User login
Diffuse Papular Eruption With Erosions and Ulcerations
The Diagnosis: Immunotherapy-Related Lichenoid Drug Eruption
Direct immunofluorescence was negative, and histopathology revealed a lichenoid interface dermatitis, minimal parakeratosis, and saw-toothed rete ridges (Figure 1). He was diagnosed with an immunotherapyrelated lichenoid drug eruption based on the morphology of the skin lesions and clinicopathologic correlation. Bullous pemphigoid and lichen planus pemphigoides were ruled out given the negative direct immunofluorescence findings. Stevens-Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN) was not consistent with the clinical presentation, especially given the lack of mucosal findings. The histology also was not consistent, as the biopsy specimen lacked apoptotic and necrotic keratinocytes to the degree seen in SJS/TEN and also had a greater degree of inflammatory infiltrate. Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome was ruled out given the lack of systemic findings, including facial swelling and lymphadenopathy and the clinical appearance of the rash. No morbilliform features were present, which is the most common presentation of DRESS syndrome.

Checkpoint inhibitor (CPI) therapy has become the cornerstone in management of certain advanced malignancies.1 Checkpoint inhibitors block cytotoxic T lymphocyte–associated protein 4, programmed cell death-1, and/or programmed cell death ligand-1, allowing activated T cells to infiltrate the tumor microenvironment and destroy malignant cells. Checkpoint inhibitors are approved for the treatment of melanoma, cutaneous squamous cell carcinoma, and Merkel cell carcinoma and are being investigated in various other cutaneous and soft tissue malignancies.1-3
Although CPIs have shown substantial efficacy in the management of advanced malignancies, immune-related adverse events (AEs) are common due to nonspecific immune activation.2 Immune-related cutaneous AEs are the most common immune-related AEs, occurring in 30% to 50% of patients who undergo treatment.2-5 Common immune-related cutaneous AEs include maculopapular, psoriasiform, and lichenoid dermatitis, as well as pruritus without dermatitis.2,3,6 Other reactions include but are not limited to bullous pemphigoid, vitiligolike depigmentation, and alopecia.2,3 Immune-related cutaneous AEs usually are self-limited; however, severe life-threatening reactions such as the spectrum of SJS/TEN and DRESS syndrome also can occur.2-4 Immune-related cutaneous AEs are graded based on the Common Terminology Criteria for Adverse Events: grade 1 reactions are asymptomatic and cover less than 10% of the patient’s body surface area (BSA), grade 2 reactions have mild symptoms and cover 10% to 30% of the patient’s BSA, grade 3 reactions have moderate to severe symptoms and cover greater than 30% of the patient’s BSA, and grade 4 reactions are life-threatening.2,3 With prompt recognition and adequate treatment, mild to moderate immune-related cutaneous AEs—grades 1 and 2—largely are reversible, and less than 5% require discontinuation of therapy.2,3,6 It has been suggested that immune-related cutaneous AEs may be a positive prognostic factor in the treatment of underlying malignancy, indicating adequate immune activation targeting the malignant cells.6
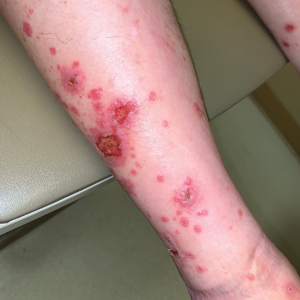
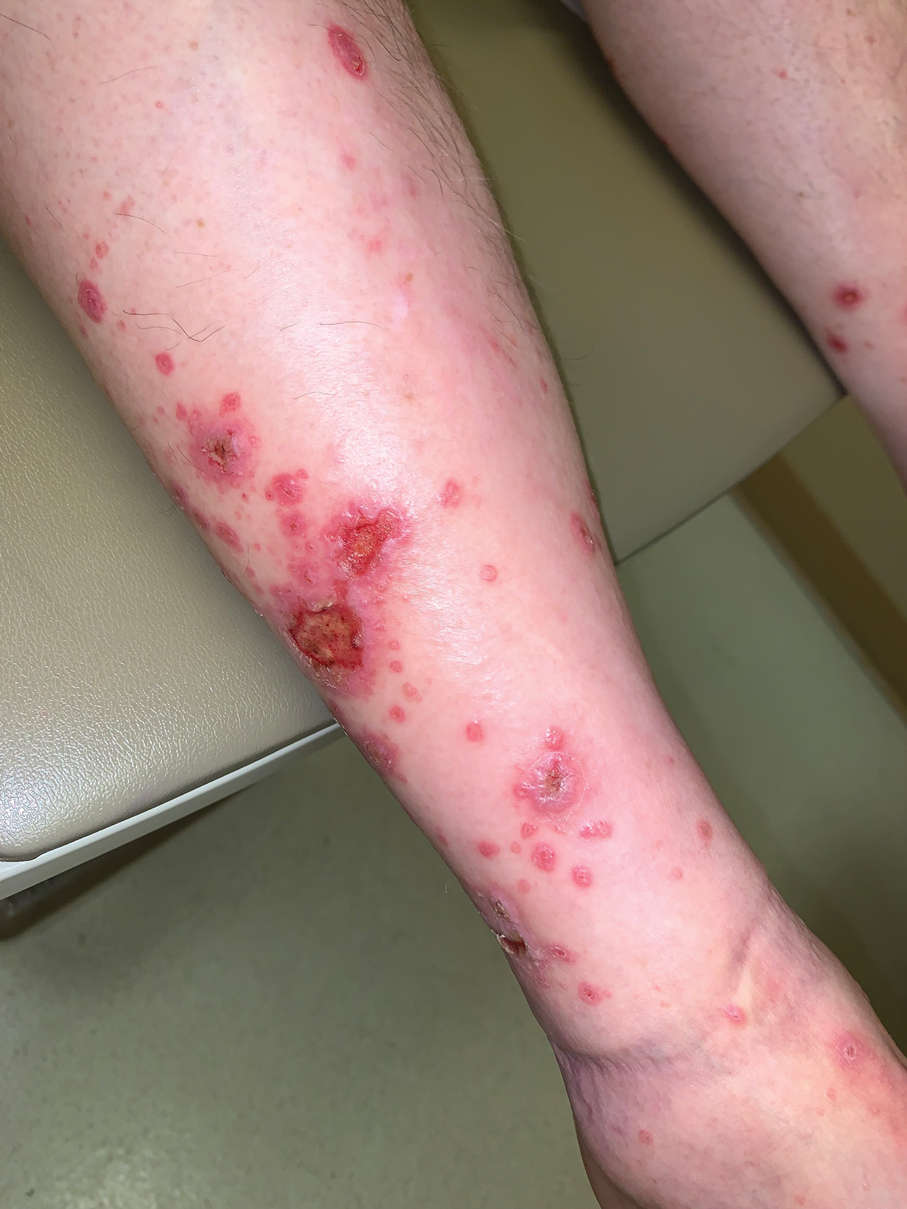
Although our patient had some typical violaceous, flat-topped papules and plaques with Wickham striae, he also had atypical findings for a lichenoid reaction. Given the endorsement of blisters, it is possible that some of these lesions initially were bullous and subsequently ruptured, leaving behind erosions. However, in other areas, there also were eroded papules and ulcerations without a reported history of excoriation, scratching, picking, or prior bullae, including difficult-to-reach areas such as the back. It is favored that these lesions represented a robust lichenoid dermatitis leading to erosive and ulcerated lesions, similar to the formation of bullous lichen planus. Lichenoid eruptions secondary to immunotherapy are well-known phenomena, but a PubMed search of articles indexed for MEDLINE using the terms ulcer, lichenoid, and immunotherapy revealed only 2 cases of ulcerative lichenoid eruptions: a localized digital erosive lichenoid dermatitis and a widespread ulcerative lichenoid drug eruption without true erosions.7,8 However, widespread erosive and ulcerated lichenoid reactions are rare.
Lichenoid eruptions most strongly are associated with anti–programmed cell death-1/ programmed cell death ligand-1 therapy, occurring in 20% of patients undergoing treatment.3 Lichenoid eruptions present as discrete, pruritic, erythematous, violaceous papules and plaques on the chest and back and rarely may involve the limbs, palmoplantar surfaces, and oral mucosa.2,3,6 Histopathologic features include a dense bandlike lymphocytic infiltrate in the dermis with scattered apoptotic keratinocytes in the basal layer of the epidermis.2,4,6 Grades 1 to 2 lesions can be managed with high-potency topical corticosteroids without CPI dose interruption, with more extensive grade 2 lesions requiring systemic corticosteroids.2,6,9 Lichenoid eruptions grade 3 or higher also require systemic corticosteroid therapy CPI therapy cessation until the eruption has receded to grade 0 to 1.2 Alternative treatment options for high-grade toxicity include phototherapy and acitretin.2,4,9
Our patient was treated with cessation of immunotherapy and initiation of a systemic corticosteroid taper, acitretin, and narrowband UVB therapy. After 6 weeks of treatment, the pain and pruritus improved and the rash had resolved in some areas while it had taken on a more classic lichenoid appearance with violaceous scaly papules and plaques (Figure 2) in areas of prior ulcers and erosions. He no longer had any bullae, erosions, or ulcers.

- Barrios DM, Do MH, Phillips GS, et al. Immune checkpoint inhibitors to treat cutaneous malignancies. J Am Acad Dermatol. 2020;83:1239-1253. doi:10.1016/j.jaad.2020.03.131
- Geisler AN, Phillips GS, Barrios DM, et al. Immune checkpoint inhibitor-related dermatologic adverse events. J Am Acad Dermatol. 2020;83:1255-1268. doi:10.1016/j.jaad.2020.03.132
- Tattersall IW, Leventhal JS. Cutaneous toxicities of immune checkpoint inhibitors: the role of the dermatologist. Yale J Biol Med. 2020;93:123-132.
- Si X, He C, Zhang L, et al. Management of immune checkpoint inhibitor-related dermatologic adverse events. Thorac Cancer. 2020;11:488-492. doi:10.1111/1759-7714.13275
- Eggermont AMM, Kicinski M, Blank CU, et al. Association between immune-related adverse events and recurrence-free survival among patients with stage III melanoma randomized to receive pembrolizumab or placebo: a secondary analysis of a randomized clinical trial. JAMA Oncol. 2020;6:519-527. doi:10.1001 /jamaoncol.2019.5570
- Sibaud V, Meyer N, Lamant L, et al. Dermatologic complications of anti-PD-1/PD-L1 immune checkpoint antibodies. Curr Opin Oncol. 2016;28:254-263. doi:10.1097/CCO.0000000000000290
- Martínez-Doménech Á, García-Legaz Martínez M, Magdaleno-Tapial J, et al. Digital ulcerative lichenoid dermatitis in a patient receiving anti-PD-1 therapy. Dermatol Online J. 2019;25:13030/qt8sm0j7t7.
- Davis MJ, Wilken R, Fung MA, et al. Debilitating erosive lichenoid interface dermatitis from checkpoint inhibitor therapy. Dermatol Online J. 2018;24:13030/qt3vq6b04v.
- Apalla Z, Papageorgiou C, Lallas A, et al. Cutaneous adverse events of immune checkpoint inhibitors: a literature review [published online January 29, 2021]. Dermatol Pract Concept. 2021;11:E2021155. doi:10.5826/dpc.1101a155
The Diagnosis: Immunotherapy-Related Lichenoid Drug Eruption
Direct immunofluorescence was negative, and histopathology revealed a lichenoid interface dermatitis, minimal parakeratosis, and saw-toothed rete ridges (Figure 1). He was diagnosed with an immunotherapyrelated lichenoid drug eruption based on the morphology of the skin lesions and clinicopathologic correlation. Bullous pemphigoid and lichen planus pemphigoides were ruled out given the negative direct immunofluorescence findings. Stevens-Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN) was not consistent with the clinical presentation, especially given the lack of mucosal findings. The histology also was not consistent, as the biopsy specimen lacked apoptotic and necrotic keratinocytes to the degree seen in SJS/TEN and also had a greater degree of inflammatory infiltrate. Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome was ruled out given the lack of systemic findings, including facial swelling and lymphadenopathy and the clinical appearance of the rash. No morbilliform features were present, which is the most common presentation of DRESS syndrome.

