User login
Appropriate laboratory testing in Lyme disease
Lyme disease is a complex multisystem bacterial infection affecting the skin, joints, heart, and nervous system. The full spectrum of disease was first recognized and the disease was named in the 1970s during an outbreak of arthritis in children in the town of Lyme, Connecticut.1
This review describes the epidemiology and pathogenesis of Lyme disease, the advantages and disadvantages of current diagnostic methods, and diagnostic algorithms.
THE MOST COMMON TICK-BORNE INFECTION IN NORTH AMERICA
Lyme disease is the most common tick-borne infection in North America.2,3 In the United States, more than 30,000 cases are reported annually. In fact, in 2017, the number of cases was about 42,000, a 16% increase from the previous year, according to the US Centers for Disease Control and Prevention (CDC).
Infected nymphs account for most cases.
The infection is caused by Borrelia burgdorferi, a particularly arthritogenic spirochete transmitted by Ixodes scapularis (the black-legged deer tick, (Figure 1) and Ixodes pacificus (the Western black-legged tick). Although the infection can occur at any time of the year, its peak incidence is in May to late September, coinciding with increased outdoor recreational activity in areas where ticks live.3,4 The typical tick habitat consists of deciduous woodland with sufficient humidity provided by a good layer of decaying vegetation. However, people can contract Lyme disease in their own backyard.3
Most cases of Lyme disease are seen in the northeastern United States, mainly in suburban and rural areas.2,3 Other areas affected include the midwestern states of Minnesota, Wisconsin, and Michigan, as well as northern California.4 Fourteen states and the District of Columbia report a high average incidence (> 10 cases per 100,000 persons) (Table 1).2
FIRST COMES IgM, THEN IgG
The pathogenesis and the different stages of infection should inform laboratory testing in Lyme disease.
It is estimated that only 5% of infected ticks that bite people actually transmit their spirochetes to the human host.5 However, once infected, the patient’s innate immune system mounts a response that results in the classic erythema migrans rash at the bite site. A rash develops in only about 85% of patients who are infected and can appear at any time between 3 and 30 days, but most commonly after 7 days. Hence, a rash occurring within the first few hours of tick contact is not erythema migrans and does not indicate infection, but rather an early reaction to tick salivary antigens.5
Antibody levels remain below the detection limits of currently available serologic tests in the first 7 days after exposure. Immunoglobulin M (IgM) antibody titers peak between 8 and 14 days after tick contact, but IgM antibodies may never develop if the patient is started on early appropriate antimicrobial therapy.5
If the infection is not treated, the spirochete may disseminate through the blood from the bite site to different tissues.3 Both cell-mediated and antibody-mediated immunity swing into action to kill the spirochetes at this stage. The IgM antibody response occurs in 1 to 2 weeks, followed by a robust IgG response in 2 to 4 weeks.6
Because IgM can also cross-react with antigens other than those associated with B burgdorferi, the IgM test is less specific than the IgG test for Lyme disease.
Once a patient is exposed and mounts an antibody-mediated response to the spirochete, the antibody profile may persist for months to years, even after successful antibiotic treatment and cure of the disease.5
Despite the immune system’s robust series of defenses, untreated B burgdorferi infection can persist, as the organism has a bag of tricks to evade destruction. It can decrease its expression of specific immunogenic surface-exposed proteins, change its antigenic properties through recombination, and bind to the patient’s extracellular matrix proteins to facilitate further dissemination.3
Certain host-genetic factors also play a role in the pathogenesis of Lyme disease, such as the HLA-DR4 allele, which has been associated with antibiotic-refractory Lyme-related arthritis.3
LYME DISEASE EVOLVES THROUGH STAGES
Lyme disease evolves through stages broadly classified as early and late infection, with significant variability in its presentation.7
Early infection
Early disease is further subdivided into “localized” infection (stage 1), characterized by a single erythema migrans lesion and local lymphadenopathy, and “disseminated” infection (stage 2), associated with multiple erythema migrans lesions distant from the bite site, facial nerve palsy, radiculoneuritis, meningitis, carditis, or migratory arthritis or arthralgia.8
Highly specific physical findings include erythema migrans, cranial nerve palsy, high-grade or progressive conduction block, and recurrent migratory polyarthritis. Less specific symptoms and signs of Lyme disease include arthralgia, myalgia, neck stiffness, palpitations, and myocarditis.5
Erythema migrans lesions are evident in at least 85% of patients with early disease.9 If they are not apparent on physical examination, they may be located at hidden sites and may be atypical in appearance or transient.5
If treatment is not started in the initial stage of the disease, 60% of infected patients may develop disseminated infection.5 Progressive, untreated infection can manifest with Lyme arthritis and neuroborreliosis.7
Noncutaneous manifestations are less common now than in the past due to increased awareness of the disease and early initiation of treatment.10
Late infection
Manifestations of late (stage 3) infection include oligoarthritis (affecting any joint but often the knee) and neuroborreliosis. Clinical signs and symptoms of Lyme disease may take months to resolve even after appropriate antimicrobial therapy is completed. This should not be interpreted as ongoing, persistent infection, but as related to host immune-mediated activity.5
INTERPRET LABORATORY RESULTS BASED ON PRETEST PROBABILITY
The usefulness of a laboratory test depends on the individual patient’s pretest probability of infection, which in turn depends on the patient’s epidemiologic risk of exposure and clinical features of Lyme disease. Patients with a high pretest probability—eg, a history of a tick bite followed by the classic erythema migrans rash—do not need testing and can start antimicrobial therapy right away.11
Serologic tests are the gold standard
Prompt diagnosis is important, as early Lyme disease is easily treatable without any future sequelae.11
Tests for Lyme disease can be divided into direct methods, which detect the spirochete itself by culture or by polymerase chain reaction (PCR), and indirect methods, which detect antibodies (Table 2). Direct tests lack sensitivity for Lyme disease; hence, serologic tests remain the gold standard. Currently recommended is a standard 2-tier testing strategy using an enzyme-linked immunosorbent assay (ELISA) followed by Western blot for confirmation.
DIRECT METHODS
Culture lacks sensitivity
A number of factors limit the sensitivity of direct culture for diagnosing Lyme disease. B burgdorferi does not grow easily in culture, requiring special media, low temperatures, and long periods of incubation. Only a relatively few spirochetes are present in human tissues and body fluids to begin with, and bacterial counts are further reduced with duration and dissemination of infection.5 All of these limit the possibility of detecting this organism.
Polymerase chain reaction may help in some situations
Molecular assays are not part of the standard evaluation and should be used only in conjunction with serologic testing.7 These tests have high specificity but lack consistent sensitivity.
That said, PCR testing may be useful:
- In early infection, before antibody responses develop
- In reinfection, when serologic tests are not reliable because the antibodies persist for many years after an infection in many patients
- In endemic areas where serologic testing has high false-positive rates due to high baseline population seropositivity for anti-Borrelia antibodies caused by subclinical infection.3
PCR assays that target plasmid-borne genes encoding outer surface proteins A and C (OspA and OspC) and VisE (variable major protein-like sequence, expressed) are more sensitive than those that detect chromosomal 16s ribosomal ribonucleic acid (rRNA) genes, as plasmid-rich “blebs” are shed in larger concentrations than chromosomal DNA during active infection.7 However, these plasmid-contained genes persist in body tissues and fluids even after the infection is cleared, and their detection may not necessarily correlate with ongoing disease.8 Detection of chromosomal 16s rRNA genes is a better predictor of true organism viability.
The sensitivity of PCR for borrelial DNA depends on the type of sample. If a skin biopsy sample is taken of the leading edge of an erythema migrans lesion, the sensitivity is 69% and the specificity is 100%. In patients with Lyme arthritis, PCR of the synovial fluid has a sensitivity of up to 80%. However, the sensitivity of PCR of the cerebrospinal fluid of patients with neurologic manifestations of Lyme disease is only 19%.7 PCR of other clinical samples, including blood and urine, is not recommended, as spirochetes are primarily confined to tissues, and very few are present in these body fluids.3,12
The disadvantage of PCR is that a positive result does not always mean active infection, as the DNA of the dead microbe persists for several months even after successful treatment.8
INDIRECT METHODS
Enzyme-linked immunosorbent assay
ELISAs detect anti-Borrelia antibodies. Early-generation ELISAs, still used in many laboratories, use whole-cell extracts of B burgdorferi. Examples are the Vidas Lyme screen (Biomérieux, biomerieux-usa.com) and the Wampole B burgdorferi IgG/M EIA II assay (Alere, www.alere.com). Newer ELISAs use recombinant proteins.13
Three major targets for ELISA antibodies are flagellin (Fla), outer surface protein C (OspC), and VisE, especially the invariable region 6 (IR6). Among these, VisE-IR6 is the most conserved region in B burgdorferi.
Early-generation assays have a sensitivity of 89% and specificity of 72%.11 However, the patient’s serum may have antibodies that cross-react with unrelated bacterial antigens, leading to false-positive results (Table 3). Whole-cell sonicate assays are not recommended as an independent test and must be confirmed with Western blot testing when assay results are indeterminate or positive.11
Newer-generation ELISAs detect antibodies targeting recombinant proteins of VisE, especially a synthetic peptide C6, within IR6.13 VisE-IR6 is the most conserved region of the B burgdorferi complex, and its detection is a highly specific finding, supporting the diagnosis of Lyme disease. Antibodies against VisE-IR6 antigen are the earliest to develop.5 An example of a newer-generation serologic test is the VisE C6 Lyme EIA kit, approved as a first-tier test by the US Food and Drug Administration in 2001. This test has a specificity of 99%,14,15 and its specificity is further increased when used in conjunction with Western blot (99.5%).15 The advantage of the C6 antibody test is that it is more sensitive than 2-tier testing during early infection (sensitivity 29%–74% vs 17%–40% in early localized infection, and 56%–90% vs 27%–78% in early disseminated infection).6
During early infection, older and newer ELISAs are less sensitive because of the limited number of antigens expressed at this stage.13 All patients suspected of having early Lyme disease who are seronegative at initial testing should have follow-up testing to look for seroconversion.13
Western blot
Western blot (immunoblot) testing identifies IgM and IgG antibodies against specific B burgdorferi antigens. It is considered positive if it detects at least 2 of a possible 3 specific IgM bands in the first 4 weeks of disease or at least 5 of 10 specific IgG bands after 4 weeks of disease (Table 4 and Figure 2).16
The nature of the bands indicates the duration of infection: Western blot bands against 23-kD OspC and 41-kD FlaB are seen in early localized infection, whereas bands against all 3 B burgdorferi proteins will be seen after several weeks of disease.17 The IgM result should be interpreted carefully, as only 2 bands are required for the test to be positive, and IgM binds to antigen less specifically than IgG.12
Interpreting the IgM Western blot test: The ‘1-month rule’
If clinical symptoms and signs of Lyme disease have been present for more than 1 month, IgM reactivity alone should not be used to support the diagnosis, in view of the likelihood of a false-positive test result in this situation.18 This is called the “1-month rule” in the diagnosis of Lyme disease.13
In early localized infection, Western blot is only half as sensitive as ELISA testing. Since the overall sensitivity of a 2-step algorithm is equal to that of its least sensitive component, 2-tiered testing is not useful in early disease.13
Although currently considered the most specific test for confirmation of Lyme disease, Western blot has limitations. It is technically and interpretively complex and is thus not universally available.13 The blots are scored by visual examination, compromising the reproducibility of the test, although densitometric blot analysis techniques and automated scanning and scoring attempt to address some of these limitations.13 Like the ELISA, Western blot can have false-positive results in healthy individuals without tick exposure, as nonspecific IgM immunoblots develop faint bands. This is because of cross-reaction between B burgdorferi antigens and antigens from other microorganisms. Around 50% of healthy adults show low-level serum IgG reactivity against the FlaB antigen, leading to false-positive results as well. In cases in which the Western blot result is indeterminate, other etiologies must be considered.
False-positive IgM Western blots are a significant problem. In a 5-year retrospective study done at 63 US Air Force healthcare facilities, 113 (53.3%) of 212 IgM Western blots were falsely positive.19 A false-positive test was defined as one that failed to meet seropositivity (a first-tier test omitted or negative, > 30 days of symptoms with negative IgG blot), lack of exposure including residing in areas without documented tick habitats, patients having atypical or no symptoms, and negative serology within 30 days of a positive test.
In a similar study done in a highly endemic area, 50 (27.5%) of 182 patients had a false-positive test.20 Physicians need to be careful when interpreting IgM Western blots. It is always important to consider locale, epidemiology, and symptoms when interpreting the test.
Limitations of serologic tests for Lyme disease
Currently available serologic tests have inherent limitations:
- Antibodies against B burgdorferi take at least 1 week to develop
- The background rate of seropositivity in endemic areas can be up to 4%, affecting the utility of a positive test result
- Serologic tests cannot be used as tests of cure because antibodies can persist for months to years even after appropriate antimicrobial therapy and cure of disease; thus, a positive serologic result could represent active infection or remote exposure21
- Antibodies can cross-react with related bacteria, including other borrelial or treponemal spirochetes
- False-positive serologic test results can also occur in association with other medical conditions such as polyclonal gammopathies and systemic lupus erythematosus.12
RECOMMENDATIONS FOR TESTING
Standard 2-tier testing
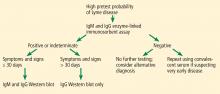
The CDC released recommendations for diagnosing Lyme disease after a second national conference of serologic diagnosis of Lyme disease in October 1994.18 The 2-tiered testing method, involving a sensitive ELISA followed by the Western blot to confirm positive and indeterminate ELISA results, was suggested as the gold standard for diagnosis (Figure 3). Of note, negative ELISA results do not require further testing.11
The sensitivity of 2-tiered testing depends on the stage of the disease. Unfortunately, this method has a wide range of sensitivity (17% to 78%) in stage 1 disease. In the same stage, the sensitivity increases from 14.1% in patients with a single erythema migrans lesion and early localized infection to 65.4% in those with multiple lesions. The algorithm has excellent sensitivity in late stage 3 infection (96% to 100%).5
A 2-step ELISA algorithm
A 2-step ELISA algorithm (without the Western blot) that includes the whole-cell sonicate assay followed by the VisE C6 peptide assay actually showed higher sensitivity and comparable specificity compared with 2-tiered testing in early localized disease (sensitivity 61%–74% vs 29%–48%, respectively; specificity 99.5% for both methods).22 This higher sensitivity was even more pronounced in early disseminated infection (sensitivity 100% vs 40%, respectively). By late infection, the sensitivities of both testing strategies reached 100%. Compared with the Western blot, the 2-step ELISA algorithm was simpler to execute in a reproducible fashion.5
The Infectious Diseases Society of America is revising its current guidelines, with an update expected late this year, which may shift the recommendation from 2-tiered testing to the 2-step ELISA algorithm.
Multiplex testing
To overcome the intrinsic problems of protein-based assays, a multiplexed, array-based assay for the diagnosis of tick-borne infections called Tick-Borne Disease Serochip (TBD-Serochip) was established using recombinant antigens that identify key immunodominant epitopes.8 More studies are needed to establish the validity and usefulness of these tests in clinical practice.
Who should not be tested?
The American College of Physicians6 recommends against testing in patients:
- Presenting with nonspecific symptoms (eg, headache, myalgia, fatigue, arthralgia) without objective signs of Lyme disease
- With low pretest probability of infection based on epidemiologic exposures and clinical features
- Living in Lyme-endemic areas with no history of tick exposure6
- Presenting less than 1 week after tick exposure5
- Seeking a test of cure for treated Lyme disease.
DIAGNOSIS IN SPECIAL SITUATIONS
Early Lyme disease
The classic erythema migrans lesion on physical examination of a patient with suspected Lyme disease is diagnostic and does not require laboratory confirmation.10
In ambiguous cases, 2-tiered testing of a serum sample during the acute presentation and again 4 to 6 weeks later can be useful. In patients who remain seronegative on paired serum samples despite symptoms lasting longer than 6 weeks and no antibiotic treatment in the interim, the diagnosis of Lyme disease is unlikely, and another diagnosis should be sought.3
Antimicrobial therapy may block the serologic response; hence, negative serologic testing in patients started on empiric antibiotics should not rule out Lyme disease.6
PCR or bacterial culture testing is not recommended in the evaluation of suspected early Lyme disease.
Central nervous system Lyme disease
Central nervous system Lyme disease is diagnosed by 2-tiered testing using peripheral blood samples because all patients with this infectious manifestation should have mounted an adequate IgG response in the blood.11
B cells migrate to and proliferate inside the central nervous system, leading to intrathecal production of anti-Borrelia antibodies. An index of cerebrospinal fluid to serum antibody greater than 1 is thus also indicative of neuroborreliosis.12 Thus, performing lumbar puncture to detect intrathecal production of antibodies may support the diagnosis of central nervous system Lyme disease; however, it is not necessary.11
Antibodies persist in the central nervous system for many years after appropriate antimicrobial treatment.
Lyme arthritis
Articular involvement in Lyme disease is characterized by a robust humoral response such that a negative IgG serologic test virtually rules out Lyme arthritis.23 PCR testing of synovial fluid for borrelial DNA has a sensitivity of 80% but may become falsely negative after 1 to 2 months of antibiotic treatment.24,25 In an algorithm suggested by Puius et al,23 PCR testing of synovial fluid should be done in patients who have minimal to no response after 2 months of appropriate oral antimicrobial therapy to determine whether intravenous antibiotics are merited.
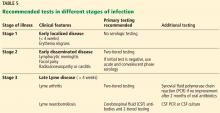
Table 5 summarizes the tests of choice in different clinical stages of infection.
Acknowledgment: The authors would like to acknowledge Anita Modi, MD, and Ceena N. Jacob, MD, for reviewing the manuscript and providing valuable suggestions, and Belinda Yen-Lieberman, PhD, for contributing pictures of the Western blot test results.
- Steere AC, Malawista SE, Snydman DR, et al. Lyme arthritis: an epidemic of oligoarticular arthritis in children and adults in three Connecticut communities. Arthritis Rheum 1977; 20(1):7–17. doi:10.1002/art.1780200102
- Centers for Disease Control and Prevention (CDC). Lyme disease: recent surveillance data. https://www.cdc.gov/lyme/datasurveillance/recent-surveillance-data.html. Accessed August 12, 2019.
- Stanek G, Wormser GP, Gray J, Strle F. Lyme borreliosis. Lancet 2012; 379(9814):461–473. doi:10.1016/S0140-6736(11)60103-7
- Arvikar SL, Steere AC. Diagnosis and treatment of Lyme arthritis. Infect Dis Clin North Am 2015; 29(2):269–280. doi:10.1016/j.idc.2015.02.004
- Schriefer ME. Lyme disease diagnosis: serology. Clin Lab Med 2015; 35(4):797–814. doi:10.1016/j.cll.2015.08.001
- Hu LT. Lyme disease. Ann Intern Med 2016; 164(9):ITC65–ITC80. doi:10.7326/AITC201605030
- Alby K, Capraro GA. Alternatives to serologic testing for diagnosis of Lyme disease. Clin Lab Med 2015; 35(4):815–825. doi:10.1016/j.cll.2015.07.005
- Dumler JS. Molecular diagnosis of Lyme disease: review and meta-analysis. Mol Diagn 2001; 6(1):1–11. doi:10.1054/modi.2001.21898
- Wormser GP, McKenna D, Carlin J, et al. Brief communication: hematogenous dissemination in early Lyme disease. Ann Intern Med 2005; 142(9):751–755. doi:10.7326/0003-4819-142-9-200505030-00011
- Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2006; 43(9):1089–1134. doi:10.1086/508667
- Guidelines for laboratory evaluation in the diagnosis of Lyme disease. American College of Physicians. Ann Intern Med 1997; 127(12):1106–1108. doi:10.7326/0003-4819-127-12-199712150-00010
- Halperin JJ. Lyme disease: a multisystem infection that affects the nervous system. Continuum (Minneap Minn) 2012; 18(6 Infectious Disease):1338–1350. doi:10.1212/01.CON.0000423850.24900.3a
- Branda JA, Body BA, Boyle J, et al. Advances in serodiagnostic testing for Lyme disease are at hand. Clin Infect Dis 2018; 66(7):1133–1139. doi:10.1093/cid/cix943
- Immunetics. Immunetics® C6 Lyme ELISA™ Kit. http://www.oxfordimmunotec.com/international/wp-content/uploads/sites/3/CF-E601-096A-C6-Pkg-Insrt.pdf. Accessed August 12, 2019.
- Civelek M, Lusis AJ. Systems genetics approaches to understand complex traits. Nat Rev Genet 2014; 15(1):34–48. doi:10.1038/nrg3575
- Centers for Disease Control and Prevention (CDC). Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. MMWR Morb Mortal Wkly Rep 1995; 44(31):590–591. pmid:7623762
- Steere AC, Mchugh G, Damle N, Sikand VK. Prospective study of serologic tests for Lyme disease. Clin Infect Dis 2008; 47(2):188–195. doi:10.1086/589242
- Centers for Disease Control and Prevention. Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. JAMA 1995; 274(12):937. pmid:7674514
- Webber BJ, Burganowski RP, Colton L, Escobar JD, Pathak SR, Gambino-Shirley KJ. Lyme disease overdiagnosis in a large healthcare system: a population-based, retrospective study. Clin Microbiol Infect 2019. doi:10.1016/j.cmi.2019.02.020. Epub ahead of print.
- Seriburi V, Ndukwe N, Chang Z, Cox ME, Wormser GP. High frequency of false positive IgM immunoblots for Borrelia burgdorferi in clinical practice. Clin Microbiol Infect 2012; 18(12):1236–1240. doi:10.1111/j.1469-0691.2011.03749.x
- Hilton E, DeVoti J, Benach JL, et al. Seroprevalence and seroconversion for tick-borne diseases in a high-risk population in the northeast United States. Am J Med 1999; 106(4):404–409. doi:10.1016/s0002-9343(99)00046-7
- Branda JA, Linskey K, Kim YA, Steere AC, Ferraro MJ. Two-tiered antibody testing for Lyme disease with use of 2 enzyme immunoassays, a whole-cell sonicate enzyme immunoassay followed by a VlsE C6 peptide enzyme immunoassay. Clin Infect Dis 2011; 53(6):541–547. doi:10.1093/cid/cir464
- Puius YA, Kalish RA. Lyme arthritis: pathogenesis, clinical presentation, and management. Infect Dis Clin North Am 2008; 22(2):289–300. doi:10.1016/j.idc.2007.12.014
- Nocton JJ, Dressler F, Rutledge BJ, Rys PN, Persing DH, Steere AC. Detection of Borrelia burgdorferi DNA by polymerase chain reaction in synovial fluid from patients with Lyme arthritis. N Engl J Med 1994; 330(4):229–234. doi:10.1056/NEJM199401273300401
- Liebling MR, Nishio MJ, Rodriguez A, Sigal LH, Jin T, Louie JS. The polymerase chain reaction for the detection of Borrelia burgdorferi in human body fluids. Arthritis Rheum 1993; 36(5):665–975. doi:10.1002/art.1780360514
Lyme disease is a complex multisystem bacterial infection affecting the skin, joints, heart, and nervous system. The full spectrum of disease was first recognized and the disease was named in the 1970s during an outbreak of arthritis in children in the town of Lyme, Connecticut.1
This review describes the epidemiology and pathogenesis of Lyme disease, the advantages and disadvantages of current diagnostic methods, and diagnostic algorithms.
THE MOST COMMON TICK-BORNE INFECTION IN NORTH AMERICA
Lyme disease is the most common tick-borne infection in North America.2,3 In the United States, more than 30,000 cases are reported annually. In fact, in 2017, the number of cases was about 42,000, a 16% increase from the previous year, according to the US Centers for Disease Control and Prevention (CDC).
Infected nymphs account for most cases.
The infection is caused by Borrelia burgdorferi, a particularly arthritogenic spirochete transmitted by Ixodes scapularis (the black-legged deer tick, (Figure 1) and Ixodes pacificus (the Western black-legged tick). Although the infection can occur at any time of the year, its peak incidence is in May to late September, coinciding with increased outdoor recreational activity in areas where ticks live.3,4 The typical tick habitat consists of deciduous woodland with sufficient humidity provided by a good layer of decaying vegetation. However, people can contract Lyme disease in their own backyard.3
Most cases of Lyme disease are seen in the northeastern United States, mainly in suburban and rural areas.2,3 Other areas affected include the midwestern states of Minnesota, Wisconsin, and Michigan, as well as northern California.4 Fourteen states and the District of Columbia report a high average incidence (> 10 cases per 100,000 persons) (Table 1).2
FIRST COMES IgM, THEN IgG
The pathogenesis and the different stages of infection should inform laboratory testing in Lyme disease.
It is estimated that only 5% of infected ticks that bite people actually transmit their spirochetes to the human host.5 However, once infected, the patient’s innate immune system mounts a response that results in the classic erythema migrans rash at the bite site. A rash develops in only about 85% of patients who are infected and can appear at any time between 3 and 30 days, but most commonly after 7 days. Hence, a rash occurring within the first few hours of tick contact is not erythema migrans and does not indicate infection, but rather an early reaction to tick salivary antigens.5
Antibody levels remain below the detection limits of currently available serologic tests in the first 7 days after exposure. Immunoglobulin M (IgM) antibody titers peak between 8 and 14 days after tick contact, but IgM antibodies may never develop if the patient is started on early appropriate antimicrobial therapy.5
If the infection is not treated, the spirochete may disseminate through the blood from the bite site to different tissues.3 Both cell-mediated and antibody-mediated immunity swing into action to kill the spirochetes at this stage. The IgM antibody response occurs in 1 to 2 weeks, followed by a robust IgG response in 2 to 4 weeks.6
Because IgM can also cross-react with antigens other than those associated with B burgdorferi, the IgM test is less specific than the IgG test for Lyme disease.
Once a patient is exposed and mounts an antibody-mediated response to the spirochete, the antibody profile may persist for months to years, even after successful antibiotic treatment and cure of the disease.5
Despite the immune system’s robust series of defenses, untreated B burgdorferi infection can persist, as the organism has a bag of tricks to evade destruction. It can decrease its expression of specific immunogenic surface-exposed proteins, change its antigenic properties through recombination, and bind to the patient’s extracellular matrix proteins to facilitate further dissemination.3
Certain host-genetic factors also play a role in the pathogenesis of Lyme disease, such as the HLA-DR4 allele, which has been associated with antibiotic-refractory Lyme-related arthritis.3
LYME DISEASE EVOLVES THROUGH STAGES
Lyme disease evolves through stages broadly classified as early and late infection, with significant variability in its presentation.7
Early infection
Early disease is further subdivided into “localized” infection (stage 1), characterized by a single erythema migrans lesion and local lymphadenopathy, and “disseminated” infection (stage 2), associated with multiple erythema migrans lesions distant from the bite site, facial nerve palsy, radiculoneuritis, meningitis, carditis, or migratory arthritis or arthralgia.8
Highly specific physical findings include erythema migrans, cranial nerve palsy, high-grade or progressive conduction block, and recurrent migratory polyarthritis. Less specific symptoms and signs of Lyme disease include arthralgia, myalgia, neck stiffness, palpitations, and myocarditis.5
Erythema migrans lesions are evident in at least 85% of patients with early disease.9 If they are not apparent on physical examination, they may be located at hidden sites and may be atypical in appearance or transient.5
If treatment is not started in the initial stage of the disease, 60% of infected patients may develop disseminated infection.5 Progressive, untreated infection can manifest with Lyme arthritis and neuroborreliosis.7
Noncutaneous manifestations are less common now than in the past due to increased awareness of the disease and early initiation of treatment.10
Late infection
Manifestations of late (stage 3) infection include oligoarthritis (affecting any joint but often the knee) and neuroborreliosis. Clinical signs and symptoms of Lyme disease may take months to resolve even after appropriate antimicrobial therapy is completed. This should not be interpreted as ongoing, persistent infection, but as related to host immune-mediated activity.5
INTERPRET LABORATORY RESULTS BASED ON PRETEST PROBABILITY
The usefulness of a laboratory test depends on the individual patient’s pretest probability of infection, which in turn depends on the patient’s epidemiologic risk of exposure and clinical features of Lyme disease. Patients with a high pretest probability—eg, a history of a tick bite followed by the classic erythema migrans rash—do not need testing and can start antimicrobial therapy right away.11
Serologic tests are the gold standard
Prompt diagnosis is important, as early Lyme disease is easily treatable without any future sequelae.11
Tests for Lyme disease can be divided into direct methods, which detect the spirochete itself by culture or by polymerase chain reaction (PCR), and indirect methods, which detect antibodies (Table 2). Direct tests lack sensitivity for Lyme disease; hence, serologic tests remain the gold standard. Currently recommended is a standard 2-tier testing strategy using an enzyme-linked immunosorbent assay (ELISA) followed by Western blot for confirmation.
