User login
Caught red‐handed
A previously healthy 58‐year‐old man presented to a community hospital's emergency department 1 day after the sudden onset of a severe headache, fever, diffuse abdominal pain, nausea, vomiting, and disorientation. The patient had a history of allergic rhinitis and his only medication was a daily multivitamin.
Key features of this patient's presentation include the abrupt onset of severe headache, disorientation, fever, and abdominal pain. The list of entities likely to make a previously healthy individual this ill this quickly is typically circumscribed. His presentation raises the possibility of bacterial meningitis (including Listeria, given his age), viral encephalitis, or other extraneural etiologies of sepsis. Noninfectious explanations seem much less likely given the rapid tempo of illness.
His proclivity for gardening and apparent tick exposure raise the question of tick‐borne illnesses. This would constitute a rather explosive onset for any of these; however, babesiosis, Rocky Mountain spotted fever (RMSF), ehrlichiosis, and anaplasmosis could present this abruptly, with dog exposure linked to RMSF.
The potential causes of fever and rash are myriad, although the severity and acuity of this patient's illness narrow the differential considerably, likely to an infectious cause. Diagnoses that typically include a generalized exanthem involving the palms and soles are meningococcal meningitis, overwhelming Staphylococcus aureus sepsis, RMSF (realizing that this disease is not common in the upper Midwest), and toxic shock syndrome. The rash described is not the classic and/or fully developed rash typical of any of these; subsequent evolution to a petechial appearance would lend further support to the first 3 diagnoses. Ehrlichiosis is still a possibility, although the palm and sole involvement would be unusual. The presence of a rash makes anaplasmosis very unlikely, although not entirely excluded. The finding of modest splenomegaly does not help further distinguish between these possibilities.
Empiric antimicrobials should be immediately administered after blood cultures, a complete blood count, and coagulation studies are obtained. Doxycycline would be appropriate to treat the possible tick‐borne diseases already mentioned, whereas antimicrobials appropriate to cover community‐acquired bacterial meningitis in a 58‐year‐old (ie, vancomycin, ampicillin, and a third‐generation cephalosporin) should also be empirically administered. Given the patient's altered mentation, a brain computed tomography (CT) should be urgently obtained. Provided this did not show evidence of increased intracranial pressure and that coagulation studies and a platelet count did not suggest a contraindication, a lumbar puncture should then be performed promptly. The patient should be placed in droplet precautions until meningococcal disease is excluded. Although most patients with bacterial meningitis will exhibit meningismus, a substantial minority will not.
These laboratory results do not significantly affect the differential diagnosis. Although nonspecific, moderate thrombocytopenia and modest elevation of hepatic transaminases are typical for tick‐borne diseases, whereas leukocytosis is somewhat atypical for these entities. Marked elevation of the C‐reactive protein with a less striking increase in the erythrocyte sedimentation rate, along with significant hypoalbuminemia, are commonly encountered early in the course of critical infectious illnesses. The elevated troponin likely reflects severe sepsis and demand ischemia, and is associated with a less favorable prognosis; an electrocardiogram and serial cardiac biomarkers are appropriate to help exclude an acute coronary syndrome. As already noted, blood cultures need to be obtained and a lumbar puncture should be performed, provided this can be safely accomplished.
Results of the lumbar puncture exclude bacterial meningitis as the explanation of this patient's illness; the mildly elevated protein is nonspecific. These studies do not otherwise change the differential diagnosis.
Supporting data for a diagnosis of pneumonia, such as pulmonary infiltrates or supplemental oxygen requirement, are lacking. Given his critical illness, broad spectrum antimicrobial coverage is indicated, and as a primary central nervous system (CNS) infection now appears unlikely, piperacillin/tazobactam (which does not have adequate CNS penetration) and vancomycin are reasonable. Empiric treatment for RMSF is appropriate, and should have been initiated earlier in the patient's course, despite the upper Midwest being out of the typical range for this disease. Doxycycline will also provide excellent coverage for ehrlichiosis and anaplasmosis.
Given the patient's deterioration, it is important to stop and reconsider the differential diagnosis in an attempt to avoid anchoring bias and premature closure. The patient's illness is almost certainly infectious in nature, and the differential is not substantially altered by the most recent information. A skin biopsy should be performed in an attempt to secure the diagnosis.
The patient's overall course, including rapid onset of severe illness and especially the apparent dramatic response to doxycycline, make tick‐borne illness very likely. Completing a course of doxycycline is certainly appropriate, typically for 7 to 14 days. The acute serologies drawn prior to discharge may well reveal the causative agent, but convalescent serology should also be obtained at the time of an outpatient follow‐up visit as immunoglobulin G has a delayed rise. Without hyponatremia or respiratory symptoms, Legionella seems unlikely.
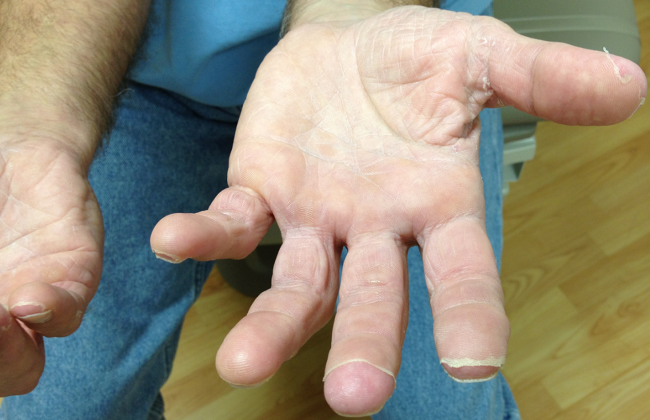
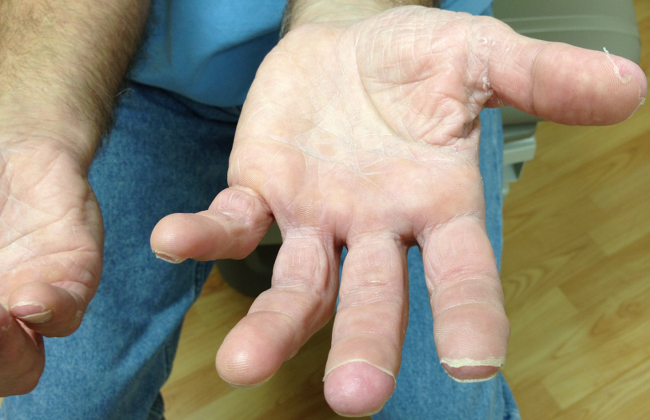
The appearance of late desquamation of the palms and soles is an unexpected and important sign. Desquamation in this pattern following an illness of this nature strongly suggests a diagnosis of staphylococcal toxic shock syndrome (TSS), and in conjunction with the negative serologies, argues that tick‐borne disease is unlikely. The list of other entities that might lead to desquamation in this setting is very short, namely adult Kawasaki disease and drug reaction. The former seems reasonably excluded based on details of the case, whereas a doxycycline‐related drug reaction, although not entirely implausible, seems quite unlikely as this medication was started after the onset of the initial rash. This patient most likely had staphylococcal TSS secondary to a minor and unappreciated skin lesion.
DISCUSSION
TSS is a systemic illness resulting in multiorgan dysfunction.[1] Infection by S aureus or Streptococcus pyogenes causes TSS by stimulating maladaptive T‐cell proliferation and cytokine release resulting in shock.[1, 2] A definitive diagnosis requires fever, a diffuse macular erythematous rash (often resembling a sunburn), with subsequent desquamation, hypotension, and involvement of at least 3 organ systems. Blood cultures, cerebrospinal cultures, and serologies for other organisms should be negative; although Staphylococcus and Streptococcus species may be isolated, they frequently are not (Table 1).[3]
| Diagnostic Criteria* | This Case |
|---|---|
| |
| Fever: Temperature 102.0F | Fever: 105.3F on admission |
| Rash: Diffuse macular erythroderma | Diffuse morbilliform rash with progression to confluent erythroderma |
| Desquamation of rash: occurs 12 weeks following rash onset | Desquamation 12 days after discharge |
| Hypotension: SBP 90 mm Hg for adults | Intermittent |
| Multisystem involvement, 3 of the following: | 4 organ systems definitively involved |
| GI: vomiting or diarrhea at disease onset | Vomiting and abdominal pain |
| Muscular: severe myalgias, or creatine phosphokinase >2 times the upper limit of normal | |
| Mucous membranes: vaginal, oropharyngeal, or conjunctival hyperemia | |
| Renal: BUN or Cr >2 times the upper limit of normal, or pyuria without evidence of infection | |
| Hepatic: total bilirubin, AST, or ALT levels >2 times the upper limit of normal | AST and ALT peaked at 128IU/L and 94 IU/L |
| Hematologic: platelets <100,000/mm3 | Platelet nadir of 80,000/mm3 |
| CNS: disorientation or altered consciousness without focal neurologic signs | Disorientation and somnolence |
| Probable case: 4 out of 5 clinical criteria present | |
| Confirmed case: 5 out of 5 clinical criteria present, or patient dies before desquamation can occur | |
A rare cause of shock, TSS is most associated with a surge of menstruation‐related cases linked to tampon use in young women in the 1980s.[4] However, in Centers for Disease Control and Prevention (CDC) surveillance between 1987 and 1996, only 59% of the 1069 cases identified were noted to be menstruation‐related, as compared to nearly 80% of all cases earlier in the decade.[4, 5] Today, the syndrome is more likely to present after musculoskeletal and cutaneous trauma, oropharyngeal infections, surgical procedures, and device implantation.[1, 6] Despite the disease's evolving epidemiology, the illness script used by physicians likely continues to focus on young women as the primary at risk population for TSS, causing physicians to neglect the diagnosis in other populations.[1, 6, 7, 8, 9] Given this change in risk factors, it is imperative that clinicians rewrite their scripts and recognize the early signs of TSS in all patients to enable quick and effective treatment.
In addition to its shifting epidemiology and rarity, the diagnosis of TSS vexes clinicians for several reasons. First, TSS cannot be quickly and definitively diagnosed because 2 diagnostic criteria cannot be fulfilled during the acute illness. The disease's hallmarka desquamative rashoccurs only if the patient survives.[3] Serologies often take weeks to return, further delaying diagnosis. During this period of diagnostic delay, the illness has usually already resolved or resulted in death. In addition, the presenting symptoms of rash, fever, and shock are nonspecific. Alternative etiologies include meningococcal meningitis, which can also present dramatically as with this patient; RMSF, which can occasionally have a fulminant presentation; bacterial sepsis, usually from Staphylococcus or Streptococcus species; acute viral syndromes; and severe drug reactions.[6, 10, 11, 12] Palmoplantar desquamation, as in this case, can further narrow the differential as this presentation is uncommon but characteristic of TSS, RMSF, and secondary syphilis.[11] Other diagnostic clues offered by the pattern of the rash may be limited by physician discomfort with diagnosing and describing rashes. Because of this lack of a definitive diagnostic test in the acute setting, it is imperative that the clinician include TSS in the differential of fever, shock, and rash, as mortality from TSS can exceed 20% in patients who are untreated.[13]
Treatment of TSS is straightforward once considered and includes the administration of antibiotics that cover both Staphylococcus and Streptococcus species, in addition to aggressive hydration and supportive care.[14] The final critical detail in this case was the appropriate arrangement of follow‐up. Given the patient's drastic improvement, the complicated process of arranging follow‐up for a transferred patient, and the current model where the hospitalists providing inpatient care do not typically follow their patients in clinic, patients such as these can easily be lost to follow‐up. Had this occurred, the desquamation would have been missed, and the patient's diagnosis would have been incomplete.
This patient was eventually diagnosed with TSS by fulfilling all 5 CDC criteria (Table 1).[3] He made a full recovery, likely aided by the administration of broad‐spectrum antibiotics (followed by doxycycline, which provided community‐acquired methicillin‐resistant S aureus coverage) and his lack of serious comorbidities. This case should serve as a reminder to hospitalists that with a discerning eye, a careful assessment of the clinical facts, and appropriate follow‐up, perhaps the next case of TSS can be caught red‐handed.
KEY POINTS
- When presented with a patient with fever, rash, and shock, hospitalists should consider meningococcal meningitis, RMSF bacterial sepsis, acute viral illness, severe drug reaction, and TSS.
- TSS, caused by S aureus or S pyogenes, is no longer predominantly associated with tampon use. Postsurgical infection and cutaneous trauma have become important present‐day risk factors.
- The initial presentation of TSS is nonspecific. Definitive diagnosis requires proper follow‐up, allowing time for infectious serologies to return negative and for the disease's hallmark desquamation to occur.
Disclosure
Nothing to report.
- . Toxic shock syndrome: major advances in pathogenesis, but not treatment. Crit Care Clin. 2013;29:651–675.
- . The toxic shock syndromes. Infect Dis Clin North Am. 1996;10(4):727–746.
- Centers for Disease Control and Prevention. National Notifiable Diseases Surveillance System. Toxic shock syndrome (other than Streptococcal) (TSS) 2011 Case Definition. Available at: http://wwwn.cdc.gov/nndss/conditions/toxic‐shock‐syndrome‐other‐than‐streptococcal/case‐definition/2011. Accessed June 4, 2015.
- Centers for Disease Control and Prevention. Update: toxic‐shock syndrome—United States. MMWR Morb Mortal Wkly Rep. 1983;32(30):398–400.
- , , , , , . Toxic shock syndrome in the United States: surveillance update, 1979–1996. Emerg Infect Dis. 1999;5(6):807–810.
- . Fever and rash. Infect Dis Clin North Am. 1996;10(1):101–110.
- , , , et al. Staphylococcal toxic shock syndrome 2000–2006: epidemiology, clinical features, and molecular characteristics. PLoS One. 2011;6(8):e22997.
- , , , et al. Toxic‐shock syndrome in menstruating women: association with tampon use and staphylococcus aureus and clinical features in 52 cases. N Engl J Med. 1980;303(25):1436–1442.
- , , , . Toxic‐shock syndrome—epidemiologic features, recurrence, risk factors, and prevention. N Engl J Med. 1980;303:1429–1435.
- , . Evaluating the febrile patient with a rash. Am Fam Physician. 2000;62(4):804–816.
- . Toxic shock syndrome: broadening the differential diagnosis. J Am Board Fam Pract. 2001;14(2):131–136.
- , , , . Spatial clustering by disease severity among reported Rocky Mountain spotted fever cases in the United States, 2001–2005. Am J Trop Med Hyg. 2009;80(1):72–77.
- , , , et al. One in five mortality in non‐menstrual toxic shock syndrome versus no mortality in menstrual cases in a balanced French series of 55 cases. Eur J Clin Microbio Infect Dis. 2008;27(1):37–43.
- , . Gram‐positive toxic shock syndromes. Lancet Infect Dis. 2009;9(5):281–290.
A previously healthy 58‐year‐old man presented to a community hospital's emergency department 1 day after the sudden onset of a severe headache, fever, diffuse abdominal pain, nausea, vomiting, and disorientation. The patient had a history of allergic rhinitis and his only medication was a daily multivitamin.
Key features of this patient's presentation include the abrupt onset of severe headache, disorientation, fever, and abdominal pain. The list of entities likely to make a previously healthy individual this ill this quickly is typically circumscribed. His presentation raises the possibility of bacterial meningitis (including Listeria, given his age), viral encephalitis, or other extraneural etiologies of sepsis. Noninfectious explanations seem much less likely given the rapid tempo of illness.
His proclivity for gardening and apparent tick exposure raise the question of tick‐borne illnesses. This would constitute a rather explosive onset for any of these; however, babesiosis, Rocky Mountain spotted fever (RMSF), ehrlichiosis, and anaplasmosis could present this abruptly, with dog exposure linked to RMSF.
The potential causes of fever and rash are myriad, although the severity and acuity of this patient's illness narrow the differential considerably, likely to an infectious cause. Diagnoses that typically include a generalized exanthem involving the palms and soles are meningococcal meningitis, overwhelming Staphylococcus aureus sepsis, RMSF (realizing that this disease is not common in the upper Midwest), and toxic shock syndrome. The rash described is not the classic and/or fully developed rash typical of any of these; subsequent evolution to a petechial appearance would lend further support to the first 3 diagnoses. Ehrlichiosis is still a possibility, although the palm and sole involvement would be unusual. The presence of a rash makes anaplasmosis very unlikely, although not entirely excluded. The finding of modest splenomegaly does not help further distinguish between these possibilities.
Empiric antimicrobials should be immediately administered after blood cultures, a complete blood count, and coagulation studies are obtained. Doxycycline would be appropriate to treat the possible tick‐borne diseases already mentioned, whereas antimicrobials appropriate to cover community‐acquired bacterial meningitis in a 58‐year‐old (ie, vancomycin, ampicillin, and a third‐generation cephalosporin) should also be empirically administered. Given the patient's altered mentation, a brain computed tomography (CT) should be urgently obtained. Provided this did not show evidence of increased intracranial pressure and that coagulation studies and a platelet count did not suggest a contraindication, a lumbar puncture should then be performed promptly. The patient should be placed in droplet precautions until meningococcal disease is excluded. Although most patients with bacterial meningitis will exhibit meningismus, a substantial minority will not.
These laboratory results do not significantly affect the differential diagnosis. Although nonspecific, moderate thrombocytopenia and modest elevation of hepatic transaminases are typical for tick‐borne diseases, whereas leukocytosis is somewhat atypical for these entities. Marked elevation of the C‐reactive protein with a less striking increase in the erythrocyte sedimentation rate, along with significant hypoalbuminemia, are commonly encountered early in the course of critical infectious illnesses. The elevated troponin likely reflects severe sepsis and demand ischemia, and is associated with a less favorable prognosis; an electrocardiogram and serial cardiac biomarkers are appropriate to help exclude an acute coronary syndrome. As already noted, blood cultures need to be obtained and a lumbar puncture should be performed, provided this can be safely accomplished.
Results of the lumbar puncture exclude bacterial meningitis as the explanation of this patient's illness; the mildly elevated protein is nonspecific. These studies do not otherwise change the differential diagnosis.
Supporting data for a diagnosis of pneumonia, such as pulmonary infiltrates or supplemental oxygen requirement, are lacking. Given his critical illness, broad spectrum antimicrobial coverage is indicated, and as a primary central nervous system (CNS) infection now appears unlikely, piperacillin/tazobactam (which does not have adequate CNS penetration) and vancomycin are reasonable. Empiric treatment for RMSF is appropriate, and should have been initiated earlier in the patient's course, despite the upper Midwest being out of the typical range for this disease. Doxycycline will also provide excellent coverage for ehrlichiosis and anaplasmosis.
Given the patient's deterioration, it is important to stop and reconsider the differential diagnosis in an attempt to avoid anchoring bias and premature closure. The patient's illness is almost certainly infectious in nature, and the differential is not substantially altered by the most recent information. A skin biopsy should be performed in an attempt to secure the diagnosis.
The patient's overall course, including rapid onset of severe illness and especially the apparent dramatic response to doxycycline, make tick‐borne illness very likely. Completing a course of doxycycline is certainly appropriate, typically for 7 to 14 days. The acute serologies drawn prior to discharge may well reveal the causative agent, but convalescent serology should also be obtained at the time of an outpatient follow‐up visit as immunoglobulin G has a delayed rise. Without hyponatremia or respiratory symptoms, Legionella seems unlikely.

The appearance of late desquamation of the palms and soles is an unexpected and important sign. Desquamation in this pattern following an illness of this nature strongly suggests a diagnosis of staphylococcal toxic shock syndrome (TSS), and in conjunction with the negative serologies, argues that tick‐borne disease is unlikely. The list of other entities that might lead to desquamation in this setting is very short, namely adult Kawasaki disease and drug reaction. The former seems reasonably excluded based on details of the case, whereas a doxycycline‐related drug reaction, although not entirely implausible, seems quite unlikely as this medication was started after the onset of the initial rash. This patient most likely had staphylococcal TSS secondary to a minor and unappreciated skin lesion.
DISCUSSION
TSS is a systemic illness resulting in multiorgan dysfunction.[1] Infection by S aureus or Streptococcus pyogenes causes TSS by stimulating maladaptive T‐cell proliferation and cytokine release resulting in shock.[1, 2] A definitive diagnosis requires fever, a diffuse macular erythematous rash (often resembling a sunburn), with subsequent desquamation, hypotension, and involvement of at least 3 organ systems. Blood cultures, cerebrospinal cultures, and serologies for other organisms should be negative; although Staphylococcus and Streptococcus species may be isolated, they frequently are not (Table 1).[3]
| Diagnostic Criteria* | This Case |
|---|---|
| |
| Fever: Temperature 102.0F | Fever: 105.3F on admission |
| Rash: Diffuse macular erythroderma | Diffuse morbilliform rash with progression to confluent erythroderma |
| Desquamation of rash: occurs 12 weeks following rash onset | Desquamation 12 days after discharge |
| Hypotension: SBP 90 mm Hg for adults | Intermittent |
| Multisystem involvement, 3 of the following: | 4 organ systems definitively involved |
| GI: vomiting or diarrhea at disease onset | Vomiting and abdominal pain |
| Muscular: severe myalgias, or creatine phosphokinase >2 times the upper limit of normal | |
| Mucous membranes: vaginal, oropharyngeal, or conjunctival hyperemia | |
| Renal: BUN or Cr >2 times the upper limit of normal, or pyuria without evidence of infection | |
| Hepatic: total bilirubin, AST, or ALT levels >2 times the upper limit of normal | AST and ALT peaked at 128IU/L and 94 IU/L |
| Hematologic: platelets <100,000/mm3 | Platelet nadir of 80,000/mm3 |
| CNS: disorientation or altered consciousness without focal neurologic signs | Disorientation and somnolence |
| Probable case: 4 out of 5 clinical criteria present | |
| Confirmed case: 5 out of 5 clinical criteria present, or patient dies before desquamation can occur | |
A rare cause of shock, TSS is most associated with a surge of menstruation‐related cases linked to tampon use in young women in the 1980s.[4] However, in Centers for Disease Control and Prevention (CDC) surveillance between 1987 and 1996, only 59% of the 1069 cases identified were noted to be menstruation‐related, as compared to nearly 80% of all cases earlier in the decade.[4, 5] Today, the syndrome is more likely to present after musculoskeletal and cutaneous trauma, oropharyngeal infections, surgical procedures, and device implantation.[1, 6] Despite the disease's evolving epidemiology, the illness script used by physicians likely continues to focus on young women as the primary at risk population for TSS, causing physicians to neglect the diagnosis in other populations.[1, 6, 7, 8, 9] Given this change in risk factors, it is imperative that clinicians rewrite their scripts and recognize the early signs of TSS in all patients to enable quick and effective treatment.
In addition to its shifting epidemiology and rarity, the diagnosis of TSS vexes clinicians for several reasons. First, TSS cannot be quickly and definitively diagnosed because 2 diagnostic criteria cannot be fulfilled during the acute illness. The disease's hallmarka desquamative rashoccurs only if the patient survives.[3] Serologies often take weeks to return, further delaying diagnosis. During this period of diagnostic delay, the illness has usually already resolved or resulted in death. In addition, the presenting symptoms of rash, fever, and shock are nonspecific. Alternative etiologies include meningococcal meningitis, which can also present dramatically as with this patient; RMSF, which can occasionally have a fulminant presentation; bacterial sepsis, usually from Staphylococcus or Streptococcus species; acute viral syndromes; and severe drug reactions.[6, 10, 11, 12] Palmoplantar desquamation, as in this case, can further narrow the differential as this presentation is uncommon but characteristic of TSS, RMSF, and secondary syphilis.[11] Other diagnostic clues offered by the pattern of the rash may be limited by physician discomfort with diagnosing and describing rashes. Because of this lack of a definitive diagnostic test in the acute setting, it is imperative that the clinician include TSS in the differential of fever, shock, and rash, as mortality from TSS can exceed 20% in patients who are untreated.[13]
Treatment of TSS is straightforward once considered and includes the administration of antibiotics that cover both Staphylococcus and Streptococcus species, in addition to aggressive hydration and supportive care.[14] The final critical detail in this case was the appropriate arrangement of follow‐up. Given the patient's drastic improvement, the complicated process of arranging follow‐up for a transferred patient, and the current model where the hospitalists providing inpatient care do not typically follow their patients in clinic, patients such as these can easily be lost to follow‐up. Had this occurred, the desquamation would have been missed, and the patient's diagnosis would have been incomplete.
This patient was eventually diagnosed with TSS by fulfilling all 5 CDC criteria (Table 1).[3] He made a full recovery, likely aided by the administration of broad‐spectrum antibiotics (followed by doxycycline, which provided community‐acquired methicillin‐resistant S aureus coverage) and his lack of serious comorbidities. This case should serve as a reminder to hospitalists that with a discerning eye, a careful assessment of the clinical facts, and appropriate follow‐up, perhaps the next case of TSS can be caught red‐handed.
KEY POINTS
- When presented with a patient with fever, rash, and shock, hospitalists should consider meningococcal meningitis, RMSF bacterial sepsis, acute viral illness, severe drug reaction, and TSS.
- TSS, caused by S aureus or S pyogenes, is no longer predominantly associated with tampon use. Postsurgical infection and cutaneous trauma have become important present‐day risk factors.
- The initial presentation of TSS is nonspecific. Definitive diagnosis requires proper follow‐up, allowing time for infectious serologies to return negative and for the disease's hallmark desquamation to occur.
Disclosure
Nothing to report.
A previously healthy 58‐year‐old man presented to a community hospital's emergency department 1 day after the sudden onset of a severe headache, fever, diffuse abdominal pain, nausea, vomiting, and disorientation. The patient had a history of allergic rhinitis and his only medication was a daily multivitamin.
Key features of this patient's presentation include the abrupt onset of severe headache, disorientation, fever, and abdominal pain. The list of entities likely to make a previously healthy individual this ill this quickly is typically circumscribed. His presentation raises the possibility of bacterial meningitis (including Listeria, given his age), viral encephalitis, or other extraneural etiologies of sepsis. Noninfectious explanations seem much less likely given the rapid tempo of illness.
His proclivity for gardening and apparent tick exposure raise the question of tick‐borne illnesses. This would constitute a rather explosive onset for any of these; however, babesiosis, Rocky Mountain spotted fever (RMSF), ehrlichiosis, and anaplasmosis could present this abruptly, with dog exposure linked to RMSF.
The potential causes of fever and rash are myriad, although the severity and acuity of this patient's illness narrow the differential considerably, likely to an infectious cause. Diagnoses that typically include a generalized exanthem involving the palms and soles are meningococcal meningitis, overwhelming Staphylococcus aureus sepsis, RMSF (realizing that this disease is not common in the upper Midwest), and toxic shock syndrome. The rash described is not the classic and/or fully developed rash typical of any of these; subsequent evolution to a petechial appearance would lend further support to the first 3 diagnoses. Ehrlichiosis is still a possibility, although the palm and sole involvement would be unusual. The presence of a rash makes anaplasmosis very unlikely, although not entirely excluded. The finding of modest splenomegaly does not help further distinguish between these possibilities.
Empiric antimicrobials should be immediately administered after blood cultures, a complete blood count, and coagulation studies are obtained. Doxycycline would be appropriate to treat the possible tick‐borne diseases already mentioned, whereas antimicrobials appropriate to cover community‐acquired bacterial meningitis in a 58‐year‐old (ie, vancomycin, ampicillin, and a third‐generation cephalosporin) should also be empirically administered. Given the patient's altered mentation, a brain computed tomography (CT) should be urgently obtained. Provided this did not show evidence of increased intracranial pressure and that coagulation studies and a platelet count did not suggest a contraindication, a lumbar puncture should then be performed promptly. The patient should be placed in droplet precautions until meningococcal disease is excluded. Although most patients with bacterial meningitis will exhibit meningismus, a substantial minority will not.
