User login
For MD-IQ on Family Practice News, but a regular topic for Rheumatology News
Study identifies distinct OA fatigue trajectories
A new study has identified three distinct trajectories of fatigue levels in patients with early symptomatic osteoarthritis (OA) in the knee and hip, report Jadran Botterman, MSc, and coauthors from the department of psychology, health, and technology at the University of Twente (the Netherlands).
Six years of data were collected from the CHECK (Cohort Hip and Cohort Knee) participants and then separated into distinct trajectories using growth mixture modeling. Three distinct fatigue trajectories were found: low fatigue, low to high fatigue, and high fatigue.
The authors found a significant association between trajectory and patient characteristics. Women, patients with comorbid disease, and patients using medications were more likely to have a high fatigue trajectory, Dr. Botterman and his colleagues reported.
“Identification of these trajectories with differing patient characteristics may warrant tailored psychosocial interventions for patients with elevated levels of fatigue,” the authors concluded.
Read the full article in the Journal of Rheumatology.
A new study has identified three distinct trajectories of fatigue levels in patients with early symptomatic osteoarthritis (OA) in the knee and hip, report Jadran Botterman, MSc, and coauthors from the department of psychology, health, and technology at the University of Twente (the Netherlands).
Six years of data were collected from the CHECK (Cohort Hip and Cohort Knee) participants and then separated into distinct trajectories using growth mixture modeling. Three distinct fatigue trajectories were found: low fatigue, low to high fatigue, and high fatigue.
The authors found a significant association between trajectory and patient characteristics. Women, patients with comorbid disease, and patients using medications were more likely to have a high fatigue trajectory, Dr. Botterman and his colleagues reported.
“Identification of these trajectories with differing patient characteristics may warrant tailored psychosocial interventions for patients with elevated levels of fatigue,” the authors concluded.
Read the full article in the Journal of Rheumatology.
A new study has identified three distinct trajectories of fatigue levels in patients with early symptomatic osteoarthritis (OA) in the knee and hip, report Jadran Botterman, MSc, and coauthors from the department of psychology, health, and technology at the University of Twente (the Netherlands).
Six years of data were collected from the CHECK (Cohort Hip and Cohort Knee) participants and then separated into distinct trajectories using growth mixture modeling. Three distinct fatigue trajectories were found: low fatigue, low to high fatigue, and high fatigue.
The authors found a significant association between trajectory and patient characteristics. Women, patients with comorbid disease, and patients using medications were more likely to have a high fatigue trajectory, Dr. Botterman and his colleagues reported.
“Identification of these trajectories with differing patient characteristics may warrant tailored psychosocial interventions for patients with elevated levels of fatigue,” the authors concluded.
Read the full article in the Journal of Rheumatology.
FROM THE JOURNAL OF RHEUMATOLOGY
Statement warns of drugs causing or exacerbating heart failure
Many commonly used prescription drugs, many OTC agents, and also several complimentary or alternative medications, can either trigger heart failure or exacerbate the disease in patients with existing heart failure according to a Scientific Statement written by a committee of the American Heart Association and released on July 11.
This first-ever authoritative U.S. overview of what is known about drugs that can affect heart failure was compiled to address an important practice issue for the large and growing number of U.S. patients with heart failure, estimated to be nearly 6 million Americans, and “provide some guidance to health care providers in how to minimize polypharmacy, improve medication safety, as well as identify the medications that could exacerbate or cause heart failure,” said Robert L. Page II, PharmD, chair of the committee and a professor of clinical pharmacy at the University of Colorado at Denver, Aurora.
Although the comprehensive statement lists 88 distinct prescription drugs or drug classes as agents that pose major or moderate threats for causing or worsening heart failure, “from the American public’s perspective, importance should be placed on educating patients regarding the impact that OTC medications can have on their heart failure,” Dr. Page said in an interview. “For example, nonsteroidal anti-inflammatory drugs like ibuprofen or naproxen can cause sodium and water retention and antagonize the effects of evidence-based heart failure pharmacotherapies. Additionally, OTC medications like pseudoephedrine, which many cough and cold products contain, can increase blood pressure and afterload,” he noted. The risks these drugs pose becomes even greater when they are taken at higher doses.
NSAIDs
The statement cites already existing guidance from the American College of Cardiology and American Heart Association that for patients with existing heart failure, use of NSAIDs should either be avoided or withdrawn when possible. The statement advises educating patients to communicate with their health care provider before taking any OTC medication or complimentary or alternative medication, avoid these agents when their efficacy and safety is uncertain, and evaluate the labels of these products for their sodium content (although the sodium content from inactive ingredients may be difficult to find in labeling).
“Currently, we teach patients to read food labels for sodium content, but we also need to educate patients on how to read OTC medication labels for both ingredients and sodium content. Many OTC antacids may have a large sodium load,” Dr. Page said. The statement includes a list of 14 prescription drugs and also highlights several OTC formulations that have an especially high sodium content.
Metformin
Among the many prescription drugs listed, one notable entry is for the oral hypoglycemic agent metformin that today is among the most widely used drugs for treating type 2 diabetes and is especially relevant for heart failure patients because, as the statement notes, 38% also have diabetes. The statement details the long history of metformin and heart failure, noting that until a decade ago, the drug had a contraindication for patients with heart failure, that metformin’s label still carries a black box warning for cautious use in heart failure patients, and that earlier in 2016, the Food and Drug Administration cautioned that metformin should not be used in patients with an estimated glomerular filtration rate of less than 30 mL/minute per 1.73 m2. The statement also endorsed a recommendation from the American Diabetes Association that metformin not be used in patients with unstable heart failure or those hospitalized for heart failure.
Antihypertensives, biologics, and more
Other notable prescription drugs listed as potentially having a major impact on causing or worsening heart failure include the antihypertensive drugs diltiazem, verapamil, and moxonidine, the tumor necrosis factor–inhibitors that are widely used to treat rheumatologic and gastroenterologic diseases, the antipsychotic clozapine, and a long list of anticancer medications, including several anthracyclines and many types of newer biologic agents.
The statement also lists several specific recommendations to health care providers for improving oversight of the drugs taken by patients with heart failure or those at risk for heart failure. These include a comprehensive medication review during each clinical encounter. The statement also suggests a “medication flow sheet” for each patient that contains the basic information regarding the regimen for each medication taken by a patient: the brand and generic name, the purpose of the medication, and its dosage. “These medication flow sheets should be used by patients as a tool to enhance safety and adherence, and they should show their flow sheets at each provider visit,” Dr. Page said.
Managing myriad meds
The statement also calls for stopping medications without a well defined indication for a patient, avoid prescribing new drugs to address side effects of other drugs, and suggests establishing a “captain” among the health care providers seen by each patient who would be particularly responsible for overseeing and keeping track of the medications the patient takes.
“Ideally, this ‘captain’ would be the patient’s primary care provider, who should be in contact with the other specialists that the patient may be seeing. However, this does not always happen,” said Dr. Page. “Therefore, I encourage each patient with heart failure to contact both their primary care provider and their health care provider who is managing their heart failure before taking or stopping any new medication including prescription, OTC, herbal, complimentary or alternative medication or supplement. Health care providers need to encourage patients to be actively engaged in their medication management.”
Dr. Page had no disclosures.
On Twitter @mitchelzoler
Many commonly used prescription drugs, many OTC agents, and also several complimentary or alternative medications, can either trigger heart failure or exacerbate the disease in patients with existing heart failure according to a Scientific Statement written by a committee of the American Heart Association and released on July 11.
This first-ever authoritative U.S. overview of what is known about drugs that can affect heart failure was compiled to address an important practice issue for the large and growing number of U.S. patients with heart failure, estimated to be nearly 6 million Americans, and “provide some guidance to health care providers in how to minimize polypharmacy, improve medication safety, as well as identify the medications that could exacerbate or cause heart failure,” said Robert L. Page II, PharmD, chair of the committee and a professor of clinical pharmacy at the University of Colorado at Denver, Aurora.
Although the comprehensive statement lists 88 distinct prescription drugs or drug classes as agents that pose major or moderate threats for causing or worsening heart failure, “from the American public’s perspective, importance should be placed on educating patients regarding the impact that OTC medications can have on their heart failure,” Dr. Page said in an interview. “For example, nonsteroidal anti-inflammatory drugs like ibuprofen or naproxen can cause sodium and water retention and antagonize the effects of evidence-based heart failure pharmacotherapies. Additionally, OTC medications like pseudoephedrine, which many cough and cold products contain, can increase blood pressure and afterload,” he noted. The risks these drugs pose becomes even greater when they are taken at higher doses.
NSAIDs
The statement cites already existing guidance from the American College of Cardiology and American Heart Association that for patients with existing heart failure, use of NSAIDs should either be avoided or withdrawn when possible. The statement advises educating patients to communicate with their health care provider before taking any OTC medication or complimentary or alternative medication, avoid these agents when their efficacy and safety is uncertain, and evaluate the labels of these products for their sodium content (although the sodium content from inactive ingredients may be difficult to find in labeling).
“Currently, we teach patients to read food labels for sodium content, but we also need to educate patients on how to read OTC medication labels for both ingredients and sodium content. Many OTC antacids may have a large sodium load,” Dr. Page said. The statement includes a list of 14 prescription drugs and also highlights several OTC formulations that have an especially high sodium content.
Metformin
Among the many prescription drugs listed, one notable entry is for the oral hypoglycemic agent metformin that today is among the most widely used drugs for treating type 2 diabetes and is especially relevant for heart failure patients because, as the statement notes, 38% also have diabetes. The statement details the long history of metformin and heart failure, noting that until a decade ago, the drug had a contraindication for patients with heart failure, that metformin’s label still carries a black box warning for cautious use in heart failure patients, and that earlier in 2016, the Food and Drug Administration cautioned that metformin should not be used in patients with an estimated glomerular filtration rate of less than 30 mL/minute per 1.73 m2. The statement also endorsed a recommendation from the American Diabetes Association that metformin not be used in patients with unstable heart failure or those hospitalized for heart failure.
Antihypertensives, biologics, and more
Other notable prescription drugs listed as potentially having a major impact on causing or worsening heart failure include the antihypertensive drugs diltiazem, verapamil, and moxonidine, the tumor necrosis factor–inhibitors that are widely used to treat rheumatologic and gastroenterologic diseases, the antipsychotic clozapine, and a long list of anticancer medications, including several anthracyclines and many types of newer biologic agents.
The statement also lists several specific recommendations to health care providers for improving oversight of the drugs taken by patients with heart failure or those at risk for heart failure. These include a comprehensive medication review during each clinical encounter. The statement also suggests a “medication flow sheet” for each patient that contains the basic information regarding the regimen for each medication taken by a patient: the brand and generic name, the purpose of the medication, and its dosage. “These medication flow sheets should be used by patients as a tool to enhance safety and adherence, and they should show their flow sheets at each provider visit,” Dr. Page said.
Managing myriad meds
The statement also calls for stopping medications without a well defined indication for a patient, avoid prescribing new drugs to address side effects of other drugs, and suggests establishing a “captain” among the health care providers seen by each patient who would be particularly responsible for overseeing and keeping track of the medications the patient takes.
“Ideally, this ‘captain’ would be the patient’s primary care provider, who should be in contact with the other specialists that the patient may be seeing. However, this does not always happen,” said Dr. Page. “Therefore, I encourage each patient with heart failure to contact both their primary care provider and their health care provider who is managing their heart failure before taking or stopping any new medication including prescription, OTC, herbal, complimentary or alternative medication or supplement. Health care providers need to encourage patients to be actively engaged in their medication management.”
Dr. Page had no disclosures.
On Twitter @mitchelzoler
Many commonly used prescription drugs, many OTC agents, and also several complimentary or alternative medications, can either trigger heart failure or exacerbate the disease in patients with existing heart failure according to a Scientific Statement written by a committee of the American Heart Association and released on July 11.
This first-ever authoritative U.S. overview of what is known about drugs that can affect heart failure was compiled to address an important practice issue for the large and growing number of U.S. patients with heart failure, estimated to be nearly 6 million Americans, and “provide some guidance to health care providers in how to minimize polypharmacy, improve medication safety, as well as identify the medications that could exacerbate or cause heart failure,” said Robert L. Page II, PharmD, chair of the committee and a professor of clinical pharmacy at the University of Colorado at Denver, Aurora.