Checkpoint inhibitor (CPI) therapy has become the cornerstone in management of certain advanced malignancies.1 Checkpoint inhibitors block cytotoxic T lymphocyte–associated protein 4, programmed cell death-1, and/or programmed cell death ligand-1, allowing activated T cells to infiltrate the tumor microenvironment and destroy malignant cells. Checkpoint inhibitors are approved for the treatment of melanoma, cutaneous squamous cell carcinoma, and Merkel cell carcinoma and are being investigated in various other cutaneous and soft tissue malignancies.1-3
Although CPIs have shown substantial efficacy in the management of advanced malignancies, immune-related adverse events (AEs) are common due to nonspecific immune activation.2 Immune-related cutaneous AEs are the most common immune-related AEs, occurring in 30% to 50% of patients who undergo treatment.2-5 Common immune-related cutaneous AEs include maculopapular, psoriasiform, and lichenoid dermatitis, as well as pruritus without dermatitis.2,3,6 Other reactions include but are not limited to bullous pemphigoid, vitiligolike depigmentation, and alopecia.2,3 Immune-related cutaneous AEs usually are self-limited; however, severe life-threatening reactions such as the spectrum of SJS/TEN and DRESS syndrome also can occur.2-4 Immune-related cutaneous AEs are graded based on the Common Terminology Criteria for Adverse Events: grade 1 reactions are asymptomatic and cover less than 10% of the patient’s body surface area (BSA), grade 2 reactions have mild symptoms and cover 10% to 30% of the patient’s BSA, grade 3 reactions have moderate to severe symptoms and cover greater than 30% of the patient’s BSA, and grade 4 reactions are life-threatening.2,3 With prompt recognition and adequate treatment, mild to moderate immune-related cutaneous AEs—grades 1 and 2—largely are reversible, and less than 5% require discontinuation of therapy.2,3,6 It has been suggested that immune-related cutaneous AEs may be a positive prognostic factor in the treatment of underlying malignancy, indicating adequate immune activation targeting the malignant cells.6
Although our patient had some typical violaceous, flat-topped papules and plaques with Wickham striae, he also had atypical findings for a lichenoid reaction. Given the endorsement of blisters, it is possible that some of these lesions initially were bullous and subsequently ruptured, leaving behind erosions. However, in other areas, there also were eroded papules and ulcerations without a reported history of excoriation, scratching, picking, or prior bullae, including difficult-to-reach areas such as the back. It is favored that these lesions represented a robust lichenoid dermatitis leading to erosive and ulcerated lesions, similar to the formation of bullous lichen planus. Lichenoid eruptions secondary to immunotherapy are well-known phenomena, but a PubMed search of articles indexed for MEDLINE using the terms ulcer, lichenoid, and immunotherapy revealed only 2 cases of ulcerative lichenoid eruptions: a localized digital erosive lichenoid dermatitis and a widespread ulcerative lichenoid drug eruption without true erosions.7,8 However, widespread erosive and ulcerated lichenoid reactions are rare.
Lichenoid eruptions most strongly are associated with anti–programmed cell death-1/ programmed cell death ligand-1 therapy, occurring in 20% of patients undergoing treatment.3 Lichenoid eruptions present as discrete, pruritic, erythematous, violaceous papules and plaques on the chest and back and rarely may involve the limbs, palmoplantar surfaces, and oral mucosa.2,3,6 Histopathologic features include a dense bandlike lymphocytic infiltrate in the dermis with scattered apoptotic keratinocytes in the basal layer of the epidermis.2,4,6 Grades 1 to 2 lesions can be managed with high-potency topical corticosteroids without CPI dose interruption, with more extensive grade 2 lesions requiring systemic corticosteroids.2,6,9 Lichenoid eruptions grade 3 or higher also require systemic corticosteroid therapy CPI therapy cessation until the eruption has receded to grade 0 to 1.2 Alternative treatment options for high-grade toxicity include phototherapy and acitretin.2,4,9
Our patient was treated with cessation of immunotherapy and initiation of a systemic corticosteroid taper, acitretin, and narrowband UVB therapy. After 6 weeks of treatment, the pain and pruritus improved and the rash had resolved in some areas while it had taken on a more classic lichenoid appearance with violaceous scaly papules and plaques (Figure 2) in areas of prior ulcers and erosions. He no longer had any bullae, erosions, or ulcers.

The Diagnosis: Immunotherapy-Related Lichenoid Drug Eruption
Direct immunofluorescence was negative, and histopathology revealed a lichenoid interface dermatitis, minimal parakeratosis, and saw-toothed rete ridges (Figure 1). He was diagnosed with an immunotherapyrelated lichenoid drug eruption based on the morphology of the skin lesions and clinicopathologic correlation. Bullous pemphigoid and lichen planus pemphigoides were ruled out given the negative direct immunofluorescence findings. Stevens-Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN) was not consistent with the clinical presentation, especially given the lack of mucosal findings. The histology also was not consistent, as the biopsy specimen lacked apoptotic and necrotic keratinocytes to the degree seen in SJS/TEN and also had a greater degree of inflammatory infiltrate. Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome was ruled out given the lack of systemic findings, including facial swelling and lymphadenopathy and the clinical appearance of the rash. No morbilliform features were present, which is the most common presentation of DRESS syndrome.

Checkpoint inhibitor (CPI) therapy has become the cornerstone in management of certain advanced malignancies.1 Checkpoint inhibitors block cytotoxic T lymphocyte–associated protein 4, programmed cell death-1, and/or programmed cell death ligand-1, allowing activated T cells to infiltrate the tumor microenvironment and destroy malignant cells. Checkpoint inhibitors are approved for the treatment of melanoma, cutaneous squamous cell carcinoma, and Merkel cell carcinoma and are being investigated in various other cutaneous and soft tissue malignancies.1-3
Although CPIs have shown substantial efficacy in the management of advanced malignancies, immune-related adverse events (AEs) are common due to nonspecific immune activation.2 Immune-related cutaneous AEs are the most common immune-related AEs, occurring in 30% to 50% of patients who undergo treatment.2-5 Common immune-related cutaneous AEs include maculopapular, psoriasiform, and lichenoid dermatitis, as well as pruritus without dermatitis.2,3,6 Other reactions include but are not limited to bullous pemphigoid, vitiligolike depigmentation, and alopecia.2,3 Immune-related cutaneous AEs usually are self-limited; however, severe life-threatening reactions such as the spectrum of SJS/TEN and DRESS syndrome also can occur.2-4 Immune-related cutaneous AEs are graded based on the Common Terminology Criteria for Adverse Events: grade 1 reactions are asymptomatic and cover less than 10% of the patient’s body surface area (BSA), grade 2 reactions have mild symptoms and cover 10% to 30% of the patient’s BSA, grade 3 reactions have moderate to severe symptoms and cover greater than 30% of the patient’s BSA, and grade 4 reactions are life-threatening.2,3 With prompt recognition and adequate treatment, mild to moderate immune-related cutaneous AEs—grades 1 and 2—largely are reversible, and less than 5% require discontinuation of therapy.2,3,6 It has been suggested that immune-related cutaneous AEs may be a positive prognostic factor in the treatment of underlying malignancy, indicating adequate immune activation targeting the malignant cells.6
Although our patient had some typical violaceous, flat-topped papules and plaques with Wickham striae, he also had atypical findings for a lichenoid reaction. Given the endorsement of blisters, it is possible that some of these lesions initially were bullous and subsequently ruptured, leaving behind erosions. However, in other areas, there also were eroded papules and ulcerations without a reported history of excoriation, scratching, picking, or prior bullae, including difficult-to-reach areas such as the back. It is favored that these lesions represented a robust lichenoid dermatitis leading to erosive and ulcerated lesions, similar to the formation of bullous lichen planus. Lichenoid eruptions secondary to immunotherapy are well-known phenomena, but a PubMed search of articles indexed for MEDLINE using the terms ulcer, lichenoid, and immunotherapy revealed only 2 cases of ulcerative lichenoid eruptions: a localized digital erosive lichenoid dermatitis and a widespread ulcerative lichenoid drug eruption without true erosions.7,8 However, widespread erosive and ulcerated lichenoid reactions are rare.
Lichenoid eruptions most strongly are associated with anti–programmed cell death-1/ programmed cell death ligand-1 therapy, occurring in 20% of patients undergoing treatment.3 Lichenoid eruptions present as discrete, pruritic, erythematous, violaceous papules and plaques on the chest and back and rarely may involve the limbs, palmoplantar surfaces, and oral mucosa.2,3,6 Histopathologic features include a dense bandlike lymphocytic infiltrate in the dermis with scattered apoptotic keratinocytes in the basal layer of the epidermis.2,4,6 Grades 1 to 2 lesions can be managed with high-potency topical corticosteroids without CPI dose interruption, with more extensive grade 2 lesions requiring systemic corticosteroids.2,6,9 Lichenoid eruptions grade 3 or higher also require systemic corticosteroid therapy CPI therapy cessation until the eruption has receded to grade 0 to 1.2 Alternative treatment options for high-grade toxicity include phototherapy and acitretin.2,4,9
Our patient was treated with cessation of immunotherapy and initiation of a systemic corticosteroid taper, acitretin, and narrowband UVB therapy. After 6 weeks of treatment, the pain and pruritus improved and the rash had resolved in some areas while it had taken on a more classic lichenoid appearance with violaceous scaly papules and plaques (Figure 2) in areas of prior ulcers and erosions. He no longer had any bullae, erosions, or ulcers.

