User login
Impact of Inpatient GCS on CI Patients
Under the Patient Protection and Affordable Care Act of 2010, commonly referred to as the Affordable Care Act, hospitals face up to a 3% penalty in Medicare reimbursements for patients readmitted within 30 days of initial discharge, and measures have been proposed for modifying payments to hospitals based on their performance on this metric.[1] Cognitive impairment (CI) is considered a major risk factor for poor postdischarge outcomes including mortality and hospital readmission.[2, 3] Hospitals are seeking strategies to reduce postdischarge mortality and rehospitalization among patients with and without CI.[4] Such strategies include use of transitional care coaches, patient and caregiver education, postdischarge follow‐up, and provision of geriatric consultative services (GCS) for the care of complex patients in the hospital setting.[5, 6, 7]
GCS utilize comprehensive geriatric assessments and multidisciplinary processes to recognize and modify risk factors that may lead to poor outcomes among hospitalized patients.[8, 9, 10, 11] Implementation of GCS models including Acute Care for Elders and, recently, the Mobile Acute Care of the Elderly services have shown many benefits among older patients including a reduction in the hospital length of stay and readmission rates.[12, 13] The benefits of such services among hospitalized elders suffering from CI, however, are not well established. The objective of this article was to evaluate the impact of GCS on the readmission and mortality rates of older adults with CI within 12 months of their hospitalization to an urban, public hospital. We hypothesized that GCS will reduce both 12‐month hospital readmissions and mortality rates among this vulnerable group of older adults.
METHODS
The study was approved by the Indiana University institutional review board, and informed consent for identifiable chart review was obtained from subjects or their legally authorized representatives.
Setting
The study was conducted at Eskenazi hospital, Indianapolis, Indiana, a 340‐bed, university‐affiliated, public hospital with over 2300 admissions of patients aged 65 years or older every year.
Population
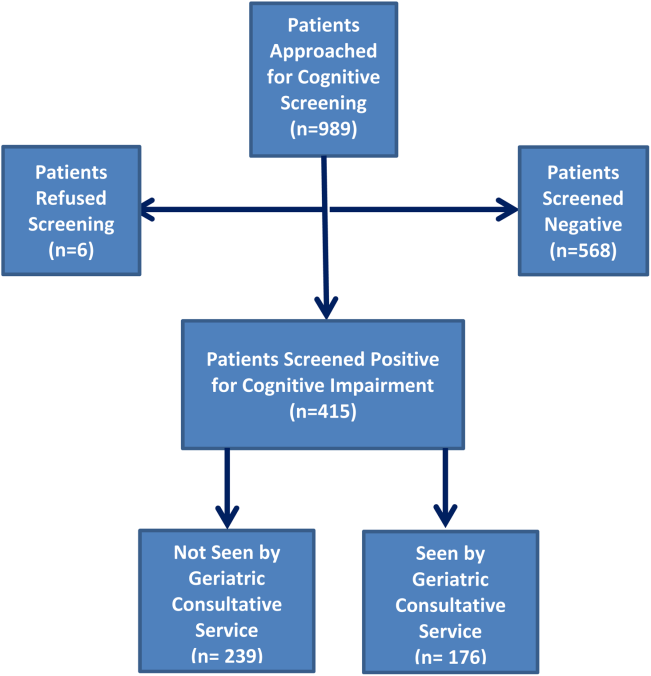
Four hundred fifteen hospitalized patients aged 65 years or older suffering from CI were enrolled into an original, randomized, controlled trial that evaluated the effect of a computerized decision support system on their quality and outcome of care between July 1, 2006 and May 30, 2008.[14] The computerized decision support included reminders for physicians to reduce the prescription of 18 anticholinergics, minimize physical restraints and Foley catheterization, and increase referral to the local GCS.[15] That previous trial neither showed an impact on quality of care nor health utilization among older patients, including mortality and hospital readmission rates. The current study uses the data from the clinical trial cohort to evaluate the effect of GCS on the 12‐month mortality and hospital readmission rates for hospitalized elders with CI (Figure 1).
Inclusion and Exclusion Criteria
Individuals were eligible for enrollment if they were aged 65 years or older, hospitalized on a medical ward, able to speak English, and had evidence of CI within 48 hours of hospital admission. Individuals were excluded if they were previously enrolled, were aphasic, or unresponsive. The presence of CI was based on the Short Portable Mental Status Questionnaire (SPMSQ),[16] a brief 10‐item screening test with a sensitivity of 86% and specificity of 99% for dementia using a score of 7 or less (maximum possible score of 10).[16] The SPMSQ scoring process adjusts for participant educational and racial status, which was a benefit to its use given the urban setting of our hospital serving a large proportion of minority and low‐education patients. A physician‐trained research assistant administered the SPMSQ within 48 hours of hospital admission.
Geriatric Consultative Services
GCS is an interdisciplinary team of a geriatrician, a geriatric pharmacist, a case manager nurse, a social worker, a medical assistant, physical therapists, and a representative of the local Area Agency on Aging. There may be a geriatric fellow and/or medicine resident available to the team based on their rotation structure. Team‐based bedside rounds are performed on new consults only, but all patients are seen individually by the team clinicians. The team emphasizes prevention of functional decline and polypharmacy, recognition and treatment of geriatric syndromes including dementia and delirium, and early discharge/transition planning. Consensus recommendations are prepared and documented in the consult notes section of the electronic medical records. Recommendations deemed critical are discussed directly with the primary teams, but no orders are placed by the GCS team. The GCS team is available on all weekdays but not on weekends or major holidays.
Study Outcomes
For this secondary analysis, we used the Regenstrief Medical Record System (RMRS) to measure 2 outcomes: hospital readmission and mortality rates up to 1 year from discharge following index hospitalization, defined as the first admission in the original clinical trial. The RMRS is the primary instrument for processing data and monitoring patient and physician activity for the hospital.[17, 18] The RMRS is linked with a state‐wide health information exchange to capture data on hospitalization outside the hospital. The RMRS also contains death certificate information for all registered patients who die in or outside the Eskenazi hospital.
Other Data Collections
Delirium was assessed at screening and then every weekday using the Confusion Assessment Method (CAM) by a trained research assistant.[19] CAM evaluates 10 symptoms of delirium specified in the Diagnostic and Statistical Manual of Mental Disorders‐III‐Revision: acute onset, fluctuating course, inattention, disorganized thinking, altered level of consciousness, disorientation, memory impairment, perceptual disturbances, psychomotor agitation or retardation, and sleep/wake disturbance. Participant demographic characteristics, including age, sex, ethnicity, and years of education, were collected from the RMRS and from interviews performed at the time of cognitive screening. Information on length of hospital stay and discharge destination (eg, home vs facility, including skilled nursing and acute rehabilitation facilities) was also obtained from the RMRS. Charlson Comorbidity Index score was calculated using International Classification of Diseases, Ninth Revision codes gathered from 1 year before admission until the time of each participant's discharge from the hospital.[20] The Acute Physiology Score (APS) from the Acute Physiology and Chronic Health Evaluation (APACHE) III was derived from data available in the RMRS to measure the severity of illness.[21] Although the APACHE III was developed in the intensive care unit using data from the first 24 hours after admission, for our study we used the worst laboratory test value during the entire hospital stay to calculate the APS.[22]
Statistical Analysis
Baseline variables are presented as means and standard deviations for continuous variables, and percentages for binary categorical variables. Comparisons between patients receiving GCS and those who did not were performed using 2 tests for categorical variables and Kruskal‐Wallis test for continuous variables. Cox proportional hazard models were used to determine the association between receiving GCS and time to hospital readmission or mortality within 30 days or 1‐year postindex admission while adjusting for other covariates. For the models using time to readmission, patients without readmission were censored either at the endpoint (30 days or 1 year) or at time of death for those who died within the time frame in each model. Because GCS was not randomly assigned, we also conducted a propensity score analysis.[23] A logistic model for the probability of receiving GCS was conducted using patient demographic variables and information collected before and at the time of GCS. Stratified Cox proportional models using quintiles of predicted probability of receiving GCS were used in a propensity‐adjusted Cox model. All data analyses were performed using SAS version 9.3 (SAS Institute, Inc., Cary, NC).
RESULTS
Between July 1, 2006 and May 30, 2008, 415 CI patients were enrolled in the original trial, with 176 receiving the GCS. As shown in Table 1, the GCS and non‐GCS groups differed significantly. The GCS group was older (79.2 years old, 8.1 standard deviation [SD] vs 75.8 years old, 7.8 SD; P0.001), scored lower on the SPMSQ (4.7, 2.7 SD vs 5.5, 2.7 SD; P=0.002), had fewer chronic conditions with a lower mean Charlson Comorbidity Index Score (2.1, 1.86 SD vs 2.8, 2.6 SD; P=0.023), but a higher percentage of delirium (48.9% vs 29.3%), a lower percentage of being discharged home (37.5% vs 56.1%), and a higher mean length of stay (6.4 days, 6.4 SD vs 5.6 days, 5.9 SD; P=0.004). They also had a lower malignancy rate (6.2% vs 14.6%; P=0.007) and a lower number of hospitalizations in the previous year (0.5 admissions, 0.9 SD vs 0.7 admissions, 1.1 SD; P=0.035). No differences were observed in regard to gender, ethnicity, history of myocardial infarctions, chronic obstructive pulmonary disease, cerebrovascular disease, peripheral vascular disease, diabetes, and use of anticholinergic medicines.
| No GCS, n=239 | GCS, n=176 | P Value* | |
|---|---|---|---|
| |||
| Baseline characteristics | |||
| Mean age (SD) | 75.8 (7.8) | 79.2 (8.1) | <0.001 |
| % Female | 66.1 [n=158] | 68.2 [n=120] | 0.657 |
| % African American | 54.8 [n=131] | 63.6 [n=112] | 0.071 |
| Mean SPMSQ score (SD) | 5.5 (2.7) | 4.7 (2.7) | 0.002 |
| Admission diagnoses | |||
| MI | 15.5 [n=37] | 13.6 [n=24] | 0.675 |
| CHF | 38.1 [n=91] | 34.7 [n=61] | 0.475 |
| PVD | 7.1 [n=17] | 9.7 [n=17] | 0.370 |
| Cerebrovascular | 13.8 [n=33] | 19.3 [n=34] | 0.140 |
| COPD | 41.0 [n=98] | 33.0 [n=58] | 0.094 |
| Diabetes | 47.7 [n=114] | 40.9 [n=72] | 0.169 |
| Malignancy | 14.6 [n=35] | 6.2 [n=11] | 0.007 |
| Metastatic cancer | 8.8 [n=21] | 1.7 [n=3] | 0.002 |
| Mean Charlson Comorbidity (SD) | 2.8 (2.6) | 2.1 (1.8) | 0.023 |
| Mean APS (SD) | 24.5 (13.8) | 25.9 (13.5) | 0.231 |
| Definite ACB Use | 35.2 [n=84] | 27.8 [n=49] | 0.136 |
| Length of stay | 5.6 (5.9) | 6.4 (6.4) | 0.004 |
| % Any delirium | 29.3 [n=70] | 48.9 [n=156] | <0.001 |
| % Discharged home | 56.1 [n=134] | 37.5 [n=66] | <0.001 |
| No. of inpatient stays prior year | 0.7 (1.1) | 0.5 (0.9) | 0.035 |
| Follow‐up outcomes | |||
| % Readmission within 30 days | 15.1 [n=36] | 22.7 [n=40] | 0.054 |
| % Readmission within 1 year | 54.4 [n=130] | 56.3 [n=99] | 0.765 |
| % Death within 30 days | 4.2 [n=10] | 1.7 [n=3] | 0.253 |
| % Death within 1 year | 26.8 [n=64] | 23.9 [n=42] | 0.569 |
| % Readmission or death within 30 days | 18.0 [n=43] | 24.4 [n=43] | 0.113 |
| % Readmission or death within 1 year | 64.8 [n=155] | 63.1 [n=111] | 0.708 |
Table 2 describes the association of various factors with receiving GCS. Patients who were positive for delirium (odds ratio [OR]=1.65; 95% confidence interval=0.98‐2.77) and were older (OR=1.04; 95% confidence interval=1.01‐1.08) had a higher propensity to receive GCS, whereas, the presence of metastatic cancer resulted in a lower propensity (OR=0.15; 95% confidence interval=0.02‐1.16) of receiving GCS. The logistic model estimated area under the receiver operating characteristic curve was 0.707.
| Adjusted OR (95% CI) | P Value | |
|---|---|---|
| ||
| Age | 1.04 (1.011.08) | 0.006 |
| Female | 1.02 (0.641.63) | 0.942 |
| African American | 1.11 (0.711.72) | 0.657 |
| Short Portable Mental Status Questionnaire score | 1.00 (0.911.10) | 0.990 |
| Acute Physiology Score | 1.00 (0.981.02) | 0.769 |
| Charlson Comorbidity Score | 1.11 (0.841.46) | 0.471 |
| Length of hospital stay | 1.02 (0.981.07) | 0.299 |
| Definite anticholinergic use* | 0.74 (0.461.20) | 0.219 |
| Any delirium during hospital stay | 1.65 (0.982.77) | 0.061 |
| Diabetes mellitus | 0.72 (0.411.26) | 0.253 |
| Myocardial infarction | 0.83 (0.411.66) | 0.593 |
| Congestive heart failure | 0.83 (0.471.47) | 0.524 |
| Peripheral vascular disease | 1.39 (0.613.18) | 0.433 |
| Cerebrovascular disease | 1.30 (0.652.59) | 0.464 |
| Malignancy | 0.45 (0.171.21) | 0.113 |
| Metastatic cancer | 0.15 (0.021.16) | 0.069 |
| Chronic obstructive pulmonary disease | 0.91 (0.531.55) | 0.727 |
Table 3 provides results from the Cox models for receiving GCS on readmission and mortality outcomes adjusting for various sets of covariates and with the propensity score adjustment. Model 1 presents unadjusted hazard ratio (HR). Model 2 presents HRs adjusting for a common set of covariates that were significantly associated with at least 1 of the outcomes, whereas model 3 presents the results adjusting for all covariates. All 4 models yielded similar results. As evident from this table, propensity‐adjusted HR for 30‐day readmission was still significantly higher among patients receiving GCS (HR=1.75; 95% confidence interval=1.06‐2.88) but not at 1 year (HR=1.19; 95% confidence interval=0.89‐1.59). There was a trend for decreased mortality for the GCS group at 30 days (HR=0.35; 95% confidence interval=0.09‐1.35), but it disappeared at 1 year (HR=0.91; 95% confidence interval=0.59‐1.40). A composite outcome of readmissions and mortality did not show any difference between the GCS and no‐GCS groups.
| Outcome Variables | Model 1 | Model 2 | Model 3 | Propensity Adjusted | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| ||||||||
| Readmission within 30 days | 1.65 (1.05, 2.59) | 0.030 | 1.73 (1.08, 2.78) | 0.024 | 1.84 (1.133.00) | 0.015 | 1.75 (1.062.88) | 0.029 |
| Readmission within 1 year | 1.13 (0.87, 1.46) | 0.373 | 1.24 (0.94, 1.63) | 0.125 | 1.26 (0.941.68) | 0.117 | 1.19 (0.891.59) | 0.245 |
| Death within 30 days | 0.43 (0.12, 1.56) | 0.199 | 0.34 (0.09, 1.28) | 0.110 | 0.25 (0.061.02) | 0.053 | 0.35 (0.091.35) | 0.126 |
| Death within 1 year | 0.95 (0.65, 1.45) | 0.806 | 0.87 (0.58 1.31) | 0.506 | 0.93 (0.601.42) | 0.724 | 0.91 (0.591.40) | 0.669 |
| Readmission or Death within 30 days | 1.48 (0.97, 2.26) | 0.070 | 1.49 (0.96, 2.33) | 0.078 | 1.56 (0.982.47) | 0.061 | 1.55 (0.972.48) | 0.069 |
| Readmission or death within 1 year | 1.05 (0.82, 1.34) | 0.699 | 1.11 (0.86, 1.43) | 0.412 | 1.15 (0.881.50) | 0.318 | 1.08 (0.831.42) | 0.569 |
DISCUSSION
To our knowledge, this is the first study to analyze the impact of GCS on hospital readmission and mortality rates of CI patients. Our results did not show any short‐term or long‐term benefits of GCS for CI patients. Recent studies exploring cost benefits of the GCS have found trends toward lower readmission, but none focused on patients with CI.[6, 24, 25] It is important to note that our study did not use random allocation to assigning the patient into the GCS or control group, thus raising the possibility that patients who received GCS were sicker and were medically and socially more complex than those who did not receive the consult. Moreover, GCS consultation is preferentially sought for and completed for patients with CI and functional limitations, consistent with our finding that GCS patients more often have delirium and are less‐often discharged home.
The nature of the GCS team is another important consideration. Our GCS model did not include unit cohorting of patients, an important component of other proposed GCS models.[26] A recent meta‐analysis found that the GCS models without unit cohorting of patients did not have an impact on 1‐ or 12‐month readmission rates.[27] Low adherence to consultant recommendations (less than 33%) was thought to be a reason for such results. Importance of cohorting with regard to accomplishing recommendations by primary teams, importance of unit staff expertise in geriatric principles, and impact of a unit model on teamwork has also been highlighted by another review.[28] These findings lend to the hypothesis that unit cohorting and direct order placement by the GCS team may improve outcomes among CI patients, including a reduction in readmission rates.
Although readmissions rates were not statistically different between GCS and control groups at 1‐year postdischarge, 30‐day readmission rates were higher among the GCS group. Previous research among older heart failure patients found that a comprehensive transitional care intervention at the time of hospital discharge significantly shortened the time to readmission in the intervention group (P=0.026).[29] The factors identified by the study authors included enhanced supervision by the transitional healthcare teams along with improved awareness and education among treated patients that may have facilitated early recognition of clinical deterioration.[29] A recent study with intensive outpatient care that resulted in increased admissions among chronically ill adults provided a similar conclusion.[30]
GCS patients showed a trend toward decreased mortality as did patients enrolled in previous studies evaluating GCS models in the inpatient setting, as suggested by a recent review.[27] A caveat to note is that these trends favored ward‐styled GCS services as compared to our open GCS model,[27, 28] although the factors cited in these dedicated units affecting mortality included prompt attention to early rehabilitation, delirium management, and prevention of pressure ulcers and are also frequently implemented for patients in our GCS service model and therefore may have produced similar results.
Our neutral results in regard to the readmissions need to be interpreted with caution. First, this study was conducted in a hospital that supports expert geriatric and palliative care teams, both in the inpatient and the ambulatory settings, that provide consultative services and train medicine teams and hospital nursing staff. On the outpatient side, the presence of a robust geriatrics house‐calls program and the Geriatric Resources for Assessment and Care of Elders team results in above‐average care for the control group, and thus may also impact apparent outcomes.[31, 32] Second, 30‐day readmissions represent a complex outcome. Two recent reviews of hospital‐initiated interventions have shown that evidence regarding best strategies to decrease 30‐day readmissions is unclear.[33] Neither review included studies that targeted patients with CI only. The 2 programs that reduced 30‐day readmissions were multifaceted and included personnel who provide bridging between the hospital and the outpatient setting.[34] The GCS does include a focus on postdischarge resources, but does that on a case‐by‐case basis and no formal posthospital follow‐ups are provided. Moreover, the value of 30‐day readmission rates as a marker of quality, even though used by policymakers as an indicator of hospital quality, remains controversial.[35, 36] Broadening the outcomes of interest to include patient‐centered outcomes including satisfaction with care, that have shown to impact other health outcomes, may help improve understanding the benefits of GCS in hospitals.[37] Other comprehensive transitional care models that failed to show a benefit on 30‐day readmissions in older patients still resulted in higher satisfaction among patients.[38] Unfortunately, our evaluation did not include an assessment of patient satisfaction and quality of transitions.
Since the study period, GCS at our hospital now has incorporated a more robust focus on advance care planning (ACP) and execution of Physician Orders for Scope of Treatment that were legislated in the state in July 2013. The GCS team members are expert in carrying out complex ACP discussions and also partner with the inpatient palliative care team. It is quite possible that a study of more recent outcomes will yield more positive results for the selected outcomes. Thus, for future trials that aim to study the impact of GCS in the inpatient settings, it may be advisable to include important quality markers such as implementation of ACP and patient satisfaction along with the health utilization outcomes.
Limitations
As mentioned previously, it is possible that our risk adjustment was insufficient to account for all the medical and psychosocial differences among groups. For example, the overall anticholinergic impact of various medications such as antipsychotic medications and histamine‐2 blockers was assessed via the Anticholinergic Burden Scale on admission, but we did not have information on medication prescribing during the stay. We were further limited by lack of baseline functional status and socioeconomic details, both of which are related to 30‐day readmissions. For example, living alone, prior use of assist devices, and belonging to lower socioeconomic status are correlated with higher readmission rates.[39, 40] Patients with available social support may receive more intense supervision and may seek medical attention sooner. On the other hand, worsening health among CI patients without any approximate social support may be unnoticed for days. Absence of details of inpatient interventions may also have resulted in unmeasurable confounders that could have impacted our study outcomes. Finally, lack of information on the uptake of GCS recommendations by the primary teams is another limitation of this analysis. Future trials should include strategies to address these information gaps.
CONCLUSION
Our results comparing inpatient geriatrics consultative services with usual care in hospitalized elders having cognitive impairment failed to demonstrate an impact on readmissions and mortality. A clinical lesson learned, however, is that much work is still required to reduce readmission and mortality rates in this especially vulnerable patient population.
Disclosures
Disclosures: This work was supported by grants from a Geriatric Academic Career Award (K01HP20517) through Health Resources and Services Administration, R01AG034205 and K23‐AG043476 from the National Institute on Aging, and the John A. Hartford Foundation Center for Excellence in Geriatric Medicine. The sponsors had no role in the study design, evaluation, or manuscript development. The authors report no conflicts of interest.
- , , , et al. Relationship between hospital readmission and mortality rates for patients hospitalized with acute myocardial infarction, heart failure, or pneumonia. JAMA. 2013;309(6):587–593.
- , , , Cognitive impairment. Can it predict the course of hospitalized patients? J Am Geriatr Soc. 1986;34(8):579–585.
- , , , , , Importance of functional measures in predicting mortality among older hospitalized patients. JAMA. 1998;279(15):1187–1193.
- , Understanding preventable hospital readmissions: masqueraders, markers, and true causal factors. J Hosp Med. 2011;6(2):51–53.
- , , , et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340(9):669–676.
- , , , et al. Effects of a multicomponent intervention on functional outcomes and process of care in hospitalized older patients: a randomized controlled trial of Acute Care for Elders (ACE) in a community hospital. J Am Geriatr Soc. 2000;48(12):1572–1581.
- , ; American Geriatrics Society Health Care Systems Committee. Improving the quality of transitional care for persons with complex care needs. J Am Geriatr Soc. 2003;51(4):556–557.
- , , , Development and implementation of a proactive geriatrics consultation model in collaboration with hospitalists. J Am Geriatr Soc. 2009;57(11):2139–2145.
- , , , et al. Screening of the risk of functional decline performed by an inpatient geriatric consultation team in a general hospital [in French]. Revue medicale de Bruxelles. 2013;34(6):462–468.
- , , , et al. Systematic detection and multidisciplinary care of delirium in older medical inpatients: a randomized trial. CMAJ. 2002;167(7):753–759.
- , , Potentially inappropriate prescribing for geriatric inpatients: an acute care of the elderly unit compared to a general medicine service. Consult Pharm. 2003;18(1):37–42, 47–39.
- , , , Evaluation of the Mobile Acute Care of the Elderly (MACE) service. JAMA Intern Med. 2013;173(11):990–996.
- , , , , , Effects of an acute care for elders unit on costs and 30‐day readmissions. JAMA Intern Med. 2013;173(11):981–987.
- , , , et al. Impact and recognition of cognitive impairment among hospitalized elders. J Hosp Med. 2010;5(2):69–75.
- , , , et al. Enhancing care for hospitalized older adults with cognitive impairment: a randomized controlled trial. J Gen Intern Med. 2012;27(5):561–567.
