investigation, the researchers found that they could prevent the buildup of large lipid droplets in oleic acid-treated human hepatoma cell lines by silencing the CIDEB gene using small interfering RNA.
The association was particularly strong among people with higher body mass indices and type 2 diabetes.
The associations with the rare protective CIDEB variants were consistent across ancestries, but people of non-European ancestry, who might be disproportionately affected by liver disease, were underrepresented in the database, the researchers note.
The study was supported by Regeneron Pharmaceuticals, which also employed several of the researchers.
A version of this article first appeared on Medscape.com.
Rare gene variants are associated with a reduced risk for multiple types of liver disease, including cirrhosis, researchers say.
People with certain variants in the gene CIDEB are one-third less likely to develop any sort of liver disease, according to Aris Baras, MD, a senior vice president at Regeneron, and colleagues.
“The unprecedented protective effect that these CIDEB genetic variants have against liver disease provides us with one of our most exciting targets and potential therapeutic approaches for a notoriously hard-to-treat disease where there are currently no approved treatments,” said Dr. Baras in a press release.
Dr. Baras and colleagues published the finding in The New England Journal of Medicine.
The finding follows on a similar discovery about a common variant in the gene HSD17B13. Treatments targeting this gene are being tested in clinical trials.
To search for more such genes, the researchers analyzed human exomes – the part of the genome that codes for proteins – to look for associations between gene variants and liver function.
The researchers used exome sequencing on 542,904 people from the UK Biobank, the Geisinger Health System MyCode cohort, and other datasets.
They found that coding variants in APOB, ABCB4, SLC30A10, and TM6SF2 were associated with increased aminotransferase levels and an increased risk for liver disease.
But variants in CIDEB were associated with decreased levels of alanine aminotransferase, a biomarker of hepatocellular injury. And they were associated with a decreased risk for liver disease of any cause (odds ratio per allele, 0.67; 95% confidence interval, 0.57-0.79).
The CIDEB variants were present in only 0.7% of the persons in the study.
Zeroing in on various kinds of liver disease, the researchers found that the CIDEB variants were associated with a reduced risk for alcoholic liver disease, nonalcoholic liver disease, any liver cirrhosis, alcoholic liver cirrhosis, nonalcoholic liver cirrhosis, and viral hepatitis.
In 3,599 patients who had undergone bariatric surgery, variants in CIDEB were associated with a reduced nonalcoholic fatty liver disease activity score of –0.98 beta per allele in score units, where scores range from 0-8, with a higher score indicating more severe disease.
In patients for whom MRI data were available, those with rare coding variants in CIDEB had lower proportions of liver fat. However, percentage of liver fat did not fully explain the reduced risk for liver disease.
Pursuing another line of investigation, the researchers found that they could prevent the buildup of large lipid droplets in oleic acid-treated human hepatoma cell lines by silencing the CIDEB gene using small interfering RNA.
The association was particularly strong among people with higher body mass indices and type 2 diabetes.
The associations with the rare protective CIDEB variants were consistent across ancestries, but people of non-European ancestry, who might be disproportionately affected by liver disease, were underrepresented in the database, the researchers note.
The study was supported by Regeneron Pharmaceuticals, which also employed several of the researchers.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Neighborhood factors contribute to liver cancer disparities in Texas
Factors operating at the community level may help explain disparities in rates of hepatocellular carcinoma (HCC) across Texas.
Researchers found that the risk for HCC is higher in neighborhoods characterized by minority populations, socioeconomic disadvantage, and blue-collar workers from specific industries.
However, these relationships are not uniform across the state, report researchers from Baylor College of Medicine, Houston.
“HCC is a serious health concern in Texas, and our foundational work is a step forward to better prevent this deadly disease,” study investigator Hashem El-Serag, MD, PhD, said in a news release.
The study was published online in Clinical Gastroenterology and Hepatology.
HCC is the most common type of liver cancer in the United States, and Texas has the highest rate of HCC. Yet, within Texas, incidence rates vary by race, ethnicity, and geographic location.
The Baylor team examined these disparities at the neighborhood level, with a focus on measures of social determinants of health and the industries where most neighborhood residents work.
They identified 11,547 Texas residents diagnosed with HCC between 2011 and 2015, at a mean age of 63 years. Roughly three-quarters were men, and 44% were non-Hispanic White, 14% non-Hispanic Black, 37% Hispanic, and 5% other.
The researchers used demographics, socioeconomic status, and employment provided by the U.S. Census Bureau to characterize the neighborhoods where these people lived when they were diagnosed with HCC.
Among their key findings, the risk for HCC among African American and Hispanic residents was highest in West Texas, South Texas, and the panhandle. However, some factors, like age and socioeconomic status, were not affected by location.
Across the entire state, however, people older than 60 years and those of low socioeconomic status had a higher relative risk for HCC.
Two areas of employment – construction and service occupations – also stood out as being associated with a higher risk for HCC, whereas employment in agriculture was associated with lower risk.
The authors caution that the ecological nature of the study precludes any firm conclusions regarding a causal link between working in these industries and HCC.
“Further research, including longitudinal studies, [is] needed to clarify the roles of specific occupations in HCC risk,” corresponding author Abiodun Oluyomi, PhD, said in the news release.
“Our findings validate factors previously associated with HCC, and our geographic analysis shows areas of Texas where specific intervention strategies may be most relevant,” Dr. Oluyomi added.
This research was supported by the Cancer Prevention & Research Institute of Texas. The authors have no relevant disclosures.
A version of this article first appeared on Medscape.com.
Factors operating at the community level may help explain disparities in rates of hepatocellular carcinoma (HCC) across Texas.
Researchers found that the risk for HCC is higher in neighborhoods characterized by minority populations, socioeconomic disadvantage, and blue-collar workers from specific industries.
However, these relationships are not uniform across the state, report researchers from Baylor College of Medicine, Houston.
“HCC is a serious health concern in Texas, and our foundational work is a step forward to better prevent this deadly disease,” study investigator Hashem El-Serag, MD, PhD, said in a news release.
The study was published online in Clinical Gastroenterology and Hepatology.
HCC is the most common type of liver cancer in the United States, and Texas has the highest rate of HCC. Yet, within Texas, incidence rates vary by race, ethnicity, and geographic location.
The Baylor team examined these disparities at the neighborhood level, with a focus on measures of social determinants of health and the industries where most neighborhood residents work.
They identified 11,547 Texas residents diagnosed with HCC between 2011 and 2015, at a mean age of 63 years. Roughly three-quarters were men, and 44% were non-Hispanic White, 14% non-Hispanic Black, 37% Hispanic, and 5% other.
The researchers used demographics, socioeconomic status, and employment provided by the U.S. Census Bureau to characterize the neighborhoods where these people lived when they were diagnosed with HCC.
Among their key findings, the risk for HCC among African American and Hispanic residents was highest in West Texas, South Texas, and the panhandle. However, some factors, like age and socioeconomic status, were not affected by location.
Across the entire state, however, people older than 60 years and those of low socioeconomic status had a higher relative risk for HCC.
Two areas of employment – construction and service occupations – also stood out as being associated with a higher risk for HCC, whereas employment in agriculture was associated with lower risk.
The authors caution that the ecological nature of the study precludes any firm conclusions regarding a causal link between working in these industries and HCC.
“Further research, including longitudinal studies, [is] needed to clarify the roles of specific occupations in HCC risk,” corresponding author Abiodun Oluyomi, PhD, said in the news release.
“Our findings validate factors previously associated with HCC, and our geographic analysis shows areas of Texas where specific intervention strategies may be most relevant,” Dr. Oluyomi added.
This research was supported by the Cancer Prevention & Research Institute of Texas. The authors have no relevant disclosures.
A version of this article first appeared on Medscape.com.
Factors operating at the community level may help explain disparities in rates of hepatocellular carcinoma (HCC) across Texas.
Researchers found that the risk for HCC is higher in neighborhoods characterized by minority populations, socioeconomic disadvantage, and blue-collar workers from specific industries.
However, these relationships are not uniform across the state, report researchers from Baylor College of Medicine, Houston.
“HCC is a serious health concern in Texas, and our foundational work is a step forward to better prevent this deadly disease,” study investigator Hashem El-Serag, MD, PhD, said in a news release.
The study was published online in Clinical Gastroenterology and Hepatology.
HCC is the most common type of liver cancer in the United States, and Texas has the highest rate of HCC. Yet, within Texas, incidence rates vary by race, ethnicity, and geographic location.
The Baylor team examined these disparities at the neighborhood level, with a focus on measures of social determinants of health and the industries where most neighborhood residents work.
They identified 11,547 Texas residents diagnosed with HCC between 2011 and 2015, at a mean age of 63 years. Roughly three-quarters were men, and 44% were non-Hispanic White, 14% non-Hispanic Black, 37% Hispanic, and 5% other.
The researchers used demographics, socioeconomic status, and employment provided by the U.S. Census Bureau to characterize the neighborhoods where these people lived when they were diagnosed with HCC.
Among their key findings, the risk for HCC among African American and Hispanic residents was highest in West Texas, South Texas, and the panhandle. However, some factors, like age and socioeconomic status, were not affected by location.
Across the entire state, however, people older than 60 years and those of low socioeconomic status had a higher relative risk for HCC.
Two areas of employment – construction and service occupations – also stood out as being associated with a higher risk for HCC, whereas employment in agriculture was associated with lower risk.
The authors caution that the ecological nature of the study precludes any firm conclusions regarding a causal link between working in these industries and HCC.
“Further research, including longitudinal studies, [is] needed to clarify the roles of specific occupations in HCC risk,” corresponding author Abiodun Oluyomi, PhD, said in the news release.
“Our findings validate factors previously associated with HCC, and our geographic analysis shows areas of Texas where specific intervention strategies may be most relevant,” Dr. Oluyomi added.
This research was supported by the Cancer Prevention & Research Institute of Texas. The authors have no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Degree of PPG reduction linked with ascites control after TIPS
A reduction in portal hepatic pressure gradient (PPG) soon after implantation of a transjugular intrahepatic portosystemic shunt (TIPS) greater than 60% was associated with improved ascites control at 6 weeks in a study published in Hepatology.
“The probability of ascites resolution is much higher if PPG reduction exceeded 60% of PPG before TIPS,” wrote the researchers, led by co–first authors Alexander Queck, MD, a postdoctoral researcher in the department of internal medicine at University Hospital Frankfurt (Germany) and Goethe University Frankfurt, and Louise Schwierz, MD, of the department of internal medicine in the University Hospital Bonn (Germany). “This study suggests that, even in patients with uncomplicated TIPS insertion, a short-term follow-up 6 weeks after TIPS should be scheduled to be able to predict their course of disease.”
The authors investigated the decrease of PPG in a single-center, retrospective analysis of 341 patients with liver cirrhosis undergoing TIPS insertion for recurrent or refractory ascites between March 1994 and July 2015. During each procedure, portal and inferior vena cava pressures were invasively measured and correlated with patients’ outcomes and ascites progression over time. In 241 patients, or 71%, chronic alcohol consumption was the reason for cirrhosis development, followed by 13% with chronic viral hepatitis (n = 43). Median survival after TIPS insertion was 102 weeks, and 19 patients received liver transplants over time.
Median portal pressure before TIPS placement was 28 mm Hg, which decreased to a median of 21 mm Hg after TIPS. Median PPG levels were 19 mm Hg before TIPS and 8 mm Hg immediately after TIPS placement.
At the time of TIPS placement, 65 patients, or 19%, had hepatic encephalopathy, and nine had severe hepatic encephalopathy. Six weeks after TIPS, two had episodes of hepatic encephalopathy.
After 6 weeks, ascites significantly improved through TIPS insertion. About 47% had a complete resolution of ascites at 6 weeks, whereas 29% had ascites detectable only by ultrasound and 24% of patients still needed large-volume paracentesis. There was an association between extent of PPG reduction and ascites resolution: Median PPG reduction was 55% of initial PPG in patients with persistence of severe ascites, 58% in patients with ascites detected by ultrasound, and 65% in patients with complete resolution of ascites at 6 weeks after TIPS.
Ascites resolved in 54% of patients with higher PPG reduction (60% or above), compared with 39% of patients with lower PPG reduction (below 60%). Ascites that was detected by ultrasound in another 27% of patients with higher PPG reduction, compared with 31% of patients with lower PPG reduction. In addition, persistent severe ascites was seen in 19% of patients with higher PPG reduction, compared with 30% of patients with lower PPG reduction.
The authors also noted the importance of timing follow-up evaluation: They noted that post-TIPS follow-up is a frequent question and not yet standardized; in this study, they found that, with follow-up at 6 weeks, they could “clearly stratify the course post TIPS” and this could “detect patients at high risk of unstable course of disease.”
PPG reduction of more than 60% after TIPS correlated with resolution of severe ascites 6 weeks after TIPS, the study authors concluded.
“This is one of the first studies that highlights the optimal goal for a portal pressure gradient in the setting of refractory ascites post TIPS procedure,” said Neeral Shah, MD, an associate professor of gastroenterology and hepatology and transplant hepatology specialist at the University of Virginia, Charlottesville.
“It is exciting to see some data from patients examining a question we have always thought to be true but have never quantified,” he said. “As a clinician who refers patients for TIPS, one of my biggest concerns is that significant shunting of blood past liver tissue through a TIPS can lead to the development of confusion.”
Dr. Shah, who wasn’t involved with the study, pointed to ongoing questions about hepatic encephalopathy around TIPS. The study authors didn’t find an issue with this among their study population, and some patients had improvements in their mental status after TIPS.
“This has not been my experience in those patients with hepatic encephalopathy at baseline pre-TIPS,” Dr. Shah said. “This point will need to be clarified further, especially if we are aiming for portal pressure gradients of 10 mm Hg or less in all patients with refractory ascites.”
The study authors declared that the research was conducted without commercial or financial relationships that could be construed as a potential conflict of interest. The authors were supported by the German Research Foundation, the German Federal Ministry of Education and Research, the European Union’s Horizon 2020 research program, and Goethe University Frankfurt. Dr. Shah reported no relevant disclosures.
A reduction in portal hepatic pressure gradient (PPG) soon after implantation of a transjugular intrahepatic portosystemic shunt (TIPS) greater than 60% was associated with improved ascites control at 6 weeks in a study published in Hepatology.
“The probability of ascites resolution is much higher if PPG reduction exceeded 60% of PPG before TIPS,” wrote the researchers, led by co–first authors Alexander Queck, MD, a postdoctoral researcher in the department of internal medicine at University Hospital Frankfurt (Germany) and Goethe University Frankfurt, and Louise Schwierz, MD, of the department of internal medicine in the University Hospital Bonn (Germany). “This study suggests that, even in patients with uncomplicated TIPS insertion, a short-term follow-up 6 weeks after TIPS should be scheduled to be able to predict their course of disease.”
The authors investigated the decrease of PPG in a single-center, retrospective analysis of 341 patients with liver cirrhosis undergoing TIPS insertion for recurrent or refractory ascites between March 1994 and July 2015. During each procedure, portal and inferior vena cava pressures were invasively measured and correlated with patients’ outcomes and ascites progression over time. In 241 patients, or 71%, chronic alcohol consumption was the reason for cirrhosis development, followed by 13% with chronic viral hepatitis (n = 43). Median survival after TIPS insertion was 102 weeks, and 19 patients received liver transplants over time.
Median portal pressure before TIPS placement was 28 mm Hg, which decreased to a median of 21 mm Hg after TIPS. Median PPG levels were 19 mm Hg before TIPS and 8 mm Hg immediately after TIPS placement.
At the time of TIPS placement, 65 patients, or 19%, had hepatic encephalopathy, and nine had severe hepatic encephalopathy. Six weeks after TIPS, two had episodes of hepatic encephalopathy.
After 6 weeks, ascites significantly improved through TIPS insertion. About 47% had a complete resolution of ascites at 6 weeks, whereas 29% had ascites detectable only by ultrasound and 24% of patients still needed large-volume paracentesis. There was an association between extent of PPG reduction and ascites resolution: Median PPG reduction was 55% of initial PPG in patients with persistence of severe ascites, 58% in patients with ascites detected by ultrasound, and 65% in patients with complete resolution of ascites at 6 weeks after TIPS.
Ascites resolved in 54% of patients with higher PPG reduction (60% or above), compared with 39% of patients with lower PPG reduction (below 60%). Ascites that was detected by ultrasound in another 27% of patients with higher PPG reduction, compared with 31% of patients with lower PPG reduction. In addition, persistent severe ascites was seen in 19% of patients with higher PPG reduction, compared with 30% of patients with lower PPG reduction.
The authors also noted the importance of timing follow-up evaluation: They noted that post-TIPS follow-up is a frequent question and not yet standardized; in this study, they found that, with follow-up at 6 weeks, they could “clearly stratify the course post TIPS” and this could “detect patients at high risk of unstable course of disease.”
PPG reduction of more than 60% after TIPS correlated with resolution of severe ascites 6 weeks after TIPS, the study authors concluded.
“This is one of the first studies that highlights the optimal goal for a portal pressure gradient in the setting of refractory ascites post TIPS procedure,” said Neeral Shah, MD, an associate professor of gastroenterology and hepatology and transplant hepatology specialist at the University of Virginia, Charlottesville.
“It is exciting to see some data from patients examining a question we have always thought to be true but have never quantified,” he said. “As a clinician who refers patients for TIPS, one of my biggest concerns is that significant shunting of blood past liver tissue through a TIPS can lead to the development of confusion.”
Dr. Shah, who wasn’t involved with the study, pointed to ongoing questions about hepatic encephalopathy around TIPS. The study authors didn’t find an issue with this among their study population, and some patients had improvements in their mental status after TIPS.
“This has not been my experience in those patients with hepatic encephalopathy at baseline pre-TIPS,” Dr. Shah said. “This point will need to be clarified further, especially if we are aiming for portal pressure gradients of 10 mm Hg or less in all patients with refractory ascites.”
The study authors declared that the research was conducted without commercial or financial relationships that could be construed as a potential conflict of interest. The authors were supported by the German Research Foundation, the German Federal Ministry of Education and Research, the European Union’s Horizon 2020 research program, and Goethe University Frankfurt. Dr. Shah reported no relevant disclosures.
A reduction in portal hepatic pressure gradient (PPG) soon after implantation of a transjugular intrahepatic portosystemic shunt (TIPS) greater than 60% was associated with improved ascites control at 6 weeks in a study published in Hepatology.
