User login
New ILD diagnostic test is available
IPF can be difficult to distinguish from other ILDs, S. Samuel Weigt, MD, of the University of California, Los Angeles, and director of UCLA Health’s Interstitial Lung Disease Center, said in a statement from Veracyte, the company marketing the test.
In fact, more than half of patients with ILDs were misdiagnosed at least once, according to a study published by the Pulmonary Fibrosis Foundation.
The new test, known as the Envisia Genomic Classifier, combines RNA sequencing and machine learning to help physicians differentiate IPF from ILDs in samples obtained through transbronchial biopsy. Its specificity and sensitivity for detecting the genomic pattern of usual interstitial pneumonia, are 88% and 70%, respectively, according to the Veracyte statement.
“Multiple studies have demonstrated that the Envisia Genomic Classifier supports more confident IPF diagnosis and optimal patient management,” Bonnie Anderson, chairman and CEO of Veracyte, said in the statement.
A benefit of the new test is that its use does not require patients to undergo risky, expensive surgery, which may not even be possible for some patients, noted Dr. Weigt. “We are pleased to be one of the few medical facilities in the country to have access to this breakthrough technology.”
To obtain more information about the Envisia Genomic Classifier and how to use the early-access program, contact Veracyte at 844-464-5864 or support@veracyte.com.
IPF can be difficult to distinguish from other ILDs, S. Samuel Weigt, MD, of the University of California, Los Angeles, and director of UCLA Health’s Interstitial Lung Disease Center, said in a statement from Veracyte, the company marketing the test.
In fact, more than half of patients with ILDs were misdiagnosed at least once, according to a study published by the Pulmonary Fibrosis Foundation.
The new test, known as the Envisia Genomic Classifier, combines RNA sequencing and machine learning to help physicians differentiate IPF from ILDs in samples obtained through transbronchial biopsy. Its specificity and sensitivity for detecting the genomic pattern of usual interstitial pneumonia, are 88% and 70%, respectively, according to the Veracyte statement.
“Multiple studies have demonstrated that the Envisia Genomic Classifier supports more confident IPF diagnosis and optimal patient management,” Bonnie Anderson, chairman and CEO of Veracyte, said in the statement.
A benefit of the new test is that its use does not require patients to undergo risky, expensive surgery, which may not even be possible for some patients, noted Dr. Weigt. “We are pleased to be one of the few medical facilities in the country to have access to this breakthrough technology.”
To obtain more information about the Envisia Genomic Classifier and how to use the early-access program, contact Veracyte at 844-464-5864 or support@veracyte.com.
IPF can be difficult to distinguish from other ILDs, S. Samuel Weigt, MD, of the University of California, Los Angeles, and director of UCLA Health’s Interstitial Lung Disease Center, said in a statement from Veracyte, the company marketing the test.
In fact, more than half of patients with ILDs were misdiagnosed at least once, according to a study published by the Pulmonary Fibrosis Foundation.
The new test, known as the Envisia Genomic Classifier, combines RNA sequencing and machine learning to help physicians differentiate IPF from ILDs in samples obtained through transbronchial biopsy. Its specificity and sensitivity for detecting the genomic pattern of usual interstitial pneumonia, are 88% and 70%, respectively, according to the Veracyte statement.
“Multiple studies have demonstrated that the Envisia Genomic Classifier supports more confident IPF diagnosis and optimal patient management,” Bonnie Anderson, chairman and CEO of Veracyte, said in the statement.
A benefit of the new test is that its use does not require patients to undergo risky, expensive surgery, which may not even be possible for some patients, noted Dr. Weigt. “We are pleased to be one of the few medical facilities in the country to have access to this breakthrough technology.”
To obtain more information about the Envisia Genomic Classifier and how to use the early-access program, contact Veracyte at 844-464-5864 or support@veracyte.com.
EPA proposal on research data to be discussed at ATS meeting
A press conference on the Environmental Protection Agency’s proposed policy on research data will be held at the American Thoracic Society International Conference on Sunday, May 20.
The conference, entitled “Silencing Science: EPA’s Proposed Policy on Research Data,” will occur at 11:15 a.m. Pacific Standard Time in the San Diego Convention Center, Meeting Room 23A (Upper Level).
For information about this press conference, contact Dacia Morris, director of communications and marketing of the ATS, at 212-315-8620.
A press conference on the Environmental Protection Agency’s proposed policy on research data will be held at the American Thoracic Society International Conference on Sunday, May 20.
The conference, entitled “Silencing Science: EPA’s Proposed Policy on Research Data,” will occur at 11:15 a.m. Pacific Standard Time in the San Diego Convention Center, Meeting Room 23A (Upper Level).
For information about this press conference, contact Dacia Morris, director of communications and marketing of the ATS, at 212-315-8620.
A press conference on the Environmental Protection Agency’s proposed policy on research data will be held at the American Thoracic Society International Conference on Sunday, May 20.
The conference, entitled “Silencing Science: EPA’s Proposed Policy on Research Data,” will occur at 11:15 a.m. Pacific Standard Time in the San Diego Convention Center, Meeting Room 23A (Upper Level).
For information about this press conference, contact Dacia Morris, director of communications and marketing of the ATS, at 212-315-8620.
FROM ATS 2018
CHEST® Physician’s preview of ATS 2018
Here is a glimpse of some of the important research that will be presented at this meeting.
The findings of several chronic obstructive pulmonary disease (COPD) drug trials will be discussed during a session entitled “ICS [Inhaled corticosteroids] in COPD: The Pendulum Keeps Swinging,” which is scheduled to occur at 9:15 a.m. in Room 14 A-B (Mezzanine Level). Among the research to be presented are the latest findings of the phase 3 IMPACT study of 10,355 symptomatic COPD patients with a history of moderate to severe exacerbations. This study compared the use of an inhaled therapy that comprised a corticosteroid, a long-acting muscarinic antagonist (LAMA), and a long-acting beta2-agonist (LABA) with the use of two other therapy combinations – a corticosteroid and a LABA, or a LABA and a LAMA. (Lipson DA et al. N Engl J Med. 2018 Apr 18;378:1671-80). Patients were randomized to receive either a once-daily combination of 100 mcg fluticasone furoate (a corticosteroid); 62.5 mcg of the LAMA, umeclidinium; and 25 mcg of the LABA, vilanterol; or dual inhaled therapy involving either 100 mcg fluticasone furoate plus 25 mcg of vilanterol, or 62.5 mcg of umeclidinium plus 25 mcg of vilanterol for 52 weeks.
One of the updates on this trial is that using the triple therapy significantly reduced on-treatment all-cause mortality over using the LAMA (62.5 mcg of umeclidinium) plus LABA (25 mcg of the vilanterol) dual therapy. Fifty of the patients who received triple therapy died (1.20%), versus 49 patients in the corticosteroid plus LABA group (1.19%) and 30 patients (1.88%) in the LAMA plus LABA group. A 42.1% reduction in risk of all-cause mortality occurred for patients who took the triple therapy, when compared with patients who took the LAMA/LABA combo (95% confidence interval, 11.9%-61.9%; P = .011), according to an abstract on the ATS International Conference’s website.
At the same time on Sunday, researchers will be presenting their research in a session entitled “Sleep Disordered Breathing, Cardiovascular Disease, and Mortality,” in Room 3 (Upper Level) of the convention center. One of the abstracts that will be discussed compared the long-term effectiveness of noninvasive ventilation (NIV) with continuous positive airway pressure (CPAP) in patients with obesity hypoventilation syndrome with severe obstructive sleep apnea. In this multicenter open-label, randomized, controlled trial, Sanchez Quiroga M et al. analyzed the results for 202 patients who used one of the two treatments for at least 3 years. Among this study’s findings were that the mortality rates and the number of cardiovascular events that occurred were similar in the two treatment groups. The mortality rate for patients who used CPAP was 14.7%, compared with 11.3% for the patients who received NIV (adjusted hazard ratio, 0.73; P = .439), and the cardiovascular events per 100 person-years were 5.1 for CPAP and 7.46 for NIV (P = .315). The researchers concluded that both treatments are equally effective for the long term, but that CPAP should be “the preferred treatment modality,” because it’s cheaper and easier to implement.