Although the comprehensive statement lists 88 distinct prescription drugs or drug classes as agents that pose major or moderate threats for causing or worsening heart failure, “from the American public’s perspective, importance should be placed on educating patients regarding the impact that OTC medications can have on their heart failure,” Dr. Page said in an interview. “For example, nonsteroidal anti-inflammatory drugs like ibuprofen or naproxen can cause sodium and water retention and antagonize the effects of evidence-based heart failure pharmacotherapies. Additionally, OTC medications like pseudoephedrine, which many cough and cold products contain, can increase blood pressure and afterload,” he noted. The risks these drugs pose becomes even greater when they are taken at higher doses.
NSAIDs
The statement cites already existing guidance from the American College of Cardiology and American Heart Association that for patients with existing heart failure, use of NSAIDs should either be avoided or withdrawn when possible. The statement advises educating patients to communicate with their health care provider before taking any OTC medication or complimentary or alternative medication, avoid these agents when their efficacy and safety is uncertain, and evaluate the labels of these products for their sodium content (although the sodium content from inactive ingredients may be difficult to find in labeling).
“Currently, we teach patients to read food labels for sodium content, but we also need to educate patients on how to read OTC medication labels for both ingredients and sodium content. Many OTC antacids may have a large sodium load,” Dr. Page said. The statement includes a list of 14 prescription drugs and also highlights several OTC formulations that have an especially high sodium content.
Metformin
Among the many prescription drugs listed, one notable entry is for the oral hypoglycemic agent metformin that today is among the most widely used drugs for treating type 2 diabetes and is especially relevant for heart failure patients because, as the statement notes, 38% also have diabetes. The statement details the long history of metformin and heart failure, noting that until a decade ago, the drug had a contraindication for patients with heart failure, that metformin’s label still carries a black box warning for cautious use in heart failure patients, and that earlier in 2016, the Food and Drug Administration cautioned that metformin should not be used in patients with an estimated glomerular filtration rate of less than 30 mL/minute per 1.73 m2. The statement also endorsed a recommendation from the American Diabetes Association that metformin not be used in patients with unstable heart failure or those hospitalized for heart failure.
Antihypertensives, biologics, and more
Other notable prescription drugs listed as potentially having a major impact on causing or worsening heart failure include the antihypertensive drugs diltiazem, verapamil, and moxonidine, the tumor necrosis factor–inhibitors that are widely used to treat rheumatologic and gastroenterologic diseases, the antipsychotic clozapine, and a long list of anticancer medications, including several anthracyclines and many types of newer biologic agents.
The statement also lists several specific recommendations to health care providers for improving oversight of the drugs taken by patients with heart failure or those at risk for heart failure. These include a comprehensive medication review during each clinical encounter. The statement also suggests a “medication flow sheet” for each patient that contains the basic information regarding the regimen for each medication taken by a patient: the brand and generic name, the purpose of the medication, and its dosage. “These medication flow sheets should be used by patients as a tool to enhance safety and adherence, and they should show their flow sheets at each provider visit,” Dr. Page said.
Managing myriad meds
The statement also calls for stopping medications without a well defined indication for a patient, avoid prescribing new drugs to address side effects of other drugs, and suggests establishing a “captain” among the health care providers seen by each patient who would be particularly responsible for overseeing and keeping track of the medications the patient takes.
“Ideally, this ‘captain’ would be the patient’s primary care provider, who should be in contact with the other specialists that the patient may be seeing. However, this does not always happen,” said Dr. Page. “Therefore, I encourage each patient with heart failure to contact both their primary care provider and their health care provider who is managing their heart failure before taking or stopping any new medication including prescription, OTC, herbal, complimentary or alternative medication or supplement. Health care providers need to encourage patients to be actively engaged in their medication management.”
Dr. Page had no disclosures.
On Twitter @mitchelzoler
Investigational Wnt inhibitor shows promise in knee osteoarthritis
LONDON – Early clinical data show that a novel injectable drug holds promise for becoming the first disease-modifying osteoarthritis drug.
The results of a randomized, placebo-controlled, double-blind phase I trial involving 61 patients showed that a single intra-articular injection of SM04690 was associated with improved Western Ontario and McMaster Universities Arthritis Index (WOMAC) function and pain scores. The investigational drug also seemed to slow joint-space narrowing, compared with baseline values, with the suggestion that it may even increase joint space width.
However, those were exploratory efficacy analyses because the primary objective of the trial was to examine the safety of SM04690, a small molecule that inhibits the Wnt signaling pathway.
“The Wnt pathway has been implicated in the development of osteoarthritis [OA],” said Dr. Yusuf Yazici during a poster presentation at the European Congress of Rheumatology.
“Overactivity of Wnt signaling leads to stem cells constantly differentiating into osteoblasts, leading to osteophyte formation,” he explained, noting that Wnt signaling also stimulates the secretion of cartilage-destroying metalloproteases (Osteoarthritis Cartilage. 2012;20:162-71). “It has been very well established in the literature that if you could somehow turn that off you, could maybe improve some of the things that are happening in osteoarthritis.”
SM04690 works by “pushing the lineage fate of progenitor stem cells in the knee towards chondrocyte formation and away from osteoblast formation,” said Dr. Yazici of New York University Langone Medical Center, New York, and the chief medical officer of Samumed, the San Diego–based company developing the novel Wnt inhibitor.
He noted preclinical data had been presented orally at the EULAR congress showing that there was cartilage growth, suppressed protease production, and reduced proinflammatory cytokine (interleukin-6 and tumor necrosis factor–alpha) production.
The phase I data represent the first in-human results, with three doses of SM04690 evaluated (0.03 mg, 0.07 mg, and 0.23 mg) versus placebo in patients with moderate to severe symptomatic OA. For inclusion, patients had to have a WOMAC total score of between 36 and 72 and Kellgren-Lawrence (KL) grade 2 or 3 knee OA, and be willing to forgo pain medication for 24 hours prior to pain assessments being performed.
At baseline, the mean age of patients ranged from 60 to 64 years, their body mass index ranged from 28.7 kg/m2 to 31.4 kg/m2, and 41%-69% had KL grade 3 knee OA.
In terms of safety, the primary objective of the trial, there were no reports of serious adverse events related to the study drug. One patient who had reported increased knee pain and paroxysmal tachycardia 2 months after the injection was found to have a history of the condition, and after unblinding, none of the patients had detectable drug levels outside of the knee.
Overall, the number of adverse events was low and no different from placebo, Dr. Yazici said. The percentage of patients reporting an adverse event with the three rising doses of SM04690 were 53%, 35%, and 44%, respectively, compared with 55% of those given placebo.
WOMAC function scores for the 0.03-mg dose declined by a mean of –18.4 at week 12 and by –20.1 at week 24 from a baseline of 39.1; for 0.07 mg, by –19.5 at week 12 and by –18.9 at 24 weeks from 37.5; for 0.23 mg, by –17.8 at week 12 and by –12.4 at week 24 from 40.4; and for placebo, by –14.9 at week 12 and by –16.0 at week 24 from 34.4.
WOMAC pain scores at baseline were a respective 10.8, 10.8, 11.4, and 9.9, and the mean changes at week 12 were –4.4, –5.8, –5.7, and –4.2. At week 24, the mean declines were –5.6, –5.3, –4.3, and –4.8.
Medial joint space width was a mean of 4.5, 3.72, 3.62, and 3.74 mm at baseline in the four treatment groups, with mean changes from baseline to 24 weeks of 0.00, 0.49, –0.15, and –0.33 for the 0.03-mg, 0.07-mg, and 0.23-mg SM04690 and placebo groups, respectively.
Although the trial was not powered to detect any statistically significant differences between the active treatment dose and placebo, there was an indication that more patients treated with SM04690 than with placebo were likely to achieve an OMERACT-OARSI strict response.
These data support the ongoing phase II trial that is being conducted in 455 patients, Dr. Yazici said. The results of that trial are expected around October 2016, which should be in time for their presentation at the annual meeting of the American College of Rheumatology.
LONDON – Early clinical data show that a novel injectable drug holds promise for becoming the first disease-modifying osteoarthritis drug.
The results of a randomized, placebo-controlled, double-blind phase I trial involving 61 patients showed that a single intra-articular injection of SM04690 was associated with improved Western Ontario and McMaster Universities Arthritis Index (WOMAC) function and pain scores. The investigational drug also seemed to slow joint-space narrowing, compared with baseline values, with the suggestion that it may even increase joint space width.
However, those were exploratory efficacy analyses because the primary objective of the trial was to examine the safety of SM04690, a small molecule that inhibits the Wnt signaling pathway.
“The Wnt pathway has been implicated in the development of osteoarthritis [OA],” said Dr. Yusuf Yazici during a poster presentation at the European Congress of Rheumatology.
“Overactivity of Wnt signaling leads to stem cells constantly differentiating into osteoblasts, leading to osteophyte formation,” he explained, noting that Wnt signaling also stimulates the secretion of cartilage-destroying metalloproteases (Osteoarthritis Cartilage. 2012;20:162-71). “It has been very well established in the literature that if you could somehow turn that off you, could maybe improve some of the things that are happening in osteoarthritis.”
SM04690 works by “pushing the lineage fate of progenitor stem cells in the knee towards chondrocyte formation and away from osteoblast formation,” said Dr. Yazici of New York University Langone Medical Center, New York, and the chief medical officer of Samumed, the San Diego–based company developing the novel Wnt inhibitor.
He noted preclinical data had been presented orally at the EULAR congress showing that there was cartilage growth, suppressed protease production, and reduced proinflammatory cytokine (interleukin-6 and tumor necrosis factor–alpha) production.
The phase I data represent the first in-human results, with three doses of SM04690 evaluated (0.03 mg, 0.07 mg, and 0.23 mg) versus placebo in patients with moderate to severe symptomatic OA. For inclusion, patients had to have a WOMAC total score of between 36 and 72 and Kellgren-Lawrence (KL) grade 2 or 3 knee OA, and be willing to forgo pain medication for 24 hours prior to pain assessments being performed.
At baseline, the mean age of patients ranged from 60 to 64 years, their body mass index ranged from 28.7 kg/m2 to 31.4 kg/m2, and 41%-69% had KL grade 3 knee OA.
In terms of safety, the primary objective of the trial, there were no reports of serious adverse events related to the study drug. One patient who had reported increased knee pain and paroxysmal tachycardia 2 months after the injection was found to have a history of the condition, and after unblinding, none of the patients had detectable drug levels outside of the knee.
Overall, the number of adverse events was low and no different from placebo, Dr. Yazici said. The percentage of patients reporting an adverse event with the three rising doses of SM04690 were 53%, 35%, and 44%, respectively, compared with 55% of those given placebo.
WOMAC function scores for the 0.03-mg dose declined by a mean of –18.4 at week 12 and by –20.1 at week 24 from a baseline of 39.1; for 0.07 mg, by –19.5 at week 12 and by –18.9 at 24 weeks from 37.5; for 0.23 mg, by –17.8 at week 12 and by –12.4 at week 24 from 40.4; and for placebo, by –14.9 at week 12 and by –16.0 at week 24 from 34.4.
WOMAC pain scores at baseline were a respective 10.8, 10.8, 11.4, and 9.9, and the mean changes at week 12 were –4.4, –5.8, –5.7, and –4.2. At week 24, the mean declines were –5.6, –5.3, –4.3, and –4.8.
Medial joint space width was a mean of 4.5, 3.72, 3.62, and 3.74 mm at baseline in the four treatment groups, with mean changes from baseline to 24 weeks of 0.00, 0.49, –0.15, and –0.33 for the 0.03-mg, 0.07-mg, and 0.23-mg SM04690 and placebo groups, respectively.
Although the trial was not powered to detect any statistically significant differences between the active treatment dose and placebo, there was an indication that more patients treated with SM04690 than with placebo were likely to achieve an OMERACT-OARSI strict response.
These data support the ongoing phase II trial that is being conducted in 455 patients, Dr. Yazici said. The results of that trial are expected around October 2016, which should be in time for their presentation at the annual meeting of the American College of Rheumatology.
LONDON – Early clinical data show that a novel injectable drug holds promise for becoming the first disease-modifying osteoarthritis drug.
The results of a randomized, placebo-controlled, double-blind phase I trial involving 61 patients showed that a single intra-articular injection of SM04690 was associated with improved Western Ontario and McMaster Universities Arthritis Index (WOMAC) function and pain scores. The investigational drug also seemed to slow joint-space narrowing, compared with baseline values, with the suggestion that it may even increase joint space width.
