User login
Length of Stay and Readmission After Total Shoulder Arthroplasty: An Analysis of 1505 Cases
Use of total shoulder arthroplasty (TSA) and reverse TSA for shoulder conditions has increased dramatically in recent years.1 Approximately 27,000 standard TSAs were performed in the United States in 2008, and this number is expected to double by 2015.2 TSA provides excellent pain relief, restoration of function, and patient satisfaction.3 The evolution of implant design over the past 25 years has contributed to excellent long-term implant survival, with rates comparable to those of total knee and hip arthroplasty.4 Similarly, compared with previous designs, contemporary designs and techniques have resulted in fewer complications.5
Several studies have investigated the long-term complications of TSA. These complications include prosthetic loosening, instability, periprosthetic fracture, rotator cuff tears, nerve injury, and deltoid dysfunction.6-11 In addition, Waterman and colleagues11 very recently assessed the influence of risk factors on short-term postoperative complications of TSA. However, none of these studies has assessed the influence of multiple risk factors on postoperative length of stay (LOS) after TSA. Only 1 study, using data from 2005 and earlier, has analyzed the potential effect of multiple patient characteristics on readmission after TSA12; other studies have been only descriptive.13-16
We conducted a retrospective cohort study to characterize the risk factors for extended LOS and readmission after TSA in a large sample of patients drawn from a national database. We hypothesized that patient factors, including age, sex, and obesity, would be significantly associated with postoperative LOS and readmission after TSA. National databases have been increasingly used in orthopedic research, as they offer particular advantages. Large sample sizes allow for powerful analyses of associations—analyses previously not possible in single-surgeon and single-institution studies. In addition, use of a large, national patient sample allows us to draw generalizable conclusions to better define patients’ and physicians’ postoperative expectations.
Methods
We conducted a retrospective cohort study using the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) database. ACS-NSQIP collects 150 patient variables from 374 participating US hospitals.17 Patients are prospectively identified, and information is collected from operative reports, medical records, and patient interviews by trained clinical reviewers.17,18 Routine auditing by the program ensures high-quality data, with reported interrater disagreement below 2% for all variables. Data are collected through the 30th postoperative day, including after discharge.
This study was granted an exemption from our institutional review board, as we used a deidentified and publicly available database. Patients who were 60 years or older and underwent TSA between 2011 and 2012 were identified in the ACS-NSQIP database. TSA patients were identified using Current Procedural Terminology (CPT) code 23472, which includes TSA and reverse TSA procedures.
Patients were divided into groups based on surgical indications, which were available as International Classification of Diseases, Ninth Revision (ICD-9) diagnosis codes. Patients with postoperative ICD-9 codes 714.0 (rheumatoid arthritis), 715.0-9 (osteoarthritis), 716.61/716.81/716.91 (unspecified arthropathy), 718.01 (articular cartilage disorder), 718.31 (recurrent dislocation of shoulder), 718.81 (other joint derangement of shoulder), 719.41/719.91 (unspecified shoulder pain/disorder), 726.0-2 (disorder of shoulder tendons and bursa), 727.61 (rotator cuff rupture), and 840.3-9 (rotator cuff sprain) were classified as having a nonfracture indication. Patients with postoperative ICD-9 codes 716.11 (traumatic arthropathy), 833.80-89 (malunion/nonunion of fracture), and 812.00-20 (fracture of proximal humerus) were classified as having a fracture-associated indication. Patients with incomplete perioperative data were excluded from the study, leaving 1505 patients for the study (out of an initial 1726).
Patient characteristics, including sex, age, height, weight, and history of smoking, were collected from the ACS-NSQIP database. Body mass index (BMI) was calculated from each patient’s height and weight. Information about medical comorbidities was also collected from the ACS-NSQIP database. History of pulmonary disease was defined as a history of dyspnea, severe chronic obstructive pulmonary disease, ventilator-assisted respiration within 48 hours before surgery, or current pneumonia. History of heart disease was defined as a history of congestive heart failure or angina within 1 month before admission, myocardial infarction within 6 months before admission, cardiac surgery, or percutaneous coronary intervention. American Society of Anesthesiologists (ASA) class 3 or higher indicates severe systemic disease. Steroid use was defined as regular administration of corticosteroid medications within 30 days before surgery. Functional status was defined as the ability to perform activities of daily living (ADLs) within 30 days before surgery, with the patient’s best functional status during this period recorded. Similar to how other variables were collected from the database, this information was obtained through medical record abstraction and patient interviews by trained personnel. ADLs are defined in the ACS-NSQIP as “activities usually performed in the course of a normal day in a person’s life” and include bathing, feeding, dressing, toileting, and mobility. An independent patient does not require assistance for any ADLs, a partially dependent patient requires assistance for some ADLs, and a totally dependent patient requires assistance in completing all ADLs. Partially and totally dependent patients were grouped for analysis. Information about a patient’s discharge destination (to home or a facility) was also available in the database.17
Extended Length of Stay
Extended LOS was defined as a binary variable that was positive when the postoperative LOS exceeded the 90th percentile LOS. The 90th percentile LOS was chosen as a cutoff to account for normal variations in LOS and differing practices of surgeons while still capturing patients with abnormally extended LOS.
Readmission
Readmission was defined as a binary variable that was positive when a patient had an unplanned readmission 1 or more times after the initial postoperative discharge.
Patient Demographics
Table 1 summarizes the demographics and comorbidities of the 1505 TSA patients who met our study inclusion criteria. Mean age was 72.8 years (range, 60-90 years). Mean BMI was 30.3 kg/m2 (range, 15.7-63.9 kg/m2); 46.7% of patients were classified as obese (BMI, ≥30 kg/m2). The cohort was 58.9% female. Four percent of patients underwent TSA for a fracture-associated indication.
Statistical Analyses
Statistical analyses were performed with Stata 11.2 (StataCorp). Bivariate and multivariate analyses were used to test patient characteristics for association with extended LOS and readmission. Discharge destination and LOS were included in the readmission analysis because this information would be available at time of discharge and would be useful to include in a model that predicts odds of readmission.
Final multivariate models were constructed using a backward stepwise process that initially included all potential variables and sequentially excluded variables with the highest P value until only those with P < .20 remained. Variables with .05 < P < .20 were left in the model to control for potential confounding but were not considered significantly associated with the outcome. All tests were 2-tailed, and the statistical difference was established at a 2-sided α level of 0.05 (P < .05).
Results
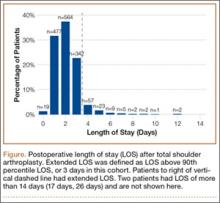
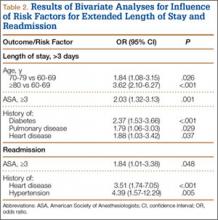
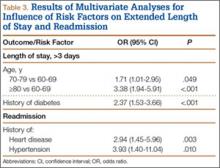
Median LOS after TSA was 2 days (interquartile range, 1-3 days), and extended LOS was defined as LOS of more than 3 days (90th percentile LOS). The distribution of LOS is depicted in the Figure. Results of the bivariate and multivariate analyses are reported in Table 2 and Table 3, respectively. Bivariate analysis revealed an association between extended LOS and increased age, ASA class 3 or higher, and history of diabetes, pulmonary disease, and heart disease. On multivariate analysis, extended LOS was associated with age 70 to 79 years (odds ratio [OR], 1.71; 95% confidence interval [CI], 1.01-2.95; P = .049), age 80 years or older (OR, 3.38; 95% CI, 1.94-5.91; P < .001), and history of diabetes (OR, 2.37; 95% CI, 1.53-3.66; P < .001).
Forty-nine patients (3.3%) were readmitted within the first 30 postoperative days. Bivariate analysis revealed an association between readmission and ASA class 3 or higher, history of heart disease, and history of hypertension. On multivariate analysis, readmission was associated only with history of heart disease (OR, 2.94; 95% CI, 1.45-5.96; P = .003) and history of hypertension (OR, 3.93; 95% CI, 1.40-11.04; P = .010).
Discussion
In the United States, TSA has become increasingly popular because of its favorable outcomes and continued implant development.1-5 However, there is a shortage of information about risk factors for short-term outcomes after TSA. In this study, we used multivariate analyses to identify patient-related factors associated with extended LOS and readmission after discharge. By identifying these factors, we can improve the preoperative discussion and postoperative planning for this procedure.
In the present study, extended LOS (>3 days) was found to be associated with older age and history of diabetes. The TSA literature has little information that can be used to compare these results, though age over 80 years was previously described as a risk factor for extended LOS after TSA.19 Uncontrolled diabetes has been identified as a risk factor for extended LOS in hip and knee arthroplasty,20 and management of diabetes may similarly complicate postoperative care, leading to extended LOS and increased costs in TSA patients. Patients with the identified risk factors for extended LOS should be counseled before surgery. In addition, this is important information for health care organizations and providers.
Readmission within 30 days after TSA was found to be independently associated with history of heart disease and history of hypertension. Similar to factors affecting LOS, patient-related risk factors for readmission are also poorly defined in the TSA literature. In total hip arthroplasty patients, heart disease has been found to be associated with readmission.21,22 Hypertension has also been associated with readmission for other orthopedic procedures.23 Results of the present study indicate these comorbidities may increase the risk for complications after discharge. It is important to note, however, that LOS did not correlate with readmission rates, indicating patients are likely being discharged at the most clinically appropriate time.
Waterman and colleagues11 very recently identified (in the ACS-NSQIP database) a patient population that underwent TSA between 2006 and 2011 to describe risk factors for postoperative complications within 30 days. They found that comorbid cardiac disease and older age were independently associated with mortality. Interestingly, the present study identified older age as associated with extended LOS, and cardiac disease as associated with readmission. Together with the results from the previous study, age and cardiac disease seem to be important patient factors to consider when planning TSA, as they are associated with a significantly worse postoperative course.
This study had several limitations. First, given the nature of the ACS-NSQIP database, readmissions are recorded only up to 30 days after surgery, including after discharge. Second, though the ACS-NSQIP tries to collect as many patient variables as possible, some information is not captured. Additional variables that could potentially affect LOS and readmission (eg, insurance status, hospital volume) were not available for analysis. However, we think the high-quality data collection process used by the ACS-NSQIP outweighs the lack of certain variables. Third, original operative notes are not available in the ACS-NSQIP database, and the only way to identify operative procedures is to check CPT codes. Unfortunately, CPT code 23472 is used for both TSA and reverse TSA, so these procedures could not be separated for analysis, and the results of this study can be used to comment only on the risks of both procedures. Another limitation is that there were not enough patients to further analyze the data by each indication.
Conclusion
With the increasing popularity of TSA for an expanding set of indications, it is important to understand the factors that can affect the postoperative course. In this study, we found several patient-related risk factors for extended LOS and readmission. Although the identified factors are generally not modifiable, this information can be used to better define the expectations of patients, providers, and organizations for this increasingly common procedure.
1. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254.
2. Day JS, Lau E, Ong KL, Williams GR, Ramsey ML, Kurtz SM. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg. 2010;19(8):1115-1120.
3. Adams JE, Sperling JW, Hoskin TL, Melton LJ 3rd, Cofield RH. Shoulder arthroplasty in Olmsted County, Minnesota, 1976–2000: a population-based study. J Shoulder Elbow Surg. 2006;15(1):50-55.
4. Farmer KW, Hammond JW, Queale WS, Keyurapan E, McFarland EG. Shoulder arthroplasty versus hip and knee arthroplasties: a comparison of outcomes. Clin Orthop Relat Res. 2007;(455):183-189.
5. Chin PY, Sperling JW, Cofield RH, Schleck C. Complications of total shoulder arthroplasty: are they fewer or different? J Shoulder Elbow Surg. 2006;15(1):19-22.
6. Bohsali KI, Wirth MA, Rockwood CA Jr. Complications of total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(10):2279-2292.
7. Torchia ME, Cofield RH, Settergren CR. Total shoulder arthroplasty with the Neer prosthesis: long-term results. J Shoulder Elbow Surg. 1997;6(6):495-505.
8. Sneppen O, Fruensgaard S, Johannsen HV, Olsen BS, Søjbjerg JO, Andersen NH. Total shoulder replacement in rheumatoid arthritis: proximal migration and loosening. J Shoulder Elbow Surg. 1996;5(1):47-52.
