User login
SIAD in Elderly Pneumonia Patients
One of the most common causes of hospitalization in the elderly is aspiration pneumonia related to dysphagia due to numerous underlying diseases.1 Thus, it is clinically important to identify prognostic factors associated with increased mortality in elderly patients with aspiration pneumonia. Hyponatremia is the most common electrolyte abnormality in hospitalized patients occurring in up to 11% of elderly patients in hospital.2 Previous studies have suggested that the presence and degree of hyponatremia is associated with the severity of pneumonia in adults and children, although the results have differed among studies.37
Hyponatremia is caused by various factors, including volume depletion, use of diuretics, hypothyroidism, adrenal insufficiency, heart failure, renal failure, and cirrhosis. Additionally, the syndrome of inappropriate antidiuresis (SIAD) is a frequent and heterogeneous disorder characterized by hyponatremia and impaired urinary dilution in the absence of any recognized stimulation of antidiuretic hormone secretion.8 Because not all patients with SIAD have elevated circulating levels of arginine vasopressin (AVP), the term SIAD is preferred to the term syndrome of inappropriate secretion of antidiuretic hormone (SIADH).9 One study has shown an association between the severity of pneumonia in children and the development of hyponatremia due to SIAD.10 To our knowledge, there have been no studies evaluating the impact of different causes of hyponatremia on mortality in elderly patients with aspiration pneumonia.
We therefore sought to investigate whether hyponatremia of all etiologies (all‐cause hyponatremia) was associated with mortality in elderly patients with aspiration pneumonia. Additionally, we compared the impact of hyponatremia due to SIAD, with hyponatremia of other etiologies, on mortality in this population
METHODS
Patients and Data Source
The Aspiration Pneumonia Dataset (APD) is the product of a retrospective analysis of elderly patients hospitalized with aspiration pneumonia from July 2004 to March 2007, performed by our second author (T.S.). The aim of the APD was to provide a dataset to allow for the development of a prediction rule for mortality, in elderly patients with aspiration pneumonia. All patients were hospitalized at Rakuwakai Otowa Hospital, in Kyoto, Japan, a 430‐bed community teaching hospital. Patients hospitalized with a diagnosis of aspiration pneumonia were identified, but those who required intensive care unit level care or intubation were excluded. The diagnosis of aspiration pneumonia was based on clinical evaluation, including a history of aspiration, a comorbidity associated with aspiration, symptoms or objective findings relevant to the respiratory system, and chest radiographic findings consistent with pneumonia.
From the APD dataset, we identified patients with hyponatremia at admission. After abstraction of the data, clinical charts were examined to obtain the data relevant to the etiologies of hyponatremia. The data were reviewed independently by 2 internal medicine physicians (J.M. and T.S.). Disagreements were resolved by consensus. Inter‐rater agreement was evaluated by using kappa statistics. The study was approved by the Ethics Committee of the Kyoto University Graduate School and Faculty of Medicine (project approval No. E290).
Definition of Hyponatremia and Its Causes
The definitions of hyponatremia and SIAD are given in Table 1. Hyponatremia was defined as serum sodium <135 mEq/L. Normonatremia was defined as serum sodium 135 mEq/L and 145 mEq/L, and hypernatremia was defined as serum sodium >145 mEq/L. Severity of hyponatremia was defined as mild (130 Na <135 mEq/L), moderate (125 Na <130 mEq/L), or severe (Na <125 mEq/L). Effective serum osmolality was calculated by the formula: [Effective serum osmolality (mOsm/kg H2O) = 2 Na (mEq/L) + glucose (mg/dL)/18].9 Hypotonic hyponatremia was defined as hyponatremia in the setting of effective serum osmolality <280 mOsm/kg H2O. Hypotonic hyponatremia was classified by extracellular fluid volume (ECFV) status as hypovolemic, SIAD euvolemic, non‐SIAD euvolemic or hypervolemic.11, 12
| Hypovolemic* | 1. Either of the terms volume depletion or hypovolemic documented in the admission notes. |
| 2. Clinical signs suggestive of volume depletion observed on physical examination and documented in admission notes (ie, dry oral cavity or dry skin in the axilla). | |
| 3. Volume depletion detected by biochemical or physiological examination (ie, metabolic alkalosis in arterial gas studies or collapsed inferior vena cava on echocardiography, respectively). | |
| 4. Increase in sodium concentration to within the normal range, together with decrease in blood urea nitrogen, following administration of hypotonic fluid. | |
| Euvolemic* | 1. The term euvolemic documented in admission notes. |
| 2. Criteria for hypovolemic and hypervolemic hyponatremia not met. | |
| Hypervolemic* | 1. The terms hypervolemic or an excess of ECFV documented in admission notes. |
| 2. Clinical signs suggestive of an excess of ECFV observed on physical examination and documented in admission notes (ie, edema or jugular venous distension). | |
| 3. Excess of ECFV detected by physiological examination (ie, dilated inferior vena cava on echocardiography). | |
| SIAD | Euvolemic hyponatremia with the following findings: |
| Urinary sodium concentration >30 mEq/L; | |
| Urinary osmolality >100 mOsm/kg H2O; | |
| Normal thyroid, adrenal, and renal function. | |
Clinical Outcomes
The primary outcome of analysis was defined as mortality within 30 days of admission (30‐day mortality). The secondary outcome was defined as mortality during the hospital stay (in‐hospital mortality).
Statistical Methods
First, for the subsequent analyses, we used a cohort from which hypernatremia and non‐hypotonic hyponatremia patients were excluded. Multivariate logistic regression was used to evaluate the impacts of hyponatremia and SIAD on the outcomes of interest. The following baseline risk factors associated with the severity of pneumonia in previous studies were considered for inclusion in the multivariate model1315: age, gender, living in a care facility, use of a feeding tube, disorientation, systolic blood pressure <90 mmHg or diastolic blood pressure <60 mmHg, heart rate >125 beats/min, body temperature <35C or >40C, respiratory failure (defined as oxygen saturation 90% or partial pressure of oxygen 60 mmHg or respiratory rate >30 breaths/min), blood glucose >250 mg/dL, anemia (defined as hematocrit <30%), blood urea nitrogen (BUN) >20 mg/dL, serum C‐reactive protein >10 mg/dL, serum albumin <3 g/dL, congestive heart failure, diabetes mellitus, chronic respiratory disease, malignancy, cirrhosis, chronic kidney failure, and central nervous system disease. The severity of pneumonia was evaluated by using the 6‐point scale of the A‐DROP (Age, Dehydration, Respiratory failure, Orientation disturbance, and low blood Pressure) scoring system proposed by the Japanese Respiratory Society.15 This is a modified version of the CURB‐65 (Confusion, Uremia, Respiratory rate, BP, age 65 years) clinical prediction rule and assesses the following parameters: age (men 70 years; women 75 years), dehydration (BUN concentration 21 mg/dL), respiratory failure (oxygen saturation 90%, partial pressure of oxygen 60 mmHg, ratio of partial pressure of oxygen to fraction of inspired oxygen 300), orientation disturbance (defined as disorientation at admission), and systolic blood pressure <90 mmHg. Patients with scores of 0 or 1 were classified as mild, patients scoring 2 as moderate, and patients scoring 3 to 5 as severe. In univariate analyses, the chi‐squared test was used. Those variables with P < 0.2 in the univariate analyses were included in the multivariate analyses. The HosmerLemeshow test was used to assess the goodness‐of‐fit for multivariate logistic regression models. Data were analyzed with STATA 10 (StataCorp, College Station, TX). Two‐tailed P values <0.05 were considered statistically significant.
RESULTS
The baseline characteristics of the study population are listed in Table 2. There were 221 elderly patients identified as having aspiration pneumonia. Of those, 65 (29%) had hyponatremia; 3 (5%) with non‐hypotonic and 62 (95%) with hypotonic hyponatremia. In the latter group, patients were characterized has having hypovolemic (39 [63%]), hypervolemic (3 [5%]), and euvolemic (20 [32%]) hyponatremia. Among the euvolemic patients, SIAD occurred in 14 (70%) of patients. Non‐SIAD euvolemic hyponatremia occurred in 6 (30%) patients and was associated with hypothyroidism (1 patient), adrenal insufficiency (1 patient), and was unclassifiable due to lack of available clinical data in 4 patients. The kappa value was 0.87 for inter‐rater agreement of the classification of hypotonic hyponatremia.
| |
| Age (yr) | 84 8.6* |
| Male | 90 (41) |
| Living in care facilities | 143 (65) |
| Use of a feeding tube | 40 (18) |
| Comorbidity | |
| Congestive heart failure | 21 (10) |
| Diabetes mellitus | 33 (15) |
| Chronic respiratory disease | 31 (14) |
| Malignancy | 14 (6) |
| Liver cirrhosis | 13 (6) |
| Chronic renal failure | 23 (10) |
| Central nervous system disease | 194 (88) |
| Disorientation | 36 (16) |
| Systolic blood pressure (mmHg) | 131 28* |
| Heart rate (beats/min) | 92 20* |
| Body temperature (C) | 37.5 1.1* |
| Respiratory rate (breaths/min) | 24 (IQR, 2030) |
| Oxygen saturation (%) | 95 (IQR, 9197) |
| pH | 7.44 (IQR, 7.407.47) |
| Glucose (mg/dL) | 140 57* |
| Hematocrit (%) | 34.7 5.9* |
| Blood urea nitrogen (mg/dL) | 22.7 15* |
| C‐reactive protein (mg/dL) | 5.2 (IQR, 1.811.7) |
| Albumin (g/dL) | 3.3 0.60* |
| A‐DROP severity class | |
| Mild (score, 0 or 1) | 83 (38) |
| Moderate (score, 2) | 84 (38) |
| Severe (score, 35) | 54 (24) |
| Sodium (mEq/L) | 137 6.98* |
| Sodium range (mEq/L) | 101162 |
| Distribution and classification of sodium concentration (mEq/L) | |
| Hypernatremia: Na >145 | 16 (7) |
| Normonatremia: 135 Na 145 | 140 (64) |
| Hyponatremia: Na <135 | 65 (29) |
| Mild: 130 Na <135 | 44 (20) |
| Moderate: 125 Na <130 | 11 (5) |
| Severe: Na <125 | 10 (4) |
| Length of stay (days) | 34.6 39* |
| 30‐day mortality | 28 (13) |
| LOS in these patients (days) | 14.7 9.6 |
| In‐hospital mortality | 63 (29) |
| LOS in these patients (days) | 41.9 33.8* |
The following variables were included in multivariate logistic analyses: congestive heart failure, cirrhosis, chronic renal failure, disorientation, body temperature <35C or >40C, anemia, and serum albumin <3 g/dL (see Supporting Information, Appendix, in the online version of this article).
In the multivariate logistic analyses, all‐cause hyponatremia was not associated with increased 30‐day mortality (odds ratio [OR] 1.85, 95% confidence interval [CI] 0.635.48; P = 0.262), but was associated with a trend toward increased risk of in‐hospital mortality (OR 2.10, 95% CI 1.004.42; P = 0.050) (Table 3). Moderate and severe hyponatremia were both significantly associated with increased in‐hospital mortality (OR 6.05, 95% CI 1.4625.0; P = 0.013 and OR 5.65, 95% CI 1.1428.1; P = 0.034, respectively). The same trends were observed for 30‐day mortality, although the results were not statistically significant. No such trend was observed for mild hyponatremia.
| Normonatremia | Hypotonic Hyponatremia | ||||
|---|---|---|---|---|---|
| All‐Cause | Mild | Moderate | Severe | ||
| n = 140 | n = 62 | n = 42 | n = 10 | n = 10 | |
| |||||
| 30‐day mortality, n (%) | 11 (8) | 11 (17) | 4 (10) | 2 (18) | 4 (60) |
| Odds ratio (95% CI) | 1 (ref) | 1.85 (0.635.48) | 1.30 (0.354.82) | 3.15 (0.5019.9) | 3.44 (0.5719.3) |
| P value | P = 0.262 | P = 0.691 | P = 0.222 | P = 0.184 | |
| In‐hospital mortality, n (%) | 28 (20) | 25 (39) | 10 (24) | 6 (55) | 7 (70) |
| Odds ratio (95% CI) | 1 (ref) | 2.10 (1.004.42) | 1.26 (0.523.07) | 6.05 (1.4625.0) | 5.65 (1.1428.1) |
| P value | P = 0.050 | P = 0.606 | P = 0.013 | P = 0.034 | |
In the multivariate logistic regression analyses, hypotonic hyponatremia due to SIAD was significantly associated with both increased risk of 30‐day mortality (OR 7.40, 95% CI 1.7331.7; P = 0.007) and increased risk of in‐hospital mortality (OR 22.3, 95% CI 4.26117; P < 0.001) (Table 4). In contrast, hypovolemic or non‐SIAD euvolemic hyponatremia was associated with neither increased risk of 30‐day mortality nor increased risk of in‐hospital mortality. There were too few hypervolemic hyponatremia patients for us to perform effective logistic analyses. The P values of the HosmerLemeshow tests were 0.45 for the multivariate logistic regression model (hypovolemic, SIAD, and non‐SIAD euvolemic vs normonatremia) with 30‐day mortality, and 0.30 for the model with in‐hospital mortality.
| Normonatremia | Hypotonic Hyponatremia | ||||
|---|---|---|---|---|---|
| Hypovolemic | Euvolemic | Hypervolemic | |||
| SIAD | non‐SIAD* | ||||
| n = 140 | n = 39 | n = 14 | n = 6 | n = 3 | |
| |||||
| 30‐day mortality, n (%) | 11 (8) | 2 (5) | 6 (43) | 1 (17) | 1 (33) |
| Odds ratio (95% CI) | 1 (ref) | 0.58 (0.113.10) | 7.40 (1.7331.7) | 2.71 (0.2430.6) | |
| P value | P = 0.525 | P = 0.007 | P = 0.421 | ||
| In‐hospital mortality, n (%) | 28 (20) | 7 (18) | 12 (86) | 1 (17) | 3 (100) |
| Odds ratio (95% CI) | 1 (ref) | 0.85 (0.322.30) | 22.3 (4.26117) | 0.93 (0.108.98) | |
| P value | P = 0.751 | P < 0.001 | P = 0.948 | ||
Six patients with SIAD were classified as having an A‐DROP severity class of mild, 4 as moderate, and 4 as severe (P = 0.908, Wilcoxon‐type test for trend). There was no association between the occurrence of SIAD and the severity of pneumonia.
DISCUSSION
We demonstrated that mortality in elderly patients with aspiration pneumonia was significantly associated with SIAD, but not with all‐cause hyponatremia. Unlike SIAD, other etiologies of hyponatremia were not associated with mortality in elderly patients with aspiration pneumonia. A recent study by Waikar and colleagues concluded that hyponatremia subgrouped by severity was not significantly associated with in‐hospital mortality in pneumonia patients, although a trend between severe hyponatremia and mortality was observed.16 Likewise, a study by Zilberberg and colleagues reported no significant increased risk of death with hyponatremia compared with normonatremia.4 These results are similar to our results for all‐cause hyponatremia and for hyponatremia subgrouped by severity. In contrast, a study by Nair and colleagues reported some increased risk of death with hyponatremia.5 Our results suggest that the heterogeneity of these previous results was probably due to the fact that SIAD was not identified in these other studies.
