User login
COVID-19 and surge capacity in U.S. hospitals
Background
As of April 2020, the United States is faced with the early stages of the coronavirus disease 2019 (COVID-19) pandemic. Experts predict up to 60% of the population will become infected with a fatality rate of 1% and a hospitalization rate of approximately 20%. Efforts to suppress viral spread have been unsuccessful as cases are reported in all 50 states, and fatalities are rising. Currently many American hospitals are ill-prepared for a significant increase in their census of critically ill and contagious patients, i.e., hospitals lack adequate surge capacity to safely handle a nationwide outbreak of COVID-19. As seen in other nations such as Italy, China, and Iran, this leads to rationing of life-saving health care and potentially preventable morbidity and mortality.
Introduction
Hospitals will be unable to provide the current standard of care to patients as the rate of infection with coronavirus disease 2019 (COVID-19) escalates. As of April 9, the World Health Organization has confirmed 1,539,118 cases and 89,998 deaths globally; and the Centers for Disease Control and Prevention has confirmed 435,941 cases and 14,865 deaths in the United States.1,2 Experts predict up to 60% of the population will eventually become infected with a fatality rate of about 1% and a hospitalization rate of approximately 20%.3,4
In the United States, with a population of 300 million people, this represents up to 180 million infected, 36 million requiring hospitalization, 11 million requiring intensive care, and 2 million fatalities over the duration of the pandemic. On March 13, President Donald Trump declared a state of national emergency, authorizing $50 billion dollars in emergency health care spending as well as asking every hospital in the country to immediately activate its emergency response plan. The use of isolation and quarantine may space out casualties over time, however high rates and volumes of hospitalizations are still expected.4,5
As the influx of patients afflicted with COVID-19 grows, needs will outstrip hospital resources forcing clinicians to ration beds and supplies. In Italy, China, and Iran, physicians are already faced with these difficult decisions. Antonio Pesenti, head of the Italian Lombardy regional crisis response unit, characterized the change in health care delivery: “We’re now being forced to set up intensive care treatment in corridors, in operating theaters, in recovery rooms. We’ve emptied entire hospital sections to make space for seriously sick people.”6
Surge capacity
Surge capacity is a hospital’s ability to adequately care for a significant influx of patients.7 Since 2011, the American College of Emergency Physicians has published guidelines calling for hospitals to have a surge capacity accounting for infectious disease outbreaks, and demands on supplies, personnel, and physical space.7 Even prior to the development of COVID-19, many hospitals faced emergency department crowding and strains on hospital capacity.8 The Organization for Economic Co-operation and Development (OECD) estimates hospital beds per 1,000 inhabitants at 2.77 for the USA, 3.18 for Italy, 4.34 for China, and 13.05 for Japan.9 Before COVID-19 many American hospitals had an insufficient number of beds. Now, in the initial phase of the pandemic, it is even more important to optimize surge capacity across the American health care system.
Requirements for COVID-19 preparation
To prepare for the increased number of seriously and critically ill patients, individual hospitals and regions must perform a needs assessment. The fundamental disease process of COVID-19 is a contagious viral pneumonia; treatment hinges on four major categories of intervention: spatial isolation (including physical space, beds, partitions, droplet precautions, food, water, and sanitation), oxygenation (including wall and portable oxygen, nasal canulae, and masks), mechanical ventilation (including ventilator machines, tubing, anesthetics, and reliable electrical power) and personnel (including physicians, nurses, technicians, and adequate personal protective equipment).10 In special circumstances and where available, extra corporeal membrane oxygenation may be considered.10 The necessary interventions are summarized in Table 1. 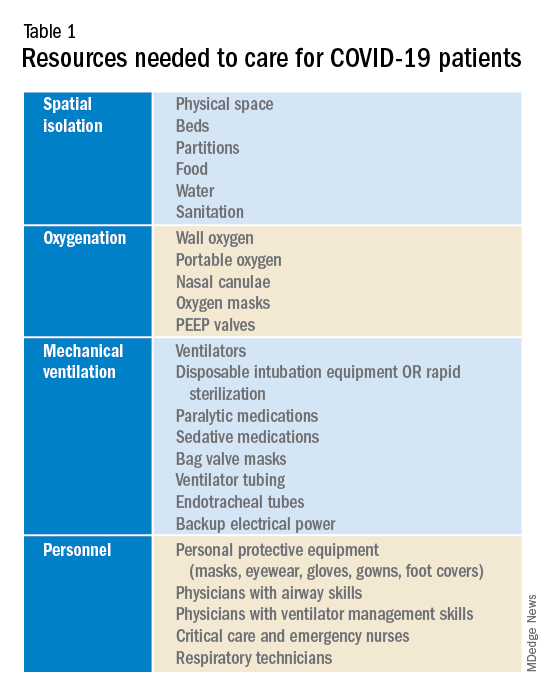
Emergency, critical care, nursing, and medical leadership should consider what sort of space, personnel, and supplies will be needed to care for a large volume of patients with contagious viral pneumonia at the same time as other hospital patients. Attention should also be given to potential need for morgue expansion. Hospitals must be proactive in procuring supplies and preparing for demands on beds and physical space. Specifically, logistics coordinators should start stockpiling ventilators, oxygen, respiratory equipment, and personal protective equipment. Reallocating supplies from other regions of the hospital such as operating rooms and ambulatory surgery centers may be considered. These resources, particularly ventilators and ventilator supplies, are already in disturbingly limited supply, and they are likely to be single most important limiting factor for survival rates. To prevent regional shortages, stockpiling efforts should ideally be aided by state and federal governments. The production and acquisition of ventilators should be immediately and significantly increased.
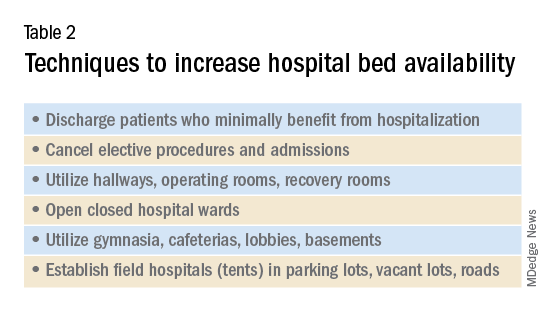
Hospitals must additionally prepare for demands for physical space and beds. Techniques to maximize space and bed availability (see Table 2) include discharging patients who do not require hospitalization, and canceling elective procedures and admissions. Additional methods would be to utilize unconventional preexisting spaces such as hallways, operating rooms, recovery rooms, hallways, closed hospital wards, basements, lobbies, cafeterias, and parking lots. Administrators should also consider establishing field hospitals or field wards, such as tents in open spaces and nearby roads. Medical care performed in unconventional environments will need to account for electricity, temperature control, oxygen delivery, and sanitation.
Conclusion
To minimize unnecessary loss of life and suffering, hospitals must expand their surge capacities in preparation for the predictable rise in demand for health care resources related to COVID-19. Numerous hospitals, particularly those that serve low-income and underserved communities, operate with a narrow financial margin.11 Independently preparing for the surge capacity needed to face COVID-19 may be infeasible for several hospitals. As a result, many health care systems will rely on government aid during this period for financial and material support. To maximize preparedness and response, hospitals should ask for and receive aid from the Federal Emergency Management Agency (FEMA), American Red Cross, state governments, and the military; these resources should be mobilized now.
Dr. Blumenberg, Dr. Noble, and Dr. Hendrickson are based in the department of emergency medicine & toxicology, Oregon Health and Science University, Portland.
References
1. Coronavirus disease 2019 (COVID-19) situation report – 60. 2020 Mar 19.
2. Coronavirus disease 2019 (COVID-19) Cases in the U.S. CDC. 2020 Apr 8.
3. Li Q et al. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med. 2020 Jan. doi: 10.1056/NEJMoa2001316.
4. Anderson RM et al. How will country-based mitigation measures influence the course of the COVID-19 epidemic? Lancet. 2020 Mar. doi: 10.1016/S0140-6736(20)30567-5.
5. Fraser C et al. Factors that make an infectious disease outbreak controllable. Proc Natl Acad Sci U S A. 2004;101(16):6146-51. doi: 10.1073/pnas.0307506101.
