User login
Don’t let a foodborne illness dampen the holiday season
According to the Centers for Disease Control and Prevention, a foodborne disease occurs in one in six persons (48 million), resulting in 128,000 hospitalizations and 3,000 deaths annually in the United States. The Foodborne Active Surveillance Network (FoodNet) of the CDC’s Emerging Infections Program monitors cases of eight laboratory diagnosed infections from 10 U.S. sites (covering 15% of the U.S. population). Monitored organisms include Campylobacter, Cyclospora, Listeria, Salmonella, Shiga toxin–producing Escherichia coli (STEC), Shigella, Vibrio, and Yersinia. In 2018, FoodNet identified 25,606 cases of infection, 5,893 hospitalizations, and 120 deaths. The incidence of infection (cases/100,000) was highest for Campylobacter (20), Salmonella (18), STEC (6), Shigella (5), Vibrio (1), Yersinia (0.9), Cyclospora (0.7), and Listeria (0.3). How might these pathogens affect your patients? First, a quick review about the four more common infections. Treatment is beyond the scope of our discussion and you are referred to the 2018-2021 Red Book for assistance. The goal of this column is to prevent your patients from becoming a statistic this holiday season.

Campylobacter
It has been the most common infection reported in FoodNet since 2013. Clinically, patients present with fever, abdominal pain, and nonbloody diarrhea. However, bloody diarrhea maybe the only symptom in neonates and young infants. Abdominal pain can mimic acute appendicitis or intussusception. Bacteremia is rare but has been reported in the elderly and in some patients with underlying conditions. During convalescence, immunoreactive complications including Guillain-Barré syndrome, reactive arthritis, and erythema nodosum may occur. In patients with diarrhea, Campylobacter jejuni and C. coli are the most frequently isolated species.
Campylobacter is present in the intestinal tract of both domestic and wild birds and animals. Transmission is via consumption of contaminated food or water. Undercooked poultry, untreated water, and unpasteurized milk are the three main vehicles of transmission. Campylobacter can be isolated in stool and blood, however isolation from stool requires special media. Rehydration is the primary therapy. Use of azithromycin or erythromycin can shorten both the duration of symptoms and bacterial shedding.
Salmonella
Nontyphoidal salmonella (NTS) are responsible for a variety of infections including asymptomatic carriage, gastroenteritis, bacteremia, and serious focal infections. Gastroenteritis is the most common illness and is manifested as diarrhea, abdominal pain, and fever. If bacteremia occurs, up to 10% of patients will develop focal infections. Invasive disease occurs most frequently in infants, persons with hemoglobinopathies, immunosuppressive disorders, and malignancies. The genus Salmonella is divided into two species, S. enterica and S. bongori with S. enterica subspecies accounting for about half of culture-confirmed Salmonella isolates reported by public health laboratories.
Although infections are more common in the summer, infections can occur year-round. In 2018, the CDC investigated at least 15 food-related NTS outbreaks and 6 have been investigated so far in 2019. In industrialized countries, acquisition usually occurs from ingestion of poultry, eggs, and milk products. Infection also has been reported after animal contact and consumption of fresh produce, meats, and contaminated water. Ground beef is the source of the November 2019 outbreak of S. dublin. Diarrhea develops within 12-72 hours. Salmonella can be isolated from stool, blood, and urine. Treatment usually is not indicated for uncomplicated gastroenteritis. While benefit has not been proven, it is recommended for those at increased risk for developing invasive disease.
Shigella
Shigella is the classic cause of colonic or dysenteric diarrhea. Humans are the primary hosts but other primates can be infected. Transmission occurs through direct person-to-person spread, from ingestion of contaminated food and water, and contact with contaminated inanimate objects. Bacteria can survive up to 6 months in food and 30 days in water. As few as 10 organisms can initiate disease. Typically mucoid or bloody diarrhea with abdominal cramps and fever occurs 1-7 days following exposure. Isolation is from stool. Bacteremia is unusual. Therapy is recommended for severe disease.
Shiga toxin–producing Escherichia coli (STEC)
STEC causes hemorrhagic colitis, which can be complicated by hemolytic uremic syndrome. While E. coli O157:H7 is the serotype most often implicated, other serotypes can cause disease. STEC is shed in feces of cattle and other animals. Infection most often is associated with ingestion of undercooked ground beef, but outbreaks also have confirmed that contaminated leafy vegetables, drinking water, peanut butter, and unpasteurized milk have been the source. Symptoms usually develop 3 to 4 days after exposure. Stools initially may be nonbloody. Abdominal pain and bloody diarrhea occur over the next 2-3 days. Fever often is absent or low grade. Stools should be sent for culture and Shiga toxin for diagnosis. Antimicrobial treatment generally is not warranted if STEC is suspected or diagnosed.
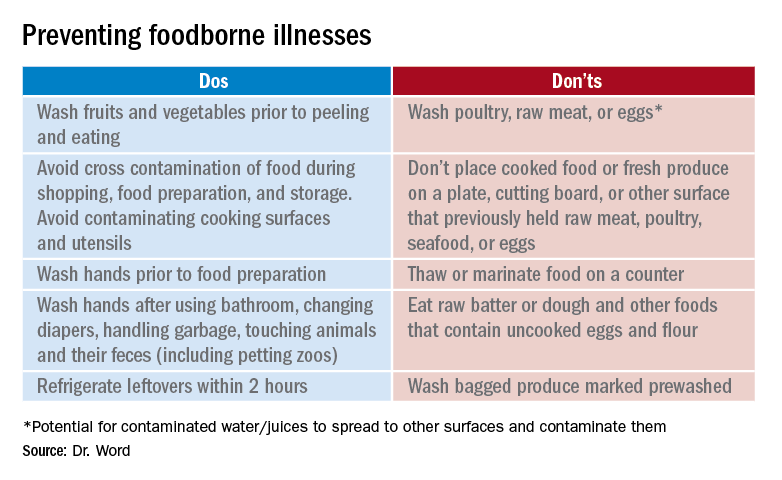
Prevention
It seems so simple. Here are the basic guidelines:
- Clean. Wash hands and surfaces frequently.
- Separate. Separate raw meats and eggs from other foods.
- Cook. Cook all meats to the right temperature.
- Chill. Refrigerate food properly.
Finally, two comments about food poisoning:
Abrupt onset of nausea, vomiting and abdominal cramping due to staphylococcal food poisoning begins 30 minutes to 6 hours after ingestion of food contaminated by enterotoxigenic strains of Staphylococcus aureus which is usually introduced by a food preparer with a purulent lesion. Food left at room temperature allows bacteria to multiply and produce a heat stable toxin. Individuals with purulent lesions of the hands, face, eyes, or nose should not be involved with food preparation.
Clostridium perfringens is the second most common bacterial cause of food poisoning. Symptoms (watery diarrhea and cramping) begin 6-24 hours after ingestion of C. perfringens spores not killed during cooking, which now have multiplied in food left at room temperature that was inadequately reheated. Illness is caused by the production of enterotoxin in the intestine. Outbreaks occur most often in November and December.
This article was updated on 11/12/19.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.
Information sources
1. foodsafety.gov
2. cdc.gov/foodsafety
3. The United States Department of Agriculture Meat and Poultry Hotline: 888-674-6854
4. Appendix VII: Clinical syndromes associated with foodborne diseases, Red Book online, 31st ed. (Washington DC: Red Book online, 2018, pp. 1086-92).
5. Foodkeeper App available at the App store. Provides appropriate food storage information; food recalls also are available.
According to the Centers for Disease Control and Prevention, a foodborne disease occurs in one in six persons (48 million), resulting in 128,000 hospitalizations and 3,000 deaths annually in the United States. The Foodborne Active Surveillance Network (FoodNet) of the CDC’s Emerging Infections Program monitors cases of eight laboratory diagnosed infections from 10 U.S. sites (covering 15% of the U.S. population). Monitored organisms include Campylobacter, Cyclospora, Listeria, Salmonella, Shiga toxin–producing Escherichia coli (STEC), Shigella, Vibrio, and Yersinia. In 2018, FoodNet identified 25,606 cases of infection, 5,893 hospitalizations, and 120 deaths. The incidence of infection (cases/100,000) was highest for Campylobacter (20), Salmonella (18), STEC (6), Shigella (5), Vibrio (1), Yersinia (0.9), Cyclospora (0.7), and Listeria (0.3). How might these pathogens affect your patients? First, a quick review about the four more common infections. Treatment is beyond the scope of our discussion and you are referred to the 2018-2021 Red Book for assistance. The goal of this column is to prevent your patients from becoming a statistic this holiday season.

Campylobacter
It has been the most common infection reported in FoodNet since 2013. Clinically, patients present with fever, abdominal pain, and nonbloody diarrhea. However, bloody diarrhea maybe the only symptom in neonates and young infants. Abdominal pain can mimic acute appendicitis or intussusception. Bacteremia is rare but has been reported in the elderly and in some patients with underlying conditions. During convalescence, immunoreactive complications including Guillain-Barré syndrome, reactive arthritis, and erythema nodosum may occur. In patients with diarrhea, Campylobacter jejuni and C. coli are the most frequently isolated species.
Campylobacter is present in the intestinal tract of both domestic and wild birds and animals. Transmission is via consumption of contaminated food or water. Undercooked poultry, untreated water, and unpasteurized milk are the three main vehicles of transmission. Campylobacter can be isolated in stool and blood, however isolation from stool requires special media. Rehydration is the primary therapy. Use of azithromycin or erythromycin can shorten both the duration of symptoms and bacterial shedding.
Salmonella
Nontyphoidal salmonella (NTS) are responsible for a variety of infections including asymptomatic carriage, gastroenteritis, bacteremia, and serious focal infections. Gastroenteritis is the most common illness and is manifested as diarrhea, abdominal pain, and fever. If bacteremia occurs, up to 10% of patients will develop focal infections. Invasive disease occurs most frequently in infants, persons with hemoglobinopathies, immunosuppressive disorders, and malignancies. The genus Salmonella is divided into two species, S. enterica and S. bongori with S. enterica subspecies accounting for about half of culture-confirmed Salmonella isolates reported by public health laboratories.
Although infections are more common in the summer, infections can occur year-round. In 2018, the CDC investigated at least 15 food-related NTS outbreaks and 6 have been investigated so far in 2019. In industrialized countries, acquisition usually occurs from ingestion of poultry, eggs, and milk products. Infection also has been reported after animal contact and consumption of fresh produce, meats, and contaminated water. Ground beef is the source of the November 2019 outbreak of S. dublin. Diarrhea develops within 12-72 hours. Salmonella can be isolated from stool, blood, and urine. Treatment usually is not indicated for uncomplicated gastroenteritis. While benefit has not been proven, it is recommended for those at increased risk for developing invasive disease.
Shigella
Shigella is the classic cause of colonic or dysenteric diarrhea. Humans are the primary hosts but other primates can be infected. Transmission occurs through direct person-to-person spread, from ingestion of contaminated food and water, and contact with contaminated inanimate objects. Bacteria can survive up to 6 months in food and 30 days in water. As few as 10 organisms can initiate disease. Typically mucoid or bloody diarrhea with abdominal cramps and fever occurs 1-7 days following exposure. Isolation is from stool. Bacteremia is unusual. Therapy is recommended for severe disease.
Shiga toxin–producing Escherichia coli (STEC)
STEC causes hemorrhagic colitis, which can be complicated by hemolytic uremic syndrome. While E. coli O157:H7 is the serotype most often implicated, other serotypes can cause disease. STEC is shed in feces of cattle and other animals. Infection most often is associated with ingestion of undercooked ground beef, but outbreaks also have confirmed that contaminated leafy vegetables, drinking water, peanut butter, and unpasteurized milk have been the source. Symptoms usually develop 3 to 4 days after exposure. Stools initially may be nonbloody. Abdominal pain and bloody diarrhea occur over the next 2-3 days. Fever often is absent or low grade. Stools should be sent for culture and Shiga toxin for diagnosis. Antimicrobial treatment generally is not warranted if STEC is suspected or diagnosed.

Prevention
It seems so simple. Here are the basic guidelines:
- Clean. Wash hands and surfaces frequently.
- Separate. Separate raw meats and eggs from other foods.
- Cook. Cook all meats to the right temperature.
- Chill. Refrigerate food properly.
Finally, two comments about food poisoning:
Abrupt onset of nausea, vomiting and abdominal cramping due to staphylococcal food poisoning begins 30 minutes to 6 hours after ingestion of food contaminated by enterotoxigenic strains of Staphylococcus aureus which is usually introduced by a food preparer with a purulent lesion. Food left at room temperature allows bacteria to multiply and produce a heat stable toxin. Individuals with purulent lesions of the hands, face, eyes, or nose should not be involved with food preparation.
Clostridium perfringens is the second most common bacterial cause of food poisoning. Symptoms (watery diarrhea and cramping) begin 6-24 hours after ingestion of C. perfringens spores not killed during cooking, which now have multiplied in food left at room temperature that was inadequately reheated. Illness is caused by the production of enterotoxin in the intestine. Outbreaks occur most often in November and December.
This article was updated on 11/12/19.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.
Information sources
1. foodsafety.gov
2. cdc.gov/foodsafety
3. The United States Department of Agriculture Meat and Poultry Hotline: 888-674-6854
4. Appendix VII: Clinical syndromes associated with foodborne diseases, Red Book online, 31st ed. (Washington DC: Red Book online, 2018, pp. 1086-92).
5. Foodkeeper App available at the App store. Provides appropriate food storage information; food recalls also are available.
According to the Centers for Disease Control and Prevention, a foodborne disease occurs in one in six persons (48 million), resulting in 128,000 hospitalizations and 3,000 deaths annually in the United States. The Foodborne Active Surveillance Network (FoodNet) of the CDC’s Emerging Infections Program monitors cases of eight laboratory diagnosed infections from 10 U.S. sites (covering 15% of the U.S. population). Monitored organisms include Campylobacter, Cyclospora, Listeria, Salmonella, Shiga toxin–producing Escherichia coli (STEC), Shigella, Vibrio, and Yersinia. In 2018, FoodNet identified 25,606 cases of infection, 5,893 hospitalizations, and 120 deaths. The incidence of infection (cases/100,000) was highest for Campylobacter (20), Salmonella (18), STEC (6), Shigella (5), Vibrio (1), Yersinia (0.9), Cyclospora (0.7), and Listeria (0.3). How might these pathogens affect your patients? First, a quick review about the four more common infections. Treatment is beyond the scope of our discussion and you are referred to the 2018-2021 Red Book for assistance. The goal of this column is to prevent your patients from becoming a statistic this holiday season.

Campylobacter
It has been the most common infection reported in FoodNet since 2013. Clinically, patients present with fever, abdominal pain, and nonbloody diarrhea. However, bloody diarrhea maybe the only symptom in neonates and young infants. Abdominal pain can mimic acute appendicitis or intussusception. Bacteremia is rare but has been reported in the elderly and in some patients with underlying conditions. During convalescence, immunoreactive complications including Guillain-Barré syndrome, reactive arthritis, and erythema nodosum may occur. In patients with diarrhea, Campylobacter jejuni and C. coli are the most frequently isolated species.
Campylobacter is present in the intestinal tract of both domestic and wild birds and animals. Transmission is via consumption of contaminated food or water. Undercooked poultry, untreated water, and unpasteurized milk are the three main vehicles of transmission. Campylobacter can be isolated in stool and blood, however isolation from stool requires special media. Rehydration is the primary therapy. Use of azithromycin or erythromycin can shorten both the duration of symptoms and bacterial shedding.
Salmonella
Nontyphoidal salmonella (NTS) are responsible for a variety of infections including asymptomatic carriage, gastroenteritis, bacteremia, and serious focal infections. Gastroenteritis is the most common illness and is manifested as diarrhea, abdominal pain, and fever. If bacteremia occurs, up to 10% of patients will develop focal infections. Invasive disease occurs most frequently in infants, persons with hemoglobinopathies, immunosuppressive disorders, and malignancies. The genus Salmonella is divided into two species, S. enterica and S. bongori with S. enterica subspecies accounting for about half of culture-confirmed Salmonella isolates reported by public health laboratories.
Although infections are more common in the summer, infections can occur year-round. In 2018, the CDC investigated at least 15 food-related NTS outbreaks and 6 have been investigated so far in 2019. In industrialized countries, acquisition usually occurs from ingestion of poultry, eggs, and milk products. Infection also has been reported after animal contact and consumption of fresh produce, meats, and contaminated water. Ground beef is the source of the November 2019 outbreak of S. dublin. Diarrhea develops within 12-72 hours. Salmonella can be isolated from stool, blood, and urine. Treatment usually is not indicated for uncomplicated gastroenteritis. While benefit has not been proven, it is recommended for those at increased risk for developing invasive disease.
Shigella
Shigella is the classic cause of colonic or dysenteric diarrhea. Humans are the primary hosts but other primates can be infected. Transmission occurs through direct person-to-person spread, from ingestion of contaminated food and water, and contact with contaminated inanimate objects. Bacteria can survive up to 6 months in food and 30 days in water. As few as 10 organisms can initiate disease. Typically mucoid or bloody diarrhea with abdominal cramps and fever occurs 1-7 days following exposure. Isolation is from stool. Bacteremia is unusual. Therapy is recommended for severe disease.
Shiga toxin–producing Escherichia coli (STEC)
STEC causes hemorrhagic colitis, which can be complicated by hemolytic uremic syndrome. While E. coli O157:H7 is the serotype most often implicated, other serotypes can cause disease. STEC is shed in feces of cattle and other animals. Infection most often is associated with ingestion of undercooked ground beef, but outbreaks also have confirmed that contaminated leafy vegetables, drinking water, peanut butter, and unpasteurized milk have been the source. Symptoms usually develop 3 to 4 days after exposure. Stools initially may be nonbloody. Abdominal pain and bloody diarrhea occur over the next 2-3 days. Fever often is absent or low grade. Stools should be sent for culture and Shiga toxin for diagnosis. Antimicrobial treatment generally is not warranted if STEC is suspected or diagnosed.

