User login
Do drug treatment POEMs report data in clinically useful ways?
Purpose To provide the best care, physicians must determine what published information is relevant, valid, and clinically useful. Patient-Oriented Evidence that Matters (POEMs) defines relevance as information that addresses clinical questions, measures clinical outcomes, and has the potential to change practice. The most useful clinical information is presented in terms of absolute risk reduction (ARR), number needed to treat (NNT), and number needed to harm (NNH). The purpose of this study was to estimate the percentage of drug treatment articles published in major medical journals that provide a calculated ARR, NNT, or NNH.
Methods We independently reviewed all drug treatment articles in 7 journals during a 6-month period for relevance, validity, and clinical usefulness. (Journals included Journal of the American Medical Association [JAMA], Archives of Internal Medicine [Arch Intern Med], British Medical Journal [BMJ], New England Journal of Medicine, Lancet, Obstetrics and Gynecology [Obstet Gynecol], and Pediatrics.) We assessed clinical usefulness by recording whether the articles reported ARR, NNT, or NNH.
Results Of the 995 articles we reviewed, only 2.4% met relevance criteria. Fewer than 1% of all drug therapy articles were POEMs with calculated ARR, NNT, or NNH. Arch Intern Med, JAMA, and BMJ published the most drug therapy POEMs: 33%, 20%, and 17%, respectively. JAMA, BMJ, and Obstet Gynecol were the only journals that published POEMs with clinically useful information.
Conclusions Most major journals that address primary care issues do not publish drug therapy POEMs; those that do rarely present information in a clinically useful manner. Editors should require authors to provide ARRs, NNTs, and NNHs to help clinicians provide the best medical care for their patients.
Medical professionals are inundated by new information, which some have described as an “information jungle.”1 Thousands of articles are published each year in hundreds of journals,2 adding to an ever-expanding knowledge base. One study suggests that the experienced primary care physician uses up to 2 million pieces of information each year to manage patients.3 To provide appropriate patient care, physicians must stay abreast of current medical knowledge.4 However, busy clinicians have little time to navigate the information jungle and sift through all of the data to determine what is relevant and clinically useful.
More than a decade has passed since Allen Shaughnessy and David Slawson developed the concept of Patient-Oriented Evidence that Matters (POEMs), “a summary of a valid piece of research that carries information that is important to patients and so to their doctors.”5 They developed a formula to classify research as a POEM: U=R×V/W, where U=usefulness of the information to doctors, R=relevance of the information to doctors, V=validity of the information, and W=work to access the information.1 The most useful information is both relevant and valid and takes little work to access. In 2002, the British Medical Journal (BMJ) proposed publishing one POEM a week based on the following criteria:
- It addresses a question that doctors encounter.
- It measures outcomes that doctors and their patients care about: symptoms, morbidity, quality of life, and mortality.
- It has the potential to change the way doctors practice.5
Even with the advent of POEMs, the true benefit of research and its application to clinical practice has yet to be determined. Physicians still have to decide which studies are valid, interpret the outcomes, and determine how they affect individual practice.
Research shows that clinicians, patients, and policy makers are more impressed by larger percentage differences than smaller ones.6 This fact is evident in the way trial results are presented in the news, by pharmaceutical representatives, and in journal articles. The relative risk reduction (RRR) is touted as suggesting either benefit or reduced harm, and the absolute numbers are largely under-reported. One study found that treatment effectiveness was perceived to be lower when the absolute risk reduction (ARR) rather than the RRR was reported.7 Perception of effectiveness decreased further when the number needed to treat (NNT) was presented.
The authors of the study concluded that ARR and NNT provide more concrete information than RRR about an intervention because they express efficacy “in a way which incorporates both the magnitude of the reduction of risk and the baseline risk without treatment.” They note that “because the exclusive reporting of relative risk may overstate the effectiveness of a treatment, actual event rates and absolute changes in risk should be reported.”7
These numbers are rarely found in journal articles and, when present, rarely appear in abstracts, tables, or graphs, where the busy clinician looks to find information quickly. Our study sought to estimate the percentage of drug treatment articles published in major medical journals that provide a calculated ARR, NNT, or number needed to harm (NNH), as demonstrated in TABLE 1.
TABLE 1
How to calculate RRR, ARR, and NNT
| Example: The rate of myocardial infarction in the control group is 4% and the rate in the treatment group is 2% | |
|---|---|
| RRR=event rate of control group - event rate of treatment group/event rate of control group | RRR=[4–2]/4=50% |
| ARR=event rate of control group - event rate of treatment group | ARR=4–2=2% |
| NNT*=100/ARR | NNT=100/2=50 |
| ARR, absolute risk reduction; NNT, number needed to treat; RRR, relative risk reduction. *This calculation is the same for the number needed to harm. | |
Methods
Pilot study
We first performed a pilot study that retrospectively reviewed all drug therapy articles published in the Journal of the American Medical Association (JAMA) from April 1, 2008, through April 1, 2009. Its purpose was to ensure concurrence in data gathering, rule out any measurement bias, and refine the analysis tool.
We applied an algorithmic approach to the review and used an Excel spreadsheet as a record-keeping tool, giving each article an abbreviated name and recording the issue, year, volume, and page numbers. We excluded case reports, review articles that were not systematic reviews or meta-analyses, letters, and editorials. We also excluded articles on cancer chemotherapy because, although family physicians need to have a working knowledge of antineoplastic drugs, they do not routinely prescribe them. Moreover, family physicians rarely write the first prescription for such a drug.
We reviewed the drug treatment articles to determine relevance—that is, whether they met POEMs criteria: addressed a question that most family medicine doctors encounter in a typical 6-month period, measured an outcome that family physicians and patients care about—such as morbidity, mortality, quality of life, or effect on clinical events—and had the potential to change clinical practice.8 Articles that met all 3 criteria were included in our analysis; articles that did not were recorded but excluded from further examination.
We analyzed articles that met relevance criteria for validity and clinical usefulness. We assessed validity based on whether the article was a randomized, controlled, double-blinded trial and whether allocation was concealed, follow-up was complete, information was analyzed on an intention-to-treat basis, and the results were statistically significant. We assessed clinical usefulness by recording whether the study reported RRR, ARR, NNT, or NNH, and if so, whether it recorded the information in free text, including the abstract, or in a graph or chart.
Review of articles in 7 journals
After the pilot study, we reviewed articles in JAMA and 6 other journals during a 6-month period from April 1, 2008 through September 30, 2008. We applied the same algorithmic analysis as in the pilot study to drug therapy articles in Archives of Internal Medicine (Arch Intern Med), BMJ, New England Journal of Medicine (N Engl J Med), Lancet, Obstetrics and Gynecology (Obstet Gynecol), and Pediatrics. We met regularly and settled disagreements about relevance, validity, or clinical usefulness by re-reviewing the article.
We recorded the total number of drug therapy articles for each journal, then tallied the number of POEMs and the total number of calculations of RRR, ARR, NNT, and NNH in articles that met POEMs criteria. We used these numbers to determine the percentage of POEMs and POEMs with clinically useful information for each journal.
Results
We identified a total of 995 articles in the 7 journals during the 6-month study period. Of these, 24 (2.4%) were classified as drug therapy POEMs and 6 (0.6%) were POEMs with clinically useful information.
The journals that published the most POEMs were Arch Intern Med, N Engl J Med, BMJ, and JAMA. Arch Intern Med had the highest percentage (33%), followed by JAMA (20%), and BMJ (17%) (TABLE 2).
When we analyzed the POEMs for clinical usefulness based on whether they provided calculated ARR, NNT, or NNH, only 3 journals published POEMs with clinically useful information: JAMA published the most (15%), followed by BMJ (9%) and Obstet Gynecol (4%) (FIGURE).
FIGURE
Percentage of POEMs with clinically useful information*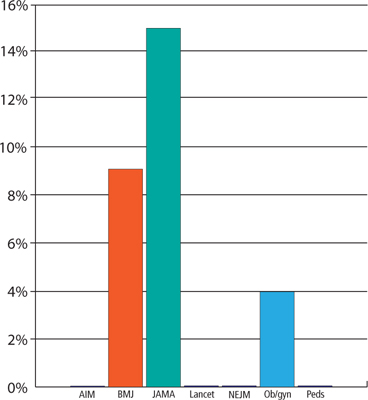
AIM, Archives of Internal Medicine; BMJ, British Medical Journal; JAMA, Journal of the American Medical Association; NEJM, New England Journal of Medicine; Ob/gyn, Obstetrics and Gynecology; Peds, Pediatrics; POEMs, Patient-Oriented Evidence that Matters.
*Clinically useful information is defined as information that includes the absolute risk reduction, number needed to treat, or number needed to harm.
TABLE 2
Few drug therapy articles are POEMs
| Journal | Articles published in 6 months, n | Drug therapy articles, n | Drug therapy articles that are POEMs, n (%) |
|---|---|---|---|
| Arch Intern Med | 123 | 24 | 8 (33) |
| BMJ | 127 | 23 | 4 (17) |
| JAMA | 85 | 20 | 4 (20) |
| Lancet | 92 | 33 | 1 (3) |
| N Engl J Med | 128 | 39 | 4 (10) |
| 0bstet Gynecol | 115 | 24 | 2 (8) |
| Pediatrics | 325 | 50 | 1 (2) |
| Arch Intern Med, Archives of Internal Medicine; BMJ, British Medical Journal; JAMA, Journal of the American Medical Association; N Engl J Med, New England Journal of Medicine; Obstet Gynecol, Obstetrics and Gynecology; POEMs, Patient-Oriented Evidence that Matters. | |||
Discussion
Our study findings are consistent with the relevance data from a previous study published in 1999.8 After more than a decade, medical journals still are not publishing drug therapy POEMs.
A disturbing scarcity of useful information. The paucity of drug therapy POEMs with clinically useful information is alarming. Based on our data we estimate that a physician would have to read on average 36 drug therapy articles to find one clinically helpful drug therapy POEM. This finding suggests that the medical literature is not helping clinicians provide the best patient care or, when it does, the busy clinician is forced to spend what little time is available in calculations to determine what can actually affect practice in positive ways.
Study limitations. Our study has a number of limitations. The study settings in the articles we reviewed ultimately determined what information was important and could potentially change clinical practice. Some studies, for example, were performed in developing countries, where the therapy being tested was not commonly used and would alter practice. In the United States, however, the same treatment would not affect clinical practice because it was either common practice or standard of care.
We reviewed only 7 major medical journals. Our results cannot necessarily be extrapolated to other major journals, although they do suggest that the findings are not limited to a few publications. Moreover, we reviewed only 2 specialty journals. It is possible that other such journals are publishing more POEMs than we observed and providing more concrete numbers that specialists can use to quickly and easily adjust their practice patterns than general journals.
We did not analyze any family medicine journals for the following reasons: American Family Physician publishes only review articles; The Journal of Family Practice does not routinely publish original research; and Annals of Family Medicine (the research journal of the discipline) is less well established than the other journals we selected for this review, having been launched in 2003.
In addition, 6 months may not have been long enough to accurately calculate the percentage of POEMs or clinically useful information in the journals we reviewed. During the pilot study, in which we analyzed a full year of JAMA, only one POEM was published in the second 6 months, and it did not contain clinically useful information. Moreover, we reviewed most journals over 6 consecutive months rather than 6 randomly chosen months.
Finally, we reviewed only drug therapy articles. Future studies could examine surgical, diagnostic, or prognostic studies.
Toward more, and more useful, POEMs
Despite the scarcity of POEMs that provide clinically useful information in major medical journals, it is important that physicians continue to practice evidence-based medicine, sifting through the available information and even calculating ARR, NNT, and NNH themselves, which most busy clinicians do not have the time or inclination to do.
How can we improve the clinical usefulness of published data? One way is for journal editors to require that authors provide ARRs and NNTs or NNHs. Another is for authors to include these calculations on their own initiative. Either way, the goal is better clinical practice and optimal patient care.
CORRESPONDENCE Adrienne Z. Ables, PharmD, Edward Via College of Osteopathic Medicine, Carolinas Campus, 350 Howard Street, Spartanburg, SC 29303; aables@carolinas.vcom.edu
1. Shaughnessy AF, Slawson DC, Bennett JH. Becoming an information master: a guidebook to the medical information jungle. J Fam Pract. 1994;39:489-499.
2. Shaughnessy AF. Evaluating and understanding articles about treatment. Am Fam Physician. 2009;79:668-670.
3. Wyatt J. Uses and sources of medical knowledge. Lancet. 1991;338:1368-1372.
4. Gonzáles-Gonzáles AI, Dawes M, Sánchez-Mateos J, et al. Information needs and information-seeking behavior of primary care physicians. Ann Fam Med. 2007;5:345-352.
5. Smith R. A POEM a week for the BMJ. BMJ. 2002;325:983.-
6. Naylor CD, Chen E, Strauss B. Measured enthusiasm: Does the method of reporting trial results alter perceptions of therapeutic effectiveness? Ann Intern Med. 1992;117:916-921.
7. Bucher HC, Weinbacher M, Gyr K. Influence of method of reporting study results on decision of physicians to prescribe drugs to lower cholesterol concentration. BMJ. 1994;309:761-764.
8. Ebell MH, Barry HC, Slawson DC, et al. Finding POEMs in the Medical Literature. J Fam Pract 1999;48:350-355.
Purpose To provide the best care, physicians must determine what published information is relevant, valid, and clinically useful. Patient-Oriented Evidence that Matters (POEMs) defines relevance as information that addresses clinical questions, measures clinical outcomes, and has the potential to change practice. The most useful clinical information is presented in terms of absolute risk reduction (ARR), number needed to treat (NNT), and number needed to harm (NNH). The purpose of this study was to estimate the percentage of drug treatment articles published in major medical journals that provide a calculated ARR, NNT, or NNH.
Methods We independently reviewed all drug treatment articles in 7 journals during a 6-month period for relevance, validity, and clinical usefulness. (Journals included Journal of the American Medical Association [JAMA], Archives of Internal Medicine [Arch Intern Med], British Medical Journal [BMJ], New England Journal of Medicine, Lancet, Obstetrics and Gynecology [Obstet Gynecol], and Pediatrics.) We assessed clinical usefulness by recording whether the articles reported ARR, NNT, or NNH.
Results Of the 995 articles we reviewed, only 2.4% met relevance criteria. Fewer than 1% of all drug therapy articles were POEMs with calculated ARR, NNT, or NNH. Arch Intern Med, JAMA, and BMJ published the most drug therapy POEMs: 33%, 20%, and 17%, respectively. JAMA, BMJ, and Obstet Gynecol were the only journals that published POEMs with clinically useful information.
Conclusions Most major journals that address primary care issues do not publish drug therapy POEMs; those that do rarely present information in a clinically useful manner. Editors should require authors to provide ARRs, NNTs, and NNHs to help clinicians provide the best medical care for their patients.
Medical professionals are inundated by new information, which some have described as an “information jungle.”1 Thousands of articles are published each year in hundreds of journals,2 adding to an ever-expanding knowledge base. One study suggests that the experienced primary care physician uses up to 2 million pieces of information each year to manage patients.3 To provide appropriate patient care, physicians must stay abreast of current medical knowledge.4 However, busy clinicians have little time to navigate the information jungle and sift through all of the data to determine what is relevant and clinically useful.
More than a decade has passed since Allen Shaughnessy and David Slawson developed the concept of Patient-Oriented Evidence that Matters (POEMs), “a summary of a valid piece of research that carries information that is important to patients and so to their doctors.”5 They developed a formula to classify research as a POEM: U=R×V/W, where U=usefulness of the information to doctors, R=relevance of the information to doctors, V=validity of the information, and W=work to access the information.1 The most useful information is both relevant and valid and takes little work to access. In 2002, the British Medical Journal (BMJ) proposed publishing one POEM a week based on the following criteria:
- It addresses a question that doctors encounter.
- It measures outcomes that doctors and their patients care about: symptoms, morbidity, quality of life, and mortality.
- It has the potential to change the way doctors practice.5
Even with the advent of POEMs, the true benefit of research and its application to clinical practice has yet to be determined. Physicians still have to decide which studies are valid, interpret the outcomes, and determine how they affect individual practice.
Research shows that clinicians, patients, and policy makers are more impressed by larger percentage differences than smaller ones.6 This fact is evident in the way trial results are presented in the news, by pharmaceutical representatives, and in journal articles. The relative risk reduction (RRR) is touted as suggesting either benefit or reduced harm, and the absolute numbers are largely under-reported. One study found that treatment effectiveness was perceived to be lower when the absolute risk reduction (ARR) rather than the RRR was reported.7 Perception of effectiveness decreased further when the number needed to treat (NNT) was presented.
