User login
For MD-IQ on Family Practice News, but a regular topic for Rheumatology News
The year in osteoarthritis
MAUI, HAWAII – One of the major happenings in the field of osteoarthritis in the past year was a disturbing report of dramatically increased risk of acute MI for at least 6 months after total knee replacement, panelists agreed at the 2016 Rheumatology Winter Clinical Symposium.
“What they found borders on frightening,” according to Dr. Martin J. Bergman of Drexel University, Philadelphia, and chief of rheumatology at Taylor Hospital in Ridley Park, Pa.
Dr. Bergman and copanelist Dr. Orrin M. Troum of the University of Southern California in Los Angeles highlighted key developments in osteoarthritis during the past year, including two major studies on total knee replacement, the Food and Drug Administration’s updated stronger warning on the cardiac and stroke risks of NSAIDs, a randomized trial which effectively takes hydroxychloroquine (Plaquenil) off the treatment menu for hand osteoarthritis, and a reassuring report on the safety of repeated intra-articular corticosteroid injections in patients with synovitic knee osteoarthritis.
Acute MI risk after total knee replacement
British investigators utilizing the U.K. National Health Service database retrospectively identified 13,849 patients who underwent total knee replacement (TKR) and an equal number of nonsurgical controls propensity-matched for cardiovascular risk factors. These two very large groups were followed for 5 years.
During the first month after TKR, the acute MI risk was 8.75-fold greater than in the matched controls. The elevated risk gradually declined thereafter, but it remained significantly higher than in controls until 1 year after surgery. At 3 months post surgery the TKR group was at fourfold increased risk of MI, compared with controls, and at 6 months their risk was still nearly double that of controls (Arthritis Rheumatol. 2015 Oct;67[10]:2771-9).
The British investigators also found a prolonged postsurgical elevated risk of MI in a large group of patients who underwent total hip replacement, although the magnitude of the increased risk, compared with matched controls, wasn’t as large as that seen after TKR.
Dr. Troum commented that the increased risk of MI during the first year after TKR identified in this study is something physicians now need to bring up in the risk/benefit discussion with patients considering TKR.
“Also, this study underscores that it may behoove us to make sure that these presurgical patients are really well worked up by a cardiologist or their primary care physician to mitigate that coronary risk as much as possible,” he added.
Another key finding in the U.K. study was that unlike the acute MI risk, the risk of venous thromboembolism following TKR remained elevated throughout the full 5 years of follow-up.
“Once you’ve had that surgery, you are at increased risk for venous thromboembolism. I think that’s something we have to keep in mind when a patient comes in with a history of total knee replacement and a complaint of calf pain or swelling – at that point, you have to think about deep venous thrombosis,” Dr. Bergman said.
TKR – Why wait?
In a Danish trial of 100 knee osteoarthritis patients deemed eligible for TKR, participants were randomized to prompt TKR followed by a 3-month regimen of exercise, dietary weight loss, physical therapy, and pain medication or to the nonsurgical regimen alone. At 12 months of follow-up, the prompt TKR group showed significantly greater improvement in a standardized score encompassing pain, symptoms, quality of life, and activities of daily living, even though one-quarter of patients in the nonsurgical treatment group bailed and underwent TKR before 12 months was up (N Engl J Med. 2015 Oct 22;373[17]:1597-606).
“My conclusion is that once you’ve determined that a patient needs and wants a total knee replacement, the patient should probably get it. Delaying – trying other modalities in an effort to lose weight and improve function – is really not going to buy you much in the way of time,” Dr. Bergman observed.
Hydroxychloroquine for hand osteoarthritis
At the 2015 European League Against Rheumatism (EULAR) meeting in Rome, Dutch investigators presented a randomized, double-blind trial in which 196 patients with symptomatic hand osteoarthritis received 6 months of hydroxychloroquine at 400 mg/day or placebo. Unlike in mild rheumatoid arthritis or lupus, hydroxychloroquine had no beneficial effect on hand osteoarthritis pain, disability, or quality of life measures.
“Plaquenil [Hydroxychloroquine] is not a good choice for patients with osteoarthritis of the hand. I think it’s a dead therapy,” Dr. Troum declared.
FDA expands warning on NSAIDs’ cardiovascular risk
On July 9, 2015, the FDA announced updated labels for NSAIDs. The new warning states that MI and stroke risk can increase as early as in the first week of NSAID use and appear to be dose- and duration-related. The agency also warned that patients who take an NSAID after a first MI are more likely to die within 1 year.
“This really brought a lot of folks to my office,” Dr. Troum recalled.
“Absolutely, this was big stuff,” Dr. Bergman agreed. “This became a nightmare for many of us because all of a sudden patients were scared to death about taking their NSAIDs.”
Intra-articular corticosteroids for knee osteoarthritis don’t accelerate cartilage deterioration
At last fall’s American College of Rheumatology meeting in San Francisco, Jeffrey B. Driban, Ph.D., of Tufts Medical Center, Boston, presented a double-blind, randomized trial of intra-articular injections of triamcinolone hexacetonide 40 mg versus saline quarterly for 2 years in 140 patients with symptomatic knee osteoarthritis with ultrasound evidence of synovitis. Participants underwent annual evaluation of periarticular bone and cartilage changes via MRI and dual-energy x-ray absorptiometry.
After 2 years, there was no difference between the two groups in terms of pain scores, walk time, or other functional measures. The injections – eight in total over 2 years – were safe, with new-onset hypertension and hyperglycemia rates of 3% in this obese population. And most important of all, there were no major differences between the two groups in terms of quantitative or semiquantitative structural endpoints; in other words, the injections didn’t increase the rate of structural disease progression. The intra-articular steroid group showed a modestly greater rate of loss of cartilage thickness, which the investigators deemed of uncertain clinical significance.
“The structural changes were minimal,” Dr. Troum noted. “This is only a 2-year study, but I can say that I now feel more comfortable giving these injections in patients who for whatever reason can’t get surgery.”
Dr. Bergman said that many orthopedic surgeons talk up the potential risk that intra-articular steroid injections will accelerate cartilage damage. They place an arbitrary limit on the number of injections a patient can receive.
“I think this study really helps us push back and say, ‘No, I think you’re fine in getting this procedure,’” the rheumatologist commented.
Dr. Bergman and Dr. Troum reported having no financial conflicts regarding their presentation.
MAUI, HAWAII – One of the major happenings in the field of osteoarthritis in the past year was a disturbing report of dramatically increased risk of acute MI for at least 6 months after total knee replacement, panelists agreed at the 2016 Rheumatology Winter Clinical Symposium.
“What they found borders on frightening,” according to Dr. Martin J. Bergman of Drexel University, Philadelphia, and chief of rheumatology at Taylor Hospital in Ridley Park, Pa.
Dr. Bergman and copanelist Dr. Orrin M. Troum of the University of Southern California in Los Angeles highlighted key developments in osteoarthritis during the past year, including two major studies on total knee replacement, the Food and Drug Administration’s updated stronger warning on the cardiac and stroke risks of NSAIDs, a randomized trial which effectively takes hydroxychloroquine (Plaquenil) off the treatment menu for hand osteoarthritis, and a reassuring report on the safety of repeated intra-articular corticosteroid injections in patients with synovitic knee osteoarthritis.
Acute MI risk after total knee replacement
British investigators utilizing the U.K. National Health Service database retrospectively identified 13,849 patients who underwent total knee replacement (TKR) and an equal number of nonsurgical controls propensity-matched for cardiovascular risk factors. These two very large groups were followed for 5 years.
During the first month after TKR, the acute MI risk was 8.75-fold greater than in the matched controls. The elevated risk gradually declined thereafter, but it remained significantly higher than in controls until 1 year after surgery. At 3 months post surgery the TKR group was at fourfold increased risk of MI, compared with controls, and at 6 months their risk was still nearly double that of controls (Arthritis Rheumatol. 2015 Oct;67[10]:2771-9).
The British investigators also found a prolonged postsurgical elevated risk of MI in a large group of patients who underwent total hip replacement, although the magnitude of the increased risk, compared with matched controls, wasn’t as large as that seen after TKR.
Dr. Troum commented that the increased risk of MI during the first year after TKR identified in this study is something physicians now need to bring up in the risk/benefit discussion with patients considering TKR.
“Also, this study underscores that it may behoove us to make sure that these presurgical patients are really well worked up by a cardiologist or their primary care physician to mitigate that coronary risk as much as possible,” he added.
Another key finding in the U.K. study was that unlike the acute MI risk, the risk of venous thromboembolism following TKR remained elevated throughout the full 5 years of follow-up.
“Once you’ve had that surgery, you are at increased risk for venous thromboembolism. I think that’s something we have to keep in mind when a patient comes in with a history of total knee replacement and a complaint of calf pain or swelling – at that point, you have to think about deep venous thrombosis,” Dr. Bergman said.
TKR – Why wait?
In a Danish trial of 100 knee osteoarthritis patients deemed eligible for TKR, participants were randomized to prompt TKR followed by a 3-month regimen of exercise, dietary weight loss, physical therapy, and pain medication or to the nonsurgical regimen alone. At 12 months of follow-up, the prompt TKR group showed significantly greater improvement in a standardized score encompassing pain, symptoms, quality of life, and activities of daily living, even though one-quarter of patients in the nonsurgical treatment group bailed and underwent TKR before 12 months was up (N Engl J Med. 2015 Oct 22;373[17]:1597-606).
“My conclusion is that once you’ve determined that a patient needs and wants a total knee replacement, the patient should probably get it. Delaying – trying other modalities in an effort to lose weight and improve function – is really not going to buy you much in the way of time,” Dr. Bergman observed.
Hydroxychloroquine for hand osteoarthritis
At the 2015 European League Against Rheumatism (EULAR) meeting in Rome, Dutch investigators presented a randomized, double-blind trial in which 196 patients with symptomatic hand osteoarthritis received 6 months of hydroxychloroquine at 400 mg/day or placebo. Unlike in mild rheumatoid arthritis or lupus, hydroxychloroquine had no beneficial effect on hand osteoarthritis pain, disability, or quality of life measures.
“Plaquenil [Hydroxychloroquine] is not a good choice for patients with osteoarthritis of the hand. I think it’s a dead therapy,” Dr. Troum declared.
FDA expands warning on NSAIDs’ cardiovascular risk
On July 9, 2015, the FDA announced updated labels for NSAIDs. The new warning states that MI and stroke risk can increase as early as in the first week of NSAID use and appear to be dose- and duration-related. The agency also warned that patients who take an NSAID after a first MI are more likely to die within 1 year.
“This really brought a lot of folks to my office,” Dr. Troum recalled.
“Absolutely, this was big stuff,” Dr. Bergman agreed. “This became a nightmare for many of us because all of a sudden patients were scared to death about taking their NSAIDs.”
Intra-articular corticosteroids for knee osteoarthritis don’t accelerate cartilage deterioration
At last fall’s American College of Rheumatology meeting in San Francisco, Jeffrey B. Driban, Ph.D., of Tufts Medical Center, Boston, presented a double-blind, randomized trial of intra-articular injections of triamcinolone hexacetonide 40 mg versus saline quarterly for 2 years in 140 patients with symptomatic knee osteoarthritis with ultrasound evidence of synovitis. Participants underwent annual evaluation of periarticular bone and cartilage changes via MRI and dual-energy x-ray absorptiometry.
After 2 years, there was no difference between the two groups in terms of pain scores, walk time, or other functional measures. The injections – eight in total over 2 years – were safe, with new-onset hypertension and hyperglycemia rates of 3% in this obese population. And most important of all, there were no major differences between the two groups in terms of quantitative or semiquantitative structural endpoints; in other words, the injections didn’t increase the rate of structural disease progression. The intra-articular steroid group showed a modestly greater rate of loss of cartilage thickness, which the investigators deemed of uncertain clinical significance.
“The structural changes were minimal,” Dr. Troum noted. “This is only a 2-year study, but I can say that I now feel more comfortable giving these injections in patients who for whatever reason can’t get surgery.”
Dr. Bergman said that many orthopedic surgeons talk up the potential risk that intra-articular steroid injections will accelerate cartilage damage. They place an arbitrary limit on the number of injections a patient can receive.
“I think this study really helps us push back and say, ‘No, I think you’re fine in getting this procedure,’” the rheumatologist commented.
Dr. Bergman and Dr. Troum reported having no financial conflicts regarding their presentation.
MAUI, HAWAII – One of the major happenings in the field of osteoarthritis in the past year was a disturbing report of dramatically increased risk of acute MI for at least 6 months after total knee replacement, panelists agreed at the 2016 Rheumatology Winter Clinical Symposium.
“What they found borders on frightening,” according to Dr. Martin J. Bergman of Drexel University, Philadelphia, and chief of rheumatology at Taylor Hospital in Ridley Park, Pa.
Dr. Bergman and copanelist Dr. Orrin M. Troum of the University of Southern California in Los Angeles highlighted key developments in osteoarthritis during the past year, including two major studies on total knee replacement, the Food and Drug Administration’s updated stronger warning on the cardiac and stroke risks of NSAIDs, a randomized trial which effectively takes hydroxychloroquine (Plaquenil) off the treatment menu for hand osteoarthritis, and a reassuring report on the safety of repeated intra-articular corticosteroid injections in patients with synovitic knee osteoarthritis.
Acute MI risk after total knee replacement
British investigators utilizing the U.K. National Health Service database retrospectively identified 13,849 patients who underwent total knee replacement (TKR) and an equal number of nonsurgical controls propensity-matched for cardiovascular risk factors. These two very large groups were followed for 5 years.
During the first month after TKR, the acute MI risk was 8.75-fold greater than in the matched controls. The elevated risk gradually declined thereafter, but it remained significantly higher than in controls until 1 year after surgery. At 3 months post surgery the TKR group was at fourfold increased risk of MI, compared with controls, and at 6 months their risk was still nearly double that of controls (Arthritis Rheumatol. 2015 Oct;67[10]:2771-9).
The British investigators also found a prolonged postsurgical elevated risk of MI in a large group of patients who underwent total hip replacement, although the magnitude of the increased risk, compared with matched controls, wasn’t as large as that seen after TKR.
Dr. Troum commented that the increased risk of MI during the first year after TKR identified in this study is something physicians now need to bring up in the risk/benefit discussion with patients considering TKR.
“Also, this study underscores that it may behoove us to make sure that these presurgical patients are really well worked up by a cardiologist or their primary care physician to mitigate that coronary risk as much as possible,” he added.
Another key finding in the U.K. study was that unlike the acute MI risk, the risk of venous thromboembolism following TKR remained elevated throughout the full 5 years of follow-up.
“Once you’ve had that surgery, you are at increased risk for venous thromboembolism. I think that’s something we have to keep in mind when a patient comes in with a history of total knee replacement and a complaint of calf pain or swelling – at that point, you have to think about deep venous thrombosis,” Dr. Bergman said.
TKR – Why wait?
In a Danish trial of 100 knee osteoarthritis patients deemed eligible for TKR, participants were randomized to prompt TKR followed by a 3-month regimen of exercise, dietary weight loss, physical therapy, and pain medication or to the nonsurgical regimen alone. At 12 months of follow-up, the prompt TKR group showed significantly greater improvement in a standardized score encompassing pain, symptoms, quality of life, and activities of daily living, even though one-quarter of patients in the nonsurgical treatment group bailed and underwent TKR before 12 months was up (N Engl J Med. 2015 Oct 22;373[17]:1597-606).
