User login
Metastatic Melanoma Mimicking Eruptive Keratoacanthomas
To the Editor:
Melanoma is the third most common skin cancer. It is estimated that 18% of melanoma patients will develop skin metastases, with skin being the first site of involvement in 56% of cases.1 Of all cancers, it is estimated that 5% will develop skin metastases. It is the presenting sign in nearly 1% of visceral cancers.2 Melanoma and nonmelanoma metastases can have sundry presentations. We present a case of metastatic melanoma with multiple keratoacanthoma (KA)–like skin lesions in a patient with a known history of nonmelanoma skin cancer (NMSC) as well as melanoma.
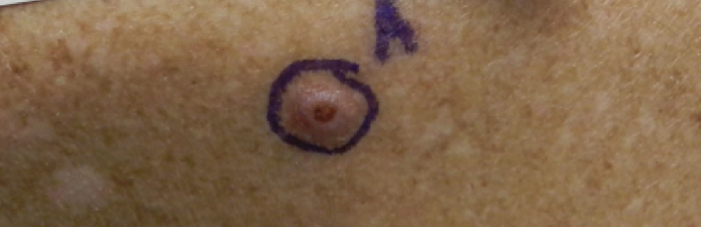
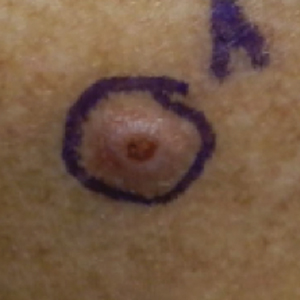
A 76-year-old man with a history of pT2aNXMX melanoma on the left upper back presented for a routine 3-month follow-up and reported several new asymptomatic bumps on the chest, back, and right upper extremity within the last 2 weeks. The melanoma was removed via wide local excision 2 years prior at an outside facility with a Breslow depth of 1.05 mm and a negative sentinel lymph node biopsy. The mitotic rate or ulceration status was unknown. He also had a history of several NMSCs, as well as a medical history of coronary artery disease, myocardial infarction, and ventricular tachycardia with cardiac defibrillator placement. Physical examination revealed 5 pink, volcano-shaped nodules with central keratotic plugs on the upper back (Figure 1), chest, and right upper extremity, in addition to 1 pink pearly nodule on the right side of the chest. The history and appearance of the lesions were suspicious for eruptive KAs. There was no evidence of cancer recurrence at the prior melanoma and NMSC sites.
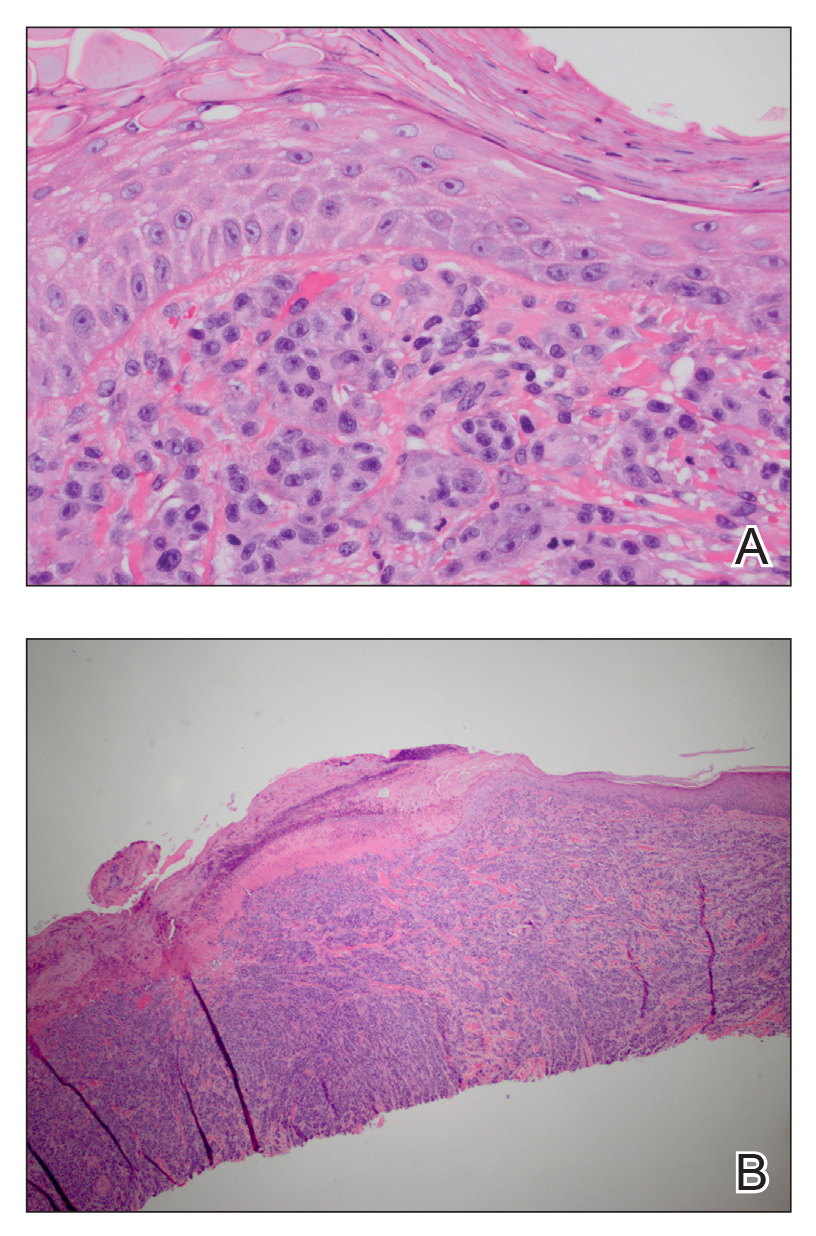
A deep shave skin biopsy was performed at all 6 sites. Histopathology showed a diffuse dermal infiltrate of elongated nests of melanocytes and nonnested melanocytes. Marked cytologic atypia and ulceration were present. Minimal connection to the overlying epidermis and a lack of junctional nests was noted. Immunohistochemical studies revealed scattered positivity for Melan-A and negative staining for AE1, AE3, cytokeratin 5, and cytokeratin 6 at all 6 sites (Figure 2). A subsequent metastatic workup showed widespread metastatic disease in the liver, bone, lung, and inferior vena cava. Computed tomography of the head was unremarkable. Magnetic resonance imaging of the brain was not performed due to the cardiac defibrillator. The patient’s lactate dehydrogenase level showed a mild increase compared to 2 months prior to the metastatic melanoma diagnosis (144 U/L vs 207 U/L [reference range, 100–200 U/L]).
