User login
Does Patient Experience Predict 30-Day Readmission? A Patient-Level Analysis of HCAHPS Data
Patient experience and 30-day readmission are important measures of quality of care for hospitalized patients. Performance on both of these measures impact hospitals financially. Performance on the Hospital Consumer Assessment of Healthcare Systems and Providers (HCAHPS) survey is linked to 25% of the incentive payment under Value Based Purchasing (VBP) Program.1 Starting in 2012, the Centers for Medicare and Medicaid Services (CMS) introduced the Readmission Reduction Program, penalizing hospitals financially for excessive readmissions.2
A relationship between patient experience and readmissions has been explored at the hospital level. Studies have mostly found that higher patient experience scores are associated with lower 30-day readmission rates. In a study of the relationship between 30-day risk-standardized readmission rates for three medical conditions (acute myocardial infarction, heart failure, and pneumonia) and patient experience, the authors noted that higher experience scores for overall care and discharge planning were associated with lower readmission rates for these conditions. They also concluded that patient experience scores were more predictive of 30-day readmission than clinical performance measures. Additionally, the authors predicted that if a hospital increased its total experience scores from the 25th percentile to the 75th percentile, there would be an associated decrease in readmissions by at least 2.3% for each of these conditions.3 Practice management companies and the media have cited this finding to conclude that higher patient experience drives clinical outcomes such as 30-day readmission and that patients are often the best judges of the quality of care delivered.4,5
Other hospital-level studies have found that high 30-day readmission rates are associated with lower overall experience scores in a mixed surgical patient population; worse reports of pain control and overall care in the colorectal surgery population; lower experience scores with discharge preparedness in vascular surgery patients; and lower experience scores with physician communication, nurse communication, and discharge preparedness.6-9 A patient-level study noted higher readmissions are associated with worse experience with physician and nursing communication along with a paradoxically better experience with discharge information.10
Because these studies used an observational design, they demonstrated associations rather than causality. An alternative hypothesis is that readmitted patients complete their patient experience survey after readmission and the low experience is the result, rather than the cause, of their readmission. For patients who are readmitted, it is unclear whether there is an opportunity to complete the survey prior to readmission and whether being readmitted may impact patient perception of quality of care. Using patient-level data, we sought to assess HCAHPS patient-experience responses linked to the index admission of the patients who were readmitted in 30 days and compare it with those patients who were not readmitted during this time period. We paid particular attention to when the surveys were returned.
METHODS
Study Design
We conducted a retrospective analysis of prospectively collected 10-year HCAHPS and Press Ganey patient survey data for a single tertiary care academic hospital.
Participants
All adult patients discharged from the hospital and who responded to the routinely sent patient-experience survey were included. Surveys were sent to a random sample of 50% of the discharged patients.
The exposure group was comprised of patients who responded to the survey and were readmitted within 30 days of discharge. After subtracting 5 days from the survey receipt date for expected delays related to mail delivery time and processing time, survey response date was calculated. The exposure group was further divided into patients who responded to the survey prior to their 30-day readmission (“Pre-readmission responders”) and those that responded to the survey after their readmission (“Postreadmission responders”). A sensitivity analysis was performed by changing the number of days subtracted from the survey receipt date by 2 days in either direction. This approach did not result in any significant changes in the results.
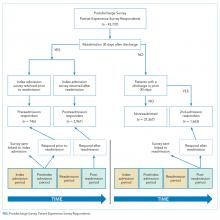
The control group comprised patients who were not readmitted to the hospital within 30 days of discharge and who did not have an admission in the previous 30 days as well (“Not readmitted” group). An additional comparison group for exploratory analysis included patients who had experienced an admission in the prior 30 days but were not readmitted after the admission linked to the survey. These patients responded to the patient-experience surveys that were linked to their second admission in 30 days (“2nd-admission responders” group; Figure).
Time Periods
All survey responders from the third quarter of 2006 to the first quarter of 2016 were included in the study. Additionally, administrative data on non-responders were available from 7/2006 to 8/2012. These data were used to estimate response rates. Patient level experience and administrative data were obtained in a linked fashion for these time periods.
Instruments
Press Ganey and HCAHPS surveys were sent via mail in the same envelope. Fifty percent of the discharged patients were randomized to receive the surveys. The Press Ganey survey contained 33 items encompassing several subdomains, including room, meal, nursing, physician, ancillary staff, visitor, discharge, and overall experience.
The HCAHPS survey contained 29 CMS-mandated items, of which 21 are related to patient experience. The development, testing, and methods for administration and reporting of the HCAHPS survey have been previously described and studies using this instrument have been reported in the literature.11 Press Ganey patient satisfaction survey results have also been reported in the literature.12
Outcome Variables and Covariates
HCAHPS and Press Ganey experience survey individual item responses were the primary outcome variables of this study. Age, self-reported health status, education, primary language spoken, service line, and time taken to respond to the surveys served as the covariates. These variables are used by CMS for patient-mix adjustment and are collected on the HCAHPS survey. Additionally, the number of days to respond to the survey were included in all regression analysis to adjust for early responder effect.13-15
Statistical Analysis
“Percent top-box” scores were calculated for each survey item for patients in each group. The percent top-box scores were calculated as the percent of patients who responded “very good” for a given item on Press Ganey survey items and “always” or “definitely yes” or “yes” or “9” or “10” on HCAHPS survey items. CMS utilizes “percent top-box scores” to calculate payments under the VBP program and to report the results publicly. Numerous studies have also reported percent top-box scores for HCAHPS survey results.12
We hypothesized that whether patients complete the HCAHPS survey before or after the readmission influences their reporting of experience. To test this hypothesis, HCAHPS and Press Ganey item top-box scores of “Pre-readmission responders” and “Postreadmission responders” were compared with those of the control group using multivariate logistic regression. “Pre-readmission responders” were also compared with “Postreadmission responders”.
“2nd-admission responders” were similarly compared with the control group for an exploratory analysis. Finally, “Postreadmission responders” and “2nd-admission responders” were compared in another exploratory analysis since both these groups responded to the survey after being exposed to the readmission, even though the “Postreadmission responders” group is administratively linked to the index admission.
The Johns Hopkins Institutional Review Board approved this study.
RESULTS
There were 43,737 survey responders, among whom 4,707 were subsequently readmitted within 30 days of discharge. Among the readmitted patients who responded to the surveys linked to their index admission, only 15.8% returned the survey before readmission (pre-readmission responders’) and 84.2% returned the survey after readmission (postreadmission responders). Additionally, 1,663 patients responded to experience surveys linked to their readmission. There were 37,365 patients in the control arm (ie, patients who responded to the survey and were not readmitted within 30 days of discharge or in the prior 30 days; Figure 1). The readmission rate among survey responders was 10.6%. Among the readmitted patients, the median number of days to readmission was 10 days while the median number of days to respond to the survey for this group was 33 days. Among the nonreadmitted patients, the median number of days to return the survey was 29 days.
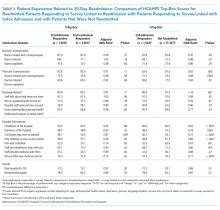
We also conducted an exploratory analysis of the postreadmission responders, comparing them with patients who received patient-experience surveys linked to their second admission in 30 days. Both of these groups were exposed to a readmission before they completed the surveys. There were no significant differences between these two groups on patient experience scores. Additionally, the patients who received the survey linked to their readmission had a broad dissatisfaction pattern on HCAHPS survey items that appeared similar to that of the postreadmission group when compared to the non-readmitted group (Table 3).
DISCUSSION
In this retrospective analysis of prospectively collected Press Ganey and HCAHPS patient-experience survey data, we found that the overwhelming majority of patients readmitted within 30 days of discharge respond to HCAHPS surveys after readmission even though the survey is sent linked to the first admission. This is not unexpected since the median time to survey response is 33 days for this group, while median time to readmission is 10 days. The dissatisfaction pattern of Postreadmission responders was similar to those who responded to the survey linked to the readmission. When a patient is readmitted prior to completing the survey, their responses appear to reflect the cumulative experience of the index admission and the readmission. The lower scores of those who respond to the survey after their readmission appear to be a driver for lower patient-experience scores related to readmissions. Overall, readmission was associated with lower scores on items in five of the nine domains used to calculate patient experience related payments under VBP.16
These findings have important implications in inferring the direction of potential causal relationship between readmissions and patient experience at the hospital level. Additionally, these patients show broad dissatisfaction with areas beyond physician communication and discharge planning. These include staff responsiveness, phlebotomy, meals, hospital cleanliness, and noise level. This pattern of dissatisfaction may represent impatience and frustration with spending additional time in the hospital environment.
Our results are consistent with findings of many of the earlier studies, but our study goes a step further by using patient-level data and incorporating survey response time in our analysis.3,7,9,10 By separating out the readmitted patients who responded to the survey prior to admission, we attempted to address the ability of patients’ perception of care to predict future readmissions. Our results do not support this idea, since pre-readmission responders had similar experience scores to non-readmitted patients. However, because of the low numbers of pre-readmission responders, the comparison lacks precision. Current HCAHPS and Press Ganey questions may lack the ability to predict future readmissions because of the timing of the survey (postdischarge) or the questions themselves.
Overall, postreadmission responders are dissatisfied with multiple domains of hospital care. Many of these survey responses may simply be related to general frustration. Alternatively, they may represent a patient population with a high degree of needs that are not as easily met by a hospital’s routine processes of care. Even though the readmission rates were 10.6% among survey responders, 14.6% of the survey responses were associated with readmissions after accounting for those who respond to surveys linked to readmission. These patients could have significant impact on cumulative experience scores.
Our study has a few limitations. First, it involves a single tertiary care academic center study, and our results may not be generalizable. Second, we did not adjust for some of the patient characteristics associated with readmissions. Patients who were admitted within 30 days are different than those not readmitted based on payor, race, length of stay, and severity of illness, and we did not adjust for these factors in our analysis. This was intentional, however. Our goal was to better understand the relationship between 30-day readmission and patient experience scores as they are used for hospital-level studies, VBP, and public reporting. For these purposes, the scores are not adjusted for factors, such as payor and length of stay. We did adjust for patient-mix adjustment factors used by CMS. Third, the response rates to the HCAHPS were low and may have biased the scores. However, HCAHPS is widely used for comparisons between hospitals has been validated, and our study results have implications with regard to comparing hospital-level performance. HCAHPS results are relevant to policy and have financial consequences.17 Fourth, our study did not directly compare whether the relationship between patient experience for the postreadmission group and nonreadmitted group was different from the relationship between the pre-readmission group and postreadmission group. It is possible that there is no difference in relationship between the groups. However, despite the small number of pre-readmission responders, these patients tended to have more favorable experience responses than those who responded after being readmitted, even after adjusting for response time. Although the P values are nonsignificant for many comparisons, the directionality of the effect is relatively consistent. Also, the vast majority of the patients fall in the postreadmission group, and these patients appear to drive the overall experience related to readmissions. Finally, since relatively few patients turned in surveys prior to readmission, we had limited power to detect a significant difference between these pre-readmission responders and nonreadmitted patients.
Our study has implications for policy makers, researchers, and providers. The HCAHPS scores of patients who are readmitted and completed the survey after being readmitted reflects their experience of both the index admission and the readmission. We did not find evidence to support that HCAHPS survey responses predict future readmissions at the patient level. Our findings do support the concept that lower readmissions rates (whether due to the patient population or processes of care that decrease readmission rates) may improve HCAHPS scores. We suggest caution in assuming that improving patient experience is likely to reduce readmission rates.
Disclosures
The authors declare no conflicts of interest.
1. Hospital value-based purchasing. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/Hospital_VBPurchasing_Fact_Sheet_ICN907664.pdf. Accessed June 25, 2016.
2. Readmissions reduction program (HRRP). Centers for Medicare & Medicaid Services. https://www.cms.gov/medicare/medicare-fee-for-service-payment/acuteinpatientpps/readmissions-reduction-program.html. Accessed June 25, 2016.
3. Boulding W, Glickman SW, Manary MP, Schulman KA, Staelin R. Relationship between patient satisfaction with inpatient care and hospital readmission within 30 days. Am J Manag Care. 2011;17(1):41-48. PubMed
4. Buum HA, Duran-Nelson AM, Menk J, Nixon LJ. Duty-hours monitoring revisited: self-report may not be adequate. Am J Med. 2013;126(4):362-365. doi: 10.1016/j.amjmed.2012.12.003 PubMed
5. Choma NN, Vasilevskis EE, Sponsler KC, Hathaway J, Kripalani S. Effect of the ACGME 16-hour rule on efficiency and quality of care: duty hours 2.0. JAMA Int Med. 2013;173(9):819-821. doi: 10.1001/jamainternmed.2013.3014 PubMed
6. Brooke BS, Samourjian E, Sarfati MR, Nguyen TT, Greer D, Kraiss LW. RR3. Patient-reported readiness at time of discharge predicts readmission following vascular surgery. J Vasc Surg. 2015;61(6):188S. doi: 10.1016/j.jvs.2015.04.356
7. Duraes LC, Merlino J, Stocchi L, et al. 756 readmission decreases patient satisfaction in colorectal surgery. Gastroenterology. 2014;146(5):S-1029. doi: 10.1016/S0016-5085(14)63751-3
8. Mitchell JP. Association of provider communication and discharge instructions on lower readmissions. J Healthc Qual. 2015;37(1):33-40. doi: 10.1097/01.JHQ.0000460126.88382.13 PubMed
9. Tsai TC, Orav EJ, Jha AK. Patient satisfaction and quality of surgical care in US hospitals. Ann Surg. 2015;261(1):2-8. doi: 10.1097/SLA.0000000000000765 PubMed
10. Hachem F, Canar J, Fullam M, Andrew S, Hohmann S, Johnson C. The relationships between HCAHPS communication and discharge satisfaction items and hospital readmissions. Patient Exp J. 2014;1(2):71-77.