On Monday morning, researchers will present their findings of the short-term cardiovascular effects of 30 pulmonary arterial hypertension patients’ use of the beta blocker carvedilol, in 3.125 mg doses taken twice a day. Right ventricular systolic pressure (RVSP) decreased by an average of 11 mmHg (P = .003) in this double-blinded, randomized, controlled open-label trial with a 1-week run-in period. Cardiac output decreased by an average of –1.8 L/min (P less than .0001), but RVSP was inversely associated with cardiac output. “Short-term carvedilol could potentially identify a subgroup for long-term therapy based on initial drop in RVSP and heart rate response,” noted Farha SY et al. in their abstract. None of the patients experienced any side effects from taking the drug. More details on this research and other studies on pulmonary hypertension will be presented at 9:15 am in Area B (Hall A-B2, Ground level) of the convention center, in the session entitled “Surf’s Up: Riding the Wave of Clinical Research in Pulmonary Hypertension.”
Look for all of our on-site coverage of the conference at mdedge.com/chestphysician next week.
Here is a glimpse of some of the important research that will be presented at this meeting.
The findings of several chronic obstructive pulmonary disease (COPD) drug trials will be discussed during a session entitled “ICS [Inhaled corticosteroids] in COPD: The Pendulum Keeps Swinging,” which is scheduled to occur at 9:15 a.m. in Room 14 A-B (Mezzanine Level). Among the research to be presented are the latest findings of the phase 3 IMPACT study of 10,355 symptomatic COPD patients with a history of moderate to severe exacerbations. This study compared the use of an inhaled therapy that comprised a corticosteroid, a long-acting muscarinic antagonist (LAMA), and a long-acting beta2-agonist (LABA) with the use of two other therapy combinations – a corticosteroid and a LABA, or a LABA and a LAMA. (Lipson DA et al. N Engl J Med. 2018 Apr 18;378:1671-80). Patients were randomized to receive either a once-daily combination of 100 mcg fluticasone furoate (a corticosteroid); 62.5 mcg of the LAMA, umeclidinium; and 25 mcg of the LABA, vilanterol; or dual inhaled therapy involving either 100 mcg fluticasone furoate plus 25 mcg of vilanterol, or 62.5 mcg of umeclidinium plus 25 mcg of vilanterol for 52 weeks.
One of the updates on this trial is that using the triple therapy significantly reduced on-treatment all-cause mortality over using the LAMA (62.5 mcg of umeclidinium) plus LABA (25 mcg of the vilanterol) dual therapy. Fifty of the patients who received triple therapy died (1.20%), versus 49 patients in the corticosteroid plus LABA group (1.19%) and 30 patients (1.88%) in the LAMA plus LABA group. A 42.1% reduction in risk of all-cause mortality occurred for patients who took the triple therapy, when compared with patients who took the LAMA/LABA combo (95% confidence interval, 11.9%-61.9%; P = .011), according to an abstract on the ATS International Conference’s website.
At the same time on Sunday, researchers will be presenting their research in a session entitled “Sleep Disordered Breathing, Cardiovascular Disease, and Mortality,” in Room 3 (Upper Level) of the convention center. One of the abstracts that will be discussed compared the long-term effectiveness of noninvasive ventilation (NIV) with continuous positive airway pressure (CPAP) in patients with obesity hypoventilation syndrome with severe obstructive sleep apnea. In this multicenter open-label, randomized, controlled trial, Sanchez Quiroga M et al. analyzed the results for 202 patients who used one of the two treatments for at least 3 years. Among this study’s findings were that the mortality rates and the number of cardiovascular events that occurred were similar in the two treatment groups. The mortality rate for patients who used CPAP was 14.7%, compared with 11.3% for the patients who received NIV (adjusted hazard ratio, 0.73; P = .439), and the cardiovascular events per 100 person-years were 5.1 for CPAP and 7.46 for NIV (P = .315). The researchers concluded that both treatments are equally effective for the long term, but that CPAP should be “the preferred treatment modality,” because it’s cheaper and easier to implement.
On Monday morning, researchers will present their findings of the short-term cardiovascular effects of 30 pulmonary arterial hypertension patients’ use of the beta blocker carvedilol, in 3.125 mg doses taken twice a day. Right ventricular systolic pressure (RVSP) decreased by an average of 11 mmHg (P = .003) in this double-blinded, randomized, controlled open-label trial with a 1-week run-in period. Cardiac output decreased by an average of –1.8 L/min (P less than .0001), but RVSP was inversely associated with cardiac output. “Short-term carvedilol could potentially identify a subgroup for long-term therapy based on initial drop in RVSP and heart rate response,” noted Farha SY et al. in their abstract. None of the patients experienced any side effects from taking the drug. More details on this research and other studies on pulmonary hypertension will be presented at 9:15 am in Area B (Hall A-B2, Ground level) of the convention center, in the session entitled “Surf’s Up: Riding the Wave of Clinical Research in Pulmonary Hypertension.”
Look for all of our on-site coverage of the conference at mdedge.com/chestphysician next week.
Here is a glimpse of some of the important research that will be presented at this meeting.
The findings of several chronic obstructive pulmonary disease (COPD) drug trials will be discussed during a session entitled “ICS [Inhaled corticosteroids] in COPD: The Pendulum Keeps Swinging,” which is scheduled to occur at 9:15 a.m. in Room 14 A-B (Mezzanine Level). Among the research to be presented are the latest findings of the phase 3 IMPACT study of 10,355 symptomatic COPD patients with a history of moderate to severe exacerbations. This study compared the use of an inhaled therapy that comprised a corticosteroid, a long-acting muscarinic antagonist (LAMA), and a long-acting beta2-agonist (LABA) with the use of two other therapy combinations – a corticosteroid and a LABA, or a LABA and a LAMA. (Lipson DA et al. N Engl J Med. 2018 Apr 18;378:1671-80). Patients were randomized to receive either a once-daily combination of 100 mcg fluticasone furoate (a corticosteroid); 62.5 mcg of the LAMA, umeclidinium; and 25 mcg of the LABA, vilanterol; or dual inhaled therapy involving either 100 mcg fluticasone furoate plus 25 mcg of vilanterol, or 62.5 mcg of umeclidinium plus 25 mcg of vilanterol for 52 weeks.
One of the updates on this trial is that using the triple therapy significantly reduced on-treatment all-cause mortality over using the LAMA (62.5 mcg of umeclidinium) plus LABA (25 mcg of the vilanterol) dual therapy. Fifty of the patients who received triple therapy died (1.20%), versus 49 patients in the corticosteroid plus LABA group (1.19%) and 30 patients (1.88%) in the LAMA plus LABA group. A 42.1% reduction in risk of all-cause mortality occurred for patients who took the triple therapy, when compared with patients who took the LAMA/LABA combo (95% confidence interval, 11.9%-61.9%; P = .011), according to an abstract on the ATS International Conference’s website.
At the same time on Sunday, researchers will be presenting their research in a session entitled “Sleep Disordered Breathing, Cardiovascular Disease, and Mortality,” in Room 3 (Upper Level) of the convention center. One of the abstracts that will be discussed compared the long-term effectiveness of noninvasive ventilation (NIV) with continuous positive airway pressure (CPAP) in patients with obesity hypoventilation syndrome with severe obstructive sleep apnea. In this multicenter open-label, randomized, controlled trial, Sanchez Quiroga M et al. analyzed the results for 202 patients who used one of the two treatments for at least 3 years. Among this study’s findings were that the mortality rates and the number of cardiovascular events that occurred were similar in the two treatment groups. The mortality rate for patients who used CPAP was 14.7%, compared with 11.3% for the patients who received NIV (adjusted hazard ratio, 0.73; P = .439), and the cardiovascular events per 100 person-years were 5.1 for CPAP and 7.46 for NIV (P = .315). The researchers concluded that both treatments are equally effective for the long term, but that CPAP should be “the preferred treatment modality,” because it’s cheaper and easier to implement.
