User login
The Arthroscopic Superior Capsular Reconstruction
Rotator cuff tears are very common, and 250,000 to 500,000 rotator cuff repairs are performed in the United States each year.1,2 In most cases, a complete repair of even large or massive tears can be achieved. However, a subset of patients exist in whom the glenohumeral joint has minimal degenerative changes and the rotator cuff tendon is either irreparable or very poor quality and unlikely to heal (ie, failed previous cuff repair). Some authors have advocated for reverse shoulder arthroplasty (RSA) in these patients despite the lack of glenohumeral arthritis. However, due to the permanent destruction of the glenohumeral articular surfaces, complication rates, and concerns about implant longevity with RSA, we believe the superior capsular reconstruction (SCR) is a viable alternative in patients in whom joint preservation is appropriate based on age limitations and/or activity requirements.3
The SCR was first described by Mihata and colleagues4 as a means to reconstruct the superior capsule in shoulders with large, irreparable posterosuperior rotator cuff tears. Originally described using a fascia lata autograft, our technique has been adapted to incorporate a dermal allograft, which limits donor site morbidity and operative time. In most cases, the dermal allograft is fixed to the normal anatomic attachments of the superior glenoid just medial to the superior labrum, laterally to the greater tuberosity, and posteriorly with side-to-side sutures to the remaining rotator cuff. If there is a robust band of “comma” tissue anteriorly, we fix the anterior margin of the dermal graft to this with side-to-side sutures. The comma tissue represents the medial sling of the biceps tendon and connects the upper subscapularis tendon to the anterior supraspinatus. In most cases, this tissue is intact after repair of the subscapularis tendon.
Technique
The patient is positioned in either the lateral decubitus or beach chair position. The arm is positioned in 20° to 30° of abduction and 20° to 30° of forward flexion. A diagnostic arthroscopy is performed through a posterior glenohumeral viewing portal. The subscapularis is visualized and repaired if torn. A biceps tenodesis is performed in most cases, as there is often a tear of the subscapularis, tear or instability of the biceps tendon, and/or a compromised attachment of the biceps root.
Attention is turned to the subacromial space. Posterior viewing and lateral working portals are established. A 10-mm flexible cannula (PassPort; Arthrex) is placed in the lateral portal to aid with suture management and graft passage. A limited subacromial decompression is performed that preserves the coracoacromial arch. The rotator cuff is carefully dissected and freed from the internal deltoid fascia. The scapular spine is identified to visualize the raphé between the supraspinatus and infraspinatus. The infraspinatus is mobilized and repaired as much as possible.
If we think that the tear might be reparable by gaining added excursion from a posterior interval slide, or if it is clearly not reparable but the remaining rim of rotator cuff obscures clear visualization of the superior glenoid, we perform a posterior interval slide. If the additional excursion that is achieved by the posterior slide is adequate for a complete repair, we proceed with the repair. However, if the tear is not reparable even after the posterior interval slide, we have found that the exposure and preparation of the superior glenoid is greatly improved after the posterior slide. After fixation of the dermal graft, we typically perform a partial side-to-side repair of the supraspinatus to the infraspinatus over the top of the graft.
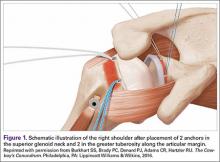
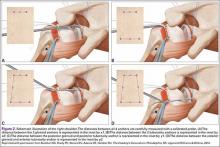
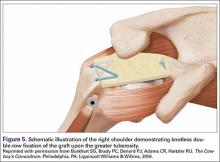
The bone beds of the greater tuberosity and just medial to the superior glenoid labrum are prepared with a shaver and motorized burr. Two anchors (3.0-mm BioComposite SutureTak; Arthrex) are placed in the superior glenoid neck at about the 10 o’clock and 2 o’clock positions approximately 5 mm medial to the superior labrum. Note: the placement medial to the labrum is chosen because this is the normal origin of the superior capsule and because of the angle of approach, these percutaneous portals are often more medial than typical portals for placing anchors during SLAP (superior labral anterior to posterior) repair. Next, 2 threaded anchors (4.75-mm BioComposite SwiveLock; Arthrex) preloaded with suture tape are placed in the greater tuberosity along the articular margin (Figure 1). However, if a biceps tenodesis with an interference screw is placed at the top of the bicipital groove, this anchor preloaded with suture tape can also serve as the anteromedial anchor in the greater tuberosity footprint. The distances between all 4 anchors are carefully measured with a calibrated probe (Figures 2A-2D).
We use a 3.0-mm acellular dermal allograft (ArthroFlex; Arthrex) to reconstruct the superior capsule. The positions of the 4 anchors are carefully marked on the dermal allograft. We routinely add an additional 5 mm of tissue to the medial, anterior, and posterior margins to decrease the risk of suture cut out. An additional 10 mm of tissue is added laterally to cover the greater tuberosity. The final contoured graft is typically trapezoidal in shape.
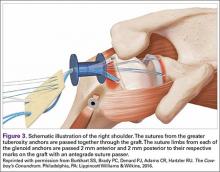
The sutures from the 4 anchors are then sequentially retrieved through the lateral cannula. The sutures from the greater tuberosity anchors are passed through their respective holes in the graft. However, the suture limbs from each of the glenoid anchors are individually passed 2 mm anterior and 2 mm posterior to their respective marks on the graft with an antegrade suture passer (Figure 3). It is important to have an assistant apply tension to each of the sutures after they are passed through the graft to decrease the chance of crossing and tangling the sutures.
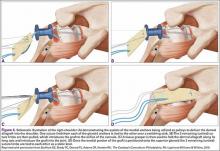
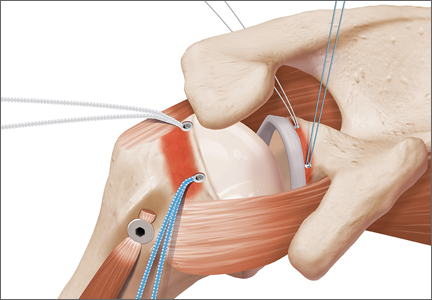
The eyelets of the medial anchors are utilized as pulleys to deliver the dermal allograft into the shoulder. One suture limb from each of the glenoid anchors is tied to the other over a switching stick (Figure 4A). The 2 remaining (untied) suture limbs are then pulled, which introduces the graft to the orifice of the cannula (Figure 4B). A tissue grasper is then used to fold the dermal allograft along its long axis and introduce the graft into the joint (Figure 4C). Once the medial portion of the graft is positioned onto the superior glenoid the 2 remaining (untied) suture limbs are tied to each other as a static knot in the subacromial space (Figure 4D).
The redundancy in the suture tapes can be removed by sequentially sliding a retriever down each suture and tensioning the suture as the nose of the instrument pushes the dermal graft down to the tuberosity bone bed. The suture tapes are crisscrossed and secured laterally with 2 additional knotless threaded anchors (Figure 5). One may also place cinch stitches at the anterolateral and posterolateral corners of the graft that are incorporated into the lateral anchors. These sutures can be useful for pulling the graft back out of the subacromial space in the event of any suture tangles, and can be used for controlling the lateral aspect of the graft during lateral anchor placement.
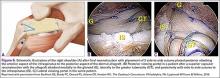
At this point in the procedure, additional glenoid anchors can be placed both anterior and posterior to the superior glenoid anchors if additional glenoid fixation is desired. Finally, 2 to 3 side-to-side sutures are placed posteriorly attaching the anterior aspect of the infraspinatus to the posterior aspect of the dermal allograft (Figures 6A-6C). If rotator interval tissue (comma tissue) is present, anterior side-to-side sutures may be placed. However, we do not recommend placing anterior side-to-side sutures directly from the dermal allograft to the subscapularis as this may deform the graft, over- constrain the shoulder, and restrict motion.
Discussion
Reconstruction of the superior capsule has been shown to restore the normal restraint to superior translation of the humeral head and reestablish a stable fulcrum at the glenohumeral joint.5 It should be mentioned that we do not perform the SCR in patients with advanced glenohumeral arthritis. The short-term results of this novel procedure have been encouraging, including our own series of patients, in which most patients have had a significant reduction in pain, improvement in function, and very few complications (P. J. Denard, MD, S. S. Burkhart, MD, P. C. Brady, MD, J. Tokish, MD, C. R. Adams, MD, unpublished data, May 2016).
The early success of this procedure suggests that a robust superior capsule is necessary, in addition to functional muscle-tendon units, to restore the stable fulcrum and force couples that are necessary for normal shoulder function. Perhaps we have not paid enough attention to the integrity of the superior capsule in the past. In cases of revision cuff repair, we pay special attention to the quality of the capsular layer deep to the cuff tendon. If the capsule is poor quality, we sometimes reconstruct the capsule with a dermal allograft (SCR) and then do a rotator cuff repair (partial or complete) over the top of the SCR to maintain the normal anatomic deep to superficial layering of the capsule and rotator cuff.
We are very conservative with our postoperative rehabilitation program after a SCR. We know that the rate of stiffness with a conservative program after an arthroscopic rotator cuff repair, even in the revision setting, is very low.6 Furthermore, both basic science on healing of soft tissue to bone and radiographic analysis of healing after postoperative rotator cuff repairs support a slow rehabilitation program.7,8 A canine model specifically evaluating acellular dermal allografts in the shoulder suggests that these grafts undergo significant remodeling and become weaker before they get stronger.9 We would rather err on the side of healing of the SCR with potentially a slight increase in the rate of shoulder stiffness than to regain early motion at the expense of graft failure. Therefore, we have the patient wear a sling with no shoulder motion for 6 weeks. Passive motion is started at 6 weeks postoperative and strengthening is delayed until 12 to 16 weeks postoperative.
1. Orr SB, Chainani A, Hippensteel KJ, et al. Aligned multilayered electrospun scaffolds for rotator cuff tendon tissue engineering. Acta Biomater. 2015;24:117-126.
2. Austin L, Black EM, Lombardi NJ, Pepe MD, Lazarus M. Arthroscopic transosseous rotator cuff repair. A prospective study on cost savings, surgical time, and outcomes. Ortho J Sports Med. 2015;3(2 Suppl). doi:10.1177/2325967115S00156.
3. Denard PJ, Lädermann A, Jiwani AZ, Burkhart SS. Functional outcome after arthroscopic repair of massive rotator cuff tears in individuals with pseudoparalysis. Arthroscopy. 2012;28(9):1214-1219.
4. Mihata T, Lee TQ, Watanabe C, et al. Clinical results of arthroscopic superior capsule reconstruction for irreparable rotator cuff tears. Arthroscopy. 2013;29(3):459-470.
5. Mihata T, McGarry MH, Pirolo JM, Kinoshita M, Lee TQ. Superior capsule reconstruction to restore superior stability in irreparable rotator cuff tears: a biomechanical cadaveric study. Am J Sports Med. 2012;40(10):2248-2255.
6. Huberty DP, Schoolfield JD, Brady PC, Vadala AP, Arrigoni P, Burkhart SS. Incidence and treatment of postoperative stiffness following arthroscopic rotator cuff repair. Arthroscopy. 2009;25(8):880-890.
7. Sonnabend DH, Howlett CR, Young AA. Histological evaluation of repair of the rotator cuff in a primate model. J Bone Joint Surg Br. 2010;92(4):586-594.
8. Lee BG, Cho NS, Rhee YG. Effect of two rehabilitation protocols on range of motion and healing rates after arthroscopic rotator cuff repair: aggressive versus limited early passive exercises. Arthroscopy. 2012;28(1):34-42.
9. Adams JE, Zobitz ME, Reach JS Jr, An KN, Steinmann SP. Rotator cuff repair using an acellular dermal matrix graft: an in vivo study in a canine model. Arthroscopy. 2006;22(7):700-709.
Rotator cuff tears are very common, and 250,000 to 500,000 rotator cuff repairs are performed in the United States each year.1,2 In most cases, a complete repair of even large or massive tears can be achieved. However, a subset of patients exist in whom the glenohumeral joint has minimal degenerative changes and the rotator cuff tendon is either irreparable or very poor quality and unlikely to heal (ie, failed previous cuff repair). Some authors have advocated for reverse shoulder arthroplasty (RSA) in these patients despite the lack of glenohumeral arthritis. However, due to the permanent destruction of the glenohumeral articular surfaces, complication rates, and concerns about implant longevity with RSA, we believe the superior capsular reconstruction (SCR) is a viable alternative in patients in whom joint preservation is appropriate based on age limitations and/or activity requirements.3
The SCR was first described by Mihata and colleagues4 as a means to reconstruct the superior capsule in shoulders with large, irreparable posterosuperior rotator cuff tears. Originally described using a fascia lata autograft, our technique has been adapted to incorporate a dermal allograft, which limits donor site morbidity and operative time. In most cases, the dermal allograft is fixed to the normal anatomic attachments of the superior glenoid just medial to the superior labrum, laterally to the greater tuberosity, and posteriorly with side-to-side sutures to the remaining rotator cuff. If there is a robust band of “comma” tissue anteriorly, we fix the anterior margin of the dermal graft to this with side-to-side sutures. The comma tissue represents the medial sling of the biceps tendon and connects the upper subscapularis tendon to the anterior supraspinatus. In most cases, this tissue is intact after repair of the subscapularis tendon.
