User login
The Hand That Feeds You
A 66‐year‐old man presented to the Emergency Department (ED) with rash and malaise in early April. He was in his usual state of good health until the morning of presentation, when he awoke feeling lethargic. Over the course of the day, his hands and feet grew cold and numb, his nose became dark red, and he developed a diffuse, net‐like red rash over his legs, hands, buttocks, and trunk. He had multiple maroon bowel movements. His wife noted that he became incoherent and brought him to the ED.
This apparently previously healthy man presented with an acute episode of fatigue and altered mental status accompanied by a prominent cutaneous eruption. The differential diagnosis will ultimately be guided by the morphology of the rash. At this stage, infectious diseases, drug or toxin exposure, and allergic processes including anaphylaxis must all be considered in this patient with rash and acute illness. The maroon bowel movements likely represent a gastrointestinal bleed that may be part of a unifying diagnosisa hematologic disorder, a vasculitis, or liver disease.
In the ED, the patient was reportedly febrile (exact temperature not recorded) with a blood pressure of 96/54 mmHg. He had pulse oximetry of 88% on room air and a diffuse purpuric rash. The patient was noted to have a leukocytosis, thrombocytopenia, coagulopathy, and an elevation of his creatinine and cardiac enzymes. He was given fluids, fresh frozen plasma, and broad‐spectrum antibiotics, and transferred directly to the intensive care unit of a tertiary medical center for further management.
Upon arrival to the intensive care unit, he complained of fatigue, progression of his nonpruritic, nonpainful rash, and worsening numbness and tingling of his extremities. He denied headache, nuchal rigidity, photophobia, vision or hearing changes, chest pain, cough, abdominal pain, myalgias, or arthralgias. While being interviewed, he had dark brown emesis and a bloody bowel movement.
The patient's past medical history included bacterial pericarditis as a teenager and remote hepatitis of unclear etiology. He rarely saw a physician, took no medications, and had no known medication allergies.
The patient worked as president of a software company and lived with his wife. He had smoked 1 to 2 packs of cigarettes a day for the past 30 years. He endorsed 2 of 4 CAGE criteria (need to Cut down, Annoyed when asked about alcohol, feel Guilty about drinking, need for an Eye opener), and his wife and had never been tested for human immunodeficiency virus (HIV). Family history was unremarkable.
The patient's presentation is concerning for a life‐threatening disease process with a rapid course. In the setting of the laboratory abnormalities demonstrating multi‐organ dysfunction, aggressive volume resuscitation and prompt initiation of broad‐spectrum antibiotics are indicated. The history does not reveal an obvious source of infection or exposure to a new drug, toxin, or allergen. His apparent gastrointestinal bleed could be explained by complications of liver disease from chronic alcohol use. For example, he could have variceal bleeding or gastropathy from portal hypertension. Alternatively, he may have bleeding secondary to a coagulopathy from decreased synthetic function of clotting factors. Other possibilities include a perforated viscus (eg, peptic ulcer) leading to bleeding and peritonitis or mesenteric ischemia, though the absence of abdominal pain makes these unlikely.
At this point, the overall presentation is most concerning for infection, especially given his chronic alcohol use and the vague history of hepatitis. The acute onset and severity of the illness are consistent with an aggressive, suppurative bacterial infection. The most likely causative organisms include gram‐negative bacteria, especially Neisseria meningitidis (with or without meningitis), as well as Staphylococcus aureus, Streptococcus pyogenes, and Rickettsia rickettsii (Rocky Mountain spotted fever).
Several months prior to presentation, he had traveled to Mexico. Two months prior to presentation, he made a trip to North Carolina and Ohio to visit his brother, who subsequently died of pneumonia. One month prior to presentation, he had traveled to urban China for work.
Because the presentation is so acute and the patient's travel took place over 1 month ago, this is unlikely to be a travel‐associated illness. Furthermore, the course is too acute to be consistent with endemic diseases of Central America and the midwestern United States, such as tuberculosis, brucellosis, and histoplasmosis.
He had a temperature of 38.7C. His heart rate was 110 beats per minute. His blood pressure was 115/78 mmHg, respiratory rate was 24 breaths per minute, and oxygen saturation was 99% on 6 liters via nasal cannula. The patient was a well‐nourished, middle‐aged man who appeared uncomfortable. He was in mild respiratory distress, though able to speak in full sentences. He was alert, coherent, and oriented to self, place, date, and time.
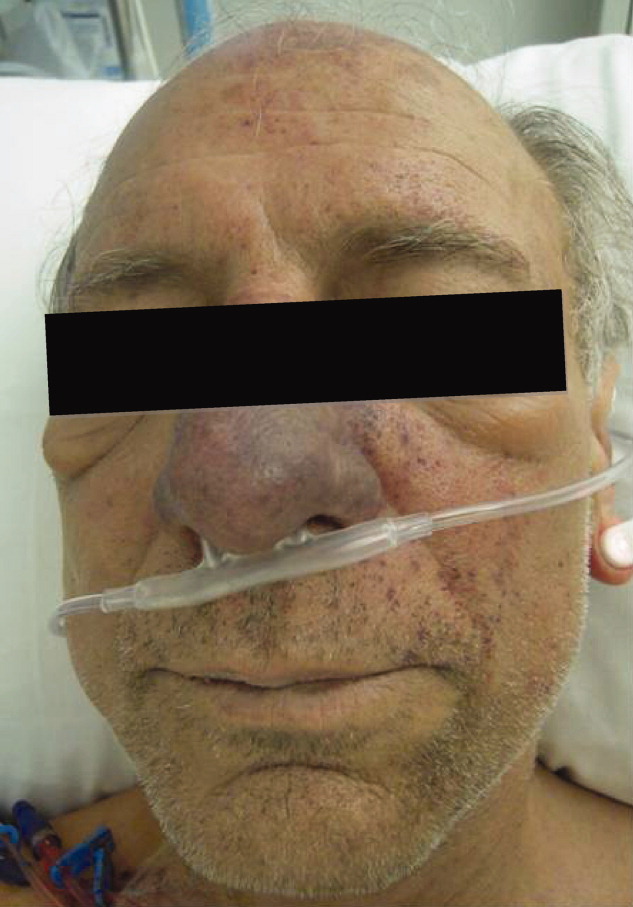
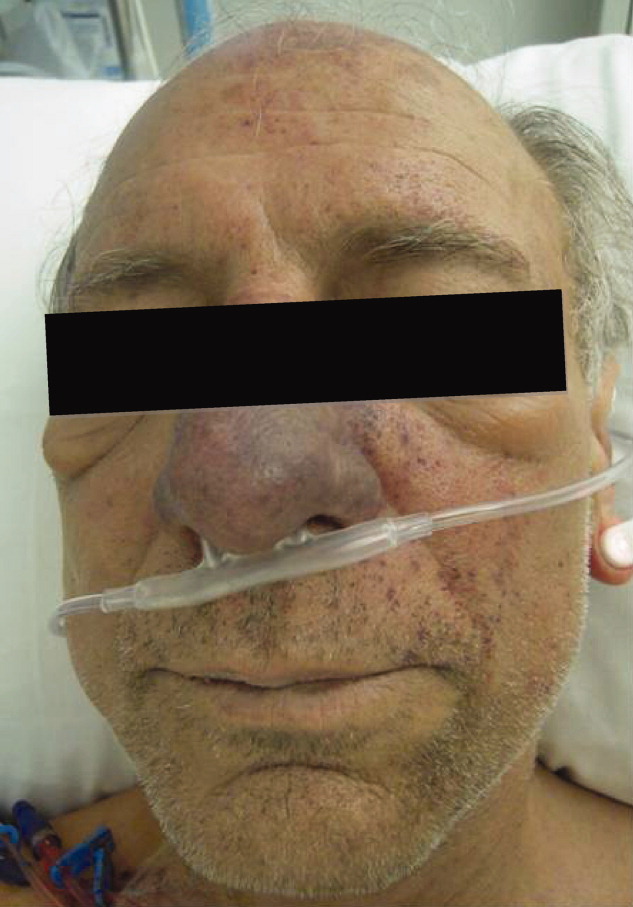
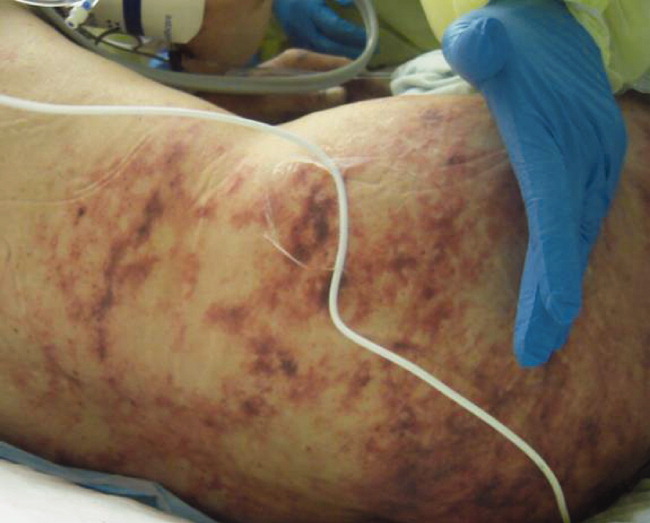
Skin examination revealed nonblanching purpuric papules coalescing into stellate plaques on his scalp, forehead, nose, cheeks, bilateral ears, hands, and feet (Figure 1). Acral surfaces, including hands and feet, were cyanotic without evidence of gangrene. He had nonblanching retiform purpuric plaques on his right flank, lower abdomen, low back, buttock, penis, scrotum, thighs, and legs (Figure 2). His right dorsal hand had 3 healing erosions of 3 to 10 mm in size without associated edema, erythema, or drainage.