- , , , Short Portable Mental Status Questionnaire as a screening test for dementia and delirium among the elderly. J Am Geriatr Soc. 1987;35(5):412–416.
- , , , et al. The Regenstrief Medical Record System: a quarter century experience. Int J Med Inform. 1999;54(3):225–253.
- , , , , , Factors determining the decision to institutionalize dementing individuals: a prospective study. Gerontologist. 1993;33(6):714–720.
- , , , , , Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941–948.
- , , , , , Resuscitation: how do we decide? A prospective study of physicians' preferences and the clinical course of hospitalized patients. JAMA. 1986;255(10):1316–1322.
- , , , et al. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991;100(6):1619–1636.
- , , , et al. Interaction between cognitive impairment and discharge destination and its effect on rehospitalization. J Am Geriatr Soc. 2013;61(11):1958–1963.
- , , , , , Propensity scores in intensive care and anaesthesiology literature: a systematic review. Intensive Care Med. 2010;36(12):1993–2003.
- , , , et al. Improving functional outcomes in older patients: lessons from an acute care for elders unit. Jt Comm J Qual Improv. 1998;24(2):63–76.
- , , , et al. Developing a stroke unit using the acute care for elders intervention and model of care. J Am Geriatr Soc. 2003;51(11):1660–1667.
- , , , A medical unit for the acute care of the elderly. J Am Geriatr Soc. 1994;42(5):545–552.
- , , , , Impact of geriatric consultation teams on clinical outcome in acute hospitals: a systematic review and meta‐analysis. BMC Med. 2013;11:48.
- , , , , Comprehensive geriatric assessment for older adults admitted to hospital: meta‐analysis of randomised controlled trials. BMJ. 2011;343:d6553.
- , , , et al. Prevention of readmission in elderly patients with congestive heart failure: results of a prospective, randomized pilot study. J Gen Intern Med. 1993;8(11):585–590.
- , , , et al. Effect of a pharmacist intervention on clinically important medication errors after hospital discharge: a randomized trial. Ann Intern Med. 2012;157(1):1–10.
- , , , , House calls for seniors: building and sustaining a model of care for homebound seniors. J Am Geriatr Soc. 2009;57(6):1103–1109.
- , , , et al. Geriatric care management for low‐income seniors: a randomized controlled trial. JAMA. 2007;298(22):2623–2633.
- , , , , Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520–528.
- , The effects of a discharge planning and home follow‐up intervention on elders hospitalized with common medical and surgical cardiac conditions. J Cardiovasc Nurs. 1999;14(1):44–54.
- Readmission to hospital: a measure of quality or outcome? Qual Saf Health Care. 2004;13(1):10–11.
- , , , , , Unintended consequences of steps to cut readmissions and reform payment may threaten care of vulnerable older adults. Health Aff (Millwood). 2012;31(7):1623–1632.
- , , Analyzing the effects of shared decision‐making, empathy and team interaction on patient satisfaction and treatment acceptance in medical rehabilitation using a structural equation modeling approach. Patient Educ Couns. 2013;91(2):167–175.
- , , , The Care Transitions Innovation (C‐TraIn) for Socioeconomically Disadvantaged Adults: results of a cluster randomized controlled trial. J Gen Intern Med. 2014;29(11):1460–1467.
- , , , Risk factors for early hospital readmission in low‐income elderly adults. J Am Geriatr Soc. 2014;62(3):489–494.
- , , , , , Understanding why patients of low socioeconomic status prefer hospitals over ambulatory care. Health Aff (Millwood). 2013;32(7):1196–1203.
Under the Patient Protection and Affordable Care Act of 2010, commonly referred to as the Affordable Care Act, hospitals face up to a 3% penalty in Medicare reimbursements for patients readmitted within 30 days of initial discharge, and measures have been proposed for modifying payments to hospitals based on their performance on this metric.[1] Cognitive impairment (CI) is considered a major risk factor for poor postdischarge outcomes including mortality and hospital readmission.[2, 3] Hospitals are seeking strategies to reduce postdischarge mortality and rehospitalization among patients with and without CI.[4] Such strategies include use of transitional care coaches, patient and caregiver education, postdischarge follow‐up, and provision of geriatric consultative services (GCS) for the care of complex patients in the hospital setting.[5, 6, 7]
GCS utilize comprehensive geriatric assessments and multidisciplinary processes to recognize and modify risk factors that may lead to poor outcomes among hospitalized patients.[8, 9, 10, 11] Implementation of GCS models including Acute Care for Elders and, recently, the Mobile Acute Care of the Elderly services have shown many benefits among older patients including a reduction in the hospital length of stay and readmission rates.[12, 13] The benefits of such services among hospitalized elders suffering from CI, however, are not well established. The objective of this article was to evaluate the impact of GCS on the readmission and mortality rates of older adults with CI within 12 months of their hospitalization to an urban, public hospital. We hypothesized that GCS will reduce both 12‐month hospital readmissions and mortality rates among this vulnerable group of older adults.
METHODS
The study was approved by the Indiana University institutional review board, and informed consent for identifiable chart review was obtained from subjects or their legally authorized representatives.
Setting
The study was conducted at Eskenazi hospital, Indianapolis, Indiana, a 340‐bed, university‐affiliated, public hospital with over 2300 admissions of patients aged 65 years or older every year.
Population
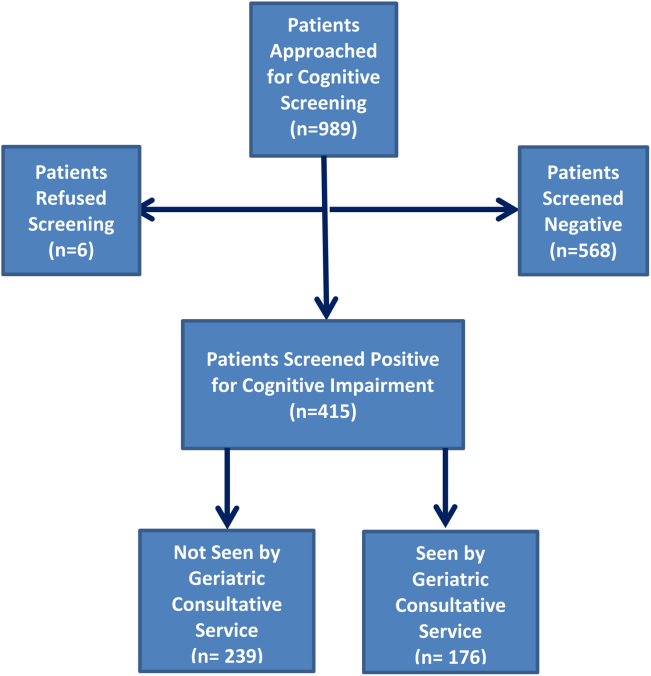
Four hundred fifteen hospitalized patients aged 65 years or older suffering from CI were enrolled into an original, randomized, controlled trial that evaluated the effect of a computerized decision support system on their quality and outcome of care between July 1, 2006 and May 30, 2008.[14] The computerized decision support included reminders for physicians to reduce the prescription of 18 anticholinergics, minimize physical restraints and Foley catheterization, and increase referral to the local GCS.[15] That previous trial neither showed an impact on quality of care nor health utilization among older patients, including mortality and hospital readmission rates. The current study uses the data from the clinical trial cohort to evaluate the effect of GCS on the 12‐month mortality and hospital readmission rates for hospitalized elders with CI (Figure 1).

Inclusion and Exclusion Criteria
Individuals were eligible for enrollment if they were aged 65 years or older, hospitalized on a medical ward, able to speak English, and had evidence of CI within 48 hours of hospital admission. Individuals were excluded if they were previously enrolled, were aphasic, or unresponsive. The presence of CI was based on the Short Portable Mental Status Questionnaire (SPMSQ),[16] a brief 10‐item screening test with a sensitivity of 86% and specificity of 99% for dementia using a score of 7 or less (maximum possible score of 10).[16] The SPMSQ scoring process adjusts for participant educational and racial status, which was a benefit to its use given the urban setting of our hospital serving a large proportion of minority and low‐education patients. A physician‐trained research assistant administered the SPMSQ within 48 hours of hospital admission.
Geriatric Consultative Services
GCS is an interdisciplinary team of a geriatrician, a geriatric pharmacist, a case manager nurse, a social worker, a medical assistant, physical therapists, and a representative of the local Area Agency on Aging. There may be a geriatric fellow and/or medicine resident available to the team based on their rotation structure. Team‐based bedside rounds are performed on new consults only, but all patients are seen individually by the team clinicians. The team emphasizes prevention of functional decline and polypharmacy, recognition and treatment of geriatric syndromes including dementia and delirium, and early discharge/transition planning. Consensus recommendations are prepared and documented in the consult notes section of the electronic medical records. Recommendations deemed critical are discussed directly with the primary teams, but no orders are placed by the GCS team. The GCS team is available on all weekdays but not on weekends or major holidays.
Study Outcomes
For this secondary analysis, we used the Regenstrief Medical Record System (RMRS) to measure 2 outcomes: hospital readmission and mortality rates up to 1 year from discharge following index hospitalization, defined as the first admission in the original clinical trial. The RMRS is the primary instrument for processing data and monitoring patient and physician activity for the hospital.[17, 18] The RMRS is linked with a state‐wide health information exchange to capture data on hospitalization outside the hospital. The RMRS also contains death certificate information for all registered patients who die in or outside the Eskenazi hospital.
Other Data Collections
Delirium was assessed at screening and then every weekday using the Confusion Assessment Method (CAM) by a trained research assistant.[19] CAM evaluates 10 symptoms of delirium specified in the Diagnostic and Statistical Manual of Mental Disorders‐III‐Revision: acute onset, fluctuating course, inattention, disorganized thinking, altered level of consciousness, disorientation, memory impairment, perceptual disturbances, psychomotor agitation or retardation, and sleep/wake disturbance. Participant demographic characteristics, including age, sex, ethnicity, and years of education, were collected from the RMRS and from interviews performed at the time of cognitive screening. Information on length of hospital stay and discharge destination (eg, home vs facility, including skilled nursing and acute rehabilitation facilities) was also obtained from the RMRS. Charlson Comorbidity Index score was calculated using International Classification of Diseases, Ninth Revision codes gathered from 1 year before admission until the time of each participant's discharge from the hospital.[20] The Acute Physiology Score (APS) from the Acute Physiology and Chronic Health Evaluation (APACHE) III was derived from data available in the RMRS to measure the severity of illness.[21] Although the APACHE III was developed in the intensive care unit using data from the first 24 hours after admission, for our study we used the worst laboratory test value during the entire hospital stay to calculate the APS.[22]
Statistical Analysis
Baseline variables are presented as means and standard deviations for continuous variables, and percentages for binary categorical variables. Comparisons between patients receiving GCS and those who did not were performed using 2 tests for categorical variables and Kruskal‐Wallis test for continuous variables. Cox proportional hazard models were used to determine the association between receiving GCS and time to hospital readmission or mortality within 30 days or 1‐year postindex admission while adjusting for other covariates. For the models using time to readmission, patients without readmission were censored either at the endpoint (30 days or 1 year) or at time of death for those who died within the time frame in each model. Because GCS was not randomly assigned, we also conducted a propensity score analysis.[23] A logistic model for the probability of receiving GCS was conducted using patient demographic variables and information collected before and at the time of GCS. Stratified Cox proportional models using quintiles of predicted probability of receiving GCS were used in a propensity‐adjusted Cox model. All data analyses were performed using SAS version 9.3 (SAS Institute, Inc., Cary, NC).
RESULTS
Between July 1, 2006 and May 30, 2008, 415 CI patients were enrolled in the original trial, with 176 receiving the GCS. As shown in Table 1, the GCS and non‐GCS groups differed significantly. The GCS group was older (79.2 years old, 8.1 standard deviation [SD] vs 75.8 years old, 7.8 SD; P0.001), scored lower on the SPMSQ (4.7, 2.7 SD vs 5.5, 2.7 SD; P=0.002), had fewer chronic conditions with a lower mean Charlson Comorbidity Index Score (2.1, 1.86 SD vs 2.8, 2.6 SD; P=0.023), but a higher percentage of delirium (48.9% vs 29.3%), a lower percentage of being discharged home (37.5% vs 56.1%), and a higher mean length of stay (6.4 days, 6.4 SD vs 5.6 days, 5.9 SD; P=0.004). They also had a lower malignancy rate (6.2% vs 14.6%; P=0.007) and a lower number of hospitalizations in the previous year (0.5 admissions, 0.9 SD vs 0.7 admissions, 1.1 SD; P=0.035). No differences were observed in regard to gender, ethnicity, history of myocardial infarctions, chronic obstructive pulmonary disease, cerebrovascular disease, peripheral vascular disease, diabetes, and use of anticholinergic medicines.
| No GCS, n=239 | GCS, n=176 | P Value* | |
|---|---|---|---|
| |||
| Baseline characteristics | |||
| Mean age (SD) | 75.8 (7.8) | 79.2 (8.1) | <0.001 |
| % Female | 66.1 [n=158] | 68.2 [n=120] | 0.657 |
| % African American | 54.8 [n=131] | 63.6 [n=112] | 0.071 |
| Mean SPMSQ score (SD) | 5.5 (2.7) | 4.7 (2.7) | 0.002 |
| Admission diagnoses | |||
| MI | 15.5 [n=37] | 13.6 [n=24] | 0.675 |
| CHF | 38.1 [n=91] | 34.7 [n=61] | 0.475 |
| PVD | 7.1 [n=17] | 9.7 [n=17] | 0.370 |
| Cerebrovascular | 13.8 [n=33] | 19.3 [n=34] | 0.140 |
| COPD | 41.0 [n=98] | 33.0 [n=58] | 0.094 |
| Diabetes | 47.7 [n=114] | 40.9 [n=72] | 0.169 |
| Malignancy | 14.6 [n=35] | 6.2 [n=11] | 0.007 |
| Metastatic cancer | 8.8 [n=21] | 1.7 [n=3] | 0.002 |
| Mean Charlson Comorbidity (SD) | 2.8 (2.6) | 2.1 (1.8) | 0.023 |
| Mean APS (SD) | 24.5 (13.8) | 25.9 (13.5) | 0.231 |
| Definite ACB Use | 35.2 [n=84] | 27.8 [n=49] | 0.136 |
| Length of stay | 5.6 (5.9) | 6.4 (6.4) | 0.004 |
| % Any delirium | 29.3 [n=70] | 48.9 [n=156] | <0.001 |
| % Discharged home | 56.1 [n=134] | 37.5 [n=66] | <0.001 |
| No. of inpatient stays prior year | 0.7 (1.1) | 0.5 (0.9) | 0.035 |
| Follow‐up outcomes | |||
| % Readmission within 30 days | 15.1 [n=36] | 22.7 [n=40] | 0.054 |
| % Readmission within 1 year | 54.4 [n=130] | 56.3 [n=99] | 0.765 |
| % Death within 30 days | 4.2 [n=10] | 1.7 [n=3] | 0.253 |
| % Death within 1 year | 26.8 [n=64] | 23.9 [n=42] | 0.569 |
| % Readmission or death within 30 days | 18.0 [n=43] | 24.4 [n=43] | 0.113 |
| % Readmission or death within 1 year | 64.8 [n=155] | 63.1 [n=111] | 0.708 |
Table 2 describes the association of various factors with receiving GCS. Patients who were positive for delirium (odds ratio [OR]=1.65; 95% confidence interval=0.98‐2.77) and were older (OR=1.04; 95% confidence interval=1.01‐1.08) had a higher propensity to receive GCS, whereas, the presence of metastatic cancer resulted in a lower propensity (OR=0.15; 95% confidence interval=0.02‐1.16) of receiving GCS. The logistic model estimated area under the receiver operating characteristic curve was 0.707.
| Adjusted OR (95% CI) | P Value | |
|---|---|---|
| ||
| Age | 1.04 (1.011.08) | 0.006 |
| Female | 1.02 (0.641.63) | 0.942 |
| African American | 1.11 (0.711.72) | 0.657 |
| Short Portable Mental Status Questionnaire score | 1.00 (0.911.10) | 0.990 |
| Acute Physiology Score | 1.00 (0.981.02) | 0.769 |
| Charlson Comorbidity Score | 1.11 (0.841.46) | 0.471 |
| Length of hospital stay | 1.02 (0.981.07) | 0.299 |
| Definite anticholinergic use* | 0.74 (0.461.20) | 0.219 |
| Any delirium during hospital stay | 1.65 (0.982.77) | 0.061 |
| Diabetes mellitus | 0.72 (0.411.26) | 0.253 |
| Myocardial infarction | 0.83 (0.411.66) | 0.593 |
| Congestive heart failure | 0.83 (0.471.47) | 0.524 |
| Peripheral vascular disease | 1.39 (0.613.18) | 0.433 |
| Cerebrovascular disease | 1.30 (0.652.59) | 0.464 |
| Malignancy | 0.45 (0.171.21) | 0.113 |
| Metastatic cancer | 0.15 (0.021.16) | 0.069 |
| Chronic obstructive pulmonary disease | 0.91 (0.531.55) | 0.727 |
Table 3 provides results from the Cox models for receiving GCS on readmission and mortality outcomes adjusting for various sets of covariates and with the propensity score adjustment. Model 1 presents unadjusted hazard ratio (HR). Model 2 presents HRs adjusting for a common set of covariates that were significantly associated with at least 1 of the outcomes, whereas model 3 presents the results adjusting for all covariates. All 4 models yielded similar results. As evident from this table, propensity‐adjusted HR for 30‐day readmission was still significantly higher among patients receiving GCS (HR=1.75; 95% confidence interval=1.06‐2.88) but not at 1 year (HR=1.19; 95% confidence interval=0.89‐1.59). There was a trend for decreased mortality for the GCS group at 30 days (HR=0.35; 95% confidence interval=0.09‐1.35), but it disappeared at 1 year (HR=0.91; 95% confidence interval=0.59‐1.40). A composite outcome of readmissions and mortality did not show any difference between the GCS and no‐GCS groups.
| Outcome Variables | Model 1 | Model 2 | Model 3 | Propensity Adjusted | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| ||||||||
| Readmission within 30 days | 1.65 (1.05, 2.59) | 0.030 | 1.73 (1.08, 2.78) | 0.024 | 1.84 (1.133.00) | 0.015 | 1.75 (1.062.88) | 0.029 |
| Readmission within 1 year | 1.13 (0.87, 1.46) | 0.373 | 1.24 (0.94, 1.63) | 0.125 | 1.26 (0.941.68) | 0.117 | 1.19 (0.891.59) | 0.245 |
| Death within 30 days | 0.43 (0.12, 1.56) | 0.199 | 0.34 (0.09, 1.28) | 0.110 | 0.25 (0.061.02) | 0.053 | 0.35 (0.091.35) | 0.126 |
| Death within 1 year | 0.95 (0.65, 1.45) | 0.806 | 0.87 (0.58 1.31) | 0.506 | 0.93 (0.601.42) | 0.724 | 0.91 (0.591.40) | 0.669 |
| Readmission or Death within 30 days | 1.48 (0.97, 2.26) | 0.070 | 1.49 (0.96, 2.33) | 0.078 | 1.56 (0.982.47) | 0.061 | 1.55 (0.972.48) | 0.069 |
| Readmission or death within 1 year | 1.05 (0.82, 1.34) | 0.699 | 1.11 (0.86, 1.43) | 0.412 | 1.15 (0.881.50) | 0.318 | 1.08 (0.831.42) | 0.569 |
DISCUSSION
To our knowledge, this is the first study to analyze the impact of GCS on hospital readmission and mortality rates of CI patients. Our results did not show any short‐term or long‐term benefits of GCS for CI patients. Recent studies exploring cost benefits of the GCS have found trends toward lower readmission, but none focused on patients with CI.[6, 24, 25] It is important to note that our study did not use random allocation to assigning the patient into the GCS or control group, thus raising the possibility that patients who received GCS were sicker and were medically and socially more complex than those who did not receive the consult. Moreover, GCS consultation is preferentially sought for and completed for patients with CI and functional limitations, consistent with our finding that GCS patients more often have delirium and are less‐often discharged home.
The nature of the GCS team is another important consideration. Our GCS model did not include unit cohorting of patients, an important component of other proposed GCS models.[26] A recent meta‐analysis found that the GCS models without unit cohorting of patients did not have an impact on 1‐ or 12‐month readmission rates.[27] Low adherence to consultant recommendations (less than 33%) was thought to be a reason for such results. Importance of cohorting with regard to accomplishing recommendations by primary teams, importance of unit staff expertise in geriatric principles, and impact of a unit model on teamwork has also been highlighted by another review.[28] These findings lend to the hypothesis that unit cohorting and direct order placement by the GCS team may improve outcomes among CI patients, including a reduction in readmission rates.
Although readmissions rates were not statistically different between GCS and control groups at 1‐year postdischarge, 30‐day readmission rates were higher among the GCS group. Previous research among older heart failure patients found that a comprehensive transitional care intervention at the time of hospital discharge significantly shortened the time to readmission in the intervention group (P=0.026).[29] The factors identified by the study authors included enhanced supervision by the transitional healthcare teams along with improved awareness and education among treated patients that may have facilitated early recognition of clinical deterioration.[29] A recent study with intensive outpatient care that resulted in increased admissions among chronically ill adults provided a similar conclusion.[30]
GCS patients showed a trend toward decreased mortality as did patients enrolled in previous studies evaluating GCS models in the inpatient setting, as suggested by a recent review.[27] A caveat to note is that these trends favored ward‐styled GCS services as compared to our open GCS model,[27, 28] although the factors cited in these dedicated units affecting mortality included prompt attention to early rehabilitation, delirium management, and prevention of pressure ulcers and are also frequently implemented for patients in our GCS service model and therefore may have produced similar results.
Our neutral results in regard to the readmissions need to be interpreted with caution. First, this study was conducted in a hospital that supports expert geriatric and palliative care teams, both in the inpatient and the ambulatory settings, that provide consultative services and train medicine teams and hospital nursing staff. On the outpatient side, the presence of a robust geriatrics house‐calls program and the Geriatric Resources for Assessment and Care of Elders team results in above‐average care for the control group, and thus may also impact apparent outcomes.[31, 32] Second, 30‐day readmissions represent a complex outcome. Two recent reviews of hospital‐initiated interventions have shown that evidence regarding best strategies to decrease 30‐day readmissions is unclear.[33] Neither review included studies that targeted patients with CI only. The 2 programs that reduced 30‐day readmissions were multifaceted and included personnel who provide bridging between the hospital and the outpatient setting.[34] The GCS does include a focus on postdischarge resources, but does that on a case‐by‐case basis and no formal posthospital follow‐ups are provided. Moreover, the value of 30‐day readmission rates as a marker of quality, even though used by policymakers as an indicator of hospital quality, remains controversial.[35, 36] Broadening the outcomes of interest to include patient‐centered outcomes including satisfaction with care, that have shown to impact other health outcomes, may help improve understanding the benefits of GCS in hospitals.[37] Other comprehensive transitional care models that failed to show a benefit on 30‐day readmissions in older patients still resulted in higher satisfaction among patients.[38] Unfortunately, our evaluation did not include an assessment of patient satisfaction and quality of transitions.
Since the study period, GCS at our hospital now has incorporated a more robust focus on advance care planning (ACP) and execution of Physician Orders for Scope of Treatment that were legislated in the state in July 2013. The GCS team members are expert in carrying out complex ACP discussions and also partner with the inpatient palliative care team. It is quite possible that a study of more recent outcomes will yield more positive results for the selected outcomes. Thus, for future trials that aim to study the impact of GCS in the inpatient settings, it may be advisable to include important quality markers such as implementation of ACP and patient satisfaction along with the health utilization outcomes.
