Article
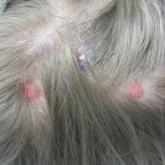
Lamotrigine-Induced Cutaneous Pseudolymphoma
- Author:
- Kelly L. Reed, DO
- Kelly B. Quinn, DO
- Anthony J. Gust, MD
Cutaneous pseudolymphomas have many causative factors, including medications, infections, tattoo ink, vaccinations, and insect bites. Lamotrigine...