Mucous membranes were dry without lesions. Cardiac examination demonstrated tachycardia without appreciable murmur. He was mildly tachypneic and his lungs were clear to auscultation without adventitious breath sounds. His abdominal examination was unremarkable. His hands and feet were cool with decreased sensation to touch. He had full range of motion and intact muscle strength, but mild bilateral dysmetria with finger‐nose‐finger testing. His radial and dorsalis pedis pulses were symmetric and brisk. Rectal exam revealed guaiac‐positive stool.
The patient's vital signs are compatible with the systemic inflammatory response syndrome. The presence of retiform purpura raises concerns for a systemic vasculitis with destruction of the vessel wall, or intravascular occlusion with thrombosis or emboli. Absence of murmur does not rule out endocarditis but makes it less likely. He has no risk factors for vasculitis, so the purpura, in conjunction with both bleeding and thrombosis, is much more suggestive of disseminated intravascular coagulation (DIC). This clotting disorder can result from a noninfectious trigger, such as acute pancreatitis or malignancy, but his presentation is more worrisome for a severe infection leading to DIC and complicated by purpura fulminans. He does not show signs of hepatic encephalopathy or cirrhosis, making decompensated liver disease a less likely inciting factor of his presentation.
Further exposure history was obtained: The patient often spent time outdoors near his rural home and used a weed‐whacker in his yard the day before admission. He owned 3 horses which he fed and often rode. He had 3 healthy dogs and had been bitten in attempts to break up fights among them, most recently 3 days prior to admission. He lived in mountain lion territory but had no direct exposure to lions. He had no known insect bites. He regularly drank well water, and consumed medium‐rare hamburgers 4 days prior to admission. One week prior to admission, a child with possible streptococcal pharyngitis visited his home.
With this history, the patient was treated with aggressive intravenous fluids and meningeal doses of ceftriaxone, vancomycin, and metronidazole.
In the summer, outdoor exposure to brush confers a risk of tick‐borne infections, including rickettsial diseases, ehrlichiosis, and spirochetal relapsing fever. However, this patient presented in the spring, and apart from rickettsial spotted fever, these illnesses tend to be indolent. It is conceivable, though unlikely, that the weed‐cutting device may have aerosolized fulminant zoonotic pathogens such as Francisella tularensis or plague that can be found in mountain lion territory.
Well water exposure suggests leptospirosis, which can present in a fulminant fashion with multi‐organ dysfunction, but is more often a subacute illness (developing over many days to a week or two). His ingestion of potentially undercooked meat raises the possibility of enterohemorrhagic infection complicated by the hemolytic uremic syndrome (HUS). However, while the purpuric rash and renal failure are compatible with HUS, the pace of illness and accompanying hypotension once again favor alternative infectious diagnoses.
The incubation period and presentation is concerning overwhelming bacterial infection related to the dog bite. Microbiological considerations include streptococcal species, Staphylococcus aureus, and gram‐negative organisms including Pasteurella species and Capnocytophaga canimorsus. The latter 2 organisms are of particular interest since they tend to cause severe sepsis in patients with alcoholism.
The antibiotic selection in this case is not straightforward. In general, empiric therapy for infections related to dog bites should include treatment for beta‐lactamaseproducing bacteria and anaerobes (eg, piperacillin/tazobactam). Yet, given the clinical presentation, severity of illness, and possible DIC, it is appropriate to be concerned about meningococcemia. Unfortunately, the tazobactam in piperacillin/tazobactam has poor central nervous system penetration so would be suboptimal treatment for meningitis. At this point, ceftriaxone, vancomycin, and metronidazole is a reasonable regimen.
Laboratory results were notable for blood urea nitrogen 50 mg/dL, creatinine 3.47 mg/dL, white cell count 21,800/L, with an absolute neutrophil count of 20,690/L, hematocrit 35.9%, platelet count 34,000/L, International Normalized Ratio 1.5, and partial thromboplastin time 44.0 seconds. His alanine aminotransferase was 356 U/L (1641 U/L), aspartate aminotransferase 959 U/L (1259 U/L), alkaline phosphatase 50 U/L (29111 U/L), and total bilirubin 1.7 mg/dL (0.31.3 mg/dL). Fibrinogen was 283 g/L (202430 g/L), lactate dehydrogenase was 1883 U/L (91185 IU/L), and uric acid was 10.5 mg/dL (3.77.7 mg/dL). His troponin I was 1.18 ng/mL (<0.05 ng/ml), and his electrocardiogram showed sinus tachycardia but no evidence of myocardial ischemia. Chest x‐ray showed no infiltrate or evidence of volume overload. Lumbar puncture was deferred out of concern for ongoing disseminated intravascular coagulation.
Transthoracic echocardiogram revealed global hypokinesis and reduced left ventricular systolic function with ejection fraction of 35%. There was no evidence of vegetations or thrombus.
The patient's thrombocytopenia and prolonged coagulation parameters further support the presence of DIC. A peripheral blood smear should be examined. If microangiopathic changes are found, other diagnoses such as thrombotic thrombocytopenic purpura might be considered, although the rapid pace of illness and presence of hypotension still make sepsis with DIC more likely.
While septic shock often causes multi‐organ system failure secondary to hypoperfusion, the presumed rapid onset of hepatic and renal abnormalities suggests that microvascular thrombosis is playing a larger role in his organ system dysfunction. Microvascular thrombosis could also contribute to his myocardial injury, though globally depressed ejection fraction and elevated troponin might also be explained by infectious myocarditis. A third possibility is that his severe sepsis caused his myocardial dysfunction. Regardless of its etiology, the patient has no clinical evidence of congestive heart failure, so no specific therapy is required at this time. However, his cardiopulmonary exam should be monitored closely, and if he survives, he should have repeat echocardiography to monitor for resolution of the global hypokinesis.
Further evaluation revealed creatine kinase of 45,000 ng/ml (55380 ng/ml) and repeat troponin of >22 ng/ml. Protein C level was low at 30%. Testing for HIV was negative. Blood smear from time of transfer had few schistocytes. Urinalysis showed muddy brown casts but no dysmorphic red blood cells or red cell casts. The patient was placed on continuous veno‐venous hemofiltration (CVVH) for worsening renal failure and oliguria from presumed acute tubular necrosis in the setting of rhabdomyolysis and sepsis.