Limitations
As mentioned previously, it is possible that our risk adjustment was insufficient to account for all the medical and psychosocial differences among groups. For example, the overall anticholinergic impact of various medications such as antipsychotic medications and histamine‐2 blockers was assessed via the Anticholinergic Burden Scale on admission, but we did not have information on medication prescribing during the stay. We were further limited by lack of baseline functional status and socioeconomic details, both of which are related to 30‐day readmissions. For example, living alone, prior use of assist devices, and belonging to lower socioeconomic status are correlated with higher readmission rates.[39, 40] Patients with available social support may receive more intense supervision and may seek medical attention sooner. On the other hand, worsening health among CI patients without any approximate social support may be unnoticed for days. Absence of details of inpatient interventions may also have resulted in unmeasurable confounders that could have impacted our study outcomes. Finally, lack of information on the uptake of GCS recommendations by the primary teams is another limitation of this analysis. Future trials should include strategies to address these information gaps.
CONCLUSION
Our results comparing inpatient geriatrics consultative services with usual care in hospitalized elders having cognitive impairment failed to demonstrate an impact on readmissions and mortality. A clinical lesson learned, however, is that much work is still required to reduce readmission and mortality rates in this especially vulnerable patient population.
Disclosures
Disclosures: This work was supported by grants from a Geriatric Academic Career Award (K01HP20517) through Health Resources and Services Administration, R01AG034205 and K23‐AG043476 from the National Institute on Aging, and the John A. Hartford Foundation Center for Excellence in Geriatric Medicine. The sponsors had no role in the study design, evaluation, or manuscript development. The authors report no conflicts of interest.
Under the Patient Protection and Affordable Care Act of 2010, commonly referred to as the Affordable Care Act, hospitals face up to a 3% penalty in Medicare reimbursements for patients readmitted within 30 days of initial discharge, and measures have been proposed for modifying payments to hospitals based on their performance on this metric.[1] Cognitive impairment (CI) is considered a major risk factor for poor postdischarge outcomes including mortality and hospital readmission.[2, 3] Hospitals are seeking strategies to reduce postdischarge mortality and rehospitalization among patients with and without CI.[4] Such strategies include use of transitional care coaches, patient and caregiver education, postdischarge follow‐up, and provision of geriatric consultative services (GCS) for the care of complex patients in the hospital setting.[5, 6, 7]
GCS utilize comprehensive geriatric assessments and multidisciplinary processes to recognize and modify risk factors that may lead to poor outcomes among hospitalized patients.[8, 9, 10, 11] Implementation of GCS models including Acute Care for Elders and, recently, the Mobile Acute Care of the Elderly services have shown many benefits among older patients including a reduction in the hospital length of stay and readmission rates.[12, 13] The benefits of such services among hospitalized elders suffering from CI, however, are not well established. The objective of this article was to evaluate the impact of GCS on the readmission and mortality rates of older adults with CI within 12 months of their hospitalization to an urban, public hospital. We hypothesized that GCS will reduce both 12‐month hospital readmissions and mortality rates among this vulnerable group of older adults.
METHODS
The study was approved by the Indiana University institutional review board, and informed consent for identifiable chart review was obtained from subjects or their legally authorized representatives.
Setting
The study was conducted at Eskenazi hospital, Indianapolis, Indiana, a 340‐bed, university‐affiliated, public hospital with over 2300 admissions of patients aged 65 years or older every year.
Population
Four hundred fifteen hospitalized patients aged 65 years or older suffering from CI were enrolled into an original, randomized, controlled trial that evaluated the effect of a computerized decision support system on their quality and outcome of care between July 1, 2006 and May 30, 2008.[14] The computerized decision support included reminders for physicians to reduce the prescription of 18 anticholinergics, minimize physical restraints and Foley catheterization, and increase referral to the local GCS.[15] That previous trial neither showed an impact on quality of care nor health utilization among older patients, including mortality and hospital readmission rates. The current study uses the data from the clinical trial cohort to evaluate the effect of GCS on the 12‐month mortality and hospital readmission rates for hospitalized elders with CI (Figure 1).

Inclusion and Exclusion Criteria
Individuals were eligible for enrollment if they were aged 65 years or older, hospitalized on a medical ward, able to speak English, and had evidence of CI within 48 hours of hospital admission. Individuals were excluded if they were previously enrolled, were aphasic, or unresponsive. The presence of CI was based on the Short Portable Mental Status Questionnaire (SPMSQ),[16] a brief 10‐item screening test with a sensitivity of 86% and specificity of 99% for dementia using a score of 7 or less (maximum possible score of 10).[16] The SPMSQ scoring process adjusts for participant educational and racial status, which was a benefit to its use given the urban setting of our hospital serving a large proportion of minority and low‐education patients. A physician‐trained research assistant administered the SPMSQ within 48 hours of hospital admission.
Geriatric Consultative Services
GCS is an interdisciplinary team of a geriatrician, a geriatric pharmacist, a case manager nurse, a social worker, a medical assistant, physical therapists, and a representative of the local Area Agency on Aging. There may be a geriatric fellow and/or medicine resident available to the team based on their rotation structure. Team‐based bedside rounds are performed on new consults only, but all patients are seen individually by the team clinicians. The team emphasizes prevention of functional decline and polypharmacy, recognition and treatment of geriatric syndromes including dementia and delirium, and early discharge/transition planning. Consensus recommendations are prepared and documented in the consult notes section of the electronic medical records. Recommendations deemed critical are discussed directly with the primary teams, but no orders are placed by the GCS team. The GCS team is available on all weekdays but not on weekends or major holidays.
Study Outcomes
For this secondary analysis, we used the Regenstrief Medical Record System (RMRS) to measure 2 outcomes: hospital readmission and mortality rates up to 1 year from discharge following index hospitalization, defined as the first admission in the original clinical trial. The RMRS is the primary instrument for processing data and monitoring patient and physician activity for the hospital.[17, 18] The RMRS is linked with a state‐wide health information exchange to capture data on hospitalization outside the hospital. The RMRS also contains death certificate information for all registered patients who die in or outside the Eskenazi hospital.
Other Data Collections
Delirium was assessed at screening and then every weekday using the Confusion Assessment Method (CAM) by a trained research assistant.[19] CAM evaluates 10 symptoms of delirium specified in the Diagnostic and Statistical Manual of Mental Disorders‐III‐Revision: acute onset, fluctuating course, inattention, disorganized thinking, altered level of consciousness, disorientation, memory impairment, perceptual disturbances, psychomotor agitation or retardation, and sleep/wake disturbance. Participant demographic characteristics, including age, sex, ethnicity, and years of education, were collected from the RMRS and from interviews performed at the time of cognitive screening. Information on length of hospital stay and discharge destination (eg, home vs facility, including skilled nursing and acute rehabilitation facilities) was also obtained from the RMRS. Charlson Comorbidity Index score was calculated using International Classification of Diseases, Ninth Revision codes gathered from 1 year before admission until the time of each participant's discharge from the hospital.[20] The Acute Physiology Score (APS) from the Acute Physiology and Chronic Health Evaluation (APACHE) III was derived from data available in the RMRS to measure the severity of illness.[21] Although the APACHE III was developed in the intensive care unit using data from the first 24 hours after admission, for our study we used the worst laboratory test value during the entire hospital stay to calculate the APS.[22]
Statistical Analysis
Baseline variables are presented as means and standard deviations for continuous variables, and percentages for binary categorical variables. Comparisons between patients receiving GCS and those who did not were performed using 2 tests for categorical variables and Kruskal‐Wallis test for continuous variables. Cox proportional hazard models were used to determine the association between receiving GCS and time to hospital readmission or mortality within 30 days or 1‐year postindex admission while adjusting for other covariates. For the models using time to readmission, patients without readmission were censored either at the endpoint (30 days or 1 year) or at time of death for those who died within the time frame in each model. Because GCS was not randomly assigned, we also conducted a propensity score analysis.[23] A logistic model for the probability of receiving GCS was conducted using patient demographic variables and information collected before and at the time of GCS. Stratified Cox proportional models using quintiles of predicted probability of receiving GCS were used in a propensity‐adjusted Cox model. All data analyses were performed using SAS version 9.3 (SAS Institute, Inc., Cary, NC).
RESULTS
Between July 1, 2006 and May 30, 2008, 415 CI patients were enrolled in the original trial, with 176 receiving the GCS. As shown in Table 1, the GCS and non‐GCS groups differed significantly. The GCS group was older (79.2 years old, 8.1 standard deviation [SD] vs 75.8 years old, 7.8 SD; P0.001), scored lower on the SPMSQ (4.7, 2.7 SD vs 5.5, 2.7 SD; P=0.002), had fewer chronic conditions with a lower mean Charlson Comorbidity Index Score (2.1, 1.86 SD vs 2.8, 2.6 SD; P=0.023), but a higher percentage of delirium (48.9% vs 29.3%), a lower percentage of being discharged home (37.5% vs 56.1%), and a higher mean length of stay (6.4 days, 6.4 SD vs 5.6 days, 5.9 SD; P=0.004). They also had a lower malignancy rate (6.2% vs 14.6%; P=0.007) and a lower number of hospitalizations in the previous year (0.5 admissions, 0.9 SD vs 0.7 admissions, 1.1 SD; P=0.035). No differences were observed in regard to gender, ethnicity, history of myocardial infarctions, chronic obstructive pulmonary disease, cerebrovascular disease, peripheral vascular disease, diabetes, and use of anticholinergic medicines.
| No GCS, n=239 | GCS, n=176 | P Value* | |
|---|---|---|---|
| |||
| Baseline characteristics | |||
| Mean age (SD) | 75.8 (7.8) | 79.2 (8.1) | <0.001 |
| % Female | 66.1 [n=158] | 68.2 [n=120] | 0.657 |
| % African American | 54.8 [n=131] | 63.6 [n=112] | 0.071 |
| Mean SPMSQ score (SD) | 5.5 (2.7) | 4.7 (2.7) | 0.002 |
| Admission diagnoses | |||
| MI | 15.5 [n=37] | 13.6 [n=24] | 0.675 |
| CHF | 38.1 [n=91] | 34.7 [n=61] | 0.475 |
| PVD | 7.1 [n=17] | 9.7 [n=17] | 0.370 |
| Cerebrovascular | 13.8 [n=33] | 19.3 [n=34] | 0.140 |
| COPD | 41.0 [n=98] | 33.0 [n=58] | 0.094 |
| Diabetes | 47.7 [n=114] | 40.9 [n=72] | 0.169 |
| Malignancy | 14.6 [n=35] | 6.2 [n=11] | 0.007 |
| Metastatic cancer | 8.8 [n=21] | 1.7 [n=3] | 0.002 |
| Mean Charlson Comorbidity (SD) | 2.8 (2.6) | 2.1 (1.8) | 0.023 |
| Mean APS (SD) | 24.5 (13.8) | 25.9 (13.5) | 0.231 |
| Definite ACB Use | 35.2 [n=84] | 27.8 [n=49] | 0.136 |
| Length of stay | 5.6 (5.9) | 6.4 (6.4) | 0.004 |
| % Any delirium | 29.3 [n=70] | 48.9 [n=156] | <0.001 |
| % Discharged home | 56.1 [n=134] | 37.5 [n=66] | <0.001 |
| No. of inpatient stays prior year | 0.7 (1.1) | 0.5 (0.9) | 0.035 |
| Follow‐up outcomes | |||
| % Readmission within 30 days | 15.1 [n=36] | 22.7 [n=40] | 0.054 |
| % Readmission within 1 year | 54.4 [n=130] | 56.3 [n=99] | 0.765 |
| % Death within 30 days | 4.2 [n=10] | 1.7 [n=3] | 0.253 |
| % Death within 1 year | 26.8 [n=64] | 23.9 [n=42] | 0.569 |
| % Readmission or death within 30 days | 18.0 [n=43] | 24.4 [n=43] | 0.113 |
| % Readmission or death within 1 year | 64.8 [n=155] | 63.1 [n=111] | 0.708 |
Table 2 describes the association of various factors with receiving GCS. Patients who were positive for delirium (odds ratio [OR]=1.65; 95% confidence interval=0.98‐2.77) and were older (OR=1.04; 95% confidence interval=1.01‐1.08) had a higher propensity to receive GCS, whereas, the presence of metastatic cancer resulted in a lower propensity (OR=0.15; 95% confidence interval=0.02‐1.16) of receiving GCS. The logistic model estimated area under the receiver operating characteristic curve was 0.707.
| Adjusted OR (95% CI) | P Value | |
|---|---|---|
| ||
| Age | 1.04 (1.011.08) | 0.006 |
| Female | 1.02 (0.641.63) | 0.942 |
| African American | 1.11 (0.711.72) | 0.657 |
| Short Portable Mental Status Questionnaire score | 1.00 (0.911.10) | 0.990 |
| Acute Physiology Score | 1.00 (0.981.02) | 0.769 |
| Charlson Comorbidity Score | 1.11 (0.841.46) | 0.471 |
| Length of hospital stay | 1.02 (0.981.07) | 0.299 |
| Definite anticholinergic use* | 0.74 (0.461.20) | 0.219 |
| Any delirium during hospital stay | 1.65 (0.982.77) | 0.061 |
| Diabetes mellitus | 0.72 (0.411.26) | 0.253 |
| Myocardial infarction | 0.83 (0.411.66) | 0.593 |
| Congestive heart failure | 0.83 (0.471.47) | 0.524 |
| Peripheral vascular disease | 1.39 (0.613.18) | 0.433 |
| Cerebrovascular disease | 1.30 (0.652.59) | 0.464 |
| Malignancy | 0.45 (0.171.21) | 0.113 |
| Metastatic cancer | 0.15 (0.021.16) | 0.069 |
| Chronic obstructive pulmonary disease | 0.91 (0.531.55) | 0.727 |
Table 3 provides results from the Cox models for receiving GCS on readmission and mortality outcomes adjusting for various sets of covariates and with the propensity score adjustment. Model 1 presents unadjusted hazard ratio (HR). Model 2 presents HRs adjusting for a common set of covariates that were significantly associated with at least 1 of the outcomes, whereas model 3 presents the results adjusting for all covariates. All 4 models yielded similar results. As evident from this table, propensity‐adjusted HR for 30‐day readmission was still significantly higher among patients receiving GCS (HR=1.75; 95% confidence interval=1.06‐2.88) but not at 1 year (HR=1.19; 95% confidence interval=0.89‐1.59). There was a trend for decreased mortality for the GCS group at 30 days (HR=0.35; 95% confidence interval=0.09‐1.35), but it disappeared at 1 year (HR=0.91; 95% confidence interval=0.59‐1.40). A composite outcome of readmissions and mortality did not show any difference between the GCS and no‐GCS groups.
| Outcome Variables | Model 1 | Model 2 | Model 3 | Propensity Adjusted | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| ||||||||
| Readmission within 30 days | 1.65 (1.05, 2.59) | 0.030 | 1.73 (1.08, 2.78) | 0.024 | 1.84 (1.133.00) | 0.015 | 1.75 (1.062.88) | 0.029 |
| Readmission within 1 year | 1.13 (0.87, 1.46) | 0.373 | 1.24 (0.94, 1.63) | 0.125 | 1.26 (0.941.68) | 0.117 | 1.19 (0.891.59) | 0.245 |
| Death within 30 days | 0.43 (0.12, 1.56) | 0.199 | 0.34 (0.09, 1.28) | 0.110 | 0.25 (0.061.02) | 0.053 | 0.35 (0.091.35) | 0.126 |
| Death within 1 year | 0.95 (0.65, 1.45) | 0.806 | 0.87 (0.58 1.31) | 0.506 | 0.93 (0.601.42) | 0.724 | 0.91 (0.591.40) | 0.669 |
| Readmission or Death within 30 days | 1.48 (0.97, 2.26) | 0.070 | 1.49 (0.96, 2.33) | 0.078 | 1.56 (0.982.47) | 0.061 | 1.55 (0.972.48) | 0.069 |
| Readmission or death within 1 year | 1.05 (0.82, 1.34) | 0.699 | 1.11 (0.86, 1.43) | 0.412 | 1.15 (0.881.50) | 0.318 | 1.08 (0.831.42) | 0.569 |
DISCUSSION
To our knowledge, this is the first study to analyze the impact of GCS on hospital readmission and mortality rates of CI patients. Our results did not show any short‐term or long‐term benefits of GCS for CI patients. Recent studies exploring cost benefits of the GCS have found trends toward lower readmission, but none focused on patients with CI.[6, 24, 25] It is important to note that our study did not use random allocation to assigning the patient into the GCS or control group, thus raising the possibility that patients who received GCS were sicker and were medically and socially more complex than those who did not receive the consult. Moreover, GCS consultation is preferentially sought for and completed for patients with CI and functional limitations, consistent with our finding that GCS patients more often have delirium and are less‐often discharged home.
The nature of the GCS team is another important consideration. Our GCS model did not include unit cohorting of patients, an important component of other proposed GCS models.[26] A recent meta‐analysis found that the GCS models without unit cohorting of patients did not have an impact on 1‐ or 12‐month readmission rates.[27] Low adherence to consultant recommendations (less than 33%) was thought to be a reason for such results. Importance of cohorting with regard to accomplishing recommendations by primary teams, importance of unit staff expertise in geriatric principles, and impact of a unit model on teamwork has also been highlighted by another review.[28] These findings lend to the hypothesis that unit cohorting and direct order placement by the GCS team may improve outcomes among CI patients, including a reduction in readmission rates.
Although readmissions rates were not statistically different between GCS and control groups at 1‐year postdischarge, 30‐day readmission rates were higher among the GCS group. Previous research among older heart failure patients found that a comprehensive transitional care intervention at the time of hospital discharge significantly shortened the time to readmission in the intervention group (P=0.026).[29] The factors identified by the study authors included enhanced supervision by the transitional healthcare teams along with improved awareness and education among treated patients that may have facilitated early recognition of clinical deterioration.[29] A recent study with intensive outpatient care that resulted in increased admissions among chronically ill adults provided a similar conclusion.[30]
GCS patients showed a trend toward decreased mortality as did patients enrolled in previous studies evaluating GCS models in the inpatient setting, as suggested by a recent review.[27] A caveat to note is that these trends favored ward‐styled GCS services as compared to our open GCS model,[27, 28] although the factors cited in these dedicated units affecting mortality included prompt attention to early rehabilitation, delirium management, and prevention of pressure ulcers and are also frequently implemented for patients in our GCS service model and therefore may have produced similar results.
Our neutral results in regard to the readmissions need to be interpreted with caution. First, this study was conducted in a hospital that supports expert geriatric and palliative care teams, both in the inpatient and the ambulatory settings, that provide consultative services and train medicine teams and hospital nursing staff. On the outpatient side, the presence of a robust geriatrics house‐calls program and the Geriatric Resources for Assessment and Care of Elders team results in above‐average care for the control group, and thus may also impact apparent outcomes.[31, 32] Second, 30‐day readmissions represent a complex outcome. Two recent reviews of hospital‐initiated interventions have shown that evidence regarding best strategies to decrease 30‐day readmissions is unclear.[33] Neither review included studies that targeted patients with CI only. The 2 programs that reduced 30‐day readmissions were multifaceted and included personnel who provide bridging between the hospital and the outpatient setting.[34] The GCS does include a focus on postdischarge resources, but does that on a case‐by‐case basis and no formal posthospital follow‐ups are provided. Moreover, the value of 30‐day readmission rates as a marker of quality, even though used by policymakers as an indicator of hospital quality, remains controversial.[35, 36] Broadening the outcomes of interest to include patient‐centered outcomes including satisfaction with care, that have shown to impact other health outcomes, may help improve understanding the benefits of GCS in hospitals.[37] Other comprehensive transitional care models that failed to show a benefit on 30‐day readmissions in older patients still resulted in higher satisfaction among patients.[38] Unfortunately, our evaluation did not include an assessment of patient satisfaction and quality of transitions.
Since the study period, GCS at our hospital now has incorporated a more robust focus on advance care planning (ACP) and execution of Physician Orders for Scope of Treatment that were legislated in the state in July 2013. The GCS team members are expert in carrying out complex ACP discussions and also partner with the inpatient palliative care team. It is quite possible that a study of more recent outcomes will yield more positive results for the selected outcomes. Thus, for future trials that aim to study the impact of GCS in the inpatient settings, it may be advisable to include important quality markers such as implementation of ACP and patient satisfaction along with the health utilization outcomes.
Limitations
As mentioned previously, it is possible that our risk adjustment was insufficient to account for all the medical and psychosocial differences among groups. For example, the overall anticholinergic impact of various medications such as antipsychotic medications and histamine‐2 blockers was assessed via the Anticholinergic Burden Scale on admission, but we did not have information on medication prescribing during the stay. We were further limited by lack of baseline functional status and socioeconomic details, both of which are related to 30‐day readmissions. For example, living alone, prior use of assist devices, and belonging to lower socioeconomic status are correlated with higher readmission rates.[39, 40] Patients with available social support may receive more intense supervision and may seek medical attention sooner. On the other hand, worsening health among CI patients without any approximate social support may be unnoticed for days. Absence of details of inpatient interventions may also have resulted in unmeasurable confounders that could have impacted our study outcomes. Finally, lack of information on the uptake of GCS recommendations by the primary teams is another limitation of this analysis. Future trials should include strategies to address these information gaps.
CONCLUSION
Our results comparing inpatient geriatrics consultative services with usual care in hospitalized elders having cognitive impairment failed to demonstrate an impact on readmissions and mortality. A clinical lesson learned, however, is that much work is still required to reduce readmission and mortality rates in this especially vulnerable patient population.
Disclosures
Disclosures: This work was supported by grants from a Geriatric Academic Career Award (K01HP20517) through Health Resources and Services Administration, R01AG034205 and K23‐AG043476 from the National Institute on Aging, and the John A. Hartford Foundation Center for Excellence in Geriatric Medicine. The sponsors had no role in the study design, evaluation, or manuscript development. The authors report no conflicts of interest.
- , , , et al. Relationship between hospital readmission and mortality rates for patients hospitalized with acute myocardial infarction, heart failure, or pneumonia. JAMA. 2013;309(6):587–593.
- , , , Cognitive impairment. Can it predict the course of hospitalized patients? J Am Geriatr Soc. 1986;34(8):579–585.
- , , , , , Importance of functional measures in predicting mortality among older hospitalized patients. JAMA. 1998;279(15):1187–1193.
- , Understanding preventable hospital readmissions: masqueraders, markers, and true causal factors. J Hosp Med. 2011;6(2):51–53.
- , , , et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340(9):669–676.
- , , , et al. Effects of a multicomponent intervention on functional outcomes and process of care in hospitalized older patients: a randomized controlled trial of Acute Care for Elders (ACE) in a community hospital. J Am Geriatr Soc. 2000;48(12):1572–1581.
- , ; American Geriatrics Society Health Care Systems Committee. Improving the quality of transitional care for persons with complex care needs. J Am Geriatr Soc. 2003;51(4):556–557.
- , , , Development and implementation of a proactive geriatrics consultation model in collaboration with hospitalists. J Am Geriatr Soc. 2009;57(11):2139–2145.
- , , , et al. Screening of the risk of functional decline performed by an inpatient geriatric consultation team in a general hospital [in French]. Revue medicale de Bruxelles. 2013;34(6):462–468.
- , , , et al. Systematic detection and multidisciplinary care of delirium in older medical inpatients: a randomized trial. CMAJ. 2002;167(7):753–759.
- , , Potentially inappropriate prescribing for geriatric inpatients: an acute care of the elderly unit compared to a general medicine service. Consult Pharm. 2003;18(1):37–42, 47–39.
- , , , Evaluation of the Mobile Acute Care of the Elderly (MACE) service. JAMA Intern Med. 2013;173(11):990–996.
- , , , , , Effects of an acute care for elders unit on costs and 30‐day readmissions. JAMA Intern Med. 2013;173(11):981–987.
- , , , et al. Impact and recognition of cognitive impairment among hospitalized elders. J Hosp Med. 2010;5(2):69–75.
- , , , et al. Enhancing care for hospitalized older adults with cognitive impairment: a randomized controlled trial. J Gen Intern Med. 2012;27(5):561–567.
- , , , Short Portable Mental Status Questionnaire as a screening test for dementia and delirium among the elderly. J Am Geriatr Soc. 1987;35(5):412–416.
- , , , et al. The Regenstrief Medical Record System: a quarter century experience. Int J Med Inform. 1999;54(3):225–253.