11. Irby DM, Cooke M, Lowenstein D, Richards B. The academy movement: a structural approach to reinvigorating the educational mission. Acad Med. 2004;79(8):729-736. doi: 10.1097/00001888-200408000-00003 PubMed
12. Siddiqui ZK, Zuccarelli R, Durkin N, Wu AW, Brotman DJ. Changes in patient satisfaction related to hospital renovation: experience with a new clinical building. J Hosp Med. 2015;10(3):165-171. doi: 10.1002/jhm.2297 PubMed
13. Nair BR, Coughlan JL, Hensley MJ. Student and patient perspectives on bedside teaching. Med Educ. 1997;31(5):341-346. doi: 10.1046/j.1365-2923.1997.00673.x PubMed
14. Elliott MN, Zaslavsky AM, Goldstein E, et al. Effects of survey mode, patient mix, and nonresponse on CAHPS® hospital survey scores. BMC Health Serv Res. 2009;44(2p1):501-518. doi: 10.1111/j.1475-6773.2008.00914.x PubMed
15. Saunders CL, Elliott MN, Lyratzopoulos G, Abel GA. Do differential response rates to patient surveys between organizations lead to unfair performance comparisons?: evidence from the English Cancer Patient Experience Survey. Medical care. 2016;54(1):45. doi: 10.1097/MLR.0000000000000457 PubMed
16. Sabel E, Archer J. “Medical education is the ugly duckling of the medical world” and other challenges to medical educators’ identity construction: a qualitative study. Acad Med. 2014;89(11):1474-1480. doi: 10.1097/ACM.0000000000000420 PubMed
17. O’Malley AJ, Zaslavsky AM, Elliott MN, Zaborski L, Cleary PD. Case‐Mix adjustment of the CAHPS® Hospital Survey. BMC Health Serv Res. 2005;40(6p2):2162-2181. doi: 10.1111/j.1475-6773.2005.00470.x
Patient experience and 30-day readmission are important measures of quality of care for hospitalized patients. Performance on both of these measures impact hospitals financially. Performance on the Hospital Consumer Assessment of Healthcare Systems and Providers (HCAHPS) survey is linked to 25% of the incentive payment under Value Based Purchasing (VBP) Program.1 Starting in 2012, the Centers for Medicare and Medicaid Services (CMS) introduced the Readmission Reduction Program, penalizing hospitals financially for excessive readmissions.2
A relationship between patient experience and readmissions has been explored at the hospital level. Studies have mostly found that higher patient experience scores are associated with lower 30-day readmission rates. In a study of the relationship between 30-day risk-standardized readmission rates for three medical conditions (acute myocardial infarction, heart failure, and pneumonia) and patient experience, the authors noted that higher experience scores for overall care and discharge planning were associated with lower readmission rates for these conditions. They also concluded that patient experience scores were more predictive of 30-day readmission than clinical performance measures. Additionally, the authors predicted that if a hospital increased its total experience scores from the 25th percentile to the 75th percentile, there would be an associated decrease in readmissions by at least 2.3% for each of these conditions.3 Practice management companies and the media have cited this finding to conclude that higher patient experience drives clinical outcomes such as 30-day readmission and that patients are often the best judges of the quality of care delivered.4,5
Other hospital-level studies have found that high 30-day readmission rates are associated with lower overall experience scores in a mixed surgical patient population; worse reports of pain control and overall care in the colorectal surgery population; lower experience scores with discharge preparedness in vascular surgery patients; and lower experience scores with physician communication, nurse communication, and discharge preparedness.6-9 A patient-level study noted higher readmissions are associated with worse experience with physician and nursing communication along with a paradoxically better experience with discharge information.10
Because these studies used an observational design, they demonstrated associations rather than causality. An alternative hypothesis is that readmitted patients complete their patient experience survey after readmission and the low experience is the result, rather than the cause, of their readmission. For patients who are readmitted, it is unclear whether there is an opportunity to complete the survey prior to readmission and whether being readmitted may impact patient perception of quality of care. Using patient-level data, we sought to assess HCAHPS patient-experience responses linked to the index admission of the patients who were readmitted in 30 days and compare it with those patients who were not readmitted during this time period. We paid particular attention to when the surveys were returned.
METHODS
Study Design
We conducted a retrospective analysis of prospectively collected 10-year HCAHPS and Press Ganey patient survey data for a single tertiary care academic hospital.
Participants
All adult patients discharged from the hospital and who responded to the routinely sent patient-experience survey were included. Surveys were sent to a random sample of 50% of the discharged patients.
The exposure group was comprised of patients who responded to the survey and were readmitted within 30 days of discharge. After subtracting 5 days from the survey receipt date for expected delays related to mail delivery time and processing time, survey response date was calculated. The exposure group was further divided into patients who responded to the survey prior to their 30-day readmission (“Pre-readmission responders”) and those that responded to the survey after their readmission (“Postreadmission responders”). A sensitivity analysis was performed by changing the number of days subtracted from the survey receipt date by 2 days in either direction. This approach did not result in any significant changes in the results.
The control group comprised patients who were not readmitted to the hospital within 30 days of discharge and who did not have an admission in the previous 30 days as well (“Not readmitted” group). An additional comparison group for exploratory analysis included patients who had experienced an admission in the prior 30 days but were not readmitted after the admission linked to the survey. These patients responded to the patient-experience surveys that were linked to their second admission in 30 days (“2nd-admission responders” group; Figure).
Time Periods
All survey responders from the third quarter of 2006 to the first quarter of 2016 were included in the study. Additionally, administrative data on non-responders were available from 7/2006 to 8/2012. These data were used to estimate response rates. Patient level experience and administrative data were obtained in a linked fashion for these time periods.
Instruments
Press Ganey and HCAHPS surveys were sent via mail in the same envelope. Fifty percent of the discharged patients were randomized to receive the surveys. The Press Ganey survey contained 33 items encompassing several subdomains, including room, meal, nursing, physician, ancillary staff, visitor, discharge, and overall experience.
The HCAHPS survey contained 29 CMS-mandated items, of which 21 are related to patient experience. The development, testing, and methods for administration and reporting of the HCAHPS survey have been previously described and studies using this instrument have been reported in the literature.11 Press Ganey patient satisfaction survey results have also been reported in the literature.12
Outcome Variables and Covariates
HCAHPS and Press Ganey experience survey individual item responses were the primary outcome variables of this study. Age, self-reported health status, education, primary language spoken, service line, and time taken to respond to the surveys served as the covariates. These variables are used by CMS for patient-mix adjustment and are collected on the HCAHPS survey. Additionally, the number of days to respond to the survey were included in all regression analysis to adjust for early responder effect.13-15
Statistical Analysis
“Percent top-box” scores were calculated for each survey item for patients in each group. The percent top-box scores were calculated as the percent of patients who responded “very good” for a given item on Press Ganey survey items and “always” or “definitely yes” or “yes” or “9” or “10” on HCAHPS survey items. CMS utilizes “percent top-box scores” to calculate payments under the VBP program and to report the results publicly. Numerous studies have also reported percent top-box scores for HCAHPS survey results.12
We hypothesized that whether patients complete the HCAHPS survey before or after the readmission influences their reporting of experience. To test this hypothesis, HCAHPS and Press Ganey item top-box scores of “Pre-readmission responders” and “Postreadmission responders” were compared with those of the control group using multivariate logistic regression. “Pre-readmission responders” were also compared with “Postreadmission responders”.
“2nd-admission responders” were similarly compared with the control group for an exploratory analysis. Finally, “Postreadmission responders” and “2nd-admission responders” were compared in another exploratory analysis since both these groups responded to the survey after being exposed to the readmission, even though the “Postreadmission responders” group is administratively linked to the index admission.
The Johns Hopkins Institutional Review Board approved this study.
RESULTS
There were 43,737 survey responders, among whom 4,707 were subsequently readmitted within 30 days of discharge. Among the readmitted patients who responded to the surveys linked to their index admission, only 15.8% returned the survey before readmission (pre-readmission responders’) and 84.2% returned the survey after readmission (postreadmission responders). Additionally, 1,663 patients responded to experience surveys linked to their readmission. There were 37,365 patients in the control arm (ie, patients who responded to the survey and were not readmitted within 30 days of discharge or in the prior 30 days; Figure 1). The readmission rate among survey responders was 10.6%. Among the readmitted patients, the median number of days to readmission was 10 days while the median number of days to respond to the survey for this group was 33 days. Among the nonreadmitted patients, the median number of days to return the survey was 29 days.
We also conducted an exploratory analysis of the postreadmission responders, comparing them with patients who received patient-experience surveys linked to their second admission in 30 days. Both of these groups were exposed to a readmission before they completed the surveys. There were no significant differences between these two groups on patient experience scores. Additionally, the patients who received the survey linked to their readmission had a broad dissatisfaction pattern on HCAHPS survey items that appeared similar to that of the postreadmission group when compared to the non-readmitted group (Table 3).
DISCUSSION
In this retrospective analysis of prospectively collected Press Ganey and HCAHPS patient-experience survey data, we found that the overwhelming majority of patients readmitted within 30 days of discharge respond to HCAHPS surveys after readmission even though the survey is sent linked to the first admission. This is not unexpected since the median time to survey response is 33 days for this group, while median time to readmission is 10 days. The dissatisfaction pattern of Postreadmission responders was similar to those who responded to the survey linked to the readmission. When a patient is readmitted prior to completing the survey, their responses appear to reflect the cumulative experience of the index admission and the readmission. The lower scores of those who respond to the survey after their readmission appear to be a driver for lower patient-experience scores related to readmissions. Overall, readmission was associated with lower scores on items in five of the nine domains used to calculate patient experience related payments under VBP.16
These findings have important implications in inferring the direction of potential causal relationship between readmissions and patient experience at the hospital level. Additionally, these patients show broad dissatisfaction with areas beyond physician communication and discharge planning. These include staff responsiveness, phlebotomy, meals, hospital cleanliness, and noise level. This pattern of dissatisfaction may represent impatience and frustration with spending additional time in the hospital environment.
Our results are consistent with findings of many of the earlier studies, but our study goes a step further by using patient-level data and incorporating survey response time in our analysis.3,7,9,10 By separating out the readmitted patients who responded to the survey prior to admission, we attempted to address the ability of patients’ perception of care to predict future readmissions. Our results do not support this idea, since pre-readmission responders had similar experience scores to non-readmitted patients. However, because of the low numbers of pre-readmission responders, the comparison lacks precision. Current HCAHPS and Press Ganey questions may lack the ability to predict future readmissions because of the timing of the survey (postdischarge) or the questions themselves.
Overall, postreadmission responders are dissatisfied with multiple domains of hospital care. Many of these survey responses may simply be related to general frustration. Alternatively, they may represent a patient population with a high degree of needs that are not as easily met by a hospital’s routine processes of care. Even though the readmission rates were 10.6% among survey responders, 14.6% of the survey responses were associated with readmissions after accounting for those who respond to surveys linked to readmission. These patients could have significant impact on cumulative experience scores.
Our study has a few limitations. First, it involves a single tertiary care academic center study, and our results may not be generalizable. Second, we did not adjust for some of the patient characteristics associated with readmissions. Patients who were admitted within 30 days are different than those not readmitted based on payor, race, length of stay, and severity of illness, and we did not adjust for these factors in our analysis. This was intentional, however. Our goal was to better understand the relationship between 30-day readmission and patient experience scores as they are used for hospital-level studies, VBP, and public reporting. For these purposes, the scores are not adjusted for factors, such as payor and length of stay. We did adjust for patient-mix adjustment factors used by CMS. Third, the response rates to the HCAHPS were low and may have biased the scores. However, HCAHPS is widely used for comparisons between hospitals has been validated, and our study results have implications with regard to comparing hospital-level performance. HCAHPS results are relevant to policy and have financial consequences.17 Fourth, our study did not directly compare whether the relationship between patient experience for the postreadmission group and nonreadmitted group was different from the relationship between the pre-readmission group and postreadmission group. It is possible that there is no difference in relationship between the groups. However, despite the small number of pre-readmission responders, these patients tended to have more favorable experience responses than those who responded after being readmitted, even after adjusting for response time. Although the P values are nonsignificant for many comparisons, the directionality of the effect is relatively consistent. Also, the vast majority of the patients fall in the postreadmission group, and these patients appear to drive the overall experience related to readmissions. Finally, since relatively few patients turned in surveys prior to readmission, we had limited power to detect a significant difference between these pre-readmission responders and nonreadmitted patients.
Our study has implications for policy makers, researchers, and providers. The HCAHPS scores of patients who are readmitted and completed the survey after being readmitted reflects their experience of both the index admission and the readmission. We did not find evidence to support that HCAHPS survey responses predict future readmissions at the patient level. Our findings do support the concept that lower readmissions rates (whether due to the patient population or processes of care that decrease readmission rates) may improve HCAHPS scores. We suggest caution in assuming that improving patient experience is likely to reduce readmission rates.
Disclosures
The authors declare no conflicts of interest.
Patient experience and 30-day readmission are important measures of quality of care for hospitalized patients. Performance on both of these measures impact hospitals financially. Performance on the Hospital Consumer Assessment of Healthcare Systems and Providers (HCAHPS) survey is linked to 25% of the incentive payment under Value Based Purchasing (VBP) Program.1 Starting in 2012, the Centers for Medicare and Medicaid Services (CMS) introduced the Readmission Reduction Program, penalizing hospitals financially for excessive readmissions.2
A relationship between patient experience and readmissions has been explored at the hospital level. Studies have mostly found that higher patient experience scores are associated with lower 30-day readmission rates. In a study of the relationship between 30-day risk-standardized readmission rates for three medical conditions (acute myocardial infarction, heart failure, and pneumonia) and patient experience, the authors noted that higher experience scores for overall care and discharge planning were associated with lower readmission rates for these conditions. They also concluded that patient experience scores were more predictive of 30-day readmission than clinical performance measures. Additionally, the authors predicted that if a hospital increased its total experience scores from the 25th percentile to the 75th percentile, there would be an associated decrease in readmissions by at least 2.3% for each of these conditions.3 Practice management companies and the media have cited this finding to conclude that higher patient experience drives clinical outcomes such as 30-day readmission and that patients are often the best judges of the quality of care delivered.4,5
Other hospital-level studies have found that high 30-day readmission rates are associated with lower overall experience scores in a mixed surgical patient population; worse reports of pain control and overall care in the colorectal surgery population; lower experience scores with discharge preparedness in vascular surgery patients; and lower experience scores with physician communication, nurse communication, and discharge preparedness.6-9 A patient-level study noted higher readmissions are associated with worse experience with physician and nursing communication along with a paradoxically better experience with discharge information.10
Because these studies used an observational design, they demonstrated associations rather than causality. An alternative hypothesis is that readmitted patients complete their patient experience survey after readmission and the low experience is the result, rather than the cause, of their readmission. For patients who are readmitted, it is unclear whether there is an opportunity to complete the survey prior to readmission and whether being readmitted may impact patient perception of quality of care. Using patient-level data, we sought to assess HCAHPS patient-experience responses linked to the index admission of the patients who were readmitted in 30 days and compare it with those patients who were not readmitted during this time period. We paid particular attention to when the surveys were returned.
METHODS
Study Design
We conducted a retrospective analysis of prospectively collected 10-year HCAHPS and Press Ganey patient survey data for a single tertiary care academic hospital.
Participants
All adult patients discharged from the hospital and who responded to the routinely sent patient-experience survey were included. Surveys were sent to a random sample of 50% of the discharged patients.
