User login
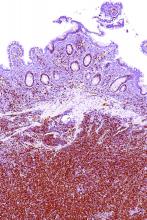
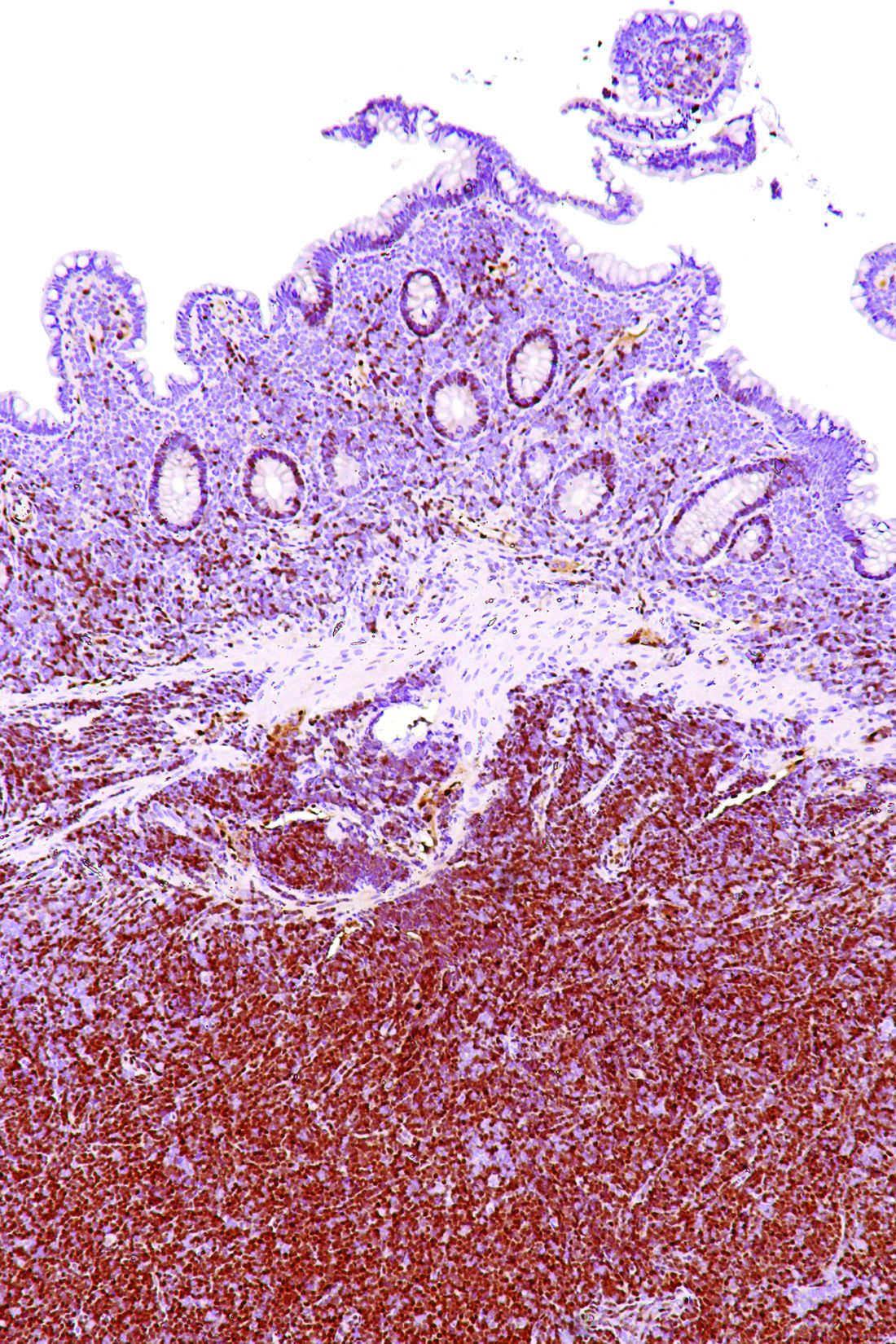
SOX11 shows value as diagnostic marker in MCL
SOX11 may be an accurate diagnostic marker for mantle cell lymphoma (MCL), allowing clinicians to distinguish it from other lymphoproliferative disorders, according to findings from a meta-analysis of 14 case-control studies.
Woojoo Lee, PhD, of Inha University, Incheon, Republic of Korea, and coinvestigators, evaluated the diagnostic accuracy of SOX11 immunohistochemistry for the diagnosis of MCL. The results were published in PLoS One.
The researchers searched major databases for studies that evaluated the use of SOX11 immunohistochemistry in patients with MCL and other lymphoproliferative disorders. After applying the search parameters, the team identified 383 studies, 14 of which were included in the meta-analysis. Various data were extracted from eligible studies, including the type and clonality of anti-SOX11 antibody, number of SOX11-positive lymphomas (MCLs and other lymphoproliferative disorders), specificity, sensitivity, and others. After combining the data, the investigators calculated pooled sensitivity, specificity, and area under the curve. Among the included studies, hairy cell leukemia, Burkitt’s lymphoma, and lymphoblastic lymphoma were common among patient populations. In total, clone MRQ-58 mouse antibodies were used in five study populations.
The researchers found that the pooled specificity was 0.95 (95% CI, 0.9-0.97), and sensitivity was 0.9 (95% CI, 0.86-0.92). There was statistically significant substantial heterogeneity observed for specificity, but not for sensitivity, the investigators reported.
“The results demonstrated that SOX11 was a potential diagnostic marker for MCL,” they wrote. “Meta-regression revealed a significant inverse relationship between specificity and proportions of [Burkitt’s lymphoma, lymphoblastic lymphoma, and hairy cell leukemia].”
With respect to antibody type, the clone MRQ-58 mouse antibody showed consistently high specificity in the clinical setting, despite observed heterogeneity.
“Future studies using MRQ-58 are needed to improve our understanding of the diagnostic accuracy of SOX11 immunohistochemistry for MCL,” the investigators wrote.
The study was funded by the Next-Generation BioGreen 21 program (Republic of Korea). The authors reported having no conflicts of interest.
SOURCE: Lee W et al. PLoS One. 2019 Nov 12. doi: 10.1371/journal.pone.0225096.
SOX11 may be an accurate diagnostic marker for mantle cell lymphoma (MCL), allowing clinicians to distinguish it from other lymphoproliferative disorders, according to findings from a meta-analysis of 14 case-control studies.
Woojoo Lee, PhD, of Inha University, Incheon, Republic of Korea, and coinvestigators, evaluated the diagnostic accuracy of SOX11 immunohistochemistry for the diagnosis of MCL. The results were published in PLoS One.
The researchers searched major databases for studies that evaluated the use of SOX11 immunohistochemistry in patients with MCL and other lymphoproliferative disorders. After applying the search parameters, the team identified 383 studies, 14 of which were included in the meta-analysis. Various data were extracted from eligible studies, including the type and clonality of anti-SOX11 antibody, number of SOX11-positive lymphomas (MCLs and other lymphoproliferative disorders), specificity, sensitivity, and others. After combining the data, the investigators calculated pooled sensitivity, specificity, and area under the curve. Among the included studies, hairy cell leukemia, Burkitt’s lymphoma, and lymphoblastic lymphoma were common among patient populations. In total, clone MRQ-58 mouse antibodies were used in five study populations.
The researchers found that the pooled specificity was 0.95 (95% CI, 0.9-0.97), and sensitivity was 0.9 (95% CI, 0.86-0.92). There was statistically significant substantial heterogeneity observed for specificity, but not for sensitivity, the investigators reported.
“The results demonstrated that SOX11 was a potential diagnostic marker for MCL,” they wrote. “Meta-regression revealed a significant inverse relationship between specificity and proportions of [Burkitt’s lymphoma, lymphoblastic lymphoma, and hairy cell leukemia].”
With respect to antibody type, the clone MRQ-58 mouse antibody showed consistently high specificity in the clinical setting, despite observed heterogeneity.
“Future studies using MRQ-58 are needed to improve our understanding of the diagnostic accuracy of SOX11 immunohistochemistry for MCL,” the investigators wrote.
The study was funded by the Next-Generation BioGreen 21 program (Republic of Korea). The authors reported having no conflicts of interest.
SOURCE: Lee W et al. PLoS One. 2019 Nov 12. doi: 10.1371/journal.pone.0225096.
SOX11 may be an accurate diagnostic marker for mantle cell lymphoma (MCL), allowing clinicians to distinguish it from other lymphoproliferative disorders, according to findings from a meta-analysis of 14 case-control studies.
Woojoo Lee, PhD, of Inha University, Incheon, Republic of Korea, and coinvestigators, evaluated the diagnostic accuracy of SOX11 immunohistochemistry for the diagnosis of MCL. The results were published in PLoS One.
The researchers searched major databases for studies that evaluated the use of SOX11 immunohistochemistry in patients with MCL and other lymphoproliferative disorders. After applying the search parameters, the team identified 383 studies, 14 of which were included in the meta-analysis. Various data were extracted from eligible studies, including the type and clonality of anti-SOX11 antibody, number of SOX11-positive lymphomas (MCLs and other lymphoproliferative disorders), specificity, sensitivity, and others. After combining the data, the investigators calculated pooled sensitivity, specificity, and area under the curve. Among the included studies, hairy cell leukemia, Burkitt’s lymphoma, and lymphoblastic lymphoma were common among patient populations. In total, clone MRQ-58 mouse antibodies were used in five study populations.
The researchers found that the pooled specificity was 0.95 (95% CI, 0.9-0.97), and sensitivity was 0.9 (95% CI, 0.86-0.92). There was statistically significant substantial heterogeneity observed for specificity, but not for sensitivity, the investigators reported.
“The results demonstrated that SOX11 was a potential diagnostic marker for MCL,” they wrote. “Meta-regression revealed a significant inverse relationship between specificity and proportions of [Burkitt’s lymphoma, lymphoblastic lymphoma, and hairy cell leukemia].”
With respect to antibody type, the clone MRQ-58 mouse antibody showed consistently high specificity in the clinical setting, despite observed heterogeneity.
“Future studies using MRQ-58 are needed to improve our understanding of the diagnostic accuracy of SOX11 immunohistochemistry for MCL,” the investigators wrote.
The study was funded by the Next-Generation BioGreen 21 program (Republic of Korea). The authors reported having no conflicts of interest.
SOURCE: Lee W et al. PLoS One. 2019 Nov 12. doi: 10.1371/journal.pone.0225096.
FROM PLOS ONE
Acalabrutinib may outperform other targeted therapies in MCL
For patients with relapsed or refractory mantle cell lymphoma (MCL), second generation Bruton’s tyrosine kinase (BTK) inhibitor acalabrutinib may offer increased response rates and better tolerability compared with other single-agent targeted therapies, based on a recent analysis.
