User login
For MD-IQ on Family Practice News, but a regular topic for Rheumatology News
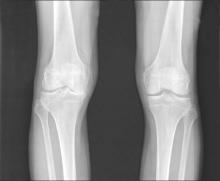
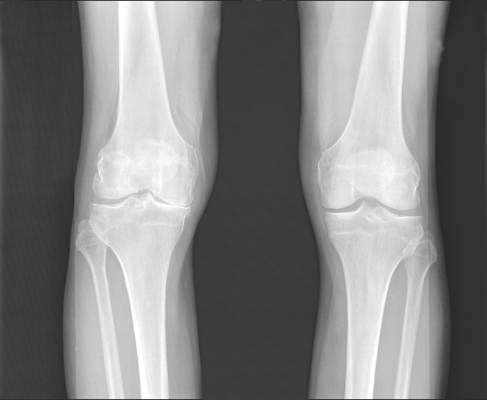
Allogeneic cell therapy fares well in phase III osteoarthritis trial
AMSTERDAM – A novel intra-articular allogeneic cell therapy improved knee osteoarthritis pain, physical activity, and patients’ quality of life in a randomized, placebo-controlled, double-blind, phase III trial reported at the World Congress on Osteoarthritis.
During a 1-year follow up, single-dose treatment with TissueGene C (Invossa) in patients with degenerative knee osteoarthritis (OA) led to significantly greater improvements in International Knee Documentation Committee (IKDC) knee pain, Likert visual analog scale (VAS) pain score, and Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) scores than in those given a saline placebo.
The positive results of the trial, which was conducted exclusively in Korea, now pave the way for a similar phase III clinical trial to be run in the United States. The study design is being finalized and will follow the usual criteria for a phase III trial, but it will add one refinement on the design used in the Korean trial in that the TissueGene C (TG-C) will be given by image-guided intra-articular injection.
TG-C consists of two different populations of chondrocytes derived from a single human subject with polydactyly finger, of which one cell population has been genetically modified to express the gene for transforming growth factor-beta 1 (TGF-B1). It is a single-dose treatment that is intended to provide symptomatic relief and delay the structural progression of knee OA.
The proposed mode of action is that it targets cartilage degradation by enhancing chondrocyte proliferation, essentially improving cartilage structure by redirecting immune responses via effects on interleukin-10 and on M2 macrophages.
The target population is patients with with Kellgren-Lawrence grade II and III knee OA, Dr. Bumsup Lee of Kolon Life Science said during a company-sponsored symposium held during the congress, which was sponsored by Osteoarthritis Research Society International. Dr. Lee is chief operating officer of TissueGene Inc. in the United States, which is collaborating with Kolon Life Science, the Korean-based biochemical company that developed the novel OA therapy.
The aim is to develop a disease-modifying osteoarthritis drug (DMOAD) that fits criteria suggested by the Food and Drug Administration and the European Medicines Agency, Dr. Lee said. The clinical development program started in 2006, with phase I, II, and III studies now completed in Korea and phase II and III studies underway in the United States.
The results of the phase III Korean trial, which involved 163 patients, were presented during a poster session and during the industry-sponsored symposium. These showed that IKDC scores progressively increased (improved) for TG-C–treated patients from baseline through months 1, 3, 6, 9, and 12 with scores of 40.3, 42.9, 48.9, 53.2, 53.6, and 55.4, respectively, versus placebo scores at the same time points of 39.6, 44.6, 45.2, 36.5, 47.1, and 44.6. The differences became significant at 6 months. Changes in IKDC scores from baseline were also only significantly in favor of TG-C versus placebo starting at 6 months (12.9 vs. 6.9), then extending to 9 months (13.3 vs. 7.5) and 12 months (15.1 vs. 5.0).
TG-C proved superior to placebo in improving VAS pain scores at 6, 9, and 12 months, and changes in WOMAC scores were significant after 12 months with a mean change of –13.9 with TG-C and –6.2 with placebo.
The OMERACT-OARSI response rate was also assessed. A responder was identified as someone with an improvement in VAS pain score of 50% or more and an absolute change in score of at least 10 points and at least a 50% improvement in IKDC score with an absolute change of at least 20 points. The response rate for TG-C and placebo was 70% versus 52% (P = .02) at 6 months, 77% versus 52% at 9 months (P = .0011), and 84% versus 45% (P less than .001) at 12 months.
The most common adverse events seen were related to the intra-articular injection, Dr. Lee noted. Two serious adverse events – arthralgia and joint swelling – occurred in one subject who received the novel treatment, but this resolved within 10 days of administration.
Biomarker analyses have been performed and it appears that patients with greater joint space width, and higher baseline levels of CTX-II and C3M may be more likely to respond to TG-C. Some changes in CTX-I and CTX-II were clinically meaningful, and although the MRI data are still being evaluated, there was one striking feature: Patients treated with TG-C did not grow any osteophytes, compared with patients on placebo.
“Invossa holds great potential for a disease-modifying osteoarthritis drug,” said Dr. Gurdyal Kalsi, chief medical officer of TissueGene Inc. Based on the evidence today, which includes a phase II trial conducted in the United States (Osteoarthritis Cartilage. 2015;23:2109-18), he said that the novel treatment “offers sustained improvement of pain and function over 2 years with a single injection.” OMERACT-OARSI response rates were 81%-86% in patients not responding to conservative therapies in the phase II study.
It is envisioned that the phase III U.S. trial will start enrolling patients from the start of next year, with the last patient enrolled around May 2018 and the first results appearing around the summer of 2019. TissueGene Inc. would then expect to have the full trial results by 2020 and make a Biologics License Application to the FDA in early 2021.
“We believe we truly have something that we hope would fulfill a DMOAD application,” Dr. Kalsi said.
Kolon Life Science and TissueGene Inc. sponsored the studies. Dr. Lee and Dr. Kalsi are employees of TissueGene Inc.
AMSTERDAM – A novel intra-articular allogeneic cell therapy improved knee osteoarthritis pain, physical activity, and patients’ quality of life in a randomized, placebo-controlled, double-blind, phase III trial reported at the World Congress on Osteoarthritis.
During a 1-year follow up, single-dose treatment with TissueGene C (Invossa) in patients with degenerative knee osteoarthritis (OA) led to significantly greater improvements in International Knee Documentation Committee (IKDC) knee pain, Likert visual analog scale (VAS) pain score, and Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) scores than in those given a saline placebo.
The positive results of the trial, which was conducted exclusively in Korea, now pave the way for a similar phase III clinical trial to be run in the United States. The study design is being finalized and will follow the usual criteria for a phase III trial, but it will add one refinement on the design used in the Korean trial in that the TissueGene C (TG-C) will be given by image-guided intra-articular injection.
TG-C consists of two different populations of chondrocytes derived from a single human subject with polydactyly finger, of which one cell population has been genetically modified to express the gene for transforming growth factor-beta 1 (TGF-B1). It is a single-dose treatment that is intended to provide symptomatic relief and delay the structural progression of knee OA.
The proposed mode of action is that it targets cartilage degradation by enhancing chondrocyte proliferation, essentially improving cartilage structure by redirecting immune responses via effects on interleukin-10 and on M2 macrophages.
The target population is patients with with Kellgren-Lawrence grade II and III knee OA, Dr. Bumsup Lee of Kolon Life Science said during a company-sponsored symposium held during the congress, which was sponsored by Osteoarthritis Research Society International. Dr. Lee is chief operating officer of TissueGene Inc. in the United States, which is collaborating with Kolon Life Science, the Korean-based biochemical company that developed the novel OA therapy.
The aim is to develop a disease-modifying osteoarthritis drug (DMOAD) that fits criteria suggested by the Food and Drug Administration and the European Medicines Agency, Dr. Lee said. The clinical development program started in 2006, with phase I, II, and III studies now completed in Korea and phase II and III studies underway in the United States.
The results of the phase III Korean trial, which involved 163 patients, were presented during a poster session and during the industry-sponsored symposium. These showed that IKDC scores progressively increased (improved) for TG-C–treated patients from baseline through months 1, 3, 6, 9, and 12 with scores of 40.3, 42.9, 48.9, 53.2, 53.6, and 55.4, respectively, versus placebo scores at the same time points of 39.6, 44.6, 45.2, 36.5, 47.1, and 44.6. The differences became significant at 6 months. Changes in IKDC scores from baseline were also only significantly in favor of TG-C versus placebo starting at 6 months (12.9 vs. 6.9), then extending to 9 months (13.3 vs. 7.5) and 12 months (15.1 vs. 5.0).
TG-C proved superior to placebo in improving VAS pain scores at 6, 9, and 12 months, and changes in WOMAC scores were significant after 12 months with a mean change of –13.9 with TG-C and –6.2 with placebo.
The OMERACT-OARSI response rate was also assessed. A responder was identified as someone with an improvement in VAS pain score of 50% or more and an absolute change in score of at least 10 points and at least a 50% improvement in IKDC score with an absolute change of at least 20 points. The response rate for TG-C and placebo was 70% versus 52% (P = .02) at 6 months, 77% versus 52% at 9 months (P = .0011), and 84% versus 45% (P less than .001) at 12 months.
The most common adverse events seen were related to the intra-articular injection, Dr. Lee noted. Two serious adverse events – arthralgia and joint swelling – occurred in one subject who received the novel treatment, but this resolved within 10 days of administration.
Biomarker analyses have been performed and it appears that patients with greater joint space width, and higher baseline levels of CTX-II and C3M may be more likely to respond to TG-C. Some changes in CTX-I and CTX-II were clinically meaningful, and although the MRI data are still being evaluated, there was one striking feature: Patients treated with TG-C did not grow any osteophytes, compared with patients on placebo.
“Invossa holds great potential for a disease-modifying osteoarthritis drug,” said Dr. Gurdyal Kalsi, chief medical officer of TissueGene Inc. Based on the evidence today, which includes a phase II trial conducted in the United States (Osteoarthritis Cartilage. 2015;23:2109-18), he said that the novel treatment “offers sustained improvement of pain and function over 2 years with a single injection.” OMERACT-OARSI response rates were 81%-86% in patients not responding to conservative therapies in the phase II study.
It is envisioned that the phase III U.S. trial will start enrolling patients from the start of next year, with the last patient enrolled around May 2018 and the first results appearing around the summer of 2019. TissueGene Inc. would then expect to have the full trial results by 2020 and make a Biologics License Application to the FDA in early 2021.
“We believe we truly have something that we hope would fulfill a DMOAD application,” Dr. Kalsi said.
Kolon Life Science and TissueGene Inc. sponsored the studies. Dr. Lee and Dr. Kalsi are employees of TissueGene Inc.
AMSTERDAM – A novel intra-articular allogeneic cell therapy improved knee osteoarthritis pain, physical activity, and patients’ quality of life in a randomized, placebo-controlled, double-blind, phase III trial reported at the World Congress on Osteoarthritis.
During a 1-year follow up, single-dose treatment with TissueGene C (Invossa) in patients with degenerative knee osteoarthritis (OA) led to significantly greater improvements in International Knee Documentation Committee (IKDC) knee pain, Likert visual analog scale (VAS) pain score, and Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) scores than in those given a saline placebo.
The positive results of the trial, which was conducted exclusively in Korea, now pave the way for a similar phase III clinical trial to be run in the United States. The study design is being finalized and will follow the usual criteria for a phase III trial, but it will add one refinement on the design used in the Korean trial in that the TissueGene C (TG-C) will be given by image-guided intra-articular injection.
TG-C consists of two different populations of chondrocytes derived from a single human subject with polydactyly finger, of which one cell population has been genetically modified to express the gene for transforming growth factor-beta 1 (TGF-B1). It is a single-dose treatment that is intended to provide symptomatic relief and delay the structural progression of knee OA.
The proposed mode of action is that it targets cartilage degradation by enhancing chondrocyte proliferation, essentially improving cartilage structure by redirecting immune responses via effects on interleukin-10 and on M2 macrophages.
The target population is patients with with Kellgren-Lawrence grade II and III knee OA, Dr. Bumsup Lee of Kolon Life Science said during a company-sponsored symposium held during the congress, which was sponsored by Osteoarthritis Research Society International. Dr. Lee is chief operating officer of TissueGene Inc. in the United States, which is collaborating with Kolon Life Science, the Korean-based biochemical company that developed the novel OA therapy.
The aim is to develop a disease-modifying osteoarthritis drug (DMOAD) that fits criteria suggested by the Food and Drug Administration and the European Medicines Agency, Dr. Lee said. The clinical development program started in 2006, with phase I, II, and III studies now completed in Korea and phase II and III studies underway in the United States.
The results of the phase III Korean trial, which involved 163 patients, were presented during a poster session and during the industry-sponsored symposium. These showed that IKDC scores progressively increased (improved) for TG-C–treated patients from baseline through months 1, 3, 6, 9, and 12 with scores of 40.3, 42.9, 48.9, 53.2, 53.6, and 55.4, respectively, versus placebo scores at the same time points of 39.6, 44.6, 45.2, 36.5, 47.1, and 44.6. The differences became significant at 6 months. Changes in IKDC scores from baseline were also only significantly in favor of TG-C versus placebo starting at 6 months (12.9 vs. 6.9), then extending to 9 months (13.3 vs. 7.5) and 12 months (15.1 vs. 5.0).
TG-C proved superior to placebo in improving VAS pain scores at 6, 9, and 12 months, and changes in WOMAC scores were significant after 12 months with a mean change of –13.9 with TG-C and –6.2 with placebo.
The OMERACT-OARSI response rate was also assessed. A responder was identified as someone with an improvement in VAS pain score of 50% or more and an absolute change in score of at least 10 points and at least a 50% improvement in IKDC score with an absolute change of at least 20 points. The response rate for TG-C and placebo was 70% versus 52% (P = .02) at 6 months, 77% versus 52% at 9 months (P = .0011), and 84% versus 45% (P less than .001) at 12 months.
The most common adverse events seen were related to the intra-articular injection, Dr. Lee noted. Two serious adverse events – arthralgia and joint swelling – occurred in one subject who received the novel treatment, but this resolved within 10 days of administration.
Biomarker analyses have been performed and it appears that patients with greater joint space width, and higher baseline levels of CTX-II and C3M may be more likely to respond to TG-C. Some changes in CTX-I and CTX-II were clinically meaningful, and although the MRI data are still being evaluated, there was one striking feature: Patients treated with TG-C did not grow any osteophytes, compared with patients on placebo.
“Invossa holds great potential for a disease-modifying osteoarthritis drug,” said Dr. Gurdyal Kalsi, chief medical officer of TissueGene Inc. Based on the evidence today, which includes a phase II trial conducted in the United States (Osteoarthritis Cartilage. 2015;23:2109-18), he said that the novel treatment “offers sustained improvement of pain and function over 2 years with a single injection.” OMERACT-OARSI response rates were 81%-86% in patients not responding to conservative therapies in the phase II study.
