User login
Telemedicine and Home Pregnancy Testing for iPLEDGE: A Survey of Clinician Perspectives
To the Editor:
In response to the challenges of the COVID-19 pandemic, iPLEDGE announced that they would accept results from home pregnancy tests and explicitly permit telemedicine.1 Given the financial and logistical burdens associated with iPLEDGE, these changes have the potential to increase access.2 However, it is unclear whether these modifications will be allowed to continue. We sought to evaluate clinician perspectives on the role of telemedicine and home pregnancy testing for iPLEDGE.
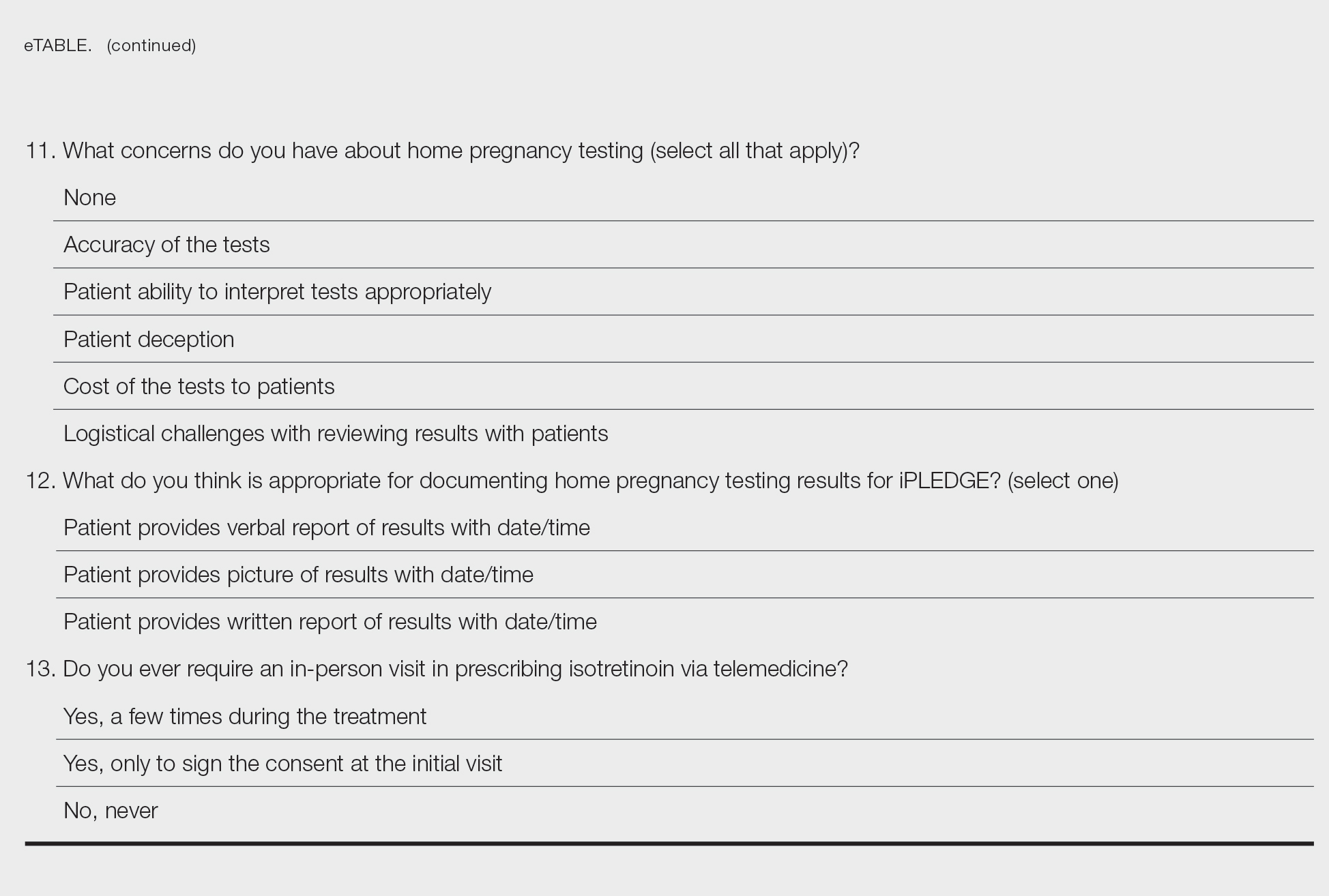
After piloting among several clinicians, a 13-question survey was distributed using the Qualtrics platform to members of the American Acne & Rosacea Society between April 14, 2021, and June 14, 2021. This survey consisted of items addressing provider practices and perspectives on telemedicine and home pregnancy testing for patients taking isotretinoin (eTable). Respondents were asked whether they think telemedicine and home pregnancy testing have improved access to care and whether they would like to continue these practices going forward. In addition, participants were asked about their concerns with home pregnancy testing and how comfortable they feel with home pregnancy testing for various contraceptive strategies (abstinence, condoms, combined oral contraceptives, and long-acting reversible contraception). This study was deemed exempt (category 2) by the University of Pennsylvania (Philadelphia, Pennsylvania) institutional review board (Protocol #844549).
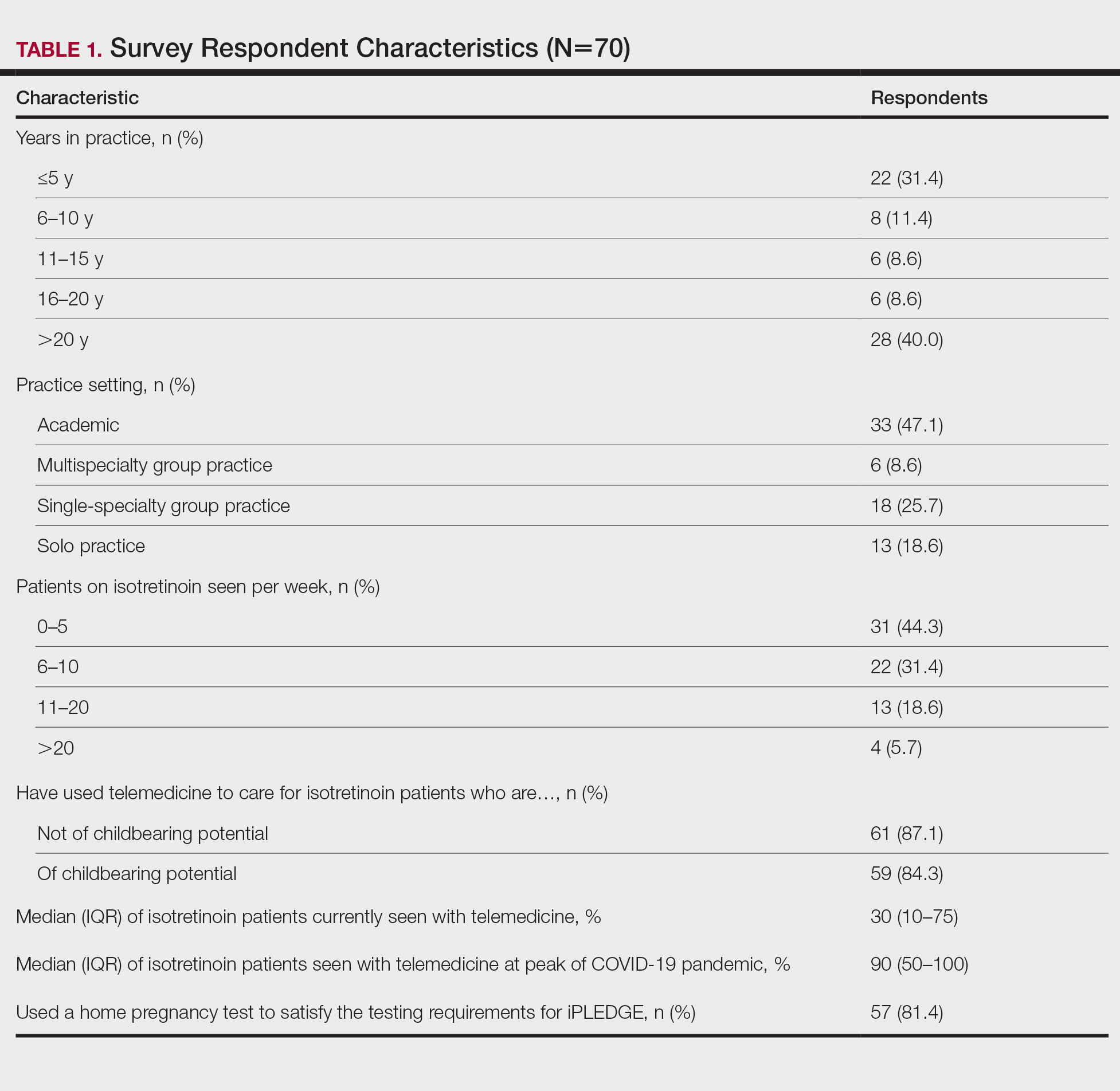
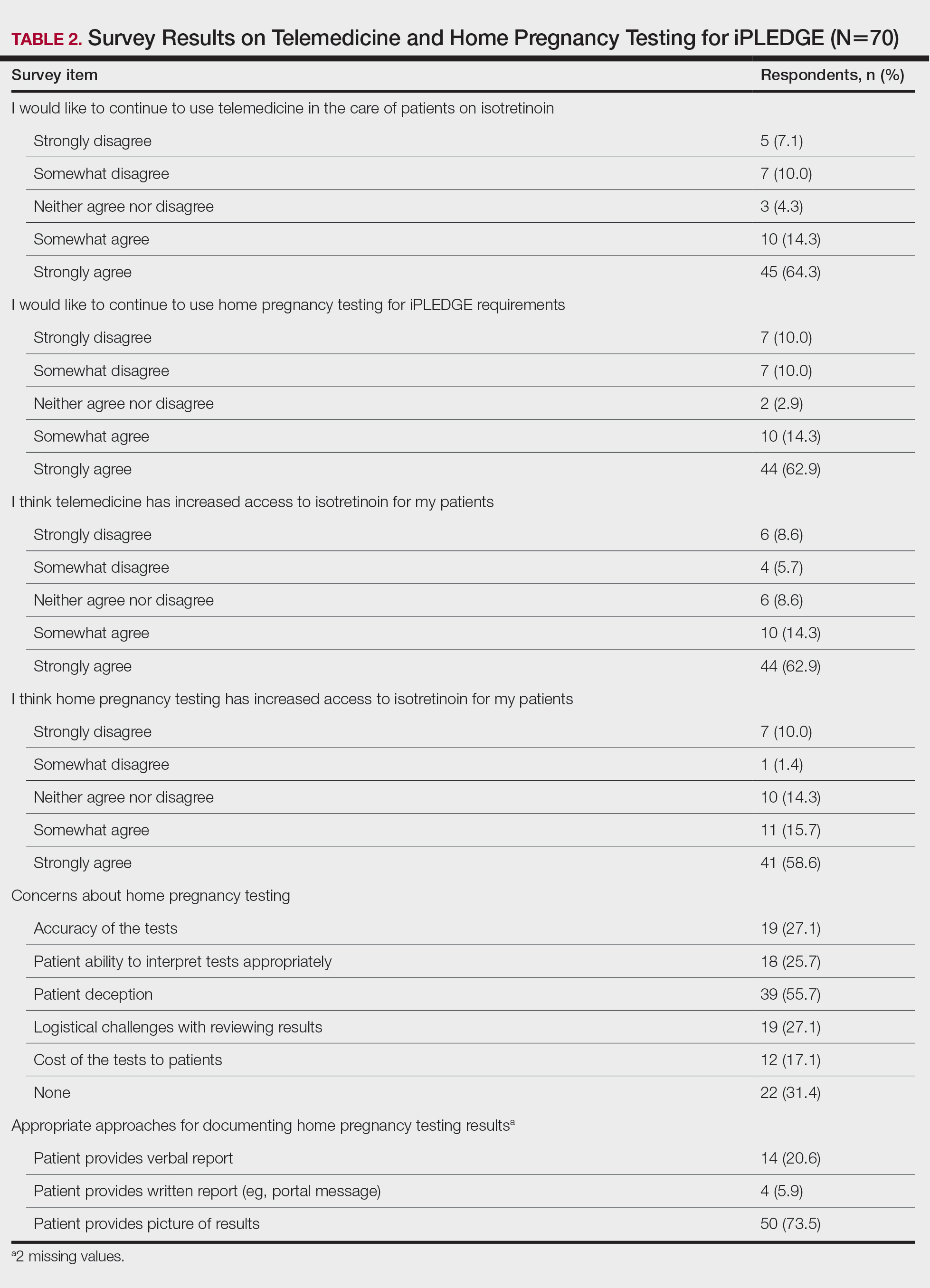
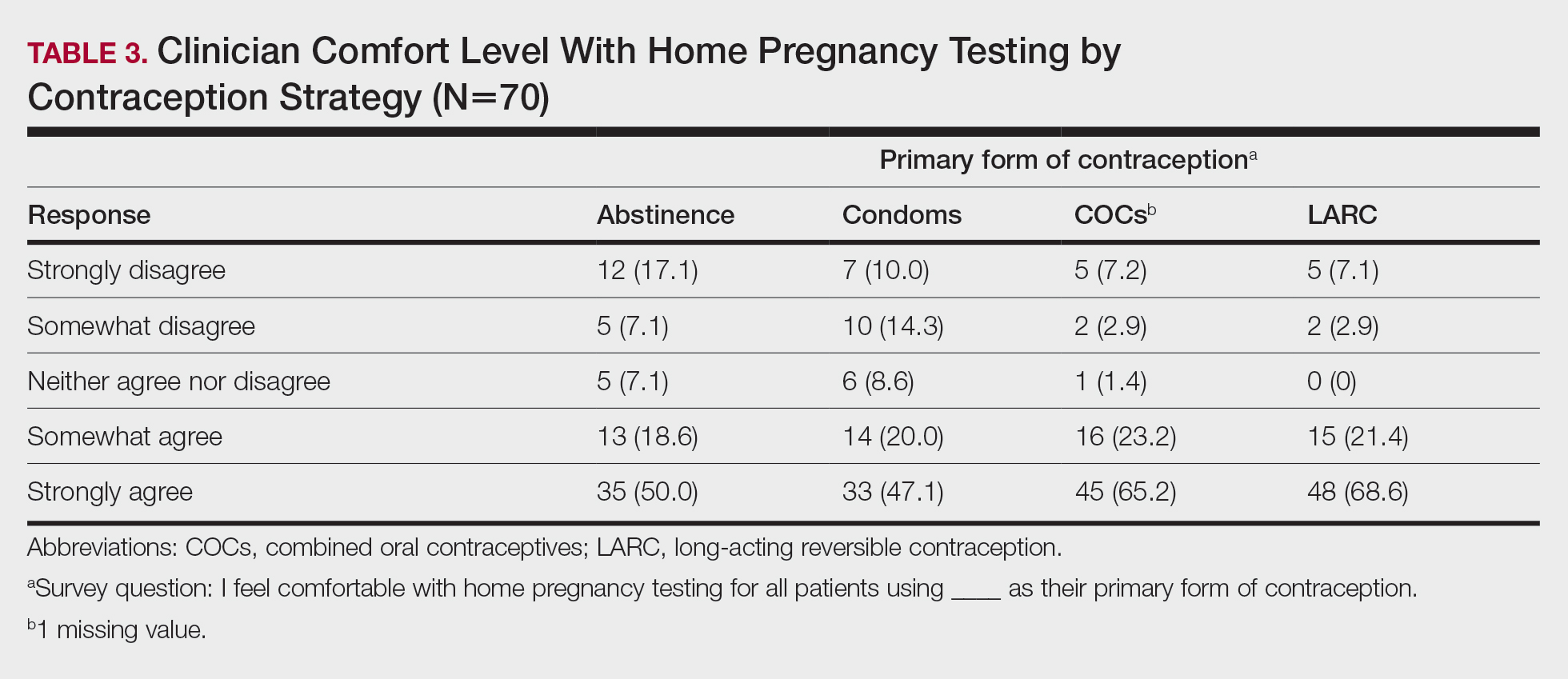
Among 70 clinicians who completed the survey (response rate, 6.4%), 33 (47.1%) practiced in an academic setting. At the peak of the COVID-19 pandemic, clinicians reported using telemedicine for a median of 90% (IQR=50%–100%) of their patients on isotretinoin, and 57 respondents (81.4%) reported having patients use a home pregnancy test for iPLEDGE (Table 1). More than 75% (55/70) agreed that they would like to continue to use telemedicine for patients on isotretinoin, and more than 75% (54/70) agreed that they would like to continue using home pregnancy testing for patients outside the setting of the COVID-19 pandemic. More than 75% (54/70) agreed that telemedicine has increased access for their patients, and more than 70% (52/70) agreed that home pregnancy testing has increased access (Table 2). Clinicians agreed that they would be comfortable using home pregnancy testing for patients choosing long-acting reversible contraception (63/70 [90.0%]), combined oral contraceptives (61/69 [88.4%]), condoms (47/70 [67.1%]), or abstinence (48/70 [68.6%])(Table 3).
The most common concerns about home pregnancy testing were patient deception (39/70 [55.7%]), logistical challenges with reviewing results (19/70 [27.1%]), accuracy of the tests (19/70 [27.1%]), and patient ability to interpret tests appropriately (18/70 [25.7%]). To document testing results, 50 respondents (73.5%) would require a picture of results, 4 (5.9%) would accept a written report from the patient, and 14 (20.6%) would accept a verbal report from the patient (Table 2).
In this survey, clinicians expressed interest in continuing to use telemedicine and home pregnancy testing to care for patients with acne treated with isotretinoin. More than 75% agreed that these changes have increased access, which is notable, as several studies have identified that female and minority patients may face iPLEDGE-associated access barriers.3,4 Continuing to allow home pregnancy testing and explicitly permitting telemedicine can enable clinicians to provide patient-centered care.2
Although clinicians felt comfortable with a variety of contraceptive strategies, particularly those with high reported effectiveness,5 there were concerns about deception and interpretation of test results. Future studies are needed to identify optimal workflows for home pregnancy testing and whether patients should be required to provide a photograph of the results.
This survey study is limited by the possibility of sampling and response bias due to the low response rate. Although the use of national listservs was employed to maximize the generalizability of the results, given the response rate, future studies are needed to evaluate whether these findings generalize to other settings. In addition, given iPLEDGE-associated access barriers, further research is needed to examine how changes such as telemedicine and home pregnancy testing influence both access to isotretinoin and pregnancy prevention.
