To the Editor:
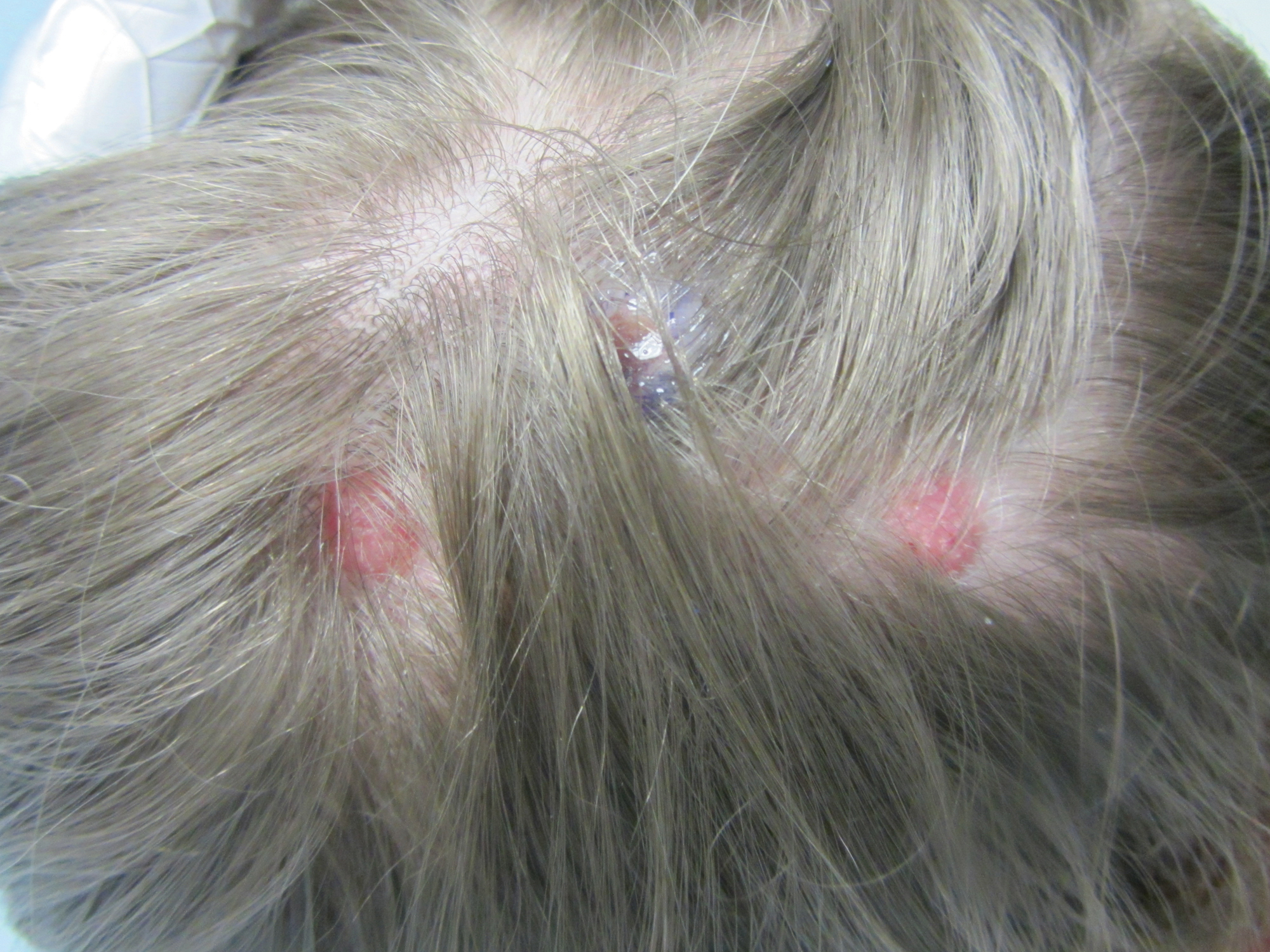
An 8-year-old girl presented with new lesions on the scalp that were mildly painful to palpation and had been increasing in size and number over the last 2 months. Her medical history was remarkable for seizures, keratosis pilaris, and seborrheic dermatitis. The seizures had been well controlled on oxcarbazepine; however, she was switched to lamotrigine 6 months prior to presentation under the care of her neurologist. The patient was not taking other oral medications, and she denied any trauma/insect bites to the affected area or systemic symptoms such as fever, fatigue, weight loss, nausea, swollen lymph nodes, or night sweats. Physical examination revealed 3 well-circumscribed, pink, slightly scaly, indurated nodules on the frontal and vertex scalp (Figure 1). She reported pain on palpation of the lesions. Treatment with ketoconazole shampoo and high-potency topical corticosteroids was ineffective.
Over a period of 2 months after the initial presentation, the patient developed a total of 9 scalp lesions. Testing was performed 4 months after presentation of lesions. Bacterial and fungal cultures of the lesional skin of the scalp were negative. Two biopsies of lesions on the scalp were performed, the first of which showed a nonspecific lymphohistiocytic infiltrate. The second biopsy revealed a dense, nodular, atypical dermal lymphoid infiltrate composed primarily of round regular lymphocytes intermixed with some larger, more irregular lymphocytes and few scattered mitoses (Figure 2).