“The probability of ascites resolution is much higher if PPG reduction exceeded 60% of PPG before TIPS,” wrote the researchers, led by co–first authors Alexander Queck, MD, a postdoctoral researcher in the department of internal medicine at University Hospital Frankfurt (Germany) and Goethe University Frankfurt, and Louise Schwierz, MD, of the department of internal medicine in the University Hospital Bonn (Germany). “This study suggests that, even in patients with uncomplicated TIPS insertion, a short-term follow-up 6 weeks after TIPS should be scheduled to be able to predict their course of disease.”
The authors investigated the decrease of PPG in a single-center, retrospective analysis of 341 patients with liver cirrhosis undergoing TIPS insertion for recurrent or refractory ascites between March 1994 and July 2015. During each procedure, portal and inferior vena cava pressures were invasively measured and correlated with patients’ outcomes and ascites progression over time. In 241 patients, or 71%, chronic alcohol consumption was the reason for cirrhosis development, followed by 13% with chronic viral hepatitis (n = 43). Median survival after TIPS insertion was 102 weeks, and 19 patients received liver transplants over time.
Median portal pressure before TIPS placement was 28 mm Hg, which decreased to a median of 21 mm Hg after TIPS. Median PPG levels were 19 mm Hg before TIPS and 8 mm Hg immediately after TIPS placement.
At the time of TIPS placement, 65 patients, or 19%, had hepatic encephalopathy, and nine had severe hepatic encephalopathy. Six weeks after TIPS, two had episodes of hepatic encephalopathy.
After 6 weeks, ascites significantly improved through TIPS insertion. About 47% had a complete resolution of ascites at 6 weeks, whereas 29% had ascites detectable only by ultrasound and 24% of patients still needed large-volume paracentesis. There was an association between extent of PPG reduction and ascites resolution: Median PPG reduction was 55% of initial PPG in patients with persistence of severe ascites, 58% in patients with ascites detected by ultrasound, and 65% in patients with complete resolution of ascites at 6 weeks after TIPS.
Ascites resolved in 54% of patients with higher PPG reduction (60% or above), compared with 39% of patients with lower PPG reduction (below 60%). Ascites that was detected by ultrasound in another 27% of patients with higher PPG reduction, compared with 31% of patients with lower PPG reduction. In addition, persistent severe ascites was seen in 19% of patients with higher PPG reduction, compared with 30% of patients with lower PPG reduction.
The authors also noted the importance of timing follow-up evaluation: They noted that post-TIPS follow-up is a frequent question and not yet standardized; in this study, they found that, with follow-up at 6 weeks, they could “clearly stratify the course post TIPS” and this could “detect patients at high risk of unstable course of disease.”
PPG reduction of more than 60% after TIPS correlated with resolution of severe ascites 6 weeks after TIPS, the study authors concluded.
“This is one of the first studies that highlights the optimal goal for a portal pressure gradient in the setting of refractory ascites post TIPS procedure,” said Neeral Shah, MD, an associate professor of gastroenterology and hepatology and transplant hepatology specialist at the University of Virginia, Charlottesville.
“It is exciting to see some data from patients examining a question we have always thought to be true but have never quantified,” he said. “As a clinician who refers patients for TIPS, one of my biggest concerns is that significant shunting of blood past liver tissue through a TIPS can lead to the development of confusion.”
Dr. Shah, who wasn’t involved with the study, pointed to ongoing questions about hepatic encephalopathy around TIPS. The study authors didn’t find an issue with this among their study population, and some patients had improvements in their mental status after TIPS.
“This has not been my experience in those patients with hepatic encephalopathy at baseline pre-TIPS,” Dr. Shah said. “This point will need to be clarified further, especially if we are aiming for portal pressure gradients of 10 mm Hg or less in all patients with refractory ascites.”
The study authors declared that the research was conducted without commercial or financial relationships that could be construed as a potential conflict of interest. The authors were supported by the German Research Foundation, the German Federal Ministry of Education and Research, the European Union’s Horizon 2020 research program, and Goethe University Frankfurt. Dr. Shah reported no relevant disclosures.
FROM HEPATOLOGY
Ultrasound on par with CT for evaluating sarcopenia in patients with cirrhosis
Using ultrasound (US) to evaluate sarcopenic obesity in patients with cirrhosis may offer accuracy on par with computed tomography (CT), according to investigators.
US-based assessment presents a more affordable point-of-care strategy that limits radiation exposure, which enables sequential monitoring, reported lead author Sukhpal Dhariwal, MBBS, MD, of the Postgraduate Institute of Medical Education and Research, Chandigarh, India.
“Preliminary data in patients with liver disease ... suggest that US muscle assessment–derived indices, especially thigh muscle thickness, identify sarcopenia CT-skeletal muscle index (SMI) and also predict hospitalization and mortality,” the investigators wrote in Journal of Clinical Gastroenterology. “However, the applicability of US-based techniques to measure muscle mass in the high-risk group of patients with cirrhosis and sarcopenic obesity has not been evaluated.”
To address this knowledge gap, the investigators performed both US- and CT-based muscle assessments in 52 patients with obesity and evidence of cirrhosis; 40 patients were male and the mean age was 50.9 years. In all, 20 (38.5%) were diagnosed with sarcopenia based on CT-determined SMI scores of less than 39 cm2/m2 for women and 50 cm2/m2 for men.
US showed that it was similarly capable of categorizing patients. The modality significantly differentiated individuals with or without sarcopenia based on high area under the curve values in four muscle indices: quadriceps muscle thickness (0.98), quadriceps muscle feather index (0.95), forearm muscle thickness (0.85), and forearm feather index (0.80).
Direct comparison of US-based assessment against CT-based SMI revealed positive correlations, with significant r values ranging from 0.40 to 0.58. These correlations were stronger in a male-only subgroup analysis, in which r values ranged from 0.52 to 0.70. R values were not calculated in the female subgroup because of the small sample size (n = 12).
The investigators adjusted indices for height, which may pose bias for overestimating muscle mass. Another limitation is the small sample size.
“US-based assessment of sarcopenia has excellent diagnostic accuracy and correlates highly with cross-sectional imaging-based SMI in cirrhosis patients with sarcopenic obesity,” the investigators concluded. “US may serve as an easy-to-use, point-of-care tool for assessing sarcopenia in sarcopenic obesity with the advantage of repeated sequential assessment.”
According to Jamile Wakim-Fleming, MD, of the Cleveland Clinic, “US-based muscle mass assessment seems to be reliable, reproducible, and simple to perform and should be encouraged along with nutrition assessments in all patients with cirrhosis and obesity.”
In a written comment, Dr. Wakim-Fleming noted the importance of timely monitoring and intervention in this patient population.
“Considering the morbidity and the poor outcomes associated with sarcopenic obesity and its frequency in cirrhosis, it is important to make early diagnosis and institute a management plan to improve muscle mass and function,” she said.
The study was supported the Patient-Centered Outcomes Research Institute, the American Medical Association, and the American Heart Association. The investigators disclosed additional relationships with Pfizer, Bristol Myers Squibb, and Novartis. Dr. Wakim-Fleming reported no relevant conflicts of interest.
Using ultrasound (US) to evaluate sarcopenic obesity in patients with cirrhosis may offer accuracy on par with computed tomography (CT), according to investigators.
US-based assessment presents a more affordable point-of-care strategy that limits radiation exposure, which enables sequential monitoring, reported lead author Sukhpal Dhariwal, MBBS, MD, of the Postgraduate Institute of Medical Education and Research, Chandigarh, India.
“Preliminary data in patients with liver disease ... suggest that US muscle assessment–derived indices, especially thigh muscle thickness, identify sarcopenia CT-skeletal muscle index (SMI) and also predict hospitalization and mortality,” the investigators wrote in Journal of Clinical Gastroenterology. “However, the applicability of US-based techniques to measure muscle mass in the high-risk group of patients with cirrhosis and sarcopenic obesity has not been evaluated.”
To address this knowledge gap, the investigators performed both US- and CT-based muscle assessments in 52 patients with obesity and evidence of cirrhosis; 40 patients were male and the mean age was 50.9 years. In all, 20 (38.5%) were diagnosed with sarcopenia based on CT-determined SMI scores of less than 39 cm2/m2 for women and 50 cm2/m2 for men.
US showed that it was similarly capable of categorizing patients. The modality significantly differentiated individuals with or without sarcopenia based on high area under the curve values in four muscle indices: quadriceps muscle thickness (0.98), quadriceps muscle feather index (0.95), forearm muscle thickness (0.85), and forearm feather index (0.80).
Direct comparison of US-based assessment against CT-based SMI revealed positive correlations, with significant r values ranging from 0.40 to 0.58. These correlations were stronger in a male-only subgroup analysis, in which r values ranged from 0.52 to 0.70. R values were not calculated in the female subgroup because of the small sample size (n = 12).
The investigators adjusted indices for height, which may pose bias for overestimating muscle mass. Another limitation is the small sample size.
“US-based assessment of sarcopenia has excellent diagnostic accuracy and correlates highly with cross-sectional imaging-based SMI in cirrhosis patients with sarcopenic obesity,” the investigators concluded. “US may serve as an easy-to-use, point-of-care tool for assessing sarcopenia in sarcopenic obesity with the advantage of repeated sequential assessment.”
According to Jamile Wakim-Fleming, MD, of the Cleveland Clinic, “US-based muscle mass assessment seems to be reliable, reproducible, and simple to perform and should be encouraged along with nutrition assessments in all patients with cirrhosis and obesity.”
In a written comment, Dr. Wakim-Fleming noted the importance of timely monitoring and intervention in this patient population.
“Considering the morbidity and the poor outcomes associated with sarcopenic obesity and its frequency in cirrhosis, it is important to make early diagnosis and institute a management plan to improve muscle mass and function,” she said.
The study was supported the Patient-Centered Outcomes Research Institute, the American Medical Association, and the American Heart Association. The investigators disclosed additional relationships with Pfizer, Bristol Myers Squibb, and Novartis. Dr. Wakim-Fleming reported no relevant conflicts of interest.
Using ultrasound (US) to evaluate sarcopenic obesity in patients with cirrhosis may offer accuracy on par with computed tomography (CT), according to investigators.
US-based assessment presents a more affordable point-of-care strategy that limits radiation exposure, which enables sequential monitoring, reported lead author Sukhpal Dhariwal, MBBS, MD, of the Postgraduate Institute of Medical Education and Research, Chandigarh, India.
“Preliminary data in patients with liver disease ... suggest that US muscle assessment–derived indices, especially thigh muscle thickness, identify sarcopenia CT-skeletal muscle index (SMI) and also predict hospitalization and mortality,” the investigators wrote in Journal of Clinical Gastroenterology. “However, the applicability of US-based techniques to measure muscle mass in the high-risk group of patients with cirrhosis and sarcopenic obesity has not been evaluated.”
To address this knowledge gap, the investigators performed both US- and CT-based muscle assessments in 52 patients with obesity and evidence of cirrhosis; 40 patients were male and the mean age was 50.9 years. In all, 20 (38.5%) were diagnosed with sarcopenia based on CT-determined SMI scores of less than 39 cm2/m2 for women and 50 cm2/m2 for men.
US showed that it was similarly capable of categorizing patients. The modality significantly differentiated individuals with or without sarcopenia based on high area under the curve values in four muscle indices: quadriceps muscle thickness (0.98), quadriceps muscle feather index (0.95), forearm muscle thickness (0.85), and forearm feather index (0.80).
Direct comparison of US-based assessment against CT-based SMI revealed positive correlations, with significant r values ranging from 0.40 to 0.58. These correlations were stronger in a male-only subgroup analysis, in which r values ranged from 0.52 to 0.70. R values were not calculated in the female subgroup because of the small sample size (n = 12).
The investigators adjusted indices for height, which may pose bias for overestimating muscle mass. Another limitation is the small sample size.
“US-based assessment of sarcopenia has excellent diagnostic accuracy and correlates highly with cross-sectional imaging-based SMI in cirrhosis patients with sarcopenic obesity,” the investigators concluded. “US may serve as an easy-to-use, point-of-care tool for assessing sarcopenia in sarcopenic obesity with the advantage of repeated sequential assessment.”
According to Jamile Wakim-Fleming, MD, of the Cleveland Clinic, “US-based muscle mass assessment seems to be reliable, reproducible, and simple to perform and should be encouraged along with nutrition assessments in all patients with cirrhosis and obesity.”
In a written comment, Dr. Wakim-Fleming noted the importance of timely monitoring and intervention in this patient population.
“Considering the morbidity and the poor outcomes associated with sarcopenic obesity and its frequency in cirrhosis, it is important to make early diagnosis and institute a management plan to improve muscle mass and function,” she said.
The study was supported the Patient-Centered Outcomes Research Institute, the American Medical Association, and the American Heart Association. The investigators disclosed additional relationships with Pfizer, Bristol Myers Squibb, and Novartis. Dr. Wakim-Fleming reported no relevant conflicts of interest.
FROM JAMA INTERNAL MEDICINE
Liver protein protects against parenteral nutrition liver injury
Hepatic protein PP2A-C-alpha may serve as a protective factor against parenteral nutrition–associated hepatic steatosis by improving liver function, according to a recent study published in Cellular and Molecular Gastroenterology and Hepatology.
Parenteral nutrition–associated hepatic steatosis likely involves the down-regulation of hepatic PP2A-C-alpha and consequent increased phosphorylation of Akt2; this in turn alters hepatic lipid metabolism, promotes triglyceride accumulation, and leads to liver injury, wrote the researchers, led by Gulisudumu Maitiabula and Feng Tian of the Research Institute of General Surgery at Jinling Hospital, Nanjing, China, and the Medical School of Nanjing University.
“Our study provides a strong rationale that PP2A-C-alpha may be involved in the pathogenesis of [parenteral nutrition–associated hepatic steatosis],” they wrote. “Further research is merited to establish whether interventions to enhance PP2A function might suppress the development of hepatic steatosis in patients receiving long-term [parenteral nutrition].”
Parenteral nutrition can be a lifesaving therapy for patients with intestinal failure caused by insufficient bowel length or function, the authors noted However, long-term use can lead to potentially fatal complications such as liver disease, but an understanding of the pathological mechanisms behind parenteral nutrition–associated hepatic steatosis limited.
The research team performed comparative proteomic/phosphoproteomic analyses of liver samples from 10 patients with parenteral nutrition–associated hepatic steatosis, as well as 8 cholelithiasis patients as controls, who were admitted to Jinling Hospital between June 2018 and June 2019. The researchers also assessed the effect of PP2A-C-alpha on liver injury from total parenteral nutrition in mice.
The research team found that PP2A-C-alpha was down-regulated in patients and mice with parenteral nutrition–associated hepatic steatosis. In addition, in patients with parenteral nutrition–associated hepatic steatosis, they found enhanced activation of serine/threonine kinase Akt2 and decreased activation of AMPK.
Mice that were given total parenteral nutrition infusion for 14 days developed hepatic steatosis, down-regulation of PP2A-C-alpha, activation of Akt2, and inhibition of AMPK. Hepatocyte-specific deletion of PP2A-C-alpha in mice given parenteral nutrition exacerbated the Akt2 activation, AMPK inhibition, and hepatic steatosis through an effect on fatty acid degradation.
On the other hand, forced expression of PP2A-C-alpha led to reductions in hepatocyte fat deposition and the pathological score for liver steatosis. Overexpression also significantly improved hepatic steatosis, suppressed Akt2, and activated AMPK. In addition, pharmacological activation of Akt2 in mice overexpressing PP2A-C-alpha led to the aggravation of hepatic steatosis.
“Collectively, these observations suggest that [parenteral nutrition] for [more than] 14 days leads to a down-regulation in PP2A-C-alpha expression that activates Akt2-dependent signaling, which would likely lead to hepatic steatosis,” the study authors wrote.
Intervention trials of PP2A-C-alpha in humans have not been performed because PP2A-C-alpha activators or effector analogs were unavailable for clinical use, they wrote. Additional clinical studies are needed to investigate the effects of PP2A-C-alpha intervention on the development of hepatic steatosis in patients receiving long-term parenteral nutrition.
The study was supported by the National Natural Science Foundation of China, the Science Foundation of Outstanding Youth in Jiangsu Province, the National Science and Technology Research Funding for Public Welfare Medical Projects, “The 13th Five-Year Plan” Foundation of Jiangsu Province for Medical Key Talents, and the Natural Science Foundation of Jiangsu Province. The study authors disclosed no conflicts of interest.
New findings may lead to novel treatments
Parenteral nutrition is a life saver for children and adults with insufficient absorptive capacity of the gastrointestinal tract. Unfortunately, up to two-thirds of patients requiring parenteral nutrition long-term develop liver disease, which can have fatal outcomes. Parenteral nutrition–associated liver disease is characterized by fibrosis and steatosis. While portal inflammation and cholestasis resolve in patients who can be weaned off parenteral nutrition, portal fibrosis and steatosis unfortunately remain in about half of the patients. The development of therapeutic strategies for this condition has thus far been hampered by the fact that the molecular mechanism of parenteral nutrition–associated liver disease was unknown.
This study by Maitiabua and colleagues from Nanjing University Medical School addresses this problem by performing a proteomic and, importantly, phospho-proteomic analysis of liver biopsies from adults treated with parenteral nutrition compared to normally-feeding controls. They discovered that levels of phosphorylated AKT2, the key signaling mediator of insulin in the liver, are increased, while protein levels of the opposing protein phosphatase 2A (PP2A) are decreased in patients receiving parenteral nutrition.
Remarkably, they could reproduce these same pathway changes in a mouse model of parenteral nutrition, which again led to a chronic activation of the insulin signaling pathway, culminating in the phosphorylation of AKT2. They show further that activation of AKT2 inhibits AMPK and alters hepatic lipid metabolism to promote triglyceride accumulation. Using the experimentally tractable mouse model, they demonstrate further that the ablation of a PP2A isoform in the liver is sufficient to cause lipid accumulation and liver injury. Conversely, restoring PP2A expression improved the hepatic phenotype in mice in the parenteral nutrition model. These findings could also be mimicked using pharmacological activation and inhibition of PP2A.
In sum, this experimental study could some day lead the way to novel treatments of parenteral nutrition-induced liver disease through the use of PP2A activators.
Klaus H. Kaestner, PhD, is with the department of genetics and Center for Molecular Studies in Digestive and Liver Diseases, Perelman School of Medicine,University of Pennsylvania, Philadelphia.