- , , , , , Factors determining the decision to institutionalize dementing individuals: a prospective study. Gerontologist. 1993;33(6):714–720.
- , , , , , Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941–948.
- , , , , , Resuscitation: how do we decide? A prospective study of physicians' preferences and the clinical course of hospitalized patients. JAMA. 1986;255(10):1316–1322.
- , , , et al. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991;100(6):1619–1636.
- , , , et al. Interaction between cognitive impairment and discharge destination and its effect on rehospitalization. J Am Geriatr Soc. 2013;61(11):1958–1963.
- , , , , , Propensity scores in intensive care and anaesthesiology literature: a systematic review. Intensive Care Med. 2010;36(12):1993–2003.
- , , , et al. Improving functional outcomes in older patients: lessons from an acute care for elders unit. Jt Comm J Qual Improv. 1998;24(2):63–76.
- , , , et al. Developing a stroke unit using the acute care for elders intervention and model of care. J Am Geriatr Soc. 2003;51(11):1660–1667.
- , , , A medical unit for the acute care of the elderly. J Am Geriatr Soc. 1994;42(5):545–552.
- , , , , Impact of geriatric consultation teams on clinical outcome in acute hospitals: a systematic review and meta‐analysis. BMC Med. 2013;11:48.
- , , , , Comprehensive geriatric assessment for older adults admitted to hospital: meta‐analysis of randomised controlled trials. BMJ. 2011;343:d6553.
- , , , et al. Prevention of readmission in elderly patients with congestive heart failure: results of a prospective, randomized pilot study. J Gen Intern Med. 1993;8(11):585–590.
- , , , et al. Effect of a pharmacist intervention on clinically important medication errors after hospital discharge: a randomized trial. Ann Intern Med. 2012;157(1):1–10.
- , , , , House calls for seniors: building and sustaining a model of care for homebound seniors. J Am Geriatr Soc. 2009;57(6):1103–1109.
- , , , et al. Geriatric care management for low‐income seniors: a randomized controlled trial. JAMA. 2007;298(22):2623–2633.
- , , , , Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520–528.
- , The effects of a discharge planning and home follow‐up intervention on elders hospitalized with common medical and surgical cardiac conditions. J Cardiovasc Nurs. 1999;14(1):44–54.
- Readmission to hospital: a measure of quality or outcome? Qual Saf Health Care. 2004;13(1):10–11.
- , , , , , Unintended consequences of steps to cut readmissions and reform payment may threaten care of vulnerable older adults. Health Aff (Millwood). 2012;31(7):1623–1632.
- , , Analyzing the effects of shared decision‐making, empathy and team interaction on patient satisfaction and treatment acceptance in medical rehabilitation using a structural equation modeling approach. Patient Educ Couns. 2013;91(2):167–175.
- , , , The Care Transitions Innovation (C‐TraIn) for Socioeconomically Disadvantaged Adults: results of a cluster randomized controlled trial. J Gen Intern Med. 2014;29(11):1460–1467.
- , , , Risk factors for early hospital readmission in low‐income elderly adults. J Am Geriatr Soc. 2014;62(3):489–494.
- , , , , , Understanding why patients of low socioeconomic status prefer hospitals over ambulatory care. Health Aff (Millwood). 2013;32(7):1196–1203.
- , , , et al. Relationship between hospital readmission and mortality rates for patients hospitalized with acute myocardial infarction, heart failure, or pneumonia. JAMA. 2013;309(6):587–593.
- , , , Cognitive impairment. Can it predict the course of hospitalized patients? J Am Geriatr Soc. 1986;34(8):579–585.
- , , , , , Importance of functional measures in predicting mortality among older hospitalized patients. JAMA. 1998;279(15):1187–1193.
- , Understanding preventable hospital readmissions: masqueraders, markers, and true causal factors. J Hosp Med. 2011;6(2):51–53.
- , , , et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340(9):669–676.
- , , , et al. Effects of a multicomponent intervention on functional outcomes and process of care in hospitalized older patients: a randomized controlled trial of Acute Care for Elders (ACE) in a community hospital. J Am Geriatr Soc. 2000;48(12):1572–1581.
- , ; American Geriatrics Society Health Care Systems Committee. Improving the quality of transitional care for persons with complex care needs. J Am Geriatr Soc. 2003;51(4):556–557.
- , , , Development and implementation of a proactive geriatrics consultation model in collaboration with hospitalists. J Am Geriatr Soc. 2009;57(11):2139–2145.
- , , , et al. Screening of the risk of functional decline performed by an inpatient geriatric consultation team in a general hospital [in French]. Revue medicale de Bruxelles. 2013;34(6):462–468.
- , , , et al. Systematic detection and multidisciplinary care of delirium in older medical inpatients: a randomized trial. CMAJ. 2002;167(7):753–759.
- , , Potentially inappropriate prescribing for geriatric inpatients: an acute care of the elderly unit compared to a general medicine service. Consult Pharm. 2003;18(1):37–42, 47–39.
- , , , Evaluation of the Mobile Acute Care of the Elderly (MACE) service. JAMA Intern Med. 2013;173(11):990–996.
- , , , , , Effects of an acute care for elders unit on costs and 30‐day readmissions. JAMA Intern Med. 2013;173(11):981–987.
- , , , et al. Impact and recognition of cognitive impairment among hospitalized elders. J Hosp Med. 2010;5(2):69–75.
- , , , et al. Enhancing care for hospitalized older adults with cognitive impairment: a randomized controlled trial. J Gen Intern Med. 2012;27(5):561–567.
- , , , Short Portable Mental Status Questionnaire as a screening test for dementia and delirium among the elderly. J Am Geriatr Soc. 1987;35(5):412–416.
- , , , et al. The Regenstrief Medical Record System: a quarter century experience. Int J Med Inform. 1999;54(3):225–253.
- , , , , , Factors determining the decision to institutionalize dementing individuals: a prospective study. Gerontologist. 1993;33(6):714–720.
- , , , , , Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941–948.
- , , , , , Resuscitation: how do we decide? A prospective study of physicians' preferences and the clinical course of hospitalized patients. JAMA. 1986;255(10):1316–1322.
- , , , et al. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991;100(6):1619–1636.
- , , , et al. Interaction between cognitive impairment and discharge destination and its effect on rehospitalization. J Am Geriatr Soc. 2013;61(11):1958–1963.
- , , , , , Propensity scores in intensive care and anaesthesiology literature: a systematic review. Intensive Care Med. 2010;36(12):1993–2003.
- , , , et al. Improving functional outcomes in older patients: lessons from an acute care for elders unit. Jt Comm J Qual Improv. 1998;24(2):63–76.
- , , , et al. Developing a stroke unit using the acute care for elders intervention and model of care. J Am Geriatr Soc. 2003;51(11):1660–1667.
- , , , A medical unit for the acute care of the elderly. J Am Geriatr Soc. 1994;42(5):545–552.
- , , , , Impact of geriatric consultation teams on clinical outcome in acute hospitals: a systematic review and meta‐analysis. BMC Med. 2013;11:48.
- , , , , Comprehensive geriatric assessment for older adults admitted to hospital: meta‐analysis of randomised controlled trials. BMJ. 2011;343:d6553.
- , , , et al. Prevention of readmission in elderly patients with congestive heart failure: results of a prospective, randomized pilot study. J Gen Intern Med. 1993;8(11):585–590.
- , , , et al. Effect of a pharmacist intervention on clinically important medication errors after hospital discharge: a randomized trial. Ann Intern Med. 2012;157(1):1–10.
- , , , , House calls for seniors: building and sustaining a model of care for homebound seniors. J Am Geriatr Soc. 2009;57(6):1103–1109.
- , , , et al. Geriatric care management for low‐income seniors: a randomized controlled trial. JAMA. 2007;298(22):2623–2633.
- , , , , Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520–528.
- , The effects of a discharge planning and home follow‐up intervention on elders hospitalized with common medical and surgical cardiac conditions. J Cardiovasc Nurs. 1999;14(1):44–54.
- Readmission to hospital: a measure of quality or outcome? Qual Saf Health Care. 2004;13(1):10–11.
- , , , , , Unintended consequences of steps to cut readmissions and reform payment may threaten care of vulnerable older adults. Health Aff (Millwood). 2012;31(7):1623–1632.
- , , Analyzing the effects of shared decision‐making, empathy and team interaction on patient satisfaction and treatment acceptance in medical rehabilitation using a structural equation modeling approach. Patient Educ Couns. 2013;91(2):167–175.
- , , , The Care Transitions Innovation (C‐TraIn) for Socioeconomically Disadvantaged Adults: results of a cluster randomized controlled trial. J Gen Intern Med. 2014;29(11):1460–1467.
- , , , Risk factors for early hospital readmission in low‐income elderly adults. J Am Geriatr Soc. 2014;62(3):489–494.
- , , , , , Understanding why patients of low socioeconomic status prefer hospitals over ambulatory care. Health Aff (Millwood). 2013;32(7):1196–1203.
© 2015 Society of Hospital Medicine
Delirium in Hospitalized Patients
Delirium is a syndrome of disturbance of consciousness, with reduced ability to focus, sustain, or shift attention, that occurs over a short period of time and fluctuates over the course of the day.1 It encompasses a variety of cognitive, behavioral, and psychological symptoms including inattention, short‐term memory loss, sleep disturbances, agitated behaviors, delusions, and visual hallucinations.2 Delirium complicates the care of 70% to 80% of mechanically ventilated patients in intensive care units (ICUs).3 Of 13 million patients aged 65 and older hospitalized in 2002, 10% to 52% had delirium at some point during their admission.4, 5
Patients experiencing delirium have a higher probability of death during their hospital stay, adjusted for age, gender, race, and comorbidities.3, 6, 7 They are more vulnerable to hospital‐acquired complications leading to prolonged ICU and hospital stay, new institutionalization, and higher healthcare costs.3, 6, 7 Even with such a range of poor outcomes, the rates of delirium recognition are low,8 resulting in inadequate management.9 There has been considerable growth in the number of articles published on delirium in recent years. Therefore, it is of value to provide a state‐of‐the‐art summary of robust evidence in the field to healthcare personnel and delirium investigators.
We systematically reviewed the literature to identify published systematic evidence reviews (SERs), which evaluated the evidence on delirium risk factors, diagnosis, pathogenesis, prevention, treatment, and outcomes. We then summarized the data from the methodologically sound SERs to provide the reader with a clinically oriented summary of delirium literature for patient care. We also identify current gaps in delirium literature, and present future directions for delirium investigators to design studies that will enhance delirium care.
DATA SOURCES AND REVIEW METHODS
The domains of risk factors, diagnosis, pathophysiology, prevention, treatment, and outcomes were selected a priori to capture all relevant SERs regarding delirium based on the framework suggested by the American Delirium Society task force.10 To maximize article retrieval, a 3‐step search strategy was applied. First, we searched the electronic database utilizing OVID Medline, PubMed, the Cochrane Library, and Cumulative Index of Nursing and Allied Health Literature (CINAHL) using the following delirium‐specific search terms: delirium, confusion, agitation, mental status change, inattention, encephalopathy, organic mental disorders, and disorientation. We combined the above terms with the following study design terms: technical report, systematic evidence review, systematic review, meta‐analysis, editorial, and clinical reviews. We limited our search to human subjects. We excluded studies that: a) enrolled patients aged <18; b) enrolled patients with current or past Diagnostic and Statistical Manual of Mental Disorders (DSM) Axis I psychotic disorders; c) did not have standardized delirium evaluation; d) evaluated alcohol or substance abuse‐related delirium; e) did not use a systematic search method for identifying delirium‐related articles; and f) evaluated delirium sub‐types. We searched articles published from January 1966 through April 2011. Second, a manual search of references of the retrieved papers plus an Internet search using Google Scholar was conducted to find additional SERs. Titles and abstracts were screened by 2 reviewers (B.A.K., M.Z.). Authors of the included studies were contacted as necessary. Third, a library professional at the Indiana University School of Medicine independently performed a literature search, and those results were compared with our search to retrieve any missing SERs.
The methodological quality of each SER was independently assessed by 2 reviewers (B.A.K., M.Z.) using the United States Preventive Services Task Force (USPSTF) Critical Appraisal for SER.11 This scale assesses parameters that are critical to the scientific credibility of an SER and categorizes the SER as poor, fair, or good (Table 1). The 2 reviewers (B.A.K., M.Z.) used a data extraction form to record the following information from each SER: primary author, publication year, number and type of studies, number of participants and their mean age, study population, method for delirium diagnosis, risk factors, preventive and therapeutic interventions, and outcomes. Any disagreement between reviewers in SER selection, data extraction, or SER appraisal was resolved through discussion with a third reviewer (M.A.B.). The conflicting findings among SERs were resolved by consensus and by including the findings from a good SER over a fair SER.
| Criteria | Rating Definition |
|---|---|
| Recent, relevant review with comprehensive sources and search strategies | Good: If all the criteria are met |
| Explicit and relevant selection criteria | |
| Standard appraisal of included studies | |
| Valid conclusion | |
| Recent, relevant review that is not clearly biased but lacks comprehensive sources and search strategies | Fair: If this criterion is met |
| Outdated, irrelevant, or biased review | Poor: If one or more of the criteria are met |
| There is no systematic search for studies | |
| There are no explicit selection criteria | |
| There is no standard appraisal of studies |
RESULTS
Our search yielded 76,060 potential citations, out of which we identified 38 SERs meeting our inclusion criteria (Table 2). Figure 1 outlines our search strategy. Based on the USPSTF criteria, 22 SERs graded as good or fair provided the data to establish our review.
| Author (Year) | Studies (n)/ Participants (n) | Mean Age (Years) | Study Type | Service | Delirium/Cognition Assessment Scales | Review Objectives* | Rating |
|---|---|---|---|---|---|---|---|
| |||||||
| Van Rompaey et al15 (2008) | 6/7,114 | 61.2 | Prospective cohort, retrospective analysis | ICU (medical, surgical, coronary, mixed) | CAM‐ICU, psychiatric interview, ICU delirium screening checklist | 1/Risk factors | Fair |
| Bryson and Wyand13 (2006) | 18/3,473 | 71.93 | RCT | Surgery | MattisKovner Verbal Recall and Recognition, GDS, DST, DSM‐III, AMT, PRT, FOMTL, DCT, FPU, GEMS, WAIS‐R, Meta Memory Questionnaire, National Adult Reading Test | 1/Risk factors | Good |
| Fong et al14 (2006) | 9/1,078 | 63.1 | RCT, case control, prospective cohort, retrospective cohort | Surgery | CAM, DSM‐III, MMSE, SPMSQ, Digit Symbol Substitution Test, Trailmaking B Test | 1/Risk factors | Fair |
| Adamis et al53 (2009) | 6/882 | 54.59 | Case control | Medicine, ICU, surgery | CAM, DRS, DSM‐III‐R, DSM‐IV, ICD‐10 | 1/Risk factors | Poor |
| Balasundaram and Holmes12 (2007) | 4/364 | 66.8 | Prospective cohort | Surgery | CAM, DRS, HDS‐R, DSM‐IV | 1/Risk factors | Good |
| Dasgupta and Dumbrell49 (2006) | 25/5,175 | 72.5 | Prospective observational | Surgery | CAM, DSM‐III/IV | 1/Risk factors | Poor |
| Elie et al50 (1998) | 27/1,365 | 75.7 | Prospective | Medicine, surgery, psychiatry | CAM, NFRD, MMSE, MSQ, SPMSQ | 1/Risk factors | Poor |
| Van Munster et al52 (2009) | 5/1,099 | 77.86 | Cohort | Medicine, surgery | CAM, DRS | 1/Risk factors | Poor |
| Van der Mast and Roest51 (1996) | 57/6,129 | 48.2 | Prospective control, retrospective | Surgery | Psychiatric interview, chart review for signs of delirium, DSM‐III, MMSE | 1/Risk factors | Poor |
| Campbell et al16 (2009) | 27/8,492 | 71.35 | Longitudinal cohort, cross‐sectional, case control | Medicine, surgery, ICU, psychiatry | CAM, CAM‐ICU, DSI, DSM‐III/III‐R/IV, SDC, MMSE, Verbal N‐Back Test, BCRS, WMS | 1/Risk factors | Fair |
| Soiza et al17 (2008) | 12/764 | 72.4 | Cohort, case control, case series | Medicine, ICU, psychiatry | CAM, DSM‐III/III‐R/IV | 1/Risk factors | Good |
| Michaud et al9 (2007) | 29/NA | 76.7 | RCT, cohort | Medicine, surgery | CAM, BOMC, DRS, MDAS, ICD‐10, DSM‐IV, MMSE | 1/Risk factors, 2/Diagnosis, 4/Prevention, 5/Treatment | Fair |
| Steis and Fick54 (2008) | 10/3,059 | 72.5 | Prospective clinical trials, retrospective, observational, case study | Medicine, surgery, ICU | DSM‐III/IV | 2/Diagnosis | Poor |
| Wei et al20 (2008) | 7/1,071 | 70.17 | Validation, adaptation, translation, application | ICU, ED, medicine, surgery | CAM, CAM‐ICU, DSM‐IV, NH‐CAM, DI | 2/Diagnosis | Good |
| Wong et al18 (2010) | 25/3,027 | 72.76 | Prospective clinical studies | Medicine, surgery | CAC, CAM, DOSS, DRS, DRS‐R‐98, Digit Span Test, GAR, MDAS, MMSE, Nu‐DESC, Vigilance A Test | 2/Diagnosis | Fair |
| Devlin et al55 (2007) | 12/2,106 | 61.8 | Validation studies | ICU | CAM, ICDSC, CTD, ROC, DSM‐III/IV, DDS, MMSE | 2/Diagnosis | Poor |
| Fick et al47 (2002) | 14/7,701 | 79.51 | Prospective cohort, retrospective cohort, cross‐sectional, clinical trials | Medicine, surgery, ED | CAM, DRS, DSM‐III/III‐R/IV, CERAD, NINCDS‐ADRDA, IQCODE, MMSE | 2/Diagnosis, 4/Prevention, 6/Prognosis | Fair |
| Siddiqi et al46 (2006) | 40/12,220 | 78.8 | Prospective cohort, cross‐sectional, case‐controlled trials | Medicine | CAM, DRS, MDAS, SPMSQ, DSM‐III/III‐R/IV, MSQ, MMSE,BPRS, IQCODE, GHQ BAS | 2/Diagnosis, 6/Prognosis | Fair |
| 28/4,915 | |||||||
| Hall et al21 (2011) | 5/315 | 71.13 | Prospective cohort | Medicine, surgery, psychogeriatric | DSM‐III/III‐R/IV, MMSE, DRS, CAM, IQCODE, GDS | 3/Pathophysiology | Good |
| Cole et al56 (1996) | 10/999 | 71.6 | Randomized and nonrandomized trials | Medicine, surgery | DSM‐III, SPMSQ | 4/Prevention | Poor |
| Siddiqi et al25 (2007) | 6/833 | 76.67 | RCT | Surgery | CAM, DRS‐R‐98, DSM‐III/IV, DSI, MDAS, AMT, MMSE, OBS | 4/Prevention | Good |
| Campbell et al27 (2009) | 13/1,305 | 65.8 | RCT | Medicine, surgery, ICU | MDAS, DRS‐R‐98 | 4/Prevention, 5/Treatment | Good |
| Weber et al41 (2004) | 13/1,650 | 73.99 | RCT, non‐RCT, clinical trials, meta‐analysis, case report | Medicine, surgery | CAM, MDAS, DSI, DRS, DSM‐III‐R/IV, MMSE | 4/Prevention, 5/Treatment | Fair |
| Milisen et al22 (2005) | 7/1,683 | 80.73 | RCT, controlled trials, beforeafter study | Medicine, surgery | CAM, DSM‐III, SPMSQ, MMSE | 4/Prevention, 5/Treatment | Good |
| Lonergan et al39 (2009) | 3/629 | 74.5 | RCT | Medicine, surgery | CAM, DRS, DRS‐R‐98, MDAS, CGI, DSM‐IV | 5/Treatment | Good |
| Jackson and Lipman40 (2004) | 1/30 | 39.2 | RCT | Medicine | DRS, DSM‐III‐R | 5/Treatment | Good |
| Lonergan et al42 (2009) | 1/106 | 54.5 | RCT | ICU | CAM‐ICU | 5/Treatment | Good |
| Bourne et al57 (2008) | 33/1,880 | 60.99 | RCT, prospective trials, comparative trials | Medicine, surgery | DRS | 4/Prevention, 5/Treatment | Poor |
| Bitsch et al58 (2004) | 12/1,823 | 79.02 | Prospective, descriptive | Surgery | CAM, MDAS, DSI, OBS, MMSE | 4/Prevention, 5/Treatment | Poor |
| Overshott et al43 (2008) | 1/80 | 67 | RCT | Surgery | CAM, DSI, DSM‐IV, MMSE | 5/Treatment | Good |
| Lacasse et al59 (2006) | 4/158 | 60.8 | RCT | Medicine, surgery | CAM, DRS‐R‐98, MDAS, DI, DSM‐III‐R/IV, MMSE | 5/Treatment | Poor |
| Peritogiannis et al60 (2009) | 23/538 | 62.84 | RCT, retrospective, open label | Medicine, surgery | DRS, DRS‐R‐98, DRS‐R‐98‐J, MDAS, DI, 10‐Point Visual Analog Scale | 5/Treatment | Poor |
| Seitz et al38 (2007) | 14/448 | 63.09 | Prospective | Medicine, surgery, ICU | DSM‐III/III‐R/IV/IV‐TR, CAM, DRS‐R‐98, MDAS, DI | 5/Treatment | Good |
| Britton and Russell37 (2001/2004) | 1/227 | 82.35 | RCT | Medicine | CAM, SPMSQ, DSM‐III‐R, MMSE | 5/Treatment | Good |
| Jackson et al6 (2004) | 9/1,885 | 77.68 | Prospective, descriptive | Medicine, surgery, ICU, psychiatry | CAM, CAM‐ICU, DRS, MMSE, DSM | 6/Prognosis | Poor |
| Cole et al44 (2009) | 18/1,322 | 81.3 | Prospective cohort | Medicine, surgery | CAM, DSM‐III/III‐R/IV, ICD‐10, OBS | 6/Prognosis | Good |
| Witlox et al45 (2010) | 42/5,777 | 79.96 | Observational | Medicine, surgery | DSM, patient interview | 6/Prognosis | Good |
| Cole and Primeau61 (1993) | 8/573 | 77.25 | Prospective trials | Medicine, surgery, psychiatry | DSM‐I/III | 6/Prognosis | Poor |
1: What Are the Risk Factors for Development of Delirium in Hospitalized Patients?
We found 6 SERs1217 that evaluated risk factors for the development of delirium. Three reviews included only surgical patients,1214 1 focused on the intensive care unit (ICU),15 and the remaining 2 had both medical and surgical patients.16, 17 Risk factors identified in an elective vascular surgery population were age >64, preoperative cognitive impairment, depression, intraoperative blood transfusions, and previous amputation.12 The risk of incident delirium conferred by general anesthesia compared to regional anesthesia in non‐cardiac surgery patients was not significantly different among both groups.13 One SER14 focused on the effects of different opioid analgesics on postoperative delirium, and whether route of administration of medicines (intravenous vs epidural) had any impact on delirium. Mepiridine was consistently associated with an increased risk of delirium in elderly surgical patients, but there were no significant differences in postoperative delirium rates among those receiving morphine, fentanyl, or hydromorphone. The rates of delirium did not differ significantly between intravenous and epidural routes of analgesic administration, except in one study where epidural route had more delirium cases, but in 85% of those cases, mepiridine was used as an epidural agent. Risk factors explored in an ICU setting found multiple predisposing and precipitating risk factors, with the surprising finding that age was not a strong predictor of delirium.15 An association between delirium and drugs with anticholinergic properties was found in 1 SER.16 There was no causal relationship between structural or functional neuroimaging findings and delirium development.17
2: What Is the Clinical Utility of Bedside Tools in Delirium Diagnosis?