The exposure group was comprised of patients who responded to the survey and were readmitted within 30 days of discharge. After subtracting 5 days from the survey receipt date for expected delays related to mail delivery time and processing time, survey response date was calculated. The exposure group was further divided into patients who responded to the survey prior to their 30-day readmission (“Pre-readmission responders”) and those that responded to the survey after their readmission (“Postreadmission responders”). A sensitivity analysis was performed by changing the number of days subtracted from the survey receipt date by 2 days in either direction. This approach did not result in any significant changes in the results.
The control group comprised patients who were not readmitted to the hospital within 30 days of discharge and who did not have an admission in the previous 30 days as well (“Not readmitted” group). An additional comparison group for exploratory analysis included patients who had experienced an admission in the prior 30 days but were not readmitted after the admission linked to the survey. These patients responded to the patient-experience surveys that were linked to their second admission in 30 days (“2nd-admission responders” group; Figure).
Time Periods
All survey responders from the third quarter of 2006 to the first quarter of 2016 were included in the study. Additionally, administrative data on non-responders were available from 7/2006 to 8/2012. These data were used to estimate response rates. Patient level experience and administrative data were obtained in a linked fashion for these time periods.
Instruments
Press Ganey and HCAHPS surveys were sent via mail in the same envelope. Fifty percent of the discharged patients were randomized to receive the surveys. The Press Ganey survey contained 33 items encompassing several subdomains, including room, meal, nursing, physician, ancillary staff, visitor, discharge, and overall experience.
The HCAHPS survey contained 29 CMS-mandated items, of which 21 are related to patient experience. The development, testing, and methods for administration and reporting of the HCAHPS survey have been previously described and studies using this instrument have been reported in the literature.11 Press Ganey patient satisfaction survey results have also been reported in the literature.12
Outcome Variables and Covariates
HCAHPS and Press Ganey experience survey individual item responses were the primary outcome variables of this study. Age, self-reported health status, education, primary language spoken, service line, and time taken to respond to the surveys served as the covariates. These variables are used by CMS for patient-mix adjustment and are collected on the HCAHPS survey. Additionally, the number of days to respond to the survey were included in all regression analysis to adjust for early responder effect.13-15
Statistical Analysis
“Percent top-box” scores were calculated for each survey item for patients in each group. The percent top-box scores were calculated as the percent of patients who responded “very good” for a given item on Press Ganey survey items and “always” or “definitely yes” or “yes” or “9” or “10” on HCAHPS survey items. CMS utilizes “percent top-box scores” to calculate payments under the VBP program and to report the results publicly. Numerous studies have also reported percent top-box scores for HCAHPS survey results.12
We hypothesized that whether patients complete the HCAHPS survey before or after the readmission influences their reporting of experience. To test this hypothesis, HCAHPS and Press Ganey item top-box scores of “Pre-readmission responders” and “Postreadmission responders” were compared with those of the control group using multivariate logistic regression. “Pre-readmission responders” were also compared with “Postreadmission responders”.
“2nd-admission responders” were similarly compared with the control group for an exploratory analysis. Finally, “Postreadmission responders” and “2nd-admission responders” were compared in another exploratory analysis since both these groups responded to the survey after being exposed to the readmission, even though the “Postreadmission responders” group is administratively linked to the index admission.
The Johns Hopkins Institutional Review Board approved this study.
RESULTS
There were 43,737 survey responders, among whom 4,707 were subsequently readmitted within 30 days of discharge. Among the readmitted patients who responded to the surveys linked to their index admission, only 15.8% returned the survey before readmission (pre-readmission responders’) and 84.2% returned the survey after readmission (postreadmission responders). Additionally, 1,663 patients responded to experience surveys linked to their readmission. There were 37,365 patients in the control arm (ie, patients who responded to the survey and were not readmitted within 30 days of discharge or in the prior 30 days; Figure 1). The readmission rate among survey responders was 10.6%. Among the readmitted patients, the median number of days to readmission was 10 days while the median number of days to respond to the survey for this group was 33 days. Among the nonreadmitted patients, the median number of days to return the survey was 29 days.
We also conducted an exploratory analysis of the postreadmission responders, comparing them with patients who received patient-experience surveys linked to their second admission in 30 days. Both of these groups were exposed to a readmission before they completed the surveys. There were no significant differences between these two groups on patient experience scores. Additionally, the patients who received the survey linked to their readmission had a broad dissatisfaction pattern on HCAHPS survey items that appeared similar to that of the postreadmission group when compared to the non-readmitted group (Table 3).
DISCUSSION
In this retrospective analysis of prospectively collected Press Ganey and HCAHPS patient-experience survey data, we found that the overwhelming majority of patients readmitted within 30 days of discharge respond to HCAHPS surveys after readmission even though the survey is sent linked to the first admission. This is not unexpected since the median time to survey response is 33 days for this group, while median time to readmission is 10 days. The dissatisfaction pattern of Postreadmission responders was similar to those who responded to the survey linked to the readmission. When a patient is readmitted prior to completing the survey, their responses appear to reflect the cumulative experience of the index admission and the readmission. The lower scores of those who respond to the survey after their readmission appear to be a driver for lower patient-experience scores related to readmissions. Overall, readmission was associated with lower scores on items in five of the nine domains used to calculate patient experience related payments under VBP.16
These findings have important implications in inferring the direction of potential causal relationship between readmissions and patient experience at the hospital level. Additionally, these patients show broad dissatisfaction with areas beyond physician communication and discharge planning. These include staff responsiveness, phlebotomy, meals, hospital cleanliness, and noise level. This pattern of dissatisfaction may represent impatience and frustration with spending additional time in the hospital environment.
Our results are consistent with findings of many of the earlier studies, but our study goes a step further by using patient-level data and incorporating survey response time in our analysis.3,7,9,10 By separating out the readmitted patients who responded to the survey prior to admission, we attempted to address the ability of patients’ perception of care to predict future readmissions. Our results do not support this idea, since pre-readmission responders had similar experience scores to non-readmitted patients. However, because of the low numbers of pre-readmission responders, the comparison lacks precision. Current HCAHPS and Press Ganey questions may lack the ability to predict future readmissions because of the timing of the survey (postdischarge) or the questions themselves.
Overall, postreadmission responders are dissatisfied with multiple domains of hospital care. Many of these survey responses may simply be related to general frustration. Alternatively, they may represent a patient population with a high degree of needs that are not as easily met by a hospital’s routine processes of care. Even though the readmission rates were 10.6% among survey responders, 14.6% of the survey responses were associated with readmissions after accounting for those who respond to surveys linked to readmission. These patients could have significant impact on cumulative experience scores.
Our study has a few limitations. First, it involves a single tertiary care academic center study, and our results may not be generalizable. Second, we did not adjust for some of the patient characteristics associated with readmissions. Patients who were admitted within 30 days are different than those not readmitted based on payor, race, length of stay, and severity of illness, and we did not adjust for these factors in our analysis. This was intentional, however. Our goal was to better understand the relationship between 30-day readmission and patient experience scores as they are used for hospital-level studies, VBP, and public reporting. For these purposes, the scores are not adjusted for factors, such as payor and length of stay. We did adjust for patient-mix adjustment factors used by CMS. Third, the response rates to the HCAHPS were low and may have biased the scores. However, HCAHPS is widely used for comparisons between hospitals has been validated, and our study results have implications with regard to comparing hospital-level performance. HCAHPS results are relevant to policy and have financial consequences.17 Fourth, our study did not directly compare whether the relationship between patient experience for the postreadmission group and nonreadmitted group was different from the relationship between the pre-readmission group and postreadmission group. It is possible that there is no difference in relationship between the groups. However, despite the small number of pre-readmission responders, these patients tended to have more favorable experience responses than those who responded after being readmitted, even after adjusting for response time. Although the P values are nonsignificant for many comparisons, the directionality of the effect is relatively consistent. Also, the vast majority of the patients fall in the postreadmission group, and these patients appear to drive the overall experience related to readmissions. Finally, since relatively few patients turned in surveys prior to readmission, we had limited power to detect a significant difference between these pre-readmission responders and nonreadmitted patients.
Our study has implications for policy makers, researchers, and providers. The HCAHPS scores of patients who are readmitted and completed the survey after being readmitted reflects their experience of both the index admission and the readmission. We did not find evidence to support that HCAHPS survey responses predict future readmissions at the patient level. Our findings do support the concept that lower readmissions rates (whether due to the patient population or processes of care that decrease readmission rates) may improve HCAHPS scores. We suggest caution in assuming that improving patient experience is likely to reduce readmission rates.
Disclosures
The authors declare no conflicts of interest.
1. Hospital value-based purchasing. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/Hospital_VBPurchasing_Fact_Sheet_ICN907664.pdf. Accessed June 25, 2016.
2. Readmissions reduction program (HRRP). Centers for Medicare & Medicaid Services. https://www.cms.gov/medicare/medicare-fee-for-service-payment/acuteinpatientpps/readmissions-reduction-program.html. Accessed June 25, 2016.
3. Boulding W, Glickman SW, Manary MP, Schulman KA, Staelin R. Relationship between patient satisfaction with inpatient care and hospital readmission within 30 days. Am J Manag Care. 2011;17(1):41-48. PubMed
4. Buum HA, Duran-Nelson AM, Menk J, Nixon LJ. Duty-hours monitoring revisited: self-report may not be adequate. Am J Med. 2013;126(4):362-365. doi: 10.1016/j.amjmed.2012.12.003 PubMed
5. Choma NN, Vasilevskis EE, Sponsler KC, Hathaway J, Kripalani S. Effect of the ACGME 16-hour rule on efficiency and quality of care: duty hours 2.0. JAMA Int Med. 2013;173(9):819-821. doi: 10.1001/jamainternmed.2013.3014 PubMed
6. Brooke BS, Samourjian E, Sarfati MR, Nguyen TT, Greer D, Kraiss LW. RR3. Patient-reported readiness at time of discharge predicts readmission following vascular surgery. J Vasc Surg. 2015;61(6):188S. doi: 10.1016/j.jvs.2015.04.356
7. Duraes LC, Merlino J, Stocchi L, et al. 756 readmission decreases patient satisfaction in colorectal surgery. Gastroenterology. 2014;146(5):S-1029. doi: 10.1016/S0016-5085(14)63751-3
8. Mitchell JP. Association of provider communication and discharge instructions on lower readmissions. J Healthc Qual. 2015;37(1):33-40. doi: 10.1097/01.JHQ.0000460126.88382.13 PubMed
9. Tsai TC, Orav EJ, Jha AK. Patient satisfaction and quality of surgical care in US hospitals. Ann Surg. 2015;261(1):2-8. doi: 10.1097/SLA.0000000000000765 PubMed
10. Hachem F, Canar J, Fullam M, Andrew S, Hohmann S, Johnson C. The relationships between HCAHPS communication and discharge satisfaction items and hospital readmissions. Patient Exp J. 2014;1(2):71-77.
11. Irby DM, Cooke M, Lowenstein D, Richards B. The academy movement: a structural approach to reinvigorating the educational mission. Acad Med. 2004;79(8):729-736. doi: 10.1097/00001888-200408000-00003 PubMed
12. Siddiqui ZK, Zuccarelli R, Durkin N, Wu AW, Brotman DJ. Changes in patient satisfaction related to hospital renovation: experience with a new clinical building. J Hosp Med. 2015;10(3):165-171. doi: 10.1002/jhm.2297 PubMed
13. Nair BR, Coughlan JL, Hensley MJ. Student and patient perspectives on bedside teaching. Med Educ. 1997;31(5):341-346. doi: 10.1046/j.1365-2923.1997.00673.x PubMed
14. Elliott MN, Zaslavsky AM, Goldstein E, et al. Effects of survey mode, patient mix, and nonresponse on CAHPS® hospital survey scores. BMC Health Serv Res. 2009;44(2p1):501-518. doi: 10.1111/j.1475-6773.2008.00914.x PubMed
15. Saunders CL, Elliott MN, Lyratzopoulos G, Abel GA. Do differential response rates to patient surveys between organizations lead to unfair performance comparisons?: evidence from the English Cancer Patient Experience Survey. Medical care. 2016;54(1):45. doi: 10.1097/MLR.0000000000000457 PubMed
16. Sabel E, Archer J. “Medical education is the ugly duckling of the medical world” and other challenges to medical educators’ identity construction: a qualitative study. Acad Med. 2014;89(11):1474-1480. doi: 10.1097/ACM.0000000000000420 PubMed
17. O’Malley AJ, Zaslavsky AM, Elliott MN, Zaborski L, Cleary PD. Case‐Mix adjustment of the CAHPS® Hospital Survey. BMC Health Serv Res. 2005;40(6p2):2162-2181. doi: 10.1111/j.1475-6773.2005.00470.x
1. Hospital value-based purchasing. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/Hospital_VBPurchasing_Fact_Sheet_ICN907664.pdf. Accessed June 25, 2016.
2. Readmissions reduction program (HRRP). Centers for Medicare & Medicaid Services. https://www.cms.gov/medicare/medicare-fee-for-service-payment/acuteinpatientpps/readmissions-reduction-program.html. Accessed June 25, 2016.
3. Boulding W, Glickman SW, Manary MP, Schulman KA, Staelin R. Relationship between patient satisfaction with inpatient care and hospital readmission within 30 days. Am J Manag Care. 2011;17(1):41-48. PubMed
4. Buum HA, Duran-Nelson AM, Menk J, Nixon LJ. Duty-hours monitoring revisited: self-report may not be adequate. Am J Med. 2013;126(4):362-365. doi: 10.1016/j.amjmed.2012.12.003 PubMed
5. Choma NN, Vasilevskis EE, Sponsler KC, Hathaway J, Kripalani S. Effect of the ACGME 16-hour rule on efficiency and quality of care: duty hours 2.0. JAMA Int Med. 2013;173(9):819-821. doi: 10.1001/jamainternmed.2013.3014 PubMed
6. Brooke BS, Samourjian E, Sarfati MR, Nguyen TT, Greer D, Kraiss LW. RR3. Patient-reported readiness at time of discharge predicts readmission following vascular surgery. J Vasc Surg. 2015;61(6):188S. doi: 10.1016/j.jvs.2015.04.356
7. Duraes LC, Merlino J, Stocchi L, et al. 756 readmission decreases patient satisfaction in colorectal surgery. Gastroenterology. 2014;146(5):S-1029. doi: 10.1016/S0016-5085(14)63751-3
8. Mitchell JP. Association of provider communication and discharge instructions on lower readmissions. J Healthc Qual. 2015;37(1):33-40. doi: 10.1097/01.JHQ.0000460126.88382.13 PubMed
9. Tsai TC, Orav EJ, Jha AK. Patient satisfaction and quality of surgical care in US hospitals. Ann Surg. 2015;261(1):2-8. doi: 10.1097/SLA.0000000000000765 PubMed
10. Hachem F, Canar J, Fullam M, Andrew S, Hohmann S, Johnson C. The relationships between HCAHPS communication and discharge satisfaction items and hospital readmissions. Patient Exp J. 2014;1(2):71-77.
