User login
Spinal cord stimulator restores Parkinson patient’s gait
The neuroprosthesis involves targeted epidural electrical stimulation of areas of the lumbosacral spinal cord that produce walking.
This new therapeutic tool offers hope to patients with PD and, combined with existing approaches, may alleviate a motor sign in PD for which there is currently “no real solution,” study investigator Eduardo Martin Moraud, PhD, who leads PD research at the Defitech Center for Interventional Neurotherapies (NeuroRestore), Lausanne, Switzerland, said in an interview.
“This is exciting for the many patients that develop gait deficits and experience frequent falls, who can only rely on physical therapy to try and minimize the consequences,” he added.
The findings were published online in Nature Medicine.
Personalized stimulation
About 90% of people with advanced PD experience gait and balance problems or freezing-of-gait episodes. These locomotor deficits typically don’t respond well to dopamine replacement therapy or deep brain stimulation (DBS) of the subthalamic nucleus, possibly because the neural origins of these motor problems involve brain circuits not related to dopamine, said Dr. Moraud.
Continuous electrical stimulation over the cervical or thoracic segments of the spinal cord reduces locomotor deficits in some people with PD, but the broader application of this strategy has led to variable and unsatisfying outcomes.
The new approach focuses on correcting abnormal activation of circuits in the lumbar spinal cord, a region that hosts all the neurons that control activation of the leg muscles used for walking.
The stimulating device is placed on the lumbar region of the spinal cord, which sends messages to leg muscles. It is wired to a small impulse generator implanted under the skin of the abdomen. Sensors placed in shoes align the stimulation to the patient’s movement.
The system can detect the beginning of a movement, immediately activate the appropriate electrode, and so facilitate the necessary movement, be that leg flexion, extension, or propulsion, said Dr. Moraud. “This allows for increased walking symmetry, reinforced balance, and increased length of steps.”
The concept of this neuroprosthesis is similar to that used to allow patients with a spinal cord injury (SCI) to walk. But unlike patients with SCI, those with PD can move their legs, indicating that there is a descending command from the brain that needs to interact with the stimulation of the spinal cord, and patients with PD can feel the stimulation.
“Both these elements imply that amplitudes of stimulation need to be much lower in PD than SCI, and that stimulation needs to be fully personalized in PD to synergistically interact with the descending commands from the brain.”
After fine-tuning this new neuroprosthesis in animal models, researchers implanted the device in a 62-year-old man with a 30-year history of PD who presented with severe gait impairments, including marked gait asymmetry, reduced stride length, and balance problems.
Gait restored to near normal
The patient had frequent freezing-of-gait episodes when turning and passing through narrow paths, which led to multiple falls a day. This was despite being treated with DBS and dopaminergic replacement therapies.
But after getting used to the neuroprosthesis, the patient now walks with a gait akin to that of people without PD.
“Our experience in the preclinical animal models and this first patient is that gait can be restored to an almost healthy level, but this, of course, may vary across patients, depending on the severity of their disease progression, and their other motor deficits,” said Dr. Moraud.
When the neuroprosthesis is turned on, freezing of gait nearly vanishes, both with and without DBS.
In addition, the neuroprosthesis augmented the impact of the patient’s rehabilitation program, which involved a variety of regular exercises, including walking on basic and complex terrains, navigating outdoors in community settings, balance training, and basic physical therapy.
Frequent use of the neuroprosthesis during gait rehabilitation also translated into “highly improved” quality of life as reported by the patient (and his wife), said Dr. Moraud.
The patient has now been using the neuroprosthesis about 8 hours a day for nearly 2 years, only switching it off when sitting for long periods of time or while sleeping.
“He regained the capacity to walk in complex or crowded environments such as shops, airports, or his own home, without falling,” said Dr. Moraud. “He went from falling five to six times per day to one or two [falls] every couple of weeks. He’s also much more confident. He can walk for many miles, run, and go on holidays, without the constant fear of falling and having related injuries.”
Dr. Moraud stressed that the device does not replace DBS, which is a “key therapy” that addresses other deficits in PD, such as rigidity or slowness of movement. “What we propose here is a fully complementary approach for the gait problems that are not well addressed by DBS.”
One of the next steps will be to evaluate the efficacy of this approach across a wider spectrum of patient profiles to fully define the best responders, said Dr. Moraud.
A ‘tour de force’
In a comment, Michael S. Okun, MD, director of the Norman Fixel Institute for Neurological Diseases, University of Florida, Gainesville, and medical director of the Parkinson’s Foundation, noted that the researchers used “a smarter device” than past approaches that failed to adequately address progressive walking challenges of patients with PD.
Although it’s “tempting to get excited” about the findings, it’s important to consider that the study included only one human subject and did not target circuits for both walking and balance, said Dr. Okun. “It’s possible that even if future studies revealed a benefit for walking, the device may or may not address falling.”
In an accompanying editorial, Aviv Mizrahi-Kliger, MD, PhD, department of neurology, University of California, San Francisco, and Karunesh Ganguly, MD, PhD, Neurology and Rehabilitation Service, San Francisco Veterans Affairs Health Care System, called the study an “impressive tour de force,” with data from the nonhuman primate model and the individual with PD “jointly” indicating that epidural electrical stimulation (EES) “is a very promising treatment for several aspects of gait, posture and balance impairments in PD.”
But although the effect in the single patient “is quite impressive,” the “next crucial step” is to test this approach in a larger cohort of patients, they said.
They noted the nonhuman model does not exhibit freezing of gait, “which precluded the ability to corroborate or further study the role of EES in alleviating this symptom of PD in an animal model.”
In addition, stimulation parameters in the patient with PD “had to rely on estimated normal activity patterns, owing to the inability to measure pre-disease patterns at the individual level,” they wrote.
The study received funding from the Defitech Foundation, ONWARD Medical, CAMS Innovation Fund for Medical Sciences, National Natural Science Foundation of China, Parkinson Schweiz Foundation, European Community’s Seventh Framework Program (NeuWalk), European Research Council, Wyss Center for Bio and Neuroengineering, Bertarelli Foundation, and Swiss National Science Foundation. Dr. Moraud and other study authors hold various patents or applications in relation to the present work. Dr. Mizrahi-Kliger has no relevant conflicts of interest; Dr. Ganguly has a patent for modulation of sensory inputs to improve motor recovery from stroke and has been a consultant to Cala Health.
A version of this article first appeared on Medscape.com.
The neuroprosthesis involves targeted epidural electrical stimulation of areas of the lumbosacral spinal cord that produce walking.
This new therapeutic tool offers hope to patients with PD and, combined with existing approaches, may alleviate a motor sign in PD for which there is currently “no real solution,” study investigator Eduardo Martin Moraud, PhD, who leads PD research at the Defitech Center for Interventional Neurotherapies (NeuroRestore), Lausanne, Switzerland, said in an interview.
“This is exciting for the many patients that develop gait deficits and experience frequent falls, who can only rely on physical therapy to try and minimize the consequences,” he added.
The findings were published online in Nature Medicine.
Personalized stimulation
About 90% of people with advanced PD experience gait and balance problems or freezing-of-gait episodes. These locomotor deficits typically don’t respond well to dopamine replacement therapy or deep brain stimulation (DBS) of the subthalamic nucleus, possibly because the neural origins of these motor problems involve brain circuits not related to dopamine, said Dr. Moraud.
Continuous electrical stimulation over the cervical or thoracic segments of the spinal cord reduces locomotor deficits in some people with PD, but the broader application of this strategy has led to variable and unsatisfying outcomes.
The new approach focuses on correcting abnormal activation of circuits in the lumbar spinal cord, a region that hosts all the neurons that control activation of the leg muscles used for walking.
The stimulating device is placed on the lumbar region of the spinal cord, which sends messages to leg muscles. It is wired to a small impulse generator implanted under the skin of the abdomen. Sensors placed in shoes align the stimulation to the patient’s movement.
The system can detect the beginning of a movement, immediately activate the appropriate electrode, and so facilitate the necessary movement, be that leg flexion, extension, or propulsion, said Dr. Moraud. “This allows for increased walking symmetry, reinforced balance, and increased length of steps.”
The concept of this neuroprosthesis is similar to that used to allow patients with a spinal cord injury (SCI) to walk. But unlike patients with SCI, those with PD can move their legs, indicating that there is a descending command from the brain that needs to interact with the stimulation of the spinal cord, and patients with PD can feel the stimulation.
“Both these elements imply that amplitudes of stimulation need to be much lower in PD than SCI, and that stimulation needs to be fully personalized in PD to synergistically interact with the descending commands from the brain.”
After fine-tuning this new neuroprosthesis in animal models, researchers implanted the device in a 62-year-old man with a 30-year history of PD who presented with severe gait impairments, including marked gait asymmetry, reduced stride length, and balance problems.
Gait restored to near normal
The patient had frequent freezing-of-gait episodes when turning and passing through narrow paths, which led to multiple falls a day. This was despite being treated with DBS and dopaminergic replacement therapies.
But after getting used to the neuroprosthesis, the patient now walks with a gait akin to that of people without PD.
“Our experience in the preclinical animal models and this first patient is that gait can be restored to an almost healthy level, but this, of course, may vary across patients, depending on the severity of their disease progression, and their other motor deficits,” said Dr. Moraud.
When the neuroprosthesis is turned on, freezing of gait nearly vanishes, both with and without DBS.
In addition, the neuroprosthesis augmented the impact of the patient’s rehabilitation program, which involved a variety of regular exercises, including walking on basic and complex terrains, navigating outdoors in community settings, balance training, and basic physical therapy.
Frequent use of the neuroprosthesis during gait rehabilitation also translated into “highly improved” quality of life as reported by the patient (and his wife), said Dr. Moraud.
The patient has now been using the neuroprosthesis about 8 hours a day for nearly 2 years, only switching it off when sitting for long periods of time or while sleeping.
“He regained the capacity to walk in complex or crowded environments such as shops, airports, or his own home, without falling,” said Dr. Moraud. “He went from falling five to six times per day to one or two [falls] every couple of weeks. He’s also much more confident. He can walk for many miles, run, and go on holidays, without the constant fear of falling and having related injuries.”
Dr. Moraud stressed that the device does not replace DBS, which is a “key therapy” that addresses other deficits in PD, such as rigidity or slowness of movement. “What we propose here is a fully complementary approach for the gait problems that are not well addressed by DBS.”
One of the next steps will be to evaluate the efficacy of this approach across a wider spectrum of patient profiles to fully define the best responders, said Dr. Moraud.
A ‘tour de force’
In a comment, Michael S. Okun, MD, director of the Norman Fixel Institute for Neurological Diseases, University of Florida, Gainesville, and medical director of the Parkinson’s Foundation, noted that the researchers used “a smarter device” than past approaches that failed to adequately address progressive walking challenges of patients with PD.
Although it’s “tempting to get excited” about the findings, it’s important to consider that the study included only one human subject and did not target circuits for both walking and balance, said Dr. Okun. “It’s possible that even if future studies revealed a benefit for walking, the device may or may not address falling.”
In an accompanying editorial, Aviv Mizrahi-Kliger, MD, PhD, department of neurology, University of California, San Francisco, and Karunesh Ganguly, MD, PhD, Neurology and Rehabilitation Service, San Francisco Veterans Affairs Health Care System, called the study an “impressive tour de force,” with data from the nonhuman primate model and the individual with PD “jointly” indicating that epidural electrical stimulation (EES) “is a very promising treatment for several aspects of gait, posture and balance impairments in PD.”
But although the effect in the single patient “is quite impressive,” the “next crucial step” is to test this approach in a larger cohort of patients, they said.
They noted the nonhuman model does not exhibit freezing of gait, “which precluded the ability to corroborate or further study the role of EES in alleviating this symptom of PD in an animal model.”
In addition, stimulation parameters in the patient with PD “had to rely on estimated normal activity patterns, owing to the inability to measure pre-disease patterns at the individual level,” they wrote.
The study received funding from the Defitech Foundation, ONWARD Medical, CAMS Innovation Fund for Medical Sciences, National Natural Science Foundation of China, Parkinson Schweiz Foundation, European Community’s Seventh Framework Program (NeuWalk), European Research Council, Wyss Center for Bio and Neuroengineering, Bertarelli Foundation, and Swiss National Science Foundation. Dr. Moraud and other study authors hold various patents or applications in relation to the present work. Dr. Mizrahi-Kliger has no relevant conflicts of interest; Dr. Ganguly has a patent for modulation of sensory inputs to improve motor recovery from stroke and has been a consultant to Cala Health.
A version of this article first appeared on Medscape.com.
The neuroprosthesis involves targeted epidural electrical stimulation of areas of the lumbosacral spinal cord that produce walking.
This new therapeutic tool offers hope to patients with PD and, combined with existing approaches, may alleviate a motor sign in PD for which there is currently “no real solution,” study investigator Eduardo Martin Moraud, PhD, who leads PD research at the Defitech Center for Interventional Neurotherapies (NeuroRestore), Lausanne, Switzerland, said in an interview.
“This is exciting for the many patients that develop gait deficits and experience frequent falls, who can only rely on physical therapy to try and minimize the consequences,” he added.
The findings were published online in Nature Medicine.
Personalized stimulation
About 90% of people with advanced PD experience gait and balance problems or freezing-of-gait episodes. These locomotor deficits typically don’t respond well to dopamine replacement therapy or deep brain stimulation (DBS) of the subthalamic nucleus, possibly because the neural origins of these motor problems involve brain circuits not related to dopamine, said Dr. Moraud.
Continuous electrical stimulation over the cervical or thoracic segments of the spinal cord reduces locomotor deficits in some people with PD, but the broader application of this strategy has led to variable and unsatisfying outcomes.
The new approach focuses on correcting abnormal activation of circuits in the lumbar spinal cord, a region that hosts all the neurons that control activation of the leg muscles used for walking.
The stimulating device is placed on the lumbar region of the spinal cord, which sends messages to leg muscles. It is wired to a small impulse generator implanted under the skin of the abdomen. Sensors placed in shoes align the stimulation to the patient’s movement.
The system can detect the beginning of a movement, immediately activate the appropriate electrode, and so facilitate the necessary movement, be that leg flexion, extension, or propulsion, said Dr. Moraud. “This allows for increased walking symmetry, reinforced balance, and increased length of steps.”
The concept of this neuroprosthesis is similar to that used to allow patients with a spinal cord injury (SCI) to walk. But unlike patients with SCI, those with PD can move their legs, indicating that there is a descending command from the brain that needs to interact with the stimulation of the spinal cord, and patients with PD can feel the stimulation.
“Both these elements imply that amplitudes of stimulation need to be much lower in PD than SCI, and that stimulation needs to be fully personalized in PD to synergistically interact with the descending commands from the brain.”
After fine-tuning this new neuroprosthesis in animal models, researchers implanted the device in a 62-year-old man with a 30-year history of PD who presented with severe gait impairments, including marked gait asymmetry, reduced stride length, and balance problems.
Gait restored to near normal
The patient had frequent freezing-of-gait episodes when turning and passing through narrow paths, which led to multiple falls a day. This was despite being treated with DBS and dopaminergic replacement therapies.
But after getting used to the neuroprosthesis, the patient now walks with a gait akin to that of people without PD.
“Our experience in the preclinical animal models and this first patient is that gait can be restored to an almost healthy level, but this, of course, may vary across patients, depending on the severity of their disease progression, and their other motor deficits,” said Dr. Moraud.
When the neuroprosthesis is turned on, freezing of gait nearly vanishes, both with and without DBS.
In addition, the neuroprosthesis augmented the impact of the patient’s rehabilitation program, which involved a variety of regular exercises, including walking on basic and complex terrains, navigating outdoors in community settings, balance training, and basic physical therapy.
Frequent use of the neuroprosthesis during gait rehabilitation also translated into “highly improved” quality of life as reported by the patient (and his wife), said Dr. Moraud.
The patient has now been using the neuroprosthesis about 8 hours a day for nearly 2 years, only switching it off when sitting for long periods of time or while sleeping.
“He regained the capacity to walk in complex or crowded environments such as shops, airports, or his own home, without falling,” said Dr. Moraud. “He went from falling five to six times per day to one or two [falls] every couple of weeks. He’s also much more confident. He can walk for many miles, run, and go on holidays, without the constant fear of falling and having related injuries.”
Dr. Moraud stressed that the device does not replace DBS, which is a “key therapy” that addresses other deficits in PD, such as rigidity or slowness of movement. “What we propose here is a fully complementary approach for the gait problems that are not well addressed by DBS.”
One of the next steps will be to evaluate the efficacy of this approach across a wider spectrum of patient profiles to fully define the best responders, said Dr. Moraud.
A ‘tour de force’
In a comment, Michael S. Okun, MD, director of the Norman Fixel Institute for Neurological Diseases, University of Florida, Gainesville, and medical director of the Parkinson’s Foundation, noted that the researchers used “a smarter device” than past approaches that failed to adequately address progressive walking challenges of patients with PD.
Although it’s “tempting to get excited” about the findings, it’s important to consider that the study included only one human subject and did not target circuits for both walking and balance, said Dr. Okun. “It’s possible that even if future studies revealed a benefit for walking, the device may or may not address falling.”