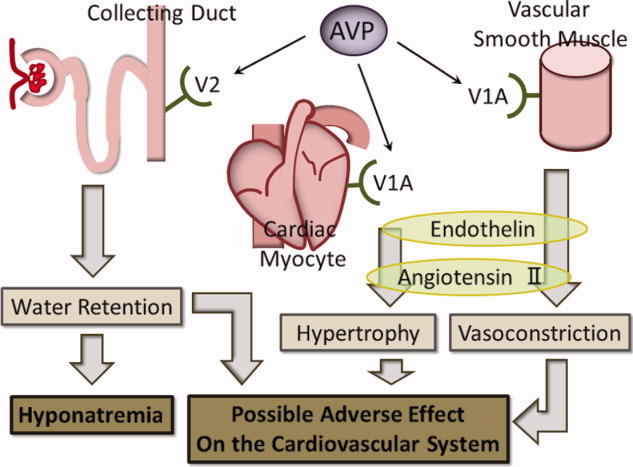
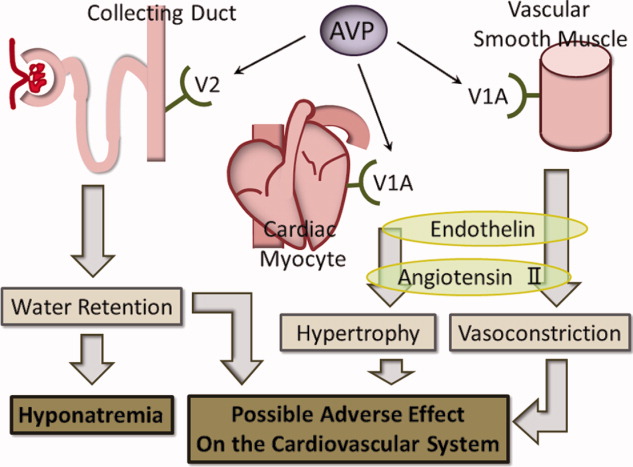
While the rationale for increased mortality in patients with pneumonia associated with SIAD is not known, it may be that there is a direct deleterious effect of elevated AVP. AVP has 3 distinct receptor subtypes, V1A, V1B, and V2. Stimulation of the V1A receptor in vascular smooth muscle promotes an increase in systemic vascular resistance, and stimulation of the same receptor in cardiac myocytes promotes myocyte hypertrophy. Stimulation of the V1B receptor in the anterior pituitary promotes adrenocorticotropic hormone release, and stimulation of the V2 receptor in the renal collecting ducts promotes an increase in water retention, which plays the main role in SIAD.1719 Our hypothesis in elderly SIAD patients with aspiration pneumonia is that increased AVP levels may lead not only to water retention and hyponatremia, but also to other effects such as vasoconstriction and myocyte hypertrophy, which may adversely influence the cardiovascular systems of elderly patients (Figure 1).
In our study, SIAD in elderly patients with aspiration pneumonia was more strongly associated with in‐hospital mortality than with 30‐day mortality. The average length of stay (LOS) of all patients dying in hospital (42 days) was significantly longer than the average LOS of those dying within 30 days of admission (15 days; P < 0.001, MannWhitney Test; Table 2). These findings suggest that SIAD was associated more strongly with longer‐term mortality than with acute‐stage mortality. The reason for the association between SIAD and longer‐term mortality remains unclear, although there may be some association between longer‐term mortality and the pathophysiologic mechanisms of AVP.
Our study has some limitations. First, because of the retrospective observational design, there is a potential for bias. We used multivariate analyses adjusted for confounding factors, however, other residual confounding factors may have remained. In addition, since the diagnosis of pneumonia was based on chart review, there may have been imprecision in the accuracy of diagnosing aspiration pneumonia. Aspiration pneumonia sometimes occurs without apparent episodes of aspiration, and this would have led to underdiagnosis. In contrast, aspiration pneumonitis can be mistaken for aspiration pneumonia; this would have led to overdiagnosis.
Second, volume status is difficult to evaluate prospectively, and thus by nature of our design, appropriate assignment of volume status was difficult. Several studies have used test infusions of isotonic saline to discriminate between these alternatives, but because our study was retrospective, we were unable to use this test.11, 20 Some studies have reported that, in patients in a state of volume depletion, volume repletion removes the stimulus for antidiuretic hormone release, allowing excess water to be excreted in a dilute urine and the serum sodium concentration to return toward normal.21, 22 According to this theory, instead of using an isotonic test infusion, we added in our study a criterion of volume depletion in which patients with a sustained increase in serum sodium concentration of 5 mEq/L and a sustained decrease in blood urea nitrogen, even with administration of hypotonic solution, were classified as volume depleted.
Third, all patients were analyzed according to status on admission, although some patients with hypovolemic hyponatremia at admission were found to have hyponatremia due to SIAD after admission.
Fourth, because the sample size of this study was small with our results revealing wide confidence intervals, an effect between other causes of hyponatremia and mortality might not have been identified. However, for 80% power, the calculated sample size was 100 non‐SIAD patients with aspiration pneumonia versus 10 SIAD patients, given that the mortality rate of elderly patients with aspiration pneumonia was, at a moderate estimate, 15% according to the studies of both Stukenborg and colleagues and Oliver and colleagues, and the mortality rate of SIAD patients was increased by 400% compared with that of non‐SIAD patients according to the study of Song and colleagues, with an alpha error of 0.05.7, 23, 24 Our sample size was therefore greater than the required size.
Fifth, because the APD dataset was compiled in 2007 for another study, it was not concurrent, and this may have led to other limitations in interpreting the data.
Finally, in Japan, the average length of hospital stay was 36.3 days in 2004 and 34.1 days in 2007much longer than other developed countries.25 Because of this situation, in‐hospital mortality, and not 30‐day mortality, represented long‐term mortality. Therefore, our results may not be easily applicable to the situation in other developed countries.
In conclusion, our results suggest that the presence of SIAD on admission in elderly patients with aspiration pneumonia is associated with increased mortality. This novel finding should be re‐evaluated, but it does raise the question of a direct, negative impact of AVP on patients' clinical outcomes. In the future, a larger prospective cohort study should be conducted to confirm the findings of this study, given the small sample size and the retrospective nature of the study. Additionally, a different population of pneumonia patients, such as those with community‐acquired pneumonia, should be examined to further evaluate the etiologies of hyponatremia in pneumonia and the association between hyponatremia of these different etiologies and mortality.
Acknowledgements
Disclosures: Jun Miyashita and Toshihiko Shimada report receiving a grant‐in‐aid from the Ministry of Health, Labour and Welfare of Japan, Development of Clinical Research Fellowship (Principal Investigator, Shunichi Fukuhara), grant H18‐001. No other potential conflict of interest relevant to this article was reported.
- ,.Aspiration pneumonia and dysphagia in the elderly.Chest.2003;124(1):328–336.
- ,.Hyponatraemia in the elderly.Age Ageing.1983;12(1):77–80.
- ,.Frequency and significance of electrolyte abnormalities in pneumonia.Indian Pediatr.1992;29(6):735–740.
- ,,, et al.Hyponatremia and hospital outcomes among patients with pneumonia: a retrospective cohort study.BMC Pulm Med.2008;8(2):16.
- ,,,.Hyponatremia in community‐acquired pneumonia.Am J Nephrol.2007;27(2):184–190.
- ,,,.Hyponatremia in pediatric community‐acquired pneumonia.Pediatr Nephrol.2008;23(12):2247–2253.
- ,,, et al.Epidemiology and clinical outcomes of community‐acquired pneumonia in adult patients in Asian countries: a prospective study by the Asian network for surveillance of resistant pathogens.Int J Antimicrob Agents.2008;31(2):107–114.
- ,.The syndrome of inappropriate secretion of antidiuretic hormone.Am J Med.1967;42(5):790–806.
- ,.Clinical practice. The syndrome of inappropriate antidiuresis.N Engl J Med.2007;356(20):2064–2072.
- ,,.Hyponatraemia and the inappropriate ADH syndrome in pneumonia.Ann Trop Paediatr.1992;12(4):455–462.
- ,,, et al.Value of fractional uric acid excretion in differential diagnosis of hyponatremic patients on diuretics.J Clin Endocrinol Metab.2008;93(8):2991–2997.
- ,.The syndrome of inappropriate antidiuretic hormone: prevalence, causes and consequences.Eur J Endocrinol.2010;162(suppl 1):S5–S12.
- ,,, et al.A prediction rule to identify low‐risk patients with community‐acquired pneumonia.N Engl J Med.1997;336(4):243–250.
- ,,, et al.Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study.Thorax.2003;58(5):377–382.
- ,,, et al.Comparison of severity scoring systems A‐DROP and CURB‐65 for community‐acquired pneumonia.Respirology.2008;13(5):731–735.
- ,,.Mortality after hospitalization with mild, moderate, and severe hyponatremia.Am J Med.2009;122(9):857–865.
- .The role of vasopressin in congestive heart failure.Cleve Clin J Med.2006;73(suppl 3):S19–S23.
- .Vasopressin antagonists—progress and promise.N Engl J Med.2006;355(20):2146–2148.
- ,,, et al.A novel vasopressin dual V1A/V2 receptor antagonist, conivaptan hydrochloride, improves hyponatremia in rats with syndrome of inappropriate secretion of antidiuretic hormone (SIADH).Biol Pharm Bull.2007;30(1):91–95.
- ,.Clinical laboratory evaluation of the syndrome of inappropriate secretion of antidiuretic hormone.Clin J Am Soc Nephrol.2008;3(4):1175–1184.
- ,,,,,.Danger of central pontine myelinolysis in hypotonic dehydration and recommendation for treatment.Am J Med Sci.1989;298(1):41–43.
- ,.Treatment of hyponatremia: a quantitative analysis.Am J Kidney Dis.1993;21(4):439–443.
- ,,, et al.Hospital discharge abstract data on comorbidity improved the prediction of death among patients hospitalized with aspiration pneumonia.J Clin Epidemiol.2004;57(5):522–532.
- ,,, et al.Comorbid disease and the effect of race and ethnicity on in‐hospital mortality from aspiration pneumonia.J Natl Med Assoc.2004;96(11):1462–1469.
- Ministry of Health, Labour and Welfare, Japan. Health Statistics in Japan 2007. Available at: http://www.mhlw.go.jp/english/database/db‐hss/hs2007.html. Accessed August 18,2010.
One of the most common causes of hospitalization in the elderly is aspiration pneumonia related to dysphagia due to numerous underlying diseases.1 Thus, it is clinically important to identify prognostic factors associated with increased mortality in elderly patients with aspiration pneumonia. Hyponatremia is the most common electrolyte abnormality in hospitalized patients occurring in up to 11% of elderly patients in hospital.2 Previous studies have suggested that the presence and degree of hyponatremia is associated with the severity of pneumonia in adults and children, although the results have differed among studies.37
Hyponatremia is caused by various factors, including volume depletion, use of diuretics, hypothyroidism, adrenal insufficiency, heart failure, renal failure, and cirrhosis. Additionally, the syndrome of inappropriate antidiuresis (SIAD) is a frequent and heterogeneous disorder characterized by hyponatremia and impaired urinary dilution in the absence of any recognized stimulation of antidiuretic hormone secretion.8 Because not all patients with SIAD have elevated circulating levels of arginine vasopressin (AVP), the term SIAD is preferred to the term syndrome of inappropriate secretion of antidiuretic hormone (SIADH).9 One study has shown an association between the severity of pneumonia in children and the development of hyponatremia due to SIAD.10 To our knowledge, there have been no studies evaluating the impact of different causes of hyponatremia on mortality in elderly patients with aspiration pneumonia.
We therefore sought to investigate whether hyponatremia of all etiologies (all‐cause hyponatremia) was associated with mortality in elderly patients with aspiration pneumonia. Additionally, we compared the impact of hyponatremia due to SIAD, with hyponatremia of other etiologies, on mortality in this population
METHODS
Patients and Data Source
The Aspiration Pneumonia Dataset (APD) is the product of a retrospective analysis of elderly patients hospitalized with aspiration pneumonia from July 2004 to March 2007, performed by our second author (T.S.). The aim of the APD was to provide a dataset to allow for the development of a prediction rule for mortality, in elderly patients with aspiration pneumonia. All patients were hospitalized at Rakuwakai Otowa Hospital, in Kyoto, Japan, a 430‐bed community teaching hospital. Patients hospitalized with a diagnosis of aspiration pneumonia were identified, but those who required intensive care unit level care or intubation were excluded. The diagnosis of aspiration pneumonia was based on clinical evaluation, including a history of aspiration, a comorbidity associated with aspiration, symptoms or objective findings relevant to the respiratory system, and chest radiographic findings consistent with pneumonia.
From the APD dataset, we identified patients with hyponatremia at admission. After abstraction of the data, clinical charts were examined to obtain the data relevant to the etiologies of hyponatremia. The data were reviewed independently by 2 internal medicine physicians (J.M. and T.S.). Disagreements were resolved by consensus. Inter‐rater agreement was evaluated by using kappa statistics. The study was approved by the Ethics Committee of the Kyoto University Graduate School and Faculty of Medicine (project approval No. E290).
Definition of Hyponatremia and Its Causes
The definitions of hyponatremia and SIAD are given in Table 1. Hyponatremia was defined as serum sodium <135 mEq/L. Normonatremia was defined as serum sodium 135 mEq/L and 145 mEq/L, and hypernatremia was defined as serum sodium >145 mEq/L. Severity of hyponatremia was defined as mild (130 Na <135 mEq/L), moderate (125 Na <130 mEq/L), or severe (Na <125 mEq/L). Effective serum osmolality was calculated by the formula: [Effective serum osmolality (mOsm/kg H2O) = 2 Na (mEq/L) + glucose (mg/dL)/18].9 Hypotonic hyponatremia was defined as hyponatremia in the setting of effective serum osmolality <280 mOsm/kg H2O. Hypotonic hyponatremia was classified by extracellular fluid volume (ECFV) status as hypovolemic, SIAD euvolemic, non‐SIAD euvolemic or hypervolemic.11, 12
| Hypovolemic* | 1. Either of the terms volume depletion or hypovolemic documented in the admission notes. |
| 2. Clinical signs suggestive of volume depletion observed on physical examination and documented in admission notes (ie, dry oral cavity or dry skin in the axilla). | |
| 3. Volume depletion detected by biochemical or physiological examination (ie, metabolic alkalosis in arterial gas studies or collapsed inferior vena cava on echocardiography, respectively). | |
| 4. Increase in sodium concentration to within the normal range, together with decrease in blood urea nitrogen, following administration of hypotonic fluid. | |
| Euvolemic* | 1. The term euvolemic documented in admission notes. |
| 2. Criteria for hypovolemic and hypervolemic hyponatremia not met. | |
| Hypervolemic* | 1. The terms hypervolemic or an excess of ECFV documented in admission notes. |
| 2. Clinical signs suggestive of an excess of ECFV observed on physical examination and documented in admission notes (ie, edema or jugular venous distension). | |
| 3. Excess of ECFV detected by physiological examination (ie, dilated inferior vena cava on echocardiography). | |
| SIAD | Euvolemic hyponatremia with the following findings: |
| Urinary sodium concentration >30 mEq/L; | |
| Urinary osmolality >100 mOsm/kg H2O; | |
| Normal thyroid, adrenal, and renal function. | |
Clinical Outcomes
The primary outcome of analysis was defined as mortality within 30 days of admission (30‐day mortality). The secondary outcome was defined as mortality during the hospital stay (in‐hospital mortality).