Acknowledgments—We would like to thank Stacey Moore (Montclair, New Jersey) and the American Acne & Rosacea Society for their help distributing the survey.
- Kane S, Admani S. COVID-19 pandemic leading to the accelerated development of a virtual health model for isotretinoin. J Dermatol Nurses Assoc. 2021;13:54-57.
- Barbieri JS, Frieden IJ, Nagler AR. Isotretinoin, patient safety, and patient-centered care-time to reform iPLEDGE. JAMA Dermatol. 2020;156:21-22.
- Barbieri JS, Shin DB, Wang S, et al. Association of race/ethnicity and sex with differences in health care use and treatment for acne. JAMA Dermatol. 2020;156:312-319.
- Charrow A, Xia FD, Lu J, et al. Differences in isotretinoin start, interruption, and early termination across race and sex in the iPLEDGE era. PloS One. 2019;14:E0210445.
- Barbieri JS, Roe AH, Mostaghimi A. Simplifying contraception requirements for iPLEDGE: a decision analysis. J Am Acad Dermatol. 2020;83:104-108.
To the Editor:
In response to the challenges of the COVID-19 pandemic, iPLEDGE announced that they would accept results from home pregnancy tests and explicitly permit telemedicine.1 Given the financial and logistical burdens associated with iPLEDGE, these changes have the potential to increase access.2 However, it is unclear whether these modifications will be allowed to continue. We sought to evaluate clinician perspectives on the role of telemedicine and home pregnancy testing for iPLEDGE.
After piloting among several clinicians, a 13-question survey was distributed using the Qualtrics platform to members of the American Acne & Rosacea Society between April 14, 2021, and June 14, 2021. This survey consisted of items addressing provider practices and perspectives on telemedicine and home pregnancy testing for patients taking isotretinoin (eTable). Respondents were asked whether they think telemedicine and home pregnancy testing have improved access to care and whether they would like to continue these practices going forward. In addition, participants were asked about their concerns with home pregnancy testing and how comfortable they feel with home pregnancy testing for various contraceptive strategies (abstinence, condoms, combined oral contraceptives, and long-acting reversible contraception). This study was deemed exempt (category 2) by the University of Pennsylvania (Philadelphia, Pennsylvania) institutional review board (Protocol #844549).
Among 70 clinicians who completed the survey (response rate, 6.4%), 33 (47.1%) practiced in an academic setting. At the peak of the COVID-19 pandemic, clinicians reported using telemedicine for a median of 90% (IQR=50%–100%) of their patients on isotretinoin, and 57 respondents (81.4%) reported having patients use a home pregnancy test for iPLEDGE (Table 1). More than 75% (55/70) agreed that they would like to continue to use telemedicine for patients on isotretinoin, and more than 75% (54/70) agreed that they would like to continue using home pregnancy testing for patients outside the setting of the COVID-19 pandemic. More than 75% (54/70) agreed that telemedicine has increased access for their patients, and more than 70% (52/70) agreed that home pregnancy testing has increased access (Table 2). Clinicians agreed that they would be comfortable using home pregnancy testing for patients choosing long-acting reversible contraception (63/70 [90.0%]), combined oral contraceptives (61/69 [88.4%]), condoms (47/70 [67.1%]), or abstinence (48/70 [68.6%])(Table 3).
The most common concerns about home pregnancy testing were patient deception (39/70 [55.7%]), logistical challenges with reviewing results (19/70 [27.1%]), accuracy of the tests (19/70 [27.1%]), and patient ability to interpret tests appropriately (18/70 [25.7%]). To document testing results, 50 respondents (73.5%) would require a picture of results, 4 (5.9%) would accept a written report from the patient, and 14 (20.6%) would accept a verbal report from the patient (Table 2).
In this survey, clinicians expressed interest in continuing to use telemedicine and home pregnancy testing to care for patients with acne treated with isotretinoin. More than 75% agreed that these changes have increased access, which is notable, as several studies have identified that female and minority patients may face iPLEDGE-associated access barriers.3,4 Continuing to allow home pregnancy testing and explicitly permitting telemedicine can enable clinicians to provide patient-centered care.2
Although clinicians felt comfortable with a variety of contraceptive strategies, particularly those with high reported effectiveness,5 there were concerns about deception and interpretation of test results. Future studies are needed to identify optimal workflows for home pregnancy testing and whether patients should be required to provide a photograph of the results.