11. Irby DM, Cooke M, Lowenstein D, Richards B. The academy movement: a structural approach to reinvigorating the educational mission. Acad Med. 2004;79(8):729-736. doi: 10.1097/00001888-200408000-00003 PubMed
12. Siddiqui ZK, Zuccarelli R, Durkin N, Wu AW, Brotman DJ. Changes in patient satisfaction related to hospital renovation: experience with a new clinical building. J Hosp Med. 2015;10(3):165-171. doi: 10.1002/jhm.2297 PubMed
13. Nair BR, Coughlan JL, Hensley MJ. Student and patient perspectives on bedside teaching. Med Educ. 1997;31(5):341-346. doi: 10.1046/j.1365-2923.1997.00673.x PubMed
14. Elliott MN, Zaslavsky AM, Goldstein E, et al. Effects of survey mode, patient mix, and nonresponse on CAHPS® hospital survey scores. BMC Health Serv Res. 2009;44(2p1):501-518. doi: 10.1111/j.1475-6773.2008.00914.x PubMed
15. Saunders CL, Elliott MN, Lyratzopoulos G, Abel GA. Do differential response rates to patient surveys between organizations lead to unfair performance comparisons?: evidence from the English Cancer Patient Experience Survey. Medical care. 2016;54(1):45. doi: 10.1097/MLR.0000000000000457 PubMed
16. Sabel E, Archer J. “Medical education is the ugly duckling of the medical world” and other challenges to medical educators’ identity construction: a qualitative study. Acad Med. 2014;89(11):1474-1480. doi: 10.1097/ACM.0000000000000420 PubMed
17. O’Malley AJ, Zaslavsky AM, Elliott MN, Zaborski L, Cleary PD. Case‐Mix adjustment of the CAHPS® Hospital Survey. BMC Health Serv Res. 2005;40(6p2):2162-2181. doi: 10.1111/j.1475-6773.2005.00470.x
© 2018 Society of Hospital Medicine
Presurgery Discontinuation of Antiplatelet Therapy in Patients
There are currently limited data to guide perioperative management of antiplatelet therapy after drug‐eluting stent (DES) implantation. The clinician must balance the risk of excessive bleeding if antiplatelet agents are continued perioperatively with the risk of stent thrombosis if antiplatelet agents are discontinued for surgerya risk that may be amplified in the perioperative period because of the prothrombotic state that accompanies the stress of surgery.
Paclitaxel‐ and sirolimus‐eluting stents have supplanted bare‐metal stents as first‐line treatment for coronary stenosis because of their efficacy in preventing in‐stent restenosis by inhibiting neointimal proliferation. However, the antiproliferative effects of DESs may also delay endothelialization, rendering them vulnerable to stent thrombosis when antiplatelet therapy is prematurely discontinued.15 Some patients with DESs may be vulnerable to stent thrombosis when antiplatelet therapy is discontinued even after a year or more of treatment.6 Although stent thrombosis is uncommon, it is deadly, with a mortality rate approaching 50%.1 Generally, antiplatelet therapy is discontinued prior to surgery. This presents a clinical dilemma for patients with DES because guidelines recommend lifelong aspirin therapy and at least 36 months of clopidogrel for patients who have undergone DES placement.79
In the bare‐metal stent era, studies demonstrated an alarming risk of stent thrombosis in the setting of noncardiac surgery within 26 weeks of stent placement.10, 11 However, the appropriate interval before elective noncardiac surgery following DES placement has not been defined and may be longer. Case reports and case series have highlighted this risk12 and have even suggested that a DES may be susceptible to stent thrombosis as long as a year after its placement.6 More recently, pooled data from controlled trials have suggested that although the overall rate of DES thrombosis may not be consistently higher than that of bare‐metal stents, the risk appears to persist far longer (probably from delayed endothelialization of the target vessel) and may be more pronounced following discontinuation of antiplatelet agents.9, 1316 This has led to recent recommendations to continue dual antiplatelet therapy (with aspirin and clopidogrel) for at least a year following DES placement and possibly indefinitely, provided that the therapy is tolerated.9 Whether this risk is accentuated in the perioperative setting independent of discontinuation of antiplatelet therapy remains unknown. In 1 registry, the strongest predictor of DES thrombosis was premature discontinuation of antiplatelet therapy (hazard ratio 90, 95% confidence interval 30270, P < .001), and noncardiac surgery was the most frequent reason for discontinuation of antiplatelet therapy.1 However, the actual incidence of stent thrombosis in patients undergoing surgery was unavailable because the denominator was unknown (ie, number of patients with stents who underwent surgery). Although it is certainly plausible that the prothrombotic and proinflammatory postoperative state augments the risk of stent thrombosis independent of discontinuation of antiplatelet therapy alone, this remains unproven.
At the time of the present study, protocol‐based clinical practice at the Cleveland Clinic Foundation's Internal Medicine Preoperative Assessment Consultation and Treatment (IMPACT) Center included routine discontinuation of all antiplatelet agents (including aspirin and clopidogrel) at least 7 days prior to noncardiac surgery, including in patients with coronary stents. Exceptions to this policy were generally made only for very minor procedures. The purpose of this study was to systematically quantify the risk of adverse cardiovascular events in patients who had DES placement and subsequently underwent elective or semielective noncardiac surgery, most of whom had discontinued all antiplatelet agents at least 7 days before surgery.
Methods
We identified all patients who had DES placement at the Cleveland Clinic who subsequently underwent preoperative evaluation for noncardiac surgery at the IMPACT Center between July 2003 and July 2005. About half the patients undergoing surgery at the Cleveland Clinic were seen in the IMPACT Center prior to surgery during the study period. Preoperative evaluation at the IMPACT Center included a standardized assessment by a hospitalist with expertise in preoperative medicine. Clinical data for each patient were contemporaneously entered into an electronic medical record. Written preoperative medication instructions were provided to each patient and documented in the electronic record, indicating specific instructions to discontinue any antiplatelet agents 710 days preoperatively.
The IMPACT Center database was crosslinked to the Cleveland Clinic Foundation Heart Center Database, which contains records of all patients who have undergone coronary stenting at the Cleveland Clinic. Computerized and written medical records of all patients in both databases were reviewed using a standardized data collection instrument. All medical data generated up to 30 days postoperatively at the Cleveland Clinic were reviewed. Social Security numbers were linked with the Social Security Death Index to verify that no patients died within 30 days of surgery.
Predefined outcomes included catheterization‐confirmed DES thrombosis, any myocardial infarction, and major bleeding within 30 days of the surgical procedure. Myocardial infarction was defined as elevation of troponin T to more than twice the upper limit of normal (0.2 mg/mL) with or without associated electrocardiographic changes or symptoms. This biochemically based definition was used with the understanding that cardiac enzyme tests are consistently ordered for patients at the Cleveland Clinic with suspected coronary events and that postoperative myocardial infarction may be atypical in presentation (eg, delirium or hypotension without chest pain). Stent thrombosis was considered present if confirmed by catheterization or autopsy and considered possible if a patient suffered from a myocardial infarction but did not have a definitive diagnostic procedure performed. DES thrombosis was considered absent if a patient underwent postoperative catheterization and the DES appeared patent. Major bleeding was defined as any bleeding requiring unplanned reoperation or bleeding in a critical location (intracranial or retroperitoneal). Invasiveness of surgery was defined prospectively according to a Cleveland Clinic bleeding classification scheme based on that of Pasternak17, 18:
Category 1. Minimal risk to patient; little or no anticipated blood loss (eg, breast biopsy, cystoscopy).
Category 2. Mild risk to patient; minimal to moderately invasive procedure; estimated blood loss < 500 cc (eg, laparoscopy, arthroscopy, hernia repair).
Category 3. Moderate risk to patient and moderate to significantly invasive; blood loss potential 5001000 cc (eg, laminectomy, total hip or knee replacement).
Category 4. Major risk to patient; highly invasive procedure; anticipated blood loss > 1500 cc (eg, major spinal reconstruction, major reconstruction of GI tract, major vascular repair without intensive care unit stay).
Category 5. Critical risk to patient; highly invasive procedure; anticipated blood loss > 1500 cc with anticipated postoperative intensive care unit stay (eg, cardiac procedure, major vascular repair with anticipated intensive care unit stay).
Statistical analyses were descriptive. We determined the rate of adverse outcomes with 95% confidence intervals (CIs) in the entire patient cohort and among prespecified patient subsets, based on timing of discontinuation of antiplatelet therapy. Predefined subsets included those who had clopidogrel and aspirin discontinued less than 3 months and less than 6 months following DES implantation. The 2 test was used to test the hypothesis that discontinuation of antiplatelet therapy was a function of the type of surgery or timing of stent placement.
The study was approved by the Cleveland Clinic Foundation's institutional review board. The requirement for informed consent was waived.
RESULTS
In total, 114 patients were evaluated in the IMPACT Center following DES placement. Baseline patient characteristics are shown in Table 1. The median age was 71 years (interquartile range 6476 years), and 66% were male. Patients had a moderate degree of comorbidity: 41% had diabetes, 12% had an ejection fraction < 45%, 34% had undergone coronary bypass, 17% had atrial fibrillation or flutter, and 20% had chronic renal insufficiency (creatinine 2.0 or end‐stage renal disease). Most patients received ‐adrenergic blockers (97%), statins (95%), and either angiotensin‐converting enzyme (ACE) inhibitors or angiotensin receptor blockers (77%) preoperatively. Patients underwent a variety of surgeries (Table 1).
| Characteristic | n (%) unless otherwise noted |
|---|---|
| |
| Demographics | |
| Age (years), median (IQR) | 71 (6476) |
| Male | 75 (65.7%) |
| White | 88 (77.2%) |
| Comorbid illnesses | |
| Diabetes mellitus | 47 (41.2%) |
| History of prior myocardial infarction | 48 (42.1%) |
| Hypertension | 108 (94.7%) |
| History of stroke or transient cerebral ischemia | 15 (13.2%) |
| Dyslipidemia or treatment with lipid‐lowering drugs | 106 (93.0%) |
| Ejection fraction < 45% | 14 (12.3%) |
| History of coronary artery bypass | 39 (34.2%) |
| Atrial fibrillation or flutter | 19 (16.7%) |
| End‐stage renal disease on dialysis | 13 (11.4%) |
| Chronic renal impairment (creatinine 2.0) without dialysis | 10 (8.8%) |
| Other medical treatments | |
| Angiotensin converting enzyme inhibitor or angiotensin receptor blocker | 88 (77.2%) |
| ‐blocker | 111 (97.3%) |
| Statin | 108 (94.7%) |
| Invasiveness of surgery* | |
| Category 1 (lowest risk) | 37 (32.5%) |
| Category 2 | 22 (19.3%) |
| Category 3 | 48 (42.1%) |
| Category 4 | 7 (6.1%) |
| Category 5 (highest risk) | 0 (0%) |
| Outpatient or short‐stay surgery | 50 (47.2%) |
| Type of surgery | |
| Major orthopedic | 39 (34.2%) |
| Minor orthopedic | 5 (4.4%) |
| Ophthalmologic | 30 (26.3%) |
| General abdominal | 8 (7.0%) |
| Gynecological | 5 (4.4%) |
| Urological | 11 (9.6%) |
| Head and neck | 5 (4.4%) |
| Vascular | 1 (0.9%) |
| Other | 10 (8.8%) |
Patients had received both paclitaxel and sirolimus stents (28% and 73% of patients, respectively); 33% of patients had had more than 1 DES (Table 2). Most patients underwent surgery within 1 year of stent placement (77%), but only 40% had surgery within 180 days of stenting and only 13% within 90 days of stenting. Most patients (77%) had antiplatelet therapy completely discontinued a median of 10 days before surgery and remained off antiplatelet therapy for a median of 14 days total. Ten of the 15 patients (67%) who underwent surgery within 90 days of stenting had all antiplatelet agents discontinued preoperatively, 24 of the 30 patients (80%) who had surgery between 91 and 180 days after stenting had antiplatelet therapy completely discontinued, and 54 of the 69 patients (78%) who had surgery more than 180 days after stenting had antiplatelet therapy completely discontinued. There was no significant relationship between timing of stent placement relative to surgery (<90, 91180, or >180 days) and decision about whether to discontinue antiplatelet therapy (P = .59). However, invasiveness of the surgery was associated with antiplatelet management: 85% of those who continued antiplatelet therapy (aspirin or aspirin and clopidogrel) during the perioperative period were patients who underwent minimally invasive surgery (P < .0001).
| Characteristic | n (%) unless otherwise noted |
|---|---|
| |
| Timing of surgery and antiplatelet agent discontinuation relative to Percutaneous coronary intervention | |
| Duration of most recent intervention relative to surgery (days), median (IQR) | 236 (125354) |
| Surgery within 90 days of DES placement | 15 (13.2%) |
| Surgery within 180 days of DES placement | 45 (39.5%) |
| Surgery within 1 year of DES placement | 88 (77.2%) |
| Percutaneous Coronary Intervention History | |
| Number of drug‐eluting stents | |
| 1 | 76 (66.7%) |
| 2 | 26 (22.8%) |
| 3+ | 12 (10.5%) |
| Paclitaxel stent 1 | 32 (28.1%) |
| Sirolimus stent 1 | 83 (72.8%) |
| Bare‐metal stent 1 | 10 (8.8%) |
| Perioperative antiplatelet treatment | |
| Clopidogrel and aspirin continued through surgery | 24 (21.1%) |
| Aspirin alone continued through surgery | 2 (1.8%) |
| Clopidogrel alone continued through surgery | 0 (0%) |
| No antiplatelet treatment at time of surgery | 88 (77.2%) |
| Among the 15 patients who had surgery within 90 days of stenting | 10 (66.7%) |
| Among the 45 patients who had surgery within 180 days of stenting | 34 (75.6%) |
| Duration of discontinuance of aspirin | |
| Median number of days discontinued preoperatively (IQR) | 10 (812) |
| Median total duration of discontinuance [days, IQR) | 14 (1019) |
| Duration of discontinuance of clopidogrel | |
| Median number of days discontinued preoperatively [days, IQR] | 10 (813) |
| Median number of days discontinued in total (IQR) | 14 (1020) |
The outcome events are presented in Table 3. Two patients (1.8%, 95% CI 0.5%6.2%) suffered a non‐ST‐elevation myocardial infarction (NSTEMI) postoperatively, and another patient (0.9%, 95% CI 0.2%4.8%) developed major bleeding, a retroperitoneal hemorrhage following kidney transplantation. This patient had been taking both aspirin and clopidogrel until 7 days prior to surgery and began to hemorrhage the day after surgery; antiplatelet agents were resumed 12 days postoperatively. No patients died (0%, 95% CI 0%3.3%). One of the 2 patients who suffered an MI was a 72‐year‐old man who had had placement of a single sirolimus‐eluting stent in the posterior descending artery 284 days prior to elective hip arthroplasty. He had no history of myocardial infarction but had undergone coronary bypass surgery 4 years earlier. Echocardiography showed he had aortic stenosis, with a calculated valve area of 0.9 cm2. He had a baseline left ventricular ejection fraction of 45%. His preoperative cardiac medications included lovastatin, lisinopril, and atenolol; he discontinued both aspirin and clopidogrel 7 days before the surgery. His NSTEMI occurred on the day of his operation, presenting with hypotension and anterolateral ST depressions. His troponin T peaked at 0.48 mg/mL, with a peak creatinine kinase of 795 U/L (MB fraction 6%). His left ventricular ejection fraction was 45% on postoperative day 2 (unchanged from baseline). He was discharged on postoperative day 8 and returned for catheterization 3 weeks later, at which time he was found to have a 70% ostial lesion in a saphenous vein graft to an obtuse marginal, which was stented. The previously placed DES was widely patent. The other patient who suffered a postoperative NSTEMI was a 68‐year‐old man with a history of carotid artery stenting and renal artery stenosis who had undergone placement of 3 sirolimus‐eluting stents in the right coronary artery 50 days prior to cervical laminectomy. He had had elective placement of the stents following a positive pharmacologic stress test. He was taking 50 mg of atenolol daily and had been taking aspirin and clopidogrel until 17 days before surgery. On postoperative day 3 he developed dyspnea, and leads V4 and V5 showed ST depressions. His troponin T peaked at 1.24 mg/mL, with a peak creatinine kinase of 879 U/L (MB fraction 6%). The patient underwent left‐heart catheterization on hospital day 10. All 3 DESs were widely patent. His left ventricular ejection fraction was estimated at 65%. He was discharged on postoperative day 15. Because neither of the patients who had a postoperative NSTEMI showed evidence of stent thrombosis on catheterization, the overall rate of stent thrombosis was 0% (95% CI 0%3.3%).
