User login
Radiofrequency Microtenotomy for Elbow Epicondylitis: Midterm Results
Elbow epicondylitis is a painful condition caused by overuse and development of tendon degeneration. It is one of the most common elbow problems in adults, occurring both laterally and medially. “Tennis elbow” or lateral epicondylitis is diagnosed 7 to 10 times more often than the medial form, “golfer’s elbow.”1 Although these injuries are often associated with racquet sports, activities such as bowling and weightlifting and the professions of carpentry, plumbing, and meat-cutting have been described as causes.2,3
Elbow epicondylitis is thought to be the result of multiple microtraumatic events that cause disruption of the internal structure of the tendon and degeneration of the cells and matrix.4 Lesions caused by chronic overuse are now commonly called tendinosis and are not considered inflammatory in nature. Although the term tendinitis is used frequently and indiscriminately, histopathologic studies have shown that specimens of tendon obtained from areas of chronic overuse do not contain large numbers of macrophages, lymphocytes, or neutrophils.5 Rather, tendinosis appears to be a degenerative process that is characterized by the presence of dense populations of fibroblasts, vascular hyperplasia, and disorganized collagen. This constellation of findings has been termed by some authors as angiofibroblastic hyperplasia.6
Conservative care for the treatment of chronic tendinosis has been well described and is often successful. Treatment consists of rest, ice, compression, and elevation in the acute phase. This can be followed with bracing, activity modification, physical therapy, oral nonsteroidal anti-inflammatory drugs, topical applications, and injections of cortisone or platelet-rich plasma. When conservative treatment fails, surgical intervention may be considered. Procedures for the treatment of lateral epicondylitis include open débridement and release, arthroscopic débridement, percutaneous release, and radiofrequency (RF) coblation. The goals of operative treatment are to resect pathological material, to stimulate neovascularization by producing focused local bleeding, and to create a healthy scar while doing the least possible structural damage to surrounding tissues.4
The efficacy of a bipolar RF-based approach for using microtenotomy was first recognized when researchers studied the effects of transmyocardial revascularization for treating congestive heart failure.7 The use of RF- and laser-based transmyocardial revascularization initiated an angiogenic response in degenerated (ischemic) heart tissue. This success led to investigating the use of a RF-based approach for performing microtenotomy. Preclinical studies demonstrated that RF-based microtenotomy was effective for stimulating an angiogenic-healing response in tendon tissue.8 Histologic evaluation of treated tendons showed an early inflammatory response, with new blood-vessel formation by 28 days. In 2005, short-term results of this technique were published.9 This preliminary prospective case series showed that the treatment was safe and effectively improved or eliminated clinical symptoms.9 In the present midterm study, we hypothesized that pain scores would improve after RF microtenotomy and that these favorable results would continue to be observed over a longer term postoperatively.
Materials and Methods
Patients
This was a prospective, nonrandomized, single-center clinical study. After receiving institutional review board approval, patients who were 18 to 65 years of age with a diagnosis of tendinosis were approached for enrollment. For inclusion, patients had to be symptomatic for at least 6 months and had to have failed extensive conservative treatments. Nonoperative treatment included activity modification, enrollment in a facility- or home-based exercise program, bracing, oral nonsteroidal anti-inflammatory medication, and cortisone injection. Candidates with diabetes, confirmed or suspected pregnancy, surgery in the same tendon, implanted hardware adjacent to the target treatment region, or who were receiving care under workers’ compensation or had litigation-related injury were excluded. A single clinician performed a thorough medical history and clinical evaluation. The clinical follow-up and data collection were performed by an independent medical technician.
Clinical Outcomes
Pain status was assessed by using a visual analog scale (VAS). Postoperative clinical assessment was conducted within the first 2 days; at 7 to 10 days; at 4 to 6 weeks; and at 3, 6, 12, and 24 months, up to 9 years postoperatively. The VAS scales were completed annually up to 9 years after the procedure.
The percent improvement of VAS score was calculated. This value represented the difference between the patient’s preoperative and most recent VAS assessments. Failure of the procedure was defined as less than 50% improvement of the VAS score.
The RF-Based Microtenotomy Device
The Topaz Microdebrider (ArthroCare), connected to a System 2000 generator at setting 4 (175 V-RMS), was used to perform the RF-based microtenotomy. The device uses a controlled plasma-mediated RF-based process (coblation). Radiofrequency energy is used to excite the electrolytes in a conductive medium, such as a saline solution, to create precisely focused plasma. The energized particles in the plasma have sufficient energy to break molecular bonds,10,11 excising or dissolving (ie, ablating) soft tissue at relatively low temperatures (typically, 40°-70° C).12,13 The diameter of the active tip of the Topaz device is 0.8 mm.
Surgical Procedure
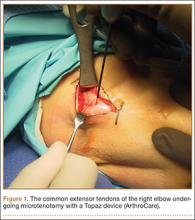
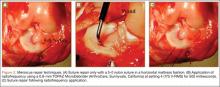
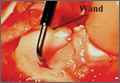
The senior author performed the majority of procedures in this study. Near the end of the series, the senior author’s associate also performed procedures. The symptomatic area of the tendon was identified and marked while the patient was alert. After the patient was positioned appropriately, light sedation was administered. A tourniquet was placed over the treatment limb and inflated to 250 mm Hg. A small incision, approximately 3 cm in length, was made over the marked treatment site to expose the involved tendon. After initiating sterile isotonic saline flow of 1 drop every 1 to 2 seconds from a line connected to the RF system, the tip of the device was placed on the tendon perpendicular to its surface (Figure 1). Using a light touch, it was activated for 500 milliseconds using a timer accessory for the control box. Five to 8 grams of pressure were applied with the device to penetrate the tendon and achieve successful ablation. The RF applications were performed at 5-mm intervals, to create a grid-like pattern on and throughout the symptomatic tendon area. The tendon was perforated to a depth of several millimeters on every second or third application throughout the treatment grid. After treatment of the symptomatic area, the wound was irrigated with copious amounts of normal saline solution and closed with interrupted nylon suture. Local anesthetic was injected only in the skin and in subcutaneous tissue. Standard wound dressings were applied. In the immediate postoperative period, the patient was advised to begin gentle active and passive range-of-motion exercises. Each patient was evaluated at 1 week postoperatively. At 6 weeks, patients were permitted to increase the intensity of their activities. Return to sports and heavy lifting was allowed once the patient was asymptomatic and had achieved full strength and range of motion; this typically occurred at 6 to 9 weeks after surgery.
Statistical Analysis
Normally distributed data were described using standard parametric statistics (ie, mean and standard deviation); non-normally distributed data were characterized using nonparametric descriptors (ie, median and quartiles). Statistical evaluation of improvement in pain status was performed by calculating 99% confidence intervals and using the Student t test for change between subsequent time points. Use of confidence intervals provides a descriptive analysis of the observed treatment effect, while permitting determination of statistical relevance. In all statistical testing, confidence bounds not including 0 were considered statistically significant. Probability of P ≤ .01 for committing type I experiment-wise error (rejecting a true null hypothesis) was selected for all statistical testing because of our lack of a control group, small sample size, and evaluation of multiple postoperative time points.
Results
Eighty consecutive patients with tendinosis of the elbow were included in this study. Sixty-nine patients were treated for lateral epicondylitis and 11 for medial epicondylitis. The average age of the patients (33 women, 47 men) was 50 years. The duration of follow-up evaluation ranged from 6 months to 9 years (mean, 2.5 years; median, 2 years). The Table presents the VAS improvement for these patients after the RF microtenotomy.
Within the lateral epicondylitis group, 91% (63/69) of the patients reported a successful outcome. The postoperative VAS improved to 1.3 from 6.9, which demonstrated an 81% improvement. Of the 6 patients that did not improve, 2 underwent repeat surgery.
Among the patients treated for medial epicondylitis, 91% (10/11) reported improvement in symptoms. The postoperative VAS improved to 1.3 from 6.1, a 79% improvement. One patient did not improve and did not undergo repeat surgery.
Discussion
For the treatment of medial and lateral elbow epicondylitis, RF microtenotomy is successful in 91% of patients. Symptomatic improvement was observed up to 9 years postoperatively. During this study, no complications were recorded; 7 treatment failures occurred. When compared with other techniques, the results with RF microtenotomy are equivalent or better.
In a retrospective study, Szabo and colleagues14 compared open, arthroscopic, and percutaneous release for lateral elbow tendinosis. They found the 3 methods to be highly effective for the treatment of tendinosis with no significant difference between them. Resection of the epicondyle and transfer of the anconeus muscle was found to be effective (94%) in a retrospective study by Almquist and colleagues.15 Dunn and coauthors16 reported a 97% success rate at 10 to 14 years postoperatively with a mini-open technique. Rubenthaler and colleagues17 showed 88% effectiveness for the open technique and 93% for the arthroscopic technique. With arthroscopic release of the extensor carpi radialis brevis tendon, Lattermann and coauthors18 reported clinical improvement in 94% of patients. In a study by Rose and colleagues,19 denervation of the lateral epicondyle was effective in relieving pain in 80% of patients who had had a positive response to a local anesthetic block. In a recently published study by Koh and coauthors,20 19 of 20 patients experienced a favorable outcome after treatment with ultrasonic microresection.
Regardless of surgical methods and their reported success rate, complications are associated with elbow surgery. Postoperative problems may include restricted function, elbow instability, persistent muscle weakness, and painful neuroma of the posterior cutaneous nerve.10,21,22 The recent introduction of arthroscopic release offers the potential for less morbidity and enables visualization of the elbow joint. However, disadvantages of the arthroscopic approach include violation of the joint for extra-articular pathology, increased operative time and cost, and neurovascular complications. Additionally, it is possible that the entire spectrum of extra-articular tendinosis cannot be effectively identified arthroscopically.23 In a prospective, randomized study, Meknas and colleagues24 compared RF microtenotomy with extensor tendon release and repair. They showed that patients treated with RF-microtenotomy experienced earlier pain relief and improved grip strength over the release group.
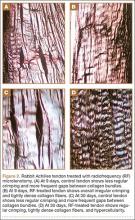
Different proposed mechanisms of action have been described to explain the favorable effects of the RF-based microtenotomy procedure, such as induced healing by an angiogenic response in the tendon tissue. In an animal study, Harwood and colleagues8 showed that low-dose RF-based plasma microtenotomy has the ability to stimulate angiogenic growth factors in tendons, such as αv integrin and vascular endothelial growth factor. These factors have been shown to be associated with healing.8 Early inflammatory response with new-vessel formation after 28 days was found in another animal study using the same method.25 Evaluation of RF-based methods in a prospective controlled laboratory study using a rabbit-tendon model showed histologic evidence of early inflammation with development of neovasculature after treatment.8 A later histologic study using an aged Achilles rabbit tendon model was performed to evaluate the effect of RF-based plasma microtenotomy on collagen remodeling.25 The degenerated tendon showed gaps, few normal crimpings, and a lack of reflectivity under polarized light. At 9 days after treatment, the treated tendon showed localized irregular crimpings, and, at 30 days, it showed regular crimping, tightly dense collagen fibers, and hypercellularity with good reflectivity. This was similar in appearance to a normal nondegenerated tendon (Figures 2A-2D). The RF-treated tendon also demonstrated an increase in production of insulin-like growth factor-1, β-fibroblast growth factor-1, αv integrin, and vascular endothelial growth factor.
Pathologic nerve ingrowth or nerve irritation in the tendon substance has been considered a possible cause of the pain experienced with tendinosis. Radiofrequency treatment has been shown to induce acute degeneration and ablation of sensory nerve fibers.26 These degenerated nerve fibers were observed to regenerate at 90 days after treatment.27 These findings provide potential evidence for early pain relief that is maintained long term as the nerves regenerate.
This midterm follow-up of patients with elbow epicondylitis has shown that RF-based microtenotomy can produce successful, durable results. Microtenotomy is a technically simple procedure to perform and is associated with a rapid and uncomplicated recovery. It is safe and can effectively eliminate or markedly reduce clinical symptoms.
Limitations
Lateral epicondylitis has been described as a self-limited disease, with resolution of symptoms at 12 to 18 months with conservative treatment. This perspective challenges the indication of any proposed surgical treatment for the condition. Although the results of this research demonstrated the benefits of RF microtenotomy, there are inherent limitations of the study design. The study lacks a control group, and randomization would improve the strength of the study. Additional outcome measures, such as Disabilities of the Arm, Shoulder, and Hand score, and grip strength could complement pain scores to provide more data. These data were collected in a preliminary study.9 Postoperative histologic analysis of treated human tissue would be ideal, but ethical considerations limit study to animal models. An additional limitation is potential examiner bias. Data collection was performed by an independent medical technician; a third-party blinded evaluation could have been performed, but this was not feasible in a clinical setting.
Conclusion
Radiofrequency-based microtenotomy is a safe and effective procedure for elbow epicondylitis. The results are durable with successful outcomes observed 9 years after surgery.
1. Leach RE, Miller JK. Lateral and medial epicondylitis of the elbow. Clin Sports Med. 1987;6(2):259-272.
2. Vangsness CT Jr, Jobe FW. Surgical technique of medial epicondylitis: Results in 35 elbows. J Bone Joint Surg Br. 1991;73(3):409-411.
3. Galloway M, DeMaio M, Mangine R. Rehabilitative techniques in the treatment of medial and lateral epicondylitis. Orthopedics. 1992;15(9):1089-1096.
4. Kraushaar BS, Nirschl RP. Tendinosis of the elbow (tennis elbow). Clinical features and findings of histological, immunohistochemical, and electron microscopy studies. J Bone Joint Surg Am. 1999;81(2):259-278.
5. Leadbetter WB. Cell-matrix response in tendon injury. Clin Sports Med. 1992;11(3):533-578.
6. Nirschl RP. Tennis elbow tendinosis: pathoanatomy, nonsurgical and surgical management. In: Fine LJ, ed. Repetitive Motion Disorders of the Upper Extremity. Rosemont, IL: American Academy of Orthopaedic Surgeons; 1995:467-479.
7. Chu V, Kuang J, Aiaid A, Korkola S, Chiu RC. Angiogenic response induced by mechanical transmyocardial revascularization. J Thorac Cardiovasc Surg 1999;118:849-856.
8. Harwood R, Bowden K, Amiel M, Tasto JP, Amiel D. Structural and angiogenic response to bipolar radiofrequency treatment of normal rabbit achilles tendon: a potential application to the treatment of tendinosis. Trans Orthop Res Soc. 2003;28:819.
9. Tasto JP, Cummings J, Medlock V, Hardesty R, Amiel D. Microtenotomy using a radiofrequency probe to treat lateral epicondylitis. Arthroscopy. 2005;21(7):851-860.
10. Woloszko J, Stalder KR, Brown IG. Plasma characteristics of repetitively-pulsed electrical discharges in saline solutions used for surgical procedures. IEEE Trans Plasma Sci. 2002;30:1376-1383.