9. Søjbjerg JO, Frich LH, Johannsen HV, Sneppen O. Late results of total shoulder replacement in patients with rheumatoid arthritis. Clin Orthop Relat Res. 1999;(366):39-45.
10. Raiss P, Bruckner T, Rickert M, Walch G. Longitudinal observational study of total shoulder replacements with cement: fifteen to twenty-year follow-up. J Bone Joint Surg Am. 2014;96(3):198-205.
11. Waterman BR, Dunn JC, Bader J, Urrea L, Schoenfeld AJ, Belmont PJ Jr. Thirty-day morbidity and mortality after elective total shoulder arthroplasty: patient-based and surgical risk factors. J Shoulder Elbow Surg. 2015;24(1):24-30.
12. Farng E, Zingmond D, Krenek L, Soohoo NF. Factors predicting complication rates after primary shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20(4):557-563.
13. Streubel PN, Simone JP, Sperling JW, Cofield R. Thirty and ninety-day reoperation rates after shoulder arthroplasty. J Bone Joint Surg Am. 2014;96(3):e17.
14. Mahoney A, Bosco JA 3rd, Zuckerman JD. Readmission after shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(3):377-381.
15. Gay DM, Lyman S, Do H, Hotchkiss RN, Marx RG, Daluiski A. Indications and reoperation rates for total elbow arthroplasty: an analysis of trends in New York state. J Bone Joint Surg Am. 2012;94(2):110-117.
16. Zumstein MA, Pinedo M, Old J, Boileau P. Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2011;20(1):146-157.
17. American College of Surgeons. User Guide for the 2012 ACS NSQIP Participant Use Data File. https://www.facs.org/~/media/files/quality%20programs/nsqip/ug12.ashx. Published October 2013. Accessed June 21, 2015.
18. Khuri SF, Henderson WG, Daley J, et al; Principal Investigators of Patient Safety in Surgery Study. Successful implementation of the Department of Veterans Affairs’ National Surgical Quality Improvement Program in the private sector: the Patient Safety in Surgery study. Ann Surg. 2008;248(2):329-336.
19. Ricchetti ET, Abboud JA, Kuntz AF, Ramsey ML, Glaser DL, Williams GR Jr. Total shoulder arthroplasty in older patients: increased perioperative morbidity? Clin Orthop Relat Res. 2011;469(4):1042-1049.
20. Marchant MH Jr, Viens NA, Cook C, Vail TP, Bolognesi MP. The impact of glycemic control and diabetes mellitus on perioperative outcomes after total joint arthroplasty. J Bone Joint Surg Am. 2009;91(7):1621-1629.
21. Schairer WW, Sing DC, Vail TP, Bozic KJ. Causes and frequency of unplanned hospital readmission after total hip arthroplasty. Clin Orthop Relat Res. 2014;472(2):464-470.
22. Vorhies JS, Wang Y, Herndon J, Maloney WJ, Huddleston JI. Readmission and length of stay after total hip arthroplasty in a national Medicare sample. J Arthroplasty. 2011;26(6 suppl):119-123.
23. Lovecchio F, Hsu WK, Smith TR, Cybulski G, Kim B, Kim JY. Predictors of thirty-day readmission after anterior cervical fusion. Spine. 2014;39(2):127-133.
Use of total shoulder arthroplasty (TSA) and reverse TSA for shoulder conditions has increased dramatically in recent years.1 Approximately 27,000 standard TSAs were performed in the United States in 2008, and this number is expected to double by 2015.2 TSA provides excellent pain relief, restoration of function, and patient satisfaction.3 The evolution of implant design over the past 25 years has contributed to excellent long-term implant survival, with rates comparable to those of total knee and hip arthroplasty.4 Similarly, compared with previous designs, contemporary designs and techniques have resulted in fewer complications.5
Several studies have investigated the long-term complications of TSA. These complications include prosthetic loosening, instability, periprosthetic fracture, rotator cuff tears, nerve injury, and deltoid dysfunction.6-11 In addition, Waterman and colleagues11 very recently assessed the influence of risk factors on short-term postoperative complications of TSA. However, none of these studies has assessed the influence of multiple risk factors on postoperative length of stay (LOS) after TSA. Only 1 study, using data from 2005 and earlier, has analyzed the potential effect of multiple patient characteristics on readmission after TSA12; other studies have been only descriptive.13-16
We conducted a retrospective cohort study to characterize the risk factors for extended LOS and readmission after TSA in a large sample of patients drawn from a national database. We hypothesized that patient factors, including age, sex, and obesity, would be significantly associated with postoperative LOS and readmission after TSA. National databases have been increasingly used in orthopedic research, as they offer particular advantages. Large sample sizes allow for powerful analyses of associations—analyses previously not possible in single-surgeon and single-institution studies. In addition, use of a large, national patient sample allows us to draw generalizable conclusions to better define patients’ and physicians’ postoperative expectations.
Methods
We conducted a retrospective cohort study using the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) database. ACS-NSQIP collects 150 patient variables from 374 participating US hospitals.17 Patients are prospectively identified, and information is collected from operative reports, medical records, and patient interviews by trained clinical reviewers.17,18 Routine auditing by the program ensures high-quality data, with reported interrater disagreement below 2% for all variables. Data are collected through the 30th postoperative day, including after discharge.
This study was granted an exemption from our institutional review board, as we used a deidentified and publicly available database. Patients who were 60 years or older and underwent TSA between 2011 and 2012 were identified in the ACS-NSQIP database. TSA patients were identified using Current Procedural Terminology (CPT) code 23472, which includes TSA and reverse TSA procedures.
Patients were divided into groups based on surgical indications, which were available as International Classification of Diseases, Ninth Revision (ICD-9) diagnosis codes. Patients with postoperative ICD-9 codes 714.0 (rheumatoid arthritis), 715.0-9 (osteoarthritis), 716.61/716.81/716.91 (unspecified arthropathy), 718.01 (articular cartilage disorder), 718.31 (recurrent dislocation of shoulder), 718.81 (other joint derangement of shoulder), 719.41/719.91 (unspecified shoulder pain/disorder), 726.0-2 (disorder of shoulder tendons and bursa), 727.61 (rotator cuff rupture), and 840.3-9 (rotator cuff sprain) were classified as having a nonfracture indication. Patients with postoperative ICD-9 codes 716.11 (traumatic arthropathy), 833.80-89 (malunion/nonunion of fracture), and 812.00-20 (fracture of proximal humerus) were classified as having a fracture-associated indication. Patients with incomplete perioperative data were excluded from the study, leaving 1505 patients for the study (out of an initial 1726).
Patient characteristics, including sex, age, height, weight, and history of smoking, were collected from the ACS-NSQIP database. Body mass index (BMI) was calculated from each patient’s height and weight. Information about medical comorbidities was also collected from the ACS-NSQIP database. History of pulmonary disease was defined as a history of dyspnea, severe chronic obstructive pulmonary disease, ventilator-assisted respiration within 48 hours before surgery, or current pneumonia. History of heart disease was defined as a history of congestive heart failure or angina within 1 month before admission, myocardial infarction within 6 months before admission, cardiac surgery, or percutaneous coronary intervention. American Society of Anesthesiologists (ASA) class 3 or higher indicates severe systemic disease. Steroid use was defined as regular administration of corticosteroid medications within 30 days before surgery. Functional status was defined as the ability to perform activities of daily living (ADLs) within 30 days before surgery, with the patient’s best functional status during this period recorded. Similar to how other variables were collected from the database, this information was obtained through medical record abstraction and patient interviews by trained personnel. ADLs are defined in the ACS-NSQIP as “activities usually performed in the course of a normal day in a person’s life” and include bathing, feeding, dressing, toileting, and mobility. An independent patient does not require assistance for any ADLs, a partially dependent patient requires assistance for some ADLs, and a totally dependent patient requires assistance in completing all ADLs. Partially and totally dependent patients were grouped for analysis. Information about a patient’s discharge destination (to home or a facility) was also available in the database.17
Extended Length of Stay
Extended LOS was defined as a binary variable that was positive when the postoperative LOS exceeded the 90th percentile LOS. The 90th percentile LOS was chosen as a cutoff to account for normal variations in LOS and differing practices of surgeons while still capturing patients with abnormally extended LOS.
Readmission
Readmission was defined as a binary variable that was positive when a patient had an unplanned readmission 1 or more times after the initial postoperative discharge.
Patient Demographics
Table 1 summarizes the demographics and comorbidities of the 1505 TSA patients who met our study inclusion criteria. Mean age was 72.8 years (range, 60-90 years). Mean BMI was 30.3 kg/m2 (range, 15.7-63.9 kg/m2); 46.7% of patients were classified as obese (BMI, ≥30 kg/m2). The cohort was 58.9% female. Four percent of patients underwent TSA for a fracture-associated indication.
Statistical Analyses
Statistical analyses were performed with Stata 11.2 (StataCorp). Bivariate and multivariate analyses were used to test patient characteristics for association with extended LOS and readmission. Discharge destination and LOS were included in the readmission analysis because this information would be available at time of discharge and would be useful to include in a model that predicts odds of readmission.
Final multivariate models were constructed using a backward stepwise process that initially included all potential variables and sequentially excluded variables with the highest P value until only those with P < .20 remained. Variables with .05 < P < .20 were left in the model to control for potential confounding but were not considered significantly associated with the outcome. All tests were 2-tailed, and the statistical difference was established at a 2-sided α level of 0.05 (P < .05).
Results
Median LOS after TSA was 2 days (interquartile range, 1-3 days), and extended LOS was defined as LOS of more than 3 days (90th percentile LOS). The distribution of LOS is depicted in the Figure. Results of the bivariate and multivariate analyses are reported in Table 2 and Table 3, respectively. Bivariate analysis revealed an association between extended LOS and increased age, ASA class 3 or higher, and history of diabetes, pulmonary disease, and heart disease. On multivariate analysis, extended LOS was associated with age 70 to 79 years (odds ratio [OR], 1.71; 95% confidence interval [CI], 1.01-2.95; P = .049), age 80 years or older (OR, 3.38; 95% CI, 1.94-5.91; P < .001), and history of diabetes (OR, 2.37; 95% CI, 1.53-3.66; P < .001).
Forty-nine patients (3.3%) were readmitted within the first 30 postoperative days. Bivariate analysis revealed an association between readmission and ASA class 3 or higher, history of heart disease, and history of hypertension. On multivariate analysis, readmission was associated only with history of heart disease (OR, 2.94; 95% CI, 1.45-5.96; P = .003) and history of hypertension (OR, 3.93; 95% CI, 1.40-11.04; P = .010).
Discussion
In the United States, TSA has become increasingly popular because of its favorable outcomes and continued implant development.1-5 However, there is a shortage of information about risk factors for short-term outcomes after TSA. In this study, we used multivariate analyses to identify patient-related factors associated with extended LOS and readmission after discharge. By identifying these factors, we can improve the preoperative discussion and postoperative planning for this procedure.
In the present study, extended LOS (>3 days) was found to be associated with older age and history of diabetes. The TSA literature has little information that can be used to compare these results, though age over 80 years was previously described as a risk factor for extended LOS after TSA.19 Uncontrolled diabetes has been identified as a risk factor for extended LOS in hip and knee arthroplasty,20 and management of diabetes may similarly complicate postoperative care, leading to extended LOS and increased costs in TSA patients. Patients with the identified risk factors for extended LOS should be counseled before surgery. In addition, this is important information for health care organizations and providers.
Readmission within 30 days after TSA was found to be independently associated with history of heart disease and history of hypertension. Similar to factors affecting LOS, patient-related risk factors for readmission are also poorly defined in the TSA literature. In total hip arthroplasty patients, heart disease has been found to be associated with readmission.21,22 Hypertension has also been associated with readmission for other orthopedic procedures.23 Results of the present study indicate these comorbidities may increase the risk for complications after discharge. It is important to note, however, that LOS did not correlate with readmission rates, indicating patients are likely being discharged at the most clinically appropriate time.