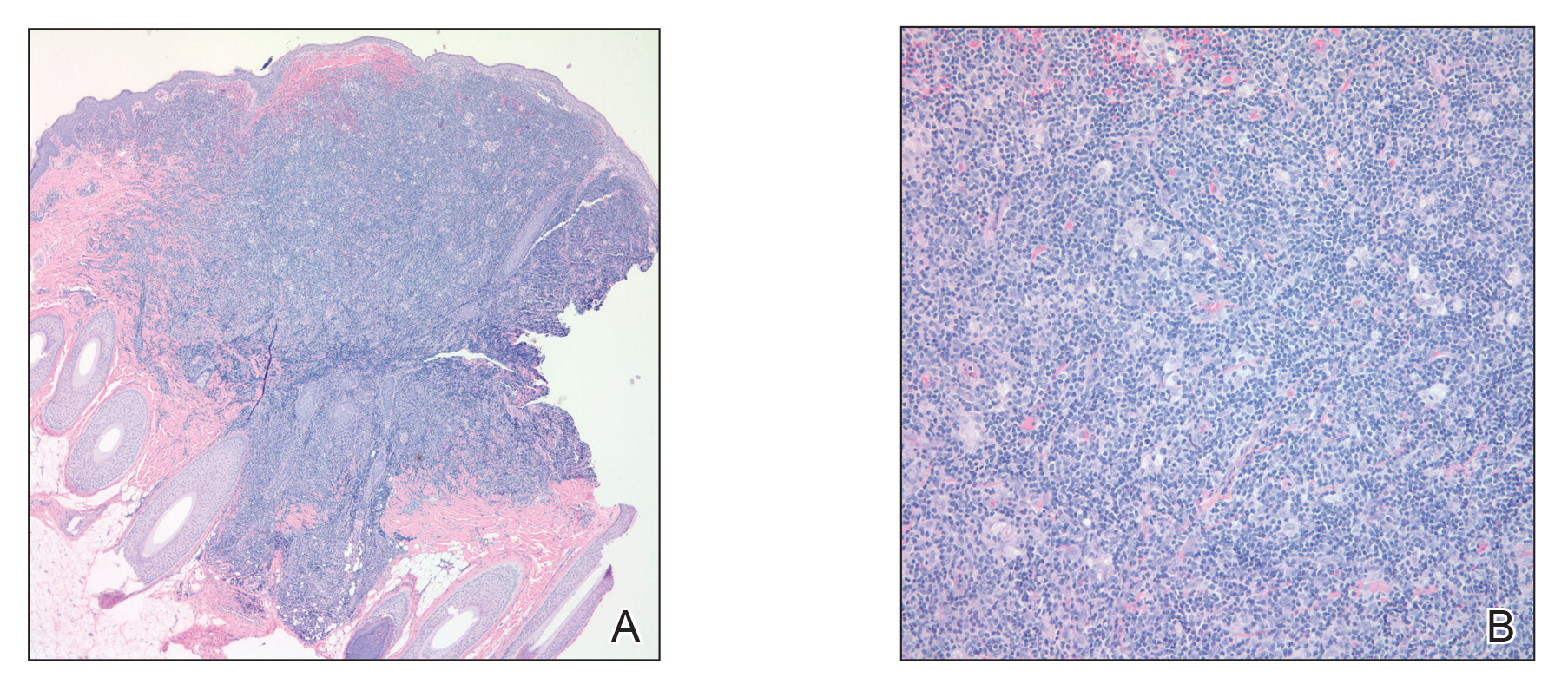
Figure 2. A, A punch biopsy of a lesion on the right lateral scalp showed an atypical dermal lymphoid infiltrate (H&E, original magnification ×4). B, A punch biopsy of a lesion on the right lateral crown of the scalp showed a closer view of the atypical dermal lymphoid infiltrate with a nodular proliferation of round regular lymphocytes intermixed with larger irregular lymphocytes and scattered mitoses (H&E, original magnification ×20).
Immunohistochemical studies revealed small B-cell lymphoma 2–positive lymphocytes with a 2:1 mixture of CD3+ T cells and CD20+CD79a+ B cells. The T cells expressed CD2, CD5, and CD43, and a subset showed a loss of CD7. The CD4:CD8 ratio was 10 to 1. No follicular dendritic networks were noted with CD21 and CD23. Rare, scattered, medium-sized CD30 cells were noted. Staining for CD10, B-cell lymphoma 6, anaplastic lymphoma kinase, Epstein-Barr virus–encoded RNA 1, IgD, and IgM were negative. The plasma cells had a κ/λ free light chain ratio of 2 to 1. Ki-67 was positive in 15% of lymphoid cells. Polymerase chain reaction analysis of T-cell receptor gene rearrangement revealed a peak at 228 bp in a predominantly polyclonal background. A thorough systemic workup including complete blood cell count, immunoglobulin assay, bone marrow transplant panel, comprehensive metabolic panel, lactate dehydrogenase test, inflammatory markers, and viral testing failed to reveal any evidence of underlying malignancy.
After conferring with the patient’s neurologist, lamotrigine was discontinued. Within a few weeks of cessation, the scalp lesions resolved without recurrence at 9-month follow-up. In addition to the lack of clinical, histological, or immunohistochemical evidence of underlying malignancy, the temporal association of the development of lesions after starting lamotrigine and rapid resolution upon its discontinuation suggested a diagnosis of lamotrigine-induced cutaneous pseudolymphoma.
Cutaneous pseudolymphoma is a term used to describe a heterogenous group of benign reactive T-cell, B-cell, or mixed-cell lymphoproliferative processes that resemble cutaneous lymphomas clinically and/or histopathologically.1 Historically, these types of proliferations have been classified under many alternative names that originally served to describe only B-cell–type proliferations. With advances in immunohistochemistry allowing for more specific cell marker identification, cutaneous pseudolymphomas often are found to contain a mixture of T-cell and B-cell populations, which also led to identifying and describing T-cell–type pseudolymphomas.2