User login
Trends in teen consumption of sports drinks are up and down
Although daily consumption of sports drinks decreased from 2010 to 2015 among teenagers, sugar-sweetened sports drinks still are popular, with numerous high school students drinking them at least weekly, said Kyla Cordery of the Steven and Alexandra Cohen Children’s Medical Center of New York, Lake Success, N.Y., and her associates.
Yet sports drink consumption in the previous week increased from 58% in 2010 to 60% in 2015 (P = .0002). And daily consumption of sports drinks also increased among teenagers watching television for more than 2 hours per day and among obese teens.
Boys were more than twice as likely as girls to drink one of more sports drinks daily (19% vs. 9%), as were more athletic/active children than those weren’t very athletic/active (18% vs. 10%).
“ Like many sugar-sweetened beverages, the excessive consumption of sports drinks is associated with weight gain, dental erosion, obesity, poor nutrition, and diabetes,” Ms. Cordery and her associates wrote. “The America Academy of Pediatrics’ Committee on Nutrition and Council on Sports Medicine and Fitness stated that the level of physical activity of the average child does not require the electrolyte replenishment offered by sports drinks.” Rehydration with water should be encouraged for most sports-related activities.
SOURCE: Cordery K et al. doi: 10.1542/peds.2017-2784.
Although daily consumption of sports drinks decreased from 2010 to 2015 among teenagers, sugar-sweetened sports drinks still are popular, with numerous high school students drinking them at least weekly, said Kyla Cordery of the Steven and Alexandra Cohen Children’s Medical Center of New York, Lake Success, N.Y., and her associates.
Yet sports drink consumption in the previous week increased from 58% in 2010 to 60% in 2015 (P = .0002). And daily consumption of sports drinks also increased among teenagers watching television for more than 2 hours per day and among obese teens.
Boys were more than twice as likely as girls to drink one of more sports drinks daily (19% vs. 9%), as were more athletic/active children than those weren’t very athletic/active (18% vs. 10%).
“ Like many sugar-sweetened beverages, the excessive consumption of sports drinks is associated with weight gain, dental erosion, obesity, poor nutrition, and diabetes,” Ms. Cordery and her associates wrote. “The America Academy of Pediatrics’ Committee on Nutrition and Council on Sports Medicine and Fitness stated that the level of physical activity of the average child does not require the electrolyte replenishment offered by sports drinks.” Rehydration with water should be encouraged for most sports-related activities.
SOURCE: Cordery K et al. doi: 10.1542/peds.2017-2784.
Although daily consumption of sports drinks decreased from 2010 to 2015 among teenagers, sugar-sweetened sports drinks still are popular, with numerous high school students drinking them at least weekly, said Kyla Cordery of the Steven and Alexandra Cohen Children’s Medical Center of New York, Lake Success, N.Y., and her associates.
Yet sports drink consumption in the previous week increased from 58% in 2010 to 60% in 2015 (P = .0002). And daily consumption of sports drinks also increased among teenagers watching television for more than 2 hours per day and among obese teens.
Boys were more than twice as likely as girls to drink one of more sports drinks daily (19% vs. 9%), as were more athletic/active children than those weren’t very athletic/active (18% vs. 10%).
“ Like many sugar-sweetened beverages, the excessive consumption of sports drinks is associated with weight gain, dental erosion, obesity, poor nutrition, and diabetes,” Ms. Cordery and her associates wrote. “The America Academy of Pediatrics’ Committee on Nutrition and Council on Sports Medicine and Fitness stated that the level of physical activity of the average child does not require the electrolyte replenishment offered by sports drinks.” Rehydration with water should be encouraged for most sports-related activities.
SOURCE: Cordery K et al. doi: 10.1542/peds.2017-2784.
FROM PEDIATRICS
New law allows Maryland students to use sunscreen at school
In early April, Maryland Governor Larry Hogan signed HB 427 into law, making Maryland the second state in 2018 to ensure that state policy allows students to possess and use sunscreen at school.
“The passing of this bill helps encourage children to develop sun-safe behaviors early on, like sunscreen application,” American Society for Dermatologic Surgery Association president Lisa Donofrio, MD, said in a statement issued by the ASDSA. “Maryland’s efforts reinforce the importance of teaching children the risks of sun exposure during outdoor activities and how to best avoid skin cancer,” she added.