| Outcome | Entire cohort (n = 114) [all antiplatelet therapy stopped in 88 patients (77%)] | Surgery < 90 days after DES (n = 15) [all antiplatelet therapy stopped in 10 patients (67%)] | Surgery < 180 days after DES (n = 45) [all antiplatelet therapy stopped in 34 patients (76%)] |
|---|---|---|---|
| |||
| Death | 0 (0%, 0%3.3%) | 0 (0%, 0%20.4%) | 0 (0%, 0%7.9%) |
| Any myocardial infarction | 2 (1.8%, 0.5%6.2%) | 1 (6.7%, 1.2%29.8%) | 1 (2.2%, 0.4%11.6%) |
| DES thrombosis | 0 (0%, 0%3.3%) | 0 (0%, 0%20.4%) | 0 (0%, 0%7.9%) |
| Major bleeding | 1 (0.9%, 0.2%4.8%) | 0 (0%, 0%20.4%) | 0 (0%, 0%7.9%) |
DISCUSSION
Although 2 patients in our study cohort suffered a postoperative myocardial infarction and underwent postoperative catheterization, neither was found to have stent thrombosis, and the MIs of both patients were NSTEMIs with modest cardiac enzyme elevations only. No patients died. A rate of myocardial infarction of less than 2% is well within that expected for patients with established coronary disease undergoing noncardiac surgery.19 That most of our patients discontinued both aspirin and clopidogrel and did not receive antiplatelet agents for a median of 14 days suggests that transient termination of antiplatelet agents in the perioperative setting is not associated with high morbidity or mortality in patients with DES, even when patients have had their stents implanted in the preceding 36 months.
Our study builds on the limited data on this topic. One small case series examined outcomes in 38 patients who had had DES placement and subsequently underwent noncardiac surgery a median of 297 days after stenting.20 None of the patients in this series suffered from stent thrombosis or myocardial infarction, but most underwent surgery without discontinuing aspirin, and 41% underwent surgery without discontinuing clopidogrel. Another recent study demonstrated a high rate of adverse cardiovascular events in patients with coronary stents who underwent noncardiac surgery up to a year after stenting, but the authors of this study did not differentiate between drug‐eluting and bare‐metal stents, and all patients were continued on antiplatelet agents and received parenteral antithrombotic treatment.21
The major strength of our study was its systematic approach. Using a computerized and comprehensive search strategy, we identified all patients who had undergone DES placement at the Cleveland Clinic who subsequently had a preoperative evaluation at the IMPACT Center. Therefore, we are confident that the number of patients in our cohort truly reflects a well‐defined at‐risk population, allowing for an accurate calculation of event rates. This approach contrasts sharply with prior case reports and case series, in which the number of patients at risk was unknown. Nevertheless, these previous reports demonstrate that DES thromboses do occur and can be devastating, so even a small risk of DES thrombosis should be taken seriously. The upper bound of the 95% confidence interval of our estimate of the rate of DES thrombosis was 3.3%, so it is entirely plausible that sampling error contributed to the low rate of thrombosis that we observed.
One major limitation of our study is its sample size. Although our cohort was more than 3 times larger than the only other published cohort of DES patients undergoing noncardiac surgery,20 we had only limited precision to quantify the risk of DES thrombosis. This limitation is particularly relevant for patients who have undergone stent implantation within 36 months of surgery, as they are the patients most likely to have incomplete reendothelialization of the stented artery. We believe that when possible it remains prudent to delay noncardiac surgery for at least 36 months and perhaps up to 12 months following DES implantation, in keeping with recent guidelines.7, 8 However, for patients with conditions such as cancer whose surgery is semielective or patients with nonsurgical bleeding problems (such as gastrointestinal bleeding), our study provides at least some reassurance that short‐term discontinuation of antiplatelet agents may not be as dangerous as some authors have suggested,1 even within 36 months of DES placement. Another important limitation of our study is potential referral bias. At the Cleveland Clinic, most patients undergoing vascular and thoracic procedures are not evaluated at the IMPACT Center. Similarly, some of the patients with severe cardiovascular disease may also have bypassed the IMPACT Center and gone to a cardiologist for preoperative evaluation. As such, we believe our findings should not be generalized to high‐risk cardiac patients or to those undergoing high‐risk procedures.
A noteworthy distinction between our cohort and the cohort reported by Compton and colleagues is that in the perioperative period, most of our patients underwent complete discontinuation of antiplatelet therapy and remained off both aspirin and clopidogrel for an average of 2 weeks, whereas most patients in the other cohort were continued on antiplatelet therapy.20 This highlights the continued controversy surrounding management of antiplatelet therapy in perioperative patients with established coronary disease, who are at substantial risk for both bleeding and myocardial infarction because of the surgery.22 Our data offer little guidance on the optimal management of antiplatelet agents perioperatively because the incidence of both bleeding and thrombosis was low and whether or not patients were continued on antiplatelet agents was not random. We advocate individualized management strategies of perioperative patients with DES. Patients undergoing procedures that carry a high risk of outcome‐affecting bleeding (such as brain surgery) should probably have their antiplatelet agents discontinued preoperatively, whereas those undergoing minor surgery may have their antiplatelet agents continued, provided the surgeon and the anesthesiologist are in agreement with this approach. The timing of DES placement should also be factored into this decision because recently placed stents carry a higher risk of thrombosis.
In summary, our findings clarify the risks of stent thrombosis and postoperative myocardial infarction in clinically stable patients with DES who undergo low‐ and intermediate‐risk noncardiac surgery. Because it is unlikely to ever be ethically appropriate or logistically feasible to conduct a randomized study of patients with DES having early versus delayed noncardiac surgery, observational cohorts will have to suffice. Additional similar studies will help to validate (or refute) our findings and to more precisely quantify the risk of adverse cardiac events when patients with DES undergo surgery, which is real, feared, and potentially catastrophic but may be overestimated.
- ,,, et al.Incidence, predictors, and outcome of thrombosis after successful implantation of drug‐eluting stents.JAMA.2005;293:2126–230.
- ,,, et al.Pathology of drug‐eluting stents in humans: delayed healing and late thrombotic risk.J Am Coll Cardiol.2006;48:193–202.
- ,,, et al.Correlates and long‐term outcomes of angiographically proven stent thrombosis with sirolimus‐ and paclitaxel‐eluting stents.Circulation.2006;113:1108–1113.
- ,,, et al.Prevalence, predictors, and outcomes of premature discontinuation of thienopyridine therapy after drug‐eluting stent placement: results from the PREMIER registry.Circulation.2006;113:2803–2809.
- .Trading restenosis for thrombosis? New questions about drug‐eluting stents.N Engl J Med.2006;355:1949–1952.
- ,,, et al.Late thrombosis in drug‐eluting coronary stents after discontinuation of antiplatelet therapy.Lancet2004;364:1519–1521.
- ,,, et al.ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/SCAI Writing Committee to Update 2001 Guidelines for Percutaneous Coronary Intervention).Circulation.2006;113:e166–e286.
- ,,, et al.ACC/AHA guidelines for the management of patients with ST‐elevation myocardial infarction—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1999 Guidelines for the Management of Patients With Acute Myocardial Infarction).Circulation.2004;110:588–636.
- ,,, et al.Prevention of premature discontinuation of dual antiplatelet therapy in patients with coronary artery stents: a science advisory from the American Heart Association, American College of Cardiology, Society for Cardiovascular Angiography and Interventions, American College of Surgeons, and American Dental Association, with representation from the American College of Physicians.Circulation.2007;115:813–818.
- ,,, et al.Clinical outcome of patients undergoing non‐cardiac surgery in the two months following coronary stenting.J Am Coll Cardiol.2003;42:234–240.
- ,,,,.Catastrophic outcomes of noncardiac surgery soon after coronary stenting.J Am Coll Cardiol.2000;35:1288–1294.
- ,.Thrombosis of sirolimus‐eluting coronary stent in the postanesthesia care unit.Anesth Analg.2005;101:971–973.
- ,,,,,.Late thrombosis of drug‐eluting stents: a meta‐analysis of randomized clinical trials.Am J Med.2006;119:1056–1061.
- ,,,,,.Long‐term outcomes with drug‐eluting stents versus bare‐metal stents in Sweden.N Engl J Med.2007;356:1009–1019.
- ,,, et al.Safety and efficacy of sirolimus‐ and paclitaxel‐eluting coronary stents.N Engl J Med.2007;356:998–1008.
- ,,,,.A pooled analysis of data comparing sirolimus‐eluting stents with bare‐metal stents.N Engl J Med.2007;356:989–997.
- .Preoperative assessment: guidelines and challenges.Acta Anaesthesiol ScandSuppl.1997;111:318–320.
- .Preoperative assessment of the ambulatory and same day admission patient.Wellcome Trends Anesthesiol.1991;9:3–11.
- ,,, et al.Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery.Circulation.1999;100:1043–1049.
- ,,,,.Risk of noncardiac surgery after coronary drug‐eluting stent implantation.Am J Cardiol.2006;98:1212–1213.
- ,,,,,.Coronary artery stenting and non‐cardiac surgery—a prospective outcome study.Br J Anaesth.2006;96:686–693.
- ,,.Double jeopardy: balance between bleeding and stent thrombosis with prolonged dual antiplatelet therapy after drug‐eluting stent implantation.Cardiovasc Revasc Med.2006;7:155–158.
There are currently limited data to guide perioperative management of antiplatelet therapy after drug‐eluting stent (DES) implantation. The clinician must balance the risk of excessive bleeding if antiplatelet agents are continued perioperatively with the risk of stent thrombosis if antiplatelet agents are discontinued for surgerya risk that may be amplified in the perioperative period because of the prothrombotic state that accompanies the stress of surgery.
Paclitaxel‐ and sirolimus‐eluting stents have supplanted bare‐metal stents as first‐line treatment for coronary stenosis because of their efficacy in preventing in‐stent restenosis by inhibiting neointimal proliferation. However, the antiproliferative effects of DESs may also delay endothelialization, rendering them vulnerable to stent thrombosis when antiplatelet therapy is prematurely discontinued.15 Some patients with DESs may be vulnerable to stent thrombosis when antiplatelet therapy is discontinued even after a year or more of treatment.6 Although stent thrombosis is uncommon, it is deadly, with a mortality rate approaching 50%.1 Generally, antiplatelet therapy is discontinued prior to surgery. This presents a clinical dilemma for patients with DES because guidelines recommend lifelong aspirin therapy and at least 36 months of clopidogrel for patients who have undergone DES placement.79
In the bare‐metal stent era, studies demonstrated an alarming risk of stent thrombosis in the setting of noncardiac surgery within 26 weeks of stent placement.10, 11 However, the appropriate interval before elective noncardiac surgery following DES placement has not been defined and may be longer. Case reports and case series have highlighted this risk12 and have even suggested that a DES may be susceptible to stent thrombosis as long as a year after its placement.6 More recently, pooled data from controlled trials have suggested that although the overall rate of DES thrombosis may not be consistently higher than that of bare‐metal stents, the risk appears to persist far longer (probably from delayed endothelialization of the target vessel) and may be more pronounced following discontinuation of antiplatelet agents.9, 1316 This has led to recent recommendations to continue dual antiplatelet therapy (with aspirin and clopidogrel) for at least a year following DES placement and possibly indefinitely, provided that the therapy is tolerated.9 Whether this risk is accentuated in the perioperative setting independent of discontinuation of antiplatelet therapy remains unknown. In 1 registry, the strongest predictor of DES thrombosis was premature discontinuation of antiplatelet therapy (hazard ratio 90, 95% confidence interval 30270, P < .001), and noncardiac surgery was the most frequent reason for discontinuation of antiplatelet therapy.1 However, the actual incidence of stent thrombosis in patients undergoing surgery was unavailable because the denominator was unknown (ie, number of patients with stents who underwent surgery). Although it is certainly plausible that the prothrombotic and proinflammatory postoperative state augments the risk of stent thrombosis independent of discontinuation of antiplatelet therapy alone, this remains unproven.
At the time of the present study, protocol‐based clinical practice at the Cleveland Clinic Foundation's Internal Medicine Preoperative Assessment Consultation and Treatment (IMPACT) Center included routine discontinuation of all antiplatelet agents (including aspirin and clopidogrel) at least 7 days prior to noncardiac surgery, including in patients with coronary stents. Exceptions to this policy were generally made only for very minor procedures. The purpose of this study was to systematically quantify the risk of adverse cardiovascular events in patients who had DES placement and subsequently underwent elective or semielective noncardiac surgery, most of whom had discontinued all antiplatelet agents at least 7 days before surgery.
Methods
We identified all patients who had DES placement at the Cleveland Clinic who subsequently underwent preoperative evaluation for noncardiac surgery at the IMPACT Center between July 2003 and July 2005. About half the patients undergoing surgery at the Cleveland Clinic were seen in the IMPACT Center prior to surgery during the study period. Preoperative evaluation at the IMPACT Center included a standardized assessment by a hospitalist with expertise in preoperative medicine. Clinical data for each patient were contemporaneously entered into an electronic medical record. Written preoperative medication instructions were provided to each patient and documented in the electronic record, indicating specific instructions to discontinue any antiplatelet agents 710 days preoperatively.
