User login
Identifying an Idle Line for Its Removal
Infections acquired in the hospital are termed healthcare‐associated infections (HAIs) and include central lineassociated blood stream infections (CLABSIs). Among HAIs, CLABSIs cause the highest number of preventable deaths.[1] Central venous catheters (CVCs) or central lines are commonly used in the hospital.[2] Each year their use is linked to 250,000 cases of CLABSIs in the United States.[3] Some CLABSIs may be prevented by the prompt removal of the line.[4] However, CVCs are often retained after their clinical indication has lapsed and are then referred to as idle lines.[5, 6] In this work, we propose and theoretically test a guideline to facilitate the safe removal of an idle line by observing the agreement and disagreement between actual practice and the proposed guideline.
METHODS
Setting
This work was conducted at a large, urban, tertiary care, academic health center in the United States as a collaborative effort to improve quality at our institution.[7]
Design and Patients
The reports linked with the electronic medical records at our institution include a daily, ward‐by‐ward listing of patients who have access other than a peripheral line in place. This central line dashboard accesses the information on intravenous access charted by bedside nurses to create a list of patients on every ward who have any kind of central access. Temporary central venous lines (CVLs), peripherally inserted central catheters (PICCs), ports, and dialysis catheters are all included. The unit charge nurses and managers use this dashboard to facilitate compliance with line care bundles. We used this source to identify patients with either type of CVC (CVLs or PICCs) on 8 days in August 2014, September 2014, and October 2014. Patients were included if they had a CVC and were on a general medical or surgical ward bed on audit day. CVLs at all sites were included (femoral, subclavian, and internal jugular). Patients in an intensive care unit (ICU) or progressive care unit on the day of the audit were excluded. Patients whose catheters were for chemotherapy and those admitted for a transplant or receiving palliative or hospice care were also excluded.
Data Collection
A protocol for data collection was written out, and a training session was held to review definitions, data sources, and methods to ensure consistency. Two authors (M.M. and J.D.) assisted by an experienced clinical nurse specialist collected data on the patients captured on audit days. Each chart was reviewed on the day of the audit, the 2 days preceding the audit day, and then followed until the patient was either discharged from the hospital or transferred to a higher level of care, died, or transitioned to palliative or hospice care. Demographics, details about the line, and the criteria for justified use were extracted from the electronic medical record.
Definitions
Justified and Idle Days
To justify the presence of a CVC on any given day, we used criteria that fell under 3 categories: intravenous (IV) access needs, unstable vitals, or meeting sepsis/systemic inflammatory response syndrome (SIRS) criteria (Table 1). For vital signs, a single abnormal reading was counted as fulfilling criteria for that day. If no criterion for justified use was met, the line was considered idle for that day.
|
| IV access needs |
| Expected duration of IV antibiotics >6 days |
| Administration of TPN |
| Anticipated requirement of home IV medications |
| Requirement of IV medications with documented difficult access |
| Hemorrhage requiring blood transfusions |
| Requiring more than 3 infusions |
| Requiring more than 2 infusions and blood transfusions |
| Abnormal vitals |
| Diastolic blood pressure >120 mm Hg |
| Systolic blood pressure <90 mm Hg |
| Systolic blood pressure >200 mm Hg |
| Heart rate >120 beats per minute |
| Heart rate <50 beats per minute |
| Respiratory rate >30 breaths per minute |
| Respiratory rate <10 breaths per minute |
| Oxygen saturation <90% as measured by pulse oximetry |
| Meeting SIRS criteria (2 or more of the following present) |
| Temp >38C, Temp <36C, heart rate >90 beats per minute, respiratory rate >20 breaths per minute, WBC >12,000/mm3, WBC <1,000/mm3, bandemia >10% |
Qualifying IV access needs were defined similarly to those previously used,[5, 6] whereas those for SIRS followed the current consensus.[8] To determine the number of IV medications or infusions, the medication administration record was reviewed. If 3 or more infusions were found, their compatibility was checked using the same database that nurses use at our institution. Difficult IV access was inferred from the indication for line placement, coupled with the absence of documentation of a peripheral IV. Clinical progress notes were reviewed to extract information on the length of proposed IV antibiotic courses, and discharge instructions were reviewed to verify whether the line was removed prior to discharge or not. The cutoffs for diastolic blood pressure, respiratory rate, and oxygen saturation used to label patients hemodynamically labile are the same as those used by previous authors and also constitute the definition of hypertensive urgency.[5, 9] However, we diverged from the values previously used for tachycardia, bradycardia, and systolic hypotension using heart rates >120 and <50 beats per minute (compared to >130 and <40 beats per minute) and systolics <90 mm Hg (compared to <80 mm Hg) to justify the line.[5] Early warning scores have been used to identify hospitalized ward patients who are at risk for clinical deterioration. Although each score utilizes different thresholds, the risk for clinical deterioration increases as the vitals worsen.[10] Bearing this in mind, the thresholds we elected to use are more clinically conservative and also parallel the nursing call orders currently used at our institution.
Proposed Guideline
We propose the guideline that a CVC may be safely removed the day after the first idle day.
RESULTS
A total of 126 lines were observed in 126 patients. Eighty‐three (65.9%) of the lines were PICCs. The remaining 43 (34.1%) were CVLs. The indications for line placement were distributed between the need for central access, total parenteral nutrition, or antibiotics (Table 2).
| Description | Value |
|---|---|
| |
| Age in yrs mean (SD) | 55.7 (18) |
| Gender, n (%) | |
| Female | 66 (52.4) |
| Male | 60 (47.6) |
| Type of line, n (%) | |
| PICC | 83 (65.9) |
| CVL | 43 (34.1) |
| Indication for line placement, n (%) | |
| Meds requiring central access or TPN | 36 (28.6) |
| Antibiotics | 34 (27.0) |
| Hemodynamic instability | 30 (23.8) |
| Poor access with multiple IV medications | 18 (14.3) |
| Unknown | 8 (6.3) |
| Line removed prior to discharge, n (%) | |
| Yes | 76 (60.3) |
| No | 50 (39.7) |
Out of the 126 patients, 50 (39.7%) were discharged from the hospital, died, were transferred to a higher level of care, or transitioned to palliative or hospice care with the line in place. In the remaining 76 patients, the audit captured 635 days, out of which a line was in place for 522 (82.2%) days. Of these 522 days, the line's presence was justified by our criteria for 351 (67.2%) days. The most common reason for a line to be justified on any given day was the need for antibiotics followed by the presence of SIRS criteria (Table 3). The remaining 171 (32.7%) days were idle.
| Criteria | N | % |
|---|---|---|
| ||
| No. of factors justifying use | ||
| 1 | 184 | 52.4% |
| 2 | 127 | 36.2% |
| >2 | 40 | 11.4 |
| Reason for justifying line* | ||
| Anticipate home or >6 days of antibiotic use | 181 | 51.6 |
| SIRS criteria | 124 | 35.3 |
| TPN | 96 | 27.4 |
| Hemodynamic instability based on hr and bp | 78 | 22.2 |
| Poor access with need for IV medications | 57 | 16.2 |
| Respiratory rate (<10 or >30/minute) | 25 | 7.1 |
| Active hemorrhage requiring transfusions | 12 | 3.4 |
| >3 infusions | 6 | 1.7 |
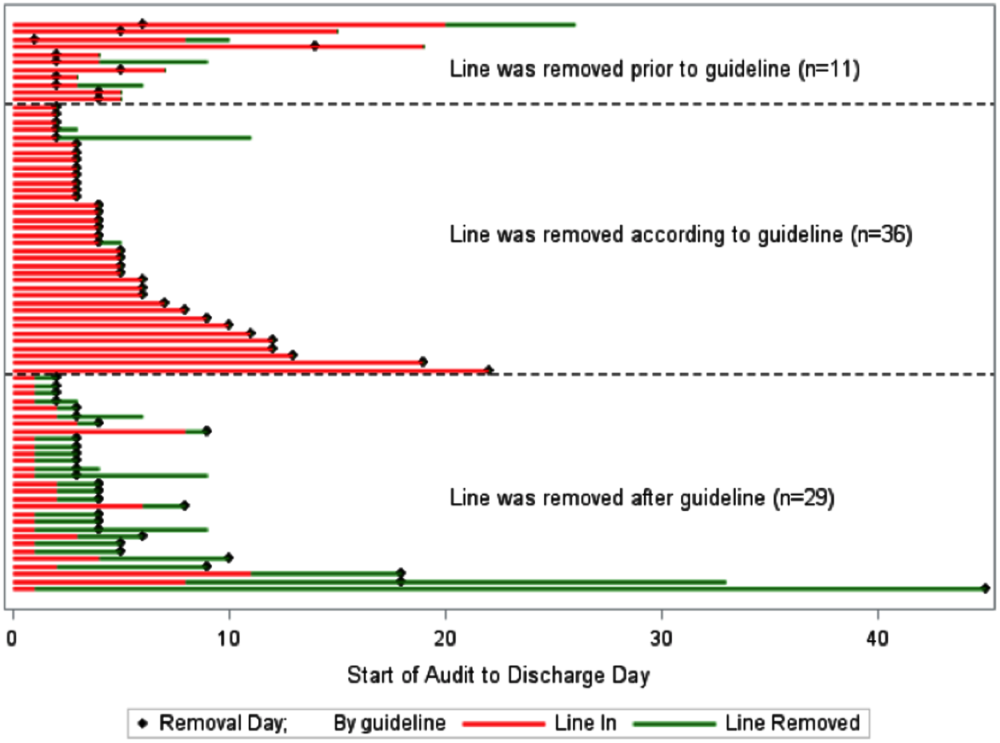
A comparison of the actual removal of the 76 central lines in practice relative to the proposed guideline of removing it the day following the first idle day is displayed in Figure 1. The central line was removed prior to our proposed guideline in 11 (14.5%) patients, and waiting for an idle day in these patients would have added 46 line days. In almost half the patients (n = 36, 47.4%), the line was removed in agreement with the proposed guideline. None of the patients in whom the line was removed prior to or in accordance with our proposed guideline required a line reinsertion. Line removal was delayed in 29 (38.2%) patients when compared to our proposed guideline. In these patients, following the guideline would have created 122 line‐free days. Most (n = 102, 83.6%) of these potential line‐free days were idle. Twenty (16.4%) were justified, of which half (n = 10) were justified by meeting SIRS criteria.

DISCUSSION
Approximately 1 in every 25 inpatients in the United States has at least 1 HAI on any given day.[11] The case fatality rate from a CLABSI may be as high as 12%, and up to 70% of these infections may be preventable.[1, 12] Interventions successful in decreasing CLABSIs have focused on patients in ICUs.[13] However, CVCs are increasingly prevalent outside the ICU, with over 4.5 million line days in non‐ICU beds reported to the National Healthcare Safety Network in 2012 compared to 2.5 million in 2010.[2, 14] However, adherence rates to infection control practices may be lower on the wards than in the ICUs.[6, 15] Consequently, although the number of CLABSIs has declined over the last decade, most are now occurring outside the ICU.[16] These trends underscore the need to develop strategies aimed at CLABSI prevention on the floors.
Analogous to the life cycle of a urinary catheter described by Meddings et al.,[17] strategies to prevent CLABSIs and other CVC‐related complications may be designed around the life cycle of a CVC. The life cycle starts with insertion and moves on to the maintenance, removal, and possible reinsertion of the line. The process thus starts with the decision to place the line. Over the last decade, this decision making has changed in part due to PICCs. This shift is reflected in PICC prevalence rates: in 2001, 11% of audited central lines were PICCs compared to 56% in 2007.[5, 6] In our audit, 66% of the CVCs were PICCs. This increase in the use of PICCs may be attributable to the ease and safety of their placement coupled with the increased availability of vascular access placement teams.[18] The risk of overuse that may result from such expediency may be countered by adhering to guidelines such as the Michigan Appropriateness Guide for Intravenous Catheters, which provides both clinically detailed guidance and an impetus for reflective decision making around intravenous access.[19]
The placement of CVCs for prolonged parenteral antibiotics may be a particular subset that bears further exploration. Similar to previous reports, we found that a large number of the CVCs were both inserted for and justified by the need for IV antibiotics.[5] Guidelines delineated by the Infectious Diseases Society of America regarding outpatient parenteral antibiotics weigh both the duration of therapy and the antimicrobial's potential for causing phlebitis when recommending the type of intravascular access.[20] Many courses may therefore be completed through peripheral or midline catheters. Developing strong partnerships between infectious disease specialists, hospitalists, and the facilities or home‐care services treating these patients may curtail the use of CVCs for antimicrobial administration.
The main focus of our work is on facilitating the safe removal of CVCs. The risk of CLABSIs increases each day a CVC is in place, and guidelines to prevent CLABSIs include recommendations to promptly remove nonessential catheters.[4, 21] There is also an emerging understanding that the risk of a PICC‐related CLABSI approaches that from a traditional central line in hospitalized patients, and PICCs confer an increased risk of venous thromboembolism.[18, 22] Although nearly half of surveyed hospitalists recently reported leaving PICCs in place until discharge day, our data suggest that this practice may be driven by the trajectory of a patient's recovery as much as by knowledge gaps related to the use of PICCs.[23] In nearly half the instances, clinical practice already mirrors our proposed guideline, with line removal coinciding with both the timing proposed by our guideline and discharge day. However, there is room for improvement, as line removal may have been expedited in the 29 patients in whom the line was retained after the first idle day. Maintaining an awareness of its presence and weighing its risks and benefits daily may facilitate the removal of a CVC. Based on the recent findings that up to a quarter of clinicians are unaware that their patients have a central line, the mere reminder of the presence of a line using such criteria may expedite its removal by triggering a purposeful reassessment of its ongoing need.[24] Premature CVC removal requiring line reinsertion is an unintended consequence that may emerge from the earlier removal of lines. In our sample, none of the patients who had lines removed either prior to or in accordance with our proposed guideline required a line reinsertion. In addition to line reinsertion, delays in laboratory testing and reporting due to the unavailability of access, increased patient discomfort, or increased workload on the bedside nurse or vascular access team must also be considered when implementing strategies aimed at decreasing line days.
We envisage using these criteria to both empower practitioners with knowledge and foster shared accountability between all team members by using a uniform tool. This can occur through partnerships between infection control, clinical nurse specialists, bedside nursing, and physicians. The electronic medical record could be leveraged to scan the record for the criteria and create a notification when the line becomes idle. In alignment with the Michigan Appropriateness Guide for Intravenous Catheters guidelines, we do not support the removal of lines by nursing staff without physician notification.[19] Such principles have been successfully harnessed in strategies to prevent both catheter‐associated urinary tract infections and CLABSIs in ICUs.[13, 25] In light of the complexity surrounding the decision making for CVCs, our criteria were focused on the wards and erred on the side of clinical caution. This clinical conservatism is apparent in the patients in whom lines were removed prior to what our guideline would propose, yet none of the patients required a line reinsertion. As concerns about recrudescent clinical instability may drive decision making around line removal, such conservatism may be warranted initially. However, the fidelity of these criteria in the clinical setting will need prospective validation. In particular, the inclusion of SIRS criteria may have led to an overestimation of justified days. Further studies may be needed to refine the criteria and find a clinical hierarchy that balances the risks and benefits of retaining a central line.
Our work has certain limitations. It is a single center's experience, and our findings may not therefore be generalizable. Except for when the indication for the line was for difficult access, we did not attempt to verify the presence of a peripheral IV. This, in combination with the inclusion of SIRS criteria, likely leads to an underestimation of idle days. In the interest of focusing on patients in whom the decision making around a line would be the least controversial, we did not continue to follow patients who were transferred to a higher level of care. It is possible, however, that these transfers were precipitated by line‐associated complications such as sepsis and would be important to track. We did not measure the agreement between data collectors, although definitions and methodologies were standardized and reviewed prior to data collection. As this was an observational assessment of a proposed guideline, we cannot predict how the recommendations generated by it will be received by clinicians. Although this may prove to be a barrier in adoption, we hope that the conversation it initiates leads to change.
Hospitalists are positioned to potentially influence the entire life cycle of a central line on the floor. Strategies can be enacted at each stage to help decrease the potential of harm from these devices to our patients. Creating and testing criteria and guidelines such as we propose represents just 1 such strategy in a multidisciplinary effort to provide the best possible care we can.
Acknowledgements
The authors thank Jennifer Dunscomb, Kristen Kelly, and their teams, and Deanna Sidwell, Todd Biggerstaff, Joan Miller, Rob Clark, and the tireless providers at Indiana University Health Methodist Hospital for their support.