DIRECT METHODS
Culture lacks sensitivity
A number of factors limit the sensitivity of direct culture for diagnosing Lyme disease. B burgdorferi does not grow easily in culture, requiring special media, low temperatures, and long periods of incubation. Only a relatively few spirochetes are present in human tissues and body fluids to begin with, and bacterial counts are further reduced with duration and dissemination of infection.5 All of these limit the possibility of detecting this organism.
Polymerase chain reaction may help in some situations
Molecular assays are not part of the standard evaluation and should be used only in conjunction with serologic testing.7 These tests have high specificity but lack consistent sensitivity.
That said, PCR testing may be useful:
- In early infection, before antibody responses develop
- In reinfection, when serologic tests are not reliable because the antibodies persist for many years after an infection in many patients
- In endemic areas where serologic testing has high false-positive rates due to high baseline population seropositivity for anti-Borrelia antibodies caused by subclinical infection.3
PCR assays that target plasmid-borne genes encoding outer surface proteins A and C (OspA and OspC) and VisE (variable major protein-like sequence, expressed) are more sensitive than those that detect chromosomal 16s ribosomal ribonucleic acid (rRNA) genes, as plasmid-rich “blebs” are shed in larger concentrations than chromosomal DNA during active infection.7 However, these plasmid-contained genes persist in body tissues and fluids even after the infection is cleared, and their detection may not necessarily correlate with ongoing disease.8 Detection of chromosomal 16s rRNA genes is a better predictor of true organism viability.
The sensitivity of PCR for borrelial DNA depends on the type of sample. If a skin biopsy sample is taken of the leading edge of an erythema migrans lesion, the sensitivity is 69% and the specificity is 100%. In patients with Lyme arthritis, PCR of the synovial fluid has a sensitivity of up to 80%. However, the sensitivity of PCR of the cerebrospinal fluid of patients with neurologic manifestations of Lyme disease is only 19%.7 PCR of other clinical samples, including blood and urine, is not recommended, as spirochetes are primarily confined to tissues, and very few are present in these body fluids.3,12
The disadvantage of PCR is that a positive result does not always mean active infection, as the DNA of the dead microbe persists for several months even after successful treatment.8
INDIRECT METHODS
Enzyme-linked immunosorbent assay
ELISAs detect anti-Borrelia antibodies. Early-generation ELISAs, still used in many laboratories, use whole-cell extracts of B burgdorferi. Examples are the Vidas Lyme screen (Biomérieux, biomerieux-usa.com) and the Wampole B burgdorferi IgG/M EIA II assay (Alere, www.alere.com). Newer ELISAs use recombinant proteins.13
Three major targets for ELISA antibodies are flagellin (Fla), outer surface protein C (OspC), and VisE, especially the invariable region 6 (IR6). Among these, VisE-IR6 is the most conserved region in B burgdorferi.
Early-generation assays have a sensitivity of 89% and specificity of 72%.11 However, the patient’s serum may have antibodies that cross-react with unrelated bacterial antigens, leading to false-positive results (Table 3). Whole-cell sonicate assays are not recommended as an independent test and must be confirmed with Western blot testing when assay results are indeterminate or positive.11
Newer-generation ELISAs detect antibodies targeting recombinant proteins of VisE, especially a synthetic peptide C6, within IR6.13 VisE-IR6 is the most conserved region of the B burgdorferi complex, and its detection is a highly specific finding, supporting the diagnosis of Lyme disease. Antibodies against VisE-IR6 antigen are the earliest to develop.5 An example of a newer-generation serologic test is the VisE C6 Lyme EIA kit, approved as a first-tier test by the US Food and Drug Administration in 2001. This test has a specificity of 99%,14,15 and its specificity is further increased when used in conjunction with Western blot (99.5%).15 The advantage of the C6 antibody test is that it is more sensitive than 2-tier testing during early infection (sensitivity 29%–74% vs 17%–40% in early localized infection, and 56%–90% vs 27%–78% in early disseminated infection).6
During early infection, older and newer ELISAs are less sensitive because of the limited number of antigens expressed at this stage.13 All patients suspected of having early Lyme disease who are seronegative at initial testing should have follow-up testing to look for seroconversion.13
Western blot
Western blot (immunoblot) testing identifies IgM and IgG antibodies against specific B burgdorferi antigens. It is considered positive if it detects at least 2 of a possible 3 specific IgM bands in the first 4 weeks of disease or at least 5 of 10 specific IgG bands after 4 weeks of disease (Table 4 and Figure 2).16
The nature of the bands indicates the duration of infection: Western blot bands against 23-kD OspC and 41-kD FlaB are seen in early localized infection, whereas bands against all 3 B burgdorferi proteins will be seen after several weeks of disease.17 The IgM result should be interpreted carefully, as only 2 bands are required for the test to be positive, and IgM binds to antigen less specifically than IgG.12
Interpreting the IgM Western blot test: The ‘1-month rule’
If clinical symptoms and signs of Lyme disease have been present for more than 1 month, IgM reactivity alone should not be used to support the diagnosis, in view of the likelihood of a false-positive test result in this situation.18 This is called the “1-month rule” in the diagnosis of Lyme disease.13
In early localized infection, Western blot is only half as sensitive as ELISA testing. Since the overall sensitivity of a 2-step algorithm is equal to that of its least sensitive component, 2-tiered testing is not useful in early disease.13
Although currently considered the most specific test for confirmation of Lyme disease, Western blot has limitations. It is technically and interpretively complex and is thus not universally available.13 The blots are scored by visual examination, compromising the reproducibility of the test, although densitometric blot analysis techniques and automated scanning and scoring attempt to address some of these limitations.13 Like the ELISA, Western blot can have false-positive results in healthy individuals without tick exposure, as nonspecific IgM immunoblots develop faint bands. This is because of cross-reaction between B burgdorferi antigens and antigens from other microorganisms. Around 50% of healthy adults show low-level serum IgG reactivity against the FlaB antigen, leading to false-positive results as well. In cases in which the Western blot result is indeterminate, other etiologies must be considered.
False-positive IgM Western blots are a significant problem. In a 5-year retrospective study done at 63 US Air Force healthcare facilities, 113 (53.3%) of 212 IgM Western blots were falsely positive.19 A false-positive test was defined as one that failed to meet seropositivity (a first-tier test omitted or negative, > 30 days of symptoms with negative IgG blot), lack of exposure including residing in areas without documented tick habitats, patients having atypical or no symptoms, and negative serology within 30 days of a positive test.
In a similar study done in a highly endemic area, 50 (27.5%) of 182 patients had a false-positive test.20 Physicians need to be careful when interpreting IgM Western blots. It is always important to consider locale, epidemiology, and symptoms when interpreting the test.
Limitations of serologic tests for Lyme disease
Currently available serologic tests have inherent limitations:
- Antibodies against B burgdorferi take at least 1 week to develop
- The background rate of seropositivity in endemic areas can be up to 4%, affecting the utility of a positive test result
- Serologic tests cannot be used as tests of cure because antibodies can persist for months to years even after appropriate antimicrobial therapy and cure of disease; thus, a positive serologic result could represent active infection or remote exposure21
- Antibodies can cross-react with related bacteria, including other borrelial or treponemal spirochetes
- False-positive serologic test results can also occur in association with other medical conditions such as polyclonal gammopathies and systemic lupus erythematosus.12
RECOMMENDATIONS FOR TESTING
Standard 2-tier testing
The CDC released recommendations for diagnosing Lyme disease after a second national conference of serologic diagnosis of Lyme disease in October 1994.18 The 2-tiered testing method, involving a sensitive ELISA followed by the Western blot to confirm positive and indeterminate ELISA results, was suggested as the gold standard for diagnosis (Figure 3). Of note, negative ELISA results do not require further testing.11
The sensitivity of 2-tiered testing depends on the stage of the disease. Unfortunately, this method has a wide range of sensitivity (17% to 78%) in stage 1 disease. In the same stage, the sensitivity increases from 14.1% in patients with a single erythema migrans lesion and early localized infection to 65.4% in those with multiple lesions. The algorithm has excellent sensitivity in late stage 3 infection (96% to 100%).5
A 2-step ELISA algorithm
A 2-step ELISA algorithm (without the Western blot) that includes the whole-cell sonicate assay followed by the VisE C6 peptide assay actually showed higher sensitivity and comparable specificity compared with 2-tiered testing in early localized disease (sensitivity 61%–74% vs 29%–48%, respectively; specificity 99.5% for both methods).22 This higher sensitivity was even more pronounced in early disseminated infection (sensitivity 100% vs 40%, respectively). By late infection, the sensitivities of both testing strategies reached 100%. Compared with the Western blot, the 2-step ELISA algorithm was simpler to execute in a reproducible fashion.5
The Infectious Diseases Society of America is revising its current guidelines, with an update expected late this year, which may shift the recommendation from 2-tiered testing to the 2-step ELISA algorithm.
Multiplex testing
To overcome the intrinsic problems of protein-based assays, a multiplexed, array-based assay for the diagnosis of tick-borne infections called Tick-Borne Disease Serochip (TBD-Serochip) was established using recombinant antigens that identify key immunodominant epitopes.8 More studies are needed to establish the validity and usefulness of these tests in clinical practice.
Who should not be tested?
The American College of Physicians6 recommends against testing in patients:
- Presenting with nonspecific symptoms (eg, headache, myalgia, fatigue, arthralgia) without objective signs of Lyme disease
- With low pretest probability of infection based on epidemiologic exposures and clinical features
- Living in Lyme-endemic areas with no history of tick exposure6
- Presenting less than 1 week after tick exposure5
- Seeking a test of cure for treated Lyme disease.
DIAGNOSIS IN SPECIAL SITUATIONS
Early Lyme disease
The classic erythema migrans lesion on physical examination of a patient with suspected Lyme disease is diagnostic and does not require laboratory confirmation.10
In ambiguous cases, 2-tiered testing of a serum sample during the acute presentation and again 4 to 6 weeks later can be useful. In patients who remain seronegative on paired serum samples despite symptoms lasting longer than 6 weeks and no antibiotic treatment in the interim, the diagnosis of Lyme disease is unlikely, and another diagnosis should be sought.3
Antimicrobial therapy may block the serologic response; hence, negative serologic testing in patients started on empiric antibiotics should not rule out Lyme disease.6
PCR or bacterial culture testing is not recommended in the evaluation of suspected early Lyme disease.
Central nervous system Lyme disease
Central nervous system Lyme disease is diagnosed by 2-tiered testing using peripheral blood samples because all patients with this infectious manifestation should have mounted an adequate IgG response in the blood.11
B cells migrate to and proliferate inside the central nervous system, leading to intrathecal production of anti-Borrelia antibodies. An index of cerebrospinal fluid to serum antibody greater than 1 is thus also indicative of neuroborreliosis.12 Thus, performing lumbar puncture to detect intrathecal production of antibodies may support the diagnosis of central nervous system Lyme disease; however, it is not necessary.11
Antibodies persist in the central nervous system for many years after appropriate antimicrobial treatment.
Lyme arthritis
Articular involvement in Lyme disease is characterized by a robust humoral response such that a negative IgG serologic test virtually rules out Lyme arthritis.23 PCR testing of synovial fluid for borrelial DNA has a sensitivity of 80% but may become falsely negative after 1 to 2 months of antibiotic treatment.24,25 In an algorithm suggested by Puius et al,23 PCR testing of synovial fluid should be done in patients who have minimal to no response after 2 months of appropriate oral antimicrobial therapy to determine whether intravenous antibiotics are merited.
Table 5 summarizes the tests of choice in different clinical stages of infection.
Acknowledgment: The authors would like to acknowledge Anita Modi, MD, and Ceena N. Jacob, MD, for reviewing the manuscript and providing valuable suggestions, and Belinda Yen-Lieberman, PhD, for contributing pictures of the Western blot test results.
Lyme disease is a complex multisystem bacterial infection affecting the skin, joints, heart, and nervous system. The full spectrum of disease was first recognized and the disease was named in the 1970s during an outbreak of arthritis in children in the town of Lyme, Connecticut.1
This review describes the epidemiology and pathogenesis of Lyme disease, the advantages and disadvantages of current diagnostic methods, and diagnostic algorithms.
THE MOST COMMON TICK-BORNE INFECTION IN NORTH AMERICA
Lyme disease is the most common tick-borne infection in North America.2,3 In the United States, more than 30,000 cases are reported annually. In fact, in 2017, the number of cases was about 42,000, a 16% increase from the previous year, according to the US Centers for Disease Control and Prevention (CDC).
Infected nymphs account for most cases.
The infection is caused by Borrelia burgdorferi, a particularly arthritogenic spirochete transmitted by Ixodes scapularis (the black-legged deer tick, (Figure 1) and Ixodes pacificus (the Western black-legged tick). Although the infection can occur at any time of the year, its peak incidence is in May to late September, coinciding with increased outdoor recreational activity in areas where ticks live.3,4 The typical tick habitat consists of deciduous woodland with sufficient humidity provided by a good layer of decaying vegetation. However, people can contract Lyme disease in their own backyard.3
Most cases of Lyme disease are seen in the northeastern United States, mainly in suburban and rural areas.2,3 Other areas affected include the midwestern states of Minnesota, Wisconsin, and Michigan, as well as northern California.4 Fourteen states and the District of Columbia report a high average incidence (> 10 cases per 100,000 persons) (Table 1).2
FIRST COMES IgM, THEN IgG
The pathogenesis and the different stages of infection should inform laboratory testing in Lyme disease.
It is estimated that only 5% of infected ticks that bite people actually transmit their spirochetes to the human host.5 However, once infected, the patient’s innate immune system mounts a response that results in the classic erythema migrans rash at the bite site. A rash develops in only about 85% of patients who are infected and can appear at any time between 3 and 30 days, but most commonly after 7 days. Hence, a rash occurring within the first few hours of tick contact is not erythema migrans and does not indicate infection, but rather an early reaction to tick salivary antigens.5
Antibody levels remain below the detection limits of currently available serologic tests in the first 7 days after exposure. Immunoglobulin M (IgM) antibody titers peak between 8 and 14 days after tick contact, but IgM antibodies may never develop if the patient is started on early appropriate antimicrobial therapy.5
If the infection is not treated, the spirochete may disseminate through the blood from the bite site to different tissues.3 Both cell-mediated and antibody-mediated immunity swing into action to kill the spirochetes at this stage. The IgM antibody response occurs in 1 to 2 weeks, followed by a robust IgG response in 2 to 4 weeks.6
Because IgM can also cross-react with antigens other than those associated with B burgdorferi, the IgM test is less specific than the IgG test for Lyme disease.
Once a patient is exposed and mounts an antibody-mediated response to the spirochete, the antibody profile may persist for months to years, even after successful antibiotic treatment and cure of the disease.5
Despite the immune system’s robust series of defenses, untreated B burgdorferi infection can persist, as the organism has a bag of tricks to evade destruction. It can decrease its expression of specific immunogenic surface-exposed proteins, change its antigenic properties through recombination, and bind to the patient’s extracellular matrix proteins to facilitate further dissemination.3
Certain host-genetic factors also play a role in the pathogenesis of Lyme disease, such as the HLA-DR4 allele, which has been associated with antibiotic-refractory Lyme-related arthritis.3
LYME DISEASE EVOLVES THROUGH STAGES
Lyme disease evolves through stages broadly classified as early and late infection, with significant variability in its presentation.7
Early infection
Early disease is further subdivided into “localized” infection (stage 1), characterized by a single erythema migrans lesion and local lymphadenopathy, and “disseminated” infection (stage 2), associated with multiple erythema migrans lesions distant from the bite site, facial nerve palsy, radiculoneuritis, meningitis, carditis, or migratory arthritis or arthralgia.8
Highly specific physical findings include erythema migrans, cranial nerve palsy, high-grade or progressive conduction block, and recurrent migratory polyarthritis. Less specific symptoms and signs of Lyme disease include arthralgia, myalgia, neck stiffness, palpitations, and myocarditis.5
Erythema migrans lesions are evident in at least 85% of patients with early disease.9 If they are not apparent on physical examination, they may be located at hidden sites and may be atypical in appearance or transient.5
If treatment is not started in the initial stage of the disease, 60% of infected patients may develop disseminated infection.5 Progressive, untreated infection can manifest with Lyme arthritis and neuroborreliosis.7
Noncutaneous manifestations are less common now than in the past due to increased awareness of the disease and early initiation of treatment.10
Late infection
Manifestations of late (stage 3) infection include oligoarthritis (affecting any joint but often the knee) and neuroborreliosis. Clinical signs and symptoms of Lyme disease may take months to resolve even after appropriate antimicrobial therapy is completed. This should not be interpreted as ongoing, persistent infection, but as related to host immune-mediated activity.5
INTERPRET LABORATORY RESULTS BASED ON PRETEST PROBABILITY
The usefulness of a laboratory test depends on the individual patient’s pretest probability of infection, which in turn depends on the patient’s epidemiologic risk of exposure and clinical features of Lyme disease. Patients with a high pretest probability—eg, a history of a tick bite followed by the classic erythema migrans rash—do not need testing and can start antimicrobial therapy right away.11
Serologic tests are the gold standard
Prompt diagnosis is important, as early Lyme disease is easily treatable without any future sequelae.11
Tests for Lyme disease can be divided into direct methods, which detect the spirochete itself by culture or by polymerase chain reaction (PCR), and indirect methods, which detect antibodies (Table 2). Direct tests lack sensitivity for Lyme disease; hence, serologic tests remain the gold standard. Currently recommended is a standard 2-tier testing strategy using an enzyme-linked immunosorbent assay (ELISA) followed by Western blot for confirmation.
DIRECT METHODS
Culture lacks sensitivity
A number of factors limit the sensitivity of direct culture for diagnosing Lyme disease. B burgdorferi does not grow easily in culture, requiring special media, low temperatures, and long periods of incubation. Only a relatively few spirochetes are present in human tissues and body fluids to begin with, and bacterial counts are further reduced with duration and dissemination of infection.5 All of these limit the possibility of detecting this organism.
Polymerase chain reaction may help in some situations
Molecular assays are not part of the standard evaluation and should be used only in conjunction with serologic testing.7 These tests have high specificity but lack consistent sensitivity.
That said, PCR testing may be useful:
- In early infection, before antibody responses develop
- In reinfection, when serologic tests are not reliable because the antibodies persist for many years after an infection in many patients
- In endemic areas where serologic testing has high false-positive rates due to high baseline population seropositivity for anti-Borrelia antibodies caused by subclinical infection.3
PCR assays that target plasmid-borne genes encoding outer surface proteins A and C (OspA and OspC) and VisE (variable major protein-like sequence, expressed) are more sensitive than those that detect chromosomal 16s ribosomal ribonucleic acid (rRNA) genes, as plasmid-rich “blebs” are shed in larger concentrations than chromosomal DNA during active infection.7 However, these plasmid-contained genes persist in body tissues and fluids even after the infection is cleared, and their detection may not necessarily correlate with ongoing disease.8 Detection of chromosomal 16s rRNA genes is a better predictor of true organism viability.
The sensitivity of PCR for borrelial DNA depends on the type of sample. If a skin biopsy sample is taken of the leading edge of an erythema migrans lesion, the sensitivity is 69% and the specificity is 100%. In patients with Lyme arthritis, PCR of the synovial fluid has a sensitivity of up to 80%. However, the sensitivity of PCR of the cerebrospinal fluid of patients with neurologic manifestations of Lyme disease is only 19%.7 PCR of other clinical samples, including blood and urine, is not recommended, as spirochetes are primarily confined to tissues, and very few are present in these body fluids.3,12
The disadvantage of PCR is that a positive result does not always mean active infection, as the DNA of the dead microbe persists for several months even after successful treatment.8
INDIRECT METHODS
Enzyme-linked immunosorbent assay
ELISAs detect anti-Borrelia antibodies. Early-generation ELISAs, still used in many laboratories, use whole-cell extracts of B burgdorferi. Examples are the Vidas Lyme screen (Biomérieux, biomerieux-usa.com) and the Wampole B burgdorferi IgG/M EIA II assay (Alere, www.alere.com). Newer ELISAs use recombinant proteins.13
Three major targets for ELISA antibodies are flagellin (Fla), outer surface protein C (OspC), and VisE, especially the invariable region 6 (IR6). Among these, VisE-IR6 is the most conserved region in B burgdorferi.
Early-generation assays have a sensitivity of 89% and specificity of 72%.11 However, the patient’s serum may have antibodies that cross-react with unrelated bacterial antigens, leading to false-positive results (Table 3). Whole-cell sonicate assays are not recommended as an independent test and must be confirmed with Western blot testing when assay results are indeterminate or positive.11
Newer-generation ELISAs detect antibodies targeting recombinant proteins of VisE, especially a synthetic peptide C6, within IR6.13 VisE-IR6 is the most conserved region of the B burgdorferi complex, and its detection is a highly specific finding, supporting the diagnosis of Lyme disease. Antibodies against VisE-IR6 antigen are the earliest to develop.5 An example of a newer-generation serologic test is the VisE C6 Lyme EIA kit, approved as a first-tier test by the US Food and Drug Administration in 2001. This test has a specificity of 99%,14,15 and its specificity is further increased when used in conjunction with Western blot (99.5%).15 The advantage of the C6 antibody test is that it is more sensitive than 2-tier testing during early infection (sensitivity 29%–74% vs 17%–40% in early localized infection, and 56%–90% vs 27%–78% in early disseminated infection).6
During early infection, older and newer ELISAs are less sensitive because of the limited number of antigens expressed at this stage.13 All patients suspected of having early Lyme disease who are seronegative at initial testing should have follow-up testing to look for seroconversion.13
Western blot
Western blot (immunoblot) testing identifies IgM and IgG antibodies against specific B burgdorferi antigens. It is considered positive if it detects at least 2 of a possible 3 specific IgM bands in the first 4 weeks of disease or at least 5 of 10 specific IgG bands after 4 weeks of disease (Table 4 and Figure 2).16
The nature of the bands indicates the duration of infection: Western blot bands against 23-kD OspC and 41-kD FlaB are seen in early localized infection, whereas bands against all 3 B burgdorferi proteins will be seen after several weeks of disease.17 The IgM result should be interpreted carefully, as only 2 bands are required for the test to be positive, and IgM binds to antigen less specifically than IgG.12
Interpreting the IgM Western blot test: The ‘1-month rule’
If clinical symptoms and signs of Lyme disease have been present for more than 1 month, IgM reactivity alone should not be used to support the diagnosis, in view of the likelihood of a false-positive test result in this situation.18 This is called the “1-month rule” in the diagnosis of Lyme disease.13
In early localized infection, Western blot is only half as sensitive as ELISA testing. Since the overall sensitivity of a 2-step algorithm is equal to that of its least sensitive component, 2-tiered testing is not useful in early disease.13
Although currently considered the most specific test for confirmation of Lyme disease, Western blot has limitations. It is technically and interpretively complex and is thus not universally available.13 The blots are scored by visual examination, compromising the reproducibility of the test, although densitometric blot analysis techniques and automated scanning and scoring attempt to address some of these limitations.13 Like the ELISA, Western blot can have false-positive results in healthy individuals without tick exposure, as nonspecific IgM immunoblots develop faint bands. This is because of cross-reaction between B burgdorferi antigens and antigens from other microorganisms. Around 50% of healthy adults show low-level serum IgG reactivity against the FlaB antigen, leading to false-positive results as well. In cases in which the Western blot result is indeterminate, other etiologies must be considered.
False-positive IgM Western blots are a significant problem. In a 5-year retrospective study done at 63 US Air Force healthcare facilities, 113 (53.3%) of 212 IgM Western blots were falsely positive.19 A false-positive test was defined as one that failed to meet seropositivity (a first-tier test omitted or negative, > 30 days of symptoms with negative IgG blot), lack of exposure including residing in areas without documented tick habitats, patients having atypical or no symptoms, and negative serology within 30 days of a positive test.
In a similar study done in a highly endemic area, 50 (27.5%) of 182 patients had a false-positive test.20 Physicians need to be careful when interpreting IgM Western blots. It is always important to consider locale, epidemiology, and symptoms when interpreting the test.
Limitations of serologic tests for Lyme disease
Currently available serologic tests have inherent limitations:
- Antibodies against B burgdorferi take at least 1 week to develop
- The background rate of seropositivity in endemic areas can be up to 4%, affecting the utility of a positive test result
- Serologic tests cannot be used as tests of cure because antibodies can persist for months to years even after appropriate antimicrobial therapy and cure of disease; thus, a positive serologic result could represent active infection or remote exposure21
- Antibodies can cross-react with related bacteria, including other borrelial or treponemal spirochetes
- False-positive serologic test results can also occur in association with other medical conditions such as polyclonal gammopathies and systemic lupus erythematosus.12
RECOMMENDATIONS FOR TESTING
Standard 2-tier testing
The CDC released recommendations for diagnosing Lyme disease after a second national conference of serologic diagnosis of Lyme disease in October 1994.18 The 2-tiered testing method, involving a sensitive ELISA followed by the Western blot to confirm positive and indeterminate ELISA results, was suggested as the gold standard for diagnosis (Figure 3). Of note, negative ELISA results do not require further testing.11
The sensitivity of 2-tiered testing depends on the stage of the disease. Unfortunately, this method has a wide range of sensitivity (17% to 78%) in stage 1 disease. In the same stage, the sensitivity increases from 14.1% in patients with a single erythema migrans lesion and early localized infection to 65.4% in those with multiple lesions. The algorithm has excellent sensitivity in late stage 3 infection (96% to 100%).5
A 2-step ELISA algorithm
A 2-step ELISA algorithm (without the Western blot) that includes the whole-cell sonicate assay followed by the VisE C6 peptide assay actually showed higher sensitivity and comparable specificity compared with 2-tiered testing in early localized disease (sensitivity 61%–74% vs 29%–48%, respectively; specificity 99.5% for both methods).22 This higher sensitivity was even more pronounced in early disseminated infection (sensitivity 100% vs 40%, respectively). By late infection, the sensitivities of both testing strategies reached 100%. Compared with the Western blot, the 2-step ELISA algorithm was simpler to execute in a reproducible fashion.5
The Infectious Diseases Society of America is revising its current guidelines, with an update expected late this year, which may shift the recommendation from 2-tiered testing to the 2-step ELISA algorithm.
Multiplex testing
To overcome the intrinsic problems of protein-based assays, a multiplexed, array-based assay for the diagnosis of tick-borne infections called Tick-Borne Disease Serochip (TBD-Serochip) was established using recombinant antigens that identify key immunodominant epitopes.8 More studies are needed to establish the validity and usefulness of these tests in clinical practice.
Who should not be tested?
The American College of Physicians6 recommends against testing in patients:
- Presenting with nonspecific symptoms (eg, headache, myalgia, fatigue, arthralgia) without objective signs of Lyme disease
- With low pretest probability of infection based on epidemiologic exposures and clinical features
- Living in Lyme-endemic areas with no history of tick exposure6
- Presenting less than 1 week after tick exposure5
- Seeking a test of cure for treated Lyme disease.
DIAGNOSIS IN SPECIAL SITUATIONS
Early Lyme disease
The classic erythema migrans lesion on physical examination of a patient with suspected Lyme disease is diagnostic and does not require laboratory confirmation.10
In ambiguous cases, 2-tiered testing of a serum sample during the acute presentation and again 4 to 6 weeks later can be useful. In patients who remain seronegative on paired serum samples despite symptoms lasting longer than 6 weeks and no antibiotic treatment in the interim, the diagnosis of Lyme disease is unlikely, and another diagnosis should be sought.3
Antimicrobial therapy may block the serologic response; hence, negative serologic testing in patients started on empiric antibiotics should not rule out Lyme disease.6
PCR or bacterial culture testing is not recommended in the evaluation of suspected early Lyme disease.