These laboratory results do not significantly affect the differential diagnosis. Although nonspecific, moderate thrombocytopenia and modest elevation of hepatic transaminases are typical for tick‐borne diseases, whereas leukocytosis is somewhat atypical for these entities. Marked elevation of the C‐reactive protein with a less striking increase in the erythrocyte sedimentation rate, along with significant hypoalbuminemia, are commonly encountered early in the course of critical infectious illnesses. The elevated troponin likely reflects severe sepsis and demand ischemia, and is associated with a less favorable prognosis; an electrocardiogram and serial cardiac biomarkers are appropriate to help exclude an acute coronary syndrome. As already noted, blood cultures need to be obtained and a lumbar puncture should be performed, provided this can be safely accomplished.
Results of the lumbar puncture exclude bacterial meningitis as the explanation of this patient's illness; the mildly elevated protein is nonspecific. These studies do not otherwise change the differential diagnosis.
Supporting data for a diagnosis of pneumonia, such as pulmonary infiltrates or supplemental oxygen requirement, are lacking. Given his critical illness, broad spectrum antimicrobial coverage is indicated, and as a primary central nervous system (CNS) infection now appears unlikely, piperacillin/tazobactam (which does not have adequate CNS penetration) and vancomycin are reasonable. Empiric treatment for RMSF is appropriate, and should have been initiated earlier in the patient's course, despite the upper Midwest being out of the typical range for this disease. Doxycycline will also provide excellent coverage for ehrlichiosis and anaplasmosis.
Given the patient's deterioration, it is important to stop and reconsider the differential diagnosis in an attempt to avoid anchoring bias and premature closure. The patient's illness is almost certainly infectious in nature, and the differential is not substantially altered by the most recent information. A skin biopsy should be performed in an attempt to secure the diagnosis.
The patient's overall course, including rapid onset of severe illness and especially the apparent dramatic response to doxycycline, make tick‐borne illness very likely. Completing a course of doxycycline is certainly appropriate, typically for 7 to 14 days. The acute serologies drawn prior to discharge may well reveal the causative agent, but convalescent serology should also be obtained at the time of an outpatient follow‐up visit as immunoglobulin G has a delayed rise. Without hyponatremia or respiratory symptoms, Legionella seems unlikely.

The appearance of late desquamation of the palms and soles is an unexpected and important sign. Desquamation in this pattern following an illness of this nature strongly suggests a diagnosis of staphylococcal toxic shock syndrome (TSS), and in conjunction with the negative serologies, argues that tick‐borne disease is unlikely. The list of other entities that might lead to desquamation in this setting is very short, namely adult Kawasaki disease and drug reaction. The former seems reasonably excluded based on details of the case, whereas a doxycycline‐related drug reaction, although not entirely implausible, seems quite unlikely as this medication was started after the onset of the initial rash. This patient most likely had staphylococcal TSS secondary to a minor and unappreciated skin lesion.
DISCUSSION
TSS is a systemic illness resulting in multiorgan dysfunction.[1] Infection by S aureus or Streptococcus pyogenes causes TSS by stimulating maladaptive T‐cell proliferation and cytokine release resulting in shock.[1, 2] A definitive diagnosis requires fever, a diffuse macular erythematous rash (often resembling a sunburn), with subsequent desquamation, hypotension, and involvement of at least 3 organ systems. Blood cultures, cerebrospinal cultures, and serologies for other organisms should be negative; although Staphylococcus and Streptococcus species may be isolated, they frequently are not (Table 1).[3]
| Diagnostic Criteria* | This Case |
|---|---|
| |
| Fever: Temperature 102.0F | Fever: 105.3F on admission |
| Rash: Diffuse macular erythroderma | Diffuse morbilliform rash with progression to confluent erythroderma |
| Desquamation of rash: occurs 12 weeks following rash onset | Desquamation 12 days after discharge |
| Hypotension: SBP 90 mm Hg for adults | Intermittent |
| Multisystem involvement, 3 of the following: | 4 organ systems definitively involved |
| GI: vomiting or diarrhea at disease onset | Vomiting and abdominal pain |
| Muscular: severe myalgias, or creatine phosphokinase >2 times the upper limit of normal | |
| Mucous membranes: vaginal, oropharyngeal, or conjunctival hyperemia | |
| Renal: BUN or Cr >2 times the upper limit of normal, or pyuria without evidence of infection | |
| Hepatic: total bilirubin, AST, or ALT levels >2 times the upper limit of normal | AST and ALT peaked at 128IU/L and 94 IU/L |
| Hematologic: platelets <100,000/mm3 | Platelet nadir of 80,000/mm3 |
| CNS: disorientation or altered consciousness without focal neurologic signs | Disorientation and somnolence |
| Probable case: 4 out of 5 clinical criteria present | |
| Confirmed case: 5 out of 5 clinical criteria present, or patient dies before desquamation can occur | |
A rare cause of shock, TSS is most associated with a surge of menstruation‐related cases linked to tampon use in young women in the 1980s.[4] However, in Centers for Disease Control and Prevention (CDC) surveillance between 1987 and 1996, only 59% of the 1069 cases identified were noted to be menstruation‐related, as compared to nearly 80% of all cases earlier in the decade.[4, 5] Today, the syndrome is more likely to present after musculoskeletal and cutaneous trauma, oropharyngeal infections, surgical procedures, and device implantation.[1, 6] Despite the disease's evolving epidemiology, the illness script used by physicians likely continues to focus on young women as the primary at risk population for TSS, causing physicians to neglect the diagnosis in other populations.[1, 6, 7, 8, 9] Given this change in risk factors, it is imperative that clinicians rewrite their scripts and recognize the early signs of TSS in all patients to enable quick and effective treatment.
In addition to its shifting epidemiology and rarity, the diagnosis of TSS vexes clinicians for several reasons. First, TSS cannot be quickly and definitively diagnosed because 2 diagnostic criteria cannot be fulfilled during the acute illness. The disease's hallmarka desquamative rashoccurs only if the patient survives.[3] Serologies often take weeks to return, further delaying diagnosis. During this period of diagnostic delay, the illness has usually already resolved or resulted in death. In addition, the presenting symptoms of rash, fever, and shock are nonspecific. Alternative etiologies include meningococcal meningitis, which can also present dramatically as with this patient; RMSF, which can occasionally have a fulminant presentation; bacterial sepsis, usually from Staphylococcus or Streptococcus species; acute viral syndromes; and severe drug reactions.[6, 10, 11, 12] Palmoplantar desquamation, as in this case, can further narrow the differential as this presentation is uncommon but characteristic of TSS, RMSF, and secondary syphilis.[11] Other diagnostic clues offered by the pattern of the rash may be limited by physician discomfort with diagnosing and describing rashes. Because of this lack of a definitive diagnostic test in the acute setting, it is imperative that the clinician include TSS in the differential of fever, shock, and rash, as mortality from TSS can exceed 20% in patients who are untreated.[13]
Treatment of TSS is straightforward once considered and includes the administration of antibiotics that cover both Staphylococcus and Streptococcus species, in addition to aggressive hydration and supportive care.[14] The final critical detail in this case was the appropriate arrangement of follow‐up. Given the patient's drastic improvement, the complicated process of arranging follow‐up for a transferred patient, and the current model where the hospitalists providing inpatient care do not typically follow their patients in clinic, patients such as these can easily be lost to follow‐up. Had this occurred, the desquamation would have been missed, and the patient's diagnosis would have been incomplete.
This patient was eventually diagnosed with TSS by fulfilling all 5 CDC criteria (Table 1).[3] He made a full recovery, likely aided by the administration of broad‐spectrum antibiotics (followed by doxycycline, which provided community‐acquired methicillin‐resistant S aureus coverage) and his lack of serious comorbidities. This case should serve as a reminder to hospitalists that with a discerning eye, a careful assessment of the clinical facts, and appropriate follow‐up, perhaps the next case of TSS can be caught red‐handed.
KEY POINTS
- When presented with a patient with fever, rash, and shock, hospitalists should consider meningococcal meningitis, RMSF bacterial sepsis, acute viral illness, severe drug reaction, and TSS.
- TSS, caused by S aureus or S pyogenes, is no longer predominantly associated with tampon use. Postsurgical infection and cutaneous trauma have become important present‐day risk factors.
- The initial presentation of TSS is nonspecific. Definitive diagnosis requires proper follow‐up, allowing time for infectious serologies to return negative and for the disease's hallmark desquamation to occur.
Disclosure
Nothing to report.
- . Toxic shock syndrome: major advances in pathogenesis, but not treatment. Crit Care Clin. 2013;29:651–675.
- . The toxic shock syndromes. Infect Dis Clin North Am. 1996;10(4):727–746.
- Centers for Disease Control and Prevention. National Notifiable Diseases Surveillance System. Toxic shock syndrome (other than Streptococcal) (TSS) 2011 Case Definition. Available at: http://wwwn.cdc.gov/nndss/conditions/toxic‐shock‐syndrome‐other‐than‐streptococcal/case‐definition/2011. Accessed June 4, 2015.
- Centers for Disease Control and Prevention. Update: toxic‐shock syndrome—United States. MMWR Morb Mortal Wkly Rep. 1983;32(30):398–400.
- , , , , , . Toxic shock syndrome in the United States: surveillance update, 1979–1996. Emerg Infect Dis. 1999;5(6):807–810.
- . Fever and rash. Infect Dis Clin North Am. 1996;10(1):101–110.
- , , , et al. Staphylococcal toxic shock syndrome 2000–2006: epidemiology, clinical features, and molecular characteristics. PLoS One. 2011;6(8):e22997.
- , , , et al. Toxic‐shock syndrome in menstruating women: association with tampon use and staphylococcus aureus and clinical features in 52 cases. N Engl J Med. 1980;303(25):1436–1442.
- , , , . Toxic‐shock syndrome—epidemiologic features, recurrence, risk factors, and prevention. N Engl J Med. 1980;303:1429–1435.
- , . Evaluating the febrile patient with a rash. Am Fam Physician. 2000;62(4):804–816.
- . Toxic shock syndrome: broadening the differential diagnosis. J Am Board Fam Pract. 2001;14(2):131–136.
- , , , . Spatial clustering by disease severity among reported Rocky Mountain spotted fever cases in the United States, 2001–2005. Am J Trop Med Hyg. 2009;80(1):72–77.
- , , , et al. One in five mortality in non‐menstrual toxic shock syndrome versus no mortality in menstrual cases in a balanced French series of 55 cases. Eur J Clin Microbio Infect Dis. 2008;27(1):37–43.
- , . Gram‐positive toxic shock syndromes. Lancet Infect Dis. 2009;9(5):281–290.
- . Toxic shock syndrome: major advances in pathogenesis, but not treatment. Crit Care Clin. 2013;29:651–675.
- . The toxic shock syndromes. Infect Dis Clin North Am. 1996;10(4):727–746.
- Centers for Disease Control and Prevention. National Notifiable Diseases Surveillance System. Toxic shock syndrome (other than Streptococcal) (TSS) 2011 Case Definition. Available at: http://wwwn.cdc.gov/nndss/conditions/toxic‐shock‐syndrome‐other‐than‐streptococcal/case‐definition/2011. Accessed June 4, 2015.
- Centers for Disease Control and Prevention. Update: toxic‐shock syndrome—United States. MMWR Morb Mortal Wkly Rep. 1983;32(30):398–400.
- , , , , , . Toxic shock syndrome in the United States: surveillance update, 1979–1996. Emerg Infect Dis. 1999;5(6):807–810.
- . Fever and rash. Infect Dis Clin North Am. 1996;10(1):101–110.
- , , , et al. Staphylococcal toxic shock syndrome 2000–2006: epidemiology, clinical features, and molecular characteristics. PLoS One. 2011;6(8):e22997.
- , , , et al. Toxic‐shock syndrome in menstruating women: association with tampon use and staphylococcus aureus and clinical features in 52 cases. N Engl J Med. 1980;303(25):1436–1442.
- , , , . Toxic‐shock syndrome—epidemiologic features, recurrence, risk factors, and prevention. N Engl J Med. 1980;303:1429–1435.
- , . Evaluating the febrile patient with a rash. Am Fam Physician. 2000;62(4):804–816.
- . Toxic shock syndrome: broadening the differential diagnosis. J Am Board Fam Pract. 2001;14(2):131–136.
- , , , . Spatial clustering by disease severity among reported Rocky Mountain spotted fever cases in the United States, 2001–2005. Am J Trop Med Hyg. 2009;80(1):72–77.
- , , , et al. One in five mortality in non‐menstrual toxic shock syndrome versus no mortality in menstrual cases in a balanced French series of 55 cases. Eur J Clin Microbio Infect Dis. 2008;27(1):37–43.
- , . Gram‐positive toxic shock syndromes. Lancet Infect Dis. 2009;9(5):281–290.
Betting the Farm
A 65‐year‐old man with a 6‐month history of diabetes mellitus presented to the emergency department in May with 1 week of fevers, headaches, myalgia, polydipsia, and polyuria.
The patient presents with symptoms suggestive of uncontrolled diabetes and infection. The broad diagnostic categories include acute infection, an emerging chronic process aggravating his diabetes, or a noninfectious mimic such as autoimmune disease or lymphoproliferative disease. New onset headache in an older patient is concerning. Although it may be attributed to fever and dehydration, primary central nervous system processes such as meningitis or encephalitis must be considered. At this stage, a detailed exposure history, including travel, food, pets, hobbies, and sick contacts as well as occupation and national origins is needed. This patient presented in May, making illnesses that peak in other seasons such as influenza and West Nile fever less likely.
He had no other medical problems except diabetes. He was not taking any medications; he had been started on glipizide but had stopped taking it 1 month prior. He denied fever, cough, chest pain, palpitations, abdominal pain, nausea, vomiting, dysuria, focal weakness, visual changes, or photophobia. He was born in Mexico and emigrated at the age of 25 years. Two months prior to presentation he visited a cattle farm in Mexico; he denied any direct contact with farm animals or dairy products. He denied ill contacts, pets, known tuberculosis exposures, and sexual partners other than his wife.
The history of recent travel to Mexico with a visit to a farm raises concerns about zoonoses. The endemic zoonoses that should be considered include parasitic (toxoplasmosis), fungal (coccidiodomycosis), and bacterial (brucellosis, Q fever, leptospirosis, tularemia, salmonellosis) infections. Nonzoonotic granulomatous infections such as cytomegalovirus (CMV) and Epstein‐Barr virus (EBV), mycobacteria, fungi (histoplasmosis, blastomycosis, cryptococcosis, aspergillosis), and bacteria (actinomycosis) should also be considered.
On examination, he was an elderly Hispanic male who appeared ill but in no acute distress. He was overweight, with a BMI of 29. His temperature was 39C, pulse 66 beats/minute, blood pressure 108/68 mm Hg, respiratory rate 18 per minute, and oxygen saturation was 96% on room air. There were no ulcerations, exudates, or erythema in the oropharynx. There was no sinus tenderness or lymphadenopathy. Cardiac examination revealed normal heart sounds with no murmurs. Respiratory examination demonstrated clear lungs. His abdomen was soft and nontender, whereas the liver and spleen were not palpable. There was no nuchal rigidity, and his mental status was normal. There were no cranial nerve deficits or weakness in his extremities. There was no skin rash or peripheral stigmata of infectious endocarditis. Genitourinary examination revealed no ulcerations, inguinal lymphadenopathy, or urethral discharge. There was no tenderness, warmth, or erythema on examination of all joints.
The physical exam is notable for temperaturepulse dissociation. Heart rate should increase by about 10 beats/minute for every 1‐degree increase in Fahrenheit temperature. The infectious causes of temperaturepulse dissociation are largely intracellular pathogens such as Salmonella, Coxiella, Chlamydia, Leptospira, Legionella, Francisella, Mycoplasma, and dengue virus. This patient is at increased risk for infection by any of these pathogens based on his recent travel to Mexico. Drug fever is the most common noninfectious cause of temperaturepulse dissociation, but this patient took no medications. At this point, a complete blood count and differential, urinalysis, blood cultures, chest x‐ray, and electrocardiogram should be ordered. Testing for human immunodeficiency virus (HIV) is appropriate, as up to 50% of patients with newly diagnosed HIV have no acknowledged risk factors. Serological studies for the aforementioned pathogens may be indicated depending on the results of these initial diagnostic tests.
Serum sodium concentration was 122 mEq/L, potassium 4.0 mEq/L, chloride 88 mEq/L, bicarbonate 14 mEq/L, blood urea nitrogen 17 mg/dL, creatinine 0.7 mg/dL, glucose 402 mg/dL, and calcium 8.5 mg/dL. Total protein was 5.4 g/dL, albumin 2.9 g/dL, total bilirubin 0.9 mg/dL, direct bilirubin 0.4 mg/dL, alkaline phosphatase 126 U/L (normal 53128), gamma‐glutamyl transferase 264 U/L (normal 360), aspartate aminotransferase 51 U/L (normal 840), alanine aminotransferase 62 U/L (normal 556), and lactate dehydrogenase 248 U/L (normal 85210). The white blood cell (WBC) count was 6800 mm3 (51% band forms, 38% segmented neutrophils, 6% monocytes, 5% lymphocytes). The hemoglobin was 15.7 g/dL, with mean corpuscular volume (MCV) of 102 fL and platelet count 59,000/mm3. Peripheral‐blood smear showed occasional macrocytes. Prothrombin time was 13.6 seconds and partial thromboplastin time was 34.5 seconds. C‐reactive protein was 11.8 mg/dL. Urinalysis revealed 80 mg of ketones per deciliter, no cells, and nitrite was negative. Hemoglobin A1c was 13%, and HIV antibody testing was negative.
Elevated circulating bands and thrombocytopenia suggest infection; however, bone marrow infiltration by infectious or neoplastic process is also possible and should be investigated. The increased gamma‐glutamyl transferase, alkaline phosphatase, and mild increases in transaminases suggest hepatic pathology. The combination of unexplained fever, hyponatremia, thrombocytopenia, elevated liver enzymes, and travel to Mexico mandates investigation for infectious diseases that often involve both the bone marrow and liver such as Brucella, Coxiella, and fungal infections such as histoplasmosis. Autoimmune diseases such as systemic lupus erythematosus and malignancy should also be considered. Blood cultures should be incubated beyond the usual 5 days because of the slower growth of Brucella or Salmonella typhi. An HIV viral load should be obtained to evaluate for acute retroviral syndrome. Serologic tests for Rickettsia, Coccidiodes, and hepatitis A, B, and C viruses should be obtained. Urine should be tested for Histoplasma and Legionella antigens. Abdominal imaging should be obtained to evaluate for hepatobiliary disease, occult intra‐abdominal abscess, or malignancy. Because the patient has unexplained fever and headache, imaging of the central nervous system and lumbar puncture are warranted.
His diabetic ketoacidosis (DKA) was treated with intravenous fluids and insulin. Lumbar puncture and cerebrospinal fluid (CSF) analysis revealed opening pressure of 18 cm H20 (normal 1025), cell count WBC 3/L (normal 05), red blood cell 204/L (normal 0), CSF protein 25 mg/dL (normal 2050), and glucose 68 mg/dL (normal 5070). Blood cultures showed no growth. HIV RNA was undetectable. Hepatitis C antibody was negative, and hepatitis A and B serologies were not consistent with an acute infection. Serum ferritin was 1147 ng/mL. Histoplasma and Legionella urine antigen tests were negative. CMV, EBV, and herpes simplex virus DNA were not detected in blood samples. Anti‐neutrophil antibody, anti‐mitochondrial antibody and anti‐neutrophil cytoplasmic antibodies were undetectable. Anti‐smooth muscle antibody was positive at a titer of 1:80. Transthoracic echocardiogram revealed normal heart valves without vegetations. A chest radiograph was normal. Brain computed tomography (CT) revealed atrophic frontal lobes. CT of his chest, abdomen, and pelvis demonstrated focal inflammatory changes of a loop of distal small bowel with surrounding fluid collection, suggesting small bowel diverticulitis. There were no pulmonary infiltrates noted, and the remainder of the CT was unremarkable.
Because the patient remains ill and additional serological test results will take time to return, a key consideration at this point is empiric treatment while awaiting test results. The CSF examination was normal. A history of travel including animal and tick exposures should be reevaluated. The timing of the trip to Mexico was outside the usual incubation period for many pathogens except for Coxiella or Brucella, and empiric therapy for both would be appropriate. The abdominal CT suggests small bowel diverticulitis, which is a rare clinical entity.
The benign abdominal examination suggests the finding is incidental. However, there are several infections that may involve the distal small bowel and proximal colon, such as yersiniosis, salmonellosis, tuberculosis, actinomycosis, histoplasmosis, and noninfectious processes including Crohn's disease and neoplasia. The absence of diarrhea or hematochezia makes yersiniosis, salmonellosis, and Crohn's disease unlikely. Histoplasmosis is unlikely given the negative urine antigen. Evaluation for neoplasia of the distal small bowel requires histologic examination. A colonoscopy with random biopsies of the colon and terminal ileum is the next step if other tests are unrevealing.
The patient was empirically treated for small bowel diverticulitis with ceftriaxone and metronidazole. Because of continued daily fevers as high as 39C, his therapy was changed to vancomycin and piperacillin‐tazobactam to cover methicillin‐resistant Staphylococcus aureus and resistant gram‐negative bacilli. The patient developed new scleral icterus on hospital day 6; the remainder of his examination was unchanged. Serum sodium concentration was 127 mEq/L, potassium 2.7 mEq/L, phosphorus 1.3 mg/dL, magnesium 1.6 mg/dL, total bilirubin 5.6 mg/dL, direct bilirubin 3.6 mg/dL, alkaline phosphatase 193 U/L, gamma‐glutamyl transferase 300 U/L, aspartate aminotransferase 91 U/L, alanine aminotransferase 52 U/L. Brucella serology was negative.
His liver enzymes remain elevated with new onset jaundice consistent with hepatitis and intrahepatic cholestasis. His persistent hypophosphatemia, hypokalemia, and hypomagnesaemia well after resolution of diabetic ketoacidosis suggests acute tubulointerstitial dysfunction, which may be a complication of empiric antibiotic treatment or renal involvement by his underlying condition. Additional blood cultures, and tissue examination and culture are the next appropriate steps. Liver or bone marrow biopsy may suggest a diagnosis that can be confirmed by tissue culture or immunohistochemistry. Histologic findings such as fibrin ringed granulomas, caseating or noncaseating granulomas, or lymphomatous infiltration may suggest Coxiella (Q fever), tuberculosis, or lymphoma respectively. Because a liver biopsy is invasive and usually provides less tissue for culture, bone marrow examination should be obtained first.
A gallium 67 scan showed nonhomogenous increased uptake in both lungs and kidneys, consistent with interstitial nephritis and bilateral pneumonia. Serum protein electrophoresis demonstrated a monoclonal immunoglobulin (Ig)G lambda band with a kappa/lambda ratio of 0.9 (normal 1.42.8). Bone marrow biopsy showed normal hematopoiesis; no plasma or malignant cells, granulomas, or evidence of hemophagocytosis; and fungal and mycobacterial stains and cultures were negative. Colonoscopy revealed normal‐appearing mucosa. Histologic examination and culture of random biopsies from the colon and terminal ileum were negative for fungi, viruses, and mycobacteria. An ultrasound‐guided liver biopsy revealed numerous noncaseating granulomas formed of histiocytes and neutrophils with occasional fibrin rings. Fungal, viral, and mycobacterial stains and cultures were negative. The patient's fever resolved after 14 days, and he was discharged home without a diagnosis and close outpatient follow‐up.
The hepatic granulomas with fibrin rings are highly suggestive of Q fever, although ring granulomas may be seen in tuberculosis, typhoid fever, lymphoma, drug reactions, sarcoidosis, and CMV infections. Competing diagnoses such as CMV have been excluded by negative serology. Microscopic examination, tissue staining, and culture from liver and bone marrow biopsies were negative for S typhi, mycobacteria, and lymphoma. Gallium scan findings are generally nonspecific and of little utility in cases such as this. The kidney involvement correlates with the biochemical evidence of tubulointerstitial dysfunction; pulmonary involvement may reflect subclinical pulmonary infection with Coxiella. Given the normal bone marrow biopsy, the monoclonal gammopathy is of undetermined significance. The positive anti‐smooth muscle antibody can be related to Q fever. Anti‐smooth muscle antibodies frequently occur in Q fever, especially in those patients with hepatitis. Given the history of exposure to cattle, unexplained fever with temperaturepulse dissociation and liver biopsy findings, Q fever is the most likely diagnosis and empiric treatment with doxycycline is warranted.
Results of serology for Coxiella burnetii sent during admission were returned after the patient's discharge. C burnetii phase I IgG and IgM antibody titers were positive (1:512 each). C burnetii phase II IgG and IgM titers also were positive (1:1024 each). The patient was seen within a week and started on doxycycline 100 mg twice daily for 2 weeks for acute Q fever. His symptoms improved; hyponatremia, liver function tests, and thrombocytopenia normalized after treatment.
DISCUSSION
Q fever was first described in 1937 as a febrile illness affecting Australian slaughterhouse workers.[1] The Q in Q fever stands for query and reflected the initial uncertainty surrounding the underlying cause of the illness. The causative organism, C burnetti, is an obligate intracellular bacterium that resides within macrophage lysosomes. It can be found in the urine, feces, milk, placenta, and amniotic fluid of ungulates (cattle, sheep, and other ruminants), and other animals such as domestic cats and dogs. C burnetii is transmitted via inhalation, ingestion, occupational, or common source exposures, and in 1 case report by person‐to‐person sexual transmission.[2] In addition to slaughterhouse workers, pregnant women and immunosuppressed patients are more susceptible to developing Q fever.[3] For patients with suspected Q fever, a detailed occupational history, including specific job duties and potential exposure to animal products, is imperative.
Q fever has both acute and chronic presentations, which are differentiated based on the clinical illness and serologies. The symptoms of acute Q fever are nonspecific and may include influenza‐like illness, fever, pneumonia, and hepatitis. It presents less commonly with hemolytic anemia, interstitial nephritis, monoclonal gammopathy, or aseptic meningitis.[4, 5, 6, 7] Symptoms typically begin between 1 and 3 weeks after animal exposure and may persist for several months. Chronic Q fever occurs when unrecognized or untreated infection persists for greater than 6 months. It commonly presents with culture‐negative endocarditis, although infected aneurysms, osteomyelitis, or other distant sites of infection may also occur.