The authors of the study concluded that ARR and NNT provide more concrete information than RRR about an intervention because they express efficacy “in a way which incorporates both the magnitude of the reduction of risk and the baseline risk without treatment.” They note that “because the exclusive reporting of relative risk may overstate the effectiveness of a treatment, actual event rates and absolute changes in risk should be reported.”7
These numbers are rarely found in journal articles and, when present, rarely appear in abstracts, tables, or graphs, where the busy clinician looks to find information quickly. Our study sought to estimate the percentage of drug treatment articles published in major medical journals that provide a calculated ARR, NNT, or number needed to harm (NNH), as demonstrated in TABLE 1.
TABLE 1
How to calculate RRR, ARR, and NNT
| Example: The rate of myocardial infarction in the control group is 4% and the rate in the treatment group is 2% | |
|---|---|
| RRR=event rate of control group - event rate of treatment group/event rate of control group | RRR=[4–2]/4=50% |
| ARR=event rate of control group - event rate of treatment group | ARR=4–2=2% |
| NNT*=100/ARR | NNT=100/2=50 |
| ARR, absolute risk reduction; NNT, number needed to treat; RRR, relative risk reduction. *This calculation is the same for the number needed to harm. | |
Methods
Pilot study
We first performed a pilot study that retrospectively reviewed all drug therapy articles published in the Journal of the American Medical Association (JAMA) from April 1, 2008, through April 1, 2009. Its purpose was to ensure concurrence in data gathering, rule out any measurement bias, and refine the analysis tool.
We applied an algorithmic approach to the review and used an Excel spreadsheet as a record-keeping tool, giving each article an abbreviated name and recording the issue, year, volume, and page numbers. We excluded case reports, review articles that were not systematic reviews or meta-analyses, letters, and editorials. We also excluded articles on cancer chemotherapy because, although family physicians need to have a working knowledge of antineoplastic drugs, they do not routinely prescribe them. Moreover, family physicians rarely write the first prescription for such a drug.
We reviewed the drug treatment articles to determine relevance—that is, whether they met POEMs criteria: addressed a question that most family medicine doctors encounter in a typical 6-month period, measured an outcome that family physicians and patients care about—such as morbidity, mortality, quality of life, or effect on clinical events—and had the potential to change clinical practice.8 Articles that met all 3 criteria were included in our analysis; articles that did not were recorded but excluded from further examination.
We analyzed articles that met relevance criteria for validity and clinical usefulness. We assessed validity based on whether the article was a randomized, controlled, double-blinded trial and whether allocation was concealed, follow-up was complete, information was analyzed on an intention-to-treat basis, and the results were statistically significant. We assessed clinical usefulness by recording whether the study reported RRR, ARR, NNT, or NNH, and if so, whether it recorded the information in free text, including the abstract, or in a graph or chart.
Review of articles in 7 journals
After the pilot study, we reviewed articles in JAMA and 6 other journals during a 6-month period from April 1, 2008 through September 30, 2008. We applied the same algorithmic analysis as in the pilot study to drug therapy articles in Archives of Internal Medicine (Arch Intern Med), BMJ, New England Journal of Medicine (N Engl J Med), Lancet, Obstetrics and Gynecology (Obstet Gynecol), and Pediatrics. We met regularly and settled disagreements about relevance, validity, or clinical usefulness by re-reviewing the article.
We recorded the total number of drug therapy articles for each journal, then tallied the number of POEMs and the total number of calculations of RRR, ARR, NNT, and NNH in articles that met POEMs criteria. We used these numbers to determine the percentage of POEMs and POEMs with clinically useful information for each journal.
Results
We identified a total of 995 articles in the 7 journals during the 6-month study period. Of these, 24 (2.4%) were classified as drug therapy POEMs and 6 (0.6%) were POEMs with clinically useful information.
The journals that published the most POEMs were Arch Intern Med, N Engl J Med, BMJ, and JAMA. Arch Intern Med had the highest percentage (33%), followed by JAMA (20%), and BMJ (17%) (TABLE 2).
When we analyzed the POEMs for clinical usefulness based on whether they provided calculated ARR, NNT, or NNH, only 3 journals published POEMs with clinically useful information: JAMA published the most (15%), followed by BMJ (9%) and Obstet Gynecol (4%) (FIGURE).
FIGURE
Percentage of POEMs with clinically useful information*
AIM, Archives of Internal Medicine; BMJ, British Medical Journal; JAMA, Journal of the American Medical Association; NEJM, New England Journal of Medicine; Ob/gyn, Obstetrics and Gynecology; Peds, Pediatrics; POEMs, Patient-Oriented Evidence that Matters.
*Clinically useful information is defined as information that includes the absolute risk reduction, number needed to treat, or number needed to harm.
TABLE 2
Few drug therapy articles are POEMs
| Journal | Articles published in 6 months, n | Drug therapy articles, n | Drug therapy articles that are POEMs, n (%) |
|---|---|---|---|
| Arch Intern Med | 123 | 24 | 8 (33) |
| BMJ | 127 | 23 | 4 (17) |
| JAMA | 85 | 20 | 4 (20) |
| Lancet | 92 | 33 | 1 (3) |
| N Engl J Med | 128 | 39 | 4 (10) |
| 0bstet Gynecol | 115 | 24 | 2 (8) |
| Pediatrics | 325 | 50 | 1 (2) |
| Arch Intern Med, Archives of Internal Medicine; BMJ, British Medical Journal; JAMA, Journal of the American Medical Association; N Engl J Med, New England Journal of Medicine; Obstet Gynecol, Obstetrics and Gynecology; POEMs, Patient-Oriented Evidence that Matters. | |||
Discussion
Our study findings are consistent with the relevance data from a previous study published in 1999.8 After more than a decade, medical journals still are not publishing drug therapy POEMs.
A disturbing scarcity of useful information. The paucity of drug therapy POEMs with clinically useful information is alarming. Based on our data we estimate that a physician would have to read on average 36 drug therapy articles to find one clinically helpful drug therapy POEM. This finding suggests that the medical literature is not helping clinicians provide the best patient care or, when it does, the busy clinician is forced to spend what little time is available in calculations to determine what can actually affect practice in positive ways.
Study limitations. Our study has a number of limitations. The study settings in the articles we reviewed ultimately determined what information was important and could potentially change clinical practice. Some studies, for example, were performed in developing countries, where the therapy being tested was not commonly used and would alter practice. In the United States, however, the same treatment would not affect clinical practice because it was either common practice or standard of care.
We reviewed only 7 major medical journals. Our results cannot necessarily be extrapolated to other major journals, although they do suggest that the findings are not limited to a few publications. Moreover, we reviewed only 2 specialty journals. It is possible that other such journals are publishing more POEMs than we observed and providing more concrete numbers that specialists can use to quickly and easily adjust their practice patterns than general journals.
We did not analyze any family medicine journals for the following reasons: American Family Physician publishes only review articles; The Journal of Family Practice does not routinely publish original research; and Annals of Family Medicine (the research journal of the discipline) is less well established than the other journals we selected for this review, having been launched in 2003.
In addition, 6 months may not have been long enough to accurately calculate the percentage of POEMs or clinically useful information in the journals we reviewed. During the pilot study, in which we analyzed a full year of JAMA, only one POEM was published in the second 6 months, and it did not contain clinically useful information. Moreover, we reviewed most journals over 6 consecutive months rather than 6 randomly chosen months.
Finally, we reviewed only drug therapy articles. Future studies could examine surgical, diagnostic, or prognostic studies.
Toward more, and more useful, POEMs
Despite the scarcity of POEMs that provide clinically useful information in major medical journals, it is important that physicians continue to practice evidence-based medicine, sifting through the available information and even calculating ARR, NNT, and NNH themselves, which most busy clinicians do not have the time or inclination to do.
How can we improve the clinical usefulness of published data? One way is for journal editors to require that authors provide ARRs and NNTs or NNHs. Another is for authors to include these calculations on their own initiative. Either way, the goal is better clinical practice and optimal patient care.
CORRESPONDENCE Adrienne Z. Ables, PharmD, Edward Via College of Osteopathic Medicine, Carolinas Campus, 350 Howard Street, Spartanburg, SC 29303; aables@carolinas.vcom.edu
Purpose To provide the best care, physicians must determine what published information is relevant, valid, and clinically useful. Patient-Oriented Evidence that Matters (POEMs) defines relevance as information that addresses clinical questions, measures clinical outcomes, and has the potential to change practice. The most useful clinical information is presented in terms of absolute risk reduction (ARR), number needed to treat (NNT), and number needed to harm (NNH). The purpose of this study was to estimate the percentage of drug treatment articles published in major medical journals that provide a calculated ARR, NNT, or NNH.
Methods We independently reviewed all drug treatment articles in 7 journals during a 6-month period for relevance, validity, and clinical usefulness. (Journals included Journal of the American Medical Association [JAMA], Archives of Internal Medicine [Arch Intern Med], British Medical Journal [BMJ], New England Journal of Medicine, Lancet, Obstetrics and Gynecology [Obstet Gynecol], and Pediatrics.) We assessed clinical usefulness by recording whether the articles reported ARR, NNT, or NNH.
Results Of the 995 articles we reviewed, only 2.4% met relevance criteria. Fewer than 1% of all drug therapy articles were POEMs with calculated ARR, NNT, or NNH. Arch Intern Med, JAMA, and BMJ published the most drug therapy POEMs: 33%, 20%, and 17%, respectively. JAMA, BMJ, and Obstet Gynecol were the only journals that published POEMs with clinically useful information.
Conclusions Most major journals that address primary care issues do not publish drug therapy POEMs; those that do rarely present information in a clinically useful manner. Editors should require authors to provide ARRs, NNTs, and NNHs to help clinicians provide the best medical care for their patients.
Medical professionals are inundated by new information, which some have described as an “information jungle.”1 Thousands of articles are published each year in hundreds of journals,2 adding to an ever-expanding knowledge base. One study suggests that the experienced primary care physician uses up to 2 million pieces of information each year to manage patients.3 To provide appropriate patient care, physicians must stay abreast of current medical knowledge.4 However, busy clinicians have little time to navigate the information jungle and sift through all of the data to determine what is relevant and clinically useful.
More than a decade has passed since Allen Shaughnessy and David Slawson developed the concept of Patient-Oriented Evidence that Matters (POEMs), “a summary of a valid piece of research that carries information that is important to patients and so to their doctors.”5 They developed a formula to classify research as a POEM: U=R×V/W, where U=usefulness of the information to doctors, R=relevance of the information to doctors, V=validity of the information, and W=work to access the information.1 The most useful information is both relevant and valid and takes little work to access. In 2002, the British Medical Journal (BMJ) proposed publishing one POEM a week based on the following criteria:
- It addresses a question that doctors encounter.
- It measures outcomes that doctors and their patients care about: symptoms, morbidity, quality of life, and mortality.
- It has the potential to change the way doctors practice.5
Even with the advent of POEMs, the true benefit of research and its application to clinical practice has yet to be determined. Physicians still have to decide which studies are valid, interpret the outcomes, and determine how they affect individual practice.
Research shows that clinicians, patients, and policy makers are more impressed by larger percentage differences than smaller ones.6 This fact is evident in the way trial results are presented in the news, by pharmaceutical representatives, and in journal articles. The relative risk reduction (RRR) is touted as suggesting either benefit or reduced harm, and the absolute numbers are largely under-reported. One study found that treatment effectiveness was perceived to be lower when the absolute risk reduction (ARR) rather than the RRR was reported.7 Perception of effectiveness decreased further when the number needed to treat (NNT) was presented.
The authors of the study concluded that ARR and NNT provide more concrete information than RRR about an intervention because they express efficacy “in a way which incorporates both the magnitude of the reduction of risk and the baseline risk without treatment.” They note that “because the exclusive reporting of relative risk may overstate the effectiveness of a treatment, actual event rates and absolute changes in risk should be reported.”7
These numbers are rarely found in journal articles and, when present, rarely appear in abstracts, tables, or graphs, where the busy clinician looks to find information quickly. Our study sought to estimate the percentage of drug treatment articles published in major medical journals that provide a calculated ARR, NNT, or number needed to harm (NNH), as demonstrated in TABLE 1.
TABLE 1
How to calculate RRR, ARR, and NNT
| Example: The rate of myocardial infarction in the control group is 4% and the rate in the treatment group is 2% | |
|---|---|
| RRR=event rate of control group - event rate of treatment group/event rate of control group | RRR=[4–2]/4=50% |
| ARR=event rate of control group - event rate of treatment group | ARR=4–2=2% |
| NNT*=100/ARR | NNT=100/2=50 |
| ARR, absolute risk reduction; NNT, number needed to treat; RRR, relative risk reduction. *This calculation is the same for the number needed to harm. | |
Methods
Pilot study
We first performed a pilot study that retrospectively reviewed all drug therapy articles published in the Journal of the American Medical Association (JAMA) from April 1, 2008, through April 1, 2009. Its purpose was to ensure concurrence in data gathering, rule out any measurement bias, and refine the analysis tool.
We applied an algorithmic approach to the review and used an Excel spreadsheet as a record-keeping tool, giving each article an abbreviated name and recording the issue, year, volume, and page numbers. We excluded case reports, review articles that were not systematic reviews or meta-analyses, letters, and editorials. We also excluded articles on cancer chemotherapy because, although family physicians need to have a working knowledge of antineoplastic drugs, they do not routinely prescribe them. Moreover, family physicians rarely write the first prescription for such a drug.
We reviewed the drug treatment articles to determine relevance—that is, whether they met POEMs criteria: addressed a question that most family medicine doctors encounter in a typical 6-month period, measured an outcome that family physicians and patients care about—such as morbidity, mortality, quality of life, or effect on clinical events—and had the potential to change clinical practice.8 Articles that met all 3 criteria were included in our analysis; articles that did not were recorded but excluded from further examination.
We analyzed articles that met relevance criteria for validity and clinical usefulness. We assessed validity based on whether the article was a randomized, controlled, double-blinded trial and whether allocation was concealed, follow-up was complete, information was analyzed on an intention-to-treat basis, and the results were statistically significant. We assessed clinical usefulness by recording whether the study reported RRR, ARR, NNT, or NNH, and if so, whether it recorded the information in free text, including the abstract, or in a graph or chart.
Review of articles in 7 journals
After the pilot study, we reviewed articles in JAMA and 6 other journals during a 6-month period from April 1, 2008 through September 30, 2008. We applied the same algorithmic analysis as in the pilot study to drug therapy articles in Archives of Internal Medicine (Arch Intern Med), BMJ, New England Journal of Medicine (N Engl J Med), Lancet, Obstetrics and Gynecology (Obstet Gynecol), and Pediatrics. We met regularly and settled disagreements about relevance, validity, or clinical usefulness by re-reviewing the article.
We recorded the total number of drug therapy articles for each journal, then tallied the number of POEMs and the total number of calculations of RRR, ARR, NNT, and NNH in articles that met POEMs criteria. We used these numbers to determine the percentage of POEMs and POEMs with clinically useful information for each journal.
Results
We identified a total of 995 articles in the 7 journals during the 6-month study period. Of these, 24 (2.4%) were classified as drug therapy POEMs and 6 (0.6%) were POEMs with clinically useful information.
The journals that published the most POEMs were Arch Intern Med, N Engl J Med, BMJ, and JAMA. Arch Intern Med had the highest percentage (33%), followed by JAMA (20%), and BMJ (17%) (TABLE 2).
When we analyzed the POEMs for clinical usefulness based on whether they provided calculated ARR, NNT, or NNH, only 3 journals published POEMs with clinically useful information: JAMA published the most (15%), followed by BMJ (9%) and Obstet Gynecol (4%) (FIGURE).
FIGURE
Percentage of POEMs with clinically useful information*
AIM, Archives of Internal Medicine; BMJ, British Medical Journal; JAMA, Journal of the American Medical Association; NEJM, New England Journal of Medicine; Ob/gyn, Obstetrics and Gynecology; Peds, Pediatrics; POEMs, Patient-Oriented Evidence that Matters.
*Clinically useful information is defined as information that includes the absolute risk reduction, number needed to treat, or number needed to harm.
TABLE 2
Few drug therapy articles are POEMs
| Journal | Articles published in 6 months, n | Drug therapy articles, n | Drug therapy articles that are POEMs, n (%) |
|---|---|---|---|
| Arch Intern Med | 123 | 24 | 8 (33) |
| BMJ | 127 | 23 | 4 (17) |
| JAMA | 85 | 20 | 4 (20) |
| Lancet | 92 | 33 | 1 (3) |
| N Engl J Med | 128 | 39 | 4 (10) |
| 0bstet Gynecol | 115 | 24 | 2 (8) |
| Pediatrics | 325 | 50 | 1 (2) |
| Arch Intern Med, Archives of Internal Medicine; BMJ, British Medical Journal; JAMA, Journal of the American Medical Association; N Engl J Med, New England Journal of Medicine; Obstet Gynecol, Obstetrics and Gynecology; POEMs, Patient-Oriented Evidence that Matters. | |||
Discussion
Our study findings are consistent with the relevance data from a previous study published in 1999.8 After more than a decade, medical journals still are not publishing drug therapy POEMs.