It is envisioned that the phase III U.S. trial will start enrolling patients from the start of next year, with the last patient enrolled around May 2018 and the first results appearing around the summer of 2019. TissueGene Inc. would then expect to have the full trial results by 2020 and make a Biologics License Application to the FDA in early 2021.
“We believe we truly have something that we hope would fulfill a DMOAD application,” Dr. Kalsi said.
Kolon Life Science and TissueGene Inc. sponsored the studies. Dr. Lee and Dr. Kalsi are employees of TissueGene Inc.
AT OARSI 2016
Key clinical point: A single intra-articular injection of TissueGene C provided symptomatic relief and could be the first disease-modifying osteoarthritis drug.
Major finding: Changes in IKDC, VAS pain, and WOMAC scores at 1 year of follow-up with TG-C and saline placebo were a respective 15.1 and 5.0 points (P less than .05), –24.5 and –0.3 points (P less than .05), and –13.9 and –6.2 points (P less than .05).
Data source: Phase III, randomized, double-blind, placebo-controlled trial conducted in Korea in 163 patients with knee osteoarthritis.
Disclosures: Kolon Life Science and TissueGene Inc. sponsored the studies. Dr. Lee and Dr. Kalsi are employees of TissueGene Inc.
Guideline change advocated on using acetaminophen for OA
AMSTERDAM – Further evidence that acetaminophen has limited benefits in patients with osteoarthritis was presented at the World Congress on Osteoarthritis, with authors of a systematic review calling for reconsideration of guidelines recommending the common analgesic as a first-line option.
“[Acetaminophen] provides minimal short-term benefits for people with hip or knee OA,” said presenting author and rheumatologist Dr. David J. Hunter of the University of Sydney. The treatment effects for both pain relief and for improving physical function were smallest in people with knee OA, he said. “In general, the small effect sizes are unlikely to be clinically relevant,” Dr. Hunter observed.
“These are mean differences across large populations in the clinical trials, and there may be certain individuals with knee or hip osteoarthritis that this may not necessarily apply to,” he conceded during a discussion following his presentation, “but I think from the perspective of the recommendations that come from guidelines, we have got to think about what would be do-able in the general population.”
The findings come shortly after the publication of a large meta-analysis of 74 trials evaluating pain-relieving medications that highlighted the ineffectiveness of acetaminophen for OA pain, particularly when compared against diclofenac and other nonsteroidal anti-inflammatory drugs (Lancet. 2016 Mar 17. doi: 10.1016/S0140-6736(16)30002-2).
Dr. Hunter and coworkers searched clinical trial and medical databases from inception to September 2015 for records relating to acetaminophen use in patients with hip or knee OA. Only placebo-controlled, randomized trials were included, and nine records were found that reported 10 trials involving 3,541 patients. Part of the analysis was published in the BMJ last year (BMJ. 2015;350:h1225. doi: 10.1136/bmj.h1225). The last prior systematic review on the topic was published in 2004 (Ann Rheum Dis. 2004;Aug;63[8]:901–7).
Pain scores were converted to a common 0-100 scale with 0 signifying no pain or disability and 100 the worst possible pain or disability and then expressed as a mean difference between the acetaminophen and placebo groups. Physical function scores were pooled to give a standardized mean difference.
There was high-quality evidence that acetaminophen given at a dose of 3-4 g per day had a significant effect on pain and physical function during a short period of more than 2 weeks to less than 3 months and a more immediate time frame of 2 weeks or less, but it was unlikely to be clinically significant, with a mean difference of just –3.14 for pain and a standardized mean difference of –0.12 to –0.15 for physical function. Differences would need to be at least 9 points for pain and greater than 0.2 for physical function to be clinically significant, Dr. Hunter explained.
Four of the trials considered knee OA only. The mean and standardized mean differences between the acetaminophen and placebo groups in those trials was just –1.09 for pain and –0.06 for physical function.
Similar numbers of patients reported being adherent to their assigned treatment group, with less rescue analgesic use in the acetaminophen-treated patients. Although no differences in adverse events, serious adverse events, or withdrawals because of adverse events were seen, there was a higher risk of liver function test (LFT) abnormalities in the acetaminophen-treated patients. The relative risk for abnormal LFTs was 3.79, but the clinical significance of this is uncertain according to the review’s authors.
“Current guidelines consistently recommend [acetaminophen] as the first line of analgesic medication for this condition,” Dr. Hunter said at the meeting, sponsored by the Osteoarthritis Research Society International. “But these results call for reconsideration of these recommendations.”
The results highlight the importance of using other, nonpharmacologic means to manage pain and physical function, the authors conclude, such as lifestyle changes, weight control, and regular physical exercise.
Dr. Hunter had no disclosures relevant to his comments.
AMSTERDAM – Further evidence that acetaminophen has limited benefits in patients with osteoarthritis was presented at the World Congress on Osteoarthritis, with authors of a systematic review calling for reconsideration of guidelines recommending the common analgesic as a first-line option.
“[Acetaminophen] provides minimal short-term benefits for people with hip or knee OA,” said presenting author and rheumatologist Dr. David J. Hunter of the University of Sydney. The treatment effects for both pain relief and for improving physical function were smallest in people with knee OA, he said. “In general, the small effect sizes are unlikely to be clinically relevant,” Dr. Hunter observed.
“These are mean differences across large populations in the clinical trials, and there may be certain individuals with knee or hip osteoarthritis that this may not necessarily apply to,” he conceded during a discussion following his presentation, “but I think from the perspective of the recommendations that come from guidelines, we have got to think about what would be do-able in the general population.”
The findings come shortly after the publication of a large meta-analysis of 74 trials evaluating pain-relieving medications that highlighted the ineffectiveness of acetaminophen for OA pain, particularly when compared against diclofenac and other nonsteroidal anti-inflammatory drugs (Lancet. 2016 Mar 17. doi: 10.1016/S0140-6736(16)30002-2).
Dr. Hunter and coworkers searched clinical trial and medical databases from inception to September 2015 for records relating to acetaminophen use in patients with hip or knee OA. Only placebo-controlled, randomized trials were included, and nine records were found that reported 10 trials involving 3,541 patients. Part of the analysis was published in the BMJ last year (BMJ. 2015;350:h1225. doi: 10.1136/bmj.h1225). The last prior systematic review on the topic was published in 2004 (Ann Rheum Dis. 2004;Aug;63[8]:901–7).
Pain scores were converted to a common 0-100 scale with 0 signifying no pain or disability and 100 the worst possible pain or disability and then expressed as a mean difference between the acetaminophen and placebo groups. Physical function scores were pooled to give a standardized mean difference.
There was high-quality evidence that acetaminophen given at a dose of 3-4 g per day had a significant effect on pain and physical function during a short period of more than 2 weeks to less than 3 months and a more immediate time frame of 2 weeks or less, but it was unlikely to be clinically significant, with a mean difference of just –3.14 for pain and a standardized mean difference of –0.12 to –0.15 for physical function. Differences would need to be at least 9 points for pain and greater than 0.2 for physical function to be clinically significant, Dr. Hunter explained.
Four of the trials considered knee OA only. The mean and standardized mean differences between the acetaminophen and placebo groups in those trials was just –1.09 for pain and –0.06 for physical function.
Similar numbers of patients reported being adherent to their assigned treatment group, with less rescue analgesic use in the acetaminophen-treated patients. Although no differences in adverse events, serious adverse events, or withdrawals because of adverse events were seen, there was a higher risk of liver function test (LFT) abnormalities in the acetaminophen-treated patients. The relative risk for abnormal LFTs was 3.79, but the clinical significance of this is uncertain according to the review’s authors.
“Current guidelines consistently recommend [acetaminophen] as the first line of analgesic medication for this condition,” Dr. Hunter said at the meeting, sponsored by the Osteoarthritis Research Society International. “But these results call for reconsideration of these recommendations.”
The results highlight the importance of using other, nonpharmacologic means to manage pain and physical function, the authors conclude, such as lifestyle changes, weight control, and regular physical exercise.
Dr. Hunter had no disclosures relevant to his comments.
AMSTERDAM – Further evidence that acetaminophen has limited benefits in patients with osteoarthritis was presented at the World Congress on Osteoarthritis, with authors of a systematic review calling for reconsideration of guidelines recommending the common analgesic as a first-line option.
“[Acetaminophen] provides minimal short-term benefits for people with hip or knee OA,” said presenting author and rheumatologist Dr. David J. Hunter of the University of Sydney. The treatment effects for both pain relief and for improving physical function were smallest in people with knee OA, he said. “In general, the small effect sizes are unlikely to be clinically relevant,” Dr. Hunter observed.
“These are mean differences across large populations in the clinical trials, and there may be certain individuals with knee or hip osteoarthritis that this may not necessarily apply to,” he conceded during a discussion following his presentation, “but I think from the perspective of the recommendations that come from guidelines, we have got to think about what would be do-able in the general population.”
The findings come shortly after the publication of a large meta-analysis of 74 trials evaluating pain-relieving medications that highlighted the ineffectiveness of acetaminophen for OA pain, particularly when compared against diclofenac and other nonsteroidal anti-inflammatory drugs (Lancet. 2016 Mar 17. doi: 10.1016/S0140-6736(16)30002-2).
Dr. Hunter and coworkers searched clinical trial and medical databases from inception to September 2015 for records relating to acetaminophen use in patients with hip or knee OA. Only placebo-controlled, randomized trials were included, and nine records were found that reported 10 trials involving 3,541 patients. Part of the analysis was published in the BMJ last year (BMJ. 2015;350:h1225. doi: 10.1136/bmj.h1225). The last prior systematic review on the topic was published in 2004 (Ann Rheum Dis. 2004;Aug;63[8]:901–7).
Pain scores were converted to a common 0-100 scale with 0 signifying no pain or disability and 100 the worst possible pain or disability and then expressed as a mean difference between the acetaminophen and placebo groups. Physical function scores were pooled to give a standardized mean difference.
There was high-quality evidence that acetaminophen given at a dose of 3-4 g per day had a significant effect on pain and physical function during a short period of more than 2 weeks to less than 3 months and a more immediate time frame of 2 weeks or less, but it was unlikely to be clinically significant, with a mean difference of just –3.14 for pain and a standardized mean difference of –0.12 to –0.15 for physical function. Differences would need to be at least 9 points for pain and greater than 0.2 for physical function to be clinically significant, Dr. Hunter explained.
Four of the trials considered knee OA only. The mean and standardized mean differences between the acetaminophen and placebo groups in those trials was just –1.09 for pain and –0.06 for physical function.
Similar numbers of patients reported being adherent to their assigned treatment group, with less rescue analgesic use in the acetaminophen-treated patients. Although no differences in adverse events, serious adverse events, or withdrawals because of adverse events were seen, there was a higher risk of liver function test (LFT) abnormalities in the acetaminophen-treated patients. The relative risk for abnormal LFTs was 3.79, but the clinical significance of this is uncertain according to the review’s authors.
“Current guidelines consistently recommend [acetaminophen] as the first line of analgesic medication for this condition,” Dr. Hunter said at the meeting, sponsored by the Osteoarthritis Research Society International. “But these results call for reconsideration of these recommendations.”
The results highlight the importance of using other, nonpharmacologic means to manage pain and physical function, the authors conclude, such as lifestyle changes, weight control, and regular physical exercise.
Dr. Hunter had no disclosures relevant to his comments.
AT OARSI 2016
Key clinical point: Acetaminophen has minimal effects on pain and physical function in patients with hip and knee osteoarthritis.
Major finding: Doses of 3-4 g of acetaminophen resulted in a mean difference of just –3.14 for pain and a standardized mean difference of –0.12 to –0.15 for physical function versus placebo.
Data source: Cochrane systematic review of 10 trials involving 3,541 patients with hip or knee OA.
Disclosures: Dr. Hunter had no disclosures relevant to his comments.
Physical therapy underused for knee osteoarthritis
AMSTERDAM – Physical therapy is underused as a first conservative treatment option for knee osteoarthritis, according to a review of data of more than 130,000 men and women serving in the U.S. military.
Data from the Military Health System Data Repository (MDR) show that clinical practice is often out of step with guidelines, as many patients are treated with intra-articular corticosteroid injections rather than physical therapy.
Within a week of an initial episode of knee OA, patients were four times more likely to be given steroid injections than receive physical therapy (14,290 vs. 3,177 patients), although a similar number of patients received injections or physical therapy within 30 days (8,228 vs. 8,407) and 90 days (9,125 vs. 10,059). Of the patients given injections early, 12,311 received them on the day of the index episode.
“What was interesting, anecdotally, was how few patients had been to physical therapy prior to surgery and how many of them had had injections before physical therapy,” Dr. Dan Rhon, director of physical therapy at Brooke Army Medical Center, Houston, said in an interview at the World Congress on Osteoarthritis.
“We wanted to look at the practice patterns because guidelines generally say that you should try physical therapy before surgery and that invasive procedures such as injections should be utilized further down the road,” he said at the meeting, which was sponsored by Osteoarthritis Research Society International.
Dr. Rhon noted that in a study of clinical practice patterns over a 5-year period from the United Healthcare Database, researchers found that physical rehabilitation was used in about one-quarter of patients, with 10% of those who went on to have surgery receiving physical therapy specific for OA, and 16% had been given intra-articular injections (Arthroscopy. 2014;30:65-71).
Results from the MDR data, which considered records from 2008 to 2013 and 1 year of follow-up, now show similar findings with 29% of patients receiving physical rehabilitation, 17.5% prior to knee arthroplasty, and 36% of patients receiving corticosteroid injections first.
What this shows, Dr. Rhon said, is that clinical practice patterns in the Military Health System do not appear to be following established guidelines. “It’s a little bit worrisome because most of these patients should at least have a trial of conservative management.” Perhaps there is some confusion over what constitutes conservative management, but when there is a choice between physical therapy and corticosteroid injections, it seems that the latter is more commonly being selected. Patients themselves could also be opting for injections over physical rehabilitation, so there may be a need for education on what options are available before surgical intervention.
Further research is needed to examine the clinical outcomes of this and perhaps look at how cost savings could be made if clinical practice more closely adhered to the OA guidelines. Looking at a longer follow-up period, say 2-5 years post diagnosis, might also give a better reflection of the use of physical therapy before surgery.
Dr. Rhon had no financial disclosures.
AMSTERDAM – Physical therapy is underused as a first conservative treatment option for knee osteoarthritis, according to a review of data of more than 130,000 men and women serving in the U.S. military.
Data from the Military Health System Data Repository (MDR) show that clinical practice is often out of step with guidelines, as many patients are treated with intra-articular corticosteroid injections rather than physical therapy.
Within a week of an initial episode of knee OA, patients were four times more likely to be given steroid injections than receive physical therapy (14,290 vs. 3,177 patients), although a similar number of patients received injections or physical therapy within 30 days (8,228 vs. 8,407) and 90 days (9,125 vs. 10,059). Of the patients given injections early, 12,311 received them on the day of the index episode.
