User login
Outpatient Parenteral Antimicrobial Therapy in Vulnerable Populations—People Who Inject Drugs and the Homeless
Outpatient parenteral antimicrobial therapy (OPAT) programs allow patients to receive antibiotic therapy at home or in other settings.1-3 Bacterial infections among people who inject drugs (PWID) and the homeless are common, leading to complicated treatment strategies. Those with opioid dependence have frequent hospitalizations.4 Bacteremia and endocarditis frequently require intravenous (IV) antibiotics5-7 and may be difficult to treat. Creating outpatient treatment plans for PWID and the homeless is challenging, and there is a paucity of data on OPAT effectiveness in these groups as they are often excluded from OPAT services.1,2,8
We evaluated treatment outcomes in PWID and the homeless in our OPAT program.
METHODS
We conducted a retrospective cohort study of hospitalized adults discharged from Harborview Medical Center (HMC) with OPAT from January 1, 2015 to April 30, 2016. HMC is a county hospital in Seattle, Washington, affiliated with the University of Washington (UW). Infectious disease specialists supervise our OPAT program and provide follow-up care. We partner with a medical respite facility, a discharge option for homeless patients.9 Respite is staffed by HMC nurses, mental health specialists, and case managers.
Patients aged >18 years were enrolled in OPAT if they were discharged with >2 weeks of IV therapy or required laboratory monitoring while on oral antibiotics. Patients with multiple hospitalizations were included for their initial OPAT encounter only. PWID discharged to respite were instructed not to use their vascular access to inject drugs, but drug abstinence was not required. A tamper-evident sticker was placed over lines that nurses evaluated daily. Patients violating line-tampering restrictions were discharged from respite, and OPAT providers developed alternative antibiotic plans.
The two primary exposures evaluated were patient-reported injection drug use and housing status, and our primary exposure measure was the four-category combination: (1) housed non-PWID, (2) housed PWID, (3) homeless non-PWID, and (4) homeless PWID. Current drug use was defined as use within three months of hospitalization. Homelessness was defined as lack of stable housing. Patients receiving chemotherapy, prolonged steroids, biologic agents, or those with organ transplant were considered immunocompromised.
The primary outcome was clinical cure, defined as completion of antibiotic therapy and resolution of infection, determined by OPAT providers. Patients who were placed on oral suppressive antibiotics or died before treatment completion were considered not cured. Unknown status, including care transfer and lost to follow-up, were noted separately. Lost to follow-up was assumed if patients did not return for care, their care was not formally transferred, and no other medical information was available.
Secondary outcomes included hospital length of stay (LOS), secondary bacteremia, line-tampering, and 30-day readmissions. Secondary bacteremia was defined as bacteremia with a different pathogen from the index illness, which occurred during the initial treatment course. Readmission included readmissions related to OPAT (ie, recurrent or worsening infection, treatment-related toxicities, line-tampering, secondary bacteremia, and line-associated complications).
Data collection was performed using REDCap, a data-capturing software program linked to the electronic medical record (EMR).10 Hospitalization dates and demographics were electronically populated from the EMR. Details regarding drug use, homelessness, comorbidities, diagnosis, discharge complications, clinical cure, and lost to follow-up were manually entered.
Statistical Analysis
Statistical calculations were performed using SAS (v. 9.4). Chi-square testing and analysis of variance were conducted to assess group differences in demographics, infection types, and clinical outcomes.
Primary and secondary outcomes were further evaluated by univariable logistic regression and presented as odds ratios, with the non-PWID housed group serving as the reference. Given the large number of PWID and homeless patients lost to follow-up, sensitivity analyses were conducted using the assumption that patients with unknown clinical outcomes did not achieve cure (ie, chronic infection or death). Multivariable regression was performed on the outcomes of cure and 30-day readmission to OPAT using backward elimination to select a final model, initially including potential confounders of age, sex, and relevant comorbidities (DM and HIV). We assumed that those lost to follow-up were not cured (or readmitted). Other secondary outcomes were either rare events or those of uncertain relevance (eg, hospital LOS) to be evaluated in the multivariable analysis.
Our study did not meet the definition of research by the UW’s institutional review board. It was a quality improvement project funded by a UW Medicine Patient Safety Innovations Program Grant.
RESULTS
Overall, 596 patients received OPAT over 16 months. OPAT patients were categorized into groups as follows: homeless PWID (9%, n = 53), housed PWID (8%, n = 48), homeless non-PWID (8%, n = 45), and housed non-PWID (75%, n = 450).
PWID were younger than non-PWID, and the majority of patients in all groups were men (Table 1). PWID were more likely to have hepatitis C. Non-PWID appeared more likely to have diabetes and be immunosuppressed.
Patients had a total of 960 types of infection (Table 1). Bacteremia was the most common infection among PWID. Osteomyelitis was the most frequent infection in non-PWID.
Discharge location varied widely (P < .001; Table 1). The majority of patients with housing (housed PWID 60.4%, housed non-PWID 59.1%) were discharged to home, although 36.7% of housed non-PWID went to nursing facilities. Among homeless patients, 58.5% of PWID and 42.2% of non-PWID were discharged to respite; 10 patients were discharged to a shelter or street. Data specific to transition from IV to oral therapy were not recorded.
Cure rates among participants with known outcomes did not differ by group (Table 1; P = .85). In a sensitivity analysis of clinical cure, assuming those with unknown outcomes were not cured, housing status and drug use were significantly associated with cure (Table 1; P < .001, in the overall test), with rates lower among housed and homeless PWID groups (50.0% and 47.2%, respectively) compared with housed and homeless non-PWID groups (73.1% and 82.2%, respectively). In the multivariable analysis after backward elimination of noninfluential measures, only PWID and housing status were associated with cure; PWID, whether housed (OR = 0.37) or not (OR = 0.33), had lower odds of cure relative to housed non-PWID (Table 2).
Secondary outcomes, evaluated on all patients regardless of cure, differed by group (Table 1). Mean LOS appeared to be shortest for homeless PWID (15.5 days versus ≥18.0 for other groups; P < .001 for overall test). Homeless PWID patients appeared more likely to have secondary bacteremia (13.2% versus <4.2% in other groups; P < .001 for overall test), line tampering (35.9% versus <2.2% in other groups; P < .001), and 30-day readmission related to OPAT (26.4% versus <16.7% in other groups; P = .004). Compared with housed non-PWID using logistic regression, homeless PWID had a higher risk of secondary bacteremia (OR = 12.9; 95% CI 3.8-37.8; P < .001), line tampering (OR 88.4; 95% CI 24.5-318.3; P < .001), and readmission for OPAT (OR 2.4; 95% CI 1.2-4.6; P = .007). After adjusting for age, sex, and comorbidities, readmission for OPAT remained elevated in homeless PWID (OR = 2.4; 95% CI 1.2-4.6). No significant differences in secondary outcomes were found between housed non-PWID and also between housed PWID and homeless non-PWID.
Among homeless persons, discharge to respite care was not associated with improved outcomes, assuming those lost to follow-up did not achieve cure. Among non-PWID discharged to respite, the cure rate was 74% (14/19) compared with 88% (23/26) discharged elsewhere (P = .20). Among PWID, 48% (15/31) discharged to respite were cured compared with 45% (10/22) discharged elsewhere (P = .83).
DISCUSSION
Our study compares the outcomes of 596 OPAT patients, including PWID and the homeless. Among those retained in care, PWID achieved similar rates of cure compared with non-PWID groups. When assuming that all lost to follow-up had poor outcomes, the cure rates were markedly lower for PWID, with no difference noted by housing status.
Data on PWID and homeless enrolled in OPAT programs are limited.5,11,12 Few studies have reported the outcomes of infections in PWID and the homeless, as these populations often experience significant loss to follow-up due to transiency, lack of care continuity, and effective means of communication.
Cure was achieved in less than half of PWID, when lack of cure was assumed for unknown outcomes. This rate was substantially less than that for non-PWID groups. The assumption that those lost to follow-up did not achieve cure dramatically alters the inference; the truth may lie somewhere between the primary and sensitivity analyses. Homeless PWID remained at the highest risk for lost to follow-up, secondary bacteremia, line-tampering, and 30-day readmission related to OPAT.
PWID have traditionally been considered as a high-risk group for OPAT,1,2,8 but to completely restrict PWID from OPAT may not be appropriate. Ho et al. studied 29 PWID who were selectively enrolled to receive OPAT, and 28 completed IV therapy without any instances of line-tampering, death, or unknown clinical status.6 Recent literature suggests that some candidates can succeed with OPAT, despite drug use.13,14
Homelessness is also considered a barrier to OPAT.1,8 Medical respite is a harm-reduction model implemented for patients who require subacute care.9 In our study, among homeless patients, PWID status was the primary determinant of whether therapy was successful, rather than respite care.
Our study may have limited generalizability to other populations. We are a single-center facility in a large, urban city. PWID and housing status were self-reported but were verified before discharge. Most of our patients were men and white; thus, outcomes may differ for others. Due to the nature of the data, cost effectiveness could not be directly calculated. LOS and readmissions serve as proxy measures.
When patients remain engaged in care, PWID and the homeless achieved comparable clinical cure rates to those of housed non-PWID. Moving forward, OPAT can be more effective in PWID and the homeless with careful patient selection and close clinical support. Access to medication-assisted therapy, such as methadone or buprenorphine,15 may improve follow-up rates and linkage to outpatient care. Additional treatment strategies to improve retention in and adherence to care may promote successful outcomes in these vulnerable populations.
Disclosures
Presented at the Poster Abstract Session: Clinical Practice Issues at ID Week, October 26–30, 2016, New Orleans, LA. No conflicts of interested related to this work for all authors.
Funding
AW and AM received NIH NIAID grant K24 AI 071113-06 and UW Medicine Patient Safety Innovations Program Grant.
1. Tice, AD, Rehm SJ, Dalovisio JR, et al. Practice guidelines for outpatient parenteral antimicrobial therapy. IDSA guidelines. Clin Infect Dis. 2004; 38(12):1651-1672. doi: 10.1086/420939. PubMed
2. Williams DN, Baker CA, Kind AC, Sannes MR. The history and evolution of outpatient parenteral antibiotic therapy (OPAT). Int J Antimicrob Agents. 2015;46(3):307-312. doi: 10.1016/j.ijantimicag.2015.07.001. PubMed
3. Gilchrist M, Seaton RA. Outpatient parenteral antimicrobial therapy and antimicrobial stewardship: challenges and checklists. J Antimicrob Chemother. 2015;70(4);965-970. doi: 10.1093/jac/dku517. PubMed
4. Ronan MV, Herzig SJ. Hospitalizations related to opioid abuse/dependence and associated serious infections from 2002-2012. Health Aff (Milwood). 2016;35(5):832-837. doi: 10.1377/hlthaff.2015.1424. PubMed
5. Beieler AM, Dellit TH, Chan JD, et al. Successful implementation of outpatient parenteral antibiotic therapy at a medical respite facility for homeless patients. J Hosp Med. 2016;11(8):531-535. doi: 10.1002/jhm.2597. PubMed
6. Ho J, Archuleta S, Sulaiman Z, Fisher D. Safe and successful treatment of intravenous drug users with a peripherally inserted central catheter in an outpatient parenteral antibiotic treatment service. J Antimicrob Chemother. 2010;65:2641-2644. doi: 10.1093/jac/dkq355. PubMed
7. Suleyman G, Kenney R, Zervos MJ, Weinmann A. Safety and efficacy of outpatient parenteral antibiotic therapy in an academic infectious disease clinic. J Clin Pharm Ther. 2017;42(1):39-43. doi: 10.1111/jcpt.12465. PubMed
8. Bhavan KP, Brown LS, Haley RW. Self-administered outpatient antimicrobial infusion by uninsured patients discharged from a safety-net hospital: a propensity-score-balanced retrospective cohort study. PLoS Med. 2015;12(12):e1001922. doi: 10.1371/journal.pmed. PubMed
9. Seattle-King County Medical Respite. https://www.kingcounty.gov/depts/health/locations/homeless-health/healthcare-for-the-homeless/services/medical-respite.aspx. Accessed October 2, 2018.
10. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377-3781. doi: 10.1016/j.jbi.2008.08.010. PubMed
11. Buerhle DJ, Shields RK, Shah N, Shoff C, Sheridan K. Risk factors associated with outpatient parenteral antibiotic therapy program failure among intravenous drug users. Open Forum Infect Dis.2017;4(3):ofx102. doi: 10.1093/ofid/ofx102. PubMed
12. Hernandez W, Price C, Knepper B, McLees M, Young H. Outpatient parenteral antimicrobial therapy administration in a homeless population. J Infus Nurs. 2016;39(2):81-85. doi: 10.1097/NAN.0000000000000165. PubMed
13. Sukuki J, Johnson J, Montgomery M, Hayden M, Price C. Outpatient parenteral antimicrobial therapy among people who inject drugs: a review of the literature. Open Forum Infect Dis. 2018;5(9):ofy194. doi: 10.1093/ofid/ofy194. PubMed
14. D’Couto HT, Robbins GK, Ard KL, Wakeman SE, Alves J, Nelson SB. Outcomes according to discharge location for persons who inject drugs receiving outpatient parenteral antimicrobial therapy. Open Forum Infect Dis. 2018;5(5):ofy056. doi: 10.1093/ofid/ofy056. PubMed
15. Rosenthal ES, Karchmer AW, Theisen-Toupal J, Castillo RA, Rowley CF. Suboptimal addiction interventions for patients hospitalized with injection drug use-associated infective endocarditis. Am J Med. 2016;129(5):481-485. doi: 10.1016/j.amjmed.2015.09.024. PubMed
Outpatient parenteral antimicrobial therapy (OPAT) programs allow patients to receive antibiotic therapy at home or in other settings.1-3 Bacterial infections among people who inject drugs (PWID) and the homeless are common, leading to complicated treatment strategies. Those with opioid dependence have frequent hospitalizations.4 Bacteremia and endocarditis frequently require intravenous (IV) antibiotics5-7 and may be difficult to treat. Creating outpatient treatment plans for PWID and the homeless is challenging, and there is a paucity of data on OPAT effectiveness in these groups as they are often excluded from OPAT services.1,2,8
We evaluated treatment outcomes in PWID and the homeless in our OPAT program.