A disturbing scarcity of useful information. The paucity of drug therapy POEMs with clinically useful information is alarming. Based on our data we estimate that a physician would have to read on average 36 drug therapy articles to find one clinically helpful drug therapy POEM. This finding suggests that the medical literature is not helping clinicians provide the best patient care or, when it does, the busy clinician is forced to spend what little time is available in calculations to determine what can actually affect practice in positive ways.
Study limitations. Our study has a number of limitations. The study settings in the articles we reviewed ultimately determined what information was important and could potentially change clinical practice. Some studies, for example, were performed in developing countries, where the therapy being tested was not commonly used and would alter practice. In the United States, however, the same treatment would not affect clinical practice because it was either common practice or standard of care.
We reviewed only 7 major medical journals. Our results cannot necessarily be extrapolated to other major journals, although they do suggest that the findings are not limited to a few publications. Moreover, we reviewed only 2 specialty journals. It is possible that other such journals are publishing more POEMs than we observed and providing more concrete numbers that specialists can use to quickly and easily adjust their practice patterns than general journals.
We did not analyze any family medicine journals for the following reasons: American Family Physician publishes only review articles; The Journal of Family Practice does not routinely publish original research; and Annals of Family Medicine (the research journal of the discipline) is less well established than the other journals we selected for this review, having been launched in 2003.
In addition, 6 months may not have been long enough to accurately calculate the percentage of POEMs or clinically useful information in the journals we reviewed. During the pilot study, in which we analyzed a full year of JAMA, only one POEM was published in the second 6 months, and it did not contain clinically useful information. Moreover, we reviewed most journals over 6 consecutive months rather than 6 randomly chosen months.
Finally, we reviewed only drug therapy articles. Future studies could examine surgical, diagnostic, or prognostic studies.
Toward more, and more useful, POEMs
Despite the scarcity of POEMs that provide clinically useful information in major medical journals, it is important that physicians continue to practice evidence-based medicine, sifting through the available information and even calculating ARR, NNT, and NNH themselves, which most busy clinicians do not have the time or inclination to do.
How can we improve the clinical usefulness of published data? One way is for journal editors to require that authors provide ARRs and NNTs or NNHs. Another is for authors to include these calculations on their own initiative. Either way, the goal is better clinical practice and optimal patient care.
CORRESPONDENCE Adrienne Z. Ables, PharmD, Edward Via College of Osteopathic Medicine, Carolinas Campus, 350 Howard Street, Spartanburg, SC 29303; aables@carolinas.vcom.edu
1. Shaughnessy AF, Slawson DC, Bennett JH. Becoming an information master: a guidebook to the medical information jungle. J Fam Pract. 1994;39:489-499.
2. Shaughnessy AF. Evaluating and understanding articles about treatment. Am Fam Physician. 2009;79:668-670.
3. Wyatt J. Uses and sources of medical knowledge. Lancet. 1991;338:1368-1372.
4. Gonzáles-Gonzáles AI, Dawes M, Sánchez-Mateos J, et al. Information needs and information-seeking behavior of primary care physicians. Ann Fam Med. 2007;5:345-352.
5. Smith R. A POEM a week for the BMJ. BMJ. 2002;325:983.-
6. Naylor CD, Chen E, Strauss B. Measured enthusiasm: Does the method of reporting trial results alter perceptions of therapeutic effectiveness? Ann Intern Med. 1992;117:916-921.
7. Bucher HC, Weinbacher M, Gyr K. Influence of method of reporting study results on decision of physicians to prescribe drugs to lower cholesterol concentration. BMJ. 1994;309:761-764.
8. Ebell MH, Barry HC, Slawson DC, et al. Finding POEMs in the Medical Literature. J Fam Pract 1999;48:350-355.
1. Shaughnessy AF, Slawson DC, Bennett JH. Becoming an information master: a guidebook to the medical information jungle. J Fam Pract. 1994;39:489-499.
2. Shaughnessy AF. Evaluating and understanding articles about treatment. Am Fam Physician. 2009;79:668-670.
3. Wyatt J. Uses and sources of medical knowledge. Lancet. 1991;338:1368-1372.
4. Gonzáles-Gonzáles AI, Dawes M, Sánchez-Mateos J, et al. Information needs and information-seeking behavior of primary care physicians. Ann Fam Med. 2007;5:345-352.
5. Smith R. A POEM a week for the BMJ. BMJ. 2002;325:983.-
6. Naylor CD, Chen E, Strauss B. Measured enthusiasm: Does the method of reporting trial results alter perceptions of therapeutic effectiveness? Ann Intern Med. 1992;117:916-921.
7. Bucher HC, Weinbacher M, Gyr K. Influence of method of reporting study results on decision of physicians to prescribe drugs to lower cholesterol concentration. BMJ. 1994;309:761-764.
8. Ebell MH, Barry HC, Slawson DC, et al. Finding POEMs in the Medical Literature. J Fam Pract 1999;48:350-355.
Preterm labor: Diagnostic and therapeutic options are not all alike
- Consider progesterone to reduce the risk of recurrent preterm delivery (A).
- Consider calcium channel blockers (CCBs) as initial therapy for preterm labor; though these are Class C agents in pregnancy, CCBs are at least as effective as other agents and cause no known fetal or neonatal side effects (A).
- Consider tocolytic therapy for preterm labor, to prolong pregnancy for 2 to 7 days and thereby permit administration of antenatal corticosteroids and transfer to a tertiary center, if needed (A). Maintenance and recurrent use of tocolytics has not proven beneficial and should be avoided (A).
Despite diagnostic and therapeutic advances in premature labor, the rate of preterm delivery has increased in the United States. Preterm delivery, defined as birth before 37 weeks gestation, occurs in about 12% of pregnancies,1 and it is the leading cause of mortality among non-anomalous fetuses.2 Among premature newborns who survive, 10% to 15% have significant handicaps.3 Long-term sequelae include visual or hearing impairment, chronic lung disease, developmental delay, and cerebral palsy.4 The annual cost of premature birth in the United States exceeds $5 billion, and the rate of preterm delivery has increased in recent years.5,6 Specific maternal attributes increase risk of preterm delivery, though these factors need not be present for premature delivery to occur.
Standard techniques of physical diagnosis may not accurately detect preterm labor, either missing the diagnosis or prompting unnecessary hospitalization and treatment. Select imaging studies can more reliably indicate a true need for intervention.
This article reviews the risk factors for preterm birth, the tests available to accurately diagnose preterm labor, and the medications available to prevent or postpone premature delivery.
Risk factors
Preterm delivery is categorized as spontaneous or indicated. Almost 75% of preterm births occur after spontaneous preterm labor or preterm premature rupture of the membranes (PPROM). The remaining 25% of early births are elective due to conditions potentially harmful to either the mother or the fetus.7
Maternal risk factors for spontaneous preterm births are listed in Table 1.7-13 The risk factors for preterm labor and PPROM are similar, with the exception that those whose membranes rupture prematurely are more likely to be indigent, to smoke cigarettes, and to have bleeding in the current pregnancy.
Not all preterm births carry the same risks. Early preterm birth—delivery before 32 weeks— is associated with clinical or subclinical infection, short cervical length, and the presence of fetal fibronectin in the cervicovaginal secretions. It tends to recur and leads to long-term fetal morbidity. Late preterm birth—delivery after 32 weeks but before 37 weeks—is associated with increased uterine contractions, tends to mimic normal labor, and is less likely to cause peripartum morbidity.7 Despite these known risk factors, women can experience preterm labor without exhibiting any of them.
TABLE 1
Maternal risk factors for spontaneous preterm birth
| Risk factor | Odds ratio |
|---|---|
| Bacterial vaginosis at <16 weeks | 7 |
| Periodontal infection | 4 |
| Prior preterm birth | 4 |
| Prepregnancy BMI <20 | 3 |
| Interpregnancy interval <6 mo | 2 |
| Bleeding in 2nd trimester | 2 |
| African-American race | 2 |
| Psychiatric disorder | 1.6 |
| Cigarette smoking | 1.5 |
| Gestational diabetes | 1.2 |
| Sources: Iams 20027 ; Leitich et al 20038 ; Jeffcoat et al 20019 ; Moutquin 200310 ; Smith et al 200311 ; Kelly et al 200212 ; Hedderson et al 2003.13 | |
Increasing the accuracy of diagnosis
Uterine contractions before 37 weeks and concomitant change in cervical dilation or effacement as detected by digital examination comprise the standard definition of preterm labor.
Caveats with clinical evaluation
The problem with the definition above is that women may not perceive contractions, and that contractions are at times difficult to differentiate from benign Braxton-Hicks contractions of normal pregnancy. Moreover, digital assessment of the cervix lacks reproducibility when the dilation is <3 cm or effacement is < 80%.7 Clinical evaluation alone can easily suggest prematurity when it is absent and miss it when it is present.7
Imaging and lab evaluation more telling
The ability to diagnose preterm labor improves with the use of transvaginal cervical sonography or measurement of fetal fibronectin.
Lesser cervical length means greater risk. Likelihood of preterm delivery is inversely proportional to cervical length measured at 18 to 28 weeks. A length of ≤2.5 cm is the threshold for abnormal condition (level of evidence [LOE]: 2).6
Fibronectin detected late may mean delivery early. Fibronectin, an extracellular glycoprotein described as the glue that attaches the fetal membrane to the underlying uterine decidua, is normally absent in cervicovaginal secretions between weeks 22 and 37 of pregnancy. Its presence (50 ng/mL) between 22 to 24 weeks is a predictor of spontaneous preterm birth; its absence makes premature delivery unlikely (LOE: 2).
Imaging and test results justify management decisions. The predictive accuracies of abnormal cervical length and of the presence of fibronectin are depicted in the Figure .7 The major benefit of these two tests is when they are normal (cervical length >2.5 cm or negative fetal fibronectin), unnecessary intervention is avoided.14 If, for example, a patient is having contractions and the cervical length is greater than 2.5 cm, or fetal fibronectin is absent, tocolytics can be discontinued and hospitalization avoided.
FIGURE
Value of transvaginal cervical imaging and fetal fibronectin measurement in predicting spontaneous preterm birth
Preventing preterm labor
Progestational agents
Many authors have advocated the use of progestational agents to inhibit premature labor. Progesterone’s presumed mechanisms are inhibition of the oxytocin effect of prostaglandin F2a and stimulation of α-adrenergic receptors, thereby increasing the α-adrenergic tocolytic response.15,16 Natural progesterone appears to be free of any untoward teratogenic, metabolic, or hemodynamic effects.17
Studies favoring progesterone. Two double-blind, placebo-controlled studies assessed the use of 17 α-hydroxyprogesterone caproate in preventing premature labor in high-risk populations. These patients had histories of preterm deliveries or spontaneous abortions.
Weekly intramuscular (IM) injections of 250 mg were started at 12 to 16 weeks gestation and given until 37 weeks or delivery, whichever came first. In both studies, the rate of premature deliveries was significantly lower in the treated group than the control group (number needed to treat [NNT]=2.4 and 4.6). In addition, neither mothers nor infants experienced adverse effects attributable to 17 α-hydroxyprogesterone caproate (LOE: 2).18,19 However, these studies were small—43 and 79 patients.
A large double-blind, placebo-controlled, randomized study established the effectiveness of 17 α-hydroxyprogesterone caproate in preventing preterm delivery.23 Four hundred fifty-nine pregnant women with a history of preterm delivery were randomized to receive weekly injections of 17 α-hydroxyprogesterone caproate 250 mg or placebo beginning at 16 to 20 weeks gestation and continuing to 36 weeks. Significantly fewer women in the treated group than the control group gave birth before 37 weeks, 36.3% v 54.9%, respectively (NNT=6; LOE: 1). Perhaps more importantly, treatment resulted in significant reductions in birth weight <2500 g (NNT=7), necrotizing enterocolitis (NNT=38), need for supplemental oxygen (NNT=11), and intraventricular hemorrhage (NNT=26). Swelling, bruising, or rash at the injection site were the most common adverse effects of 17 α-hydroxyprogesterone caproate administration.
Vaginal progesterone suppositories have also been shown to decrease the rate of preterm birth in patients at increased risk.17 da Fonseca et al noted that among 142 women who had 1 prior preterm birth, prophylactic cerclage, or uterine malformation, daily use of a 100-mg vaginal progesterone suppository compared with placebo significantly decreased the likelihood of delivery prior to 37 weeks, 14% v 28% (NNT=7; LOE: 1). Adverse effects of progesterone suppositories were not mentioned.
Studies lacking clear benefit. Another investigator enrolled 168 active-duty military women into a randomized double-blind study that evaluated weekly IM injections of 1000 mg 17 α-hydroxyprogesterone caproate or placebo beginning between 16 and 20 weeks gestation.20 The study population was chosen based on a report that active-duty pregnant military personnel had an increased number of pregnancy complications including low birth-weight infants and increased premature delivery.21 In contrast to the previous studies, no significant difference in premature labor or perinatal mortality was seen between the 2 groups (LOE: 2). Perhaps although active-duty personnel are at a higher risk for preterm labor, the risk is not as high as it is with previous preterm birth, prior spontaneous abortion, and so forth.
An additional small study of 77 women with twin pregnancies investigated the use of 17 α-hydroxyprogesterone caproate to prevent preterm delivery.22 Patients were randomized to receive weekly injections of 17 α-hydroxyprogesterone caproate 250 mg or placebo from the 28th gestational week until the 37th week or delivery. The mean duration of pregnancy did not differ between the two groups of women, nor did perinatal mortality (LOE: 2).
Evidence generally supports use of progesterone. Based on available data, consider administering progesterone to women at high risk for preterm delivery to prevent recurrent preterm birth (SOR: A). These data are summarized in Table 2 .
Hydroxyprogesterone caproate in oil is commercially available in the United States in 125 mg/mL and 250 mg/mL strengths. It is not, however, approved by the Food and Drug Administration (FDA) for the prevention of preterm labor.
Progesterone suppositories are also not FDA approved, and they are commercially unavailable in the US. Therefore, they have to be extemporaneously compounded.
TABLE 2
Evidence generally supports progesterone in prevention of preterm labor
| Study | Treatment | Comment | NNT | LOE |
|---|---|---|---|---|
| Johnson18 | Weekly IM 250 mg injections of 17 α-hydroxyprogesterone caproate | Decreased preterm delivery | 2.4 | 2 |
| Yemeni19 | Weekly IM 250 mg injections of 17 α-hydroxyprogesterone caproate | Decreased preterm delivery | 4.6 | 2 |
| Hauth20 | Weekly IM 1000 mg injections of 17 α-hydroxyprogesterone caproate | Did not decrease preterm delivery | N/A | 2 |
| Hartikainen-Sorri22 | Weekly IM 250 mg injections of 17 α-hydroxyprogesterone caproate | Did not decrease preterm delivery | N/A | 2 |
| Meis23 | Weekly IM 250 mg injections of 17 α-hydroxyprogesterone caproate | Decreased preterm Delivery | 6 | 1 |
| da Fonseca17 | Daily 100 mg progesterone vaginal suppositories | Decreased preterm delivery | 7 | 1 |
| NNT, number needed to treat; LOE, level of evidence; IM, intramuscular; N/A, not applicable. | ||||
Treating bacterial vaginosis may not prevent preterm labor
Bacterial vaginosis (BV) during pregnancy has been associated with preterm birth, and antibiotics have been thought perhaps to reduce this risk. A systematic review of 10 trials including 4249 pregnant women with BV showed that, although antibiotics eradicated BV, they did not significantly reduce the risk of birth before 32, 34, or 37 weeks (LOE: 1).24 This was true even for women with a history of preterm delivery. However, antibiotics did decrease the risk of PPROM. The authors concluded that evidence does not support screening all pregnant women for asymptomatic BV to prevent preterm birth.
Treating asymptomatic bacteriuria may prevent preterm labor
Asymptomatic bacteriuria in pregnant women has also been associated with preterm birth. One review of 14 trials comparing antibiotic treatment with placebo in this patient population—though not of high quality—demonstrated decreased incidences of pyelonephritis and preterm birth or low birth weight (LOE: 1).25
Treating preterm labor
The primary goal of using tocolytics in preterm labor is to prolong pregnancy for 2 to 7 days, permitting administration of corticosteroids and transfer to a tertiary care center when necessary. Recommendations for the treatment of preterm labor are summarized in Table 3 .
TABLE 3
Evaluation of proposed treatments for preterm labor
| Treatment | Comment | NNT | LOE | References |
|---|---|---|---|---|
| Calcium channel blockers | Reduce preterm delivery, decrease neonatal morbidity, well-tolerated | 11 | 1 | 26, 27 |
| Beta-agonists | Prolong pregnancy but no beneficial effect on neonatal morbidity; maternal side effects often lead to discontinuation | N/A | 1 | 33, 34 |
| Magnesium sulfate | Does not prolong pregnancy; maternal side effects often lead to discontinuation | N/A | 1 | 33, 40 |
| Prostaglandin inhibitors | Prolong pregnancy but no beneficial effect on neonatal morbidity; increase risk of postpartum hemorrhage | N/A | 1 | 33 |
| 2 | 43 | |||
| Antibiotics | No evidence to support use | N/A | 1 | 44 |
| Corticosteroids | Strongly recommended; decrease neonatal morbidity, well-tolerated | U | 1 | 46 |
| NNT, number needed to treat; LOE, level of evidence; N/A, not applicable; U, unavailable data. | ||||
Calcium channel blockers safe, effective
Calcium channel blockers (CCBs) are nonspecific smooth muscle relaxants. They prevent the influx of extracellular calcium ions into the myometrial cell, thereby exerting their tocolytic effect. Nifedipine is the most widely studied CCB in the management of preterm labor.