Statistical Methods
First, for the subsequent analyses, we used a cohort from which hypernatremia and non‐hypotonic hyponatremia patients were excluded. Multivariate logistic regression was used to evaluate the impacts of hyponatremia and SIAD on the outcomes of interest. The following baseline risk factors associated with the severity of pneumonia in previous studies were considered for inclusion in the multivariate model1315: age, gender, living in a care facility, use of a feeding tube, disorientation, systolic blood pressure <90 mmHg or diastolic blood pressure <60 mmHg, heart rate >125 beats/min, body temperature <35C or >40C, respiratory failure (defined as oxygen saturation 90% or partial pressure of oxygen 60 mmHg or respiratory rate >30 breaths/min), blood glucose >250 mg/dL, anemia (defined as hematocrit <30%), blood urea nitrogen (BUN) >20 mg/dL, serum C‐reactive protein >10 mg/dL, serum albumin <3 g/dL, congestive heart failure, diabetes mellitus, chronic respiratory disease, malignancy, cirrhosis, chronic kidney failure, and central nervous system disease. The severity of pneumonia was evaluated by using the 6‐point scale of the A‐DROP (Age, Dehydration, Respiratory failure, Orientation disturbance, and low blood Pressure) scoring system proposed by the Japanese Respiratory Society.15 This is a modified version of the CURB‐65 (Confusion, Uremia, Respiratory rate, BP, age 65 years) clinical prediction rule and assesses the following parameters: age (men 70 years; women 75 years), dehydration (BUN concentration 21 mg/dL), respiratory failure (oxygen saturation 90%, partial pressure of oxygen 60 mmHg, ratio of partial pressure of oxygen to fraction of inspired oxygen 300), orientation disturbance (defined as disorientation at admission), and systolic blood pressure <90 mmHg. Patients with scores of 0 or 1 were classified as mild, patients scoring 2 as moderate, and patients scoring 3 to 5 as severe. In univariate analyses, the chi‐squared test was used. Those variables with P < 0.2 in the univariate analyses were included in the multivariate analyses. The HosmerLemeshow test was used to assess the goodness‐of‐fit for multivariate logistic regression models. Data were analyzed with STATA 10 (StataCorp, College Station, TX). Two‐tailed P values <0.05 were considered statistically significant.
RESULTS
The baseline characteristics of the study population are listed in Table 2. There were 221 elderly patients identified as having aspiration pneumonia. Of those, 65 (29%) had hyponatremia; 3 (5%) with non‐hypotonic and 62 (95%) with hypotonic hyponatremia. In the latter group, patients were characterized has having hypovolemic (39 [63%]), hypervolemic (3 [5%]), and euvolemic (20 [32%]) hyponatremia. Among the euvolemic patients, SIAD occurred in 14 (70%) of patients. Non‐SIAD euvolemic hyponatremia occurred in 6 (30%) patients and was associated with hypothyroidism (1 patient), adrenal insufficiency (1 patient), and was unclassifiable due to lack of available clinical data in 4 patients. The kappa value was 0.87 for inter‐rater agreement of the classification of hypotonic hyponatremia.
| |
| Age (yr) | 84 8.6* |
| Male | 90 (41) |
| Living in care facilities | 143 (65) |
| Use of a feeding tube | 40 (18) |
| Comorbidity | |
| Congestive heart failure | 21 (10) |
| Diabetes mellitus | 33 (15) |
| Chronic respiratory disease | 31 (14) |
| Malignancy | 14 (6) |
| Liver cirrhosis | 13 (6) |
| Chronic renal failure | 23 (10) |
| Central nervous system disease | 194 (88) |
| Disorientation | 36 (16) |
| Systolic blood pressure (mmHg) | 131 28* |
| Heart rate (beats/min) | 92 20* |
| Body temperature (C) | 37.5 1.1* |
| Respiratory rate (breaths/min) | 24 (IQR, 2030) |
| Oxygen saturation (%) | 95 (IQR, 9197) |
| pH | 7.44 (IQR, 7.407.47) |
| Glucose (mg/dL) | 140 57* |
| Hematocrit (%) | 34.7 5.9* |
| Blood urea nitrogen (mg/dL) | 22.7 15* |
| C‐reactive protein (mg/dL) | 5.2 (IQR, 1.811.7) |
| Albumin (g/dL) | 3.3 0.60* |
| A‐DROP severity class | |
| Mild (score, 0 or 1) | 83 (38) |
| Moderate (score, 2) | 84 (38) |
| Severe (score, 35) | 54 (24) |
| Sodium (mEq/L) | 137 6.98* |
| Sodium range (mEq/L) | 101162 |
| Distribution and classification of sodium concentration (mEq/L) | |
| Hypernatremia: Na >145 | 16 (7) |
| Normonatremia: 135 Na 145 | 140 (64) |
| Hyponatremia: Na <135 | 65 (29) |
| Mild: 130 Na <135 | 44 (20) |
| Moderate: 125 Na <130 | 11 (5) |
| Severe: Na <125 | 10 (4) |
| Length of stay (days) | 34.6 39* |
| 30‐day mortality | 28 (13) |
| LOS in these patients (days) | 14.7 9.6 |
| In‐hospital mortality | 63 (29) |
| LOS in these patients (days) | 41.9 33.8* |
The following variables were included in multivariate logistic analyses: congestive heart failure, cirrhosis, chronic renal failure, disorientation, body temperature <35C or >40C, anemia, and serum albumin <3 g/dL (see Supporting Information, Appendix, in the online version of this article).
In the multivariate logistic analyses, all‐cause hyponatremia was not associated with increased 30‐day mortality (odds ratio [OR] 1.85, 95% confidence interval [CI] 0.635.48; P = 0.262), but was associated with a trend toward increased risk of in‐hospital mortality (OR 2.10, 95% CI 1.004.42; P = 0.050) (Table 3). Moderate and severe hyponatremia were both significantly associated with increased in‐hospital mortality (OR 6.05, 95% CI 1.4625.0; P = 0.013 and OR 5.65, 95% CI 1.1428.1; P = 0.034, respectively). The same trends were observed for 30‐day mortality, although the results were not statistically significant. No such trend was observed for mild hyponatremia.
| Normonatremia | Hypotonic Hyponatremia | ||||
|---|---|---|---|---|---|
| All‐Cause | Mild | Moderate | Severe | ||
| n = 140 | n = 62 | n = 42 | n = 10 | n = 10 | |
| |||||
| 30‐day mortality, n (%) | 11 (8) | 11 (17) | 4 (10) | 2 (18) | 4 (60) |
| Odds ratio (95% CI) | 1 (ref) | 1.85 (0.635.48) | 1.30 (0.354.82) | 3.15 (0.5019.9) | 3.44 (0.5719.3) |
| P value | P = 0.262 | P = 0.691 | P = 0.222 | P = 0.184 | |
| In‐hospital mortality, n (%) | 28 (20) | 25 (39) | 10 (24) | 6 (55) | 7 (70) |
| Odds ratio (95% CI) | 1 (ref) | 2.10 (1.004.42) | 1.26 (0.523.07) | 6.05 (1.4625.0) | 5.65 (1.1428.1) |
| P value | P = 0.050 | P = 0.606 | P = 0.013 | P = 0.034 | |
In the multivariate logistic regression analyses, hypotonic hyponatremia due to SIAD was significantly associated with both increased risk of 30‐day mortality (OR 7.40, 95% CI 1.7331.7; P = 0.007) and increased risk of in‐hospital mortality (OR 22.3, 95% CI 4.26117; P < 0.001) (Table 4). In contrast, hypovolemic or non‐SIAD euvolemic hyponatremia was associated with neither increased risk of 30‐day mortality nor increased risk of in‐hospital mortality. There were too few hypervolemic hyponatremia patients for us to perform effective logistic analyses. The P values of the HosmerLemeshow tests were 0.45 for the multivariate logistic regression model (hypovolemic, SIAD, and non‐SIAD euvolemic vs normonatremia) with 30‐day mortality, and 0.30 for the model with in‐hospital mortality.
| Normonatremia | Hypotonic Hyponatremia | ||||
|---|---|---|---|---|---|
| Hypovolemic | Euvolemic | Hypervolemic | |||
| SIAD | non‐SIAD* | ||||
| n = 140 | n = 39 | n = 14 | n = 6 | n = 3 | |
| |||||
| 30‐day mortality, n (%) | 11 (8) | 2 (5) | 6 (43) | 1 (17) | 1 (33) |
| Odds ratio (95% CI) | 1 (ref) | 0.58 (0.113.10) | 7.40 (1.7331.7) | 2.71 (0.2430.6) | |
| P value | P = 0.525 | P = 0.007 | P = 0.421 | ||
| In‐hospital mortality, n (%) | 28 (20) | 7 (18) | 12 (86) | 1 (17) | 3 (100) |
| Odds ratio (95% CI) | 1 (ref) | 0.85 (0.322.30) | 22.3 (4.26117) | 0.93 (0.108.98) | |
| P value | P = 0.751 | P < 0.001 | P = 0.948 | ||
Six patients with SIAD were classified as having an A‐DROP severity class of mild, 4 as moderate, and 4 as severe (P = 0.908, Wilcoxon‐type test for trend). There was no association between the occurrence of SIAD and the severity of pneumonia.
DISCUSSION
We demonstrated that mortality in elderly patients with aspiration pneumonia was significantly associated with SIAD, but not with all‐cause hyponatremia. Unlike SIAD, other etiologies of hyponatremia were not associated with mortality in elderly patients with aspiration pneumonia. A recent study by Waikar and colleagues concluded that hyponatremia subgrouped by severity was not significantly associated with in‐hospital mortality in pneumonia patients, although a trend between severe hyponatremia and mortality was observed.16 Likewise, a study by Zilberberg and colleagues reported no significant increased risk of death with hyponatremia compared with normonatremia.4 These results are similar to our results for all‐cause hyponatremia and for hyponatremia subgrouped by severity. In contrast, a study by Nair and colleagues reported some increased risk of death with hyponatremia.5 Our results suggest that the heterogeneity of these previous results was probably due to the fact that SIAD was not identified in these other studies.
While the rationale for increased mortality in patients with pneumonia associated with SIAD is not known, it may be that there is a direct deleterious effect of elevated AVP. AVP has 3 distinct receptor subtypes, V1A, V1B, and V2. Stimulation of the V1A receptor in vascular smooth muscle promotes an increase in systemic vascular resistance, and stimulation of the same receptor in cardiac myocytes promotes myocyte hypertrophy. Stimulation of the V1B receptor in the anterior pituitary promotes adrenocorticotropic hormone release, and stimulation of the V2 receptor in the renal collecting ducts promotes an increase in water retention, which plays the main role in SIAD.1719 Our hypothesis in elderly SIAD patients with aspiration pneumonia is that increased AVP levels may lead not only to water retention and hyponatremia, but also to other effects such as vasoconstriction and myocyte hypertrophy, which may adversely influence the cardiovascular systems of elderly patients (Figure 1).

In our study, SIAD in elderly patients with aspiration pneumonia was more strongly associated with in‐hospital mortality than with 30‐day mortality. The average length of stay (LOS) of all patients dying in hospital (42 days) was significantly longer than the average LOS of those dying within 30 days of admission (15 days; P < 0.001, MannWhitney Test; Table 2). These findings suggest that SIAD was associated more strongly with longer‐term mortality than with acute‐stage mortality. The reason for the association between SIAD and longer‐term mortality remains unclear, although there may be some association between longer‐term mortality and the pathophysiologic mechanisms of AVP.
Our study has some limitations. First, because of the retrospective observational design, there is a potential for bias. We used multivariate analyses adjusted for confounding factors, however, other residual confounding factors may have remained. In addition, since the diagnosis of pneumonia was based on chart review, there may have been imprecision in the accuracy of diagnosing aspiration pneumonia. Aspiration pneumonia sometimes occurs without apparent episodes of aspiration, and this would have led to underdiagnosis. In contrast, aspiration pneumonitis can be mistaken for aspiration pneumonia; this would have led to overdiagnosis.
Second, volume status is difficult to evaluate prospectively, and thus by nature of our design, appropriate assignment of volume status was difficult. Several studies have used test infusions of isotonic saline to discriminate between these alternatives, but because our study was retrospective, we were unable to use this test.11, 20 Some studies have reported that, in patients in a state of volume depletion, volume repletion removes the stimulus for antidiuretic hormone release, allowing excess water to be excreted in a dilute urine and the serum sodium concentration to return toward normal.21, 22 According to this theory, instead of using an isotonic test infusion, we added in our study a criterion of volume depletion in which patients with a sustained increase in serum sodium concentration of 5 mEq/L and a sustained decrease in blood urea nitrogen, even with administration of hypotonic solution, were classified as volume depleted.
Third, all patients were analyzed according to status on admission, although some patients with hypovolemic hyponatremia at admission were found to have hyponatremia due to SIAD after admission.
Fourth, because the sample size of this study was small with our results revealing wide confidence intervals, an effect between other causes of hyponatremia and mortality might not have been identified. However, for 80% power, the calculated sample size was 100 non‐SIAD patients with aspiration pneumonia versus 10 SIAD patients, given that the mortality rate of elderly patients with aspiration pneumonia was, at a moderate estimate, 15% according to the studies of both Stukenborg and colleagues and Oliver and colleagues, and the mortality rate of SIAD patients was increased by 400% compared with that of non‐SIAD patients according to the study of Song and colleagues, with an alpha error of 0.05.7, 23, 24 Our sample size was therefore greater than the required size.
Fifth, because the APD dataset was compiled in 2007 for another study, it was not concurrent, and this may have led to other limitations in interpreting the data.
Finally, in Japan, the average length of hospital stay was 36.3 days in 2004 and 34.1 days in 2007much longer than other developed countries.25 Because of this situation, in‐hospital mortality, and not 30‐day mortality, represented long‐term mortality. Therefore, our results may not be easily applicable to the situation in other developed countries.
In conclusion, our results suggest that the presence of SIAD on admission in elderly patients with aspiration pneumonia is associated with increased mortality. This novel finding should be re‐evaluated, but it does raise the question of a direct, negative impact of AVP on patients' clinical outcomes. In the future, a larger prospective cohort study should be conducted to confirm the findings of this study, given the small sample size and the retrospective nature of the study. Additionally, a different population of pneumonia patients, such as those with community‐acquired pneumonia, should be examined to further evaluate the etiologies of hyponatremia in pneumonia and the association between hyponatremia of these different etiologies and mortality.
Acknowledgements
Disclosures: Jun Miyashita and Toshihiko Shimada report receiving a grant‐in‐aid from the Ministry of Health, Labour and Welfare of Japan, Development of Clinical Research Fellowship (Principal Investigator, Shunichi Fukuhara), grant H18‐001. No other potential conflict of interest relevant to this article was reported.
One of the most common causes of hospitalization in the elderly is aspiration pneumonia related to dysphagia due to numerous underlying diseases.1 Thus, it is clinically important to identify prognostic factors associated with increased mortality in elderly patients with aspiration pneumonia. Hyponatremia is the most common electrolyte abnormality in hospitalized patients occurring in up to 11% of elderly patients in hospital.2 Previous studies have suggested that the presence and degree of hyponatremia is associated with the severity of pneumonia in adults and children, although the results have differed among studies.37
Hyponatremia is caused by various factors, including volume depletion, use of diuretics, hypothyroidism, adrenal insufficiency, heart failure, renal failure, and cirrhosis. Additionally, the syndrome of inappropriate antidiuresis (SIAD) is a frequent and heterogeneous disorder characterized by hyponatremia and impaired urinary dilution in the absence of any recognized stimulation of antidiuretic hormone secretion.8 Because not all patients with SIAD have elevated circulating levels of arginine vasopressin (AVP), the term SIAD is preferred to the term syndrome of inappropriate secretion of antidiuretic hormone (SIADH).9 One study has shown an association between the severity of pneumonia in children and the development of hyponatremia due to SIAD.10 To our knowledge, there have been no studies evaluating the impact of different causes of hyponatremia on mortality in elderly patients with aspiration pneumonia.
We therefore sought to investigate whether hyponatremia of all etiologies (all‐cause hyponatremia) was associated with mortality in elderly patients with aspiration pneumonia. Additionally, we compared the impact of hyponatremia due to SIAD, with hyponatremia of other etiologies, on mortality in this population
METHODS
Patients and Data Source
The Aspiration Pneumonia Dataset (APD) is the product of a retrospective analysis of elderly patients hospitalized with aspiration pneumonia from July 2004 to March 2007, performed by our second author (T.S.). The aim of the APD was to provide a dataset to allow for the development of a prediction rule for mortality, in elderly patients with aspiration pneumonia. All patients were hospitalized at Rakuwakai Otowa Hospital, in Kyoto, Japan, a 430‐bed community teaching hospital. Patients hospitalized with a diagnosis of aspiration pneumonia were identified, but those who required intensive care unit level care or intubation were excluded. The diagnosis of aspiration pneumonia was based on clinical evaluation, including a history of aspiration, a comorbidity associated with aspiration, symptoms or objective findings relevant to the respiratory system, and chest radiographic findings consistent with pneumonia.