11. Stalder KR, Woloszko J, Brown IG, Smith CD. Repetitive plasma discharges in saline solutions. Appl Phys Lett. 2001;79:4503-4505.
12. Woloszko J, Gilbride C. Coblation technology (plasma mediated ablation for otolaryngology applications). Proc SPIE. 2000;3907:306–316.
13. Woloszko J, Kwende MM, Stalder KR. Coblation in otolaryngology. Proc SPIE. 2003;4949:341–352.
14. Szabo SJ, Savoie FH 3rd, Field LD, Ramsey JR, Hosemann CD. Tendinosis of the extensor carpi radialis brevis: an evaluation of three methods of operative treatment. J Shoulder Elbow Surg Am. 2006;15(6):721-727.
15. Almquist EE, Necking L, Bach AW. Epicondylar resection with anconeus transfer for chronic lateral epicondylitis. J Hand Surg Am. 1998;23(4):723-731.
16. Dunn JH, Kim JJ, Davis L, Nirschl RP. Ten- to 14-year follow-up of the Nirschl surgical technique for lateral epicondylitis. Am J Sports Med. 2008;36(2):261-266.
17. Rubenthaler F, Wiese M, Senge A, Keller L, Wittenberg RH. Long-term follow-up of open and endoscopic Hohmann procedures for lateral epicondylitis. Arthroscopy. 2005;21(6):684-690.
18. Lattermann C, Romeo AA, Anbari A, et al. Arthroscopic debridement of the extensor carpi radialis brevis for the treatment of recalcitrant lateral epicondylitis. J Shoulder Elbow Surg. 2010;19(5):651-656.
19. Rose NE, Forman SK, Dellon AL. Denervation of the lateral epicondyle for treatment of chronic lateral epicondylitis. J Hand Surg Am. 2013;38(2):344-349.
20. Koh JS, Mohan PC, Howe TS, et al. Fasciotomy and surgical tenotomy for recalcitrant lateral elbow tendonopathy: early clinical experience with a novel device for minimally invasive percutaneous microresection. Am J Sports Med. 2013;41(3):636-644.
21. Nirschl RP, Ashman ES. Elbow tendonopathy: tennis elbow. Clin Sports Med. 2003;22(4):813-836.
22. Dellon AL, Kim J, Ducic I. Painful neuroma of the posterior cutaneous nerve of the forearm after surgery for lateral humeral epicondylitis. J Hand Surg Am. 2004;29(3):387-390.
23. Cummins CA. Lateral epicondylitis: in-vivo assessment of arthroscopic debridement and correlation with patient outcomes. Am J Sports Med. 2006;34(9):1486-1491.
24. Meknas K, Odden-Miland A, Mercer JB, Castillejo M, Johansen O. Radiofrequency microtenotomy: a promising method for treatment of recalcitrant lateral epicondylitis. Am J Sports Med. 2008;36(10):1960-1965.
25. Takahashi N, Tasto JP, Locke J, et al. The use of radiofrequency (RF) for the treatment of chronic tendinosis. Paper presented at: 6th Biennial Congress of the International Society of Arthroscopy, Knee Surgery, and Orthopaedic Sports Medicine Congress; May 2007; Florence, Italy. Abstract 1433.
26. Takahashi N, Tasto JP, Ritter M, et al. Pain relief through an antinociceptive effect after radiofrequency application. Am J Sports Med. 2007;35(5):805-810.
27. Ochiai N, Tasto JP, Ohtori S, Takahashi N, Moriya H, Amiel D. Nerve regeneration after radiofrequency ablation. Am J Sports Med. 2007;35(11):1940-1944.
Elbow epicondylitis is a painful condition caused by overuse and development of tendon degeneration. It is one of the most common elbow problems in adults, occurring both laterally and medially. “Tennis elbow” or lateral epicondylitis is diagnosed 7 to 10 times more often than the medial form, “golfer’s elbow.”1 Although these injuries are often associated with racquet sports, activities such as bowling and weightlifting and the professions of carpentry, plumbing, and meat-cutting have been described as causes.2,3
Elbow epicondylitis is thought to be the result of multiple microtraumatic events that cause disruption of the internal structure of the tendon and degeneration of the cells and matrix.4 Lesions caused by chronic overuse are now commonly called tendinosis and are not considered inflammatory in nature. Although the term tendinitis is used frequently and indiscriminately, histopathologic studies have shown that specimens of tendon obtained from areas of chronic overuse do not contain large numbers of macrophages, lymphocytes, or neutrophils.5 Rather, tendinosis appears to be a degenerative process that is characterized by the presence of dense populations of fibroblasts, vascular hyperplasia, and disorganized collagen. This constellation of findings has been termed by some authors as angiofibroblastic hyperplasia.6
Conservative care for the treatment of chronic tendinosis has been well described and is often successful. Treatment consists of rest, ice, compression, and elevation in the acute phase. This can be followed with bracing, activity modification, physical therapy, oral nonsteroidal anti-inflammatory drugs, topical applications, and injections of cortisone or platelet-rich plasma. When conservative treatment fails, surgical intervention may be considered. Procedures for the treatment of lateral epicondylitis include open débridement and release, arthroscopic débridement, percutaneous release, and radiofrequency (RF) coblation. The goals of operative treatment are to resect pathological material, to stimulate neovascularization by producing focused local bleeding, and to create a healthy scar while doing the least possible structural damage to surrounding tissues.4
The efficacy of a bipolar RF-based approach for using microtenotomy was first recognized when researchers studied the effects of transmyocardial revascularization for treating congestive heart failure.7 The use of RF- and laser-based transmyocardial revascularization initiated an angiogenic response in degenerated (ischemic) heart tissue. This success led to investigating the use of a RF-based approach for performing microtenotomy. Preclinical studies demonstrated that RF-based microtenotomy was effective for stimulating an angiogenic-healing response in tendon tissue.8 Histologic evaluation of treated tendons showed an early inflammatory response, with new blood-vessel formation by 28 days. In 2005, short-term results of this technique were published.9 This preliminary prospective case series showed that the treatment was safe and effectively improved or eliminated clinical symptoms.9 In the present midterm study, we hypothesized that pain scores would improve after RF microtenotomy and that these favorable results would continue to be observed over a longer term postoperatively.
Materials and Methods
Patients
This was a prospective, nonrandomized, single-center clinical study. After receiving institutional review board approval, patients who were 18 to 65 years of age with a diagnosis of tendinosis were approached for enrollment. For inclusion, patients had to be symptomatic for at least 6 months and had to have failed extensive conservative treatments. Nonoperative treatment included activity modification, enrollment in a facility- or home-based exercise program, bracing, oral nonsteroidal anti-inflammatory medication, and cortisone injection. Candidates with diabetes, confirmed or suspected pregnancy, surgery in the same tendon, implanted hardware adjacent to the target treatment region, or who were receiving care under workers’ compensation or had litigation-related injury were excluded. A single clinician performed a thorough medical history and clinical evaluation. The clinical follow-up and data collection were performed by an independent medical technician.
Clinical Outcomes
Pain status was assessed by using a visual analog scale (VAS). Postoperative clinical assessment was conducted within the first 2 days; at 7 to 10 days; at 4 to 6 weeks; and at 3, 6, 12, and 24 months, up to 9 years postoperatively. The VAS scales were completed annually up to 9 years after the procedure.
The percent improvement of VAS score was calculated. This value represented the difference between the patient’s preoperative and most recent VAS assessments. Failure of the procedure was defined as less than 50% improvement of the VAS score.
The RF-Based Microtenotomy Device
The Topaz Microdebrider (ArthroCare), connected to a System 2000 generator at setting 4 (175 V-RMS), was used to perform the RF-based microtenotomy. The device uses a controlled plasma-mediated RF-based process (coblation). Radiofrequency energy is used to excite the electrolytes in a conductive medium, such as a saline solution, to create precisely focused plasma. The energized particles in the plasma have sufficient energy to break molecular bonds,10,11 excising or dissolving (ie, ablating) soft tissue at relatively low temperatures (typically, 40°-70° C).12,13 The diameter of the active tip of the Topaz device is 0.8 mm.
Surgical Procedure
The senior author performed the majority of procedures in this study. Near the end of the series, the senior author’s associate also performed procedures. The symptomatic area of the tendon was identified and marked while the patient was alert. After the patient was positioned appropriately, light sedation was administered. A tourniquet was placed over the treatment limb and inflated to 250 mm Hg. A small incision, approximately 3 cm in length, was made over the marked treatment site to expose the involved tendon. After initiating sterile isotonic saline flow of 1 drop every 1 to 2 seconds from a line connected to the RF system, the tip of the device was placed on the tendon perpendicular to its surface (Figure 1). Using a light touch, it was activated for 500 milliseconds using a timer accessory for the control box. Five to 8 grams of pressure were applied with the device to penetrate the tendon and achieve successful ablation. The RF applications were performed at 5-mm intervals, to create a grid-like pattern on and throughout the symptomatic tendon area. The tendon was perforated to a depth of several millimeters on every second or third application throughout the treatment grid. After treatment of the symptomatic area, the wound was irrigated with copious amounts of normal saline solution and closed with interrupted nylon suture. Local anesthetic was injected only in the skin and in subcutaneous tissue. Standard wound dressings were applied. In the immediate postoperative period, the patient was advised to begin gentle active and passive range-of-motion exercises. Each patient was evaluated at 1 week postoperatively. At 6 weeks, patients were permitted to increase the intensity of their activities. Return to sports and heavy lifting was allowed once the patient was asymptomatic and had achieved full strength and range of motion; this typically occurred at 6 to 9 weeks after surgery.
Statistical Analysis
Normally distributed data were described using standard parametric statistics (ie, mean and standard deviation); non-normally distributed data were characterized using nonparametric descriptors (ie, median and quartiles). Statistical evaluation of improvement in pain status was performed by calculating 99% confidence intervals and using the Student t test for change between subsequent time points. Use of confidence intervals provides a descriptive analysis of the observed treatment effect, while permitting determination of statistical relevance. In all statistical testing, confidence bounds not including 0 were considered statistically significant. Probability of P ≤ .01 for committing type I experiment-wise error (rejecting a true null hypothesis) was selected for all statistical testing because of our lack of a control group, small sample size, and evaluation of multiple postoperative time points.
Results
Eighty consecutive patients with tendinosis of the elbow were included in this study. Sixty-nine patients were treated for lateral epicondylitis and 11 for medial epicondylitis. The average age of the patients (33 women, 47 men) was 50 years. The duration of follow-up evaluation ranged from 6 months to 9 years (mean, 2.5 years; median, 2 years). The Table presents the VAS improvement for these patients after the RF microtenotomy.
Within the lateral epicondylitis group, 91% (63/69) of the patients reported a successful outcome. The postoperative VAS improved to 1.3 from 6.9, which demonstrated an 81% improvement. Of the 6 patients that did not improve, 2 underwent repeat surgery.
Among the patients treated for medial epicondylitis, 91% (10/11) reported improvement in symptoms. The postoperative VAS improved to 1.3 from 6.1, a 79% improvement. One patient did not improve and did not undergo repeat surgery.
Discussion
For the treatment of medial and lateral elbow epicondylitis, RF microtenotomy is successful in 91% of patients. Symptomatic improvement was observed up to 9 years postoperatively. During this study, no complications were recorded; 7 treatment failures occurred. When compared with other techniques, the results with RF microtenotomy are equivalent or better.
In a retrospective study, Szabo and colleagues14 compared open, arthroscopic, and percutaneous release for lateral elbow tendinosis. They found the 3 methods to be highly effective for the treatment of tendinosis with no significant difference between them. Resection of the epicondyle and transfer of the anconeus muscle was found to be effective (94%) in a retrospective study by Almquist and colleagues.15 Dunn and coauthors16 reported a 97% success rate at 10 to 14 years postoperatively with a mini-open technique. Rubenthaler and colleagues17 showed 88% effectiveness for the open technique and 93% for the arthroscopic technique. With arthroscopic release of the extensor carpi radialis brevis tendon, Lattermann and coauthors18 reported clinical improvement in 94% of patients. In a study by Rose and colleagues,19 denervation of the lateral epicondyle was effective in relieving pain in 80% of patients who had had a positive response to a local anesthetic block. In a recently published study by Koh and coauthors,20 19 of 20 patients experienced a favorable outcome after treatment with ultrasonic microresection.
Regardless of surgical methods and their reported success rate, complications are associated with elbow surgery. Postoperative problems may include restricted function, elbow instability, persistent muscle weakness, and painful neuroma of the posterior cutaneous nerve.10,21,22 The recent introduction of arthroscopic release offers the potential for less morbidity and enables visualization of the elbow joint. However, disadvantages of the arthroscopic approach include violation of the joint for extra-articular pathology, increased operative time and cost, and neurovascular complications. Additionally, it is possible that the entire spectrum of extra-articular tendinosis cannot be effectively identified arthroscopically.23 In a prospective, randomized study, Meknas and colleagues24 compared RF microtenotomy with extensor tendon release and repair. They showed that patients treated with RF-microtenotomy experienced earlier pain relief and improved grip strength over the release group.
Different proposed mechanisms of action have been described to explain the favorable effects of the RF-based microtenotomy procedure, such as induced healing by an angiogenic response in the tendon tissue. In an animal study, Harwood and colleagues8 showed that low-dose RF-based plasma microtenotomy has the ability to stimulate angiogenic growth factors in tendons, such as αv integrin and vascular endothelial growth factor. These factors have been shown to be associated with healing.8 Early inflammatory response with new-vessel formation after 28 days was found in another animal study using the same method.25 Evaluation of RF-based methods in a prospective controlled laboratory study using a rabbit-tendon model showed histologic evidence of early inflammation with development of neovasculature after treatment.8 A later histologic study using an aged Achilles rabbit tendon model was performed to evaluate the effect of RF-based plasma microtenotomy on collagen remodeling.25 The degenerated tendon showed gaps, few normal crimpings, and a lack of reflectivity under polarized light. At 9 days after treatment, the treated tendon showed localized irregular crimpings, and, at 30 days, it showed regular crimping, tightly dense collagen fibers, and hypercellularity with good reflectivity. This was similar in appearance to a normal nondegenerated tendon (Figures 2A-2D). The RF-treated tendon also demonstrated an increase in production of insulin-like growth factor-1, β-fibroblast growth factor-1, αv integrin, and vascular endothelial growth factor.
Pathologic nerve ingrowth or nerve irritation in the tendon substance has been considered a possible cause of the pain experienced with tendinosis. Radiofrequency treatment has been shown to induce acute degeneration and ablation of sensory nerve fibers.26 These degenerated nerve fibers were observed to regenerate at 90 days after treatment.27 These findings provide potential evidence for early pain relief that is maintained long term as the nerves regenerate.