Disclosures: This work was supported by the Indiana University Health Values Grant for research. The authors have no conflicts of interests to report.
- , , , , , . Estimating the proportion of healthcare‐associated infections that are reasonably preventable and the related mortality and costs. Infect Control Hosp Epidemiol. 2011;32(2):101–114.
- , , , et al. National Healthcare Safety Network (NHSN) report, data summary for 2012, device‐associated module. Am J Infect Control. 2013;41(12):1148–1166.
- , , . The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc. 2006;81(9):1159–1171.
- , , , et al. Guidelines for the prevention of intravascular catheter‐related infections. Clin Infect Dis. 2011;52(9):e162–e193.
- , , , et al. Temporary central venous catheter utilization patterns in a large tertiary care center: tracking the “idle central venous catheter.” Infect Control Hosp Epidemiol. 2012;33(1):50–57.
- , , , , , . Unnecessary use of central venous catheters: the need to look outside the intensive care unit. Infect Control Hosp Epidemiol. 2004;25(3):266–268.
- IU Health Methodist Hospital website. Available at: http://iuhealth.org/methodist/aboIut. Accessed October 20, 2014.
- , , , et al. Definitions for Sepsis and Organ Failure and Guidelines for the Use of Innovative Therapies in Sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 2009;136(5 suppl):e28.
- , , , , , . Acute hypertension: a systematic review and appraisal of guidelines. Ochsner J. 2014;14(4):655–663.
- , , . Predicting clinical deterioration in the hospital: the impact of outcome selection. Resuscitation. 2013;84(5):564–568.
- , , , et al. Multistate point‐prevalence survey of health care–associated infections. N Engl J Med. 2014;370(13):1198–1208.
- , , , et al. Estimating health care‐associated infections and deaths in U.S. hospitals, 2002. Public Health Rep. 2007;122(2):160–166.
- , , , et al. An intervention to decrease catheter‐related bloodstream infections in the ICU. N Engl J Med. 2006;355(26):2725–2732.
- , , , et al. Data summary for 2011, device‐associated module. Centers for Disease Control and Prevention. National Healthcare Safety Network (NHSN) Report. Available at: http://www.cdc.gov/nhsn/PDFs/dataStat/NHSN‐Report‐2011‐Data‐Summary.pdf. Published April 1, 2013. Last accessed January 2015.
- , , . Idle central venous catheter‐days pose infection risk for patients after discharge from intensive care. Am J Infect Control. 2014;42(4):453–455.
- , . Update on emerging infections: news from the Centers for Disease Control and Prevention. Vital signs: central line‐associated blood stream infections—United States, 2001, 2008, and 2009. Ann Emerg Med. 2011;58(5):447–451.
- , , , , , . Reducing unnecessary urinary catheter use and other strategies to prevent catheter‐associated urinary tract infection: an integrative review. BMJ Qual Saf. 2014;23(4):277–289.
- , , , , . The risk of bloodstream infection associated with peripherally inserted central catheters compared with central venous catheters in adults: a systematic review and meta‐analysis. Infect Control Hosp Epidemiol. 2013;34(9):908–918.
- , , , et al. The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC): results from a multispecialty panel using the RAND/UCLA Appropriateness Method. Ann Intern Med. 2015;163(6 suppl):S1–S40.
- , , , et al. Practice guidelines for outpatient parenteral antimicrobial therapy. IDSA guidelines. Clin Infect Dis. 2004;38(12):1651–1672.
- , . Nonuniform risk of bloodstream infection with increasing central venous catheter‐days. Infect Control Hosp Epidemiol. 2005;26(8):715–719.
- , , , et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta‐analysis. Lancet. 2013;382(9889):311–325.
- , , , , . Hospitalist experiences, practice, opinions, and knowledge regarding peripherally inserted central catheters: results of a national survey. J Hosp Med. 2013;8(11):635–638.
- , , , et al. Do clinicians know which of their patients have central venous catheters? Ann Intern Med. 2014;161(8):562.
- , , , , , . Reducing foley catheter device days in an intensive care unit: using the evidence to change practice. AACN Adv Crit Care. 2006;17(3):272–283.
Infections acquired in the hospital are termed healthcare‐associated infections (HAIs) and include central lineassociated blood stream infections (CLABSIs). Among HAIs, CLABSIs cause the highest number of preventable deaths.[1] Central venous catheters (CVCs) or central lines are commonly used in the hospital.[2] Each year their use is linked to 250,000 cases of CLABSIs in the United States.[3] Some CLABSIs may be prevented by the prompt removal of the line.[4] However, CVCs are often retained after their clinical indication has lapsed and are then referred to as idle lines.[5, 6] In this work, we propose and theoretically test a guideline to facilitate the safe removal of an idle line by observing the agreement and disagreement between actual practice and the proposed guideline.
METHODS
Setting
This work was conducted at a large, urban, tertiary care, academic health center in the United States as a collaborative effort to improve quality at our institution.[7]
Design and Patients
The reports linked with the electronic medical records at our institution include a daily, ward‐by‐ward listing of patients who have access other than a peripheral line in place. This central line dashboard accesses the information on intravenous access charted by bedside nurses to create a list of patients on every ward who have any kind of central access. Temporary central venous lines (CVLs), peripherally inserted central catheters (PICCs), ports, and dialysis catheters are all included. The unit charge nurses and managers use this dashboard to facilitate compliance with line care bundles. We used this source to identify patients with either type of CVC (CVLs or PICCs) on 8 days in August 2014, September 2014, and October 2014. Patients were included if they had a CVC and were on a general medical or surgical ward bed on audit day. CVLs at all sites were included (femoral, subclavian, and internal jugular). Patients in an intensive care unit (ICU) or progressive care unit on the day of the audit were excluded. Patients whose catheters were for chemotherapy and those admitted for a transplant or receiving palliative or hospice care were also excluded.
Data Collection
A protocol for data collection was written out, and a training session was held to review definitions, data sources, and methods to ensure consistency. Two authors (M.M. and J.D.) assisted by an experienced clinical nurse specialist collected data on the patients captured on audit days. Each chart was reviewed on the day of the audit, the 2 days preceding the audit day, and then followed until the patient was either discharged from the hospital or transferred to a higher level of care, died, or transitioned to palliative or hospice care. Demographics, details about the line, and the criteria for justified use were extracted from the electronic medical record.
Definitions
Justified and Idle Days
To justify the presence of a CVC on any given day, we used criteria that fell under 3 categories: intravenous (IV) access needs, unstable vitals, or meeting sepsis/systemic inflammatory response syndrome (SIRS) criteria (Table 1). For vital signs, a single abnormal reading was counted as fulfilling criteria for that day. If no criterion for justified use was met, the line was considered idle for that day.
|
| IV access needs |
| Expected duration of IV antibiotics >6 days |
| Administration of TPN |
| Anticipated requirement of home IV medications |
| Requirement of IV medications with documented difficult access |
| Hemorrhage requiring blood transfusions |
| Requiring more than 3 infusions |
| Requiring more than 2 infusions and blood transfusions |
| Abnormal vitals |
| Diastolic blood pressure >120 mm Hg |
| Systolic blood pressure <90 mm Hg |
| Systolic blood pressure >200 mm Hg |
| Heart rate >120 beats per minute |
| Heart rate <50 beats per minute |
| Respiratory rate >30 breaths per minute |
| Respiratory rate <10 breaths per minute |
| Oxygen saturation <90% as measured by pulse oximetry |
| Meeting SIRS criteria (2 or more of the following present) |
| Temp >38C, Temp <36C, heart rate >90 beats per minute, respiratory rate >20 breaths per minute, WBC >12,000/mm3, WBC <1,000/mm3, bandemia >10% |
Qualifying IV access needs were defined similarly to those previously used,[5, 6] whereas those for SIRS followed the current consensus.[8] To determine the number of IV medications or infusions, the medication administration record was reviewed. If 3 or more infusions were found, their compatibility was checked using the same database that nurses use at our institution. Difficult IV access was inferred from the indication for line placement, coupled with the absence of documentation of a peripheral IV. Clinical progress notes were reviewed to extract information on the length of proposed IV antibiotic courses, and discharge instructions were reviewed to verify whether the line was removed prior to discharge or not. The cutoffs for diastolic blood pressure, respiratory rate, and oxygen saturation used to label patients hemodynamically labile are the same as those used by previous authors and also constitute the definition of hypertensive urgency.[5, 9] However, we diverged from the values previously used for tachycardia, bradycardia, and systolic hypotension using heart rates >120 and <50 beats per minute (compared to >130 and <40 beats per minute) and systolics <90 mm Hg (compared to <80 mm Hg) to justify the line.[5] Early warning scores have been used to identify hospitalized ward patients who are at risk for clinical deterioration. Although each score utilizes different thresholds, the risk for clinical deterioration increases as the vitals worsen.[10] Bearing this in mind, the thresholds we elected to use are more clinically conservative and also parallel the nursing call orders currently used at our institution.
Proposed Guideline
We propose the guideline that a CVC may be safely removed the day after the first idle day.
RESULTS
A total of 126 lines were observed in 126 patients. Eighty‐three (65.9%) of the lines were PICCs. The remaining 43 (34.1%) were CVLs. The indications for line placement were distributed between the need for central access, total parenteral nutrition, or antibiotics (Table 2).
| Description | Value |
|---|---|
| |
| Age in yrs mean (SD) | 55.7 (18) |
| Gender, n (%) | |
| Female | 66 (52.4) |
| Male | 60 (47.6) |
| Type of line, n (%) | |
| PICC | 83 (65.9) |
| CVL | 43 (34.1) |
| Indication for line placement, n (%) | |
| Meds requiring central access or TPN | 36 (28.6) |
| Antibiotics | 34 (27.0) |
| Hemodynamic instability | 30 (23.8) |
| Poor access with multiple IV medications | 18 (14.3) |
| Unknown | 8 (6.3) |
| Line removed prior to discharge, n (%) | |
| Yes | 76 (60.3) |
| No | 50 (39.7) |
Out of the 126 patients, 50 (39.7%) were discharged from the hospital, died, were transferred to a higher level of care, or transitioned to palliative or hospice care with the line in place. In the remaining 76 patients, the audit captured 635 days, out of which a line was in place for 522 (82.2%) days. Of these 522 days, the line's presence was justified by our criteria for 351 (67.2%) days. The most common reason for a line to be justified on any given day was the need for antibiotics followed by the presence of SIRS criteria (Table 3). The remaining 171 (32.7%) days were idle.
| Criteria | N | % |
|---|---|---|
| ||
| No. of factors justifying use | ||
| 1 | 184 | 52.4% |
| 2 | 127 | 36.2% |
| >2 | 40 | 11.4 |
| Reason for justifying line* | ||
| Anticipate home or >6 days of antibiotic use | 181 | 51.6 |
| SIRS criteria | 124 | 35.3 |
| TPN | 96 | 27.4 |
| Hemodynamic instability based on hr and bp | 78 | 22.2 |
| Poor access with need for IV medications | 57 | 16.2 |
| Respiratory rate (<10 or >30/minute) | 25 | 7.1 |
| Active hemorrhage requiring transfusions | 12 | 3.4 |
| >3 infusions | 6 | 1.7 |
A comparison of the actual removal of the 76 central lines in practice relative to the proposed guideline of removing it the day following the first idle day is displayed in Figure 1. The central line was removed prior to our proposed guideline in 11 (14.5%) patients, and waiting for an idle day in these patients would have added 46 line days. In almost half the patients (n = 36, 47.4%), the line was removed in agreement with the proposed guideline. None of the patients in whom the line was removed prior to or in accordance with our proposed guideline required a line reinsertion. Line removal was delayed in 29 (38.2%) patients when compared to our proposed guideline. In these patients, following the guideline would have created 122 line‐free days. Most (n = 102, 83.6%) of these potential line‐free days were idle. Twenty (16.4%) were justified, of which half (n = 10) were justified by meeting SIRS criteria.

DISCUSSION
Approximately 1 in every 25 inpatients in the United States has at least 1 HAI on any given day.[11] The case fatality rate from a CLABSI may be as high as 12%, and up to 70% of these infections may be preventable.[1, 12] Interventions successful in decreasing CLABSIs have focused on patients in ICUs.[13] However, CVCs are increasingly prevalent outside the ICU, with over 4.5 million line days in non‐ICU beds reported to the National Healthcare Safety Network in 2012 compared to 2.5 million in 2010.[2, 14] However, adherence rates to infection control practices may be lower on the wards than in the ICUs.[6, 15] Consequently, although the number of CLABSIs has declined over the last decade, most are now occurring outside the ICU.[16] These trends underscore the need to develop strategies aimed at CLABSI prevention on the floors.
Analogous to the life cycle of a urinary catheter described by Meddings et al.,[17] strategies to prevent CLABSIs and other CVC‐related complications may be designed around the life cycle of a CVC. The life cycle starts with insertion and moves on to the maintenance, removal, and possible reinsertion of the line. The process thus starts with the decision to place the line. Over the last decade, this decision making has changed in part due to PICCs. This shift is reflected in PICC prevalence rates: in 2001, 11% of audited central lines were PICCs compared to 56% in 2007.[5, 6] In our audit, 66% of the CVCs were PICCs. This increase in the use of PICCs may be attributable to the ease and safety of their placement coupled with the increased availability of vascular access placement teams.[18] The risk of overuse that may result from such expediency may be countered by adhering to guidelines such as the Michigan Appropriateness Guide for Intravenous Catheters, which provides both clinically detailed guidance and an impetus for reflective decision making around intravenous access.[19]
The placement of CVCs for prolonged parenteral antibiotics may be a particular subset that bears further exploration. Similar to previous reports, we found that a large number of the CVCs were both inserted for and justified by the need for IV antibiotics.[5] Guidelines delineated by the Infectious Diseases Society of America regarding outpatient parenteral antibiotics weigh both the duration of therapy and the antimicrobial's potential for causing phlebitis when recommending the type of intravascular access.[20] Many courses may therefore be completed through peripheral or midline catheters. Developing strong partnerships between infectious disease specialists, hospitalists, and the facilities or home‐care services treating these patients may curtail the use of CVCs for antimicrobial administration.
The main focus of our work is on facilitating the safe removal of CVCs. The risk of CLABSIs increases each day a CVC is in place, and guidelines to prevent CLABSIs include recommendations to promptly remove nonessential catheters.[4, 21] There is also an emerging understanding that the risk of a PICC‐related CLABSI approaches that from a traditional central line in hospitalized patients, and PICCs confer an increased risk of venous thromboembolism.[18, 22] Although nearly half of surveyed hospitalists recently reported leaving PICCs in place until discharge day, our data suggest that this practice may be driven by the trajectory of a patient's recovery as much as by knowledge gaps related to the use of PICCs.[23] In nearly half the instances, clinical practice already mirrors our proposed guideline, with line removal coinciding with both the timing proposed by our guideline and discharge day. However, there is room for improvement, as line removal may have been expedited in the 29 patients in whom the line was retained after the first idle day. Maintaining an awareness of its presence and weighing its risks and benefits daily may facilitate the removal of a CVC. Based on the recent findings that up to a quarter of clinicians are unaware that their patients have a central line, the mere reminder of the presence of a line using such criteria may expedite its removal by triggering a purposeful reassessment of its ongoing need.[24] Premature CVC removal requiring line reinsertion is an unintended consequence that may emerge from the earlier removal of lines. In our sample, none of the patients who had lines removed either prior to or in accordance with our proposed guideline required a line reinsertion. In addition to line reinsertion, delays in laboratory testing and reporting due to the unavailability of access, increased patient discomfort, or increased workload on the bedside nurse or vascular access team must also be considered when implementing strategies aimed at decreasing line days.
We envisage using these criteria to both empower practitioners with knowledge and foster shared accountability between all team members by using a uniform tool. This can occur through partnerships between infection control, clinical nurse specialists, bedside nursing, and physicians. The electronic medical record could be leveraged to scan the record for the criteria and create a notification when the line becomes idle. In alignment with the Michigan Appropriateness Guide for Intravenous Catheters guidelines, we do not support the removal of lines by nursing staff without physician notification.[19] Such principles have been successfully harnessed in strategies to prevent both catheter‐associated urinary tract infections and CLABSIs in ICUs.[13, 25] In light of the complexity surrounding the decision making for CVCs, our criteria were focused on the wards and erred on the side of clinical caution. This clinical conservatism is apparent in the patients in whom lines were removed prior to what our guideline would propose, yet none of the patients required a line reinsertion. As concerns about recrudescent clinical instability may drive decision making around line removal, such conservatism may be warranted initially. However, the fidelity of these criteria in the clinical setting will need prospective validation. In particular, the inclusion of SIRS criteria may have led to an overestimation of justified days. Further studies may be needed to refine the criteria and find a clinical hierarchy that balances the risks and benefits of retaining a central line.