In an accompanying editorial, Aviv Mizrahi-Kliger, MD, PhD, department of neurology, University of California, San Francisco, and Karunesh Ganguly, MD, PhD, Neurology and Rehabilitation Service, San Francisco Veterans Affairs Health Care System, called the study an “impressive tour de force,” with data from the nonhuman primate model and the individual with PD “jointly” indicating that epidural electrical stimulation (EES) “is a very promising treatment for several aspects of gait, posture and balance impairments in PD.”
But although the effect in the single patient “is quite impressive,” the “next crucial step” is to test this approach in a larger cohort of patients, they said.
They noted the nonhuman model does not exhibit freezing of gait, “which precluded the ability to corroborate or further study the role of EES in alleviating this symptom of PD in an animal model.”
In addition, stimulation parameters in the patient with PD “had to rely on estimated normal activity patterns, owing to the inability to measure pre-disease patterns at the individual level,” they wrote.
The study received funding from the Defitech Foundation, ONWARD Medical, CAMS Innovation Fund for Medical Sciences, National Natural Science Foundation of China, Parkinson Schweiz Foundation, European Community’s Seventh Framework Program (NeuWalk), European Research Council, Wyss Center for Bio and Neuroengineering, Bertarelli Foundation, and Swiss National Science Foundation. Dr. Moraud and other study authors hold various patents or applications in relation to the present work. Dr. Mizrahi-Kliger has no relevant conflicts of interest; Dr. Ganguly has a patent for modulation of sensory inputs to improve motor recovery from stroke and has been a consultant to Cala Health.
A version of this article first appeared on Medscape.com.
FROM NATURE MEDICINE
Artificial intelligence presents opportunities, challenges in neurologic practice
PHOENIX – and it presents opportunities for increased production and automation of some tasks. However, it is prone to error and ‘hallucinations’ despite an authoritative tone, so its conclusions must be verified.
Those were some of the messages from a talk by John Morren, MD, an associate professor of neurology at Case Western Reserve University, Cleveland, who spoke about AI at the 2023 annual meeting of the American Association for Neuromuscular and Electrodiagnostic Medicine (AANEM).
He encouraged attendees to get involved in the conversation of AI, because it is here to stay and will have a big impact on health care. “If we’re not around the table making decisions, decisions will be made for us in our absence and won’t be in our favor,” said Dr. Morren.
He started out his talk by asking if anyone in the room had used AI. After about half raised their hands, he countered that nearly everyone likely had. Voice assistants like SIRI and Alexa, social media with curated feeds, online shopping tools that provide product suggestions, and content recommendations from streaming services like Netflix all rely on AI technology.
Within medicine, AI is already playing a role in various fields, including medical imaging, disease diagnosis, drug discovery and development, predictive analytics, personalized medicine, telemedicine, and health care management.
It also has potential to be used on the job. For example, ChatGPT can generate and refine conversations towards a specific length, format, style, and level of detail. Alternatives include Bing AI from Microsoft, Bard AI from Google, Writesonic, Copy.ai, SpinBot, HIX.AI, and Chatsonic.
Specific to medicine, Consensus is a search engine that uses AI to search for, summarize, and synthesize studies from peer-reviewed literature.
Trust, but verify
Dr. Morren presented some specific use cases, including patient education and responses to patient inquiries, as well as generating letters to insurance companies appealing denial of coverage claims. He also showed an example where he asked Bing AI to explain to a patient, at a sixth- to seventh-grade reading level, the red-flag symptoms of myasthenic crisis.
AI can generate summaries of clinical evidence of previous studies. Asked by this reporter how to trust the accuracies of the summaries if the user hasn’t thoroughly read the papers, he acknowledged the imperfection of AI. “I would say that if you’re going to make a decision that you would not have made normally based on the summary that it’s giving, if you can find the fact that you’re anchoring the decision on, go into the article yourself and make sure that it’s well vetted. The AI is just good to tap you on your shoulder and say, ‘hey, just consider this.’ That’s all it is. You should always trust, but verify. If the AI is forcing you to say something new that you would not say, maybe don’t do it – or at least research it to know that it’s the truth and then you elevate yourself and get yourself to the next level.”
Limitations
The need to verify can create its own burden, according to one attendee. “I often find I end up spending more time verifying [what ChatGPT has provided]. This seems to take more time than a traditional way of going to PubMed or UpToDate or any of the other human generated consensus way,” he said.
Dr. Morren replied that he wouldn’t recommend using ChatGPT to query medical literature. Instead he recommended Consensus, which only searches the peer-reviewed medical literature.
Another key limitation is that most AI programs are date limited: For example, ChatGPT doesn’t include information after September 2021, though this may change with paid subscriptions. He also starkly warned the audience to never enter sensitive information, including patient identifiers.
There are legal and ethical considerations to AI. Dr. Morren warned against overreliance on AI, as this could undermine compassion and lead to erosion of trust, which makes it important to disclose any use of AI-generated content.
Another attendee raised concerns that AI may be generating research content, including slides for presentations, abstracts, titles, or article text. Dr. Morren said that some organizations, such as the International Committee of Medical Journal Editors, have incorporated AI in their recommendations, stating that authors should disclose any contributions of AI to their publications. However, there is little that can be done to identify AI-generated content, leaving it up to the honor code.
Asked to make predictions about how AI will evolve in the clinic over the next 2-3 years, Dr. Morren suggested that it will likely be embedded in electronic medical records. He anticipated that it will save physicians time so that they can spend more time interacting directly with patients. He quoted Eric Topol, MD, professor of medicine at Scripps Research Translational Institute, La Jolla, Calif., as saying that AI could save 20% of a physician’s time, which could be spent with patients. Dr. Morren saw it differently. “I know where that 20% of time liberated is going to go. I’m going to see 20% more patients. I’m a realist,” he said, to audience laughter.
He also predicted that AI will be found in wearables and devices, allowing health care to expand into the patient’s home in real time. “A lot of what we’re wearing is going to be an extension of the doctor’s office,” he said.
For those hoping for more guidance, Dr. Morren noted that he is the chairman of the professional practice committee of AANEM, and the group will be putting out a position statement within the next couple of months. “It will be a little bit of a blueprint for the path going forward. There are specific things that need to be done. In research, for example, you have to ensure that datasets are diverse enough. To do that we need to have inter-institutional collaboration. We have to ensure patient privacy. Consent for this needs to be a little more explicit because this is a novel area. Those are things that need to be stipulated and ratified through a task force.”
Dr. Morren has no relevant financial disclosures.
PHOENIX – and it presents opportunities for increased production and automation of some tasks. However, it is prone to error and ‘hallucinations’ despite an authoritative tone, so its conclusions must be verified.
Those were some of the messages from a talk by John Morren, MD, an associate professor of neurology at Case Western Reserve University, Cleveland, who spoke about AI at the 2023 annual meeting of the American Association for Neuromuscular and Electrodiagnostic Medicine (AANEM).
He encouraged attendees to get involved in the conversation of AI, because it is here to stay and will have a big impact on health care. “If we’re not around the table making decisions, decisions will be made for us in our absence and won’t be in our favor,” said Dr. Morren.
He started out his talk by asking if anyone in the room had used AI. After about half raised their hands, he countered that nearly everyone likely had. Voice assistants like SIRI and Alexa, social media with curated feeds, online shopping tools that provide product suggestions, and content recommendations from streaming services like Netflix all rely on AI technology.
Within medicine, AI is already playing a role in various fields, including medical imaging, disease diagnosis, drug discovery and development, predictive analytics, personalized medicine, telemedicine, and health care management.
It also has potential to be used on the job. For example, ChatGPT can generate and refine conversations towards a specific length, format, style, and level of detail. Alternatives include Bing AI from Microsoft, Bard AI from Google, Writesonic, Copy.ai, SpinBot, HIX.AI, and Chatsonic.
Specific to medicine, Consensus is a search engine that uses AI to search for, summarize, and synthesize studies from peer-reviewed literature.
Trust, but verify
Dr. Morren presented some specific use cases, including patient education and responses to patient inquiries, as well as generating letters to insurance companies appealing denial of coverage claims. He also showed an example where he asked Bing AI to explain to a patient, at a sixth- to seventh-grade reading level, the red-flag symptoms of myasthenic crisis.
AI can generate summaries of clinical evidence of previous studies. Asked by this reporter how to trust the accuracies of the summaries if the user hasn’t thoroughly read the papers, he acknowledged the imperfection of AI. “I would say that if you’re going to make a decision that you would not have made normally based on the summary that it’s giving, if you can find the fact that you’re anchoring the decision on, go into the article yourself and make sure that it’s well vetted. The AI is just good to tap you on your shoulder and say, ‘hey, just consider this.’ That’s all it is. You should always trust, but verify. If the AI is forcing you to say something new that you would not say, maybe don’t do it – or at least research it to know that it’s the truth and then you elevate yourself and get yourself to the next level.”
Limitations
The need to verify can create its own burden, according to one attendee. “I often find I end up spending more time verifying [what ChatGPT has provided]. This seems to take more time than a traditional way of going to PubMed or UpToDate or any of the other human generated consensus way,” he said.
Dr. Morren replied that he wouldn’t recommend using ChatGPT to query medical literature. Instead he recommended Consensus, which only searches the peer-reviewed medical literature.
Another key limitation is that most AI programs are date limited: For example, ChatGPT doesn’t include information after September 2021, though this may change with paid subscriptions. He also starkly warned the audience to never enter sensitive information, including patient identifiers.
There are legal and ethical considerations to AI. Dr. Morren warned against overreliance on AI, as this could undermine compassion and lead to erosion of trust, which makes it important to disclose any use of AI-generated content.
Another attendee raised concerns that AI may be generating research content, including slides for presentations, abstracts, titles, or article text. Dr. Morren said that some organizations, such as the International Committee of Medical Journal Editors, have incorporated AI in their recommendations, stating that authors should disclose any contributions of AI to their publications. However, there is little that can be done to identify AI-generated content, leaving it up to the honor code.
Asked to make predictions about how AI will evolve in the clinic over the next 2-3 years, Dr. Morren suggested that it will likely be embedded in electronic medical records. He anticipated that it will save physicians time so that they can spend more time interacting directly with patients. He quoted Eric Topol, MD, professor of medicine at Scripps Research Translational Institute, La Jolla, Calif., as saying that AI could save 20% of a physician’s time, which could be spent with patients. Dr. Morren saw it differently. “I know where that 20% of time liberated is going to go. I’m going to see 20% more patients. I’m a realist,” he said, to audience laughter.
He also predicted that AI will be found in wearables and devices, allowing health care to expand into the patient’s home in real time. “A lot of what we’re wearing is going to be an extension of the doctor’s office,” he said.
For those hoping for more guidance, Dr. Morren noted that he is the chairman of the professional practice committee of AANEM, and the group will be putting out a position statement within the next couple of months. “It will be a little bit of a blueprint for the path going forward. There are specific things that need to be done. In research, for example, you have to ensure that datasets are diverse enough. To do that we need to have inter-institutional collaboration. We have to ensure patient privacy. Consent for this needs to be a little more explicit because this is a novel area. Those are things that need to be stipulated and ratified through a task force.”
Dr. Morren has no relevant financial disclosures.
PHOENIX – and it presents opportunities for increased production and automation of some tasks. However, it is prone to error and ‘hallucinations’ despite an authoritative tone, so its conclusions must be verified.
Those were some of the messages from a talk by John Morren, MD, an associate professor of neurology at Case Western Reserve University, Cleveland, who spoke about AI at the 2023 annual meeting of the American Association for Neuromuscular and Electrodiagnostic Medicine (AANEM).
He encouraged attendees to get involved in the conversation of AI, because it is here to stay and will have a big impact on health care. “If we’re not around the table making decisions, decisions will be made for us in our absence and won’t be in our favor,” said Dr. Morren.
He started out his talk by asking if anyone in the room had used AI. After about half raised their hands, he countered that nearly everyone likely had. Voice assistants like SIRI and Alexa, social media with curated feeds, online shopping tools that provide product suggestions, and content recommendations from streaming services like Netflix all rely on AI technology.
Within medicine, AI is already playing a role in various fields, including medical imaging, disease diagnosis, drug discovery and development, predictive analytics, personalized medicine, telemedicine, and health care management.
It also has potential to be used on the job. For example, ChatGPT can generate and refine conversations towards a specific length, format, style, and level of detail. Alternatives include Bing AI from Microsoft, Bard AI from Google, Writesonic, Copy.ai, SpinBot, HIX.AI, and Chatsonic.
Specific to medicine, Consensus is a search engine that uses AI to search for, summarize, and synthesize studies from peer-reviewed literature.
Trust, but verify
Dr. Morren presented some specific use cases, including patient education and responses to patient inquiries, as well as generating letters to insurance companies appealing denial of coverage claims. He also showed an example where he asked Bing AI to explain to a patient, at a sixth- to seventh-grade reading level, the red-flag symptoms of myasthenic crisis.
AI can generate summaries of clinical evidence of previous studies. Asked by this reporter how to trust the accuracies of the summaries if the user hasn’t thoroughly read the papers, he acknowledged the imperfection of AI. “I would say that if you’re going to make a decision that you would not have made normally based on the summary that it’s giving, if you can find the fact that you’re anchoring the decision on, go into the article yourself and make sure that it’s well vetted. The AI is just good to tap you on your shoulder and say, ‘hey, just consider this.’ That’s all it is. You should always trust, but verify. If the AI is forcing you to say something new that you would not say, maybe don’t do it – or at least research it to know that it’s the truth and then you elevate yourself and get yourself to the next level.”
Limitations
The need to verify can create its own burden, according to one attendee. “I often find I end up spending more time verifying [what ChatGPT has provided]. This seems to take more time than a traditional way of going to PubMed or UpToDate or any of the other human generated consensus way,” he said.
Dr. Morren replied that he wouldn’t recommend using ChatGPT to query medical literature. Instead he recommended Consensus, which only searches the peer-reviewed medical literature.
Another key limitation is that most AI programs are date limited: For example, ChatGPT doesn’t include information after September 2021, though this may change with paid subscriptions. He also starkly warned the audience to never enter sensitive information, including patient identifiers.
There are legal and ethical considerations to AI. Dr. Morren warned against overreliance on AI, as this could undermine compassion and lead to erosion of trust, which makes it important to disclose any use of AI-generated content.
Another attendee raised concerns that AI may be generating research content, including slides for presentations, abstracts, titles, or article text. Dr. Morren said that some organizations, such as the International Committee of Medical Journal Editors, have incorporated AI in their recommendations, stating that authors should disclose any contributions of AI to their publications. However, there is little that can be done to identify AI-generated content, leaving it up to the honor code.
Asked to make predictions about how AI will evolve in the clinic over the next 2-3 years, Dr. Morren suggested that it will likely be embedded in electronic medical records. He anticipated that it will save physicians time so that they can spend more time interacting directly with patients. He quoted Eric Topol, MD, professor of medicine at Scripps Research Translational Institute, La Jolla, Calif., as saying that AI could save 20% of a physician’s time, which could be spent with patients. Dr. Morren saw it differently. “I know where that 20% of time liberated is going to go. I’m going to see 20% more patients. I’m a realist,” he said, to audience laughter.
He also predicted that AI will be found in wearables and devices, allowing health care to expand into the patient’s home in real time. “A lot of what we’re wearing is going to be an extension of the doctor’s office,” he said.
For those hoping for more guidance, Dr. Morren noted that he is the chairman of the professional practice committee of AANEM, and the group will be putting out a position statement within the next couple of months. “It will be a little bit of a blueprint for the path going forward. There are specific things that need to be done. In research, for example, you have to ensure that datasets are diverse enough. To do that we need to have inter-institutional collaboration. We have to ensure patient privacy. Consent for this needs to be a little more explicit because this is a novel area. Those are things that need to be stipulated and ratified through a task force.”
Dr. Morren has no relevant financial disclosures.
AT AANEM 2023
When digestive symptoms signal Parkinson’s disease
The enteric nervous system (ENS), which is regarded as our second brain, is the part of the autonomic nervous system that controls the digestive tract. Housed along the entire length of the digestive tract, it is made up of more than 100 million neurons. It plays a central role in controlling the regulation of gastrointestinal motility, absorption of nutrients, and control of the intestinal barrier that protects the body from external pathogens.
Braak’s hypothesis suggests that the digestive tract could be the starting point for Parkinson’s disease. The fact that nearly all patients with Parkinson’s disease experience digestive problems and have neuropathological lesions in intrinsic and extrinsic innervation of the gastrointestinal tract suggests that Parkinson’s disease also has a gastrointestinal component.
Besides the ascending pathway formulated by Braak, a descending etiology in which gastrointestinal symptoms are present in early stages when neurological signposts have not yet been noticed is supported by evidence from trials. These gastrointestinal symptoms then represent a risk factor. Links have also been described between a history of gastrointestinal symptoms and Alzheimer’s disease and cerebrovascular diseases (CVD), thus justifying studies on a larger scale.
Large combined study
The authors have conducted a combined case-control and cohort study using TriNetX, a national network of medical records based in the United States. They identified 24,624 patients with idiopathic Parkinson’s disease in the case-control analysis and compared them with control subjects without neurological disease. They also identified subjects with Alzheimer’s disease and CVD, to study previous gastrointestinal signs. Secondly, 18 cohorts with each exposure (various gastrointestinal symptoms, appendectomy, vagotomy) were compared with their negative controls (NC) for the development of Parkinson’s disease, Alzheimer’s disease, or CVD in 5 years.