The IMPACT Center database was crosslinked to the Cleveland Clinic Foundation Heart Center Database, which contains records of all patients who have undergone coronary stenting at the Cleveland Clinic. Computerized and written medical records of all patients in both databases were reviewed using a standardized data collection instrument. All medical data generated up to 30 days postoperatively at the Cleveland Clinic were reviewed. Social Security numbers were linked with the Social Security Death Index to verify that no patients died within 30 days of surgery.
Predefined outcomes included catheterization‐confirmed DES thrombosis, any myocardial infarction, and major bleeding within 30 days of the surgical procedure. Myocardial infarction was defined as elevation of troponin T to more than twice the upper limit of normal (0.2 mg/mL) with or without associated electrocardiographic changes or symptoms. This biochemically based definition was used with the understanding that cardiac enzyme tests are consistently ordered for patients at the Cleveland Clinic with suspected coronary events and that postoperative myocardial infarction may be atypical in presentation (eg, delirium or hypotension without chest pain). Stent thrombosis was considered present if confirmed by catheterization or autopsy and considered possible if a patient suffered from a myocardial infarction but did not have a definitive diagnostic procedure performed. DES thrombosis was considered absent if a patient underwent postoperative catheterization and the DES appeared patent. Major bleeding was defined as any bleeding requiring unplanned reoperation or bleeding in a critical location (intracranial or retroperitoneal). Invasiveness of surgery was defined prospectively according to a Cleveland Clinic bleeding classification scheme based on that of Pasternak17, 18:
Category 1. Minimal risk to patient; little or no anticipated blood loss (eg, breast biopsy, cystoscopy).
Category 2. Mild risk to patient; minimal to moderately invasive procedure; estimated blood loss < 500 cc (eg, laparoscopy, arthroscopy, hernia repair).
Category 3. Moderate risk to patient and moderate to significantly invasive; blood loss potential 5001000 cc (eg, laminectomy, total hip or knee replacement).
Category 4. Major risk to patient; highly invasive procedure; anticipated blood loss > 1500 cc (eg, major spinal reconstruction, major reconstruction of GI tract, major vascular repair without intensive care unit stay).
Category 5. Critical risk to patient; highly invasive procedure; anticipated blood loss > 1500 cc with anticipated postoperative intensive care unit stay (eg, cardiac procedure, major vascular repair with anticipated intensive care unit stay).
Statistical analyses were descriptive. We determined the rate of adverse outcomes with 95% confidence intervals (CIs) in the entire patient cohort and among prespecified patient subsets, based on timing of discontinuation of antiplatelet therapy. Predefined subsets included those who had clopidogrel and aspirin discontinued less than 3 months and less than 6 months following DES implantation. The 2 test was used to test the hypothesis that discontinuation of antiplatelet therapy was a function of the type of surgery or timing of stent placement.
The study was approved by the Cleveland Clinic Foundation's institutional review board. The requirement for informed consent was waived.
RESULTS
In total, 114 patients were evaluated in the IMPACT Center following DES placement. Baseline patient characteristics are shown in Table 1. The median age was 71 years (interquartile range 6476 years), and 66% were male. Patients had a moderate degree of comorbidity: 41% had diabetes, 12% had an ejection fraction < 45%, 34% had undergone coronary bypass, 17% had atrial fibrillation or flutter, and 20% had chronic renal insufficiency (creatinine 2.0 or end‐stage renal disease). Most patients received ‐adrenergic blockers (97%), statins (95%), and either angiotensin‐converting enzyme (ACE) inhibitors or angiotensin receptor blockers (77%) preoperatively. Patients underwent a variety of surgeries (Table 1).
| Characteristic | n (%) unless otherwise noted |
|---|---|
| |
| Demographics | |
| Age (years), median (IQR) | 71 (6476) |
| Male | 75 (65.7%) |
| White | 88 (77.2%) |
| Comorbid illnesses | |
| Diabetes mellitus | 47 (41.2%) |
| History of prior myocardial infarction | 48 (42.1%) |
| Hypertension | 108 (94.7%) |
| History of stroke or transient cerebral ischemia | 15 (13.2%) |
| Dyslipidemia or treatment with lipid‐lowering drugs | 106 (93.0%) |
| Ejection fraction < 45% | 14 (12.3%) |
| History of coronary artery bypass | 39 (34.2%) |
| Atrial fibrillation or flutter | 19 (16.7%) |
| End‐stage renal disease on dialysis | 13 (11.4%) |
| Chronic renal impairment (creatinine 2.0) without dialysis | 10 (8.8%) |
| Other medical treatments | |
| Angiotensin converting enzyme inhibitor or angiotensin receptor blocker | 88 (77.2%) |
| ‐blocker | 111 (97.3%) |
| Statin | 108 (94.7%) |
| Invasiveness of surgery* | |
| Category 1 (lowest risk) | 37 (32.5%) |
| Category 2 | 22 (19.3%) |
| Category 3 | 48 (42.1%) |
| Category 4 | 7 (6.1%) |
| Category 5 (highest risk) | 0 (0%) |
| Outpatient or short‐stay surgery | 50 (47.2%) |
| Type of surgery | |
| Major orthopedic | 39 (34.2%) |
| Minor orthopedic | 5 (4.4%) |
| Ophthalmologic | 30 (26.3%) |
| General abdominal | 8 (7.0%) |
| Gynecological | 5 (4.4%) |
| Urological | 11 (9.6%) |
| Head and neck | 5 (4.4%) |
| Vascular | 1 (0.9%) |
| Other | 10 (8.8%) |
Patients had received both paclitaxel and sirolimus stents (28% and 73% of patients, respectively); 33% of patients had had more than 1 DES (Table 2). Most patients underwent surgery within 1 year of stent placement (77%), but only 40% had surgery within 180 days of stenting and only 13% within 90 days of stenting. Most patients (77%) had antiplatelet therapy completely discontinued a median of 10 days before surgery and remained off antiplatelet therapy for a median of 14 days total. Ten of the 15 patients (67%) who underwent surgery within 90 days of stenting had all antiplatelet agents discontinued preoperatively, 24 of the 30 patients (80%) who had surgery between 91 and 180 days after stenting had antiplatelet therapy completely discontinued, and 54 of the 69 patients (78%) who had surgery more than 180 days after stenting had antiplatelet therapy completely discontinued. There was no significant relationship between timing of stent placement relative to surgery (<90, 91180, or >180 days) and decision about whether to discontinue antiplatelet therapy (P = .59). However, invasiveness of the surgery was associated with antiplatelet management: 85% of those who continued antiplatelet therapy (aspirin or aspirin and clopidogrel) during the perioperative period were patients who underwent minimally invasive surgery (P < .0001).
| Characteristic | n (%) unless otherwise noted |
|---|---|
| |
| Timing of surgery and antiplatelet agent discontinuation relative to Percutaneous coronary intervention | |
| Duration of most recent intervention relative to surgery (days), median (IQR) | 236 (125354) |
| Surgery within 90 days of DES placement | 15 (13.2%) |
| Surgery within 180 days of DES placement | 45 (39.5%) |
| Surgery within 1 year of DES placement | 88 (77.2%) |
| Percutaneous Coronary Intervention History | |
| Number of drug‐eluting stents | |
| 1 | 76 (66.7%) |
| 2 | 26 (22.8%) |
| 3+ | 12 (10.5%) |
| Paclitaxel stent 1 | 32 (28.1%) |
| Sirolimus stent 1 | 83 (72.8%) |
| Bare‐metal stent 1 | 10 (8.8%) |
| Perioperative antiplatelet treatment | |
| Clopidogrel and aspirin continued through surgery | 24 (21.1%) |
| Aspirin alone continued through surgery | 2 (1.8%) |
| Clopidogrel alone continued through surgery | 0 (0%) |
| No antiplatelet treatment at time of surgery | 88 (77.2%) |
| Among the 15 patients who had surgery within 90 days of stenting | 10 (66.7%) |
| Among the 45 patients who had surgery within 180 days of stenting | 34 (75.6%) |
| Duration of discontinuance of aspirin | |
| Median number of days discontinued preoperatively (IQR) | 10 (812) |
| Median total duration of discontinuance [days, IQR) | 14 (1019) |
| Duration of discontinuance of clopidogrel | |
| Median number of days discontinued preoperatively [days, IQR] | 10 (813) |
| Median number of days discontinued in total (IQR) | 14 (1020) |
The outcome events are presented in Table 3. Two patients (1.8%, 95% CI 0.5%6.2%) suffered a non‐ST‐elevation myocardial infarction (NSTEMI) postoperatively, and another patient (0.9%, 95% CI 0.2%4.8%) developed major bleeding, a retroperitoneal hemorrhage following kidney transplantation. This patient had been taking both aspirin and clopidogrel until 7 days prior to surgery and began to hemorrhage the day after surgery; antiplatelet agents were resumed 12 days postoperatively. No patients died (0%, 95% CI 0%3.3%). One of the 2 patients who suffered an MI was a 72‐year‐old man who had had placement of a single sirolimus‐eluting stent in the posterior descending artery 284 days prior to elective hip arthroplasty. He had no history of myocardial infarction but had undergone coronary bypass surgery 4 years earlier. Echocardiography showed he had aortic stenosis, with a calculated valve area of 0.9 cm2. He had a baseline left ventricular ejection fraction of 45%. His preoperative cardiac medications included lovastatin, lisinopril, and atenolol; he discontinued both aspirin and clopidogrel 7 days before the surgery. His NSTEMI occurred on the day of his operation, presenting with hypotension and anterolateral ST depressions. His troponin T peaked at 0.48 mg/mL, with a peak creatinine kinase of 795 U/L (MB fraction 6%). His left ventricular ejection fraction was 45% on postoperative day 2 (unchanged from baseline). He was discharged on postoperative day 8 and returned for catheterization 3 weeks later, at which time he was found to have a 70% ostial lesion in a saphenous vein graft to an obtuse marginal, which was stented. The previously placed DES was widely patent. The other patient who suffered a postoperative NSTEMI was a 68‐year‐old man with a history of carotid artery stenting and renal artery stenosis who had undergone placement of 3 sirolimus‐eluting stents in the right coronary artery 50 days prior to cervical laminectomy. He had had elective placement of the stents following a positive pharmacologic stress test. He was taking 50 mg of atenolol daily and had been taking aspirin and clopidogrel until 17 days before surgery. On postoperative day 3 he developed dyspnea, and leads V4 and V5 showed ST depressions. His troponin T peaked at 1.24 mg/mL, with a peak creatinine kinase of 879 U/L (MB fraction 6%). The patient underwent left‐heart catheterization on hospital day 10. All 3 DESs were widely patent. His left ventricular ejection fraction was estimated at 65%. He was discharged on postoperative day 15. Because neither of the patients who had a postoperative NSTEMI showed evidence of stent thrombosis on catheterization, the overall rate of stent thrombosis was 0% (95% CI 0%3.3%).
| Outcome | Entire cohort (n = 114) [all antiplatelet therapy stopped in 88 patients (77%)] | Surgery < 90 days after DES (n = 15) [all antiplatelet therapy stopped in 10 patients (67%)] | Surgery < 180 days after DES (n = 45) [all antiplatelet therapy stopped in 34 patients (76%)] |
|---|---|---|---|
| |||
| Death | 0 (0%, 0%3.3%) | 0 (0%, 0%20.4%) | 0 (0%, 0%7.9%) |
| Any myocardial infarction | 2 (1.8%, 0.5%6.2%) | 1 (6.7%, 1.2%29.8%) | 1 (2.2%, 0.4%11.6%) |
| DES thrombosis | 0 (0%, 0%3.3%) | 0 (0%, 0%20.4%) | 0 (0%, 0%7.9%) |
| Major bleeding | 1 (0.9%, 0.2%4.8%) | 0 (0%, 0%20.4%) | 0 (0%, 0%7.9%) |
DISCUSSION
Although 2 patients in our study cohort suffered a postoperative myocardial infarction and underwent postoperative catheterization, neither was found to have stent thrombosis, and the MIs of both patients were NSTEMIs with modest cardiac enzyme elevations only. No patients died. A rate of myocardial infarction of less than 2% is well within that expected for patients with established coronary disease undergoing noncardiac surgery.19 That most of our patients discontinued both aspirin and clopidogrel and did not receive antiplatelet agents for a median of 14 days suggests that transient termination of antiplatelet agents in the perioperative setting is not associated with high morbidity or mortality in patients with DES, even when patients have had their stents implanted in the preceding 36 months.
Our study builds on the limited data on this topic. One small case series examined outcomes in 38 patients who had had DES placement and subsequently underwent noncardiac surgery a median of 297 days after stenting.20 None of the patients in this series suffered from stent thrombosis or myocardial infarction, but most underwent surgery without discontinuing aspirin, and 41% underwent surgery without discontinuing clopidogrel. Another recent study demonstrated a high rate of adverse cardiovascular events in patients with coronary stents who underwent noncardiac surgery up to a year after stenting, but the authors of this study did not differentiate between drug‐eluting and bare‐metal stents, and all patients were continued on antiplatelet agents and received parenteral antithrombotic treatment.21
The major strength of our study was its systematic approach. Using a computerized and comprehensive search strategy, we identified all patients who had undergone DES placement at the Cleveland Clinic who subsequently had a preoperative evaluation at the IMPACT Center. Therefore, we are confident that the number of patients in our cohort truly reflects a well‐defined at‐risk population, allowing for an accurate calculation of event rates. This approach contrasts sharply with prior case reports and case series, in which the number of patients at risk was unknown. Nevertheless, these previous reports demonstrate that DES thromboses do occur and can be devastating, so even a small risk of DES thrombosis should be taken seriously. The upper bound of the 95% confidence interval of our estimate of the rate of DES thrombosis was 3.3%, so it is entirely plausible that sampling error contributed to the low rate of thrombosis that we observed.
One major limitation of our study is its sample size. Although our cohort was more than 3 times larger than the only other published cohort of DES patients undergoing noncardiac surgery,20 we had only limited precision to quantify the risk of DES thrombosis. This limitation is particularly relevant for patients who have undergone stent implantation within 36 months of surgery, as they are the patients most likely to have incomplete reendothelialization of the stented artery. We believe that when possible it remains prudent to delay noncardiac surgery for at least 36 months and perhaps up to 12 months following DES implantation, in keeping with recent guidelines.7, 8 However, for patients with conditions such as cancer whose surgery is semielective or patients with nonsurgical bleeding problems (such as gastrointestinal bleeding), our study provides at least some reassurance that short‐term discontinuation of antiplatelet agents may not be as dangerous as some authors have suggested,1 even within 36 months of DES placement. Another important limitation of our study is potential referral bias. At the Cleveland Clinic, most patients undergoing vascular and thoracic procedures are not evaluated at the IMPACT Center. Similarly, some of the patients with severe cardiovascular disease may also have bypassed the IMPACT Center and gone to a cardiologist for preoperative evaluation. As such, we believe our findings should not be generalized to high‐risk cardiac patients or to those undergoing high‐risk procedures.