This midterm follow-up of patients with elbow epicondylitis has shown that RF-based microtenotomy can produce successful, durable results. Microtenotomy is a technically simple procedure to perform and is associated with a rapid and uncomplicated recovery. It is safe and can effectively eliminate or markedly reduce clinical symptoms.
Limitations
Lateral epicondylitis has been described as a self-limited disease, with resolution of symptoms at 12 to 18 months with conservative treatment. This perspective challenges the indication of any proposed surgical treatment for the condition. Although the results of this research demonstrated the benefits of RF microtenotomy, there are inherent limitations of the study design. The study lacks a control group, and randomization would improve the strength of the study. Additional outcome measures, such as Disabilities of the Arm, Shoulder, and Hand score, and grip strength could complement pain scores to provide more data. These data were collected in a preliminary study.9 Postoperative histologic analysis of treated human tissue would be ideal, but ethical considerations limit study to animal models. An additional limitation is potential examiner bias. Data collection was performed by an independent medical technician; a third-party blinded evaluation could have been performed, but this was not feasible in a clinical setting.
Conclusion
Radiofrequency-based microtenotomy is a safe and effective procedure for elbow epicondylitis. The results are durable with successful outcomes observed 9 years after surgery.
Elbow epicondylitis is a painful condition caused by overuse and development of tendon degeneration. It is one of the most common elbow problems in adults, occurring both laterally and medially. “Tennis elbow” or lateral epicondylitis is diagnosed 7 to 10 times more often than the medial form, “golfer’s elbow.”1 Although these injuries are often associated with racquet sports, activities such as bowling and weightlifting and the professions of carpentry, plumbing, and meat-cutting have been described as causes.2,3
Elbow epicondylitis is thought to be the result of multiple microtraumatic events that cause disruption of the internal structure of the tendon and degeneration of the cells and matrix.4 Lesions caused by chronic overuse are now commonly called tendinosis and are not considered inflammatory in nature. Although the term tendinitis is used frequently and indiscriminately, histopathologic studies have shown that specimens of tendon obtained from areas of chronic overuse do not contain large numbers of macrophages, lymphocytes, or neutrophils.5 Rather, tendinosis appears to be a degenerative process that is characterized by the presence of dense populations of fibroblasts, vascular hyperplasia, and disorganized collagen. This constellation of findings has been termed by some authors as angiofibroblastic hyperplasia.6
Conservative care for the treatment of chronic tendinosis has been well described and is often successful. Treatment consists of rest, ice, compression, and elevation in the acute phase. This can be followed with bracing, activity modification, physical therapy, oral nonsteroidal anti-inflammatory drugs, topical applications, and injections of cortisone or platelet-rich plasma. When conservative treatment fails, surgical intervention may be considered. Procedures for the treatment of lateral epicondylitis include open débridement and release, arthroscopic débridement, percutaneous release, and radiofrequency (RF) coblation. The goals of operative treatment are to resect pathological material, to stimulate neovascularization by producing focused local bleeding, and to create a healthy scar while doing the least possible structural damage to surrounding tissues.4
The efficacy of a bipolar RF-based approach for using microtenotomy was first recognized when researchers studied the effects of transmyocardial revascularization for treating congestive heart failure.7 The use of RF- and laser-based transmyocardial revascularization initiated an angiogenic response in degenerated (ischemic) heart tissue. This success led to investigating the use of a RF-based approach for performing microtenotomy. Preclinical studies demonstrated that RF-based microtenotomy was effective for stimulating an angiogenic-healing response in tendon tissue.8 Histologic evaluation of treated tendons showed an early inflammatory response, with new blood-vessel formation by 28 days. In 2005, short-term results of this technique were published.9 This preliminary prospective case series showed that the treatment was safe and effectively improved or eliminated clinical symptoms.9 In the present midterm study, we hypothesized that pain scores would improve after RF microtenotomy and that these favorable results would continue to be observed over a longer term postoperatively.
Materials and Methods
Patients
This was a prospective, nonrandomized, single-center clinical study. After receiving institutional review board approval, patients who were 18 to 65 years of age with a diagnosis of tendinosis were approached for enrollment. For inclusion, patients had to be symptomatic for at least 6 months and had to have failed extensive conservative treatments. Nonoperative treatment included activity modification, enrollment in a facility- or home-based exercise program, bracing, oral nonsteroidal anti-inflammatory medication, and cortisone injection. Candidates with diabetes, confirmed or suspected pregnancy, surgery in the same tendon, implanted hardware adjacent to the target treatment region, or who were receiving care under workers’ compensation or had litigation-related injury were excluded. A single clinician performed a thorough medical history and clinical evaluation. The clinical follow-up and data collection were performed by an independent medical technician.
Clinical Outcomes
Pain status was assessed by using a visual analog scale (VAS). Postoperative clinical assessment was conducted within the first 2 days; at 7 to 10 days; at 4 to 6 weeks; and at 3, 6, 12, and 24 months, up to 9 years postoperatively. The VAS scales were completed annually up to 9 years after the procedure.
The percent improvement of VAS score was calculated. This value represented the difference between the patient’s preoperative and most recent VAS assessments. Failure of the procedure was defined as less than 50% improvement of the VAS score.
The RF-Based Microtenotomy Device
The Topaz Microdebrider (ArthroCare), connected to a System 2000 generator at setting 4 (175 V-RMS), was used to perform the RF-based microtenotomy. The device uses a controlled plasma-mediated RF-based process (coblation). Radiofrequency energy is used to excite the electrolytes in a conductive medium, such as a saline solution, to create precisely focused plasma. The energized particles in the plasma have sufficient energy to break molecular bonds,10,11 excising or dissolving (ie, ablating) soft tissue at relatively low temperatures (typically, 40°-70° C).12,13 The diameter of the active tip of the Topaz device is 0.8 mm.
Surgical Procedure
The senior author performed the majority of procedures in this study. Near the end of the series, the senior author’s associate also performed procedures. The symptomatic area of the tendon was identified and marked while the patient was alert. After the patient was positioned appropriately, light sedation was administered. A tourniquet was placed over the treatment limb and inflated to 250 mm Hg. A small incision, approximately 3 cm in length, was made over the marked treatment site to expose the involved tendon. After initiating sterile isotonic saline flow of 1 drop every 1 to 2 seconds from a line connected to the RF system, the tip of the device was placed on the tendon perpendicular to its surface (Figure 1). Using a light touch, it was activated for 500 milliseconds using a timer accessory for the control box. Five to 8 grams of pressure were applied with the device to penetrate the tendon and achieve successful ablation. The RF applications were performed at 5-mm intervals, to create a grid-like pattern on and throughout the symptomatic tendon area. The tendon was perforated to a depth of several millimeters on every second or third application throughout the treatment grid. After treatment of the symptomatic area, the wound was irrigated with copious amounts of normal saline solution and closed with interrupted nylon suture. Local anesthetic was injected only in the skin and in subcutaneous tissue. Standard wound dressings were applied. In the immediate postoperative period, the patient was advised to begin gentle active and passive range-of-motion exercises. Each patient was evaluated at 1 week postoperatively. At 6 weeks, patients were permitted to increase the intensity of their activities. Return to sports and heavy lifting was allowed once the patient was asymptomatic and had achieved full strength and range of motion; this typically occurred at 6 to 9 weeks after surgery.
Statistical Analysis
Normally distributed data were described using standard parametric statistics (ie, mean and standard deviation); non-normally distributed data were characterized using nonparametric descriptors (ie, median and quartiles). Statistical evaluation of improvement in pain status was performed by calculating 99% confidence intervals and using the Student t test for change between subsequent time points. Use of confidence intervals provides a descriptive analysis of the observed treatment effect, while permitting determination of statistical relevance. In all statistical testing, confidence bounds not including 0 were considered statistically significant. Probability of P ≤ .01 for committing type I experiment-wise error (rejecting a true null hypothesis) was selected for all statistical testing because of our lack of a control group, small sample size, and evaluation of multiple postoperative time points.
Results
Eighty consecutive patients with tendinosis of the elbow were included in this study. Sixty-nine patients were treated for lateral epicondylitis and 11 for medial epicondylitis. The average age of the patients (33 women, 47 men) was 50 years. The duration of follow-up evaluation ranged from 6 months to 9 years (mean, 2.5 years; median, 2 years). The Table presents the VAS improvement for these patients after the RF microtenotomy.
Within the lateral epicondylitis group, 91% (63/69) of the patients reported a successful outcome. The postoperative VAS improved to 1.3 from 6.9, which demonstrated an 81% improvement. Of the 6 patients that did not improve, 2 underwent repeat surgery.
Among the patients treated for medial epicondylitis, 91% (10/11) reported improvement in symptoms. The postoperative VAS improved to 1.3 from 6.1, a 79% improvement. One patient did not improve and did not undergo repeat surgery.
Discussion
For the treatment of medial and lateral elbow epicondylitis, RF microtenotomy is successful in 91% of patients. Symptomatic improvement was observed up to 9 years postoperatively. During this study, no complications were recorded; 7 treatment failures occurred. When compared with other techniques, the results with RF microtenotomy are equivalent or better.
In a retrospective study, Szabo and colleagues14 compared open, arthroscopic, and percutaneous release for lateral elbow tendinosis. They found the 3 methods to be highly effective for the treatment of tendinosis with no significant difference between them. Resection of the epicondyle and transfer of the anconeus muscle was found to be effective (94%) in a retrospective study by Almquist and colleagues.15 Dunn and coauthors16 reported a 97% success rate at 10 to 14 years postoperatively with a mini-open technique. Rubenthaler and colleagues17 showed 88% effectiveness for the open technique and 93% for the arthroscopic technique. With arthroscopic release of the extensor carpi radialis brevis tendon, Lattermann and coauthors18 reported clinical improvement in 94% of patients. In a study by Rose and colleagues,19 denervation of the lateral epicondyle was effective in relieving pain in 80% of patients who had had a positive response to a local anesthetic block. In a recently published study by Koh and coauthors,20 19 of 20 patients experienced a favorable outcome after treatment with ultrasonic microresection.
Regardless of surgical methods and their reported success rate, complications are associated with elbow surgery. Postoperative problems may include restricted function, elbow instability, persistent muscle weakness, and painful neuroma of the posterior cutaneous nerve.10,21,22 The recent introduction of arthroscopic release offers the potential for less morbidity and enables visualization of the elbow joint. However, disadvantages of the arthroscopic approach include violation of the joint for extra-articular pathology, increased operative time and cost, and neurovascular complications. Additionally, it is possible that the entire spectrum of extra-articular tendinosis cannot be effectively identified arthroscopically.23 In a prospective, randomized study, Meknas and colleagues24 compared RF microtenotomy with extensor tendon release and repair. They showed that patients treated with RF-microtenotomy experienced earlier pain relief and improved grip strength over the release group.
Different proposed mechanisms of action have been described to explain the favorable effects of the RF-based microtenotomy procedure, such as induced healing by an angiogenic response in the tendon tissue. In an animal study, Harwood and colleagues8 showed that low-dose RF-based plasma microtenotomy has the ability to stimulate angiogenic growth factors in tendons, such as αv integrin and vascular endothelial growth factor. These factors have been shown to be associated with healing.8 Early inflammatory response with new-vessel formation after 28 days was found in another animal study using the same method.25 Evaluation of RF-based methods in a prospective controlled laboratory study using a rabbit-tendon model showed histologic evidence of early inflammation with development of neovasculature after treatment.8 A later histologic study using an aged Achilles rabbit tendon model was performed to evaluate the effect of RF-based plasma microtenotomy on collagen remodeling.25 The degenerated tendon showed gaps, few normal crimpings, and a lack of reflectivity under polarized light. At 9 days after treatment, the treated tendon showed localized irregular crimpings, and, at 30 days, it showed regular crimping, tightly dense collagen fibers, and hypercellularity with good reflectivity. This was similar in appearance to a normal nondegenerated tendon (Figures 2A-2D). The RF-treated tendon also demonstrated an increase in production of insulin-like growth factor-1, β-fibroblast growth factor-1, αv integrin, and vascular endothelial growth factor.
Pathologic nerve ingrowth or nerve irritation in the tendon substance has been considered a possible cause of the pain experienced with tendinosis. Radiofrequency treatment has been shown to induce acute degeneration and ablation of sensory nerve fibers.26 These degenerated nerve fibers were observed to regenerate at 90 days after treatment.27 These findings provide potential evidence for early pain relief that is maintained long term as the nerves regenerate.
This midterm follow-up of patients with elbow epicondylitis has shown that RF-based microtenotomy can produce successful, durable results. Microtenotomy is a technically simple procedure to perform and is associated with a rapid and uncomplicated recovery. It is safe and can effectively eliminate or markedly reduce clinical symptoms.
Limitations
Lateral epicondylitis has been described as a self-limited disease, with resolution of symptoms at 12 to 18 months with conservative treatment. This perspective challenges the indication of any proposed surgical treatment for the condition. Although the results of this research demonstrated the benefits of RF microtenotomy, there are inherent limitations of the study design. The study lacks a control group, and randomization would improve the strength of the study. Additional outcome measures, such as Disabilities of the Arm, Shoulder, and Hand score, and grip strength could complement pain scores to provide more data. These data were collected in a preliminary study.9 Postoperative histologic analysis of treated human tissue would be ideal, but ethical considerations limit study to animal models. An additional limitation is potential examiner bias. Data collection was performed by an independent medical technician; a third-party blinded evaluation could have been performed, but this was not feasible in a clinical setting.
Conclusion
Radiofrequency-based microtenotomy is a safe and effective procedure for elbow epicondylitis. The results are durable with successful outcomes observed 9 years after surgery.
1. Leach RE, Miller JK. Lateral and medial epicondylitis of the elbow. Clin Sports Med. 1987;6(2):259-272.
2. Vangsness CT Jr, Jobe FW. Surgical technique of medial epicondylitis: Results in 35 elbows. J Bone Joint Surg Br. 1991;73(3):409-411.
3. Galloway M, DeMaio M, Mangine R. Rehabilitative techniques in the treatment of medial and lateral epicondylitis. Orthopedics. 1992;15(9):1089-1096.
4. Kraushaar BS, Nirschl RP. Tendinosis of the elbow (tennis elbow). Clinical features and findings of histological, immunohistochemical, and electron microscopy studies. J Bone Joint Surg Am. 1999;81(2):259-278.
5. Leadbetter WB. Cell-matrix response in tendon injury. Clin Sports Med. 1992;11(3):533-578.
6. Nirschl RP. Tennis elbow tendinosis: pathoanatomy, nonsurgical and surgical management. In: Fine LJ, ed. Repetitive Motion Disorders of the Upper Extremity. Rosemont, IL: American Academy of Orthopaedic Surgeons; 1995:467-479.
7. Chu V, Kuang J, Aiaid A, Korkola S, Chiu RC. Angiogenic response induced by mechanical transmyocardial revascularization. J Thorac Cardiovasc Surg 1999;118:849-856.