However, those were exploratory efficacy analyses because the primary objective of the trial was to examine the safety of SM04690, a small molecule that inhibits the Wnt signaling pathway.
“The Wnt pathway has been implicated in the development of osteoarthritis [OA],” said Dr. Yusuf Yazici during a poster presentation at the European Congress of Rheumatology.
“Overactivity of Wnt signaling leads to stem cells constantly differentiating into osteoblasts, leading to osteophyte formation,” he explained, noting that Wnt signaling also stimulates the secretion of cartilage-destroying metalloproteases (Osteoarthritis Cartilage. 2012;20:162-71). “It has been very well established in the literature that if you could somehow turn that off you, could maybe improve some of the things that are happening in osteoarthritis.”
SM04690 works by “pushing the lineage fate of progenitor stem cells in the knee towards chondrocyte formation and away from osteoblast formation,” said Dr. Yazici of New York University Langone Medical Center, New York, and the chief medical officer of Samumed, the San Diego–based company developing the novel Wnt inhibitor.
He noted preclinical data had been presented orally at the EULAR congress showing that there was cartilage growth, suppressed protease production, and reduced proinflammatory cytokine (interleukin-6 and tumor necrosis factor–alpha) production.
The phase I data represent the first in-human results, with three doses of SM04690 evaluated (0.03 mg, 0.07 mg, and 0.23 mg) versus placebo in patients with moderate to severe symptomatic OA. For inclusion, patients had to have a WOMAC total score of between 36 and 72 and Kellgren-Lawrence (KL) grade 2 or 3 knee OA, and be willing to forgo pain medication for 24 hours prior to pain assessments being performed.
At baseline, the mean age of patients ranged from 60 to 64 years, their body mass index ranged from 28.7 kg/m2 to 31.4 kg/m2, and 41%-69% had KL grade 3 knee OA.
In terms of safety, the primary objective of the trial, there were no reports of serious adverse events related to the study drug. One patient who had reported increased knee pain and paroxysmal tachycardia 2 months after the injection was found to have a history of the condition, and after unblinding, none of the patients had detectable drug levels outside of the knee.
Overall, the number of adverse events was low and no different from placebo, Dr. Yazici said. The percentage of patients reporting an adverse event with the three rising doses of SM04690 were 53%, 35%, and 44%, respectively, compared with 55% of those given placebo.
WOMAC function scores for the 0.03-mg dose declined by a mean of –18.4 at week 12 and by –20.1 at week 24 from a baseline of 39.1; for 0.07 mg, by –19.5 at week 12 and by –18.9 at 24 weeks from 37.5; for 0.23 mg, by –17.8 at week 12 and by –12.4 at week 24 from 40.4; and for placebo, by –14.9 at week 12 and by –16.0 at week 24 from 34.4.
WOMAC pain scores at baseline were a respective 10.8, 10.8, 11.4, and 9.9, and the mean changes at week 12 were –4.4, –5.8, –5.7, and –4.2. At week 24, the mean declines were –5.6, –5.3, –4.3, and –4.8.
Medial joint space width was a mean of 4.5, 3.72, 3.62, and 3.74 mm at baseline in the four treatment groups, with mean changes from baseline to 24 weeks of 0.00, 0.49, –0.15, and –0.33 for the 0.03-mg, 0.07-mg, and 0.23-mg SM04690 and placebo groups, respectively.
Although the trial was not powered to detect any statistically significant differences between the active treatment dose and placebo, there was an indication that more patients treated with SM04690 than with placebo were likely to achieve an OMERACT-OARSI strict response.
These data support the ongoing phase II trial that is being conducted in 455 patients, Dr. Yazici said. The results of that trial are expected around October 2016, which should be in time for their presentation at the annual meeting of the American College of Rheumatology.
AT THE EULAR 2016 CONGRESS
Key clinical point: Early clinical data show that a novel injectable drug holds promise for becoming the first disease-modifying osteoarthritis drug.
Major finding: SM04690 was well tolerated, and exploratory efficacy analyses showed improved function, pain, and joint space width.
Data source: A multicenter, randomized, placebo-controlled, double-blind phase I trial involving 61 patients with knee osteoarthritis.
Disclosures: Dr. Yazici is chief medical officer of Samumed, the company that funded the study.
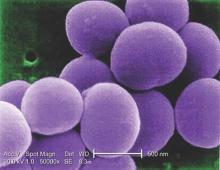
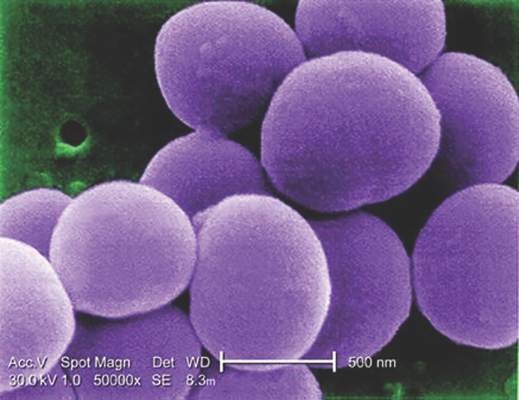
Glucocorticoids increase risk of S. aureus bacteremia
Use of systemic glucocorticoids significantly increased risk for community-acquired Staphylococcus aureus bacteremia (CA-SAB) in a dose-dependent fashion, based on data from a large Danish registry.
On average, current users of systemic glucocorticoids had an adjusted 2.5-fold increased risk of CA-SAB, compared with nonusers. The risk was most pronounced in long-term users of glucocorticoids, including patients with connective tissue disease and patients with chronic pulmonary disease. Among new users of glucocorticoids, the risk of CA-SAB was highest for patients with cancer, in the retrospective, case-control study published by Mayo Clinic Proceedings.
Dr. Jesper Smit of Aalborg (Denmark) University and his colleagues, looked at all 2,638 patients admitted with first-time CA-SAB and 26,379 matched population controls in Northern Denmark medical databases between January 1, 2000, and December 31, 2011.
New glucocorticoid users had an odds ratio for CA-SAB of 2.7, slightly higher than the OR of 2.3 for long-term users. Former glucocorticoid users had a considerably lower OR for CA-SAB of 1.3.
Risk of CA-SAB rose in a dose-dependent fashion as 90-day cumulative doses increased. For subjects taking a cumulative dose of 150 mg or less, the adjusted OR for CA-SAB was 1.3. At a cumulative dose of 500-1000 mg, OR rose to 2.4. At a cumulative dose greater than 1000 mg, OR was 6.2.
Risk did not differ based on individuals’ sex, age group, or the severity of any comorbidity.
“This is the first study to specifically investigate whether the use of glucocorticoids is associated with increased risk of CA-SAB,” the authors concluded, adding that “these results extend the current knowledge of risk factors for CA-SAB and may serve as a reminder for clinicians to carefully weigh the elevated risk against the potential beneficial effect of glucocorticoid therapy, particularly in patients with concomitant CA-SAB risk factors.”
This study was supported by grants from Heinrich Kopp, Hertha Christensen, and North Denmark Health Sciences Research foundation. The authors did not report any relevant financial disclosures.
Use of systemic glucocorticoids significantly increased risk for community-acquired Staphylococcus aureus bacteremia (CA-SAB) in a dose-dependent fashion, based on data from a large Danish registry.
On average, current users of systemic glucocorticoids had an adjusted 2.5-fold increased risk of CA-SAB, compared with nonusers. The risk was most pronounced in long-term users of glucocorticoids, including patients with connective tissue disease and patients with chronic pulmonary disease. Among new users of glucocorticoids, the risk of CA-SAB was highest for patients with cancer, in the retrospective, case-control study published by Mayo Clinic Proceedings.
Dr. Jesper Smit of Aalborg (Denmark) University and his colleagues, looked at all 2,638 patients admitted with first-time CA-SAB and 26,379 matched population controls in Northern Denmark medical databases between January 1, 2000, and December 31, 2011.
New glucocorticoid users had an odds ratio for CA-SAB of 2.7, slightly higher than the OR of 2.3 for long-term users. Former glucocorticoid users had a considerably lower OR for CA-SAB of 1.3.
Risk of CA-SAB rose in a dose-dependent fashion as 90-day cumulative doses increased. For subjects taking a cumulative dose of 150 mg or less, the adjusted OR for CA-SAB was 1.3. At a cumulative dose of 500-1000 mg, OR rose to 2.4. At a cumulative dose greater than 1000 mg, OR was 6.2.
Risk did not differ based on individuals’ sex, age group, or the severity of any comorbidity.
“This is the first study to specifically investigate whether the use of glucocorticoids is associated with increased risk of CA-SAB,” the authors concluded, adding that “these results extend the current knowledge of risk factors for CA-SAB and may serve as a reminder for clinicians to carefully weigh the elevated risk against the potential beneficial effect of glucocorticoid therapy, particularly in patients with concomitant CA-SAB risk factors.”
This study was supported by grants from Heinrich Kopp, Hertha Christensen, and North Denmark Health Sciences Research foundation. The authors did not report any relevant financial disclosures.
Use of systemic glucocorticoids significantly increased risk for community-acquired Staphylococcus aureus bacteremia (CA-SAB) in a dose-dependent fashion, based on data from a large Danish registry.
On average, current users of systemic glucocorticoids had an adjusted 2.5-fold increased risk of CA-SAB, compared with nonusers. The risk was most pronounced in long-term users of glucocorticoids, including patients with connective tissue disease and patients with chronic pulmonary disease. Among new users of glucocorticoids, the risk of CA-SAB was highest for patients with cancer, in the retrospective, case-control study published by Mayo Clinic Proceedings.
Dr. Jesper Smit of Aalborg (Denmark) University and his colleagues, looked at all 2,638 patients admitted with first-time CA-SAB and 26,379 matched population controls in Northern Denmark medical databases between January 1, 2000, and December 31, 2011.
New glucocorticoid users had an odds ratio for CA-SAB of 2.7, slightly higher than the OR of 2.3 for long-term users. Former glucocorticoid users had a considerably lower OR for CA-SAB of 1.3.
Risk of CA-SAB rose in a dose-dependent fashion as 90-day cumulative doses increased. For subjects taking a cumulative dose of 150 mg or less, the adjusted OR for CA-SAB was 1.3. At a cumulative dose of 500-1000 mg, OR rose to 2.4. At a cumulative dose greater than 1000 mg, OR was 6.2.
Risk did not differ based on individuals’ sex, age group, or the severity of any comorbidity.
“This is the first study to specifically investigate whether the use of glucocorticoids is associated with increased risk of CA-SAB,” the authors concluded, adding that “these results extend the current knowledge of risk factors for CA-SAB and may serve as a reminder for clinicians to carefully weigh the elevated risk against the potential beneficial effect of glucocorticoid therapy, particularly in patients with concomitant CA-SAB risk factors.”
This study was supported by grants from Heinrich Kopp, Hertha Christensen, and North Denmark Health Sciences Research foundation. The authors did not report any relevant financial disclosures.
FROM MAYO CLINIC PROCEEDINGS
Key clinical point: Taking glucocorticoids can significantly increase the risk of contracting community-acquired Staphylococcus aureus bacteremia (CA-SAB).
Major finding: New glucocorticoid users had an odds ratio for CA-SAB of 2.7, slightly higher than the OR of 2.3 for long-term users. Former glucocorticoid users had a considerably lower OR for CA-SAB of 1.3.
Data source: Retrospective, case-control study of all adults with first-time CA-SAB in Northern Denmark medical registries between 2000 and 2011.
Disclosures: Study supported by grants from Heinrich Kopp, Hertha Christensen, and North Denmark Health Sciences Research foundation. The authors did not report any relevant financial disclosures.
Study: TNF inhibitors improve extraintestinal IBD manifestations
SAN DIEGO – Tumor necrosis factor inhibitors improved the extraintestinal manifestations of inflammatory bowel disease (IBD) among more than half of affected patients, according to a national cohort study.
“The best response rates were for psoriasis, aphthous stomatitis, uveitis, and peripheral arthritis,” said Dr. Thomas Greuter of University Hospital in Zürich. Patients responded similarly whether they received oral infliximab or subcutaneous adalimumab or certolizumab, he noted.
IBD often is associated with debilitating disorders of the skin, joints, eyes, and hepatobiliary tract, but “due to the lack of randomized, controlled trials, the therapy of extraintestinal manifestations remains rather empirical,” Dr. Greuter said at the annual Digestive Disease Week.