The patient has severe rhabdomyolysis that cannot fully be explained by his initial hypoperfusion and is more likely related to the overwhelming infection and microthrombosis. Rhabdomyolysis probably contributed to his acute tubular necrosis and renal failure.
Dermatology consultation identified the rash as likely purpura fulminans. They recommended a skin biopsy to rule out vasculitis. Three skin biopsies revealed micro‐vascular thrombosis; direct immunofluorescence test was negative for vasculitis; his skin tissue culture was negative for bacterial, mycobacterial, and fungal organisms.
Input from the dermatology service was key in identifying the rash. Purpura fulminans has a limited differential that includes severe infection from gram‐negative organisms and protein C and S deficiency. Since the biopsy results made vasculitis unlikely, the team was able to focus greater attention on potential pathogens such as Pasteurella species and C. canimorsus.
The biopsy also confirms the clinical suspicion that microvascular thrombosis is causing the patient's acute kidney injury, rhabdomyolysis, and myocardial ischemia. The presence of microvascular thrombosis prompts consideration of antithrombotic therapy such as heparin, but benefits of this therapy must be weighed against contraindications including bleeding and thrombocytopenia.
Ultimately out of concerns for recurrent gastrointestinal bleeding, the primary team decided not to treat with heparin or other antithrombotic therapy.
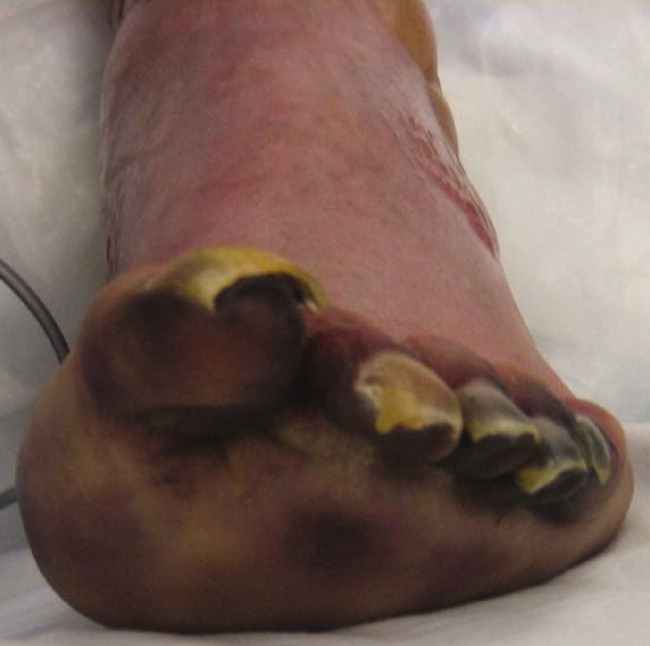
After several days of supportive care with antibiotics and renal replacement therapy, the patient showed gradual improvement of his retiform purpura, sensory neuropathy, laboratory data, and other markers of end‐organ dysfunction. Purpura of his fingertips, feet, and toes progressed to dry gangrene (Figure 3), which was monitored for potential need for amputation. He remained dependent on intermittent hemodialysis.
His initial antibiotic regimen was narrowed to ceftriaxone monotherapy. Five days after initial presentation, blood cultures drawn from the outside emergency department grew a gram‐negative rod in the anaerobic broth. Ten days later, this gram‐negative rod was identified as Capnocytophaga canimorsus. He was ultimately discharged to a skilled nursing facility.
Generally growth of an organism in broth only suggests either a very low inoculum or that the isolate is a contaminant. In this case, it was because the causative organism, C. canimorsus, is an obligate anaerobe and quite fastidious, so unlikely to grow easily. The identification of C. canimorsus from the initial blood culture is not surprising in this patient who presented with severe sepsis, DIC, and purpura fulminans after a recent dog bite. While the patient's chronic alcohol use may explain his fulminant infection from an atypical organism, one should always consider occult underlying malignancy as a predisposing factor, particularly in patients of this age group.
With the appropriate course of antibiotics, C. canimorsus infection should be completely cured. However, recovery of kidney and cardiac function could take weeks to months, and his dry gangrene may or may not resolve.
COMMENTARY
Capnocytophaga canimorsis sepsis is a rare and potentially deadly complication of dog bites that can present with rash, cellulitis, purpura fulminans, arthritis, meningitis, and endocarditis. The discussant considered a broad differential for the presentation of fever, rash, and acute illness. While the travel history was intriguing, the severity and pace of illness allowed him to focus attention on more recent infectious exposures. The ultimate key to the diagnosis was the patient's history of dog bite, an important but underrecognized source of serious infection in the United States.
According to the Centers for Disease Control and Prevention, there are approximately 4 million dog bites in the country each year. Of these, 300,000 bite victims seek care in the emergency department, resulting in 13,000 hospitalizations and 20 deaths annually.1 Infected dog bite wounds often grow polymicrobial flora. Pasteurella species are the most frequently found organisms in both dog and cat bite wounds. However, other aerobes such as streptococci, staphylococci, Moraxella, and Neisseria, as well as anaerobes including Fusobacterium and Bacteroides species, are also common.2
C. canimorsis is a facultative, fastidious gram‐negative bacillus found in the mouth flora of not only dogs but also cats and humans. It is often mistaken for other gram‐negative rod species.3 As with the patient described in this report, systemic infection from C. canimorsis can follow even superficial or well‐healed bite wounds.
Since this bacterium was first described in the literature 30 years ago, more than 100 cases of C. canimorsus infection have been described, with a mortality rate of nearly 30%.4 C. canimorsus occurs more frequently in males and in patients 50 to 70 years of age. Traditional risk factors include alcohol abuse, asplenia, immunosuppression, and corticosteroid treatment. However, in a case series of 56 isolates in California, only 10% of patients with Capnocytophaga sepsis were asplenic and none had alcohol abuse reported in their medical charts. In this series, median time from dog bite to the onset of symptoms was 3 days. Eighty‐five percent of patients presented with fever, while 32% had sepsis and 13% had DIC or septic shock.3
While C. canimorsus was once susceptible to a range of antibiotics, several reports from Canada and Europe document rising rates of beta‐lactamaseproducing strains that have caused clinically significant disease.5, 6 Individual susceptibility data take days to obtain, so it is important to start with empiric therapy. In general, empiric therapy for all serious dog bites should cover beta‐lactamaseproducing bacteria and anaerobes, for example, with amoxicillin/clavulanate, ampicillin/sulbactam, or piperacillin/tazobactam. If the patient is allergic to penicillin, clindamycin plus a fluoroquinolone can be used instead.
There are previous reports of purpura fulminans and symmetric peripheral gangrene following Capnocytophaga infection from dog bites.7, 8 Purpura fulminans is defined as rapidly progressive skin necrosis due to dermal vascular thrombosis, often in the setting of DIC. Early involvement occurs at acral sites, such as the nose, ears, fingers, and toes. Purpuric lesions often progress to skin necrosis or dry gangrene within 24 to 48 hours. In a review of 12 patients with purpura fulminans, only 9 survived. Eight of the 9 survivors required amputation of at least 1 limb, and 4 of them required 4‐limb amputation.7
In this patient who presented with fever and rash, the discussant recognized early on an underlying infectious etiology. Although the patient's exposure history led the discussant to consider a host of possibilities, the recognition of purpura fulminans allowed him to narrow his differential. Ultimately, the dog's bite clinched the diagnosis.
KEY TEACHING POINTS
-
Sepsis caused by C. canimorsus is often characterized by rash, cellulitis, arthritis, meningitis, and endocarditis. In some instances, infection can progress to purpura fulminans.
-
In cases where fastidious organisms are suspected as an infectious source, microbiology labs should be notified of suspected organisms so they can extend incubation periods or use special media to maximize culture yield and the likelihood of accurate identification.
-
Empiric therapy for serious dog bites should cover beta‐lactamaseproducing bacteria and anaerobes. Consider using amoxicillin/clavulanate, ampicillin/sulbactam, or piperacillin/tazobactam.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
This icon represents the patient's case. Each paragraph that follows represents the discussant's thoughts.