6. Mackenzie J and Balmer C. Italy locks down millions as its coronavirus deaths jump. Reuters. 2020 Mar 9.
7. Health care system surge capacity recognition, preparedness, and response. Ann Emerg Med. 2012;59(3):240-1. doi: 10.1016/j.annemergmed.2011.11.030.
8. Pitts SR et al. A cross-sectional study of emergency department boarding practices in the United States. Acad Emerg Med. 2014;21(5):497-503. doi: 10.1111/acem.12375.
9. Health at a Glance 2019. OECD; 2019. doi: 10.1787/4dd50c09-en.
10. Murthy S et al. Care for critically ill patients with COVID-19. JAMA. 2020 Mar. doi: 10.1001/jama.2020.3633.
11. Ly DP et al. The association between hospital margins, quality of care, and closure or other change in operating status. J Gen Intern Med. 2011;26(11):1291-6. doi: 10.1007/s11606-011-1815-5.
Background
As of April 2020, the United States is faced with the early stages of the coronavirus disease 2019 (COVID-19) pandemic. Experts predict up to 60% of the population will become infected with a fatality rate of 1% and a hospitalization rate of approximately 20%. Efforts to suppress viral spread have been unsuccessful as cases are reported in all 50 states, and fatalities are rising. Currently many American hospitals are ill-prepared for a significant increase in their census of critically ill and contagious patients, i.e., hospitals lack adequate surge capacity to safely handle a nationwide outbreak of COVID-19. As seen in other nations such as Italy, China, and Iran, this leads to rationing of life-saving health care and potentially preventable morbidity and mortality.
Introduction
Hospitals will be unable to provide the current standard of care to patients as the rate of infection with coronavirus disease 2019 (COVID-19) escalates. As of April 9, the World Health Organization has confirmed 1,539,118 cases and 89,998 deaths globally; and the Centers for Disease Control and Prevention has confirmed 435,941 cases and 14,865 deaths in the United States.1,2 Experts predict up to 60% of the population will eventually become infected with a fatality rate of about 1% and a hospitalization rate of approximately 20%.3,4
In the United States, with a population of 300 million people, this represents up to 180 million infected, 36 million requiring hospitalization, 11 million requiring intensive care, and 2 million fatalities over the duration of the pandemic. On March 13, President Donald Trump declared a state of national emergency, authorizing $50 billion dollars in emergency health care spending as well as asking every hospital in the country to immediately activate its emergency response plan. The use of isolation and quarantine may space out casualties over time, however high rates and volumes of hospitalizations are still expected.4,5
As the influx of patients afflicted with COVID-19 grows, needs will outstrip hospital resources forcing clinicians to ration beds and supplies. In Italy, China, and Iran, physicians are already faced with these difficult decisions. Antonio Pesenti, head of the Italian Lombardy regional crisis response unit, characterized the change in health care delivery: “We’re now being forced to set up intensive care treatment in corridors, in operating theaters, in recovery rooms. We’ve emptied entire hospital sections to make space for seriously sick people.”6
Surge capacity
Surge capacity is a hospital’s ability to adequately care for a significant influx of patients.7 Since 2011, the American College of Emergency Physicians has published guidelines calling for hospitals to have a surge capacity accounting for infectious disease outbreaks, and demands on supplies, personnel, and physical space.7 Even prior to the development of COVID-19, many hospitals faced emergency department crowding and strains on hospital capacity.8 The Organization for Economic Co-operation and Development (OECD) estimates hospital beds per 1,000 inhabitants at 2.77 for the USA, 3.18 for Italy, 4.34 for China, and 13.05 for Japan.9 Before COVID-19 many American hospitals had an insufficient number of beds. Now, in the initial phase of the pandemic, it is even more important to optimize surge capacity across the American health care system.
Requirements for COVID-19 preparation
To prepare for the increased number of seriously and critically ill patients, individual hospitals and regions must perform a needs assessment. The fundamental disease process of COVID-19 is a contagious viral pneumonia; treatment hinges on four major categories of intervention: spatial isolation (including physical space, beds, partitions, droplet precautions, food, water, and sanitation), oxygenation (including wall and portable oxygen, nasal canulae, and masks), mechanical ventilation (including ventilator machines, tubing, anesthetics, and reliable electrical power) and personnel (including physicians, nurses, technicians, and adequate personal protective equipment).10 In special circumstances and where available, extra corporeal membrane oxygenation may be considered.10 The necessary interventions are summarized in Table 1. 
Emergency, critical care, nursing, and medical leadership should consider what sort of space, personnel, and supplies will be needed to care for a large volume of patients with contagious viral pneumonia at the same time as other hospital patients. Attention should also be given to potential need for morgue expansion. Hospitals must be proactive in procuring supplies and preparing for demands on beds and physical space. Specifically, logistics coordinators should start stockpiling ventilators, oxygen, respiratory equipment, and personal protective equipment. Reallocating supplies from other regions of the hospital such as operating rooms and ambulatory surgery centers may be considered. These resources, particularly ventilators and ventilator supplies, are already in disturbingly limited supply, and they are likely to be single most important limiting factor for survival rates. To prevent regional shortages, stockpiling efforts should ideally be aided by state and federal governments. The production and acquisition of ventilators should be immediately and significantly increased.
Hospitals must additionally prepare for demands for physical space and beds. Techniques to maximize space and bed availability (see Table 2) include discharging patients who do not require hospitalization, and canceling elective procedures and admissions. Additional methods would be to utilize unconventional preexisting spaces such as hallways, operating rooms, recovery rooms, hallways, closed hospital wards, basements, lobbies, cafeterias, and parking lots. Administrators should also consider establishing field hospitals or field wards, such as tents in open spaces and nearby roads. Medical care performed in unconventional environments will need to account for electricity, temperature control, oxygen delivery, and sanitation.

Conclusion
To minimize unnecessary loss of life and suffering, hospitals must expand their surge capacities in preparation for the predictable rise in demand for health care resources related to COVID-19. Numerous hospitals, particularly those that serve low-income and underserved communities, operate with a narrow financial margin.11 Independently preparing for the surge capacity needed to face COVID-19 may be infeasible for several hospitals. As a result, many health care systems will rely on government aid during this period for financial and material support. To maximize preparedness and response, hospitals should ask for and receive aid from the Federal Emergency Management Agency (FEMA), American Red Cross, state governments, and the military; these resources should be mobilized now.
Dr. Blumenberg, Dr. Noble, and Dr. Hendrickson are based in the department of emergency medicine & toxicology, Oregon Health and Science University, Portland.
References
1. Coronavirus disease 2019 (COVID-19) situation report – 60. 2020 Mar 19.
2. Coronavirus disease 2019 (COVID-19) Cases in the U.S. CDC. 2020 Apr 8.
3. Li Q et al. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med. 2020 Jan. doi: 10.1056/NEJMoa2001316.
4. Anderson RM et al. How will country-based mitigation measures influence the course of the COVID-19 epidemic? Lancet. 2020 Mar. doi: 10.1016/S0140-6736(20)30567-5.
5. Fraser C et al. Factors that make an infectious disease outbreak controllable. Proc Natl Acad Sci U S A. 2004;101(16):6146-51. doi: 10.1073/pnas.0307506101.
6. Mackenzie J and Balmer C. Italy locks down millions as its coronavirus deaths jump. Reuters. 2020 Mar 9.
7. Health care system surge capacity recognition, preparedness, and response. Ann Emerg Med. 2012;59(3):240-1. doi: 10.1016/j.annemergmed.2011.11.030.
8. Pitts SR et al. A cross-sectional study of emergency department boarding practices in the United States. Acad Emerg Med. 2014;21(5):497-503. doi: 10.1111/acem.12375.
9. Health at a Glance 2019. OECD; 2019. doi: 10.1787/4dd50c09-en.
10. Murthy S et al. Care for critically ill patients with COVID-19. JAMA. 2020 Mar. doi: 10.1001/jama.2020.3633.
11. Ly DP et al. The association between hospital margins, quality of care, and closure or other change in operating status. J Gen Intern Med. 2011;26(11):1291-6. doi: 10.1007/s11606-011-1815-5.