Gastroparesis, dysphagia, and irritable bowel syndrome (IBS) without diarrhea or constipation were shown to have specific associations with Parkinson’s disease (vs. NC, Alzheimer’s disease, and CVD) in both case-controls (odds ratios all P < .0001) and cohort analyses (relative risks all P < .05). While functional dyspepsia, IBS with diarrhea, diarrhea, and fecal incontinence were not specific to Parkinson’s disease, IBS with constipation and intestinal pseudo-obstruction showed specificity to Parkinson’s disease in the case-control (OR, 4.11) and cohort (RR, 1.84) analyses. Appendectomy reduced the risk of Parkinson’s disease in the cohort study (RR, 0.48). Neither inflammatory bowel disease nor vagotomy was associated with Parkinson’s disease.
A ‘second brain’
This broad study attempted to explore the gut-brain axis by looking for associations between neurological diagnoses and prior gastrointestinal symptoms and later development of Parkinson’s disease. After adjustment to account for multiple comparisons and acknowledgment of the initial risk in patients with Alzheimer’s disease and CVD, only dysphagia, gastroparesis, IBS without diarrhea, and isolated constipation were significantly and specifically associated with Parkinson’s disease.
Numerous literature reviews mention that ENS lesions are responsible for gastrointestinal disorders observed in patients with Parkinson’s disease. Tests on gastrointestinal autopsy and biopsy specimens have established that alpha synuclein clusters, which are morphologically similar to Lewy bodies in the CNS, are seen in the vagus nerve and in the ENS in most subjects with Parkinson’s disease. However, these studies have not shown any loss of neurons in the ENS in Parkinson’s disease, and the presence of alpha synuclein deposits in the ENS is not sufficient in itself to explain these gastrointestinal disorders.
It therefore remains to be determined whether vagal nerve damage alone can explain gastrointestinal disorders or whether dysfunction of enteric neurons without neuronal loss is occurring. So, damage to the ENS from alpha synuclein deposits would be early and would precede damage to the CNS, thus affording evidence in support of Braak’s hypothesis, which relies on autopsy data that does not allow for longitudinal monitoring in a single individual.
Appendectomy appeared to be protective, leading to additional speculation about its role in the pathophysiology of Parkinson’s disease. Additional mechanistic studies are therefore needed to establish causality and confirm the gut-brain axis or the role of dysbiosis and of intestinal permeability problems.
In conclusion, this large, first-of-its-kind multicenter study conducted on a national scale shows that Subject to future longitudinal mechanistic studies, early detection of these gastrointestinal disorders could aid in identifying patients at risk of Parkinson’s, and it could then be assumed that disease-modifying treatments could, at this early stage, halt progression of the disease linked to toxic clusters of alpha synuclein.
This article was translated from JIM, which is part of the Medscape professional network.
A version of this article first appeared on Medscape.com.
The enteric nervous system (ENS), which is regarded as our second brain, is the part of the autonomic nervous system that controls the digestive tract. Housed along the entire length of the digestive tract, it is made up of more than 100 million neurons. It plays a central role in controlling the regulation of gastrointestinal motility, absorption of nutrients, and control of the intestinal barrier that protects the body from external pathogens.
Braak’s hypothesis suggests that the digestive tract could be the starting point for Parkinson’s disease. The fact that nearly all patients with Parkinson’s disease experience digestive problems and have neuropathological lesions in intrinsic and extrinsic innervation of the gastrointestinal tract suggests that Parkinson’s disease also has a gastrointestinal component.
Besides the ascending pathway formulated by Braak, a descending etiology in which gastrointestinal symptoms are present in early stages when neurological signposts have not yet been noticed is supported by evidence from trials. These gastrointestinal symptoms then represent a risk factor. Links have also been described between a history of gastrointestinal symptoms and Alzheimer’s disease and cerebrovascular diseases (CVD), thus justifying studies on a larger scale.
Large combined study
The authors have conducted a combined case-control and cohort study using TriNetX, a national network of medical records based in the United States. They identified 24,624 patients with idiopathic Parkinson’s disease in the case-control analysis and compared them with control subjects without neurological disease. They also identified subjects with Alzheimer’s disease and CVD, to study previous gastrointestinal signs. Secondly, 18 cohorts with each exposure (various gastrointestinal symptoms, appendectomy, vagotomy) were compared with their negative controls (NC) for the development of Parkinson’s disease, Alzheimer’s disease, or CVD in 5 years.
Gastroparesis, dysphagia, and irritable bowel syndrome (IBS) without diarrhea or constipation were shown to have specific associations with Parkinson’s disease (vs. NC, Alzheimer’s disease, and CVD) in both case-controls (odds ratios all P < .0001) and cohort analyses (relative risks all P < .05). While functional dyspepsia, IBS with diarrhea, diarrhea, and fecal incontinence were not specific to Parkinson’s disease, IBS with constipation and intestinal pseudo-obstruction showed specificity to Parkinson’s disease in the case-control (OR, 4.11) and cohort (RR, 1.84) analyses. Appendectomy reduced the risk of Parkinson’s disease in the cohort study (RR, 0.48). Neither inflammatory bowel disease nor vagotomy was associated with Parkinson’s disease.
A ‘second brain’
This broad study attempted to explore the gut-brain axis by looking for associations between neurological diagnoses and prior gastrointestinal symptoms and later development of Parkinson’s disease. After adjustment to account for multiple comparisons and acknowledgment of the initial risk in patients with Alzheimer’s disease and CVD, only dysphagia, gastroparesis, IBS without diarrhea, and isolated constipation were significantly and specifically associated with Parkinson’s disease.
Numerous literature reviews mention that ENS lesions are responsible for gastrointestinal disorders observed in patients with Parkinson’s disease. Tests on gastrointestinal autopsy and biopsy specimens have established that alpha synuclein clusters, which are morphologically similar to Lewy bodies in the CNS, are seen in the vagus nerve and in the ENS in most subjects with Parkinson’s disease. However, these studies have not shown any loss of neurons in the ENS in Parkinson’s disease, and the presence of alpha synuclein deposits in the ENS is not sufficient in itself to explain these gastrointestinal disorders.
It therefore remains to be determined whether vagal nerve damage alone can explain gastrointestinal disorders or whether dysfunction of enteric neurons without neuronal loss is occurring. So, damage to the ENS from alpha synuclein deposits would be early and would precede damage to the CNS, thus affording evidence in support of Braak’s hypothesis, which relies on autopsy data that does not allow for longitudinal monitoring in a single individual.
Appendectomy appeared to be protective, leading to additional speculation about its role in the pathophysiology of Parkinson’s disease. Additional mechanistic studies are therefore needed to establish causality and confirm the gut-brain axis or the role of dysbiosis and of intestinal permeability problems.
In conclusion, this large, first-of-its-kind multicenter study conducted on a national scale shows that Subject to future longitudinal mechanistic studies, early detection of these gastrointestinal disorders could aid in identifying patients at risk of Parkinson’s, and it could then be assumed that disease-modifying treatments could, at this early stage, halt progression of the disease linked to toxic clusters of alpha synuclein.
This article was translated from JIM, which is part of the Medscape professional network.
A version of this article first appeared on Medscape.com.
The enteric nervous system (ENS), which is regarded as our second brain, is the part of the autonomic nervous system that controls the digestive tract. Housed along the entire length of the digestive tract, it is made up of more than 100 million neurons. It plays a central role in controlling the regulation of gastrointestinal motility, absorption of nutrients, and control of the intestinal barrier that protects the body from external pathogens.
Braak’s hypothesis suggests that the digestive tract could be the starting point for Parkinson’s disease. The fact that nearly all patients with Parkinson’s disease experience digestive problems and have neuropathological lesions in intrinsic and extrinsic innervation of the gastrointestinal tract suggests that Parkinson’s disease also has a gastrointestinal component.
Besides the ascending pathway formulated by Braak, a descending etiology in which gastrointestinal symptoms are present in early stages when neurological signposts have not yet been noticed is supported by evidence from trials. These gastrointestinal symptoms then represent a risk factor. Links have also been described between a history of gastrointestinal symptoms and Alzheimer’s disease and cerebrovascular diseases (CVD), thus justifying studies on a larger scale.
Large combined study
The authors have conducted a combined case-control and cohort study using TriNetX, a national network of medical records based in the United States. They identified 24,624 patients with idiopathic Parkinson’s disease in the case-control analysis and compared them with control subjects without neurological disease. They also identified subjects with Alzheimer’s disease and CVD, to study previous gastrointestinal signs. Secondly, 18 cohorts with each exposure (various gastrointestinal symptoms, appendectomy, vagotomy) were compared with their negative controls (NC) for the development of Parkinson’s disease, Alzheimer’s disease, or CVD in 5 years.
Gastroparesis, dysphagia, and irritable bowel syndrome (IBS) without diarrhea or constipation were shown to have specific associations with Parkinson’s disease (vs. NC, Alzheimer’s disease, and CVD) in both case-controls (odds ratios all P < .0001) and cohort analyses (relative risks all P < .05). While functional dyspepsia, IBS with diarrhea, diarrhea, and fecal incontinence were not specific to Parkinson’s disease, IBS with constipation and intestinal pseudo-obstruction showed specificity to Parkinson’s disease in the case-control (OR, 4.11) and cohort (RR, 1.84) analyses. Appendectomy reduced the risk of Parkinson’s disease in the cohort study (RR, 0.48). Neither inflammatory bowel disease nor vagotomy was associated with Parkinson’s disease.
A ‘second brain’
This broad study attempted to explore the gut-brain axis by looking for associations between neurological diagnoses and prior gastrointestinal symptoms and later development of Parkinson’s disease. After adjustment to account for multiple comparisons and acknowledgment of the initial risk in patients with Alzheimer’s disease and CVD, only dysphagia, gastroparesis, IBS without diarrhea, and isolated constipation were significantly and specifically associated with Parkinson’s disease.
Numerous literature reviews mention that ENS lesions are responsible for gastrointestinal disorders observed in patients with Parkinson’s disease. Tests on gastrointestinal autopsy and biopsy specimens have established that alpha synuclein clusters, which are morphologically similar to Lewy bodies in the CNS, are seen in the vagus nerve and in the ENS in most subjects with Parkinson’s disease. However, these studies have not shown any loss of neurons in the ENS in Parkinson’s disease, and the presence of alpha synuclein deposits in the ENS is not sufficient in itself to explain these gastrointestinal disorders.
It therefore remains to be determined whether vagal nerve damage alone can explain gastrointestinal disorders or whether dysfunction of enteric neurons without neuronal loss is occurring. So, damage to the ENS from alpha synuclein deposits would be early and would precede damage to the CNS, thus affording evidence in support of Braak’s hypothesis, which relies on autopsy data that does not allow for longitudinal monitoring in a single individual.
Appendectomy appeared to be protective, leading to additional speculation about its role in the pathophysiology of Parkinson’s disease. Additional mechanistic studies are therefore needed to establish causality and confirm the gut-brain axis or the role of dysbiosis and of intestinal permeability problems.
In conclusion, this large, first-of-its-kind multicenter study conducted on a national scale shows that Subject to future longitudinal mechanistic studies, early detection of these gastrointestinal disorders could aid in identifying patients at risk of Parkinson’s, and it could then be assumed that disease-modifying treatments could, at this early stage, halt progression of the disease linked to toxic clusters of alpha synuclein.
This article was translated from JIM, which is part of the Medscape professional network.
A version of this article first appeared on Medscape.com.
A new clue into the cause, spread of Parkinson’s disease?
.
While defects in mitochondrial functions and in mitochondrial DNA have been implicated in PD in the past, the current study demonstrates “for the first time how damaged mitochondrial DNA can underlie the mechanisms of PD initiation and spread in brain,” lead investigator Shohreh Issazadeh-Navikas, PhD, with the University of Copenhagen, told this news organization.
“This has direct implication for clinical diagnosis” – if damaged mtDNA can be detected in blood, it could serve as an early biomarker for disease, she explained.
The study was published online in Molecular Psychiatry.
“Infectious-like” spread of PD pathology
In earlier work, the researchers identified dysregulated interferon-beta (IFN-beta) signaling as a “top candidate pathway” associated with sporadic PD and its progression to PD with dementia (PDD).
In mice PD models that were deficient in IFN-beta signaling, the investigators showed that neuronal IFN-beta is required to maintain mitochondrial homeostasis and metabolism.
Lack of neuronal IFN-beta or disruption of its downstream signaling causes the accumulation of damaged mitochondria with excessive oxidative stress and insufficient adenosine triphosphate production.
In the current study, using postmortem brain tissue samples from patients with sporadic PD, they confirmed that there were deletions of mtDNA in the medial frontal gyrus, a region implicated in cognitive impairments in PD, suggesting a potential role of damaged mtDNA in disease pathophysiology.
They also identified mtDNA deletions in a “hotspot” in complex I respiratory chain subunits that were associated with dysregulation of oxidative stress and DNA damage response pathways in cohorts with sporadic PD and PDD.
They confirmed the contribution of mtDNA damage to PD pathology in the PD mouse models. They showed that lack of neuronal IFN-beta signaling leads to oxidative damage and mutations in mtDNA in neurons, which are subsequently released outside the neurons.
Injecting damaged mtDNA into mouse brain induced PDD-like behavioral symptoms, including neuropsychiatric, motor, and cognitive impairments. It also caused neurodegeneration in brain regions distant from the injection site, suggesting that damaged mtDNA triggers spread of PDD characteristics in an “infectious-like” manner, the researchers report.
Further study revealed that the mechanism through which damaged mtDNA causes pathology in healthy neurons involves dual activation of Toll-like receptor (TLR) 9 and 4 pathways, leading to increased oxidative stress and neuronal cell death, respectively.
“Our proteomic analysis of extracellular vesicles containing damaged mtDNA identified the TLR4 activator, ribosomal protein S3, as a key protein involved in recognizing and extruding damaged mtDNA,” the investigators write.
In the future they plan to investigate how mtDNA damage can serve as a predictive marker for different disease stages and progression and to explore potential therapeutic strategies aimed at restoring normal mitochondrial function to rectify the mitochondrial dysfunctions implicated in PD.
Making a comeback?
Commenting on the research for this news organization, James Beck, PhD, chief scientific officer at the Parkinson’s Foundation, noted that the role of mitochondria in PD is “like a starlet that burst onto the scene in the 80s, faded into obscurity, and through diligence and continued research has moved beyond being a solid character actor and is reemerging as a force to reckon with.
“This paper only adds to the allure that mitochondria may have in contributing to PD by providing evidence of a novel process by which mitochondria may be not only contributing to PD and loss of dopamine neurons but may play a larger role in the subsequent effects that many people with PD experience – dementia,” Dr. Beck said.
He noted that the authors identified several proteins as facilitating the neurodegeneration that is wrought by damaged mitochondrial DNA.
“These could be potential targets for future drug development. In addition, this work implicates alterations in immune signaling and drugs in development to target inflammatory responses may also bring ancillary benefit,” Dr. Beck said.
However, he said, “while very interesting findings, this is really the first effort that demonstrates how damaged mitochondrial DNA may contribute to neurodegeneration in the context of PD and PD dementia. Further work needs to validate these findings as well as to elucidate mechanisms underlying the propagation of the mitochondrial DNA from cell to cell.”
Funding for this research was provided by the European Union’s Horizon 2020 Research and Innovation Program, the Lundbeck Foundation, and the Danish Council for Independent Research–Medicine. Dr. Issazadeh-Navikas and Dr. Beck have disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
.
While defects in mitochondrial functions and in mitochondrial DNA have been implicated in PD in the past, the current study demonstrates “for the first time how damaged mitochondrial DNA can underlie the mechanisms of PD initiation and spread in brain,” lead investigator Shohreh Issazadeh-Navikas, PhD, with the University of Copenhagen, told this news organization.
“This has direct implication for clinical diagnosis” – if damaged mtDNA can be detected in blood, it could serve as an early biomarker for disease, she explained.
The study was published online in Molecular Psychiatry.
“Infectious-like” spread of PD pathology
In earlier work, the researchers identified dysregulated interferon-beta (IFN-beta) signaling as a “top candidate pathway” associated with sporadic PD and its progression to PD with dementia (PDD).
In mice PD models that were deficient in IFN-beta signaling, the investigators showed that neuronal IFN-beta is required to maintain mitochondrial homeostasis and metabolism.
Lack of neuronal IFN-beta or disruption of its downstream signaling causes the accumulation of damaged mitochondria with excessive oxidative stress and insufficient adenosine triphosphate production.
In the current study, using postmortem brain tissue samples from patients with sporadic PD, they confirmed that there were deletions of mtDNA in the medial frontal gyrus, a region implicated in cognitive impairments in PD, suggesting a potential role of damaged mtDNA in disease pathophysiology.
They also identified mtDNA deletions in a “hotspot” in complex I respiratory chain subunits that were associated with dysregulation of oxidative stress and DNA damage response pathways in cohorts with sporadic PD and PDD.
They confirmed the contribution of mtDNA damage to PD pathology in the PD mouse models. They showed that lack of neuronal IFN-beta signaling leads to oxidative damage and mutations in mtDNA in neurons, which are subsequently released outside the neurons.
Injecting damaged mtDNA into mouse brain induced PDD-like behavioral symptoms, including neuropsychiatric, motor, and cognitive impairments. It also caused neurodegeneration in brain regions distant from the injection site, suggesting that damaged mtDNA triggers spread of PDD characteristics in an “infectious-like” manner, the researchers report.