Prevention
It seems so simple. Here are the basic guidelines:
- Clean. Wash hands and surfaces frequently.
- Separate. Separate raw meats and eggs from other foods.
- Cook. Cook all meats to the right temperature.
- Chill. Refrigerate food properly.
Finally, two comments about food poisoning:
Abrupt onset of nausea, vomiting and abdominal cramping due to staphylococcal food poisoning begins 30 minutes to 6 hours after ingestion of food contaminated by enterotoxigenic strains of Staphylococcus aureus which is usually introduced by a food preparer with a purulent lesion. Food left at room temperature allows bacteria to multiply and produce a heat stable toxin. Individuals with purulent lesions of the hands, face, eyes, or nose should not be involved with food preparation.
Clostridium perfringens is the second most common bacterial cause of food poisoning. Symptoms (watery diarrhea and cramping) begin 6-24 hours after ingestion of C. perfringens spores not killed during cooking, which now have multiplied in food left at room temperature that was inadequately reheated. Illness is caused by the production of enterotoxin in the intestine. Outbreaks occur most often in November and December.
This article was updated on 11/12/19.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.
Information sources
1. foodsafety.gov
2. cdc.gov/foodsafety
3. The United States Department of Agriculture Meat and Poultry Hotline: 888-674-6854
4. Appendix VII: Clinical syndromes associated with foodborne diseases, Red Book online, 31st ed. (Washington DC: Red Book online, 2018, pp. 1086-92).
5. Foodkeeper App available at the App store. Provides appropriate food storage information; food recalls also are available.
Get patients vaccinated: Avoid unwelcome international travel souvenirs
Summer officially began June 21, 2019, but many of your patients already may have departed or will soon be headed to international destinations. Reasons for travel are as variable as their destinations and include but are not limited to family vacations, mission trips, study abroad, parental job relocation, and visiting friends and relatives. The majority of the trips are planned at least 3 months in advance; however, for many travelers and their parents, they suddenly get an aha moment and realize there is/are specific vaccines required to obtain a visa or entry to their final destination. Unfortunately, too much emphasis is focused on required vaccines. The well-informed traveler knows that they may be exposed to multiple diseases and many are vaccine preventable.
The accompanying table lists vaccines traditionally considered to be travel vaccines. Several require multiple doses administered over 21-28 days to provide protection. Others such as cholera and yellow fever must be completed at least 10 days prior to departure to be effective. Typhoid has two formulations: The oral and injectable typhoid vaccines should be completed 1 and 2 weeks, respectively, prior to travel. Several vaccines have age limitations. Routine immunization of all infants against hepatitis A was recommended in 2006. Depending on your region, there may be adolescents who have not been immunized. Fortunately, hepatitis A vaccine works immediately.
One of the challenges you face is identifying someone in your area that provides travel medicine advice and immunizations to children and adolescents. Most children and teens travel with their parents, but today many adolescents travel independently with organized groups. Most of the vaccines listed are not routinely administered at your office, yet you most likely will be the first call a parent makes seeking travel advice.
Let me tell you about a few vaccines in particular.
Japanese encephalitis
This is most common cause of encephalitis in Asia and parts of the western Pacific. Risk generally is limited to rural agricultural areas where the causative virus is transmitted by a mosquito. Fatality rates are 20%-30%. Among survivors, 30%-50% have significant neurologic, cognitive, and psychiatric sequelae. Candidates for this vaccine are long-term travelers and short-term travelers with extensive outdoor rural activities.
Meningococcal conjugate vaccines (MCV4)
All travelers to the Hajj Pilgrimage (Aug. 9-14, 2019) and/or Umrah must show proof of immunization. Vaccine must be received at least 10 days prior to and no greater than 5 years prior to arrival to Saudi Arabia. Conjugate vaccine must clearly be documented for validity of 5 years. For all health entry requirements, go to www.moh.gov.sa/en/hajj/pages/healthregulations.aspx.
Measles
The Advisory Committee on Immunization Practices recommends all infants 6-11 months old receive one dose of MMR prior to international travel regardless of the destination. This should be followed by two additional countable doses. All persons at least 12 months of age and born after 1956 should receive two doses of MMR at least 28 days apart prior to international travel.
Rabies
Rabies is a viral disease endemic in more than 150 countries with approximately 60,000 fatal cases worldwide each year. Asia and Africa are the areas with the highest risk of exposure, and dogs are the principal hosts. Human rabies is almost always fatal once symptoms develop. Preexposure vaccine is recommended for persons with prolonged and/or remote travel to countries where rabies immunoglobulin is unavailable and the occurrence of animal rabies is high. Post exposure vaccination on days 0 and 3 still would be required.*
Typhoid
A bacterial infection caused by Salmonella enterica serotype Typhi and Paratyphi manifests with fever, headache, abdominal pain, diarrhea, or constipation. When bacteremia occurs, it usually is referred to as enteric fever. It is acquired by consumption of food/water contaminated with human feces. Highest risk areas include Africa, Southern Asia, and Southeast Asia
Yellow fever
Risk is limited to sub-Saharan Africa and the tropical areas of South America. It is transmitted by the bite of an infected mosquito. The vaccine is required for entry into at least 16 countries. In a country where yellow fever is present, persons transiting through for more than 12 hours to reach their final destination may actually cause a change in the entry requirements for the destination country. For example, travel from the United States to Tanzania requires no yellow fever vaccine while travel from the United States to Nairobi (more than 12 hours) to Tanzania requires yellow fever vaccine for entry into Tanzania. Travel sequence and duration is extremely important. Check the Centers for Disease Control and Prevention yellow fever site and/or the consulate for the most up-to-date yellow fever vaccine requirements.
YF-Vax (yellow fever vaccine) produced by Sanofi Pasteur in the United States currently is unavailable. The company is building a new facility, and vaccine will not be available for the remainder of 2019. To assure vaccine for U.S. travelers, Stamaril, a yellow fever vaccine produced by Sanofi Pasteur in France has been made available at more than 250 sites nationwide. Because Stamaril is offered at a limited number of locations, persons in need of vaccine should not delay seeking it. Because of increased demand related to summer travel, travelers in some areas have reported delays of several weeks in scheduling an appointment. To locate a Stamaril site in your area, go to wwwnc.cdc.gov/travel/page/search-for-stamaril-clinics.
There are several other diseases transmitted by mosquitoes and ticks including malaria, dengue, Zika and rickettsial diseases. Vigilant use of mosquito repellents is a must. Prophylactic medication is available for only malaria and should be initiated prior to exposure. Frequency and duration depends on the medication selected.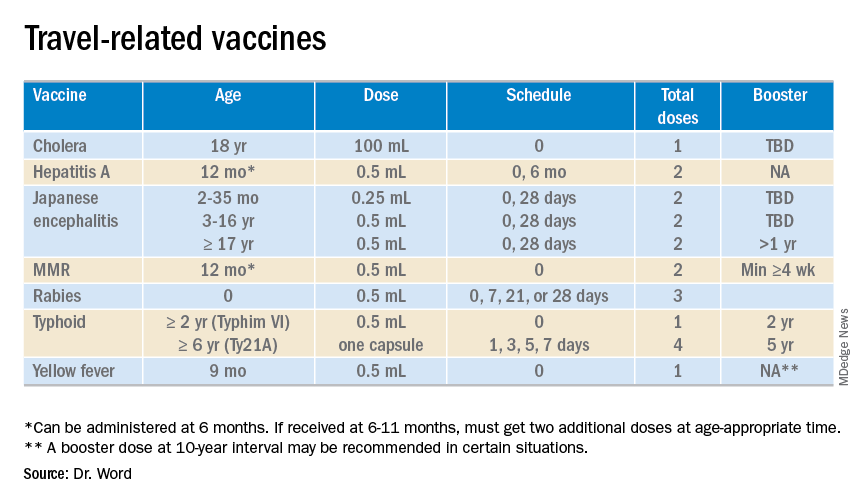
So how do you assist your patients?
Once you’ve identified a travel medicine facility in your area, encourage them to seek pretravel advice 4-6 weeks prior to international travel and make sure their routine immunizations are up to date. Generally, this is not an issue. One challenge is the early administration of MMR. While most practitioners know that early administration for international travel has been recommended for years, many office staff are accustomed to administration at only the 12 month and 4 year visit. When parents call requesting immunization, they often are informed that is it unnecessary and the appointment denied. This is a challenge, especially when coordination of administration of another live vaccine, such as yellow fever, is planned. Familiarizing all members of the health care team with current vaccine recommendations is critical.
For country-specific information, up-to-date travel alerts, and to locate a travel medicine clinic, visit www.cdc.gov/travel.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She had no relevant financial disclosures. Email her at pdnews@mdedge.com.
*This article was updated 6/18/2019.
Summer officially began June 21, 2019, but many of your patients already may have departed or will soon be headed to international destinations. Reasons for travel are as variable as their destinations and include but are not limited to family vacations, mission trips, study abroad, parental job relocation, and visiting friends and relatives. The majority of the trips are planned at least 3 months in advance; however, for many travelers and their parents, they suddenly get an aha moment and realize there is/are specific vaccines required to obtain a visa or entry to their final destination. Unfortunately, too much emphasis is focused on required vaccines. The well-informed traveler knows that they may be exposed to multiple diseases and many are vaccine preventable.
The accompanying table lists vaccines traditionally considered to be travel vaccines. Several require multiple doses administered over 21-28 days to provide protection. Others such as cholera and yellow fever must be completed at least 10 days prior to departure to be effective. Typhoid has two formulations: The oral and injectable typhoid vaccines should be completed 1 and 2 weeks, respectively, prior to travel. Several vaccines have age limitations. Routine immunization of all infants against hepatitis A was recommended in 2006. Depending on your region, there may be adolescents who have not been immunized. Fortunately, hepatitis A vaccine works immediately.
One of the challenges you face is identifying someone in your area that provides travel medicine advice and immunizations to children and adolescents. Most children and teens travel with their parents, but today many adolescents travel independently with organized groups. Most of the vaccines listed are not routinely administered at your office, yet you most likely will be the first call a parent makes seeking travel advice.
Let me tell you about a few vaccines in particular.
Japanese encephalitis
This is most common cause of encephalitis in Asia and parts of the western Pacific. Risk generally is limited to rural agricultural areas where the causative virus is transmitted by a mosquito. Fatality rates are 20%-30%. Among survivors, 30%-50% have significant neurologic, cognitive, and psychiatric sequelae. Candidates for this vaccine are long-term travelers and short-term travelers with extensive outdoor rural activities.
Meningococcal conjugate vaccines (MCV4)
All travelers to the Hajj Pilgrimage (Aug. 9-14, 2019) and/or Umrah must show proof of immunization. Vaccine must be received at least 10 days prior to and no greater than 5 years prior to arrival to Saudi Arabia. Conjugate vaccine must clearly be documented for validity of 5 years. For all health entry requirements, go to www.moh.gov.sa/en/hajj/pages/healthregulations.aspx.
Measles
The Advisory Committee on Immunization Practices recommends all infants 6-11 months old receive one dose of MMR prior to international travel regardless of the destination. This should be followed by two additional countable doses. All persons at least 12 months of age and born after 1956 should receive two doses of MMR at least 28 days apart prior to international travel.
Rabies
Rabies is a viral disease endemic in more than 150 countries with approximately 60,000 fatal cases worldwide each year. Asia and Africa are the areas with the highest risk of exposure, and dogs are the principal hosts. Human rabies is almost always fatal once symptoms develop. Preexposure vaccine is recommended for persons with prolonged and/or remote travel to countries where rabies immunoglobulin is unavailable and the occurrence of animal rabies is high. Post exposure vaccination on days 0 and 3 still would be required.*
Typhoid
A bacterial infection caused by Salmonella enterica serotype Typhi and Paratyphi manifests with fever, headache, abdominal pain, diarrhea, or constipation. When bacteremia occurs, it usually is referred to as enteric fever. It is acquired by consumption of food/water contaminated with human feces. Highest risk areas include Africa, Southern Asia, and Southeast Asia
Yellow fever
Risk is limited to sub-Saharan Africa and the tropical areas of South America. It is transmitted by the bite of an infected mosquito. The vaccine is required for entry into at least 16 countries. In a country where yellow fever is present, persons transiting through for more than 12 hours to reach their final destination may actually cause a change in the entry requirements for the destination country. For example, travel from the United States to Tanzania requires no yellow fever vaccine while travel from the United States to Nairobi (more than 12 hours) to Tanzania requires yellow fever vaccine for entry into Tanzania. Travel sequence and duration is extremely important. Check the Centers for Disease Control and Prevention yellow fever site and/or the consulate for the most up-to-date yellow fever vaccine requirements.
YF-Vax (yellow fever vaccine) produced by Sanofi Pasteur in the United States currently is unavailable. The company is building a new facility, and vaccine will not be available for the remainder of 2019. To assure vaccine for U.S. travelers, Stamaril, a yellow fever vaccine produced by Sanofi Pasteur in France has been made available at more than 250 sites nationwide. Because Stamaril is offered at a limited number of locations, persons in need of vaccine should not delay seeking it. Because of increased demand related to summer travel, travelers in some areas have reported delays of several weeks in scheduling an appointment. To locate a Stamaril site in your area, go to wwwnc.cdc.gov/travel/page/search-for-stamaril-clinics.
There are several other diseases transmitted by mosquitoes and ticks including malaria, dengue, Zika and rickettsial diseases. Vigilant use of mosquito repellents is a must. Prophylactic medication is available for only malaria and should be initiated prior to exposure. Frequency and duration depends on the medication selected.
So how do you assist your patients?
Once you’ve identified a travel medicine facility in your area, encourage them to seek pretravel advice 4-6 weeks prior to international travel and make sure their routine immunizations are up to date. Generally, this is not an issue. One challenge is the early administration of MMR. While most practitioners know that early administration for international travel has been recommended for years, many office staff are accustomed to administration at only the 12 month and 4 year visit. When parents call requesting immunization, they often are informed that is it unnecessary and the appointment denied. This is a challenge, especially when coordination of administration of another live vaccine, such as yellow fever, is planned. Familiarizing all members of the health care team with current vaccine recommendations is critical.
For country-specific information, up-to-date travel alerts, and to locate a travel medicine clinic, visit www.cdc.gov/travel.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She had no relevant financial disclosures. Email her at pdnews@mdedge.com.
*This article was updated 6/18/2019.
Summer officially began June 21, 2019, but many of your patients already may have departed or will soon be headed to international destinations. Reasons for travel are as variable as their destinations and include but are not limited to family vacations, mission trips, study abroad, parental job relocation, and visiting friends and relatives. The majority of the trips are planned at least 3 months in advance; however, for many travelers and their parents, they suddenly get an aha moment and realize there is/are specific vaccines required to obtain a visa or entry to their final destination. Unfortunately, too much emphasis is focused on required vaccines. The well-informed traveler knows that they may be exposed to multiple diseases and many are vaccine preventable.
The accompanying table lists vaccines traditionally considered to be travel vaccines. Several require multiple doses administered over 21-28 days to provide protection. Others such as cholera and yellow fever must be completed at least 10 days prior to departure to be effective. Typhoid has two formulations: The oral and injectable typhoid vaccines should be completed 1 and 2 weeks, respectively, prior to travel. Several vaccines have age limitations. Routine immunization of all infants against hepatitis A was recommended in 2006. Depending on your region, there may be adolescents who have not been immunized. Fortunately, hepatitis A vaccine works immediately.
One of the challenges you face is identifying someone in your area that provides travel medicine advice and immunizations to children and adolescents. Most children and teens travel with their parents, but today many adolescents travel independently with organized groups. Most of the vaccines listed are not routinely administered at your office, yet you most likely will be the first call a parent makes seeking travel advice.
Let me tell you about a few vaccines in particular.
Japanese encephalitis
This is most common cause of encephalitis in Asia and parts of the western Pacific. Risk generally is limited to rural agricultural areas where the causative virus is transmitted by a mosquito. Fatality rates are 20%-30%. Among survivors, 30%-50% have significant neurologic, cognitive, and psychiatric sequelae. Candidates for this vaccine are long-term travelers and short-term travelers with extensive outdoor rural activities.
Meningococcal conjugate vaccines (MCV4)
All travelers to the Hajj Pilgrimage (Aug. 9-14, 2019) and/or Umrah must show proof of immunization. Vaccine must be received at least 10 days prior to and no greater than 5 years prior to arrival to Saudi Arabia. Conjugate vaccine must clearly be documented for validity of 5 years. For all health entry requirements, go to www.moh.gov.sa/en/hajj/pages/healthregulations.aspx.
Measles
The Advisory Committee on Immunization Practices recommends all infants 6-11 months old receive one dose of MMR prior to international travel regardless of the destination. This should be followed by two additional countable doses. All persons at least 12 months of age and born after 1956 should receive two doses of MMR at least 28 days apart prior to international travel.
Rabies
Rabies is a viral disease endemic in more than 150 countries with approximately 60,000 fatal cases worldwide each year. Asia and Africa are the areas with the highest risk of exposure, and dogs are the principal hosts. Human rabies is almost always fatal once symptoms develop. Preexposure vaccine is recommended for persons with prolonged and/or remote travel to countries where rabies immunoglobulin is unavailable and the occurrence of animal rabies is high. Post exposure vaccination on days 0 and 3 still would be required.*
Typhoid
A bacterial infection caused by Salmonella enterica serotype Typhi and Paratyphi manifests with fever, headache, abdominal pain, diarrhea, or constipation. When bacteremia occurs, it usually is referred to as enteric fever. It is acquired by consumption of food/water contaminated with human feces. Highest risk areas include Africa, Southern Asia, and Southeast Asia
Yellow fever
Risk is limited to sub-Saharan Africa and the tropical areas of South America. It is transmitted by the bite of an infected mosquito. The vaccine is required for entry into at least 16 countries. In a country where yellow fever is present, persons transiting through for more than 12 hours to reach their final destination may actually cause a change in the entry requirements for the destination country. For example, travel from the United States to Tanzania requires no yellow fever vaccine while travel from the United States to Nairobi (more than 12 hours) to Tanzania requires yellow fever vaccine for entry into Tanzania. Travel sequence and duration is extremely important. Check the Centers for Disease Control and Prevention yellow fever site and/or the consulate for the most up-to-date yellow fever vaccine requirements.
YF-Vax (yellow fever vaccine) produced by Sanofi Pasteur in the United States currently is unavailable. The company is building a new facility, and vaccine will not be available for the remainder of 2019. To assure vaccine for U.S. travelers, Stamaril, a yellow fever vaccine produced by Sanofi Pasteur in France has been made available at more than 250 sites nationwide. Because Stamaril is offered at a limited number of locations, persons in need of vaccine should not delay seeking it. Because of increased demand related to summer travel, travelers in some areas have reported delays of several weeks in scheduling an appointment. To locate a Stamaril site in your area, go to wwwnc.cdc.gov/travel/page/search-for-stamaril-clinics.
There are several other diseases transmitted by mosquitoes and ticks including malaria, dengue, Zika and rickettsial diseases. Vigilant use of mosquito repellents is a must. Prophylactic medication is available for only malaria and should be initiated prior to exposure. Frequency and duration depends on the medication selected.
So how do you assist your patients?
Once you’ve identified a travel medicine facility in your area, encourage them to seek pretravel advice 4-6 weeks prior to international travel and make sure their routine immunizations are up to date. Generally, this is not an issue. One challenge is the early administration of MMR. While most practitioners know that early administration for international travel has been recommended for years, many office staff are accustomed to administration at only the 12 month and 4 year visit. When parents call requesting immunization, they often are informed that is it unnecessary and the appointment denied. This is a challenge, especially when coordination of administration of another live vaccine, such as yellow fever, is planned. Familiarizing all members of the health care team with current vaccine recommendations is critical.
For country-specific information, up-to-date travel alerts, and to locate a travel medicine clinic, visit www.cdc.gov/travel.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She had no relevant financial disclosures. Email her at pdnews@mdedge.com.
*This article was updated 6/18/2019.
Recommending HPV vaccination: How would you grade yourself?
A few weeks ago, a patient asked whether he could get my opinion on something unrelated to his yellow fever vaccine visit: He asked what I thought about the human papillomavirus (HPV) vaccine. His daughter’s primary care physician (PCP) had recommended it, but he “heard that it wasn’t safe.” We had a brief discussion.
My pediatric training days have long since ended, but I was taught never to miss an opportunity to immunize. In this case, it was to help a parent decide to immunize. This type of encounter is not unusual because, as part of preparing persons for international travel, I review their routine immunizations. When documentation of a vaccine is absent, it is pointed out and often remedied after a brief discussion.
Unfortunately, with HPV, too often parents state “my primary care physician said” it was optional, it was not required, or it was never recommended. Some were told to wait until their child was older, and several have safety concerns as did the parent above. I sometimes hear, “it’s not necessary for my child”; this is usually a clue indicating that the issue is more likely about how HPV is transmitted than what HPV vaccine can prevent. Most have welcomed the opportunity to discuss the vaccine, hear about its benefits, and have their questions answered. All leave with HPV information and are directed to websites that provide accurate information. They are referred to their PCP – hopefully to be immunized.
Three vaccines – meningococcal conjugate vaccine (MCV), Tdap, and HPV vaccine – all are recommended for administration at 11-12 years of age. A booster of MCV is recommended at 16 years. However, let’s focus on HPV. In 2007, HPV administration was recommended by the Advisory Committee on Immunization Practices (ACIP) for girls; by 2011, the recommendation was extended to boys. It was a three-dose schedule expected to be completed by age 13 years. In December 2016, a two-dose schedule administered at least 6 months apart was recommended for teens who initiated immunization at less than 15 years. Three doses were still recommended for those initiating HPV after 15 years. This was the only time the number of doses to complete a vaccine series had been decreased based on postlicensure data. So
Vaccine coverage
The National Immunization Survey–Teen (NIS-Teen) monitors vaccine coverage annually amongst adolescents aged 13-17 years. Data are obtained from individuals from every state, as well as the District of Columbia, the U.S. Virgin Islands, and six major urban areas.
According to the Centers for Disease Control and Prevention’s Morbidity and Mortality Weekly Report (2018 Aug 24;67[33]:909-17), HPV vaccination continues to lag behind Tdap and MCV in 2018. Among all adolescents, coverage with one or more doses of HPV was 66%, with up-to-date HPV status in 49%. In contrast, 82% received a dose of MCV, and 89% received a dose of Tdap.
Coverage for receiving one or more doses of HPV among females was 69%, and up-to-date HPV status was 53%; among males, coverage with one or more doses was 63%, and up-to-date HPV status was 44%.
Up-to-date HPV coverage status differed geographically, ranging from 29% in Mississippi to 78% in DC. Overall, eight states and the District of Columbia reported increases in up-to-date status (District of Columbia, Louisiana, Massachusetts, Nebraska, North Carolina, South Carolina, Texas, Vermont, and Virginia). Kudos to Virginia for having the largest increase (20 percentage points).
Coverage also differed between urban and rural areas: one or more doses at 70% vs. 59% and up-to-date status at 52% vs. 42%.
HPV coverage differed by poverty level as well. It was higher for persons living below the poverty level, with one or more doses in 73% and up-to-date status in 54%, compared with persons living at or above poverty level at 63% and 47%, respectively.
HPV-related cancers

The most recent CDC data regarding types of HPV-associated cancers during 2011-2015 suggest that HPV types 16 and 18 account for the majority of cervical (78%) and oropharyngeal (86%) cancers.