Acknowledgements
The authors thank Snigdha Vallabhaneni, MD, from the UCSF Division of Infectious Diseases, for her contributions to the discussion on C. canimorsus. They also thank Kanade Shinkai, MD, PhD, from the UCSF Department of Dermatology, and Heather Nye, MD, PhD, from the UCSF Division of Hospital Medicine, for their review of the manuscript.
Disclosure: Nothing to report.
- ,,.Incidence of dog bite injuries treated in emergency departments.JAMA.1998;279:51–53.
- ,,,,.Bacteriologic analysis of infected dog and cat bites. Emergency Medicine Animal Bite Infection Study Group.N Engl J Med.1999;340:85–92.
- ,,,.Diagnosing Capnocytophaga canimorsus infections.Emerg Infect Dis.2006;12:340–342.
- ,,.Capnocytophaga canimorsus infections in human: review of the literature and cases report.Eur J Epidemiol.1996;12:521–533.
- ,,,,.Antimicrobial susceptibilities and beta‐lactamase characterization of Capnocytophaga species.Antimicrob Agents Chemother.1992;36:2197–2200.
- ,,,,,.Bacteremia due to Capnocytophaga species in patients with neutropenia: high frequency of beta‐lactamase‐producing strains.Clin Infect Dis.1999;28:1172–1174.
- ,,.Presentation and outcome of purpura fulminans associated with peripheral gangrene in 12 patients at Mayo Clinic.J Am Acad Dermatol.2007;57:944–956.
- ,,,.Capnocytophaga canimorsus sepsis with purpura fulminans and symmetrical gangrene following a dog bite in a shelter employee.Am J Med Sci.2004:327:369–372.
A 66‐year‐old man presented to the Emergency Department (ED) with rash and malaise in early April. He was in his usual state of good health until the morning of presentation, when he awoke feeling lethargic. Over the course of the day, his hands and feet grew cold and numb, his nose became dark red, and he developed a diffuse, net‐like red rash over his legs, hands, buttocks, and trunk. He had multiple maroon bowel movements. His wife noted that he became incoherent and brought him to the ED.
This apparently previously healthy man presented with an acute episode of fatigue and altered mental status accompanied by a prominent cutaneous eruption. The differential diagnosis will ultimately be guided by the morphology of the rash. At this stage, infectious diseases, drug or toxin exposure, and allergic processes including anaphylaxis must all be considered in this patient with rash and acute illness. The maroon bowel movements likely represent a gastrointestinal bleed that may be part of a unifying diagnosisa hematologic disorder, a vasculitis, or liver disease.
In the ED, the patient was reportedly febrile (exact temperature not recorded) with a blood pressure of 96/54 mmHg. He had pulse oximetry of 88% on room air and a diffuse purpuric rash. The patient was noted to have a leukocytosis, thrombocytopenia, coagulopathy, and an elevation of his creatinine and cardiac enzymes. He was given fluids, fresh frozen plasma, and broad‐spectrum antibiotics, and transferred directly to the intensive care unit of a tertiary medical center for further management.
Upon arrival to the intensive care unit, he complained of fatigue, progression of his nonpruritic, nonpainful rash, and worsening numbness and tingling of his extremities. He denied headache, nuchal rigidity, photophobia, vision or hearing changes, chest pain, cough, abdominal pain, myalgias, or arthralgias. While being interviewed, he had dark brown emesis and a bloody bowel movement.
The patient's past medical history included bacterial pericarditis as a teenager and remote hepatitis of unclear etiology. He rarely saw a physician, took no medications, and had no known medication allergies.
The patient worked as president of a software company and lived with his wife. He had smoked 1 to 2 packs of cigarettes a day for the past 30 years. He endorsed 2 of 4 CAGE criteria (need to Cut down, Annoyed when asked about alcohol, feel Guilty about drinking, need for an Eye opener), and his wife and had never been tested for human immunodeficiency virus (HIV). Family history was unremarkable.
The patient's presentation is concerning for a life‐threatening disease process with a rapid course. In the setting of the laboratory abnormalities demonstrating multi‐organ dysfunction, aggressive volume resuscitation and prompt initiation of broad‐spectrum antibiotics are indicated. The history does not reveal an obvious source of infection or exposure to a new drug, toxin, or allergen. His apparent gastrointestinal bleed could be explained by complications of liver disease from chronic alcohol use. For example, he could have variceal bleeding or gastropathy from portal hypertension. Alternatively, he may have bleeding secondary to a coagulopathy from decreased synthetic function of clotting factors. Other possibilities include a perforated viscus (eg, peptic ulcer) leading to bleeding and peritonitis or mesenteric ischemia, though the absence of abdominal pain makes these unlikely.
At this point, the overall presentation is most concerning for infection, especially given his chronic alcohol use and the vague history of hepatitis. The acute onset and severity of the illness are consistent with an aggressive, suppurative bacterial infection. The most likely causative organisms include gram‐negative bacteria, especially Neisseria meningitidis (with or without meningitis), as well as Staphylococcus aureus, Streptococcus pyogenes, and Rickettsia rickettsii (Rocky Mountain spotted fever).
Several months prior to presentation, he had traveled to Mexico. Two months prior to presentation, he made a trip to North Carolina and Ohio to visit his brother, who subsequently died of pneumonia. One month prior to presentation, he had traveled to urban China for work.
Because the presentation is so acute and the patient's travel took place over 1 month ago, this is unlikely to be a travel‐associated illness. Furthermore, the course is too acute to be consistent with endemic diseases of Central America and the midwestern United States, such as tuberculosis, brucellosis, and histoplasmosis.
He had a temperature of 38.7C. His heart rate was 110 beats per minute. His blood pressure was 115/78 mmHg, respiratory rate was 24 breaths per minute, and oxygen saturation was 99% on 6 liters via nasal cannula. The patient was a well‐nourished, middle‐aged man who appeared uncomfortable. He was in mild respiratory distress, though able to speak in full sentences. He was alert, coherent, and oriented to self, place, date, and time.
Skin examination revealed nonblanching purpuric papules coalescing into stellate plaques on his scalp, forehead, nose, cheeks, bilateral ears, hands, and feet (Figure 1). Acral surfaces, including hands and feet, were cyanotic without evidence of gangrene. He had nonblanching retiform purpuric plaques on his right flank, lower abdomen, low back, buttock, penis, scrotum, thighs, and legs (Figure 2). His right dorsal hand had 3 healing erosions of 3 to 10 mm in size without associated edema, erythema, or drainage.


Mucous membranes were dry without lesions. Cardiac examination demonstrated tachycardia without appreciable murmur. He was mildly tachypneic and his lungs were clear to auscultation without adventitious breath sounds. His abdominal examination was unremarkable. His hands and feet were cool with decreased sensation to touch. He had full range of motion and intact muscle strength, but mild bilateral dysmetria with finger‐nose‐finger testing. His radial and dorsalis pedis pulses were symmetric and brisk. Rectal exam revealed guaiac‐positive stool.
The patient's vital signs are compatible with the systemic inflammatory response syndrome. The presence of retiform purpura raises concerns for a systemic vasculitis with destruction of the vessel wall, or intravascular occlusion with thrombosis or emboli. Absence of murmur does not rule out endocarditis but makes it less likely. He has no risk factors for vasculitis, so the purpura, in conjunction with both bleeding and thrombosis, is much more suggestive of disseminated intravascular coagulation (DIC). This clotting disorder can result from a noninfectious trigger, such as acute pancreatitis or malignancy, but his presentation is more worrisome for a severe infection leading to DIC and complicated by purpura fulminans. He does not show signs of hepatic encephalopathy or cirrhosis, making decompensated liver disease a less likely inciting factor of his presentation.
Further exposure history was obtained: The patient often spent time outdoors near his rural home and used a weed‐whacker in his yard the day before admission. He owned 3 horses which he fed and often rode. He had 3 healthy dogs and had been bitten in attempts to break up fights among them, most recently 3 days prior to admission. He lived in mountain lion territory but had no direct exposure to lions. He had no known insect bites. He regularly drank well water, and consumed medium‐rare hamburgers 4 days prior to admission. One week prior to admission, a child with possible streptococcal pharyngitis visited his home.