“What was interesting, anecdotally, was how few patients had been to physical therapy prior to surgery and how many of them had had injections before physical therapy,” Dr. Dan Rhon, director of physical therapy at Brooke Army Medical Center, Houston, said in an interview at the World Congress on Osteoarthritis.
“We wanted to look at the practice patterns because guidelines generally say that you should try physical therapy before surgery and that invasive procedures such as injections should be utilized further down the road,” he said at the meeting, which was sponsored by Osteoarthritis Research Society International.
Dr. Rhon noted that in a study of clinical practice patterns over a 5-year period from the United Healthcare Database, researchers found that physical rehabilitation was used in about one-quarter of patients, with 10% of those who went on to have surgery receiving physical therapy specific for OA, and 16% had been given intra-articular injections (Arthroscopy. 2014;30:65-71).
Results from the MDR data, which considered records from 2008 to 2013 and 1 year of follow-up, now show similar findings with 29% of patients receiving physical rehabilitation, 17.5% prior to knee arthroplasty, and 36% of patients receiving corticosteroid injections first.
What this shows, Dr. Rhon said, is that clinical practice patterns in the Military Health System do not appear to be following established guidelines. “It’s a little bit worrisome because most of these patients should at least have a trial of conservative management.” Perhaps there is some confusion over what constitutes conservative management, but when there is a choice between physical therapy and corticosteroid injections, it seems that the latter is more commonly being selected. Patients themselves could also be opting for injections over physical rehabilitation, so there may be a need for education on what options are available before surgical intervention.
Further research is needed to examine the clinical outcomes of this and perhaps look at how cost savings could be made if clinical practice more closely adhered to the OA guidelines. Looking at a longer follow-up period, say 2-5 years post diagnosis, might also give a better reflection of the use of physical therapy before surgery.
Dr. Rhon had no financial disclosures.
AMSTERDAM – Physical therapy is underused as a first conservative treatment option for knee osteoarthritis, according to a review of data of more than 130,000 men and women serving in the U.S. military.
Data from the Military Health System Data Repository (MDR) show that clinical practice is often out of step with guidelines, as many patients are treated with intra-articular corticosteroid injections rather than physical therapy.
Within a week of an initial episode of knee OA, patients were four times more likely to be given steroid injections than receive physical therapy (14,290 vs. 3,177 patients), although a similar number of patients received injections or physical therapy within 30 days (8,228 vs. 8,407) and 90 days (9,125 vs. 10,059). Of the patients given injections early, 12,311 received them on the day of the index episode.
“What was interesting, anecdotally, was how few patients had been to physical therapy prior to surgery and how many of them had had injections before physical therapy,” Dr. Dan Rhon, director of physical therapy at Brooke Army Medical Center, Houston, said in an interview at the World Congress on Osteoarthritis.
“We wanted to look at the practice patterns because guidelines generally say that you should try physical therapy before surgery and that invasive procedures such as injections should be utilized further down the road,” he said at the meeting, which was sponsored by Osteoarthritis Research Society International.
Dr. Rhon noted that in a study of clinical practice patterns over a 5-year period from the United Healthcare Database, researchers found that physical rehabilitation was used in about one-quarter of patients, with 10% of those who went on to have surgery receiving physical therapy specific for OA, and 16% had been given intra-articular injections (Arthroscopy. 2014;30:65-71).
Results from the MDR data, which considered records from 2008 to 2013 and 1 year of follow-up, now show similar findings with 29% of patients receiving physical rehabilitation, 17.5% prior to knee arthroplasty, and 36% of patients receiving corticosteroid injections first.
What this shows, Dr. Rhon said, is that clinical practice patterns in the Military Health System do not appear to be following established guidelines. “It’s a little bit worrisome because most of these patients should at least have a trial of conservative management.” Perhaps there is some confusion over what constitutes conservative management, but when there is a choice between physical therapy and corticosteroid injections, it seems that the latter is more commonly being selected. Patients themselves could also be opting for injections over physical rehabilitation, so there may be a need for education on what options are available before surgical intervention.
Further research is needed to examine the clinical outcomes of this and perhaps look at how cost savings could be made if clinical practice more closely adhered to the OA guidelines. Looking at a longer follow-up period, say 2-5 years post diagnosis, might also give a better reflection of the use of physical therapy before surgery.
Dr. Rhon had no financial disclosures.
AT OARSI 2016
Key clinical point: Contrary to guidelines, physical therapy is being underused as a first treatment option for osteoarthritis.
Major finding: Only 29% of patients received physical rehabilitation while 36% were given intra-articular steroid injections first.
Data source: Retrospective review of data from the U.S. Military Health Care System Data Repository from 2008 to 2013 on more than 130,000 patients with OA.
Disclosures: Dr. Rhon had no financial disclosures.
Blood biomarker panel studies aim to predict knee osteoarthritis, progression
AMSTERDAM – Results of separate U.S. and European studies hold promise that predictive blood tests for the diagnosis and progression of knee osteoarthritis could soon be developed.
Researchers at Duke University in Durham, N.C., and at the Hospital Universitario de A Coruña, Spain, reported their studies’ findings at the World Congress on Osteoarthritis sponsored by the Osteoarthritis Research Society International.
Clinical trials are often statistically underpowered to measure progression of knee osteoarthritis, noted Dr. Virginia B. Kraus of Duke University, because of inefficient methods for identifying patients who would progress.
“This is because, with the exception of generalized OA, traditional risk factors are very poor predictors, and that includes age, gender, BMI [body mass index], knee pain, and baseline joint space width,” she explained. Radiographic changes and changes in Kellgren-Lawrence grade are often slow to appear and may only occur in a few subjects at a time. Imaging biomarkers have been identified but are a “rather expensive way of predicting progression,” Dr. Kraus said. There have been some associations between OA progression and biomarkers in the urine and serum, she said, but still not at a level that could be put to clinical use.
“We hypothesized that we could improve the utility of OA biomarkers by using a nonbiased, nontargeted [mass spectrometry] approach,” Dr. Kraus said, explaining that this allowed them to create a Multiple Reaction Monitoring (MRM) panel of 146 serum peptides that were based on analyses of serum, synovial fluid, and urine samples from knee OA radiographic progressors and nonprogressors. The MRM panel was used first to identify candidate biomarkers and then confirm their differential expression patterns, followed by a process of verifying and validating the selected serum biomarkers.
The main aim of the study was to look for serum biomarkers in data from the Prediction of Osteoarthritis Progression (POP) and Genetics of Generalized Osteoarthritis (GOGO) patient cohorts that would be highly predictive for knee OA progression, with a secondary aim to look at potential early diagnostic biomarkers. The investigators used data from 83 patients with symptomatic knee progression to search for prognostic biomarkers and 126 with symptoms but without knee progression to find diagnostic biomarkers. The mean age of patients was 64 years, and 82% were female. The mean BMI was 28 kg/m2.
As expected, clinical predictors alone – age, gender, and BMI – had low predictive power for OA progression, with an area under the curve (AUC) of about 0.7. However, these clinical parameters in conjunction with a panel of 10 markers identified via MRM improved the AUC to 0.9 in the verification set and 0.8 in the validation set. For diagnosis, the AUC for clinical covariates alone was 0.8, but combined with a set of 19 MRM-identified markers, the AUC was 1.0 for the verification set and 0.9 for the validation set.
Dr. Kraus said that the serum peptide biomarkers identified from the MRM panel predicted that OA progression pathways would involve aggregation of cells, complement activation, cell movement of leukocytes, and cellular infiltration of leukocytes. The pathways predicted to be involved in a diagnosis of OA included skeletal function, cell movement, formation of filaments, cellular protrusions, and hemostasis.
“Results from this work in progress suggest that a select set of biomarkers from nontargeted analyses could significantly improve prediction of knee OA progression,” Dr. Kraus concluded. They also suggest a set of biomarkers could be used for diagnosis because of their distinction from those for progression, with one exception. Future work will look at whether the diagnostic panel could be used in patients with nonradiographic OA to potentially diagnose and perhaps help treat OA early before structural damage is apparent, she said.
The proteomics group at the Hospital Universitario de A Coruña collaborated with researchers at the KTH-Royal Institute of Technology in Stockholm and the University of Oxford (England) to also identify a serum protein biomarker panel that might prove useful for the diagnosis of OA.
Their study compared serum samples taken from 461 patients with knee OA with those taken from 412 patients with rheumatoid arthritis and 159 nonarthritic individuals. Serum samples from each of these groups were divided into a screening (n = 593) and a verification set (n = 439). The data were adjusted for variations in participant’s age, BMI, and gender.
In the screening phase, the investigators used an antibody suspension bead array composed of 174 antibodies to examine 78 protein targets to try to identify the best serum protein biomarker candidates. They replicated the findings in an adapted array of 79 antibodies and 34 protein targets and then used this array in the verification set of serum samples.
There were three proteins that could potentially differentiate patients with OA from controls and perhaps be used to diagnose OA. These were: complement 3, which is involved in inflammation; inter-alpha-trypsin inhibitor heavy chain I, which is involved in the stability of the extracellular matrix; and S100 calcium-binding protein A6, which has a role in chondrocyte differentiation and thus bone remodeling.
“Compared to rheumatoid arthritis patients, we observed that the level of complement 3 and inter-alpha-trypsin inhibitor heavy chain I are significantly increased in osteoarthritis patients,” said the presenting study author Lucía Maria Lourido Salas, PhD. “This suggests the potential of these two proteins as specific proteins for osteoarthritis,” she proposed. A further 28 proteins were found that might also help to differentiate OA from RA; 16 of these were increased and 12 decreased in OA patients. Together these 30 proteins could potentially help identify a specific serum protein signature of OA, Dr. Salas said.
Dr. Kraus and Dr. Salas did not report having any financial disclosures. Dr. Kraus’ research is funded by the Duke Biomarker Factory.
AMSTERDAM – Results of separate U.S. and European studies hold promise that predictive blood tests for the diagnosis and progression of knee osteoarthritis could soon be developed.
Researchers at Duke University in Durham, N.C., and at the Hospital Universitario de A Coruña, Spain, reported their studies’ findings at the World Congress on Osteoarthritis sponsored by the Osteoarthritis Research Society International.
Clinical trials are often statistically underpowered to measure progression of knee osteoarthritis, noted Dr. Virginia B. Kraus of Duke University, because of inefficient methods for identifying patients who would progress.
“This is because, with the exception of generalized OA, traditional risk factors are very poor predictors, and that includes age, gender, BMI [body mass index], knee pain, and baseline joint space width,” she explained. Radiographic changes and changes in Kellgren-Lawrence grade are often slow to appear and may only occur in a few subjects at a time. Imaging biomarkers have been identified but are a “rather expensive way of predicting progression,” Dr. Kraus said. There have been some associations between OA progression and biomarkers in the urine and serum, she said, but still not at a level that could be put to clinical use.
“We hypothesized that we could improve the utility of OA biomarkers by using a nonbiased, nontargeted [mass spectrometry] approach,” Dr. Kraus said, explaining that this allowed them to create a Multiple Reaction Monitoring (MRM) panel of 146 serum peptides that were based on analyses of serum, synovial fluid, and urine samples from knee OA radiographic progressors and nonprogressors. The MRM panel was used first to identify candidate biomarkers and then confirm their differential expression patterns, followed by a process of verifying and validating the selected serum biomarkers.
The main aim of the study was to look for serum biomarkers in data from the Prediction of Osteoarthritis Progression (POP) and Genetics of Generalized Osteoarthritis (GOGO) patient cohorts that would be highly predictive for knee OA progression, with a secondary aim to look at potential early diagnostic biomarkers. The investigators used data from 83 patients with symptomatic knee progression to search for prognostic biomarkers and 126 with symptoms but without knee progression to find diagnostic biomarkers. The mean age of patients was 64 years, and 82% were female. The mean BMI was 28 kg/m2.
As expected, clinical predictors alone – age, gender, and BMI – had low predictive power for OA progression, with an area under the curve (AUC) of about 0.7. However, these clinical parameters in conjunction with a panel of 10 markers identified via MRM improved the AUC to 0.9 in the verification set and 0.8 in the validation set. For diagnosis, the AUC for clinical covariates alone was 0.8, but combined with a set of 19 MRM-identified markers, the AUC was 1.0 for the verification set and 0.9 for the validation set.
Dr. Kraus said that the serum peptide biomarkers identified from the MRM panel predicted that OA progression pathways would involve aggregation of cells, complement activation, cell movement of leukocytes, and cellular infiltration of leukocytes. The pathways predicted to be involved in a diagnosis of OA included skeletal function, cell movement, formation of filaments, cellular protrusions, and hemostasis.
“Results from this work in progress suggest that a select set of biomarkers from nontargeted analyses could significantly improve prediction of knee OA progression,” Dr. Kraus concluded. They also suggest a set of biomarkers could be used for diagnosis because of their distinction from those for progression, with one exception. Future work will look at whether the diagnostic panel could be used in patients with nonradiographic OA to potentially diagnose and perhaps help treat OA early before structural damage is apparent, she said.
The proteomics group at the Hospital Universitario de A Coruña collaborated with researchers at the KTH-Royal Institute of Technology in Stockholm and the University of Oxford (England) to also identify a serum protein biomarker panel that might prove useful for the diagnosis of OA.
Their study compared serum samples taken from 461 patients with knee OA with those taken from 412 patients with rheumatoid arthritis and 159 nonarthritic individuals. Serum samples from each of these groups were divided into a screening (n = 593) and a verification set (n = 439). The data were adjusted for variations in participant’s age, BMI, and gender.
In the screening phase, the investigators used an antibody suspension bead array composed of 174 antibodies to examine 78 protein targets to try to identify the best serum protein biomarker candidates. They replicated the findings in an adapted array of 79 antibodies and 34 protein targets and then used this array in the verification set of serum samples.
There were three proteins that could potentially differentiate patients with OA from controls and perhaps be used to diagnose OA. These were: complement 3, which is involved in inflammation; inter-alpha-trypsin inhibitor heavy chain I, which is involved in the stability of the extracellular matrix; and S100 calcium-binding protein A6, which has a role in chondrocyte differentiation and thus bone remodeling.
“Compared to rheumatoid arthritis patients, we observed that the level of complement 3 and inter-alpha-trypsin inhibitor heavy chain I are significantly increased in osteoarthritis patients,” said the presenting study author Lucía Maria Lourido Salas, PhD. “This suggests the potential of these two proteins as specific proteins for osteoarthritis,” she proposed. A further 28 proteins were found that might also help to differentiate OA from RA; 16 of these were increased and 12 decreased in OA patients. Together these 30 proteins could potentially help identify a specific serum protein signature of OA, Dr. Salas said.
Dr. Kraus and Dr. Salas did not report having any financial disclosures. Dr. Kraus’ research is funded by the Duke Biomarker Factory.