A systematic review of 12 randomized controlled trials including 1029 women demonstrated that CCBs significantly reduced the rate of preterm delivery within 7 days of the start of treatment and before 34 weeks gestation (LOE: 1).26 Compared with betamimetics, CCBs caused fewer maternal adverse effects, and decreased the frequency of neonatal respiratory distress syndrome, necrotizing enterocolitis, intraventricular hemorrhage, and jaundice. The authors concluded that CCBs are preferable to betamimetics for tocolysis.
Calcium channel blockers have also been compared with magnesium. Eighty women at 20 to 34 weeks gestation with documented cervical change were randomized to receive nifedipine or magnesium.27 No difference in tocolytic efficacy was observed between the treatments, but there were significantly fewer maternal side effects among the nifedipine-treated patients (LOE: 2).
CCBs appear not to adversely affect the human fetus.28,29 There was no significant increase in congenital anomalies among 586 mothers who had been exposed to CCBs compared with 907 controls: 2.6% vs 2.4%, respectively (LOE: 2).30 CCBs are effective at reducing the rate of preterm delivery, decreasing neonatal morbidity and mortality, and are well tolerated by both mother and baby. Although some other tocolytics (such as beta-agonists and prostaglandin inhibitors) prolong pregnancy, they have not demonstrated a beneficial effect on neonatal morbidity.
Dosing recommendations for nifedipine are a 30 mg loading dose, followed by 10 to 20 mg every 4 to 6 hours.31 Maternal side effects may include flushing, headache, nausea, dizziness, and transient hypotension. Neonatal side effects have not been documented. Nifedipine should not be used in conjunction with magnesium sulfate because the combination has resulted in cardiac collapse (LOE: 3).32
Beta-agonists marginally useful
Beta-agonists increase cyclic AMP, thereby causing smooth muscle relaxation. Betamimetics are widely used as tocolytic agents. Although they have been shown to prolong pregnancy by 24 to 48 hours, they have not decreased adverse perinatal or neonatal outcomes (LOE: 1).33,34 In addition, they have been associated with maternal side effects of palpitations, arrhythmias, nausea, tremor, chorioamnionitis, hyperglycemia, hypokalemia, and pulmonary edema. The risk of pulmonary edema increases if beta-agonists are used with magnesium sulfate.33,35 Fetal and neonatal side effects may include tachycardia, hyperinsulinemia, and hyperglycemia.14,35
Terbutaline is the preferred beta-agonist for preterm labor due to low cost and multiple dose forms. It may be administered subcutaneously, 0.25 mg every 30 minutes up to 1 mg in 4 hours, or by intravenous infusion, 2.5 to 10 μg per minute up to an effective maximum dose of no more than 30 μg per minute. Once stabilized, the patient is often maintained on oral terbutaline, 2.5 to 5 μg by mouth every 4 to 6 hours until term. This practice, however, has not been shown to significantly prolong pregnancy (LOE: 1).36
The major benefit of terbutaline may be in the management of preterm uterine contractions without cervical changes. Among patients with premature contractions at 20 to 34 weeks, who were randomized to hydration or observation or a single subcutaneous dose of 0.25 mg of terbutaline, the shortest length of stay in triage was for the women who received the terbutaline (LOE: 1).37
Magnesium sulfate: No good rationale for use
Magnesium sulfate in the management of preterm labor is controversial. Although widely used in North America, it is generally avoided in Europe, Australia, and the United Kingdom38,39 due to the absence of clear evidence showing efficacy in the face of its adverse effect profile. Data from systematic reviews indicate it does not delay or prevent preterm birth (LOE: 1).33,40 In addition, maternal side effects may require discontinuation of treatment. Side effects include flushing, nausea, headache, ileus, and hypocalcemia. Pulmonary edema is a serious maternal side effect and is increased when magnesium is co-administered with other tocolytic agents. Cardiac arrest is rare.14,33 Not only does evidence suggest magnesium sulfate does not delay or prevent preterm birth, but one study suggests its use may increase infant mortality (LOE: 2).41
In one trial of 1062 women, magnesium sulfate or placebo was given at 30 weeks’ gestation. Two years following birth, children of mothers who received magnesium sulfate exhibited less gross motor dysfunction than those born to mothers who received placebo, 18% vs 34% respectively (LOE: 1).42 However, the rate of pediatric mortality and cerebral palsy, the primary endpoints of the study, did not differ between the groups. Further studies are necessary before magnesium sulfate can be recommended for neuroprotection.
Prostaglandin inhibitors may be preferred for hydramnios
Indomethacin is the prostaglandin synthetase inhibitor most studied in preterm labor, though the numbers of patients in these studies was generally small.33 Indomethacin reduced the rate of preterm delivery within 7 days of treatment and before 37 weeks gestation. However, it did not decrease the rate of neonatal morbidity. Importantly, indomethacin was associated with an increased risk of postpartum hemorrhage (LOE: 1).33 A retrospective review demonstrated an increase in incidence and severity of postnatal patent ductus arteriosus in neonates whose mothers were treated with indomethacin (LOE: 2).43 Most prospective studies, however, have not found this complication. Prostaglandin inhibitors may be the preferred choice of tocolytics if hydramnios is suspected in conjunction with preterm labor.
Antibiotics: one indication only
It is hypothesized that women who experience preterm labor have infections of the upper genital tract, and that infection or inflammation leads to contractions. A meta-analysis of 11 randomized trials with 7428 women in preterm labor and intact membranes demonstrated that, although prophylactic antibiotics decreased the incidence of maternal infection, there was no benefit in neonatal outcomes (LOE: 1).44 Although it did not reach statistical significance, there was a trend towards an increase in neonatal deaths in the antibiotic group, raising concerns about their use. Therefore, administration of prophylactic antibiotics in this patient population is not recommended. Conversely, antibiotic prophylaxis is recommended for all women colonized with group B streptococcus, unless a cesarean delivery is planned, to prevent perinatal disease.45
Corticosteroids beneficial for preterm infants
Antenatal corticosteroids reduce mortality, incidence and severity of respiratory distress syndrome, and intraventricular hemorrhage in preterm infants (LOE: 1).46 Women at risk for preterm delivery between 24 and 34 weeks of gestation should be given betamethasone 12 mg IM, two doses 24 hours apart, or dexamethasone 6 mg IM, two doses 12 hours apart. There are no significant maternal or neonatal adverse effects with these regimens. Administration of tocolytic drugs may be necessary to prolong gestation and provide time for steroids to act.
Corresponding author
Adrienne Z. Ables, PharmD, Spartanburg Family Medicine Residency Program, 853 N Church St Suite 510, Spartanburg, SC 29303. E-mail: azables@srhs.com.
1. Ventura SJ, Martin JA, Curtin SC, Menacker F, Hamilton BE. Births: final data for 1999. Natl Vital Stat Rep 2001;49:1-100.
2. Rush RW, Davey DA, Segal ML. The effect of preterm delivery on perinatal mortality. Br J Obstet Gynaecol 1978;85:806-811.
3. Ehrenhaft PM, Wagner JL, Herdman RC. Changing prognosis for very low birth weight infants. Obstet Gynecol 1989;74:528.-
4. Hack M, Taylor HG, Klein N, Eiben R, Schatschneider C, Mercuri-Munich N. School-age outcomes in children with birth weight under 750 g. N Engl J Med 1994;331:753-759.
5. Rogowski JA. The economics of preterm delivery. Prenat Neonat Med 1998;3:16-20.
6. Iams JD, Goldenberg RL, Meis PJ, et al. The length of the cervix and the risk of spontaneous premature delivery. N Engl J Med 1996;334:567-572.
7. Iams JD. Preterm birth. In: Gabbe SG, Niebyl JR, Simpson JL, eds. Obstetrics: Normal and Problem Pregnancies 4th ed. New York, NY: Churchill Livingstone; 2002.
8. Leitich H, Bodner-Adler B, Brunbauer M, Egarter C, Husslei P. Bacterial vaginosis as a risk factor for preterm delivery: A meta-analysis. Am J Obstet Gynecol 2003;189:139-147.
9. Jeffcoat MK, Geurs NC, Reddy MS, Cliver SP, Goldenberg RL, Hauth JC. Periodontal infection and preterm birth: Results of prospective study. J Am Dent Assoc. 2001;132:875-880.
10. Moutquin JM. Socio-economic and psychosocial factors in the management and prevention of preterm labour. Br J Obstet Gynaecol 2003;110:56-60.
11. Smith GC, Pell JP, Dobbie R. Interpregnancy interval and risk of preterm birth and neonatal death: Retrospective cohort study. BMJ 2003;327:313-319.
12. Kelly RH, Russo J, Holt VL, Danielsen BH, Zatzick DF, Walker E, Kato W. Psychiatric and substance use disorders as risk factors for low birth weight and preterm delivery. Obstet Gynecol 2002;100:297-304.
13. Hedderson MM, Ferrara A, Sacks DA. Gestational diabetes mellitus and lesser degrees of pregnancy hyperglycemia: Association with increased risk of spontaneous preterm birth. Obstet Gynecol 2003;102:850-856.
14. American College of Obstetricians and Gynecologists Management of preterm labor. ACOG practical bulletin no. 43. Washington, DC: ACOG, 2003.
15. Lockwood CJ, Senyei AE, Dischie MR, et al. Fetal fibronectin in cervical and vaginal secretions as a predictor of preterm delivery. N Engl J Med 1991;325:669-674.
16. Fuchs AR, Fuchs R. Endocrinology of human parturition: a review. Br J Obstet Gynaecol 1984;91:948-67.
17. da Fonseca EB, Bittar R, Carvalho MHB, et al. Prophylactic administration of progesterone by vaginal suppository to reduce the incidence of spontaneous preterm birth in women at increased risk: A randomized placebo-controlled double blind study. Am J Obstet Gynecol 2003;188:419-424.
18. Johnson JW, Austin KL, Jones GS, et al. Efficacy of 17 a-hydroxyprogesterone caproate in the prevention of premature labor. N Engl J Med 1975;293:675-689.
19. Yemeni M, Borenstein R, Dreazen E, et al. Prevention of premature labor by 17 a-hydroxyprogesterone caproate. Am J Obstet Gynecol 1985;151:574-577.
20. Hauth JC, Gilstrap LC, Brekken AL, et al. The effect of 17 a-hydroxyprogesterone caproate on pregnancy outcome in an active-duty military population. Am J Obstet Gynecol 1983;146:187-190.
21. Fox ME, Harris RE, Brekken AL. The active duty military pregnancy: a new high-risk category. Am J Obstet Gynecol 1977;129:705-707.
22. Hartikainen-Sorri AL, Kauppila A, Tuimala R. Inefficacy of 17 a-hydroxyprogesterone caproate in the prevention of prematurity in twin pregnancy. Obstet Gynecol 1980;56:692-695.
23. Meis PJ. 17 Alpha hydroxyprogesterone caproate prevents recurrent preterm birth. N Engl J Med 2003;348:2379-2385.
24. McDonald H, Brocklehurst P, Parsons J, Vigneswaran R. Antibiotics for treating bacterial vaginosis in pregnancy (Cochrane Review).In: The Cochrane Library, Issue 2, 2004. Chichester, UK: John Wiley & Sons, Ltd.
25. Smaill F. Antibiotics for asymptomatic bacteriuria in pregnancy (Cochrane Review).In: The Cochrane Library, Issue 2, 2004. Chichester, UK: John Wiley & Sons, Ltd.
26. King JF, Flenady VJ, Papatsonis DNM, et al. Calcium channel blockers for inhibiting preterm labour (Cochrane Review).In: The Cochrane Library, Issue 3, 2003. Oxford: Update Software.
27. Glock, JL, Morales WJ. Efficacy and safety of nifedipine versus magnesium sulfate in the management of preterm labor: a randomized study. Am J Obstet Gynecol 1993;169:960-964.
28. Sorensen HT, Steffensen FH, Olesen C, et al. Pregnancy outcome in women exposed to calcium channel blockers. Reprod Toxicol 1998;12:383-384.
29. Magee LA, Schick B, Donnefeld AE, et al. The safety of calcium channel blockers in human pregnancy: a prospective, multicenter cohort study. Am J Obstet Gynecol 1996;174:823-828.
30. Sorensen HT, Czeizel AE, Rockenbauer M, et al. The risk of limb deficiencies and other congenital abnormalities in children exposed in utero to calcium channel blockers. Acta Obstet Gynecol Scand 2001;80:397-401.
31. Hearne AE, Nagey DA. Therapeutic agents in preterm labor: tocolytic agents. Clin Obstet Gynecol 2000;43:787-801.
32. Ben-Ami M, Giladi Y, Shalev E. The combination of magnesium sulphate and nifedipine: a cause of neuromuscular blockade. Br J Obstet Gynaecol 1994;101:262-263.
33. Gyetvai K, Hannah M E, Hodnett E D, et al. Tocolytics for preterm labor: a systematic review. Obstet Gynecol 1999;94:869-877.
34. King JF, Grant A, Keirse MJNC, et al. Beta-mimetics in preterm labor: an overview of the randomized controlled trials. Br J Obstet Gynaecol 1988;95:211-222.
35. Jeyabalan A, Caritis SN. Pharmacologic inhibition of preterm labor. Clin Obstset Gynecol 2002;45:99-113.
36. Macones GA, Berlin M, Berlin J. Efficacy of oral beta-ago-nist maintenance therapy in preterm labor: a meta-analysis. Obstet Gynecol 1995;85:313-317.
37. Guinn DA, Goepfert AR, Owen J, Brumfield C, Hauth JC. Management options in women with preterm uterine contractions: A randomized clinical trial. Am J Obstet Gynecol 1997;177:814-818.
38. Norwitz ER, Robinson JN, Challis JRG. The control of labor. N Engl J Med 1999;341:660-666.
39. Bennett P, Edwards D. Use of magnesium sulphate in obstetrics. Lancet 1997;350:1491.-
40. Crowther CA, Hiller JE, Doyle LW. Magnesium sulphate for preventing preterm birth in threatened preterm labour (Cochrane Review).In: The Cochrane Library, Issue 3, 2003. Oxford: Update Software.
41. Mittendorf R, Covert R, Boman J, et al. Is tocolytic magnesium sulfate associated with increased total paediatric mortality? Lancet 1997;350:1517-1518.
42. Crowther CA, Hiller JE, Haslam RR. Effect of magnesium sulfate given for neuroprotection before preterm birth. A randomized controlled trial. JAMA 2003;290:2669-2676.
43. Hammerman C, Glaser J, Kaplan M, et al. Indomethacin tocolysis increases postnatal patent ductus arteriosus severity. Pediatrics 1998;102:E56.-
44. King J, Flenady V. Prophylactic antibiotics for inhibiting preterm labour with intact membranes (Cochrane Review).In: The Cochrane Library, Issue 3, 2003. Oxford: Update Software.
45. Schrag S, Gorwitz R, Fultz-Butts K, Schuchat A. Prevention of perinatal group B streptococcal disease. Revised guidelines from CDC. MMWR Recomm Rep 2002;51(RR-11):1-22.Available at:www.cdc.gov/mmwr/PDF/RR/RR5111.pdf. Accessed January 21, 2005.
46. Crowley P. Prophylactic corticosteroids for preterm birth (Cochrane Review).In: The Cochrane Library, Issue 4, 2003. Oxford: Update Software.
- Consider progesterone to reduce the risk of recurrent preterm delivery (A).
- Consider calcium channel blockers (CCBs) as initial therapy for preterm labor; though these are Class C agents in pregnancy, CCBs are at least as effective as other agents and cause no known fetal or neonatal side effects (A).
- Consider tocolytic therapy for preterm labor, to prolong pregnancy for 2 to 7 days and thereby permit administration of antenatal corticosteroids and transfer to a tertiary center, if needed (A). Maintenance and recurrent use of tocolytics has not proven beneficial and should be avoided (A).
Despite diagnostic and therapeutic advances in premature labor, the rate of preterm delivery has increased in the United States. Preterm delivery, defined as birth before 37 weeks gestation, occurs in about 12% of pregnancies,1 and it is the leading cause of mortality among non-anomalous fetuses.2 Among premature newborns who survive, 10% to 15% have significant handicaps.3 Long-term sequelae include visual or hearing impairment, chronic lung disease, developmental delay, and cerebral palsy.4 The annual cost of premature birth in the United States exceeds $5 billion, and the rate of preterm delivery has increased in recent years.5,6 Specific maternal attributes increase risk of preterm delivery, though these factors need not be present for premature delivery to occur.