New findings may lead to novel treatments
Parenteral nutrition is a life saver for children and adults with insufficient absorptive capacity of the gastrointestinal tract. Unfortunately, up to two-thirds of patients requiring parenteral nutrition long-term develop liver disease, which can have fatal outcomes. Parenteral nutrition–associated liver disease is characterized by fibrosis and steatosis. While portal inflammation and cholestasis resolve in patients who can be weaned off parenteral nutrition, portal fibrosis and steatosis unfortunately remain in about half of the patients. The development of therapeutic strategies for this condition has thus far been hampered by the fact that the molecular mechanism of parenteral nutrition–associated liver disease was unknown.
This study by Maitiabua and colleagues from Nanjing University Medical School addresses this problem by performing a proteomic and, importantly, phospho-proteomic analysis of liver biopsies from adults treated with parenteral nutrition compared to normally-feeding controls. They discovered that levels of phosphorylated AKT2, the key signaling mediator of insulin in the liver, are increased, while protein levels of the opposing protein phosphatase 2A (PP2A) are decreased in patients receiving parenteral nutrition.
Remarkably, they could reproduce these same pathway changes in a mouse model of parenteral nutrition, which again led to a chronic activation of the insulin signaling pathway, culminating in the phosphorylation of AKT2. They show further that activation of AKT2 inhibits AMPK and alters hepatic lipid metabolism to promote triglyceride accumulation. Using the experimentally tractable mouse model, they demonstrate further that the ablation of a PP2A isoform in the liver is sufficient to cause lipid accumulation and liver injury. Conversely, restoring PP2A expression improved the hepatic phenotype in mice in the parenteral nutrition model. These findings could also be mimicked using pharmacological activation and inhibition of PP2A.
In sum, this experimental study could some day lead the way to novel treatments of parenteral nutrition-induced liver disease through the use of PP2A activators.
Klaus H. Kaestner, PhD, is with the department of genetics and Center for Molecular Studies in Digestive and Liver Diseases, Perelman School of Medicine,University of Pennsylvania, Philadelphia.
New findings may lead to novel treatments
Parenteral nutrition is a life saver for children and adults with insufficient absorptive capacity of the gastrointestinal tract. Unfortunately, up to two-thirds of patients requiring parenteral nutrition long-term develop liver disease, which can have fatal outcomes. Parenteral nutrition–associated liver disease is characterized by fibrosis and steatosis. While portal inflammation and cholestasis resolve in patients who can be weaned off parenteral nutrition, portal fibrosis and steatosis unfortunately remain in about half of the patients. The development of therapeutic strategies for this condition has thus far been hampered by the fact that the molecular mechanism of parenteral nutrition–associated liver disease was unknown.
This study by Maitiabua and colleagues from Nanjing University Medical School addresses this problem by performing a proteomic and, importantly, phospho-proteomic analysis of liver biopsies from adults treated with parenteral nutrition compared to normally-feeding controls. They discovered that levels of phosphorylated AKT2, the key signaling mediator of insulin in the liver, are increased, while protein levels of the opposing protein phosphatase 2A (PP2A) are decreased in patients receiving parenteral nutrition.
Remarkably, they could reproduce these same pathway changes in a mouse model of parenteral nutrition, which again led to a chronic activation of the insulin signaling pathway, culminating in the phosphorylation of AKT2. They show further that activation of AKT2 inhibits AMPK and alters hepatic lipid metabolism to promote triglyceride accumulation. Using the experimentally tractable mouse model, they demonstrate further that the ablation of a PP2A isoform in the liver is sufficient to cause lipid accumulation and liver injury. Conversely, restoring PP2A expression improved the hepatic phenotype in mice in the parenteral nutrition model. These findings could also be mimicked using pharmacological activation and inhibition of PP2A.
In sum, this experimental study could some day lead the way to novel treatments of parenteral nutrition-induced liver disease through the use of PP2A activators.
Klaus H. Kaestner, PhD, is with the department of genetics and Center for Molecular Studies in Digestive and Liver Diseases, Perelman School of Medicine,University of Pennsylvania, Philadelphia.
Hepatic protein PP2A-C-alpha may serve as a protective factor against parenteral nutrition–associated hepatic steatosis by improving liver function, according to a recent study published in Cellular and Molecular Gastroenterology and Hepatology.
Parenteral nutrition–associated hepatic steatosis likely involves the down-regulation of hepatic PP2A-C-alpha and consequent increased phosphorylation of Akt2; this in turn alters hepatic lipid metabolism, promotes triglyceride accumulation, and leads to liver injury, wrote the researchers, led by Gulisudumu Maitiabula and Feng Tian of the Research Institute of General Surgery at Jinling Hospital, Nanjing, China, and the Medical School of Nanjing University.
“Our study provides a strong rationale that PP2A-C-alpha may be involved in the pathogenesis of [parenteral nutrition–associated hepatic steatosis],” they wrote. “Further research is merited to establish whether interventions to enhance PP2A function might suppress the development of hepatic steatosis in patients receiving long-term [parenteral nutrition].”
Parenteral nutrition can be a lifesaving therapy for patients with intestinal failure caused by insufficient bowel length or function, the authors noted However, long-term use can lead to potentially fatal complications such as liver disease, but an understanding of the pathological mechanisms behind parenteral nutrition–associated hepatic steatosis limited.
The research team performed comparative proteomic/phosphoproteomic analyses of liver samples from 10 patients with parenteral nutrition–associated hepatic steatosis, as well as 8 cholelithiasis patients as controls, who were admitted to Jinling Hospital between June 2018 and June 2019. The researchers also assessed the effect of PP2A-C-alpha on liver injury from total parenteral nutrition in mice.
The research team found that PP2A-C-alpha was down-regulated in patients and mice with parenteral nutrition–associated hepatic steatosis. In addition, in patients with parenteral nutrition–associated hepatic steatosis, they found enhanced activation of serine/threonine kinase Akt2 and decreased activation of AMPK.
Mice that were given total parenteral nutrition infusion for 14 days developed hepatic steatosis, down-regulation of PP2A-C-alpha, activation of Akt2, and inhibition of AMPK. Hepatocyte-specific deletion of PP2A-C-alpha in mice given parenteral nutrition exacerbated the Akt2 activation, AMPK inhibition, and hepatic steatosis through an effect on fatty acid degradation.
On the other hand, forced expression of PP2A-C-alpha led to reductions in hepatocyte fat deposition and the pathological score for liver steatosis. Overexpression also significantly improved hepatic steatosis, suppressed Akt2, and activated AMPK. In addition, pharmacological activation of Akt2 in mice overexpressing PP2A-C-alpha led to the aggravation of hepatic steatosis.
“Collectively, these observations suggest that [parenteral nutrition] for [more than] 14 days leads to a down-regulation in PP2A-C-alpha expression that activates Akt2-dependent signaling, which would likely lead to hepatic steatosis,” the study authors wrote.
Intervention trials of PP2A-C-alpha in humans have not been performed because PP2A-C-alpha activators or effector analogs were unavailable for clinical use, they wrote. Additional clinical studies are needed to investigate the effects of PP2A-C-alpha intervention on the development of hepatic steatosis in patients receiving long-term parenteral nutrition.
The study was supported by the National Natural Science Foundation of China, the Science Foundation of Outstanding Youth in Jiangsu Province, the National Science and Technology Research Funding for Public Welfare Medical Projects, “The 13th Five-Year Plan” Foundation of Jiangsu Province for Medical Key Talents, and the Natural Science Foundation of Jiangsu Province. The study authors disclosed no conflicts of interest.
Hepatic protein PP2A-C-alpha may serve as a protective factor against parenteral nutrition–associated hepatic steatosis by improving liver function, according to a recent study published in Cellular and Molecular Gastroenterology and Hepatology.
Parenteral nutrition–associated hepatic steatosis likely involves the down-regulation of hepatic PP2A-C-alpha and consequent increased phosphorylation of Akt2; this in turn alters hepatic lipid metabolism, promotes triglyceride accumulation, and leads to liver injury, wrote the researchers, led by Gulisudumu Maitiabula and Feng Tian of the Research Institute of General Surgery at Jinling Hospital, Nanjing, China, and the Medical School of Nanjing University.
“Our study provides a strong rationale that PP2A-C-alpha may be involved in the pathogenesis of [parenteral nutrition–associated hepatic steatosis],” they wrote. “Further research is merited to establish whether interventions to enhance PP2A function might suppress the development of hepatic steatosis in patients receiving long-term [parenteral nutrition].”
Parenteral nutrition can be a lifesaving therapy for patients with intestinal failure caused by insufficient bowel length or function, the authors noted However, long-term use can lead to potentially fatal complications such as liver disease, but an understanding of the pathological mechanisms behind parenteral nutrition–associated hepatic steatosis limited.
The research team performed comparative proteomic/phosphoproteomic analyses of liver samples from 10 patients with parenteral nutrition–associated hepatic steatosis, as well as 8 cholelithiasis patients as controls, who were admitted to Jinling Hospital between June 2018 and June 2019. The researchers also assessed the effect of PP2A-C-alpha on liver injury from total parenteral nutrition in mice.
The research team found that PP2A-C-alpha was down-regulated in patients and mice with parenteral nutrition–associated hepatic steatosis. In addition, in patients with parenteral nutrition–associated hepatic steatosis, they found enhanced activation of serine/threonine kinase Akt2 and decreased activation of AMPK.
Mice that were given total parenteral nutrition infusion for 14 days developed hepatic steatosis, down-regulation of PP2A-C-alpha, activation of Akt2, and inhibition of AMPK. Hepatocyte-specific deletion of PP2A-C-alpha in mice given parenteral nutrition exacerbated the Akt2 activation, AMPK inhibition, and hepatic steatosis through an effect on fatty acid degradation.
On the other hand, forced expression of PP2A-C-alpha led to reductions in hepatocyte fat deposition and the pathological score for liver steatosis. Overexpression also significantly improved hepatic steatosis, suppressed Akt2, and activated AMPK. In addition, pharmacological activation of Akt2 in mice overexpressing PP2A-C-alpha led to the aggravation of hepatic steatosis.
“Collectively, these observations suggest that [parenteral nutrition] for [more than] 14 days leads to a down-regulation in PP2A-C-alpha expression that activates Akt2-dependent signaling, which would likely lead to hepatic steatosis,” the study authors wrote.
Intervention trials of PP2A-C-alpha in humans have not been performed because PP2A-C-alpha activators or effector analogs were unavailable for clinical use, they wrote. Additional clinical studies are needed to investigate the effects of PP2A-C-alpha intervention on the development of hepatic steatosis in patients receiving long-term parenteral nutrition.
The study was supported by the National Natural Science Foundation of China, the Science Foundation of Outstanding Youth in Jiangsu Province, the National Science and Technology Research Funding for Public Welfare Medical Projects, “The 13th Five-Year Plan” Foundation of Jiangsu Province for Medical Key Talents, and the Natural Science Foundation of Jiangsu Province. The study authors disclosed no conflicts of interest.
FROM CELLULAR AND MOLECULAR GASTROENTEROLOGY AND HEPATOLOGY
Model may predict age-related mortality after TIPS implantation
Mortality after implantation of a transjugular intrahepatic portosystemic shunt (TIPS) is increased for patients aged 70 and older with cirrhosis, but creatinine and sodium levels can help with decision-making, according to a study published in Hepatology.
TIPS can improve survival in cirrhotic patients with refractory ascites or portal hypertensive bleeding, and age alone shouldn’t preclude older patients from receiving TIPS, wrote the researchers led by Francesco Vizzutti, MD, of the department of experimental and clinical medicine at the University of Florence in Italy.
“However, the indication for TIPS in older adult patients (70 years and over) is debated, and a specific prediction model developed in this particular setting is lacking,” they wrote.
Dr. Vizzutti and colleagues aimed to develop and validate a multivariable model to accurately predict mortality in older adults. They prospectively enrolled 411 patients at four Italian referral centers with de novo TIPS implantation for refractory ascites or secondary prophylaxis of variceal bleeding between October 2020 and March 2021.
All patients underwent TIPS placement using Viatorr-covered stent grafts. All patients had follow-up outpatient appointments every 6 months until the end of the study or when clinically indicated, such as recurrence of portal hypertension complications or TIPS dysfunction.
The research team created a competing risks model to predict liver-related mortality attributable to liver failure, portal hypertensive bleeding, hepatorenal syndrome, or hepatocellular carcinoma, with orthotopic liver transplant and death from extrahepatic causes considered as competing events. In older adults, the only competing event was death from extrahepatic causes because this age group could not receive orthotopic liver transplant.
Alcohol use disorder was the most common etiology at 37%, followed by viral infection at 30%. At the time of TIPS placement, alcohol use disorder was present as a main or concomitant etiology of liver disease in 181 patients, including 36 with active alcohol consumption.
Compared with younger patients, older adults had significantly higher prevalence of viral etiology (at 41%) and lower prevalence of alcohol use disorder (at 18%). In terms of liver function, older adults had significantly less advanced liver disease based on international normalized ratio levels, likely “reflecting a more careful selection by physicians when managing older adults,” the study authors wrote. However, older adults had significantly higher creatinine levels than younger patients, “underlining the importance of the assessment of kidney function when selecting patients for TIPS placement,” the authors wrote.
During a median follow-up time of about 20 months after TIPS placement, 99 of 411 (or 24%) of patients died of liver-related causes, 49 underwent a transplant, and 17 died of extrahepatic causes. Among the 99 older adults, 44 (or 44%) died of liver-related causes, and 7 patients died of extrahepatic causes.
In the overall cohort, the probabilities of liver-related death were 13% after 1 year, 17% after 2 years, and 24% after 3 years. The probabilities were higher in older adults, at 19% after 1 year, 30% after 2 years, and 41% after 3 years.
According to the model, age, alcoholic etiology, creatinine levels, and international normalized ratio levels were independently associated with a higher risk of liver-related death. In older adults, creatinine and sodium levels were the only independent risk factors for death.
Notably, older adult patients with favorable creatinine and sodium levels (1.2 mg/dL and 140 mEq/L, respectively) had survival probabilities of liver-related death at 1, 2, and 3 years from TIPS placement of 14%, 26%, and 34%, respectively, the authors wrote. In contrast, older adults with creatinine levels of 2.5 mg/dL and sodium levels of 130 mEq/L had worse outcomes, with risks of liver-related death of 71%, 92%, and 96%, respectively.
“These results suggest that older adult patients with preserved renal function and normal sodium levels could obtain a survival outcome after TIPS placement similar to younger patients,” they wrote. “Moreover, the occurrence of [hepatic encephalopathy] and/or recurrence of ascites or bleeding was not significantly different comparing the two groups of patients according to age.”
Future research should update the prediction model with larger sample sizes, the study authors wrote.
“The decision for or against TIPS should be made only after carefully weighing the risks and benefits, taking into consideration the available literature,” said Bubu Banini, MD, PhD, an assistant professor of digestive diseases and translational research director of the Metabolic Health and Weight Loss Program at Yale University, New Haven, Conn.
Dr. Banini, who wasn’t involved with the study, said the prediction model could be a useful tool to guide the decision-making process.
“As is usually the case with management of portal hypertension–related complications, a multidisciplinary discussion with evaluation of a multitude of factors, including quality of life, comorbidities, risks, and benefits, should guide decision-making,” she said.
Dr. Banini highlighted the finding that alcohol etiology for cirrhosis was associated with higher mortality compared with viral etiology.
“This is important in the context of unfortunately increasing trends in alcohol consumption in the pre- and peri-COVID era and the increased prevalence of alcohol-associated liver disease, especially in women,” she said.
The study was supported by grants from the University of Florence and the University of Modena and Reggio Emilia. The study authors have received lecture fees from Gore Medical, which creates stent grafts. Dr. Banini reported no relevant disclosures.
Mortality after implantation of a transjugular intrahepatic portosystemic shunt (TIPS) is increased for patients aged 70 and older with cirrhosis, but creatinine and sodium levels can help with decision-making, according to a study published in Hepatology.
TIPS can improve survival in cirrhotic patients with refractory ascites or portal hypertensive bleeding, and age alone shouldn’t preclude older patients from receiving TIPS, wrote the researchers led by Francesco Vizzutti, MD, of the department of experimental and clinical medicine at the University of Florence in Italy.
“However, the indication for TIPS in older adult patients (70 years and over) is debated, and a specific prediction model developed in this particular setting is lacking,” they wrote.
Dr. Vizzutti and colleagues aimed to develop and validate a multivariable model to accurately predict mortality in older adults. They prospectively enrolled 411 patients at four Italian referral centers with de novo TIPS implantation for refractory ascites or secondary prophylaxis of variceal bleeding between October 2020 and March 2021.
All patients underwent TIPS placement using Viatorr-covered stent grafts. All patients had follow-up outpatient appointments every 6 months until the end of the study or when clinically indicated, such as recurrence of portal hypertension complications or TIPS dysfunction.
The research team created a competing risks model to predict liver-related mortality attributable to liver failure, portal hypertensive bleeding, hepatorenal syndrome, or hepatocellular carcinoma, with orthotopic liver transplant and death from extrahepatic causes considered as competing events. In older adults, the only competing event was death from extrahepatic causes because this age group could not receive orthotopic liver transplant.
Alcohol use disorder was the most common etiology at 37%, followed by viral infection at 30%. At the time of TIPS placement, alcohol use disorder was present as a main or concomitant etiology of liver disease in 181 patients, including 36 with active alcohol consumption.
Compared with younger patients, older adults had significantly higher prevalence of viral etiology (at 41%) and lower prevalence of alcohol use disorder (at 18%). In terms of liver function, older adults had significantly less advanced liver disease based on international normalized ratio levels, likely “reflecting a more careful selection by physicians when managing older adults,” the study authors wrote. However, older adults had significantly higher creatinine levels than younger patients, “underlining the importance of the assessment of kidney function when selecting patients for TIPS placement,” the authors wrote.
During a median follow-up time of about 20 months after TIPS placement, 99 of 411 (or 24%) of patients died of liver-related causes, 49 underwent a transplant, and 17 died of extrahepatic causes. Among the 99 older adults, 44 (or 44%) died of liver-related causes, and 7 patients died of extrahepatic causes.
In the overall cohort, the probabilities of liver-related death were 13% after 1 year, 17% after 2 years, and 24% after 3 years. The probabilities were higher in older adults, at 19% after 1 year, 30% after 2 years, and 41% after 3 years.