Further study revealed that the mechanism through which damaged mtDNA causes pathology in healthy neurons involves dual activation of Toll-like receptor (TLR) 9 and 4 pathways, leading to increased oxidative stress and neuronal cell death, respectively.
“Our proteomic analysis of extracellular vesicles containing damaged mtDNA identified the TLR4 activator, ribosomal protein S3, as a key protein involved in recognizing and extruding damaged mtDNA,” the investigators write.
In the future they plan to investigate how mtDNA damage can serve as a predictive marker for different disease stages and progression and to explore potential therapeutic strategies aimed at restoring normal mitochondrial function to rectify the mitochondrial dysfunctions implicated in PD.
Making a comeback?
Commenting on the research for this news organization, James Beck, PhD, chief scientific officer at the Parkinson’s Foundation, noted that the role of mitochondria in PD is “like a starlet that burst onto the scene in the 80s, faded into obscurity, and through diligence and continued research has moved beyond being a solid character actor and is reemerging as a force to reckon with.
“This paper only adds to the allure that mitochondria may have in contributing to PD by providing evidence of a novel process by which mitochondria may be not only contributing to PD and loss of dopamine neurons but may play a larger role in the subsequent effects that many people with PD experience – dementia,” Dr. Beck said.
He noted that the authors identified several proteins as facilitating the neurodegeneration that is wrought by damaged mitochondrial DNA.
“These could be potential targets for future drug development. In addition, this work implicates alterations in immune signaling and drugs in development to target inflammatory responses may also bring ancillary benefit,” Dr. Beck said.
However, he said, “while very interesting findings, this is really the first effort that demonstrates how damaged mitochondrial DNA may contribute to neurodegeneration in the context of PD and PD dementia. Further work needs to validate these findings as well as to elucidate mechanisms underlying the propagation of the mitochondrial DNA from cell to cell.”
Funding for this research was provided by the European Union’s Horizon 2020 Research and Innovation Program, the Lundbeck Foundation, and the Danish Council for Independent Research–Medicine. Dr. Issazadeh-Navikas and Dr. Beck have disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
.
While defects in mitochondrial functions and in mitochondrial DNA have been implicated in PD in the past, the current study demonstrates “for the first time how damaged mitochondrial DNA can underlie the mechanisms of PD initiation and spread in brain,” lead investigator Shohreh Issazadeh-Navikas, PhD, with the University of Copenhagen, told this news organization.
“This has direct implication for clinical diagnosis” – if damaged mtDNA can be detected in blood, it could serve as an early biomarker for disease, she explained.
The study was published online in Molecular Psychiatry.
“Infectious-like” spread of PD pathology
In earlier work, the researchers identified dysregulated interferon-beta (IFN-beta) signaling as a “top candidate pathway” associated with sporadic PD and its progression to PD with dementia (PDD).
In mice PD models that were deficient in IFN-beta signaling, the investigators showed that neuronal IFN-beta is required to maintain mitochondrial homeostasis and metabolism.
Lack of neuronal IFN-beta or disruption of its downstream signaling causes the accumulation of damaged mitochondria with excessive oxidative stress and insufficient adenosine triphosphate production.
In the current study, using postmortem brain tissue samples from patients with sporadic PD, they confirmed that there were deletions of mtDNA in the medial frontal gyrus, a region implicated in cognitive impairments in PD, suggesting a potential role of damaged mtDNA in disease pathophysiology.
They also identified mtDNA deletions in a “hotspot” in complex I respiratory chain subunits that were associated with dysregulation of oxidative stress and DNA damage response pathways in cohorts with sporadic PD and PDD.
They confirmed the contribution of mtDNA damage to PD pathology in the PD mouse models. They showed that lack of neuronal IFN-beta signaling leads to oxidative damage and mutations in mtDNA in neurons, which are subsequently released outside the neurons.
Injecting damaged mtDNA into mouse brain induced PDD-like behavioral symptoms, including neuropsychiatric, motor, and cognitive impairments. It also caused neurodegeneration in brain regions distant from the injection site, suggesting that damaged mtDNA triggers spread of PDD characteristics in an “infectious-like” manner, the researchers report.
Further study revealed that the mechanism through which damaged mtDNA causes pathology in healthy neurons involves dual activation of Toll-like receptor (TLR) 9 and 4 pathways, leading to increased oxidative stress and neuronal cell death, respectively.
“Our proteomic analysis of extracellular vesicles containing damaged mtDNA identified the TLR4 activator, ribosomal protein S3, as a key protein involved in recognizing and extruding damaged mtDNA,” the investigators write.
In the future they plan to investigate how mtDNA damage can serve as a predictive marker for different disease stages and progression and to explore potential therapeutic strategies aimed at restoring normal mitochondrial function to rectify the mitochondrial dysfunctions implicated in PD.
Making a comeback?
Commenting on the research for this news organization, James Beck, PhD, chief scientific officer at the Parkinson’s Foundation, noted that the role of mitochondria in PD is “like a starlet that burst onto the scene in the 80s, faded into obscurity, and through diligence and continued research has moved beyond being a solid character actor and is reemerging as a force to reckon with.
“This paper only adds to the allure that mitochondria may have in contributing to PD by providing evidence of a novel process by which mitochondria may be not only contributing to PD and loss of dopamine neurons but may play a larger role in the subsequent effects that many people with PD experience – dementia,” Dr. Beck said.
He noted that the authors identified several proteins as facilitating the neurodegeneration that is wrought by damaged mitochondrial DNA.
“These could be potential targets for future drug development. In addition, this work implicates alterations in immune signaling and drugs in development to target inflammatory responses may also bring ancillary benefit,” Dr. Beck said.
However, he said, “while very interesting findings, this is really the first effort that demonstrates how damaged mitochondrial DNA may contribute to neurodegeneration in the context of PD and PD dementia. Further work needs to validate these findings as well as to elucidate mechanisms underlying the propagation of the mitochondrial DNA from cell to cell.”
Funding for this research was provided by the European Union’s Horizon 2020 Research and Innovation Program, the Lundbeck Foundation, and the Danish Council for Independent Research–Medicine. Dr. Issazadeh-Navikas and Dr. Beck have disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
FROM MOLECULAR PSYCHIATRY
Loneliness tied to increased risk for Parkinson’s disease
TOPLINE:
Loneliness is associated with a higher risk of developing Parkinson’s disease (PD) across demographic groups and independent of other risk factors, data from nearly 500,000 U.K. adults suggest.
METHODOLOGY:
- Loneliness is associated with illness and death, including higher risk of neurodegenerative diseases, but no study has examined whether the association between loneliness and detrimental outcomes extends to PD.
- The current analysis included 491,603 U.K. Biobank participants (mean age, 56; 54% women) without a diagnosis of PD at baseline.
- Loneliness was assessed by a single question at baseline and incident PD was ascertained via health records over 15 years.
- Researchers assessed whether the association between loneliness and PD was moderated by age, sex, or genetic risk and whether the association was accounted for by sociodemographic factors; behavioral, mental, physical, or social factors; or genetic risk.
TAKEAWAY:
- Roughly 19% of the cohort reported being lonely. Compared with those who were not lonely, those who did report being lonely were slightly younger and were more likely to be women. They also had fewer resources, more health risk behaviors (current smoker and physically inactive), and worse physical and mental health.
- Over 15+ years of follow-up, 2,822 participants developed PD (incidence rate: 47 per 100,000 person-years). Compared with those who did not develop PD, those who did were older and more likely to be male, former smokers, have higher BMI and PD polygenetic risk score, and to have diabetes, hypertension, myocardial infarction or stroke, anxiety, or depression.
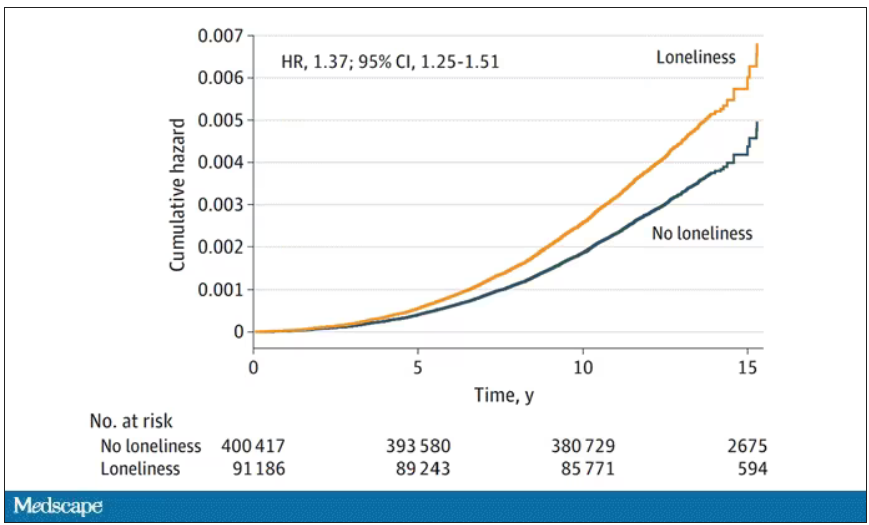
- In the primary analysis, individuals who reported being lonely had a higher risk for PD (hazard ratio, 1.37) – an association that remained after accounting for demographic and socioeconomic status, social isolation, PD polygenetic risk score, smoking, physical activity, BMI, diabetes, hypertension, stroke, myocardial infarction, depression, and having ever seen a psychiatrist (fully adjusted HR, 1.25).
- The association between loneliness and incident PD was not moderated by sex, age, or polygenetic risk score.
- Contrary to expectations for a prodromal syndrome, loneliness was not associated with incident PD in the first 5 years after baseline but was associated with PD risk in the subsequent 10 years of follow-up (HR, 1.32).
IN PRACTICE:
“Our findings complement other evidence that loneliness is a psychosocial determinant of health associated with increased risk of morbidity and mortality [and] supports recent calls for the protective and healing effects of personally meaningful social connection,” the authors write.
SOURCE:
The study, with first author Antonio Terracciano, PhD, of Florida State University College of Medicine, Tallahassee, was published online in JAMA Neurology.
LIMITATIONS:
This observational study could not determine causality or whether reverse causality could explain the association. Loneliness was assessed by a single yes/no question. PD diagnosis relied on hospital admission and death records and may have missed early PD diagnoses.
DISCLOSURES:
Funding for the study was provided by the National Institutes of Health and National Institute on Aging. The authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
Loneliness is associated with a higher risk of developing Parkinson’s disease (PD) across demographic groups and independent of other risk factors, data from nearly 500,000 U.K. adults suggest.
METHODOLOGY:
- Loneliness is associated with illness and death, including higher risk of neurodegenerative diseases, but no study has examined whether the association between loneliness and detrimental outcomes extends to PD.
- The current analysis included 491,603 U.K. Biobank participants (mean age, 56; 54% women) without a diagnosis of PD at baseline.
- Loneliness was assessed by a single question at baseline and incident PD was ascertained via health records over 15 years.
- Researchers assessed whether the association between loneliness and PD was moderated by age, sex, or genetic risk and whether the association was accounted for by sociodemographic factors; behavioral, mental, physical, or social factors; or genetic risk.
TAKEAWAY:
- Roughly 19% of the cohort reported being lonely. Compared with those who were not lonely, those who did report being lonely were slightly younger and were more likely to be women. They also had fewer resources, more health risk behaviors (current smoker and physically inactive), and worse physical and mental health.
- Over 15+ years of follow-up, 2,822 participants developed PD (incidence rate: 47 per 100,000 person-years). Compared with those who did not develop PD, those who did were older and more likely to be male, former smokers, have higher BMI and PD polygenetic risk score, and to have diabetes, hypertension, myocardial infarction or stroke, anxiety, or depression.
- In the primary analysis, individuals who reported being lonely had a higher risk for PD (hazard ratio, 1.37) – an association that remained after accounting for demographic and socioeconomic status, social isolation, PD polygenetic risk score, smoking, physical activity, BMI, diabetes, hypertension, stroke, myocardial infarction, depression, and having ever seen a psychiatrist (fully adjusted HR, 1.25).
- The association between loneliness and incident PD was not moderated by sex, age, or polygenetic risk score.
- Contrary to expectations for a prodromal syndrome, loneliness was not associated with incident PD in the first 5 years after baseline but was associated with PD risk in the subsequent 10 years of follow-up (HR, 1.32).
IN PRACTICE:
“Our findings complement other evidence that loneliness is a psychosocial determinant of health associated with increased risk of morbidity and mortality [and] supports recent calls for the protective and healing effects of personally meaningful social connection,” the authors write.
SOURCE:
The study, with first author Antonio Terracciano, PhD, of Florida State University College of Medicine, Tallahassee, was published online in JAMA Neurology.
LIMITATIONS:
This observational study could not determine causality or whether reverse causality could explain the association. Loneliness was assessed by a single yes/no question. PD diagnosis relied on hospital admission and death records and may have missed early PD diagnoses.
DISCLOSURES:
Funding for the study was provided by the National Institutes of Health and National Institute on Aging. The authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
Loneliness is associated with a higher risk of developing Parkinson’s disease (PD) across demographic groups and independent of other risk factors, data from nearly 500,000 U.K. adults suggest.
METHODOLOGY:
- Loneliness is associated with illness and death, including higher risk of neurodegenerative diseases, but no study has examined whether the association between loneliness and detrimental outcomes extends to PD.
- The current analysis included 491,603 U.K. Biobank participants (mean age, 56; 54% women) without a diagnosis of PD at baseline.
- Loneliness was assessed by a single question at baseline and incident PD was ascertained via health records over 15 years.
- Researchers assessed whether the association between loneliness and PD was moderated by age, sex, or genetic risk and whether the association was accounted for by sociodemographic factors; behavioral, mental, physical, or social factors; or genetic risk.
TAKEAWAY:
- Roughly 19% of the cohort reported being lonely. Compared with those who were not lonely, those who did report being lonely were slightly younger and were more likely to be women. They also had fewer resources, more health risk behaviors (current smoker and physically inactive), and worse physical and mental health.
- Over 15+ years of follow-up, 2,822 participants developed PD (incidence rate: 47 per 100,000 person-years). Compared with those who did not develop PD, those who did were older and more likely to be male, former smokers, have higher BMI and PD polygenetic risk score, and to have diabetes, hypertension, myocardial infarction or stroke, anxiety, or depression.
- In the primary analysis, individuals who reported being lonely had a higher risk for PD (hazard ratio, 1.37) – an association that remained after accounting for demographic and socioeconomic status, social isolation, PD polygenetic risk score, smoking, physical activity, BMI, diabetes, hypertension, stroke, myocardial infarction, depression, and having ever seen a psychiatrist (fully adjusted HR, 1.25).
- The association between loneliness and incident PD was not moderated by sex, age, or polygenetic risk score.
- Contrary to expectations for a prodromal syndrome, loneliness was not associated with incident PD in the first 5 years after baseline but was associated with PD risk in the subsequent 10 years of follow-up (HR, 1.32).
IN PRACTICE:
“Our findings complement other evidence that loneliness is a psychosocial determinant of health associated with increased risk of morbidity and mortality [and] supports recent calls for the protective and healing effects of personally meaningful social connection,” the authors write.
SOURCE:
The study, with first author Antonio Terracciano, PhD, of Florida State University College of Medicine, Tallahassee, was published online in JAMA Neurology.
LIMITATIONS:
This observational study could not determine causality or whether reverse causality could explain the association. Loneliness was assessed by a single yes/no question. PD diagnosis relied on hospital admission and death records and may have missed early PD diagnoses.
DISCLOSURES:
Funding for the study was provided by the National Institutes of Health and National Institute on Aging. The authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The surprising link between loneliness and Parkinson’s disease
This transcript has been edited for clarity.
On May 3, 2023, Surgeon General Vivek Murthy issued an advisory raising an alarm about what he called an “epidemic of loneliness” in the United States.
Now, I am not saying that Vivek Murthy read my book, “How Medicine Works and When It Doesn’t” – released in January and available in bookstores now – where, in chapter 11, I call attention to the problem of loneliness and its relationship to the exponential rise in deaths of despair. But Vivek, if you did, let me know. I could use the publicity.
No, of course the idea that loneliness is a public health issue is not new, but I’m glad to see it finally getting attention. At this point, studies have linked loneliness to heart disease, stroke, dementia, and premature death.
The UK Biobank is really a treasure trove of data for epidemiologists. I must see three to four studies a week coming out of this mega-dataset. This one, appearing in JAMA Neurology, caught my eye for its focus specifically on loneliness as a risk factor – something I’m hoping to see more of in the future.
The study examines data from just under 500,000 individuals in the United Kingdom who answered a survey including the question “Do you often feel lonely?” between 2006 and 2010; 18.4% of people answered yes. Individuals’ electronic health record data were then monitored over time to see who would get a new diagnosis code consistent with Parkinson’s disease. Through 2021, 2822 people did – that’s just over half a percent.
So, now we do the statistics thing. Of the nonlonely folks, 2,273 went on to develop Parkinson’s disease. Of those who said they often feel lonely, 549 people did. The raw numbers here, to be honest, aren’t that compelling. Lonely people had an absolute risk for Parkinson’s disease about 0.03% higher than that of nonlonely people. Put another way, you’d need to take over 3,000 lonely souls and make them not lonely to prevent 1 case of Parkinson’s disease.