With this history, the patient was treated with aggressive intravenous fluids and meningeal doses of ceftriaxone, vancomycin, and metronidazole.
In the summer, outdoor exposure to brush confers a risk of tick‐borne infections, including rickettsial diseases, ehrlichiosis, and spirochetal relapsing fever. However, this patient presented in the spring, and apart from rickettsial spotted fever, these illnesses tend to be indolent. It is conceivable, though unlikely, that the weed‐cutting device may have aerosolized fulminant zoonotic pathogens such as Francisella tularensis or plague that can be found in mountain lion territory.
Well water exposure suggests leptospirosis, which can present in a fulminant fashion with multi‐organ dysfunction, but is more often a subacute illness (developing over many days to a week or two). His ingestion of potentially undercooked meat raises the possibility of enterohemorrhagic infection complicated by the hemolytic uremic syndrome (HUS). However, while the purpuric rash and renal failure are compatible with HUS, the pace of illness and accompanying hypotension once again favor alternative infectious diagnoses.
The incubation period and presentation is concerning overwhelming bacterial infection related to the dog bite. Microbiological considerations include streptococcal species, Staphylococcus aureus, and gram‐negative organisms including Pasteurella species and Capnocytophaga canimorsus. The latter 2 organisms are of particular interest since they tend to cause severe sepsis in patients with alcoholism.
The antibiotic selection in this case is not straightforward. In general, empiric therapy for infections related to dog bites should include treatment for beta‐lactamaseproducing bacteria and anaerobes (eg, piperacillin/tazobactam). Yet, given the clinical presentation, severity of illness, and possible DIC, it is appropriate to be concerned about meningococcemia. Unfortunately, the tazobactam in piperacillin/tazobactam has poor central nervous system penetration so would be suboptimal treatment for meningitis. At this point, ceftriaxone, vancomycin, and metronidazole is a reasonable regimen.
Laboratory results were notable for blood urea nitrogen 50 mg/dL, creatinine 3.47 mg/dL, white cell count 21,800/L, with an absolute neutrophil count of 20,690/L, hematocrit 35.9%, platelet count 34,000/L, International Normalized Ratio 1.5, and partial thromboplastin time 44.0 seconds. His alanine aminotransferase was 356 U/L (1641 U/L), aspartate aminotransferase 959 U/L (1259 U/L), alkaline phosphatase 50 U/L (29111 U/L), and total bilirubin 1.7 mg/dL (0.31.3 mg/dL). Fibrinogen was 283 g/L (202430 g/L), lactate dehydrogenase was 1883 U/L (91185 IU/L), and uric acid was 10.5 mg/dL (3.77.7 mg/dL). His troponin I was 1.18 ng/mL (<0.05 ng/ml), and his electrocardiogram showed sinus tachycardia but no evidence of myocardial ischemia. Chest x‐ray showed no infiltrate or evidence of volume overload. Lumbar puncture was deferred out of concern for ongoing disseminated intravascular coagulation.
Transthoracic echocardiogram revealed global hypokinesis and reduced left ventricular systolic function with ejection fraction of 35%. There was no evidence of vegetations or thrombus.
The patient's thrombocytopenia and prolonged coagulation parameters further support the presence of DIC. A peripheral blood smear should be examined. If microangiopathic changes are found, other diagnoses such as thrombotic thrombocytopenic purpura might be considered, although the rapid pace of illness and presence of hypotension still make sepsis with DIC more likely.
While septic shock often causes multi‐organ system failure secondary to hypoperfusion, the presumed rapid onset of hepatic and renal abnormalities suggests that microvascular thrombosis is playing a larger role in his organ system dysfunction. Microvascular thrombosis could also contribute to his myocardial injury, though globally depressed ejection fraction and elevated troponin might also be explained by infectious myocarditis. A third possibility is that his severe sepsis caused his myocardial dysfunction. Regardless of its etiology, the patient has no clinical evidence of congestive heart failure, so no specific therapy is required at this time. However, his cardiopulmonary exam should be monitored closely, and if he survives, he should have repeat echocardiography to monitor for resolution of the global hypokinesis.
Further evaluation revealed creatine kinase of 45,000 ng/ml (55380 ng/ml) and repeat troponin of >22 ng/ml. Protein C level was low at 30%. Testing for HIV was negative. Blood smear from time of transfer had few schistocytes. Urinalysis showed muddy brown casts but no dysmorphic red blood cells or red cell casts. The patient was placed on continuous veno‐venous hemofiltration (CVVH) for worsening renal failure and oliguria from presumed acute tubular necrosis in the setting of rhabdomyolysis and sepsis.
The patient has severe rhabdomyolysis that cannot fully be explained by his initial hypoperfusion and is more likely related to the overwhelming infection and microthrombosis. Rhabdomyolysis probably contributed to his acute tubular necrosis and renal failure.
Dermatology consultation identified the rash as likely purpura fulminans. They recommended a skin biopsy to rule out vasculitis. Three skin biopsies revealed micro‐vascular thrombosis; direct immunofluorescence test was negative for vasculitis; his skin tissue culture was negative for bacterial, mycobacterial, and fungal organisms.
Input from the dermatology service was key in identifying the rash. Purpura fulminans has a limited differential that includes severe infection from gram‐negative organisms and protein C and S deficiency. Since the biopsy results made vasculitis unlikely, the team was able to focus greater attention on potential pathogens such as Pasteurella species and C. canimorsus.
The biopsy also confirms the clinical suspicion that microvascular thrombosis is causing the patient's acute kidney injury, rhabdomyolysis, and myocardial ischemia. The presence of microvascular thrombosis prompts consideration of antithrombotic therapy such as heparin, but benefits of this therapy must be weighed against contraindications including bleeding and thrombocytopenia.
Ultimately out of concerns for recurrent gastrointestinal bleeding, the primary team decided not to treat with heparin or other antithrombotic therapy.
After several days of supportive care with antibiotics and renal replacement therapy, the patient showed gradual improvement of his retiform purpura, sensory neuropathy, laboratory data, and other markers of end‐organ dysfunction. Purpura of his fingertips, feet, and toes progressed to dry gangrene (Figure 3), which was monitored for potential need for amputation. He remained dependent on intermittent hemodialysis.

His initial antibiotic regimen was narrowed to ceftriaxone monotherapy. Five days after initial presentation, blood cultures drawn from the outside emergency department grew a gram‐negative rod in the anaerobic broth. Ten days later, this gram‐negative rod was identified as Capnocytophaga canimorsus. He was ultimately discharged to a skilled nursing facility.
Generally growth of an organism in broth only suggests either a very low inoculum or that the isolate is a contaminant. In this case, it was because the causative organism, C. canimorsus, is an obligate anaerobe and quite fastidious, so unlikely to grow easily. The identification of C. canimorsus from the initial blood culture is not surprising in this patient who presented with severe sepsis, DIC, and purpura fulminans after a recent dog bite. While the patient's chronic alcohol use may explain his fulminant infection from an atypical organism, one should always consider occult underlying malignancy as a predisposing factor, particularly in patients of this age group.
With the appropriate course of antibiotics, C. canimorsus infection should be completely cured. However, recovery of kidney and cardiac function could take weeks to months, and his dry gangrene may or may not resolve.
COMMENTARY
Capnocytophaga canimorsis sepsis is a rare and potentially deadly complication of dog bites that can present with rash, cellulitis, purpura fulminans, arthritis, meningitis, and endocarditis. The discussant considered a broad differential for the presentation of fever, rash, and acute illness. While the travel history was intriguing, the severity and pace of illness allowed him to focus attention on more recent infectious exposures. The ultimate key to the diagnosis was the patient's history of dog bite, an important but underrecognized source of serious infection in the United States.