Waterman and colleagues11 very recently identified (in the ACS-NSQIP database) a patient population that underwent TSA between 2006 and 2011 to describe risk factors for postoperative complications within 30 days. They found that comorbid cardiac disease and older age were independently associated with mortality. Interestingly, the present study identified older age as associated with extended LOS, and cardiac disease as associated with readmission. Together with the results from the previous study, age and cardiac disease seem to be important patient factors to consider when planning TSA, as they are associated with a significantly worse postoperative course.
This study had several limitations. First, given the nature of the ACS-NSQIP database, readmissions are recorded only up to 30 days after surgery, including after discharge. Second, though the ACS-NSQIP tries to collect as many patient variables as possible, some information is not captured. Additional variables that could potentially affect LOS and readmission (eg, insurance status, hospital volume) were not available for analysis. However, we think the high-quality data collection process used by the ACS-NSQIP outweighs the lack of certain variables. Third, original operative notes are not available in the ACS-NSQIP database, and the only way to identify operative procedures is to check CPT codes. Unfortunately, CPT code 23472 is used for both TSA and reverse TSA, so these procedures could not be separated for analysis, and the results of this study can be used to comment only on the risks of both procedures. Another limitation is that there were not enough patients to further analyze the data by each indication.
Conclusion
With the increasing popularity of TSA for an expanding set of indications, it is important to understand the factors that can affect the postoperative course. In this study, we found several patient-related risk factors for extended LOS and readmission. Although the identified factors are generally not modifiable, this information can be used to better define the expectations of patients, providers, and organizations for this increasingly common procedure.
Use of total shoulder arthroplasty (TSA) and reverse TSA for shoulder conditions has increased dramatically in recent years.1 Approximately 27,000 standard TSAs were performed in the United States in 2008, and this number is expected to double by 2015.2 TSA provides excellent pain relief, restoration of function, and patient satisfaction.3 The evolution of implant design over the past 25 years has contributed to excellent long-term implant survival, with rates comparable to those of total knee and hip arthroplasty.4 Similarly, compared with previous designs, contemporary designs and techniques have resulted in fewer complications.5
Several studies have investigated the long-term complications of TSA. These complications include prosthetic loosening, instability, periprosthetic fracture, rotator cuff tears, nerve injury, and deltoid dysfunction.6-11 In addition, Waterman and colleagues11 very recently assessed the influence of risk factors on short-term postoperative complications of TSA. However, none of these studies has assessed the influence of multiple risk factors on postoperative length of stay (LOS) after TSA. Only 1 study, using data from 2005 and earlier, has analyzed the potential effect of multiple patient characteristics on readmission after TSA12; other studies have been only descriptive.13-16
We conducted a retrospective cohort study to characterize the risk factors for extended LOS and readmission after TSA in a large sample of patients drawn from a national database. We hypothesized that patient factors, including age, sex, and obesity, would be significantly associated with postoperative LOS and readmission after TSA. National databases have been increasingly used in orthopedic research, as they offer particular advantages. Large sample sizes allow for powerful analyses of associations—analyses previously not possible in single-surgeon and single-institution studies. In addition, use of a large, national patient sample allows us to draw generalizable conclusions to better define patients’ and physicians’ postoperative expectations.
Methods
We conducted a retrospective cohort study using the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) database. ACS-NSQIP collects 150 patient variables from 374 participating US hospitals.17 Patients are prospectively identified, and information is collected from operative reports, medical records, and patient interviews by trained clinical reviewers.17,18 Routine auditing by the program ensures high-quality data, with reported interrater disagreement below 2% for all variables. Data are collected through the 30th postoperative day, including after discharge.
This study was granted an exemption from our institutional review board, as we used a deidentified and publicly available database. Patients who were 60 years or older and underwent TSA between 2011 and 2012 were identified in the ACS-NSQIP database. TSA patients were identified using Current Procedural Terminology (CPT) code 23472, which includes TSA and reverse TSA procedures.
Patients were divided into groups based on surgical indications, which were available as International Classification of Diseases, Ninth Revision (ICD-9) diagnosis codes. Patients with postoperative ICD-9 codes 714.0 (rheumatoid arthritis), 715.0-9 (osteoarthritis), 716.61/716.81/716.91 (unspecified arthropathy), 718.01 (articular cartilage disorder), 718.31 (recurrent dislocation of shoulder), 718.81 (other joint derangement of shoulder), 719.41/719.91 (unspecified shoulder pain/disorder), 726.0-2 (disorder of shoulder tendons and bursa), 727.61 (rotator cuff rupture), and 840.3-9 (rotator cuff sprain) were classified as having a nonfracture indication. Patients with postoperative ICD-9 codes 716.11 (traumatic arthropathy), 833.80-89 (malunion/nonunion of fracture), and 812.00-20 (fracture of proximal humerus) were classified as having a fracture-associated indication. Patients with incomplete perioperative data were excluded from the study, leaving 1505 patients for the study (out of an initial 1726).
Patient characteristics, including sex, age, height, weight, and history of smoking, were collected from the ACS-NSQIP database. Body mass index (BMI) was calculated from each patient’s height and weight. Information about medical comorbidities was also collected from the ACS-NSQIP database. History of pulmonary disease was defined as a history of dyspnea, severe chronic obstructive pulmonary disease, ventilator-assisted respiration within 48 hours before surgery, or current pneumonia. History of heart disease was defined as a history of congestive heart failure or angina within 1 month before admission, myocardial infarction within 6 months before admission, cardiac surgery, or percutaneous coronary intervention. American Society of Anesthesiologists (ASA) class 3 or higher indicates severe systemic disease. Steroid use was defined as regular administration of corticosteroid medications within 30 days before surgery. Functional status was defined as the ability to perform activities of daily living (ADLs) within 30 days before surgery, with the patient’s best functional status during this period recorded. Similar to how other variables were collected from the database, this information was obtained through medical record abstraction and patient interviews by trained personnel. ADLs are defined in the ACS-NSQIP as “activities usually performed in the course of a normal day in a person’s life” and include bathing, feeding, dressing, toileting, and mobility. An independent patient does not require assistance for any ADLs, a partially dependent patient requires assistance for some ADLs, and a totally dependent patient requires assistance in completing all ADLs. Partially and totally dependent patients were grouped for analysis. Information about a patient’s discharge destination (to home or a facility) was also available in the database.17
Extended Length of Stay
Extended LOS was defined as a binary variable that was positive when the postoperative LOS exceeded the 90th percentile LOS. The 90th percentile LOS was chosen as a cutoff to account for normal variations in LOS and differing practices of surgeons while still capturing patients with abnormally extended LOS.
Readmission
Readmission was defined as a binary variable that was positive when a patient had an unplanned readmission 1 or more times after the initial postoperative discharge.
Patient Demographics
Table 1 summarizes the demographics and comorbidities of the 1505 TSA patients who met our study inclusion criteria. Mean age was 72.8 years (range, 60-90 years). Mean BMI was 30.3 kg/m2 (range, 15.7-63.9 kg/m2); 46.7% of patients were classified as obese (BMI, ≥30 kg/m2). The cohort was 58.9% female. Four percent of patients underwent TSA for a fracture-associated indication.
Statistical Analyses
Statistical analyses were performed with Stata 11.2 (StataCorp). Bivariate and multivariate analyses were used to test patient characteristics for association with extended LOS and readmission. Discharge destination and LOS were included in the readmission analysis because this information would be available at time of discharge and would be useful to include in a model that predicts odds of readmission.
Final multivariate models were constructed using a backward stepwise process that initially included all potential variables and sequentially excluded variables with the highest P value until only those with P < .20 remained. Variables with .05 < P < .20 were left in the model to control for potential confounding but were not considered significantly associated with the outcome. All tests were 2-tailed, and the statistical difference was established at a 2-sided α level of 0.05 (P < .05).
Results
Median LOS after TSA was 2 days (interquartile range, 1-3 days), and extended LOS was defined as LOS of more than 3 days (90th percentile LOS). The distribution of LOS is depicted in the Figure. Results of the bivariate and multivariate analyses are reported in Table 2 and Table 3, respectively. Bivariate analysis revealed an association between extended LOS and increased age, ASA class 3 or higher, and history of diabetes, pulmonary disease, and heart disease. On multivariate analysis, extended LOS was associated with age 70 to 79 years (odds ratio [OR], 1.71; 95% confidence interval [CI], 1.01-2.95; P = .049), age 80 years or older (OR, 3.38; 95% CI, 1.94-5.91; P < .001), and history of diabetes (OR, 2.37; 95% CI, 1.53-3.66; P < .001).
Forty-nine patients (3.3%) were readmitted within the first 30 postoperative days. Bivariate analysis revealed an association between readmission and ASA class 3 or higher, history of heart disease, and history of hypertension. On multivariate analysis, readmission was associated only with history of heart disease (OR, 2.94; 95% CI, 1.45-5.96; P = .003) and history of hypertension (OR, 3.93; 95% CI, 1.40-11.04; P = .010).
Discussion
In the United States, TSA has become increasingly popular because of its favorable outcomes and continued implant development.1-5 However, there is a shortage of information about risk factors for short-term outcomes after TSA. In this study, we used multivariate analyses to identify patient-related factors associated with extended LOS and readmission after discharge. By identifying these factors, we can improve the preoperative discussion and postoperative planning for this procedure.
In the present study, extended LOS (>3 days) was found to be associated with older age and history of diabetes. The TSA literature has little information that can be used to compare these results, though age over 80 years was previously described as a risk factor for extended LOS after TSA.19 Uncontrolled diabetes has been identified as a risk factor for extended LOS in hip and knee arthroplasty,20 and management of diabetes may similarly complicate postoperative care, leading to extended LOS and increased costs in TSA patients. Patients with the identified risk factors for extended LOS should be counseled before surgery. In addition, this is important information for health care organizations and providers.
Readmission within 30 days after TSA was found to be independently associated with history of heart disease and history of hypertension. Similar to factors affecting LOS, patient-related risk factors for readmission are also poorly defined in the TSA literature. In total hip arthroplasty patients, heart disease has been found to be associated with readmission.21,22 Hypertension has also been associated with readmission for other orthopedic procedures.23 Results of the present study indicate these comorbidities may increase the risk for complications after discharge. It is important to note, however, that LOS did not correlate with readmission rates, indicating patients are likely being discharged at the most clinically appropriate time.
Waterman and colleagues11 very recently identified (in the ACS-NSQIP database) a patient population that underwent TSA between 2006 and 2011 to describe risk factors for postoperative complications within 30 days. They found that comorbid cardiac disease and older age were independently associated with mortality. Interestingly, the present study identified older age as associated with extended LOS, and cardiac disease as associated with readmission. Together with the results from the previous study, age and cardiac disease seem to be important patient factors to consider when planning TSA, as they are associated with a significantly worse postoperative course.
This study had several limitations. First, given the nature of the ACS-NSQIP database, readmissions are recorded only up to 30 days after surgery, including after discharge. Second, though the ACS-NSQIP tries to collect as many patient variables as possible, some information is not captured. Additional variables that could potentially affect LOS and readmission (eg, insurance status, hospital volume) were not available for analysis. However, we think the high-quality data collection process used by the ACS-NSQIP outweighs the lack of certain variables. Third, original operative notes are not available in the ACS-NSQIP database, and the only way to identify operative procedures is to check CPT codes. Unfortunately, CPT code 23472 is used for both TSA and reverse TSA, so these procedures could not be separated for analysis, and the results of this study can be used to comment only on the risks of both procedures. Another limitation is that there were not enough patients to further analyze the data by each indication.
Conclusion
With the increasing popularity of TSA for an expanding set of indications, it is important to understand the factors that can affect the postoperative course. In this study, we found several patient-related risk factors for extended LOS and readmission. Although the identified factors are generally not modifiable, this information can be used to better define the expectations of patients, providers, and organizations for this increasingly common procedure.
1. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254.
2. Day JS, Lau E, Ong KL, Williams GR, Ramsey ML, Kurtz SM. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg. 2010;19(8):1115-1120.
3. Adams JE, Sperling JW, Hoskin TL, Melton LJ 3rd, Cofield RH. Shoulder arthroplasty in Olmsted County, Minnesota, 1976–2000: a population-based study. J Shoulder Elbow Surg. 2006;15(1):50-55.
4. Farmer KW, Hammond JW, Queale WS, Keyurapan E, McFarland EG. Shoulder arthroplasty versus hip and knee arthroplasties: a comparison of outcomes. Clin Orthop Relat Res. 2007;(455):183-189.