Our work has certain limitations. It is a single center's experience, and our findings may not therefore be generalizable. Except for when the indication for the line was for difficult access, we did not attempt to verify the presence of a peripheral IV. This, in combination with the inclusion of SIRS criteria, likely leads to an underestimation of idle days. In the interest of focusing on patients in whom the decision making around a line would be the least controversial, we did not continue to follow patients who were transferred to a higher level of care. It is possible, however, that these transfers were precipitated by line‐associated complications such as sepsis and would be important to track. We did not measure the agreement between data collectors, although definitions and methodologies were standardized and reviewed prior to data collection. As this was an observational assessment of a proposed guideline, we cannot predict how the recommendations generated by it will be received by clinicians. Although this may prove to be a barrier in adoption, we hope that the conversation it initiates leads to change.
Hospitalists are positioned to potentially influence the entire life cycle of a central line on the floor. Strategies can be enacted at each stage to help decrease the potential of harm from these devices to our patients. Creating and testing criteria and guidelines such as we propose represents just 1 such strategy in a multidisciplinary effort to provide the best possible care we can.
Acknowledgements
The authors thank Jennifer Dunscomb, Kristen Kelly, and their teams, and Deanna Sidwell, Todd Biggerstaff, Joan Miller, Rob Clark, and the tireless providers at Indiana University Health Methodist Hospital for their support.
Disclosures: This work was supported by the Indiana University Health Values Grant for research. The authors have no conflicts of interests to report.
Infections acquired in the hospital are termed healthcare‐associated infections (HAIs) and include central lineassociated blood stream infections (CLABSIs). Among HAIs, CLABSIs cause the highest number of preventable deaths.[1] Central venous catheters (CVCs) or central lines are commonly used in the hospital.[2] Each year their use is linked to 250,000 cases of CLABSIs in the United States.[3] Some CLABSIs may be prevented by the prompt removal of the line.[4] However, CVCs are often retained after their clinical indication has lapsed and are then referred to as idle lines.[5, 6] In this work, we propose and theoretically test a guideline to facilitate the safe removal of an idle line by observing the agreement and disagreement between actual practice and the proposed guideline.
METHODS
Setting
This work was conducted at a large, urban, tertiary care, academic health center in the United States as a collaborative effort to improve quality at our institution.[7]
Design and Patients
The reports linked with the electronic medical records at our institution include a daily, ward‐by‐ward listing of patients who have access other than a peripheral line in place. This central line dashboard accesses the information on intravenous access charted by bedside nurses to create a list of patients on every ward who have any kind of central access. Temporary central venous lines (CVLs), peripherally inserted central catheters (PICCs), ports, and dialysis catheters are all included. The unit charge nurses and managers use this dashboard to facilitate compliance with line care bundles. We used this source to identify patients with either type of CVC (CVLs or PICCs) on 8 days in August 2014, September 2014, and October 2014. Patients were included if they had a CVC and were on a general medical or surgical ward bed on audit day. CVLs at all sites were included (femoral, subclavian, and internal jugular). Patients in an intensive care unit (ICU) or progressive care unit on the day of the audit were excluded. Patients whose catheters were for chemotherapy and those admitted for a transplant or receiving palliative or hospice care were also excluded.
Data Collection
A protocol for data collection was written out, and a training session was held to review definitions, data sources, and methods to ensure consistency. Two authors (M.M. and J.D.) assisted by an experienced clinical nurse specialist collected data on the patients captured on audit days. Each chart was reviewed on the day of the audit, the 2 days preceding the audit day, and then followed until the patient was either discharged from the hospital or transferred to a higher level of care, died, or transitioned to palliative or hospice care. Demographics, details about the line, and the criteria for justified use were extracted from the electronic medical record.
Definitions
Justified and Idle Days
To justify the presence of a CVC on any given day, we used criteria that fell under 3 categories: intravenous (IV) access needs, unstable vitals, or meeting sepsis/systemic inflammatory response syndrome (SIRS) criteria (Table 1). For vital signs, a single abnormal reading was counted as fulfilling criteria for that day. If no criterion for justified use was met, the line was considered idle for that day.
|
| IV access needs |
| Expected duration of IV antibiotics >6 days |
| Administration of TPN |
| Anticipated requirement of home IV medications |
| Requirement of IV medications with documented difficult access |
| Hemorrhage requiring blood transfusions |
| Requiring more than 3 infusions |
| Requiring more than 2 infusions and blood transfusions |
| Abnormal vitals |
| Diastolic blood pressure >120 mm Hg |
| Systolic blood pressure <90 mm Hg |
| Systolic blood pressure >200 mm Hg |
| Heart rate >120 beats per minute |
| Heart rate <50 beats per minute |
| Respiratory rate >30 breaths per minute |
| Respiratory rate <10 breaths per minute |
| Oxygen saturation <90% as measured by pulse oximetry |
| Meeting SIRS criteria (2 or more of the following present) |
| Temp >38C, Temp <36C, heart rate >90 beats per minute, respiratory rate >20 breaths per minute, WBC >12,000/mm3, WBC <1,000/mm3, bandemia >10% |
Qualifying IV access needs were defined similarly to those previously used,[5, 6] whereas those for SIRS followed the current consensus.[8] To determine the number of IV medications or infusions, the medication administration record was reviewed. If 3 or more infusions were found, their compatibility was checked using the same database that nurses use at our institution. Difficult IV access was inferred from the indication for line placement, coupled with the absence of documentation of a peripheral IV. Clinical progress notes were reviewed to extract information on the length of proposed IV antibiotic courses, and discharge instructions were reviewed to verify whether the line was removed prior to discharge or not. The cutoffs for diastolic blood pressure, respiratory rate, and oxygen saturation used to label patients hemodynamically labile are the same as those used by previous authors and also constitute the definition of hypertensive urgency.[5, 9] However, we diverged from the values previously used for tachycardia, bradycardia, and systolic hypotension using heart rates >120 and <50 beats per minute (compared to >130 and <40 beats per minute) and systolics <90 mm Hg (compared to <80 mm Hg) to justify the line.[5] Early warning scores have been used to identify hospitalized ward patients who are at risk for clinical deterioration. Although each score utilizes different thresholds, the risk for clinical deterioration increases as the vitals worsen.[10] Bearing this in mind, the thresholds we elected to use are more clinically conservative and also parallel the nursing call orders currently used at our institution.
Proposed Guideline
We propose the guideline that a CVC may be safely removed the day after the first idle day.
RESULTS
A total of 126 lines were observed in 126 patients. Eighty‐three (65.9%) of the lines were PICCs. The remaining 43 (34.1%) were CVLs. The indications for line placement were distributed between the need for central access, total parenteral nutrition, or antibiotics (Table 2).
| Description | Value |
|---|---|
| |
| Age in yrs mean (SD) | 55.7 (18) |
| Gender, n (%) | |
| Female | 66 (52.4) |
| Male | 60 (47.6) |
| Type of line, n (%) | |
| PICC | 83 (65.9) |
| CVL | 43 (34.1) |
| Indication for line placement, n (%) | |
| Meds requiring central access or TPN | 36 (28.6) |
| Antibiotics | 34 (27.0) |
| Hemodynamic instability | 30 (23.8) |
| Poor access with multiple IV medications | 18 (14.3) |
| Unknown | 8 (6.3) |
| Line removed prior to discharge, n (%) | |
| Yes | 76 (60.3) |
| No | 50 (39.7) |
Out of the 126 patients, 50 (39.7%) were discharged from the hospital, died, were transferred to a higher level of care, or transitioned to palliative or hospice care with the line in place. In the remaining 76 patients, the audit captured 635 days, out of which a line was in place for 522 (82.2%) days. Of these 522 days, the line's presence was justified by our criteria for 351 (67.2%) days. The most common reason for a line to be justified on any given day was the need for antibiotics followed by the presence of SIRS criteria (Table 3). The remaining 171 (32.7%) days were idle.
| Criteria | N | % |
|---|---|---|
| ||
| No. of factors justifying use | ||
| 1 | 184 | 52.4% |
| 2 | 127 | 36.2% |
| >2 | 40 | 11.4 |
| Reason for justifying line* | ||
| Anticipate home or >6 days of antibiotic use | 181 | 51.6 |
| SIRS criteria | 124 | 35.3 |
| TPN | 96 | 27.4 |
| Hemodynamic instability based on hr and bp | 78 | 22.2 |
| Poor access with need for IV medications | 57 | 16.2 |
| Respiratory rate (<10 or >30/minute) | 25 | 7.1 |
| Active hemorrhage requiring transfusions | 12 | 3.4 |
| >3 infusions | 6 | 1.7 |
A comparison of the actual removal of the 76 central lines in practice relative to the proposed guideline of removing it the day following the first idle day is displayed in Figure 1. The central line was removed prior to our proposed guideline in 11 (14.5%) patients, and waiting for an idle day in these patients would have added 46 line days. In almost half the patients (n = 36, 47.4%), the line was removed in agreement with the proposed guideline. None of the patients in whom the line was removed prior to or in accordance with our proposed guideline required a line reinsertion. Line removal was delayed in 29 (38.2%) patients when compared to our proposed guideline. In these patients, following the guideline would have created 122 line‐free days. Most (n = 102, 83.6%) of these potential line‐free days were idle. Twenty (16.4%) were justified, of which half (n = 10) were justified by meeting SIRS criteria.

DISCUSSION
Approximately 1 in every 25 inpatients in the United States has at least 1 HAI on any given day.[11] The case fatality rate from a CLABSI may be as high as 12%, and up to 70% of these infections may be preventable.[1, 12] Interventions successful in decreasing CLABSIs have focused on patients in ICUs.[13] However, CVCs are increasingly prevalent outside the ICU, with over 4.5 million line days in non‐ICU beds reported to the National Healthcare Safety Network in 2012 compared to 2.5 million in 2010.[2, 14] However, adherence rates to infection control practices may be lower on the wards than in the ICUs.[6, 15] Consequently, although the number of CLABSIs has declined over the last decade, most are now occurring outside the ICU.[16] These trends underscore the need to develop strategies aimed at CLABSI prevention on the floors.
Analogous to the life cycle of a urinary catheter described by Meddings et al.,[17] strategies to prevent CLABSIs and other CVC‐related complications may be designed around the life cycle of a CVC. The life cycle starts with insertion and moves on to the maintenance, removal, and possible reinsertion of the line. The process thus starts with the decision to place the line. Over the last decade, this decision making has changed in part due to PICCs. This shift is reflected in PICC prevalence rates: in 2001, 11% of audited central lines were PICCs compared to 56% in 2007.[5, 6] In our audit, 66% of the CVCs were PICCs. This increase in the use of PICCs may be attributable to the ease and safety of their placement coupled with the increased availability of vascular access placement teams.[18] The risk of overuse that may result from such expediency may be countered by adhering to guidelines such as the Michigan Appropriateness Guide for Intravenous Catheters, which provides both clinically detailed guidance and an impetus for reflective decision making around intravenous access.[19]
The placement of CVCs for prolonged parenteral antibiotics may be a particular subset that bears further exploration. Similar to previous reports, we found that a large number of the CVCs were both inserted for and justified by the need for IV antibiotics.[5] Guidelines delineated by the Infectious Diseases Society of America regarding outpatient parenteral antibiotics weigh both the duration of therapy and the antimicrobial's potential for causing phlebitis when recommending the type of intravascular access.[20] Many courses may therefore be completed through peripheral or midline catheters. Developing strong partnerships between infectious disease specialists, hospitalists, and the facilities or home‐care services treating these patients may curtail the use of CVCs for antimicrobial administration.
The main focus of our work is on facilitating the safe removal of CVCs. The risk of CLABSIs increases each day a CVC is in place, and guidelines to prevent CLABSIs include recommendations to promptly remove nonessential catheters.[4, 21] There is also an emerging understanding that the risk of a PICC‐related CLABSI approaches that from a traditional central line in hospitalized patients, and PICCs confer an increased risk of venous thromboembolism.[18, 22] Although nearly half of surveyed hospitalists recently reported leaving PICCs in place until discharge day, our data suggest that this practice may be driven by the trajectory of a patient's recovery as much as by knowledge gaps related to the use of PICCs.[23] In nearly half the instances, clinical practice already mirrors our proposed guideline, with line removal coinciding with both the timing proposed by our guideline and discharge day. However, there is room for improvement, as line removal may have been expedited in the 29 patients in whom the line was retained after the first idle day. Maintaining an awareness of its presence and weighing its risks and benefits daily may facilitate the removal of a CVC. Based on the recent findings that up to a quarter of clinicians are unaware that their patients have a central line, the mere reminder of the presence of a line using such criteria may expedite its removal by triggering a purposeful reassessment of its ongoing need.[24] Premature CVC removal requiring line reinsertion is an unintended consequence that may emerge from the earlier removal of lines. In our sample, none of the patients who had lines removed either prior to or in accordance with our proposed guideline required a line reinsertion. In addition to line reinsertion, delays in laboratory testing and reporting due to the unavailability of access, increased patient discomfort, or increased workload on the bedside nurse or vascular access team must also be considered when implementing strategies aimed at decreasing line days.
We envisage using these criteria to both empower practitioners with knowledge and foster shared accountability between all team members by using a uniform tool. This can occur through partnerships between infection control, clinical nurse specialists, bedside nursing, and physicians. The electronic medical record could be leveraged to scan the record for the criteria and create a notification when the line becomes idle. In alignment with the Michigan Appropriateness Guide for Intravenous Catheters guidelines, we do not support the removal of lines by nursing staff without physician notification.[19] Such principles have been successfully harnessed in strategies to prevent both catheter‐associated urinary tract infections and CLABSIs in ICUs.[13, 25] In light of the complexity surrounding the decision making for CVCs, our criteria were focused on the wards and erred on the side of clinical caution. This clinical conservatism is apparent in the patients in whom lines were removed prior to what our guideline would propose, yet none of the patients required a line reinsertion. As concerns about recrudescent clinical instability may drive decision making around line removal, such conservatism may be warranted initially. However, the fidelity of these criteria in the clinical setting will need prospective validation. In particular, the inclusion of SIRS criteria may have led to an overestimation of justified days. Further studies may be needed to refine the criteria and find a clinical hierarchy that balances the risks and benefits of retaining a central line.
Our work has certain limitations. It is a single center's experience, and our findings may not therefore be generalizable. Except for when the indication for the line was for difficult access, we did not attempt to verify the presence of a peripheral IV. This, in combination with the inclusion of SIRS criteria, likely leads to an underestimation of idle days. In the interest of focusing on patients in whom the decision making around a line would be the least controversial, we did not continue to follow patients who were transferred to a higher level of care. It is possible, however, that these transfers were precipitated by line‐associated complications such as sepsis and would be important to track. We did not measure the agreement between data collectors, although definitions and methodologies were standardized and reviewed prior to data collection. As this was an observational assessment of a proposed guideline, we cannot predict how the recommendations generated by it will be received by clinicians. Although this may prove to be a barrier in adoption, we hope that the conversation it initiates leads to change.
Hospitalists are positioned to potentially influence the entire life cycle of a central line on the floor. Strategies can be enacted at each stage to help decrease the potential of harm from these devices to our patients. Creating and testing criteria and guidelines such as we propose represents just 1 such strategy in a multidisciplinary effort to provide the best possible care we can.
Acknowledgements
The authors thank Jennifer Dunscomb, Kristen Kelly, and their teams, and Deanna Sidwell, Todd Biggerstaff, Joan Miller, Rob Clark, and the tireless providers at Indiana University Health Methodist Hospital for their support.
Disclosures: This work was supported by the Indiana University Health Values Grant for research. The authors have no conflicts of interests to report.
- , , , , , . Estimating the proportion of healthcare‐associated infections that are reasonably preventable and the related mortality and costs. Infect Control Hosp Epidemiol. 2011;32(2):101–114.
- , , , et al. National Healthcare Safety Network (NHSN) report, data summary for 2012, device‐associated module. Am J Infect Control. 2013;41(12):1148–1166.
- , , . The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc. 2006;81(9):1159–1171.