According to the Centers for Disease Control and Prevention, there are approximately 4 million dog bites in the country each year. Of these, 300,000 bite victims seek care in the emergency department, resulting in 13,000 hospitalizations and 20 deaths annually.1 Infected dog bite wounds often grow polymicrobial flora. Pasteurella species are the most frequently found organisms in both dog and cat bite wounds. However, other aerobes such as streptococci, staphylococci, Moraxella, and Neisseria, as well as anaerobes including Fusobacterium and Bacteroides species, are also common.2
C. canimorsis is a facultative, fastidious gram‐negative bacillus found in the mouth flora of not only dogs but also cats and humans. It is often mistaken for other gram‐negative rod species.3 As with the patient described in this report, systemic infection from C. canimorsis can follow even superficial or well‐healed bite wounds.
Since this bacterium was first described in the literature 30 years ago, more than 100 cases of C. canimorsus infection have been described, with a mortality rate of nearly 30%.4 C. canimorsus occurs more frequently in males and in patients 50 to 70 years of age. Traditional risk factors include alcohol abuse, asplenia, immunosuppression, and corticosteroid treatment. However, in a case series of 56 isolates in California, only 10% of patients with Capnocytophaga sepsis were asplenic and none had alcohol abuse reported in their medical charts. In this series, median time from dog bite to the onset of symptoms was 3 days. Eighty‐five percent of patients presented with fever, while 32% had sepsis and 13% had DIC or septic shock.3
While C. canimorsus was once susceptible to a range of antibiotics, several reports from Canada and Europe document rising rates of beta‐lactamaseproducing strains that have caused clinically significant disease.5, 6 Individual susceptibility data take days to obtain, so it is important to start with empiric therapy. In general, empiric therapy for all serious dog bites should cover beta‐lactamaseproducing bacteria and anaerobes, for example, with amoxicillin/clavulanate, ampicillin/sulbactam, or piperacillin/tazobactam. If the patient is allergic to penicillin, clindamycin plus a fluoroquinolone can be used instead.
There are previous reports of purpura fulminans and symmetric peripheral gangrene following Capnocytophaga infection from dog bites.7, 8 Purpura fulminans is defined as rapidly progressive skin necrosis due to dermal vascular thrombosis, often in the setting of DIC. Early involvement occurs at acral sites, such as the nose, ears, fingers, and toes. Purpuric lesions often progress to skin necrosis or dry gangrene within 24 to 48 hours. In a review of 12 patients with purpura fulminans, only 9 survived. Eight of the 9 survivors required amputation of at least 1 limb, and 4 of them required 4‐limb amputation.7
In this patient who presented with fever and rash, the discussant recognized early on an underlying infectious etiology. Although the patient's exposure history led the discussant to consider a host of possibilities, the recognition of purpura fulminans allowed him to narrow his differential. Ultimately, the dog's bite clinched the diagnosis.
KEY TEACHING POINTS
-
Sepsis caused by C. canimorsus is often characterized by rash, cellulitis, arthritis, meningitis, and endocarditis. In some instances, infection can progress to purpura fulminans.
-
In cases where fastidious organisms are suspected as an infectious source, microbiology labs should be notified of suspected organisms so they can extend incubation periods or use special media to maximize culture yield and the likelihood of accurate identification.
-
Empiric therapy for serious dog bites should cover beta‐lactamaseproducing bacteria and anaerobes. Consider using amoxicillin/clavulanate, ampicillin/sulbactam, or piperacillin/tazobactam.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
This icon represents the patient's case. Each paragraph that follows represents the discussant's thoughts.
Acknowledgements
The authors thank Snigdha Vallabhaneni, MD, from the UCSF Division of Infectious Diseases, for her contributions to the discussion on C. canimorsus. They also thank Kanade Shinkai, MD, PhD, from the UCSF Department of Dermatology, and Heather Nye, MD, PhD, from the UCSF Division of Hospital Medicine, for their review of the manuscript.
Disclosure: Nothing to report.
A 66‐year‐old man presented to the Emergency Department (ED) with rash and malaise in early April. He was in his usual state of good health until the morning of presentation, when he awoke feeling lethargic. Over the course of the day, his hands and feet grew cold and numb, his nose became dark red, and he developed a diffuse, net‐like red rash over his legs, hands, buttocks, and trunk. He had multiple maroon bowel movements. His wife noted that he became incoherent and brought him to the ED.
This apparently previously healthy man presented with an acute episode of fatigue and altered mental status accompanied by a prominent cutaneous eruption. The differential diagnosis will ultimately be guided by the morphology of the rash. At this stage, infectious diseases, drug or toxin exposure, and allergic processes including anaphylaxis must all be considered in this patient with rash and acute illness. The maroon bowel movements likely represent a gastrointestinal bleed that may be part of a unifying diagnosisa hematologic disorder, a vasculitis, or liver disease.
In the ED, the patient was reportedly febrile (exact temperature not recorded) with a blood pressure of 96/54 mmHg. He had pulse oximetry of 88% on room air and a diffuse purpuric rash. The patient was noted to have a leukocytosis, thrombocytopenia, coagulopathy, and an elevation of his creatinine and cardiac enzymes. He was given fluids, fresh frozen plasma, and broad‐spectrum antibiotics, and transferred directly to the intensive care unit of a tertiary medical center for further management.
Upon arrival to the intensive care unit, he complained of fatigue, progression of his nonpruritic, nonpainful rash, and worsening numbness and tingling of his extremities. He denied headache, nuchal rigidity, photophobia, vision or hearing changes, chest pain, cough, abdominal pain, myalgias, or arthralgias. While being interviewed, he had dark brown emesis and a bloody bowel movement.
The patient's past medical history included bacterial pericarditis as a teenager and remote hepatitis of unclear etiology. He rarely saw a physician, took no medications, and had no known medication allergies.
The patient worked as president of a software company and lived with his wife. He had smoked 1 to 2 packs of cigarettes a day for the past 30 years. He endorsed 2 of 4 CAGE criteria (need to Cut down, Annoyed when asked about alcohol, feel Guilty about drinking, need for an Eye opener), and his wife and had never been tested for human immunodeficiency virus (HIV). Family history was unremarkable.
The patient's presentation is concerning for a life‐threatening disease process with a rapid course. In the setting of the laboratory abnormalities demonstrating multi‐organ dysfunction, aggressive volume resuscitation and prompt initiation of broad‐spectrum antibiotics are indicated. The history does not reveal an obvious source of infection or exposure to a new drug, toxin, or allergen. His apparent gastrointestinal bleed could be explained by complications of liver disease from chronic alcohol use. For example, he could have variceal bleeding or gastropathy from portal hypertension. Alternatively, he may have bleeding secondary to a coagulopathy from decreased synthetic function of clotting factors. Other possibilities include a perforated viscus (eg, peptic ulcer) leading to bleeding and peritonitis or mesenteric ischemia, though the absence of abdominal pain makes these unlikely.
At this point, the overall presentation is most concerning for infection, especially given his chronic alcohol use and the vague history of hepatitis. The acute onset and severity of the illness are consistent with an aggressive, suppurative bacterial infection. The most likely causative organisms include gram‐negative bacteria, especially Neisseria meningitidis (with or without meningitis), as well as Staphylococcus aureus, Streptococcus pyogenes, and Rickettsia rickettsii (Rocky Mountain spotted fever).
Several months prior to presentation, he had traveled to Mexico. Two months prior to presentation, he made a trip to North Carolina and Ohio to visit his brother, who subsequently died of pneumonia. One month prior to presentation, he had traveled to urban China for work.
Because the presentation is so acute and the patient's travel took place over 1 month ago, this is unlikely to be a travel‐associated illness. Furthermore, the course is too acute to be consistent with endemic diseases of Central America and the midwestern United States, such as tuberculosis, brucellosis, and histoplasmosis.
He had a temperature of 38.7C. His heart rate was 110 beats per minute. His blood pressure was 115/78 mmHg, respiratory rate was 24 breaths per minute, and oxygen saturation was 99% on 6 liters via nasal cannula. The patient was a well‐nourished, middle‐aged man who appeared uncomfortable. He was in mild respiratory distress, though able to speak in full sentences. He was alert, coherent, and oriented to self, place, date, and time.
