News

Summer Is Not Over: Let's Talk About Recreational Water–Associated Illnesses
- Author:
- Bonnie M. Word, MD
Infections acquired during recreational water activity can lead to illnesses involving the gastrointestinal tract, central nervous system,...
News

Summertime and Mosquitoes Are Breeding
- Author:
- Bonnie M. Word, MD
Because some mosquitoes can carry and transmit infectious diseases, prevention of bites is important, as is recognition of mosquito-borne...
News

Preparing for the viral trifecta: RSV, influenza, and COVID-19
- Author:
- Bonnie M. Word, MD
We are now armed with the most up-to-date interventions to help prevent the acquisition of RSV, influenza, and...
Opinion

Rabies: How to respond to parents’ questions
- Author:
- Bonnie M. Word, MD
Rabies is one of the most fatal diseases but can be prevented by avoiding contact with wild animals, maintenance of high immunization rates in...
Opinion

Congenital syphilis: It’s still a significant public health problem
- Author:
- Bonnie M. Word, MD
One result of syphilis during pregnancy is intrauterine infection and resultant congenital disease in the infant.
Opinion

Measles outbreaks: Protecting your patients during international travel
- Author:
- Bonnie M. Word, MD
Most countries with current measles outbreaks may not be typical travel destinations for tourists; however, they are common destinations for...
Opinion

Adolescent immunizations and protecting our children from COVID-19
- Author:
- Bonnie M. Word, MD
Now, we have another adolescent vaccine to discuss and encourage our patients to receive.
Opinion

Tick talk for families and pediatricians
- Author:
- Bonnie M. Word, MD
Ticks are responsible for most vector-transmitted diseases in the United States, with Lyme disease most frequently reported.
Opinion

Should our patients really go home for the holidays?
- Author:
- Bonnie M. Word, MD
Risks to warn patients of include high-risk status, risk of SARS-CoV-2 transmission and how to mitigate it, and mode of transportation.
Opinion
COVID-19 and its impact on the pediatric patient
- Author:
- Bonnie M. Word, MD
Strategies to mitigate additional spread such as social distancing, wearing facial masks, and hand washing still...
Opinion
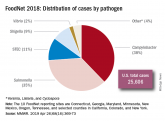
Don’t let a foodborne illness dampen the holiday season
- Author:
- Bonnie M. Word, MD
Tell your patients the four guides to safe food preparation and storage: clean, separate, cook, and chill....
Opinion

Get patients vaccinated: Avoid unwelcome international travel souvenirs
- Author:
- Bonnie M. Word, MD
A trip to a travel medicine physician can protect patients from returning from international travel with an unwelcome infection.
Opinion
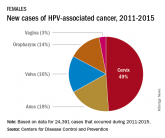
Recommending HPV vaccination: How would you grade yourself?
- Author:
- Bonnie M. Word, MD
HPV vaccination is cancer prevention.
Opinion

International travel updates
- Author:
- Bonnie M. Word, MD
No matter the destination, you want to make sure your patients are medically prepared and only return home with one thing: souvenirs.
Opinion
Artemisinin: Its global impact on the treatment of malaria
- Author:
- Bonnie M. Word, MD
Although not endemic in the United States, malaria still is brought in by travelers.