- , , , et al. Guidelines for the prevention of intravascular catheter‐related infections. Clin Infect Dis. 2011;52(9):e162–e193.
- , , , et al. Temporary central venous catheter utilization patterns in a large tertiary care center: tracking the “idle central venous catheter.” Infect Control Hosp Epidemiol. 2012;33(1):50–57.
- , , , , , . Unnecessary use of central venous catheters: the need to look outside the intensive care unit. Infect Control Hosp Epidemiol. 2004;25(3):266–268.
- IU Health Methodist Hospital website. Available at: http://iuhealth.org/methodist/aboIut. Accessed October 20, 2014.
- , , , et al. Definitions for Sepsis and Organ Failure and Guidelines for the Use of Innovative Therapies in Sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 2009;136(5 suppl):e28.
- , , , , , . Acute hypertension: a systematic review and appraisal of guidelines. Ochsner J. 2014;14(4):655–663.
- , , . Predicting clinical deterioration in the hospital: the impact of outcome selection. Resuscitation. 2013;84(5):564–568.
- , , , et al. Multistate point‐prevalence survey of health care–associated infections. N Engl J Med. 2014;370(13):1198–1208.
- , , , et al. Estimating health care‐associated infections and deaths in U.S. hospitals, 2002. Public Health Rep. 2007;122(2):160–166.
- , , , et al. An intervention to decrease catheter‐related bloodstream infections in the ICU. N Engl J Med. 2006;355(26):2725–2732.
- , , , et al. Data summary for 2011, device‐associated module. Centers for Disease Control and Prevention. National Healthcare Safety Network (NHSN) Report. Available at: http://www.cdc.gov/nhsn/PDFs/dataStat/NHSN‐Report‐2011‐Data‐Summary.pdf. Published April 1, 2013. Last accessed January 2015.
- , , . Idle central venous catheter‐days pose infection risk for patients after discharge from intensive care. Am J Infect Control. 2014;42(4):453–455.
- , . Update on emerging infections: news from the Centers for Disease Control and Prevention. Vital signs: central line‐associated blood stream infections—United States, 2001, 2008, and 2009. Ann Emerg Med. 2011;58(5):447–451.
- , , , , , . Reducing unnecessary urinary catheter use and other strategies to prevent catheter‐associated urinary tract infection: an integrative review. BMJ Qual Saf. 2014;23(4):277–289.
- , , , , . The risk of bloodstream infection associated with peripherally inserted central catheters compared with central venous catheters in adults: a systematic review and meta‐analysis. Infect Control Hosp Epidemiol. 2013;34(9):908–918.
- , , , et al. The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC): results from a multispecialty panel using the RAND/UCLA Appropriateness Method. Ann Intern Med. 2015;163(6 suppl):S1–S40.
- , , , et al. Practice guidelines for outpatient parenteral antimicrobial therapy. IDSA guidelines. Clin Infect Dis. 2004;38(12):1651–1672.
- , . Nonuniform risk of bloodstream infection with increasing central venous catheter‐days. Infect Control Hosp Epidemiol. 2005;26(8):715–719.
- , , , et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta‐analysis. Lancet. 2013;382(9889):311–325.
- , , , , . Hospitalist experiences, practice, opinions, and knowledge regarding peripherally inserted central catheters: results of a national survey. J Hosp Med. 2013;8(11):635–638.
- , , , et al. Do clinicians know which of their patients have central venous catheters? Ann Intern Med. 2014;161(8):562.
- , , , , , . Reducing foley catheter device days in an intensive care unit: using the evidence to change practice. AACN Adv Crit Care. 2006;17(3):272–283.
- , , , , , . Estimating the proportion of healthcare‐associated infections that are reasonably preventable and the related mortality and costs. Infect Control Hosp Epidemiol. 2011;32(2):101–114.
- , , , et al. National Healthcare Safety Network (NHSN) report, data summary for 2012, device‐associated module. Am J Infect Control. 2013;41(12):1148–1166.
- , , . The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc. 2006;81(9):1159–1171.
- , , , et al. Guidelines for the prevention of intravascular catheter‐related infections. Clin Infect Dis. 2011;52(9):e162–e193.
- , , , et al. Temporary central venous catheter utilization patterns in a large tertiary care center: tracking the “idle central venous catheter.” Infect Control Hosp Epidemiol. 2012;33(1):50–57.
- , , , , , . Unnecessary use of central venous catheters: the need to look outside the intensive care unit. Infect Control Hosp Epidemiol. 2004;25(3):266–268.
- IU Health Methodist Hospital website. Available at: http://iuhealth.org/methodist/aboIut. Accessed October 20, 2014.
- , , , et al. Definitions for Sepsis and Organ Failure and Guidelines for the Use of Innovative Therapies in Sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 2009;136(5 suppl):e28.
- , , , , , . Acute hypertension: a systematic review and appraisal of guidelines. Ochsner J. 2014;14(4):655–663.
- , , . Predicting clinical deterioration in the hospital: the impact of outcome selection. Resuscitation. 2013;84(5):564–568.
- , , , et al. Multistate point‐prevalence survey of health care–associated infections. N Engl J Med. 2014;370(13):1198–1208.
- , , , et al. Estimating health care‐associated infections and deaths in U.S. hospitals, 2002. Public Health Rep. 2007;122(2):160–166.
- , , , et al. An intervention to decrease catheter‐related bloodstream infections in the ICU. N Engl J Med. 2006;355(26):2725–2732.
- , , , et al. Data summary for 2011, device‐associated module. Centers for Disease Control and Prevention. National Healthcare Safety Network (NHSN) Report. Available at: http://www.cdc.gov/nhsn/PDFs/dataStat/NHSN‐Report‐2011‐Data‐Summary.pdf. Published April 1, 2013. Last accessed January 2015.
- , , . Idle central venous catheter‐days pose infection risk for patients after discharge from intensive care. Am J Infect Control. 2014;42(4):453–455.
- , . Update on emerging infections: news from the Centers for Disease Control and Prevention. Vital signs: central line‐associated blood stream infections—United States, 2001, 2008, and 2009. Ann Emerg Med. 2011;58(5):447–451.
- , , , , , . Reducing unnecessary urinary catheter use and other strategies to prevent catheter‐associated urinary tract infection: an integrative review. BMJ Qual Saf. 2014;23(4):277–289.
- , , , , . The risk of bloodstream infection associated with peripherally inserted central catheters compared with central venous catheters in adults: a systematic review and meta‐analysis. Infect Control Hosp Epidemiol. 2013;34(9):908–918.
- , , , et al. The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC): results from a multispecialty panel using the RAND/UCLA Appropriateness Method. Ann Intern Med. 2015;163(6 suppl):S1–S40.
- , , , et al. Practice guidelines for outpatient parenteral antimicrobial therapy. IDSA guidelines. Clin Infect Dis. 2004;38(12):1651–1672.
- , . Nonuniform risk of bloodstream infection with increasing central venous catheter‐days. Infect Control Hosp Epidemiol. 2005;26(8):715–719.
- , , , et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta‐analysis. Lancet. 2013;382(9889):311–325.
- , , , , . Hospitalist experiences, practice, opinions, and knowledge regarding peripherally inserted central catheters: results of a national survey. J Hosp Med. 2013;8(11):635–638.
- , , , et al. Do clinicians know which of their patients have central venous catheters? Ann Intern Med. 2014;161(8):562.
- , , , , , . Reducing foley catheter device days in an intensive care unit: using the evidence to change practice. AACN Adv Crit Care. 2006;17(3):272–283.
Redesigning Inpatient Care
Despite an estimated annual $2.6 trillion expenditure on healthcare, the United States performs poorly on indicators of health and harm during care.[1, 2, 3] Hospitals around the nation are working to improve the care they deliver. We describe a model developed at our institution and report the evaluation of the outcomes associated with its implementation on the general medical and surgical units. The Indiana University Institutional Review Board approved this work.
SETTING AND DEFINITIONS
Indiana University Health Methodist Hospital (MH) is an academic center in Indianapolis, Indiana, serving over 30,000 patients annually.[4] In 2012, responding to the coexisting needs to improve quality and contain costs, the MH leadership team redesigned care in the hospital. The new model centers around accountable care teams (ACTs). Each ACT is a geographically defined set of providers accepting ownership for the clinical, service, and financial outcomes of their respective inpatient unit. The units studied are described in Table 1.
| Unit | No. of Beds | Predominant Diagnosis (Maximum Domain Score)* | |
|---|---|---|---|
| |||
| Medical units with progressive‐care beds | 1 | 33 | Pulmonary (3.4, 3.5, 5) |
| 2 | 28 | Cardiology (4.8, 3.5, 4) | |
| 3 | 24 | General medical (4.8, 3.5, 4) | |
| Medical units without progressive‐care beds | 4 | 36 | Renal/diabetic (4, 3.5, 5) |
| 5 | 24 | General medical (3.75, 4, 5) | |
| Surgical units with progressive‐care beds | 6 | 51 | Cardiothoracic surgery/cardiology (4, 4, 5) |
| 7 | 29 | Trauma/general surgery (3.75, 3.5, 5) | |
| 8 | 23 | Neurosurgical/neurological (4.8, 5, 5) | |
| 9 | 24 | Neurosurgical/neurological (4.4, 4.5, 5) | |
| Surgical units without progressive‐care beds | 10 | 29 | General/urologic/gynecologic/plastic surgery (3.4, 3, 2) |
| 11 | 26 | Orthopedic surgery (4.6, 4, 5) | |
THE ACT MODEL
The model comprises 8 interventions rooted in 3 foundational domains: (1) enhancing interprofessional collaboration (IPC), (2) enabling data‐driven decisions, and (3) providing leadership. Each intervention is briefly described under its main focus (see Supporting Information, Appendix A, in the online version of this article for further details).
Enhancing IPC
Geographical Cohorting of Patients and Providers
Hospitalist providers are localized for 4 consecutive months to 1 unit. An interdisciplinary team including a case manager, clinical nurse specialist, pharmacist, nutritionist, and social worker also serve each unit. Learners (residents, pharmacy, and medical students) are embedded in the team when rotating on the hospital medicine service. The presence of unit‐based nurse managers and charge nurses predates the model and is retained.
Bedside Collaborative Rounding
Geographically cohorted providers round on their patients with the bedside nurse guided by a customizable script.
Daily Huddle
The hospitalist, learners, and the interdisciplinary team for the unit meet each weekday to discuss patients' needs for a safe transition out of the hospital. Each unit determined the timing, location, and script for the huddle while retaining the focus on discharge planning (see Supporting Information, Appendix A2, in the online version of this article for a sample script).
Hospitalist and Specialty Comanagement Agreements
Guidelines delineating responsibilities for providers of each specialty were developed. Examples include orders pertaining to the management of a dialysis catheter in a patient with end‐stage renal disease, the removal of drains in postsurgical patients, and wound care.
Unit White Board
Each unit has a white board at the nursing station. Similar to the huddle, it is focused on discharge planning.
Enabling Data‐Driven Decisions
Monthly Review of Unit‐Level Data
Data analytics at our institution developed a data dashboard. Key metrics including length of stay (LOS), patient satisfaction scores, readmission rates, and costs are tracked and attributed to the discharging unit. The data are collated monthly by the ACT program director and distributed to each unit's leadership. Monthly interdisciplinary meetings are held to review trends. Learners are encouraged but not required to attend.
Weekly Patient Satisfaction Rounding
The unit's nurse manager and physician leader conduct weekly satisfaction rounds on patients. The conversation is open‐ended and focused on eliciting positive and negative experiences.
Providing Leadership
Designated hospitalist and, where relevant, specialty leaders are committed to serve each unit for at least 1 year as a resource for both medical and operational problem solving. The leader stays closely connected with the unit's nurse manager. In addition to day‐to‐day troubleshooting, the leader is responsible for monitoring outcome trends. There is currently no stipend, training, or other incentive offered for the role.
Implementation Timelines and ACT Scores
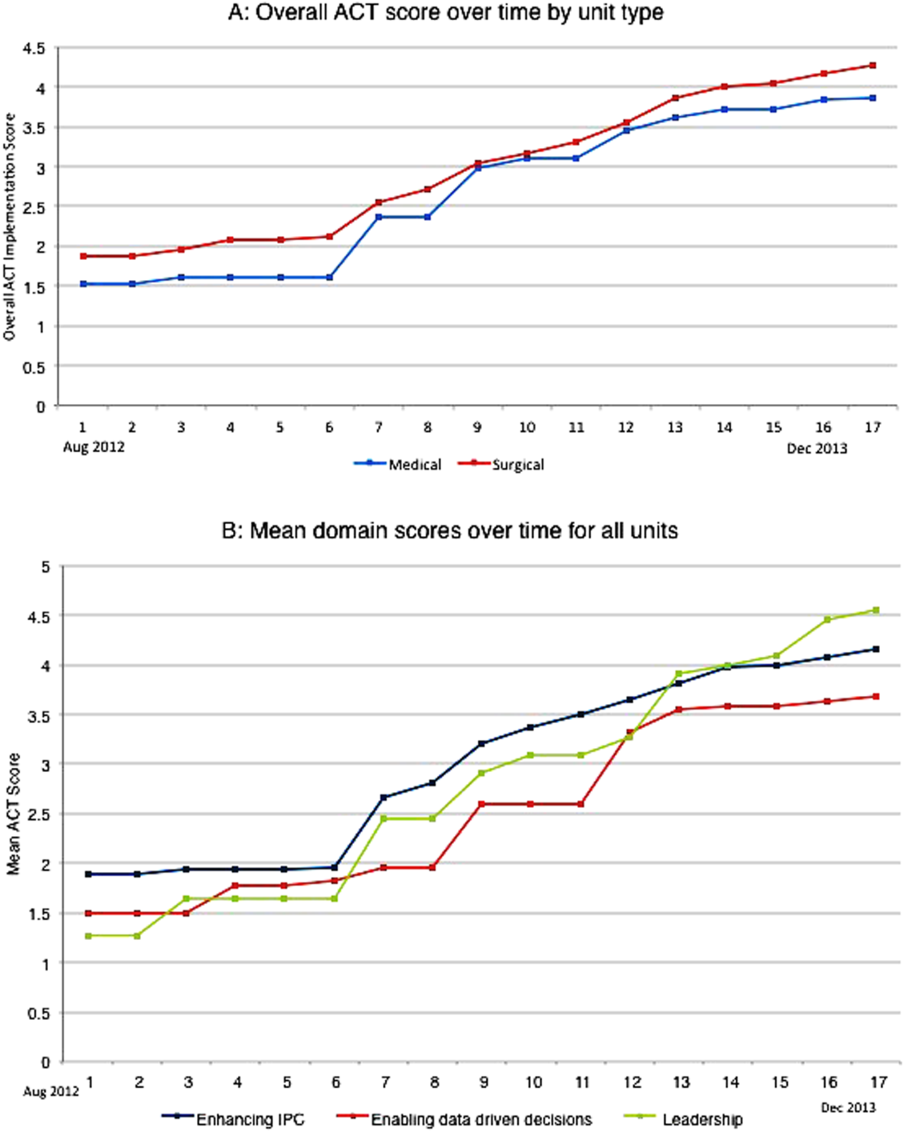
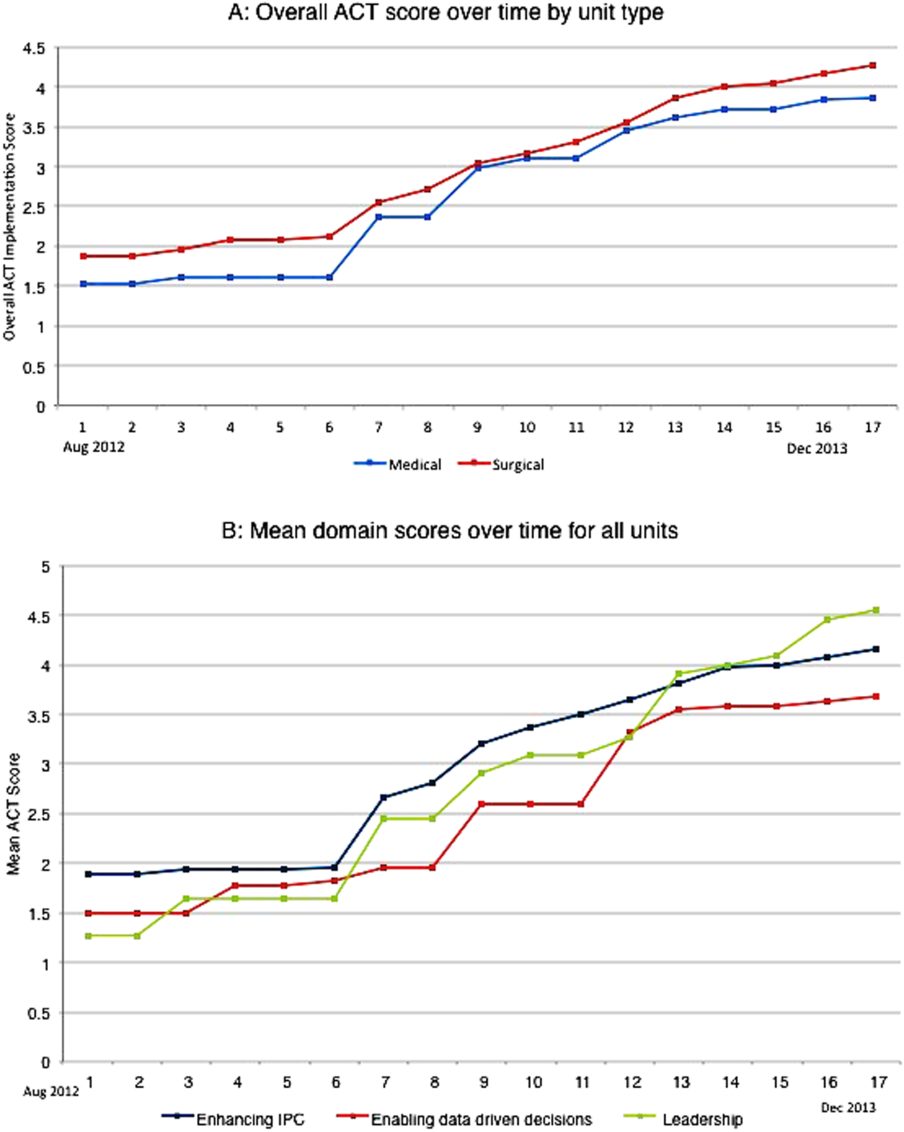
The development of the ACTs started in the spring of 2012. Physician, nursing, and pharmacy support was sought, and a pilot unit was formed in August 2012. The model was cascaded hospital wide by December 2013, with support from the ACT program director (A.N.). The program director observed and scored the uptake of each intervention by each unit monthly. A score of 1 denoted no implementation, whereas 5 denoted complete implementation. The criteria for scoring are presented in Table 2. The monthly scores for all 8 interventions in each of the 11 units were averaged as an overall ACT score, which reflects the implementation dose of the ACT model. Monthly domain scores for enhancing IPC and enabling data‐driven decisions were also calculated as the average score within each domain. This yielded 3 domain scores. Figure 1A plots by month the overall ACT score for the medical and surgical units, and Figure 1B plots the implementation score for the 3 domains between August 2012 and December 2013 for all units. The uptake of the interventions varied between units. This allowed our analysis to explore the dose relationships between the model and outcomes independent of underlying time trends that may be affected by concomitant initiatives.