Improved safety could lead to long-term benefits resulting from extended treatment duration, according to lead author Claire Telford, PhD, of AstraZeneca in Gaithersburg, Md., and colleagues. AstraZeneca manufactures acalabrutinib (Calquence).
Currently, treatment for MCL is guided by a number of clinical considerations, the investigators explained in Clinical Therapeutics.
“The type of treatment recommended for relapsed/refractory MCL depends on multiple factors, namely time to the relapse, extent of disease, previous regimens, candidacy for allogeneic stem cell transplantation, and the patient’s overall health,” they wrote.
To determine how acalabrutinib stacks up with other options, the investigators drew data from the phase 2 ACE-LY-004 trial, which tested acalabrutinib in 124 patients with relapsed or refractory MCL. After matching, the investigators compared the ACE-LY-004 outcomes from 12 other trials, in which patients received different targeted therapies.
Results pointed to higher overall response and complete response rates for acalabrutinib, compared with other single-agent targeted therapy. Specifically, acalabrutinib had a higher overall response rate, compared with ibrutinib (9.3% higher), lenalidomide (38.1% higher), temsirolimus (40.7% higher), and bortezomib (50.6% higher). For each of these, complete responses also were higher.
There was no significant difference in overall response or complete response rates between acalabrutinib and rituximab combinations – bendamustine plus rituximab, ibrutinib plus rituximab, and lenalidomide plus rituximab.
The investigators also highlighted a number of safety advantages. Compared with ibrutinib, acalabrutinib was associated with significantly fewer instances of grade 3 or 4 atrial fibrillation. Risk of grade 3 or 4 thrombocytopenia was significantly lower with acalabrutinib than with ibrutinib, bortezomib, lenalidomide, and temsirolimus.
Still, in some instances, acalabrutinib was comparatively less tolerable. It was associated with a higher risk of grade 3 or 4 infections than bendamustine plus rituximab; and anemia was more common among patients receiving acalabrutinib than among those who had lenalidomide plus rituximab or ibrutinib plus rituximab.
“This comparison of targeted therapies used in the treatment of relapsed/refractory MCL has shown that acalabrutinib has the potential to provide higher response rates, with trends for longer [progression-free survival] and [overall survival], and an improved safety profile,” the investigators wrote.
The study was funded by AstraZeneca. Dr. Telford is an employee of AstraZeneca and other authors reported financial relationships with the company.
SOURCE: Telford C et al. Clin Ther. 2019 Nov 4. doi: 10.1016/j.clinthera.2019.09.012 .
For patients with relapsed or refractory mantle cell lymphoma (MCL), second generation Bruton’s tyrosine kinase (BTK) inhibitor acalabrutinib may offer increased response rates and better tolerability compared with other single-agent targeted therapies, based on a recent analysis.
Improved safety could lead to long-term benefits resulting from extended treatment duration, according to lead author Claire Telford, PhD, of AstraZeneca in Gaithersburg, Md., and colleagues. AstraZeneca manufactures acalabrutinib (Calquence).
Currently, treatment for MCL is guided by a number of clinical considerations, the investigators explained in Clinical Therapeutics.
“The type of treatment recommended for relapsed/refractory MCL depends on multiple factors, namely time to the relapse, extent of disease, previous regimens, candidacy for allogeneic stem cell transplantation, and the patient’s overall health,” they wrote.
To determine how acalabrutinib stacks up with other options, the investigators drew data from the phase 2 ACE-LY-004 trial, which tested acalabrutinib in 124 patients with relapsed or refractory MCL. After matching, the investigators compared the ACE-LY-004 outcomes from 12 other trials, in which patients received different targeted therapies.
Results pointed to higher overall response and complete response rates for acalabrutinib, compared with other single-agent targeted therapy. Specifically, acalabrutinib had a higher overall response rate, compared with ibrutinib (9.3% higher), lenalidomide (38.1% higher), temsirolimus (40.7% higher), and bortezomib (50.6% higher). For each of these, complete responses also were higher.
There was no significant difference in overall response or complete response rates between acalabrutinib and rituximab combinations – bendamustine plus rituximab, ibrutinib plus rituximab, and lenalidomide plus rituximab.
The investigators also highlighted a number of safety advantages. Compared with ibrutinib, acalabrutinib was associated with significantly fewer instances of grade 3 or 4 atrial fibrillation. Risk of grade 3 or 4 thrombocytopenia was significantly lower with acalabrutinib than with ibrutinib, bortezomib, lenalidomide, and temsirolimus.
Still, in some instances, acalabrutinib was comparatively less tolerable. It was associated with a higher risk of grade 3 or 4 infections than bendamustine plus rituximab; and anemia was more common among patients receiving acalabrutinib than among those who had lenalidomide plus rituximab or ibrutinib plus rituximab.
“This comparison of targeted therapies used in the treatment of relapsed/refractory MCL has shown that acalabrutinib has the potential to provide higher response rates, with trends for longer [progression-free survival] and [overall survival], and an improved safety profile,” the investigators wrote.
The study was funded by AstraZeneca. Dr. Telford is an employee of AstraZeneca and other authors reported financial relationships with the company.
SOURCE: Telford C et al. Clin Ther. 2019 Nov 4. doi: 10.1016/j.clinthera.2019.09.012 .
For patients with relapsed or refractory mantle cell lymphoma (MCL), second generation Bruton’s tyrosine kinase (BTK) inhibitor acalabrutinib may offer increased response rates and better tolerability compared with other single-agent targeted therapies, based on a recent analysis.
Improved safety could lead to long-term benefits resulting from extended treatment duration, according to lead author Claire Telford, PhD, of AstraZeneca in Gaithersburg, Md., and colleagues. AstraZeneca manufactures acalabrutinib (Calquence).
Currently, treatment for MCL is guided by a number of clinical considerations, the investigators explained in Clinical Therapeutics.
“The type of treatment recommended for relapsed/refractory MCL depends on multiple factors, namely time to the relapse, extent of disease, previous regimens, candidacy for allogeneic stem cell transplantation, and the patient’s overall health,” they wrote.
To determine how acalabrutinib stacks up with other options, the investigators drew data from the phase 2 ACE-LY-004 trial, which tested acalabrutinib in 124 patients with relapsed or refractory MCL. After matching, the investigators compared the ACE-LY-004 outcomes from 12 other trials, in which patients received different targeted therapies.
Results pointed to higher overall response and complete response rates for acalabrutinib, compared with other single-agent targeted therapy. Specifically, acalabrutinib had a higher overall response rate, compared with ibrutinib (9.3% higher), lenalidomide (38.1% higher), temsirolimus (40.7% higher), and bortezomib (50.6% higher). For each of these, complete responses also were higher.
There was no significant difference in overall response or complete response rates between acalabrutinib and rituximab combinations – bendamustine plus rituximab, ibrutinib plus rituximab, and lenalidomide plus rituximab.
The investigators also highlighted a number of safety advantages. Compared with ibrutinib, acalabrutinib was associated with significantly fewer instances of grade 3 or 4 atrial fibrillation. Risk of grade 3 or 4 thrombocytopenia was significantly lower with acalabrutinib than with ibrutinib, bortezomib, lenalidomide, and temsirolimus.
Still, in some instances, acalabrutinib was comparatively less tolerable. It was associated with a higher risk of grade 3 or 4 infections than bendamustine plus rituximab; and anemia was more common among patients receiving acalabrutinib than among those who had lenalidomide plus rituximab or ibrutinib plus rituximab.
“This comparison of targeted therapies used in the treatment of relapsed/refractory MCL has shown that acalabrutinib has the potential to provide higher response rates, with trends for longer [progression-free survival] and [overall survival], and an improved safety profile,” the investigators wrote.
The study was funded by AstraZeneca. Dr. Telford is an employee of AstraZeneca and other authors reported financial relationships with the company.
SOURCE: Telford C et al. Clin Ther. 2019 Nov 4. doi: 10.1016/j.clinthera.2019.09.012 .
FROM CLINICAL THERAPEUTICS
FDA approves Brukinsa for relapsed, refractory MCL
The Food and Drug Administration has approved zanubrutinib (Brukinsa) for the treatment of mantle cell lymphoma (MCL) in adult patients who have received at least one prior therapy.
The approval is based on results from two separate studies; in a global phase 1/2 trial, patients with relapsed or refractory MCL who received zanubrutinib had an overall response rate of 84%, with 22% experiencing a complete response and 62% experiencing partial response. Median duration of response was 18.5 months. The ORR in the second study – a multicenter phase 2 trial – was also 84%, but with 59% experiencing a complete response and 24% experiencing partial response; duration of response was 19.5 months.
The most common adverse events reported during the trials were decreased neutrophil count, decreased platelet count, upper respiratory tract infection, decreased white blood cell count, decreased hemoglobin, rash, bruising, diarrhea, cough, musculoskeletal pain, pneumonia, urinary tract infection, hematuria, fatigue, constipation, and hemorrhage. The most common serious adverse events were pneumonia and hemorrhage.
Of the 118 patients with MCL treated with zanubrutinib over the two trials, 8 had to be discontinued because of adverse events.
The recommended dose of zanubrutinib is 320 mg, taken orally 160 mg twice daily or 320 mg once daily, with or without food.
“BTK [Bruton kinase] inhibition is an established mode of treatment for patients with MCL, but many patients treated with previously approved BTK inhibitors do not fully respond to BTK therapy or are forced to discontinue treatment early due to side effects. Today we have a new option for our adult patients who have received one prior systemic or targeted therapy and are living with MCL,” Luhua (Michael) Wang, MD, clinical trial investigator and professor in the department of lymphoma and myeloma at the University of Texas MD Anderson Cancer Center, Houston, said in a statement.
The Food and Drug Administration has approved zanubrutinib (Brukinsa) for the treatment of mantle cell lymphoma (MCL) in adult patients who have received at least one prior therapy.
The approval is based on results from two separate studies; in a global phase 1/2 trial, patients with relapsed or refractory MCL who received zanubrutinib had an overall response rate of 84%, with 22% experiencing a complete response and 62% experiencing partial response. Median duration of response was 18.5 months. The ORR in the second study – a multicenter phase 2 trial – was also 84%, but with 59% experiencing a complete response and 24% experiencing partial response; duration of response was 19.5 months.
The most common adverse events reported during the trials were decreased neutrophil count, decreased platelet count, upper respiratory tract infection, decreased white blood cell count, decreased hemoglobin, rash, bruising, diarrhea, cough, musculoskeletal pain, pneumonia, urinary tract infection, hematuria, fatigue, constipation, and hemorrhage. The most common serious adverse events were pneumonia and hemorrhage.