5. Chin PY, Sperling JW, Cofield RH, Schleck C. Complications of total shoulder arthroplasty: are they fewer or different? J Shoulder Elbow Surg. 2006;15(1):19-22.
6. Bohsali KI, Wirth MA, Rockwood CA Jr. Complications of total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(10):2279-2292.
7. Torchia ME, Cofield RH, Settergren CR. Total shoulder arthroplasty with the Neer prosthesis: long-term results. J Shoulder Elbow Surg. 1997;6(6):495-505.
8. Sneppen O, Fruensgaard S, Johannsen HV, Olsen BS, Søjbjerg JO, Andersen NH. Total shoulder replacement in rheumatoid arthritis: proximal migration and loosening. J Shoulder Elbow Surg. 1996;5(1):47-52.
9. Søjbjerg JO, Frich LH, Johannsen HV, Sneppen O. Late results of total shoulder replacement in patients with rheumatoid arthritis. Clin Orthop Relat Res. 1999;(366):39-45.
10. Raiss P, Bruckner T, Rickert M, Walch G. Longitudinal observational study of total shoulder replacements with cement: fifteen to twenty-year follow-up. J Bone Joint Surg Am. 2014;96(3):198-205.
11. Waterman BR, Dunn JC, Bader J, Urrea L, Schoenfeld AJ, Belmont PJ Jr. Thirty-day morbidity and mortality after elective total shoulder arthroplasty: patient-based and surgical risk factors. J Shoulder Elbow Surg. 2015;24(1):24-30.
12. Farng E, Zingmond D, Krenek L, Soohoo NF. Factors predicting complication rates after primary shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20(4):557-563.
13. Streubel PN, Simone JP, Sperling JW, Cofield R. Thirty and ninety-day reoperation rates after shoulder arthroplasty. J Bone Joint Surg Am. 2014;96(3):e17.
14. Mahoney A, Bosco JA 3rd, Zuckerman JD. Readmission after shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(3):377-381.
15. Gay DM, Lyman S, Do H, Hotchkiss RN, Marx RG, Daluiski A. Indications and reoperation rates for total elbow arthroplasty: an analysis of trends in New York state. J Bone Joint Surg Am. 2012;94(2):110-117.
16. Zumstein MA, Pinedo M, Old J, Boileau P. Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2011;20(1):146-157.
17. American College of Surgeons. User Guide for the 2012 ACS NSQIP Participant Use Data File. https://www.facs.org/~/media/files/quality%20programs/nsqip/ug12.ashx. Published October 2013. Accessed June 21, 2015.
18. Khuri SF, Henderson WG, Daley J, et al; Principal Investigators of Patient Safety in Surgery Study. Successful implementation of the Department of Veterans Affairs’ National Surgical Quality Improvement Program in the private sector: the Patient Safety in Surgery study. Ann Surg. 2008;248(2):329-336.
19. Ricchetti ET, Abboud JA, Kuntz AF, Ramsey ML, Glaser DL, Williams GR Jr. Total shoulder arthroplasty in older patients: increased perioperative morbidity? Clin Orthop Relat Res. 2011;469(4):1042-1049.
20. Marchant MH Jr, Viens NA, Cook C, Vail TP, Bolognesi MP. The impact of glycemic control and diabetes mellitus on perioperative outcomes after total joint arthroplasty. J Bone Joint Surg Am. 2009;91(7):1621-1629.
21. Schairer WW, Sing DC, Vail TP, Bozic KJ. Causes and frequency of unplanned hospital readmission after total hip arthroplasty. Clin Orthop Relat Res. 2014;472(2):464-470.
22. Vorhies JS, Wang Y, Herndon J, Maloney WJ, Huddleston JI. Readmission and length of stay after total hip arthroplasty in a national Medicare sample. J Arthroplasty. 2011;26(6 suppl):119-123.
23. Lovecchio F, Hsu WK, Smith TR, Cybulski G, Kim B, Kim JY. Predictors of thirty-day readmission after anterior cervical fusion. Spine. 2014;39(2):127-133.
1. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254.
2. Day JS, Lau E, Ong KL, Williams GR, Ramsey ML, Kurtz SM. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg. 2010;19(8):1115-1120.
3. Adams JE, Sperling JW, Hoskin TL, Melton LJ 3rd, Cofield RH. Shoulder arthroplasty in Olmsted County, Minnesota, 1976–2000: a population-based study. J Shoulder Elbow Surg. 2006;15(1):50-55.
4. Farmer KW, Hammond JW, Queale WS, Keyurapan E, McFarland EG. Shoulder arthroplasty versus hip and knee arthroplasties: a comparison of outcomes. Clin Orthop Relat Res. 2007;(455):183-189.
5. Chin PY, Sperling JW, Cofield RH, Schleck C. Complications of total shoulder arthroplasty: are they fewer or different? J Shoulder Elbow Surg. 2006;15(1):19-22.
6. Bohsali KI, Wirth MA, Rockwood CA Jr. Complications of total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(10):2279-2292.
7. Torchia ME, Cofield RH, Settergren CR. Total shoulder arthroplasty with the Neer prosthesis: long-term results. J Shoulder Elbow Surg. 1997;6(6):495-505.
8. Sneppen O, Fruensgaard S, Johannsen HV, Olsen BS, Søjbjerg JO, Andersen NH. Total shoulder replacement in rheumatoid arthritis: proximal migration and loosening. J Shoulder Elbow Surg. 1996;5(1):47-52.
9. Søjbjerg JO, Frich LH, Johannsen HV, Sneppen O. Late results of total shoulder replacement in patients with rheumatoid arthritis. Clin Orthop Relat Res. 1999;(366):39-45.
10. Raiss P, Bruckner T, Rickert M, Walch G. Longitudinal observational study of total shoulder replacements with cement: fifteen to twenty-year follow-up. J Bone Joint Surg Am. 2014;96(3):198-205.
11. Waterman BR, Dunn JC, Bader J, Urrea L, Schoenfeld AJ, Belmont PJ Jr. Thirty-day morbidity and mortality after elective total shoulder arthroplasty: patient-based and surgical risk factors. J Shoulder Elbow Surg. 2015;24(1):24-30.
12. Farng E, Zingmond D, Krenek L, Soohoo NF. Factors predicting complication rates after primary shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20(4):557-563.
13. Streubel PN, Simone JP, Sperling JW, Cofield R. Thirty and ninety-day reoperation rates after shoulder arthroplasty. J Bone Joint Surg Am. 2014;96(3):e17.
14. Mahoney A, Bosco JA 3rd, Zuckerman JD. Readmission after shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(3):377-381.
15. Gay DM, Lyman S, Do H, Hotchkiss RN, Marx RG, Daluiski A. Indications and reoperation rates for total elbow arthroplasty: an analysis of trends in New York state. J Bone Joint Surg Am. 2012;94(2):110-117.
16. Zumstein MA, Pinedo M, Old J, Boileau P. Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2011;20(1):146-157.
17. American College of Surgeons. User Guide for the 2012 ACS NSQIP Participant Use Data File. https://www.facs.org/~/media/files/quality%20programs/nsqip/ug12.ashx. Published October 2013. Accessed June 21, 2015.
18. Khuri SF, Henderson WG, Daley J, et al; Principal Investigators of Patient Safety in Surgery Study. Successful implementation of the Department of Veterans Affairs’ National Surgical Quality Improvement Program in the private sector: the Patient Safety in Surgery study. Ann Surg. 2008;248(2):329-336.
19. Ricchetti ET, Abboud JA, Kuntz AF, Ramsey ML, Glaser DL, Williams GR Jr. Total shoulder arthroplasty in older patients: increased perioperative morbidity? Clin Orthop Relat Res. 2011;469(4):1042-1049.
20. Marchant MH Jr, Viens NA, Cook C, Vail TP, Bolognesi MP. The impact of glycemic control and diabetes mellitus on perioperative outcomes after total joint arthroplasty. J Bone Joint Surg Am. 2009;91(7):1621-1629.
21. Schairer WW, Sing DC, Vail TP, Bozic KJ. Causes and frequency of unplanned hospital readmission after total hip arthroplasty. Clin Orthop Relat Res. 2014;472(2):464-470.
22. Vorhies JS, Wang Y, Herndon J, Maloney WJ, Huddleston JI. Readmission and length of stay after total hip arthroplasty in a national Medicare sample. J Arthroplasty. 2011;26(6 suppl):119-123.
23. Lovecchio F, Hsu WK, Smith TR, Cybulski G, Kim B, Kim JY. Predictors of thirty-day readmission after anterior cervical fusion. Spine. 2014;39(2):127-133.
Risk Factors for Thromboembolic Events After Surgery for Ankle Fractures
Venous thromboembolic events (VTEs), encompassing both deep vein thrombosis (DVT) and pulmonary embolism (PE), are potentially fatal events that can occur after orthopedic surgery.1 In patients who do not receive prophylaxis, VTE incidence can be as high as 70% for total hip arthroplasty,2 26% for hip fracture,3 and 5% for ankle fracture.4 Based on the relatively low incidence of VTE after ankle fractures and insufficient evidence for VTE prophylaxis in this population, the American Orthopaedic Foot and Ankle Society and the American College of Chest Physicians do not recommend routine screening or prophylaxis for VTE in patients with ankle fractures.1,5 Nevertheless, certain patients may be at increased risk for VTE after open reduction and internal fixation (ORIF) of an ankle fracture. In such cases, further consideration for prophylaxis may be warranted.
Other studies of VTEs have identified general risk factors of increased age, obesity, prior thromboembolic disease, oral contraceptive use, multitrauma, varicose veins, and prolonged immobilization, among others.1,6,7 In orthopedics, most of this research comes from total joint arthroplasty and hip fracture studies. However, there is relatively limited data for ankle fracture. The best studies directly addressing VTE after ORIF of ankle fractures have had important limitations, including missing patient data and suboptimal capture of VTE occurrences,8-10 possibly leading to underestimates of the incidence of VTEs.
Given the limited data available, we conducted a retrospective national-cohort study to determine the incidence of and independent risk factors for VTEs after ankle fracture ORIF. If patients who are at higher risk for VTE can be identified, they can and should be carefully monitored and be considered for VTE prophylaxis. This information is needed for patient counseling and clinical decision-making.
Materials and Methods
This retrospective study used the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) database, which captures data from more than 370 participating US hospitals.11 In ACS-NSQIP, 150 patient variables are collected from operative reports, medical records, and patient interviews by trained clinical reviewers.11,12 Patients are identified prospectively and randomly sampled at participating hospitals. Routine auditing is performed to ensure high-quality data. Clinical data are collected for the entire 30-day postoperative period, regardless of discharge status during this time.
Patients who underwent ankle fracture ORIF between 2005 and 2012 were identified in the ACS-NSQIP database. They were initially selected by the postoperative diagnosis of ankle fracture (International Classification of Diseases, Ninth Revision codes 824.0-824.9). Of these patients, only those with primary Current Procedural Terminology codes 27766 (ORIF of medial malleolus fracture), 27769 (ORIF of posterior malleolus fracture), 27792 (ORIF of lateral malleolus fracture), 27814 (ORIF of bimalleollar fracture), and 27822/27823 (ORIF of trimalleollar fracture) were included in the analysis. Patients with incomplete perioperative data were excluded, leaving 4412 patients (out of the initial 4785) for analysis.
Patient characteristics, including sex, age, height, weight, and history of smoking, were collected from the ACS-NSQIP database. Body mass index (BMI) was calculated from each patient’s height and weight. Age was divided into approximately 20-year increments, beginning with age 18 years, in order to compare younger, middle-aged, and elderly groups of patients with ankle fractures. BMI was divided into categories based on the World Health Organization definitions of obesity: under 25 kg/m2 (normal weight), 25 to 30 kg/m2 (overweight), 30 to 35 kg/m2 (class I obesity), and 35 kg/m2 or over (class II and class III obesity).13
Information about medical comorbidities is also available in the ACS-NSQIP database. History of pulmonary disease was defined as a history of dyspnea, severe chronic obstructive pulmonary disease, ventilator-assisted respiration within 48 hours before surgery, or current pneumonia. History of heart disease was defined as a history of congestive heart failure (CHF) or angina within 1 month before admission, myocardial infarction within 6 months before admission, cardiac surgery, or percutaneous coronary intervention. American Society of Anesthesiologists (ASA) classes 3 and above signify severe systemic disease. Steroid use was defined as requiring regular administration of corticosteroid medications within 1 month before surgery. Disseminated cancer was defined as a malignancy that has spread to 1 or more sites besides the primary site.