METHODS
We conducted a retrospective cohort study of hospitalized adults discharged from Harborview Medical Center (HMC) with OPAT from January 1, 2015 to April 30, 2016. HMC is a county hospital in Seattle, Washington, affiliated with the University of Washington (UW). Infectious disease specialists supervise our OPAT program and provide follow-up care. We partner with a medical respite facility, a discharge option for homeless patients.9 Respite is staffed by HMC nurses, mental health specialists, and case managers.
Patients aged >18 years were enrolled in OPAT if they were discharged with >2 weeks of IV therapy or required laboratory monitoring while on oral antibiotics. Patients with multiple hospitalizations were included for their initial OPAT encounter only. PWID discharged to respite were instructed not to use their vascular access to inject drugs, but drug abstinence was not required. A tamper-evident sticker was placed over lines that nurses evaluated daily. Patients violating line-tampering restrictions were discharged from respite, and OPAT providers developed alternative antibiotic plans.
The two primary exposures evaluated were patient-reported injection drug use and housing status, and our primary exposure measure was the four-category combination: (1) housed non-PWID, (2) housed PWID, (3) homeless non-PWID, and (4) homeless PWID. Current drug use was defined as use within three months of hospitalization. Homelessness was defined as lack of stable housing. Patients receiving chemotherapy, prolonged steroids, biologic agents, or those with organ transplant were considered immunocompromised.
The primary outcome was clinical cure, defined as completion of antibiotic therapy and resolution of infection, determined by OPAT providers. Patients who were placed on oral suppressive antibiotics or died before treatment completion were considered not cured. Unknown status, including care transfer and lost to follow-up, were noted separately. Lost to follow-up was assumed if patients did not return for care, their care was not formally transferred, and no other medical information was available.
Secondary outcomes included hospital length of stay (LOS), secondary bacteremia, line-tampering, and 30-day readmissions. Secondary bacteremia was defined as bacteremia with a different pathogen from the index illness, which occurred during the initial treatment course. Readmission included readmissions related to OPAT (ie, recurrent or worsening infection, treatment-related toxicities, line-tampering, secondary bacteremia, and line-associated complications).
Data collection was performed using REDCap, a data-capturing software program linked to the electronic medical record (EMR).10 Hospitalization dates and demographics were electronically populated from the EMR. Details regarding drug use, homelessness, comorbidities, diagnosis, discharge complications, clinical cure, and lost to follow-up were manually entered.
Statistical Analysis
Statistical calculations were performed using SAS (v. 9.4). Chi-square testing and analysis of variance were conducted to assess group differences in demographics, infection types, and clinical outcomes.
Primary and secondary outcomes were further evaluated by univariable logistic regression and presented as odds ratios, with the non-PWID housed group serving as the reference. Given the large number of PWID and homeless patients lost to follow-up, sensitivity analyses were conducted using the assumption that patients with unknown clinical outcomes did not achieve cure (ie, chronic infection or death). Multivariable regression was performed on the outcomes of cure and 30-day readmission to OPAT using backward elimination to select a final model, initially including potential confounders of age, sex, and relevant comorbidities (DM and HIV). We assumed that those lost to follow-up were not cured (or readmitted). Other secondary outcomes were either rare events or those of uncertain relevance (eg, hospital LOS) to be evaluated in the multivariable analysis.
Our study did not meet the definition of research by the UW’s institutional review board. It was a quality improvement project funded by a UW Medicine Patient Safety Innovations Program Grant.
RESULTS
Overall, 596 patients received OPAT over 16 months. OPAT patients were categorized into groups as follows: homeless PWID (9%, n = 53), housed PWID (8%, n = 48), homeless non-PWID (8%, n = 45), and housed non-PWID (75%, n = 450).
PWID were younger than non-PWID, and the majority of patients in all groups were men (Table 1). PWID were more likely to have hepatitis C. Non-PWID appeared more likely to have diabetes and be immunosuppressed.
Patients had a total of 960 types of infection (Table 1). Bacteremia was the most common infection among PWID. Osteomyelitis was the most frequent infection in non-PWID.
Discharge location varied widely (P < .001; Table 1). The majority of patients with housing (housed PWID 60.4%, housed non-PWID 59.1%) were discharged to home, although 36.7% of housed non-PWID went to nursing facilities. Among homeless patients, 58.5% of PWID and 42.2% of non-PWID were discharged to respite; 10 patients were discharged to a shelter or street. Data specific to transition from IV to oral therapy were not recorded.
Cure rates among participants with known outcomes did not differ by group (Table 1; P = .85). In a sensitivity analysis of clinical cure, assuming those with unknown outcomes were not cured, housing status and drug use were significantly associated with cure (Table 1; P < .001, in the overall test), with rates lower among housed and homeless PWID groups (50.0% and 47.2%, respectively) compared with housed and homeless non-PWID groups (73.1% and 82.2%, respectively). In the multivariable analysis after backward elimination of noninfluential measures, only PWID and housing status were associated with cure; PWID, whether housed (OR = 0.37) or not (OR = 0.33), had lower odds of cure relative to housed non-PWID (Table 2).
Secondary outcomes, evaluated on all patients regardless of cure, differed by group (Table 1). Mean LOS appeared to be shortest for homeless PWID (15.5 days versus ≥18.0 for other groups; P < .001 for overall test). Homeless PWID patients appeared more likely to have secondary bacteremia (13.2% versus <4.2% in other groups; P < .001 for overall test), line tampering (35.9% versus <2.2% in other groups; P < .001), and 30-day readmission related to OPAT (26.4% versus <16.7% in other groups; P = .004). Compared with housed non-PWID using logistic regression, homeless PWID had a higher risk of secondary bacteremia (OR = 12.9; 95% CI 3.8-37.8; P < .001), line tampering (OR 88.4; 95% CI 24.5-318.3; P < .001), and readmission for OPAT (OR 2.4; 95% CI 1.2-4.6; P = .007). After adjusting for age, sex, and comorbidities, readmission for OPAT remained elevated in homeless PWID (OR = 2.4; 95% CI 1.2-4.6). No significant differences in secondary outcomes were found between housed non-PWID and also between housed PWID and homeless non-PWID.
Among homeless persons, discharge to respite care was not associated with improved outcomes, assuming those lost to follow-up did not achieve cure. Among non-PWID discharged to respite, the cure rate was 74% (14/19) compared with 88% (23/26) discharged elsewhere (P = .20). Among PWID, 48% (15/31) discharged to respite were cured compared with 45% (10/22) discharged elsewhere (P = .83).
DISCUSSION
Our study compares the outcomes of 596 OPAT patients, including PWID and the homeless. Among those retained in care, PWID achieved similar rates of cure compared with non-PWID groups. When assuming that all lost to follow-up had poor outcomes, the cure rates were markedly lower for PWID, with no difference noted by housing status.
Data on PWID and homeless enrolled in OPAT programs are limited.5,11,12 Few studies have reported the outcomes of infections in PWID and the homeless, as these populations often experience significant loss to follow-up due to transiency, lack of care continuity, and effective means of communication.
Cure was achieved in less than half of PWID, when lack of cure was assumed for unknown outcomes. This rate was substantially less than that for non-PWID groups. The assumption that those lost to follow-up did not achieve cure dramatically alters the inference; the truth may lie somewhere between the primary and sensitivity analyses. Homeless PWID remained at the highest risk for lost to follow-up, secondary bacteremia, line-tampering, and 30-day readmission related to OPAT.
PWID have traditionally been considered as a high-risk group for OPAT,1,2,8 but to completely restrict PWID from OPAT may not be appropriate. Ho et al. studied 29 PWID who were selectively enrolled to receive OPAT, and 28 completed IV therapy without any instances of line-tampering, death, or unknown clinical status.6 Recent literature suggests that some candidates can succeed with OPAT, despite drug use.13,14
Homelessness is also considered a barrier to OPAT.1,8 Medical respite is a harm-reduction model implemented for patients who require subacute care.9 In our study, among homeless patients, PWID status was the primary determinant of whether therapy was successful, rather than respite care.
Our study may have limited generalizability to other populations. We are a single-center facility in a large, urban city. PWID and housing status were self-reported but were verified before discharge. Most of our patients were men and white; thus, outcomes may differ for others. Due to the nature of the data, cost effectiveness could not be directly calculated. LOS and readmissions serve as proxy measures.
When patients remain engaged in care, PWID and the homeless achieved comparable clinical cure rates to those of housed non-PWID. Moving forward, OPAT can be more effective in PWID and the homeless with careful patient selection and close clinical support. Access to medication-assisted therapy, such as methadone or buprenorphine,15 may improve follow-up rates and linkage to outpatient care. Additional treatment strategies to improve retention in and adherence to care may promote successful outcomes in these vulnerable populations.
Disclosures
Presented at the Poster Abstract Session: Clinical Practice Issues at ID Week, October 26–30, 2016, New Orleans, LA. No conflicts of interested related to this work for all authors.
Funding
AW and AM received NIH NIAID grant K24 AI 071113-06 and UW Medicine Patient Safety Innovations Program Grant.
Outpatient parenteral antimicrobial therapy (OPAT) programs allow patients to receive antibiotic therapy at home or in other settings.1-3 Bacterial infections among people who inject drugs (PWID) and the homeless are common, leading to complicated treatment strategies. Those with opioid dependence have frequent hospitalizations.4 Bacteremia and endocarditis frequently require intravenous (IV) antibiotics5-7 and may be difficult to treat. Creating outpatient treatment plans for PWID and the homeless is challenging, and there is a paucity of data on OPAT effectiveness in these groups as they are often excluded from OPAT services.1,2,8
We evaluated treatment outcomes in PWID and the homeless in our OPAT program.
METHODS
We conducted a retrospective cohort study of hospitalized adults discharged from Harborview Medical Center (HMC) with OPAT from January 1, 2015 to April 30, 2016. HMC is a county hospital in Seattle, Washington, affiliated with the University of Washington (UW). Infectious disease specialists supervise our OPAT program and provide follow-up care. We partner with a medical respite facility, a discharge option for homeless patients.9 Respite is staffed by HMC nurses, mental health specialists, and case managers.
Patients aged >18 years were enrolled in OPAT if they were discharged with >2 weeks of IV therapy or required laboratory monitoring while on oral antibiotics. Patients with multiple hospitalizations were included for their initial OPAT encounter only. PWID discharged to respite were instructed not to use their vascular access to inject drugs, but drug abstinence was not required. A tamper-evident sticker was placed over lines that nurses evaluated daily. Patients violating line-tampering restrictions were discharged from respite, and OPAT providers developed alternative antibiotic plans.
The two primary exposures evaluated were patient-reported injection drug use and housing status, and our primary exposure measure was the four-category combination: (1) housed non-PWID, (2) housed PWID, (3) homeless non-PWID, and (4) homeless PWID. Current drug use was defined as use within three months of hospitalization. Homelessness was defined as lack of stable housing. Patients receiving chemotherapy, prolonged steroids, biologic agents, or those with organ transplant were considered immunocompromised.
The primary outcome was clinical cure, defined as completion of antibiotic therapy and resolution of infection, determined by OPAT providers. Patients who were placed on oral suppressive antibiotics or died before treatment completion were considered not cured. Unknown status, including care transfer and lost to follow-up, were noted separately. Lost to follow-up was assumed if patients did not return for care, their care was not formally transferred, and no other medical information was available.
Secondary outcomes included hospital length of stay (LOS), secondary bacteremia, line-tampering, and 30-day readmissions. Secondary bacteremia was defined as bacteremia with a different pathogen from the index illness, which occurred during the initial treatment course. Readmission included readmissions related to OPAT (ie, recurrent or worsening infection, treatment-related toxicities, line-tampering, secondary bacteremia, and line-associated complications).
Data collection was performed using REDCap, a data-capturing software program linked to the electronic medical record (EMR).10 Hospitalization dates and demographics were electronically populated from the EMR. Details regarding drug use, homelessness, comorbidities, diagnosis, discharge complications, clinical cure, and lost to follow-up were manually entered.
Statistical Analysis
Statistical calculations were performed using SAS (v. 9.4). Chi-square testing and analysis of variance were conducted to assess group differences in demographics, infection types, and clinical outcomes.