Still, the costs of loneliness are not measured exclusively in Parkinson’s disease, and I would argue that the real risks here come from other sources: alcohol abuse, drug abuse, and suicide. Nevertheless, the weak but significant association with Parkinson’s disease reminds us that loneliness is a neurologic phenomenon. There is something about social connection that affects our brain in a way that is not just spiritual; it is actually biological.
Of course, people who say they are often lonely are different in other ways from people who report not being lonely. Lonely people, in this dataset, were younger, more likely to be female, less likely to have a college degree, in worse physical health, and engaged in more high-risk health behaviors like smoking.
The authors adjusted for all of these factors and found that, on the relative scale, lonely people were still about 20%-30% more likely to develop Parkinson’s disease.
So, what do we do about this? There is no pill for loneliness, and God help us if there ever is. Recognizing the problem is a good start. But there are some policy things we can do to reduce loneliness. We can invest in public spaces that bring people together – parks, museums, libraries – and public transportation. We can deal with tech companies that are so optimized at capturing our attention that we cease to engage with other humans. And, individually, we can just reach out a bit more. We’ve spent the past few pandemic years with our attention focused sharply inward. It’s time to look out again.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale University’s Clinical and Translational Research Accelerator in New Haven, Conn. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
On May 3, 2023, Surgeon General Vivek Murthy issued an advisory raising an alarm about what he called an “epidemic of loneliness” in the United States.
Now, I am not saying that Vivek Murthy read my book, “How Medicine Works and When It Doesn’t” – released in January and available in bookstores now – where, in chapter 11, I call attention to the problem of loneliness and its relationship to the exponential rise in deaths of despair. But Vivek, if you did, let me know. I could use the publicity.
No, of course the idea that loneliness is a public health issue is not new, but I’m glad to see it finally getting attention. At this point, studies have linked loneliness to heart disease, stroke, dementia, and premature death.
The UK Biobank is really a treasure trove of data for epidemiologists. I must see three to four studies a week coming out of this mega-dataset. This one, appearing in JAMA Neurology, caught my eye for its focus specifically on loneliness as a risk factor – something I’m hoping to see more of in the future.
The study examines data from just under 500,000 individuals in the United Kingdom who answered a survey including the question “Do you often feel lonely?” between 2006 and 2010; 18.4% of people answered yes. Individuals’ electronic health record data were then monitored over time to see who would get a new diagnosis code consistent with Parkinson’s disease. Through 2021, 2822 people did – that’s just over half a percent.
So, now we do the statistics thing. Of the nonlonely folks, 2,273 went on to develop Parkinson’s disease. Of those who said they often feel lonely, 549 people did. The raw numbers here, to be honest, aren’t that compelling. Lonely people had an absolute risk for Parkinson’s disease about 0.03% higher than that of nonlonely people. Put another way, you’d need to take over 3,000 lonely souls and make them not lonely to prevent 1 case of Parkinson’s disease.
Still, the costs of loneliness are not measured exclusively in Parkinson’s disease, and I would argue that the real risks here come from other sources: alcohol abuse, drug abuse, and suicide. Nevertheless, the weak but significant association with Parkinson’s disease reminds us that loneliness is a neurologic phenomenon. There is something about social connection that affects our brain in a way that is not just spiritual; it is actually biological.
Of course, people who say they are often lonely are different in other ways from people who report not being lonely. Lonely people, in this dataset, were younger, more likely to be female, less likely to have a college degree, in worse physical health, and engaged in more high-risk health behaviors like smoking.
The authors adjusted for all of these factors and found that, on the relative scale, lonely people were still about 20%-30% more likely to develop Parkinson’s disease.
So, what do we do about this? There is no pill for loneliness, and God help us if there ever is. Recognizing the problem is a good start. But there are some policy things we can do to reduce loneliness. We can invest in public spaces that bring people together – parks, museums, libraries – and public transportation. We can deal with tech companies that are so optimized at capturing our attention that we cease to engage with other humans. And, individually, we can just reach out a bit more. We’ve spent the past few pandemic years with our attention focused sharply inward. It’s time to look out again.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale University’s Clinical and Translational Research Accelerator in New Haven, Conn. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
On May 3, 2023, Surgeon General Vivek Murthy issued an advisory raising an alarm about what he called an “epidemic of loneliness” in the United States.
Now, I am not saying that Vivek Murthy read my book, “How Medicine Works and When It Doesn’t” – released in January and available in bookstores now – where, in chapter 11, I call attention to the problem of loneliness and its relationship to the exponential rise in deaths of despair. But Vivek, if you did, let me know. I could use the publicity.
No, of course the idea that loneliness is a public health issue is not new, but I’m glad to see it finally getting attention. At this point, studies have linked loneliness to heart disease, stroke, dementia, and premature death.
The UK Biobank is really a treasure trove of data for epidemiologists. I must see three to four studies a week coming out of this mega-dataset. This one, appearing in JAMA Neurology, caught my eye for its focus specifically on loneliness as a risk factor – something I’m hoping to see more of in the future.
The study examines data from just under 500,000 individuals in the United Kingdom who answered a survey including the question “Do you often feel lonely?” between 2006 and 2010; 18.4% of people answered yes. Individuals’ electronic health record data were then monitored over time to see who would get a new diagnosis code consistent with Parkinson’s disease. Through 2021, 2822 people did – that’s just over half a percent.
So, now we do the statistics thing. Of the nonlonely folks, 2,273 went on to develop Parkinson’s disease. Of those who said they often feel lonely, 549 people did. The raw numbers here, to be honest, aren’t that compelling. Lonely people had an absolute risk for Parkinson’s disease about 0.03% higher than that of nonlonely people. Put another way, you’d need to take over 3,000 lonely souls and make them not lonely to prevent 1 case of Parkinson’s disease.
Still, the costs of loneliness are not measured exclusively in Parkinson’s disease, and I would argue that the real risks here come from other sources: alcohol abuse, drug abuse, and suicide. Nevertheless, the weak but significant association with Parkinson’s disease reminds us that loneliness is a neurologic phenomenon. There is something about social connection that affects our brain in a way that is not just spiritual; it is actually biological.
Of course, people who say they are often lonely are different in other ways from people who report not being lonely. Lonely people, in this dataset, were younger, more likely to be female, less likely to have a college degree, in worse physical health, and engaged in more high-risk health behaviors like smoking.
The authors adjusted for all of these factors and found that, on the relative scale, lonely people were still about 20%-30% more likely to develop Parkinson’s disease.
So, what do we do about this? There is no pill for loneliness, and God help us if there ever is. Recognizing the problem is a good start. But there are some policy things we can do to reduce loneliness. We can invest in public spaces that bring people together – parks, museums, libraries – and public transportation. We can deal with tech companies that are so optimized at capturing our attention that we cease to engage with other humans. And, individually, we can just reach out a bit more. We’ve spent the past few pandemic years with our attention focused sharply inward. It’s time to look out again.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale University’s Clinical and Translational Research Accelerator in New Haven, Conn. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
These four GI conditions may predict Parkinson’s disease
Early detection of these conditions might help identify patients at risk for PD, potentially prompting preventive strategies, the researchers suggest.
The results of previous experimental studies by the team supported the Braak hypothesis, which states that idiopathic PD originates in the gut in a subset of patients. However, no previous study had investigated a broad range of gastrointestinal symptoms and syndromes that might occur prior to a PD diagnosis.
Given their preclinical work, the authors were not surprised to find that certain GI syndromes were specifically associated with PD, even when compared with Alzheimer’s disease (AD) and cerebrovascular disease (CVD), principal author Pankaj Jay Pasricha, MBBS, MD, of Mayo Clinic Arizona, Scottsdale, said in an interview. However, they were “impressed by the strength of the associations.”
“Experts have known for a very long time that constipation is a potential risk factor for PD, so this study adds to the list of GI conditions that could potentially be risk factors,” he said.
The study was published online in Gut.
Studies converge
To determine the incidence of GI syndromes and interventions preceding PD, the investigators performed a combined case-control and cohort study using a U.S.-based nationwide medical record network.
First, they compared 24,624 individuals with new-onset idiopathic PD with the same number of matched negative controls (NCs), as well as 19,046 people with AD and 23,942 with CVD to investigate the presence of preexisting GI conditions, which the researchers referred to as “exposures.” Overall, the mean age was about 70, and about half of those studied were women.
Eighteen conditions covering the entire GI tract were investigated. These included achalasia, dysphagia, gastroesophageal reflux disease, gastroparesis, functional dyspepsia, paralytic ileus, diarrhea, irritable bowel syndrome (IBS) with and without diarrhea, intestinal pseudo-obstruction, fecal incontinence, Crohn’s disease, ulcerative colitis, and microscopic colitis, as well as appendectomy and vagotomy.
All GI syndromes were significantly increased in the PD group, compared with NCs (odds ratio > 1). However, only preexisting dysphagia (OR, 3.58), gastroparesis (OR, 4.64), functional dyspepsia (OR, 3.39), intestinal pseudo-obstruction (OR, 3.01), diarrhea (OR, 2.85), constipation (OR, 3.32), IBS with constipation (OR, 4.11), IBS with diarrhea (OR, 4.31), IBS without diarrhea (OR, 3.53), and fecal incontinence (OR, 3.76) produced ORs that were numerically greater than the upper limit of the negative exposures.
In addition, only gastroparesis, dysphagia, IBS with constipation, IBS without diarrhea, and constipation were specific for PD, compared with the AD and CVD groups (OR > 1). After correction for false discovery rate, though, gastroparesis and constipation did not remain significantly different, compared with the AD and CVD groups.
Other preexisting GI conditions not only were significantly associated with PD but also showed strong associations with the AD and CVD groups.
To validate the case-control analyses, the team set up a complementary cohort study. Eighteen cohorts – each diagnosed with one of the GI conditions in the case-control analysis – were compared with their respective NC cohorts for the prospective risk of developing PD, AD, or CVD within 5 years.
Gastroparesis, dysphagia, IBS without diarrhea, and constipation showed specific associations with PD versus NCs, AD, and CVD in the cohort analysis. Their relative risks versus NCs were 2.43, 2.27, 1.17, and 2.38, respectively.
Functional dyspepsia, IBS with diarrhea, diarrhea, and fecal incontinence were not PD specific, but IBS with constipation and intestinal pseudo-obstruction showed PD specificity in both the case-control (OR, 4.11) and cohort analyses (RR, 1.84).
Appendectomy decreased the risk for PD in the cohort analysis (RR, 0.48), but neither inflammatory bowel disease nor vagotomy was associated with PD.
“This study is the first to establish substantial observational evidence that the clinical diagnosis of not only constipation but also dysphagia, gastroparesis, and IBS without diarrhea might specifically predict the development of PD, whereas other exposures were less specific,” the researchers wrote.
However, Dr. Pasricha said, “there is no need for alarm.” Clinicians should reassure patients that “the overall risk for developing PD is low. The overwhelming majority of patients with these GI conditions will never develop PD.”
His team will be doing experimental work on the biological mechanisms that might explain the current study’s findings. “In addition, the U.S. National Institutes of Health has issued a call for proposals to perform research in patients that could help understand these associations better,” he said.
Body or brain?
The Parkinson’s Foundation’s National Medical Advisor, Michael S. Okun, MD, called the study “fascinating.”
The findings “confirm many other studies showing that GI symptoms can precede a Parkinson’s disease diagnosis,” he said in an interview.
Although the study was designed to test the Braak hypothesis, “the dataset really cannot confirm or refute Braak pathology, which can only be accomplished with comparison to postmortem samples,” he added.
“The raging debate in the field of body-first versus brain-first Parkinson’s may be somewhat artificial, especially if we consider that Parkinson’s is not one disease,” Dr. Okun noted. “It will take clinical data, pathology, and the collaboration of many researchers to solve the puzzle.”
“The Foundation continues to monitor all the advancements in the ‘gut’ Parkinson field,” he said. “We do not recommend at this time changing the approach to clinical care based on this data.”
No funding or competing interests were declared. Dr. Okun declared no relevant disclosures.
A version of this article first appeared on Medscape.com.
Early detection of these conditions might help identify patients at risk for PD, potentially prompting preventive strategies, the researchers suggest.
The results of previous experimental studies by the team supported the Braak hypothesis, which states that idiopathic PD originates in the gut in a subset of patients. However, no previous study had investigated a broad range of gastrointestinal symptoms and syndromes that might occur prior to a PD diagnosis.
Given their preclinical work, the authors were not surprised to find that certain GI syndromes were specifically associated with PD, even when compared with Alzheimer’s disease (AD) and cerebrovascular disease (CVD), principal author Pankaj Jay Pasricha, MBBS, MD, of Mayo Clinic Arizona, Scottsdale, said in an interview. However, they were “impressed by the strength of the associations.”
“Experts have known for a very long time that constipation is a potential risk factor for PD, so this study adds to the list of GI conditions that could potentially be risk factors,” he said.
The study was published online in Gut.
Studies converge
To determine the incidence of GI syndromes and interventions preceding PD, the investigators performed a combined case-control and cohort study using a U.S.-based nationwide medical record network.
First, they compared 24,624 individuals with new-onset idiopathic PD with the same number of matched negative controls (NCs), as well as 19,046 people with AD and 23,942 with CVD to investigate the presence of preexisting GI conditions, which the researchers referred to as “exposures.” Overall, the mean age was about 70, and about half of those studied were women.
Eighteen conditions covering the entire GI tract were investigated. These included achalasia, dysphagia, gastroesophageal reflux disease, gastroparesis, functional dyspepsia, paralytic ileus, diarrhea, irritable bowel syndrome (IBS) with and without diarrhea, intestinal pseudo-obstruction, fecal incontinence, Crohn’s disease, ulcerative colitis, and microscopic colitis, as well as appendectomy and vagotomy.
All GI syndromes were significantly increased in the PD group, compared with NCs (odds ratio > 1). However, only preexisting dysphagia (OR, 3.58), gastroparesis (OR, 4.64), functional dyspepsia (OR, 3.39), intestinal pseudo-obstruction (OR, 3.01), diarrhea (OR, 2.85), constipation (OR, 3.32), IBS with constipation (OR, 4.11), IBS with diarrhea (OR, 4.31), IBS without diarrhea (OR, 3.53), and fecal incontinence (OR, 3.76) produced ORs that were numerically greater than the upper limit of the negative exposures.
In addition, only gastroparesis, dysphagia, IBS with constipation, IBS without diarrhea, and constipation were specific for PD, compared with the AD and CVD groups (OR > 1). After correction for false discovery rate, though, gastroparesis and constipation did not remain significantly different, compared with the AD and CVD groups.
Other preexisting GI conditions not only were significantly associated with PD but also showed strong associations with the AD and CVD groups.
To validate the case-control analyses, the team set up a complementary cohort study. Eighteen cohorts – each diagnosed with one of the GI conditions in the case-control analysis – were compared with their respective NC cohorts for the prospective risk of developing PD, AD, or CVD within 5 years.
Gastroparesis, dysphagia, IBS without diarrhea, and constipation showed specific associations with PD versus NCs, AD, and CVD in the cohort analysis. Their relative risks versus NCs were 2.43, 2.27, 1.17, and 2.38, respectively.
Functional dyspepsia, IBS with diarrhea, diarrhea, and fecal incontinence were not PD specific, but IBS with constipation and intestinal pseudo-obstruction showed PD specificity in both the case-control (OR, 4.11) and cohort analyses (RR, 1.84).
Appendectomy decreased the risk for PD in the cohort analysis (RR, 0.48), but neither inflammatory bowel disease nor vagotomy was associated with PD.
“This study is the first to establish substantial observational evidence that the clinical diagnosis of not only constipation but also dysphagia, gastroparesis, and IBS without diarrhea might specifically predict the development of PD, whereas other exposures were less specific,” the researchers wrote.
However, Dr. Pasricha said, “there is no need for alarm.” Clinicians should reassure patients that “the overall risk for developing PD is low. The overwhelming majority of patients with these GI conditions will never develop PD.”
His team will be doing experimental work on the biological mechanisms that might explain the current study’s findings. “In addition, the U.S. National Institutes of Health has issued a call for proposals to perform research in patients that could help understand these associations better,” he said.
Body or brain?
The Parkinson’s Foundation’s National Medical Advisor, Michael S. Okun, MD, called the study “fascinating.”
The findings “confirm many other studies showing that GI symptoms can precede a Parkinson’s disease diagnosis,” he said in an interview.
Although the study was designed to test the Braak hypothesis, “the dataset really cannot confirm or refute Braak pathology, which can only be accomplished with comparison to postmortem samples,” he added.
“The raging debate in the field of body-first versus brain-first Parkinson’s may be somewhat artificial, especially if we consider that Parkinson’s is not one disease,” Dr. Okun noted. “It will take clinical data, pathology, and the collaboration of many researchers to solve the puzzle.”
“The Foundation continues to monitor all the advancements in the ‘gut’ Parkinson field,” he said. “We do not recommend at this time changing the approach to clinical care based on this data.”
No funding or competing interests were declared. Dr. Okun declared no relevant disclosures.
A version of this article first appeared on Medscape.com.
Early detection of these conditions might help identify patients at risk for PD, potentially prompting preventive strategies, the researchers suggest.
The results of previous experimental studies by the team supported the Braak hypothesis, which states that idiopathic PD originates in the gut in a subset of patients. However, no previous study had investigated a broad range of gastrointestinal symptoms and syndromes that might occur prior to a PD diagnosis.