Currently, there are more cases of oropharyngeal cancer than cervical, and we have no screening tool for the former.
Safety
Safety has been well documented. Since licensure, no serious safety concerns have been identified, contrary to what has been reported on various social and news media outlets. Yet it remains a concern for many parents who have delayed initiation of vaccine. Efficacy also has been documented in the United States and abroad.
Suggestions for improving HPV immunization coverage
Here are eight suggestions to help you recommend the vaccine and convince hesitant parents of its necessity:
1. Focus on your delivery of the HPV immunization recommendation. Clinician recommendation is the No. 1 reason parents vaccinate. The tone you use and how you make the recommendation can affect how the parent perceives the importance of this vaccine. The following are components of a high-quality recommendation (Academic Pediatrics. 2018;18:S23-S27):
- Routinely recommend vaccine at 11-12 years.
- Recommend vaccine for all preteens, not just those you feel are at risk for infection.
- Recommend the vaccine be given the same day it is discussed.
- Use language that expresses the importance of the HPV vaccine.
2. Use the “announcement or presumptive approach.” You expect the parent to agree with your recommendation. You don’t want to convey that it is an option.
3. Remind parents that immunizing on time means only two doses of HPV.
4. Revisit the topic again during another visit if a parent declines. Data suggest secondary acceptance can be as high as 66%.
5. Consider using a motivational interviewing approach for parents who are very hesitant to vaccinate. Most people want to comply with recommended health interventions.
6. Educate your staff about the importance of HPV vaccine and how it prevents cancer.
7. Determine how well your practice immunizes adolescents. This would be a perfect quality improvement project.
8. Explore “Answering Parents’ Questions” and other resources at www.cdc.gov/hpv to find quick answers to HPV vaccine–related questions .
Why is HPV coverage, a vaccine to prevent cancer, still lagging behind Tdap and MCV? I am as puzzled as others. What I do know is this: Our children will mature and one day become sexually active. They can be exposed to and get infected with HPV, and we can’t predict which ones will not clear the virus and end up developing an HPV-related cancer in the future. At the end of the day, HPV vaccination is cancer prevention.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.
A few weeks ago, a patient asked whether he could get my opinion on something unrelated to his yellow fever vaccine visit: He asked what I thought about the human papillomavirus (HPV) vaccine. His daughter’s primary care physician (PCP) had recommended it, but he “heard that it wasn’t safe.” We had a brief discussion.
My pediatric training days have long since ended, but I was taught never to miss an opportunity to immunize. In this case, it was to help a parent decide to immunize. This type of encounter is not unusual because, as part of preparing persons for international travel, I review their routine immunizations. When documentation of a vaccine is absent, it is pointed out and often remedied after a brief discussion.
Unfortunately, with HPV, too often parents state “my primary care physician said” it was optional, it was not required, or it was never recommended. Some were told to wait until their child was older, and several have safety concerns as did the parent above. I sometimes hear, “it’s not necessary for my child”; this is usually a clue indicating that the issue is more likely about how HPV is transmitted than what HPV vaccine can prevent. Most have welcomed the opportunity to discuss the vaccine, hear about its benefits, and have their questions answered. All leave with HPV information and are directed to websites that provide accurate information. They are referred to their PCP – hopefully to be immunized.
Three vaccines – meningococcal conjugate vaccine (MCV), Tdap, and HPV vaccine – all are recommended for administration at 11-12 years of age. A booster of MCV is recommended at 16 years. However, let’s focus on HPV. In 2007, HPV administration was recommended by the Advisory Committee on Immunization Practices (ACIP) for girls; by 2011, the recommendation was extended to boys. It was a three-dose schedule expected to be completed by age 13 years. In December 2016, a two-dose schedule administered at least 6 months apart was recommended for teens who initiated immunization at less than 15 years. Three doses were still recommended for those initiating HPV after 15 years. This was the only time the number of doses to complete a vaccine series had been decreased based on postlicensure data. So
Vaccine coverage
The National Immunization Survey–Teen (NIS-Teen) monitors vaccine coverage annually amongst adolescents aged 13-17 years. Data are obtained from individuals from every state, as well as the District of Columbia, the U.S. Virgin Islands, and six major urban areas.
According to the Centers for Disease Control and Prevention’s Morbidity and Mortality Weekly Report (2018 Aug 24;67[33]:909-17), HPV vaccination continues to lag behind Tdap and MCV in 2018. Among all adolescents, coverage with one or more doses of HPV was 66%, with up-to-date HPV status in 49%. In contrast, 82% received a dose of MCV, and 89% received a dose of Tdap.
Coverage for receiving one or more doses of HPV among females was 69%, and up-to-date HPV status was 53%; among males, coverage with one or more doses was 63%, and up-to-date HPV status was 44%.
Up-to-date HPV coverage status differed geographically, ranging from 29% in Mississippi to 78% in DC. Overall, eight states and the District of Columbia reported increases in up-to-date status (District of Columbia, Louisiana, Massachusetts, Nebraska, North Carolina, South Carolina, Texas, Vermont, and Virginia). Kudos to Virginia for having the largest increase (20 percentage points).
Coverage also differed between urban and rural areas: one or more doses at 70% vs. 59% and up-to-date status at 52% vs. 42%.
HPV coverage differed by poverty level as well. It was higher for persons living below the poverty level, with one or more doses in 73% and up-to-date status in 54%, compared with persons living at or above poverty level at 63% and 47%, respectively.
HPV-related cancers

The most recent CDC data regarding types of HPV-associated cancers during 2011-2015 suggest that HPV types 16 and 18 account for the majority of cervical (78%) and oropharyngeal (86%) cancers.

Currently, there are more cases of oropharyngeal cancer than cervical, and we have no screening tool for the former.
Safety
Safety has been well documented. Since licensure, no serious safety concerns have been identified, contrary to what has been reported on various social and news media outlets. Yet it remains a concern for many parents who have delayed initiation of vaccine. Efficacy also has been documented in the United States and abroad.
Suggestions for improving HPV immunization coverage
Here are eight suggestions to help you recommend the vaccine and convince hesitant parents of its necessity:
1. Focus on your delivery of the HPV immunization recommendation. Clinician recommendation is the No. 1 reason parents vaccinate. The tone you use and how you make the recommendation can affect how the parent perceives the importance of this vaccine. The following are components of a high-quality recommendation (Academic Pediatrics. 2018;18:S23-S27):
- Routinely recommend vaccine at 11-12 years.
- Recommend vaccine for all preteens, not just those you feel are at risk for infection.
- Recommend the vaccine be given the same day it is discussed.
- Use language that expresses the importance of the HPV vaccine.
2. Use the “announcement or presumptive approach.” You expect the parent to agree with your recommendation. You don’t want to convey that it is an option.
3. Remind parents that immunizing on time means only two doses of HPV.
4. Revisit the topic again during another visit if a parent declines. Data suggest secondary acceptance can be as high as 66%.
5. Consider using a motivational interviewing approach for parents who are very hesitant to vaccinate. Most people want to comply with recommended health interventions.
6. Educate your staff about the importance of HPV vaccine and how it prevents cancer.
7. Determine how well your practice immunizes adolescents. This would be a perfect quality improvement project.
8. Explore “Answering Parents’ Questions” and other resources at www.cdc.gov/hpv to find quick answers to HPV vaccine–related questions .
Why is HPV coverage, a vaccine to prevent cancer, still lagging behind Tdap and MCV? I am as puzzled as others. What I do know is this: Our children will mature and one day become sexually active. They can be exposed to and get infected with HPV, and we can’t predict which ones will not clear the virus and end up developing an HPV-related cancer in the future. At the end of the day, HPV vaccination is cancer prevention.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.
A few weeks ago, a patient asked whether he could get my opinion on something unrelated to his yellow fever vaccine visit: He asked what I thought about the human papillomavirus (HPV) vaccine. His daughter’s primary care physician (PCP) had recommended it, but he “heard that it wasn’t safe.” We had a brief discussion.
My pediatric training days have long since ended, but I was taught never to miss an opportunity to immunize. In this case, it was to help a parent decide to immunize. This type of encounter is not unusual because, as part of preparing persons for international travel, I review their routine immunizations. When documentation of a vaccine is absent, it is pointed out and often remedied after a brief discussion.
Unfortunately, with HPV, too often parents state “my primary care physician said” it was optional, it was not required, or it was never recommended. Some were told to wait until their child was older, and several have safety concerns as did the parent above. I sometimes hear, “it’s not necessary for my child”; this is usually a clue indicating that the issue is more likely about how HPV is transmitted than what HPV vaccine can prevent. Most have welcomed the opportunity to discuss the vaccine, hear about its benefits, and have their questions answered. All leave with HPV information and are directed to websites that provide accurate information. They are referred to their PCP – hopefully to be immunized.
Three vaccines – meningococcal conjugate vaccine (MCV), Tdap, and HPV vaccine – all are recommended for administration at 11-12 years of age. A booster of MCV is recommended at 16 years. However, let’s focus on HPV. In 2007, HPV administration was recommended by the Advisory Committee on Immunization Practices (ACIP) for girls; by 2011, the recommendation was extended to boys. It was a three-dose schedule expected to be completed by age 13 years. In December 2016, a two-dose schedule administered at least 6 months apart was recommended for teens who initiated immunization at less than 15 years. Three doses were still recommended for those initiating HPV after 15 years. This was the only time the number of doses to complete a vaccine series had been decreased based on postlicensure data. So
Vaccine coverage
The National Immunization Survey–Teen (NIS-Teen) monitors vaccine coverage annually amongst adolescents aged 13-17 years. Data are obtained from individuals from every state, as well as the District of Columbia, the U.S. Virgin Islands, and six major urban areas.
According to the Centers for Disease Control and Prevention’s Morbidity and Mortality Weekly Report (2018 Aug 24;67[33]:909-17), HPV vaccination continues to lag behind Tdap and MCV in 2018. Among all adolescents, coverage with one or more doses of HPV was 66%, with up-to-date HPV status in 49%. In contrast, 82% received a dose of MCV, and 89% received a dose of Tdap.
Coverage for receiving one or more doses of HPV among females was 69%, and up-to-date HPV status was 53%; among males, coverage with one or more doses was 63%, and up-to-date HPV status was 44%.
Up-to-date HPV coverage status differed geographically, ranging from 29% in Mississippi to 78% in DC. Overall, eight states and the District of Columbia reported increases in up-to-date status (District of Columbia, Louisiana, Massachusetts, Nebraska, North Carolina, South Carolina, Texas, Vermont, and Virginia). Kudos to Virginia for having the largest increase (20 percentage points).
Coverage also differed between urban and rural areas: one or more doses at 70% vs. 59% and up-to-date status at 52% vs. 42%.
HPV coverage differed by poverty level as well. It was higher for persons living below the poverty level, with one or more doses in 73% and up-to-date status in 54%, compared with persons living at or above poverty level at 63% and 47%, respectively.
HPV-related cancers

The most recent CDC data regarding types of HPV-associated cancers during 2011-2015 suggest that HPV types 16 and 18 account for the majority of cervical (78%) and oropharyngeal (86%) cancers.