Technique
The patient is positioned in either the lateral decubitus or beach chair position. The arm is positioned in 20° to 30° of abduction and 20° to 30° of forward flexion. A diagnostic arthroscopy is performed through a posterior glenohumeral viewing portal. The subscapularis is visualized and repaired if torn. A biceps tenodesis is performed in most cases, as there is often a tear of the subscapularis, tear or instability of the biceps tendon, and/or a compromised attachment of the biceps root.
Attention is turned to the subacromial space. Posterior viewing and lateral working portals are established. A 10-mm flexible cannula (PassPort; Arthrex) is placed in the lateral portal to aid with suture management and graft passage. A limited subacromial decompression is performed that preserves the coracoacromial arch. The rotator cuff is carefully dissected and freed from the internal deltoid fascia. The scapular spine is identified to visualize the raphé between the supraspinatus and infraspinatus. The infraspinatus is mobilized and repaired as much as possible.
If we think that the tear might be reparable by gaining added excursion from a posterior interval slide, or if it is clearly not reparable but the remaining rim of rotator cuff obscures clear visualization of the superior glenoid, we perform a posterior interval slide. If the additional excursion that is achieved by the posterior slide is adequate for a complete repair, we proceed with the repair. However, if the tear is not reparable even after the posterior interval slide, we have found that the exposure and preparation of the superior glenoid is greatly improved after the posterior slide. After fixation of the dermal graft, we typically perform a partial side-to-side repair of the supraspinatus to the infraspinatus over the top of the graft.
The bone beds of the greater tuberosity and just medial to the superior glenoid labrum are prepared with a shaver and motorized burr. Two anchors (3.0-mm BioComposite SutureTak; Arthrex) are placed in the superior glenoid neck at about the 10 o’clock and 2 o’clock positions approximately 5 mm medial to the superior labrum. Note: the placement medial to the labrum is chosen because this is the normal origin of the superior capsule and because of the angle of approach, these percutaneous portals are often more medial than typical portals for placing anchors during SLAP (superior labral anterior to posterior) repair. Next, 2 threaded anchors (4.75-mm BioComposite SwiveLock; Arthrex) preloaded with suture tape are placed in the greater tuberosity along the articular margin (Figure 1). However, if a biceps tenodesis with an interference screw is placed at the top of the bicipital groove, this anchor preloaded with suture tape can also serve as the anteromedial anchor in the greater tuberosity footprint. The distances between all 4 anchors are carefully measured with a calibrated probe (Figures 2A-2D).
We use a 3.0-mm acellular dermal allograft (ArthroFlex; Arthrex) to reconstruct the superior capsule. The positions of the 4 anchors are carefully marked on the dermal allograft. We routinely add an additional 5 mm of tissue to the medial, anterior, and posterior margins to decrease the risk of suture cut out. An additional 10 mm of tissue is added laterally to cover the greater tuberosity. The final contoured graft is typically trapezoidal in shape.
The sutures from the 4 anchors are then sequentially retrieved through the lateral cannula. The sutures from the greater tuberosity anchors are passed through their respective holes in the graft. However, the suture limbs from each of the glenoid anchors are individually passed 2 mm anterior and 2 mm posterior to their respective marks on the graft with an antegrade suture passer (Figure 3). It is important to have an assistant apply tension to each of the sutures after they are passed through the graft to decrease the chance of crossing and tangling the sutures.
The eyelets of the medial anchors are utilized as pulleys to deliver the dermal allograft into the shoulder. One suture limb from each of the glenoid anchors is tied to the other over a switching stick (Figure 4A). The 2 remaining (untied) suture limbs are then pulled, which introduces the graft to the orifice of the cannula (Figure 4B). A tissue grasper is then used to fold the dermal allograft along its long axis and introduce the graft into the joint (Figure 4C). Once the medial portion of the graft is positioned onto the superior glenoid the 2 remaining (untied) suture limbs are tied to each other as a static knot in the subacromial space (Figure 4D).
The redundancy in the suture tapes can be removed by sequentially sliding a retriever down each suture and tensioning the suture as the nose of the instrument pushes the dermal graft down to the tuberosity bone bed. The suture tapes are crisscrossed and secured laterally with 2 additional knotless threaded anchors (Figure 5). One may also place cinch stitches at the anterolateral and posterolateral corners of the graft that are incorporated into the lateral anchors. These sutures can be useful for pulling the graft back out of the subacromial space in the event of any suture tangles, and can be used for controlling the lateral aspect of the graft during lateral anchor placement.
At this point in the procedure, additional glenoid anchors can be placed both anterior and posterior to the superior glenoid anchors if additional glenoid fixation is desired. Finally, 2 to 3 side-to-side sutures are placed posteriorly attaching the anterior aspect of the infraspinatus to the posterior aspect of the dermal allograft (Figures 6A-6C). If rotator interval tissue (comma tissue) is present, anterior side-to-side sutures may be placed. However, we do not recommend placing anterior side-to-side sutures directly from the dermal allograft to the subscapularis as this may deform the graft, over- constrain the shoulder, and restrict motion.
Discussion
Reconstruction of the superior capsule has been shown to restore the normal restraint to superior translation of the humeral head and reestablish a stable fulcrum at the glenohumeral joint.5 It should be mentioned that we do not perform the SCR in patients with advanced glenohumeral arthritis. The short-term results of this novel procedure have been encouraging, including our own series of patients, in which most patients have had a significant reduction in pain, improvement in function, and very few complications (P. J. Denard, MD, S. S. Burkhart, MD, P. C. Brady, MD, J. Tokish, MD, C. R. Adams, MD, unpublished data, May 2016).
The early success of this procedure suggests that a robust superior capsule is necessary, in addition to functional muscle-tendon units, to restore the stable fulcrum and force couples that are necessary for normal shoulder function. Perhaps we have not paid enough attention to the integrity of the superior capsule in the past. In cases of revision cuff repair, we pay special attention to the quality of the capsular layer deep to the cuff tendon. If the capsule is poor quality, we sometimes reconstruct the capsule with a dermal allograft (SCR) and then do a rotator cuff repair (partial or complete) over the top of the SCR to maintain the normal anatomic deep to superficial layering of the capsule and rotator cuff.
We are very conservative with our postoperative rehabilitation program after a SCR. We know that the rate of stiffness with a conservative program after an arthroscopic rotator cuff repair, even in the revision setting, is very low.6 Furthermore, both basic science on healing of soft tissue to bone and radiographic analysis of healing after postoperative rotator cuff repairs support a slow rehabilitation program.7,8 A canine model specifically evaluating acellular dermal allografts in the shoulder suggests that these grafts undergo significant remodeling and become weaker before they get stronger.9 We would rather err on the side of healing of the SCR with potentially a slight increase in the rate of shoulder stiffness than to regain early motion at the expense of graft failure. Therefore, we have the patient wear a sling with no shoulder motion for 6 weeks. Passive motion is started at 6 weeks postoperative and strengthening is delayed until 12 to 16 weeks postoperative.
Rotator cuff tears are very common, and 250,000 to 500,000 rotator cuff repairs are performed in the United States each year.1,2 In most cases, a complete repair of even large or massive tears can be achieved. However, a subset of patients exist in whom the glenohumeral joint has minimal degenerative changes and the rotator cuff tendon is either irreparable or very poor quality and unlikely to heal (ie, failed previous cuff repair). Some authors have advocated for reverse shoulder arthroplasty (RSA) in these patients despite the lack of glenohumeral arthritis. However, due to the permanent destruction of the glenohumeral articular surfaces, complication rates, and concerns about implant longevity with RSA, we believe the superior capsular reconstruction (SCR) is a viable alternative in patients in whom joint preservation is appropriate based on age limitations and/or activity requirements.3
The SCR was first described by Mihata and colleagues4 as a means to reconstruct the superior capsule in shoulders with large, irreparable posterosuperior rotator cuff tears. Originally described using a fascia lata autograft, our technique has been adapted to incorporate a dermal allograft, which limits donor site morbidity and operative time. In most cases, the dermal allograft is fixed to the normal anatomic attachments of the superior glenoid just medial to the superior labrum, laterally to the greater tuberosity, and posteriorly with side-to-side sutures to the remaining rotator cuff. If there is a robust band of “comma” tissue anteriorly, we fix the anterior margin of the dermal graft to this with side-to-side sutures. The comma tissue represents the medial sling of the biceps tendon and connects the upper subscapularis tendon to the anterior supraspinatus. In most cases, this tissue is intact after repair of the subscapularis tendon.
Technique
The patient is positioned in either the lateral decubitus or beach chair position. The arm is positioned in 20° to 30° of abduction and 20° to 30° of forward flexion. A diagnostic arthroscopy is performed through a posterior glenohumeral viewing portal. The subscapularis is visualized and repaired if torn. A biceps tenodesis is performed in most cases, as there is often a tear of the subscapularis, tear or instability of the biceps tendon, and/or a compromised attachment of the biceps root.
Attention is turned to the subacromial space. Posterior viewing and lateral working portals are established. A 10-mm flexible cannula (PassPort; Arthrex) is placed in the lateral portal to aid with suture management and graft passage. A limited subacromial decompression is performed that preserves the coracoacromial arch. The rotator cuff is carefully dissected and freed from the internal deltoid fascia. The scapular spine is identified to visualize the raphé between the supraspinatus and infraspinatus. The infraspinatus is mobilized and repaired as much as possible.
If we think that the tear might be reparable by gaining added excursion from a posterior interval slide, or if it is clearly not reparable but the remaining rim of rotator cuff obscures clear visualization of the superior glenoid, we perform a posterior interval slide. If the additional excursion that is achieved by the posterior slide is adequate for a complete repair, we proceed with the repair. However, if the tear is not reparable even after the posterior interval slide, we have found that the exposure and preparation of the superior glenoid is greatly improved after the posterior slide. After fixation of the dermal graft, we typically perform a partial side-to-side repair of the supraspinatus to the infraspinatus over the top of the graft.
The bone beds of the greater tuberosity and just medial to the superior glenoid labrum are prepared with a shaver and motorized burr. Two anchors (3.0-mm BioComposite SutureTak; Arthrex) are placed in the superior glenoid neck at about the 10 o’clock and 2 o’clock positions approximately 5 mm medial to the superior labrum. Note: the placement medial to the labrum is chosen because this is the normal origin of the superior capsule and because of the angle of approach, these percutaneous portals are often more medial than typical portals for placing anchors during SLAP (superior labral anterior to posterior) repair. Next, 2 threaded anchors (4.75-mm BioComposite SwiveLock; Arthrex) preloaded with suture tape are placed in the greater tuberosity along the articular margin (Figure 1). However, if a biceps tenodesis with an interference screw is placed at the top of the bicipital groove, this anchor preloaded with suture tape can also serve as the anteromedial anchor in the greater tuberosity footprint. The distances between all 4 anchors are carefully measured with a calibrated probe (Figures 2A-2D).
We use a 3.0-mm acellular dermal allograft (ArthroFlex; Arthrex) to reconstruct the superior capsule. The positions of the 4 anchors are carefully marked on the dermal allograft. We routinely add an additional 5 mm of tissue to the medial, anterior, and posterior margins to decrease the risk of suture cut out. An additional 10 mm of tissue is added laterally to cover the greater tuberosity. The final contoured graft is typically trapezoidal in shape.
The sutures from the 4 anchors are then sequentially retrieved through the lateral cannula. The sutures from the greater tuberosity anchors are passed through their respective holes in the graft. However, the suture limbs from each of the glenoid anchors are individually passed 2 mm anterior and 2 mm posterior to their respective marks on the graft with an antegrade suture passer (Figure 3). It is important to have an assistant apply tension to each of the sutures after they are passed through the graft to decrease the chance of crossing and tangling the sutures.
The eyelets of the medial anchors are utilized as pulleys to deliver the dermal allograft into the shoulder. One suture limb from each of the glenoid anchors is tied to the other over a switching stick (Figure 4A). The 2 remaining (untied) suture limbs are then pulled, which introduces the graft to the orifice of the cannula (Figure 4B). A tissue grasper is then used to fold the dermal allograft along its long axis and introduce the graft into the joint (Figure 4C). Once the medial portion of the graft is positioned onto the superior glenoid the 2 remaining (untied) suture limbs are tied to each other as a static knot in the subacromial space (Figure 4D).
The redundancy in the suture tapes can be removed by sequentially sliding a retriever down each suture and tensioning the suture as the nose of the instrument pushes the dermal graft down to the tuberosity bone bed. The suture tapes are crisscrossed and secured laterally with 2 additional knotless threaded anchors (Figure 5). One may also place cinch stitches at the anterolateral and posterolateral corners of the graft that are incorporated into the lateral anchors. These sutures can be useful for pulling the graft back out of the subacromial space in the event of any suture tangles, and can be used for controlling the lateral aspect of the graft during lateral anchor placement.
At this point in the procedure, additional glenoid anchors can be placed both anterior and posterior to the superior glenoid anchors if additional glenoid fixation is desired. Finally, 2 to 3 side-to-side sutures are placed posteriorly attaching the anterior aspect of the infraspinatus to the posterior aspect of the dermal allograft (Figures 6A-6C). If rotator interval tissue (comma tissue) is present, anterior side-to-side sutures may be placed. However, we do not recommend placing anterior side-to-side sutures directly from the dermal allograft to the subscapularis as this may deform the graft, over- constrain the shoulder, and restrict motion.