From the APD dataset, we identified patients with hyponatremia at admission. After abstraction of the data, clinical charts were examined to obtain the data relevant to the etiologies of hyponatremia. The data were reviewed independently by 2 internal medicine physicians (J.M. and T.S.). Disagreements were resolved by consensus. Inter‐rater agreement was evaluated by using kappa statistics. The study was approved by the Ethics Committee of the Kyoto University Graduate School and Faculty of Medicine (project approval No. E290).
Definition of Hyponatremia and Its Causes
The definitions of hyponatremia and SIAD are given in Table 1. Hyponatremia was defined as serum sodium <135 mEq/L. Normonatremia was defined as serum sodium 135 mEq/L and 145 mEq/L, and hypernatremia was defined as serum sodium >145 mEq/L. Severity of hyponatremia was defined as mild (130 Na <135 mEq/L), moderate (125 Na <130 mEq/L), or severe (Na <125 mEq/L). Effective serum osmolality was calculated by the formula: [Effective serum osmolality (mOsm/kg H2O) = 2 Na (mEq/L) + glucose (mg/dL)/18].9 Hypotonic hyponatremia was defined as hyponatremia in the setting of effective serum osmolality <280 mOsm/kg H2O. Hypotonic hyponatremia was classified by extracellular fluid volume (ECFV) status as hypovolemic, SIAD euvolemic, non‐SIAD euvolemic or hypervolemic.11, 12
| Hypovolemic* | 1. Either of the terms volume depletion or hypovolemic documented in the admission notes. |
| 2. Clinical signs suggestive of volume depletion observed on physical examination and documented in admission notes (ie, dry oral cavity or dry skin in the axilla). | |
| 3. Volume depletion detected by biochemical or physiological examination (ie, metabolic alkalosis in arterial gas studies or collapsed inferior vena cava on echocardiography, respectively). | |
| 4. Increase in sodium concentration to within the normal range, together with decrease in blood urea nitrogen, following administration of hypotonic fluid. | |
| Euvolemic* | 1. The term euvolemic documented in admission notes. |
| 2. Criteria for hypovolemic and hypervolemic hyponatremia not met. | |
| Hypervolemic* | 1. The terms hypervolemic or an excess of ECFV documented in admission notes. |
| 2. Clinical signs suggestive of an excess of ECFV observed on physical examination and documented in admission notes (ie, edema or jugular venous distension). | |
| 3. Excess of ECFV detected by physiological examination (ie, dilated inferior vena cava on echocardiography). | |
| SIAD | Euvolemic hyponatremia with the following findings: |
| Urinary sodium concentration >30 mEq/L; | |
| Urinary osmolality >100 mOsm/kg H2O; | |
| Normal thyroid, adrenal, and renal function. | |
Clinical Outcomes
The primary outcome of analysis was defined as mortality within 30 days of admission (30‐day mortality). The secondary outcome was defined as mortality during the hospital stay (in‐hospital mortality).
Statistical Methods
First, for the subsequent analyses, we used a cohort from which hypernatremia and non‐hypotonic hyponatremia patients were excluded. Multivariate logistic regression was used to evaluate the impacts of hyponatremia and SIAD on the outcomes of interest. The following baseline risk factors associated with the severity of pneumonia in previous studies were considered for inclusion in the multivariate model1315: age, gender, living in a care facility, use of a feeding tube, disorientation, systolic blood pressure <90 mmHg or diastolic blood pressure <60 mmHg, heart rate >125 beats/min, body temperature <35C or >40C, respiratory failure (defined as oxygen saturation 90% or partial pressure of oxygen 60 mmHg or respiratory rate >30 breaths/min), blood glucose >250 mg/dL, anemia (defined as hematocrit <30%), blood urea nitrogen (BUN) >20 mg/dL, serum C‐reactive protein >10 mg/dL, serum albumin <3 g/dL, congestive heart failure, diabetes mellitus, chronic respiratory disease, malignancy, cirrhosis, chronic kidney failure, and central nervous system disease. The severity of pneumonia was evaluated by using the 6‐point scale of the A‐DROP (Age, Dehydration, Respiratory failure, Orientation disturbance, and low blood Pressure) scoring system proposed by the Japanese Respiratory Society.15 This is a modified version of the CURB‐65 (Confusion, Uremia, Respiratory rate, BP, age 65 years) clinical prediction rule and assesses the following parameters: age (men 70 years; women 75 years), dehydration (BUN concentration 21 mg/dL), respiratory failure (oxygen saturation 90%, partial pressure of oxygen 60 mmHg, ratio of partial pressure of oxygen to fraction of inspired oxygen 300), orientation disturbance (defined as disorientation at admission), and systolic blood pressure <90 mmHg. Patients with scores of 0 or 1 were classified as mild, patients scoring 2 as moderate, and patients scoring 3 to 5 as severe. In univariate analyses, the chi‐squared test was used. Those variables with P < 0.2 in the univariate analyses were included in the multivariate analyses. The HosmerLemeshow test was used to assess the goodness‐of‐fit for multivariate logistic regression models. Data were analyzed with STATA 10 (StataCorp, College Station, TX). Two‐tailed P values <0.05 were considered statistically significant.
RESULTS
The baseline characteristics of the study population are listed in Table 2. There were 221 elderly patients identified as having aspiration pneumonia. Of those, 65 (29%) had hyponatremia; 3 (5%) with non‐hypotonic and 62 (95%) with hypotonic hyponatremia. In the latter group, patients were characterized has having hypovolemic (39 [63%]), hypervolemic (3 [5%]), and euvolemic (20 [32%]) hyponatremia. Among the euvolemic patients, SIAD occurred in 14 (70%) of patients. Non‐SIAD euvolemic hyponatremia occurred in 6 (30%) patients and was associated with hypothyroidism (1 patient), adrenal insufficiency (1 patient), and was unclassifiable due to lack of available clinical data in 4 patients. The kappa value was 0.87 for inter‐rater agreement of the classification of hypotonic hyponatremia.
| |
| Age (yr) | 84 8.6* |
| Male | 90 (41) |
| Living in care facilities | 143 (65) |
| Use of a feeding tube | 40 (18) |
| Comorbidity | |
| Congestive heart failure | 21 (10) |
| Diabetes mellitus | 33 (15) |
| Chronic respiratory disease | 31 (14) |
| Malignancy | 14 (6) |
| Liver cirrhosis | 13 (6) |
| Chronic renal failure | 23 (10) |
| Central nervous system disease | 194 (88) |
| Disorientation | 36 (16) |
| Systolic blood pressure (mmHg) | 131 28* |
| Heart rate (beats/min) | 92 20* |
| Body temperature (C) | 37.5 1.1* |
| Respiratory rate (breaths/min) | 24 (IQR, 2030) |
| Oxygen saturation (%) | 95 (IQR, 9197) |
| pH | 7.44 (IQR, 7.407.47) |
| Glucose (mg/dL) | 140 57* |
| Hematocrit (%) | 34.7 5.9* |
| Blood urea nitrogen (mg/dL) | 22.7 15* |
| C‐reactive protein (mg/dL) | 5.2 (IQR, 1.811.7) |
| Albumin (g/dL) | 3.3 0.60* |
| A‐DROP severity class | |
| Mild (score, 0 or 1) | 83 (38) |
| Moderate (score, 2) | 84 (38) |
| Severe (score, 35) | 54 (24) |
| Sodium (mEq/L) | 137 6.98* |
| Sodium range (mEq/L) | 101162 |
| Distribution and classification of sodium concentration (mEq/L) | |
| Hypernatremia: Na >145 | 16 (7) |
| Normonatremia: 135 Na 145 | 140 (64) |
| Hyponatremia: Na <135 | 65 (29) |
| Mild: 130 Na <135 | 44 (20) |
| Moderate: 125 Na <130 | 11 (5) |
| Severe: Na <125 | 10 (4) |
| Length of stay (days) | 34.6 39* |
| 30‐day mortality | 28 (13) |
| LOS in these patients (days) | 14.7 9.6 |
| In‐hospital mortality | 63 (29) |
| LOS in these patients (days) | 41.9 33.8* |
The following variables were included in multivariate logistic analyses: congestive heart failure, cirrhosis, chronic renal failure, disorientation, body temperature <35C or >40C, anemia, and serum albumin <3 g/dL (see Supporting Information, Appendix, in the online version of this article).
In the multivariate logistic analyses, all‐cause hyponatremia was not associated with increased 30‐day mortality (odds ratio [OR] 1.85, 95% confidence interval [CI] 0.635.48; P = 0.262), but was associated with a trend toward increased risk of in‐hospital mortality (OR 2.10, 95% CI 1.004.42; P = 0.050) (Table 3). Moderate and severe hyponatremia were both significantly associated with increased in‐hospital mortality (OR 6.05, 95% CI 1.4625.0; P = 0.013 and OR 5.65, 95% CI 1.1428.1; P = 0.034, respectively). The same trends were observed for 30‐day mortality, although the results were not statistically significant. No such trend was observed for mild hyponatremia.
| Normonatremia | Hypotonic Hyponatremia | ||||
|---|---|---|---|---|---|
| All‐Cause | Mild | Moderate | Severe | ||
| n = 140 | n = 62 | n = 42 | n = 10 | n = 10 | |
| |||||
| 30‐day mortality, n (%) | 11 (8) | 11 (17) | 4 (10) | 2 (18) | 4 (60) |
| Odds ratio (95% CI) | 1 (ref) | 1.85 (0.635.48) | 1.30 (0.354.82) | 3.15 (0.5019.9) | 3.44 (0.5719.3) |
| P value | P = 0.262 | P = 0.691 | P = 0.222 | P = 0.184 | |
| In‐hospital mortality, n (%) | 28 (20) | 25 (39) | 10 (24) | 6 (55) | 7 (70) |
| Odds ratio (95% CI) | 1 (ref) | 2.10 (1.004.42) | 1.26 (0.523.07) | 6.05 (1.4625.0) | 5.65 (1.1428.1) |
| P value | P = 0.050 | P = 0.606 | P = 0.013 | P = 0.034 | |
In the multivariate logistic regression analyses, hypotonic hyponatremia due to SIAD was significantly associated with both increased risk of 30‐day mortality (OR 7.40, 95% CI 1.7331.7; P = 0.007) and increased risk of in‐hospital mortality (OR 22.3, 95% CI 4.26117; P < 0.001) (Table 4). In contrast, hypovolemic or non‐SIAD euvolemic hyponatremia was associated with neither increased risk of 30‐day mortality nor increased risk of in‐hospital mortality. There were too few hypervolemic hyponatremia patients for us to perform effective logistic analyses. The P values of the HosmerLemeshow tests were 0.45 for the multivariate logistic regression model (hypovolemic, SIAD, and non‐SIAD euvolemic vs normonatremia) with 30‐day mortality, and 0.30 for the model with in‐hospital mortality.
| Normonatremia | Hypotonic Hyponatremia | ||||
|---|---|---|---|---|---|
| Hypovolemic | Euvolemic | Hypervolemic | |||
| SIAD | non‐SIAD* | ||||
| n = 140 | n = 39 | n = 14 | n = 6 | n = 3 | |
| |||||
| 30‐day mortality, n (%) | 11 (8) | 2 (5) | 6 (43) | 1 (17) | 1 (33) |
| Odds ratio (95% CI) | 1 (ref) | 0.58 (0.113.10) | 7.40 (1.7331.7) | 2.71 (0.2430.6) | |
| P value | P = 0.525 | P = 0.007 | P = 0.421 | ||
| In‐hospital mortality, n (%) | 28 (20) | 7 (18) | 12 (86) | 1 (17) | 3 (100) |
| Odds ratio (95% CI) | 1 (ref) | 0.85 (0.322.30) | 22.3 (4.26117) | 0.93 (0.108.98) | |
| P value | P = 0.751 | P < 0.001 | P = 0.948 | ||
Six patients with SIAD were classified as having an A‐DROP severity class of mild, 4 as moderate, and 4 as severe (P = 0.908, Wilcoxon‐type test for trend). There was no association between the occurrence of SIAD and the severity of pneumonia.
DISCUSSION
We demonstrated that mortality in elderly patients with aspiration pneumonia was significantly associated with SIAD, but not with all‐cause hyponatremia. Unlike SIAD, other etiologies of hyponatremia were not associated with mortality in elderly patients with aspiration pneumonia. A recent study by Waikar and colleagues concluded that hyponatremia subgrouped by severity was not significantly associated with in‐hospital mortality in pneumonia patients, although a trend between severe hyponatremia and mortality was observed.16 Likewise, a study by Zilberberg and colleagues reported no significant increased risk of death with hyponatremia compared with normonatremia.4 These results are similar to our results for all‐cause hyponatremia and for hyponatremia subgrouped by severity. In contrast, a study by Nair and colleagues reported some increased risk of death with hyponatremia.5 Our results suggest that the heterogeneity of these previous results was probably due to the fact that SIAD was not identified in these other studies.
While the rationale for increased mortality in patients with pneumonia associated with SIAD is not known, it may be that there is a direct deleterious effect of elevated AVP. AVP has 3 distinct receptor subtypes, V1A, V1B, and V2. Stimulation of the V1A receptor in vascular smooth muscle promotes an increase in systemic vascular resistance, and stimulation of the same receptor in cardiac myocytes promotes myocyte hypertrophy. Stimulation of the V1B receptor in the anterior pituitary promotes adrenocorticotropic hormone release, and stimulation of the V2 receptor in the renal collecting ducts promotes an increase in water retention, which plays the main role in SIAD.1719 Our hypothesis in elderly SIAD patients with aspiration pneumonia is that increased AVP levels may lead not only to water retention and hyponatremia, but also to other effects such as vasoconstriction and myocyte hypertrophy, which may adversely influence the cardiovascular systems of elderly patients (Figure 1).

In our study, SIAD in elderly patients with aspiration pneumonia was more strongly associated with in‐hospital mortality than with 30‐day mortality. The average length of stay (LOS) of all patients dying in hospital (42 days) was significantly longer than the average LOS of those dying within 30 days of admission (15 days; P < 0.001, MannWhitney Test; Table 2). These findings suggest that SIAD was associated more strongly with longer‐term mortality than with acute‐stage mortality. The reason for the association between SIAD and longer‐term mortality remains unclear, although there may be some association between longer‐term mortality and the pathophysiologic mechanisms of AVP.
Our study has some limitations. First, because of the retrospective observational design, there is a potential for bias. We used multivariate analyses adjusted for confounding factors, however, other residual confounding factors may have remained. In addition, since the diagnosis of pneumonia was based on chart review, there may have been imprecision in the accuracy of diagnosing aspiration pneumonia. Aspiration pneumonia sometimes occurs without apparent episodes of aspiration, and this would have led to underdiagnosis. In contrast, aspiration pneumonitis can be mistaken for aspiration pneumonia; this would have led to overdiagnosis.
Second, volume status is difficult to evaluate prospectively, and thus by nature of our design, appropriate assignment of volume status was difficult. Several studies have used test infusions of isotonic saline to discriminate between these alternatives, but because our study was retrospective, we were unable to use this test.11, 20 Some studies have reported that, in patients in a state of volume depletion, volume repletion removes the stimulus for antidiuretic hormone release, allowing excess water to be excreted in a dilute urine and the serum sodium concentration to return toward normal.21, 22 According to this theory, instead of using an isotonic test infusion, we added in our study a criterion of volume depletion in which patients with a sustained increase in serum sodium concentration of 5 mEq/L and a sustained decrease in blood urea nitrogen, even with administration of hypotonic solution, were classified as volume depleted.
Third, all patients were analyzed according to status on admission, although some patients with hypovolemic hyponatremia at admission were found to have hyponatremia due to SIAD after admission.