Primary and secondary outcomes were further evaluated by univariable logistic regression and presented as odds ratios, with the non-PWID housed group serving as the reference. Given the large number of PWID and homeless patients lost to follow-up, sensitivity analyses were conducted using the assumption that patients with unknown clinical outcomes did not achieve cure (ie, chronic infection or death). Multivariable regression was performed on the outcomes of cure and 30-day readmission to OPAT using backward elimination to select a final model, initially including potential confounders of age, sex, and relevant comorbidities (DM and HIV). We assumed that those lost to follow-up were not cured (or readmitted). Other secondary outcomes were either rare events or those of uncertain relevance (eg, hospital LOS) to be evaluated in the multivariable analysis.
Our study did not meet the definition of research by the UW’s institutional review board. It was a quality improvement project funded by a UW Medicine Patient Safety Innovations Program Grant.
RESULTS
Overall, 596 patients received OPAT over 16 months. OPAT patients were categorized into groups as follows: homeless PWID (9%, n = 53), housed PWID (8%, n = 48), homeless non-PWID (8%, n = 45), and housed non-PWID (75%, n = 450).
PWID were younger than non-PWID, and the majority of patients in all groups were men (Table 1). PWID were more likely to have hepatitis C. Non-PWID appeared more likely to have diabetes and be immunosuppressed.
Patients had a total of 960 types of infection (Table 1). Bacteremia was the most common infection among PWID. Osteomyelitis was the most frequent infection in non-PWID.
Discharge location varied widely (P < .001; Table 1). The majority of patients with housing (housed PWID 60.4%, housed non-PWID 59.1%) were discharged to home, although 36.7% of housed non-PWID went to nursing facilities. Among homeless patients, 58.5% of PWID and 42.2% of non-PWID were discharged to respite; 10 patients were discharged to a shelter or street. Data specific to transition from IV to oral therapy were not recorded.
Cure rates among participants with known outcomes did not differ by group (Table 1; P = .85). In a sensitivity analysis of clinical cure, assuming those with unknown outcomes were not cured, housing status and drug use were significantly associated with cure (Table 1; P < .001, in the overall test), with rates lower among housed and homeless PWID groups (50.0% and 47.2%, respectively) compared with housed and homeless non-PWID groups (73.1% and 82.2%, respectively). In the multivariable analysis after backward elimination of noninfluential measures, only PWID and housing status were associated with cure; PWID, whether housed (OR = 0.37) or not (OR = 0.33), had lower odds of cure relative to housed non-PWID (Table 2).
Secondary outcomes, evaluated on all patients regardless of cure, differed by group (Table 1). Mean LOS appeared to be shortest for homeless PWID (15.5 days versus ≥18.0 for other groups; P < .001 for overall test). Homeless PWID patients appeared more likely to have secondary bacteremia (13.2% versus <4.2% in other groups; P < .001 for overall test), line tampering (35.9% versus <2.2% in other groups; P < .001), and 30-day readmission related to OPAT (26.4% versus <16.7% in other groups; P = .004). Compared with housed non-PWID using logistic regression, homeless PWID had a higher risk of secondary bacteremia (OR = 12.9; 95% CI 3.8-37.8; P < .001), line tampering (OR 88.4; 95% CI 24.5-318.3; P < .001), and readmission for OPAT (OR 2.4; 95% CI 1.2-4.6; P = .007). After adjusting for age, sex, and comorbidities, readmission for OPAT remained elevated in homeless PWID (OR = 2.4; 95% CI 1.2-4.6). No significant differences in secondary outcomes were found between housed non-PWID and also between housed PWID and homeless non-PWID.
Among homeless persons, discharge to respite care was not associated with improved outcomes, assuming those lost to follow-up did not achieve cure. Among non-PWID discharged to respite, the cure rate was 74% (14/19) compared with 88% (23/26) discharged elsewhere (P = .20). Among PWID, 48% (15/31) discharged to respite were cured compared with 45% (10/22) discharged elsewhere (P = .83).
DISCUSSION
Our study compares the outcomes of 596 OPAT patients, including PWID and the homeless. Among those retained in care, PWID achieved similar rates of cure compared with non-PWID groups. When assuming that all lost to follow-up had poor outcomes, the cure rates were markedly lower for PWID, with no difference noted by housing status.
Data on PWID and homeless enrolled in OPAT programs are limited.5,11,12 Few studies have reported the outcomes of infections in PWID and the homeless, as these populations often experience significant loss to follow-up due to transiency, lack of care continuity, and effective means of communication.
Cure was achieved in less than half of PWID, when lack of cure was assumed for unknown outcomes. This rate was substantially less than that for non-PWID groups. The assumption that those lost to follow-up did not achieve cure dramatically alters the inference; the truth may lie somewhere between the primary and sensitivity analyses. Homeless PWID remained at the highest risk for lost to follow-up, secondary bacteremia, line-tampering, and 30-day readmission related to OPAT.
PWID have traditionally been considered as a high-risk group for OPAT,1,2,8 but to completely restrict PWID from OPAT may not be appropriate. Ho et al. studied 29 PWID who were selectively enrolled to receive OPAT, and 28 completed IV therapy without any instances of line-tampering, death, or unknown clinical status.6 Recent literature suggests that some candidates can succeed with OPAT, despite drug use.13,14
Homelessness is also considered a barrier to OPAT.1,8 Medical respite is a harm-reduction model implemented for patients who require subacute care.9 In our study, among homeless patients, PWID status was the primary determinant of whether therapy was successful, rather than respite care.
Our study may have limited generalizability to other populations. We are a single-center facility in a large, urban city. PWID and housing status were self-reported but were verified before discharge. Most of our patients were men and white; thus, outcomes may differ for others. Due to the nature of the data, cost effectiveness could not be directly calculated. LOS and readmissions serve as proxy measures.
When patients remain engaged in care, PWID and the homeless achieved comparable clinical cure rates to those of housed non-PWID. Moving forward, OPAT can be more effective in PWID and the homeless with careful patient selection and close clinical support. Access to medication-assisted therapy, such as methadone or buprenorphine,15 may improve follow-up rates and linkage to outpatient care. Additional treatment strategies to improve retention in and adherence to care may promote successful outcomes in these vulnerable populations.
Disclosures
Presented at the Poster Abstract Session: Clinical Practice Issues at ID Week, October 26–30, 2016, New Orleans, LA. No conflicts of interested related to this work for all authors.
Funding
AW and AM received NIH NIAID grant K24 AI 071113-06 and UW Medicine Patient Safety Innovations Program Grant.
1. Tice, AD, Rehm SJ, Dalovisio JR, et al. Practice guidelines for outpatient parenteral antimicrobial therapy. IDSA guidelines. Clin Infect Dis. 2004; 38(12):1651-1672. doi: 10.1086/420939. PubMed
2. Williams DN, Baker CA, Kind AC, Sannes MR. The history and evolution of outpatient parenteral antibiotic therapy (OPAT). Int J Antimicrob Agents. 2015;46(3):307-312. doi: 10.1016/j.ijantimicag.2015.07.001. PubMed
3. Gilchrist M, Seaton RA. Outpatient parenteral antimicrobial therapy and antimicrobial stewardship: challenges and checklists. J Antimicrob Chemother. 2015;70(4);965-970. doi: 10.1093/jac/dku517. PubMed
4. Ronan MV, Herzig SJ. Hospitalizations related to opioid abuse/dependence and associated serious infections from 2002-2012. Health Aff (Milwood). 2016;35(5):832-837. doi: 10.1377/hlthaff.2015.1424. PubMed
5. Beieler AM, Dellit TH, Chan JD, et al. Successful implementation of outpatient parenteral antibiotic therapy at a medical respite facility for homeless patients. J Hosp Med. 2016;11(8):531-535. doi: 10.1002/jhm.2597. PubMed
6. Ho J, Archuleta S, Sulaiman Z, Fisher D. Safe and successful treatment of intravenous drug users with a peripherally inserted central catheter in an outpatient parenteral antibiotic treatment service. J Antimicrob Chemother. 2010;65:2641-2644. doi: 10.1093/jac/dkq355. PubMed
7. Suleyman G, Kenney R, Zervos MJ, Weinmann A. Safety and efficacy of outpatient parenteral antibiotic therapy in an academic infectious disease clinic. J Clin Pharm Ther. 2017;42(1):39-43. doi: 10.1111/jcpt.12465. PubMed
8. Bhavan KP, Brown LS, Haley RW. Self-administered outpatient antimicrobial infusion by uninsured patients discharged from a safety-net hospital: a propensity-score-balanced retrospective cohort study. PLoS Med. 2015;12(12):e1001922. doi: 10.1371/journal.pmed. PubMed
9. Seattle-King County Medical Respite. https://www.kingcounty.gov/depts/health/locations/homeless-health/healthcare-for-the-homeless/services/medical-respite.aspx. Accessed October 2, 2018.
10. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377-3781. doi: 10.1016/j.jbi.2008.08.010. PubMed
11. Buerhle DJ, Shields RK, Shah N, Shoff C, Sheridan K. Risk factors associated with outpatient parenteral antibiotic therapy program failure among intravenous drug users. Open Forum Infect Dis.2017;4(3):ofx102. doi: 10.1093/ofid/ofx102. PubMed
12. Hernandez W, Price C, Knepper B, McLees M, Young H. Outpatient parenteral antimicrobial therapy administration in a homeless population. J Infus Nurs. 2016;39(2):81-85. doi: 10.1097/NAN.0000000000000165. PubMed
13. Sukuki J, Johnson J, Montgomery M, Hayden M, Price C. Outpatient parenteral antimicrobial therapy among people who inject drugs: a review of the literature. Open Forum Infect Dis. 2018;5(9):ofy194. doi: 10.1093/ofid/ofy194. PubMed
14. D’Couto HT, Robbins GK, Ard KL, Wakeman SE, Alves J, Nelson SB. Outcomes according to discharge location for persons who inject drugs receiving outpatient parenteral antimicrobial therapy. Open Forum Infect Dis. 2018;5(5):ofy056. doi: 10.1093/ofid/ofy056. PubMed
15. Rosenthal ES, Karchmer AW, Theisen-Toupal J, Castillo RA, Rowley CF. Suboptimal addiction interventions for patients hospitalized with injection drug use-associated infective endocarditis. Am J Med. 2016;129(5):481-485. doi: 10.1016/j.amjmed.2015.09.024. PubMed
1. Tice, AD, Rehm SJ, Dalovisio JR, et al. Practice guidelines for outpatient parenteral antimicrobial therapy. IDSA guidelines. Clin Infect Dis. 2004; 38(12):1651-1672. doi: 10.1086/420939. PubMed
2. Williams DN, Baker CA, Kind AC, Sannes MR. The history and evolution of outpatient parenteral antibiotic therapy (OPAT). Int J Antimicrob Agents. 2015;46(3):307-312. doi: 10.1016/j.ijantimicag.2015.07.001. PubMed
3. Gilchrist M, Seaton RA. Outpatient parenteral antimicrobial therapy and antimicrobial stewardship: challenges and checklists. J Antimicrob Chemother. 2015;70(4);965-970. doi: 10.1093/jac/dku517. PubMed
4. Ronan MV, Herzig SJ. Hospitalizations related to opioid abuse/dependence and associated serious infections from 2002-2012. Health Aff (Milwood). 2016;35(5):832-837. doi: 10.1377/hlthaff.2015.1424. PubMed
5. Beieler AM, Dellit TH, Chan JD, et al. Successful implementation of outpatient parenteral antibiotic therapy at a medical respite facility for homeless patients. J Hosp Med. 2016;11(8):531-535. doi: 10.1002/jhm.2597. PubMed
6. Ho J, Archuleta S, Sulaiman Z, Fisher D. Safe and successful treatment of intravenous drug users with a peripherally inserted central catheter in an outpatient parenteral antibiotic treatment service. J Antimicrob Chemother. 2010;65:2641-2644. doi: 10.1093/jac/dkq355. PubMed
7. Suleyman G, Kenney R, Zervos MJ, Weinmann A. Safety and efficacy of outpatient parenteral antibiotic therapy in an academic infectious disease clinic. J Clin Pharm Ther. 2017;42(1):39-43. doi: 10.1111/jcpt.12465. PubMed
8. Bhavan KP, Brown LS, Haley RW. Self-administered outpatient antimicrobial infusion by uninsured patients discharged from a safety-net hospital: a propensity-score-balanced retrospective cohort study. PLoS Med. 2015;12(12):e1001922. doi: 10.1371/journal.pmed. PubMed
9. Seattle-King County Medical Respite. https://www.kingcounty.gov/depts/health/locations/homeless-health/healthcare-for-the-homeless/services/medical-respite.aspx. Accessed October 2, 2018.
10. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377-3781. doi: 10.1016/j.jbi.2008.08.010. PubMed
11. Buerhle DJ, Shields RK, Shah N, Shoff C, Sheridan K. Risk factors associated with outpatient parenteral antibiotic therapy program failure among intravenous drug users. Open Forum Infect Dis.2017;4(3):ofx102. doi: 10.1093/ofid/ofx102. PubMed
12. Hernandez W, Price C, Knepper B, McLees M, Young H. Outpatient parenteral antimicrobial therapy administration in a homeless population. J Infus Nurs. 2016;39(2):81-85. doi: 10.1097/NAN.0000000000000165. PubMed
13. Sukuki J, Johnson J, Montgomery M, Hayden M, Price C. Outpatient parenteral antimicrobial therapy among people who inject drugs: a review of the literature. Open Forum Infect Dis. 2018;5(9):ofy194. doi: 10.1093/ofid/ofy194. PubMed
14. D’Couto HT, Robbins GK, Ard KL, Wakeman SE, Alves J, Nelson SB. Outcomes according to discharge location for persons who inject drugs receiving outpatient parenteral antimicrobial therapy. Open Forum Infect Dis. 2018;5(5):ofy056. doi: 10.1093/ofid/ofy056. PubMed
15. Rosenthal ES, Karchmer AW, Theisen-Toupal J, Castillo RA, Rowley CF. Suboptimal addiction interventions for patients hospitalized with injection drug use-associated infective endocarditis. Am J Med. 2016;129(5):481-485. doi: 10.1016/j.amjmed.2015.09.024. PubMed
© 2019 Society of Hospital Medicine
Genital herpes: Diagnostic and management considerations in pregnant women
Genital herpes is a common infection caused by herpes simplex virus type 1 (HSV-1) or herpes simplex virus type 2 (HSV-2). Although life-threatening health consequences of HSV infection after infancy are uncommon, women with genital herpes remain at risk for recurrent symptoms, which can be associated with significant physical and psychosocial distress. These patients also can transmit the disease to their partners and neonates, and have a 2- to 3-fold increased risk of HIV acquisition. In this article, we review the diagnosis and management of genital herpes in pregnant women.
CASE Asymptomatic pregnant patient tests positive for herpes
Sarah is a healthy 32-year-old (G1P0) presenting at 8 weeks’ gestation for her first prenatal visit. She requests HSV testing as she learned that genital herpes is common and it can be transmitted to the baby. You order the HSV-2 IgG assay from your laboratory, which performs the HerpeSelect HSV-2 enzyme immunoassay as the standard test. The test result is positive, with an index value of 2.2 (the manufacturer defines an index value >1.1 as positive). Repeat testing in 4 weeks returns positive results again, with an index value of 2.8.
The patient is distressed at this news. She has no history of genital lesions or symptoms consistent with genital herpes and is worried that her husband has been unfaithful. How would you manage this case?
How prevalent is HSV?
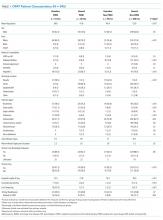
Genital herpes is a chronic viral infection transmitted through close contact with a person who is shedding the virus from genital or oral mucosa. In the United States, the National Health and Nutrition Examination Survey indicated an HSV-2 seroprevalence of 16% among persons aged 14 to 49 in 2005–2010, a decline from 21% in 1988–1991.1 The prevalence among women is twice as high as among men, at 20% versus 11%, respectively. Among those with HSV-2, 87% are not aware that they are infected; they are at risk of infecting their partners, however.1
In the same age group, the prevalence of HSV-1 is 54%.2 The seroprevalence of HSV-1 in adolescents declined from 39% in 1999–2004 to 30% in 2005–2010, resulting in a high number of young people who are seronegative at the time of sexual debut. Concurrently, genital HSV-1 has emerged as a frequent cause of first-episode genital herpes, often associated with oral-genital contact during sexual debut.2,3
When evaluating patients for possible genital herpes provide general educational messages regarding HSV infection and obtain a detailed medical and sexual history to determine the best diagnostic approach.
What are the clinical features of genital HSV infection?
The clinical manifestations of genital herpes vary according to whether the infection is primary, nonprimary first episode, or recurrent.
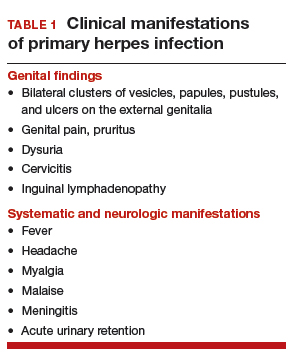
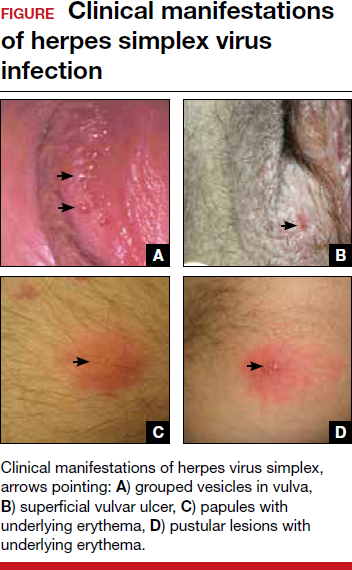
Primary infection. During primary infection,which occurs 4 to 12 days after sexual exposure and in the absence of pre-existing antibodies to HSV-1 or HSV-2, patients may experience genital and systemic symptoms (FIGURE and TABLE 1). Since this infection usually occurs in otherwise healthy people, for many, this is the most severe disease that they have experienced. However, most patients with primary infection develop mild, atypical, or completely asymptomatic presentation and are not diagnosed at the time of HSV acquisition. Whether primary infection is caused by HSV-1 or HSV-2 cannot be differentiated based on the clinical presentation alone.
Nonprimary first episode infection. In a nonprimary infection, newly acquired infection with HSV-1 or HSV-2 occurs in a person with pre-existing antibodies to the other virus. Almost always, this means new HSV-2 infection in a HSV-1 seropositive person, as prior HSV-2 infection appears to protect against HSV-1 acquisition. In general, the clinical presentation of nonprimary infection is somewhat milder and the rate of complications is lower, but clinically the overlap is great, and antibody tests are needed to define whether the patient has primary or nonprimary infection.4
Recurrent genital herpes infection occurs in most patients with genital herpes. The rate of recurrence is low in patients with genital HSV-1 and often high in patients with genital HSV-2 infection. The median number of recurrences is 1 in the first year of genital HSV-1 infection, and many patients will not have any recurrences following the first year. By contrast, in patients with genital HSV-2 infection, the median number of recurrences is 4, and a high rate of recurrences can continue for many years. Prodromal symptoms (localized irritation, paresthesias, and pruritus) can precede recurrences, which usually present with fewer lesions and last a shorter time than primary infection. Recurrent genital lesions tend to heal in approximately 5 to 10 days in the absence of antiviral treatment, and systemic symptoms are uncommon.5
Asymptomatic viral shedding. After resolution of a primary HSV infection, people shed the virus in the genital tract despite symptom absence. Asymptomatic shedding tends to be more frequent and prolonged with primary genital HSV-2 infection compared with HSV-1 infection.6,7 The frequency of HSV shedding is highest in the first year of infection, and decreases subsequently.8 However, it is likely to persist intermittently for many years. Because the natural history is so strikingly different in genital HSV-1 versus HSV-2, identification of the viral type is important for prognostic information.
The first HSV episode does not necessarily indicate a new or recent infection—in about 25% of persons it represents the first recognized genital herpes episode. Additional serologic and virologic evaluation can be pursued to determine if the first episode represents a new infection.
Read about the diagnostic tests for genital HSV.
What diagnostic tests are available for genital herpes?
Most HSV infections are clinically silent. Therefore, laboratory tests are required to diagnose the infection. Even if symptoms are present, diagnoses based only on clinical presentation have a 20% false-positive rate. Always confirm diagnosis by laboratory assay.9 Furthermore, couples that are discordant for HSV-2 by history are often concordant by serologic assays, as the transmission already has occurred but was not recognized. In these cases, the direction of transmission cannot be determined, and stable couples often experience relief learning that they are not discordant.
Related article:
Effective treatment of recurrent bacterial vaginosis
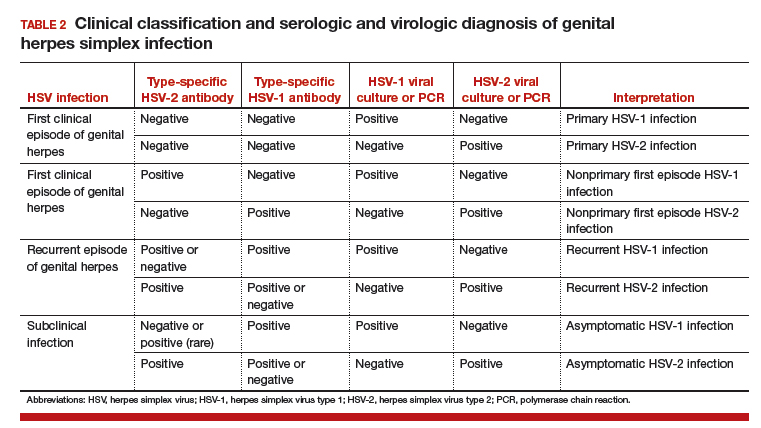
Several laboratory tools for HSV diagnosis based on direct viral detection and antibody detection can be used in clinical settings (TABLE 2). Among patients with symptomatic genital herpes, a sample from the lesion can be used to confirm and identify viral type. Because polymerase chain reaction (PCR) is substantially more sensitive than viral culture and increasingly available it has emerged as the preferred test.9 Viral culture is highly specific (>99%), but sensitivity varies according to collection technique and stage of the lesions. (The test is less sensitive when lesions are healing.)9,10 Antigen detection by immunofluorescence (direct fluorescent antibody) detects HSV from active lesions with high specificity, but sensitivity is low. Cytologic identification of infected cells (using Tzanck or Pap test) has limited utility for diagnosis due to low sensitivity and specificity.9
Type-specific antibodies to HSV develop during the first several weeks after acquisition and persist indefinitely.11 Most accurate type-specific serologic tests are based on detection of glycoprotein G1 and glycoprotein G2 for HSV-1 and HSV-2, respectively.
HerpeSelect HSV-2 enzyme immunoassay (EIA) is one of the most commonly used tests in the United States. The manufacturer considers results with index values 1.1 or greater as showing HSV-2 infection. Unfortunately, low positive results, often with a defined index value of 1.1 to 3.5, are frequently false positive. These low positive values should be confirmed with another test, such as Western blot.9
Western blot has been considered the gold standard assay for HSV-1 and HSV-2 antibody detection; this test is available at the University of Washington in Seattle. When comparing the HSV-1 EIA and HSV-2 EIA with the Western blot assay in clinical practice, the estimated sensitivity and specificity are 70.2% and 91.6%, respectively, for HSV-1 and 91.9% and 57.4%, respectively, for HSV-2.12
HerpeSelect HSV-2 Immunoblot testing should not be considered as confirmatory because this assay detects the same antigen as the HSV-2 EIA. Serologic tests based on detection of HSV-IgM should not be used for diagnosis of genital herpes as IgM response can present during a new infection or HSV reactivation and because IgM responses are not type-specific. Clearly, more accurate commercial type-specific antibody tests are needed.
Specific HSV antibodies can take up to 12 weeks to develop. Therefore, repeat serologic testing for patients in whom initial HSV antibody results are negative yet recent genital herpes acquisition is suspected.11 A confirmed positive HSV-2 antibody test indicates anogenital infection, even in a person who lacks genital symptoms. This finding became evident through a study of 53 HSV-2 seropositive patients who lacked a history of genital herpes. Patients were followed for 3 months, and all but 1 developed either virologic or clinical (or both) evidence of genital herpes.13
In the absence of genital or orolabial symptoms among individuals with positive HSV-1, serologic testing cannot distinguish anogenital from orolabial infection. Most of these infections may represent oral HSV-1 infection; however, given increasing occurrence of genital HSV-1 infection, this could also represent a genital infection.
What are the clinical uses of type-specific HSV serology?
Type-specific serologic tests are helpful in diagnosing patients with atypical or asymptomatic infection and managing the care of persons whose sex partners have genital herpes. Serologic testing can be useful to confirm a clinical diagnosis of HSV, to determine whether atypical lesions or symptoms are attributable to HSV, and as part of evaluation for sexually transmitted diseases in select patients. Screening for HSV-1 and HSV-2 in the general population is not supported by the Centers for Disease Control and Prevention (CDC) or the US Preventive Services Task Force (USPSTF) for several reasons9,10:
- suboptimal performance of commercial HSV antibody tests
- low positive predictive value of these tests in low prevalence HSV settings
- lack of widely available confirmatory testing
- lack of cost-effectiveness
- potential for psychological harm.
Read about treating HSV infection during pregnancy.
Case Continued…
Because Sarah did not have a history of genital herpes, a serum sample was tested by the University of Washington Western blot. The results indicated that Sarah is seronegative for HSV-1 and HSV-2.
Sarah, who is now at 16 weeks’ gestation, returns for evaluation of new genital pain. On examination, she has several shallow ulcerations on the labia and bilateral tender inguinal adenopathy. Her husband recently had cold sores. She is anxious and would like to know if she has genital herpes and if her baby is at risk for HSV infection. You swab the base of a lesion for HSV PCR testing and start antiviral treatment.