Background
As of April 2020, the United States is faced with the early stages of the coronavirus disease 2019 (COVID-19) pandemic. Experts predict up to 60% of the population will become infected with a fatality rate of 1% and a hospitalization rate of approximately 20%. Efforts to suppress viral spread have been unsuccessful as cases are reported in all 50 states, and fatalities are rising. Currently many American hospitals are ill-prepared for a significant increase in their census of critically ill and contagious patients, i.e., hospitals lack adequate surge capacity to safely handle a nationwide outbreak of COVID-19. As seen in other nations such as Italy, China, and Iran, this leads to rationing of life-saving health care and potentially preventable morbidity and mortality.
Introduction
Hospitals will be unable to provide the current standard of care to patients as the rate of infection with coronavirus disease 2019 (COVID-19) escalates. As of April 9, the World Health Organization has confirmed 1,539,118 cases and 89,998 deaths globally; and the Centers for Disease Control and Prevention has confirmed 435,941 cases and 14,865 deaths in the United States.1,2 Experts predict up to 60% of the population will eventually become infected with a fatality rate of about 1% and a hospitalization rate of approximately 20%.3,4
In the United States, with a population of 300 million people, this represents up to 180 million infected, 36 million requiring hospitalization, 11 million requiring intensive care, and 2 million fatalities over the duration of the pandemic. On March 13, President Donald Trump declared a state of national emergency, authorizing $50 billion dollars in emergency health care spending as well as asking every hospital in the country to immediately activate its emergency response plan. The use of isolation and quarantine may space out casualties over time, however high rates and volumes of hospitalizations are still expected.4,5
As the influx of patients afflicted with COVID-19 grows, needs will outstrip hospital resources forcing clinicians to ration beds and supplies. In Italy, China, and Iran, physicians are already faced with these difficult decisions. Antonio Pesenti, head of the Italian Lombardy regional crisis response unit, characterized the change in health care delivery: “We’re now being forced to set up intensive care treatment in corridors, in operating theaters, in recovery rooms. We’ve emptied entire hospital sections to make space for seriously sick people.”6
Surge capacity
Surge capacity is a hospital’s ability to adequately care for a significant influx of patients.7 Since 2011, the American College of Emergency Physicians has published guidelines calling for hospitals to have a surge capacity accounting for infectious disease outbreaks, and demands on supplies, personnel, and physical space.7 Even prior to the development of COVID-19, many hospitals faced emergency department crowding and strains on hospital capacity.8 The Organization for Economic Co-operation and Development (OECD) estimates hospital beds per 1,000 inhabitants at 2.77 for the USA, 3.18 for Italy, 4.34 for China, and 13.05 for Japan.9 Before COVID-19 many American hospitals had an insufficient number of beds. Now, in the initial phase of the pandemic, it is even more important to optimize surge capacity across the American health care system.
Requirements for COVID-19 preparation
To prepare for the increased number of seriously and critically ill patients, individual hospitals and regions must perform a needs assessment. The fundamental disease process of COVID-19 is a contagious viral pneumonia; treatment hinges on four major categories of intervention: spatial isolation (including physical space, beds, partitions, droplet precautions, food, water, and sanitation), oxygenation (including wall and portable oxygen, nasal canulae, and masks), mechanical ventilation (including ventilator machines, tubing, anesthetics, and reliable electrical power) and personnel (including physicians, nurses, technicians, and adequate personal protective equipment).10 In special circumstances and where available, extra corporeal membrane oxygenation may be considered.10 The necessary interventions are summarized in Table 1. 
Emergency, critical care, nursing, and medical leadership should consider what sort of space, personnel, and supplies will be needed to care for a large volume of patients with contagious viral pneumonia at the same time as other hospital patients. Attention should also be given to potential need for morgue expansion. Hospitals must be proactive in procuring supplies and preparing for demands on beds and physical space. Specifically, logistics coordinators should start stockpiling ventilators, oxygen, respiratory equipment, and personal protective equipment. Reallocating supplies from other regions of the hospital such as operating rooms and ambulatory surgery centers may be considered. These resources, particularly ventilators and ventilator supplies, are already in disturbingly limited supply, and they are likely to be single most important limiting factor for survival rates. To prevent regional shortages, stockpiling efforts should ideally be aided by state and federal governments. The production and acquisition of ventilators should be immediately and significantly increased.
Hospitals must additionally prepare for demands for physical space and beds. Techniques to maximize space and bed availability (see Table 2) include discharging patients who do not require hospitalization, and canceling elective procedures and admissions. Additional methods would be to utilize unconventional preexisting spaces such as hallways, operating rooms, recovery rooms, hallways, closed hospital wards, basements, lobbies, cafeterias, and parking lots. Administrators should also consider establishing field hospitals or field wards, such as tents in open spaces and nearby roads. Medical care performed in unconventional environments will need to account for electricity, temperature control, oxygen delivery, and sanitation.

Conclusion
To minimize unnecessary loss of life and suffering, hospitals must expand their surge capacities in preparation for the predictable rise in demand for health care resources related to COVID-19. Numerous hospitals, particularly those that serve low-income and underserved communities, operate with a narrow financial margin.11 Independently preparing for the surge capacity needed to face COVID-19 may be infeasible for several hospitals. As a result, many health care systems will rely on government aid during this period for financial and material support. To maximize preparedness and response, hospitals should ask for and receive aid from the Federal Emergency Management Agency (FEMA), American Red Cross, state governments, and the military; these resources should be mobilized now.
Dr. Blumenberg, Dr. Noble, and Dr. Hendrickson are based in the department of emergency medicine & toxicology, Oregon Health and Science University, Portland.
References
1. Coronavirus disease 2019 (COVID-19) situation report – 60. 2020 Mar 19.
2. Coronavirus disease 2019 (COVID-19) Cases in the U.S. CDC. 2020 Apr 8.
3. Li Q et al. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med. 2020 Jan. doi: 10.1056/NEJMoa2001316.
4. Anderson RM et al. How will country-based mitigation measures influence the course of the COVID-19 epidemic? Lancet. 2020 Mar. doi: 10.1016/S0140-6736(20)30567-5.
5. Fraser C et al. Factors that make an infectious disease outbreak controllable. Proc Natl Acad Sci U S A. 2004;101(16):6146-51. doi: 10.1073/pnas.0307506101.
6. Mackenzie J and Balmer C. Italy locks down millions as its coronavirus deaths jump. Reuters. 2020 Mar 9.
7. Health care system surge capacity recognition, preparedness, and response. Ann Emerg Med. 2012;59(3):240-1. doi: 10.1016/j.annemergmed.2011.11.030.
8. Pitts SR et al. A cross-sectional study of emergency department boarding practices in the United States. Acad Emerg Med. 2014;21(5):497-503. doi: 10.1111/acem.12375.
9. Health at a Glance 2019. OECD; 2019. doi: 10.1787/4dd50c09-en.
10. Murthy S et al. Care for critically ill patients with COVID-19. JAMA. 2020 Mar. doi: 10.1001/jama.2020.3633.
11. Ly DP et al. The association between hospital margins, quality of care, and closure or other change in operating status. J Gen Intern Med. 2011;26(11):1291-6. doi: 10.1007/s11606-011-1815-5.
Sarcoidosis Resulting in Exsanguinating Esophageal Variceal Hemorrhage
Sarcoidosis is a systemic disorder of unknown etiology and is characterized by the formation of granulomas throughout various organs in the body. The most common form is pulmonary sarcoidosis, which affects 90% of patients; the second most common form is oculocutaneous sarcoidosis;1 and the third most common form is hepatic sarcoidosis, which affects 63% to 90% of patients.2 Although the liver is frequently involved in all forms of sarcoidosis, only a fraction of patients present with clinically evident liver disease.1 Approximately 20% to 30% of patients have abnormalities on liver function tests, whereas only about 1% of patients show evidence of portal hypertension and cirrhosis.3 In fact, in the English literature, there were 35 reported cases of portal hypertension due to sarcoidosis between 1949 to 2001, of which 16 of the patients had no evidence of cirrhosis.4
The diagnosis of sarcoidosis is usually made by a compilation of clinical signs and symptoms, imaging studies, and biopsies demonstrating noncaseating granulomas. This case report describes a patient who presented with portal hypertension and esophageal variceal bleeding secondary to sarcoidosis of the liver without cirrhotic changes.