ASDSA worked with the Maryland Dermatologic Society in advocating for passage of the law. In the statement, ASDSA Board Member Lawrence Green, MD, of Rockville, Maryland, who testified in favor of SB 217, said, “allowing children to put on sunscreen before recess … would really help protect them from the dangers of the sun.”
More information about SUNucate is available here.
In early April, Maryland Governor Larry Hogan signed HB 427 into law, making Maryland the second state in 2018 to ensure that state policy allows students to possess and use sunscreen at school.
“The passing of this bill helps encourage children to develop sun-safe behaviors early on, like sunscreen application,” American Society for Dermatologic Surgery Association president Lisa Donofrio, MD, said in a statement issued by the ASDSA. “Maryland’s efforts reinforce the importance of teaching children the risks of sun exposure during outdoor activities and how to best avoid skin cancer,” she added.
ASDSA worked with the Maryland Dermatologic Society in advocating for passage of the law. In the statement, ASDSA Board Member Lawrence Green, MD, of Rockville, Maryland, who testified in favor of SB 217, said, “allowing children to put on sunscreen before recess … would really help protect them from the dangers of the sun.”
More information about SUNucate is available here.
In early April, Maryland Governor Larry Hogan signed HB 427 into law, making Maryland the second state in 2018 to ensure that state policy allows students to possess and use sunscreen at school.
“The passing of this bill helps encourage children to develop sun-safe behaviors early on, like sunscreen application,” American Society for Dermatologic Surgery Association president Lisa Donofrio, MD, said in a statement issued by the ASDSA. “Maryland’s efforts reinforce the importance of teaching children the risks of sun exposure during outdoor activities and how to best avoid skin cancer,” she added.
ASDSA worked with the Maryland Dermatologic Society in advocating for passage of the law. In the statement, ASDSA Board Member Lawrence Green, MD, of Rockville, Maryland, who testified in favor of SB 217, said, “allowing children to put on sunscreen before recess … would really help protect them from the dangers of the sun.”
More information about SUNucate is available here.
Liquid nicotine for e-cigarettes may poison young children
said Preethi Govindarajan of the Research Institute at Nationwide Children’s Hospital in Columbus, Ohio, and his associates.
Since January 2015, pediatric exposures to liquid nicotine have decreased, which may be attributable to legislation requiring child-resistant packaging for liquid nicotine containers and also greater public awareness of the risks associated with e-cigarette products, they noted.
There was a significant increase in the monthly number of liquid nicotine exposures of 2,390% (P less than .001) from November 2012 through January 2015, and a significant decrease of 48% (P less than .001) from January 2015 through April 2017. “From August 2016 (175 exposures), the first month after the federal Child Nicotine Poisoning Prevention Act went into effect, to April 2017 (142 exposures), there was an 18.9% decrease in the number of monthly liquid nicotine exposures,” Mr. Govindarajan and his associates said.
“Child-resistant e-cigarette devices, use of flow restrictors on liquid nicotine containers, and regulations on e-cigarette liquid flavoring, labeling, and concentrations could further reduce the incidence of these exposures and the likelihood of serious medical outcomes when exposures do occur,” they concluded.
SOURCE: Govindarajan P et al. Pediatrics. 2018;141(5):e20173361.
said Preethi Govindarajan of the Research Institute at Nationwide Children’s Hospital in Columbus, Ohio, and his associates.
Since January 2015, pediatric exposures to liquid nicotine have decreased, which may be attributable to legislation requiring child-resistant packaging for liquid nicotine containers and also greater public awareness of the risks associated with e-cigarette products, they noted.
There was a significant increase in the monthly number of liquid nicotine exposures of 2,390% (P less than .001) from November 2012 through January 2015, and a significant decrease of 48% (P less than .001) from January 2015 through April 2017. “From August 2016 (175 exposures), the first month after the federal Child Nicotine Poisoning Prevention Act went into effect, to April 2017 (142 exposures), there was an 18.9% decrease in the number of monthly liquid nicotine exposures,” Mr. Govindarajan and his associates said.
“Child-resistant e-cigarette devices, use of flow restrictors on liquid nicotine containers, and regulations on e-cigarette liquid flavoring, labeling, and concentrations could further reduce the incidence of these exposures and the likelihood of serious medical outcomes when exposures do occur,” they concluded.
SOURCE: Govindarajan P et al. Pediatrics. 2018;141(5):e20173361.
said Preethi Govindarajan of the Research Institute at Nationwide Children’s Hospital in Columbus, Ohio, and his associates.