According to the model, age, alcoholic etiology, creatinine levels, and international normalized ratio levels were independently associated with a higher risk of liver-related death. In older adults, creatinine and sodium levels were the only independent risk factors for death.
Notably, older adult patients with favorable creatinine and sodium levels (1.2 mg/dL and 140 mEq/L, respectively) had survival probabilities of liver-related death at 1, 2, and 3 years from TIPS placement of 14%, 26%, and 34%, respectively, the authors wrote. In contrast, older adults with creatinine levels of 2.5 mg/dL and sodium levels of 130 mEq/L had worse outcomes, with risks of liver-related death of 71%, 92%, and 96%, respectively.
“These results suggest that older adult patients with preserved renal function and normal sodium levels could obtain a survival outcome after TIPS placement similar to younger patients,” they wrote. “Moreover, the occurrence of [hepatic encephalopathy] and/or recurrence of ascites or bleeding was not significantly different comparing the two groups of patients according to age.”
Future research should update the prediction model with larger sample sizes, the study authors wrote.
“The decision for or against TIPS should be made only after carefully weighing the risks and benefits, taking into consideration the available literature,” said Bubu Banini, MD, PhD, an assistant professor of digestive diseases and translational research director of the Metabolic Health and Weight Loss Program at Yale University, New Haven, Conn.
Dr. Banini, who wasn’t involved with the study, said the prediction model could be a useful tool to guide the decision-making process.
“As is usually the case with management of portal hypertension–related complications, a multidisciplinary discussion with evaluation of a multitude of factors, including quality of life, comorbidities, risks, and benefits, should guide decision-making,” she said.
Dr. Banini highlighted the finding that alcohol etiology for cirrhosis was associated with higher mortality compared with viral etiology.
“This is important in the context of unfortunately increasing trends in alcohol consumption in the pre- and peri-COVID era and the increased prevalence of alcohol-associated liver disease, especially in women,” she said.
The study was supported by grants from the University of Florence and the University of Modena and Reggio Emilia. The study authors have received lecture fees from Gore Medical, which creates stent grafts. Dr. Banini reported no relevant disclosures.
Mortality after implantation of a transjugular intrahepatic portosystemic shunt (TIPS) is increased for patients aged 70 and older with cirrhosis, but creatinine and sodium levels can help with decision-making, according to a study published in Hepatology.
TIPS can improve survival in cirrhotic patients with refractory ascites or portal hypertensive bleeding, and age alone shouldn’t preclude older patients from receiving TIPS, wrote the researchers led by Francesco Vizzutti, MD, of the department of experimental and clinical medicine at the University of Florence in Italy.
“However, the indication for TIPS in older adult patients (70 years and over) is debated, and a specific prediction model developed in this particular setting is lacking,” they wrote.
Dr. Vizzutti and colleagues aimed to develop and validate a multivariable model to accurately predict mortality in older adults. They prospectively enrolled 411 patients at four Italian referral centers with de novo TIPS implantation for refractory ascites or secondary prophylaxis of variceal bleeding between October 2020 and March 2021.
All patients underwent TIPS placement using Viatorr-covered stent grafts. All patients had follow-up outpatient appointments every 6 months until the end of the study or when clinically indicated, such as recurrence of portal hypertension complications or TIPS dysfunction.
The research team created a competing risks model to predict liver-related mortality attributable to liver failure, portal hypertensive bleeding, hepatorenal syndrome, or hepatocellular carcinoma, with orthotopic liver transplant and death from extrahepatic causes considered as competing events. In older adults, the only competing event was death from extrahepatic causes because this age group could not receive orthotopic liver transplant.
Alcohol use disorder was the most common etiology at 37%, followed by viral infection at 30%. At the time of TIPS placement, alcohol use disorder was present as a main or concomitant etiology of liver disease in 181 patients, including 36 with active alcohol consumption.
Compared with younger patients, older adults had significantly higher prevalence of viral etiology (at 41%) and lower prevalence of alcohol use disorder (at 18%). In terms of liver function, older adults had significantly less advanced liver disease based on international normalized ratio levels, likely “reflecting a more careful selection by physicians when managing older adults,” the study authors wrote. However, older adults had significantly higher creatinine levels than younger patients, “underlining the importance of the assessment of kidney function when selecting patients for TIPS placement,” the authors wrote.
During a median follow-up time of about 20 months after TIPS placement, 99 of 411 (or 24%) of patients died of liver-related causes, 49 underwent a transplant, and 17 died of extrahepatic causes. Among the 99 older adults, 44 (or 44%) died of liver-related causes, and 7 patients died of extrahepatic causes.
In the overall cohort, the probabilities of liver-related death were 13% after 1 year, 17% after 2 years, and 24% after 3 years. The probabilities were higher in older adults, at 19% after 1 year, 30% after 2 years, and 41% after 3 years.
According to the model, age, alcoholic etiology, creatinine levels, and international normalized ratio levels were independently associated with a higher risk of liver-related death. In older adults, creatinine and sodium levels were the only independent risk factors for death.
Notably, older adult patients with favorable creatinine and sodium levels (1.2 mg/dL and 140 mEq/L, respectively) had survival probabilities of liver-related death at 1, 2, and 3 years from TIPS placement of 14%, 26%, and 34%, respectively, the authors wrote. In contrast, older adults with creatinine levels of 2.5 mg/dL and sodium levels of 130 mEq/L had worse outcomes, with risks of liver-related death of 71%, 92%, and 96%, respectively.
“These results suggest that older adult patients with preserved renal function and normal sodium levels could obtain a survival outcome after TIPS placement similar to younger patients,” they wrote. “Moreover, the occurrence of [hepatic encephalopathy] and/or recurrence of ascites or bleeding was not significantly different comparing the two groups of patients according to age.”
Future research should update the prediction model with larger sample sizes, the study authors wrote.
“The decision for or against TIPS should be made only after carefully weighing the risks and benefits, taking into consideration the available literature,” said Bubu Banini, MD, PhD, an assistant professor of digestive diseases and translational research director of the Metabolic Health and Weight Loss Program at Yale University, New Haven, Conn.
Dr. Banini, who wasn’t involved with the study, said the prediction model could be a useful tool to guide the decision-making process.
“As is usually the case with management of portal hypertension–related complications, a multidisciplinary discussion with evaluation of a multitude of factors, including quality of life, comorbidities, risks, and benefits, should guide decision-making,” she said.
Dr. Banini highlighted the finding that alcohol etiology for cirrhosis was associated with higher mortality compared with viral etiology.
“This is important in the context of unfortunately increasing trends in alcohol consumption in the pre- and peri-COVID era and the increased prevalence of alcohol-associated liver disease, especially in women,” she said.
The study was supported by grants from the University of Florence and the University of Modena and Reggio Emilia. The study authors have received lecture fees from Gore Medical, which creates stent grafts. Dr. Banini reported no relevant disclosures.
FROM HEPATOLOGY
NAFLD linked with increased heart failure risk
The risk of developing incident heart failure is 1.5-times higher in people with nonalcoholic fatty liver disease (NAFLD) during a median follow-up of 10 years, according to a new meta-analysis.
The risk appears to increase with greater liver disease severity and was independent of age, sex, ethnicity, obesity, and the presence of diabetes, hypertension, and other common cardiovascular risk factors.
“Health care professionals should be aware that the risk of new-onset heart failure is moderately higher in patients with NAFLD,” senior author Giovanni Targher, MD, said in an interview.
“Because of the link between the two conditions, more careful surveillance of these patients will be needed,” said Dr. Targher, who is an associate professor of diabetes and endocrinology at the University of Verona (Italy). “In particular, the results of this meta-analysis highlight the need for a patient-centered, multidisciplinary, and holistic approach to manage both liver disease and cardiovascular risk in patients with NAFLD.”
The study was published online in Gut.
Risk calculations
NAFLD has become one of the most common causes of chronic liver disease worldwide (affecting up to about 30% of the world’s adults), and is expected to rise sharply in the next decade, the study authors write. The disease is linked with liver-related conditions, such as nonalcoholic steatohepatitis, cirrhosis, and hepatocellular carcinoma, as well as complications in other organs.
Previous meta-analyses have found an association between NAFLD and a higher risk of heart failure, though the analyses included a relatively small number of studies and a relatively modest sample size, Dr. Targher and colleagues write.
Since then, several new cohort studies have examined the association, which inspired a new meta-analysis.
The research team analyzed 11 observational cohort studies with aggregate data on more than 11 million middle-aged people from different countries, including nearly 3 million with NAFLD and nearly 98,000 cases of incident heart failure over a median follow-up of 10 years.
In the studies, NAFLD was diagnosed by serum liver enzyme levels, serum biomarkers or scores, diagnostic codes, imaging techniques, or liver histology. Four studies were conducted in the United States, three were conducted in South Korea, and four were carried out in Europe, including Finland, Sweden, and the United Kingdom.
Dr. Targher and colleagues found that the presence of NAFLD was associated with a moderately higher risk of new-onset heart failure, with a pooled random-effects hazard ratio of 1.5. The risk was independent of age, sex, ethnicity, adiposity measures, diabetes, hypertension, and other typical cardiovascular risk factors.
The association between NAFLD and heart failure risk was consistent even when the comparison was stratified by study country, follow-up length, modality of heart failure diagnosis, and modality of NAFLD diagnosis.
In addition, sensitivity analyses didn’t change the results, and a funnel plot suggested that publication bias was unlikely.
“Accumulating evidence supports that NAFLD is part of a multisystem disease that adversely affects several extrahepatic organs, including the heart,” Dr. Targher said.
“NAFLD not only promotes accelerated coronary atherosclerosis but also confers a higher risk of myocardial abnormalities (cardiac remodeling and hypertrophy) and certain arrhythmias (mostly atrial fibrillation), which may precede and promote the development of new-onset heart failure over time,” he said.
Future research
Dr. Targher and colleagues also found that the risk of incident heart failure appeared to further increase with more advanced liver disease, particularly with higher levels of liver fibrosis, as assessed by noninvasive fibrosis biomarkers or histology. With only two cohort studies that examined the association, the authors judged there was insufficient data available to combine the studies into a meta-analysis.
But the observations are consistent with other recent meta-analyses that reported a significant association between the presence and severity of NAFLD and the risk of developing adverse cardiovascular outcomes, atrial fibrillation, chronic kidney disease, or other non-liver complications.
“It’s reassuring that the observations that have come from single studies hold true when you look at the totality of evidence,” Ambarish Pandey, MD, a cardiologist and assistant professor of internal medicine at the University of Texas Southwestern Medical Center, Dallas, told this news organization.
Dr. Pandey, who wasn’t involved with this study, conducted one of the recent meta-analyses that found a 1.6-times increased risk of heart failure associated with NAFLD, as well as a further increased risk with more advanced liver disease.
Now Dr. Pandey and colleagues are studying the underlying mechanisms for the link between NAFLD and heart failure risk, including cardiac structure and function, biomarkers of injury and stress, and how proportions of liver fat influence risk. Additional studies should investigate whether resolving NAFLD could reduce the risk of heart failure, he said.
“It’s really important to look for patients with NAFLD in primary care and think about cardiovascular disease in our liver patients,” he said. “Early strategies to implement the prevention of heart failure would go a long way in reducing long-term risks for these patients.”
The study authors did not declare a specific grant for this research from any funding agency in the public, commercial, or nonprofit sectors. Dr. Targher and Dr. Pandey report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The risk of developing incident heart failure is 1.5-times higher in people with nonalcoholic fatty liver disease (NAFLD) during a median follow-up of 10 years, according to a new meta-analysis.
The risk appears to increase with greater liver disease severity and was independent of age, sex, ethnicity, obesity, and the presence of diabetes, hypertension, and other common cardiovascular risk factors.
“Health care professionals should be aware that the risk of new-onset heart failure is moderately higher in patients with NAFLD,” senior author Giovanni Targher, MD, said in an interview.
“Because of the link between the two conditions, more careful surveillance of these patients will be needed,” said Dr. Targher, who is an associate professor of diabetes and endocrinology at the University of Verona (Italy). “In particular, the results of this meta-analysis highlight the need for a patient-centered, multidisciplinary, and holistic approach to manage both liver disease and cardiovascular risk in patients with NAFLD.”
The study was published online in Gut.
Risk calculations
NAFLD has become one of the most common causes of chronic liver disease worldwide (affecting up to about 30% of the world’s adults), and is expected to rise sharply in the next decade, the study authors write. The disease is linked with liver-related conditions, such as nonalcoholic steatohepatitis, cirrhosis, and hepatocellular carcinoma, as well as complications in other organs.
Previous meta-analyses have found an association between NAFLD and a higher risk of heart failure, though the analyses included a relatively small number of studies and a relatively modest sample size, Dr. Targher and colleagues write.
Since then, several new cohort studies have examined the association, which inspired a new meta-analysis.
The research team analyzed 11 observational cohort studies with aggregate data on more than 11 million middle-aged people from different countries, including nearly 3 million with NAFLD and nearly 98,000 cases of incident heart failure over a median follow-up of 10 years.
In the studies, NAFLD was diagnosed by serum liver enzyme levels, serum biomarkers or scores, diagnostic codes, imaging techniques, or liver histology. Four studies were conducted in the United States, three were conducted in South Korea, and four were carried out in Europe, including Finland, Sweden, and the United Kingdom.
Dr. Targher and colleagues found that the presence of NAFLD was associated with a moderately higher risk of new-onset heart failure, with a pooled random-effects hazard ratio of 1.5. The risk was independent of age, sex, ethnicity, adiposity measures, diabetes, hypertension, and other typical cardiovascular risk factors.
The association between NAFLD and heart failure risk was consistent even when the comparison was stratified by study country, follow-up length, modality of heart failure diagnosis, and modality of NAFLD diagnosis.
In addition, sensitivity analyses didn’t change the results, and a funnel plot suggested that publication bias was unlikely.
“Accumulating evidence supports that NAFLD is part of a multisystem disease that adversely affects several extrahepatic organs, including the heart,” Dr. Targher said.
“NAFLD not only promotes accelerated coronary atherosclerosis but also confers a higher risk of myocardial abnormalities (cardiac remodeling and hypertrophy) and certain arrhythmias (mostly atrial fibrillation), which may precede and promote the development of new-onset heart failure over time,” he said.
Future research
Dr. Targher and colleagues also found that the risk of incident heart failure appeared to further increase with more advanced liver disease, particularly with higher levels of liver fibrosis, as assessed by noninvasive fibrosis biomarkers or histology. With only two cohort studies that examined the association, the authors judged there was insufficient data available to combine the studies into a meta-analysis.
But the observations are consistent with other recent meta-analyses that reported a significant association between the presence and severity of NAFLD and the risk of developing adverse cardiovascular outcomes, atrial fibrillation, chronic kidney disease, or other non-liver complications.
“It’s reassuring that the observations that have come from single studies hold true when you look at the totality of evidence,” Ambarish Pandey, MD, a cardiologist and assistant professor of internal medicine at the University of Texas Southwestern Medical Center, Dallas, told this news organization.
Dr. Pandey, who wasn’t involved with this study, conducted one of the recent meta-analyses that found a 1.6-times increased risk of heart failure associated with NAFLD, as well as a further increased risk with more advanced liver disease.
Now Dr. Pandey and colleagues are studying the underlying mechanisms for the link between NAFLD and heart failure risk, including cardiac structure and function, biomarkers of injury and stress, and how proportions of liver fat influence risk. Additional studies should investigate whether resolving NAFLD could reduce the risk of heart failure, he said.
“It’s really important to look for patients with NAFLD in primary care and think about cardiovascular disease in our liver patients,” he said. “Early strategies to implement the prevention of heart failure would go a long way in reducing long-term risks for these patients.”
The study authors did not declare a specific grant for this research from any funding agency in the public, commercial, or nonprofit sectors. Dr. Targher and Dr. Pandey report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The risk of developing incident heart failure is 1.5-times higher in people with nonalcoholic fatty liver disease (NAFLD) during a median follow-up of 10 years, according to a new meta-analysis.
The risk appears to increase with greater liver disease severity and was independent of age, sex, ethnicity, obesity, and the presence of diabetes, hypertension, and other common cardiovascular risk factors.
“Health care professionals should be aware that the risk of new-onset heart failure is moderately higher in patients with NAFLD,” senior author Giovanni Targher, MD, said in an interview.
“Because of the link between the two conditions, more careful surveillance of these patients will be needed,” said Dr. Targher, who is an associate professor of diabetes and endocrinology at the University of Verona (Italy). “In particular, the results of this meta-analysis highlight the need for a patient-centered, multidisciplinary, and holistic approach to manage both liver disease and cardiovascular risk in patients with NAFLD.”
The study was published online in Gut.
Risk calculations
NAFLD has become one of the most common causes of chronic liver disease worldwide (affecting up to about 30% of the world’s adults), and is expected to rise sharply in the next decade, the study authors write. The disease is linked with liver-related conditions, such as nonalcoholic steatohepatitis, cirrhosis, and hepatocellular carcinoma, as well as complications in other organs.
Previous meta-analyses have found an association between NAFLD and a higher risk of heart failure, though the analyses included a relatively small number of studies and a relatively modest sample size, Dr. Targher and colleagues write.
Since then, several new cohort studies have examined the association, which inspired a new meta-analysis.
The research team analyzed 11 observational cohort studies with aggregate data on more than 11 million middle-aged people from different countries, including nearly 3 million with NAFLD and nearly 98,000 cases of incident heart failure over a median follow-up of 10 years.
In the studies, NAFLD was diagnosed by serum liver enzyme levels, serum biomarkers or scores, diagnostic codes, imaging techniques, or liver histology. Four studies were conducted in the United States, three were conducted in South Korea, and four were carried out in Europe, including Finland, Sweden, and the United Kingdom.
Dr. Targher and colleagues found that the presence of NAFLD was associated with a moderately higher risk of new-onset heart failure, with a pooled random-effects hazard ratio of 1.5. The risk was independent of age, sex, ethnicity, adiposity measures, diabetes, hypertension, and other typical cardiovascular risk factors.
The association between NAFLD and heart failure risk was consistent even when the comparison was stratified by study country, follow-up length, modality of heart failure diagnosis, and modality of NAFLD diagnosis.
In addition, sensitivity analyses didn’t change the results, and a funnel plot suggested that publication bias was unlikely.