To study the role of anti-TNF agents in treating these disorders, he and his associates analyzed data for 1,249 patients from the national Swiss IBD Cohort Study between 2006 and 2010. Patients were typically in their mid-30s and had lived with IBD for about 9 years, he said.
A total of 366 patients (29%) had at least one extraintestinal manifestation of IBD – most commonly peripheral arthritis (75%), followed by aphthous stomatitis (24%), and ankylosing spondylitis (22%). In all, 213 (58%) patients received at least one anti-TNF agent, and 40% received the prescription specifically for extraintestinal manifestations. Nearly two-thirds of the patients received infliximab, while 22% received adalimumab and 15% received certolizumab.
About 55% of patients improved on anti-TNF therapy over an average of 7 years of follow-up, Dr. Greuter and his associates reported. Among all three anti-TNF agents, response rates ranged from 100% for psoriasis, to 80% for erythema nodosum and stomatitis, to 73% for arthritis and uveitis, to 50% for pyoderma granulosum. Overall rates of improvement were slightly higher for infliximab than for the other two drugs, but “adalimumab and certolizumab were used mostly as a second or a third-line anti-TNF agent, and the response rate to a second or third-line treatment was lower than for the first one,” Dr. Greuter said. Some patients also received corticosteroids and immunomodulators, but excluding this subgroup had little effect on rates of response to anti-TNF therapy, he added.
Dr. Greuter also reported that 11 patients (about 5% of the cohort) developed 14 new extraintestinal manifestations after starting anti-TNF agents – usually peripheral arthritis, but also pyoderma granulosum, aphthous stomatitis, psoriasis, and uveitis. “We cannot say if this was primary, or a side effect of treatment,” he said. These disorders usually improved if patients stayed on their anti-TNF agent, he added.
About two-thirds of patients in the cohort were female, more than three-quarters had Crohn’s disease, 19% had ulcerative colitis, and 3% had indeterminate colitis, he noted.
A research grant from the Swiss National Science Foundation funded the study. Dr. Greuter had no disclosures.
SAN DIEGO – Tumor necrosis factor inhibitors improved the extraintestinal manifestations of inflammatory bowel disease (IBD) among more than half of affected patients, according to a national cohort study.
“The best response rates were for psoriasis, aphthous stomatitis, uveitis, and peripheral arthritis,” said Dr. Thomas Greuter of University Hospital in Zürich. Patients responded similarly whether they received oral infliximab or subcutaneous adalimumab or certolizumab, he noted.
IBD often is associated with debilitating disorders of the skin, joints, eyes, and hepatobiliary tract, but “due to the lack of randomized, controlled trials, the therapy of extraintestinal manifestations remains rather empirical,” Dr. Greuter said at the annual Digestive Disease Week.
To study the role of anti-TNF agents in treating these disorders, he and his associates analyzed data for 1,249 patients from the national Swiss IBD Cohort Study between 2006 and 2010. Patients were typically in their mid-30s and had lived with IBD for about 9 years, he said.
A total of 366 patients (29%) had at least one extraintestinal manifestation of IBD – most commonly peripheral arthritis (75%), followed by aphthous stomatitis (24%), and ankylosing spondylitis (22%). In all, 213 (58%) patients received at least one anti-TNF agent, and 40% received the prescription specifically for extraintestinal manifestations. Nearly two-thirds of the patients received infliximab, while 22% received adalimumab and 15% received certolizumab.
About 55% of patients improved on anti-TNF therapy over an average of 7 years of follow-up, Dr. Greuter and his associates reported. Among all three anti-TNF agents, response rates ranged from 100% for psoriasis, to 80% for erythema nodosum and stomatitis, to 73% for arthritis and uveitis, to 50% for pyoderma granulosum. Overall rates of improvement were slightly higher for infliximab than for the other two drugs, but “adalimumab and certolizumab were used mostly as a second or a third-line anti-TNF agent, and the response rate to a second or third-line treatment was lower than for the first one,” Dr. Greuter said. Some patients also received corticosteroids and immunomodulators, but excluding this subgroup had little effect on rates of response to anti-TNF therapy, he added.
Dr. Greuter also reported that 11 patients (about 5% of the cohort) developed 14 new extraintestinal manifestations after starting anti-TNF agents – usually peripheral arthritis, but also pyoderma granulosum, aphthous stomatitis, psoriasis, and uveitis. “We cannot say if this was primary, or a side effect of treatment,” he said. These disorders usually improved if patients stayed on their anti-TNF agent, he added.
About two-thirds of patients in the cohort were female, more than three-quarters had Crohn’s disease, 19% had ulcerative colitis, and 3% had indeterminate colitis, he noted.
A research grant from the Swiss National Science Foundation funded the study. Dr. Greuter had no disclosures.
SAN DIEGO – Tumor necrosis factor inhibitors improved the extraintestinal manifestations of inflammatory bowel disease (IBD) among more than half of affected patients, according to a national cohort study.
“The best response rates were for psoriasis, aphthous stomatitis, uveitis, and peripheral arthritis,” said Dr. Thomas Greuter of University Hospital in Zürich. Patients responded similarly whether they received oral infliximab or subcutaneous adalimumab or certolizumab, he noted.
IBD often is associated with debilitating disorders of the skin, joints, eyes, and hepatobiliary tract, but “due to the lack of randomized, controlled trials, the therapy of extraintestinal manifestations remains rather empirical,” Dr. Greuter said at the annual Digestive Disease Week.
To study the role of anti-TNF agents in treating these disorders, he and his associates analyzed data for 1,249 patients from the national Swiss IBD Cohort Study between 2006 and 2010. Patients were typically in their mid-30s and had lived with IBD for about 9 years, he said.
A total of 366 patients (29%) had at least one extraintestinal manifestation of IBD – most commonly peripheral arthritis (75%), followed by aphthous stomatitis (24%), and ankylosing spondylitis (22%). In all, 213 (58%) patients received at least one anti-TNF agent, and 40% received the prescription specifically for extraintestinal manifestations. Nearly two-thirds of the patients received infliximab, while 22% received adalimumab and 15% received certolizumab.
About 55% of patients improved on anti-TNF therapy over an average of 7 years of follow-up, Dr. Greuter and his associates reported. Among all three anti-TNF agents, response rates ranged from 100% for psoriasis, to 80% for erythema nodosum and stomatitis, to 73% for arthritis and uveitis, to 50% for pyoderma granulosum. Overall rates of improvement were slightly higher for infliximab than for the other two drugs, but “adalimumab and certolizumab were used mostly as a second or a third-line anti-TNF agent, and the response rate to a second or third-line treatment was lower than for the first one,” Dr. Greuter said. Some patients also received corticosteroids and immunomodulators, but excluding this subgroup had little effect on rates of response to anti-TNF therapy, he added.
Dr. Greuter also reported that 11 patients (about 5% of the cohort) developed 14 new extraintestinal manifestations after starting anti-TNF agents – usually peripheral arthritis, but also pyoderma granulosum, aphthous stomatitis, psoriasis, and uveitis. “We cannot say if this was primary, or a side effect of treatment,” he said. These disorders usually improved if patients stayed on their anti-TNF agent, he added.
About two-thirds of patients in the cohort were female, more than three-quarters had Crohn’s disease, 19% had ulcerative colitis, and 3% had indeterminate colitis, he noted.
A research grant from the Swiss National Science Foundation funded the study. Dr. Greuter had no disclosures.
AT DDW® 2016
Key clinical point: Tumor necrosis factor inhibitors improved the extraintestinal manifestations of inflammatory bowel disease among more than half of affected patients.
Major finding: About 55% of patients who received infliximab, adalimumab, or certolizumab had a clinical response.
Data source: A study of 1,249 patients with IBD from a national cohort.
Disclosures: A research grant from the Swiss National Science Foundation funded the study. Dr. Greuter had no disclosures.
Jury still out on mortality benefits of knee replacement in OA
People with osteoarthritis who go on to have a total or partial knee replacement do not appear to have an increased risk of all-cause mortality, but the jury is still out on whether they gain any improvement, a study showed.
In their research published in the Annals of the Rheumatic Diseases [2016 May 17. doi: 10.1136/annrheumdis-2016-209167], Dr. Devyani Misra of Boston University and colleagues noted that knee replacement (KR) was thought to decrease long-term mortality risk because of the relief from pain and improvement in function that typically comes with surgery. However, studies on the topic had been conflicting, largely because of the challenges associated with studying mortality with KR surgery in observational settings.
In the current study the research team sought to evaluate the relation of KR to the risk of all-cause mortality among subjects with knee OA, while at the same time giving particular attention to “potential sources of confounding bias that may account for [the] effect of KR on mortality.”
Using patient data from the U.K. primary care electronic database THIN, the investigators compared the risk of mortality among 14,042 subjects who had OA, were aged 50-89 years old, and had had or had not had KR.
They discovered a strong protective effect of KR on all-cause long-term mortality risk, particularly among the adults over 63 years of age.
For example, people who had undergone KR had a 28% lower risk of mortality than did non-KR subjects (hazard ratio, 0.72; 95% confidence interval, 0.66-0.78).
In the overall propensity score–matched study sample, crude mortality per 1,000 person-years (total person-years) for the KR and non-KR cohorts were 19 (61,015) and 25 (58,294), respectively.
However, despite their best efforts, the researchers said the results showed evidence of residual confounding.
“For example, the observation of improved survival immediately after KR, despite the expectation of potential short-term increased postoperative mortality risk supports the presence of residual confounding,” they wrote.
Another finding suggestive of confounding was that the protective effect was seen only in older patients (over 63) when the authors stratified study participants by age.
“While it is possible that survival benefit seen in older patients with KR is a true effect because it is in this group that greater physical activity is particularly important to survival, more likely it is a result of residual confounding because subject selection is rigorous in this age group due to vulnerability,” the authors wrote.
They concluded that knee replacement “did not appear to be associated with an increased risk of all-cause mortality.”
“While we cannot rule out that KR may potentially reduce the risk of mortality over the long term, the true extent of that potential benefit is difficult to discern due to confounding by indication in observational studies using administrative data or electronic health records,” they added.
This study was funded by the Arthritis Foundation Postdoctoral Fellowship Award, the ACR Rheumatology Research Foundation Investigator Award, and a Boston University scholarship grant.
People with osteoarthritis who go on to have a total or partial knee replacement do not appear to have an increased risk of all-cause mortality, but the jury is still out on whether they gain any improvement, a study showed.
In their research published in the Annals of the Rheumatic Diseases [2016 May 17. doi: 10.1136/annrheumdis-2016-209167], Dr. Devyani Misra of Boston University and colleagues noted that knee replacement (KR) was thought to decrease long-term mortality risk because of the relief from pain and improvement in function that typically comes with surgery. However, studies on the topic had been conflicting, largely because of the challenges associated with studying mortality with KR surgery in observational settings.
In the current study the research team sought to evaluate the relation of KR to the risk of all-cause mortality among subjects with knee OA, while at the same time giving particular attention to “potential sources of confounding bias that may account for [the] effect of KR on mortality.”
Using patient data from the U.K. primary care electronic database THIN, the investigators compared the risk of mortality among 14,042 subjects who had OA, were aged 50-89 years old, and had had or had not had KR.
They discovered a strong protective effect of KR on all-cause long-term mortality risk, particularly among the adults over 63 years of age.
For example, people who had undergone KR had a 28% lower risk of mortality than did non-KR subjects (hazard ratio, 0.72; 95% confidence interval, 0.66-0.78).
In the overall propensity score–matched study sample, crude mortality per 1,000 person-years (total person-years) for the KR and non-KR cohorts were 19 (61,015) and 25 (58,294), respectively.
However, despite their best efforts, the researchers said the results showed evidence of residual confounding.
“For example, the observation of improved survival immediately after KR, despite the expectation of potential short-term increased postoperative mortality risk supports the presence of residual confounding,” they wrote.
Another finding suggestive of confounding was that the protective effect was seen only in older patients (over 63) when the authors stratified study participants by age.
“While it is possible that survival benefit seen in older patients with KR is a true effect because it is in this group that greater physical activity is particularly important to survival, more likely it is a result of residual confounding because subject selection is rigorous in this age group due to vulnerability,” the authors wrote.
They concluded that knee replacement “did not appear to be associated with an increased risk of all-cause mortality.”
“While we cannot rule out that KR may potentially reduce the risk of mortality over the long term, the true extent of that potential benefit is difficult to discern due to confounding by indication in observational studies using administrative data or electronic health records,” they added.