Since January 2015, pediatric exposures to liquid nicotine have decreased, which may be attributable to legislation requiring child-resistant packaging for liquid nicotine containers and also greater public awareness of the risks associated with e-cigarette products, they noted.
There was a significant increase in the monthly number of liquid nicotine exposures of 2,390% (P less than .001) from November 2012 through January 2015, and a significant decrease of 48% (P less than .001) from January 2015 through April 2017. “From August 2016 (175 exposures), the first month after the federal Child Nicotine Poisoning Prevention Act went into effect, to April 2017 (142 exposures), there was an 18.9% decrease in the number of monthly liquid nicotine exposures,” Mr. Govindarajan and his associates said.
“Child-resistant e-cigarette devices, use of flow restrictors on liquid nicotine containers, and regulations on e-cigarette liquid flavoring, labeling, and concentrations could further reduce the incidence of these exposures and the likelihood of serious medical outcomes when exposures do occur,” they concluded.
SOURCE: Govindarajan P et al. Pediatrics. 2018;141(5):e20173361.
Language difficulties persist until age 13 for very preterm infants
Children born very preterm continue to display language difficulties compared with term controls at 13 years of age, with no evidence of developmental “catch up.”
Given the functional implications associated with language deficits, early language-based interventions should be considered for children born VP, investigators wrote in Pediatrics.
“Difficulties in language functioning in this cohort of children born VP remained stable from 2 to 13 years of age. Although generalized language deficits were observed, the VP group had marked difficulties on expressive components of language,” wrote Thi-Nhu-Ngoc Nguyen, BSc, of Monash University, Melbourne, and her associates.
Read the full story here.
Children born very preterm continue to display language difficulties compared with term controls at 13 years of age, with no evidence of developmental “catch up.”
Given the functional implications associated with language deficits, early language-based interventions should be considered for children born VP, investigators wrote in Pediatrics.
“Difficulties in language functioning in this cohort of children born VP remained stable from 2 to 13 years of age. Although generalized language deficits were observed, the VP group had marked difficulties on expressive components of language,” wrote Thi-Nhu-Ngoc Nguyen, BSc, of Monash University, Melbourne, and her associates.
Read the full story here.
Children born very preterm continue to display language difficulties compared with term controls at 13 years of age, with no evidence of developmental “catch up.”
Given the functional implications associated with language deficits, early language-based interventions should be considered for children born VP, investigators wrote in Pediatrics.
“Difficulties in language functioning in this cohort of children born VP remained stable from 2 to 13 years of age. Although generalized language deficits were observed, the VP group had marked difficulties on expressive components of language,” wrote Thi-Nhu-Ngoc Nguyen, BSc, of Monash University, Melbourne, and her associates.
Read the full story here.
FROM PEDIATRICS
Therapeutic horseback riding may lower veterans’ PTSD symptoms
Veterans with posttraumatic stress disorder might benefit from therapeutic horseback riding, a small study suggests.
“Our findings provide empirical evidence that [therapeutic horseback riding] is effective at improving coping skills and in lessening one’s difficulty with emotional regulation, especially with longer riding interventions,” wrote Rebecca A. Johnson, PhD, of the University of Missouri, Columbia, and her associates.
Overall, 57 veterans were recruited and 28 enrolled in the randomized trial at baseline. Those individuals were randomized into two groups: a wait-list control group and a treatment group. Eventually, all of the veterans participated in the therapeutic riding program. Meanwhile, the riding center staff were not aware of which veterans had been assigned to either group. The Professional Association of Therapeutic Horsemanship, a nonprofit group that promotes equine-related activities for people with special needs, selected the horses that were used in the study. During the data collection periods, PTSD symptoms were measured via the PTSD Checklist–Military Version, or PCL-M. This self-report measure asks patients about problems in response to “stressful military experiences,” the researchers wrote. The Coping Self-Efficacy Scale and the Difficulties in Emotion Regulation Scale were among the other instruments used.
While riding, the results showed, participants had a statistically significant decrease in PTSD symptoms over the course of the 6-week program. “,” Dr. Johnson and her associates wrote. “Further detailed examination showed that participants had a 66.7% likelihood of having lower PTSD scores at 3 weeks, and an 87.5% likelihood at 6 weeks.”
Anecdotally, some of the veterans wanted to continue therapeutic riding after the end of the program, and they were able to do so.
