User login
ART Linked With Congenital Heart Defects in Newborns
The rate of congenital heart defects is higher in newborns conceived using assisted reproductive technologies (ART) than in newborns conceived without assistance. This finding comes from a population-based cohort study led by Dr. Nona Sargisian, a gynecologist at the University of Gothenburg, Sweden, and colleagues, which was published in the European Heart Journal.
The researchers analyzed more than 7 million results of all live-born children in Denmark, Finland, Sweden, and Norway between 1984 and 2015. They found that congenital heart defects occurred more frequently in the ART newborn group (1.85%) than in naturally conceived newborns (1.15%).
The study also revealed that the risk for congenital heart defects in multiple births is higher than in single births, with and without the use of ART. However, the result that congenital heart defects occur more often in ART newborns remained significant when comparing single births from both groups (1.62% vs 1.11%).
Relatively Low Prevalence
Barbara Sonntag, MD, PhD, a gynecologist at Amedes Fertility Center in Hamburg, Germany, referred to a “clinically relevant risk increase” with a relatively low prevalence of the condition.
“When 1000 children are born, an abnormality occurs in 18 children after ART, compared with 11 children born after natural conception,” she told the Science Media Center.
Dr. Sonntag emphasized that the risk is particularly increased by a multiple pregnancy. A statement about causality is not possible based on the study, but multiple pregnancies are generally associated with increased risks during pregnancy and for the children.
The large and robust dataset confirms long-known findings, said Georg Griesinger, MD, PhD, medical director of the fertility centers of the University Medical Center Schleswig-Holstein in Lübeck and Manhagen, Germany.
The key figures can be found in single births, he explained. “Among single births conceived by ART, the rate of severe congenital heart defects was 1.62% compared with 1.11% in spontaneously conceived single births, an increase in risk by 1.19 times. For severe heart defects, the rate was 0.31% in ART single births, compared with 0.25% in spontaneously conceived single births.”
The increased risks are consistent with existing literature. Therefore, the current study does not reveal any new risk signals, said Dr. Griesinger.
Single Embryo Transfer
The “risks are small but present,” according to Michael von Wolff, MD, head of gynecological endocrinology and reproductive medicine at Bern University Hospital in Switzerland. “Therefore, ART therapy should only be carried out after exhausting conservative treatments,” he recommended. For example, ovarian stimulation with low-dose hormone preparations could be an option.
Dr. Griesinger pointed out that, in absolute numbers, all maternal and fetal or neonatal risks are significantly increased in twins and higher-order multiples, compared with the estimated risk association within the actual ART treatment.
“For this reason, reproductive medicine specialists have been advocating for single-embryo transfer for years to promote the occurrence of single pregnancies through ART,” said Dr. Griesinger.
The study “emphasizes the importance of single embryo transfer to avoid the higher risks associated with multiple pregnancies,” according to Rocío Núñez Calonge, PhD, scientific director of the International Reproduction Unit in Alicante, Spain.
Dr. Sonntag also sees a “strong additional call to avoid multiple pregnancies through a predominant strategy of single-embryo transfer in the data. The increased rate of childhood birth defects is already part of the information provided before assisted reproduction.”
This story was translated from the Medscape German edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
The rate of congenital heart defects is higher in newborns conceived using assisted reproductive technologies (ART) than in newborns conceived without assistance. This finding comes from a population-based cohort study led by Dr. Nona Sargisian, a gynecologist at the University of Gothenburg, Sweden, and colleagues, which was published in the European Heart Journal.
The researchers analyzed more than 7 million results of all live-born children in Denmark, Finland, Sweden, and Norway between 1984 and 2015. They found that congenital heart defects occurred more frequently in the ART newborn group (1.85%) than in naturally conceived newborns (1.15%).
The study also revealed that the risk for congenital heart defects in multiple births is higher than in single births, with and without the use of ART. However, the result that congenital heart defects occur more often in ART newborns remained significant when comparing single births from both groups (1.62% vs 1.11%).
Relatively Low Prevalence
Barbara Sonntag, MD, PhD, a gynecologist at Amedes Fertility Center in Hamburg, Germany, referred to a “clinically relevant risk increase” with a relatively low prevalence of the condition.
“When 1000 children are born, an abnormality occurs in 18 children after ART, compared with 11 children born after natural conception,” she told the Science Media Center.
Dr. Sonntag emphasized that the risk is particularly increased by a multiple pregnancy. A statement about causality is not possible based on the study, but multiple pregnancies are generally associated with increased risks during pregnancy and for the children.
The large and robust dataset confirms long-known findings, said Georg Griesinger, MD, PhD, medical director of the fertility centers of the University Medical Center Schleswig-Holstein in Lübeck and Manhagen, Germany.
The key figures can be found in single births, he explained. “Among single births conceived by ART, the rate of severe congenital heart defects was 1.62% compared with 1.11% in spontaneously conceived single births, an increase in risk by 1.19 times. For severe heart defects, the rate was 0.31% in ART single births, compared with 0.25% in spontaneously conceived single births.”
The increased risks are consistent with existing literature. Therefore, the current study does not reveal any new risk signals, said Dr. Griesinger.
Single Embryo Transfer
The “risks are small but present,” according to Michael von Wolff, MD, head of gynecological endocrinology and reproductive medicine at Bern University Hospital in Switzerland. “Therefore, ART therapy should only be carried out after exhausting conservative treatments,” he recommended. For example, ovarian stimulation with low-dose hormone preparations could be an option.
Dr. Griesinger pointed out that, in absolute numbers, all maternal and fetal or neonatal risks are significantly increased in twins and higher-order multiples, compared with the estimated risk association within the actual ART treatment.
“For this reason, reproductive medicine specialists have been advocating for single-embryo transfer for years to promote the occurrence of single pregnancies through ART,” said Dr. Griesinger.
The study “emphasizes the importance of single embryo transfer to avoid the higher risks associated with multiple pregnancies,” according to Rocío Núñez Calonge, PhD, scientific director of the International Reproduction Unit in Alicante, Spain.
Dr. Sonntag also sees a “strong additional call to avoid multiple pregnancies through a predominant strategy of single-embryo transfer in the data. The increased rate of childhood birth defects is already part of the information provided before assisted reproduction.”
This story was translated from the Medscape German edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
The rate of congenital heart defects is higher in newborns conceived using assisted reproductive technologies (ART) than in newborns conceived without assistance. This finding comes from a population-based cohort study led by Dr. Nona Sargisian, a gynecologist at the University of Gothenburg, Sweden, and colleagues, which was published in the European Heart Journal.
The researchers analyzed more than 7 million results of all live-born children in Denmark, Finland, Sweden, and Norway between 1984 and 2015. They found that congenital heart defects occurred more frequently in the ART newborn group (1.85%) than in naturally conceived newborns (1.15%).
The study also revealed that the risk for congenital heart defects in multiple births is higher than in single births, with and without the use of ART. However, the result that congenital heart defects occur more often in ART newborns remained significant when comparing single births from both groups (1.62% vs 1.11%).
Relatively Low Prevalence
Barbara Sonntag, MD, PhD, a gynecologist at Amedes Fertility Center in Hamburg, Germany, referred to a “clinically relevant risk increase” with a relatively low prevalence of the condition.
“When 1000 children are born, an abnormality occurs in 18 children after ART, compared with 11 children born after natural conception,” she told the Science Media Center.
Dr. Sonntag emphasized that the risk is particularly increased by a multiple pregnancy. A statement about causality is not possible based on the study, but multiple pregnancies are generally associated with increased risks during pregnancy and for the children.
The large and robust dataset confirms long-known findings, said Georg Griesinger, MD, PhD, medical director of the fertility centers of the University Medical Center Schleswig-Holstein in Lübeck and Manhagen, Germany.
The key figures can be found in single births, he explained. “Among single births conceived by ART, the rate of severe congenital heart defects was 1.62% compared with 1.11% in spontaneously conceived single births, an increase in risk by 1.19 times. For severe heart defects, the rate was 0.31% in ART single births, compared with 0.25% in spontaneously conceived single births.”
The increased risks are consistent with existing literature. Therefore, the current study does not reveal any new risk signals, said Dr. Griesinger.
Single Embryo Transfer
The “risks are small but present,” according to Michael von Wolff, MD, head of gynecological endocrinology and reproductive medicine at Bern University Hospital in Switzerland. “Therefore, ART therapy should only be carried out after exhausting conservative treatments,” he recommended. For example, ovarian stimulation with low-dose hormone preparations could be an option.
Dr. Griesinger pointed out that, in absolute numbers, all maternal and fetal or neonatal risks are significantly increased in twins and higher-order multiples, compared with the estimated risk association within the actual ART treatment.
“For this reason, reproductive medicine specialists have been advocating for single-embryo transfer for years to promote the occurrence of single pregnancies through ART,” said Dr. Griesinger.
The study “emphasizes the importance of single embryo transfer to avoid the higher risks associated with multiple pregnancies,” according to Rocío Núñez Calonge, PhD, scientific director of the International Reproduction Unit in Alicante, Spain.
Dr. Sonntag also sees a “strong additional call to avoid multiple pregnancies through a predominant strategy of single-embryo transfer in the data. The increased rate of childhood birth defects is already part of the information provided before assisted reproduction.”
This story was translated from the Medscape German edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
FROM EUROPEAN HEART JOURNAL
CBD Use in Pregnant People Double That of Nonpregnant Counterparts
Pregnant women in a large North American sample reported nearly double the rate of cannabidiol (CBD) use compared with nonpregnant women, new data published in a research letter in Obstetrics & Gynecology indicates.
Healthcare providers should be aware of the high rate of CBD use in pregnancy, especially as legal use of cannabis is increasing faster than evidence on outcomes for exposed offspring, note the researchers, led by Devika Bhatia, MD, from the Department of Psychiatry, Colorado School of Medicine, University of Colorado Anschutz Medical Campus in Aurora.
In an accompanying editorial, Torri D. Metz, MD, MS, deputy editor for obstetrics for Obstetrics & Gynecology, writes that the study “is critically important.” She points out that pregnant individuals may perceive that CBD is a safe drug to use in pregnancy, despite there being essentially no data examining whether or not this is the case.
Large Dataset From United States and Canada
Researchers used data from the International Cannabis Policy Study (2019-2021), a repeated cross-sectional survey of people aged 16-65 years in the United States and Canada. There were 66,457 women in the sample, including 1096 pregnant women.
Particularly concerning, the authors write, is the prenatal use of CBD-only products. Those products are advertised to contain only CBD, rather than tetrahydrocannabinol (THC). They point out CBD-only products are often legal in North America and often marketed as supplements.
The prevalence of CBD-only use in pregnant women in the study was 20.4% compared with 11.3% among nonpregnant women, P < .001. The top reason for use by pregnant women was anxiety (58.4%). Other top reasons included depression (40.3%), posttraumatic stress disorder (32.1%), pain (52.3%), headache (35.6%), and nausea or vomiting (31.9%).
“Nonpregnant women were significantly more likely to report using CBD for pain, sleep, general well-being, and ‘other’ physical or mental health reasons, or to not use CBD for mental health,” the authors write, adding that the reasons for CBD use highlight drivers that may be important to address in treating pregnant patients.
Provider Endorsement in Some Cases
Dr. Metz, associate professor of obstetrics and gynecology with the University of Utah Health in Salt Lake City, says in some cases women may be getting endorsement of CBD use from their provider or at least implied support when CBD is prescribed. In the study, pregnant women had 2.33 times greater adjusted odds of having a CBD prescription than nonpregnant women (95% confidence interval, 1.27-2.88).
She points to another cross-sectional study of more than 10,000 participants using PRAMS (Pregnancy Risk Assessment Monitoring System) data that found that “from 2017 to 2019, 63% of pregnant women reported that they were not told to avoid cannabis use in pregnancy, and 8% noted that they were advised to use cannabis by their prenatal care practitioner.”
The American College of Obstetricians and Gynecologists recommends against prescribing cannabis products for pregnant or lactating women.
Studies that have explored THC and its metabolites have shown “a consistent association between cannabis use and decreased fetal growth,” Dr. Metz noted. “There also remain persistent concerns about the long-term neurodevelopmental effects of maternal cannabis use on the fetus and, subsequently, the newborn.”
Limitations of the study include the self-reported responses and participants’ ability to accurately distinguish between CBD-only and THC-containing products.
Because self-reports of CBD use in pregnancy may be drastically underestimated and nonreliable, Dr. Metz writes, development of blood and urine screens to help detect CBD product use “will be helpful in moving the field forward.”
Study senior author David Hammond, PhD, has been a paid expert witness on behalf of public health authorities in response to legal challenges from the cannabis, tobacco, vaping, and food industries. Other authors did not report any potential conflicts. Dr. Metz reports personal fees from Pfizer, and grants from Pfizer for her role as a site principal investigator for SARS-CoV-2 vaccination and for her role as a site PI for RSV vaccination in pregnancy study.
Pregnant women in a large North American sample reported nearly double the rate of cannabidiol (CBD) use compared with nonpregnant women, new data published in a research letter in Obstetrics & Gynecology indicates.
Healthcare providers should be aware of the high rate of CBD use in pregnancy, especially as legal use of cannabis is increasing faster than evidence on outcomes for exposed offspring, note the researchers, led by Devika Bhatia, MD, from the Department of Psychiatry, Colorado School of Medicine, University of Colorado Anschutz Medical Campus in Aurora.
In an accompanying editorial, Torri D. Metz, MD, MS, deputy editor for obstetrics for Obstetrics & Gynecology, writes that the study “is critically important.” She points out that pregnant individuals may perceive that CBD is a safe drug to use in pregnancy, despite there being essentially no data examining whether or not this is the case.
Large Dataset From United States and Canada
Researchers used data from the International Cannabis Policy Study (2019-2021), a repeated cross-sectional survey of people aged 16-65 years in the United States and Canada. There were 66,457 women in the sample, including 1096 pregnant women.
Particularly concerning, the authors write, is the prenatal use of CBD-only products. Those products are advertised to contain only CBD, rather than tetrahydrocannabinol (THC). They point out CBD-only products are often legal in North America and often marketed as supplements.
The prevalence of CBD-only use in pregnant women in the study was 20.4% compared with 11.3% among nonpregnant women, P < .001. The top reason for use by pregnant women was anxiety (58.4%). Other top reasons included depression (40.3%), posttraumatic stress disorder (32.1%), pain (52.3%), headache (35.6%), and nausea or vomiting (31.9%).
“Nonpregnant women were significantly more likely to report using CBD for pain, sleep, general well-being, and ‘other’ physical or mental health reasons, or to not use CBD for mental health,” the authors write, adding that the reasons for CBD use highlight drivers that may be important to address in treating pregnant patients.
Provider Endorsement in Some Cases
Dr. Metz, associate professor of obstetrics and gynecology with the University of Utah Health in Salt Lake City, says in some cases women may be getting endorsement of CBD use from their provider or at least implied support when CBD is prescribed. In the study, pregnant women had 2.33 times greater adjusted odds of having a CBD prescription than nonpregnant women (95% confidence interval, 1.27-2.88).
She points to another cross-sectional study of more than 10,000 participants using PRAMS (Pregnancy Risk Assessment Monitoring System) data that found that “from 2017 to 2019, 63% of pregnant women reported that they were not told to avoid cannabis use in pregnancy, and 8% noted that they were advised to use cannabis by their prenatal care practitioner.”
The American College of Obstetricians and Gynecologists recommends against prescribing cannabis products for pregnant or lactating women.
Studies that have explored THC and its metabolites have shown “a consistent association between cannabis use and decreased fetal growth,” Dr. Metz noted. “There also remain persistent concerns about the long-term neurodevelopmental effects of maternal cannabis use on the fetus and, subsequently, the newborn.”
Limitations of the study include the self-reported responses and participants’ ability to accurately distinguish between CBD-only and THC-containing products.
Because self-reports of CBD use in pregnancy may be drastically underestimated and nonreliable, Dr. Metz writes, development of blood and urine screens to help detect CBD product use “will be helpful in moving the field forward.”
Study senior author David Hammond, PhD, has been a paid expert witness on behalf of public health authorities in response to legal challenges from the cannabis, tobacco, vaping, and food industries. Other authors did not report any potential conflicts. Dr. Metz reports personal fees from Pfizer, and grants from Pfizer for her role as a site principal investigator for SARS-CoV-2 vaccination and for her role as a site PI for RSV vaccination in pregnancy study.
Pregnant women in a large North American sample reported nearly double the rate of cannabidiol (CBD) use compared with nonpregnant women, new data published in a research letter in Obstetrics & Gynecology indicates.
Healthcare providers should be aware of the high rate of CBD use in pregnancy, especially as legal use of cannabis is increasing faster than evidence on outcomes for exposed offspring, note the researchers, led by Devika Bhatia, MD, from the Department of Psychiatry, Colorado School of Medicine, University of Colorado Anschutz Medical Campus in Aurora.
In an accompanying editorial, Torri D. Metz, MD, MS, deputy editor for obstetrics for Obstetrics & Gynecology, writes that the study “is critically important.” She points out that pregnant individuals may perceive that CBD is a safe drug to use in pregnancy, despite there being essentially no data examining whether or not this is the case.
Large Dataset From United States and Canada
Researchers used data from the International Cannabis Policy Study (2019-2021), a repeated cross-sectional survey of people aged 16-65 years in the United States and Canada. There were 66,457 women in the sample, including 1096 pregnant women.
Particularly concerning, the authors write, is the prenatal use of CBD-only products. Those products are advertised to contain only CBD, rather than tetrahydrocannabinol (THC). They point out CBD-only products are often legal in North America and often marketed as supplements.
The prevalence of CBD-only use in pregnant women in the study was 20.4% compared with 11.3% among nonpregnant women, P < .001. The top reason for use by pregnant women was anxiety (58.4%). Other top reasons included depression (40.3%), posttraumatic stress disorder (32.1%), pain (52.3%), headache (35.6%), and nausea or vomiting (31.9%).
“Nonpregnant women were significantly more likely to report using CBD for pain, sleep, general well-being, and ‘other’ physical or mental health reasons, or to not use CBD for mental health,” the authors write, adding that the reasons for CBD use highlight drivers that may be important to address in treating pregnant patients.
Provider Endorsement in Some Cases
Dr. Metz, associate professor of obstetrics and gynecology with the University of Utah Health in Salt Lake City, says in some cases women may be getting endorsement of CBD use from their provider or at least implied support when CBD is prescribed. In the study, pregnant women had 2.33 times greater adjusted odds of having a CBD prescription than nonpregnant women (95% confidence interval, 1.27-2.88).