AMSTERDAM – Results of separate U.S. and European studies hold promise that predictive blood tests for the diagnosis and progression of knee osteoarthritis could soon be developed.
Researchers at Duke University in Durham, N.C., and at the Hospital Universitario de A Coruña, Spain, reported their studies’ findings at the World Congress on Osteoarthritis sponsored by the Osteoarthritis Research Society International.
Clinical trials are often statistically underpowered to measure progression of knee osteoarthritis, noted Dr. Virginia B. Kraus of Duke University, because of inefficient methods for identifying patients who would progress.
“This is because, with the exception of generalized OA, traditional risk factors are very poor predictors, and that includes age, gender, BMI [body mass index], knee pain, and baseline joint space width,” she explained. Radiographic changes and changes in Kellgren-Lawrence grade are often slow to appear and may only occur in a few subjects at a time. Imaging biomarkers have been identified but are a “rather expensive way of predicting progression,” Dr. Kraus said. There have been some associations between OA progression and biomarkers in the urine and serum, she said, but still not at a level that could be put to clinical use.
“We hypothesized that we could improve the utility of OA biomarkers by using a nonbiased, nontargeted [mass spectrometry] approach,” Dr. Kraus said, explaining that this allowed them to create a Multiple Reaction Monitoring (MRM) panel of 146 serum peptides that were based on analyses of serum, synovial fluid, and urine samples from knee OA radiographic progressors and nonprogressors. The MRM panel was used first to identify candidate biomarkers and then confirm their differential expression patterns, followed by a process of verifying and validating the selected serum biomarkers.
The main aim of the study was to look for serum biomarkers in data from the Prediction of Osteoarthritis Progression (POP) and Genetics of Generalized Osteoarthritis (GOGO) patient cohorts that would be highly predictive for knee OA progression, with a secondary aim to look at potential early diagnostic biomarkers. The investigators used data from 83 patients with symptomatic knee progression to search for prognostic biomarkers and 126 with symptoms but without knee progression to find diagnostic biomarkers. The mean age of patients was 64 years, and 82% were female. The mean BMI was 28 kg/m2.
As expected, clinical predictors alone – age, gender, and BMI – had low predictive power for OA progression, with an area under the curve (AUC) of about 0.7. However, these clinical parameters in conjunction with a panel of 10 markers identified via MRM improved the AUC to 0.9 in the verification set and 0.8 in the validation set. For diagnosis, the AUC for clinical covariates alone was 0.8, but combined with a set of 19 MRM-identified markers, the AUC was 1.0 for the verification set and 0.9 for the validation set.
Dr. Kraus said that the serum peptide biomarkers identified from the MRM panel predicted that OA progression pathways would involve aggregation of cells, complement activation, cell movement of leukocytes, and cellular infiltration of leukocytes. The pathways predicted to be involved in a diagnosis of OA included skeletal function, cell movement, formation of filaments, cellular protrusions, and hemostasis.
“Results from this work in progress suggest that a select set of biomarkers from nontargeted analyses could significantly improve prediction of knee OA progression,” Dr. Kraus concluded. They also suggest a set of biomarkers could be used for diagnosis because of their distinction from those for progression, with one exception. Future work will look at whether the diagnostic panel could be used in patients with nonradiographic OA to potentially diagnose and perhaps help treat OA early before structural damage is apparent, she said.
The proteomics group at the Hospital Universitario de A Coruña collaborated with researchers at the KTH-Royal Institute of Technology in Stockholm and the University of Oxford (England) to also identify a serum protein biomarker panel that might prove useful for the diagnosis of OA.
Their study compared serum samples taken from 461 patients with knee OA with those taken from 412 patients with rheumatoid arthritis and 159 nonarthritic individuals. Serum samples from each of these groups were divided into a screening (n = 593) and a verification set (n = 439). The data were adjusted for variations in participant’s age, BMI, and gender.
In the screening phase, the investigators used an antibody suspension bead array composed of 174 antibodies to examine 78 protein targets to try to identify the best serum protein biomarker candidates. They replicated the findings in an adapted array of 79 antibodies and 34 protein targets and then used this array in the verification set of serum samples.
There were three proteins that could potentially differentiate patients with OA from controls and perhaps be used to diagnose OA. These were: complement 3, which is involved in inflammation; inter-alpha-trypsin inhibitor heavy chain I, which is involved in the stability of the extracellular matrix; and S100 calcium-binding protein A6, which has a role in chondrocyte differentiation and thus bone remodeling.
“Compared to rheumatoid arthritis patients, we observed that the level of complement 3 and inter-alpha-trypsin inhibitor heavy chain I are significantly increased in osteoarthritis patients,” said the presenting study author Lucía Maria Lourido Salas, PhD. “This suggests the potential of these two proteins as specific proteins for osteoarthritis,” she proposed. A further 28 proteins were found that might also help to differentiate OA from RA; 16 of these were increased and 12 decreased in OA patients. Together these 30 proteins could potentially help identify a specific serum protein signature of OA, Dr. Salas said.
Dr. Kraus and Dr. Salas did not report having any financial disclosures. Dr. Kraus’ research is funded by the Duke Biomarker Factory.
AT OARSI 2016
Key clinical point: Distinct serum biomarker panels have been identified for OA progression and diagnosis.
Major finding: The predictive power of clinical variables was improved by using serum biomarker panels in one U.S. study, with area under the curve of 0.7 for progression and 0.8 for diagnosis increasing to 0.9 and 1.0.
Data source: U.S. and European studies investigating serum biomarkers for OA diagnosis and progression.
Disclosures: Dr. Kraus and Dr. Salas did not report having any financial disclosures.
Osteoarthritis hip pain follows four distinct paths
AMSTERDAM – There are four distinct patterns of pain in patients with early presumed hip osteoarthritis, and several factors could help identify patients most likely to experience more severe pain, according to analysis from a Dutch study.
The majority of the 545 patients included in the analysis from the Cohort Hip and Knee (CHECK) study experienced a low or a mild constant hip pain over a 5-year follow-up period (231 patients, 42.4%). Around one-quarter had moderate hip pain with moderate pain progression (132 patients, 24.2%), about one in six (88 patients, 16.1%) had moderate hip pain with moderate pain regression, and the remainder (94 patients, 17.2%) had constant, severe hip pain.
Factors associated with these pain trajectories included the level of education achieved, ability to cope with pain, the Western Ontario and McMaster Universities Arthritis Index (WOMAC) physical function subscale, and pain experienced from internal rotation of the hip.
The findings were reported at the World Congress on Osteoarthritis sponsored by the Osteoarthritis Research Society International. They were also published online in Osteoarthritis and Cartilage (2106 Feb 5. doi: 10.1016/j.joca.2015.11.023).
Although prior studies examined progression predictors in hip OA, none looked specifically at how pain changes over time, noted study author Dr. Alex Bastick of Erasmus University Medical Center, Rotterdam, the Netherlands. Thus, the current aim was to define the trajectory of hip pain among individuals with early symptoms of hip OA, and identify any baseline factors that might help clinicians predict which pain trajectory patients may follow.
The CHECK study is a 10-year nationwide cohort study of 1,002 participants in the Netherlands with assumed early symptomatic OA of the knee, hip, or both. For the current analysis, Dr. Bastick and his associates considered only those patients with hip pain or stiffness who had completed 5 years of follow-up. At baseline, the mean age of the total population was 55.7 years, and 81% of participants were female. About one-quarter (26%) of participants had a clinical OA diagnosis according to American College of Rheumatology criteria.
Baseline radiographic severity was not associated with the various pain trajectories identified, Dr. Bastick observed, but patients who had achieved a lower level of education were roughly three times more likely to experience severe pain than mild or low constant pain. Patients experiencing severe pain were more likely to use pain transformation as a coping strategy, have higher activity limitation scores, and experience painful internal hip rotation.
Because around 60% of patients follow the mild or moderately progressing pain trajectories, conservative treatment in the early stages of hip OA appears appropriate, Dr. Bastick and his associates suggested. Their findings highlight the importance of a physical examination, and that reassessment of clinical symptoms should perhaps be undertaken in the first year of follow-up.
“Future research should be aimed at measuring symptomatic progression of hip OA with even more frequent symptom assessment,” the researchers proposed.
Reumafonds, the Dutch Arthritis Foundation, funded the study. Dr. Bastick did not have financial disclosures.
AMSTERDAM – There are four distinct patterns of pain in patients with early presumed hip osteoarthritis, and several factors could help identify patients most likely to experience more severe pain, according to analysis from a Dutch study.
The majority of the 545 patients included in the analysis from the Cohort Hip and Knee (CHECK) study experienced a low or a mild constant hip pain over a 5-year follow-up period (231 patients, 42.4%). Around one-quarter had moderate hip pain with moderate pain progression (132 patients, 24.2%), about one in six (88 patients, 16.1%) had moderate hip pain with moderate pain regression, and the remainder (94 patients, 17.2%) had constant, severe hip pain.
Factors associated with these pain trajectories included the level of education achieved, ability to cope with pain, the Western Ontario and McMaster Universities Arthritis Index (WOMAC) physical function subscale, and pain experienced from internal rotation of the hip.
The findings were reported at the World Congress on Osteoarthritis sponsored by the Osteoarthritis Research Society International. They were also published online in Osteoarthritis and Cartilage (2106 Feb 5. doi: 10.1016/j.joca.2015.11.023).
Although prior studies examined progression predictors in hip OA, none looked specifically at how pain changes over time, noted study author Dr. Alex Bastick of Erasmus University Medical Center, Rotterdam, the Netherlands. Thus, the current aim was to define the trajectory of hip pain among individuals with early symptoms of hip OA, and identify any baseline factors that might help clinicians predict which pain trajectory patients may follow.
The CHECK study is a 10-year nationwide cohort study of 1,002 participants in the Netherlands with assumed early symptomatic OA of the knee, hip, or both. For the current analysis, Dr. Bastick and his associates considered only those patients with hip pain or stiffness who had completed 5 years of follow-up. At baseline, the mean age of the total population was 55.7 years, and 81% of participants were female. About one-quarter (26%) of participants had a clinical OA diagnosis according to American College of Rheumatology criteria.
Baseline radiographic severity was not associated with the various pain trajectories identified, Dr. Bastick observed, but patients who had achieved a lower level of education were roughly three times more likely to experience severe pain than mild or low constant pain. Patients experiencing severe pain were more likely to use pain transformation as a coping strategy, have higher activity limitation scores, and experience painful internal hip rotation.
Because around 60% of patients follow the mild or moderately progressing pain trajectories, conservative treatment in the early stages of hip OA appears appropriate, Dr. Bastick and his associates suggested. Their findings highlight the importance of a physical examination, and that reassessment of clinical symptoms should perhaps be undertaken in the first year of follow-up.
“Future research should be aimed at measuring symptomatic progression of hip OA with even more frequent symptom assessment,” the researchers proposed.
Reumafonds, the Dutch Arthritis Foundation, funded the study. Dr. Bastick did not have financial disclosures.
AMSTERDAM – There are four distinct patterns of pain in patients with early presumed hip osteoarthritis, and several factors could help identify patients most likely to experience more severe pain, according to analysis from a Dutch study.
The majority of the 545 patients included in the analysis from the Cohort Hip and Knee (CHECK) study experienced a low or a mild constant hip pain over a 5-year follow-up period (231 patients, 42.4%). Around one-quarter had moderate hip pain with moderate pain progression (132 patients, 24.2%), about one in six (88 patients, 16.1%) had moderate hip pain with moderate pain regression, and the remainder (94 patients, 17.2%) had constant, severe hip pain.
Factors associated with these pain trajectories included the level of education achieved, ability to cope with pain, the Western Ontario and McMaster Universities Arthritis Index (WOMAC) physical function subscale, and pain experienced from internal rotation of the hip.
The findings were reported at the World Congress on Osteoarthritis sponsored by the Osteoarthritis Research Society International. They were also published online in Osteoarthritis and Cartilage (2106 Feb 5. doi: 10.1016/j.joca.2015.11.023).
Although prior studies examined progression predictors in hip OA, none looked specifically at how pain changes over time, noted study author Dr. Alex Bastick of Erasmus University Medical Center, Rotterdam, the Netherlands. Thus, the current aim was to define the trajectory of hip pain among individuals with early symptoms of hip OA, and identify any baseline factors that might help clinicians predict which pain trajectory patients may follow.
The CHECK study is a 10-year nationwide cohort study of 1,002 participants in the Netherlands with assumed early symptomatic OA of the knee, hip, or both. For the current analysis, Dr. Bastick and his associates considered only those patients with hip pain or stiffness who had completed 5 years of follow-up. At baseline, the mean age of the total population was 55.7 years, and 81% of participants were female. About one-quarter (26%) of participants had a clinical OA diagnosis according to American College of Rheumatology criteria.
Baseline radiographic severity was not associated with the various pain trajectories identified, Dr. Bastick observed, but patients who had achieved a lower level of education were roughly three times more likely to experience severe pain than mild or low constant pain. Patients experiencing severe pain were more likely to use pain transformation as a coping strategy, have higher activity limitation scores, and experience painful internal hip rotation.
Because around 60% of patients follow the mild or moderately progressing pain trajectories, conservative treatment in the early stages of hip OA appears appropriate, Dr. Bastick and his associates suggested. Their findings highlight the importance of a physical examination, and that reassessment of clinical symptoms should perhaps be undertaken in the first year of follow-up.
“Future research should be aimed at measuring symptomatic progression of hip OA with even more frequent symptom assessment,” the researchers proposed.
Reumafonds, the Dutch Arthritis Foundation, funded the study. Dr. Bastick did not have financial disclosures.
AT OARSI 2016
Key clinical point: Understanding how osteoarthritis hip pain changes over time may help manage those who experience severe pain.
Major finding: Four hip pain trajectories were identified: low, mild constant pain (42.4%, n = 231); moderate, with moderate pain progression (24.2%, n = 132); moderate, with moderate pain regression (16.1%, n = 88); and constant severe pain (17.2%, n = 94).
Data source: The Cohort Hip and Knee (CHECK) study, a Dutch, prospective, nationwide study of 1,002 participants with assumed early symptomatic osteoarthritis of the knee, hip, or both.
Disclosures: Reumafonds, the Dutch Arthritis Foundation, funded the study. Dr. Bastick did not have financial disclosures.
Sustained-release steroid provides prolonged OA pain relief
AMSTERDAM – A single intra-articular injection of a novel corticosteroid formulation provided substantial and persistent pain relief for at least 12 weeks in patients with knee osteoarthritis in a phase III trial that was presented at the World Congress on Osteoarthritis.