This survey study is limited by the possibility of sampling and response bias due to the low response rate. Although the use of national listservs was employed to maximize the generalizability of the results, given the response rate, future studies are needed to evaluate whether these findings generalize to other settings. In addition, given iPLEDGE-associated access barriers, further research is needed to examine how changes such as telemedicine and home pregnancy testing influence both access to isotretinoin and pregnancy prevention.
Acknowledgments—We would like to thank Stacey Moore (Montclair, New Jersey) and the American Acne & Rosacea Society for their help distributing the survey.
To the Editor:
In response to the challenges of the COVID-19 pandemic, iPLEDGE announced that they would accept results from home pregnancy tests and explicitly permit telemedicine.1 Given the financial and logistical burdens associated with iPLEDGE, these changes have the potential to increase access.2 However, it is unclear whether these modifications will be allowed to continue. We sought to evaluate clinician perspectives on the role of telemedicine and home pregnancy testing for iPLEDGE.
After piloting among several clinicians, a 13-question survey was distributed using the Qualtrics platform to members of the American Acne & Rosacea Society between April 14, 2021, and June 14, 2021. This survey consisted of items addressing provider practices and perspectives on telemedicine and home pregnancy testing for patients taking isotretinoin (eTable). Respondents were asked whether they think telemedicine and home pregnancy testing have improved access to care and whether they would like to continue these practices going forward. In addition, participants were asked about their concerns with home pregnancy testing and how comfortable they feel with home pregnancy testing for various contraceptive strategies (abstinence, condoms, combined oral contraceptives, and long-acting reversible contraception). This study was deemed exempt (category 2) by the University of Pennsylvania (Philadelphia, Pennsylvania) institutional review board (Protocol #844549).
Among 70 clinicians who completed the survey (response rate, 6.4%), 33 (47.1%) practiced in an academic setting. At the peak of the COVID-19 pandemic, clinicians reported using telemedicine for a median of 90% (IQR=50%–100%) of their patients on isotretinoin, and 57 respondents (81.4%) reported having patients use a home pregnancy test for iPLEDGE (Table 1). More than 75% (55/70) agreed that they would like to continue to use telemedicine for patients on isotretinoin, and more than 75% (54/70) agreed that they would like to continue using home pregnancy testing for patients outside the setting of the COVID-19 pandemic. More than 75% (54/70) agreed that telemedicine has increased access for their patients, and more than 70% (52/70) agreed that home pregnancy testing has increased access (Table 2). Clinicians agreed that they would be comfortable using home pregnancy testing for patients choosing long-acting reversible contraception (63/70 [90.0%]), combined oral contraceptives (61/69 [88.4%]), condoms (47/70 [67.1%]), or abstinence (48/70 [68.6%])(Table 3).
The most common concerns about home pregnancy testing were patient deception (39/70 [55.7%]), logistical challenges with reviewing results (19/70 [27.1%]), accuracy of the tests (19/70 [27.1%]), and patient ability to interpret tests appropriately (18/70 [25.7%]). To document testing results, 50 respondents (73.5%) would require a picture of results, 4 (5.9%) would accept a written report from the patient, and 14 (20.6%) would accept a verbal report from the patient (Table 2).
In this survey, clinicians expressed interest in continuing to use telemedicine and home pregnancy testing to care for patients with acne treated with isotretinoin. More than 75% agreed that these changes have increased access, which is notable, as several studies have identified that female and minority patients may face iPLEDGE-associated access barriers.3,4 Continuing to allow home pregnancy testing and explicitly permitting telemedicine can enable clinicians to provide patient-centered care.2
Although clinicians felt comfortable with a variety of contraceptive strategies, particularly those with high reported effectiveness,5 there were concerns about deception and interpretation of test results. Future studies are needed to identify optimal workflows for home pregnancy testing and whether patients should be required to provide a photograph of the results.
This survey study is limited by the possibility of sampling and response bias due to the low response rate. Although the use of national listservs was employed to maximize the generalizability of the results, given the response rate, future studies are needed to evaluate whether these findings generalize to other settings. In addition, given iPLEDGE-associated access barriers, further research is needed to examine how changes such as telemedicine and home pregnancy testing influence both access to isotretinoin and pregnancy prevention.
Acknowledgments—We would like to thank Stacey Moore (Montclair, New Jersey) and the American Acne & Rosacea Society for their help distributing the survey.
- Kane S, Admani S. COVID-19 pandemic leading to the accelerated development of a virtual health model for isotretinoin. J Dermatol Nurses Assoc. 2021;13:54-57.
- Barbieri JS, Frieden IJ, Nagler AR. Isotretinoin, patient safety, and patient-centered care-time to reform iPLEDGE. JAMA Dermatol. 2020;156:21-22.
- Barbieri JS, Shin DB, Wang S, et al. Association of race/ethnicity and sex with differences in health care use and treatment for acne. JAMA Dermatol. 2020;156:312-319.
- Charrow A, Xia FD, Lu J, et al. Differences in isotretinoin start, interruption, and early termination across race and sex in the iPLEDGE era. PloS One. 2019;14:E0210445.
- Barbieri JS, Roe AH, Mostaghimi A. Simplifying contraception requirements for iPLEDGE: a decision analysis. J Am Acad Dermatol. 2020;83:104-108.
- Kane S, Admani S. COVID-19 pandemic leading to the accelerated development of a virtual health model for isotretinoin. J Dermatol Nurses Assoc. 2021;13:54-57.
- Barbieri JS, Frieden IJ, Nagler AR. Isotretinoin, patient safety, and patient-centered care-time to reform iPLEDGE. JAMA Dermatol. 2020;156:21-22.
- Barbieri JS, Shin DB, Wang S, et al. Association of race/ethnicity and sex with differences in health care use and treatment for acne. JAMA Dermatol. 2020;156:312-319.
- Charrow A, Xia FD, Lu J, et al. Differences in isotretinoin start, interruption, and early termination across race and sex in the iPLEDGE era. PloS One. 2019;14:E0210445.
- Barbieri JS, Roe AH, Mostaghimi A. Simplifying contraception requirements for iPLEDGE: a decision analysis. J Am Acad Dermatol. 2020;83:104-108.
PRACTICE POINTS
- The majority of clinicians report that the use of telemedicine and home pregnancy testing for iPLEDGE has improved access to care and that they would like to continue these practices.
- Continuing to allow home pregnancy testing and explicitly permitting telemedicine can enable clinicians to provide patient-centered care for patients treated with isotretinoin.
Delayed Cutaneous Reactions to Iodinated Contrast
Case Report
A 67-year-old woman with a history of allergic rhinitis presented in the spring with a pruritic eruption of 2 days’ duration that began on the abdomen and spread to the chest, back, and bilateral arms. Six days prior to the onset of the symptoms she underwent computed tomography (CT) of the abdomen and pelvis to evaluate abdominal pain and peripheral eosinophilia. Two iodinated contrast (IC) agents were used: intravenous iohexol and oral diatrizoate meglumine–diatrizoate sodium. The eruption was not preceded by fever, malaise, sore throat, rhinorrhea, cough, arthralgia, headache, diarrhea, or new medication or supplement use. The patient denied any history of drug allergy or cutaneous eruptions. Her current medications, which she had been taking long-term, were aspirin, lisinopril, diltiazem, levothyroxine, esomeprazole, paroxetine, gabapentin, and diphenhydramine.
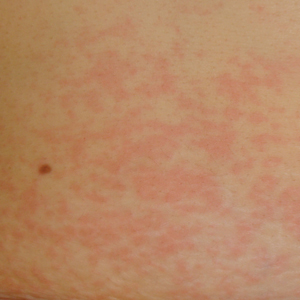
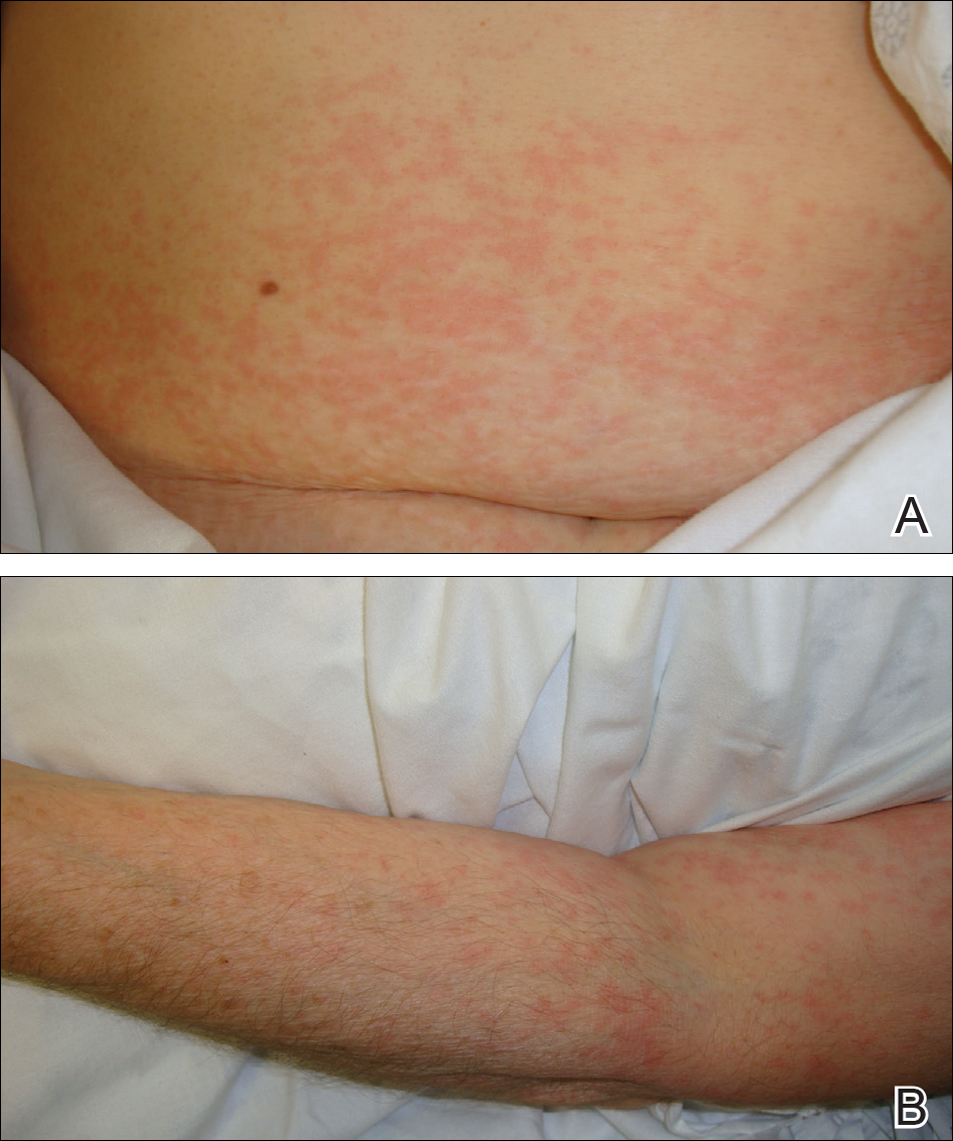
Physical examination was notable for erythematous, blanchable, nontender macules coalescing into patches on the trunk and bilateral arms (Figure). There was slight erythema on the nasolabial folds and ears. The mucosal surfaces and distal legs were clear. The patient was afebrile. Her white blood cell count was 12.5×109/L with 32.3% eosinophils (baseline: white blood cell count, 14.8×109/L; 22% eosinophils)(reference range, 4.8–10.8×109/L; 1%–4% eosinophils). Her comprehensive metabolic panel was within reference range. The human immunodeficiency virus 1/2 antibody immunoassay was nonreactive.