She points to another cross-sectional study of more than 10,000 participants using PRAMS (Pregnancy Risk Assessment Monitoring System) data that found that “from 2017 to 2019, 63% of pregnant women reported that they were not told to avoid cannabis use in pregnancy, and 8% noted that they were advised to use cannabis by their prenatal care practitioner.”
The American College of Obstetricians and Gynecologists recommends against prescribing cannabis products for pregnant or lactating women.
Studies that have explored THC and its metabolites have shown “a consistent association between cannabis use and decreased fetal growth,” Dr. Metz noted. “There also remain persistent concerns about the long-term neurodevelopmental effects of maternal cannabis use on the fetus and, subsequently, the newborn.”
Limitations of the study include the self-reported responses and participants’ ability to accurately distinguish between CBD-only and THC-containing products.
Because self-reports of CBD use in pregnancy may be drastically underestimated and nonreliable, Dr. Metz writes, development of blood and urine screens to help detect CBD product use “will be helpful in moving the field forward.”
Study senior author David Hammond, PhD, has been a paid expert witness on behalf of public health authorities in response to legal challenges from the cannabis, tobacco, vaping, and food industries. Other authors did not report any potential conflicts. Dr. Metz reports personal fees from Pfizer, and grants from Pfizer for her role as a site principal investigator for SARS-CoV-2 vaccination and for her role as a site PI for RSV vaccination in pregnancy study.
FROM OBSTETRICS & GYNECOLOGY
Syphilis Treatment Falls Short for Pregnant Patients
Approximately one third of pregnant individuals with syphilis were inadequately treated or not treated for syphilis despite receiving timely prenatal care, based on data from nearly 1500 patients.
Although congenital syphilis is preventable with treatment before or early in pregnancy, data from the Centers for Disease Control and Prevention (CDC) show a doubling of syphilis rates in the United States between 2018 and 2021 wrote Ayzsa Tannis, MPH, of the Centers for Disease Control and Prevention, Atlanta, and colleagues.
To better understand factors contributing to inadequate syphilis treatment during pregnancy, the researchers examined data from 1476 individuals with syphilis during pregnancy. The study population came from six jurisdictions that participated in the Surveillance for Emerging Threats to Pregnant People and Infants Network, and sources included case investigations, medical records, and links between laboratory data and vital records.
The researchers characterized the status of syphilis during pregnancy as adequate, inadequate, or not treated based on the CDC’s Sexually Transmitted Infections Treatment Guidelines, 2021. Prenatal care was defined as timely (at least 30 days prior to pregnancy outcome), nontimely (less than 30 days before pregnancy outcome), and no prenatal care. The findings were published in Obstetrics & Gynecology.
Of the 1476 individuals studied, 855 (57.9%) were adequately treated for syphilis and 621 (42.1%) were inadequately or not treated.
Overall, 82% of the study population received timely prenatal care. However, 32.1% of those who received timely prenatal care were inadequately treated, including 14.8% who received no syphilis treatment. Individuals with nontimely or no prenatal care were significantly more likely to receive inadequate or no treatment for syphilis than those who received timely care (risk ratio, 2.50 and 2.73, respectively).
The findings were consistent with previous studies of missed opportunities for prevention and treatment, the researchers noted. Factors behind nontimely treatment (less than 30 days before pregnancy outcome) may include intermittent shortages of benzathine penicillin G, the standard treatment for syphilis, as well as the lack of time and administrative support for clinicians to communicate with patients and health departments, and to expedite treatment, the researchers wrote.
The results were limited by several factors including the use of data from six US jurisdictions that may not generalize to other areas, the variations in reporting years for the different jurisdictions, and variation in mandates for syphilis screening during pregnancy, the researchers noted.
More research is needed to improve syphilis testing itself, and to develop more treatment options, the researchers concluded. Partnerships among public health, patient advocacy groups, prenatal care clinicians, and other clinicians outside the prenatal care setting also are needed for effective intervention in pregnant individuals with syphilis, they said.
The study was carried out as part of the regular work of the CDC, supported by the Epidemiology and Laboratory Capacity for Prevention and Control of Emerging Infectious Diseases Cooperative Agreement and through contractual mechanisms including the Local Health Department Initiative to Chickasaw Health Consulting. The researchers had no financial conflicts to disclose.
Approximately one third of pregnant individuals with syphilis were inadequately treated or not treated for syphilis despite receiving timely prenatal care, based on data from nearly 1500 patients.
Although congenital syphilis is preventable with treatment before or early in pregnancy, data from the Centers for Disease Control and Prevention (CDC) show a doubling of syphilis rates in the United States between 2018 and 2021 wrote Ayzsa Tannis, MPH, of the Centers for Disease Control and Prevention, Atlanta, and colleagues.
To better understand factors contributing to inadequate syphilis treatment during pregnancy, the researchers examined data from 1476 individuals with syphilis during pregnancy. The study population came from six jurisdictions that participated in the Surveillance for Emerging Threats to Pregnant People and Infants Network, and sources included case investigations, medical records, and links between laboratory data and vital records.
The researchers characterized the status of syphilis during pregnancy as adequate, inadequate, or not treated based on the CDC’s Sexually Transmitted Infections Treatment Guidelines, 2021. Prenatal care was defined as timely (at least 30 days prior to pregnancy outcome), nontimely (less than 30 days before pregnancy outcome), and no prenatal care. The findings were published in Obstetrics & Gynecology.
Of the 1476 individuals studied, 855 (57.9%) were adequately treated for syphilis and 621 (42.1%) were inadequately or not treated.
Overall, 82% of the study population received timely prenatal care. However, 32.1% of those who received timely prenatal care were inadequately treated, including 14.8% who received no syphilis treatment. Individuals with nontimely or no prenatal care were significantly more likely to receive inadequate or no treatment for syphilis than those who received timely care (risk ratio, 2.50 and 2.73, respectively).
The findings were consistent with previous studies of missed opportunities for prevention and treatment, the researchers noted. Factors behind nontimely treatment (less than 30 days before pregnancy outcome) may include intermittent shortages of benzathine penicillin G, the standard treatment for syphilis, as well as the lack of time and administrative support for clinicians to communicate with patients and health departments, and to expedite treatment, the researchers wrote.
The results were limited by several factors including the use of data from six US jurisdictions that may not generalize to other areas, the variations in reporting years for the different jurisdictions, and variation in mandates for syphilis screening during pregnancy, the researchers noted.
More research is needed to improve syphilis testing itself, and to develop more treatment options, the researchers concluded. Partnerships among public health, patient advocacy groups, prenatal care clinicians, and other clinicians outside the prenatal care setting also are needed for effective intervention in pregnant individuals with syphilis, they said.
The study was carried out as part of the regular work of the CDC, supported by the Epidemiology and Laboratory Capacity for Prevention and Control of Emerging Infectious Diseases Cooperative Agreement and through contractual mechanisms including the Local Health Department Initiative to Chickasaw Health Consulting. The researchers had no financial conflicts to disclose.
Approximately one third of pregnant individuals with syphilis were inadequately treated or not treated for syphilis despite receiving timely prenatal care, based on data from nearly 1500 patients.
Although congenital syphilis is preventable with treatment before or early in pregnancy, data from the Centers for Disease Control and Prevention (CDC) show a doubling of syphilis rates in the United States between 2018 and 2021 wrote Ayzsa Tannis, MPH, of the Centers for Disease Control and Prevention, Atlanta, and colleagues.
To better understand factors contributing to inadequate syphilis treatment during pregnancy, the researchers examined data from 1476 individuals with syphilis during pregnancy. The study population came from six jurisdictions that participated in the Surveillance for Emerging Threats to Pregnant People and Infants Network, and sources included case investigations, medical records, and links between laboratory data and vital records.
The researchers characterized the status of syphilis during pregnancy as adequate, inadequate, or not treated based on the CDC’s Sexually Transmitted Infections Treatment Guidelines, 2021. Prenatal care was defined as timely (at least 30 days prior to pregnancy outcome), nontimely (less than 30 days before pregnancy outcome), and no prenatal care. The findings were published in Obstetrics & Gynecology.
Of the 1476 individuals studied, 855 (57.9%) were adequately treated for syphilis and 621 (42.1%) were inadequately or not treated.
Overall, 82% of the study population received timely prenatal care. However, 32.1% of those who received timely prenatal care were inadequately treated, including 14.8% who received no syphilis treatment. Individuals with nontimely or no prenatal care were significantly more likely to receive inadequate or no treatment for syphilis than those who received timely care (risk ratio, 2.50 and 2.73, respectively).
The findings were consistent with previous studies of missed opportunities for prevention and treatment, the researchers noted. Factors behind nontimely treatment (less than 30 days before pregnancy outcome) may include intermittent shortages of benzathine penicillin G, the standard treatment for syphilis, as well as the lack of time and administrative support for clinicians to communicate with patients and health departments, and to expedite treatment, the researchers wrote.
The results were limited by several factors including the use of data from six US jurisdictions that may not generalize to other areas, the variations in reporting years for the different jurisdictions, and variation in mandates for syphilis screening during pregnancy, the researchers noted.
More research is needed to improve syphilis testing itself, and to develop more treatment options, the researchers concluded. Partnerships among public health, patient advocacy groups, prenatal care clinicians, and other clinicians outside the prenatal care setting also are needed for effective intervention in pregnant individuals with syphilis, they said.
The study was carried out as part of the regular work of the CDC, supported by the Epidemiology and Laboratory Capacity for Prevention and Control of Emerging Infectious Diseases Cooperative Agreement and through contractual mechanisms including the Local Health Department Initiative to Chickasaw Health Consulting. The researchers had no financial conflicts to disclose.
FROM OBSTETRICS & GYNECOLOGY
New Research Dissects Transgenerational Obesity and Diabetes
FAIRFAX, VIRGINIA — Nearly 30 years ago, in a 1995 paper, the British physician-epidemiologist David Barker, MD, PhD, wrote about his fetal origins hypothesis — the idea that programs to address fetal undernutrition and low birth weight produced later coronary heart disease (BMJ 1995;311:171-4).
His hypothesis and subsequent research led to the concept of adult diseases of fetal origins, which today extends beyond low birth weight and implicates the in utero environment as a significant determinant of risk for adverse childhood and adult metabolic outcomes and for major chronic diseases, including diabetes and obesity. Studies have shown that the offspring of pregnant mothers with diabetes have a higher risk of developing obesity and diabetes themselves.
“It’s a whole discipline [of research],” E. Albert Reece, MD, PhD, MBA, of the University of Maryland School of Medicine (UMSOM), said in an interview. “But what we’ve never quite understood is the ‘how’ and ‘why’? What are the mechanisms driving the fetal origins of such adverse outcomes in offspring?
At the biennial meeting of the Diabetes in Pregnancy Study Group of North America (DPSG), investigators described studies underway that are digging deeper into the associations between the intrauterine milieu and longer-term offspring health — and that are searching for biological and molecular processes that may be involved.
The studies are like “branches of the Barker hypothesis,” said Dr. Reece, former dean of UMSOM and current director of the UMSOM Center for Advanced Research Training and Innovation, who co-organized the DPSG meeting. “They’re taking the hypothesis and dissecting it by asking, for instance, it is possible that transgenerational obesity may align with the Barker hypothesis? Is it possible that it involves epigenetics regulation? Could we find biomarkers?”
The need for a better understanding of the fetal origins framework — and its subsequent transgenerational impact — is urgent. From 2000 to 2018, the prevalence of childhood obesity increased from 14.7% to 19.2% (a 31% increase) and the prevalence of severe childhood obesity rose from 3.9% to 6.1% (a 56% increase), according to data from the U.S. National Health and Nutrition Examination Survey (Obes Facts. 2022;15[4]:560-9).
Children aged 2-5 years have had an especially sharp increase in obesity (Pediatrics 2018;141[3]:e20173459), Christine Wey Hockett, PhD, of the University of South Dakota School of Medicine, said at the DPSG meeting (Figure 1). 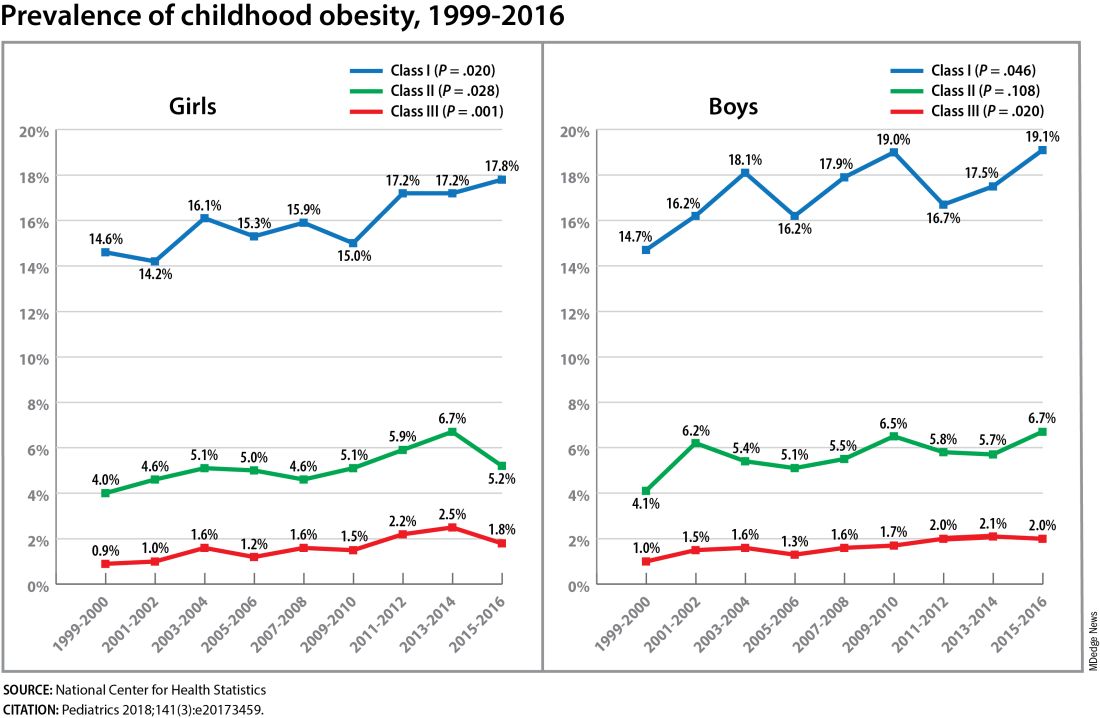
Also notable, she said, is that one-quarter of today’s pediatric diabetes cases are type 2 diabetes, which “is significant as there is a higher prevalence of early complications and comorbidities in youth with type 2 diabetes compared to type 1 diabetes.”
Moreover, recent projections estimate that 57% of today’s children will be obese at 35 years of age (N Engl J Med. 2017;377[22]:2145-53) and that 45% will have diabetes or prediabetes by 2030 (Popul Health Manag. 2017;20[1]:6-12), said Dr. Hockett, assistant professor in the university’s department of pediatrics. An investigator of the Exploring Perinatal Outcomes Among Children (EPOCH) study, which looked at gestational diabetes (GDM) and offspring cardiometabolic risks, she said more chronic disease “at increasingly younger ages [points toward] prebirth influences.”
She noted that there are critical periods postnatally — such as infancy and puberty — that can “impact or further shift the trajectory of chronic disease.” The developmental origins theory posits that life events and biological and environmental processes during the lifespan can modify the effects of intrauterine exposures.
The transgenerational implications “are clear,” she said. “As the number of reproductive-aged individuals with chronic diseases rises, the number of exposed offspring also rises ... It leads to a vicious cycle.”
Deeper Dives Into Associations, Potential Mechanisms
The EPOCH prospective cohort study with which Dr. Hockett was involved gave her a front-seat view of the transgenerational adverse effects of in utero exposure to hyperglycemia. The study recruited ethnically diverse maternal/child dyads from the Kaiser Permanente of Colorado perinatal database from 1992 to 2002 and assessed 418 offspring at two points — a mean age of 10.5 years and 16.5 years — for fasting blood glucose, adiposity, and diet and physical activity. The second visit also involved an oral glucose tolerance test.
The 77 offspring who had been exposed in utero to GDM had a homeostatic model assessment of insulin resistance (HOMA-IR) that was 18% higher, a 19% lower Matsuda index, and a 9% greater HOMA of β-cell function (HOMA-β) than the 341 offspring whose mothers did not have diabetes. Each 5-kg/m2 increase in prepregnancy body mass index predicted increased insulin resistance, but there was no combined effect of both maternal obesity and diabetes in utero.
Exposed offspring had a higher BMI and increased adiposity, but when BMI was controlled for in the analysis of metabolic outcomes, maternal diabetes was still associated with 12% higher HOMA-IR and a 17% lower Matsuda index. “So [the metabolic outcomes] are a direct effect of maternal diabetes,” Dr. Hockett said at the DPSG meeting, noting the fetal overnutrition hypothesis in which maternal glucose, but not maternal insulin, freely passes through the placenta, promoting growth and adiposity in the fetus.
[The EPOCH results on metabolic outcomes and offspring adiposity were published in 2017 and 2019, respectively (Diabet Med. 2017;34:1392-9; Diabetologia. 2019;62:2017-24). In 2020, EPOCH researchers reported sex-specific effects on cardiovascular outcomes, with GDM exposure associated with higher total and LDL cholesterol in girls and higher systolic blood pressure in boys (Pediatr Obes. 2020;15[5]:e12611).]
Now, a new longitudinal cohort study underway in Phoenix, is taking a deeper dive, trying to pinpoint what exactly influences childhood obesity and metabolic risk by following Hispanic and American Indian maternal/child dyads from pregnancy until 18 years postpartum. Researchers are looking not only at associations between maternal risk factors (pregnancy BMI, gestational weight gain, and diabetes in pregnancy) and offspring BMI, adiposity, and growth patterns, but also how various factors during pregnancy — clinical, genetic, lifestyle, biochemical — ”may mediate the associations,” said lead investigator Madhumita Sinha, MD.
“We need a better understanding at the molecular level of the biological processes that lead to obesity in children and that cause metabolic dysfunction,” said Dr. Sinha, who heads the Diabetes Epidemiology and Clinical Research Section of the of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) branch in Phoenix.
The populations being enrolled in the ETCHED study (for Early Tracking of Childhood Health Determinants) are at especially high risk of childhood obesity and metabolic dysfunction. Research conducted decades ago by the NIDDK in Phoenix showed that approximately 50% of Pima Indian children from diabetic pregnancies develop type 2 diabetes by age 25 (N Engl J Med. 1983;308:242-5). Years later, to tease out possible genetic factors, researchers compared siblings born before and after their mother was found to have type 2 diabetes, and found significantly higher rates of diabetes in those born after the mother’s diagnosis, affirming the role of in utero toxicity (Diabetes 2000;49:2208-11).