FX006 (Zilretta), given at a dose of 40 mg, significantly (P less than .05) reduced the mean daily pain intensity score from around 2 weeks onwards in the region of 3.0 to 3.5 points on a 0-10 scale compared with a reduction of around 1.5 to 2.0 points for placebo. There were also significant reductions in additional efficacy outcomes such as Western Ontario and McMaster Universities Index (WOMAC) pain and function versus placebo and a standard formulation of triamcinolone acetate (TCA).
Data from a phase IIb trial had originally been scheduled to be presented at the meeting – which is sponsored by the Osteoarthritis Research Society International – but the phase III findings had just become available, said Dr. Philip Conaghan of the University of Leeds (England). The phase II findings showed that there was a substantial and persistent pain relieving effect of FX006 versus placebo and that there was evidence of a dose response.
These data provide “proof of concept that we can develop long-acting steroids that have real potential for changing how we might manage knee OA,” said Dr. Conaghan, professor of musculoskeletal medicine and deputy director of the Leeds Musculoskeletal Biomedical Research Unit.
Intra-articular steroids have long been used to treat OA and they can be effective, albeit for a short period of time. FX006 is an investigational formulation of TCA in polylactic-co-glycolic acid microspheres that has been designed to try to extend the analgesic effects of the steroid and to reduce overall systemic exposure and thus side effects. Each microsphere is around 45 microns in diameter.
The aim of the phase IIb study was to examine the efficacy of two doses of FX006 (20 mg and 40 mg) versus placebo in patients with moderate to severe OA knee pain. The double-blind, randomized trial involved 310 patients who were followed for 24 weeks. The primary outcome was the change from baseline to week 12 in the weekly mean of the average daily pain intensity scores, compared with placebo.
There was evidence of a dose response, with the 40-mg dose of FX006 producing statistically significantly greater changes in pain scores versus placebo. Although the primary endpoint of a statistical difference at 12 weeks was not met, there were significant differences at all other time points from week 1 to 13. WOMAC pain and function scores were also significantly reduced with the 40-mg dose versus placebo.
The phase III trial involved 484 patients who were randomized to intra-articular injections of FX006 40 mg, standard TCA 40 mg, or placebo. As in the phase IIb trial patients were well matched at baseline, with a mean overall age of 62 years, a body mass index of 30 kg/m2 and around two-thirds having OA in both knees.
Dr. Conaghan reported that there were “no unexpected safety signals” during the trials. A combined safety summary showed that treatment-emergent adverse events occurred in 42.2% and 50.9% of patients treated with FX006 20 mg and 40 mg, respectively, in 49.2% of placebo-treated patients, and in 56.5% of TCA-treated patients. Serious adverse event rates were 1%, 3%, 1.1%, and 2.5%, respectively, and were assessed as being unrelated to treatment. Adverse events related to the knee occurred in 14.7%, 16.6%, 14.1%, and 9.9%, and injection-related adverse events in 2%, 1.9%, 4.2%, and 1.9%, respectively.
The studies were funded by Flexion Therapeutics. Dr. Conaghan did not report his financial disclosures.
AMSTERDAM – A single intra-articular injection of a novel corticosteroid formulation provided substantial and persistent pain relief for at least 12 weeks in patients with knee osteoarthritis in a phase III trial that was presented at the World Congress on Osteoarthritis.
FX006 (Zilretta), given at a dose of 40 mg, significantly (P less than .05) reduced the mean daily pain intensity score from around 2 weeks onwards in the region of 3.0 to 3.5 points on a 0-10 scale compared with a reduction of around 1.5 to 2.0 points for placebo. There were also significant reductions in additional efficacy outcomes such as Western Ontario and McMaster Universities Index (WOMAC) pain and function versus placebo and a standard formulation of triamcinolone acetate (TCA).
Data from a phase IIb trial had originally been scheduled to be presented at the meeting – which is sponsored by the Osteoarthritis Research Society International – but the phase III findings had just become available, said Dr. Philip Conaghan of the University of Leeds (England). The phase II findings showed that there was a substantial and persistent pain relieving effect of FX006 versus placebo and that there was evidence of a dose response.
These data provide “proof of concept that we can develop long-acting steroids that have real potential for changing how we might manage knee OA,” said Dr. Conaghan, professor of musculoskeletal medicine and deputy director of the Leeds Musculoskeletal Biomedical Research Unit.
Intra-articular steroids have long been used to treat OA and they can be effective, albeit for a short period of time. FX006 is an investigational formulation of TCA in polylactic-co-glycolic acid microspheres that has been designed to try to extend the analgesic effects of the steroid and to reduce overall systemic exposure and thus side effects. Each microsphere is around 45 microns in diameter.
The aim of the phase IIb study was to examine the efficacy of two doses of FX006 (20 mg and 40 mg) versus placebo in patients with moderate to severe OA knee pain. The double-blind, randomized trial involved 310 patients who were followed for 24 weeks. The primary outcome was the change from baseline to week 12 in the weekly mean of the average daily pain intensity scores, compared with placebo.
There was evidence of a dose response, with the 40-mg dose of FX006 producing statistically significantly greater changes in pain scores versus placebo. Although the primary endpoint of a statistical difference at 12 weeks was not met, there were significant differences at all other time points from week 1 to 13. WOMAC pain and function scores were also significantly reduced with the 40-mg dose versus placebo.
The phase III trial involved 484 patients who were randomized to intra-articular injections of FX006 40 mg, standard TCA 40 mg, or placebo. As in the phase IIb trial patients were well matched at baseline, with a mean overall age of 62 years, a body mass index of 30 kg/m2 and around two-thirds having OA in both knees.
Dr. Conaghan reported that there were “no unexpected safety signals” during the trials. A combined safety summary showed that treatment-emergent adverse events occurred in 42.2% and 50.9% of patients treated with FX006 20 mg and 40 mg, respectively, in 49.2% of placebo-treated patients, and in 56.5% of TCA-treated patients. Serious adverse event rates were 1%, 3%, 1.1%, and 2.5%, respectively, and were assessed as being unrelated to treatment. Adverse events related to the knee occurred in 14.7%, 16.6%, 14.1%, and 9.9%, and injection-related adverse events in 2%, 1.9%, 4.2%, and 1.9%, respectively.
The studies were funded by Flexion Therapeutics. Dr. Conaghan did not report his financial disclosures.
AMSTERDAM – A single intra-articular injection of a novel corticosteroid formulation provided substantial and persistent pain relief for at least 12 weeks in patients with knee osteoarthritis in a phase III trial that was presented at the World Congress on Osteoarthritis.
FX006 (Zilretta), given at a dose of 40 mg, significantly (P less than .05) reduced the mean daily pain intensity score from around 2 weeks onwards in the region of 3.0 to 3.5 points on a 0-10 scale compared with a reduction of around 1.5 to 2.0 points for placebo. There were also significant reductions in additional efficacy outcomes such as Western Ontario and McMaster Universities Index (WOMAC) pain and function versus placebo and a standard formulation of triamcinolone acetate (TCA).
Data from a phase IIb trial had originally been scheduled to be presented at the meeting – which is sponsored by the Osteoarthritis Research Society International – but the phase III findings had just become available, said Dr. Philip Conaghan of the University of Leeds (England). The phase II findings showed that there was a substantial and persistent pain relieving effect of FX006 versus placebo and that there was evidence of a dose response.
These data provide “proof of concept that we can develop long-acting steroids that have real potential for changing how we might manage knee OA,” said Dr. Conaghan, professor of musculoskeletal medicine and deputy director of the Leeds Musculoskeletal Biomedical Research Unit.
Intra-articular steroids have long been used to treat OA and they can be effective, albeit for a short period of time. FX006 is an investigational formulation of TCA in polylactic-co-glycolic acid microspheres that has been designed to try to extend the analgesic effects of the steroid and to reduce overall systemic exposure and thus side effects. Each microsphere is around 45 microns in diameter.
The aim of the phase IIb study was to examine the efficacy of two doses of FX006 (20 mg and 40 mg) versus placebo in patients with moderate to severe OA knee pain. The double-blind, randomized trial involved 310 patients who were followed for 24 weeks. The primary outcome was the change from baseline to week 12 in the weekly mean of the average daily pain intensity scores, compared with placebo.
There was evidence of a dose response, with the 40-mg dose of FX006 producing statistically significantly greater changes in pain scores versus placebo. Although the primary endpoint of a statistical difference at 12 weeks was not met, there were significant differences at all other time points from week 1 to 13. WOMAC pain and function scores were also significantly reduced with the 40-mg dose versus placebo.
The phase III trial involved 484 patients who were randomized to intra-articular injections of FX006 40 mg, standard TCA 40 mg, or placebo. As in the phase IIb trial patients were well matched at baseline, with a mean overall age of 62 years, a body mass index of 30 kg/m2 and around two-thirds having OA in both knees.
Dr. Conaghan reported that there were “no unexpected safety signals” during the trials. A combined safety summary showed that treatment-emergent adverse events occurred in 42.2% and 50.9% of patients treated with FX006 20 mg and 40 mg, respectively, in 49.2% of placebo-treated patients, and in 56.5% of TCA-treated patients. Serious adverse event rates were 1%, 3%, 1.1%, and 2.5%, respectively, and were assessed as being unrelated to treatment. Adverse events related to the knee occurred in 14.7%, 16.6%, 14.1%, and 9.9%, and injection-related adverse events in 2%, 1.9%, 4.2%, and 1.9%, respectively.
The studies were funded by Flexion Therapeutics. Dr. Conaghan did not report his financial disclosures.
AT OARSI 2016
Key clinical point: A single intra-articular injection of FX006 provided pain relief for up to 12 weeks.
Major finding: The mean daily pain intensity score was significantly reduced by 3.0 to 3.5 points with FX006 versus 1.5 to 2.0 points for placebo.
Data source: A phase IIb (n = 310) and a phase III (n = 454) study conducted in patients with moderate to severe knee OA.
Disclosures: The studies were funded by Flexion Therapeutics. Dr. Conaghan did not report his financial disclosures.
Persistent knee pain predicts structural osteoarthritis early
AMSTERDAM – Persistent knee pain is an important predictor of structural joint damage and could potentially be used to predict knee osteoarthritis (OA) earlier, according to Dutch research reported at the World Congress on Osteoarthritis.
The analysis found that women participating in the Rotterdam Study who had knee pain on most days of the preceding month were more than four times more likely to develop knee OA within 5 years on MRI (odds ratio, 4.53) than were those without frequent knee pain.
Other signs and symptoms predictive of later OA on MRI included a history of knee injury (OR, 2.52), pain on palpation (OR, 2.30) or during activity (OR, 2.13), the feeling of the knee giving way (OR, 2.13), joint line tenderness (OR, 2.04), crepitus (or grating sounds or sensations; OR, 1.85), and Heberden’s nodes (OR, 1.67).
“Signs and symptoms can predict structural knee OA in 5 years in women without knee OA at baseline,” said the presenting study author Dieuwke Schiphof, Ph.D., a postdoctoral researcher at Erasmus University Medical Center in Rotterdam, at the meeting sponsored by the Osteoarthritis Research Society International.
“Knee pain on most days of the last month is an important predictive symptom for all structural OA definitions,” she said, adding that crepitus was an important sign of patellofemoral OA, and the feeling of giving way and joint line tenderness were important signs for tibiofemoral OA.
The aim of their study was to try to determine the value of previously identified clinical signs and symptoms for determining the onset of structural knee OA. Around 600 women aged 45-60 years without OA at baseline were included from the population-based cohort Rotterdam study. Participants had completed a standard questionnaire and had physical, radiographic, and MRI examination of both knees at baseline and at a mean follow-up of 5 years later.
The mean age of women was 59.5 years and 33 of 1,137 knees had isolated patellofemoral OA and 69 had isolated tibiofemoral OA, 111 one or the other, and 22 had radiographic knee OA.
Results showed that knee pain on most days of the last month (OR, 7.57) and crepitus (OR, 3.05) were most predictive of isolated patellofemoral OA on MRI. The prevalence of patellofemoral OA was 3% but this increased to 20% if both these signs and symptoms were present.
Knee pain on most days of the last month (OR, 4.13) was also predictive of isolated tibiofemoral OA on MRI, together with a history of knee pain (OR, 2.48), knee pain during activities (OR, 2.04), and the feeling of the knee giving way (OR, 3.15). If two or three or more signs and symptoms were present, the predictive value increased from a prevalence of 6% to 12% to 18%.
Knee pain on most days of the last month was also highly predictive of radiographic OA (OR, 7.77), as was pain at palpation (OR, 4.36), prior injury (OR, 3.64), and the feeling of the knee giving way (OR, 2.75). Again, when additional signs or symptoms were present the prevalence increased from a prevalence of 3% at follow-up to 4% with one, 8% with two, and up to 14% for three or more.
These signs and symptoms could potentially be used to help identify patients that may benefit from preventive treatment for knee OA, the researchers believe.
The study was funded by The Netherlands Organisation for Health Research and Development and Reumafonds, the Dutch Arthritis Association. Dr. Schiphof had no financial disclosures.
AMSTERDAM – Persistent knee pain is an important predictor of structural joint damage and could potentially be used to predict knee osteoarthritis (OA) earlier, according to Dutch research reported at the World Congress on Osteoarthritis.
The analysis found that women participating in the Rotterdam Study who had knee pain on most days of the preceding month were more than four times more likely to develop knee OA within 5 years on MRI (odds ratio, 4.53) than were those without frequent knee pain.
Other signs and symptoms predictive of later OA on MRI included a history of knee injury (OR, 2.52), pain on palpation (OR, 2.30) or during activity (OR, 2.13), the feeling of the knee giving way (OR, 2.13), joint line tenderness (OR, 2.04), crepitus (or grating sounds or sensations; OR, 1.85), and Heberden’s nodes (OR, 1.67).
“Signs and symptoms can predict structural knee OA in 5 years in women without knee OA at baseline,” said the presenting study author Dieuwke Schiphof, Ph.D., a postdoctoral researcher at Erasmus University Medical Center in Rotterdam, at the meeting sponsored by the Osteoarthritis Research Society International.
“Knee pain on most days of the last month is an important predictive symptom for all structural OA definitions,” she said, adding that crepitus was an important sign of patellofemoral OA, and the feeling of giving way and joint line tenderness were important signs for tibiofemoral OA.
The aim of their study was to try to determine the value of previously identified clinical signs and symptoms for determining the onset of structural knee OA. Around 600 women aged 45-60 years without OA at baseline were included from the population-based cohort Rotterdam study. Participants had completed a standard questionnaire and had physical, radiographic, and MRI examination of both knees at baseline and at a mean follow-up of 5 years later.
The mean age of women was 59.5 years and 33 of 1,137 knees had isolated patellofemoral OA and 69 had isolated tibiofemoral OA, 111 one or the other, and 22 had radiographic knee OA.
Results showed that knee pain on most days of the last month (OR, 7.57) and crepitus (OR, 3.05) were most predictive of isolated patellofemoral OA on MRI. The prevalence of patellofemoral OA was 3% but this increased to 20% if both these signs and symptoms were present.