“My conclusion is that once you’ve determined that a patient needs and wants a total knee replacement, the patient should probably get it. Delaying – trying other modalities in an effort to lose weight and improve function – is really not going to buy you much in the way of time,” Dr. Bergman observed.
Hydroxychloroquine for hand osteoarthritis
At the 2015 European League Against Rheumatism (EULAR) meeting in Rome, Dutch investigators presented a randomized, double-blind trial in which 196 patients with symptomatic hand osteoarthritis received 6 months of hydroxychloroquine at 400 mg/day or placebo. Unlike in mild rheumatoid arthritis or lupus, hydroxychloroquine had no beneficial effect on hand osteoarthritis pain, disability, or quality of life measures.
“Plaquenil [Hydroxychloroquine] is not a good choice for patients with osteoarthritis of the hand. I think it’s a dead therapy,” Dr. Troum declared.
FDA expands warning on NSAIDs’ cardiovascular risk
On July 9, 2015, the FDA announced updated labels for NSAIDs. The new warning states that MI and stroke risk can increase as early as in the first week of NSAID use and appear to be dose- and duration-related. The agency also warned that patients who take an NSAID after a first MI are more likely to die within 1 year.
“This really brought a lot of folks to my office,” Dr. Troum recalled.
“Absolutely, this was big stuff,” Dr. Bergman agreed. “This became a nightmare for many of us because all of a sudden patients were scared to death about taking their NSAIDs.”
Intra-articular corticosteroids for knee osteoarthritis don’t accelerate cartilage deterioration
At last fall’s American College of Rheumatology meeting in San Francisco, Jeffrey B. Driban, Ph.D., of Tufts Medical Center, Boston, presented a double-blind, randomized trial of intra-articular injections of triamcinolone hexacetonide 40 mg versus saline quarterly for 2 years in 140 patients with symptomatic knee osteoarthritis with ultrasound evidence of synovitis. Participants underwent annual evaluation of periarticular bone and cartilage changes via MRI and dual-energy x-ray absorptiometry.
After 2 years, there was no difference between the two groups in terms of pain scores, walk time, or other functional measures. The injections – eight in total over 2 years – were safe, with new-onset hypertension and hyperglycemia rates of 3% in this obese population. And most important of all, there were no major differences between the two groups in terms of quantitative or semiquantitative structural endpoints; in other words, the injections didn’t increase the rate of structural disease progression. The intra-articular steroid group showed a modestly greater rate of loss of cartilage thickness, which the investigators deemed of uncertain clinical significance.
“The structural changes were minimal,” Dr. Troum noted. “This is only a 2-year study, but I can say that I now feel more comfortable giving these injections in patients who for whatever reason can’t get surgery.”
Dr. Bergman said that many orthopedic surgeons talk up the potential risk that intra-articular steroid injections will accelerate cartilage damage. They place an arbitrary limit on the number of injections a patient can receive.
“I think this study really helps us push back and say, ‘No, I think you’re fine in getting this procedure,’” the rheumatologist commented.
Dr. Bergman and Dr. Troum reported having no financial conflicts regarding their presentation.
EXPERT ANALYSIS FROM RWCS 2016
USPSTF: Screen all adults for depression
All adults, including pregnant and postpartum women, should be screened for depression, according to new recommendations of the U.S. Preventive Services Task Force.
The recommendation also calls for screening to be coupled with “adequate systems” to ensure diagnosis, treatment, and follow-up (JAMA. 2016 Jan 26;315[4]:380-7).
The depression screening recommendation, authored by Dr. Albert L. Siu and the other members of the USPSTF, is a level B recommendation, meaning that it has either high certainty of moderate net benefit, or moderate certainty of moderate to substantial net benefit.
The new guidance in screening for depression helps address a disorder that is “the leading cause of disability among adults in high-income countries,” said Dr. Siu and his coauthors. Lost productivity attributable to depression cost $23 billion in the United States in 2011, and $22.8 billion was spent on treatments for depression in 2009, the last year for which figures are available.
Dr. Siu, chair of geriatrics and palliative medicine at Icahn School of Medicine at Mount Sinai, New York, and his coauthors cited “convincing evidence that screening improves the accurate identification of adult patients with depression in primary care settings, including pregnant and postpartum women.”
In addition, the task force found convincing evidence that for older adults as well as the general adult population, treatment of “depression identified through screening in primary care settings with antidepressants, psychotherapy, or both decreases clinical morbidity.”
For pregnant and postpartum women with depression, Dr. Siu and his coauthors found “adequate” evidence that cognitive behavioral therapy (CBT) improves outcomes.
The recommendation does not identify optimal timing and intervals for depression screening, citing a need for more research in this area. However, “a pragmatic approach might include screening all adults who have not been screened previously and using clinical judgment in consideration of risk factors, comorbid conditions, and life events to determine if additional screening of high-risk patients is warranted,” explained Dr. Siu and his coauthors.
The new depression screening recommendation from USPSTF updates the 2009 recommendation, which recommended universal screening if “staff-assisted depression care supports” were in place, and targeted screening based on clinical judgment and patient preference if such support were unavailable.
The rationale for the current recommendation of universal screening for those 18 years and older is the “recognition that such support is now much more widely available and accepted as part of mental health care,” the task force members said.
Any potential harms of screening, said Dr. Siu and his coauthors, were minimal to nonexistent.
Overall, the USPSTF assigned a small to moderate risk to the use of medication in depression. However, the use of “second-generation” antidepressants – mostly SSRIs – was associated with some harms, including increased risk of suicidal behavior in young adults and of gastrointestinal bleeding in older adults, as well as potential fetal harms in pregnant women taking antidepressants.
Using CBT to treat depression in pregnant and postpartum women was also associated with minimal to no harm.
The USPSTF screening recommendation is aligned with the American Academy of Family Physicians’ recommendation to screen the general adult population for depression, and with the American Academy of Pediatrics’ recommendation that pediatricians screen mothers for depression at their babies’ 1-, 2-, and 4-month office visits.
Released in draft form in July 2015, the depression screening recommendation was available for public comment for a period of 4 weeks. In response to public input, the final recommendation’s implementation section clarifies and characterizes an “adequate system” of screening, and gives more resources for evidence-based depression screening and treatment.
The Agency for Healthcare Research and Quality supports the operations of the USPSTF, but the task force’s recommendations are independent of the federal government. Dr. Siu and the other task force members reported no conflicts of interest.
On Twitter @karioakes
Arthritis affects one in five adults and is one of the most frequent reasons for ambulatory visits to the primary care physician. Arthritis affects patients both physically and psychologically and often leads to depressed mood with subsequent worse health outcomes, including increased mortality. Specifically, depression in patients with arthritis is an independent risk factor for cardiovascular disease, myocardial infarction, and suicide. Patients with arthritis and associated depression have increased health service utilization and are less likely to be adherent with their medications. In addition to these negative health consequences, depression may contribute to unemployment, loss of work productivity, and increased healthcare costs in persons with arthritis.
Depression screening guidelines for adults with chronic musculoskeletal diseases such as arthritis have been endorsed by the U.K. National Institute of Clinical Excellence. The U.S. Preventive Service Task Force and the Canadian Task Force for Preventive Health Care recommend depression screening in all adults. However, before screening for depression in specific patient groups can be recommended, well-established criteria should be met. Generally, screening is reasonable if the condition, depression in this case, is important and prevalent, can be effectively treated, and cannot be readily detected without screening. Comorbid depression in patients with arthritis meets these criteria. It is highly prevalent with rates ranging from 18%-42%. Depression with inflammatory arthritis, such as rheumatoid arthritis (RA), occurs more frequently than with osteoarthritis but even though it is more prevalent, depression with RA is often unrecognized and/or untreated.
Performing depression screening should not unduly burden physicians because, on average, depression screening adds less than 3 minutes to a visit. Asking two simple questions about mood and anhedonia (“Over the past 2 weeks, have you felt down, depressed, or hopeless?” and “Over the past 2 weeks, have you felt little interest or pleasure in doing things?”) is as effective as using more formal instruments. Implicit in the use of depression screening is the assumption that screening will increase recognition of depression and that recognized patients would benefit from treatment. It has been shown that patients who screen positive but were not in treatment had high rates of depression and overall poor mental health outcomes. Thus, provision of or referral to treatment is a necessary follow-up to screening.
It has been shown that there is no difference in depression screening rates in patients with arthritis, compared with the general population, despite patients with arthritis being considered “high risk.” Given the endorsement of national guidelines for depression screening, quality improvement initiatives should target physicians and non-physicians to increase the recognition of depression in high-risk groups and the use of appropriate interventions, such as mental health referrals and/or treatment with antidepressants.
Dr. Mary Margaretten is an associate professor of medicine in the division of rheumatology at the University of California, San Francisco. She has no relevant disclosures.
Arthritis affects one in five adults and is one of the most frequent reasons for ambulatory visits to the primary care physician. Arthritis affects patients both physically and psychologically and often leads to depressed mood with subsequent worse health outcomes, including increased mortality. Specifically, depression in patients with arthritis is an independent risk factor for cardiovascular disease, myocardial infarction, and suicide. Patients with arthritis and associated depression have increased health service utilization and are less likely to be adherent with their medications. In addition to these negative health consequences, depression may contribute to unemployment, loss of work productivity, and increased healthcare costs in persons with arthritis.
Depression screening guidelines for adults with chronic musculoskeletal diseases such as arthritis have been endorsed by the U.K. National Institute of Clinical Excellence. The U.S. Preventive Service Task Force and the Canadian Task Force for Preventive Health Care recommend depression screening in all adults. However, before screening for depression in specific patient groups can be recommended, well-established criteria should be met. Generally, screening is reasonable if the condition, depression in this case, is important and prevalent, can be effectively treated, and cannot be readily detected without screening. Comorbid depression in patients with arthritis meets these criteria. It is highly prevalent with rates ranging from 18%-42%. Depression with inflammatory arthritis, such as rheumatoid arthritis (RA), occurs more frequently than with osteoarthritis but even though it is more prevalent, depression with RA is often unrecognized and/or untreated.
Performing depression screening should not unduly burden physicians because, on average, depression screening adds less than 3 minutes to a visit. Asking two simple questions about mood and anhedonia (“Over the past 2 weeks, have you felt down, depressed, or hopeless?” and “Over the past 2 weeks, have you felt little interest or pleasure in doing things?”) is as effective as using more formal instruments. Implicit in the use of depression screening is the assumption that screening will increase recognition of depression and that recognized patients would benefit from treatment. It has been shown that patients who screen positive but were not in treatment had high rates of depression and overall poor mental health outcomes. Thus, provision of or referral to treatment is a necessary follow-up to screening.
It has been shown that there is no difference in depression screening rates in patients with arthritis, compared with the general population, despite patients with arthritis being considered “high risk.” Given the endorsement of national guidelines for depression screening, quality improvement initiatives should target physicians and non-physicians to increase the recognition of depression in high-risk groups and the use of appropriate interventions, such as mental health referrals and/or treatment with antidepressants.
Dr. Mary Margaretten is an associate professor of medicine in the division of rheumatology at the University of California, San Francisco. She has no relevant disclosures.
Arthritis affects one in five adults and is one of the most frequent reasons for ambulatory visits to the primary care physician. Arthritis affects patients both physically and psychologically and often leads to depressed mood with subsequent worse health outcomes, including increased mortality. Specifically, depression in patients with arthritis is an independent risk factor for cardiovascular disease, myocardial infarction, and suicide. Patients with arthritis and associated depression have increased health service utilization and are less likely to be adherent with their medications. In addition to these negative health consequences, depression may contribute to unemployment, loss of work productivity, and increased healthcare costs in persons with arthritis.
Depression screening guidelines for adults with chronic musculoskeletal diseases such as arthritis have been endorsed by the U.K. National Institute of Clinical Excellence. The U.S. Preventive Service Task Force and the Canadian Task Force for Preventive Health Care recommend depression screening in all adults. However, before screening for depression in specific patient groups can be recommended, well-established criteria should be met. Generally, screening is reasonable if the condition, depression in this case, is important and prevalent, can be effectively treated, and cannot be readily detected without screening. Comorbid depression in patients with arthritis meets these criteria. It is highly prevalent with rates ranging from 18%-42%. Depression with inflammatory arthritis, such as rheumatoid arthritis (RA), occurs more frequently than with osteoarthritis but even though it is more prevalent, depression with RA is often unrecognized and/or untreated.
Performing depression screening should not unduly burden physicians because, on average, depression screening adds less than 3 minutes to a visit. Asking two simple questions about mood and anhedonia (“Over the past 2 weeks, have you felt down, depressed, or hopeless?” and “Over the past 2 weeks, have you felt little interest or pleasure in doing things?”) is as effective as using more formal instruments. Implicit in the use of depression screening is the assumption that screening will increase recognition of depression and that recognized patients would benefit from treatment. It has been shown that patients who screen positive but were not in treatment had high rates of depression and overall poor mental health outcomes. Thus, provision of or referral to treatment is a necessary follow-up to screening.
It has been shown that there is no difference in depression screening rates in patients with arthritis, compared with the general population, despite patients with arthritis being considered “high risk.” Given the endorsement of national guidelines for depression screening, quality improvement initiatives should target physicians and non-physicians to increase the recognition of depression in high-risk groups and the use of appropriate interventions, such as mental health referrals and/or treatment with antidepressants.
Dr. Mary Margaretten is an associate professor of medicine in the division of rheumatology at the University of California, San Francisco. She has no relevant disclosures.
All adults, including pregnant and postpartum women, should be screened for depression, according to new recommendations of the U.S. Preventive Services Task Force.
The recommendation also calls for screening to be coupled with “adequate systems” to ensure diagnosis, treatment, and follow-up (JAMA. 2016 Jan 26;315[4]:380-7).
The depression screening recommendation, authored by Dr. Albert L. Siu and the other members of the USPSTF, is a level B recommendation, meaning that it has either high certainty of moderate net benefit, or moderate certainty of moderate to substantial net benefit.
The new guidance in screening for depression helps address a disorder that is “the leading cause of disability among adults in high-income countries,” said Dr. Siu and his coauthors. Lost productivity attributable to depression cost $23 billion in the United States in 2011, and $22.8 billion was spent on treatments for depression in 2009, the last year for which figures are available.
Dr. Siu, chair of geriatrics and palliative medicine at Icahn School of Medicine at Mount Sinai, New York, and his coauthors cited “convincing evidence that screening improves the accurate identification of adult patients with depression in primary care settings, including pregnant and postpartum women.”
In addition, the task force found convincing evidence that for older adults as well as the general adult population, treatment of “depression identified through screening in primary care settings with antidepressants, psychotherapy, or both decreases clinical morbidity.”
For pregnant and postpartum women with depression, Dr. Siu and his coauthors found “adequate” evidence that cognitive behavioral therapy (CBT) improves outcomes.
The recommendation does not identify optimal timing and intervals for depression screening, citing a need for more research in this area. However, “a pragmatic approach might include screening all adults who have not been screened previously and using clinical judgment in consideration of risk factors, comorbid conditions, and life events to determine if additional screening of high-risk patients is warranted,” explained Dr. Siu and his coauthors.