Discussion
Reconstruction of the superior capsule has been shown to restore the normal restraint to superior translation of the humeral head and reestablish a stable fulcrum at the glenohumeral joint.5 It should be mentioned that we do not perform the SCR in patients with advanced glenohumeral arthritis. The short-term results of this novel procedure have been encouraging, including our own series of patients, in which most patients have had a significant reduction in pain, improvement in function, and very few complications (P. J. Denard, MD, S. S. Burkhart, MD, P. C. Brady, MD, J. Tokish, MD, C. R. Adams, MD, unpublished data, May 2016).
The early success of this procedure suggests that a robust superior capsule is necessary, in addition to functional muscle-tendon units, to restore the stable fulcrum and force couples that are necessary for normal shoulder function. Perhaps we have not paid enough attention to the integrity of the superior capsule in the past. In cases of revision cuff repair, we pay special attention to the quality of the capsular layer deep to the cuff tendon. If the capsule is poor quality, we sometimes reconstruct the capsule with a dermal allograft (SCR) and then do a rotator cuff repair (partial or complete) over the top of the SCR to maintain the normal anatomic deep to superficial layering of the capsule and rotator cuff.
We are very conservative with our postoperative rehabilitation program after a SCR. We know that the rate of stiffness with a conservative program after an arthroscopic rotator cuff repair, even in the revision setting, is very low.6 Furthermore, both basic science on healing of soft tissue to bone and radiographic analysis of healing after postoperative rotator cuff repairs support a slow rehabilitation program.7,8 A canine model specifically evaluating acellular dermal allografts in the shoulder suggests that these grafts undergo significant remodeling and become weaker before they get stronger.9 We would rather err on the side of healing of the SCR with potentially a slight increase in the rate of shoulder stiffness than to regain early motion at the expense of graft failure. Therefore, we have the patient wear a sling with no shoulder motion for 6 weeks. Passive motion is started at 6 weeks postoperative and strengthening is delayed until 12 to 16 weeks postoperative.
1. Orr SB, Chainani A, Hippensteel KJ, et al. Aligned multilayered electrospun scaffolds for rotator cuff tendon tissue engineering. Acta Biomater. 2015;24:117-126.
2. Austin L, Black EM, Lombardi NJ, Pepe MD, Lazarus M. Arthroscopic transosseous rotator cuff repair. A prospective study on cost savings, surgical time, and outcomes. Ortho J Sports Med. 2015;3(2 Suppl). doi:10.1177/2325967115S00156.
3. Denard PJ, Lädermann A, Jiwani AZ, Burkhart SS. Functional outcome after arthroscopic repair of massive rotator cuff tears in individuals with pseudoparalysis. Arthroscopy. 2012;28(9):1214-1219.
4. Mihata T, Lee TQ, Watanabe C, et al. Clinical results of arthroscopic superior capsule reconstruction for irreparable rotator cuff tears. Arthroscopy. 2013;29(3):459-470.
5. Mihata T, McGarry MH, Pirolo JM, Kinoshita M, Lee TQ. Superior capsule reconstruction to restore superior stability in irreparable rotator cuff tears: a biomechanical cadaveric study. Am J Sports Med. 2012;40(10):2248-2255.
6. Huberty DP, Schoolfield JD, Brady PC, Vadala AP, Arrigoni P, Burkhart SS. Incidence and treatment of postoperative stiffness following arthroscopic rotator cuff repair. Arthroscopy. 2009;25(8):880-890.
7. Sonnabend DH, Howlett CR, Young AA. Histological evaluation of repair of the rotator cuff in a primate model. J Bone Joint Surg Br. 2010;92(4):586-594.
8. Lee BG, Cho NS, Rhee YG. Effect of two rehabilitation protocols on range of motion and healing rates after arthroscopic rotator cuff repair: aggressive versus limited early passive exercises. Arthroscopy. 2012;28(1):34-42.
9. Adams JE, Zobitz ME, Reach JS Jr, An KN, Steinmann SP. Rotator cuff repair using an acellular dermal matrix graft: an in vivo study in a canine model. Arthroscopy. 2006;22(7):700-709.
1. Orr SB, Chainani A, Hippensteel KJ, et al. Aligned multilayered electrospun scaffolds for rotator cuff tendon tissue engineering. Acta Biomater. 2015;24:117-126.
2. Austin L, Black EM, Lombardi NJ, Pepe MD, Lazarus M. Arthroscopic transosseous rotator cuff repair. A prospective study on cost savings, surgical time, and outcomes. Ortho J Sports Med. 2015;3(2 Suppl). doi:10.1177/2325967115S00156.
3. Denard PJ, Lädermann A, Jiwani AZ, Burkhart SS. Functional outcome after arthroscopic repair of massive rotator cuff tears in individuals with pseudoparalysis. Arthroscopy. 2012;28(9):1214-1219.
4. Mihata T, Lee TQ, Watanabe C, et al. Clinical results of arthroscopic superior capsule reconstruction for irreparable rotator cuff tears. Arthroscopy. 2013;29(3):459-470.
5. Mihata T, McGarry MH, Pirolo JM, Kinoshita M, Lee TQ. Superior capsule reconstruction to restore superior stability in irreparable rotator cuff tears: a biomechanical cadaveric study. Am J Sports Med. 2012;40(10):2248-2255.
6. Huberty DP, Schoolfield JD, Brady PC, Vadala AP, Arrigoni P, Burkhart SS. Incidence and treatment of postoperative stiffness following arthroscopic rotator cuff repair. Arthroscopy. 2009;25(8):880-890.
7. Sonnabend DH, Howlett CR, Young AA. Histological evaluation of repair of the rotator cuff in a primate model. J Bone Joint Surg Br. 2010;92(4):586-594.
8. Lee BG, Cho NS, Rhee YG. Effect of two rehabilitation protocols on range of motion and healing rates after arthroscopic rotator cuff repair: aggressive versus limited early passive exercises. Arthroscopy. 2012;28(1):34-42.
9. Adams JE, Zobitz ME, Reach JS Jr, An KN, Steinmann SP. Rotator cuff repair using an acellular dermal matrix graft: an in vivo study in a canine model. Arthroscopy. 2006;22(7):700-709.
The Burden of Craft in Arthroscopic Rotator Cuff Repair: Where We Have Been and Where We Are Going
I am very honored that Dr. Rob Bell, past president of the American Shoulder and Elbow Surgeons, invited me to give last year’s Neer Lecture. Dr. Bell asked me to specifically address my role in the development of arthroscopic rotator cuff repair and to recount the significant resistance that the early arthroscopic shoulder surgeons faced from the shoulder establishment as we struggled to achieve mainstream acceptance for this new technology. Tasked with such a personal topic, I find myself in a position analogous to that of Winston Churchill at the end of World War II. When a journalist asked him to speculate on how historians would portray his role in the war, he replied without hesitation, “History will be kind to me because I intend to write it.”
So let’s start at the beginning. And for me it makes the most sense to travel back to the year I started my practice: 1981. The world then was very different from today’s world. On January 20, 1981, Ronald Reagan was inaugurated President of the United States. The same day, 52 US hostages in Iran were released after having been held captive for 442 days. In March 1981, Reagan survived an assassination attempt; 3 months earlier, John Lennon had not been so lucky. Lennon’s hit song “Starting Over” garnered the highest musical awards posthumously.
The world of shoulder surgery was also very different in 1981. The arthroscope was the “instrument of the devil,” according to Dr. Rockwood. And shoulder surgery was ruled by the Charlies—Dr. Charles Neer, Dr. Charlie Rockwood, and any other Charlie who felt compelled to marginalize shoulder arthroscopy.
My personal world in the early 1980s was daunting as well. I had just completed my residency at the Mayo Clinic and my sports medicine fellowship in Eugene, Oregon. I had a young son, a new daughter, and a new job with the San Antonio Orthopaedic Group. I had a new house with a 21% mortgage loan and a “new” used car with a 23% car loan.
I was simultaneously energized and intimidated by my new job, where I was doing general orthopedics with a “special interest” in shoulder surgery and sports medicine. I was initially very proud and humbled by the fact that my senior partners had entrusted me with the care of the most difficult shoulder cases within the practice. But that pride got cut down to its appropriate size the day after I had thanked one of my partners, Dr. Lamar Collie, for his confidence in my potential as a shoulder surgeon. Dr. Collie replied matter-of-factly, “Sure … but you need to understand that we always make the new guy the shoulder expert because shoulders never do worth a damn.”
For shoulder arthroscopy, the early 1980s were exciting. Most of us who were scoping shoulders had already been doing knee arthroscopy and were trying to adapt knee instruments to the shoulder. This worked for some simple excisional cases. For example, I recall excising the bucket-handle portion of a type III SLAP (superior labral tear from anterior to posterior) lesion in 1983. In general, however, shoulder problems were different from knee problems and usually involved repair rather than excision of damaged tissues. Therefore, the technology used in knee arthroscopy was often not directly transferable to the shoulder. Furthermore, treatment of the rotator cuff necessitated development of arthroscopic techniques in a virtual space, the subacromial space, and this was an entirely new arthroscopic concept.
Development of Arthroscopic Rotator Cuff Repair
A major mind-expanding turning point for me occurred in 1984 when I attended one of Dr. Jim Esch’s early San Diego shoulder courses. During that course, Dr. Harvard Ellman of Los Angeles demonstrated to me on a cadaver shoulder how he created a virtual subacromial working space that allowed enough visualization for an arthroscopic acromioplasty. At that moment, I knew that arthroscopic rotator cuff repair was just around the corner. Up until then, I had not been able to envision complex extra-articular reconstructive surgery, as all previous arthroscopic surgery had been intra-articular. But now, having realized a virtual working space could always be created, I knew it would be relatively straightforward to develop the portals to approach the cuff as well as the implants and the instruments to repair it. But I also knew that progression to all-arthroscopic repair techniques would have to be stepwise and that the final repair constructs would need to be at least as strong as those of open repair in order to be acceptable. With an undergraduate degree in mechanical engineering, I had a reasonably clear idea of the concepts I wanted to apply to the instrumentation and techniques, though I could never have envisioned how circuitous the route to the end result would be.
First Steps
I sketched out my ideas for arthroscopic suture passers and knot-tying instruments and presented them to a couple of the major arthroscopy companies in the United States, but the companies were not interested. They did not believe arthroscopy would have any meaningful applications in the shoulder. So, I enlisted the services of a local San Antonio aircraft machinist to fabricate instruments for me. By 1987, I was doing arthroscopic side-to-side margin convergence1 cuff repairs for U-shape tears on a regular basis. And I was doing these at the most hostile point in the universe for arthroscopic shoulder surgery: San Antonio, Texas.
Only a few surgeons were doing arthroscopic shoulder surgery in the 1980s and early 1990s, and without exception these surgeons became the leader-pioneers in the new discipline. In general, these were young surgeons who were in private practice and removed from academia and professional organizations, and thus relatively sheltered from the actions of the shoulder rule-makers of the day. They accepted their status as pariahs as they developed their techniques out of the view of mainstream orthopedics. These leaders included Jim Esch, Steve Snyder, Dick Caspari, Lanny Johnson, Gene Wolf, Gary Gartsman, Rob Bell, and Howard Sweeney. We shared our techniques and our ideas with one another, encouraged one another, and generally became good friends.
Thomas Kuhn, in his classic book The Structure of Scientific Revolutions,2 observed that paradigm shifts within a given field were usually achieved by practitioners who were either very young (naïve) or outside the established hierarchy in the field. The surgeons who contributed most to the shift of shoulder surgery from open to arthroscopic techniques were generally young men who were in private practice and had little to lose by inciting the disdain of the shoulder establishment. Predictably, resistance from the mainstream open shoulder surgeons increased as arthroscopic techniques became more successful and more threatening to the primacy of the open shoulder surgeons. The disdain yielded to disruption and finally to transformation as the paradigm shift occurred. The conflict between the open shoulder surgeons and the arthroscopic shoulder surgeons passed through all the phases that Mahatma Gandhi had described many years before. “First they ignore you; then they laugh at you; then they fight you; then you win.”
Building a Ship in a Bottle
At the start of the 1990s, I recognized that my progress in arthroscopic rotator cuff repair would be extremely slow unless I could find an industry partner who shared my vision for full-scale conversion to arthroscopic means of repair and would be willing to help make it a reality. In 1991, I happened to meet Reinhold Schmieding, the owner of Arthrex, a small arthroscopic device company in Naples, Florida. Reinhold invited me to visit him to discuss the feasibility of developing arthroscopic repair systems for the shoulder. At the time, the world headquarters of Arthrex was a 20×30-ft storage room in an office service center, and there were 2 employees. One employee, Don Grafton, was a talented engineer without medical experience. By the end of my first day there, Reinhold and Don and I had agreed that developing arthroscopic repair systems for shoulder instability and rotator cuff repair would become a top priority for Arthrex.