| 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|
| |||||
| Geographical cohorting of patients and the ACT* | None | At least 1 discipline comprising the ACT is unit based | All disciplines comprising the ACT except the hospitalist unit based | All disciplines including the hospitalist unit based | 4 + 80% of hospitalist provider's patients on the unit |
| Bedside collaborative rounding | None | Occurring 1 day a week on at least 25% of the patients on the unit | Occurring 2 to 3 days a week on at least 50% of the patients on the unit | Occurring 3 to 4 days a week on at least 75% of the patients on the unit | Occurring MondayFriday on all patients on the unit |
| Daily huddle | None | Occurring daily, 1 out of 4 ACT disciplines represented, at least 25% of patients on the unit discussed | Occurring daily, 2 out of 4 ACT disciplines represented, at least 50% of patients on the unit discussed | Occurring daily, 3 out of 4 ACT disciplines represented, at least 75% of patients on the unit discussed | Occurring daily, all disciplines of the ACT represented, all patients on the unit discussed |
| Hospitalist and specialty comanagement agreements | None | One out of 3 specialists represented on the unit collaborating with the hospitalists on at least 25% of relevant patients | One out of 3 specialists represented on the unit collaborating with the hospitalists on at least 50% of relevant patients | Two out of 3 specialists on the unit collaborating with the hospitalists on at least 75% of relevant patients | All specialists on the unit collaborating with the hospitalists on all relevant patients on the unit |
| Unit white board | None | Present but only used by nursing | Present and used by all ACT disciplines except physician providers | Present and used by entire ACT; use inconsistent | Present and used MondayFriday by all disciplines of ACT |
| Monthly review of unit level data | None | Nurse manager reviewing data with ACT program director | Nurse manager and unit leader reviewing data with ACT program director | Meeting either not consistently occurring monthly or not consistently attended by entire ACT | Monthly meeting with entire ACT |
| Weekly patient satisfaction rounding | None | Nurse manager performing up to 1 week a month | Nurse manager performing weekly | Nurse and physician leader performing up to 3 times a month | Nurse and physician leader performing weekly |
| Leadership | None | For units with specialties, either hospitalist or specialist leader identified | Both hospitalist and specialist leader Identified | Both hospitalist and specialist leaders (where applicable) identified and partially engaged in leadership role | Both hospitalist and specialist leaders (where applicable) identified and engaged in leadership role |
Outcomes
Monthly data between August 2012 and December 2013 were analyzed.
Measures of Value
MH is a member of the University Health Consortium, which measures outcomes of participants relative to their peers. MH measures LOS index as a ratio of observed LOS to expected LOS that is adjusted for severity of illness.[5]
Variable direct costs (VDCs) are costs that a hospital can save if a service is not provided.[6] A hospital's case‐mix index (CMI) represents the average diagnosis‐related group relative weight for that hospital. We track VDCs adjusted for CMI (CMI‐adjusted VDC).[7]
Thirty‐day readmission rate is the percentage of cases that are readmitted to MH within 30 days of discharge from the index admission.[8]
Measures of Patient Satisfaction
The Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey covers topics relevant to a patient's experience in the hospital.[9] Patient satisfaction scores are tracked by responses to the HCAHPS survey.
Measures of Provider Satisfaction
Hospitalist and specialty providers, leadership, and case management teams were surveyed via email through SurveyMonkey in July 2014. The survey included Likert responses that elicited opinions and comments about the ACT model.
Statistical Methods
The primary predictor of interest was the monthly overall ACT score. We also explored the domain scores as well as the individual scores for each intervention. Generalized linear mixed models were fit to investigate the association between each predictor (overall ACT score, ACT domain scores, and individual implementation scores) and each outcome (LOS index, CMI‐adjusted VDC, 30‐day readmission rate, and overall patient satisfaction). The model for testing each ACT score also included covariates of inpatient units as a random effect, as well as date and type of unit as fixed effects. We set the statistical significance level at 0.01 and reported 99% confidence intervals.
Descriptive statistics were used to report the provider satisfaction survey results.
RESULTS
The overall ACT score was associated with LOS index and CMI‐adjusted VDC (both P < 0.001). For every 1‐unit increase in the overall ACT score, LOS index decreased by 0.078 and CMI‐adjusted VDC decreased by $273.99 (Table 3).
| Length of Stay Index | CMI Adjusted VDC | |||
|---|---|---|---|---|
| Estimate (99% CI)* | P Value | Estimate (99% CI)* | P Value | |
| ||||
| Overall ACT Score | 0.078 (0.123 to 0.032) | <0.001 | 274.0 (477.31 to 70.68) | <0.001 |
| Enhancing IPC | 0.071 (0.117 to 0.026) | <0.001 | 284.7 (488.08 to 81.23) | <0.001 |
| Enabling data‐driven decisions | 0.044 (0.080 to 0.009) | 0.002 | 145.4 (304.57 to 13.81) | 0.02 |
| Providing leadership | 0.027 (0.049 to 0.005) | 0.001 | 69.9 (169.00 to 29.26) | 0.07 |
Looking at domains, enhancing IPC resulted in statistically significant decreases in both LOS index and CMI‐adjusted VDC, but providing leadership and enabling data‐driven decisions decreased only the LOS index. Most of the 8 individual interventions were associated with at least 1 of these 2 outcomes. (Even where the associations were not significant, they were all in the direction of decreasing LOS and cost). In these models, the covariate of type of units (medical vs surgical) was not associated with LOS or cost. There was no significant time trend in LOS or cost, except in models where an intervention had no association with either outcome. Inclusion of all individual effective interventions in the same statistical model to assess their relative contributions was not possible because they were highly correlated (correlations 0.450.89).
Thirty‐day readmissions and patient satisfaction were not significantly associated with the overall ACT score, but exploratory analyses showed that patient satisfaction increased with the implementation of geographical cohorting (P = 0.007).
Survey Results
The response rate was 87% (96/110). Between 85% and 96% of respondents either agreed or strongly agreed that the ACT model had improved the quality and safety of the care delivered, improved communication between providers and patients, and improved their own engagement and job satisfaction. Overall, 78% of the respondents either agreed or strongly agreed that the model improved efficiency (Table 4). Suggestions for improvements revolved around increasing the emphasis on patient centeredness and bedside nursing engagement.
| The ACT Model | Strongly Agree, n (%) | Agree, n (%) | Disagree, n (%) | Strongly Disagree, n (%) |
|---|---|---|---|---|
| ||||
| Has improved the quality and safety of patient care | 46 (47.9) | 46 (47.9) | 2 (2.1) | 2 (2.1) |
| Has improved communication with patients and families | 42 (43.7) | 47 (49.0) | 5 (5.2) | 2 (2.1) |
| Has improved your efficiency/productivity | 31 (32.6) | 43 (45.3) | 17 (17.9) | 4 (4.2) |
| Has improved your engagement and job satisfaction | 33 (34.4) | 49 (51.0) | 10 (10.4) | 4 (4.2) |
| Is a better model of delivering patient care | 45 (47.4) | 44 (46.3) | 2 (2.1) | 4 (4.2) |
DISCUSSION
The serious problems in US healthcare constitute an urgent imperative to innovate and reform.[10] Inpatient care reflects 31% of the expenditure on healthcare, and in 2010, 35.1 million patients were discharged from the hospital after spending an average of 4.8 days as an inpatient.[11] These figures represent an immense opportunity to intervene. Measuring the impact of quality improvement efforts is often complicated by concomitant changes that affect outcomes over the interval studied. Our approach allowed us to detect statistically significant changes in LOS index and CMI‐adjusted VDC associated with the ACT implementation dose that could be separated from the underlying time trends.
The ACT model we describe is rooted in improving 3 foundational domains; quantifying each intervention's compartmentalized contribution, however, proved difficult. Each intervention intertwines with the others to create changes in attitudes, knowledge, and culture that are difficult to measure yet may synergistically affect outcomes. For example, although geographical cohorting appears to have the strongest statistical association with outcomes, this may be mediated by how it enables other processes to take place more effectively. Based on this analysis, therefore, the ACT model may best be considered a bundled intervention.
The team caring for a patient during hospitalization is so complex that fewer than a quarter of patients know their physician's or nurse's name.[12] This complexity impairs communication between patients and providers and between the providers themselves. Communication failures are consistently identified as root causes in sentinel events reported to the Joint Commission.[13] IPC is the process by which different professional groups work together to positively impact health care. IPC overlaps with communication, coordination, and teamwork, and improvements in IPC may improve care.[14] Some elements of the model we describe have been tested previously.[15, 16, 17] Localization of teams may increase productivity and the frequency with which physicians and nurses communicate. Localization also decreases the number of pages received and steps walked by providers during a workday.[15, 16, 17] However, these studies reported a trend toward an increase in the LOS and neutral effects on cost and readmission rates. We found statistically significant decreases in both LOS and cost associated with the geographic cohorting of patients and providers. Notably, our model localized not only the physician providers but also the interdisciplinary team of pharmacists, clinical nurse specialists, case managers, and social workers. This proximity may facilitate IPC between all members that culminates in improved efficiency. The possibility of delays in discharges to avoid new admissions in a geographically structured team has previously been raised to explain the associated increases in LOS.[16, 17] The accountability of each unit for its metrics, the communication between nursing and physicians, and the timely availability of the unit's performance data aligns everyone toward a shared goal and provides some protection from an unintended consequence.
Structured interdisciplinary rounds decrease adverse events and improve teamwork ratings.[18, 19] The huddle in our model is a forum to collaborate between disciplines that proved to be effective in decreasing LOS and costs. Our huddle aims to discuss all the patients on the unit. This allows the team to assist each other in problem solving for the entire unit and not just the patients on the geographically cohorted team. This approach, in addition to the improved IPC fostered by the ACT model, may help explain how benefits in LOS and costs permeated across all 11 diverse units despite the presence of patients who are not directly served by the geographically cohorted team.
High‐performing clinical systems maintain an awareness of their overarching mission and unit‐based leaders can influence the frontline by reiterating the organizational mission and aligning efforts with outcomes.[20] Our leadership model is similar to those described by other institutions in the strong partnerships between physicians and nursing.[21] As outlined by Kim et al., investing in the professional development of the unit leaders may help them fulfill their roles and serve the organization better.[21]
The fragmentation and lack of ownership over the continuum of patient care causes duplication and waste. The proposal in the Accountable Care Act to create accountable care organizations is rooted in the understanding that providers and organizations will seek out new ways of improving quality when held accountable for their outcomes.[22] To foster ownership and accountability, reporting of metrics at the unit level is needed. Furthermore, an informational infrastructure is critical, as improvements cannot occur without the availability of data to both monitor performance and measure the effect of interventions.[10, 23] Even without any other interventions, providing feedback alone is an effective way of changing practices.[24] According to Berwick et al., this phenomenon reflects practitioners' intrinsic motivation to simply want to be better.[25] Our monthly review of each unit's data is an effective way to provide timely feedback to the frontline that sparks pride, ownership, and innovative thinking.
Based on our mean ACT score and CMI‐adjusted VDC reductions alone, we estimate savings of $649.36 per hospitalization (mean increase in ACT implementation of 2.37 times reduction in cost index of $273.99 per unit increase in overall ACT score). This figure does not include savings realized through reductions in LOS. This is a small decrease relative to the mean cost of hospitalization, yet when compounded over the annual MH census, it would result in substantial savings. The model relied on the restructuring of the existing workforce and the only direct additional cost was the early salary support for the ACT program director.
Limitations
We recognize several limitations. It is a single center's experience and may not be generalizable. The diffusion of knowledge and culture carried between units and the relatively rapid implementation timeline did not allow for a control unit. A single observer assigned our implementation scores, and therefore we cannot report measures of inter‐rater reliability. However, defined criteria and direct observations were used wherever possible. Although administratively available data have their limitations, where available, we used measurements that are adjusted for severity of illness and CMI. We therefore feel that this dataset is an accurate representation of currently reported national quality indicators.
FURTHER DIRECTIONS
Although there is a need to improve our healthcare system, interventions should be deliberate and evidence based wherever possible.[26] Geographic cohorting may decrease the frequency of paging interruptions for physicians and practitioners while increasing face‐to‐face interruptions.[27] The net effect on safety with this trade‐off should be investigated.
The presence of an intervention does not guarantee its success. Despite geographic cohorting and interdisciplinary meetings, communication that influences physician decision making may not improve.[28] Although instruments to measure ratings of team work and collaboration are available, focusing on clinically relevant outcomes of teamwork, such as prevention of harm, may be more empowering feedback for the frontline. Formal cost‐benefit analyses and outcomes related to physician and nursing retention will be equally important for assessing the sustainability of the model. Involving patients and their caregivers and inviting their perspectives as care is redesigned will also be critical in maintaining patient centeredness. Research addressing interventions to mediate preventable readmission risk and understanding the drivers of patient satisfaction is also needed.
The true value of the model may be in its potential to monitor and drive change within itself. Continuously aligning aims, incentives, performance measures, and feedback will help support this innovation and drive. This affects not only patient care but creates microcosms within which research and education can thrive. We hope that our experience will help guide other institutions as we all strive in our journey to improve the care we deliver.
Acknowledgements
The authors thank the Indiana University Health Physicians hospitalists at MH, Sandy Janitz and Decision Support, the Indiana University Health executive leadership team, Robert Clark, Malaz Boustani, Dennis Watson, Nadia Adams, Todd Biggerstaff, Deanne Kashiwagi, and the tireless providers at MH for their support.
Disclosure: This work was supported by a grant from the Indiana University Health Values Fund. The authors have no conflicts of interest to disclose.