“We conclude that [therapeutic horseback riding] shows promise as a beneficial intervention for veterans with PTSD, but did not measure functional ability,” they wrote.
Veterans with posttraumatic stress disorder might benefit from therapeutic horseback riding, a small study suggests.
“Our findings provide empirical evidence that [therapeutic horseback riding] is effective at improving coping skills and in lessening one’s difficulty with emotional regulation, especially with longer riding interventions,” wrote Rebecca A. Johnson, PhD, of the University of Missouri, Columbia, and her associates.
Overall, 57 veterans were recruited and 28 enrolled in the randomized trial at baseline. Those individuals were randomized into two groups: a wait-list control group and a treatment group. Eventually, all of the veterans participated in the therapeutic riding program. Meanwhile, the riding center staff were not aware of which veterans had been assigned to either group. The Professional Association of Therapeutic Horsemanship, a nonprofit group that promotes equine-related activities for people with special needs, selected the horses that were used in the study. During the data collection periods, PTSD symptoms were measured via the PTSD Checklist–Military Version, or PCL-M. This self-report measure asks patients about problems in response to “stressful military experiences,” the researchers wrote. The Coping Self-Efficacy Scale and the Difficulties in Emotion Regulation Scale were among the other instruments used.
While riding, the results showed, participants had a statistically significant decrease in PTSD symptoms over the course of the 6-week program. “,” Dr. Johnson and her associates wrote. “Further detailed examination showed that participants had a 66.7% likelihood of having lower PTSD scores at 3 weeks, and an 87.5% likelihood at 6 weeks.”
Anecdotally, some of the veterans wanted to continue therapeutic riding after the end of the program, and they were able to do so.
“We conclude that [therapeutic horseback riding] shows promise as a beneficial intervention for veterans with PTSD, but did not measure functional ability,” they wrote.
Veterans with posttraumatic stress disorder might benefit from therapeutic horseback riding, a small study suggests.
“Our findings provide empirical evidence that [therapeutic horseback riding] is effective at improving coping skills and in lessening one’s difficulty with emotional regulation, especially with longer riding interventions,” wrote Rebecca A. Johnson, PhD, of the University of Missouri, Columbia, and her associates.
Overall, 57 veterans were recruited and 28 enrolled in the randomized trial at baseline. Those individuals were randomized into two groups: a wait-list control group and a treatment group. Eventually, all of the veterans participated in the therapeutic riding program. Meanwhile, the riding center staff were not aware of which veterans had been assigned to either group. The Professional Association of Therapeutic Horsemanship, a nonprofit group that promotes equine-related activities for people with special needs, selected the horses that were used in the study. During the data collection periods, PTSD symptoms were measured via the PTSD Checklist–Military Version, or PCL-M. This self-report measure asks patients about problems in response to “stressful military experiences,” the researchers wrote. The Coping Self-Efficacy Scale and the Difficulties in Emotion Regulation Scale were among the other instruments used.
While riding, the results showed, participants had a statistically significant decrease in PTSD symptoms over the course of the 6-week program. “,” Dr. Johnson and her associates wrote. “Further detailed examination showed that participants had a 66.7% likelihood of having lower PTSD scores at 3 weeks, and an 87.5% likelihood at 6 weeks.”
Anecdotally, some of the veterans wanted to continue therapeutic riding after the end of the program, and they were able to do so.
“We conclude that [therapeutic horseback riding] shows promise as a beneficial intervention for veterans with PTSD, but did not measure functional ability,” they wrote.
FROM MILITARY MEDICAL RESEARCH
NIMH launches interactive statistics section on its website
The National Institute of Mental Health (NIMH) has announced the creation of a redesigned statistics section on its website that feature data interactive visualization tools.
In addition to providing statistics on mental illness in general, . It also includes data on suicide, which is among the country’s top 10 causes of death.
“Each [person with mental illness] has a singular, compelling story that conveys an understanding of the depth of suffering,” said Joshua A. Gordon, MD, PhD, director of the NIMH, in a statement announcing the tool. “Statistics build on this foundation by helping us better understand the broader scope and impact of mental illnesses on society.”
The NIMH said the section would be updated as new data from agencies, such as the Centers for Disease Control and Prevention and the Substance Abuse and Mental Health Services Administration, are released.
Read the full announcement here
The National Institute of Mental Health (NIMH) has announced the creation of a redesigned statistics section on its website that feature data interactive visualization tools.