On Monday morning, researchers will present their findings of the short-term cardiovascular effects of 30 pulmonary arterial hypertension patients’ use of the beta blocker carvedilol, in 3.125 mg doses taken twice a day. Right ventricular systolic pressure (RVSP) decreased by an average of 11 mmHg (P = .003) in this double-blinded, randomized, controlled open-label trial with a 1-week run-in period. Cardiac output decreased by an average of –1.8 L/min (P less than .0001), but RVSP was inversely associated with cardiac output. “Short-term carvedilol could potentially identify a subgroup for long-term therapy based on initial drop in RVSP and heart rate response,” noted Farha SY et al. in their abstract. None of the patients experienced any side effects from taking the drug. More details on this research and other studies on pulmonary hypertension will be presented at 9:15 am in Area B (Hall A-B2, Ground level) of the convention center, in the session entitled “Surf’s Up: Riding the Wave of Clinical Research in Pulmonary Hypertension.”
Look for all of our on-site coverage of the conference at mdedge.com/chestphysician next week.
FROM ATS 2018
Don’t use cannabis to treat OSA, AASM recommends
, according to a position statement published in the Journal of Clinical Sleep Medicine’s April issue.
In the statement, the professional society recommends that state legislators, regulators, and health departments exclude obstructive sleep apnea (OSA) as an indication for medical cannabis programs.
The “unreliable delivery methods and insufficient evidence of treatment effectiveness, tolerability, and safety” of medical cannabis and its synthetic extracts are among the reasons the AASM gave for making its recommendations. “Further research is needed to better understand the mechanistic actions of medical cannabis and its synthetic extracts, the long-term role of these synthetic extracts on OSA treatment, and harms and benefits,” the AASM concluded in its statement, authored by Kannan Ramar, MD, and other members of a panel of experts on sleep medicine.
Dronabinol is the only cannabis product that has been tested on patients with OSA for the treatment of this disorder. While some synthetic cannabis products are approved by the Food and Drug Administration for other medical indications, the synthetic-based cannabis product dronabinol has not received FDA approval for the treatment of OSA.
Researchers have examined dronabinol’s use for treating OSA in small pilot and proof-of-concept studies and most patients in these studies reported experiencing treatment-related side effects, such as somnolence, wrote Dr. Ramar, of the division of pulmonary and critical care medicine at the Center for Sleep Medicine, Mayo Clinic, Rochester Minn., and his colleagues.
These trials involved patients having taken dronabinol pills in strengths ranging from 2.5 mg to 10 mg. One such study (Front Psychiatry. 2013 Jan 22. doi: 10.3389/fpsyt.2013.00001), authored by Bharati Prasad of the University of Illinois, Chicago, and colleagues, showed a significant improvement in apnea-hypopnea index (AHI) of 32%, after 17 patients used dronabinol for 3 weeks, when compared with baseline AHIs (–14.1; P = .007).
A placebo-controlled randomized study of 73 adults with moderate or severe OSA similarly found a 33% decline in AHI in patients following 6 weeks of treatment with 10-mg doses of dronabinol (Sleep. 2018 Jan 1. doi: 10.1093/sleep/zsx184).
In the placebo-controlled study, 73 patients were randomized to receive 2.5 mg of dronabinol or 10 mg of dronabinol daily for up to 6 weeks, or placebo. At the end of treatment, researchers saw significant increases in the AHI among the patients on placebo, while those who received dronabinol showed decreases in the number of apnea and hypopnea events per hour. Patients given the 2.5-mg dose of dronabinol had a mean decrease of 10.7 events per hour, and those on the 10-mg dose had a mean decrease of 12.9 events per hour compared with placebo. The difference between the placebo and treatment arms was significant for both dosages, and the AHI decreases were similar between the two dosages of dronabinol.
These effects were largely due to reductions in apnea events; the largest reduction was seen in the REM apnea index in patients treated with the 10-mg dose of dronabinol. However, there were few effects on the expression of hypopneas, except in the higher-dose group.
After adjustment for age, race, ethnicity, and baseline AHI, the increases seen in the placebo group were no longer significant, but the decreases from baseline seen in the treatment arms were greater. Dronabinol treatment also was associated with significant decreases, compared with placebo, in non-REM AHI and REM AHI.
Overall, nearly 90% of patients in this trial reported at least one adverse event, with the rates having not differed significantly between the treatment and placebo arms. The most frequently reported adverse events were “sleepiness/drowsiness” (n = 25; 8% of total adverse events reported), headache (n = 24; 8%), “nausea/vomiting” (n = 23; 8%), and “dizziness/lightheadedness” (n = 12; 4%). In addition, one patient experienced diarrhea and vomiting that required admission to a hospital, which was judged as possibly related to the study medication. There were six other withdrawals due to adverse events, including dizziness and vision changes, vertigo, ECG arrhythmias, and headache with dizziness and vomiting.
“Synthetic medical cannabis may have differential side effects, with variable efficacy and side effects in the treatment of OSA. Therefore, it is the position of the American Academy of Sleep Medicine that medical cannabis and/or its synthetic extracts should not be used for the treatment of OSA,” Dr. Ramar and his associates wrote.
, according to a position statement published in the Journal of Clinical Sleep Medicine’s April issue.
In the statement, the professional society recommends that state legislators, regulators, and health departments exclude obstructive sleep apnea (OSA) as an indication for medical cannabis programs.
The “unreliable delivery methods and insufficient evidence of treatment effectiveness, tolerability, and safety” of medical cannabis and its synthetic extracts are among the reasons the AASM gave for making its recommendations. “Further research is needed to better understand the mechanistic actions of medical cannabis and its synthetic extracts, the long-term role of these synthetic extracts on OSA treatment, and harms and benefits,” the AASM concluded in its statement, authored by Kannan Ramar, MD, and other members of a panel of experts on sleep medicine.
Dronabinol is the only cannabis product that has been tested on patients with OSA for the treatment of this disorder. While some synthetic cannabis products are approved by the Food and Drug Administration for other medical indications, the synthetic-based cannabis product dronabinol has not received FDA approval for the treatment of OSA.
Researchers have examined dronabinol’s use for treating OSA in small pilot and proof-of-concept studies and most patients in these studies reported experiencing treatment-related side effects, such as somnolence, wrote Dr. Ramar, of the division of pulmonary and critical care medicine at the Center for Sleep Medicine, Mayo Clinic, Rochester Minn., and his colleagues.
These trials involved patients having taken dronabinol pills in strengths ranging from 2.5 mg to 10 mg. One such study (Front Psychiatry. 2013 Jan 22. doi: 10.3389/fpsyt.2013.00001), authored by Bharati Prasad of the University of Illinois, Chicago, and colleagues, showed a significant improvement in apnea-hypopnea index (AHI) of 32%, after 17 patients used dronabinol for 3 weeks, when compared with baseline AHIs (–14.1; P = .007).
A placebo-controlled randomized study of 73 adults with moderate or severe OSA similarly found a 33% decline in AHI in patients following 6 weeks of treatment with 10-mg doses of dronabinol (Sleep. 2018 Jan 1. doi: 10.1093/sleep/zsx184).