Central nervous system Lyme disease
Central nervous system Lyme disease is diagnosed by 2-tiered testing using peripheral blood samples because all patients with this infectious manifestation should have mounted an adequate IgG response in the blood.11
B cells migrate to and proliferate inside the central nervous system, leading to intrathecal production of anti-Borrelia antibodies. An index of cerebrospinal fluid to serum antibody greater than 1 is thus also indicative of neuroborreliosis.12 Thus, performing lumbar puncture to detect intrathecal production of antibodies may support the diagnosis of central nervous system Lyme disease; however, it is not necessary.11
Antibodies persist in the central nervous system for many years after appropriate antimicrobial treatment.
Lyme arthritis
Articular involvement in Lyme disease is characterized by a robust humoral response such that a negative IgG serologic test virtually rules out Lyme arthritis.23 PCR testing of synovial fluid for borrelial DNA has a sensitivity of 80% but may become falsely negative after 1 to 2 months of antibiotic treatment.24,25 In an algorithm suggested by Puius et al,23 PCR testing of synovial fluid should be done in patients who have minimal to no response after 2 months of appropriate oral antimicrobial therapy to determine whether intravenous antibiotics are merited.
Table 5 summarizes the tests of choice in different clinical stages of infection.
Acknowledgment: The authors would like to acknowledge Anita Modi, MD, and Ceena N. Jacob, MD, for reviewing the manuscript and providing valuable suggestions, and Belinda Yen-Lieberman, PhD, for contributing pictures of the Western blot test results.
- Steere AC, Malawista SE, Snydman DR, et al. Lyme arthritis: an epidemic of oligoarticular arthritis in children and adults in three Connecticut communities. Arthritis Rheum 1977; 20(1):7–17. doi:10.1002/art.1780200102
- Centers for Disease Control and Prevention (CDC). Lyme disease: recent surveillance data. https://www.cdc.gov/lyme/datasurveillance/recent-surveillance-data.html. Accessed August 12, 2019.
- Stanek G, Wormser GP, Gray J, Strle F. Lyme borreliosis. Lancet 2012; 379(9814):461–473. doi:10.1016/S0140-6736(11)60103-7
- Arvikar SL, Steere AC. Diagnosis and treatment of Lyme arthritis. Infect Dis Clin North Am 2015; 29(2):269–280. doi:10.1016/j.idc.2015.02.004
- Schriefer ME. Lyme disease diagnosis: serology. Clin Lab Med 2015; 35(4):797–814. doi:10.1016/j.cll.2015.08.001
- Hu LT. Lyme disease. Ann Intern Med 2016; 164(9):ITC65–ITC80. doi:10.7326/AITC201605030
- Alby K, Capraro GA. Alternatives to serologic testing for diagnosis of Lyme disease. Clin Lab Med 2015; 35(4):815–825. doi:10.1016/j.cll.2015.07.005
- Dumler JS. Molecular diagnosis of Lyme disease: review and meta-analysis. Mol Diagn 2001; 6(1):1–11. doi:10.1054/modi.2001.21898
- Wormser GP, McKenna D, Carlin J, et al. Brief communication: hematogenous dissemination in early Lyme disease. Ann Intern Med 2005; 142(9):751–755. doi:10.7326/0003-4819-142-9-200505030-00011
- Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2006; 43(9):1089–1134. doi:10.1086/508667
- Guidelines for laboratory evaluation in the diagnosis of Lyme disease. American College of Physicians. Ann Intern Med 1997; 127(12):1106–1108. doi:10.7326/0003-4819-127-12-199712150-00010
- Halperin JJ. Lyme disease: a multisystem infection that affects the nervous system. Continuum (Minneap Minn) 2012; 18(6 Infectious Disease):1338–1350. doi:10.1212/01.CON.0000423850.24900.3a
- Branda JA, Body BA, Boyle J, et al. Advances in serodiagnostic testing for Lyme disease are at hand. Clin Infect Dis 2018; 66(7):1133–1139. doi:10.1093/cid/cix943
- Immunetics. Immunetics® C6 Lyme ELISA™ Kit. http://www.oxfordimmunotec.com/international/wp-content/uploads/sites/3/CF-E601-096A-C6-Pkg-Insrt.pdf. Accessed August 12, 2019.
- Civelek M, Lusis AJ. Systems genetics approaches to understand complex traits. Nat Rev Genet 2014; 15(1):34–48. doi:10.1038/nrg3575
- Centers for Disease Control and Prevention (CDC). Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. MMWR Morb Mortal Wkly Rep 1995; 44(31):590–591. pmid:7623762
- Steere AC, Mchugh G, Damle N, Sikand VK. Prospective study of serologic tests for Lyme disease. Clin Infect Dis 2008; 47(2):188–195. doi:10.1086/589242
- Centers for Disease Control and Prevention. Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. JAMA 1995; 274(12):937. pmid:7674514
- Webber BJ, Burganowski RP, Colton L, Escobar JD, Pathak SR, Gambino-Shirley KJ. Lyme disease overdiagnosis in a large healthcare system: a population-based, retrospective study. Clin Microbiol Infect 2019. doi:10.1016/j.cmi.2019.02.020. Epub ahead of print.
- Seriburi V, Ndukwe N, Chang Z, Cox ME, Wormser GP. High frequency of false positive IgM immunoblots for Borrelia burgdorferi in clinical practice. Clin Microbiol Infect 2012; 18(12):1236–1240. doi:10.1111/j.1469-0691.2011.03749.x
- Hilton E, DeVoti J, Benach JL, et al. Seroprevalence and seroconversion for tick-borne diseases in a high-risk population in the northeast United States. Am J Med 1999; 106(4):404–409. doi:10.1016/s0002-9343(99)00046-7
- Branda JA, Linskey K, Kim YA, Steere AC, Ferraro MJ. Two-tiered antibody testing for Lyme disease with use of 2 enzyme immunoassays, a whole-cell sonicate enzyme immunoassay followed by a VlsE C6 peptide enzyme immunoassay. Clin Infect Dis 2011; 53(6):541–547. doi:10.1093/cid/cir464
- Puius YA, Kalish RA. Lyme arthritis: pathogenesis, clinical presentation, and management. Infect Dis Clin North Am 2008; 22(2):289–300. doi:10.1016/j.idc.2007.12.014
- Nocton JJ, Dressler F, Rutledge BJ, Rys PN, Persing DH, Steere AC. Detection of Borrelia burgdorferi DNA by polymerase chain reaction in synovial fluid from patients with Lyme arthritis. N Engl J Med 1994; 330(4):229–234. doi:10.1056/NEJM199401273300401
- Liebling MR, Nishio MJ, Rodriguez A, Sigal LH, Jin T, Louie JS. The polymerase chain reaction for the detection of Borrelia burgdorferi in human body fluids. Arthritis Rheum 1993; 36(5):665–975. doi:10.1002/art.1780360514
- Steere AC, Malawista SE, Snydman DR, et al. Lyme arthritis: an epidemic of oligoarticular arthritis in children and adults in three Connecticut communities. Arthritis Rheum 1977; 20(1):7–17. doi:10.1002/art.1780200102
- Centers for Disease Control and Prevention (CDC). Lyme disease: recent surveillance data. https://www.cdc.gov/lyme/datasurveillance/recent-surveillance-data.html. Accessed August 12, 2019.
- Stanek G, Wormser GP, Gray J, Strle F. Lyme borreliosis. Lancet 2012; 379(9814):461–473. doi:10.1016/S0140-6736(11)60103-7
- Arvikar SL, Steere AC. Diagnosis and treatment of Lyme arthritis. Infect Dis Clin North Am 2015; 29(2):269–280. doi:10.1016/j.idc.2015.02.004
- Schriefer ME. Lyme disease diagnosis: serology. Clin Lab Med 2015; 35(4):797–814. doi:10.1016/j.cll.2015.08.001
- Hu LT. Lyme disease. Ann Intern Med 2016; 164(9):ITC65–ITC80. doi:10.7326/AITC201605030
- Alby K, Capraro GA. Alternatives to serologic testing for diagnosis of Lyme disease. Clin Lab Med 2015; 35(4):815–825. doi:10.1016/j.cll.2015.07.005
- Dumler JS. Molecular diagnosis of Lyme disease: review and meta-analysis. Mol Diagn 2001; 6(1):1–11. doi:10.1054/modi.2001.21898
- Wormser GP, McKenna D, Carlin J, et al. Brief communication: hematogenous dissemination in early Lyme disease. Ann Intern Med 2005; 142(9):751–755. doi:10.7326/0003-4819-142-9-200505030-00011
- Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2006; 43(9):1089–1134. doi:10.1086/508667
- Guidelines for laboratory evaluation in the diagnosis of Lyme disease. American College of Physicians. Ann Intern Med 1997; 127(12):1106–1108. doi:10.7326/0003-4819-127-12-199712150-00010
- Halperin JJ. Lyme disease: a multisystem infection that affects the nervous system. Continuum (Minneap Minn) 2012; 18(6 Infectious Disease):1338–1350. doi:10.1212/01.CON.0000423850.24900.3a
- Branda JA, Body BA, Boyle J, et al. Advances in serodiagnostic testing for Lyme disease are at hand. Clin Infect Dis 2018; 66(7):1133–1139. doi:10.1093/cid/cix943
- Immunetics. Immunetics® C6 Lyme ELISA™ Kit. http://www.oxfordimmunotec.com/international/wp-content/uploads/sites/3/CF-E601-096A-C6-Pkg-Insrt.pdf. Accessed August 12, 2019.
- Civelek M, Lusis AJ. Systems genetics approaches to understand complex traits. Nat Rev Genet 2014; 15(1):34–48. doi:10.1038/nrg3575
- Centers for Disease Control and Prevention (CDC). Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. MMWR Morb Mortal Wkly Rep 1995; 44(31):590–591. pmid:7623762
- Steere AC, Mchugh G, Damle N, Sikand VK. Prospective study of serologic tests for Lyme disease. Clin Infect Dis 2008; 47(2):188–195. doi:10.1086/589242
- Centers for Disease Control and Prevention. Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. JAMA 1995; 274(12):937. pmid:7674514
- Webber BJ, Burganowski RP, Colton L, Escobar JD, Pathak SR, Gambino-Shirley KJ. Lyme disease overdiagnosis in a large healthcare system: a population-based, retrospective study. Clin Microbiol Infect 2019. doi:10.1016/j.cmi.2019.02.020. Epub ahead of print.
- Seriburi V, Ndukwe N, Chang Z, Cox ME, Wormser GP. High frequency of false positive IgM immunoblots for Borrelia burgdorferi in clinical practice. Clin Microbiol Infect 2012; 18(12):1236–1240. doi:10.1111/j.1469-0691.2011.03749.x
- Hilton E, DeVoti J, Benach JL, et al. Seroprevalence and seroconversion for tick-borne diseases in a high-risk population in the northeast United States. Am J Med 1999; 106(4):404–409. doi:10.1016/s0002-9343(99)00046-7
- Branda JA, Linskey K, Kim YA, Steere AC, Ferraro MJ. Two-tiered antibody testing for Lyme disease with use of 2 enzyme immunoassays, a whole-cell sonicate enzyme immunoassay followed by a VlsE C6 peptide enzyme immunoassay. Clin Infect Dis 2011; 53(6):541–547. doi:10.1093/cid/cir464
- Puius YA, Kalish RA. Lyme arthritis: pathogenesis, clinical presentation, and management. Infect Dis Clin North Am 2008; 22(2):289–300. doi:10.1016/j.idc.2007.12.014
- Nocton JJ, Dressler F, Rutledge BJ, Rys PN, Persing DH, Steere AC. Detection of Borrelia burgdorferi DNA by polymerase chain reaction in synovial fluid from patients with Lyme arthritis. N Engl J Med 1994; 330(4):229–234. doi:10.1056/NEJM199401273300401
- Liebling MR, Nishio MJ, Rodriguez A, Sigal LH, Jin T, Louie JS. The polymerase chain reaction for the detection of Borrelia burgdorferi in human body fluids. Arthritis Rheum 1993; 36(5):665–975. doi:10.1002/art.1780360514
KEY POINTS
- Lyme disease, the most common tick-borne infection in North America, is a complex multisystem bacterial disease caused by Borrelia burgdorferi.
- Lyme disease preferably affects the skin, joints, and nervous system and presents with typical and atypical features. Certain clinical features are diagnostic. Its diagnosis is mainly clinical and epidemiologic and, when doubtful, is supported by serologic testing.
- Standard 2-tiered testing is the diagnostic testing method of choice—enzyme-linked immunoassay followed by Western blot. Interpretation of the bands depends on the duration of infection.
- When interpreting the test results, be aware of false-positives and the reasons for them.
Common infectious complications of liver transplant
The immunosuppressed state of liver transplant recipients makes them vulnerable to infections after surgery.1 These infections are directly correlated with the net state of immunosuppression. Higher levels of immunosuppression mean a higher risk of infection, with rates of infection typically highest in the early posttransplant period.
Common infections during this period include operative and perioperative nosocomial bacterial and fungal infections, reactivation of latent infections, and invasive fungal infections such as candidiasis, aspergillosis, and pneumocystosis. Donor-derived infections also must be considered. As time passes and the level of immunosuppression is reduced, liver recipients are less prone to infection.1
The risk of infection can be minimized by appropriate antimicrobial prophylaxis, strategies for safe living after transplant,2 vaccination,3 careful balancing of immunosuppressive therapy,4 and thoughtful donor selection.5 Drug-drug interactions are common and must be carefully considered to minimize the risk.
This review highlights common infectious complications encountered after liver transplant.
INTRA-ABDOMINAL INFECTIONS
Intra-abdominal infections are common in the early postoperative period.6,7
Risk factors include:
- Pretransplant ascites
- Posttransplant dialysis
- Wound infection
- Reoperation8
- Hepatic artery thrombosis
- Roux-en-Y choledochojejunostomy anastomosis.9
Signs that may indicate intra-abdominal infection include fever, abdominal pain, leukocytosis, and elevated liver enzymes. But because of their immunosuppressed state, transplant recipients may not manifest fever as readily as the general population. They should be evaluated for cholangitis, peritonitis, biloma, and intra-abdominal abscess.
Organisms. Intra-abdominal infections are often polymicrobial. Enterococci, Staphylococcus aureus, gram-negative species including Pseudomonas, Klebsiella, and Acinetobacter, and Candida species are the most common pathogens. Strains are often resistant to multiple drugs, especially in patients who received antibiotics in the weeks before transplant.8,10
Liver transplant recipients are also particularly susceptible to Clostridium difficile-associated colitis as a result of immunosuppression and frequent use of antibiotics perioperatively and postoperatively.11 The spectrum of C difficile infection ranges from mild diarrhea to life-threatening colitis, and the course in liver transplant patients tends to be more complicated than in immunocompetent patients.12
Diagnosis. Intra-abdominal infections should be looked for and treated promptly, as they are associated with a higher mortality rate, a greater risk of graft loss, and a higher incidence of retransplant.6,10 Abdominal ultrasonography or computed tomography (CT) can confirm the presence of fluid collections.
Treatment. Infected collections can be treated with percutaneous or surgical drainage and antimicrobial therapy. In the case of biliary tract complications, retransplant or surgical correction of biliary leakage or stenosis decreases the risk of death.6
Suspicion should be high for C difficile-associated colitis in cases of posttransplant diarrhea. C difficile toxin stool assays help confirm the diagnosis.12 Oral metronidazole is recommended in mild to moderate C difficile infection, with oral vancomycin and intravenous metronidazole reserved for severe cases. Colectomy may be necessary in patients with toxic megacolon.
CYTOMEGALOVIRUS INFECTION
Cytomegalovirus is an important opportunistic pathogen in liver transplant recipients.13 It causes a range of manifestations, from infection (viremia with or without symptoms) to cytomegalovirus syndrome (fever, malaise, and cell-line cytopenias) to tissue-invasive disease with end-organ disease.14 Without preventive measures and treatment, cytomegalovirus disease can increase the risk of morbidity, allograft loss and death.15,16
Risk factors for cytomegalovirus infection (Table 1) include:
- Discordant serostatus of the donor and recipient (the risk is highest in seronegative recipients of organs from seropositive donors)
- Higher levels of immunosuppression, especially when antilymphocyte antibodies are used
- Treatment of graft rejection
- Coinfection with other human herpesviruses, such as Epstein-Barr virus.4,17
Preventing cytomegalovirus infection
The strategy to prevent cytomegalovirus infection depends on the serologic status of the donor and recipient and may include antiviral prophylaxis or preemptive treatment (Table 2).18
Prophylaxis involves giving antiviral drugs during the early high-risk period, with the goal of preventing the development of cytomegalovirus viremia. The alternative preemptive strategy emphasizes serial testing for cytomegalovirus viremia, with the goal of intervening with antiviral medications while viremia is at a low level, thus avoiding potential progression to cytomegalovirus disease. Both strategies have pros and cons that should be considered by each transplant center when setting institutional policy.
A prophylactic approach seems very effective at preventing both infection and disease from cytomegalovirus and has been shown to reduce graft rejection and the risk of death.18 It is preferred in cytomegalovirus-negative recipients when the donor was cytomegalovirus-positive—a high-risk situation.19 However, these patients are also at higher risk of late-onset cytomegalovirus disease. Higher cost and potential drug toxicity, mainly neutropenia from ganciclovir-based regimens, are additional considerations.
Preemptive treatment, in contrast, reserves drug treatment for patients who are actually infected with cytomegalovirus, thus resulting in fewer adverse drug events and lower cost; but it requires regular monitoring. Preemptive methods, by definition, cannot prevent infection, and with this strategy tissue-invasive disease not associated with viremia does occasionally occur.20 As such, patients with a clinical presentation that suggests cytomegalovirus but have negative results on blood testing should be considered for tissue biopsy with culture and immunohistochemical stain.
The most commonly used regimens for antiviral prophylaxis and treatment in liver transplant recipients are intravenous ganciclovir and oral valganciclovir.21 Although valganciclovir is the most commonly used agent in this setting because of ease of administration, it has not been approved by the US Food and Drug Administration in liver transplant patients, as it was associated with higher rates of cytomegalovirus tissue-invasive disease.22–24 Additionally, drug-resistant cytomegalovirus strains have been associated with valganciclovir prophylaxis in cytomegalovirus-negative recipients of solid organs from cytomegalovirus-positive donors.25
Prophylaxis typically consists of therapy for 3 months from the time of transplant. In higher-risk patients (donor-positive, recipient-negative), longer courses of prophylaxis have been extrapolated from data in kidney transplant recipients.26 Extension or reinstitution of prophylaxis should also be considered in liver transplant patients receiving treatment for rejection with antilymphocyte therapy.
Routine screening for cytomegalovirus is not recommended while patients are receiving prophylaxis. High-risk patients who are not receiving prophylaxis should be monitored with nucleic acid or pp65 antigenemia testing as part of the preemptive strategy protocol.
Treatment of cytomegalovirus disease
Although no specific threshold has been established, treatment is generally indicated if a patient has a consistent clinical syndrome, evidence of tissue injury, and persistent or increasing viremia.
Treatment involves giving antiviral drugs and also reducing the level of immunosuppression, if possible, until symptoms and viremia have resolved.
The choice of antiviral therapy depends on the severity of disease. Intravenous ganciclovir (5 mg/kg twice daily adjusted for renal impairment) or oral valganciclovir (900 mg twice daily, also renally dose-adjusted when necessary) can be used for mild to moderate disease if no significant gastrointestinal involvement is reported. Intravenous ganciclovir is preferred for patients with more severe disease or gastrointestinal involvement. The minimum duration of treatment is 2 weeks and may need to be prolonged until both symptoms and viremia completely resolve.18
Drug resistance can occur and should be considered in patients who have a history of prolonged ganciclovir or valganciclovir exposure who do not clinically improve or have persistent or rising viremia. In such cases, genotype assays are helpful, and initiation of alternative therapy should be considered. Mutations conferring resistance to ganciclovir are often associated with cross-resistance to cidofovir. Cidofovir can therefore be considered only when genotype assays demonstrate specific mutations conferring an isolated resistance to ganciclovir.27 The addition of foscarnet to the ganciclovir regimen or substitution of foscarnet for ganciclovir are accepted approaches.
Although cytomegalovirus hyperimmunoglobulin has been used in prophylaxis and invasive disease treatment, its role in the management of ganciclovir-resistant cytomegalovirus infections remains controversial.28
EPSTEIN-BARR VIRUS POSTTRANSPLANT LYMPHOPROLIFERATIVE DISEASE
Epstein-Barr virus-associated posttransplant lymphoproliferative disease is a spectrum of disorders ranging from an infectious mononucleosis syndrome to aggressive malignancy with the potential for death and significant morbidity after liver transplant.29 The timeline of risk varies, but the disease is most common in the first year after transplant.
Risk factors for this disease (Table 1) are:
- Primary Epstein-Barr virus infection
- Cytomegalovirus donor-recipient mismatch
- Cytomegalovirus disease
- Higher levels of immunosuppression, especially with antilymphocyte antibodies.30
The likelihood of Epstein-Barr virus playing a contributing role is lower in later-onset posttransplant lymphoproliferative disease. Patients who are older at the time of transplant, who receive highly immunogenic allografts including a liver as a component of a multivisceral transplant, and who receive increased immunosuppression to treat rejection are at even greater risk of late posttransplant lymphoproliferative disease.31 This is in contrast to early posttransplant lymphoproliferative disease, which is seen more commonly in children as a result of primary Epstein-Barr virus infection.
Recognition and diagnosis. Heightened suspicion is required when considering posttransplant lymphoproliferative disease, and careful evaluation of consistent symptoms and allograft dysfunction are required.
Clinically, posttransplant lymphoproliferative disease should be suspected if a liver transplant recipient develops unexplained fever, weight loss, lymphadenopathy, or cell-line cytopenias.30,32 Other signs and symptoms may be related to the organ involved and may include evidence of hepatitis, pneumonitis, and gastrointestinal disease.31
Adjunctive diagnostic testing includes donor and recipient serology to characterize overall risk before transplantation and quantification of Epstein-Barr viral load, but confirmation relies on tissue histopathology.
Treatment focuses on reducing immunosuppression.30,32 Adding antiviral agents does not seem to improve outcome in all cases.33 Depending on clinical response and histologic classification, additional therapies such as anti-CD20 humanized chimeric monoclonal antibodies, surgery, radiation, and conventional chemotherapy may be required.34
Preventive approaches remain controversial. Chemoprophylaxis with an antiviral such as ganciclovir is occasionally used but has not been shown to consistently decrease rates of posttransplant lymphoproliferative disease. These agents may act in an indirect manner, leading to decreased rates of cytomegalovirus infection, a major cofactor for posttransplant lymphoproliferative disease.24
Passive immunoprophylaxis with immunoglobulin targeting cytomegalovirus has shown to decrease rates of non-Hodgkin lymphoma from posttransplant lymphoproliferative disease in renal transplant recipients in the first year after transplant,35 but data are lacking regarding its use in liver transplant recipients. Monitoring of the viral load and subsequent reduction of immunosuppression remain the most efficient measures to date.36
FUNGAL INFECTIONS
Candida species account for more than half of fungal infections in liver transplant recipients.37 However, a change has been noted in the past 20 years, with a decrease in Candida infections accompanied by an increase in Aspergillus infections.38 Endemic mycoses such as coccidioidomycosis, blastomycosis, and histoplasmosis should be considered with the appropriate epidemiologic history or if disease develops early after transplant and the donor came from a highly endemic region.39Cryptococcus may also be encountered.
Diagnosis. One of the most challenging aspects of fungal infection in liver transplant recipients is timely diagnosis. Heightened suspicion and early biopsy for pathological and microbiological confirmation are necessary. Although available noninvasive diagnostic tools often lack specificity, early detection of fungal markers may be of great use in guiding further diagnostic workup or empiric treatment in the critically ill.
Noninvasive tests include galactomannan, cryptococcal antigen, histoplasma antigen, (1-3)-beta-D-glucan assay and various antibody tests. Galactomannan testing has been widely used to aid in the diagnosis of invasive aspergillosis. Similarly, the (1-3)-beta-D-glucan assay is a non–culture-based tool for diagnosing and monitoring the treatment of invasive fungal infections. However, a definite diagnosis cannot be made on the basis of a positive test alone.40 The complementary diagnostic characteristics of combining noninvasive assays have yet to be fully elucidated.41 Cultures and tissue histopathology are also used when possible.
Treatment is based on targeted specific antifungal drug therapy and reduction of immunosuppressive therapy, when possible. The choice of antifungal agent varies with the pathogen, the site of involvement, and the severity of the disease. A focus on potential drug interactions, their management, and therapeutic drug monitoring when using antifungal medications is essential in the posttransplant period. Combination therapy can be considered in some situations to enhance synergy. The following sections discuss in greater detail Candida species, Aspergillus species, and Pneumocystis jirovecii infections.
Candida infections
Candidiasis after liver transplant is typically nosocomial, especially when diagnosed during the first 3 months (Table 3).37
Risk factors for invasive candidiasis include perioperative colonization, prolonged operative time, retransplant, greater transfusion requirements, and postoperative renal failure.37,42,43 Invasive candidiasis is of concern for its effects on morbidity, mortality, and cost of care.43–46
Organisms. The frequency of implicated species, in particular those with a natural resistance to fluconazole, differs in various reports.37,45,46Candida albicans remains the most commonly isolated pathogen; however, non-albicans species including those resistant to fluconazole have been reported more frequently and include Candida glabrata and Candida krusei.47,48
Signs and diagnosis. Invasive candidiasis in liver transplant recipients generally manifests itself in catheter-related blood stream infections, urinary tract infections, or intra-abdominal infections. Diagnosis can be made by isolating Candida from blood cultures, recovering organisms in culture of a normally sterile site, or finding direct microscopic evidence of the fungus on tissue specimens.49
Disseminated candidiasis refers to the involvement of distant anatomic sites. Clinical manifestations may cause vision changes, abdominal pain or skin nodules with findings of candidemia, hepatosplenic abscesses, or retinal exudates on funduscopy.49
Treatment of invasive candidiasis in liver recipients often involves antifungal therapy and reduction of immunosuppression. Broad-spectrum antifungals are initially advocated in an empirical approach to cover fluconazole-resistant strains of the non-albicans subgroups.50 Depending on antifungal susceptibility, treatment can later be adjusted.
Fluconazole remains the agent of choice in most C albicans infections.47 However, attention should be paid to the possibility of resistance in patients who have received fluconazole prophylaxis within the past 30 days. Additional agents used in treatment may include echinocandins, amphotericin, and additional azoles.
Antifungal prophylaxis is recommended in high-risk liver transplant patients, although its optimal duration remains undetermined.44 Antifungal prophylaxis has been associated with decreased incidence of both superficial and invasive candidiasis.51
Aspergillus infection
Aspergillus, the second most common fungal pathogen, has become a more common concern in liver transplant recipients. Aspergillus fumigatus is the most frequently encountered species.38,52
Risk factors. These infections typically occur in the first year, during intense immunosuppression. Retransplant, renal failure, and fulminant hepatic failure are major risk factors.52 In the presence of risk factors and a suggestive clinical setting, invasive aspergillosis should be considered and the diagnosis pursued.
Diagnosis is suggested by positive findings on CT accompanied by lower respiratory tract symptoms, focal lesions on neuroimaging, or demonstration of the fungus on cultures.49 However, Aspergillus is rarely grown in blood culture. The galactomannan antigen is a noninvasive test that can provide supporting evidence for the diagnosis.41,52 False-positive results do occur in the setting of certain antibiotics and cross-reacting fungi.53
Treatment consists of antifungal therapy and immunosuppression reduction.52
Voriconazole is the first-line agent for invasive aspergillosis. Monitoring for potential drug-drug interactions and side effects is required.54,55 Amphotericin B is considered a second-line choice due to toxicity and lack of an oral formulation. In refractory cases, combined antifungal therapy could be considered.52 The duration of treatment is generally a minimum of 12 weeks.
Prophylaxis. Specific prophylaxis against invasive aspergillosis is not currently recommended; however, some authors suggest a prophylactic approach using echinocandins or liposomal amphotericin B in high-risk patients.51,52 Aspergillosis is associated with a considerable increase in mortality in liver transplant recipients, which highlights the importance of timely management.52,56
Pneumocystis jirovecii
P jirovecii remains a common opportunistic pathogen in people with impaired immunity, including transplant and human immunodeficiency virus patients.