My initial bias toward arthroscopic cuff repair was that a transosseous bone tunnel technique not only would be possible but would be superior to suture anchor fixation. In fact, my first 2 patents with Arthrex were for instrumentation for an arthroscopic transosseous repair technique. I tested my hypothesis with 2 successive biomechanical studies. The first examined cyclic loading of bone tunnel repairs, and the second examined cyclic loading of anchor-based repairs.3,4 Evaluating the data from these 2 studies, I was surprised to find that anchor-based repairs were significantly stronger than bone tunnel repairs. In addition, anchors shifted the weak link from the bone–suture interface to the tendon–suture interface; in essence, anchors optimized bone fixation by shifting the weak link in the construct to the tendon. I was then completely convinced of the superiority of suture anchors over bone tunnels, and that conviction has become even stronger over the years. After these 2 cyclic loading studies, I shifted my focus, and that of Arthrex, toward arthroscopic suture anchor repair of the rotator cuff.
Reconciling Technique and Instrumentation With Anatomy and Biomechanics
Having recognized the importance of the rotator cable attachments both anatomically5 and biomechanically,6,7 I thought it important to reinforce them as a routine part of performing rotator cuff repairs. Our anatomical and biomechanical studies had had great translational implications in the development of our techniques and instrumentation.
As mentioned earlier, Don Grafton was the chief (and for a long time only) engineer at Arthrex. As he had no medical experience, I invited him to come to San Antonio to observe surgery. During Don’s many visits, I showed him pathology in the operating room and pointed out what I could do with the instruments I had and what I could not do. Then in the evening we went to my house and brainstormed how to perform the “missing” surgical manipulations, how to improve manipulations that were suboptimal, and how to optimize final surgical constructs.
Passing suture through tendon was an early challenge. One must remember that, in the early 1990s, it was not possible for machinists to fabricate complex shapes. Therefore, straight tubular retrograde suture passers were the logical first option. We initially developed spring-loaded retrograde hook retrievers (Figure 1) and then curved suture hooks with shuttling wires (Lasso). To me, the most unappealing feature of retrograde suture passage was the oblique angle of approach through the tendon, which caused a length–tension mismatch between the upper fibers and the lower fibers of the muscle–tendon unit. We recognized we could eliminate the mismatch if we passed the suture antegrade, such that it would pass perpendicular to the tendon fibers. These insights and efforts culminated in development of the Viper suture passer and then the FastPass Scorpion suture passer, which has a spring-loaded trapdoor on the upper jaw for ergonomic self-retrieving of the suture once it is passed through the tendon.
To develop a knot pusher that optimized knot tying (yielding the highest knot security and the tightest loop security), we used prototype instruments to tie and test literally thousands of knots in the laboratory. We were thus able to verify that the Surgeon’s Sixth Finger Knot Pusher (Arthrex) reproducibly tied optimized knots8,9 and also optimized knot fixation and bone fixation. However, our suture was not yet optimized and was prone to breakage, and our suture–tendon interface was not yet optimized. Clearly, improvement was needed in 2 more areas.
Don came up with the idea for a virtually unbreakable suture and developed that idea into FiberWire.10 Shortly thereafter, I contributed the idea and design for FiberTape, which dramatically enhanced suture pullout strength and footprint compression.
Anchor designs improved rapidly and dramatically. We made the second-generation BioCorkscrew fully threaded, which virtually eliminated anchor failure, even in soft bone.
Optimization of the suture–tendon interface took a giant step forward when Park and colleagues11,12 introduced linked double-row rotator cuff repair. Much as with a Chinese finger trap, the harder you pull, the stronger it becomes, with yield load approaching ultimate load.
At this point, it seemed we had optimized virtually every segment of the rotator cuff repair construct. Each component was just about as good as it could be. Or was it?
The Accidental Quest for Knotless Fixation
In November 1998, I made my first trip to China as a guest speaker at the Congress of the Hong Kong Orthopaedic Association. My first view of the magnificent Hong Kong skyline across Victoria Harbour was truly breathtaking. As I admired the gleaming glass towers and the concrete canyons of the city, I had no idea that the very next day these modern skyscrapers would reveal an ancient secret that would change my approach to arthroscopic rotator cuff repair.
The day after my arrival, Dr. James Lam took me to lunch. As we approached the restaurant, he pointed across the street to a tall building that was being renovated and had scaffolding supporting workers alongside the first 9 stories of the exterior wall. Dr. Lam said that, after lunch, he would take me to the construction site for a closer look at the scaffolding.
After lunch, we walked to the base of the scaffolding. Dr. Lam told me it was constructed entirely of bamboo poles held together with lashings but no knots (Figure 2). Lashings were secured by turning them back on themselves and wrapping them in an entirely knotless manner.13 I found it incredible that this knotless fixation was so secure that it could support the weight of workers many stories above the ground. I resolved to determine how this fixation method worked and see if the same mechanism might help us achieve reliable knotless fixation in surgery.
When I returned home, I broke out my college engineering books and reacquainted myself with the concept of cable friction. As has happened so often in the past, however, it took a practical lesson from the ranch to truly illustrate for me how cable friction works.
Every cowboy knows that a spirited horse cannot be restrained with only one lead rope. However, a cowboy can wrap a lead rope around a “snubbing post” and thereby gain complete control over the animal, despite the horse’s superior size and strength. The cable friction between the rope and the post creates such a large restraining force that the cowboy can easily hold the animal without the help of a knotted rope (Figure 3). In similar fashion in the Hong Kong scaffolding, fixation strength results from the significant amount of cable friction produced when the lashings wrap around one another and around the bamboo poles.
The cable friction concept was pivotal in the development of knotless fixation in arthroscopic rotator cuff repair. In lateral row fixation, the eyelet of the PushLock and SwiveLock suture anchors (Arthrex) produces significant cable friction at the eyelet–suture interface, in addition to frictional force wedging the suture between anchor and bone.
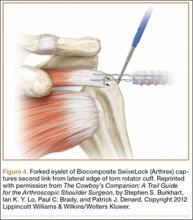
As with so many other devices in shoulder arthroscopy, the SwiveLock suture anchor developed in stages. In the first stage, a chainlike suture with consecutive intersecting links was used (FiberChain). The idea for an adjustable fixation construct came to me because I thought that a forked eyelet on a SwiveLock would provide a firm fixation point when inserted into the appropriate suture link, yet would be totally adjustable simply by choosing a tighter or looser link (Figure 4). Although the system worked very well, it was technically challenging. The process was greatly simplified after Don Grafton and I developed FiberTape and recognized that the power of cable friction was dramatically increased by the larger contact area between the eyelet and the braided FiberTape. The SpeedBridge construct (Arthrex), which enhanced cable friction fixation by means of passing FiberTape through the anchor eyelets, also provided a larger compressive interface at the repair site by using FiberTape rather than conventional suture. These incremental improvements led to what I would characterize as today’s gold standard for arthroscopic rotator cuff repair: a largely knotless linked double-row construct using FiberTape, with cinch-loop sutures at the anterior and posterior margins of the tear to reinforce the cable attachments and simultaneously reduce the dog-ears that typically occur in those locations, and a double-pulley medial mattress if tendon quality is poor (Figure 5).
The Burden of Craft
With all the recent enthusiasm for level I studies, I think we need to examine whether they will accelerate technological advancement in rotator cuff repair. The answer, in my opinion, is a resounding no. This answer is based on a major disconnect I have detected in how we evaluate these studies in rotator cuff disease and repair.
An irony related to technological advancement in surgery is that the more technically advanced the surgery becomes, the more skill is required. This fact is completely at odds with the public’s perception that technological advances make procedures easier. In arthroscopic rotator cuff repair, the surgeon must look, feel, and be aware to a greater degree than in open surgery.
Edward Tenner, in his book Why Things Bite Back, described the burden of the practitioner of any advanced technology as the burden of craft.14 The burden of craft is the inherent demand on all craftspeople, but particularly surgeons, to “up our game” if we are to be successful in our craft. For arthroscopic rotator cuff repair, the burden of craft requires patience, attention to detail, and the ability to work in a virtual space. Not everyone has these skills. But anyone who wants to practice in this discipline has an obligation to learn the skills required, and then to teach them to others and assess how well they are being applied.
The problem with relying on level I studies to assess the efficacy of a surgical procedure is that they are inherently biased by the surgeons involved. As results depend on surgeons’ skills, and surgeons’ skill levels are not equal, level I studies cannot prove what is possible, cannot demonstrate the limits of a technique, and cannot demonstrate the equivalence of techniques.
Amazingly enough, there are still rotator cuff repair “deniers” who confidently assert from the podium that a large percentage of massive cuff tears cannot be repaired and that, even if they can be repaired, they do not have the biological potential to heal. Given the disparity in surgeons’ skills and results, however, one must ask whether poor results are a consequence of a biological deficit in the patient, or of a skill deficit in the surgeon.
What I know is that we have techniques for predictable arthroscopic repair and healing of the vast majority of rotator cuff tears, even massive tears,15-17 and patients do very well clinically. Yet, among many orthopedic surgeons, there is a trend to go straight to reverse total shoulder arthroplasty (rTSA) for massive tears—despite the evidence against it. As reported in the literature, rTSA results are not as good as arthroscopic cuff repair results, and the complication rate for rTSA is much higher.
Why has this trend toward rTSA for massive tears gained so much momentum? The only reason I can surmise is that, for the average surgeon, rTSA is easier and quicker than arthroscopic repair for massive tears. But the reason for choosing a specific type of surgery for a given problem should not be that it is easiest for the surgeon; it should be that it is best for the patient.
The surgeon should start by asking what procedure he or she would want if the roles were reversed—if the surgeon were the patient with the massive rotator cuff tear. If a surgeon does not have the skill set for the best procedure for a particular patient, he or she is obligated to send that patient to a surgeon who does have the skills. In addition, given that infection is the most feared complication in most shoulder surgeries, the surgeon should ask which infection rate would be personally acceptable. Arthroscopic rotator cuff repair has a reported infection rate of 1.6 per 1000, or .0016,18 whereas rTSA has an infection rate about 25 times higher, or .04.19 Further, the surgeon must consider the relative severity of the consequences of infection. By any measure, an infected arthroscopy is a straightforward treatable complication, but an infected shoulder replacement is a human tragedy. Patients vastly prefer the minimally invasive arthroscopic approach, and through online searches can easily identify who can offer an arthroscopic solution.
To reproducibly achieve successful arthroscopic repair of massive rotator cuff tears, the surgeon must know advanced techniques, including subscapularis repair techniques,20,21 interval slides,22,23 and self-reinforcing constructs.24,25
“It’s a poor carpenter who blames his tools.” This 18th-century English proverb is as true today as it was 300 years ago. The tools for arthroscopic cuff repair exist, and they are excellent. The burden of craft is the surgeon’s burden and obligation. As surgeons, we must accept that obligation and the responsibility of that burden.
As mentioned earlier, Dr. Rob Bell’s charge to me when he invited me to give the Neer Lecture was to sum up my involvement in the development of arthroscopic shoulder surgery. The short version is that I have been doing shoulder arthroscopy for 31 years; have received 28 US patents related to shoulder instruments and implants and have 12 US patents pending; have published 167 peer-reviewed articles, a couple dozen book chapters, and 2 textbooks on shoulder arthroscopy; have trained 25 fellows; and have hosted approximately 3000 visiting surgeons in my operating room. My greatest professional dream was to see the standard of care for rotator cuff repair and shoulder instability transition from open to arthroscopic techniques, and I have been fortunate enough to have observed that paradigm shift during my career.
What do I envision over the next 31 years? As we all know, history runs in both directions, and some things simply have not happened yet. In terms of rotator cuff treatment, I think over the next few years the guiding principle of treatment will be joint preservation. All rotator cuff tears, even massive tears, will be repaired arthroscopically. Patients and insurers will demand arthroscopic repair, and surgeons without the skill set will migrate to other subspecialties. As for the role of arthroplasty in the treatment of rotator cuff tears, rTSA will be indicated only for pseudoparalysis after failed cuff repair in low-demand elderly patients.
In rotator cuff treatment, I envision a standard of care that is almost entirely arthroscopic. This standard will demand that surgeons who treat rotator cuff tears be proficient in arthroscopic repair of the full range of tears. Acquiring the skills for arthroscopic repair may not be easy, but then “there’s the easy way, and there’s the Cowboy Way.” As my dad used to tell me when I complained about working too hard, “No man ever drowned in his own sweat.” We shoulder surgeons must accept the burden of craft that accompanies the new standard of arthroscopic cuff repair, and we must offer our patients the same level of care we would choose for ourselves.
Happy trails!
1. Burkhart SS, Athanasiou KA, Wirth MA. Margin convergence: a method of reducing strain in massive rotator cuff tears. Arthroscopy. 1996:12(3):335-338.
2. Kuhn TS. The Structure of Scientific Revolutions. Chicago, IL: University of Chicago Press; 1962.
3. Burkhart SS, Johnson TC, Wirth MA, Athanasiou KA. Cyclic loading of transosseous rotator cuff repairs: tension overload as a possible cause of failure. Arthroscopy. 1997;13(2):172-176.
4. Burkhart SS, Diaz Pagàn JL, Wirth MA, Athansiou KA. Cyclic loading of anchor-based rotator cuff repairs: confirmation of the tension overload phenomenon and comparison of suture anchor fixation with transosseous fixation. Arthroscopy. 1997;13(6):720-724.