Fourth, because the sample size of this study was small with our results revealing wide confidence intervals, an effect between other causes of hyponatremia and mortality might not have been identified. However, for 80% power, the calculated sample size was 100 non‐SIAD patients with aspiration pneumonia versus 10 SIAD patients, given that the mortality rate of elderly patients with aspiration pneumonia was, at a moderate estimate, 15% according to the studies of both Stukenborg and colleagues and Oliver and colleagues, and the mortality rate of SIAD patients was increased by 400% compared with that of non‐SIAD patients according to the study of Song and colleagues, with an alpha error of 0.05.7, 23, 24 Our sample size was therefore greater than the required size.
Fifth, because the APD dataset was compiled in 2007 for another study, it was not concurrent, and this may have led to other limitations in interpreting the data.
Finally, in Japan, the average length of hospital stay was 36.3 days in 2004 and 34.1 days in 2007much longer than other developed countries.25 Because of this situation, in‐hospital mortality, and not 30‐day mortality, represented long‐term mortality. Therefore, our results may not be easily applicable to the situation in other developed countries.
In conclusion, our results suggest that the presence of SIAD on admission in elderly patients with aspiration pneumonia is associated with increased mortality. This novel finding should be re‐evaluated, but it does raise the question of a direct, negative impact of AVP on patients' clinical outcomes. In the future, a larger prospective cohort study should be conducted to confirm the findings of this study, given the small sample size and the retrospective nature of the study. Additionally, a different population of pneumonia patients, such as those with community‐acquired pneumonia, should be examined to further evaluate the etiologies of hyponatremia in pneumonia and the association between hyponatremia of these different etiologies and mortality.
Acknowledgements
Disclosures: Jun Miyashita and Toshihiko Shimada report receiving a grant‐in‐aid from the Ministry of Health, Labour and Welfare of Japan, Development of Clinical Research Fellowship (Principal Investigator, Shunichi Fukuhara), grant H18‐001. No other potential conflict of interest relevant to this article was reported.
- ,.Aspiration pneumonia and dysphagia in the elderly.Chest.2003;124(1):328–336.
- ,.Hyponatraemia in the elderly.Age Ageing.1983;12(1):77–80.
- ,.Frequency and significance of electrolyte abnormalities in pneumonia.Indian Pediatr.1992;29(6):735–740.
- ,,, et al.Hyponatremia and hospital outcomes among patients with pneumonia: a retrospective cohort study.BMC Pulm Med.2008;8(2):16.
- ,,,.Hyponatremia in community‐acquired pneumonia.Am J Nephrol.2007;27(2):184–190.
- ,,,.Hyponatremia in pediatric community‐acquired pneumonia.Pediatr Nephrol.2008;23(12):2247–2253.
- ,,, et al.Epidemiology and clinical outcomes of community‐acquired pneumonia in adult patients in Asian countries: a prospective study by the Asian network for surveillance of resistant pathogens.Int J Antimicrob Agents.2008;31(2):107–114.
- ,.The syndrome of inappropriate secretion of antidiuretic hormone.Am J Med.1967;42(5):790–806.
- ,.Clinical practice. The syndrome of inappropriate antidiuresis.N Engl J Med.2007;356(20):2064–2072.
- ,,.Hyponatraemia and the inappropriate ADH syndrome in pneumonia.Ann Trop Paediatr.1992;12(4):455–462.
- ,,, et al.Value of fractional uric acid excretion in differential diagnosis of hyponatremic patients on diuretics.J Clin Endocrinol Metab.2008;93(8):2991–2997.
- ,.The syndrome of inappropriate antidiuretic hormone: prevalence, causes and consequences.Eur J Endocrinol.2010;162(suppl 1):S5–S12.
- ,,, et al.A prediction rule to identify low‐risk patients with community‐acquired pneumonia.N Engl J Med.1997;336(4):243–250.
- ,,, et al.Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study.Thorax.2003;58(5):377–382.
- ,,, et al.Comparison of severity scoring systems A‐DROP and CURB‐65 for community‐acquired pneumonia.Respirology.2008;13(5):731–735.
- ,,.Mortality after hospitalization with mild, moderate, and severe hyponatremia.Am J Med.2009;122(9):857–865.
- .The role of vasopressin in congestive heart failure.Cleve Clin J Med.2006;73(suppl 3):S19–S23.
- .Vasopressin antagonists—progress and promise.N Engl J Med.2006;355(20):2146–2148.
- ,,, et al.A novel vasopressin dual V1A/V2 receptor antagonist, conivaptan hydrochloride, improves hyponatremia in rats with syndrome of inappropriate secretion of antidiuretic hormone (SIADH).Biol Pharm Bull.2007;30(1):91–95.
- ,.Clinical laboratory evaluation of the syndrome of inappropriate secretion of antidiuretic hormone.Clin J Am Soc Nephrol.2008;3(4):1175–1184.
- ,,,,,.Danger of central pontine myelinolysis in hypotonic dehydration and recommendation for treatment.Am J Med Sci.1989;298(1):41–43.
- ,.Treatment of hyponatremia: a quantitative analysis.Am J Kidney Dis.1993;21(4):439–443.
- ,,, et al.Hospital discharge abstract data on comorbidity improved the prediction of death among patients hospitalized with aspiration pneumonia.J Clin Epidemiol.2004;57(5):522–532.
- ,,, et al.Comorbid disease and the effect of race and ethnicity on in‐hospital mortality from aspiration pneumonia.J Natl Med Assoc.2004;96(11):1462–1469.
- Ministry of Health, Labour and Welfare, Japan. Health Statistics in Japan 2007. Available at: http://www.mhlw.go.jp/english/database/db‐hss/hs2007.html. Accessed August 18,2010.
- ,.Aspiration pneumonia and dysphagia in the elderly.Chest.2003;124(1):328–336.
- ,.Hyponatraemia in the elderly.Age Ageing.1983;12(1):77–80.
- ,.Frequency and significance of electrolyte abnormalities in pneumonia.Indian Pediatr.1992;29(6):735–740.
- ,,, et al.Hyponatremia and hospital outcomes among patients with pneumonia: a retrospective cohort study.BMC Pulm Med.2008;8(2):16.
- ,,,.Hyponatremia in community‐acquired pneumonia.Am J Nephrol.2007;27(2):184–190.
- ,,,.Hyponatremia in pediatric community‐acquired pneumonia.Pediatr Nephrol.2008;23(12):2247–2253.
- ,,, et al.Epidemiology and clinical outcomes of community‐acquired pneumonia in adult patients in Asian countries: a prospective study by the Asian network for surveillance of resistant pathogens.Int J Antimicrob Agents.2008;31(2):107–114.
- ,.The syndrome of inappropriate secretion of antidiuretic hormone.Am J Med.1967;42(5):790–806.
- ,.Clinical practice. The syndrome of inappropriate antidiuresis.N Engl J Med.2007;356(20):2064–2072.
- ,,.Hyponatraemia and the inappropriate ADH syndrome in pneumonia.Ann Trop Paediatr.1992;12(4):455–462.
- ,,, et al.Value of fractional uric acid excretion in differential diagnosis of hyponatremic patients on diuretics.J Clin Endocrinol Metab.2008;93(8):2991–2997.
- ,.The syndrome of inappropriate antidiuretic hormone: prevalence, causes and consequences.Eur J Endocrinol.2010;162(suppl 1):S5–S12.
- ,,, et al.A prediction rule to identify low‐risk patients with community‐acquired pneumonia.N Engl J Med.1997;336(4):243–250.
- ,,, et al.Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study.Thorax.2003;58(5):377–382.
- ,,, et al.Comparison of severity scoring systems A‐DROP and CURB‐65 for community‐acquired pneumonia.Respirology.2008;13(5):731–735.
- ,,.Mortality after hospitalization with mild, moderate, and severe hyponatremia.Am J Med.2009;122(9):857–865.
- .The role of vasopressin in congestive heart failure.Cleve Clin J Med.2006;73(suppl 3):S19–S23.
- .Vasopressin antagonists—progress and promise.N Engl J Med.2006;355(20):2146–2148.
- ,,, et al.A novel vasopressin dual V1A/V2 receptor antagonist, conivaptan hydrochloride, improves hyponatremia in rats with syndrome of inappropriate secretion of antidiuretic hormone (SIADH).Biol Pharm Bull.2007;30(1):91–95.
- ,.Clinical laboratory evaluation of the syndrome of inappropriate secretion of antidiuretic hormone.Clin J Am Soc Nephrol.2008;3(4):1175–1184.
- ,,,,,.Danger of central pontine myelinolysis in hypotonic dehydration and recommendation for treatment.Am J Med Sci.1989;298(1):41–43.
- ,.Treatment of hyponatremia: a quantitative analysis.Am J Kidney Dis.1993;21(4):439–443.
- ,,, et al.Hospital discharge abstract data on comorbidity improved the prediction of death among patients hospitalized with aspiration pneumonia.J Clin Epidemiol.2004;57(5):522–532.
- ,,, et al.Comorbid disease and the effect of race and ethnicity on in‐hospital mortality from aspiration pneumonia.J Natl Med Assoc.2004;96(11):1462–1469.
- Ministry of Health, Labour and Welfare, Japan. Health Statistics in Japan 2007. Available at: http://www.mhlw.go.jp/english/database/db‐hss/hs2007.html. Accessed August 18,2010.
Copyright © 2012 Society of Hospital Medicine
Survey of Academic Hospitalist Leaders
Hospitalists are hospital‐based physicians whose primary professional focus is patient care, education, research, and administrative activities related to hospital medicine.1 Initially, community‐based hospitals were far more likely to employ hospitalists than academic centers. However, today most academic centers employ hospitalist models and it is now a fully recognized entity in academic settings.2
While much has been written about the structure, business operations, and potential benefits of nonteaching (clinical) hospitalist programs,3, 4 there is little known about the current state of academic hospitalist programs or their challenges. For example, who are the leaders of academic hospitalist medicine groups? Given the youth of the field, are academic hospitalists receiving adequate mentorship and are they advancing academically? What are future directions and goals for academic hospitalist groups?
To better understand academic hospitalist programs, we surveyed division chiefs and academic hospitalist leaders to explore existing business models and operations, the status of mentorship, and key issues in growth and retention.
Methods
Sites and Subjects
We targeted potential hospital medicine group leaders by identifying academic medical centers using Association of American Medical Colleges (AAMC), the Accreditation Council for Graduate Medical Education (ACGME), the Association of Chiefs of General Internal Medicine (ACGIM), and the Society of Hospital Medicine (SHM) lists of sites with teaching missions. We then used publicly available data (eg, from websites maintained by the sites) to identify physician leaders who: 1) self identified as a leader of a hospitalist group at an academic medical center (or a Chief of Division of General Internal Medicine which managed a hospitalist group) in the SHM database, 2) were listed as such on the website, or 3) were members of ACGIM and listed as a hospitalist group leader at a university based medical center.
Survey Development
Our survey was based on questions used in previous research by the authors,5 with additional questions regarding operations of academic hospitalist programs, growth and retention of hospitalists, and mentorship developed by the study authors. Questions were pretested among a selected group of members of the Society of General Internal Medicine (SGIM) Academic Hospitalist Task Force and the SHM Academic Hospitalist Interest Group, after which the survey was refined and converted into its electronic form.
Survey Methods
The email survey process began in April 2007 with an initial survey sent to those physicians identified using preexisting data, as described. Our survey asked first if recipients were directly responsible for the oversight of a hospitalist group (eg, the division chief or director of the hospital medicine group) and if they practiced at an academic medical center. Only respondents who answered yes to both of these criteria were invited to respond to our survey. Those who felt the survey did not apply to them were invited to forward the email survey on to the appropriate person at their site or respond that their hospital had no hospital medicine service. Subsequent reminder emails were sent to nonrespondents at 10‐day intervals up to a total of four times. This survey was granted exempt status from the UCSF Institutional Review Board.
Statistical Methods
Response rates and frequencies and distribution of survey responses were analyzed using univariable statistics.
Results
Characteristics of Responding Sites
We received responses from 57 (40%) of the academic sites identified as having an academic hospital medicine group. Hospitalist group leaders at responding sites had been in their current position 3.8 years, graduated medical school approximately 15 years prior, and were either Assistant (40%), Associate (32%), or Full Professors (23%). Group leaders reported that the vast majority (91%) of group full‐time members were in junior faculty positions (Instructor or Assistant Professor), who were working full‐time. On average, responding programs were 6 years old (formed in 2001) and currently had 10.0 total full time equivalents (FTEs). A total of 38 of the groups (67%) were part of the larger Division of General Internal Medicine, whereas 9 groups (16 %) were their own division within the Department of Medicine. The remaining 17% were part of another division.
Mentorship Practices In Academic Hospital Medicine Groups
As one mechanism of mentorship, annual performance reviews were offered in most programs (88%). These were usually performed by the general medicine division chief or hospitalist leader. Mentoring relationships for clinician investigators (CI) were most often from personnel outside the hospitalist group, whereas clinician‐educators (CE) most often were mentored by faculty inside the group.