- Barrios DM, Do MH, Phillips GS, et al. Immune checkpoint inhibitors to treat cutaneous malignancies. J Am Acad Dermatol. 2020;83:1239-1253. doi:10.1016/j.jaad.2020.03.131
- Geisler AN, Phillips GS, Barrios DM, et al. Immune checkpoint inhibitor-related dermatologic adverse events. J Am Acad Dermatol. 2020;83:1255-1268. doi:10.1016/j.jaad.2020.03.132
- Tattersall IW, Leventhal JS. Cutaneous toxicities of immune checkpoint inhibitors: the role of the dermatologist. Yale J Biol Med. 2020;93:123-132.
- Si X, He C, Zhang L, et al. Management of immune checkpoint inhibitor-related dermatologic adverse events. Thorac Cancer. 2020;11:488-492. doi:10.1111/1759-7714.13275
- Eggermont AMM, Kicinski M, Blank CU, et al. Association between immune-related adverse events and recurrence-free survival among patients with stage III melanoma randomized to receive pembrolizumab or placebo: a secondary analysis of a randomized clinical trial. JAMA Oncol. 2020;6:519-527. doi:10.1001 /jamaoncol.2019.5570
- Sibaud V, Meyer N, Lamant L, et al. Dermatologic complications of anti-PD-1/PD-L1 immune checkpoint antibodies. Curr Opin Oncol. 2016;28:254-263. doi:10.1097/CCO.0000000000000290
- Martínez-Doménech Á, García-Legaz Martínez M, Magdaleno-Tapial J, et al. Digital ulcerative lichenoid dermatitis in a patient receiving anti-PD-1 therapy. Dermatol Online J. 2019;25:13030/qt8sm0j7t7.
- Davis MJ, Wilken R, Fung MA, et al. Debilitating erosive lichenoid interface dermatitis from checkpoint inhibitor therapy. Dermatol Online J. 2018;24:13030/qt3vq6b04v.
- Apalla Z, Papageorgiou C, Lallas A, et al. Cutaneous adverse events of immune checkpoint inhibitors: a literature review [published online January 29, 2021]. Dermatol Pract Concept. 2021;11:E2021155. doi:10.5826/dpc.1101a155
- Barrios DM, Do MH, Phillips GS, et al. Immune checkpoint inhibitors to treat cutaneous malignancies. J Am Acad Dermatol. 2020;83:1239-1253. doi:10.1016/j.jaad.2020.03.131
- Geisler AN, Phillips GS, Barrios DM, et al. Immune checkpoint inhibitor-related dermatologic adverse events. J Am Acad Dermatol. 2020;83:1255-1268. doi:10.1016/j.jaad.2020.03.132
- Tattersall IW, Leventhal JS. Cutaneous toxicities of immune checkpoint inhibitors: the role of the dermatologist. Yale J Biol Med. 2020;93:123-132.
- Si X, He C, Zhang L, et al. Management of immune checkpoint inhibitor-related dermatologic adverse events. Thorac Cancer. 2020;11:488-492. doi:10.1111/1759-7714.13275
- Eggermont AMM, Kicinski M, Blank CU, et al. Association between immune-related adverse events and recurrence-free survival among patients with stage III melanoma randomized to receive pembrolizumab or placebo: a secondary analysis of a randomized clinical trial. JAMA Oncol. 2020;6:519-527. doi:10.1001 /jamaoncol.2019.5570
- Sibaud V, Meyer N, Lamant L, et al. Dermatologic complications of anti-PD-1/PD-L1 immune checkpoint antibodies. Curr Opin Oncol. 2016;28:254-263. doi:10.1097/CCO.0000000000000290
- Martínez-Doménech Á, García-Legaz Martínez M, Magdaleno-Tapial J, et al. Digital ulcerative lichenoid dermatitis in a patient receiving anti-PD-1 therapy. Dermatol Online J. 2019;25:13030/qt8sm0j7t7.
- Davis MJ, Wilken R, Fung MA, et al. Debilitating erosive lichenoid interface dermatitis from checkpoint inhibitor therapy. Dermatol Online J. 2018;24:13030/qt3vq6b04v.
- Apalla Z, Papageorgiou C, Lallas A, et al. Cutaneous adverse events of immune checkpoint inhibitors: a literature review [published online January 29, 2021]. Dermatol Pract Concept. 2021;11:E2021155. doi:10.5826/dpc.1101a155
A 70-year-old man presented with a painful, pruritic, diffuse eruption on the trunk, legs, and arms of 2 months’ duration. He had a history of stage IV pleomorphic cell sarcoma of the retroperitoneum and was started on pembrolizumab therapy 6 weeks prior to the eruption. Physical examination revealed violaceous papules and plaques with shiny reticulated scaling as well as multiple scattered eroded papules and shallow ulcerations. The oral mucosa and genitals were spared. The patient endorsed blisters followed by open sores that were both itchy and painful. He denied self-infliction. Both the patient and his wife denied scratching. Two biopsies for direct immunofluorescence and histopathology were performed.
Variations in Preference for Topical Vehicles Among Demographic Groups
Topical medication is a mainstay in the treatment of dermatologic conditions. Adherence to medication regimens can be challenging in patients requiring long-term topical treatment, and nonadherence is multifactorial. A major modifiable contributing factor is patient dissatisfaction with the vehicle used. Medications often have options for different topical preparations. Therefore, it is important to consider patient preference when prescribing topical treatments to maximize adherence, ensure patient satisfaction, and optimize outcomes.
We hypothesized that notable differences exist among demographic groups regarding preference for topical vehicles. Little research has been conducted to delineate trends. This study aimed to identify variations in preference for creams, lotions, and ointments by age, gender, and ethnicity.
Methods
Data were collected through surveys distributed to all patients seen at the Truman Medical Center University Health Dermatology Clinic in Kansas City, Missouri, between September 2018 and June 2019. The study was approved by the University of Missouri Kansas City institutional review board. An estimated response rate of 95% was achieved. Each patient was informed that the survey was voluntary and anonymous, and declining to complete the survey had no effect on the care provided. Each patient completed only 1 survey and returned it to a collection box before departing from clinic.
In the survey, patients provided demographic information, including age, gender, and ethnicity. Age groups included patients younger than 40 years, 40 to 60 years, and older than 60 years. Gender groups included male and female. Ethnicity included white, black, Hispanic/Latino, and Asian/Pacific Islander or other. Patients then chose 1 of 3 options for topical vehicle preference: cream, lotion, or ointment. Each of these options was accompanied by a brief description of the vehicle, a photograph, and examples of common commercial products to aid in decision-making. The expected values were calculated based on a probability distribution under the assumption that variables have no association. Therefore, the discrepancy between the expected value and the observed value was used to describe the significance of the association between variables.
Data were analyzed using χ2 tests with the aid of a statistician. P<.05 was considered statistically significant.
Results
A total of 404 surveys were collected and recorded. Data showed statistically significant trends in each demographic parameter.
Age
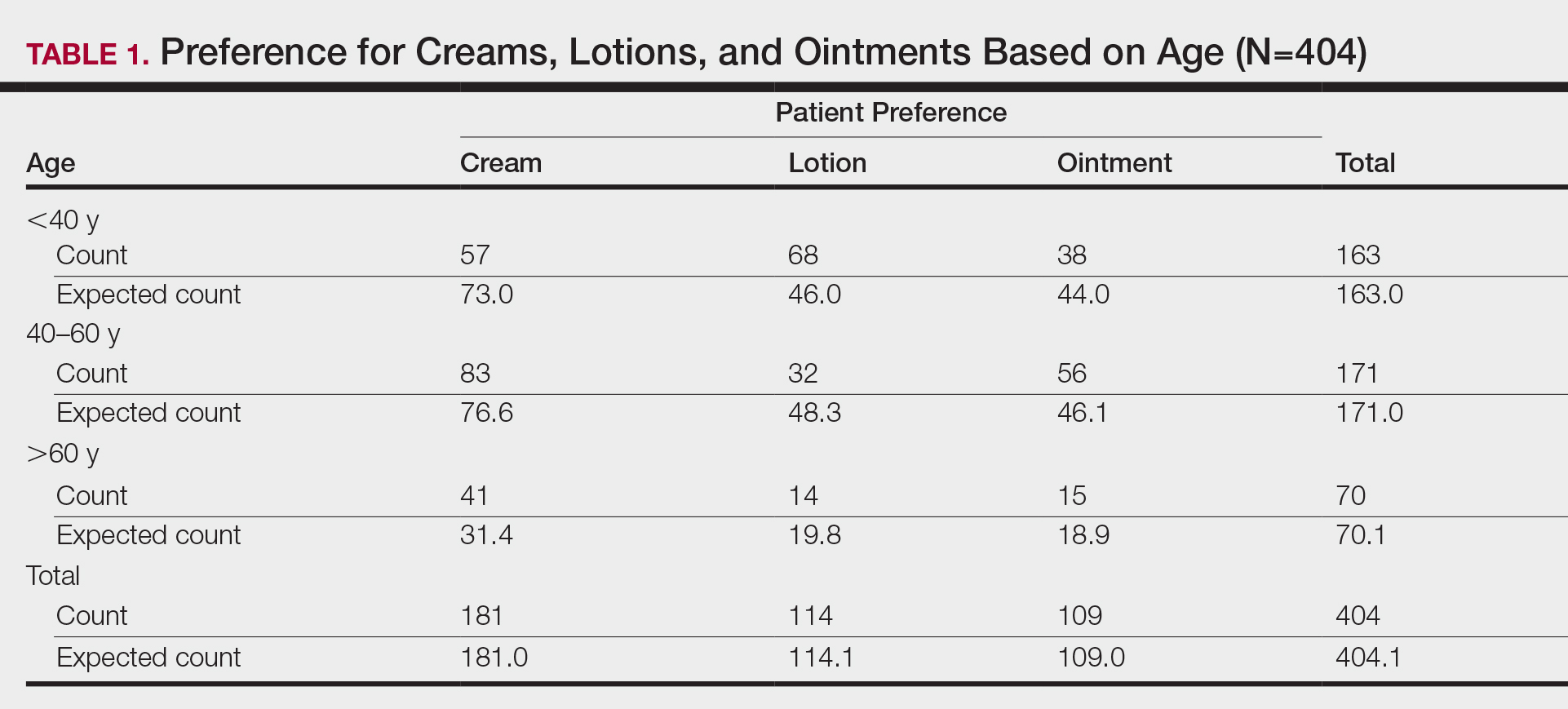
First, we analyzed differences in preference based on age (Table 1). Of 404 patients, 163 were younger than 40 years, 171 were aged 40 to 60 years, and 70 were older than 60 years. Patients younger than 40 years preferred lotion (68 vs 46.0 expected). Patients aged 40 to 60 years showed preference for cream (83 vs 76.6 expected) and ointment (56 vs 46.1 expected). Patients older than 60 years preferred cream (41 vs 31.4 expected). These findings were statistically significant (P<.0001).
Gender
Next, we evaluated variations based on gender (Table 2). Of 404 patients, 254 were female and 150 were male. Females preferred cream (127 vs 113.8 expected). Males exhibited preference for lotion (50 vs 42.3 expected) and ointment (46 vs 40.5 expected). Differences between genders were statistically significant (P=.023).

Ethnicity
We then analyzed preferences based on ethnicity (Table 3). Of 404 patients, 30 were Hispanic/Latino, 26 were Asian/Pacific Islander or other, 227 were white, and 121 were black. Hispanic/Latino patients showed equivocal findings, aligning with expected counts. Asian/Pacific Islander or other patients exhibited slight preferences for cream (14 vs 11.6 expected) and lotion (10 vs 7.3 expected). White patients preferred cream (119 vs 101.7 expected) and lotion (82 vs 64.1 expected). Black patients showed strong preference for ointment (72 vs 32.6 expected). Differences in preferences based on ethnicity were statistically significant (P<.0001).

Comment
Topical medication is a mainstay of dermatologic therapy. Many topical preparations (or vehicles) exist, including ointments, creams, lotions, gels, solutions, and foams. Vehicle type not only influences bioavailability of the prepared medication but also has a notable impact on adherence and subsequent efficacy of the topical therapy.
Medication adherence is especially challenging in dermatology, as topical medications play a central role in treatment. Compliance with the medication regimen is paramount in treatment efficacy.1 In dermatology, adherence with oral medications is higher than it is for topical medications2; various factors contribute to this difference. Compliance may decline with topical treatment due to time-consuming application, misunderstanding about the disease or the treatment regimen, frequency of administration, dissatisfaction with efficacy or appearance, and other variables.3
Other factors have been found to be important to topical medication adherence; younger age, female gender, marriage, employment, nonsmoking, nondrinking, and higher cognitive ability were associated with higher topical medication adherence.4 Our study focused on one factor: identification of demographic-specific preferences that might have implications on adherence within the studied demographic groups.
It is known that individual preferences exist when patients are choosing a topical preparation. However, a PubMed search of articles indexed for MEDLINE using the terms topical, vehicle, preparation, adherence, and preference revealed few studies that examined the preference for topical vehicle by age, gender, or ethnicity.
Existing studies have examined preferences for topical preparations based on specific disease states; this literature, albeit limited, demonstrates that preferences for topical product formulations vary among acne, atopic dermatitis, and plaque psoriasis patients.5 Other studies focus on specific patient populations or medications. For example, one study found that preference for corticosteroid vehicles among psoriasis patients was highly variable and choice of vehicle was critical to adherence.6 Another study highlighted differences in vehicle choice between younger and older age groups with psoriasis.7
Given the limited data overall, it was our goal to determine if any patterns of preference existed by age, gender, or ethnicity, regardless of disease state or indication for topical product. Importantly, over-the-counter products—cosmetic or otherwise—were not differentiated from prescribed topical medications. Our survey elucidated significant differences in preference by age, gender, and ethnicity.
Notable Findings
Regarding age, patients younger than 40 years preferred lotion, patients aged 40 to 60 years preferred cream, and patients older than 60 years preferred cream. Analysis based on gender showed that females preferred cream, and males preferred lotion and ointment. Analysis based on ethnicity most notably demonstrated a strong preference for ointment in black patients while showing preference for cream in white patients.
Potential Biases and Pitfalls
Limitations of this study included the small Hispanic/Latino and Asian/Pacific Islander populations surveyed, possible misunderstanding of the survey by respondents, and the potential for surveys being filled out twice by the same patient. Future surveys could be conducted over a longer period to increase the total sample size and to better characterize less-represented populations, such as Hispanic and Asian patients. To avoid repeat participation, the first question of the survey asked patients to indicate if they had previously completed the survey and instructed patients who had to return the repeat survey to the front desk.
To limit other errors, our survey included concise accessible descriptions of each preparation along with clear representative photographs and examples of common brands. Still, it is possible that some mistakes could have been made while patients filled out the survey based on comprehension deficits, oversight, or other reasons. It also is possible that preference might vary individually depending on the indication of the topical product—cosmetic or therapeutic—or even by anatomic site of application. Neither of these considerations was assessed specifically in our survey.
Conclusion
Our hope is that this study helps practitioners better anticipate topical preferences among patients with the ultimate goal of increasing medication adherence and patient outcomes. Nevertheless, although these general trends can provide helpful guidance, we acknowledge that individual preferences vary, and care should always be patient centered.
Acknowledgment
We thank An-Lin Cheng, PhD (Kansas City, Missouri), for assistance with the statistical analysis.
- Kircik LH. Vehicles always matter. J Drugs Dermatol. 2019;18:s99.
- Furue M, Onozuka D, Takeuchi S, et al. Poor adherence to oral andtopical medication in 3096 dermatological patients as assessed by the Morisky Medication Adherence Scale-8. Br J Dermatol. 2015;172:272-275.
- Tan X, Feldman SR, Chang, J, et al. Topical drug delivery systems in dermatology: a review of patient adherence issues. Expert Opin Drug Deliv. 2012;9:1263-1271.
- Ahn CS, Culp L, Huang WW, et al. Adherence in dermatology. J Dermatolog Treat. 2017;28:94-103.
- Eastman WJ, Malahias S, Delconte J, et al. Assessing attributes of topical vehicles for the treatment of acne, atopic dermatitis, and plaque psoriasis. Cutis. 2014;94:46-53.
- Felix K, Unrue E, Inyang M, et al. Patients preferences for different corticosteroid vehicles are highly variable. J Dermatolog Treat. 2019;31:147-151.
- Hong C-H, Papp KA, Lophaven KW, et al. Patients with psoriasis have different preferences for topical therapy, highlighting the importance of individualized treatment approaches: randomized phase IIIb PSO-INSIGHTFUL study. J Eur Acad Dermatol Venereol. 2017;31:1876-1883.
Topical medication is a mainstay in the treatment of dermatologic conditions. Adherence to medication regimens can be challenging in patients requiring long-term topical treatment, and nonadherence is multifactorial. A major modifiable contributing factor is patient dissatisfaction with the vehicle used. Medications often have options for different topical preparations. Therefore, it is important to consider patient preference when prescribing topical treatments to maximize adherence, ensure patient satisfaction, and optimize outcomes.
We hypothesized that notable differences exist among demographic groups regarding preference for topical vehicles. Little research has been conducted to delineate trends. This study aimed to identify variations in preference for creams, lotions, and ointments by age, gender, and ethnicity.
Methods
Data were collected through surveys distributed to all patients seen at the Truman Medical Center University Health Dermatology Clinic in Kansas City, Missouri, between September 2018 and June 2019. The study was approved by the University of Missouri Kansas City institutional review board. An estimated response rate of 95% was achieved. Each patient was informed that the survey was voluntary and anonymous, and declining to complete the survey had no effect on the care provided. Each patient completed only 1 survey and returned it to a collection box before departing from clinic.
In the survey, patients provided demographic information, including age, gender, and ethnicity. Age groups included patients younger than 40 years, 40 to 60 years, and older than 60 years. Gender groups included male and female. Ethnicity included white, black, Hispanic/Latino, and Asian/Pacific Islander or other. Patients then chose 1 of 3 options for topical vehicle preference: cream, lotion, or ointment. Each of these options was accompanied by a brief description of the vehicle, a photograph, and examples of common commercial products to aid in decision-making. The expected values were calculated based on a probability distribution under the assumption that variables have no association. Therefore, the discrepancy between the expected value and the observed value was used to describe the significance of the association between variables.
Data were analyzed using χ2 tests with the aid of a statistician. P<.05 was considered statistically significant.
Results
A total of 404 surveys were collected and recorded. Data showed statistically significant trends in each demographic parameter.
Age
First, we analyzed differences in preference based on age (Table 1). Of 404 patients, 163 were younger than 40 years, 171 were aged 40 to 60 years, and 70 were older than 60 years. Patients younger than 40 years preferred lotion (68 vs 46.0 expected). Patients aged 40 to 60 years showed preference for cream (83 vs 76.6 expected) and ointment (56 vs 46.1 expected). Patients older than 60 years preferred cream (41 vs 31.4 expected). These findings were statistically significant (P<.0001).