8. Harwood R, Bowden K, Amiel M, Tasto JP, Amiel D. Structural and angiogenic response to bipolar radiofrequency treatment of normal rabbit achilles tendon: a potential application to the treatment of tendinosis. Trans Orthop Res Soc. 2003;28:819.
9. Tasto JP, Cummings J, Medlock V, Hardesty R, Amiel D. Microtenotomy using a radiofrequency probe to treat lateral epicondylitis. Arthroscopy. 2005;21(7):851-860.
10. Woloszko J, Stalder KR, Brown IG. Plasma characteristics of repetitively-pulsed electrical discharges in saline solutions used for surgical procedures. IEEE Trans Plasma Sci. 2002;30:1376-1383.
11. Stalder KR, Woloszko J, Brown IG, Smith CD. Repetitive plasma discharges in saline solutions. Appl Phys Lett. 2001;79:4503-4505.
12. Woloszko J, Gilbride C. Coblation technology (plasma mediated ablation for otolaryngology applications). Proc SPIE. 2000;3907:306–316.
13. Woloszko J, Kwende MM, Stalder KR. Coblation in otolaryngology. Proc SPIE. 2003;4949:341–352.
14. Szabo SJ, Savoie FH 3rd, Field LD, Ramsey JR, Hosemann CD. Tendinosis of the extensor carpi radialis brevis: an evaluation of three methods of operative treatment. J Shoulder Elbow Surg Am. 2006;15(6):721-727.
15. Almquist EE, Necking L, Bach AW. Epicondylar resection with anconeus transfer for chronic lateral epicondylitis. J Hand Surg Am. 1998;23(4):723-731.
16. Dunn JH, Kim JJ, Davis L, Nirschl RP. Ten- to 14-year follow-up of the Nirschl surgical technique for lateral epicondylitis. Am J Sports Med. 2008;36(2):261-266.
17. Rubenthaler F, Wiese M, Senge A, Keller L, Wittenberg RH. Long-term follow-up of open and endoscopic Hohmann procedures for lateral epicondylitis. Arthroscopy. 2005;21(6):684-690.
18. Lattermann C, Romeo AA, Anbari A, et al. Arthroscopic debridement of the extensor carpi radialis brevis for the treatment of recalcitrant lateral epicondylitis. J Shoulder Elbow Surg. 2010;19(5):651-656.
19. Rose NE, Forman SK, Dellon AL. Denervation of the lateral epicondyle for treatment of chronic lateral epicondylitis. J Hand Surg Am. 2013;38(2):344-349.
20. Koh JS, Mohan PC, Howe TS, et al. Fasciotomy and surgical tenotomy for recalcitrant lateral elbow tendonopathy: early clinical experience with a novel device for minimally invasive percutaneous microresection. Am J Sports Med. 2013;41(3):636-644.
21. Nirschl RP, Ashman ES. Elbow tendonopathy: tennis elbow. Clin Sports Med. 2003;22(4):813-836.
22. Dellon AL, Kim J, Ducic I. Painful neuroma of the posterior cutaneous nerve of the forearm after surgery for lateral humeral epicondylitis. J Hand Surg Am. 2004;29(3):387-390.
23. Cummins CA. Lateral epicondylitis: in-vivo assessment of arthroscopic debridement and correlation with patient outcomes. Am J Sports Med. 2006;34(9):1486-1491.
24. Meknas K, Odden-Miland A, Mercer JB, Castillejo M, Johansen O. Radiofrequency microtenotomy: a promising method for treatment of recalcitrant lateral epicondylitis. Am J Sports Med. 2008;36(10):1960-1965.
25. Takahashi N, Tasto JP, Locke J, et al. The use of radiofrequency (RF) for the treatment of chronic tendinosis. Paper presented at: 6th Biennial Congress of the International Society of Arthroscopy, Knee Surgery, and Orthopaedic Sports Medicine Congress; May 2007; Florence, Italy. Abstract 1433.
26. Takahashi N, Tasto JP, Ritter M, et al. Pain relief through an antinociceptive effect after radiofrequency application. Am J Sports Med. 2007;35(5):805-810.
27. Ochiai N, Tasto JP, Ohtori S, Takahashi N, Moriya H, Amiel D. Nerve regeneration after radiofrequency ablation. Am J Sports Med. 2007;35(11):1940-1944.
1. Leach RE, Miller JK. Lateral and medial epicondylitis of the elbow. Clin Sports Med. 1987;6(2):259-272.
2. Vangsness CT Jr, Jobe FW. Surgical technique of medial epicondylitis: Results in 35 elbows. J Bone Joint Surg Br. 1991;73(3):409-411.
3. Galloway M, DeMaio M, Mangine R. Rehabilitative techniques in the treatment of medial and lateral epicondylitis. Orthopedics. 1992;15(9):1089-1096.
4. Kraushaar BS, Nirschl RP. Tendinosis of the elbow (tennis elbow). Clinical features and findings of histological, immunohistochemical, and electron microscopy studies. J Bone Joint Surg Am. 1999;81(2):259-278.
5. Leadbetter WB. Cell-matrix response in tendon injury. Clin Sports Med. 1992;11(3):533-578.
6. Nirschl RP. Tennis elbow tendinosis: pathoanatomy, nonsurgical and surgical management. In: Fine LJ, ed. Repetitive Motion Disorders of the Upper Extremity. Rosemont, IL: American Academy of Orthopaedic Surgeons; 1995:467-479.
7. Chu V, Kuang J, Aiaid A, Korkola S, Chiu RC. Angiogenic response induced by mechanical transmyocardial revascularization. J Thorac Cardiovasc Surg 1999;118:849-856.
8. Harwood R, Bowden K, Amiel M, Tasto JP, Amiel D. Structural and angiogenic response to bipolar radiofrequency treatment of normal rabbit achilles tendon: a potential application to the treatment of tendinosis. Trans Orthop Res Soc. 2003;28:819.
9. Tasto JP, Cummings J, Medlock V, Hardesty R, Amiel D. Microtenotomy using a radiofrequency probe to treat lateral epicondylitis. Arthroscopy. 2005;21(7):851-860.
10. Woloszko J, Stalder KR, Brown IG. Plasma characteristics of repetitively-pulsed electrical discharges in saline solutions used for surgical procedures. IEEE Trans Plasma Sci. 2002;30:1376-1383.
11. Stalder KR, Woloszko J, Brown IG, Smith CD. Repetitive plasma discharges in saline solutions. Appl Phys Lett. 2001;79:4503-4505.
12. Woloszko J, Gilbride C. Coblation technology (plasma mediated ablation for otolaryngology applications). Proc SPIE. 2000;3907:306–316.
13. Woloszko J, Kwende MM, Stalder KR. Coblation in otolaryngology. Proc SPIE. 2003;4949:341–352.
14. Szabo SJ, Savoie FH 3rd, Field LD, Ramsey JR, Hosemann CD. Tendinosis of the extensor carpi radialis brevis: an evaluation of three methods of operative treatment. J Shoulder Elbow Surg Am. 2006;15(6):721-727.
15. Almquist EE, Necking L, Bach AW. Epicondylar resection with anconeus transfer for chronic lateral epicondylitis. J Hand Surg Am. 1998;23(4):723-731.
16. Dunn JH, Kim JJ, Davis L, Nirschl RP. Ten- to 14-year follow-up of the Nirschl surgical technique for lateral epicondylitis. Am J Sports Med. 2008;36(2):261-266.
17. Rubenthaler F, Wiese M, Senge A, Keller L, Wittenberg RH. Long-term follow-up of open and endoscopic Hohmann procedures for lateral epicondylitis. Arthroscopy. 2005;21(6):684-690.
18. Lattermann C, Romeo AA, Anbari A, et al. Arthroscopic debridement of the extensor carpi radialis brevis for the treatment of recalcitrant lateral epicondylitis. J Shoulder Elbow Surg. 2010;19(5):651-656.
19. Rose NE, Forman SK, Dellon AL. Denervation of the lateral epicondyle for treatment of chronic lateral epicondylitis. J Hand Surg Am. 2013;38(2):344-349.
20. Koh JS, Mohan PC, Howe TS, et al. Fasciotomy and surgical tenotomy for recalcitrant lateral elbow tendonopathy: early clinical experience with a novel device for minimally invasive percutaneous microresection. Am J Sports Med. 2013;41(3):636-644.
21. Nirschl RP, Ashman ES. Elbow tendonopathy: tennis elbow. Clin Sports Med. 2003;22(4):813-836.
22. Dellon AL, Kim J, Ducic I. Painful neuroma of the posterior cutaneous nerve of the forearm after surgery for lateral humeral epicondylitis. J Hand Surg Am. 2004;29(3):387-390.
23. Cummins CA. Lateral epicondylitis: in-vivo assessment of arthroscopic debridement and correlation with patient outcomes. Am J Sports Med. 2006;34(9):1486-1491.
24. Meknas K, Odden-Miland A, Mercer JB, Castillejo M, Johansen O. Radiofrequency microtenotomy: a promising method for treatment of recalcitrant lateral epicondylitis. Am J Sports Med. 2008;36(10):1960-1965.
25. Takahashi N, Tasto JP, Locke J, et al. The use of radiofrequency (RF) for the treatment of chronic tendinosis. Paper presented at: 6th Biennial Congress of the International Society of Arthroscopy, Knee Surgery, and Orthopaedic Sports Medicine Congress; May 2007; Florence, Italy. Abstract 1433.
26. Takahashi N, Tasto JP, Ritter M, et al. Pain relief through an antinociceptive effect after radiofrequency application. Am J Sports Med. 2007;35(5):805-810.
27. Ochiai N, Tasto JP, Ohtori S, Takahashi N, Moriya H, Amiel D. Nerve regeneration after radiofrequency ablation. Am J Sports Med. 2007;35(11):1940-1944.
Radiofrequency Stimulation for Potential Healing of Meniscal Injuries in the Avascular Zone
Partial meniscectomy of tears in the avascular zone remains one of the most common orthopedic procedures. While results of partial meniscectomy in younger patients have excellent short- to medium-term results, the long-term clinical outcomes are often less favorable.1-3 Repair in the avascular “white-white” zone has resulted in lower patient satisfaction scores and higher revision surgery rates.4-7 Consequently, most tears in this region have been treated with partial meniscectomy.
The inability to repair rather than resect menisci with avascular tears has led to extensive research. Techniques such as trephination and rasping to initiate an angiogenic response have had inconsistent and unreliable results when applied to the white-white zone.8-13 In contrast, Tasto and colleagues14 have shown that radiofrequency (RF) applied to hypovascular tissue can not only stimulate tissue vascularity, but also increase organization of fibroblastic cells. In addition, Tasto and colleagues15,16 have shown that RF application can significantly improve histologic healing and clinical outcomes in refractory cases of Achilles tendinopathy and lateral epicondylitis. In Japanese white rabbit menisci, Higuchi and coauthors17 applied monopolar RF at 60°C and 40W to avascular zone tears to fuse the tissue. They found a significant increase in fibroblast proliferation and fusion of collagen fibers at 2, 4, and 12 weeks after surgery. They also found significant acellular zones of meniscus tissue and attributed these findings to fibrochondrocyte death because of thermal treatment.
This body of research led to the present study, which evaluates the effect of low-temperature, bipolar RF stimulation, in conjunction with suture repair, on the healing of tears in the avascular white-white zone of the meniscus both in vivo and ex vivo. We performed gross and histologic analyses of the treatment groups for the in vivo aspect of the study and biochemical analyses to study the ex vivo effects of RF treatment. 3H-thymidine incorporation has been shown to be a reliable indicator of cellular proliferation in several studies, and this was measured in our treatment groups.18-21 In addition, the response of mitogenic growth factors (IGF-1, bFGF) and angiogenic markers (VEGF, αV) to RF treatment was studied.22 We hypothesized that bipolar RF application would show increased gross, histologic, and biochemical healing when combined with suture repair of longitudinal avascular zone meniscus tears.
Materials and Methods
Creation of Meniscal Tears
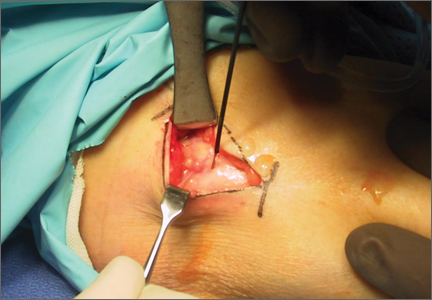
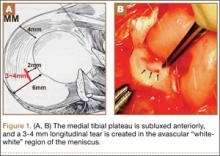
Fifty-four healthy, skeletally mature male and female adult New Zealand white rabbits aged 7 to 18 months were used for the study. All procedures conformed to the guidelines of our university’s institutional animal care and use committee and the American Association for Accreditation of Laboratory Animal Care. All rabbits underwent a surgical procedure in which a longitudinal tear was created in the avascular white-white zone of the medial meniscus. Using sterile technique and instrumentation, a medial parapatellar incision was made on the left knee of each rabbit. The patella was retracted laterally, exposing the medial meniscus. The tibia was then externally rotated to sublux the anterior horn of the medial meniscus anteriorly. A longitudinal full-thickness meniscal tear (3-4 mm in length) was created in the avascular zone (inner third) of the anterior horn of the medial meniscus using an 11-blade scalpel (Figures 1A, 1B). The location of the tear was grossly performed in the same location in each meniscus. The rabbits were randomly divided into 3 treatment groups: 1, 2, and 3 (Table 1). Group 1 (n = 6) served as a control with no repair or RF treatment applied. Group 2 (n = 15) underwent suture repair only of the meniscal tear using 5-0 nylon suture in a horizontal mattress pattern (Figure 2A). Group 3 (n = 33) underwent suture repair after RF stimulation was applied to both sides of the meniscus tear (Figures 2B, 2C). RF was applied using a 0.8-mm TOPAZ MicroDebrider (ArthroCare, Sunnyvale, California) set at level 4 (175 V-RMS) for 500 milliseconds. Lactated ringer’s solution was continuously infused through the probe via sterile tubing to prevent overheating.
After meniscal treatment, hemostasis of the surrounding surgical dissection was achieved to prevent hematoma formation, and the wounds were irrigated. The patellae were relocated and the arthrotomies were closed with a running 2-0 vicryl suture. Fascial and subcutaneous layers were closed with a running 3-0 vicryl suture, and skin was closed with subcuticular 4‑0 vicryl sutures. The rabbit limbs were allowed weight-bearing with unrestricted range of motion within 2x2x2-ft cages.
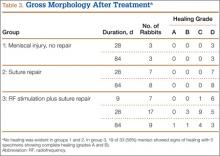
For all groups, menisci were explanted at 28 and 84 days for gross and histologic analysis. For biochemical assessments, menisci were explanted at 9, 28, and 84 days (Table 1).