Of the 118 patients with MCL treated with zanubrutinib over the two trials, 8 had to be discontinued because of adverse events.
The recommended dose of zanubrutinib is 320 mg, taken orally 160 mg twice daily or 320 mg once daily, with or without food.
“BTK [Bruton kinase] inhibition is an established mode of treatment for patients with MCL, but many patients treated with previously approved BTK inhibitors do not fully respond to BTK therapy or are forced to discontinue treatment early due to side effects. Today we have a new option for our adult patients who have received one prior systemic or targeted therapy and are living with MCL,” Luhua (Michael) Wang, MD, clinical trial investigator and professor in the department of lymphoma and myeloma at the University of Texas MD Anderson Cancer Center, Houston, said in a statement.
The Food and Drug Administration has approved zanubrutinib (Brukinsa) for the treatment of mantle cell lymphoma (MCL) in adult patients who have received at least one prior therapy.
The approval is based on results from two separate studies; in a global phase 1/2 trial, patients with relapsed or refractory MCL who received zanubrutinib had an overall response rate of 84%, with 22% experiencing a complete response and 62% experiencing partial response. Median duration of response was 18.5 months. The ORR in the second study – a multicenter phase 2 trial – was also 84%, but with 59% experiencing a complete response and 24% experiencing partial response; duration of response was 19.5 months.
The most common adverse events reported during the trials were decreased neutrophil count, decreased platelet count, upper respiratory tract infection, decreased white blood cell count, decreased hemoglobin, rash, bruising, diarrhea, cough, musculoskeletal pain, pneumonia, urinary tract infection, hematuria, fatigue, constipation, and hemorrhage. The most common serious adverse events were pneumonia and hemorrhage.
Of the 118 patients with MCL treated with zanubrutinib over the two trials, 8 had to be discontinued because of adverse events.
The recommended dose of zanubrutinib is 320 mg, taken orally 160 mg twice daily or 320 mg once daily, with or without food.
“BTK [Bruton kinase] inhibition is an established mode of treatment for patients with MCL, but many patients treated with previously approved BTK inhibitors do not fully respond to BTK therapy or are forced to discontinue treatment early due to side effects. Today we have a new option for our adult patients who have received one prior systemic or targeted therapy and are living with MCL,” Luhua (Michael) Wang, MD, clinical trial investigator and professor in the department of lymphoma and myeloma at the University of Texas MD Anderson Cancer Center, Houston, said in a statement.
Survival ‘excellent’ after rituximab-bendamustine induction in transplant-eligible MCL
The combination of rituximab and bendamustine (RB) provided “excellent” survival with less toxicity, compared with a cytarabine-based induction regimen, in transplant-eligible patients with mantle cell lymphoma, according to a long-term follow-up report from randomized phase 2 trial.
The 5-year survival rates for RB were “provocatively similar” to what was achieved with the standard, intensive R-hyperCVAD regimen, investigators said in this update on the Southwest Oncology Group (SWOG) S1106 study.
By contrast, the R-hyperCVAD regimen was associated with more toxicity and higher failure rates for stem cell mobilization, according to the report’s lead author, Manali Kamdar, MD, of the University of Colorado, Denver, and coauthors.
“Overall, S1106 demonstrated that an outpatient-based, less intensive induction therapy of bendamustine plus rituximab is highly effective, safe, and durable in untreated transplant-eligible MCL patients,” Dr. Kamdar and her colleagues reported in Blood Advances.
The results have guided the design of an upcoming study, EA4181, in which patients with mantle cell lymphoma will be treated with an RB backbone plus cytarabine, the BTK inhibitor acalabrutinib, or both, according to the authors.
In the present study, S1106, patients with mantle cell lymphoma were randomized to receive RB or the R-hyperCVAD regimen, which consisted of rituximab with hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone, alternating with high-dose cytarabine and methotrexate. Both regimens were followed by autologous hematopoietic stem cell transplant.
The stem cell mobilization failure rate was 29% in the R-hyperCVAD arm in an interim analysis conducted after 53 of a planned 160 patients had been enrolled, including 35 in the RB arm and 17 in the R-hyperCVAD arm, according to a report published in the British Journal of Haematology (2016 Dec 19. doi: 10.1111/bjh.14480). That analysis triggered a shutdown of the study, based on a rule stating that either arm would be deemed “unacceptably toxic” if the mobilization rate exceeded 10%.
Accordingly, R-hyperCVAD is “not an ideal platform” for future trials, the investigators said. At that time, the estimated 2-year progression-free survival (PFS) was 81% versus 82% for RB and R-hyperCVAD, respectively, while overall survival (OS) was 87% versus 88%.
With additional follow-up, the 5-year PFS is 66% and 62% in the RB and R-hyperCVAD arms, respectively, while 5-year OS is 80% and 74%, according to the investigators.
The RB regimen also results in “excellent” minimal residual disease (MRD) negativity, they added.
MRD status was evaluated in 12 paired pre- and postinduction therapy specimens, of which 2 pairs were from patients in the R-hyperCVAD arm, and 10 pairs were from patients in the RB arm.
In the R-hyperCVAD arm, both patients were MRD positive at baseline, and MRD negative after induction, according to the investigators. Similarly, 9 of 10 patients in the RB arm were MRD positive at baseline, and of those, 7 converted to MRD negative following induction.
The research was supported by the National Cancer Institute, and in part by Sequenta (Adaptive Biotechnologies). Dr. Kamdar reported being on the speakers bureau of Seattle Genetics and receiving consultancy fees from AstraZeneca, Celgene, and Genentech. Co-authors of the study provided disclosures related to Millennium Pharmaceuticals, Affimed, Seattle Genetics, Pharmacyclics, and Merck, among others.
SOURCE: Kamdar M et al. Blood Adv. 2019 Oct 22;3(20):3132-5.
The combination of rituximab and bendamustine (RB) provided “excellent” survival with less toxicity, compared with a cytarabine-based induction regimen, in transplant-eligible patients with mantle cell lymphoma, according to a long-term follow-up report from randomized phase 2 trial.
The 5-year survival rates for RB were “provocatively similar” to what was achieved with the standard, intensive R-hyperCVAD regimen, investigators said in this update on the Southwest Oncology Group (SWOG) S1106 study.
By contrast, the R-hyperCVAD regimen was associated with more toxicity and higher failure rates for stem cell mobilization, according to the report’s lead author, Manali Kamdar, MD, of the University of Colorado, Denver, and coauthors.
“Overall, S1106 demonstrated that an outpatient-based, less intensive induction therapy of bendamustine plus rituximab is highly effective, safe, and durable in untreated transplant-eligible MCL patients,” Dr. Kamdar and her colleagues reported in Blood Advances.
The results have guided the design of an upcoming study, EA4181, in which patients with mantle cell lymphoma will be treated with an RB backbone plus cytarabine, the BTK inhibitor acalabrutinib, or both, according to the authors.
In the present study, S1106, patients with mantle cell lymphoma were randomized to receive RB or the R-hyperCVAD regimen, which consisted of rituximab with hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone, alternating with high-dose cytarabine and methotrexate. Both regimens were followed by autologous hematopoietic stem cell transplant.
The stem cell mobilization failure rate was 29% in the R-hyperCVAD arm in an interim analysis conducted after 53 of a planned 160 patients had been enrolled, including 35 in the RB arm and 17 in the R-hyperCVAD arm, according to a report published in the British Journal of Haematology (2016 Dec 19. doi: 10.1111/bjh.14480). That analysis triggered a shutdown of the study, based on a rule stating that either arm would be deemed “unacceptably toxic” if the mobilization rate exceeded 10%.
Accordingly, R-hyperCVAD is “not an ideal platform” for future trials, the investigators said. At that time, the estimated 2-year progression-free survival (PFS) was 81% versus 82% for RB and R-hyperCVAD, respectively, while overall survival (OS) was 87% versus 88%.
With additional follow-up, the 5-year PFS is 66% and 62% in the RB and R-hyperCVAD arms, respectively, while 5-year OS is 80% and 74%, according to the investigators.
The RB regimen also results in “excellent” minimal residual disease (MRD) negativity, they added.
MRD status was evaluated in 12 paired pre- and postinduction therapy specimens, of which 2 pairs were from patients in the R-hyperCVAD arm, and 10 pairs were from patients in the RB arm.
In the R-hyperCVAD arm, both patients were MRD positive at baseline, and MRD negative after induction, according to the investigators. Similarly, 9 of 10 patients in the RB arm were MRD positive at baseline, and of those, 7 converted to MRD negative following induction.
The research was supported by the National Cancer Institute, and in part by Sequenta (Adaptive Biotechnologies). Dr. Kamdar reported being on the speakers bureau of Seattle Genetics and receiving consultancy fees from AstraZeneca, Celgene, and Genentech. Co-authors of the study provided disclosures related to Millennium Pharmaceuticals, Affimed, Seattle Genetics, Pharmacyclics, and Merck, among others.
SOURCE: Kamdar M et al. Blood Adv. 2019 Oct 22;3(20):3132-5.
The combination of rituximab and bendamustine (RB) provided “excellent” survival with less toxicity, compared with a cytarabine-based induction regimen, in transplant-eligible patients with mantle cell lymphoma, according to a long-term follow-up report from randomized phase 2 trial.
The 5-year survival rates for RB were “provocatively similar” to what was achieved with the standard, intensive R-hyperCVAD regimen, investigators said in this update on the Southwest Oncology Group (SWOG) S1106 study.
By contrast, the R-hyperCVAD regimen was associated with more toxicity and higher failure rates for stem cell mobilization, according to the report’s lead author, Manali Kamdar, MD, of the University of Colorado, Denver, and coauthors.
“Overall, S1106 demonstrated that an outpatient-based, less intensive induction therapy of bendamustine plus rituximab is highly effective, safe, and durable in untreated transplant-eligible MCL patients,” Dr. Kamdar and her colleagues reported in Blood Advances.
The results have guided the design of an upcoming study, EA4181, in which patients with mantle cell lymphoma will be treated with an RB backbone plus cytarabine, the BTK inhibitor acalabrutinib, or both, according to the authors.
In the present study, S1106, patients with mantle cell lymphoma were randomized to receive RB or the R-hyperCVAD regimen, which consisted of rituximab with hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone, alternating with high-dose cytarabine and methotrexate. Both regimens were followed by autologous hematopoietic stem cell transplant.
The stem cell mobilization failure rate was 29% in the R-hyperCVAD arm in an interim analysis conducted after 53 of a planned 160 patients had been enrolled, including 35 in the RB arm and 17 in the R-hyperCVAD arm, according to a report published in the British Journal of Haematology (2016 Dec 19. doi: 10.1111/bjh.14480). That analysis triggered a shutdown of the study, based on a rule stating that either arm would be deemed “unacceptably toxic” if the mobilization rate exceeded 10%.