Gender
Next, we evaluated variations based on gender (Table 2). Of 404 patients, 254 were female and 150 were male. Females preferred cream (127 vs 113.8 expected). Males exhibited preference for lotion (50 vs 42.3 expected) and ointment (46 vs 40.5 expected). Differences between genders were statistically significant (P=.023).

Ethnicity
We then analyzed preferences based on ethnicity (Table 3). Of 404 patients, 30 were Hispanic/Latino, 26 were Asian/Pacific Islander or other, 227 were white, and 121 were black. Hispanic/Latino patients showed equivocal findings, aligning with expected counts. Asian/Pacific Islander or other patients exhibited slight preferences for cream (14 vs 11.6 expected) and lotion (10 vs 7.3 expected). White patients preferred cream (119 vs 101.7 expected) and lotion (82 vs 64.1 expected). Black patients showed strong preference for ointment (72 vs 32.6 expected). Differences in preferences based on ethnicity were statistically significant (P<.0001).

Comment
Topical medication is a mainstay of dermatologic therapy. Many topical preparations (or vehicles) exist, including ointments, creams, lotions, gels, solutions, and foams. Vehicle type not only influences bioavailability of the prepared medication but also has a notable impact on adherence and subsequent efficacy of the topical therapy.
Medication adherence is especially challenging in dermatology, as topical medications play a central role in treatment. Compliance with the medication regimen is paramount in treatment efficacy.1 In dermatology, adherence with oral medications is higher than it is for topical medications2; various factors contribute to this difference. Compliance may decline with topical treatment due to time-consuming application, misunderstanding about the disease or the treatment regimen, frequency of administration, dissatisfaction with efficacy or appearance, and other variables.3
Other factors have been found to be important to topical medication adherence; younger age, female gender, marriage, employment, nonsmoking, nondrinking, and higher cognitive ability were associated with higher topical medication adherence.4 Our study focused on one factor: identification of demographic-specific preferences that might have implications on adherence within the studied demographic groups.
It is known that individual preferences exist when patients are choosing a topical preparation. However, a PubMed search of articles indexed for MEDLINE using the terms topical, vehicle, preparation, adherence, and preference revealed few studies that examined the preference for topical vehicle by age, gender, or ethnicity.
Existing studies have examined preferences for topical preparations based on specific disease states; this literature, albeit limited, demonstrates that preferences for topical product formulations vary among acne, atopic dermatitis, and plaque psoriasis patients.5 Other studies focus on specific patient populations or medications. For example, one study found that preference for corticosteroid vehicles among psoriasis patients was highly variable and choice of vehicle was critical to adherence.6 Another study highlighted differences in vehicle choice between younger and older age groups with psoriasis.7
Given the limited data overall, it was our goal to determine if any patterns of preference existed by age, gender, or ethnicity, regardless of disease state or indication for topical product. Importantly, over-the-counter products—cosmetic or otherwise—were not differentiated from prescribed topical medications. Our survey elucidated significant differences in preference by age, gender, and ethnicity.
Notable Findings
Regarding age, patients younger than 40 years preferred lotion, patients aged 40 to 60 years preferred cream, and patients older than 60 years preferred cream. Analysis based on gender showed that females preferred cream, and males preferred lotion and ointment. Analysis based on ethnicity most notably demonstrated a strong preference for ointment in black patients while showing preference for cream in white patients.
Potential Biases and Pitfalls
Limitations of this study included the small Hispanic/Latino and Asian/Pacific Islander populations surveyed, possible misunderstanding of the survey by respondents, and the potential for surveys being filled out twice by the same patient. Future surveys could be conducted over a longer period to increase the total sample size and to better characterize less-represented populations, such as Hispanic and Asian patients. To avoid repeat participation, the first question of the survey asked patients to indicate if they had previously completed the survey and instructed patients who had to return the repeat survey to the front desk.
To limit other errors, our survey included concise accessible descriptions of each preparation along with clear representative photographs and examples of common brands. Still, it is possible that some mistakes could have been made while patients filled out the survey based on comprehension deficits, oversight, or other reasons. It also is possible that preference might vary individually depending on the indication of the topical product—cosmetic or therapeutic—or even by anatomic site of application. Neither of these considerations was assessed specifically in our survey.
Conclusion
Our hope is that this study helps practitioners better anticipate topical preferences among patients with the ultimate goal of increasing medication adherence and patient outcomes. Nevertheless, although these general trends can provide helpful guidance, we acknowledge that individual preferences vary, and care should always be patient centered.
Acknowledgment
We thank An-Lin Cheng, PhD (Kansas City, Missouri), for assistance with the statistical analysis.
Topical medication is a mainstay in the treatment of dermatologic conditions. Adherence to medication regimens can be challenging in patients requiring long-term topical treatment, and nonadherence is multifactorial. A major modifiable contributing factor is patient dissatisfaction with the vehicle used. Medications often have options for different topical preparations. Therefore, it is important to consider patient preference when prescribing topical treatments to maximize adherence, ensure patient satisfaction, and optimize outcomes.
We hypothesized that notable differences exist among demographic groups regarding preference for topical vehicles. Little research has been conducted to delineate trends. This study aimed to identify variations in preference for creams, lotions, and ointments by age, gender, and ethnicity.
Methods
Data were collected through surveys distributed to all patients seen at the Truman Medical Center University Health Dermatology Clinic in Kansas City, Missouri, between September 2018 and June 2019. The study was approved by the University of Missouri Kansas City institutional review board. An estimated response rate of 95% was achieved. Each patient was informed that the survey was voluntary and anonymous, and declining to complete the survey had no effect on the care provided. Each patient completed only 1 survey and returned it to a collection box before departing from clinic.
In the survey, patients provided demographic information, including age, gender, and ethnicity. Age groups included patients younger than 40 years, 40 to 60 years, and older than 60 years. Gender groups included male and female. Ethnicity included white, black, Hispanic/Latino, and Asian/Pacific Islander or other. Patients then chose 1 of 3 options for topical vehicle preference: cream, lotion, or ointment. Each of these options was accompanied by a brief description of the vehicle, a photograph, and examples of common commercial products to aid in decision-making. The expected values were calculated based on a probability distribution under the assumption that variables have no association. Therefore, the discrepancy between the expected value and the observed value was used to describe the significance of the association between variables.
Data were analyzed using χ2 tests with the aid of a statistician. P<.05 was considered statistically significant.
Results
A total of 404 surveys were collected and recorded. Data showed statistically significant trends in each demographic parameter.
Age
First, we analyzed differences in preference based on age (Table 1). Of 404 patients, 163 were younger than 40 years, 171 were aged 40 to 60 years, and 70 were older than 60 years. Patients younger than 40 years preferred lotion (68 vs 46.0 expected). Patients aged 40 to 60 years showed preference for cream (83 vs 76.6 expected) and ointment (56 vs 46.1 expected). Patients older than 60 years preferred cream (41 vs 31.4 expected). These findings were statistically significant (P<.0001).

Gender
Next, we evaluated variations based on gender (Table 2). Of 404 patients, 254 were female and 150 were male. Females preferred cream (127 vs 113.8 expected). Males exhibited preference for lotion (50 vs 42.3 expected) and ointment (46 vs 40.5 expected). Differences between genders were statistically significant (P=.023).

Ethnicity
We then analyzed preferences based on ethnicity (Table 3). Of 404 patients, 30 were Hispanic/Latino, 26 were Asian/Pacific Islander or other, 227 were white, and 121 were black. Hispanic/Latino patients showed equivocal findings, aligning with expected counts. Asian/Pacific Islander or other patients exhibited slight preferences for cream (14 vs 11.6 expected) and lotion (10 vs 7.3 expected). White patients preferred cream (119 vs 101.7 expected) and lotion (82 vs 64.1 expected). Black patients showed strong preference for ointment (72 vs 32.6 expected). Differences in preferences based on ethnicity were statistically significant (P<.0001).