In the new study, the researchers will look at adipokines and inflammatory biomarkers in the mothers and offspring in addition to traditional anthropometric and glycemic measures. They’ll analyze placental tissue, breast milk, and the gut microbiome longitudinally, and they’ll lean heavily on genomics/epigenomics, proteomics, and metabolomics. “There’s potential,” Dr. Sinha said, “to develop a more accurate predictive and prognostic model of childhood obesity.”
The researchers also will study the role of family, socioeconomics, and environmental factors in influencing child growth patterns and they’ll look at neurodevelopment in infancy and childhood. As of October 2023, almost 80 pregnant women, most with obesity and almost one-third with type 2 diabetes, had enrolled in the study. Over the next several years, the study aims to enroll 750 dyads.
The Timing of In Utero Exposure
Shelley Ehrlich, MD, ScD, MPH, of the University of Cincinnati and Cincinnati Children’s Hospital Medical Center, is aiming, meanwhile, to learn how the timing of in utero exposure to hyperglycemia predicts specific metabolic and cardiovascular morbidities in the adult offspring of diabetic mothers.
“While we know that exposure to maternal diabetes, regardless of type, increases the risk of obesity, insulin resistance, diabetes, renal compromise, and cardiovascular disease in the offspring, there is little known about the level and timing of hyperglycemic exposure during fetal development that triggers these adverse outcomes,” said Dr. Ehrlich. A goal, she said, is to identify gestational profiles that predict phenotypes of offspring at risk for morbidity in later life.
She and other investigators with the TEAM (Transgenerational Effect on Adult Morbidity) study have recruited over 170 offspring of mothers who participated in the Diabetes in Pregnancy Program Project Grant (PPG) at the University of Cincinnati Medical Center from 1978 to 1995 — a landmark study that demonstrated the effect of strict glucose control in reducing major congenital malformations.
The women in the PPG study had frequent glucose monitoring (up to 6-8 times a day) throughout their pregnancies, and now, their recruited offspring, who are up to 43 years of age, are being assessed for obesity, diabetes/metabolic health, cardiovascular disease/cardiac and peripheral vascular structure and function, and other outcomes including those that may be amenable to secondary prevention (J Diabetes Res. Nov 1;2021:6590431).
Preliminary findings from over 170 offspring recruited between 2017 and 2022 suggest that in utero exposure to dysglycemia (as measured by standard deviations of glycohemoglobin) in the third trimester appears to increase the risk of morbid obesity in adulthood, while exposure to dysglycemia in the first trimester increases the risk of impaired glucose tolerance. The risk of B-cell dysfunction, meanwhile, appears to be linked to dysglycemia in the first and third trimesters — particularly the first — Dr. Ehrlich reported.
Cognitive outcomes in offspring have also been assessed and here it appears that dysglycemia in the third trimester is linked to worse scores on the Wechsler Abbreviated Scale of Intelligence (WASI-II), said Katherine Bowers, PhD, MPH, a TEAM study coinvestigator, also of Cincinnati Children’s Hospital Medical Center.
“We’ve already observed [an association between] diabetes in pregnancy and cognition in early childhood and through adolescence, but [the question has been] does this association persist into adulthood?” she said.
Preliminary analyses of 104 offspring show no statistically significant associations between maternal dysglycemia in the first or second trimesters and offspring cognition, but “consistent inverse associations between maternal glycohemoglobin in the third trimester across two [WASI-II] subscales and composite measures of cognition,” Dr. Bowers said.
Their analysis adjusted for a variety of factors, including maternal age, prepregnancy and first trimester BMI, race, family history of diabetes, and diabetes severity/macrovascular complications.
Back In The Laboratory
At the other end of the research spectrum, basic research scientists are also investigating the mechanisms and sequelae of in utero hyperglycemia and other injuries, including congenital malformations, placental adaptive responses and fetal programming. Researchers are asking, for instance, what does placental metabolic reprogramming entail? What role do placental extracellular vesicles play in GDM? Can we alter the in utero environment and thus improve the short and long-term fetal/infant outcomes?
Animal research done at the UMSOM Center for Birth Defects Research, led by Dr. Reece and Peixin Yang, PhD, suggests that “a good portion of in utero injury is due to epigenetics,” Dr. Reece said in the interview. “We’ve shown that under conditions of hyperglycemia, for example, genetic regulation and genetic function can be altered.”
Through in vivo research, they have also shown that antioxidants or membrane stabilizers such as arachidonic acid or myo-inositol, or experimental inhibitors to certain pro-apoptotic intermediates, can individually or collectively result in reduced malformations. “It is highly likely that understanding the biological impact of various altered in utero environments, and then modifying or reversing those environments, will result in short and long-term outcome improvements similar to those shown with congenital malformations,” Dr. Reece said.
FAIRFAX, VIRGINIA — Nearly 30 years ago, in a 1995 paper, the British physician-epidemiologist David Barker, MD, PhD, wrote about his fetal origins hypothesis — the idea that programs to address fetal undernutrition and low birth weight produced later coronary heart disease (BMJ 1995;311:171-4).
His hypothesis and subsequent research led to the concept of adult diseases of fetal origins, which today extends beyond low birth weight and implicates the in utero environment as a significant determinant of risk for adverse childhood and adult metabolic outcomes and for major chronic diseases, including diabetes and obesity. Studies have shown that the offspring of pregnant mothers with diabetes have a higher risk of developing obesity and diabetes themselves.
“It’s a whole discipline [of research],” E. Albert Reece, MD, PhD, MBA, of the University of Maryland School of Medicine (UMSOM), said in an interview. “But what we’ve never quite understood is the ‘how’ and ‘why’? What are the mechanisms driving the fetal origins of such adverse outcomes in offspring?
At the biennial meeting of the Diabetes in Pregnancy Study Group of North America (DPSG), investigators described studies underway that are digging deeper into the associations between the intrauterine milieu and longer-term offspring health — and that are searching for biological and molecular processes that may be involved.
The studies are like “branches of the Barker hypothesis,” said Dr. Reece, former dean of UMSOM and current director of the UMSOM Center for Advanced Research Training and Innovation, who co-organized the DPSG meeting. “They’re taking the hypothesis and dissecting it by asking, for instance, it is possible that transgenerational obesity may align with the Barker hypothesis? Is it possible that it involves epigenetics regulation? Could we find biomarkers?”
The need for a better understanding of the fetal origins framework — and its subsequent transgenerational impact — is urgent. From 2000 to 2018, the prevalence of childhood obesity increased from 14.7% to 19.2% (a 31% increase) and the prevalence of severe childhood obesity rose from 3.9% to 6.1% (a 56% increase), according to data from the U.S. National Health and Nutrition Examination Survey (Obes Facts. 2022;15[4]:560-9).
Children aged 2-5 years have had an especially sharp increase in obesity (Pediatrics 2018;141[3]:e20173459), Christine Wey Hockett, PhD, of the University of South Dakota School of Medicine, said at the DPSG meeting (Figure 1). 
Also notable, she said, is that one-quarter of today’s pediatric diabetes cases are type 2 diabetes, which “is significant as there is a higher prevalence of early complications and comorbidities in youth with type 2 diabetes compared to type 1 diabetes.”
Moreover, recent projections estimate that 57% of today’s children will be obese at 35 years of age (N Engl J Med. 2017;377[22]:2145-53) and that 45% will have diabetes or prediabetes by 2030 (Popul Health Manag. 2017;20[1]:6-12), said Dr. Hockett, assistant professor in the university’s department of pediatrics. An investigator of the Exploring Perinatal Outcomes Among Children (EPOCH) study, which looked at gestational diabetes (GDM) and offspring cardiometabolic risks, she said more chronic disease “at increasingly younger ages [points toward] prebirth influences.”
She noted that there are critical periods postnatally — such as infancy and puberty — that can “impact or further shift the trajectory of chronic disease.” The developmental origins theory posits that life events and biological and environmental processes during the lifespan can modify the effects of intrauterine exposures.
The transgenerational implications “are clear,” she said. “As the number of reproductive-aged individuals with chronic diseases rises, the number of exposed offspring also rises ... It leads to a vicious cycle.”
Deeper Dives Into Associations, Potential Mechanisms
The EPOCH prospective cohort study with which Dr. Hockett was involved gave her a front-seat view of the transgenerational adverse effects of in utero exposure to hyperglycemia. The study recruited ethnically diverse maternal/child dyads from the Kaiser Permanente of Colorado perinatal database from 1992 to 2002 and assessed 418 offspring at two points — a mean age of 10.5 years and 16.5 years — for fasting blood glucose, adiposity, and diet and physical activity. The second visit also involved an oral glucose tolerance test.
The 77 offspring who had been exposed in utero to GDM had a homeostatic model assessment of insulin resistance (HOMA-IR) that was 18% higher, a 19% lower Matsuda index, and a 9% greater HOMA of β-cell function (HOMA-β) than the 341 offspring whose mothers did not have diabetes. Each 5-kg/m2 increase in prepregnancy body mass index predicted increased insulin resistance, but there was no combined effect of both maternal obesity and diabetes in utero.
Exposed offspring had a higher BMI and increased adiposity, but when BMI was controlled for in the analysis of metabolic outcomes, maternal diabetes was still associated with 12% higher HOMA-IR and a 17% lower Matsuda index. “So [the metabolic outcomes] are a direct effect of maternal diabetes,” Dr. Hockett said at the DPSG meeting, noting the fetal overnutrition hypothesis in which maternal glucose, but not maternal insulin, freely passes through the placenta, promoting growth and adiposity in the fetus.
[The EPOCH results on metabolic outcomes and offspring adiposity were published in 2017 and 2019, respectively (Diabet Med. 2017;34:1392-9; Diabetologia. 2019;62:2017-24). In 2020, EPOCH researchers reported sex-specific effects on cardiovascular outcomes, with GDM exposure associated with higher total and LDL cholesterol in girls and higher systolic blood pressure in boys (Pediatr Obes. 2020;15[5]:e12611).]
Now, a new longitudinal cohort study underway in Phoenix, is taking a deeper dive, trying to pinpoint what exactly influences childhood obesity and metabolic risk by following Hispanic and American Indian maternal/child dyads from pregnancy until 18 years postpartum. Researchers are looking not only at associations between maternal risk factors (pregnancy BMI, gestational weight gain, and diabetes in pregnancy) and offspring BMI, adiposity, and growth patterns, but also how various factors during pregnancy — clinical, genetic, lifestyle, biochemical — ”may mediate the associations,” said lead investigator Madhumita Sinha, MD.
“We need a better understanding at the molecular level of the biological processes that lead to obesity in children and that cause metabolic dysfunction,” said Dr. Sinha, who heads the Diabetes Epidemiology and Clinical Research Section of the of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) branch in Phoenix.
The populations being enrolled in the ETCHED study (for Early Tracking of Childhood Health Determinants) are at especially high risk of childhood obesity and metabolic dysfunction. Research conducted decades ago by the NIDDK in Phoenix showed that approximately 50% of Pima Indian children from diabetic pregnancies develop type 2 diabetes by age 25 (N Engl J Med. 1983;308:242-5). Years later, to tease out possible genetic factors, researchers compared siblings born before and after their mother was found to have type 2 diabetes, and found significantly higher rates of diabetes in those born after the mother’s diagnosis, affirming the role of in utero toxicity (Diabetes 2000;49:2208-11).
In the new study, the researchers will look at adipokines and inflammatory biomarkers in the mothers and offspring in addition to traditional anthropometric and glycemic measures. They’ll analyze placental tissue, breast milk, and the gut microbiome longitudinally, and they’ll lean heavily on genomics/epigenomics, proteomics, and metabolomics. “There’s potential,” Dr. Sinha said, “to develop a more accurate predictive and prognostic model of childhood obesity.”
The researchers also will study the role of family, socioeconomics, and environmental factors in influencing child growth patterns and they’ll look at neurodevelopment in infancy and childhood. As of October 2023, almost 80 pregnant women, most with obesity and almost one-third with type 2 diabetes, had enrolled in the study. Over the next several years, the study aims to enroll 750 dyads.
The Timing of In Utero Exposure
Shelley Ehrlich, MD, ScD, MPH, of the University of Cincinnati and Cincinnati Children’s Hospital Medical Center, is aiming, meanwhile, to learn how the timing of in utero exposure to hyperglycemia predicts specific metabolic and cardiovascular morbidities in the adult offspring of diabetic mothers.
“While we know that exposure to maternal diabetes, regardless of type, increases the risk of obesity, insulin resistance, diabetes, renal compromise, and cardiovascular disease in the offspring, there is little known about the level and timing of hyperglycemic exposure during fetal development that triggers these adverse outcomes,” said Dr. Ehrlich. A goal, she said, is to identify gestational profiles that predict phenotypes of offspring at risk for morbidity in later life.
She and other investigators with the TEAM (Transgenerational Effect on Adult Morbidity) study have recruited over 170 offspring of mothers who participated in the Diabetes in Pregnancy Program Project Grant (PPG) at the University of Cincinnati Medical Center from 1978 to 1995 — a landmark study that demonstrated the effect of strict glucose control in reducing major congenital malformations.
The women in the PPG study had frequent glucose monitoring (up to 6-8 times a day) throughout their pregnancies, and now, their recruited offspring, who are up to 43 years of age, are being assessed for obesity, diabetes/metabolic health, cardiovascular disease/cardiac and peripheral vascular structure and function, and other outcomes including those that may be amenable to secondary prevention (J Diabetes Res. Nov 1;2021:6590431).
Preliminary findings from over 170 offspring recruited between 2017 and 2022 suggest that in utero exposure to dysglycemia (as measured by standard deviations of glycohemoglobin) in the third trimester appears to increase the risk of morbid obesity in adulthood, while exposure to dysglycemia in the first trimester increases the risk of impaired glucose tolerance. The risk of B-cell dysfunction, meanwhile, appears to be linked to dysglycemia in the first and third trimesters — particularly the first — Dr. Ehrlich reported.
Cognitive outcomes in offspring have also been assessed and here it appears that dysglycemia in the third trimester is linked to worse scores on the Wechsler Abbreviated Scale of Intelligence (WASI-II), said Katherine Bowers, PhD, MPH, a TEAM study coinvestigator, also of Cincinnati Children’s Hospital Medical Center.
“We’ve already observed [an association between] diabetes in pregnancy and cognition in early childhood and through adolescence, but [the question has been] does this association persist into adulthood?” she said.
Preliminary analyses of 104 offspring show no statistically significant associations between maternal dysglycemia in the first or second trimesters and offspring cognition, but “consistent inverse associations between maternal glycohemoglobin in the third trimester across two [WASI-II] subscales and composite measures of cognition,” Dr. Bowers said.
Their analysis adjusted for a variety of factors, including maternal age, prepregnancy and first trimester BMI, race, family history of diabetes, and diabetes severity/macrovascular complications.
Back In The Laboratory
At the other end of the research spectrum, basic research scientists are also investigating the mechanisms and sequelae of in utero hyperglycemia and other injuries, including congenital malformations, placental adaptive responses and fetal programming. Researchers are asking, for instance, what does placental metabolic reprogramming entail? What role do placental extracellular vesicles play in GDM? Can we alter the in utero environment and thus improve the short and long-term fetal/infant outcomes?
Animal research done at the UMSOM Center for Birth Defects Research, led by Dr. Reece and Peixin Yang, PhD, suggests that “a good portion of in utero injury is due to epigenetics,” Dr. Reece said in the interview. “We’ve shown that under conditions of hyperglycemia, for example, genetic regulation and genetic function can be altered.”
Through in vivo research, they have also shown that antioxidants or membrane stabilizers such as arachidonic acid or myo-inositol, or experimental inhibitors to certain pro-apoptotic intermediates, can individually or collectively result in reduced malformations. “It is highly likely that understanding the biological impact of various altered in utero environments, and then modifying or reversing those environments, will result in short and long-term outcome improvements similar to those shown with congenital malformations,” Dr. Reece said.
FAIRFAX, VIRGINIA — Nearly 30 years ago, in a 1995 paper, the British physician-epidemiologist David Barker, MD, PhD, wrote about his fetal origins hypothesis — the idea that programs to address fetal undernutrition and low birth weight produced later coronary heart disease (BMJ 1995;311:171-4).
His hypothesis and subsequent research led to the concept of adult diseases of fetal origins, which today extends beyond low birth weight and implicates the in utero environment as a significant determinant of risk for adverse childhood and adult metabolic outcomes and for major chronic diseases, including diabetes and obesity. Studies have shown that the offspring of pregnant mothers with diabetes have a higher risk of developing obesity and diabetes themselves.
“It’s a whole discipline [of research],” E. Albert Reece, MD, PhD, MBA, of the University of Maryland School of Medicine (UMSOM), said in an interview. “But what we’ve never quite understood is the ‘how’ and ‘why’? What are the mechanisms driving the fetal origins of such adverse outcomes in offspring?
At the biennial meeting of the Diabetes in Pregnancy Study Group of North America (DPSG), investigators described studies underway that are digging deeper into the associations between the intrauterine milieu and longer-term offspring health — and that are searching for biological and molecular processes that may be involved.
The studies are like “branches of the Barker hypothesis,” said Dr. Reece, former dean of UMSOM and current director of the UMSOM Center for Advanced Research Training and Innovation, who co-organized the DPSG meeting. “They’re taking the hypothesis and dissecting it by asking, for instance, it is possible that transgenerational obesity may align with the Barker hypothesis? Is it possible that it involves epigenetics regulation? Could we find biomarkers?”
The need for a better understanding of the fetal origins framework — and its subsequent transgenerational impact — is urgent. From 2000 to 2018, the prevalence of childhood obesity increased from 14.7% to 19.2% (a 31% increase) and the prevalence of severe childhood obesity rose from 3.9% to 6.1% (a 56% increase), according to data from the U.S. National Health and Nutrition Examination Survey (Obes Facts. 2022;15[4]:560-9).
Children aged 2-5 years have had an especially sharp increase in obesity (Pediatrics 2018;141[3]:e20173459), Christine Wey Hockett, PhD, of the University of South Dakota School of Medicine, said at the DPSG meeting (Figure 1). 
Also notable, she said, is that one-quarter of today’s pediatric diabetes cases are type 2 diabetes, which “is significant as there is a higher prevalence of early complications and comorbidities in youth with type 2 diabetes compared to type 1 diabetes.”
Moreover, recent projections estimate that 57% of today’s children will be obese at 35 years of age (N Engl J Med. 2017;377[22]:2145-53) and that 45% will have diabetes or prediabetes by 2030 (Popul Health Manag. 2017;20[1]:6-12), said Dr. Hockett, assistant professor in the university’s department of pediatrics. An investigator of the Exploring Perinatal Outcomes Among Children (EPOCH) study, which looked at gestational diabetes (GDM) and offspring cardiometabolic risks, she said more chronic disease “at increasingly younger ages [points toward] prebirth influences.”