C burnetti is present in 2 antigenic forms that can be assessed by serology. Phase I is the more virulent, infectious form of C burnetti, which transitions to the avirulent phase II form during laboratory handling. In acute Q fever, phase II serologies are typically elevated out of proportion to phase I serologies, whereas this pattern is reversed in chronic Q fever. The diagnostic gold standard of acute Q fever is a 4‐fold rise in phase II antibody titers taken 3 to 6 weeks apart.[8] Histologic examination of affected organs can support a diagnosis of Q fever. The presence of ringed granulomas on liver or bone marrow biopsy specimens is highly suggestive, but not pathognomonic, of Q fever.[9]
Q fever is highly susceptible to several classes of antibiotics. For acute Q fever, doxycycline and tetracycline are typically used, with fluoroquinolones and chloramphenicol as alternatives.[8, 10] Patients with chronic Q fever should be treated with doxycycline and hydroxychloroquine. The addition of hydroxychloroquine alkalinizes the macrophage lysosome and enhances bacterial eradication.[8] For patients with acute Q fever, physicians should determine the risk of progression to chronic Q fever because closer monitoring is necessary. Patients with valvular heart lesions, immunosuppression, and pregnant women are at elevated risk of chronic Q fever. Trimethoprim/sulfamethoxazole can be used in place of doxycycline in pregnant women, as doxycycline and fluoroquinolones are contraindicated in pregnancy.[8]
This patient presented with a nonspecific febrile illness. Although the treating clinicians obtained a history of exposure to cattle early in his course, both the diagnosis and treatment were delayed. There are several possible explanations for the delay. First, although Q fever is a relatively common zoonosis, it remains an uncommon diagnosis, particularly among hospitalized patients. As a result, clinicians often focus on more common conditions. In this case, typical infections, malignancies, and inflammatory diseases were considered more likely. Second, the patient presented with hepatitis, an uncommon presentation of Q fever. Classical clinical reasoning suggests that atypical presentations of common diseases will occur more frequently than typical presentations of uncommon diseases. This case presented with an atypical presentation of an uncommon disease. The resultant lower pretest probability further dissuaded the patient's physicians from consideration of Q fever. Third, the finding of small bowel diverticulitis was a potential distractor. In patients with nonspecific febrile illnesses, it is common for physicians to anchor on any abnormal findings. In this case, the small bowel diverticulitis led to antibiotic treatment that was ineffective against C burnetti.
There were several clues to the diagnosis of Q fever in this patient's presentation. First, the pulsetemperature dissociation suggested infection with an intracellular pathogen. Hospitalists should recognize this association and be mindful of this often‐subtle clinical finding when faced with diagnostic uncertainty. Second, the patient was exposed to cattle prior to the onset of his illness. The fact that he did not have a direct exposure to animals underscores the infectivity of C burnetti. Finally, elevated alkaline phosphatase and transaminases were suggestive of an infiltrative disease; in the setting of a nonspecific febrile illness, Q fever was an important diagnostic consideration.
The key treatment decision in this case was the initiation and choice of antibiotics. Because of this patient's history of exposure to cattle and lack of a compelling alternative diagnosis, empiric treatment with doxycycline would have been appropriate. Hospitalists must weigh the potential benefit of early treatment of Q fever against the risks associated with antibiotic overuse. In patients presenting with a febrile illness after ungulate exposure, the decision to bet the farm with empiric doxycycline therapy may lead to clinical improvement, obviating a more invasive or extensive diagnostic evaluation.
TEACHING POINTS
- Acute Q fever typically presents 2 to 3 weeks after ungulate exposure with a febrile illness, pneumonia, and granulomatous hepatitis.
- Pulsetemperature dissociation is suggestive of infection by intracellular pathogens such as Coxiella, Salmonella, Leptospira, Legionella, and Mycoplasma.
- Clinicians should consider empiric doxycycline therapy in patients with suspected zoonosis (eg, Q fever, brucellosis, anaplasmosis, leptospirosis, Rocky Mountain spotted fever) while awaiting confirmatory tests, as improvement may obviate invasive testing.
Disclosure: Nothing to report.
- . “Q” fever, a new fever entity: clinical features, diagnosis, and laboratory investigation. Rev Infect Dis. 1983;5(4):790–800.
- , , , . Q fever: a biological weapon in your backyard. Lancet Infect Dis. 2003;3(11):709–721.
- , , , . Role of sex, age, previous valve lesion, and pregnancy in the clinical expression and outcome of Q fever after a large outbreak. Clin Infect Dis. 2007;15:44(2):232–237.
- , , , , . Unusual manifestations of acute Q fever: autoimmune hemolytic anemia and tubulointerstitial nephritis. Ann of Clin Microbiol Antimicrob. 2012;11:14.
- , , . Q fever. Lancet. 2006;367(9511):679–688.
- , , , , , . Transitory monoclonal gammopathy and acute Q fever. Enferm Infecc Microbiol Clin. 1995;13(7):442.
- , . Q fever. Clin Microbiol Rev. 1999;12(4):518–553.
- , , , et al. Diagnosis and management of Q fever—United States, 2013: recommendations from CDC and the Q Fever Working Group. MMWR Recomm Rep. 2013;62(RR‐03):1–30.
- , , , et al. Hepatic fibrin‐ring granulomas: a clinicopathologic study of 23 patients. Hum Pathol. 1991;22(6):607–613.
- , , . Travel‐associated zoonotic bacterial diseases. Curr Opin Infect Dis. 2011;24(5):457–463.
A 65‐year‐old man with a 6‐month history of diabetes mellitus presented to the emergency department in May with 1 week of fevers, headaches, myalgia, polydipsia, and polyuria.
The patient presents with symptoms suggestive of uncontrolled diabetes and infection. The broad diagnostic categories include acute infection, an emerging chronic process aggravating his diabetes, or a noninfectious mimic such as autoimmune disease or lymphoproliferative disease. New onset headache in an older patient is concerning. Although it may be attributed to fever and dehydration, primary central nervous system processes such as meningitis or encephalitis must be considered. At this stage, a detailed exposure history, including travel, food, pets, hobbies, and sick contacts as well as occupation and national origins is needed. This patient presented in May, making illnesses that peak in other seasons such as influenza and West Nile fever less likely.
He had no other medical problems except diabetes. He was not taking any medications; he had been started on glipizide but had stopped taking it 1 month prior. He denied fever, cough, chest pain, palpitations, abdominal pain, nausea, vomiting, dysuria, focal weakness, visual changes, or photophobia. He was born in Mexico and emigrated at the age of 25 years. Two months prior to presentation he visited a cattle farm in Mexico; he denied any direct contact with farm animals or dairy products. He denied ill contacts, pets, known tuberculosis exposures, and sexual partners other than his wife.
The history of recent travel to Mexico with a visit to a farm raises concerns about zoonoses. The endemic zoonoses that should be considered include parasitic (toxoplasmosis), fungal (coccidiodomycosis), and bacterial (brucellosis, Q fever, leptospirosis, tularemia, salmonellosis) infections. Nonzoonotic granulomatous infections such as cytomegalovirus (CMV) and Epstein‐Barr virus (EBV), mycobacteria, fungi (histoplasmosis, blastomycosis, cryptococcosis, aspergillosis), and bacteria (actinomycosis) should also be considered.
On examination, he was an elderly Hispanic male who appeared ill but in no acute distress. He was overweight, with a BMI of 29. His temperature was 39C, pulse 66 beats/minute, blood pressure 108/68 mm Hg, respiratory rate 18 per minute, and oxygen saturation was 96% on room air. There were no ulcerations, exudates, or erythema in the oropharynx. There was no sinus tenderness or lymphadenopathy. Cardiac examination revealed normal heart sounds with no murmurs. Respiratory examination demonstrated clear lungs. His abdomen was soft and nontender, whereas the liver and spleen were not palpable. There was no nuchal rigidity, and his mental status was normal. There were no cranial nerve deficits or weakness in his extremities. There was no skin rash or peripheral stigmata of infectious endocarditis. Genitourinary examination revealed no ulcerations, inguinal lymphadenopathy, or urethral discharge. There was no tenderness, warmth, or erythema on examination of all joints.
The physical exam is notable for temperaturepulse dissociation. Heart rate should increase by about 10 beats/minute for every 1‐degree increase in Fahrenheit temperature. The infectious causes of temperaturepulse dissociation are largely intracellular pathogens such as Salmonella, Coxiella, Chlamydia, Leptospira, Legionella, Francisella, Mycoplasma, and dengue virus. This patient is at increased risk for infection by any of these pathogens based on his recent travel to Mexico. Drug fever is the most common noninfectious cause of temperaturepulse dissociation, but this patient took no medications. At this point, a complete blood count and differential, urinalysis, blood cultures, chest x‐ray, and electrocardiogram should be ordered. Testing for human immunodeficiency virus (HIV) is appropriate, as up to 50% of patients with newly diagnosed HIV have no acknowledged risk factors. Serological studies for the aforementioned pathogens may be indicated depending on the results of these initial diagnostic tests.
Serum sodium concentration was 122 mEq/L, potassium 4.0 mEq/L, chloride 88 mEq/L, bicarbonate 14 mEq/L, blood urea nitrogen 17 mg/dL, creatinine 0.7 mg/dL, glucose 402 mg/dL, and calcium 8.5 mg/dL. Total protein was 5.4 g/dL, albumin 2.9 g/dL, total bilirubin 0.9 mg/dL, direct bilirubin 0.4 mg/dL, alkaline phosphatase 126 U/L (normal 53128), gamma‐glutamyl transferase 264 U/L (normal 360), aspartate aminotransferase 51 U/L (normal 840), alanine aminotransferase 62 U/L (normal 556), and lactate dehydrogenase 248 U/L (normal 85210). The white blood cell (WBC) count was 6800 mm3 (51% band forms, 38% segmented neutrophils, 6% monocytes, 5% lymphocytes). The hemoglobin was 15.7 g/dL, with mean corpuscular volume (MCV) of 102 fL and platelet count 59,000/mm3. Peripheral‐blood smear showed occasional macrocytes. Prothrombin time was 13.6 seconds and partial thromboplastin time was 34.5 seconds. C‐reactive protein was 11.8 mg/dL. Urinalysis revealed 80 mg of ketones per deciliter, no cells, and nitrite was negative. Hemoglobin A1c was 13%, and HIV antibody testing was negative.
Elevated circulating bands and thrombocytopenia suggest infection; however, bone marrow infiltration by infectious or neoplastic process is also possible and should be investigated. The increased gamma‐glutamyl transferase, alkaline phosphatase, and mild increases in transaminases suggest hepatic pathology. The combination of unexplained fever, hyponatremia, thrombocytopenia, elevated liver enzymes, and travel to Mexico mandates investigation for infectious diseases that often involve both the bone marrow and liver such as Brucella, Coxiella, and fungal infections such as histoplasmosis. Autoimmune diseases such as systemic lupus erythematosus and malignancy should also be considered. Blood cultures should be incubated beyond the usual 5 days because of the slower growth of Brucella or Salmonella typhi. An HIV viral load should be obtained to evaluate for acute retroviral syndrome. Serologic tests for Rickettsia, Coccidiodes, and hepatitis A, B, and C viruses should be obtained. Urine should be tested for Histoplasma and Legionella antigens. Abdominal imaging should be obtained to evaluate for hepatobiliary disease, occult intra‐abdominal abscess, or malignancy. Because the patient has unexplained fever and headache, imaging of the central nervous system and lumbar puncture are warranted.
His diabetic ketoacidosis (DKA) was treated with intravenous fluids and insulin. Lumbar puncture and cerebrospinal fluid (CSF) analysis revealed opening pressure of 18 cm H20 (normal 1025), cell count WBC 3/L (normal 05), red blood cell 204/L (normal 0), CSF protein 25 mg/dL (normal 2050), and glucose 68 mg/dL (normal 5070). Blood cultures showed no growth. HIV RNA was undetectable. Hepatitis C antibody was negative, and hepatitis A and B serologies were not consistent with an acute infection. Serum ferritin was 1147 ng/mL. Histoplasma and Legionella urine antigen tests were negative. CMV, EBV, and herpes simplex virus DNA were not detected in blood samples. Anti‐neutrophil antibody, anti‐mitochondrial antibody and anti‐neutrophil cytoplasmic antibodies were undetectable. Anti‐smooth muscle antibody was positive at a titer of 1:80. Transthoracic echocardiogram revealed normal heart valves without vegetations. A chest radiograph was normal. Brain computed tomography (CT) revealed atrophic frontal lobes. CT of his chest, abdomen, and pelvis demonstrated focal inflammatory changes of a loop of distal small bowel with surrounding fluid collection, suggesting small bowel diverticulitis. There were no pulmonary infiltrates noted, and the remainder of the CT was unremarkable.
Because the patient remains ill and additional serological test results will take time to return, a key consideration at this point is empiric treatment while awaiting test results. The CSF examination was normal. A history of travel including animal and tick exposures should be reevaluated. The timing of the trip to Mexico was outside the usual incubation period for many pathogens except for Coxiella or Brucella, and empiric therapy for both would be appropriate. The abdominal CT suggests small bowel diverticulitis, which is a rare clinical entity.
The benign abdominal examination suggests the finding is incidental. However, there are several infections that may involve the distal small bowel and proximal colon, such as yersiniosis, salmonellosis, tuberculosis, actinomycosis, histoplasmosis, and noninfectious processes including Crohn's disease and neoplasia. The absence of diarrhea or hematochezia makes yersiniosis, salmonellosis, and Crohn's disease unlikely. Histoplasmosis is unlikely given the negative urine antigen. Evaluation for neoplasia of the distal small bowel requires histologic examination. A colonoscopy with random biopsies of the colon and terminal ileum is the next step if other tests are unrevealing.
The patient was empirically treated for small bowel diverticulitis with ceftriaxone and metronidazole. Because of continued daily fevers as high as 39C, his therapy was changed to vancomycin and piperacillin‐tazobactam to cover methicillin‐resistant Staphylococcus aureus and resistant gram‐negative bacilli. The patient developed new scleral icterus on hospital day 6; the remainder of his examination was unchanged. Serum sodium concentration was 127 mEq/L, potassium 2.7 mEq/L, phosphorus 1.3 mg/dL, magnesium 1.6 mg/dL, total bilirubin 5.6 mg/dL, direct bilirubin 3.6 mg/dL, alkaline phosphatase 193 U/L, gamma‐glutamyl transferase 300 U/L, aspartate aminotransferase 91 U/L, alanine aminotransferase 52 U/L. Brucella serology was negative.
His liver enzymes remain elevated with new onset jaundice consistent with hepatitis and intrahepatic cholestasis. His persistent hypophosphatemia, hypokalemia, and hypomagnesaemia well after resolution of diabetic ketoacidosis suggests acute tubulointerstitial dysfunction, which may be a complication of empiric antibiotic treatment or renal involvement by his underlying condition. Additional blood cultures, and tissue examination and culture are the next appropriate steps. Liver or bone marrow biopsy may suggest a diagnosis that can be confirmed by tissue culture or immunohistochemistry. Histologic findings such as fibrin ringed granulomas, caseating or noncaseating granulomas, or lymphomatous infiltration may suggest Coxiella (Q fever), tuberculosis, or lymphoma respectively. Because a liver biopsy is invasive and usually provides less tissue for culture, bone marrow examination should be obtained first.
A gallium 67 scan showed nonhomogenous increased uptake in both lungs and kidneys, consistent with interstitial nephritis and bilateral pneumonia. Serum protein electrophoresis demonstrated a monoclonal immunoglobulin (Ig)G lambda band with a kappa/lambda ratio of 0.9 (normal 1.42.8). Bone marrow biopsy showed normal hematopoiesis; no plasma or malignant cells, granulomas, or evidence of hemophagocytosis; and fungal and mycobacterial stains and cultures were negative. Colonoscopy revealed normal‐appearing mucosa. Histologic examination and culture of random biopsies from the colon and terminal ileum were negative for fungi, viruses, and mycobacteria. An ultrasound‐guided liver biopsy revealed numerous noncaseating granulomas formed of histiocytes and neutrophils with occasional fibrin rings. Fungal, viral, and mycobacterial stains and cultures were negative. The patient's fever resolved after 14 days, and he was discharged home without a diagnosis and close outpatient follow‐up.
The hepatic granulomas with fibrin rings are highly suggestive of Q fever, although ring granulomas may be seen in tuberculosis, typhoid fever, lymphoma, drug reactions, sarcoidosis, and CMV infections. Competing diagnoses such as CMV have been excluded by negative serology. Microscopic examination, tissue staining, and culture from liver and bone marrow biopsies were negative for S typhi, mycobacteria, and lymphoma. Gallium scan findings are generally nonspecific and of little utility in cases such as this. The kidney involvement correlates with the biochemical evidence of tubulointerstitial dysfunction; pulmonary involvement may reflect subclinical pulmonary infection with Coxiella. Given the normal bone marrow biopsy, the monoclonal gammopathy is of undetermined significance. The positive anti‐smooth muscle antibody can be related to Q fever. Anti‐smooth muscle antibodies frequently occur in Q fever, especially in those patients with hepatitis. Given the history of exposure to cattle, unexplained fever with temperaturepulse dissociation and liver biopsy findings, Q fever is the most likely diagnosis and empiric treatment with doxycycline is warranted.
Results of serology for Coxiella burnetii sent during admission were returned after the patient's discharge. C burnetii phase I IgG and IgM antibody titers were positive (1:512 each). C burnetii phase II IgG and IgM titers also were positive (1:1024 each). The patient was seen within a week and started on doxycycline 100 mg twice daily for 2 weeks for acute Q fever. His symptoms improved; hyponatremia, liver function tests, and thrombocytopenia normalized after treatment.
DISCUSSION
Q fever was first described in 1937 as a febrile illness affecting Australian slaughterhouse workers.[1] The Q in Q fever stands for query and reflected the initial uncertainty surrounding the underlying cause of the illness. The causative organism, C burnetti, is an obligate intracellular bacterium that resides within macrophage lysosomes. It can be found in the urine, feces, milk, placenta, and amniotic fluid of ungulates (cattle, sheep, and other ruminants), and other animals such as domestic cats and dogs. C burnetii is transmitted via inhalation, ingestion, occupational, or common source exposures, and in 1 case report by person‐to‐person sexual transmission.[2] In addition to slaughterhouse workers, pregnant women and immunosuppressed patients are more susceptible to developing Q fever.[3] For patients with suspected Q fever, a detailed occupational history, including specific job duties and potential exposure to animal products, is imperative.
Q fever has both acute and chronic presentations, which are differentiated based on the clinical illness and serologies. The symptoms of acute Q fever are nonspecific and may include influenza‐like illness, fever, pneumonia, and hepatitis. It presents less commonly with hemolytic anemia, interstitial nephritis, monoclonal gammopathy, or aseptic meningitis.[4, 5, 6, 7] Symptoms typically begin between 1 and 3 weeks after animal exposure and may persist for several months. Chronic Q fever occurs when unrecognized or untreated infection persists for greater than 6 months. It commonly presents with culture‐negative endocarditis, although infected aneurysms, osteomyelitis, or other distant sites of infection may also occur.
C burnetti is present in 2 antigenic forms that can be assessed by serology. Phase I is the more virulent, infectious form of C burnetti, which transitions to the avirulent phase II form during laboratory handling. In acute Q fever, phase II serologies are typically elevated out of proportion to phase I serologies, whereas this pattern is reversed in chronic Q fever. The diagnostic gold standard of acute Q fever is a 4‐fold rise in phase II antibody titers taken 3 to 6 weeks apart.[8] Histologic examination of affected organs can support a diagnosis of Q fever. The presence of ringed granulomas on liver or bone marrow biopsy specimens is highly suggestive, but not pathognomonic, of Q fever.[9]
Q fever is highly susceptible to several classes of antibiotics. For acute Q fever, doxycycline and tetracycline are typically used, with fluoroquinolones and chloramphenicol as alternatives.[8, 10] Patients with chronic Q fever should be treated with doxycycline and hydroxychloroquine. The addition of hydroxychloroquine alkalinizes the macrophage lysosome and enhances bacterial eradication.[8] For patients with acute Q fever, physicians should determine the risk of progression to chronic Q fever because closer monitoring is necessary. Patients with valvular heart lesions, immunosuppression, and pregnant women are at elevated risk of chronic Q fever. Trimethoprim/sulfamethoxazole can be used in place of doxycycline in pregnant women, as doxycycline and fluoroquinolones are contraindicated in pregnancy.[8]
This patient presented with a nonspecific febrile illness. Although the treating clinicians obtained a history of exposure to cattle early in his course, both the diagnosis and treatment were delayed. There are several possible explanations for the delay. First, although Q fever is a relatively common zoonosis, it remains an uncommon diagnosis, particularly among hospitalized patients. As a result, clinicians often focus on more common conditions. In this case, typical infections, malignancies, and inflammatory diseases were considered more likely. Second, the patient presented with hepatitis, an uncommon presentation of Q fever. Classical clinical reasoning suggests that atypical presentations of common diseases will occur more frequently than typical presentations of uncommon diseases. This case presented with an atypical presentation of an uncommon disease. The resultant lower pretest probability further dissuaded the patient's physicians from consideration of Q fever. Third, the finding of small bowel diverticulitis was a potential distractor. In patients with nonspecific febrile illnesses, it is common for physicians to anchor on any abnormal findings. In this case, the small bowel diverticulitis led to antibiotic treatment that was ineffective against C burnetti.
There were several clues to the diagnosis of Q fever in this patient's presentation. First, the pulsetemperature dissociation suggested infection with an intracellular pathogen. Hospitalists should recognize this association and be mindful of this often‐subtle clinical finding when faced with diagnostic uncertainty. Second, the patient was exposed to cattle prior to the onset of his illness. The fact that he did not have a direct exposure to animals underscores the infectivity of C burnetti. Finally, elevated alkaline phosphatase and transaminases were suggestive of an infiltrative disease; in the setting of a nonspecific febrile illness, Q fever was an important diagnostic consideration.
The key treatment decision in this case was the initiation and choice of antibiotics. Because of this patient's history of exposure to cattle and lack of a compelling alternative diagnosis, empiric treatment with doxycycline would have been appropriate. Hospitalists must weigh the potential benefit of early treatment of Q fever against the risks associated with antibiotic overuse. In patients presenting with a febrile illness after ungulate exposure, the decision to bet the farm with empiric doxycycline therapy may lead to clinical improvement, obviating a more invasive or extensive diagnostic evaluation.
TEACHING POINTS
- Acute Q fever typically presents 2 to 3 weeks after ungulate exposure with a febrile illness, pneumonia, and granulomatous hepatitis.
- Pulsetemperature dissociation is suggestive of infection by intracellular pathogens such as Coxiella, Salmonella, Leptospira, Legionella, and Mycoplasma.
- Clinicians should consider empiric doxycycline therapy in patients with suspected zoonosis (eg, Q fever, brucellosis, anaplasmosis, leptospirosis, Rocky Mountain spotted fever) while awaiting confirmatory tests, as improvement may obviate invasive testing.
Disclosure: Nothing to report.
A 65‐year‐old man with a 6‐month history of diabetes mellitus presented to the emergency department in May with 1 week of fevers, headaches, myalgia, polydipsia, and polyuria.
The patient presents with symptoms suggestive of uncontrolled diabetes and infection. The broad diagnostic categories include acute infection, an emerging chronic process aggravating his diabetes, or a noninfectious mimic such as autoimmune disease or lymphoproliferative disease. New onset headache in an older patient is concerning. Although it may be attributed to fever and dehydration, primary central nervous system processes such as meningitis or encephalitis must be considered. At this stage, a detailed exposure history, including travel, food, pets, hobbies, and sick contacts as well as occupation and national origins is needed. This patient presented in May, making illnesses that peak in other seasons such as influenza and West Nile fever less likely.
He had no other medical problems except diabetes. He was not taking any medications; he had been started on glipizide but had stopped taking it 1 month prior. He denied fever, cough, chest pain, palpitations, abdominal pain, nausea, vomiting, dysuria, focal weakness, visual changes, or photophobia. He was born in Mexico and emigrated at the age of 25 years. Two months prior to presentation he visited a cattle farm in Mexico; he denied any direct contact with farm animals or dairy products. He denied ill contacts, pets, known tuberculosis exposures, and sexual partners other than his wife.
The history of recent travel to Mexico with a visit to a farm raises concerns about zoonoses. The endemic zoonoses that should be considered include parasitic (toxoplasmosis), fungal (coccidiodomycosis), and bacterial (brucellosis, Q fever, leptospirosis, tularemia, salmonellosis) infections. Nonzoonotic granulomatous infections such as cytomegalovirus (CMV) and Epstein‐Barr virus (EBV), mycobacteria, fungi (histoplasmosis, blastomycosis, cryptococcosis, aspergillosis), and bacteria (actinomycosis) should also be considered.
On examination, he was an elderly Hispanic male who appeared ill but in no acute distress. He was overweight, with a BMI of 29. His temperature was 39C, pulse 66 beats/minute, blood pressure 108/68 mm Hg, respiratory rate 18 per minute, and oxygen saturation was 96% on room air. There were no ulcerations, exudates, or erythema in the oropharynx. There was no sinus tenderness or lymphadenopathy. Cardiac examination revealed normal heart sounds with no murmurs. Respiratory examination demonstrated clear lungs. His abdomen was soft and nontender, whereas the liver and spleen were not palpable. There was no nuchal rigidity, and his mental status was normal. There were no cranial nerve deficits or weakness in his extremities. There was no skin rash or peripheral stigmata of infectious endocarditis. Genitourinary examination revealed no ulcerations, inguinal lymphadenopathy, or urethral discharge. There was no tenderness, warmth, or erythema on examination of all joints.
The physical exam is notable for temperaturepulse dissociation. Heart rate should increase by about 10 beats/minute for every 1‐degree increase in Fahrenheit temperature. The infectious causes of temperaturepulse dissociation are largely intracellular pathogens such as Salmonella, Coxiella, Chlamydia, Leptospira, Legionella, Francisella, Mycoplasma, and dengue virus. This patient is at increased risk for infection by any of these pathogens based on his recent travel to Mexico. Drug fever is the most common noninfectious cause of temperaturepulse dissociation, but this patient took no medications. At this point, a complete blood count and differential, urinalysis, blood cultures, chest x‐ray, and electrocardiogram should be ordered. Testing for human immunodeficiency virus (HIV) is appropriate, as up to 50% of patients with newly diagnosed HIV have no acknowledged risk factors. Serological studies for the aforementioned pathogens may be indicated depending on the results of these initial diagnostic tests.
Serum sodium concentration was 122 mEq/L, potassium 4.0 mEq/L, chloride 88 mEq/L, bicarbonate 14 mEq/L, blood urea nitrogen 17 mg/dL, creatinine 0.7 mg/dL, glucose 402 mg/dL, and calcium 8.5 mg/dL. Total protein was 5.4 g/dL, albumin 2.9 g/dL, total bilirubin 0.9 mg/dL, direct bilirubin 0.4 mg/dL, alkaline phosphatase 126 U/L (normal 53128), gamma‐glutamyl transferase 264 U/L (normal 360), aspartate aminotransferase 51 U/L (normal 840), alanine aminotransferase 62 U/L (normal 556), and lactate dehydrogenase 248 U/L (normal 85210). The white blood cell (WBC) count was 6800 mm3 (51% band forms, 38% segmented neutrophils, 6% monocytes, 5% lymphocytes). The hemoglobin was 15.7 g/dL, with mean corpuscular volume (MCV) of 102 fL and platelet count 59,000/mm3. Peripheral‐blood smear showed occasional macrocytes. Prothrombin time was 13.6 seconds and partial thromboplastin time was 34.5 seconds. C‐reactive protein was 11.8 mg/dL. Urinalysis revealed 80 mg of ketones per deciliter, no cells, and nitrite was negative. Hemoglobin A1c was 13%, and HIV antibody testing was negative.
Elevated circulating bands and thrombocytopenia suggest infection; however, bone marrow infiltration by infectious or neoplastic process is also possible and should be investigated. The increased gamma‐glutamyl transferase, alkaline phosphatase, and mild increases in transaminases suggest hepatic pathology. The combination of unexplained fever, hyponatremia, thrombocytopenia, elevated liver enzymes, and travel to Mexico mandates investigation for infectious diseases that often involve both the bone marrow and liver such as Brucella, Coxiella, and fungal infections such as histoplasmosis. Autoimmune diseases such as systemic lupus erythematosus and malignancy should also be considered. Blood cultures should be incubated beyond the usual 5 days because of the slower growth of Brucella or Salmonella typhi. An HIV viral load should be obtained to evaluate for acute retroviral syndrome. Serologic tests for Rickettsia, Coccidiodes, and hepatitis A, B, and C viruses should be obtained. Urine should be tested for Histoplasma and Legionella antigens. Abdominal imaging should be obtained to evaluate for hepatobiliary disease, occult intra‐abdominal abscess, or malignancy. Because the patient has unexplained fever and headache, imaging of the central nervous system and lumbar puncture are warranted.