In the placebo-controlled study, 73 patients were randomized to receive 2.5 mg of dronabinol or 10 mg of dronabinol daily for up to 6 weeks, or placebo. At the end of treatment, researchers saw significant increases in the AHI among the patients on placebo, while those who received dronabinol showed decreases in the number of apnea and hypopnea events per hour. Patients given the 2.5-mg dose of dronabinol had a mean decrease of 10.7 events per hour, and those on the 10-mg dose had a mean decrease of 12.9 events per hour compared with placebo. The difference between the placebo and treatment arms was significant for both dosages, and the AHI decreases were similar between the two dosages of dronabinol.
These effects were largely due to reductions in apnea events; the largest reduction was seen in the REM apnea index in patients treated with the 10-mg dose of dronabinol. However, there were few effects on the expression of hypopneas, except in the higher-dose group.
After adjustment for age, race, ethnicity, and baseline AHI, the increases seen in the placebo group were no longer significant, but the decreases from baseline seen in the treatment arms were greater. Dronabinol treatment also was associated with significant decreases, compared with placebo, in non-REM AHI and REM AHI.
Overall, nearly 90% of patients in this trial reported at least one adverse event, with the rates having not differed significantly between the treatment and placebo arms. The most frequently reported adverse events were “sleepiness/drowsiness” (n = 25; 8% of total adverse events reported), headache (n = 24; 8%), “nausea/vomiting” (n = 23; 8%), and “dizziness/lightheadedness” (n = 12; 4%). In addition, one patient experienced diarrhea and vomiting that required admission to a hospital, which was judged as possibly related to the study medication. There were six other withdrawals due to adverse events, including dizziness and vision changes, vertigo, ECG arrhythmias, and headache with dizziness and vomiting.
“Synthetic medical cannabis may have differential side effects, with variable efficacy and side effects in the treatment of OSA. Therefore, it is the position of the American Academy of Sleep Medicine that medical cannabis and/or its synthetic extracts should not be used for the treatment of OSA,” Dr. Ramar and his associates wrote.
, according to a position statement published in the Journal of Clinical Sleep Medicine’s April issue.
In the statement, the professional society recommends that state legislators, regulators, and health departments exclude obstructive sleep apnea (OSA) as an indication for medical cannabis programs.
The “unreliable delivery methods and insufficient evidence of treatment effectiveness, tolerability, and safety” of medical cannabis and its synthetic extracts are among the reasons the AASM gave for making its recommendations. “Further research is needed to better understand the mechanistic actions of medical cannabis and its synthetic extracts, the long-term role of these synthetic extracts on OSA treatment, and harms and benefits,” the AASM concluded in its statement, authored by Kannan Ramar, MD, and other members of a panel of experts on sleep medicine.
Dronabinol is the only cannabis product that has been tested on patients with OSA for the treatment of this disorder. While some synthetic cannabis products are approved by the Food and Drug Administration for other medical indications, the synthetic-based cannabis product dronabinol has not received FDA approval for the treatment of OSA.
Researchers have examined dronabinol’s use for treating OSA in small pilot and proof-of-concept studies and most patients in these studies reported experiencing treatment-related side effects, such as somnolence, wrote Dr. Ramar, of the division of pulmonary and critical care medicine at the Center for Sleep Medicine, Mayo Clinic, Rochester Minn., and his colleagues.
These trials involved patients having taken dronabinol pills in strengths ranging from 2.5 mg to 10 mg. One such study (Front Psychiatry. 2013 Jan 22. doi: 10.3389/fpsyt.2013.00001), authored by Bharati Prasad of the University of Illinois, Chicago, and colleagues, showed a significant improvement in apnea-hypopnea index (AHI) of 32%, after 17 patients used dronabinol for 3 weeks, when compared with baseline AHIs (–14.1; P = .007).
A placebo-controlled randomized study of 73 adults with moderate or severe OSA similarly found a 33% decline in AHI in patients following 6 weeks of treatment with 10-mg doses of dronabinol (Sleep. 2018 Jan 1. doi: 10.1093/sleep/zsx184).
In the placebo-controlled study, 73 patients were randomized to receive 2.5 mg of dronabinol or 10 mg of dronabinol daily for up to 6 weeks, or placebo. At the end of treatment, researchers saw significant increases in the AHI among the patients on placebo, while those who received dronabinol showed decreases in the number of apnea and hypopnea events per hour. Patients given the 2.5-mg dose of dronabinol had a mean decrease of 10.7 events per hour, and those on the 10-mg dose had a mean decrease of 12.9 events per hour compared with placebo. The difference between the placebo and treatment arms was significant for both dosages, and the AHI decreases were similar between the two dosages of dronabinol.
These effects were largely due to reductions in apnea events; the largest reduction was seen in the REM apnea index in patients treated with the 10-mg dose of dronabinol. However, there were few effects on the expression of hypopneas, except in the higher-dose group.
After adjustment for age, race, ethnicity, and baseline AHI, the increases seen in the placebo group were no longer significant, but the decreases from baseline seen in the treatment arms were greater. Dronabinol treatment also was associated with significant decreases, compared with placebo, in non-REM AHI and REM AHI.
Overall, nearly 90% of patients in this trial reported at least one adverse event, with the rates having not differed significantly between the treatment and placebo arms. The most frequently reported adverse events were “sleepiness/drowsiness” (n = 25; 8% of total adverse events reported), headache (n = 24; 8%), “nausea/vomiting” (n = 23; 8%), and “dizziness/lightheadedness” (n = 12; 4%). In addition, one patient experienced diarrhea and vomiting that required admission to a hospital, which was judged as possibly related to the study medication. There were six other withdrawals due to adverse events, including dizziness and vision changes, vertigo, ECG arrhythmias, and headache with dizziness and vomiting.
“Synthetic medical cannabis may have differential side effects, with variable efficacy and side effects in the treatment of OSA. Therefore, it is the position of the American Academy of Sleep Medicine that medical cannabis and/or its synthetic extracts should not be used for the treatment of OSA,” Dr. Ramar and his associates wrote.
FROM THE JOURNAL OF CLINICAL SLEEP MEDICINE
FDA meeting on medical devices for sleep apnea scheduled
In a statement sent to members, CHEST invited all to attend this open meeting, which will be held at the FDA White Oak Campus in Silver Spring, Md.
In a statement sent to members, CHEST invited all to attend this open meeting, which will be held at the FDA White Oak Campus in Silver Spring, Md.
In a statement sent to members, CHEST invited all to attend this open meeting, which will be held at the FDA White Oak Campus in Silver Spring, Md.
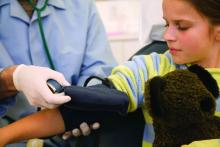
Adenotonsillectomy reduced hypertension in OSA subgroup
after surgery, according to a retrospective analysis.
This is one of the few studies to have ever examined whether adenotonsillectomy for children with OSA had any effects on blood pressure (BP) and was based on “one of the largest cohorts for evaluating postoperative BP changes in nonobese children with OSA,” noted Cho-Hsueh Lee, MD, and colleagues. The report was published in JAMA Otolaryngology–Head & Neck Surgery. Among the previous studies that evaluated BP in children with OSA before and after having this surgery, the results varied, they added.
The researchers analyzed the medical records of 240 nonobese children with clinical symptoms and polysomnography-confirmed OSA (having an apnea-hypopnea index of greater than 1) who underwent adenotonsillectomy. Prior to surgery, 169 patients (70.4%) of the patients were classified as nonhypertensive, while 71 (29.6%) were classified as hypertensive. The children had a mean age of 7.3 years, and 160 were males.
Patients participated in full-night polysomnography (PSG) before surgery and at 3-6 months after adenotonsillectomy in the National Taiwan University Hospital Sleep Center. Apnea episodes were defined as a 90% decrease in airflow for two consecutive breaths. Sleep center staff measured the study participants’ systolic and diastolic BP in a sleep center using an electronic sphygmomanometer, in the evening, prior to the PSG study, and in the morning. Pediatric hypertension was based on the nocturnal BP measurement and was defined as having mean systolic and diastolic BP greater or equal to the 95th percentile for age, sex, and height.