She noted that there are critical periods postnatally — such as infancy and puberty — that can “impact or further shift the trajectory of chronic disease.” The developmental origins theory posits that life events and biological and environmental processes during the lifespan can modify the effects of intrauterine exposures.
The transgenerational implications “are clear,” she said. “As the number of reproductive-aged individuals with chronic diseases rises, the number of exposed offspring also rises ... It leads to a vicious cycle.”
Deeper Dives Into Associations, Potential Mechanisms
The EPOCH prospective cohort study with which Dr. Hockett was involved gave her a front-seat view of the transgenerational adverse effects of in utero exposure to hyperglycemia. The study recruited ethnically diverse maternal/child dyads from the Kaiser Permanente of Colorado perinatal database from 1992 to 2002 and assessed 418 offspring at two points — a mean age of 10.5 years and 16.5 years — for fasting blood glucose, adiposity, and diet and physical activity. The second visit also involved an oral glucose tolerance test.
The 77 offspring who had been exposed in utero to GDM had a homeostatic model assessment of insulin resistance (HOMA-IR) that was 18% higher, a 19% lower Matsuda index, and a 9% greater HOMA of β-cell function (HOMA-β) than the 341 offspring whose mothers did not have diabetes. Each 5-kg/m2 increase in prepregnancy body mass index predicted increased insulin resistance, but there was no combined effect of both maternal obesity and diabetes in utero.
Exposed offspring had a higher BMI and increased adiposity, but when BMI was controlled for in the analysis of metabolic outcomes, maternal diabetes was still associated with 12% higher HOMA-IR and a 17% lower Matsuda index. “So [the metabolic outcomes] are a direct effect of maternal diabetes,” Dr. Hockett said at the DPSG meeting, noting the fetal overnutrition hypothesis in which maternal glucose, but not maternal insulin, freely passes through the placenta, promoting growth and adiposity in the fetus.
[The EPOCH results on metabolic outcomes and offspring adiposity were published in 2017 and 2019, respectively (Diabet Med. 2017;34:1392-9; Diabetologia. 2019;62:2017-24). In 2020, EPOCH researchers reported sex-specific effects on cardiovascular outcomes, with GDM exposure associated with higher total and LDL cholesterol in girls and higher systolic blood pressure in boys (Pediatr Obes. 2020;15[5]:e12611).]
Now, a new longitudinal cohort study underway in Phoenix, is taking a deeper dive, trying to pinpoint what exactly influences childhood obesity and metabolic risk by following Hispanic and American Indian maternal/child dyads from pregnancy until 18 years postpartum. Researchers are looking not only at associations between maternal risk factors (pregnancy BMI, gestational weight gain, and diabetes in pregnancy) and offspring BMI, adiposity, and growth patterns, but also how various factors during pregnancy — clinical, genetic, lifestyle, biochemical — ”may mediate the associations,” said lead investigator Madhumita Sinha, MD.
“We need a better understanding at the molecular level of the biological processes that lead to obesity in children and that cause metabolic dysfunction,” said Dr. Sinha, who heads the Diabetes Epidemiology and Clinical Research Section of the of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) branch in Phoenix.
The populations being enrolled in the ETCHED study (for Early Tracking of Childhood Health Determinants) are at especially high risk of childhood obesity and metabolic dysfunction. Research conducted decades ago by the NIDDK in Phoenix showed that approximately 50% of Pima Indian children from diabetic pregnancies develop type 2 diabetes by age 25 (N Engl J Med. 1983;308:242-5). Years later, to tease out possible genetic factors, researchers compared siblings born before and after their mother was found to have type 2 diabetes, and found significantly higher rates of diabetes in those born after the mother’s diagnosis, affirming the role of in utero toxicity (Diabetes 2000;49:2208-11).
In the new study, the researchers will look at adipokines and inflammatory biomarkers in the mothers and offspring in addition to traditional anthropometric and glycemic measures. They’ll analyze placental tissue, breast milk, and the gut microbiome longitudinally, and they’ll lean heavily on genomics/epigenomics, proteomics, and metabolomics. “There’s potential,” Dr. Sinha said, “to develop a more accurate predictive and prognostic model of childhood obesity.”
The researchers also will study the role of family, socioeconomics, and environmental factors in influencing child growth patterns and they’ll look at neurodevelopment in infancy and childhood. As of October 2023, almost 80 pregnant women, most with obesity and almost one-third with type 2 diabetes, had enrolled in the study. Over the next several years, the study aims to enroll 750 dyads.
The Timing of In Utero Exposure
Shelley Ehrlich, MD, ScD, MPH, of the University of Cincinnati and Cincinnati Children’s Hospital Medical Center, is aiming, meanwhile, to learn how the timing of in utero exposure to hyperglycemia predicts specific metabolic and cardiovascular morbidities in the adult offspring of diabetic mothers.
“While we know that exposure to maternal diabetes, regardless of type, increases the risk of obesity, insulin resistance, diabetes, renal compromise, and cardiovascular disease in the offspring, there is little known about the level and timing of hyperglycemic exposure during fetal development that triggers these adverse outcomes,” said Dr. Ehrlich. A goal, she said, is to identify gestational profiles that predict phenotypes of offspring at risk for morbidity in later life.
She and other investigators with the TEAM (Transgenerational Effect on Adult Morbidity) study have recruited over 170 offspring of mothers who participated in the Diabetes in Pregnancy Program Project Grant (PPG) at the University of Cincinnati Medical Center from 1978 to 1995 — a landmark study that demonstrated the effect of strict glucose control in reducing major congenital malformations.
The women in the PPG study had frequent glucose monitoring (up to 6-8 times a day) throughout their pregnancies, and now, their recruited offspring, who are up to 43 years of age, are being assessed for obesity, diabetes/metabolic health, cardiovascular disease/cardiac and peripheral vascular structure and function, and other outcomes including those that may be amenable to secondary prevention (J Diabetes Res. Nov 1;2021:6590431).
Preliminary findings from over 170 offspring recruited between 2017 and 2022 suggest that in utero exposure to dysglycemia (as measured by standard deviations of glycohemoglobin) in the third trimester appears to increase the risk of morbid obesity in adulthood, while exposure to dysglycemia in the first trimester increases the risk of impaired glucose tolerance. The risk of B-cell dysfunction, meanwhile, appears to be linked to dysglycemia in the first and third trimesters — particularly the first — Dr. Ehrlich reported.
Cognitive outcomes in offspring have also been assessed and here it appears that dysglycemia in the third trimester is linked to worse scores on the Wechsler Abbreviated Scale of Intelligence (WASI-II), said Katherine Bowers, PhD, MPH, a TEAM study coinvestigator, also of Cincinnati Children’s Hospital Medical Center.
“We’ve already observed [an association between] diabetes in pregnancy and cognition in early childhood and through adolescence, but [the question has been] does this association persist into adulthood?” she said.
Preliminary analyses of 104 offspring show no statistically significant associations between maternal dysglycemia in the first or second trimesters and offspring cognition, but “consistent inverse associations between maternal glycohemoglobin in the third trimester across two [WASI-II] subscales and composite measures of cognition,” Dr. Bowers said.
Their analysis adjusted for a variety of factors, including maternal age, prepregnancy and first trimester BMI, race, family history of diabetes, and diabetes severity/macrovascular complications.
Back In The Laboratory
At the other end of the research spectrum, basic research scientists are also investigating the mechanisms and sequelae of in utero hyperglycemia and other injuries, including congenital malformations, placental adaptive responses and fetal programming. Researchers are asking, for instance, what does placental metabolic reprogramming entail? What role do placental extracellular vesicles play in GDM? Can we alter the in utero environment and thus improve the short and long-term fetal/infant outcomes?
Animal research done at the UMSOM Center for Birth Defects Research, led by Dr. Reece and Peixin Yang, PhD, suggests that “a good portion of in utero injury is due to epigenetics,” Dr. Reece said in the interview. “We’ve shown that under conditions of hyperglycemia, for example, genetic regulation and genetic function can be altered.”
Through in vivo research, they have also shown that antioxidants or membrane stabilizers such as arachidonic acid or myo-inositol, or experimental inhibitors to certain pro-apoptotic intermediates, can individually or collectively result in reduced malformations. “It is highly likely that understanding the biological impact of various altered in utero environments, and then modifying or reversing those environments, will result in short and long-term outcome improvements similar to those shown with congenital malformations,” Dr. Reece said.
FROM DPSG-NA 2023
Targeting Fetus-derived Gdf15 May Curb Nausea and Vomiting of Pregnancy
, and targeting the hormone prophylactically may reduce this common gestational condition.
This protein acts on the brainstem to cause emesis, and, significantly, a mother’s prior exposure to it determines the degree of NVP severity she will experience, according to international researchers including Marlena Fejzo, PhD, a clinical assistant professor of population and public Health at Keck School of Medicine, University of Southern California, Los Angeles.
“GDF15 is at the mechanistic heart of NVP and HG [hyperemesis gravidarum],” Dr. Fejzo and colleagues wrote in Nature, pointing to the need for preventive and therapeutic strategies.
“My previous research showed an association between variation in the GDF15 gene and nausea and vomiting of pregnancy and HG, and this study takes it one step further by elucidating the mechanism. It confirms that the nausea and vomiting (N/V) hormone GDF15 is a major cause of NVP and HG,” Dr. Fejzo said.
The etiology of NVP remains poorly understood although it affects up to 80% of pregnancies. In the US, its severe form, HG, is the leading cause of hospitalization in early pregnancy and the second-leading reason for pregnancy hospitalization overall.
The immunoassay-based study showed that the majority of GDF15 in maternal blood during pregnancy comes from the fetal part of the placenta, and confirms previous studies reporting higher levels in pregnancies with more severe NVP, said Dr. Fejzo, who is who is a board member of the Hyperemesis Education and Research Foundation.
“However, what was really fascinating and surprising is that prior to pregnancy the women who have more severe NVP symptoms actually have lower levels of the hormone.”
Although the gene variant linked to HG was previously associated with higher circulating levels in maternal blood, counterintuitively, this new research showed that women with abnormally high levels prior to pregnancy have either no or very little NVP, said Dr. Fejzo. “That suggests that in humans higher levels may lead to a desensitization to the high levels of the hormone in pregnancy. Then we also proved that desensitization can occur in a mouse model.”
According to Erin Higgins, MD, a clinical assistant professor of obgyn and reproductive biology at the Cleveland Clinic, Cleveland, Ohio, who was not involved in the study, “This is an exciting finding that may help us to better target treatment of N/V in pregnancy. Factors for NVP have been identified, but to my knowledge there has not been a clear etiology.”
Dr. Higgins cautioned, however, that the GDF15 gene seems important in normal placentation, “so it’s not as simple as blocking the gene or its receptor.” But since preconception exposure to GDF15 might decrease nausea and vomiting once a woman is pregnant, prophylactic treatment may be possible, and metformin has been suggested as a possibility, she said.
The study findings emerged from immunoassays on maternal blood samples collected at about 15 weeks (first trimester and early second trimester), from women with NVP (n = 168) or seen at a hospital for HG (n = 57). Results were compared with those from controls having similar characteristics but no significant symptoms.
Interestingly, GDF15 is also associated with cachexia, a condition similar to HG and characterized by loss of appetite and weight loss, Dr. Fejzo noted. “The hormone can be produced by malignant tumors at levels similar to those seen in pregnancy, and symptoms can be reduced by blocking GDF15 or its receptor, GFRAL. Clinical trials are already underway in cancer patients to test this.”
She is seeking funding to test the impact of increasing GDF15 levels prior to pregnancy in patients who previously experienced HG. “I am confident that desensitizing patients by increasing GDF15 prior to pregnancy and by lowering GDF15 levels during pregnancy will work. But we need to make sure we do safety studies and get the dosing and duration right, and that will take some time.”
Desensitization will need testing first in HG, where the risk for adverse maternal and fetal outcomes is high, so the benefit will outweigh any possible risk of testing medication in pregnancy, she continued. “It will take some time before we get to patients with normal NVP, but I do believe eventually the new findings will result in game-changing therapeutics for the condition.”
Dr. Higgins added, “Even if this isn’t the golden ticket, researchers and clinicians are working toward improvements in the treatment of NVP. We’ve already come a long way in recent years with the development of treatment algorithms and the advent of doxylamine/pyridoxine.”
This study was supported primarily by the Medical Research Council UK and National Institute for Health and Care Research UK, with additional support from various research funding organizations, including Novo Nordisk Foundation.
Dr. Fejzo is a paid consultant for Materna Biosciences and NGM Biopharmaceuticals, and a board member and science adviser for the Hyperemesis Education and Research Foundation.
Numerous study co-authors disclosed financial relationships with private-sector companies, including employment and patent ownership.
Dr. Higgins disclosed no competing interests relevant to her comments but is an instructor for Organon.
, and targeting the hormone prophylactically may reduce this common gestational condition.
This protein acts on the brainstem to cause emesis, and, significantly, a mother’s prior exposure to it determines the degree of NVP severity she will experience, according to international researchers including Marlena Fejzo, PhD, a clinical assistant professor of population and public Health at Keck School of Medicine, University of Southern California, Los Angeles.
“GDF15 is at the mechanistic heart of NVP and HG [hyperemesis gravidarum],” Dr. Fejzo and colleagues wrote in Nature, pointing to the need for preventive and therapeutic strategies.
“My previous research showed an association between variation in the GDF15 gene and nausea and vomiting of pregnancy and HG, and this study takes it one step further by elucidating the mechanism. It confirms that the nausea and vomiting (N/V) hormone GDF15 is a major cause of NVP and HG,” Dr. Fejzo said.
The etiology of NVP remains poorly understood although it affects up to 80% of pregnancies. In the US, its severe form, HG, is the leading cause of hospitalization in early pregnancy and the second-leading reason for pregnancy hospitalization overall.
The immunoassay-based study showed that the majority of GDF15 in maternal blood during pregnancy comes from the fetal part of the placenta, and confirms previous studies reporting higher levels in pregnancies with more severe NVP, said Dr. Fejzo, who is who is a board member of the Hyperemesis Education and Research Foundation.
“However, what was really fascinating and surprising is that prior to pregnancy the women who have more severe NVP symptoms actually have lower levels of the hormone.”
Although the gene variant linked to HG was previously associated with higher circulating levels in maternal blood, counterintuitively, this new research showed that women with abnormally high levels prior to pregnancy have either no or very little NVP, said Dr. Fejzo. “That suggests that in humans higher levels may lead to a desensitization to the high levels of the hormone in pregnancy. Then we also proved that desensitization can occur in a mouse model.”
According to Erin Higgins, MD, a clinical assistant professor of obgyn and reproductive biology at the Cleveland Clinic, Cleveland, Ohio, who was not involved in the study, “This is an exciting finding that may help us to better target treatment of N/V in pregnancy. Factors for NVP have been identified, but to my knowledge there has not been a clear etiology.”
Dr. Higgins cautioned, however, that the GDF15 gene seems important in normal placentation, “so it’s not as simple as blocking the gene or its receptor.” But since preconception exposure to GDF15 might decrease nausea and vomiting once a woman is pregnant, prophylactic treatment may be possible, and metformin has been suggested as a possibility, she said.
The study findings emerged from immunoassays on maternal blood samples collected at about 15 weeks (first trimester and early second trimester), from women with NVP (n = 168) or seen at a hospital for HG (n = 57). Results were compared with those from controls having similar characteristics but no significant symptoms.
Interestingly, GDF15 is also associated with cachexia, a condition similar to HG and characterized by loss of appetite and weight loss, Dr. Fejzo noted. “The hormone can be produced by malignant tumors at levels similar to those seen in pregnancy, and symptoms can be reduced by blocking GDF15 or its receptor, GFRAL. Clinical trials are already underway in cancer patients to test this.”
She is seeking funding to test the impact of increasing GDF15 levels prior to pregnancy in patients who previously experienced HG. “I am confident that desensitizing patients by increasing GDF15 prior to pregnancy and by lowering GDF15 levels during pregnancy will work. But we need to make sure we do safety studies and get the dosing and duration right, and that will take some time.”
Desensitization will need testing first in HG, where the risk for adverse maternal and fetal outcomes is high, so the benefit will outweigh any possible risk of testing medication in pregnancy, she continued. “It will take some time before we get to patients with normal NVP, but I do believe eventually the new findings will result in game-changing therapeutics for the condition.”
Dr. Higgins added, “Even if this isn’t the golden ticket, researchers and clinicians are working toward improvements in the treatment of NVP. We’ve already come a long way in recent years with the development of treatment algorithms and the advent of doxylamine/pyridoxine.”
This study was supported primarily by the Medical Research Council UK and National Institute for Health and Care Research UK, with additional support from various research funding organizations, including Novo Nordisk Foundation.
Dr. Fejzo is a paid consultant for Materna Biosciences and NGM Biopharmaceuticals, and a board member and science adviser for the Hyperemesis Education and Research Foundation.
Numerous study co-authors disclosed financial relationships with private-sector companies, including employment and patent ownership.
Dr. Higgins disclosed no competing interests relevant to her comments but is an instructor for Organon.
, and targeting the hormone prophylactically may reduce this common gestational condition.
This protein acts on the brainstem to cause emesis, and, significantly, a mother’s prior exposure to it determines the degree of NVP severity she will experience, according to international researchers including Marlena Fejzo, PhD, a clinical assistant professor of population and public Health at Keck School of Medicine, University of Southern California, Los Angeles.
“GDF15 is at the mechanistic heart of NVP and HG [hyperemesis gravidarum],” Dr. Fejzo and colleagues wrote in Nature, pointing to the need for preventive and therapeutic strategies.
“My previous research showed an association between variation in the GDF15 gene and nausea and vomiting of pregnancy and HG, and this study takes it one step further by elucidating the mechanism. It confirms that the nausea and vomiting (N/V) hormone GDF15 is a major cause of NVP and HG,” Dr. Fejzo said.
The etiology of NVP remains poorly understood although it affects up to 80% of pregnancies. In the US, its severe form, HG, is the leading cause of hospitalization in early pregnancy and the second-leading reason for pregnancy hospitalization overall.
The immunoassay-based study showed that the majority of GDF15 in maternal blood during pregnancy comes from the fetal part of the placenta, and confirms previous studies reporting higher levels in pregnancies with more severe NVP, said Dr. Fejzo, who is who is a board member of the Hyperemesis Education and Research Foundation.
“However, what was really fascinating and surprising is that prior to pregnancy the women who have more severe NVP symptoms actually have lower levels of the hormone.”