The patient was diagnosed with an exanthematous eruption caused by IC and was treated with oral hydroxyzine and triamcinolone acetonide cream 0.1%. The eruption resolved within 2 weeks without recurrence at 3-month follow-up.
Comment
Del
Clinical Presentation of Delayed Reactions
Most delayed cutaneous reactions to IC present as exanthematous eruptions in the week following a contrast-enhanced CT scan or coronary angiogram.2,12 The reactions tend to resolve within 2 weeks of onset, and the treatment is largely supportive with antihistamines and/or corticosteroids for the associated pruritus.2,5,6 In a study of 98 patients with a history of delayed reactions to IC, delayed-onset urticaria and angioedema also have been reported with incidence rates of 19% and 24%, respectively.2 Other reactions are less common. In the same study, 7% of patients developed palpable purpura; acute generalized exanthematous pustulosis; bullous, flexural, or psoriasislike exanthema; exfoliative eruptions; or purpura and a maculopapular eruption combined with eosinophilia.2 There also have been reports of IC-induced erythema multiforme,3 fixed drug eruptions,10,11 symmetrical drug-related intertriginous and flexural exanthema,13 cutaneous vasculitis,14 drug reactions with eosinophilia and systemic symptoms,15 Stevens-Johnson syndrome/TEN,7,8,16,17 and iododerma.18
IC Agents
Virtually all delayed cutaneous reactions to IC reportedly are due to intravascular rather than oral agents,1,2,19 with the exception of iododerma18 and 1 reported case of TEN.17 Intravenous iohexol was most likely the offending drug in our case. In a prospective cohort study of 539 patients undergoing CT scans, the absolute risk for developing a delayed cutaneous reaction (defined as rash, itching, or skin redness or swelling) to intravascular iohexol was 9.4%.20 Randomized, double-blind studies have found that the risk for delayed cutaneous eruptions is similar among various types of IC, except for iodixanol, which confers a higher risk.5,6,21
Risk Factors
Interestingly, analyses have shown that delayed reactions to IC are more common in atopic patients and during high pollen season.22 Our patient displayed these risk factors, as she had allergic rhinitis and presented for evaluation in late spring when local pollen counts were high. Additionally, patients who develop delayed reactions to IC are notably more likely than controls to have a history of other cutaneous drug reactions, serum creatinine levels greater than 2.0 mg/dL (reference range, 0.6–1.2 mg/dL),3 or history of treatment with recombinant interleukin 2.19
Patients with a history of delayed reactions to IC are not at increased risk for immediate reactions and vice versa.2,3 This finding is consistent with the evidence that delayed and immediate reactions to IC are mechanistically unrelated.23 Additionally, seafood allergy is not a risk factor for either immediate or delayed reactions to IC, despite a common misconception among physicians and patients because seafood is iodinated.24,25
Reexposure to IC
Patients who have had delayed cutaneous reactions to IC are at risk for similar eruptions upon reexposure. Although the reactions are believed to be cell mediated, skin testing with IC is not sensitive enough to reliably identify tolerable alternatives.12 Consequently, gadolinium-based agents have been recommended in patients with a history of reactions to IC if additional contrast-enhanced studies are needed.13,26 Iodinated and gadolinium-based contrast agents do not cross-react, and gadolinium is compatible with studies other than magnetic resonance imaging.1,27
Premedication
Despite the absence of cross-reactivity, the American College of Radiology considers patients with hypersensitivity reactions to IC to be at increased risk for reactions to gadolinium but does not make specific recommendations regarding premedication given the dearth of data.1 As a result, premedication may be considered prior to gadolinium administration depending on the severity of the delayed reaction to IC. Additionally, premedication may be beneficial in cases in which gadolinium is contraindicated and IC must be reused. In a retrospective study, all patients with suspected delayed reactions to IC tolerated IC or gadolinium contrast when pretreated with corticosteroids with or without antihistamines.28 Regimens with corticosteroids and either cyclosporine or intravenous immunoglobulin also have prevented the recurrence of IC-induced exanthematous eruptions and Stevens-Johnson syndrome.29,30 Despite these reports, delayed cutaneous reactions to IC have recurred in other patients receiving corticosteroids, antihistamines, and/or cyclosporine for premedication or concurrent treatment of an underlying condition.16,29-31
Conclusion
It is important for dermatologists to recognize IC as a cause of delayed drug reactions. Current awareness is limited, and as a result, patients often are reexposed to the offending contrast agents unsuspectingly. In addition to diagnosing these eruptions, dermatologists may help prevent their recurrence if future contrast-enhanced studies are required by recommending gadolinium-based agents and/or premedication.
- Cohan RH, Davenport MS, Dillman JR, et al; ACR Committee on Drugs and Contrast Media. ACR Manual on Contrast Media. 9th ed. Reston, VA: American College of Radiology; 2013.
- Brockow K, Romano A, Aberer W, et al; European Network of Drug Allergy and the EAACI Interest Group on Drug Hypersensitivity. Skin testing in patients with hypersensitivity reactions to iodinated contrast media—a European multicenter study. Allergy. 2009;64:234-241.
Hosoya T, Yamaguchi K, Akutsu T, et al. Delayed adverse reactions to iodinated contrast media and their risk factors. Radiat Med. 2000;18:39-45. - Rydberg J, Charles J, Aspelin P. Frequency of late allergy-like adverse reactions following injection of intravascular non-ionic contrast media: a retrospective study comparing a non-ionic monomeric contrast medium with a non-ionic dimeric contrast medium. Acta Radiol. 1998;39:219-222.
- Sutton AG, Finn P, Grech ED, et al. Early and late reactions after the use of iopamidol 340, ioxaglate 320, and iodixanol 320 in cardiac catheterization. Am Heart J. 2001;141:677-683.
- Sutton AG, Finn P, Campbell PG, et al. Early and late reactions following the use of iopamidol 340, iomeprol 350 and iodixanol 320 in cardiac catheterization. J Invasive Cardiol. 2003;15:133-138.
- Brown M, Yowler C, Brandt C. Recurrent toxic epidermal necrolysis secondary to iopromide contrast. J Burn Care Res. 2013;34:E53-E56.
- Rosado A, Canto G, Veleiro B, et al. Toxic epidermal necrolysis after repeated injections of iohexol. AJR Am J Roentgenol. 2001;176:262-263.
- Peterson A, Katzberg RW, Fung MA, et al. Acute generalized exanthematous pustulosis as a delayed dermatotoxic reaction to IV-administered nonionic contrast media. AJR Am J Roentgenol. 2006;187:W198-W201.
- Good AE, Novak E, Sonda LP III. Fixed eruption and fever after urography. South Med J. 1980;73:948-949.
- Benson PM, Giblin WJ, Douglas DM. Transient, nonpigmenting fixed drug eruption caused by radiopaque contrast media. J Am Acad Dermatol. 1990;23(2, pt 2):379-381.
- Torres MJ, Gomez F, Doña I, et al. Diagnostic evaluation of patients with nonimmediate cutaneous hypersensitivity reactions to iodinated contrast media. Allergy. 2012;67:929-935.
- Scherer K, Harr T, Bach S, et al. The role of iodine in hypersensitivity reactions to radio contrast media. Clin Exp Allergy. 2010;40:468-475.
- Reynolds NJ, Wallington TB, Burton JL. Hydralazine predisposes to acute cutaneous vasculitis following urography with iopamidol. Br J Dermatol. 1993;129:82-85.
- Belhadjali H, Bouzgarrou L, Youssef M, et al. DRESS syndrome induced by sodium meglumine ioxitalamate. Allergy. 2008;63:786-787.
- Baldwin BT, Lien MH, Khan H, et al. Case of fatal toxic epidermal necrolysis due to cardiac catheterization dye. J Drugs Dermatol. 2010;9:837-840.
- Schmidt BJ, Foley WD, Bohorfoush AG. Toxic epidermal necrolysis related to oral administration of diluted diatrizoate meglumine and diatrizoate sodium. AJR Am J Roentgenol. 1998;171:1215-1216.
- Young AL, Grossman ME. Acute iododerma secondary to iodinated contrast media. Br J Dermatol. 2014;170:1377-1379.
- Choyke PL, Miller DL, Lotze MT, et al. Delayed reactions to contrast media after interleukin-2 immunotherapy. Radiology. 1992;183:111-114.
- Loh S, Bagheri S, Katzberg RW, et al. Delayed adverse reaction to contrast-enhanced CT: a prospective single-center study comparison to control group without enhancement. Radiology. 2010;255:764-771.
- Bertrand P, Delhommais A, Alison D, et al. Immediate and delayed tolerance of iohexol and ioxaglate in lower limb phlebography: a double-blind comparative study in humans. Acad Radiol. 1995;2:683-686.
- Munechika H, Hiramatsu Y, Kudo S, et al. A prospective survey of delayed adverse reactions to iohexol in urography and computed tomography. Eur Radiol. 2003;13:185-194.
- Guéant-Rodriguez RM, Romano A, Barbaud A, et al. Hypersensitivity reactions to iodinated contrast media. Curr Pharm Des. 2006;12:3359-3372.
- H
uang SW. Seafood and iodine: an analysis of a medical myth. Allergy Asthma Proc. 2005;26:468-469. - B
aig M, Farag A, Sajid J, et al. Shellfish allergy and relation to iodinated contrast media: United Kingdom survey. World J Cardiol. 2014;6:107-111. - B
öhm I, Schild HH. A practical guide to diagnose lesser-known immediate and delayed contrast media-induced adverse cutaneous reactions. Eur Radiol. 2006;16:1570-1579. - Ose K, Doue T, Zen K, et al. “Gadolinium” as an alternative to iodinated contrast media for X-ray angiography in patients with severe allergy. Circ J. 2005;69:507-509.
- Ji
ngu A, Fukuda J, Taketomi-Takahashi A, et al. Breakthrough reactions of iodinated and gadolinium contrast media after oral steroid premedication protocol. BMC Med Imaging. 2014;14:34. - Ro
mano A, Artesani MC, Andriolo M, et al. Effective prophylactic protocol in delayed hypersensitivity to contrast media: report of a case involving lymphocyte transformation studies with different compounds. Radiology. 2002;225:466-470. - He
bert AA, Bogle MA. Intravenous immunoglobulin prophylaxis for recurrent Stevens-Johnson syndrome. J Am Acad Dermatol. 2004;50:286-288. - Ha
sdenteufel F, Waton J, Cordebar V, et al. Delayed hypersensitivity reactions caused by iodixanol: an assessment of cross-reactivity in 22 patients. J Allergy Clin Immunol. 2011;128:1356-1357.