His diabetic ketoacidosis (DKA) was treated with intravenous fluids and insulin. Lumbar puncture and cerebrospinal fluid (CSF) analysis revealed opening pressure of 18 cm H20 (normal 1025), cell count WBC 3/L (normal 05), red blood cell 204/L (normal 0), CSF protein 25 mg/dL (normal 2050), and glucose 68 mg/dL (normal 5070). Blood cultures showed no growth. HIV RNA was undetectable. Hepatitis C antibody was negative, and hepatitis A and B serologies were not consistent with an acute infection. Serum ferritin was 1147 ng/mL. Histoplasma and Legionella urine antigen tests were negative. CMV, EBV, and herpes simplex virus DNA were not detected in blood samples. Anti‐neutrophil antibody, anti‐mitochondrial antibody and anti‐neutrophil cytoplasmic antibodies were undetectable. Anti‐smooth muscle antibody was positive at a titer of 1:80. Transthoracic echocardiogram revealed normal heart valves without vegetations. A chest radiograph was normal. Brain computed tomography (CT) revealed atrophic frontal lobes. CT of his chest, abdomen, and pelvis demonstrated focal inflammatory changes of a loop of distal small bowel with surrounding fluid collection, suggesting small bowel diverticulitis. There were no pulmonary infiltrates noted, and the remainder of the CT was unremarkable.
Because the patient remains ill and additional serological test results will take time to return, a key consideration at this point is empiric treatment while awaiting test results. The CSF examination was normal. A history of travel including animal and tick exposures should be reevaluated. The timing of the trip to Mexico was outside the usual incubation period for many pathogens except for Coxiella or Brucella, and empiric therapy for both would be appropriate. The abdominal CT suggests small bowel diverticulitis, which is a rare clinical entity.
The benign abdominal examination suggests the finding is incidental. However, there are several infections that may involve the distal small bowel and proximal colon, such as yersiniosis, salmonellosis, tuberculosis, actinomycosis, histoplasmosis, and noninfectious processes including Crohn's disease and neoplasia. The absence of diarrhea or hematochezia makes yersiniosis, salmonellosis, and Crohn's disease unlikely. Histoplasmosis is unlikely given the negative urine antigen. Evaluation for neoplasia of the distal small bowel requires histologic examination. A colonoscopy with random biopsies of the colon and terminal ileum is the next step if other tests are unrevealing.
The patient was empirically treated for small bowel diverticulitis with ceftriaxone and metronidazole. Because of continued daily fevers as high as 39C, his therapy was changed to vancomycin and piperacillin‐tazobactam to cover methicillin‐resistant Staphylococcus aureus and resistant gram‐negative bacilli. The patient developed new scleral icterus on hospital day 6; the remainder of his examination was unchanged. Serum sodium concentration was 127 mEq/L, potassium 2.7 mEq/L, phosphorus 1.3 mg/dL, magnesium 1.6 mg/dL, total bilirubin 5.6 mg/dL, direct bilirubin 3.6 mg/dL, alkaline phosphatase 193 U/L, gamma‐glutamyl transferase 300 U/L, aspartate aminotransferase 91 U/L, alanine aminotransferase 52 U/L. Brucella serology was negative.
His liver enzymes remain elevated with new onset jaundice consistent with hepatitis and intrahepatic cholestasis. His persistent hypophosphatemia, hypokalemia, and hypomagnesaemia well after resolution of diabetic ketoacidosis suggests acute tubulointerstitial dysfunction, which may be a complication of empiric antibiotic treatment or renal involvement by his underlying condition. Additional blood cultures, and tissue examination and culture are the next appropriate steps. Liver or bone marrow biopsy may suggest a diagnosis that can be confirmed by tissue culture or immunohistochemistry. Histologic findings such as fibrin ringed granulomas, caseating or noncaseating granulomas, or lymphomatous infiltration may suggest Coxiella (Q fever), tuberculosis, or lymphoma respectively. Because a liver biopsy is invasive and usually provides less tissue for culture, bone marrow examination should be obtained first.
A gallium 67 scan showed nonhomogenous increased uptake in both lungs and kidneys, consistent with interstitial nephritis and bilateral pneumonia. Serum protein electrophoresis demonstrated a monoclonal immunoglobulin (Ig)G lambda band with a kappa/lambda ratio of 0.9 (normal 1.42.8). Bone marrow biopsy showed normal hematopoiesis; no plasma or malignant cells, granulomas, or evidence of hemophagocytosis; and fungal and mycobacterial stains and cultures were negative. Colonoscopy revealed normal‐appearing mucosa. Histologic examination and culture of random biopsies from the colon and terminal ileum were negative for fungi, viruses, and mycobacteria. An ultrasound‐guided liver biopsy revealed numerous noncaseating granulomas formed of histiocytes and neutrophils with occasional fibrin rings. Fungal, viral, and mycobacterial stains and cultures were negative. The patient's fever resolved after 14 days, and he was discharged home without a diagnosis and close outpatient follow‐up.
The hepatic granulomas with fibrin rings are highly suggestive of Q fever, although ring granulomas may be seen in tuberculosis, typhoid fever, lymphoma, drug reactions, sarcoidosis, and CMV infections. Competing diagnoses such as CMV have been excluded by negative serology. Microscopic examination, tissue staining, and culture from liver and bone marrow biopsies were negative for S typhi, mycobacteria, and lymphoma. Gallium scan findings are generally nonspecific and of little utility in cases such as this. The kidney involvement correlates with the biochemical evidence of tubulointerstitial dysfunction; pulmonary involvement may reflect subclinical pulmonary infection with Coxiella. Given the normal bone marrow biopsy, the monoclonal gammopathy is of undetermined significance. The positive anti‐smooth muscle antibody can be related to Q fever. Anti‐smooth muscle antibodies frequently occur in Q fever, especially in those patients with hepatitis. Given the history of exposure to cattle, unexplained fever with temperaturepulse dissociation and liver biopsy findings, Q fever is the most likely diagnosis and empiric treatment with doxycycline is warranted.
Results of serology for Coxiella burnetii sent during admission were returned after the patient's discharge. C burnetii phase I IgG and IgM antibody titers were positive (1:512 each). C burnetii phase II IgG and IgM titers also were positive (1:1024 each). The patient was seen within a week and started on doxycycline 100 mg twice daily for 2 weeks for acute Q fever. His symptoms improved; hyponatremia, liver function tests, and thrombocytopenia normalized after treatment.
DISCUSSION
Q fever was first described in 1937 as a febrile illness affecting Australian slaughterhouse workers.[1] The Q in Q fever stands for query and reflected the initial uncertainty surrounding the underlying cause of the illness. The causative organism, C burnetti, is an obligate intracellular bacterium that resides within macrophage lysosomes. It can be found in the urine, feces, milk, placenta, and amniotic fluid of ungulates (cattle, sheep, and other ruminants), and other animals such as domestic cats and dogs. C burnetii is transmitted via inhalation, ingestion, occupational, or common source exposures, and in 1 case report by person‐to‐person sexual transmission.[2] In addition to slaughterhouse workers, pregnant women and immunosuppressed patients are more susceptible to developing Q fever.[3] For patients with suspected Q fever, a detailed occupational history, including specific job duties and potential exposure to animal products, is imperative.
Q fever has both acute and chronic presentations, which are differentiated based on the clinical illness and serologies. The symptoms of acute Q fever are nonspecific and may include influenza‐like illness, fever, pneumonia, and hepatitis. It presents less commonly with hemolytic anemia, interstitial nephritis, monoclonal gammopathy, or aseptic meningitis.[4, 5, 6, 7] Symptoms typically begin between 1 and 3 weeks after animal exposure and may persist for several months. Chronic Q fever occurs when unrecognized or untreated infection persists for greater than 6 months. It commonly presents with culture‐negative endocarditis, although infected aneurysms, osteomyelitis, or other distant sites of infection may also occur.
C burnetti is present in 2 antigenic forms that can be assessed by serology. Phase I is the more virulent, infectious form of C burnetti, which transitions to the avirulent phase II form during laboratory handling. In acute Q fever, phase II serologies are typically elevated out of proportion to phase I serologies, whereas this pattern is reversed in chronic Q fever. The diagnostic gold standard of acute Q fever is a 4‐fold rise in phase II antibody titers taken 3 to 6 weeks apart.[8] Histologic examination of affected organs can support a diagnosis of Q fever. The presence of ringed granulomas on liver or bone marrow biopsy specimens is highly suggestive, but not pathognomonic, of Q fever.[9]
Q fever is highly susceptible to several classes of antibiotics. For acute Q fever, doxycycline and tetracycline are typically used, with fluoroquinolones and chloramphenicol as alternatives.[8, 10] Patients with chronic Q fever should be treated with doxycycline and hydroxychloroquine. The addition of hydroxychloroquine alkalinizes the macrophage lysosome and enhances bacterial eradication.[8] For patients with acute Q fever, physicians should determine the risk of progression to chronic Q fever because closer monitoring is necessary. Patients with valvular heart lesions, immunosuppression, and pregnant women are at elevated risk of chronic Q fever. Trimethoprim/sulfamethoxazole can be used in place of doxycycline in pregnant women, as doxycycline and fluoroquinolones are contraindicated in pregnancy.[8]
This patient presented with a nonspecific febrile illness. Although the treating clinicians obtained a history of exposure to cattle early in his course, both the diagnosis and treatment were delayed. There are several possible explanations for the delay. First, although Q fever is a relatively common zoonosis, it remains an uncommon diagnosis, particularly among hospitalized patients. As a result, clinicians often focus on more common conditions. In this case, typical infections, malignancies, and inflammatory diseases were considered more likely. Second, the patient presented with hepatitis, an uncommon presentation of Q fever. Classical clinical reasoning suggests that atypical presentations of common diseases will occur more frequently than typical presentations of uncommon diseases. This case presented with an atypical presentation of an uncommon disease. The resultant lower pretest probability further dissuaded the patient's physicians from consideration of Q fever. Third, the finding of small bowel diverticulitis was a potential distractor. In patients with nonspecific febrile illnesses, it is common for physicians to anchor on any abnormal findings. In this case, the small bowel diverticulitis led to antibiotic treatment that was ineffective against C burnetti.
There were several clues to the diagnosis of Q fever in this patient's presentation. First, the pulsetemperature dissociation suggested infection with an intracellular pathogen. Hospitalists should recognize this association and be mindful of this often‐subtle clinical finding when faced with diagnostic uncertainty. Second, the patient was exposed to cattle prior to the onset of his illness. The fact that he did not have a direct exposure to animals underscores the infectivity of C burnetti. Finally, elevated alkaline phosphatase and transaminases were suggestive of an infiltrative disease; in the setting of a nonspecific febrile illness, Q fever was an important diagnostic consideration.
The key treatment decision in this case was the initiation and choice of antibiotics. Because of this patient's history of exposure to cattle and lack of a compelling alternative diagnosis, empiric treatment with doxycycline would have been appropriate. Hospitalists must weigh the potential benefit of early treatment of Q fever against the risks associated with antibiotic overuse. In patients presenting with a febrile illness after ungulate exposure, the decision to bet the farm with empiric doxycycline therapy may lead to clinical improvement, obviating a more invasive or extensive diagnostic evaluation.
TEACHING POINTS
- Acute Q fever typically presents 2 to 3 weeks after ungulate exposure with a febrile illness, pneumonia, and granulomatous hepatitis.
- Pulsetemperature dissociation is suggestive of infection by intracellular pathogens such as Coxiella, Salmonella, Leptospira, Legionella, and Mycoplasma.
- Clinicians should consider empiric doxycycline therapy in patients with suspected zoonosis (eg, Q fever, brucellosis, anaplasmosis, leptospirosis, Rocky Mountain spotted fever) while awaiting confirmatory tests, as improvement may obviate invasive testing.
Disclosure: Nothing to report.
- . “Q” fever, a new fever entity: clinical features, diagnosis, and laboratory investigation. Rev Infect Dis. 1983;5(4):790–800.
- , , , . Q fever: a biological weapon in your backyard. Lancet Infect Dis. 2003;3(11):709–721.
- , , , . Role of sex, age, previous valve lesion, and pregnancy in the clinical expression and outcome of Q fever after a large outbreak. Clin Infect Dis. 2007;15:44(2):232–237.
- , , , , . Unusual manifestations of acute Q fever: autoimmune hemolytic anemia and tubulointerstitial nephritis. Ann of Clin Microbiol Antimicrob. 2012;11:14.
- , , . Q fever. Lancet. 2006;367(9511):679–688.
- , , , , , . Transitory monoclonal gammopathy and acute Q fever. Enferm Infecc Microbiol Clin. 1995;13(7):442.
- , . Q fever. Clin Microbiol Rev. 1999;12(4):518–553.
- , , , et al. Diagnosis and management of Q fever—United States, 2013: recommendations from CDC and the Q Fever Working Group. MMWR Recomm Rep. 2013;62(RR‐03):1–30.
- , , , et al. Hepatic fibrin‐ring granulomas: a clinicopathologic study of 23 patients. Hum Pathol. 1991;22(6):607–613.
- , , . Travel‐associated zoonotic bacterial diseases. Curr Opin Infect Dis. 2011;24(5):457–463.
- . “Q” fever, a new fever entity: clinical features, diagnosis, and laboratory investigation. Rev Infect Dis. 1983;5(4):790–800.
- , , , . Q fever: a biological weapon in your backyard. Lancet Infect Dis. 2003;3(11):709–721.
- , , , . Role of sex, age, previous valve lesion, and pregnancy in the clinical expression and outcome of Q fever after a large outbreak. Clin Infect Dis. 2007;15:44(2):232–237.
- , , , , . Unusual manifestations of acute Q fever: autoimmune hemolytic anemia and tubulointerstitial nephritis. Ann of Clin Microbiol Antimicrob. 2012;11:14.
- , , . Q fever. Lancet. 2006;367(9511):679–688.
- , , , , , . Transitory monoclonal gammopathy and acute Q fever. Enferm Infecc Microbiol Clin. 1995;13(7):442.
- , . Q fever. Clin Microbiol Rev. 1999;12(4):518–553.
- , , , et al. Diagnosis and management of Q fever—United States, 2013: recommendations from CDC and the Q Fever Working Group. MMWR Recomm Rep. 2013;62(RR‐03):1–30.
- , , , et al. Hepatic fibrin‐ring granulomas: a clinicopathologic study of 23 patients. Hum Pathol. 1991;22(6):607–613.
- , , . Travel‐associated zoonotic bacterial diseases. Curr Opin Infect Dis. 2011;24(5):457–463.
Mobilization in Severe Sepsis
Severe sepsis, defined as an infection leading to systemic inflammatory response and acute organ dysfunction, is a significant cause of morbidity and mortality.[1, 2, 3] Although it has been a condition classically attributed to patients in the intensive care unit (ICU), accumulating data suggest that a substantial proportion of patients with severe sepsis are managed by hospitalists and floor teams in non‐ICU, general ward settings.[1, 4, 5] Although the incidence of severe sepsis continues to rise both in the United States and other developed nations,[2, 6, 7] advances in early recognition, management, and care of this condition have resulted in improved rates of survival.[8] The resultant increase in a severe sepsis survivor population[6] make the long‐term sequelae of this condition an important public health problem.[9]
In both the ICU and on general wards, severe sepsis survivors suffer from decreased functional status, worsened quality of life, increased cognitive dysfunction, and sarcopenia.[4, 6, 10, 11, 12, 13, 14] Not surprisingly, many such patients are discharged to long‐term care facilities for physical rehabilitation,[15] with escalating utilization of resources[16] and cost.[17, 18] Inexpensive interventions that improve outcomes following sepsis would thus be welcomed.
It is well known that physical therapy (PT) and early mobilization are beneficial in mitigating functional decline in a number of conditions.[19, 20, 21, 22] PT can improve outcomes in several ways: prevention of bed rest deconditioning, mitigation of mechanisms that lead to sarcopenia, increased pulmonary and tissue aerobic capacity, and improved sense of well‐being. Indeed, among the population cared for in ICU settings, early mobility and PT lead to more ventilator‐free days, better functional status at discharge, shorter duration of delirium, and even a potentially reduced risk of central line‐associated bloodstream infection (CLABSI).[23, 24] However, whether initiating early PT can improve outcomes in patients with severe sepsis treated by either intensivists or hospitalists/floor teams outside the ICU is unknown.
Therefore, to better understand this phenomenon, we systematically reviewed and integrated the literature regarding early mobilization and PT for severe sepsis outside the ICU. To be more inclusive, a secondary review including populations with any infectious etiology and severe sepsis treated within the ICU was also conducted. Our review begins by providing an overview of the pathophysiology behind functional decline in severe sepsis, along with existing evidence on early mobilization efficacy in other patient populations. We then proceed with a review of the extant literature on the aforementioned topic. We conclude with an evaluation of the current evidence on the subject, along with assertions regarding future research in the area.
PATHOPHYSIOLOGY OF DISABILITY FOLLOWING HOSPITALIZATION FOR SEVERE SEPSIS
The pathophysiology behind functional decline in patients hospitalized with severe sepsis is multifactorial (Figure 1). During hospitalization, it is well known that patients suffer from restricted mobility,[25] and that this impediment is linked to poor functional outcomes.[26] Described as far back as Hippocrates,[27] more recent studies have elucidated how prolonged bed rest leads to a multitude of physiological changes that promote deconditioning.[28] Specifically, skeletal muscle atrophy and decreased protein synthesis, independent of ongoing disease processes and acute illness, have been demonstrated in both animal and human models of prolonged inactivity.[29, 30] Additionally, bed rest leading to insensible fluid losses, a decline in stroke volume and effective cardiac output, bone loss, and decreased insulin sensitivity has been reported.[28, 31] There is little doubt that the aforementioned issues pertain to severe sepsis patients outside the ICU. In fact, nearly all of the acute mechanisms driving Creditor's hazards of hospitalization are noted among patients with severe sepsis.[32]
Furthermore, several factors preceding hospitalization may increase risk of disability. For example, Covinsky et al. described a number of risk factors, such as comorbid conditions, cognitive impairment, and various psychosocial aspects such as depression and limited social support, as being associated with increased risk of functional decline.[33] Thus, both in‐hospital and prehospital factors likely combine within an individual patient's context to determine risk of physical decline.
On this backdrop and the inherent immobilization associated with hospitalization, sepsis and inflammation catalyze physiologic changes that further propagate deconditioning.[7] Implicated pathways and proteins for this process include the mammalian target of rapamycin, human growth hormone, insulin‐like growth factors, interleukin‐1, and tumor necrosis factor‐. Through several metabolic alterations, sepsis independently promotes skeletal muscle breakdown and impairs skeletal muscle synthesis.[34, 35, 36] Inflammation associated with sepsis also increases oxidant burden, further leading to muscle dysfunction and dysregulation.[7, 31, 37, 38]
EFFECTS OF PHYSICAL THERAPY AND MOBILIZATION ON CLINICAL OUTCOMES
In patients with nonsepsis conditions who are at risk for functional decline, the effectiveness of physical therapy has been studied in multiple settings with positive outcomes. For example, in hospitalized elderly patients with general deconditioning, PT‐based interventions have demonstrated reductions in length of hospital stay.[39] Additionally, exercise in healthy subjects who have been subjected to bed rest has been shown to attenuate physiological changes, and maintain plasma and red cell volume and work capacity.[40] Adequate safety and improved outcomes have also been demonstrated in the general population of critically ill patients who receive early PT and mobilization. Improved functional capacity at discharge, decreases in duration of delirium, increased ventilator‐free days, decreased risk for CLABSI, and a better general sense of well‐being following these interventions have been widely reported in the literature.[14, 19, 23, 24, 41, 42, 43, 44, 45] Interestingly, critically ill patients may have a dose‐ and time‐dependent response to PT; that is, high intensity and early onset mobility‐based interventions are often associated with more ventilator‐free time and improved functional outcomes, resulting in shorter ICU and hospital length of stay.[42, 46, 47, 48]
Moderate intensity exercise has also been shown to improve 6‐minute walking distance in patients convalescing from coronary artery bypass grafting surgery.[49] Furthermore, in the postoperative setting, patients suffering traumatic hip fractures are known to benefit from physical and occupational therapies with shorter time to ambulation and improved locomotion in the recovery period.[21, 50, 51] Among patients with stroke, PT and gait training has led to improvements in speed, gait, independence during walking, activities of daily living, and extended activities of daily living.[52, 53, 54] A recent meta‐analysis also suggested that extra PT compared to regular treatment in patients with acute and subacute conditions such as stroke and postoperative states improved mobility and quality of life, while reducing length of hospital stay.[22]
Although this evidence suggests potential benefits for PT and mobilization, it is important to note that the effect of these treatments in dissimilar populations is unknown and may not necessarily be positive. For example, a recent study examining PT and its impact on patients with hip osteoarthritis showed no clinical benefit.[55] Mobilizing patients in severe illness may be associated with important risks, including falls, worsening of their clinical status, or moral discouragement in the setting of limited capacity. Therefore, understanding which elements of mobilization efforts create the greatest impact in the context of delivery of the intervention is critical to assessing the risk, benefit, and efficacy of PT‐based interventions.
EARLY PHYSICAL THERAPY FOR SEVERE SEPSIS OUTSIDE THE ICU: LITERATURE REVIEW
Given the functional decline associated with severe sepsis and the evidence of PT efficacy in other populations, we reviewed the current literature for studies evaluating physical therapy in severe sepsis patients outside the ICU. With the assistance of medical reference librarians, we searched MEDLINE via PubMed (1950present), EMBASE (1946present), Cochrane CENTRAL Register of Controlled Trials, and the Cochrane Database of Reviews of Effectiveness (1960present via Ovid). The search was last updated in June 2014.
We searched for studies that (1) involved human patients 18 years of age, (2) included patients with a primary diagnosis of sepsis or severe sepsis being treated outside the ICU, (3) featured a primary intervention that included PT or an early mobilization‐based initiative, and (4) reported a primary clinical or functional outcome of interest. Early was defined based on the included studies' definition. To be fully inclusive, we also conducted a secondary review with inclusion criteria expanded to studies of either any infectious pathology or severe sepsis patient in the ICU that employed PT interventions.
Our electronic search retrieved 815 records (Figure 2). Despite this approach, no publications met our primary inclusion criteria as we found no study that implemented a mobility intervention directed toward patients with sepsis treated outside the ICU. Our expanded secondary review included patients with any infectious pathology or those with severe sepsis in the ICU treated with PT; in this review, 2 studies met eligibility criteria.[56] In a 2003 cluster‐randomized trial, Mundy and colleagues randomized patients admitted with pneumonia to receive early PT or usual care. The outcomes of interest were hospital length of stay, mortality, number of chest radiographs, emergency department visits, and readmissions at 30 and 90 days after hospital admission. Although the study has important limitations (including patient‐level difference between trial arms, subjective definition of early mobilization), the authors found a significant decrease in length of stay among patients with pneumonia who received early PT compared to controls (5.8 vs 6.9 days, absolute difference 1.1 days, 95% confidence interval: 02.2 days). The study also reported a substantial decrease in adjusted mean hospital charges for the early mobilization group versus the usual care group ($10,159 per patient vs. $12,868 per patient, P=0.05). In the second study, Sossdorf et al. retrospectively evaluated a cohort of 999 patients with severe sepsis and septic shock and assessed whether onset and frequency of PT‐based interventions was associated with clinical benefit. After multivariate analysis, the authors reported a small mortality benefit associated with the relative number of PT interventions (hazard ratio: 0.982, P<0.001).[45]
EXPLAINING THE VOID
Our integrative review of the current literature reveals a gap in our understanding of the role of early mobilization in severe sepsis both within and beyond the ICU. Given the promise of PT‐based interventions and the toll of severe sepsis, one must ask: why may this be so?
First, the understanding that severe sepsis leads to significant, long‐term consequences for survivors has only been identified recently. Thus, it is possible that the burden and consequences related to this condition have not been fully recognized in clinical settings, leading to a paucity of research and interventions. Although the association between sepsis and mortality has been known since the 1990s,[57] long‐term complications and enduring morbidity of this disease continue to be realized. Indeed, many studies delineating the longer‐term effects of sepsis have been only recently published.[6, 10, 11, 12, 13]
Second, it is likely that many clinicians ascribe to the viewpoint that severe sepsis is an ICU‐only condition, a myth that has been discounted by multiple studies.[1, 4, 5] Although our study shows a paucity of evidence in both ICU and nonICU‐based severe sepsis, almost half of severe sepsis occurs outside the ICU, carrying with it many of the same clinical implications. Additionally, increased morbidity, mortality, and resource utilization are known to be true in all patients with severe sepsis, irrespective of where they receive treatment in the hospital.[4, 5, 6] Recent evidence has also shown that severe sepsis treated on the floor may be clinically, epidemiologically, and even prognostically unique from its ICU counterpart.[5, 58, 59] Therefore, it appears that research domains with tailored interventions to both ICU and non‐ICU severe sepsis patients are important areas of inquiry for clinicians. Such research may serve the purpose of assessing impact of early mobilization and unmasking any treatment heterogeneity that may exist when dealing with severe sepsis. Though trials of PT in ICU‐based severe sepsis are underway,[60] it is prudent that these also extend beyond the ICU‐setting.
Third, variability in early mobility practices and billing documentation for severe sepsis patients may exist, adding barriers to performing high‐quality research on this topic. In fact, administrative billing records for PT may offer insufficient granularity about services provided or therapies administered, particularly in the ICU where variability in early mobilization practices have been shown despite common employment of physiotherapists.[61]
Finally, many hospitalists may believe that patients with severe sepsis are simply too sick for early mobilization or PT, possibly limiting their participation in clinical or research‐based interventions. This perception has been well described in ICU populations, where it has been well studied and shown to be false.[41, 42, 43] Nevertheless, if severe sepsis patients are viewed as relatively sick hospitalized patients, it is plausible that resistance against early mobilization interventions may exist.[62] Understanding these biases and being mindful of such barriers when conducting studies in this area would be important.
CONCLUSION AND FUTURE DIRECTIONS
The cost burdens of severe sepsis are substantial. Elixhauser et al. suggest that it is currently the single most expensive cause of acute hospitalization in the United States.[63] Importantly, a large proportion of patients with severe sepsis receive care from hospitalists and/or floor teams on the general wards. Our integrative review has demonstrated a knowledge gap when it comes to rigorous assessments of PT and mobilization treatments in patients with severe sepsis within and beyond the ICU. Existing evidence provides a strong rationale for why functional decline occurs in patients with severe sepsis. A reasonable argument for PT‐based interventions to mitigate functional decline in this subset exists, but rigorous evaluation of such interventions is necessary. Physical and mobilization‐based treatments are routinely available and efficacious in several other settings and populations. It could be rapidly deployed and potentially improve outcomes in those with severe sepsis. Research would be welcomed to establish optimal dosing, efficacy, and cost effectiveness of PT and early mobilization for severe sepsis, particularly in patients treated on the general wards by hospitalists and floor teams.
How may such a research agenda be launched? A balanced multipronged approach is necessary. First, large‐scale epidemiological data to understand variation in practice are needed. Focused studies carried out by community and academic hospitalists on septic patients treated outside the ICU are the call of the hour. These data, in turn, can help create registries that assess for risk factors, quality of treatment, and long‐term outcomes among survivors of this condition. Second, evaluation and improvement of the coding and precision of physical and occupational therapy billing records is necessary so that their added value can be assessed and tracked using administrative data. Third, targeted prospective studies and clinical trials to directly evaluate the effect of PT in well‐defined patient populations with sepsis outside the ICU are needed. In this arena, hospitalist expertise and trained physical therapists will be crucial. The focus of this work should be directed toward both short‐term and long‐term functional outcomes, as well as mortality and morbidity assessments. Fourth, these patient‐centered efforts should loop back and inform the foundational biology of severe sepsis, thus illuminating patient‐centered end points, from biomarker analysis to physiometric measurements in basic and translational research.