Although the gene variant linked to HG was previously associated with higher circulating levels in maternal blood, counterintuitively, this new research showed that women with abnormally high levels prior to pregnancy have either no or very little NVP, said Dr. Fejzo. “That suggests that in humans higher levels may lead to a desensitization to the high levels of the hormone in pregnancy. Then we also proved that desensitization can occur in a mouse model.”
According to Erin Higgins, MD, a clinical assistant professor of obgyn and reproductive biology at the Cleveland Clinic, Cleveland, Ohio, who was not involved in the study, “This is an exciting finding that may help us to better target treatment of N/V in pregnancy. Factors for NVP have been identified, but to my knowledge there has not been a clear etiology.”
Dr. Higgins cautioned, however, that the GDF15 gene seems important in normal placentation, “so it’s not as simple as blocking the gene or its receptor.” But since preconception exposure to GDF15 might decrease nausea and vomiting once a woman is pregnant, prophylactic treatment may be possible, and metformin has been suggested as a possibility, she said.
The study findings emerged from immunoassays on maternal blood samples collected at about 15 weeks (first trimester and early second trimester), from women with NVP (n = 168) or seen at a hospital for HG (n = 57). Results were compared with those from controls having similar characteristics but no significant symptoms.
Interestingly, GDF15 is also associated with cachexia, a condition similar to HG and characterized by loss of appetite and weight loss, Dr. Fejzo noted. “The hormone can be produced by malignant tumors at levels similar to those seen in pregnancy, and symptoms can be reduced by blocking GDF15 or its receptor, GFRAL. Clinical trials are already underway in cancer patients to test this.”
She is seeking funding to test the impact of increasing GDF15 levels prior to pregnancy in patients who previously experienced HG. “I am confident that desensitizing patients by increasing GDF15 prior to pregnancy and by lowering GDF15 levels during pregnancy will work. But we need to make sure we do safety studies and get the dosing and duration right, and that will take some time.”
Desensitization will need testing first in HG, where the risk for adverse maternal and fetal outcomes is high, so the benefit will outweigh any possible risk of testing medication in pregnancy, she continued. “It will take some time before we get to patients with normal NVP, but I do believe eventually the new findings will result in game-changing therapeutics for the condition.”
Dr. Higgins added, “Even if this isn’t the golden ticket, researchers and clinicians are working toward improvements in the treatment of NVP. We’ve already come a long way in recent years with the development of treatment algorithms and the advent of doxylamine/pyridoxine.”
This study was supported primarily by the Medical Research Council UK and National Institute for Health and Care Research UK, with additional support from various research funding organizations, including Novo Nordisk Foundation.
Dr. Fejzo is a paid consultant for Materna Biosciences and NGM Biopharmaceuticals, and a board member and science adviser for the Hyperemesis Education and Research Foundation.
Numerous study co-authors disclosed financial relationships with private-sector companies, including employment and patent ownership.
Dr. Higgins disclosed no competing interests relevant to her comments but is an instructor for Organon.
FROM NATURE
A Tale of Two Babies and the ‘Family Tragedy’ of Congenital Syphilis
Delivered at 34 weeks’ gestation, Baby “Alex” had an enlarged liver and spleen on his initial newborn exam, poor tone, and a diffuse, peeling rash. Baby “Aaliyah” was born at term and appeared healthy. By 1 month of age, she was gaining weight poorly and developed copious nasal drainage and a salmon-colored rash on the soles of her feet.
The connection? Both babies were ultimately diagnosed with congenital syphilis. Infections in both babies could have been prevented if their mothers had been tested for syphilis and treated during pregnancy. Alex’s mom had no prenatal care. Aaliyah’s mom had tested negative for syphilis during her first trimester but had not been re-tested, despite sharing with her health care provider that she had a new sexual partner.
Alex and Aaliyah are representative of what Centers for Disease Control and Prevention (CDC) Chief Medical Officer Debra Houry, MD, MPH, calls a “family tragedy.” Cases of congenital syphilis are rising rapidly in the United States, reaching a 30-year high in 2021.1 Cases increased by 755% between 2012 and 2021, from 335 in 2012 to 2,865 in 2021. In 2022, cases rose again: 3,761 cases of congenital syphilis were reported, including 231 stillbirths and 51 infant deaths. Infants with congenital syphilis are at risk for lifelong complications, including deafness, blindness, and intellectual disability.
Most of these cases were preventable. Congenital syphilis is rare when pregnant people complete adequate treatment at least 30 days before delivery. In 2022, lack of testing or timely testing contributed to 36.8% of congenital syphilis cases. Nearly 40% of birth parents of infected babies received inadequate treatment during pregnancy, and 11.2% received no treatment or treatment was not documented.
, suggesting ongoing barriers to care related to social determinants of health. In 2021, the highest rates of congenital syphilis were among babies born to individuals who were non-Hispanic American Indian or Alaska Native (384 cases per 100,000 live births), non-Hispanic Native Hawaiian or other Pacific Islander (192 cases per 100,000 live births), and non-Hispanic Black or African American (169 cases per 100,000 live births). Six states had rates of congenital syphilis that exceeded 160 cases per 100,000 population, including Arizona, New Mexico, Louisiana, Mississippi, Texas, and Oklahoma. That is more than twice the national rate of 77.9 cases/100,000.
Reducing the Risk
To reduce rates of congenital syphilis in all people, barriers to testing must be eliminated. The CDC recommends that all pregnant people be tested early in pregnancy, with repeat testing at 28 weeks and at delivery for those at increased risk for infection based on individual risk factors or residence in a high-prevalence community. Rapid syphilis testing and treatment during pregnancy is recommended in settings such as emergency departments, syringe service programs, prisons/jails, and maternal and child health programs to minimize missed opportunities for care.
While pediatric clinicians rarely care for pregnant patients, they also have an essential role to play in reducing the adverse health outcomes associated with congenital syphilis. No infant should be discharged from the newborn nursery without confirming that the birth parent was tested for syphilis at least once and was treated appropriately if positive. Appropriate treatment during pregnancy is a single dose of benzathine penicillin G for primary, secondary, or early latent syphilis. Late-latent syphilis or syphilis of unknown duration is treated with three doses of benzathine penicillin G spaced 7-9 days apart. If the doses are given further than 9 days apart, treatment is considered inadequate, and the series of doses must be restarted. Benzathine penicillin G remains in short supply in the United States, but is the only drug recommended to treat syphilis during pregnancy.
Collaboration between obstetrical and newborn care providers is essential. Those who care for newborns need easy access to birthing parents’ syphilis treatment results. As more health care facilities implement routine syphilis testing at delivery, rapid syphilis testing must be available to avoid prolonging newborn hospital stays.
Pediatricians need to maintain an index of suspicion for congenital syphilis, regardless of maternal history, because symptomatic congenital syphilis can mimic a variety of infectious and noninfectious conditions. Most infected infants look normal at birth. While the majority of cases of congenital syphilis are identified in the newborn period, a 2021 paper published in Pediatrics described 84 infants born between 2014 and 2018 who were diagnosed beyond a month of age.2 These represented 2.2% of all infants born with congenital syphilis. Common symptoms included rash, snuffles, and hepatomegaly. Sixty-nine percent of infants who had long bone radiographs obtained had findings consistent with congenital syphilis. Typical imaging findings include periostitis and demineralization of the metaphysis and diaphysis of long bones, although fractures can also occur. Case reports describe infants who presented with fractures and were initially evaluated for nonaccidental trauma.3
Another critical approach is to treat syphilis in people of childbearing age before pregnancy occurs. The CDC recommends syphilis testing for sexually active females 18-44 years of age and living in communities with high rates of syphilis. County-specific specific rates of syphilis rates are available at https://www.cdc.gov/nchhstp/atlas/syphilis/. Point-of-care tests are now available for syphilis and may facilitate timely treatment.
Additional resources describing syphilis testing and treatment are available from the CDC and the American Academy of Pediatrics.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022-2023. The opinions expressed in this article are her own. Dr. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta, and Gilead. Email her at pdnews@mdedge.com. (Also kristina.bryant@louisville.edu.)
References
1. McDonald R et al. Vital Signs: Missed Opportunities for Preventing Congenital Syphilis — United States, 2022. MMWR Morb Mortal Wkly Rep. 2023 Nov 17;72(46):1269-74. doi: 10.15585/mmwr.mm7246e1.
2. Kimball A et al. Congenital Syphilis Diagnosed Beyond the Neonatal Period in the United States: 2014-2018. Pediatrics. 2021 Sep;148(3):e2020049080. doi: 10.1542/peds.2020-049080.
3. Jacobs K et al. Congenital Syphilis Misdiagnosed as Suspected Nonaccidental Trauma. Pediatrics. 2019 Oct;144(4):e20191564. doi: 10.1542/peds.2019-1564.
Delivered at 34 weeks’ gestation, Baby “Alex” had an enlarged liver and spleen on his initial newborn exam, poor tone, and a diffuse, peeling rash. Baby “Aaliyah” was born at term and appeared healthy. By 1 month of age, she was gaining weight poorly and developed copious nasal drainage and a salmon-colored rash on the soles of her feet.
The connection? Both babies were ultimately diagnosed with congenital syphilis. Infections in both babies could have been prevented if their mothers had been tested for syphilis and treated during pregnancy. Alex’s mom had no prenatal care. Aaliyah’s mom had tested negative for syphilis during her first trimester but had not been re-tested, despite sharing with her health care provider that she had a new sexual partner.
Alex and Aaliyah are representative of what Centers for Disease Control and Prevention (CDC) Chief Medical Officer Debra Houry, MD, MPH, calls a “family tragedy.” Cases of congenital syphilis are rising rapidly in the United States, reaching a 30-year high in 2021.1 Cases increased by 755% between 2012 and 2021, from 335 in 2012 to 2,865 in 2021. In 2022, cases rose again: 3,761 cases of congenital syphilis were reported, including 231 stillbirths and 51 infant deaths. Infants with congenital syphilis are at risk for lifelong complications, including deafness, blindness, and intellectual disability.
Most of these cases were preventable. Congenital syphilis is rare when pregnant people complete adequate treatment at least 30 days before delivery. In 2022, lack of testing or timely testing contributed to 36.8% of congenital syphilis cases. Nearly 40% of birth parents of infected babies received inadequate treatment during pregnancy, and 11.2% received no treatment or treatment was not documented.
, suggesting ongoing barriers to care related to social determinants of health. In 2021, the highest rates of congenital syphilis were among babies born to individuals who were non-Hispanic American Indian or Alaska Native (384 cases per 100,000 live births), non-Hispanic Native Hawaiian or other Pacific Islander (192 cases per 100,000 live births), and non-Hispanic Black or African American (169 cases per 100,000 live births). Six states had rates of congenital syphilis that exceeded 160 cases per 100,000 population, including Arizona, New Mexico, Louisiana, Mississippi, Texas, and Oklahoma. That is more than twice the national rate of 77.9 cases/100,000.
Reducing the Risk
To reduce rates of congenital syphilis in all people, barriers to testing must be eliminated. The CDC recommends that all pregnant people be tested early in pregnancy, with repeat testing at 28 weeks and at delivery for those at increased risk for infection based on individual risk factors or residence in a high-prevalence community. Rapid syphilis testing and treatment during pregnancy is recommended in settings such as emergency departments, syringe service programs, prisons/jails, and maternal and child health programs to minimize missed opportunities for care.
While pediatric clinicians rarely care for pregnant patients, they also have an essential role to play in reducing the adverse health outcomes associated with congenital syphilis. No infant should be discharged from the newborn nursery without confirming that the birth parent was tested for syphilis at least once and was treated appropriately if positive. Appropriate treatment during pregnancy is a single dose of benzathine penicillin G for primary, secondary, or early latent syphilis. Late-latent syphilis or syphilis of unknown duration is treated with three doses of benzathine penicillin G spaced 7-9 days apart. If the doses are given further than 9 days apart, treatment is considered inadequate, and the series of doses must be restarted. Benzathine penicillin G remains in short supply in the United States, but is the only drug recommended to treat syphilis during pregnancy.
Collaboration between obstetrical and newborn care providers is essential. Those who care for newborns need easy access to birthing parents’ syphilis treatment results. As more health care facilities implement routine syphilis testing at delivery, rapid syphilis testing must be available to avoid prolonging newborn hospital stays.
Pediatricians need to maintain an index of suspicion for congenital syphilis, regardless of maternal history, because symptomatic congenital syphilis can mimic a variety of infectious and noninfectious conditions. Most infected infants look normal at birth. While the majority of cases of congenital syphilis are identified in the newborn period, a 2021 paper published in Pediatrics described 84 infants born between 2014 and 2018 who were diagnosed beyond a month of age.2 These represented 2.2% of all infants born with congenital syphilis. Common symptoms included rash, snuffles, and hepatomegaly. Sixty-nine percent of infants who had long bone radiographs obtained had findings consistent with congenital syphilis. Typical imaging findings include periostitis and demineralization of the metaphysis and diaphysis of long bones, although fractures can also occur. Case reports describe infants who presented with fractures and were initially evaluated for nonaccidental trauma.3
Another critical approach is to treat syphilis in people of childbearing age before pregnancy occurs. The CDC recommends syphilis testing for sexually active females 18-44 years of age and living in communities with high rates of syphilis. County-specific specific rates of syphilis rates are available at https://www.cdc.gov/nchhstp/atlas/syphilis/. Point-of-care tests are now available for syphilis and may facilitate timely treatment.
Additional resources describing syphilis testing and treatment are available from the CDC and the American Academy of Pediatrics.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022-2023. The opinions expressed in this article are her own. Dr. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta, and Gilead. Email her at pdnews@mdedge.com. (Also kristina.bryant@louisville.edu.)
References
1. McDonald R et al. Vital Signs: Missed Opportunities for Preventing Congenital Syphilis — United States, 2022. MMWR Morb Mortal Wkly Rep. 2023 Nov 17;72(46):1269-74. doi: 10.15585/mmwr.mm7246e1.
2. Kimball A et al. Congenital Syphilis Diagnosed Beyond the Neonatal Period in the United States: 2014-2018. Pediatrics. 2021 Sep;148(3):e2020049080. doi: 10.1542/peds.2020-049080.
3. Jacobs K et al. Congenital Syphilis Misdiagnosed as Suspected Nonaccidental Trauma. Pediatrics. 2019 Oct;144(4):e20191564. doi: 10.1542/peds.2019-1564.
Delivered at 34 weeks’ gestation, Baby “Alex” had an enlarged liver and spleen on his initial newborn exam, poor tone, and a diffuse, peeling rash. Baby “Aaliyah” was born at term and appeared healthy. By 1 month of age, she was gaining weight poorly and developed copious nasal drainage and a salmon-colored rash on the soles of her feet.
The connection? Both babies were ultimately diagnosed with congenital syphilis. Infections in both babies could have been prevented if their mothers had been tested for syphilis and treated during pregnancy. Alex’s mom had no prenatal care. Aaliyah’s mom had tested negative for syphilis during her first trimester but had not been re-tested, despite sharing with her health care provider that she had a new sexual partner.
Alex and Aaliyah are representative of what Centers for Disease Control and Prevention (CDC) Chief Medical Officer Debra Houry, MD, MPH, calls a “family tragedy.” Cases of congenital syphilis are rising rapidly in the United States, reaching a 30-year high in 2021.1 Cases increased by 755% between 2012 and 2021, from 335 in 2012 to 2,865 in 2021. In 2022, cases rose again: 3,761 cases of congenital syphilis were reported, including 231 stillbirths and 51 infant deaths. Infants with congenital syphilis are at risk for lifelong complications, including deafness, blindness, and intellectual disability.
Most of these cases were preventable. Congenital syphilis is rare when pregnant people complete adequate treatment at least 30 days before delivery. In 2022, lack of testing or timely testing contributed to 36.8% of congenital syphilis cases. Nearly 40% of birth parents of infected babies received inadequate treatment during pregnancy, and 11.2% received no treatment or treatment was not documented.
, suggesting ongoing barriers to care related to social determinants of health. In 2021, the highest rates of congenital syphilis were among babies born to individuals who were non-Hispanic American Indian or Alaska Native (384 cases per 100,000 live births), non-Hispanic Native Hawaiian or other Pacific Islander (192 cases per 100,000 live births), and non-Hispanic Black or African American (169 cases per 100,000 live births). Six states had rates of congenital syphilis that exceeded 160 cases per 100,000 population, including Arizona, New Mexico, Louisiana, Mississippi, Texas, and Oklahoma. That is more than twice the national rate of 77.9 cases/100,000.
Reducing the Risk
To reduce rates of congenital syphilis in all people, barriers to testing must be eliminated. The CDC recommends that all pregnant people be tested early in pregnancy, with repeat testing at 28 weeks and at delivery for those at increased risk for infection based on individual risk factors or residence in a high-prevalence community. Rapid syphilis testing and treatment during pregnancy is recommended in settings such as emergency departments, syringe service programs, prisons/jails, and maternal and child health programs to minimize missed opportunities for care.
While pediatric clinicians rarely care for pregnant patients, they also have an essential role to play in reducing the adverse health outcomes associated with congenital syphilis. No infant should be discharged from the newborn nursery without confirming that the birth parent was tested for syphilis at least once and was treated appropriately if positive. Appropriate treatment during pregnancy is a single dose of benzathine penicillin G for primary, secondary, or early latent syphilis. Late-latent syphilis or syphilis of unknown duration is treated with three doses of benzathine penicillin G spaced 7-9 days apart. If the doses are given further than 9 days apart, treatment is considered inadequate, and the series of doses must be restarted. Benzathine penicillin G remains in short supply in the United States, but is the only drug recommended to treat syphilis during pregnancy.
Collaboration between obstetrical and newborn care providers is essential. Those who care for newborns need easy access to birthing parents’ syphilis treatment results. As more health care facilities implement routine syphilis testing at delivery, rapid syphilis testing must be available to avoid prolonging newborn hospital stays.
Pediatricians need to maintain an index of suspicion for congenital syphilis, regardless of maternal history, because symptomatic congenital syphilis can mimic a variety of infectious and noninfectious conditions. Most infected infants look normal at birth. While the majority of cases of congenital syphilis are identified in the newborn period, a 2021 paper published in Pediatrics described 84 infants born between 2014 and 2018 who were diagnosed beyond a month of age.2 These represented 2.2% of all infants born with congenital syphilis. Common symptoms included rash, snuffles, and hepatomegaly. Sixty-nine percent of infants who had long bone radiographs obtained had findings consistent with congenital syphilis. Typical imaging findings include periostitis and demineralization of the metaphysis and diaphysis of long bones, although fractures can also occur. Case reports describe infants who presented with fractures and were initially evaluated for nonaccidental trauma.3
Another critical approach is to treat syphilis in people of childbearing age before pregnancy occurs. The CDC recommends syphilis testing for sexually active females 18-44 years of age and living in communities with high rates of syphilis. County-specific specific rates of syphilis rates are available at https://www.cdc.gov/nchhstp/atlas/syphilis/. Point-of-care tests are now available for syphilis and may facilitate timely treatment.