This study was funded by the Arthritis Foundation Postdoctoral Fellowship Award, the ACR Rheumatology Research Foundation Investigator Award, and a Boston University scholarship grant.
People with osteoarthritis who go on to have a total or partial knee replacement do not appear to have an increased risk of all-cause mortality, but the jury is still out on whether they gain any improvement, a study showed.
In their research published in the Annals of the Rheumatic Diseases [2016 May 17. doi: 10.1136/annrheumdis-2016-209167], Dr. Devyani Misra of Boston University and colleagues noted that knee replacement (KR) was thought to decrease long-term mortality risk because of the relief from pain and improvement in function that typically comes with surgery. However, studies on the topic had been conflicting, largely because of the challenges associated with studying mortality with KR surgery in observational settings.
In the current study the research team sought to evaluate the relation of KR to the risk of all-cause mortality among subjects with knee OA, while at the same time giving particular attention to “potential sources of confounding bias that may account for [the] effect of KR on mortality.”
Using patient data from the U.K. primary care electronic database THIN, the investigators compared the risk of mortality among 14,042 subjects who had OA, were aged 50-89 years old, and had had or had not had KR.
They discovered a strong protective effect of KR on all-cause long-term mortality risk, particularly among the adults over 63 years of age.
For example, people who had undergone KR had a 28% lower risk of mortality than did non-KR subjects (hazard ratio, 0.72; 95% confidence interval, 0.66-0.78).
In the overall propensity score–matched study sample, crude mortality per 1,000 person-years (total person-years) for the KR and non-KR cohorts were 19 (61,015) and 25 (58,294), respectively.
However, despite their best efforts, the researchers said the results showed evidence of residual confounding.
“For example, the observation of improved survival immediately after KR, despite the expectation of potential short-term increased postoperative mortality risk supports the presence of residual confounding,” they wrote.
Another finding suggestive of confounding was that the protective effect was seen only in older patients (over 63) when the authors stratified study participants by age.
“While it is possible that survival benefit seen in older patients with KR is a true effect because it is in this group that greater physical activity is particularly important to survival, more likely it is a result of residual confounding because subject selection is rigorous in this age group due to vulnerability,” the authors wrote.
They concluded that knee replacement “did not appear to be associated with an increased risk of all-cause mortality.”
“While we cannot rule out that KR may potentially reduce the risk of mortality over the long term, the true extent of that potential benefit is difficult to discern due to confounding by indication in observational studies using administrative data or electronic health records,” they added.
This study was funded by the Arthritis Foundation Postdoctoral Fellowship Award, the ACR Rheumatology Research Foundation Investigator Award, and a Boston University scholarship grant.
FROM ANNALS OF THE RHEUMATIC DISEASES
Key clinical point:Knee replacement surgery in people with OA showed a protective effect on mortality, but residual confounding in the study makes it challenging to definitively conclude whether the surgery conferred a long-term mortality benefit.
Major finding: Subjects who had undergone a knee replacement had a 28% lower risk of mortality than non-KR subjects (HR, 0.72; 95% CI, 0.66-0.78).
Data source: Population-based time-varying propensity score–matched cohort of 14,042 subjects with OA aged 50-89 years with and without knee replacement.
Disclosures: This study was funded by the Arthritis Foundation Postdoctoral Fellowship Award, the ACR Rheumatology Research Foundation Investigator Award, and a Boston University scholarship grant.
Anti-TNF agents may slow erosive hand osteoarthritis
LONDON – Tumor necrosis factor may play a role in erosive hand osteoarthritis, and treatments such as etanercept that target this cytokine may help prevent progression of the condition, according to the results of two studies presented at the European Congress of Rheumatology.
In one of the studies, immunoscintigraphic detection of radiolabeled certolizumab pegol was used to show that tumor necrosis factor (TNF) was present in swollen finger joints. In another study, researchers looked to see if treatment with etanercept would have any specific effects on the joints of patients with erosive hand osteoarthritis (OA) and performed a separate analysis of the potential effect on synovitis and the effect on bone marrow lesions.
“We previously had the idea that TNF is an important cytokine in the pathogenesis of erosive [hand] osteoarthritis; but there have been no animal studies, and it’s very difficult to take biopsies or fluid aspiration from these small finger joints,” Dr. Ruth Wittoek, a staff rheumatologist at Ghent University Hospital in Belgium and a coauthor of all three studies, explained in a precongress interview. “We needed to look for other possibilities to really identify the presence of TNF in those affected joints.”
Dr. Wittoek and her associates used immunoscintigraphy to take static images of both hands of five patients with erosive OA immediately (less than 15 minutes) after administration of radiolabeled certolizumab pegol (early phase) and 4-6 hours following the injection (late phase).
The patients studied had erosive OA for a median of 8.4 years, and their median age was 55.6 years. All patients underwent clinical examination for presence of tenderness and palpable swelling of the joints and ultrasound 1 day prior to undergoing immunoscintigraphy.
All 18 interphalangeal (IP) finger joints were scored according to the anatomical phase scoring system on x-ray, and 90 IP finger joints were studied in total. The uptake of radiolabeled certolizumab pegol was semiquantitatively described as being absent, weak, or strong.
During the early phase following administration, uptake of the radiolabeled TNF inhibitor was seen in seven (7.8%) joints, although the uptake was described as weak in all cases. The radiolabeled TNF inhibitor was seen in 24 (26.7%) joints during the late phase following administration, with five instances described as strong uptake and the remaining 19 instances being weak uptake. No uptake of the radiolabeled TNF inhibitor was seen in metacarpophalangeal joints.
Uptake of the radiolabeled TNF inhibitor was linked to signs of disease activity, including tender, swollen, and radiographically active joints.
Late uptake was present in 12 (36.4%) of 33 tender joints and in 12 (21.1%) of 57 nontender joints (odds ratio, 2.1; 95% confidence interval, 0.8-5.6; P was nonsignificant).
The relationship was most pronounced with palpable joint swelling: Late uptake was present in 14 (61%) of 23 swollen joints and 10 (14.9%) of 67 nonswollen joints (OR, 8.9; 95% CI, 3.3-26.0; P less than .001).
Late uptake was present in 18 (29%) of 62 sonographically active joints (defined as any presence of effusion or synovial proliferation) but just 6 (21.4%) of 28 noninflamed joints (OR, 1.5; 95% CI, 0.5-4.3; P was nonsignificant).
Uptake of the radiolabeled TNF inhibitor was observed in all anatomical phases of erosive hand OA, Dr. Wittoek noted, but the strongest association was found during the final remodeling (R) phase.
“Soft-tissue swelling strongly correlated with uptake of certolizumab, meaning in these joints a lot of TNF was present,” Dr. Wittoek said. “These data further solidify the rationale for cytokine-directed therapies in erosive OA.”
Although the data provide proof of concept that TNF may be involved in erosive hand OA, the lack of a control tracer was noted as a limitation of the study after the investigator’s presentation. Dr. Wittoek conceded that it would be interesting to examine that in a future study.
So, if TNF is present, what effect does anti-TNF therapy have on the joints in erosive hand OA?
That question was addressed in a multicenter, double-blind, randomized, placebo-controlled trial involving 90 patients who were randomized to receive either 50 mg of subcutaneous etanercept weekly for 24 weeks, then 25 mg weekly for the remainder of 1 year (45 patients), or placebo (45 patients). Participants were a mean age of 60 years, 81% were women, and 96% fulfilled the American College of Rheumatology hand OA criteria.
“Synovial inflammation is often present in erosive hand OA; moreover, synovitis is associated with pain and with structural damage after around 2 and a half years,” the lead study author, Dr. Margreet Kloppenburg, a professor of rheumatology at Leiden University Medical Centre in the Netherlands, said in an interview.
“Therefore, we wanted to know whether blocking of synovial inflammation by a well-known drug such as etanercept would also have a positive effect on outcomes in erosive hand OA,” Dr. Kloppenburg explained.
The primary outcome measure was the level of OA pain assessed on a visual analog scale (VAS) at 24 weeks.
Secondary endpoints included assessment of hand function, quality of life, the number of tender joints, and grip strength after 4, 8, 12, 24, and 36 weeks, and after 1 year. Radiographic progression of IP joints was scored blindly at baseline, 24 weeks, and 1 year following the quantitative Ghent University Scoring System (GUSS). VAS pain was compared between treatment groups at 24 weeks and 1 year in intention-to-treat analyses.
Although etanercept was not superior to placebo on VAS pain at 24 weeks, it was superior to placebo both on pain and structural damage assessed by GUSS in the symptomatic and inflammatory patients who completed the study. The drug was especially effective in joints with signs of inflammation.
Overall, VAS pain in all patients decreased by 24.8 mm (95% CI, –29.2 to –20.5; P less than .001) at 24 weeks. In intention-to-treat analysis, differences in pain between the groups were in favor of etanercept but did not reach statistical significance. The per-protocol analysis of GUSS showed a mean difference in favor of etanercept, indicating more remodeling in the etanercept group.
Additional analyses showed an interaction between soft swelling/erythema and etanercept treatment on GUSS, resulting in a statistically significant (P less than .05) mean difference between the two treatment groups. More patients dropped out on placebo than on etanercept (six vs. three) because of inefficacy, whereas more dropped out on etanercept than on placebo (six vs. one) because of adverse effects.
“Synovial inflammation is an interesting target for treatment in OA patients with an inflammatory hand osteoarthritis phenotype,” Dr. Kloppenburg said.
A separate analysis of the same multicenter study suggests that etanercept is effective in inhibiting bone marrow lesions (BMLs) in patients with erosive hand OA.
The researchers studied 20 participants with symptomatic erosive OA with clinical and ultrasonographic signs of inflammation in at least one IP joint. The patients underwent contrast-enhanced MRI of the eight distal and proximal IP joints of one hand at baseline and 1 year. Images were scored for synovitis and BMLs (0-3 per joint, total score 0-24), blinded for patient characteristics.
Radiographs of the same hand were scored according to the Verbruggen-Veys system. Logistic regression was used to associate the presence of an MRI feature in a joint with being in an erosive versus nonerosive anatomical phase.
“Although erosive hand OA is a condition with a high disease burden, no disease-modifying treatments are available yet,” explained Féline Kroon, a PhD student in the department of rheumatology at Leiden University Medical Center in the Netherlands, in an interview. “Research to find a new form of therapy is partly hindered by our limited knowledge on the pathophysiology of the disease.”
However, “new imaging modalities like MRI enable us to study the pathophysiology of erosive OA more closely,” she said. “This study also gave us the unique opportunity to investigate whether anti-TNF, which is known to lead to clinical improvement and improvement of inflammatory lesions on MRI in other rheumatic diseases like rheumatoid arthritis, might also be effective in erosive OA.”
The presence of BMLs, but not synovitis, was associated with the presence of an erosive anatomical phase in a joint, and treatment with etanercept appeared to be effective in inhibiting these lesions. That suggests a role for TNF in the pathophysiology of erosive OA.
The inhibitory effect of etanercept on BMLs was more pronounced in IP joints with severe synovitis at baseline, suggesting that inflamed synovial tissue could be a source of TNF production in erosive OA.
The total synovitis score was similar at baseline and 1 year in both study groups. For BMLs, the median total score at baseline was 5.4 (range 2-9) and 7.0 (0-9) at 1 year in the placebo group, versus 4.5 (3-9) and 3.7 (0-8), respectively, in the etanercept group.
The presence of BMLs was associated with being in the erosive and remodeling anatomical phases of erosive hand OA. Synovitis was not associated with those phases.
“We think that TNF-alpha plays a role in the pathophysiology of erosive OA via an effect on the subchondral bone,” Ms. Kroon said. “Because we saw that the beneficial effect of etanercept on BMLs was more pronounced in joints with synovitis at baseline, we think that, in an inflamed synovial hand joint, an interaction takes place between synovium and subchondral bone, which could be influenced by blocking TNF.”
Pfizer and UCB supported the investigator-initiated studies and provided the study drugs. Dr. Kloppenburg has received lecturing, consultancy, and investigator fees or grants from AbbVie, APPROACH, GlaxoSmithKline, Levicept, Pfizer, Servier, and UCB, all paid to her institution. All other authors declared no conflicts of interests.
LONDON – Tumor necrosis factor may play a role in erosive hand osteoarthritis, and treatments such as etanercept that target this cytokine may help prevent progression of the condition, according to the results of two studies presented at the European Congress of Rheumatology.