Hospitalist Leaders' Priorities and Impressions of Growth, Opportunities, Career Development and Barriers
Hospitalist leaders reported the highest priorities for hospitalist leaders were developing research and teaching programs, and minimizing turnover. Other priorities included achieving financial stability, applying for extramural funding, and reducing clinical workload (Table 2). Only 14% of respondents noted that becoming a separate division was a priority.0
| Characteristic | n (%) |
|---|---|
| |
| Group leader characteristics | |
| Academic rank | |
| Assistant professor/other | 26 (45) |
| Associate professor | 18 (32) |
| Full professor | 13 (23) |
| Years in position (mean, range) | 3.8 (2.07.0) |
| Group characteristics | |
| Hospital medicine place in school of medicine | |
| Within the department of medicine | 55 (98) |
| Separate division | 9 (16) |
| Within division of general medicine | 38 (67) |
| Other | 9 (16) |
| Not in the department of medicine | 1 (2) |
| Program size (mean, range) | |
| Number of hospitalists in program | 10 (718) |
| Number of FTE | 11 (3.512) |
| FTE's hired in past 2 years (July 2005 to survey date) | 4.0 (2.27.0) |
| Hospitalist activities | |
| Medicine consultation | 52 (91) |
| Quality improvement projects | 52 (91) |
| Nonteaching attending | 44 (77) |
| Comanagement of surgical patients | 44 (77) |
| 24‐hour coverage | 24 (61) |
| Manage patient transfer requests | 32 (56) |
| Peer review/morbidity and mortality | 31 (54) |
| Education program leadership | 29 (51) |
| Medical student program leadership | 29 (51) |
| Palliative care program | 23 (40) |
| Preoperative clinic | 23 (40) |
| Emergency department triage | 14 (25) |
| Post discharge follow‐up clinic | 13 (23) |
| Skill nursing facility coverage | 4 (7) |
| Other | 15 (26) |
| Mentorship Activity | n (%) |
|---|---|
| Programs performing annual reviews with faculty | 50 (88) |
| Who performs the annual review? | |
| General Medicine Division Chief | 9 (18) |
| Hospitalist leader | 18 (36) |
| Both | 13 (26) |
| Other (eg, Department Chair, Chief Medical Officer) | 10 (20) |
| Who is the primary source of mentorship for clinician‐educators? | |
| Senior faculty within the group | 43 (77) |
| Generalist faculty outside the group, but within the institution | 6 (11) |
| Subspecialty Internal Medicine faculty outside the group, but within the institution | 3 (5) |
| Non‐Internal Medicine (eg, surgeon, epidemiologist) outside the group, but within the institution | 0 (0) |
| Faculty from another institution | 0 (0) |
| Don't know | 4 (7) |
| Who is the primary source of mentorship for clinician‐investigators? | |
| Senior faculty within the group | 6 (12) |
| Generalist faculty outside the group, but within the institution | 13 (25) |
| Subspecialty internal medicine faculty outside the group, but within the institution | 6 (12) |
| Non‐Internal Medicine (eg, surgeon, epidemiologist) outside the group, but within the institution | 2 (4) |
| Faculty from another institution | 3 (6) |
| Don't know | 2 (4) |
| Not applicable; no clinician investigators | 20 (38) |
| Highest Priority, n (%) | Intermediate Priority, n (%) | Lowest Priority, n (%) | Not a Priority, n (%) | NA, n (%) | |
|---|---|---|---|---|---|
| Reducing individual faculty clinical workload | 9 (16) | 22 (3) | 11 (2) | 14 (2) | 0 (0) |
| Achieving financial stability | 13 (24) | 30 (55) | 6 (11) | 6 (11) | 0 (0) |
| Minimizing turnover | 22 (39) | 27 (48) | 6 (11) | 1 (2) | 0 (0) |
| Developing teaching programs | 22 (39) | 29 (52) | 3 (5) | 2 (4) | 0 (0) |
| Becoming a separate division | 3 (5) | 5 (9) | 11 (20) | 23 (41) | 14 (25) |
| Developing research | 25 (45) | 18 (32) | 5 (9) | 6 (11) | 2 (4) |
| Applying for extramural funding | 10 (18) | 24 (43) | 10 (18) | 8 (14) | 4 (7) |
| Strongly Agree, n (%) | Agree, n (%) | Neutral, n (%) | Disagree, n (%) | Strongly Disagree, n (%) | NA, n (%) | |
|---|---|---|---|---|---|---|
| Growth and sustainability | ||||||
| Availability of funds is limiting expansion of academic functions (eg, education and research) | 20 (36) | 21 (38) | 5 (9) | 7 (12) | 3 (5) | 0 (0) |
| Availability of funds is limiting expansion of clinical functions (eg, development of new services) | 11 (20) | 17 (30) | 14 (25) | 10 (18) | 4 (7) | 0 (0) |
| My faculty are developing sustainable nonclinical activities | 9 (16) | 23 (41) | 12 (21) | 9 (16) | 3 (5) | 0 (0) |
| Career development | ||||||
| Mentorship is a major issue for my clinician‐educator faculty | 14 (25) | 28 (50) | 7 (12) | 4 (7) | 1 (2) | 2 (4) |
| Mentorship is a major issue for my research faculty | 22 (40) | 10 (18) | 4 (7) | 3 (5) | 2 (4) | 14 (25) |
| External support for hospital medicine group | ||||||
| There is investment in the development of academic functions of our hospitalist program from my hospital | 4 (7) | 12 (21) | 10 (18) | 22 (39) | 8 (14) | 0 (0) |
| There is investment in the development of academic functions of our hospitalist program from the Department of Medicine | 22 (40) | 17 (31) | 8 (15) | 4 (7) | 2 (4) | 2 (4) |
In general, academic hospitalist leaders reported that Departments of Medicine and Divisions of General Medicine (where applicable) were invested in the development of their academic functions. Yet, more than half of program directors reported that hospitals were not supportive. Moreover, lack of funds limited the expansion of their academic or clinical functions (Table 3). Additionally, while the majority either strongly agree or agree that their faculty are developing sustainable nonclinical activities (57%), they perceive that they are at risk for burnout (69%), and that lack of mentorship is a major issue for both CE (75%) and research faculty (58%). Lastly, while program directors strongly agree or agree (71%) that their hospitalist groups are respected by other academic physicians, they additionally strongly agree or agree that their Departments of Medicine (58%) and other Divisions (78%) view their hospitalist program as a clinical service rather than an academic program.
Discussion
Our survey provides a unique snapshot of academic hospitalist groups, highlighting a perceived lack of support and respect for their programs, a need to increase education and scholarly activities, and a desire to better prepare faculty for academic promotion.
Academic hospitalist groups and leaders reflected what one would expect from a field that is just over a decade old. Program leaders were relatively new to their position, as were their division group members. As a result, it is not surprising that most of the academic hospitalist leaders identified mentorship as a major issue. We were encouraged to see that most programs were offering annual reviews. However, the majority of these annual reviews were performed by the group leaders, many of whom are relatively junior (40% Assistant Professors) and may not be experienced in mentoring and performing annual reviews. Importantly, the absence of a mentor (or a high‐quality, experienced one) among physicians, and specifically hospitalists, may result in fewer peer‐reviewed first author and non‐peer‐reviewed publications, and less experience leading a teaching session at a national meeting.6 Research suggests that effective mentoring may help faculty increase career satisfaction and productivity and reduce their risk for burn‐out.7 Hospitalist groups might benefit nationally from focusing specifically on finding adequate mentorship either within or outside their groups. In addition, national organizations such as the SHM and the SGIM could potentially help these groups and individual hospitalists in creating mentorship networks and a mentoring infrastructure.
Academic hospitalist leaders were concerned about the ability of their faculty to develop sustainable nonclinical activities and scholarship. Notably, more than 40% of surveyed leaders agreed or strongly agreed that their faculty were not developing sustainable nonclinical activities. For individual faculty, the inability to develop scholarly activities and engage in academic pursuits may create challenges in getting promoted by traditional academic pathways. Some have recognized this issue and tried to develop practical solutions.2 In addition, academic hospitalists often engage in nonclinical activities such as quality improvement or patient safety which do not fit in the traditional tripartite mission of academics (clinical care, education, and research). In this survey, more than 90% of groups were engaged in quality improvement projects and over half in peer review exercises (Table 1). As many of these scholarly activities require innovation, sophisticated data analysis, and can have far‐reaching and substantial impacts on healthcare, some have argued these should be considered as part of the promotion process.8 Notably, the SGIM Academic Hospitalist Taskforce has created the Quality Portfolio, a structured adjunct to promotions packets to organize and document work in quality improvement and patient safety.9
While there were few CI in the divisions surveyed, building CI programs was a major priority of programs. In programs reporting the presence of CI's, they report limited access to research support. This highlights the potential role and benefit of post residency training in designing and conducting clinical research whether in a traditional general internal medicine fellowship or in 1 of the many growing hospital medicine fellowships.10 There also appears to be a need for funding to support the research careers of junior hospitalists. While access to effective mentorship is integrally linked to achieving increased academic accomplishments, there is certainly an ample call for research in the areas of quality improvement, patient safety; systems‐based practice, hospital efficiency, transitions of care,11 perioperative medicine,12 and education.2, 13, 14 While providing lower costs per admission and lower lengths of stay, hospitalists seem well‐positioned to spearhead active research in cost‐effectiveness in the hospital.14 Additionally, a quality portfolio, documenting such quality improvement projects, has been suggested as an effective means to provide a record of this work for academic promotion.9
The diverse activities of today's hospitalists are transforming the traditional view of academic work and are critical to the growth of hospitals, patient care, and development of the field of hospital medicine itself. Until these areas are fully embraced as legitimate areas of academic productivity and scholarship, the academic advancement of hospitalists will be slow.
It is unclear from our survey if academic hospitalist programs are truly getting the support they need to succeed. On one hand, there was general agreement that the Departments of Medicine and Divisions of General and Hospital Medicine were invested in the development of the academic accomplishments. Yet, the majority of program directors believed that they are viewed by the Department or Division as a clinical rather than an academic program. Moreover, over half of program directors report that their hospital was not supportive and therefore have limited the expansion of their hospitalist groups' educational and research activities. Lastly, for a large majority of programs, unavailable funding also acted to limit growth and expansion of academic functions. In a mere 2 decades, Emergency Medicine has become one of the largest US specialties and yet research and funding in the field have been lagging and are limiting academic expansion. Junior faculty seeking research careers struggled to find support and mentorship within their emergency medicine divisions.15 Challenges faced by academic emergency medicine provide important historical perspective for the even more rapidly growing field of academic hospital medicine. Learning from the Academic Emergency Medicine experience, academic hospitalists should proactively identify scholarship and research opportunities unique to hospitalist and fitting the needs of academic institutions. Involvement in national medical organizations, such as SGIM‐SHM‐ACGIM Academic Hospitalist Academy, or the SGIM Academic Hospitalist Task Force, where skill and career development is the focus, will undoubtedly promote the success of academic hospitalist. Expanding valuable niches of expertise, such as quality control, perioperative medicine and care transitions, create an indispensible component of hospital care. Lastly mentoring programs for academic hospitalist within SHM and SGIM are also essential for networking and career development. There are several limitations to our study. Our response rate of 40% was relatively low, and our results may not be representative of all academic hospitalist division chiefs and their programs, may be overstating the perceived difficulties of the survey sample, or conversely missing a large portion too overwhelmed by current duties who lacked the time to complete the survey. Having said this, our survey methodology targeted sites where we could identify potentialnot confirmedhospitalist groups and hospitalist group leaders. For this reason, our response rate could be higher (if some of our contacts were in error). Our results are a cross‐sectional survey based on self report and are subject to recall bias. In addition, our study was carried out in 2007, and while issues such as mentorship may remain important, our results regarding financial arrangements may not be applicable to the current economic climate. Finally, while improving mentorship was identified as a principle objective for program leaders, we did not explore the existing quality of mentorship, nor perceived shortfalls. This should be the subject of future exploration.
The vast majority of academic hospital medicine programs continue to view inadequate support, expanding research, mentorship, and academic promotion as critical issues for the future. Thus, further understanding of these features, and interventions to allow for success, are of crucial importance in the continued development of academic hospitalists. Our study supports the need for mentoring and career development programs, targeting academic hospitalists and their leaders. In addition, attention should be paid to activities that support career fit, creating sustainable and viable job descriptions for academic hospitalists, and preventing burnout.16 At the same time we must expand the traditional view of scholarship and training and advocate for promotion criteria that value the unique contributions of hospitalists to become in line with the broad areas that hospitalists work.
- Anonymous. Definition of a hospitalist.2009. Society of Hospital Medicine Homepage/General Information. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Hospitalist_Definition4:240–246.
- ,,,,.Resource‐based relative value scale analysis between teaching and nonteaching hospitalist services.Health Care Management.2009;1(28):81–85.
- ,,, et al.Effects of physician experience on costs and outcomes on an academic general medicine service.Ann Intern Med.2002;137:866–874.
- ,,,.Hospitalists and the practice of inpatient medicine.Ann Intern Med.1999;130:343–349.
- ,,,,,.Rates, predictors and consequences of low career satisfaction and burnout in academic hospital medicine.J Hosp Med.2009;4(S1):24–25.
- ,.Mentoring faculty in academic medicine: a new paradigm?.J Gen Intern Med.2005;20(9):866–870.
- ,.Clinicians in quality improvement: a new career pathway in academic medicine.JAMA.2009;301(7):766–768.
- ,,,.Academic hospitalist taskforce quality portfolio rationale and development. 02/23/2009; Quality portfolio introduction. Available at:http://www.sgim.org/index.cfm?pageId=846. Accessed July 2010.
- ,,,.Hospital medicine fellowships: works in progress.Am J Med.2006;119:72e1–72e7.
- ,,,.Promoting effective care transitions of hospital discharge. A review of key issues for hospitalists.J. Hosp Med.2007;2:314–323.
- ,.Hospitalists and anesthesiologists as perioperative physicians: are their roles complementary?Proc (Bayl Univ Med Cent).2007;20(2):140–142.
- .A systematic review of outcomes and quality measures in adult patients cared for by hospitalists vs nonhospitalist.Mayo Clin Proc.2009;84(3):248–254.
- .Reflections: the hospitalist movement a decade later.J Hosp Med.2006;1:248–252.
- .Anyone, Anything, Anytime A History of Emergency Medicine.1st ed.Philadelphia, PA:Mosby‐Elsevier;2006.
- ,,, et al.Career fit and burnout among academic faculty.Arch Intern Med.2009;169(10):990–995.
Hospitalists are hospital‐based physicians whose primary professional focus is patient care, education, research, and administrative activities related to hospital medicine.1 Initially, community‐based hospitals were far more likely to employ hospitalists than academic centers. However, today most academic centers employ hospitalist models and it is now a fully recognized entity in academic settings.2
While much has been written about the structure, business operations, and potential benefits of nonteaching (clinical) hospitalist programs,3, 4 there is little known about the current state of academic hospitalist programs or their challenges. For example, who are the leaders of academic hospitalist medicine groups? Given the youth of the field, are academic hospitalists receiving adequate mentorship and are they advancing academically? What are future directions and goals for academic hospitalist groups?
To better understand academic hospitalist programs, we surveyed division chiefs and academic hospitalist leaders to explore existing business models and operations, the status of mentorship, and key issues in growth and retention.
Methods
Sites and Subjects
We targeted potential hospital medicine group leaders by identifying academic medical centers using Association of American Medical Colleges (AAMC), the Accreditation Council for Graduate Medical Education (ACGME), the Association of Chiefs of General Internal Medicine (ACGIM), and the Society of Hospital Medicine (SHM) lists of sites with teaching missions. We then used publicly available data (eg, from websites maintained by the sites) to identify physician leaders who: 1) self identified as a leader of a hospitalist group at an academic medical center (or a Chief of Division of General Internal Medicine which managed a hospitalist group) in the SHM database, 2) were listed as such on the website, or 3) were members of ACGIM and listed as a hospitalist group leader at a university based medical center.
Survey Development
Our survey was based on questions used in previous research by the authors,5 with additional questions regarding operations of academic hospitalist programs, growth and retention of hospitalists, and mentorship developed by the study authors. Questions were pretested among a selected group of members of the Society of General Internal Medicine (SGIM) Academic Hospitalist Task Force and the SHM Academic Hospitalist Interest Group, after which the survey was refined and converted into its electronic form.
Survey Methods
The email survey process began in April 2007 with an initial survey sent to those physicians identified using preexisting data, as described. Our survey asked first if recipients were directly responsible for the oversight of a hospitalist group (eg, the division chief or director of the hospital medicine group) and if they practiced at an academic medical center. Only respondents who answered yes to both of these criteria were invited to respond to our survey. Those who felt the survey did not apply to them were invited to forward the email survey on to the appropriate person at their site or respond that their hospital had no hospital medicine service. Subsequent reminder emails were sent to nonrespondents at 10‐day intervals up to a total of four times. This survey was granted exempt status from the UCSF Institutional Review Board.
Statistical Methods
Response rates and frequencies and distribution of survey responses were analyzed using univariable statistics.
Results
Characteristics of Responding Sites
We received responses from 57 (40%) of the academic sites identified as having an academic hospital medicine group. Hospitalist group leaders at responding sites had been in their current position 3.8 years, graduated medical school approximately 15 years prior, and were either Assistant (40%), Associate (32%), or Full Professors (23%). Group leaders reported that the vast majority (91%) of group full‐time members were in junior faculty positions (Instructor or Assistant Professor), who were working full‐time. On average, responding programs were 6 years old (formed in 2001) and currently had 10.0 total full time equivalents (FTEs). A total of 38 of the groups (67%) were part of the larger Division of General Internal Medicine, whereas 9 groups (16 %) were their own division within the Department of Medicine. The remaining 17% were part of another division.
Mentorship Practices In Academic Hospital Medicine Groups
As one mechanism of mentorship, annual performance reviews were offered in most programs (88%). These were usually performed by the general medicine division chief or hospitalist leader. Mentoring relationships for clinician investigators (CI) were most often from personnel outside the hospitalist group, whereas clinician‐educators (CE) most often were mentored by faculty inside the group.