The patient had no systemic symptoms at follow-up 5 weeks later. He was already evaluated by an oncologist and received his first dose of ipilimumab. He was BRAF-mutation negative. He had developed 2 new skin metastases. Five of 6 initially biopsied metastases returned and were growing; they were tender and friable with intermittent bleeding. He was subsequently referred to surgical oncology for excision of symptomatic nodules as palliative care.
Although melanoma is well known to metastasize years and even decades later, KA-like lesions have not been reported as manifestations of metastatic melanoma.4,5 Our patient likely had a primary amelanotic melanoma, as the medical records from the outside facility stated that basal cell carcinoma was ruled out via biopsy. The amelanotic nature of the primary melanoma may have influenced the amelanotic appearance of the metastases. Our patient had no signs of immunosuppression that could have contributed to the sudden skin metastases.
Depending on the subtype of cutaneous metastases (eg, satellitosis, in-transit disease, distant cutaneous metastases), the location prevalence of the primary melanoma varies. In a study of 4865 melanoma patients who were diagnosed and followed prospectively over a 30-year period, skin metastases were mostly locoregional and presentation on the leg and foot were disproportionate.1 In contrast, the trunk was overrepresented for distant metastases. Distant metastases also were more associated with concurrent metastases to the viscera.1 Accordingly, a patient’s prognosis and management will differ depending on the subtype of cutaneous metastases.
Eruptive or multiple KAs classically have been associated with the Grzybowski variant, the Ferguson-Smith familial variant, and Muir-Torre syndrome. It was reported as a paraneoplastic syndrome associated with colon cancer, ovarian cancer, and once with myelodysplastic syndrome.3 Keratoacanthomas are being classified as well-differentiated squamous cell carcinomas and have metastatic potential. A biopsy is recommended to diagnose KAs as opposed to historically being monitored for resolution. A skin biopsy is the standard of care in management of KAs.