Comment
Topical medication is a mainstay of dermatologic therapy. Many topical preparations (or vehicles) exist, including ointments, creams, lotions, gels, solutions, and foams. Vehicle type not only influences bioavailability of the prepared medication but also has a notable impact on adherence and subsequent efficacy of the topical therapy.
Medication adherence is especially challenging in dermatology, as topical medications play a central role in treatment. Compliance with the medication regimen is paramount in treatment efficacy.1 In dermatology, adherence with oral medications is higher than it is for topical medications2; various factors contribute to this difference. Compliance may decline with topical treatment due to time-consuming application, misunderstanding about the disease or the treatment regimen, frequency of administration, dissatisfaction with efficacy or appearance, and other variables.3
Other factors have been found to be important to topical medication adherence; younger age, female gender, marriage, employment, nonsmoking, nondrinking, and higher cognitive ability were associated with higher topical medication adherence.4 Our study focused on one factor: identification of demographic-specific preferences that might have implications on adherence within the studied demographic groups.
It is known that individual preferences exist when patients are choosing a topical preparation. However, a PubMed search of articles indexed for MEDLINE using the terms topical, vehicle, preparation, adherence, and preference revealed few studies that examined the preference for topical vehicle by age, gender, or ethnicity.
Existing studies have examined preferences for topical preparations based on specific disease states; this literature, albeit limited, demonstrates that preferences for topical product formulations vary among acne, atopic dermatitis, and plaque psoriasis patients.5 Other studies focus on specific patient populations or medications. For example, one study found that preference for corticosteroid vehicles among psoriasis patients was highly variable and choice of vehicle was critical to adherence.6 Another study highlighted differences in vehicle choice between younger and older age groups with psoriasis.7
Given the limited data overall, it was our goal to determine if any patterns of preference existed by age, gender, or ethnicity, regardless of disease state or indication for topical product. Importantly, over-the-counter products—cosmetic or otherwise—were not differentiated from prescribed topical medications. Our survey elucidated significant differences in preference by age, gender, and ethnicity.
Notable Findings
Regarding age, patients younger than 40 years preferred lotion, patients aged 40 to 60 years preferred cream, and patients older than 60 years preferred cream. Analysis based on gender showed that females preferred cream, and males preferred lotion and ointment. Analysis based on ethnicity most notably demonstrated a strong preference for ointment in black patients while showing preference for cream in white patients.
Potential Biases and Pitfalls
Limitations of this study included the small Hispanic/Latino and Asian/Pacific Islander populations surveyed, possible misunderstanding of the survey by respondents, and the potential for surveys being filled out twice by the same patient. Future surveys could be conducted over a longer period to increase the total sample size and to better characterize less-represented populations, such as Hispanic and Asian patients. To avoid repeat participation, the first question of the survey asked patients to indicate if they had previously completed the survey and instructed patients who had to return the repeat survey to the front desk.
To limit other errors, our survey included concise accessible descriptions of each preparation along with clear representative photographs and examples of common brands. Still, it is possible that some mistakes could have been made while patients filled out the survey based on comprehension deficits, oversight, or other reasons. It also is possible that preference might vary individually depending on the indication of the topical product—cosmetic or therapeutic—or even by anatomic site of application. Neither of these considerations was assessed specifically in our survey.
Conclusion
Our hope is that this study helps practitioners better anticipate topical preferences among patients with the ultimate goal of increasing medication adherence and patient outcomes. Nevertheless, although these general trends can provide helpful guidance, we acknowledge that individual preferences vary, and care should always be patient centered.
Acknowledgment
We thank An-Lin Cheng, PhD (Kansas City, Missouri), for assistance with the statistical analysis.
- Kircik LH. Vehicles always matter. J Drugs Dermatol. 2019;18:s99.
- Furue M, Onozuka D, Takeuchi S, et al. Poor adherence to oral andtopical medication in 3096 dermatological patients as assessed by the Morisky Medication Adherence Scale-8. Br J Dermatol. 2015;172:272-275.
- Tan X, Feldman SR, Chang, J, et al. Topical drug delivery systems in dermatology: a review of patient adherence issues. Expert Opin Drug Deliv. 2012;9:1263-1271.
- Ahn CS, Culp L, Huang WW, et al. Adherence in dermatology. J Dermatolog Treat. 2017;28:94-103.
- Eastman WJ, Malahias S, Delconte J, et al. Assessing attributes of topical vehicles for the treatment of acne, atopic dermatitis, and plaque psoriasis. Cutis. 2014;94:46-53.
- Felix K, Unrue E, Inyang M, et al. Patients preferences for different corticosteroid vehicles are highly variable. J Dermatolog Treat. 2019;31:147-151.
- Hong C-H, Papp KA, Lophaven KW, et al. Patients with psoriasis have different preferences for topical therapy, highlighting the importance of individualized treatment approaches: randomized phase IIIb PSO-INSIGHTFUL study. J Eur Acad Dermatol Venereol. 2017;31:1876-1883.
- Kircik LH. Vehicles always matter. J Drugs Dermatol. 2019;18:s99.
- Furue M, Onozuka D, Takeuchi S, et al. Poor adherence to oral andtopical medication in 3096 dermatological patients as assessed by the Morisky Medication Adherence Scale-8. Br J Dermatol. 2015;172:272-275.
- Tan X, Feldman SR, Chang, J, et al. Topical drug delivery systems in dermatology: a review of patient adherence issues. Expert Opin Drug Deliv. 2012;9:1263-1271.
- Ahn CS, Culp L, Huang WW, et al. Adherence in dermatology. J Dermatolog Treat. 2017;28:94-103.
- Eastman WJ, Malahias S, Delconte J, et al. Assessing attributes of topical vehicles for the treatment of acne, atopic dermatitis, and plaque psoriasis. Cutis. 2014;94:46-53.
- Felix K, Unrue E, Inyang M, et al. Patients preferences for different corticosteroid vehicles are highly variable. J Dermatolog Treat. 2019;31:147-151.
- Hong C-H, Papp KA, Lophaven KW, et al. Patients with psoriasis have different preferences for topical therapy, highlighting the importance of individualized treatment approaches: randomized phase IIIb PSO-INSIGHTFUL study. J Eur Acad Dermatol Venereol. 2017;31:1876-1883.
Practice Points
- Variations exist in preference for topical vehicles by age group, gender, and ethnicity.
- Identifying and utilizing preferred treatment options can help maximize patient outcomes.
Hand-foot-and-mouth Disease Caused by Coxsackievirus A6 on the Rise
Hand-foot-and-mouth disease (HFMD) is a viral illness caused by several enteroviruses, most commonly coxsackievirus A16 (CVA16) and enterovirus 71 (EV71). The disease is generally seen in children younger than 5 years, characterized by lesions of the oral mucosa, palms, and soles, usually lasting 7 to 10 days. Other coxsackie type A viruses, including CVA6, CVA9, and CVA10, also are associated with HFMD.1-5 Although CVA16 has traditionally been the primary strain causing HFMD, CVA6 has become a major cause of HFMD outbreaks in the United States and worldwide in recent years.6-12 Interestingly, CVA6 also has been found to be associated with adult HFMD, which has increased in incidence. The CVA6 strain was first identified in association with the disease during HFMD outbreaks in Finland and Singapore in 2008,13,14 with similar strains detected in subsequent outbreaks in Taiwan, Japan, Spain, France, China, India, and the United States.12,15-25 Most cases took place in warmer months, with one winter outbreak in Massachusetts in 2012.24
Herein, we review the incidence of CVA6, as well as its atypical presentation, diagnosis, and treatment to aid dermatologists. Given the increasing incidence of HFMD caused by CVA6 and its often atypical presentation, it is important for dermatologists to be aware of this increasingly notable disease state and its viral cause.
Incidence of CVA6
Coxsackievirus A6 has been identified as the cause of many reported outbreaks of HFMD since it was first identified in 2008, and it is known to cause both pediatric and adult outbreaks.7-12 It may even be surpassing other strains in frequency in certain areas. In Tianjin, China, for example, EV71 and CVA16 were the most common serotypes causing HFMD from 2008 to 2012; however, in 2013, CVA6 was the most prevalent strain.26
The incidence of CVA6 also has been increasing in other areas.28
In 2015, an outbreak of HFMD took place at Lackland Air Force Base in Texas during a basic military training. Eight cases were confirmed and 45 cases were suspected. The rate of infection was 0.4% (50/12,270) among trainees and 0.3% (2/602) among instructors.7 Eight of 12 nasopharyngeal swabs tested positive for EV by way of local real-time reverse transcription–polymerase chain reaction (RT-PCR). Four nasopharyngeal swabs were sent to the CDC for evaluation and all were positive for CVA6.7
Presentation
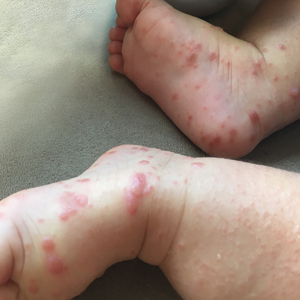
Because the prevalence of CVA6 has increased, it is important to be able to identify the presentation of HFMD caused by this strain. Coxsackievirus A6 has been found to affect a broader demographic and cause more severe cases of HFMD with its unique constellation of findings compared to other known strains. Patients present with flulike symptoms; higher fever than present in typical HFMD; and a longer duration of disease, typically lasting 2 weeks. Patients also may present with more severe skin disease compared to classic HFMD, not only including vesicles but also large bullae, erosions, and ulcers on the dorsal and plantar feet (Figure 1).
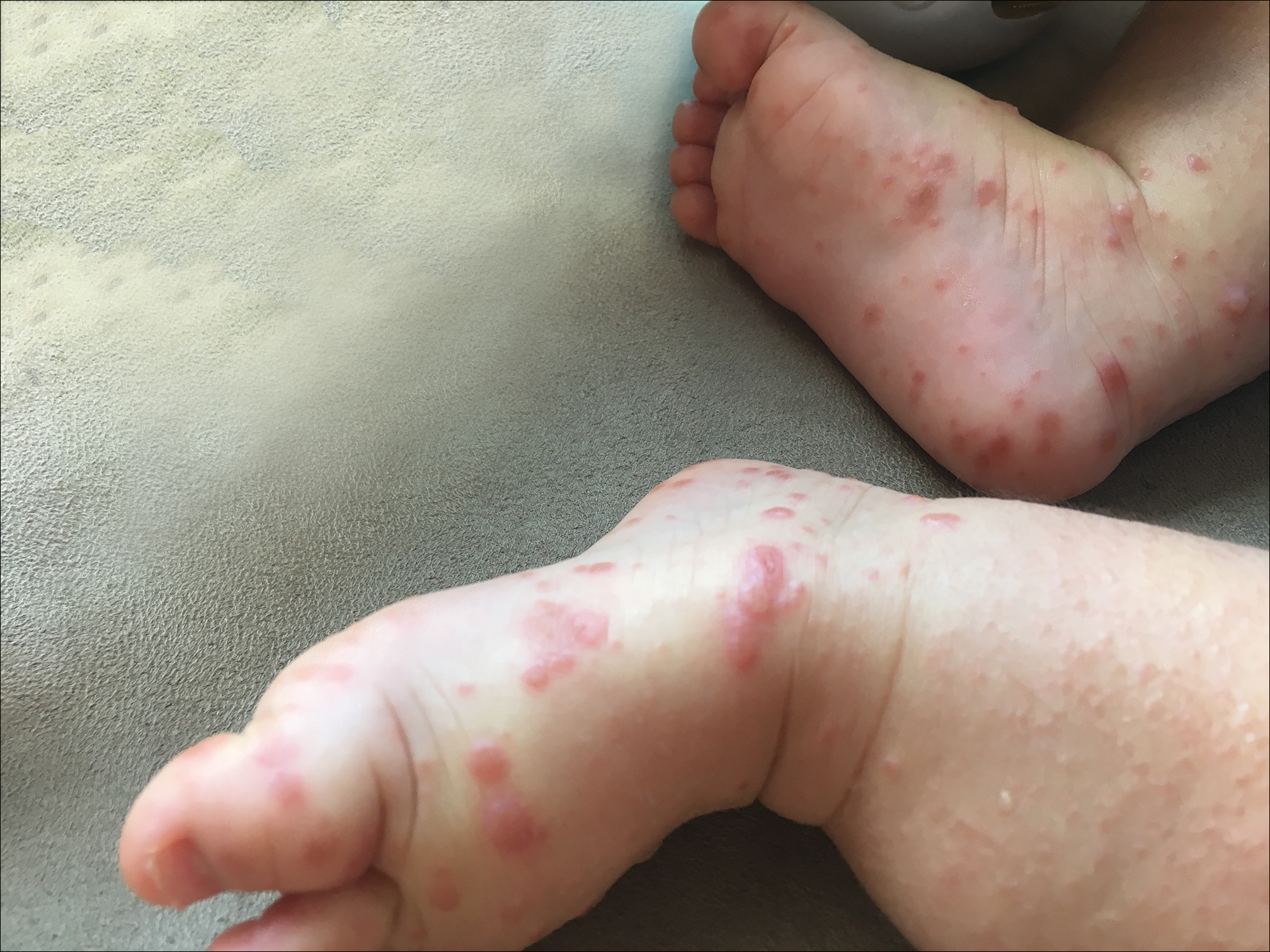
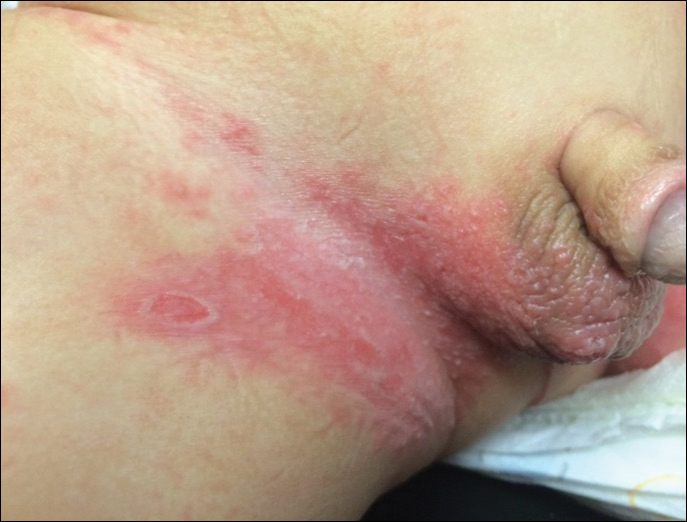
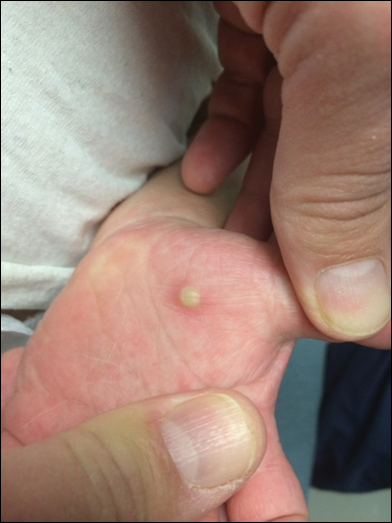
In patients with atopic dermatitis, CVA6 also shows a predilection to appear in areas of skin disease, such as the flexural regions of the arms and legs, and is referred to as eczema coxsackium.24,38,39 It can mimic eczema herpeticum or varicella superinfection, which are important considerations to include in the differential diagnosis. Additionally, CVA6-induced lesions often show up in previously irritated or traumatized areas such as sunburns, fungal infections, and diaper dermatitis in children. Lesions have been described to sometimes mimic Gianotti-Crosti syndrome, with involvement of the extensor surfaces, buttocks, and cheeks, and sparing of the trunk.24
Clinical Diagnosis
Because HFMD is uncommon and atypical in adults, skin biopsies may be used in the initial workup and evaluation of patients. It is important to understand the histologic features associated with HFMD, including spongiosis with exocytosis of neutrophils as well as keratinocyte necrosis and pallor with associated shadow cells.6 In one series, the most extensively involved areas of keratinocyte necrosis were the stratum granulosum and upper half of the stratum spinosum.40 In the dermis, vascular involvement may be present on a spectrum with the extravasation of red blood cells and leukocytoclasis or true leukocytoclastic vasculitis.6,40 Vesicular lesions show severe dermal edema and inflammatory infiltrate.6,41 CD3+ and CD8+ lymphocytes predominate. Cytotoxic T lymphocytes are present and express granzyme B and granulysin, both important mediators of apoptosis in virally infected keratinocytes.6