Hospitalist Leaders' Priorities and Impressions of Growth, Opportunities, Career Development and Barriers
Hospitalist leaders reported the highest priorities for hospitalist leaders were developing research and teaching programs, and minimizing turnover. Other priorities included achieving financial stability, applying for extramural funding, and reducing clinical workload (Table 2). Only 14% of respondents noted that becoming a separate division was a priority.0
| Characteristic | n (%) |
|---|---|
| |
| Group leader characteristics | |
| Academic rank | |
| Assistant professor/other | 26 (45) |
| Associate professor | 18 (32) |
| Full professor | 13 (23) |
| Years in position (mean, range) | 3.8 (2.07.0) |
| Group characteristics | |
| Hospital medicine place in school of medicine | |
| Within the department of medicine | 55 (98) |
| Separate division | 9 (16) |
| Within division of general medicine | 38 (67) |
| Other | 9 (16) |
| Not in the department of medicine | 1 (2) |
| Program size (mean, range) | |
| Number of hospitalists in program | 10 (718) |
| Number of FTE | 11 (3.512) |
| FTE's hired in past 2 years (July 2005 to survey date) | 4.0 (2.27.0) |
| Hospitalist activities | |
| Medicine consultation | 52 (91) |
| Quality improvement projects | 52 (91) |
| Nonteaching attending | 44 (77) |
| Comanagement of surgical patients | 44 (77) |
| 24‐hour coverage | 24 (61) |
| Manage patient transfer requests | 32 (56) |
| Peer review/morbidity and mortality | 31 (54) |
| Education program leadership | 29 (51) |
| Medical student program leadership | 29 (51) |
| Palliative care program | 23 (40) |
| Preoperative clinic | 23 (40) |
| Emergency department triage | 14 (25) |
| Post discharge follow‐up clinic | 13 (23) |
| Skill nursing facility coverage | 4 (7) |
| Other | 15 (26) |
| Mentorship Activity | n (%) |
|---|---|
| Programs performing annual reviews with faculty | 50 (88) |
| Who performs the annual review? | |
| General Medicine Division Chief | 9 (18) |
| Hospitalist leader | 18 (36) |
| Both | 13 (26) |
| Other (eg, Department Chair, Chief Medical Officer) | 10 (20) |
| Who is the primary source of mentorship for clinician‐educators? | |
| Senior faculty within the group | 43 (77) |
| Generalist faculty outside the group, but within the institution | 6 (11) |
| Subspecialty Internal Medicine faculty outside the group, but within the institution | 3 (5) |
| Non‐Internal Medicine (eg, surgeon, epidemiologist) outside the group, but within the institution | 0 (0) |
| Faculty from another institution | 0 (0) |
| Don't know | 4 (7) |
| Who is the primary source of mentorship for clinician‐investigators? | |
| Senior faculty within the group | 6 (12) |
| Generalist faculty outside the group, but within the institution | 13 (25) |
| Subspecialty internal medicine faculty outside the group, but within the institution | 6 (12) |
| Non‐Internal Medicine (eg, surgeon, epidemiologist) outside the group, but within the institution | 2 (4) |
| Faculty from another institution | 3 (6) |
| Don't know | 2 (4) |
| Not applicable; no clinician investigators | 20 (38) |
| Highest Priority, n (%) | Intermediate Priority, n (%) | Lowest Priority, n (%) | Not a Priority, n (%) | NA, n (%) | |
|---|---|---|---|---|---|
| Reducing individual faculty clinical workload | 9 (16) | 22 (3) | 11 (2) | 14 (2) | 0 (0) |
| Achieving financial stability | 13 (24) | 30 (55) | 6 (11) | 6 (11) | 0 (0) |
| Minimizing turnover | 22 (39) | 27 (48) | 6 (11) | 1 (2) | 0 (0) |
| Developing teaching programs | 22 (39) | 29 (52) | 3 (5) | 2 (4) | 0 (0) |
| Becoming a separate division | 3 (5) | 5 (9) | 11 (20) | 23 (41) | 14 (25) |
| Developing research | 25 (45) | 18 (32) | 5 (9) | 6 (11) | 2 (4) |
| Applying for extramural funding | 10 (18) | 24 (43) | 10 (18) | 8 (14) | 4 (7) |
| Strongly Agree, n (%) | Agree, n (%) | Neutral, n (%) | Disagree, n (%) | Strongly Disagree, n (%) | NA, n (%) | |
|---|---|---|---|---|---|---|
| Growth and sustainability | ||||||
| Availability of funds is limiting expansion of academic functions (eg, education and research) | 20 (36) | 21 (38) | 5 (9) | 7 (12) | 3 (5) | 0 (0) |
| Availability of funds is limiting expansion of clinical functions (eg, development of new services) | 11 (20) | 17 (30) | 14 (25) | 10 (18) | 4 (7) | 0 (0) |
| My faculty are developing sustainable nonclinical activities | 9 (16) | 23 (41) | 12 (21) | 9 (16) | 3 (5) | 0 (0) |
| Career development | ||||||
| Mentorship is a major issue for my clinician‐educator faculty | 14 (25) | 28 (50) | 7 (12) | 4 (7) | 1 (2) | 2 (4) |
| Mentorship is a major issue for my research faculty | 22 (40) | 10 (18) | 4 (7) | 3 (5) | 2 (4) | 14 (25) |
| External support for hospital medicine group | ||||||
| There is investment in the development of academic functions of our hospitalist program from my hospital | 4 (7) | 12 (21) | 10 (18) | 22 (39) | 8 (14) | 0 (0) |
| There is investment in the development of academic functions of our hospitalist program from the Department of Medicine | 22 (40) | 17 (31) | 8 (15) | 4 (7) | 2 (4) | 2 (4) |
In general, academic hospitalist leaders reported that Departments of Medicine and Divisions of General Medicine (where applicable) were invested in the development of their academic functions. Yet, more than half of program directors reported that hospitals were not supportive. Moreover, lack of funds limited the expansion of their academic or clinical functions (Table 3). Additionally, while the majority either strongly agree or agree that their faculty are developing sustainable nonclinical activities (57%), they perceive that they are at risk for burnout (69%), and that lack of mentorship is a major issue for both CE (75%) and research faculty (58%). Lastly, while program directors strongly agree or agree (71%) that their hospitalist groups are respected by other academic physicians, they additionally strongly agree or agree that their Departments of Medicine (58%) and other Divisions (78%) view their hospitalist program as a clinical service rather than an academic program.
Discussion
Our survey provides a unique snapshot of academic hospitalist groups, highlighting a perceived lack of support and respect for their programs, a need to increase education and scholarly activities, and a desire to better prepare faculty for academic promotion.
Academic hospitalist groups and leaders reflected what one would expect from a field that is just over a decade old. Program leaders were relatively new to their position, as were their division group members. As a result, it is not surprising that most of the academic hospitalist leaders identified mentorship as a major issue. We were encouraged to see that most programs were offering annual reviews. However, the majority of these annual reviews were performed by the group leaders, many of whom are relatively junior (40% Assistant Professors) and may not be experienced in mentoring and performing annual reviews. Importantly, the absence of a mentor (or a high‐quality, experienced one) among physicians, and specifically hospitalists, may result in fewer peer‐reviewed first author and non‐peer‐reviewed publications, and less experience leading a teaching session at a national meeting.6 Research suggests that effective mentoring may help faculty increase career satisfaction and productivity and reduce their risk for burn‐out.7 Hospitalist groups might benefit nationally from focusing specifically on finding adequate mentorship either within or outside their groups. In addition, national organizations such as the SHM and the SGIM could potentially help these groups and individual hospitalists in creating mentorship networks and a mentoring infrastructure.
Academic hospitalist leaders were concerned about the ability of their faculty to develop sustainable nonclinical activities and scholarship. Notably, more than 40% of surveyed leaders agreed or strongly agreed that their faculty were not developing sustainable nonclinical activities. For individual faculty, the inability to develop scholarly activities and engage in academic pursuits may create challenges in getting promoted by traditional academic pathways. Some have recognized this issue and tried to develop practical solutions.2 In addition, academic hospitalists often engage in nonclinical activities such as quality improvement or patient safety which do not fit in the traditional tripartite mission of academics (clinical care, education, and research). In this survey, more than 90% of groups were engaged in quality improvement projects and over half in peer review exercises (Table 1). As many of these scholarly activities require innovation, sophisticated data analysis, and can have far‐reaching and substantial impacts on healthcare, some have argued these should be considered as part of the promotion process.8 Notably, the SGIM Academic Hospitalist Taskforce has created the Quality Portfolio, a structured adjunct to promotions packets to organize and document work in quality improvement and patient safety.9
While there were few CI in the divisions surveyed, building CI programs was a major priority of programs. In programs reporting the presence of CI's, they report limited access to research support. This highlights the potential role and benefit of post residency training in designing and conducting clinical research whether in a traditional general internal medicine fellowship or in 1 of the many growing hospital medicine fellowships.10 There also appears to be a need for funding to support the research careers of junior hospitalists. While access to effective mentorship is integrally linked to achieving increased academic accomplishments, there is certainly an ample call for research in the areas of quality improvement, patient safety; systems‐based practice, hospital efficiency, transitions of care,11 perioperative medicine,12 and education.2, 13, 14 While providing lower costs per admission and lower lengths of stay, hospitalists seem well‐positioned to spearhead active research in cost‐effectiveness in the hospital.14 Additionally, a quality portfolio, documenting such quality improvement projects, has been suggested as an effective means to provide a record of this work for academic promotion.9
The diverse activities of today's hospitalists are transforming the traditional view of academic work and are critical to the growth of hospitals, patient care, and development of the field of hospital medicine itself. Until these areas are fully embraced as legitimate areas of academic productivity and scholarship, the academic advancement of hospitalists will be slow.
It is unclear from our survey if academic hospitalist programs are truly getting the support they need to succeed. On one hand, there was general agreement that the Departments of Medicine and Divisions of General and Hospital Medicine were invested in the development of the academic accomplishments. Yet, the majority of program directors believed that they are viewed by the Department or Division as a clinical rather than an academic program. Moreover, over half of program directors report that their hospital was not supportive and therefore have limited the expansion of their hospitalist groups' educational and research activities. Lastly, for a large majority of programs, unavailable funding also acted to limit growth and expansion of academic functions. In a mere 2 decades, Emergency Medicine has become one of the largest US specialties and yet research and funding in the field have been lagging and are limiting academic expansion. Junior faculty seeking research careers struggled to find support and mentorship within their emergency medicine divisions.15 Challenges faced by academic emergency medicine provide important historical perspective for the even more rapidly growing field of academic hospital medicine. Learning from the Academic Emergency Medicine experience, academic hospitalists should proactively identify scholarship and research opportunities unique to hospitalist and fitting the needs of academic institutions. Involvement in national medical organizations, such as SGIM‐SHM‐ACGIM Academic Hospitalist Academy, or the SGIM Academic Hospitalist Task Force, where skill and career development is the focus, will undoubtedly promote the success of academic hospitalist. Expanding valuable niches of expertise, such as quality control, perioperative medicine and care transitions, create an indispensible component of hospital care. Lastly mentoring programs for academic hospitalist within SHM and SGIM are also essential for networking and career development. There are several limitations to our study. Our response rate of 40% was relatively low, and our results may not be representative of all academic hospitalist division chiefs and their programs, may be overstating the perceived difficulties of the survey sample, or conversely missing a large portion too overwhelmed by current duties who lacked the time to complete the survey. Having said this, our survey methodology targeted sites where we could identify potentialnot confirmedhospitalist groups and hospitalist group leaders. For this reason, our response rate could be higher (if some of our contacts were in error). Our results are a cross‐sectional survey based on self report and are subject to recall bias. In addition, our study was carried out in 2007, and while issues such as mentorship may remain important, our results regarding financial arrangements may not be applicable to the current economic climate. Finally, while improving mentorship was identified as a principle objective for program leaders, we did not explore the existing quality of mentorship, nor perceived shortfalls. This should be the subject of future exploration.
The vast majority of academic hospital medicine programs continue to view inadequate support, expanding research, mentorship, and academic promotion as critical issues for the future. Thus, further understanding of these features, and interventions to allow for success, are of crucial importance in the continued development of academic hospitalists. Our study supports the need for mentoring and career development programs, targeting academic hospitalists and their leaders. In addition, attention should be paid to activities that support career fit, creating sustainable and viable job descriptions for academic hospitalists, and preventing burnout.16 At the same time we must expand the traditional view of scholarship and training and advocate for promotion criteria that value the unique contributions of hospitalists to become in line with the broad areas that hospitalists work.
Hospitalists are hospital‐based physicians whose primary professional focus is patient care, education, research, and administrative activities related to hospital medicine.1 Initially, community‐based hospitals were far more likely to employ hospitalists than academic centers. However, today most academic centers employ hospitalist models and it is now a fully recognized entity in academic settings.2
While much has been written about the structure, business operations, and potential benefits of nonteaching (clinical) hospitalist programs,3, 4 there is little known about the current state of academic hospitalist programs or their challenges. For example, who are the leaders of academic hospitalist medicine groups? Given the youth of the field, are academic hospitalists receiving adequate mentorship and are they advancing academically? What are future directions and goals for academic hospitalist groups?
To better understand academic hospitalist programs, we surveyed division chiefs and academic hospitalist leaders to explore existing business models and operations, the status of mentorship, and key issues in growth and retention.
Methods
Sites and Subjects
We targeted potential hospital medicine group leaders by identifying academic medical centers using Association of American Medical Colleges (AAMC), the Accreditation Council for Graduate Medical Education (ACGME), the Association of Chiefs of General Internal Medicine (ACGIM), and the Society of Hospital Medicine (SHM) lists of sites with teaching missions. We then used publicly available data (eg, from websites maintained by the sites) to identify physician leaders who: 1) self identified as a leader of a hospitalist group at an academic medical center (or a Chief of Division of General Internal Medicine which managed a hospitalist group) in the SHM database, 2) were listed as such on the website, or 3) were members of ACGIM and listed as a hospitalist group leader at a university based medical center.
Survey Development
Our survey was based on questions used in previous research by the authors,5 with additional questions regarding operations of academic hospitalist programs, growth and retention of hospitalists, and mentorship developed by the study authors. Questions were pretested among a selected group of members of the Society of General Internal Medicine (SGIM) Academic Hospitalist Task Force and the SHM Academic Hospitalist Interest Group, after which the survey was refined and converted into its electronic form.
Survey Methods
The email survey process began in April 2007 with an initial survey sent to those physicians identified using preexisting data, as described. Our survey asked first if recipients were directly responsible for the oversight of a hospitalist group (eg, the division chief or director of the hospital medicine group) and if they practiced at an academic medical center. Only respondents who answered yes to both of these criteria were invited to respond to our survey. Those who felt the survey did not apply to them were invited to forward the email survey on to the appropriate person at their site or respond that their hospital had no hospital medicine service. Subsequent reminder emails were sent to nonrespondents at 10‐day intervals up to a total of four times. This survey was granted exempt status from the UCSF Institutional Review Board.
Statistical Methods
Response rates and frequencies and distribution of survey responses were analyzed using univariable statistics.
Results
Characteristics of Responding Sites
We received responses from 57 (40%) of the academic sites identified as having an academic hospital medicine group. Hospitalist group leaders at responding sites had been in their current position 3.8 years, graduated medical school approximately 15 years prior, and were either Assistant (40%), Associate (32%), or Full Professors (23%). Group leaders reported that the vast majority (91%) of group full‐time members were in junior faculty positions (Instructor or Assistant Professor), who were working full‐time. On average, responding programs were 6 years old (formed in 2001) and currently had 10.0 total full time equivalents (FTEs). A total of 38 of the groups (67%) were part of the larger Division of General Internal Medicine, whereas 9 groups (16 %) were their own division within the Department of Medicine. The remaining 17% were part of another division.
Mentorship Practices In Academic Hospital Medicine Groups
As one mechanism of mentorship, annual performance reviews were offered in most programs (88%). These were usually performed by the general medicine division chief or hospitalist leader. Mentoring relationships for clinician investigators (CI) were most often from personnel outside the hospitalist group, whereas clinician‐educators (CE) most often were mentored by faculty inside the group.