Case
A 47-year-old woman presented to the ED via emergency medical services with a 1-hour history of hematemesis and melena. The patient stated that she felt fatigued, nauseated, and light-headed, but had no pain or focal weakness. Her medical history was significant for pulmonary and renal sarcoidosis. She underwent a liver biopsy 1 week prior to presentation, with a 6-day hospitalization period, due to new ascites found on examination.
The patient’s vital signs at presentation were: blood pressure (BP), 72/56 mm Hg; heart rate (HR), 133 beats/min, respiratory rate, 24 breaths/min; and temperature, 97.0oF. Oxygen saturation was 99% on room air. Physical examination revealed an alert and oriented middle-aged woman in extremis who was vomiting dark-colored blood. The cardiac and pulmonary examination revealed no extraneous sounds; the abdominal examination showed ascites with a liver edge palpable 4 cm beneath the right costal margin. The patient had no scleral icterus, palmar erythema, spider angiomata, fetor hepaticus, caput medusa, cutaneous ecchymoses, or any other stigmata of cirrhosis.
Two large-bore peripheral intravenous (IV) catheters were placed and a massive blood transfusion protocol was initiated. Packed red blood cells (PRBCs) from the resuscitation-area refrigerator were infused immediately via a pressurized fluid warmer.
After consultation with gastroenterology and general surgery services, the patient was given 1 g ceftriaxone IV, 1 g tranexamic acid IV, 20 mcg desmopressin IV, 50 mcg octreotide IV, 40 mg pantoprazole IV, 8 mg ondansetron IV, 4 g calcium gluconate IV, and 100 mg hydrocortisone IV.
Throughout the patient’s first 10 minutes in the ED, she remained persistently hypotensive and continued to vomit. Since the patient’s sensorium was intact, the team quickly discussed goals of care with her. The patient’s wishes were for maximal life-sustaining therapy, including endotracheal intubation and chest compressions, if necessary.
After this discussion, the patient was given IV etomidate and rocuronium and was intubated using video-assisted laryngoscopy. Following intubation, she was sedated with an infusion of fentanyl and underwent orogastric tube placement to aspirate stomach contents. A total of 2.5 L of frank blood were drained from the patient’s stomach.
A size 9 French single lumen left-femoral central venous catheter also was placed, through which additional blood products were infused. The patient received a total of 28 U PRBCs, fresh frozen plasma, and platelets over a 3-hour period. During transfusion, the patient’s vital signs improved to a systolic BP ranging between 110 to 120 mm Hg and an HR ranging between 90 to 110 beats/min; she did not experience any further hypotensive episodes throughout her stay in the ED.
Laboratory studies were significant for metabolic acidosis, hyperkalemia, acute on chronic anemia, leukocytosis, and acute on chronic renal failure. Synthetic function of the liver and transaminases appeared normal (Table).
The patient’s hyperkalemia was treated with 1 g calcium chloride IV, 50 g dextrose IV, and 10 U regular insulin IV. A portable chest radiograph showed an appropriately positioned endotracheal tube, and an electrocardiogram revealed sinus tachycardia without signs of hyperkalemia. A computed tomography (CT) scan of the abdomen and pelvis from the patient’s recent hospitalization, 1 week prior to presentation, showed hepatomegaly, liver granulomas, ascites, and periportal lymphadenopathy (Figure 1).
A review of the patient’s recent liver biopsy and ascitic fluid analysis revealed noncaseating granulomas compressing the hepatic sinusoids, and a serum ascites albumin gradient greater than 1.1 g/dL, implying portal hypertension without cirrhosis. The surgical team attempted to place a Sengstaken-Blakemore tube, but the device could not be positioned properly due to the patient’s narrowed esophagus.
The ED nurses cleaned the patient, preserving her dignity; thereafter the patient’s adult children visited with her briefly before she was taken for an upper endoscopy, which was performed in the ED. The endoscopy revealed actively hemorrhaging esophageal varices at the gastroesophageal junction (Figure 2). The varices were treated with endoscopic ligation; the gastroenterologist placed a total of 11 bands, resulting in cessation of bleeding.
After the endoscopy, the patient was admitted to the medical intensive care unit (ICU). Approximately 1.5 hours after arriving at the ICU, she developed renewed hematemesis. Despite efforts to control bleeding and provide hemodynamic support, the patient died 1 hour later.
Discussion
Etiology
Esophageal variceal hemorrhage is caused by pressure elevation in the portal venous system, leading to engorged esophageal veins that can bleed spontaneously. Approximately 90% of portal hypertension is due to liver cirrhosis.5 The remaining 10% of cases are primarily vascular in etiology, with endothelial dysfunction and thrombosis leading to increased portal resistance. Noncirrhotic causes of portal hypertension include malignancy, congenital diseases, viral hepatitides, vascular thromboses or fistulae, constrictive pericarditis, fatty liver of pregnancy, drugs, radiation injury, and infiltrative diseases.5
Sarcoidosis may cause noncaseating granulomas to form in the liver, leading to portal hypertension and fatal exsanguination from esophageal variceal hemorrhage. Although the lesions of sarcoidosis classically form in the lungs, any organ system may be affected.6,7 Frank cirrhosis of the liver occurs in only 1% of sarcoidosis patients; however, radiographic involvement of the liver is seen in 5% to 15% of patients.8
There are several mechanisms which may be responsible for portal hypertension in patients with sarcoidosis, including granulomas causing mass effect on the hepatic sinusoids; arteriovenous shunts within the granuloma; granulomatous phlebitis within the sinusoids; or compressive periportal lymphadenopathy.9 Regardless of the mechanism, a review of the literature demonstrates an association between sarcoidosis and symptomatic portal hypertension.2,4,10,11Although our patient ultimately died, early initiation of massive blood transfusion protocol, airway protection, attention to electrolytes, and endoscopic control of the hemorrhage source provided the best chance for survival.
Medical Therapy
The first priority in managing and treating esophageal varices is to secure the patient’s airways to prevent aspiration. Two large bore IV lines should be placed to permit rapid infusion of crystalloid fluids or blood products. Initiating antibiotics, specifically IV ceftriaxone, to patients with variceal bleeding is a class I recommendation, as this is the only intervention shown to increase patient survival.12 Although proton pump inhibitors (PPI) and somatostatin analogues (typically octreotide) are frequently given, they are both class II recommendations because there is limited evidence supporting the benefit of their use.12 However, current guidelines recommend treating patients for variceal bleeding with an initial bolus of a PPI, followed by a continuous infusion of PPI for 72 hours. As previously noted, multiple studies, have failed to show any decrease in mortality associated with this treatment.12
Other agents that are used to treat variceal bleeding include octreotide and vasopressin. Octreotide, a somatostatin analog, is generally given as an initial IV bolus followed by continuous infusion, and has been shown to decrease transfusion requirements without mortality benefit.12 Vasopressin is generally given to critically ill patients, and is considered a third-line treatment for variceal bleeding.
Since our patient had a history of chronic kidney disease, desmopressin was empirically administered in the event platelet dysfunction was a contributing factor to bleeding.13 The absence of cirrhosis was significant because our patient was unlikely to have a bleeding diathesis caused by coagulation factor deficiency. Therefore, the goal transfusion ratio of blood products should be balanced, similar to that in traumatic exsanguination, rather than favoring an increased ratio of plasma to other blood products. Similarly, tranexamic acid was administered because insufficient tamponade rather than coagulopathy was the presumed cause of sustained hemorrhage.
An additional complicating factor in our patient’s care was the potential effect of the massive transfusion on electrolytes. Packed RBCs have a pH of approximately 6.8 and may carry up to 25 mmol/L of potassium, which may have exacerbated our patient’s underlying hyperkalemia.14 Rapid blood transfusion also places patients at risk for acute hypocalcemia secondary to citrate toxicity; this did not occur in our patient in part because the metabolic function of her liver was preserved and citrate could be broken down in the hepatocyte Krebs cycle.15 Calcium therapy doubled as treatment for the hyperkalemia and as prophylaxis against further hypocalcemia. No dysrhythmias were observed.