Gross Analysis
Immediately after specimen removal, all medial menisci were evaluated for gross morphology. A grading system was used for organization and classification of data (Table 2). Three blinded orthopedic surgeon-observers performed all grading. Grade A was considered complete healing of the meniscus. Grade B involved complete healing with a trace of injury remaining on the surface of the meniscus. Grade C represented incomplete healing with a full-thickness injury that was stable to stress of the repair site with an arthroscopic probe. Grade D had no healing with the injured region unstable to stress of the repair site with an arthroscopic probe.
Histologic Analysis and Microscopic Grading of Meniscal Healing
After gross evaluation by the 3 blinded observers, each meniscus was fixed for 24 hours in 10% buffered formalin. Each specimen was then embedded in paraffin and cut into 6-µm slices along the radial plane. The tissue samples were stained with hematoxylin-eosin, and microscopic grading was assigned. The grading system was the same as that used for gross morphologic analysis.
Biochemical Analysis
To determine whether RF treatment stimulated a healing response in the avascular zone of the meniscus, measurements of specific biochemical markers were analyzed at 9, 28, and 84 days after treatment. As a control, unrepaired meniscal tissue from the contralateral knee was also analyzed. 3H-thymidine incorporation into the meniscus was measured to assess cell proliferation.23 At sacrifice, control and treated menisci were dissected and immediately placed into sterile culture media (Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum, antibiotic, and fungicide). 3H-thymidine was added at a concentration of 5µCi/mL of media to each tube. After incubation for 48 hours at 37°C under 5% CO2, the menisci were removed and dialyzed against water for 24 hours to remove unincorporated thymidine. After washing, the menisci were lyophilized, aliquots weighed, and radioactivity determined by liquid scintillation spectrometry. Results are expressed as counts per minute per mg dry tissue weight.
Semiquantitative reverse transcription polymerase chain reaction (RT-PCR) was used to determine mRNA expression of mitogenic growth factors, IGF-1 and bFGF, and angiogenic markers, αV and VEFG.24 National Institutes of Health (NIH) image-analysis software (version 1.61; NIH, Bethesda, Maryland) was used to quantitatively scan RT-PCR profiles after agarose gel electrophoresis and ethidium bromide visualization. Values were normalized to the housekeeping gene, GAPDH.
Statistical Analysis
Data are expressed as mean (SD) and evaluated using an unpaired Student t test between groups. Statistical significance was established at P < .05.
Results
Gross Morphology
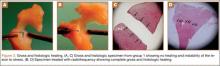
Analysis of gross morphology showed signs of healing only in the group treated with suture repair combined with RF treatment (Table 3). In group 1 (meniscal injury only) and group 2 (suture repair only), no healing occurred at 28 and 84 days (Figure 3A). A meniscal grading system was developed to better describe the varying levels of healing shown in the suture-plus-RF-treatment group (Table 2). Of the specimens that showed healing in group 3, 1 had complete healing (grade A) within the avascular zone of the meniscus at 84 days (Figure 3B). In addition, 4 specimens subjected to suture repair and RF treatment had complete healing with only a trace of injured tissue remaining (grade B). Fourteen specimens in group 3 had incomplete healing with lesions stable to stress suggesting early signs of healing (grade C). In total, 58% of menisci treated with RF showed signs of healing while the remaining 14 specimens in group 3 showed none (grade D).
Histologic Examination
The histology correlated well with gross analysis. No microscopic evidence of healing was seen in groups 1 and 2 (Figure 3C). Of the specimens treated with suture repair combined with RF, 19 (58%) showed varying degrees of histologic healing. While gross morphologic examination showed that only 1 specimen had complete healing, microscopic analysis showed that 1 specimen from group 3 had grade B healing on gross analysis but grade A healing on histologic analysis. Thus, upon histologic examination, 2 specimens showed complete healing of injuries in the avascular zone of the meniscus when treated with suture repair combined with RF treatment rather than the 1 specimen seen on gross morphology (Figure 3D).
Biochemical Analysis
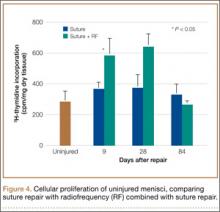
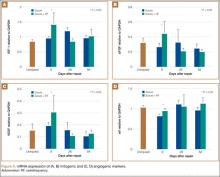
Biochemical assessments were performed at 9, 28, and 84 days after surgery. 3H-thymidine incorporation was studied as a marker for cellular proliferation, and its levels were significantly higher in meniscus explants treated with RF (Figure 4). The mean (SD) rate of incorporation for meniscal tears treated with suture repair plus RF was 590 (80) cpm/mg dry tissue at 9 days. This value was approximately 40% greater than the menisci treated with suture repair only, which had a mean (SD) value of 380 (30) cpm/mg (P < .05). Normal, unrepaired meniscal tissue had a mean (SD) 3H-thymidine incorporation rate of 250 (35) cpm/mg. By 84 days, thymidine levels returned to uninjured levels in both suture-only and RF-treated menisci. Semiquantitative RT-PCR analysis showed that, 9 days after repair, the RF-treated menisci had increased mRNA expression of IGF-1, bFGF, VEGF, and αV relative to untreated repairs (Figure 5). There was a statistically significant acute phase response in IGF-1, bFGF, VEGF and αV in groups treated with RF at 9 days (P > .05).
Adverse Outcomes
There were no surgical complications. During the histologic evaluation, there were no incidences of fibrochondrocyte cell death or damage from the application of RF treatment.
Discussion
RF treatments have been used for many years in various medical and surgical applications. Presently, the most common implementation of RF is for cutting and coagulating tissue during surgery. More recently, however, several publications have shown that when used properly and safely, RF can be an effective surgical adjunct for tendinosis recalcitrant to conservative therapy.15-17,25-32
Many have suggested that RF coblation is successful in these clinical scenarios because of its ability to promote an increased angiogenic and fibroblastic response in hypovascular tissue.29,33,34
This body of literature led to the evaluation of RF coblation in treating meniscal tears in the avascular zone. Studies have shown poor success of meniscus repairs done in the avascular zone; however, our data demonstrate that supplementing suture repair with RF treatment may improve the acute-phase healing response. Although the control and suture-repair groups showed no signs of healing, the suture-repair-combined-with-RF-treatment group had 2 specimens in which complete gross and histologic healing occurred. In addition, 19 (58%) specimens in the RF group showed gross or histologic signs of healing.
Biochemically, 3H-thymidine incorporation was examined to assess cellular proliferation. Mitogenic (IGF, bFGF) and angiogenic (VEGF, αV) growth factors were measured as markers of an increased healing response. Compared with noninjured meniscal tissue, 3H-thymidine incorporation was significantly increased in both the suture and suture-combined-with-RF-treatment groups at 9 and 28 days after surgery. Between the suture and suture-RF groups, RF treatment led to a 40% greater increase in 3H-thymidine incorporation suggesting greater cellular proliferation in the immediate postoperative period. With respect to mitogenic and angiogenic factors, IGF, bFGF, VEGF, and αV were only significantly increased when RF was combined with suture repair. All 4 factors are important regulators of vasculogenesis, angiogenesis, wound healing, bone remodeling, and neurogenesis. The suture repair–only group showed no upregulation of these factors compared with uninjured controls.
Our study has several strengths. Using an animal model with menisci grossly similar to that of humans, we performed a controlled study comparing 2 treatment options, suture repair only and suture repair combined with RF treatment.35,36 The animal model also enabled second-look examinations at designated intervals. We analyzed the effect of RF treatment on concrete measures, such as gross, histologic, and biochemical healing. In particular, the biochemical analysis may indicate that RF treatment can increase the proliferative, mitogenic, and angiogenic capabilities of surrounding progenitor cells. This was evidenced by the statistically significant increase we saw in IGF-1, bFGF, VEGF, and αV at 9 and 28 days compared with controls.
Meniscal tears in the avascular zone represent a significant treatment dilemma for the physiologically young patient population. While partial meniscectomy provides excellent short-term relief, the long-term outcome of this intervention is degenerative joint disease. Meniscal repair in the central two-thirds of the meniscus has shown poor results. Our study presents data that show supplementing suture repair of avascular meniscal tears with RF can lead to increased gross, histologic, and biochemical healing in the New Zealand white rabbit. While these results are encouraging, studies with longer follow-up and specimens that represent the human menisci are necessary to determine whether these preliminary results would translate to human meniscal tears in the avascular zone.
Weaknesses of our study include the use of an animal model and the location of the tear created in the menisci. While using an animal model had many strengths, the results of our study are probably not strong enough to immediately extrapolate the use of RF in human meniscal repairs. However, the data we obtained are very encouraging and perhaps suggest that RF warrants human trials. Our open surgical technique made it difficult to create and repair a tear on the posterior horn of the medical meniscus without completely dislocating the knee anteriorly. As a result, the knees were subluxed anteriorly, and the meniscal tears and repairs were performed more anteriorly. The more anterior aspects of the menisci do not undergo the same rotational and axial loads as the posterior horn, and it is unclear whether this difference would contribute to the results we obtained from RF treatment. In addition, the tears were surgically created and the repair was done during the same procedure. Patients do not present in this manner, and this further underscores the need for a clinical trial to determine the effectiveness of this treatment option in humans.
Conclusion
RF-based supplementation of meniscal repairs in the avascular zone showed acute signs of biochemical healing in 58% of New Zealand white rabbit specimens. In addition, gross and histologic evaluations showed an increase in healing compared with controls. Two specimens treated with RF in combination with suture repair had complete healing. These results illustrate the effectiveness of RF in stimulating a healing response in hypovascular tissue. Clinical trials are necessary to determine the effectiveness of this treatment in humans.
1. Fauno P, Nielsen AB. Arthroscopic partial meniscectomy: a long-term follow-up. Arthroscopy. 1992;8(3):345-349.
2. Lynch MA, Henning CE, Glick KR, Jr. Knee joint surface changes. Long-term follow-up meniscus tear treatment in stable anterior cruciate ligament reconstructions. Clin Orthop. 1983;172:148-153.
3. Roos H, Lauren M, Adalberth T, Roos EM, Jonsson K, Lohmander LS. Knee osteoarthritis after meniscectomy: prevalence of radiographic changes after twenty-one years, compared with matched controls. Arthritis Rheum. 1998;41(4):687-693.
4. Hennerbichler A, Moutos FT, Hennerbichler D, Weinberg JB, Guilak F. Repair response of the inner and outer regions of the porcine meniscus in vitro. Am J Sports Med. 2007;35(5):754‑762.
5. Gershuni DH, Hargens AR, Danzig LA. Regional nutrition and cellularity of the meniscus. Implications for tear and repair. Sports Med. 1988;5(5):322-327.
6. Gershuni DH, Skyhar MJ, Danzig LA, Camp J, Hargens AR, Akeson WH. Experimental models to promote healing of tears in the avascular segment of canine knee menisci. J Bone Joint Surg Am. 1989;71(9):1363-1370.
7. Papachristou G, Efstathopoulos N, Plessas S, Levidiotis C, Chronopoulos E, Sourlas J. Isolated meniscal repair in the avascular area. Acta Orthop Belg. 2003;69(4):341-345.
8. Fox JM, Rintz KG, Ferkel RD. Trephination of incomplete meniscal tears. Arthroscopy. 1993;9(4):451-455.
9. Zhang Z, Arnold JA, Williams T, McCann B. Repairs by trephination and suturing of longitudinal injuries in the avascular area of the meniscus in goats. Am J Sports Med. 1995;23(1):35-41.
10. Zhang ZN, Tu KY, Xu YK, Zhang WM, Liu ZT, Ou SH. Treatment of longitudinal injuries in avascular area of meniscus in dogs by trephination. Arthroscopy. 1988;4(3):151-159.
11. Ochi M, Uchio Y, Okuda K, Shu N, Yamaguchi H, Sakai Y. Expression of cytokines after meniscal rasping to promote meniscal healing. Arthroscopy. 2001;17(7):724-731.
12. Okuda K, Ochi M, Shu N, Uchio Y. Meniscal rasping for repair of meniscal tear in the avascular zone. Arthroscopy. 1999;15(3):281-286.
13. Uchio Y, Ochi M, Adachi N, Kawasaki K, Iwasa J. Results of rasping of meniscal tears with and without anterior cruciate ligament injury as evaluated by second-look arthroscopy. Arthroscopy. 2003;19(5):463-469.
14. Tasto JP, Cummings J, Medlock V, Harwood F, Hardesty R, Amiel D. The tendon treatment center: new horizons in the treatment of tendinosis. Arthroscopy. 2003;19(suppl 1):213-223.
15. Tasto JP. The role of radiofrequency-based devices in shaping the future of orthopedic surgery. Orthopedics. 2006;29(10):874-875.
16. Tasto JP, Cummings J, Medlock V, Hardesty R, Amiel D. Microtenotomy using a radiofrequency probe to treat lateral epicondylitis. Arthroscopy. 2005;21(7):851-860.
17. Higuchi H, Kimura M, Kobayashi A, Hatayama K, Takagishi K. A novel treatment of hypermobile lateral meniscus with monopolar radiofrequency energy. Arthroscopy 2004;20 (suppl 2):1-5.
18. Tonna EA, Cronkite EP. The periosteum. Autoradiographic studies on cellular proliferation and transformation utilizing tritiated thymidine. Clin Orthop. 1963;30:218-233.
19. Madewell BR. Serum thymidine kinase activity: an alternative to histologic markers of cellular proliferation in canine lymphoma. J Vet Intern Med. 2004;18(5):595-596.
20. Mujoomdar M, Bennett A, Hoskin D, Blay J. Adenosine stimulation of proliferation of breast carcinoma cell lines: evaluation of the [3H]thymidine assay system and modulatory effects of the cellular microenvironment in vitro. J Cell Physiol. 2004;201(3):429-438.
21. Vander Borght T, Labar D, Pauwels S, Lambotte L. Production of [2-11C]thymidine for quantification of cellular proliferation with PET. Int J Rad Appl Instrum A. 1991;42(1):103-104.
22. Spindler KP, Mayes CE, Miller RR, Imro AK, Davidson JM. Regional mitogenic response of the meniscus to platelet-derived growth factor (PDGF-AB). J Orthop Res. 1995;13(2):201-207.
23. Thomopoulos S, Zaegel M, Das R, et al. PDGF-BB released in tendon repair using a novel delivery system promotes cell proliferation and collagen remodeling. J Orthop Res. 2007;25(10):1358-1368.
24. Pennock AT, Robertson CM, Emmerson BC, Harwood FL, Amiel D. Role of apoptotic and matrix-degrading genes in articular cartilage and meniscus of mature and aged rabbits during development of osteoarthritis. Arthritis Rheum. 2007;56(5):1529-1536.