Knee pain on most days of the last month (OR, 4.13) was also predictive of isolated tibiofemoral OA on MRI, together with a history of knee pain (OR, 2.48), knee pain during activities (OR, 2.04), and the feeling of the knee giving way (OR, 3.15). If two or three or more signs and symptoms were present, the predictive value increased from a prevalence of 6% to 12% to 18%.
Knee pain on most days of the last month was also highly predictive of radiographic OA (OR, 7.77), as was pain at palpation (OR, 4.36), prior injury (OR, 3.64), and the feeling of the knee giving way (OR, 2.75). Again, when additional signs or symptoms were present the prevalence increased from a prevalence of 3% at follow-up to 4% with one, 8% with two, and up to 14% for three or more.
These signs and symptoms could potentially be used to help identify patients that may benefit from preventive treatment for knee OA, the researchers believe.
The study was funded by The Netherlands Organisation for Health Research and Development and Reumafonds, the Dutch Arthritis Association. Dr. Schiphof had no financial disclosures.
AMSTERDAM – Persistent knee pain is an important predictor of structural joint damage and could potentially be used to predict knee osteoarthritis (OA) earlier, according to Dutch research reported at the World Congress on Osteoarthritis.
The analysis found that women participating in the Rotterdam Study who had knee pain on most days of the preceding month were more than four times more likely to develop knee OA within 5 years on MRI (odds ratio, 4.53) than were those without frequent knee pain.
Other signs and symptoms predictive of later OA on MRI included a history of knee injury (OR, 2.52), pain on palpation (OR, 2.30) or during activity (OR, 2.13), the feeling of the knee giving way (OR, 2.13), joint line tenderness (OR, 2.04), crepitus (or grating sounds or sensations; OR, 1.85), and Heberden’s nodes (OR, 1.67).
“Signs and symptoms can predict structural knee OA in 5 years in women without knee OA at baseline,” said the presenting study author Dieuwke Schiphof, Ph.D., a postdoctoral researcher at Erasmus University Medical Center in Rotterdam, at the meeting sponsored by the Osteoarthritis Research Society International.
“Knee pain on most days of the last month is an important predictive symptom for all structural OA definitions,” she said, adding that crepitus was an important sign of patellofemoral OA, and the feeling of giving way and joint line tenderness were important signs for tibiofemoral OA.
The aim of their study was to try to determine the value of previously identified clinical signs and symptoms for determining the onset of structural knee OA. Around 600 women aged 45-60 years without OA at baseline were included from the population-based cohort Rotterdam study. Participants had completed a standard questionnaire and had physical, radiographic, and MRI examination of both knees at baseline and at a mean follow-up of 5 years later.
The mean age of women was 59.5 years and 33 of 1,137 knees had isolated patellofemoral OA and 69 had isolated tibiofemoral OA, 111 one or the other, and 22 had radiographic knee OA.
Results showed that knee pain on most days of the last month (OR, 7.57) and crepitus (OR, 3.05) were most predictive of isolated patellofemoral OA on MRI. The prevalence of patellofemoral OA was 3% but this increased to 20% if both these signs and symptoms were present.
Knee pain on most days of the last month (OR, 4.13) was also predictive of isolated tibiofemoral OA on MRI, together with a history of knee pain (OR, 2.48), knee pain during activities (OR, 2.04), and the feeling of the knee giving way (OR, 3.15). If two or three or more signs and symptoms were present, the predictive value increased from a prevalence of 6% to 12% to 18%.
Knee pain on most days of the last month was also highly predictive of radiographic OA (OR, 7.77), as was pain at palpation (OR, 4.36), prior injury (OR, 3.64), and the feeling of the knee giving way (OR, 2.75). Again, when additional signs or symptoms were present the prevalence increased from a prevalence of 3% at follow-up to 4% with one, 8% with two, and up to 14% for three or more.
These signs and symptoms could potentially be used to help identify patients that may benefit from preventive treatment for knee OA, the researchers believe.
The study was funded by The Netherlands Organisation for Health Research and Development and Reumafonds, the Dutch Arthritis Association. Dr. Schiphof had no financial disclosures.
AT OARSI 2016
Key clinical point: Several signs and symptoms could be used to help identify patients with osteoarthritis (OA) up to 5 years before joint damage occurs.
Major finding: Knee pain on most days of the last month was predictive of both MRI and radiographic knee damage.
Data source: 592 women from the Rotterdam Study without knee OA at baseline.
Disclosures: The study was funded by The Netherlands Organisation for Health Research and Development and Reumafonds, the Dutch Arthritis Association. Dr. Schiphof had no financial disclosures.
Less symptomatic patients ‘worse off’ after knee surgery
AMSTERDAM – Patients with milder knee osteoarthritis symptoms or better quality of life before undergoing total knee replacement surgery gained less benefit from the surgery than did those who had more severe symptoms in two separate analyses of British and U.S. patients.
Additional evidence from total knee replacements (TKRs) performed on U.S. participants of the Osteoarthritis Initiative also suggest that as the use of TKR has increased to include less symptomatic patients, the overall cost-effectiveness of the procedure has declined.
“Knee replacements are one of those interventions that are known to be very effective and very cost-effective,” Rafael Pinedo-Villanueva, Ph.D., of the University of Oxford (England) said during his presentation of National Health Service data from England at the World Congress on Osteoarthritis. Indeed, knee replacements are associated with significant improvements in pain, function, and quality of life, he said, but that is if you look at the mean values.
As the deciles for baseline knee pain and function decrease in severity, there are diminishing mean improvements and an increasing proportion of patients who do worse after the operation, he reported. Up to 17% of patients had unchanged or improved knee pain scores and up to 27% had lower quality of life scores. If minimally important differences were considered, these percentages rose to 40% and 48% of patients being worse off, respectively.
“The significant improvements seem to be overshadowing what happens to those patients who are doing worse,” Dr. Pinedo-Villanueva suggested. “So essentially cost-effectiveness is being driven by the magnitude of the change in those who do improve, and we don’t really see much about what is happening to those who are doing worse.”
Of over 215,000 records of knee replacement collected from all patients undergoing TKR in England during 2008-2012, Dr. Pinedo-Villanueva and his study coauthors found 117,844 had data on pre- and post-operative knee pain assessed using the Oxford Knee Score (OKS) and quality of life measured with the EQ-5D instrument. The majority of replacements were in women (55%) and almost three quarters of patients had one or no comorbidities. Overall, the mean change in OKS was 15 points, improving from 19 to 34 (the higher the score the lesser the knee pain). EQ-5D scores also improved by a mean difference of 0.30 (from 0.41 to 0.70 where 1.0 is perfect health). Although the vast majority of patients had improved OKS and EQ-5D scores after surgery, unchanged or decreased scores were seen in 8% and 22% of patients, respectively.
“As we breakdown these data by deciles of baseline pain and function we see clearly that those starting at the lower decile improved the most, and that’s to be expected; they’ve a lot more to improve than the ones that came into the operation at the higher decile,” Dr. Pinedo-Villanueva said at the Congress, sponsored by the Osteoarthritis Research Society International. But there were patients who fared worse at every decile, he noted.
Dr. Pinedo-Villanueva concluded that outcome prediction models were needed to try to reduce the number of patients who are apparently worse off after knee replacement and improve the efficiency of resource allocation.
The value of TKR in a contemporary U.S. population was the focus of a separate presentation by Dr. Bart Ferket of Mount Sinai Hospital in New York. Dr. Ferket reported the results of a study looking at the impact of TKR on patients’ quality of life, lifetime costs, and quality-adjusted life years (QALYs) while varying the use of TKR by patients’ functional status at baseline.
“In the United States, the rate at which total knee replacement is performed has doubled in the last two decades,” Dr. Ferket observed. This “disproportionate” increase has been attributed to expanding the eligibility criteria to include less symptomatic patients.
Using data collected over an 8-year period on 1,327 participants from the Osteoarthritis Initiative, Dr. Ferket and his associates at Mount Sinai and Erasmus University Medical Center in Rotterdam (The Netherlands) discovered that the increased uptake of TKR might have affected the likely benefit and reduced the overall cost-effectiveness of the procedure.
At baseline, 17% of the participants, who all had knee osteoarthritis, had had a prior knee replacement.
Quality of life measured on the physical component scores (PCS) of the 12-item Short Form (SF-12) were generally improved after TKR but decreased in those who did not have a knee replaced. The effect on the mental component of the SF-12 was less clear, with possibly a decrease seen in some patients. Changes on the Western Ontario and McMaster Universities Arthritis Index (WOMAC) and Knee injury and Osteoarthritis Outcome Score (KOOS) showed a considerable benefit for knee replacement and there was a general decrease in pain medication over time in those who had surgery. The overall effect was more pronounced if patients with greater baseline symptoms were considered.
Cost-effectiveness modeling showed that reserving TKR for more seriously affected patients may make it more economically attractive. The QALYs gained from TKR was about 11 but as the number of QALYs increased, so did the relative lifetime cost, with increasing incremental cost-effectiveness ratios (ICERs) as SF-12 PCS rose. ICERs were around $143,000, $160,000, $217,000, $385,000, and $1,175,000 considering patients with SF-12 PCS of less than 30, 35, 40, 45, and 50, respectively.
“The more lenient the eligibility criteria are, the higher the effectiveness, but also the higher the costs,” Dr. Ferket said. “The most cost-effective scenarios are actually more restrictive that what is currently seen in current practice in the U.S.”
Dr. Pinedo-Villanueva and Dr. Ferket reported having no financial disclosures.
AMSTERDAM – Patients with milder knee osteoarthritis symptoms or better quality of life before undergoing total knee replacement surgery gained less benefit from the surgery than did those who had more severe symptoms in two separate analyses of British and U.S. patients.
Additional evidence from total knee replacements (TKRs) performed on U.S. participants of the Osteoarthritis Initiative also suggest that as the use of TKR has increased to include less symptomatic patients, the overall cost-effectiveness of the procedure has declined.
“Knee replacements are one of those interventions that are known to be very effective and very cost-effective,” Rafael Pinedo-Villanueva, Ph.D., of the University of Oxford (England) said during his presentation of National Health Service data from England at the World Congress on Osteoarthritis. Indeed, knee replacements are associated with significant improvements in pain, function, and quality of life, he said, but that is if you look at the mean values.
As the deciles for baseline knee pain and function decrease in severity, there are diminishing mean improvements and an increasing proportion of patients who do worse after the operation, he reported. Up to 17% of patients had unchanged or improved knee pain scores and up to 27% had lower quality of life scores. If minimally important differences were considered, these percentages rose to 40% and 48% of patients being worse off, respectively.
“The significant improvements seem to be overshadowing what happens to those patients who are doing worse,” Dr. Pinedo-Villanueva suggested. “So essentially cost-effectiveness is being driven by the magnitude of the change in those who do improve, and we don’t really see much about what is happening to those who are doing worse.”
Of over 215,000 records of knee replacement collected from all patients undergoing TKR in England during 2008-2012, Dr. Pinedo-Villanueva and his study coauthors found 117,844 had data on pre- and post-operative knee pain assessed using the Oxford Knee Score (OKS) and quality of life measured with the EQ-5D instrument. The majority of replacements were in women (55%) and almost three quarters of patients had one or no comorbidities. Overall, the mean change in OKS was 15 points, improving from 19 to 34 (the higher the score the lesser the knee pain). EQ-5D scores also improved by a mean difference of 0.30 (from 0.41 to 0.70 where 1.0 is perfect health). Although the vast majority of patients had improved OKS and EQ-5D scores after surgery, unchanged or decreased scores were seen in 8% and 22% of patients, respectively.
“As we breakdown these data by deciles of baseline pain and function we see clearly that those starting at the lower decile improved the most, and that’s to be expected; they’ve a lot more to improve than the ones that came into the operation at the higher decile,” Dr. Pinedo-Villanueva said at the Congress, sponsored by the Osteoarthritis Research Society International. But there were patients who fared worse at every decile, he noted.
Dr. Pinedo-Villanueva concluded that outcome prediction models were needed to try to reduce the number of patients who are apparently worse off after knee replacement and improve the efficiency of resource allocation.
The value of TKR in a contemporary U.S. population was the focus of a separate presentation by Dr. Bart Ferket of Mount Sinai Hospital in New York. Dr. Ferket reported the results of a study looking at the impact of TKR on patients’ quality of life, lifetime costs, and quality-adjusted life years (QALYs) while varying the use of TKR by patients’ functional status at baseline.
“In the United States, the rate at which total knee replacement is performed has doubled in the last two decades,” Dr. Ferket observed. This “disproportionate” increase has been attributed to expanding the eligibility criteria to include less symptomatic patients.
Using data collected over an 8-year period on 1,327 participants from the Osteoarthritis Initiative, Dr. Ferket and his associates at Mount Sinai and Erasmus University Medical Center in Rotterdam (The Netherlands) discovered that the increased uptake of TKR might have affected the likely benefit and reduced the overall cost-effectiveness of the procedure.
At baseline, 17% of the participants, who all had knee osteoarthritis, had had a prior knee replacement.
Quality of life measured on the physical component scores (PCS) of the 12-item Short Form (SF-12) were generally improved after TKR but decreased in those who did not have a knee replaced. The effect on the mental component of the SF-12 was less clear, with possibly a decrease seen in some patients. Changes on the Western Ontario and McMaster Universities Arthritis Index (WOMAC) and Knee injury and Osteoarthritis Outcome Score (KOOS) showed a considerable benefit for knee replacement and there was a general decrease in pain medication over time in those who had surgery. The overall effect was more pronounced if patients with greater baseline symptoms were considered.
Cost-effectiveness modeling showed that reserving TKR for more seriously affected patients may make it more economically attractive. The QALYs gained from TKR was about 11 but as the number of QALYs increased, so did the relative lifetime cost, with increasing incremental cost-effectiveness ratios (ICERs) as SF-12 PCS rose. ICERs were around $143,000, $160,000, $217,000, $385,000, and $1,175,000 considering patients with SF-12 PCS of less than 30, 35, 40, 45, and 50, respectively.
“The more lenient the eligibility criteria are, the higher the effectiveness, but also the higher the costs,” Dr. Ferket said. “The most cost-effective scenarios are actually more restrictive that what is currently seen in current practice in the U.S.”
Dr. Pinedo-Villanueva and Dr. Ferket reported having no financial disclosures.
AMSTERDAM – Patients with milder knee osteoarthritis symptoms or better quality of life before undergoing total knee replacement surgery gained less benefit from the surgery than did those who had more severe symptoms in two separate analyses of British and U.S. patients.
Additional evidence from total knee replacements (TKRs) performed on U.S. participants of the Osteoarthritis Initiative also suggest that as the use of TKR has increased to include less symptomatic patients, the overall cost-effectiveness of the procedure has declined.