5. Burkhart SS, Esch JC, Jolson RS. The rotator crescent and rotator cable: an anatomic description of the shoulder’s “suspension bridge.” Arthroscopy. 1993;9(6):611-616.
6. Burkhart SS, Nottage WM, Ogilvie-Harris DJ, Kohn HS, Pachelli A. Partial repair of irreparable rotator cuff tears. Arthroscopy. 1994;10(4):363-370.
7. Halder AM, O’Driscoll SW, Heers G, et al. Biomechanical comparison of effects of supraspinatus tendon detachments, tendon defects, and muscle retractions. J Bone Joint Surg Am. 2002;84(5):780-785.
8. Lo IK, Burkhart SS, Chan KC, Athanasiou K. Arthroscopic knots: determining the optimal balance of loop security and knot security. Arthroscopy. 2004;20(5):489-502.
9. Lo IK, Ochoa E Jr, Burkhart SS. A comparison of knot security and loop security in arthroscopic knots tied with newer high-strength suture materials. Arthroscopy. 2010;26(9 suppl):S120-S126.
10. Lo IK, Burkhart SS, Athanasiou K. Abrasion resistance of two types of nonabsorbable braided suture. Arthroscopy. 2004;20(4):407-413.
11. Park MC, ElAttrache NS, Tibone JE, Ahmad CS, Jun BJ, Lee TQ. Part I: footprint contact characteristics for a “transosseous-equivalent” rotator cuff repair technique compared to a double-row technique. J Shoulder Elbow Surg. 2007;16(4):461-468.
12. Park MC, Tibone JE, ElAttrache NS, Ahmad CS, Jun BJ, Lee TQ. Part II: biomechanical assessment for a footprint-restoring transosseous-equivalent rotator cuff repair technique compared with a double-row repair technique. J Shoulder Elbow Surg. 2007;16(4):469-476.
13. Burkhart SS, Athanasiou KA. The twist-lock concept of tissue transport and suture fixation without knots: observations along the Hong Kong skyline. Arthroscopy. 2003;19(6):613-625.
14. Tenner E. Why Things Bite Back. New York, NY: Random House; 1996.
15. Denard PJ, Jiwani AZ, Lädermann A, Burkhart SS. Long-term outcome of arthroscopic massive rotator cuff repair: the importance of double-row fixation. Arthroscopy. 2012;28(7):909-915.
16. Denard PJ, Lädermann A, Jiwani AZ, Burkhart SS. Functional outcome after arthroscopic repair of massive rotator cuff tears in individuals with pseudoparalysis. Arthroscopy. 2012;28(9):1214-1219.
17. Lädermann A, Denard PJ, Burkhart SS. Revision arthroscopic rotator cuff repair: systematic review and authors’ preferred surgical technique. Arthroscopy. 2012;28(8):1160-1169.
18. Randelli P, Castagna A, Cabitza F, Cabitza P, Arrigoni P, Denti M. Infectious and thromboembolic complications of arthroscopic shoulder surgery. J Shoulder Elbow Surg. 2010;19(1):97-101.
19. Zumstein MA, Pinedo M, Old J, Boileau P. Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2011;20(1):146-157.
20. Adams CR, Schoolfield JD, Burkhart SS. The results of arthroscopic subscapularis tendon repairs. Arthroscopy. 2008;24(12):1381-1389.
21. Denard PJ, Jiwani AZ, Lädermann A, Burkhart SS. Long-term outcome of a consecutive series of subscapularis tendon tears repaired arthroscopically. Arthroscopy. 2012;28(11):1587-1591.
22. Lo IK, Burkhart SS. Arthroscopic repair of massive, contracted, immobile rotator cuff tears using single and double interval slides: technique and preliminary results. Arthroscopy. 2004;20(1):22-33.
23. Lo IK, Burkhart SS. The interval slide in continuity: a method of mobilizing the anterosuperior rotator cuff without disrupting the tear margins. Arthroscopy. 2004;20(4):435-441.
24. Denard PJ, Burkhart SS. Techniques for managing poor quality tissue and bone during arthroscopic rotator cuff repair. Arthroscopy. 2011;27(10):1409-1421.
25. Burkhart SS, Denard PJ, Konicek J, Hanypsiak BT. Biomechanical validation of load-sharing rip-stop fixation for the repair of tissue-deficient rotator cuff tears. Am J Sports Med. 2014;42(2):457-462.
I am very honored that Dr. Rob Bell, past president of the American Shoulder and Elbow Surgeons, invited me to give last year’s Neer Lecture. Dr. Bell asked me to specifically address my role in the development of arthroscopic rotator cuff repair and to recount the significant resistance that the early arthroscopic shoulder surgeons faced from the shoulder establishment as we struggled to achieve mainstream acceptance for this new technology. Tasked with such a personal topic, I find myself in a position analogous to that of Winston Churchill at the end of World War II. When a journalist asked him to speculate on how historians would portray his role in the war, he replied without hesitation, “History will be kind to me because I intend to write it.”
So let’s start at the beginning. And for me it makes the most sense to travel back to the year I started my practice: 1981. The world then was very different from today’s world. On January 20, 1981, Ronald Reagan was inaugurated President of the United States. The same day, 52 US hostages in Iran were released after having been held captive for 442 days. In March 1981, Reagan survived an assassination attempt; 3 months earlier, John Lennon had not been so lucky. Lennon’s hit song “Starting Over” garnered the highest musical awards posthumously.
The world of shoulder surgery was also very different in 1981. The arthroscope was the “instrument of the devil,” according to Dr. Rockwood. And shoulder surgery was ruled by the Charlies—Dr. Charles Neer, Dr. Charlie Rockwood, and any other Charlie who felt compelled to marginalize shoulder arthroscopy.
My personal world in the early 1980s was daunting as well. I had just completed my residency at the Mayo Clinic and my sports medicine fellowship in Eugene, Oregon. I had a young son, a new daughter, and a new job with the San Antonio Orthopaedic Group. I had a new house with a 21% mortgage loan and a “new” used car with a 23% car loan.
I was simultaneously energized and intimidated by my new job, where I was doing general orthopedics with a “special interest” in shoulder surgery and sports medicine. I was initially very proud and humbled by the fact that my senior partners had entrusted me with the care of the most difficult shoulder cases within the practice. But that pride got cut down to its appropriate size the day after I had thanked one of my partners, Dr. Lamar Collie, for his confidence in my potential as a shoulder surgeon. Dr. Collie replied matter-of-factly, “Sure … but you need to understand that we always make the new guy the shoulder expert because shoulders never do worth a damn.”
For shoulder arthroscopy, the early 1980s were exciting. Most of us who were scoping shoulders had already been doing knee arthroscopy and were trying to adapt knee instruments to the shoulder. This worked for some simple excisional cases. For example, I recall excising the bucket-handle portion of a type III SLAP (superior labral tear from anterior to posterior) lesion in 1983. In general, however, shoulder problems were different from knee problems and usually involved repair rather than excision of damaged tissues. Therefore, the technology used in knee arthroscopy was often not directly transferable to the shoulder. Furthermore, treatment of the rotator cuff necessitated development of arthroscopic techniques in a virtual space, the subacromial space, and this was an entirely new arthroscopic concept.
Development of Arthroscopic Rotator Cuff Repair
A major mind-expanding turning point for me occurred in 1984 when I attended one of Dr. Jim Esch’s early San Diego shoulder courses. During that course, Dr. Harvard Ellman of Los Angeles demonstrated to me on a cadaver shoulder how he created a virtual subacromial working space that allowed enough visualization for an arthroscopic acromioplasty. At that moment, I knew that arthroscopic rotator cuff repair was just around the corner. Up until then, I had not been able to envision complex extra-articular reconstructive surgery, as all previous arthroscopic surgery had been intra-articular. But now, having realized a virtual working space could always be created, I knew it would be relatively straightforward to develop the portals to approach the cuff as well as the implants and the instruments to repair it. But I also knew that progression to all-arthroscopic repair techniques would have to be stepwise and that the final repair constructs would need to be at least as strong as those of open repair in order to be acceptable. With an undergraduate degree in mechanical engineering, I had a reasonably clear idea of the concepts I wanted to apply to the instrumentation and techniques, though I could never have envisioned how circuitous the route to the end result would be.
First Steps
I sketched out my ideas for arthroscopic suture passers and knot-tying instruments and presented them to a couple of the major arthroscopy companies in the United States, but the companies were not interested. They did not believe arthroscopy would have any meaningful applications in the shoulder. So, I enlisted the services of a local San Antonio aircraft machinist to fabricate instruments for me. By 1987, I was doing arthroscopic side-to-side margin convergence1 cuff repairs for U-shape tears on a regular basis. And I was doing these at the most hostile point in the universe for arthroscopic shoulder surgery: San Antonio, Texas.
Only a few surgeons were doing arthroscopic shoulder surgery in the 1980s and early 1990s, and without exception these surgeons became the leader-pioneers in the new discipline. In general, these were young surgeons who were in private practice and removed from academia and professional organizations, and thus relatively sheltered from the actions of the shoulder rule-makers of the day. They accepted their status as pariahs as they developed their techniques out of the view of mainstream orthopedics. These leaders included Jim Esch, Steve Snyder, Dick Caspari, Lanny Johnson, Gene Wolf, Gary Gartsman, Rob Bell, and Howard Sweeney. We shared our techniques and our ideas with one another, encouraged one another, and generally became good friends.
Thomas Kuhn, in his classic book The Structure of Scientific Revolutions,2 observed that paradigm shifts within a given field were usually achieved by practitioners who were either very young (naïve) or outside the established hierarchy in the field. The surgeons who contributed most to the shift of shoulder surgery from open to arthroscopic techniques were generally young men who were in private practice and had little to lose by inciting the disdain of the shoulder establishment. Predictably, resistance from the mainstream open shoulder surgeons increased as arthroscopic techniques became more successful and more threatening to the primacy of the open shoulder surgeons. The disdain yielded to disruption and finally to transformation as the paradigm shift occurred. The conflict between the open shoulder surgeons and the arthroscopic shoulder surgeons passed through all the phases that Mahatma Gandhi had described many years before. “First they ignore you; then they laugh at you; then they fight you; then you win.”
Building a Ship in a Bottle
At the start of the 1990s, I recognized that my progress in arthroscopic rotator cuff repair would be extremely slow unless I could find an industry partner who shared my vision for full-scale conversion to arthroscopic means of repair and would be willing to help make it a reality. In 1991, I happened to meet Reinhold Schmieding, the owner of Arthrex, a small arthroscopic device company in Naples, Florida. Reinhold invited me to visit him to discuss the feasibility of developing arthroscopic repair systems for the shoulder. At the time, the world headquarters of Arthrex was a 20×30-ft storage room in an office service center, and there were 2 employees. One employee, Don Grafton, was a talented engineer without medical experience. By the end of my first day there, Reinhold and Don and I had agreed that developing arthroscopic repair systems for shoulder instability and rotator cuff repair would become a top priority for Arthrex.
My initial bias toward arthroscopic cuff repair was that a transosseous bone tunnel technique not only would be possible but would be superior to suture anchor fixation. In fact, my first 2 patents with Arthrex were for instrumentation for an arthroscopic transosseous repair technique. I tested my hypothesis with 2 successive biomechanical studies. The first examined cyclic loading of bone tunnel repairs, and the second examined cyclic loading of anchor-based repairs.3,4 Evaluating the data from these 2 studies, I was surprised to find that anchor-based repairs were significantly stronger than bone tunnel repairs. In addition, anchors shifted the weak link from the bone–suture interface to the tendon–suture interface; in essence, anchors optimized bone fixation by shifting the weak link in the construct to the tendon. I was then completely convinced of the superiority of suture anchors over bone tunnels, and that conviction has become even stronger over the years. After these 2 cyclic loading studies, I shifted my focus, and that of Arthrex, toward arthroscopic suture anchor repair of the rotator cuff.
Reconciling Technique and Instrumentation With Anatomy and Biomechanics
Having recognized the importance of the rotator cable attachments both anatomically5 and biomechanically,6,7 I thought it important to reinforce them as a routine part of performing rotator cuff repairs. Our anatomical and biomechanical studies had had great translational implications in the development of our techniques and instrumentation.
As mentioned earlier, Don Grafton was the chief (and for a long time only) engineer at Arthrex. As he had no medical experience, I invited him to come to San Antonio to observe surgery. During Don’s many visits, I showed him pathology in the operating room and pointed out what I could do with the instruments I had and what I could not do. Then in the evening we went to my house and brainstormed how to perform the “missing” surgical manipulations, how to improve manipulations that were suboptimal, and how to optimize final surgical constructs.
Passing suture through tendon was an early challenge. One must remember that, in the early 1990s, it was not possible for machinists to fabricate complex shapes. Therefore, straight tubular retrograde suture passers were the logical first option. We initially developed spring-loaded retrograde hook retrievers (Figure 1) and then curved suture hooks with shuttling wires (Lasso). To me, the most unappealing feature of retrograde suture passage was the oblique angle of approach through the tendon, which caused a length–tension mismatch between the upper fibers and the lower fibers of the muscle–tendon unit. We recognized we could eliminate the mismatch if we passed the suture antegrade, such that it would pass perpendicular to the tendon fibers. These insights and efforts culminated in development of the Viper suture passer and then the FastPass Scorpion suture passer, which has a spring-loaded trapdoor on the upper jaw for ergonomic self-retrieving of the suture once it is passed through the tendon.