25. Allen RT, Tasto JP, Cummings J, Robertson CM, Amiel D. Meniscal debridement with an arthroscopic radiofrequency wand versus an arthroscopic shaver: comparative effects on menisci and underlying articular cartilage. Arthroscopy. 2006;22(4):385-393.
26. Figueroa D, Calvo R, Vaisman A, et al. Bipolar radiofrequency in the human meniscus. Comparative study between patients younger and older than 40 years of age. Knee. 2007;14(5):357-360.
27. Friedman M, LoSavio P, Ibrahim H, Ramakrishnan V. Radiofrequency tonsil reduction: safety, morbidity, and efficacy. Laryngoscope. 2003;113(5):882-887.
28. Hall DJ, Littlefield PD, Birkmire-Peters DP, Holtel MR. Radiofrequency ablation versus electrocautery in tonsillectomy. Otolaryngol Head Neck Surg. 2004;130(3):300-305.
29. Kaplan H, Gat A. Clinical and histopathological results following TriPollar radiofrequency skin treatments. J Cosmet Laser Ther. 2009;11(2):78-84.
30. Mancini PF. Coblation: a new technology and technique for skin resurfacing and other aesthetic surgical procedures. Aesthetic Plast Surg. 2001;2595):372-377.
31. Penka I, Kaplan Z, Sefr R, Sirotek L, Eber Z, Ondrák M. Use of radiofrequency ablation in the treatment of malignant liver lesions. Hepatogastroenterology. 2008;55(82-83):562-567.
32. Tasto JP, Ash SA. Current uses of radiofrequency in arthroscopic knee surgery. Am J Knee Surg. 1999;12(3):186-191.
33. Amiel D, Ball ST, Tasto JP. Chondrocyte viability and metabolic activity after treatment of bovine articular cartilage with bipolar radiofrequency: an in vitro study. Arthroscopy. 2004;20(5):503-510.
34. Barry KJ, Kaplan J, Connolly RJ, et al. The effect of radiofrequency-generated thermal energy on the mechanical and histologic characteristics of the arterial wall in vivo: implications for radiofrequency angioplasty. Am Heart J. 1989;117(2):332-341.
35. Hoch DH, Grodzinsky AJ, Koob TJ, Albert ML, Eyre DR. Early changes in material properties of rabbit articular cartilage after meniscectomy. J Orthop Res. 1983;1(1):4-12.
36. Thompson AM, Stockwell RA. An ultrastructural study of the marginal transitional zone in the rabbit knee joint. J Anat. 1983;136(Pt 4):701-713.
Partial meniscectomy of tears in the avascular zone remains one of the most common orthopedic procedures. While results of partial meniscectomy in younger patients have excellent short- to medium-term results, the long-term clinical outcomes are often less favorable.1-3 Repair in the avascular “white-white” zone has resulted in lower patient satisfaction scores and higher revision surgery rates.4-7 Consequently, most tears in this region have been treated with partial meniscectomy.
The inability to repair rather than resect menisci with avascular tears has led to extensive research. Techniques such as trephination and rasping to initiate an angiogenic response have had inconsistent and unreliable results when applied to the white-white zone.8-13 In contrast, Tasto and colleagues14 have shown that radiofrequency (RF) applied to hypovascular tissue can not only stimulate tissue vascularity, but also increase organization of fibroblastic cells. In addition, Tasto and colleagues15,16 have shown that RF application can significantly improve histologic healing and clinical outcomes in refractory cases of Achilles tendinopathy and lateral epicondylitis. In Japanese white rabbit menisci, Higuchi and coauthors17 applied monopolar RF at 60°C and 40W to avascular zone tears to fuse the tissue. They found a significant increase in fibroblast proliferation and fusion of collagen fibers at 2, 4, and 12 weeks after surgery. They also found significant acellular zones of meniscus tissue and attributed these findings to fibrochondrocyte death because of thermal treatment.
This body of research led to the present study, which evaluates the effect of low-temperature, bipolar RF stimulation, in conjunction with suture repair, on the healing of tears in the avascular white-white zone of the meniscus both in vivo and ex vivo. We performed gross and histologic analyses of the treatment groups for the in vivo aspect of the study and biochemical analyses to study the ex vivo effects of RF treatment. 3H-thymidine incorporation has been shown to be a reliable indicator of cellular proliferation in several studies, and this was measured in our treatment groups.18-21 In addition, the response of mitogenic growth factors (IGF-1, bFGF) and angiogenic markers (VEGF, αV) to RF treatment was studied.22 We hypothesized that bipolar RF application would show increased gross, histologic, and biochemical healing when combined with suture repair of longitudinal avascular zone meniscus tears.
Materials and Methods
Creation of Meniscal Tears
Fifty-four healthy, skeletally mature male and female adult New Zealand white rabbits aged 7 to 18 months were used for the study. All procedures conformed to the guidelines of our university’s institutional animal care and use committee and the American Association for Accreditation of Laboratory Animal Care. All rabbits underwent a surgical procedure in which a longitudinal tear was created in the avascular white-white zone of the medial meniscus. Using sterile technique and instrumentation, a medial parapatellar incision was made on the left knee of each rabbit. The patella was retracted laterally, exposing the medial meniscus. The tibia was then externally rotated to sublux the anterior horn of the medial meniscus anteriorly. A longitudinal full-thickness meniscal tear (3-4 mm in length) was created in the avascular zone (inner third) of the anterior horn of the medial meniscus using an 11-blade scalpel (Figures 1A, 1B). The location of the tear was grossly performed in the same location in each meniscus. The rabbits were randomly divided into 3 treatment groups: 1, 2, and 3 (Table 1). Group 1 (n = 6) served as a control with no repair or RF treatment applied. Group 2 (n = 15) underwent suture repair only of the meniscal tear using 5-0 nylon suture in a horizontal mattress pattern (Figure 2A). Group 3 (n = 33) underwent suture repair after RF stimulation was applied to both sides of the meniscus tear (Figures 2B, 2C). RF was applied using a 0.8-mm TOPAZ MicroDebrider (ArthroCare, Sunnyvale, California) set at level 4 (175 V-RMS) for 500 milliseconds. Lactated ringer’s solution was continuously infused through the probe via sterile tubing to prevent overheating.
After meniscal treatment, hemostasis of the surrounding surgical dissection was achieved to prevent hematoma formation, and the wounds were irrigated. The patellae were relocated and the arthrotomies were closed with a running 2-0 vicryl suture. Fascial and subcutaneous layers were closed with a running 3-0 vicryl suture, and skin was closed with subcuticular 4‑0 vicryl sutures. The rabbit limbs were allowed weight-bearing with unrestricted range of motion within 2x2x2-ft cages.
For all groups, menisci were explanted at 28 and 84 days for gross and histologic analysis. For biochemical assessments, menisci were explanted at 9, 28, and 84 days (Table 1).
Gross Analysis
Immediately after specimen removal, all medial menisci were evaluated for gross morphology. A grading system was used for organization and classification of data (Table 2). Three blinded orthopedic surgeon-observers performed all grading. Grade A was considered complete healing of the meniscus. Grade B involved complete healing with a trace of injury remaining on the surface of the meniscus. Grade C represented incomplete healing with a full-thickness injury that was stable to stress of the repair site with an arthroscopic probe. Grade D had no healing with the injured region unstable to stress of the repair site with an arthroscopic probe.
Histologic Analysis and Microscopic Grading of Meniscal Healing
After gross evaluation by the 3 blinded observers, each meniscus was fixed for 24 hours in 10% buffered formalin. Each specimen was then embedded in paraffin and cut into 6-µm slices along the radial plane. The tissue samples were stained with hematoxylin-eosin, and microscopic grading was assigned. The grading system was the same as that used for gross morphologic analysis.
Biochemical Analysis
To determine whether RF treatment stimulated a healing response in the avascular zone of the meniscus, measurements of specific biochemical markers were analyzed at 9, 28, and 84 days after treatment. As a control, unrepaired meniscal tissue from the contralateral knee was also analyzed. 3H-thymidine incorporation into the meniscus was measured to assess cell proliferation.23 At sacrifice, control and treated menisci were dissected and immediately placed into sterile culture media (Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum, antibiotic, and fungicide). 3H-thymidine was added at a concentration of 5µCi/mL of media to each tube. After incubation for 48 hours at 37°C under 5% CO2, the menisci were removed and dialyzed against water for 24 hours to remove unincorporated thymidine. After washing, the menisci were lyophilized, aliquots weighed, and radioactivity determined by liquid scintillation spectrometry. Results are expressed as counts per minute per mg dry tissue weight.
Semiquantitative reverse transcription polymerase chain reaction (RT-PCR) was used to determine mRNA expression of mitogenic growth factors, IGF-1 and bFGF, and angiogenic markers, αV and VEFG.24 National Institutes of Health (NIH) image-analysis software (version 1.61; NIH, Bethesda, Maryland) was used to quantitatively scan RT-PCR profiles after agarose gel electrophoresis and ethidium bromide visualization. Values were normalized to the housekeeping gene, GAPDH.
Statistical Analysis
Data are expressed as mean (SD) and evaluated using an unpaired Student t test between groups. Statistical significance was established at P < .05.
Results
Gross Morphology
Analysis of gross morphology showed signs of healing only in the group treated with suture repair combined with RF treatment (Table 3). In group 1 (meniscal injury only) and group 2 (suture repair only), no healing occurred at 28 and 84 days (Figure 3A). A meniscal grading system was developed to better describe the varying levels of healing shown in the suture-plus-RF-treatment group (Table 2). Of the specimens that showed healing in group 3, 1 had complete healing (grade A) within the avascular zone of the meniscus at 84 days (Figure 3B). In addition, 4 specimens subjected to suture repair and RF treatment had complete healing with only a trace of injured tissue remaining (grade B). Fourteen specimens in group 3 had incomplete healing with lesions stable to stress suggesting early signs of healing (grade C). In total, 58% of menisci treated with RF showed signs of healing while the remaining 14 specimens in group 3 showed none (grade D).
Histologic Examination
The histology correlated well with gross analysis. No microscopic evidence of healing was seen in groups 1 and 2 (Figure 3C). Of the specimens treated with suture repair combined with RF, 19 (58%) showed varying degrees of histologic healing. While gross morphologic examination showed that only 1 specimen had complete healing, microscopic analysis showed that 1 specimen from group 3 had grade B healing on gross analysis but grade A healing on histologic analysis. Thus, upon histologic examination, 2 specimens showed complete healing of injuries in the avascular zone of the meniscus when treated with suture repair combined with RF treatment rather than the 1 specimen seen on gross morphology (Figure 3D).
Biochemical Analysis
Biochemical assessments were performed at 9, 28, and 84 days after surgery. 3H-thymidine incorporation was studied as a marker for cellular proliferation, and its levels were significantly higher in meniscus explants treated with RF (Figure 4). The mean (SD) rate of incorporation for meniscal tears treated with suture repair plus RF was 590 (80) cpm/mg dry tissue at 9 days. This value was approximately 40% greater than the menisci treated with suture repair only, which had a mean (SD) value of 380 (30) cpm/mg (P < .05). Normal, unrepaired meniscal tissue had a mean (SD) 3H-thymidine incorporation rate of 250 (35) cpm/mg. By 84 days, thymidine levels returned to uninjured levels in both suture-only and RF-treated menisci. Semiquantitative RT-PCR analysis showed that, 9 days after repair, the RF-treated menisci had increased mRNA expression of IGF-1, bFGF, VEGF, and αV relative to untreated repairs (Figure 5). There was a statistically significant acute phase response in IGF-1, bFGF, VEGF and αV in groups treated with RF at 9 days (P > .05).
Adverse Outcomes
There were no surgical complications. During the histologic evaluation, there were no incidences of fibrochondrocyte cell death or damage from the application of RF treatment.
Discussion
RF treatments have been used for many years in various medical and surgical applications. Presently, the most common implementation of RF is for cutting and coagulating tissue during surgery. More recently, however, several publications have shown that when used properly and safely, RF can be an effective surgical adjunct for tendinosis recalcitrant to conservative therapy.15-17,25-32
Many have suggested that RF coblation is successful in these clinical scenarios because of its ability to promote an increased angiogenic and fibroblastic response in hypovascular tissue.29,33,34
This body of literature led to the evaluation of RF coblation in treating meniscal tears in the avascular zone. Studies have shown poor success of meniscus repairs done in the avascular zone; however, our data demonstrate that supplementing suture repair with RF treatment may improve the acute-phase healing response. Although the control and suture-repair groups showed no signs of healing, the suture-repair-combined-with-RF-treatment group had 2 specimens in which complete gross and histologic healing occurred. In addition, 19 (58%) specimens in the RF group showed gross or histologic signs of healing.
Biochemically, 3H-thymidine incorporation was examined to assess cellular proliferation. Mitogenic (IGF, bFGF) and angiogenic (VEGF, αV) growth factors were measured as markers of an increased healing response. Compared with noninjured meniscal tissue, 3H-thymidine incorporation was significantly increased in both the suture and suture-combined-with-RF-treatment groups at 9 and 28 days after surgery. Between the suture and suture-RF groups, RF treatment led to a 40% greater increase in 3H-thymidine incorporation suggesting greater cellular proliferation in the immediate postoperative period. With respect to mitogenic and angiogenic factors, IGF, bFGF, VEGF, and αV were only significantly increased when RF was combined with suture repair. All 4 factors are important regulators of vasculogenesis, angiogenesis, wound healing, bone remodeling, and neurogenesis. The suture repair–only group showed no upregulation of these factors compared with uninjured controls.
Our study has several strengths. Using an animal model with menisci grossly similar to that of humans, we performed a controlled study comparing 2 treatment options, suture repair only and suture repair combined with RF treatment.35,36 The animal model also enabled second-look examinations at designated intervals. We analyzed the effect of RF treatment on concrete measures, such as gross, histologic, and biochemical healing. In particular, the biochemical analysis may indicate that RF treatment can increase the proliferative, mitogenic, and angiogenic capabilities of surrounding progenitor cells. This was evidenced by the statistically significant increase we saw in IGF-1, bFGF, VEGF, and αV at 9 and 28 days compared with controls.
Meniscal tears in the avascular zone represent a significant treatment dilemma for the physiologically young patient population. While partial meniscectomy provides excellent short-term relief, the long-term outcome of this intervention is degenerative joint disease. Meniscal repair in the central two-thirds of the meniscus has shown poor results. Our study presents data that show supplementing suture repair of avascular meniscal tears with RF can lead to increased gross, histologic, and biochemical healing in the New Zealand white rabbit. While these results are encouraging, studies with longer follow-up and specimens that represent the human menisci are necessary to determine whether these preliminary results would translate to human meniscal tears in the avascular zone.