“Postoperatively, hypertensive children had a significant decrease in all BP measures, including nocturnal and morning [systolic] BP ... A total of 47 hypertensive patients (66.2%) became nonhypertensive after surgery,” the researchers said.
For patients who were hypertensive before surgery, the average nocturnal (before PSG) preop systolic BP was 114.3 mm Hg, versus 107.5 mm Hg after surgery. The mean nocturnal diastolic BP for this same group of patients decreased to 65.1 mm Hg from 74.3 mm Hg. Similarly, the average morning (after PSG) systolic BP and diastolic BP were 106.0 mm Hg and 64.4 mm Hg after these patients underwent adenotonsillectomy, compared with 111.8 mm Hg and 71.7 mm Hg prior to surgery, respectively.
The adenotonsillectomy didn’t improve all patients’ BP. For some who were nonhypertensive before surgery, blood pressure increased, with 36 (21.3%) of this group having become hypersensitive after surgery, the researchers acknowledged.
Overall, the cohort experienced significant improvements in several PSG measures, including the average apnea-hypopnea index, which decreased from 12.1 events per hour to 1.7. The total arousal index also declined, going from 6.1 events per hour to 4.2. In addition, the mean oxygen saturation improved from 96.8% to 97.7%.
The investigators described several limitations of the study, including their inability to collect patients’ arterial stiffness, carotid intima thickness, and other cardiovascular measures beyond BP.
They recommended a follow-up study. “Although we observed improvements in BP measures within 6 months after surgery for hypertensive children with OSA, the long-term effects of surgery on BP remain uncertain,” they explained.
The study was supported by grants from the Ministry of Science and Technology, Republic of China (Taiwan). The researchers disclosed no potential conflicts of interest.
SOURCE: Lee, C-H et al. JAMA Otolaryngol Head Neck Surg. 2018 Feb 15. doi: 10.1001/jamaoto.2017.3127.
after surgery, according to a retrospective analysis.
This is one of the few studies to have ever examined whether adenotonsillectomy for children with OSA had any effects on blood pressure (BP) and was based on “one of the largest cohorts for evaluating postoperative BP changes in nonobese children with OSA,” noted Cho-Hsueh Lee, MD, and colleagues. The report was published in JAMA Otolaryngology–Head & Neck Surgery. Among the previous studies that evaluated BP in children with OSA before and after having this surgery, the results varied, they added.
The researchers analyzed the medical records of 240 nonobese children with clinical symptoms and polysomnography-confirmed OSA (having an apnea-hypopnea index of greater than 1) who underwent adenotonsillectomy. Prior to surgery, 169 patients (70.4%) of the patients were classified as nonhypertensive, while 71 (29.6%) were classified as hypertensive. The children had a mean age of 7.3 years, and 160 were males.
Patients participated in full-night polysomnography (PSG) before surgery and at 3-6 months after adenotonsillectomy in the National Taiwan University Hospital Sleep Center. Apnea episodes were defined as a 90% decrease in airflow for two consecutive breaths. Sleep center staff measured the study participants’ systolic and diastolic BP in a sleep center using an electronic sphygmomanometer, in the evening, prior to the PSG study, and in the morning. Pediatric hypertension was based on the nocturnal BP measurement and was defined as having mean systolic and diastolic BP greater or equal to the 95th percentile for age, sex, and height.
“Postoperatively, hypertensive children had a significant decrease in all BP measures, including nocturnal and morning [systolic] BP ... A total of 47 hypertensive patients (66.2%) became nonhypertensive after surgery,” the researchers said.
For patients who were hypertensive before surgery, the average nocturnal (before PSG) preop systolic BP was 114.3 mm Hg, versus 107.5 mm Hg after surgery. The mean nocturnal diastolic BP for this same group of patients decreased to 65.1 mm Hg from 74.3 mm Hg. Similarly, the average morning (after PSG) systolic BP and diastolic BP were 106.0 mm Hg and 64.4 mm Hg after these patients underwent adenotonsillectomy, compared with 111.8 mm Hg and 71.7 mm Hg prior to surgery, respectively.
The adenotonsillectomy didn’t improve all patients’ BP. For some who were nonhypertensive before surgery, blood pressure increased, with 36 (21.3%) of this group having become hypersensitive after surgery, the researchers acknowledged.
Overall, the cohort experienced significant improvements in several PSG measures, including the average apnea-hypopnea index, which decreased from 12.1 events per hour to 1.7. The total arousal index also declined, going from 6.1 events per hour to 4.2. In addition, the mean oxygen saturation improved from 96.8% to 97.7%.
The investigators described several limitations of the study, including their inability to collect patients’ arterial stiffness, carotid intima thickness, and other cardiovascular measures beyond BP.
They recommended a follow-up study. “Although we observed improvements in BP measures within 6 months after surgery for hypertensive children with OSA, the long-term effects of surgery on BP remain uncertain,” they explained.
The study was supported by grants from the Ministry of Science and Technology, Republic of China (Taiwan). The researchers disclosed no potential conflicts of interest.
SOURCE: Lee, C-H et al. JAMA Otolaryngol Head Neck Surg. 2018 Feb 15. doi: 10.1001/jamaoto.2017.3127.
after surgery, according to a retrospective analysis.
This is one of the few studies to have ever examined whether adenotonsillectomy for children with OSA had any effects on blood pressure (BP) and was based on “one of the largest cohorts for evaluating postoperative BP changes in nonobese children with OSA,” noted Cho-Hsueh Lee, MD, and colleagues. The report was published in JAMA Otolaryngology–Head & Neck Surgery. Among the previous studies that evaluated BP in children with OSA before and after having this surgery, the results varied, they added.
The researchers analyzed the medical records of 240 nonobese children with clinical symptoms and polysomnography-confirmed OSA (having an apnea-hypopnea index of greater than 1) who underwent adenotonsillectomy. Prior to surgery, 169 patients (70.4%) of the patients were classified as nonhypertensive, while 71 (29.6%) were classified as hypertensive. The children had a mean age of 7.3 years, and 160 were males.
Patients participated in full-night polysomnography (PSG) before surgery and at 3-6 months after adenotonsillectomy in the National Taiwan University Hospital Sleep Center. Apnea episodes were defined as a 90% decrease in airflow for two consecutive breaths. Sleep center staff measured the study participants’ systolic and diastolic BP in a sleep center using an electronic sphygmomanometer, in the evening, prior to the PSG study, and in the morning. Pediatric hypertension was based on the nocturnal BP measurement and was defined as having mean systolic and diastolic BP greater or equal to the 95th percentile for age, sex, and height.
“Postoperatively, hypertensive children had a significant decrease in all BP measures, including nocturnal and morning [systolic] BP ... A total of 47 hypertensive patients (66.2%) became nonhypertensive after surgery,” the researchers said.
For patients who were hypertensive before surgery, the average nocturnal (before PSG) preop systolic BP was 114.3 mm Hg, versus 107.5 mm Hg after surgery. The mean nocturnal diastolic BP for this same group of patients decreased to 65.1 mm Hg from 74.3 mm Hg. Similarly, the average morning (after PSG) systolic BP and diastolic BP were 106.0 mm Hg and 64.4 mm Hg after these patients underwent adenotonsillectomy, compared with 111.8 mm Hg and 71.7 mm Hg prior to surgery, respectively.
The adenotonsillectomy didn’t improve all patients’ BP. For some who were nonhypertensive before surgery, blood pressure increased, with 36 (21.3%) of this group having become hypersensitive after surgery, the researchers acknowledged.
Overall, the cohort experienced significant improvements in several PSG measures, including the average apnea-hypopnea index, which decreased from 12.1 events per hour to 1.7. The total arousal index also declined, going from 6.1 events per hour to 4.2. In addition, the mean oxygen saturation improved from 96.8% to 97.7%.