“Accumulating evidence supports that NAFLD is part of a multisystem disease that adversely affects several extrahepatic organs, including the heart,” Dr. Targher said.
“NAFLD not only promotes accelerated coronary atherosclerosis but also confers a higher risk of myocardial abnormalities (cardiac remodeling and hypertrophy) and certain arrhythmias (mostly atrial fibrillation), which may precede and promote the development of new-onset heart failure over time,” he said.
Future research
Dr. Targher and colleagues also found that the risk of incident heart failure appeared to further increase with more advanced liver disease, particularly with higher levels of liver fibrosis, as assessed by noninvasive fibrosis biomarkers or histology. With only two cohort studies that examined the association, the authors judged there was insufficient data available to combine the studies into a meta-analysis.
But the observations are consistent with other recent meta-analyses that reported a significant association between the presence and severity of NAFLD and the risk of developing adverse cardiovascular outcomes, atrial fibrillation, chronic kidney disease, or other non-liver complications.
“It’s reassuring that the observations that have come from single studies hold true when you look at the totality of evidence,” Ambarish Pandey, MD, a cardiologist and assistant professor of internal medicine at the University of Texas Southwestern Medical Center, Dallas, told this news organization.
Dr. Pandey, who wasn’t involved with this study, conducted one of the recent meta-analyses that found a 1.6-times increased risk of heart failure associated with NAFLD, as well as a further increased risk with more advanced liver disease.
Now Dr. Pandey and colleagues are studying the underlying mechanisms for the link between NAFLD and heart failure risk, including cardiac structure and function, biomarkers of injury and stress, and how proportions of liver fat influence risk. Additional studies should investigate whether resolving NAFLD could reduce the risk of heart failure, he said.
“It’s really important to look for patients with NAFLD in primary care and think about cardiovascular disease in our liver patients,” he said. “Early strategies to implement the prevention of heart failure would go a long way in reducing long-term risks for these patients.”
The study authors did not declare a specific grant for this research from any funding agency in the public, commercial, or nonprofit sectors. Dr. Targher and Dr. Pandey report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM GUT
Therapeutic management of NAFLD
Nonalcoholic fatty liver disease (NAFLD) is defined by the presence of hepatic steatosis detected on either imaging or histology in the absence of secondary causes of fatty liver (e.g., excessive alcohol consumption) or other chronic liver diseases.1 For practical NAFLD diagnosis purposes, excessive alcohol intake can be defined as an active or recent history of more than 21 standard drinks per week in men and more than14 standard drinks per week in women. For the sake of terminology, NAFLD is characterized by fatty liver infiltration, affecting at least 5% of hepatocytes, with no evidence of hepatocyte injury, whereas nonalcoholic steatohepatitis (NASH) is defined as the presence of necroinflammation with or without fibrosis in a background of fatty liver.1
Natural history
NASH and the degree of fibrosis are the two most important determinants of the natural history of NAFLD. NASH can evolve into fibrosis and cirrhosis, whereas advanced fibrosis and cirrhosis (stages 3 or 4 of fibrosis) significantly increase the risk of liver-related decompensation and mortality. NAFLD, per se, has been associated with an increased risk of overall mortality, compared with that of the general population.2 The three most common causes of mortality for patients with NAFLD are cardiovascular diseases (CVD), extrahepatic malignancies, and liver-related deaths. Mortality and liver-related events, including hepatic decompensation and hepatocellular carcinoma (HCC), may significantly increase in a dose-dependent manner with increasing fibrosis stages, and stages 3 or 4 of fibrosis may display the highest rates of all-cause mortality and liver-related events.3,4 It is important to note, however, that almost 15% of HCCs occur in patients with NAFLD who do not have cirrhosis.5 The presence of commonly associated comorbidities such as obesity, insulin resistance or diabetes, dyslipidemia, hypothyroidism, polycystic ovary syndrome, and sleep apnea may contribute to an increased risk of NASH and advanced fibrosis and, therefore, an accelerated clinical course of NAFLD.
Nonpharmacological interventions
Lifestyle modification
Lifestyle modification to achieve weight loss remains a first-line intervention in patients with NAFLD. Weight loss achieved either by hypocaloric diet alone or in conjunction with increased physical activity can be beneficial for all patients with NAFLD. The benefits extend not only to those who are overweight and obese but also to those within normal body weight (lean NAFLD).1,6,7 Weight loss of approximately 3%-5% is necessary to improve hepatic steatosis, but a greater weight loss (7%-10%) is required to improve other histopathological features like necroinflammatory lesions and fibrosis.8-10 Individuals with higher BMI and/or type 2 diabetes (T2D) will require a larger weight reduction to achieve a similar benefit on NAFLD-related features.7,8 Weight loss via lifestyle changes can also decrease hepatic venous pressure gradient (HVPG), with greater declines reported among those with more than 10% weight loss.11
Weight loss can be achieved through a variety of modalities, but long-term maintenance of lost weight is much more challenging. A combination of a hypocaloric diet with a caloric deficit of 500-1,000 kcal/d, alongside moderate-intensity exercise and intensive on-site behavioral treatment, will likely increase the possibility of a sustained weight loss over time.1,12 A growing body of scientific evidence indicates that a healthy diet that includes a reduction of high-glycemic-index foods and refined carbohydrates; increased consumption of monounsaturated fatty acids, omega-3 fatty acids, and fibers; and high intakes of olive oil, nuts, vegetables, fruits, legumes, whole grains, and fish can have beneficial effects on NAFLD and its severity.13-16 Adherence to these healthy dietary patterns has been associated with a marked reduction in CVD morbidity and mortality and is, thus, a strategic lifestyle recommendation for patients with NAFLD in whom the leading cause of morbidity and death is CVD.1,3
Exercise alone in adults with NAFLD may reduce hepatic steatosis, but its ability to improve inflammation and fibrosis has not been proven in well-designed RCTs.17,18 Physical activity and exercise have been shown to curb both the development and the progression of NAFLD, and beneficial effects could be achieved independent of weight loss.17,19,20 Most importantly, moderate-to-vigorous physical activity is likely associated with lower all-cause and cardiovascular mortality in patients with NAFLD.21
Heavy alcohol intake should be avoided by patients with NAFLD or NASH, and those with cirrhotic NASH should avoid any alcohol consumption given the risk of HCC and hepatic decompensation.1,4,22 Limiting light-to-moderate alcohol intake among patients without cirrhosis is still under debate.1 People with NAFLD may be advised to drink an equivalent of two to three 8-oz cups of regular brewed coffee daily as it has shown certain antifibrotic effects in NAFLD patients.23
Bariatric surgery
Bariatric surgery is an attractive therapeutic option for eligible obese patients with NAFLD. Bariatric surgery has the potential for inducing great weight loss and, therefore, reverses not only the steatosis, inflammation, and fibrosis among NAFLD individuals but also important comorbid conditions like T2D. A recent systematic review and meta-analysis examining data on the effects of bariatric surgery on histologic features of NAFLD from 32 cohort studies (no RCTs included) showed that bariatric surgery was associated with significant improvements in steatosis (66%), lobular inflammation (50%), ballooning degeneration (76%), and fibrosis (40%), and the benefits were significantly higher in those who underwent Roux-en-Y gastric bypass (RYGB). Of note, worsening of liver histology, including fibrosis, could be seen in up to 12% of patients who underwent bariatric surgery.24 The postsurgical weight regained after RYGB could explain partly the lack of fibrosis improvement or even worsening of fibrosis, although further research is needed to clarify these controversial findings.
RYGB and sleeve gastrectomy (SG) are the most commonly performed bariatric surgeries worldwide. Patients who undergo RYGB achieve higher weight loss when compared with those treated with SG.25 Among all bariatric procedures, RYGB could result in a higher proportion of complete resolution of NAFLD than SG, although evidence is inconclusive on fibrosis improvement rates.24,26 Most recently, a single-center RCT has compared the effects of RYGB vs. SG on liver fat content and fibrosis in patients with severe obesity and T2D.27 Data showed that both surgical procedures were highly and equally effective in reducing fatty liver content (quantified by magnetic resonance imaging), with an almost complete resolution of the fatty liver at 1 year of both surgical interventions. The beneficial effects of both GB and SG on fibrosis (assessed by enhanced liver test [ELF]) were less evident with no substantial difference between the two groups. Importantly, 69% of participants had an increase in their ELF scores during the study, despite the majority of participants achieving significant reductions in their body weights and better glycemic control at the end of the study. These findings might be considered with caution as several factors, such as the duration of the study (only 1 year) and lack of a liver biopsy to confirm fibrosis changes over time, could be influencing the study results.
Among all NAFLD phenotypes, those with cirrhosis and, most importantly, hepatic decompensation appear to be at increased risk of perioperative mortality and inpatient hospital stays than those without cirrhosis.28-29 Bariatric surgery is an absolute contraindication in patients with decompensated cirrhosis (Child B and Child C). Among compensated -Child A- cirrhotics, those with portal hypertension are at increased risk of morbidity and perioperative mortality.30 A recent analysis of National Inpatient Sample data suggested that the rates of complications in those with cirrhosis have decreased with time, which could be due to a better selection process and the use of more restrictive bariatric surgery in those with cirrhosis. Low volume centers (defined as less than 50 procedures per year) and nonrestrictive bariatric surgery were associated with a higher mortality rate. These data may suggest that patients with cirrhosis should undergo bariatric surgery only in high-volume centers after a multidisciplinary evaluation.31 Bariatric endoscopy is emerging as a new treatment for obesity, but the long-term durability of its effects remains to be determined.
A recent retrospective cohort study, including 1,158 adult patients with biopsy-proven NASH, has investigated the benefits of bariatric surgery on the occurrence of major adverse liver and cardiovascular outcomes in 650 patients who underwent bariatric surgery, compared with 508 patients who received nonsurgical usual care. This study showed that bariatric surgery was associated with 88% lower risk of progression of fatty liver to cirrhosis, liver cancer, or liver-related death, and 70% lower risk of serious CVD events during a follow-up period of 10 years.32 Within 1 year after surgery, 0.6% of patients died from surgical complications. The potential benefits of bariatric surgery in patients with NAFLD must be balanced against surgical risk, especially in eligible obese individuals with established cirrhosis. Data from a retrospective cohort study have shown that bariatric surgery in obese cirrhotic patients does not seem to associate with excessive mortality, compared with noncirrhotic obese patients.33 More data on immediate complication rates and long-term outcomes in patients with NAFLD by type of bariatric surgery is also required.
NAFLD as a standalone is not an indication for bariatric surgery. However, it could be considered in NAFLD patients who have a BMI of 40 kg/m2 or more without coexisting comorbidities or with a BMI of 35 kg/m2 or more and one or more severe obesity-related comorbidities, including T2D, hypertension, hyperlipidemia, or obstructive sleep apnea. Bariatric surgery must always be offered in centers with an experienced bariatric surgery program.1
Management of comorbidities
Given the multiple comorbidities associated with NAFLD and the potential to influence its severity, a comprehensive and multidisciplinary approach is needed to ameliorate not only the progression of liver disease but also those complications related to metabolic syndrome, hyperlipidemia, hypertension, diabetes, and other related conditions. Of note, all patients with NAFLD should receive aggressive management of comorbidities regardless of the severity of NAFLD. Ideally, a multidisciplinary team – including a primary care provider, an endocrinologist for patients with T2D, and a gastroenterologist/hepatologist – is needed to successfully manage patients with NAFLD.
It is well recognized that individuals with biopsy-proven NAFLD are at a higher risk of coronary heart disease, stroke, congestive heart failure, and death resulting from CVD when compared with the non-NAFLD population, and excess in CVD morbidity and mortality is evident across all stages of NAFLD and increases with worsening disease severity.34 The strong association between CVD and NAFLD has important clinical implications that may influence the decision to initiate treatment for primary prevention, including lipid-lowering, antihypertensive, or antiplatelet therapies.35 Statins are widely used to reduce LDL cholesterol and have been proven to be safe in NAFLD, including for those with elevated liver enzymes and even in compensated cirrhosis, in several studies conducted during the last 15 years.36 Statins are characterized by anti-inflammatory, anti-oxidative, antifibrotic, and plaque-stabilizing effects, whereby they may improve vascular and hepatic function among patients with NAFLD and reduce cardiovascular risk.37 Statin use for the treatment of NAFLD is still controversial and off-label and is not specifically recommended to treat NASH, but positive results have been shown for reductions in liver enzymes.1 A recent meta-analysis of 13 studies showed that continued use of statin in cirrhosis was associated with a 46% and 44% risk reduction in hepatic decompensation and mortality, respectively.38
The Food and Drug Administration has approved omega-3 (n-3) fatty acid agents and fibrates for the treatment of very high triglycerides (500 mg/dL or higher); however, no specific indications exist to treat NAFLD.1 Fenofibrate is related to mild aminotransferase elevations and, in some cases, severe liver injury, so caution must be paid, especially within 2 days of taking the drug.39-40
NAFLD phenotypes that need liver pharmacotherapy
There are still no FDA-approved drugs or biological treatments for NASH. Pharmacological interventions aiming primarily at improving liver disease should generally be limited to those with biopsy-proven NASH and clinically significant fibrosis (fibrosis stages of 2 or greater).1,4 For FDA approval, medications used for treating NAFLD with fibrosis need to meet one of the following endpoint criteria: resolution of NASH without worsening of fibrosis, improvement in fibrosis without worsening of NASH, or both. In addition to those criteria, a new medication might improve the metabolic profile and have a tolerable safety profile. Table 1 displays those NAFLD phenotypes that will likely benefit from liver-directed therapy.
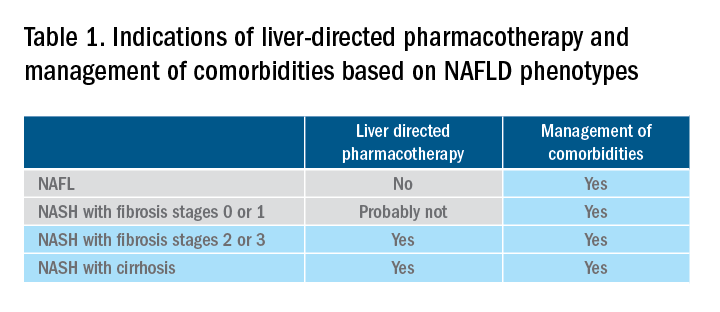
Obeticholic acid as an experimental therapy for NASH
A planned month-18 interim analysis of a multicentre, phase III RCT examined the efficacy and safety of obeticholic acid (OCA), a farnesoid X receptor agonist, in patients with NASH and stages 1-3 of fibrosis. The primary endpoint (fibrosis reduction 1 stage or more with no worsening of NASH) was met by 12% of patients in the placebo group, 18% of patients receiving OCA 10 mg (P = .045), and 23% of those receiving OCA 25 mg (P = .0002). An alternative primary endpoint of NASH resolution with no worsening of fibrosis was not met. OCA 25 mg led to the highest rates of pruritus and hyperlipidemia, compared with OCA 10 mg.42 These side effects seem to be related to the activation of the farnesoid X receptor.43
Currently available but off label medications
Vitamin E, an antioxidant, administered at a daily dose of 800 IU/day improves steatosis, inflammation, and ballooning, but not fibrosis in nondiabetic adults with biopsy-proven NASH.44 Vitamin E for 96 weeks was associated with a significantly higher rate of improvement in NASH (43% vs. 19%, P less than .01), compared with placebo.44 In the Treatment of Nonalcoholic Fatty Liver Disease in Children trial (TONIC), which examined vitamin E (800 IU/day) or metformin (500 mg twice daily) against placebo in children with biopsy-proven NAFLD, resolution of NASH was significantly greater in children treated with vitamin E than in children treated with placebo (58% vs. 28%, P less than .01). Metformin did not significantly improve the NASH resolution rates, compared with placebo (41% vs. 28%, P = .23). Vitamin E could be recommended for nondiabetic adults or children if lifestyle modifications do not produce the expected results as a result of noncompliance or ineffectiveness. Since continued use of vitamin E has been suggested to be associated with a very small increase in the risk for prostate cancer (an absolute increase of 1.6 per 1,000 person-years of vitamin E use) in men, risks and benefits should be discussed with each patient before starting therapy. A meta-analysis of nine placebo-controlled trials including roughly 119,000 patients reported that vitamin E supplementation increases the risk of hemorrhagic stroke by 20% while reducing ischemic stroke by 10%. It was estimated that vitamin E supplementation would prevent one ischemic stroke per 476 treated patients while inducing one hemorrhagic stroke for every 1,250 patients. It is noteworthy that the combination of vitamin E with anticoagulant and/or antiplatelet therapy was not examined in this trial, so we could not determine how combination therapy might affect the risk of ischemic or hemorrhagic stroke.45
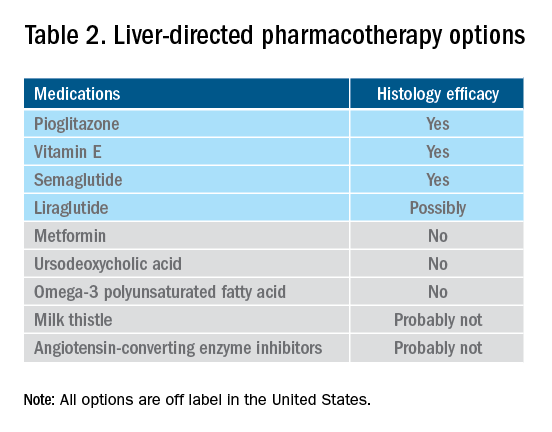
Thiazolidinediones drugs have been reported to be effective in improving NAFLD in many human studies. Evidence from RCTs suggests that pioglitazone could significantly improve glucose metabolism, alanine aminotransferase, and liver histology – such as hepatic steatosis, lobular inflammation, and ballooning degeneration – among patients with or without T2D. However, the beneficial effects on improving fibrosis remain to be verified.1,46 Because of safety concerns, the risk/benefit balance of using pioglitazone to treat NASH should be discussed with each patient.47-48 Pioglitazone has been associated with long-term risk of bladder cancer,49 congestive heart failure,50 and bone fractures.51 Data from the Pioglitazone, Vitamin E, or Placebo for Nonalcoholic Steatohepatitis (PIVENS) trial showed that pioglitazone was significantly associated with weight gain but with no other serious adverse events. However, this study was not powered to test any safety-related hypotheses.44
Glucagon-like peptide 1 analogs have been reported to induce weight loss and reduce insulin resistance, which may lead to improvements in NAFLD. Phase II RCTs of glucagon-like peptide 1 receptor agonists (liraglutide and semaglutide) for the treatment of biopsy-proven NASH showed significant improvements in serum liver enzymes, steatosis, and inflammation, as well as NASH resolution without worsening liver fibrosis, although no direct benefit was observed in reversing fibrosis.52-53 One of these studies explores the efficacy and safety of different doses of daily subcutaneous semaglutide vs. placebo on the rates of resolution of NASH with no worsening of fibrosis. The highest dose (0.4 mg) showed the greatest difference (59% vs. 17%, P less than .01), compared with the placebo arm. However, there was no difference in improvement in fibrosis stage between the two groups (43% in the 0.4-mg group vs. 33% in the placebo group, P = .48).53 Gastrointestinal adverse events were common in the semaglutide arm.