Currently, there are more cases of oropharyngeal cancer than cervical, and we have no screening tool for the former.
Safety
Safety has been well documented. Since licensure, no serious safety concerns have been identified, contrary to what has been reported on various social and news media outlets. Yet it remains a concern for many parents who have delayed initiation of vaccine. Efficacy also has been documented in the United States and abroad.
Suggestions for improving HPV immunization coverage
Here are eight suggestions to help you recommend the vaccine and convince hesitant parents of its necessity:
1. Focus on your delivery of the HPV immunization recommendation. Clinician recommendation is the No. 1 reason parents vaccinate. The tone you use and how you make the recommendation can affect how the parent perceives the importance of this vaccine. The following are components of a high-quality recommendation (Academic Pediatrics. 2018;18:S23-S27):
- Routinely recommend vaccine at 11-12 years.
- Recommend vaccine for all preteens, not just those you feel are at risk for infection.
- Recommend the vaccine be given the same day it is discussed.
- Use language that expresses the importance of the HPV vaccine.
2. Use the “announcement or presumptive approach.” You expect the parent to agree with your recommendation. You don’t want to convey that it is an option.
3. Remind parents that immunizing on time means only two doses of HPV.
4. Revisit the topic again during another visit if a parent declines. Data suggest secondary acceptance can be as high as 66%.
5. Consider using a motivational interviewing approach for parents who are very hesitant to vaccinate. Most people want to comply with recommended health interventions.
6. Educate your staff about the importance of HPV vaccine and how it prevents cancer.
7. Determine how well your practice immunizes adolescents. This would be a perfect quality improvement project.
8. Explore “Answering Parents’ Questions” and other resources at www.cdc.gov/hpv to find quick answers to HPV vaccine–related questions .
Why is HPV coverage, a vaccine to prevent cancer, still lagging behind Tdap and MCV? I am as puzzled as others. What I do know is this: Our children will mature and one day become sexually active. They can be exposed to and get infected with HPV, and we can’t predict which ones will not clear the virus and end up developing an HPV-related cancer in the future. At the end of the day, HPV vaccination is cancer prevention.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.
International travel updates
It’s that time of year again. Many of your patients will join the 80.2 million Americans with plans for international travel this summer.
In 2016, Mexico (31.2 million) and Canada (13.9 million) were the top two destinations of U.S. residents. Based on 2016 U.S. Commerce data, an additional 35.1 million Americans headed to overseas destinations, including 9% who traveled with children. Vacation and visiting friends and relatives accounted for 55% and 27% of the reasons for all travel, respectively. Education accounted for 4% of travelers.
Required versus recommended vaccines
The goal of a required vaccine is to prevent international spread of disease. The host country is protecting its citizens from visitors importing and facilitating the spread of a disease. Yellow fever and meningococcal disease are the only vaccines required for entry into any country. Entry requirements vary by country. Yellow fever may be an entry requirement for all travelers or it may be limited to those who have been in, or have had transit through, a country where yellow fever can be transmitted at least 6 days prior to the arrival at their final destination – a reminder that the sequence of the patient’s itinerary is important. In addition, just because a vaccine is not required for entry does not mean the risk for exposure and acquisition is nonexistent.
In contrast, recommended vaccines are for the protection of the individual. Travelers may be exposed to vaccine-preventable diseases that do not exist in their country (such as measles, typhoid fever, and yellow fever). They are at risk for acquisition and may return home infected, which could create the potential to spread the disease to susceptible contacts.
Most travelers comprehend required vaccines but often fail to understand the importance of receiving recommended vaccines. Lammert et al. reported that, of 24,478 persons who received pretravel advice between July 2012 and June 2014 through Global TravEpiNet, a national consortium of U.S. clinics, 97% were eligible for at least one vaccine. The majority were eligible for typhoid (n = 20,092) and hepatitis A (n = 12,990). Of patients included in the study, 25% (6,573) refused one or more vaccines. The most common reason cited for refusal was a lack of concern about the illness. Travelers visiting friends and relatives were less likely to accept all recommended vaccines, compared with those who were not visiting friends and relatives (odds ratio, 0.74) (J Trav Med. 2017 Jan. doi: 10.1093/jtm/taw075). In the United States, international travel remains the most common risk factor for acquisition of both typhoid fever and hepatitis A.
What’s new
The U.S. Advisory Committee on Immunization Practices recommends administering the hepatitis A vaccine to infants aged 6-11 months with travel to or living in developing countries and areas with high to moderate risk for hepatitis A virus transmission. Any dose received at less than 12 months of age does not count, and the administration of two age-appropriate doses should occur following this dose.
Old but still relevant
Measles: The Advisory Committee on Immunization Practices recommends all infants aged 6-11 months receive one dose of MMR prior to international travel regardless of the destination. This should be followed by two additional countable doses. All persons at least 12 months of age and born after 1956 should receive two doses of MMR at least 28 days apart prior to international travel.
Prior to administering, determine whether your patient will travel to a yellow fever–endemic area because both are live vaccines and should be received the same day. Otherwise, administer MMR doses 28 days apart; coordination between facilities or receipt of both at one facility may be necessary.
Yellow fever vaccine: The U.S. supplies of YF-Vax by Sanofi Pasteur are not expected to be available again until the end of 2018. To provide vaccines for U.S. travelers, Stamaril – a yellow fever vaccine produced by Sanofi Pasteur in France – has been made available at more than 250 sites through an Expanded Access Investigational New Drug Program.
Since Stamaril is offered at a limited number of locations, persons with anticipated travel to a country where receipt of yellow fever vaccine is either required for entry or recommended for their protection should not wait until the last minute to obtain it. Postponing a trip or changing a destination is preferred if vaccine is not received, especially when the person is traveling to countries with an ongoing outbreak.
The vaccination does not become valid until 10 days after receipt. Infants aged at least 9 months may receive the vaccine. Since the yellow fever vaccine is a live vaccine, administration may be contraindicated in certain individuals. Exemption letters are provided for those with medical contraindication.
To locate a Stamaril site in your area: https://wwwnc.cdc.gov/travel/page/search-for-stamaril-clinics.
Current disease outbreaks
Yellow fever: Brazil
Since Dec. 2017, more than 1,100 laboratory-confirmed cases of yellow fever have been reported, including 17 reported in unvaccinated international travelers. Fatal cases also have been reported. In addition to areas in Brazil where yellow fever vaccination had been recommended prior to the recent outbreaks, the vaccine now also is recommended for people who are traveling to or living in all of Espírito Santo State, São Paulo State, and Rio de Janeiro State, as well as several cities in Bahia State. Unvaccinated travelers should avoid travel to areas where vaccination is recommended. Those previously vaccinated at 10 years ago or longer should consider a booster.
Listeria: South Africa
An ongoing outbreak has been reported since Jan. 2017. Around 1,000 people have been infected. Avoid consumption of processed meats including “Polony” (South African bologna).
Measles: Belarus, Japan, Liberia, and Taiwan
All countries have reported an increase in cases since April 2018. Measles outbreaks have been reported in an additional 13 countries since Jan. 2018, including France, Ireland, Italy, the Philippines, and the United Kingdom.
Norovirus: Canada
More than 120 cases have been linked to consumption of raw or lightly cooked oysters from western Canada.
For more country-specific information and up to date travel alerts, visit http://www.cdc.gov/travel.
Dr. Word is a pediatric infectious disease specialist and the director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures.
It’s that time of year again. Many of your patients will join the 80.2 million Americans with plans for international travel this summer.
In 2016, Mexico (31.2 million) and Canada (13.9 million) were the top two destinations of U.S. residents. Based on 2016 U.S. Commerce data, an additional 35.1 million Americans headed to overseas destinations, including 9% who traveled with children. Vacation and visiting friends and relatives accounted for 55% and 27% of the reasons for all travel, respectively. Education accounted for 4% of travelers.
Required versus recommended vaccines
The goal of a required vaccine is to prevent international spread of disease. The host country is protecting its citizens from visitors importing and facilitating the spread of a disease. Yellow fever and meningococcal disease are the only vaccines required for entry into any country. Entry requirements vary by country. Yellow fever may be an entry requirement for all travelers or it may be limited to those who have been in, or have had transit through, a country where yellow fever can be transmitted at least 6 days prior to the arrival at their final destination – a reminder that the sequence of the patient’s itinerary is important. In addition, just because a vaccine is not required for entry does not mean the risk for exposure and acquisition is nonexistent.
In contrast, recommended vaccines are for the protection of the individual. Travelers may be exposed to vaccine-preventable diseases that do not exist in their country (such as measles, typhoid fever, and yellow fever). They are at risk for acquisition and may return home infected, which could create the potential to spread the disease to susceptible contacts.
Most travelers comprehend required vaccines but often fail to understand the importance of receiving recommended vaccines. Lammert et al. reported that, of 24,478 persons who received pretravel advice between July 2012 and June 2014 through Global TravEpiNet, a national consortium of U.S. clinics, 97% were eligible for at least one vaccine. The majority were eligible for typhoid (n = 20,092) and hepatitis A (n = 12,990). Of patients included in the study, 25% (6,573) refused one or more vaccines. The most common reason cited for refusal was a lack of concern about the illness. Travelers visiting friends and relatives were less likely to accept all recommended vaccines, compared with those who were not visiting friends and relatives (odds ratio, 0.74) (J Trav Med. 2017 Jan. doi: 10.1093/jtm/taw075). In the United States, international travel remains the most common risk factor for acquisition of both typhoid fever and hepatitis A.
What’s new
The U.S. Advisory Committee on Immunization Practices recommends administering the hepatitis A vaccine to infants aged 6-11 months with travel to or living in developing countries and areas with high to moderate risk for hepatitis A virus transmission. Any dose received at less than 12 months of age does not count, and the administration of two age-appropriate doses should occur following this dose.
Old but still relevant
Measles: The Advisory Committee on Immunization Practices recommends all infants aged 6-11 months receive one dose of MMR prior to international travel regardless of the destination. This should be followed by two additional countable doses. All persons at least 12 months of age and born after 1956 should receive two doses of MMR at least 28 days apart prior to international travel.
Prior to administering, determine whether your patient will travel to a yellow fever–endemic area because both are live vaccines and should be received the same day. Otherwise, administer MMR doses 28 days apart; coordination between facilities or receipt of both at one facility may be necessary.
Yellow fever vaccine: The U.S. supplies of YF-Vax by Sanofi Pasteur are not expected to be available again until the end of 2018. To provide vaccines for U.S. travelers, Stamaril – a yellow fever vaccine produced by Sanofi Pasteur in France – has been made available at more than 250 sites through an Expanded Access Investigational New Drug Program.
Since Stamaril is offered at a limited number of locations, persons with anticipated travel to a country where receipt of yellow fever vaccine is either required for entry or recommended for their protection should not wait until the last minute to obtain it. Postponing a trip or changing a destination is preferred if vaccine is not received, especially when the person is traveling to countries with an ongoing outbreak.
The vaccination does not become valid until 10 days after receipt. Infants aged at least 9 months may receive the vaccine. Since the yellow fever vaccine is a live vaccine, administration may be contraindicated in certain individuals. Exemption letters are provided for those with medical contraindication.
To locate a Stamaril site in your area: https://wwwnc.cdc.gov/travel/page/search-for-stamaril-clinics.
Current disease outbreaks
Yellow fever: Brazil
Since Dec. 2017, more than 1,100 laboratory-confirmed cases of yellow fever have been reported, including 17 reported in unvaccinated international travelers. Fatal cases also have been reported. In addition to areas in Brazil where yellow fever vaccination had been recommended prior to the recent outbreaks, the vaccine now also is recommended for people who are traveling to or living in all of Espírito Santo State, São Paulo State, and Rio de Janeiro State, as well as several cities in Bahia State. Unvaccinated travelers should avoid travel to areas where vaccination is recommended. Those previously vaccinated at 10 years ago or longer should consider a booster.
Listeria: South Africa
An ongoing outbreak has been reported since Jan. 2017. Around 1,000 people have been infected. Avoid consumption of processed meats including “Polony” (South African bologna).
Measles: Belarus, Japan, Liberia, and Taiwan
All countries have reported an increase in cases since April 2018. Measles outbreaks have been reported in an additional 13 countries since Jan. 2018, including France, Ireland, Italy, the Philippines, and the United Kingdom.
Norovirus: Canada
More than 120 cases have been linked to consumption of raw or lightly cooked oysters from western Canada.
For more country-specific information and up to date travel alerts, visit http://www.cdc.gov/travel.
Dr. Word is a pediatric infectious disease specialist and the director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures.
It’s that time of year again. Many of your patients will join the 80.2 million Americans with plans for international travel this summer.
In 2016, Mexico (31.2 million) and Canada (13.9 million) were the top two destinations of U.S. residents. Based on 2016 U.S. Commerce data, an additional 35.1 million Americans headed to overseas destinations, including 9% who traveled with children. Vacation and visiting friends and relatives accounted for 55% and 27% of the reasons for all travel, respectively. Education accounted for 4% of travelers.
Required versus recommended vaccines
The goal of a required vaccine is to prevent international spread of disease. The host country is protecting its citizens from visitors importing and facilitating the spread of a disease. Yellow fever and meningococcal disease are the only vaccines required for entry into any country. Entry requirements vary by country. Yellow fever may be an entry requirement for all travelers or it may be limited to those who have been in, or have had transit through, a country where yellow fever can be transmitted at least 6 days prior to the arrival at their final destination – a reminder that the sequence of the patient’s itinerary is important. In addition, just because a vaccine is not required for entry does not mean the risk for exposure and acquisition is nonexistent.
In contrast, recommended vaccines are for the protection of the individual. Travelers may be exposed to vaccine-preventable diseases that do not exist in their country (such as measles, typhoid fever, and yellow fever). They are at risk for acquisition and may return home infected, which could create the potential to spread the disease to susceptible contacts.
Most travelers comprehend required vaccines but often fail to understand the importance of receiving recommended vaccines. Lammert et al. reported that, of 24,478 persons who received pretravel advice between July 2012 and June 2014 through Global TravEpiNet, a national consortium of U.S. clinics, 97% were eligible for at least one vaccine. The majority were eligible for typhoid (n = 20,092) and hepatitis A (n = 12,990). Of patients included in the study, 25% (6,573) refused one or more vaccines. The most common reason cited for refusal was a lack of concern about the illness. Travelers visiting friends and relatives were less likely to accept all recommended vaccines, compared with those who were not visiting friends and relatives (odds ratio, 0.74) (J Trav Med. 2017 Jan. doi: 10.1093/jtm/taw075). In the United States, international travel remains the most common risk factor for acquisition of both typhoid fever and hepatitis A.
What’s new
The U.S. Advisory Committee on Immunization Practices recommends administering the hepatitis A vaccine to infants aged 6-11 months with travel to or living in developing countries and areas with high to moderate risk for hepatitis A virus transmission. Any dose received at less than 12 months of age does not count, and the administration of two age-appropriate doses should occur following this dose.
Old but still relevant
Measles: The Advisory Committee on Immunization Practices recommends all infants aged 6-11 months receive one dose of MMR prior to international travel regardless of the destination. This should be followed by two additional countable doses. All persons at least 12 months of age and born after 1956 should receive two doses of MMR at least 28 days apart prior to international travel.
Prior to administering, determine whether your patient will travel to a yellow fever–endemic area because both are live vaccines and should be received the same day. Otherwise, administer MMR doses 28 days apart; coordination between facilities or receipt of both at one facility may be necessary.
Yellow fever vaccine: The U.S. supplies of YF-Vax by Sanofi Pasteur are not expected to be available again until the end of 2018. To provide vaccines for U.S. travelers, Stamaril – a yellow fever vaccine produced by Sanofi Pasteur in France – has been made available at more than 250 sites through an Expanded Access Investigational New Drug Program.
Since Stamaril is offered at a limited number of locations, persons with anticipated travel to a country where receipt of yellow fever vaccine is either required for entry or recommended for their protection should not wait until the last minute to obtain it. Postponing a trip or changing a destination is preferred if vaccine is not received, especially when the person is traveling to countries with an ongoing outbreak.
The vaccination does not become valid until 10 days after receipt. Infants aged at least 9 months may receive the vaccine. Since the yellow fever vaccine is a live vaccine, administration may be contraindicated in certain individuals. Exemption letters are provided for those with medical contraindication.
To locate a Stamaril site in your area: https://wwwnc.cdc.gov/travel/page/search-for-stamaril-clinics.
Current disease outbreaks
Yellow fever: Brazil
Since Dec. 2017, more than 1,100 laboratory-confirmed cases of yellow fever have been reported, including 17 reported in unvaccinated international travelers. Fatal cases also have been reported. In addition to areas in Brazil where yellow fever vaccination had been recommended prior to the recent outbreaks, the vaccine now also is recommended for people who are traveling to or living in all of Espírito Santo State, São Paulo State, and Rio de Janeiro State, as well as several cities in Bahia State. Unvaccinated travelers should avoid travel to areas where vaccination is recommended. Those previously vaccinated at 10 years ago or longer should consider a booster.
Listeria: South Africa
An ongoing outbreak has been reported since Jan. 2017. Around 1,000 people have been infected. Avoid consumption of processed meats including “Polony” (South African bologna).
Measles: Belarus, Japan, Liberia, and Taiwan
All countries have reported an increase in cases since April 2018. Measles outbreaks have been reported in an additional 13 countries since Jan. 2018, including France, Ireland, Italy, the Philippines, and the United Kingdom.
Norovirus: Canada
More than 120 cases have been linked to consumption of raw or lightly cooked oysters from western Canada.
For more country-specific information and up to date travel alerts, visit http://www.cdc.gov/travel.
Dr. Word is a pediatric infectious disease specialist and the director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures.
Artemisinin: Its global impact on the treatment of malaria
Malaria remains a major international public health concern. In 2015, the World Health Organization estimated that 212 million individuals were infected and that there were 429,000 deaths. This represents a 21% decline in incidence globally and a 29% decline in global mortality between 2010 and 2015. In 2016, malaria was endemic in 91 countries and territories, down from 108 in 2000. Although malaria has been eliminated from the United States since the early 1950s, approximately 1,700 cases are reported annually, most of which occur in returned travelers, according to the Centers for Disease Control and Prevention.
Five species of Plasmodium (P. falciparum, P. vivax, P. malariae, P. ovale, and, more recently, P. knowelsi) account for most of the infections in humans and are transmitted by the bite of an infected female Anopheles mosquito. The disease is rarely acquired by blood transfusion, by needle sharing, by organ transplantation, or congenitally. Once diagnosed, malaria can be treated; however, delay in initiating therapy can lead to both serious and fatal outcomes.
Treatment
Historically, drug development was driven by the need to protect the military. While quinine was isolated from the bark of the cinchona tree in 1820, chloroquine, proguanil, mefloquine, and atovaquone each were developed during or after a military conflict during 1945-1985. Tetracycline/doxycycline and clindamycin also have antimalarial activity. Use of any of these agents as monotherapy has led to drug resistance and treatment failure.
Artemisinin
Artemisinin (also known as qinghao su) and its derivatives are a new class of antimalarials derived from the sweet wormwood plant Artemisia annua. Initially developed in China in the 1970s, this class gained global attention in the 1990s. and have the fastest parasite clearance time, rapid resolution of symptoms, and an excellent safety profile. They have activity against all Plasmodium species.
Because of artemisinins’ rapid elimination, they are used in combination with an agent that also kills blood parasites but has a slower elimination rate and a different mechanism of action. The goal is to prevent and delay the development of resistance and reduce recrudescence. The superiority of artemisinin-based combination therapy (ACT) over monotherapies has been documented.
Resistance, always a concern, has remained limited to specific areas in Southeast Asia since reported in 2008. Monitoring drug efficacy, safety, quality of antimalarials is ongoing, as is discouraging monotherapy use of these agents. Globally, artemisinins are the mainstay of treatment. Spread of resistance would be a major setback for both malaria control and elimination.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Email her at pdnews@frontlinemedcom.com.
Malaria remains a major international public health concern. In 2015, the World Health Organization estimated that 212 million individuals were infected and that there were 429,000 deaths. This represents a 21% decline in incidence globally and a 29% decline in global mortality between 2010 and 2015. In 2016, malaria was endemic in 91 countries and territories, down from 108 in 2000. Although malaria has been eliminated from the United States since the early 1950s, approximately 1,700 cases are reported annually, most of which occur in returned travelers, according to the Centers for Disease Control and Prevention.
Five species of Plasmodium (P. falciparum, P. vivax, P. malariae, P. ovale, and, more recently, P. knowelsi) account for most of the infections in humans and are transmitted by the bite of an infected female Anopheles mosquito. The disease is rarely acquired by blood transfusion, by needle sharing, by organ transplantation, or congenitally. Once diagnosed, malaria can be treated; however, delay in initiating therapy can lead to both serious and fatal outcomes.
Treatment
Historically, drug development was driven by the need to protect the military. While quinine was isolated from the bark of the cinchona tree in 1820, chloroquine, proguanil, mefloquine, and atovaquone each were developed during or after a military conflict during 1945-1985. Tetracycline/doxycycline and clindamycin also have antimalarial activity. Use of any of these agents as monotherapy has led to drug resistance and treatment failure.
Artemisinin
Artemisinin (also known as qinghao su) and its derivatives are a new class of antimalarials derived from the sweet wormwood plant Artemisia annua. Initially developed in China in the 1970s, this class gained global attention in the 1990s. and have the fastest parasite clearance time, rapid resolution of symptoms, and an excellent safety profile. They have activity against all Plasmodium species.
Because of artemisinins’ rapid elimination, they are used in combination with an agent that also kills blood parasites but has a slower elimination rate and a different mechanism of action. The goal is to prevent and delay the development of resistance and reduce recrudescence. The superiority of artemisinin-based combination therapy (ACT) over monotherapies has been documented.
Resistance, always a concern, has remained limited to specific areas in Southeast Asia since reported in 2008. Monitoring drug efficacy, safety, quality of antimalarials is ongoing, as is discouraging monotherapy use of these agents. Globally, artemisinins are the mainstay of treatment. Spread of resistance would be a major setback for both malaria control and elimination.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Email her at pdnews@frontlinemedcom.com.
Malaria remains a major international public health concern. In 2015, the World Health Organization estimated that 212 million individuals were infected and that there were 429,000 deaths. This represents a 21% decline in incidence globally and a 29% decline in global mortality between 2010 and 2015. In 2016, malaria was endemic in 91 countries and territories, down from 108 in 2000. Although malaria has been eliminated from the United States since the early 1950s, approximately 1,700 cases are reported annually, most of which occur in returned travelers, according to the Centers for Disease Control and Prevention.
Five species of Plasmodium (P. falciparum, P. vivax, P. malariae, P. ovale, and, more recently, P. knowelsi) account for most of the infections in humans and are transmitted by the bite of an infected female Anopheles mosquito. The disease is rarely acquired by blood transfusion, by needle sharing, by organ transplantation, or congenitally. Once diagnosed, malaria can be treated; however, delay in initiating therapy can lead to both serious and fatal outcomes.
Treatment
Historically, drug development was driven by the need to protect the military. While quinine was isolated from the bark of the cinchona tree in 1820, chloroquine, proguanil, mefloquine, and atovaquone each were developed during or after a military conflict during 1945-1985. Tetracycline/doxycycline and clindamycin also have antimalarial activity. Use of any of these agents as monotherapy has led to drug resistance and treatment failure.
Artemisinin
Artemisinin (also known as qinghao su) and its derivatives are a new class of antimalarials derived from the sweet wormwood plant Artemisia annua. Initially developed in China in the 1970s, this class gained global attention in the 1990s. and have the fastest parasite clearance time, rapid resolution of symptoms, and an excellent safety profile. They have activity against all Plasmodium species.
Because of artemisinins’ rapid elimination, they are used in combination with an agent that also kills blood parasites but has a slower elimination rate and a different mechanism of action. The goal is to prevent and delay the development of resistance and reduce recrudescence. The superiority of artemisinin-based combination therapy (ACT) over monotherapies has been documented.
Resistance, always a concern, has remained limited to specific areas in Southeast Asia since reported in 2008. Monitoring drug efficacy, safety, quality of antimalarials is ongoing, as is discouraging monotherapy use of these agents. Globally, artemisinins are the mainstay of treatment. Spread of resistance would be a major setback for both malaria control and elimination.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Email her at pdnews@frontlinemedcom.com.
Salmonella infections: The source may be as close as your patient’s backyard
I recently received a group text from a friend voicing her frustration that her neighbor had acquired chickens, and she shared a photo of some roaming freely in the front yard. Naturally, my response was related to the potential infectious disease exposure and infections. Another friend chimed in “fresh eggs, and these are free range chickens. They don’t get sick. ... Many people in my area have chickens.” Unbeknownst to my friends, they had helped me select the ID Consult topic for this month.
Nontyphoidal Salmonella bacteria are associated with a wide spectrum of infections which range from asymptomatic gastrointestinal carriage to bacteremia, meningitis, osteomyelitis, and focal infections. Invasive disease is seen most often in children younger than 5 years of age, persons aged 65 years or older, and individuals with hemoglobinopathies including sickle cell disease and those with immunodeficiencies. Annually, the Centers for Disease Control and Prevention estimates that nontyphoidal salmonellosis is responsible for 1.2 million illnesses, 23,000 hospitalizations, and 450 deaths in the United States. Gastroenteritis is the most common manifestation of the disease and is characterized by abdominal cramps, diarrhea, and fever that develops 12-72 hours after exposure. It is usually self-limited. As previously reported in this column (June, 2017), Salmonella is one of the top two foodborne pathogens in the United States, and most outbreaks have been associated with consumption of contaminated food. But wait, contaminated food is not the only cause of some of our most recent outbreaks.
Live poultry-associated salmonellosis (LPAS)
LPAS was first reported in the 1950s. More recent epidemiologic data was published by C. Basler et al. (Emerging Infect Dis. 2016;22[10]:1705-11). LPAS was defined as two or more culture confirmed human Salmonella infections with a combination of epidemiologic, laboratory, or traceback evidence linking illnesses to contact with live poultry. The median outbreak size involved 26 cases (range, 4-363) and 77% (41 of 53) were multistate. The median age of the patients was 9 years (range, less than 1 to 92 years), and 31% were aged 5 years or younger. Exposure to chicks and ducklings was reported in 85% and 38%, respectively. High-risk practices included keeping poultry inside of the home (46%), snuggling baby birds (49%), and kissing baby birds (13%). The median time from purchase of poultry to onset of illness was 17 days (range, 1-672), and 66% reported onset of illness less than 30 days after purchase. Almost 52% reported owning poultry for less than 1 year.
The number of outbreaks continued to increase. From 1990 to 2005, there were a total of 17 outbreaks, compared with 36 between 2006 and 2014. Historically, outbreaks occurred in children around Easter when brightly colored dyed chicks were purchased. In the above review, 80% of outbreaks began in February, March, or April with an average duration of 4.9 months (range, 1-12).
Salmonella isolates
Backyard flocks and LPAS
More recently outbreaks have been associated with backyard flocks occurring year round and affecting both adults and children in contrast to seasonal peaks. The first multistate backyard flock outbreak was documented in 2007. Currently, the CDC is investigating 10 separate multistate outbreaks that began on Jan. 4, 2017. It involves 48 states, 961 infected individuals, 215 hospitalizations, and 1 death. At least 5 salmonella serotypes have been isolated.
What about the hatcheries?
It’s estimated that 50 million live poultry are sold annually. Birds are shipped within 24 hours after hatching via the U.S. Postal Service in boxes containing up to 100 chicks. Delivery occurs within 72 hours of hatching. Approximately 20 mail order hatcheries provide the majority of poultry sold to the general public. The National Poultry Improvement Plan (NPIP) is a voluntary state and federal testing and certification program whose goal is to eliminate poultry disease from breeder flocks to prevent egg-transmitted and hatchery-disseminated diseases. All hatcheries may participate. They also may participate in the voluntary Salmonella monitoring program. Note participation is not mandatory.