- Committee on Quality of Health Care in America; Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: The National Academies Press; 2001.
- . Is US health really the best in the world? JAMA. 2000;284(4):483–485.
- , , , , , . Temporal trends in rates of patient harm resulting from medical care. N Engl J Med. 2010;363(22):2124–2134.
- Indiana University Health. Available at: http://iuhealth.org/methodist/aboIut/. Accessed October 20, 2014.
- University Health Consortium. Available at: https://www.uhc.edu/docs/45014769_QSS_dashboard_FAQs.pdf. Accessed October 23, 2014.
- , , , et al. Distribution of variable vs fixed costs of hospital care. JAMA. 1999;281(7):644–649.
- Centers for Medicare and Medicaid Services. Case mix index. Available at: http://www.cms.gov/Medicare/Medicare‐Fee‐for‐Service‐Payment/AcuteInpatientPPS/Acute‐Inpatient‐Files‐for‐Download‐Items/CMS022630.html. Accessed May 4, 2015.
- University Health Consortium. Available at: https://www.uhc.edu. Accessed October 23, 2014.
- Centers for Medicare and Medicaid Services. Hospital Consumer Assessment of Healthcare Providers and Systems. HCAHPS survey content and administration. Centers for Medicare 280(11):1000–1005.
- Centers for Disease Control and Prevention. FastStats. Available at: http://www.cdc.gov/nchs/fastats/default.htm. Accessed October 27, 2014.
- , . Does your patient know your name? An approach to enhancing patients' awareness of their caretaker's name. J Healthc Qual. 2005;27(4):53–56.
- The Joint Commission. Sentinel event data: root causes by event type 2004‐third quarter. Available at: http://www.jointcommissionorg. Available at: http://www.jointcommission.org/assets/1/18/Root_Causes_by_Event_Type_2004-2Q2013.pdf. Accessed March 26, 2014.
- , , . Interprofessional collaboration: effects of practice‐based interventions on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2009;(3):CD000072.
- , , , et al. Impact of localizing physicians to hospital units on nurse–physician communication and agreement on the plan of care. J Gen Intern Med. 2009;24(11):1223–1227.
- , , , et al. Impact of localizing general medical teams to a single nursing unit. J Hosp Med. 2012;7(7):551–556.
- , , , et al. Implementation of a physician assistant/hospitalist service in an academic medical center: impact on efficiency and patient outcomes. J Hosp Med. 2008;3(5):361–368.
- , , , , , . Improving teamwork: impact of structured interdisciplinary rounds on a medical teaching unit. J Gen Intern Med. 2010;25(8):826–832.
- , , , ; High Performance Teams and the Hospital of the Future Project Team. Interdisciplinary teamwork in hospitals: a review and practical recommendations for improvement. J Hosp Med. 2011;7(1):48–54.
- , , , , , . Microsystems in health care: part 8. Developing people and improving work life: what front‐line staff told us. Jt Comm J Qual Saf. 2003;29(10):512–522.
- , , , , , . Unit‐based interprofessional leadership models in six US hospitals. J Hosp Med. 2014;9(8):545–550.
- , , , . Creating accountable care organizations: the extended hospital medical staff. Health Aff (Millwood). 2007;26(1):w44–w57.
- , . Using performance measurement to drive improvement: a road map for change. Med Care. 2003;41(1 suppl):I48–I60.
- , . Changing physicians' practices. N Engl J Med. 1993;329(17):1271–1273.
- , , . Connections between quality measurement and improvement. Med Care. 2003;41(1 suppl):I30–I38.
- , , . The tension between needing to improve care and knowing how to do it. N Engl J Med. 2007;357(6):608–613.
- , . A qualitative evaluation of geographical localization of hospitalists: how unintended consequences may impact quality. J Gen Intern Med. 2014;29(7):1009–1016.
- , , , , . Disengaged: a qualitative study of communication and collaboration between physicians and other professions on general internal medicine wards. BMC Health Serv Res. 2013;13:494.
Despite an estimated annual $2.6 trillion expenditure on healthcare, the United States performs poorly on indicators of health and harm during care.[1, 2, 3] Hospitals around the nation are working to improve the care they deliver. We describe a model developed at our institution and report the evaluation of the outcomes associated with its implementation on the general medical and surgical units. The Indiana University Institutional Review Board approved this work.
SETTING AND DEFINITIONS
Indiana University Health Methodist Hospital (MH) is an academic center in Indianapolis, Indiana, serving over 30,000 patients annually.[4] In 2012, responding to the coexisting needs to improve quality and contain costs, the MH leadership team redesigned care in the hospital. The new model centers around accountable care teams (ACTs). Each ACT is a geographically defined set of providers accepting ownership for the clinical, service, and financial outcomes of their respective inpatient unit. The units studied are described in Table 1.
| Unit | No. of Beds | Predominant Diagnosis (Maximum Domain Score)* | |
|---|---|---|---|
| |||
| Medical units with progressive‐care beds | 1 | 33 | Pulmonary (3.4, 3.5, 5) |
| 2 | 28 | Cardiology (4.8, 3.5, 4) | |
| 3 | 24 | General medical (4.8, 3.5, 4) | |
| Medical units without progressive‐care beds | 4 | 36 | Renal/diabetic (4, 3.5, 5) |
| 5 | 24 | General medical (3.75, 4, 5) | |
| Surgical units with progressive‐care beds | 6 | 51 | Cardiothoracic surgery/cardiology (4, 4, 5) |
| 7 | 29 | Trauma/general surgery (3.75, 3.5, 5) | |
| 8 | 23 | Neurosurgical/neurological (4.8, 5, 5) | |
| 9 | 24 | Neurosurgical/neurological (4.4, 4.5, 5) | |
| Surgical units without progressive‐care beds | 10 | 29 | General/urologic/gynecologic/plastic surgery (3.4, 3, 2) |
| 11 | 26 | Orthopedic surgery (4.6, 4, 5) | |
THE ACT MODEL
The model comprises 8 interventions rooted in 3 foundational domains: (1) enhancing interprofessional collaboration (IPC), (2) enabling data‐driven decisions, and (3) providing leadership. Each intervention is briefly described under its main focus (see Supporting Information, Appendix A, in the online version of this article for further details).
Enhancing IPC
Geographical Cohorting of Patients and Providers
Hospitalist providers are localized for 4 consecutive months to 1 unit. An interdisciplinary team including a case manager, clinical nurse specialist, pharmacist, nutritionist, and social worker also serve each unit. Learners (residents, pharmacy, and medical students) are embedded in the team when rotating on the hospital medicine service. The presence of unit‐based nurse managers and charge nurses predates the model and is retained.
Bedside Collaborative Rounding
Geographically cohorted providers round on their patients with the bedside nurse guided by a customizable script.
Daily Huddle
The hospitalist, learners, and the interdisciplinary team for the unit meet each weekday to discuss patients' needs for a safe transition out of the hospital. Each unit determined the timing, location, and script for the huddle while retaining the focus on discharge planning (see Supporting Information, Appendix A2, in the online version of this article for a sample script).
Hospitalist and Specialty Comanagement Agreements
Guidelines delineating responsibilities for providers of each specialty were developed. Examples include orders pertaining to the management of a dialysis catheter in a patient with end‐stage renal disease, the removal of drains in postsurgical patients, and wound care.
Unit White Board
Each unit has a white board at the nursing station. Similar to the huddle, it is focused on discharge planning.
Enabling Data‐Driven Decisions
Monthly Review of Unit‐Level Data
Data analytics at our institution developed a data dashboard. Key metrics including length of stay (LOS), patient satisfaction scores, readmission rates, and costs are tracked and attributed to the discharging unit. The data are collated monthly by the ACT program director and distributed to each unit's leadership. Monthly interdisciplinary meetings are held to review trends. Learners are encouraged but not required to attend.
Weekly Patient Satisfaction Rounding
The unit's nurse manager and physician leader conduct weekly satisfaction rounds on patients. The conversation is open‐ended and focused on eliciting positive and negative experiences.
Providing Leadership
Designated hospitalist and, where relevant, specialty leaders are committed to serve each unit for at least 1 year as a resource for both medical and operational problem solving. The leader stays closely connected with the unit's nurse manager. In addition to day‐to‐day troubleshooting, the leader is responsible for monitoring outcome trends. There is currently no stipend, training, or other incentive offered for the role.
Implementation Timelines and ACT Scores
The development of the ACTs started in the spring of 2012. Physician, nursing, and pharmacy support was sought, and a pilot unit was formed in August 2012. The model was cascaded hospital wide by December 2013, with support from the ACT program director (A.N.). The program director observed and scored the uptake of each intervention by each unit monthly. A score of 1 denoted no implementation, whereas 5 denoted complete implementation. The criteria for scoring are presented in Table 2. The monthly scores for all 8 interventions in each of the 11 units were averaged as an overall ACT score, which reflects the implementation dose of the ACT model. Monthly domain scores for enhancing IPC and enabling data‐driven decisions were also calculated as the average score within each domain. This yielded 3 domain scores. Figure 1A plots by month the overall ACT score for the medical and surgical units, and Figure 1B plots the implementation score for the 3 domains between August 2012 and December 2013 for all units. The uptake of the interventions varied between units. This allowed our analysis to explore the dose relationships between the model and outcomes independent of underlying time trends that may be affected by concomitant initiatives.
| 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|
| |||||
| Geographical cohorting of patients and the ACT* | None | At least 1 discipline comprising the ACT is unit based | All disciplines comprising the ACT except the hospitalist unit based | All disciplines including the hospitalist unit based | 4 + 80% of hospitalist provider's patients on the unit |
| Bedside collaborative rounding | None | Occurring 1 day a week on at least 25% of the patients on the unit | Occurring 2 to 3 days a week on at least 50% of the patients on the unit | Occurring 3 to 4 days a week on at least 75% of the patients on the unit | Occurring MondayFriday on all patients on the unit |
| Daily huddle | None | Occurring daily, 1 out of 4 ACT disciplines represented, at least 25% of patients on the unit discussed | Occurring daily, 2 out of 4 ACT disciplines represented, at least 50% of patients on the unit discussed | Occurring daily, 3 out of 4 ACT disciplines represented, at least 75% of patients on the unit discussed | Occurring daily, all disciplines of the ACT represented, all patients on the unit discussed |
| Hospitalist and specialty comanagement agreements | None | One out of 3 specialists represented on the unit collaborating with the hospitalists on at least 25% of relevant patients | One out of 3 specialists represented on the unit collaborating with the hospitalists on at least 50% of relevant patients | Two out of 3 specialists on the unit collaborating with the hospitalists on at least 75% of relevant patients | All specialists on the unit collaborating with the hospitalists on all relevant patients on the unit |
| Unit white board | None | Present but only used by nursing | Present and used by all ACT disciplines except physician providers | Present and used by entire ACT; use inconsistent | Present and used MondayFriday by all disciplines of ACT |
| Monthly review of unit level data | None | Nurse manager reviewing data with ACT program director | Nurse manager and unit leader reviewing data with ACT program director | Meeting either not consistently occurring monthly or not consistently attended by entire ACT | Monthly meeting with entire ACT |
| Weekly patient satisfaction rounding | None | Nurse manager performing up to 1 week a month | Nurse manager performing weekly | Nurse and physician leader performing up to 3 times a month | Nurse and physician leader performing weekly |
| Leadership | None | For units with specialties, either hospitalist or specialist leader identified | Both hospitalist and specialist leader Identified | Both hospitalist and specialist leaders (where applicable) identified and partially engaged in leadership role | Both hospitalist and specialist leaders (where applicable) identified and engaged in leadership role |

Outcomes
Monthly data between August 2012 and December 2013 were analyzed.
Measures of Value
MH is a member of the University Health Consortium, which measures outcomes of participants relative to their peers. MH measures LOS index as a ratio of observed LOS to expected LOS that is adjusted for severity of illness.[5]
Variable direct costs (VDCs) are costs that a hospital can save if a service is not provided.[6] A hospital's case‐mix index (CMI) represents the average diagnosis‐related group relative weight for that hospital. We track VDCs adjusted for CMI (CMI‐adjusted VDC).[7]
Thirty‐day readmission rate is the percentage of cases that are readmitted to MH within 30 days of discharge from the index admission.[8]
Measures of Patient Satisfaction
The Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey covers topics relevant to a patient's experience in the hospital.[9] Patient satisfaction scores are tracked by responses to the HCAHPS survey.
Measures of Provider Satisfaction
Hospitalist and specialty providers, leadership, and case management teams were surveyed via email through SurveyMonkey in July 2014. The survey included Likert responses that elicited opinions and comments about the ACT model.
Statistical Methods
The primary predictor of interest was the monthly overall ACT score. We also explored the domain scores as well as the individual scores for each intervention. Generalized linear mixed models were fit to investigate the association between each predictor (overall ACT score, ACT domain scores, and individual implementation scores) and each outcome (LOS index, CMI‐adjusted VDC, 30‐day readmission rate, and overall patient satisfaction). The model for testing each ACT score also included covariates of inpatient units as a random effect, as well as date and type of unit as fixed effects. We set the statistical significance level at 0.01 and reported 99% confidence intervals.
Descriptive statistics were used to report the provider satisfaction survey results.
RESULTS
The overall ACT score was associated with LOS index and CMI‐adjusted VDC (both P < 0.001). For every 1‐unit increase in the overall ACT score, LOS index decreased by 0.078 and CMI‐adjusted VDC decreased by $273.99 (Table 3).
| Length of Stay Index | CMI Adjusted VDC | |||
|---|---|---|---|---|
| Estimate (99% CI)* | P Value | Estimate (99% CI)* | P Value | |
| ||||
| Overall ACT Score | 0.078 (0.123 to 0.032) | <0.001 | 274.0 (477.31 to 70.68) | <0.001 |
| Enhancing IPC | 0.071 (0.117 to 0.026) | <0.001 | 284.7 (488.08 to 81.23) | <0.001 |
| Enabling data‐driven decisions | 0.044 (0.080 to 0.009) | 0.002 | 145.4 (304.57 to 13.81) | 0.02 |
| Providing leadership | 0.027 (0.049 to 0.005) | 0.001 | 69.9 (169.00 to 29.26) | 0.07 |
Looking at domains, enhancing IPC resulted in statistically significant decreases in both LOS index and CMI‐adjusted VDC, but providing leadership and enabling data‐driven decisions decreased only the LOS index. Most of the 8 individual interventions were associated with at least 1 of these 2 outcomes. (Even where the associations were not significant, they were all in the direction of decreasing LOS and cost). In these models, the covariate of type of units (medical vs surgical) was not associated with LOS or cost. There was no significant time trend in LOS or cost, except in models where an intervention had no association with either outcome. Inclusion of all individual effective interventions in the same statistical model to assess their relative contributions was not possible because they were highly correlated (correlations 0.450.89).
Thirty‐day readmissions and patient satisfaction were not significantly associated with the overall ACT score, but exploratory analyses showed that patient satisfaction increased with the implementation of geographical cohorting (P = 0.007).
Survey Results
The response rate was 87% (96/110). Between 85% and 96% of respondents either agreed or strongly agreed that the ACT model had improved the quality and safety of the care delivered, improved communication between providers and patients, and improved their own engagement and job satisfaction. Overall, 78% of the respondents either agreed or strongly agreed that the model improved efficiency (Table 4). Suggestions for improvements revolved around increasing the emphasis on patient centeredness and bedside nursing engagement.
| The ACT Model | Strongly Agree, n (%) | Agree, n (%) | Disagree, n (%) | Strongly Disagree, n (%) |
|---|---|---|---|---|
| ||||
| Has improved the quality and safety of patient care | 46 (47.9) | 46 (47.9) | 2 (2.1) | 2 (2.1) |
| Has improved communication with patients and families | 42 (43.7) | 47 (49.0) | 5 (5.2) | 2 (2.1) |
| Has improved your efficiency/productivity | 31 (32.6) | 43 (45.3) | 17 (17.9) | 4 (4.2) |
| Has improved your engagement and job satisfaction | 33 (34.4) | 49 (51.0) | 10 (10.4) | 4 (4.2) |
| Is a better model of delivering patient care | 45 (47.4) | 44 (46.3) | 2 (2.1) | 4 (4.2) |
DISCUSSION
The serious problems in US healthcare constitute an urgent imperative to innovate and reform.[10] Inpatient care reflects 31% of the expenditure on healthcare, and in 2010, 35.1 million patients were discharged from the hospital after spending an average of 4.8 days as an inpatient.[11] These figures represent an immense opportunity to intervene. Measuring the impact of quality improvement efforts is often complicated by concomitant changes that affect outcomes over the interval studied. Our approach allowed us to detect statistically significant changes in LOS index and CMI‐adjusted VDC associated with the ACT implementation dose that could be separated from the underlying time trends.