In one of the studies, immunoscintigraphic detection of radiolabeled certolizumab pegol was used to show that tumor necrosis factor (TNF) was present in swollen finger joints. In another study, researchers looked to see if treatment with etanercept would have any specific effects on the joints of patients with erosive hand osteoarthritis (OA) and performed a separate analysis of the potential effect on synovitis and the effect on bone marrow lesions.
“We previously had the idea that TNF is an important cytokine in the pathogenesis of erosive [hand] osteoarthritis; but there have been no animal studies, and it’s very difficult to take biopsies or fluid aspiration from these small finger joints,” Dr. Ruth Wittoek, a staff rheumatologist at Ghent University Hospital in Belgium and a coauthor of all three studies, explained in a precongress interview. “We needed to look for other possibilities to really identify the presence of TNF in those affected joints.”
Dr. Wittoek and her associates used immunoscintigraphy to take static images of both hands of five patients with erosive OA immediately (less than 15 minutes) after administration of radiolabeled certolizumab pegol (early phase) and 4-6 hours following the injection (late phase).
The patients studied had erosive OA for a median of 8.4 years, and their median age was 55.6 years. All patients underwent clinical examination for presence of tenderness and palpable swelling of the joints and ultrasound 1 day prior to undergoing immunoscintigraphy.
All 18 interphalangeal (IP) finger joints were scored according to the anatomical phase scoring system on x-ray, and 90 IP finger joints were studied in total. The uptake of radiolabeled certolizumab pegol was semiquantitatively described as being absent, weak, or strong.
During the early phase following administration, uptake of the radiolabeled TNF inhibitor was seen in seven (7.8%) joints, although the uptake was described as weak in all cases. The radiolabeled TNF inhibitor was seen in 24 (26.7%) joints during the late phase following administration, with five instances described as strong uptake and the remaining 19 instances being weak uptake. No uptake of the radiolabeled TNF inhibitor was seen in metacarpophalangeal joints.
Uptake of the radiolabeled TNF inhibitor was linked to signs of disease activity, including tender, swollen, and radiographically active joints.
Late uptake was present in 12 (36.4%) of 33 tender joints and in 12 (21.1%) of 57 nontender joints (odds ratio, 2.1; 95% confidence interval, 0.8-5.6; P was nonsignificant).
The relationship was most pronounced with palpable joint swelling: Late uptake was present in 14 (61%) of 23 swollen joints and 10 (14.9%) of 67 nonswollen joints (OR, 8.9; 95% CI, 3.3-26.0; P less than .001).
Late uptake was present in 18 (29%) of 62 sonographically active joints (defined as any presence of effusion or synovial proliferation) but just 6 (21.4%) of 28 noninflamed joints (OR, 1.5; 95% CI, 0.5-4.3; P was nonsignificant).
Uptake of the radiolabeled TNF inhibitor was observed in all anatomical phases of erosive hand OA, Dr. Wittoek noted, but the strongest association was found during the final remodeling (R) phase.
“Soft-tissue swelling strongly correlated with uptake of certolizumab, meaning in these joints a lot of TNF was present,” Dr. Wittoek said. “These data further solidify the rationale for cytokine-directed therapies in erosive OA.”
Although the data provide proof of concept that TNF may be involved in erosive hand OA, the lack of a control tracer was noted as a limitation of the study after the investigator’s presentation. Dr. Wittoek conceded that it would be interesting to examine that in a future study.
So, if TNF is present, what effect does anti-TNF therapy have on the joints in erosive hand OA?
That question was addressed in a multicenter, double-blind, randomized, placebo-controlled trial involving 90 patients who were randomized to receive either 50 mg of subcutaneous etanercept weekly for 24 weeks, then 25 mg weekly for the remainder of 1 year (45 patients), or placebo (45 patients). Participants were a mean age of 60 years, 81% were women, and 96% fulfilled the American College of Rheumatology hand OA criteria.
“Synovial inflammation is often present in erosive hand OA; moreover, synovitis is associated with pain and with structural damage after around 2 and a half years,” the lead study author, Dr. Margreet Kloppenburg, a professor of rheumatology at Leiden University Medical Centre in the Netherlands, said in an interview.
“Therefore, we wanted to know whether blocking of synovial inflammation by a well-known drug such as etanercept would also have a positive effect on outcomes in erosive hand OA,” Dr. Kloppenburg explained.
The primary outcome measure was the level of OA pain assessed on a visual analog scale (VAS) at 24 weeks.
Secondary endpoints included assessment of hand function, quality of life, the number of tender joints, and grip strength after 4, 8, 12, 24, and 36 weeks, and after 1 year. Radiographic progression of IP joints was scored blindly at baseline, 24 weeks, and 1 year following the quantitative Ghent University Scoring System (GUSS). VAS pain was compared between treatment groups at 24 weeks and 1 year in intention-to-treat analyses.
Although etanercept was not superior to placebo on VAS pain at 24 weeks, it was superior to placebo both on pain and structural damage assessed by GUSS in the symptomatic and inflammatory patients who completed the study. The drug was especially effective in joints with signs of inflammation.
Overall, VAS pain in all patients decreased by 24.8 mm (95% CI, –29.2 to –20.5; P less than .001) at 24 weeks. In intention-to-treat analysis, differences in pain between the groups were in favor of etanercept but did not reach statistical significance. The per-protocol analysis of GUSS showed a mean difference in favor of etanercept, indicating more remodeling in the etanercept group.
Additional analyses showed an interaction between soft swelling/erythema and etanercept treatment on GUSS, resulting in a statistically significant (P less than .05) mean difference between the two treatment groups. More patients dropped out on placebo than on etanercept (six vs. three) because of inefficacy, whereas more dropped out on etanercept than on placebo (six vs. one) because of adverse effects.
“Synovial inflammation is an interesting target for treatment in OA patients with an inflammatory hand osteoarthritis phenotype,” Dr. Kloppenburg said.
A separate analysis of the same multicenter study suggests that etanercept is effective in inhibiting bone marrow lesions (BMLs) in patients with erosive hand OA.
The researchers studied 20 participants with symptomatic erosive OA with clinical and ultrasonographic signs of inflammation in at least one IP joint. The patients underwent contrast-enhanced MRI of the eight distal and proximal IP joints of one hand at baseline and 1 year. Images were scored for synovitis and BMLs (0-3 per joint, total score 0-24), blinded for patient characteristics.
Radiographs of the same hand were scored according to the Verbruggen-Veys system. Logistic regression was used to associate the presence of an MRI feature in a joint with being in an erosive versus nonerosive anatomical phase.
“Although erosive hand OA is a condition with a high disease burden, no disease-modifying treatments are available yet,” explained Féline Kroon, a PhD student in the department of rheumatology at Leiden University Medical Center in the Netherlands, in an interview. “Research to find a new form of therapy is partly hindered by our limited knowledge on the pathophysiology of the disease.”
However, “new imaging modalities like MRI enable us to study the pathophysiology of erosive OA more closely,” she said. “This study also gave us the unique opportunity to investigate whether anti-TNF, which is known to lead to clinical improvement and improvement of inflammatory lesions on MRI in other rheumatic diseases like rheumatoid arthritis, might also be effective in erosive OA.”
The presence of BMLs, but not synovitis, was associated with the presence of an erosive anatomical phase in a joint, and treatment with etanercept appeared to be effective in inhibiting these lesions. That suggests a role for TNF in the pathophysiology of erosive OA.
The inhibitory effect of etanercept on BMLs was more pronounced in IP joints with severe synovitis at baseline, suggesting that inflamed synovial tissue could be a source of TNF production in erosive OA.
The total synovitis score was similar at baseline and 1 year in both study groups. For BMLs, the median total score at baseline was 5.4 (range 2-9) and 7.0 (0-9) at 1 year in the placebo group, versus 4.5 (3-9) and 3.7 (0-8), respectively, in the etanercept group.
The presence of BMLs was associated with being in the erosive and remodeling anatomical phases of erosive hand OA. Synovitis was not associated with those phases.
“We think that TNF-alpha plays a role in the pathophysiology of erosive OA via an effect on the subchondral bone,” Ms. Kroon said. “Because we saw that the beneficial effect of etanercept on BMLs was more pronounced in joints with synovitis at baseline, we think that, in an inflamed synovial hand joint, an interaction takes place between synovium and subchondral bone, which could be influenced by blocking TNF.”
Pfizer and UCB supported the investigator-initiated studies and provided the study drugs. Dr. Kloppenburg has received lecturing, consultancy, and investigator fees or grants from AbbVie, APPROACH, GlaxoSmithKline, Levicept, Pfizer, Servier, and UCB, all paid to her institution. All other authors declared no conflicts of interests.
LONDON – Tumor necrosis factor may play a role in erosive hand osteoarthritis, and treatments such as etanercept that target this cytokine may help prevent progression of the condition, according to the results of two studies presented at the European Congress of Rheumatology.
In one of the studies, immunoscintigraphic detection of radiolabeled certolizumab pegol was used to show that tumor necrosis factor (TNF) was present in swollen finger joints. In another study, researchers looked to see if treatment with etanercept would have any specific effects on the joints of patients with erosive hand osteoarthritis (OA) and performed a separate analysis of the potential effect on synovitis and the effect on bone marrow lesions.
“We previously had the idea that TNF is an important cytokine in the pathogenesis of erosive [hand] osteoarthritis; but there have been no animal studies, and it’s very difficult to take biopsies or fluid aspiration from these small finger joints,” Dr. Ruth Wittoek, a staff rheumatologist at Ghent University Hospital in Belgium and a coauthor of all three studies, explained in a precongress interview. “We needed to look for other possibilities to really identify the presence of TNF in those affected joints.”
Dr. Wittoek and her associates used immunoscintigraphy to take static images of both hands of five patients with erosive OA immediately (less than 15 minutes) after administration of radiolabeled certolizumab pegol (early phase) and 4-6 hours following the injection (late phase).
The patients studied had erosive OA for a median of 8.4 years, and their median age was 55.6 years. All patients underwent clinical examination for presence of tenderness and palpable swelling of the joints and ultrasound 1 day prior to undergoing immunoscintigraphy.
All 18 interphalangeal (IP) finger joints were scored according to the anatomical phase scoring system on x-ray, and 90 IP finger joints were studied in total. The uptake of radiolabeled certolizumab pegol was semiquantitatively described as being absent, weak, or strong.
During the early phase following administration, uptake of the radiolabeled TNF inhibitor was seen in seven (7.8%) joints, although the uptake was described as weak in all cases. The radiolabeled TNF inhibitor was seen in 24 (26.7%) joints during the late phase following administration, with five instances described as strong uptake and the remaining 19 instances being weak uptake. No uptake of the radiolabeled TNF inhibitor was seen in metacarpophalangeal joints.
Uptake of the radiolabeled TNF inhibitor was linked to signs of disease activity, including tender, swollen, and radiographically active joints.
Late uptake was present in 12 (36.4%) of 33 tender joints and in 12 (21.1%) of 57 nontender joints (odds ratio, 2.1; 95% confidence interval, 0.8-5.6; P was nonsignificant).
The relationship was most pronounced with palpable joint swelling: Late uptake was present in 14 (61%) of 23 swollen joints and 10 (14.9%) of 67 nonswollen joints (OR, 8.9; 95% CI, 3.3-26.0; P less than .001).
Late uptake was present in 18 (29%) of 62 sonographically active joints (defined as any presence of effusion or synovial proliferation) but just 6 (21.4%) of 28 noninflamed joints (OR, 1.5; 95% CI, 0.5-4.3; P was nonsignificant).
Uptake of the radiolabeled TNF inhibitor was observed in all anatomical phases of erosive hand OA, Dr. Wittoek noted, but the strongest association was found during the final remodeling (R) phase.
“Soft-tissue swelling strongly correlated with uptake of certolizumab, meaning in these joints a lot of TNF was present,” Dr. Wittoek said. “These data further solidify the rationale for cytokine-directed therapies in erosive OA.”
Although the data provide proof of concept that TNF may be involved in erosive hand OA, the lack of a control tracer was noted as a limitation of the study after the investigator’s presentation. Dr. Wittoek conceded that it would be interesting to examine that in a future study.
So, if TNF is present, what effect does anti-TNF therapy have on the joints in erosive hand OA?