The new depression screening recommendation from USPSTF updates the 2009 recommendation, which recommended universal screening if “staff-assisted depression care supports” were in place, and targeted screening based on clinical judgment and patient preference if such support were unavailable.
The rationale for the current recommendation of universal screening for those 18 years and older is the “recognition that such support is now much more widely available and accepted as part of mental health care,” the task force members said.
Any potential harms of screening, said Dr. Siu and his coauthors, were minimal to nonexistent.
Overall, the USPSTF assigned a small to moderate risk to the use of medication in depression. However, the use of “second-generation” antidepressants – mostly SSRIs – was associated with some harms, including increased risk of suicidal behavior in young adults and of gastrointestinal bleeding in older adults, as well as potential fetal harms in pregnant women taking antidepressants.
Using CBT to treat depression in pregnant and postpartum women was also associated with minimal to no harm.
The USPSTF screening recommendation is aligned with the American Academy of Family Physicians’ recommendation to screen the general adult population for depression, and with the American Academy of Pediatrics’ recommendation that pediatricians screen mothers for depression at their babies’ 1-, 2-, and 4-month office visits.
Released in draft form in July 2015, the depression screening recommendation was available for public comment for a period of 4 weeks. In response to public input, the final recommendation’s implementation section clarifies and characterizes an “adequate system” of screening, and gives more resources for evidence-based depression screening and treatment.
The Agency for Healthcare Research and Quality supports the operations of the USPSTF, but the task force’s recommendations are independent of the federal government. Dr. Siu and the other task force members reported no conflicts of interest.
On Twitter @karioakes
All adults, including pregnant and postpartum women, should be screened for depression, according to new recommendations of the U.S. Preventive Services Task Force.
The recommendation also calls for screening to be coupled with “adequate systems” to ensure diagnosis, treatment, and follow-up (JAMA. 2016 Jan 26;315[4]:380-7).
The depression screening recommendation, authored by Dr. Albert L. Siu and the other members of the USPSTF, is a level B recommendation, meaning that it has either high certainty of moderate net benefit, or moderate certainty of moderate to substantial net benefit.
The new guidance in screening for depression helps address a disorder that is “the leading cause of disability among adults in high-income countries,” said Dr. Siu and his coauthors. Lost productivity attributable to depression cost $23 billion in the United States in 2011, and $22.8 billion was spent on treatments for depression in 2009, the last year for which figures are available.
Dr. Siu, chair of geriatrics and palliative medicine at Icahn School of Medicine at Mount Sinai, New York, and his coauthors cited “convincing evidence that screening improves the accurate identification of adult patients with depression in primary care settings, including pregnant and postpartum women.”
In addition, the task force found convincing evidence that for older adults as well as the general adult population, treatment of “depression identified through screening in primary care settings with antidepressants, psychotherapy, or both decreases clinical morbidity.”
For pregnant and postpartum women with depression, Dr. Siu and his coauthors found “adequate” evidence that cognitive behavioral therapy (CBT) improves outcomes.
The recommendation does not identify optimal timing and intervals for depression screening, citing a need for more research in this area. However, “a pragmatic approach might include screening all adults who have not been screened previously and using clinical judgment in consideration of risk factors, comorbid conditions, and life events to determine if additional screening of high-risk patients is warranted,” explained Dr. Siu and his coauthors.
The new depression screening recommendation from USPSTF updates the 2009 recommendation, which recommended universal screening if “staff-assisted depression care supports” were in place, and targeted screening based on clinical judgment and patient preference if such support were unavailable.
The rationale for the current recommendation of universal screening for those 18 years and older is the “recognition that such support is now much more widely available and accepted as part of mental health care,” the task force members said.
Any potential harms of screening, said Dr. Siu and his coauthors, were minimal to nonexistent.
Overall, the USPSTF assigned a small to moderate risk to the use of medication in depression. However, the use of “second-generation” antidepressants – mostly SSRIs – was associated with some harms, including increased risk of suicidal behavior in young adults and of gastrointestinal bleeding in older adults, as well as potential fetal harms in pregnant women taking antidepressants.
Using CBT to treat depression in pregnant and postpartum women was also associated with minimal to no harm.
The USPSTF screening recommendation is aligned with the American Academy of Family Physicians’ recommendation to screen the general adult population for depression, and with the American Academy of Pediatrics’ recommendation that pediatricians screen mothers for depression at their babies’ 1-, 2-, and 4-month office visits.
Released in draft form in July 2015, the depression screening recommendation was available for public comment for a period of 4 weeks. In response to public input, the final recommendation’s implementation section clarifies and characterizes an “adequate system” of screening, and gives more resources for evidence-based depression screening and treatment.
The Agency for Healthcare Research and Quality supports the operations of the USPSTF, but the task force’s recommendations are independent of the federal government. Dr. Siu and the other task force members reported no conflicts of interest.
On Twitter @karioakes
FROM JAMA
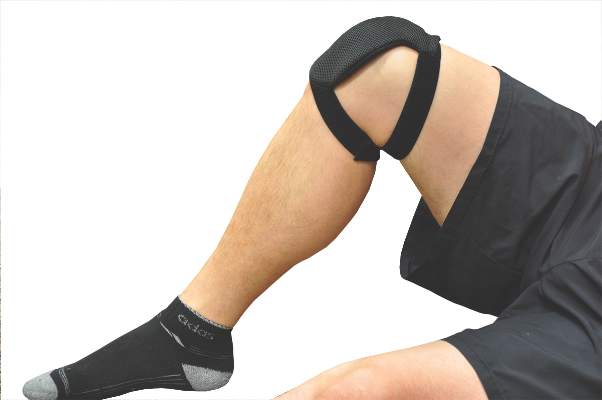
Wearable device offers home-based knee OA pain relief
A wearable pulsed electromagnetic fields device reduced pain intensity and improved physical functioning in patients with painful knee osteoarthritis (OA) in a double-blind, randomized trial.
The commercially available device (ActiPatch, Bioelectronics Corp.) did not improve patients’ mental health, but significantly reduced patients’ intake of NSAIDs and analgesics, compared with placebo.
“Although NSAIDs remain the gold standard for the treatment of pain in OA, there is increasing need to find conservative and alternative approaches, in order to avoid the toxicity associated with the chronic use of the analgesics, mostly in the elderly population,” wrote Dr. Gian Luca Bagnato of the University of Messina (Italy) and his colleagues (Rheumatology [Oxford]. 2015 Dec 24. doi: 10.1093/rheumatology/kev426).
Pulsed electromagnetic fields (PEMF) therapy has been shown to reduce chondrocyte apoptosis and MMP-13 expression of knee cartilage and favorably affect cartilage homeostasis in animal models, but data regarding osteoarthritis (OA) pain and function in humans are mixed.
A recent systematic review found no effect in all 14 trials analyzed, but when only high-quality randomized clinical trials were included, PEMF provided significantly better pain relief at 4 and 8 weeks and better function at 8 weeks than did placebo (Rheumatology [Oxford]. 2013;52[5]:815-24).
Not only has the quality of trials varied, so has the PEMF pulse frequency and duration used in trials, “further limiting the possibility of comparing efficacy and safety,” Dr. Bagnato and associates observed.
The current study evenly randomized 60 patients with radiologic evidence of knee OA and persistent pain to wear the PEMF or a placebo device for a minimum of 12 hours, mainly at night, with the device kept in place with a wrap. The active device emits a form of non-ionizing electromagnetic radiation at a frequency of 27.12 MHz, a pulse rate of 1,000 Hz, and a burst width of 100 microsec.
Persistent pain was defined as a minimal mean score of 40 mm for global pain on the VAS (visual analog scale) and daily pain during the month prior to enrollment despite maximal tolerated doses of conventional medical therapy, including acetaminophen and/or an NSAID. The patients’ mean age was 67.7 years and mean OA duration 12 years.
The primary efficacy endpoint was reduction in pain intensity at 1 month on the VAS and WOMAC (Western Ontario and McMaster Universities Arthritis Index). The mean WOMAC total score at baseline was 132.9.
At 1 month, VAS pain scores were reduced 25.5% with the PEMF device and 3.6% with the placebo device. The standardized treatment effect size induced by PEMF therapy was –0.73 (95% confidence interval, –1.24 to –0.19), the investigators reported.
WOMAC pain subscale and total scores fell 23.4% and 18.4% with the PEMF device versus a 2.3% reduction for both scores with the placebo device. The standardized effect size was –0.61 for WOMAC pain (95% CI, –1.12 to –0.09) and –0.34 for WOMAC total score (95% CI, –0.85 to 0.17).
At 1 month, the mean Short Form-36 physical health score was significantly better in the PEMF group than in the placebo group (55.8 vs. 53.1; P = .024), while SF-36 mental health scores were nearly identical (43.8 vs. 43.6; P = .6).
Patients were allowed per protocol to take prescribed analgesic therapy as needed, but eight patients from the PEMF group stopped these medications, while one patient from the placebo group stopped medication and three started a new therapy for chronic pain. No adverse events were reported during the study.
“Given that our data are limited to a low number of participants and the long-term efficacy of the wearable device is unknown, the generalizability of the results needs to be confirmed in a larger clinical trial with a longer duration of treatment,” Dr. Bagnato and his coauthors concluded. “However, the use of a wearable PEMF therapy in knee OA can be considered as an alternative safe and effective therapy in knee OA, providing the possibility for home-based management of pain, compared with previous studies.”
Nonpharmacologic therapies and pharmacologic agents are helpful for a large segment of the population with knee osteoarthritis (OA). In contrast to rheumatoid arthritis for which there are now many truly effective agents, the physician and patient are frustrated with the borderline effective therapies for a proportion of those poorly responsive patients on present day therapy with knee OA. Joint replacement continues to be the most effective treatment for hip and knee OA, but many have postoperative joint pain. In addition, the population of patients with pain from knee OA is growing with the aging population and already exceeds the number that can be accommodated by our present physicians, without even considering the financial burden.
 |
Dr. Roy D. Altman |
Until something is of proven benefit, researchers continue to fine-tune existing programs to maximize their benefit. One of the nonpharmacologic therapies is a wearable device that delivers pulsed electromagnetic fields (PEMF). Clinical trials supporting pulsed electrical stimulation for knee OA have been present for more than 20 years (J Rheumatol. 1993 Mar;20:456-60 and J Rheumatol. 1995 Sep;22:1757-61). Indeed, the devices have been commercially available for more than 10 years.
In the conclusions of a 2013 Cochrane review, “... electromagnetic field treatment may provide moderate benefit for osteoarthritis sufferers in terms of pain relief,” with more data needed for physical function and quality of life (Cochrane Database Syst Rev. 2013;Dec 14;12:CD003523). The studies tended to be small, as there were nine studies including 636 patients in the review. One of the problems in performing a systematic review is that there have been a variety of devices tested that vary in their functions. Examples of devices that have been tested in knee OA are the ActiPatch (used in the study by Dr. Bagnato and his colleagues), BioniCare, EarthPulse, MAGCELL ARTHRO, and Magnetofield devices. They vary in structure, size, frequency (Hz) per area, magnetic flux density, time intervals of each frequency (burst milliseconds), voltage, decibel level, duty cycle, contact time and intervals, wearing device for minutes/hours, etc. Blinding of when the device is on or off in studies has been complicated.
Dr. Bagnato and his associates add to the limited literature with a well-designed and well-conducted but relatively small trial. However, until there are more data, it will be difficult to use these devices on a regular basis, as they tend to be quite expensive and require a strong commitment of time and energy by the patient, who often thinks of the device as a form of alternative medicine.
Dr. Roy D. Altman is professor emeritus of medicine in the division of rheumatology and immunology at the University of California, Los Angeles. He has no relevant disclosures.
Nonpharmacologic therapies and pharmacologic agents are helpful for a large segment of the population with knee osteoarthritis (OA). In contrast to rheumatoid arthritis for which there are now many truly effective agents, the physician and patient are frustrated with the borderline effective therapies for a proportion of those poorly responsive patients on present day therapy with knee OA. Joint replacement continues to be the most effective treatment for hip and knee OA, but many have postoperative joint pain. In addition, the population of patients with pain from knee OA is growing with the aging population and already exceeds the number that can be accommodated by our present physicians, without even considering the financial burden.
 |
Dr. Roy D. Altman |
Until something is of proven benefit, researchers continue to fine-tune existing programs to maximize their benefit. One of the nonpharmacologic therapies is a wearable device that delivers pulsed electromagnetic fields (PEMF). Clinical trials supporting pulsed electrical stimulation for knee OA have been present for more than 20 years (J Rheumatol. 1993 Mar;20:456-60 and J Rheumatol. 1995 Sep;22:1757-61). Indeed, the devices have been commercially available for more than 10 years.
In the conclusions of a 2013 Cochrane review, “... electromagnetic field treatment may provide moderate benefit for osteoarthritis sufferers in terms of pain relief,” with more data needed for physical function and quality of life (Cochrane Database Syst Rev. 2013;Dec 14;12:CD003523). The studies tended to be small, as there were nine studies including 636 patients in the review. One of the problems in performing a systematic review is that there have been a variety of devices tested that vary in their functions. Examples of devices that have been tested in knee OA are the ActiPatch (used in the study by Dr. Bagnato and his colleagues), BioniCare, EarthPulse, MAGCELL ARTHRO, and Magnetofield devices. They vary in structure, size, frequency (Hz) per area, magnetic flux density, time intervals of each frequency (burst milliseconds), voltage, decibel level, duty cycle, contact time and intervals, wearing device for minutes/hours, etc. Blinding of when the device is on or off in studies has been complicated.
Dr. Bagnato and his associates add to the limited literature with a well-designed and well-conducted but relatively small trial. However, until there are more data, it will be difficult to use these devices on a regular basis, as they tend to be quite expensive and require a strong commitment of time and energy by the patient, who often thinks of the device as a form of alternative medicine.
Dr. Roy D. Altman is professor emeritus of medicine in the division of rheumatology and immunology at the University of California, Los Angeles. He has no relevant disclosures.
Nonpharmacologic therapies and pharmacologic agents are helpful for a large segment of the population with knee osteoarthritis (OA). In contrast to rheumatoid arthritis for which there are now many truly effective agents, the physician and patient are frustrated with the borderline effective therapies for a proportion of those poorly responsive patients on present day therapy with knee OA. Joint replacement continues to be the most effective treatment for hip and knee OA, but many have postoperative joint pain. In addition, the population of patients with pain from knee OA is growing with the aging population and already exceeds the number that can be accommodated by our present physicians, without even considering the financial burden.
 |
Dr. Roy D. Altman |
Until something is of proven benefit, researchers continue to fine-tune existing programs to maximize their benefit. One of the nonpharmacologic therapies is a wearable device that delivers pulsed electromagnetic fields (PEMF). Clinical trials supporting pulsed electrical stimulation for knee OA have been present for more than 20 years (J Rheumatol. 1993 Mar;20:456-60 and J Rheumatol. 1995 Sep;22:1757-61). Indeed, the devices have been commercially available for more than 10 years.