To develop a knot pusher that optimized knot tying (yielding the highest knot security and the tightest loop security), we used prototype instruments to tie and test literally thousands of knots in the laboratory. We were thus able to verify that the Surgeon’s Sixth Finger Knot Pusher (Arthrex) reproducibly tied optimized knots8,9 and also optimized knot fixation and bone fixation. However, our suture was not yet optimized and was prone to breakage, and our suture–tendon interface was not yet optimized. Clearly, improvement was needed in 2 more areas.
Don came up with the idea for a virtually unbreakable suture and developed that idea into FiberWire.10 Shortly thereafter, I contributed the idea and design for FiberTape, which dramatically enhanced suture pullout strength and footprint compression.
Anchor designs improved rapidly and dramatically. We made the second-generation BioCorkscrew fully threaded, which virtually eliminated anchor failure, even in soft bone.
Optimization of the suture–tendon interface took a giant step forward when Park and colleagues11,12 introduced linked double-row rotator cuff repair. Much as with a Chinese finger trap, the harder you pull, the stronger it becomes, with yield load approaching ultimate load.
At this point, it seemed we had optimized virtually every segment of the rotator cuff repair construct. Each component was just about as good as it could be. Or was it?
The Accidental Quest for Knotless Fixation
In November 1998, I made my first trip to China as a guest speaker at the Congress of the Hong Kong Orthopaedic Association. My first view of the magnificent Hong Kong skyline across Victoria Harbour was truly breathtaking. As I admired the gleaming glass towers and the concrete canyons of the city, I had no idea that the very next day these modern skyscrapers would reveal an ancient secret that would change my approach to arthroscopic rotator cuff repair.
The day after my arrival, Dr. James Lam took me to lunch. As we approached the restaurant, he pointed across the street to a tall building that was being renovated and had scaffolding supporting workers alongside the first 9 stories of the exterior wall. Dr. Lam said that, after lunch, he would take me to the construction site for a closer look at the scaffolding.
After lunch, we walked to the base of the scaffolding. Dr. Lam told me it was constructed entirely of bamboo poles held together with lashings but no knots (Figure 2). Lashings were secured by turning them back on themselves and wrapping them in an entirely knotless manner.13 I found it incredible that this knotless fixation was so secure that it could support the weight of workers many stories above the ground. I resolved to determine how this fixation method worked and see if the same mechanism might help us achieve reliable knotless fixation in surgery.
When I returned home, I broke out my college engineering books and reacquainted myself with the concept of cable friction. As has happened so often in the past, however, it took a practical lesson from the ranch to truly illustrate for me how cable friction works.
Every cowboy knows that a spirited horse cannot be restrained with only one lead rope. However, a cowboy can wrap a lead rope around a “snubbing post” and thereby gain complete control over the animal, despite the horse’s superior size and strength. The cable friction between the rope and the post creates such a large restraining force that the cowboy can easily hold the animal without the help of a knotted rope (Figure 3). In similar fashion in the Hong Kong scaffolding, fixation strength results from the significant amount of cable friction produced when the lashings wrap around one another and around the bamboo poles.
The cable friction concept was pivotal in the development of knotless fixation in arthroscopic rotator cuff repair. In lateral row fixation, the eyelet of the PushLock and SwiveLock suture anchors (Arthrex) produces significant cable friction at the eyelet–suture interface, in addition to frictional force wedging the suture between anchor and bone.
As with so many other devices in shoulder arthroscopy, the SwiveLock suture anchor developed in stages. In the first stage, a chainlike suture with consecutive intersecting links was used (FiberChain). The idea for an adjustable fixation construct came to me because I thought that a forked eyelet on a SwiveLock would provide a firm fixation point when inserted into the appropriate suture link, yet would be totally adjustable simply by choosing a tighter or looser link (Figure 4). Although the system worked very well, it was technically challenging. The process was greatly simplified after Don Grafton and I developed FiberTape and recognized that the power of cable friction was dramatically increased by the larger contact area between the eyelet and the braided FiberTape. The SpeedBridge construct (Arthrex), which enhanced cable friction fixation by means of passing FiberTape through the anchor eyelets, also provided a larger compressive interface at the repair site by using FiberTape rather than conventional suture. These incremental improvements led to what I would characterize as today’s gold standard for arthroscopic rotator cuff repair: a largely knotless linked double-row construct using FiberTape, with cinch-loop sutures at the anterior and posterior margins of the tear to reinforce the cable attachments and simultaneously reduce the dog-ears that typically occur in those locations, and a double-pulley medial mattress if tendon quality is poor (Figure 5).
The Burden of Craft
With all the recent enthusiasm for level I studies, I think we need to examine whether they will accelerate technological advancement in rotator cuff repair. The answer, in my opinion, is a resounding no. This answer is based on a major disconnect I have detected in how we evaluate these studies in rotator cuff disease and repair.
An irony related to technological advancement in surgery is that the more technically advanced the surgery becomes, the more skill is required. This fact is completely at odds with the public’s perception that technological advances make procedures easier. In arthroscopic rotator cuff repair, the surgeon must look, feel, and be aware to a greater degree than in open surgery.
Edward Tenner, in his book Why Things Bite Back, described the burden of the practitioner of any advanced technology as the burden of craft.14 The burden of craft is the inherent demand on all craftspeople, but particularly surgeons, to “up our game” if we are to be successful in our craft. For arthroscopic rotator cuff repair, the burden of craft requires patience, attention to detail, and the ability to work in a virtual space. Not everyone has these skills. But anyone who wants to practice in this discipline has an obligation to learn the skills required, and then to teach them to others and assess how well they are being applied.
The problem with relying on level I studies to assess the efficacy of a surgical procedure is that they are inherently biased by the surgeons involved. As results depend on surgeons’ skills, and surgeons’ skill levels are not equal, level I studies cannot prove what is possible, cannot demonstrate the limits of a technique, and cannot demonstrate the equivalence of techniques.
Amazingly enough, there are still rotator cuff repair “deniers” who confidently assert from the podium that a large percentage of massive cuff tears cannot be repaired and that, even if they can be repaired, they do not have the biological potential to heal. Given the disparity in surgeons’ skills and results, however, one must ask whether poor results are a consequence of a biological deficit in the patient, or of a skill deficit in the surgeon.
What I know is that we have techniques for predictable arthroscopic repair and healing of the vast majority of rotator cuff tears, even massive tears,15-17 and patients do very well clinically. Yet, among many orthopedic surgeons, there is a trend to go straight to reverse total shoulder arthroplasty (rTSA) for massive tears—despite the evidence against it. As reported in the literature, rTSA results are not as good as arthroscopic cuff repair results, and the complication rate for rTSA is much higher.
Why has this trend toward rTSA for massive tears gained so much momentum? The only reason I can surmise is that, for the average surgeon, rTSA is easier and quicker than arthroscopic repair for massive tears. But the reason for choosing a specific type of surgery for a given problem should not be that it is easiest for the surgeon; it should be that it is best for the patient.
The surgeon should start by asking what procedure he or she would want if the roles were reversed—if the surgeon were the patient with the massive rotator cuff tear. If a surgeon does not have the skill set for the best procedure for a particular patient, he or she is obligated to send that patient to a surgeon who does have the skills. In addition, given that infection is the most feared complication in most shoulder surgeries, the surgeon should ask which infection rate would be personally acceptable. Arthroscopic rotator cuff repair has a reported infection rate of 1.6 per 1000, or .0016,18 whereas rTSA has an infection rate about 25 times higher, or .04.19 Further, the surgeon must consider the relative severity of the consequences of infection. By any measure, an infected arthroscopy is a straightforward treatable complication, but an infected shoulder replacement is a human tragedy. Patients vastly prefer the minimally invasive arthroscopic approach, and through online searches can easily identify who can offer an arthroscopic solution.
To reproducibly achieve successful arthroscopic repair of massive rotator cuff tears, the surgeon must know advanced techniques, including subscapularis repair techniques,20,21 interval slides,22,23 and self-reinforcing constructs.24,25
“It’s a poor carpenter who blames his tools.” This 18th-century English proverb is as true today as it was 300 years ago. The tools for arthroscopic cuff repair exist, and they are excellent. The burden of craft is the surgeon’s burden and obligation. As surgeons, we must accept that obligation and the responsibility of that burden.
As mentioned earlier, Dr. Rob Bell’s charge to me when he invited me to give the Neer Lecture was to sum up my involvement in the development of arthroscopic shoulder surgery. The short version is that I have been doing shoulder arthroscopy for 31 years; have received 28 US patents related to shoulder instruments and implants and have 12 US patents pending; have published 167 peer-reviewed articles, a couple dozen book chapters, and 2 textbooks on shoulder arthroscopy; have trained 25 fellows; and have hosted approximately 3000 visiting surgeons in my operating room. My greatest professional dream was to see the standard of care for rotator cuff repair and shoulder instability transition from open to arthroscopic techniques, and I have been fortunate enough to have observed that paradigm shift during my career.
What do I envision over the next 31 years? As we all know, history runs in both directions, and some things simply have not happened yet. In terms of rotator cuff treatment, I think over the next few years the guiding principle of treatment will be joint preservation. All rotator cuff tears, even massive tears, will be repaired arthroscopically. Patients and insurers will demand arthroscopic repair, and surgeons without the skill set will migrate to other subspecialties. As for the role of arthroplasty in the treatment of rotator cuff tears, rTSA will be indicated only for pseudoparalysis after failed cuff repair in low-demand elderly patients.
In rotator cuff treatment, I envision a standard of care that is almost entirely arthroscopic. This standard will demand that surgeons who treat rotator cuff tears be proficient in arthroscopic repair of the full range of tears. Acquiring the skills for arthroscopic repair may not be easy, but then “there’s the easy way, and there’s the Cowboy Way.” As my dad used to tell me when I complained about working too hard, “No man ever drowned in his own sweat.” We shoulder surgeons must accept the burden of craft that accompanies the new standard of arthroscopic cuff repair, and we must offer our patients the same level of care we would choose for ourselves.
Happy trails!
I am very honored that Dr. Rob Bell, past president of the American Shoulder and Elbow Surgeons, invited me to give last year’s Neer Lecture. Dr. Bell asked me to specifically address my role in the development of arthroscopic rotator cuff repair and to recount the significant resistance that the early arthroscopic shoulder surgeons faced from the shoulder establishment as we struggled to achieve mainstream acceptance for this new technology. Tasked with such a personal topic, I find myself in a position analogous to that of Winston Churchill at the end of World War II. When a journalist asked him to speculate on how historians would portray his role in the war, he replied without hesitation, “History will be kind to me because I intend to write it.”
So let’s start at the beginning. And for me it makes the most sense to travel back to the year I started my practice: 1981. The world then was very different from today’s world. On January 20, 1981, Ronald Reagan was inaugurated President of the United States. The same day, 52 US hostages in Iran were released after having been held captive for 442 days. In March 1981, Reagan survived an assassination attempt; 3 months earlier, John Lennon had not been so lucky. Lennon’s hit song “Starting Over” garnered the highest musical awards posthumously.
The world of shoulder surgery was also very different in 1981. The arthroscope was the “instrument of the devil,” according to Dr. Rockwood. And shoulder surgery was ruled by the Charlies—Dr. Charles Neer, Dr. Charlie Rockwood, and any other Charlie who felt compelled to marginalize shoulder arthroscopy.
My personal world in the early 1980s was daunting as well. I had just completed my residency at the Mayo Clinic and my sports medicine fellowship in Eugene, Oregon. I had a young son, a new daughter, and a new job with the San Antonio Orthopaedic Group. I had a new house with a 21% mortgage loan and a “new” used car with a 23% car loan.
I was simultaneously energized and intimidated by my new job, where I was doing general orthopedics with a “special interest” in shoulder surgery and sports medicine. I was initially very proud and humbled by the fact that my senior partners had entrusted me with the care of the most difficult shoulder cases within the practice. But that pride got cut down to its appropriate size the day after I had thanked one of my partners, Dr. Lamar Collie, for his confidence in my potential as a shoulder surgeon. Dr. Collie replied matter-of-factly, “Sure … but you need to understand that we always make the new guy the shoulder expert because shoulders never do worth a damn.”
For shoulder arthroscopy, the early 1980s were exciting. Most of us who were scoping shoulders had already been doing knee arthroscopy and were trying to adapt knee instruments to the shoulder. This worked for some simple excisional cases. For example, I recall excising the bucket-handle portion of a type III SLAP (superior labral tear from anterior to posterior) lesion in 1983. In general, however, shoulder problems were different from knee problems and usually involved repair rather than excision of damaged tissues. Therefore, the technology used in knee arthroscopy was often not directly transferable to the shoulder. Furthermore, treatment of the rotator cuff necessitated development of arthroscopic techniques in a virtual space, the subacromial space, and this was an entirely new arthroscopic concept.