Standard techniques of physical diagnosis may not accurately detect preterm labor, either missing the diagnosis or prompting unnecessary hospitalization and treatment. Select imaging studies can more reliably indicate a true need for intervention.
This article reviews the risk factors for preterm birth, the tests available to accurately diagnose preterm labor, and the medications available to prevent or postpone premature delivery.
Risk factors
Preterm delivery is categorized as spontaneous or indicated. Almost 75% of preterm births occur after spontaneous preterm labor or preterm premature rupture of the membranes (PPROM). The remaining 25% of early births are elective due to conditions potentially harmful to either the mother or the fetus.7
Maternal risk factors for spontaneous preterm births are listed in Table 1.7-13 The risk factors for preterm labor and PPROM are similar, with the exception that those whose membranes rupture prematurely are more likely to be indigent, to smoke cigarettes, and to have bleeding in the current pregnancy.
Not all preterm births carry the same risks. Early preterm birth—delivery before 32 weeks— is associated with clinical or subclinical infection, short cervical length, and the presence of fetal fibronectin in the cervicovaginal secretions. It tends to recur and leads to long-term fetal morbidity. Late preterm birth—delivery after 32 weeks but before 37 weeks—is associated with increased uterine contractions, tends to mimic normal labor, and is less likely to cause peripartum morbidity.7 Despite these known risk factors, women can experience preterm labor without exhibiting any of them.
TABLE 1
Maternal risk factors for spontaneous preterm birth
| Risk factor | Odds ratio |
|---|---|
| Bacterial vaginosis at <16 weeks | 7 |
| Periodontal infection | 4 |
| Prior preterm birth | 4 |
| Prepregnancy BMI <20 | 3 |
| Interpregnancy interval <6 mo | 2 |
| Bleeding in 2nd trimester | 2 |
| African-American race | 2 |
| Psychiatric disorder | 1.6 |
| Cigarette smoking | 1.5 |
| Gestational diabetes | 1.2 |
| Sources: Iams 20027 ; Leitich et al 20038 ; Jeffcoat et al 20019 ; Moutquin 200310 ; Smith et al 200311 ; Kelly et al 200212 ; Hedderson et al 2003.13 | |
Increasing the accuracy of diagnosis
Uterine contractions before 37 weeks and concomitant change in cervical dilation or effacement as detected by digital examination comprise the standard definition of preterm labor.
Caveats with clinical evaluation
The problem with the definition above is that women may not perceive contractions, and that contractions are at times difficult to differentiate from benign Braxton-Hicks contractions of normal pregnancy. Moreover, digital assessment of the cervix lacks reproducibility when the dilation is <3 cm or effacement is < 80%.7 Clinical evaluation alone can easily suggest prematurity when it is absent and miss it when it is present.7
Imaging and lab evaluation more telling
The ability to diagnose preterm labor improves with the use of transvaginal cervical sonography or measurement of fetal fibronectin.
Lesser cervical length means greater risk. Likelihood of preterm delivery is inversely proportional to cervical length measured at 18 to 28 weeks. A length of ≤2.5 cm is the threshold for abnormal condition (level of evidence [LOE]: 2).6
Fibronectin detected late may mean delivery early. Fibronectin, an extracellular glycoprotein described as the glue that attaches the fetal membrane to the underlying uterine decidua, is normally absent in cervicovaginal secretions between weeks 22 and 37 of pregnancy. Its presence (50 ng/mL) between 22 to 24 weeks is a predictor of spontaneous preterm birth; its absence makes premature delivery unlikely (LOE: 2).
Imaging and test results justify management decisions. The predictive accuracies of abnormal cervical length and of the presence of fibronectin are depicted in the Figure .7 The major benefit of these two tests is when they are normal (cervical length >2.5 cm or negative fetal fibronectin), unnecessary intervention is avoided.14 If, for example, a patient is having contractions and the cervical length is greater than 2.5 cm, or fetal fibronectin is absent, tocolytics can be discontinued and hospitalization avoided.
FIGURE
Value of transvaginal cervical imaging and fetal fibronectin measurement in predicting spontaneous preterm birth
Preventing preterm labor
Progestational agents
Many authors have advocated the use of progestational agents to inhibit premature labor. Progesterone’s presumed mechanisms are inhibition of the oxytocin effect of prostaglandin F2a and stimulation of α-adrenergic receptors, thereby increasing the α-adrenergic tocolytic response.15,16 Natural progesterone appears to be free of any untoward teratogenic, metabolic, or hemodynamic effects.17
Studies favoring progesterone. Two double-blind, placebo-controlled studies assessed the use of 17 α-hydroxyprogesterone caproate in preventing premature labor in high-risk populations. These patients had histories of preterm deliveries or spontaneous abortions.
Weekly intramuscular (IM) injections of 250 mg were started at 12 to 16 weeks gestation and given until 37 weeks or delivery, whichever came first. In both studies, the rate of premature deliveries was significantly lower in the treated group than the control group (number needed to treat [NNT]=2.4 and 4.6). In addition, neither mothers nor infants experienced adverse effects attributable to 17 α-hydroxyprogesterone caproate (LOE: 2).18,19 However, these studies were small—43 and 79 patients.
A large double-blind, placebo-controlled, randomized study established the effectiveness of 17 α-hydroxyprogesterone caproate in preventing preterm delivery.23 Four hundred fifty-nine pregnant women with a history of preterm delivery were randomized to receive weekly injections of 17 α-hydroxyprogesterone caproate 250 mg or placebo beginning at 16 to 20 weeks gestation and continuing to 36 weeks. Significantly fewer women in the treated group than the control group gave birth before 37 weeks, 36.3% v 54.9%, respectively (NNT=6; LOE: 1). Perhaps more importantly, treatment resulted in significant reductions in birth weight <2500 g (NNT=7), necrotizing enterocolitis (NNT=38), need for supplemental oxygen (NNT=11), and intraventricular hemorrhage (NNT=26). Swelling, bruising, or rash at the injection site were the most common adverse effects of 17 α-hydroxyprogesterone caproate administration.
Vaginal progesterone suppositories have also been shown to decrease the rate of preterm birth in patients at increased risk.17 da Fonseca et al noted that among 142 women who had 1 prior preterm birth, prophylactic cerclage, or uterine malformation, daily use of a 100-mg vaginal progesterone suppository compared with placebo significantly decreased the likelihood of delivery prior to 37 weeks, 14% v 28% (NNT=7; LOE: 1). Adverse effects of progesterone suppositories were not mentioned.
Studies lacking clear benefit. Another investigator enrolled 168 active-duty military women into a randomized double-blind study that evaluated weekly IM injections of 1000 mg 17 α-hydroxyprogesterone caproate or placebo beginning between 16 and 20 weeks gestation.20 The study population was chosen based on a report that active-duty pregnant military personnel had an increased number of pregnancy complications including low birth-weight infants and increased premature delivery.21 In contrast to the previous studies, no significant difference in premature labor or perinatal mortality was seen between the 2 groups (LOE: 2). Perhaps although active-duty personnel are at a higher risk for preterm labor, the risk is not as high as it is with previous preterm birth, prior spontaneous abortion, and so forth.
An additional small study of 77 women with twin pregnancies investigated the use of 17 α-hydroxyprogesterone caproate to prevent preterm delivery.22 Patients were randomized to receive weekly injections of 17 α-hydroxyprogesterone caproate 250 mg or placebo from the 28th gestational week until the 37th week or delivery. The mean duration of pregnancy did not differ between the two groups of women, nor did perinatal mortality (LOE: 2).
Evidence generally supports use of progesterone. Based on available data, consider administering progesterone to women at high risk for preterm delivery to prevent recurrent preterm birth (SOR: A). These data are summarized in Table 2 .
Hydroxyprogesterone caproate in oil is commercially available in the United States in 125 mg/mL and 250 mg/mL strengths. It is not, however, approved by the Food and Drug Administration (FDA) for the prevention of preterm labor.
Progesterone suppositories are also not FDA approved, and they are commercially unavailable in the US. Therefore, they have to be extemporaneously compounded.
TABLE 2
Evidence generally supports progesterone in prevention of preterm labor
| Study | Treatment | Comment | NNT | LOE |
|---|---|---|---|---|
| Johnson18 | Weekly IM 250 mg injections of 17 α-hydroxyprogesterone caproate | Decreased preterm delivery | 2.4 | 2 |
| Yemeni19 | Weekly IM 250 mg injections of 17 α-hydroxyprogesterone caproate | Decreased preterm delivery | 4.6 | 2 |
| Hauth20 | Weekly IM 1000 mg injections of 17 α-hydroxyprogesterone caproate | Did not decrease preterm delivery | N/A | 2 |
| Hartikainen-Sorri22 | Weekly IM 250 mg injections of 17 α-hydroxyprogesterone caproate | Did not decrease preterm delivery | N/A | 2 |
| Meis23 | Weekly IM 250 mg injections of 17 α-hydroxyprogesterone caproate | Decreased preterm Delivery | 6 | 1 |
| da Fonseca17 | Daily 100 mg progesterone vaginal suppositories | Decreased preterm delivery | 7 | 1 |
| NNT, number needed to treat; LOE, level of evidence; IM, intramuscular; N/A, not applicable. | ||||
Treating bacterial vaginosis may not prevent preterm labor
Bacterial vaginosis (BV) during pregnancy has been associated with preterm birth, and antibiotics have been thought perhaps to reduce this risk. A systematic review of 10 trials including 4249 pregnant women with BV showed that, although antibiotics eradicated BV, they did not significantly reduce the risk of birth before 32, 34, or 37 weeks (LOE: 1).24 This was true even for women with a history of preterm delivery. However, antibiotics did decrease the risk of PPROM. The authors concluded that evidence does not support screening all pregnant women for asymptomatic BV to prevent preterm birth.
Treating asymptomatic bacteriuria may prevent preterm labor
Asymptomatic bacteriuria in pregnant women has also been associated with preterm birth. One review of 14 trials comparing antibiotic treatment with placebo in this patient population—though not of high quality—demonstrated decreased incidences of pyelonephritis and preterm birth or low birth weight (LOE: 1).25
Treating preterm labor
The primary goal of using tocolytics in preterm labor is to prolong pregnancy for 2 to 7 days, permitting administration of corticosteroids and transfer to a tertiary care center when necessary. Recommendations for the treatment of preterm labor are summarized in Table 3 .
TABLE 3
Evaluation of proposed treatments for preterm labor
| Treatment | Comment | NNT | LOE | References |
|---|---|---|---|---|
| Calcium channel blockers | Reduce preterm delivery, decrease neonatal morbidity, well-tolerated | 11 | 1 | 26, 27 |
| Beta-agonists | Prolong pregnancy but no beneficial effect on neonatal morbidity; maternal side effects often lead to discontinuation | N/A | 1 | 33, 34 |
| Magnesium sulfate | Does not prolong pregnancy; maternal side effects often lead to discontinuation | N/A | 1 | 33, 40 |
| Prostaglandin inhibitors | Prolong pregnancy but no beneficial effect on neonatal morbidity; increase risk of postpartum hemorrhage | N/A | 1 | 33 |
| 2 | 43 | |||
| Antibiotics | No evidence to support use | N/A | 1 | 44 |
| Corticosteroids | Strongly recommended; decrease neonatal morbidity, well-tolerated | U | 1 | 46 |
| NNT, number needed to treat; LOE, level of evidence; N/A, not applicable; U, unavailable data. | ||||
Calcium channel blockers safe, effective
Calcium channel blockers (CCBs) are nonspecific smooth muscle relaxants. They prevent the influx of extracellular calcium ions into the myometrial cell, thereby exerting their tocolytic effect. Nifedipine is the most widely studied CCB in the management of preterm labor.
A systematic review of 12 randomized controlled trials including 1029 women demonstrated that CCBs significantly reduced the rate of preterm delivery within 7 days of the start of treatment and before 34 weeks gestation (LOE: 1).26 Compared with betamimetics, CCBs caused fewer maternal adverse effects, and decreased the frequency of neonatal respiratory distress syndrome, necrotizing enterocolitis, intraventricular hemorrhage, and jaundice. The authors concluded that CCBs are preferable to betamimetics for tocolysis.
Calcium channel blockers have also been compared with magnesium. Eighty women at 20 to 34 weeks gestation with documented cervical change were randomized to receive nifedipine or magnesium.27 No difference in tocolytic efficacy was observed between the treatments, but there were significantly fewer maternal side effects among the nifedipine-treated patients (LOE: 2).
CCBs appear not to adversely affect the human fetus.28,29 There was no significant increase in congenital anomalies among 586 mothers who had been exposed to CCBs compared with 907 controls: 2.6% vs 2.4%, respectively (LOE: 2).30 CCBs are effective at reducing the rate of preterm delivery, decreasing neonatal morbidity and mortality, and are well tolerated by both mother and baby. Although some other tocolytics (such as beta-agonists and prostaglandin inhibitors) prolong pregnancy, they have not demonstrated a beneficial effect on neonatal morbidity.
Dosing recommendations for nifedipine are a 30 mg loading dose, followed by 10 to 20 mg every 4 to 6 hours.31 Maternal side effects may include flushing, headache, nausea, dizziness, and transient hypotension. Neonatal side effects have not been documented. Nifedipine should not be used in conjunction with magnesium sulfate because the combination has resulted in cardiac collapse (LOE: 3).32
Beta-agonists marginally useful
Beta-agonists increase cyclic AMP, thereby causing smooth muscle relaxation. Betamimetics are widely used as tocolytic agents. Although they have been shown to prolong pregnancy by 24 to 48 hours, they have not decreased adverse perinatal or neonatal outcomes (LOE: 1).33,34 In addition, they have been associated with maternal side effects of palpitations, arrhythmias, nausea, tremor, chorioamnionitis, hyperglycemia, hypokalemia, and pulmonary edema. The risk of pulmonary edema increases if beta-agonists are used with magnesium sulfate.33,35 Fetal and neonatal side effects may include tachycardia, hyperinsulinemia, and hyperglycemia.14,35
Terbutaline is the preferred beta-agonist for preterm labor due to low cost and multiple dose forms. It may be administered subcutaneously, 0.25 mg every 30 minutes up to 1 mg in 4 hours, or by intravenous infusion, 2.5 to 10 μg per minute up to an effective maximum dose of no more than 30 μg per minute. Once stabilized, the patient is often maintained on oral terbutaline, 2.5 to 5 μg by mouth every 4 to 6 hours until term. This practice, however, has not been shown to significantly prolong pregnancy (LOE: 1).36
The major benefit of terbutaline may be in the management of preterm uterine contractions without cervical changes. Among patients with premature contractions at 20 to 34 weeks, who were randomized to hydration or observation or a single subcutaneous dose of 0.25 mg of terbutaline, the shortest length of stay in triage was for the women who received the terbutaline (LOE: 1).37
Magnesium sulfate: No good rationale for use
Magnesium sulfate in the management of preterm labor is controversial. Although widely used in North America, it is generally avoided in Europe, Australia, and the United Kingdom38,39 due to the absence of clear evidence showing efficacy in the face of its adverse effect profile. Data from systematic reviews indicate it does not delay or prevent preterm birth (LOE: 1).33,40 In addition, maternal side effects may require discontinuation of treatment. Side effects include flushing, nausea, headache, ileus, and hypocalcemia. Pulmonary edema is a serious maternal side effect and is increased when magnesium is co-administered with other tocolytic agents. Cardiac arrest is rare.14,33 Not only does evidence suggest magnesium sulfate does not delay or prevent preterm birth, but one study suggests its use may increase infant mortality (LOE: 2).41
In one trial of 1062 women, magnesium sulfate or placebo was given at 30 weeks’ gestation. Two years following birth, children of mothers who received magnesium sulfate exhibited less gross motor dysfunction than those born to mothers who received placebo, 18% vs 34% respectively (LOE: 1).42 However, the rate of pediatric mortality and cerebral palsy, the primary endpoints of the study, did not differ between the groups. Further studies are necessary before magnesium sulfate can be recommended for neuroprotection.
Prostaglandin inhibitors may be preferred for hydramnios
Indomethacin is the prostaglandin synthetase inhibitor most studied in preterm labor, though the numbers of patients in these studies was generally small.33 Indomethacin reduced the rate of preterm delivery within 7 days of treatment and before 37 weeks gestation. However, it did not decrease the rate of neonatal morbidity. Importantly, indomethacin was associated with an increased risk of postpartum hemorrhage (LOE: 1).33 A retrospective review demonstrated an increase in incidence and severity of postnatal patent ductus arteriosus in neonates whose mothers were treated with indomethacin (LOE: 2).43 Most prospective studies, however, have not found this complication. Prostaglandin inhibitors may be the preferred choice of tocolytics if hydramnios is suspected in conjunction with preterm labor.