Case Report
A 67-year-old woman with a history of allergic rhinitis presented in the spring with a pruritic eruption of 2 days’ duration that began on the abdomen and spread to the chest, back, and bilateral arms. Six days prior to the onset of the symptoms she underwent computed tomography (CT) of the abdomen and pelvis to evaluate abdominal pain and peripheral eosinophilia. Two iodinated contrast (IC) agents were used: intravenous iohexol and oral diatrizoate meglumine–diatrizoate sodium. The eruption was not preceded by fever, malaise, sore throat, rhinorrhea, cough, arthralgia, headache, diarrhea, or new medication or supplement use. The patient denied any history of drug allergy or cutaneous eruptions. Her current medications, which she had been taking long-term, were aspirin, lisinopril, diltiazem, levothyroxine, esomeprazole, paroxetine, gabapentin, and diphenhydramine.
Physical examination was notable for erythematous, blanchable, nontender macules coalescing into patches on the trunk and bilateral arms (Figure). There was slight erythema on the nasolabial folds and ears. The mucosal surfaces and distal legs were clear. The patient was afebrile. Her white blood cell count was 12.5×109/L with 32.3% eosinophils (baseline: white blood cell count, 14.8×109/L; 22% eosinophils)(reference range, 4.8–10.8×109/L; 1%–4% eosinophils). Her comprehensive metabolic panel was within reference range. The human immunodeficiency virus 1/2 antibody immunoassay was nonreactive.

The patient was diagnosed with an exanthematous eruption caused by IC and was treated with oral hydroxyzine and triamcinolone acetonide cream 0.1%. The eruption resolved within 2 weeks without recurrence at 3-month follow-up.
Comment
Del
Clinical Presentation of Delayed Reactions
Most delayed cutaneous reactions to IC present as exanthematous eruptions in the week following a contrast-enhanced CT scan or coronary angiogram.2,12 The reactions tend to resolve within 2 weeks of onset, and the treatment is largely supportive with antihistamines and/or corticosteroids for the associated pruritus.2,5,6 In a study of 98 patients with a history of delayed reactions to IC, delayed-onset urticaria and angioedema also have been reported with incidence rates of 19% and 24%, respectively.2 Other reactions are less common. In the same study, 7% of patients developed palpable purpura; acute generalized exanthematous pustulosis; bullous, flexural, or psoriasislike exanthema; exfoliative eruptions; or purpura and a maculopapular eruption combined with eosinophilia.2 There also have been reports of IC-induced erythema multiforme,3 fixed drug eruptions,10,11 symmetrical drug-related intertriginous and flexural exanthema,13 cutaneous vasculitis,14 drug reactions with eosinophilia and systemic symptoms,15 Stevens-Johnson syndrome/TEN,7,8,16,17 and iododerma.18
IC Agents
Virtually all delayed cutaneous reactions to IC reportedly are due to intravascular rather than oral agents,1,2,19 with the exception of iododerma18 and 1 reported case of TEN.17 Intravenous iohexol was most likely the offending drug in our case. In a prospective cohort study of 539 patients undergoing CT scans, the absolute risk for developing a delayed cutaneous reaction (defined as rash, itching, or skin redness or swelling) to intravascular iohexol was 9.4%.20 Randomized, double-blind studies have found that the risk for delayed cutaneous eruptions is similar among various types of IC, except for iodixanol, which confers a higher risk.5,6,21
Risk Factors
Interestingly, analyses have shown that delayed reactions to IC are more common in atopic patients and during high pollen season.22 Our patient displayed these risk factors, as she had allergic rhinitis and presented for evaluation in late spring when local pollen counts were high. Additionally, patients who develop delayed reactions to IC are notably more likely than controls to have a history of other cutaneous drug reactions, serum creatinine levels greater than 2.0 mg/dL (reference range, 0.6–1.2 mg/dL),3 or history of treatment with recombinant interleukin 2.19
Patients with a history of delayed reactions to IC are not at increased risk for immediate reactions and vice versa.2,3 This finding is consistent with the evidence that delayed and immediate reactions to IC are mechanistically unrelated.23 Additionally, seafood allergy is not a risk factor for either immediate or delayed reactions to IC, despite a common misconception among physicians and patients because seafood is iodinated.24,25
Reexposure to IC
Patients who have had delayed cutaneous reactions to IC are at risk for similar eruptions upon reexposure. Although the reactions are believed to be cell mediated, skin testing with IC is not sensitive enough to reliably identify tolerable alternatives.12 Consequently, gadolinium-based agents have been recommended in patients with a history of reactions to IC if additional contrast-enhanced studies are needed.13,26 Iodinated and gadolinium-based contrast agents do not cross-react, and gadolinium is compatible with studies other than magnetic resonance imaging.1,27
Premedication
Despite the absence of cross-reactivity, the American College of Radiology considers patients with hypersensitivity reactions to IC to be at increased risk for reactions to gadolinium but does not make specific recommendations regarding premedication given the dearth of data.1 As a result, premedication may be considered prior to gadolinium administration depending on the severity of the delayed reaction to IC. Additionally, premedication may be beneficial in cases in which gadolinium is contraindicated and IC must be reused. In a retrospective study, all patients with suspected delayed reactions to IC tolerated IC or gadolinium contrast when pretreated with corticosteroids with or without antihistamines.28 Regimens with corticosteroids and either cyclosporine or intravenous immunoglobulin also have prevented the recurrence of IC-induced exanthematous eruptions and Stevens-Johnson syndrome.29,30 Despite these reports, delayed cutaneous reactions to IC have recurred in other patients receiving corticosteroids, antihistamines, and/or cyclosporine for premedication or concurrent treatment of an underlying condition.16,29-31
Conclusion
It is important for dermatologists to recognize IC as a cause of delayed drug reactions. Current awareness is limited, and as a result, patients often are reexposed to the offending contrast agents unsuspectingly. In addition to diagnosing these eruptions, dermatologists may help prevent their recurrence if future contrast-enhanced studies are required by recommending gadolinium-based agents and/or premedication.
Case Report
A 67-year-old woman with a history of allergic rhinitis presented in the spring with a pruritic eruption of 2 days’ duration that began on the abdomen and spread to the chest, back, and bilateral arms. Six days prior to the onset of the symptoms she underwent computed tomography (CT) of the abdomen and pelvis to evaluate abdominal pain and peripheral eosinophilia. Two iodinated contrast (IC) agents were used: intravenous iohexol and oral diatrizoate meglumine–diatrizoate sodium. The eruption was not preceded by fever, malaise, sore throat, rhinorrhea, cough, arthralgia, headache, diarrhea, or new medication or supplement use. The patient denied any history of drug allergy or cutaneous eruptions. Her current medications, which she had been taking long-term, were aspirin, lisinopril, diltiazem, levothyroxine, esomeprazole, paroxetine, gabapentin, and diphenhydramine.
Physical examination was notable for erythematous, blanchable, nontender macules coalescing into patches on the trunk and bilateral arms (Figure). There was slight erythema on the nasolabial folds and ears. The mucosal surfaces and distal legs were clear. The patient was afebrile. Her white blood cell count was 12.5×109/L with 32.3% eosinophils (baseline: white blood cell count, 14.8×109/L; 22% eosinophils)(reference range, 4.8–10.8×109/L; 1%–4% eosinophils). Her comprehensive metabolic panel was within reference range. The human immunodeficiency virus 1/2 antibody immunoassay was nonreactive.

The patient was diagnosed with an exanthematous eruption caused by IC and was treated with oral hydroxyzine and triamcinolone acetonide cream 0.1%. The eruption resolved within 2 weeks without recurrence at 3-month follow-up.
Comment
Del
Clinical Presentation of Delayed Reactions
Most delayed cutaneous reactions to IC present as exanthematous eruptions in the week following a contrast-enhanced CT scan or coronary angiogram.2,12 The reactions tend to resolve within 2 weeks of onset, and the treatment is largely supportive with antihistamines and/or corticosteroids for the associated pruritus.2,5,6 In a study of 98 patients with a history of delayed reactions to IC, delayed-onset urticaria and angioedema also have been reported with incidence rates of 19% and 24%, respectively.2 Other reactions are less common. In the same study, 7% of patients developed palpable purpura; acute generalized exanthematous pustulosis; bullous, flexural, or psoriasislike exanthema; exfoliative eruptions; or purpura and a maculopapular eruption combined with eosinophilia.2 There also have been reports of IC-induced erythema multiforme,3 fixed drug eruptions,10,11 symmetrical drug-related intertriginous and flexural exanthema,13 cutaneous vasculitis,14 drug reactions with eosinophilia and systemic symptoms,15 Stevens-Johnson syndrome/TEN,7,8,16,17 and iododerma.18
IC Agents
Virtually all delayed cutaneous reactions to IC reportedly are due to intravascular rather than oral agents,1,2,19 with the exception of iododerma18 and 1 reported case of TEN.17 Intravenous iohexol was most likely the offending drug in our case. In a prospective cohort study of 539 patients undergoing CT scans, the absolute risk for developing a delayed cutaneous reaction (defined as rash, itching, or skin redness or swelling) to intravascular iohexol was 9.4%.20 Randomized, double-blind studies have found that the risk for delayed cutaneous eruptions is similar among various types of IC, except for iodixanol, which confers a higher risk.5,6,21
Risk Factors
Interestingly, analyses have shown that delayed reactions to IC are more common in atopic patients and during high pollen season.22 Our patient displayed these risk factors, as she had allergic rhinitis and presented for evaluation in late spring when local pollen counts were high. Additionally, patients who develop delayed reactions to IC are notably more likely than controls to have a history of other cutaneous drug reactions, serum creatinine levels greater than 2.0 mg/dL (reference range, 0.6–1.2 mg/dL),3 or history of treatment with recombinant interleukin 2.19
Patients with a history of delayed reactions to IC are not at increased risk for immediate reactions and vice versa.2,3 This finding is consistent with the evidence that delayed and immediate reactions to IC are mechanistically unrelated.23 Additionally, seafood allergy is not a risk factor for either immediate or delayed reactions to IC, despite a common misconception among physicians and patients because seafood is iodinated.24,25
Reexposure to IC
Patients who have had delayed cutaneous reactions to IC are at risk for similar eruptions upon reexposure. Although the reactions are believed to be cell mediated, skin testing with IC is not sensitive enough to reliably identify tolerable alternatives.12 Consequently, gadolinium-based agents have been recommended in patients with a history of reactions to IC if additional contrast-enhanced studies are needed.13,26 Iodinated and gadolinium-based contrast agents do not cross-react, and gadolinium is compatible with studies other than magnetic resonance imaging.1,27
Premedication
Despite the absence of cross-reactivity, the American College of Radiology considers patients with hypersensitivity reactions to IC to be at increased risk for reactions to gadolinium but does not make specific recommendations regarding premedication given the dearth of data.1 As a result, premedication may be considered prior to gadolinium administration depending on the severity of the delayed reaction to IC. Additionally, premedication may be beneficial in cases in which gadolinium is contraindicated and IC must be reused. In a retrospective study, all patients with suspected delayed reactions to IC tolerated IC or gadolinium contrast when pretreated with corticosteroids with or without antihistamines.28 Regimens with corticosteroids and either cyclosporine or intravenous immunoglobulin also have prevented the recurrence of IC-induced exanthematous eruptions and Stevens-Johnson syndrome.29,30 Despite these reports, delayed cutaneous reactions to IC have recurred in other patients receiving corticosteroids, antihistamines, and/or cyclosporine for premedication or concurrent treatment of an underlying condition.16,29-31
Conclusion
It is important for dermatologists to recognize IC as a cause of delayed drug reactions. Current awareness is limited, and as a result, patients often are reexposed to the offending contrast agents unsuspectingly. In addition to diagnosing these eruptions, dermatologists may help prevent their recurrence if future contrast-enhanced studies are required by recommending gadolinium-based agents and/or premedication.