Preventing future outbreaks: patient/parental education is mandatory
1. Make sure your parents know about the association of Salmonella and live poultry. Reinforce these are farm animals, not pets. Purchase birds from hatcheries that participate in NPIP and the Salmonella monitoring programs.
2. Chicks, ducklings, or other live poultry should not be taken to schools, day care facilities, or nursing homes. Poultry should not be allowed in the home or in areas where food or drink is being prepared or consumed.
3. Poultry should not be snuggled, kissed, or allowed to touch one’s mouth. Hand washing with soap and water should occur after touching live poultry or any object touched in areas where they live or roam.
4. Contact with live poultry should be avoided in those at risk for developing serious infections including persons aged 5 years or younger, 65 years or older, immunocompromised individuals, and those with hemoglobinopathies.
5. All equipment used to care for live birds should be washed outdoors. Owners should have designated shoes when caring for poultry which should never be worn inside the home.
Hopefully, the next time you see a patient with fever and diarrhea you will recall this topic and ask about their contact with live poultry.
Additional resources to facilitate discussions can be found at www.cdc.gov/salmonella.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Email her at pdnews@frontlinemedcom.com.
I recently received a group text from a friend voicing her frustration that her neighbor had acquired chickens, and she shared a photo of some roaming freely in the front yard. Naturally, my response was related to the potential infectious disease exposure and infections. Another friend chimed in “fresh eggs, and these are free range chickens. They don’t get sick. ... Many people in my area have chickens.” Unbeknownst to my friends, they had helped me select the ID Consult topic for this month.
Nontyphoidal Salmonella bacteria are associated with a wide spectrum of infections which range from asymptomatic gastrointestinal carriage to bacteremia, meningitis, osteomyelitis, and focal infections. Invasive disease is seen most often in children younger than 5 years of age, persons aged 65 years or older, and individuals with hemoglobinopathies including sickle cell disease and those with immunodeficiencies. Annually, the Centers for Disease Control and Prevention estimates that nontyphoidal salmonellosis is responsible for 1.2 million illnesses, 23,000 hospitalizations, and 450 deaths in the United States. Gastroenteritis is the most common manifestation of the disease and is characterized by abdominal cramps, diarrhea, and fever that develops 12-72 hours after exposure. It is usually self-limited. As previously reported in this column (June, 2017), Salmonella is one of the top two foodborne pathogens in the United States, and most outbreaks have been associated with consumption of contaminated food. But wait, contaminated food is not the only cause of some of our most recent outbreaks.
Live poultry-associated salmonellosis (LPAS)
LPAS was first reported in the 1950s. More recent epidemiologic data was published by C. Basler et al. (Emerging Infect Dis. 2016;22[10]:1705-11). LPAS was defined as two or more culture confirmed human Salmonella infections with a combination of epidemiologic, laboratory, or traceback evidence linking illnesses to contact with live poultry. The median outbreak size involved 26 cases (range, 4-363) and 77% (41 of 53) were multistate. The median age of the patients was 9 years (range, less than 1 to 92 years), and 31% were aged 5 years or younger. Exposure to chicks and ducklings was reported in 85% and 38%, respectively. High-risk practices included keeping poultry inside of the home (46%), snuggling baby birds (49%), and kissing baby birds (13%). The median time from purchase of poultry to onset of illness was 17 days (range, 1-672), and 66% reported onset of illness less than 30 days after purchase. Almost 52% reported owning poultry for less than 1 year.
The number of outbreaks continued to increase. From 1990 to 2005, there were a total of 17 outbreaks, compared with 36 between 2006 and 2014. Historically, outbreaks occurred in children around Easter when brightly colored dyed chicks were purchased. In the above review, 80% of outbreaks began in February, March, or April with an average duration of 4.9 months (range, 1-12).
Salmonella isolates
Backyard flocks and LPAS
More recently outbreaks have been associated with backyard flocks occurring year round and affecting both adults and children in contrast to seasonal peaks. The first multistate backyard flock outbreak was documented in 2007. Currently, the CDC is investigating 10 separate multistate outbreaks that began on Jan. 4, 2017. It involves 48 states, 961 infected individuals, 215 hospitalizations, and 1 death. At least 5 salmonella serotypes have been isolated.
What about the hatcheries?
It’s estimated that 50 million live poultry are sold annually. Birds are shipped within 24 hours after hatching via the U.S. Postal Service in boxes containing up to 100 chicks. Delivery occurs within 72 hours of hatching. Approximately 20 mail order hatcheries provide the majority of poultry sold to the general public. The National Poultry Improvement Plan (NPIP) is a voluntary state and federal testing and certification program whose goal is to eliminate poultry disease from breeder flocks to prevent egg-transmitted and hatchery-disseminated diseases. All hatcheries may participate. They also may participate in the voluntary Salmonella monitoring program. Note participation is not mandatory.
Preventing future outbreaks: patient/parental education is mandatory
1. Make sure your parents know about the association of Salmonella and live poultry. Reinforce these are farm animals, not pets. Purchase birds from hatcheries that participate in NPIP and the Salmonella monitoring programs.
2. Chicks, ducklings, or other live poultry should not be taken to schools, day care facilities, or nursing homes. Poultry should not be allowed in the home or in areas where food or drink is being prepared or consumed.
3. Poultry should not be snuggled, kissed, or allowed to touch one’s mouth. Hand washing with soap and water should occur after touching live poultry or any object touched in areas where they live or roam.
4. Contact with live poultry should be avoided in those at risk for developing serious infections including persons aged 5 years or younger, 65 years or older, immunocompromised individuals, and those with hemoglobinopathies.
5. All equipment used to care for live birds should be washed outdoors. Owners should have designated shoes when caring for poultry which should never be worn inside the home.
Hopefully, the next time you see a patient with fever and diarrhea you will recall this topic and ask about their contact with live poultry.
Additional resources to facilitate discussions can be found at www.cdc.gov/salmonella.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Email her at pdnews@frontlinemedcom.com.
I recently received a group text from a friend voicing her frustration that her neighbor had acquired chickens, and she shared a photo of some roaming freely in the front yard. Naturally, my response was related to the potential infectious disease exposure and infections. Another friend chimed in “fresh eggs, and these are free range chickens. They don’t get sick. ... Many people in my area have chickens.” Unbeknownst to my friends, they had helped me select the ID Consult topic for this month.
Nontyphoidal Salmonella bacteria are associated with a wide spectrum of infections which range from asymptomatic gastrointestinal carriage to bacteremia, meningitis, osteomyelitis, and focal infections. Invasive disease is seen most often in children younger than 5 years of age, persons aged 65 years or older, and individuals with hemoglobinopathies including sickle cell disease and those with immunodeficiencies. Annually, the Centers for Disease Control and Prevention estimates that nontyphoidal salmonellosis is responsible for 1.2 million illnesses, 23,000 hospitalizations, and 450 deaths in the United States. Gastroenteritis is the most common manifestation of the disease and is characterized by abdominal cramps, diarrhea, and fever that develops 12-72 hours after exposure. It is usually self-limited. As previously reported in this column (June, 2017), Salmonella is one of the top two foodborne pathogens in the United States, and most outbreaks have been associated with consumption of contaminated food. But wait, contaminated food is not the only cause of some of our most recent outbreaks.
Live poultry-associated salmonellosis (LPAS)
LPAS was first reported in the 1950s. More recent epidemiologic data was published by C. Basler et al. (Emerging Infect Dis. 2016;22[10]:1705-11). LPAS was defined as two or more culture confirmed human Salmonella infections with a combination of epidemiologic, laboratory, or traceback evidence linking illnesses to contact with live poultry. The median outbreak size involved 26 cases (range, 4-363) and 77% (41 of 53) were multistate. The median age of the patients was 9 years (range, less than 1 to 92 years), and 31% were aged 5 years or younger. Exposure to chicks and ducklings was reported in 85% and 38%, respectively. High-risk practices included keeping poultry inside of the home (46%), snuggling baby birds (49%), and kissing baby birds (13%). The median time from purchase of poultry to onset of illness was 17 days (range, 1-672), and 66% reported onset of illness less than 30 days after purchase. Almost 52% reported owning poultry for less than 1 year.
The number of outbreaks continued to increase. From 1990 to 2005, there were a total of 17 outbreaks, compared with 36 between 2006 and 2014. Historically, outbreaks occurred in children around Easter when brightly colored dyed chicks were purchased. In the above review, 80% of outbreaks began in February, March, or April with an average duration of 4.9 months (range, 1-12).
Salmonella isolates
Backyard flocks and LPAS
More recently outbreaks have been associated with backyard flocks occurring year round and affecting both adults and children in contrast to seasonal peaks. The first multistate backyard flock outbreak was documented in 2007. Currently, the CDC is investigating 10 separate multistate outbreaks that began on Jan. 4, 2017. It involves 48 states, 961 infected individuals, 215 hospitalizations, and 1 death. At least 5 salmonella serotypes have been isolated.
What about the hatcheries?
It’s estimated that 50 million live poultry are sold annually. Birds are shipped within 24 hours after hatching via the U.S. Postal Service in boxes containing up to 100 chicks. Delivery occurs within 72 hours of hatching. Approximately 20 mail order hatcheries provide the majority of poultry sold to the general public. The National Poultry Improvement Plan (NPIP) is a voluntary state and federal testing and certification program whose goal is to eliminate poultry disease from breeder flocks to prevent egg-transmitted and hatchery-disseminated diseases. All hatcheries may participate. They also may participate in the voluntary Salmonella monitoring program. Note participation is not mandatory.
Preventing future outbreaks: patient/parental education is mandatory
1. Make sure your parents know about the association of Salmonella and live poultry. Reinforce these are farm animals, not pets. Purchase birds from hatcheries that participate in NPIP and the Salmonella monitoring programs.
2. Chicks, ducklings, or other live poultry should not be taken to schools, day care facilities, or nursing homes. Poultry should not be allowed in the home or in areas where food or drink is being prepared or consumed.
3. Poultry should not be snuggled, kissed, or allowed to touch one’s mouth. Hand washing with soap and water should occur after touching live poultry or any object touched in areas where they live or roam.
4. Contact with live poultry should be avoided in those at risk for developing serious infections including persons aged 5 years or younger, 65 years or older, immunocompromised individuals, and those with hemoglobinopathies.
5. All equipment used to care for live birds should be washed outdoors. Owners should have designated shoes when caring for poultry which should never be worn inside the home.
Hopefully, the next time you see a patient with fever and diarrhea you will recall this topic and ask about their contact with live poultry.
Additional resources to facilitate discussions can be found at www.cdc.gov/salmonella.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Email her at pdnews@frontlinemedcom.com.
Mycobacterium tuberculosis: Overcoming one obstacle on the road to elimination
March 24 is World TB Day. It was on this date in 1882 that physician Robert Koch announced the discovery of Mycobacterium tuberculosis, the causative agent of tuberculosis. Worldwide, activities are planned to raise awareness of TB and to support initiatives for prevention, better control, and ultimately the elimination of this disease.
Globally in 2015, the World Health Organization estimated there were 10.4 million new cases of TB, including 1 million in children. Data from the United States reveal that after 20 years of annual decline, the incidence of TB has plateaued. In 2015, 9,563 cases of TB disease were reported, including 440 cases in children less than 15 years of age. While the overall incidence was 3 cases per 100,000, the incidence among foreign-born persons was 15.1 cases per 100,000. There were 3,201 cases (33.5%) among U.S.-born individuals. Foreign-born persons accounted for 66.2% of cases; however, the majority of those cases were diagnosed several years after their arrival in the United States. The top five countries of origin of these individuals were China, India, Mexico, the Philippines, and Vietnam. In contrast, only one-quarter of all pediatric cases occurred in foreign-born children. Four states (California, Florida, New York, and Texas) reported more than 500 cases each in 2015, as they have for the last 7 consecutive years. In 2015, these states accounted for slightly more than half (4,839) of all cases (MMWR 2016 Mar 25;65[11]:273-8).
Why as pediatricians should we be concerned? TB in a child is a sentinel event and represents recent or ongoing transmission. Young children who are infected are more likely to progress to TB disease and develop severe manifestations such as miliary TB or meningitis. Children less than 4 years old and those with certain underlying disorders, including those with an immunodeficiency or who are receiving immunosuppressive agents, also are at greater risk for progression from infection to disease. Other predictors of disease progression include diagnosis of the infection within the past 2 years, use of chemotherapy and high-dose corticosteroids, as well as certain cancers, diabetes, and chronic renal failure.
Once infected, most children and adolescents remain asymptomatic. If disease occurs, symptoms develop 1-6 months after infection and include fever, cough, weight loss or failure to thrive, night sweats, and chills. Chest radiographic findings are nonspecific. Infiltrates and intrathoracic lymph node enlargement may or may not be present. However, our goal is to diagnose at-risk children with infection, treat them, and avoid their progression to TB disease.
Screening tests
The interferon-gamma release assay is a blood test that has a greater specificity than TST and requires only one visit. A positive test is seen in both latent TB infection and TB disease. There is no cross-reaction with BCG. This is the ideal test for prior BCG recipients and others who are unlikely to return for TST readings and are at least 5 years of age.
A chest radiograph is required to differentiate latent TB infection from TB disease. Latent TB infection is diagnosed when there is an absence of parenchymal disease, opacification, or intrathoracic adenopathy.
Treatment of latent TB infection versus TB disease is beyond the scope of this article. Consultation with an infectious disease expert is recommended.
For additional information and resources, go to www.cdc.gov/tb, and for a sample TB risk assessment tool, go to www.cdc.gov/tb/publications/ltbi/appendixa.htm.
As we mark the passing of another World TB Day, we have one goal – to identify, screen, and treat children and adolescents at risk for latent TB infection and help eliminate future cases of TB disease.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures.
March 24 is World TB Day. It was on this date in 1882 that physician Robert Koch announced the discovery of Mycobacterium tuberculosis, the causative agent of tuberculosis. Worldwide, activities are planned to raise awareness of TB and to support initiatives for prevention, better control, and ultimately the elimination of this disease.
Globally in 2015, the World Health Organization estimated there were 10.4 million new cases of TB, including 1 million in children. Data from the United States reveal that after 20 years of annual decline, the incidence of TB has plateaued. In 2015, 9,563 cases of TB disease were reported, including 440 cases in children less than 15 years of age. While the overall incidence was 3 cases per 100,000, the incidence among foreign-born persons was 15.1 cases per 100,000. There were 3,201 cases (33.5%) among U.S.-born individuals. Foreign-born persons accounted for 66.2% of cases; however, the majority of those cases were diagnosed several years after their arrival in the United States. The top five countries of origin of these individuals were China, India, Mexico, the Philippines, and Vietnam. In contrast, only one-quarter of all pediatric cases occurred in foreign-born children. Four states (California, Florida, New York, and Texas) reported more than 500 cases each in 2015, as they have for the last 7 consecutive years. In 2015, these states accounted for slightly more than half (4,839) of all cases (MMWR 2016 Mar 25;65[11]:273-8).
Why as pediatricians should we be concerned? TB in a child is a sentinel event and represents recent or ongoing transmission. Young children who are infected are more likely to progress to TB disease and develop severe manifestations such as miliary TB or meningitis. Children less than 4 years old and those with certain underlying disorders, including those with an immunodeficiency or who are receiving immunosuppressive agents, also are at greater risk for progression from infection to disease. Other predictors of disease progression include diagnosis of the infection within the past 2 years, use of chemotherapy and high-dose corticosteroids, as well as certain cancers, diabetes, and chronic renal failure.
Once infected, most children and adolescents remain asymptomatic. If disease occurs, symptoms develop 1-6 months after infection and include fever, cough, weight loss or failure to thrive, night sweats, and chills. Chest radiographic findings are nonspecific. Infiltrates and intrathoracic lymph node enlargement may or may not be present. However, our goal is to diagnose at-risk children with infection, treat them, and avoid their progression to TB disease.
Screening tests
The interferon-gamma release assay is a blood test that has a greater specificity than TST and requires only one visit. A positive test is seen in both latent TB infection and TB disease. There is no cross-reaction with BCG. This is the ideal test for prior BCG recipients and others who are unlikely to return for TST readings and are at least 5 years of age.
A chest radiograph is required to differentiate latent TB infection from TB disease. Latent TB infection is diagnosed when there is an absence of parenchymal disease, opacification, or intrathoracic adenopathy.
Treatment of latent TB infection versus TB disease is beyond the scope of this article. Consultation with an infectious disease expert is recommended.
For additional information and resources, go to www.cdc.gov/tb, and for a sample TB risk assessment tool, go to www.cdc.gov/tb/publications/ltbi/appendixa.htm.
As we mark the passing of another World TB Day, we have one goal – to identify, screen, and treat children and adolescents at risk for latent TB infection and help eliminate future cases of TB disease.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures.
March 24 is World TB Day. It was on this date in 1882 that physician Robert Koch announced the discovery of Mycobacterium tuberculosis, the causative agent of tuberculosis. Worldwide, activities are planned to raise awareness of TB and to support initiatives for prevention, better control, and ultimately the elimination of this disease.
Globally in 2015, the World Health Organization estimated there were 10.4 million new cases of TB, including 1 million in children. Data from the United States reveal that after 20 years of annual decline, the incidence of TB has plateaued. In 2015, 9,563 cases of TB disease were reported, including 440 cases in children less than 15 years of age. While the overall incidence was 3 cases per 100,000, the incidence among foreign-born persons was 15.1 cases per 100,000. There were 3,201 cases (33.5%) among U.S.-born individuals. Foreign-born persons accounted for 66.2% of cases; however, the majority of those cases were diagnosed several years after their arrival in the United States. The top five countries of origin of these individuals were China, India, Mexico, the Philippines, and Vietnam. In contrast, only one-quarter of all pediatric cases occurred in foreign-born children. Four states (California, Florida, New York, and Texas) reported more than 500 cases each in 2015, as they have for the last 7 consecutive years. In 2015, these states accounted for slightly more than half (4,839) of all cases (MMWR 2016 Mar 25;65[11]:273-8).
Why as pediatricians should we be concerned? TB in a child is a sentinel event and represents recent or ongoing transmission. Young children who are infected are more likely to progress to TB disease and develop severe manifestations such as miliary TB or meningitis. Children less than 4 years old and those with certain underlying disorders, including those with an immunodeficiency or who are receiving immunosuppressive agents, also are at greater risk for progression from infection to disease. Other predictors of disease progression include diagnosis of the infection within the past 2 years, use of chemotherapy and high-dose corticosteroids, as well as certain cancers, diabetes, and chronic renal failure.
Once infected, most children and adolescents remain asymptomatic. If disease occurs, symptoms develop 1-6 months after infection and include fever, cough, weight loss or failure to thrive, night sweats, and chills. Chest radiographic findings are nonspecific. Infiltrates and intrathoracic lymph node enlargement may or may not be present. However, our goal is to diagnose at-risk children with infection, treat them, and avoid their progression to TB disease.
Screening tests
The interferon-gamma release assay is a blood test that has a greater specificity than TST and requires only one visit. A positive test is seen in both latent TB infection and TB disease. There is no cross-reaction with BCG. This is the ideal test for prior BCG recipients and others who are unlikely to return for TST readings and are at least 5 years of age.
A chest radiograph is required to differentiate latent TB infection from TB disease. Latent TB infection is diagnosed when there is an absence of parenchymal disease, opacification, or intrathoracic adenopathy.
Treatment of latent TB infection versus TB disease is beyond the scope of this article. Consultation with an infectious disease expert is recommended.
For additional information and resources, go to www.cdc.gov/tb, and for a sample TB risk assessment tool, go to www.cdc.gov/tb/publications/ltbi/appendixa.htm.
As we mark the passing of another World TB Day, we have one goal – to identify, screen, and treat children and adolescents at risk for latent TB infection and help eliminate future cases of TB disease.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures.
HPV vaccine and adolescents: What we say really does matter
It has been almost 10 years since the Advisory Committee on Immunization Practices (ACIP) recommended administration of human papillomavirus (HPV) vaccine for 11- to 12-year-old girls and young women up to 26 years of age. Routine administration in preteen boys and young adult males up to 21 years of age was recommended in 2011. An HPV series should be completed by 13 years. So how well are we protecting our patients?
Vaccine coverage
The National Immunization Survey–Teen (NIS-Teen) monitors vaccine coverage annually among adolescents 13-17 years. Data are obtained from individuals from the 50 states, the District of Columbia, the U.S. Virgin Islands, and six major urban areas (MMWR. 2016 Aug 26;65[33]:850-8).
HPV vaccination continues to lag behind Tdap and the meningococcal conjugate vaccine (MCV), although each one is recommended to be administered at the 11- to 12-year visit. In 2015, coverage for receiving at least one dose of HPV vaccine among females was almost 62.8 % and for at least three doses was 41.9%; among males, coverage with at least one dose was 49.8% and for at least three doses was 28.1%. Compared with 2014, coverage for at least one dose of HPV vaccine increased 2.8% in females and 8.1% in males. Males also had a 7.6% for receipt of at least two doses of HPV vaccine, compared with 2014. HPV vaccine coverage in females aged 13 and younger also was lower than for those aged 15 and older. Coverage did not differ for males based on age.
HPV vaccination coverage also differed by state. In 2015, 28 states reported increased coverage in males, but only 7 states had increased coverage in females. Among all adolescents, coverage with at least one dose of HPV vaccine was 56.1%, at least two doses was 45.4%, and at least three doses was 34.9%. In contrast, 86.4% of all adolescents received at least one dose of Tdap, and 81.3% received at least one dose of MCV.
HPV-associated cancers
HPV is the most common sexually transmitted infection in both men and women. It is estimated that 79 million Americans are infected and 14 million new infections occur annually, usually in teens and young adults. Although most infections are asymptomatic and clear spontaneously, persistent infection with oncogenic types can progress to cancer. Cervical and oropharyngeal cancer were the most common HPV-associated cancers in women and men, respectively, in 2008-2012 (MMWR 2016;65:661-6).
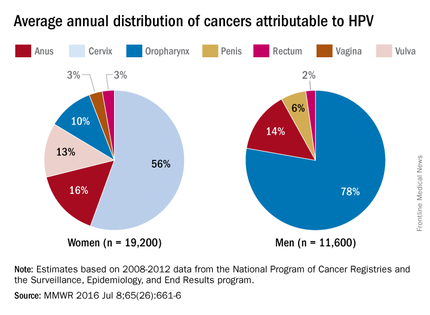
All three HPV vaccines protect against HPV types 16 and 18. These types are estimated to account for the majority of cervical and oropharyngeal cancers, 66% and 62%, respectively. The additional types in the 9-valent HPV will protect against HPV types that cause approximately 15% of cervical cancers.
The association between HPV and cancer is clear. So why isn’t this vaccine being embraced? HPV vaccine is all about cancer prevention. Isn’t it? What are the barriers to HPV vaccination? Are parental concerns the only barrier? Are we recommending this vaccine as strongly as others?
Vaccine safety and efficacy
Safety has been a concern voiced by some parents. Collectively, HPV vaccines were studied in more than 95,000 individuals prior to licensure. Almost 90 million doses of vaccine have been distributed in the United States and more than 183 million, worldwide. The federal government utilizes three systems to monitor vaccine safety once a vaccine is licensed: The Vaccine Adverse Event Reporting System (VAERS), the Vaccine Safety Datalink (VSD), and the Clinical Immunization Safety Assessment (CISA) Network. Ongoing safety studies also are conducted by vaccine manufacturers. Since licensure, no serious safety concerns have been identified. Postvaccination syncope, first identified in the VAERS database in 2006, has declined since observation post injection was recommended by ACIP. Multiple studies in the United States and abroad have not demonstrated a causal association with HPV vaccine and any autoimmune and/or neurologic condition or increased risk for thromboembolism.
Mélanie Drolet, PhD, and her colleagues reviewed 20 studies in nine countries with at least 50% coverage in female adolescents aged 13-19 years. There was a 68% reduction in the prevalence of HPV types 16 and 18 and a 61% reduction in anal warts in the postvaccine era (Lancet Infect Dis. 2015 May;15[5]:565-80). Studies also indicate there is no indication of waning immunity.
Parental perceptions
Some parents feel the vaccine is not necessary because their child is not sexually active and/or is not at risk for acquiring a sexually transmitted infection. Others opt to delay initiation. NHANES (National Health and Nutrition Examination Survey) data from 2011 to 2014 revealed that among females aged 14-26 years whose age was known at the time of their first dose of HPV vaccine, 43% had reported having sex before or in the same year that they received their first dose.
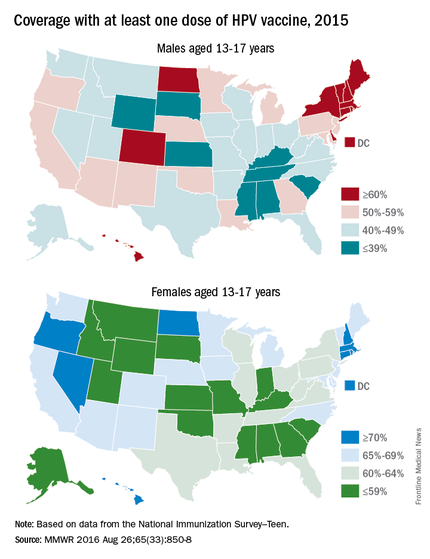
One consistent reason parents indicate for not vaccinating is the lack of recommendation from their child’s health provider. Differences in age and sex recommendations also are reported. NIS-Teen 2013 demonstrated that parents of girls were more likely than parents of boys to receive a provider recommendation (65% vs.42%.) Only 29% of female parents indicated they’d received a provider recommendation to have their child vaccinated with HPV by ages 11-12 years.
Mandy A. Allison, MD, and her colleagues reviewed primary care physician perspectives about HPV vaccine in a national survey among 364 pediatricians and 218 family physicians (FPs). Although 84% of pediatricians and 75% of FPs indicated they always discuss HPV vaccination, only 60% of pediatricians and 59% of FPs strongly recommend HPV vaccine for 11- to 12-year-old girls; for boys it was 52% and 41%. More than half reported parental deferral. For pediatricians who almost never discussed the topic, the reasons included that the patient was not sexually active (54%), the child was young (38%), and the patient was already receiving other vaccines (35%) (Pediatrics. 2016 Feb;137[2]:e20152488).
Providers can be influenced by their perceptions of what value parents place on vaccines. In one study, parents were asked to put a value on specific vaccines. Providers were then asked to estimate how parents ranked the vaccines on a scale of 0-10. Providers underestimated the value placed on HPV vaccine (9.3 vs 5.2) (Vaccine 2014;32:579-84).
Improving HPV coverage: Preventing future HPV-related cancers
HPV vaccine should be recommended with as much conviction as Tdap and MCV at the 11- to 12-year visit for both girls and boys. Administration of all three should occur on the same day. Clinician recommendation is the No. 1 reason parents decide to vaccinate. The mantra “same way, same day” should become synonymous with the 11- to 12-year visit. All who have contact with the patient, beginning with the front desk staff, should know the importance of HPV vaccine, and when and why it is recommended. Often, families spend more time with support staff and have discussions prior to interacting with you.
Anticipate questions about HPV. Why give the vaccine when the child is so young and not sexually active? Is my child really at risk? Is it safe? I read on the Internet. … Questions should be interpreted as a need for additional information and reassurance from you.
Remember to emphasize that HPV vaccine is important because it prevents cancer and it is most effective prior to exposure to HPV.
Additional resources to facilitate your discussions about HPV can be found at www.cdc.gov/hpv.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures.
It has been almost 10 years since the Advisory Committee on Immunization Practices (ACIP) recommended administration of human papillomavirus (HPV) vaccine for 11- to 12-year-old girls and young women up to 26 years of age. Routine administration in preteen boys and young adult males up to 21 years of age was recommended in 2011. An HPV series should be completed by 13 years. So how well are we protecting our patients?
Vaccine coverage
The National Immunization Survey–Teen (NIS-Teen) monitors vaccine coverage annually among adolescents 13-17 years. Data are obtained from individuals from the 50 states, the District of Columbia, the U.S. Virgin Islands, and six major urban areas (MMWR. 2016 Aug 26;65[33]:850-8).
HPV vaccination continues to lag behind Tdap and the meningococcal conjugate vaccine (MCV), although each one is recommended to be administered at the 11- to 12-year visit. In 2015, coverage for receiving at least one dose of HPV vaccine among females was almost 62.8 % and for at least three doses was 41.9%; among males, coverage with at least one dose was 49.8% and for at least three doses was 28.1%. Compared with 2014, coverage for at least one dose of HPV vaccine increased 2.8% in females and 8.1% in males. Males also had a 7.6% for receipt of at least two doses of HPV vaccine, compared with 2014. HPV vaccine coverage in females aged 13 and younger also was lower than for those aged 15 and older. Coverage did not differ for males based on age.
HPV vaccination coverage also differed by state. In 2015, 28 states reported increased coverage in males, but only 7 states had increased coverage in females. Among all adolescents, coverage with at least one dose of HPV vaccine was 56.1%, at least two doses was 45.4%, and at least three doses was 34.9%. In contrast, 86.4% of all adolescents received at least one dose of Tdap, and 81.3% received at least one dose of MCV.
HPV-associated cancers
HPV is the most common sexually transmitted infection in both men and women. It is estimated that 79 million Americans are infected and 14 million new infections occur annually, usually in teens and young adults. Although most infections are asymptomatic and clear spontaneously, persistent infection with oncogenic types can progress to cancer. Cervical and oropharyngeal cancer were the most common HPV-associated cancers in women and men, respectively, in 2008-2012 (MMWR 2016;65:661-6).

