Article
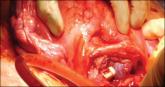
Steps to minimize morbidity from unanticipated placenta accreta spectrum
- Author:
- Daniela Carusi, MD, MSc
- Brett Einerson, MD, MPH
New quality indicators and novel disposable duodenoscopes may meet this need, but several questions regarding these interventions remain...
Article
Reduce the use of perioperative opioids with a multimodal pain management strategy
- Author:
- Michaela Farber, MD, MS
- Daniela Carusi, MD, MSc
- Robert L. Barbieri, MD
Patients undergoing cesarean surgery who were previously naïve to opioid medications have a 0.33% to 2.2% probability of transitioning to the...
Article

Uterus-sparing interventions to treat postpartum hemorrhage during cesarean delivery surgery
- Author:
- Daniela Carusi, MD, MSc
- Robert L. Barbieri, MD
Cesarean delivery is often associated with postpartum hemorrhage. Frequent use of uterine-sparing interventions can help reduce...