Functional status was defined as the ability to perform activities of daily living (ADLs) within 30 days before surgery. Best functional status during this period was recorded. ACS-NSQIP defines ADLs as the “activities usually performed in the course of a normal day in a person’s life,” including bathing, feeding, dressing, toileting, and mobility. An independent patient does not require assistance for any ADLs; a partially dependent patient requires assistance for some ADLs; and a totally dependent patient requires assistance in all ADLs. Partially and totally dependent patients were grouped for analysis. Anesthesia type was separated into general and nongeneral, which includes monitored anesthesia care, spinal anesthesia, and regional anesthesia.
ACS-NSQIP also records the occurrence of multiple events up to 30 days after surgery. For our study, VTE was defined as the occurrence of a DVT or a PE during this period. ACS-NSQIP defines DVT as a new blood clot or thrombus identified within a vein—with confirmation by duplex ultrasonography, venogram, or computed tomography (CT)—that required therapy (anticoagulation, placement of vena cava filter, and/or clipping of vena cava). PE is recorded if ventilation/perfusion (VQ) scan, CT examination, transesophageal echocardiogram, pulmonary arteriogram, CT angiogram, or any other definitive modality is positive.
Statistical analyses were performed with Stata Version 11.2 (StataCorp). Demographic and comorbidity variables were tested for association with occurrence of VTE using bivariate and multivariate logistic regression.
Final multivariate models were constructed with a backward stepwise process that initially included all potential variables and sequentially excluded variables with the highest P value until only those with P < .200 remained. Variables with .050 < P < .200 were left in the model to control for potential confounding but are not considered significantly associated with the outcome. Statistical significance was established at a 2-sided α of 0.050 (P < .050). The fitness of the final logistic regression model was assessed with the C statistic and the Hosmer-Lemeshow goodness-of-fit test.
Results
For the 4412 ankle fracture patients who met the inclusion criteria, mean (SD) age was 50.9 (18.2) years, and mean (SD) BMI was 30.4 (7.6) kg/m2. The cohort was 40.4% male. Surgery was performed on 235 patients (5.3%) with medial malleolus fracture, 1143 patients (25.9%) with lateral malleolus fracture, 1705 patients (38.6%) with bimalleollar fracture, and 1329 patients (30.1%) with trimalleollar fracture. Table 1 summarizes the patient characteristics.
Of the 33 patients (0.8%) with a VTE recorded within the first 30 postoperative days, 16 (0.4% of all patients) had a DVT recorded, 14 (0.3% of all patients) had a PE recorded, and 3 (0.1% of all patients) had both a DVT and a PE recorded. In 13 (39.4%) of the 33 patients with a VTE, the event occurred after discharge. VTEs were reported a mean (SD) of 11.5 (9.6) days after surgery. No patient in this study died of VTE.
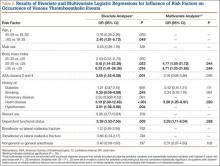
Bivariate logistic regressions were performed to test the association of each patient variable with the occurrence of a VTE. Results are listed in Table 2. The bivariate analyses revealed significant associations between VTE after ankle fracture ORIF and the patient variables of age 60 years or older (odds ratio [OR], 2.40; 95% confidence interval [CI], 1.01-5.72), class I obesity (BMI, 30-35 kg/m2: OR, 5.15, 95% CI, 1.14-23.28), class II and class III obesity (BMI, ≥35 kg/m2: OR, 6.33, 95% CI, 1.41-28.38), ASA classes 3 and 4 (OR, 3.05; 95% CI, 1.53-6.08), history of heart disease (OR, 5.10; 95% CI, 2.08-12.49), history of hypertension (OR, 2.81; 95% CI, 1.39-5.66), and dependent functional status (OR, 3.39; 95% CI, 1.52-7.56).
Multivariate logistic regression was used to control for potential confounding variables and determine which factors were independently associated with VTEs. Results of this analysis are listed in Table 2 as well. The multivariate analysis revealed that the patient variables of class I obesity (BMI, 30-35 kg/m2: OR, 4.77; 95% CI, 1.05-21.72; P = .044), class II and class III obesity (BMI, ≥35 kg/m2: OR, 4.71; 95% CI, 1.03-21.68; P = .046), history of heart disease (OR, 3.28; 95% CI, 1.20-8.97; P = .020), and dependent functional status (OR, 2.59; 95% CI, 1.11-6.04; P = .028) were independently associated with an increased rate of VTEs. Of note, anesthesia type was not significantly associated with occurrence of VTE on bivariate or multivariate analysis.
The C statistic of the final multivariate model was 0.76, indicating very good distinguishing ability. The Hosmer-Lemeshow goodness-of-fit test showed no evidence of lack of fit.
Discussion
Citing the lack of conclusive evidence and the low incidence of VTE after ankle fracture surgery, current recommendations are to avoid routine VTE prophylaxis in the postoperative management of patients who undergo this surgery.1,5 However, it is important to identify patients who are at increased risk, as some may benefit from VTE prophylaxis. In the present study, we used the large, high-quality ACS-NSQIP database collecting information from multiple US hospitals to examine risk factors for VTE after ankle fracture ORIF. We identified 4412 patients who underwent ankle fracture ORIF between 2005 and 2012, and found an overall VTE incidence of 0.8%. Multivariate analysis identified obesity, history of heart disease, and dependent functional status as independent risk factors for VTE after ankle fracture ORIF.
This study’s 0.8% incidence of VTE after ankle fracture ORIF is consistent with the range (0.29%-5%) reported in other ankle fracture studies.4,8-10,14-18 We found that VTEs occurred a mean of about 11 days after surgery, and no patient died of VTE.
Obesity (BMI, ≥30 kg/m2) had the strongest association with VTEs in this study. Obesity, which is a growing public health concern, can make postoperative care and mobilization more difficult.19 Obesity has previously been associated with VTEs after ankle fractures, and BMI of over 25 kg/m2 is one of the Caprini criteria for thrombosis risk factor assessment.6,10 In our study, however, BMI of 25 to 30 kg/m2 was not associated with an increased VTE rate, indicating that moderately overweight patients may not be at significantly higher risk for VTE (compared with patients with normal BMI) and may not need VTE prophylaxis. VTE prophylaxis after ankle fracture surgery may be considered in patients with BMI over 30 kg/m2.
History of heart disease was also associated with VTEs in this study. Patients with a history of heart disease were at 3 times the risk for VTE within 30 days of ankle fracture surgery. This association is also consistent with the Caprini criteria, which include acute myocardial infarction and CHF as risk factors for venous thrombosis.6 Other studies have found associations between CHF and VTE and between cardiovascular risk factors and VTE.7,20 The association between cardiovascular disease and VTE may derive from the decreased venous flow rate associated with CHF or an overall vascular disease state. These patients may benefit from heightened surveillance and postoperative prophylaxis for VTE.
Dependent functional status was the final risk factor found to be associated with VTE after ankle fracture ORIF. This association likely derives from an inability to mobilize independently, leading to increased venous stasis. Immobilization has been previously associated with increased risk for VTE after ankle surgery.7,14,16,20 Caretakers should be aware of this increased risk during the postoperative period and diligently monitor these patients for signs and symptoms of VTE. Prophylaxis may also be considered in this patient population.
Several risk factors that were significant on bivariate analysis (increased age; increased ASA class; history of diabetes, pulmonary disease, hypertension) were not significant in the final multivariate model. This finding suggests covariance between these factors and those that were significant in the final multivariate model. In particular, age and increased overall comorbidity (represented by increased ASA class) were not significant in our multivariate model—contrary to findings of other studies.8-10 It is possible that history of heart disease alone was responsible for the association between overall comorbidity and VTE in those studies. In the present study, separating and controlling for individual comorbidities could have allowed this association to be more precisely characterized.
The characteristics of the ACS-NSQIP database limited our study in several ways. First, although ACS-NSQIP makes significant efforts to collect as many patient variables as possible, some information is not captured. Data about additional factors that may affect VTE risk (eg, history of previous VTE, hypercoagulable state, history of malignancy other than disseminated cancer, tourniquet time, patient position in operating room) were not available. Second, data are collected only on those postoperative adverse events that occur within 30 days after surgery; data on VTEs that occur later are not captured. However, it has been shown that the majority of VTEs occur within the first 30 days after lower extremity trauma and surgery,21,22 so this follow-up interval was deemed adequate for capture of VTE data. Third, the database does not include information on the prophylactic regimens used for these patients—which may have weakened the associations between predictor variables and VTE risk and led to an underestimated effect size. VTE incidence, as well as the odds of developing a VTE with one of the identified risk factors, may actually be higher than reported in this study.
Conclusion
VTEs are serious complications that can occur after ORIF of ankle fractures. In this study, the overall incidence of VTE after ankle fracture ORIF was 0.8%. Although the American Orthopaedic Foot and Ankle Society and the American College of Chest Physicians do not recommend routine screening or prophylaxis for VTE in patients with ankle fractures,1,5 the results of this study showed there may be a benefit in emphasizing VTE prophylaxis after ankle fracture ORIF in patients with obesity, history of heart disease, or dependent functional status. At minimum, these patients should be more carefully monitored for development of VTEs.
1. American Orthopaedic Foot and Ankle Society. Position statement: the use of VTED prophylaxis in foot and ankle surgery. http://www.aofas.org/medical-community/health-policy/Documents/VTED-Position-Statement-Approv-7-9-13-FINAL.pdf. Updated 2013. Accessed May 10, 2015.
2. Grady-Benson JC, Oishi CS, Hanson PB, Colwell CW Jr, Otis SM, Walker RH. Routine postoperative duplex ultrasonography screening and monitoring for the detection of deep vein thrombosis. A survey of 110 total hip arthroplasties. Clin Orthop Relat Res. 1994;(307):130-141.
3. Salzman EW, Harris WH, DeSanctis RW. Anticoagulation for prevention of thromboembolism following fractures of the hip. New Engl J Med. 1966;275(3):122-130.
4. Patil S, Gandhi J, Curzon I, Hui AC. Incidence of deep-vein thrombosis in patients with fractures of the ankle treated in a plaster cast. J Bone Joint Surg Br. 2007;89(10):1340-1343.
5. Falck-Ytter Y, Francis CW, Johanson NA, et al; American College of Chest Physicians. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e278S-e325S.
6. Caprini JA. Thrombosis risk assessment as a guide to quality patient care. Dis Mon. 2005;51(2-3):70-78.
7. Mayle RE Jr, DiGiovanni CW, Lin SS, Tabrizi P, Chou LB. Current concepts review: venous thromboembolic disease in foot and ankle surgery. Foot Ankle Int. 2007;28(11):1207-1216.
8. Jameson SS, Augustine A, James P, et al. Venous thromboembolic events following foot and ankle surgery in the English National Health Service. J Bone Joint Surg Br. 2011;93(4):490-497.
9. SooHoo NF, Eagan M, Krenek L, Zingmond DS. Incidence and factors predicting pulmonary embolism and deep venous thrombosis following surgical treatment of ankle fractures. Foot Ankle Surg. 2011;17(4):259-262.
10. Shibuya N, Frost CH, Campbell JD, Davis ML, Jupiter DC. Incidence of acute deep vein thrombosis and pulmonary embolism in foot and ankle trauma: analysis of the National Trauma Data Bank. J Foot Ankle Surg. 2012;51(1):63-68.
11. American College of Surgeons National Surgical Quality Improvement Program. User Guide for the 2012 ACS NSQIP Participant Use Data File. http://site.acsnsqip.org/wp-content/uploads/2013/10/ACSNSQIP.PUF_.UserGuide.2012.pdf. Published October 2013. Accessed May 10, 2015.
12. Khuri SF, Henderson WG, Daley J, et al; Principal Investigators of Patient Safety in Surgery Study. Successful implementation of the Department of Veterans Affairs’ National Surgical Quality Improvement Program in the private sector: the Patient Safety in Surgery study. Ann Surg. 2008;248(2):329-336.
13. Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA. 1999;282(16):1523-1529.
14. Mizel MS, Temple HT, Michelson JD, et al. Thromboembolism after foot and ankle surgery. A multicenter study. Clin Orthop Relat Res. 1998;(348):180-185.
15. Solis G, Saxby T. Incidence of DVT following surgery of the foot and ankle. Foot Ankle Int. 2002;23(5):411-414.
16. Hanslow SS, Grujic L, Slater HK, Chen D. Thromboembolic disease after foot and ankle surgery. Foot Ankle Int. 2006;27(9):693-695.
17. Pelet S, Roger ME, Belzile EL, Bouchard M. The incidence of thromboembolic events in surgically treated ankle fracture. J Bone Joint Surg Am. 2012;94(6):502-506.
18. Manafi Rasi A, Kazemian G, Emami Moghadam M, et al. Deep vein thrombosis following below knee immobilization: the need for chemoprophylaxis. Trauma Mon. 2013;17(4):367-369.
19. Sabharwal S, Root MZ. Impact of obesity on orthopaedics. J Bone Joint Surg Am. 2012;94(11):1045-1052.
20. Kadous A, Abdelgawad AA, Kanlic E. Deep venous thrombosis and pulmonary embolism after surgical treatment of ankle fractures: a case report and review of literature. J Foot Ankle Surg. 2012;51(4):457-463.
21. Forsythe RM, Peitzman AB, DeCato T, et al. Early lower extremity fracture fixation and the risk of early pulmonary embolus: filter before fixation? J Trauma. 2011;70(6):1381-1388.
22. Bjørnarå BT, Gudmundsen TE, Dahl OE. Frequency and timing of clinical venous thromboembolism after major joint surgery. J Bone Joint Surg Br. 2006;88(3):386-391.
Venous thromboembolic events (VTEs), encompassing both deep vein thrombosis (DVT) and pulmonary embolism (PE), are potentially fatal events that can occur after orthopedic surgery.1 In patients who do not receive prophylaxis, VTE incidence can be as high as 70% for total hip arthroplasty,2 26% for hip fracture,3 and 5% for ankle fracture.4 Based on the relatively low incidence of VTE after ankle fractures and insufficient evidence for VTE prophylaxis in this population, the American Orthopaedic Foot and Ankle Society and the American College of Chest Physicians do not recommend routine screening or prophylaxis for VTE in patients with ankle fractures.1,5 Nevertheless, certain patients may be at increased risk for VTE after open reduction and internal fixation (ORIF) of an ankle fracture. In such cases, further consideration for prophylaxis may be warranted.
Other studies of VTEs have identified general risk factors of increased age, obesity, prior thromboembolic disease, oral contraceptive use, multitrauma, varicose veins, and prolonged immobilization, among others.1,6,7 In orthopedics, most of this research comes from total joint arthroplasty and hip fracture studies. However, there is relatively limited data for ankle fracture. The best studies directly addressing VTE after ORIF of ankle fractures have had important limitations, including missing patient data and suboptimal capture of VTE occurrences,8-10 possibly leading to underestimates of the incidence of VTEs.
Given the limited data available, we conducted a retrospective national-cohort study to determine the incidence of and independent risk factors for VTEs after ankle fracture ORIF. If patients who are at higher risk for VTE can be identified, they can and should be carefully monitored and be considered for VTE prophylaxis. This information is needed for patient counseling and clinical decision-making.
Materials and Methods
This retrospective study used the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) database, which captures data from more than 370 participating US hospitals.11 In ACS-NSQIP, 150 patient variables are collected from operative reports, medical records, and patient interviews by trained clinical reviewers.11,12 Patients are identified prospectively and randomly sampled at participating hospitals. Routine auditing is performed to ensure high-quality data. Clinical data are collected for the entire 30-day postoperative period, regardless of discharge status during this time.
Patients who underwent ankle fracture ORIF between 2005 and 2012 were identified in the ACS-NSQIP database. They were initially selected by the postoperative diagnosis of ankle fracture (International Classification of Diseases, Ninth Revision codes 824.0-824.9). Of these patients, only those with primary Current Procedural Terminology codes 27766 (ORIF of medial malleolus fracture), 27769 (ORIF of posterior malleolus fracture), 27792 (ORIF of lateral malleolus fracture), 27814 (ORIF of bimalleollar fracture), and 27822/27823 (ORIF of trimalleollar fracture) were included in the analysis. Patients with incomplete perioperative data were excluded, leaving 4412 patients (out of the initial 4785) for analysis.
Patient characteristics, including sex, age, height, weight, and history of smoking, were collected from the ACS-NSQIP database. Body mass index (BMI) was calculated from each patient’s height and weight. Age was divided into approximately 20-year increments, beginning with age 18 years, in order to compare younger, middle-aged, and elderly groups of patients with ankle fractures. BMI was divided into categories based on the World Health Organization definitions of obesity: under 25 kg/m2 (normal weight), 25 to 30 kg/m2 (overweight), 30 to 35 kg/m2 (class I obesity), and 35 kg/m2 or over (class II and class III obesity).13
Information about medical comorbidities is also available in the ACS-NSQIP database. History of pulmonary disease was defined as a history of dyspnea, severe chronic obstructive pulmonary disease, ventilator-assisted respiration within 48 hours before surgery, or current pneumonia. History of heart disease was defined as a history of congestive heart failure (CHF) or angina within 1 month before admission, myocardial infarction within 6 months before admission, cardiac surgery, or percutaneous coronary intervention. American Society of Anesthesiologists (ASA) classes 3 and above signify severe systemic disease. Steroid use was defined as requiring regular administration of corticosteroid medications within 1 month before surgery. Disseminated cancer was defined as a malignancy that has spread to 1 or more sites besides the primary site.
Functional status was defined as the ability to perform activities of daily living (ADLs) within 30 days before surgery. Best functional status during this period was recorded. ACS-NSQIP defines ADLs as the “activities usually performed in the course of a normal day in a person’s life,” including bathing, feeding, dressing, toileting, and mobility. An independent patient does not require assistance for any ADLs; a partially dependent patient requires assistance for some ADLs; and a totally dependent patient requires assistance in all ADLs. Partially and totally dependent patients were grouped for analysis. Anesthesia type was separated into general and nongeneral, which includes monitored anesthesia care, spinal anesthesia, and regional anesthesia.
ACS-NSQIP also records the occurrence of multiple events up to 30 days after surgery. For our study, VTE was defined as the occurrence of a DVT or a PE during this period. ACS-NSQIP defines DVT as a new blood clot or thrombus identified within a vein—with confirmation by duplex ultrasonography, venogram, or computed tomography (CT)—that required therapy (anticoagulation, placement of vena cava filter, and/or clipping of vena cava). PE is recorded if ventilation/perfusion (VQ) scan, CT examination, transesophageal echocardiogram, pulmonary arteriogram, CT angiogram, or any other definitive modality is positive.
Statistical analyses were performed with Stata Version 11.2 (StataCorp). Demographic and comorbidity variables were tested for association with occurrence of VTE using bivariate and multivariate logistic regression.
Final multivariate models were constructed with a backward stepwise process that initially included all potential variables and sequentially excluded variables with the highest P value until only those with P < .200 remained. Variables with .050 < P < .200 were left in the model to control for potential confounding but are not considered significantly associated with the outcome. Statistical significance was established at a 2-sided α of 0.050 (P < .050). The fitness of the final logistic regression model was assessed with the C statistic and the Hosmer-Lemeshow goodness-of-fit test.
Results
For the 4412 ankle fracture patients who met the inclusion criteria, mean (SD) age was 50.9 (18.2) years, and mean (SD) BMI was 30.4 (7.6) kg/m2. The cohort was 40.4% male. Surgery was performed on 235 patients (5.3%) with medial malleolus fracture, 1143 patients (25.9%) with lateral malleolus fracture, 1705 patients (38.6%) with bimalleollar fracture, and 1329 patients (30.1%) with trimalleollar fracture. Table 1 summarizes the patient characteristics.
Of the 33 patients (0.8%) with a VTE recorded within the first 30 postoperative days, 16 (0.4% of all patients) had a DVT recorded, 14 (0.3% of all patients) had a PE recorded, and 3 (0.1% of all patients) had both a DVT and a PE recorded. In 13 (39.4%) of the 33 patients with a VTE, the event occurred after discharge. VTEs were reported a mean (SD) of 11.5 (9.6) days after surgery. No patient in this study died of VTE.
Bivariate logistic regressions were performed to test the association of each patient variable with the occurrence of a VTE. Results are listed in Table 2. The bivariate analyses revealed significant associations between VTE after ankle fracture ORIF and the patient variables of age 60 years or older (odds ratio [OR], 2.40; 95% confidence interval [CI], 1.01-5.72), class I obesity (BMI, 30-35 kg/m2: OR, 5.15, 95% CI, 1.14-23.28), class II and class III obesity (BMI, ≥35 kg/m2: OR, 6.33, 95% CI, 1.41-28.38), ASA classes 3 and 4 (OR, 3.05; 95% CI, 1.53-6.08), history of heart disease (OR, 5.10; 95% CI, 2.08-12.49), history of hypertension (OR, 2.81; 95% CI, 1.39-5.66), and dependent functional status (OR, 3.39; 95% CI, 1.52-7.56).
Multivariate logistic regression was used to control for potential confounding variables and determine which factors were independently associated with VTEs. Results of this analysis are listed in Table 2 as well. The multivariate analysis revealed that the patient variables of class I obesity (BMI, 30-35 kg/m2: OR, 4.77; 95% CI, 1.05-21.72; P = .044), class II and class III obesity (BMI, ≥35 kg/m2: OR, 4.71; 95% CI, 1.03-21.68; P = .046), history of heart disease (OR, 3.28; 95% CI, 1.20-8.97; P = .020), and dependent functional status (OR, 2.59; 95% CI, 1.11-6.04; P = .028) were independently associated with an increased rate of VTEs. Of note, anesthesia type was not significantly associated with occurrence of VTE on bivariate or multivariate analysis.
The C statistic of the final multivariate model was 0.76, indicating very good distinguishing ability. The Hosmer-Lemeshow goodness-of-fit test showed no evidence of lack of fit.
Discussion
Citing the lack of conclusive evidence and the low incidence of VTE after ankle fracture surgery, current recommendations are to avoid routine VTE prophylaxis in the postoperative management of patients who undergo this surgery.1,5 However, it is important to identify patients who are at increased risk, as some may benefit from VTE prophylaxis. In the present study, we used the large, high-quality ACS-NSQIP database collecting information from multiple US hospitals to examine risk factors for VTE after ankle fracture ORIF. We identified 4412 patients who underwent ankle fracture ORIF between 2005 and 2012, and found an overall VTE incidence of 0.8%. Multivariate analysis identified obesity, history of heart disease, and dependent functional status as independent risk factors for VTE after ankle fracture ORIF.
This study’s 0.8% incidence of VTE after ankle fracture ORIF is consistent with the range (0.29%-5%) reported in other ankle fracture studies.4,8-10,14-18 We found that VTEs occurred a mean of about 11 days after surgery, and no patient died of VTE.
Obesity (BMI, ≥30 kg/m2) had the strongest association with VTEs in this study. Obesity, which is a growing public health concern, can make postoperative care and mobilization more difficult.19 Obesity has previously been associated with VTEs after ankle fractures, and BMI of over 25 kg/m2 is one of the Caprini criteria for thrombosis risk factor assessment.6,10 In our study, however, BMI of 25 to 30 kg/m2 was not associated with an increased VTE rate, indicating that moderately overweight patients may not be at significantly higher risk for VTE (compared with patients with normal BMI) and may not need VTE prophylaxis. VTE prophylaxis after ankle fracture surgery may be considered in patients with BMI over 30 kg/m2.
History of heart disease was also associated with VTEs in this study. Patients with a history of heart disease were at 3 times the risk for VTE within 30 days of ankle fracture surgery. This association is also consistent with the Caprini criteria, which include acute myocardial infarction and CHF as risk factors for venous thrombosis.6 Other studies have found associations between CHF and VTE and between cardiovascular risk factors and VTE.7,20 The association between cardiovascular disease and VTE may derive from the decreased venous flow rate associated with CHF or an overall vascular disease state. These patients may benefit from heightened surveillance and postoperative prophylaxis for VTE.