Accordingly, R-hyperCVAD is “not an ideal platform” for future trials, the investigators said. At that time, the estimated 2-year progression-free survival (PFS) was 81% versus 82% for RB and R-hyperCVAD, respectively, while overall survival (OS) was 87% versus 88%.
With additional follow-up, the 5-year PFS is 66% and 62% in the RB and R-hyperCVAD arms, respectively, while 5-year OS is 80% and 74%, according to the investigators.
The RB regimen also results in “excellent” minimal residual disease (MRD) negativity, they added.
MRD status was evaluated in 12 paired pre- and postinduction therapy specimens, of which 2 pairs were from patients in the R-hyperCVAD arm, and 10 pairs were from patients in the RB arm.
In the R-hyperCVAD arm, both patients were MRD positive at baseline, and MRD negative after induction, according to the investigators. Similarly, 9 of 10 patients in the RB arm were MRD positive at baseline, and of those, 7 converted to MRD negative following induction.
The research was supported by the National Cancer Institute, and in part by Sequenta (Adaptive Biotechnologies). Dr. Kamdar reported being on the speakers bureau of Seattle Genetics and receiving consultancy fees from AstraZeneca, Celgene, and Genentech. Co-authors of the study provided disclosures related to Millennium Pharmaceuticals, Affimed, Seattle Genetics, Pharmacyclics, and Merck, among others.
SOURCE: Kamdar M et al. Blood Adv. 2019 Oct 22;3(20):3132-5.
FROM BLOOD ADVANCES
ASCO to award $50,000 young investigator grant to study MCL
Early-career researchers who are interested in studying
The young investigator grant is for a 1-year period and the award is used to fund a project focused on clinical or translational research on the clinical biology, natural history, prevention, screening, diagnosis, therapy, or epidemiology of MCL.
The purpose of this annual award, according to ASCO, is to fund physicians during the transition from a fellowship program to a faculty appointment.
Eligible applicants must be physicians currently in the last 2 years of final subspecialty training and within 10 years of having obtained his or her medical degree. Additionally, applicants must be planning a research career in clinical oncology, with a focus on MCL.
The grant selection committee’s primary criteria include the significance and originality of the proposed study and hypothesis, the feasibility of the experiment and methodology, whether it has an appropriate and detailed statistical analysis plan, and if the research is patient oriented.
The application deadline is Jan. 7, 2020, and the award term is July 1, 2020–June 30, 2021.
Application instructions are available on the ASCO website.
Early-career researchers who are interested in studying
The young investigator grant is for a 1-year period and the award is used to fund a project focused on clinical or translational research on the clinical biology, natural history, prevention, screening, diagnosis, therapy, or epidemiology of MCL.
The purpose of this annual award, according to ASCO, is to fund physicians during the transition from a fellowship program to a faculty appointment.
Eligible applicants must be physicians currently in the last 2 years of final subspecialty training and within 10 years of having obtained his or her medical degree. Additionally, applicants must be planning a research career in clinical oncology, with a focus on MCL.
The grant selection committee’s primary criteria include the significance and originality of the proposed study and hypothesis, the feasibility of the experiment and methodology, whether it has an appropriate and detailed statistical analysis plan, and if the research is patient oriented.
The application deadline is Jan. 7, 2020, and the award term is July 1, 2020–June 30, 2021.
Application instructions are available on the ASCO website.
Early-career researchers who are interested in studying
The young investigator grant is for a 1-year period and the award is used to fund a project focused on clinical or translational research on the clinical biology, natural history, prevention, screening, diagnosis, therapy, or epidemiology of MCL.
The purpose of this annual award, according to ASCO, is to fund physicians during the transition from a fellowship program to a faculty appointment.
Eligible applicants must be physicians currently in the last 2 years of final subspecialty training and within 10 years of having obtained his or her medical degree. Additionally, applicants must be planning a research career in clinical oncology, with a focus on MCL.
The grant selection committee’s primary criteria include the significance and originality of the proposed study and hypothesis, the feasibility of the experiment and methodology, whether it has an appropriate and detailed statistical analysis plan, and if the research is patient oriented.
The application deadline is Jan. 7, 2020, and the award term is July 1, 2020–June 30, 2021.
Application instructions are available on the ASCO website.
Follow-up shows favorable results with acalabrutinib in MCL
Acalabrutinib monotherapy can produce durable responses in relapsed/refractory mantle cell lymphoma (MCL), according to updated results from a phase 2 trial.
The drug produced an overall response rate (ORR) of 81%, and the median duration of response was 26 months.
These are the highest such figures reported “among all approved single-agent therapies for the treatment of relapsed/refractory MCL,” Michael Wang, MD, of the MD Anderson Cancer Center at the University of Texas in Houston and colleagues wrote in a letter in Leukemia.
Dr. Wang and colleagues reported updated results in 124 patients treated on the ACE-LY-004 trial. At baseline, the patients had a median age of 68 years (range, 42-90 years), and 80% were men. Three-quarters of patients had stage IV disease, 72% had extranodal disease, 21% had blastoid/pleomorphic MCL, and 26% had a Ki-67 proliferation index of 50% or greater.
At a median follow-up of 26 months, 40% (n = 49) of patients were still on acalabrutinib, and 61% (n = 76) were still in follow-up for survival. Six patients went on to allogeneic transplant at a median of 19 days after stopping acalabrutinib.
The ORR was 81% (100/124), and the complete response (CR) rate was 43% (n = 53). Four patients who initially had a partial response converted to a CR with longer follow-up. The estimated 24-month duration of response was 52.4%.
“ORR was consistent across patients with refractory disease and those with blastoid/pleomorphic MCL, despite those patients having a higher mean Ki-67 index [of 50% or greater], suggesting that some patients with poorer prognosis may also benefit from acalabrutinib,” Dr. Wang and colleagues wrote.
There were 29 patients evaluable for minimal residual disease (MRD) assessment. Seven patients (24%) had MRD-negative disease in the peripheral blood after they achieved a CR. An additional patient with a CR became MRD negative when a second blood sample was taken about 6 months after the first.
“Despite limited samples, these results demonstrate that continued use of acalabrutinib can lead to undetectable MRD in patients with CR,” Dr. Wang and his colleagues wrote. “Since most patients with MRD data are still on treatment (27/29), relationships between MRD negativity and durability of response cannot be made at this time.”
The median progression-free survival was 20 months, and the median overall survival was not reached. The estimated 24-month progression-free survival rate was 49.0%, and the estimated 24-month overall survival rate was 72.4%. Patients with low/intermediate Mantle Cell Lymphoma International Prognostic Index scores, classical MCL, and a Ki-67 index less than 50% had a longer duration of response and survival.
The adverse event profile was “largely consistent with earlier reporting,” Dr. Wang and colleagues wrote. The most frequent adverse events were headache (38%), diarrhea (36%), fatigue (28%), cough (22%), and myalgia (21%). The most common grade 3/4 adverse events were anemia (10%), neutropenia (10%), and pneumonia (6%).
Ten patients developed second primary cancers. There were no new atrial fibrillation events and no new hypertension events. The frequency of infections decreased over time, as did the number of bleeding events. However, two of three major hemorrhage events occurred after the previous report was published.
There were 43 deaths (35%), 29 of them because of disease progression. Six patients died of adverse events, two died of unknown causes, and two died of secondary acute myeloid leukemia. Other causes of death included multiorgan failure, intestinal obstruction, lung cancer, and graft-versus-host disease.
This study was sponsored by Acerta Pharma, a member of the AstraZeneca Group. The researchers reported relationships with AstraZeneca/Acerta Pharma and many other companies.
SOURCE: Wang M et al. Leukemia. 2019 Sep 26. doi: 10.1038/s41375-019-0575-9.
Acalabrutinib monotherapy can produce durable responses in relapsed/refractory mantle cell lymphoma (MCL), according to updated results from a phase 2 trial.
The drug produced an overall response rate (ORR) of 81%, and the median duration of response was 26 months.
These are the highest such figures reported “among all approved single-agent therapies for the treatment of relapsed/refractory MCL,” Michael Wang, MD, of the MD Anderson Cancer Center at the University of Texas in Houston and colleagues wrote in a letter in Leukemia.
Dr. Wang and colleagues reported updated results in 124 patients treated on the ACE-LY-004 trial. At baseline, the patients had a median age of 68 years (range, 42-90 years), and 80% were men. Three-quarters of patients had stage IV disease, 72% had extranodal disease, 21% had blastoid/pleomorphic MCL, and 26% had a Ki-67 proliferation index of 50% or greater.
At a median follow-up of 26 months, 40% (n = 49) of patients were still on acalabrutinib, and 61% (n = 76) were still in follow-up for survival. Six patients went on to allogeneic transplant at a median of 19 days after stopping acalabrutinib.
The ORR was 81% (100/124), and the complete response (CR) rate was 43% (n = 53). Four patients who initially had a partial response converted to a CR with longer follow-up. The estimated 24-month duration of response was 52.4%.
“ORR was consistent across patients with refractory disease and those with blastoid/pleomorphic MCL, despite those patients having a higher mean Ki-67 index [of 50% or greater], suggesting that some patients with poorer prognosis may also benefit from acalabrutinib,” Dr. Wang and colleagues wrote.
There were 29 patients evaluable for minimal residual disease (MRD) assessment. Seven patients (24%) had MRD-negative disease in the peripheral blood after they achieved a CR. An additional patient with a CR became MRD negative when a second blood sample was taken about 6 months after the first.
“Despite limited samples, these results demonstrate that continued use of acalabrutinib can lead to undetectable MRD in patients with CR,” Dr. Wang and his colleagues wrote. “Since most patients with MRD data are still on treatment (27/29), relationships between MRD negativity and durability of response cannot be made at this time.”
The median progression-free survival was 20 months, and the median overall survival was not reached. The estimated 24-month progression-free survival rate was 49.0%, and the estimated 24-month overall survival rate was 72.4%. Patients with low/intermediate Mantle Cell Lymphoma International Prognostic Index scores, classical MCL, and a Ki-67 index less than 50% had a longer duration of response and survival.
The adverse event profile was “largely consistent with earlier reporting,” Dr. Wang and colleagues wrote. The most frequent adverse events were headache (38%), diarrhea (36%), fatigue (28%), cough (22%), and myalgia (21%). The most common grade 3/4 adverse events were anemia (10%), neutropenia (10%), and pneumonia (6%).
Ten patients developed second primary cancers. There were no new atrial fibrillation events and no new hypertension events. The frequency of infections decreased over time, as did the number of bleeding events. However, two of three major hemorrhage events occurred after the previous report was published.