That question was addressed in a multicenter, double-blind, randomized, placebo-controlled trial involving 90 patients who were randomized to receive either 50 mg of subcutaneous etanercept weekly for 24 weeks, then 25 mg weekly for the remainder of 1 year (45 patients), or placebo (45 patients). Participants were a mean age of 60 years, 81% were women, and 96% fulfilled the American College of Rheumatology hand OA criteria.
“Synovial inflammation is often present in erosive hand OA; moreover, synovitis is associated with pain and with structural damage after around 2 and a half years,” the lead study author, Dr. Margreet Kloppenburg, a professor of rheumatology at Leiden University Medical Centre in the Netherlands, said in an interview.
“Therefore, we wanted to know whether blocking of synovial inflammation by a well-known drug such as etanercept would also have a positive effect on outcomes in erosive hand OA,” Dr. Kloppenburg explained.
The primary outcome measure was the level of OA pain assessed on a visual analog scale (VAS) at 24 weeks.
Secondary endpoints included assessment of hand function, quality of life, the number of tender joints, and grip strength after 4, 8, 12, 24, and 36 weeks, and after 1 year. Radiographic progression of IP joints was scored blindly at baseline, 24 weeks, and 1 year following the quantitative Ghent University Scoring System (GUSS). VAS pain was compared between treatment groups at 24 weeks and 1 year in intention-to-treat analyses.
Although etanercept was not superior to placebo on VAS pain at 24 weeks, it was superior to placebo both on pain and structural damage assessed by GUSS in the symptomatic and inflammatory patients who completed the study. The drug was especially effective in joints with signs of inflammation.
Overall, VAS pain in all patients decreased by 24.8 mm (95% CI, –29.2 to –20.5; P less than .001) at 24 weeks. In intention-to-treat analysis, differences in pain between the groups were in favor of etanercept but did not reach statistical significance. The per-protocol analysis of GUSS showed a mean difference in favor of etanercept, indicating more remodeling in the etanercept group.
Additional analyses showed an interaction between soft swelling/erythema and etanercept treatment on GUSS, resulting in a statistically significant (P less than .05) mean difference between the two treatment groups. More patients dropped out on placebo than on etanercept (six vs. three) because of inefficacy, whereas more dropped out on etanercept than on placebo (six vs. one) because of adverse effects.
“Synovial inflammation is an interesting target for treatment in OA patients with an inflammatory hand osteoarthritis phenotype,” Dr. Kloppenburg said.
A separate analysis of the same multicenter study suggests that etanercept is effective in inhibiting bone marrow lesions (BMLs) in patients with erosive hand OA.
The researchers studied 20 participants with symptomatic erosive OA with clinical and ultrasonographic signs of inflammation in at least one IP joint. The patients underwent contrast-enhanced MRI of the eight distal and proximal IP joints of one hand at baseline and 1 year. Images were scored for synovitis and BMLs (0-3 per joint, total score 0-24), blinded for patient characteristics.
Radiographs of the same hand were scored according to the Verbruggen-Veys system. Logistic regression was used to associate the presence of an MRI feature in a joint with being in an erosive versus nonerosive anatomical phase.
“Although erosive hand OA is a condition with a high disease burden, no disease-modifying treatments are available yet,” explained Féline Kroon, a PhD student in the department of rheumatology at Leiden University Medical Center in the Netherlands, in an interview. “Research to find a new form of therapy is partly hindered by our limited knowledge on the pathophysiology of the disease.”
However, “new imaging modalities like MRI enable us to study the pathophysiology of erosive OA more closely,” she said. “This study also gave us the unique opportunity to investigate whether anti-TNF, which is known to lead to clinical improvement and improvement of inflammatory lesions on MRI in other rheumatic diseases like rheumatoid arthritis, might also be effective in erosive OA.”
The presence of BMLs, but not synovitis, was associated with the presence of an erosive anatomical phase in a joint, and treatment with etanercept appeared to be effective in inhibiting these lesions. That suggests a role for TNF in the pathophysiology of erosive OA.
The inhibitory effect of etanercept on BMLs was more pronounced in IP joints with severe synovitis at baseline, suggesting that inflamed synovial tissue could be a source of TNF production in erosive OA.
The total synovitis score was similar at baseline and 1 year in both study groups. For BMLs, the median total score at baseline was 5.4 (range 2-9) and 7.0 (0-9) at 1 year in the placebo group, versus 4.5 (3-9) and 3.7 (0-8), respectively, in the etanercept group.
The presence of BMLs was associated with being in the erosive and remodeling anatomical phases of erosive hand OA. Synovitis was not associated with those phases.
“We think that TNF-alpha plays a role in the pathophysiology of erosive OA via an effect on the subchondral bone,” Ms. Kroon said. “Because we saw that the beneficial effect of etanercept on BMLs was more pronounced in joints with synovitis at baseline, we think that, in an inflamed synovial hand joint, an interaction takes place between synovium and subchondral bone, which could be influenced by blocking TNF.”
Pfizer and UCB supported the investigator-initiated studies and provided the study drugs. Dr. Kloppenburg has received lecturing, consultancy, and investigator fees or grants from AbbVie, APPROACH, GlaxoSmithKline, Levicept, Pfizer, Servier, and UCB, all paid to her institution. All other authors declared no conflicts of interests.
AT THE EULAR 2016 CONGRESS
Key clinical point: Early data suggest targeting tumor necrosis factor could be of benefit in patients with erosive hand disease.
Major finding: TNF was found in the hand joints, and anti-TNF therapy reduced synovial inflammation and bone marrow lesions.
Data source: Two studies looking at the effects of anti-TNF treatments in patients with erosive hand osteoarthritis.
Disclosures: Pfizer and UCB supported the investigator-initiated studies and provided the study drugs. Dr. Kloppenburg has received lecturing, consultancy, and investigator fees or grants from AbbVie, APPROACH, GlaxoSmithKline, Levicept, Pfizer, Servier, and UCB, all paid to her institution. All other authors declared no conflicts of interests.
Phone coaching adds limited benefits in knee osteoarthritis
Adding telephone coaching to a home-based physiotherapist-prescribed activity program does not increase the pain and function benefits of such a program alone in knee osteoarthritis, a randomized controlled trial shows.
Kim L. Bennell, Ph.D., and her associates recruited volunteers who were aged 50 years and older, had knee pain rated at 4 or higher on an 11-point scale, and were classified as sedentary. One group participated in both coaching and the home-based physiotherapy program; the other participated in the home-based physiotherapy program alone.
Overall,142 (85%), 136 (81%), and 128 (76%) participants completed 6-, 12-, and 18-month measurements. In the 6-month outcomes, no significance differences were found between the two groups in average pain (mean difference, 0.4 units) or in Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) function (1.8 units). In addition, there was no change between group differences observed at either 12 or 18 months. At 6 months, however, both groups showed large significant and clinically important improvements from baseline in the primary outcomes of pain and function, reported Dr. Bennell of the Centre for Health, Exercise and Sports Medicine at the University of Melbourne.
Reseachers also examined secondary outcomes and found there was no significant difference for change in numeric rating scale walking pain, WOMAC pain, or quality-of-life scores at any time.
“Improving exercise adherence was an aim of our coaching intervention given that adherence is positively linked to clinical outcomes in knee OA,” the reseachers concluded. “Our study provides novel information about the effects of telephone coaching alongside a physiotherapy prescribed physical activity and exercise program and extends the limited research in telephone coaching for OA.”
Read the full study in Arthritis Care & Research (doi: 10.1002/acr.22915).
Adding telephone coaching to a home-based physiotherapist-prescribed activity program does not increase the pain and function benefits of such a program alone in knee osteoarthritis, a randomized controlled trial shows.
Kim L. Bennell, Ph.D., and her associates recruited volunteers who were aged 50 years and older, had knee pain rated at 4 or higher on an 11-point scale, and were classified as sedentary. One group participated in both coaching and the home-based physiotherapy program; the other participated in the home-based physiotherapy program alone.
Overall,142 (85%), 136 (81%), and 128 (76%) participants completed 6-, 12-, and 18-month measurements. In the 6-month outcomes, no significance differences were found between the two groups in average pain (mean difference, 0.4 units) or in Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) function (1.8 units). In addition, there was no change between group differences observed at either 12 or 18 months. At 6 months, however, both groups showed large significant and clinically important improvements from baseline in the primary outcomes of pain and function, reported Dr. Bennell of the Centre for Health, Exercise and Sports Medicine at the University of Melbourne.
Reseachers also examined secondary outcomes and found there was no significant difference for change in numeric rating scale walking pain, WOMAC pain, or quality-of-life scores at any time.
“Improving exercise adherence was an aim of our coaching intervention given that adherence is positively linked to clinical outcomes in knee OA,” the reseachers concluded. “Our study provides novel information about the effects of telephone coaching alongside a physiotherapy prescribed physical activity and exercise program and extends the limited research in telephone coaching for OA.”
Read the full study in Arthritis Care & Research (doi: 10.1002/acr.22915).
Adding telephone coaching to a home-based physiotherapist-prescribed activity program does not increase the pain and function benefits of such a program alone in knee osteoarthritis, a randomized controlled trial shows.
Kim L. Bennell, Ph.D., and her associates recruited volunteers who were aged 50 years and older, had knee pain rated at 4 or higher on an 11-point scale, and were classified as sedentary. One group participated in both coaching and the home-based physiotherapy program; the other participated in the home-based physiotherapy program alone.
Overall,142 (85%), 136 (81%), and 128 (76%) participants completed 6-, 12-, and 18-month measurements. In the 6-month outcomes, no significance differences were found between the two groups in average pain (mean difference, 0.4 units) or in Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) function (1.8 units). In addition, there was no change between group differences observed at either 12 or 18 months. At 6 months, however, both groups showed large significant and clinically important improvements from baseline in the primary outcomes of pain and function, reported Dr. Bennell of the Centre for Health, Exercise and Sports Medicine at the University of Melbourne.
Reseachers also examined secondary outcomes and found there was no significant difference for change in numeric rating scale walking pain, WOMAC pain, or quality-of-life scores at any time.
“Improving exercise adherence was an aim of our coaching intervention given that adherence is positively linked to clinical outcomes in knee OA,” the reseachers concluded. “Our study provides novel information about the effects of telephone coaching alongside a physiotherapy prescribed physical activity and exercise program and extends the limited research in telephone coaching for OA.”
Read the full study in Arthritis Care & Research (doi: 10.1002/acr.22915).
FROM ARTHRITIS CARE & RESEARCH
Adolescent knee pain ‘not benign,’ linked to later OA
GLASGOW – Older adults are more than seven times as likely to develop knee osteoarthritis if they had anterior knee pain as adolescents, according to the results of a case-control study.
The adjusted odds ratio for patellofemoral osteoarthritis (PFOA) was 7.5 if there was prior adolescent anterior knee pain. Although the 95% confidence interval was wide (1.51-36.94) the association was significant (P = .014). Adjustment had been made for the potential confounding factors of previous patellar dislocation, prior surgery, and patient-reported knee instability; before this adjustment the OR was 20.2 (95% CI, 3.34-11.67).
Patellar dislocation during adolescence also was found to be a significant risk factor for later PFOA (aOR, 3.2; 95% CI, 1.25-9.18; P = .016).
“Adolescent anterior knee pain represents a constellation of symptoms and had always been thought of as benign and self-limiting,” Henry Conchie, a medical student at the University of Bristol (England), said at the British Society for Rheumatology annual conference.
“I think the take-home message from our research is really that this traditional view of benign pathology associated with adolescent anterior knee pain and patellar dislocation must be challenged and when seen in clinical practice we now encourage the acknowledgment of the potentially severe consequences in the future,” Mr. Conchie said.
A link between adolescent anterior knee pain and later PFOA has previously been suggested but there are few data to support this observation, he explained. So the aim of the current study was to look at this in more detail in a group of patients from the knee arthroplasty database at Southmead Hospital in Bristol.
Questionnaires that asked about a variety of symptoms and knee pain were sent to 190 patients in the database who had undergone patellofemoral arthroplasty and so had severe, isolated, and radiologically confirmed PFOA. Questionnaires also were sent to 445 patients who had undergone arthroplasty for unicompartmental tibiofemoral arthritis to serve as the control group.
A subanalysis was performed to look at the mean age of the first dislocation and the investigators found that patients with PFOA were likely to be much younger than controls, with a 44-year difference observed between the groups.
“This adds some weight to the theory that this process [PFOA] begins much earlier than once thought – at a younger age,” Mr. Conchie suggested.