Adult HFMD primarily is a clinical diagnosis, and histopathologic analysis can be a useful tool in certain cases. Coxsackievirus A6 does not grow well on culture and is not detected by standard serologic testing laboratories, necessitating the use of quantitative RT-PCR analysis.41,42 In one study, culture was able to detect only 14% to 16% of samples that tested positive by quantitative RT-PCR.43 This form of PCR identifies viral subtype through amplification of enterovirus viral protein 1 capsid gene sequence.24 Unfortunately, this testing often is not offered in most readily available laboratories and often necessitates being sent out to more well-equipped laboratories.2,24
Treatment
Hand-foot-and-mouth disease is a self-limited illness and requires only supportive care with a focus on hydration and pain management. Lesions heal without scarring but may leave notable postinflammatory pigment alteration that may last months to years, depending on extent of disease and skin type. Secondarily infected individuals should be treated with appropriate antibiotics or antivirals depending on the infectious agent. Hand hygiene is of great importance, and hospitalized patients should be put on strict contact precautions. It also is important to isolate patients from vulnerable individuals, especially pregnant women, as coxsackievirus has been linked to intrauterine infections and loss of pregnancy.24
Genetic Analysis
Genetic studies of the virus have suggested that nonstructural genes may be playing an interesting role in clinical phenotypes and outcomes of CVA6 infection.44 These genetic studies also are being implemented into the understanding of the virus’ evolution as well as the construction of vaccinations.27,44
Conclusion
With the increasing prevalence of CVA6-associated HFMD, it is important to understand the clinical presentation and histologic findings associated with this atypical presentation of the disease as well as the changing epidemiology of the viral strains causing HFMD.
- Galen WK. Cutaneous manifestations of enterovirus infections. In: Tyring SK, ed. Mucocutaneous Manifestations of Viral Diseases. New York, NY: Marcel Dekker; 2002:455-467.
- Ramirez-Fort M, Downing C, Doan H, et al. Coxsackievirus A6 associated hand, foot and mouth disease in adults: clinical presentation and review of the literature. J Clin Virol. 2014;60:381-386.
- Khetsuriani N, Lamonte-Fowlkes A, Oberst S, et al. Enterovirus surveillance—United States, 1970-2005. MMWR Surveill Summ. 2006;55:1-20.
- Yang F, Zhang T, Hu Y, et al. Survey of enterovirus infections from hand, foot and mouth disease outbreak in China, 2009. Virol J. 2011;8:508.
- Ho M, Chen ER, Hsu KH, et al. An epidemic of enterovirus 71 infection in Taiwan. Taiwan Enterovirus Epidemic Working Group. N Engl J Med. 1999;341:929-935.
- Second J, Velter C, Calès S, et al. Clinicopathologic analysis of atypical hand, foot, and mouth disease in adult patients. J Am Acad Dermatol. 2016;76:722-729.
- Banta J, Lenz B, Pawlak M, et al. Notes from the field: outbreak of hand, foot, and mouth disease caused by coxsackievirus A6 among basic military trainees—Texas, 2015. MMWR Morb Mortal Wkly Rep. 2016;65.26:678-680.
- Bian L, Wang Y, Yao X, et al. Coxsackievirus A6: a new emerging pathogen causing hand, foot and mouth disease outbreaks worldwide. Expert Rev Anti Infect Ther. 2015;13:1061-1071.
- Buttery VW, Kenyon C, Grunewald S, et al. Notes from the field: atypical presentations of hand, foot, and mouth disease caused by coxsackievirus A6—Minnesota, 2014. MMWR Morb Mortal Wkly Rep. 2015;64:805.
- Puenpa J, Chieochansin T, Linsuwanon P, et al. Hand, foot, and mouth disease caused by coxsackievirus A6, Thailand, 2012. Emerg Infect Dis. 2013;19:641-643.
- Flett K, Youngster I, Huang J, et al. Hand, foot, and mouth disease caused by coxsackievirus A6. Emerg Infect Dis. 2012;18:1702-1704.
- Centers for Disease Control and Prevention. Notes from the field: severe hand, foot, and mouth disease associated with coxsackievirus A6—Alabama, Connecticut, California, and Nevada, November 2011-February 2012. MMWR Morb Mortal Wkly Rep. 2012;61:213-214.
- Blomqvist S, Klemola P, Kaijalainen S, et al. Co-circulation of coxsackieviruses A6 and A10 in hand, foot and mouth disease outbreak in Finland. J Clin Virol. 2010;48:49-54.
- Osterback R, Vuorinen T, Linna M, et al. Coxsackievirus A6 and hand, foot, and mouth disease, Finland. Emerg Infect Dis. 2009;15:1485-1488.
- Zeng H, Lu J, Zheng H, et al. The epidemiological study of coxsackievirus A6 revealing hand, foot and mouth disease epidemic patterns in Guandong, China. Sci Rep. 2015;5:10550.
- Mirand A, Henquell C, Archimbaud C, et al. Outbreak of hand, foot and mouth disease/herpangina associated with coxsackievirus A6 andA10 infections in 2010, France: a large citywide, prospective observational study. Clin Microbiol Infect. 2012;18:E110-E118.
- Wei SH, Huang YP, Liu MC, et al. An outbreak of coxsackievirus A6 hand, foot, and mouth disease associated with onychomadesis in Taiwan, 2010. BMC Infect Dis. 2011;11:346.
- Fujimoto T, Iizuka S, Enomoto M, et al. Hand, foot, and mouth disease caused by coxsackievirus A6, Japan, 2011. Emerg Infect Dis. 2012;18:337-339.
- Bracho MA, Gonzalez-Candelas F, Valero A, et al. Enterovirus co-infections and onychomadesis after hand, foot, and mouth disease, Spain, 2008. Emerg Infect Dis. 2011;17:2223-2231.
- Gopalkrishna V, Patil PR, Patil GP, et al. Circulation of multiple enterovirus serotypes causing hand, foot and mouth disease in India. J Med Microbiol. 2012;61:420-425.
- Lo SH, Huang YC, Huang CG, et al. Clinical and epidemiologic features of coxsackievirus A6 infection in children in northern Taiwan between 2004 and 2009. J Microbiol Immunol Infect. 2011;44:252-257.
- Lu QB, Zhang XA, Wo Y, et al. Circulation of coxsackievirus A10 and A6 in hand-foot-mouth disease in China, 2009-2011. PLoS One. 2012;7:E52073.
- Wu Y, Yeo A, Phoon MC, et al. The largest outbreak of hand; foot and mouth disease in Singapore in 2008: the role of enterovirus 71 and coxsackievirus A strains. Int J Infect Dis. 2010;14:E1076-E1081.
- Ventarola D, Bordone L, Silverberg N. Update on hand-foot-and-mouth disease. Clin Dermatol. 2015;33:340-346.
- Li Y, Chang Z, Wu P, et al. Emerging enteroviruses causing hand, foot and mouth disease, China. 2010-2016. Emerg Infect Dis. 2018;24:1902-1906.
- Tan X, Li L, Zhang B, et al. Molecular epidemiology of coxsackievirus A6 associated with outbreaks of hand, foot, and mouth disease in Tianjin, China, in 2013. Arch Virol. 2015;160:1097-1104.
- Li Y, Bao H, Zhang X, et al. Epidemiological and genetic analysis concerning the non-enterovirus 71 and non-coxsackievirus A16 causative agents related to hand, foot and mouth disease in Anyang City, Henan Province, China, from 2011 to 2015. J Med Virol. 2017;89:1749-1758.
- Guan H, Wang J, Wang C, et al. Etiology of multiple non-EV71 and non-CVA16 enteroviruses associated with hand, foot, and mouth disease in Jinan, China, 2009-2013. PLoS One. 2015;10:E0142733.
- Cabrerizo M, Tarrago´ D, Muñoz-Almagro C, et al. Mollecular epidemiology of enterovirus 71, coxsackievirus A16 and A6 associated with hand, foot and mouth disease in Spain. Clin Microbiol Infect. 2014;20:O150-O156.
- Lønnberg A, Elberling J, Fischer T, et al. Two cases of hand, foot, and mouth disease involving the scalp. Acta Derm Venereol. 2013;93:467-468.
- Lott JP, Liu K, Landry ML, et al. Atypical hand-foot-and-mouth disease associated with coxsackievirus A6 infection. J Am Acad Dermatol. 2013;69:736-741.
- Kaminska K, Martinetti G, Lucchini R, et al. Coxsackievirus A6 and hand, foot and mouth disease: three case reports of familial child-to-immunocompetent adult transmission and a literature review. Case Rep Dermatol. 2013;5:203-209.
- Shin JU, Oh SH, Lee JH. A case of hand-foot-mouth disease in an immunocompetent adult. Ann Dermatol. 2010;22:216-218.
- Osterback R, Vuorinen T, Linna M, et al. Coxsackievirus A6 and hand, foot, and mouth disease, Finland. Emerg Infect Dis. 2009;15:1485-1488.
- Feder HM, Bennett N, Modlin JF. Atypical hand, foot, and mouth disease: a vesiculobullous eruption caused by coxsackie virus A6. Lancet Infect Dis. 2014;14:83-86.
- Wei SH, Huang YP, Liu MC, et al. An outbreak of coxsackievirus A6 hand, foot, and mouth disease associated with onychomadesis in Taiwan, 2010. BMC Infect Dis. 2011;11:346.
- Kim M, Kim B, Byun S, et al. Beau’s lines and onychomadesis after hand-foot-mouth disease. Clin Pediatr Dermatol. 2015;1:1.
- Mathes EF, Oza V, Frieden IJ, et al. “Eczema coxsackium” and unusual cutaneous findings in an enterovirus outbreak. Pediatrics. 2013;132:E149-E157.
- Lynch M, Sears A, Cookson H, et al. Disseminated coxsackievirus A6 affecting children with atopic dermatitis. Clin Exp Dermatol. 2015;40:525-528.
- Laga A, Shroba S, Hanna J. Atypical hand, foot and mouth disease in adults associated with coxsackievirus A6: a clinicopathologic study. J Cutan Pathol. 2016;43:940-945.
- Schmidt NJ, Ho HH, Lennette EH. Propagation and isolation of group A coxsackieviruses in RD cells. J Clin Microbiol. 1975;2:183-185.
- Oberste MS, Penaranda S, Rogers SL, et al. Comparative evaluation of Taqman real-time PCR and semi-nested VP1 PCR for detection of enteroviruses in clinical specimens. J Clin Virol. 2010;49:73-74.
- Lee MK, Chan PK, Ho II, et al. Enterovirus infection among patients admitted to hospital in Hong Kong in 2010: epidemiology, clinical characteristics, and importance of molecular diagnosis. J Med Virol. 2013;85:1811-1817.
- Yee PTI, Laa Poh C. Impact of genetic changes, pathogenicity and antigenicity on enterovirus A71 vaccine development. Virology. 2017;506:121-129.
Hand-foot-and-mouth disease (HFMD) is a viral illness caused by several enteroviruses, most commonly coxsackievirus A16 (CVA16) and enterovirus 71 (EV71). The disease is generally seen in children younger than 5 years, characterized by lesions of the oral mucosa, palms, and soles, usually lasting 7 to 10 days. Other coxsackie type A viruses, including CVA6, CVA9, and CVA10, also are associated with HFMD.1-5 Although CVA16 has traditionally been the primary strain causing HFMD, CVA6 has become a major cause of HFMD outbreaks in the United States and worldwide in recent years.6-12 Interestingly, CVA6 also has been found to be associated with adult HFMD, which has increased in incidence. The CVA6 strain was first identified in association with the disease during HFMD outbreaks in Finland and Singapore in 2008,13,14 with similar strains detected in subsequent outbreaks in Taiwan, Japan, Spain, France, China, India, and the United States.12,15-25 Most cases took place in warmer months, with one winter outbreak in Massachusetts in 2012.24
Herein, we review the incidence of CVA6, as well as its atypical presentation, diagnosis, and treatment to aid dermatologists. Given the increasing incidence of HFMD caused by CVA6 and its often atypical presentation, it is important for dermatologists to be aware of this increasingly notable disease state and its viral cause.
Incidence of CVA6
Coxsackievirus A6 has been identified as the cause of many reported outbreaks of HFMD since it was first identified in 2008, and it is known to cause both pediatric and adult outbreaks.7-12 It may even be surpassing other strains in frequency in certain areas. In Tianjin, China, for example, EV71 and CVA16 were the most common serotypes causing HFMD from 2008 to 2012; however, in 2013, CVA6 was the most prevalent strain.26
The incidence of CVA6 also has been increasing in other areas.28
In 2015, an outbreak of HFMD took place at Lackland Air Force Base in Texas during a basic military training. Eight cases were confirmed and 45 cases were suspected. The rate of infection was 0.4% (50/12,270) among trainees and 0.3% (2/602) among instructors.7 Eight of 12 nasopharyngeal swabs tested positive for EV by way of local real-time reverse transcription–polymerase chain reaction (RT-PCR). Four nasopharyngeal swabs were sent to the CDC for evaluation and all were positive for CVA6.7
Presentation
Because the prevalence of CVA6 has increased, it is important to be able to identify the presentation of HFMD caused by this strain. Coxsackievirus A6 has been found to affect a broader demographic and cause more severe cases of HFMD with its unique constellation of findings compared to other known strains. Patients present with flulike symptoms; higher fever than present in typical HFMD; and a longer duration of disease, typically lasting 2 weeks. Patients also may present with more severe skin disease compared to classic HFMD, not only including vesicles but also large bullae, erosions, and ulcers on the dorsal and plantar feet (Figure 1).