The accuracy of bedside instruments in diagnosing delirium was assessed in an SER of 25 prospective studies.18 Among the 11 scales reviewed, the Confusion Assessment Method (CAM) had the most evidence supporting its use as a bedside tool (+likelihood ratio [LR], 9.6; 95% CI [confidence interval], 5.816.0; LR, 0.16; 95% CI, 0.090.29). The Folstein mini‐mental status examination (MMSE)19 (score <24) was the least useful test for identifying delirium (LR, 1.6; 95% CI, 1.22.0). Another SER evaluating the psychometric properties of CAM demonstrated a sensitivity of 94% (CI, 91%97%) and specificity of 89% (CI, 85%94%).20 CAM also showed prognostic value with worsening of delirium outcomes depending on the number of CAM items present.20
3: What Is the Underlying Pathophysiology of Delirium and Is There a Role of Measuring Biomarkers for Delirium?
We found only 1 SER which examined the associations between cerebrospinal fluid biomarkers and delirium.21 Delirium was associated with raised levels of serotonin metabolites, interleukin‐8, cortisol, lactate, and protein. Additionally, higher acetylcholinesterase predicted poor outcome after delirium, and higher dopamine metabolites were associated with psychotic features. Delirium was also associated with reduced levels of somatostatin, ‐endorphin, and neuron‐specific enolase.
4: Can Delirium Be Prevented?
Nonpharmacologic Interventions
An SER22 reviewing multicomponent interventions to prevent delirium identified 2 studies23, 24 showing statistically significant results. In the Yale Delirium Prevention Trial,23 the intervention was targeted toward minimizing 6 risk factors in elderly patients (70 years of age) admitted to a general medicine service, who did not have delirium at the time of admission, but were at risk for delirium development. The interventions included: orientation activities for the cognitively impaired, early mobilization, preventing sleep deprivation, minimizing the use of psychoactive drugs, use of eyeglasses and hearing aids, and treating volume depletion. The incidence of delirium was 9.9% with this intervention compared with 15% in the usual care group (OR [odds ratio], 0.60; 95% CI, 0.390.92).23 The other studied patients with hip fractures, randomized to either standard care versus the addition of a geriatrics consultation preoperatively or immediately after hip repair, providing recommendations based on a structured protocol.24 The incidence of delirium during hospitalization was 32% in the geriatrics consultation group versus 50% in the standard care group (OR, 0.48; 95% CI, 0.230.98; relative risk [RR], 0.64; 95% CI, 0.370.98), but there was no difference in duration of delirium.24
Pharmacologic Interventions
A Cochrane review found 6 randomized controlled trials for preventing delirium in hospitalized surgical patients.25 Low‐dose haloperidol prophylaxis was found to be effective in reducing the severity (mean difference in delirium rating scale score of 4.0 (95% CI, 2.05.8) and duration of delirium (RR, 6.44; 95% CI, 7.64 to 5.24), along with shortening the length of hospital stay (mean difference in hospital days, 5.5; 95% CI, 1.42.3) in hip surgery patients, but it did not prevent delirium occurrence.26 A review by Campbell et al evaluated 9 studies testing pharmacological interventions in preventing delirium in surgical patients.27 Use of a single‐dose risperidone after cardiac surgery decreased delirium incidence compared to placebo.28 Donepezil and citicoline showed no benefit in preventing delirium.2931 Early restoration of sleep cycles with the use of a benzodiazepine/opiate combination and pain control with gabapentin postoperatively reduced delirium incidence.32, 33 Interventions started on day of surgery and continued for up to 3 days postoperatively were found to be effective in reducing delirium incidence.27
5: How Should Delirium Be Treated?
Nonpharmacologic Interventions
The multicomponent intervention SER22 mentioned above evaluated the efficacy of interventions ranging from a geriatric psychiatric consultation and a nursing liaison to assess patients' daily pain management, to treating hypoxemia and other metabolic derangements along with a standardized screening tool for early detection of delirium. Delirious patients randomized to a geriatrician or a geriatric psychiatrist's consultation making treatment decisions, along with daily visits by a nursing liaison, resulted in improvement in short portable mental status questionnaire scores (SPMSQ) from 8.2 to 7.9, two weeks after admission, whereas the usual care group showed a deterioration in scores (8.4 to 9.1).34 Though by week 8, the difference between both groups disappeared. While the severity and recurrence rates of delirium were unchanged, the trial by Inouye et al23 evaluating 6 standardized intervention protocols showed a significant reduction in the total number of hospital days with delirium (105 vs 161 days, P = 0.02). Training of nurses to use a delirium screening instrument to identify delirium in hip fracture patients, along with prompt implementation of interventions based on a nursing guide for evaluation of causes of delirium, resulted in a shorter duration of delirium (median = 1 day vs 4 days, P = 0.03) and severity, compared to the usual care group.35 Daily assessment by a gerontological nurse resulted in greater improvement in functional status (21% vs 10%).36 No difference in patients' length of stay or mortality was demonstrated in any of the studies included in the review.22 A Cochrane review assessing efficacy of multidisciplinary interventions for reducing delirium in cognitively impaired patients did not identify any studies.37
Pharmacologic Interventions
We identified 7 SERs,27, 3843 addressing the efficacy and safety of various pharmacological interventions to treat delirium. Campbell et al suggested that blocking the dopaminergic system with neuroleptics, and reducing the exposure to lorazepam, might reduce delirium severity and duration among hospitalized elders, including those in the ICU.27 There was no advantage of using atypical neuroleptics over haloperidol. Low‐dose haloperidol use was associated with reduced delirium severity and duration in hip surgery patients.26 Seitz et al38 evaluated the efficacy and safety of antipsychotics (haloperidol, olanzapine, quetiapine, risperidone, mianserin, and lorazepam) in treating delirium symptoms. They evaluated prospective single‐agent and comparison trials. None of the studies included a placebo group. An improvement in delirium severity was observed in the majority of studies, but there was no advantage of one agent over the other in comparison trials. Most trials were underpowered to detect a clinically significant difference and are of short duration (<7 days) to adequately assess for delirium resolution.
A Cochrane review39 comparing the efficacy of haloperidol over risperidone and olanzapine for treating delirium showed similar findings as Campbell and colleagues' SER.27 The decrease in delirium severity scores was not significantly different using low‐dose haloperidol (<3.0 mg per day) compared with olanzapine and risperidone (OR, 0.63; 95% CI, 0.291.38; P = 0.25). High‐dose haloperidol (>4.5 mg per day) was associated with an increased incidence of extrapyramidal adverse effects. The role of drug therapy for delirium in terminally ill adult patients was evaluated in a Cochrane review40 and by Weber et al.41 They suggested the use of haloperidol or chlorpromazine in reducing delirium in acquired immune deficiency syndrome (AIDS) patients. Benzodiazepines were ineffective for treatment of non‐alcohol withdrawal delirium.42 In mechanically ventilated ICU patients, dexmedetomidine treatment increased number of delirium/coma‐free days compared with lorazepam (7 vs 3 days, P = 0.01).42 Cholinesterase inhibitor donepezil did not decrease duration of delirium compared to placebo in postoperative orthopedic patients.43
6: What Is the Impact of Delirium on Patient Outcomes?
We found 4 SERs.4447 Persistent delirium defined as delirium present on admission and at the time of discharge or beyond, and its impact on outcomes in older hospitalized patients, was evaluated in 1 SER. The combined proportions of patients with persistent delirium at discharge, 1, 3, and 6 months were 44.7%, 32.8%, 25.6%, and 21%, respectively.44 Evaluation of prognosis was complicated by small number of subjects and differences in length of follow up.
Delirium in elderly (>65 years) patients was associated with an increased risk of death45, 46 compared with controls, with a mortality rate of 38% in delirious patients compared to 27.5% in controls (hazard ratio[HR], 1.95; 95% CI, 1.512.52).45 This association persisted independent of preexisting dementia. Patients with delirium compared to controls were also at increased risk of institutionalization (33.4% vs 10.7%) (OR, 2.41; 95% CI, 1.773.29) and dementia (62.5% vs 8.1%) (OR, 12.52; 95% CI, 1.8684.21).45 In patients with dementia, delirium increased the risk of 30‐day rehospitalization and admission to long‐term care, compared to patients with dementia or delirium alone.47
DISCUSSION AND CLINICAL IMPLICATIONS
Our study identified age, cognitive impairment, depression, and mepiridine use for analgesia as risk factors for delirium in surgical patients. Drugs with anticholinergic properties were implicated in delirium development in both medical and surgical patients. The CAM has the best available data to be used as a diagnostic tool for delirium. Multicomponent interventions to prevent delirium occurrence are effective in a non‐cognitively impaired population, and low‐dose haloperidol prophylaxis decreases delirium duration and severity without affecting delirium incidence in hip surgery patients. There is no advantage of using atypical antipsychotics over haloperidol in treating delirium, and low‐dose haloperidol is as effective as a higher dose without unwarranted extrapyramidal side effects. Delirium carries a poor prognosis with an increased risk of death, institutionalization, and dementia.
Hospitals may benefit from implementing multicomponent strategies, focusing on at‐risk elderly medical and surgical patients, administered by a multidisciplinary team to reduce delirium incidence. For ICU physicians and administrators, development of sedation guidelines minimizing the use of benzodiazepines will decrease the risk of delirium development.
A structured approach in diagnosing delirium is required to maximize identification. Use of the CAM, based on best available data is recommended. However, the length of time in doing the CAM (more than 10 minutes with the requisite mental status examination) and insensitivity in nonexpert hands suggest a need for alternative screening tools. Haloperidol should be the preferred first‐line pharmacological therapy for delirium, with atypical antipsychotics reserved for patients with contraindications to haloperidol or those who are refractory to therapy with haloperidol. Figure 2 delineates a clinical model for delirium management derived from the findings in the Results section.
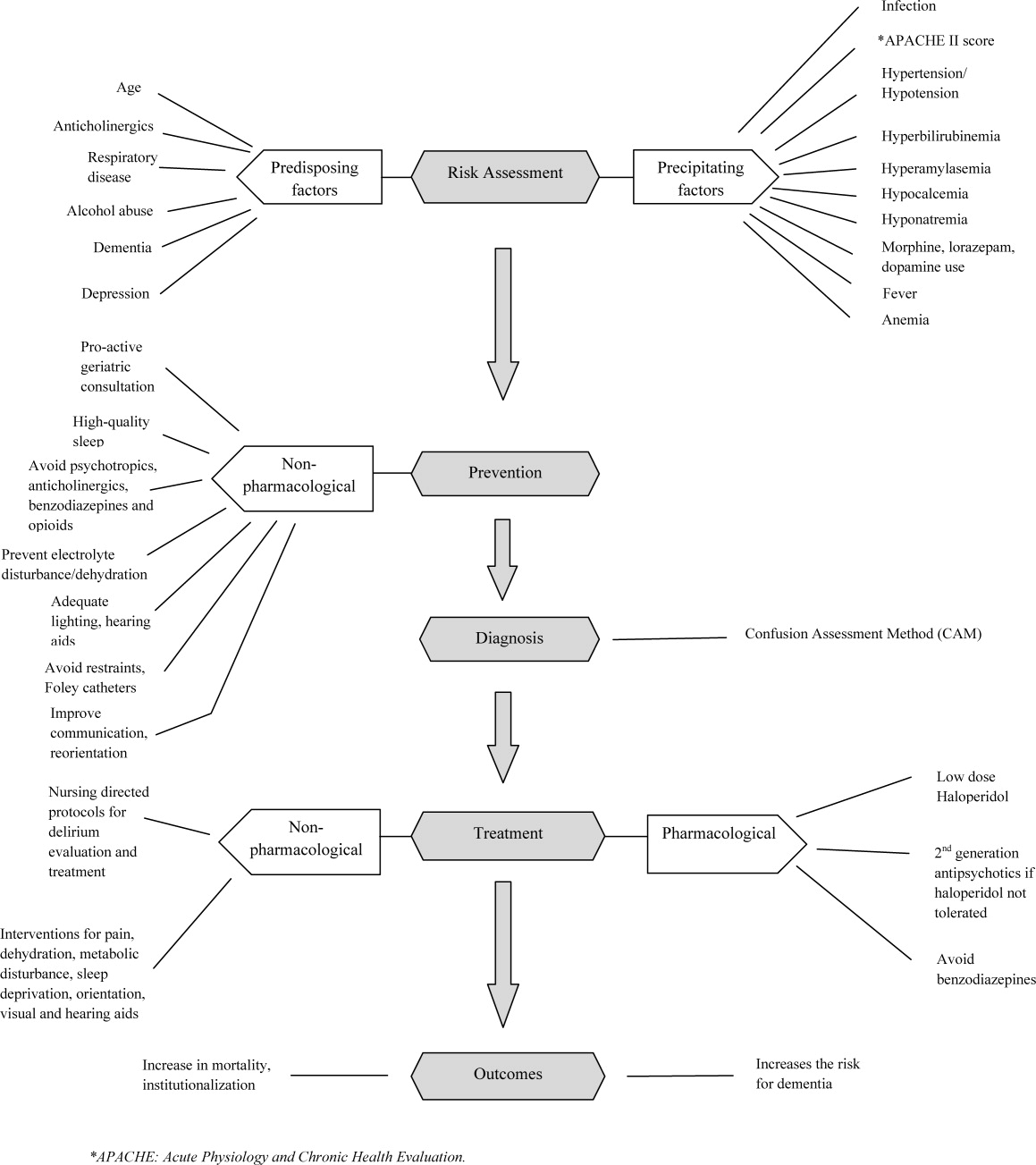
FUTURE RESEARCH DIRECTIONS
We identified multiple areas without clear guidelines that could provide opportunities for future research. A role for routine delirium screening can be clarified through a well‐designed delirium screening trial investigating the benefits of delirium screening, coupled with a multicomponent intervention versus usual care. Use of pharmacotherapy in delirium prevention needs to be explored further in a large randomized trial, with 3 arms to compare typical antipsychotics, atypical antipsychotics, and placebo in patients at risk for delirium with a primary outcome of delirium incidence. In regard to delirium treatment, a large randomized trial to compare haloperidol with atypical antipsychotics, with a placebo arm focusing not only on delirium duration and severity, but also on long‐term outcomes such as rehospitalizations, institutionalization, cognitive impairment, and mortality, is warranted. Figure 3 points out potential areas for researchers to investigate hypotheses generated by our review and thereby improve delirium care.
To our knowledge, our SER presents the first summary of SERs in delirium. Prior to this review, Michaud et al9 and National Institute for Health and Clinical Excellence48 published delirium guidelines, but in both of these guidelines, evidence was collected from a multitude of studies ranging in methodology from scientific review and meta‐analysis to observational studies, and the majority of recommendations were based on expert opinion. On the contrary, our review was limited to rigorously conducted SERs; hence, we utilized the highest level, critically appraised evidence to provide guidance to clinicians and researchers.
Limitations include a diverse group of studies with a heterogeneous population of patients, preventing pooling of results. We did not review each individual study included in the 38 SERs. We excluded non‐English language SERs, studies evaluating delirium subtypes, alcohol or substance abuse‐related delirium, or delirium associated with psychiatric disorders. As we only reviewed SERs, some notable studies not included in the SERs may have been missed.
CONCLUSION
Delirium among hospitalized patients is a common syndrome with a significant burden to the healthcare system and society. The field of delirium has seen considerable advances in diagnosis, prevention, and treatment over the last decade. Even with this advancement, there are still areas of uncertainty, such as: the benefits and costs of delirium screening; the benefits and harms of single or combined pharmacological agents for delirium prevention and treatment; the development of a set of reliable biomarkers for delirium diagnosis, prognosis, and response to therapy; the long‐term effect of delirium‐specific therapeutics on patients' cognitive, physical, and psychological functions; and the relationship between delirium and the development of Alzheimer's disease. As our understanding of delirium's impact on patients and healthcare improves, delirium should be identified as an indicator of poor long‐term prognosis, and should prompt immediate and effective evidence‐based management strategies, like any other critical illness.
Note Added in Proof
Disclosure: This study was supported by the National Institute on Aging (NIA), grant R01AG054205‐02; and the National Institute of Mental Health (NIMH), grant R24MH080827‐04.
- ,,,,,.Clarifying delirium: the confusion assessment method. A new method for detection of delirium.Ann Intern Med.1990;113(12):941–948.
- ,,.Delirium; a subcortical phenomenon?J Neuropsychiatry Clin Neurosci.1989;1(3):283–290.
- ,,, et al.The impact of delirium on the survival of mechanically ventilated patients.Crit Care Med.2004;32(11):2254–2259.
- ,.2002 National Hospital Discharge Survey.Adv Data.2004;342:1–29.
- ,.Delirium in hospitalized older adults. In: Ham R, Sloane P, Warshaw G, Bernard M, Flaherty E, eds.Primary Care Geriatrics, a Case‐Based Approach.5th ed.Philadelphia, PA:Mosby/Elsevier;2007:210–218.
- ,,,,.The association between delirium and cognitive decline: a review of the empirical literature.Neuropsychol Rev.2004;14(2):87–98.
- ,,, et al.Costs associated with delirium in mechanically ventilated patients.Crit Care Med.2004;32(4):955–962.
- ,,,.Detection in delirium in the acute hospital.Age Ageing.2010;39(1):131–135.
- ,,, et al;for the Delirium Guidelines Development Group.Delirium: guidelines for general hospitals.J Psychosom Res.2007;62(3):371–383.
- ,,,,.Delirium: a strategic plan to bring an ancient disease into the 21st century.J Am Geriatr Soc.2011;59:S237–S240.
- ,,, et al;for the Methods Work Group.Third US Preventive Services Task Force. Current methods of the US Preventive Services Task Force: a review of the process.Am J Prev Med.2001;20:21–35.
- ,.Delirium in vascular surgery.Eur J Vasc Endovasc Surg.2007;34(2):131–134.
- ,.Evidence‐based clinical update: general anesthesia and the risk of delirium and postoperative cognitive dysfunction.Can J Anaesth.2006;53(7):669–677.
- ,,.The role of postoperative analgesia in delirium and cognitive decline in elderly patients: a systematic review.Anesth Analg.2006(4):1255–1266.
- ,,,,.Risk factors for intensive care delirium: a systematic review.Intensive Crit Care Nurs.2008;24(2):98–107.
- ,,, et al.The cognitive impact of anticholinergics: a clinical review.Clin Interv Aging.2009;4:225–233.
- ,,,,,.Neuroimaging studies of delirium: a systematic review.J Psychosom Res.2008;65(3):239–248.
- ,,,.Does this patient have delirium? Value of bedside instruments.JAMA.2010;304(7):779–786.
- ,,.“Mini‐mental state.” A practical method for grading the cognitive state of patients for the clinician.J Psychiatr Res.1975;12(3):189–198.
- ,,,.The confusion assessment method: a systematic review of current usage.J Am Geriatr Soc.2008;56(5):823–830.
- ,,.A systematic literature review of cerebrospinal fluid biomarkers in delirium.Dement Geriatr Cogn Disord.2011;32:9–93.
- ,,,.Multicomponent intervention strategies for managing delirium in hospitalized older people; systematic review.J Adv Nurs.2005;52(1):79–90.
- ,,, et al.A multicomponent intervention to prevent delirium in hospitalized older patients.N Engl J Med.1999;340(9):669–676.
- ,,,.Reducing delirium after hip fracture: a randomized trial.J Am Geriatr Soc.2001;49(5):516–522.
- ,,,.Interventions for preventing delirium in hospitalized patients.Cochrane Database Syst Rev.2007;2:CD005563. DOI: 10.1002/14651858.CD005563.
- ,,, et al.Haloperidol prophylaxis for elderly hip‐surgery patients at risk for delirium: a randomized placebo‐controlled study.J Am Geriatr Soc.2005;53(10):1658–1666.
- ,,, et al.Pharmacological management of delirium in hospitalized adults: a systematic evidence review.J Gen Intern Med.2009;24:848–853.
- ,.Efficacy of risperidone for prevention of postoperative delirium in cardiac surgery.Anaesth Intensive Care.2007;35(5):714–719.
- ,,,,.Donepezil in the prevention and treatment of post‐surgical delirium.Am J Geriatr Psychiatry.2005;13:1100–1106.
- ,,, et al.A randomized,doubleblind, placebo‐controlled trial of donepezil hydrochloride (Aricept) for reducing the incidence of postoperative delirium after elective total hip replacement.Int J Geriatr Psychiatry.2007;22:343–349.
- ,,, et al.Use of procholinergics in the prevention of postoperative delirium in hip fracture surgery in the elderly. A randomized controlled trial [in Spanish].Rev Neurol.2001;33(8):716–719.
- ,,, et al.A novel approach to the prevention of postoperative delirium in the elderly after gastrointestinal surgery.Surg Today.2002;32:310–314.
- ,,, et al.Pilot clinical trial of gabapentin to decrease postoperative delirium in older patients.Neurology.2006;67(7):1251–1253.
- ,,, et al.Systematic intervention for elderly inpatients with delirium: a randomized clinical trial.Can Med Assoc J.1994;151:965–970.
- ,,, et al.A nurse‐led interdisciplinary intervention program for delirium in elderly hip‐fracture patients.J Am Geriatr Soc.2001;49:523–532.
- ,,,.Functional status outcomes of a nursing intervention in hospitalized elderly.Image J Nurs Sch.1992;24:201–220.
- ,.Multidisciplinary team interventions for delirium in patients with chronic cognitive impairment.Cochrane Database Syst Rev.2001;1:CD000395. Update in: Cochrane Database Syst Rev. year="2004"2004;2:CD000395.
- ,,.Antipsychotics in the treatment of delirium: a systematic review.J Clin Psychiatry.2007;68(1):11–21.
- ,,.Antipsychotics for delirium. The Cochrane Collaboration.The Cochrane Library.2009;1:1–117.
- ,.Drug therapy for delirium in terminally ill patients.Cochrane Database Syst Rev.2004;2:CD004770.
- ,,.Delirium: current trends in prevention and treatment.J Intern Med.2004;34(3):115–121.
- ,,,.Benzodiazepines for delirium.Cochrane Database Syst Rev.2009;1:CD006379. Update in: Cochrane Database Syst Rev.year="2009"2009;4:CD006379.
- ,,.Cholinesterase inhibitors for delirium.Cochrane Database Syst Rev.2008;1:CD005317.
- ,,,.Persistent delirium in older hospital patients: a systematic review of frequency and prognosis.Age Ageing.2009;38(1):19–26.
- ,,,,,.Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta‐analysis.JAMA.2010;304(4):443–451.
- ,,.Occurrence and outcome of delirium in medical in‐patients: a systematic literature review.Age Ageing.2006;35(4):350–364.
- ,,.Delirium superimposed on dementia: a systematic review.J Am Geriatr Soc.2002;50(10):1723–1732.
- National Institute for Health and Clinical Excellence. NICE guidelines for delirium diagnosis, prevention and management. Available at: http://www.ice.ork.uk/guidelines. Accessed October 1,2011.
- ,.Preoperative risk assessment for delirium after noncardiac surgery: a systematic review.J Am Geriatr Soc.2006;54(10):1578–1589.
- ,,,.Delirium risk factors in elderly hospitalized patients.J Gen Intern Med.1998;13(3):204–212.
- ,.Delirium after cardiac surgery: a critical review.J Psychosom Res.1996;41(1):13–30.
- ,,,,.The association between delirium and the apolipoprotein E epsilon 4 allele: new study results and a meta‐analysis.Am J Geriatr Psychiatry.2009;17:856–862.
- ,,.The genetics of deliria.Int Rev Psychiatry.2009;21(1):20–29.
- ,.Are nurses recognizing delirium? A systematic review.J Gerontol Nurs.2008;34(9):40–48.
- ,,,.Delirium assessment in the critically ill.Intensive Care Med.2007;33(6):929–940.
- ,,.Effectiveness of interventions to prevent delirium in hospitalized patients: a systematic review.Can Med Assoc J.1996;155(9):1263–1268.
- ,,,.Drug treatment of delirium: past, present and future.J Psychosom Res.2008;65(3):273–282.
- ,,,.Pathogensis of and management strategies for postoperative delirium after hip fracture: a review.Acta Orthop Scand.2004;75(4):378–389.