Prophylaxis. Widespread adoption of antimicrobial prophylaxis by transplant centers has decreased the rates of P jirovecii infection in liver transplant recipients.57,58 Commonly used prophylactic regimens after liver transplantation include a single-strength trimethoprim-sulfamethoxazole tablet daily or a double-strength tablet three times per week for a minimum of 6 to 12 months after transplant. Atovaquone and dapsone can be used as alternatives in cases of intolerance to trimethoprim-sulfamethoxazole (Table 2).
Inhaled pentamidine is clearly inferior and should be used only when the other medications are contraindicated.59
Signs and diagnosis. P jirovecii pneumonia is characterized by fever, cough, dyspnea, and chest pain. Insidious hypoxemia, abnormal chest examination, and bilateral interstitial pneumonia on chest radiography are common.
CT may be more sensitive than chest radiography.57 Findings suggestive of P jirovecii pneumonia on chest CT are extensive bilateral and symmetrical ground-glass attenuations. Other less-characteristic findings include upper lobar parenchymal opacities and spontaneous pneumothorax.57,60
The serum (1,3)-beta-D-glucan assay derived from major cell-wall components of P jirovecii might be helpful. Studies report a sensitivity for P jirovecii pneumonia as high as 96% and a negative predictive value of 99.8%.61,62
Definitive diagnosis requires identification of the pathogen. Routine expectorated sputum sampling is generally associated with a poor diagnostic yield. Bronchoscopy and bronchoalveolar lavage with silver or fluorescent antibody staining of samples, polymerase chain reaction testing, or both significantly improves diagnosis. Transbronchial or open lung biopsy are often unnecessary.57
Treatment. Trimethoprim-sulfamethoxazole is the first-line agent for treating P jirovecii pneumonia.57 The minimum duration of treatment is 14 days, with extended courses for severe infection.
Intravenous pentamidine or clindamycin plus primaquine are alternatives for patients who cannot tolerate trimethoprim-sulfamethoxazole. The major concern with intravenous pentamidine is renal dysfunction. Hypoglycemia or hyperglycemia, neutropenia, thrombocytopenia, nausea, dysgeusia, and pancreatitis may also occur.63
Atovaquone might also be beneficial in mild to moderate P jirovecii pneumonia. The main side effects include skin rashes, gastrointestinal intolerance, and elevation of transaminases.64
A corticosteroid (40–60 mg of prednisone or its equivalent) may be beneficial in conjunction with antimicrobial therapy in patients with significant hypoxia (partial pressure of arterial oxygen < 70 mm Hg on room air) in decreasing the risk of respiratory failure and need for intubation.
With appropriate and timely antimicrobial prophylaxis, cases of P jirovecii pneumonia should continue to decrease.
TUBERCULOSIS
Development of tuberculosis after transplantation is a catastrophic complication, with mortality rates of up to 30%.65 Most cases of posttransplant tuberculosis represent reactivation of latent disease.66 Screening with tuberculin skin tests or interferon-gamma-release assays is recommended in all liver transplant candidates. Chest radiography before transplant is necessary when assessing a positive screening test.67
The optimal management of latent tuberculosis in these cases remains controversial. Patients at high risk or those with positive screening results on chest radiography warrant treatment for latent tuberculosis infection with isoniazid unless contraindicated.67,68
The ideal time to initiate prophylactic isoniazid therapy is unclear. Some authors suggest delaying it, as it might be associated with poor tolerance and hepatotoxicity.69 Others have found that early isoniazid use was not associated with negative outcomes.70
Risk factors for symptomatic tuberculosis after liver transplant include previous infection with tuberculosis, intensified immunosuppression (especially anti-T-lymphocyte therapies), diabetes mellitus, and other co-infections (Table 1).71
The increased incidence of atypical presentations in recent years makes the diagnosis of active tuberculosis among liver transplant recipients challenging. Sputum smears can be negative due to low mycobacterial burdens, and tuberculin skin testing and interferon-gamma-release assays may be falsely negative due to immunosuppression.67
Treatment of active tuberculosis consists initially of a four-drug regimen using isoniazid, rifampin, pyrazinamide, and ethambutol for 2 months. Adjustments are made in accordance with culture and sensitivity results. Treatment can then be tapered to two drugs (isoniazid and rifampin) for a minimum of 4 additional months. Prolonged treatment may be required in instances of extrapulmonary or disseminated disease.65,72
Tuberculosis treatment can be complicated by hepatotoxicity in liver transplant recipients because of direct drug effects and drug-drug interactions with immunosuppressive agents. Close monitoring for rejection and hepatotoxicity is therefore imperative while liver transplant recipients are receiving antituberculosis therapy. Drug-drug interactions may also be responsible for marked reductions in immunosuppression levels, especially with regimens containing rifampin.71 Substitution of rifabutin for rifampin reduces the effect of drug interactions.66
VIRAL HEPATITIS
Hepatitis B virus
Hepatitis B virus-related end-stage liver disease and hepatocellular carcinoma are common indications for liver transplant in Asia. It is less common in the United States and Europe, accounting for less than 10% of all liver transplant cases. Prognosis is favorable in recipients undergoing liver transplant for hepatitis B virus, with excellent survival rates. Prevention of reinfection is crucial in these patients.
Treatment with combination antiviral agents and hepatitis B immunoglobulin (HBIG) is effective.73 Lamivudine was the first nucleoside analogue found to be effective against hepatitis B virus. Its low cost and relative safety are strong arguments in favor of its continued use in liver transplant recipients.74 In patients without evidence of hepatitis B viral replication at the time of transplant, monotherapy with lamivudine has led to low recurrence rates, and adefovir can be added to control resistant viral strains.75
The frequent emergence of resistance with lamivudine favors newer agents such as entecavir or tenofovir. These nucleoside and nucleotide analogues have a higher barrier to resistance, and thus resistance to them is rare. They are also more efficient, potentially allowing use of an HBIG-sparing protocol.76 However, they are associated with a higher risk of nephrotoxicity and require dose adjustments in renal insufficiency. Data directly comparing entecavir and tenofovir are scarce.
Prophylaxis. Most studies support an individualized approach for prevention of hepatitis B virus reinfection. High-risk patients, ie, those positive for HBe antigen or with high viral loads (> 100,000 copies/mL) are generally treated with both HBIG and antiviral agents.77 Low-risk patients are those with a negative HBe antigen, low hepatitis B virus DNA levels, hepatitis B virus-related acute liver failure, and cirrhosis resulting from coinfection with both hepatitis B and hepatitis D virus.75 In low-risk patients, discontinuation of HBIG after 1 to 2 years of treatment is appropriate, and long-term prophylaxis with antiviral agents alone is an option. However, levels of hepatitis B DNA should be monitored closely.78,79
Hepatitis C virus
Recurrence of hepatitis C virus infection is the rule among patients who are viremic at the time of liver transplant.80,81 Most of these patients will show histologic evidence of recurrent hepatitis within the first year after liver transplant. It is often difficult to distinguish between the histopathological appearance of a recurrent hepatitis C virus infection and acute cellular rejection.
Progression to fibrosis and subsequently cirrhosis and decompensation is highly variable in hepatitis C virus-infected liver transplant recipients. Diabetes, insulin resistance, and possibly hepatitis steatosis have been associated with a rapid progression to advanced fibrosis. The contribution of immunosuppression to the progression of hepatitis C virus remains an area of active study. Some studies point to antilymphocyte immunosuppressive agents as a potential cause.82 Liver biopsy is a useful tool in this situation. It allows monitoring of disease severity and progression and may distinguish recurrent hepatitis C virus disease from other causes of liver enzyme elevation.
The major concern with the recurrence of hepatitis C virus infection after liver transplant is allograft loss. Rates of patient and graft survival are reduced in infected patients compared with hepatitis C virus-negative patients.83,84 Prophylactic antiviral therapy has no current role in the management of hepatitis C virus disease. Those manifesting moderate to severe necroinflammation or mild to moderate fibrosis indicative of progressive disease should be treated.81,85
Sustained viral clearance with antiviral agents confers a graft survival benefit.
The combination of peg-interferon and weight-based ribavirin has been the standard of treatment but may be associated with increased rates of rejection.86,87 The sustained virologic response rates for hepatitis C virus range from 60% in genotypes 4, 5, and 6 after 48 weeks of treatment to 60% to 80% in genotypes 2 and 3 after 24 weeks, but only about 30% in genotype 1.88
Treatment with the newer agents, especially protease inhibitors, in genotype 1 (peg-interferon, ribavirin, and either telaprevir or boceprevir) has been evaluated. Success rates reaching 70% have been achieved.89 Adverse effects can be a major setback. Serious complications include severe anemia, renal dysfunction, increased risk of infection, and death.
Triple therapy should be carefully considered in liver transplant patients with genotype 1 hepatitis C virus.90 Significant drug-drug interactions are reported between hepatitis C virus protease inhibitors and immunosuppression regimens. Additional new oral direct- acting antivirals have been investigated. They bring promising advances in hepatitis C virus treatment and pave the way for interferon-free regimens with pangenotypic activity.
IMMUNIZATION
Immunization can decrease the risk of infectious complications in liver transplant recipients, as well as in close contacts and healthcare professionals.3
Influenza. Pretransplant influenza vaccine and posttransplant annual influenza vaccines are necessary.
Pneumococcal immunization should additionally be provided prior to transplant and repeated every 3 to 5 years thereafter.3,91
A number of other vaccinations should also be completed before transplant, including the hepatitis A and B vaccines and the tetanus/diphtheria/acellular pertussis vaccines. However, these vaccinations have not been shown to be detrimental to patients after transplant.91
Varicella and zoster vaccines should be given before liver transplant—zoster in patients over age 60, and varicella in patients with no immunity. Live vaccines, including varicella and zoster vaccines, are contraindicated after liver transplant.3
Human papillomavirus. The bivalent human papillomavirus vaccine can be given before transplant in females ages 9 to 26; the quadrivalent vaccine is beneficial in those ages 9 to 26 and in women under age 45.3,91
IMMUNOSUPPRESSION CARRIES RISK OF INFECTION
Most liver transplant patients require prolonged immunosuppressive therapy. This comes with an increased risk of new or recurrent infections, potentially causing death and significant morbidity.
Evaluation of existing risk factors, appropriate prophylaxis and immunization, timely diagnosis, and treatment of such infections are therefore essential steps for the successful management of liver transplant recipients.
- Fishman JA. Infection in solid-organ transplant recipients. N Engl J Med 2007; 357:2601–2614.
- Avery RK, Michaels MG; AST Infectious Diseases Community of Practice. Strategies for safe living after solid organ transplantation. Am J Transplant 2013; 13(suppl 4):304–310.
- Danziger-Isakov L, Kumar D; AST Infectious Diseases Community of Practice. Vaccination in solid organ transplantation. Am J Transplant 2013; 13(suppl 4):311–317.
- San Juan R, Aguado JM, Lumbreras C, et al; RESITRA Network, Spain. Incidence, clinical characteristics and risk factors of late infection in solid organ transplant recipients: data from the RESITRA study group. Am J Transplant 2007; 7:964–971.
- Ison MG, Grossi P; AST Infectious Diseases Community of Practice. Donor-derived infections in solid organ transplantation. Am J Transplant 2013; 13(suppl 4):22–30.
- Kim YJ, Kim SI, Wie SH, et al. Infectious complications in living-donor liver transplant recipients: a 9-year single-center experience. Transpl Infect Dis 2008; 10:316–324.
- Arnow PM. Infections following orthotopic liver transplantation. HPB Surg 1991; 3:221–233.
- Reid GE, Grim SA, Sankary H, Benedetti E, Oberholzer J, Clark NM. Early intra-abdominal infections associated with orthotopic liver transplantation. Transplantation 2009; 87:1706–1711.
- Said A, Safdar N, Lucey MR, et al. Infected bilomas in liver transplant recipients, incidence, risk factors and implications for prevention. Am J Transplant 2004; 4:574–582.
- Safdar N, Said A, Lucey MR, et al. Infected bilomas in liver transplant recipients: clinical features, optimal management, and risk factors for mortality. Clin Infect Dis 2004; 39:517–525.
- Niemczyk M, Leszczyniski P, Wyzgał J, Paczek L, Krawczyk M, Luczak M. Infections caused by Clostridium difficile in kidney or liver graft recipients. Ann Transplant 2005; 10:70–74.
- Albright JB, Bonatti H, Mendez J, et al. Early and late onset Clostridium difficile-associated colitis following liver transplantation. Transpl Int 2007; 20:856–866.
- Lee SO, Razonable RR. Current concepts on cytomegalovirus infection after liver transplantation. World J Hepatol 2010; 2:325–336.
- Ljungman P, Griffiths P, Paya C. Definitions of cytomegalovirus infection and disease in transplant recipients. Clin Infect Dis 2002; 34:1094–1097.
- Beam E, Razonable RR. Cytomegalovirus in solid organ transplantation: epidemiology, prevention, and treatment. Curr Infect Dis Rep 2012; 14:633–641.
- Bodro M, Sabé N, Lladó L, et al. Prophylaxis versus preemptive therapy for cytomegalovirus disease in high-risk liver transplant recipients. Liver Transpl 2012; 18:1093–1099.
- Weigand K, Schnitzler P, Schmidt J, et al. Cytomegalovirus infection after liver transplantation incidence, risks, and benefits of prophylaxis. Transplant Proc 2010; 42:2634–2641.
- Razonable RR, Humar A; AST Infectious Diseases Community of Practice. Cytomegalovirus in solid organ transplantation. Am J Transplant 2013; 13(suppl 4):93–106.
- Meije Y, Fortún J, Len Ó, et al; Spanish Network for Research on Infection in Transplantation (RESITRA) and the Spanish Network for Research on Infectious Diseases (REIPI). Prevention strategies for cytomegalovirus disease and long-term outcomes in the high-risk transplant patient (D+/R-): experience from the RESITRA-REIPI cohort. Transpl Infect Dis 2014; 16:387–396.
- Durand CM, Marr KA, Arnold CA, et al. Detection of cytomegalovirus DNA in plasma as an adjunct diagnostic for gastrointestinal tract disease in kidney and liver transplant recipients. Clin Infect Dis 2013; 57:1550–1559.
- Levitsky J, Singh N, Wagener MM, Stosor V, Abecassis M, Ison MG. A survey of CMV prevention strategies after liver transplantation. Am J Transplant 2008; 8:158–161.
- Marcelin JR, Beam E, Razonable RR. Cytomegalovirus infection in liver transplant recipients: updates on clinical management. World J Gastroenterol 2014; 20:10658–10667.
- Kalil AC, Freifeld AG, Lyden ER, Stoner JA. Valganciclovir for cytomegalovirus prevention in solid organ transplant patients: an evidence-based reassessment of safety and efficacy. PLoS One 2009; 4:e5512.
- Kalil AC, Mindru C, Botha JF, et al. Risk of cytomegalovirus disease in high-risk liver transplant recipients on valganciclovir prophylaxis: a systematic review and meta-analysis. Liver Transpl 2012; 18:1440–1447.
- Eid AJ, Arthurs SK, Deziel PJ, Wilhelm MP, Razonable RR. Emergence of drug-resistant cytomegalovirus in the era of valganciclovir prophylaxis: therapeutic implications and outcomes. Clin Transplant 2008; 22:162–170.
- Kumar D, Humar A. Cytomegalovirus prophylaxis: how long is enough? Nat Rev Nephrol 2010; 6:13–14.
- Lurain NS, Chou S. Antiviral drug resistance of human cytomegalovirus. Clin Microbiol Rev 2010; 23:689–712.
- Torres-Madriz G, Boucher HW. Immunocompromised hosts: perspectives in the treatment and prophylaxis of cytomegalovirus disease in solid-organ transplant recipients. Clin Infect Dis 2008; 47:702–711.
- Burra P, Buda A, Livi U, et al. Occurrence of post-transplant lymphoproliferative disorders among over thousand adult recipients: any role for hepatitis C infection? Eur J Gastroenterol Hepatol 2006; 18:1065–1070.
- Jain A, Nalesnik M, Reyes J, et al. Posttransplant lymphoproliferative disorders in liver transplantation: a 20-year experience. Ann Surg 2002; 236:429–437.
- Allen UD, Preiksaitis JK; AST Infectious Diseases Community of Practice. Epstein-Barr virus and posttransplant lymphoproliferative disorder in solid organ transplantation. Am J Transplant 2013; 13(suppl 4):107–120.
- Allen U, Preiksaitis J; AST Infectious Diseases Community of Practice. Epstein-Barr virus and posttransplant lymphoproliferative disorder in solid organ transplant recipients. Am J Transplant 2009; 9(suppl 4):S87–S96.
- Perrine SP, Hermine O, Small T, et al. A phase 1/2 trial of arginine butyrate and ganciclovir in patients with Epstein-Barr virus-associated lymphoid malignancies. Blood 2007; 109:2571–2578.
- Jagadeesh D, Woda BA, Draper J, Evens AM. Post transplant lymphoproliferative disorders: risk, classification, and therapeutic recommendations. Curr Treat Options Oncol 2012; 13:122–136.
- Opelz G, Daniel V, Naujokat C, Fickenscher H, Döhler B. Effect of cytomegalovirus prophylaxis with immunoglobulin or with antiviral drugs on post-transplant non-Hodgkin lymphoma: a multicentre retrospective analysis. Lancet Oncol 2007; 8:212–218.
- Nowalk AJ, Green M. Epstein-Barr virus–associated posttransplant lymphoproliferative disorder: strategies for prevention and cure. Liver Transpl 2010; 16(suppl S2):S54–S59.
- Pappas PG, Silveira FP; AST Infectious Diseases Community of Practice. Candida in solid organ transplant recipients. Am J Transplant 2009; 9(suppl 4):S173–S179.
- Singh N, Wagener MM, Marino IR, Gayowski T. Trends in invasive fungal infections in liver transplant recipients: correlation with evolution in transplantation practices. Transplantation 2002; 73:63–67.
- Miller R, Assi M; AST Infectious Diseases Community of Practice. Endemic fungal infections in solid organ transplantation. Am J Transplant 2013; 13(suppl 4):250–261.
- Fontana C, Gaziano R, Favaro M, Casalinuovo IA, Pistoia E, Di Francesco P. (1-3)-beta-D-glucan vs galactomannan antigen in diagnosing invasive fungal infections (IFIs). Open Microbiol J 2012; 6:70–73.
- Aydogan S, Kustimur S, Kalkancı A. Comparison of glucan and galactomannan tests with real-time PCR for diagnosis of invasive aspergillosis in a neutropenic rat model [Turkish]. Mikrobiyol Bul 2010; 44:441–452.
- Hadley S, Huckabee C, Pappas PG, et al. Outcomes of antifungal prophylaxis in high-risk liver transplant recipients. Transpl Infect Dis 2009; 11:40–48.
- Pappas PG, Kauffman CA, Andes D, et al; Infectious Diseases Society of America. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 2009; 48:503–535.
- Person AK, Kontoyiannis DP, Alexander BD. Fungal infections in transplant and oncology patients. Infect Dis Clin North Am 2010; 24:439–459.
- Van Hal SJ, Marriott DJE, Chen SCA, et al; Australian Candidaemia Study. Candidemia following solid organ transplantation in the era of antifungal prophylaxis: the Australian experience. Transpl Infect Dis 2009; 11:122–127.
- Singh N. Fungal infections in the recipients of solid organ transplantation. Infect Dis Clin North Am 2003; 17:113–134,
- Liu X, Ling Z, Li L, Ruan B. Invasive fungal infections in liver transplantation. Int J Infect Dis 2011; 15:e298–e304.
- Raghuram A, Restrepo A, Safadjou S, et al. Invasive fungal infections following liver transplantation: incidence, risk factors, survival, and impact of fluconazole-resistant Candida parapsilosis (2003-2007). Liver Transpl 2012; 18:1100–1109.
- De Pauw B, Walsh TJ, Donnelly JP, et al; European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group; National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 2008; 46:1813–1821.
- Moreno A, Cervera C, Gavaldá J, et al. Bloodstream infections among transplant recipients: results of a nationwide surveillance in Spain. Am J Transplant 2007; 7:2579–2586.
- Cruciani M, Mengoli C, Malena M, Bosco O, Serpelloni G, Grossi P. Antifungal prophylaxis in liver transplant patients: a systematic review and meta-analysis. Liver Transpl 2006; 12:850–858.
- Singh N, Husain S; AST Infectious Diseases Community of Practice. Invasive aspergillosis in solid organ transplant recipients. Am J Transplant 2009; 9(suppl 4):S180–S191.
- Fortún J, Martín-Dávila P, Alvarez ME, et al. False-positive results of Aspergillus galactomannan antigenemia in liver transplant recipients. Transplantation 2009; 87:256–260.
- Cherian T, Giakoustidis A, Yokoyama S, et al. Treatment of refractory cerebral aspergillosis in a liver transplant recipient with voriconazole: case report and review of the literature. Exp Clin Transplant 2012; 10:482–486.
- Luong ML, Hosseini-Moghaddam SM, Singer LG, et al. Risk factors for voriconazole hepatotoxicity at 12 weeks in lung transplant recipients. Am J Transplant 2012; 12:1929–1935.
- Neofytos D, Fishman JA, Horn D, et al. Epidemiology and outcome of invasive fungal infections in solid organ transplant recipients. Transpl Infect Dis 2010; 12:220–229.
- Martin SI, Fishman JA; AST Infectious Diseases Community of Practice. Pneumocystis pneumonia in solid organ transplant recipients. Am J Transplant 2009; 9(suppl 4):S227–S233.
- Levine SJ, Masur H, Gill VJ, et al. Effect of aerosolized pentamidine prophylaxis on the diagnosis of Pneumocystis carinii pneumonia by induced sputum examination in patients infected with the human immunodeficiency virus. Am Rev Respir Dis 1991; 144:760–764.
- Rodriguez M, Sifri CD, Fishman JA. Failure of low-dose atovaquone prophylaxis against Pneumocystis jiroveci infection in transplant recipients. Clin Infect Dis 2004; 38:e76–e78.
- Crans CA Jr, Boiselle PM. Imaging features of Pneumocystis carinii pneumonia. Crit Rev Diagn Imaging 1999; 40:251–284.
- Onishi A, Sugiyama D, Kogata Y, et al. Diagnostic accuracy of serum 1,3-beta-D-glucan for Pneumocystis jiroveci pneumonia, invasive candidiasis, and invasive aspergillosis: systematic review and meta-analysis. J Clin Microbiol 2012; 50:7–15.
- Held J, Koch MS, Reischl U, Danner T, Serr A. Serum (1→3)-ß-D-glucan measurement as an early indicator of Pneumocystis jirovecii pneumonia and evaluation of its prognostic value. Clin Microbiol Infect 2011; 17:595–602.
- Fishman JA. Prevention of infection caused by Pneumocystis carinii in transplant recipients. Clin Infect Dis 2001; 33:1397–1405.
- Colby C, McAfee S, Sackstein R, Finkelstein D, Fishman J, Spitzer T. A prospective randomized trial comparing the toxicity and safety of atovaquone with trimethoprim/sulfamethoxazole as Pneumocystis carinii pneumonia prophylaxis following autologous peripheral blood stem cell transplantation. Bone Marrow Transplant 1999; 24:897–902.
- Subramanian A, Dorman S; AST Infectious Diseases Community of Practice. Mycobacterium tuberculosis in solid organ transplant recipients. Am J Transplant 2009; 9(suppl 4):S57–S62.
- Subramanian AK, Morris MI; AST Infectious Diseases Community of Practice. Mycobacterium tuberculosis infections in solid organ transplantation. Am J Transplant 2013; 13(suppl 4):68–76.
- Horne DJ, Narita M, Spitters CL, Parimi S, Dodson S, Limaye AP. Challenging issues in tuberculosis in solid organ transplantation. Clin Infect Dis 2013; 57:1473–1482.
- Holty JE, Gould MK, Meinke L, Keeffe EB, Ruoss SJ. Tuberculosis in liver transplant recipients: a systematic review and meta-analysis of individual patient data. Liver Transpl 2009; 15:894–906.
- Jafri SM, Singal AG, Kaul D, Fontana RJ. Detection and management of latent tuberculosis in liver transplant patients. Liver Transpl 2011; 17:306–314.
- Fábrega E, Sampedro B, Cabezas J, et al. Chemoprophylaxis with isoniazid in liver transplant recipients. Liver Transpl 2012; 18:1110–1117.
- Aguado JM, Torre-Cisneros J, Fortún J, et al. Tuberculosis in solid-organ transplant recipients: consensus statement of the group for the study of infection in transplant recipients (GESITRA) of the Spanish Society of Infectious Diseases and Clinical Microbiology. Clin Infect Dis 2009; 48:1276–1284.
- Yehia BR, Blumberg EA. Mycobacterium tuberculosis infection in liver transplantation. Liver Transpl 2010; 16:1129–1135.
- Katz LH, Paul M, Guy DG, Tur-Kaspa R. Prevention of recurrent hepatitis B virus infection after liver transplantation: hepatitis B immunoglobulin, antiviral drugs, or both? Systematic review and meta-analysis. Transpl Infect Dis 2010; 12:292–308.
- Jiang L, Jiang LS, Cheng NS, Yan LN. Current prophylactic strategies against hepatitis B virus recurrence after liver transplantation. World J Gastroenterol 2009; 15:2489–2499.
- Riediger C, Berberat PO, Sauer P, et al. Prophylaxis and treatment of recurrent viral hepatitis after liver transplantation. Nephrol Dial Transplant 2007; 22(suppl 8):viii37–viii46.
- Cholongitas E, Vasiliadis T, Antoniadis N, Goulis I, Papanikolaou V, Akriviadis E. Hepatitis B prophylaxis post liver transplantation with newer nucleos(t)ide analogues after hepatitis B immunoglobulin discontinuation. Transpl Infect Dis 2012; 14:479–487.
- Fox AN, Terrault NA. Individualizing hepatitis B infection prophylaxis in liver transplant recipients. J Hepatol 2011; 55:507–509.
- Fox AN, Terrault NA. The option of HBIG-free prophylaxis against recurrent HBV. J Hepatol 2012; 56:1189–1197.
- Wesdorp DJ, Knoester M, Braat AE, et al. Nucleoside plus nucleotide analogs and cessation of hepatitis B immunoglobulin after liver transplantation in chronic hepatitis B is safe and effective. J Clin Virol 2013; 58:67–73.
- Terrault NA, Berenguer M. Treating hepatitis C infection in liver transplant recipients. Liver Transpl 2006; 12:1192–1204.
- Ciria R, Pleguezuelo M, Khorsandi SE, et al. Strategies to reduce hepatitis C virus recurrence after liver transplantation. World J Hepatol 2013; 5:237–250.
- Issa NC, Fishman JA. Infectious complications of antilymphocyte therapies in solid organ transplantation. Clin Infect Dis 2009; 48:772–786.
- Kalambokis G, Manousou P, Samonakis D, et al. Clinical outcome of HCV-related graft cirrhosis and prognostic value of hepatic venous pressure gradient. Transpl Int 2009; 22:172–181.
- Neumann UP, Berg T, Bahra M, et al. Long-term outcome of liver transplants for chronic hepatitis C: a 10-year follow-up. Transplantation 2004; 77:226–231.
- Wiesner RH, Sorrell M, Villamil F; International Liver Transplantation Society Expert Panel. Report of the first International Liver Transplantation Society expert panel consensus conference on liver transplantation and hepatitis C. Liver Transpl 2003; 9:S1–S9.
- Dinges S, Morard I, Heim M, et al; Swiss Association for the Study of the Liver (SASL 17). Pegylated interferon-alpha2a/ribavirin treatment of recurrent hepatitis C after liver transplantation. Transpl Infect Dis 2009; 11:33–39.
- Veldt BJ, Poterucha JJ, Watt KD, et al. Impact of pegylated interferon and ribavirin treatment on graft survival in liver transplant patients with recurrent hepatitis C infection. Am J Transplant 2008; 8:2426–2433.
- Faisal N, Yoshida EM, Bilodeau M, et al. Protease inhibitor-based triple therapy is highly effective for hepatitis C recurrence after liver transplant: a multicenter experience. Ann Hepatol 2014; 13:525–532.