Development of Arthroscopic Rotator Cuff Repair
A major mind-expanding turning point for me occurred in 1984 when I attended one of Dr. Jim Esch’s early San Diego shoulder courses. During that course, Dr. Harvard Ellman of Los Angeles demonstrated to me on a cadaver shoulder how he created a virtual subacromial working space that allowed enough visualization for an arthroscopic acromioplasty. At that moment, I knew that arthroscopic rotator cuff repair was just around the corner. Up until then, I had not been able to envision complex extra-articular reconstructive surgery, as all previous arthroscopic surgery had been intra-articular. But now, having realized a virtual working space could always be created, I knew it would be relatively straightforward to develop the portals to approach the cuff as well as the implants and the instruments to repair it. But I also knew that progression to all-arthroscopic repair techniques would have to be stepwise and that the final repair constructs would need to be at least as strong as those of open repair in order to be acceptable. With an undergraduate degree in mechanical engineering, I had a reasonably clear idea of the concepts I wanted to apply to the instrumentation and techniques, though I could never have envisioned how circuitous the route to the end result would be.
First Steps
I sketched out my ideas for arthroscopic suture passers and knot-tying instruments and presented them to a couple of the major arthroscopy companies in the United States, but the companies were not interested. They did not believe arthroscopy would have any meaningful applications in the shoulder. So, I enlisted the services of a local San Antonio aircraft machinist to fabricate instruments for me. By 1987, I was doing arthroscopic side-to-side margin convergence1 cuff repairs for U-shape tears on a regular basis. And I was doing these at the most hostile point in the universe for arthroscopic shoulder surgery: San Antonio, Texas.
Only a few surgeons were doing arthroscopic shoulder surgery in the 1980s and early 1990s, and without exception these surgeons became the leader-pioneers in the new discipline. In general, these were young surgeons who were in private practice and removed from academia and professional organizations, and thus relatively sheltered from the actions of the shoulder rule-makers of the day. They accepted their status as pariahs as they developed their techniques out of the view of mainstream orthopedics. These leaders included Jim Esch, Steve Snyder, Dick Caspari, Lanny Johnson, Gene Wolf, Gary Gartsman, Rob Bell, and Howard Sweeney. We shared our techniques and our ideas with one another, encouraged one another, and generally became good friends.
Thomas Kuhn, in his classic book The Structure of Scientific Revolutions,2 observed that paradigm shifts within a given field were usually achieved by practitioners who were either very young (naïve) or outside the established hierarchy in the field. The surgeons who contributed most to the shift of shoulder surgery from open to arthroscopic techniques were generally young men who were in private practice and had little to lose by inciting the disdain of the shoulder establishment. Predictably, resistance from the mainstream open shoulder surgeons increased as arthroscopic techniques became more successful and more threatening to the primacy of the open shoulder surgeons. The disdain yielded to disruption and finally to transformation as the paradigm shift occurred. The conflict between the open shoulder surgeons and the arthroscopic shoulder surgeons passed through all the phases that Mahatma Gandhi had described many years before. “First they ignore you; then they laugh at you; then they fight you; then you win.”
Building a Ship in a Bottle
At the start of the 1990s, I recognized that my progress in arthroscopic rotator cuff repair would be extremely slow unless I could find an industry partner who shared my vision for full-scale conversion to arthroscopic means of repair and would be willing to help make it a reality. In 1991, I happened to meet Reinhold Schmieding, the owner of Arthrex, a small arthroscopic device company in Naples, Florida. Reinhold invited me to visit him to discuss the feasibility of developing arthroscopic repair systems for the shoulder. At the time, the world headquarters of Arthrex was a 20×30-ft storage room in an office service center, and there were 2 employees. One employee, Don Grafton, was a talented engineer without medical experience. By the end of my first day there, Reinhold and Don and I had agreed that developing arthroscopic repair systems for shoulder instability and rotator cuff repair would become a top priority for Arthrex.
My initial bias toward arthroscopic cuff repair was that a transosseous bone tunnel technique not only would be possible but would be superior to suture anchor fixation. In fact, my first 2 patents with Arthrex were for instrumentation for an arthroscopic transosseous repair technique. I tested my hypothesis with 2 successive biomechanical studies. The first examined cyclic loading of bone tunnel repairs, and the second examined cyclic loading of anchor-based repairs.3,4 Evaluating the data from these 2 studies, I was surprised to find that anchor-based repairs were significantly stronger than bone tunnel repairs. In addition, anchors shifted the weak link from the bone–suture interface to the tendon–suture interface; in essence, anchors optimized bone fixation by shifting the weak link in the construct to the tendon. I was then completely convinced of the superiority of suture anchors over bone tunnels, and that conviction has become even stronger over the years. After these 2 cyclic loading studies, I shifted my focus, and that of Arthrex, toward arthroscopic suture anchor repair of the rotator cuff.
Reconciling Technique and Instrumentation With Anatomy and Biomechanics
Having recognized the importance of the rotator cable attachments both anatomically5 and biomechanically,6,7 I thought it important to reinforce them as a routine part of performing rotator cuff repairs. Our anatomical and biomechanical studies had had great translational implications in the development of our techniques and instrumentation.
As mentioned earlier, Don Grafton was the chief (and for a long time only) engineer at Arthrex. As he had no medical experience, I invited him to come to San Antonio to observe surgery. During Don’s many visits, I showed him pathology in the operating room and pointed out what I could do with the instruments I had and what I could not do. Then in the evening we went to my house and brainstormed how to perform the “missing” surgical manipulations, how to improve manipulations that were suboptimal, and how to optimize final surgical constructs.
Passing suture through tendon was an early challenge. One must remember that, in the early 1990s, it was not possible for machinists to fabricate complex shapes. Therefore, straight tubular retrograde suture passers were the logical first option. We initially developed spring-loaded retrograde hook retrievers (Figure 1) and then curved suture hooks with shuttling wires (Lasso). To me, the most unappealing feature of retrograde suture passage was the oblique angle of approach through the tendon, which caused a length–tension mismatch between the upper fibers and the lower fibers of the muscle–tendon unit. We recognized we could eliminate the mismatch if we passed the suture antegrade, such that it would pass perpendicular to the tendon fibers. These insights and efforts culminated in development of the Viper suture passer and then the FastPass Scorpion suture passer, which has a spring-loaded trapdoor on the upper jaw for ergonomic self-retrieving of the suture once it is passed through the tendon.
To develop a knot pusher that optimized knot tying (yielding the highest knot security and the tightest loop security), we used prototype instruments to tie and test literally thousands of knots in the laboratory. We were thus able to verify that the Surgeon’s Sixth Finger Knot Pusher (Arthrex) reproducibly tied optimized knots8,9 and also optimized knot fixation and bone fixation. However, our suture was not yet optimized and was prone to breakage, and our suture–tendon interface was not yet optimized. Clearly, improvement was needed in 2 more areas.
Don came up with the idea for a virtually unbreakable suture and developed that idea into FiberWire.10 Shortly thereafter, I contributed the idea and design for FiberTape, which dramatically enhanced suture pullout strength and footprint compression.
Anchor designs improved rapidly and dramatically. We made the second-generation BioCorkscrew fully threaded, which virtually eliminated anchor failure, even in soft bone.
Optimization of the suture–tendon interface took a giant step forward when Park and colleagues11,12 introduced linked double-row rotator cuff repair. Much as with a Chinese finger trap, the harder you pull, the stronger it becomes, with yield load approaching ultimate load.
At this point, it seemed we had optimized virtually every segment of the rotator cuff repair construct. Each component was just about as good as it could be. Or was it?
The Accidental Quest for Knotless Fixation
In November 1998, I made my first trip to China as a guest speaker at the Congress of the Hong Kong Orthopaedic Association. My first view of the magnificent Hong Kong skyline across Victoria Harbour was truly breathtaking. As I admired the gleaming glass towers and the concrete canyons of the city, I had no idea that the very next day these modern skyscrapers would reveal an ancient secret that would change my approach to arthroscopic rotator cuff repair.
The day after my arrival, Dr. James Lam took me to lunch. As we approached the restaurant, he pointed across the street to a tall building that was being renovated and had scaffolding supporting workers alongside the first 9 stories of the exterior wall. Dr. Lam said that, after lunch, he would take me to the construction site for a closer look at the scaffolding.
After lunch, we walked to the base of the scaffolding. Dr. Lam told me it was constructed entirely of bamboo poles held together with lashings but no knots (Figure 2). Lashings were secured by turning them back on themselves and wrapping them in an entirely knotless manner.13 I found it incredible that this knotless fixation was so secure that it could support the weight of workers many stories above the ground. I resolved to determine how this fixation method worked and see if the same mechanism might help us achieve reliable knotless fixation in surgery.
When I returned home, I broke out my college engineering books and reacquainted myself with the concept of cable friction. As has happened so often in the past, however, it took a practical lesson from the ranch to truly illustrate for me how cable friction works.
Every cowboy knows that a spirited horse cannot be restrained with only one lead rope. However, a cowboy can wrap a lead rope around a “snubbing post” and thereby gain complete control over the animal, despite the horse’s superior size and strength. The cable friction between the rope and the post creates such a large restraining force that the cowboy can easily hold the animal without the help of a knotted rope (Figure 3). In similar fashion in the Hong Kong scaffolding, fixation strength results from the significant amount of cable friction produced when the lashings wrap around one another and around the bamboo poles.
The cable friction concept was pivotal in the development of knotless fixation in arthroscopic rotator cuff repair. In lateral row fixation, the eyelet of the PushLock and SwiveLock suture anchors (Arthrex) produces significant cable friction at the eyelet–suture interface, in addition to frictional force wedging the suture between anchor and bone.
As with so many other devices in shoulder arthroscopy, the SwiveLock suture anchor developed in stages. In the first stage, a chainlike suture with consecutive intersecting links was used (FiberChain). The idea for an adjustable fixation construct came to me because I thought that a forked eyelet on a SwiveLock would provide a firm fixation point when inserted into the appropriate suture link, yet would be totally adjustable simply by choosing a tighter or looser link (Figure 4). Although the system worked very well, it was technically challenging. The process was greatly simplified after Don Grafton and I developed FiberTape and recognized that the power of cable friction was dramatically increased by the larger contact area between the eyelet and the braided FiberTape. The SpeedBridge construct (Arthrex), which enhanced cable friction fixation by means of passing FiberTape through the anchor eyelets, also provided a larger compressive interface at the repair site by using FiberTape rather than conventional suture. These incremental improvements led to what I would characterize as today’s gold standard for arthroscopic rotator cuff repair: a largely knotless linked double-row construct using FiberTape, with cinch-loop sutures at the anterior and posterior margins of the tear to reinforce the cable attachments and simultaneously reduce the dog-ears that typically occur in those locations, and a double-pulley medial mattress if tendon quality is poor (Figure 5).
The Burden of Craft
With all the recent enthusiasm for level I studies, I think we need to examine whether they will accelerate technological advancement in rotator cuff repair. The answer, in my opinion, is a resounding no. This answer is based on a major disconnect I have detected in how we evaluate these studies in rotator cuff disease and repair.
An irony related to technological advancement in surgery is that the more technically advanced the surgery becomes, the more skill is required. This fact is completely at odds with the public’s perception that technological advances make procedures easier. In arthroscopic rotator cuff repair, the surgeon must look, feel, and be aware to a greater degree than in open surgery.
Edward Tenner, in his book Why Things Bite Back, described the burden of the practitioner of any advanced technology as the burden of craft.14 The burden of craft is the inherent demand on all craftspeople, but particularly surgeons, to “up our game” if we are to be successful in our craft. For arthroscopic rotator cuff repair, the burden of craft requires patience, attention to detail, and the ability to work in a virtual space. Not everyone has these skills. But anyone who wants to practice in this discipline has an obligation to learn the skills required, and then to teach them to others and assess how well they are being applied.
The problem with relying on level I studies to assess the efficacy of a surgical procedure is that they are inherently biased by the surgeons involved. As results depend on surgeons’ skills, and surgeons’ skill levels are not equal, level I studies cannot prove what is possible, cannot demonstrate the limits of a technique, and cannot demonstrate the equivalence of techniques.
Amazingly enough, there are still rotator cuff repair “deniers” who confidently assert from the podium that a large percentage of massive cuff tears cannot be repaired and that, even if they can be repaired, they do not have the biological potential to heal. Given the disparity in surgeons’ skills and results, however, one must ask whether poor results are a consequence of a biological deficit in the patient, or of a skill deficit in the surgeon.
What I know is that we have techniques for predictable arthroscopic repair and healing of the vast majority of rotator cuff tears, even massive tears,15-17 and patients do very well clinically. Yet, among many orthopedic surgeons, there is a trend to go straight to reverse total shoulder arthroplasty (rTSA) for massive tears—despite the evidence against it. As reported in the literature, rTSA results are not as good as arthroscopic cuff repair results, and the complication rate for rTSA is much higher.
Why has this trend toward rTSA for massive tears gained so much momentum? The only reason I can surmise is that, for the average surgeon, rTSA is easier and quicker than arthroscopic repair for massive tears. But the reason for choosing a specific type of surgery for a given problem should not be that it is easiest for the surgeon; it should be that it is best for the patient.