In the conclusions of a 2013 Cochrane review, “... electromagnetic field treatment may provide moderate benefit for osteoarthritis sufferers in terms of pain relief,” with more data needed for physical function and quality of life (Cochrane Database Syst Rev. 2013;Dec 14;12:CD003523). The studies tended to be small, as there were nine studies including 636 patients in the review. One of the problems in performing a systematic review is that there have been a variety of devices tested that vary in their functions. Examples of devices that have been tested in knee OA are the ActiPatch (used in the study by Dr. Bagnato and his colleagues), BioniCare, EarthPulse, MAGCELL ARTHRO, and Magnetofield devices. They vary in structure, size, frequency (Hz) per area, magnetic flux density, time intervals of each frequency (burst milliseconds), voltage, decibel level, duty cycle, contact time and intervals, wearing device for minutes/hours, etc. Blinding of when the device is on or off in studies has been complicated.
Dr. Bagnato and his associates add to the limited literature with a well-designed and well-conducted but relatively small trial. However, until there are more data, it will be difficult to use these devices on a regular basis, as they tend to be quite expensive and require a strong commitment of time and energy by the patient, who often thinks of the device as a form of alternative medicine.
Dr. Roy D. Altman is professor emeritus of medicine in the division of rheumatology and immunology at the University of California, Los Angeles. He has no relevant disclosures.
A wearable pulsed electromagnetic fields device reduced pain intensity and improved physical functioning in patients with painful knee osteoarthritis (OA) in a double-blind, randomized trial.
The commercially available device (ActiPatch, Bioelectronics Corp.) did not improve patients’ mental health, but significantly reduced patients’ intake of NSAIDs and analgesics, compared with placebo.
“Although NSAIDs remain the gold standard for the treatment of pain in OA, there is increasing need to find conservative and alternative approaches, in order to avoid the toxicity associated with the chronic use of the analgesics, mostly in the elderly population,” wrote Dr. Gian Luca Bagnato of the University of Messina (Italy) and his colleagues (Rheumatology [Oxford]. 2015 Dec 24. doi: 10.1093/rheumatology/kev426).
Pulsed electromagnetic fields (PEMF) therapy has been shown to reduce chondrocyte apoptosis and MMP-13 expression of knee cartilage and favorably affect cartilage homeostasis in animal models, but data regarding osteoarthritis (OA) pain and function in humans are mixed.
A recent systematic review found no effect in all 14 trials analyzed, but when only high-quality randomized clinical trials were included, PEMF provided significantly better pain relief at 4 and 8 weeks and better function at 8 weeks than did placebo (Rheumatology [Oxford]. 2013;52[5]:815-24).
Not only has the quality of trials varied, so has the PEMF pulse frequency and duration used in trials, “further limiting the possibility of comparing efficacy and safety,” Dr. Bagnato and associates observed.
The current study evenly randomized 60 patients with radiologic evidence of knee OA and persistent pain to wear the PEMF or a placebo device for a minimum of 12 hours, mainly at night, with the device kept in place with a wrap. The active device emits a form of non-ionizing electromagnetic radiation at a frequency of 27.12 MHz, a pulse rate of 1,000 Hz, and a burst width of 100 microsec.
Persistent pain was defined as a minimal mean score of 40 mm for global pain on the VAS (visual analog scale) and daily pain during the month prior to enrollment despite maximal tolerated doses of conventional medical therapy, including acetaminophen and/or an NSAID. The patients’ mean age was 67.7 years and mean OA duration 12 years.
The primary efficacy endpoint was reduction in pain intensity at 1 month on the VAS and WOMAC (Western Ontario and McMaster Universities Arthritis Index). The mean WOMAC total score at baseline was 132.9.
At 1 month, VAS pain scores were reduced 25.5% with the PEMF device and 3.6% with the placebo device. The standardized treatment effect size induced by PEMF therapy was –0.73 (95% confidence interval, –1.24 to –0.19), the investigators reported.
WOMAC pain subscale and total scores fell 23.4% and 18.4% with the PEMF device versus a 2.3% reduction for both scores with the placebo device. The standardized effect size was –0.61 for WOMAC pain (95% CI, –1.12 to –0.09) and –0.34 for WOMAC total score (95% CI, –0.85 to 0.17).
At 1 month, the mean Short Form-36 physical health score was significantly better in the PEMF group than in the placebo group (55.8 vs. 53.1; P = .024), while SF-36 mental health scores were nearly identical (43.8 vs. 43.6; P = .6).
Patients were allowed per protocol to take prescribed analgesic therapy as needed, but eight patients from the PEMF group stopped these medications, while one patient from the placebo group stopped medication and three started a new therapy for chronic pain. No adverse events were reported during the study.
“Given that our data are limited to a low number of participants and the long-term efficacy of the wearable device is unknown, the generalizability of the results needs to be confirmed in a larger clinical trial with a longer duration of treatment,” Dr. Bagnato and his coauthors concluded. “However, the use of a wearable PEMF therapy in knee OA can be considered as an alternative safe and effective therapy in knee OA, providing the possibility for home-based management of pain, compared with previous studies.”
A wearable pulsed electromagnetic fields device reduced pain intensity and improved physical functioning in patients with painful knee osteoarthritis (OA) in a double-blind, randomized trial.
The commercially available device (ActiPatch, Bioelectronics Corp.) did not improve patients’ mental health, but significantly reduced patients’ intake of NSAIDs and analgesics, compared with placebo.
“Although NSAIDs remain the gold standard for the treatment of pain in OA, there is increasing need to find conservative and alternative approaches, in order to avoid the toxicity associated with the chronic use of the analgesics, mostly in the elderly population,” wrote Dr. Gian Luca Bagnato of the University of Messina (Italy) and his colleagues (Rheumatology [Oxford]. 2015 Dec 24. doi: 10.1093/rheumatology/kev426).
Pulsed electromagnetic fields (PEMF) therapy has been shown to reduce chondrocyte apoptosis and MMP-13 expression of knee cartilage and favorably affect cartilage homeostasis in animal models, but data regarding osteoarthritis (OA) pain and function in humans are mixed.
A recent systematic review found no effect in all 14 trials analyzed, but when only high-quality randomized clinical trials were included, PEMF provided significantly better pain relief at 4 and 8 weeks and better function at 8 weeks than did placebo (Rheumatology [Oxford]. 2013;52[5]:815-24).
Not only has the quality of trials varied, so has the PEMF pulse frequency and duration used in trials, “further limiting the possibility of comparing efficacy and safety,” Dr. Bagnato and associates observed.
The current study evenly randomized 60 patients with radiologic evidence of knee OA and persistent pain to wear the PEMF or a placebo device for a minimum of 12 hours, mainly at night, with the device kept in place with a wrap. The active device emits a form of non-ionizing electromagnetic radiation at a frequency of 27.12 MHz, a pulse rate of 1,000 Hz, and a burst width of 100 microsec.
Persistent pain was defined as a minimal mean score of 40 mm for global pain on the VAS (visual analog scale) and daily pain during the month prior to enrollment despite maximal tolerated doses of conventional medical therapy, including acetaminophen and/or an NSAID. The patients’ mean age was 67.7 years and mean OA duration 12 years.
The primary efficacy endpoint was reduction in pain intensity at 1 month on the VAS and WOMAC (Western Ontario and McMaster Universities Arthritis Index). The mean WOMAC total score at baseline was 132.9.
At 1 month, VAS pain scores were reduced 25.5% with the PEMF device and 3.6% with the placebo device. The standardized treatment effect size induced by PEMF therapy was –0.73 (95% confidence interval, –1.24 to –0.19), the investigators reported.
WOMAC pain subscale and total scores fell 23.4% and 18.4% with the PEMF device versus a 2.3% reduction for both scores with the placebo device. The standardized effect size was –0.61 for WOMAC pain (95% CI, –1.12 to –0.09) and –0.34 for WOMAC total score (95% CI, –0.85 to 0.17).
At 1 month, the mean Short Form-36 physical health score was significantly better in the PEMF group than in the placebo group (55.8 vs. 53.1; P = .024), while SF-36 mental health scores were nearly identical (43.8 vs. 43.6; P = .6).
Patients were allowed per protocol to take prescribed analgesic therapy as needed, but eight patients from the PEMF group stopped these medications, while one patient from the placebo group stopped medication and three started a new therapy for chronic pain. No adverse events were reported during the study.
“Given that our data are limited to a low number of participants and the long-term efficacy of the wearable device is unknown, the generalizability of the results needs to be confirmed in a larger clinical trial with a longer duration of treatment,” Dr. Bagnato and his coauthors concluded. “However, the use of a wearable PEMF therapy in knee OA can be considered as an alternative safe and effective therapy in knee OA, providing the possibility for home-based management of pain, compared with previous studies.”
FROM RHEUMATOLOGY
Key clinical point: Pulsed electromagnetic fields therapy is safe and effective in improving knee osteoarthritis symptoms.
Major finding: The mean treatment effect size was –0.73 in the VAS score and –0.34 in the WOMAC score.
Data source: Double-blind, randomized trial in 60 patients with knee osteoarthritis and persistent pain.
Disclosures: Bioelectronics provided the pulsed electromagnetic fields and placebo devices. The authors reported having no conflicts of interest.
Rheumatology trends, research, concerns highlighted for 2016
The coming year in rheumatology brings with it a variety of trends and concerns about how rheumatologists can chart the best course for their practices and patients amid mounting fiscal and regulatory pressures.
Questions also arise as to how rheumatology can improve its attractiveness to students and residents in 2016 with the current level of effort in mentoring, outreach, and competition against higher-paying subspecialties.
There’s also high interest and expectations in the new year for studies on systemic sclerosis and microbiome research, as well as questions about what the future holds for intra-articular hyaluronic acid and over-the-counter topical nonsteroidal anti-inflammatory drugs for osteoarthritis (OA).
Rheumatology News editorial advisory board members gave their thoughts on these areas of rheumatology in 2016.
Insurance and reimbursement problems
The changing landscape in insurance plans, brought about largely by the Affordable Care Act, is having a big impact on patients and physicians, particularly in Florida, where more than 1.5 million people signed up for an ACA federal marketplace plan in 2015. Difficulty in accessing and affording care in 2016 figures to be an even greater problem, said Dr. Norman Gaylis, who is in private practice in Aventura, Fla.
In general, many policies are passing an increased burden onto patients in regard to deductibles, copayments, and costs of medications, he said. “That’s putting tremendous stress on practices. I think this is nationwide, where we’re finding that rheumatology patients are not getting access to the drugs for [several] reasons: they’ve become unaffordable, the various pharmaceutical support programs have run out of money, and the amount of work that practices are now performing in trying to get authorization for the patients far exceeds any type of revenue [it] could be generating or should be generating to cover these increased costs. So essentially there’s a reduction in reimbursement and a reduction in revenue going along at the same time.”
Dr. Gaylis noted that there is “tremendous pressure” from all sides to reduce access to rheumatology drugs, which have rising costs. For instance, the cost of a monthly supply of generic celebrex in his practice’s area is on average $120-$200, “which is almost prohibitive for many of our patients.”
“We’re finding that this year [2015] alone, 20% of patients who are on standard infusion therapy as a routine part of their management of rheumatoid arthritis have basically dropped out,” he said. “If that’s equal across the board, that means a very high number of patients are not getting optimal care.”
The trend for rheumatologists, particularly those in solo practice, to make contracts with fewer insurance companies could accelerate in 2016, Dr. Gaylis said.
Some rheumatologists are beginning to not accept insured patients with coverage from managed care companies or the lower-tier payers, and “that’s a significant trend if it starts evolving because it will create a two-tiered system in that you’ll have the more affluent patient going to one of these practices, and then you’ll have clinics where you’ll have a totally different level of care.” Whether it builds up enough to where rheumatologists begin to develop hybrid concierge practices is a fair question, he said. “It’s very difficult to conceive of a patient paying for both primary care and subspecialist concierge service. But I am starting to see signals where there may well be some integration between concierge primary care and concierge subspecialties.”
Training, mentoring more rheumatologists
Another issue going into 2016 is the lack of mentoring and assistance to medical students and residents to draw them to the subspecialty and keep them there, as well as the viability of rheumatology as an attractive subspecialty. “It’s difficult to see how we can attract medical students and residents to the specialty when the cost of their education leaves them with staggering bills to be paid. You’ve got to be extremely passionate to want to be a rheumatologist,” Dr. Gaylis said.
Dr. Elizabeth Volkmann, clinical instructor in rheumatology at the University of California, Los Angeles, agreed and said she looked forward to seeing how the future of mentoring programs in rheumatology will progress in 2016 and beyond. She noted that the American College of Rheumatology (ACR)/Childhood Arthritis and Rheumatology Research Alliance Mentoring Interest Group (AMIGO), a career-mentoring program that serves most fellows and many junior faculty in pediatric rheumatology across the United States and Canada, recently reported success in establishing mentor contact, suitability of mentor-mentee pairing, as well as benefit with respect to career development, scholarship, and work-life balance, and was especially useful to fellows, compared with junior faculty (Arthritis Care Res. 2015 Sep 28. doi: 10.1002/acr.22732).
Dr. Volkmann expressed interest in seeing how the ACR’s Choose Rheumatology! mentorship program is performing. The program seeks to pair students and residents with rheumatologist mentors who will offer advice and help to guide them. She also pointed to the European League Against Rheumatism’s working group for young rheumatologists, the Emerging EULAR Network (EMEUNET), which seeks to promote the educational, research, and mentoring needs of young clinicians and researchers in rheumatology in Europe. as a potential model for the ACR to use in the United States.
Some of the conference-based resources available to rheumatology fellows include the ACR’s 2016 State-of-the-Art Clinical Symposium, which provides a presymposium course specifically for fellows and has sessions on choosing career paths, and the Rheumatology Research Workshop, which targets rheumatologists interested in pursuing an academic research career. Fellows-in-training travel scholarships are available for both conferences.
New systemic sclerosis and microbiome research
Many new studies in systemic sclerosis will build on results obtained in earlier-phase trials or unanswered questions arising out of treatment comparison studies. “It is a very exciting time in the world of scleroderma,” said Dr. Virginia Steen, professor of medicine at Georgetown University, Washington.
At least four new trials are studying treatments for skin manifestations of systemic sclerosis, noted Dr. Steen. In patients with diffuse cutaneous systemic sclerosis, the phase II ASSET study is testing abatacept (Orencia) against placebo and another phase II study is testing riociguat (Adempas). Roche has started enrolling patients for a phase III trial of tocilizumab (Actemra) on the heels of positive findings from its phase II study. Dr. Steen and colleagues at Johns Hopkins University are also finishing up a pilot study of the effects of intravenous immunoglobulin (Privigen) on skin disease in systemic sclerosis and should have results ready for 2016.
Two additional trials will be investigating treatments for interstitial lung disease associated with systemic sclerosis: the Scleroderma Lung Study III, testing the use of mycophenolate mofetil in all patients with the addition of pirfenidone (Esbriet) or placebo, and a separate phase III study of nintedanib (Ofev) vs. placebo that just began enrolling patients.
There are also several trials of add-on therapies, including topical nitroglycerin for Raynaud’s phenomenon or riociguat for digital ulcers, and another that is testing autologous adipose–derived regenerative cells for the treatment of hand dysfunction.
In addition to these trials now underway, the expected 2016 publication of the validation of the Combined Response Index for Systemic Sclerosis will hopefully “lead to real movement forward in the treatment of systemic sclerosis,” said Dr. Daniel E. Furst, the Carl Pearson Professor of Medicine at University of California, Los Angeles.