- Mariño Z, van Bömmel F, Forns X, Berg T. New concepts of sofosbuvir-based treatment regimens in patients with hepatitis C. Gut 2014; 63:207–215.
- Coilly A, Roche B, Dumortier J, et al. Safety and efficacy of protease inhibitors to treat hepatitis C after liver transplantation: a multicenter experience. J Hepatol 2014; 60:78–86.
- Lucey MR, Terrault N, Ojo L, et al. Long-term management of the successful adult liver transplant: 2012 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Liver Transpl 2013; 19:3–26.
The immunosuppressed state of liver transplant recipients makes them vulnerable to infections after surgery.1 These infections are directly correlated with the net state of immunosuppression. Higher levels of immunosuppression mean a higher risk of infection, with rates of infection typically highest in the early posttransplant period.
Common infections during this period include operative and perioperative nosocomial bacterial and fungal infections, reactivation of latent infections, and invasive fungal infections such as candidiasis, aspergillosis, and pneumocystosis. Donor-derived infections also must be considered. As time passes and the level of immunosuppression is reduced, liver recipients are less prone to infection.1
The risk of infection can be minimized by appropriate antimicrobial prophylaxis, strategies for safe living after transplant,2 vaccination,3 careful balancing of immunosuppressive therapy,4 and thoughtful donor selection.5 Drug-drug interactions are common and must be carefully considered to minimize the risk.
This review highlights common infectious complications encountered after liver transplant.
INTRA-ABDOMINAL INFECTIONS
Intra-abdominal infections are common in the early postoperative period.6,7
Risk factors include:
- Pretransplant ascites
- Posttransplant dialysis
- Wound infection
- Reoperation8
- Hepatic artery thrombosis
- Roux-en-Y choledochojejunostomy anastomosis.9
Signs that may indicate intra-abdominal infection include fever, abdominal pain, leukocytosis, and elevated liver enzymes. But because of their immunosuppressed state, transplant recipients may not manifest fever as readily as the general population. They should be evaluated for cholangitis, peritonitis, biloma, and intra-abdominal abscess.
Organisms. Intra-abdominal infections are often polymicrobial. Enterococci, Staphylococcus aureus, gram-negative species including Pseudomonas, Klebsiella, and Acinetobacter, and Candida species are the most common pathogens. Strains are often resistant to multiple drugs, especially in patients who received antibiotics in the weeks before transplant.8,10
Liver transplant recipients are also particularly susceptible to Clostridium difficile-associated colitis as a result of immunosuppression and frequent use of antibiotics perioperatively and postoperatively.11 The spectrum of C difficile infection ranges from mild diarrhea to life-threatening colitis, and the course in liver transplant patients tends to be more complicated than in immunocompetent patients.12
Diagnosis. Intra-abdominal infections should be looked for and treated promptly, as they are associated with a higher mortality rate, a greater risk of graft loss, and a higher incidence of retransplant.6,10 Abdominal ultrasonography or computed tomography (CT) can confirm the presence of fluid collections.
Treatment. Infected collections can be treated with percutaneous or surgical drainage and antimicrobial therapy. In the case of biliary tract complications, retransplant or surgical correction of biliary leakage or stenosis decreases the risk of death.6
Suspicion should be high for C difficile-associated colitis in cases of posttransplant diarrhea. C difficile toxin stool assays help confirm the diagnosis.12 Oral metronidazole is recommended in mild to moderate C difficile infection, with oral vancomycin and intravenous metronidazole reserved for severe cases. Colectomy may be necessary in patients with toxic megacolon.
CYTOMEGALOVIRUS INFECTION
Cytomegalovirus is an important opportunistic pathogen in liver transplant recipients.13 It causes a range of manifestations, from infection (viremia with or without symptoms) to cytomegalovirus syndrome (fever, malaise, and cell-line cytopenias) to tissue-invasive disease with end-organ disease.14 Without preventive measures and treatment, cytomegalovirus disease can increase the risk of morbidity, allograft loss and death.15,16
Risk factors for cytomegalovirus infection (Table 1) include:
- Discordant serostatus of the donor and recipient (the risk is highest in seronegative recipients of organs from seropositive donors)
- Higher levels of immunosuppression, especially when antilymphocyte antibodies are used
- Treatment of graft rejection
- Coinfection with other human herpesviruses, such as Epstein-Barr virus.4,17
Preventing cytomegalovirus infection
The strategy to prevent cytomegalovirus infection depends on the serologic status of the donor and recipient and may include antiviral prophylaxis or preemptive treatment (Table 2).18
Prophylaxis involves giving antiviral drugs during the early high-risk period, with the goal of preventing the development of cytomegalovirus viremia. The alternative preemptive strategy emphasizes serial testing for cytomegalovirus viremia, with the goal of intervening with antiviral medications while viremia is at a low level, thus avoiding potential progression to cytomegalovirus disease. Both strategies have pros and cons that should be considered by each transplant center when setting institutional policy.
A prophylactic approach seems very effective at preventing both infection and disease from cytomegalovirus and has been shown to reduce graft rejection and the risk of death.18 It is preferred in cytomegalovirus-negative recipients when the donor was cytomegalovirus-positive—a high-risk situation.19 However, these patients are also at higher risk of late-onset cytomegalovirus disease. Higher cost and potential drug toxicity, mainly neutropenia from ganciclovir-based regimens, are additional considerations.
Preemptive treatment, in contrast, reserves drug treatment for patients who are actually infected with cytomegalovirus, thus resulting in fewer adverse drug events and lower cost; but it requires regular monitoring. Preemptive methods, by definition, cannot prevent infection, and with this strategy tissue-invasive disease not associated with viremia does occasionally occur.20 As such, patients with a clinical presentation that suggests cytomegalovirus but have negative results on blood testing should be considered for tissue biopsy with culture and immunohistochemical stain.
The most commonly used regimens for antiviral prophylaxis and treatment in liver transplant recipients are intravenous ganciclovir and oral valganciclovir.21 Although valganciclovir is the most commonly used agent in this setting because of ease of administration, it has not been approved by the US Food and Drug Administration in liver transplant patients, as it was associated with higher rates of cytomegalovirus tissue-invasive disease.22–24 Additionally, drug-resistant cytomegalovirus strains have been associated with valganciclovir prophylaxis in cytomegalovirus-negative recipients of solid organs from cytomegalovirus-positive donors.25
Prophylaxis typically consists of therapy for 3 months from the time of transplant. In higher-risk patients (donor-positive, recipient-negative), longer courses of prophylaxis have been extrapolated from data in kidney transplant recipients.26 Extension or reinstitution of prophylaxis should also be considered in liver transplant patients receiving treatment for rejection with antilymphocyte therapy.
Routine screening for cytomegalovirus is not recommended while patients are receiving prophylaxis. High-risk patients who are not receiving prophylaxis should be monitored with nucleic acid or pp65 antigenemia testing as part of the preemptive strategy protocol.
Treatment of cytomegalovirus disease
Although no specific threshold has been established, treatment is generally indicated if a patient has a consistent clinical syndrome, evidence of tissue injury, and persistent or increasing viremia.
Treatment involves giving antiviral drugs and also reducing the level of immunosuppression, if possible, until symptoms and viremia have resolved.
The choice of antiviral therapy depends on the severity of disease. Intravenous ganciclovir (5 mg/kg twice daily adjusted for renal impairment) or oral valganciclovir (900 mg twice daily, also renally dose-adjusted when necessary) can be used for mild to moderate disease if no significant gastrointestinal involvement is reported. Intravenous ganciclovir is preferred for patients with more severe disease or gastrointestinal involvement. The minimum duration of treatment is 2 weeks and may need to be prolonged until both symptoms and viremia completely resolve.18
Drug resistance can occur and should be considered in patients who have a history of prolonged ganciclovir or valganciclovir exposure who do not clinically improve or have persistent or rising viremia. In such cases, genotype assays are helpful, and initiation of alternative therapy should be considered. Mutations conferring resistance to ganciclovir are often associated with cross-resistance to cidofovir. Cidofovir can therefore be considered only when genotype assays demonstrate specific mutations conferring an isolated resistance to ganciclovir.27 The addition of foscarnet to the ganciclovir regimen or substitution of foscarnet for ganciclovir are accepted approaches.
Although cytomegalovirus hyperimmunoglobulin has been used in prophylaxis and invasive disease treatment, its role in the management of ganciclovir-resistant cytomegalovirus infections remains controversial.28
EPSTEIN-BARR VIRUS POSTTRANSPLANT LYMPHOPROLIFERATIVE DISEASE
Epstein-Barr virus-associated posttransplant lymphoproliferative disease is a spectrum of disorders ranging from an infectious mononucleosis syndrome to aggressive malignancy with the potential for death and significant morbidity after liver transplant.29 The timeline of risk varies, but the disease is most common in the first year after transplant.
Risk factors for this disease (Table 1) are:
- Primary Epstein-Barr virus infection
- Cytomegalovirus donor-recipient mismatch
- Cytomegalovirus disease
- Higher levels of immunosuppression, especially with antilymphocyte antibodies.30
The likelihood of Epstein-Barr virus playing a contributing role is lower in later-onset posttransplant lymphoproliferative disease. Patients who are older at the time of transplant, who receive highly immunogenic allografts including a liver as a component of a multivisceral transplant, and who receive increased immunosuppression to treat rejection are at even greater risk of late posttransplant lymphoproliferative disease.31 This is in contrast to early posttransplant lymphoproliferative disease, which is seen more commonly in children as a result of primary Epstein-Barr virus infection.
Recognition and diagnosis. Heightened suspicion is required when considering posttransplant lymphoproliferative disease, and careful evaluation of consistent symptoms and allograft dysfunction are required.
Clinically, posttransplant lymphoproliferative disease should be suspected if a liver transplant recipient develops unexplained fever, weight loss, lymphadenopathy, or cell-line cytopenias.30,32 Other signs and symptoms may be related to the organ involved and may include evidence of hepatitis, pneumonitis, and gastrointestinal disease.31
Adjunctive diagnostic testing includes donor and recipient serology to characterize overall risk before transplantation and quantification of Epstein-Barr viral load, but confirmation relies on tissue histopathology.
Treatment focuses on reducing immunosuppression.30,32 Adding antiviral agents does not seem to improve outcome in all cases.33 Depending on clinical response and histologic classification, additional therapies such as anti-CD20 humanized chimeric monoclonal antibodies, surgery, radiation, and conventional chemotherapy may be required.34
Preventive approaches remain controversial. Chemoprophylaxis with an antiviral such as ganciclovir is occasionally used but has not been shown to consistently decrease rates of posttransplant lymphoproliferative disease. These agents may act in an indirect manner, leading to decreased rates of cytomegalovirus infection, a major cofactor for posttransplant lymphoproliferative disease.24
Passive immunoprophylaxis with immunoglobulin targeting cytomegalovirus has shown to decrease rates of non-Hodgkin lymphoma from posttransplant lymphoproliferative disease in renal transplant recipients in the first year after transplant,35 but data are lacking regarding its use in liver transplant recipients. Monitoring of the viral load and subsequent reduction of immunosuppression remain the most efficient measures to date.36
FUNGAL INFECTIONS
Candida species account for more than half of fungal infections in liver transplant recipients.37 However, a change has been noted in the past 20 years, with a decrease in Candida infections accompanied by an increase in Aspergillus infections.38 Endemic mycoses such as coccidioidomycosis, blastomycosis, and histoplasmosis should be considered with the appropriate epidemiologic history or if disease develops early after transplant and the donor came from a highly endemic region.39Cryptococcus may also be encountered.
Diagnosis. One of the most challenging aspects of fungal infection in liver transplant recipients is timely diagnosis. Heightened suspicion and early biopsy for pathological and microbiological confirmation are necessary. Although available noninvasive diagnostic tools often lack specificity, early detection of fungal markers may be of great use in guiding further diagnostic workup or empiric treatment in the critically ill.
Noninvasive tests include galactomannan, cryptococcal antigen, histoplasma antigen, (1-3)-beta-D-glucan assay and various antibody tests. Galactomannan testing has been widely used to aid in the diagnosis of invasive aspergillosis. Similarly, the (1-3)-beta-D-glucan assay is a non–culture-based tool for diagnosing and monitoring the treatment of invasive fungal infections. However, a definite diagnosis cannot be made on the basis of a positive test alone.40 The complementary diagnostic characteristics of combining noninvasive assays have yet to be fully elucidated.41 Cultures and tissue histopathology are also used when possible.
Treatment is based on targeted specific antifungal drug therapy and reduction of immunosuppressive therapy, when possible. The choice of antifungal agent varies with the pathogen, the site of involvement, and the severity of the disease. A focus on potential drug interactions, their management, and therapeutic drug monitoring when using antifungal medications is essential in the posttransplant period. Combination therapy can be considered in some situations to enhance synergy. The following sections discuss in greater detail Candida species, Aspergillus species, and Pneumocystis jirovecii infections.
Candida infections
Candidiasis after liver transplant is typically nosocomial, especially when diagnosed during the first 3 months (Table 3).37
Risk factors for invasive candidiasis include perioperative colonization, prolonged operative time, retransplant, greater transfusion requirements, and postoperative renal failure.37,42,43 Invasive candidiasis is of concern for its effects on morbidity, mortality, and cost of care.43–46
Organisms. The frequency of implicated species, in particular those with a natural resistance to fluconazole, differs in various reports.37,45,46Candida albicans remains the most commonly isolated pathogen; however, non-albicans species including those resistant to fluconazole have been reported more frequently and include Candida glabrata and Candida krusei.47,48
Signs and diagnosis. Invasive candidiasis in liver transplant recipients generally manifests itself in catheter-related blood stream infections, urinary tract infections, or intra-abdominal infections. Diagnosis can be made by isolating Candida from blood cultures, recovering organisms in culture of a normally sterile site, or finding direct microscopic evidence of the fungus on tissue specimens.49
Disseminated candidiasis refers to the involvement of distant anatomic sites. Clinical manifestations may cause vision changes, abdominal pain or skin nodules with findings of candidemia, hepatosplenic abscesses, or retinal exudates on funduscopy.49
Treatment of invasive candidiasis in liver recipients often involves antifungal therapy and reduction of immunosuppression. Broad-spectrum antifungals are initially advocated in an empirical approach to cover fluconazole-resistant strains of the non-albicans subgroups.50 Depending on antifungal susceptibility, treatment can later be adjusted.
Fluconazole remains the agent of choice in most C albicans infections.47 However, attention should be paid to the possibility of resistance in patients who have received fluconazole prophylaxis within the past 30 days. Additional agents used in treatment may include echinocandins, amphotericin, and additional azoles.
Antifungal prophylaxis is recommended in high-risk liver transplant patients, although its optimal duration remains undetermined.44 Antifungal prophylaxis has been associated with decreased incidence of both superficial and invasive candidiasis.51
Aspergillus infection
Aspergillus, the second most common fungal pathogen, has become a more common concern in liver transplant recipients. Aspergillus fumigatus is the most frequently encountered species.38,52
Risk factors. These infections typically occur in the first year, during intense immunosuppression. Retransplant, renal failure, and fulminant hepatic failure are major risk factors.52 In the presence of risk factors and a suggestive clinical setting, invasive aspergillosis should be considered and the diagnosis pursued.
Diagnosis is suggested by positive findings on CT accompanied by lower respiratory tract symptoms, focal lesions on neuroimaging, or demonstration of the fungus on cultures.49 However, Aspergillus is rarely grown in blood culture. The galactomannan antigen is a noninvasive test that can provide supporting evidence for the diagnosis.41,52 False-positive results do occur in the setting of certain antibiotics and cross-reacting fungi.53
Treatment consists of antifungal therapy and immunosuppression reduction.52
Voriconazole is the first-line agent for invasive aspergillosis. Monitoring for potential drug-drug interactions and side effects is required.54,55 Amphotericin B is considered a second-line choice due to toxicity and lack of an oral formulation. In refractory cases, combined antifungal therapy could be considered.52 The duration of treatment is generally a minimum of 12 weeks.
Prophylaxis. Specific prophylaxis against invasive aspergillosis is not currently recommended; however, some authors suggest a prophylactic approach using echinocandins or liposomal amphotericin B in high-risk patients.51,52 Aspergillosis is associated with a considerable increase in mortality in liver transplant recipients, which highlights the importance of timely management.52,56
Pneumocystis jirovecii
P jirovecii remains a common opportunistic pathogen in people with impaired immunity, including transplant and human immunodeficiency virus patients.
Prophylaxis. Widespread adoption of antimicrobial prophylaxis by transplant centers has decreased the rates of P jirovecii infection in liver transplant recipients.57,58 Commonly used prophylactic regimens after liver transplantation include a single-strength trimethoprim-sulfamethoxazole tablet daily or a double-strength tablet three times per week for a minimum of 6 to 12 months after transplant. Atovaquone and dapsone can be used as alternatives in cases of intolerance to trimethoprim-sulfamethoxazole (Table 2).
Inhaled pentamidine is clearly inferior and should be used only when the other medications are contraindicated.59
Signs and diagnosis. P jirovecii pneumonia is characterized by fever, cough, dyspnea, and chest pain. Insidious hypoxemia, abnormal chest examination, and bilateral interstitial pneumonia on chest radiography are common.
CT may be more sensitive than chest radiography.57 Findings suggestive of P jirovecii pneumonia on chest CT are extensive bilateral and symmetrical ground-glass attenuations. Other less-characteristic findings include upper lobar parenchymal opacities and spontaneous pneumothorax.57,60
The serum (1,3)-beta-D-glucan assay derived from major cell-wall components of P jirovecii might be helpful. Studies report a sensitivity for P jirovecii pneumonia as high as 96% and a negative predictive value of 99.8%.61,62
Definitive diagnosis requires identification of the pathogen. Routine expectorated sputum sampling is generally associated with a poor diagnostic yield. Bronchoscopy and bronchoalveolar lavage with silver or fluorescent antibody staining of samples, polymerase chain reaction testing, or both significantly improves diagnosis. Transbronchial or open lung biopsy are often unnecessary.57
Treatment. Trimethoprim-sulfamethoxazole is the first-line agent for treating P jirovecii pneumonia.57 The minimum duration of treatment is 14 days, with extended courses for severe infection.
Intravenous pentamidine or clindamycin plus primaquine are alternatives for patients who cannot tolerate trimethoprim-sulfamethoxazole. The major concern with intravenous pentamidine is renal dysfunction. Hypoglycemia or hyperglycemia, neutropenia, thrombocytopenia, nausea, dysgeusia, and pancreatitis may also occur.63
Atovaquone might also be beneficial in mild to moderate P jirovecii pneumonia. The main side effects include skin rashes, gastrointestinal intolerance, and elevation of transaminases.64
A corticosteroid (40–60 mg of prednisone or its equivalent) may be beneficial in conjunction with antimicrobial therapy in patients with significant hypoxia (partial pressure of arterial oxygen < 70 mm Hg on room air) in decreasing the risk of respiratory failure and need for intubation.
With appropriate and timely antimicrobial prophylaxis, cases of P jirovecii pneumonia should continue to decrease.
TUBERCULOSIS
Development of tuberculosis after transplantation is a catastrophic complication, with mortality rates of up to 30%.65 Most cases of posttransplant tuberculosis represent reactivation of latent disease.66 Screening with tuberculin skin tests or interferon-gamma-release assays is recommended in all liver transplant candidates. Chest radiography before transplant is necessary when assessing a positive screening test.67
The optimal management of latent tuberculosis in these cases remains controversial. Patients at high risk or those with positive screening results on chest radiography warrant treatment for latent tuberculosis infection with isoniazid unless contraindicated.67,68
The ideal time to initiate prophylactic isoniazid therapy is unclear. Some authors suggest delaying it, as it might be associated with poor tolerance and hepatotoxicity.69 Others have found that early isoniazid use was not associated with negative outcomes.70
Risk factors for symptomatic tuberculosis after liver transplant include previous infection with tuberculosis, intensified immunosuppression (especially anti-T-lymphocyte therapies), diabetes mellitus, and other co-infections (Table 1).71
The increased incidence of atypical presentations in recent years makes the diagnosis of active tuberculosis among liver transplant recipients challenging. Sputum smears can be negative due to low mycobacterial burdens, and tuberculin skin testing and interferon-gamma-release assays may be falsely negative due to immunosuppression.67
Treatment of active tuberculosis consists initially of a four-drug regimen using isoniazid, rifampin, pyrazinamide, and ethambutol for 2 months. Adjustments are made in accordance with culture and sensitivity results. Treatment can then be tapered to two drugs (isoniazid and rifampin) for a minimum of 4 additional months. Prolonged treatment may be required in instances of extrapulmonary or disseminated disease.65,72
Tuberculosis treatment can be complicated by hepatotoxicity in liver transplant recipients because of direct drug effects and drug-drug interactions with immunosuppressive agents. Close monitoring for rejection and hepatotoxicity is therefore imperative while liver transplant recipients are receiving antituberculosis therapy. Drug-drug interactions may also be responsible for marked reductions in immunosuppression levels, especially with regimens containing rifampin.71 Substitution of rifabutin for rifampin reduces the effect of drug interactions.66
VIRAL HEPATITIS
Hepatitis B virus
Hepatitis B virus-related end-stage liver disease and hepatocellular carcinoma are common indications for liver transplant in Asia. It is less common in the United States and Europe, accounting for less than 10% of all liver transplant cases. Prognosis is favorable in recipients undergoing liver transplant for hepatitis B virus, with excellent survival rates. Prevention of reinfection is crucial in these patients.
Treatment with combination antiviral agents and hepatitis B immunoglobulin (HBIG) is effective.73 Lamivudine was the first nucleoside analogue found to be effective against hepatitis B virus. Its low cost and relative safety are strong arguments in favor of its continued use in liver transplant recipients.74 In patients without evidence of hepatitis B viral replication at the time of transplant, monotherapy with lamivudine has led to low recurrence rates, and adefovir can be added to control resistant viral strains.75
The frequent emergence of resistance with lamivudine favors newer agents such as entecavir or tenofovir. These nucleoside and nucleotide analogues have a higher barrier to resistance, and thus resistance to them is rare. They are also more efficient, potentially allowing use of an HBIG-sparing protocol.76 However, they are associated with a higher risk of nephrotoxicity and require dose adjustments in renal insufficiency. Data directly comparing entecavir and tenofovir are scarce.
Prophylaxis. Most studies support an individualized approach for prevention of hepatitis B virus reinfection. High-risk patients, ie, those positive for HBe antigen or with high viral loads (> 100,000 copies/mL) are generally treated with both HBIG and antiviral agents.77 Low-risk patients are those with a negative HBe antigen, low hepatitis B virus DNA levels, hepatitis B virus-related acute liver failure, and cirrhosis resulting from coinfection with both hepatitis B and hepatitis D virus.75 In low-risk patients, discontinuation of HBIG after 1 to 2 years of treatment is appropriate, and long-term prophylaxis with antiviral agents alone is an option. However, levels of hepatitis B DNA should be monitored closely.78,79
Hepatitis C virus
Recurrence of hepatitis C virus infection is the rule among patients who are viremic at the time of liver transplant.80,81 Most of these patients will show histologic evidence of recurrent hepatitis within the first year after liver transplant. It is often difficult to distinguish between the histopathological appearance of a recurrent hepatitis C virus infection and acute cellular rejection.
Progression to fibrosis and subsequently cirrhosis and decompensation is highly variable in hepatitis C virus-infected liver transplant recipients. Diabetes, insulin resistance, and possibly hepatitis steatosis have been associated with a rapid progression to advanced fibrosis. The contribution of immunosuppression to the progression of hepatitis C virus remains an area of active study. Some studies point to antilymphocyte immunosuppressive agents as a potential cause.82 Liver biopsy is a useful tool in this situation. It allows monitoring of disease severity and progression and may distinguish recurrent hepatitis C virus disease from other causes of liver enzyme elevation.
The major concern with the recurrence of hepatitis C virus infection after liver transplant is allograft loss. Rates of patient and graft survival are reduced in infected patients compared with hepatitis C virus-negative patients.83,84 Prophylactic antiviral therapy has no current role in the management of hepatitis C virus disease. Those manifesting moderate to severe necroinflammation or mild to moderate fibrosis indicative of progressive disease should be treated.81,85
Sustained viral clearance with antiviral agents confers a graft survival benefit.
The combination of peg-interferon and weight-based ribavirin has been the standard of treatment but may be associated with increased rates of rejection.86,87 The sustained virologic response rates for hepatitis C virus range from 60% in genotypes 4, 5, and 6 after 48 weeks of treatment to 60% to 80% in genotypes 2 and 3 after 24 weeks, but only about 30% in genotype 1.88
Treatment with the newer agents, especially protease inhibitors, in genotype 1 (peg-interferon, ribavirin, and either telaprevir or boceprevir) has been evaluated. Success rates reaching 70% have been achieved.89 Adverse effects can be a major setback. Serious complications include severe anemia, renal dysfunction, increased risk of infection, and death.
Triple therapy should be carefully considered in liver transplant patients with genotype 1 hepatitis C virus.90 Significant drug-drug interactions are reported between hepatitis C virus protease inhibitors and immunosuppression regimens. Additional new oral direct- acting antivirals have been investigated. They bring promising advances in hepatitis C virus treatment and pave the way for interferon-free regimens with pangenotypic activity.
IMMUNIZATION
Immunization can decrease the risk of infectious complications in liver transplant recipients, as well as in close contacts and healthcare professionals.3
Influenza. Pretransplant influenza vaccine and posttransplant annual influenza vaccines are necessary.
Pneumococcal immunization should additionally be provided prior to transplant and repeated every 3 to 5 years thereafter.3,91
A number of other vaccinations should also be completed before transplant, including the hepatitis A and B vaccines and the tetanus/diphtheria/acellular pertussis vaccines. However, these vaccinations have not been shown to be detrimental to patients after transplant.91
Varicella and zoster vaccines should be given before liver transplant—zoster in patients over age 60, and varicella in patients with no immunity. Live vaccines, including varicella and zoster vaccines, are contraindicated after liver transplant.3
Human papillomavirus. The bivalent human papillomavirus vaccine can be given before transplant in females ages 9 to 26; the quadrivalent vaccine is beneficial in those ages 9 to 26 and in women under age 45.3,91
IMMUNOSUPPRESSION CARRIES RISK OF INFECTION
Most liver transplant patients require prolonged immunosuppressive therapy. This comes with an increased risk of new or recurrent infections, potentially causing death and significant morbidity.
Evaluation of existing risk factors, appropriate prophylaxis and immunization, timely diagnosis, and treatment of such infections are therefore essential steps for the successful management of liver transplant recipients.
The immunosuppressed state of liver transplant recipients makes them vulnerable to infections after surgery.1 These infections are directly correlated with the net state of immunosuppression. Higher levels of immunosuppression mean a higher risk of infection, with rates of infection typically highest in the early posttransplant period.
Common infections during this period include operative and perioperative nosocomial bacterial and fungal infections, reactivation of latent infections, and invasive fungal infections such as candidiasis, aspergillosis, and pneumocystosis. Donor-derived infections also must be considered. As time passes and the level of immunosuppression is reduced, liver recipients are less prone to infection.1
The risk of infection can be minimized by appropriate antimicrobial prophylaxis, strategies for safe living after transplant,2 vaccination,3 careful balancing of immunosuppressive therapy,4 and thoughtful donor selection.5 Drug-drug interactions are common and must be carefully considered to minimize the risk.
This review highlights common infectious complications encountered after liver transplant.
INTRA-ABDOMINAL INFECTIONS
Intra-abdominal infections are common in the early postoperative period.6,7
Risk factors include:
- Pretransplant ascites
- Posttransplant dialysis
- Wound infection
- Reoperation8
- Hepatic artery thrombosis
- Roux-en-Y choledochojejunostomy anastomosis.9
Signs that may indicate intra-abdominal infection include fever, abdominal pain, leukocytosis, and elevated liver enzymes. But because of their immunosuppressed state, transplant recipients may not manifest fever as readily as the general population. They should be evaluated for cholangitis, peritonitis, biloma, and intra-abdominal abscess.