All three HPV vaccines protect against HPV types 16 and 18. These types are estimated to account for the majority of cervical and oropharyngeal cancers, 66% and 62%, respectively. The additional types in the 9-valent HPV will protect against HPV types that cause approximately 15% of cervical cancers.
The association between HPV and cancer is clear. So why isn’t this vaccine being embraced? HPV vaccine is all about cancer prevention. Isn’t it? What are the barriers to HPV vaccination? Are parental concerns the only barrier? Are we recommending this vaccine as strongly as others?
Vaccine safety and efficacy
Safety has been a concern voiced by some parents. Collectively, HPV vaccines were studied in more than 95,000 individuals prior to licensure. Almost 90 million doses of vaccine have been distributed in the United States and more than 183 million, worldwide. The federal government utilizes three systems to monitor vaccine safety once a vaccine is licensed: The Vaccine Adverse Event Reporting System (VAERS), the Vaccine Safety Datalink (VSD), and the Clinical Immunization Safety Assessment (CISA) Network. Ongoing safety studies also are conducted by vaccine manufacturers. Since licensure, no serious safety concerns have been identified. Postvaccination syncope, first identified in the VAERS database in 2006, has declined since observation post injection was recommended by ACIP. Multiple studies in the United States and abroad have not demonstrated a causal association with HPV vaccine and any autoimmune and/or neurologic condition or increased risk for thromboembolism.
Mélanie Drolet, PhD, and her colleagues reviewed 20 studies in nine countries with at least 50% coverage in female adolescents aged 13-19 years. There was a 68% reduction in the prevalence of HPV types 16 and 18 and a 61% reduction in anal warts in the postvaccine era (Lancet Infect Dis. 2015 May;15[5]:565-80). Studies also indicate there is no indication of waning immunity.
Parental perceptions
Some parents feel the vaccine is not necessary because their child is not sexually active and/or is not at risk for acquiring a sexually transmitted infection. Others opt to delay initiation. NHANES (National Health and Nutrition Examination Survey) data from 2011 to 2014 revealed that among females aged 14-26 years whose age was known at the time of their first dose of HPV vaccine, 43% had reported having sex before or in the same year that they received their first dose.