Hospitalist Leaders' Priorities and Impressions of Growth, Opportunities, Career Development and Barriers
Hospitalist leaders reported the highest priorities for hospitalist leaders were developing research and teaching programs, and minimizing turnover. Other priorities included achieving financial stability, applying for extramural funding, and reducing clinical workload (Table 2). Only 14% of respondents noted that becoming a separate division was a priority.0
| Characteristic | n (%) |
|---|---|
| |
| Group leader characteristics | |
| Academic rank | |
| Assistant professor/other | 26 (45) |
| Associate professor | 18 (32) |
| Full professor | 13 (23) |
| Years in position (mean, range) | 3.8 (2.07.0) |
| Group characteristics | |
| Hospital medicine place in school of medicine | |
| Within the department of medicine | 55 (98) |
| Separate division | 9 (16) |
| Within division of general medicine | 38 (67) |
| Other | 9 (16) |
| Not in the department of medicine | 1 (2) |
| Program size (mean, range) | |
| Number of hospitalists in program | 10 (718) |
| Number of FTE | 11 (3.512) |
| FTE's hired in past 2 years (July 2005 to survey date) | 4.0 (2.27.0) |
| Hospitalist activities | |
| Medicine consultation | 52 (91) |
| Quality improvement projects | 52 (91) |
| Nonteaching attending | 44 (77) |
| Comanagement of surgical patients | 44 (77) |
| 24‐hour coverage | 24 (61) |
| Manage patient transfer requests | 32 (56) |
| Peer review/morbidity and mortality | 31 (54) |
| Education program leadership | 29 (51) |
| Medical student program leadership | 29 (51) |
| Palliative care program | 23 (40) |
| Preoperative clinic | 23 (40) |
| Emergency department triage | 14 (25) |
| Post discharge follow‐up clinic | 13 (23) |
| Skill nursing facility coverage | 4 (7) |
| Other | 15 (26) |
| Mentorship Activity | n (%) |
|---|---|
| Programs performing annual reviews with faculty | 50 (88) |
| Who performs the annual review? | |
| General Medicine Division Chief | 9 (18) |
| Hospitalist leader | 18 (36) |
| Both | 13 (26) |
| Other (eg, Department Chair, Chief Medical Officer) | 10 (20) |
| Who is the primary source of mentorship for clinician‐educators? | |
| Senior faculty within the group | 43 (77) |
| Generalist faculty outside the group, but within the institution | 6 (11) |
| Subspecialty Internal Medicine faculty outside the group, but within the institution | 3 (5) |
| Non‐Internal Medicine (eg, surgeon, epidemiologist) outside the group, but within the institution | 0 (0) |
| Faculty from another institution | 0 (0) |
| Don't know | 4 (7) |
| Who is the primary source of mentorship for clinician‐investigators? | |
| Senior faculty within the group | 6 (12) |
| Generalist faculty outside the group, but within the institution | 13 (25) |
| Subspecialty internal medicine faculty outside the group, but within the institution | 6 (12) |
| Non‐Internal Medicine (eg, surgeon, epidemiologist) outside the group, but within the institution | 2 (4) |
| Faculty from another institution | 3 (6) |
| Don't know | 2 (4) |
| Not applicable; no clinician investigators | 20 (38) |
| Highest Priority, n (%) | Intermediate Priority, n (%) | Lowest Priority, n (%) | Not a Priority, n (%) | NA, n (%) | |
|---|---|---|---|---|---|
| Reducing individual faculty clinical workload | 9 (16) | 22 (3) | 11 (2) | 14 (2) | 0 (0) |
| Achieving financial stability | 13 (24) | 30 (55) | 6 (11) | 6 (11) | 0 (0) |
| Minimizing turnover | 22 (39) | 27 (48) | 6 (11) | 1 (2) | 0 (0) |
| Developing teaching programs | 22 (39) | 29 (52) | 3 (5) | 2 (4) | 0 (0) |
| Becoming a separate division | 3 (5) | 5 (9) | 11 (20) | 23 (41) | 14 (25) |
| Developing research | 25 (45) | 18 (32) | 5 (9) | 6 (11) | 2 (4) |
| Applying for extramural funding | 10 (18) | 24 (43) | 10 (18) | 8 (14) | 4 (7) |
| Strongly Agree, n (%) | Agree, n (%) | Neutral, n (%) | Disagree, n (%) | Strongly Disagree, n (%) | NA, n (%) | |
|---|---|---|---|---|---|---|
| Growth and sustainability | ||||||
| Availability of funds is limiting expansion of academic functions (eg, education and research) | 20 (36) | 21 (38) | 5 (9) | 7 (12) | 3 (5) | 0 (0) |
| Availability of funds is limiting expansion of clinical functions (eg, development of new services) | 11 (20) | 17 (30) | 14 (25) | 10 (18) | 4 (7) | 0 (0) |
| My faculty are developing sustainable nonclinical activities | 9 (16) | 23 (41) | 12 (21) | 9 (16) | 3 (5) | 0 (0) |
| Career development | ||||||
| Mentorship is a major issue for my clinician‐educator faculty | 14 (25) | 28 (50) | 7 (12) | 4 (7) | 1 (2) | 2 (4) |
| Mentorship is a major issue for my research faculty | 22 (40) | 10 (18) | 4 (7) | 3 (5) | 2 (4) | 14 (25) |
| External support for hospital medicine group | ||||||
| There is investment in the development of academic functions of our hospitalist program from my hospital | 4 (7) | 12 (21) | 10 (18) | 22 (39) | 8 (14) | 0 (0) |
| There is investment in the development of academic functions of our hospitalist program from the Department of Medicine | 22 (40) | 17 (31) | 8 (15) | 4 (7) | 2 (4) | 2 (4) |
In general, academic hospitalist leaders reported that Departments of Medicine and Divisions of General Medicine (where applicable) were invested in the development of their academic functions. Yet, more than half of program directors reported that hospitals were not supportive. Moreover, lack of funds limited the expansion of their academic or clinical functions (Table 3). Additionally, while the majority either strongly agree or agree that their faculty are developing sustainable nonclinical activities (57%), they perceive that they are at risk for burnout (69%), and that lack of mentorship is a major issue for both CE (75%) and research faculty (58%). Lastly, while program directors strongly agree or agree (71%) that their hospitalist groups are respected by other academic physicians, they additionally strongly agree or agree that their Departments of Medicine (58%) and other Divisions (78%) view their hospitalist program as a clinical service rather than an academic program.
Discussion
Our survey provides a unique snapshot of academic hospitalist groups, highlighting a perceived lack of support and respect for their programs, a need to increase education and scholarly activities, and a desire to better prepare faculty for academic promotion.
Academic hospitalist groups and leaders reflected what one would expect from a field that is just over a decade old. Program leaders were relatively new to their position, as were their division group members. As a result, it is not surprising that most of the academic hospitalist leaders identified mentorship as a major issue. We were encouraged to see that most programs were offering annual reviews. However, the majority of these annual reviews were performed by the group leaders, many of whom are relatively junior (40% Assistant Professors) and may not be experienced in mentoring and performing annual reviews. Importantly, the absence of a mentor (or a high‐quality, experienced one) among physicians, and specifically hospitalists, may result in fewer peer‐reviewed first author and non‐peer‐reviewed publications, and less experience leading a teaching session at a national meeting.6 Research suggests that effective mentoring may help faculty increase career satisfaction and productivity and reduce their risk for burn‐out.7 Hospitalist groups might benefit nationally from focusing specifically on finding adequate mentorship either within or outside their groups. In addition, national organizations such as the SHM and the SGIM could potentially help these groups and individual hospitalists in creating mentorship networks and a mentoring infrastructure.
Academic hospitalist leaders were concerned about the ability of their faculty to develop sustainable nonclinical activities and scholarship. Notably, more than 40% of surveyed leaders agreed or strongly agreed that their faculty were not developing sustainable nonclinical activities. For individual faculty, the inability to develop scholarly activities and engage in academic pursuits may create challenges in getting promoted by traditional academic pathways. Some have recognized this issue and tried to develop practical solutions.2 In addition, academic hospitalists often engage in nonclinical activities such as quality improvement or patient safety which do not fit in the traditional tripartite mission of academics (clinical care, education, and research). In this survey, more than 90% of groups were engaged in quality improvement projects and over half in peer review exercises (Table 1). As many of these scholarly activities require innovation, sophisticated data analysis, and can have far‐reaching and substantial impacts on healthcare, some have argued these should be considered as part of the promotion process.8 Notably, the SGIM Academic Hospitalist Taskforce has created the Quality Portfolio, a structured adjunct to promotions packets to organize and document work in quality improvement and patient safety.9
While there were few CI in the divisions surveyed, building CI programs was a major priority of programs. In programs reporting the presence of CI's, they report limited access to research support. This highlights the potential role and benefit of post residency training in designing and conducting clinical research whether in a traditional general internal medicine fellowship or in 1 of the many growing hospital medicine fellowships.10 There also appears to be a need for funding to support the research careers of junior hospitalists. While access to effective mentorship is integrally linked to achieving increased academic accomplishments, there is certainly an ample call for research in the areas of quality improvement, patient safety; systems‐based practice, hospital efficiency, transitions of care,11 perioperative medicine,12 and education.2, 13, 14 While providing lower costs per admission and lower lengths of stay, hospitalists seem well‐positioned to spearhead active research in cost‐effectiveness in the hospital.14 Additionally, a quality portfolio, documenting such quality improvement projects, has been suggested as an effective means to provide a record of this work for academic promotion.9
The diverse activities of today's hospitalists are transforming the traditional view of academic work and are critical to the growth of hospitals, patient care, and development of the field of hospital medicine itself. Until these areas are fully embraced as legitimate areas of academic productivity and scholarship, the academic advancement of hospitalists will be slow.
It is unclear from our survey if academic hospitalist programs are truly getting the support they need to succeed. On one hand, there was general agreement that the Departments of Medicine and Divisions of General and Hospital Medicine were invested in the development of the academic accomplishments. Yet, the majority of program directors believed that they are viewed by the Department or Division as a clinical rather than an academic program. Moreover, over half of program directors report that their hospital was not supportive and therefore have limited the expansion of their hospitalist groups' educational and research activities. Lastly, for a large majority of programs, unavailable funding also acted to limit growth and expansion of academic functions. In a mere 2 decades, Emergency Medicine has become one of the largest US specialties and yet research and funding in the field have been lagging and are limiting academic expansion. Junior faculty seeking research careers struggled to find support and mentorship within their emergency medicine divisions.15 Challenges faced by academic emergency medicine provide important historical perspective for the even more rapidly growing field of academic hospital medicine. Learning from the Academic Emergency Medicine experience, academic hospitalists should proactively identify scholarship and research opportunities unique to hospitalist and fitting the needs of academic institutions. Involvement in national medical organizations, such as SGIM‐SHM‐ACGIM Academic Hospitalist Academy, or the SGIM Academic Hospitalist Task Force, where skill and career development is the focus, will undoubtedly promote the success of academic hospitalist. Expanding valuable niches of expertise, such as quality control, perioperative medicine and care transitions, create an indispensible component of hospital care. Lastly mentoring programs for academic hospitalist within SHM and SGIM are also essential for networking and career development. There are several limitations to our study. Our response rate of 40% was relatively low, and our results may not be representative of all academic hospitalist division chiefs and their programs, may be overstating the perceived difficulties of the survey sample, or conversely missing a large portion too overwhelmed by current duties who lacked the time to complete the survey. Having said this, our survey methodology targeted sites where we could identify potentialnot confirmedhospitalist groups and hospitalist group leaders. For this reason, our response rate could be higher (if some of our contacts were in error). Our results are a cross‐sectional survey based on self report and are subject to recall bias. In addition, our study was carried out in 2007, and while issues such as mentorship may remain important, our results regarding financial arrangements may not be applicable to the current economic climate. Finally, while improving mentorship was identified as a principle objective for program leaders, we did not explore the existing quality of mentorship, nor perceived shortfalls. This should be the subject of future exploration.
The vast majority of academic hospital medicine programs continue to view inadequate support, expanding research, mentorship, and academic promotion as critical issues for the future. Thus, further understanding of these features, and interventions to allow for success, are of crucial importance in the continued development of academic hospitalists. Our study supports the need for mentoring and career development programs, targeting academic hospitalists and their leaders. In addition, attention should be paid to activities that support career fit, creating sustainable and viable job descriptions for academic hospitalists, and preventing burnout.16 At the same time we must expand the traditional view of scholarship and training and advocate for promotion criteria that value the unique contributions of hospitalists to become in line with the broad areas that hospitalists work.
- Anonymous. Definition of a hospitalist.2009. Society of Hospital Medicine Homepage/General Information. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Hospitalist_Definition4:240–246.
- ,,,,.Resource‐based relative value scale analysis between teaching and nonteaching hospitalist services.Health Care Management.2009;1(28):81–85.
- ,,, et al.Effects of physician experience on costs and outcomes on an academic general medicine service.Ann Intern Med.2002;137:866–874.
- ,,,.Hospitalists and the practice of inpatient medicine.Ann Intern Med.1999;130:343–349.
- ,,,,,.Rates, predictors and consequences of low career satisfaction and burnout in academic hospital medicine.J Hosp Med.2009;4(S1):24–25.
- ,.Mentoring faculty in academic medicine: a new paradigm?.J Gen Intern Med.2005;20(9):866–870.
- ,.Clinicians in quality improvement: a new career pathway in academic medicine.JAMA.2009;301(7):766–768.
- ,,,.Academic hospitalist taskforce quality portfolio rationale and development. 02/23/2009; Quality portfolio introduction. Available at:http://www.sgim.org/index.cfm?pageId=846. Accessed July 2010.
- ,,,.Hospital medicine fellowships: works in progress.Am J Med.2006;119:72e1–72e7.
- ,,,.Promoting effective care transitions of hospital discharge. A review of key issues for hospitalists.J. Hosp Med.2007;2:314–323.
- ,.Hospitalists and anesthesiologists as perioperative physicians: are their roles complementary?Proc (Bayl Univ Med Cent).2007;20(2):140–142.
- .A systematic review of outcomes and quality measures in adult patients cared for by hospitalists vs nonhospitalist.Mayo Clin Proc.2009;84(3):248–254.
- .Reflections: the hospitalist movement a decade later.J Hosp Med.2006;1:248–252.
- .Anyone, Anything, Anytime A History of Emergency Medicine.1st ed.Philadelphia, PA:Mosby‐Elsevier;2006.
- ,,, et al.Career fit and burnout among academic faculty.Arch Intern Med.2009;169(10):990–995.
- Anonymous. Definition of a hospitalist.2009. Society of Hospital Medicine Homepage/General Information. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Hospitalist_Definition4:240–246.
- ,,,,.Resource‐based relative value scale analysis between teaching and nonteaching hospitalist services.Health Care Management.2009;1(28):81–85.
- ,,, et al.Effects of physician experience on costs and outcomes on an academic general medicine service.Ann Intern Med.2002;137:866–874.
- ,,,.Hospitalists and the practice of inpatient medicine.Ann Intern Med.1999;130:343–349.
- ,,,,,.Rates, predictors and consequences of low career satisfaction and burnout in academic hospital medicine.J Hosp Med.2009;4(S1):24–25.
- ,.Mentoring faculty in academic medicine: a new paradigm?.J Gen Intern Med.2005;20(9):866–870.
- ,.Clinicians in quality improvement: a new career pathway in academic medicine.JAMA.2009;301(7):766–768.
- ,,,.Academic hospitalist taskforce quality portfolio rationale and development. 02/23/2009; Quality portfolio introduction. Available at:http://www.sgim.org/index.cfm?pageId=846. Accessed July 2010.
- ,,,.Hospital medicine fellowships: works in progress.Am J Med.2006;119:72e1–72e7.
- ,,,.Promoting effective care transitions of hospital discharge. A review of key issues for hospitalists.J. Hosp Med.2007;2:314–323.
- ,.Hospitalists and anesthesiologists as perioperative physicians: are their roles complementary?Proc (Bayl Univ Med Cent).2007;20(2):140–142.
- .A systematic review of outcomes and quality measures in adult patients cared for by hospitalists vs nonhospitalist.Mayo Clin Proc.2009;84(3):248–254.
- .Reflections: the hospitalist movement a decade later.J Hosp Med.2006;1:248–252.
- .Anyone, Anything, Anytime A History of Emergency Medicine.1st ed.Philadelphia, PA:Mosby‐Elsevier;2006.
- ,,, et al.Career fit and burnout among academic faculty.Arch Intern Med.2009;169(10):990–995.
Copyright © 2011 Society of Hospital Medicine