Organisms. Intra-abdominal infections are often polymicrobial. Enterococci, Staphylococcus aureus, gram-negative species including Pseudomonas, Klebsiella, and Acinetobacter, and Candida species are the most common pathogens. Strains are often resistant to multiple drugs, especially in patients who received antibiotics in the weeks before transplant.8,10
Liver transplant recipients are also particularly susceptible to Clostridium difficile-associated colitis as a result of immunosuppression and frequent use of antibiotics perioperatively and postoperatively.11 The spectrum of C difficile infection ranges from mild diarrhea to life-threatening colitis, and the course in liver transplant patients tends to be more complicated than in immunocompetent patients.12
Diagnosis. Intra-abdominal infections should be looked for and treated promptly, as they are associated with a higher mortality rate, a greater risk of graft loss, and a higher incidence of retransplant.6,10 Abdominal ultrasonography or computed tomography (CT) can confirm the presence of fluid collections.
Treatment. Infected collections can be treated with percutaneous or surgical drainage and antimicrobial therapy. In the case of biliary tract complications, retransplant or surgical correction of biliary leakage or stenosis decreases the risk of death.6
Suspicion should be high for C difficile-associated colitis in cases of posttransplant diarrhea. C difficile toxin stool assays help confirm the diagnosis.12 Oral metronidazole is recommended in mild to moderate C difficile infection, with oral vancomycin and intravenous metronidazole reserved for severe cases. Colectomy may be necessary in patients with toxic megacolon.
CYTOMEGALOVIRUS INFECTION
Cytomegalovirus is an important opportunistic pathogen in liver transplant recipients.13 It causes a range of manifestations, from infection (viremia with or without symptoms) to cytomegalovirus syndrome (fever, malaise, and cell-line cytopenias) to tissue-invasive disease with end-organ disease.14 Without preventive measures and treatment, cytomegalovirus disease can increase the risk of morbidity, allograft loss and death.15,16
Risk factors for cytomegalovirus infection (Table 1) include:
- Discordant serostatus of the donor and recipient (the risk is highest in seronegative recipients of organs from seropositive donors)
- Higher levels of immunosuppression, especially when antilymphocyte antibodies are used
- Treatment of graft rejection
- Coinfection with other human herpesviruses, such as Epstein-Barr virus.4,17
Preventing cytomegalovirus infection
The strategy to prevent cytomegalovirus infection depends on the serologic status of the donor and recipient and may include antiviral prophylaxis or preemptive treatment (Table 2).18
Prophylaxis involves giving antiviral drugs during the early high-risk period, with the goal of preventing the development of cytomegalovirus viremia. The alternative preemptive strategy emphasizes serial testing for cytomegalovirus viremia, with the goal of intervening with antiviral medications while viremia is at a low level, thus avoiding potential progression to cytomegalovirus disease. Both strategies have pros and cons that should be considered by each transplant center when setting institutional policy.
A prophylactic approach seems very effective at preventing both infection and disease from cytomegalovirus and has been shown to reduce graft rejection and the risk of death.18 It is preferred in cytomegalovirus-negative recipients when the donor was cytomegalovirus-positive—a high-risk situation.19 However, these patients are also at higher risk of late-onset cytomegalovirus disease. Higher cost and potential drug toxicity, mainly neutropenia from ganciclovir-based regimens, are additional considerations.
Preemptive treatment, in contrast, reserves drug treatment for patients who are actually infected with cytomegalovirus, thus resulting in fewer adverse drug events and lower cost; but it requires regular monitoring. Preemptive methods, by definition, cannot prevent infection, and with this strategy tissue-invasive disease not associated with viremia does occasionally occur.20 As such, patients with a clinical presentation that suggests cytomegalovirus but have negative results on blood testing should be considered for tissue biopsy with culture and immunohistochemical stain.
The most commonly used regimens for antiviral prophylaxis and treatment in liver transplant recipients are intravenous ganciclovir and oral valganciclovir.21 Although valganciclovir is the most commonly used agent in this setting because of ease of administration, it has not been approved by the US Food and Drug Administration in liver transplant patients, as it was associated with higher rates of cytomegalovirus tissue-invasive disease.22–24 Additionally, drug-resistant cytomegalovirus strains have been associated with valganciclovir prophylaxis in cytomegalovirus-negative recipients of solid organs from cytomegalovirus-positive donors.25
Prophylaxis typically consists of therapy for 3 months from the time of transplant. In higher-risk patients (donor-positive, recipient-negative), longer courses of prophylaxis have been extrapolated from data in kidney transplant recipients.26 Extension or reinstitution of prophylaxis should also be considered in liver transplant patients receiving treatment for rejection with antilymphocyte therapy.
Routine screening for cytomegalovirus is not recommended while patients are receiving prophylaxis. High-risk patients who are not receiving prophylaxis should be monitored with nucleic acid or pp65 antigenemia testing as part of the preemptive strategy protocol.
Treatment of cytomegalovirus disease
Although no specific threshold has been established, treatment is generally indicated if a patient has a consistent clinical syndrome, evidence of tissue injury, and persistent or increasing viremia.
Treatment involves giving antiviral drugs and also reducing the level of immunosuppression, if possible, until symptoms and viremia have resolved.
The choice of antiviral therapy depends on the severity of disease. Intravenous ganciclovir (5 mg/kg twice daily adjusted for renal impairment) or oral valganciclovir (900 mg twice daily, also renally dose-adjusted when necessary) can be used for mild to moderate disease if no significant gastrointestinal involvement is reported. Intravenous ganciclovir is preferred for patients with more severe disease or gastrointestinal involvement. The minimum duration of treatment is 2 weeks and may need to be prolonged until both symptoms and viremia completely resolve.18
Drug resistance can occur and should be considered in patients who have a history of prolonged ganciclovir or valganciclovir exposure who do not clinically improve or have persistent or rising viremia. In such cases, genotype assays are helpful, and initiation of alternative therapy should be considered. Mutations conferring resistance to ganciclovir are often associated with cross-resistance to cidofovir. Cidofovir can therefore be considered only when genotype assays demonstrate specific mutations conferring an isolated resistance to ganciclovir.27 The addition of foscarnet to the ganciclovir regimen or substitution of foscarnet for ganciclovir are accepted approaches.
Although cytomegalovirus hyperimmunoglobulin has been used in prophylaxis and invasive disease treatment, its role in the management of ganciclovir-resistant cytomegalovirus infections remains controversial.28
EPSTEIN-BARR VIRUS POSTTRANSPLANT LYMPHOPROLIFERATIVE DISEASE
Epstein-Barr virus-associated posttransplant lymphoproliferative disease is a spectrum of disorders ranging from an infectious mononucleosis syndrome to aggressive malignancy with the potential for death and significant morbidity after liver transplant.29 The timeline of risk varies, but the disease is most common in the first year after transplant.
Risk factors for this disease (Table 1) are:
- Primary Epstein-Barr virus infection
- Cytomegalovirus donor-recipient mismatch
- Cytomegalovirus disease
- Higher levels of immunosuppression, especially with antilymphocyte antibodies.30
The likelihood of Epstein-Barr virus playing a contributing role is lower in later-onset posttransplant lymphoproliferative disease. Patients who are older at the time of transplant, who receive highly immunogenic allografts including a liver as a component of a multivisceral transplant, and who receive increased immunosuppression to treat rejection are at even greater risk of late posttransplant lymphoproliferative disease.31 This is in contrast to early posttransplant lymphoproliferative disease, which is seen more commonly in children as a result of primary Epstein-Barr virus infection.
Recognition and diagnosis. Heightened suspicion is required when considering posttransplant lymphoproliferative disease, and careful evaluation of consistent symptoms and allograft dysfunction are required.
Clinically, posttransplant lymphoproliferative disease should be suspected if a liver transplant recipient develops unexplained fever, weight loss, lymphadenopathy, or cell-line cytopenias.30,32 Other signs and symptoms may be related to the organ involved and may include evidence of hepatitis, pneumonitis, and gastrointestinal disease.31
Adjunctive diagnostic testing includes donor and recipient serology to characterize overall risk before transplantation and quantification of Epstein-Barr viral load, but confirmation relies on tissue histopathology.
Treatment focuses on reducing immunosuppression.30,32 Adding antiviral agents does not seem to improve outcome in all cases.33 Depending on clinical response and histologic classification, additional therapies such as anti-CD20 humanized chimeric monoclonal antibodies, surgery, radiation, and conventional chemotherapy may be required.34
Preventive approaches remain controversial. Chemoprophylaxis with an antiviral such as ganciclovir is occasionally used but has not been shown to consistently decrease rates of posttransplant lymphoproliferative disease. These agents may act in an indirect manner, leading to decreased rates of cytomegalovirus infection, a major cofactor for posttransplant lymphoproliferative disease.24
Passive immunoprophylaxis with immunoglobulin targeting cytomegalovirus has shown to decrease rates of non-Hodgkin lymphoma from posttransplant lymphoproliferative disease in renal transplant recipients in the first year after transplant,35 but data are lacking regarding its use in liver transplant recipients. Monitoring of the viral load and subsequent reduction of immunosuppression remain the most efficient measures to date.36
FUNGAL INFECTIONS
Candida species account for more than half of fungal infections in liver transplant recipients.37 However, a change has been noted in the past 20 years, with a decrease in Candida infections accompanied by an increase in Aspergillus infections.38 Endemic mycoses such as coccidioidomycosis, blastomycosis, and histoplasmosis should be considered with the appropriate epidemiologic history or if disease develops early after transplant and the donor came from a highly endemic region.39Cryptococcus may also be encountered.
Diagnosis. One of the most challenging aspects of fungal infection in liver transplant recipients is timely diagnosis. Heightened suspicion and early biopsy for pathological and microbiological confirmation are necessary. Although available noninvasive diagnostic tools often lack specificity, early detection of fungal markers may be of great use in guiding further diagnostic workup or empiric treatment in the critically ill.
Noninvasive tests include galactomannan, cryptococcal antigen, histoplasma antigen, (1-3)-beta-D-glucan assay and various antibody tests. Galactomannan testing has been widely used to aid in the diagnosis of invasive aspergillosis. Similarly, the (1-3)-beta-D-glucan assay is a non–culture-based tool for diagnosing and monitoring the treatment of invasive fungal infections. However, a definite diagnosis cannot be made on the basis of a positive test alone.40 The complementary diagnostic characteristics of combining noninvasive assays have yet to be fully elucidated.41 Cultures and tissue histopathology are also used when possible.
Treatment is based on targeted specific antifungal drug therapy and reduction of immunosuppressive therapy, when possible. The choice of antifungal agent varies with the pathogen, the site of involvement, and the severity of the disease. A focus on potential drug interactions, their management, and therapeutic drug monitoring when using antifungal medications is essential in the posttransplant period. Combination therapy can be considered in some situations to enhance synergy. The following sections discuss in greater detail Candida species, Aspergillus species, and Pneumocystis jirovecii infections.
Candida infections
Candidiasis after liver transplant is typically nosocomial, especially when diagnosed during the first 3 months (Table 3).37
Risk factors for invasive candidiasis include perioperative colonization, prolonged operative time, retransplant, greater transfusion requirements, and postoperative renal failure.37,42,43 Invasive candidiasis is of concern for its effects on morbidity, mortality, and cost of care.43–46
Organisms. The frequency of implicated species, in particular those with a natural resistance to fluconazole, differs in various reports.37,45,46Candida albicans remains the most commonly isolated pathogen; however, non-albicans species including those resistant to fluconazole have been reported more frequently and include Candida glabrata and Candida krusei.47,48
Signs and diagnosis. Invasive candidiasis in liver transplant recipients generally manifests itself in catheter-related blood stream infections, urinary tract infections, or intra-abdominal infections. Diagnosis can be made by isolating Candida from blood cultures, recovering organisms in culture of a normally sterile site, or finding direct microscopic evidence of the fungus on tissue specimens.49
Disseminated candidiasis refers to the involvement of distant anatomic sites. Clinical manifestations may cause vision changes, abdominal pain or skin nodules with findings of candidemia, hepatosplenic abscesses, or retinal exudates on funduscopy.49
Treatment of invasive candidiasis in liver recipients often involves antifungal therapy and reduction of immunosuppression. Broad-spectrum antifungals are initially advocated in an empirical approach to cover fluconazole-resistant strains of the non-albicans subgroups.50 Depending on antifungal susceptibility, treatment can later be adjusted.
Fluconazole remains the agent of choice in most C albicans infections.47 However, attention should be paid to the possibility of resistance in patients who have received fluconazole prophylaxis within the past 30 days. Additional agents used in treatment may include echinocandins, amphotericin, and additional azoles.
Antifungal prophylaxis is recommended in high-risk liver transplant patients, although its optimal duration remains undetermined.44 Antifungal prophylaxis has been associated with decreased incidence of both superficial and invasive candidiasis.51
Aspergillus infection
Aspergillus, the second most common fungal pathogen, has become a more common concern in liver transplant recipients. Aspergillus fumigatus is the most frequently encountered species.38,52
Risk factors. These infections typically occur in the first year, during intense immunosuppression. Retransplant, renal failure, and fulminant hepatic failure are major risk factors.52 In the presence of risk factors and a suggestive clinical setting, invasive aspergillosis should be considered and the diagnosis pursued.
Diagnosis is suggested by positive findings on CT accompanied by lower respiratory tract symptoms, focal lesions on neuroimaging, or demonstration of the fungus on cultures.49 However, Aspergillus is rarely grown in blood culture. The galactomannan antigen is a noninvasive test that can provide supporting evidence for the diagnosis.41,52 False-positive results do occur in the setting of certain antibiotics and cross-reacting fungi.53
Treatment consists of antifungal therapy and immunosuppression reduction.52
Voriconazole is the first-line agent for invasive aspergillosis. Monitoring for potential drug-drug interactions and side effects is required.54,55 Amphotericin B is considered a second-line choice due to toxicity and lack of an oral formulation. In refractory cases, combined antifungal therapy could be considered.52 The duration of treatment is generally a minimum of 12 weeks.
Prophylaxis. Specific prophylaxis against invasive aspergillosis is not currently recommended; however, some authors suggest a prophylactic approach using echinocandins or liposomal amphotericin B in high-risk patients.51,52 Aspergillosis is associated with a considerable increase in mortality in liver transplant recipients, which highlights the importance of timely management.52,56
Pneumocystis jirovecii
P jirovecii remains a common opportunistic pathogen in people with impaired immunity, including transplant and human immunodeficiency virus patients.
Prophylaxis. Widespread adoption of antimicrobial prophylaxis by transplant centers has decreased the rates of P jirovecii infection in liver transplant recipients.57,58 Commonly used prophylactic regimens after liver transplantation include a single-strength trimethoprim-sulfamethoxazole tablet daily or a double-strength tablet three times per week for a minimum of 6 to 12 months after transplant. Atovaquone and dapsone can be used as alternatives in cases of intolerance to trimethoprim-sulfamethoxazole (Table 2).
Inhaled pentamidine is clearly inferior and should be used only when the other medications are contraindicated.59
Signs and diagnosis. P jirovecii pneumonia is characterized by fever, cough, dyspnea, and chest pain. Insidious hypoxemia, abnormal chest examination, and bilateral interstitial pneumonia on chest radiography are common.
CT may be more sensitive than chest radiography.57 Findings suggestive of P jirovecii pneumonia on chest CT are extensive bilateral and symmetrical ground-glass attenuations. Other less-characteristic findings include upper lobar parenchymal opacities and spontaneous pneumothorax.57,60
The serum (1,3)-beta-D-glucan assay derived from major cell-wall components of P jirovecii might be helpful. Studies report a sensitivity for P jirovecii pneumonia as high as 96% and a negative predictive value of 99.8%.61,62
Definitive diagnosis requires identification of the pathogen. Routine expectorated sputum sampling is generally associated with a poor diagnostic yield. Bronchoscopy and bronchoalveolar lavage with silver or fluorescent antibody staining of samples, polymerase chain reaction testing, or both significantly improves diagnosis. Transbronchial or open lung biopsy are often unnecessary.57
Treatment. Trimethoprim-sulfamethoxazole is the first-line agent for treating P jirovecii pneumonia.57 The minimum duration of treatment is 14 days, with extended courses for severe infection.
Intravenous pentamidine or clindamycin plus primaquine are alternatives for patients who cannot tolerate trimethoprim-sulfamethoxazole. The major concern with intravenous pentamidine is renal dysfunction. Hypoglycemia or hyperglycemia, neutropenia, thrombocytopenia, nausea, dysgeusia, and pancreatitis may also occur.63
Atovaquone might also be beneficial in mild to moderate P jirovecii pneumonia. The main side effects include skin rashes, gastrointestinal intolerance, and elevation of transaminases.64
A corticosteroid (40–60 mg of prednisone or its equivalent) may be beneficial in conjunction with antimicrobial therapy in patients with significant hypoxia (partial pressure of arterial oxygen < 70 mm Hg on room air) in decreasing the risk of respiratory failure and need for intubation.
With appropriate and timely antimicrobial prophylaxis, cases of P jirovecii pneumonia should continue to decrease.
TUBERCULOSIS
Development of tuberculosis after transplantation is a catastrophic complication, with mortality rates of up to 30%.65 Most cases of posttransplant tuberculosis represent reactivation of latent disease.66 Screening with tuberculin skin tests or interferon-gamma-release assays is recommended in all liver transplant candidates. Chest radiography before transplant is necessary when assessing a positive screening test.67
The optimal management of latent tuberculosis in these cases remains controversial. Patients at high risk or those with positive screening results on chest radiography warrant treatment for latent tuberculosis infection with isoniazid unless contraindicated.67,68
The ideal time to initiate prophylactic isoniazid therapy is unclear. Some authors suggest delaying it, as it might be associated with poor tolerance and hepatotoxicity.69 Others have found that early isoniazid use was not associated with negative outcomes.70
Risk factors for symptomatic tuberculosis after liver transplant include previous infection with tuberculosis, intensified immunosuppression (especially anti-T-lymphocyte therapies), diabetes mellitus, and other co-infections (Table 1).71
The increased incidence of atypical presentations in recent years makes the diagnosis of active tuberculosis among liver transplant recipients challenging. Sputum smears can be negative due to low mycobacterial burdens, and tuberculin skin testing and interferon-gamma-release assays may be falsely negative due to immunosuppression.67
Treatment of active tuberculosis consists initially of a four-drug regimen using isoniazid, rifampin, pyrazinamide, and ethambutol for 2 months. Adjustments are made in accordance with culture and sensitivity results. Treatment can then be tapered to two drugs (isoniazid and rifampin) for a minimum of 4 additional months. Prolonged treatment may be required in instances of extrapulmonary or disseminated disease.65,72
Tuberculosis treatment can be complicated by hepatotoxicity in liver transplant recipients because of direct drug effects and drug-drug interactions with immunosuppressive agents. Close monitoring for rejection and hepatotoxicity is therefore imperative while liver transplant recipients are receiving antituberculosis therapy. Drug-drug interactions may also be responsible for marked reductions in immunosuppression levels, especially with regimens containing rifampin.71 Substitution of rifabutin for rifampin reduces the effect of drug interactions.66
VIRAL HEPATITIS
Hepatitis B virus
Hepatitis B virus-related end-stage liver disease and hepatocellular carcinoma are common indications for liver transplant in Asia. It is less common in the United States and Europe, accounting for less than 10% of all liver transplant cases. Prognosis is favorable in recipients undergoing liver transplant for hepatitis B virus, with excellent survival rates. Prevention of reinfection is crucial in these patients.
Treatment with combination antiviral agents and hepatitis B immunoglobulin (HBIG) is effective.73 Lamivudine was the first nucleoside analogue found to be effective against hepatitis B virus. Its low cost and relative safety are strong arguments in favor of its continued use in liver transplant recipients.74 In patients without evidence of hepatitis B viral replication at the time of transplant, monotherapy with lamivudine has led to low recurrence rates, and adefovir can be added to control resistant viral strains.75
The frequent emergence of resistance with lamivudine favors newer agents such as entecavir or tenofovir. These nucleoside and nucleotide analogues have a higher barrier to resistance, and thus resistance to them is rare. They are also more efficient, potentially allowing use of an HBIG-sparing protocol.76 However, they are associated with a higher risk of nephrotoxicity and require dose adjustments in renal insufficiency. Data directly comparing entecavir and tenofovir are scarce.
Prophylaxis. Most studies support an individualized approach for prevention of hepatitis B virus reinfection. High-risk patients, ie, those positive for HBe antigen or with high viral loads (> 100,000 copies/mL) are generally treated with both HBIG and antiviral agents.77 Low-risk patients are those with a negative HBe antigen, low hepatitis B virus DNA levels, hepatitis B virus-related acute liver failure, and cirrhosis resulting from coinfection with both hepatitis B and hepatitis D virus.75 In low-risk patients, discontinuation of HBIG after 1 to 2 years of treatment is appropriate, and long-term prophylaxis with antiviral agents alone is an option. However, levels of hepatitis B DNA should be monitored closely.78,79
Hepatitis C virus
Recurrence of hepatitis C virus infection is the rule among patients who are viremic at the time of liver transplant.80,81 Most of these patients will show histologic evidence of recurrent hepatitis within the first year after liver transplant. It is often difficult to distinguish between the histopathological appearance of a recurrent hepatitis C virus infection and acute cellular rejection.
Progression to fibrosis and subsequently cirrhosis and decompensation is highly variable in hepatitis C virus-infected liver transplant recipients. Diabetes, insulin resistance, and possibly hepatitis steatosis have been associated with a rapid progression to advanced fibrosis. The contribution of immunosuppression to the progression of hepatitis C virus remains an area of active study. Some studies point to antilymphocyte immunosuppressive agents as a potential cause.82 Liver biopsy is a useful tool in this situation. It allows monitoring of disease severity and progression and may distinguish recurrent hepatitis C virus disease from other causes of liver enzyme elevation.
The major concern with the recurrence of hepatitis C virus infection after liver transplant is allograft loss. Rates of patient and graft survival are reduced in infected patients compared with hepatitis C virus-negative patients.83,84 Prophylactic antiviral therapy has no current role in the management of hepatitis C virus disease. Those manifesting moderate to severe necroinflammation or mild to moderate fibrosis indicative of progressive disease should be treated.81,85
Sustained viral clearance with antiviral agents confers a graft survival benefit.
The combination of peg-interferon and weight-based ribavirin has been the standard of treatment but may be associated with increased rates of rejection.86,87 The sustained virologic response rates for hepatitis C virus range from 60% in genotypes 4, 5, and 6 after 48 weeks of treatment to 60% to 80% in genotypes 2 and 3 after 24 weeks, but only about 30% in genotype 1.88
Treatment with the newer agents, especially protease inhibitors, in genotype 1 (peg-interferon, ribavirin, and either telaprevir or boceprevir) has been evaluated. Success rates reaching 70% have been achieved.89 Adverse effects can be a major setback. Serious complications include severe anemia, renal dysfunction, increased risk of infection, and death.
Triple therapy should be carefully considered in liver transplant patients with genotype 1 hepatitis C virus.90 Significant drug-drug interactions are reported between hepatitis C virus protease inhibitors and immunosuppression regimens. Additional new oral direct- acting antivirals have been investigated. They bring promising advances in hepatitis C virus treatment and pave the way for interferon-free regimens with pangenotypic activity.
IMMUNIZATION
Immunization can decrease the risk of infectious complications in liver transplant recipients, as well as in close contacts and healthcare professionals.3
Influenza. Pretransplant influenza vaccine and posttransplant annual influenza vaccines are necessary.
Pneumococcal immunization should additionally be provided prior to transplant and repeated every 3 to 5 years thereafter.3,91
A number of other vaccinations should also be completed before transplant, including the hepatitis A and B vaccines and the tetanus/diphtheria/acellular pertussis vaccines. However, these vaccinations have not been shown to be detrimental to patients after transplant.91
Varicella and zoster vaccines should be given before liver transplant—zoster in patients over age 60, and varicella in patients with no immunity. Live vaccines, including varicella and zoster vaccines, are contraindicated after liver transplant.3
Human papillomavirus. The bivalent human papillomavirus vaccine can be given before transplant in females ages 9 to 26; the quadrivalent vaccine is beneficial in those ages 9 to 26 and in women under age 45.3,91
IMMUNOSUPPRESSION CARRIES RISK OF INFECTION
Most liver transplant patients require prolonged immunosuppressive therapy. This comes with an increased risk of new or recurrent infections, potentially causing death and significant morbidity.
Evaluation of existing risk factors, appropriate prophylaxis and immunization, timely diagnosis, and treatment of such infections are therefore essential steps for the successful management of liver transplant recipients.
- Fishman JA. Infection in solid-organ transplant recipients. N Engl J Med 2007; 357:2601–2614.
- Avery RK, Michaels MG; AST Infectious Diseases Community of Practice. Strategies for safe living after solid organ transplantation. Am J Transplant 2013; 13(suppl 4):304–310.
- Danziger-Isakov L, Kumar D; AST Infectious Diseases Community of Practice. Vaccination in solid organ transplantation. Am J Transplant 2013; 13(suppl 4):311–317.
- San Juan R, Aguado JM, Lumbreras C, et al; RESITRA Network, Spain. Incidence, clinical characteristics and risk factors of late infection in solid organ transplant recipients: data from the RESITRA study group. Am J Transplant 2007; 7:964–971.
- Ison MG, Grossi P; AST Infectious Diseases Community of Practice. Donor-derived infections in solid organ transplantation. Am J Transplant 2013; 13(suppl 4):22–30.
- Kim YJ, Kim SI, Wie SH, et al. Infectious complications in living-donor liver transplant recipients: a 9-year single-center experience. Transpl Infect Dis 2008; 10:316–324.
- Arnow PM. Infections following orthotopic liver transplantation. HPB Surg 1991; 3:221–233.
- Reid GE, Grim SA, Sankary H, Benedetti E, Oberholzer J, Clark NM. Early intra-abdominal infections associated with orthotopic liver transplantation. Transplantation 2009; 87:1706–1711.
- Said A, Safdar N, Lucey MR, et al. Infected bilomas in liver transplant recipients, incidence, risk factors and implications for prevention. Am J Transplant 2004; 4:574–582.
- Safdar N, Said A, Lucey MR, et al. Infected bilomas in liver transplant recipients: clinical features, optimal management, and risk factors for mortality. Clin Infect Dis 2004; 39:517–525.
- Niemczyk M, Leszczyniski P, Wyzgał J, Paczek L, Krawczyk M, Luczak M. Infections caused by Clostridium difficile in kidney or liver graft recipients. Ann Transplant 2005; 10:70–74.
- Albright JB, Bonatti H, Mendez J, et al. Early and late onset Clostridium difficile-associated colitis following liver transplantation. Transpl Int 2007; 20:856–866.
- Lee SO, Razonable RR. Current concepts on cytomegalovirus infection after liver transplantation. World J Hepatol 2010; 2:325–336.
- Ljungman P, Griffiths P, Paya C. Definitions of cytomegalovirus infection and disease in transplant recipients. Clin Infect Dis 2002; 34:1094–1097.
- Beam E, Razonable RR. Cytomegalovirus in solid organ transplantation: epidemiology, prevention, and treatment. Curr Infect Dis Rep 2012; 14:633–641.
- Bodro M, Sabé N, Lladó L, et al. Prophylaxis versus preemptive therapy for cytomegalovirus disease in high-risk liver transplant recipients. Liver Transpl 2012; 18:1093–1099.
- Weigand K, Schnitzler P, Schmidt J, et al. Cytomegalovirus infection after liver transplantation incidence, risks, and benefits of prophylaxis. Transplant Proc 2010; 42:2634–2641.
- Razonable RR, Humar A; AST Infectious Diseases Community of Practice. Cytomegalovirus in solid organ transplantation. Am J Transplant 2013; 13(suppl 4):93–106.
- Meije Y, Fortún J, Len Ó, et al; Spanish Network for Research on Infection in Transplantation (RESITRA) and the Spanish Network for Research on Infectious Diseases (REIPI). Prevention strategies for cytomegalovirus disease and long-term outcomes in the high-risk transplant patient (D+/R-): experience from the RESITRA-REIPI cohort. Transpl Infect Dis 2014; 16:387–396.
- Durand CM, Marr KA, Arnold CA, et al. Detection of cytomegalovirus DNA in plasma as an adjunct diagnostic for gastrointestinal tract disease in kidney and liver transplant recipients. Clin Infect Dis 2013; 57:1550–1559.
- Levitsky J, Singh N, Wagener MM, Stosor V, Abecassis M, Ison MG. A survey of CMV prevention strategies after liver transplantation. Am J Transplant 2008; 8:158–161.