One consistent reason parents indicate for not vaccinating is the lack of recommendation from their child’s health provider. Differences in age and sex recommendations also are reported. NIS-Teen 2013 demonstrated that parents of girls were more likely than parents of boys to receive a provider recommendation (65% vs.42%.) Only 29% of female parents indicated they’d received a provider recommendation to have their child vaccinated with HPV by ages 11-12 years.
Mandy A. Allison, MD, and her colleagues reviewed primary care physician perspectives about HPV vaccine in a national survey among 364 pediatricians and 218 family physicians (FPs). Although 84% of pediatricians and 75% of FPs indicated they always discuss HPV vaccination, only 60% of pediatricians and 59% of FPs strongly recommend HPV vaccine for 11- to 12-year-old girls; for boys it was 52% and 41%. More than half reported parental deferral. For pediatricians who almost never discussed the topic, the reasons included that the patient was not sexually active (54%), the child was young (38%), and the patient was already receiving other vaccines (35%) (Pediatrics. 2016 Feb;137[2]:e20152488).
Providers can be influenced by their perceptions of what value parents place on vaccines. In one study, parents were asked to put a value on specific vaccines. Providers were then asked to estimate how parents ranked the vaccines on a scale of 0-10. Providers underestimated the value placed on HPV vaccine (9.3 vs 5.2) (Vaccine 2014;32:579-84).
Improving HPV coverage: Preventing future HPV-related cancers
HPV vaccine should be recommended with as much conviction as Tdap and MCV at the 11- to 12-year visit for both girls and boys. Administration of all three should occur on the same day. Clinician recommendation is the No. 1 reason parents decide to vaccinate. The mantra “same way, same day” should become synonymous with the 11- to 12-year visit. All who have contact with the patient, beginning with the front desk staff, should know the importance of HPV vaccine, and when and why it is recommended. Often, families spend more time with support staff and have discussions prior to interacting with you.
Anticipate questions about HPV. Why give the vaccine when the child is so young and not sexually active? Is my child really at risk? Is it safe? I read on the Internet. … Questions should be interpreted as a need for additional information and reassurance from you.
Remember to emphasize that HPV vaccine is important because it prevents cancer and it is most effective prior to exposure to HPV.
Additional resources to facilitate your discussions about HPV can be found at www.cdc.gov/hpv.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures.
It has been almost 10 years since the Advisory Committee on Immunization Practices (ACIP) recommended administration of human papillomavirus (HPV) vaccine for 11- to 12-year-old girls and young women up to 26 years of age. Routine administration in preteen boys and young adult males up to 21 years of age was recommended in 2011. An HPV series should be completed by 13 years. So how well are we protecting our patients?
Vaccine coverage
The National Immunization Survey–Teen (NIS-Teen) monitors vaccine coverage annually among adolescents 13-17 years. Data are obtained from individuals from the 50 states, the District of Columbia, the U.S. Virgin Islands, and six major urban areas (MMWR. 2016 Aug 26;65[33]:850-8).
HPV vaccination continues to lag behind Tdap and the meningococcal conjugate vaccine (MCV), although each one is recommended to be administered at the 11- to 12-year visit. In 2015, coverage for receiving at least one dose of HPV vaccine among females was almost 62.8 % and for at least three doses was 41.9%; among males, coverage with at least one dose was 49.8% and for at least three doses was 28.1%. Compared with 2014, coverage for at least one dose of HPV vaccine increased 2.8% in females and 8.1% in males. Males also had a 7.6% for receipt of at least two doses of HPV vaccine, compared with 2014. HPV vaccine coverage in females aged 13 and younger also was lower than for those aged 15 and older. Coverage did not differ for males based on age.
HPV vaccination coverage also differed by state. In 2015, 28 states reported increased coverage in males, but only 7 states had increased coverage in females. Among all adolescents, coverage with at least one dose of HPV vaccine was 56.1%, at least two doses was 45.4%, and at least three doses was 34.9%. In contrast, 86.4% of all adolescents received at least one dose of Tdap, and 81.3% received at least one dose of MCV.
HPV-associated cancers
HPV is the most common sexually transmitted infection in both men and women. It is estimated that 79 million Americans are infected and 14 million new infections occur annually, usually in teens and young adults. Although most infections are asymptomatic and clear spontaneously, persistent infection with oncogenic types can progress to cancer. Cervical and oropharyngeal cancer were the most common HPV-associated cancers in women and men, respectively, in 2008-2012 (MMWR 2016;65:661-6).

All three HPV vaccines protect against HPV types 16 and 18. These types are estimated to account for the majority of cervical and oropharyngeal cancers, 66% and 62%, respectively. The additional types in the 9-valent HPV will protect against HPV types that cause approximately 15% of cervical cancers.
The association between HPV and cancer is clear. So why isn’t this vaccine being embraced? HPV vaccine is all about cancer prevention. Isn’t it? What are the barriers to HPV vaccination? Are parental concerns the only barrier? Are we recommending this vaccine as strongly as others?
Vaccine safety and efficacy
Safety has been a concern voiced by some parents. Collectively, HPV vaccines were studied in more than 95,000 individuals prior to licensure. Almost 90 million doses of vaccine have been distributed in the United States and more than 183 million, worldwide. The federal government utilizes three systems to monitor vaccine safety once a vaccine is licensed: The Vaccine Adverse Event Reporting System (VAERS), the Vaccine Safety Datalink (VSD), and the Clinical Immunization Safety Assessment (CISA) Network. Ongoing safety studies also are conducted by vaccine manufacturers. Since licensure, no serious safety concerns have been identified. Postvaccination syncope, first identified in the VAERS database in 2006, has declined since observation post injection was recommended by ACIP. Multiple studies in the United States and abroad have not demonstrated a causal association with HPV vaccine and any autoimmune and/or neurologic condition or increased risk for thromboembolism.
Mélanie Drolet, PhD, and her colleagues reviewed 20 studies in nine countries with at least 50% coverage in female adolescents aged 13-19 years. There was a 68% reduction in the prevalence of HPV types 16 and 18 and a 61% reduction in anal warts in the postvaccine era (Lancet Infect Dis. 2015 May;15[5]:565-80). Studies also indicate there is no indication of waning immunity.
Parental perceptions
Some parents feel the vaccine is not necessary because their child is not sexually active and/or is not at risk for acquiring a sexually transmitted infection. Others opt to delay initiation. NHANES (National Health and Nutrition Examination Survey) data from 2011 to 2014 revealed that among females aged 14-26 years whose age was known at the time of their first dose of HPV vaccine, 43% had reported having sex before or in the same year that they received their first dose.