Surgical Intervention
Emergency physicians should consult with gastroenterology services so that an endoscopy can be performed as soon as possible to evaluate for and control bleeding. When an endoscopy cannot be performed rapidly, there are multiple balloon tamponade devices available that can be used to temporize the bleeding, such as the Sengstaken-Blakemore tube.12
Although balloon tamponade devices are typically reserved for the last line of therapy, endoscopy rather than transjugular intrahepatic portosystemic shunt (TIPS) was the preferred method of hemorrhage source control in our patient for several reasons. First, although the working diagnosis of varices was based on the patient’s history, we wanted to evaluate for other causes of upper gastrointestinal bleeding since our patient had no history of endoscopy. Therefore, endoscopy had both a therapeutic and diagnostic value. Secondly, though TIPS may decrease pressure within the bleeding varix, only endoscopy permits direct hemostasis. Also, endoscopy also was preferred over TIPS because our patient was too unstable to move to the interventional radiology suite.16
Conclusion
Although life-threatening esophageal variceal hemorrhage is a rare manifestation of an uncommon disease, it should be considered in the differential diagnosis of a patient who has sarcoidosis and presents with gastrointestinal bleeding. Additionally, when caring for a patient with massive hematemesis without evidence of liver cirrhosis, other etiologies of portal hypertension and esophageal varices, such as sarcoidosis, should be considered.
1. Rao DA, Dellaripa PF. Extrapulmonary manifestations of sarcoidosis. Rheum Dis Clin North Am. 2013;39(2):277-297. doi:10.1016/j.rdc.2013.02.007.
2. Mistilis SP, Green JR, Schiff L. Hepatic sarcoidosis with portal hypertension. Am J Med. 1964;36(3):470-475. doi:10.1016/0002-9343(64)90175-5.
3. Tekeste H, Latour F, Levitt RE. Portal hypertension complicating sarcoid liver disease: case report and review of the literature. Am J Gastroenterol. 1984;79(5):389-396.
4. Ivonye C, Elhammali B, Henriques-Forsythe M, Bennett-Gittens R, Oderinde A. Disseminated sarcoidosis resulting in portal hypertension and gastrointestinal bleeding: a rare presentation. Can J Gastroenterol. 2012;26(8):508-509. http://www.ncbi.nlm.nih.gov/pubmed/22891173. Accessed May 16, 2018.
5. Tetangco EP, Silva RG, Lerma EV. Portal hypertension: etiology, evaluation, and management. Dis Mon. 2016;62(12):411-426. doi:10.1016/j.disamonth.2016.08.001.
6. Valeyre D, Prasse A, Nunes H, Uzunhan Y, Brillet PY, Müller-Quernheim J. Sarcoidosis. Lancet. 2014;383(9923):1155-1167. doi:10.1016/S0140-6736(13)60680-7.
7. Al-Kofahi K, Korsten P, Ascoli C, et al. Management of extrapulmonary sarcoidosis: challenges and solutions. Ther Clin Risk Manag. 2016;12:1623-1634. doi:10.2147/TCRM.S74476.
8. Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med. 2007;357(21):2153-2165. doi:10.1056/NEJMra071714.
9. Ebert EC, Kierson M, Hagspiel KD. Gastrointestinal and hepatic manifestations of sarcoidosis. Am J Gastroenterol. 2008;103(12):3184-3192. doi:10.1111/j.1572-0241.2008.02202.x.
10. Fraimow W, Myerson RM. Portal hypertension and bleeding esophageal varices secondary to sarcoidosis of the liver. Am J Med. 1957;23(6):995-998.
11. Saito H, Ohmori M, Iwamuro M, et al. Hepatic and gastric involvement in a case of systemic sarcoidosis presenting with rupture of esophageal varices. Intern Med. 2018;56(19):2583-2588. doi:10.2169/internalmedicine.8768-16.
12. DeLaney M, Greene CJ. Emergency Department evaluation and management of patients with upper gastrointestinal bleeding. Emerg Med Pract. 2015;17(4):1-18; quiz 19.
13. Ozgönenel B, Rajpurkar M, Lusher JM. How do you treat bleeding disorders with desmopressin? Postgrad Med J. 2007;83(977):159-163. doi:10.1136/pgmj.2006.052118.
14. Sümpelmann R, Schürholz T, Thorns E, Hausdörfer J. Acid-base, electrolyte and metabolite concentrations in packed red blood cells for major transfusion in infants. Paediatr Anaesth. 2001;11(2):169-173. doi:10.1046/j.1460-9592.2001.00637.x.
15. Monchi M. Citrate pathophysiology and metabolism. Transfus Apher Sci. 2018;56(1):28-30. doi:10.1016/j.transci.2016.12.013.
16. Shah RP, Sze DY. Complications during transjugular intrahepatic portosystemic shunt creation. Tech Vasc Interv Radiol. 2016;19(1):61-73. doi:10.1053/j.tvir.2016.01.007.
Sarcoidosis is a systemic disorder of unknown etiology and is characterized by the formation of granulomas throughout various organs in the body. The most common form is pulmonary sarcoidosis, which affects 90% of patients; the second most common form is oculocutaneous sarcoidosis;1 and the third most common form is hepatic sarcoidosis, which affects 63% to 90% of patients.2 Although the liver is frequently involved in all forms of sarcoidosis, only a fraction of patients present with clinically evident liver disease.1 Approximately 20% to 30% of patients have abnormalities on liver function tests, whereas only about 1% of patients show evidence of portal hypertension and cirrhosis.3 In fact, in the English literature, there were 35 reported cases of portal hypertension due to sarcoidosis between 1949 to 2001, of which 16 of the patients had no evidence of cirrhosis.4
The diagnosis of sarcoidosis is usually made by a compilation of clinical signs and symptoms, imaging studies, and biopsies demonstrating noncaseating granulomas. This case report describes a patient who presented with portal hypertension and esophageal variceal bleeding secondary to sarcoidosis of the liver without cirrhotic changes.
Case
A 47-year-old woman presented to the ED via emergency medical services with a 1-hour history of hematemesis and melena. The patient stated that she felt fatigued, nauseated, and light-headed, but had no pain or focal weakness. Her medical history was significant for pulmonary and renal sarcoidosis. She underwent a liver biopsy 1 week prior to presentation, with a 6-day hospitalization period, due to new ascites found on examination.
The patient’s vital signs at presentation were: blood pressure (BP), 72/56 mm Hg; heart rate (HR), 133 beats/min, respiratory rate, 24 breaths/min; and temperature, 97.0oF. Oxygen saturation was 99% on room air. Physical examination revealed an alert and oriented middle-aged woman in extremis who was vomiting dark-colored blood. The cardiac and pulmonary examination revealed no extraneous sounds; the abdominal examination showed ascites with a liver edge palpable 4 cm beneath the right costal margin. The patient had no scleral icterus, palmar erythema, spider angiomata, fetor hepaticus, caput medusa, cutaneous ecchymoses, or any other stigmata of cirrhosis.
Two large-bore peripheral intravenous (IV) catheters were placed and a massive blood transfusion protocol was initiated. Packed red blood cells (PRBCs) from the resuscitation-area refrigerator were infused immediately via a pressurized fluid warmer.
After consultation with gastroenterology and general surgery services, the patient was given 1 g ceftriaxone IV, 1 g tranexamic acid IV, 20 mcg desmopressin IV, 50 mcg octreotide IV, 40 mg pantoprazole IV, 8 mg ondansetron IV, 4 g calcium gluconate IV, and 100 mg hydrocortisone IV.
Throughout the patient’s first 10 minutes in the ED, she remained persistently hypotensive and continued to vomit. Since the patient’s sensorium was intact, the team quickly discussed goals of care with her. The patient’s wishes were for maximal life-sustaining therapy, including endotracheal intubation and chest compressions, if necessary.
After this discussion, the patient was given IV etomidate and rocuronium and was intubated using video-assisted laryngoscopy. Following intubation, she was sedated with an infusion of fentanyl and underwent orogastric tube placement to aspirate stomach contents. A total of 2.5 L of frank blood were drained from the patient’s stomach.