Additional resources describing syphilis testing and treatment are available from the CDC and the American Academy of Pediatrics.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022-2023. The opinions expressed in this article are her own. Dr. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta, and Gilead. Email her at pdnews@mdedge.com. (Also kristina.bryant@louisville.edu.)
References
1. McDonald R et al. Vital Signs: Missed Opportunities for Preventing Congenital Syphilis — United States, 2022. MMWR Morb Mortal Wkly Rep. 2023 Nov 17;72(46):1269-74. doi: 10.15585/mmwr.mm7246e1.
2. Kimball A et al. Congenital Syphilis Diagnosed Beyond the Neonatal Period in the United States: 2014-2018. Pediatrics. 2021 Sep;148(3):e2020049080. doi: 10.1542/peds.2020-049080.
3. Jacobs K et al. Congenital Syphilis Misdiagnosed as Suspected Nonaccidental Trauma. Pediatrics. 2019 Oct;144(4):e20191564. doi: 10.1542/peds.2019-1564.
Two biomarkers promising for preeclampsia prediction
Two biomarkers – pregnancy-associated plasma protein A2 (PAPP-A2) and activin A – when added to relevant clinical information have a better positive predictive value than and a comparable negative predictive value to the currently used ratio of soluble fms-like tyrosine kinase 1 (sFlt-1) to placental growth factor (PlGF), new research suggests.
The third-trimester sFlt-1:PlGF ratio can predict short-term absence of preeclampsia. By contrast, , according to the authors.
Preeclampsia has “potentially devastating maternal and fetal complications, [including] significantly increased cardiovascular risk for affected women later in life,” study author Stella S. Daskalopoulou, MD, PhD, associate professor of medicine at McGill University Health Centre in Montreal, said in an interview.
“A more accurate prediction of preeclampsia is expected to improve risk stratification and clinical care and shape clinical practice guidelines,” she said.
The study was published online in the Canadian Journal of Cardiology.
Better predictive value
For a prospective cohort study, the investigators recruited 192 women with first-trimester high-risk singleton pregnancies from tertiary obstetric clinics in Montreal.
At baseline, they collected clinical information, including height, prepregnancy weight, personal and family medical history, and medication use.
At each trimester, blood pressure was measured, and blood samples were collected to quantify sFlt-1, PlGF, PAPP-A2, PAPP-A, activin A, inhibin A, follistatin, and glycosylated fibronectin. For the sFlt-1:PlGF ratio, the researchers used a cutoff point of 38, based on prior evidence. Because there are no agreed-upon cutoff points for the other biomarkers, they chose cutoff points that maximized sensitivity and specificity.
Pregnancies were considered high risk if the mother had any of the following conditions: prepregnancy BMI ≥ 25, maternal age ≥ 35 years, chronic hypertension, diabetes, renal disease, conception via in vitro fertilization, or maternal or first-degree family history of preeclampsia.
The primary outcome was preeclampsia, which was defined according to the Society of Obstetrics and Gynecology guidelines as systolic blood pressure ≥ 140 mm Hg or diastolic blood pressure of ≥ mm Hg together with either proteinuria or maternal end-organ dysfunction.
A total of 18 women (9.38%) developed preeclampsia. Those women had higher blood pressure at baseline (although it was within normal limits) and were more likely to have preexisting diabetes or a previous pregnancy with preeclampsia. They were also more likely to report Black race. Serum levels of PAPP-A, PAPP-A2, activin A, and inhibin A were significantly different between patients who developed preeclampsia and those who did not. These levels were increased throughout pregnancy.
Alongside the sFlt-1:PlGF ratio, two biomarkers, PAPP-A2 (odds ratio, 1.78) and activin A (OR, 1.84), were significantly associated with the primary outcome after adjustment for age, prepregnancy BMI, race, and mean arterial pressure.
When added to a model that included those clinical factors, a positive third-trimester result for both PAPP-A2 and activin A had a better positive predictive value than the sFlt-1:PlGF ratio added to the clinical model (91.67% vs. 66.67%). The two biomarkers also had a negative predictive value that was comparable to that of the sFlt-1:PlGF ratio (97.69% vs. 96%).
Study limitations include the small sample size and missing covariates for some participants. Furthermore, the findings cannot be generalized to low-risk populations.
“Whereas the third-trimester sFlt-1:PlGF ratio can predict short-term absence of preeclampsia, PAPP-A2 and activin A had both high positive and negative predictive values and thus could serve as biomarkers to predict the occurrence (and absence) of preeclampsia; these findings will be validated in future studies,” the authors concluded.
Dr. Daskalopoulou said that her group is currently performing a large multinational study, PULSE, “which will be the ideal platform to validate and extend our findings. The aim of the study is to predict preeclampsia using a multimodal approach that includes arterial stiffness measurements and blood biomarkers.”
She expanded on the potential benefits of this research. “Finding an accurate predictive tool would not only help design appropriate early care plans for truly high-risk pregnant women, including monitoring and delivery planning, but also facilitate the development of novel strategies for the prevention and treatment of preeclampsia, improving the life of millions of young mothers and their offspring around the world.”
Promising biomarkers
Commenting on the study, Nieca Goldberg, MD, clinical associate professor of medicine at NYU Langone Health and medical director of Atria, both in New York, said, “These biomarkers are promising, as the current biomarker, sFlt-1:PlGF, is good at ruling out preeclampsia in the short term, while the new biomarkers show that they are better at ruling in preeclampsia” as well as ruling it out. Dr. Goldberg was not involved in the research.
“The current study is small, some participant data points are missing, and the researchers only studied high-risk pregnancies,” she added. “We need larger studies of all the risk markers, in both high- and low-risk pregnancies that are followed throughout pregnancy.”
This work was supported by the Fonds de recherche du Québec Santé (FRQS), Heart and Stroke Foundation of Canada, McGill University Department of Obstetrics and Gynecology Academic Enrichment Fund, and Canadian Foundation for Women›s Health. Dr. Daskalopoulou is a senior clinician-scientist supported by a FRQS Clinician Scientist-Senior salary award. Dr. Daskalopoulou and Dr. Goldberg disclosed no conflicts of interest.
A version of this article appeared on Medscape.com.
Two biomarkers – pregnancy-associated plasma protein A2 (PAPP-A2) and activin A – when added to relevant clinical information have a better positive predictive value than and a comparable negative predictive value to the currently used ratio of soluble fms-like tyrosine kinase 1 (sFlt-1) to placental growth factor (PlGF), new research suggests.
The third-trimester sFlt-1:PlGF ratio can predict short-term absence of preeclampsia. By contrast, , according to the authors.
Preeclampsia has “potentially devastating maternal and fetal complications, [including] significantly increased cardiovascular risk for affected women later in life,” study author Stella S. Daskalopoulou, MD, PhD, associate professor of medicine at McGill University Health Centre in Montreal, said in an interview.
“A more accurate prediction of preeclampsia is expected to improve risk stratification and clinical care and shape clinical practice guidelines,” she said.
The study was published online in the Canadian Journal of Cardiology.
Better predictive value
For a prospective cohort study, the investigators recruited 192 women with first-trimester high-risk singleton pregnancies from tertiary obstetric clinics in Montreal.
At baseline, they collected clinical information, including height, prepregnancy weight, personal and family medical history, and medication use.
At each trimester, blood pressure was measured, and blood samples were collected to quantify sFlt-1, PlGF, PAPP-A2, PAPP-A, activin A, inhibin A, follistatin, and glycosylated fibronectin. For the sFlt-1:PlGF ratio, the researchers used a cutoff point of 38, based on prior evidence. Because there are no agreed-upon cutoff points for the other biomarkers, they chose cutoff points that maximized sensitivity and specificity.
Pregnancies were considered high risk if the mother had any of the following conditions: prepregnancy BMI ≥ 25, maternal age ≥ 35 years, chronic hypertension, diabetes, renal disease, conception via in vitro fertilization, or maternal or first-degree family history of preeclampsia.
The primary outcome was preeclampsia, which was defined according to the Society of Obstetrics and Gynecology guidelines as systolic blood pressure ≥ 140 mm Hg or diastolic blood pressure of ≥ mm Hg together with either proteinuria or maternal end-organ dysfunction.
A total of 18 women (9.38%) developed preeclampsia. Those women had higher blood pressure at baseline (although it was within normal limits) and were more likely to have preexisting diabetes or a previous pregnancy with preeclampsia. They were also more likely to report Black race. Serum levels of PAPP-A, PAPP-A2, activin A, and inhibin A were significantly different between patients who developed preeclampsia and those who did not. These levels were increased throughout pregnancy.
Alongside the sFlt-1:PlGF ratio, two biomarkers, PAPP-A2 (odds ratio, 1.78) and activin A (OR, 1.84), were significantly associated with the primary outcome after adjustment for age, prepregnancy BMI, race, and mean arterial pressure.
When added to a model that included those clinical factors, a positive third-trimester result for both PAPP-A2 and activin A had a better positive predictive value than the sFlt-1:PlGF ratio added to the clinical model (91.67% vs. 66.67%). The two biomarkers also had a negative predictive value that was comparable to that of the sFlt-1:PlGF ratio (97.69% vs. 96%).
Study limitations include the small sample size and missing covariates for some participants. Furthermore, the findings cannot be generalized to low-risk populations.
“Whereas the third-trimester sFlt-1:PlGF ratio can predict short-term absence of preeclampsia, PAPP-A2 and activin A had both high positive and negative predictive values and thus could serve as biomarkers to predict the occurrence (and absence) of preeclampsia; these findings will be validated in future studies,” the authors concluded.
Dr. Daskalopoulou said that her group is currently performing a large multinational study, PULSE, “which will be the ideal platform to validate and extend our findings. The aim of the study is to predict preeclampsia using a multimodal approach that includes arterial stiffness measurements and blood biomarkers.”
She expanded on the potential benefits of this research. “Finding an accurate predictive tool would not only help design appropriate early care plans for truly high-risk pregnant women, including monitoring and delivery planning, but also facilitate the development of novel strategies for the prevention and treatment of preeclampsia, improving the life of millions of young mothers and their offspring around the world.”
Promising biomarkers
Commenting on the study, Nieca Goldberg, MD, clinical associate professor of medicine at NYU Langone Health and medical director of Atria, both in New York, said, “These biomarkers are promising, as the current biomarker, sFlt-1:PlGF, is good at ruling out preeclampsia in the short term, while the new biomarkers show that they are better at ruling in preeclampsia” as well as ruling it out. Dr. Goldberg was not involved in the research.
“The current study is small, some participant data points are missing, and the researchers only studied high-risk pregnancies,” she added. “We need larger studies of all the risk markers, in both high- and low-risk pregnancies that are followed throughout pregnancy.”
This work was supported by the Fonds de recherche du Québec Santé (FRQS), Heart and Stroke Foundation of Canada, McGill University Department of Obstetrics and Gynecology Academic Enrichment Fund, and Canadian Foundation for Women›s Health. Dr. Daskalopoulou is a senior clinician-scientist supported by a FRQS Clinician Scientist-Senior salary award. Dr. Daskalopoulou and Dr. Goldberg disclosed no conflicts of interest.
A version of this article appeared on Medscape.com.
Two biomarkers – pregnancy-associated plasma protein A2 (PAPP-A2) and activin A – when added to relevant clinical information have a better positive predictive value than and a comparable negative predictive value to the currently used ratio of soluble fms-like tyrosine kinase 1 (sFlt-1) to placental growth factor (PlGF), new research suggests.
The third-trimester sFlt-1:PlGF ratio can predict short-term absence of preeclampsia. By contrast, , according to the authors.
Preeclampsia has “potentially devastating maternal and fetal complications, [including] significantly increased cardiovascular risk for affected women later in life,” study author Stella S. Daskalopoulou, MD, PhD, associate professor of medicine at McGill University Health Centre in Montreal, said in an interview.
“A more accurate prediction of preeclampsia is expected to improve risk stratification and clinical care and shape clinical practice guidelines,” she said.
The study was published online in the Canadian Journal of Cardiology.
Better predictive value
For a prospective cohort study, the investigators recruited 192 women with first-trimester high-risk singleton pregnancies from tertiary obstetric clinics in Montreal.
At baseline, they collected clinical information, including height, prepregnancy weight, personal and family medical history, and medication use.
At each trimester, blood pressure was measured, and blood samples were collected to quantify sFlt-1, PlGF, PAPP-A2, PAPP-A, activin A, inhibin A, follistatin, and glycosylated fibronectin. For the sFlt-1:PlGF ratio, the researchers used a cutoff point of 38, based on prior evidence. Because there are no agreed-upon cutoff points for the other biomarkers, they chose cutoff points that maximized sensitivity and specificity.
Pregnancies were considered high risk if the mother had any of the following conditions: prepregnancy BMI ≥ 25, maternal age ≥ 35 years, chronic hypertension, diabetes, renal disease, conception via in vitro fertilization, or maternal or first-degree family history of preeclampsia.
The primary outcome was preeclampsia, which was defined according to the Society of Obstetrics and Gynecology guidelines as systolic blood pressure ≥ 140 mm Hg or diastolic blood pressure of ≥ mm Hg together with either proteinuria or maternal end-organ dysfunction.
A total of 18 women (9.38%) developed preeclampsia. Those women had higher blood pressure at baseline (although it was within normal limits) and were more likely to have preexisting diabetes or a previous pregnancy with preeclampsia. They were also more likely to report Black race. Serum levels of PAPP-A, PAPP-A2, activin A, and inhibin A were significantly different between patients who developed preeclampsia and those who did not. These levels were increased throughout pregnancy.
Alongside the sFlt-1:PlGF ratio, two biomarkers, PAPP-A2 (odds ratio, 1.78) and activin A (OR, 1.84), were significantly associated with the primary outcome after adjustment for age, prepregnancy BMI, race, and mean arterial pressure.
When added to a model that included those clinical factors, a positive third-trimester result for both PAPP-A2 and activin A had a better positive predictive value than the sFlt-1:PlGF ratio added to the clinical model (91.67% vs. 66.67%). The two biomarkers also had a negative predictive value that was comparable to that of the sFlt-1:PlGF ratio (97.69% vs. 96%).
Study limitations include the small sample size and missing covariates for some participants. Furthermore, the findings cannot be generalized to low-risk populations.
“Whereas the third-trimester sFlt-1:PlGF ratio can predict short-term absence of preeclampsia, PAPP-A2 and activin A had both high positive and negative predictive values and thus could serve as biomarkers to predict the occurrence (and absence) of preeclampsia; these findings will be validated in future studies,” the authors concluded.
Dr. Daskalopoulou said that her group is currently performing a large multinational study, PULSE, “which will be the ideal platform to validate and extend our findings. The aim of the study is to predict preeclampsia using a multimodal approach that includes arterial stiffness measurements and blood biomarkers.”
She expanded on the potential benefits of this research. “Finding an accurate predictive tool would not only help design appropriate early care plans for truly high-risk pregnant women, including monitoring and delivery planning, but also facilitate the development of novel strategies for the prevention and treatment of preeclampsia, improving the life of millions of young mothers and their offspring around the world.”
Promising biomarkers
Commenting on the study, Nieca Goldberg, MD, clinical associate professor of medicine at NYU Langone Health and medical director of Atria, both in New York, said, “These biomarkers are promising, as the current biomarker, sFlt-1:PlGF, is good at ruling out preeclampsia in the short term, while the new biomarkers show that they are better at ruling in preeclampsia” as well as ruling it out. Dr. Goldberg was not involved in the research.
“The current study is small, some participant data points are missing, and the researchers only studied high-risk pregnancies,” she added. “We need larger studies of all the risk markers, in both high- and low-risk pregnancies that are followed throughout pregnancy.”
This work was supported by the Fonds de recherche du Québec Santé (FRQS), Heart and Stroke Foundation of Canada, McGill University Department of Obstetrics and Gynecology Academic Enrichment Fund, and Canadian Foundation for Women›s Health. Dr. Daskalopoulou is a senior clinician-scientist supported by a FRQS Clinician Scientist-Senior salary award. Dr. Daskalopoulou and Dr. Goldberg disclosed no conflicts of interest.
A version of this article appeared on Medscape.com.
FROM THE CANADIAN JOURNAL OF CARDIOLOGY
Test all perinatally exposed infants for HCV: CDC
In utero–exposed infants should be tested at 2-6 months of life, much earlier than the current strategy of testing at 18 months.
HCV infection, which can lead to liver fibrosis and cirrhosis, liver failure, hepatic cancer, and transplant, will develop in 6%-7% of all perinatally exposed infants and children. Curative therapy with direct-acting antivirals can be administered starting at age 3, the CDC noted in Morbidity and Mortality Week Report (MMWR).
About 70% of children 18 months and older are not being tested with the current strategy of anti-HCV testing.
This current MMWR report supplements the 2020 CDC recommendations for adult HCV screening, which includes universal screening among pregnant persons during each pregnancy.
The new recommendations
- Perinatally exposed infants should receive a nucleic acid amplification test for HCV RNA at 2-6 months of age to identify those who might develop chronic HCV infection if not treated.
- Those with detectable HCV RNA should be managed in consultation with an expert in pediatric HCV.
- Infants with undetectable HCV RNA do not require further follow-up unless clinically warranted.
“Testing perinatally exposed infants beginning at age 2 months with a NAT for HCV RNA is cost-effective and allows for earlier linkage to care, appropriate evaluation, and the opportunity to provide curative, life-saving therapy,” the MMWR report said.
A growing problem
The CDC noted that rates of HCV infections during pregnancy are on the rise, corresponding with the ongoing opioid crisis and intravenous drug use.
Yet most perinatally exposed children are not tested for HCV infection and are not referred for hepatitis C care. Reasons might include lack of awareness of perinatal exposure by pediatric providers, lack of regular pediatric care among exposed children, and switching of health care providers before the former recommended testing age of 18 months.
The CDC’s testing recommendation is welcome news to Dawnette A. Lewis, MD, a maternal fetal medicine specialist at Northwell Health in New Hyde Park, N.Y. “As opposed to data for hep B and HIV, we have traditionally had little information and experience regarding the transmission and impact of hep C in pregnant women and their babies. We’ve been having that conversation about the lack of information for some time, and now there’s an opportunity to get evolving data on hep C and how it affects the baby, ” she said.