- ,,.Systematic review of antipsychotics for the treatment of hospital‐associated delirium in medically or surgically ill patients.Ann Pharmacother.2006;40(11):1966–1973.
- ,,,,.Atypical antipsychotics in the treatment of delirium.Psychiatry Clin Neurosci.2009;63(5):623–631.
- ,.Prognosis of delirium in elderly hospital patients.Can Med Assoc J.1993;149(1):41–46.
Delirium is a syndrome of disturbance of consciousness, with reduced ability to focus, sustain, or shift attention, that occurs over a short period of time and fluctuates over the course of the day.1 It encompasses a variety of cognitive, behavioral, and psychological symptoms including inattention, short‐term memory loss, sleep disturbances, agitated behaviors, delusions, and visual hallucinations.2 Delirium complicates the care of 70% to 80% of mechanically ventilated patients in intensive care units (ICUs).3 Of 13 million patients aged 65 and older hospitalized in 2002, 10% to 52% had delirium at some point during their admission.4, 5
Patients experiencing delirium have a higher probability of death during their hospital stay, adjusted for age, gender, race, and comorbidities.3, 6, 7 They are more vulnerable to hospital‐acquired complications leading to prolonged ICU and hospital stay, new institutionalization, and higher healthcare costs.3, 6, 7 Even with such a range of poor outcomes, the rates of delirium recognition are low,8 resulting in inadequate management.9 There has been considerable growth in the number of articles published on delirium in recent years. Therefore, it is of value to provide a state‐of‐the‐art summary of robust evidence in the field to healthcare personnel and delirium investigators.
We systematically reviewed the literature to identify published systematic evidence reviews (SERs), which evaluated the evidence on delirium risk factors, diagnosis, pathogenesis, prevention, treatment, and outcomes. We then summarized the data from the methodologically sound SERs to provide the reader with a clinically oriented summary of delirium literature for patient care. We also identify current gaps in delirium literature, and present future directions for delirium investigators to design studies that will enhance delirium care.
DATA SOURCES AND REVIEW METHODS
The domains of risk factors, diagnosis, pathophysiology, prevention, treatment, and outcomes were selected a priori to capture all relevant SERs regarding delirium based on the framework suggested by the American Delirium Society task force.10 To maximize article retrieval, a 3‐step search strategy was applied. First, we searched the electronic database utilizing OVID Medline, PubMed, the Cochrane Library, and Cumulative Index of Nursing and Allied Health Literature (CINAHL) using the following delirium‐specific search terms: delirium, confusion, agitation, mental status change, inattention, encephalopathy, organic mental disorders, and disorientation. We combined the above terms with the following study design terms: technical report, systematic evidence review, systematic review, meta‐analysis, editorial, and clinical reviews. We limited our search to human subjects. We excluded studies that: a) enrolled patients aged <18; b) enrolled patients with current or past Diagnostic and Statistical Manual of Mental Disorders (DSM) Axis I psychotic disorders; c) did not have standardized delirium evaluation; d) evaluated alcohol or substance abuse‐related delirium; e) did not use a systematic search method for identifying delirium‐related articles; and f) evaluated delirium sub‐types. We searched articles published from January 1966 through April 2011. Second, a manual search of references of the retrieved papers plus an Internet search using Google Scholar was conducted to find additional SERs. Titles and abstracts were screened by 2 reviewers (B.A.K., M.Z.). Authors of the included studies were contacted as necessary. Third, a library professional at the Indiana University School of Medicine independently performed a literature search, and those results were compared with our search to retrieve any missing SERs.
The methodological quality of each SER was independently assessed by 2 reviewers (B.A.K., M.Z.) using the United States Preventive Services Task Force (USPSTF) Critical Appraisal for SER.11 This scale assesses parameters that are critical to the scientific credibility of an SER and categorizes the SER as poor, fair, or good (Table 1). The 2 reviewers (B.A.K., M.Z.) used a data extraction form to record the following information from each SER: primary author, publication year, number and type of studies, number of participants and their mean age, study population, method for delirium diagnosis, risk factors, preventive and therapeutic interventions, and outcomes. Any disagreement between reviewers in SER selection, data extraction, or SER appraisal was resolved through discussion with a third reviewer (M.A.B.). The conflicting findings among SERs were resolved by consensus and by including the findings from a good SER over a fair SER.
| Criteria | Rating Definition |
|---|---|
| Recent, relevant review with comprehensive sources and search strategies | Good: If all the criteria are met |
| Explicit and relevant selection criteria | |
| Standard appraisal of included studies | |
| Valid conclusion | |
| Recent, relevant review that is not clearly biased but lacks comprehensive sources and search strategies | Fair: If this criterion is met |
| Outdated, irrelevant, or biased review | Poor: If one or more of the criteria are met |
| There is no systematic search for studies | |
| There are no explicit selection criteria | |
| There is no standard appraisal of studies |
RESULTS
Our search yielded 76,060 potential citations, out of which we identified 38 SERs meeting our inclusion criteria (Table 2). Figure 1 outlines our search strategy. Based on the USPSTF criteria, 22 SERs graded as good or fair provided the data to establish our review.

| Author (Year) | Studies (n)/ Participants (n) | Mean Age (Years) | Study Type | Service | Delirium/Cognition Assessment Scales | Review Objectives* | Rating |
|---|---|---|---|---|---|---|---|
| |||||||
| Van Rompaey et al15 (2008) | 6/7,114 | 61.2 | Prospective cohort, retrospective analysis | ICU (medical, surgical, coronary, mixed) | CAM‐ICU, psychiatric interview, ICU delirium screening checklist | 1/Risk factors | Fair |
| Bryson and Wyand13 (2006) | 18/3,473 | 71.93 | RCT | Surgery | MattisKovner Verbal Recall and Recognition, GDS, DST, DSM‐III, AMT, PRT, FOMTL, DCT, FPU, GEMS, WAIS‐R, Meta Memory Questionnaire, National Adult Reading Test | 1/Risk factors | Good |
| Fong et al14 (2006) | 9/1,078 | 63.1 | RCT, case control, prospective cohort, retrospective cohort | Surgery | CAM, DSM‐III, MMSE, SPMSQ, Digit Symbol Substitution Test, Trailmaking B Test | 1/Risk factors | Fair |
| Adamis et al53 (2009) | 6/882 | 54.59 | Case control | Medicine, ICU, surgery | CAM, DRS, DSM‐III‐R, DSM‐IV, ICD‐10 | 1/Risk factors | Poor |
| Balasundaram and Holmes12 (2007) | 4/364 | 66.8 | Prospective cohort | Surgery | CAM, DRS, HDS‐R, DSM‐IV | 1/Risk factors | Good |
| Dasgupta and Dumbrell49 (2006) | 25/5,175 | 72.5 | Prospective observational | Surgery | CAM, DSM‐III/IV | 1/Risk factors | Poor |
| Elie et al50 (1998) | 27/1,365 | 75.7 | Prospective | Medicine, surgery, psychiatry | CAM, NFRD, MMSE, MSQ, SPMSQ | 1/Risk factors | Poor |
| Van Munster et al52 (2009) | 5/1,099 | 77.86 | Cohort | Medicine, surgery | CAM, DRS | 1/Risk factors | Poor |
| Van der Mast and Roest51 (1996) | 57/6,129 | 48.2 | Prospective control, retrospective | Surgery | Psychiatric interview, chart review for signs of delirium, DSM‐III, MMSE | 1/Risk factors | Poor |
| Campbell et al16 (2009) | 27/8,492 | 71.35 | Longitudinal cohort, cross‐sectional, case control | Medicine, surgery, ICU, psychiatry | CAM, CAM‐ICU, DSI, DSM‐III/III‐R/IV, SDC, MMSE, Verbal N‐Back Test, BCRS, WMS | 1/Risk factors | Fair |
| Soiza et al17 (2008) | 12/764 | 72.4 | Cohort, case control, case series | Medicine, ICU, psychiatry | CAM, DSM‐III/III‐R/IV | 1/Risk factors | Good |
| Michaud et al9 (2007) | 29/NA | 76.7 | RCT, cohort | Medicine, surgery | CAM, BOMC, DRS, MDAS, ICD‐10, DSM‐IV, MMSE | 1/Risk factors, 2/Diagnosis, 4/Prevention, 5/Treatment | Fair |
| Steis and Fick54 (2008) | 10/3,059 | 72.5 | Prospective clinical trials, retrospective, observational, case study | Medicine, surgery, ICU | DSM‐III/IV | 2/Diagnosis | Poor |
| Wei et al20 (2008) | 7/1,071 | 70.17 | Validation, adaptation, translation, application | ICU, ED, medicine, surgery | CAM, CAM‐ICU, DSM‐IV, NH‐CAM, DI | 2/Diagnosis | Good |
| Wong et al18 (2010) | 25/3,027 | 72.76 | Prospective clinical studies | Medicine, surgery | CAC, CAM, DOSS, DRS, DRS‐R‐98, Digit Span Test, GAR, MDAS, MMSE, Nu‐DESC, Vigilance A Test | 2/Diagnosis | Fair |
| Devlin et al55 (2007) | 12/2,106 | 61.8 | Validation studies | ICU | CAM, ICDSC, CTD, ROC, DSM‐III/IV, DDS, MMSE | 2/Diagnosis | Poor |
| Fick et al47 (2002) | 14/7,701 | 79.51 | Prospective cohort, retrospective cohort, cross‐sectional, clinical trials | Medicine, surgery, ED | CAM, DRS, DSM‐III/III‐R/IV, CERAD, NINCDS‐ADRDA, IQCODE, MMSE | 2/Diagnosis, 4/Prevention, 6/Prognosis | Fair |
| Siddiqi et al46 (2006) | 40/12,220 | 78.8 | Prospective cohort, cross‐sectional, case‐controlled trials | Medicine | CAM, DRS, MDAS, SPMSQ, DSM‐III/III‐R/IV, MSQ, MMSE,BPRS, IQCODE, GHQ BAS | 2/Diagnosis, 6/Prognosis | Fair |
| 28/4,915 | |||||||
| Hall et al21 (2011) | 5/315 | 71.13 | Prospective cohort | Medicine, surgery, psychogeriatric | DSM‐III/III‐R/IV, MMSE, DRS, CAM, IQCODE, GDS | 3/Pathophysiology | Good |
| Cole et al56 (1996) | 10/999 | 71.6 | Randomized and nonrandomized trials | Medicine, surgery | DSM‐III, SPMSQ | 4/Prevention | Poor |
| Siddiqi et al25 (2007) | 6/833 | 76.67 | RCT | Surgery | CAM, DRS‐R‐98, DSM‐III/IV, DSI, MDAS, AMT, MMSE, OBS | 4/Prevention | Good |
| Campbell et al27 (2009) | 13/1,305 | 65.8 | RCT | Medicine, surgery, ICU | MDAS, DRS‐R‐98 | 4/Prevention, 5/Treatment | Good |
| Weber et al41 (2004) | 13/1,650 | 73.99 | RCT, non‐RCT, clinical trials, meta‐analysis, case report | Medicine, surgery | CAM, MDAS, DSI, DRS, DSM‐III‐R/IV, MMSE | 4/Prevention, 5/Treatment | Fair |
| Milisen et al22 (2005) | 7/1,683 | 80.73 | RCT, controlled trials, beforeafter study | Medicine, surgery | CAM, DSM‐III, SPMSQ, MMSE | 4/Prevention, 5/Treatment | Good |
| Lonergan et al39 (2009) | 3/629 | 74.5 | RCT | Medicine, surgery | CAM, DRS, DRS‐R‐98, MDAS, CGI, DSM‐IV | 5/Treatment | Good |
| Jackson and Lipman40 (2004) | 1/30 | 39.2 | RCT | Medicine | DRS, DSM‐III‐R | 5/Treatment | Good |
| Lonergan et al42 (2009) | 1/106 | 54.5 | RCT | ICU | CAM‐ICU | 5/Treatment | Good |
| Bourne et al57 (2008) | 33/1,880 | 60.99 | RCT, prospective trials, comparative trials | Medicine, surgery | DRS | 4/Prevention, 5/Treatment | Poor |
| Bitsch et al58 (2004) | 12/1,823 | 79.02 | Prospective, descriptive | Surgery | CAM, MDAS, DSI, OBS, MMSE | 4/Prevention, 5/Treatment | Poor |
| Overshott et al43 (2008) | 1/80 | 67 | RCT | Surgery | CAM, DSI, DSM‐IV, MMSE | 5/Treatment | Good |
| Lacasse et al59 (2006) | 4/158 | 60.8 | RCT | Medicine, surgery | CAM, DRS‐R‐98, MDAS, DI, DSM‐III‐R/IV, MMSE | 5/Treatment | Poor |
| Peritogiannis et al60 (2009) | 23/538 | 62.84 | RCT, retrospective, open label | Medicine, surgery | DRS, DRS‐R‐98, DRS‐R‐98‐J, MDAS, DI, 10‐Point Visual Analog Scale | 5/Treatment | Poor |
| Seitz et al38 (2007) | 14/448 | 63.09 | Prospective | Medicine, surgery, ICU | DSM‐III/III‐R/IV/IV‐TR, CAM, DRS‐R‐98, MDAS, DI | 5/Treatment | Good |
| Britton and Russell37 (2001/2004) | 1/227 | 82.35 | RCT | Medicine | CAM, SPMSQ, DSM‐III‐R, MMSE | 5/Treatment | Good |
| Jackson et al6 (2004) | 9/1,885 | 77.68 | Prospective, descriptive | Medicine, surgery, ICU, psychiatry | CAM, CAM‐ICU, DRS, MMSE, DSM | 6/Prognosis | Poor |
| Cole et al44 (2009) | 18/1,322 | 81.3 | Prospective cohort | Medicine, surgery | CAM, DSM‐III/III‐R/IV, ICD‐10, OBS | 6/Prognosis | Good |
| Witlox et al45 (2010) | 42/5,777 | 79.96 | Observational | Medicine, surgery | DSM, patient interview | 6/Prognosis | Good |
| Cole and Primeau61 (1993) | 8/573 | 77.25 | Prospective trials | Medicine, surgery, psychiatry | DSM‐I/III | 6/Prognosis | Poor |
1: What Are the Risk Factors for Development of Delirium in Hospitalized Patients?
We found 6 SERs1217 that evaluated risk factors for the development of delirium. Three reviews included only surgical patients,1214 1 focused on the intensive care unit (ICU),15 and the remaining 2 had both medical and surgical patients.16, 17 Risk factors identified in an elective vascular surgery population were age >64, preoperative cognitive impairment, depression, intraoperative blood transfusions, and previous amputation.12 The risk of incident delirium conferred by general anesthesia compared to regional anesthesia in non‐cardiac surgery patients was not significantly different among both groups.13 One SER14 focused on the effects of different opioid analgesics on postoperative delirium, and whether route of administration of medicines (intravenous vs epidural) had any impact on delirium. Mepiridine was consistently associated with an increased risk of delirium in elderly surgical patients, but there were no significant differences in postoperative delirium rates among those receiving morphine, fentanyl, or hydromorphone. The rates of delirium did not differ significantly between intravenous and epidural routes of analgesic administration, except in one study where epidural route had more delirium cases, but in 85% of those cases, mepiridine was used as an epidural agent. Risk factors explored in an ICU setting found multiple predisposing and precipitating risk factors, with the surprising finding that age was not a strong predictor of delirium.15 An association between delirium and drugs with anticholinergic properties was found in 1 SER.16 There was no causal relationship between structural or functional neuroimaging findings and delirium development.17
2: What Is the Clinical Utility of Bedside Tools in Delirium Diagnosis?
The accuracy of bedside instruments in diagnosing delirium was assessed in an SER of 25 prospective studies.18 Among the 11 scales reviewed, the Confusion Assessment Method (CAM) had the most evidence supporting its use as a bedside tool (+likelihood ratio [LR], 9.6; 95% CI [confidence interval], 5.816.0; LR, 0.16; 95% CI, 0.090.29). The Folstein mini‐mental status examination (MMSE)19 (score <24) was the least useful test for identifying delirium (LR, 1.6; 95% CI, 1.22.0). Another SER evaluating the psychometric properties of CAM demonstrated a sensitivity of 94% (CI, 91%97%) and specificity of 89% (CI, 85%94%).20 CAM also showed prognostic value with worsening of delirium outcomes depending on the number of CAM items present.20
3: What Is the Underlying Pathophysiology of Delirium and Is There a Role of Measuring Biomarkers for Delirium?
We found only 1 SER which examined the associations between cerebrospinal fluid biomarkers and delirium.21 Delirium was associated with raised levels of serotonin metabolites, interleukin‐8, cortisol, lactate, and protein. Additionally, higher acetylcholinesterase predicted poor outcome after delirium, and higher dopamine metabolites were associated with psychotic features. Delirium was also associated with reduced levels of somatostatin, ‐endorphin, and neuron‐specific enolase.
4: Can Delirium Be Prevented?
Nonpharmacologic Interventions
An SER22 reviewing multicomponent interventions to prevent delirium identified 2 studies23, 24 showing statistically significant results. In the Yale Delirium Prevention Trial,23 the intervention was targeted toward minimizing 6 risk factors in elderly patients (70 years of age) admitted to a general medicine service, who did not have delirium at the time of admission, but were at risk for delirium development. The interventions included: orientation activities for the cognitively impaired, early mobilization, preventing sleep deprivation, minimizing the use of psychoactive drugs, use of eyeglasses and hearing aids, and treating volume depletion. The incidence of delirium was 9.9% with this intervention compared with 15% in the usual care group (OR [odds ratio], 0.60; 95% CI, 0.390.92).23 The other studied patients with hip fractures, randomized to either standard care versus the addition of a geriatrics consultation preoperatively or immediately after hip repair, providing recommendations based on a structured protocol.24 The incidence of delirium during hospitalization was 32% in the geriatrics consultation group versus 50% in the standard care group (OR, 0.48; 95% CI, 0.230.98; relative risk [RR], 0.64; 95% CI, 0.370.98), but there was no difference in duration of delirium.24
Pharmacologic Interventions
A Cochrane review found 6 randomized controlled trials for preventing delirium in hospitalized surgical patients.25 Low‐dose haloperidol prophylaxis was found to be effective in reducing the severity (mean difference in delirium rating scale score of 4.0 (95% CI, 2.05.8) and duration of delirium (RR, 6.44; 95% CI, 7.64 to 5.24), along with shortening the length of hospital stay (mean difference in hospital days, 5.5; 95% CI, 1.42.3) in hip surgery patients, but it did not prevent delirium occurrence.26 A review by Campbell et al evaluated 9 studies testing pharmacological interventions in preventing delirium in surgical patients.27 Use of a single‐dose risperidone after cardiac surgery decreased delirium incidence compared to placebo.28 Donepezil and citicoline showed no benefit in preventing delirium.2931 Early restoration of sleep cycles with the use of a benzodiazepine/opiate combination and pain control with gabapentin postoperatively reduced delirium incidence.32, 33 Interventions started on day of surgery and continued for up to 3 days postoperatively were found to be effective in reducing delirium incidence.27
5: How Should Delirium Be Treated?
Nonpharmacologic Interventions
The multicomponent intervention SER22 mentioned above evaluated the efficacy of interventions ranging from a geriatric psychiatric consultation and a nursing liaison to assess patients' daily pain management, to treating hypoxemia and other metabolic derangements along with a standardized screening tool for early detection of delirium. Delirious patients randomized to a geriatrician or a geriatric psychiatrist's consultation making treatment decisions, along with daily visits by a nursing liaison, resulted in improvement in short portable mental status questionnaire scores (SPMSQ) from 8.2 to 7.9, two weeks after admission, whereas the usual care group showed a deterioration in scores (8.4 to 9.1).34 Though by week 8, the difference between both groups disappeared. While the severity and recurrence rates of delirium were unchanged, the trial by Inouye et al23 evaluating 6 standardized intervention protocols showed a significant reduction in the total number of hospital days with delirium (105 vs 161 days, P = 0.02). Training of nurses to use a delirium screening instrument to identify delirium in hip fracture patients, along with prompt implementation of interventions based on a nursing guide for evaluation of causes of delirium, resulted in a shorter duration of delirium (median = 1 day vs 4 days, P = 0.03) and severity, compared to the usual care group.35 Daily assessment by a gerontological nurse resulted in greater improvement in functional status (21% vs 10%).36 No difference in patients' length of stay or mortality was demonstrated in any of the studies included in the review.22 A Cochrane review assessing efficacy of multidisciplinary interventions for reducing delirium in cognitively impaired patients did not identify any studies.37
Pharmacologic Interventions
We identified 7 SERs,27, 3843 addressing the efficacy and safety of various pharmacological interventions to treat delirium. Campbell et al suggested that blocking the dopaminergic system with neuroleptics, and reducing the exposure to lorazepam, might reduce delirium severity and duration among hospitalized elders, including those in the ICU.27 There was no advantage of using atypical neuroleptics over haloperidol. Low‐dose haloperidol use was associated with reduced delirium severity and duration in hip surgery patients.26 Seitz et al38 evaluated the efficacy and safety of antipsychotics (haloperidol, olanzapine, quetiapine, risperidone, mianserin, and lorazepam) in treating delirium symptoms. They evaluated prospective single‐agent and comparison trials. None of the studies included a placebo group. An improvement in delirium severity was observed in the majority of studies, but there was no advantage of one agent over the other in comparison trials. Most trials were underpowered to detect a clinically significant difference and are of short duration (<7 days) to adequately assess for delirium resolution.
A Cochrane review39 comparing the efficacy of haloperidol over risperidone and olanzapine for treating delirium showed similar findings as Campbell and colleagues' SER.27 The decrease in delirium severity scores was not significantly different using low‐dose haloperidol (<3.0 mg per day) compared with olanzapine and risperidone (OR, 0.63; 95% CI, 0.291.38; P = 0.25). High‐dose haloperidol (>4.5 mg per day) was associated with an increased incidence of extrapyramidal adverse effects. The role of drug therapy for delirium in terminally ill adult patients was evaluated in a Cochrane review40 and by Weber et al.41 They suggested the use of haloperidol or chlorpromazine in reducing delirium in acquired immune deficiency syndrome (AIDS) patients. Benzodiazepines were ineffective for treatment of non‐alcohol withdrawal delirium.42 In mechanically ventilated ICU patients, dexmedetomidine treatment increased number of delirium/coma‐free days compared with lorazepam (7 vs 3 days, P = 0.01).42 Cholinesterase inhibitor donepezil did not decrease duration of delirium compared to placebo in postoperative orthopedic patients.43
6: What Is the Impact of Delirium on Patient Outcomes?
We found 4 SERs.4447 Persistent delirium defined as delirium present on admission and at the time of discharge or beyond, and its impact on outcomes in older hospitalized patients, was evaluated in 1 SER. The combined proportions of patients with persistent delirium at discharge, 1, 3, and 6 months were 44.7%, 32.8%, 25.6%, and 21%, respectively.44 Evaluation of prognosis was complicated by small number of subjects and differences in length of follow up.
Delirium in elderly (>65 years) patients was associated with an increased risk of death45, 46 compared with controls, with a mortality rate of 38% in delirious patients compared to 27.5% in controls (hazard ratio[HR], 1.95; 95% CI, 1.512.52).45 This association persisted independent of preexisting dementia. Patients with delirium compared to controls were also at increased risk of institutionalization (33.4% vs 10.7%) (OR, 2.41; 95% CI, 1.773.29) and dementia (62.5% vs 8.1%) (OR, 12.52; 95% CI, 1.8684.21).45 In patients with dementia, delirium increased the risk of 30‐day rehospitalization and admission to long‐term care, compared to patients with dementia or delirium alone.47
DISCUSSION AND CLINICAL IMPLICATIONS
Our study identified age, cognitive impairment, depression, and mepiridine use for analgesia as risk factors for delirium in surgical patients. Drugs with anticholinergic properties were implicated in delirium development in both medical and surgical patients. The CAM has the best available data to be used as a diagnostic tool for delirium. Multicomponent interventions to prevent delirium occurrence are effective in a non‐cognitively impaired population, and low‐dose haloperidol prophylaxis decreases delirium duration and severity without affecting delirium incidence in hip surgery patients. There is no advantage of using atypical antipsychotics over haloperidol in treating delirium, and low‐dose haloperidol is as effective as a higher dose without unwarranted extrapyramidal side effects. Delirium carries a poor prognosis with an increased risk of death, institutionalization, and dementia.