“Spontaneous” NASH resolution and fibrosis improvement are commonly seen in participants assigned to placebo arms in clinical trials. A recent meta-analysis of 43 RCTs including 2649 placebo-treated patients showed a pooled estimate of NASH resolution without worsening of fibrosis and 1 stage reduction or more in fibrosis of 12% and 19%, respectively. Relevant factors involved in “spontaneous” NASH improvement are unknown but could be related to changes in BMI resulting from lifestyle changes, race and ethnicity, age, and, likely, NAFLD-related genetic variations, although more data is needed to better understand the histologic response in placebo-treated patients.54
Semaglutide injections (2.4 mg once weekly) or (2.0 mg once weekly) have been recently approved by the FDA for chronic weight management in adults with obesity or overweight with at least one weight-related condition or glucose control of T2D, respectively. Of note, the semaglutide dose used in the NASH trial is not currently available for the treatment of patients who are overweight/obese or have T2D, but the beneficial effects on body weight reductions and glucose control are similar overall to the effects seen with currently available doses for management of obesity or diabetes. One may consider using semaglutide in patients who are overweight/obese or have T2D with NASH, but in the senior author’s experience, it has been quite challenging to receive the payer’s approval, as its use is not specifically approved to treat liver disease.1
How to follow patients with NAFLD in the clinic
Once a diagnosis of NAFLD is made, the use of noninvasive testing may aid to identify which patients are at high risk of fibrosis. Easy to use clinical tools, such as the NAFLD Fibrosis Score and the Fib-4 index, and liver stiffness measurements using vibration-controlled transient elastography (FibroScan) or magnetic resonance elastography (MRE) are clinically useful noninvasive tools for identifying patients with NAFLD who have a higher likelihood of progressing to advanced fibrosis.1,55 The use of either NAFLD Fibrosis Score (less than -1.455) or Fib-4 index (less than 1.30) low cutoffs may be particularly useful to rule out advanced fibrosis. People with a NAFLD Fibrosis Score (greater than –1.455) or Fib-4 index (greater than 1.30) should undergo liver stiffness measurement (LSM) via FibroScan. Those with an LSM of 8 kPa or higher should be referred to specialized care, where a decision to perform a liver biopsy and initiate monitoring and therapy will be taken. MRE is the most accurate noninvasive method for the estimation of liver fibrosis. When MRE is available, it can be a diagnostic alternative to accurately rule in and rule out patients with advanced fibrosis. This technique can be preferred in clinical trials, but it is rarely used in clinical practice because it is expensive and not easily available. Reassessment by noninvasive scores at 1-3 years’ follow-up will be considered for those with an LSM less than 8 kPa. Patients with NASH cirrhosis should be screened for both gastroesophageal varix and HCC according to the American Association for the Study of Liver Diseases guidelines.56-57
Dr. Vilar-Gomez is assistant professor in the division of gastroenterology and hepatology at Indiana University, Indianapolis. Dr. Chalasani is vice president for academic affairs at Indiana University Health, Indianapolis, and the David W. Crabb Professor of Gastroenterology and Hepatology and an adjunct professor of anatomy, cell biology, and physiology in the division of gastroenterology and hepatology at Indiana University. Dr. Vilar-Gomez reports no financial conflicts of interest. Dr. Chalasani serves as a paid consultant to AbbVie, Boehringer-Ingelheim, Altimmune, Madrigal, Lilly, Zydus, and Galectin. He receives research support from Galectin and DSM.
References
1. Chalasani N et al. Hepatology 2018;67:328-57.
2. Söderberg C et al. Hepatology 2010;51:595-602.
3. Sanyal AJ et al. N Engl J Med 2021;385:1559-69.
4. Vilar-Gomez E et al. Gastroenterology 2018;155:443-57.e17.
5. Younossi ZM et al. Hepatology 2016;64:73-84.
6. EASL-EASD-EASO. J Hepatol 2016;64:1388-402.
7. Wong VW et al. J Hepatol 2018; 69:1349-56.
8. Vilar-Gomez E et al. Gastroenterology 2015;149:367-78.e5; quiz e14-5.
9. Promrat K et al. Hepatology 2010;51:121-9.
10. Wong VW et al. J Hepatol 2013;59:536-42.
11. Berzigotti A et al. Hepatology 2017;65:1293-1305.
12. Sacks FM et al. N Engl J Med 2009;360:859-73.
13. Vilar-Gomez E et al. Hepatology 2022 Jun;75(6):1491-1506.
14. Zelber-Sagi S et al. Liver Int 2017;37:936-49.
15. Hassani Zadeh S et al. J Gastroenterol Hepatol 2021;36:1470-8.
16. Yaskolka Meir A et al. Gut 2021;70:2085-95.
17. Sung KC et al. J Hepatol 2016;65:791-7.
18. Orci LA et al. Clin Gastroenterol Hepatol 2016;14:1398-411.
19. Ryu S et al. J Hepatol 2015;63:1229-37.
20. Kim D et al. Hepatology 2020;72:1556-68.
21. Kim D et al. Clin Gastroenterol Hepatol 2021;19:1240-7.e5.
22. Ascha MS et al. Hepatology 2010;51:1972-8.
23. Bambha K et al. Liver Int 2014;34:1250-8.
24. Lee Y et al. Clin Gastroenterol Hepatol 2019;17:1040-60.e11.
25. Grönroos S et al. JAMA Surg 2021;156:137-46.
26. Fakhry TK et al. Surg Obes Relat Dis 2019;15:502-11.
27. Seeberg KA et al. Ann Intern Med 2022;175:74-83.
28. Bower G et al. Obes Surg 2015;25:2280-9.
29. Jan A et al. Obes Surg 2015;25:1518-26.
30. Hanipah ZN et al. Obes Surg 2018;28:3431-8.
31. Are VS et al. Am J Gastroenterol 2020;115:1849-56.
32. Aminian A et al. JAMA 2021;326:2031-42.
33. Vuppalanchi R et al. Ann Surg 2022;275:e174-80.
34. Simon TG et al. Gut 2021. doi: 10.1136/gutjnl-2021-325724.
35. Lonardo A et al. J Hepatol 2018;68:335-52.
36. Chalasani N et al. Gastroenterology 2004;126:1287-92.
37. Pastori D et al. Dig Liver Dis 2015;47:4-11.
38. Kim RG et al. Clin Gastroenterol Hepatol 2017;15:1521-30.e8.
39. Ahmad J et al. Dig Dis Sci 2017;62:3596-604.
40. Chalasani NP et al. Am J Gastroenterol 2021;116(5):878-98.
41. Rinella ME et al. Hepatology 2019;70:1424-36.
42. Younossi ZM et al. Lancet 2019;394:2184-96.
43. Ratziu V. Clin Liver Dis (Hoboken) 2021;17:398-400.
44. Sanyal AJ et al. N Engl J Med 2010;341:1675-85.
45. Schürks M et al. BMJ 2010;341:c5702.
46. Cusi K et al. Ann Intern Med 2016;165:305-15.
47. Lewis JD et al. JAMA 2015;314:265-77.
48. Billington EO et al. Diabetologia 2015;58:2238-46.
49. Lewis JD et al. Diabetes Care 2011;34:916-22.
50. Erdmann E et al. Diabetes Care 2007;30:2773-8.
51. Viscoli CM et al. J Clin Endocrinol Metab 2017;102:914-22.
52. Armstong MJ et al. Lancet 2016;387:679-90.
53. Newsome PN et al. N Engl J Med 2021;384:1113-24.
54. Ng CH et al. Hepatology 2022;75:1647-61.
55. Kanwal F et al. Gastroenterology 2021;161:1030-1042.e8.
56. Garcia-Tsao G et al. Hepatology 2017;65:310-35.
57. Heimbach JK et al. Hepatology 2018;67:358-80.
Nonalcoholic fatty liver disease (NAFLD) is defined by the presence of hepatic steatosis detected on either imaging or histology in the absence of secondary causes of fatty liver (e.g., excessive alcohol consumption) or other chronic liver diseases.1 For practical NAFLD diagnosis purposes, excessive alcohol intake can be defined as an active or recent history of more than 21 standard drinks per week in men and more than14 standard drinks per week in women. For the sake of terminology, NAFLD is characterized by fatty liver infiltration, affecting at least 5% of hepatocytes, with no evidence of hepatocyte injury, whereas nonalcoholic steatohepatitis (NASH) is defined as the presence of necroinflammation with or without fibrosis in a background of fatty liver.1
Natural history
NASH and the degree of fibrosis are the two most important determinants of the natural history of NAFLD. NASH can evolve into fibrosis and cirrhosis, whereas advanced fibrosis and cirrhosis (stages 3 or 4 of fibrosis) significantly increase the risk of liver-related decompensation and mortality. NAFLD, per se, has been associated with an increased risk of overall mortality, compared with that of the general population.2 The three most common causes of mortality for patients with NAFLD are cardiovascular diseases (CVD), extrahepatic malignancies, and liver-related deaths. Mortality and liver-related events, including hepatic decompensation and hepatocellular carcinoma (HCC), may significantly increase in a dose-dependent manner with increasing fibrosis stages, and stages 3 or 4 of fibrosis may display the highest rates of all-cause mortality and liver-related events.3,4 It is important to note, however, that almost 15% of HCCs occur in patients with NAFLD who do not have cirrhosis.5 The presence of commonly associated comorbidities such as obesity, insulin resistance or diabetes, dyslipidemia, hypothyroidism, polycystic ovary syndrome, and sleep apnea may contribute to an increased risk of NASH and advanced fibrosis and, therefore, an accelerated clinical course of NAFLD.
Nonpharmacological interventions
Lifestyle modification
Lifestyle modification to achieve weight loss remains a first-line intervention in patients with NAFLD. Weight loss achieved either by hypocaloric diet alone or in conjunction with increased physical activity can be beneficial for all patients with NAFLD. The benefits extend not only to those who are overweight and obese but also to those within normal body weight (lean NAFLD).1,6,7 Weight loss of approximately 3%-5% is necessary to improve hepatic steatosis, but a greater weight loss (7%-10%) is required to improve other histopathological features like necroinflammatory lesions and fibrosis.8-10 Individuals with higher BMI and/or type 2 diabetes (T2D) will require a larger weight reduction to achieve a similar benefit on NAFLD-related features.7,8 Weight loss via lifestyle changes can also decrease hepatic venous pressure gradient (HVPG), with greater declines reported among those with more than 10% weight loss.11
Weight loss can be achieved through a variety of modalities, but long-term maintenance of lost weight is much more challenging. A combination of a hypocaloric diet with a caloric deficit of 500-1,000 kcal/d, alongside moderate-intensity exercise and intensive on-site behavioral treatment, will likely increase the possibility of a sustained weight loss over time.1,12 A growing body of scientific evidence indicates that a healthy diet that includes a reduction of high-glycemic-index foods and refined carbohydrates; increased consumption of monounsaturated fatty acids, omega-3 fatty acids, and fibers; and high intakes of olive oil, nuts, vegetables, fruits, legumes, whole grains, and fish can have beneficial effects on NAFLD and its severity.13-16 Adherence to these healthy dietary patterns has been associated with a marked reduction in CVD morbidity and mortality and is, thus, a strategic lifestyle recommendation for patients with NAFLD in whom the leading cause of morbidity and death is CVD.1,3
Exercise alone in adults with NAFLD may reduce hepatic steatosis, but its ability to improve inflammation and fibrosis has not been proven in well-designed RCTs.17,18 Physical activity and exercise have been shown to curb both the development and the progression of NAFLD, and beneficial effects could be achieved independent of weight loss.17,19,20 Most importantly, moderate-to-vigorous physical activity is likely associated with lower all-cause and cardiovascular mortality in patients with NAFLD.21
Heavy alcohol intake should be avoided by patients with NAFLD or NASH, and those with cirrhotic NASH should avoid any alcohol consumption given the risk of HCC and hepatic decompensation.1,4,22 Limiting light-to-moderate alcohol intake among patients without cirrhosis is still under debate.1 People with NAFLD may be advised to drink an equivalent of two to three 8-oz cups of regular brewed coffee daily as it has shown certain antifibrotic effects in NAFLD patients.23
Bariatric surgery
Bariatric surgery is an attractive therapeutic option for eligible obese patients with NAFLD. Bariatric surgery has the potential for inducing great weight loss and, therefore, reverses not only the steatosis, inflammation, and fibrosis among NAFLD individuals but also important comorbid conditions like T2D. A recent systematic review and meta-analysis examining data on the effects of bariatric surgery on histologic features of NAFLD from 32 cohort studies (no RCTs included) showed that bariatric surgery was associated with significant improvements in steatosis (66%), lobular inflammation (50%), ballooning degeneration (76%), and fibrosis (40%), and the benefits were significantly higher in those who underwent Roux-en-Y gastric bypass (RYGB). Of note, worsening of liver histology, including fibrosis, could be seen in up to 12% of patients who underwent bariatric surgery.24 The postsurgical weight regained after RYGB could explain partly the lack of fibrosis improvement or even worsening of fibrosis, although further research is needed to clarify these controversial findings.
RYGB and sleeve gastrectomy (SG) are the most commonly performed bariatric surgeries worldwide. Patients who undergo RYGB achieve higher weight loss when compared with those treated with SG.25 Among all bariatric procedures, RYGB could result in a higher proportion of complete resolution of NAFLD than SG, although evidence is inconclusive on fibrosis improvement rates.24,26 Most recently, a single-center RCT has compared the effects of RYGB vs. SG on liver fat content and fibrosis in patients with severe obesity and T2D.27 Data showed that both surgical procedures were highly and equally effective in reducing fatty liver content (quantified by magnetic resonance imaging), with an almost complete resolution of the fatty liver at 1 year of both surgical interventions. The beneficial effects of both GB and SG on fibrosis (assessed by enhanced liver test [ELF]) were less evident with no substantial difference between the two groups. Importantly, 69% of participants had an increase in their ELF scores during the study, despite the majority of participants achieving significant reductions in their body weights and better glycemic control at the end of the study. These findings might be considered with caution as several factors, such as the duration of the study (only 1 year) and lack of a liver biopsy to confirm fibrosis changes over time, could be influencing the study results.
Among all NAFLD phenotypes, those with cirrhosis and, most importantly, hepatic decompensation appear to be at increased risk of perioperative mortality and inpatient hospital stays than those without cirrhosis.28-29 Bariatric surgery is an absolute contraindication in patients with decompensated cirrhosis (Child B and Child C). Among compensated -Child A- cirrhotics, those with portal hypertension are at increased risk of morbidity and perioperative mortality.30 A recent analysis of National Inpatient Sample data suggested that the rates of complications in those with cirrhosis have decreased with time, which could be due to a better selection process and the use of more restrictive bariatric surgery in those with cirrhosis. Low volume centers (defined as less than 50 procedures per year) and nonrestrictive bariatric surgery were associated with a higher mortality rate. These data may suggest that patients with cirrhosis should undergo bariatric surgery only in high-volume centers after a multidisciplinary evaluation.31 Bariatric endoscopy is emerging as a new treatment for obesity, but the long-term durability of its effects remains to be determined.
A recent retrospective cohort study, including 1,158 adult patients with biopsy-proven NASH, has investigated the benefits of bariatric surgery on the occurrence of major adverse liver and cardiovascular outcomes in 650 patients who underwent bariatric surgery, compared with 508 patients who received nonsurgical usual care. This study showed that bariatric surgery was associated with 88% lower risk of progression of fatty liver to cirrhosis, liver cancer, or liver-related death, and 70% lower risk of serious CVD events during a follow-up period of 10 years.32 Within 1 year after surgery, 0.6% of patients died from surgical complications. The potential benefits of bariatric surgery in patients with NAFLD must be balanced against surgical risk, especially in eligible obese individuals with established cirrhosis. Data from a retrospective cohort study have shown that bariatric surgery in obese cirrhotic patients does not seem to associate with excessive mortality, compared with noncirrhotic obese patients.33 More data on immediate complication rates and long-term outcomes in patients with NAFLD by type of bariatric surgery is also required.
NAFLD as a standalone is not an indication for bariatric surgery. However, it could be considered in NAFLD patients who have a BMI of 40 kg/m2 or more without coexisting comorbidities or with a BMI of 35 kg/m2 or more and one or more severe obesity-related comorbidities, including T2D, hypertension, hyperlipidemia, or obstructive sleep apnea. Bariatric surgery must always be offered in centers with an experienced bariatric surgery program.1
Management of comorbidities
Given the multiple comorbidities associated with NAFLD and the potential to influence its severity, a comprehensive and multidisciplinary approach is needed to ameliorate not only the progression of liver disease but also those complications related to metabolic syndrome, hyperlipidemia, hypertension, diabetes, and other related conditions. Of note, all patients with NAFLD should receive aggressive management of comorbidities regardless of the severity of NAFLD. Ideally, a multidisciplinary team – including a primary care provider, an endocrinologist for patients with T2D, and a gastroenterologist/hepatologist – is needed to successfully manage patients with NAFLD.