In patients with atopic dermatitis, CVA6 also shows a predilection to appear in areas of skin disease, such as the flexural regions of the arms and legs, and is referred to as eczema coxsackium.24,38,39 It can mimic eczema herpeticum or varicella superinfection, which are important considerations to include in the differential diagnosis. Additionally, CVA6-induced lesions often show up in previously irritated or traumatized areas such as sunburns, fungal infections, and diaper dermatitis in children. Lesions have been described to sometimes mimic Gianotti-Crosti syndrome, with involvement of the extensor surfaces, buttocks, and cheeks, and sparing of the trunk.24
Clinical Diagnosis
Because HFMD is uncommon and atypical in adults, skin biopsies may be used in the initial workup and evaluation of patients. It is important to understand the histologic features associated with HFMD, including spongiosis with exocytosis of neutrophils as well as keratinocyte necrosis and pallor with associated shadow cells.6 In one series, the most extensively involved areas of keratinocyte necrosis were the stratum granulosum and upper half of the stratum spinosum.40 In the dermis, vascular involvement may be present on a spectrum with the extravasation of red blood cells and leukocytoclasis or true leukocytoclastic vasculitis.6,40 Vesicular lesions show severe dermal edema and inflammatory infiltrate.6,41 CD3+ and CD8+ lymphocytes predominate. Cytotoxic T lymphocytes are present and express granzyme B and granulysin, both important mediators of apoptosis in virally infected keratinocytes.6
Adult HFMD primarily is a clinical diagnosis, and histopathologic analysis can be a useful tool in certain cases. Coxsackievirus A6 does not grow well on culture and is not detected by standard serologic testing laboratories, necessitating the use of quantitative RT-PCR analysis.41,42 In one study, culture was able to detect only 14% to 16% of samples that tested positive by quantitative RT-PCR.43 This form of PCR identifies viral subtype through amplification of enterovirus viral protein 1 capsid gene sequence.24 Unfortunately, this testing often is not offered in most readily available laboratories and often necessitates being sent out to more well-equipped laboratories.2,24
Treatment
Hand-foot-and-mouth disease is a self-limited illness and requires only supportive care with a focus on hydration and pain management. Lesions heal without scarring but may leave notable postinflammatory pigment alteration that may last months to years, depending on extent of disease and skin type. Secondarily infected individuals should be treated with appropriate antibiotics or antivirals depending on the infectious agent. Hand hygiene is of great importance, and hospitalized patients should be put on strict contact precautions. It also is important to isolate patients from vulnerable individuals, especially pregnant women, as coxsackievirus has been linked to intrauterine infections and loss of pregnancy.24
Genetic Analysis
Genetic studies of the virus have suggested that nonstructural genes may be playing an interesting role in clinical phenotypes and outcomes of CVA6 infection.44 These genetic studies also are being implemented into the understanding of the virus’ evolution as well as the construction of vaccinations.27,44
Conclusion
With the increasing prevalence of CVA6-associated HFMD, it is important to understand the clinical presentation and histologic findings associated with this atypical presentation of the disease as well as the changing epidemiology of the viral strains causing HFMD.
Hand-foot-and-mouth disease (HFMD) is a viral illness caused by several enteroviruses, most commonly coxsackievirus A16 (CVA16) and enterovirus 71 (EV71). The disease is generally seen in children younger than 5 years, characterized by lesions of the oral mucosa, palms, and soles, usually lasting 7 to 10 days. Other coxsackie type A viruses, including CVA6, CVA9, and CVA10, also are associated with HFMD.1-5 Although CVA16 has traditionally been the primary strain causing HFMD, CVA6 has become a major cause of HFMD outbreaks in the United States and worldwide in recent years.6-12 Interestingly, CVA6 also has been found to be associated with adult HFMD, which has increased in incidence. The CVA6 strain was first identified in association with the disease during HFMD outbreaks in Finland and Singapore in 2008,13,14 with similar strains detected in subsequent outbreaks in Taiwan, Japan, Spain, France, China, India, and the United States.12,15-25 Most cases took place in warmer months, with one winter outbreak in Massachusetts in 2012.24
Herein, we review the incidence of CVA6, as well as its atypical presentation, diagnosis, and treatment to aid dermatologists. Given the increasing incidence of HFMD caused by CVA6 and its often atypical presentation, it is important for dermatologists to be aware of this increasingly notable disease state and its viral cause.
Incidence of CVA6
Coxsackievirus A6 has been identified as the cause of many reported outbreaks of HFMD since it was first identified in 2008, and it is known to cause both pediatric and adult outbreaks.7-12 It may even be surpassing other strains in frequency in certain areas. In Tianjin, China, for example, EV71 and CVA16 were the most common serotypes causing HFMD from 2008 to 2012; however, in 2013, CVA6 was the most prevalent strain.26
The incidence of CVA6 also has been increasing in other areas.28
In 2015, an outbreak of HFMD took place at Lackland Air Force Base in Texas during a basic military training. Eight cases were confirmed and 45 cases were suspected. The rate of infection was 0.4% (50/12,270) among trainees and 0.3% (2/602) among instructors.7 Eight of 12 nasopharyngeal swabs tested positive for EV by way of local real-time reverse transcription–polymerase chain reaction (RT-PCR). Four nasopharyngeal swabs were sent to the CDC for evaluation and all were positive for CVA6.7
Presentation
Because the prevalence of CVA6 has increased, it is important to be able to identify the presentation of HFMD caused by this strain. Coxsackievirus A6 has been found to affect a broader demographic and cause more severe cases of HFMD with its unique constellation of findings compared to other known strains. Patients present with flulike symptoms; higher fever than present in typical HFMD; and a longer duration of disease, typically lasting 2 weeks. Patients also may present with more severe skin disease compared to classic HFMD, not only including vesicles but also large bullae, erosions, and ulcers on the dorsal and plantar feet (Figure 1).