Antibiotics: one indication only
It is hypothesized that women who experience preterm labor have infections of the upper genital tract, and that infection or inflammation leads to contractions. A meta-analysis of 11 randomized trials with 7428 women in preterm labor and intact membranes demonstrated that, although prophylactic antibiotics decreased the incidence of maternal infection, there was no benefit in neonatal outcomes (LOE: 1).44 Although it did not reach statistical significance, there was a trend towards an increase in neonatal deaths in the antibiotic group, raising concerns about their use. Therefore, administration of prophylactic antibiotics in this patient population is not recommended. Conversely, antibiotic prophylaxis is recommended for all women colonized with group B streptococcus, unless a cesarean delivery is planned, to prevent perinatal disease.45
Corticosteroids beneficial for preterm infants
Antenatal corticosteroids reduce mortality, incidence and severity of respiratory distress syndrome, and intraventricular hemorrhage in preterm infants (LOE: 1).46 Women at risk for preterm delivery between 24 and 34 weeks of gestation should be given betamethasone 12 mg IM, two doses 24 hours apart, or dexamethasone 6 mg IM, two doses 12 hours apart. There are no significant maternal or neonatal adverse effects with these regimens. Administration of tocolytic drugs may be necessary to prolong gestation and provide time for steroids to act.
Corresponding author
Adrienne Z. Ables, PharmD, Spartanburg Family Medicine Residency Program, 853 N Church St Suite 510, Spartanburg, SC 29303. E-mail: azables@srhs.com.
- Consider progesterone to reduce the risk of recurrent preterm delivery (A).
- Consider calcium channel blockers (CCBs) as initial therapy for preterm labor; though these are Class C agents in pregnancy, CCBs are at least as effective as other agents and cause no known fetal or neonatal side effects (A).
- Consider tocolytic therapy for preterm labor, to prolong pregnancy for 2 to 7 days and thereby permit administration of antenatal corticosteroids and transfer to a tertiary center, if needed (A). Maintenance and recurrent use of tocolytics has not proven beneficial and should be avoided (A).
Despite diagnostic and therapeutic advances in premature labor, the rate of preterm delivery has increased in the United States. Preterm delivery, defined as birth before 37 weeks gestation, occurs in about 12% of pregnancies,1 and it is the leading cause of mortality among non-anomalous fetuses.2 Among premature newborns who survive, 10% to 15% have significant handicaps.3 Long-term sequelae include visual or hearing impairment, chronic lung disease, developmental delay, and cerebral palsy.4 The annual cost of premature birth in the United States exceeds $5 billion, and the rate of preterm delivery has increased in recent years.5,6 Specific maternal attributes increase risk of preterm delivery, though these factors need not be present for premature delivery to occur.
Standard techniques of physical diagnosis may not accurately detect preterm labor, either missing the diagnosis or prompting unnecessary hospitalization and treatment. Select imaging studies can more reliably indicate a true need for intervention.
This article reviews the risk factors for preterm birth, the tests available to accurately diagnose preterm labor, and the medications available to prevent or postpone premature delivery.
Risk factors
Preterm delivery is categorized as spontaneous or indicated. Almost 75% of preterm births occur after spontaneous preterm labor or preterm premature rupture of the membranes (PPROM). The remaining 25% of early births are elective due to conditions potentially harmful to either the mother or the fetus.7
Maternal risk factors for spontaneous preterm births are listed in Table 1.7-13 The risk factors for preterm labor and PPROM are similar, with the exception that those whose membranes rupture prematurely are more likely to be indigent, to smoke cigarettes, and to have bleeding in the current pregnancy.
Not all preterm births carry the same risks. Early preterm birth—delivery before 32 weeks— is associated with clinical or subclinical infection, short cervical length, and the presence of fetal fibronectin in the cervicovaginal secretions. It tends to recur and leads to long-term fetal morbidity. Late preterm birth—delivery after 32 weeks but before 37 weeks—is associated with increased uterine contractions, tends to mimic normal labor, and is less likely to cause peripartum morbidity.7 Despite these known risk factors, women can experience preterm labor without exhibiting any of them.
TABLE 1
Maternal risk factors for spontaneous preterm birth
| Risk factor | Odds ratio |
|---|---|
| Bacterial vaginosis at <16 weeks | 7 |
| Periodontal infection | 4 |
| Prior preterm birth | 4 |
| Prepregnancy BMI <20 | 3 |
| Interpregnancy interval <6 mo | 2 |
| Bleeding in 2nd trimester | 2 |
| African-American race | 2 |
| Psychiatric disorder | 1.6 |
| Cigarette smoking | 1.5 |
| Gestational diabetes | 1.2 |
| Sources: Iams 20027 ; Leitich et al 20038 ; Jeffcoat et al 20019 ; Moutquin 200310 ; Smith et al 200311 ; Kelly et al 200212 ; Hedderson et al 2003.13 | |
Increasing the accuracy of diagnosis
Uterine contractions before 37 weeks and concomitant change in cervical dilation or effacement as detected by digital examination comprise the standard definition of preterm labor.
Caveats with clinical evaluation
The problem with the definition above is that women may not perceive contractions, and that contractions are at times difficult to differentiate from benign Braxton-Hicks contractions of normal pregnancy. Moreover, digital assessment of the cervix lacks reproducibility when the dilation is <3 cm or effacement is < 80%.7 Clinical evaluation alone can easily suggest prematurity when it is absent and miss it when it is present.7
Imaging and lab evaluation more telling
The ability to diagnose preterm labor improves with the use of transvaginal cervical sonography or measurement of fetal fibronectin.
Lesser cervical length means greater risk. Likelihood of preterm delivery is inversely proportional to cervical length measured at 18 to 28 weeks. A length of ≤2.5 cm is the threshold for abnormal condition (level of evidence [LOE]: 2).6
Fibronectin detected late may mean delivery early. Fibronectin, an extracellular glycoprotein described as the glue that attaches the fetal membrane to the underlying uterine decidua, is normally absent in cervicovaginal secretions between weeks 22 and 37 of pregnancy. Its presence (50 ng/mL) between 22 to 24 weeks is a predictor of spontaneous preterm birth; its absence makes premature delivery unlikely (LOE: 2).
Imaging and test results justify management decisions. The predictive accuracies of abnormal cervical length and of the presence of fibronectin are depicted in the Figure .7 The major benefit of these two tests is when they are normal (cervical length >2.5 cm or negative fetal fibronectin), unnecessary intervention is avoided.14 If, for example, a patient is having contractions and the cervical length is greater than 2.5 cm, or fetal fibronectin is absent, tocolytics can be discontinued and hospitalization avoided.
FIGURE
Value of transvaginal cervical imaging and fetal fibronectin measurement in predicting spontaneous preterm birth
Preventing preterm labor
Progestational agents
Many authors have advocated the use of progestational agents to inhibit premature labor. Progesterone’s presumed mechanisms are inhibition of the oxytocin effect of prostaglandin F2a and stimulation of α-adrenergic receptors, thereby increasing the α-adrenergic tocolytic response.15,16 Natural progesterone appears to be free of any untoward teratogenic, metabolic, or hemodynamic effects.17
Studies favoring progesterone. Two double-blind, placebo-controlled studies assessed the use of 17 α-hydroxyprogesterone caproate in preventing premature labor in high-risk populations. These patients had histories of preterm deliveries or spontaneous abortions.
Weekly intramuscular (IM) injections of 250 mg were started at 12 to 16 weeks gestation and given until 37 weeks or delivery, whichever came first. In both studies, the rate of premature deliveries was significantly lower in the treated group than the control group (number needed to treat [NNT]=2.4 and 4.6). In addition, neither mothers nor infants experienced adverse effects attributable to 17 α-hydroxyprogesterone caproate (LOE: 2).18,19 However, these studies were small—43 and 79 patients.
A large double-blind, placebo-controlled, randomized study established the effectiveness of 17 α-hydroxyprogesterone caproate in preventing preterm delivery.23 Four hundred fifty-nine pregnant women with a history of preterm delivery were randomized to receive weekly injections of 17 α-hydroxyprogesterone caproate 250 mg or placebo beginning at 16 to 20 weeks gestation and continuing to 36 weeks. Significantly fewer women in the treated group than the control group gave birth before 37 weeks, 36.3% v 54.9%, respectively (NNT=6; LOE: 1). Perhaps more importantly, treatment resulted in significant reductions in birth weight <2500 g (NNT=7), necrotizing enterocolitis (NNT=38), need for supplemental oxygen (NNT=11), and intraventricular hemorrhage (NNT=26). Swelling, bruising, or rash at the injection site were the most common adverse effects of 17 α-hydroxyprogesterone caproate administration.
Vaginal progesterone suppositories have also been shown to decrease the rate of preterm birth in patients at increased risk.17 da Fonseca et al noted that among 142 women who had 1 prior preterm birth, prophylactic cerclage, or uterine malformation, daily use of a 100-mg vaginal progesterone suppository compared with placebo significantly decreased the likelihood of delivery prior to 37 weeks, 14% v 28% (NNT=7; LOE: 1). Adverse effects of progesterone suppositories were not mentioned.
Studies lacking clear benefit. Another investigator enrolled 168 active-duty military women into a randomized double-blind study that evaluated weekly IM injections of 1000 mg 17 α-hydroxyprogesterone caproate or placebo beginning between 16 and 20 weeks gestation.20 The study population was chosen based on a report that active-duty pregnant military personnel had an increased number of pregnancy complications including low birth-weight infants and increased premature delivery.21 In contrast to the previous studies, no significant difference in premature labor or perinatal mortality was seen between the 2 groups (LOE: 2). Perhaps although active-duty personnel are at a higher risk for preterm labor, the risk is not as high as it is with previous preterm birth, prior spontaneous abortion, and so forth.
An additional small study of 77 women with twin pregnancies investigated the use of 17 α-hydroxyprogesterone caproate to prevent preterm delivery.22 Patients were randomized to receive weekly injections of 17 α-hydroxyprogesterone caproate 250 mg or placebo from the 28th gestational week until the 37th week or delivery. The mean duration of pregnancy did not differ between the two groups of women, nor did perinatal mortality (LOE: 2).
Evidence generally supports use of progesterone. Based on available data, consider administering progesterone to women at high risk for preterm delivery to prevent recurrent preterm birth (SOR: A). These data are summarized in Table 2 .
Hydroxyprogesterone caproate in oil is commercially available in the United States in 125 mg/mL and 250 mg/mL strengths. It is not, however, approved by the Food and Drug Administration (FDA) for the prevention of preterm labor.
Progesterone suppositories are also not FDA approved, and they are commercially unavailable in the US. Therefore, they have to be extemporaneously compounded.
TABLE 2
Evidence generally supports progesterone in prevention of preterm labor
| Study | Treatment | Comment | NNT | LOE |
|---|---|---|---|---|
| Johnson18 | Weekly IM 250 mg injections of 17 α-hydroxyprogesterone caproate | Decreased preterm delivery | 2.4 | 2 |
| Yemeni19 | Weekly IM 250 mg injections of 17 α-hydroxyprogesterone caproate | Decreased preterm delivery | 4.6 | 2 |
| Hauth20 | Weekly IM 1000 mg injections of 17 α-hydroxyprogesterone caproate | Did not decrease preterm delivery | N/A | 2 |
| Hartikainen-Sorri22 | Weekly IM 250 mg injections of 17 α-hydroxyprogesterone caproate | Did not decrease preterm delivery | N/A | 2 |
| Meis23 | Weekly IM 250 mg injections of 17 α-hydroxyprogesterone caproate | Decreased preterm Delivery | 6 | 1 |
| da Fonseca17 | Daily 100 mg progesterone vaginal suppositories | Decreased preterm delivery | 7 | 1 |
| NNT, number needed to treat; LOE, level of evidence; IM, intramuscular; N/A, not applicable. | ||||
Treating bacterial vaginosis may not prevent preterm labor
Bacterial vaginosis (BV) during pregnancy has been associated with preterm birth, and antibiotics have been thought perhaps to reduce this risk. A systematic review of 10 trials including 4249 pregnant women with BV showed that, although antibiotics eradicated BV, they did not significantly reduce the risk of birth before 32, 34, or 37 weeks (LOE: 1).24 This was true even for women with a history of preterm delivery. However, antibiotics did decrease the risk of PPROM. The authors concluded that evidence does not support screening all pregnant women for asymptomatic BV to prevent preterm birth.
Treating asymptomatic bacteriuria may prevent preterm labor
Asymptomatic bacteriuria in pregnant women has also been associated with preterm birth. One review of 14 trials comparing antibiotic treatment with placebo in this patient population—though not of high quality—demonstrated decreased incidences of pyelonephritis and preterm birth or low birth weight (LOE: 1).25
Treating preterm labor
The primary goal of using tocolytics in preterm labor is to prolong pregnancy for 2 to 7 days, permitting administration of corticosteroids and transfer to a tertiary care center when necessary. Recommendations for the treatment of preterm labor are summarized in Table 3 .
TABLE 3
Evaluation of proposed treatments for preterm labor
| Treatment | Comment | NNT | LOE | References |
|---|---|---|---|---|
| Calcium channel blockers | Reduce preterm delivery, decrease neonatal morbidity, well-tolerated | 11 | 1 | 26, 27 |
| Beta-agonists | Prolong pregnancy but no beneficial effect on neonatal morbidity; maternal side effects often lead to discontinuation | N/A | 1 | 33, 34 |
| Magnesium sulfate | Does not prolong pregnancy; maternal side effects often lead to discontinuation | N/A | 1 | 33, 40 |
| Prostaglandin inhibitors | Prolong pregnancy but no beneficial effect on neonatal morbidity; increase risk of postpartum hemorrhage | N/A | 1 | 33 |
| 2 | 43 | |||
| Antibiotics | No evidence to support use | N/A | 1 | 44 |
| Corticosteroids | Strongly recommended; decrease neonatal morbidity, well-tolerated | U | 1 | 46 |
| NNT, number needed to treat; LOE, level of evidence; N/A, not applicable; U, unavailable data. | ||||
Calcium channel blockers safe, effective
Calcium channel blockers (CCBs) are nonspecific smooth muscle relaxants. They prevent the influx of extracellular calcium ions into the myometrial cell, thereby exerting their tocolytic effect. Nifedipine is the most widely studied CCB in the management of preterm labor.
A systematic review of 12 randomized controlled trials including 1029 women demonstrated that CCBs significantly reduced the rate of preterm delivery within 7 days of the start of treatment and before 34 weeks gestation (LOE: 1).26 Compared with betamimetics, CCBs caused fewer maternal adverse effects, and decreased the frequency of neonatal respiratory distress syndrome, necrotizing enterocolitis, intraventricular hemorrhage, and jaundice. The authors concluded that CCBs are preferable to betamimetics for tocolysis.
Calcium channel blockers have also been compared with magnesium. Eighty women at 20 to 34 weeks gestation with documented cervical change were randomized to receive nifedipine or magnesium.27 No difference in tocolytic efficacy was observed between the treatments, but there were significantly fewer maternal side effects among the nifedipine-treated patients (LOE: 2).
CCBs appear not to adversely affect the human fetus.28,29 There was no significant increase in congenital anomalies among 586 mothers who had been exposed to CCBs compared with 907 controls: 2.6% vs 2.4%, respectively (LOE: 2).30 CCBs are effective at reducing the rate of preterm delivery, decreasing neonatal morbidity and mortality, and are well tolerated by both mother and baby. Although some other tocolytics (such as beta-agonists and prostaglandin inhibitors) prolong pregnancy, they have not demonstrated a beneficial effect on neonatal morbidity.
