Opinion

Is Being ‘Manly’ a Threat to a Man’s Health?
- Author:
- F. Perry Wilson, MD, MSCE
We have empirical evidence that men downplay their medical symptoms — and that manlier men downplay them even more.
News

Why Cardiac Biomarkers Don’t Help Predict Heart Disease
- Author:
- F. Perry Wilson, MD, MSCE
Physician discusses recent study findings that cardiac biomarkers will not likely help risk-stratify patients.
Opinion
It Would Be Nice if Olive Oil Really Did Prevent Dementia
- Author:
- F. Perry Wilson, MD, MSCE
Does olive oil prevent dementia? Let’s weigh the evidence.
Opinion

Intermittent Fasting + HIIT: Fitness Fad or Fix?
- Author:
- F. Perry Wilson, MD, MSCE
Any lifestyle change is hard, but with persistence the changes become habits and, eventually, those habits do become pleasurable.
News

Are Women Better Doctors Than Men?
- Author:
- F. Perry Wilson, MD, MSCE
Study finds female hospitalists provided better care, defined as lower 30-day mortality, than male hospitalists.
News
‘Difficult Patient’: Stigmatizing Words and Medical Error
- Author:
- F. Perry Wilson, MD, MSCE
Dr. F. Perry Wilson comments on the potential of stigmatized language in medical records to lead to missed diagnoses and poor outcomes.
Opinion

A Banned Chemical That Is Still Causing Cancer
- Author:
- F. Perry Wilson, MD, MSCE
This carcinogen ‘is still around: in our soil, in our food, and in our blood.’
Opinion

Vitamin D Supplements May Be a Double-Edged Sword
- Author:
- F. Perry Wilson, MD, MSCE
I can tell you that for your “average woman,” vitamin D supplementation likely has no effect on mortality.
Opinion

COVID-19 Is a Very Weird Virus
- Author:
- F. Perry Wilson, MD, MSCE
COVID may cause immune system dysfunction that puts patients at risk for autoimmunity.
News

It Sure Looks Like Cannabis Is Bad for the Heart, Doesn’t It?
- Author:
- F. Perry Wilson, MD, MSCE
Dr. Wilson discusses recent research on cannabis and heart disease.
News

Bivalent Vaccines Protect Even Children Who’ve Had COVID
- Author:
- F. Perry Wilson, MD, MSCE
Doctor discusses the progression of understanding of COVID-19 and current vaccines.
News
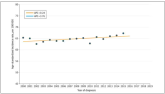
More Young Women Being Diagnosed With Breast Cancer Than Ever Before
- Author:
- F. Perry Wilson, MD, MSCE
Rates of breast cancer are up as well as among young women, according to study.
News

Even Intentional Weight Loss Linked With Cancer
- Author:
- F. Perry Wilson, MD, MSCE
Significant weight loss, even intentionally, can be indicative of cancer.
News
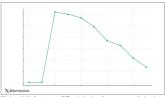
Testosterone Replacement May Cause ... Fracture?
- Author:
- F. Perry Wilson, MD, MSCE
Testosterone replacement linked to possible increased in fractures.
News
Yes, Patients Are Getting More Complicated
- Author:
- F. Perry Wilson, MD, MSCE
The average hospitalized patient is older, more likely to have kidney disease or diabetes, is on more medications, and has spent more time in the...