In addition to providing statistics on mental illness in general, . It also includes data on suicide, which is among the country’s top 10 causes of death.
“Each [person with mental illness] has a singular, compelling story that conveys an understanding of the depth of suffering,” said Joshua A. Gordon, MD, PhD, director of the NIMH, in a statement announcing the tool. “Statistics build on this foundation by helping us better understand the broader scope and impact of mental illnesses on society.”
The NIMH said the section would be updated as new data from agencies, such as the Centers for Disease Control and Prevention and the Substance Abuse and Mental Health Services Administration, are released.
Read the full announcement here
The National Institute of Mental Health (NIMH) has announced the creation of a redesigned statistics section on its website that feature data interactive visualization tools.
In addition to providing statistics on mental illness in general, . It also includes data on suicide, which is among the country’s top 10 causes of death.
“Each [person with mental illness] has a singular, compelling story that conveys an understanding of the depth of suffering,” said Joshua A. Gordon, MD, PhD, director of the NIMH, in a statement announcing the tool. “Statistics build on this foundation by helping us better understand the broader scope and impact of mental illnesses on society.”
The NIMH said the section would be updated as new data from agencies, such as the Centers for Disease Control and Prevention and the Substance Abuse and Mental Health Services Administration, are released.
Read the full announcement here
APOE4: Elders with allele benefit from lifestyle changes
allele, Alina Solomon, MD, PhD, reported in JAMA Neurology.
“Whether such benefits are more pronounced in APOE4 carriers, compared with noncarriers, should be further investigated,” wrote Dr. Solomon of the Institute of Clinical Medicine/Neurology at the University of Eastern Finland, Kuopio, and her associates.
The investigators analyzed data of 1,109 participants in the multicenter Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER), a randomized, controlled trial of at-risk individuals from the general population. Participants were aged 60-77 years, and 362 of them were carriers of the APOE4 allele.
Those randomized to the intervention group received targeted information about nutrition, instructions about physical exercise, and cognitive training – including group sessions led by a psychologist. The control group received “regular health advice” (JAMA Neurol. 2018 Jan 22. doi: 10.1001/jamaneurol.2017.4365).
After the interventions, participants underwent a battery of neuropsychological testing. Dr. Solomon and her associates found that the per-year difference between the intervention and control groups in the total score change was 0.037 (95% confidence interval, 0.001-0.073) among APOE4 carriers and 0.014 (95% CI, −0.011-0.039) among noncarriers.
Among other things, the findings stress the importance of early prevention strategies targeting simultaneously many risk factors that are modifiable, the investigators said.
To read the full story, click here.
allele, Alina Solomon, MD, PhD, reported in JAMA Neurology.
“Whether such benefits are more pronounced in APOE4 carriers, compared with noncarriers, should be further investigated,” wrote Dr. Solomon of the Institute of Clinical Medicine/Neurology at the University of Eastern Finland, Kuopio, and her associates.
The investigators analyzed data of 1,109 participants in the multicenter Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER), a randomized, controlled trial of at-risk individuals from the general population. Participants were aged 60-77 years, and 362 of them were carriers of the APOE4 allele.
Those randomized to the intervention group received targeted information about nutrition, instructions about physical exercise, and cognitive training – including group sessions led by a psychologist. The control group received “regular health advice” (JAMA Neurol. 2018 Jan 22. doi: 10.1001/jamaneurol.2017.4365).
After the interventions, participants underwent a battery of neuropsychological testing. Dr. Solomon and her associates found that the per-year difference between the intervention and control groups in the total score change was 0.037 (95% confidence interval, 0.001-0.073) among APOE4 carriers and 0.014 (95% CI, −0.011-0.039) among noncarriers.
Among other things, the findings stress the importance of early prevention strategies targeting simultaneously many risk factors that are modifiable, the investigators said.
To read the full story, click here.
allele, Alina Solomon, MD, PhD, reported in JAMA Neurology.
“Whether such benefits are more pronounced in APOE4 carriers, compared with noncarriers, should be further investigated,” wrote Dr. Solomon of the Institute of Clinical Medicine/Neurology at the University of Eastern Finland, Kuopio, and her associates.
The investigators analyzed data of 1,109 participants in the multicenter Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER), a randomized, controlled trial of at-risk individuals from the general population. Participants were aged 60-77 years, and 362 of them were carriers of the APOE4 allele.