Treating HSV infection during pregnancy
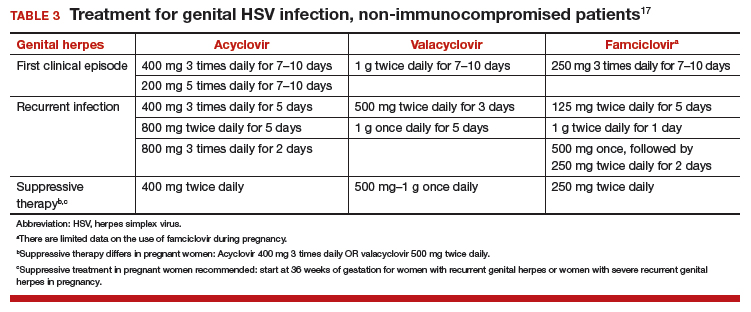
Women presenting with a new genital ulcer consistent with HSV should receive empiric antiviral treatment while awaiting confirmatory diagnostic laboratory testing, even during pregnancy. Antiviral therapy with acyclovir, valacyclovir, and famciclovir is the backbone of management of most symptomatic patients with herpes. Antiviral drugs can reduce signs and symptoms of first or recurrent genital herpes and can be used for daily suppressive therapy to prevent recurrences. These drugs do not eradicate the infection or alter the risk of frequency or severity after the drug is discontinued.
Antiviral advantages/disadvantages. Acyclovir is the least expensive drug, but valacyclovir is the most convenient therapy given its less frequent dosing. Acyclovir and valacyclovir are equally efficacious in treating first-episode genital herpes infection with respect to duration of viral shedding, time of healing, duration of pain, and time to symptom clearance. Two randomized clinical trials showed similar benefits of acyclovir and valacyclovir for suppressive therapy management of genital herpes.14,15 Only 1 study compared the efficacy of famciclovir to valacyclovir for suppression and showed that valacyclovir was more effective.16 The cost of famciclovir is usually higher, and it has the least data on use in pregnant women. Acyclovir therapy can be safely used throughout pregnancy and during breastfeeding.9 Antiviral regimens for the treatment of genital HSV in pregnant and nonpregnant women recommended by the CDC are summarized in TABLE 3.17
Related article:
5 ways to reduce infection risk during pregnancy
Will your patient’s infant develop neonatal herpes infection?
Neonatal herpes is a potentially devastating infection that results from exposure to HSV from the maternal genital tract at vaginal delivery. Most cases occur in infants born to women who lack a history of genital herpes.18 In a large cohort study conducted in Washington State, isolation of HSV at the time of labor was strongly associated with vertical transmission (odds ratio [OR], 346).19 The risk of neonatal herpes increased among women shedding HSV-1 compared with HSV-2 (OR, 16.5). The highest risk of transmission to the neonate is in women who acquire genital herpes in a period close to the delivery (30% to 50% risk of transmission), compared with women with a prenatal history of herpes or who acquired herpes early in pregnancy (about 1% to 3% risk of transmission), most likely due to protective HSV-specific maternal antibodies and lower viral load during reactivation versus primary infection.18
Neonatal HSV-1 infection also has been reported in neonates born to women with primary HSV-1 gingivostomatitis during pregnancy; 70% of these women had oral clinical symptoms during the peripartum period.20 Potential mechanisms are exposure to infected genital secretions, direct maternal hematogenous spread, or oral shedding from close contacts.
Although prenatal HSV screening is not recommended by the CDC or USPSTF, serologic testing could be helpful when identifying appropriate pregnancy management for women with a prior history of HSV infection. It also could be beneficial in identifying women without HSV to guide counseling prevention for HSV acquisition. In patients presenting with active genital lesions, viral-specific diagnostic evaluation should be obtained. In those with a history of laboratory confirmed genital herpes, no additional testing is warranted.
Preventing neonatal herpes
There are no prevention strategies for neonatal herpes in the United States, and the incidence of neonatal herpes has not changed in several decades.10 The current treatment guidelines focus on managing women who may be at risk for HSV acquisition during pregnancy and the management of genital lesions in women during pregnancy.9,10,21
When the partner has HSV. Women who have no history of genital herpes or who are seronegative for HSV-2 should avoid intercourse during the third trimester with a partner known to have genital herpes.9 Those who have no history of orolabial herpes or who are seronegative for HSV-1 and have a seropositive partner should avoid receptive oral-genital contact and genital intercourse.9 Condoms can reduce but not eliminate the risk of HSV transmission; to effectively avoid genital herpes infection, abstinence is recommended.
When the patient has HSV. When managing the care of a pregnant woman with genital herpes evaluate for clinical symptoms and timing of infection or recurrence relative to time of delivery:
- Monitor women with a mild recurrence of HSV during the first 35 weeks of pregnancy without antiviral treatment, as most of the recurrent episodes of genital herpes are short.
- Consider antivirals for women with severe symptoms or multiple recurrences.
- Offer women with a history of genital lesions suppressive antiviral therapy at 36 weeks of gestation until delivery.21
In a meta-analysis of 7 randomized trials, 1,249 women with a history of genital herpes prior to or during pregnancy received prophylaxis with either acyclovir or valacyclovir versus placebo or no treatment at 36 weeks of gestation. Antiviral therapy reduced the risk of HSV recurrence at delivery (relative risk [RR], 0.28), cesarean delivery in those with recurrent genital herpes (RR, 0.3), and asymptomatic shedding at delivery (RR, 0.14).22 No data are available regarding the effectiveness of this approach to prevention of neonatal HSV, and case reports confirm neonatal HSV in infants born to women who received suppressive antiviral therapy at the end of pregnancy.23
When cesarean delivery is warranted. At the time of delivery, ask all women about symptoms of genital herpes, including prodromal symptoms, and examine them for genital lesions. For women with active lesions or prodromal symptoms, offer cesarean delivery at the onset of labor or rupture of membranes—this recommendation is supported by the CDC and the American College of Obstetricians and Gynecologists.9,21 The protective effect of cesarean delivery was evaluated in a large cohort study that found: among women who were shedding HSV at the time of delivery, neonates born by cesarean delivery were less likely to develop HSV infection compared with those born through vaginal delivery (1.2% vs 7.7%, respectively).19 Cesarean delivery is not indicated in patients with a history of HSV without clinical recurrence or prodrome at delivery, as such women have a very low risk of transmitting the infection to the neonate.24
Avoid transcervical antepartum obstetric procedures to reduce the risk of placenta or membrane HSV infection; however, transabdominal invasive procedures can be performed safely, even in the presence of active genital lesions.21 Intrapartum procedures that can cause fetal skin disruption, such as use of fetal scalp electrode or forceps, are risk factors for HSV transmission and should be avoided in women with a history of genital herpes.
Related articles:
8 common questions about newborn circumcision
Case Resolved
Sarah’s genital lesion PCR results returned positive for HSV-1. She probably acquired the infection from oral-genital sex with her husband who likely has oral HSV-1, given the history of cold sores. You treat Sarah with acyclovir 400 mg 3 times per day for 7 days. At 36 weeks’ gestation, Sarah begins suppressive antiviral therapy until delivery. She spontaneously labors at 39 weeks’ gestation; at that time, she has no genital lesions and she delivers vaginally a healthy baby.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Fanfair RN, Zaidi A, Taylor LD, Xu F, Gottlieb S, Markowitz L. Trends in seroprevalence of herpes simplex virus type 2 among non-Hispanic blacks and non-Hispanic whites aged 14 to 49 years–United States, 1988 to 2010. Sex Transm Dis. 2013;40(11):860–864.
- Bradley H, Markowitz LE, Gibson T, McQuillan GM. Seroprevalence of herpes simplex virus types 1 and 2–United States, 1999-2010. J Infect Dis. 2014;209(3):325–333.
- Bernstein DI, Bellamy AR, Hook EW, 3rd, et al. Epidemiology, clinical presentation, and antibody response to primary infection with herpes simplex virus type 1 and type 2 in young women. Clin Infect Dis. 2013;56(3):344–351.
- Kimberlin DW, Rouse DJ. Clinical practice. Genital herpes. N Engl J Med. 2004;350(19):1970–1977.
- Corey L, Adams HG, Brown ZA, Holmes KK. Genital herpes simplex virus infections: clinical manifestations, course, and complications. Ann Intern Med. 1983;98(6):958–972.
- Wald A, Zeh J, Selke S, Ashley RL, Corey L. Virologic characteristics of subclinical and symptomatic genital herpes infections. N Engl J Med. 1995;333(12):770–775.
- Reeves WC, Corey L, Adams HG, Vontver LA, Holmes KK. Risk of recurrence after first episodes of genital herpes. Relation to HSV type and antibody response. N Engl J Med. 1981;305(6):315–319.
- Phipps W, Saracino M, Magaret A, et al. Persistent genital herpes simplex virus-2 shedding years following the first clinical episode. J Infect Dis. 2011;203(2):180–187.
- Workowski KA, Bolan GA; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1–137.
- Bibbins-Domingo K, Grossman DC, Curry SJ, et al; US Preventive Task Force. Serologic screening for genital herpes infection: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;316(23):2525–2530.
- Gupta R, Warren T, Wald A. Genital herpes. Lancet. 2007;370(9605):2127–2137.
- Agyemang E, Le QA, Warren T, et al. Performance of commercial enzyme-linked immunoassays 1 (EIA) for diagnosis of herpes simplex virus-1 and herpes simplex virus-2 infection in a clinical setting. Sex Transm Dis. 2017; doi:10.1097/olq.0000000000000689.
- Wald A, Zeh J, Selke S, et al. Reactivation of genital herpes simplex virus type 2 infection in asymptomatic seropositive persons. N Engl J Med. 2000;342(12):844–850.
- Gupta R, Wald A, Krantz E, et al. Valacyclovir and acyclovir for suppression of shedding of herpes simplex virus in the genital tract. J Infect Dis. 2004;190(8):1374–1381.
- Reitano M, Tyring S, Lang W, et al. Valaciclovir for the suppression of recurrent genital herpes simplex virus infection: a large-scale dose range-finding study. International Valaciclovir HSV Study Group. J Infect Dis. 1998;178(3): 603–610.
- Wald A, Selke S, Warren T, et al. Comparative efficacy of famciclovir and valacyclovir for suppression of recurrent genital herpes and viral shedding. Sex Transm Dis. 2006;33(9):529–533.
- Workowski KA, Bolan GA; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015 [published correction appears in MMWR Recomm Rep. 2015;64(33):924]. MMWR Recomm Rep. 2015;64(RR-03):1–137.
- Corey L, Wald A. Maternal and neonatal herpes simplex virus infections. N Engl J Med. 2009;361(14):1376–1385.
- Brown ZA, Wald A, Morrow RA, Selke S, Zeh J, Corey L. Effect of serologic status and cesarean delivery on transmission rates of herpes simplex virus from mother to infant. JAMA. 2003;289(2):203–209.
- Healy SA, Mohan KM, Melvin AJ, Wald A. Primary maternal herpes simplex virus-1 gingivostomatitis during pregnancy and neonatal herpes: case series and literature review. J Pediatric Infect Dis Soc. 2012;1(4):299–305.
- American College of Obstetricians and Gynecoloigsts Committee on Practice Bulletins. ACOG Practice Bulletin No. 82: Management of herpes in pregnancy. Obstet Gynecol. 2007;109(6):1489–1498.
- Hollier LM, Wendel GD. Third trimester antiviral prophylaxis for preventing maternal genital herpes simplex virus (HSV) recurrences and neonatal infection. Cochrane Database Syst Rev. 2008(1):CD004946.
- Pinninti SG, Angara R, Feja KN, et al. Neonatal herpes disease following maternal antenatal antiviral suppressive therapy: a multicenter case series. J Pediatr. 2012;161(1):134–138.e1–e3.
- Vontver LA, Hickok DE, Brown Z, Reid L, Corey L. Recurrent genital herpes simplex virus infection in pregnancy: infant outcome and frequency of asymptomatic recurrences. American journal of obstetrics and gynecology. 1982;143(1):75–84.
Genital herpes is a common infection caused by herpes simplex virus type 1 (HSV-1) or herpes simplex virus type 2 (HSV-2). Although life-threatening health consequences of HSV infection after infancy are uncommon, women with genital herpes remain at risk for recurrent symptoms, which can be associated with significant physical and psychosocial distress. These patients also can transmit the disease to their partners and neonates, and have a 2- to 3-fold increased risk of HIV acquisition. In this article, we review the diagnosis and management of genital herpes in pregnant women.
CASE Asymptomatic pregnant patient tests positive for herpes
Sarah is a healthy 32-year-old (G1P0) presenting at 8 weeks’ gestation for her first prenatal visit. She requests HSV testing as she learned that genital herpes is common and it can be transmitted to the baby. You order the HSV-2 IgG assay from your laboratory, which performs the HerpeSelect HSV-2 enzyme immunoassay as the standard test. The test result is positive, with an index value of 2.2 (the manufacturer defines an index value >1.1 as positive). Repeat testing in 4 weeks returns positive results again, with an index value of 2.8.
The patient is distressed at this news. She has no history of genital lesions or symptoms consistent with genital herpes and is worried that her husband has been unfaithful. How would you manage this case?
How prevalent is HSV?
Genital herpes is a chronic viral infection transmitted through close contact with a person who is shedding the virus from genital or oral mucosa. In the United States, the National Health and Nutrition Examination Survey indicated an HSV-2 seroprevalence of 16% among persons aged 14 to 49 in 2005–2010, a decline from 21% in 1988–1991.1 The prevalence among women is twice as high as among men, at 20% versus 11%, respectively. Among those with HSV-2, 87% are not aware that they are infected; they are at risk of infecting their partners, however.1
In the same age group, the prevalence of HSV-1 is 54%.2 The seroprevalence of HSV-1 in adolescents declined from 39% in 1999–2004 to 30% in 2005–2010, resulting in a high number of young people who are seronegative at the time of sexual debut. Concurrently, genital HSV-1 has emerged as a frequent cause of first-episode genital herpes, often associated with oral-genital contact during sexual debut.2,3
When evaluating patients for possible genital herpes provide general educational messages regarding HSV infection and obtain a detailed medical and sexual history to determine the best diagnostic approach.