Weaknesses of our study include the use of an animal model and the location of the tear created in the menisci. While using an animal model had many strengths, the results of our study are probably not strong enough to immediately extrapolate the use of RF in human meniscal repairs. However, the data we obtained are very encouraging and perhaps suggest that RF warrants human trials. Our open surgical technique made it difficult to create and repair a tear on the posterior horn of the medical meniscus without completely dislocating the knee anteriorly. As a result, the knees were subluxed anteriorly, and the meniscal tears and repairs were performed more anteriorly. The more anterior aspects of the menisci do not undergo the same rotational and axial loads as the posterior horn, and it is unclear whether this difference would contribute to the results we obtained from RF treatment. In addition, the tears were surgically created and the repair was done during the same procedure. Patients do not present in this manner, and this further underscores the need for a clinical trial to determine the effectiveness of this treatment option in humans.
Conclusion
RF-based supplementation of meniscal repairs in the avascular zone showed acute signs of biochemical healing in 58% of New Zealand white rabbit specimens. In addition, gross and histologic evaluations showed an increase in healing compared with controls. Two specimens treated with RF in combination with suture repair had complete healing. These results illustrate the effectiveness of RF in stimulating a healing response in hypovascular tissue. Clinical trials are necessary to determine the effectiveness of this treatment in humans.
Partial meniscectomy of tears in the avascular zone remains one of the most common orthopedic procedures. While results of partial meniscectomy in younger patients have excellent short- to medium-term results, the long-term clinical outcomes are often less favorable.1-3 Repair in the avascular “white-white” zone has resulted in lower patient satisfaction scores and higher revision surgery rates.4-7 Consequently, most tears in this region have been treated with partial meniscectomy.
The inability to repair rather than resect menisci with avascular tears has led to extensive research. Techniques such as trephination and rasping to initiate an angiogenic response have had inconsistent and unreliable results when applied to the white-white zone.8-13 In contrast, Tasto and colleagues14 have shown that radiofrequency (RF) applied to hypovascular tissue can not only stimulate tissue vascularity, but also increase organization of fibroblastic cells. In addition, Tasto and colleagues15,16 have shown that RF application can significantly improve histologic healing and clinical outcomes in refractory cases of Achilles tendinopathy and lateral epicondylitis. In Japanese white rabbit menisci, Higuchi and coauthors17 applied monopolar RF at 60°C and 40W to avascular zone tears to fuse the tissue. They found a significant increase in fibroblast proliferation and fusion of collagen fibers at 2, 4, and 12 weeks after surgery. They also found significant acellular zones of meniscus tissue and attributed these findings to fibrochondrocyte death because of thermal treatment.
This body of research led to the present study, which evaluates the effect of low-temperature, bipolar RF stimulation, in conjunction with suture repair, on the healing of tears in the avascular white-white zone of the meniscus both in vivo and ex vivo. We performed gross and histologic analyses of the treatment groups for the in vivo aspect of the study and biochemical analyses to study the ex vivo effects of RF treatment. 3H-thymidine incorporation has been shown to be a reliable indicator of cellular proliferation in several studies, and this was measured in our treatment groups.18-21 In addition, the response of mitogenic growth factors (IGF-1, bFGF) and angiogenic markers (VEGF, αV) to RF treatment was studied.22 We hypothesized that bipolar RF application would show increased gross, histologic, and biochemical healing when combined with suture repair of longitudinal avascular zone meniscus tears.
Materials and Methods
Creation of Meniscal Tears
Fifty-four healthy, skeletally mature male and female adult New Zealand white rabbits aged 7 to 18 months were used for the study. All procedures conformed to the guidelines of our university’s institutional animal care and use committee and the American Association for Accreditation of Laboratory Animal Care. All rabbits underwent a surgical procedure in which a longitudinal tear was created in the avascular white-white zone of the medial meniscus. Using sterile technique and instrumentation, a medial parapatellar incision was made on the left knee of each rabbit. The patella was retracted laterally, exposing the medial meniscus. The tibia was then externally rotated to sublux the anterior horn of the medial meniscus anteriorly. A longitudinal full-thickness meniscal tear (3-4 mm in length) was created in the avascular zone (inner third) of the anterior horn of the medial meniscus using an 11-blade scalpel (Figures 1A, 1B). The location of the tear was grossly performed in the same location in each meniscus. The rabbits were randomly divided into 3 treatment groups: 1, 2, and 3 (Table 1). Group 1 (n = 6) served as a control with no repair or RF treatment applied. Group 2 (n = 15) underwent suture repair only of the meniscal tear using 5-0 nylon suture in a horizontal mattress pattern (Figure 2A). Group 3 (n = 33) underwent suture repair after RF stimulation was applied to both sides of the meniscus tear (Figures 2B, 2C). RF was applied using a 0.8-mm TOPAZ MicroDebrider (ArthroCare, Sunnyvale, California) set at level 4 (175 V-RMS) for 500 milliseconds. Lactated ringer’s solution was continuously infused through the probe via sterile tubing to prevent overheating.
After meniscal treatment, hemostasis of the surrounding surgical dissection was achieved to prevent hematoma formation, and the wounds were irrigated. The patellae were relocated and the arthrotomies were closed with a running 2-0 vicryl suture. Fascial and subcutaneous layers were closed with a running 3-0 vicryl suture, and skin was closed with subcuticular 4‑0 vicryl sutures. The rabbit limbs were allowed weight-bearing with unrestricted range of motion within 2x2x2-ft cages.
For all groups, menisci were explanted at 28 and 84 days for gross and histologic analysis. For biochemical assessments, menisci were explanted at 9, 28, and 84 days (Table 1).
Gross Analysis
Immediately after specimen removal, all medial menisci were evaluated for gross morphology. A grading system was used for organization and classification of data (Table 2). Three blinded orthopedic surgeon-observers performed all grading. Grade A was considered complete healing of the meniscus. Grade B involved complete healing with a trace of injury remaining on the surface of the meniscus. Grade C represented incomplete healing with a full-thickness injury that was stable to stress of the repair site with an arthroscopic probe. Grade D had no healing with the injured region unstable to stress of the repair site with an arthroscopic probe.
Histologic Analysis and Microscopic Grading of Meniscal Healing
After gross evaluation by the 3 blinded observers, each meniscus was fixed for 24 hours in 10% buffered formalin. Each specimen was then embedded in paraffin and cut into 6-µm slices along the radial plane. The tissue samples were stained with hematoxylin-eosin, and microscopic grading was assigned. The grading system was the same as that used for gross morphologic analysis.
Biochemical Analysis
To determine whether RF treatment stimulated a healing response in the avascular zone of the meniscus, measurements of specific biochemical markers were analyzed at 9, 28, and 84 days after treatment. As a control, unrepaired meniscal tissue from the contralateral knee was also analyzed. 3H-thymidine incorporation into the meniscus was measured to assess cell proliferation.23 At sacrifice, control and treated menisci were dissected and immediately placed into sterile culture media (Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum, antibiotic, and fungicide). 3H-thymidine was added at a concentration of 5µCi/mL of media to each tube. After incubation for 48 hours at 37°C under 5% CO2, the menisci were removed and dialyzed against water for 24 hours to remove unincorporated thymidine. After washing, the menisci were lyophilized, aliquots weighed, and radioactivity determined by liquid scintillation spectrometry. Results are expressed as counts per minute per mg dry tissue weight.
Semiquantitative reverse transcription polymerase chain reaction (RT-PCR) was used to determine mRNA expression of mitogenic growth factors, IGF-1 and bFGF, and angiogenic markers, αV and VEFG.24 National Institutes of Health (NIH) image-analysis software (version 1.61; NIH, Bethesda, Maryland) was used to quantitatively scan RT-PCR profiles after agarose gel electrophoresis and ethidium bromide visualization. Values were normalized to the housekeeping gene, GAPDH.
Statistical Analysis
Data are expressed as mean (SD) and evaluated using an unpaired Student t test between groups. Statistical significance was established at P < .05.
Results
Gross Morphology
Analysis of gross morphology showed signs of healing only in the group treated with suture repair combined with RF treatment (Table 3). In group 1 (meniscal injury only) and group 2 (suture repair only), no healing occurred at 28 and 84 days (Figure 3A). A meniscal grading system was developed to better describe the varying levels of healing shown in the suture-plus-RF-treatment group (Table 2). Of the specimens that showed healing in group 3, 1 had complete healing (grade A) within the avascular zone of the meniscus at 84 days (Figure 3B). In addition, 4 specimens subjected to suture repair and RF treatment had complete healing with only a trace of injured tissue remaining (grade B). Fourteen specimens in group 3 had incomplete healing with lesions stable to stress suggesting early signs of healing (grade C). In total, 58% of menisci treated with RF showed signs of healing while the remaining 14 specimens in group 3 showed none (grade D).
Histologic Examination
The histology correlated well with gross analysis. No microscopic evidence of healing was seen in groups 1 and 2 (Figure 3C). Of the specimens treated with suture repair combined with RF, 19 (58%) showed varying degrees of histologic healing. While gross morphologic examination showed that only 1 specimen had complete healing, microscopic analysis showed that 1 specimen from group 3 had grade B healing on gross analysis but grade A healing on histologic analysis. Thus, upon histologic examination, 2 specimens showed complete healing of injuries in the avascular zone of the meniscus when treated with suture repair combined with RF treatment rather than the 1 specimen seen on gross morphology (Figure 3D).
Biochemical Analysis
Biochemical assessments were performed at 9, 28, and 84 days after surgery. 3H-thymidine incorporation was studied as a marker for cellular proliferation, and its levels were significantly higher in meniscus explants treated with RF (Figure 4). The mean (SD) rate of incorporation for meniscal tears treated with suture repair plus RF was 590 (80) cpm/mg dry tissue at 9 days. This value was approximately 40% greater than the menisci treated with suture repair only, which had a mean (SD) value of 380 (30) cpm/mg (P < .05). Normal, unrepaired meniscal tissue had a mean (SD) 3H-thymidine incorporation rate of 250 (35) cpm/mg. By 84 days, thymidine levels returned to uninjured levels in both suture-only and RF-treated menisci. Semiquantitative RT-PCR analysis showed that, 9 days after repair, the RF-treated menisci had increased mRNA expression of IGF-1, bFGF, VEGF, and αV relative to untreated repairs (Figure 5). There was a statistically significant acute phase response in IGF-1, bFGF, VEGF and αV in groups treated with RF at 9 days (P > .05).
Adverse Outcomes
There were no surgical complications. During the histologic evaluation, there were no incidences of fibrochondrocyte cell death or damage from the application of RF treatment.
Discussion
RF treatments have been used for many years in various medical and surgical applications. Presently, the most common implementation of RF is for cutting and coagulating tissue during surgery. More recently, however, several publications have shown that when used properly and safely, RF can be an effective surgical adjunct for tendinosis recalcitrant to conservative therapy.15-17,25-32
Many have suggested that RF coblation is successful in these clinical scenarios because of its ability to promote an increased angiogenic and fibroblastic response in hypovascular tissue.29,33,34
This body of literature led to the evaluation of RF coblation in treating meniscal tears in the avascular zone. Studies have shown poor success of meniscus repairs done in the avascular zone; however, our data demonstrate that supplementing suture repair with RF treatment may improve the acute-phase healing response. Although the control and suture-repair groups showed no signs of healing, the suture-repair-combined-with-RF-treatment group had 2 specimens in which complete gross and histologic healing occurred. In addition, 19 (58%) specimens in the RF group showed gross or histologic signs of healing.
Biochemically, 3H-thymidine incorporation was examined to assess cellular proliferation. Mitogenic (IGF, bFGF) and angiogenic (VEGF, αV) growth factors were measured as markers of an increased healing response. Compared with noninjured meniscal tissue, 3H-thymidine incorporation was significantly increased in both the suture and suture-combined-with-RF-treatment groups at 9 and 28 days after surgery. Between the suture and suture-RF groups, RF treatment led to a 40% greater increase in 3H-thymidine incorporation suggesting greater cellular proliferation in the immediate postoperative period. With respect to mitogenic and angiogenic factors, IGF, bFGF, VEGF, and αV were only significantly increased when RF was combined with suture repair. All 4 factors are important regulators of vasculogenesis, angiogenesis, wound healing, bone remodeling, and neurogenesis. The suture repair–only group showed no upregulation of these factors compared with uninjured controls.
Our study has several strengths. Using an animal model with menisci grossly similar to that of humans, we performed a controlled study comparing 2 treatment options, suture repair only and suture repair combined with RF treatment.35,36 The animal model also enabled second-look examinations at designated intervals. We analyzed the effect of RF treatment on concrete measures, such as gross, histologic, and biochemical healing. In particular, the biochemical analysis may indicate that RF treatment can increase the proliferative, mitogenic, and angiogenic capabilities of surrounding progenitor cells. This was evidenced by the statistically significant increase we saw in IGF-1, bFGF, VEGF, and αV at 9 and 28 days compared with controls.
Meniscal tears in the avascular zone represent a significant treatment dilemma for the physiologically young patient population. While partial meniscectomy provides excellent short-term relief, the long-term outcome of this intervention is degenerative joint disease. Meniscal repair in the central two-thirds of the meniscus has shown poor results. Our study presents data that show supplementing suture repair of avascular meniscal tears with RF can lead to increased gross, histologic, and biochemical healing in the New Zealand white rabbit. While these results are encouraging, studies with longer follow-up and specimens that represent the human menisci are necessary to determine whether these preliminary results would translate to human meniscal tears in the avascular zone.
Weaknesses of our study include the use of an animal model and the location of the tear created in the menisci. While using an animal model had many strengths, the results of our study are probably not strong enough to immediately extrapolate the use of RF in human meniscal repairs. However, the data we obtained are very encouraging and perhaps suggest that RF warrants human trials. Our open surgical technique made it difficult to create and repair a tear on the posterior horn of the medical meniscus without completely dislocating the knee anteriorly. As a result, the knees were subluxed anteriorly, and the meniscal tears and repairs were performed more anteriorly. The more anterior aspects of the menisci do not undergo the same rotational and axial loads as the posterior horn, and it is unclear whether this difference would contribute to the results we obtained from RF treatment. In addition, the tears were surgically created and the repair was done during the same procedure. Patients do not present in this manner, and this further underscores the need for a clinical trial to determine the effectiveness of this treatment option in humans.
Conclusion
RF-based supplementation of meniscal repairs in the avascular zone showed acute signs of biochemical healing in 58% of New Zealand white rabbit specimens. In addition, gross and histologic evaluations showed an increase in healing compared with controls. Two specimens treated with RF in combination with suture repair had complete healing. These results illustrate the effectiveness of RF in stimulating a healing response in hypovascular tissue. Clinical trials are necessary to determine the effectiveness of this treatment in humans.
1. Fauno P, Nielsen AB. Arthroscopic partial meniscectomy: a long-term follow-up. Arthroscopy. 1992;8(3):345-349.
2. Lynch MA, Henning CE, Glick KR, Jr. Knee joint surface changes. Long-term follow-up meniscus tear treatment in stable anterior cruciate ligament reconstructions. Clin Orthop. 1983;172:148-153.