Dependent functional status was the final risk factor found to be associated with VTE after ankle fracture ORIF. This association likely derives from an inability to mobilize independently, leading to increased venous stasis. Immobilization has been previously associated with increased risk for VTE after ankle surgery.7,14,16,20 Caretakers should be aware of this increased risk during the postoperative period and diligently monitor these patients for signs and symptoms of VTE. Prophylaxis may also be considered in this patient population.
Several risk factors that were significant on bivariate analysis (increased age; increased ASA class; history of diabetes, pulmonary disease, hypertension) were not significant in the final multivariate model. This finding suggests covariance between these factors and those that were significant in the final multivariate model. In particular, age and increased overall comorbidity (represented by increased ASA class) were not significant in our multivariate model—contrary to findings of other studies.8-10 It is possible that history of heart disease alone was responsible for the association between overall comorbidity and VTE in those studies. In the present study, separating and controlling for individual comorbidities could have allowed this association to be more precisely characterized.
The characteristics of the ACS-NSQIP database limited our study in several ways. First, although ACS-NSQIP makes significant efforts to collect as many patient variables as possible, some information is not captured. Data about additional factors that may affect VTE risk (eg, history of previous VTE, hypercoagulable state, history of malignancy other than disseminated cancer, tourniquet time, patient position in operating room) were not available. Second, data are collected only on those postoperative adverse events that occur within 30 days after surgery; data on VTEs that occur later are not captured. However, it has been shown that the majority of VTEs occur within the first 30 days after lower extremity trauma and surgery,21,22 so this follow-up interval was deemed adequate for capture of VTE data. Third, the database does not include information on the prophylactic regimens used for these patients—which may have weakened the associations between predictor variables and VTE risk and led to an underestimated effect size. VTE incidence, as well as the odds of developing a VTE with one of the identified risk factors, may actually be higher than reported in this study.
Conclusion
VTEs are serious complications that can occur after ORIF of ankle fractures. In this study, the overall incidence of VTE after ankle fracture ORIF was 0.8%. Although the American Orthopaedic Foot and Ankle Society and the American College of Chest Physicians do not recommend routine screening or prophylaxis for VTE in patients with ankle fractures,1,5 the results of this study showed there may be a benefit in emphasizing VTE prophylaxis after ankle fracture ORIF in patients with obesity, history of heart disease, or dependent functional status. At minimum, these patients should be more carefully monitored for development of VTEs.
Venous thromboembolic events (VTEs), encompassing both deep vein thrombosis (DVT) and pulmonary embolism (PE), are potentially fatal events that can occur after orthopedic surgery.1 In patients who do not receive prophylaxis, VTE incidence can be as high as 70% for total hip arthroplasty,2 26% for hip fracture,3 and 5% for ankle fracture.4 Based on the relatively low incidence of VTE after ankle fractures and insufficient evidence for VTE prophylaxis in this population, the American Orthopaedic Foot and Ankle Society and the American College of Chest Physicians do not recommend routine screening or prophylaxis for VTE in patients with ankle fractures.1,5 Nevertheless, certain patients may be at increased risk for VTE after open reduction and internal fixation (ORIF) of an ankle fracture. In such cases, further consideration for prophylaxis may be warranted.
Other studies of VTEs have identified general risk factors of increased age, obesity, prior thromboembolic disease, oral contraceptive use, multitrauma, varicose veins, and prolonged immobilization, among others.1,6,7 In orthopedics, most of this research comes from total joint arthroplasty and hip fracture studies. However, there is relatively limited data for ankle fracture. The best studies directly addressing VTE after ORIF of ankle fractures have had important limitations, including missing patient data and suboptimal capture of VTE occurrences,8-10 possibly leading to underestimates of the incidence of VTEs.
Given the limited data available, we conducted a retrospective national-cohort study to determine the incidence of and independent risk factors for VTEs after ankle fracture ORIF. If patients who are at higher risk for VTE can be identified, they can and should be carefully monitored and be considered for VTE prophylaxis. This information is needed for patient counseling and clinical decision-making.
Materials and Methods
This retrospective study used the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) database, which captures data from more than 370 participating US hospitals.11 In ACS-NSQIP, 150 patient variables are collected from operative reports, medical records, and patient interviews by trained clinical reviewers.11,12 Patients are identified prospectively and randomly sampled at participating hospitals. Routine auditing is performed to ensure high-quality data. Clinical data are collected for the entire 30-day postoperative period, regardless of discharge status during this time.
Patients who underwent ankle fracture ORIF between 2005 and 2012 were identified in the ACS-NSQIP database. They were initially selected by the postoperative diagnosis of ankle fracture (International Classification of Diseases, Ninth Revision codes 824.0-824.9). Of these patients, only those with primary Current Procedural Terminology codes 27766 (ORIF of medial malleolus fracture), 27769 (ORIF of posterior malleolus fracture), 27792 (ORIF of lateral malleolus fracture), 27814 (ORIF of bimalleollar fracture), and 27822/27823 (ORIF of trimalleollar fracture) were included in the analysis. Patients with incomplete perioperative data were excluded, leaving 4412 patients (out of the initial 4785) for analysis.
Patient characteristics, including sex, age, height, weight, and history of smoking, were collected from the ACS-NSQIP database. Body mass index (BMI) was calculated from each patient’s height and weight. Age was divided into approximately 20-year increments, beginning with age 18 years, in order to compare younger, middle-aged, and elderly groups of patients with ankle fractures. BMI was divided into categories based on the World Health Organization definitions of obesity: under 25 kg/m2 (normal weight), 25 to 30 kg/m2 (overweight), 30 to 35 kg/m2 (class I obesity), and 35 kg/m2 or over (class II and class III obesity).13
Information about medical comorbidities is also available in the ACS-NSQIP database. History of pulmonary disease was defined as a history of dyspnea, severe chronic obstructive pulmonary disease, ventilator-assisted respiration within 48 hours before surgery, or current pneumonia. History of heart disease was defined as a history of congestive heart failure (CHF) or angina within 1 month before admission, myocardial infarction within 6 months before admission, cardiac surgery, or percutaneous coronary intervention. American Society of Anesthesiologists (ASA) classes 3 and above signify severe systemic disease. Steroid use was defined as requiring regular administration of corticosteroid medications within 1 month before surgery. Disseminated cancer was defined as a malignancy that has spread to 1 or more sites besides the primary site.
Functional status was defined as the ability to perform activities of daily living (ADLs) within 30 days before surgery. Best functional status during this period was recorded. ACS-NSQIP defines ADLs as the “activities usually performed in the course of a normal day in a person’s life,” including bathing, feeding, dressing, toileting, and mobility. An independent patient does not require assistance for any ADLs; a partially dependent patient requires assistance for some ADLs; and a totally dependent patient requires assistance in all ADLs. Partially and totally dependent patients were grouped for analysis. Anesthesia type was separated into general and nongeneral, which includes monitored anesthesia care, spinal anesthesia, and regional anesthesia.
ACS-NSQIP also records the occurrence of multiple events up to 30 days after surgery. For our study, VTE was defined as the occurrence of a DVT or a PE during this period. ACS-NSQIP defines DVT as a new blood clot or thrombus identified within a vein—with confirmation by duplex ultrasonography, venogram, or computed tomography (CT)—that required therapy (anticoagulation, placement of vena cava filter, and/or clipping of vena cava). PE is recorded if ventilation/perfusion (VQ) scan, CT examination, transesophageal echocardiogram, pulmonary arteriogram, CT angiogram, or any other definitive modality is positive.
Statistical analyses were performed with Stata Version 11.2 (StataCorp). Demographic and comorbidity variables were tested for association with occurrence of VTE using bivariate and multivariate logistic regression.
Final multivariate models were constructed with a backward stepwise process that initially included all potential variables and sequentially excluded variables with the highest P value until only those with P < .200 remained. Variables with .050 < P < .200 were left in the model to control for potential confounding but are not considered significantly associated with the outcome. Statistical significance was established at a 2-sided α of 0.050 (P < .050). The fitness of the final logistic regression model was assessed with the C statistic and the Hosmer-Lemeshow goodness-of-fit test.
Results
For the 4412 ankle fracture patients who met the inclusion criteria, mean (SD) age was 50.9 (18.2) years, and mean (SD) BMI was 30.4 (7.6) kg/m2. The cohort was 40.4% male. Surgery was performed on 235 patients (5.3%) with medial malleolus fracture, 1143 patients (25.9%) with lateral malleolus fracture, 1705 patients (38.6%) with bimalleollar fracture, and 1329 patients (30.1%) with trimalleollar fracture. Table 1 summarizes the patient characteristics.
Of the 33 patients (0.8%) with a VTE recorded within the first 30 postoperative days, 16 (0.4% of all patients) had a DVT recorded, 14 (0.3% of all patients) had a PE recorded, and 3 (0.1% of all patients) had both a DVT and a PE recorded. In 13 (39.4%) of the 33 patients with a VTE, the event occurred after discharge. VTEs were reported a mean (SD) of 11.5 (9.6) days after surgery. No patient in this study died of VTE.
Bivariate logistic regressions were performed to test the association of each patient variable with the occurrence of a VTE. Results are listed in Table 2. The bivariate analyses revealed significant associations between VTE after ankle fracture ORIF and the patient variables of age 60 years or older (odds ratio [OR], 2.40; 95% confidence interval [CI], 1.01-5.72), class I obesity (BMI, 30-35 kg/m2: OR, 5.15, 95% CI, 1.14-23.28), class II and class III obesity (BMI, ≥35 kg/m2: OR, 6.33, 95% CI, 1.41-28.38), ASA classes 3 and 4 (OR, 3.05; 95% CI, 1.53-6.08), history of heart disease (OR, 5.10; 95% CI, 2.08-12.49), history of hypertension (OR, 2.81; 95% CI, 1.39-5.66), and dependent functional status (OR, 3.39; 95% CI, 1.52-7.56).
Multivariate logistic regression was used to control for potential confounding variables and determine which factors were independently associated with VTEs. Results of this analysis are listed in Table 2 as well. The multivariate analysis revealed that the patient variables of class I obesity (BMI, 30-35 kg/m2: OR, 4.77; 95% CI, 1.05-21.72; P = .044), class II and class III obesity (BMI, ≥35 kg/m2: OR, 4.71; 95% CI, 1.03-21.68; P = .046), history of heart disease (OR, 3.28; 95% CI, 1.20-8.97; P = .020), and dependent functional status (OR, 2.59; 95% CI, 1.11-6.04; P = .028) were independently associated with an increased rate of VTEs. Of note, anesthesia type was not significantly associated with occurrence of VTE on bivariate or multivariate analysis.
The C statistic of the final multivariate model was 0.76, indicating very good distinguishing ability. The Hosmer-Lemeshow goodness-of-fit test showed no evidence of lack of fit.
Discussion
Citing the lack of conclusive evidence and the low incidence of VTE after ankle fracture surgery, current recommendations are to avoid routine VTE prophylaxis in the postoperative management of patients who undergo this surgery.1,5 However, it is important to identify patients who are at increased risk, as some may benefit from VTE prophylaxis. In the present study, we used the large, high-quality ACS-NSQIP database collecting information from multiple US hospitals to examine risk factors for VTE after ankle fracture ORIF. We identified 4412 patients who underwent ankle fracture ORIF between 2005 and 2012, and found an overall VTE incidence of 0.8%. Multivariate analysis identified obesity, history of heart disease, and dependent functional status as independent risk factors for VTE after ankle fracture ORIF.
This study’s 0.8% incidence of VTE after ankle fracture ORIF is consistent with the range (0.29%-5%) reported in other ankle fracture studies.4,8-10,14-18 We found that VTEs occurred a mean of about 11 days after surgery, and no patient died of VTE.
Obesity (BMI, ≥30 kg/m2) had the strongest association with VTEs in this study. Obesity, which is a growing public health concern, can make postoperative care and mobilization more difficult.19 Obesity has previously been associated with VTEs after ankle fractures, and BMI of over 25 kg/m2 is one of the Caprini criteria for thrombosis risk factor assessment.6,10 In our study, however, BMI of 25 to 30 kg/m2 was not associated with an increased VTE rate, indicating that moderately overweight patients may not be at significantly higher risk for VTE (compared with patients with normal BMI) and may not need VTE prophylaxis. VTE prophylaxis after ankle fracture surgery may be considered in patients with BMI over 30 kg/m2.