Those randomized to the intervention group received targeted information about nutrition, instructions about physical exercise, and cognitive training – including group sessions led by a psychologist. The control group received “regular health advice” (JAMA Neurol. 2018 Jan 22. doi: 10.1001/jamaneurol.2017.4365).
After the interventions, participants underwent a battery of neuropsychological testing. Dr. Solomon and her associates found that the per-year difference between the intervention and control groups in the total score change was 0.037 (95% confidence interval, 0.001-0.073) among APOE4 carriers and 0.014 (95% CI, −0.011-0.039) among noncarriers.
Among other things, the findings stress the importance of early prevention strategies targeting simultaneously many risk factors that are modifiable, the investigators said.
To read the full story, click here.
FROM JAMA NEUROLOGY
CBT cost effective for depressed teens refusing antidepressants
“In this study, we demonstrate that brief primary care CBT is a cost-effective treatment option for adolescents with depression and likely generates cost savings over 2 years,” said John F. Dickerson, PhD, of Kaiser Permanente Center for Health Research, Portland, Ore., and his associates
Youth randomly assigned to CBT plus TAU had 26.8 more depression-free days (P = .043) and 0.067 more quality-adjusted life-years (P = .043) on average, compared with patients receiving TAU over a 12-month period. Also, patients in the CBT group had fewer hospitalizations, compared with patients in the TAU group (1.1% vs. 8.8% during 12 months, and 4.4% vs. 12.1% during 24 months), reported Dr. Dickerson and his colleagues.
By the end of the 24-month follow-up, average total costs were $2,811 among youth randomly assigned to CBT plus TAU and $7,354 among youth assigned TAU (adjusted to 2008 U.S. dollars).
“Many adolescents with depression choose to not initiate or continue antidepressant therapy, which limits their options for depression treatment,” the investigators said. “In this evaluation, it is demonstrated that brief, primary care–based CBT is a cost-effective option for the treatment of depression among adolescents with depression who decline or quickly discontinue pharmacotherapy.”
Read more at Pediatrics (2018. doi: 10.1542/peds.2017-1969).
“In this study, we demonstrate that brief primary care CBT is a cost-effective treatment option for adolescents with depression and likely generates cost savings over 2 years,” said John F. Dickerson, PhD, of Kaiser Permanente Center for Health Research, Portland, Ore., and his associates
Youth randomly assigned to CBT plus TAU had 26.8 more depression-free days (P = .043) and 0.067 more quality-adjusted life-years (P = .043) on average, compared with patients receiving TAU over a 12-month period. Also, patients in the CBT group had fewer hospitalizations, compared with patients in the TAU group (1.1% vs. 8.8% during 12 months, and 4.4% vs. 12.1% during 24 months), reported Dr. Dickerson and his colleagues.
By the end of the 24-month follow-up, average total costs were $2,811 among youth randomly assigned to CBT plus TAU and $7,354 among youth assigned TAU (adjusted to 2008 U.S. dollars).
“Many adolescents with depression choose to not initiate or continue antidepressant therapy, which limits their options for depression treatment,” the investigators said. “In this evaluation, it is demonstrated that brief, primary care–based CBT is a cost-effective option for the treatment of depression among adolescents with depression who decline or quickly discontinue pharmacotherapy.”
Read more at Pediatrics (2018. doi: 10.1542/peds.2017-1969).
“In this study, we demonstrate that brief primary care CBT is a cost-effective treatment option for adolescents with depression and likely generates cost savings over 2 years,” said John F. Dickerson, PhD, of Kaiser Permanente Center for Health Research, Portland, Ore., and his associates
Youth randomly assigned to CBT plus TAU had 26.8 more depression-free days (P = .043) and 0.067 more quality-adjusted life-years (P = .043) on average, compared with patients receiving TAU over a 12-month period. Also, patients in the CBT group had fewer hospitalizations, compared with patients in the TAU group (1.1% vs. 8.8% during 12 months, and 4.4% vs. 12.1% during 24 months), reported Dr. Dickerson and his colleagues.
By the end of the 24-month follow-up, average total costs were $2,811 among youth randomly assigned to CBT plus TAU and $7,354 among youth assigned TAU (adjusted to 2008 U.S. dollars).
“Many adolescents with depression choose to not initiate or continue antidepressant therapy, which limits their options for depression treatment,” the investigators said. “In this evaluation, it is demonstrated that brief, primary care–based CBT is a cost-effective option for the treatment of depression among adolescents with depression who decline or quickly discontinue pharmacotherapy.”