Further examination of the Wnt signaling pathway in systemic sclerosis should also bring better insights into the disease’s pathogenesis and potential for treatment, Dr. Furst noted, as recent experimental results have shown that its activation induces fibroblast activation with subsequent myofibroblast differentiation and excessive collagen release. Small-molecule inhibitors of Wnt signaling in early clinical trials have shown promising results.
Dr. Furst and Dr. Volkmann said they also hope for studies describing better and more specific data regarding the microbiome in rheumatic disease, especially systemic sclerosis and rheumatoid arthritis, both of which have microbiome data linked with clinically meaningful outcomes (Nat Med. 2015 Aug;21[8]:895-905).
An important unanswered question, Dr. Volkmann said, is whether differences found in GI microbial composition between diseases and between different disease subtypes are clinically meaningful.
OA treatments: AAOS guidelines, OTC topical NSAIDs
The repercussions of the American Academy of Orthopaedic Surgeons 2013 clinical practice guideline’s statement on intra-articular hyaluronic acid treatment of knee OA will continue to be felt in 2016, according to Dr. Roy D. Altman, professor emeritus of medicine at University of California, Los Angeles.
The AAOS took a different stance from all other recent treatment guidelines for knee OA by stating: “Intra-articular hyaluronic acid is no longer recommended as a method of treatment for patients with symptomatic osteoarthritis of the knee.” The AAOS’s recommendation didn’t create much confusion with physicians and patients because its use continues to increase, but instead it has contributed to “a plethora of contradictory publications and increasing resistance on coverage by insurance carriers,” Dr. Altman said.
Another unresolved issue in OA treatment is “the resistance of the FDA to approve over-the-counter topical NSAIDs,” Dr. Altman said. The FDA requires a new drug application for OTC topical NSAIDs demonstrating efficacy and safety, “as if they were completely new,” when the safety exceeds presently approved OTC oral NSAIDs and has already been shown for prescription topical NSAIDs, he said.
The coming year in rheumatology brings with it a variety of trends and concerns about how rheumatologists can chart the best course for their practices and patients amid mounting fiscal and regulatory pressures.
Questions also arise as to how rheumatology can improve its attractiveness to students and residents in 2016 with the current level of effort in mentoring, outreach, and competition against higher-paying subspecialties.
There’s also high interest and expectations in the new year for studies on systemic sclerosis and microbiome research, as well as questions about what the future holds for intra-articular hyaluronic acid and over-the-counter topical nonsteroidal anti-inflammatory drugs for osteoarthritis (OA).
Rheumatology News editorial advisory board members gave their thoughts on these areas of rheumatology in 2016.
Insurance and reimbursement problems
The changing landscape in insurance plans, brought about largely by the Affordable Care Act, is having a big impact on patients and physicians, particularly in Florida, where more than 1.5 million people signed up for an ACA federal marketplace plan in 2015. Difficulty in accessing and affording care in 2016 figures to be an even greater problem, said Dr. Norman Gaylis, who is in private practice in Aventura, Fla.
In general, many policies are passing an increased burden onto patients in regard to deductibles, copayments, and costs of medications, he said. “That’s putting tremendous stress on practices. I think this is nationwide, where we’re finding that rheumatology patients are not getting access to the drugs for [several] reasons: they’ve become unaffordable, the various pharmaceutical support programs have run out of money, and the amount of work that practices are now performing in trying to get authorization for the patients far exceeds any type of revenue [it] could be generating or should be generating to cover these increased costs. So essentially there’s a reduction in reimbursement and a reduction in revenue going along at the same time.”
Dr. Gaylis noted that there is “tremendous pressure” from all sides to reduce access to rheumatology drugs, which have rising costs. For instance, the cost of a monthly supply of generic celebrex in his practice’s area is on average $120-$200, “which is almost prohibitive for many of our patients.”
“We’re finding that this year [2015] alone, 20% of patients who are on standard infusion therapy as a routine part of their management of rheumatoid arthritis have basically dropped out,” he said. “If that’s equal across the board, that means a very high number of patients are not getting optimal care.”
The trend for rheumatologists, particularly those in solo practice, to make contracts with fewer insurance companies could accelerate in 2016, Dr. Gaylis said.
Some rheumatologists are beginning to not accept insured patients with coverage from managed care companies or the lower-tier payers, and “that’s a significant trend if it starts evolving because it will create a two-tiered system in that you’ll have the more affluent patient going to one of these practices, and then you’ll have clinics where you’ll have a totally different level of care.” Whether it builds up enough to where rheumatologists begin to develop hybrid concierge practices is a fair question, he said. “It’s very difficult to conceive of a patient paying for both primary care and subspecialist concierge service. But I am starting to see signals where there may well be some integration between concierge primary care and concierge subspecialties.”
Training, mentoring more rheumatologists
Another issue going into 2016 is the lack of mentoring and assistance to medical students and residents to draw them to the subspecialty and keep them there, as well as the viability of rheumatology as an attractive subspecialty. “It’s difficult to see how we can attract medical students and residents to the specialty when the cost of their education leaves them with staggering bills to be paid. You’ve got to be extremely passionate to want to be a rheumatologist,” Dr. Gaylis said.
Dr. Elizabeth Volkmann, clinical instructor in rheumatology at the University of California, Los Angeles, agreed and said she looked forward to seeing how the future of mentoring programs in rheumatology will progress in 2016 and beyond. She noted that the American College of Rheumatology (ACR)/Childhood Arthritis and Rheumatology Research Alliance Mentoring Interest Group (AMIGO), a career-mentoring program that serves most fellows and many junior faculty in pediatric rheumatology across the United States and Canada, recently reported success in establishing mentor contact, suitability of mentor-mentee pairing, as well as benefit with respect to career development, scholarship, and work-life balance, and was especially useful to fellows, compared with junior faculty (Arthritis Care Res. 2015 Sep 28. doi: 10.1002/acr.22732).
Dr. Volkmann expressed interest in seeing how the ACR’s Choose Rheumatology! mentorship program is performing. The program seeks to pair students and residents with rheumatologist mentors who will offer advice and help to guide them. She also pointed to the European League Against Rheumatism’s working group for young rheumatologists, the Emerging EULAR Network (EMEUNET), which seeks to promote the educational, research, and mentoring needs of young clinicians and researchers in rheumatology in Europe. as a potential model for the ACR to use in the United States.
Some of the conference-based resources available to rheumatology fellows include the ACR’s 2016 State-of-the-Art Clinical Symposium, which provides a presymposium course specifically for fellows and has sessions on choosing career paths, and the Rheumatology Research Workshop, which targets rheumatologists interested in pursuing an academic research career. Fellows-in-training travel scholarships are available for both conferences.
New systemic sclerosis and microbiome research
Many new studies in systemic sclerosis will build on results obtained in earlier-phase trials or unanswered questions arising out of treatment comparison studies. “It is a very exciting time in the world of scleroderma,” said Dr. Virginia Steen, professor of medicine at Georgetown University, Washington.
At least four new trials are studying treatments for skin manifestations of systemic sclerosis, noted Dr. Steen. In patients with diffuse cutaneous systemic sclerosis, the phase II ASSET study is testing abatacept (Orencia) against placebo and another phase II study is testing riociguat (Adempas). Roche has started enrolling patients for a phase III trial of tocilizumab (Actemra) on the heels of positive findings from its phase II study. Dr. Steen and colleagues at Johns Hopkins University are also finishing up a pilot study of the effects of intravenous immunoglobulin (Privigen) on skin disease in systemic sclerosis and should have results ready for 2016.
Two additional trials will be investigating treatments for interstitial lung disease associated with systemic sclerosis: the Scleroderma Lung Study III, testing the use of mycophenolate mofetil in all patients with the addition of pirfenidone (Esbriet) or placebo, and a separate phase III study of nintedanib (Ofev) vs. placebo that just began enrolling patients.
There are also several trials of add-on therapies, including topical nitroglycerin for Raynaud’s phenomenon or riociguat for digital ulcers, and another that is testing autologous adipose–derived regenerative cells for the treatment of hand dysfunction.
In addition to these trials now underway, the expected 2016 publication of the validation of the Combined Response Index for Systemic Sclerosis will hopefully “lead to real movement forward in the treatment of systemic sclerosis,” said Dr. Daniel E. Furst, the Carl Pearson Professor of Medicine at University of California, Los Angeles.
Further examination of the Wnt signaling pathway in systemic sclerosis should also bring better insights into the disease’s pathogenesis and potential for treatment, Dr. Furst noted, as recent experimental results have shown that its activation induces fibroblast activation with subsequent myofibroblast differentiation and excessive collagen release. Small-molecule inhibitors of Wnt signaling in early clinical trials have shown promising results.
Dr. Furst and Dr. Volkmann said they also hope for studies describing better and more specific data regarding the microbiome in rheumatic disease, especially systemic sclerosis and rheumatoid arthritis, both of which have microbiome data linked with clinically meaningful outcomes (Nat Med. 2015 Aug;21[8]:895-905).
An important unanswered question, Dr. Volkmann said, is whether differences found in GI microbial composition between diseases and between different disease subtypes are clinically meaningful.
OA treatments: AAOS guidelines, OTC topical NSAIDs
The repercussions of the American Academy of Orthopaedic Surgeons 2013 clinical practice guideline’s statement on intra-articular hyaluronic acid treatment of knee OA will continue to be felt in 2016, according to Dr. Roy D. Altman, professor emeritus of medicine at University of California, Los Angeles.
The AAOS took a different stance from all other recent treatment guidelines for knee OA by stating: “Intra-articular hyaluronic acid is no longer recommended as a method of treatment for patients with symptomatic osteoarthritis of the knee.” The AAOS’s recommendation didn’t create much confusion with physicians and patients because its use continues to increase, but instead it has contributed to “a plethora of contradictory publications and increasing resistance on coverage by insurance carriers,” Dr. Altman said.
Another unresolved issue in OA treatment is “the resistance of the FDA to approve over-the-counter topical NSAIDs,” Dr. Altman said. The FDA requires a new drug application for OTC topical NSAIDs demonstrating efficacy and safety, “as if they were completely new,” when the safety exceeds presently approved OTC oral NSAIDs and has already been shown for prescription topical NSAIDs, he said.
The coming year in rheumatology brings with it a variety of trends and concerns about how rheumatologists can chart the best course for their practices and patients amid mounting fiscal and regulatory pressures.
Questions also arise as to how rheumatology can improve its attractiveness to students and residents in 2016 with the current level of effort in mentoring, outreach, and competition against higher-paying subspecialties.
There’s also high interest and expectations in the new year for studies on systemic sclerosis and microbiome research, as well as questions about what the future holds for intra-articular hyaluronic acid and over-the-counter topical nonsteroidal anti-inflammatory drugs for osteoarthritis (OA).
Rheumatology News editorial advisory board members gave their thoughts on these areas of rheumatology in 2016.
Insurance and reimbursement problems
The changing landscape in insurance plans, brought about largely by the Affordable Care Act, is having a big impact on patients and physicians, particularly in Florida, where more than 1.5 million people signed up for an ACA federal marketplace plan in 2015. Difficulty in accessing and affording care in 2016 figures to be an even greater problem, said Dr. Norman Gaylis, who is in private practice in Aventura, Fla.
In general, many policies are passing an increased burden onto patients in regard to deductibles, copayments, and costs of medications, he said. “That’s putting tremendous stress on practices. I think this is nationwide, where we’re finding that rheumatology patients are not getting access to the drugs for [several] reasons: they’ve become unaffordable, the various pharmaceutical support programs have run out of money, and the amount of work that practices are now performing in trying to get authorization for the patients far exceeds any type of revenue [it] could be generating or should be generating to cover these increased costs. So essentially there’s a reduction in reimbursement and a reduction in revenue going along at the same time.”
Dr. Gaylis noted that there is “tremendous pressure” from all sides to reduce access to rheumatology drugs, which have rising costs. For instance, the cost of a monthly supply of generic celebrex in his practice’s area is on average $120-$200, “which is almost prohibitive for many of our patients.”
“We’re finding that this year [2015] alone, 20% of patients who are on standard infusion therapy as a routine part of their management of rheumatoid arthritis have basically dropped out,” he said. “If that’s equal across the board, that means a very high number of patients are not getting optimal care.”
The trend for rheumatologists, particularly those in solo practice, to make contracts with fewer insurance companies could accelerate in 2016, Dr. Gaylis said.
Some rheumatologists are beginning to not accept insured patients with coverage from managed care companies or the lower-tier payers, and “that’s a significant trend if it starts evolving because it will create a two-tiered system in that you’ll have the more affluent patient going to one of these practices, and then you’ll have clinics where you’ll have a totally different level of care.” Whether it builds up enough to where rheumatologists begin to develop hybrid concierge practices is a fair question, he said. “It’s very difficult to conceive of a patient paying for both primary care and subspecialist concierge service. But I am starting to see signals where there may well be some integration between concierge primary care and concierge subspecialties.”
Training, mentoring more rheumatologists
Another issue going into 2016 is the lack of mentoring and assistance to medical students and residents to draw them to the subspecialty and keep them there, as well as the viability of rheumatology as an attractive subspecialty. “It’s difficult to see how we can attract medical students and residents to the specialty when the cost of their education leaves them with staggering bills to be paid. You’ve got to be extremely passionate to want to be a rheumatologist,” Dr. Gaylis said.
Dr. Elizabeth Volkmann, clinical instructor in rheumatology at the University of California, Los Angeles, agreed and said she looked forward to seeing how the future of mentoring programs in rheumatology will progress in 2016 and beyond. She noted that the American College of Rheumatology (ACR)/Childhood Arthritis and Rheumatology Research Alliance Mentoring Interest Group (AMIGO), a career-mentoring program that serves most fellows and many junior faculty in pediatric rheumatology across the United States and Canada, recently reported success in establishing mentor contact, suitability of mentor-mentee pairing, as well as benefit with respect to career development, scholarship, and work-life balance, and was especially useful to fellows, compared with junior faculty (Arthritis Care Res. 2015 Sep 28. doi: 10.1002/acr.22732).
Dr. Volkmann expressed interest in seeing how the ACR’s Choose Rheumatology! mentorship program is performing. The program seeks to pair students and residents with rheumatologist mentors who will offer advice and help to guide them. She also pointed to the European League Against Rheumatism’s working group for young rheumatologists, the Emerging EULAR Network (EMEUNET), which seeks to promote the educational, research, and mentoring needs of young clinicians and researchers in rheumatology in Europe. as a potential model for the ACR to use in the United States.
Some of the conference-based resources available to rheumatology fellows include the ACR’s 2016 State-of-the-Art Clinical Symposium, which provides a presymposium course specifically for fellows and has sessions on choosing career paths, and the Rheumatology Research Workshop, which targets rheumatologists interested in pursuing an academic research career. Fellows-in-training travel scholarships are available for both conferences.
New systemic sclerosis and microbiome research
Many new studies in systemic sclerosis will build on results obtained in earlier-phase trials or unanswered questions arising out of treatment comparison studies. “It is a very exciting time in the world of scleroderma,” said Dr. Virginia Steen, professor of medicine at Georgetown University, Washington.
At least four new trials are studying treatments for skin manifestations of systemic sclerosis, noted Dr. Steen. In patients with diffuse cutaneous systemic sclerosis, the phase II ASSET study is testing abatacept (Orencia) against placebo and another phase II study is testing riociguat (Adempas). Roche has started enrolling patients for a phase III trial of tocilizumab (Actemra) on the heels of positive findings from its phase II study. Dr. Steen and colleagues at Johns Hopkins University are also finishing up a pilot study of the effects of intravenous immunoglobulin (Privigen) on skin disease in systemic sclerosis and should have results ready for 2016.