A noteworthy distinction between our cohort and the cohort reported by Compton and colleagues is that in the perioperative period, most of our patients underwent complete discontinuation of antiplatelet therapy and remained off both aspirin and clopidogrel for an average of 2 weeks, whereas most patients in the other cohort were continued on antiplatelet therapy.20 This highlights the continued controversy surrounding management of antiplatelet therapy in perioperative patients with established coronary disease, who are at substantial risk for both bleeding and myocardial infarction because of the surgery.22 Our data offer little guidance on the optimal management of antiplatelet agents perioperatively because the incidence of both bleeding and thrombosis was low and whether or not patients were continued on antiplatelet agents was not random. We advocate individualized management strategies of perioperative patients with DES. Patients undergoing procedures that carry a high risk of outcome‐affecting bleeding (such as brain surgery) should probably have their antiplatelet agents discontinued preoperatively, whereas those undergoing minor surgery may have their antiplatelet agents continued, provided the surgeon and the anesthesiologist are in agreement with this approach. The timing of DES placement should also be factored into this decision because recently placed stents carry a higher risk of thrombosis.
In summary, our findings clarify the risks of stent thrombosis and postoperative myocardial infarction in clinically stable patients with DES who undergo low‐ and intermediate‐risk noncardiac surgery. Because it is unlikely to ever be ethically appropriate or logistically feasible to conduct a randomized study of patients with DES having early versus delayed noncardiac surgery, observational cohorts will have to suffice. Additional similar studies will help to validate (or refute) our findings and to more precisely quantify the risk of adverse cardiac events when patients with DES undergo surgery, which is real, feared, and potentially catastrophic but may be overestimated.
There are currently limited data to guide perioperative management of antiplatelet therapy after drug‐eluting stent (DES) implantation. The clinician must balance the risk of excessive bleeding if antiplatelet agents are continued perioperatively with the risk of stent thrombosis if antiplatelet agents are discontinued for surgerya risk that may be amplified in the perioperative period because of the prothrombotic state that accompanies the stress of surgery.
Paclitaxel‐ and sirolimus‐eluting stents have supplanted bare‐metal stents as first‐line treatment for coronary stenosis because of their efficacy in preventing in‐stent restenosis by inhibiting neointimal proliferation. However, the antiproliferative effects of DESs may also delay endothelialization, rendering them vulnerable to stent thrombosis when antiplatelet therapy is prematurely discontinued.15 Some patients with DESs may be vulnerable to stent thrombosis when antiplatelet therapy is discontinued even after a year or more of treatment.6 Although stent thrombosis is uncommon, it is deadly, with a mortality rate approaching 50%.1 Generally, antiplatelet therapy is discontinued prior to surgery. This presents a clinical dilemma for patients with DES because guidelines recommend lifelong aspirin therapy and at least 36 months of clopidogrel for patients who have undergone DES placement.79
In the bare‐metal stent era, studies demonstrated an alarming risk of stent thrombosis in the setting of noncardiac surgery within 26 weeks of stent placement.10, 11 However, the appropriate interval before elective noncardiac surgery following DES placement has not been defined and may be longer. Case reports and case series have highlighted this risk12 and have even suggested that a DES may be susceptible to stent thrombosis as long as a year after its placement.6 More recently, pooled data from controlled trials have suggested that although the overall rate of DES thrombosis may not be consistently higher than that of bare‐metal stents, the risk appears to persist far longer (probably from delayed endothelialization of the target vessel) and may be more pronounced following discontinuation of antiplatelet agents.9, 1316 This has led to recent recommendations to continue dual antiplatelet therapy (with aspirin and clopidogrel) for at least a year following DES placement and possibly indefinitely, provided that the therapy is tolerated.9 Whether this risk is accentuated in the perioperative setting independent of discontinuation of antiplatelet therapy remains unknown. In 1 registry, the strongest predictor of DES thrombosis was premature discontinuation of antiplatelet therapy (hazard ratio 90, 95% confidence interval 30270, P < .001), and noncardiac surgery was the most frequent reason for discontinuation of antiplatelet therapy.1 However, the actual incidence of stent thrombosis in patients undergoing surgery was unavailable because the denominator was unknown (ie, number of patients with stents who underwent surgery). Although it is certainly plausible that the prothrombotic and proinflammatory postoperative state augments the risk of stent thrombosis independent of discontinuation of antiplatelet therapy alone, this remains unproven.
At the time of the present study, protocol‐based clinical practice at the Cleveland Clinic Foundation's Internal Medicine Preoperative Assessment Consultation and Treatment (IMPACT) Center included routine discontinuation of all antiplatelet agents (including aspirin and clopidogrel) at least 7 days prior to noncardiac surgery, including in patients with coronary stents. Exceptions to this policy were generally made only for very minor procedures. The purpose of this study was to systematically quantify the risk of adverse cardiovascular events in patients who had DES placement and subsequently underwent elective or semielective noncardiac surgery, most of whom had discontinued all antiplatelet agents at least 7 days before surgery.
Methods
We identified all patients who had DES placement at the Cleveland Clinic who subsequently underwent preoperative evaluation for noncardiac surgery at the IMPACT Center between July 2003 and July 2005. About half the patients undergoing surgery at the Cleveland Clinic were seen in the IMPACT Center prior to surgery during the study period. Preoperative evaluation at the IMPACT Center included a standardized assessment by a hospitalist with expertise in preoperative medicine. Clinical data for each patient were contemporaneously entered into an electronic medical record. Written preoperative medication instructions were provided to each patient and documented in the electronic record, indicating specific instructions to discontinue any antiplatelet agents 710 days preoperatively.
The IMPACT Center database was crosslinked to the Cleveland Clinic Foundation Heart Center Database, which contains records of all patients who have undergone coronary stenting at the Cleveland Clinic. Computerized and written medical records of all patients in both databases were reviewed using a standardized data collection instrument. All medical data generated up to 30 days postoperatively at the Cleveland Clinic were reviewed. Social Security numbers were linked with the Social Security Death Index to verify that no patients died within 30 days of surgery.
Predefined outcomes included catheterization‐confirmed DES thrombosis, any myocardial infarction, and major bleeding within 30 days of the surgical procedure. Myocardial infarction was defined as elevation of troponin T to more than twice the upper limit of normal (0.2 mg/mL) with or without associated electrocardiographic changes or symptoms. This biochemically based definition was used with the understanding that cardiac enzyme tests are consistently ordered for patients at the Cleveland Clinic with suspected coronary events and that postoperative myocardial infarction may be atypical in presentation (eg, delirium or hypotension without chest pain). Stent thrombosis was considered present if confirmed by catheterization or autopsy and considered possible if a patient suffered from a myocardial infarction but did not have a definitive diagnostic procedure performed. DES thrombosis was considered absent if a patient underwent postoperative catheterization and the DES appeared patent. Major bleeding was defined as any bleeding requiring unplanned reoperation or bleeding in a critical location (intracranial or retroperitoneal). Invasiveness of surgery was defined prospectively according to a Cleveland Clinic bleeding classification scheme based on that of Pasternak17, 18:
Category 1. Minimal risk to patient; little or no anticipated blood loss (eg, breast biopsy, cystoscopy).
Category 2. Mild risk to patient; minimal to moderately invasive procedure; estimated blood loss < 500 cc (eg, laparoscopy, arthroscopy, hernia repair).
Category 3. Moderate risk to patient and moderate to significantly invasive; blood loss potential 5001000 cc (eg, laminectomy, total hip or knee replacement).
Category 4. Major risk to patient; highly invasive procedure; anticipated blood loss > 1500 cc (eg, major spinal reconstruction, major reconstruction of GI tract, major vascular repair without intensive care unit stay).
Category 5. Critical risk to patient; highly invasive procedure; anticipated blood loss > 1500 cc with anticipated postoperative intensive care unit stay (eg, cardiac procedure, major vascular repair with anticipated intensive care unit stay).
Statistical analyses were descriptive. We determined the rate of adverse outcomes with 95% confidence intervals (CIs) in the entire patient cohort and among prespecified patient subsets, based on timing of discontinuation of antiplatelet therapy. Predefined subsets included those who had clopidogrel and aspirin discontinued less than 3 months and less than 6 months following DES implantation. The 2 test was used to test the hypothesis that discontinuation of antiplatelet therapy was a function of the type of surgery or timing of stent placement.
The study was approved by the Cleveland Clinic Foundation's institutional review board. The requirement for informed consent was waived.
RESULTS
In total, 114 patients were evaluated in the IMPACT Center following DES placement. Baseline patient characteristics are shown in Table 1. The median age was 71 years (interquartile range 6476 years), and 66% were male. Patients had a moderate degree of comorbidity: 41% had diabetes, 12% had an ejection fraction < 45%, 34% had undergone coronary bypass, 17% had atrial fibrillation or flutter, and 20% had chronic renal insufficiency (creatinine 2.0 or end‐stage renal disease). Most patients received ‐adrenergic blockers (97%), statins (95%), and either angiotensin‐converting enzyme (ACE) inhibitors or angiotensin receptor blockers (77%) preoperatively. Patients underwent a variety of surgeries (Table 1).
| Characteristic | n (%) unless otherwise noted |
|---|---|
| |
| Demographics | |
| Age (years), median (IQR) | 71 (6476) |
| Male | 75 (65.7%) |
| White | 88 (77.2%) |
| Comorbid illnesses | |
| Diabetes mellitus | 47 (41.2%) |
| History of prior myocardial infarction | 48 (42.1%) |
| Hypertension | 108 (94.7%) |
| History of stroke or transient cerebral ischemia | 15 (13.2%) |
| Dyslipidemia or treatment with lipid‐lowering drugs | 106 (93.0%) |
| Ejection fraction < 45% | 14 (12.3%) |
| History of coronary artery bypass | 39 (34.2%) |
| Atrial fibrillation or flutter | 19 (16.7%) |
| End‐stage renal disease on dialysis | 13 (11.4%) |
| Chronic renal impairment (creatinine 2.0) without dialysis | 10 (8.8%) |
| Other medical treatments | |
| Angiotensin converting enzyme inhibitor or angiotensin receptor blocker | 88 (77.2%) |
| ‐blocker | 111 (97.3%) |
| Statin | 108 (94.7%) |
| Invasiveness of surgery* | |
| Category 1 (lowest risk) | 37 (32.5%) |
| Category 2 | 22 (19.3%) |
| Category 3 | 48 (42.1%) |
| Category 4 | 7 (6.1%) |
| Category 5 (highest risk) | 0 (0%) |
| Outpatient or short‐stay surgery | 50 (47.2%) |
| Type of surgery | |
| Major orthopedic | 39 (34.2%) |
| Minor orthopedic | 5 (4.4%) |
| Ophthalmologic | 30 (26.3%) |
| General abdominal | 8 (7.0%) |
| Gynecological | 5 (4.4%) |
| Urological | 11 (9.6%) |
| Head and neck | 5 (4.4%) |
| Vascular | 1 (0.9%) |
| Other | 10 (8.8%) |
Patients had received both paclitaxel and sirolimus stents (28% and 73% of patients, respectively); 33% of patients had had more than 1 DES (Table 2). Most patients underwent surgery within 1 year of stent placement (77%), but only 40% had surgery within 180 days of stenting and only 13% within 90 days of stenting. Most patients (77%) had antiplatelet therapy completely discontinued a median of 10 days before surgery and remained off antiplatelet therapy for a median of 14 days total. Ten of the 15 patients (67%) who underwent surgery within 90 days of stenting had all antiplatelet agents discontinued preoperatively, 24 of the 30 patients (80%) who had surgery between 91 and 180 days after stenting had antiplatelet therapy completely discontinued, and 54 of the 69 patients (78%) who had surgery more than 180 days after stenting had antiplatelet therapy completely discontinued. There was no significant relationship between timing of stent placement relative to surgery (<90, 91180, or >180 days) and decision about whether to discontinue antiplatelet therapy (P = .59). However, invasiveness of the surgery was associated with antiplatelet management: 85% of those who continued antiplatelet therapy (aspirin or aspirin and clopidogrel) during the perioperative period were patients who underwent minimally invasive surgery (P < .0001).
| Characteristic | n (%) unless otherwise noted |
|---|---|
| |
| Timing of surgery and antiplatelet agent discontinuation relative to Percutaneous coronary intervention | |
| Duration of most recent intervention relative to surgery (days), median (IQR) | 236 (125354) |
| Surgery within 90 days of DES placement | 15 (13.2%) |
| Surgery within 180 days of DES placement | 45 (39.5%) |
| Surgery within 1 year of DES placement | 88 (77.2%) |
| Percutaneous Coronary Intervention History | |
| Number of drug‐eluting stents | |
| 1 | 76 (66.7%) |
| 2 | 26 (22.8%) |
| 3+ | 12 (10.5%) |
| Paclitaxel stent 1 | 32 (28.1%) |
| Sirolimus stent 1 | 83 (72.8%) |
| Bare‐metal stent 1 | 10 (8.8%) |
| Perioperative antiplatelet treatment | |
| Clopidogrel and aspirin continued through surgery | 24 (21.1%) |
| Aspirin alone continued through surgery | 2 (1.8%) |
| Clopidogrel alone continued through surgery | 0 (0%) |
| No antiplatelet treatment at time of surgery | 88 (77.2%) |
| Among the 15 patients who had surgery within 90 days of stenting | 10 (66.7%) |
| Among the 45 patients who had surgery within 180 days of stenting | 34 (75.6%) |
| Duration of discontinuance of aspirin | |
| Median number of days discontinued preoperatively (IQR) | 10 (812) |
| Median total duration of discontinuance [days, IQR) | 14 (1019) |
| Duration of discontinuance of clopidogrel | |
| Median number of days discontinued preoperatively [days, IQR] | 10 (813) |
| Median number of days discontinued in total (IQR) | 14 (1020) |
The outcome events are presented in Table 3. Two patients (1.8%, 95% CI 0.5%6.2%) suffered a non‐ST‐elevation myocardial infarction (NSTEMI) postoperatively, and another patient (0.9%, 95% CI 0.2%4.8%) developed major bleeding, a retroperitoneal hemorrhage following kidney transplantation. This patient had been taking both aspirin and clopidogrel until 7 days prior to surgery and began to hemorrhage the day after surgery; antiplatelet agents were resumed 12 days postoperatively. No patients died (0%, 95% CI 0%3.3%). One of the 2 patients who suffered an MI was a 72‐year‐old man who had had placement of a single sirolimus‐eluting stent in the posterior descending artery 284 days prior to elective hip arthroplasty. He had no history of myocardial infarction but had undergone coronary bypass surgery 4 years earlier. Echocardiography showed he had aortic stenosis, with a calculated valve area of 0.9 cm2. He had a baseline left ventricular ejection fraction of 45%. His preoperative cardiac medications included lovastatin, lisinopril, and atenolol; he discontinued both aspirin and clopidogrel 7 days before the surgery. His NSTEMI occurred on the day of his operation, presenting with hypotension and anterolateral ST depressions. His troponin T peaked at 0.48 mg/mL, with a peak creatinine kinase of 795 U/L (MB fraction 6%). His left ventricular ejection fraction was 45% on postoperative day 2 (unchanged from baseline). He was discharged on postoperative day 8 and returned for catheterization 3 weeks later, at which time he was found to have a 70% ostial lesion in a saphenous vein graft to an obtuse marginal, which was stented. The previously placed DES was widely patent. The other patient who suffered a postoperative NSTEMI was a 68‐year‐old man with a history of carotid artery stenting and renal artery stenosis who had undergone placement of 3 sirolimus‐eluting stents in the right coronary artery 50 days prior to cervical laminectomy. He had had elective placement of the stents following a positive pharmacologic stress test. He was taking 50 mg of atenolol daily and had been taking aspirin and clopidogrel until 17 days before surgery. On postoperative day 3 he developed dyspnea, and leads V4 and V5 showed ST depressions. His troponin T peaked at 1.24 mg/mL, with a peak creatinine kinase of 879 U/L (MB fraction 6%). The patient underwent left‐heart catheterization on hospital day 10. All 3 DESs were widely patent. His left ventricular ejection fraction was estimated at 65%. He was discharged on postoperative day 15. Because neither of the patients who had a postoperative NSTEMI showed evidence of stent thrombosis on catheterization, the overall rate of stent thrombosis was 0% (95% CI 0%3.3%).