One consistent reason parents indicate for not vaccinating is the lack of recommendation from their child’s health provider. Differences in age and sex recommendations also are reported. NIS-Teen 2013 demonstrated that parents of girls were more likely than parents of boys to receive a provider recommendation (65% vs.42%.) Only 29% of female parents indicated they’d received a provider recommendation to have their child vaccinated with HPV by ages 11-12 years.
Mandy A. Allison, MD, and her colleagues reviewed primary care physician perspectives about HPV vaccine in a national survey among 364 pediatricians and 218 family physicians (FPs). Although 84% of pediatricians and 75% of FPs indicated they always discuss HPV vaccination, only 60% of pediatricians and 59% of FPs strongly recommend HPV vaccine for 11- to 12-year-old girls; for boys it was 52% and 41%. More than half reported parental deferral. For pediatricians who almost never discussed the topic, the reasons included that the patient was not sexually active (54%), the child was young (38%), and the patient was already receiving other vaccines (35%) (Pediatrics. 2016 Feb;137[2]:e20152488).
Providers can be influenced by their perceptions of what value parents place on vaccines. In one study, parents were asked to put a value on specific vaccines. Providers were then asked to estimate how parents ranked the vaccines on a scale of 0-10. Providers underestimated the value placed on HPV vaccine (9.3 vs 5.2) (Vaccine 2014;32:579-84).
Improving HPV coverage: Preventing future HPV-related cancers
HPV vaccine should be recommended with as much conviction as Tdap and MCV at the 11- to 12-year visit for both girls and boys. Administration of all three should occur on the same day. Clinician recommendation is the No. 1 reason parents decide to vaccinate. The mantra “same way, same day” should become synonymous with the 11- to 12-year visit. All who have contact with the patient, beginning with the front desk staff, should know the importance of HPV vaccine, and when and why it is recommended. Often, families spend more time with support staff and have discussions prior to interacting with you.
Anticipate questions about HPV. Why give the vaccine when the child is so young and not sexually active? Is my child really at risk? Is it safe? I read on the Internet. … Questions should be interpreted as a need for additional information and reassurance from you.
Remember to emphasize that HPV vaccine is important because it prevents cancer and it is most effective prior to exposure to HPV.
Additional resources to facilitate your discussions about HPV can be found at www.cdc.gov/hpv.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures.
Arboviral and other vector-borne diseases
May has arrived, and for the majority of your patients it signals the end of the school year and the beginning of summer vacation. Zika virus is on the minds of most people since its arrival to the Western Hemisphere in March 2015. With the fluidity of this outbreak and almost daily news updates and recommendations, many parents have voiced or will be voicing concerns regarding summer travel destinations.
Many concerns about Zika virus have been previously addressed in this column (“Zika virus: More questions than answers?” by Dr. Kristina Bryant). However, if the decision is to avoid international travel because of the ongoing Zika outbreak, it doesn’t mean your patients get a free pass and will not have to be concerned about acquiring any infectious diseases. They still need to be vigilant about avoiding those pesky vectors that transmit arboviruses and other vector-borne diseases that occur in the United States.
Arboviruses are transmitted by mosquitoes, ticks, or fleas. Most infections are subclinical. If symptoms develop, they are manifested by a generalized febrile illness including fever, headache, myalgia, arthralgia, and rash. Hemorrhagic fever (dengue) or neuroinvasive disease can include aseptic meningitis, encephalitis, or acute flaccid paralysis. Neuroinvasive disease rarely occurs with dengue, Colorado tick fever, and chikungunya infections.
While more than 100 arboviruses can cause infection, some of the more common arboviruses associated with human disease include West Nile, first detected in the United States in 1999 and chikungunya, first reported in the Americas in 2013 with local transmission documented in Florida, Puerto Rico, and the U.S. Virgin Islands in 2014. It is estimated that dengue causes over 100 million cases worldwide annually. Almost 40% of the world’s inhabitants live in endemic areas. The majority of cases on the U.S. mainland are imported. However, it is endemic in all U.S. territories including Guam, American Samoa, the U.S. Virgin Islands, and Puerto Rico. Between September 2015 and March 2016, Hawaii experienced a dengue outbreak involving 264 individuals including 46 children. As of April 16, 2016, there were no infectious individuals on the island.
Other domestic arboviruses causing disease include St. Louis, Eastern, and Western Equine encephalitis, La Crosse encephalitis, Colorado tick fever, and Powassan virus. All are transmitted by mosquitoes with the exception of Powassan and Colorado tick fever, which are transmitted by ticks. The numbers of cases nationally are much lower for these diseases, compared with West Nile, dengue, and chikungunya. National and state-specific information is available for domestic arboviruses at diseasemaps.usgs.gov/mapviewer. Data is compiled by ArboNET, a national arboviral surveillance system that is managed by the Centers for Disease Control and Prevention (CDC) in conjunction with state health departments. Not only is human disease monitored, but it also maintains data on viremic blood donors, dead birds, mosquitoes, veterinary disease cases, and sentinel animals.
Spring and summer are the most active seasons for ticks. Bacterial and spirochetal diseases transmitted by them include rickettsial diseases such as Rocky Mountain Spotted Fever, ehrlichiosis, and anaplasmosis. Tularemia in addition to Lyme and tick-borne relapsing fever are also transmitted by ticks. Babesiosis, which is due to a parasite, and southern tick-associated rash illness (STARI), whose causative agent is yet to be determined, are two additional tick-related diagnoses.
Of note, dengue, chikungunya, and Zika are all transmitted by infected Aedes mosquitoes. There is no enzootic cycle. Just human-mosquito-human transmission. In contrast, West Nile virus is transmitted by Culex mosquitoes in an enzootic cycle between an avian reservoir and humans.
Treatment
There is no specific treatment for arboviral infections. The primary goal is relief of symptoms with fluids, bed rest, and analgesics. For bacterial vector-borne diseases, antibiotic therapy is indicated and is based on the specific pathogen. Doxycycline is the drug of choice for treatment of suspected and confirmed Rocky Mountain Spotted Fever, ehrlichiosis, and anaplasmosis even in children less than 8 years of age. Delay in initiation of antimicrobial therapy pending definitive diagnosis may lead to an adverse outcome. It is also the drug of choice for tick-borne relapsing fever.
Lyme disease is also responsive to antibiotic treatment. Therapy is based on the disease category. (Lyme disease in “Red Book: 2015 Report of the Committee on Infectious Diseases,” [Elk Grove Village, Ill.: American Academy of Pediatrics, 2015, pp. 516-25]).
STARI clinically presents with a lesion that resembles erythema migrans in southern and southeastern states. However, it has not been associated with any of the complications reported with disseminated Lyme disease. Treatment is not recommended.
Tularemia and babesiosis are both responsive to antimicrobial therapy and would best be managed in consultation with an infectious disease physician.
A handy, concise, up to date reference guide about all of the tick-borne diseases including photographs is available at the App Store. The Tickborne Diseases App was developed by the CDC and it is free!
Prevention
The cornerstone of disease prevention is avoidance of mosquito and tick bites, in addition to eliminating mosquito breeding sites. Ticks are generally found near the ground, in brushy or wooded areas. They usually wait for a potential host to brush against them. When this happens, they climb onto the host and find a site to attach.
Is there a role for antimicrobial prophylaxis once a tick has been discovered? There is no data to support antimicrobial prophylaxis to prevent Rocky Mountain spotted fever, ehrlichiosis, and anaplasmosis. Prophylaxis with doxycycline or ciprofloxacin is recommended for children and adults after exposure to an intentional release of tularemia and for laboratory workers after inadvertent exposure. For prevention of Lyme disease, a single dose of doxycycline (4 mg/kg, max dose 200 mg) may be offered under limited conditions: The patient is at least 8 years of age, resides in an area where Lyme is highly endemic, the tick removed was engorged, therapy can be initiated within 72 hours after tick removal, and the estimated time of attachment was at least 36 hours. There is inadequate data on the use of amoxicillin.
Remember, not all mosquitoes are alike. Those that transmit chikungunya, dengue, and Zika (Aedes mosquitoes) are primarily daytime mosquitoes, but also can bite at night. West Nile is transmitted by Culex mosquitoes, which feed from dusk to dawn.
Here are some tips to share with your patients that should decrease their chances of acquiring a mosquito or tick-borne disease:
• Apply mosquito repellent only to intact exposed skin when outdoors. Most repellents can be safely used on children at least 2 months of age and older. Avoid applying repellent directly on the child’s hand. Use at least a 20% DEET (N,N-diethyl-meta-toluamide) containing product. Other Environmental Protection Agency–registered repellents are an alternative (Additional information is available at http://www2.epa.gov/insect-repellents). Products containing oil of lemon eucalyptus (OLE) or p-Menthane-3,8-diol (PMD) should not be used on children under 3 years of age.
• Apply permethrin to clothing, hats, boots, and so on. It is designed to repel mosquitoes and ticks. It can last for several washings. It is ideal to spray over nets covering carriers in children younger than 2 months of age.
• Wear long-sleeved shirts and long pants tucked inside of socks when hiking.
• Check for ticks daily, especially under the arms, behind the ears, around the waist, behind the knees, and inside belly buttons after outdoor activities.
• Have your patients learn how to effectively remove a tick. With a fine tipped tweezer, grasp the tick as close to the skin as possible and pull straight up with even pressure. Do not twist or jerk the tick. Do not squash the tick. Place it in a bag and dispose of it. Clean the site after removal with alcohol, iodine, or soap and water.
• Encourage families to mosquito proof their home by using screens on windows and doors, and using air conditioning when available.
• Empty and scrub all items that contain water such as birdbaths, planters, or wading pools around the outside of the home at least weekly because mosquitoes lay eggs in or near free standing water.
• Dogs and cats should be treated for ticks as recommended by the veterinarian.
The impact of the ongoing Zika virus outbreak is uncertain. While it may have an impact on those planning international travel now and in the near future, several arboviral and vector-borne diseases currently exist in the United States. Encouraging our patients to practice interventions to prevent mosquito and tick bites now will also serve to protect them if Zika virus becomes established in the Aedes mosquitoes here in the future and/or if they have plans for international travel. For up to date information on Zika virus for yourself and your patients, visit www.cdc.gov/zika.
Bonnie M. Word, M.D., is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Email Dr. Word at pdnews@frontlinemedcom.com.
May has arrived, and for the majority of your patients it signals the end of the school year and the beginning of summer vacation. Zika virus is on the minds of most people since its arrival to the Western Hemisphere in March 2015. With the fluidity of this outbreak and almost daily news updates and recommendations, many parents have voiced or will be voicing concerns regarding summer travel destinations.
Many concerns about Zika virus have been previously addressed in this column (“Zika virus: More questions than answers?” by Dr. Kristina Bryant). However, if the decision is to avoid international travel because of the ongoing Zika outbreak, it doesn’t mean your patients get a free pass and will not have to be concerned about acquiring any infectious diseases. They still need to be vigilant about avoiding those pesky vectors that transmit arboviruses and other vector-borne diseases that occur in the United States.
Arboviruses are transmitted by mosquitoes, ticks, or fleas. Most infections are subclinical. If symptoms develop, they are manifested by a generalized febrile illness including fever, headache, myalgia, arthralgia, and rash. Hemorrhagic fever (dengue) or neuroinvasive disease can include aseptic meningitis, encephalitis, or acute flaccid paralysis. Neuroinvasive disease rarely occurs with dengue, Colorado tick fever, and chikungunya infections.
While more than 100 arboviruses can cause infection, some of the more common arboviruses associated with human disease include West Nile, first detected in the United States in 1999 and chikungunya, first reported in the Americas in 2013 with local transmission documented in Florida, Puerto Rico, and the U.S. Virgin Islands in 2014. It is estimated that dengue causes over 100 million cases worldwide annually. Almost 40% of the world’s inhabitants live in endemic areas. The majority of cases on the U.S. mainland are imported. However, it is endemic in all U.S. territories including Guam, American Samoa, the U.S. Virgin Islands, and Puerto Rico. Between September 2015 and March 2016, Hawaii experienced a dengue outbreak involving 264 individuals including 46 children. As of April 16, 2016, there were no infectious individuals on the island.
Other domestic arboviruses causing disease include St. Louis, Eastern, and Western Equine encephalitis, La Crosse encephalitis, Colorado tick fever, and Powassan virus. All are transmitted by mosquitoes with the exception of Powassan and Colorado tick fever, which are transmitted by ticks. The numbers of cases nationally are much lower for these diseases, compared with West Nile, dengue, and chikungunya. National and state-specific information is available for domestic arboviruses at diseasemaps.usgs.gov/mapviewer. Data is compiled by ArboNET, a national arboviral surveillance system that is managed by the Centers for Disease Control and Prevention (CDC) in conjunction with state health departments. Not only is human disease monitored, but it also maintains data on viremic blood donors, dead birds, mosquitoes, veterinary disease cases, and sentinel animals.
Spring and summer are the most active seasons for ticks. Bacterial and spirochetal diseases transmitted by them include rickettsial diseases such as Rocky Mountain Spotted Fever, ehrlichiosis, and anaplasmosis. Tularemia in addition to Lyme and tick-borne relapsing fever are also transmitted by ticks. Babesiosis, which is due to a parasite, and southern tick-associated rash illness (STARI), whose causative agent is yet to be determined, are two additional tick-related diagnoses.
Of note, dengue, chikungunya, and Zika are all transmitted by infected Aedes mosquitoes. There is no enzootic cycle. Just human-mosquito-human transmission. In contrast, West Nile virus is transmitted by Culex mosquitoes in an enzootic cycle between an avian reservoir and humans.
Treatment
There is no specific treatment for arboviral infections. The primary goal is relief of symptoms with fluids, bed rest, and analgesics. For bacterial vector-borne diseases, antibiotic therapy is indicated and is based on the specific pathogen. Doxycycline is the drug of choice for treatment of suspected and confirmed Rocky Mountain Spotted Fever, ehrlichiosis, and anaplasmosis even in children less than 8 years of age. Delay in initiation of antimicrobial therapy pending definitive diagnosis may lead to an adverse outcome. It is also the drug of choice for tick-borne relapsing fever.
Lyme disease is also responsive to antibiotic treatment. Therapy is based on the disease category. (Lyme disease in “Red Book: 2015 Report of the Committee on Infectious Diseases,” [Elk Grove Village, Ill.: American Academy of Pediatrics, 2015, pp. 516-25]).
STARI clinically presents with a lesion that resembles erythema migrans in southern and southeastern states. However, it has not been associated with any of the complications reported with disseminated Lyme disease. Treatment is not recommended.
Tularemia and babesiosis are both responsive to antimicrobial therapy and would best be managed in consultation with an infectious disease physician.
A handy, concise, up to date reference guide about all of the tick-borne diseases including photographs is available at the App Store. The Tickborne Diseases App was developed by the CDC and it is free!
Prevention
The cornerstone of disease prevention is avoidance of mosquito and tick bites, in addition to eliminating mosquito breeding sites. Ticks are generally found near the ground, in brushy or wooded areas. They usually wait for a potential host to brush against them. When this happens, they climb onto the host and find a site to attach.
Is there a role for antimicrobial prophylaxis once a tick has been discovered? There is no data to support antimicrobial prophylaxis to prevent Rocky Mountain spotted fever, ehrlichiosis, and anaplasmosis. Prophylaxis with doxycycline or ciprofloxacin is recommended for children and adults after exposure to an intentional release of tularemia and for laboratory workers after inadvertent exposure. For prevention of Lyme disease, a single dose of doxycycline (4 mg/kg, max dose 200 mg) may be offered under limited conditions: The patient is at least 8 years of age, resides in an area where Lyme is highly endemic, the tick removed was engorged, therapy can be initiated within 72 hours after tick removal, and the estimated time of attachment was at least 36 hours. There is inadequate data on the use of amoxicillin.
Remember, not all mosquitoes are alike. Those that transmit chikungunya, dengue, and Zika (Aedes mosquitoes) are primarily daytime mosquitoes, but also can bite at night. West Nile is transmitted by Culex mosquitoes, which feed from dusk to dawn.
Here are some tips to share with your patients that should decrease their chances of acquiring a mosquito or tick-borne disease:
• Apply mosquito repellent only to intact exposed skin when outdoors. Most repellents can be safely used on children at least 2 months of age and older. Avoid applying repellent directly on the child’s hand. Use at least a 20% DEET (N,N-diethyl-meta-toluamide) containing product. Other Environmental Protection Agency–registered repellents are an alternative (Additional information is available at http://www2.epa.gov/insect-repellents). Products containing oil of lemon eucalyptus (OLE) or p-Menthane-3,8-diol (PMD) should not be used on children under 3 years of age.
• Apply permethrin to clothing, hats, boots, and so on. It is designed to repel mosquitoes and ticks. It can last for several washings. It is ideal to spray over nets covering carriers in children younger than 2 months of age.
• Wear long-sleeved shirts and long pants tucked inside of socks when hiking.
• Check for ticks daily, especially under the arms, behind the ears, around the waist, behind the knees, and inside belly buttons after outdoor activities.
• Have your patients learn how to effectively remove a tick. With a fine tipped tweezer, grasp the tick as close to the skin as possible and pull straight up with even pressure. Do not twist or jerk the tick. Do not squash the tick. Place it in a bag and dispose of it. Clean the site after removal with alcohol, iodine, or soap and water.
• Encourage families to mosquito proof their home by using screens on windows and doors, and using air conditioning when available.
• Empty and scrub all items that contain water such as birdbaths, planters, or wading pools around the outside of the home at least weekly because mosquitoes lay eggs in or near free standing water.
• Dogs and cats should be treated for ticks as recommended by the veterinarian.
The impact of the ongoing Zika virus outbreak is uncertain. While it may have an impact on those planning international travel now and in the near future, several arboviral and vector-borne diseases currently exist in the United States. Encouraging our patients to practice interventions to prevent mosquito and tick bites now will also serve to protect them if Zika virus becomes established in the Aedes mosquitoes here in the future and/or if they have plans for international travel. For up to date information on Zika virus for yourself and your patients, visit www.cdc.gov/zika.
Bonnie M. Word, M.D., is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Email Dr. Word at pdnews@frontlinemedcom.com.
May has arrived, and for the majority of your patients it signals the end of the school year and the beginning of summer vacation. Zika virus is on the minds of most people since its arrival to the Western Hemisphere in March 2015. With the fluidity of this outbreak and almost daily news updates and recommendations, many parents have voiced or will be voicing concerns regarding summer travel destinations.
Many concerns about Zika virus have been previously addressed in this column (“Zika virus: More questions than answers?” by Dr. Kristina Bryant). However, if the decision is to avoid international travel because of the ongoing Zika outbreak, it doesn’t mean your patients get a free pass and will not have to be concerned about acquiring any infectious diseases. They still need to be vigilant about avoiding those pesky vectors that transmit arboviruses and other vector-borne diseases that occur in the United States.
Arboviruses are transmitted by mosquitoes, ticks, or fleas. Most infections are subclinical. If symptoms develop, they are manifested by a generalized febrile illness including fever, headache, myalgia, arthralgia, and rash. Hemorrhagic fever (dengue) or neuroinvasive disease can include aseptic meningitis, encephalitis, or acute flaccid paralysis. Neuroinvasive disease rarely occurs with dengue, Colorado tick fever, and chikungunya infections.
While more than 100 arboviruses can cause infection, some of the more common arboviruses associated with human disease include West Nile, first detected in the United States in 1999 and chikungunya, first reported in the Americas in 2013 with local transmission documented in Florida, Puerto Rico, and the U.S. Virgin Islands in 2014. It is estimated that dengue causes over 100 million cases worldwide annually. Almost 40% of the world’s inhabitants live in endemic areas. The majority of cases on the U.S. mainland are imported. However, it is endemic in all U.S. territories including Guam, American Samoa, the U.S. Virgin Islands, and Puerto Rico. Between September 2015 and March 2016, Hawaii experienced a dengue outbreak involving 264 individuals including 46 children. As of April 16, 2016, there were no infectious individuals on the island.
Other domestic arboviruses causing disease include St. Louis, Eastern, and Western Equine encephalitis, La Crosse encephalitis, Colorado tick fever, and Powassan virus. All are transmitted by mosquitoes with the exception of Powassan and Colorado tick fever, which are transmitted by ticks. The numbers of cases nationally are much lower for these diseases, compared with West Nile, dengue, and chikungunya. National and state-specific information is available for domestic arboviruses at diseasemaps.usgs.gov/mapviewer. Data is compiled by ArboNET, a national arboviral surveillance system that is managed by the Centers for Disease Control and Prevention (CDC) in conjunction with state health departments. Not only is human disease monitored, but it also maintains data on viremic blood donors, dead birds, mosquitoes, veterinary disease cases, and sentinel animals.
Spring and summer are the most active seasons for ticks. Bacterial and spirochetal diseases transmitted by them include rickettsial diseases such as Rocky Mountain Spotted Fever, ehrlichiosis, and anaplasmosis. Tularemia in addition to Lyme and tick-borne relapsing fever are also transmitted by ticks. Babesiosis, which is due to a parasite, and southern tick-associated rash illness (STARI), whose causative agent is yet to be determined, are two additional tick-related diagnoses.
Of note, dengue, chikungunya, and Zika are all transmitted by infected Aedes mosquitoes. There is no enzootic cycle. Just human-mosquito-human transmission. In contrast, West Nile virus is transmitted by Culex mosquitoes in an enzootic cycle between an avian reservoir and humans.
Treatment
There is no specific treatment for arboviral infections. The primary goal is relief of symptoms with fluids, bed rest, and analgesics. For bacterial vector-borne diseases, antibiotic therapy is indicated and is based on the specific pathogen. Doxycycline is the drug of choice for treatment of suspected and confirmed Rocky Mountain Spotted Fever, ehrlichiosis, and anaplasmosis even in children less than 8 years of age. Delay in initiation of antimicrobial therapy pending definitive diagnosis may lead to an adverse outcome. It is also the drug of choice for tick-borne relapsing fever.
Lyme disease is also responsive to antibiotic treatment. Therapy is based on the disease category. (Lyme disease in “Red Book: 2015 Report of the Committee on Infectious Diseases,” [Elk Grove Village, Ill.: American Academy of Pediatrics, 2015, pp. 516-25]).
STARI clinically presents with a lesion that resembles erythema migrans in southern and southeastern states. However, it has not been associated with any of the complications reported with disseminated Lyme disease. Treatment is not recommended.
Tularemia and babesiosis are both responsive to antimicrobial therapy and would best be managed in consultation with an infectious disease physician.
A handy, concise, up to date reference guide about all of the tick-borne diseases including photographs is available at the App Store. The Tickborne Diseases App was developed by the CDC and it is free!
Prevention
The cornerstone of disease prevention is avoidance of mosquito and tick bites, in addition to eliminating mosquito breeding sites. Ticks are generally found near the ground, in brushy or wooded areas. They usually wait for a potential host to brush against them. When this happens, they climb onto the host and find a site to attach.
Is there a role for antimicrobial prophylaxis once a tick has been discovered? There is no data to support antimicrobial prophylaxis to prevent Rocky Mountain spotted fever, ehrlichiosis, and anaplasmosis. Prophylaxis with doxycycline or ciprofloxacin is recommended for children and adults after exposure to an intentional release of tularemia and for laboratory workers after inadvertent exposure. For prevention of Lyme disease, a single dose of doxycycline (4 mg/kg, max dose 200 mg) may be offered under limited conditions: The patient is at least 8 years of age, resides in an area where Lyme is highly endemic, the tick removed was engorged, therapy can be initiated within 72 hours after tick removal, and the estimated time of attachment was at least 36 hours. There is inadequate data on the use of amoxicillin.
Remember, not all mosquitoes are alike. Those that transmit chikungunya, dengue, and Zika (Aedes mosquitoes) are primarily daytime mosquitoes, but also can bite at night. West Nile is transmitted by Culex mosquitoes, which feed from dusk to dawn.
Here are some tips to share with your patients that should decrease their chances of acquiring a mosquito or tick-borne disease:
• Apply mosquito repellent only to intact exposed skin when outdoors. Most repellents can be safely used on children at least 2 months of age and older. Avoid applying repellent directly on the child’s hand. Use at least a 20% DEET (N,N-diethyl-meta-toluamide) containing product. Other Environmental Protection Agency–registered repellents are an alternative (Additional information is available at http://www2.epa.gov/insect-repellents). Products containing oil of lemon eucalyptus (OLE) or p-Menthane-3,8-diol (PMD) should not be used on children under 3 years of age.
• Apply permethrin to clothing, hats, boots, and so on. It is designed to repel mosquitoes and ticks. It can last for several washings. It is ideal to spray over nets covering carriers in children younger than 2 months of age.
• Wear long-sleeved shirts and long pants tucked inside of socks when hiking.
• Check for ticks daily, especially under the arms, behind the ears, around the waist, behind the knees, and inside belly buttons after outdoor activities.
• Have your patients learn how to effectively remove a tick. With a fine tipped tweezer, grasp the tick as close to the skin as possible and pull straight up with even pressure. Do not twist or jerk the tick. Do not squash the tick. Place it in a bag and dispose of it. Clean the site after removal with alcohol, iodine, or soap and water.
• Encourage families to mosquito proof their home by using screens on windows and doors, and using air conditioning when available.
• Empty and scrub all items that contain water such as birdbaths, planters, or wading pools around the outside of the home at least weekly because mosquitoes lay eggs in or near free standing water.
• Dogs and cats should be treated for ticks as recommended by the veterinarian.
The impact of the ongoing Zika virus outbreak is uncertain. While it may have an impact on those planning international travel now and in the near future, several arboviral and vector-borne diseases currently exist in the United States. Encouraging our patients to practice interventions to prevent mosquito and tick bites now will also serve to protect them if Zika virus becomes established in the Aedes mosquitoes here in the future and/or if they have plans for international travel. For up to date information on Zika virus for yourself and your patients, visit www.cdc.gov/zika.
Bonnie M. Word, M.D., is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Email Dr. Word at pdnews@frontlinemedcom.com.
Water woes: Recognizing and treating recreational water illness
Most of our patients have been or will be exposed to water in a recreational setting this summer. As health care providers, we might not routinely consider illnesses associated with recreational water exposure or discuss preventive strategies; however, the Centers for Disease Control and Prevention has been actively promoting awareness about recreational water illnesses for years. May 18-24, 2015, was the 11th annual observance of Healthy and Safe Swimming Week, formerly known as Recreational Illness and Injury Prevention Week. The focus for 2015 was promoting the role of swimmers, residential pool owners, public health officials, and beach staff in the prevention of drownings, chemical injuries, and outbreaks of illness. One goal was for the swimmer to take a more active role in protecting themselves and preventing the spread of infections to others. For our colleagues, that means educating both parents and children.
To begin our discussion, let’s define recreational water illnesses (RWI). RWIs are caused by a variety of infectious pathogens transmitted by ingestion, inhalation of aerosols or mists, or having contact with contaminated water from both treated (swimming pools, hot tubs, water parks, and fountains) and untreated (lakes, rivers, and oceans) sources of water in recreational venues. RWIs also can be caused by chemicals that have evaporated from water leading to poor indoor air quality. However, I am focusing on the infectious etiologies.
A broad spectrum of infections are associated with RWIs, including infections of the gastrointestinal tract, ear, skin, eye, central nervous system, and wounds. Diarrhea is the most common infection. Implicated pathogens include Giardia, Shigella, norovirus, and Escherichia coli O157:H7, but it is Cryptosporidium that has emerged as the pathogen implicated most often in swimming pool–related outbreaks. Recently published data from the CDC revealed that in 2011-2012, there were 90 recreational-associated outbreaks reported from 32 states and Puerto Rico resulting in 1,788 infections, with 69 outbreaks occurring in treated water venues. Of these, 36 (51%) were caused by Cryptosporidium. Among 21 outbreaks occurring in untreated recreational water, E. coli was responsible for 7 (33%) (MMWR Morb. Mortal. Wkly Rep. 2015;64:668-72)
It’s no surprise diarrhea is the most common illness. Infection can easily occur after swallowing contaminated water. Many erroneously think chlorine kills all pathogens. Cryptosporidium is chlorine tolerant and can persist in treated water with the current recommended levels of chlorine for more than 10 days (J. Water Health 2008;6:513-20). For chlorine-sensitive pathogens, maintenance of the disinfection process must remain intact. What role do swimmers play? Most people have about 0.4 g of feces on their bottoms that can contaminate water when rinsed off. How many people enter a pool with a diarrheal illness? How many may go swimming after having recently recovered from a diarrheal illness and may have asymptomatic shedding? We all have cringed when we see a diapered child in the water. All of these are potential ways for the swimmer to contaminate an adequately treated pool. Additionally, while Cryptosporidium infections are usually self-limited, some individuals, including the immunocompromised host and especially those with advanced HIV and those who are solid organ transplant recipients, may have a protracted course of profuse diarrhea if infected.
While diarrhea maybe the most common RWI, it is not the only one. Acute otitis externa (AOE), more commonly known as “swimmer’s ear,” is one of the most frequent reasons for summer health care encounters. It has been estimated that in the United States in 2007, 2.4 million health care visits resulted in the diagnosis of AOE (MMWR Morb. Mortal. Wkly. Rep. 2011;60:605-9). Visits were highest among children aged 5-9 years; however, adults accounted for 53% of the encounters. Inflammation and infection of the external auditory canal is usually caused by bacteria. Pseudomonas aeruginosa or Staphylococcus aureus are the two most common etiologies. Water is easily introduced into the external auditory canal with recreational water activities, leading to maceration and subsequent infection of the canal. Simply reminding parents to thoroughly dry their child’s ears after water exposure can help prevent AOE.
P. aeruginosa also is the agent causing the self-limiting conditions hot tub folliculitis and hot-foot syndrome. Hot tub folliculitis is characterized by the development of tender, pruritic papules and papulopustules on the hips, buttocks, and axillae, usually developing 8-48 hours after exposure to water that has been contaminated because of inadequate chlorination. Hot-foot syndrome is characterized by painful planter nodules (N. Engl. J. Med. 2001;345:335).
Serious diseases are encountered infrequently, but there are some that require more urgent interventions. Primary amebic meningoencephalitis (PAM) is an extremely rare, progressive, and almost always fatal infection of the brain caused by Naegleria fowleri. The pathogen is found in warm freshwater including lakes, rivers, streams, and hot springs. It enters the body through the nose and travels via the olfactory nerve to the brain. Infection usually occurs when individuals swim or dive in warm freshwater. Most cases have been reported in children from Southern states. In 2010, the first case in a northern state was reported from Minnesota, and three additional cases have since been reported in Kansas and Indiana (J. Ped. Infect. Dis. 2014 [doi: 10.1093/jpids/piu103]). Cases also have been reported in two individuals who were regular users of neti pots for sinus irrigation because the irrigating solution was prepared with contaminated tap water (Clin. Infect. Dis. 2012;55:e79-85). Clinical presentation is similar to bacterial meningitis. Helpful diagnostic clues may come from obtaining a history of swimming in freshwater within the 2 weeks prior to presentation, especially during the summer, or the use of nasal or sinus irrigation with untreated tap water. Consultation with an infectious disease specialist is recommended.
Acanthamoeba keratitis is a potentially blinding infection of the cornea that primarily occurs in individuals who wear contact lenses. Risk factors for the infection include swimming, showering, and use of hot tubs while wearing contact lenses. Improper storage and cleansing contacts with tap water are other risk factors. Anyone with corneal trauma and similar water exposures also would be at risk. Clinically, the history combined with a foreign-body sensation, pain, and decreased visual acuity should make one include this infection in the differential diagnosis. Referral to an ophthalmologist is required.
Finally, swimming with an open wound is a portal of entry for Vibrio vulnificus. It usually is associated with consumption of contaminated seafood, especially oysters. In immunocompromised individuals, especially those with chronic liver disease, this bacteria can cause a life-threatening illness leading to bacteremia, septic shock, and development of blistering skin lesions. Infections are fatal in approximately 50% of cases.
The goal of this brief review was not to discourage swimming, but to make your patients and their families healthy swimmers. Here are a few things the CDC is recommending to help them achieve that goal:
• Shower prior to going swimming.
• Do not swallow or drink pool water.
• Take bathroom breaks every hour and rinse off before going back into the water.
• Do not swim if you have diarrhea.
• Wait at least 2 weeks to go swimming if you have had diarrhea.
• Change swim diapers frequently and away from the water.
• Suggest patients download the free CDC app Healthy Swimming for more detailed information and suggest they visit cdc.gov/healthywater/swimming.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She had no relevant financial disclosures. Write to Dr. Word at pdnews@frontlinemedcom.com.
Most of our patients have been or will be exposed to water in a recreational setting this summer. As health care providers, we might not routinely consider illnesses associated with recreational water exposure or discuss preventive strategies; however, the Centers for Disease Control and Prevention has been actively promoting awareness about recreational water illnesses for years. May 18-24, 2015, was the 11th annual observance of Healthy and Safe Swimming Week, formerly known as Recreational Illness and Injury Prevention Week. The focus for 2015 was promoting the role of swimmers, residential pool owners, public health officials, and beach staff in the prevention of drownings, chemical injuries, and outbreaks of illness. One goal was for the swimmer to take a more active role in protecting themselves and preventing the spread of infections to others. For our colleagues, that means educating both parents and children.
To begin our discussion, let’s define recreational water illnesses (RWI). RWIs are caused by a variety of infectious pathogens transmitted by ingestion, inhalation of aerosols or mists, or having contact with contaminated water from both treated (swimming pools, hot tubs, water parks, and fountains) and untreated (lakes, rivers, and oceans) sources of water in recreational venues. RWIs also can be caused by chemicals that have evaporated from water leading to poor indoor air quality. However, I am focusing on the infectious etiologies.
A broad spectrum of infections are associated with RWIs, including infections of the gastrointestinal tract, ear, skin, eye, central nervous system, and wounds. Diarrhea is the most common infection. Implicated pathogens include Giardia, Shigella, norovirus, and Escherichia coli O157:H7, but it is Cryptosporidium that has emerged as the pathogen implicated most often in swimming pool–related outbreaks. Recently published data from the CDC revealed that in 2011-2012, there were 90 recreational-associated outbreaks reported from 32 states and Puerto Rico resulting in 1,788 infections, with 69 outbreaks occurring in treated water venues. Of these, 36 (51%) were caused by Cryptosporidium. Among 21 outbreaks occurring in untreated recreational water, E. coli was responsible for 7 (33%) (MMWR Morb. Mortal. Wkly Rep. 2015;64:668-72)
It’s no surprise diarrhea is the most common illness. Infection can easily occur after swallowing contaminated water. Many erroneously think chlorine kills all pathogens. Cryptosporidium is chlorine tolerant and can persist in treated water with the current recommended levels of chlorine for more than 10 days (J. Water Health 2008;6:513-20). For chlorine-sensitive pathogens, maintenance of the disinfection process must remain intact. What role do swimmers play? Most people have about 0.4 g of feces on their bottoms that can contaminate water when rinsed off. How many people enter a pool with a diarrheal illness? How many may go swimming after having recently recovered from a diarrheal illness and may have asymptomatic shedding? We all have cringed when we see a diapered child in the water. All of these are potential ways for the swimmer to contaminate an adequately treated pool. Additionally, while Cryptosporidium infections are usually self-limited, some individuals, including the immunocompromised host and especially those with advanced HIV and those who are solid organ transplant recipients, may have a protracted course of profuse diarrhea if infected.
While diarrhea maybe the most common RWI, it is not the only one. Acute otitis externa (AOE), more commonly known as “swimmer’s ear,” is one of the most frequent reasons for summer health care encounters. It has been estimated that in the United States in 2007, 2.4 million health care visits resulted in the diagnosis of AOE (MMWR Morb. Mortal. Wkly. Rep. 2011;60:605-9). Visits were highest among children aged 5-9 years; however, adults accounted for 53% of the encounters. Inflammation and infection of the external auditory canal is usually caused by bacteria. Pseudomonas aeruginosa or Staphylococcus aureus are the two most common etiologies. Water is easily introduced into the external auditory canal with recreational water activities, leading to maceration and subsequent infection of the canal. Simply reminding parents to thoroughly dry their child’s ears after water exposure can help prevent AOE.
P. aeruginosa also is the agent causing the self-limiting conditions hot tub folliculitis and hot-foot syndrome. Hot tub folliculitis is characterized by the development of tender, pruritic papules and papulopustules on the hips, buttocks, and axillae, usually developing 8-48 hours after exposure to water that has been contaminated because of inadequate chlorination. Hot-foot syndrome is characterized by painful planter nodules (N. Engl. J. Med. 2001;345:335).
Serious diseases are encountered infrequently, but there are some that require more urgent interventions. Primary amebic meningoencephalitis (PAM) is an extremely rare, progressive, and almost always fatal infection of the brain caused by Naegleria fowleri. The pathogen is found in warm freshwater including lakes, rivers, streams, and hot springs. It enters the body through the nose and travels via the olfactory nerve to the brain. Infection usually occurs when individuals swim or dive in warm freshwater. Most cases have been reported in children from Southern states. In 2010, the first case in a northern state was reported from Minnesota, and three additional cases have since been reported in Kansas and Indiana (J. Ped. Infect. Dis. 2014 [doi: 10.1093/jpids/piu103]). Cases also have been reported in two individuals who were regular users of neti pots for sinus irrigation because the irrigating solution was prepared with contaminated tap water (Clin. Infect. Dis. 2012;55:e79-85). Clinical presentation is similar to bacterial meningitis. Helpful diagnostic clues may come from obtaining a history of swimming in freshwater within the 2 weeks prior to presentation, especially during the summer, or the use of nasal or sinus irrigation with untreated tap water. Consultation with an infectious disease specialist is recommended.
Acanthamoeba keratitis is a potentially blinding infection of the cornea that primarily occurs in individuals who wear contact lenses. Risk factors for the infection include swimming, showering, and use of hot tubs while wearing contact lenses. Improper storage and cleansing contacts with tap water are other risk factors. Anyone with corneal trauma and similar water exposures also would be at risk. Clinically, the history combined with a foreign-body sensation, pain, and decreased visual acuity should make one include this infection in the differential diagnosis. Referral to an ophthalmologist is required.
Finally, swimming with an open wound is a portal of entry for Vibrio vulnificus. It usually is associated with consumption of contaminated seafood, especially oysters. In immunocompromised individuals, especially those with chronic liver disease, this bacteria can cause a life-threatening illness leading to bacteremia, septic shock, and development of blistering skin lesions. Infections are fatal in approximately 50% of cases.
The goal of this brief review was not to discourage swimming, but to make your patients and their families healthy swimmers. Here are a few things the CDC is recommending to help them achieve that goal:
• Shower prior to going swimming.
• Do not swallow or drink pool water.
• Take bathroom breaks every hour and rinse off before going back into the water.
• Do not swim if you have diarrhea.
• Wait at least 2 weeks to go swimming if you have had diarrhea.
• Change swim diapers frequently and away from the water.
• Suggest patients download the free CDC app Healthy Swimming for more detailed information and suggest they visit cdc.gov/healthywater/swimming.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She had no relevant financial disclosures. Write to Dr. Word at pdnews@frontlinemedcom.com.
Most of our patients have been or will be exposed to water in a recreational setting this summer. As health care providers, we might not routinely consider illnesses associated with recreational water exposure or discuss preventive strategies; however, the Centers for Disease Control and Prevention has been actively promoting awareness about recreational water illnesses for years. May 18-24, 2015, was the 11th annual observance of Healthy and Safe Swimming Week, formerly known as Recreational Illness and Injury Prevention Week. The focus for 2015 was promoting the role of swimmers, residential pool owners, public health officials, and beach staff in the prevention of drownings, chemical injuries, and outbreaks of illness. One goal was for the swimmer to take a more active role in protecting themselves and preventing the spread of infections to others. For our colleagues, that means educating both parents and children.
To begin our discussion, let’s define recreational water illnesses (RWI). RWIs are caused by a variety of infectious pathogens transmitted by ingestion, inhalation of aerosols or mists, or having contact with contaminated water from both treated (swimming pools, hot tubs, water parks, and fountains) and untreated (lakes, rivers, and oceans) sources of water in recreational venues. RWIs also can be caused by chemicals that have evaporated from water leading to poor indoor air quality. However, I am focusing on the infectious etiologies.
A broad spectrum of infections are associated with RWIs, including infections of the gastrointestinal tract, ear, skin, eye, central nervous system, and wounds. Diarrhea is the most common infection. Implicated pathogens include Giardia, Shigella, norovirus, and Escherichia coli O157:H7, but it is Cryptosporidium that has emerged as the pathogen implicated most often in swimming pool–related outbreaks. Recently published data from the CDC revealed that in 2011-2012, there were 90 recreational-associated outbreaks reported from 32 states and Puerto Rico resulting in 1,788 infections, with 69 outbreaks occurring in treated water venues. Of these, 36 (51%) were caused by Cryptosporidium. Among 21 outbreaks occurring in untreated recreational water, E. coli was responsible for 7 (33%) (MMWR Morb. Mortal. Wkly Rep. 2015;64:668-72)
It’s no surprise diarrhea is the most common illness. Infection can easily occur after swallowing contaminated water. Many erroneously think chlorine kills all pathogens. Cryptosporidium is chlorine tolerant and can persist in treated water with the current recommended levels of chlorine for more than 10 days (J. Water Health 2008;6:513-20). For chlorine-sensitive pathogens, maintenance of the disinfection process must remain intact. What role do swimmers play? Most people have about 0.4 g of feces on their bottoms that can contaminate water when rinsed off. How many people enter a pool with a diarrheal illness? How many may go swimming after having recently recovered from a diarrheal illness and may have asymptomatic shedding? We all have cringed when we see a diapered child in the water. All of these are potential ways for the swimmer to contaminate an adequately treated pool. Additionally, while Cryptosporidium infections are usually self-limited, some individuals, including the immunocompromised host and especially those with advanced HIV and those who are solid organ transplant recipients, may have a protracted course of profuse diarrhea if infected.
While diarrhea maybe the most common RWI, it is not the only one. Acute otitis externa (AOE), more commonly known as “swimmer’s ear,” is one of the most frequent reasons for summer health care encounters. It has been estimated that in the United States in 2007, 2.4 million health care visits resulted in the diagnosis of AOE (MMWR Morb. Mortal. Wkly. Rep. 2011;60:605-9). Visits were highest among children aged 5-9 years; however, adults accounted for 53% of the encounters. Inflammation and infection of the external auditory canal is usually caused by bacteria. Pseudomonas aeruginosa or Staphylococcus aureus are the two most common etiologies. Water is easily introduced into the external auditory canal with recreational water activities, leading to maceration and subsequent infection of the canal. Simply reminding parents to thoroughly dry their child’s ears after water exposure can help prevent AOE.
P. aeruginosa also is the agent causing the self-limiting conditions hot tub folliculitis and hot-foot syndrome. Hot tub folliculitis is characterized by the development of tender, pruritic papules and papulopustules on the hips, buttocks, and axillae, usually developing 8-48 hours after exposure to water that has been contaminated because of inadequate chlorination. Hot-foot syndrome is characterized by painful planter nodules (N. Engl. J. Med. 2001;345:335).
Serious diseases are encountered infrequently, but there are some that require more urgent interventions. Primary amebic meningoencephalitis (PAM) is an extremely rare, progressive, and almost always fatal infection of the brain caused by Naegleria fowleri. The pathogen is found in warm freshwater including lakes, rivers, streams, and hot springs. It enters the body through the nose and travels via the olfactory nerve to the brain. Infection usually occurs when individuals swim or dive in warm freshwater. Most cases have been reported in children from Southern states. In 2010, the first case in a northern state was reported from Minnesota, and three additional cases have since been reported in Kansas and Indiana (J. Ped. Infect. Dis. 2014 [doi: 10.1093/jpids/piu103]). Cases also have been reported in two individuals who were regular users of neti pots for sinus irrigation because the irrigating solution was prepared with contaminated tap water (Clin. Infect. Dis. 2012;55:e79-85). Clinical presentation is similar to bacterial meningitis. Helpful diagnostic clues may come from obtaining a history of swimming in freshwater within the 2 weeks prior to presentation, especially during the summer, or the use of nasal or sinus irrigation with untreated tap water. Consultation with an infectious disease specialist is recommended.
Acanthamoeba keratitis is a potentially blinding infection of the cornea that primarily occurs in individuals who wear contact lenses. Risk factors for the infection include swimming, showering, and use of hot tubs while wearing contact lenses. Improper storage and cleansing contacts with tap water are other risk factors. Anyone with corneal trauma and similar water exposures also would be at risk. Clinically, the history combined with a foreign-body sensation, pain, and decreased visual acuity should make one include this infection in the differential diagnosis. Referral to an ophthalmologist is required.
Finally, swimming with an open wound is a portal of entry for Vibrio vulnificus. It usually is associated with consumption of contaminated seafood, especially oysters. In immunocompromised individuals, especially those with chronic liver disease, this bacteria can cause a life-threatening illness leading to bacteremia, septic shock, and development of blistering skin lesions. Infections are fatal in approximately 50% of cases.
The goal of this brief review was not to discourage swimming, but to make your patients and their families healthy swimmers. Here are a few things the CDC is recommending to help them achieve that goal:
• Shower prior to going swimming.
• Do not swallow or drink pool water.
• Take bathroom breaks every hour and rinse off before going back into the water.
• Do not swim if you have diarrhea.
• Wait at least 2 weeks to go swimming if you have had diarrhea.
• Change swim diapers frequently and away from the water.
• Suggest patients download the free CDC app Healthy Swimming for more detailed information and suggest they visit cdc.gov/healthywater/swimming.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She had no relevant financial disclosures. Write to Dr. Word at pdnews@frontlinemedcom.com.




