- Cohan RH, Davenport MS, Dillman JR, et al; ACR Committee on Drugs and Contrast Media. ACR Manual on Contrast Media. 9th ed. Reston, VA: American College of Radiology; 2013.
- Brockow K, Romano A, Aberer W, et al; European Network of Drug Allergy and the EAACI Interest Group on Drug Hypersensitivity. Skin testing in patients with hypersensitivity reactions to iodinated contrast media—a European multicenter study. Allergy. 2009;64:234-241.
Hosoya T, Yamaguchi K, Akutsu T, et al. Delayed adverse reactions to iodinated contrast media and their risk factors. Radiat Med. 2000;18:39-45. - Rydberg J, Charles J, Aspelin P. Frequency of late allergy-like adverse reactions following injection of intravascular non-ionic contrast media: a retrospective study comparing a non-ionic monomeric contrast medium with a non-ionic dimeric contrast medium. Acta Radiol. 1998;39:219-222.
- Sutton AG, Finn P, Grech ED, et al. Early and late reactions after the use of iopamidol 340, ioxaglate 320, and iodixanol 320 in cardiac catheterization. Am Heart J. 2001;141:677-683.
- Sutton AG, Finn P, Campbell PG, et al. Early and late reactions following the use of iopamidol 340, iomeprol 350 and iodixanol 320 in cardiac catheterization. J Invasive Cardiol. 2003;15:133-138.
- Brown M, Yowler C, Brandt C. Recurrent toxic epidermal necrolysis secondary to iopromide contrast. J Burn Care Res. 2013;34:E53-E56.
- Rosado A, Canto G, Veleiro B, et al. Toxic epidermal necrolysis after repeated injections of iohexol. AJR Am J Roentgenol. 2001;176:262-263.
- Peterson A, Katzberg RW, Fung MA, et al. Acute generalized exanthematous pustulosis as a delayed dermatotoxic reaction to IV-administered nonionic contrast media. AJR Am J Roentgenol. 2006;187:W198-W201.
- Good AE, Novak E, Sonda LP III. Fixed eruption and fever after urography. South Med J. 1980;73:948-949.
- Benson PM, Giblin WJ, Douglas DM. Transient, nonpigmenting fixed drug eruption caused by radiopaque contrast media. J Am Acad Dermatol. 1990;23(2, pt 2):379-381.
- Torres MJ, Gomez F, Doña I, et al. Diagnostic evaluation of patients with nonimmediate cutaneous hypersensitivity reactions to iodinated contrast media. Allergy. 2012;67:929-935.
- Scherer K, Harr T, Bach S, et al. The role of iodine in hypersensitivity reactions to radio contrast media. Clin Exp Allergy. 2010;40:468-475.
- Reynolds NJ, Wallington TB, Burton JL. Hydralazine predisposes to acute cutaneous vasculitis following urography with iopamidol. Br J Dermatol. 1993;129:82-85.
- Belhadjali H, Bouzgarrou L, Youssef M, et al. DRESS syndrome induced by sodium meglumine ioxitalamate. Allergy. 2008;63:786-787.
- Baldwin BT, Lien MH, Khan H, et al. Case of fatal toxic epidermal necrolysis due to cardiac catheterization dye. J Drugs Dermatol. 2010;9:837-840.
- Schmidt BJ, Foley WD, Bohorfoush AG. Toxic epidermal necrolysis related to oral administration of diluted diatrizoate meglumine and diatrizoate sodium. AJR Am J Roentgenol. 1998;171:1215-1216.
- Young AL, Grossman ME. Acute iododerma secondary to iodinated contrast media. Br J Dermatol. 2014;170:1377-1379.
- Choyke PL, Miller DL, Lotze MT, et al. Delayed reactions to contrast media after interleukin-2 immunotherapy. Radiology. 1992;183:111-114.
- Loh S, Bagheri S, Katzberg RW, et al. Delayed adverse reaction to contrast-enhanced CT: a prospective single-center study comparison to control group without enhancement. Radiology. 2010;255:764-771.
- Bertrand P, Delhommais A, Alison D, et al. Immediate and delayed tolerance of iohexol and ioxaglate in lower limb phlebography: a double-blind comparative study in humans. Acad Radiol. 1995;2:683-686.
- Munechika H, Hiramatsu Y, Kudo S, et al. A prospective survey of delayed adverse reactions to iohexol in urography and computed tomography. Eur Radiol. 2003;13:185-194.
- Guéant-Rodriguez RM, Romano A, Barbaud A, et al. Hypersensitivity reactions to iodinated contrast media. Curr Pharm Des. 2006;12:3359-3372.
- H
uang SW. Seafood and iodine: an analysis of a medical myth. Allergy Asthma Proc. 2005;26:468-469. - B
aig M, Farag A, Sajid J, et al. Shellfish allergy and relation to iodinated contrast media: United Kingdom survey. World J Cardiol. 2014;6:107-111. - B
öhm I, Schild HH. A practical guide to diagnose lesser-known immediate and delayed contrast media-induced adverse cutaneous reactions. Eur Radiol. 2006;16:1570-1579. - Ose K, Doue T, Zen K, et al. “Gadolinium” as an alternative to iodinated contrast media for X-ray angiography in patients with severe allergy. Circ J. 2005;69:507-509.
- Ji
ngu A, Fukuda J, Taketomi-Takahashi A, et al. Breakthrough reactions of iodinated and gadolinium contrast media after oral steroid premedication protocol. BMC Med Imaging. 2014;14:34. - Ro
mano A, Artesani MC, Andriolo M, et al. Effective prophylactic protocol in delayed hypersensitivity to contrast media: report of a case involving lymphocyte transformation studies with different compounds. Radiology. 2002;225:466-470. - He
bert AA, Bogle MA. Intravenous immunoglobulin prophylaxis for recurrent Stevens-Johnson syndrome. J Am Acad Dermatol. 2004;50:286-288. - Ha
sdenteufel F, Waton J, Cordebar V, et al. Delayed hypersensitivity reactions caused by iodixanol: an assessment of cross-reactivity in 22 patients. J Allergy Clin Immunol. 2011;128:1356-1357.
- Cohan RH, Davenport MS, Dillman JR, et al; ACR Committee on Drugs and Contrast Media. ACR Manual on Contrast Media. 9th ed. Reston, VA: American College of Radiology; 2013.
- Brockow K, Romano A, Aberer W, et al; European Network of Drug Allergy and the EAACI Interest Group on Drug Hypersensitivity. Skin testing in patients with hypersensitivity reactions to iodinated contrast media—a European multicenter study. Allergy. 2009;64:234-241.
Hosoya T, Yamaguchi K, Akutsu T, et al. Delayed adverse reactions to iodinated contrast media and their risk factors. Radiat Med. 2000;18:39-45. - Rydberg J, Charles J, Aspelin P. Frequency of late allergy-like adverse reactions following injection of intravascular non-ionic contrast media: a retrospective study comparing a non-ionic monomeric contrast medium with a non-ionic dimeric contrast medium. Acta Radiol. 1998;39:219-222.
- Sutton AG, Finn P, Grech ED, et al. Early and late reactions after the use of iopamidol 340, ioxaglate 320, and iodixanol 320 in cardiac catheterization. Am Heart J. 2001;141:677-683.
- Sutton AG, Finn P, Campbell PG, et al. Early and late reactions following the use of iopamidol 340, iomeprol 350 and iodixanol 320 in cardiac catheterization. J Invasive Cardiol. 2003;15:133-138.
- Brown M, Yowler C, Brandt C. Recurrent toxic epidermal necrolysis secondary to iopromide contrast. J Burn Care Res. 2013;34:E53-E56.
- Rosado A, Canto G, Veleiro B, et al. Toxic epidermal necrolysis after repeated injections of iohexol. AJR Am J Roentgenol. 2001;176:262-263.
- Peterson A, Katzberg RW, Fung MA, et al. Acute generalized exanthematous pustulosis as a delayed dermatotoxic reaction to IV-administered nonionic contrast media. AJR Am J Roentgenol. 2006;187:W198-W201.
- Good AE, Novak E, Sonda LP III. Fixed eruption and fever after urography. South Med J. 1980;73:948-949.
- Benson PM, Giblin WJ, Douglas DM. Transient, nonpigmenting fixed drug eruption caused by radiopaque contrast media. J Am Acad Dermatol. 1990;23(2, pt 2):379-381.
- Torres MJ, Gomez F, Doña I, et al. Diagnostic evaluation of patients with nonimmediate cutaneous hypersensitivity reactions to iodinated contrast media. Allergy. 2012;67:929-935.
- Scherer K, Harr T, Bach S, et al. The role of iodine in hypersensitivity reactions to radio contrast media. Clin Exp Allergy. 2010;40:468-475.
- Reynolds NJ, Wallington TB, Burton JL. Hydralazine predisposes to acute cutaneous vasculitis following urography with iopamidol. Br J Dermatol. 1993;129:82-85.
- Belhadjali H, Bouzgarrou L, Youssef M, et al. DRESS syndrome induced by sodium meglumine ioxitalamate. Allergy. 2008;63:786-787.
- Baldwin BT, Lien MH, Khan H, et al. Case of fatal toxic epidermal necrolysis due to cardiac catheterization dye. J Drugs Dermatol. 2010;9:837-840.
- Schmidt BJ, Foley WD, Bohorfoush AG. Toxic epidermal necrolysis related to oral administration of diluted diatrizoate meglumine and diatrizoate sodium. AJR Am J Roentgenol. 1998;171:1215-1216.
- Young AL, Grossman ME. Acute iododerma secondary to iodinated contrast media. Br J Dermatol. 2014;170:1377-1379.
- Choyke PL, Miller DL, Lotze MT, et al. Delayed reactions to contrast media after interleukin-2 immunotherapy. Radiology. 1992;183:111-114.
- Loh S, Bagheri S, Katzberg RW, et al. Delayed adverse reaction to contrast-enhanced CT: a prospective single-center study comparison to control group without enhancement. Radiology. 2010;255:764-771.
- Bertrand P, Delhommais A, Alison D, et al. Immediate and delayed tolerance of iohexol and ioxaglate in lower limb phlebography: a double-blind comparative study in humans. Acad Radiol. 1995;2:683-686.
- Munechika H, Hiramatsu Y, Kudo S, et al. A prospective survey of delayed adverse reactions to iohexol in urography and computed tomography. Eur Radiol. 2003;13:185-194.
- Guéant-Rodriguez RM, Romano A, Barbaud A, et al. Hypersensitivity reactions to iodinated contrast media. Curr Pharm Des. 2006;12:3359-3372.
- H
uang SW. Seafood and iodine: an analysis of a medical myth. Allergy Asthma Proc. 2005;26:468-469. - B
aig M, Farag A, Sajid J, et al. Shellfish allergy and relation to iodinated contrast media: United Kingdom survey. World J Cardiol. 2014;6:107-111. - B
öhm I, Schild HH. A practical guide to diagnose lesser-known immediate and delayed contrast media-induced adverse cutaneous reactions. Eur Radiol. 2006;16:1570-1579. - Ose K, Doue T, Zen K, et al. “Gadolinium” as an alternative to iodinated contrast media for X-ray angiography in patients with severe allergy. Circ J. 2005;69:507-509.