The investigators described several limitations of the study, including their inability to collect patients’ arterial stiffness, carotid intima thickness, and other cardiovascular measures beyond BP.
They recommended a follow-up study. “Although we observed improvements in BP measures within 6 months after surgery for hypertensive children with OSA, the long-term effects of surgery on BP remain uncertain,” they explained.
The study was supported by grants from the Ministry of Science and Technology, Republic of China (Taiwan). The researchers disclosed no potential conflicts of interest.
SOURCE: Lee, C-H et al. JAMA Otolaryngol Head Neck Surg. 2018 Feb 15. doi: 10.1001/jamaoto.2017.3127.
FROM JAMA OTOLARYNGOLOGY-HEAD & NECK SURGERY
Key clinical point: Hypertensive children with obstructive sleep apnea (OSA) who had an adenotonsillectomy experienced significant improvements in their blood pressure after surgery.
Major finding: Sixty-six percent of hypertensive patients with OSA became nonhypertensive after adenotonsillectomy.
Study details: A retrospective analysis of 240 nonobese children with OSA who underwent adenotonsillectomy.
Disclosures: The study was supported by grants from the Ministry of Science and Technology, Republic of China (Taiwan). The researchers disclosed no potential conflicts of interest.
Source: Lee, C-H et al. JAMA Otolaryngol Head Neck Surg. 2018 Feb 15. doi: 10.1001/jamaoto.2017.3127.
FDA approves starting dose of roflumilast
The Food and Drug Administration has approved the use of a 250-mcg dose of roflumilast for patients with chronic obstructive pulmonary disease (COPD) for 4 weeks, followed by the use of 500-mcg therapeutic doses, according to a statement from the drug’s marketer, AstraZeneca.
The larger doses of roflumilast (Daliresp) are currently indicated for reducing the risk of COPD exacerbations in patients with severe COPD associated with chronic bronchitis and a history of exacerbations, according to the statement. The selective phosphodiesterase-4 inhibitor, roflumilast, was approved for this use in 500-mcg doses in 2011. The new smaller doses of the drug are being offered to help reduce the rate of treatment discontinuation with use of the higher therapeutic dosing. The 250-mcg doses of roflumilast are not to be used as treatment for COPD.
“As the only once-daily tablet to provide enhanced protection against COPD exacerbations when added to current bronchodilator therapy, this is an important new dosing option to help patients start and stay on treatment. Exacerbations are associated with hospitalizations and an accelerated decline in lung function, and these patients living with COPD need effective treatment options,” Tosh Butt, vice president, respiratory, at AstraZeneca, said in the press release.
The approval of use of the 250-mcg doses was based on data from the OPTIMIZE study (Evaluation of Tolerability and Pharmacokinetics of Roflumilast trial, 250 mcg and 500 mcg, as an add-on to Standard COPD Treatment to Treat Severe COPD), according to the statement.
Over 12 weeks, the percentage of patients stopping treatment was significantly lower in those first given 250 mcg of roflumilast daily for 4 weeks, followed by 500 mcg once a week for 8 weeks (18.4%), compared with those given 500 mcg of roflumilast daily for 12 weeks (24.6%; odds ratio, 0.66; 95% confidence interval, 0.47-0.93; P = .017).
In eight controlled clinical trials, the most common adverse effects were diarrhea, weight loss, nausea, headache, back pain, influenza, insomnia, dizziness, and decreased appetite.
The Food and Drug Administration has approved the use of a 250-mcg dose of roflumilast for patients with chronic obstructive pulmonary disease (COPD) for 4 weeks, followed by the use of 500-mcg therapeutic doses, according to a statement from the drug’s marketer, AstraZeneca.
The larger doses of roflumilast (Daliresp) are currently indicated for reducing the risk of COPD exacerbations in patients with severe COPD associated with chronic bronchitis and a history of exacerbations, according to the statement. The selective phosphodiesterase-4 inhibitor, roflumilast, was approved for this use in 500-mcg doses in 2011. The new smaller doses of the drug are being offered to help reduce the rate of treatment discontinuation with use of the higher therapeutic dosing. The 250-mcg doses of roflumilast are not to be used as treatment for COPD.
“As the only once-daily tablet to provide enhanced protection against COPD exacerbations when added to current bronchodilator therapy, this is an important new dosing option to help patients start and stay on treatment. Exacerbations are associated with hospitalizations and an accelerated decline in lung function, and these patients living with COPD need effective treatment options,” Tosh Butt, vice president, respiratory, at AstraZeneca, said in the press release.
The approval of use of the 250-mcg doses was based on data from the OPTIMIZE study (Evaluation of Tolerability and Pharmacokinetics of Roflumilast trial, 250 mcg and 500 mcg, as an add-on to Standard COPD Treatment to Treat Severe COPD), according to the statement.
Over 12 weeks, the percentage of patients stopping treatment was significantly lower in those first given 250 mcg of roflumilast daily for 4 weeks, followed by 500 mcg once a week for 8 weeks (18.4%), compared with those given 500 mcg of roflumilast daily for 12 weeks (24.6%; odds ratio, 0.66; 95% confidence interval, 0.47-0.93; P = .017).
In eight controlled clinical trials, the most common adverse effects were diarrhea, weight loss, nausea, headache, back pain, influenza, insomnia, dizziness, and decreased appetite.
The Food and Drug Administration has approved the use of a 250-mcg dose of roflumilast for patients with chronic obstructive pulmonary disease (COPD) for 4 weeks, followed by the use of 500-mcg therapeutic doses, according to a statement from the drug’s marketer, AstraZeneca.
The larger doses of roflumilast (Daliresp) are currently indicated for reducing the risk of COPD exacerbations in patients with severe COPD associated with chronic bronchitis and a history of exacerbations, according to the statement. The selective phosphodiesterase-4 inhibitor, roflumilast, was approved for this use in 500-mcg doses in 2011. The new smaller doses of the drug are being offered to help reduce the rate of treatment discontinuation with use of the higher therapeutic dosing. The 250-mcg doses of roflumilast are not to be used as treatment for COPD.
“As the only once-daily tablet to provide enhanced protection against COPD exacerbations when added to current bronchodilator therapy, this is an important new dosing option to help patients start and stay on treatment. Exacerbations are associated with hospitalizations and an accelerated decline in lung function, and these patients living with COPD need effective treatment options,” Tosh Butt, vice president, respiratory, at AstraZeneca, said in the press release.
The approval of use of the 250-mcg doses was based on data from the OPTIMIZE study (Evaluation of Tolerability and Pharmacokinetics of Roflumilast trial, 250 mcg and 500 mcg, as an add-on to Standard COPD Treatment to Treat Severe COPD), according to the statement.
Over 12 weeks, the percentage of patients stopping treatment was significantly lower in those first given 250 mcg of roflumilast daily for 4 weeks, followed by 500 mcg once a week for 8 weeks (18.4%), compared with those given 500 mcg of roflumilast daily for 12 weeks (24.6%; odds ratio, 0.66; 95% confidence interval, 0.47-0.93; P = .017).
In eight controlled clinical trials, the most common adverse effects were diarrhea, weight loss, nausea, headache, back pain, influenza, insomnia, dizziness, and decreased appetite.
FDA axes asthma drugs’ boxed warning
The Food and Drug Administration has eliminated the boxed warning for risk of asthma-related death from the labels of products containing both an inhaled corticosteroid (ICS) and a long-acting beta agonist (LABA), the agency announced.
In 2011, the FDA required companies manufacturing fixed-dose LABA-ICS combination products to conduct 26-week clinical safety trials to evaluate the risks of serious adverse asthma-related events in patients treated with these drugs. Specifically, the companies had to compare the follows the FDA’s review of these trials, which found that treating asthma with LABAs in combination with ICS did not result in patients experiencing significantly more serious asthma-related side effects and asthma-related deaths, compared with those being treated with an ICS alone, according to the FDA announcement. “Results of subgroup analyses for gender, adolescents 12-18 years, and African Americans are consistent with the primary endpoint results,” the statement added.