There were 43 deaths (35%), 29 of them because of disease progression. Six patients died of adverse events, two died of unknown causes, and two died of secondary acute myeloid leukemia. Other causes of death included multiorgan failure, intestinal obstruction, lung cancer, and graft-versus-host disease.
This study was sponsored by Acerta Pharma, a member of the AstraZeneca Group. The researchers reported relationships with AstraZeneca/Acerta Pharma and many other companies.
SOURCE: Wang M et al. Leukemia. 2019 Sep 26. doi: 10.1038/s41375-019-0575-9.
Acalabrutinib monotherapy can produce durable responses in relapsed/refractory mantle cell lymphoma (MCL), according to updated results from a phase 2 trial.
The drug produced an overall response rate (ORR) of 81%, and the median duration of response was 26 months.
These are the highest such figures reported “among all approved single-agent therapies for the treatment of relapsed/refractory MCL,” Michael Wang, MD, of the MD Anderson Cancer Center at the University of Texas in Houston and colleagues wrote in a letter in Leukemia.
Dr. Wang and colleagues reported updated results in 124 patients treated on the ACE-LY-004 trial. At baseline, the patients had a median age of 68 years (range, 42-90 years), and 80% were men. Three-quarters of patients had stage IV disease, 72% had extranodal disease, 21% had blastoid/pleomorphic MCL, and 26% had a Ki-67 proliferation index of 50% or greater.
At a median follow-up of 26 months, 40% (n = 49) of patients were still on acalabrutinib, and 61% (n = 76) were still in follow-up for survival. Six patients went on to allogeneic transplant at a median of 19 days after stopping acalabrutinib.
The ORR was 81% (100/124), and the complete response (CR) rate was 43% (n = 53). Four patients who initially had a partial response converted to a CR with longer follow-up. The estimated 24-month duration of response was 52.4%.
“ORR was consistent across patients with refractory disease and those with blastoid/pleomorphic MCL, despite those patients having a higher mean Ki-67 index [of 50% or greater], suggesting that some patients with poorer prognosis may also benefit from acalabrutinib,” Dr. Wang and colleagues wrote.
There were 29 patients evaluable for minimal residual disease (MRD) assessment. Seven patients (24%) had MRD-negative disease in the peripheral blood after they achieved a CR. An additional patient with a CR became MRD negative when a second blood sample was taken about 6 months after the first.
“Despite limited samples, these results demonstrate that continued use of acalabrutinib can lead to undetectable MRD in patients with CR,” Dr. Wang and his colleagues wrote. “Since most patients with MRD data are still on treatment (27/29), relationships between MRD negativity and durability of response cannot be made at this time.”
The median progression-free survival was 20 months, and the median overall survival was not reached. The estimated 24-month progression-free survival rate was 49.0%, and the estimated 24-month overall survival rate was 72.4%. Patients with low/intermediate Mantle Cell Lymphoma International Prognostic Index scores, classical MCL, and a Ki-67 index less than 50% had a longer duration of response and survival.
The adverse event profile was “largely consistent with earlier reporting,” Dr. Wang and colleagues wrote. The most frequent adverse events were headache (38%), diarrhea (36%), fatigue (28%), cough (22%), and myalgia (21%). The most common grade 3/4 adverse events were anemia (10%), neutropenia (10%), and pneumonia (6%).
Ten patients developed second primary cancers. There were no new atrial fibrillation events and no new hypertension events. The frequency of infections decreased over time, as did the number of bleeding events. However, two of three major hemorrhage events occurred after the previous report was published.
There were 43 deaths (35%), 29 of them because of disease progression. Six patients died of adverse events, two died of unknown causes, and two died of secondary acute myeloid leukemia. Other causes of death included multiorgan failure, intestinal obstruction, lung cancer, and graft-versus-host disease.
This study was sponsored by Acerta Pharma, a member of the AstraZeneca Group. The researchers reported relationships with AstraZeneca/Acerta Pharma and many other companies.
SOURCE: Wang M et al. Leukemia. 2019 Sep 26. doi: 10.1038/s41375-019-0575-9.
FROM LEUKEMIA
Staging PET/CT better defines extent of mantle cell lymphoma
Use of staging PET/CT better defines the extent of mantle cell lymphoma (MCL) in certain compartments, adding important information for treatment decisions, a cohort study suggests. However, patient selection and evaluation criteria are important for its utility.
“A correct and early identification of initial disease could be crucial because it could affect patient management and therapeutic choice,” Domenico Albano, MD, a nuclear medicine physician at the University of Brescia (Italy) and Spedali Civili Brescia, and colleagues wrote in Clinical Lymphoma, Myeloma, & Leukemia.
Using retrospective data from two centers and 122 patients with MCL, the investigators compared the utility of staging 18F-fluorodeoxyglucose (18F-FDG) PET/CT with that of other modalities used for detecting disease in specific compartments. They also assessed its impact on patient management.
The study results showed that, with the exception of a single patient having bone marrow involvement, all patients had positive PET/CT results, with detection of at least one hypermetabolic lesion.
For assessing nodal involvement, compared with CT alone, PET/CT detected additional lesions in 21% of patients. Splenic lesions were detected by PET/CT in 49% of patients and by CT alone in 47%.
For assessing bone marrow involvement, compared with bone marrow biopsy, PET/CT had a sensitivity of 52%, a specificity of 98%, a positive predictive value of 97%, a negative predictive value of 65%, and an overall accuracy of 74%.
For gastrointestinal involvement, compared with endoscopy, PET/CT had a sensitivity of 64% (78% after excluding diabetic patients taking metformin), a specificity of 91% (92%), a positive predictive value of 69% (72%), a negative predictive value of 90% (94%), and an overall accuracy of 85% (89%).
Ultimately, relative to CT alone, PET/CT altered stage and management in 19% of patients. Specifically, this imaging led to up-staging in 17% of patients, prompting clinicians to switch to more-aggressive chemotherapy, and down-staging in 2% of patients, prompting clinicians to skip unnecessary invasive therapies.
“We demonstrated that 18F-FDG pathologic uptake in MCL occurred in almost all patients, with excellent detection rate in nodal and splenic disease – indeed, better than CT,” the researchers wrote. “When we analyzed bone marrow and gastrointestinal involvement, the selection of patients excluding all conditions potentially affecting organ uptake and the use of specific criteria for the evaluation of these organs seemed to be crucial. Within these caveats, PET/CT showed good specificity for bone marrow and gastrointestinal evaluation.”
Study funding was not disclosed. Dr. Albano reported having no relevant conflicts of interest.
SOURCE: Albano D et al. Clin Lymphoma Myeloma Leuk. 2019 Aug;19(8):e457-64.
Use of staging PET/CT better defines the extent of mantle cell lymphoma (MCL) in certain compartments, adding important information for treatment decisions, a cohort study suggests. However, patient selection and evaluation criteria are important for its utility.
“A correct and early identification of initial disease could be crucial because it could affect patient management and therapeutic choice,” Domenico Albano, MD, a nuclear medicine physician at the University of Brescia (Italy) and Spedali Civili Brescia, and colleagues wrote in Clinical Lymphoma, Myeloma, & Leukemia.
Using retrospective data from two centers and 122 patients with MCL, the investigators compared the utility of staging 18F-fluorodeoxyglucose (18F-FDG) PET/CT with that of other modalities used for detecting disease in specific compartments. They also assessed its impact on patient management.
The study results showed that, with the exception of a single patient having bone marrow involvement, all patients had positive PET/CT results, with detection of at least one hypermetabolic lesion.
For assessing nodal involvement, compared with CT alone, PET/CT detected additional lesions in 21% of patients. Splenic lesions were detected by PET/CT in 49% of patients and by CT alone in 47%.
For assessing bone marrow involvement, compared with bone marrow biopsy, PET/CT had a sensitivity of 52%, a specificity of 98%, a positive predictive value of 97%, a negative predictive value of 65%, and an overall accuracy of 74%.
For gastrointestinal involvement, compared with endoscopy, PET/CT had a sensitivity of 64% (78% after excluding diabetic patients taking metformin), a specificity of 91% (92%), a positive predictive value of 69% (72%), a negative predictive value of 90% (94%), and an overall accuracy of 85% (89%).
Ultimately, relative to CT alone, PET/CT altered stage and management in 19% of patients. Specifically, this imaging led to up-staging in 17% of patients, prompting clinicians to switch to more-aggressive chemotherapy, and down-staging in 2% of patients, prompting clinicians to skip unnecessary invasive therapies.
“We demonstrated that 18F-FDG pathologic uptake in MCL occurred in almost all patients, with excellent detection rate in nodal and splenic disease – indeed, better than CT,” the researchers wrote. “When we analyzed bone marrow and gastrointestinal involvement, the selection of patients excluding all conditions potentially affecting organ uptake and the use of specific criteria for the evaluation of these organs seemed to be crucial. Within these caveats, PET/CT showed good specificity for bone marrow and gastrointestinal evaluation.”
Study funding was not disclosed. Dr. Albano reported having no relevant conflicts of interest.
SOURCE: Albano D et al. Clin Lymphoma Myeloma Leuk. 2019 Aug;19(8):e457-64.
Use of staging PET/CT better defines the extent of mantle cell lymphoma (MCL) in certain compartments, adding important information for treatment decisions, a cohort study suggests. However, patient selection and evaluation criteria are important for its utility.
“A correct and early identification of initial disease could be crucial because it could affect patient management and therapeutic choice,” Domenico Albano, MD, a nuclear medicine physician at the University of Brescia (Italy) and Spedali Civili Brescia, and colleagues wrote in Clinical Lymphoma, Myeloma, & Leukemia.
Using retrospective data from two centers and 122 patients with MCL, the investigators compared the utility of staging 18F-fluorodeoxyglucose (18F-FDG) PET/CT with that of other modalities used for detecting disease in specific compartments. They also assessed its impact on patient management.
The study results showed that, with the exception of a single patient having bone marrow involvement, all patients had positive PET/CT results, with detection of at least one hypermetabolic lesion.
For assessing nodal involvement, compared with CT alone, PET/CT detected additional lesions in 21% of patients. Splenic lesions were detected by PET/CT in 49% of patients and by CT alone in 47%.
For assessing bone marrow involvement, compared with bone marrow biopsy, PET/CT had a sensitivity of 52%, a specificity of 98%, a positive predictive value of 97%, a negative predictive value of 65%, and an overall accuracy of 74%.
For gastrointestinal involvement, compared with endoscopy, PET/CT had a sensitivity of 64% (78% after excluding diabetic patients taking metformin), a specificity of 91% (92%), a positive predictive value of 69% (72%), a negative predictive value of 90% (94%), and an overall accuracy of 85% (89%).