History of heart disease was also associated with VTEs in this study. Patients with a history of heart disease were at 3 times the risk for VTE within 30 days of ankle fracture surgery. This association is also consistent with the Caprini criteria, which include acute myocardial infarction and CHF as risk factors for venous thrombosis.6 Other studies have found associations between CHF and VTE and between cardiovascular risk factors and VTE.7,20 The association between cardiovascular disease and VTE may derive from the decreased venous flow rate associated with CHF or an overall vascular disease state. These patients may benefit from heightened surveillance and postoperative prophylaxis for VTE.
Dependent functional status was the final risk factor found to be associated with VTE after ankle fracture ORIF. This association likely derives from an inability to mobilize independently, leading to increased venous stasis. Immobilization has been previously associated with increased risk for VTE after ankle surgery.7,14,16,20 Caretakers should be aware of this increased risk during the postoperative period and diligently monitor these patients for signs and symptoms of VTE. Prophylaxis may also be considered in this patient population.
Several risk factors that were significant on bivariate analysis (increased age; increased ASA class; history of diabetes, pulmonary disease, hypertension) were not significant in the final multivariate model. This finding suggests covariance between these factors and those that were significant in the final multivariate model. In particular, age and increased overall comorbidity (represented by increased ASA class) were not significant in our multivariate model—contrary to findings of other studies.8-10 It is possible that history of heart disease alone was responsible for the association between overall comorbidity and VTE in those studies. In the present study, separating and controlling for individual comorbidities could have allowed this association to be more precisely characterized.
The characteristics of the ACS-NSQIP database limited our study in several ways. First, although ACS-NSQIP makes significant efforts to collect as many patient variables as possible, some information is not captured. Data about additional factors that may affect VTE risk (eg, history of previous VTE, hypercoagulable state, history of malignancy other than disseminated cancer, tourniquet time, patient position in operating room) were not available. Second, data are collected only on those postoperative adverse events that occur within 30 days after surgery; data on VTEs that occur later are not captured. However, it has been shown that the majority of VTEs occur within the first 30 days after lower extremity trauma and surgery,21,22 so this follow-up interval was deemed adequate for capture of VTE data. Third, the database does not include information on the prophylactic regimens used for these patients—which may have weakened the associations between predictor variables and VTE risk and led to an underestimated effect size. VTE incidence, as well as the odds of developing a VTE with one of the identified risk factors, may actually be higher than reported in this study.
Conclusion
VTEs are serious complications that can occur after ORIF of ankle fractures. In this study, the overall incidence of VTE after ankle fracture ORIF was 0.8%. Although the American Orthopaedic Foot and Ankle Society and the American College of Chest Physicians do not recommend routine screening or prophylaxis for VTE in patients with ankle fractures,1,5 the results of this study showed there may be a benefit in emphasizing VTE prophylaxis after ankle fracture ORIF in patients with obesity, history of heart disease, or dependent functional status. At minimum, these patients should be more carefully monitored for development of VTEs.
1. American Orthopaedic Foot and Ankle Society. Position statement: the use of VTED prophylaxis in foot and ankle surgery. http://www.aofas.org/medical-community/health-policy/Documents/VTED-Position-Statement-Approv-7-9-13-FINAL.pdf. Updated 2013. Accessed May 10, 2015.
2. Grady-Benson JC, Oishi CS, Hanson PB, Colwell CW Jr, Otis SM, Walker RH. Routine postoperative duplex ultrasonography screening and monitoring for the detection of deep vein thrombosis. A survey of 110 total hip arthroplasties. Clin Orthop Relat Res. 1994;(307):130-141.
3. Salzman EW, Harris WH, DeSanctis RW. Anticoagulation for prevention of thromboembolism following fractures of the hip. New Engl J Med. 1966;275(3):122-130.
4. Patil S, Gandhi J, Curzon I, Hui AC. Incidence of deep-vein thrombosis in patients with fractures of the ankle treated in a plaster cast. J Bone Joint Surg Br. 2007;89(10):1340-1343.
5. Falck-Ytter Y, Francis CW, Johanson NA, et al; American College of Chest Physicians. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e278S-e325S.
6. Caprini JA. Thrombosis risk assessment as a guide to quality patient care. Dis Mon. 2005;51(2-3):70-78.
7. Mayle RE Jr, DiGiovanni CW, Lin SS, Tabrizi P, Chou LB. Current concepts review: venous thromboembolic disease in foot and ankle surgery. Foot Ankle Int. 2007;28(11):1207-1216.
8. Jameson SS, Augustine A, James P, et al. Venous thromboembolic events following foot and ankle surgery in the English National Health Service. J Bone Joint Surg Br. 2011;93(4):490-497.
9. SooHoo NF, Eagan M, Krenek L, Zingmond DS. Incidence and factors predicting pulmonary embolism and deep venous thrombosis following surgical treatment of ankle fractures. Foot Ankle Surg. 2011;17(4):259-262.
10. Shibuya N, Frost CH, Campbell JD, Davis ML, Jupiter DC. Incidence of acute deep vein thrombosis and pulmonary embolism in foot and ankle trauma: analysis of the National Trauma Data Bank. J Foot Ankle Surg. 2012;51(1):63-68.
11. American College of Surgeons National Surgical Quality Improvement Program. User Guide for the 2012 ACS NSQIP Participant Use Data File. http://site.acsnsqip.org/wp-content/uploads/2013/10/ACSNSQIP.PUF_.UserGuide.2012.pdf. Published October 2013. Accessed May 10, 2015.
12. Khuri SF, Henderson WG, Daley J, et al; Principal Investigators of Patient Safety in Surgery Study. Successful implementation of the Department of Veterans Affairs’ National Surgical Quality Improvement Program in the private sector: the Patient Safety in Surgery study. Ann Surg. 2008;248(2):329-336.
13. Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA. 1999;282(16):1523-1529.
14. Mizel MS, Temple HT, Michelson JD, et al. Thromboembolism after foot and ankle surgery. A multicenter study. Clin Orthop Relat Res. 1998;(348):180-185.
15. Solis G, Saxby T. Incidence of DVT following surgery of the foot and ankle. Foot Ankle Int. 2002;23(5):411-414.
16. Hanslow SS, Grujic L, Slater HK, Chen D. Thromboembolic disease after foot and ankle surgery. Foot Ankle Int. 2006;27(9):693-695.
17. Pelet S, Roger ME, Belzile EL, Bouchard M. The incidence of thromboembolic events in surgically treated ankle fracture. J Bone Joint Surg Am. 2012;94(6):502-506.
18. Manafi Rasi A, Kazemian G, Emami Moghadam M, et al. Deep vein thrombosis following below knee immobilization: the need for chemoprophylaxis. Trauma Mon. 2013;17(4):367-369.
19. Sabharwal S, Root MZ. Impact of obesity on orthopaedics. J Bone Joint Surg Am. 2012;94(11):1045-1052.
20. Kadous A, Abdelgawad AA, Kanlic E. Deep venous thrombosis and pulmonary embolism after surgical treatment of ankle fractures: a case report and review of literature. J Foot Ankle Surg. 2012;51(4):457-463.
21. Forsythe RM, Peitzman AB, DeCato T, et al. Early lower extremity fracture fixation and the risk of early pulmonary embolus: filter before fixation? J Trauma. 2011;70(6):1381-1388.
22. Bjørnarå BT, Gudmundsen TE, Dahl OE. Frequency and timing of clinical venous thromboembolism after major joint surgery. J Bone Joint Surg Br. 2006;88(3):386-391.
1. American Orthopaedic Foot and Ankle Society. Position statement: the use of VTED prophylaxis in foot and ankle surgery. http://www.aofas.org/medical-community/health-policy/Documents/VTED-Position-Statement-Approv-7-9-13-FINAL.pdf. Updated 2013. Accessed May 10, 2015.
2. Grady-Benson JC, Oishi CS, Hanson PB, Colwell CW Jr, Otis SM, Walker RH. Routine postoperative duplex ultrasonography screening and monitoring for the detection of deep vein thrombosis. A survey of 110 total hip arthroplasties. Clin Orthop Relat Res. 1994;(307):130-141.
3. Salzman EW, Harris WH, DeSanctis RW. Anticoagulation for prevention of thromboembolism following fractures of the hip. New Engl J Med. 1966;275(3):122-130.
4. Patil S, Gandhi J, Curzon I, Hui AC. Incidence of deep-vein thrombosis in patients with fractures of the ankle treated in a plaster cast. J Bone Joint Surg Br. 2007;89(10):1340-1343.
5. Falck-Ytter Y, Francis CW, Johanson NA, et al; American College of Chest Physicians. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e278S-e325S.
6. Caprini JA. Thrombosis risk assessment as a guide to quality patient care. Dis Mon. 2005;51(2-3):70-78.
7. Mayle RE Jr, DiGiovanni CW, Lin SS, Tabrizi P, Chou LB. Current concepts review: venous thromboembolic disease in foot and ankle surgery. Foot Ankle Int. 2007;28(11):1207-1216.
8. Jameson SS, Augustine A, James P, et al. Venous thromboembolic events following foot and ankle surgery in the English National Health Service. J Bone Joint Surg Br. 2011;93(4):490-497.
9. SooHoo NF, Eagan M, Krenek L, Zingmond DS. Incidence and factors predicting pulmonary embolism and deep venous thrombosis following surgical treatment of ankle fractures. Foot Ankle Surg. 2011;17(4):259-262.
10. Shibuya N, Frost CH, Campbell JD, Davis ML, Jupiter DC. Incidence of acute deep vein thrombosis and pulmonary embolism in foot and ankle trauma: analysis of the National Trauma Data Bank. J Foot Ankle Surg. 2012;51(1):63-68.
11. American College of Surgeons National Surgical Quality Improvement Program. User Guide for the 2012 ACS NSQIP Participant Use Data File. http://site.acsnsqip.org/wp-content/uploads/2013/10/ACSNSQIP.PUF_.UserGuide.2012.pdf. Published October 2013. Accessed May 10, 2015.
12. Khuri SF, Henderson WG, Daley J, et al; Principal Investigators of Patient Safety in Surgery Study. Successful implementation of the Department of Veterans Affairs’ National Surgical Quality Improvement Program in the private sector: the Patient Safety in Surgery study. Ann Surg. 2008;248(2):329-336.
13. Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA. 1999;282(16):1523-1529.
14. Mizel MS, Temple HT, Michelson JD, et al. Thromboembolism after foot and ankle surgery. A multicenter study. Clin Orthop Relat Res. 1998;(348):180-185.
15. Solis G, Saxby T. Incidence of DVT following surgery of the foot and ankle. Foot Ankle Int. 2002;23(5):411-414.
16. Hanslow SS, Grujic L, Slater HK, Chen D. Thromboembolic disease after foot and ankle surgery. Foot Ankle Int. 2006;27(9):693-695.
17. Pelet S, Roger ME, Belzile EL, Bouchard M. The incidence of thromboembolic events in surgically treated ankle fracture. J Bone Joint Surg Am. 2012;94(6):502-506.
18. Manafi Rasi A, Kazemian G, Emami Moghadam M, et al. Deep vein thrombosis following below knee immobilization: the need for chemoprophylaxis. Trauma Mon. 2013;17(4):367-369.
19. Sabharwal S, Root MZ. Impact of obesity on orthopaedics. J Bone Joint Surg Am. 2012;94(11):1045-1052.
20. Kadous A, Abdelgawad AA, Kanlic E. Deep venous thrombosis and pulmonary embolism after surgical treatment of ankle fractures: a case report and review of literature. J Foot Ankle Surg. 2012;51(4):457-463.
21. Forsythe RM, Peitzman AB, DeCato T, et al. Early lower extremity fracture fixation and the risk of early pulmonary embolus: filter before fixation? J Trauma. 2011;70(6):1381-1388.
22. Bjørnarå BT, Gudmundsen TE, Dahl OE. Frequency and timing of clinical venous thromboembolism after major joint surgery. J Bone Joint Surg Br. 2006;88(3):386-391.