In addition to being associated with Muir-Torre syndrome and classified as a paraneoplastic syndrome,3 eruptive KAs can occur following skin resurfacing for actinic damage, fractional photothermolysis, cryotherapy, Jessner peels, and trichloroacetic acid peels.6 A couple other uncommon settings include a case report of an arc welder with job-associated radiation and multiple reports of tattoo-induced KAs.7,8 There is the new increasingly common association of squamous cell carcinomas with BRAF inhibitors, such as vemurafenib, for metastatic melanoma.9
In a 2012 review article on cutaneous metastases, Riahi and Cohen10 found 8 cases of cutaneous metastases presenting as KA-like lesions; none were metastatic melanoma. All were solitary lesions, not multiple lesions, as in our patient. The sources were lung (3 cases), breast, esophagus, chondrosarcoma, bronchial, and mesothelioma. The most common location was the upper lip. Additionally, similar to our patient, they behaved clinically as KAs with rapid growth and keratotic plugs and were asymptomatic.10
Metastatic melanoma may mimic many other cutaneous processes that may make the diagnosis more difficult. Our case indicates that cutaneous metastases may mimic KAs. Although multiple KA-like lesions can spontaneously occur, a paraneoplastic syndrome and other underlying etiologies should be considered.
- Savoia P, Fava P, Nardò T, et al. Skin metastases of malignant melanoma: a clinical and prognostic study. Melanoma Res. 2009;19:321-326.
- Lookingbill DP, Spangler N, Sexton FM. Skin involvement as the presenting sign of internal carcinoma. J Am Acad Dermatol. 1990;22:19-26.
- Behzad M, Michl C, Pfützner W. Multiple eruptive keratoacanthomas associated with myelodysplastic syndrome. J Dtsch Dermatol Ges. 2012;10:359-360.
- Cheung WL, Patel RR, Leonard A, et al. Amelanotic melanoma: a detailed morphologic analysis with clinicopathologic correlation of 75 cases. J Cutan Pathol. 2012;39:33-39.
- Ferrari A, Piccolo D, Fargnoli MC, et al. Cutaneous amelanotic melanoma metastasis and dermatofibromas showing a dotted vascular pattern. Acta Dermato Venereologica. 2004;84:164-165.
- Mohr B, Fernandez MP, Krejci-Manwaring J. Eruptive keratoacanthoma after Jessner’s and trichloroacetic acid peel for actinic keratosis. Dermatol Surg. 2013;39:331-333.
- Wolfe CM, Green WH, Cognetta AB, et al. Multiple squamous cell carcinomas and eruptive keratoacanthomas in an arc welder. Dermatol Surg. 2013;39:328-330.
- Kluger N, Phan A, Debarbieux S, et al. Skin cancers arising in tattoos: coincidental or not? Dermatology. 2008;217:219-221.
- Mays R, Curry J, Kim K, et al. Eruptive squamous cell carcinomas after vemurafenib therapy. J Cutan Med Surg. 2013;17:419-422.
- Riahi RR, Cohen PR. Clinical manifestations of cutaneous metastases: a review with special emphasis on cutaneous metastases mimicking keratoacanthoma. Am J Clin Dermatol. 2012;13:103-112.
To the Editor:
Melanoma is the third most common skin cancer. It is estimated that 18% of melanoma patients will develop skin metastases, with skin being the first site of involvement in 56% of cases.1 Of all cancers, it is estimated that 5% will develop skin metastases. It is the presenting sign in nearly 1% of visceral cancers.2 Melanoma and nonmelanoma metastases can have sundry presentations. We present a case of metastatic melanoma with multiple keratoacanthoma (KA)–like skin lesions in a patient with a known history of nonmelanoma skin cancer (NMSC) as well as melanoma.
A 76-year-old man with a history of pT2aNXMX melanoma on the left upper back presented for a routine 3-month follow-up and reported several new asymptomatic bumps on the chest, back, and right upper extremity within the last 2 weeks. The melanoma was removed via wide local excision 2 years prior at an outside facility with a Breslow depth of 1.05 mm and a negative sentinel lymph node biopsy. The mitotic rate or ulceration status was unknown. He also had a history of several NMSCs, as well as a medical history of coronary artery disease, myocardial infarction, and ventricular tachycardia with cardiac defibrillator placement. Physical examination revealed 5 pink, volcano-shaped nodules with central keratotic plugs on the upper back (Figure 1), chest, and right upper extremity, in addition to 1 pink pearly nodule on the right side of the chest. The history and appearance of the lesions were suspicious for eruptive KAs. There was no evidence of cancer recurrence at the prior melanoma and NMSC sites.