Given their preclinical work, the authors were not surprised to find that certain GI syndromes were specifically associated with PD, even when compared with Alzheimer’s disease (AD) and cerebrovascular disease (CVD), principal author Pankaj Jay Pasricha, MBBS, MD, of Mayo Clinic Arizona, Scottsdale, said in an interview. However, they were “impressed by the strength of the associations.”
“Experts have known for a very long time that constipation is a potential risk factor for PD, so this study adds to the list of GI conditions that could potentially be risk factors,” he said.
The study was published online in Gut.
Studies converge
To determine the incidence of GI syndromes and interventions preceding PD, the investigators performed a combined case-control and cohort study using a U.S.-based nationwide medical record network.
First, they compared 24,624 individuals with new-onset idiopathic PD with the same number of matched negative controls (NCs), as well as 19,046 people with AD and 23,942 with CVD to investigate the presence of preexisting GI conditions, which the researchers referred to as “exposures.” Overall, the mean age was about 70, and about half of those studied were women.
Eighteen conditions covering the entire GI tract were investigated. These included achalasia, dysphagia, gastroesophageal reflux disease, gastroparesis, functional dyspepsia, paralytic ileus, diarrhea, irritable bowel syndrome (IBS) with and without diarrhea, intestinal pseudo-obstruction, fecal incontinence, Crohn’s disease, ulcerative colitis, and microscopic colitis, as well as appendectomy and vagotomy.
All GI syndromes were significantly increased in the PD group, compared with NCs (odds ratio > 1). However, only preexisting dysphagia (OR, 3.58), gastroparesis (OR, 4.64), functional dyspepsia (OR, 3.39), intestinal pseudo-obstruction (OR, 3.01), diarrhea (OR, 2.85), constipation (OR, 3.32), IBS with constipation (OR, 4.11), IBS with diarrhea (OR, 4.31), IBS without diarrhea (OR, 3.53), and fecal incontinence (OR, 3.76) produced ORs that were numerically greater than the upper limit of the negative exposures.
In addition, only gastroparesis, dysphagia, IBS with constipation, IBS without diarrhea, and constipation were specific for PD, compared with the AD and CVD groups (OR > 1). After correction for false discovery rate, though, gastroparesis and constipation did not remain significantly different, compared with the AD and CVD groups.
Other preexisting GI conditions not only were significantly associated with PD but also showed strong associations with the AD and CVD groups.
To validate the case-control analyses, the team set up a complementary cohort study. Eighteen cohorts – each diagnosed with one of the GI conditions in the case-control analysis – were compared with their respective NC cohorts for the prospective risk of developing PD, AD, or CVD within 5 years.
Gastroparesis, dysphagia, IBS without diarrhea, and constipation showed specific associations with PD versus NCs, AD, and CVD in the cohort analysis. Their relative risks versus NCs were 2.43, 2.27, 1.17, and 2.38, respectively.
Functional dyspepsia, IBS with diarrhea, diarrhea, and fecal incontinence were not PD specific, but IBS with constipation and intestinal pseudo-obstruction showed PD specificity in both the case-control (OR, 4.11) and cohort analyses (RR, 1.84).
Appendectomy decreased the risk for PD in the cohort analysis (RR, 0.48), but neither inflammatory bowel disease nor vagotomy was associated with PD.
“This study is the first to establish substantial observational evidence that the clinical diagnosis of not only constipation but also dysphagia, gastroparesis, and IBS without diarrhea might specifically predict the development of PD, whereas other exposures were less specific,” the researchers wrote.
However, Dr. Pasricha said, “there is no need for alarm.” Clinicians should reassure patients that “the overall risk for developing PD is low. The overwhelming majority of patients with these GI conditions will never develop PD.”
His team will be doing experimental work on the biological mechanisms that might explain the current study’s findings. “In addition, the U.S. National Institutes of Health has issued a call for proposals to perform research in patients that could help understand these associations better,” he said.
Body or brain?
The Parkinson’s Foundation’s National Medical Advisor, Michael S. Okun, MD, called the study “fascinating.”
The findings “confirm many other studies showing that GI symptoms can precede a Parkinson’s disease diagnosis,” he said in an interview.
Although the study was designed to test the Braak hypothesis, “the dataset really cannot confirm or refute Braak pathology, which can only be accomplished with comparison to postmortem samples,” he added.
“The raging debate in the field of body-first versus brain-first Parkinson’s may be somewhat artificial, especially if we consider that Parkinson’s is not one disease,” Dr. Okun noted. “It will take clinical data, pathology, and the collaboration of many researchers to solve the puzzle.”
“The Foundation continues to monitor all the advancements in the ‘gut’ Parkinson field,” he said. “We do not recommend at this time changing the approach to clinical care based on this data.”
No funding or competing interests were declared. Dr. Okun declared no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM GUT
Could retinal changes be a harbinger of Parkinson’s?
Changes in retinal tissues known to be associated with Parkinson’s disease (PD) may occur up to 7 years before clinical symptoms of the disease appear, a new study suggests.
Researchers used artificial intelligence (AI) to analyze data from two population-level data sets and the world’s largest database of retinal images and associated clinical data to detect the retinal changes in patients with PD and in healthy individuals who developed the disease years later.
Prior research had shown that PD is associated with a thinning of the ganglion cell-inner plexiform layer (GCIPL) in the retina, something that investigators confirmed in this new study. But they also identified changes in the inner nuclear layer (INL), which is a new finding.
The study is the largest to date on retinal markers in PD and the first to show these changes in living patients.
“I think we are still several years away from converting these findings into individual level prediction for patients,” lead author, Siegfried Wagner, MD, MsC, Honorary Clinical Senior Research Fellow at Moorfields Eye Hospital and University College of London Institute of Ophthalmology in London, told this news organization. “The most important takeaway is that there are observable differences in the retina of individuals who go on to develop Parkinson’s disease.”
The findings were published online in Neurology.
Another look at OCT
Researchers used data from retinal eye scans taken by optical coherence tomography (OCT), a noninvasive three-dimensional imaging technology that is widely used by opticians.
Other studies have used OCT to detect retinal changes in multiple sclerosis and cognitive decline.
For this research, investigators identified markers in people with PD using ophthalmic imaging data from 700 patients and 105,770 controls who participated in the retrospective AlzEye study.
After adjustment for age, sex, ethnicity, hypertension, and diabetes, individuals with PD had significantly thinner GCIPL and reduced thickness of the INL.
To evaluate retinal changes in patients before a PD diagnosis, researchers then turned to 50,405 participants in the UK Biobank with no history of PD who received a retinal scan as part of their baseline visit. Of that group, 53 were diagnosed with PD during the study period.
Researchers found an association between new diagnoses of PD and reduced thickness of the GCIPL (hazard ratio [HR], 0.62; P = .002) and thinner INL, especially at the inferior subfield (HR, 0.66; P = .002). That association persisted even in people whose clinical symptoms developed within 2 years of the retinal scan.
“We wonder if the reduced INL thickness is indicating a direct dopaminergic impairment occurring within the inner retina,” Dr. Wagner said. “Dopaminergic amacrine cells only account for a small proportion of the cells in this layer but previous work in the laboratory shows observable abnormalities in Parkinson’s disease.”
Too early for diagnostics?
Commenting on the findings, Rebecca Gilbert, MD, PhD, chief scientific officer, American Parkinson Disease Association, noted that the changes in the retinal thickness identified in the study were too small to be useful in the clinic as a screening tool for early PD.
“In order for that to happen, the specificity and sensitivity needs to be established,” she said. “Both specificity and sensitivity need to be high enough so that the test can be used to give clinically meaningful results – and reliably tell an individual with PD that he or she does have the disease and individual without PD that he or she doesn’t have the disease.”
Authors of an accompanying editorial agreed. Valeria Koska, MD, and Philipp Albrecht, MD, both of Heinrich Heine University Düsseldorf in Germany, noted that though the effect sizes of retinal changes were small, the study “sets new standards for the role of retinal morphology as potential biomarker in neurodegenerative disease.”
The study was funded by Fight for Sight UK, Medical Research Council, UK Research & Innovation, Basque Health Department, and the Wellcome Trust Study. Dr. Wagner reported funding from the Medical Research Council and the Rank Prize. Dr. Gilbert is employed by the American Parkinson Disease Association. Dr. Albrecht has received grant and personal fees and nonfinancial support from Allergan, Biogen, Celgene, Ipsen, Janssen Cilag, Merck, Merz Pharmaceuticals, Novartis, Roche, and Teva, outside the submitted work. Dr. Koska reported no relevant disclosures.
A version of this article appeared on Medscape.com.
Changes in retinal tissues known to be associated with Parkinson’s disease (PD) may occur up to 7 years before clinical symptoms of the disease appear, a new study suggests.
Researchers used artificial intelligence (AI) to analyze data from two population-level data sets and the world’s largest database of retinal images and associated clinical data to detect the retinal changes in patients with PD and in healthy individuals who developed the disease years later.
Prior research had shown that PD is associated with a thinning of the ganglion cell-inner plexiform layer (GCIPL) in the retina, something that investigators confirmed in this new study. But they also identified changes in the inner nuclear layer (INL), which is a new finding.
The study is the largest to date on retinal markers in PD and the first to show these changes in living patients.
“I think we are still several years away from converting these findings into individual level prediction for patients,” lead author, Siegfried Wagner, MD, MsC, Honorary Clinical Senior Research Fellow at Moorfields Eye Hospital and University College of London Institute of Ophthalmology in London, told this news organization. “The most important takeaway is that there are observable differences in the retina of individuals who go on to develop Parkinson’s disease.”
The findings were published online in Neurology.
Another look at OCT
Researchers used data from retinal eye scans taken by optical coherence tomography (OCT), a noninvasive three-dimensional imaging technology that is widely used by opticians.
Other studies have used OCT to detect retinal changes in multiple sclerosis and cognitive decline.
For this research, investigators identified markers in people with PD using ophthalmic imaging data from 700 patients and 105,770 controls who participated in the retrospective AlzEye study.
After adjustment for age, sex, ethnicity, hypertension, and diabetes, individuals with PD had significantly thinner GCIPL and reduced thickness of the INL.
To evaluate retinal changes in patients before a PD diagnosis, researchers then turned to 50,405 participants in the UK Biobank with no history of PD who received a retinal scan as part of their baseline visit. Of that group, 53 were diagnosed with PD during the study period.
Researchers found an association between new diagnoses of PD and reduced thickness of the GCIPL (hazard ratio [HR], 0.62; P = .002) and thinner INL, especially at the inferior subfield (HR, 0.66; P = .002). That association persisted even in people whose clinical symptoms developed within 2 years of the retinal scan.
“We wonder if the reduced INL thickness is indicating a direct dopaminergic impairment occurring within the inner retina,” Dr. Wagner said. “Dopaminergic amacrine cells only account for a small proportion of the cells in this layer but previous work in the laboratory shows observable abnormalities in Parkinson’s disease.”
Too early for diagnostics?
Commenting on the findings, Rebecca Gilbert, MD, PhD, chief scientific officer, American Parkinson Disease Association, noted that the changes in the retinal thickness identified in the study were too small to be useful in the clinic as a screening tool for early PD.
“In order for that to happen, the specificity and sensitivity needs to be established,” she said. “Both specificity and sensitivity need to be high enough so that the test can be used to give clinically meaningful results – and reliably tell an individual with PD that he or she does have the disease and individual without PD that he or she doesn’t have the disease.”
Authors of an accompanying editorial agreed. Valeria Koska, MD, and Philipp Albrecht, MD, both of Heinrich Heine University Düsseldorf in Germany, noted that though the effect sizes of retinal changes were small, the study “sets new standards for the role of retinal morphology as potential biomarker in neurodegenerative disease.”
The study was funded by Fight for Sight UK, Medical Research Council, UK Research & Innovation, Basque Health Department, and the Wellcome Trust Study. Dr. Wagner reported funding from the Medical Research Council and the Rank Prize. Dr. Gilbert is employed by the American Parkinson Disease Association. Dr. Albrecht has received grant and personal fees and nonfinancial support from Allergan, Biogen, Celgene, Ipsen, Janssen Cilag, Merck, Merz Pharmaceuticals, Novartis, Roche, and Teva, outside the submitted work. Dr. Koska reported no relevant disclosures.
A version of this article appeared on Medscape.com.
Changes in retinal tissues known to be associated with Parkinson’s disease (PD) may occur up to 7 years before clinical symptoms of the disease appear, a new study suggests.
Researchers used artificial intelligence (AI) to analyze data from two population-level data sets and the world’s largest database of retinal images and associated clinical data to detect the retinal changes in patients with PD and in healthy individuals who developed the disease years later.
Prior research had shown that PD is associated with a thinning of the ganglion cell-inner plexiform layer (GCIPL) in the retina, something that investigators confirmed in this new study. But they also identified changes in the inner nuclear layer (INL), which is a new finding.
The study is the largest to date on retinal markers in PD and the first to show these changes in living patients.
“I think we are still several years away from converting these findings into individual level prediction for patients,” lead author, Siegfried Wagner, MD, MsC, Honorary Clinical Senior Research Fellow at Moorfields Eye Hospital and University College of London Institute of Ophthalmology in London, told this news organization. “The most important takeaway is that there are observable differences in the retina of individuals who go on to develop Parkinson’s disease.”
The findings were published online in Neurology.
Another look at OCT
Researchers used data from retinal eye scans taken by optical coherence tomography (OCT), a noninvasive three-dimensional imaging technology that is widely used by opticians.
Other studies have used OCT to detect retinal changes in multiple sclerosis and cognitive decline.
For this research, investigators identified markers in people with PD using ophthalmic imaging data from 700 patients and 105,770 controls who participated in the retrospective AlzEye study.
After adjustment for age, sex, ethnicity, hypertension, and diabetes, individuals with PD had significantly thinner GCIPL and reduced thickness of the INL.
To evaluate retinal changes in patients before a PD diagnosis, researchers then turned to 50,405 participants in the UK Biobank with no history of PD who received a retinal scan as part of their baseline visit. Of that group, 53 were diagnosed with PD during the study period.
Researchers found an association between new diagnoses of PD and reduced thickness of the GCIPL (hazard ratio [HR], 0.62; P = .002) and thinner INL, especially at the inferior subfield (HR, 0.66; P = .002). That association persisted even in people whose clinical symptoms developed within 2 years of the retinal scan.
“We wonder if the reduced INL thickness is indicating a direct dopaminergic impairment occurring within the inner retina,” Dr. Wagner said. “Dopaminergic amacrine cells only account for a small proportion of the cells in this layer but previous work in the laboratory shows observable abnormalities in Parkinson’s disease.”
Too early for diagnostics?
Commenting on the findings, Rebecca Gilbert, MD, PhD, chief scientific officer, American Parkinson Disease Association, noted that the changes in the retinal thickness identified in the study were too small to be useful in the clinic as a screening tool for early PD.
“In order for that to happen, the specificity and sensitivity needs to be established,” she said. “Both specificity and sensitivity need to be high enough so that the test can be used to give clinically meaningful results – and reliably tell an individual with PD that he or she does have the disease and individual without PD that he or she doesn’t have the disease.”
Authors of an accompanying editorial agreed. Valeria Koska, MD, and Philipp Albrecht, MD, both of Heinrich Heine University Düsseldorf in Germany, noted that though the effect sizes of retinal changes were small, the study “sets new standards for the role of retinal morphology as potential biomarker in neurodegenerative disease.”
The study was funded by Fight for Sight UK, Medical Research Council, UK Research & Innovation, Basque Health Department, and the Wellcome Trust Study. Dr. Wagner reported funding from the Medical Research Council and the Rank Prize. Dr. Gilbert is employed by the American Parkinson Disease Association. Dr. Albrecht has received grant and personal fees and nonfinancial support from Allergan, Biogen, Celgene, Ipsen, Janssen Cilag, Merck, Merz Pharmaceuticals, Novartis, Roche, and Teva, outside the submitted work. Dr. Koska reported no relevant disclosures.
A version of this article appeared on Medscape.com.
Playing football linked to higher Parkinson’s risk
In a cross-sectional study of older men, former tackle football players had a 61% higher likelihood of reporting a diagnosis of parkinsonism or PD, compared with men who played non-football sports.
Longer duration of football participation and higher level of play (college and professional) were associated with higher risk.
Lead researcher Michael L. Alosco, PhD, director of the Boston University Alzheimer’s Disease Research Center, said it’s important to note that the findings are from a cohort of men “enriched” for having PD.
“These are people who are likely already concerned for or at risk for having this disease. We don’t yet know how our findings translate to the general population,” Dr. Alosco said in an interview.
The study was published online in JAMA Network Open.
Repetitive head impacts
Dating back to the 1920s, PD and parkinsonism an umbrella term that refers to motor symptoms associated with PD and other conditions have long been described in boxers who suffer repetitive head impacts.
Multiple studies have linked tackle football with progressive brain diseases such as chronic traumatic encephalopathy. Few studies, however, have investigated the association between participation in football and PD.
For their study, Dr. Alosco and colleagues leveraged data from Fox Insight, a longitudinal online study of some people with and some without PD that is sponsored by the Michael J. Fox Foundation for Parkinson’s Research.
They focused their analyses on 1,875 men (mean age, 67 years) who reported playing any organized sport. As noted, the cohort was enriched for parkinsonism or PD. A total of 1,602 (85%) had received a diagnosis of parkinsonism/PD, and 273 had not.