In conclusion, this review sheds light on the fact that interventions that may mitigate the functional and cognitive decline in survivors of severe sepsis appear underdeveloped. Although the precise benefit of such interventions remains unclear, the low‐cost, widespread availability and generalizability of PT‐based interventions make it a worthy candidate for future research. As the numbers of survivors of sepsis expand, an unmet public health need for interventions to improve the long‐term outcomes of this population exists. Hospitalists and intensivists caring for severe sepsis patients must rise to meet this need. Together, we can help improve the lives of patients afflicted with severe sepsis, wherever they may receive care in the hospital.
Acknowledgements
The authors acknowledge the efforts of medical research librarians Andy Hickner, MSI, and Marissa Conte, MSI, on this project.
Disclosures
This work was supported by the National Institutes of HealthK08, HL091249 (T.J.I.) and VA HSR&D IIR‐11109 (T.J.I.). The views expressed here are the authors' own and do not necessarily represent the views of the US government or the Department of Veterans' Affairs. The authors report no conflicts of interest.
- , . Epidemiology of sepsis: an update. Crit Care Med. 2001;29:S109–S116.
- , , , et al. Nationwide trends of severe sepsis in the 21st century (2000–2007). Chest. 2011;140:1223–1231.
- , , , et al. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554.
- , , , et al. Functional outcomes of general medical patients with severe sepsis. BMC Infect Dis. 2013;13:588.
- , , , et al. The epidemiology of acute organ system dysfunction from severe sepsis outside of the intensive care unit. J Hosp Med. 2013;8:243–247.
- , , , et al. Population burden of long‐term survivorship after severe sepsis in older Americans. J Am Geriatr Soc. 2012;60:1070–1077.
- , , , et al. Systemic inflammatory response syndrome increases immobility‐induced neuromuscular weakness. Crit Care Med. 2008;36:910–916.
- , , , et al. Early goal‐directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377.
- . The lingering consequences of sepsis: a hidden public health disaster? JAMA. 2010;304:1833–1834.
- , , , et al. Long‐term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304:1787–1794.
- , , , et al. Spurious inferences about long‐term outcomes: the case of severe sepsis and geriatric conditions. Am J Respir Crit Care Med. 2012;185:835–841.
- , , , et al. Long‐term outcome and quality‐adjusted life years after severe sepsis. Crit Care Med. 2009;37:1268–1274.
- , , , et al. Long‐term mortality and quality of life in sepsis: a systematic review. Crit Care Med. 2010;38:1276–1283.
- , , , et al. Improving post‐intensive care unit neuropsychiatric outcomes: understanding cognitive effects of physical activity. Am J Respir Crit Care Med. 2012;186:1220–1228.
- , , , et al. Hospitalizations, costs, and outcomes of severe sepsis in the United States 2003 to 2007. Crit Care Med. 2012;40:754–761.
- , , , et al. Long‐term acute care hospital utilization after critical illness. JAMA. 2010;303:2253–2259.
- , , , et al. Long‐term survival and healthcare utilization outcomes attributable to sepsis and pneumonia. BMC Health Serv Res. 2012;12:432.
- , , , et al. Long‐term mortality and medical care charges in patients with severe sepsis. Crit Care Med. 2003;31:2316–2323.
- , , , et al. Early exercise in critically ill patients enhances short‐term functional recovery. Crit Care Med. 2009;37:2499–2505.
- , , , et al. Exercise‐based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev. 2011;(7):CD001800.
- , , , et al. What is the role of timing in the surgical and rehabilitative care of community‐dwelling older persons with acute hip fracture? Arch Intern Med. 1997;157:513–520.
- , , . Extra physical therapy reduces patient length of stay and improves functional outcomes and quality of life in people with acute or subacute conditions: a systematic review. Arch Phys Med Rehabilil. 2011;92:1490–1500.
- , , . Reduction of intensive care unit length of stay: the case of early mobilization. Health Care Manag (Frederick). 2014;33:128–135.
- , , , et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373:1874–1882.
- , , , et al. Hospitalization, restricted activity, and the development of disability among older persons. JAMA. 2004;292:2115–2124.
- , , , et al. Low mobility during hospitalization and functional decline in older adults. J Am Geriatr Soc. 2011;59:266–273.
- , . The Medical Works of Hippocrates. Oxford, United Kingdom: Blackwell; 1950.
- , , . An overview of the issues: physiological effects of bed rest and restricted physical activity. Med Sci Sports Exerc. 1997;29:187–190.
- , , , et al. Prolonged bed rest decreases skeletal muscle and whole body protein synthesis. Am J Physiol. 1996;270:E627–E633.
- , . Metabolic consequences of muscle disuse atrophy. J Nutr. 2005;135:1824S–1828S.
- . Inactivity and inflammation in the critically ill patient. Crit Care Clin. 2007;23:21–34.
- . Hazards of hospitalization of the elderly. Ann Intern Med. 1993;118:219–223.
- , , . Hospitalization‐associated disability: “She was probably able to ambulate, but I'm not sure”. JAMA. 2011;306:1782–1793.
- , , , et al. A sustained rat model for studying the long‐lasting catabolic state of sepsis. Infect Immun. 1999;67:1079–1085.
- . Regulation of skeletal muscle protein turnover during sepsis. Curr Opin Clin Nutr. Metab Care. 1998;1:217–224.
- , , . Regulation of muscle protein synthesis during sepsis and inflammation. Am J Physiol Endocrinol Metab. 2007;293:E453–E459.
- , . From muscle disuse to myopathy in COPD: potential contribution of oxidative stress. Eur Respir J. 2005;26:703–719.
- , , . Oxidative stress and gene expression in sepsis. Br J Anaesth. 2003;90:221–232.
- , , , et al. Early ambulation and length of stay in older adults hospitalized for acute illness. Arch Intern Med. 2010;170:1942–1943.
- . Intensive exercise training during bed rest attenuates deconditioning. Med Sci Sports Exerc. 1997;29:207–215.
- , , , et al. Early activity is feasible and safe in respiratory failure patients. Crit Care Med. 2007;35:139–145.
- , , , et al. Early intensive care unit mobility therapy in the treatment of acute respiratory failure. Crit Care Med. 2008;36:2238–2243.
- . Clinical trials of early mobilization of critically ill patients. Crit Care Med. 2009;37:S442–S447.
- . Mobilizing patients in the intensive care unit: improving neuromuscular weakness and physical function. JAMA. 2008;300:1685–1690.
- , , , et al. Potential effect of physiotherapeutic treatment on mortality rate in patients with severe sepsis and septic shock: a retrospective cohort analysis. J Crit Care. 2013;28:954–958.
- , , , et al. Effects of physical training on functional status in patients with prolonged mechanical ventilation. Phys Ther. 2006;86:1271–1281.
- , , , et al. Impact of whole‐body rehabilitation in patients receiving chronic mechanical ventilation. Crit Care Med. 2005;33:2259–2265.
- . Rehabilitation of patients admitted to a respiratory intensive care unit. Arch Phys Med Rehabil. 1998;79:849–854.
- , , , et al. Supervised moderate intensity exercise improves distance walked at hospital discharge following coronary artery bypass graft surgery—a randomised controlled trial. Heart Lung Circ. 2008;17:129–138.
- , , , et al. Systematic review of hip fracture rehabilitation practices in the elderly. Arch Phys Med Rehabil. 2009;90:246–262.
- , , , et al. Physical therapy and mobility 2 and 6 months after hip fracture. J Am Geriatr Soc. 2004;52:1114–1120.
- , , , et al. Physical fitness training for stroke patients. Cochrane Database Syst Rev. 2011;(11):CD003316.
- , , , et al. Effects of augmented exercise therapy on outcome of gait and gait‐related activities in the first 6 months after stroke: a meta‐analysis. Stroke. 2011;42:3311–3315.
- , , , et al. Effects of augmented exercise therapy time after stroke: a meta‐analysis. Stroke. 2004;35:2529–2539.
- , , , et al. Effect of physical therapy on pain and function in patients with hip osteoarthritis: a randomized clinical trial. JAMA. 2014;311:1987–1997.
- , , , et al. Early mobilization of patients hospitalized with community‐acquired pneumonia. Chest. 2003;124:883–889.
- , , , et al. Magnitude and duration of the effect of sepsis on survival. Department of Veterans Affairs Systemic Sepsis Cooperative Studies Group. JAMA. 1997;277:1058–1063.
- , , , et al. Epidemiology of sepsis in Victoria, Australia. Crit Care Med. 2005;33:71–80.
- , , , et al. Sepsis incidence and outcome: contrasting the intensive care unit with the hospital ward. Crit Care Med. 2007;35:1284–1289.
- , , . Early rehabilitation in sepsis: a prospective randomised controlled trial investigating functional and physiological outcomes The i‐PERFORM Trial (Protocol Article). BMC Anesthesiol. 2011;11:21.
- , , , et al. TEAM: a prospective multi‐centre cohort study of early activity and mobilisation in ICU. In: American Thoracic Society 2013 International Conference; May 17–22, 2013; Philadelphia, PA. Am J Respir Crit Care Med. 2013;187:A3625.
- , , , et al. Improving long‐term outcomes after discharge from intensive care unit: report from a stakeholders' conference. Crit Care Med. 2012;40:502–509.
- , , . Septicemia in U.S. hospitals, 2009: statistical brief #122. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville, MD; 2006.
Severe sepsis, defined as an infection leading to systemic inflammatory response and acute organ dysfunction, is a significant cause of morbidity and mortality.[1, 2, 3] Although it has been a condition classically attributed to patients in the intensive care unit (ICU), accumulating data suggest that a substantial proportion of patients with severe sepsis are managed by hospitalists and floor teams in non‐ICU, general ward settings.[1, 4, 5] Although the incidence of severe sepsis continues to rise both in the United States and other developed nations,[2, 6, 7] advances in early recognition, management, and care of this condition have resulted in improved rates of survival.[8] The resultant increase in a severe sepsis survivor population[6] make the long‐term sequelae of this condition an important public health problem.[9]
In both the ICU and on general wards, severe sepsis survivors suffer from decreased functional status, worsened quality of life, increased cognitive dysfunction, and sarcopenia.[4, 6, 10, 11, 12, 13, 14] Not surprisingly, many such patients are discharged to long‐term care facilities for physical rehabilitation,[15] with escalating utilization of resources[16] and cost.[17, 18] Inexpensive interventions that improve outcomes following sepsis would thus be welcomed.
It is well known that physical therapy (PT) and early mobilization are beneficial in mitigating functional decline in a number of conditions.[19, 20, 21, 22] PT can improve outcomes in several ways: prevention of bed rest deconditioning, mitigation of mechanisms that lead to sarcopenia, increased pulmonary and tissue aerobic capacity, and improved sense of well‐being. Indeed, among the population cared for in ICU settings, early mobility and PT lead to more ventilator‐free days, better functional status at discharge, shorter duration of delirium, and even a potentially reduced risk of central line‐associated bloodstream infection (CLABSI).[23, 24] However, whether initiating early PT can improve outcomes in patients with severe sepsis treated by either intensivists or hospitalists/floor teams outside the ICU is unknown.
Therefore, to better understand this phenomenon, we systematically reviewed and integrated the literature regarding early mobilization and PT for severe sepsis outside the ICU. To be more inclusive, a secondary review including populations with any infectious etiology and severe sepsis treated within the ICU was also conducted. Our review begins by providing an overview of the pathophysiology behind functional decline in severe sepsis, along with existing evidence on early mobilization efficacy in other patient populations. We then proceed with a review of the extant literature on the aforementioned topic. We conclude with an evaluation of the current evidence on the subject, along with assertions regarding future research in the area.
PATHOPHYSIOLOGY OF DISABILITY FOLLOWING HOSPITALIZATION FOR SEVERE SEPSIS
The pathophysiology behind functional decline in patients hospitalized with severe sepsis is multifactorial (Figure 1). During hospitalization, it is well known that patients suffer from restricted mobility,[25] and that this impediment is linked to poor functional outcomes.[26] Described as far back as Hippocrates,[27] more recent studies have elucidated how prolonged bed rest leads to a multitude of physiological changes that promote deconditioning.[28] Specifically, skeletal muscle atrophy and decreased protein synthesis, independent of ongoing disease processes and acute illness, have been demonstrated in both animal and human models of prolonged inactivity.[29, 30] Additionally, bed rest leading to insensible fluid losses, a decline in stroke volume and effective cardiac output, bone loss, and decreased insulin sensitivity has been reported.[28, 31] There is little doubt that the aforementioned issues pertain to severe sepsis patients outside the ICU. In fact, nearly all of the acute mechanisms driving Creditor's hazards of hospitalization are noted among patients with severe sepsis.[32]

Furthermore, several factors preceding hospitalization may increase risk of disability. For example, Covinsky et al. described a number of risk factors, such as comorbid conditions, cognitive impairment, and various psychosocial aspects such as depression and limited social support, as being associated with increased risk of functional decline.[33] Thus, both in‐hospital and prehospital factors likely combine within an individual patient's context to determine risk of physical decline.
On this backdrop and the inherent immobilization associated with hospitalization, sepsis and inflammation catalyze physiologic changes that further propagate deconditioning.[7] Implicated pathways and proteins for this process include the mammalian target of rapamycin, human growth hormone, insulin‐like growth factors, interleukin‐1, and tumor necrosis factor‐. Through several metabolic alterations, sepsis independently promotes skeletal muscle breakdown and impairs skeletal muscle synthesis.[34, 35, 36] Inflammation associated with sepsis also increases oxidant burden, further leading to muscle dysfunction and dysregulation.[7, 31, 37, 38]
EFFECTS OF PHYSICAL THERAPY AND MOBILIZATION ON CLINICAL OUTCOMES
In patients with nonsepsis conditions who are at risk for functional decline, the effectiveness of physical therapy has been studied in multiple settings with positive outcomes. For example, in hospitalized elderly patients with general deconditioning, PT‐based interventions have demonstrated reductions in length of hospital stay.[39] Additionally, exercise in healthy subjects who have been subjected to bed rest has been shown to attenuate physiological changes, and maintain plasma and red cell volume and work capacity.[40] Adequate safety and improved outcomes have also been demonstrated in the general population of critically ill patients who receive early PT and mobilization. Improved functional capacity at discharge, decreases in duration of delirium, increased ventilator‐free days, decreased risk for CLABSI, and a better general sense of well‐being following these interventions have been widely reported in the literature.[14, 19, 23, 24, 41, 42, 43, 44, 45] Interestingly, critically ill patients may have a dose‐ and time‐dependent response to PT; that is, high intensity and early onset mobility‐based interventions are often associated with more ventilator‐free time and improved functional outcomes, resulting in shorter ICU and hospital length of stay.[42, 46, 47, 48]
Moderate intensity exercise has also been shown to improve 6‐minute walking distance in patients convalescing from coronary artery bypass grafting surgery.[49] Furthermore, in the postoperative setting, patients suffering traumatic hip fractures are known to benefit from physical and occupational therapies with shorter time to ambulation and improved locomotion in the recovery period.[21, 50, 51] Among patients with stroke, PT and gait training has led to improvements in speed, gait, independence during walking, activities of daily living, and extended activities of daily living.[52, 53, 54] A recent meta‐analysis also suggested that extra PT compared to regular treatment in patients with acute and subacute conditions such as stroke and postoperative states improved mobility and quality of life, while reducing length of hospital stay.[22]
Although this evidence suggests potential benefits for PT and mobilization, it is important to note that the effect of these treatments in dissimilar populations is unknown and may not necessarily be positive. For example, a recent study examining PT and its impact on patients with hip osteoarthritis showed no clinical benefit.[55] Mobilizing patients in severe illness may be associated with important risks, including falls, worsening of their clinical status, or moral discouragement in the setting of limited capacity. Therefore, understanding which elements of mobilization efforts create the greatest impact in the context of delivery of the intervention is critical to assessing the risk, benefit, and efficacy of PT‐based interventions.
EARLY PHYSICAL THERAPY FOR SEVERE SEPSIS OUTSIDE THE ICU: LITERATURE REVIEW
Given the functional decline associated with severe sepsis and the evidence of PT efficacy in other populations, we reviewed the current literature for studies evaluating physical therapy in severe sepsis patients outside the ICU. With the assistance of medical reference librarians, we searched MEDLINE via PubMed (1950present), EMBASE (1946present), Cochrane CENTRAL Register of Controlled Trials, and the Cochrane Database of Reviews of Effectiveness (1960present via Ovid). The search was last updated in June 2014.
We searched for studies that (1) involved human patients 18 years of age, (2) included patients with a primary diagnosis of sepsis or severe sepsis being treated outside the ICU, (3) featured a primary intervention that included PT or an early mobilization‐based initiative, and (4) reported a primary clinical or functional outcome of interest. Early was defined based on the included studies' definition. To be fully inclusive, we also conducted a secondary review with inclusion criteria expanded to studies of either any infectious pathology or severe sepsis patient in the ICU that employed PT interventions.
Our electronic search retrieved 815 records (Figure 2). Despite this approach, no publications met our primary inclusion criteria as we found no study that implemented a mobility intervention directed toward patients with sepsis treated outside the ICU. Our expanded secondary review included patients with any infectious pathology or those with severe sepsis in the ICU treated with PT; in this review, 2 studies met eligibility criteria.[56] In a 2003 cluster‐randomized trial, Mundy and colleagues randomized patients admitted with pneumonia to receive early PT or usual care. The outcomes of interest were hospital length of stay, mortality, number of chest radiographs, emergency department visits, and readmissions at 30 and 90 days after hospital admission. Although the study has important limitations (including patient‐level difference between trial arms, subjective definition of early mobilization), the authors found a significant decrease in length of stay among patients with pneumonia who received early PT compared to controls (5.8 vs 6.9 days, absolute difference 1.1 days, 95% confidence interval: 02.2 days). The study also reported a substantial decrease in adjusted mean hospital charges for the early mobilization group versus the usual care group ($10,159 per patient vs. $12,868 per patient, P=0.05). In the second study, Sossdorf et al. retrospectively evaluated a cohort of 999 patients with severe sepsis and septic shock and assessed whether onset and frequency of PT‐based interventions was associated with clinical benefit. After multivariate analysis, the authors reported a small mortality benefit associated with the relative number of PT interventions (hazard ratio: 0.982, P<0.001).[45]

EXPLAINING THE VOID
Our integrative review of the current literature reveals a gap in our understanding of the role of early mobilization in severe sepsis both within and beyond the ICU. Given the promise of PT‐based interventions and the toll of severe sepsis, one must ask: why may this be so?
First, the understanding that severe sepsis leads to significant, long‐term consequences for survivors has only been identified recently. Thus, it is possible that the burden and consequences related to this condition have not been fully recognized in clinical settings, leading to a paucity of research and interventions. Although the association between sepsis and mortality has been known since the 1990s,[57] long‐term complications and enduring morbidity of this disease continue to be realized. Indeed, many studies delineating the longer‐term effects of sepsis have been only recently published.[6, 10, 11, 12, 13]
Second, it is likely that many clinicians ascribe to the viewpoint that severe sepsis is an ICU‐only condition, a myth that has been discounted by multiple studies.[1, 4, 5] Although our study shows a paucity of evidence in both ICU and nonICU‐based severe sepsis, almost half of severe sepsis occurs outside the ICU, carrying with it many of the same clinical implications. Additionally, increased morbidity, mortality, and resource utilization are known to be true in all patients with severe sepsis, irrespective of where they receive treatment in the hospital.[4, 5, 6] Recent evidence has also shown that severe sepsis treated on the floor may be clinically, epidemiologically, and even prognostically unique from its ICU counterpart.[5, 58, 59] Therefore, it appears that research domains with tailored interventions to both ICU and non‐ICU severe sepsis patients are important areas of inquiry for clinicians. Such research may serve the purpose of assessing impact of early mobilization and unmasking any treatment heterogeneity that may exist when dealing with severe sepsis. Though trials of PT in ICU‐based severe sepsis are underway,[60] it is prudent that these also extend beyond the ICU‐setting.
Third, variability in early mobility practices and billing documentation for severe sepsis patients may exist, adding barriers to performing high‐quality research on this topic. In fact, administrative billing records for PT may offer insufficient granularity about services provided or therapies administered, particularly in the ICU where variability in early mobilization practices have been shown despite common employment of physiotherapists.[61]
Finally, many hospitalists may believe that patients with severe sepsis are simply too sick for early mobilization or PT, possibly limiting their participation in clinical or research‐based interventions. This perception has been well described in ICU populations, where it has been well studied and shown to be false.[41, 42, 43] Nevertheless, if severe sepsis patients are viewed as relatively sick hospitalized patients, it is plausible that resistance against early mobilization interventions may exist.[62] Understanding these biases and being mindful of such barriers when conducting studies in this area would be important.
CONCLUSION AND FUTURE DIRECTIONS
The cost burdens of severe sepsis are substantial. Elixhauser et al. suggest that it is currently the single most expensive cause of acute hospitalization in the United States.[63] Importantly, a large proportion of patients with severe sepsis receive care from hospitalists and/or floor teams on the general wards. Our integrative review has demonstrated a knowledge gap when it comes to rigorous assessments of PT and mobilization treatments in patients with severe sepsis within and beyond the ICU. Existing evidence provides a strong rationale for why functional decline occurs in patients with severe sepsis. A reasonable argument for PT‐based interventions to mitigate functional decline in this subset exists, but rigorous evaluation of such interventions is necessary. Physical and mobilization‐based treatments are routinely available and efficacious in several other settings and populations. It could be rapidly deployed and potentially improve outcomes in those with severe sepsis. Research would be welcomed to establish optimal dosing, efficacy, and cost effectiveness of PT and early mobilization for severe sepsis, particularly in patients treated on the general wards by hospitalists and floor teams.
How may such a research agenda be launched? A balanced multipronged approach is necessary. First, large‐scale epidemiological data to understand variation in practice are needed. Focused studies carried out by community and academic hospitalists on septic patients treated outside the ICU are the call of the hour. These data, in turn, can help create registries that assess for risk factors, quality of treatment, and long‐term outcomes among survivors of this condition. Second, evaluation and improvement of the coding and precision of physical and occupational therapy billing records is necessary so that their added value can be assessed and tracked using administrative data. Third, targeted prospective studies and clinical trials to directly evaluate the effect of PT in well‐defined patient populations with sepsis outside the ICU are needed. In this arena, hospitalist expertise and trained physical therapists will be crucial. The focus of this work should be directed toward both short‐term and long‐term functional outcomes, as well as mortality and morbidity assessments. Fourth, these patient‐centered efforts should loop back and inform the foundational biology of severe sepsis, thus illuminating patient‐centered end points, from biomarker analysis to physiometric measurements in basic and translational research.
In conclusion, this review sheds light on the fact that interventions that may mitigate the functional and cognitive decline in survivors of severe sepsis appear underdeveloped. Although the precise benefit of such interventions remains unclear, the low‐cost, widespread availability and generalizability of PT‐based interventions make it a worthy candidate for future research. As the numbers of survivors of sepsis expand, an unmet public health need for interventions to improve the long‐term outcomes of this population exists. Hospitalists and intensivists caring for severe sepsis patients must rise to meet this need. Together, we can help improve the lives of patients afflicted with severe sepsis, wherever they may receive care in the hospital.
Acknowledgements
The authors acknowledge the efforts of medical research librarians Andy Hickner, MSI, and Marissa Conte, MSI, on this project.
Disclosures
This work was supported by the National Institutes of HealthK08, HL091249 (T.J.I.) and VA HSR&D IIR‐11109 (T.J.I.). The views expressed here are the authors' own and do not necessarily represent the views of the US government or the Department of Veterans' Affairs. The authors report no conflicts of interest.
Severe sepsis, defined as an infection leading to systemic inflammatory response and acute organ dysfunction, is a significant cause of morbidity and mortality.[1, 2, 3] Although it has been a condition classically attributed to patients in the intensive care unit (ICU), accumulating data suggest that a substantial proportion of patients with severe sepsis are managed by hospitalists and floor teams in non‐ICU, general ward settings.[1, 4, 5] Although the incidence of severe sepsis continues to rise both in the United States and other developed nations,[2, 6, 7] advances in early recognition, management, and care of this condition have resulted in improved rates of survival.[8] The resultant increase in a severe sepsis survivor population[6] make the long‐term sequelae of this condition an important public health problem.[9]
In both the ICU and on general wards, severe sepsis survivors suffer from decreased functional status, worsened quality of life, increased cognitive dysfunction, and sarcopenia.[4, 6, 10, 11, 12, 13, 14] Not surprisingly, many such patients are discharged to long‐term care facilities for physical rehabilitation,[15] with escalating utilization of resources[16] and cost.[17, 18] Inexpensive interventions that improve outcomes following sepsis would thus be welcomed.
It is well known that physical therapy (PT) and early mobilization are beneficial in mitigating functional decline in a number of conditions.[19, 20, 21, 22] PT can improve outcomes in several ways: prevention of bed rest deconditioning, mitigation of mechanisms that lead to sarcopenia, increased pulmonary and tissue aerobic capacity, and improved sense of well‐being. Indeed, among the population cared for in ICU settings, early mobility and PT lead to more ventilator‐free days, better functional status at discharge, shorter duration of delirium, and even a potentially reduced risk of central line‐associated bloodstream infection (CLABSI).[23, 24] However, whether initiating early PT can improve outcomes in patients with severe sepsis treated by either intensivists or hospitalists/floor teams outside the ICU is unknown.
Therefore, to better understand this phenomenon, we systematically reviewed and integrated the literature regarding early mobilization and PT for severe sepsis outside the ICU. To be more inclusive, a secondary review including populations with any infectious etiology and severe sepsis treated within the ICU was also conducted. Our review begins by providing an overview of the pathophysiology behind functional decline in severe sepsis, along with existing evidence on early mobilization efficacy in other patient populations. We then proceed with a review of the extant literature on the aforementioned topic. We conclude with an evaluation of the current evidence on the subject, along with assertions regarding future research in the area.
PATHOPHYSIOLOGY OF DISABILITY FOLLOWING HOSPITALIZATION FOR SEVERE SEPSIS
The pathophysiology behind functional decline in patients hospitalized with severe sepsis is multifactorial (Figure 1). During hospitalization, it is well known that patients suffer from restricted mobility,[25] and that this impediment is linked to poor functional outcomes.[26] Described as far back as Hippocrates,[27] more recent studies have elucidated how prolonged bed rest leads to a multitude of physiological changes that promote deconditioning.[28] Specifically, skeletal muscle atrophy and decreased protein synthesis, independent of ongoing disease processes and acute illness, have been demonstrated in both animal and human models of prolonged inactivity.[29, 30] Additionally, bed rest leading to insensible fluid losses, a decline in stroke volume and effective cardiac output, bone loss, and decreased insulin sensitivity has been reported.[28, 31] There is little doubt that the aforementioned issues pertain to severe sepsis patients outside the ICU. In fact, nearly all of the acute mechanisms driving Creditor's hazards of hospitalization are noted among patients with severe sepsis.[32]

Furthermore, several factors preceding hospitalization may increase risk of disability. For example, Covinsky et al. described a number of risk factors, such as comorbid conditions, cognitive impairment, and various psychosocial aspects such as depression and limited social support, as being associated with increased risk of functional decline.[33] Thus, both in‐hospital and prehospital factors likely combine within an individual patient's context to determine risk of physical decline.