A deep shave skin biopsy was performed at all 6 sites. Histopathology showed a diffuse dermal infiltrate of elongated nests of melanocytes and nonnested melanocytes. Marked cytologic atypia and ulceration were present. Minimal connection to the overlying epidermis and a lack of junctional nests was noted. Immunohistochemical studies revealed scattered positivity for Melan-A and negative staining for AE1, AE3, cytokeratin 5, and cytokeratin 6 at all 6 sites (Figure 2). A subsequent metastatic workup showed widespread metastatic disease in the liver, bone, lung, and inferior vena cava. Computed tomography of the head was unremarkable. Magnetic resonance imaging of the brain was not performed due to the cardiac defibrillator. The patient’s lactate dehydrogenase level showed a mild increase compared to 2 months prior to the metastatic melanoma diagnosis (144 U/L vs 207 U/L [reference range, 100–200 U/L]).
The patient had no systemic symptoms at follow-up 5 weeks later. He was already evaluated by an oncologist and received his first dose of ipilimumab. He was BRAF-mutation negative. He had developed 2 new skin metastases. Five of 6 initially biopsied metastases returned and were growing; they were tender and friable with intermittent bleeding. He was subsequently referred to surgical oncology for excision of symptomatic nodules as palliative care.
Although melanoma is well known to metastasize years and even decades later, KA-like lesions have not been reported as manifestations of metastatic melanoma.4,5 Our patient likely had a primary amelanotic melanoma, as the medical records from the outside facility stated that basal cell carcinoma was ruled out via biopsy. The amelanotic nature of the primary melanoma may have influenced the amelanotic appearance of the metastases. Our patient had no signs of immunosuppression that could have contributed to the sudden skin metastases.
Depending on the subtype of cutaneous metastases (eg, satellitosis, in-transit disease, distant cutaneous metastases), the location prevalence of the primary melanoma varies. In a study of 4865 melanoma patients who were diagnosed and followed prospectively over a 30-year period, skin metastases were mostly locoregional and presentation on the leg and foot were disproportionate.1 In contrast, the trunk was overrepresented for distant metastases. Distant metastases also were more associated with concurrent metastases to the viscera.1 Accordingly, a patient’s prognosis and management will differ depending on the subtype of cutaneous metastases.
Eruptive or multiple KAs classically have been associated with the Grzybowski variant, the Ferguson-Smith familial variant, and Muir-Torre syndrome. It was reported as a paraneoplastic syndrome associated with colon cancer, ovarian cancer, and once with myelodysplastic syndrome.3 Keratoacanthomas are being classified as well-differentiated squamous cell carcinomas and have metastatic potential. A biopsy is recommended to diagnose KAs as opposed to historically being monitored for resolution. A skin biopsy is the standard of care in management of KAs.
In addition to being associated with Muir-Torre syndrome and classified as a paraneoplastic syndrome,3 eruptive KAs can occur following skin resurfacing for actinic damage, fractional photothermolysis, cryotherapy, Jessner peels, and trichloroacetic acid peels.6 A couple other uncommon settings include a case report of an arc welder with job-associated radiation and multiple reports of tattoo-induced KAs.7,8 There is the new increasingly common association of squamous cell carcinomas with BRAF inhibitors, such as vemurafenib, for metastatic melanoma.9
In a 2012 review article on cutaneous metastases, Riahi and Cohen10 found 8 cases of cutaneous metastases presenting as KA-like lesions; none were metastatic melanoma. All were solitary lesions, not multiple lesions, as in our patient. The sources were lung (3 cases), breast, esophagus, chondrosarcoma, bronchial, and mesothelioma. The most common location was the upper lip. Additionally, similar to our patient, they behaved clinically as KAs with rapid growth and keratotic plugs and were asymptomatic.10
Metastatic melanoma may mimic many other cutaneous processes that may make the diagnosis more difficult. Our case indicates that cutaneous metastases may mimic KAs. Although multiple KA-like lesions can spontaneously occur, a paraneoplastic syndrome and other underlying etiologies should be considered.
To the Editor:
Melanoma is the third most common skin cancer. It is estimated that 18% of melanoma patients will develop skin metastases, with skin being the first site of involvement in 56% of cases.1 Of all cancers, it is estimated that 5% will develop skin metastases. It is the presenting sign in nearly 1% of visceral cancers.2 Melanoma and nonmelanoma metastases can have sundry presentations. We present a case of metastatic melanoma with multiple keratoacanthoma (KA)–like skin lesions in a patient with a known history of nonmelanoma skin cancer (NMSC) as well as melanoma.