“These trials showed that LABAs, when used with ICS, did not significantly increase the risk of asthma-related hospitalizations, the need to insert a breathing tube known as intubation, or asthma-related deaths, compared to ICS alone,” the FDA said in the statement.
The trials also demonstrated that using the combination reduced asthma exacerbations, compared with using ICS alone, and that most of the exacerbations “were those that required at least 3 days of systemic corticosteroids” – information that is being added the product labels, according to the FDA.
The products that will no longer carry this boxed warning in their labels include AstraZeneca’s budesonide/formoterol fumarate dihydrate (Symbicort) and GlaxoSmithKline’s fluticasone furoate/vilanterol (Breo Ellipta) and fluticasone propionate/salmeterol (Advair Diskus and Advair HFA).
The FDA also approved updates to the Warnings and Precautions section of labeling for the ICS/LABA class, which now includes a description of the four trials. Information on the efficacy of the drugs, found in the trials, has been added to the Clinical Studies section of the labels as well.
In a related safety announcement, the FDA stated the following: “Using LABAs alone to treat asthma without an ICS to treat lung inflammation is associated with an increased risk of asthma-related death. Therefore, the Boxed Warning stating this will remain in the labels of all single-ingredient LABA medicines, which are approved to treat asthma, chronic obstructive pulmonary disease (COPD), and wheezing caused by exercise. The labels of medicines that contain both an ICS and LABA also retain a Warning and Precaution related to the increased risk of asthma-related death when LABAs are used without an ICS to treat asthma.
The Food and Drug Administration has eliminated the boxed warning for risk of asthma-related death from the labels of products containing both an inhaled corticosteroid (ICS) and a long-acting beta agonist (LABA), the agency announced.
In 2011, the FDA required companies manufacturing fixed-dose LABA-ICS combination products to conduct 26-week clinical safety trials to evaluate the risks of serious adverse asthma-related events in patients treated with these drugs. Specifically, the companies had to compare the follows the FDA’s review of these trials, which found that treating asthma with LABAs in combination with ICS did not result in patients experiencing significantly more serious asthma-related side effects and asthma-related deaths, compared with those being treated with an ICS alone, according to the FDA announcement. “Results of subgroup analyses for gender, adolescents 12-18 years, and African Americans are consistent with the primary endpoint results,” the statement added.
“These trials showed that LABAs, when used with ICS, did not significantly increase the risk of asthma-related hospitalizations, the need to insert a breathing tube known as intubation, or asthma-related deaths, compared to ICS alone,” the FDA said in the statement.
The trials also demonstrated that using the combination reduced asthma exacerbations, compared with using ICS alone, and that most of the exacerbations “were those that required at least 3 days of systemic corticosteroids” – information that is being added the product labels, according to the FDA.
The products that will no longer carry this boxed warning in their labels include AstraZeneca’s budesonide/formoterol fumarate dihydrate (Symbicort) and GlaxoSmithKline’s fluticasone furoate/vilanterol (Breo Ellipta) and fluticasone propionate/salmeterol (Advair Diskus and Advair HFA).
The FDA also approved updates to the Warnings and Precautions section of labeling for the ICS/LABA class, which now includes a description of the four trials. Information on the efficacy of the drugs, found in the trials, has been added to the Clinical Studies section of the labels as well.
In a related safety announcement, the FDA stated the following: “Using LABAs alone to treat asthma without an ICS to treat lung inflammation is associated with an increased risk of asthma-related death. Therefore, the Boxed Warning stating this will remain in the labels of all single-ingredient LABA medicines, which are approved to treat asthma, chronic obstructive pulmonary disease (COPD), and wheezing caused by exercise. The labels of medicines that contain both an ICS and LABA also retain a Warning and Precaution related to the increased risk of asthma-related death when LABAs are used without an ICS to treat asthma.
The Food and Drug Administration has eliminated the boxed warning for risk of asthma-related death from the labels of products containing both an inhaled corticosteroid (ICS) and a long-acting beta agonist (LABA), the agency announced.
In 2011, the FDA required companies manufacturing fixed-dose LABA-ICS combination products to conduct 26-week clinical safety trials to evaluate the risks of serious adverse asthma-related events in patients treated with these drugs. Specifically, the companies had to compare the follows the FDA’s review of these trials, which found that treating asthma with LABAs in combination with ICS did not result in patients experiencing significantly more serious asthma-related side effects and asthma-related deaths, compared with those being treated with an ICS alone, according to the FDA announcement. “Results of subgroup analyses for gender, adolescents 12-18 years, and African Americans are consistent with the primary endpoint results,” the statement added.
“These trials showed that LABAs, when used with ICS, did not significantly increase the risk of asthma-related hospitalizations, the need to insert a breathing tube known as intubation, or asthma-related deaths, compared to ICS alone,” the FDA said in the statement.
The trials also demonstrated that using the combination reduced asthma exacerbations, compared with using ICS alone, and that most of the exacerbations “were those that required at least 3 days of systemic corticosteroids” – information that is being added the product labels, according to the FDA.
The products that will no longer carry this boxed warning in their labels include AstraZeneca’s budesonide/formoterol fumarate dihydrate (Symbicort) and GlaxoSmithKline’s fluticasone furoate/vilanterol (Breo Ellipta) and fluticasone propionate/salmeterol (Advair Diskus and Advair HFA).
The FDA also approved updates to the Warnings and Precautions section of labeling for the ICS/LABA class, which now includes a description of the four trials. Information on the efficacy of the drugs, found in the trials, has been added to the Clinical Studies section of the labels as well.
In a related safety announcement, the FDA stated the following: “Using LABAs alone to treat asthma without an ICS to treat lung inflammation is associated with an increased risk of asthma-related death. Therefore, the Boxed Warning stating this will remain in the labels of all single-ingredient LABA medicines, which are approved to treat asthma, chronic obstructive pulmonary disease (COPD), and wheezing caused by exercise. The labels of medicines that contain both an ICS and LABA also retain a Warning and Precaution related to the increased risk of asthma-related death when LABAs are used without an ICS to treat asthma.
FDA asked to approve add-on drug for eosinophilic COPD
GlaxoSmithKline asked the Food and Drug Administration to approve an interleuklin-5 antagonist as an add-on maintenance therapy for patients with eosinophilic chronic obstructive pulmonary disease (COPD).
The pharmaceutical and health care company is seeking approval of mepolizumab to be used specifically to treat COPD patients with an eosinophilic phenotype. The drug currently is indicated to treat patients aged 12 years or older with severe asthma and asthma with an eosinophilic phenotype and is sold under the name Nucala, according to a GlaxoSmithKline statement issued November 7.
Headache, injection site reaction, back pain, and fatigue are the most common adverse reactions seen in patients who took mepolizumab during clinical trials.
Mepolizumab is not approved for the treatment of COPD anywhere in the world, and GlaxoSmithKline intends to also ask other countries’ regulatory authorities to allow this drug to be sold as a therapy for COPD.
GlaxoSmithKline asked the Food and Drug Administration to approve an interleuklin-5 antagonist as an add-on maintenance therapy for patients with eosinophilic chronic obstructive pulmonary disease (COPD).
The pharmaceutical and health care company is seeking approval of mepolizumab to be used specifically to treat COPD patients with an eosinophilic phenotype. The drug currently is indicated to treat patients aged 12 years or older with severe asthma and asthma with an eosinophilic phenotype and is sold under the name Nucala, according to a GlaxoSmithKline statement issued November 7.
Headache, injection site reaction, back pain, and fatigue are the most common adverse reactions seen in patients who took mepolizumab during clinical trials.