A size 9 French single lumen left-femoral central venous catheter also was placed, through which additional blood products were infused. The patient received a total of 28 U PRBCs, fresh frozen plasma, and platelets over a 3-hour period. During transfusion, the patient’s vital signs improved to a systolic BP ranging between 110 to 120 mm Hg and an HR ranging between 90 to 110 beats/min; she did not experience any further hypotensive episodes throughout her stay in the ED.
Laboratory studies were significant for metabolic acidosis, hyperkalemia, acute on chronic anemia, leukocytosis, and acute on chronic renal failure. Synthetic function of the liver and transaminases appeared normal (Table).
The patient’s hyperkalemia was treated with 1 g calcium chloride IV, 50 g dextrose IV, and 10 U regular insulin IV. A portable chest radiograph showed an appropriately positioned endotracheal tube, and an electrocardiogram revealed sinus tachycardia without signs of hyperkalemia. A computed tomography (CT) scan of the abdomen and pelvis from the patient’s recent hospitalization, 1 week prior to presentation, showed hepatomegaly, liver granulomas, ascites, and periportal lymphadenopathy (Figure 1).
A review of the patient’s recent liver biopsy and ascitic fluid analysis revealed noncaseating granulomas compressing the hepatic sinusoids, and a serum ascites albumin gradient greater than 1.1 g/dL, implying portal hypertension without cirrhosis. The surgical team attempted to place a Sengstaken-Blakemore tube, but the device could not be positioned properly due to the patient’s narrowed esophagus.
The ED nurses cleaned the patient, preserving her dignity; thereafter the patient’s adult children visited with her briefly before she was taken for an upper endoscopy, which was performed in the ED. The endoscopy revealed actively hemorrhaging esophageal varices at the gastroesophageal junction (Figure 2). The varices were treated with endoscopic ligation; the gastroenterologist placed a total of 11 bands, resulting in cessation of bleeding.
After the endoscopy, the patient was admitted to the medical intensive care unit (ICU). Approximately 1.5 hours after arriving at the ICU, she developed renewed hematemesis. Despite efforts to control bleeding and provide hemodynamic support, the patient died 1 hour later.
Discussion
Etiology
Esophageal variceal hemorrhage is caused by pressure elevation in the portal venous system, leading to engorged esophageal veins that can bleed spontaneously. Approximately 90% of portal hypertension is due to liver cirrhosis.5 The remaining 10% of cases are primarily vascular in etiology, with endothelial dysfunction and thrombosis leading to increased portal resistance. Noncirrhotic causes of portal hypertension include malignancy, congenital diseases, viral hepatitides, vascular thromboses or fistulae, constrictive pericarditis, fatty liver of pregnancy, drugs, radiation injury, and infiltrative diseases.5
Sarcoidosis may cause noncaseating granulomas to form in the liver, leading to portal hypertension and fatal exsanguination from esophageal variceal hemorrhage. Although the lesions of sarcoidosis classically form in the lungs, any organ system may be affected.6,7 Frank cirrhosis of the liver occurs in only 1% of sarcoidosis patients; however, radiographic involvement of the liver is seen in 5% to 15% of patients.8
There are several mechanisms which may be responsible for portal hypertension in patients with sarcoidosis, including granulomas causing mass effect on the hepatic sinusoids; arteriovenous shunts within the granuloma; granulomatous phlebitis within the sinusoids; or compressive periportal lymphadenopathy.9 Regardless of the mechanism, a review of the literature demonstrates an association between sarcoidosis and symptomatic portal hypertension.2,4,10,11Although our patient ultimately died, early initiation of massive blood transfusion protocol, airway protection, attention to electrolytes, and endoscopic control of the hemorrhage source provided the best chance for survival.
Medical Therapy
The first priority in managing and treating esophageal varices is to secure the patient’s airways to prevent aspiration. Two large bore IV lines should be placed to permit rapid infusion of crystalloid fluids or blood products. Initiating antibiotics, specifically IV ceftriaxone, to patients with variceal bleeding is a class I recommendation, as this is the only intervention shown to increase patient survival.12 Although proton pump inhibitors (PPI) and somatostatin analogues (typically octreotide) are frequently given, they are both class II recommendations because there is limited evidence supporting the benefit of their use.12 However, current guidelines recommend treating patients for variceal bleeding with an initial bolus of a PPI, followed by a continuous infusion of PPI for 72 hours. As previously noted, multiple studies, have failed to show any decrease in mortality associated with this treatment.12
Other agents that are used to treat variceal bleeding include octreotide and vasopressin. Octreotide, a somatostatin analog, is generally given as an initial IV bolus followed by continuous infusion, and has been shown to decrease transfusion requirements without mortality benefit.12 Vasopressin is generally given to critically ill patients, and is considered a third-line treatment for variceal bleeding.
Since our patient had a history of chronic kidney disease, desmopressin was empirically administered in the event platelet dysfunction was a contributing factor to bleeding.13 The absence of cirrhosis was significant because our patient was unlikely to have a bleeding diathesis caused by coagulation factor deficiency. Therefore, the goal transfusion ratio of blood products should be balanced, similar to that in traumatic exsanguination, rather than favoring an increased ratio of plasma to other blood products. Similarly, tranexamic acid was administered because insufficient tamponade rather than coagulopathy was the presumed cause of sustained hemorrhage.
An additional complicating factor in our patient’s care was the potential effect of the massive transfusion on electrolytes. Packed RBCs have a pH of approximately 6.8 and may carry up to 25 mmol/L of potassium, which may have exacerbated our patient’s underlying hyperkalemia.14 Rapid blood transfusion also places patients at risk for acute hypocalcemia secondary to citrate toxicity; this did not occur in our patient in part because the metabolic function of her liver was preserved and citrate could be broken down in the hepatocyte Krebs cycle.15 Calcium therapy doubled as treatment for the hyperkalemia and as prophylaxis against further hypocalcemia. No dysrhythmias were observed.
Surgical Intervention
Emergency physicians should consult with gastroenterology services so that an endoscopy can be performed as soon as possible to evaluate for and control bleeding. When an endoscopy cannot be performed rapidly, there are multiple balloon tamponade devices available that can be used to temporize the bleeding, such as the Sengstaken-Blakemore tube.12
Although balloon tamponade devices are typically reserved for the last line of therapy, endoscopy rather than transjugular intrahepatic portosystemic shunt (TIPS) was the preferred method of hemorrhage source control in our patient for several reasons. First, although the working diagnosis of varices was based on the patient’s history, we wanted to evaluate for other causes of upper gastrointestinal bleeding since our patient had no history of endoscopy. Therefore, endoscopy had both a therapeutic and diagnostic value. Secondly, though TIPS may decrease pressure within the bleeding varix, only endoscopy permits direct hemostasis. Also, endoscopy also was preferred over TIPS because our patient was too unstable to move to the interventional radiology suite.16
Conclusion
Although life-threatening esophageal variceal hemorrhage is a rare manifestation of an uncommon disease, it should be considered in the differential diagnosis of a patient who has sarcoidosis and presents with gastrointestinal bleeding. Additionally, when caring for a patient with massive hematemesis without evidence of liver cirrhosis, other etiologies of portal hypertension and esophageal varices, such as sarcoidosis, should be considered.
Sarcoidosis is a systemic disorder of unknown etiology and is characterized by the formation of granulomas throughout various organs in the body. The most common form is pulmonary sarcoidosis, which affects 90% of patients; the second most common form is oculocutaneous sarcoidosis;1 and the third most common form is hepatic sarcoidosis, which affects 63% to 90% of patients.2 Although the liver is frequently involved in all forms of sarcoidosis, only a fraction of patients present with clinically evident liver disease.1 Approximately 20% to 30% of patients have abnormalities on liver function tests, whereas only about 1% of patients show evidence of portal hypertension and cirrhosis.3 In fact, in the English literature, there were 35 reported cases of portal hypertension due to sarcoidosis between 1949 to 2001, of which 16 of the patients had no evidence of cirrhosis.4
The diagnosis of sarcoidosis is usually made by a compilation of clinical signs and symptoms, imaging studies, and biopsies demonstrating noncaseating granulomas. This case report describes a patient who presented with portal hypertension and esophageal variceal bleeding secondary to sarcoidosis of the liver without cirrhotic changes.