The ACT model we describe is rooted in improving 3 foundational domains; quantifying each intervention's compartmentalized contribution, however, proved difficult. Each intervention intertwines with the others to create changes in attitudes, knowledge, and culture that are difficult to measure yet may synergistically affect outcomes. For example, although geographical cohorting appears to have the strongest statistical association with outcomes, this may be mediated by how it enables other processes to take place more effectively. Based on this analysis, therefore, the ACT model may best be considered a bundled intervention.
The team caring for a patient during hospitalization is so complex that fewer than a quarter of patients know their physician's or nurse's name.[12] This complexity impairs communication between patients and providers and between the providers themselves. Communication failures are consistently identified as root causes in sentinel events reported to the Joint Commission.[13] IPC is the process by which different professional groups work together to positively impact health care. IPC overlaps with communication, coordination, and teamwork, and improvements in IPC may improve care.[14] Some elements of the model we describe have been tested previously.[15, 16, 17] Localization of teams may increase productivity and the frequency with which physicians and nurses communicate. Localization also decreases the number of pages received and steps walked by providers during a workday.[15, 16, 17] However, these studies reported a trend toward an increase in the LOS and neutral effects on cost and readmission rates. We found statistically significant decreases in both LOS and cost associated with the geographic cohorting of patients and providers. Notably, our model localized not only the physician providers but also the interdisciplinary team of pharmacists, clinical nurse specialists, case managers, and social workers. This proximity may facilitate IPC between all members that culminates in improved efficiency. The possibility of delays in discharges to avoid new admissions in a geographically structured team has previously been raised to explain the associated increases in LOS.[16, 17] The accountability of each unit for its metrics, the communication between nursing and physicians, and the timely availability of the unit's performance data aligns everyone toward a shared goal and provides some protection from an unintended consequence.
Structured interdisciplinary rounds decrease adverse events and improve teamwork ratings.[18, 19] The huddle in our model is a forum to collaborate between disciplines that proved to be effective in decreasing LOS and costs. Our huddle aims to discuss all the patients on the unit. This allows the team to assist each other in problem solving for the entire unit and not just the patients on the geographically cohorted team. This approach, in addition to the improved IPC fostered by the ACT model, may help explain how benefits in LOS and costs permeated across all 11 diverse units despite the presence of patients who are not directly served by the geographically cohorted team.
High‐performing clinical systems maintain an awareness of their overarching mission and unit‐based leaders can influence the frontline by reiterating the organizational mission and aligning efforts with outcomes.[20] Our leadership model is similar to those described by other institutions in the strong partnerships between physicians and nursing.[21] As outlined by Kim et al., investing in the professional development of the unit leaders may help them fulfill their roles and serve the organization better.[21]
The fragmentation and lack of ownership over the continuum of patient care causes duplication and waste. The proposal in the Accountable Care Act to create accountable care organizations is rooted in the understanding that providers and organizations will seek out new ways of improving quality when held accountable for their outcomes.[22] To foster ownership and accountability, reporting of metrics at the unit level is needed. Furthermore, an informational infrastructure is critical, as improvements cannot occur without the availability of data to both monitor performance and measure the effect of interventions.[10, 23] Even without any other interventions, providing feedback alone is an effective way of changing practices.[24] According to Berwick et al., this phenomenon reflects practitioners' intrinsic motivation to simply want to be better.[25] Our monthly review of each unit's data is an effective way to provide timely feedback to the frontline that sparks pride, ownership, and innovative thinking.
Based on our mean ACT score and CMI‐adjusted VDC reductions alone, we estimate savings of $649.36 per hospitalization (mean increase in ACT implementation of 2.37 times reduction in cost index of $273.99 per unit increase in overall ACT score). This figure does not include savings realized through reductions in LOS. This is a small decrease relative to the mean cost of hospitalization, yet when compounded over the annual MH census, it would result in substantial savings. The model relied on the restructuring of the existing workforce and the only direct additional cost was the early salary support for the ACT program director.
Limitations
We recognize several limitations. It is a single center's experience and may not be generalizable. The diffusion of knowledge and culture carried between units and the relatively rapid implementation timeline did not allow for a control unit. A single observer assigned our implementation scores, and therefore we cannot report measures of inter‐rater reliability. However, defined criteria and direct observations were used wherever possible. Although administratively available data have their limitations, where available, we used measurements that are adjusted for severity of illness and CMI. We therefore feel that this dataset is an accurate representation of currently reported national quality indicators.
FURTHER DIRECTIONS
Although there is a need to improve our healthcare system, interventions should be deliberate and evidence based wherever possible.[26] Geographic cohorting may decrease the frequency of paging interruptions for physicians and practitioners while increasing face‐to‐face interruptions.[27] The net effect on safety with this trade‐off should be investigated.
The presence of an intervention does not guarantee its success. Despite geographic cohorting and interdisciplinary meetings, communication that influences physician decision making may not improve.[28] Although instruments to measure ratings of team work and collaboration are available, focusing on clinically relevant outcomes of teamwork, such as prevention of harm, may be more empowering feedback for the frontline. Formal cost‐benefit analyses and outcomes related to physician and nursing retention will be equally important for assessing the sustainability of the model. Involving patients and their caregivers and inviting their perspectives as care is redesigned will also be critical in maintaining patient centeredness. Research addressing interventions to mediate preventable readmission risk and understanding the drivers of patient satisfaction is also needed.
The true value of the model may be in its potential to monitor and drive change within itself. Continuously aligning aims, incentives, performance measures, and feedback will help support this innovation and drive. This affects not only patient care but creates microcosms within which research and education can thrive. We hope that our experience will help guide other institutions as we all strive in our journey to improve the care we deliver.
Acknowledgements
The authors thank the Indiana University Health Physicians hospitalists at MH, Sandy Janitz and Decision Support, the Indiana University Health executive leadership team, Robert Clark, Malaz Boustani, Dennis Watson, Nadia Adams, Todd Biggerstaff, Deanne Kashiwagi, and the tireless providers at MH for their support.
Disclosure: This work was supported by a grant from the Indiana University Health Values Fund. The authors have no conflicts of interest to disclose.
Despite an estimated annual $2.6 trillion expenditure on healthcare, the United States performs poorly on indicators of health and harm during care.[1, 2, 3] Hospitals around the nation are working to improve the care they deliver. We describe a model developed at our institution and report the evaluation of the outcomes associated with its implementation on the general medical and surgical units. The Indiana University Institutional Review Board approved this work.
SETTING AND DEFINITIONS
Indiana University Health Methodist Hospital (MH) is an academic center in Indianapolis, Indiana, serving over 30,000 patients annually.[4] In 2012, responding to the coexisting needs to improve quality and contain costs, the MH leadership team redesigned care in the hospital. The new model centers around accountable care teams (ACTs). Each ACT is a geographically defined set of providers accepting ownership for the clinical, service, and financial outcomes of their respective inpatient unit. The units studied are described in Table 1.
| Unit | No. of Beds | Predominant Diagnosis (Maximum Domain Score)* | |
|---|---|---|---|
| |||
| Medical units with progressive‐care beds | 1 | 33 | Pulmonary (3.4, 3.5, 5) |
| 2 | 28 | Cardiology (4.8, 3.5, 4) | |
| 3 | 24 | General medical (4.8, 3.5, 4) | |
| Medical units without progressive‐care beds | 4 | 36 | Renal/diabetic (4, 3.5, 5) |
| 5 | 24 | General medical (3.75, 4, 5) | |
| Surgical units with progressive‐care beds | 6 | 51 | Cardiothoracic surgery/cardiology (4, 4, 5) |
| 7 | 29 | Trauma/general surgery (3.75, 3.5, 5) | |
| 8 | 23 | Neurosurgical/neurological (4.8, 5, 5) | |
| 9 | 24 | Neurosurgical/neurological (4.4, 4.5, 5) | |
| Surgical units without progressive‐care beds | 10 | 29 | General/urologic/gynecologic/plastic surgery (3.4, 3, 2) |
| 11 | 26 | Orthopedic surgery (4.6, 4, 5) | |
THE ACT MODEL
The model comprises 8 interventions rooted in 3 foundational domains: (1) enhancing interprofessional collaboration (IPC), (2) enabling data‐driven decisions, and (3) providing leadership. Each intervention is briefly described under its main focus (see Supporting Information, Appendix A, in the online version of this article for further details).
Enhancing IPC
Geographical Cohorting of Patients and Providers
Hospitalist providers are localized for 4 consecutive months to 1 unit. An interdisciplinary team including a case manager, clinical nurse specialist, pharmacist, nutritionist, and social worker also serve each unit. Learners (residents, pharmacy, and medical students) are embedded in the team when rotating on the hospital medicine service. The presence of unit‐based nurse managers and charge nurses predates the model and is retained.
Bedside Collaborative Rounding
Geographically cohorted providers round on their patients with the bedside nurse guided by a customizable script.
Daily Huddle
The hospitalist, learners, and the interdisciplinary team for the unit meet each weekday to discuss patients' needs for a safe transition out of the hospital. Each unit determined the timing, location, and script for the huddle while retaining the focus on discharge planning (see Supporting Information, Appendix A2, in the online version of this article for a sample script).
Hospitalist and Specialty Comanagement Agreements
Guidelines delineating responsibilities for providers of each specialty were developed. Examples include orders pertaining to the management of a dialysis catheter in a patient with end‐stage renal disease, the removal of drains in postsurgical patients, and wound care.
Unit White Board
Each unit has a white board at the nursing station. Similar to the huddle, it is focused on discharge planning.
Enabling Data‐Driven Decisions
Monthly Review of Unit‐Level Data
Data analytics at our institution developed a data dashboard. Key metrics including length of stay (LOS), patient satisfaction scores, readmission rates, and costs are tracked and attributed to the discharging unit. The data are collated monthly by the ACT program director and distributed to each unit's leadership. Monthly interdisciplinary meetings are held to review trends. Learners are encouraged but not required to attend.
Weekly Patient Satisfaction Rounding
The unit's nurse manager and physician leader conduct weekly satisfaction rounds on patients. The conversation is open‐ended and focused on eliciting positive and negative experiences.
Providing Leadership
Designated hospitalist and, where relevant, specialty leaders are committed to serve each unit for at least 1 year as a resource for both medical and operational problem solving. The leader stays closely connected with the unit's nurse manager. In addition to day‐to‐day troubleshooting, the leader is responsible for monitoring outcome trends. There is currently no stipend, training, or other incentive offered for the role.
Implementation Timelines and ACT Scores
The development of the ACTs started in the spring of 2012. Physician, nursing, and pharmacy support was sought, and a pilot unit was formed in August 2012. The model was cascaded hospital wide by December 2013, with support from the ACT program director (A.N.). The program director observed and scored the uptake of each intervention by each unit monthly. A score of 1 denoted no implementation, whereas 5 denoted complete implementation. The criteria for scoring are presented in Table 2. The monthly scores for all 8 interventions in each of the 11 units were averaged as an overall ACT score, which reflects the implementation dose of the ACT model. Monthly domain scores for enhancing IPC and enabling data‐driven decisions were also calculated as the average score within each domain. This yielded 3 domain scores. Figure 1A plots by month the overall ACT score for the medical and surgical units, and Figure 1B plots the implementation score for the 3 domains between August 2012 and December 2013 for all units. The uptake of the interventions varied between units. This allowed our analysis to explore the dose relationships between the model and outcomes independent of underlying time trends that may be affected by concomitant initiatives.
| 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|
| |||||
| Geographical cohorting of patients and the ACT* | None | At least 1 discipline comprising the ACT is unit based | All disciplines comprising the ACT except the hospitalist unit based | All disciplines including the hospitalist unit based | 4 + 80% of hospitalist provider's patients on the unit |
| Bedside collaborative rounding | None | Occurring 1 day a week on at least 25% of the patients on the unit | Occurring 2 to 3 days a week on at least 50% of the patients on the unit | Occurring 3 to 4 days a week on at least 75% of the patients on the unit | Occurring MondayFriday on all patients on the unit |
| Daily huddle | None | Occurring daily, 1 out of 4 ACT disciplines represented, at least 25% of patients on the unit discussed | Occurring daily, 2 out of 4 ACT disciplines represented, at least 50% of patients on the unit discussed | Occurring daily, 3 out of 4 ACT disciplines represented, at least 75% of patients on the unit discussed | Occurring daily, all disciplines of the ACT represented, all patients on the unit discussed |
| Hospitalist and specialty comanagement agreements | None | One out of 3 specialists represented on the unit collaborating with the hospitalists on at least 25% of relevant patients | One out of 3 specialists represented on the unit collaborating with the hospitalists on at least 50% of relevant patients | Two out of 3 specialists on the unit collaborating with the hospitalists on at least 75% of relevant patients | All specialists on the unit collaborating with the hospitalists on all relevant patients on the unit |
| Unit white board | None | Present but only used by nursing | Present and used by all ACT disciplines except physician providers | Present and used by entire ACT; use inconsistent | Present and used MondayFriday by all disciplines of ACT |
| Monthly review of unit level data | None | Nurse manager reviewing data with ACT program director | Nurse manager and unit leader reviewing data with ACT program director | Meeting either not consistently occurring monthly or not consistently attended by entire ACT | Monthly meeting with entire ACT |
| Weekly patient satisfaction rounding | None | Nurse manager performing up to 1 week a month | Nurse manager performing weekly | Nurse and physician leader performing up to 3 times a month | Nurse and physician leader performing weekly |
| Leadership | None | For units with specialties, either hospitalist or specialist leader identified | Both hospitalist and specialist leader Identified | Both hospitalist and specialist leaders (where applicable) identified and partially engaged in leadership role | Both hospitalist and specialist leaders (where applicable) identified and engaged in leadership role |

Outcomes
Monthly data between August 2012 and December 2013 were analyzed.
Measures of Value
MH is a member of the University Health Consortium, which measures outcomes of participants relative to their peers. MH measures LOS index as a ratio of observed LOS to expected LOS that is adjusted for severity of illness.[5]
Variable direct costs (VDCs) are costs that a hospital can save if a service is not provided.[6] A hospital's case‐mix index (CMI) represents the average diagnosis‐related group relative weight for that hospital. We track VDCs adjusted for CMI (CMI‐adjusted VDC).[7]
Thirty‐day readmission rate is the percentage of cases that are readmitted to MH within 30 days of discharge from the index admission.[8]
Measures of Patient Satisfaction
The Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey covers topics relevant to a patient's experience in the hospital.[9] Patient satisfaction scores are tracked by responses to the HCAHPS survey.
Measures of Provider Satisfaction
Hospitalist and specialty providers, leadership, and case management teams were surveyed via email through SurveyMonkey in July 2014. The survey included Likert responses that elicited opinions and comments about the ACT model.
Statistical Methods
The primary predictor of interest was the monthly overall ACT score. We also explored the domain scores as well as the individual scores for each intervention. Generalized linear mixed models were fit to investigate the association between each predictor (overall ACT score, ACT domain scores, and individual implementation scores) and each outcome (LOS index, CMI‐adjusted VDC, 30‐day readmission rate, and overall patient satisfaction). The model for testing each ACT score also included covariates of inpatient units as a random effect, as well as date and type of unit as fixed effects. We set the statistical significance level at 0.01 and reported 99% confidence intervals.
Descriptive statistics were used to report the provider satisfaction survey results.
RESULTS
The overall ACT score was associated with LOS index and CMI‐adjusted VDC (both P < 0.001). For every 1‐unit increase in the overall ACT score, LOS index decreased by 0.078 and CMI‐adjusted VDC decreased by $273.99 (Table 3).
| Length of Stay Index | CMI Adjusted VDC | |||
|---|---|---|---|---|
| Estimate (99% CI)* | P Value | Estimate (99% CI)* | P Value | |
| ||||
| Overall ACT Score | 0.078 (0.123 to 0.032) | <0.001 | 274.0 (477.31 to 70.68) | <0.001 |
| Enhancing IPC | 0.071 (0.117 to 0.026) | <0.001 | 284.7 (488.08 to 81.23) | <0.001 |
| Enabling data‐driven decisions | 0.044 (0.080 to 0.009) | 0.002 | 145.4 (304.57 to 13.81) | 0.02 |
| Providing leadership | 0.027 (0.049 to 0.005) | 0.001 | 69.9 (169.00 to 29.26) | 0.07 |
Looking at domains, enhancing IPC resulted in statistically significant decreases in both LOS index and CMI‐adjusted VDC, but providing leadership and enabling data‐driven decisions decreased only the LOS index. Most of the 8 individual interventions were associated with at least 1 of these 2 outcomes. (Even where the associations were not significant, they were all in the direction of decreasing LOS and cost). In these models, the covariate of type of units (medical vs surgical) was not associated with LOS or cost. There was no significant time trend in LOS or cost, except in models where an intervention had no association with either outcome. Inclusion of all individual effective interventions in the same statistical model to assess their relative contributions was not possible because they were highly correlated (correlations 0.450.89).