The surgeon should start by asking what procedure he or she would want if the roles were reversed—if the surgeon were the patient with the massive rotator cuff tear. If a surgeon does not have the skill set for the best procedure for a particular patient, he or she is obligated to send that patient to a surgeon who does have the skills. In addition, given that infection is the most feared complication in most shoulder surgeries, the surgeon should ask which infection rate would be personally acceptable. Arthroscopic rotator cuff repair has a reported infection rate of 1.6 per 1000, or .0016,18 whereas rTSA has an infection rate about 25 times higher, or .04.19 Further, the surgeon must consider the relative severity of the consequences of infection. By any measure, an infected arthroscopy is a straightforward treatable complication, but an infected shoulder replacement is a human tragedy. Patients vastly prefer the minimally invasive arthroscopic approach, and through online searches can easily identify who can offer an arthroscopic solution.
To reproducibly achieve successful arthroscopic repair of massive rotator cuff tears, the surgeon must know advanced techniques, including subscapularis repair techniques,20,21 interval slides,22,23 and self-reinforcing constructs.24,25
“It’s a poor carpenter who blames his tools.” This 18th-century English proverb is as true today as it was 300 years ago. The tools for arthroscopic cuff repair exist, and they are excellent. The burden of craft is the surgeon’s burden and obligation. As surgeons, we must accept that obligation and the responsibility of that burden.
As mentioned earlier, Dr. Rob Bell’s charge to me when he invited me to give the Neer Lecture was to sum up my involvement in the development of arthroscopic shoulder surgery. The short version is that I have been doing shoulder arthroscopy for 31 years; have received 28 US patents related to shoulder instruments and implants and have 12 US patents pending; have published 167 peer-reviewed articles, a couple dozen book chapters, and 2 textbooks on shoulder arthroscopy; have trained 25 fellows; and have hosted approximately 3000 visiting surgeons in my operating room. My greatest professional dream was to see the standard of care for rotator cuff repair and shoulder instability transition from open to arthroscopic techniques, and I have been fortunate enough to have observed that paradigm shift during my career.
What do I envision over the next 31 years? As we all know, history runs in both directions, and some things simply have not happened yet. In terms of rotator cuff treatment, I think over the next few years the guiding principle of treatment will be joint preservation. All rotator cuff tears, even massive tears, will be repaired arthroscopically. Patients and insurers will demand arthroscopic repair, and surgeons without the skill set will migrate to other subspecialties. As for the role of arthroplasty in the treatment of rotator cuff tears, rTSA will be indicated only for pseudoparalysis after failed cuff repair in low-demand elderly patients.
In rotator cuff treatment, I envision a standard of care that is almost entirely arthroscopic. This standard will demand that surgeons who treat rotator cuff tears be proficient in arthroscopic repair of the full range of tears. Acquiring the skills for arthroscopic repair may not be easy, but then “there’s the easy way, and there’s the Cowboy Way.” As my dad used to tell me when I complained about working too hard, “No man ever drowned in his own sweat.” We shoulder surgeons must accept the burden of craft that accompanies the new standard of arthroscopic cuff repair, and we must offer our patients the same level of care we would choose for ourselves.
Happy trails!
1. Burkhart SS, Athanasiou KA, Wirth MA. Margin convergence: a method of reducing strain in massive rotator cuff tears. Arthroscopy. 1996:12(3):335-338.
2. Kuhn TS. The Structure of Scientific Revolutions. Chicago, IL: University of Chicago Press; 1962.
3. Burkhart SS, Johnson TC, Wirth MA, Athanasiou KA. Cyclic loading of transosseous rotator cuff repairs: tension overload as a possible cause of failure. Arthroscopy. 1997;13(2):172-176.
4. Burkhart SS, Diaz Pagàn JL, Wirth MA, Athansiou KA. Cyclic loading of anchor-based rotator cuff repairs: confirmation of the tension overload phenomenon and comparison of suture anchor fixation with transosseous fixation. Arthroscopy. 1997;13(6):720-724.
5. Burkhart SS, Esch JC, Jolson RS. The rotator crescent and rotator cable: an anatomic description of the shoulder’s “suspension bridge.” Arthroscopy. 1993;9(6):611-616.
6. Burkhart SS, Nottage WM, Ogilvie-Harris DJ, Kohn HS, Pachelli A. Partial repair of irreparable rotator cuff tears. Arthroscopy. 1994;10(4):363-370.
7. Halder AM, O’Driscoll SW, Heers G, et al. Biomechanical comparison of effects of supraspinatus tendon detachments, tendon defects, and muscle retractions. J Bone Joint Surg Am. 2002;84(5):780-785.
8. Lo IK, Burkhart SS, Chan KC, Athanasiou K. Arthroscopic knots: determining the optimal balance of loop security and knot security. Arthroscopy. 2004;20(5):489-502.
9. Lo IK, Ochoa E Jr, Burkhart SS. A comparison of knot security and loop security in arthroscopic knots tied with newer high-strength suture materials. Arthroscopy. 2010;26(9 suppl):S120-S126.
10. Lo IK, Burkhart SS, Athanasiou K. Abrasion resistance of two types of nonabsorbable braided suture. Arthroscopy. 2004;20(4):407-413.
11. Park MC, ElAttrache NS, Tibone JE, Ahmad CS, Jun BJ, Lee TQ. Part I: footprint contact characteristics for a “transosseous-equivalent” rotator cuff repair technique compared to a double-row technique. J Shoulder Elbow Surg. 2007;16(4):461-468.
12. Park MC, Tibone JE, ElAttrache NS, Ahmad CS, Jun BJ, Lee TQ. Part II: biomechanical assessment for a footprint-restoring transosseous-equivalent rotator cuff repair technique compared with a double-row repair technique. J Shoulder Elbow Surg. 2007;16(4):469-476.
13. Burkhart SS, Athanasiou KA. The twist-lock concept of tissue transport and suture fixation without knots: observations along the Hong Kong skyline. Arthroscopy. 2003;19(6):613-625.
14. Tenner E. Why Things Bite Back. New York, NY: Random House; 1996.
15. Denard PJ, Jiwani AZ, Lädermann A, Burkhart SS. Long-term outcome of arthroscopic massive rotator cuff repair: the importance of double-row fixation. Arthroscopy. 2012;28(7):909-915.
16. Denard PJ, Lädermann A, Jiwani AZ, Burkhart SS. Functional outcome after arthroscopic repair of massive rotator cuff tears in individuals with pseudoparalysis. Arthroscopy. 2012;28(9):1214-1219.
17. Lädermann A, Denard PJ, Burkhart SS. Revision arthroscopic rotator cuff repair: systematic review and authors’ preferred surgical technique. Arthroscopy. 2012;28(8):1160-1169.
18. Randelli P, Castagna A, Cabitza F, Cabitza P, Arrigoni P, Denti M. Infectious and thromboembolic complications of arthroscopic shoulder surgery. J Shoulder Elbow Surg. 2010;19(1):97-101.
19. Zumstein MA, Pinedo M, Old J, Boileau P. Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2011;20(1):146-157.
20. Adams CR, Schoolfield JD, Burkhart SS. The results of arthroscopic subscapularis tendon repairs. Arthroscopy. 2008;24(12):1381-1389.
21. Denard PJ, Jiwani AZ, Lädermann A, Burkhart SS. Long-term outcome of a consecutive series of subscapularis tendon tears repaired arthroscopically. Arthroscopy. 2012;28(11):1587-1591.
22. Lo IK, Burkhart SS. Arthroscopic repair of massive, contracted, immobile rotator cuff tears using single and double interval slides: technique and preliminary results. Arthroscopy. 2004;20(1):22-33.
23. Lo IK, Burkhart SS. The interval slide in continuity: a method of mobilizing the anterosuperior rotator cuff without disrupting the tear margins. Arthroscopy. 2004;20(4):435-441.
24. Denard PJ, Burkhart SS. Techniques for managing poor quality tissue and bone during arthroscopic rotator cuff repair. Arthroscopy. 2011;27(10):1409-1421.
25. Burkhart SS, Denard PJ, Konicek J, Hanypsiak BT. Biomechanical validation of load-sharing rip-stop fixation for the repair of tissue-deficient rotator cuff tears. Am J Sports Med. 2014;42(2):457-462.
1. Burkhart SS, Athanasiou KA, Wirth MA. Margin convergence: a method of reducing strain in massive rotator cuff tears. Arthroscopy. 1996:12(3):335-338.
2. Kuhn TS. The Structure of Scientific Revolutions. Chicago, IL: University of Chicago Press; 1962.
3. Burkhart SS, Johnson TC, Wirth MA, Athanasiou KA. Cyclic loading of transosseous rotator cuff repairs: tension overload as a possible cause of failure. Arthroscopy. 1997;13(2):172-176.
4. Burkhart SS, Diaz Pagàn JL, Wirth MA, Athansiou KA. Cyclic loading of anchor-based rotator cuff repairs: confirmation of the tension overload phenomenon and comparison of suture anchor fixation with transosseous fixation. Arthroscopy. 1997;13(6):720-724.
5. Burkhart SS, Esch JC, Jolson RS. The rotator crescent and rotator cable: an anatomic description of the shoulder’s “suspension bridge.” Arthroscopy. 1993;9(6):611-616.
6. Burkhart SS, Nottage WM, Ogilvie-Harris DJ, Kohn HS, Pachelli A. Partial repair of irreparable rotator cuff tears. Arthroscopy. 1994;10(4):363-370.
7. Halder AM, O’Driscoll SW, Heers G, et al. Biomechanical comparison of effects of supraspinatus tendon detachments, tendon defects, and muscle retractions. J Bone Joint Surg Am. 2002;84(5):780-785.
8. Lo IK, Burkhart SS, Chan KC, Athanasiou K. Arthroscopic knots: determining the optimal balance of loop security and knot security. Arthroscopy. 2004;20(5):489-502.
9. Lo IK, Ochoa E Jr, Burkhart SS. A comparison of knot security and loop security in arthroscopic knots tied with newer high-strength suture materials. Arthroscopy. 2010;26(9 suppl):S120-S126.
10. Lo IK, Burkhart SS, Athanasiou K. Abrasion resistance of two types of nonabsorbable braided suture. Arthroscopy. 2004;20(4):407-413.
11. Park MC, ElAttrache NS, Tibone JE, Ahmad CS, Jun BJ, Lee TQ. Part I: footprint contact characteristics for a “transosseous-equivalent” rotator cuff repair technique compared to a double-row technique. J Shoulder Elbow Surg. 2007;16(4):461-468.
12. Park MC, Tibone JE, ElAttrache NS, Ahmad CS, Jun BJ, Lee TQ. Part II: biomechanical assessment for a footprint-restoring transosseous-equivalent rotator cuff repair technique compared with a double-row repair technique. J Shoulder Elbow Surg. 2007;16(4):469-476.
13. Burkhart SS, Athanasiou KA. The twist-lock concept of tissue transport and suture fixation without knots: observations along the Hong Kong skyline. Arthroscopy. 2003;19(6):613-625.
14. Tenner E. Why Things Bite Back. New York, NY: Random House; 1996.
15. Denard PJ, Jiwani AZ, Lädermann A, Burkhart SS. Long-term outcome of arthroscopic massive rotator cuff repair: the importance of double-row fixation. Arthroscopy. 2012;28(7):909-915.
16. Denard PJ, Lädermann A, Jiwani AZ, Burkhart SS. Functional outcome after arthroscopic repair of massive rotator cuff tears in individuals with pseudoparalysis. Arthroscopy. 2012;28(9):1214-1219.
17. Lädermann A, Denard PJ, Burkhart SS. Revision arthroscopic rotator cuff repair: systematic review and authors’ preferred surgical technique. Arthroscopy. 2012;28(8):1160-1169.
18. Randelli P, Castagna A, Cabitza F, Cabitza P, Arrigoni P, Denti M. Infectious and thromboembolic complications of arthroscopic shoulder surgery. J Shoulder Elbow Surg. 2010;19(1):97-101.
19. Zumstein MA, Pinedo M, Old J, Boileau P. Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2011;20(1):146-157.
20. Adams CR, Schoolfield JD, Burkhart SS. The results of arthroscopic subscapularis tendon repairs. Arthroscopy. 2008;24(12):1381-1389.
21. Denard PJ, Jiwani AZ, Lädermann A, Burkhart SS. Long-term outcome of a consecutive series of subscapularis tendon tears repaired arthroscopically. Arthroscopy. 2012;28(11):1587-1591.
22. Lo IK, Burkhart SS. Arthroscopic repair of massive, contracted, immobile rotator cuff tears using single and double interval slides: technique and preliminary results. Arthroscopy. 2004;20(1):22-33.
23. Lo IK, Burkhart SS. The interval slide in continuity: a method of mobilizing the anterosuperior rotator cuff without disrupting the tear margins. Arthroscopy. 2004;20(4):435-441.
24. Denard PJ, Burkhart SS. Techniques for managing poor quality tissue and bone during arthroscopic rotator cuff repair. Arthroscopy. 2011;27(10):1409-1421.
25. Burkhart SS, Denard PJ, Konicek J, Hanypsiak BT. Biomechanical validation of load-sharing rip-stop fixation for the repair of tissue-deficient rotator cuff tears. Am J Sports Med. 2014;42(2):457-462.