- Marcelin JR, Beam E, Razonable RR. Cytomegalovirus infection in liver transplant recipients: updates on clinical management. World J Gastroenterol 2014; 20:10658–10667.
- Kalil AC, Freifeld AG, Lyden ER, Stoner JA. Valganciclovir for cytomegalovirus prevention in solid organ transplant patients: an evidence-based reassessment of safety and efficacy. PLoS One 2009; 4:e5512.
- Kalil AC, Mindru C, Botha JF, et al. Risk of cytomegalovirus disease in high-risk liver transplant recipients on valganciclovir prophylaxis: a systematic review and meta-analysis. Liver Transpl 2012; 18:1440–1447.
- Eid AJ, Arthurs SK, Deziel PJ, Wilhelm MP, Razonable RR. Emergence of drug-resistant cytomegalovirus in the era of valganciclovir prophylaxis: therapeutic implications and outcomes. Clin Transplant 2008; 22:162–170.
- Kumar D, Humar A. Cytomegalovirus prophylaxis: how long is enough? Nat Rev Nephrol 2010; 6:13–14.
- Lurain NS, Chou S. Antiviral drug resistance of human cytomegalovirus. Clin Microbiol Rev 2010; 23:689–712.
- Torres-Madriz G, Boucher HW. Immunocompromised hosts: perspectives in the treatment and prophylaxis of cytomegalovirus disease in solid-organ transplant recipients. Clin Infect Dis 2008; 47:702–711.
- Burra P, Buda A, Livi U, et al. Occurrence of post-transplant lymphoproliferative disorders among over thousand adult recipients: any role for hepatitis C infection? Eur J Gastroenterol Hepatol 2006; 18:1065–1070.
- Jain A, Nalesnik M, Reyes J, et al. Posttransplant lymphoproliferative disorders in liver transplantation: a 20-year experience. Ann Surg 2002; 236:429–437.
- Allen UD, Preiksaitis JK; AST Infectious Diseases Community of Practice. Epstein-Barr virus and posttransplant lymphoproliferative disorder in solid organ transplantation. Am J Transplant 2013; 13(suppl 4):107–120.
- Allen U, Preiksaitis J; AST Infectious Diseases Community of Practice. Epstein-Barr virus and posttransplant lymphoproliferative disorder in solid organ transplant recipients. Am J Transplant 2009; 9(suppl 4):S87–S96.
- Perrine SP, Hermine O, Small T, et al. A phase 1/2 trial of arginine butyrate and ganciclovir in patients with Epstein-Barr virus-associated lymphoid malignancies. Blood 2007; 109:2571–2578.
- Jagadeesh D, Woda BA, Draper J, Evens AM. Post transplant lymphoproliferative disorders: risk, classification, and therapeutic recommendations. Curr Treat Options Oncol 2012; 13:122–136.
- Opelz G, Daniel V, Naujokat C, Fickenscher H, Döhler B. Effect of cytomegalovirus prophylaxis with immunoglobulin or with antiviral drugs on post-transplant non-Hodgkin lymphoma: a multicentre retrospective analysis. Lancet Oncol 2007; 8:212–218.
- Nowalk AJ, Green M. Epstein-Barr virus–associated posttransplant lymphoproliferative disorder: strategies for prevention and cure. Liver Transpl 2010; 16(suppl S2):S54–S59.
- Pappas PG, Silveira FP; AST Infectious Diseases Community of Practice. Candida in solid organ transplant recipients. Am J Transplant 2009; 9(suppl 4):S173–S179.
- Singh N, Wagener MM, Marino IR, Gayowski T. Trends in invasive fungal infections in liver transplant recipients: correlation with evolution in transplantation practices. Transplantation 2002; 73:63–67.
- Miller R, Assi M; AST Infectious Diseases Community of Practice. Endemic fungal infections in solid organ transplantation. Am J Transplant 2013; 13(suppl 4):250–261.
- Fontana C, Gaziano R, Favaro M, Casalinuovo IA, Pistoia E, Di Francesco P. (1-3)-beta-D-glucan vs galactomannan antigen in diagnosing invasive fungal infections (IFIs). Open Microbiol J 2012; 6:70–73.
- Aydogan S, Kustimur S, Kalkancı A. Comparison of glucan and galactomannan tests with real-time PCR for diagnosis of invasive aspergillosis in a neutropenic rat model [Turkish]. Mikrobiyol Bul 2010; 44:441–452.
- Hadley S, Huckabee C, Pappas PG, et al. Outcomes of antifungal prophylaxis in high-risk liver transplant recipients. Transpl Infect Dis 2009; 11:40–48.
- Pappas PG, Kauffman CA, Andes D, et al; Infectious Diseases Society of America. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 2009; 48:503–535.
- Person AK, Kontoyiannis DP, Alexander BD. Fungal infections in transplant and oncology patients. Infect Dis Clin North Am 2010; 24:439–459.
- Van Hal SJ, Marriott DJE, Chen SCA, et al; Australian Candidaemia Study. Candidemia following solid organ transplantation in the era of antifungal prophylaxis: the Australian experience. Transpl Infect Dis 2009; 11:122–127.
- Singh N. Fungal infections in the recipients of solid organ transplantation. Infect Dis Clin North Am 2003; 17:113–134,
- Liu X, Ling Z, Li L, Ruan B. Invasive fungal infections in liver transplantation. Int J Infect Dis 2011; 15:e298–e304.
- Raghuram A, Restrepo A, Safadjou S, et al. Invasive fungal infections following liver transplantation: incidence, risk factors, survival, and impact of fluconazole-resistant Candida parapsilosis (2003-2007). Liver Transpl 2012; 18:1100–1109.
- De Pauw B, Walsh TJ, Donnelly JP, et al; European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group; National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 2008; 46:1813–1821.
- Moreno A, Cervera C, Gavaldá J, et al. Bloodstream infections among transplant recipients: results of a nationwide surveillance in Spain. Am J Transplant 2007; 7:2579–2586.
- Cruciani M, Mengoli C, Malena M, Bosco O, Serpelloni G, Grossi P. Antifungal prophylaxis in liver transplant patients: a systematic review and meta-analysis. Liver Transpl 2006; 12:850–858.
- Singh N, Husain S; AST Infectious Diseases Community of Practice. Invasive aspergillosis in solid organ transplant recipients. Am J Transplant 2009; 9(suppl 4):S180–S191.
- Fortún J, Martín-Dávila P, Alvarez ME, et al. False-positive results of Aspergillus galactomannan antigenemia in liver transplant recipients. Transplantation 2009; 87:256–260.
- Cherian T, Giakoustidis A, Yokoyama S, et al. Treatment of refractory cerebral aspergillosis in a liver transplant recipient with voriconazole: case report and review of the literature. Exp Clin Transplant 2012; 10:482–486.
- Luong ML, Hosseini-Moghaddam SM, Singer LG, et al. Risk factors for voriconazole hepatotoxicity at 12 weeks in lung transplant recipients. Am J Transplant 2012; 12:1929–1935.
- Neofytos D, Fishman JA, Horn D, et al. Epidemiology and outcome of invasive fungal infections in solid organ transplant recipients. Transpl Infect Dis 2010; 12:220–229.
- Martin SI, Fishman JA; AST Infectious Diseases Community of Practice. Pneumocystis pneumonia in solid organ transplant recipients. Am J Transplant 2009; 9(suppl 4):S227–S233.
- Levine SJ, Masur H, Gill VJ, et al. Effect of aerosolized pentamidine prophylaxis on the diagnosis of Pneumocystis carinii pneumonia by induced sputum examination in patients infected with the human immunodeficiency virus. Am Rev Respir Dis 1991; 144:760–764.
- Rodriguez M, Sifri CD, Fishman JA. Failure of low-dose atovaquone prophylaxis against Pneumocystis jiroveci infection in transplant recipients. Clin Infect Dis 2004; 38:e76–e78.
- Crans CA Jr, Boiselle PM. Imaging features of Pneumocystis carinii pneumonia. Crit Rev Diagn Imaging 1999; 40:251–284.
- Onishi A, Sugiyama D, Kogata Y, et al. Diagnostic accuracy of serum 1,3-beta-D-glucan for Pneumocystis jiroveci pneumonia, invasive candidiasis, and invasive aspergillosis: systematic review and meta-analysis. J Clin Microbiol 2012; 50:7–15.
- Held J, Koch MS, Reischl U, Danner T, Serr A. Serum (1→3)-ß-D-glucan measurement as an early indicator of Pneumocystis jirovecii pneumonia and evaluation of its prognostic value. Clin Microbiol Infect 2011; 17:595–602.
- Fishman JA. Prevention of infection caused by Pneumocystis carinii in transplant recipients. Clin Infect Dis 2001; 33:1397–1405.
- Colby C, McAfee S, Sackstein R, Finkelstein D, Fishman J, Spitzer T. A prospective randomized trial comparing the toxicity and safety of atovaquone with trimethoprim/sulfamethoxazole as Pneumocystis carinii pneumonia prophylaxis following autologous peripheral blood stem cell transplantation. Bone Marrow Transplant 1999; 24:897–902.
- Subramanian A, Dorman S; AST Infectious Diseases Community of Practice. Mycobacterium tuberculosis in solid organ transplant recipients. Am J Transplant 2009; 9(suppl 4):S57–S62.
- Subramanian AK, Morris MI; AST Infectious Diseases Community of Practice. Mycobacterium tuberculosis infections in solid organ transplantation. Am J Transplant 2013; 13(suppl 4):68–76.
- Horne DJ, Narita M, Spitters CL, Parimi S, Dodson S, Limaye AP. Challenging issues in tuberculosis in solid organ transplantation. Clin Infect Dis 2013; 57:1473–1482.
- Holty JE, Gould MK, Meinke L, Keeffe EB, Ruoss SJ. Tuberculosis in liver transplant recipients: a systematic review and meta-analysis of individual patient data. Liver Transpl 2009; 15:894–906.
- Jafri SM, Singal AG, Kaul D, Fontana RJ. Detection and management of latent tuberculosis in liver transplant patients. Liver Transpl 2011; 17:306–314.
- Fábrega E, Sampedro B, Cabezas J, et al. Chemoprophylaxis with isoniazid in liver transplant recipients. Liver Transpl 2012; 18:1110–1117.
- Aguado JM, Torre-Cisneros J, Fortún J, et al. Tuberculosis in solid-organ transplant recipients: consensus statement of the group for the study of infection in transplant recipients (GESITRA) of the Spanish Society of Infectious Diseases and Clinical Microbiology. Clin Infect Dis 2009; 48:1276–1284.
- Yehia BR, Blumberg EA. Mycobacterium tuberculosis infection in liver transplantation. Liver Transpl 2010; 16:1129–1135.
- Katz LH, Paul M, Guy DG, Tur-Kaspa R. Prevention of recurrent hepatitis B virus infection after liver transplantation: hepatitis B immunoglobulin, antiviral drugs, or both? Systematic review and meta-analysis. Transpl Infect Dis 2010; 12:292–308.
- Jiang L, Jiang LS, Cheng NS, Yan LN. Current prophylactic strategies against hepatitis B virus recurrence after liver transplantation. World J Gastroenterol 2009; 15:2489–2499.
- Riediger C, Berberat PO, Sauer P, et al. Prophylaxis and treatment of recurrent viral hepatitis after liver transplantation. Nephrol Dial Transplant 2007; 22(suppl 8):viii37–viii46.
- Cholongitas E, Vasiliadis T, Antoniadis N, Goulis I, Papanikolaou V, Akriviadis E. Hepatitis B prophylaxis post liver transplantation with newer nucleos(t)ide analogues after hepatitis B immunoglobulin discontinuation. Transpl Infect Dis 2012; 14:479–487.
- Fox AN, Terrault NA. Individualizing hepatitis B infection prophylaxis in liver transplant recipients. J Hepatol 2011; 55:507–509.
- Fox AN, Terrault NA. The option of HBIG-free prophylaxis against recurrent HBV. J Hepatol 2012; 56:1189–1197.
- Wesdorp DJ, Knoester M, Braat AE, et al. Nucleoside plus nucleotide analogs and cessation of hepatitis B immunoglobulin after liver transplantation in chronic hepatitis B is safe and effective. J Clin Virol 2013; 58:67–73.
- Terrault NA, Berenguer M. Treating hepatitis C infection in liver transplant recipients. Liver Transpl 2006; 12:1192–1204.
- Ciria R, Pleguezuelo M, Khorsandi SE, et al. Strategies to reduce hepatitis C virus recurrence after liver transplantation. World J Hepatol 2013; 5:237–250.
- Issa NC, Fishman JA. Infectious complications of antilymphocyte therapies in solid organ transplantation. Clin Infect Dis 2009; 48:772–786.
- Kalambokis G, Manousou P, Samonakis D, et al. Clinical outcome of HCV-related graft cirrhosis and prognostic value of hepatic venous pressure gradient. Transpl Int 2009; 22:172–181.
- Neumann UP, Berg T, Bahra M, et al. Long-term outcome of liver transplants for chronic hepatitis C: a 10-year follow-up. Transplantation 2004; 77:226–231.
- Wiesner RH, Sorrell M, Villamil F; International Liver Transplantation Society Expert Panel. Report of the first International Liver Transplantation Society expert panel consensus conference on liver transplantation and hepatitis C. Liver Transpl 2003; 9:S1–S9.
- Dinges S, Morard I, Heim M, et al; Swiss Association for the Study of the Liver (SASL 17). Pegylated interferon-alpha2a/ribavirin treatment of recurrent hepatitis C after liver transplantation. Transpl Infect Dis 2009; 11:33–39.
- Veldt BJ, Poterucha JJ, Watt KD, et al. Impact of pegylated interferon and ribavirin treatment on graft survival in liver transplant patients with recurrent hepatitis C infection. Am J Transplant 2008; 8:2426–2433.
- Faisal N, Yoshida EM, Bilodeau M, et al. Protease inhibitor-based triple therapy is highly effective for hepatitis C recurrence after liver transplant: a multicenter experience. Ann Hepatol 2014; 13:525–532.
- Mariño Z, van Bömmel F, Forns X, Berg T. New concepts of sofosbuvir-based treatment regimens in patients with hepatitis C. Gut 2014; 63:207–215.
- Coilly A, Roche B, Dumortier J, et al. Safety and efficacy of protease inhibitors to treat hepatitis C after liver transplantation: a multicenter experience. J Hepatol 2014; 60:78–86.
- Lucey MR, Terrault N, Ojo L, et al. Long-term management of the successful adult liver transplant: 2012 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Liver Transpl 2013; 19:3–26.
- Fishman JA. Infection in solid-organ transplant recipients. N Engl J Med 2007; 357:2601–2614.
- Avery RK, Michaels MG; AST Infectious Diseases Community of Practice. Strategies for safe living after solid organ transplantation. Am J Transplant 2013; 13(suppl 4):304–310.
- Danziger-Isakov L, Kumar D; AST Infectious Diseases Community of Practice. Vaccination in solid organ transplantation. Am J Transplant 2013; 13(suppl 4):311–317.
- San Juan R, Aguado JM, Lumbreras C, et al; RESITRA Network, Spain. Incidence, clinical characteristics and risk factors of late infection in solid organ transplant recipients: data from the RESITRA study group. Am J Transplant 2007; 7:964–971.
- Ison MG, Grossi P; AST Infectious Diseases Community of Practice. Donor-derived infections in solid organ transplantation. Am J Transplant 2013; 13(suppl 4):22–30.
- Kim YJ, Kim SI, Wie SH, et al. Infectious complications in living-donor liver transplant recipients: a 9-year single-center experience. Transpl Infect Dis 2008; 10:316–324.
- Arnow PM. Infections following orthotopic liver transplantation. HPB Surg 1991; 3:221–233.
- Reid GE, Grim SA, Sankary H, Benedetti E, Oberholzer J, Clark NM. Early intra-abdominal infections associated with orthotopic liver transplantation. Transplantation 2009; 87:1706–1711.
- Said A, Safdar N, Lucey MR, et al. Infected bilomas in liver transplant recipients, incidence, risk factors and implications for prevention. Am J Transplant 2004; 4:574–582.
- Safdar N, Said A, Lucey MR, et al. Infected bilomas in liver transplant recipients: clinical features, optimal management, and risk factors for mortality. Clin Infect Dis 2004; 39:517–525.
- Niemczyk M, Leszczyniski P, Wyzgał J, Paczek L, Krawczyk M, Luczak M. Infections caused by Clostridium difficile in kidney or liver graft recipients. Ann Transplant 2005; 10:70–74.
- Albright JB, Bonatti H, Mendez J, et al. Early and late onset Clostridium difficile-associated colitis following liver transplantation. Transpl Int 2007; 20:856–866.
- Lee SO, Razonable RR. Current concepts on cytomegalovirus infection after liver transplantation. World J Hepatol 2010; 2:325–336.
- Ljungman P, Griffiths P, Paya C. Definitions of cytomegalovirus infection and disease in transplant recipients. Clin Infect Dis 2002; 34:1094–1097.
- Beam E, Razonable RR. Cytomegalovirus in solid organ transplantation: epidemiology, prevention, and treatment. Curr Infect Dis Rep 2012; 14:633–641.
- Bodro M, Sabé N, Lladó L, et al. Prophylaxis versus preemptive therapy for cytomegalovirus disease in high-risk liver transplant recipients. Liver Transpl 2012; 18:1093–1099.
- Weigand K, Schnitzler P, Schmidt J, et al. Cytomegalovirus infection after liver transplantation incidence, risks, and benefits of prophylaxis. Transplant Proc 2010; 42:2634–2641.
- Razonable RR, Humar A; AST Infectious Diseases Community of Practice. Cytomegalovirus in solid organ transplantation. Am J Transplant 2013; 13(suppl 4):93–106.
- Meije Y, Fortún J, Len Ó, et al; Spanish Network for Research on Infection in Transplantation (RESITRA) and the Spanish Network for Research on Infectious Diseases (REIPI). Prevention strategies for cytomegalovirus disease and long-term outcomes in the high-risk transplant patient (D+/R-): experience from the RESITRA-REIPI cohort. Transpl Infect Dis 2014; 16:387–396.
- Durand CM, Marr KA, Arnold CA, et al. Detection of cytomegalovirus DNA in plasma as an adjunct diagnostic for gastrointestinal tract disease in kidney and liver transplant recipients. Clin Infect Dis 2013; 57:1550–1559.
- Levitsky J, Singh N, Wagener MM, Stosor V, Abecassis M, Ison MG. A survey of CMV prevention strategies after liver transplantation. Am J Transplant 2008; 8:158–161.
- Marcelin JR, Beam E, Razonable RR. Cytomegalovirus infection in liver transplant recipients: updates on clinical management. World J Gastroenterol 2014; 20:10658–10667.
- Kalil AC, Freifeld AG, Lyden ER, Stoner JA. Valganciclovir for cytomegalovirus prevention in solid organ transplant patients: an evidence-based reassessment of safety and efficacy. PLoS One 2009; 4:e5512.
- Kalil AC, Mindru C, Botha JF, et al. Risk of cytomegalovirus disease in high-risk liver transplant recipients on valganciclovir prophylaxis: a systematic review and meta-analysis. Liver Transpl 2012; 18:1440–1447.
- Eid AJ, Arthurs SK, Deziel PJ, Wilhelm MP, Razonable RR. Emergence of drug-resistant cytomegalovirus in the era of valganciclovir prophylaxis: therapeutic implications and outcomes. Clin Transplant 2008; 22:162–170.
- Kumar D, Humar A. Cytomegalovirus prophylaxis: how long is enough? Nat Rev Nephrol 2010; 6:13–14.
- Lurain NS, Chou S. Antiviral drug resistance of human cytomegalovirus. Clin Microbiol Rev 2010; 23:689–712.
- Torres-Madriz G, Boucher HW. Immunocompromised hosts: perspectives in the treatment and prophylaxis of cytomegalovirus disease in solid-organ transplant recipients. Clin Infect Dis 2008; 47:702–711.
- Burra P, Buda A, Livi U, et al. Occurrence of post-transplant lymphoproliferative disorders among over thousand adult recipients: any role for hepatitis C infection? Eur J Gastroenterol Hepatol 2006; 18:1065–1070.
- Jain A, Nalesnik M, Reyes J, et al. Posttransplant lymphoproliferative disorders in liver transplantation: a 20-year experience. Ann Surg 2002; 236:429–437.
- Allen UD, Preiksaitis JK; AST Infectious Diseases Community of Practice. Epstein-Barr virus and posttransplant lymphoproliferative disorder in solid organ transplantation. Am J Transplant 2013; 13(suppl 4):107–120.
- Allen U, Preiksaitis J; AST Infectious Diseases Community of Practice. Epstein-Barr virus and posttransplant lymphoproliferative disorder in solid organ transplant recipients. Am J Transplant 2009; 9(suppl 4):S87–S96.
- Perrine SP, Hermine O, Small T, et al. A phase 1/2 trial of arginine butyrate and ganciclovir in patients with Epstein-Barr virus-associated lymphoid malignancies. Blood 2007; 109:2571–2578.
- Jagadeesh D, Woda BA, Draper J, Evens AM. Post transplant lymphoproliferative disorders: risk, classification, and therapeutic recommendations. Curr Treat Options Oncol 2012; 13:122–136.
- Opelz G, Daniel V, Naujokat C, Fickenscher H, Döhler B. Effect of cytomegalovirus prophylaxis with immunoglobulin or with antiviral drugs on post-transplant non-Hodgkin lymphoma: a multicentre retrospective analysis. Lancet Oncol 2007; 8:212–218.
- Nowalk AJ, Green M. Epstein-Barr virus–associated posttransplant lymphoproliferative disorder: strategies for prevention and cure. Liver Transpl 2010; 16(suppl S2):S54–S59.
- Pappas PG, Silveira FP; AST Infectious Diseases Community of Practice. Candida in solid organ transplant recipients. Am J Transplant 2009; 9(suppl 4):S173–S179.
- Singh N, Wagener MM, Marino IR, Gayowski T. Trends in invasive fungal infections in liver transplant recipients: correlation with evolution in transplantation practices. Transplantation 2002; 73:63–67.
- Miller R, Assi M; AST Infectious Diseases Community of Practice. Endemic fungal infections in solid organ transplantation. Am J Transplant 2013; 13(suppl 4):250–261.
- Fontana C, Gaziano R, Favaro M, Casalinuovo IA, Pistoia E, Di Francesco P. (1-3)-beta-D-glucan vs galactomannan antigen in diagnosing invasive fungal infections (IFIs). Open Microbiol J 2012; 6:70–73.
- Aydogan S, Kustimur S, Kalkancı A. Comparison of glucan and galactomannan tests with real-time PCR for diagnosis of invasive aspergillosis in a neutropenic rat model [Turkish]. Mikrobiyol Bul 2010; 44:441–452.
- Hadley S, Huckabee C, Pappas PG, et al. Outcomes of antifungal prophylaxis in high-risk liver transplant recipients. Transpl Infect Dis 2009; 11:40–48.
- Pappas PG, Kauffman CA, Andes D, et al; Infectious Diseases Society of America. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 2009; 48:503–535.
- Person AK, Kontoyiannis DP, Alexander BD. Fungal infections in transplant and oncology patients. Infect Dis Clin North Am 2010; 24:439–459.
- Van Hal SJ, Marriott DJE, Chen SCA, et al; Australian Candidaemia Study. Candidemia following solid organ transplantation in the era of antifungal prophylaxis: the Australian experience. Transpl Infect Dis 2009; 11:122–127.
- Singh N. Fungal infections in the recipients of solid organ transplantation. Infect Dis Clin North Am 2003; 17:113–134,
- Liu X, Ling Z, Li L, Ruan B. Invasive fungal infections in liver transplantation. Int J Infect Dis 2011; 15:e298–e304.
- Raghuram A, Restrepo A, Safadjou S, et al. Invasive fungal infections following liver transplantation: incidence, risk factors, survival, and impact of fluconazole-resistant Candida parapsilosis (2003-2007). Liver Transpl 2012; 18:1100–1109.
- De Pauw B, Walsh TJ, Donnelly JP, et al; European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group; National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 2008; 46:1813–1821.
- Moreno A, Cervera C, Gavaldá J, et al. Bloodstream infections among transplant recipients: results of a nationwide surveillance in Spain. Am J Transplant 2007; 7:2579–2586.
- Cruciani M, Mengoli C, Malena M, Bosco O, Serpelloni G, Grossi P. Antifungal prophylaxis in liver transplant patients: a systematic review and meta-analysis. Liver Transpl 2006; 12:850–858.
- Singh N, Husain S; AST Infectious Diseases Community of Practice. Invasive aspergillosis in solid organ transplant recipients. Am J Transplant 2009; 9(suppl 4):S180–S191.
- Fortún J, Martín-Dávila P, Alvarez ME, et al. False-positive results of Aspergillus galactomannan antigenemia in liver transplant recipients. Transplantation 2009; 87:256–260.
- Cherian T, Giakoustidis A, Yokoyama S, et al. Treatment of refractory cerebral aspergillosis in a liver transplant recipient with voriconazole: case report and review of the literature. Exp Clin Transplant 2012; 10:482–486.
- Luong ML, Hosseini-Moghaddam SM, Singer LG, et al. Risk factors for voriconazole hepatotoxicity at 12 weeks in lung transplant recipients. Am J Transplant 2012; 12:1929–1935.
- Neofytos D, Fishman JA, Horn D, et al. Epidemiology and outcome of invasive fungal infections in solid organ transplant recipients. Transpl Infect Dis 2010; 12:220–229.
- Martin SI, Fishman JA; AST Infectious Diseases Community of Practice. Pneumocystis pneumonia in solid organ transplant recipients. Am J Transplant 2009; 9(suppl 4):S227–S233.
- Levine SJ, Masur H, Gill VJ, et al. Effect of aerosolized pentamidine prophylaxis on the diagnosis of Pneumocystis carinii pneumonia by induced sputum examination in patients infected with the human immunodeficiency virus. Am Rev Respir Dis 1991; 144:760–764.
- Rodriguez M, Sifri CD, Fishman JA. Failure of low-dose atovaquone prophylaxis against Pneumocystis jiroveci infection in transplant recipients. Clin Infect Dis 2004; 38:e76–e78.
- Crans CA Jr, Boiselle PM. Imaging features of Pneumocystis carinii pneumonia. Crit Rev Diagn Imaging 1999; 40:251–284.
- Onishi A, Sugiyama D, Kogata Y, et al. Diagnostic accuracy of serum 1,3-beta-D-glucan for Pneumocystis jiroveci pneumonia, invasive candidiasis, and invasive aspergillosis: systematic review and meta-analysis. J Clin Microbiol 2012; 50:7–15.
- Held J, Koch MS, Reischl U, Danner T, Serr A. Serum (1→3)-ß-D-glucan measurement as an early indicator of Pneumocystis jirovecii pneumonia and evaluation of its prognostic value. Clin Microbiol Infect 2011; 17:595–602.
- Fishman JA. Prevention of infection caused by Pneumocystis carinii in transplant recipients. Clin Infect Dis 2001; 33:1397–1405.
- Colby C, McAfee S, Sackstein R, Finkelstein D, Fishman J, Spitzer T. A prospective randomized trial comparing the toxicity and safety of atovaquone with trimethoprim/sulfamethoxazole as Pneumocystis carinii pneumonia prophylaxis following autologous peripheral blood stem cell transplantation. Bone Marrow Transplant 1999; 24:897–902.
- Subramanian A, Dorman S; AST Infectious Diseases Community of Practice. Mycobacterium tuberculosis in solid organ transplant recipients. Am J Transplant 2009; 9(suppl 4):S57–S62.
- Subramanian AK, Morris MI; AST Infectious Diseases Community of Practice. Mycobacterium tuberculosis infections in solid organ transplantation. Am J Transplant 2013; 13(suppl 4):68–76.
- Horne DJ, Narita M, Spitters CL, Parimi S, Dodson S, Limaye AP. Challenging issues in tuberculosis in solid organ transplantation. Clin Infect Dis 2013; 57:1473–1482.
- Holty JE, Gould MK, Meinke L, Keeffe EB, Ruoss SJ. Tuberculosis in liver transplant recipients: a systematic review and meta-analysis of individual patient data. Liver Transpl 2009; 15:894–906.