Altogether, 729 men had a history of playing tackle football, and 1,146 men played non-football sports (control group). Among the football players, 82% played at youth sports or at the high school level; 17% played at the college level; and fewer than 1% played at the pro or semi-pro level.
Among the football players, 648 (89%) reported a parkinsonism/PD diagnosis.
A history of playing football was associated with higher odds of reporting a parkinsonism/PD diagnosis (odds ratio, 1.61; 95% confidence interval, 1.19-2.17) after accounting for age, education level, history of diabetes and heart disease, body mass index (BMI), traumatic brain injury with loss of consciousness, and family history of PD.
Football players who had longer careers and who played at higher levels of competition were at increased risk of having parkinsonism or PD.
Playing one to four seasons yielded an OR of 1.39 (95% CI, 0.98-1.98). The OR was 2.18 (95% CI, 1.36-3.49) for playing five or more seasons.
Football players who competed at the college or professional level had nearly triple the odds of reporting a parkinsonism/PD diagnosis (OR, 2.93; 95% CI, 1.28-6.73), compared with athletes who played at the youth or high school level.
Age at first exposure to football was not associated with a parkinsonism/PD diagnosis.
The researchers cautioned that this was a convenience sample of mostly White people, and the sample was enriched for having PD – factors that limit the generalizability of the findings.
Also, diagnosis of PD was self-reported by participants through online assessments, and objective in-person evaluations were not conducted.
Unequivocal link?
“This is among the first and largest to look at the relationship between football and having a diagnosis of PD in a large cohort of people from the Fox Insight online study,” Dr. Alosco said.
He cautioned that “not all people who play football will develop later-life neurological problems. That being said, the study adds to the accumulating evidence that suggests playing football is one risk factor for the development of later-life brain diseases.
“This represents an opportunity to educate the communities on the potential risks of playing football (short and long term), including what we know and what we don’t know, so that people can make informed decisions on participating in tackle football and develop additional ways to mitigate risk,” Dr. Alosco said.
In a comment, Shaheen Lakhan, MD, PhD, a neurologist and researcher from Boston, said: “The emerging body of research leaves little doubt that engaging in football raises the risk of developing Parkinson’s disease and parkinsonism.
“This progressive line of investigation serves to enhance our understanding, unequivocally demonstrating that even participation in amateur football, including at the youth and high school levels, constitutes a significant risk factor for the onset of Parkinson’s disease,” said Dr. Lakhan, who was not involved in the study.
However, he said it’s “crucial to underscore that the statistics reveal a notable distinction: individuals who have a history of college or professional football play face odds nearly three times higher of receiving a diagnosis of parkinsonism or Parkinson’s disease when compared to their counterparts who engaged in football during their youth or high-school years.
“Ultimately, determinations regarding involvement in sports should be a collaborative endeavor involving parents, young athletes, and health care providers. It is incumbent upon physicians to equip parents and youth with a comprehensive comprehension of the potential risks and rewards inherent in football participation,” Dr. Lakhan said.
He added, though, that there are multifaceted advantages to playing football. “This pursuit nurtures cardiovascular well-being, fosters invaluable social interactions, cultivates teamwork, instills discipline through regimented routines, and hones a spectrum of physical proficiencies,” Dr. Lakhan said.
“It’s worth noting that a constellation of alternative sports, including track and field, swimming, soccer, baseball, and tennis, can be cogently discussed as substitutes, all while preserving the manifold benefits of athletic engagement,” Dr. Lakhan added.
The Fox Insight Study is funded by the Michael J. Fox Foundation for Parkinson’s Research. The study was conducted in collaboration with the Michael J. Fox Foundation for Parkinson’s Research, the sponsor of the Fox Insight study, which collected and aggregated data used in the study. It was also supported by the National Institute of Neurological Disorders and Stroke. Dr. Alosco received grants from the National Institutes of Health during the conduct of the study, an honorarium from the Michael J. Fox Foundation for work unrelated to the study, and royalties from Oxford University Press outside the submitted work. Dr. Lakhan disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a cross-sectional study of older men, former tackle football players had a 61% higher likelihood of reporting a diagnosis of parkinsonism or PD, compared with men who played non-football sports.
Longer duration of football participation and higher level of play (college and professional) were associated with higher risk.
Lead researcher Michael L. Alosco, PhD, director of the Boston University Alzheimer’s Disease Research Center, said it’s important to note that the findings are from a cohort of men “enriched” for having PD.
“These are people who are likely already concerned for or at risk for having this disease. We don’t yet know how our findings translate to the general population,” Dr. Alosco said in an interview.
The study was published online in JAMA Network Open.
Repetitive head impacts
Dating back to the 1920s, PD and parkinsonism an umbrella term that refers to motor symptoms associated with PD and other conditions have long been described in boxers who suffer repetitive head impacts.
Multiple studies have linked tackle football with progressive brain diseases such as chronic traumatic encephalopathy. Few studies, however, have investigated the association between participation in football and PD.
For their study, Dr. Alosco and colleagues leveraged data from Fox Insight, a longitudinal online study of some people with and some without PD that is sponsored by the Michael J. Fox Foundation for Parkinson’s Research.
They focused their analyses on 1,875 men (mean age, 67 years) who reported playing any organized sport. As noted, the cohort was enriched for parkinsonism or PD. A total of 1,602 (85%) had received a diagnosis of parkinsonism/PD, and 273 had not.
Altogether, 729 men had a history of playing tackle football, and 1,146 men played non-football sports (control group). Among the football players, 82% played at youth sports or at the high school level; 17% played at the college level; and fewer than 1% played at the pro or semi-pro level.
Among the football players, 648 (89%) reported a parkinsonism/PD diagnosis.
A history of playing football was associated with higher odds of reporting a parkinsonism/PD diagnosis (odds ratio, 1.61; 95% confidence interval, 1.19-2.17) after accounting for age, education level, history of diabetes and heart disease, body mass index (BMI), traumatic brain injury with loss of consciousness, and family history of PD.
Football players who had longer careers and who played at higher levels of competition were at increased risk of having parkinsonism or PD.
Playing one to four seasons yielded an OR of 1.39 (95% CI, 0.98-1.98). The OR was 2.18 (95% CI, 1.36-3.49) for playing five or more seasons.
Football players who competed at the college or professional level had nearly triple the odds of reporting a parkinsonism/PD diagnosis (OR, 2.93; 95% CI, 1.28-6.73), compared with athletes who played at the youth or high school level.
Age at first exposure to football was not associated with a parkinsonism/PD diagnosis.
The researchers cautioned that this was a convenience sample of mostly White people, and the sample was enriched for having PD – factors that limit the generalizability of the findings.
Also, diagnosis of PD was self-reported by participants through online assessments, and objective in-person evaluations were not conducted.
Unequivocal link?
“This is among the first and largest to look at the relationship between football and having a diagnosis of PD in a large cohort of people from the Fox Insight online study,” Dr. Alosco said.
He cautioned that “not all people who play football will develop later-life neurological problems. That being said, the study adds to the accumulating evidence that suggests playing football is one risk factor for the development of later-life brain diseases.
“This represents an opportunity to educate the communities on the potential risks of playing football (short and long term), including what we know and what we don’t know, so that people can make informed decisions on participating in tackle football and develop additional ways to mitigate risk,” Dr. Alosco said.
In a comment, Shaheen Lakhan, MD, PhD, a neurologist and researcher from Boston, said: “The emerging body of research leaves little doubt that engaging in football raises the risk of developing Parkinson’s disease and parkinsonism.
“This progressive line of investigation serves to enhance our understanding, unequivocally demonstrating that even participation in amateur football, including at the youth and high school levels, constitutes a significant risk factor for the onset of Parkinson’s disease,” said Dr. Lakhan, who was not involved in the study.
However, he said it’s “crucial to underscore that the statistics reveal a notable distinction: individuals who have a history of college or professional football play face odds nearly three times higher of receiving a diagnosis of parkinsonism or Parkinson’s disease when compared to their counterparts who engaged in football during their youth or high-school years.
“Ultimately, determinations regarding involvement in sports should be a collaborative endeavor involving parents, young athletes, and health care providers. It is incumbent upon physicians to equip parents and youth with a comprehensive comprehension of the potential risks and rewards inherent in football participation,” Dr. Lakhan said.
He added, though, that there are multifaceted advantages to playing football. “This pursuit nurtures cardiovascular well-being, fosters invaluable social interactions, cultivates teamwork, instills discipline through regimented routines, and hones a spectrum of physical proficiencies,” Dr. Lakhan said.
“It’s worth noting that a constellation of alternative sports, including track and field, swimming, soccer, baseball, and tennis, can be cogently discussed as substitutes, all while preserving the manifold benefits of athletic engagement,” Dr. Lakhan added.
The Fox Insight Study is funded by the Michael J. Fox Foundation for Parkinson’s Research. The study was conducted in collaboration with the Michael J. Fox Foundation for Parkinson’s Research, the sponsor of the Fox Insight study, which collected and aggregated data used in the study. It was also supported by the National Institute of Neurological Disorders and Stroke. Dr. Alosco received grants from the National Institutes of Health during the conduct of the study, an honorarium from the Michael J. Fox Foundation for work unrelated to the study, and royalties from Oxford University Press outside the submitted work. Dr. Lakhan disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a cross-sectional study of older men, former tackle football players had a 61% higher likelihood of reporting a diagnosis of parkinsonism or PD, compared with men who played non-football sports.
Longer duration of football participation and higher level of play (college and professional) were associated with higher risk.
Lead researcher Michael L. Alosco, PhD, director of the Boston University Alzheimer’s Disease Research Center, said it’s important to note that the findings are from a cohort of men “enriched” for having PD.
“These are people who are likely already concerned for or at risk for having this disease. We don’t yet know how our findings translate to the general population,” Dr. Alosco said in an interview.
The study was published online in JAMA Network Open.
Repetitive head impacts
Dating back to the 1920s, PD and parkinsonism an umbrella term that refers to motor symptoms associated with PD and other conditions have long been described in boxers who suffer repetitive head impacts.
Multiple studies have linked tackle football with progressive brain diseases such as chronic traumatic encephalopathy. Few studies, however, have investigated the association between participation in football and PD.
For their study, Dr. Alosco and colleagues leveraged data from Fox Insight, a longitudinal online study of some people with and some without PD that is sponsored by the Michael J. Fox Foundation for Parkinson’s Research.
They focused their analyses on 1,875 men (mean age, 67 years) who reported playing any organized sport. As noted, the cohort was enriched for parkinsonism or PD. A total of 1,602 (85%) had received a diagnosis of parkinsonism/PD, and 273 had not.
Altogether, 729 men had a history of playing tackle football, and 1,146 men played non-football sports (control group). Among the football players, 82% played at youth sports or at the high school level; 17% played at the college level; and fewer than 1% played at the pro or semi-pro level.
Among the football players, 648 (89%) reported a parkinsonism/PD diagnosis.
A history of playing football was associated with higher odds of reporting a parkinsonism/PD diagnosis (odds ratio, 1.61; 95% confidence interval, 1.19-2.17) after accounting for age, education level, history of diabetes and heart disease, body mass index (BMI), traumatic brain injury with loss of consciousness, and family history of PD.
Football players who had longer careers and who played at higher levels of competition were at increased risk of having parkinsonism or PD.
Playing one to four seasons yielded an OR of 1.39 (95% CI, 0.98-1.98). The OR was 2.18 (95% CI, 1.36-3.49) for playing five or more seasons.
Football players who competed at the college or professional level had nearly triple the odds of reporting a parkinsonism/PD diagnosis (OR, 2.93; 95% CI, 1.28-6.73), compared with athletes who played at the youth or high school level.
Age at first exposure to football was not associated with a parkinsonism/PD diagnosis.
The researchers cautioned that this was a convenience sample of mostly White people, and the sample was enriched for having PD – factors that limit the generalizability of the findings.
Also, diagnosis of PD was self-reported by participants through online assessments, and objective in-person evaluations were not conducted.
Unequivocal link?
“This is among the first and largest to look at the relationship between football and having a diagnosis of PD in a large cohort of people from the Fox Insight online study,” Dr. Alosco said.
He cautioned that “not all people who play football will develop later-life neurological problems. That being said, the study adds to the accumulating evidence that suggests playing football is one risk factor for the development of later-life brain diseases.
“This represents an opportunity to educate the communities on the potential risks of playing football (short and long term), including what we know and what we don’t know, so that people can make informed decisions on participating in tackle football and develop additional ways to mitigate risk,” Dr. Alosco said.
In a comment, Shaheen Lakhan, MD, PhD, a neurologist and researcher from Boston, said: “The emerging body of research leaves little doubt that engaging in football raises the risk of developing Parkinson’s disease and parkinsonism.
“This progressive line of investigation serves to enhance our understanding, unequivocally demonstrating that even participation in amateur football, including at the youth and high school levels, constitutes a significant risk factor for the onset of Parkinson’s disease,” said Dr. Lakhan, who was not involved in the study.
However, he said it’s “crucial to underscore that the statistics reveal a notable distinction: individuals who have a history of college or professional football play face odds nearly three times higher of receiving a diagnosis of parkinsonism or Parkinson’s disease when compared to their counterparts who engaged in football during their youth or high-school years.
“Ultimately, determinations regarding involvement in sports should be a collaborative endeavor involving parents, young athletes, and health care providers. It is incumbent upon physicians to equip parents and youth with a comprehensive comprehension of the potential risks and rewards inherent in football participation,” Dr. Lakhan said.
He added, though, that there are multifaceted advantages to playing football. “This pursuit nurtures cardiovascular well-being, fosters invaluable social interactions, cultivates teamwork, instills discipline through regimented routines, and hones a spectrum of physical proficiencies,” Dr. Lakhan said.
“It’s worth noting that a constellation of alternative sports, including track and field, swimming, soccer, baseball, and tennis, can be cogently discussed as substitutes, all while preserving the manifold benefits of athletic engagement,” Dr. Lakhan added.
The Fox Insight Study is funded by the Michael J. Fox Foundation for Parkinson’s Research. The study was conducted in collaboration with the Michael J. Fox Foundation for Parkinson’s Research, the sponsor of the Fox Insight study, which collected and aggregated data used in the study. It was also supported by the National Institute of Neurological Disorders and Stroke. Dr. Alosco received grants from the National Institutes of Health during the conduct of the study, an honorarium from the Michael J. Fox Foundation for work unrelated to the study, and royalties from Oxford University Press outside the submitted work. Dr. Lakhan disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Why genetic testing may be our best shot at progress in Parkinson’s disease
In 2017, Sanofi Genzyme launched a phase 2 clinical trial of a drug designed to target a specific genetic mutation in some patients with Parkinson’s disease. Researchers hoped the drug would slow or even stop disease progression.
Like many before it, the trial yielded disappointing results and the company shut it down in 2021. It was the latest in a string of unsuccessful clinical trials testing disease-modifying Parkinson’s disease drugs.
Although it failed, the Sanofi Genzyme study was different: It was the first to enroll patients with Parkinson’s disease who had a specific genotype and marked the earliest days of precision medicine and gene-specific drug development for the disease.
Once thought to play only a small role in a small number of patients with Parkinson’s disease,
“We’re about to enter this era of precision medicine for Parkinson’s disease, which makes genetic testing important,” said James Beck, PhD, senior vice president and chief scientific officer for the Parkinson’s Foundation.
“A number of companies have clinical trials or are in preparation for clinical trials to test some specific therapies that would depend upon people having a specific genetic mutation,” he said.
Today, at least four clinical trials of drugs that target specific Parkinson’s disease-related gene variants on LRRK2 and GBA are under way, and more are in the pipeline. Whether these drugs will be effective at modifying the course of the disease remains to be seen. First, the trials must enroll enough patients. And therein lies the challenge: Genetic testing isn’t part of routine Parkinson’s disease care and isn’t covered by most insurance policies. Most patients don’t know their genotype.
It’s a significant roadblock to the future of a precision medicine approach that is based on a patient’s individual genotype, which some experts argue offers the best shot at slowing disease progression.
“To enroll in clinical trials for precision drugs people with Parkinson’s disease have to be aware of their genetic status,” said Roy N. Alcalay, MD, chief of the movement disorders division at Tel Aviv Medical Center in Israel and part-time associate professor at Columbia University in New York. “How can a person with Parkinson’s and a LRRK2 mutation join a precision medicine trial for LRRK2 if she does not know she is a LRRK2 carrier?”
Free genetic testing
Previous studies have shown that some genetic variants increase the risk for Parkinson’s disease after exposure to environmental factors such as pesticides. Research has also shown that a patient’s genotype can predict survival time and that certain medications may prove more effective at slowing disease progression in patients with specific genotypes. All of this points to a significant role for genetics in a disorder that is rapidly increasing.
This makes expanding patient access to genetic testing even more important, Dr. Alcalay said, noting that it’s equally important that patients are informed of their genotype, something that doesn’t usually happen in blinded clinical trials.
To that end, Dr. Alcalay hopes a national genetics study he is leading will address access and need-to-know issues. PD GENEration, a project launched in 2019 by the Parkinson’s Foundation, offers patients free genetic testing for seven clinically relevant Parkinson’s disease-related genes.
Testing is done at home or in a nearby clinic and the results are shared with patients during a free genetic counseling session and with site investigators. Patient samples are stored in a genetic data bank that is open to researchers around the world.