Thirty‐day readmissions and patient satisfaction were not significantly associated with the overall ACT score, but exploratory analyses showed that patient satisfaction increased with the implementation of geographical cohorting (P = 0.007).
Survey Results
The response rate was 87% (96/110). Between 85% and 96% of respondents either agreed or strongly agreed that the ACT model had improved the quality and safety of the care delivered, improved communication between providers and patients, and improved their own engagement and job satisfaction. Overall, 78% of the respondents either agreed or strongly agreed that the model improved efficiency (Table 4). Suggestions for improvements revolved around increasing the emphasis on patient centeredness and bedside nursing engagement.
| The ACT Model | Strongly Agree, n (%) | Agree, n (%) | Disagree, n (%) | Strongly Disagree, n (%) |
|---|---|---|---|---|
| ||||
| Has improved the quality and safety of patient care | 46 (47.9) | 46 (47.9) | 2 (2.1) | 2 (2.1) |
| Has improved communication with patients and families | 42 (43.7) | 47 (49.0) | 5 (5.2) | 2 (2.1) |
| Has improved your efficiency/productivity | 31 (32.6) | 43 (45.3) | 17 (17.9) | 4 (4.2) |
| Has improved your engagement and job satisfaction | 33 (34.4) | 49 (51.0) | 10 (10.4) | 4 (4.2) |
| Is a better model of delivering patient care | 45 (47.4) | 44 (46.3) | 2 (2.1) | 4 (4.2) |
DISCUSSION
The serious problems in US healthcare constitute an urgent imperative to innovate and reform.[10] Inpatient care reflects 31% of the expenditure on healthcare, and in 2010, 35.1 million patients were discharged from the hospital after spending an average of 4.8 days as an inpatient.[11] These figures represent an immense opportunity to intervene. Measuring the impact of quality improvement efforts is often complicated by concomitant changes that affect outcomes over the interval studied. Our approach allowed us to detect statistically significant changes in LOS index and CMI‐adjusted VDC associated with the ACT implementation dose that could be separated from the underlying time trends.
The ACT model we describe is rooted in improving 3 foundational domains; quantifying each intervention's compartmentalized contribution, however, proved difficult. Each intervention intertwines with the others to create changes in attitudes, knowledge, and culture that are difficult to measure yet may synergistically affect outcomes. For example, although geographical cohorting appears to have the strongest statistical association with outcomes, this may be mediated by how it enables other processes to take place more effectively. Based on this analysis, therefore, the ACT model may best be considered a bundled intervention.
The team caring for a patient during hospitalization is so complex that fewer than a quarter of patients know their physician's or nurse's name.[12] This complexity impairs communication between patients and providers and between the providers themselves. Communication failures are consistently identified as root causes in sentinel events reported to the Joint Commission.[13] IPC is the process by which different professional groups work together to positively impact health care. IPC overlaps with communication, coordination, and teamwork, and improvements in IPC may improve care.[14] Some elements of the model we describe have been tested previously.[15, 16, 17] Localization of teams may increase productivity and the frequency with which physicians and nurses communicate. Localization also decreases the number of pages received and steps walked by providers during a workday.[15, 16, 17] However, these studies reported a trend toward an increase in the LOS and neutral effects on cost and readmission rates. We found statistically significant decreases in both LOS and cost associated with the geographic cohorting of patients and providers. Notably, our model localized not only the physician providers but also the interdisciplinary team of pharmacists, clinical nurse specialists, case managers, and social workers. This proximity may facilitate IPC between all members that culminates in improved efficiency. The possibility of delays in discharges to avoid new admissions in a geographically structured team has previously been raised to explain the associated increases in LOS.[16, 17] The accountability of each unit for its metrics, the communication between nursing and physicians, and the timely availability of the unit's performance data aligns everyone toward a shared goal and provides some protection from an unintended consequence.
Structured interdisciplinary rounds decrease adverse events and improve teamwork ratings.[18, 19] The huddle in our model is a forum to collaborate between disciplines that proved to be effective in decreasing LOS and costs. Our huddle aims to discuss all the patients on the unit. This allows the team to assist each other in problem solving for the entire unit and not just the patients on the geographically cohorted team. This approach, in addition to the improved IPC fostered by the ACT model, may help explain how benefits in LOS and costs permeated across all 11 diverse units despite the presence of patients who are not directly served by the geographically cohorted team.
High‐performing clinical systems maintain an awareness of their overarching mission and unit‐based leaders can influence the frontline by reiterating the organizational mission and aligning efforts with outcomes.[20] Our leadership model is similar to those described by other institutions in the strong partnerships between physicians and nursing.[21] As outlined by Kim et al., investing in the professional development of the unit leaders may help them fulfill their roles and serve the organization better.[21]
The fragmentation and lack of ownership over the continuum of patient care causes duplication and waste. The proposal in the Accountable Care Act to create accountable care organizations is rooted in the understanding that providers and organizations will seek out new ways of improving quality when held accountable for their outcomes.[22] To foster ownership and accountability, reporting of metrics at the unit level is needed. Furthermore, an informational infrastructure is critical, as improvements cannot occur without the availability of data to both monitor performance and measure the effect of interventions.[10, 23] Even without any other interventions, providing feedback alone is an effective way of changing practices.[24] According to Berwick et al., this phenomenon reflects practitioners' intrinsic motivation to simply want to be better.[25] Our monthly review of each unit's data is an effective way to provide timely feedback to the frontline that sparks pride, ownership, and innovative thinking.
Based on our mean ACT score and CMI‐adjusted VDC reductions alone, we estimate savings of $649.36 per hospitalization (mean increase in ACT implementation of 2.37 times reduction in cost index of $273.99 per unit increase in overall ACT score). This figure does not include savings realized through reductions in LOS. This is a small decrease relative to the mean cost of hospitalization, yet when compounded over the annual MH census, it would result in substantial savings. The model relied on the restructuring of the existing workforce and the only direct additional cost was the early salary support for the ACT program director.
Limitations
We recognize several limitations. It is a single center's experience and may not be generalizable. The diffusion of knowledge and culture carried between units and the relatively rapid implementation timeline did not allow for a control unit. A single observer assigned our implementation scores, and therefore we cannot report measures of inter‐rater reliability. However, defined criteria and direct observations were used wherever possible. Although administratively available data have their limitations, where available, we used measurements that are adjusted for severity of illness and CMI. We therefore feel that this dataset is an accurate representation of currently reported national quality indicators.
FURTHER DIRECTIONS
Although there is a need to improve our healthcare system, interventions should be deliberate and evidence based wherever possible.[26] Geographic cohorting may decrease the frequency of paging interruptions for physicians and practitioners while increasing face‐to‐face interruptions.[27] The net effect on safety with this trade‐off should be investigated.
The presence of an intervention does not guarantee its success. Despite geographic cohorting and interdisciplinary meetings, communication that influences physician decision making may not improve.[28] Although instruments to measure ratings of team work and collaboration are available, focusing on clinically relevant outcomes of teamwork, such as prevention of harm, may be more empowering feedback for the frontline. Formal cost‐benefit analyses and outcomes related to physician and nursing retention will be equally important for assessing the sustainability of the model. Involving patients and their caregivers and inviting their perspectives as care is redesigned will also be critical in maintaining patient centeredness. Research addressing interventions to mediate preventable readmission risk and understanding the drivers of patient satisfaction is also needed.
The true value of the model may be in its potential to monitor and drive change within itself. Continuously aligning aims, incentives, performance measures, and feedback will help support this innovation and drive. This affects not only patient care but creates microcosms within which research and education can thrive. We hope that our experience will help guide other institutions as we all strive in our journey to improve the care we deliver.
Acknowledgements
The authors thank the Indiana University Health Physicians hospitalists at MH, Sandy Janitz and Decision Support, the Indiana University Health executive leadership team, Robert Clark, Malaz Boustani, Dennis Watson, Nadia Adams, Todd Biggerstaff, Deanne Kashiwagi, and the tireless providers at MH for their support.
Disclosure: This work was supported by a grant from the Indiana University Health Values Fund. The authors have no conflicts of interest to disclose.
- Committee on Quality of Health Care in America; Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: The National Academies Press; 2001.
- . Is US health really the best in the world? JAMA. 2000;284(4):483–485.
- , , , , , . Temporal trends in rates of patient harm resulting from medical care. N Engl J Med. 2010;363(22):2124–2134.
- Indiana University Health. Available at: http://iuhealth.org/methodist/aboIut/. Accessed October 20, 2014.
- University Health Consortium. Available at: https://www.uhc.edu/docs/45014769_QSS_dashboard_FAQs.pdf. Accessed October 23, 2014.
- , , , et al. Distribution of variable vs fixed costs of hospital care. JAMA. 1999;281(7):644–649.
- Centers for Medicare and Medicaid Services. Case mix index. Available at: http://www.cms.gov/Medicare/Medicare‐Fee‐for‐Service‐Payment/AcuteInpatientPPS/Acute‐Inpatient‐Files‐for‐Download‐Items/CMS022630.html. Accessed May 4, 2015.
- University Health Consortium. Available at: https://www.uhc.edu. Accessed October 23, 2014.
- Centers for Medicare and Medicaid Services. Hospital Consumer Assessment of Healthcare Providers and Systems. HCAHPS survey content and administration. Centers for Medicare 280(11):1000–1005.
- Centers for Disease Control and Prevention. FastStats. Available at: http://www.cdc.gov/nchs/fastats/default.htm. Accessed October 27, 2014.
- , . Does your patient know your name? An approach to enhancing patients' awareness of their caretaker's name. J Healthc Qual. 2005;27(4):53–56.
- The Joint Commission. Sentinel event data: root causes by event type 2004‐third quarter. Available at: http://www.jointcommissionorg. Available at: http://www.jointcommission.org/assets/1/18/Root_Causes_by_Event_Type_2004-2Q2013.pdf. Accessed March 26, 2014.
- , , . Interprofessional collaboration: effects of practice‐based interventions on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2009;(3):CD000072.
- , , , et al. Impact of localizing physicians to hospital units on nurse–physician communication and agreement on the plan of care. J Gen Intern Med. 2009;24(11):1223–1227.
- , , , et al. Impact of localizing general medical teams to a single nursing unit. J Hosp Med. 2012;7(7):551–556.
- , , , et al. Implementation of a physician assistant/hospitalist service in an academic medical center: impact on efficiency and patient outcomes. J Hosp Med. 2008;3(5):361–368.
- , , , , , . Improving teamwork: impact of structured interdisciplinary rounds on a medical teaching unit. J Gen Intern Med. 2010;25(8):826–832.
- , , , ; High Performance Teams and the Hospital of the Future Project Team. Interdisciplinary teamwork in hospitals: a review and practical recommendations for improvement. J Hosp Med. 2011;7(1):48–54.
- , , , , , . Microsystems in health care: part 8. Developing people and improving work life: what front‐line staff told us. Jt Comm J Qual Saf. 2003;29(10):512–522.
- , , , , , . Unit‐based interprofessional leadership models in six US hospitals. J Hosp Med. 2014;9(8):545–550.
- , , , . Creating accountable care organizations: the extended hospital medical staff. Health Aff (Millwood). 2007;26(1):w44–w57.
- , . Using performance measurement to drive improvement: a road map for change. Med Care. 2003;41(1 suppl):I48–I60.
- , . Changing physicians' practices. N Engl J Med. 1993;329(17):1271–1273.
- , , . Connections between quality measurement and improvement. Med Care. 2003;41(1 suppl):I30–I38.
- , , . The tension between needing to improve care and knowing how to do it. N Engl J Med. 2007;357(6):608–613.
- , . A qualitative evaluation of geographical localization of hospitalists: how unintended consequences may impact quality. J Gen Intern Med. 2014;29(7):1009–1016.
- , , , , . Disengaged: a qualitative study of communication and collaboration between physicians and other professions on general internal medicine wards. BMC Health Serv Res. 2013;13:494.
- Committee on Quality of Health Care in America; Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: The National Academies Press; 2001.
- . Is US health really the best in the world? JAMA. 2000;284(4):483–485.
- , , , , , . Temporal trends in rates of patient harm resulting from medical care. N Engl J Med. 2010;363(22):2124–2134.
- Indiana University Health. Available at: http://iuhealth.org/methodist/aboIut/. Accessed October 20, 2014.
- University Health Consortium. Available at: https://www.uhc.edu/docs/45014769_QSS_dashboard_FAQs.pdf. Accessed October 23, 2014.
- , , , et al. Distribution of variable vs fixed costs of hospital care. JAMA. 1999;281(7):644–649.
- Centers for Medicare and Medicaid Services. Case mix index. Available at: http://www.cms.gov/Medicare/Medicare‐Fee‐for‐Service‐Payment/AcuteInpatientPPS/Acute‐Inpatient‐Files‐for‐Download‐Items/CMS022630.html. Accessed May 4, 2015.
- University Health Consortium. Available at: https://www.uhc.edu. Accessed October 23, 2014.
- Centers for Medicare and Medicaid Services. Hospital Consumer Assessment of Healthcare Providers and Systems. HCAHPS survey content and administration. Centers for Medicare 280(11):1000–1005.
- Centers for Disease Control and Prevention. FastStats. Available at: http://www.cdc.gov/nchs/fastats/default.htm. Accessed October 27, 2014.
- , . Does your patient know your name? An approach to enhancing patients' awareness of their caretaker's name. J Healthc Qual. 2005;27(4):53–56.
- The Joint Commission. Sentinel event data: root causes by event type 2004‐third quarter. Available at: http://www.jointcommissionorg. Available at: http://www.jointcommission.org/assets/1/18/Root_Causes_by_Event_Type_2004-2Q2013.pdf. Accessed March 26, 2014.
- , , . Interprofessional collaboration: effects of practice‐based interventions on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2009;(3):CD000072.
- , , , et al. Impact of localizing physicians to hospital units on nurse–physician communication and agreement on the plan of care. J Gen Intern Med. 2009;24(11):1223–1227.
- , , , et al. Impact of localizing general medical teams to a single nursing unit. J Hosp Med. 2012;7(7):551–556.
- , , , et al. Implementation of a physician assistant/hospitalist service in an academic medical center: impact on efficiency and patient outcomes. J Hosp Med. 2008;3(5):361–368.
- , , , , , . Improving teamwork: impact of structured interdisciplinary rounds on a medical teaching unit. J Gen Intern Med. 2010;25(8):826–832.
- , , , ; High Performance Teams and the Hospital of the Future Project Team. Interdisciplinary teamwork in hospitals: a review and practical recommendations for improvement. J Hosp Med. 2011;7(1):48–54.
- , , , , , . Microsystems in health care: part 8. Developing people and improving work life: what front‐line staff told us. Jt Comm J Qual Saf. 2003;29(10):512–522.
- , , , , , . Unit‐based interprofessional leadership models in six US hospitals. J Hosp Med. 2014;9(8):545–550.
- , , , . Creating accountable care organizations: the extended hospital medical staff. Health Aff (Millwood). 2007;26(1):w44–w57.
- , . Using performance measurement to drive improvement: a road map for change. Med Care. 2003;41(1 suppl):I48–I60.
- , . Changing physicians' practices. N Engl J Med. 1993;329(17):1271–1273.
- , , . Connections between quality measurement and improvement. Med Care. 2003;41(1 suppl):I30–I38.
- , , . The tension between needing to improve care and knowing how to do it. N Engl J Med. 2007;357(6):608–613.
- , . A qualitative evaluation of geographical localization of hospitalists: how unintended consequences may impact quality. J Gen Intern Med. 2014;29(7):1009–1016.
- , , , , . Disengaged: a qualitative study of communication and collaboration between physicians and other professions on general internal medicine wards. BMC Health Serv Res. 2013;13:494.
© 2015 Society of Hospital Medicine