- Jafri SM, Singal AG, Kaul D, Fontana RJ. Detection and management of latent tuberculosis in liver transplant patients. Liver Transpl 2011; 17:306–314.
- Fábrega E, Sampedro B, Cabezas J, et al. Chemoprophylaxis with isoniazid in liver transplant recipients. Liver Transpl 2012; 18:1110–1117.
- Aguado JM, Torre-Cisneros J, Fortún J, et al. Tuberculosis in solid-organ transplant recipients: consensus statement of the group for the study of infection in transplant recipients (GESITRA) of the Spanish Society of Infectious Diseases and Clinical Microbiology. Clin Infect Dis 2009; 48:1276–1284.
- Yehia BR, Blumberg EA. Mycobacterium tuberculosis infection in liver transplantation. Liver Transpl 2010; 16:1129–1135.
- Katz LH, Paul M, Guy DG, Tur-Kaspa R. Prevention of recurrent hepatitis B virus infection after liver transplantation: hepatitis B immunoglobulin, antiviral drugs, or both? Systematic review and meta-analysis. Transpl Infect Dis 2010; 12:292–308.
- Jiang L, Jiang LS, Cheng NS, Yan LN. Current prophylactic strategies against hepatitis B virus recurrence after liver transplantation. World J Gastroenterol 2009; 15:2489–2499.
- Riediger C, Berberat PO, Sauer P, et al. Prophylaxis and treatment of recurrent viral hepatitis after liver transplantation. Nephrol Dial Transplant 2007; 22(suppl 8):viii37–viii46.
- Cholongitas E, Vasiliadis T, Antoniadis N, Goulis I, Papanikolaou V, Akriviadis E. Hepatitis B prophylaxis post liver transplantation with newer nucleos(t)ide analogues after hepatitis B immunoglobulin discontinuation. Transpl Infect Dis 2012; 14:479–487.
- Fox AN, Terrault NA. Individualizing hepatitis B infection prophylaxis in liver transplant recipients. J Hepatol 2011; 55:507–509.
- Fox AN, Terrault NA. The option of HBIG-free prophylaxis against recurrent HBV. J Hepatol 2012; 56:1189–1197.
- Wesdorp DJ, Knoester M, Braat AE, et al. Nucleoside plus nucleotide analogs and cessation of hepatitis B immunoglobulin after liver transplantation in chronic hepatitis B is safe and effective. J Clin Virol 2013; 58:67–73.
- Terrault NA, Berenguer M. Treating hepatitis C infection in liver transplant recipients. Liver Transpl 2006; 12:1192–1204.
- Ciria R, Pleguezuelo M, Khorsandi SE, et al. Strategies to reduce hepatitis C virus recurrence after liver transplantation. World J Hepatol 2013; 5:237–250.
- Issa NC, Fishman JA. Infectious complications of antilymphocyte therapies in solid organ transplantation. Clin Infect Dis 2009; 48:772–786.
- Kalambokis G, Manousou P, Samonakis D, et al. Clinical outcome of HCV-related graft cirrhosis and prognostic value of hepatic venous pressure gradient. Transpl Int 2009; 22:172–181.
- Neumann UP, Berg T, Bahra M, et al. Long-term outcome of liver transplants for chronic hepatitis C: a 10-year follow-up. Transplantation 2004; 77:226–231.
- Wiesner RH, Sorrell M, Villamil F; International Liver Transplantation Society Expert Panel. Report of the first International Liver Transplantation Society expert panel consensus conference on liver transplantation and hepatitis C. Liver Transpl 2003; 9:S1–S9.
- Dinges S, Morard I, Heim M, et al; Swiss Association for the Study of the Liver (SASL 17). Pegylated interferon-alpha2a/ribavirin treatment of recurrent hepatitis C after liver transplantation. Transpl Infect Dis 2009; 11:33–39.
- Veldt BJ, Poterucha JJ, Watt KD, et al. Impact of pegylated interferon and ribavirin treatment on graft survival in liver transplant patients with recurrent hepatitis C infection. Am J Transplant 2008; 8:2426–2433.
- Faisal N, Yoshida EM, Bilodeau M, et al. Protease inhibitor-based triple therapy is highly effective for hepatitis C recurrence after liver transplant: a multicenter experience. Ann Hepatol 2014; 13:525–532.
- Mariño Z, van Bömmel F, Forns X, Berg T. New concepts of sofosbuvir-based treatment regimens in patients with hepatitis C. Gut 2014; 63:207–215.
- Coilly A, Roche B, Dumortier J, et al. Safety and efficacy of protease inhibitors to treat hepatitis C after liver transplantation: a multicenter experience. J Hepatol 2014; 60:78–86.
- Lucey MR, Terrault N, Ojo L, et al. Long-term management of the successful adult liver transplant: 2012 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Liver Transpl 2013; 19:3–26.
KEY POINTS
- After liver transplant, the risk of infection and the likely causal organisms vary with the patient’s state of immunosuppression and the time of infection.
- Recurrent or newly acquired infections may jeopardize the survival of the graft and the recipient.
- Because infections with viruses, fungi, and atypical pathogens can alter the prognosis, they need to be prevented and carefully managed.
- An ongoing assessment of each patient’s risk of infection allows the clinician to constantly and efficiently adapt immunosuppressive, prophylactic, and therapeutic strategies.
Women and HIV: An expanded perspective
In this issue of the Cleveland Clinic Journal of Medicine, Drs. Short and Anderson give an overview of the epidemic of human immunodeficiency virus (HIV) infection in US women and the various aspects of health care of this group, including pregnancy.1 They introduce a much broader topic and bring to light a number of additional concerns.
HIV PREYS ON THE VULNERABLE
The authors review epidemiologic trends and the evolving demographics of HIV, which deserve specific discussion.
In the early years of the epidemic, ie, the early 1980s, HIV infection in women was overshadowed by the epidemic in men, particularly men who have sex with men. The epidemic in men who have sex with men remains the larger component of the HIV picture in the United States. But worldwide, HIV is an evenly balanced problem, with nearly half of all infections occurring in women.2 Women have received much more attention recently.
In the United States, about 300,000 women are living with HIV, and 10% of them are unaware of it. Between 1985 and 2013, the number of HIV cases in US women tripled.
The epidemic continues to disproportionately affect women of color. Two-thirds of all women with HIV are African American,2 and estimates suggest that 1 of every 32 African American women will acquire HIV during her lifetime. On a positive note, there was a 20% reduction in new infections among African American women between 2008 and 2010.3
The epidemic preys on the vulnerable and is fueled by poverty, lack of education (general and health literacy), substance abuse, and restricted access to health care. Major metropolitan areas such as New York, Washington, DC, Miami, and Los Angeles are “hot spots,” where high concentrations of infected people reside.4
Many women underestimate or do not perceive their susceptibility. They unknowingly acquire HIV infection from their male partners, many of whom are unaware of their infection. Some of their partners may lead a dual life of bisexuality. In some areas, an estimated 20% of men who have sex with men also engage in sex with women.5 If these women contract the disease, they may be diagnosed at a late stage and when they are symptomatic, or coincidentally during pregnancy and childbirth.
Negotiating safe sex practices can be difficult for a woman. She may perceive or lack empowerment to do so, fearing rejection, isolation, or violence. Sexual violence may have been initiated in childhood, through intimate partners, rape, sex trafficking, or prostitution. Patterns vary throughout the world, but sexual violence is more common than perceived.6 Because of shame, embarrassment, and isolation, many victims do not seek medical care and so may carry undiagnosed infections. Even when they access care, they are less likely to remain in the HIV care system.7 Greater efforts are needed to reach these women, make them feel supported in care, and keep them in the system.
TESTING IS CRUCIAL
Diagnosis remains a weak link in the chain of care for both men and women. Success has been noted in the form of a marked reduction in cases of mother-to-child transmission, thanks to near-universal opt-out screening during pregnancy or at delivery.
If appropriate routine testing were done for all people, as advocated by the US Centers for Disease Control and Prevention guidelines,8 more cases could be diagnosed, behaviors changed, and treatment offered. Control of HIV through treatment can lead to a 96% reduction in transmission between serodiscordant partners, as demonstrated in HPTN 052, an ongoing phase 3 trial.9 Early diagnosis and treatment offer the potential for improved immune regeneration and healthier lives.
PRE-EXPOSURE PROPHYLAXIS
Pre-exposure prophylaxis (PrEP) is one approach to empowering women and preventing HIV infection. Studies have demonstrated the efficacy of this approach, although some studies have not.10,11
An important finding in the failed studies appeared to be a lack of adherence to the regimen.11 Unless taken faithfully, PrEP will not succeed. Additionally, there may be inherent differences in outcomes for unknown reasons. Lack of access to the necessary two-drug combination regimen is another barrier.
PrEP is expensive, requires regular monitoring, and requires patients to remain engaged in medical care. Currently, not all medical programs offer PrEP, and not all insurance policies cover it. Further insight into long-term side effects and complications is needed.
Although PrEP is an attractive concept and a reality for some, it is an incomplete solution to prevention at this time.
MEN AND WOMEN ARE DIFFERENT
Men and women are different physiologically and psychologically. Women typically have a lower body mass, lower bone mass, and higher content of body fat. As a result, women may differ from men in their ability to tolerate medications, and long-term side effects may be more pronounced.
Women are also more likely to place family responsibilities above self-preservation and personal health concerns. As a result, providing for and taking care of their children takes precedence over care of their own health.
Providing care to women presents many challenges and opportunities to improve their health. Health care access, transportation, assistance with child care during medical visits, the availability of counseling to deal with shame, guilt, and depression, and maintaining women within the care system are but a few examples.
AGING WITH HIV: STUDY NEEDED
Antiretroviral therapy has enabled patients to survive and often to reach a normal life expectancy if the infection is diagnosed and treated early. As a result, HIV-associated causes of death have been replaced by non-HIV comorbidities typical of aging, such as cardiovascular disease, organ failure (heart, lung, kidney, liver), non-HIV cancers, and bone disease.
Women face unique aspects of aging with menopause, including an accelerated rate of bone loss resulting in osteoporosis. HIV itself and some antiretroviral drugs may increase the loss of bone mineral density. Alcohol abuse, sedentary lifestyle, smoking, hepatitis C co-infection, and poor nutrition also contribute to this problem. Bone disease and many other aspects of aging and HIV in women require more research and intervention.
Other areas that need to be studied are the unique mucosal immune system of the female genital tract, the interplay of sex hormones and the immune system, the role of genital tract inflammation in increasing the risk of HIV acquisition, sexual violence and HIV acquisition, and the safety and efficacy of PrEP for women. This will require prioritization and ongoing funding, which is becoming scarcer. If there is to be hope of containing this disease, our efforts to understand it must not diminish.
- Short WR, Anderson JR. Caring for women with HIV: unique needs and challenges. Cleve Clin J Med 2014; 81:691–701.
- UNAIDS. Women out loud: how women living with HIV will help the world end AIDS. www.unaids.org/en/media/unaids/contentassets/documents/unaidspublication/2012/20121211_Women_Out_Loud_en.pdf. Accessed October 2, 2014.
- Centers for Disease Control and Prevention (CDC). HIV among women. www.cdc.gov/hiv/risk/gender/women/index.html. Accessed October 2, 2014.
- Hodder SL, Justman J, Hughes JP, et al; HIV Prevention Trials Network 064; Women’s HIV SeroIncidence Study Team. HIV acquisition among women from selected areas of the United States: a cohort study. Ann Intern Med 2013; 158:10–18.
- UNAIDS. Fact Sheet: women, girls, gender equality and HIV. www.unaids.org/en/media/unaids/contentassets/documents/factsheet/2012/20120217_FS_WomenGirls_en.pdf. Accessed October 2, 2014.
- Centers for Disease Control and Prevention (CDC). National intimate partner and sexual violence survey: 2010 summary report. www.cdc.gov/violenceprevention/pdf/nisvs_executive_summary-a.pdf. Accessed October 2, 2014.
- Siemieniuk RA, Krentz HB, Miller P, Woodman K, Ko K, Gill MJ. The clinical implications of high rates of intimate partner violence against HIV-positive women. J Acquir Immune Defic Syndr 2013; 64:32–38.
- Branson BM, Handsfield HH, Lampe MA, et al; Centers for Disease Control and Prevention (CDC). Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep 2006; 55:1–17.
- Cohen MS, Chen YQ, McCauley M, et al; HPTN 052 Study Team. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365:493–505.
- Grant RM, Lama JR, Anderson PL, et al; iPrEx Study Team. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010; 363:2587–2599.
- Haberer JE, Baeten JM, Campbell J, et al. Adherence to antiretroviral prophylaxis for HIV prevention: a substudy cohort within a clinical trial of serodiscordant couples in East Africa. PLoS Med 2013; 10 9:e1001511.
In this issue of the Cleveland Clinic Journal of Medicine, Drs. Short and Anderson give an overview of the epidemic of human immunodeficiency virus (HIV) infection in US women and the various aspects of health care of this group, including pregnancy.1 They introduce a much broader topic and bring to light a number of additional concerns.
HIV PREYS ON THE VULNERABLE
The authors review epidemiologic trends and the evolving demographics of HIV, which deserve specific discussion.
In the early years of the epidemic, ie, the early 1980s, HIV infection in women was overshadowed by the epidemic in men, particularly men who have sex with men. The epidemic in men who have sex with men remains the larger component of the HIV picture in the United States. But worldwide, HIV is an evenly balanced problem, with nearly half of all infections occurring in women.2 Women have received much more attention recently.
In the United States, about 300,000 women are living with HIV, and 10% of them are unaware of it. Between 1985 and 2013, the number of HIV cases in US women tripled.
The epidemic continues to disproportionately affect women of color. Two-thirds of all women with HIV are African American,2 and estimates suggest that 1 of every 32 African American women will acquire HIV during her lifetime. On a positive note, there was a 20% reduction in new infections among African American women between 2008 and 2010.3
The epidemic preys on the vulnerable and is fueled by poverty, lack of education (general and health literacy), substance abuse, and restricted access to health care. Major metropolitan areas such as New York, Washington, DC, Miami, and Los Angeles are “hot spots,” where high concentrations of infected people reside.4
Many women underestimate or do not perceive their susceptibility. They unknowingly acquire HIV infection from their male partners, many of whom are unaware of their infection. Some of their partners may lead a dual life of bisexuality. In some areas, an estimated 20% of men who have sex with men also engage in sex with women.5 If these women contract the disease, they may be diagnosed at a late stage and when they are symptomatic, or coincidentally during pregnancy and childbirth.
Negotiating safe sex practices can be difficult for a woman. She may perceive or lack empowerment to do so, fearing rejection, isolation, or violence. Sexual violence may have been initiated in childhood, through intimate partners, rape, sex trafficking, or prostitution. Patterns vary throughout the world, but sexual violence is more common than perceived.6 Because of shame, embarrassment, and isolation, many victims do not seek medical care and so may carry undiagnosed infections. Even when they access care, they are less likely to remain in the HIV care system.7 Greater efforts are needed to reach these women, make them feel supported in care, and keep them in the system.
TESTING IS CRUCIAL
Diagnosis remains a weak link in the chain of care for both men and women. Success has been noted in the form of a marked reduction in cases of mother-to-child transmission, thanks to near-universal opt-out screening during pregnancy or at delivery.
If appropriate routine testing were done for all people, as advocated by the US Centers for Disease Control and Prevention guidelines,8 more cases could be diagnosed, behaviors changed, and treatment offered. Control of HIV through treatment can lead to a 96% reduction in transmission between serodiscordant partners, as demonstrated in HPTN 052, an ongoing phase 3 trial.9 Early diagnosis and treatment offer the potential for improved immune regeneration and healthier lives.
PRE-EXPOSURE PROPHYLAXIS
Pre-exposure prophylaxis (PrEP) is one approach to empowering women and preventing HIV infection. Studies have demonstrated the efficacy of this approach, although some studies have not.10,11
An important finding in the failed studies appeared to be a lack of adherence to the regimen.11 Unless taken faithfully, PrEP will not succeed. Additionally, there may be inherent differences in outcomes for unknown reasons. Lack of access to the necessary two-drug combination regimen is another barrier.
PrEP is expensive, requires regular monitoring, and requires patients to remain engaged in medical care. Currently, not all medical programs offer PrEP, and not all insurance policies cover it. Further insight into long-term side effects and complications is needed.
Although PrEP is an attractive concept and a reality for some, it is an incomplete solution to prevention at this time.
MEN AND WOMEN ARE DIFFERENT
Men and women are different physiologically and psychologically. Women typically have a lower body mass, lower bone mass, and higher content of body fat. As a result, women may differ from men in their ability to tolerate medications, and long-term side effects may be more pronounced.
Women are also more likely to place family responsibilities above self-preservation and personal health concerns. As a result, providing for and taking care of their children takes precedence over care of their own health.
Providing care to women presents many challenges and opportunities to improve their health. Health care access, transportation, assistance with child care during medical visits, the availability of counseling to deal with shame, guilt, and depression, and maintaining women within the care system are but a few examples.
AGING WITH HIV: STUDY NEEDED
Antiretroviral therapy has enabled patients to survive and often to reach a normal life expectancy if the infection is diagnosed and treated early. As a result, HIV-associated causes of death have been replaced by non-HIV comorbidities typical of aging, such as cardiovascular disease, organ failure (heart, lung, kidney, liver), non-HIV cancers, and bone disease.
Women face unique aspects of aging with menopause, including an accelerated rate of bone loss resulting in osteoporosis. HIV itself and some antiretroviral drugs may increase the loss of bone mineral density. Alcohol abuse, sedentary lifestyle, smoking, hepatitis C co-infection, and poor nutrition also contribute to this problem. Bone disease and many other aspects of aging and HIV in women require more research and intervention.
Other areas that need to be studied are the unique mucosal immune system of the female genital tract, the interplay of sex hormones and the immune system, the role of genital tract inflammation in increasing the risk of HIV acquisition, sexual violence and HIV acquisition, and the safety and efficacy of PrEP for women. This will require prioritization and ongoing funding, which is becoming scarcer. If there is to be hope of containing this disease, our efforts to understand it must not diminish.
In this issue of the Cleveland Clinic Journal of Medicine, Drs. Short and Anderson give an overview of the epidemic of human immunodeficiency virus (HIV) infection in US women and the various aspects of health care of this group, including pregnancy.1 They introduce a much broader topic and bring to light a number of additional concerns.
HIV PREYS ON THE VULNERABLE
The authors review epidemiologic trends and the evolving demographics of HIV, which deserve specific discussion.
In the early years of the epidemic, ie, the early 1980s, HIV infection in women was overshadowed by the epidemic in men, particularly men who have sex with men. The epidemic in men who have sex with men remains the larger component of the HIV picture in the United States. But worldwide, HIV is an evenly balanced problem, with nearly half of all infections occurring in women.2 Women have received much more attention recently.
In the United States, about 300,000 women are living with HIV, and 10% of them are unaware of it. Between 1985 and 2013, the number of HIV cases in US women tripled.
The epidemic continues to disproportionately affect women of color. Two-thirds of all women with HIV are African American,2 and estimates suggest that 1 of every 32 African American women will acquire HIV during her lifetime. On a positive note, there was a 20% reduction in new infections among African American women between 2008 and 2010.3
The epidemic preys on the vulnerable and is fueled by poverty, lack of education (general and health literacy), substance abuse, and restricted access to health care. Major metropolitan areas such as New York, Washington, DC, Miami, and Los Angeles are “hot spots,” where high concentrations of infected people reside.4
Many women underestimate or do not perceive their susceptibility. They unknowingly acquire HIV infection from their male partners, many of whom are unaware of their infection. Some of their partners may lead a dual life of bisexuality. In some areas, an estimated 20% of men who have sex with men also engage in sex with women.5 If these women contract the disease, they may be diagnosed at a late stage and when they are symptomatic, or coincidentally during pregnancy and childbirth.
Negotiating safe sex practices can be difficult for a woman. She may perceive or lack empowerment to do so, fearing rejection, isolation, or violence. Sexual violence may have been initiated in childhood, through intimate partners, rape, sex trafficking, or prostitution. Patterns vary throughout the world, but sexual violence is more common than perceived.6 Because of shame, embarrassment, and isolation, many victims do not seek medical care and so may carry undiagnosed infections. Even when they access care, they are less likely to remain in the HIV care system.7 Greater efforts are needed to reach these women, make them feel supported in care, and keep them in the system.
TESTING IS CRUCIAL
Diagnosis remains a weak link in the chain of care for both men and women. Success has been noted in the form of a marked reduction in cases of mother-to-child transmission, thanks to near-universal opt-out screening during pregnancy or at delivery.
If appropriate routine testing were done for all people, as advocated by the US Centers for Disease Control and Prevention guidelines,8 more cases could be diagnosed, behaviors changed, and treatment offered. Control of HIV through treatment can lead to a 96% reduction in transmission between serodiscordant partners, as demonstrated in HPTN 052, an ongoing phase 3 trial.9 Early diagnosis and treatment offer the potential for improved immune regeneration and healthier lives.
PRE-EXPOSURE PROPHYLAXIS
Pre-exposure prophylaxis (PrEP) is one approach to empowering women and preventing HIV infection. Studies have demonstrated the efficacy of this approach, although some studies have not.10,11
An important finding in the failed studies appeared to be a lack of adherence to the regimen.11 Unless taken faithfully, PrEP will not succeed. Additionally, there may be inherent differences in outcomes for unknown reasons. Lack of access to the necessary two-drug combination regimen is another barrier.
PrEP is expensive, requires regular monitoring, and requires patients to remain engaged in medical care. Currently, not all medical programs offer PrEP, and not all insurance policies cover it. Further insight into long-term side effects and complications is needed.
Although PrEP is an attractive concept and a reality for some, it is an incomplete solution to prevention at this time.
MEN AND WOMEN ARE DIFFERENT
Men and women are different physiologically and psychologically. Women typically have a lower body mass, lower bone mass, and higher content of body fat. As a result, women may differ from men in their ability to tolerate medications, and long-term side effects may be more pronounced.
Women are also more likely to place family responsibilities above self-preservation and personal health concerns. As a result, providing for and taking care of their children takes precedence over care of their own health.
Providing care to women presents many challenges and opportunities to improve their health. Health care access, transportation, assistance with child care during medical visits, the availability of counseling to deal with shame, guilt, and depression, and maintaining women within the care system are but a few examples.
AGING WITH HIV: STUDY NEEDED
Antiretroviral therapy has enabled patients to survive and often to reach a normal life expectancy if the infection is diagnosed and treated early. As a result, HIV-associated causes of death have been replaced by non-HIV comorbidities typical of aging, such as cardiovascular disease, organ failure (heart, lung, kidney, liver), non-HIV cancers, and bone disease.
Women face unique aspects of aging with menopause, including an accelerated rate of bone loss resulting in osteoporosis. HIV itself and some antiretroviral drugs may increase the loss of bone mineral density. Alcohol abuse, sedentary lifestyle, smoking, hepatitis C co-infection, and poor nutrition also contribute to this problem. Bone disease and many other aspects of aging and HIV in women require more research and intervention.
Other areas that need to be studied are the unique mucosal immune system of the female genital tract, the interplay of sex hormones and the immune system, the role of genital tract inflammation in increasing the risk of HIV acquisition, sexual violence and HIV acquisition, and the safety and efficacy of PrEP for women. This will require prioritization and ongoing funding, which is becoming scarcer. If there is to be hope of containing this disease, our efforts to understand it must not diminish.
- Short WR, Anderson JR. Caring for women with HIV: unique needs and challenges. Cleve Clin J Med 2014; 81:691–701.
- UNAIDS. Women out loud: how women living with HIV will help the world end AIDS. www.unaids.org/en/media/unaids/contentassets/documents/unaidspublication/2012/20121211_Women_Out_Loud_en.pdf. Accessed October 2, 2014.
- Centers for Disease Control and Prevention (CDC). HIV among women. www.cdc.gov/hiv/risk/gender/women/index.html. Accessed October 2, 2014.
- Hodder SL, Justman J, Hughes JP, et al; HIV Prevention Trials Network 064; Women’s HIV SeroIncidence Study Team. HIV acquisition among women from selected areas of the United States: a cohort study. Ann Intern Med 2013; 158:10–18.
- UNAIDS. Fact Sheet: women, girls, gender equality and HIV. www.unaids.org/en/media/unaids/contentassets/documents/factsheet/2012/20120217_FS_WomenGirls_en.pdf. Accessed October 2, 2014.
- Centers for Disease Control and Prevention (CDC). National intimate partner and sexual violence survey: 2010 summary report. www.cdc.gov/violenceprevention/pdf/nisvs_executive_summary-a.pdf. Accessed October 2, 2014.
- Siemieniuk RA, Krentz HB, Miller P, Woodman K, Ko K, Gill MJ. The clinical implications of high rates of intimate partner violence against HIV-positive women. J Acquir Immune Defic Syndr 2013; 64:32–38.
- Branson BM, Handsfield HH, Lampe MA, et al; Centers for Disease Control and Prevention (CDC). Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep 2006; 55:1–17.
- Cohen MS, Chen YQ, McCauley M, et al; HPTN 052 Study Team. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365:493–505.
- Grant RM, Lama JR, Anderson PL, et al; iPrEx Study Team. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010; 363:2587–2599.
- Haberer JE, Baeten JM, Campbell J, et al. Adherence to antiretroviral prophylaxis for HIV prevention: a substudy cohort within a clinical trial of serodiscordant couples in East Africa. PLoS Med 2013; 10 9:e1001511.
- Short WR, Anderson JR. Caring for women with HIV: unique needs and challenges. Cleve Clin J Med 2014; 81:691–701.
- UNAIDS. Women out loud: how women living with HIV will help the world end AIDS. www.unaids.org/en/media/unaids/contentassets/documents/unaidspublication/2012/20121211_Women_Out_Loud_en.pdf. Accessed October 2, 2014.
- Centers for Disease Control and Prevention (CDC). HIV among women. www.cdc.gov/hiv/risk/gender/women/index.html. Accessed October 2, 2014.
- Hodder SL, Justman J, Hughes JP, et al; HIV Prevention Trials Network 064; Women’s HIV SeroIncidence Study Team. HIV acquisition among women from selected areas of the United States: a cohort study. Ann Intern Med 2013; 158:10–18.
- UNAIDS. Fact Sheet: women, girls, gender equality and HIV. www.unaids.org/en/media/unaids/contentassets/documents/factsheet/2012/20120217_FS_WomenGirls_en.pdf. Accessed October 2, 2014.
- Centers for Disease Control and Prevention (CDC). National intimate partner and sexual violence survey: 2010 summary report. www.cdc.gov/violenceprevention/pdf/nisvs_executive_summary-a.pdf. Accessed October 2, 2014.
- Siemieniuk RA, Krentz HB, Miller P, Woodman K, Ko K, Gill MJ. The clinical implications of high rates of intimate partner violence against HIV-positive women. J Acquir Immune Defic Syndr 2013; 64:32–38.
- Branson BM, Handsfield HH, Lampe MA, et al; Centers for Disease Control and Prevention (CDC). Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep 2006; 55:1–17.
- Cohen MS, Chen YQ, McCauley M, et al; HPTN 052 Study Team. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365:493–505.
- Grant RM, Lama JR, Anderson PL, et al; iPrEx Study Team. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010; 363:2587–2599.
- Haberer JE, Baeten JM, Campbell J, et al. Adherence to antiretroviral prophylaxis for HIV prevention: a substudy cohort within a clinical trial of serodiscordant couples in East Africa. PLoS Med 2013; 10 9:e1001511.



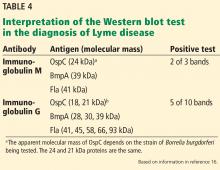
![Positive Western blot test (Borrelia B31 ViraStripe [Viramed Diagnostics]) in a patient who presented with rash and arthritis. This test uses purified specific antigens of strain B31 of Borrelia burgdorferi sensu stricto. Positive Western blot test (Borrelia B31 ViraStripe [Viramed Diagnostics]) in a patient who presented with rash and arthritis. This test uses purified specific antigens of strain B31 of Borrelia burgdorferi sensu stricto.](https://cdn.mdedge.com/files/s3fs-public/styles/medium/public/756fig2.jpg?itok=fMD7ruHJ)