Two additional trials will be investigating treatments for interstitial lung disease associated with systemic sclerosis: the Scleroderma Lung Study III, testing the use of mycophenolate mofetil in all patients with the addition of pirfenidone (Esbriet) or placebo, and a separate phase III study of nintedanib (Ofev) vs. placebo that just began enrolling patients.
There are also several trials of add-on therapies, including topical nitroglycerin for Raynaud’s phenomenon or riociguat for digital ulcers, and another that is testing autologous adipose–derived regenerative cells for the treatment of hand dysfunction.
In addition to these trials now underway, the expected 2016 publication of the validation of the Combined Response Index for Systemic Sclerosis will hopefully “lead to real movement forward in the treatment of systemic sclerosis,” said Dr. Daniel E. Furst, the Carl Pearson Professor of Medicine at University of California, Los Angeles.
Further examination of the Wnt signaling pathway in systemic sclerosis should also bring better insights into the disease’s pathogenesis and potential for treatment, Dr. Furst noted, as recent experimental results have shown that its activation induces fibroblast activation with subsequent myofibroblast differentiation and excessive collagen release. Small-molecule inhibitors of Wnt signaling in early clinical trials have shown promising results.
Dr. Furst and Dr. Volkmann said they also hope for studies describing better and more specific data regarding the microbiome in rheumatic disease, especially systemic sclerosis and rheumatoid arthritis, both of which have microbiome data linked with clinically meaningful outcomes (Nat Med. 2015 Aug;21[8]:895-905).
An important unanswered question, Dr. Volkmann said, is whether differences found in GI microbial composition between diseases and between different disease subtypes are clinically meaningful.
OA treatments: AAOS guidelines, OTC topical NSAIDs
The repercussions of the American Academy of Orthopaedic Surgeons 2013 clinical practice guideline’s statement on intra-articular hyaluronic acid treatment of knee OA will continue to be felt in 2016, according to Dr. Roy D. Altman, professor emeritus of medicine at University of California, Los Angeles.
The AAOS took a different stance from all other recent treatment guidelines for knee OA by stating: “Intra-articular hyaluronic acid is no longer recommended as a method of treatment for patients with symptomatic osteoarthritis of the knee.” The AAOS’s recommendation didn’t create much confusion with physicians and patients because its use continues to increase, but instead it has contributed to “a plethora of contradictory publications and increasing resistance on coverage by insurance carriers,” Dr. Altman said.
Another unresolved issue in OA treatment is “the resistance of the FDA to approve over-the-counter topical NSAIDs,” Dr. Altman said. The FDA requires a new drug application for OTC topical NSAIDs demonstrating efficacy and safety, “as if they were completely new,” when the safety exceeds presently approved OTC oral NSAIDs and has already been shown for prescription topical NSAIDs, he said.
Thigh muscle weakness a risk factor for knee replacement in women
Women with knee osteoarthritis who had low thigh muscle strength were more likely to need a knee replacement in a case-control study of participants in the Osteoarthritis Initiative (OAI).
In particular, predictors of knee replacement included knee extensor weakness in the year prior to knee replacement and longitudinal deterioration in knee extensor strength over a 2-year observation period prior to surgery. Measurement of knee extensor strength in women with knee osteoarthritis may then indicate who could benefit from weight training exercises to potentially delay or prevent the need for knee replacement surgery, said the researchers, led by Dr. Adam Culvenor of Paracelsus Medical University in Salzburg, Austria (Arthritis Rheumatol. 2015 Dec 14. doi: 10.1002/art.39540).
The optimal knee extensor strength threshold for differentiating those with and without knee replacement risk was approximately 200 N or 0.9 Nm/kg; or prevention of any loss of knee extensor strength over 2 years.
“There appears to be a considerable window for women below this threshold to obtain realistic strength gains and potentially lower the risk of knee replacement,” the study authors concluded.
In the multicenter, longitudinal, case-control study of 4,796 participants in the OAI (60% of whom were women), the investigators identified 136 participants who had received a knee replacement and matched them with controls who had not received a knee replacement and were similar in age, body mass index (BMI), and radiographic stage. The mean age of the women was 65 years and the mean BMI was 29 kg/m2.
The results showed that knee extensor strength at the examination prior to knee replacement (time T0), which occurred 2 years or less before surgery, was significantly lower in females who had received a knee replacement than in matched controls (pain-adjusted odds ratio, 1.72; 95% confidence interval, 1.16-2.56; P = .007). Measurement of the longitudinal change in knee extensor and flexor strength between T0 and 2 years prior to T0 (T-2) also provided similar results (pain-adjusted OR, 4.30; 95% CI, 1.34-13.79; P = .014). The findings were independent of age, BMI, and radiographic disease severity, the researchers noted.
The investigators found no relationship between knee extensor or flexor muscle strength in men and subsequent need for knee replacement surgery. The relationship between thigh muscle strength and knee replacement for women did not extend to measurements made at T-2 or T-4 or the change in thigh muscle strength between T-2 and T-4.
The OAI receives funding from the National Institutes of Health, Merck Research Laboratories, Novartis, GlaxoSmithKline, and Pfizer. The work was also funded by a grant from the European Union Seventh Framework Programme. One author disclosed consulting or preparing educational sessions for pharmaceutical companies and for receiving research support. Two authors reported being employees of Chondrometrics GmbH, a company providing MR image analysis services to academic researchers and to industry.
Women with knee osteoarthritis who had low thigh muscle strength were more likely to need a knee replacement in a case-control study of participants in the Osteoarthritis Initiative (OAI).
In particular, predictors of knee replacement included knee extensor weakness in the year prior to knee replacement and longitudinal deterioration in knee extensor strength over a 2-year observation period prior to surgery. Measurement of knee extensor strength in women with knee osteoarthritis may then indicate who could benefit from weight training exercises to potentially delay or prevent the need for knee replacement surgery, said the researchers, led by Dr. Adam Culvenor of Paracelsus Medical University in Salzburg, Austria (Arthritis Rheumatol. 2015 Dec 14. doi: 10.1002/art.39540).
The optimal knee extensor strength threshold for differentiating those with and without knee replacement risk was approximately 200 N or 0.9 Nm/kg; or prevention of any loss of knee extensor strength over 2 years.
“There appears to be a considerable window for women below this threshold to obtain realistic strength gains and potentially lower the risk of knee replacement,” the study authors concluded.
In the multicenter, longitudinal, case-control study of 4,796 participants in the OAI (60% of whom were women), the investigators identified 136 participants who had received a knee replacement and matched them with controls who had not received a knee replacement and were similar in age, body mass index (BMI), and radiographic stage. The mean age of the women was 65 years and the mean BMI was 29 kg/m2.
The results showed that knee extensor strength at the examination prior to knee replacement (time T0), which occurred 2 years or less before surgery, was significantly lower in females who had received a knee replacement than in matched controls (pain-adjusted odds ratio, 1.72; 95% confidence interval, 1.16-2.56; P = .007). Measurement of the longitudinal change in knee extensor and flexor strength between T0 and 2 years prior to T0 (T-2) also provided similar results (pain-adjusted OR, 4.30; 95% CI, 1.34-13.79; P = .014). The findings were independent of age, BMI, and radiographic disease severity, the researchers noted.
The investigators found no relationship between knee extensor or flexor muscle strength in men and subsequent need for knee replacement surgery. The relationship between thigh muscle strength and knee replacement for women did not extend to measurements made at T-2 or T-4 or the change in thigh muscle strength between T-2 and T-4.
The OAI receives funding from the National Institutes of Health, Merck Research Laboratories, Novartis, GlaxoSmithKline, and Pfizer. The work was also funded by a grant from the European Union Seventh Framework Programme. One author disclosed consulting or preparing educational sessions for pharmaceutical companies and for receiving research support. Two authors reported being employees of Chondrometrics GmbH, a company providing MR image analysis services to academic researchers and to industry.
Women with knee osteoarthritis who had low thigh muscle strength were more likely to need a knee replacement in a case-control study of participants in the Osteoarthritis Initiative (OAI).
In particular, predictors of knee replacement included knee extensor weakness in the year prior to knee replacement and longitudinal deterioration in knee extensor strength over a 2-year observation period prior to surgery. Measurement of knee extensor strength in women with knee osteoarthritis may then indicate who could benefit from weight training exercises to potentially delay or prevent the need for knee replacement surgery, said the researchers, led by Dr. Adam Culvenor of Paracelsus Medical University in Salzburg, Austria (Arthritis Rheumatol. 2015 Dec 14. doi: 10.1002/art.39540).
The optimal knee extensor strength threshold for differentiating those with and without knee replacement risk was approximately 200 N or 0.9 Nm/kg; or prevention of any loss of knee extensor strength over 2 years.
“There appears to be a considerable window for women below this threshold to obtain realistic strength gains and potentially lower the risk of knee replacement,” the study authors concluded.
In the multicenter, longitudinal, case-control study of 4,796 participants in the OAI (60% of whom were women), the investigators identified 136 participants who had received a knee replacement and matched them with controls who had not received a knee replacement and were similar in age, body mass index (BMI), and radiographic stage. The mean age of the women was 65 years and the mean BMI was 29 kg/m2.
The results showed that knee extensor strength at the examination prior to knee replacement (time T0), which occurred 2 years or less before surgery, was significantly lower in females who had received a knee replacement than in matched controls (pain-adjusted odds ratio, 1.72; 95% confidence interval, 1.16-2.56; P = .007). Measurement of the longitudinal change in knee extensor and flexor strength between T0 and 2 years prior to T0 (T-2) also provided similar results (pain-adjusted OR, 4.30; 95% CI, 1.34-13.79; P = .014). The findings were independent of age, BMI, and radiographic disease severity, the researchers noted.
The investigators found no relationship between knee extensor or flexor muscle strength in men and subsequent need for knee replacement surgery. The relationship between thigh muscle strength and knee replacement for women did not extend to measurements made at T-2 or T-4 or the change in thigh muscle strength between T-2 and T-4.
The OAI receives funding from the National Institutes of Health, Merck Research Laboratories, Novartis, GlaxoSmithKline, and Pfizer. The work was also funded by a grant from the European Union Seventh Framework Programme. One author disclosed consulting or preparing educational sessions for pharmaceutical companies and for receiving research support. Two authors reported being employees of Chondrometrics GmbH, a company providing MR image analysis services to academic researchers and to industry.
FROM ARTHRITIS & RHEUMATOLOGY
Key clinical point: A window of opportunity exists for women with knee osteoarthritis and low thigh muscle strength to potentially lower their risk of knee replacement.
Major finding: Women who underwent knee replacement had significantly lower thigh muscle strength compared to matched controls who had not had knee surgery.
Data source: A multicenter longitudinal case control study involving 4,796 participants in the Osteoarthritis Initiative (OAI).
Disclosures: The OAI receives funding from the National Institutes of Health, Merck Research Laboratories, Novartis, GlaxoSmithKline, and Pfizer. The work was also funded by a grant from the European Union Seventh Framework Programme. One author disclosed consulting or preparing educational sessions for pharmaceutical companies and for receiving research support. Two authors reported being employees of Chondrometrics GmbH, a company providing MR image analysis services to academic researchers and to industry.
Radiography missed most clinical cases of hip osteoarthritis
Radiography detected up to 16% of cases of hip osteoarthritis among older patients with frequent hip pain in an analysis of participants in the Framingham Osteoarthritis Study and the Osteoarthritis Initiative.
“In older patients, inadequate recognition of osteoarthritis has consequences. Decreased functional status from osteoarthritis significantly increases morbidity from coronary heart disease, lung disease, diabetes, obesity, falls, frailty, and various other ailments,” said Dr. Chan Kim of Boston University and his associates. “Because many patients with hip pain do not have radiographic hip osteoarthritis, a health professional should continue with the evaluation and treatment of osteoarthritis, despite negative radiographic findings.”
Radiographic pathology often is detected late in the course of knee OA and correlates poorly with knee pain, but few studies have examined these trends for the hip. The researchers analyzed pelvic radiographs and hip pain among 946 participants in the Framingham Osteoarthritis Study and 4,366 participants in the Osteoarthritis Initiative. They defined radiographic hip OA as a Kellgren-Lawrence grade of 2 or more – that is, definite superolateral or superomedial joint space narrowing and a definite osteophyte. They used various clinical symptoms of hip OA for comparison. Participants in both studies were older than 45 years, and tended to be in their early 60s (BMJ 2015 Dec 2. doi: 10.1136/bmj.h5983).
The most sensitive criterion in the study was groin pain, for which radiography was positive in 37% of hips in the Framingham Study and 17% of hips in the Osteoarthritis Initiative, the researchers said. Other clinical criteria were less sensitive, including anterior thigh pain, frequent hip pain, and painful internal rotation. Moreover, about 21%-24% of hips with radiographic OA were frequently painful.
The study did not evaluate MRI findings, the investigators noted. They suggested that such results would resemble those for the knee, in which MRI is “more sensitive than radiography, [but] it is far less specific for abnormalities suggestive of osteoarthritis in most middle-aged and older people.”
The National Institute of Arthritis and Musculoskeletal and Skin Diseases funded the study. The Osteoarthritis Initiative is funded by the National Institutes of Health, Merck Research Laboratories, Novartis, GlaxoSmithKline, and Pfizer. The researchers had no disclosures.
Radiography detected up to 16% of cases of hip osteoarthritis among older patients with frequent hip pain in an analysis of participants in the Framingham Osteoarthritis Study and the Osteoarthritis Initiative.
“In older patients, inadequate recognition of osteoarthritis has consequences. Decreased functional status from osteoarthritis significantly increases morbidity from coronary heart disease, lung disease, diabetes, obesity, falls, frailty, and various other ailments,” said Dr. Chan Kim of Boston University and his associates. “Because many patients with hip pain do not have radiographic hip osteoarthritis, a health professional should continue with the evaluation and treatment of osteoarthritis, despite negative radiographic findings.”
Radiographic pathology often is detected late in the course of knee OA and correlates poorly with knee pain, but few studies have examined these trends for the hip. The researchers analyzed pelvic radiographs and hip pain among 946 participants in the Framingham Osteoarthritis Study and 4,366 participants in the Osteoarthritis Initiative. They defined radiographic hip OA as a Kellgren-Lawrence grade of 2 or more – that is, definite superolateral or superomedial joint space narrowing and a definite osteophyte. They used various clinical symptoms of hip OA for comparison. Participants in both studies were older than 45 years, and tended to be in their early 60s (BMJ 2015 Dec 2. doi: 10.1136/bmj.h5983).
The most sensitive criterion in the study was groin pain, for which radiography was positive in 37% of hips in the Framingham Study and 17% of hips in the Osteoarthritis Initiative, the researchers said. Other clinical criteria were less sensitive, including anterior thigh pain, frequent hip pain, and painful internal rotation. Moreover, about 21%-24% of hips with radiographic OA were frequently painful.
The study did not evaluate MRI findings, the investigators noted. They suggested that such results would resemble those for the knee, in which MRI is “more sensitive than radiography, [but] it is far less specific for abnormalities suggestive of osteoarthritis in most middle-aged and older people.”