“We surveyed clinical trialists in the Parkinson’s disease field prior to initiation of PD GENEration and estimated that over 90% of people with Parkinson’s disease prior to the effort were not aware of their genetic status,” Dr. Alcalay said.
“I think precision medicine in Parkinson’s disease will not happen without PD GENEration or similar efforts.”
‘Overwhelming’ patient interest
Participants in the study are screened for variants in seven genes known to be involved in Parkinson’s disease risk: GBA, LRRK2, PRKN, PINK1, SNCA, PARK7, and VPS35.
In less than 3 years, the study has already produced what is thought to be the largest genetic data bank of sequenced sets of Parkinson’s disease-risk genes made accessible to patients. Since the end of 2020, the first year of patient enrollment, the number of participants has increased from 676 to 10,515 and the number of participating clinical sites rose from 12 to 101.
The foundation has spent nearly $20 million on the project so far and plans to spend another $10 million to reach a goal of 15,000 patients. The study, which is funded by private donors, is so successful that the foundation has had to scale back enrollment.
“When we were at a peak, we had over 700 participants enrolling each month,” Dr. Beck said. Beginning in April, the program capped new sign-ups to 200 patients per month and created a waiting list for future enrollment. The waiting list is hundreds of patients long.
“The participants’ response to enroll in PD GENEration demonstrates there is an overwhelming interest by people with Parkinson’s disease to learn more about their genetic risk factors,” Dr. Alcalay said.
A research driver
Nearly 60% of participants enrolled so far are male and close to 80% are White. The average age is 69 years and 44% were diagnosed in the past 5 years. Close to 75% had never participated in a clinical trial.
Nearly 13% have tested positive for mutations on at least one of the seven target genes. Previous studies had suggested genetics were involved in only about 10% of cases.
The majority of those with positive results had early-onset Parkinson’s disease, high-risk ancestry, or a first-degree relative with the disease. However, 9% of people who tested positive weren’t in any of those categories.
Genetic information collected by the project is shared with the Global Parkinson’s Genetics Program (GP2), a resource program of the Aligning Science Across Parkinson’s initiative that is focused on the disease’s genetic architecture. Researchers around the world have access to GP2 data to study known gene variants and identify new ones.
PD GENEration participants can choose to be notified if they are carriers of gene variants discovered in the future.
“All DNA samples shared by participants are undergoing research-grade testing,” Dr. Beck said. “Not only do we want to be able to inform people with Parkinson’s disease about their genetic status, but we also want to be able to use this precious resource to further drive research into the genetics of Parkinson’s disease.”
Early success
Patient recruitment has long been one of the biggest challenges to any clinical trial’s success. Research suggests that 90% of all clinical trials fail to reach recruitment milestones in their allotted time frame and two-thirds of multicenter trials fold because too few patients sign up. Data from the Parkinson’s Foundation show that only about 1% of all patients with Parkinson’s disease participate in clinical trials.
Increasing those numbers is the primary goal of PD GENEration, Dr. Beck said. And there’s evidence it’s already paying off.
Earlier this year, one of the program’s participating clinical sites, Intermountain Health, in Salt Lake City, Utah, joined a phase 2 clinical trial of an experimental drug that targets a mutation on the GBA1 gene.
“One of the reasons we were able to participate was when we got the call about joining, we were able to say that we had patients with that specific gene mutation, and we could only say that because the patients had been genotyped through PD GENEration,” said Kathleen E. McKee, MD, director of movement disorders, associate medical director of neurosciences research, and PD GENEration principal investigator at Intermountain Health.
Since 2021, Dr. McKee has enrolled hundreds of patients in the foundation’s gene study and hopes to enroll even more. Few patients turn down the opportunity to participate, she added. Knowing their genotype has proven empowering for her patients, most of whom could not afford genetic testing on their own.
“Previously I would tell patients this is not going to change your immediate management,” Dr. McKee said. “Now I tell my patients that these trials are out there, it may actually change how I treat you and what I recommend.”
A version of this article appeared on Medscape.com.
In 2017, Sanofi Genzyme launched a phase 2 clinical trial of a drug designed to target a specific genetic mutation in some patients with Parkinson’s disease. Researchers hoped the drug would slow or even stop disease progression.
Like many before it, the trial yielded disappointing results and the company shut it down in 2021. It was the latest in a string of unsuccessful clinical trials testing disease-modifying Parkinson’s disease drugs.
Although it failed, the Sanofi Genzyme study was different: It was the first to enroll patients with Parkinson’s disease who had a specific genotype and marked the earliest days of precision medicine and gene-specific drug development for the disease.
Once thought to play only a small role in a small number of patients with Parkinson’s disease,
“We’re about to enter this era of precision medicine for Parkinson’s disease, which makes genetic testing important,” said James Beck, PhD, senior vice president and chief scientific officer for the Parkinson’s Foundation.
“A number of companies have clinical trials or are in preparation for clinical trials to test some specific therapies that would depend upon people having a specific genetic mutation,” he said.
Today, at least four clinical trials of drugs that target specific Parkinson’s disease-related gene variants on LRRK2 and GBA are under way, and more are in the pipeline. Whether these drugs will be effective at modifying the course of the disease remains to be seen. First, the trials must enroll enough patients. And therein lies the challenge: Genetic testing isn’t part of routine Parkinson’s disease care and isn’t covered by most insurance policies. Most patients don’t know their genotype.
It’s a significant roadblock to the future of a precision medicine approach that is based on a patient’s individual genotype, which some experts argue offers the best shot at slowing disease progression.
“To enroll in clinical trials for precision drugs people with Parkinson’s disease have to be aware of their genetic status,” said Roy N. Alcalay, MD, chief of the movement disorders division at Tel Aviv Medical Center in Israel and part-time associate professor at Columbia University in New York. “How can a person with Parkinson’s and a LRRK2 mutation join a precision medicine trial for LRRK2 if she does not know she is a LRRK2 carrier?”
Free genetic testing
Previous studies have shown that some genetic variants increase the risk for Parkinson’s disease after exposure to environmental factors such as pesticides. Research has also shown that a patient’s genotype can predict survival time and that certain medications may prove more effective at slowing disease progression in patients with specific genotypes. All of this points to a significant role for genetics in a disorder that is rapidly increasing.
This makes expanding patient access to genetic testing even more important, Dr. Alcalay said, noting that it’s equally important that patients are informed of their genotype, something that doesn’t usually happen in blinded clinical trials.
To that end, Dr. Alcalay hopes a national genetics study he is leading will address access and need-to-know issues. PD GENEration, a project launched in 2019 by the Parkinson’s Foundation, offers patients free genetic testing for seven clinically relevant Parkinson’s disease-related genes.
Testing is done at home or in a nearby clinic and the results are shared with patients during a free genetic counseling session and with site investigators. Patient samples are stored in a genetic data bank that is open to researchers around the world.
“We surveyed clinical trialists in the Parkinson’s disease field prior to initiation of PD GENEration and estimated that over 90% of people with Parkinson’s disease prior to the effort were not aware of their genetic status,” Dr. Alcalay said.
“I think precision medicine in Parkinson’s disease will not happen without PD GENEration or similar efforts.”
‘Overwhelming’ patient interest
Participants in the study are screened for variants in seven genes known to be involved in Parkinson’s disease risk: GBA, LRRK2, PRKN, PINK1, SNCA, PARK7, and VPS35.
In less than 3 years, the study has already produced what is thought to be the largest genetic data bank of sequenced sets of Parkinson’s disease-risk genes made accessible to patients. Since the end of 2020, the first year of patient enrollment, the number of participants has increased from 676 to 10,515 and the number of participating clinical sites rose from 12 to 101.
The foundation has spent nearly $20 million on the project so far and plans to spend another $10 million to reach a goal of 15,000 patients. The study, which is funded by private donors, is so successful that the foundation has had to scale back enrollment.
“When we were at a peak, we had over 700 participants enrolling each month,” Dr. Beck said. Beginning in April, the program capped new sign-ups to 200 patients per month and created a waiting list for future enrollment. The waiting list is hundreds of patients long.
“The participants’ response to enroll in PD GENEration demonstrates there is an overwhelming interest by people with Parkinson’s disease to learn more about their genetic risk factors,” Dr. Alcalay said.
A research driver
Nearly 60% of participants enrolled so far are male and close to 80% are White. The average age is 69 years and 44% were diagnosed in the past 5 years. Close to 75% had never participated in a clinical trial.
Nearly 13% have tested positive for mutations on at least one of the seven target genes. Previous studies had suggested genetics were involved in only about 10% of cases.
The majority of those with positive results had early-onset Parkinson’s disease, high-risk ancestry, or a first-degree relative with the disease. However, 9% of people who tested positive weren’t in any of those categories.
Genetic information collected by the project is shared with the Global Parkinson’s Genetics Program (GP2), a resource program of the Aligning Science Across Parkinson’s initiative that is focused on the disease’s genetic architecture. Researchers around the world have access to GP2 data to study known gene variants and identify new ones.
PD GENEration participants can choose to be notified if they are carriers of gene variants discovered in the future.
“All DNA samples shared by participants are undergoing research-grade testing,” Dr. Beck said. “Not only do we want to be able to inform people with Parkinson’s disease about their genetic status, but we also want to be able to use this precious resource to further drive research into the genetics of Parkinson’s disease.”
Early success
Patient recruitment has long been one of the biggest challenges to any clinical trial’s success. Research suggests that 90% of all clinical trials fail to reach recruitment milestones in their allotted time frame and two-thirds of multicenter trials fold because too few patients sign up. Data from the Parkinson’s Foundation show that only about 1% of all patients with Parkinson’s disease participate in clinical trials.
Increasing those numbers is the primary goal of PD GENEration, Dr. Beck said. And there’s evidence it’s already paying off.
Earlier this year, one of the program’s participating clinical sites, Intermountain Health, in Salt Lake City, Utah, joined a phase 2 clinical trial of an experimental drug that targets a mutation on the GBA1 gene.
“One of the reasons we were able to participate was when we got the call about joining, we were able to say that we had patients with that specific gene mutation, and we could only say that because the patients had been genotyped through PD GENEration,” said Kathleen E. McKee, MD, director of movement disorders, associate medical director of neurosciences research, and PD GENEration principal investigator at Intermountain Health.
Since 2021, Dr. McKee has enrolled hundreds of patients in the foundation’s gene study and hopes to enroll even more. Few patients turn down the opportunity to participate, she added. Knowing their genotype has proven empowering for her patients, most of whom could not afford genetic testing on their own.
“Previously I would tell patients this is not going to change your immediate management,” Dr. McKee said. “Now I tell my patients that these trials are out there, it may actually change how I treat you and what I recommend.”
A version of this article appeared on Medscape.com.
In 2017, Sanofi Genzyme launched a phase 2 clinical trial of a drug designed to target a specific genetic mutation in some patients with Parkinson’s disease. Researchers hoped the drug would slow or even stop disease progression.
Like many before it, the trial yielded disappointing results and the company shut it down in 2021. It was the latest in a string of unsuccessful clinical trials testing disease-modifying Parkinson’s disease drugs.
Although it failed, the Sanofi Genzyme study was different: It was the first to enroll patients with Parkinson’s disease who had a specific genotype and marked the earliest days of precision medicine and gene-specific drug development for the disease.
Once thought to play only a small role in a small number of patients with Parkinson’s disease,
“We’re about to enter this era of precision medicine for Parkinson’s disease, which makes genetic testing important,” said James Beck, PhD, senior vice president and chief scientific officer for the Parkinson’s Foundation.
“A number of companies have clinical trials or are in preparation for clinical trials to test some specific therapies that would depend upon people having a specific genetic mutation,” he said.
Today, at least four clinical trials of drugs that target specific Parkinson’s disease-related gene variants on LRRK2 and GBA are under way, and more are in the pipeline. Whether these drugs will be effective at modifying the course of the disease remains to be seen. First, the trials must enroll enough patients. And therein lies the challenge: Genetic testing isn’t part of routine Parkinson’s disease care and isn’t covered by most insurance policies. Most patients don’t know their genotype.
It’s a significant roadblock to the future of a precision medicine approach that is based on a patient’s individual genotype, which some experts argue offers the best shot at slowing disease progression.
“To enroll in clinical trials for precision drugs people with Parkinson’s disease have to be aware of their genetic status,” said Roy N. Alcalay, MD, chief of the movement disorders division at Tel Aviv Medical Center in Israel and part-time associate professor at Columbia University in New York. “How can a person with Parkinson’s and a LRRK2 mutation join a precision medicine trial for LRRK2 if she does not know she is a LRRK2 carrier?”
Free genetic testing
Previous studies have shown that some genetic variants increase the risk for Parkinson’s disease after exposure to environmental factors such as pesticides. Research has also shown that a patient’s genotype can predict survival time and that certain medications may prove more effective at slowing disease progression in patients with specific genotypes. All of this points to a significant role for genetics in a disorder that is rapidly increasing.
This makes expanding patient access to genetic testing even more important, Dr. Alcalay said, noting that it’s equally important that patients are informed of their genotype, something that doesn’t usually happen in blinded clinical trials.
To that end, Dr. Alcalay hopes a national genetics study he is leading will address access and need-to-know issues. PD GENEration, a project launched in 2019 by the Parkinson’s Foundation, offers patients free genetic testing for seven clinically relevant Parkinson’s disease-related genes.
Testing is done at home or in a nearby clinic and the results are shared with patients during a free genetic counseling session and with site investigators. Patient samples are stored in a genetic data bank that is open to researchers around the world.
“We surveyed clinical trialists in the Parkinson’s disease field prior to initiation of PD GENEration and estimated that over 90% of people with Parkinson’s disease prior to the effort were not aware of their genetic status,” Dr. Alcalay said.
“I think precision medicine in Parkinson’s disease will not happen without PD GENEration or similar efforts.”
‘Overwhelming’ patient interest
Participants in the study are screened for variants in seven genes known to be involved in Parkinson’s disease risk: GBA, LRRK2, PRKN, PINK1, SNCA, PARK7, and VPS35.
In less than 3 years, the study has already produced what is thought to be the largest genetic data bank of sequenced sets of Parkinson’s disease-risk genes made accessible to patients. Since the end of 2020, the first year of patient enrollment, the number of participants has increased from 676 to 10,515 and the number of participating clinical sites rose from 12 to 101.
The foundation has spent nearly $20 million on the project so far and plans to spend another $10 million to reach a goal of 15,000 patients. The study, which is funded by private donors, is so successful that the foundation has had to scale back enrollment.
“When we were at a peak, we had over 700 participants enrolling each month,” Dr. Beck said. Beginning in April, the program capped new sign-ups to 200 patients per month and created a waiting list for future enrollment. The waiting list is hundreds of patients long.
“The participants’ response to enroll in PD GENEration demonstrates there is an overwhelming interest by people with Parkinson’s disease to learn more about their genetic risk factors,” Dr. Alcalay said.
A research driver
Nearly 60% of participants enrolled so far are male and close to 80% are White. The average age is 69 years and 44% were diagnosed in the past 5 years. Close to 75% had never participated in a clinical trial.
Nearly 13% have tested positive for mutations on at least one of the seven target genes. Previous studies had suggested genetics were involved in only about 10% of cases.
The majority of those with positive results had early-onset Parkinson’s disease, high-risk ancestry, or a first-degree relative with the disease. However, 9% of people who tested positive weren’t in any of those categories.
Genetic information collected by the project is shared with the Global Parkinson’s Genetics Program (GP2), a resource program of the Aligning Science Across Parkinson’s initiative that is focused on the disease’s genetic architecture. Researchers around the world have access to GP2 data to study known gene variants and identify new ones.
PD GENEration participants can choose to be notified if they are carriers of gene variants discovered in the future.
“All DNA samples shared by participants are undergoing research-grade testing,” Dr. Beck said. “Not only do we want to be able to inform people with Parkinson’s disease about their genetic status, but we also want to be able to use this precious resource to further drive research into the genetics of Parkinson’s disease.”
Early success
Patient recruitment has long been one of the biggest challenges to any clinical trial’s success. Research suggests that 90% of all clinical trials fail to reach recruitment milestones in their allotted time frame and two-thirds of multicenter trials fold because too few patients sign up. Data from the Parkinson’s Foundation show that only about 1% of all patients with Parkinson’s disease participate in clinical trials.
Increasing those numbers is the primary goal of PD GENEration, Dr. Beck said. And there’s evidence it’s already paying off.
Earlier this year, one of the program’s participating clinical sites, Intermountain Health, in Salt Lake City, Utah, joined a phase 2 clinical trial of an experimental drug that targets a mutation on the GBA1 gene.
“One of the reasons we were able to participate was when we got the call about joining, we were able to say that we had patients with that specific gene mutation, and we could only say that because the patients had been genotyped through PD GENEration,” said Kathleen E. McKee, MD, director of movement disorders, associate medical director of neurosciences research, and PD GENEration principal investigator at Intermountain Health.
Since 2021, Dr. McKee has enrolled hundreds of patients in the foundation’s gene study and hopes to enroll even more. Few patients turn down the opportunity to participate, she added. Knowing their genotype has proven empowering for her patients, most of whom could not afford genetic testing on their own.
“Previously I would tell patients this is not going to change your immediate management,” Dr. McKee said. “Now I tell my patients that these trials are out there, it may actually change how I treat you and what I recommend.”
A version of this article appeared on Medscape.com.