“Knee replacements are one of those interventions that are known to be very effective and very cost-effective,” Rafael Pinedo-Villanueva, Ph.D., of the University of Oxford (England) said during his presentation of National Health Service data from England at the World Congress on Osteoarthritis. Indeed, knee replacements are associated with significant improvements in pain, function, and quality of life, he said, but that is if you look at the mean values.
As the deciles for baseline knee pain and function decrease in severity, there are diminishing mean improvements and an increasing proportion of patients who do worse after the operation, he reported. Up to 17% of patients had unchanged or improved knee pain scores and up to 27% had lower quality of life scores. If minimally important differences were considered, these percentages rose to 40% and 48% of patients being worse off, respectively.
“The significant improvements seem to be overshadowing what happens to those patients who are doing worse,” Dr. Pinedo-Villanueva suggested. “So essentially cost-effectiveness is being driven by the magnitude of the change in those who do improve, and we don’t really see much about what is happening to those who are doing worse.”
Of over 215,000 records of knee replacement collected from all patients undergoing TKR in England during 2008-2012, Dr. Pinedo-Villanueva and his study coauthors found 117,844 had data on pre- and post-operative knee pain assessed using the Oxford Knee Score (OKS) and quality of life measured with the EQ-5D instrument. The majority of replacements were in women (55%) and almost three quarters of patients had one or no comorbidities. Overall, the mean change in OKS was 15 points, improving from 19 to 34 (the higher the score the lesser the knee pain). EQ-5D scores also improved by a mean difference of 0.30 (from 0.41 to 0.70 where 1.0 is perfect health). Although the vast majority of patients had improved OKS and EQ-5D scores after surgery, unchanged or decreased scores were seen in 8% and 22% of patients, respectively.
“As we breakdown these data by deciles of baseline pain and function we see clearly that those starting at the lower decile improved the most, and that’s to be expected; they’ve a lot more to improve than the ones that came into the operation at the higher decile,” Dr. Pinedo-Villanueva said at the Congress, sponsored by the Osteoarthritis Research Society International. But there were patients who fared worse at every decile, he noted.
Dr. Pinedo-Villanueva concluded that outcome prediction models were needed to try to reduce the number of patients who are apparently worse off after knee replacement and improve the efficiency of resource allocation.
The value of TKR in a contemporary U.S. population was the focus of a separate presentation by Dr. Bart Ferket of Mount Sinai Hospital in New York. Dr. Ferket reported the results of a study looking at the impact of TKR on patients’ quality of life, lifetime costs, and quality-adjusted life years (QALYs) while varying the use of TKR by patients’ functional status at baseline.
“In the United States, the rate at which total knee replacement is performed has doubled in the last two decades,” Dr. Ferket observed. This “disproportionate” increase has been attributed to expanding the eligibility criteria to include less symptomatic patients.
Using data collected over an 8-year period on 1,327 participants from the Osteoarthritis Initiative, Dr. Ferket and his associates at Mount Sinai and Erasmus University Medical Center in Rotterdam (The Netherlands) discovered that the increased uptake of TKR might have affected the likely benefit and reduced the overall cost-effectiveness of the procedure.
At baseline, 17% of the participants, who all had knee osteoarthritis, had had a prior knee replacement.
Quality of life measured on the physical component scores (PCS) of the 12-item Short Form (SF-12) were generally improved after TKR but decreased in those who did not have a knee replaced. The effect on the mental component of the SF-12 was less clear, with possibly a decrease seen in some patients. Changes on the Western Ontario and McMaster Universities Arthritis Index (WOMAC) and Knee injury and Osteoarthritis Outcome Score (KOOS) showed a considerable benefit for knee replacement and there was a general decrease in pain medication over time in those who had surgery. The overall effect was more pronounced if patients with greater baseline symptoms were considered.
Cost-effectiveness modeling showed that reserving TKR for more seriously affected patients may make it more economically attractive. The QALYs gained from TKR was about 11 but as the number of QALYs increased, so did the relative lifetime cost, with increasing incremental cost-effectiveness ratios (ICERs) as SF-12 PCS rose. ICERs were around $143,000, $160,000, $217,000, $385,000, and $1,175,000 considering patients with SF-12 PCS of less than 30, 35, 40, 45, and 50, respectively.
“The more lenient the eligibility criteria are, the higher the effectiveness, but also the higher the costs,” Dr. Ferket said. “The most cost-effective scenarios are actually more restrictive that what is currently seen in current practice in the U.S.”
Dr. Pinedo-Villanueva and Dr. Ferket reported having no financial disclosures.
AT OARSI 2016
Key clinical point: Patients with milder osteoarthritis can fare worse after knee replacement surgery, and the cost-effectiveness of the procedure is lower.
Major finding: Knee pain and quality of life scores were unchanged or worse after the operation in 8% and 22% of patients, respectively.
Data source: Two separate studies looking at the value of knee replacement in patients with knee osteoarthritis.
Disclosures: Dr. Pinedo-Villanueva and Dr. Ferket reported having no financial disclosures.
ACR’s 2016-2020 research agenda built through consensus
Therapeutic goals set the tone for the American College of Rheumatology National Research Agenda 2016-2020 by calling for the discovery and development of new therapies for rheumatic disease; finding predictors of response and nonresponse to, and adverse events from therapy; and improving the understanding of how therapies should be used.
Those are the top 3 out of 15 goals facilitated by the ACR’s Committee on Research, which finalized the agenda after seeking input from members of the ACR and Association of Rheumatology Health Professionals (ARHP) living in the United States, and going through several rounds of refining and prioritizing the importance of goals through the input of clinicians, researchers, patients, and stakeholders. The Committee on Research uses the agenda to “set the compass for the organization in terms of research initiatives and facilitate the ACR’s advocacy for the research goals identified.”
Dr. Alexis R. Ogdie-Beatty, who jointly led the development of the agenda for the Committee on Research along with Dr. S. Louis Bridges, said that while the goals for 2016-2020 had a great deal of overlap with those of 2011-2015, “some of the topics that came up were different. Some of the topics were more specific than in the previous agenda. We have some idea how important these issues were to rheumatologists, given that rheumatologists (and patients) rated the importance of the items. Defining new therapeutic targets and developing new therapies for rheumatic diseases was by far the most highly rated goal by rheumatologists. Next most highly rated was to advocate for increased support for rheumatology research and rheumatology investigators – this was included as a supplementary goal that supports the rest of the agenda. Other newer items were those around determining how the changing health care landscape affects rheumatology patients and clinicians. In addition, nonpharmacologic therapy, adult outcomes of pediatric disease, and optimizing patient engagement were topics that were felt to be important. I think these highlight the input of clinicians in identifying research objectives.”
The 2016-2020 agenda is the third set of goals developed by the committee since 2005, and the first to “crowdsource” the important questions to ACR and ARHP members rather than be assembled solely by the committee.
The agenda arose from a multistage process that began with a web-based survey to the ACR/ARHP membership that asked respondents to “list the five most important research questions that need to be addressed over the next 5 years in order to improve the care for patients with rheumatic disease.” A selected group of 100 individuals representing patients, clinicians (academic and community), research (all types with diverse areas/diseases of interest), allied health professionals, pediatric and adult rheumatology, men and women, all career stages, and all regions of the country, used a Delphi exercise to rate 30 statements generated from the survey on a scale from 1 (not important) to 10 (very important). They had the option to provide comments. At a Leadership Summit, stakeholders from various nonprofit foundations associated with rheumatic diseases, the National Institutes of Health, and the president of the Rheumatology Research Foundation gave comments on a draft agenda to the Committee on Research, after which the committee discussed the results and input and then solicited further 1-10 ratings and comments on preliminary agenda goals from the same group of 100 individuals as in the second phase, plus an additional 17 clinicians.
Up next in the rank-ordering after therapeutic goals were three goals about understanding:
• The etiology, pathogenesis, and genetic basis of rheumatic diseases.
• Early disease states to improve early diagnosis, develop biomarkers for early detection, and determine how earlier treatment changes outcomes.
• The immune system and autoimmunity by defining autoimmunity triggers and determining how epigenetics affect disease susceptibility and inflammation.
The 5-year plan proposed developing improved outcome measures that incorporate patient self-reports, imaging, and measures of clinical response and disease activity. The agenda also seeks to gain better understanding of how patients with rheumatic disease, rheumatologists, and rheumatology health professionals are being affected by the changing U.S. health care landscape.
The plan calls for determining the role of nonpharmacologic therapy in the management of rheumatic disease (promoting and improving adherence to physical activity, finding optimal exercise prescriptions, and determining the role of diet on disease activity), as well as evaluating the role of regenerative medicine.
The agenda spells out the need for better engagement of patients in their care as well as for understanding how comorbidities are influenced by rheumatic disease and how pain and fatigue arise in rheumatic disease.
In two separate goals, committee members listed the importance of determining adult outcomes of pediatric rheumatic diseases and the effect of aging on the development, progression, and management of rheumatic diseases.
The Committee on Research identified three supplemental goals that support the others:
• Advocating for increased support for rheumatology research and rheumatology investigators.
• Harmonizing data from existing cohorts and registries to optimize research capabilities.
• Improving patient research partner involvement in research protocols.
Therapeutic goals set the tone for the American College of Rheumatology National Research Agenda 2016-2020 by calling for the discovery and development of new therapies for rheumatic disease; finding predictors of response and nonresponse to, and adverse events from therapy; and improving the understanding of how therapies should be used.
Those are the top 3 out of 15 goals facilitated by the ACR’s Committee on Research, which finalized the agenda after seeking input from members of the ACR and Association of Rheumatology Health Professionals (ARHP) living in the United States, and going through several rounds of refining and prioritizing the importance of goals through the input of clinicians, researchers, patients, and stakeholders. The Committee on Research uses the agenda to “set the compass for the organization in terms of research initiatives and facilitate the ACR’s advocacy for the research goals identified.”
Dr. Alexis R. Ogdie-Beatty, who jointly led the development of the agenda for the Committee on Research along with Dr. S. Louis Bridges, said that while the goals for 2016-2020 had a great deal of overlap with those of 2011-2015, “some of the topics that came up were different. Some of the topics were more specific than in the previous agenda. We have some idea how important these issues were to rheumatologists, given that rheumatologists (and patients) rated the importance of the items. Defining new therapeutic targets and developing new therapies for rheumatic diseases was by far the most highly rated goal by rheumatologists. Next most highly rated was to advocate for increased support for rheumatology research and rheumatology investigators – this was included as a supplementary goal that supports the rest of the agenda. Other newer items were those around determining how the changing health care landscape affects rheumatology patients and clinicians. In addition, nonpharmacologic therapy, adult outcomes of pediatric disease, and optimizing patient engagement were topics that were felt to be important. I think these highlight the input of clinicians in identifying research objectives.”
The 2016-2020 agenda is the third set of goals developed by the committee since 2005, and the first to “crowdsource” the important questions to ACR and ARHP members rather than be assembled solely by the committee.
The agenda arose from a multistage process that began with a web-based survey to the ACR/ARHP membership that asked respondents to “list the five most important research questions that need to be addressed over the next 5 years in order to improve the care for patients with rheumatic disease.” A selected group of 100 individuals representing patients, clinicians (academic and community), research (all types with diverse areas/diseases of interest), allied health professionals, pediatric and adult rheumatology, men and women, all career stages, and all regions of the country, used a Delphi exercise to rate 30 statements generated from the survey on a scale from 1 (not important) to 10 (very important). They had the option to provide comments. At a Leadership Summit, stakeholders from various nonprofit foundations associated with rheumatic diseases, the National Institutes of Health, and the president of the Rheumatology Research Foundation gave comments on a draft agenda to the Committee on Research, after which the committee discussed the results and input and then solicited further 1-10 ratings and comments on preliminary agenda goals from the same group of 100 individuals as in the second phase, plus an additional 17 clinicians.
Up next in the rank-ordering after therapeutic goals were three goals about understanding:
• The etiology, pathogenesis, and genetic basis of rheumatic diseases.
• Early disease states to improve early diagnosis, develop biomarkers for early detection, and determine how earlier treatment changes outcomes.
• The immune system and autoimmunity by defining autoimmunity triggers and determining how epigenetics affect disease susceptibility and inflammation.
The 5-year plan proposed developing improved outcome measures that incorporate patient self-reports, imaging, and measures of clinical response and disease activity. The agenda also seeks to gain better understanding of how patients with rheumatic disease, rheumatologists, and rheumatology health professionals are being affected by the changing U.S. health care landscape.
The plan calls for determining the role of nonpharmacologic therapy in the management of rheumatic disease (promoting and improving adherence to physical activity, finding optimal exercise prescriptions, and determining the role of diet on disease activity), as well as evaluating the role of regenerative medicine.
The agenda spells out the need for better engagement of patients in their care as well as for understanding how comorbidities are influenced by rheumatic disease and how pain and fatigue arise in rheumatic disease.
In two separate goals, committee members listed the importance of determining adult outcomes of pediatric rheumatic diseases and the effect of aging on the development, progression, and management of rheumatic diseases.
The Committee on Research identified three supplemental goals that support the others:
• Advocating for increased support for rheumatology research and rheumatology investigators.
• Harmonizing data from existing cohorts and registries to optimize research capabilities.
• Improving patient research partner involvement in research protocols.
Therapeutic goals set the tone for the American College of Rheumatology National Research Agenda 2016-2020 by calling for the discovery and development of new therapies for rheumatic disease; finding predictors of response and nonresponse to, and adverse events from therapy; and improving the understanding of how therapies should be used.
Those are the top 3 out of 15 goals facilitated by the ACR’s Committee on Research, which finalized the agenda after seeking input from members of the ACR and Association of Rheumatology Health Professionals (ARHP) living in the United States, and going through several rounds of refining and prioritizing the importance of goals through the input of clinicians, researchers, patients, and stakeholders. The Committee on Research uses the agenda to “set the compass for the organization in terms of research initiatives and facilitate the ACR’s advocacy for the research goals identified.”
Dr. Alexis R. Ogdie-Beatty, who jointly led the development of the agenda for the Committee on Research along with Dr. S. Louis Bridges, said that while the goals for 2016-2020 had a great deal of overlap with those of 2011-2015, “some of the topics that came up were different. Some of the topics were more specific than in the previous agenda. We have some idea how important these issues were to rheumatologists, given that rheumatologists (and patients) rated the importance of the items. Defining new therapeutic targets and developing new therapies for rheumatic diseases was by far the most highly rated goal by rheumatologists. Next most highly rated was to advocate for increased support for rheumatology research and rheumatology investigators – this was included as a supplementary goal that supports the rest of the agenda. Other newer items were those around determining how the changing health care landscape affects rheumatology patients and clinicians. In addition, nonpharmacologic therapy, adult outcomes of pediatric disease, and optimizing patient engagement were topics that were felt to be important. I think these highlight the input of clinicians in identifying research objectives.”