What are the clinical features of genital HSV infection?
The clinical manifestations of genital herpes vary according to whether the infection is primary, nonprimary first episode, or recurrent.
Primary infection. During primary infection,which occurs 4 to 12 days after sexual exposure and in the absence of pre-existing antibodies to HSV-1 or HSV-2, patients may experience genital and systemic symptoms (FIGURE and TABLE 1). Since this infection usually occurs in otherwise healthy people, for many, this is the most severe disease that they have experienced. However, most patients with primary infection develop mild, atypical, or completely asymptomatic presentation and are not diagnosed at the time of HSV acquisition. Whether primary infection is caused by HSV-1 or HSV-2 cannot be differentiated based on the clinical presentation alone.

Nonprimary first episode infection. In a nonprimary infection, newly acquired infection with HSV-1 or HSV-2 occurs in a person with pre-existing antibodies to the other virus. Almost always, this means new HSV-2 infection in a HSV-1 seropositive person, as prior HSV-2 infection appears to protect against HSV-1 acquisition. In general, the clinical presentation of nonprimary infection is somewhat milder and the rate of complications is lower, but clinically the overlap is great, and antibody tests are needed to define whether the patient has primary or nonprimary infection.4
Recurrent genital herpes infection occurs in most patients with genital herpes. The rate of recurrence is low in patients with genital HSV-1 and often high in patients with genital HSV-2 infection. The median number of recurrences is 1 in the first year of genital HSV-1 infection, and many patients will not have any recurrences following the first year. By contrast, in patients with genital HSV-2 infection, the median number of recurrences is 4, and a high rate of recurrences can continue for many years. Prodromal symptoms (localized irritation, paresthesias, and pruritus) can precede recurrences, which usually present with fewer lesions and last a shorter time than primary infection. Recurrent genital lesions tend to heal in approximately 5 to 10 days in the absence of antiviral treatment, and systemic symptoms are uncommon.5
Asymptomatic viral shedding. After resolution of a primary HSV infection, people shed the virus in the genital tract despite symptom absence. Asymptomatic shedding tends to be more frequent and prolonged with primary genital HSV-2 infection compared with HSV-1 infection.6,7 The frequency of HSV shedding is highest in the first year of infection, and decreases subsequently.8 However, it is likely to persist intermittently for many years. Because the natural history is so strikingly different in genital HSV-1 versus HSV-2, identification of the viral type is important for prognostic information.
The first HSV episode does not necessarily indicate a new or recent infection—in about 25% of persons it represents the first recognized genital herpes episode. Additional serologic and virologic evaluation can be pursued to determine if the first episode represents a new infection.
Read about the diagnostic tests for genital HSV.
What diagnostic tests are available for genital herpes?
Most HSV infections are clinically silent. Therefore, laboratory tests are required to diagnose the infection. Even if symptoms are present, diagnoses based only on clinical presentation have a 20% false-positive rate. Always confirm diagnosis by laboratory assay.9 Furthermore, couples that are discordant for HSV-2 by history are often concordant by serologic assays, as the transmission already has occurred but was not recognized. In these cases, the direction of transmission cannot be determined, and stable couples often experience relief learning that they are not discordant.
Related article:
Effective treatment of recurrent bacterial vaginosis
Several laboratory tools for HSV diagnosis based on direct viral detection and antibody detection can be used in clinical settings (TABLE 2). Among patients with symptomatic genital herpes, a sample from the lesion can be used to confirm and identify viral type. Because polymerase chain reaction (PCR) is substantially more sensitive than viral culture and increasingly available it has emerged as the preferred test.9 Viral culture is highly specific (>99%), but sensitivity varies according to collection technique and stage of the lesions. (The test is less sensitive when lesions are healing.)9,10 Antigen detection by immunofluorescence (direct fluorescent antibody) detects HSV from active lesions with high specificity, but sensitivity is low. Cytologic identification of infected cells (using Tzanck or Pap test) has limited utility for diagnosis due to low sensitivity and specificity.9
Type-specific antibodies to HSV develop during the first several weeks after acquisition and persist indefinitely.11 Most accurate type-specific serologic tests are based on detection of glycoprotein G1 and glycoprotein G2 for HSV-1 and HSV-2, respectively.
HerpeSelect HSV-2 enzyme immunoassay (EIA) is one of the most commonly used tests in the United States. The manufacturer considers results with index values 1.1 or greater as showing HSV-2 infection. Unfortunately, low positive results, often with a defined index value of 1.1 to 3.5, are frequently false positive. These low positive values should be confirmed with another test, such as Western blot.9
Western blot has been considered the gold standard assay for HSV-1 and HSV-2 antibody detection; this test is available at the University of Washington in Seattle. When comparing the HSV-1 EIA and HSV-2 EIA with the Western blot assay in clinical practice, the estimated sensitivity and specificity are 70.2% and 91.6%, respectively, for HSV-1 and 91.9% and 57.4%, respectively, for HSV-2.12
HerpeSelect HSV-2 Immunoblot testing should not be considered as confirmatory because this assay detects the same antigen as the HSV-2 EIA. Serologic tests based on detection of HSV-IgM should not be used for diagnosis of genital herpes as IgM response can present during a new infection or HSV reactivation and because IgM responses are not type-specific. Clearly, more accurate commercial type-specific antibody tests are needed.
Specific HSV antibodies can take up to 12 weeks to develop. Therefore, repeat serologic testing for patients in whom initial HSV antibody results are negative yet recent genital herpes acquisition is suspected.11 A confirmed positive HSV-2 antibody test indicates anogenital infection, even in a person who lacks genital symptoms. This finding became evident through a study of 53 HSV-2 seropositive patients who lacked a history of genital herpes. Patients were followed for 3 months, and all but 1 developed either virologic or clinical (or both) evidence of genital herpes.13
In the absence of genital or orolabial symptoms among individuals with positive HSV-1, serologic testing cannot distinguish anogenital from orolabial infection. Most of these infections may represent oral HSV-1 infection; however, given increasing occurrence of genital HSV-1 infection, this could also represent a genital infection.
What are the clinical uses of type-specific HSV serology?
Type-specific serologic tests are helpful in diagnosing patients with atypical or asymptomatic infection and managing the care of persons whose sex partners have genital herpes. Serologic testing can be useful to confirm a clinical diagnosis of HSV, to determine whether atypical lesions or symptoms are attributable to HSV, and as part of evaluation for sexually transmitted diseases in select patients. Screening for HSV-1 and HSV-2 in the general population is not supported by the Centers for Disease Control and Prevention (CDC) or the US Preventive Services Task Force (USPSTF) for several reasons9,10:
- suboptimal performance of commercial HSV antibody tests
- low positive predictive value of these tests in low prevalence HSV settings
- lack of widely available confirmatory testing
- lack of cost-effectiveness
- potential for psychological harm.
Read about treating HSV infection during pregnancy.
Case Continued…
Because Sarah did not have a history of genital herpes, a serum sample was tested by the University of Washington Western blot. The results indicated that Sarah is seronegative for HSV-1 and HSV-2.
Sarah, who is now at 16 weeks’ gestation, returns for evaluation of new genital pain. On examination, she has several shallow ulcerations on the labia and bilateral tender inguinal adenopathy. Her husband recently had cold sores. She is anxious and would like to know if she has genital herpes and if her baby is at risk for HSV infection. You swab the base of a lesion for HSV PCR testing and start antiviral treatment.
Treating HSV infection during pregnancy
Women presenting with a new genital ulcer consistent with HSV should receive empiric antiviral treatment while awaiting confirmatory diagnostic laboratory testing, even during pregnancy. Antiviral therapy with acyclovir, valacyclovir, and famciclovir is the backbone of management of most symptomatic patients with herpes. Antiviral drugs can reduce signs and symptoms of first or recurrent genital herpes and can be used for daily suppressive therapy to prevent recurrences. These drugs do not eradicate the infection or alter the risk of frequency or severity after the drug is discontinued.
Antiviral advantages/disadvantages. Acyclovir is the least expensive drug, but valacyclovir is the most convenient therapy given its less frequent dosing. Acyclovir and valacyclovir are equally efficacious in treating first-episode genital herpes infection with respect to duration of viral shedding, time of healing, duration of pain, and time to symptom clearance. Two randomized clinical trials showed similar benefits of acyclovir and valacyclovir for suppressive therapy management of genital herpes.14,15 Only 1 study compared the efficacy of famciclovir to valacyclovir for suppression and showed that valacyclovir was more effective.16 The cost of famciclovir is usually higher, and it has the least data on use in pregnant women. Acyclovir therapy can be safely used throughout pregnancy and during breastfeeding.9 Antiviral regimens for the treatment of genital HSV in pregnant and nonpregnant women recommended by the CDC are summarized in TABLE 3.17
Related article:
5 ways to reduce infection risk during pregnancy
Will your patient’s infant develop neonatal herpes infection?
Neonatal herpes is a potentially devastating infection that results from exposure to HSV from the maternal genital tract at vaginal delivery. Most cases occur in infants born to women who lack a history of genital herpes.18 In a large cohort study conducted in Washington State, isolation of HSV at the time of labor was strongly associated with vertical transmission (odds ratio [OR], 346).19 The risk of neonatal herpes increased among women shedding HSV-1 compared with HSV-2 (OR, 16.5). The highest risk of transmission to the neonate is in women who acquire genital herpes in a period close to the delivery (30% to 50% risk of transmission), compared with women with a prenatal history of herpes or who acquired herpes early in pregnancy (about 1% to 3% risk of transmission), most likely due to protective HSV-specific maternal antibodies and lower viral load during reactivation versus primary infection.18
Neonatal HSV-1 infection also has been reported in neonates born to women with primary HSV-1 gingivostomatitis during pregnancy; 70% of these women had oral clinical symptoms during the peripartum period.20 Potential mechanisms are exposure to infected genital secretions, direct maternal hematogenous spread, or oral shedding from close contacts.
Although prenatal HSV screening is not recommended by the CDC or USPSTF, serologic testing could be helpful when identifying appropriate pregnancy management for women with a prior history of HSV infection. It also could be beneficial in identifying women without HSV to guide counseling prevention for HSV acquisition. In patients presenting with active genital lesions, viral-specific diagnostic evaluation should be obtained. In those with a history of laboratory confirmed genital herpes, no additional testing is warranted.
Preventing neonatal herpes
There are no prevention strategies for neonatal herpes in the United States, and the incidence of neonatal herpes has not changed in several decades.10 The current treatment guidelines focus on managing women who may be at risk for HSV acquisition during pregnancy and the management of genital lesions in women during pregnancy.9,10,21
When the partner has HSV. Women who have no history of genital herpes or who are seronegative for HSV-2 should avoid intercourse during the third trimester with a partner known to have genital herpes.9 Those who have no history of orolabial herpes or who are seronegative for HSV-1 and have a seropositive partner should avoid receptive oral-genital contact and genital intercourse.9 Condoms can reduce but not eliminate the risk of HSV transmission; to effectively avoid genital herpes infection, abstinence is recommended.
When the patient has HSV. When managing the care of a pregnant woman with genital herpes evaluate for clinical symptoms and timing of infection or recurrence relative to time of delivery:
- Monitor women with a mild recurrence of HSV during the first 35 weeks of pregnancy without antiviral treatment, as most of the recurrent episodes of genital herpes are short.
- Consider antivirals for women with severe symptoms or multiple recurrences.
- Offer women with a history of genital lesions suppressive antiviral therapy at 36 weeks of gestation until delivery.21
In a meta-analysis of 7 randomized trials, 1,249 women with a history of genital herpes prior to or during pregnancy received prophylaxis with either acyclovir or valacyclovir versus placebo or no treatment at 36 weeks of gestation. Antiviral therapy reduced the risk of HSV recurrence at delivery (relative risk [RR], 0.28), cesarean delivery in those with recurrent genital herpes (RR, 0.3), and asymptomatic shedding at delivery (RR, 0.14).22 No data are available regarding the effectiveness of this approach to prevention of neonatal HSV, and case reports confirm neonatal HSV in infants born to women who received suppressive antiviral therapy at the end of pregnancy.23
When cesarean delivery is warranted. At the time of delivery, ask all women about symptoms of genital herpes, including prodromal symptoms, and examine them for genital lesions. For women with active lesions or prodromal symptoms, offer cesarean delivery at the onset of labor or rupture of membranes—this recommendation is supported by the CDC and the American College of Obstetricians and Gynecologists.9,21 The protective effect of cesarean delivery was evaluated in a large cohort study that found: among women who were shedding HSV at the time of delivery, neonates born by cesarean delivery were less likely to develop HSV infection compared with those born through vaginal delivery (1.2% vs 7.7%, respectively).19 Cesarean delivery is not indicated in patients with a history of HSV without clinical recurrence or prodrome at delivery, as such women have a very low risk of transmitting the infection to the neonate.24
Avoid transcervical antepartum obstetric procedures to reduce the risk of placenta or membrane HSV infection; however, transabdominal invasive procedures can be performed safely, even in the presence of active genital lesions.21 Intrapartum procedures that can cause fetal skin disruption, such as use of fetal scalp electrode or forceps, are risk factors for HSV transmission and should be avoided in women with a history of genital herpes.