The National Institute of Arthritis and Musculoskeletal and Skin Diseases funded the study. The Osteoarthritis Initiative is funded by the National Institutes of Health, Merck Research Laboratories, Novartis, GlaxoSmithKline, and Pfizer. The researchers had no disclosures.
Radiography detected up to 16% of cases of hip osteoarthritis among older patients with frequent hip pain in an analysis of participants in the Framingham Osteoarthritis Study and the Osteoarthritis Initiative.
“In older patients, inadequate recognition of osteoarthritis has consequences. Decreased functional status from osteoarthritis significantly increases morbidity from coronary heart disease, lung disease, diabetes, obesity, falls, frailty, and various other ailments,” said Dr. Chan Kim of Boston University and his associates. “Because many patients with hip pain do not have radiographic hip osteoarthritis, a health professional should continue with the evaluation and treatment of osteoarthritis, despite negative radiographic findings.”
Radiographic pathology often is detected late in the course of knee OA and correlates poorly with knee pain, but few studies have examined these trends for the hip. The researchers analyzed pelvic radiographs and hip pain among 946 participants in the Framingham Osteoarthritis Study and 4,366 participants in the Osteoarthritis Initiative. They defined radiographic hip OA as a Kellgren-Lawrence grade of 2 or more – that is, definite superolateral or superomedial joint space narrowing and a definite osteophyte. They used various clinical symptoms of hip OA for comparison. Participants in both studies were older than 45 years, and tended to be in their early 60s (BMJ 2015 Dec 2. doi: 10.1136/bmj.h5983).
The most sensitive criterion in the study was groin pain, for which radiography was positive in 37% of hips in the Framingham Study and 17% of hips in the Osteoarthritis Initiative, the researchers said. Other clinical criteria were less sensitive, including anterior thigh pain, frequent hip pain, and painful internal rotation. Moreover, about 21%-24% of hips with radiographic OA were frequently painful.
The study did not evaluate MRI findings, the investigators noted. They suggested that such results would resemble those for the knee, in which MRI is “more sensitive than radiography, [but] it is far less specific for abnormalities suggestive of osteoarthritis in most middle-aged and older people.”
The National Institute of Arthritis and Musculoskeletal and Skin Diseases funded the study. The Osteoarthritis Initiative is funded by the National Institutes of Health, Merck Research Laboratories, Novartis, GlaxoSmithKline, and Pfizer. The researchers had no disclosures.
FROM BMJ
Key clinical point: Radiographic hip osteoarthritis correlates poorly with hip pain, even among older patients with a high index of suspicion for hip OA.
Major finding: Radiography detected up to 16% of cases of hip OA among older patients with frequent hip pain.
Data source: An analysis of pelvic radiographs and hip pain reported by 946 participants in the Framingham Osteoarthritis Study and 4,366 participants in the Osteoarthritis Initiative.
Disclosures: The National Institute of Arthritis and Musculoskeletal and Skin Diseases funded the study. The Osteoarthritis Initiative is funded by the National Institutes of Health, Merck Research Laboratories, Novartis, GlaxoSmithKline, and Pfizer. The researchers had no disclosures.
Lateral wedge insoles provide minimal biomechanical help in knee OA
Lateral wedge insoles worn by people with medial knee osteoarthritis (OA) provide a limited amount of immediate biomechanical improvement during walking and may be best suited to people who have biomechanical phenotypes that would benefit the most, according to findings from a systematic review and meta-analysis of studies examining the intraindividual effects of the insoles.
“This review is ... the most definitive, up-to-date and comprehensive analysis on this issue to clarify the effects of lateral wedge insoles on biomechanical risk factors for knee OA progression,” wrote lead investigator John Arnold, Ph.D., of the University of South Australia, Adelaide, and his colleagues (Arthritis Care Res. 2015 Nov 25. doi: 10.1002/acr.22797).
The investigators reviewed 18 studies with a total of 534 participants and found small, but statistically significant reductions in estimates of knee joint loading based on the surrogate measures of external knee adduction moment (EKAM) and the knee adduction angular impulse (KAAI).
Most studies (14) tested full-length insoles, and the remaining four allowed a customized amount based on comfort and/or pain level. Another two used heel wedges, and two others tested both. The inclination angle of the insoles was most commonly 5 degrees, but ranged from 4 to 11 degrees. Some studies used a concomitant medial arch support; these were of a generic design in four studies and were made to order in another three. The lateral wedge insoles were compared against flat insoles, the patients’ own footwear, or standardized footwear.
The pooled effect sizes of both the first and second peak EKAM reductions were small, with standard mean differences of –0.20 to –0.25. For the first EKAM, the effect sizes did not vary according to whether studies used flat insoles or shoes only as comparators, whereas for the eight studies that reported second EKAM outcomes, there was a larger pooled effect size for comparisons against shoe-only than for one study that made flat insole comparisons. The pooled estimate for the standard mean difference in nine studies that reported KAAI was –0.14.
There was only weak evidence for publication bias in all the comparisons for the surrogate measures, and most had a low level of statistical heterogeneity between the outcomes of the studies.
The investigators noted that this meta-analysis of surrogate measures for knee joint loading does not take cumulative loading into account, so that even though the reduction in peak EKAM and KAAI was small, it may amount “to a large cumulative effect imparted on the knee over the course of the day. This should be considered when interpreting the findings of this review and future research on load modifying interventions in knee osteoarthritis.” They said that while EKAM has been associated with OA progression, KAAI has been thought to be a better measure of the duration and magnitude of loading in knee OA and has been associated with medial tibiofemoral cartilage loss over 1-2 years.
“Prescription [for lateral wedge insoles] based on biomechanical response and use of insoles only in individuals who show reductions in knee joint loading (biomechanical phenotypes) appears more appropriate to increase the likelihood of a favorable long-term response regarding the attenuation of structural changes. This would limit their application and benefit to a smaller number of individuals, but is still likely to be significant considering the overall prevalence of knee OA and projected rise due to population aging and rising obesity levels,” the authors concluded.
The investigators had no outside funding source for their systematic review. One of the authors may receive royalties from Salford Insole, a manufacturer of lateral wedge insoles.
Lateral wedge insoles worn by people with medial knee osteoarthritis (OA) provide a limited amount of immediate biomechanical improvement during walking and may be best suited to people who have biomechanical phenotypes that would benefit the most, according to findings from a systematic review and meta-analysis of studies examining the intraindividual effects of the insoles.
“This review is ... the most definitive, up-to-date and comprehensive analysis on this issue to clarify the effects of lateral wedge insoles on biomechanical risk factors for knee OA progression,” wrote lead investigator John Arnold, Ph.D., of the University of South Australia, Adelaide, and his colleagues (Arthritis Care Res. 2015 Nov 25. doi: 10.1002/acr.22797).
The investigators reviewed 18 studies with a total of 534 participants and found small, but statistically significant reductions in estimates of knee joint loading based on the surrogate measures of external knee adduction moment (EKAM) and the knee adduction angular impulse (KAAI).
Most studies (14) tested full-length insoles, and the remaining four allowed a customized amount based on comfort and/or pain level. Another two used heel wedges, and two others tested both. The inclination angle of the insoles was most commonly 5 degrees, but ranged from 4 to 11 degrees. Some studies used a concomitant medial arch support; these were of a generic design in four studies and were made to order in another three. The lateral wedge insoles were compared against flat insoles, the patients’ own footwear, or standardized footwear.
The pooled effect sizes of both the first and second peak EKAM reductions were small, with standard mean differences of –0.20 to –0.25. For the first EKAM, the effect sizes did not vary according to whether studies used flat insoles or shoes only as comparators, whereas for the eight studies that reported second EKAM outcomes, there was a larger pooled effect size for comparisons against shoe-only than for one study that made flat insole comparisons. The pooled estimate for the standard mean difference in nine studies that reported KAAI was –0.14.
There was only weak evidence for publication bias in all the comparisons for the surrogate measures, and most had a low level of statistical heterogeneity between the outcomes of the studies.
The investigators noted that this meta-analysis of surrogate measures for knee joint loading does not take cumulative loading into account, so that even though the reduction in peak EKAM and KAAI was small, it may amount “to a large cumulative effect imparted on the knee over the course of the day. This should be considered when interpreting the findings of this review and future research on load modifying interventions in knee osteoarthritis.” They said that while EKAM has been associated with OA progression, KAAI has been thought to be a better measure of the duration and magnitude of loading in knee OA and has been associated with medial tibiofemoral cartilage loss over 1-2 years.
“Prescription [for lateral wedge insoles] based on biomechanical response and use of insoles only in individuals who show reductions in knee joint loading (biomechanical phenotypes) appears more appropriate to increase the likelihood of a favorable long-term response regarding the attenuation of structural changes. This would limit their application and benefit to a smaller number of individuals, but is still likely to be significant considering the overall prevalence of knee OA and projected rise due to population aging and rising obesity levels,” the authors concluded.
The investigators had no outside funding source for their systematic review. One of the authors may receive royalties from Salford Insole, a manufacturer of lateral wedge insoles.
Lateral wedge insoles worn by people with medial knee osteoarthritis (OA) provide a limited amount of immediate biomechanical improvement during walking and may be best suited to people who have biomechanical phenotypes that would benefit the most, according to findings from a systematic review and meta-analysis of studies examining the intraindividual effects of the insoles.
“This review is ... the most definitive, up-to-date and comprehensive analysis on this issue to clarify the effects of lateral wedge insoles on biomechanical risk factors for knee OA progression,” wrote lead investigator John Arnold, Ph.D., of the University of South Australia, Adelaide, and his colleagues (Arthritis Care Res. 2015 Nov 25. doi: 10.1002/acr.22797).
The investigators reviewed 18 studies with a total of 534 participants and found small, but statistically significant reductions in estimates of knee joint loading based on the surrogate measures of external knee adduction moment (EKAM) and the knee adduction angular impulse (KAAI).
Most studies (14) tested full-length insoles, and the remaining four allowed a customized amount based on comfort and/or pain level. Another two used heel wedges, and two others tested both. The inclination angle of the insoles was most commonly 5 degrees, but ranged from 4 to 11 degrees. Some studies used a concomitant medial arch support; these were of a generic design in four studies and were made to order in another three. The lateral wedge insoles were compared against flat insoles, the patients’ own footwear, or standardized footwear.
The pooled effect sizes of both the first and second peak EKAM reductions were small, with standard mean differences of –0.20 to –0.25. For the first EKAM, the effect sizes did not vary according to whether studies used flat insoles or shoes only as comparators, whereas for the eight studies that reported second EKAM outcomes, there was a larger pooled effect size for comparisons against shoe-only than for one study that made flat insole comparisons. The pooled estimate for the standard mean difference in nine studies that reported KAAI was –0.14.
There was only weak evidence for publication bias in all the comparisons for the surrogate measures, and most had a low level of statistical heterogeneity between the outcomes of the studies.
The investigators noted that this meta-analysis of surrogate measures for knee joint loading does not take cumulative loading into account, so that even though the reduction in peak EKAM and KAAI was small, it may amount “to a large cumulative effect imparted on the knee over the course of the day. This should be considered when interpreting the findings of this review and future research on load modifying interventions in knee osteoarthritis.” They said that while EKAM has been associated with OA progression, KAAI has been thought to be a better measure of the duration and magnitude of loading in knee OA and has been associated with medial tibiofemoral cartilage loss over 1-2 years.
“Prescription [for lateral wedge insoles] based on biomechanical response and use of insoles only in individuals who show reductions in knee joint loading (biomechanical phenotypes) appears more appropriate to increase the likelihood of a favorable long-term response regarding the attenuation of structural changes. This would limit their application and benefit to a smaller number of individuals, but is still likely to be significant considering the overall prevalence of knee OA and projected rise due to population aging and rising obesity levels,” the authors concluded.
The investigators had no outside funding source for their systematic review. One of the authors may receive royalties from Salford Insole, a manufacturer of lateral wedge insoles.
FROM ARTHRITIS CARE & RESEARCH
Key clinical point: Make sure that patients with medial knee OA have an appropriate biomechanical phenotype to use lateral wedge insoles.
Major finding: The pooled effect sizes of both the first and second peak external knee adduction moment reductions were small, with standard mean differences of –0.20 to –0.25.
Data source: A systematic review and meta-analysis of 18 studies involving 534 patients with medial knee OA.
Disclosures: The investigators had no outside funding source for their systematic review. One of the authors may receive royalties from Salford Insole, a manufacturer of lateral wedge insoles.
ACR: Don’t be fooled by contaminated synovial fluid
SAN FRANCISCO – Hold off on surgery in patients with presumed septic arthritis if they’re not otherwise too sick and their cultures don’t grow out a pathogenic organism within 48 hours.
The reason is because those patients are likely to have synovial fluid that was contaminated during collection, not a true joint infection.
The advice comes from investigators at Beth Israel Deaconess Medical Center, Boston, who compared 425 monoarticular septic arthritis cases with 25 cases that turned out to be false positives due to synovial fluid contamination; most of the false positives got antibiotics, and three (12%) had joint operations that they did not need.
“Rushing off to the operating room isn’t” always warranted. “You can suspect contamination if patients have milder disease manifestations and cultures grow late,” said investigator Dr. Robert H. Shmerling, clinical chief of Beth Israel’s division of rheumatology.
The findings help determine when – and when not – to be aggressive with patients who present with what looks to be septic arthritis. “No one’s ever really looked at this before,” he said at the annual meeting of the American College of Rheumatology.
“These are very different sorts of patients. Look at the full range of clinical characteristics and lab values, not just the synovial fluid tap. If contamination is suspected, you can wait until the cultures come back or possibly do serial taps before going to the operating room,” said coinvestigator Clara Zhu, a medical student at Boston University.
Patients with true joint infections had higher mean peripheral polymorphonuclear neutrophil percentages (78% vs. 68% in false positives) and synovial fluid polymorphonuclear cell percentages (88% vs. 74% in false positives). True cases also had substantially higher mean synovial fluid white blood cell counts (88,000 vs. 29,000).
Unlike true cases, contaminated synovial fluid took about 4 days to grow out a positive culture, and the most common organisms by far were coagulase-negative staphylococci, typically normal skin bacteria.
Patients with contaminated fluid also tended to be older (71 vs. 59 years), with fewer prior admissions. They were far less likely to have had recent joint procedures and histories of septic arthritis but were more likely to have synovial fluid crystals, as in gout. False positives also left the hospital sooner (7 vs. 11 days) and were less likely to be readmitted within 2 months. They were also less likely to present with fever (19% vs. 37%) but not significantly so.
This “study suggests that contaminated synovial fluid is found in up to 6% of patients with suspected septic arthritis and positive synovial fluid or synovial biopsy cultures. We recommend a conservative approach for patients with ... mild disease manifestations and no growth of pathogenic organisms within the first 48 hours,” the investigators concluded.
The authors have no disclosures, and there was no outside funding for the work.
SAN FRANCISCO – Hold off on surgery in patients with presumed septic arthritis if they’re not otherwise too sick and their cultures don’t grow out a pathogenic organism within 48 hours.
The reason is because those patients are likely to have synovial fluid that was contaminated during collection, not a true joint infection.
The advice comes from investigators at Beth Israel Deaconess Medical Center, Boston, who compared 425 monoarticular septic arthritis cases with 25 cases that turned out to be false positives due to synovial fluid contamination; most of the false positives got antibiotics, and three (12%) had joint operations that they did not need.