Case
A 47-year-old woman presented to the ED via emergency medical services with a 1-hour history of hematemesis and melena. The patient stated that she felt fatigued, nauseated, and light-headed, but had no pain or focal weakness. Her medical history was significant for pulmonary and renal sarcoidosis. She underwent a liver biopsy 1 week prior to presentation, with a 6-day hospitalization period, due to new ascites found on examination.
The patient’s vital signs at presentation were: blood pressure (BP), 72/56 mm Hg; heart rate (HR), 133 beats/min, respiratory rate, 24 breaths/min; and temperature, 97.0oF. Oxygen saturation was 99% on room air. Physical examination revealed an alert and oriented middle-aged woman in extremis who was vomiting dark-colored blood. The cardiac and pulmonary examination revealed no extraneous sounds; the abdominal examination showed ascites with a liver edge palpable 4 cm beneath the right costal margin. The patient had no scleral icterus, palmar erythema, spider angiomata, fetor hepaticus, caput medusa, cutaneous ecchymoses, or any other stigmata of cirrhosis.
Two large-bore peripheral intravenous (IV) catheters were placed and a massive blood transfusion protocol was initiated. Packed red blood cells (PRBCs) from the resuscitation-area refrigerator were infused immediately via a pressurized fluid warmer.
After consultation with gastroenterology and general surgery services, the patient was given 1 g ceftriaxone IV, 1 g tranexamic acid IV, 20 mcg desmopressin IV, 50 mcg octreotide IV, 40 mg pantoprazole IV, 8 mg ondansetron IV, 4 g calcium gluconate IV, and 100 mg hydrocortisone IV.
Throughout the patient’s first 10 minutes in the ED, she remained persistently hypotensive and continued to vomit. Since the patient’s sensorium was intact, the team quickly discussed goals of care with her. The patient’s wishes were for maximal life-sustaining therapy, including endotracheal intubation and chest compressions, if necessary.
After this discussion, the patient was given IV etomidate and rocuronium and was intubated using video-assisted laryngoscopy. Following intubation, she was sedated with an infusion of fentanyl and underwent orogastric tube placement to aspirate stomach contents. A total of 2.5 L of frank blood were drained from the patient’s stomach.
A size 9 French single lumen left-femoral central venous catheter also was placed, through which additional blood products were infused. The patient received a total of 28 U PRBCs, fresh frozen plasma, and platelets over a 3-hour period. During transfusion, the patient’s vital signs improved to a systolic BP ranging between 110 to 120 mm Hg and an HR ranging between 90 to 110 beats/min; she did not experience any further hypotensive episodes throughout her stay in the ED.
Laboratory studies were significant for metabolic acidosis, hyperkalemia, acute on chronic anemia, leukocytosis, and acute on chronic renal failure. Synthetic function of the liver and transaminases appeared normal (Table).
The patient’s hyperkalemia was treated with 1 g calcium chloride IV, 50 g dextrose IV, and 10 U regular insulin IV. A portable chest radiograph showed an appropriately positioned endotracheal tube, and an electrocardiogram revealed sinus tachycardia without signs of hyperkalemia. A computed tomography (CT) scan of the abdomen and pelvis from the patient’s recent hospitalization, 1 week prior to presentation, showed hepatomegaly, liver granulomas, ascites, and periportal lymphadenopathy (Figure 1).
A review of the patient’s recent liver biopsy and ascitic fluid analysis revealed noncaseating granulomas compressing the hepatic sinusoids, and a serum ascites albumin gradient greater than 1.1 g/dL, implying portal hypertension without cirrhosis. The surgical team attempted to place a Sengstaken-Blakemore tube, but the device could not be positioned properly due to the patient’s narrowed esophagus.
The ED nurses cleaned the patient, preserving her dignity; thereafter the patient’s adult children visited with her briefly before she was taken for an upper endoscopy, which was performed in the ED. The endoscopy revealed actively hemorrhaging esophageal varices at the gastroesophageal junction (Figure 2). The varices were treated with endoscopic ligation; the gastroenterologist placed a total of 11 bands, resulting in cessation of bleeding.
After the endoscopy, the patient was admitted to the medical intensive care unit (ICU). Approximately 1.5 hours after arriving at the ICU, she developed renewed hematemesis. Despite efforts to control bleeding and provide hemodynamic support, the patient died 1 hour later.
Discussion
Etiology
Esophageal variceal hemorrhage is caused by pressure elevation in the portal venous system, leading to engorged esophageal veins that can bleed spontaneously. Approximately 90% of portal hypertension is due to liver cirrhosis.5 The remaining 10% of cases are primarily vascular in etiology, with endothelial dysfunction and thrombosis leading to increased portal resistance. Noncirrhotic causes of portal hypertension include malignancy, congenital diseases, viral hepatitides, vascular thromboses or fistulae, constrictive pericarditis, fatty liver of pregnancy, drugs, radiation injury, and infiltrative diseases.5
Sarcoidosis may cause noncaseating granulomas to form in the liver, leading to portal hypertension and fatal exsanguination from esophageal variceal hemorrhage. Although the lesions of sarcoidosis classically form in the lungs, any organ system may be affected.6,7 Frank cirrhosis of the liver occurs in only 1% of sarcoidosis patients; however, radiographic involvement of the liver is seen in 5% to 15% of patients.8
There are several mechanisms which may be responsible for portal hypertension in patients with sarcoidosis, including granulomas causing mass effect on the hepatic sinusoids; arteriovenous shunts within the granuloma; granulomatous phlebitis within the sinusoids; or compressive periportal lymphadenopathy.9 Regardless of the mechanism, a review of the literature demonstrates an association between sarcoidosis and symptomatic portal hypertension.2,4,10,11Although our patient ultimately died, early initiation of massive blood transfusion protocol, airway protection, attention to electrolytes, and endoscopic control of the hemorrhage source provided the best chance for survival.
Medical Therapy
The first priority in managing and treating esophageal varices is to secure the patient’s airways to prevent aspiration. Two large bore IV lines should be placed to permit rapid infusion of crystalloid fluids or blood products. Initiating antibiotics, specifically IV ceftriaxone, to patients with variceal bleeding is a class I recommendation, as this is the only intervention shown to increase patient survival.12 Although proton pump inhibitors (PPI) and somatostatin analogues (typically octreotide) are frequently given, they are both class II recommendations because there is limited evidence supporting the benefit of their use.12 However, current guidelines recommend treating patients for variceal bleeding with an initial bolus of a PPI, followed by a continuous infusion of PPI for 72 hours. As previously noted, multiple studies, have failed to show any decrease in mortality associated with this treatment.12
Other agents that are used to treat variceal bleeding include octreotide and vasopressin. Octreotide, a somatostatin analog, is generally given as an initial IV bolus followed by continuous infusion, and has been shown to decrease transfusion requirements without mortality benefit.12 Vasopressin is generally given to critically ill patients, and is considered a third-line treatment for variceal bleeding.
Since our patient had a history of chronic kidney disease, desmopressin was empirically administered in the event platelet dysfunction was a contributing factor to bleeding.13 The absence of cirrhosis was significant because our patient was unlikely to have a bleeding diathesis caused by coagulation factor deficiency. Therefore, the goal transfusion ratio of blood products should be balanced, similar to that in traumatic exsanguination, rather than favoring an increased ratio of plasma to other blood products. Similarly, tranexamic acid was administered because insufficient tamponade rather than coagulopathy was the presumed cause of sustained hemorrhage.
An additional complicating factor in our patient’s care was the potential effect of the massive transfusion on electrolytes. Packed RBCs have a pH of approximately 6.8 and may carry up to 25 mmol/L of potassium, which may have exacerbated our patient’s underlying hyperkalemia.14 Rapid blood transfusion also places patients at risk for acute hypocalcemia secondary to citrate toxicity; this did not occur in our patient in part because the metabolic function of her liver was preserved and citrate could be broken down in the hepatocyte Krebs cycle.15 Calcium therapy doubled as treatment for the hyperkalemia and as prophylaxis against further hypocalcemia. No dysrhythmias were observed.