In her view, mothers will likely be quite accepting of testing for their infants. “It could be integrated into the routine newborn screening panel, so there should not be barriers to accessibility if they’re getting prenatal and neonatal care.”
Commenting on HCV testing for babies in an interview at his institution, Ravi R. Jhaveri, MD, division head of pediatric infectious diseases at Northwestern Medicine’s Ann & Robert H. Lurie Children’s Hospital of Chicago, said, “This is a terrific way to capitalize on the fact that infants already come to the doctor for many visits during the first months of life for their vaccines and their well-child check. And so this should be an easy way to streamline our testing strategy and hopefully lose many fewer patients.”
Northwestern Medicine is an innovative clinic offering HCV testing and treatment outside of clinical trials for pregnant women and their infants with the goal of preventing transmission from mother to child.
Northwestern is launching a clinical trial of treatment for HCV-positive pregnant patients during regular prenatal care. “With very simple treatments similar to taking a prenatal vitamin, it would be easy and seamless to fit into the existing schedule,” said Lyn Yee, MD, a Northwestern maternal-fetal medicine specialist.
Dr. Yee stressed that eliminating hepatitis C will likely be one of the most significant health advancements of the decade.
Dr. Lewis, Dr. Jhaveri, and Dr. Yee had no relevant conflicts of interest to declare with regard to their comments.
In utero–exposed infants should be tested at 2-6 months of life, much earlier than the current strategy of testing at 18 months.
HCV infection, which can lead to liver fibrosis and cirrhosis, liver failure, hepatic cancer, and transplant, will develop in 6%-7% of all perinatally exposed infants and children. Curative therapy with direct-acting antivirals can be administered starting at age 3, the CDC noted in Morbidity and Mortality Week Report (MMWR).
About 70% of children 18 months and older are not being tested with the current strategy of anti-HCV testing.
This current MMWR report supplements the 2020 CDC recommendations for adult HCV screening, which includes universal screening among pregnant persons during each pregnancy.
The new recommendations
- Perinatally exposed infants should receive a nucleic acid amplification test for HCV RNA at 2-6 months of age to identify those who might develop chronic HCV infection if not treated.
- Those with detectable HCV RNA should be managed in consultation with an expert in pediatric HCV.
- Infants with undetectable HCV RNA do not require further follow-up unless clinically warranted.
“Testing perinatally exposed infants beginning at age 2 months with a NAT for HCV RNA is cost-effective and allows for earlier linkage to care, appropriate evaluation, and the opportunity to provide curative, life-saving therapy,” the MMWR report said.
A growing problem
The CDC noted that rates of HCV infections during pregnancy are on the rise, corresponding with the ongoing opioid crisis and intravenous drug use.
Yet most perinatally exposed children are not tested for HCV infection and are not referred for hepatitis C care. Reasons might include lack of awareness of perinatal exposure by pediatric providers, lack of regular pediatric care among exposed children, and switching of health care providers before the former recommended testing age of 18 months.
The CDC’s testing recommendation is welcome news to Dawnette A. Lewis, MD, a maternal fetal medicine specialist at Northwell Health in New Hyde Park, N.Y. “As opposed to data for hep B and HIV, we have traditionally had little information and experience regarding the transmission and impact of hep C in pregnant women and their babies. We’ve been having that conversation about the lack of information for some time, and now there’s an opportunity to get evolving data on hep C and how it affects the baby, ” she said.
In her view, mothers will likely be quite accepting of testing for their infants. “It could be integrated into the routine newborn screening panel, so there should not be barriers to accessibility if they’re getting prenatal and neonatal care.”
Commenting on HCV testing for babies in an interview at his institution, Ravi R. Jhaveri, MD, division head of pediatric infectious diseases at Northwestern Medicine’s Ann & Robert H. Lurie Children’s Hospital of Chicago, said, “This is a terrific way to capitalize on the fact that infants already come to the doctor for many visits during the first months of life for their vaccines and their well-child check. And so this should be an easy way to streamline our testing strategy and hopefully lose many fewer patients.”
Northwestern Medicine is an innovative clinic offering HCV testing and treatment outside of clinical trials for pregnant women and their infants with the goal of preventing transmission from mother to child.
Northwestern is launching a clinical trial of treatment for HCV-positive pregnant patients during regular prenatal care. “With very simple treatments similar to taking a prenatal vitamin, it would be easy and seamless to fit into the existing schedule,” said Lyn Yee, MD, a Northwestern maternal-fetal medicine specialist.
Dr. Yee stressed that eliminating hepatitis C will likely be one of the most significant health advancements of the decade.
Dr. Lewis, Dr. Jhaveri, and Dr. Yee had no relevant conflicts of interest to declare with regard to their comments.
In utero–exposed infants should be tested at 2-6 months of life, much earlier than the current strategy of testing at 18 months.
HCV infection, which can lead to liver fibrosis and cirrhosis, liver failure, hepatic cancer, and transplant, will develop in 6%-7% of all perinatally exposed infants and children. Curative therapy with direct-acting antivirals can be administered starting at age 3, the CDC noted in Morbidity and Mortality Week Report (MMWR).
About 70% of children 18 months and older are not being tested with the current strategy of anti-HCV testing.
This current MMWR report supplements the 2020 CDC recommendations for adult HCV screening, which includes universal screening among pregnant persons during each pregnancy.
The new recommendations
- Perinatally exposed infants should receive a nucleic acid amplification test for HCV RNA at 2-6 months of age to identify those who might develop chronic HCV infection if not treated.
- Those with detectable HCV RNA should be managed in consultation with an expert in pediatric HCV.
- Infants with undetectable HCV RNA do not require further follow-up unless clinically warranted.
“Testing perinatally exposed infants beginning at age 2 months with a NAT for HCV RNA is cost-effective and allows for earlier linkage to care, appropriate evaluation, and the opportunity to provide curative, life-saving therapy,” the MMWR report said.
A growing problem
The CDC noted that rates of HCV infections during pregnancy are on the rise, corresponding with the ongoing opioid crisis and intravenous drug use.
Yet most perinatally exposed children are not tested for HCV infection and are not referred for hepatitis C care. Reasons might include lack of awareness of perinatal exposure by pediatric providers, lack of regular pediatric care among exposed children, and switching of health care providers before the former recommended testing age of 18 months.
The CDC’s testing recommendation is welcome news to Dawnette A. Lewis, MD, a maternal fetal medicine specialist at Northwell Health in New Hyde Park, N.Y. “As opposed to data for hep B and HIV, we have traditionally had little information and experience regarding the transmission and impact of hep C in pregnant women and their babies. We’ve been having that conversation about the lack of information for some time, and now there’s an opportunity to get evolving data on hep C and how it affects the baby, ” she said.
In her view, mothers will likely be quite accepting of testing for their infants. “It could be integrated into the routine newborn screening panel, so there should not be barriers to accessibility if they’re getting prenatal and neonatal care.”
Commenting on HCV testing for babies in an interview at his institution, Ravi R. Jhaveri, MD, division head of pediatric infectious diseases at Northwestern Medicine’s Ann & Robert H. Lurie Children’s Hospital of Chicago, said, “This is a terrific way to capitalize on the fact that infants already come to the doctor for many visits during the first months of life for their vaccines and their well-child check. And so this should be an easy way to streamline our testing strategy and hopefully lose many fewer patients.”
Northwestern Medicine is an innovative clinic offering HCV testing and treatment outside of clinical trials for pregnant women and their infants with the goal of preventing transmission from mother to child.
Northwestern is launching a clinical trial of treatment for HCV-positive pregnant patients during regular prenatal care. “With very simple treatments similar to taking a prenatal vitamin, it would be easy and seamless to fit into the existing schedule,” said Lyn Yee, MD, a Northwestern maternal-fetal medicine specialist.
Dr. Yee stressed that eliminating hepatitis C will likely be one of the most significant health advancements of the decade.
Dr. Lewis, Dr. Jhaveri, and Dr. Yee had no relevant conflicts of interest to declare with regard to their comments.
A dozen genes emerge as dangerous during pregnancy
Single gene disorders remain a leading cause of morbidity and mortality in newborns and children, but carrier screening for such disorders was limited until recent advances in DNA sequencing, wrote Vivienne Souter, MD, of Natera in Austin, Tex., and colleagues.
Identifying single gene disorders in carrier screening also includes the discovery of genetic variants that could affect the carrier parent during pregnancy, they said.
In a study published in Obstetrics and Gynecology, the researchers reviewed data from 91,637 female patients who underwent testing via a 274-gene carrier screening panel. The median age of the participants was 32.8 years, and approximately half were pregnant at the time of the testing.
Based on previously published reports, the researchers identified 12 genes with potential for carrier manifestations during pregnancy; of these, 9 had manifestations whether or not the fetus was affected by the genetic condition (ABCB11, COL4A3, COL4A4, COL4A5, DMD, F9, F11, GLA, and OTC) and 3 had manifestations only if the fetus was affected by the condition (CPT1A, CYP19A1, and HADHA).
Overall, 66% of the tests were positive for at least one of the 274 genes; the frequency of potentially pathogenic variants for the 12 genes that could manifest as complications during pregnancy ranged from 1 in 117 individuals for the F11 gene to 1 in 8,331 for the OTC gene.
A total of 2.3% of the participant tests were associated a pathogenic or likely pathogenic variant in at least 1 of the 12 genes, which accounted for 3.5% of all positive samples, and 2.0% were identified as carriers for 1 of the 9 genes that could affect women during pregnancy regardless of fetal genetic status.
“People of Ashkenazi Jewish heritage were over-represented in the carrier group, representing 6.0% of carriers but only 1.9% of the entire study cohort,” the researchers noted.
Manifestations related to the 12 genes included cardiomyopathy, hemorrhage, gestational hypertensive disorders, cholestasis of pregnancy, acute fatty liver, hyperammonemic crisis, and maternal virilization.
“The reported incidence of pregnancy complications in carriers ranged from 10% to 62% depending on the gene involved, but information was limited for most of the conditions,” and published literature identified management recommendations for 11 of the 12 genes, the researchers wrote.
The findings were limited by several factors including the use of cases received by the laboratory, which might have yielded more women with above-average risk because of family history, the researchers noted. Other limitations included a lack of data on further evaluation or counseling after the screening, and the lack of separation of the results according to the specific variant, they said. Also, the study population was limited to those who had access to carrier screening, and may not be generalizable to the population at large.
However, the results support the value of carrier screening, and pretest counseling should inform individuals of the potential identification of genes that might increase their risk of complications during pregnancy, the researchers said.
“Obstetric care professionals should also be aware that carrier status for certain conditions can be important for risk assessment and management in pregnancy,” and post-test genetic counseling, follow-up testing, and clinical management can help reduce risks, which could potentially be identified prior to pregnancy, they concluded.
The study was funded by Natera. Dr. Souter is an employee of Natera.
Single gene disorders remain a leading cause of morbidity and mortality in newborns and children, but carrier screening for such disorders was limited until recent advances in DNA sequencing, wrote Vivienne Souter, MD, of Natera in Austin, Tex., and colleagues.
Identifying single gene disorders in carrier screening also includes the discovery of genetic variants that could affect the carrier parent during pregnancy, they said.
In a study published in Obstetrics and Gynecology, the researchers reviewed data from 91,637 female patients who underwent testing via a 274-gene carrier screening panel. The median age of the participants was 32.8 years, and approximately half were pregnant at the time of the testing.
Based on previously published reports, the researchers identified 12 genes with potential for carrier manifestations during pregnancy; of these, 9 had manifestations whether or not the fetus was affected by the genetic condition (ABCB11, COL4A3, COL4A4, COL4A5, DMD, F9, F11, GLA, and OTC) and 3 had manifestations only if the fetus was affected by the condition (CPT1A, CYP19A1, and HADHA).
Overall, 66% of the tests were positive for at least one of the 274 genes; the frequency of potentially pathogenic variants for the 12 genes that could manifest as complications during pregnancy ranged from 1 in 117 individuals for the F11 gene to 1 in 8,331 for the OTC gene.
A total of 2.3% of the participant tests were associated a pathogenic or likely pathogenic variant in at least 1 of the 12 genes, which accounted for 3.5% of all positive samples, and 2.0% were identified as carriers for 1 of the 9 genes that could affect women during pregnancy regardless of fetal genetic status.
“People of Ashkenazi Jewish heritage were over-represented in the carrier group, representing 6.0% of carriers but only 1.9% of the entire study cohort,” the researchers noted.
Manifestations related to the 12 genes included cardiomyopathy, hemorrhage, gestational hypertensive disorders, cholestasis of pregnancy, acute fatty liver, hyperammonemic crisis, and maternal virilization.
“The reported incidence of pregnancy complications in carriers ranged from 10% to 62% depending on the gene involved, but information was limited for most of the conditions,” and published literature identified management recommendations for 11 of the 12 genes, the researchers wrote.
The findings were limited by several factors including the use of cases received by the laboratory, which might have yielded more women with above-average risk because of family history, the researchers noted. Other limitations included a lack of data on further evaluation or counseling after the screening, and the lack of separation of the results according to the specific variant, they said. Also, the study population was limited to those who had access to carrier screening, and may not be generalizable to the population at large.
However, the results support the value of carrier screening, and pretest counseling should inform individuals of the potential identification of genes that might increase their risk of complications during pregnancy, the researchers said.
“Obstetric care professionals should also be aware that carrier status for certain conditions can be important for risk assessment and management in pregnancy,” and post-test genetic counseling, follow-up testing, and clinical management can help reduce risks, which could potentially be identified prior to pregnancy, they concluded.
The study was funded by Natera. Dr. Souter is an employee of Natera.
Single gene disorders remain a leading cause of morbidity and mortality in newborns and children, but carrier screening for such disorders was limited until recent advances in DNA sequencing, wrote Vivienne Souter, MD, of Natera in Austin, Tex., and colleagues.
Identifying single gene disorders in carrier screening also includes the discovery of genetic variants that could affect the carrier parent during pregnancy, they said.
In a study published in Obstetrics and Gynecology, the researchers reviewed data from 91,637 female patients who underwent testing via a 274-gene carrier screening panel. The median age of the participants was 32.8 years, and approximately half were pregnant at the time of the testing.
Based on previously published reports, the researchers identified 12 genes with potential for carrier manifestations during pregnancy; of these, 9 had manifestations whether or not the fetus was affected by the genetic condition (ABCB11, COL4A3, COL4A4, COL4A5, DMD, F9, F11, GLA, and OTC) and 3 had manifestations only if the fetus was affected by the condition (CPT1A, CYP19A1, and HADHA).
Overall, 66% of the tests were positive for at least one of the 274 genes; the frequency of potentially pathogenic variants for the 12 genes that could manifest as complications during pregnancy ranged from 1 in 117 individuals for the F11 gene to 1 in 8,331 for the OTC gene.
A total of 2.3% of the participant tests were associated a pathogenic or likely pathogenic variant in at least 1 of the 12 genes, which accounted for 3.5% of all positive samples, and 2.0% were identified as carriers for 1 of the 9 genes that could affect women during pregnancy regardless of fetal genetic status.
“People of Ashkenazi Jewish heritage were over-represented in the carrier group, representing 6.0% of carriers but only 1.9% of the entire study cohort,” the researchers noted.
Manifestations related to the 12 genes included cardiomyopathy, hemorrhage, gestational hypertensive disorders, cholestasis of pregnancy, acute fatty liver, hyperammonemic crisis, and maternal virilization.
“The reported incidence of pregnancy complications in carriers ranged from 10% to 62% depending on the gene involved, but information was limited for most of the conditions,” and published literature identified management recommendations for 11 of the 12 genes, the researchers wrote.
The findings were limited by several factors including the use of cases received by the laboratory, which might have yielded more women with above-average risk because of family history, the researchers noted. Other limitations included a lack of data on further evaluation or counseling after the screening, and the lack of separation of the results according to the specific variant, they said. Also, the study population was limited to those who had access to carrier screening, and may not be generalizable to the population at large.
However, the results support the value of carrier screening, and pretest counseling should inform individuals of the potential identification of genes that might increase their risk of complications during pregnancy, the researchers said.
“Obstetric care professionals should also be aware that carrier status for certain conditions can be important for risk assessment and management in pregnancy,” and post-test genetic counseling, follow-up testing, and clinical management can help reduce risks, which could potentially be identified prior to pregnancy, they concluded.
The study was funded by Natera. Dr. Souter is an employee of Natera.
FROM OBSTETRICS & GYNECOLOGY
Hypertensive disorders screening recommended for all pregnant women
Hypertensive disorders of pregnancy in the United States increased from approximately 500 cases per 10,000 deliveries to 1,021 cases per 10,000 deliveries from 1993 to 2016-2017, and remain a leading cause of maternal morbidity and mortality, wrote Task Force Chair Michael J. Barry, MD, of Massachusetts General Hospital, Boston, and colleagues in the final recommendation statement published in JAMA.
The USPSTF commissioned a systematic review to assess the risks and benefits of hypertensive screening for asymptomatic pregnant women. The resulting grade B recommendation indicates that screening for hypertensive disorders in pregnancy using blood pressure measurements yields a substantial net benefit.
The recommendation applies to “all pregnant women and pregnant persons of all genders without a known diagnosis of a hypertensive disorder of pregnancy or chronic hypertension,” the authors said.
The recommendation calls for the use of blood pressure measurements to evaluate hypertensive disorders, with measurements taken at each prenatal visit. A positive result for new-onset hypertension was defined as systolic blood pressure of 140 mm Hg or diastolic blood pressure 90 mm Hg in the absence of chronic hypertension, based on two measurements at least 4 hours apart. Regular review of blood pressure can help identify and manage potentially fatal conditions.
However, screening alone is insufficient to improve inequities in health outcomes associated with hypertensive disorders of pregnancy, the authors emphasized. Data from previous studies have shown that Black patients are at increased risk for hypertensive disorders of pregnancy and severe complications, and that Black and Hispanic patients have twice the risk of stroke with hypertensive disorders of pregnancy as White patients.
In the evidence report that supported the recommendation, Jillian T. Henderson, PhD, of Kaiser Permanente in Portland, Ore., and colleagues reviewed six studies including 10,165 individuals. The studies (five clinical trials and one nonrandomized study) compared changes in prenatal screening with usual care.
Overall, the review yielded no evidence that any other screening strategies were more useful than routine blood pressure measurement to identify hypertensive disorders of pregnancy in asymptomatic women.
The findings cited to support the recommendation were limited by several factors, including the lack of power to detect pregnancy health outcomes and potential harms of different screening programs, and the lack of power to evaluate outcomes for American Indian, Alaska Native, or Black individuals, who have disproportionately high rates of hypertensive disorders of pregnancy, the authors said.