The 2016-2020 agenda is the third set of goals developed by the committee since 2005, and the first to “crowdsource” the important questions to ACR and ARHP members rather than be assembled solely by the committee.
The agenda arose from a multistage process that began with a web-based survey to the ACR/ARHP membership that asked respondents to “list the five most important research questions that need to be addressed over the next 5 years in order to improve the care for patients with rheumatic disease.” A selected group of 100 individuals representing patients, clinicians (academic and community), research (all types with diverse areas/diseases of interest), allied health professionals, pediatric and adult rheumatology, men and women, all career stages, and all regions of the country, used a Delphi exercise to rate 30 statements generated from the survey on a scale from 1 (not important) to 10 (very important). They had the option to provide comments. At a Leadership Summit, stakeholders from various nonprofit foundations associated with rheumatic diseases, the National Institutes of Health, and the president of the Rheumatology Research Foundation gave comments on a draft agenda to the Committee on Research, after which the committee discussed the results and input and then solicited further 1-10 ratings and comments on preliminary agenda goals from the same group of 100 individuals as in the second phase, plus an additional 17 clinicians.
Up next in the rank-ordering after therapeutic goals were three goals about understanding:
• The etiology, pathogenesis, and genetic basis of rheumatic diseases.
• Early disease states to improve early diagnosis, develop biomarkers for early detection, and determine how earlier treatment changes outcomes.
• The immune system and autoimmunity by defining autoimmunity triggers and determining how epigenetics affect disease susceptibility and inflammation.
The 5-year plan proposed developing improved outcome measures that incorporate patient self-reports, imaging, and measures of clinical response and disease activity. The agenda also seeks to gain better understanding of how patients with rheumatic disease, rheumatologists, and rheumatology health professionals are being affected by the changing U.S. health care landscape.
The plan calls for determining the role of nonpharmacologic therapy in the management of rheumatic disease (promoting and improving adherence to physical activity, finding optimal exercise prescriptions, and determining the role of diet on disease activity), as well as evaluating the role of regenerative medicine.
The agenda spells out the need for better engagement of patients in their care as well as for understanding how comorbidities are influenced by rheumatic disease and how pain and fatigue arise in rheumatic disease.
In two separate goals, committee members listed the importance of determining adult outcomes of pediatric rheumatic diseases and the effect of aging on the development, progression, and management of rheumatic diseases.
The Committee on Research identified three supplemental goals that support the others:
• Advocating for increased support for rheumatology research and rheumatology investigators.
• Harmonizing data from existing cohorts and registries to optimize research capabilities.
• Improving patient research partner involvement in research protocols.
Acetaminophen ineffective against osteoarthritis pain
The widely used painkiller acetaminophen has little effect on osteoarthritic pain even at high doses, according to results from a new meta-analysis, while several other agents, including diclofenac, improve pain more robustly.
The study, published online March 17 in The Lancet (doi: 10.1016/S0140-6736(16)30002-2), reviewed results from 74 randomized trials enrolling nearly 60,000 patients with knee or hip osteoarthritis.
Patients were assigned different single-agent treatment regimens, comprising various dosages of seven nonsteroidal anti-inflammatory drugs (rofecoxib, lumiracoxib, etoricoxib, diclofenac, celecoxib, naproxen, and ibuprofen) or acetaminophen; in the included trials some treatments were compared head to head, and others to placebo.
First author Bruno R. da Costa, Ph.D., of the University of Bern (Switzerland) and his colleagues found to their surprise that acetaminophen had a nearly null effect on pain symptoms at doses ranging from under 2,000 mg a day to as much as 4,000 mg.
The study’s preestablished cutoff for clinically important pain reduction was an effect size of –0.37. While the most effective regimens in the study had effect sizes approaching –0.6, compared with placebo, acetaminophen’s effect size was only –0.17 across the doses studied.
Acetaminophen “is clinically ineffective and should not be recommended for the symptomatic treatment of osteoarthritis, irrespective of the dose,” the researchers concluded.
Diclofenac, meanwhile, had one of the greatest effect sizes at the maximum dose of 150 mg per day (–0.57), and etoricoxib 60 mg and rofecoxib 25 mg were comparably effective. All three agents at these maximum daily doses had 100% probability to reach the minimum clinically important difference established in the study when used to reduce osteoarthritic pain.
By comparison, maximum daily doses of ibuprofen (2,400 mg) and naproxen (1,000 mg) had 83% and 78% probability, respectively, of achieving clinically important reductions. Treatment effects increased with dosage, but reached statistical significance only for celecoxib, diclofenac, and naproxen.
“Although our findings suggest that some NSAIDs have a clinically relevant treatment effect on osteoarthritis pain,” the investigators wrote, their benefit has to be weighed against their potential harmful effects, which include cardiovascular risk associated with diclofenac and gastrointestinal complications linked to naproxen.
“Appropriate drug selection is a major challenge in patients with osteoarthritis, who are often elderly with polypharmacy. Our study will help to put the available safety data into perspective,” they wrote.
The agents were used short-term, reflecting real-life practice, with average follow-up in the study less than 3 months. However, trials with longer-term follow-up may be needed to compare effectiveness of regimens “on a continuous fixed-dose versus NSAIDs on an as-needed basis,” the researchers acknowledged.
The Swiss National Science Foundation and the Arco Foundation of Switzerland sponsored the study. One investigator disclosed institutional research support from AstraZeneca, Biotronik, Biosensors International, Eli Lilly, and the Medicines Company, while another is currently employed by Novartis and holds shares in Cogitars.
In this network meta-analysis, the most remarkable result is that acetaminophen does not seem to confer any demonstrable effect or benefit in osteoarthritis, at any dose. This finding is not entirely unexpected. It has been on the market for as long as most of us remember. Its efficacy has never been properly established or quantified in chronic diseases, and is probably not as great as many would believe. Its safety is also questioned, not just in overdose. Is recommending it as the universal first-line analgesic in osteoarthritis still tenable? Many patients could be suffering needlessly because of perceived NSAIDs risks and acetaminophen benefits (which might not be real). Perhaps researchers need to reassess both these perceptions (or misconceptions) and the use of other analgesic options that have been discarded over time.
These comments are taken from an editorial by Dr. Nicholas Moore and associates from the department of pharmacology at the University of Bordeaux (France) that accompanied the report by Dr. da Costa and colleagues (Lancet. 2016 Mar 17. doi: 10.1016/S0140-6736(15)01170-8). Dr. Moore disclosed past research support from Boots, Reckitt-Benckiser, Novartis, Pfizer, Roche, Rhone Poulenc, Sanofi, and Helsinn.
In this network meta-analysis, the most remarkable result is that acetaminophen does not seem to confer any demonstrable effect or benefit in osteoarthritis, at any dose. This finding is not entirely unexpected. It has been on the market for as long as most of us remember. Its efficacy has never been properly established or quantified in chronic diseases, and is probably not as great as many would believe. Its safety is also questioned, not just in overdose. Is recommending it as the universal first-line analgesic in osteoarthritis still tenable? Many patients could be suffering needlessly because of perceived NSAIDs risks and acetaminophen benefits (which might not be real). Perhaps researchers need to reassess both these perceptions (or misconceptions) and the use of other analgesic options that have been discarded over time.
These comments are taken from an editorial by Dr. Nicholas Moore and associates from the department of pharmacology at the University of Bordeaux (France) that accompanied the report by Dr. da Costa and colleagues (Lancet. 2016 Mar 17. doi: 10.1016/S0140-6736(15)01170-8). Dr. Moore disclosed past research support from Boots, Reckitt-Benckiser, Novartis, Pfizer, Roche, Rhone Poulenc, Sanofi, and Helsinn.
In this network meta-analysis, the most remarkable result is that acetaminophen does not seem to confer any demonstrable effect or benefit in osteoarthritis, at any dose. This finding is not entirely unexpected. It has been on the market for as long as most of us remember. Its efficacy has never been properly established or quantified in chronic diseases, and is probably not as great as many would believe. Its safety is also questioned, not just in overdose. Is recommending it as the universal first-line analgesic in osteoarthritis still tenable? Many patients could be suffering needlessly because of perceived NSAIDs risks and acetaminophen benefits (which might not be real). Perhaps researchers need to reassess both these perceptions (or misconceptions) and the use of other analgesic options that have been discarded over time.
These comments are taken from an editorial by Dr. Nicholas Moore and associates from the department of pharmacology at the University of Bordeaux (France) that accompanied the report by Dr. da Costa and colleagues (Lancet. 2016 Mar 17. doi: 10.1016/S0140-6736(15)01170-8). Dr. Moore disclosed past research support from Boots, Reckitt-Benckiser, Novartis, Pfizer, Roche, Rhone Poulenc, Sanofi, and Helsinn.
The widely used painkiller acetaminophen has little effect on osteoarthritic pain even at high doses, according to results from a new meta-analysis, while several other agents, including diclofenac, improve pain more robustly.
The study, published online March 17 in The Lancet (doi: 10.1016/S0140-6736(16)30002-2), reviewed results from 74 randomized trials enrolling nearly 60,000 patients with knee or hip osteoarthritis.
Patients were assigned different single-agent treatment regimens, comprising various dosages of seven nonsteroidal anti-inflammatory drugs (rofecoxib, lumiracoxib, etoricoxib, diclofenac, celecoxib, naproxen, and ibuprofen) or acetaminophen; in the included trials some treatments were compared head to head, and others to placebo.
First author Bruno R. da Costa, Ph.D., of the University of Bern (Switzerland) and his colleagues found to their surprise that acetaminophen had a nearly null effect on pain symptoms at doses ranging from under 2,000 mg a day to as much as 4,000 mg.
The study’s preestablished cutoff for clinically important pain reduction was an effect size of –0.37. While the most effective regimens in the study had effect sizes approaching –0.6, compared with placebo, acetaminophen’s effect size was only –0.17 across the doses studied.
Acetaminophen “is clinically ineffective and should not be recommended for the symptomatic treatment of osteoarthritis, irrespective of the dose,” the researchers concluded.
Diclofenac, meanwhile, had one of the greatest effect sizes at the maximum dose of 150 mg per day (–0.57), and etoricoxib 60 mg and rofecoxib 25 mg were comparably effective. All three agents at these maximum daily doses had 100% probability to reach the minimum clinically important difference established in the study when used to reduce osteoarthritic pain.
By comparison, maximum daily doses of ibuprofen (2,400 mg) and naproxen (1,000 mg) had 83% and 78% probability, respectively, of achieving clinically important reductions. Treatment effects increased with dosage, but reached statistical significance only for celecoxib, diclofenac, and naproxen.
“Although our findings suggest that some NSAIDs have a clinically relevant treatment effect on osteoarthritis pain,” the investigators wrote, their benefit has to be weighed against their potential harmful effects, which include cardiovascular risk associated with diclofenac and gastrointestinal complications linked to naproxen.
“Appropriate drug selection is a major challenge in patients with osteoarthritis, who are often elderly with polypharmacy. Our study will help to put the available safety data into perspective,” they wrote.
The agents were used short-term, reflecting real-life practice, with average follow-up in the study less than 3 months. However, trials with longer-term follow-up may be needed to compare effectiveness of regimens “on a continuous fixed-dose versus NSAIDs on an as-needed basis,” the researchers acknowledged.
The Swiss National Science Foundation and the Arco Foundation of Switzerland sponsored the study. One investigator disclosed institutional research support from AstraZeneca, Biotronik, Biosensors International, Eli Lilly, and the Medicines Company, while another is currently employed by Novartis and holds shares in Cogitars.
The widely used painkiller acetaminophen has little effect on osteoarthritic pain even at high doses, according to results from a new meta-analysis, while several other agents, including diclofenac, improve pain more robustly.
The study, published online March 17 in The Lancet (doi: 10.1016/S0140-6736(16)30002-2), reviewed results from 74 randomized trials enrolling nearly 60,000 patients with knee or hip osteoarthritis.
Patients were assigned different single-agent treatment regimens, comprising various dosages of seven nonsteroidal anti-inflammatory drugs (rofecoxib, lumiracoxib, etoricoxib, diclofenac, celecoxib, naproxen, and ibuprofen) or acetaminophen; in the included trials some treatments were compared head to head, and others to placebo.
First author Bruno R. da Costa, Ph.D., of the University of Bern (Switzerland) and his colleagues found to their surprise that acetaminophen had a nearly null effect on pain symptoms at doses ranging from under 2,000 mg a day to as much as 4,000 mg.
The study’s preestablished cutoff for clinically important pain reduction was an effect size of –0.37. While the most effective regimens in the study had effect sizes approaching –0.6, compared with placebo, acetaminophen’s effect size was only –0.17 across the doses studied.
Acetaminophen “is clinically ineffective and should not be recommended for the symptomatic treatment of osteoarthritis, irrespective of the dose,” the researchers concluded.
Diclofenac, meanwhile, had one of the greatest effect sizes at the maximum dose of 150 mg per day (–0.57), and etoricoxib 60 mg and rofecoxib 25 mg were comparably effective. All three agents at these maximum daily doses had 100% probability to reach the minimum clinically important difference established in the study when used to reduce osteoarthritic pain.
By comparison, maximum daily doses of ibuprofen (2,400 mg) and naproxen (1,000 mg) had 83% and 78% probability, respectively, of achieving clinically important reductions. Treatment effects increased with dosage, but reached statistical significance only for celecoxib, diclofenac, and naproxen.
“Although our findings suggest that some NSAIDs have a clinically relevant treatment effect on osteoarthritis pain,” the investigators wrote, their benefit has to be weighed against their potential harmful effects, which include cardiovascular risk associated with diclofenac and gastrointestinal complications linked to naproxen.
“Appropriate drug selection is a major challenge in patients with osteoarthritis, who are often elderly with polypharmacy. Our study will help to put the available safety data into perspective,” they wrote.
The agents were used short-term, reflecting real-life practice, with average follow-up in the study less than 3 months. However, trials with longer-term follow-up may be needed to compare effectiveness of regimens “on a continuous fixed-dose versus NSAIDs on an as-needed basis,” the researchers acknowledged.
The Swiss National Science Foundation and the Arco Foundation of Switzerland sponsored the study. One investigator disclosed institutional research support from AstraZeneca, Biotronik, Biosensors International, Eli Lilly, and the Medicines Company, while another is currently employed by Novartis and holds shares in Cogitars.
FROM THE LANCET
Key clinical point:Acetaminophen, even at high doses, is largely ineffective at reducing pain in knee or hip osteoarthritis.
Major finding: Effect size for acetaminophen was –0.17 vs. placebo (not reaching clinical threshold of –0.37), compared with –0.57 for the maximum dose of diclofenac.
Data source: A meta-analysis of 74 randomized trials evaluating 23 treatment regimens including seven NSAIDS and acetaminophen in 58,566 patients
Disclosures: The Swiss National Science Foundation and the Arco Foundation of Switzerland sponsored the study. Two investigators disclosed industry relationships.