On this backdrop and the inherent immobilization associated with hospitalization, sepsis and inflammation catalyze physiologic changes that further propagate deconditioning.[7] Implicated pathways and proteins for this process include the mammalian target of rapamycin, human growth hormone, insulin‐like growth factors, interleukin‐1, and tumor necrosis factor‐. Through several metabolic alterations, sepsis independently promotes skeletal muscle breakdown and impairs skeletal muscle synthesis.[34, 35, 36] Inflammation associated with sepsis also increases oxidant burden, further leading to muscle dysfunction and dysregulation.[7, 31, 37, 38]
EFFECTS OF PHYSICAL THERAPY AND MOBILIZATION ON CLINICAL OUTCOMES
In patients with nonsepsis conditions who are at risk for functional decline, the effectiveness of physical therapy has been studied in multiple settings with positive outcomes. For example, in hospitalized elderly patients with general deconditioning, PT‐based interventions have demonstrated reductions in length of hospital stay.[39] Additionally, exercise in healthy subjects who have been subjected to bed rest has been shown to attenuate physiological changes, and maintain plasma and red cell volume and work capacity.[40] Adequate safety and improved outcomes have also been demonstrated in the general population of critically ill patients who receive early PT and mobilization. Improved functional capacity at discharge, decreases in duration of delirium, increased ventilator‐free days, decreased risk for CLABSI, and a better general sense of well‐being following these interventions have been widely reported in the literature.[14, 19, 23, 24, 41, 42, 43, 44, 45] Interestingly, critically ill patients may have a dose‐ and time‐dependent response to PT; that is, high intensity and early onset mobility‐based interventions are often associated with more ventilator‐free time and improved functional outcomes, resulting in shorter ICU and hospital length of stay.[42, 46, 47, 48]
Moderate intensity exercise has also been shown to improve 6‐minute walking distance in patients convalescing from coronary artery bypass grafting surgery.[49] Furthermore, in the postoperative setting, patients suffering traumatic hip fractures are known to benefit from physical and occupational therapies with shorter time to ambulation and improved locomotion in the recovery period.[21, 50, 51] Among patients with stroke, PT and gait training has led to improvements in speed, gait, independence during walking, activities of daily living, and extended activities of daily living.[52, 53, 54] A recent meta‐analysis also suggested that extra PT compared to regular treatment in patients with acute and subacute conditions such as stroke and postoperative states improved mobility and quality of life, while reducing length of hospital stay.[22]
Although this evidence suggests potential benefits for PT and mobilization, it is important to note that the effect of these treatments in dissimilar populations is unknown and may not necessarily be positive. For example, a recent study examining PT and its impact on patients with hip osteoarthritis showed no clinical benefit.[55] Mobilizing patients in severe illness may be associated with important risks, including falls, worsening of their clinical status, or moral discouragement in the setting of limited capacity. Therefore, understanding which elements of mobilization efforts create the greatest impact in the context of delivery of the intervention is critical to assessing the risk, benefit, and efficacy of PT‐based interventions.
EARLY PHYSICAL THERAPY FOR SEVERE SEPSIS OUTSIDE THE ICU: LITERATURE REVIEW
Given the functional decline associated with severe sepsis and the evidence of PT efficacy in other populations, we reviewed the current literature for studies evaluating physical therapy in severe sepsis patients outside the ICU. With the assistance of medical reference librarians, we searched MEDLINE via PubMed (1950present), EMBASE (1946present), Cochrane CENTRAL Register of Controlled Trials, and the Cochrane Database of Reviews of Effectiveness (1960present via Ovid). The search was last updated in June 2014.
We searched for studies that (1) involved human patients 18 years of age, (2) included patients with a primary diagnosis of sepsis or severe sepsis being treated outside the ICU, (3) featured a primary intervention that included PT or an early mobilization‐based initiative, and (4) reported a primary clinical or functional outcome of interest. Early was defined based on the included studies' definition. To be fully inclusive, we also conducted a secondary review with inclusion criteria expanded to studies of either any infectious pathology or severe sepsis patient in the ICU that employed PT interventions.
Our electronic search retrieved 815 records (Figure 2). Despite this approach, no publications met our primary inclusion criteria as we found no study that implemented a mobility intervention directed toward patients with sepsis treated outside the ICU. Our expanded secondary review included patients with any infectious pathology or those with severe sepsis in the ICU treated with PT; in this review, 2 studies met eligibility criteria.[56] In a 2003 cluster‐randomized trial, Mundy and colleagues randomized patients admitted with pneumonia to receive early PT or usual care. The outcomes of interest were hospital length of stay, mortality, number of chest radiographs, emergency department visits, and readmissions at 30 and 90 days after hospital admission. Although the study has important limitations (including patient‐level difference between trial arms, subjective definition of early mobilization), the authors found a significant decrease in length of stay among patients with pneumonia who received early PT compared to controls (5.8 vs 6.9 days, absolute difference 1.1 days, 95% confidence interval: 02.2 days). The study also reported a substantial decrease in adjusted mean hospital charges for the early mobilization group versus the usual care group ($10,159 per patient vs. $12,868 per patient, P=0.05). In the second study, Sossdorf et al. retrospectively evaluated a cohort of 999 patients with severe sepsis and septic shock and assessed whether onset and frequency of PT‐based interventions was associated with clinical benefit. After multivariate analysis, the authors reported a small mortality benefit associated with the relative number of PT interventions (hazard ratio: 0.982, P<0.001).[45]

EXPLAINING THE VOID
Our integrative review of the current literature reveals a gap in our understanding of the role of early mobilization in severe sepsis both within and beyond the ICU. Given the promise of PT‐based interventions and the toll of severe sepsis, one must ask: why may this be so?
First, the understanding that severe sepsis leads to significant, long‐term consequences for survivors has only been identified recently. Thus, it is possible that the burden and consequences related to this condition have not been fully recognized in clinical settings, leading to a paucity of research and interventions. Although the association between sepsis and mortality has been known since the 1990s,[57] long‐term complications and enduring morbidity of this disease continue to be realized. Indeed, many studies delineating the longer‐term effects of sepsis have been only recently published.[6, 10, 11, 12, 13]
Second, it is likely that many clinicians ascribe to the viewpoint that severe sepsis is an ICU‐only condition, a myth that has been discounted by multiple studies.[1, 4, 5] Although our study shows a paucity of evidence in both ICU and nonICU‐based severe sepsis, almost half of severe sepsis occurs outside the ICU, carrying with it many of the same clinical implications. Additionally, increased morbidity, mortality, and resource utilization are known to be true in all patients with severe sepsis, irrespective of where they receive treatment in the hospital.[4, 5, 6] Recent evidence has also shown that severe sepsis treated on the floor may be clinically, epidemiologically, and even prognostically unique from its ICU counterpart.[5, 58, 59] Therefore, it appears that research domains with tailored interventions to both ICU and non‐ICU severe sepsis patients are important areas of inquiry for clinicians. Such research may serve the purpose of assessing impact of early mobilization and unmasking any treatment heterogeneity that may exist when dealing with severe sepsis. Though trials of PT in ICU‐based severe sepsis are underway,[60] it is prudent that these also extend beyond the ICU‐setting.
Third, variability in early mobility practices and billing documentation for severe sepsis patients may exist, adding barriers to performing high‐quality research on this topic. In fact, administrative billing records for PT may offer insufficient granularity about services provided or therapies administered, particularly in the ICU where variability in early mobilization practices have been shown despite common employment of physiotherapists.[61]
Finally, many hospitalists may believe that patients with severe sepsis are simply too sick for early mobilization or PT, possibly limiting their participation in clinical or research‐based interventions. This perception has been well described in ICU populations, where it has been well studied and shown to be false.[41, 42, 43] Nevertheless, if severe sepsis patients are viewed as relatively sick hospitalized patients, it is plausible that resistance against early mobilization interventions may exist.[62] Understanding these biases and being mindful of such barriers when conducting studies in this area would be important.
CONCLUSION AND FUTURE DIRECTIONS
The cost burdens of severe sepsis are substantial. Elixhauser et al. suggest that it is currently the single most expensive cause of acute hospitalization in the United States.[63] Importantly, a large proportion of patients with severe sepsis receive care from hospitalists and/or floor teams on the general wards. Our integrative review has demonstrated a knowledge gap when it comes to rigorous assessments of PT and mobilization treatments in patients with severe sepsis within and beyond the ICU. Existing evidence provides a strong rationale for why functional decline occurs in patients with severe sepsis. A reasonable argument for PT‐based interventions to mitigate functional decline in this subset exists, but rigorous evaluation of such interventions is necessary. Physical and mobilization‐based treatments are routinely available and efficacious in several other settings and populations. It could be rapidly deployed and potentially improve outcomes in those with severe sepsis. Research would be welcomed to establish optimal dosing, efficacy, and cost effectiveness of PT and early mobilization for severe sepsis, particularly in patients treated on the general wards by hospitalists and floor teams.
How may such a research agenda be launched? A balanced multipronged approach is necessary. First, large‐scale epidemiological data to understand variation in practice are needed. Focused studies carried out by community and academic hospitalists on septic patients treated outside the ICU are the call of the hour. These data, in turn, can help create registries that assess for risk factors, quality of treatment, and long‐term outcomes among survivors of this condition. Second, evaluation and improvement of the coding and precision of physical and occupational therapy billing records is necessary so that their added value can be assessed and tracked using administrative data. Third, targeted prospective studies and clinical trials to directly evaluate the effect of PT in well‐defined patient populations with sepsis outside the ICU are needed. In this arena, hospitalist expertise and trained physical therapists will be crucial. The focus of this work should be directed toward both short‐term and long‐term functional outcomes, as well as mortality and morbidity assessments. Fourth, these patient‐centered efforts should loop back and inform the foundational biology of severe sepsis, thus illuminating patient‐centered end points, from biomarker analysis to physiometric measurements in basic and translational research.
In conclusion, this review sheds light on the fact that interventions that may mitigate the functional and cognitive decline in survivors of severe sepsis appear underdeveloped. Although the precise benefit of such interventions remains unclear, the low‐cost, widespread availability and generalizability of PT‐based interventions make it a worthy candidate for future research. As the numbers of survivors of sepsis expand, an unmet public health need for interventions to improve the long‐term outcomes of this population exists. Hospitalists and intensivists caring for severe sepsis patients must rise to meet this need. Together, we can help improve the lives of patients afflicted with severe sepsis, wherever they may receive care in the hospital.
Acknowledgements
The authors acknowledge the efforts of medical research librarians Andy Hickner, MSI, and Marissa Conte, MSI, on this project.
Disclosures
This work was supported by the National Institutes of HealthK08, HL091249 (T.J.I.) and VA HSR&D IIR‐11109 (T.J.I.). The views expressed here are the authors' own and do not necessarily represent the views of the US government or the Department of Veterans' Affairs. The authors report no conflicts of interest.
- , . Epidemiology of sepsis: an update. Crit Care Med. 2001;29:S109–S116.
- , , , et al. Nationwide trends of severe sepsis in the 21st century (2000–2007). Chest. 2011;140:1223–1231.
- , , , et al. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554.
- , , , et al. Functional outcomes of general medical patients with severe sepsis. BMC Infect Dis. 2013;13:588.
- , , , et al. The epidemiology of acute organ system dysfunction from severe sepsis outside of the intensive care unit. J Hosp Med. 2013;8:243–247.
- , , , et al. Population burden of long‐term survivorship after severe sepsis in older Americans. J Am Geriatr Soc. 2012;60:1070–1077.
- , , , et al. Systemic inflammatory response syndrome increases immobility‐induced neuromuscular weakness. Crit Care Med. 2008;36:910–916.
- , , , et al. Early goal‐directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377.
- . The lingering consequences of sepsis: a hidden public health disaster? JAMA. 2010;304:1833–1834.
- , , , et al. Long‐term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304:1787–1794.
- , , , et al. Spurious inferences about long‐term outcomes: the case of severe sepsis and geriatric conditions. Am J Respir Crit Care Med. 2012;185:835–841.
- , , , et al. Long‐term outcome and quality‐adjusted life years after severe sepsis. Crit Care Med. 2009;37:1268–1274.
- , , , et al. Long‐term mortality and quality of life in sepsis: a systematic review. Crit Care Med. 2010;38:1276–1283.
- , , , et al. Improving post‐intensive care unit neuropsychiatric outcomes: understanding cognitive effects of physical activity. Am J Respir Crit Care Med. 2012;186:1220–1228.
- , , , et al. Hospitalizations, costs, and outcomes of severe sepsis in the United States 2003 to 2007. Crit Care Med. 2012;40:754–761.
- , , , et al. Long‐term acute care hospital utilization after critical illness. JAMA. 2010;303:2253–2259.
- , , , et al. Long‐term survival and healthcare utilization outcomes attributable to sepsis and pneumonia. BMC Health Serv Res. 2012;12:432.
- , , , et al. Long‐term mortality and medical care charges in patients with severe sepsis. Crit Care Med. 2003;31:2316–2323.
- , , , et al. Early exercise in critically ill patients enhances short‐term functional recovery. Crit Care Med. 2009;37:2499–2505.
- , , , et al. Exercise‐based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev. 2011;(7):CD001800.
- , , , et al. What is the role of timing in the surgical and rehabilitative care of community‐dwelling older persons with acute hip fracture? Arch Intern Med. 1997;157:513–520.
- , , . Extra physical therapy reduces patient length of stay and improves functional outcomes and quality of life in people with acute or subacute conditions: a systematic review. Arch Phys Med Rehabilil. 2011;92:1490–1500.
- , , . Reduction of intensive care unit length of stay: the case of early mobilization. Health Care Manag (Frederick). 2014;33:128–135.
- , , , et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373:1874–1882.
- , , , et al. Hospitalization, restricted activity, and the development of disability among older persons. JAMA. 2004;292:2115–2124.
- , , , et al. Low mobility during hospitalization and functional decline in older adults. J Am Geriatr Soc. 2011;59:266–273.
- , . The Medical Works of Hippocrates. Oxford, United Kingdom: Blackwell; 1950.
- , , . An overview of the issues: physiological effects of bed rest and restricted physical activity. Med Sci Sports Exerc. 1997;29:187–190.
- , , , et al. Prolonged bed rest decreases skeletal muscle and whole body protein synthesis. Am J Physiol. 1996;270:E627–E633.
- , . Metabolic consequences of muscle disuse atrophy. J Nutr. 2005;135:1824S–1828S.
- . Inactivity and inflammation in the critically ill patient. Crit Care Clin. 2007;23:21–34.
- . Hazards of hospitalization of the elderly. Ann Intern Med. 1993;118:219–223.
- , , . Hospitalization‐associated disability: “She was probably able to ambulate, but I'm not sure”. JAMA. 2011;306:1782–1793.
- , , , et al. A sustained rat model for studying the long‐lasting catabolic state of sepsis. Infect Immun. 1999;67:1079–1085.
- . Regulation of skeletal muscle protein turnover during sepsis. Curr Opin Clin Nutr. Metab Care. 1998;1:217–224.
- , , . Regulation of muscle protein synthesis during sepsis and inflammation. Am J Physiol Endocrinol Metab. 2007;293:E453–E459.
- , . From muscle disuse to myopathy in COPD: potential contribution of oxidative stress. Eur Respir J. 2005;26:703–719.
- , , . Oxidative stress and gene expression in sepsis. Br J Anaesth. 2003;90:221–232.
- , , , et al. Early ambulation and length of stay in older adults hospitalized for acute illness. Arch Intern Med. 2010;170:1942–1943.
- . Intensive exercise training during bed rest attenuates deconditioning. Med Sci Sports Exerc. 1997;29:207–215.
- , , , et al. Early activity is feasible and safe in respiratory failure patients. Crit Care Med. 2007;35:139–145.
- , , , et al. Early intensive care unit mobility therapy in the treatment of acute respiratory failure. Crit Care Med. 2008;36:2238–2243.
- . Clinical trials of early mobilization of critically ill patients. Crit Care Med. 2009;37:S442–S447.
- . Mobilizing patients in the intensive care unit: improving neuromuscular weakness and physical function. JAMA. 2008;300:1685–1690.
- , , , et al. Potential effect of physiotherapeutic treatment on mortality rate in patients with severe sepsis and septic shock: a retrospective cohort analysis. J Crit Care. 2013;28:954–958.
- , , , et al. Effects of physical training on functional status in patients with prolonged mechanical ventilation. Phys Ther. 2006;86:1271–1281.
- , , , et al. Impact of whole‐body rehabilitation in patients receiving chronic mechanical ventilation. Crit Care Med. 2005;33:2259–2265.
- . Rehabilitation of patients admitted to a respiratory intensive care unit. Arch Phys Med Rehabil. 1998;79:849–854.
- , , , et al. Supervised moderate intensity exercise improves distance walked at hospital discharge following coronary artery bypass graft surgery—a randomised controlled trial. Heart Lung Circ. 2008;17:129–138.
- , , , et al. Systematic review of hip fracture rehabilitation practices in the elderly. Arch Phys Med Rehabil. 2009;90:246–262.
- , , , et al. Physical therapy and mobility 2 and 6 months after hip fracture. J Am Geriatr Soc. 2004;52:1114–1120.
- , , , et al. Physical fitness training for stroke patients. Cochrane Database Syst Rev. 2011;(11):CD003316.
- , , , et al. Effects of augmented exercise therapy on outcome of gait and gait‐related activities in the first 6 months after stroke: a meta‐analysis. Stroke. 2011;42:3311–3315.
- , , , et al. Effects of augmented exercise therapy time after stroke: a meta‐analysis. Stroke. 2004;35:2529–2539.
- , , , et al. Effect of physical therapy on pain and function in patients with hip osteoarthritis: a randomized clinical trial. JAMA. 2014;311:1987–1997.
- , , , et al. Early mobilization of patients hospitalized with community‐acquired pneumonia. Chest. 2003;124:883–889.
- , , , et al. Magnitude and duration of the effect of sepsis on survival. Department of Veterans Affairs Systemic Sepsis Cooperative Studies Group. JAMA. 1997;277:1058–1063.
- , , , et al. Epidemiology of sepsis in Victoria, Australia. Crit Care Med. 2005;33:71–80.
- , , , et al. Sepsis incidence and outcome: contrasting the intensive care unit with the hospital ward. Crit Care Med. 2007;35:1284–1289.
- , , . Early rehabilitation in sepsis: a prospective randomised controlled trial investigating functional and physiological outcomes The i‐PERFORM Trial (Protocol Article). BMC Anesthesiol. 2011;11:21.
- , , , et al. TEAM: a prospective multi‐centre cohort study of early activity and mobilisation in ICU. In: American Thoracic Society 2013 International Conference; May 17–22, 2013; Philadelphia, PA. Am J Respir Crit Care Med. 2013;187:A3625.
- , , , et al. Improving long‐term outcomes after discharge from intensive care unit: report from a stakeholders' conference. Crit Care Med. 2012;40:502–509.
- , , . Septicemia in U.S. hospitals, 2009: statistical brief #122. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville, MD; 2006.
- , . Epidemiology of sepsis: an update. Crit Care Med. 2001;29:S109–S116.
- , , , et al. Nationwide trends of severe sepsis in the 21st century (2000–2007). Chest. 2011;140:1223–1231.
- , , , et al. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554.
- , , , et al. Functional outcomes of general medical patients with severe sepsis. BMC Infect Dis. 2013;13:588.
- , , , et al. The epidemiology of acute organ system dysfunction from severe sepsis outside of the intensive care unit. J Hosp Med. 2013;8:243–247.
- , , , et al. Population burden of long‐term survivorship after severe sepsis in older Americans. J Am Geriatr Soc. 2012;60:1070–1077.
- , , , et al. Systemic inflammatory response syndrome increases immobility‐induced neuromuscular weakness. Crit Care Med. 2008;36:910–916.
- , , , et al. Early goal‐directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377.
- . The lingering consequences of sepsis: a hidden public health disaster? JAMA. 2010;304:1833–1834.
- , , , et al. Long‐term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304:1787–1794.
- , , , et al. Spurious inferences about long‐term outcomes: the case of severe sepsis and geriatric conditions. Am J Respir Crit Care Med. 2012;185:835–841.
- , , , et al. Long‐term outcome and quality‐adjusted life years after severe sepsis. Crit Care Med. 2009;37:1268–1274.
- , , , et al. Long‐term mortality and quality of life in sepsis: a systematic review. Crit Care Med. 2010;38:1276–1283.
- , , , et al. Improving post‐intensive care unit neuropsychiatric outcomes: understanding cognitive effects of physical activity. Am J Respir Crit Care Med. 2012;186:1220–1228.
- , , , et al. Hospitalizations, costs, and outcomes of severe sepsis in the United States 2003 to 2007. Crit Care Med. 2012;40:754–761.
- , , , et al. Long‐term acute care hospital utilization after critical illness. JAMA. 2010;303:2253–2259.
- , , , et al. Long‐term survival and healthcare utilization outcomes attributable to sepsis and pneumonia. BMC Health Serv Res. 2012;12:432.
- , , , et al. Long‐term mortality and medical care charges in patients with severe sepsis. Crit Care Med. 2003;31:2316–2323.
- , , , et al. Early exercise in critically ill patients enhances short‐term functional recovery. Crit Care Med. 2009;37:2499–2505.
- , , , et al. Exercise‐based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev. 2011;(7):CD001800.
- , , , et al. What is the role of timing in the surgical and rehabilitative care of community‐dwelling older persons with acute hip fracture? Arch Intern Med. 1997;157:513–520.
- , , . Extra physical therapy reduces patient length of stay and improves functional outcomes and quality of life in people with acute or subacute conditions: a systematic review. Arch Phys Med Rehabilil. 2011;92:1490–1500.
- , , . Reduction of intensive care unit length of stay: the case of early mobilization. Health Care Manag (Frederick). 2014;33:128–135.
- , , , et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373:1874–1882.
- , , , et al. Hospitalization, restricted activity, and the development of disability among older persons. JAMA. 2004;292:2115–2124.
- , , , et al. Low mobility during hospitalization and functional decline in older adults. J Am Geriatr Soc. 2011;59:266–273.
- , . The Medical Works of Hippocrates. Oxford, United Kingdom: Blackwell; 1950.
- , , . An overview of the issues: physiological effects of bed rest and restricted physical activity. Med Sci Sports Exerc. 1997;29:187–190.
- , , , et al. Prolonged bed rest decreases skeletal muscle and whole body protein synthesis. Am J Physiol. 1996;270:E627–E633.
- , . Metabolic consequences of muscle disuse atrophy. J Nutr. 2005;135:1824S–1828S.
- . Inactivity and inflammation in the critically ill patient. Crit Care Clin. 2007;23:21–34.
- . Hazards of hospitalization of the elderly. Ann Intern Med. 1993;118:219–223.
- , , . Hospitalization‐associated disability: “She was probably able to ambulate, but I'm not sure”. JAMA. 2011;306:1782–1793.
- , , , et al. A sustained rat model for studying the long‐lasting catabolic state of sepsis. Infect Immun. 1999;67:1079–1085.
- . Regulation of skeletal muscle protein turnover during sepsis. Curr Opin Clin Nutr. Metab Care. 1998;1:217–224.
- , , . Regulation of muscle protein synthesis during sepsis and inflammation. Am J Physiol Endocrinol Metab. 2007;293:E453–E459.
- , . From muscle disuse to myopathy in COPD: potential contribution of oxidative stress. Eur Respir J. 2005;26:703–719.
- , , . Oxidative stress and gene expression in sepsis. Br J Anaesth. 2003;90:221–232.
- , , , et al. Early ambulation and length of stay in older adults hospitalized for acute illness. Arch Intern Med. 2010;170:1942–1943.
- . Intensive exercise training during bed rest attenuates deconditioning. Med Sci Sports Exerc. 1997;29:207–215.
- , , , et al. Early activity is feasible and safe in respiratory failure patients. Crit Care Med. 2007;35:139–145.
- , , , et al. Early intensive care unit mobility therapy in the treatment of acute respiratory failure. Crit Care Med. 2008;36:2238–2243.
- . Clinical trials of early mobilization of critically ill patients. Crit Care Med. 2009;37:S442–S447.
- . Mobilizing patients in the intensive care unit: improving neuromuscular weakness and physical function. JAMA. 2008;300:1685–1690.
- , , , et al. Potential effect of physiotherapeutic treatment on mortality rate in patients with severe sepsis and septic shock: a retrospective cohort analysis. J Crit Care. 2013;28:954–958.
- , , , et al. Effects of physical training on functional status in patients with prolonged mechanical ventilation. Phys Ther. 2006;86:1271–1281.
- , , , et al. Impact of whole‐body rehabilitation in patients receiving chronic mechanical ventilation. Crit Care Med. 2005;33:2259–2265.
- . Rehabilitation of patients admitted to a respiratory intensive care unit. Arch Phys Med Rehabil. 1998;79:849–854.
- , , , et al. Supervised moderate intensity exercise improves distance walked at hospital discharge following coronary artery bypass graft surgery—a randomised controlled trial. Heart Lung Circ. 2008;17:129–138.
- , , , et al. Systematic review of hip fracture rehabilitation practices in the elderly. Arch Phys Med Rehabil. 2009;90:246–262.
- , , , et al. Physical therapy and mobility 2 and 6 months after hip fracture. J Am Geriatr Soc. 2004;52:1114–1120.
- , , , et al. Physical fitness training for stroke patients. Cochrane Database Syst Rev. 2011;(11):CD003316.
- , , , et al. Effects of augmented exercise therapy on outcome of gait and gait‐related activities in the first 6 months after stroke: a meta‐analysis. Stroke. 2011;42:3311–3315.
- , , , et al. Effects of augmented exercise therapy time after stroke: a meta‐analysis. Stroke. 2004;35:2529–2539.
- , , , et al. Effect of physical therapy on pain and function in patients with hip osteoarthritis: a randomized clinical trial. JAMA. 2014;311:1987–1997.
- , , , et al. Early mobilization of patients hospitalized with community‐acquired pneumonia. Chest. 2003;124:883–889.
- , , , et al. Magnitude and duration of the effect of sepsis on survival. Department of Veterans Affairs Systemic Sepsis Cooperative Studies Group. JAMA. 1997;277:1058–1063.
- , , , et al. Epidemiology of sepsis in Victoria, Australia. Crit Care Med. 2005;33:71–80.
- , , , et al. Sepsis incidence and outcome: contrasting the intensive care unit with the hospital ward. Crit Care Med. 2007;35:1284–1289.
- , , . Early rehabilitation in sepsis: a prospective randomised controlled trial investigating functional and physiological outcomes The i‐PERFORM Trial (Protocol Article). BMC Anesthesiol. 2011;11:21.
- , , , et al. TEAM: a prospective multi‐centre cohort study of early activity and mobilisation in ICU. In: American Thoracic Society 2013 International Conference; May 17–22, 2013; Philadelphia, PA. Am J Respir Crit Care Med. 2013;187:A3625.
- , , , et al. Improving long‐term outcomes after discharge from intensive care unit: report from a stakeholders' conference. Crit Care Med. 2012;40:502–509.
- , , . Septicemia in U.S. hospitals, 2009: statistical brief #122. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville, MD; 2006.
Bigger Than His Bite
A 58‐year‐old male presented to a local community hospital emergency department with fever and altered mental status. Earlier in the day he had complained of chills, swollen tongue, numbness and tingling in his extremities with associated burning pain, and generalized weakness. En route to the emergency department, he was extremely agitated and moving uncontrollably. On arrival, he was noted to be in respiratory distress and was intubated for hypoxic respiratory failure. He was subsequently transferred to an academic medical center, and in transit was noted to have sustained supraventricular tachycardia with a heart rate of 160 beats per minute.