“Rushing off to the operating room isn’t” always warranted. “You can suspect contamination if patients have milder disease manifestations and cultures grow late,” said investigator Dr. Robert H. Shmerling, clinical chief of Beth Israel’s division of rheumatology.
The findings help determine when – and when not – to be aggressive with patients who present with what looks to be septic arthritis. “No one’s ever really looked at this before,” he said at the annual meeting of the American College of Rheumatology.
“These are very different sorts of patients. Look at the full range of clinical characteristics and lab values, not just the synovial fluid tap. If contamination is suspected, you can wait until the cultures come back or possibly do serial taps before going to the operating room,” said coinvestigator Clara Zhu, a medical student at Boston University.
Patients with true joint infections had higher mean peripheral polymorphonuclear neutrophil percentages (78% vs. 68% in false positives) and synovial fluid polymorphonuclear cell percentages (88% vs. 74% in false positives). True cases also had substantially higher mean synovial fluid white blood cell counts (88,000 vs. 29,000).
Unlike true cases, contaminated synovial fluid took about 4 days to grow out a positive culture, and the most common organisms by far were coagulase-negative staphylococci, typically normal skin bacteria.
Patients with contaminated fluid also tended to be older (71 vs. 59 years), with fewer prior admissions. They were far less likely to have had recent joint procedures and histories of septic arthritis but were more likely to have synovial fluid crystals, as in gout. False positives also left the hospital sooner (7 vs. 11 days) and were less likely to be readmitted within 2 months. They were also less likely to present with fever (19% vs. 37%) but not significantly so.
This “study suggests that contaminated synovial fluid is found in up to 6% of patients with suspected septic arthritis and positive synovial fluid or synovial biopsy cultures. We recommend a conservative approach for patients with ... mild disease manifestations and no growth of pathogenic organisms within the first 48 hours,” the investigators concluded.
The authors have no disclosures, and there was no outside funding for the work.
SAN FRANCISCO – Hold off on surgery in patients with presumed septic arthritis if they’re not otherwise too sick and their cultures don’t grow out a pathogenic organism within 48 hours.
The reason is because those patients are likely to have synovial fluid that was contaminated during collection, not a true joint infection.
The advice comes from investigators at Beth Israel Deaconess Medical Center, Boston, who compared 425 monoarticular septic arthritis cases with 25 cases that turned out to be false positives due to synovial fluid contamination; most of the false positives got antibiotics, and three (12%) had joint operations that they did not need.
“Rushing off to the operating room isn’t” always warranted. “You can suspect contamination if patients have milder disease manifestations and cultures grow late,” said investigator Dr. Robert H. Shmerling, clinical chief of Beth Israel’s division of rheumatology.
The findings help determine when – and when not – to be aggressive with patients who present with what looks to be septic arthritis. “No one’s ever really looked at this before,” he said at the annual meeting of the American College of Rheumatology.
“These are very different sorts of patients. Look at the full range of clinical characteristics and lab values, not just the synovial fluid tap. If contamination is suspected, you can wait until the cultures come back or possibly do serial taps before going to the operating room,” said coinvestigator Clara Zhu, a medical student at Boston University.
Patients with true joint infections had higher mean peripheral polymorphonuclear neutrophil percentages (78% vs. 68% in false positives) and synovial fluid polymorphonuclear cell percentages (88% vs. 74% in false positives). True cases also had substantially higher mean synovial fluid white blood cell counts (88,000 vs. 29,000).
Unlike true cases, contaminated synovial fluid took about 4 days to grow out a positive culture, and the most common organisms by far were coagulase-negative staphylococci, typically normal skin bacteria.
Patients with contaminated fluid also tended to be older (71 vs. 59 years), with fewer prior admissions. They were far less likely to have had recent joint procedures and histories of septic arthritis but were more likely to have synovial fluid crystals, as in gout. False positives also left the hospital sooner (7 vs. 11 days) and were less likely to be readmitted within 2 months. They were also less likely to present with fever (19% vs. 37%) but not significantly so.
This “study suggests that contaminated synovial fluid is found in up to 6% of patients with suspected septic arthritis and positive synovial fluid or synovial biopsy cultures. We recommend a conservative approach for patients with ... mild disease manifestations and no growth of pathogenic organisms within the first 48 hours,” the investigators concluded.
The authors have no disclosures, and there was no outside funding for the work.
AT THE ACR ANNUAL MEETING
Key clinical point: It’s probably not really septic arthritis if patients have mild disease manifestations and slow-growing synovial fluid cultures.
Major finding: True cases of septic arthritis had substantially higher mean synovial fluid white blood cell counts than did false-positive cases (88,000 vs. 29,000).
Data source: Review of 450 patients with presumed septic arthritis.
Disclosures: The authors have no disclosures, and there was no outside funding for the work.
Synovitis, effusion associated with increased pain sensitivity
Synovitis and effusion were associated with increases in pain sensitivity at the patella and wrist, respectively, in a study of 1,111 patients with or at risk of knee osteoarthritis (OA).
Radiographs and MRIs were taken of the patients’ knees, and the patients wrists and patellae were subjected to standardized quantitative sensory testing (QST) measures. The QST measures included temporal summation, which is a measure of central pain amplification based on “an augmented response to repetitive mechanical stimulation,” and pressure pain threshold (PPT), a measure of sensitivity to pain evoked by mechanical stimulation of nociceptors. (Lower PPTs represent a greater degree of sensitization or pain sensitivity.) All tests were conducted at baseline and 2 years later.
Synovitis was associated with a significant decrease in PPT at the patella, while effusion was associated with a decrease in PPT at the wrist. Effusion was additionally associated with risk of incident temporal summation. In contrast to synovitis and effusion, bone marrow lesions were not associated with either temporal summation or decreased pressure pain threshold.
“Our findings support the potential relevance of inflammation in the development and heightening of sensitization in knee osteoarthritis in humans. We found that synovitis was associated with lower PPT and a decrease in PPT at the patella over time, indicating increased pain sensitization or sensitivity. Effusion was associated with development of new temporal summation at the patella, and with a decrease in PPT at the wrist, a site distant to the pathology; both findings suggest the involvement of central sensitization. Thus inflammation appears to influence the development of and perhaps amplification of sensitization,” said Dr. Tuhina Neogi, of the department of medicine at Boston University and her colleagues.
Read the full study in Arthritis & Rheumatology (doi: 10.1002/art.39488).
Synovitis and effusion were associated with increases in pain sensitivity at the patella and wrist, respectively, in a study of 1,111 patients with or at risk of knee osteoarthritis (OA).
Radiographs and MRIs were taken of the patients’ knees, and the patients wrists and patellae were subjected to standardized quantitative sensory testing (QST) measures. The QST measures included temporal summation, which is a measure of central pain amplification based on “an augmented response to repetitive mechanical stimulation,” and pressure pain threshold (PPT), a measure of sensitivity to pain evoked by mechanical stimulation of nociceptors. (Lower PPTs represent a greater degree of sensitization or pain sensitivity.) All tests were conducted at baseline and 2 years later.
Synovitis was associated with a significant decrease in PPT at the patella, while effusion was associated with a decrease in PPT at the wrist. Effusion was additionally associated with risk of incident temporal summation. In contrast to synovitis and effusion, bone marrow lesions were not associated with either temporal summation or decreased pressure pain threshold.
“Our findings support the potential relevance of inflammation in the development and heightening of sensitization in knee osteoarthritis in humans. We found that synovitis was associated with lower PPT and a decrease in PPT at the patella over time, indicating increased pain sensitization or sensitivity. Effusion was associated with development of new temporal summation at the patella, and with a decrease in PPT at the wrist, a site distant to the pathology; both findings suggest the involvement of central sensitization. Thus inflammation appears to influence the development of and perhaps amplification of sensitization,” said Dr. Tuhina Neogi, of the department of medicine at Boston University and her colleagues.
Read the full study in Arthritis & Rheumatology (doi: 10.1002/art.39488).
Synovitis and effusion were associated with increases in pain sensitivity at the patella and wrist, respectively, in a study of 1,111 patients with or at risk of knee osteoarthritis (OA).
Radiographs and MRIs were taken of the patients’ knees, and the patients wrists and patellae were subjected to standardized quantitative sensory testing (QST) measures. The QST measures included temporal summation, which is a measure of central pain amplification based on “an augmented response to repetitive mechanical stimulation,” and pressure pain threshold (PPT), a measure of sensitivity to pain evoked by mechanical stimulation of nociceptors. (Lower PPTs represent a greater degree of sensitization or pain sensitivity.) All tests were conducted at baseline and 2 years later.
Synovitis was associated with a significant decrease in PPT at the patella, while effusion was associated with a decrease in PPT at the wrist. Effusion was additionally associated with risk of incident temporal summation. In contrast to synovitis and effusion, bone marrow lesions were not associated with either temporal summation or decreased pressure pain threshold.
“Our findings support the potential relevance of inflammation in the development and heightening of sensitization in knee osteoarthritis in humans. We found that synovitis was associated with lower PPT and a decrease in PPT at the patella over time, indicating increased pain sensitization or sensitivity. Effusion was associated with development of new temporal summation at the patella, and with a decrease in PPT at the wrist, a site distant to the pathology; both findings suggest the involvement of central sensitization. Thus inflammation appears to influence the development of and perhaps amplification of sensitization,” said Dr. Tuhina Neogi, of the department of medicine at Boston University and her colleagues.
Read the full study in Arthritis & Rheumatology (doi: 10.1002/art.39488).
FROM ARTHRITIS & RHEUMATOLOGY
Overweight, obese patients at greater risk for knee replacement surgery
Both overweight and obese patients with knee osteoarthritis (OA) are more likely to get knee replacement surgery, compared with normal-weight patients with knee OA, results of a population-based cohort study of people in Catalonia, Spain, suggest.
The study included 105,189 patients, who had been diagnosed with knee OA between 2006 and 2011. Patients with a history of knee OA or knee replacement in either knee before Jan. 1, 2006, and patients with a history inflammatory arthritis were not included in the study.
The patients were followed from the date of knee OA diagnosis until the date they underwent elective knee replacement surgery or until Dec. 31, 2011. (The researchers were unable to follow up with all individuals initially enrolled in the study.) The participants were broken up into the following categories based on their body mass index: normal (BMI was less than 25 kg/m2), overweight (BMI was 25 to less than 30 kg/m2), obese class I (BMI was 30 to less than 35 kg/m2), obese class II (BMI was 35 to less than 40 kg/m2), and obese class III (BMI was greater than or equal to 40 kg/m2).
The risk of knee replacement increased with BMI. For patients with a normal weight, the incidence rates of surgery were 1.35/100 person-years, compared with 3.49/100 person-years in patients in obese class III. Adjusted hazard ratios for knee replacement surgery were 1.41 for overweight, 1.97 for obese class I, 2.39 for obese class II, and 2.67 for obese class III, compared with normal-weight study participants.
An additional finding was a significant interaction between BMI and age on the risk of knee replacement (P is less than .001), with a higher relative hazard associated with obesity among patients aged less than 68 years.
“This research demonstrates that overweight and obesity are strong independent predictors of the clinical progression of knee OA, from disease onset/diagnosis to joint failure and subsequent [knee replacement]. Overweight subjects are at over 40% increased risk of surgery, and those who are obese have a more than doubled risk when compared to subjects with normal weight,” said Kristen M. Leyland, D.Phil., and her colleagues.
Read the full study in Arthritis & Rheumatology (doi: 10.1002/art.39486).
Both overweight and obese patients with knee osteoarthritis (OA) are more likely to get knee replacement surgery, compared with normal-weight patients with knee OA, results of a population-based cohort study of people in Catalonia, Spain, suggest.
The study included 105,189 patients, who had been diagnosed with knee OA between 2006 and 2011. Patients with a history of knee OA or knee replacement in either knee before Jan. 1, 2006, and patients with a history inflammatory arthritis were not included in the study.
The patients were followed from the date of knee OA diagnosis until the date they underwent elective knee replacement surgery or until Dec. 31, 2011. (The researchers were unable to follow up with all individuals initially enrolled in the study.) The participants were broken up into the following categories based on their body mass index: normal (BMI was less than 25 kg/m2), overweight (BMI was 25 to less than 30 kg/m2), obese class I (BMI was 30 to less than 35 kg/m2), obese class II (BMI was 35 to less than 40 kg/m2), and obese class III (BMI was greater than or equal to 40 kg/m2).
The risk of knee replacement increased with BMI. For patients with a normal weight, the incidence rates of surgery were 1.35/100 person-years, compared with 3.49/100 person-years in patients in obese class III. Adjusted hazard ratios for knee replacement surgery were 1.41 for overweight, 1.97 for obese class I, 2.39 for obese class II, and 2.67 for obese class III, compared with normal-weight study participants.
An additional finding was a significant interaction between BMI and age on the risk of knee replacement (P is less than .001), with a higher relative hazard associated with obesity among patients aged less than 68 years.
“This research demonstrates that overweight and obesity are strong independent predictors of the clinical progression of knee OA, from disease onset/diagnosis to joint failure and subsequent [knee replacement]. Overweight subjects are at over 40% increased risk of surgery, and those who are obese have a more than doubled risk when compared to subjects with normal weight,” said Kristen M. Leyland, D.Phil., and her colleagues.
Read the full study in Arthritis & Rheumatology (doi: 10.1002/art.39486).
Both overweight and obese patients with knee osteoarthritis (OA) are more likely to get knee replacement surgery, compared with normal-weight patients with knee OA, results of a population-based cohort study of people in Catalonia, Spain, suggest.
The study included 105,189 patients, who had been diagnosed with knee OA between 2006 and 2011. Patients with a history of knee OA or knee replacement in either knee before Jan. 1, 2006, and patients with a history inflammatory arthritis were not included in the study.
The patients were followed from the date of knee OA diagnosis until the date they underwent elective knee replacement surgery or until Dec. 31, 2011. (The researchers were unable to follow up with all individuals initially enrolled in the study.) The participants were broken up into the following categories based on their body mass index: normal (BMI was less than 25 kg/m2), overweight (BMI was 25 to less than 30 kg/m2), obese class I (BMI was 30 to less than 35 kg/m2), obese class II (BMI was 35 to less than 40 kg/m2), and obese class III (BMI was greater than or equal to 40 kg/m2).
The risk of knee replacement increased with BMI. For patients with a normal weight, the incidence rates of surgery were 1.35/100 person-years, compared with 3.49/100 person-years in patients in obese class III. Adjusted hazard ratios for knee replacement surgery were 1.41 for overweight, 1.97 for obese class I, 2.39 for obese class II, and 2.67 for obese class III, compared with normal-weight study participants.
An additional finding was a significant interaction between BMI and age on the risk of knee replacement (P is less than .001), with a higher relative hazard associated with obesity among patients aged less than 68 years.
“This research demonstrates that overweight and obesity are strong independent predictors of the clinical progression of knee OA, from disease onset/diagnosis to joint failure and subsequent [knee replacement]. Overweight subjects are at over 40% increased risk of surgery, and those who are obese have a more than doubled risk when compared to subjects with normal weight,” said Kristen M. Leyland, D.Phil., and her colleagues.
Read the full study in Arthritis & Rheumatology (doi: 10.1002/art.39486).
FROM ARTHRITIS & RHEUMATOLOGY