| Outcome | Entire cohort (n = 114) [all antiplatelet therapy stopped in 88 patients (77%)] | Surgery < 90 days after DES (n = 15) [all antiplatelet therapy stopped in 10 patients (67%)] | Surgery < 180 days after DES (n = 45) [all antiplatelet therapy stopped in 34 patients (76%)] |
|---|---|---|---|
| |||
| Death | 0 (0%, 0%3.3%) | 0 (0%, 0%20.4%) | 0 (0%, 0%7.9%) |
| Any myocardial infarction | 2 (1.8%, 0.5%6.2%) | 1 (6.7%, 1.2%29.8%) | 1 (2.2%, 0.4%11.6%) |
| DES thrombosis | 0 (0%, 0%3.3%) | 0 (0%, 0%20.4%) | 0 (0%, 0%7.9%) |
| Major bleeding | 1 (0.9%, 0.2%4.8%) | 0 (0%, 0%20.4%) | 0 (0%, 0%7.9%) |
DISCUSSION
Although 2 patients in our study cohort suffered a postoperative myocardial infarction and underwent postoperative catheterization, neither was found to have stent thrombosis, and the MIs of both patients were NSTEMIs with modest cardiac enzyme elevations only. No patients died. A rate of myocardial infarction of less than 2% is well within that expected for patients with established coronary disease undergoing noncardiac surgery.19 That most of our patients discontinued both aspirin and clopidogrel and did not receive antiplatelet agents for a median of 14 days suggests that transient termination of antiplatelet agents in the perioperative setting is not associated with high morbidity or mortality in patients with DES, even when patients have had their stents implanted in the preceding 36 months.
Our study builds on the limited data on this topic. One small case series examined outcomes in 38 patients who had had DES placement and subsequently underwent noncardiac surgery a median of 297 days after stenting.20 None of the patients in this series suffered from stent thrombosis or myocardial infarction, but most underwent surgery without discontinuing aspirin, and 41% underwent surgery without discontinuing clopidogrel. Another recent study demonstrated a high rate of adverse cardiovascular events in patients with coronary stents who underwent noncardiac surgery up to a year after stenting, but the authors of this study did not differentiate between drug‐eluting and bare‐metal stents, and all patients were continued on antiplatelet agents and received parenteral antithrombotic treatment.21
The major strength of our study was its systematic approach. Using a computerized and comprehensive search strategy, we identified all patients who had undergone DES placement at the Cleveland Clinic who subsequently had a preoperative evaluation at the IMPACT Center. Therefore, we are confident that the number of patients in our cohort truly reflects a well‐defined at‐risk population, allowing for an accurate calculation of event rates. This approach contrasts sharply with prior case reports and case series, in which the number of patients at risk was unknown. Nevertheless, these previous reports demonstrate that DES thromboses do occur and can be devastating, so even a small risk of DES thrombosis should be taken seriously. The upper bound of the 95% confidence interval of our estimate of the rate of DES thrombosis was 3.3%, so it is entirely plausible that sampling error contributed to the low rate of thrombosis that we observed.
One major limitation of our study is its sample size. Although our cohort was more than 3 times larger than the only other published cohort of DES patients undergoing noncardiac surgery,20 we had only limited precision to quantify the risk of DES thrombosis. This limitation is particularly relevant for patients who have undergone stent implantation within 36 months of surgery, as they are the patients most likely to have incomplete reendothelialization of the stented artery. We believe that when possible it remains prudent to delay noncardiac surgery for at least 36 months and perhaps up to 12 months following DES implantation, in keeping with recent guidelines.7, 8 However, for patients with conditions such as cancer whose surgery is semielective or patients with nonsurgical bleeding problems (such as gastrointestinal bleeding), our study provides at least some reassurance that short‐term discontinuation of antiplatelet agents may not be as dangerous as some authors have suggested,1 even within 36 months of DES placement. Another important limitation of our study is potential referral bias. At the Cleveland Clinic, most patients undergoing vascular and thoracic procedures are not evaluated at the IMPACT Center. Similarly, some of the patients with severe cardiovascular disease may also have bypassed the IMPACT Center and gone to a cardiologist for preoperative evaluation. As such, we believe our findings should not be generalized to high‐risk cardiac patients or to those undergoing high‐risk procedures.
A noteworthy distinction between our cohort and the cohort reported by Compton and colleagues is that in the perioperative period, most of our patients underwent complete discontinuation of antiplatelet therapy and remained off both aspirin and clopidogrel for an average of 2 weeks, whereas most patients in the other cohort were continued on antiplatelet therapy.20 This highlights the continued controversy surrounding management of antiplatelet therapy in perioperative patients with established coronary disease, who are at substantial risk for both bleeding and myocardial infarction because of the surgery.22 Our data offer little guidance on the optimal management of antiplatelet agents perioperatively because the incidence of both bleeding and thrombosis was low and whether or not patients were continued on antiplatelet agents was not random. We advocate individualized management strategies of perioperative patients with DES. Patients undergoing procedures that carry a high risk of outcome‐affecting bleeding (such as brain surgery) should probably have their antiplatelet agents discontinued preoperatively, whereas those undergoing minor surgery may have their antiplatelet agents continued, provided the surgeon and the anesthesiologist are in agreement with this approach. The timing of DES placement should also be factored into this decision because recently placed stents carry a higher risk of thrombosis.
In summary, our findings clarify the risks of stent thrombosis and postoperative myocardial infarction in clinically stable patients with DES who undergo low‐ and intermediate‐risk noncardiac surgery. Because it is unlikely to ever be ethically appropriate or logistically feasible to conduct a randomized study of patients with DES having early versus delayed noncardiac surgery, observational cohorts will have to suffice. Additional similar studies will help to validate (or refute) our findings and to more precisely quantify the risk of adverse cardiac events when patients with DES undergo surgery, which is real, feared, and potentially catastrophic but may be overestimated.
- ,,, et al.Incidence, predictors, and outcome of thrombosis after successful implantation of drug‐eluting stents.JAMA.2005;293:2126–230.
- ,,, et al.Pathology of drug‐eluting stents in humans: delayed healing and late thrombotic risk.J Am Coll Cardiol.2006;48:193–202.
- ,,, et al.Correlates and long‐term outcomes of angiographically proven stent thrombosis with sirolimus‐ and paclitaxel‐eluting stents.Circulation.2006;113:1108–1113.
- ,,, et al.Prevalence, predictors, and outcomes of premature discontinuation of thienopyridine therapy after drug‐eluting stent placement: results from the PREMIER registry.Circulation.2006;113:2803–2809.
- .Trading restenosis for thrombosis? New questions about drug‐eluting stents.N Engl J Med.2006;355:1949–1952.
- ,,, et al.Late thrombosis in drug‐eluting coronary stents after discontinuation of antiplatelet therapy.Lancet2004;364:1519–1521.
- ,,, et al.ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/SCAI Writing Committee to Update 2001 Guidelines for Percutaneous Coronary Intervention).Circulation.2006;113:e166–e286.
- ,,, et al.ACC/AHA guidelines for the management of patients with ST‐elevation myocardial infarction—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1999 Guidelines for the Management of Patients With Acute Myocardial Infarction).Circulation.2004;110:588–636.
- ,,, et al.Prevention of premature discontinuation of dual antiplatelet therapy in patients with coronary artery stents: a science advisory from the American Heart Association, American College of Cardiology, Society for Cardiovascular Angiography and Interventions, American College of Surgeons, and American Dental Association, with representation from the American College of Physicians.Circulation.2007;115:813–818.
- ,,, et al.Clinical outcome of patients undergoing non‐cardiac surgery in the two months following coronary stenting.J Am Coll Cardiol.2003;42:234–240.
- ,,,,.Catastrophic outcomes of noncardiac surgery soon after coronary stenting.J Am Coll Cardiol.2000;35:1288–1294.
- ,.Thrombosis of sirolimus‐eluting coronary stent in the postanesthesia care unit.Anesth Analg.2005;101:971–973.
- ,,,,,.Late thrombosis of drug‐eluting stents: a meta‐analysis of randomized clinical trials.Am J Med.2006;119:1056–1061.
- ,,,,,.Long‐term outcomes with drug‐eluting stents versus bare‐metal stents in Sweden.N Engl J Med.2007;356:1009–1019.
- ,,, et al.Safety and efficacy of sirolimus‐ and paclitaxel‐eluting coronary stents.N Engl J Med.2007;356:998–1008.
- ,,,,.A pooled analysis of data comparing sirolimus‐eluting stents with bare‐metal stents.N Engl J Med.2007;356:989–997.
- .Preoperative assessment: guidelines and challenges.Acta Anaesthesiol ScandSuppl.1997;111:318–320.
- .Preoperative assessment of the ambulatory and same day admission patient.Wellcome Trends Anesthesiol.1991;9:3–11.
- ,,, et al.Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery.Circulation.1999;100:1043–1049.
- ,,,,.Risk of noncardiac surgery after coronary drug‐eluting stent implantation.Am J Cardiol.2006;98:1212–1213.
- ,,,,,.Coronary artery stenting and non‐cardiac surgery—a prospective outcome study.Br J Anaesth.2006;96:686–693.
- ,,.Double jeopardy: balance between bleeding and stent thrombosis with prolonged dual antiplatelet therapy after drug‐eluting stent implantation.Cardiovasc Revasc Med.2006;7:155–158.
- ,,, et al.Incidence, predictors, and outcome of thrombosis after successful implantation of drug‐eluting stents.JAMA.2005;293:2126–230.
- ,,, et al.Pathology of drug‐eluting stents in humans: delayed healing and late thrombotic risk.J Am Coll Cardiol.2006;48:193–202.
- ,,, et al.Correlates and long‐term outcomes of angiographically proven stent thrombosis with sirolimus‐ and paclitaxel‐eluting stents.Circulation.2006;113:1108–1113.
- ,,, et al.Prevalence, predictors, and outcomes of premature discontinuation of thienopyridine therapy after drug‐eluting stent placement: results from the PREMIER registry.Circulation.2006;113:2803–2809.
- .Trading restenosis for thrombosis? New questions about drug‐eluting stents.N Engl J Med.2006;355:1949–1952.
- ,,, et al.Late thrombosis in drug‐eluting coronary stents after discontinuation of antiplatelet therapy.Lancet2004;364:1519–1521.
- ,,, et al.ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/SCAI Writing Committee to Update 2001 Guidelines for Percutaneous Coronary Intervention).Circulation.2006;113:e166–e286.
- ,,, et al.ACC/AHA guidelines for the management of patients with ST‐elevation myocardial infarction—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1999 Guidelines for the Management of Patients With Acute Myocardial Infarction).Circulation.2004;110:588–636.
- ,,, et al.Prevention of premature discontinuation of dual antiplatelet therapy in patients with coronary artery stents: a science advisory from the American Heart Association, American College of Cardiology, Society for Cardiovascular Angiography and Interventions, American College of Surgeons, and American Dental Association, with representation from the American College of Physicians.Circulation.2007;115:813–818.
- ,,, et al.Clinical outcome of patients undergoing non‐cardiac surgery in the two months following coronary stenting.J Am Coll Cardiol.2003;42:234–240.
- ,,,,.Catastrophic outcomes of noncardiac surgery soon after coronary stenting.J Am Coll Cardiol.2000;35:1288–1294.
- ,.Thrombosis of sirolimus‐eluting coronary stent in the postanesthesia care unit.Anesth Analg.2005;101:971–973.
- ,,,,,.Late thrombosis of drug‐eluting stents: a meta‐analysis of randomized clinical trials.Am J Med.2006;119:1056–1061.
- ,,,,,.Long‐term outcomes with drug‐eluting stents versus bare‐metal stents in Sweden.N Engl J Med.2007;356:1009–1019.
- ,,, et al.Safety and efficacy of sirolimus‐ and paclitaxel‐eluting coronary stents.N Engl J Med.2007;356:998–1008.
- ,,,,.A pooled analysis of data comparing sirolimus‐eluting stents with bare‐metal stents.N Engl J Med.2007;356:989–997.
- .Preoperative assessment: guidelines and challenges.Acta Anaesthesiol ScandSuppl.1997;111:318–320.
- .Preoperative assessment of the ambulatory and same day admission patient.Wellcome Trends Anesthesiol.1991;9:3–11.
- ,,, et al.Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery.Circulation.1999;100:1043–1049.
- ,,,,.Risk of noncardiac surgery after coronary drug‐eluting stent implantation.Am J Cardiol.2006;98:1212–1213.
- ,,,,,.Coronary artery stenting and non‐cardiac surgery—a prospective outcome study.Br J Anaesth.2006;96:686–693.
- ,,.Double jeopardy: balance between bleeding and stent thrombosis with prolonged dual antiplatelet therapy after drug‐eluting stent implantation.Cardiovasc Revasc Med.2006;7:155–158.
Copyright © 2007 Society of Hospital Medicine