Read more at Pediatrics (2018. doi: 10.1542/peds.2017-1969).
FROM PEDIATRICS
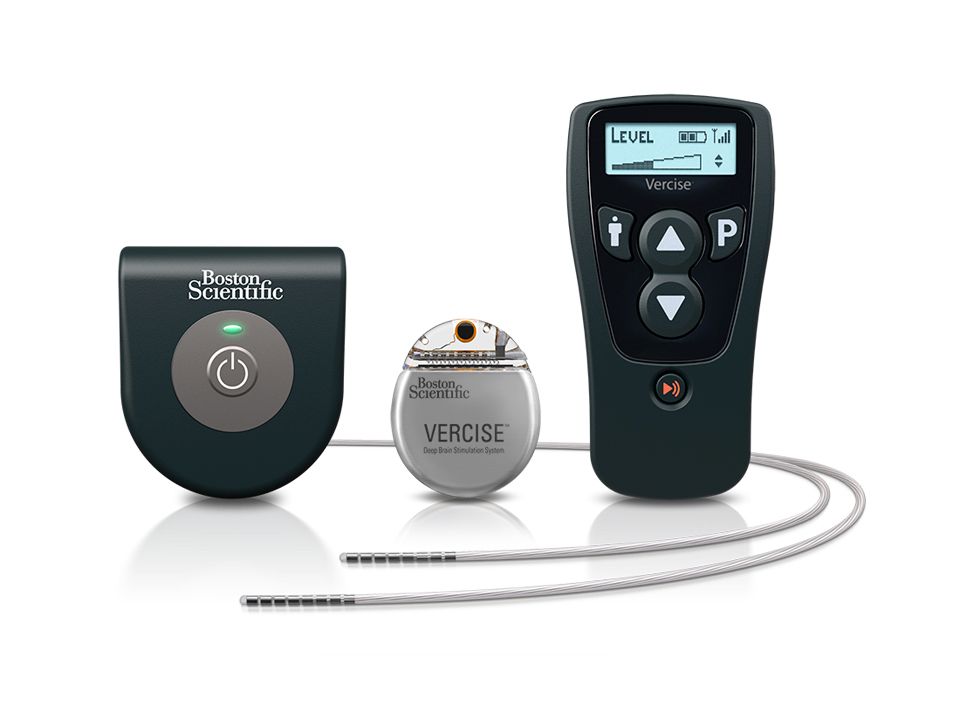
New DBS device gains approval for Parkinson’s disease
The approval is based on as-yet unpublished results of the INTREPID trial, which successfully met its primary endpoint of mean change in waking hours with good symptom control, according to an announcement from the device’s manufacturer, Boston Scientific. The INTREPID study is the first multicenter, prospective, double-blind, randomized, sham-controlled study of DBS for Parkinson’s disease in the United States and enrolled in 292 patients at 23 sites.
The implantable pulse generator of the device is “the smallest, rechargeable DBS device available in the U.S.,” according to Boston Scientific, and can independently control the amount of current delivered by each of the electrodes on the implanted leads, allowing them “to work together to address common challenges in DBS therapy such as fluctuations in symptoms and the progressive nature of the condition by offering more adaptable delivery of stimulation.”
“The Vercise DBS System changes the landscape of what physicians can do to help improve the quality of life for people living with Parkinson’s disease,” Jerry Vitek, MD, PhD, professor and chair of the department of neurology at the University of Minnesota, Minneapolis, and coordinating principal investigator for the INTREPID study said in the Boston Scientific announcement. “This system provides an ability to sculpt the current field in the DBS target using novel technology that offers flexibility in programming. This flexibility allows us to target different regions of the subthalamic nucleus, which we believe will improve outcomes while reducing side effects.”
The approval is based on as-yet unpublished results of the INTREPID trial, which successfully met its primary endpoint of mean change in waking hours with good symptom control, according to an announcement from the device’s manufacturer, Boston Scientific. The INTREPID study is the first multicenter, prospective, double-blind, randomized, sham-controlled study of DBS for Parkinson’s disease in the United States and enrolled in 292 patients at 23 sites.
The implantable pulse generator of the device is “the smallest, rechargeable DBS device available in the U.S.,” according to Boston Scientific, and can independently control the amount of current delivered by each of the electrodes on the implanted leads, allowing them “to work together to address common challenges in DBS therapy such as fluctuations in symptoms and the progressive nature of the condition by offering more adaptable delivery of stimulation.”