In patients with atopic dermatitis, CVA6 also shows a predilection to appear in areas of skin disease, such as the flexural regions of the arms and legs, and is referred to as eczema coxsackium.24,38,39 It can mimic eczema herpeticum or varicella superinfection, which are important considerations to include in the differential diagnosis. Additionally, CVA6-induced lesions often show up in previously irritated or traumatized areas such as sunburns, fungal infections, and diaper dermatitis in children. Lesions have been described to sometimes mimic Gianotti-Crosti syndrome, with involvement of the extensor surfaces, buttocks, and cheeks, and sparing of the trunk.24
Clinical Diagnosis
Because HFMD is uncommon and atypical in adults, skin biopsies may be used in the initial workup and evaluation of patients. It is important to understand the histologic features associated with HFMD, including spongiosis with exocytosis of neutrophils as well as keratinocyte necrosis and pallor with associated shadow cells.6 In one series, the most extensively involved areas of keratinocyte necrosis were the stratum granulosum and upper half of the stratum spinosum.40 In the dermis, vascular involvement may be present on a spectrum with the extravasation of red blood cells and leukocytoclasis or true leukocytoclastic vasculitis.6,40 Vesicular lesions show severe dermal edema and inflammatory infiltrate.6,41 CD3+ and CD8+ lymphocytes predominate. Cytotoxic T lymphocytes are present and express granzyme B and granulysin, both important mediators of apoptosis in virally infected keratinocytes.6
Adult HFMD primarily is a clinical diagnosis, and histopathologic analysis can be a useful tool in certain cases. Coxsackievirus A6 does not grow well on culture and is not detected by standard serologic testing laboratories, necessitating the use of quantitative RT-PCR analysis.41,42 In one study, culture was able to detect only 14% to 16% of samples that tested positive by quantitative RT-PCR.43 This form of PCR identifies viral subtype through amplification of enterovirus viral protein 1 capsid gene sequence.24 Unfortunately, this testing often is not offered in most readily available laboratories and often necessitates being sent out to more well-equipped laboratories.2,24
Treatment
Hand-foot-and-mouth disease is a self-limited illness and requires only supportive care with a focus on hydration and pain management. Lesions heal without scarring but may leave notable postinflammatory pigment alteration that may last months to years, depending on extent of disease and skin type. Secondarily infected individuals should be treated with appropriate antibiotics or antivirals depending on the infectious agent. Hand hygiene is of great importance, and hospitalized patients should be put on strict contact precautions. It also is important to isolate patients from vulnerable individuals, especially pregnant women, as coxsackievirus has been linked to intrauterine infections and loss of pregnancy.24
Genetic Analysis
Genetic studies of the virus have suggested that nonstructural genes may be playing an interesting role in clinical phenotypes and outcomes of CVA6 infection.44 These genetic studies also are being implemented into the understanding of the virus’ evolution as well as the construction of vaccinations.27,44
Conclusion
With the increasing prevalence of CVA6-associated HFMD, it is important to understand the clinical presentation and histologic findings associated with this atypical presentation of the disease as well as the changing epidemiology of the viral strains causing HFMD.
- Galen WK. Cutaneous manifestations of enterovirus infections. In: Tyring SK, ed. Mucocutaneous Manifestations of Viral Diseases. New York, NY: Marcel Dekker; 2002:455-467.
- Ramirez-Fort M, Downing C, Doan H, et al. Coxsackievirus A6 associated hand, foot and mouth disease in adults: clinical presentation and review of the literature. J Clin Virol. 2014;60:381-386.
- Khetsuriani N, Lamonte-Fowlkes A, Oberst S, et al. Enterovirus surveillance—United States, 1970-2005. MMWR Surveill Summ. 2006;55:1-20.
- Yang F, Zhang T, Hu Y, et al. Survey of enterovirus infections from hand, foot and mouth disease outbreak in China, 2009. Virol J. 2011;8:508.
- Ho M, Chen ER, Hsu KH, et al. An epidemic of enterovirus 71 infection in Taiwan. Taiwan Enterovirus Epidemic Working Group. N Engl J Med. 1999;341:929-935.
- Second J, Velter C, Calès S, et al. Clinicopathologic analysis of atypical hand, foot, and mouth disease in adult patients. J Am Acad Dermatol. 2016;76:722-729.
- Banta J, Lenz B, Pawlak M, et al. Notes from the field: outbreak of hand, foot, and mouth disease caused by coxsackievirus A6 among basic military trainees—Texas, 2015. MMWR Morb Mortal Wkly Rep. 2016;65.26:678-680.
- Bian L, Wang Y, Yao X, et al. Coxsackievirus A6: a new emerging pathogen causing hand, foot and mouth disease outbreaks worldwide. Expert Rev Anti Infect Ther. 2015;13:1061-1071.
- Buttery VW, Kenyon C, Grunewald S, et al. Notes from the field: atypical presentations of hand, foot, and mouth disease caused by coxsackievirus A6—Minnesota, 2014. MMWR Morb Mortal Wkly Rep. 2015;64:805.
- Puenpa J, Chieochansin T, Linsuwanon P, et al. Hand, foot, and mouth disease caused by coxsackievirus A6, Thailand, 2012. Emerg Infect Dis. 2013;19:641-643.
- Flett K, Youngster I, Huang J, et al. Hand, foot, and mouth disease caused by coxsackievirus A6. Emerg Infect Dis. 2012;18:1702-1704.
- Centers for Disease Control and Prevention. Notes from the field: severe hand, foot, and mouth disease associated with coxsackievirus A6—Alabama, Connecticut, California, and Nevada, November 2011-February 2012. MMWR Morb Mortal Wkly Rep. 2012;61:213-214.
- Blomqvist S, Klemola P, Kaijalainen S, et al. Co-circulation of coxsackieviruses A6 and A10 in hand, foot and mouth disease outbreak in Finland. J Clin Virol. 2010;48:49-54.
- Osterback R, Vuorinen T, Linna M, et al. Coxsackievirus A6 and hand, foot, and mouth disease, Finland. Emerg Infect Dis. 2009;15:1485-1488.
- Zeng H, Lu J, Zheng H, et al. The epidemiological study of coxsackievirus A6 revealing hand, foot and mouth disease epidemic patterns in Guandong, China. Sci Rep. 2015;5:10550.
- Mirand A, Henquell C, Archimbaud C, et al. Outbreak of hand, foot and mouth disease/herpangina associated with coxsackievirus A6 andA10 infections in 2010, France: a large citywide, prospective observational study. Clin Microbiol Infect. 2012;18:E110-E118.
- Wei SH, Huang YP, Liu MC, et al. An outbreak of coxsackievirus A6 hand, foot, and mouth disease associated with onychomadesis in Taiwan, 2010. BMC Infect Dis. 2011;11:346.
- Fujimoto T, Iizuka S, Enomoto M, et al. Hand, foot, and mouth disease caused by coxsackievirus A6, Japan, 2011. Emerg Infect Dis. 2012;18:337-339.
- Bracho MA, Gonzalez-Candelas F, Valero A, et al. Enterovirus co-infections and onychomadesis after hand, foot, and mouth disease, Spain, 2008. Emerg Infect Dis. 2011;17:2223-2231.
- Gopalkrishna V, Patil PR, Patil GP, et al. Circulation of multiple enterovirus serotypes causing hand, foot and mouth disease in India. J Med Microbiol. 2012;61:420-425.
- Lo SH, Huang YC, Huang CG, et al. Clinical and epidemiologic features of coxsackievirus A6 infection in children in northern Taiwan between 2004 and 2009. J Microbiol Immunol Infect. 2011;44:252-257.
- Lu QB, Zhang XA, Wo Y, et al. Circulation of coxsackievirus A10 and A6 in hand-foot-mouth disease in China, 2009-2011. PLoS One. 2012;7:E52073.
- Wu Y, Yeo A, Phoon MC, et al. The largest outbreak of hand; foot and mouth disease in Singapore in 2008: the role of enterovirus 71 and coxsackievirus A strains. Int J Infect Dis. 2010;14:E1076-E1081.
- Ventarola D, Bordone L, Silverberg N. Update on hand-foot-and-mouth disease. Clin Dermatol. 2015;33:340-346.
- Li Y, Chang Z, Wu P, et al. Emerging enteroviruses causing hand, foot and mouth disease, China. 2010-2016. Emerg Infect Dis. 2018;24:1902-1906.
- Tan X, Li L, Zhang B, et al. Molecular epidemiology of coxsackievirus A6 associated with outbreaks of hand, foot, and mouth disease in Tianjin, China, in 2013. Arch Virol. 2015;160:1097-1104.
- Li Y, Bao H, Zhang X, et al. Epidemiological and genetic analysis concerning the non-enterovirus 71 and non-coxsackievirus A16 causative agents related to hand, foot and mouth disease in Anyang City, Henan Province, China, from 2011 to 2015. J Med Virol. 2017;89:1749-1758.
- Guan H, Wang J, Wang C, et al. Etiology of multiple non-EV71 and non-CVA16 enteroviruses associated with hand, foot, and mouth disease in Jinan, China, 2009-2013. PLoS One. 2015;10:E0142733.
- Cabrerizo M, Tarrago´ D, Muñoz-Almagro C, et al. Mollecular epidemiology of enterovirus 71, coxsackievirus A16 and A6 associated with hand, foot and mouth disease in Spain. Clin Microbiol Infect. 2014;20:O150-O156.
- Lønnberg A, Elberling J, Fischer T, et al. Two cases of hand, foot, and mouth disease involving the scalp. Acta Derm Venereol. 2013;93:467-468.
- Lott JP, Liu K, Landry ML, et al. Atypical hand-foot-and-mouth disease associated with coxsackievirus A6 infection. J Am Acad Dermatol. 2013;69:736-741.
- Kaminska K, Martinetti G, Lucchini R, et al. Coxsackievirus A6 and hand, foot and mouth disease: three case reports of familial child-to-immunocompetent adult transmission and a literature review. Case Rep Dermatol. 2013;5:203-209.
- Shin JU, Oh SH, Lee JH. A case of hand-foot-mouth disease in an immunocompetent adult. Ann Dermatol. 2010;22:216-218.
- Osterback R, Vuorinen T, Linna M, et al. Coxsackievirus A6 and hand, foot, and mouth disease, Finland. Emerg Infect Dis. 2009;15:1485-1488.
- Feder HM, Bennett N, Modlin JF. Atypical hand, foot, and mouth disease: a vesiculobullous eruption caused by coxsackie virus A6. Lancet Infect Dis. 2014;14:83-86.
- Wei SH, Huang YP, Liu MC, et al. An outbreak of coxsackievirus A6 hand, foot, and mouth disease associated with onychomadesis in Taiwan, 2010. BMC Infect Dis. 2011;11:346.
- Kim M, Kim B, Byun S, et al. Beau’s lines and onychomadesis after hand-foot-mouth disease. Clin Pediatr Dermatol. 2015;1:1.
- Mathes EF, Oza V, Frieden IJ, et al. “Eczema coxsackium” and unusual cutaneous findings in an enterovirus outbreak. Pediatrics. 2013;132:E149-E157.
- Lynch M, Sears A, Cookson H, et al. Disseminated coxsackievirus A6 affecting children with atopic dermatitis. Clin Exp Dermatol. 2015;40:525-528.
- Laga A, Shroba S, Hanna J. Atypical hand, foot and mouth disease in adults associated with coxsackievirus A6: a clinicopathologic study. J Cutan Pathol. 2016;43:940-945.
- Schmidt NJ, Ho HH, Lennette EH. Propagation and isolation of group A coxsackieviruses in RD cells. J Clin Microbiol. 1975;2:183-185.
- Oberste MS, Penaranda S, Rogers SL, et al. Comparative evaluation of Taqman real-time PCR and semi-nested VP1 PCR for detection of enteroviruses in clinical specimens. J Clin Virol. 2010;49:73-74.
- Lee MK, Chan PK, Ho II, et al. Enterovirus infection among patients admitted to hospital in Hong Kong in 2010: epidemiology, clinical characteristics, and importance of molecular diagnosis. J Med Virol. 2013;85:1811-1817.
- Yee PTI, Laa Poh C. Impact of genetic changes, pathogenicity and antigenicity on enterovirus A71 vaccine development. Virology. 2017;506:121-129.
- Galen WK. Cutaneous manifestations of enterovirus infections. In: Tyring SK, ed. Mucocutaneous Manifestations of Viral Diseases. New York, NY: Marcel Dekker; 2002:455-467.
- Ramirez-Fort M, Downing C, Doan H, et al. Coxsackievirus A6 associated hand, foot and mouth disease in adults: clinical presentation and review of the literature. J Clin Virol. 2014;60:381-386.
- Khetsuriani N, Lamonte-Fowlkes A, Oberst S, et al. Enterovirus surveillance—United States, 1970-2005. MMWR Surveill Summ. 2006;55:1-20.
- Yang F, Zhang T, Hu Y, et al. Survey of enterovirus infections from hand, foot and mouth disease outbreak in China, 2009. Virol J. 2011;8:508.
- Ho M, Chen ER, Hsu KH, et al. An epidemic of enterovirus 71 infection in Taiwan. Taiwan Enterovirus Epidemic Working Group. N Engl J Med. 1999;341:929-935.
- Second J, Velter C, Calès S, et al. Clinicopathologic analysis of atypical hand, foot, and mouth disease in adult patients. J Am Acad Dermatol. 2016;76:722-729.
- Banta J, Lenz B, Pawlak M, et al. Notes from the field: outbreak of hand, foot, and mouth disease caused by coxsackievirus A6 among basic military trainees—Texas, 2015. MMWR Morb Mortal Wkly Rep. 2016;65.26:678-680.
- Bian L, Wang Y, Yao X, et al. Coxsackievirus A6: a new emerging pathogen causing hand, foot and mouth disease outbreaks worldwide. Expert Rev Anti Infect Ther. 2015;13:1061-1071.
- Buttery VW, Kenyon C, Grunewald S, et al. Notes from the field: atypical presentations of hand, foot, and mouth disease caused by coxsackievirus A6—Minnesota, 2014. MMWR Morb Mortal Wkly Rep. 2015;64:805.
- Puenpa J, Chieochansin T, Linsuwanon P, et al. Hand, foot, and mouth disease caused by coxsackievirus A6, Thailand, 2012. Emerg Infect Dis. 2013;19:641-643.
- Flett K, Youngster I, Huang J, et al. Hand, foot, and mouth disease caused by coxsackievirus A6. Emerg Infect Dis. 2012;18:1702-1704.
- Centers for Disease Control and Prevention. Notes from the field: severe hand, foot, and mouth disease associated with coxsackievirus A6—Alabama, Connecticut, California, and Nevada, November 2011-February 2012. MMWR Morb Mortal Wkly Rep. 2012;61:213-214.
- Blomqvist S, Klemola P, Kaijalainen S, et al. Co-circulation of coxsackieviruses A6 and A10 in hand, foot and mouth disease outbreak in Finland. J Clin Virol. 2010;48:49-54.
- Osterback R, Vuorinen T, Linna M, et al. Coxsackievirus A6 and hand, foot, and mouth disease, Finland. Emerg Infect Dis. 2009;15:1485-1488.
- Zeng H, Lu J, Zheng H, et al. The epidemiological study of coxsackievirus A6 revealing hand, foot and mouth disease epidemic patterns in Guandong, China. Sci Rep. 2015;5:10550.
- Mirand A, Henquell C, Archimbaud C, et al. Outbreak of hand, foot and mouth disease/herpangina associated with coxsackievirus A6 andA10 infections in 2010, France: a large citywide, prospective observational study. Clin Microbiol Infect. 2012;18:E110-E118.
- Wei SH, Huang YP, Liu MC, et al. An outbreak of coxsackievirus A6 hand, foot, and mouth disease associated with onychomadesis in Taiwan, 2010. BMC Infect Dis. 2011;11:346.
- Fujimoto T, Iizuka S, Enomoto M, et al. Hand, foot, and mouth disease caused by coxsackievirus A6, Japan, 2011. Emerg Infect Dis. 2012;18:337-339.
- Bracho MA, Gonzalez-Candelas F, Valero A, et al. Enterovirus co-infections and onychomadesis after hand, foot, and mouth disease, Spain, 2008. Emerg Infect Dis. 2011;17:2223-2231.
- Gopalkrishna V, Patil PR, Patil GP, et al. Circulation of multiple enterovirus serotypes causing hand, foot and mouth disease in India. J Med Microbiol. 2012;61:420-425.
- Lo SH, Huang YC, Huang CG, et al. Clinical and epidemiologic features of coxsackievirus A6 infection in children in northern Taiwan between 2004 and 2009. J Microbiol Immunol Infect. 2011;44:252-257.
- Lu QB, Zhang XA, Wo Y, et al. Circulation of coxsackievirus A10 and A6 in hand-foot-mouth disease in China, 2009-2011. PLoS One. 2012;7:E52073.
- Wu Y, Yeo A, Phoon MC, et al. The largest outbreak of hand; foot and mouth disease in Singapore in 2008: the role of enterovirus 71 and coxsackievirus A strains. Int J Infect Dis. 2010;14:E1076-E1081.
- Ventarola D, Bordone L, Silverberg N. Update on hand-foot-and-mouth disease. Clin Dermatol. 2015;33:340-346.
- Li Y, Chang Z, Wu P, et al. Emerging enteroviruses causing hand, foot and mouth disease, China. 2010-2016. Emerg Infect Dis. 2018;24:1902-1906.
- Tan X, Li L, Zhang B, et al. Molecular epidemiology of coxsackievirus A6 associated with outbreaks of hand, foot, and mouth disease in Tianjin, China, in 2013. Arch Virol. 2015;160:1097-1104.
- Li Y, Bao H, Zhang X, et al. Epidemiological and genetic analysis concerning the non-enterovirus 71 and non-coxsackievirus A16 causative agents related to hand, foot and mouth disease in Anyang City, Henan Province, China, from 2011 to 2015. J Med Virol. 2017;89:1749-1758.
- Guan H, Wang J, Wang C, et al. Etiology of multiple non-EV71 and non-CVA16 enteroviruses associated with hand, foot, and mouth disease in Jinan, China, 2009-2013. PLoS One. 2015;10:E0142733.
- Cabrerizo M, Tarrago´ D, Muñoz-Almagro C, et al. Mollecular epidemiology of enterovirus 71, coxsackievirus A16 and A6 associated with hand, foot and mouth disease in Spain. Clin Microbiol Infect. 2014;20:O150-O156.
- Lønnberg A, Elberling J, Fischer T, et al. Two cases of hand, foot, and mouth disease involving the scalp. Acta Derm Venereol. 2013;93:467-468.
- Lott JP, Liu K, Landry ML, et al. Atypical hand-foot-and-mouth disease associated with coxsackievirus A6 infection. J Am Acad Dermatol. 2013;69:736-741.
- Kaminska K, Martinetti G, Lucchini R, et al. Coxsackievirus A6 and hand, foot and mouth disease: three case reports of familial child-to-immunocompetent adult transmission and a literature review. Case Rep Dermatol. 2013;5:203-209.
- Shin JU, Oh SH, Lee JH. A case of hand-foot-mouth disease in an immunocompetent adult. Ann Dermatol. 2010;22:216-218.
- Osterback R, Vuorinen T, Linna M, et al. Coxsackievirus A6 and hand, foot, and mouth disease, Finland. Emerg Infect Dis. 2009;15:1485-1488.
- Feder HM, Bennett N, Modlin JF. Atypical hand, foot, and mouth disease: a vesiculobullous eruption caused by coxsackie virus A6. Lancet Infect Dis. 2014;14:83-86.
- Wei SH, Huang YP, Liu MC, et al. An outbreak of coxsackievirus A6 hand, foot, and mouth disease associated with onychomadesis in Taiwan, 2010. BMC Infect Dis. 2011;11:346.
- Kim M, Kim B, Byun S, et al. Beau’s lines and onychomadesis after hand-foot-mouth disease. Clin Pediatr Dermatol. 2015;1:1.
- Mathes EF, Oza V, Frieden IJ, et al. “Eczema coxsackium” and unusual cutaneous findings in an enterovirus outbreak. Pediatrics. 2013;132:E149-E157.
- Lynch M, Sears A, Cookson H, et al. Disseminated coxsackievirus A6 affecting children with atopic dermatitis. Clin Exp Dermatol. 2015;40:525-528.
- Laga A, Shroba S, Hanna J. Atypical hand, foot and mouth disease in adults associated with coxsackievirus A6: a clinicopathologic study. J Cutan Pathol. 2016;43:940-945.
- Schmidt NJ, Ho HH, Lennette EH. Propagation and isolation of group A coxsackieviruses in RD cells. J Clin Microbiol. 1975;2:183-185.
- Oberste MS, Penaranda S, Rogers SL, et al. Comparative evaluation of Taqman real-time PCR and semi-nested VP1 PCR for detection of enteroviruses in clinical specimens. J Clin Virol. 2010;49:73-74.
- Lee MK, Chan PK, Ho II, et al. Enterovirus infection among patients admitted to hospital in Hong Kong in 2010: epidemiology, clinical characteristics, and importance of molecular diagnosis. J Med Virol. 2013;85:1811-1817.
- Yee PTI, Laa Poh C. Impact of genetic changes, pathogenicity and antigenicity on enterovirus A71 vaccine development. Virology. 2017;506:121-129.
Practice Points
- Coxsackievirus A6 is an increasingly more common cause of hand-foot-and-mouth disease (HFMD), often with atypical presentation, more severe disease, and association with HFMD in adults.
- Coxsackievirus A6 has become a major cause of HFMD outbreak in the United States and worldwide.