Although the differential for altered mental status is broad, associated fever limits the main diagnostic considerations to infectious, toxic, and some inflammatory disorders. Confusion and fever are most concerning for a central nervous system infection, either meningitis or encephalitis. Sepsis from a broader range of infectious etiologies may also present with these symptoms. His respiratory failure could represent acute respiratory distress syndrome (ARDS) due to sepsis, aspiration, or a manifestation of a multisystem inflammatory disease.
He did not have any significant past medical or surgical history. Three days before his initial presentation, the patient was bitten on the left hand and forearm while breaking up a dogfight. The dogs that bit him belonged to his son, but were unvaccinated. He did not seek medical attention and it was unclear how he treated his wounds at home.
Dogs may serve as vectors for a number of zoonoses. Species of both Pasteurella and Capnocytophaga may cause sepsis and rarely meningitis as a consequence of dog bites. The incubation period of 3 days, though brief, does not exclude either infection. Rabies encephalitis is also possible, particularly given the dogs' unvaccinated status. However, the typical incubation period for rabies is on the order of months, and a 3‐day interval from inoculation to symptoms would be highly unusual. Although other explanations for his symptoms are more likely, he should still be considered for vaccination and rabies immune globulin. The dogs should be observed for clinical manifestations of rabies. Despite the patient's history of dog bite, a broad differential diagnosis must be maintained.
The patient lived in Michigan and worked in a chemical factory driving equipment without any hazardous exposures. He did not have any allergies. He drank 6 beers per day; he did not smoke cigarettes and had no history of illicit drug use. He was single and had 4 adult children.
His history of heavy alcohol consumption raises several additional possibilities. Delirium tremens, alcohol withdrawal seizures, or hepatic encephalopathy as a consequence of alcoholic cirrhosis are both potential contributors to his presentation. Furthermore, the physiologic signs of alcohol withdrawal are similar to many critical illnesses, which may present a diagnostic challenge. The patient's history of employment at a chemical factory is intriguing, though the details of any potential occupational exposures are unknown. Carbon monoxide poisoning can present with altered mental status and agitation, whereas anticholinergic toxicity can present with fever, tachycardia, and altered mental status; however, there is no obvious source of exposure to either.
On physical examination, the patient was intubated with a Glasgow Coma Scale of 11 without sedation; serial examinations revealed a fluctuating level of consciousness. His temperature was 38.1C, heart rate was 158 beats per minute, and blood pressure was 93/68 mm Hg. Mechanical ventilation was provided with assist control mode, a respiratory rate of 28 breaths per minute, tidal volume 466 mL, and positive end expiratory pressure of 20 cm of water. His oxygen saturation was 81% on 100% oxygen. Examination of his neck exhibited a large left neck hematoma from the unsuccessful placement of an external jugular intravenous catheter. Pupils were 4 mm in diameter and minimally reactive. There was no scleral icterus. Cardiac exam revealed tachycardia and regular rhythm without murmurs, rubs, or gallops. Lung exam was significant for bilateral rhonchi and minimal tracheal secretions. Extremity exam revealed 0.25 to 1.5 cm in diameter puncture bite marks with abrasions on his left third and fourth upper extremity digits as well as on his left forearm. Skin exam was diffusely cool with a mottled appearance. Neurologic exam revealed absent deep tendon reflexes throughout and apparent flaccid paralysis of all 4 extremities. Examination of the abdomen, lymph nodes, mouth, and throat were unremarkable.
The shock associated with sepsis is typically distributive, with intense vasodilation that classically leads to warm extremities. His mottled, cool extremities raise concern for disseminated intravascular coagulation (DIC), which can be seen in patients with septic shock, particularly cases caused by meningococcal disease and Capnocytophaga infections. His neurologic examination is typical of lower motor neuron disease, although acute upper motor neuron lesions can also be associated with hyporeflexia. Rabies can manifest as flaccid paralysis, but this would classically predate the mental status changes. Rabies remains a consideration, albeit a less likely one. Zoonoses, particularly Capnocytophaga and Pasteurella, are possible; however, a thorough search for other infections leading to sepsis is still indicated. His lung findings suggest severe ARDS.
The white blood cell count was 5,900/mm3, with 91% neutrophils, 6.6% lymphocytes, and 0.5% monocytes. The hemoglobin level was 13.0 g/dL, and the platelet count was 12,000/mm3. The fibrinogen level was 89 mg/dL (normal range 200400 mg/dL), international normalized ratio and partial‐thromboplastin time were 4.6 (normal range 0.8 to 1.1) and greater than 120.0 seconds (normal range 2535 seconds), respectively. Lactate dehydrogenase level was 698 IU/L (normal 120240 IU/L), and haptoglobin was 54 mg/dL (normal 41165 mg/dL). Serum sodium was 136 mmol/L, potassium 4.6 mmol/L, chloride 101 mmol/L, bicarbonate 16 mmol/L, blood urea nitrogen 29 mg/dL, creatinine 2.28 mg/dL, glucose 123 mg/dL, calcium 7.0 mg/dL, magnesium 1.7 mg/dL, and phosphorus 7.2 mg/dL. Total protein was 4.3 g/dL (normal 6.08.3 g/dL), albumin 2.5 g/dL (normal 3.54.9 g/dL), total bilirubin 2.3 mg/dL (normal 0.21.2 mg/dL), aspartate aminotransferase 71 IU/L (normal 830 IU/L), alanine aminotransferase 29 IU/L (normal 735 IU/L), and alkaline phosphatase 107 IU/L (normal 30130 IU/L). The serum troponin‐I level was 0.76 ng/mL, creatine phosphokinase 397 ng/mL, and creatine kinase‐myocardial band 3.5 ng/mL. Initial arterial blood gas analysis revealed a pH of 7.00, pCO2 57 mm Hg, pO2 98 mm Hg, and a lactic acid of 6.5 mmol/L (normal 0.52.2 mmol/L).
The patient has a normal absolute white blood cell count in the setting of septic shock. He has a relative neutrophilia and a marked leukopenia, both of which can be seen in overwhelming infections. The patient's arterial blood gas analysis indicates he has a mixed metabolic and respiratory acidosis. The normal physiologic response to metabolic acidosis is to increase minute ventilation and induce a compensatory respiratory alkalosis. His concomitant respiratory acidosis in the face of mechanical ventilation and presumed adequate minute ventilation suggests severely impaired alveolar gas exchange, most likely from ARDS. He has numerous other metabolic abnormalities, including acute kidney injury, DIC, and hemolytic anemia, all of which may be seen with severe bacterial infections or septic shock. Neisseria meningitidis and other gram‐negative infections would be of particular concern in this case. The combination of fever, altered mental status, thrombocytopenia, hemolytic anemia, and renal failure could be consistent with thrombotic thrombocytopenic purpura. However, the prolonged coagulation studies are much more consistent with DIC.
Intravenous antimicrobials were administered including ceftriaxone (initiated in the emergency department of the transferring hospital), ampicillin, vancomycin, piperacillin/tazobactam, clindamycin, metronidazole, doxycycline, and acyclovir. He received tetanus and rabies vaccines as well as tetanus and rabies immune globulin. The patient was given aggressive intravenous crystalloid fluids with minimal response in blood pressure. Intravenous norepinephrine was initiated to maintain a mean arterial pressure above 65 mm Hg. A plain chest radiograph (Figure 1) revealed perihilar airspace opacities. Head computed tomography without contrast revealed global cerebral volume loss greater than expected for the patient's age; no evidence of intracranial hemorrhage, mass effect, or edema; and proptosis of the eyes with adjacent preseptal soft tissue swelling without evidence of retrobulbar hemorrhage or vascular engorgement. Ultrasound of the left neck hematoma was negative for pulsatile mass. Electrocardiogram (ECG) revealed sinus tachycardia without evidence of ischemic changes. A bedside transthoracic echocardiogram showed hyperdynamic changes without evidence of hypokinesis but with inspiratory collapse of the inferior vena cava. Abdominal ultrasound was normal. Plain radiographs of the left hand (Figure 2) identified only mild soft tissue swelling over the dorsum of the hand. An ultrasound of the left hand and left forearm did not identify any abnormal fluid collection. A dialysis catheter was placed after the patient received platelets and fresh frozen plasma for initiation of continuous renal replacement therapy.
Given this patient's fulminant presentation, he was appropriately started on a very broad anti‐infective regimen. Although fungal infections are less likely, his current antimicrobial regimen lacks antifungal coverage. His finding of proptosis raises concern for mucormycosis, although the time course and clinical presentation are somewhat atypical. Because of the severity of his presentation, initiation of amphotericin B could be considered if he fails to quickly respond to the current regimen. There is no known effective treatment for rabies. Thus, if his presentation is due to rabies encephalitis, rabies vaccine and immunoglobulin will not be effective at treating active rabies infection. However, given his exposure history and the dogs' unvaccinated status, postexposure prophylaxis was appropriate to prevent future development of rabies. The inspiratory collapse and hyperdynamic ventricular response seen on his bedside echocardiogram is consistent with decreased effective circulating volume from sepsis or severe hypovolemia rather than acute heart failure.
Less than 36 hours after admission (60 hours after his symptoms began), the patient's oxygenation status had not improved. He developed diffuse cutaneous purpura with hemorrhagic bullae. Liver, renal, and cardiac function markers were all markedly abnormal. All cultures from the transferring hospital, collected before antibiotics were initiated, were negative to date. However, Gram stain of blood cultures performed at the academic medical center revealed possible gram‐negative rods. The patient remained unresponsive without sedation. ECG revealed evidence of inferior and anterolateral ischemia. The patient's family was informed of his persistently deteriorating condition and elected to pursue comfort measures. Two hours later the patient expired. The family agreed to an autopsy.
This patient succumbed to overwhelming sepsis and multiorgan failure. Although the etiologic pathogen is not immediately clear, several clues point to a likely unifying diagnosis. First, he has a history of a recent dog bite with minimal local evidence of infection. Second, he presented with fulminant sepsis with DIC, hemolytic anemia, and diffuse mottling that progressed to purpura fulminans. Third, a possible gram‐negative rod was isolated on blood Gram stain. Fourth, he has a history of heavy alcohol use. For these reasons, Capnocytophaga canimorsus is the most likely underlying etiology. C canimorsus is a fastidious gram‐negative coccobacillus that is an uncommon cause of fulminant sepsis in patients with dog bites. It is difficult to isolate due to culture growth requirements, which may explain the negative blood cultures in this case. Patients with alcoholism are predisposed to fulminant sepsis from C canimorsus, which often presents with hepatic and renal failure. The myocardial ischemia may be secondary to the metabolic and thrombotic complications of sepsis.
On autopsy, there was purpura fulminans involving over 90% of the total body surface area as well as skin slippage and loose bullae of greater than 75% of the total body surface area. There was infarction of the kidneys, liver, spleen, and adrenal glands as well as focal contraction bands of necrosis of the myocardium. The lungs showed diffuse alveolar damage. There was hemorrhage, edema, and necrosis seen in sections taken from the puncture wounds. Following the patient's death, it was reported by the transferring institution that C canimorsus was identified from 2 of 2 antemortem blood cultures, and pan‐sensitive Acinetobacter lwoffii in 1 of 2 blood cultures, though no sensitivities were performed on the C canimorsus isolate. In addition, antemortem cultures obtained at the academic medical center identified Capnocytophaga species in 1 of 2 peripheral blood culture specimens; sensitivities were not performed. Autopsy determined the cause of death in this patient to be septic complications of dog bite.
COMMENTARY
Dog bites are frequent, with over 12,000 occurring daily in the United States; of these, approximately 20% require medical attention.[1] Although most patients rapidly recover with conservative management, even initially benign‐appearing injuries can lead to long‐term morbidity or death. The hands are most often affected and are associated with more frequent need for both antibiotics and surgical intervention.[2, 3] The severity of injury does not correlate with subsequent infections.[3]
Management of dog bite injuries includes careful wound management. All patients with moderate to severe injury should be assessed within 48 hours by physical examination and radiography to assess the degree of injury and any associated nerve, tendon, joint, or bone damage. If there is concern for rabies based on history or vaccination status of the animal, prompt irrigation and debridement is crucial. Antimicrobial prophylaxis, typically with amoxicillinclavulanate, should be given to high‐risk patients, such as those with cirrhosis, asplenia, or other immunosuppressing conditions.[4] Most infections are caused by Pasteurella and Bacteroides, whereas Capnocytophaga may cause severe disease, particularly in patients with immunosuppression or excessive alcohol intake.[5] This patient was at increased risk of infection due to his late presentation following the initial bite and consequent delayed wound care, injury to the hand, and his history of alcoholism.[4]
Several members of the genus Capnocytophaga have been found in the oral cavities of both humans and canines. C canimorsus, found only in canine or feline oral cavities, is the only member of the genus known to cause human disease.[6] It is a fastidious gram‐negative rod requiring an environment enriched with carbon dioxide, making it notoriously difficult to isolate. Cultures typically do not show growth for 5 to 7 days; thus, it is not surprising all cultures were initially negative in this case.[4, 7] C canimorsus is a well‐described cause of sepsis related to dog bites, with some cases bearing similarity to fulminant meningococcal disease.[8] Severe illness typically occurs in immunosuppressed patients, particularly those with asplenia or cirrhosis.[9, 10] The pathophysiology of fulminant C canimorsus infections is not well described. It has been suggested that certain strains may produce a toxin that inhibits macrophages and inactivates tumor necrosis factor in humans, although this is not yet widely accepted.[11] Treatment of C canimorsus involves early administration of effective antimicrobials, supportive care, and standard management of the bite injury. C canimorsus is susceptible to several classes of antibiotics; ‐lactams, such as penicillin derivatives and cephalosporins, and potentiated sulphonamides, such as trimethoprim/sulfamethoxazole, typically have the best in vitro activity.[12] As illustrated in this case, even with prompt, effective antibiotic administration, C canimorsus infection can progress to DIC, multisystem organ failure, and death.[9]
A lwoffii was also identified, but was almost certainly a contaminant. It is a gram‐negative bacillus that is widely distributed throughout the environment. Commonly found on human skin and within the human oropharynx, it rarely causes human disease. Clinical manifestations of infection with A lwoffii are typically mild, and include superficial skin and soft tissue infection, urinary tract infection, and rarely bacteremia. Because of the severe presentation in this case and the compelling alternative explanation of C canimorsus, A lwoffii was almost certainly a contaminant.
Rabies was an intriguing possibility in this case given the unvaccinated status of the dogs and the patient's prominent neurologic findings. Clinicians must consider the possibility of rabies in any patient with a bite injury from an unvaccinated dog. However, rabies remains extremely rare in the developed world as a result of the overwhelming success of animal vaccination and postexposure prophylaxis. Furthermore, rabies typically has an incubation period of several months. If rabies had caused this patient's presentation, rabies immunoglobulin would have been ineffective. Nevertheless, rabies prophylaxis with rabies immunoglobulin and vaccination is appropriate to prevent subsequent disease unless rabies infection can be definitively excluded.[13]
This patient presented with septic shock, DIC, and multisystem organ failure after a dog bite. The discussant quickly recognized the propensity of Capnocytophaga to cause this constellation of findings in alcoholic patients after dog bites. This patient did not have cirrhosis or asplenia, both of which are known risk factors for C canimorsus infection; however, the fulminant presentation made C canimorsus a necessary consideration. Ultimately, the dramatic nature of the patient's presentation combined with his history of heavy alcohol intake led the discussant to the correct diagnosis of septic shock secondary to C canimorsus infection complicating a benign‐appearing dog bite. Clinicians caring for patients who present with sepsis after a recent dog bite should consider C canimorsus, remembering that on occasion, a dog's bark may not be bigger than his bite.
TEACHING POINTS
- The initial management of moderate or severe dog‐bite injuries includes careful wound assessment and radiography to exclude any associated bone, nerve, joint, or tendon injury.
- Immunosuppressed patients with dog bites, including those with cirrhosis or asplenia, should receive amoxicillin/clavulanate prophylaxis.
- C canimorsus is a fastidious gram‐negative bacillus that may cause fulminant sepsis after dog bites. It is associated with DIC, purpura fulminans, and multisystem organ failure.
- ‐lactam antibiotics, such as penicillin derivatives or cephalosporins, or sulphonamides, are the treatment of choice for C canimorsus.
Disclosure
Nothing to report.
- , , , . Dog bites: still a problem? Injury Prev. 2008;14(5):296–301.
- , , , . Dog bite injuries: primary and secondary emergency department presentations—a retrospective cohort study. ScientificWorldJournal. 2013;2013:393176.
- , , , et al. Management of vascular trauma from dog bites. J Vascular Surg. 2013;58(5):1346–1352.
- , . Dog bites. BMJ. 2007;334(7590):413–417.
- , , , . Bacterial infections as complications of dog bites [in Danish]. Ugeskrift Laeger. 1998;160(34):4860–4863.
- , , , , . Bite‐related and septic syndromes caused by cats and dogs. Lancet Infect Dis. 2009;9(7):439–447.
- , , , , . Bacteriologic analysis of infected dog and cat bites. Emergency Medicine Animal Bite Infection Study Group. N Engl J Med. 1999;340(2):85–92.
- , , , . Diagnosing Capnocytophaga canimorsus infections. Emerg Infect Dis. 2006;12(2):340–342.
- , , . Capnocytophaga canimorsus septicemia in Denmark, 1982–1995: review of 39 cases. Clinical Infect Dis. 1996;23(1):71–75.
- . Consequences of alcohol consumption on host defence. Alcohol Alcohol. 1999;34(6):830–841.
- , , , , , . Molecular characterization of Capnocytophaga canimorsus and other canine Capnocytophaga spp. and assessment by PCR of their frequencies in dogs. J Clin Microbiol. 2009;47(10):3218–3225.
- , , , . The bacteriology and antimicrobial susceptibility of infected and non‐infected dog bite wounds: fifty cases. Vet Microbiol. 2008;127(3‐4):360–368.
- U.S. Department of Health and Human Services. Centers for Disease Control and Prevention. Human rabies—Alabama, Tennessee, and Texas, 1994. Morbidity and Mortality Weekly Report; 1995. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/00036736.htm. Accessed March 1, 2014.
A 58‐year‐old male presented to a local community hospital emergency department with fever and altered mental status. Earlier in the day he had complained of chills, swollen tongue, numbness and tingling in his extremities with associated burning pain, and generalized weakness. En route to the emergency department, he was extremely agitated and moving uncontrollably. On arrival, he was noted to be in respiratory distress and was intubated for hypoxic respiratory failure. He was subsequently transferred to an academic medical center, and in transit was noted to have sustained supraventricular tachycardia with a heart rate of 160 beats per minute.
Although the differential for altered mental status is broad, associated fever limits the main diagnostic considerations to infectious, toxic, and some inflammatory disorders. Confusion and fever are most concerning for a central nervous system infection, either meningitis or encephalitis. Sepsis from a broader range of infectious etiologies may also present with these symptoms. His respiratory failure could represent acute respiratory distress syndrome (ARDS) due to sepsis, aspiration, or a manifestation of a multisystem inflammatory disease.
He did not have any significant past medical or surgical history. Three days before his initial presentation, the patient was bitten on the left hand and forearm while breaking up a dogfight. The dogs that bit him belonged to his son, but were unvaccinated. He did not seek medical attention and it was unclear how he treated his wounds at home.
Dogs may serve as vectors for a number of zoonoses. Species of both Pasteurella and Capnocytophaga may cause sepsis and rarely meningitis as a consequence of dog bites. The incubation period of 3 days, though brief, does not exclude either infection. Rabies encephalitis is also possible, particularly given the dogs' unvaccinated status. However, the typical incubation period for rabies is on the order of months, and a 3‐day interval from inoculation to symptoms would be highly unusual. Although other explanations for his symptoms are more likely, he should still be considered for vaccination and rabies immune globulin. The dogs should be observed for clinical manifestations of rabies. Despite the patient's history of dog bite, a broad differential diagnosis must be maintained.
The patient lived in Michigan and worked in a chemical factory driving equipment without any hazardous exposures. He did not have any allergies. He drank 6 beers per day; he did not smoke cigarettes and had no history of illicit drug use. He was single and had 4 adult children.
His history of heavy alcohol consumption raises several additional possibilities. Delirium tremens, alcohol withdrawal seizures, or hepatic encephalopathy as a consequence of alcoholic cirrhosis are both potential contributors to his presentation. Furthermore, the physiologic signs of alcohol withdrawal are similar to many critical illnesses, which may present a diagnostic challenge. The patient's history of employment at a chemical factory is intriguing, though the details of any potential occupational exposures are unknown. Carbon monoxide poisoning can present with altered mental status and agitation, whereas anticholinergic toxicity can present with fever, tachycardia, and altered mental status; however, there is no obvious source of exposure to either.
On physical examination, the patient was intubated with a Glasgow Coma Scale of 11 without sedation; serial examinations revealed a fluctuating level of consciousness. His temperature was 38.1C, heart rate was 158 beats per minute, and blood pressure was 93/68 mm Hg. Mechanical ventilation was provided with assist control mode, a respiratory rate of 28 breaths per minute, tidal volume 466 mL, and positive end expiratory pressure of 20 cm of water. His oxygen saturation was 81% on 100% oxygen. Examination of his neck exhibited a large left neck hematoma from the unsuccessful placement of an external jugular intravenous catheter. Pupils were 4 mm in diameter and minimally reactive. There was no scleral icterus. Cardiac exam revealed tachycardia and regular rhythm without murmurs, rubs, or gallops. Lung exam was significant for bilateral rhonchi and minimal tracheal secretions. Extremity exam revealed 0.25 to 1.5 cm in diameter puncture bite marks with abrasions on his left third and fourth upper extremity digits as well as on his left forearm. Skin exam was diffusely cool with a mottled appearance. Neurologic exam revealed absent deep tendon reflexes throughout and apparent flaccid paralysis of all 4 extremities. Examination of the abdomen, lymph nodes, mouth, and throat were unremarkable.
The shock associated with sepsis is typically distributive, with intense vasodilation that classically leads to warm extremities. His mottled, cool extremities raise concern for disseminated intravascular coagulation (DIC), which can be seen in patients with septic shock, particularly cases caused by meningococcal disease and Capnocytophaga infections. His neurologic examination is typical of lower motor neuron disease, although acute upper motor neuron lesions can also be associated with hyporeflexia. Rabies can manifest as flaccid paralysis, but this would classically predate the mental status changes. Rabies remains a consideration, albeit a less likely one. Zoonoses, particularly Capnocytophaga and Pasteurella, are possible; however, a thorough search for other infections leading to sepsis is still indicated. His lung findings suggest severe ARDS.
The white blood cell count was 5,900/mm3, with 91% neutrophils, 6.6% lymphocytes, and 0.5% monocytes. The hemoglobin level was 13.0 g/dL, and the platelet count was 12,000/mm3. The fibrinogen level was 89 mg/dL (normal range 200400 mg/dL), international normalized ratio and partial‐thromboplastin time were 4.6 (normal range 0.8 to 1.1) and greater than 120.0 seconds (normal range 2535 seconds), respectively. Lactate dehydrogenase level was 698 IU/L (normal 120240 IU/L), and haptoglobin was 54 mg/dL (normal 41165 mg/dL). Serum sodium was 136 mmol/L, potassium 4.6 mmol/L, chloride 101 mmol/L, bicarbonate 16 mmol/L, blood urea nitrogen 29 mg/dL, creatinine 2.28 mg/dL, glucose 123 mg/dL, calcium 7.0 mg/dL, magnesium 1.7 mg/dL, and phosphorus 7.2 mg/dL. Total protein was 4.3 g/dL (normal 6.08.3 g/dL), albumin 2.5 g/dL (normal 3.54.9 g/dL), total bilirubin 2.3 mg/dL (normal 0.21.2 mg/dL), aspartate aminotransferase 71 IU/L (normal 830 IU/L), alanine aminotransferase 29 IU/L (normal 735 IU/L), and alkaline phosphatase 107 IU/L (normal 30130 IU/L). The serum troponin‐I level was 0.76 ng/mL, creatine phosphokinase 397 ng/mL, and creatine kinase‐myocardial band 3.5 ng/mL. Initial arterial blood gas analysis revealed a pH of 7.00, pCO2 57 mm Hg, pO2 98 mm Hg, and a lactic acid of 6.5 mmol/L (normal 0.52.2 mmol/L).
The patient has a normal absolute white blood cell count in the setting of septic shock. He has a relative neutrophilia and a marked leukopenia, both of which can be seen in overwhelming infections. The patient's arterial blood gas analysis indicates he has a mixed metabolic and respiratory acidosis. The normal physiologic response to metabolic acidosis is to increase minute ventilation and induce a compensatory respiratory alkalosis. His concomitant respiratory acidosis in the face of mechanical ventilation and presumed adequate minute ventilation suggests severely impaired alveolar gas exchange, most likely from ARDS. He has numerous other metabolic abnormalities, including acute kidney injury, DIC, and hemolytic anemia, all of which may be seen with severe bacterial infections or septic shock. Neisseria meningitidis and other gram‐negative infections would be of particular concern in this case. The combination of fever, altered mental status, thrombocytopenia, hemolytic anemia, and renal failure could be consistent with thrombotic thrombocytopenic purpura. However, the prolonged coagulation studies are much more consistent with DIC.
Intravenous antimicrobials were administered including ceftriaxone (initiated in the emergency department of the transferring hospital), ampicillin, vancomycin, piperacillin/tazobactam, clindamycin, metronidazole, doxycycline, and acyclovir. He received tetanus and rabies vaccines as well as tetanus and rabies immune globulin. The patient was given aggressive intravenous crystalloid fluids with minimal response in blood pressure. Intravenous norepinephrine was initiated to maintain a mean arterial pressure above 65 mm Hg. A plain chest radiograph (Figure 1) revealed perihilar airspace opacities. Head computed tomography without contrast revealed global cerebral volume loss greater than expected for the patient's age; no evidence of intracranial hemorrhage, mass effect, or edema; and proptosis of the eyes with adjacent preseptal soft tissue swelling without evidence of retrobulbar hemorrhage or vascular engorgement. Ultrasound of the left neck hematoma was negative for pulsatile mass. Electrocardiogram (ECG) revealed sinus tachycardia without evidence of ischemic changes. A bedside transthoracic echocardiogram showed hyperdynamic changes without evidence of hypokinesis but with inspiratory collapse of the inferior vena cava. Abdominal ultrasound was normal. Plain radiographs of the left hand (Figure 2) identified only mild soft tissue swelling over the dorsum of the hand. An ultrasound of the left hand and left forearm did not identify any abnormal fluid collection. A dialysis catheter was placed after the patient received platelets and fresh frozen plasma for initiation of continuous renal replacement therapy.


Given this patient's fulminant presentation, he was appropriately started on a very broad anti‐infective regimen. Although fungal infections are less likely, his current antimicrobial regimen lacks antifungal coverage. His finding of proptosis raises concern for mucormycosis, although the time course and clinical presentation are somewhat atypical. Because of the severity of his presentation, initiation of amphotericin B could be considered if he fails to quickly respond to the current regimen. There is no known effective treatment for rabies. Thus, if his presentation is due to rabies encephalitis, rabies vaccine and immunoglobulin will not be effective at treating active rabies infection. However, given his exposure history and the dogs' unvaccinated status, postexposure prophylaxis was appropriate to prevent future development of rabies. The inspiratory collapse and hyperdynamic ventricular response seen on his bedside echocardiogram is consistent with decreased effective circulating volume from sepsis or severe hypovolemia rather than acute heart failure.