Dosing recommendations for nifedipine are a 30 mg loading dose, followed by 10 to 20 mg every 4 to 6 hours.31 Maternal side effects may include flushing, headache, nausea, dizziness, and transient hypotension. Neonatal side effects have not been documented. Nifedipine should not be used in conjunction with magnesium sulfate because the combination has resulted in cardiac collapse (LOE: 3).32
Beta-agonists marginally useful
Beta-agonists increase cyclic AMP, thereby causing smooth muscle relaxation. Betamimetics are widely used as tocolytic agents. Although they have been shown to prolong pregnancy by 24 to 48 hours, they have not decreased adverse perinatal or neonatal outcomes (LOE: 1).33,34 In addition, they have been associated with maternal side effects of palpitations, arrhythmias, nausea, tremor, chorioamnionitis, hyperglycemia, hypokalemia, and pulmonary edema. The risk of pulmonary edema increases if beta-agonists are used with magnesium sulfate.33,35 Fetal and neonatal side effects may include tachycardia, hyperinsulinemia, and hyperglycemia.14,35
Terbutaline is the preferred beta-agonist for preterm labor due to low cost and multiple dose forms. It may be administered subcutaneously, 0.25 mg every 30 minutes up to 1 mg in 4 hours, or by intravenous infusion, 2.5 to 10 μg per minute up to an effective maximum dose of no more than 30 μg per minute. Once stabilized, the patient is often maintained on oral terbutaline, 2.5 to 5 μg by mouth every 4 to 6 hours until term. This practice, however, has not been shown to significantly prolong pregnancy (LOE: 1).36
The major benefit of terbutaline may be in the management of preterm uterine contractions without cervical changes. Among patients with premature contractions at 20 to 34 weeks, who were randomized to hydration or observation or a single subcutaneous dose of 0.25 mg of terbutaline, the shortest length of stay in triage was for the women who received the terbutaline (LOE: 1).37
Magnesium sulfate: No good rationale for use
Magnesium sulfate in the management of preterm labor is controversial. Although widely used in North America, it is generally avoided in Europe, Australia, and the United Kingdom38,39 due to the absence of clear evidence showing efficacy in the face of its adverse effect profile. Data from systematic reviews indicate it does not delay or prevent preterm birth (LOE: 1).33,40 In addition, maternal side effects may require discontinuation of treatment. Side effects include flushing, nausea, headache, ileus, and hypocalcemia. Pulmonary edema is a serious maternal side effect and is increased when magnesium is co-administered with other tocolytic agents. Cardiac arrest is rare.14,33 Not only does evidence suggest magnesium sulfate does not delay or prevent preterm birth, but one study suggests its use may increase infant mortality (LOE: 2).41
In one trial of 1062 women, magnesium sulfate or placebo was given at 30 weeks’ gestation. Two years following birth, children of mothers who received magnesium sulfate exhibited less gross motor dysfunction than those born to mothers who received placebo, 18% vs 34% respectively (LOE: 1).42 However, the rate of pediatric mortality and cerebral palsy, the primary endpoints of the study, did not differ between the groups. Further studies are necessary before magnesium sulfate can be recommended for neuroprotection.
Prostaglandin inhibitors may be preferred for hydramnios
Indomethacin is the prostaglandin synthetase inhibitor most studied in preterm labor, though the numbers of patients in these studies was generally small.33 Indomethacin reduced the rate of preterm delivery within 7 days of treatment and before 37 weeks gestation. However, it did not decrease the rate of neonatal morbidity. Importantly, indomethacin was associated with an increased risk of postpartum hemorrhage (LOE: 1).33 A retrospective review demonstrated an increase in incidence and severity of postnatal patent ductus arteriosus in neonates whose mothers were treated with indomethacin (LOE: 2).43 Most prospective studies, however, have not found this complication. Prostaglandin inhibitors may be the preferred choice of tocolytics if hydramnios is suspected in conjunction with preterm labor.
Antibiotics: one indication only
It is hypothesized that women who experience preterm labor have infections of the upper genital tract, and that infection or inflammation leads to contractions. A meta-analysis of 11 randomized trials with 7428 women in preterm labor and intact membranes demonstrated that, although prophylactic antibiotics decreased the incidence of maternal infection, there was no benefit in neonatal outcomes (LOE: 1).44 Although it did not reach statistical significance, there was a trend towards an increase in neonatal deaths in the antibiotic group, raising concerns about their use. Therefore, administration of prophylactic antibiotics in this patient population is not recommended. Conversely, antibiotic prophylaxis is recommended for all women colonized with group B streptococcus, unless a cesarean delivery is planned, to prevent perinatal disease.45
Corticosteroids beneficial for preterm infants
Antenatal corticosteroids reduce mortality, incidence and severity of respiratory distress syndrome, and intraventricular hemorrhage in preterm infants (LOE: 1).46 Women at risk for preterm delivery between 24 and 34 weeks of gestation should be given betamethasone 12 mg IM, two doses 24 hours apart, or dexamethasone 6 mg IM, two doses 12 hours apart. There are no significant maternal or neonatal adverse effects with these regimens. Administration of tocolytic drugs may be necessary to prolong gestation and provide time for steroids to act.
Corresponding author
Adrienne Z. Ables, PharmD, Spartanburg Family Medicine Residency Program, 853 N Church St Suite 510, Spartanburg, SC 29303. E-mail: azables@srhs.com.
1. Ventura SJ, Martin JA, Curtin SC, Menacker F, Hamilton BE. Births: final data for 1999. Natl Vital Stat Rep 2001;49:1-100.
2. Rush RW, Davey DA, Segal ML. The effect of preterm delivery on perinatal mortality. Br J Obstet Gynaecol 1978;85:806-811.
3. Ehrenhaft PM, Wagner JL, Herdman RC. Changing prognosis for very low birth weight infants. Obstet Gynecol 1989;74:528.-
4. Hack M, Taylor HG, Klein N, Eiben R, Schatschneider C, Mercuri-Munich N. School-age outcomes in children with birth weight under 750 g. N Engl J Med 1994;331:753-759.
5. Rogowski JA. The economics of preterm delivery. Prenat Neonat Med 1998;3:16-20.
6. Iams JD, Goldenberg RL, Meis PJ, et al. The length of the cervix and the risk of spontaneous premature delivery. N Engl J Med 1996;334:567-572.
7. Iams JD. Preterm birth. In: Gabbe SG, Niebyl JR, Simpson JL, eds. Obstetrics: Normal and Problem Pregnancies 4th ed. New York, NY: Churchill Livingstone; 2002.
8. Leitich H, Bodner-Adler B, Brunbauer M, Egarter C, Husslei P. Bacterial vaginosis as a risk factor for preterm delivery: A meta-analysis. Am J Obstet Gynecol 2003;189:139-147.
9. Jeffcoat MK, Geurs NC, Reddy MS, Cliver SP, Goldenberg RL, Hauth JC. Periodontal infection and preterm birth: Results of prospective study. J Am Dent Assoc. 2001;132:875-880.
10. Moutquin JM. Socio-economic and psychosocial factors in the management and prevention of preterm labour. Br J Obstet Gynaecol 2003;110:56-60.
11. Smith GC, Pell JP, Dobbie R. Interpregnancy interval and risk of preterm birth and neonatal death: Retrospective cohort study. BMJ 2003;327:313-319.
12. Kelly RH, Russo J, Holt VL, Danielsen BH, Zatzick DF, Walker E, Kato W. Psychiatric and substance use disorders as risk factors for low birth weight and preterm delivery. Obstet Gynecol 2002;100:297-304.
13. Hedderson MM, Ferrara A, Sacks DA. Gestational diabetes mellitus and lesser degrees of pregnancy hyperglycemia: Association with increased risk of spontaneous preterm birth. Obstet Gynecol 2003;102:850-856.
14. American College of Obstetricians and Gynecologists Management of preterm labor. ACOG practical bulletin no. 43. Washington, DC: ACOG, 2003.
15. Lockwood CJ, Senyei AE, Dischie MR, et al. Fetal fibronectin in cervical and vaginal secretions as a predictor of preterm delivery. N Engl J Med 1991;325:669-674.
16. Fuchs AR, Fuchs R. Endocrinology of human parturition: a review. Br J Obstet Gynaecol 1984;91:948-67.
17. da Fonseca EB, Bittar R, Carvalho MHB, et al. Prophylactic administration of progesterone by vaginal suppository to reduce the incidence of spontaneous preterm birth in women at increased risk: A randomized placebo-controlled double blind study. Am J Obstet Gynecol 2003;188:419-424.
18. Johnson JW, Austin KL, Jones GS, et al. Efficacy of 17 a-hydroxyprogesterone caproate in the prevention of premature labor. N Engl J Med 1975;293:675-689.
19. Yemeni M, Borenstein R, Dreazen E, et al. Prevention of premature labor by 17 a-hydroxyprogesterone caproate. Am J Obstet Gynecol 1985;151:574-577.
20. Hauth JC, Gilstrap LC, Brekken AL, et al. The effect of 17 a-hydroxyprogesterone caproate on pregnancy outcome in an active-duty military population. Am J Obstet Gynecol 1983;146:187-190.
21. Fox ME, Harris RE, Brekken AL. The active duty military pregnancy: a new high-risk category. Am J Obstet Gynecol 1977;129:705-707.
22. Hartikainen-Sorri AL, Kauppila A, Tuimala R. Inefficacy of 17 a-hydroxyprogesterone caproate in the prevention of prematurity in twin pregnancy. Obstet Gynecol 1980;56:692-695.
23. Meis PJ. 17 Alpha hydroxyprogesterone caproate prevents recurrent preterm birth. N Engl J Med 2003;348:2379-2385.
24. McDonald H, Brocklehurst P, Parsons J, Vigneswaran R. Antibiotics for treating bacterial vaginosis in pregnancy (Cochrane Review).In: The Cochrane Library, Issue 2, 2004. Chichester, UK: John Wiley & Sons, Ltd.
25. Smaill F. Antibiotics for asymptomatic bacteriuria in pregnancy (Cochrane Review).In: The Cochrane Library, Issue 2, 2004. Chichester, UK: John Wiley & Sons, Ltd.
26. King JF, Flenady VJ, Papatsonis DNM, et al. Calcium channel blockers for inhibiting preterm labour (Cochrane Review).In: The Cochrane Library, Issue 3, 2003. Oxford: Update Software.
27. Glock, JL, Morales WJ. Efficacy and safety of nifedipine versus magnesium sulfate in the management of preterm labor: a randomized study. Am J Obstet Gynecol 1993;169:960-964.
28. Sorensen HT, Steffensen FH, Olesen C, et al. Pregnancy outcome in women exposed to calcium channel blockers. Reprod Toxicol 1998;12:383-384.
29. Magee LA, Schick B, Donnefeld AE, et al. The safety of calcium channel blockers in human pregnancy: a prospective, multicenter cohort study. Am J Obstet Gynecol 1996;174:823-828.
30. Sorensen HT, Czeizel AE, Rockenbauer M, et al. The risk of limb deficiencies and other congenital abnormalities in children exposed in utero to calcium channel blockers. Acta Obstet Gynecol Scand 2001;80:397-401.
31. Hearne AE, Nagey DA. Therapeutic agents in preterm labor: tocolytic agents. Clin Obstet Gynecol 2000;43:787-801.
32. Ben-Ami M, Giladi Y, Shalev E. The combination of magnesium sulphate and nifedipine: a cause of neuromuscular blockade. Br J Obstet Gynaecol 1994;101:262-263.
33. Gyetvai K, Hannah M E, Hodnett E D, et al. Tocolytics for preterm labor: a systematic review. Obstet Gynecol 1999;94:869-877.
34. King JF, Grant A, Keirse MJNC, et al. Beta-mimetics in preterm labor: an overview of the randomized controlled trials. Br J Obstet Gynaecol 1988;95:211-222.
35. Jeyabalan A, Caritis SN. Pharmacologic inhibition of preterm labor. Clin Obstset Gynecol 2002;45:99-113.
36. Macones GA, Berlin M, Berlin J. Efficacy of oral beta-ago-nist maintenance therapy in preterm labor: a meta-analysis. Obstet Gynecol 1995;85:313-317.
37. Guinn DA, Goepfert AR, Owen J, Brumfield C, Hauth JC. Management options in women with preterm uterine contractions: A randomized clinical trial. Am J Obstet Gynecol 1997;177:814-818.
38. Norwitz ER, Robinson JN, Challis JRG. The control of labor. N Engl J Med 1999;341:660-666.
39. Bennett P, Edwards D. Use of magnesium sulphate in obstetrics. Lancet 1997;350:1491.-
40. Crowther CA, Hiller JE, Doyle LW. Magnesium sulphate for preventing preterm birth in threatened preterm labour (Cochrane Review).In: The Cochrane Library, Issue 3, 2003. Oxford: Update Software.
41. Mittendorf R, Covert R, Boman J, et al. Is tocolytic magnesium sulfate associated with increased total paediatric mortality? Lancet 1997;350:1517-1518.
42. Crowther CA, Hiller JE, Haslam RR. Effect of magnesium sulfate given for neuroprotection before preterm birth. A randomized controlled trial. JAMA 2003;290:2669-2676.
43. Hammerman C, Glaser J, Kaplan M, et al. Indomethacin tocolysis increases postnatal patent ductus arteriosus severity. Pediatrics 1998;102:E56.-
44. King J, Flenady V. Prophylactic antibiotics for inhibiting preterm labour with intact membranes (Cochrane Review).In: The Cochrane Library, Issue 3, 2003. Oxford: Update Software.
45. Schrag S, Gorwitz R, Fultz-Butts K, Schuchat A. Prevention of perinatal group B streptococcal disease. Revised guidelines from CDC. MMWR Recomm Rep 2002;51(RR-11):1-22.Available at:www.cdc.gov/mmwr/PDF/RR/RR5111.pdf. Accessed January 21, 2005.
46. Crowley P. Prophylactic corticosteroids for preterm birth (Cochrane Review).In: The Cochrane Library, Issue 4, 2003. Oxford: Update Software.
1. Ventura SJ, Martin JA, Curtin SC, Menacker F, Hamilton BE. Births: final data for 1999. Natl Vital Stat Rep 2001;49:1-100.
2. Rush RW, Davey DA, Segal ML. The effect of preterm delivery on perinatal mortality. Br J Obstet Gynaecol 1978;85:806-811.
3. Ehrenhaft PM, Wagner JL, Herdman RC. Changing prognosis for very low birth weight infants. Obstet Gynecol 1989;74:528.-
4. Hack M, Taylor HG, Klein N, Eiben R, Schatschneider C, Mercuri-Munich N. School-age outcomes in children with birth weight under 750 g. N Engl J Med 1994;331:753-759.
5. Rogowski JA. The economics of preterm delivery. Prenat Neonat Med 1998;3:16-20.
6. Iams JD, Goldenberg RL, Meis PJ, et al. The length of the cervix and the risk of spontaneous premature delivery. N Engl J Med 1996;334:567-572.
7. Iams JD. Preterm birth. In: Gabbe SG, Niebyl JR, Simpson JL, eds. Obstetrics: Normal and Problem Pregnancies 4th ed. New York, NY: Churchill Livingstone; 2002.
8. Leitich H, Bodner-Adler B, Brunbauer M, Egarter C, Husslei P. Bacterial vaginosis as a risk factor for preterm delivery: A meta-analysis. Am J Obstet Gynecol 2003;189:139-147.
9. Jeffcoat MK, Geurs NC, Reddy MS, Cliver SP, Goldenberg RL, Hauth JC. Periodontal infection and preterm birth: Results of prospective study. J Am Dent Assoc. 2001;132:875-880.
10. Moutquin JM. Socio-economic and psychosocial factors in the management and prevention of preterm labour. Br J Obstet Gynaecol 2003;110:56-60.
11. Smith GC, Pell JP, Dobbie R. Interpregnancy interval and risk of preterm birth and neonatal death: Retrospective cohort study. BMJ 2003;327:313-319.
12. Kelly RH, Russo J, Holt VL, Danielsen BH, Zatzick DF, Walker E, Kato W. Psychiatric and substance use disorders as risk factors for low birth weight and preterm delivery. Obstet Gynecol 2002;100:297-304.
13. Hedderson MM, Ferrara A, Sacks DA. Gestational diabetes mellitus and lesser degrees of pregnancy hyperglycemia: Association with increased risk of spontaneous preterm birth. Obstet Gynecol 2003;102:850-856.
14. American College of Obstetricians and Gynecologists Management of preterm labor. ACOG practical bulletin no. 43. Washington, DC: ACOG, 2003.
15. Lockwood CJ, Senyei AE, Dischie MR, et al. Fetal fibronectin in cervical and vaginal secretions as a predictor of preterm delivery. N Engl J Med 1991;325:669-674.
16. Fuchs AR, Fuchs R. Endocrinology of human parturition: a review. Br J Obstet Gynaecol 1984;91:948-67.
17. da Fonseca EB, Bittar R, Carvalho MHB, et al. Prophylactic administration of progesterone by vaginal suppository to reduce the incidence of spontaneous preterm birth in women at increased risk: A randomized placebo-controlled double blind study. Am J Obstet Gynecol 2003;188:419-424.
18. Johnson JW, Austin KL, Jones GS, et al. Efficacy of 17 a-hydroxyprogesterone caproate in the prevention of premature labor. N Engl J Med 1975;293:675-689.
19. Yemeni M, Borenstein R, Dreazen E, et al. Prevention of premature labor by 17 a-hydroxyprogesterone caproate. Am J Obstet Gynecol 1985;151:574-577.
20. Hauth JC, Gilstrap LC, Brekken AL, et al. The effect of 17 a-hydroxyprogesterone caproate on pregnancy outcome in an active-duty military population. Am J Obstet Gynecol 1983;146:187-190.
21. Fox ME, Harris RE, Brekken AL. The active duty military pregnancy: a new high-risk category. Am J Obstet Gynecol 1977;129:705-707.
22. Hartikainen-Sorri AL, Kauppila A, Tuimala R. Inefficacy of 17 a-hydroxyprogesterone caproate in the prevention of prematurity in twin pregnancy. Obstet Gynecol 1980;56:692-695.
23. Meis PJ. 17 Alpha hydroxyprogesterone caproate prevents recurrent preterm birth. N Engl J Med 2003;348:2379-2385.
24. McDonald H, Brocklehurst P, Parsons J, Vigneswaran R. Antibiotics for treating bacterial vaginosis in pregnancy (Cochrane Review).In: The Cochrane Library, Issue 2, 2004. Chichester, UK: John Wiley & Sons, Ltd.