A 76-year-old man with a history of pT2aNXMX melanoma on the left upper back presented for a routine 3-month follow-up and reported several new asymptomatic bumps on the chest, back, and right upper extremity within the last 2 weeks. The melanoma was removed via wide local excision 2 years prior at an outside facility with a Breslow depth of 1.05 mm and a negative sentinel lymph node biopsy. The mitotic rate or ulceration status was unknown. He also had a history of several NMSCs, as well as a medical history of coronary artery disease, myocardial infarction, and ventricular tachycardia with cardiac defibrillator placement. Physical examination revealed 5 pink, volcano-shaped nodules with central keratotic plugs on the upper back (Figure 1), chest, and right upper extremity, in addition to 1 pink pearly nodule on the right side of the chest. The history and appearance of the lesions were suspicious for eruptive KAs. There was no evidence of cancer recurrence at the prior melanoma and NMSC sites.
A deep shave skin biopsy was performed at all 6 sites. Histopathology showed a diffuse dermal infiltrate of elongated nests of melanocytes and nonnested melanocytes. Marked cytologic atypia and ulceration were present. Minimal connection to the overlying epidermis and a lack of junctional nests was noted. Immunohistochemical studies revealed scattered positivity for Melan-A and negative staining for AE1, AE3, cytokeratin 5, and cytokeratin 6 at all 6 sites (Figure 2). A subsequent metastatic workup showed widespread metastatic disease in the liver, bone, lung, and inferior vena cava. Computed tomography of the head was unremarkable. Magnetic resonance imaging of the brain was not performed due to the cardiac defibrillator. The patient’s lactate dehydrogenase level showed a mild increase compared to 2 months prior to the metastatic melanoma diagnosis (144 U/L vs 207 U/L [reference range, 100–200 U/L]).
The patient had no systemic symptoms at follow-up 5 weeks later. He was already evaluated by an oncologist and received his first dose of ipilimumab. He was BRAF-mutation negative. He had developed 2 new skin metastases. Five of 6 initially biopsied metastases returned and were growing; they were tender and friable with intermittent bleeding. He was subsequently referred to surgical oncology for excision of symptomatic nodules as palliative care.
Although melanoma is well known to metastasize years and even decades later, KA-like lesions have not been reported as manifestations of metastatic melanoma.4,5 Our patient likely had a primary amelanotic melanoma, as the medical records from the outside facility stated that basal cell carcinoma was ruled out via biopsy. The amelanotic nature of the primary melanoma may have influenced the amelanotic appearance of the metastases. Our patient had no signs of immunosuppression that could have contributed to the sudden skin metastases.
Depending on the subtype of cutaneous metastases (eg, satellitosis, in-transit disease, distant cutaneous metastases), the location prevalence of the primary melanoma varies. In a study of 4865 melanoma patients who were diagnosed and followed prospectively over a 30-year period, skin metastases were mostly locoregional and presentation on the leg and foot were disproportionate.1 In contrast, the trunk was overrepresented for distant metastases. Distant metastases also were more associated with concurrent metastases to the viscera.1 Accordingly, a patient’s prognosis and management will differ depending on the subtype of cutaneous metastases.
Eruptive or multiple KAs classically have been associated with the Grzybowski variant, the Ferguson-Smith familial variant, and Muir-Torre syndrome. It was reported as a paraneoplastic syndrome associated with colon cancer, ovarian cancer, and once with myelodysplastic syndrome.3 Keratoacanthomas are being classified as well-differentiated squamous cell carcinomas and have metastatic potential. A biopsy is recommended to diagnose KAs as opposed to historically being monitored for resolution. A skin biopsy is the standard of care in management of KAs.
In addition to being associated with Muir-Torre syndrome and classified as a paraneoplastic syndrome,3 eruptive KAs can occur following skin resurfacing for actinic damage, fractional photothermolysis, cryotherapy, Jessner peels, and trichloroacetic acid peels.6 A couple other uncommon settings include a case report of an arc welder with job-associated radiation and multiple reports of tattoo-induced KAs.7,8 There is the new increasingly common association of squamous cell carcinomas with BRAF inhibitors, such as vemurafenib, for metastatic melanoma.9
In a 2012 review article on cutaneous metastases, Riahi and Cohen10 found 8 cases of cutaneous metastases presenting as KA-like lesions; none were metastatic melanoma. All were solitary lesions, not multiple lesions, as in our patient. The sources were lung (3 cases), breast, esophagus, chondrosarcoma, bronchial, and mesothelioma. The most common location was the upper lip. Additionally, similar to our patient, they behaved clinically as KAs with rapid growth and keratotic plugs and were asymptomatic.10
Metastatic melanoma may mimic many other cutaneous processes that may make the diagnosis more difficult. Our case indicates that cutaneous metastases may mimic KAs. Although multiple KA-like lesions can spontaneously occur, a paraneoplastic syndrome and other underlying etiologies should be considered.