Related articles:
8 common questions about newborn circumcision
Case Resolved
Sarah’s genital lesion PCR results returned positive for HSV-1. She probably acquired the infection from oral-genital sex with her husband who likely has oral HSV-1, given the history of cold sores. You treat Sarah with acyclovir 400 mg 3 times per day for 7 days. At 36 weeks’ gestation, Sarah begins suppressive antiviral therapy until delivery. She spontaneously labors at 39 weeks’ gestation; at that time, she has no genital lesions and she delivers vaginally a healthy baby.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Genital herpes is a common infection caused by herpes simplex virus type 1 (HSV-1) or herpes simplex virus type 2 (HSV-2). Although life-threatening health consequences of HSV infection after infancy are uncommon, women with genital herpes remain at risk for recurrent symptoms, which can be associated with significant physical and psychosocial distress. These patients also can transmit the disease to their partners and neonates, and have a 2- to 3-fold increased risk of HIV acquisition. In this article, we review the diagnosis and management of genital herpes in pregnant women.
CASE Asymptomatic pregnant patient tests positive for herpes
Sarah is a healthy 32-year-old (G1P0) presenting at 8 weeks’ gestation for her first prenatal visit. She requests HSV testing as she learned that genital herpes is common and it can be transmitted to the baby. You order the HSV-2 IgG assay from your laboratory, which performs the HerpeSelect HSV-2 enzyme immunoassay as the standard test. The test result is positive, with an index value of 2.2 (the manufacturer defines an index value >1.1 as positive). Repeat testing in 4 weeks returns positive results again, with an index value of 2.8.
The patient is distressed at this news. She has no history of genital lesions or symptoms consistent with genital herpes and is worried that her husband has been unfaithful. How would you manage this case?
How prevalent is HSV?
Genital herpes is a chronic viral infection transmitted through close contact with a person who is shedding the virus from genital or oral mucosa. In the United States, the National Health and Nutrition Examination Survey indicated an HSV-2 seroprevalence of 16% among persons aged 14 to 49 in 2005–2010, a decline from 21% in 1988–1991.1 The prevalence among women is twice as high as among men, at 20% versus 11%, respectively. Among those with HSV-2, 87% are not aware that they are infected; they are at risk of infecting their partners, however.1
In the same age group, the prevalence of HSV-1 is 54%.2 The seroprevalence of HSV-1 in adolescents declined from 39% in 1999–2004 to 30% in 2005–2010, resulting in a high number of young people who are seronegative at the time of sexual debut. Concurrently, genital HSV-1 has emerged as a frequent cause of first-episode genital herpes, often associated with oral-genital contact during sexual debut.2,3
When evaluating patients for possible genital herpes provide general educational messages regarding HSV infection and obtain a detailed medical and sexual history to determine the best diagnostic approach.
What are the clinical features of genital HSV infection?
The clinical manifestations of genital herpes vary according to whether the infection is primary, nonprimary first episode, or recurrent.
Primary infection. During primary infection,which occurs 4 to 12 days after sexual exposure and in the absence of pre-existing antibodies to HSV-1 or HSV-2, patients may experience genital and systemic symptoms (FIGURE and TABLE 1). Since this infection usually occurs in otherwise healthy people, for many, this is the most severe disease that they have experienced. However, most patients with primary infection develop mild, atypical, or completely asymptomatic presentation and are not diagnosed at the time of HSV acquisition. Whether primary infection is caused by HSV-1 or HSV-2 cannot be differentiated based on the clinical presentation alone.

Nonprimary first episode infection. In a nonprimary infection, newly acquired infection with HSV-1 or HSV-2 occurs in a person with pre-existing antibodies to the other virus. Almost always, this means new HSV-2 infection in a HSV-1 seropositive person, as prior HSV-2 infection appears to protect against HSV-1 acquisition. In general, the clinical presentation of nonprimary infection is somewhat milder and the rate of complications is lower, but clinically the overlap is great, and antibody tests are needed to define whether the patient has primary or nonprimary infection.4
Recurrent genital herpes infection occurs in most patients with genital herpes. The rate of recurrence is low in patients with genital HSV-1 and often high in patients with genital HSV-2 infection. The median number of recurrences is 1 in the first year of genital HSV-1 infection, and many patients will not have any recurrences following the first year. By contrast, in patients with genital HSV-2 infection, the median number of recurrences is 4, and a high rate of recurrences can continue for many years. Prodromal symptoms (localized irritation, paresthesias, and pruritus) can precede recurrences, which usually present with fewer lesions and last a shorter time than primary infection. Recurrent genital lesions tend to heal in approximately 5 to 10 days in the absence of antiviral treatment, and systemic symptoms are uncommon.5
Asymptomatic viral shedding. After resolution of a primary HSV infection, people shed the virus in the genital tract despite symptom absence. Asymptomatic shedding tends to be more frequent and prolonged with primary genital HSV-2 infection compared with HSV-1 infection.6,7 The frequency of HSV shedding is highest in the first year of infection, and decreases subsequently.8 However, it is likely to persist intermittently for many years. Because the natural history is so strikingly different in genital HSV-1 versus HSV-2, identification of the viral type is important for prognostic information.
The first HSV episode does not necessarily indicate a new or recent infection—in about 25% of persons it represents the first recognized genital herpes episode. Additional serologic and virologic evaluation can be pursued to determine if the first episode represents a new infection.
Read about the diagnostic tests for genital HSV.
What diagnostic tests are available for genital herpes?
Most HSV infections are clinically silent. Therefore, laboratory tests are required to diagnose the infection. Even if symptoms are present, diagnoses based only on clinical presentation have a 20% false-positive rate. Always confirm diagnosis by laboratory assay.9 Furthermore, couples that are discordant for HSV-2 by history are often concordant by serologic assays, as the transmission already has occurred but was not recognized. In these cases, the direction of transmission cannot be determined, and stable couples often experience relief learning that they are not discordant.
Related article:
Effective treatment of recurrent bacterial vaginosis
Several laboratory tools for HSV diagnosis based on direct viral detection and antibody detection can be used in clinical settings (TABLE 2). Among patients with symptomatic genital herpes, a sample from the lesion can be used to confirm and identify viral type. Because polymerase chain reaction (PCR) is substantially more sensitive than viral culture and increasingly available it has emerged as the preferred test.9 Viral culture is highly specific (>99%), but sensitivity varies according to collection technique and stage of the lesions. (The test is less sensitive when lesions are healing.)9,10 Antigen detection by immunofluorescence (direct fluorescent antibody) detects HSV from active lesions with high specificity, but sensitivity is low. Cytologic identification of infected cells (using Tzanck or Pap test) has limited utility for diagnosis due to low sensitivity and specificity.9
Type-specific antibodies to HSV develop during the first several weeks after acquisition and persist indefinitely.11 Most accurate type-specific serologic tests are based on detection of glycoprotein G1 and glycoprotein G2 for HSV-1 and HSV-2, respectively.
HerpeSelect HSV-2 enzyme immunoassay (EIA) is one of the most commonly used tests in the United States. The manufacturer considers results with index values 1.1 or greater as showing HSV-2 infection. Unfortunately, low positive results, often with a defined index value of 1.1 to 3.5, are frequently false positive. These low positive values should be confirmed with another test, such as Western blot.9
Western blot has been considered the gold standard assay for HSV-1 and HSV-2 antibody detection; this test is available at the University of Washington in Seattle. When comparing the HSV-1 EIA and HSV-2 EIA with the Western blot assay in clinical practice, the estimated sensitivity and specificity are 70.2% and 91.6%, respectively, for HSV-1 and 91.9% and 57.4%, respectively, for HSV-2.12
HerpeSelect HSV-2 Immunoblot testing should not be considered as confirmatory because this assay detects the same antigen as the HSV-2 EIA. Serologic tests based on detection of HSV-IgM should not be used for diagnosis of genital herpes as IgM response can present during a new infection or HSV reactivation and because IgM responses are not type-specific. Clearly, more accurate commercial type-specific antibody tests are needed.
Specific HSV antibodies can take up to 12 weeks to develop. Therefore, repeat serologic testing for patients in whom initial HSV antibody results are negative yet recent genital herpes acquisition is suspected.11 A confirmed positive HSV-2 antibody test indicates anogenital infection, even in a person who lacks genital symptoms. This finding became evident through a study of 53 HSV-2 seropositive patients who lacked a history of genital herpes. Patients were followed for 3 months, and all but 1 developed either virologic or clinical (or both) evidence of genital herpes.13
In the absence of genital or orolabial symptoms among individuals with positive HSV-1, serologic testing cannot distinguish anogenital from orolabial infection. Most of these infections may represent oral HSV-1 infection; however, given increasing occurrence of genital HSV-1 infection, this could also represent a genital infection.
What are the clinical uses of type-specific HSV serology?
Type-specific serologic tests are helpful in diagnosing patients with atypical or asymptomatic infection and managing the care of persons whose sex partners have genital herpes. Serologic testing can be useful to confirm a clinical diagnosis of HSV, to determine whether atypical lesions or symptoms are attributable to HSV, and as part of evaluation for sexually transmitted diseases in select patients. Screening for HSV-1 and HSV-2 in the general population is not supported by the Centers for Disease Control and Prevention (CDC) or the US Preventive Services Task Force (USPSTF) for several reasons9,10:
- suboptimal performance of commercial HSV antibody tests
- low positive predictive value of these tests in low prevalence HSV settings
- lack of widely available confirmatory testing
- lack of cost-effectiveness
- potential for psychological harm.
Read about treating HSV infection during pregnancy.
Case Continued…
Because Sarah did not have a history of genital herpes, a serum sample was tested by the University of Washington Western blot. The results indicated that Sarah is seronegative for HSV-1 and HSV-2.
Sarah, who is now at 16 weeks’ gestation, returns for evaluation of new genital pain. On examination, she has several shallow ulcerations on the labia and bilateral tender inguinal adenopathy. Her husband recently had cold sores. She is anxious and would like to know if she has genital herpes and if her baby is at risk for HSV infection. You swab the base of a lesion for HSV PCR testing and start antiviral treatment.
Treating HSV infection during pregnancy
Women presenting with a new genital ulcer consistent with HSV should receive empiric antiviral treatment while awaiting confirmatory diagnostic laboratory testing, even during pregnancy. Antiviral therapy with acyclovir, valacyclovir, and famciclovir is the backbone of management of most symptomatic patients with herpes. Antiviral drugs can reduce signs and symptoms of first or recurrent genital herpes and can be used for daily suppressive therapy to prevent recurrences. These drugs do not eradicate the infection or alter the risk of frequency or severity after the drug is discontinued.
Antiviral advantages/disadvantages. Acyclovir is the least expensive drug, but valacyclovir is the most convenient therapy given its less frequent dosing. Acyclovir and valacyclovir are equally efficacious in treating first-episode genital herpes infection with respect to duration of viral shedding, time of healing, duration of pain, and time to symptom clearance. Two randomized clinical trials showed similar benefits of acyclovir and valacyclovir for suppressive therapy management of genital herpes.14,15 Only 1 study compared the efficacy of famciclovir to valacyclovir for suppression and showed that valacyclovir was more effective.16 The cost of famciclovir is usually higher, and it has the least data on use in pregnant women. Acyclovir therapy can be safely used throughout pregnancy and during breastfeeding.9 Antiviral regimens for the treatment of genital HSV in pregnant and nonpregnant women recommended by the CDC are summarized in TABLE 3.17
Related article:
5 ways to reduce infection risk during pregnancy
Will your patient’s infant develop neonatal herpes infection?
Neonatal herpes is a potentially devastating infection that results from exposure to HSV from the maternal genital tract at vaginal delivery. Most cases occur in infants born to women who lack a history of genital herpes.18 In a large cohort study conducted in Washington State, isolation of HSV at the time of labor was strongly associated with vertical transmission (odds ratio [OR], 346).19 The risk of neonatal herpes increased among women shedding HSV-1 compared with HSV-2 (OR, 16.5). The highest risk of transmission to the neonate is in women who acquire genital herpes in a period close to the delivery (30% to 50% risk of transmission), compared with women with a prenatal history of herpes or who acquired herpes early in pregnancy (about 1% to 3% risk of transmission), most likely due to protective HSV-specific maternal antibodies and lower viral load during reactivation versus primary infection.18
Neonatal HSV-1 infection also has been reported in neonates born to women with primary HSV-1 gingivostomatitis during pregnancy; 70% of these women had oral clinical symptoms during the peripartum period.20 Potential mechanisms are exposure to infected genital secretions, direct maternal hematogenous spread, or oral shedding from close contacts.
Although prenatal HSV screening is not recommended by the CDC or USPSTF, serologic testing could be helpful when identifying appropriate pregnancy management for women with a prior history of HSV infection. It also could be beneficial in identifying women without HSV to guide counseling prevention for HSV acquisition. In patients presenting with active genital lesions, viral-specific diagnostic evaluation should be obtained. In those with a history of laboratory confirmed genital herpes, no additional testing is warranted.