3. Roos H, Lauren M, Adalberth T, Roos EM, Jonsson K, Lohmander LS. Knee osteoarthritis after meniscectomy: prevalence of radiographic changes after twenty-one years, compared with matched controls. Arthritis Rheum. 1998;41(4):687-693.
4. Hennerbichler A, Moutos FT, Hennerbichler D, Weinberg JB, Guilak F. Repair response of the inner and outer regions of the porcine meniscus in vitro. Am J Sports Med. 2007;35(5):754‑762.
5. Gershuni DH, Hargens AR, Danzig LA. Regional nutrition and cellularity of the meniscus. Implications for tear and repair. Sports Med. 1988;5(5):322-327.
6. Gershuni DH, Skyhar MJ, Danzig LA, Camp J, Hargens AR, Akeson WH. Experimental models to promote healing of tears in the avascular segment of canine knee menisci. J Bone Joint Surg Am. 1989;71(9):1363-1370.
7. Papachristou G, Efstathopoulos N, Plessas S, Levidiotis C, Chronopoulos E, Sourlas J. Isolated meniscal repair in the avascular area. Acta Orthop Belg. 2003;69(4):341-345.
8. Fox JM, Rintz KG, Ferkel RD. Trephination of incomplete meniscal tears. Arthroscopy. 1993;9(4):451-455.
9. Zhang Z, Arnold JA, Williams T, McCann B. Repairs by trephination and suturing of longitudinal injuries in the avascular area of the meniscus in goats. Am J Sports Med. 1995;23(1):35-41.
10. Zhang ZN, Tu KY, Xu YK, Zhang WM, Liu ZT, Ou SH. Treatment of longitudinal injuries in avascular area of meniscus in dogs by trephination. Arthroscopy. 1988;4(3):151-159.
11. Ochi M, Uchio Y, Okuda K, Shu N, Yamaguchi H, Sakai Y. Expression of cytokines after meniscal rasping to promote meniscal healing. Arthroscopy. 2001;17(7):724-731.
12. Okuda K, Ochi M, Shu N, Uchio Y. Meniscal rasping for repair of meniscal tear in the avascular zone. Arthroscopy. 1999;15(3):281-286.
13. Uchio Y, Ochi M, Adachi N, Kawasaki K, Iwasa J. Results of rasping of meniscal tears with and without anterior cruciate ligament injury as evaluated by second-look arthroscopy. Arthroscopy. 2003;19(5):463-469.
14. Tasto JP, Cummings J, Medlock V, Harwood F, Hardesty R, Amiel D. The tendon treatment center: new horizons in the treatment of tendinosis. Arthroscopy. 2003;19(suppl 1):213-223.
15. Tasto JP. The role of radiofrequency-based devices in shaping the future of orthopedic surgery. Orthopedics. 2006;29(10):874-875.
16. Tasto JP, Cummings J, Medlock V, Hardesty R, Amiel D. Microtenotomy using a radiofrequency probe to treat lateral epicondylitis. Arthroscopy. 2005;21(7):851-860.
17. Higuchi H, Kimura M, Kobayashi A, Hatayama K, Takagishi K. A novel treatment of hypermobile lateral meniscus with monopolar radiofrequency energy. Arthroscopy 2004;20 (suppl 2):1-5.
18. Tonna EA, Cronkite EP. The periosteum. Autoradiographic studies on cellular proliferation and transformation utilizing tritiated thymidine. Clin Orthop. 1963;30:218-233.
19. Madewell BR. Serum thymidine kinase activity: an alternative to histologic markers of cellular proliferation in canine lymphoma. J Vet Intern Med. 2004;18(5):595-596.
20. Mujoomdar M, Bennett A, Hoskin D, Blay J. Adenosine stimulation of proliferation of breast carcinoma cell lines: evaluation of the [3H]thymidine assay system and modulatory effects of the cellular microenvironment in vitro. J Cell Physiol. 2004;201(3):429-438.
21. Vander Borght T, Labar D, Pauwels S, Lambotte L. Production of [2-11C]thymidine for quantification of cellular proliferation with PET. Int J Rad Appl Instrum A. 1991;42(1):103-104.
22. Spindler KP, Mayes CE, Miller RR, Imro AK, Davidson JM. Regional mitogenic response of the meniscus to platelet-derived growth factor (PDGF-AB). J Orthop Res. 1995;13(2):201-207.
23. Thomopoulos S, Zaegel M, Das R, et al. PDGF-BB released in tendon repair using a novel delivery system promotes cell proliferation and collagen remodeling. J Orthop Res. 2007;25(10):1358-1368.
24. Pennock AT, Robertson CM, Emmerson BC, Harwood FL, Amiel D. Role of apoptotic and matrix-degrading genes in articular cartilage and meniscus of mature and aged rabbits during development of osteoarthritis. Arthritis Rheum. 2007;56(5):1529-1536.
25. Allen RT, Tasto JP, Cummings J, Robertson CM, Amiel D. Meniscal debridement with an arthroscopic radiofrequency wand versus an arthroscopic shaver: comparative effects on menisci and underlying articular cartilage. Arthroscopy. 2006;22(4):385-393.
26. Figueroa D, Calvo R, Vaisman A, et al. Bipolar radiofrequency in the human meniscus. Comparative study between patients younger and older than 40 years of age. Knee. 2007;14(5):357-360.
27. Friedman M, LoSavio P, Ibrahim H, Ramakrishnan V. Radiofrequency tonsil reduction: safety, morbidity, and efficacy. Laryngoscope. 2003;113(5):882-887.
28. Hall DJ, Littlefield PD, Birkmire-Peters DP, Holtel MR. Radiofrequency ablation versus electrocautery in tonsillectomy. Otolaryngol Head Neck Surg. 2004;130(3):300-305.
29. Kaplan H, Gat A. Clinical and histopathological results following TriPollar radiofrequency skin treatments. J Cosmet Laser Ther. 2009;11(2):78-84.
30. Mancini PF. Coblation: a new technology and technique for skin resurfacing and other aesthetic surgical procedures. Aesthetic Plast Surg. 2001;2595):372-377.
31. Penka I, Kaplan Z, Sefr R, Sirotek L, Eber Z, Ondrák M. Use of radiofrequency ablation in the treatment of malignant liver lesions. Hepatogastroenterology. 2008;55(82-83):562-567.
32. Tasto JP, Ash SA. Current uses of radiofrequency in arthroscopic knee surgery. Am J Knee Surg. 1999;12(3):186-191.
33. Amiel D, Ball ST, Tasto JP. Chondrocyte viability and metabolic activity after treatment of bovine articular cartilage with bipolar radiofrequency: an in vitro study. Arthroscopy. 2004;20(5):503-510.
34. Barry KJ, Kaplan J, Connolly RJ, et al. The effect of radiofrequency-generated thermal energy on the mechanical and histologic characteristics of the arterial wall in vivo: implications for radiofrequency angioplasty. Am Heart J. 1989;117(2):332-341.
35. Hoch DH, Grodzinsky AJ, Koob TJ, Albert ML, Eyre DR. Early changes in material properties of rabbit articular cartilage after meniscectomy. J Orthop Res. 1983;1(1):4-12.
36. Thompson AM, Stockwell RA. An ultrastructural study of the marginal transitional zone in the rabbit knee joint. J Anat. 1983;136(Pt 4):701-713.
1. Fauno P, Nielsen AB. Arthroscopic partial meniscectomy: a long-term follow-up. Arthroscopy. 1992;8(3):345-349.
2. Lynch MA, Henning CE, Glick KR, Jr. Knee joint surface changes. Long-term follow-up meniscus tear treatment in stable anterior cruciate ligament reconstructions. Clin Orthop. 1983;172:148-153.
3. Roos H, Lauren M, Adalberth T, Roos EM, Jonsson K, Lohmander LS. Knee osteoarthritis after meniscectomy: prevalence of radiographic changes after twenty-one years, compared with matched controls. Arthritis Rheum. 1998;41(4):687-693.
4. Hennerbichler A, Moutos FT, Hennerbichler D, Weinberg JB, Guilak F. Repair response of the inner and outer regions of the porcine meniscus in vitro. Am J Sports Med. 2007;35(5):754‑762.
5. Gershuni DH, Hargens AR, Danzig LA. Regional nutrition and cellularity of the meniscus. Implications for tear and repair. Sports Med. 1988;5(5):322-327.
6. Gershuni DH, Skyhar MJ, Danzig LA, Camp J, Hargens AR, Akeson WH. Experimental models to promote healing of tears in the avascular segment of canine knee menisci. J Bone Joint Surg Am. 1989;71(9):1363-1370.
7. Papachristou G, Efstathopoulos N, Plessas S, Levidiotis C, Chronopoulos E, Sourlas J. Isolated meniscal repair in the avascular area. Acta Orthop Belg. 2003;69(4):341-345.
8. Fox JM, Rintz KG, Ferkel RD. Trephination of incomplete meniscal tears. Arthroscopy. 1993;9(4):451-455.
9. Zhang Z, Arnold JA, Williams T, McCann B. Repairs by trephination and suturing of longitudinal injuries in the avascular area of the meniscus in goats. Am J Sports Med. 1995;23(1):35-41.
10. Zhang ZN, Tu KY, Xu YK, Zhang WM, Liu ZT, Ou SH. Treatment of longitudinal injuries in avascular area of meniscus in dogs by trephination. Arthroscopy. 1988;4(3):151-159.
11. Ochi M, Uchio Y, Okuda K, Shu N, Yamaguchi H, Sakai Y. Expression of cytokines after meniscal rasping to promote meniscal healing. Arthroscopy. 2001;17(7):724-731.
12. Okuda K, Ochi M, Shu N, Uchio Y. Meniscal rasping for repair of meniscal tear in the avascular zone. Arthroscopy. 1999;15(3):281-286.
13. Uchio Y, Ochi M, Adachi N, Kawasaki K, Iwasa J. Results of rasping of meniscal tears with and without anterior cruciate ligament injury as evaluated by second-look arthroscopy. Arthroscopy. 2003;19(5):463-469.
14. Tasto JP, Cummings J, Medlock V, Harwood F, Hardesty R, Amiel D. The tendon treatment center: new horizons in the treatment of tendinosis. Arthroscopy. 2003;19(suppl 1):213-223.
15. Tasto JP. The role of radiofrequency-based devices in shaping the future of orthopedic surgery. Orthopedics. 2006;29(10):874-875.
16. Tasto JP, Cummings J, Medlock V, Hardesty R, Amiel D. Microtenotomy using a radiofrequency probe to treat lateral epicondylitis. Arthroscopy. 2005;21(7):851-860.
17. Higuchi H, Kimura M, Kobayashi A, Hatayama K, Takagishi K. A novel treatment of hypermobile lateral meniscus with monopolar radiofrequency energy. Arthroscopy 2004;20 (suppl 2):1-5.
18. Tonna EA, Cronkite EP. The periosteum. Autoradiographic studies on cellular proliferation and transformation utilizing tritiated thymidine. Clin Orthop. 1963;30:218-233.
19. Madewell BR. Serum thymidine kinase activity: an alternative to histologic markers of cellular proliferation in canine lymphoma. J Vet Intern Med. 2004;18(5):595-596.
20. Mujoomdar M, Bennett A, Hoskin D, Blay J. Adenosine stimulation of proliferation of breast carcinoma cell lines: evaluation of the [3H]thymidine assay system and modulatory effects of the cellular microenvironment in vitro. J Cell Physiol. 2004;201(3):429-438.
21. Vander Borght T, Labar D, Pauwels S, Lambotte L. Production of [2-11C]thymidine for quantification of cellular proliferation with PET. Int J Rad Appl Instrum A. 1991;42(1):103-104.
22. Spindler KP, Mayes CE, Miller RR, Imro AK, Davidson JM. Regional mitogenic response of the meniscus to platelet-derived growth factor (PDGF-AB). J Orthop Res. 1995;13(2):201-207.
23. Thomopoulos S, Zaegel M, Das R, et al. PDGF-BB released in tendon repair using a novel delivery system promotes cell proliferation and collagen remodeling. J Orthop Res. 2007;25(10):1358-1368.
24. Pennock AT, Robertson CM, Emmerson BC, Harwood FL, Amiel D. Role of apoptotic and matrix-degrading genes in articular cartilage and meniscus of mature and aged rabbits during development of osteoarthritis. Arthritis Rheum. 2007;56(5):1529-1536.
25. Allen RT, Tasto JP, Cummings J, Robertson CM, Amiel D. Meniscal debridement with an arthroscopic radiofrequency wand versus an arthroscopic shaver: comparative effects on menisci and underlying articular cartilage. Arthroscopy. 2006;22(4):385-393.
26. Figueroa D, Calvo R, Vaisman A, et al. Bipolar radiofrequency in the human meniscus. Comparative study between patients younger and older than 40 years of age. Knee. 2007;14(5):357-360.
27. Friedman M, LoSavio P, Ibrahim H, Ramakrishnan V. Radiofrequency tonsil reduction: safety, morbidity, and efficacy. Laryngoscope. 2003;113(5):882-887.
28. Hall DJ, Littlefield PD, Birkmire-Peters DP, Holtel MR. Radiofrequency ablation versus electrocautery in tonsillectomy. Otolaryngol Head Neck Surg. 2004;130(3):300-305.
29. Kaplan H, Gat A. Clinical and histopathological results following TriPollar radiofrequency skin treatments. J Cosmet Laser Ther. 2009;11(2):78-84.
30. Mancini PF. Coblation: a new technology and technique for skin resurfacing and other aesthetic surgical procedures. Aesthetic Plast Surg. 2001;2595):372-377.
31. Penka I, Kaplan Z, Sefr R, Sirotek L, Eber Z, Ondrák M. Use of radiofrequency ablation in the treatment of malignant liver lesions. Hepatogastroenterology. 2008;55(82-83):562-567.
32. Tasto JP, Ash SA. Current uses of radiofrequency in arthroscopic knee surgery. Am J Knee Surg. 1999;12(3):186-191.
33. Amiel D, Ball ST, Tasto JP. Chondrocyte viability and metabolic activity after treatment of bovine articular cartilage with bipolar radiofrequency: an in vitro study. Arthroscopy. 2004;20(5):503-510.
34. Barry KJ, Kaplan J, Connolly RJ, et al. The effect of radiofrequency-generated thermal energy on the mechanical and histologic characteristics of the arterial wall in vivo: implications for radiofrequency angioplasty. Am Heart J. 1989;117(2):332-341.
35. Hoch DH, Grodzinsky AJ, Koob TJ, Albert ML, Eyre DR. Early changes in material properties of rabbit articular cartilage after meniscectomy. J Orthop Res. 1983;1(1):4-12.
36. Thompson AM, Stockwell RA. An ultrastructural study of the marginal transitional zone in the rabbit knee joint. J Anat. 1983;136(Pt 4):701-713.