Hospitals may benefit from implementing multicomponent strategies, focusing on at‐risk elderly medical and surgical patients, administered by a multidisciplinary team to reduce delirium incidence. For ICU physicians and administrators, development of sedation guidelines minimizing the use of benzodiazepines will decrease the risk of delirium development.
A structured approach in diagnosing delirium is required to maximize identification. Use of the CAM, based on best available data is recommended. However, the length of time in doing the CAM (more than 10 minutes with the requisite mental status examination) and insensitivity in nonexpert hands suggest a need for alternative screening tools. Haloperidol should be the preferred first‐line pharmacological therapy for delirium, with atypical antipsychotics reserved for patients with contraindications to haloperidol or those who are refractory to therapy with haloperidol. Figure 2 delineates a clinical model for delirium management derived from the findings in the Results section.

FUTURE RESEARCH DIRECTIONS
We identified multiple areas without clear guidelines that could provide opportunities for future research. A role for routine delirium screening can be clarified through a well‐designed delirium screening trial investigating the benefits of delirium screening, coupled with a multicomponent intervention versus usual care. Use of pharmacotherapy in delirium prevention needs to be explored further in a large randomized trial, with 3 arms to compare typical antipsychotics, atypical antipsychotics, and placebo in patients at risk for delirium with a primary outcome of delirium incidence. In regard to delirium treatment, a large randomized trial to compare haloperidol with atypical antipsychotics, with a placebo arm focusing not only on delirium duration and severity, but also on long‐term outcomes such as rehospitalizations, institutionalization, cognitive impairment, and mortality, is warranted. Figure 3 points out potential areas for researchers to investigate hypotheses generated by our review and thereby improve delirium care.

To our knowledge, our SER presents the first summary of SERs in delirium. Prior to this review, Michaud et al9 and National Institute for Health and Clinical Excellence48 published delirium guidelines, but in both of these guidelines, evidence was collected from a multitude of studies ranging in methodology from scientific review and meta‐analysis to observational studies, and the majority of recommendations were based on expert opinion. On the contrary, our review was limited to rigorously conducted SERs; hence, we utilized the highest level, critically appraised evidence to provide guidance to clinicians and researchers.
Limitations include a diverse group of studies with a heterogeneous population of patients, preventing pooling of results. We did not review each individual study included in the 38 SERs. We excluded non‐English language SERs, studies evaluating delirium subtypes, alcohol or substance abuse‐related delirium, or delirium associated with psychiatric disorders. As we only reviewed SERs, some notable studies not included in the SERs may have been missed.
CONCLUSION
Delirium among hospitalized patients is a common syndrome with a significant burden to the healthcare system and society. The field of delirium has seen considerable advances in diagnosis, prevention, and treatment over the last decade. Even with this advancement, there are still areas of uncertainty, such as: the benefits and costs of delirium screening; the benefits and harms of single or combined pharmacological agents for delirium prevention and treatment; the development of a set of reliable biomarkers for delirium diagnosis, prognosis, and response to therapy; the long‐term effect of delirium‐specific therapeutics on patients' cognitive, physical, and psychological functions; and the relationship between delirium and the development of Alzheimer's disease. As our understanding of delirium's impact on patients and healthcare improves, delirium should be identified as an indicator of poor long‐term prognosis, and should prompt immediate and effective evidence‐based management strategies, like any other critical illness.
Note Added in Proof
Disclosure: This study was supported by the National Institute on Aging (NIA), grant R01AG054205‐02; and the National Institute of Mental Health (NIMH), grant R24MH080827‐04.
Delirium is a syndrome of disturbance of consciousness, with reduced ability to focus, sustain, or shift attention, that occurs over a short period of time and fluctuates over the course of the day.1 It encompasses a variety of cognitive, behavioral, and psychological symptoms including inattention, short‐term memory loss, sleep disturbances, agitated behaviors, delusions, and visual hallucinations.2 Delirium complicates the care of 70% to 80% of mechanically ventilated patients in intensive care units (ICUs).3 Of 13 million patients aged 65 and older hospitalized in 2002, 10% to 52% had delirium at some point during their admission.4, 5
Patients experiencing delirium have a higher probability of death during their hospital stay, adjusted for age, gender, race, and comorbidities.3, 6, 7 They are more vulnerable to hospital‐acquired complications leading to prolonged ICU and hospital stay, new institutionalization, and higher healthcare costs.3, 6, 7 Even with such a range of poor outcomes, the rates of delirium recognition are low,8 resulting in inadequate management.9 There has been considerable growth in the number of articles published on delirium in recent years. Therefore, it is of value to provide a state‐of‐the‐art summary of robust evidence in the field to healthcare personnel and delirium investigators.
We systematically reviewed the literature to identify published systematic evidence reviews (SERs), which evaluated the evidence on delirium risk factors, diagnosis, pathogenesis, prevention, treatment, and outcomes. We then summarized the data from the methodologically sound SERs to provide the reader with a clinically oriented summary of delirium literature for patient care. We also identify current gaps in delirium literature, and present future directions for delirium investigators to design studies that will enhance delirium care.
DATA SOURCES AND REVIEW METHODS
The domains of risk factors, diagnosis, pathophysiology, prevention, treatment, and outcomes were selected a priori to capture all relevant SERs regarding delirium based on the framework suggested by the American Delirium Society task force.10 To maximize article retrieval, a 3‐step search strategy was applied. First, we searched the electronic database utilizing OVID Medline, PubMed, the Cochrane Library, and Cumulative Index of Nursing and Allied Health Literature (CINAHL) using the following delirium‐specific search terms: delirium, confusion, agitation, mental status change, inattention, encephalopathy, organic mental disorders, and disorientation. We combined the above terms with the following study design terms: technical report, systematic evidence review, systematic review, meta‐analysis, editorial, and clinical reviews. We limited our search to human subjects. We excluded studies that: a) enrolled patients aged <18; b) enrolled patients with current or past Diagnostic and Statistical Manual of Mental Disorders (DSM) Axis I psychotic disorders; c) did not have standardized delirium evaluation; d) evaluated alcohol or substance abuse‐related delirium; e) did not use a systematic search method for identifying delirium‐related articles; and f) evaluated delirium sub‐types. We searched articles published from January 1966 through April 2011. Second, a manual search of references of the retrieved papers plus an Internet search using Google Scholar was conducted to find additional SERs. Titles and abstracts were screened by 2 reviewers (B.A.K., M.Z.). Authors of the included studies were contacted as necessary. Third, a library professional at the Indiana University School of Medicine independently performed a literature search, and those results were compared with our search to retrieve any missing SERs.
The methodological quality of each SER was independently assessed by 2 reviewers (B.A.K., M.Z.) using the United States Preventive Services Task Force (USPSTF) Critical Appraisal for SER.11 This scale assesses parameters that are critical to the scientific credibility of an SER and categorizes the SER as poor, fair, or good (Table 1). The 2 reviewers (B.A.K., M.Z.) used a data extraction form to record the following information from each SER: primary author, publication year, number and type of studies, number of participants and their mean age, study population, method for delirium diagnosis, risk factors, preventive and therapeutic interventions, and outcomes. Any disagreement between reviewers in SER selection, data extraction, or SER appraisal was resolved through discussion with a third reviewer (M.A.B.). The conflicting findings among SERs were resolved by consensus and by including the findings from a good SER over a fair SER.
| Criteria | Rating Definition |
|---|---|
| Recent, relevant review with comprehensive sources and search strategies | Good: If all the criteria are met |
| Explicit and relevant selection criteria | |
| Standard appraisal of included studies | |
| Valid conclusion | |
| Recent, relevant review that is not clearly biased but lacks comprehensive sources and search strategies | Fair: If this criterion is met |
| Outdated, irrelevant, or biased review | Poor: If one or more of the criteria are met |
| There is no systematic search for studies | |
| There are no explicit selection criteria | |
| There is no standard appraisal of studies |
RESULTS
Our search yielded 76,060 potential citations, out of which we identified 38 SERs meeting our inclusion criteria (Table 2). Figure 1 outlines our search strategy. Based on the USPSTF criteria, 22 SERs graded as good or fair provided the data to establish our review.

| Author (Year) | Studies (n)/ Participants (n) | Mean Age (Years) | Study Type | Service | Delirium/Cognition Assessment Scales | Review Objectives* | Rating |
|---|---|---|---|---|---|---|---|
| |||||||
| Van Rompaey et al15 (2008) | 6/7,114 | 61.2 | Prospective cohort, retrospective analysis | ICU (medical, surgical, coronary, mixed) | CAM‐ICU, psychiatric interview, ICU delirium screening checklist | 1/Risk factors | Fair |
| Bryson and Wyand13 (2006) | 18/3,473 | 71.93 | RCT | Surgery | MattisKovner Verbal Recall and Recognition, GDS, DST, DSM‐III, AMT, PRT, FOMTL, DCT, FPU, GEMS, WAIS‐R, Meta Memory Questionnaire, National Adult Reading Test | 1/Risk factors | Good |
| Fong et al14 (2006) | 9/1,078 | 63.1 | RCT, case control, prospective cohort, retrospective cohort | Surgery | CAM, DSM‐III, MMSE, SPMSQ, Digit Symbol Substitution Test, Trailmaking B Test | 1/Risk factors | Fair |
| Adamis et al53 (2009) | 6/882 | 54.59 | Case control | Medicine, ICU, surgery | CAM, DRS, DSM‐III‐R, DSM‐IV, ICD‐10 | 1/Risk factors | Poor |
| Balasundaram and Holmes12 (2007) | 4/364 | 66.8 | Prospective cohort | Surgery | CAM, DRS, HDS‐R, DSM‐IV | 1/Risk factors | Good |
| Dasgupta and Dumbrell49 (2006) | 25/5,175 | 72.5 | Prospective observational | Surgery | CAM, DSM‐III/IV | 1/Risk factors | Poor |
| Elie et al50 (1998) | 27/1,365 | 75.7 | Prospective | Medicine, surgery, psychiatry | CAM, NFRD, MMSE, MSQ, SPMSQ | 1/Risk factors | Poor |
| Van Munster et al52 (2009) | 5/1,099 | 77.86 | Cohort | Medicine, surgery | CAM, DRS | 1/Risk factors | Poor |
| Van der Mast and Roest51 (1996) | 57/6,129 | 48.2 | Prospective control, retrospective | Surgery | Psychiatric interview, chart review for signs of delirium, DSM‐III, MMSE | 1/Risk factors | Poor |
| Campbell et al16 (2009) | 27/8,492 | 71.35 | Longitudinal cohort, cross‐sectional, case control | Medicine, surgery, ICU, psychiatry | CAM, CAM‐ICU, DSI, DSM‐III/III‐R/IV, SDC, MMSE, Verbal N‐Back Test, BCRS, WMS | 1/Risk factors | Fair |
| Soiza et al17 (2008) | 12/764 | 72.4 | Cohort, case control, case series | Medicine, ICU, psychiatry | CAM, DSM‐III/III‐R/IV | 1/Risk factors | Good |
| Michaud et al9 (2007) | 29/NA | 76.7 | RCT, cohort | Medicine, surgery | CAM, BOMC, DRS, MDAS, ICD‐10, DSM‐IV, MMSE | 1/Risk factors, 2/Diagnosis, 4/Prevention, 5/Treatment | Fair |
| Steis and Fick54 (2008) | 10/3,059 | 72.5 | Prospective clinical trials, retrospective, observational, case study | Medicine, surgery, ICU | DSM‐III/IV | 2/Diagnosis | Poor |
| Wei et al20 (2008) | 7/1,071 | 70.17 | Validation, adaptation, translation, application | ICU, ED, medicine, surgery | CAM, CAM‐ICU, DSM‐IV, NH‐CAM, DI | 2/Diagnosis | Good |
| Wong et al18 (2010) | 25/3,027 | 72.76 | Prospective clinical studies | Medicine, surgery | CAC, CAM, DOSS, DRS, DRS‐R‐98, Digit Span Test, GAR, MDAS, MMSE, Nu‐DESC, Vigilance A Test | 2/Diagnosis | Fair |
| Devlin et al55 (2007) | 12/2,106 | 61.8 | Validation studies | ICU | CAM, ICDSC, CTD, ROC, DSM‐III/IV, DDS, MMSE | 2/Diagnosis | Poor |
| Fick et al47 (2002) | 14/7,701 | 79.51 | Prospective cohort, retrospective cohort, cross‐sectional, clinical trials | Medicine, surgery, ED | CAM, DRS, DSM‐III/III‐R/IV, CERAD, NINCDS‐ADRDA, IQCODE, MMSE | 2/Diagnosis, 4/Prevention, 6/Prognosis | Fair |
| Siddiqi et al46 (2006) | 40/12,220 | 78.8 | Prospective cohort, cross‐sectional, case‐controlled trials | Medicine | CAM, DRS, MDAS, SPMSQ, DSM‐III/III‐R/IV, MSQ, MMSE,BPRS, IQCODE, GHQ BAS | 2/Diagnosis, 6/Prognosis | Fair |
| 28/4,915 | |||||||
| Hall et al21 (2011) | 5/315 | 71.13 | Prospective cohort | Medicine, surgery, psychogeriatric | DSM‐III/III‐R/IV, MMSE, DRS, CAM, IQCODE, GDS | 3/Pathophysiology | Good |
| Cole et al56 (1996) | 10/999 | 71.6 | Randomized and nonrandomized trials | Medicine, surgery | DSM‐III, SPMSQ | 4/Prevention | Poor |
| Siddiqi et al25 (2007) | 6/833 | 76.67 | RCT | Surgery | CAM, DRS‐R‐98, DSM‐III/IV, DSI, MDAS, AMT, MMSE, OBS | 4/Prevention | Good |
| Campbell et al27 (2009) | 13/1,305 | 65.8 | RCT | Medicine, surgery, ICU | MDAS, DRS‐R‐98 | 4/Prevention, 5/Treatment | Good |
| Weber et al41 (2004) | 13/1,650 | 73.99 | RCT, non‐RCT, clinical trials, meta‐analysis, case report | Medicine, surgery | CAM, MDAS, DSI, DRS, DSM‐III‐R/IV, MMSE | 4/Prevention, 5/Treatment | Fair |
| Milisen et al22 (2005) | 7/1,683 | 80.73 | RCT, controlled trials, beforeafter study | Medicine, surgery | CAM, DSM‐III, SPMSQ, MMSE | 4/Prevention, 5/Treatment | Good |
| Lonergan et al39 (2009) | 3/629 | 74.5 | RCT | Medicine, surgery | CAM, DRS, DRS‐R‐98, MDAS, CGI, DSM‐IV | 5/Treatment | Good |
| Jackson and Lipman40 (2004) | 1/30 | 39.2 | RCT | Medicine | DRS, DSM‐III‐R | 5/Treatment | Good |
| Lonergan et al42 (2009) | 1/106 | 54.5 | RCT | ICU | CAM‐ICU | 5/Treatment | Good |
| Bourne et al57 (2008) | 33/1,880 | 60.99 | RCT, prospective trials, comparative trials | Medicine, surgery | DRS | 4/Prevention, 5/Treatment | Poor |
| Bitsch et al58 (2004) | 12/1,823 | 79.02 | Prospective, descriptive | Surgery | CAM, MDAS, DSI, OBS, MMSE | 4/Prevention, 5/Treatment | Poor |
| Overshott et al43 (2008) | 1/80 | 67 | RCT | Surgery | CAM, DSI, DSM‐IV, MMSE | 5/Treatment | Good |
| Lacasse et al59 (2006) | 4/158 | 60.8 | RCT | Medicine, surgery | CAM, DRS‐R‐98, MDAS, DI, DSM‐III‐R/IV, MMSE | 5/Treatment | Poor |
| Peritogiannis et al60 (2009) | 23/538 | 62.84 | RCT, retrospective, open label | Medicine, surgery | DRS, DRS‐R‐98, DRS‐R‐98‐J, MDAS, DI, 10‐Point Visual Analog Scale | 5/Treatment | Poor |
| Seitz et al38 (2007) | 14/448 | 63.09 | Prospective | Medicine, surgery, ICU | DSM‐III/III‐R/IV/IV‐TR, CAM, DRS‐R‐98, MDAS, DI | 5/Treatment | Good |
| Britton and Russell37 (2001/2004) | 1/227 | 82.35 | RCT | Medicine | CAM, SPMSQ, DSM‐III‐R, MMSE | 5/Treatment | Good |
| Jackson et al6 (2004) | 9/1,885 | 77.68 | Prospective, descriptive | Medicine, surgery, ICU, psychiatry | CAM, CAM‐ICU, DRS, MMSE, DSM | 6/Prognosis | Poor |
| Cole et al44 (2009) | 18/1,322 | 81.3 | Prospective cohort | Medicine, surgery | CAM, DSM‐III/III‐R/IV, ICD‐10, OBS | 6/Prognosis | Good |
| Witlox et al45 (2010) | 42/5,777 | 79.96 | Observational | Medicine, surgery | DSM, patient interview | 6/Prognosis | Good |
| Cole and Primeau61 (1993) | 8/573 | 77.25 | Prospective trials | Medicine, surgery, psychiatry | DSM‐I/III | 6/Prognosis | Poor |
1: What Are the Risk Factors for Development of Delirium in Hospitalized Patients?
We found 6 SERs1217 that evaluated risk factors for the development of delirium. Three reviews included only surgical patients,1214 1 focused on the intensive care unit (ICU),15 and the remaining 2 had both medical and surgical patients.16, 17 Risk factors identified in an elective vascular surgery population were age >64, preoperative cognitive impairment, depression, intraoperative blood transfusions, and previous amputation.12 The risk of incident delirium conferred by general anesthesia compared to regional anesthesia in non‐cardiac surgery patients was not significantly different among both groups.13 One SER14 focused on the effects of different opioid analgesics on postoperative delirium, and whether route of administration of medicines (intravenous vs epidural) had any impact on delirium. Mepiridine was consistently associated with an increased risk of delirium in elderly surgical patients, but there were no significant differences in postoperative delirium rates among those receiving morphine, fentanyl, or hydromorphone. The rates of delirium did not differ significantly between intravenous and epidural routes of analgesic administration, except in one study where epidural route had more delirium cases, but in 85% of those cases, mepiridine was used as an epidural agent. Risk factors explored in an ICU setting found multiple predisposing and precipitating risk factors, with the surprising finding that age was not a strong predictor of delirium.15 An association between delirium and drugs with anticholinergic properties was found in 1 SER.16 There was no causal relationship between structural or functional neuroimaging findings and delirium development.17
2: What Is the Clinical Utility of Bedside Tools in Delirium Diagnosis?
The accuracy of bedside instruments in diagnosing delirium was assessed in an SER of 25 prospective studies.18 Among the 11 scales reviewed, the Confusion Assessment Method (CAM) had the most evidence supporting its use as a bedside tool (+likelihood ratio [LR], 9.6; 95% CI [confidence interval], 5.816.0; LR, 0.16; 95% CI, 0.090.29). The Folstein mini‐mental status examination (MMSE)19 (score <24) was the least useful test for identifying delirium (LR, 1.6; 95% CI, 1.22.0). Another SER evaluating the psychometric properties of CAM demonstrated a sensitivity of 94% (CI, 91%97%) and specificity of 89% (CI, 85%94%).20 CAM also showed prognostic value with worsening of delirium outcomes depending on the number of CAM items present.20
3: What Is the Underlying Pathophysiology of Delirium and Is There a Role of Measuring Biomarkers for Delirium?
We found only 1 SER which examined the associations between cerebrospinal fluid biomarkers and delirium.21 Delirium was associated with raised levels of serotonin metabolites, interleukin‐8, cortisol, lactate, and protein. Additionally, higher acetylcholinesterase predicted poor outcome after delirium, and higher dopamine metabolites were associated with psychotic features. Delirium was also associated with reduced levels of somatostatin, ‐endorphin, and neuron‐specific enolase.
4: Can Delirium Be Prevented?
Nonpharmacologic Interventions
An SER22 reviewing multicomponent interventions to prevent delirium identified 2 studies23, 24 showing statistically significant results. In the Yale Delirium Prevention Trial,23 the intervention was targeted toward minimizing 6 risk factors in elderly patients (70 years of age) admitted to a general medicine service, who did not have delirium at the time of admission, but were at risk for delirium development. The interventions included: orientation activities for the cognitively impaired, early mobilization, preventing sleep deprivation, minimizing the use of psychoactive drugs, use of eyeglasses and hearing aids, and treating volume depletion. The incidence of delirium was 9.9% with this intervention compared with 15% in the usual care group (OR [odds ratio], 0.60; 95% CI, 0.390.92).23 The other studied patients with hip fractures, randomized to either standard care versus the addition of a geriatrics consultation preoperatively or immediately after hip repair, providing recommendations based on a structured protocol.24 The incidence of delirium during hospitalization was 32% in the geriatrics consultation group versus 50% in the standard care group (OR, 0.48; 95% CI, 0.230.98; relative risk [RR], 0.64; 95% CI, 0.370.98), but there was no difference in duration of delirium.24
Pharmacologic Interventions
A Cochrane review found 6 randomized controlled trials for preventing delirium in hospitalized surgical patients.25 Low‐dose haloperidol prophylaxis was found to be effective in reducing the severity (mean difference in delirium rating scale score of 4.0 (95% CI, 2.05.8) and duration of delirium (RR, 6.44; 95% CI, 7.64 to 5.24), along with shortening the length of hospital stay (mean difference in hospital days, 5.5; 95% CI, 1.42.3) in hip surgery patients, but it did not prevent delirium occurrence.26 A review by Campbell et al evaluated 9 studies testing pharmacological interventions in preventing delirium in surgical patients.27 Use of a single‐dose risperidone after cardiac surgery decreased delirium incidence compared to placebo.28 Donepezil and citicoline showed no benefit in preventing delirium.2931 Early restoration of sleep cycles with the use of a benzodiazepine/opiate combination and pain control with gabapentin postoperatively reduced delirium incidence.32, 33 Interventions started on day of surgery and continued for up to 3 days postoperatively were found to be effective in reducing delirium incidence.27
5: How Should Delirium Be Treated?
Nonpharmacologic Interventions
The multicomponent intervention SER22 mentioned above evaluated the efficacy of interventions ranging from a geriatric psychiatric consultation and a nursing liaison to assess patients' daily pain management, to treating hypoxemia and other metabolic derangements along with a standardized screening tool for early detection of delirium. Delirious patients randomized to a geriatrician or a geriatric psychiatrist's consultation making treatment decisions, along with daily visits by a nursing liaison, resulted in improvement in short portable mental status questionnaire scores (SPMSQ) from 8.2 to 7.9, two weeks after admission, whereas the usual care group showed a deterioration in scores (8.4 to 9.1).34 Though by week 8, the difference between both groups disappeared. While the severity and recurrence rates of delirium were unchanged, the trial by Inouye et al23 evaluating 6 standardized intervention protocols showed a significant reduction in the total number of hospital days with delirium (105 vs 161 days, P = 0.02). Training of nurses to use a delirium screening instrument to identify delirium in hip fracture patients, along with prompt implementation of interventions based on a nursing guide for evaluation of causes of delirium, resulted in a shorter duration of delirium (median = 1 day vs 4 days, P = 0.03) and severity, compared to the usual care group.35 Daily assessment by a gerontological nurse resulted in greater improvement in functional status (21% vs 10%).36 No difference in patients' length of stay or mortality was demonstrated in any of the studies included in the review.22 A Cochrane review assessing efficacy of multidisciplinary interventions for reducing delirium in cognitively impaired patients did not identify any studies.37
Pharmacologic Interventions
We identified 7 SERs,27, 3843 addressing the efficacy and safety of various pharmacological interventions to treat delirium. Campbell et al suggested that blocking the dopaminergic system with neuroleptics, and reducing the exposure to lorazepam, might reduce delirium severity and duration among hospitalized elders, including those in the ICU.27 There was no advantage of using atypical neuroleptics over haloperidol. Low‐dose haloperidol use was associated with reduced delirium severity and duration in hip surgery patients.26 Seitz et al38 evaluated the efficacy and safety of antipsychotics (haloperidol, olanzapine, quetiapine, risperidone, mianserin, and lorazepam) in treating delirium symptoms. They evaluated prospective single‐agent and comparison trials. None of the studies included a placebo group. An improvement in delirium severity was observed in the majority of studies, but there was no advantage of one agent over the other in comparison trials. Most trials were underpowered to detect a clinically significant difference and are of short duration (<7 days) to adequately assess for delirium resolution.