- Savoia P, Fava P, Nardò T, et al. Skin metastases of malignant melanoma: a clinical and prognostic study. Melanoma Res. 2009;19:321-326.
- Lookingbill DP, Spangler N, Sexton FM. Skin involvement as the presenting sign of internal carcinoma. J Am Acad Dermatol. 1990;22:19-26.
- Behzad M, Michl C, Pfützner W. Multiple eruptive keratoacanthomas associated with myelodysplastic syndrome. J Dtsch Dermatol Ges. 2012;10:359-360.
- Cheung WL, Patel RR, Leonard A, et al. Amelanotic melanoma: a detailed morphologic analysis with clinicopathologic correlation of 75 cases. J Cutan Pathol. 2012;39:33-39.
- Ferrari A, Piccolo D, Fargnoli MC, et al. Cutaneous amelanotic melanoma metastasis and dermatofibromas showing a dotted vascular pattern. Acta Dermato Venereologica. 2004;84:164-165.
- Mohr B, Fernandez MP, Krejci-Manwaring J. Eruptive keratoacanthoma after Jessner’s and trichloroacetic acid peel for actinic keratosis. Dermatol Surg. 2013;39:331-333.
- Wolfe CM, Green WH, Cognetta AB, et al. Multiple squamous cell carcinomas and eruptive keratoacanthomas in an arc welder. Dermatol Surg. 2013;39:328-330.
- Kluger N, Phan A, Debarbieux S, et al. Skin cancers arising in tattoos: coincidental or not? Dermatology. 2008;217:219-221.
- Mays R, Curry J, Kim K, et al. Eruptive squamous cell carcinomas after vemurafenib therapy. J Cutan Med Surg. 2013;17:419-422.
- Riahi RR, Cohen PR. Clinical manifestations of cutaneous metastases: a review with special emphasis on cutaneous metastases mimicking keratoacanthoma. Am J Clin Dermatol. 2012;13:103-112.
- Savoia P, Fava P, Nardò T, et al. Skin metastases of malignant melanoma: a clinical and prognostic study. Melanoma Res. 2009;19:321-326.
- Lookingbill DP, Spangler N, Sexton FM. Skin involvement as the presenting sign of internal carcinoma. J Am Acad Dermatol. 1990;22:19-26.
- Behzad M, Michl C, Pfützner W. Multiple eruptive keratoacanthomas associated with myelodysplastic syndrome. J Dtsch Dermatol Ges. 2012;10:359-360.
- Cheung WL, Patel RR, Leonard A, et al. Amelanotic melanoma: a detailed morphologic analysis with clinicopathologic correlation of 75 cases. J Cutan Pathol. 2012;39:33-39.
- Ferrari A, Piccolo D, Fargnoli MC, et al. Cutaneous amelanotic melanoma metastasis and dermatofibromas showing a dotted vascular pattern. Acta Dermato Venereologica. 2004;84:164-165.
- Mohr B, Fernandez MP, Krejci-Manwaring J. Eruptive keratoacanthoma after Jessner’s and trichloroacetic acid peel for actinic keratosis. Dermatol Surg. 2013;39:331-333.
- Wolfe CM, Green WH, Cognetta AB, et al. Multiple squamous cell carcinomas and eruptive keratoacanthomas in an arc welder. Dermatol Surg. 2013;39:328-330.
- Kluger N, Phan A, Debarbieux S, et al. Skin cancers arising in tattoos: coincidental or not? Dermatology. 2008;217:219-221.
- Mays R, Curry J, Kim K, et al. Eruptive squamous cell carcinomas after vemurafenib therapy. J Cutan Med Surg. 2013;17:419-422.
- Riahi RR, Cohen PR. Clinical manifestations of cutaneous metastases: a review with special emphasis on cutaneous metastases mimicking keratoacanthoma. Am J Clin Dermatol. 2012;13:103-112.
Practice Points
- Cutaneous metastatic melanoma can have variable clinical presentations.
- Patients with a history of melanoma should be monitored closely with a low threshold for biopsy of new skin lesions.