Surgical Intervention
Emergency physicians should consult with gastroenterology services so that an endoscopy can be performed as soon as possible to evaluate for and control bleeding. When an endoscopy cannot be performed rapidly, there are multiple balloon tamponade devices available that can be used to temporize the bleeding, such as the Sengstaken-Blakemore tube.12
Although balloon tamponade devices are typically reserved for the last line of therapy, endoscopy rather than transjugular intrahepatic portosystemic shunt (TIPS) was the preferred method of hemorrhage source control in our patient for several reasons. First, although the working diagnosis of varices was based on the patient’s history, we wanted to evaluate for other causes of upper gastrointestinal bleeding since our patient had no history of endoscopy. Therefore, endoscopy had both a therapeutic and diagnostic value. Secondly, though TIPS may decrease pressure within the bleeding varix, only endoscopy permits direct hemostasis. Also, endoscopy also was preferred over TIPS because our patient was too unstable to move to the interventional radiology suite.16
Conclusion
Although life-threatening esophageal variceal hemorrhage is a rare manifestation of an uncommon disease, it should be considered in the differential diagnosis of a patient who has sarcoidosis and presents with gastrointestinal bleeding. Additionally, when caring for a patient with massive hematemesis without evidence of liver cirrhosis, other etiologies of portal hypertension and esophageal varices, such as sarcoidosis, should be considered.
1. Rao DA, Dellaripa PF. Extrapulmonary manifestations of sarcoidosis. Rheum Dis Clin North Am. 2013;39(2):277-297. doi:10.1016/j.rdc.2013.02.007.
2. Mistilis SP, Green JR, Schiff L. Hepatic sarcoidosis with portal hypertension. Am J Med. 1964;36(3):470-475. doi:10.1016/0002-9343(64)90175-5.
3. Tekeste H, Latour F, Levitt RE. Portal hypertension complicating sarcoid liver disease: case report and review of the literature. Am J Gastroenterol. 1984;79(5):389-396.
4. Ivonye C, Elhammali B, Henriques-Forsythe M, Bennett-Gittens R, Oderinde A. Disseminated sarcoidosis resulting in portal hypertension and gastrointestinal bleeding: a rare presentation. Can J Gastroenterol. 2012;26(8):508-509. http://www.ncbi.nlm.nih.gov/pubmed/22891173. Accessed May 16, 2018.
5. Tetangco EP, Silva RG, Lerma EV. Portal hypertension: etiology, evaluation, and management. Dis Mon. 2016;62(12):411-426. doi:10.1016/j.disamonth.2016.08.001.
6. Valeyre D, Prasse A, Nunes H, Uzunhan Y, Brillet PY, Müller-Quernheim J. Sarcoidosis. Lancet. 2014;383(9923):1155-1167. doi:10.1016/S0140-6736(13)60680-7.
7. Al-Kofahi K, Korsten P, Ascoli C, et al. Management of extrapulmonary sarcoidosis: challenges and solutions. Ther Clin Risk Manag. 2016;12:1623-1634. doi:10.2147/TCRM.S74476.
8. Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med. 2007;357(21):2153-2165. doi:10.1056/NEJMra071714.
9. Ebert EC, Kierson M, Hagspiel KD. Gastrointestinal and hepatic manifestations of sarcoidosis. Am J Gastroenterol. 2008;103(12):3184-3192. doi:10.1111/j.1572-0241.2008.02202.x.
10. Fraimow W, Myerson RM. Portal hypertension and bleeding esophageal varices secondary to sarcoidosis of the liver. Am J Med. 1957;23(6):995-998.
11. Saito H, Ohmori M, Iwamuro M, et al. Hepatic and gastric involvement in a case of systemic sarcoidosis presenting with rupture of esophageal varices. Intern Med. 2018;56(19):2583-2588. doi:10.2169/internalmedicine.8768-16.
12. DeLaney M, Greene CJ. Emergency Department evaluation and management of patients with upper gastrointestinal bleeding. Emerg Med Pract. 2015;17(4):1-18; quiz 19.
13. Ozgönenel B, Rajpurkar M, Lusher JM. How do you treat bleeding disorders with desmopressin? Postgrad Med J. 2007;83(977):159-163. doi:10.1136/pgmj.2006.052118.
14. Sümpelmann R, Schürholz T, Thorns E, Hausdörfer J. Acid-base, electrolyte and metabolite concentrations in packed red blood cells for major transfusion in infants. Paediatr Anaesth. 2001;11(2):169-173. doi:10.1046/j.1460-9592.2001.00637.x.
15. Monchi M. Citrate pathophysiology and metabolism. Transfus Apher Sci. 2018;56(1):28-30. doi:10.1016/j.transci.2016.12.013.
16. Shah RP, Sze DY. Complications during transjugular intrahepatic portosystemic shunt creation. Tech Vasc Interv Radiol. 2016;19(1):61-73. doi:10.1053/j.tvir.2016.01.007.
1. Rao DA, Dellaripa PF. Extrapulmonary manifestations of sarcoidosis. Rheum Dis Clin North Am. 2013;39(2):277-297. doi:10.1016/j.rdc.2013.02.007.
2. Mistilis SP, Green JR, Schiff L. Hepatic sarcoidosis with portal hypertension. Am J Med. 1964;36(3):470-475. doi:10.1016/0002-9343(64)90175-5.
3. Tekeste H, Latour F, Levitt RE. Portal hypertension complicating sarcoid liver disease: case report and review of the literature. Am J Gastroenterol. 1984;79(5):389-396.
4. Ivonye C, Elhammali B, Henriques-Forsythe M, Bennett-Gittens R, Oderinde A. Disseminated sarcoidosis resulting in portal hypertension and gastrointestinal bleeding: a rare presentation. Can J Gastroenterol. 2012;26(8):508-509. http://www.ncbi.nlm.nih.gov/pubmed/22891173. Accessed May 16, 2018.
5. Tetangco EP, Silva RG, Lerma EV. Portal hypertension: etiology, evaluation, and management. Dis Mon. 2016;62(12):411-426. doi:10.1016/j.disamonth.2016.08.001.
6. Valeyre D, Prasse A, Nunes H, Uzunhan Y, Brillet PY, Müller-Quernheim J. Sarcoidosis. Lancet. 2014;383(9923):1155-1167. doi:10.1016/S0140-6736(13)60680-7.
7. Al-Kofahi K, Korsten P, Ascoli C, et al. Management of extrapulmonary sarcoidosis: challenges and solutions. Ther Clin Risk Manag. 2016;12:1623-1634. doi:10.2147/TCRM.S74476.
8. Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med. 2007;357(21):2153-2165. doi:10.1056/NEJMra071714.
9. Ebert EC, Kierson M, Hagspiel KD. Gastrointestinal and hepatic manifestations of sarcoidosis. Am J Gastroenterol. 2008;103(12):3184-3192. doi:10.1111/j.1572-0241.2008.02202.x.
10. Fraimow W, Myerson RM. Portal hypertension and bleeding esophageal varices secondary to sarcoidosis of the liver. Am J Med. 1957;23(6):995-998.
11. Saito H, Ohmori M, Iwamuro M, et al. Hepatic and gastric involvement in a case of systemic sarcoidosis presenting with rupture of esophageal varices. Intern Med. 2018;56(19):2583-2588. doi:10.2169/internalmedicine.8768-16.
12. DeLaney M, Greene CJ. Emergency Department evaluation and management of patients with upper gastrointestinal bleeding. Emerg Med Pract. 2015;17(4):1-18; quiz 19.
13. Ozgönenel B, Rajpurkar M, Lusher JM. How do you treat bleeding disorders with desmopressin? Postgrad Med J. 2007;83(977):159-163. doi:10.1136/pgmj.2006.052118.
14. Sümpelmann R, Schürholz T, Thorns E, Hausdörfer J. Acid-base, electrolyte and metabolite concentrations in packed red blood cells for major transfusion in infants. Paediatr Anaesth. 2001;11(2):169-173. doi:10.1046/j.1460-9592.2001.00637.x.
15. Monchi M. Citrate pathophysiology and metabolism. Transfus Apher Sci. 2018;56(1):28-30. doi:10.1016/j.transci.2016.12.013.
16. Shah RP, Sze DY. Complications during transjugular intrahepatic portosystemic shunt creation. Tech Vasc Interv Radiol. 2016;19(1):61-73. doi:10.1053/j.tvir.2016.01.007.