It is well recognized that individuals with biopsy-proven NAFLD are at a higher risk of coronary heart disease, stroke, congestive heart failure, and death resulting from CVD when compared with the non-NAFLD population, and excess in CVD morbidity and mortality is evident across all stages of NAFLD and increases with worsening disease severity.34 The strong association between CVD and NAFLD has important clinical implications that may influence the decision to initiate treatment for primary prevention, including lipid-lowering, antihypertensive, or antiplatelet therapies.35 Statins are widely used to reduce LDL cholesterol and have been proven to be safe in NAFLD, including for those with elevated liver enzymes and even in compensated cirrhosis, in several studies conducted during the last 15 years.36 Statins are characterized by anti-inflammatory, anti-oxidative, antifibrotic, and plaque-stabilizing effects, whereby they may improve vascular and hepatic function among patients with NAFLD and reduce cardiovascular risk.37 Statin use for the treatment of NAFLD is still controversial and off-label and is not specifically recommended to treat NASH, but positive results have been shown for reductions in liver enzymes.1 A recent meta-analysis of 13 studies showed that continued use of statin in cirrhosis was associated with a 46% and 44% risk reduction in hepatic decompensation and mortality, respectively.38
The Food and Drug Administration has approved omega-3 (n-3) fatty acid agents and fibrates for the treatment of very high triglycerides (500 mg/dL or higher); however, no specific indications exist to treat NAFLD.1 Fenofibrate is related to mild aminotransferase elevations and, in some cases, severe liver injury, so caution must be paid, especially within 2 days of taking the drug.39-40
NAFLD phenotypes that need liver pharmacotherapy
There are still no FDA-approved drugs or biological treatments for NASH. Pharmacological interventions aiming primarily at improving liver disease should generally be limited to those with biopsy-proven NASH and clinically significant fibrosis (fibrosis stages of 2 or greater).1,4 For FDA approval, medications used for treating NAFLD with fibrosis need to meet one of the following endpoint criteria: resolution of NASH without worsening of fibrosis, improvement in fibrosis without worsening of NASH, or both. In addition to those criteria, a new medication might improve the metabolic profile and have a tolerable safety profile. Table 1 displays those NAFLD phenotypes that will likely benefit from liver-directed therapy.

Obeticholic acid as an experimental therapy for NASH
A planned month-18 interim analysis of a multicentre, phase III RCT examined the efficacy and safety of obeticholic acid (OCA), a farnesoid X receptor agonist, in patients with NASH and stages 1-3 of fibrosis. The primary endpoint (fibrosis reduction 1 stage or more with no worsening of NASH) was met by 12% of patients in the placebo group, 18% of patients receiving OCA 10 mg (P = .045), and 23% of those receiving OCA 25 mg (P = .0002). An alternative primary endpoint of NASH resolution with no worsening of fibrosis was not met. OCA 25 mg led to the highest rates of pruritus and hyperlipidemia, compared with OCA 10 mg.42 These side effects seem to be related to the activation of the farnesoid X receptor.43
Currently available but off label medications
Vitamin E, an antioxidant, administered at a daily dose of 800 IU/day improves steatosis, inflammation, and ballooning, but not fibrosis in nondiabetic adults with biopsy-proven NASH.44 Vitamin E for 96 weeks was associated with a significantly higher rate of improvement in NASH (43% vs. 19%, P less than .01), compared with placebo.44 In the Treatment of Nonalcoholic Fatty Liver Disease in Children trial (TONIC), which examined vitamin E (800 IU/day) or metformin (500 mg twice daily) against placebo in children with biopsy-proven NAFLD, resolution of NASH was significantly greater in children treated with vitamin E than in children treated with placebo (58% vs. 28%, P less than .01). Metformin did not significantly improve the NASH resolution rates, compared with placebo (41% vs. 28%, P = .23). Vitamin E could be recommended for nondiabetic adults or children if lifestyle modifications do not produce the expected results as a result of noncompliance or ineffectiveness. Since continued use of vitamin E has been suggested to be associated with a very small increase in the risk for prostate cancer (an absolute increase of 1.6 per 1,000 person-years of vitamin E use) in men, risks and benefits should be discussed with each patient before starting therapy. A meta-analysis of nine placebo-controlled trials including roughly 119,000 patients reported that vitamin E supplementation increases the risk of hemorrhagic stroke by 20% while reducing ischemic stroke by 10%. It was estimated that vitamin E supplementation would prevent one ischemic stroke per 476 treated patients while inducing one hemorrhagic stroke for every 1,250 patients. It is noteworthy that the combination of vitamin E with anticoagulant and/or antiplatelet therapy was not examined in this trial, so we could not determine how combination therapy might affect the risk of ischemic or hemorrhagic stroke.45

Thiazolidinediones drugs have been reported to be effective in improving NAFLD in many human studies. Evidence from RCTs suggests that pioglitazone could significantly improve glucose metabolism, alanine aminotransferase, and liver histology – such as hepatic steatosis, lobular inflammation, and ballooning degeneration – among patients with or without T2D. However, the beneficial effects on improving fibrosis remain to be verified.1,46 Because of safety concerns, the risk/benefit balance of using pioglitazone to treat NASH should be discussed with each patient.47-48 Pioglitazone has been associated with long-term risk of bladder cancer,49 congestive heart failure,50 and bone fractures.51 Data from the Pioglitazone, Vitamin E, or Placebo for Nonalcoholic Steatohepatitis (PIVENS) trial showed that pioglitazone was significantly associated with weight gain but with no other serious adverse events. However, this study was not powered to test any safety-related hypotheses.44
Glucagon-like peptide 1 analogs have been reported to induce weight loss and reduce insulin resistance, which may lead to improvements in NAFLD. Phase II RCTs of glucagon-like peptide 1 receptor agonists (liraglutide and semaglutide) for the treatment of biopsy-proven NASH showed significant improvements in serum liver enzymes, steatosis, and inflammation, as well as NASH resolution without worsening liver fibrosis, although no direct benefit was observed in reversing fibrosis.52-53 One of these studies explores the efficacy and safety of different doses of daily subcutaneous semaglutide vs. placebo on the rates of resolution of NASH with no worsening of fibrosis. The highest dose (0.4 mg) showed the greatest difference (59% vs. 17%, P less than .01), compared with the placebo arm. However, there was no difference in improvement in fibrosis stage between the two groups (43% in the 0.4-mg group vs. 33% in the placebo group, P = .48).53 Gastrointestinal adverse events were common in the semaglutide arm.
“Spontaneous” NASH resolution and fibrosis improvement are commonly seen in participants assigned to placebo arms in clinical trials. A recent meta-analysis of 43 RCTs including 2649 placebo-treated patients showed a pooled estimate of NASH resolution without worsening of fibrosis and 1 stage reduction or more in fibrosis of 12% and 19%, respectively. Relevant factors involved in “spontaneous” NASH improvement are unknown but could be related to changes in BMI resulting from lifestyle changes, race and ethnicity, age, and, likely, NAFLD-related genetic variations, although more data is needed to better understand the histologic response in placebo-treated patients.54
Semaglutide injections (2.4 mg once weekly) or (2.0 mg once weekly) have been recently approved by the FDA for chronic weight management in adults with obesity or overweight with at least one weight-related condition or glucose control of T2D, respectively. Of note, the semaglutide dose used in the NASH trial is not currently available for the treatment of patients who are overweight/obese or have T2D, but the beneficial effects on body weight reductions and glucose control are similar overall to the effects seen with currently available doses for management of obesity or diabetes. One may consider using semaglutide in patients who are overweight/obese or have T2D with NASH, but in the senior author’s experience, it has been quite challenging to receive the payer’s approval, as its use is not specifically approved to treat liver disease.1
How to follow patients with NAFLD in the clinic
Once a diagnosis of NAFLD is made, the use of noninvasive testing may aid to identify which patients are at high risk of fibrosis. Easy to use clinical tools, such as the NAFLD Fibrosis Score and the Fib-4 index, and liver stiffness measurements using vibration-controlled transient elastography (FibroScan) or magnetic resonance elastography (MRE) are clinically useful noninvasive tools for identifying patients with NAFLD who have a higher likelihood of progressing to advanced fibrosis.1,55 The use of either NAFLD Fibrosis Score (less than -1.455) or Fib-4 index (less than 1.30) low cutoffs may be particularly useful to rule out advanced fibrosis. People with a NAFLD Fibrosis Score (greater than –1.455) or Fib-4 index (greater than 1.30) should undergo liver stiffness measurement (LSM) via FibroScan. Those with an LSM of 8 kPa or higher should be referred to specialized care, where a decision to perform a liver biopsy and initiate monitoring and therapy will be taken. MRE is the most accurate noninvasive method for the estimation of liver fibrosis. When MRE is available, it can be a diagnostic alternative to accurately rule in and rule out patients with advanced fibrosis. This technique can be preferred in clinical trials, but it is rarely used in clinical practice because it is expensive and not easily available. Reassessment by noninvasive scores at 1-3 years’ follow-up will be considered for those with an LSM less than 8 kPa. Patients with NASH cirrhosis should be screened for both gastroesophageal varix and HCC according to the American Association for the Study of Liver Diseases guidelines.56-57
Dr. Vilar-Gomez is assistant professor in the division of gastroenterology and hepatology at Indiana University, Indianapolis. Dr. Chalasani is vice president for academic affairs at Indiana University Health, Indianapolis, and the David W. Crabb Professor of Gastroenterology and Hepatology and an adjunct professor of anatomy, cell biology, and physiology in the division of gastroenterology and hepatology at Indiana University. Dr. Vilar-Gomez reports no financial conflicts of interest. Dr. Chalasani serves as a paid consultant to AbbVie, Boehringer-Ingelheim, Altimmune, Madrigal, Lilly, Zydus, and Galectin. He receives research support from Galectin and DSM.
References
1. Chalasani N et al. Hepatology 2018;67:328-57.
2. Söderberg C et al. Hepatology 2010;51:595-602.
3. Sanyal AJ et al. N Engl J Med 2021;385:1559-69.
4. Vilar-Gomez E et al. Gastroenterology 2018;155:443-57.e17.
5. Younossi ZM et al. Hepatology 2016;64:73-84.
6. EASL-EASD-EASO. J Hepatol 2016;64:1388-402.
7. Wong VW et al. J Hepatol 2018; 69:1349-56.
8. Vilar-Gomez E et al. Gastroenterology 2015;149:367-78.e5; quiz e14-5.
9. Promrat K et al. Hepatology 2010;51:121-9.
10. Wong VW et al. J Hepatol 2013;59:536-42.
11. Berzigotti A et al. Hepatology 2017;65:1293-1305.
12. Sacks FM et al. N Engl J Med 2009;360:859-73.
13. Vilar-Gomez E et al. Hepatology 2022 Jun;75(6):1491-1506.
14. Zelber-Sagi S et al. Liver Int 2017;37:936-49.
15. Hassani Zadeh S et al. J Gastroenterol Hepatol 2021;36:1470-8.
16. Yaskolka Meir A et al. Gut 2021;70:2085-95.
17. Sung KC et al. J Hepatol 2016;65:791-7.
18. Orci LA et al. Clin Gastroenterol Hepatol 2016;14:1398-411.
19. Ryu S et al. J Hepatol 2015;63:1229-37.
20. Kim D et al. Hepatology 2020;72:1556-68.
21. Kim D et al. Clin Gastroenterol Hepatol 2021;19:1240-7.e5.
22. Ascha MS et al. Hepatology 2010;51:1972-8.
23. Bambha K et al. Liver Int 2014;34:1250-8.
24. Lee Y et al. Clin Gastroenterol Hepatol 2019;17:1040-60.e11.
25. Grönroos S et al. JAMA Surg 2021;156:137-46.
26. Fakhry TK et al. Surg Obes Relat Dis 2019;15:502-11.
27. Seeberg KA et al. Ann Intern Med 2022;175:74-83.
28. Bower G et al. Obes Surg 2015;25:2280-9.
29. Jan A et al. Obes Surg 2015;25:1518-26.
30. Hanipah ZN et al. Obes Surg 2018;28:3431-8.
31. Are VS et al. Am J Gastroenterol 2020;115:1849-56.
32. Aminian A et al. JAMA 2021;326:2031-42.
33. Vuppalanchi R et al. Ann Surg 2022;275:e174-80.
34. Simon TG et al. Gut 2021. doi: 10.1136/gutjnl-2021-325724.
35. Lonardo A et al. J Hepatol 2018;68:335-52.
36. Chalasani N et al. Gastroenterology 2004;126:1287-92.
37. Pastori D et al. Dig Liver Dis 2015;47:4-11.
38. Kim RG et al. Clin Gastroenterol Hepatol 2017;15:1521-30.e8.
39. Ahmad J et al. Dig Dis Sci 2017;62:3596-604.
40. Chalasani NP et al. Am J Gastroenterol 2021;116(5):878-98.
41. Rinella ME et al. Hepatology 2019;70:1424-36.
42. Younossi ZM et al. Lancet 2019;394:2184-96.
43. Ratziu V. Clin Liver Dis (Hoboken) 2021;17:398-400.
44. Sanyal AJ et al. N Engl J Med 2010;341:1675-85.
45. Schürks M et al. BMJ 2010;341:c5702.
46. Cusi K et al. Ann Intern Med 2016;165:305-15.
47. Lewis JD et al. JAMA 2015;314:265-77.
48. Billington EO et al. Diabetologia 2015;58:2238-46.
49. Lewis JD et al. Diabetes Care 2011;34:916-22.
50. Erdmann E et al. Diabetes Care 2007;30:2773-8.
51. Viscoli CM et al. J Clin Endocrinol Metab 2017;102:914-22.
52. Armstong MJ et al. Lancet 2016;387:679-90.
53. Newsome PN et al. N Engl J Med 2021;384:1113-24.
54. Ng CH et al. Hepatology 2022;75:1647-61.
55. Kanwal F et al. Gastroenterology 2021;161:1030-1042.e8.
56. Garcia-Tsao G et al. Hepatology 2017;65:310-35.
57. Heimbach JK et al. Hepatology 2018;67:358-80.
Nonalcoholic fatty liver disease (NAFLD) is defined by the presence of hepatic steatosis detected on either imaging or histology in the absence of secondary causes of fatty liver (e.g., excessive alcohol consumption) or other chronic liver diseases.1 For practical NAFLD diagnosis purposes, excessive alcohol intake can be defined as an active or recent history of more than 21 standard drinks per week in men and more than14 standard drinks per week in women. For the sake of terminology, NAFLD is characterized by fatty liver infiltration, affecting at least 5% of hepatocytes, with no evidence of hepatocyte injury, whereas nonalcoholic steatohepatitis (NASH) is defined as the presence of necroinflammation with or without fibrosis in a background of fatty liver.1
Natural history
NASH and the degree of fibrosis are the two most important determinants of the natural history of NAFLD. NASH can evolve into fibrosis and cirrhosis, whereas advanced fibrosis and cirrhosis (stages 3 or 4 of fibrosis) significantly increase the risk of liver-related decompensation and mortality. NAFLD, per se, has been associated with an increased risk of overall mortality, compared with that of the general population.2 The three most common causes of mortality for patients with NAFLD are cardiovascular diseases (CVD), extrahepatic malignancies, and liver-related deaths. Mortality and liver-related events, including hepatic decompensation and hepatocellular carcinoma (HCC), may significantly increase in a dose-dependent manner with increasing fibrosis stages, and stages 3 or 4 of fibrosis may display the highest rates of all-cause mortality and liver-related events.3,4 It is important to note, however, that almost 15% of HCCs occur in patients with NAFLD who do not have cirrhosis.5 The presence of commonly associated comorbidities such as obesity, insulin resistance or diabetes, dyslipidemia, hypothyroidism, polycystic ovary syndrome, and sleep apnea may contribute to an increased risk of NASH and advanced fibrosis and, therefore, an accelerated clinical course of NAFLD.
Nonpharmacological interventions
Lifestyle modification
Lifestyle modification to achieve weight loss remains a first-line intervention in patients with NAFLD. Weight loss achieved either by hypocaloric diet alone or in conjunction with increased physical activity can be beneficial for all patients with NAFLD. The benefits extend not only to those who are overweight and obese but also to those within normal body weight (lean NAFLD).1,6,7 Weight loss of approximately 3%-5% is necessary to improve hepatic steatosis, but a greater weight loss (7%-10%) is required to improve other histopathological features like necroinflammatory lesions and fibrosis.8-10 Individuals with higher BMI and/or type 2 diabetes (T2D) will require a larger weight reduction to achieve a similar benefit on NAFLD-related features.7,8 Weight loss via lifestyle changes can also decrease hepatic venous pressure gradient (HVPG), with greater declines reported among those with more than 10% weight loss.11
Weight loss can be achieved through a variety of modalities, but long-term maintenance of lost weight is much more challenging. A combination of a hypocaloric diet with a caloric deficit of 500-1,000 kcal/d, alongside moderate-intensity exercise and intensive on-site behavioral treatment, will likely increase the possibility of a sustained weight loss over time.1,12 A growing body of scientific evidence indicates that a healthy diet that includes a reduction of high-glycemic-index foods and refined carbohydrates; increased consumption of monounsaturated fatty acids, omega-3 fatty acids, and fibers; and high intakes of olive oil, nuts, vegetables, fruits, legumes, whole grains, and fish can have beneficial effects on NAFLD and its severity.13-16 Adherence to these healthy dietary patterns has been associated with a marked reduction in CVD morbidity and mortality and is, thus, a strategic lifestyle recommendation for patients with NAFLD in whom the leading cause of morbidity and death is CVD.1,3
Exercise alone in adults with NAFLD may reduce hepatic steatosis, but its ability to improve inflammation and fibrosis has not been proven in well-designed RCTs.17,18 Physical activity and exercise have been shown to curb both the development and the progression of NAFLD, and beneficial effects could be achieved independent of weight loss.17,19,20 Most importantly, moderate-to-vigorous physical activity is likely associated with lower all-cause and cardiovascular mortality in patients with NAFLD.21
Heavy alcohol intake should be avoided by patients with NAFLD or NASH, and those with cirrhotic NASH should avoid any alcohol consumption given the risk of HCC and hepatic decompensation.1,4,22 Limiting light-to-moderate alcohol intake among patients without cirrhosis is still under debate.1 People with NAFLD may be advised to drink an equivalent of two to three 8-oz cups of regular brewed coffee daily as it has shown certain antifibrotic effects in NAFLD patients.23
Bariatric surgery
Bariatric surgery is an attractive therapeutic option for eligible obese patients with NAFLD. Bariatric surgery has the potential for inducing great weight loss and, therefore, reverses not only the steatosis, inflammation, and fibrosis among NAFLD individuals but also important comorbid conditions like T2D. A recent systematic review and meta-analysis examining data on the effects of bariatric surgery on histologic features of NAFLD from 32 cohort studies (no RCTs included) showed that bariatric surgery was associated with significant improvements in steatosis (66%), lobular inflammation (50%), ballooning degeneration (76%), and fibrosis (40%), and the benefits were significantly higher in those who underwent Roux-en-Y gastric bypass (RYGB). Of note, worsening of liver histology, including fibrosis, could be seen in up to 12% of patients who underwent bariatric surgery.24 The postsurgical weight regained after RYGB could explain partly the lack of fibrosis improvement or even worsening of fibrosis, although further research is needed to clarify these controversial findings.