- Ji
ngu A, Fukuda J, Taketomi-Takahashi A, et al. Breakthrough reactions of iodinated and gadolinium contrast media after oral steroid premedication protocol. BMC Med Imaging. 2014;14:34. - Ro
mano A, Artesani MC, Andriolo M, et al. Effective prophylactic protocol in delayed hypersensitivity to contrast media: report of a case involving lymphocyte transformation studies with different compounds. Radiology. 2002;225:466-470. - He
bert AA, Bogle MA. Intravenous immunoglobulin prophylaxis for recurrent Stevens-Johnson syndrome. J Am Acad Dermatol. 2004;50:286-288. - Ha
sdenteufel F, Waton J, Cordebar V, et al. Delayed hypersensitivity reactions caused by iodixanol: an assessment of cross-reactivity in 22 patients. J Allergy Clin Immunol. 2011;128:1356-1357.
Practice Points
- Delayed cutaneous reactions to iodinated contrast (IC) are common, but patients frequently are misdiagnosed and inadvertently readministered the offending agent.
- The most common IC-induced delayed reactions are self-limited exanthematous eruptions that develop within 1 week of exposure.
- Risk factors for delayed reactions to IC include atopy, contrast exposure during high pollen season, use of the agent iodixanol, a history of other cutaneous drug eruptions, elevated serum creatinine levels, and treatment with recombinant interleukin 2.
- Dermatologists can help prevent recurrent reactions in patients who require repeated exposure to IC by recommending gadolinium-based contrast agents and/or premedication.
Verrucous Nodule on the Upper Lip
The Diagnosis: Disseminated Coccidioidomycosis
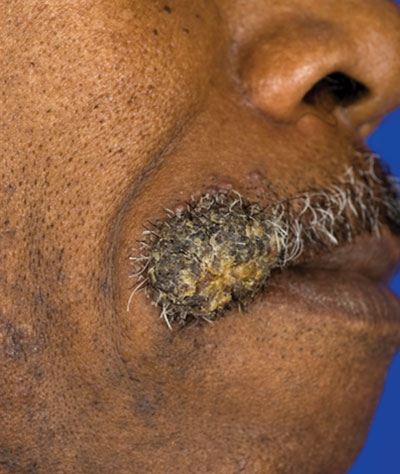
Fungi of the genus Coccidioides cause coccidioidomycosis and live in the soil of endemic areas including the southwestern United States (eg, Arizona, New Mexico, California) and Mexico. Coccidioides is a dimorphic fungus with parasitic and infectious saprophytic phases. Each year there are approximately 150,000 new infections of coccidioidomycosis in the United States, almost exclusively in the southwest.1 Coccidioidomycosis typically is an asymptomatic or mild infection in an immunocompetent patient. Although the lungs are nearly always the primary sites of infection, common sites of dissemination include the skin, meninges, bones, and joints. The skin is the most common site of disseminated, or secondary, coccidioidomycosis.2 Less commonly and usually caused by traumatic implantation, the skin is the site of primary infection.
Disseminated coccidioidomycosis occurs in approximately 1 in 200 infected individuals.2,3 Certain populations of patients are more likely to be affected by disseminated coccidioidomycosis, including specific ethnic groups such as black individuals, Filipinos, and Mexicans4,5; pregnant women6; and immunosuppressed patients such as those with human immunodeficiency virus, hematogenous malignancy, or organ transplantation.7-9 When skin lesions are present, they usually develop after the initial lung manifestation. The location of the lesion in cutaneous disseminated disease can be highly variable, but the face and head are the most common locations (30%).10
Cutaneous manifestations of coccidioidomycosis may be classified as being caused by the presence of the organism in the skin (organism specific) or a reactive process. Organism-specific cutaneous lesions are commonly due to systemic disease with secondary skin involvement, but they also may be due to a primary infection. These organism-specific lesions can present as papules, nodules, macules, verrucous plaques, abscesses, or pustules. Reactive cutaneous manifestations are only associated with disseminated disease; do not contain any organisms; and include manifestations such as erythema nodosum, acute exanthem, erythema multiforme, and possibly Sweet syndrome.11
The clinical differential diagnosis of cutaneous coccidioidomycosis includes other
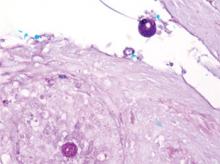
mycoses such as histoplasmosis and blastomycosis, as well as tuberculosis, sarcoidosis, basal cell and squamous cell carcinoma, and verruca vulgaris. The diagnosis of cutaneous coccidioidomycosis can be made with skin biopsy, culture, and serologic tests. The characteristic spherules can be visualized on routine hematoxylin and eosin stain or more readily with fungal stains (Figure). Spherules are thick walled and distinguishable from other fungi because of the characteristic endospores inside as well as their larger size. Organisms also may be detected via culture within 2 to 5 days.
Distinguishing primary cutaneous from disseminated skin lesions can be challenging but can have notable treatment implications. Although histology typically cannot distinguish primary from disseminated cutaneous infections, clinical history and serologic studies have been found to be useful. In disseminated disease, IgG antibodies are elevated, while in primary cutaneous disease, IgM antibodies are elevated but typically not IgG.12 Therefore, tube precipitin and latex particle agglutination tests that detect IgM antibodies should be positive in primary infections.13 Primary lesions can spontaneously resolve within months to years and may not require treatment if symptomatic, while secondary lesions must be therapeutically addressed.12 Despite the lack of treatment needed, primary cutaneous infections often are treated with azoles.14 In contrast, disseminated cutaneous infection requires systemic therapy. Treatment of disseminated infection with cutaneous coccidioidomycosis typically includes amphotericin B until a clinical response is achieved and antibody titers decline. Amphotericin B can then be replaced with an oral azole such as itraconozole or fluconazole.15
The patient discussed in this case demonstrates the typical clinical presentation of disseminated coccidioidomycosis with classical and diagnostic pathology. This case also highlights the importance of a detailed travel history; in the era of globalization, patients often present with diseases in nonendemic areas. Clinicians must consider the diagnosis of coccidioidomycosis, even in immunocompetent patients in nonendemic areas when their history and presentation are appropriate. The diagnosis should be confirmed with biopsy, culture, and/or serology.
1. Galgiani JN, Ampel NM, Blair JE, et al. Coccidioidomycosis [published online ahead of print September 20, 2005]. Clin Infect Dis. 2005;41:1217-1223.
2. Chiller TM, Galgiani JN, Stevens DA. Coccidioidomycosis. Infect Dis Clin North Am. 2003;17:41-57, vii.
3. Rance BR, Elston DM. Disseminated coccidioidomyclosis discovered during routine skin cancer screening. Cutis. 2002;70:70-72.
4. Einstein HE, Johnson RH. Coccidioidomycosis: new aspects of epidemiology and therapy [comment in Clin Infect Dis. 1994;18:470]. Clin Infect Dis. 1993;16:349- 354.
5. Crum NF, Ballon-Landa G. Coccidioidomycosis in pregnancy: case report and review of the literature. Am J Med. 2006;119:e11-e17.
6. Caldwell JW, Arsura EL, Kilgore WB, et al. Coccidioidomycosis in pregnancy during an epidemic in California. Obstet Gynecol. 2000;95:236-239.
7. Singh VR, Smith DK, Lawerence J, et al. Coccidioidomy-cosis in patients infected with human immunodeficiency virus: review of 91 cases at a single institution. Clin Infect Dis. 1996;23:563-568.
8. Blair JE, Logan JL. Coccidioidomycosis in solid organ transplantation [published online ahead of print October 4, 2001]. Clin Infect Dis. 2001;33:1536-1544.
9. Riley DK, Galgiani JN, O’Donnell MR, et al. Coccidioidomycosis in bone marrow transplant recipients. Transplantation. 1993;56:1531-1533.
10. Carpenter JB, Feldman JS, Leyva WH, et al. Clinical and pathologic characteristics of disseminated cutaneous coccidioidomycosis. J Am Acad Dermatol. 2010;62:831-837.
11. DiCaudo DJ. Coccidioidomycosis: a review and update. J Am Acad Dermatol. 2006;55:929-942; quiz 943-945.
12. Chang A, Tung RC, McGillis TS, et al. Primary cutaneous coccidioidomycosis. J Am Acad Dermatol. 2003;49:944-949.
13. Wilson JW, Smith CE, Plunkett OA. Primary cutaneous coccidioidomycosis: the criteria for diagnosis and report of a case. Calif Med. 1953;79:233-239.
14. Blair JE. State-of-the-art treatment of coccidioidomycosis: skin and soft-tissue infections [published online ahead of print March 1, 2007]. Ann N Y Acad Sci. 2007;1111:411-421.
15. Galgiani JN, Ampel NM, Catanzaro A, et al. Practice guidelines for the treatment of coccidioidomycosis. Infectious Diseases Society of America [published online ahead of print April 20, 2000]. Clin Infect Dis. 2000;30:658-661.
The Diagnosis: Disseminated Coccidioidomycosis
Fungi of the genus Coccidioides cause coccidioidomycosis and live in the soil of endemic areas including the southwestern United States (eg, Arizona, New Mexico, California) and Mexico. Coccidioides is a dimorphic fungus with parasitic and infectious saprophytic phases. Each year there are approximately 150,000 new infections of coccidioidomycosis in the United States, almost exclusively in the southwest.1 Coccidioidomycosis typically is an asymptomatic or mild infection in an immunocompetent patient. Although the lungs are nearly always the primary sites of infection, common sites of dissemination include the skin, meninges, bones, and joints. The skin is the most common site of disseminated, or secondary, coccidioidomycosis.2 Less commonly and usually caused by traumatic implantation, the skin is the site of primary infection.
Disseminated coccidioidomycosis occurs in approximately 1 in 200 infected individuals.2,3 Certain populations of patients are more likely to be affected by disseminated coccidioidomycosis, including specific ethnic groups such as black individuals, Filipinos, and Mexicans4,5; pregnant women6; and immunosuppressed patients such as those with human immunodeficiency virus, hematogenous malignancy, or organ transplantation.7-9 When skin lesions are present, they usually develop after the initial lung manifestation. The location of the lesion in cutaneous disseminated disease can be highly variable, but the face and head are the most common locations (30%).10
Cutaneous manifestations of coccidioidomycosis may be classified as being caused by the presence of the organism in the skin (organism specific) or a reactive process. Organism-specific cutaneous lesions are commonly due to systemic disease with secondary skin involvement, but they also may be due to a primary infection. These organism-specific lesions can present as papules, nodules, macules, verrucous plaques, abscesses, or pustules. Reactive cutaneous manifestations are only associated with disseminated disease; do not contain any organisms; and include manifestations such as erythema nodosum, acute exanthem, erythema multiforme, and possibly Sweet syndrome.11
The clinical differential diagnosis of cutaneous coccidioidomycosis includes other
mycoses such as histoplasmosis and blastomycosis, as well as tuberculosis, sarcoidosis, basal cell and squamous cell carcinoma, and verruca vulgaris. The diagnosis of cutaneous coccidioidomycosis can be made with skin biopsy, culture, and serologic tests. The characteristic spherules can be visualized on routine hematoxylin and eosin stain or more readily with fungal stains (Figure). Spherules are thick walled and distinguishable from other fungi because of the characteristic endospores inside as well as their larger size. Organisms also may be detected via culture within 2 to 5 days.