More research is needed to identify which screening approaches may lead to improved disease detection and better health outcomes, but the results of the review support the grade B recommendation for hypertensive screening of all pregnant women, they concluded.
Early identification makes a difference
The new recommendation is important because it can help all moms and babies to be healthier, said Wanda Nicholson, MD, vice chair of the task force, in an interview.
“We are recommending that all pregnant persons have a blood pressure check at every visit throughout pregnancy,” said Dr. Nicholson, an ob.gyn. by training who also serves as professor of prevention and community health at George Washington University in Washington. “We know that there is a maternal health crisis in this country, and we know that hypertensive disorders of pregnancy are one of the key factors related to that,” she said.
Unfortunately, barriers to routine screening for hypertensive disorders of pregnancy persist, said Dr. Nicholson. The incidence of hypertensive disorders of pregnancy is higher in many of the same populations who also have challenges in accessing regular prenatal care, notably those who are Black, Native American, or Alaska Native, she noted.
The new recommendation also serves as an opportunity to call attention to the health care disparities for these populations, not only during pregnancy, but in general, she emphasized.
In clinical practice, the definition of hypertensive disorders of pregnancy involves three different diagnoses – gestational hypertension, preeclampsia, and eclampsia – that can be seen as points on a continuum, said Dr. Nicholson. The sooner patients are identified with hypertensive disorders of pregnancy, the sooner intervention and treatment can begin, she said. To that end, she added the clinical pearl of using a properly sized blood pressure cuff to obtain an accurate reading and avoid missed diagnoses.
The task force also outlined several key areas for additional research, said Dr. Nicholson. First, more research is needed on alternative screening strategies, such as at-home blood pressure monitoring for patients, as well as teleheath visits. Second, more studies are needed to address the disparities in prenatal care and include more diverse populations in clinical research. Third, future studies need to consider social determinants of health and other factors that might impact maternal health outcomes. “These steps will help achieve the larger goal of healthier mothers and babies,” Dr. Nicholson said.
Back to basics to improve women’s health
Some clinicians may be disappointed by the Evidence Report’s primary finding that no alternative screening strategies outperformed routine blood pressure measurement, wrote Anne E. Denoble, MD, and Christian M. Pettker, MD, both of Yale University, New Haven, Conn., in an accompanying editorial.
While potentially frustrating at first glance, the findings of the Evidence Report provide a foundation for improvement and reassurance that the best existing screening methods are basic and fundamental: regular prenatal visits with routine, in-office blood pressure measurements, and urine protein screening when clinically indicated, they said.
However, the USPSTF review also noted persistent research gaps that must be addressed to significantly improve maternal health outcomes, they said. Notable gaps include the disproportionately low numbers of Black patients in current studies, and the need for studies of alternate models of prenatal care, including the use of remote blood pressure monitoring, and the use of biomarkers to screen for and predict hypertensive disorders of pregnancy.
The most striking limitation may be the focus on prenatal care, with lack of attention to postpartum mortality risk, given that more than half of pregnancy-related deaths occur postpartum, the authors noted.
Although current screening tools may be used in practice “with skill and might,” more effort at multiple levels is needed to address the larger maternal health crisis in the United States, they said.
Expand screening, engage primary care for long-term benefits
Screening for hypertensive disorders of pregnancy “can and should be within the purview of internists,” wrote Srilakshmi Mitta, MD; Cary P. Gross, MD; Melissa A. Simon, MD, of Brown University, Yale University, and Northwestern University, respectively, in a separate editorial. The recommendation to extend screening beyond preeclampsia is timely, given the consistent increase in all hypertensive disorders of pregnancy since 1990, the authors said.
Pregnancy is not the only time for screening, counseling, and management of hypertensive disorders, they emphasized. “All persons who have reproductive capacity and/or are planning pregnancy, along with those who are post partum, should be screened for hypertensive disorders, aligning the USPSTF with guidelines from the American College of Obstetricians and Gynecologists, the American College of Cardiology, and the American Heart Association,” they said, and all clinicians should be on board to identify and treat hypertensive disorders of pregnancy, especially in underserved racial and ethnic minorities for whom primary care may be their only source of health care.
“Pregnancy is a window of opportunity to influence current and future life course, not just of the individual, but also of the fetus(es),other children, and family,” and timely intervention has the potential for great public health impact, they said.
Dr. Denoble disclosed grants from the HealthPartners Institute for Education and Research and from the Patient-Centered Outcomes Research Institute. Dr. Simon serves on the Advisory Committee for Research on Women’s Health for the National Institutes of Health Office of Research on Women’s Health and serves as a member of the Centers for Disease Control and Prevention Community Preventive Services Task Force; she was a member of the USPSTF from 2017 to 2020. Dr. Gross disclosed grants from Johnson and Johnson and the National Comprehensive Cancer Network (through a grant to the NCCN from AstraZeneca) and personal fees from Genentech.
Hypertensive disorders of pregnancy in the United States increased from approximately 500 cases per 10,000 deliveries to 1,021 cases per 10,000 deliveries from 1993 to 2016-2017, and remain a leading cause of maternal morbidity and mortality, wrote Task Force Chair Michael J. Barry, MD, of Massachusetts General Hospital, Boston, and colleagues in the final recommendation statement published in JAMA.
The USPSTF commissioned a systematic review to assess the risks and benefits of hypertensive screening for asymptomatic pregnant women. The resulting grade B recommendation indicates that screening for hypertensive disorders in pregnancy using blood pressure measurements yields a substantial net benefit.
The recommendation applies to “all pregnant women and pregnant persons of all genders without a known diagnosis of a hypertensive disorder of pregnancy or chronic hypertension,” the authors said.
The recommendation calls for the use of blood pressure measurements to evaluate hypertensive disorders, with measurements taken at each prenatal visit. A positive result for new-onset hypertension was defined as systolic blood pressure of 140 mm Hg or diastolic blood pressure 90 mm Hg in the absence of chronic hypertension, based on two measurements at least 4 hours apart. Regular review of blood pressure can help identify and manage potentially fatal conditions.
However, screening alone is insufficient to improve inequities in health outcomes associated with hypertensive disorders of pregnancy, the authors emphasized. Data from previous studies have shown that Black patients are at increased risk for hypertensive disorders of pregnancy and severe complications, and that Black and Hispanic patients have twice the risk of stroke with hypertensive disorders of pregnancy as White patients.
In the evidence report that supported the recommendation, Jillian T. Henderson, PhD, of Kaiser Permanente in Portland, Ore., and colleagues reviewed six studies including 10,165 individuals. The studies (five clinical trials and one nonrandomized study) compared changes in prenatal screening with usual care.
Overall, the review yielded no evidence that any other screening strategies were more useful than routine blood pressure measurement to identify hypertensive disorders of pregnancy in asymptomatic women.
The findings cited to support the recommendation were limited by several factors, including the lack of power to detect pregnancy health outcomes and potential harms of different screening programs, and the lack of power to evaluate outcomes for American Indian, Alaska Native, or Black individuals, who have disproportionately high rates of hypertensive disorders of pregnancy, the authors said.
More research is needed to identify which screening approaches may lead to improved disease detection and better health outcomes, but the results of the review support the grade B recommendation for hypertensive screening of all pregnant women, they concluded.
Early identification makes a difference
The new recommendation is important because it can help all moms and babies to be healthier, said Wanda Nicholson, MD, vice chair of the task force, in an interview.
“We are recommending that all pregnant persons have a blood pressure check at every visit throughout pregnancy,” said Dr. Nicholson, an ob.gyn. by training who also serves as professor of prevention and community health at George Washington University in Washington. “We know that there is a maternal health crisis in this country, and we know that hypertensive disorders of pregnancy are one of the key factors related to that,” she said.
Unfortunately, barriers to routine screening for hypertensive disorders of pregnancy persist, said Dr. Nicholson. The incidence of hypertensive disorders of pregnancy is higher in many of the same populations who also have challenges in accessing regular prenatal care, notably those who are Black, Native American, or Alaska Native, she noted.
The new recommendation also serves as an opportunity to call attention to the health care disparities for these populations, not only during pregnancy, but in general, she emphasized.
In clinical practice, the definition of hypertensive disorders of pregnancy involves three different diagnoses – gestational hypertension, preeclampsia, and eclampsia – that can be seen as points on a continuum, said Dr. Nicholson. The sooner patients are identified with hypertensive disorders of pregnancy, the sooner intervention and treatment can begin, she said. To that end, she added the clinical pearl of using a properly sized blood pressure cuff to obtain an accurate reading and avoid missed diagnoses.
The task force also outlined several key areas for additional research, said Dr. Nicholson. First, more research is needed on alternative screening strategies, such as at-home blood pressure monitoring for patients, as well as teleheath visits. Second, more studies are needed to address the disparities in prenatal care and include more diverse populations in clinical research. Third, future studies need to consider social determinants of health and other factors that might impact maternal health outcomes. “These steps will help achieve the larger goal of healthier mothers and babies,” Dr. Nicholson said.
Back to basics to improve women’s health
Some clinicians may be disappointed by the Evidence Report’s primary finding that no alternative screening strategies outperformed routine blood pressure measurement, wrote Anne E. Denoble, MD, and Christian M. Pettker, MD, both of Yale University, New Haven, Conn., in an accompanying editorial.
While potentially frustrating at first glance, the findings of the Evidence Report provide a foundation for improvement and reassurance that the best existing screening methods are basic and fundamental: regular prenatal visits with routine, in-office blood pressure measurements, and urine protein screening when clinically indicated, they said.
However, the USPSTF review also noted persistent research gaps that must be addressed to significantly improve maternal health outcomes, they said. Notable gaps include the disproportionately low numbers of Black patients in current studies, and the need for studies of alternate models of prenatal care, including the use of remote blood pressure monitoring, and the use of biomarkers to screen for and predict hypertensive disorders of pregnancy.
The most striking limitation may be the focus on prenatal care, with lack of attention to postpartum mortality risk, given that more than half of pregnancy-related deaths occur postpartum, the authors noted.
Although current screening tools may be used in practice “with skill and might,” more effort at multiple levels is needed to address the larger maternal health crisis in the United States, they said.
Expand screening, engage primary care for long-term benefits
Screening for hypertensive disorders of pregnancy “can and should be within the purview of internists,” wrote Srilakshmi Mitta, MD; Cary P. Gross, MD; Melissa A. Simon, MD, of Brown University, Yale University, and Northwestern University, respectively, in a separate editorial. The recommendation to extend screening beyond preeclampsia is timely, given the consistent increase in all hypertensive disorders of pregnancy since 1990, the authors said.
Pregnancy is not the only time for screening, counseling, and management of hypertensive disorders, they emphasized. “All persons who have reproductive capacity and/or are planning pregnancy, along with those who are post partum, should be screened for hypertensive disorders, aligning the USPSTF with guidelines from the American College of Obstetricians and Gynecologists, the American College of Cardiology, and the American Heart Association,” they said, and all clinicians should be on board to identify and treat hypertensive disorders of pregnancy, especially in underserved racial and ethnic minorities for whom primary care may be their only source of health care.
“Pregnancy is a window of opportunity to influence current and future life course, not just of the individual, but also of the fetus(es),other children, and family,” and timely intervention has the potential for great public health impact, they said.
Dr. Denoble disclosed grants from the HealthPartners Institute for Education and Research and from the Patient-Centered Outcomes Research Institute. Dr. Simon serves on the Advisory Committee for Research on Women’s Health for the National Institutes of Health Office of Research on Women’s Health and serves as a member of the Centers for Disease Control and Prevention Community Preventive Services Task Force; she was a member of the USPSTF from 2017 to 2020. Dr. Gross disclosed grants from Johnson and Johnson and the National Comprehensive Cancer Network (through a grant to the NCCN from AstraZeneca) and personal fees from Genentech.
Hypertensive disorders of pregnancy in the United States increased from approximately 500 cases per 10,000 deliveries to 1,021 cases per 10,000 deliveries from 1993 to 2016-2017, and remain a leading cause of maternal morbidity and mortality, wrote Task Force Chair Michael J. Barry, MD, of Massachusetts General Hospital, Boston, and colleagues in the final recommendation statement published in JAMA.
The USPSTF commissioned a systematic review to assess the risks and benefits of hypertensive screening for asymptomatic pregnant women. The resulting grade B recommendation indicates that screening for hypertensive disorders in pregnancy using blood pressure measurements yields a substantial net benefit.
The recommendation applies to “all pregnant women and pregnant persons of all genders without a known diagnosis of a hypertensive disorder of pregnancy or chronic hypertension,” the authors said.
The recommendation calls for the use of blood pressure measurements to evaluate hypertensive disorders, with measurements taken at each prenatal visit. A positive result for new-onset hypertension was defined as systolic blood pressure of 140 mm Hg or diastolic blood pressure 90 mm Hg in the absence of chronic hypertension, based on two measurements at least 4 hours apart. Regular review of blood pressure can help identify and manage potentially fatal conditions.
However, screening alone is insufficient to improve inequities in health outcomes associated with hypertensive disorders of pregnancy, the authors emphasized. Data from previous studies have shown that Black patients are at increased risk for hypertensive disorders of pregnancy and severe complications, and that Black and Hispanic patients have twice the risk of stroke with hypertensive disorders of pregnancy as White patients.
In the evidence report that supported the recommendation, Jillian T. Henderson, PhD, of Kaiser Permanente in Portland, Ore., and colleagues reviewed six studies including 10,165 individuals. The studies (five clinical trials and one nonrandomized study) compared changes in prenatal screening with usual care.
Overall, the review yielded no evidence that any other screening strategies were more useful than routine blood pressure measurement to identify hypertensive disorders of pregnancy in asymptomatic women.
The findings cited to support the recommendation were limited by several factors, including the lack of power to detect pregnancy health outcomes and potential harms of different screening programs, and the lack of power to evaluate outcomes for American Indian, Alaska Native, or Black individuals, who have disproportionately high rates of hypertensive disorders of pregnancy, the authors said.
More research is needed to identify which screening approaches may lead to improved disease detection and better health outcomes, but the results of the review support the grade B recommendation for hypertensive screening of all pregnant women, they concluded.
Early identification makes a difference
The new recommendation is important because it can help all moms and babies to be healthier, said Wanda Nicholson, MD, vice chair of the task force, in an interview.
“We are recommending that all pregnant persons have a blood pressure check at every visit throughout pregnancy,” said Dr. Nicholson, an ob.gyn. by training who also serves as professor of prevention and community health at George Washington University in Washington. “We know that there is a maternal health crisis in this country, and we know that hypertensive disorders of pregnancy are one of the key factors related to that,” she said.
Unfortunately, barriers to routine screening for hypertensive disorders of pregnancy persist, said Dr. Nicholson. The incidence of hypertensive disorders of pregnancy is higher in many of the same populations who also have challenges in accessing regular prenatal care, notably those who are Black, Native American, or Alaska Native, she noted.
The new recommendation also serves as an opportunity to call attention to the health care disparities for these populations, not only during pregnancy, but in general, she emphasized.
In clinical practice, the definition of hypertensive disorders of pregnancy involves three different diagnoses – gestational hypertension, preeclampsia, and eclampsia – that can be seen as points on a continuum, said Dr. Nicholson. The sooner patients are identified with hypertensive disorders of pregnancy, the sooner intervention and treatment can begin, she said. To that end, she added the clinical pearl of using a properly sized blood pressure cuff to obtain an accurate reading and avoid missed diagnoses.
The task force also outlined several key areas for additional research, said Dr. Nicholson. First, more research is needed on alternative screening strategies, such as at-home blood pressure monitoring for patients, as well as teleheath visits. Second, more studies are needed to address the disparities in prenatal care and include more diverse populations in clinical research. Third, future studies need to consider social determinants of health and other factors that might impact maternal health outcomes. “These steps will help achieve the larger goal of healthier mothers and babies,” Dr. Nicholson said.
Back to basics to improve women’s health
Some clinicians may be disappointed by the Evidence Report’s primary finding that no alternative screening strategies outperformed routine blood pressure measurement, wrote Anne E. Denoble, MD, and Christian M. Pettker, MD, both of Yale University, New Haven, Conn., in an accompanying editorial.
While potentially frustrating at first glance, the findings of the Evidence Report provide a foundation for improvement and reassurance that the best existing screening methods are basic and fundamental: regular prenatal visits with routine, in-office blood pressure measurements, and urine protein screening when clinically indicated, they said.
However, the USPSTF review also noted persistent research gaps that must be addressed to significantly improve maternal health outcomes, they said. Notable gaps include the disproportionately low numbers of Black patients in current studies, and the need for studies of alternate models of prenatal care, including the use of remote blood pressure monitoring, and the use of biomarkers to screen for and predict hypertensive disorders of pregnancy.
The most striking limitation may be the focus on prenatal care, with lack of attention to postpartum mortality risk, given that more than half of pregnancy-related deaths occur postpartum, the authors noted.
Although current screening tools may be used in practice “with skill and might,” more effort at multiple levels is needed to address the larger maternal health crisis in the United States, they said.
Expand screening, engage primary care for long-term benefits
Screening for hypertensive disorders of pregnancy “can and should be within the purview of internists,” wrote Srilakshmi Mitta, MD; Cary P. Gross, MD; Melissa A. Simon, MD, of Brown University, Yale University, and Northwestern University, respectively, in a separate editorial. The recommendation to extend screening beyond preeclampsia is timely, given the consistent increase in all hypertensive disorders of pregnancy since 1990, the authors said.
Pregnancy is not the only time for screening, counseling, and management of hypertensive disorders, they emphasized. “All persons who have reproductive capacity and/or are planning pregnancy, along with those who are post partum, should be screened for hypertensive disorders, aligning the USPSTF with guidelines from the American College of Obstetricians and Gynecologists, the American College of Cardiology, and the American Heart Association,” they said, and all clinicians should be on board to identify and treat hypertensive disorders of pregnancy, especially in underserved racial and ethnic minorities for whom primary care may be their only source of health care.
“Pregnancy is a window of opportunity to influence current and future life course, not just of the individual, but also of the fetus(es),other children, and family,” and timely intervention has the potential for great public health impact, they said.
Dr. Denoble disclosed grants from the HealthPartners Institute for Education and Research and from the Patient-Centered Outcomes Research Institute. Dr. Simon serves on the Advisory Committee for Research on Women’s Health for the National Institutes of Health Office of Research on Women’s Health and serves as a member of the Centers for Disease Control and Prevention Community Preventive Services Task Force; she was a member of the USPSTF from 2017 to 2020. Dr. Gross disclosed grants from Johnson and Johnson and the National Comprehensive Cancer Network (through a grant to the NCCN from AstraZeneca) and personal fees from Genentech.
FROM JAMA