25. Smaill F. Antibiotics for asymptomatic bacteriuria in pregnancy (Cochrane Review).In: The Cochrane Library, Issue 2, 2004. Chichester, UK: John Wiley & Sons, Ltd.
26. King JF, Flenady VJ, Papatsonis DNM, et al. Calcium channel blockers for inhibiting preterm labour (Cochrane Review).In: The Cochrane Library, Issue 3, 2003. Oxford: Update Software.
27. Glock, JL, Morales WJ. Efficacy and safety of nifedipine versus magnesium sulfate in the management of preterm labor: a randomized study. Am J Obstet Gynecol 1993;169:960-964.
28. Sorensen HT, Steffensen FH, Olesen C, et al. Pregnancy outcome in women exposed to calcium channel blockers. Reprod Toxicol 1998;12:383-384.
29. Magee LA, Schick B, Donnefeld AE, et al. The safety of calcium channel blockers in human pregnancy: a prospective, multicenter cohort study. Am J Obstet Gynecol 1996;174:823-828.
30. Sorensen HT, Czeizel AE, Rockenbauer M, et al. The risk of limb deficiencies and other congenital abnormalities in children exposed in utero to calcium channel blockers. Acta Obstet Gynecol Scand 2001;80:397-401.
31. Hearne AE, Nagey DA. Therapeutic agents in preterm labor: tocolytic agents. Clin Obstet Gynecol 2000;43:787-801.
32. Ben-Ami M, Giladi Y, Shalev E. The combination of magnesium sulphate and nifedipine: a cause of neuromuscular blockade. Br J Obstet Gynaecol 1994;101:262-263.
33. Gyetvai K, Hannah M E, Hodnett E D, et al. Tocolytics for preterm labor: a systematic review. Obstet Gynecol 1999;94:869-877.
34. King JF, Grant A, Keirse MJNC, et al. Beta-mimetics in preterm labor: an overview of the randomized controlled trials. Br J Obstet Gynaecol 1988;95:211-222.
35. Jeyabalan A, Caritis SN. Pharmacologic inhibition of preterm labor. Clin Obstset Gynecol 2002;45:99-113.
36. Macones GA, Berlin M, Berlin J. Efficacy of oral beta-ago-nist maintenance therapy in preterm labor: a meta-analysis. Obstet Gynecol 1995;85:313-317.
37. Guinn DA, Goepfert AR, Owen J, Brumfield C, Hauth JC. Management options in women with preterm uterine contractions: A randomized clinical trial. Am J Obstet Gynecol 1997;177:814-818.
38. Norwitz ER, Robinson JN, Challis JRG. The control of labor. N Engl J Med 1999;341:660-666.
39. Bennett P, Edwards D. Use of magnesium sulphate in obstetrics. Lancet 1997;350:1491.-
40. Crowther CA, Hiller JE, Doyle LW. Magnesium sulphate for preventing preterm birth in threatened preterm labour (Cochrane Review).In: The Cochrane Library, Issue 3, 2003. Oxford: Update Software.
41. Mittendorf R, Covert R, Boman J, et al. Is tocolytic magnesium sulfate associated with increased total paediatric mortality? Lancet 1997;350:1517-1518.
42. Crowther CA, Hiller JE, Haslam RR. Effect of magnesium sulfate given for neuroprotection before preterm birth. A randomized controlled trial. JAMA 2003;290:2669-2676.
43. Hammerman C, Glaser J, Kaplan M, et al. Indomethacin tocolysis increases postnatal patent ductus arteriosus severity. Pediatrics 1998;102:E56.-
44. King J, Flenady V. Prophylactic antibiotics for inhibiting preterm labour with intact membranes (Cochrane Review).In: The Cochrane Library, Issue 3, 2003. Oxford: Update Software.
45. Schrag S, Gorwitz R, Fultz-Butts K, Schuchat A. Prevention of perinatal group B streptococcal disease. Revised guidelines from CDC. MMWR Recomm Rep 2002;51(RR-11):1-22.Available at:www.cdc.gov/mmwr/PDF/RR/RR5111.pdf. Accessed January 21, 2005.
46. Crowley P. Prophylactic corticosteroids for preterm birth (Cochrane Review).In: The Cochrane Library, Issue 4, 2003. Oxford: Update Software.
High-dose azithromycin or amoxicillin-clavulanate for recurrent otitis media?
Use high-dose azithromycin for 3 days if antibiotics are needed, instead of a 10-day course of high-dose amoxicillin-clavulanate for the treatment of recurrent or persistent acute otitis media. For every 10 children using azithromycin instead of amoxicillin-clavulanate, there is 1 additional clinical cure at 1 month and 1 less episode of diarrhea. However, no difference in clinical success is seen at 2 weeks.
Use high-dose azithromycin for 3 days if antibiotics are needed, instead of a 10-day course of high-dose amoxicillin-clavulanate for the treatment of recurrent or persistent acute otitis media. For every 10 children using azithromycin instead of amoxicillin-clavulanate, there is 1 additional clinical cure at 1 month and 1 less episode of diarrhea. However, no difference in clinical success is seen at 2 weeks.
Use high-dose azithromycin for 3 days if antibiotics are needed, instead of a 10-day course of high-dose amoxicillin-clavulanate for the treatment of recurrent or persistent acute otitis media. For every 10 children using azithromycin instead of amoxicillin-clavulanate, there is 1 additional clinical cure at 1 month and 1 less episode of diarrhea. However, no difference in clinical success is seen at 2 weeks.
Extended-release oxybutynin and tolterodine treat overactive bladder
Extended-release versions of oxybutynin and tolterodine are similarly effective and tolerable in the treatment of women with overactive bladder. No differences are seen in reduction of weekly episodes of urge incontinence and total incontinence after 3 months of treatment. Extended-release oxybutynin is more effective than extended-release tolterodine in promoting total dryness (no episodes of incontinence) after 12 weeks of treatment. Dry mouth is more common with oxybutynin; however, other side effects are similar.
Extended-release versions of oxybutynin and tolterodine are similarly effective and tolerable in the treatment of women with overactive bladder. No differences are seen in reduction of weekly episodes of urge incontinence and total incontinence after 3 months of treatment. Extended-release oxybutynin is more effective than extended-release tolterodine in promoting total dryness (no episodes of incontinence) after 12 weeks of treatment. Dry mouth is more common with oxybutynin; however, other side effects are similar.
Extended-release versions of oxybutynin and tolterodine are similarly effective and tolerable in the treatment of women with overactive bladder. No differences are seen in reduction of weekly episodes of urge incontinence and total incontinence after 3 months of treatment. Extended-release oxybutynin is more effective than extended-release tolterodine in promoting total dryness (no episodes of incontinence) after 12 weeks of treatment. Dry mouth is more common with oxybutynin; however, other side effects are similar.
Is imipramine or buspirone treatment effective in patients wishing to discontinue long-term benzodiazepine use?
BACKGROUND: Discontinuation of benzodiazepines in patients on long-term treatment may be associated with restlessness, agitation, increased anxiety, insomnia, irritability, palpitations, and many other troublesome symptoms. Thus, patients with anxiety disorders taking long-term benzodiazepine therapy have difficulty successfully discontinuing treatment. Approaches to discontinuation include a gradual taper, cognitive-behavioral therapy, and adjunctive pharmacologic treatment.
POPULATION STUDIED: A total of 107 adult patients with generalized anxiety disorder were recruited from physician offices and by notices in the media. The patients had taken benzodiazepines for an average of 8.5 years (range=1-31 years); 91% had previously attempted discontinuation (average=3.4 attempts). Interestingly, only 24% of the patients were satisfied with their benzodiazepine therapy. The mean age was 48 years (range=22-77 years); 45% were women. All were treated at a psychopharmacology research unit and returned to the care of their family physicians at the end of the study.
STUDY DESIGN AND VALIDITY: After 2 to 4 weeks taking a steady dose of their benzodiazepine, the patients were assigned to receive double-blind treatment with imipramine 25 mg, buspirone 5 mg, or placebo. Each study drug was titrated over 2 weeks to a goal of 6 capsules daily in divided doses. After 4 weeks of concomitant treatment with a benzodiazepine and assigned study drug, the benzodiazepine dose was tapered by approximately 25% each week for 4 to 6 weeks. This taper was followed by a 5-week benzodiazepine-free phase; treatment was continued for 3 weeks followed by 2 weeks of placebo treatment. Patients were monitored weekly for withdrawal symptoms, anxiety, and depression. There are several limitations in the validity of this study. There is no mention of the method of randomization or of concealed allocation of study patients. Thirty-two patients (30%) did not complete the taper phase, and these patients were not all accounted for, that is, there was no intention-to-treat analysis. Additionally, the total number of patients remaining in each treatment arm was small, and the study may not have been large enough to find differences if they actually exist. Also, there was no comparison of demographics or clinical characteristics of the 3 treatment groups.
OUTCOMES MEASURED: The main outcomes measured were: (1) whether the taper was successful as defined by a benzodiazepine-free state at 12 weeks post-taper and (2) severity of symptoms of benzodiazepine discontinuation as rated by both physicians and patients.
RESULTS: At 3 months after benzodiazepine discontinuation, 82.5% of the imipramine-treated patients were benzodiazepine-free compared with 37.5% of the placebo-treated patients (P >.01; number needed to treat=2). Buspirone was less effective, and the success rate was not statistically different from placebo. However, the severity of withdrawal symptoms was worse in the imipramine-treated patients than with the buspirone- and placebo-treated patients (16.6 vs 8.9 and 10.4, respectively) on the 38-item physician-rated checklist (P >.03). Similar results were obtained using the patient-rated checklist, although these results were not shown. Dry mouth occurred significantly more frequently in imipramine-treated patients than in buspirone- and placebo-treated patients (number needed to harm=2).
This small study indicates that imipramine is a viable adjunctive agent in promoting benzodiazepine discontinuation in motivated patients who are dissatisfied with their treatment. It does not, however, decrease the severity of withdrawal symptoms compared with placebo. Buspirone did not affect withdrawal rates, although the study probably did not have sufficient power to detect a benefit if one truly exists. Imipramine may be a useful therapy to help patients discontinue benzodiazepine use, though a confirmatory study would be useful before making a firm recommendation.
BACKGROUND: Discontinuation of benzodiazepines in patients on long-term treatment may be associated with restlessness, agitation, increased anxiety, insomnia, irritability, palpitations, and many other troublesome symptoms. Thus, patients with anxiety disorders taking long-term benzodiazepine therapy have difficulty successfully discontinuing treatment. Approaches to discontinuation include a gradual taper, cognitive-behavioral therapy, and adjunctive pharmacologic treatment.
POPULATION STUDIED: A total of 107 adult patients with generalized anxiety disorder were recruited from physician offices and by notices in the media. The patients had taken benzodiazepines for an average of 8.5 years (range=1-31 years); 91% had previously attempted discontinuation (average=3.4 attempts). Interestingly, only 24% of the patients were satisfied with their benzodiazepine therapy. The mean age was 48 years (range=22-77 years); 45% were women. All were treated at a psychopharmacology research unit and returned to the care of their family physicians at the end of the study.
STUDY DESIGN AND VALIDITY: After 2 to 4 weeks taking a steady dose of their benzodiazepine, the patients were assigned to receive double-blind treatment with imipramine 25 mg, buspirone 5 mg, or placebo. Each study drug was titrated over 2 weeks to a goal of 6 capsules daily in divided doses. After 4 weeks of concomitant treatment with a benzodiazepine and assigned study drug, the benzodiazepine dose was tapered by approximately 25% each week for 4 to 6 weeks. This taper was followed by a 5-week benzodiazepine-free phase; treatment was continued for 3 weeks followed by 2 weeks of placebo treatment. Patients were monitored weekly for withdrawal symptoms, anxiety, and depression. There are several limitations in the validity of this study. There is no mention of the method of randomization or of concealed allocation of study patients. Thirty-two patients (30%) did not complete the taper phase, and these patients were not all accounted for, that is, there was no intention-to-treat analysis. Additionally, the total number of patients remaining in each treatment arm was small, and the study may not have been large enough to find differences if they actually exist. Also, there was no comparison of demographics or clinical characteristics of the 3 treatment groups.
OUTCOMES MEASURED: The main outcomes measured were: (1) whether the taper was successful as defined by a benzodiazepine-free state at 12 weeks post-taper and (2) severity of symptoms of benzodiazepine discontinuation as rated by both physicians and patients.
RESULTS: At 3 months after benzodiazepine discontinuation, 82.5% of the imipramine-treated patients were benzodiazepine-free compared with 37.5% of the placebo-treated patients (P >.01; number needed to treat=2). Buspirone was less effective, and the success rate was not statistically different from placebo. However, the severity of withdrawal symptoms was worse in the imipramine-treated patients than with the buspirone- and placebo-treated patients (16.6 vs 8.9 and 10.4, respectively) on the 38-item physician-rated checklist (P >.03). Similar results were obtained using the patient-rated checklist, although these results were not shown. Dry mouth occurred significantly more frequently in imipramine-treated patients than in buspirone- and placebo-treated patients (number needed to harm=2).
This small study indicates that imipramine is a viable adjunctive agent in promoting benzodiazepine discontinuation in motivated patients who are dissatisfied with their treatment. It does not, however, decrease the severity of withdrawal symptoms compared with placebo. Buspirone did not affect withdrawal rates, although the study probably did not have sufficient power to detect a benefit if one truly exists. Imipramine may be a useful therapy to help patients discontinue benzodiazepine use, though a confirmatory study would be useful before making a firm recommendation.
BACKGROUND: Discontinuation of benzodiazepines in patients on long-term treatment may be associated with restlessness, agitation, increased anxiety, insomnia, irritability, palpitations, and many other troublesome symptoms. Thus, patients with anxiety disorders taking long-term benzodiazepine therapy have difficulty successfully discontinuing treatment. Approaches to discontinuation include a gradual taper, cognitive-behavioral therapy, and adjunctive pharmacologic treatment.
POPULATION STUDIED: A total of 107 adult patients with generalized anxiety disorder were recruited from physician offices and by notices in the media. The patients had taken benzodiazepines for an average of 8.5 years (range=1-31 years); 91% had previously attempted discontinuation (average=3.4 attempts). Interestingly, only 24% of the patients were satisfied with their benzodiazepine therapy. The mean age was 48 years (range=22-77 years); 45% were women. All were treated at a psychopharmacology research unit and returned to the care of their family physicians at the end of the study.
STUDY DESIGN AND VALIDITY: After 2 to 4 weeks taking a steady dose of their benzodiazepine, the patients were assigned to receive double-blind treatment with imipramine 25 mg, buspirone 5 mg, or placebo. Each study drug was titrated over 2 weeks to a goal of 6 capsules daily in divided doses. After 4 weeks of concomitant treatment with a benzodiazepine and assigned study drug, the benzodiazepine dose was tapered by approximately 25% each week for 4 to 6 weeks. This taper was followed by a 5-week benzodiazepine-free phase; treatment was continued for 3 weeks followed by 2 weeks of placebo treatment. Patients were monitored weekly for withdrawal symptoms, anxiety, and depression. There are several limitations in the validity of this study. There is no mention of the method of randomization or of concealed allocation of study patients. Thirty-two patients (30%) did not complete the taper phase, and these patients were not all accounted for, that is, there was no intention-to-treat analysis. Additionally, the total number of patients remaining in each treatment arm was small, and the study may not have been large enough to find differences if they actually exist. Also, there was no comparison of demographics or clinical characteristics of the 3 treatment groups.
OUTCOMES MEASURED: The main outcomes measured were: (1) whether the taper was successful as defined by a benzodiazepine-free state at 12 weeks post-taper and (2) severity of symptoms of benzodiazepine discontinuation as rated by both physicians and patients.
RESULTS: At 3 months after benzodiazepine discontinuation, 82.5% of the imipramine-treated patients were benzodiazepine-free compared with 37.5% of the placebo-treated patients (P >.01; number needed to treat=2). Buspirone was less effective, and the success rate was not statistically different from placebo. However, the severity of withdrawal symptoms was worse in the imipramine-treated patients than with the buspirone- and placebo-treated patients (16.6 vs 8.9 and 10.4, respectively) on the 38-item physician-rated checklist (P >.03). Similar results were obtained using the patient-rated checklist, although these results were not shown. Dry mouth occurred significantly more frequently in imipramine-treated patients than in buspirone- and placebo-treated patients (number needed to harm=2).
This small study indicates that imipramine is a viable adjunctive agent in promoting benzodiazepine discontinuation in motivated patients who are dissatisfied with their treatment. It does not, however, decrease the severity of withdrawal symptoms compared with placebo. Buspirone did not affect withdrawal rates, although the study probably did not have sufficient power to detect a benefit if one truly exists. Imipramine may be a useful therapy to help patients discontinue benzodiazepine use, though a confirmatory study would be useful before making a firm recommendation.