Distinguishing primary cutaneous from disseminated skin lesions can be challenging but can have notable treatment implications. Although histology typically cannot distinguish primary from disseminated cutaneous infections, clinical history and serologic studies have been found to be useful. In disseminated disease, IgG antibodies are elevated, while in primary cutaneous disease, IgM antibodies are elevated but typically not IgG.12 Therefore, tube precipitin and latex particle agglutination tests that detect IgM antibodies should be positive in primary infections.13 Primary lesions can spontaneously resolve within months to years and may not require treatment if symptomatic, while secondary lesions must be therapeutically addressed.12 Despite the lack of treatment needed, primary cutaneous infections often are treated with azoles.14 In contrast, disseminated cutaneous infection requires systemic therapy. Treatment of disseminated infection with cutaneous coccidioidomycosis typically includes amphotericin B until a clinical response is achieved and antibody titers decline. Amphotericin B can then be replaced with an oral azole such as itraconozole or fluconazole.15
The patient discussed in this case demonstrates the typical clinical presentation of disseminated coccidioidomycosis with classical and diagnostic pathology. This case also highlights the importance of a detailed travel history; in the era of globalization, patients often present with diseases in nonendemic areas. Clinicians must consider the diagnosis of coccidioidomycosis, even in immunocompetent patients in nonendemic areas when their history and presentation are appropriate. The diagnosis should be confirmed with biopsy, culture, and/or serology.
The Diagnosis: Disseminated Coccidioidomycosis
Fungi of the genus Coccidioides cause coccidioidomycosis and live in the soil of endemic areas including the southwestern United States (eg, Arizona, New Mexico, California) and Mexico. Coccidioides is a dimorphic fungus with parasitic and infectious saprophytic phases. Each year there are approximately 150,000 new infections of coccidioidomycosis in the United States, almost exclusively in the southwest.1 Coccidioidomycosis typically is an asymptomatic or mild infection in an immunocompetent patient. Although the lungs are nearly always the primary sites of infection, common sites of dissemination include the skin, meninges, bones, and joints. The skin is the most common site of disseminated, or secondary, coccidioidomycosis.2 Less commonly and usually caused by traumatic implantation, the skin is the site of primary infection.
Disseminated coccidioidomycosis occurs in approximately 1 in 200 infected individuals.2,3 Certain populations of patients are more likely to be affected by disseminated coccidioidomycosis, including specific ethnic groups such as black individuals, Filipinos, and Mexicans4,5; pregnant women6; and immunosuppressed patients such as those with human immunodeficiency virus, hematogenous malignancy, or organ transplantation.7-9 When skin lesions are present, they usually develop after the initial lung manifestation. The location of the lesion in cutaneous disseminated disease can be highly variable, but the face and head are the most common locations (30%).10
Cutaneous manifestations of coccidioidomycosis may be classified as being caused by the presence of the organism in the skin (organism specific) or a reactive process. Organism-specific cutaneous lesions are commonly due to systemic disease with secondary skin involvement, but they also may be due to a primary infection. These organism-specific lesions can present as papules, nodules, macules, verrucous plaques, abscesses, or pustules. Reactive cutaneous manifestations are only associated with disseminated disease; do not contain any organisms; and include manifestations such as erythema nodosum, acute exanthem, erythema multiforme, and possibly Sweet syndrome.11
The clinical differential diagnosis of cutaneous coccidioidomycosis includes other
mycoses such as histoplasmosis and blastomycosis, as well as tuberculosis, sarcoidosis, basal cell and squamous cell carcinoma, and verruca vulgaris. The diagnosis of cutaneous coccidioidomycosis can be made with skin biopsy, culture, and serologic tests. The characteristic spherules can be visualized on routine hematoxylin and eosin stain or more readily with fungal stains (Figure). Spherules are thick walled and distinguishable from other fungi because of the characteristic endospores inside as well as their larger size. Organisms also may be detected via culture within 2 to 5 days.
Distinguishing primary cutaneous from disseminated skin lesions can be challenging but can have notable treatment implications. Although histology typically cannot distinguish primary from disseminated cutaneous infections, clinical history and serologic studies have been found to be useful. In disseminated disease, IgG antibodies are elevated, while in primary cutaneous disease, IgM antibodies are elevated but typically not IgG.12 Therefore, tube precipitin and latex particle agglutination tests that detect IgM antibodies should be positive in primary infections.13 Primary lesions can spontaneously resolve within months to years and may not require treatment if symptomatic, while secondary lesions must be therapeutically addressed.12 Despite the lack of treatment needed, primary cutaneous infections often are treated with azoles.14 In contrast, disseminated cutaneous infection requires systemic therapy. Treatment of disseminated infection with cutaneous coccidioidomycosis typically includes amphotericin B until a clinical response is achieved and antibody titers decline. Amphotericin B can then be replaced with an oral azole such as itraconozole or fluconazole.15
The patient discussed in this case demonstrates the typical clinical presentation of disseminated coccidioidomycosis with classical and diagnostic pathology. This case also highlights the importance of a detailed travel history; in the era of globalization, patients often present with diseases in nonendemic areas. Clinicians must consider the diagnosis of coccidioidomycosis, even in immunocompetent patients in nonendemic areas when their history and presentation are appropriate. The diagnosis should be confirmed with biopsy, culture, and/or serology.
1. Galgiani JN, Ampel NM, Blair JE, et al. Coccidioidomycosis [published online ahead of print September 20, 2005]. Clin Infect Dis. 2005;41:1217-1223.
2. Chiller TM, Galgiani JN, Stevens DA. Coccidioidomycosis. Infect Dis Clin North Am. 2003;17:41-57, vii.
3. Rance BR, Elston DM. Disseminated coccidioidomyclosis discovered during routine skin cancer screening. Cutis. 2002;70:70-72.
4. Einstein HE, Johnson RH. Coccidioidomycosis: new aspects of epidemiology and therapy [comment in Clin Infect Dis. 1994;18:470]. Clin Infect Dis. 1993;16:349- 354.
5. Crum NF, Ballon-Landa G. Coccidioidomycosis in pregnancy: case report and review of the literature. Am J Med. 2006;119:e11-e17.
6. Caldwell JW, Arsura EL, Kilgore WB, et al. Coccidioidomycosis in pregnancy during an epidemic in California. Obstet Gynecol. 2000;95:236-239.
7. Singh VR, Smith DK, Lawerence J, et al. Coccidioidomy-cosis in patients infected with human immunodeficiency virus: review of 91 cases at a single institution. Clin Infect Dis. 1996;23:563-568.
8. Blair JE, Logan JL. Coccidioidomycosis in solid organ transplantation [published online ahead of print October 4, 2001]. Clin Infect Dis. 2001;33:1536-1544.
9. Riley DK, Galgiani JN, O’Donnell MR, et al. Coccidioidomycosis in bone marrow transplant recipients. Transplantation. 1993;56:1531-1533.
10. Carpenter JB, Feldman JS, Leyva WH, et al. Clinical and pathologic characteristics of disseminated cutaneous coccidioidomycosis. J Am Acad Dermatol. 2010;62:831-837.
11. DiCaudo DJ. Coccidioidomycosis: a review and update. J Am Acad Dermatol. 2006;55:929-942; quiz 943-945.
12. Chang A, Tung RC, McGillis TS, et al. Primary cutaneous coccidioidomycosis. J Am Acad Dermatol. 2003;49:944-949.
13. Wilson JW, Smith CE, Plunkett OA. Primary cutaneous coccidioidomycosis: the criteria for diagnosis and report of a case. Calif Med. 1953;79:233-239.
14. Blair JE. State-of-the-art treatment of coccidioidomycosis: skin and soft-tissue infections [published online ahead of print March 1, 2007]. Ann N Y Acad Sci. 2007;1111:411-421.
15. Galgiani JN, Ampel NM, Catanzaro A, et al. Practice guidelines for the treatment of coccidioidomycosis. Infectious Diseases Society of America [published online ahead of print April 20, 2000]. Clin Infect Dis. 2000;30:658-661.
1. Galgiani JN, Ampel NM, Blair JE, et al. Coccidioidomycosis [published online ahead of print September 20, 2005]. Clin Infect Dis. 2005;41:1217-1223.
2. Chiller TM, Galgiani JN, Stevens DA. Coccidioidomycosis. Infect Dis Clin North Am. 2003;17:41-57, vii.
3. Rance BR, Elston DM. Disseminated coccidioidomyclosis discovered during routine skin cancer screening. Cutis. 2002;70:70-72.
4. Einstein HE, Johnson RH. Coccidioidomycosis: new aspects of epidemiology and therapy [comment in Clin Infect Dis. 1994;18:470]. Clin Infect Dis. 1993;16:349- 354.
5. Crum NF, Ballon-Landa G. Coccidioidomycosis in pregnancy: case report and review of the literature. Am J Med. 2006;119:e11-e17.
6. Caldwell JW, Arsura EL, Kilgore WB, et al. Coccidioidomycosis in pregnancy during an epidemic in California. Obstet Gynecol. 2000;95:236-239.
7. Singh VR, Smith DK, Lawerence J, et al. Coccidioidomy-cosis in patients infected with human immunodeficiency virus: review of 91 cases at a single institution. Clin Infect Dis. 1996;23:563-568.
8. Blair JE, Logan JL. Coccidioidomycosis in solid organ transplantation [published online ahead of print October 4, 2001]. Clin Infect Dis. 2001;33:1536-1544.
9. Riley DK, Galgiani JN, O’Donnell MR, et al. Coccidioidomycosis in bone marrow transplant recipients. Transplantation. 1993;56:1531-1533.
10. Carpenter JB, Feldman JS, Leyva WH, et al. Clinical and pathologic characteristics of disseminated cutaneous coccidioidomycosis. J Am Acad Dermatol. 2010;62:831-837.
11. DiCaudo DJ. Coccidioidomycosis: a review and update. J Am Acad Dermatol. 2006;55:929-942; quiz 943-945.
12. Chang A, Tung RC, McGillis TS, et al. Primary cutaneous coccidioidomycosis. J Am Acad Dermatol. 2003;49:944-949.
13. Wilson JW, Smith CE, Plunkett OA. Primary cutaneous coccidioidomycosis: the criteria for diagnosis and report of a case. Calif Med. 1953;79:233-239.
14. Blair JE. State-of-the-art treatment of coccidioidomycosis: skin and soft-tissue infections [published online ahead of print March 1, 2007]. Ann N Y Acad Sci. 2007;1111:411-421.
15. Galgiani JN, Ampel NM, Catanzaro A, et al. Practice guidelines for the treatment of coccidioidomycosis. Infectious Diseases Society of America [published online ahead of print April 20, 2000]. Clin Infect Dis. 2000;30:658-661.
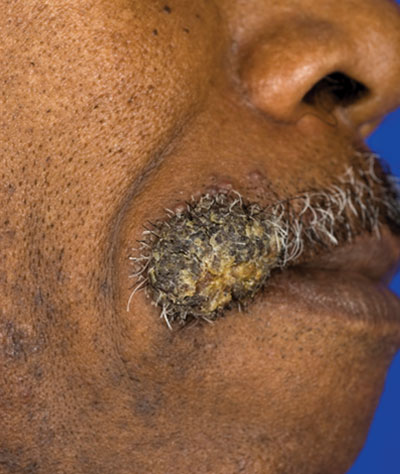
A 62-year-old black man presented to a dermatologist in the northeastern United States with a verrucous nontender nodule on the upper lip after traveling to southern California 1 month prior. The patient was not immunocompromised but reported a recent febrile upper respiratory illness.