A Cochrane review39 comparing the efficacy of haloperidol over risperidone and olanzapine for treating delirium showed similar findings as Campbell and colleagues' SER.27 The decrease in delirium severity scores was not significantly different using low‐dose haloperidol (<3.0 mg per day) compared with olanzapine and risperidone (OR, 0.63; 95% CI, 0.291.38; P = 0.25). High‐dose haloperidol (>4.5 mg per day) was associated with an increased incidence of extrapyramidal adverse effects. The role of drug therapy for delirium in terminally ill adult patients was evaluated in a Cochrane review40 and by Weber et al.41 They suggested the use of haloperidol or chlorpromazine in reducing delirium in acquired immune deficiency syndrome (AIDS) patients. Benzodiazepines were ineffective for treatment of non‐alcohol withdrawal delirium.42 In mechanically ventilated ICU patients, dexmedetomidine treatment increased number of delirium/coma‐free days compared with lorazepam (7 vs 3 days, P = 0.01).42 Cholinesterase inhibitor donepezil did not decrease duration of delirium compared to placebo in postoperative orthopedic patients.43
6: What Is the Impact of Delirium on Patient Outcomes?
We found 4 SERs.4447 Persistent delirium defined as delirium present on admission and at the time of discharge or beyond, and its impact on outcomes in older hospitalized patients, was evaluated in 1 SER. The combined proportions of patients with persistent delirium at discharge, 1, 3, and 6 months were 44.7%, 32.8%, 25.6%, and 21%, respectively.44 Evaluation of prognosis was complicated by small number of subjects and differences in length of follow up.
Delirium in elderly (>65 years) patients was associated with an increased risk of death45, 46 compared with controls, with a mortality rate of 38% in delirious patients compared to 27.5% in controls (hazard ratio[HR], 1.95; 95% CI, 1.512.52).45 This association persisted independent of preexisting dementia. Patients with delirium compared to controls were also at increased risk of institutionalization (33.4% vs 10.7%) (OR, 2.41; 95% CI, 1.773.29) and dementia (62.5% vs 8.1%) (OR, 12.52; 95% CI, 1.8684.21).45 In patients with dementia, delirium increased the risk of 30‐day rehospitalization and admission to long‐term care, compared to patients with dementia or delirium alone.47
DISCUSSION AND CLINICAL IMPLICATIONS
Our study identified age, cognitive impairment, depression, and mepiridine use for analgesia as risk factors for delirium in surgical patients. Drugs with anticholinergic properties were implicated in delirium development in both medical and surgical patients. The CAM has the best available data to be used as a diagnostic tool for delirium. Multicomponent interventions to prevent delirium occurrence are effective in a non‐cognitively impaired population, and low‐dose haloperidol prophylaxis decreases delirium duration and severity without affecting delirium incidence in hip surgery patients. There is no advantage of using atypical antipsychotics over haloperidol in treating delirium, and low‐dose haloperidol is as effective as a higher dose without unwarranted extrapyramidal side effects. Delirium carries a poor prognosis with an increased risk of death, institutionalization, and dementia.
Hospitals may benefit from implementing multicomponent strategies, focusing on at‐risk elderly medical and surgical patients, administered by a multidisciplinary team to reduce delirium incidence. For ICU physicians and administrators, development of sedation guidelines minimizing the use of benzodiazepines will decrease the risk of delirium development.
A structured approach in diagnosing delirium is required to maximize identification. Use of the CAM, based on best available data is recommended. However, the length of time in doing the CAM (more than 10 minutes with the requisite mental status examination) and insensitivity in nonexpert hands suggest a need for alternative screening tools. Haloperidol should be the preferred first‐line pharmacological therapy for delirium, with atypical antipsychotics reserved for patients with contraindications to haloperidol or those who are refractory to therapy with haloperidol. Figure 2 delineates a clinical model for delirium management derived from the findings in the Results section.

FUTURE RESEARCH DIRECTIONS
We identified multiple areas without clear guidelines that could provide opportunities for future research. A role for routine delirium screening can be clarified through a well‐designed delirium screening trial investigating the benefits of delirium screening, coupled with a multicomponent intervention versus usual care. Use of pharmacotherapy in delirium prevention needs to be explored further in a large randomized trial, with 3 arms to compare typical antipsychotics, atypical antipsychotics, and placebo in patients at risk for delirium with a primary outcome of delirium incidence. In regard to delirium treatment, a large randomized trial to compare haloperidol with atypical antipsychotics, with a placebo arm focusing not only on delirium duration and severity, but also on long‐term outcomes such as rehospitalizations, institutionalization, cognitive impairment, and mortality, is warranted. Figure 3 points out potential areas for researchers to investigate hypotheses generated by our review and thereby improve delirium care.

To our knowledge, our SER presents the first summary of SERs in delirium. Prior to this review, Michaud et al9 and National Institute for Health and Clinical Excellence48 published delirium guidelines, but in both of these guidelines, evidence was collected from a multitude of studies ranging in methodology from scientific review and meta‐analysis to observational studies, and the majority of recommendations were based on expert opinion. On the contrary, our review was limited to rigorously conducted SERs; hence, we utilized the highest level, critically appraised evidence to provide guidance to clinicians and researchers.
Limitations include a diverse group of studies with a heterogeneous population of patients, preventing pooling of results. We did not review each individual study included in the 38 SERs. We excluded non‐English language SERs, studies evaluating delirium subtypes, alcohol or substance abuse‐related delirium, or delirium associated with psychiatric disorders. As we only reviewed SERs, some notable studies not included in the SERs may have been missed.
CONCLUSION
Delirium among hospitalized patients is a common syndrome with a significant burden to the healthcare system and society. The field of delirium has seen considerable advances in diagnosis, prevention, and treatment over the last decade. Even with this advancement, there are still areas of uncertainty, such as: the benefits and costs of delirium screening; the benefits and harms of single or combined pharmacological agents for delirium prevention and treatment; the development of a set of reliable biomarkers for delirium diagnosis, prognosis, and response to therapy; the long‐term effect of delirium‐specific therapeutics on patients' cognitive, physical, and psychological functions; and the relationship between delirium and the development of Alzheimer's disease. As our understanding of delirium's impact on patients and healthcare improves, delirium should be identified as an indicator of poor long‐term prognosis, and should prompt immediate and effective evidence‐based management strategies, like any other critical illness.
Note Added in Proof
Disclosure: This study was supported by the National Institute on Aging (NIA), grant R01AG054205‐02; and the National Institute of Mental Health (NIMH), grant R24MH080827‐04.
- ,,,,,.Clarifying delirium: the confusion assessment method. A new method for detection of delirium.Ann Intern Med.1990;113(12):941–948.
- ,,.Delirium; a subcortical phenomenon?J Neuropsychiatry Clin Neurosci.1989;1(3):283–290.
- ,,, et al.The impact of delirium on the survival of mechanically ventilated patients.Crit Care Med.2004;32(11):2254–2259.
- ,.2002 National Hospital Discharge Survey.Adv Data.2004;342:1–29.
- ,.Delirium in hospitalized older adults. In: Ham R, Sloane P, Warshaw G, Bernard M, Flaherty E, eds.Primary Care Geriatrics, a Case‐Based Approach.5th ed.Philadelphia, PA:Mosby/Elsevier;2007:210–218.
- ,,,,.The association between delirium and cognitive decline: a review of the empirical literature.Neuropsychol Rev.2004;14(2):87–98.
- ,,, et al.Costs associated with delirium in mechanically ventilated patients.Crit Care Med.2004;32(4):955–962.
- ,,,.Detection in delirium in the acute hospital.Age Ageing.2010;39(1):131–135.
- ,,, et al;for the Delirium Guidelines Development Group.Delirium: guidelines for general hospitals.J Psychosom Res.2007;62(3):371–383.
- ,,,,.Delirium: a strategic plan to bring an ancient disease into the 21st century.J Am Geriatr Soc.2011;59:S237–S240.
- ,,, et al;for the Methods Work Group.Third US Preventive Services Task Force. Current methods of the US Preventive Services Task Force: a review of the process.Am J Prev Med.2001;20:21–35.
- ,.Delirium in vascular surgery.Eur J Vasc Endovasc Surg.2007;34(2):131–134.
- ,.Evidence‐based clinical update: general anesthesia and the risk of delirium and postoperative cognitive dysfunction.Can J Anaesth.2006;53(7):669–677.
- ,,.The role of postoperative analgesia in delirium and cognitive decline in elderly patients: a systematic review.Anesth Analg.2006(4):1255–1266.
- ,,,,.Risk factors for intensive care delirium: a systematic review.Intensive Crit Care Nurs.2008;24(2):98–107.
- ,,, et al.The cognitive impact of anticholinergics: a clinical review.Clin Interv Aging.2009;4:225–233.
- ,,,,,.Neuroimaging studies of delirium: a systematic review.J Psychosom Res.2008;65(3):239–248.
- ,,,.Does this patient have delirium? Value of bedside instruments.JAMA.2010;304(7):779–786.
- ,,.“Mini‐mental state.” A practical method for grading the cognitive state of patients for the clinician.J Psychiatr Res.1975;12(3):189–198.
- ,,,.The confusion assessment method: a systematic review of current usage.J Am Geriatr Soc.2008;56(5):823–830.
- ,,.A systematic literature review of cerebrospinal fluid biomarkers in delirium.Dement Geriatr Cogn Disord.2011;32:9–93.
- ,,,.Multicomponent intervention strategies for managing delirium in hospitalized older people; systematic review.J Adv Nurs.2005;52(1):79–90.
- ,,, et al.A multicomponent intervention to prevent delirium in hospitalized older patients.N Engl J Med.1999;340(9):669–676.
- ,,,.Reducing delirium after hip fracture: a randomized trial.J Am Geriatr Soc.2001;49(5):516–522.
- ,,,.Interventions for preventing delirium in hospitalized patients.Cochrane Database Syst Rev.2007;2:CD005563. DOI: 10.1002/14651858.CD005563.
- ,,, et al.Haloperidol prophylaxis for elderly hip‐surgery patients at risk for delirium: a randomized placebo‐controlled study.J Am Geriatr Soc.2005;53(10):1658–1666.
- ,,, et al.Pharmacological management of delirium in hospitalized adults: a systematic evidence review.J Gen Intern Med.2009;24:848–853.
- ,.Efficacy of risperidone for prevention of postoperative delirium in cardiac surgery.Anaesth Intensive Care.2007;35(5):714–719.
- ,,,,.Donepezil in the prevention and treatment of post‐surgical delirium.Am J Geriatr Psychiatry.2005;13:1100–1106.
- ,,, et al.A randomized,doubleblind, placebo‐controlled trial of donepezil hydrochloride (Aricept) for reducing the incidence of postoperative delirium after elective total hip replacement.Int J Geriatr Psychiatry.2007;22:343–349.
- ,,, et al.Use of procholinergics in the prevention of postoperative delirium in hip fracture surgery in the elderly. A randomized controlled trial [in Spanish].Rev Neurol.2001;33(8):716–719.
- ,,, et al.A novel approach to the prevention of postoperative delirium in the elderly after gastrointestinal surgery.Surg Today.2002;32:310–314.
- ,,, et al.Pilot clinical trial of gabapentin to decrease postoperative delirium in older patients.Neurology.2006;67(7):1251–1253.
- ,,, et al.Systematic intervention for elderly inpatients with delirium: a randomized clinical trial.Can Med Assoc J.1994;151:965–970.
- ,,, et al.A nurse‐led interdisciplinary intervention program for delirium in elderly hip‐fracture patients.J Am Geriatr Soc.2001;49:523–532.
- ,,,.Functional status outcomes of a nursing intervention in hospitalized elderly.Image J Nurs Sch.1992;24:201–220.
- ,.Multidisciplinary team interventions for delirium in patients with chronic cognitive impairment.Cochrane Database Syst Rev.2001;1:CD000395. Update in: Cochrane Database Syst Rev. year="2004"2004;2:CD000395.
- ,,.Antipsychotics in the treatment of delirium: a systematic review.J Clin Psychiatry.2007;68(1):11–21.
- ,,.Antipsychotics for delirium. The Cochrane Collaboration.The Cochrane Library.2009;1:1–117.
- ,.Drug therapy for delirium in terminally ill patients.Cochrane Database Syst Rev.2004;2:CD004770.
- ,,.Delirium: current trends in prevention and treatment.J Intern Med.2004;34(3):115–121.
- ,,,.Benzodiazepines for delirium.Cochrane Database Syst Rev.2009;1:CD006379. Update in: Cochrane Database Syst Rev.year="2009"2009;4:CD006379.
- ,,.Cholinesterase inhibitors for delirium.Cochrane Database Syst Rev.2008;1:CD005317.
- ,,,.Persistent delirium in older hospital patients: a systematic review of frequency and prognosis.Age Ageing.2009;38(1):19–26.
- ,,,,,.Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta‐analysis.JAMA.2010;304(4):443–451.
- ,,.Occurrence and outcome of delirium in medical in‐patients: a systematic literature review.Age Ageing.2006;35(4):350–364.
- ,,.Delirium superimposed on dementia: a systematic review.J Am Geriatr Soc.2002;50(10):1723–1732.
- National Institute for Health and Clinical Excellence. NICE guidelines for delirium diagnosis, prevention and management. Available at: http://www.ice.ork.uk/guidelines. Accessed October 1,2011.
- ,.Preoperative risk assessment for delirium after noncardiac surgery: a systematic review.J Am Geriatr Soc.2006;54(10):1578–1589.
- ,,,.Delirium risk factors in elderly hospitalized patients.J Gen Intern Med.1998;13(3):204–212.
- ,.Delirium after cardiac surgery: a critical review.J Psychosom Res.1996;41(1):13–30.
- ,,,,.The association between delirium and the apolipoprotein E epsilon 4 allele: new study results and a meta‐analysis.Am J Geriatr Psychiatry.2009;17:856–862.
- ,,.The genetics of deliria.Int Rev Psychiatry.2009;21(1):20–29.
- ,.Are nurses recognizing delirium? A systematic review.J Gerontol Nurs.2008;34(9):40–48.
- ,,,.Delirium assessment in the critically ill.Intensive Care Med.2007;33(6):929–940.
- ,,.Effectiveness of interventions to prevent delirium in hospitalized patients: a systematic review.Can Med Assoc J.1996;155(9):1263–1268.
- ,,,.Drug treatment of delirium: past, present and future.J Psychosom Res.2008;65(3):273–282.
- ,,,.Pathogensis of and management strategies for postoperative delirium after hip fracture: a review.Acta Orthop Scand.2004;75(4):378–389.
- ,,.Systematic review of antipsychotics for the treatment of hospital‐associated delirium in medically or surgically ill patients.Ann Pharmacother.2006;40(11):1966–1973.
- ,,,,.Atypical antipsychotics in the treatment of delirium.Psychiatry Clin Neurosci.2009;63(5):623–631.
- ,.Prognosis of delirium in elderly hospital patients.Can Med Assoc J.1993;149(1):41–46.
- ,,,,,.Clarifying delirium: the confusion assessment method. A new method for detection of delirium.Ann Intern Med.1990;113(12):941–948.
- ,,.Delirium; a subcortical phenomenon?J Neuropsychiatry Clin Neurosci.1989;1(3):283–290.
- ,,, et al.The impact of delirium on the survival of mechanically ventilated patients.Crit Care Med.2004;32(11):2254–2259.
- ,.2002 National Hospital Discharge Survey.Adv Data.2004;342:1–29.
- ,.Delirium in hospitalized older adults. In: Ham R, Sloane P, Warshaw G, Bernard M, Flaherty E, eds.Primary Care Geriatrics, a Case‐Based Approach.5th ed.Philadelphia, PA:Mosby/Elsevier;2007:210–218.
- ,,,,.The association between delirium and cognitive decline: a review of the empirical literature.Neuropsychol Rev.2004;14(2):87–98.
- ,,, et al.Costs associated with delirium in mechanically ventilated patients.Crit Care Med.2004;32(4):955–962.
- ,,,.Detection in delirium in the acute hospital.Age Ageing.2010;39(1):131–135.
- ,,, et al;for the Delirium Guidelines Development Group.Delirium: guidelines for general hospitals.J Psychosom Res.2007;62(3):371–383.
- ,,,,.Delirium: a strategic plan to bring an ancient disease into the 21st century.J Am Geriatr Soc.2011;59:S237–S240.
- ,,, et al;for the Methods Work Group.Third US Preventive Services Task Force. Current methods of the US Preventive Services Task Force: a review of the process.Am J Prev Med.2001;20:21–35.
- ,.Delirium in vascular surgery.Eur J Vasc Endovasc Surg.2007;34(2):131–134.
- ,.Evidence‐based clinical update: general anesthesia and the risk of delirium and postoperative cognitive dysfunction.Can J Anaesth.2006;53(7):669–677.
- ,,.The role of postoperative analgesia in delirium and cognitive decline in elderly patients: a systematic review.Anesth Analg.2006(4):1255–1266.
- ,,,,.Risk factors for intensive care delirium: a systematic review.Intensive Crit Care Nurs.2008;24(2):98–107.
- ,,, et al.The cognitive impact of anticholinergics: a clinical review.Clin Interv Aging.2009;4:225–233.
- ,,,,,.Neuroimaging studies of delirium: a systematic review.J Psychosom Res.2008;65(3):239–248.
- ,,,.Does this patient have delirium? Value of bedside instruments.JAMA.2010;304(7):779–786.
- ,,.“Mini‐mental state.” A practical method for grading the cognitive state of patients for the clinician.J Psychiatr Res.1975;12(3):189–198.
- ,,,.The confusion assessment method: a systematic review of current usage.J Am Geriatr Soc.2008;56(5):823–830.
- ,,.A systematic literature review of cerebrospinal fluid biomarkers in delirium.Dement Geriatr Cogn Disord.2011;32:9–93.
- ,,,.Multicomponent intervention strategies for managing delirium in hospitalized older people; systematic review.J Adv Nurs.2005;52(1):79–90.
- ,,, et al.A multicomponent intervention to prevent delirium in hospitalized older patients.N Engl J Med.1999;340(9):669–676.
- ,,,.Reducing delirium after hip fracture: a randomized trial.J Am Geriatr Soc.2001;49(5):516–522.
- ,,,.Interventions for preventing delirium in hospitalized patients.Cochrane Database Syst Rev.2007;2:CD005563. DOI: 10.1002/14651858.CD005563.
- ,,, et al.Haloperidol prophylaxis for elderly hip‐surgery patients at risk for delirium: a randomized placebo‐controlled study.J Am Geriatr Soc.2005;53(10):1658–1666.
- ,,, et al.Pharmacological management of delirium in hospitalized adults: a systematic evidence review.J Gen Intern Med.2009;24:848–853.
- ,.Efficacy of risperidone for prevention of postoperative delirium in cardiac surgery.Anaesth Intensive Care.2007;35(5):714–719.
- ,,,,.Donepezil in the prevention and treatment of post‐surgical delirium.Am J Geriatr Psychiatry.2005;13:1100–1106.
- ,,, et al.A randomized,doubleblind, placebo‐controlled trial of donepezil hydrochloride (Aricept) for reducing the incidence of postoperative delirium after elective total hip replacement.Int J Geriatr Psychiatry.2007;22:343–349.
- ,,, et al.Use of procholinergics in the prevention of postoperative delirium in hip fracture surgery in the elderly. A randomized controlled trial [in Spanish].Rev Neurol.2001;33(8):716–719.
- ,,, et al.A novel approach to the prevention of postoperative delirium in the elderly after gastrointestinal surgery.Surg Today.2002;32:310–314.
- ,,, et al.Pilot clinical trial of gabapentin to decrease postoperative delirium in older patients.Neurology.2006;67(7):1251–1253.
- ,,, et al.Systematic intervention for elderly inpatients with delirium: a randomized clinical trial.Can Med Assoc J.1994;151:965–970.
- ,,, et al.A nurse‐led interdisciplinary intervention program for delirium in elderly hip‐fracture patients.J Am Geriatr Soc.2001;49:523–532.
- ,,,.Functional status outcomes of a nursing intervention in hospitalized elderly.Image J Nurs Sch.1992;24:201–220.
- ,.Multidisciplinary team interventions for delirium in patients with chronic cognitive impairment.Cochrane Database Syst Rev.2001;1:CD000395. Update in: Cochrane Database Syst Rev. year="2004"2004;2:CD000395.
- ,,.Antipsychotics in the treatment of delirium: a systematic review.J Clin Psychiatry.2007;68(1):11–21.
- ,,.Antipsychotics for delirium. The Cochrane Collaboration.The Cochrane Library.2009;1:1–117.
- ,.Drug therapy for delirium in terminally ill patients.Cochrane Database Syst Rev.2004;2:CD004770.
- ,,.Delirium: current trends in prevention and treatment.J Intern Med.2004;34(3):115–121.
- ,,,.Benzodiazepines for delirium.Cochrane Database Syst Rev.2009;1:CD006379. Update in: Cochrane Database Syst Rev.year="2009"2009;4:CD006379.
- ,,.Cholinesterase inhibitors for delirium.Cochrane Database Syst Rev.2008;1:CD005317.
- ,,,.Persistent delirium in older hospital patients: a systematic review of frequency and prognosis.Age Ageing.2009;38(1):19–26.
- ,,,,,.Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta‐analysis.JAMA.2010;304(4):443–451.
- ,,.Occurrence and outcome of delirium in medical in‐patients: a systematic literature review.Age Ageing.2006;35(4):350–364.
- ,,.Delirium superimposed on dementia: a systematic review.J Am Geriatr Soc.2002;50(10):1723–1732.
- National Institute for Health and Clinical Excellence. NICE guidelines for delirium diagnosis, prevention and management. Available at: http://www.ice.ork.uk/guidelines. Accessed October 1,2011.
- ,.Preoperative risk assessment for delirium after noncardiac surgery: a systematic review.J Am Geriatr Soc.2006;54(10):1578–1589.
- ,,,.Delirium risk factors in elderly hospitalized patients.J Gen Intern Med.1998;13(3):204–212.
- ,.Delirium after cardiac surgery: a critical review.J Psychosom Res.1996;41(1):13–30.
- ,,,,.The association between delirium and the apolipoprotein E epsilon 4 allele: new study results and a meta‐analysis.Am J Geriatr Psychiatry.2009;17:856–862.
- ,,.The genetics of deliria.Int Rev Psychiatry.2009;21(1):20–29.
- ,.Are nurses recognizing delirium? A systematic review.J Gerontol Nurs.2008;34(9):40–48.
- ,,,.Delirium assessment in the critically ill.Intensive Care Med.2007;33(6):929–940.
- ,,.Effectiveness of interventions to prevent delirium in hospitalized patients: a systematic review.Can Med Assoc J.1996;155(9):1263–1268.
- ,,,.Drug treatment of delirium: past, present and future.J Psychosom Res.2008;65(3):273–282.
- ,,,.Pathogensis of and management strategies for postoperative delirium after hip fracture: a review.Acta Orthop Scand.2004;75(4):378–389.
- ,,.Systematic review of antipsychotics for the treatment of hospital‐associated delirium in medically or surgically ill patients.Ann Pharmacother.2006;40(11):1966–1973.
- ,,,,.Atypical antipsychotics in the treatment of delirium.Psychiatry Clin Neurosci.2009;63(5):623–631.
- ,.Prognosis of delirium in elderly hospital patients.Can Med Assoc J.1993;149(1):41–46.