Skin examination revealed nonblanching purpuric papules coalescing into stellate plaques on his scalp, forehead, nose, cheeks, bilateral ears, hands, and feet (Figure 1). Acral surfaces, including hands and feet, were cyanotic without evidence of gangrene. He had nonblanching retiform purpuric plaques on his right flank, lower abdomen, low back, buttock, penis, scrotum, thighs, and legs (Figure 2). His right dorsal hand had 3 healing erosions of 3 to 10 mm in size without associated edema, erythema, or drainage.


Mucous membranes were dry without lesions. Cardiac examination demonstrated tachycardia without appreciable murmur. He was mildly tachypneic and his lungs were clear to auscultation without adventitious breath sounds. His abdominal examination was unremarkable. His hands and feet were cool with decreased sensation to touch. He had full range of motion and intact muscle strength, but mild bilateral dysmetria with finger‐nose‐finger testing. His radial and dorsalis pedis pulses were symmetric and brisk. Rectal exam revealed guaiac‐positive stool.
The patient's vital signs are compatible with the systemic inflammatory response syndrome. The presence of retiform purpura raises concerns for a systemic vasculitis with destruction of the vessel wall, or intravascular occlusion with thrombosis or emboli. Absence of murmur does not rule out endocarditis but makes it less likely. He has no risk factors for vasculitis, so the purpura, in conjunction with both bleeding and thrombosis, is much more suggestive of disseminated intravascular coagulation (DIC). This clotting disorder can result from a noninfectious trigger, such as acute pancreatitis or malignancy, but his presentation is more worrisome for a severe infection leading to DIC and complicated by purpura fulminans. He does not show signs of hepatic encephalopathy or cirrhosis, making decompensated liver disease a less likely inciting factor of his presentation.
Further exposure history was obtained: The patient often spent time outdoors near his rural home and used a weed‐whacker in his yard the day before admission. He owned 3 horses which he fed and often rode. He had 3 healthy dogs and had been bitten in attempts to break up fights among them, most recently 3 days prior to admission. He lived in mountain lion territory but had no direct exposure to lions. He had no known insect bites. He regularly drank well water, and consumed medium‐rare hamburgers 4 days prior to admission. One week prior to admission, a child with possible streptococcal pharyngitis visited his home.
With this history, the patient was treated with aggressive intravenous fluids and meningeal doses of ceftriaxone, vancomycin, and metronidazole.
In the summer, outdoor exposure to brush confers a risk of tick‐borne infections, including rickettsial diseases, ehrlichiosis, and spirochetal relapsing fever. However, this patient presented in the spring, and apart from rickettsial spotted fever, these illnesses tend to be indolent. It is conceivable, though unlikely, that the weed‐cutting device may have aerosolized fulminant zoonotic pathogens such as Francisella tularensis or plague that can be found in mountain lion territory.
Well water exposure suggests leptospirosis, which can present in a fulminant fashion with multi‐organ dysfunction, but is more often a subacute illness (developing over many days to a week or two). His ingestion of potentially undercooked meat raises the possibility of enterohemorrhagic infection complicated by the hemolytic uremic syndrome (HUS). However, while the purpuric rash and renal failure are compatible with HUS, the pace of illness and accompanying hypotension once again favor alternative infectious diagnoses.
The incubation period and presentation is concerning overwhelming bacterial infection related to the dog bite. Microbiological considerations include streptococcal species, Staphylococcus aureus, and gram‐negative organisms including Pasteurella species and Capnocytophaga canimorsus. The latter 2 organisms are of particular interest since they tend to cause severe sepsis in patients with alcoholism.
The antibiotic selection in this case is not straightforward. In general, empiric therapy for infections related to dog bites should include treatment for beta‐lactamaseproducing bacteria and anaerobes (eg, piperacillin/tazobactam). Yet, given the clinical presentation, severity of illness, and possible DIC, it is appropriate to be concerned about meningococcemia. Unfortunately, the tazobactam in piperacillin/tazobactam has poor central nervous system penetration so would be suboptimal treatment for meningitis. At this point, ceftriaxone, vancomycin, and metronidazole is a reasonable regimen.
Laboratory results were notable for blood urea nitrogen 50 mg/dL, creatinine 3.47 mg/dL, white cell count 21,800/L, with an absolute neutrophil count of 20,690/L, hematocrit 35.9%, platelet count 34,000/L, International Normalized Ratio 1.5, and partial thromboplastin time 44.0 seconds. His alanine aminotransferase was 356 U/L (1641 U/L), aspartate aminotransferase 959 U/L (1259 U/L), alkaline phosphatase 50 U/L (29111 U/L), and total bilirubin 1.7 mg/dL (0.31.3 mg/dL). Fibrinogen was 283 g/L (202430 g/L), lactate dehydrogenase was 1883 U/L (91185 IU/L), and uric acid was 10.5 mg/dL (3.77.7 mg/dL). His troponin I was 1.18 ng/mL (<0.05 ng/ml), and his electrocardiogram showed sinus tachycardia but no evidence of myocardial ischemia. Chest x‐ray showed no infiltrate or evidence of volume overload. Lumbar puncture was deferred out of concern for ongoing disseminated intravascular coagulation.
Transthoracic echocardiogram revealed global hypokinesis and reduced left ventricular systolic function with ejection fraction of 35%. There was no evidence of vegetations or thrombus.
The patient's thrombocytopenia and prolonged coagulation parameters further support the presence of DIC. A peripheral blood smear should be examined. If microangiopathic changes are found, other diagnoses such as thrombotic thrombocytopenic purpura might be considered, although the rapid pace of illness and presence of hypotension still make sepsis with DIC more likely.
While septic shock often causes multi‐organ system failure secondary to hypoperfusion, the presumed rapid onset of hepatic and renal abnormalities suggests that microvascular thrombosis is playing a larger role in his organ system dysfunction. Microvascular thrombosis could also contribute to his myocardial injury, though globally depressed ejection fraction and elevated troponin might also be explained by infectious myocarditis. A third possibility is that his severe sepsis caused his myocardial dysfunction. Regardless of its etiology, the patient has no clinical evidence of congestive heart failure, so no specific therapy is required at this time. However, his cardiopulmonary exam should be monitored closely, and if he survives, he should have repeat echocardiography to monitor for resolution of the global hypokinesis.
Further evaluation revealed creatine kinase of 45,000 ng/ml (55380 ng/ml) and repeat troponin of >22 ng/ml. Protein C level was low at 30%. Testing for HIV was negative. Blood smear from time of transfer had few schistocytes. Urinalysis showed muddy brown casts but no dysmorphic red blood cells or red cell casts. The patient was placed on continuous veno‐venous hemofiltration (CVVH) for worsening renal failure and oliguria from presumed acute tubular necrosis in the setting of rhabdomyolysis and sepsis.
The patient has severe rhabdomyolysis that cannot fully be explained by his initial hypoperfusion and is more likely related to the overwhelming infection and microthrombosis. Rhabdomyolysis probably contributed to his acute tubular necrosis and renal failure.
Dermatology consultation identified the rash as likely purpura fulminans. They recommended a skin biopsy to rule out vasculitis. Three skin biopsies revealed micro‐vascular thrombosis; direct immunofluorescence test was negative for vasculitis; his skin tissue culture was negative for bacterial, mycobacterial, and fungal organisms.
Input from the dermatology service was key in identifying the rash. Purpura fulminans has a limited differential that includes severe infection from gram‐negative organisms and protein C and S deficiency. Since the biopsy results made vasculitis unlikely, the team was able to focus greater attention on potential pathogens such as Pasteurella species and C. canimorsus.
The biopsy also confirms the clinical suspicion that microvascular thrombosis is causing the patient's acute kidney injury, rhabdomyolysis, and myocardial ischemia. The presence of microvascular thrombosis prompts consideration of antithrombotic therapy such as heparin, but benefits of this therapy must be weighed against contraindications including bleeding and thrombocytopenia.
Ultimately out of concerns for recurrent gastrointestinal bleeding, the primary team decided not to treat with heparin or other antithrombotic therapy.
After several days of supportive care with antibiotics and renal replacement therapy, the patient showed gradual improvement of his retiform purpura, sensory neuropathy, laboratory data, and other markers of end‐organ dysfunction. Purpura of his fingertips, feet, and toes progressed to dry gangrene (Figure 3), which was monitored for potential need for amputation. He remained dependent on intermittent hemodialysis.