RYGB and sleeve gastrectomy (SG) are the most commonly performed bariatric surgeries worldwide. Patients who undergo RYGB achieve higher weight loss when compared with those treated with SG.25 Among all bariatric procedures, RYGB could result in a higher proportion of complete resolution of NAFLD than SG, although evidence is inconclusive on fibrosis improvement rates.24,26 Most recently, a single-center RCT has compared the effects of RYGB vs. SG on liver fat content and fibrosis in patients with severe obesity and T2D.27 Data showed that both surgical procedures were highly and equally effective in reducing fatty liver content (quantified by magnetic resonance imaging), with an almost complete resolution of the fatty liver at 1 year of both surgical interventions. The beneficial effects of both GB and SG on fibrosis (assessed by enhanced liver test [ELF]) were less evident with no substantial difference between the two groups. Importantly, 69% of participants had an increase in their ELF scores during the study, despite the majority of participants achieving significant reductions in their body weights and better glycemic control at the end of the study. These findings might be considered with caution as several factors, such as the duration of the study (only 1 year) and lack of a liver biopsy to confirm fibrosis changes over time, could be influencing the study results.
Among all NAFLD phenotypes, those with cirrhosis and, most importantly, hepatic decompensation appear to be at increased risk of perioperative mortality and inpatient hospital stays than those without cirrhosis.28-29 Bariatric surgery is an absolute contraindication in patients with decompensated cirrhosis (Child B and Child C). Among compensated -Child A- cirrhotics, those with portal hypertension are at increased risk of morbidity and perioperative mortality.30 A recent analysis of National Inpatient Sample data suggested that the rates of complications in those with cirrhosis have decreased with time, which could be due to a better selection process and the use of more restrictive bariatric surgery in those with cirrhosis. Low volume centers (defined as less than 50 procedures per year) and nonrestrictive bariatric surgery were associated with a higher mortality rate. These data may suggest that patients with cirrhosis should undergo bariatric surgery only in high-volume centers after a multidisciplinary evaluation.31 Bariatric endoscopy is emerging as a new treatment for obesity, but the long-term durability of its effects remains to be determined.
A recent retrospective cohort study, including 1,158 adult patients with biopsy-proven NASH, has investigated the benefits of bariatric surgery on the occurrence of major adverse liver and cardiovascular outcomes in 650 patients who underwent bariatric surgery, compared with 508 patients who received nonsurgical usual care. This study showed that bariatric surgery was associated with 88% lower risk of progression of fatty liver to cirrhosis, liver cancer, or liver-related death, and 70% lower risk of serious CVD events during a follow-up period of 10 years.32 Within 1 year after surgery, 0.6% of patients died from surgical complications. The potential benefits of bariatric surgery in patients with NAFLD must be balanced against surgical risk, especially in eligible obese individuals with established cirrhosis. Data from a retrospective cohort study have shown that bariatric surgery in obese cirrhotic patients does not seem to associate with excessive mortality, compared with noncirrhotic obese patients.33 More data on immediate complication rates and long-term outcomes in patients with NAFLD by type of bariatric surgery is also required.
NAFLD as a standalone is not an indication for bariatric surgery. However, it could be considered in NAFLD patients who have a BMI of 40 kg/m2 or more without coexisting comorbidities or with a BMI of 35 kg/m2 or more and one or more severe obesity-related comorbidities, including T2D, hypertension, hyperlipidemia, or obstructive sleep apnea. Bariatric surgery must always be offered in centers with an experienced bariatric surgery program.1
Management of comorbidities
Given the multiple comorbidities associated with NAFLD and the potential to influence its severity, a comprehensive and multidisciplinary approach is needed to ameliorate not only the progression of liver disease but also those complications related to metabolic syndrome, hyperlipidemia, hypertension, diabetes, and other related conditions. Of note, all patients with NAFLD should receive aggressive management of comorbidities regardless of the severity of NAFLD. Ideally, a multidisciplinary team – including a primary care provider, an endocrinologist for patients with T2D, and a gastroenterologist/hepatologist – is needed to successfully manage patients with NAFLD.
It is well recognized that individuals with biopsy-proven NAFLD are at a higher risk of coronary heart disease, stroke, congestive heart failure, and death resulting from CVD when compared with the non-NAFLD population, and excess in CVD morbidity and mortality is evident across all stages of NAFLD and increases with worsening disease severity.34 The strong association between CVD and NAFLD has important clinical implications that may influence the decision to initiate treatment for primary prevention, including lipid-lowering, antihypertensive, or antiplatelet therapies.35 Statins are widely used to reduce LDL cholesterol and have been proven to be safe in NAFLD, including for those with elevated liver enzymes and even in compensated cirrhosis, in several studies conducted during the last 15 years.36 Statins are characterized by anti-inflammatory, anti-oxidative, antifibrotic, and plaque-stabilizing effects, whereby they may improve vascular and hepatic function among patients with NAFLD and reduce cardiovascular risk.37 Statin use for the treatment of NAFLD is still controversial and off-label and is not specifically recommended to treat NASH, but positive results have been shown for reductions in liver enzymes.1 A recent meta-analysis of 13 studies showed that continued use of statin in cirrhosis was associated with a 46% and 44% risk reduction in hepatic decompensation and mortality, respectively.38
The Food and Drug Administration has approved omega-3 (n-3) fatty acid agents and fibrates for the treatment of very high triglycerides (500 mg/dL or higher); however, no specific indications exist to treat NAFLD.1 Fenofibrate is related to mild aminotransferase elevations and, in some cases, severe liver injury, so caution must be paid, especially within 2 days of taking the drug.39-40
NAFLD phenotypes that need liver pharmacotherapy
There are still no FDA-approved drugs or biological treatments for NASH. Pharmacological interventions aiming primarily at improving liver disease should generally be limited to those with biopsy-proven NASH and clinically significant fibrosis (fibrosis stages of 2 or greater).1,4 For FDA approval, medications used for treating NAFLD with fibrosis need to meet one of the following endpoint criteria: resolution of NASH without worsening of fibrosis, improvement in fibrosis without worsening of NASH, or both. In addition to those criteria, a new medication might improve the metabolic profile and have a tolerable safety profile. Table 1 displays those NAFLD phenotypes that will likely benefit from liver-directed therapy.

Obeticholic acid as an experimental therapy for NASH
A planned month-18 interim analysis of a multicentre, phase III RCT examined the efficacy and safety of obeticholic acid (OCA), a farnesoid X receptor agonist, in patients with NASH and stages 1-3 of fibrosis. The primary endpoint (fibrosis reduction 1 stage or more with no worsening of NASH) was met by 12% of patients in the placebo group, 18% of patients receiving OCA 10 mg (P = .045), and 23% of those receiving OCA 25 mg (P = .0002). An alternative primary endpoint of NASH resolution with no worsening of fibrosis was not met. OCA 25 mg led to the highest rates of pruritus and hyperlipidemia, compared with OCA 10 mg.42 These side effects seem to be related to the activation of the farnesoid X receptor.43
Currently available but off label medications
Vitamin E, an antioxidant, administered at a daily dose of 800 IU/day improves steatosis, inflammation, and ballooning, but not fibrosis in nondiabetic adults with biopsy-proven NASH.44 Vitamin E for 96 weeks was associated with a significantly higher rate of improvement in NASH (43% vs. 19%, P less than .01), compared with placebo.44 In the Treatment of Nonalcoholic Fatty Liver Disease in Children trial (TONIC), which examined vitamin E (800 IU/day) or metformin (500 mg twice daily) against placebo in children with biopsy-proven NAFLD, resolution of NASH was significantly greater in children treated with vitamin E than in children treated with placebo (58% vs. 28%, P less than .01). Metformin did not significantly improve the NASH resolution rates, compared with placebo (41% vs. 28%, P = .23). Vitamin E could be recommended for nondiabetic adults or children if lifestyle modifications do not produce the expected results as a result of noncompliance or ineffectiveness. Since continued use of vitamin E has been suggested to be associated with a very small increase in the risk for prostate cancer (an absolute increase of 1.6 per 1,000 person-years of vitamin E use) in men, risks and benefits should be discussed with each patient before starting therapy. A meta-analysis of nine placebo-controlled trials including roughly 119,000 patients reported that vitamin E supplementation increases the risk of hemorrhagic stroke by 20% while reducing ischemic stroke by 10%. It was estimated that vitamin E supplementation would prevent one ischemic stroke per 476 treated patients while inducing one hemorrhagic stroke for every 1,250 patients. It is noteworthy that the combination of vitamin E with anticoagulant and/or antiplatelet therapy was not examined in this trial, so we could not determine how combination therapy might affect the risk of ischemic or hemorrhagic stroke.45

Thiazolidinediones drugs have been reported to be effective in improving NAFLD in many human studies. Evidence from RCTs suggests that pioglitazone could significantly improve glucose metabolism, alanine aminotransferase, and liver histology – such as hepatic steatosis, lobular inflammation, and ballooning degeneration – among patients with or without T2D. However, the beneficial effects on improving fibrosis remain to be verified.1,46 Because of safety concerns, the risk/benefit balance of using pioglitazone to treat NASH should be discussed with each patient.47-48 Pioglitazone has been associated with long-term risk of bladder cancer,49 congestive heart failure,50 and bone fractures.51 Data from the Pioglitazone, Vitamin E, or Placebo for Nonalcoholic Steatohepatitis (PIVENS) trial showed that pioglitazone was significantly associated with weight gain but with no other serious adverse events. However, this study was not powered to test any safety-related hypotheses.44
Glucagon-like peptide 1 analogs have been reported to induce weight loss and reduce insulin resistance, which may lead to improvements in NAFLD. Phase II RCTs of glucagon-like peptide 1 receptor agonists (liraglutide and semaglutide) for the treatment of biopsy-proven NASH showed significant improvements in serum liver enzymes, steatosis, and inflammation, as well as NASH resolution without worsening liver fibrosis, although no direct benefit was observed in reversing fibrosis.52-53 One of these studies explores the efficacy and safety of different doses of daily subcutaneous semaglutide vs. placebo on the rates of resolution of NASH with no worsening of fibrosis. The highest dose (0.4 mg) showed the greatest difference (59% vs. 17%, P less than .01), compared with the placebo arm. However, there was no difference in improvement in fibrosis stage between the two groups (43% in the 0.4-mg group vs. 33% in the placebo group, P = .48).53 Gastrointestinal adverse events were common in the semaglutide arm.
“Spontaneous” NASH resolution and fibrosis improvement are commonly seen in participants assigned to placebo arms in clinical trials. A recent meta-analysis of 43 RCTs including 2649 placebo-treated patients showed a pooled estimate of NASH resolution without worsening of fibrosis and 1 stage reduction or more in fibrosis of 12% and 19%, respectively. Relevant factors involved in “spontaneous” NASH improvement are unknown but could be related to changes in BMI resulting from lifestyle changes, race and ethnicity, age, and, likely, NAFLD-related genetic variations, although more data is needed to better understand the histologic response in placebo-treated patients.54
Semaglutide injections (2.4 mg once weekly) or (2.0 mg once weekly) have been recently approved by the FDA for chronic weight management in adults with obesity or overweight with at least one weight-related condition or glucose control of T2D, respectively. Of note, the semaglutide dose used in the NASH trial is not currently available for the treatment of patients who are overweight/obese or have T2D, but the beneficial effects on body weight reductions and glucose control are similar overall to the effects seen with currently available doses for management of obesity or diabetes. One may consider using semaglutide in patients who are overweight/obese or have T2D with NASH, but in the senior author’s experience, it has been quite challenging to receive the payer’s approval, as its use is not specifically approved to treat liver disease.1
How to follow patients with NAFLD in the clinic
Once a diagnosis of NAFLD is made, the use of noninvasive testing may aid to identify which patients are at high risk of fibrosis. Easy to use clinical tools, such as the NAFLD Fibrosis Score and the Fib-4 index, and liver stiffness measurements using vibration-controlled transient elastography (FibroScan) or magnetic resonance elastography (MRE) are clinically useful noninvasive tools for identifying patients with NAFLD who have a higher likelihood of progressing to advanced fibrosis.1,55 The use of either NAFLD Fibrosis Score (less than -1.455) or Fib-4 index (less than 1.30) low cutoffs may be particularly useful to rule out advanced fibrosis. People with a NAFLD Fibrosis Score (greater than –1.455) or Fib-4 index (greater than 1.30) should undergo liver stiffness measurement (LSM) via FibroScan. Those with an LSM of 8 kPa or higher should be referred to specialized care, where a decision to perform a liver biopsy and initiate monitoring and therapy will be taken. MRE is the most accurate noninvasive method for the estimation of liver fibrosis. When MRE is available, it can be a diagnostic alternative to accurately rule in and rule out patients with advanced fibrosis. This technique can be preferred in clinical trials, but it is rarely used in clinical practice because it is expensive and not easily available. Reassessment by noninvasive scores at 1-3 years’ follow-up will be considered for those with an LSM less than 8 kPa. Patients with NASH cirrhosis should be screened for both gastroesophageal varix and HCC according to the American Association for the Study of Liver Diseases guidelines.56-57
Dr. Vilar-Gomez is assistant professor in the division of gastroenterology and hepatology at Indiana University, Indianapolis. Dr. Chalasani is vice president for academic affairs at Indiana University Health, Indianapolis, and the David W. Crabb Professor of Gastroenterology and Hepatology and an adjunct professor of anatomy, cell biology, and physiology in the division of gastroenterology and hepatology at Indiana University. Dr. Vilar-Gomez reports no financial conflicts of interest. Dr. Chalasani serves as a paid consultant to AbbVie, Boehringer-Ingelheim, Altimmune, Madrigal, Lilly, Zydus, and Galectin. He receives research support from Galectin and DSM.
References
1. Chalasani N et al. Hepatology 2018;67:328-57.
2. Söderberg C et al. Hepatology 2010;51:595-602.
3. Sanyal AJ et al. N Engl J Med 2021;385:1559-69.
4. Vilar-Gomez E et al. Gastroenterology 2018;155:443-57.e17.
5. Younossi ZM et al. Hepatology 2016;64:73-84.
6. EASL-EASD-EASO. J Hepatol 2016;64:1388-402.
7. Wong VW et al. J Hepatol 2018; 69:1349-56.
8. Vilar-Gomez E et al. Gastroenterology 2015;149:367-78.e5; quiz e14-5.
9. Promrat K et al. Hepatology 2010;51:121-9.
10. Wong VW et al. J Hepatol 2013;59:536-42.
11. Berzigotti A et al. Hepatology 2017;65:1293-1305.
12. Sacks FM et al. N Engl J Med 2009;360:859-73.
13. Vilar-Gomez E et al. Hepatology 2022 Jun;75(6):1491-1506.
14. Zelber-Sagi S et al. Liver Int 2017;37:936-49.
15. Hassani Zadeh S et al. J Gastroenterol Hepatol 2021;36:1470-8.
16. Yaskolka Meir A et al. Gut 2021;70:2085-95.
17. Sung KC et al. J Hepatol 2016;65:791-7.
18. Orci LA et al. Clin Gastroenterol Hepatol 2016;14:1398-411.
19. Ryu S et al. J Hepatol 2015;63:1229-37.
20. Kim D et al. Hepatology 2020;72:1556-68.
21. Kim D et al. Clin Gastroenterol Hepatol 2021;19:1240-7.e5.
22. Ascha MS et al. Hepatology 2010;51:1972-8.
23. Bambha K et al. Liver Int 2014;34:1250-8.
24. Lee Y et al. Clin Gastroenterol Hepatol 2019;17:1040-60.e11.
25. Grönroos S et al. JAMA Surg 2021;156:137-46.
26. Fakhry TK et al. Surg Obes Relat Dis 2019;15:502-11.
27. Seeberg KA et al. Ann Intern Med 2022;175:74-83.
28. Bower G et al. Obes Surg 2015;25:2280-9.
29. Jan A et al. Obes Surg 2015;25:1518-26.
30. Hanipah ZN et al. Obes Surg 2018;28:3431-8.
31. Are VS et al. Am J Gastroenterol 2020;115:1849-56.
32. Aminian A et al. JAMA 2021;326:2031-42.
33. Vuppalanchi R et al. Ann Surg 2022;275:e174-80.
34. Simon TG et al. Gut 2021. doi: 10.1136/gutjnl-2021-325724.
35. Lonardo A et al. J Hepatol 2018;68:335-52.
36. Chalasani N et al. Gastroenterology 2004;126:1287-92.
37. Pastori D et al. Dig Liver Dis 2015;47:4-11.
38. Kim RG et al. Clin Gastroenterol Hepatol 2017;15:1521-30.e8.
39. Ahmad J et al. Dig Dis Sci 2017;62:3596-604.
40. Chalasani NP et al. Am J Gastroenterol 2021;116(5):878-98.
41. Rinella ME et al. Hepatology 2019;70:1424-36.
42. Younossi ZM et al. Lancet 2019;394:2184-96.
43. Ratziu V. Clin Liver Dis (Hoboken) 2021;17:398-400.
44. Sanyal AJ et al. N Engl J Med 2010;341:1675-85.
45. Schürks M et al. BMJ 2010;341:c5702.
46. Cusi K et al. Ann Intern Med 2016;165:305-15.
47. Lewis JD et al. JAMA 2015;314:265-77.
48. Billington EO et al. Diabetologia 2015;58:2238-46.
49. Lewis JD et al. Diabetes Care 2011;34:916-22.
50. Erdmann E et al. Diabetes Care 2007;30:2773-8.
51. Viscoli CM et al. J Clin Endocrinol Metab 2017;102:914-22.
52. Armstong MJ et al. Lancet 2016;387:679-90.
53. Newsome PN et al. N Engl J Med 2021;384:1113-24.
54. Ng CH et al. Hepatology 2022;75:1647-61.
55. Kanwal F et al. Gastroenterology 2021;161:1030-1042.e8.
56. Garcia-Tsao G et al. Hepatology 2017;65:310-35.
57. Heimbach JK et al. Hepatology 2018;67:358-80.