Ultimately, relative to CT alone, PET/CT altered stage and management in 19% of patients. Specifically, this imaging led to up-staging in 17% of patients, prompting clinicians to switch to more-aggressive chemotherapy, and down-staging in 2% of patients, prompting clinicians to skip unnecessary invasive therapies.
“We demonstrated that 18F-FDG pathologic uptake in MCL occurred in almost all patients, with excellent detection rate in nodal and splenic disease – indeed, better than CT,” the researchers wrote. “When we analyzed bone marrow and gastrointestinal involvement, the selection of patients excluding all conditions potentially affecting organ uptake and the use of specific criteria for the evaluation of these organs seemed to be crucial. Within these caveats, PET/CT showed good specificity for bone marrow and gastrointestinal evaluation.”
Study funding was not disclosed. Dr. Albano reported having no relevant conflicts of interest.
SOURCE: Albano D et al. Clin Lymphoma Myeloma Leuk. 2019 Aug;19(8):e457-64.
FROM CLINICAL LYMPHOMA, MYELOMA, & LEUKEMIA
Rituximab, bendamustine look better than chemo alone in MCL
In older patients with newly diagnosed mantle cell lymphoma (MCL), first-line therapy with rituximab- and bendamustine-based regimens significantly reduced 1-year mortality rates versus chemotherapy alone, according to a retrospective analysis.
“This study evaluated the comparative effectiveness of [rituximab, bortezomib, or bendamustine] in elderly patients newly diagnosed with MCL,” wrote Shuangshuang Fu, PhD, of the University of Texas MD Anderson Cancer Center, Houston, and colleagues. The findings were reported in Clinical Lymphoma, Myeloma & Leukemia.
The researchers studied population-based data from the Surveillance, Epidemiology, and End Results (SEER)-Medicare linked database. They identified all patients over age 65 years who received a new diagnosis of MCL between Jan. 1, 1999, and Dec. 31, 2013.
The study cohort included a total of 1,215 patients. Participants were classified into four different groups according to treatment regimen: chemotherapy alone, rituximab plus or minus chemotherapy, bendamustine plus or minus chemotherapy, and bortezomib plus or minus chemotherapy.
At 1-year follow-up, the team analyzed various mortality outcomes, including MCL-specific, all-cause, and noncancer mortality. The bortezomib results were not included in the primary analysis because of small sample size, according to the researchers.
After multivariable analysis, Dr. Fu and colleagues found that 1-year all-cause mortality rate was significantly lower for patients receiving rituximab-based regimens, compared with chemotherapy alone (hazard ratio, 0.38; 95% confidence interval, 0.25-0.59). There was a similar decline for MCL-specific mortality (HR, 0.38; 95% CI, 0.24-0.60).
The 1-year MCL-specific mortality was also significantly reduced in the bendamustine group, compared with chemotherapy alone (HR, 0.49; 95% CI, 0.24-0.99).
“Our findings comparing rituximab with chemotherapy alone further confirmed the benefit of adding rituximab to chemotherapy in newly diagnosed older MCL patients,” they wrote.
The researchers acknowledged that a key limitation of the study was the observational design. As a result, selection bias and unmeasured confounding could have influenced the results.
“Future studies evaluating the comparative effectiveness of those newly approved novel agents for MCL patients were warranted as more data are available,” they concluded.
The study was funded by the Duncan Family Institute, the Cancer Prevention Research Institute of Texas, and the National Institutes of Health. The authors reported having no conflicts of interest.
SOURCE: Fu S et al. Clin Lymphoma Myeloma Leuk. 2019 Aug 30. doi: 10.1016/j.clml.2019.08.014.
In older patients with newly diagnosed mantle cell lymphoma (MCL), first-line therapy with rituximab- and bendamustine-based regimens significantly reduced 1-year mortality rates versus chemotherapy alone, according to a retrospective analysis.
“This study evaluated the comparative effectiveness of [rituximab, bortezomib, or bendamustine] in elderly patients newly diagnosed with MCL,” wrote Shuangshuang Fu, PhD, of the University of Texas MD Anderson Cancer Center, Houston, and colleagues. The findings were reported in Clinical Lymphoma, Myeloma & Leukemia.
The researchers studied population-based data from the Surveillance, Epidemiology, and End Results (SEER)-Medicare linked database. They identified all patients over age 65 years who received a new diagnosis of MCL between Jan. 1, 1999, and Dec. 31, 2013.
The study cohort included a total of 1,215 patients. Participants were classified into four different groups according to treatment regimen: chemotherapy alone, rituximab plus or minus chemotherapy, bendamustine plus or minus chemotherapy, and bortezomib plus or minus chemotherapy.
At 1-year follow-up, the team analyzed various mortality outcomes, including MCL-specific, all-cause, and noncancer mortality. The bortezomib results were not included in the primary analysis because of small sample size, according to the researchers.
After multivariable analysis, Dr. Fu and colleagues found that 1-year all-cause mortality rate was significantly lower for patients receiving rituximab-based regimens, compared with chemotherapy alone (hazard ratio, 0.38; 95% confidence interval, 0.25-0.59). There was a similar decline for MCL-specific mortality (HR, 0.38; 95% CI, 0.24-0.60).
The 1-year MCL-specific mortality was also significantly reduced in the bendamustine group, compared with chemotherapy alone (HR, 0.49; 95% CI, 0.24-0.99).
“Our findings comparing rituximab with chemotherapy alone further confirmed the benefit of adding rituximab to chemotherapy in newly diagnosed older MCL patients,” they wrote.
The researchers acknowledged that a key limitation of the study was the observational design. As a result, selection bias and unmeasured confounding could have influenced the results.
“Future studies evaluating the comparative effectiveness of those newly approved novel agents for MCL patients were warranted as more data are available,” they concluded.
The study was funded by the Duncan Family Institute, the Cancer Prevention Research Institute of Texas, and the National Institutes of Health. The authors reported having no conflicts of interest.
SOURCE: Fu S et al. Clin Lymphoma Myeloma Leuk. 2019 Aug 30. doi: 10.1016/j.clml.2019.08.014.
In older patients with newly diagnosed mantle cell lymphoma (MCL), first-line therapy with rituximab- and bendamustine-based regimens significantly reduced 1-year mortality rates versus chemotherapy alone, according to a retrospective analysis.
“This study evaluated the comparative effectiveness of [rituximab, bortezomib, or bendamustine] in elderly patients newly diagnosed with MCL,” wrote Shuangshuang Fu, PhD, of the University of Texas MD Anderson Cancer Center, Houston, and colleagues. The findings were reported in Clinical Lymphoma, Myeloma & Leukemia.
The researchers studied population-based data from the Surveillance, Epidemiology, and End Results (SEER)-Medicare linked database. They identified all patients over age 65 years who received a new diagnosis of MCL between Jan. 1, 1999, and Dec. 31, 2013.
The study cohort included a total of 1,215 patients. Participants were classified into four different groups according to treatment regimen: chemotherapy alone, rituximab plus or minus chemotherapy, bendamustine plus or minus chemotherapy, and bortezomib plus or minus chemotherapy.
At 1-year follow-up, the team analyzed various mortality outcomes, including MCL-specific, all-cause, and noncancer mortality. The bortezomib results were not included in the primary analysis because of small sample size, according to the researchers.
After multivariable analysis, Dr. Fu and colleagues found that 1-year all-cause mortality rate was significantly lower for patients receiving rituximab-based regimens, compared with chemotherapy alone (hazard ratio, 0.38; 95% confidence interval, 0.25-0.59). There was a similar decline for MCL-specific mortality (HR, 0.38; 95% CI, 0.24-0.60).
The 1-year MCL-specific mortality was also significantly reduced in the bendamustine group, compared with chemotherapy alone (HR, 0.49; 95% CI, 0.24-0.99).
“Our findings comparing rituximab with chemotherapy alone further confirmed the benefit of adding rituximab to chemotherapy in newly diagnosed older MCL patients,” they wrote.
The researchers acknowledged that a key limitation of the study was the observational design. As a result, selection bias and unmeasured confounding could have influenced the results.
“Future studies evaluating the comparative effectiveness of those newly approved novel agents for MCL patients were warranted as more data are available,” they concluded.
The study was funded by the Duncan Family Institute, the Cancer Prevention Research Institute of Texas, and the National Institutes of Health. The authors reported having no conflicts of interest.
SOURCE: Fu S et al. Clin Lymphoma Myeloma Leuk. 2019 Aug 30. doi: 10.1016/j.clml.2019.08.014.
FROM CLINICAL LYMPHOMA, MYELOMA & LEUKEMIA
Ibrutinib-rituximab induction yields ‘unprecedented’ responses in MCL
LUGANO, Switzerland – In younger patients with previously untreated mantle cell lymphoma, the chemotherapy-free combination of ibrutinib and rituximab followed by a short course of chemotherapy was associated with an “unprecedented” 3-year progression-free survival rate, investigators in the phase 2 WINDOW-1 trial reported.
Among 50 patients aged 65 years and younger who received ibrutinib and rituximab until they achieved a complete or partial response, followed by four cycles of chemotherapy with rituximab plus hyper-CVAD (cyclophosphamide, vincristine, doxorubicin and dexamethasone) and rituximab plus methotrexate, the 3-year progression-free survival (PFS) rate was 88%, said Michael Wang, MD, from the University of Texas MD Anderson Cancer Center in Houston.
Additionally, for patients with the low-risk features, the 3-year PFS rate was 90%.
“Chemo-free ibrutinib-rituximab induced unprecedented – unprecedented – efficacy before chemo consolidation,” he said at the International Conference on Malignant Lymphoma.
Dr. Wang presented data from an interim analysis of the investigator-initiated single-center trial. Fifty patients aged 65 years or younger with untreated mantle cell lymphoma (MCL), good performance status, and good organ function were enrolled.
The patients were treated with ibrutinib and rituximab for two cycles and then evaluated for response with PET-CT scan, bone marrow biopsy, and for some patients, esophagogastroduodenoscopy (EGD) and colonoscopy with random biopsies.
In the induction phase, patients received ibrutinib daily on days 1-28 and rituximab intravenously over 6-8 hours on days 1, 8, 15, and 22 of cycle 1, and then over 4 hours on day 1 of cycles 3-12. The treatment was repeated every 28 days for up to 12 cycles in the absence of disease progression or unacceptable toxicity, or until patients achieved a complete response.
In the consolidation phase, patients received rituximab IV over 6 hours on day 1; oral or IV dexamethasone on days 1-4; cyclophosphamide IV over 3 hours twice daily on days 2-4; doxorubicin IV over 15-30 minutes on day 5; and vincristine IV over 15-30 minutes on day 5 of cycles one, three, five, and seven. Patients also received rituximab IV over 6 hours on day 1; methotrexate IV over 24 hours on day 2; and cytarabine IV over 2 hours twice daily on days 3 and 4 of cycles two, four, six, and eight. Treatments were repeated every 28 days for up to eight cycles in the absence of disease progression or unacceptable toxicity.
