Opinion

Really? Cancer screening doesn’t save lives?
- Author:
- F. Perry Wilson, MD, MSCE
The screening tests are unlikely to help most people because most people will not die of the particular type of cancer being screened for. But it...
News

On the best way to exercise
- Author:
- F. Perry Wilson, MD, MSCE
If you are going to get activity, push on the vigorous activity if you’re physically able to do it. And of course, layering in the muscle-...
Opinion
A new and completely different pain medicine
- Author:
- F. Perry Wilson, MD, MSCE
Patients assigned to the highest dose of VX-548 had a statistically significant lower cumulative amount of pain in the 48 hours after surgery.
Opinion
What AI can see in CT scans that humans can’t
- Author:
- F. Perry Wilson, MD, MSCE
This is a space where AI can make some massive gains – not by trying to do radiologists’ jobs better than radiologists, but by extracting...
Opinion
The surprising occupations with higher-than-expected ovarian cancer rates
- Author:
- F. Perry Wilson, MD, MSCE
This is how these types of studies work; they tend to raise more questions than they answer.
Opinion
The most important question in medicine
- Author:
- F. Perry Wilson, MD, MSCE
“What else could this be?”
Opinion

The cardiopulmonary effects of mask wearing
- Author:
- F. Perry Wilson, MD, MSCE
Clearly, this study lets us conclude that mask wearing at rest has less of an effect than mask wearing during exercise. But remember that, in...
Opinion

COVID boosters effective, but not for long
- Author:
- F. Perry Wilson, MD, MSCE
The question is: Does receipt of a bivalent COVID vaccine booster prevent hospitalizations, ICU stay, or death?
Opinion

The 30th-birthday gift that could save a life
- Author:
- F. Perry Wilson, MD, MSCE
Imagine a future when, at 30, you get your genome sequenced and doctors tell you what needs to be done to save your life.
Opinion

Surprising brain activity moments before death
- Author:
- F. Perry Wilson, MD, MSCE
The electrical patterns of the brains in these patients looked very similar to the patterns seen in dreaming humans, as well as in patients with...
Opinion
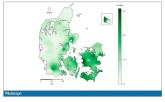
Autism: Is it in the water?
- Author:
- F. Perry Wilson, MD, MSCE
The results show a rather steady and consistent association between higher lithium levels in groundwater and the prevalence of ASD in children.
Opinion

‘Excess’ deaths surging, but why?
- Author:
- F. Perry Wilson, MD, MSCE
‘The excess death gap between the United States and Western Europe has never been higher,’ according to a new study.
Opinion

Celebrity death finally solved – with locks of hair
- Author:
- F. Perry Wilson, MD, MSCE
Case: A 56-year-old musician presents with diffuse abdominal pain, cramping, and jaundice. His medical history is notable for years of diffuse...
Opinion
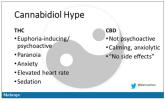
Joint effort: CBD not just innocent bystander in weed
- Author:
- F. Perry Wilson, MD, MSCE
‘I visited a legal cannabis dispensary in Massachusetts a few years ago, mostly to see what the hype was about.’
Opinion

A new (old) drug joins the COVID fray, and guess what? It works
- Author:
- F. Perry Wilson, MD, MSCE
Part of the reason the monoclonals have failed lately is because of their specificity.