Mepolizumab is not approved for the treatment of COPD anywhere in the world, and GlaxoSmithKline intends to also ask other countries’ regulatory authorities to allow this drug to be sold as a therapy for COPD.
GlaxoSmithKline asked the Food and Drug Administration to approve an interleuklin-5 antagonist as an add-on maintenance therapy for patients with eosinophilic chronic obstructive pulmonary disease (COPD).
The pharmaceutical and health care company is seeking approval of mepolizumab to be used specifically to treat COPD patients with an eosinophilic phenotype. The drug currently is indicated to treat patients aged 12 years or older with severe asthma and asthma with an eosinophilic phenotype and is sold under the name Nucala, according to a GlaxoSmithKline statement issued November 7.
Headache, injection site reaction, back pain, and fatigue are the most common adverse reactions seen in patients who took mepolizumab during clinical trials.
Mepolizumab is not approved for the treatment of COPD anywhere in the world, and GlaxoSmithKline intends to also ask other countries’ regulatory authorities to allow this drug to be sold as a therapy for COPD.
Aspirin responsiveness improved in some with OSA
Obstructive sleep apnea patients with endothelial dysfunction gained aspirin responsiveness after using continuous positive airway pressure (CPAP) therapy, according to the findings from a small study scheduled to be presented at CHEST 2017.
“Endothelial dysfunction is an important phenomenon implicated in cardiovascular morbidity in obstructive sleep apnea (OSA) patients. While it has been demonstrated that CPAP improves endothelial function, our understanding of the pathophysiologic links between CPAP therapy and cardiovascular outcomes remain limited,” researchers wrote in the study’s abstract.
The researchers examined 18 patients’ endothelial function before and after using CPAP therapy for a median of 37 days, along with the relationship between endothelial function and aspirin responsiveness in these same patients. All study participants had been recently diagnosed with moderate to severe OSA and underwent modified peripheral artery tonometry and platelet aggregometry before and after beginning CPAP therapy. Most of the patients (14) demonstrated aspirin resistance at baseline.
Endothelial dysfunction was defined as having a reactive hyperemia index (RHI) of less than or equal to 1.67, while aspirin resistance was defined as having a reading of at least 550 aspirin reaction units (ARU).
At baseline, the average RHI of patients was 1.79 (standard deviation = 0.3), with 8 of the patients having had endothelial dysfunction. Following CPAP use, patients’ mean RHI increased to 1.94 (SD = 0.36), and endothelial dysfunction was present in just 5 of the study participants.*
After using CPAP, those patients with endothelial dysfunction at baseline were responsive to aspirin, with their average ARU reading at 520 following therapy. In contrast, those patients with normal endothelial function at baseline remained resistant to aspirin following CPAP use, based on mean ARU values before and after therapy.
Lirim Krveshi, DO, of Danbury (Conn.) Hospital, is scheduled to present this study, “A Prospective Cohort Study of Endothelial Function and its Relationship to Aspirin Responsiveness in OSA Patients,” on Sunday, Oct. 29, at 1:45 p.m. in Convention Center, room 601A. This presentation is part of the Obstructive Sleep Apnea: Insights & Management session, running from 1:30 p.m. to 3:00 p.m.
The study’s authors reported no conflicts of interest.
*This article was updated Oct. 27, 2017.
Obstructive sleep apnea patients with endothelial dysfunction gained aspirin responsiveness after using continuous positive airway pressure (CPAP) therapy, according to the findings from a small study scheduled to be presented at CHEST 2017.
“Endothelial dysfunction is an important phenomenon implicated in cardiovascular morbidity in obstructive sleep apnea (OSA) patients. While it has been demonstrated that CPAP improves endothelial function, our understanding of the pathophysiologic links between CPAP therapy and cardiovascular outcomes remain limited,” researchers wrote in the study’s abstract.
The researchers examined 18 patients’ endothelial function before and after using CPAP therapy for a median of 37 days, along with the relationship between endothelial function and aspirin responsiveness in these same patients. All study participants had been recently diagnosed with moderate to severe OSA and underwent modified peripheral artery tonometry and platelet aggregometry before and after beginning CPAP therapy. Most of the patients (14) demonstrated aspirin resistance at baseline.
Endothelial dysfunction was defined as having a reactive hyperemia index (RHI) of less than or equal to 1.67, while aspirin resistance was defined as having a reading of at least 550 aspirin reaction units (ARU).
At baseline, the average RHI of patients was 1.79 (standard deviation = 0.3), with 8 of the patients having had endothelial dysfunction. Following CPAP use, patients’ mean RHI increased to 1.94 (SD = 0.36), and endothelial dysfunction was present in just 5 of the study participants.*
After using CPAP, those patients with endothelial dysfunction at baseline were responsive to aspirin, with their average ARU reading at 520 following therapy. In contrast, those patients with normal endothelial function at baseline remained resistant to aspirin following CPAP use, based on mean ARU values before and after therapy.
Lirim Krveshi, DO, of Danbury (Conn.) Hospital, is scheduled to present this study, “A Prospective Cohort Study of Endothelial Function and its Relationship to Aspirin Responsiveness in OSA Patients,” on Sunday, Oct. 29, at 1:45 p.m. in Convention Center, room 601A. This presentation is part of the Obstructive Sleep Apnea: Insights & Management session, running from 1:30 p.m. to 3:00 p.m.
The study’s authors reported no conflicts of interest.
*This article was updated Oct. 27, 2017.
Obstructive sleep apnea patients with endothelial dysfunction gained aspirin responsiveness after using continuous positive airway pressure (CPAP) therapy, according to the findings from a small study scheduled to be presented at CHEST 2017.
“Endothelial dysfunction is an important phenomenon implicated in cardiovascular morbidity in obstructive sleep apnea (OSA) patients. While it has been demonstrated that CPAP improves endothelial function, our understanding of the pathophysiologic links between CPAP therapy and cardiovascular outcomes remain limited,” researchers wrote in the study’s abstract.
The researchers examined 18 patients’ endothelial function before and after using CPAP therapy for a median of 37 days, along with the relationship between endothelial function and aspirin responsiveness in these same patients. All study participants had been recently diagnosed with moderate to severe OSA and underwent modified peripheral artery tonometry and platelet aggregometry before and after beginning CPAP therapy. Most of the patients (14) demonstrated aspirin resistance at baseline.
Endothelial dysfunction was defined as having a reactive hyperemia index (RHI) of less than or equal to 1.67, while aspirin resistance was defined as having a reading of at least 550 aspirin reaction units (ARU).
At baseline, the average RHI of patients was 1.79 (standard deviation = 0.3), with 8 of the patients having had endothelial dysfunction. Following CPAP use, patients’ mean RHI increased to 1.94 (SD = 0.36), and endothelial dysfunction was present in just 5 of the study participants.*
After using CPAP, those patients with endothelial dysfunction at baseline were responsive to aspirin, with their average ARU reading at 520 following therapy. In contrast, those patients with normal endothelial function at baseline remained resistant to aspirin following CPAP use, based on mean ARU values before and after therapy.
Lirim Krveshi, DO, of Danbury (Conn.) Hospital, is scheduled to present this study, “A Prospective Cohort Study of Endothelial Function and its Relationship to Aspirin Responsiveness in OSA Patients,” on Sunday, Oct. 29, at 1:45 p.m. in Convention Center, room 601A. This presentation is part of the Obstructive Sleep Apnea: Insights & Management session, running from 1:30 p.m. to 3:00 p.m.
The study’s authors reported no conflicts of interest.
*This article was updated Oct. 27, 2017.
FROM CHEST 2017
Key clinical point:
Major finding: The average aspirin reaction units reading for patients who had endothelial dysfunction at baseline was 520 following therapy.
Data source: A prospective cohort study of 18 patients with newly diagnosed moderate to severe OSA.
Disclosures: The study’s authors reported no conflicts of interest.