Patients who had a complete response (CR) after two cycles of induction and those who had disease progression on induction went on to consolidation. Patients with partial responses (PR) to induction continued on ibrutinib/rituximab until either the loss of a PR or best response for up to 12 cycles, with those who achieved a CR then moving on to consolidation.
Patients who had a CR after induction received four cycles of R-hyperCVAD, no subsequent stem cell transplant, and no maintenance therapy. Patients who had a PR after induction received two cycles of R-hyperCVAD, were reassessed, and then continued on R-hyperCVAD until CR or for up to eight total cycles.
Patients with either stable disease or progression during R-hyperCVAD were taken off the study.
Of the 50 patients enrolled, all 50 were evaluable for part A (induction), and 48 were evaluable after induction and consolidation (two patients withdrew for personal reasons).
After a median follow-up of 36 months, the overall response rate (ORR) following induction was 100%, consisting of 46 CRs (92%) and four PRs (8%).
In an intention-to-treat analysis (including the two patients who withdrew), the ORR was 96%, consisting of CRs in 47 patients (94%) and a PR in 1 patient (2%).
Neither the median PFS nor median overall survival had been reached at the time of data cutoff, and no patients have died.
Of the 50 enrolled patients, four experienced disease progression after 17, 24, 34, and 35 months of treatment. The patients with disease progression included one with Ki-67 of less than 30%, and three with KI-67 of 30% or greater.
Grade 3-4 toxicities during induction including myelosuppression in 4%; fatigue, myalgia, and rashes in 8% each; and oral mucositis in 4%.
Dr. Wang said that future studies on minimal residual disease and clonal evolution are ongoing, and that data on more patients will be presented at the next annual meeting of the American Society of Hematology, scheduled for December 2019.
He also noted that the WINDOW-2 trial, in which ibrutinib and rituximab are followed by veneotclax and hyper-CVAD chemotherapy in patients with newly diagnosed MCL, is open and rapidly enrolling patients.
The study is supported by the National Cancer Institute. Dr. Wang reported financial relationships with Janssen, Pharmacyclics, and other companies.
SOURCE: Wang M et al. ICML-15, Abstract 12.
LUGANO, Switzerland – In younger patients with previously untreated mantle cell lymphoma, the chemotherapy-free combination of ibrutinib and rituximab followed by a short course of chemotherapy was associated with an “unprecedented” 3-year progression-free survival rate, investigators in the phase 2 WINDOW-1 trial reported.
Among 50 patients aged 65 years and younger who received ibrutinib and rituximab until they achieved a complete or partial response, followed by four cycles of chemotherapy with rituximab plus hyper-CVAD (cyclophosphamide, vincristine, doxorubicin and dexamethasone) and rituximab plus methotrexate, the 3-year progression-free survival (PFS) rate was 88%, said Michael Wang, MD, from the University of Texas MD Anderson Cancer Center in Houston.
Additionally, for patients with the low-risk features, the 3-year PFS rate was 90%.
“Chemo-free ibrutinib-rituximab induced unprecedented – unprecedented – efficacy before chemo consolidation,” he said at the International Conference on Malignant Lymphoma.
Dr. Wang presented data from an interim analysis of the investigator-initiated single-center trial. Fifty patients aged 65 years or younger with untreated mantle cell lymphoma (MCL), good performance status, and good organ function were enrolled.
The patients were treated with ibrutinib and rituximab for two cycles and then evaluated for response with PET-CT scan, bone marrow biopsy, and for some patients, esophagogastroduodenoscopy (EGD) and colonoscopy with random biopsies.
In the induction phase, patients received ibrutinib daily on days 1-28 and rituximab intravenously over 6-8 hours on days 1, 8, 15, and 22 of cycle 1, and then over 4 hours on day 1 of cycles 3-12. The treatment was repeated every 28 days for up to 12 cycles in the absence of disease progression or unacceptable toxicity, or until patients achieved a complete response.
In the consolidation phase, patients received rituximab IV over 6 hours on day 1; oral or IV dexamethasone on days 1-4; cyclophosphamide IV over 3 hours twice daily on days 2-4; doxorubicin IV over 15-30 minutes on day 5; and vincristine IV over 15-30 minutes on day 5 of cycles one, three, five, and seven. Patients also received rituximab IV over 6 hours on day 1; methotrexate IV over 24 hours on day 2; and cytarabine IV over 2 hours twice daily on days 3 and 4 of cycles two, four, six, and eight. Treatments were repeated every 28 days for up to eight cycles in the absence of disease progression or unacceptable toxicity.
Patients who had a complete response (CR) after two cycles of induction and those who had disease progression on induction went on to consolidation. Patients with partial responses (PR) to induction continued on ibrutinib/rituximab until either the loss of a PR or best response for up to 12 cycles, with those who achieved a CR then moving on to consolidation.
Patients who had a CR after induction received four cycles of R-hyperCVAD, no subsequent stem cell transplant, and no maintenance therapy. Patients who had a PR after induction received two cycles of R-hyperCVAD, were reassessed, and then continued on R-hyperCVAD until CR or for up to eight total cycles.
Patients with either stable disease or progression during R-hyperCVAD were taken off the study.
Of the 50 patients enrolled, all 50 were evaluable for part A (induction), and 48 were evaluable after induction and consolidation (two patients withdrew for personal reasons).
After a median follow-up of 36 months, the overall response rate (ORR) following induction was 100%, consisting of 46 CRs (92%) and four PRs (8%).
In an intention-to-treat analysis (including the two patients who withdrew), the ORR was 96%, consisting of CRs in 47 patients (94%) and a PR in 1 patient (2%).
Neither the median PFS nor median overall survival had been reached at the time of data cutoff, and no patients have died.
Of the 50 enrolled patients, four experienced disease progression after 17, 24, 34, and 35 months of treatment. The patients with disease progression included one with Ki-67 of less than 30%, and three with KI-67 of 30% or greater.
Grade 3-4 toxicities during induction including myelosuppression in 4%; fatigue, myalgia, and rashes in 8% each; and oral mucositis in 4%.
Dr. Wang said that future studies on minimal residual disease and clonal evolution are ongoing, and that data on more patients will be presented at the next annual meeting of the American Society of Hematology, scheduled for December 2019.
He also noted that the WINDOW-2 trial, in which ibrutinib and rituximab are followed by veneotclax and hyper-CVAD chemotherapy in patients with newly diagnosed MCL, is open and rapidly enrolling patients.
The study is supported by the National Cancer Institute. Dr. Wang reported financial relationships with Janssen, Pharmacyclics, and other companies.
SOURCE: Wang M et al. ICML-15, Abstract 12.
LUGANO, Switzerland – In younger patients with previously untreated mantle cell lymphoma, the chemotherapy-free combination of ibrutinib and rituximab followed by a short course of chemotherapy was associated with an “unprecedented” 3-year progression-free survival rate, investigators in the phase 2 WINDOW-1 trial reported.
Among 50 patients aged 65 years and younger who received ibrutinib and rituximab until they achieved a complete or partial response, followed by four cycles of chemotherapy with rituximab plus hyper-CVAD (cyclophosphamide, vincristine, doxorubicin and dexamethasone) and rituximab plus methotrexate, the 3-year progression-free survival (PFS) rate was 88%, said Michael Wang, MD, from the University of Texas MD Anderson Cancer Center in Houston.
Additionally, for patients with the low-risk features, the 3-year PFS rate was 90%.
“Chemo-free ibrutinib-rituximab induced unprecedented – unprecedented – efficacy before chemo consolidation,” he said at the International Conference on Malignant Lymphoma.
Dr. Wang presented data from an interim analysis of the investigator-initiated single-center trial. Fifty patients aged 65 years or younger with untreated mantle cell lymphoma (MCL), good performance status, and good organ function were enrolled.
The patients were treated with ibrutinib and rituximab for two cycles and then evaluated for response with PET-CT scan, bone marrow biopsy, and for some patients, esophagogastroduodenoscopy (EGD) and colonoscopy with random biopsies.
In the induction phase, patients received ibrutinib daily on days 1-28 and rituximab intravenously over 6-8 hours on days 1, 8, 15, and 22 of cycle 1, and then over 4 hours on day 1 of cycles 3-12. The treatment was repeated every 28 days for up to 12 cycles in the absence of disease progression or unacceptable toxicity, or until patients achieved a complete response.
In the consolidation phase, patients received rituximab IV over 6 hours on day 1; oral or IV dexamethasone on days 1-4; cyclophosphamide IV over 3 hours twice daily on days 2-4; doxorubicin IV over 15-30 minutes on day 5; and vincristine IV over 15-30 minutes on day 5 of cycles one, three, five, and seven. Patients also received rituximab IV over 6 hours on day 1; methotrexate IV over 24 hours on day 2; and cytarabine IV over 2 hours twice daily on days 3 and 4 of cycles two, four, six, and eight. Treatments were repeated every 28 days for up to eight cycles in the absence of disease progression or unacceptable toxicity.
Patients who had a complete response (CR) after two cycles of induction and those who had disease progression on induction went on to consolidation. Patients with partial responses (PR) to induction continued on ibrutinib/rituximab until either the loss of a PR or best response for up to 12 cycles, with those who achieved a CR then moving on to consolidation.
Patients who had a CR after induction received four cycles of R-hyperCVAD, no subsequent stem cell transplant, and no maintenance therapy. Patients who had a PR after induction received two cycles of R-hyperCVAD, were reassessed, and then continued on R-hyperCVAD until CR or for up to eight total cycles.
Patients with either stable disease or progression during R-hyperCVAD were taken off the study.
Of the 50 patients enrolled, all 50 were evaluable for part A (induction), and 48 were evaluable after induction and consolidation (two patients withdrew for personal reasons).
After a median follow-up of 36 months, the overall response rate (ORR) following induction was 100%, consisting of 46 CRs (92%) and four PRs (8%).
In an intention-to-treat analysis (including the two patients who withdrew), the ORR was 96%, consisting of CRs in 47 patients (94%) and a PR in 1 patient (2%).
Neither the median PFS nor median overall survival had been reached at the time of data cutoff, and no patients have died.
Of the 50 enrolled patients, four experienced disease progression after 17, 24, 34, and 35 months of treatment. The patients with disease progression included one with Ki-67 of less than 30%, and three with KI-67 of 30% or greater.
Grade 3-4 toxicities during induction including myelosuppression in 4%; fatigue, myalgia, and rashes in 8% each; and oral mucositis in 4%.