The study subjects were surveyed 1-4 years after their operation, so patient recall could have affected the results, but the use of the unicompartmental tibiofemoral arthritis patients as controls should have reduced this potential bias, he said. The fact that they had gone through an arthroplasty meant that they would have had very similar experiences to the PFOA group in terms of pain.
Although only severe OA cases and arthritis controls were used, the team believes that the findings are robust as these were clearly defined patient groups, albeit at the end of the disease spectrum.
“Thought can now turn to etiological mechanisms underlying these relationships, and I think it is likely that anatomical etiologies such as patellar outer and trochlear dysplasia can define both the pain and instability in youth as well as the patellofemoral osteoarthritis in later life,” Mr. Conchie proposed. Further research to look at this would be needed in future.
During the Q&A following his presentation, Dr. Eileen Baildam of Alder Hey Children’s Hospital in Liverpool, England, commented that she had looked at the persistence of pain in patients with adolescent anterior knee pain some years ago and found that, 10-20 years later, 60% were still experiencing pain.
The chair of the session, Dr. Joyce Davidson of the Royal Hospital for Sick Children in Glasgow, summed up by saying: “I think we do see lots of patients and maybe we just need to be aware that this may not be as benign as we think, and certainly we should be looking for abnormal patellae and being very aware of it in young people.”
Mr. Conchie and his coauthors had nothing to disclose.
GLASGOW – Older adults are more than seven times as likely to develop knee osteoarthritis if they had anterior knee pain as adolescents, according to the results of a case-control study.
The adjusted odds ratio for patellofemoral osteoarthritis (PFOA) was 7.5 if there was prior adolescent anterior knee pain. Although the 95% confidence interval was wide (1.51-36.94) the association was significant (P = .014). Adjustment had been made for the potential confounding factors of previous patellar dislocation, prior surgery, and patient-reported knee instability; before this adjustment the OR was 20.2 (95% CI, 3.34-11.67).
Patellar dislocation during adolescence also was found to be a significant risk factor for later PFOA (aOR, 3.2; 95% CI, 1.25-9.18; P = .016).
“Adolescent anterior knee pain represents a constellation of symptoms and had always been thought of as benign and self-limiting,” Henry Conchie, a medical student at the University of Bristol (England), said at the British Society for Rheumatology annual conference.
“I think the take-home message from our research is really that this traditional view of benign pathology associated with adolescent anterior knee pain and patellar dislocation must be challenged and when seen in clinical practice we now encourage the acknowledgment of the potentially severe consequences in the future,” Mr. Conchie said.
A link between adolescent anterior knee pain and later PFOA has previously been suggested but there are few data to support this observation, he explained. So the aim of the current study was to look at this in more detail in a group of patients from the knee arthroplasty database at Southmead Hospital in Bristol.
Questionnaires that asked about a variety of symptoms and knee pain were sent to 190 patients in the database who had undergone patellofemoral arthroplasty and so had severe, isolated, and radiologically confirmed PFOA. Questionnaires also were sent to 445 patients who had undergone arthroplasty for unicompartmental tibiofemoral arthritis to serve as the control group.
A subanalysis was performed to look at the mean age of the first dislocation and the investigators found that patients with PFOA were likely to be much younger than controls, with a 44-year difference observed between the groups.
“This adds some weight to the theory that this process [PFOA] begins much earlier than once thought – at a younger age,” Mr. Conchie suggested.
The study subjects were surveyed 1-4 years after their operation, so patient recall could have affected the results, but the use of the unicompartmental tibiofemoral arthritis patients as controls should have reduced this potential bias, he said. The fact that they had gone through an arthroplasty meant that they would have had very similar experiences to the PFOA group in terms of pain.
Although only severe OA cases and arthritis controls were used, the team believes that the findings are robust as these were clearly defined patient groups, albeit at the end of the disease spectrum.
“Thought can now turn to etiological mechanisms underlying these relationships, and I think it is likely that anatomical etiologies such as patellar outer and trochlear dysplasia can define both the pain and instability in youth as well as the patellofemoral osteoarthritis in later life,” Mr. Conchie proposed. Further research to look at this would be needed in future.
During the Q&A following his presentation, Dr. Eileen Baildam of Alder Hey Children’s Hospital in Liverpool, England, commented that she had looked at the persistence of pain in patients with adolescent anterior knee pain some years ago and found that, 10-20 years later, 60% were still experiencing pain.
The chair of the session, Dr. Joyce Davidson of the Royal Hospital for Sick Children in Glasgow, summed up by saying: “I think we do see lots of patients and maybe we just need to be aware that this may not be as benign as we think, and certainly we should be looking for abnormal patellae and being very aware of it in young people.”
Mr. Conchie and his coauthors had nothing to disclose.
GLASGOW – Older adults are more than seven times as likely to develop knee osteoarthritis if they had anterior knee pain as adolescents, according to the results of a case-control study.
The adjusted odds ratio for patellofemoral osteoarthritis (PFOA) was 7.5 if there was prior adolescent anterior knee pain. Although the 95% confidence interval was wide (1.51-36.94) the association was significant (P = .014). Adjustment had been made for the potential confounding factors of previous patellar dislocation, prior surgery, and patient-reported knee instability; before this adjustment the OR was 20.2 (95% CI, 3.34-11.67).
Patellar dislocation during adolescence also was found to be a significant risk factor for later PFOA (aOR, 3.2; 95% CI, 1.25-9.18; P = .016).
“Adolescent anterior knee pain represents a constellation of symptoms and had always been thought of as benign and self-limiting,” Henry Conchie, a medical student at the University of Bristol (England), said at the British Society for Rheumatology annual conference.
“I think the take-home message from our research is really that this traditional view of benign pathology associated with adolescent anterior knee pain and patellar dislocation must be challenged and when seen in clinical practice we now encourage the acknowledgment of the potentially severe consequences in the future,” Mr. Conchie said.
A link between adolescent anterior knee pain and later PFOA has previously been suggested but there are few data to support this observation, he explained. So the aim of the current study was to look at this in more detail in a group of patients from the knee arthroplasty database at Southmead Hospital in Bristol.
Questionnaires that asked about a variety of symptoms and knee pain were sent to 190 patients in the database who had undergone patellofemoral arthroplasty and so had severe, isolated, and radiologically confirmed PFOA. Questionnaires also were sent to 445 patients who had undergone arthroplasty for unicompartmental tibiofemoral arthritis to serve as the control group.
A subanalysis was performed to look at the mean age of the first dislocation and the investigators found that patients with PFOA were likely to be much younger than controls, with a 44-year difference observed between the groups.
“This adds some weight to the theory that this process [PFOA] begins much earlier than once thought – at a younger age,” Mr. Conchie suggested.
The study subjects were surveyed 1-4 years after their operation, so patient recall could have affected the results, but the use of the unicompartmental tibiofemoral arthritis patients as controls should have reduced this potential bias, he said. The fact that they had gone through an arthroplasty meant that they would have had very similar experiences to the PFOA group in terms of pain.
Although only severe OA cases and arthritis controls were used, the team believes that the findings are robust as these were clearly defined patient groups, albeit at the end of the disease spectrum.
“Thought can now turn to etiological mechanisms underlying these relationships, and I think it is likely that anatomical etiologies such as patellar outer and trochlear dysplasia can define both the pain and instability in youth as well as the patellofemoral osteoarthritis in later life,” Mr. Conchie proposed. Further research to look at this would be needed in future.
During the Q&A following his presentation, Dr. Eileen Baildam of Alder Hey Children’s Hospital in Liverpool, England, commented that she had looked at the persistence of pain in patients with adolescent anterior knee pain some years ago and found that, 10-20 years later, 60% were still experiencing pain.
The chair of the session, Dr. Joyce Davidson of the Royal Hospital for Sick Children in Glasgow, summed up by saying: “I think we do see lots of patients and maybe we just need to be aware that this may not be as benign as we think, and certainly we should be looking for abnormal patellae and being very aware of it in young people.”
Mr. Conchie and his coauthors had nothing to disclose.
AT RHEUMATOLOGY 2016
Key clinical point: Knee pain in adolescence was directly linked to later development of knee osteoarthritis.
Major finding: Adolescent knee pain increased the likelihood for developing OA, with an adjusted odds ratio of 7.5 (P = .014).
Data source: Case-control study of 190 adults with patellofemoral OA and 445 controls without patellofemoral OA who had arthroplasty.
Disclosures: Mr. Conchie and his coauthors had nothing to disclose.
Opioid-based therapies reduce TKA needs for OA patients, but not costs
Treatment with opioids is not cost effective in osteoarthritis patients without comorbidities, according to Savannah R. Smith and her associates.
When a 10% reduction in total knee arthroplasty (TKA) effectiveness from opioid-based therapies was assumed, tramadol therapy delayed TKA by 7 years and tramadol plus oxycodone therapy delayed TKA by 9 years. Opioid-based therapy reduced primary TKA utilization by 4% for tramadol and by 10% for tramadol plus oxycodone, and reduced revision TKA use by 23% and 39%, respectively.
While both opioid-based therapies reduced dependence on TKA, treatment was more expensive and it reduced quality of life, compared with an opioid-sparing therapy. For a 60-year-old OA patient for whom TKA was not an option, the incremental cost-effectiveness ratio for tramadol was $39,600 per quality-adjusted life-year, compared with a therapy without opioids, and the incremental cost-effectiveness ratio for tramadol plus oxycodone was $116,800 per quality-adjusted life-year.
“Given the risk of diversion and its associated cost for potent opioids, policy makers may consider limiting the use of potent opioids in knee OA patients. From a cost-effectiveness standpoint, both opioid-based strategies led to higher costs without providing additional benefits, unless patients were unwilling or unable to undergo TKA later,” the investigators noted.
Find the full study in Arthritis Care and Research (doi: 10.1002/acr.22916).
Treatment with opioids is not cost effective in osteoarthritis patients without comorbidities, according to Savannah R. Smith and her associates.
When a 10% reduction in total knee arthroplasty (TKA) effectiveness from opioid-based therapies was assumed, tramadol therapy delayed TKA by 7 years and tramadol plus oxycodone therapy delayed TKA by 9 years. Opioid-based therapy reduced primary TKA utilization by 4% for tramadol and by 10% for tramadol plus oxycodone, and reduced revision TKA use by 23% and 39%, respectively.
While both opioid-based therapies reduced dependence on TKA, treatment was more expensive and it reduced quality of life, compared with an opioid-sparing therapy. For a 60-year-old OA patient for whom TKA was not an option, the incremental cost-effectiveness ratio for tramadol was $39,600 per quality-adjusted life-year, compared with a therapy without opioids, and the incremental cost-effectiveness ratio for tramadol plus oxycodone was $116,800 per quality-adjusted life-year.
“Given the risk of diversion and its associated cost for potent opioids, policy makers may consider limiting the use of potent opioids in knee OA patients. From a cost-effectiveness standpoint, both opioid-based strategies led to higher costs without providing additional benefits, unless patients were unwilling or unable to undergo TKA later,” the investigators noted.
Find the full study in Arthritis Care and Research (doi: 10.1002/acr.22916).
Treatment with opioids is not cost effective in osteoarthritis patients without comorbidities, according to Savannah R. Smith and her associates.
When a 10% reduction in total knee arthroplasty (TKA) effectiveness from opioid-based therapies was assumed, tramadol therapy delayed TKA by 7 years and tramadol plus oxycodone therapy delayed TKA by 9 years. Opioid-based therapy reduced primary TKA utilization by 4% for tramadol and by 10% for tramadol plus oxycodone, and reduced revision TKA use by 23% and 39%, respectively.
While both opioid-based therapies reduced dependence on TKA, treatment was more expensive and it reduced quality of life, compared with an opioid-sparing therapy. For a 60-year-old OA patient for whom TKA was not an option, the incremental cost-effectiveness ratio for tramadol was $39,600 per quality-adjusted life-year, compared with a therapy without opioids, and the incremental cost-effectiveness ratio for tramadol plus oxycodone was $116,800 per quality-adjusted life-year.
“Given the risk of diversion and its associated cost for potent opioids, policy makers may consider limiting the use of potent opioids in knee OA patients. From a cost-effectiveness standpoint, both opioid-based strategies led to higher costs without providing additional benefits, unless patients were unwilling or unable to undergo TKA later,” the investigators noted.
Find the full study in Arthritis Care and Research (doi: 10.1002/acr.22916).
FROM ARTHRITIS CARE AND RESEARCH