Less than 36 hours after admission (60 hours after his symptoms began), the patient's oxygenation status had not improved. He developed diffuse cutaneous purpura with hemorrhagic bullae. Liver, renal, and cardiac function markers were all markedly abnormal. All cultures from the transferring hospital, collected before antibiotics were initiated, were negative to date. However, Gram stain of blood cultures performed at the academic medical center revealed possible gram‐negative rods. The patient remained unresponsive without sedation. ECG revealed evidence of inferior and anterolateral ischemia. The patient's family was informed of his persistently deteriorating condition and elected to pursue comfort measures. Two hours later the patient expired. The family agreed to an autopsy.
This patient succumbed to overwhelming sepsis and multiorgan failure. Although the etiologic pathogen is not immediately clear, several clues point to a likely unifying diagnosis. First, he has a history of a recent dog bite with minimal local evidence of infection. Second, he presented with fulminant sepsis with DIC, hemolytic anemia, and diffuse mottling that progressed to purpura fulminans. Third, a possible gram‐negative rod was isolated on blood Gram stain. Fourth, he has a history of heavy alcohol use. For these reasons, Capnocytophaga canimorsus is the most likely underlying etiology. C canimorsus is a fastidious gram‐negative coccobacillus that is an uncommon cause of fulminant sepsis in patients with dog bites. It is difficult to isolate due to culture growth requirements, which may explain the negative blood cultures in this case. Patients with alcoholism are predisposed to fulminant sepsis from C canimorsus, which often presents with hepatic and renal failure. The myocardial ischemia may be secondary to the metabolic and thrombotic complications of sepsis.
On autopsy, there was purpura fulminans involving over 90% of the total body surface area as well as skin slippage and loose bullae of greater than 75% of the total body surface area. There was infarction of the kidneys, liver, spleen, and adrenal glands as well as focal contraction bands of necrosis of the myocardium. The lungs showed diffuse alveolar damage. There was hemorrhage, edema, and necrosis seen in sections taken from the puncture wounds. Following the patient's death, it was reported by the transferring institution that C canimorsus was identified from 2 of 2 antemortem blood cultures, and pan‐sensitive Acinetobacter lwoffii in 1 of 2 blood cultures, though no sensitivities were performed on the C canimorsus isolate. In addition, antemortem cultures obtained at the academic medical center identified Capnocytophaga species in 1 of 2 peripheral blood culture specimens; sensitivities were not performed. Autopsy determined the cause of death in this patient to be septic complications of dog bite.
COMMENTARY
Dog bites are frequent, with over 12,000 occurring daily in the United States; of these, approximately 20% require medical attention.[1] Although most patients rapidly recover with conservative management, even initially benign‐appearing injuries can lead to long‐term morbidity or death. The hands are most often affected and are associated with more frequent need for both antibiotics and surgical intervention.[2, 3] The severity of injury does not correlate with subsequent infections.[3]
Management of dog bite injuries includes careful wound management. All patients with moderate to severe injury should be assessed within 48 hours by physical examination and radiography to assess the degree of injury and any associated nerve, tendon, joint, or bone damage. If there is concern for rabies based on history or vaccination status of the animal, prompt irrigation and debridement is crucial. Antimicrobial prophylaxis, typically with amoxicillinclavulanate, should be given to high‐risk patients, such as those with cirrhosis, asplenia, or other immunosuppressing conditions.[4] Most infections are caused by Pasteurella and Bacteroides, whereas Capnocytophaga may cause severe disease, particularly in patients with immunosuppression or excessive alcohol intake.[5] This patient was at increased risk of infection due to his late presentation following the initial bite and consequent delayed wound care, injury to the hand, and his history of alcoholism.[4]
Several members of the genus Capnocytophaga have been found in the oral cavities of both humans and canines. C canimorsus, found only in canine or feline oral cavities, is the only member of the genus known to cause human disease.[6] It is a fastidious gram‐negative rod requiring an environment enriched with carbon dioxide, making it notoriously difficult to isolate. Cultures typically do not show growth for 5 to 7 days; thus, it is not surprising all cultures were initially negative in this case.[4, 7] C canimorsus is a well‐described cause of sepsis related to dog bites, with some cases bearing similarity to fulminant meningococcal disease.[8] Severe illness typically occurs in immunosuppressed patients, particularly those with asplenia or cirrhosis.[9, 10] The pathophysiology of fulminant C canimorsus infections is not well described. It has been suggested that certain strains may produce a toxin that inhibits macrophages and inactivates tumor necrosis factor in humans, although this is not yet widely accepted.[11] Treatment of C canimorsus involves early administration of effective antimicrobials, supportive care, and standard management of the bite injury. C canimorsus is susceptible to several classes of antibiotics; ‐lactams, such as penicillin derivatives and cephalosporins, and potentiated sulphonamides, such as trimethoprim/sulfamethoxazole, typically have the best in vitro activity.[12] As illustrated in this case, even with prompt, effective antibiotic administration, C canimorsus infection can progress to DIC, multisystem organ failure, and death.[9]
A lwoffii was also identified, but was almost certainly a contaminant. It is a gram‐negative bacillus that is widely distributed throughout the environment. Commonly found on human skin and within the human oropharynx, it rarely causes human disease. Clinical manifestations of infection with A lwoffii are typically mild, and include superficial skin and soft tissue infection, urinary tract infection, and rarely bacteremia. Because of the severe presentation in this case and the compelling alternative explanation of C canimorsus, A lwoffii was almost certainly a contaminant.
Rabies was an intriguing possibility in this case given the unvaccinated status of the dogs and the patient's prominent neurologic findings. Clinicians must consider the possibility of rabies in any patient with a bite injury from an unvaccinated dog. However, rabies remains extremely rare in the developed world as a result of the overwhelming success of animal vaccination and postexposure prophylaxis. Furthermore, rabies typically has an incubation period of several months. If rabies had caused this patient's presentation, rabies immunoglobulin would have been ineffective. Nevertheless, rabies prophylaxis with rabies immunoglobulin and vaccination is appropriate to prevent subsequent disease unless rabies infection can be definitively excluded.[13]
This patient presented with septic shock, DIC, and multisystem organ failure after a dog bite. The discussant quickly recognized the propensity of Capnocytophaga to cause this constellation of findings in alcoholic patients after dog bites. This patient did not have cirrhosis or asplenia, both of which are known risk factors for C canimorsus infection; however, the fulminant presentation made C canimorsus a necessary consideration. Ultimately, the dramatic nature of the patient's presentation combined with his history of heavy alcohol intake led the discussant to the correct diagnosis of septic shock secondary to C canimorsus infection complicating a benign‐appearing dog bite. Clinicians caring for patients who present with sepsis after a recent dog bite should consider C canimorsus, remembering that on occasion, a dog's bark may not be bigger than his bite.
TEACHING POINTS
- The initial management of moderate or severe dog‐bite injuries includes careful wound assessment and radiography to exclude any associated bone, nerve, joint, or tendon injury.
- Immunosuppressed patients with dog bites, including those with cirrhosis or asplenia, should receive amoxicillin/clavulanate prophylaxis.
- C canimorsus is a fastidious gram‐negative bacillus that may cause fulminant sepsis after dog bites. It is associated with DIC, purpura fulminans, and multisystem organ failure.
- ‐lactam antibiotics, such as penicillin derivatives or cephalosporins, or sulphonamides, are the treatment of choice for C canimorsus.
Disclosure
Nothing to report.
A 58‐year‐old male presented to a local community hospital emergency department with fever and altered mental status. Earlier in the day he had complained of chills, swollen tongue, numbness and tingling in his extremities with associated burning pain, and generalized weakness. En route to the emergency department, he was extremely agitated and moving uncontrollably. On arrival, he was noted to be in respiratory distress and was intubated for hypoxic respiratory failure. He was subsequently transferred to an academic medical center, and in transit was noted to have sustained supraventricular tachycardia with a heart rate of 160 beats per minute.
Although the differential for altered mental status is broad, associated fever limits the main diagnostic considerations to infectious, toxic, and some inflammatory disorders. Confusion and fever are most concerning for a central nervous system infection, either meningitis or encephalitis. Sepsis from a broader range of infectious etiologies may also present with these symptoms. His respiratory failure could represent acute respiratory distress syndrome (ARDS) due to sepsis, aspiration, or a manifestation of a multisystem inflammatory disease.
He did not have any significant past medical or surgical history. Three days before his initial presentation, the patient was bitten on the left hand and forearm while breaking up a dogfight. The dogs that bit him belonged to his son, but were unvaccinated. He did not seek medical attention and it was unclear how he treated his wounds at home.
Dogs may serve as vectors for a number of zoonoses. Species of both Pasteurella and Capnocytophaga may cause sepsis and rarely meningitis as a consequence of dog bites. The incubation period of 3 days, though brief, does not exclude either infection. Rabies encephalitis is also possible, particularly given the dogs' unvaccinated status. However, the typical incubation period for rabies is on the order of months, and a 3‐day interval from inoculation to symptoms would be highly unusual. Although other explanations for his symptoms are more likely, he should still be considered for vaccination and rabies immune globulin. The dogs should be observed for clinical manifestations of rabies. Despite the patient's history of dog bite, a broad differential diagnosis must be maintained.
The patient lived in Michigan and worked in a chemical factory driving equipment without any hazardous exposures. He did not have any allergies. He drank 6 beers per day; he did not smoke cigarettes and had no history of illicit drug use. He was single and had 4 adult children.
His history of heavy alcohol consumption raises several additional possibilities. Delirium tremens, alcohol withdrawal seizures, or hepatic encephalopathy as a consequence of alcoholic cirrhosis are both potential contributors to his presentation. Furthermore, the physiologic signs of alcohol withdrawal are similar to many critical illnesses, which may present a diagnostic challenge. The patient's history of employment at a chemical factory is intriguing, though the details of any potential occupational exposures are unknown. Carbon monoxide poisoning can present with altered mental status and agitation, whereas anticholinergic toxicity can present with fever, tachycardia, and altered mental status; however, there is no obvious source of exposure to either.
On physical examination, the patient was intubated with a Glasgow Coma Scale of 11 without sedation; serial examinations revealed a fluctuating level of consciousness. His temperature was 38.1C, heart rate was 158 beats per minute, and blood pressure was 93/68 mm Hg. Mechanical ventilation was provided with assist control mode, a respiratory rate of 28 breaths per minute, tidal volume 466 mL, and positive end expiratory pressure of 20 cm of water. His oxygen saturation was 81% on 100% oxygen. Examination of his neck exhibited a large left neck hematoma from the unsuccessful placement of an external jugular intravenous catheter. Pupils were 4 mm in diameter and minimally reactive. There was no scleral icterus. Cardiac exam revealed tachycardia and regular rhythm without murmurs, rubs, or gallops. Lung exam was significant for bilateral rhonchi and minimal tracheal secretions. Extremity exam revealed 0.25 to 1.5 cm in diameter puncture bite marks with abrasions on his left third and fourth upper extremity digits as well as on his left forearm. Skin exam was diffusely cool with a mottled appearance. Neurologic exam revealed absent deep tendon reflexes throughout and apparent flaccid paralysis of all 4 extremities. Examination of the abdomen, lymph nodes, mouth, and throat were unremarkable.
The shock associated with sepsis is typically distributive, with intense vasodilation that classically leads to warm extremities. His mottled, cool extremities raise concern for disseminated intravascular coagulation (DIC), which can be seen in patients with septic shock, particularly cases caused by meningococcal disease and Capnocytophaga infections. His neurologic examination is typical of lower motor neuron disease, although acute upper motor neuron lesions can also be associated with hyporeflexia. Rabies can manifest as flaccid paralysis, but this would classically predate the mental status changes. Rabies remains a consideration, albeit a less likely one. Zoonoses, particularly Capnocytophaga and Pasteurella, are possible; however, a thorough search for other infections leading to sepsis is still indicated. His lung findings suggest severe ARDS.
The white blood cell count was 5,900/mm3, with 91% neutrophils, 6.6% lymphocytes, and 0.5% monocytes. The hemoglobin level was 13.0 g/dL, and the platelet count was 12,000/mm3. The fibrinogen level was 89 mg/dL (normal range 200400 mg/dL), international normalized ratio and partial‐thromboplastin time were 4.6 (normal range 0.8 to 1.1) and greater than 120.0 seconds (normal range 2535 seconds), respectively. Lactate dehydrogenase level was 698 IU/L (normal 120240 IU/L), and haptoglobin was 54 mg/dL (normal 41165 mg/dL). Serum sodium was 136 mmol/L, potassium 4.6 mmol/L, chloride 101 mmol/L, bicarbonate 16 mmol/L, blood urea nitrogen 29 mg/dL, creatinine 2.28 mg/dL, glucose 123 mg/dL, calcium 7.0 mg/dL, magnesium 1.7 mg/dL, and phosphorus 7.2 mg/dL. Total protein was 4.3 g/dL (normal 6.08.3 g/dL), albumin 2.5 g/dL (normal 3.54.9 g/dL), total bilirubin 2.3 mg/dL (normal 0.21.2 mg/dL), aspartate aminotransferase 71 IU/L (normal 830 IU/L), alanine aminotransferase 29 IU/L (normal 735 IU/L), and alkaline phosphatase 107 IU/L (normal 30130 IU/L). The serum troponin‐I level was 0.76 ng/mL, creatine phosphokinase 397 ng/mL, and creatine kinase‐myocardial band 3.5 ng/mL. Initial arterial blood gas analysis revealed a pH of 7.00, pCO2 57 mm Hg, pO2 98 mm Hg, and a lactic acid of 6.5 mmol/L (normal 0.52.2 mmol/L).
The patient has a normal absolute white blood cell count in the setting of septic shock. He has a relative neutrophilia and a marked leukopenia, both of which can be seen in overwhelming infections. The patient's arterial blood gas analysis indicates he has a mixed metabolic and respiratory acidosis. The normal physiologic response to metabolic acidosis is to increase minute ventilation and induce a compensatory respiratory alkalosis. His concomitant respiratory acidosis in the face of mechanical ventilation and presumed adequate minute ventilation suggests severely impaired alveolar gas exchange, most likely from ARDS. He has numerous other metabolic abnormalities, including acute kidney injury, DIC, and hemolytic anemia, all of which may be seen with severe bacterial infections or septic shock. Neisseria meningitidis and other gram‐negative infections would be of particular concern in this case. The combination of fever, altered mental status, thrombocytopenia, hemolytic anemia, and renal failure could be consistent with thrombotic thrombocytopenic purpura. However, the prolonged coagulation studies are much more consistent with DIC.
Intravenous antimicrobials were administered including ceftriaxone (initiated in the emergency department of the transferring hospital), ampicillin, vancomycin, piperacillin/tazobactam, clindamycin, metronidazole, doxycycline, and acyclovir. He received tetanus and rabies vaccines as well as tetanus and rabies immune globulin. The patient was given aggressive intravenous crystalloid fluids with minimal response in blood pressure. Intravenous norepinephrine was initiated to maintain a mean arterial pressure above 65 mm Hg. A plain chest radiograph (Figure 1) revealed perihilar airspace opacities. Head computed tomography without contrast revealed global cerebral volume loss greater than expected for the patient's age; no evidence of intracranial hemorrhage, mass effect, or edema; and proptosis of the eyes with adjacent preseptal soft tissue swelling without evidence of retrobulbar hemorrhage or vascular engorgement. Ultrasound of the left neck hematoma was negative for pulsatile mass. Electrocardiogram (ECG) revealed sinus tachycardia without evidence of ischemic changes. A bedside transthoracic echocardiogram showed hyperdynamic changes without evidence of hypokinesis but with inspiratory collapse of the inferior vena cava. Abdominal ultrasound was normal. Plain radiographs of the left hand (Figure 2) identified only mild soft tissue swelling over the dorsum of the hand. An ultrasound of the left hand and left forearm did not identify any abnormal fluid collection. A dialysis catheter was placed after the patient received platelets and fresh frozen plasma for initiation of continuous renal replacement therapy.


Given this patient's fulminant presentation, he was appropriately started on a very broad anti‐infective regimen. Although fungal infections are less likely, his current antimicrobial regimen lacks antifungal coverage. His finding of proptosis raises concern for mucormycosis, although the time course and clinical presentation are somewhat atypical. Because of the severity of his presentation, initiation of amphotericin B could be considered if he fails to quickly respond to the current regimen. There is no known effective treatment for rabies. Thus, if his presentation is due to rabies encephalitis, rabies vaccine and immunoglobulin will not be effective at treating active rabies infection. However, given his exposure history and the dogs' unvaccinated status, postexposure prophylaxis was appropriate to prevent future development of rabies. The inspiratory collapse and hyperdynamic ventricular response seen on his bedside echocardiogram is consistent with decreased effective circulating volume from sepsis or severe hypovolemia rather than acute heart failure.
Less than 36 hours after admission (60 hours after his symptoms began), the patient's oxygenation status had not improved. He developed diffuse cutaneous purpura with hemorrhagic bullae. Liver, renal, and cardiac function markers were all markedly abnormal. All cultures from the transferring hospital, collected before antibiotics were initiated, were negative to date. However, Gram stain of blood cultures performed at the academic medical center revealed possible gram‐negative rods. The patient remained unresponsive without sedation. ECG revealed evidence of inferior and anterolateral ischemia. The patient's family was informed of his persistently deteriorating condition and elected to pursue comfort measures. Two hours later the patient expired. The family agreed to an autopsy.
This patient succumbed to overwhelming sepsis and multiorgan failure. Although the etiologic pathogen is not immediately clear, several clues point to a likely unifying diagnosis. First, he has a history of a recent dog bite with minimal local evidence of infection. Second, he presented with fulminant sepsis with DIC, hemolytic anemia, and diffuse mottling that progressed to purpura fulminans. Third, a possible gram‐negative rod was isolated on blood Gram stain. Fourth, he has a history of heavy alcohol use. For these reasons, Capnocytophaga canimorsus is the most likely underlying etiology. C canimorsus is a fastidious gram‐negative coccobacillus that is an uncommon cause of fulminant sepsis in patients with dog bites. It is difficult to isolate due to culture growth requirements, which may explain the negative blood cultures in this case. Patients with alcoholism are predisposed to fulminant sepsis from C canimorsus, which often presents with hepatic and renal failure. The myocardial ischemia may be secondary to the metabolic and thrombotic complications of sepsis.
On autopsy, there was purpura fulminans involving over 90% of the total body surface area as well as skin slippage and loose bullae of greater than 75% of the total body surface area. There was infarction of the kidneys, liver, spleen, and adrenal glands as well as focal contraction bands of necrosis of the myocardium. The lungs showed diffuse alveolar damage. There was hemorrhage, edema, and necrosis seen in sections taken from the puncture wounds. Following the patient's death, it was reported by the transferring institution that C canimorsus was identified from 2 of 2 antemortem blood cultures, and pan‐sensitive Acinetobacter lwoffii in 1 of 2 blood cultures, though no sensitivities were performed on the C canimorsus isolate. In addition, antemortem cultures obtained at the academic medical center identified Capnocytophaga species in 1 of 2 peripheral blood culture specimens; sensitivities were not performed. Autopsy determined the cause of death in this patient to be septic complications of dog bite.
COMMENTARY
Dog bites are frequent, with over 12,000 occurring daily in the United States; of these, approximately 20% require medical attention.[1] Although most patients rapidly recover with conservative management, even initially benign‐appearing injuries can lead to long‐term morbidity or death. The hands are most often affected and are associated with more frequent need for both antibiotics and surgical intervention.[2, 3] The severity of injury does not correlate with subsequent infections.[3]
Management of dog bite injuries includes careful wound management. All patients with moderate to severe injury should be assessed within 48 hours by physical examination and radiography to assess the degree of injury and any associated nerve, tendon, joint, or bone damage. If there is concern for rabies based on history or vaccination status of the animal, prompt irrigation and debridement is crucial. Antimicrobial prophylaxis, typically with amoxicillinclavulanate, should be given to high‐risk patients, such as those with cirrhosis, asplenia, or other immunosuppressing conditions.[4] Most infections are caused by Pasteurella and Bacteroides, whereas Capnocytophaga may cause severe disease, particularly in patients with immunosuppression or excessive alcohol intake.[5] This patient was at increased risk of infection due to his late presentation following the initial bite and consequent delayed wound care, injury to the hand, and his history of alcoholism.[4]
Several members of the genus Capnocytophaga have been found in the oral cavities of both humans and canines. C canimorsus, found only in canine or feline oral cavities, is the only member of the genus known to cause human disease.[6] It is a fastidious gram‐negative rod requiring an environment enriched with carbon dioxide, making it notoriously difficult to isolate. Cultures typically do not show growth for 5 to 7 days; thus, it is not surprising all cultures were initially negative in this case.[4, 7] C canimorsus is a well‐described cause of sepsis related to dog bites, with some cases bearing similarity to fulminant meningococcal disease.[8] Severe illness typically occurs in immunosuppressed patients, particularly those with asplenia or cirrhosis.[9, 10] The pathophysiology of fulminant C canimorsus infections is not well described. It has been suggested that certain strains may produce a toxin that inhibits macrophages and inactivates tumor necrosis factor in humans, although this is not yet widely accepted.[11] Treatment of C canimorsus involves early administration of effective antimicrobials, supportive care, and standard management of the bite injury. C canimorsus is susceptible to several classes of antibiotics; ‐lactams, such as penicillin derivatives and cephalosporins, and potentiated sulphonamides, such as trimethoprim/sulfamethoxazole, typically have the best in vitro activity.[12] As illustrated in this case, even with prompt, effective antibiotic administration, C canimorsus infection can progress to DIC, multisystem organ failure, and death.[9]
A lwoffii was also identified, but was almost certainly a contaminant. It is a gram‐negative bacillus that is widely distributed throughout the environment. Commonly found on human skin and within the human oropharynx, it rarely causes human disease. Clinical manifestations of infection with A lwoffii are typically mild, and include superficial skin and soft tissue infection, urinary tract infection, and rarely bacteremia. Because of the severe presentation in this case and the compelling alternative explanation of C canimorsus, A lwoffii was almost certainly a contaminant.
Rabies was an intriguing possibility in this case given the unvaccinated status of the dogs and the patient's prominent neurologic findings. Clinicians must consider the possibility of rabies in any patient with a bite injury from an unvaccinated dog. However, rabies remains extremely rare in the developed world as a result of the overwhelming success of animal vaccination and postexposure prophylaxis. Furthermore, rabies typically has an incubation period of several months. If rabies had caused this patient's presentation, rabies immunoglobulin would have been ineffective. Nevertheless, rabies prophylaxis with rabies immunoglobulin and vaccination is appropriate to prevent subsequent disease unless rabies infection can be definitively excluded.[13]
This patient presented with septic shock, DIC, and multisystem organ failure after a dog bite. The discussant quickly recognized the propensity of Capnocytophaga to cause this constellation of findings in alcoholic patients after dog bites. This patient did not have cirrhosis or asplenia, both of which are known risk factors for C canimorsus infection; however, the fulminant presentation made C canimorsus a necessary consideration. Ultimately, the dramatic nature of the patient's presentation combined with his history of heavy alcohol intake led the discussant to the correct diagnosis of septic shock secondary to C canimorsus infection complicating a benign‐appearing dog bite. Clinicians caring for patients who present with sepsis after a recent dog bite should consider C canimorsus, remembering that on occasion, a dog's bark may not be bigger than his bite.
TEACHING POINTS
- The initial management of moderate or severe dog‐bite injuries includes careful wound assessment and radiography to exclude any associated bone, nerve, joint, or tendon injury.
- Immunosuppressed patients with dog bites, including those with cirrhosis or asplenia, should receive amoxicillin/clavulanate prophylaxis.
- C canimorsus is a fastidious gram‐negative bacillus that may cause fulminant sepsis after dog bites. It is associated with DIC, purpura fulminans, and multisystem organ failure.
- ‐lactam antibiotics, such as penicillin derivatives or cephalosporins, or sulphonamides, are the treatment of choice for C canimorsus.
Disclosure
Nothing to report.
- , , , . Dog bites: still a problem? Injury Prev. 2008;14(5):296–301.
- , , , . Dog bite injuries: primary and secondary emergency department presentations—a retrospective cohort study. ScientificWorldJournal. 2013;2013:393176.
- , , , et al. Management of vascular trauma from dog bites. J Vascular Surg. 2013;58(5):1346–1352.
- , . Dog bites. BMJ. 2007;334(7590):413–417.
- , , , . Bacterial infections as complications of dog bites [in Danish]. Ugeskrift Laeger. 1998;160(34):4860–4863.
- , , , , . Bite‐related and septic syndromes caused by cats and dogs. Lancet Infect Dis. 2009;9(7):439–447.
- , , , , . Bacteriologic analysis of infected dog and cat bites. Emergency Medicine Animal Bite Infection Study Group. N Engl J Med. 1999;340(2):85–92.
- , , , . Diagnosing Capnocytophaga canimorsus infections. Emerg Infect Dis. 2006;12(2):340–342.
- , , . Capnocytophaga canimorsus septicemia in Denmark, 1982–1995: review of 39 cases. Clinical Infect Dis. 1996;23(1):71–75.
- . Consequences of alcohol consumption on host defence. Alcohol Alcohol. 1999;34(6):830–841.
- , , , , , . Molecular characterization of Capnocytophaga canimorsus and other canine Capnocytophaga spp. and assessment by PCR of their frequencies in dogs. J Clin Microbiol. 2009;47(10):3218–3225.
- , , , . The bacteriology and antimicrobial susceptibility of infected and non‐infected dog bite wounds: fifty cases. Vet Microbiol. 2008;127(3‐4):360–368.
- U.S. Department of Health and Human Services. Centers for Disease Control and Prevention. Human rabies—Alabama, Tennessee, and Texas, 1994. Morbidity and Mortality Weekly Report; 1995. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/00036736.htm. Accessed March 1, 2014.
- , , , . Dog bites: still a problem? Injury Prev. 2008;14(5):296–301.
- , , , . Dog bite injuries: primary and secondary emergency department presentations—a retrospective cohort study. ScientificWorldJournal. 2013;2013:393176.
- , , , et al. Management of vascular trauma from dog bites. J Vascular Surg. 2013;58(5):1346–1352.
- , . Dog bites. BMJ. 2007;334(7590):413–417.
- , , , . Bacterial infections as complications of dog bites [in Danish]. Ugeskrift Laeger. 1998;160(34):4860–4863.
- , , , , . Bite‐related and septic syndromes caused by cats and dogs. Lancet Infect Dis. 2009;9(7):439–447.
- , , , , . Bacteriologic analysis of infected dog and cat bites. Emergency Medicine Animal Bite Infection Study Group. N Engl J Med. 1999;340(2):85–92.
- , , , . Diagnosing Capnocytophaga canimorsus infections. Emerg Infect Dis. 2006;12(2):340–342.
- , , . Capnocytophaga canimorsus septicemia in Denmark, 1982–1995: review of 39 cases. Clinical Infect Dis. 1996;23(1):71–75.
- . Consequences of alcohol consumption on host defence. Alcohol Alcohol. 1999;34(6):830–841.
- , , , , , . Molecular characterization of Capnocytophaga canimorsus and other canine Capnocytophaga spp. and assessment by PCR of their frequencies in dogs. J Clin Microbiol. 2009;47(10):3218–3225.
- , , , . The bacteriology and antimicrobial susceptibility of infected and non‐infected dog bite wounds: fifty cases. Vet Microbiol. 2008;127(3‐4):360–368.
- U.S. Department of Health and Human Services. Centers for Disease Control and Prevention. Human rabies—Alabama, Tennessee, and Texas, 1994. Morbidity and Mortality Weekly Report; 1995. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/00036736.htm. Accessed March 1, 2014.