His initial antibiotic regimen was narrowed to ceftriaxone monotherapy. Five days after initial presentation, blood cultures drawn from the outside emergency department grew a gram‐negative rod in the anaerobic broth. Ten days later, this gram‐negative rod was identified as Capnocytophaga canimorsus. He was ultimately discharged to a skilled nursing facility.
Generally growth of an organism in broth only suggests either a very low inoculum or that the isolate is a contaminant. In this case, it was because the causative organism, C. canimorsus, is an obligate anaerobe and quite fastidious, so unlikely to grow easily. The identification of C. canimorsus from the initial blood culture is not surprising in this patient who presented with severe sepsis, DIC, and purpura fulminans after a recent dog bite. While the patient's chronic alcohol use may explain his fulminant infection from an atypical organism, one should always consider occult underlying malignancy as a predisposing factor, particularly in patients of this age group.
With the appropriate course of antibiotics, C. canimorsus infection should be completely cured. However, recovery of kidney and cardiac function could take weeks to months, and his dry gangrene may or may not resolve.
COMMENTARY
Capnocytophaga canimorsis sepsis is a rare and potentially deadly complication of dog bites that can present with rash, cellulitis, purpura fulminans, arthritis, meningitis, and endocarditis. The discussant considered a broad differential for the presentation of fever, rash, and acute illness. While the travel history was intriguing, the severity and pace of illness allowed him to focus attention on more recent infectious exposures. The ultimate key to the diagnosis was the patient's history of dog bite, an important but underrecognized source of serious infection in the United States.
According to the Centers for Disease Control and Prevention, there are approximately 4 million dog bites in the country each year. Of these, 300,000 bite victims seek care in the emergency department, resulting in 13,000 hospitalizations and 20 deaths annually.1 Infected dog bite wounds often grow polymicrobial flora. Pasteurella species are the most frequently found organisms in both dog and cat bite wounds. However, other aerobes such as streptococci, staphylococci, Moraxella, and Neisseria, as well as anaerobes including Fusobacterium and Bacteroides species, are also common.2
C. canimorsis is a facultative, fastidious gram‐negative bacillus found in the mouth flora of not only dogs but also cats and humans. It is often mistaken for other gram‐negative rod species.3 As with the patient described in this report, systemic infection from C. canimorsis can follow even superficial or well‐healed bite wounds.
Since this bacterium was first described in the literature 30 years ago, more than 100 cases of C. canimorsus infection have been described, with a mortality rate of nearly 30%.4 C. canimorsus occurs more frequently in males and in patients 50 to 70 years of age. Traditional risk factors include alcohol abuse, asplenia, immunosuppression, and corticosteroid treatment. However, in a case series of 56 isolates in California, only 10% of patients with Capnocytophaga sepsis were asplenic and none had alcohol abuse reported in their medical charts. In this series, median time from dog bite to the onset of symptoms was 3 days. Eighty‐five percent of patients presented with fever, while 32% had sepsis and 13% had DIC or septic shock.3
While C. canimorsus was once susceptible to a range of antibiotics, several reports from Canada and Europe document rising rates of beta‐lactamaseproducing strains that have caused clinically significant disease.5, 6 Individual susceptibility data take days to obtain, so it is important to start with empiric therapy. In general, empiric therapy for all serious dog bites should cover beta‐lactamaseproducing bacteria and anaerobes, for example, with amoxicillin/clavulanate, ampicillin/sulbactam, or piperacillin/tazobactam. If the patient is allergic to penicillin, clindamycin plus a fluoroquinolone can be used instead.
There are previous reports of purpura fulminans and symmetric peripheral gangrene following Capnocytophaga infection from dog bites.7, 8 Purpura fulminans is defined as rapidly progressive skin necrosis due to dermal vascular thrombosis, often in the setting of DIC. Early involvement occurs at acral sites, such as the nose, ears, fingers, and toes. Purpuric lesions often progress to skin necrosis or dry gangrene within 24 to 48 hours. In a review of 12 patients with purpura fulminans, only 9 survived. Eight of the 9 survivors required amputation of at least 1 limb, and 4 of them required 4‐limb amputation.7
In this patient who presented with fever and rash, the discussant recognized early on an underlying infectious etiology. Although the patient's exposure history led the discussant to consider a host of possibilities, the recognition of purpura fulminans allowed him to narrow his differential. Ultimately, the dog's bite clinched the diagnosis.
KEY TEACHING POINTS
-
Sepsis caused by C. canimorsus is often characterized by rash, cellulitis, arthritis, meningitis, and endocarditis. In some instances, infection can progress to purpura fulminans.
-
In cases where fastidious organisms are suspected as an infectious source, microbiology labs should be notified of suspected organisms so they can extend incubation periods or use special media to maximize culture yield and the likelihood of accurate identification.
-
Empiric therapy for serious dog bites should cover beta‐lactamaseproducing bacteria and anaerobes. Consider using amoxicillin/clavulanate, ampicillin/sulbactam, or piperacillin/tazobactam.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
This icon represents the patient's case. Each paragraph that follows represents the discussant's thoughts.
Acknowledgements
The authors thank Snigdha Vallabhaneni, MD, from the UCSF Division of Infectious Diseases, for her contributions to the discussion on C. canimorsus. They also thank Kanade Shinkai, MD, PhD, from the UCSF Department of Dermatology, and Heather Nye, MD, PhD, from the UCSF Division of Hospital Medicine, for their review of the manuscript.
Disclosure: Nothing to report.
- ,,.Incidence of dog bite injuries treated in emergency departments.JAMA.1998;279:51–53.
- ,,,,.Bacteriologic analysis of infected dog and cat bites. Emergency Medicine Animal Bite Infection Study Group.N Engl J Med.1999;340:85–92.
- ,,,.Diagnosing Capnocytophaga canimorsus infections.Emerg Infect Dis.2006;12:340–342.
- ,,.Capnocytophaga canimorsus infections in human: review of the literature and cases report.Eur J Epidemiol.1996;12:521–533.
- ,,,,.Antimicrobial susceptibilities and beta‐lactamase characterization of Capnocytophaga species.Antimicrob Agents Chemother.1992;36:2197–2200.
- ,,,,,.Bacteremia due to Capnocytophaga species in patients with neutropenia: high frequency of beta‐lactamase‐producing strains.Clin Infect Dis.1999;28:1172–1174.
- ,,.Presentation and outcome of purpura fulminans associated with peripheral gangrene in 12 patients at Mayo Clinic.J Am Acad Dermatol.2007;57:944–956.
- ,,,.Capnocytophaga canimorsus sepsis with purpura fulminans and symmetrical gangrene following a dog bite in a shelter employee.Am J Med Sci.2004:327:369–372.
- ,,.Incidence of dog bite injuries treated in emergency departments.JAMA.1998;279:51–53.
- ,,,,.Bacteriologic analysis of infected dog and cat bites. Emergency Medicine Animal Bite Infection Study Group.N Engl J Med.1999;340:85–92.
- ,,,.Diagnosing Capnocytophaga canimorsus infections.Emerg Infect Dis.2006;12:340–342.
- ,,.Capnocytophaga canimorsus infections in human: review of the literature and cases report.Eur J Epidemiol.1996;12:521–533.
- ,,,,.Antimicrobial susceptibilities and beta‐lactamase characterization of Capnocytophaga species.Antimicrob Agents Chemother.1992;36:2197–2200.
- ,,,,,.Bacteremia due to Capnocytophaga species in patients with neutropenia: high frequency of beta‐lactamase‐producing strains.Clin Infect Dis.1999;28:1172–1174.
- ,,.Presentation and outcome of purpura fulminans associated with peripheral gangrene in 12 patients at Mayo Clinic.J Am Acad Dermatol.2007;57:944–956.
- ,,,.Capnocytophaga canimorsus sepsis with purpura fulminans and symmetrical gangrene following a dog bite in a shelter employee.Am J Med Sci.2004:327:369–372.