“The Vercise DBS System changes the landscape of what physicians can do to help improve the quality of life for people living with Parkinson’s disease,” Jerry Vitek, MD, PhD, professor and chair of the department of neurology at the University of Minnesota, Minneapolis, and coordinating principal investigator for the INTREPID study said in the Boston Scientific announcement. “This system provides an ability to sculpt the current field in the DBS target using novel technology that offers flexibility in programming. This flexibility allows us to target different regions of the subthalamic nucleus, which we believe will improve outcomes while reducing side effects.”
The approval is based on as-yet unpublished results of the INTREPID trial, which successfully met its primary endpoint of mean change in waking hours with good symptom control, according to an announcement from the device’s manufacturer, Boston Scientific. The INTREPID study is the first multicenter, prospective, double-blind, randomized, sham-controlled study of DBS for Parkinson’s disease in the United States and enrolled in 292 patients at 23 sites.
The implantable pulse generator of the device is “the smallest, rechargeable DBS device available in the U.S.,” according to Boston Scientific, and can independently control the amount of current delivered by each of the electrodes on the implanted leads, allowing them “to work together to address common challenges in DBS therapy such as fluctuations in symptoms and the progressive nature of the condition by offering more adaptable delivery of stimulation.”
“The Vercise DBS System changes the landscape of what physicians can do to help improve the quality of life for people living with Parkinson’s disease,” Jerry Vitek, MD, PhD, professor and chair of the department of neurology at the University of Minnesota, Minneapolis, and coordinating principal investigator for the INTREPID study said in the Boston Scientific announcement. “This system provides an ability to sculpt the current field in the DBS target using novel technology that offers flexibility in programming. This flexibility allows us to target different regions of the subthalamic nucleus, which we believe will improve outcomes while reducing side effects.”
Peer-to-peer learning about robotic surgery is happening on social media
Social media is now being used by surgeons for interaction among peers, informal learning, exchange of technical information, and diffusion of ideas.
A study has examined posting and membership data from a closed Facebook page, the “Robotic Surgery Collaboration,” created by surgeons who practice robotic-assisted procedures. Overall, the findings show exponential growth in membership in January 2015 through August 2016, some signs of stagnating engagement, and use of the platform for peer-to-peer learning and discussion.
“The growth in this group over time suggests that surgeons found it useful for engaging in informal interactions and learning vicariously from one another, but also reveals that not all users were actively engaged in these interactions each month and that growth in active membership differed from growth in overall group membership (as evident in the stagnating growth of active members, despite continued growth in total members),” the investigators concluded. Read the full study at Ann Surg. 2017 Aug 29. doi: 10.1097/SLA.0000000000002479. (Epub ahead of print).
Social media is now being used by surgeons for interaction among peers, informal learning, exchange of technical information, and diffusion of ideas.
A study has examined posting and membership data from a closed Facebook page, the “Robotic Surgery Collaboration,” created by surgeons who practice robotic-assisted procedures. Overall, the findings show exponential growth in membership in January 2015 through August 2016, some signs of stagnating engagement, and use of the platform for peer-to-peer learning and discussion.
“The growth in this group over time suggests that surgeons found it useful for engaging in informal interactions and learning vicariously from one another, but also reveals that not all users were actively engaged in these interactions each month and that growth in active membership differed from growth in overall group membership (as evident in the stagnating growth of active members, despite continued growth in total members),” the investigators concluded. Read the full study at Ann Surg. 2017 Aug 29. doi: 10.1097/SLA.0000000000002479. (Epub ahead of print).
Social media is now being used by surgeons for interaction among peers, informal learning, exchange of technical information, and diffusion of ideas.
A study has examined posting and membership data from a closed Facebook page, the “Robotic Surgery Collaboration,” created by surgeons who practice robotic-assisted procedures. Overall, the findings show exponential growth in membership in January 2015 through August 2016, some signs of stagnating engagement, and use of the platform for peer-to-peer learning and discussion.
“The growth in this group over time suggests that surgeons found it useful for engaging in informal interactions and learning vicariously from one another, but also reveals that not all users were actively engaged in these interactions each month and that growth in active membership differed from growth in overall group membership (as evident in the stagnating growth of active members, despite continued growth in total members),” the investigators concluded. Read the full study at Ann Surg. 2017 Aug 29. doi: 10.1097/SLA.0000000000002479. (Epub ahead of print).