Preventing neonatal herpes
There are no prevention strategies for neonatal herpes in the United States, and the incidence of neonatal herpes has not changed in several decades.10 The current treatment guidelines focus on managing women who may be at risk for HSV acquisition during pregnancy and the management of genital lesions in women during pregnancy.9,10,21
When the partner has HSV. Women who have no history of genital herpes or who are seronegative for HSV-2 should avoid intercourse during the third trimester with a partner known to have genital herpes.9 Those who have no history of orolabial herpes or who are seronegative for HSV-1 and have a seropositive partner should avoid receptive oral-genital contact and genital intercourse.9 Condoms can reduce but not eliminate the risk of HSV transmission; to effectively avoid genital herpes infection, abstinence is recommended.
When the patient has HSV. When managing the care of a pregnant woman with genital herpes evaluate for clinical symptoms and timing of infection or recurrence relative to time of delivery:
- Monitor women with a mild recurrence of HSV during the first 35 weeks of pregnancy without antiviral treatment, as most of the recurrent episodes of genital herpes are short.
- Consider antivirals for women with severe symptoms or multiple recurrences.
- Offer women with a history of genital lesions suppressive antiviral therapy at 36 weeks of gestation until delivery.21
In a meta-analysis of 7 randomized trials, 1,249 women with a history of genital herpes prior to or during pregnancy received prophylaxis with either acyclovir or valacyclovir versus placebo or no treatment at 36 weeks of gestation. Antiviral therapy reduced the risk of HSV recurrence at delivery (relative risk [RR], 0.28), cesarean delivery in those with recurrent genital herpes (RR, 0.3), and asymptomatic shedding at delivery (RR, 0.14).22 No data are available regarding the effectiveness of this approach to prevention of neonatal HSV, and case reports confirm neonatal HSV in infants born to women who received suppressive antiviral therapy at the end of pregnancy.23
When cesarean delivery is warranted. At the time of delivery, ask all women about symptoms of genital herpes, including prodromal symptoms, and examine them for genital lesions. For women with active lesions or prodromal symptoms, offer cesarean delivery at the onset of labor or rupture of membranes—this recommendation is supported by the CDC and the American College of Obstetricians and Gynecologists.9,21 The protective effect of cesarean delivery was evaluated in a large cohort study that found: among women who were shedding HSV at the time of delivery, neonates born by cesarean delivery were less likely to develop HSV infection compared with those born through vaginal delivery (1.2% vs 7.7%, respectively).19 Cesarean delivery is not indicated in patients with a history of HSV without clinical recurrence or prodrome at delivery, as such women have a very low risk of transmitting the infection to the neonate.24
Avoid transcervical antepartum obstetric procedures to reduce the risk of placenta or membrane HSV infection; however, transabdominal invasive procedures can be performed safely, even in the presence of active genital lesions.21 Intrapartum procedures that can cause fetal skin disruption, such as use of fetal scalp electrode or forceps, are risk factors for HSV transmission and should be avoided in women with a history of genital herpes.
Related articles:
8 common questions about newborn circumcision
Case Resolved
Sarah’s genital lesion PCR results returned positive for HSV-1. She probably acquired the infection from oral-genital sex with her husband who likely has oral HSV-1, given the history of cold sores. You treat Sarah with acyclovir 400 mg 3 times per day for 7 days. At 36 weeks’ gestation, Sarah begins suppressive antiviral therapy until delivery. She spontaneously labors at 39 weeks’ gestation; at that time, she has no genital lesions and she delivers vaginally a healthy baby.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Fanfair RN, Zaidi A, Taylor LD, Xu F, Gottlieb S, Markowitz L. Trends in seroprevalence of herpes simplex virus type 2 among non-Hispanic blacks and non-Hispanic whites aged 14 to 49 years–United States, 1988 to 2010. Sex Transm Dis. 2013;40(11):860–864.
- Bradley H, Markowitz LE, Gibson T, McQuillan GM. Seroprevalence of herpes simplex virus types 1 and 2–United States, 1999-2010. J Infect Dis. 2014;209(3):325–333.
- Bernstein DI, Bellamy AR, Hook EW, 3rd, et al. Epidemiology, clinical presentation, and antibody response to primary infection with herpes simplex virus type 1 and type 2 in young women. Clin Infect Dis. 2013;56(3):344–351.
- Kimberlin DW, Rouse DJ. Clinical practice. Genital herpes. N Engl J Med. 2004;350(19):1970–1977.
- Corey L, Adams HG, Brown ZA, Holmes KK. Genital herpes simplex virus infections: clinical manifestations, course, and complications. Ann Intern Med. 1983;98(6):958–972.
- Wald A, Zeh J, Selke S, Ashley RL, Corey L. Virologic characteristics of subclinical and symptomatic genital herpes infections. N Engl J Med. 1995;333(12):770–775.
- Reeves WC, Corey L, Adams HG, Vontver LA, Holmes KK. Risk of recurrence after first episodes of genital herpes. Relation to HSV type and antibody response. N Engl J Med. 1981;305(6):315–319.
- Phipps W, Saracino M, Magaret A, et al. Persistent genital herpes simplex virus-2 shedding years following the first clinical episode. J Infect Dis. 2011;203(2):180–187.
- Workowski KA, Bolan GA; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1–137.
- Bibbins-Domingo K, Grossman DC, Curry SJ, et al; US Preventive Task Force. Serologic screening for genital herpes infection: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;316(23):2525–2530.
- Gupta R, Warren T, Wald A. Genital herpes. Lancet. 2007;370(9605):2127–2137.
- Agyemang E, Le QA, Warren T, et al. Performance of commercial enzyme-linked immunoassays 1 (EIA) for diagnosis of herpes simplex virus-1 and herpes simplex virus-2 infection in a clinical setting. Sex Transm Dis. 2017; doi:10.1097/olq.0000000000000689.
- Wald A, Zeh J, Selke S, et al. Reactivation of genital herpes simplex virus type 2 infection in asymptomatic seropositive persons. N Engl J Med. 2000;342(12):844–850.
- Gupta R, Wald A, Krantz E, et al. Valacyclovir and acyclovir for suppression of shedding of herpes simplex virus in the genital tract. J Infect Dis. 2004;190(8):1374–1381.
- Reitano M, Tyring S, Lang W, et al. Valaciclovir for the suppression of recurrent genital herpes simplex virus infection: a large-scale dose range-finding study. International Valaciclovir HSV Study Group. J Infect Dis. 1998;178(3): 603–610.
- Wald A, Selke S, Warren T, et al. Comparative efficacy of famciclovir and valacyclovir for suppression of recurrent genital herpes and viral shedding. Sex Transm Dis. 2006;33(9):529–533.
- Workowski KA, Bolan GA; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015 [published correction appears in MMWR Recomm Rep. 2015;64(33):924]. MMWR Recomm Rep. 2015;64(RR-03):1–137.
- Corey L, Wald A. Maternal and neonatal herpes simplex virus infections. N Engl J Med. 2009;361(14):1376–1385.
- Brown ZA, Wald A, Morrow RA, Selke S, Zeh J, Corey L. Effect of serologic status and cesarean delivery on transmission rates of herpes simplex virus from mother to infant. JAMA. 2003;289(2):203–209.
- Healy SA, Mohan KM, Melvin AJ, Wald A. Primary maternal herpes simplex virus-1 gingivostomatitis during pregnancy and neonatal herpes: case series and literature review. J Pediatric Infect Dis Soc. 2012;1(4):299–305.
- American College of Obstetricians and Gynecoloigsts Committee on Practice Bulletins. ACOG Practice Bulletin No. 82: Management of herpes in pregnancy. Obstet Gynecol. 2007;109(6):1489–1498.
- Hollier LM, Wendel GD. Third trimester antiviral prophylaxis for preventing maternal genital herpes simplex virus (HSV) recurrences and neonatal infection. Cochrane Database Syst Rev. 2008(1):CD004946.
- Pinninti SG, Angara R, Feja KN, et al. Neonatal herpes disease following maternal antenatal antiviral suppressive therapy: a multicenter case series. J Pediatr. 2012;161(1):134–138.e1–e3.
- Vontver LA, Hickok DE, Brown Z, Reid L, Corey L. Recurrent genital herpes simplex virus infection in pregnancy: infant outcome and frequency of asymptomatic recurrences. American journal of obstetrics and gynecology. 1982;143(1):75–84.
- Fanfair RN, Zaidi A, Taylor LD, Xu F, Gottlieb S, Markowitz L. Trends in seroprevalence of herpes simplex virus type 2 among non-Hispanic blacks and non-Hispanic whites aged 14 to 49 years–United States, 1988 to 2010. Sex Transm Dis. 2013;40(11):860–864.
- Bradley H, Markowitz LE, Gibson T, McQuillan GM. Seroprevalence of herpes simplex virus types 1 and 2–United States, 1999-2010. J Infect Dis. 2014;209(3):325–333.
- Bernstein DI, Bellamy AR, Hook EW, 3rd, et al. Epidemiology, clinical presentation, and antibody response to primary infection with herpes simplex virus type 1 and type 2 in young women. Clin Infect Dis. 2013;56(3):344–351.
- Kimberlin DW, Rouse DJ. Clinical practice. Genital herpes. N Engl J Med. 2004;350(19):1970–1977.
- Corey L, Adams HG, Brown ZA, Holmes KK. Genital herpes simplex virus infections: clinical manifestations, course, and complications. Ann Intern Med. 1983;98(6):958–972.
- Wald A, Zeh J, Selke S, Ashley RL, Corey L. Virologic characteristics of subclinical and symptomatic genital herpes infections. N Engl J Med. 1995;333(12):770–775.
- Reeves WC, Corey L, Adams HG, Vontver LA, Holmes KK. Risk of recurrence after first episodes of genital herpes. Relation to HSV type and antibody response. N Engl J Med. 1981;305(6):315–319.
- Phipps W, Saracino M, Magaret A, et al. Persistent genital herpes simplex virus-2 shedding years following the first clinical episode. J Infect Dis. 2011;203(2):180–187.
- Workowski KA, Bolan GA; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1–137.
- Bibbins-Domingo K, Grossman DC, Curry SJ, et al; US Preventive Task Force. Serologic screening for genital herpes infection: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;316(23):2525–2530.
- Gupta R, Warren T, Wald A. Genital herpes. Lancet. 2007;370(9605):2127–2137.
- Agyemang E, Le QA, Warren T, et al. Performance of commercial enzyme-linked immunoassays 1 (EIA) for diagnosis of herpes simplex virus-1 and herpes simplex virus-2 infection in a clinical setting. Sex Transm Dis. 2017; doi:10.1097/olq.0000000000000689.
- Wald A, Zeh J, Selke S, et al. Reactivation of genital herpes simplex virus type 2 infection in asymptomatic seropositive persons. N Engl J Med. 2000;342(12):844–850.
- Gupta R, Wald A, Krantz E, et al. Valacyclovir and acyclovir for suppression of shedding of herpes simplex virus in the genital tract. J Infect Dis. 2004;190(8):1374–1381.
- Reitano M, Tyring S, Lang W, et al. Valaciclovir for the suppression of recurrent genital herpes simplex virus infection: a large-scale dose range-finding study. International Valaciclovir HSV Study Group. J Infect Dis. 1998;178(3): 603–610.
- Wald A, Selke S, Warren T, et al. Comparative efficacy of famciclovir and valacyclovir for suppression of recurrent genital herpes and viral shedding. Sex Transm Dis. 2006;33(9):529–533.
- Workowski KA, Bolan GA; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015 [published correction appears in MMWR Recomm Rep. 2015;64(33):924]. MMWR Recomm Rep. 2015;64(RR-03):1–137.
- Corey L, Wald A. Maternal and neonatal herpes simplex virus infections. N Engl J Med. 2009;361(14):1376–1385.
- Brown ZA, Wald A, Morrow RA, Selke S, Zeh J, Corey L. Effect of serologic status and cesarean delivery on transmission rates of herpes simplex virus from mother to infant. JAMA. 2003;289(2):203–209.
- Healy SA, Mohan KM, Melvin AJ, Wald A. Primary maternal herpes simplex virus-1 gingivostomatitis during pregnancy and neonatal herpes: case series and literature review. J Pediatric Infect Dis Soc. 2012;1(4):299–305.
- American College of Obstetricians and Gynecoloigsts Committee on Practice Bulletins. ACOG Practice Bulletin No. 82: Management of herpes in pregnancy. Obstet Gynecol. 2007;109(6):1489–1498.
- Hollier LM, Wendel GD. Third trimester antiviral prophylaxis for preventing maternal genital herpes simplex virus (HSV) recurrences and neonatal infection. Cochrane Database Syst Rev. 2008(1):CD004946.
- Pinninti SG, Angara R, Feja KN, et al. Neonatal herpes disease following maternal antenatal antiviral suppressive therapy: a multicenter case series. J Pediatr. 2012;161(1):134–138.e1–e3.
- Vontver LA, Hickok DE, Brown Z, Reid L, Corey L. Recurrent genital herpes simplex virus infection in pregnancy: infant outcome and frequency of asymptomatic recurrences. American journal of obstetrics and gynecology. 1982;143(1):75–84.