Dr. Wang said that future studies on minimal residual disease and clonal evolution are ongoing, and that data on more patients will be presented at the next annual meeting of the American Society of Hematology, scheduled for December 2019.
He also noted that the WINDOW-2 trial, in which ibrutinib and rituximab are followed by veneotclax and hyper-CVAD chemotherapy in patients with newly diagnosed MCL, is open and rapidly enrolling patients.
The study is supported by the National Cancer Institute. Dr. Wang reported financial relationships with Janssen, Pharmacyclics, and other companies.
SOURCE: Wang M et al. ICML-15, Abstract 12.
REPORTING FROM 15-ICML
Ibrutinib/rituximab effective, safe as frontline treatment for older patients with MCL
LUGANO, SWITZERLAND – The chemotherapy-free combination of ibrutinib (Imbruvica) and rituximab is highly effective as frontline therapy for older, transplant-ineligible patients with nonblastoid mantle cell lymphoma, according to investigators.
In a phase 2 study of patients with a median age of 71 years, 38 of 41 patients (93%) had an objective response, and the regimen was both safe and easy to administer, reported Preetesh Jain, MD, PhD, of the University of Texas MD Anderson Cancer Center in Houston.
“The adverse event profile was generally favorable, with specific monitoring recommended for patients with cardiovascular comorbidities and a history of atrial fibrillation,” he said at the International Conference on Malignant Lymphoma.
The investigators enrolled 48 patients aged 65 years and older with previously untreated mantle cell lymphoma (MCL), of whom 41 were evaluable for the primary endpoints of overall response rate (ORR) and safety. The patients had good performance status and normal organ function, with the largest tumor size less than 10 cm. Patients with atrial fibrillation could participate, if the fibrillation was controlled. Patients with Ki-67 protein levels of 50% or greater and blastoid/pleomorphic histology were excluded.
Patients were treated with ibrutinib 560 mg orally daily for each 28-day cycle, with therapy continued until disease progression, or until therapy was stopped for any other reason. Patients also received intravenous rituximab 375 mg/m2 on days 1, 8, 15 and 22, plus or minus one day for cycle 1, on day 1 of cycles 3-8, and on day 1 of every other cycle for up to 2 years.
Of the 41 patients evaluable for response, 26 (64%) had a complete response (CR) and 12 (29%) had a partial response. Three additional patients had stable disease, for an objective response rate of 93%.
Of 34 patients with PET scans, all had negative scans. Of 37 patients evaluable for minimal residual disease (MRD) by flow cytometry, 21 (58%) were MRD negative.
Patients with low or intermediate Mantle Cell Lymphoma International Prognostic Index (MIPI) scores had a higher ORR (100% vs. 89% for patients with high MIPI scores), and patients with low Ki-67 levels had a higher response rate than that of patients with KI-67 of 30% or greater (80% vs. 87%).
Neither median 3-year progression-free survival nor median 3-year overall survival have been reached, with respective 3-year rates of 87% and 95%.
Four patients experienced disease progression at 4, 10, 13 and 33 months of treatment. Three of these patients had disease that had transformed to blastoid/pleomorphic variant, two had Ki-67 of 30% or greater, one had mutations in TP53, and one had FAT1 and SF3B1 mutations.
Two patients died after ibrutinib therapy, one who had discontinued therapy because of bleeding, and the other who died on treatment at 13 months from transformed disease. Both of these patients had high Ki-67 levels.
Grade 3 or 4 hematological adverse events were neutropenia in four patients, and thrombocytopenia in two patients. There were no cases of grade 3 or 4 anemia.
Grade 3 or 4 nonhematological adverse events were fatigue, myalgia, and atrial fibrillation in seven patients each, diarrhea in six patients, and petechiae/bleeding in three patients.
Patients will continue to be followed for late adverse events, secondary cancers, and relapses, and further studies on clonal evolution, mutation profiling, and MRD are ongoing and will be reported at a later date, Dr. Jain said.
The National Cancer Institute supported the study. Dr. Jain reported having no financial disclosures.
SOURCE: Jain P et al. 15-ICML. Abstract 011.
LUGANO, SWITZERLAND – The chemotherapy-free combination of ibrutinib (Imbruvica) and rituximab is highly effective as frontline therapy for older, transplant-ineligible patients with nonblastoid mantle cell lymphoma, according to investigators.
In a phase 2 study of patients with a median age of 71 years, 38 of 41 patients (93%) had an objective response, and the regimen was both safe and easy to administer, reported Preetesh Jain, MD, PhD, of the University of Texas MD Anderson Cancer Center in Houston.
“The adverse event profile was generally favorable, with specific monitoring recommended for patients with cardiovascular comorbidities and a history of atrial fibrillation,” he said at the International Conference on Malignant Lymphoma.
The investigators enrolled 48 patients aged 65 years and older with previously untreated mantle cell lymphoma (MCL), of whom 41 were evaluable for the primary endpoints of overall response rate (ORR) and safety. The patients had good performance status and normal organ function, with the largest tumor size less than 10 cm. Patients with atrial fibrillation could participate, if the fibrillation was controlled. Patients with Ki-67 protein levels of 50% or greater and blastoid/pleomorphic histology were excluded.
Patients were treated with ibrutinib 560 mg orally daily for each 28-day cycle, with therapy continued until disease progression, or until therapy was stopped for any other reason. Patients also received intravenous rituximab 375 mg/m2 on days 1, 8, 15 and 22, plus or minus one day for cycle 1, on day 1 of cycles 3-8, and on day 1 of every other cycle for up to 2 years.
Of the 41 patients evaluable for response, 26 (64%) had a complete response (CR) and 12 (29%) had a partial response. Three additional patients had stable disease, for an objective response rate of 93%.
Of 34 patients with PET scans, all had negative scans. Of 37 patients evaluable for minimal residual disease (MRD) by flow cytometry, 21 (58%) were MRD negative.
Patients with low or intermediate Mantle Cell Lymphoma International Prognostic Index (MIPI) scores had a higher ORR (100% vs. 89% for patients with high MIPI scores), and patients with low Ki-67 levels had a higher response rate than that of patients with KI-67 of 30% or greater (80% vs. 87%).
Neither median 3-year progression-free survival nor median 3-year overall survival have been reached, with respective 3-year rates of 87% and 95%.
Four patients experienced disease progression at 4, 10, 13 and 33 months of treatment. Three of these patients had disease that had transformed to blastoid/pleomorphic variant, two had Ki-67 of 30% or greater, one had mutations in TP53, and one had FAT1 and SF3B1 mutations.
Two patients died after ibrutinib therapy, one who had discontinued therapy because of bleeding, and the other who died on treatment at 13 months from transformed disease. Both of these patients had high Ki-67 levels.
Grade 3 or 4 hematological adverse events were neutropenia in four patients, and thrombocytopenia in two patients. There were no cases of grade 3 or 4 anemia.
Grade 3 or 4 nonhematological adverse events were fatigue, myalgia, and atrial fibrillation in seven patients each, diarrhea in six patients, and petechiae/bleeding in three patients.
Patients will continue to be followed for late adverse events, secondary cancers, and relapses, and further studies on clonal evolution, mutation profiling, and MRD are ongoing and will be reported at a later date, Dr. Jain said.
The National Cancer Institute supported the study. Dr. Jain reported having no financial disclosures.
SOURCE: Jain P et al. 15-ICML. Abstract 011.
LUGANO, SWITZERLAND – The chemotherapy-free combination of ibrutinib (Imbruvica) and rituximab is highly effective as frontline therapy for older, transplant-ineligible patients with nonblastoid mantle cell lymphoma, according to investigators.
In a phase 2 study of patients with a median age of 71 years, 38 of 41 patients (93%) had an objective response, and the regimen was both safe and easy to administer, reported Preetesh Jain, MD, PhD, of the University of Texas MD Anderson Cancer Center in Houston.
“The adverse event profile was generally favorable, with specific monitoring recommended for patients with cardiovascular comorbidities and a history of atrial fibrillation,” he said at the International Conference on Malignant Lymphoma.
The investigators enrolled 48 patients aged 65 years and older with previously untreated mantle cell lymphoma (MCL), of whom 41 were evaluable for the primary endpoints of overall response rate (ORR) and safety. The patients had good performance status and normal organ function, with the largest tumor size less than 10 cm. Patients with atrial fibrillation could participate, if the fibrillation was controlled. Patients with Ki-67 protein levels of 50% or greater and blastoid/pleomorphic histology were excluded.
Patients were treated with ibrutinib 560 mg orally daily for each 28-day cycle, with therapy continued until disease progression, or until therapy was stopped for any other reason. Patients also received intravenous rituximab 375 mg/m2 on days 1, 8, 15 and 22, plus or minus one day for cycle 1, on day 1 of cycles 3-8, and on day 1 of every other cycle for up to 2 years.
Of the 41 patients evaluable for response, 26 (64%) had a complete response (CR) and 12 (29%) had a partial response. Three additional patients had stable disease, for an objective response rate of 93%.
Of 34 patients with PET scans, all had negative scans. Of 37 patients evaluable for minimal residual disease (MRD) by flow cytometry, 21 (58%) were MRD negative.
Patients with low or intermediate Mantle Cell Lymphoma International Prognostic Index (MIPI) scores had a higher ORR (100% vs. 89% for patients with high MIPI scores), and patients with low Ki-67 levels had a higher response rate than that of patients with KI-67 of 30% or greater (80% vs. 87%).
Neither median 3-year progression-free survival nor median 3-year overall survival have been reached, with respective 3-year rates of 87% and 95%.
Four patients experienced disease progression at 4, 10, 13 and 33 months of treatment. Three of these patients had disease that had transformed to blastoid/pleomorphic variant, two had Ki-67 of 30% or greater, one had mutations in TP53, and one had FAT1 and SF3B1 mutations.
Two patients died after ibrutinib therapy, one who had discontinued therapy because of bleeding, and the other who died on treatment at 13 months from transformed disease. Both of these patients had high Ki-67 levels.
Grade 3 or 4 hematological adverse events were neutropenia in four patients, and thrombocytopenia in two patients. There were no cases of grade 3 or 4 anemia.
Grade 3 or 4 nonhematological adverse events were fatigue, myalgia, and atrial fibrillation in seven patients each, diarrhea in six patients, and petechiae/bleeding in three patients.
Patients will continue to be followed for late adverse events, secondary cancers, and relapses, and further studies on clonal evolution, mutation profiling, and MRD are ongoing and will be reported at a later date, Dr. Jain said.
The National Cancer Institute supported the study. Dr. Jain reported having no financial disclosures.
SOURCE: Jain P et al. 15-ICML. Abstract 011.
REPORTING FROM 15-ICML