User login
Treatment of Elephantiasic Pretibial Myxedema With Rituximab Therapy
To the Editor:
Pretibial myxedema (PTM) is bilateral, nonpitting, scaly thickening and induration of the skin that most commonly occurs on the anterior aspects of the legs and feet. Pretibial myxedema occurs in approximately 0.5% to 4.3% of patients with hyperthyroidism.1 Thyroid dermopathy often is thought of as the classic nonpitting PTM with skin induration and color change. However, rarer forms of PTM, including plaque, nodular, and elephantiasic, also are important to note.2
Elephantiasic PTM is extremely rare, occurring in less than 1% of patients with PTM.2 Elephantiasic PTM is characterized by the persistent swelling of 1 or both legs; thickening of the skin overlying the dorsum of the feet, ankles, and toes; and verrucous irregular plaques that often are fleshy and flattened. The clinical differential diagnosis of elephantiasic PTM includes elephantiasis nostra verrucosa, a late-stage complication of chronic lymphedema that can be related to a variety of infectious or noninfectious obstructive processes. Few effective therapeutic modalities exist in the treatment of elephantiasic PTM. We present a case of elephantiasic PTM.
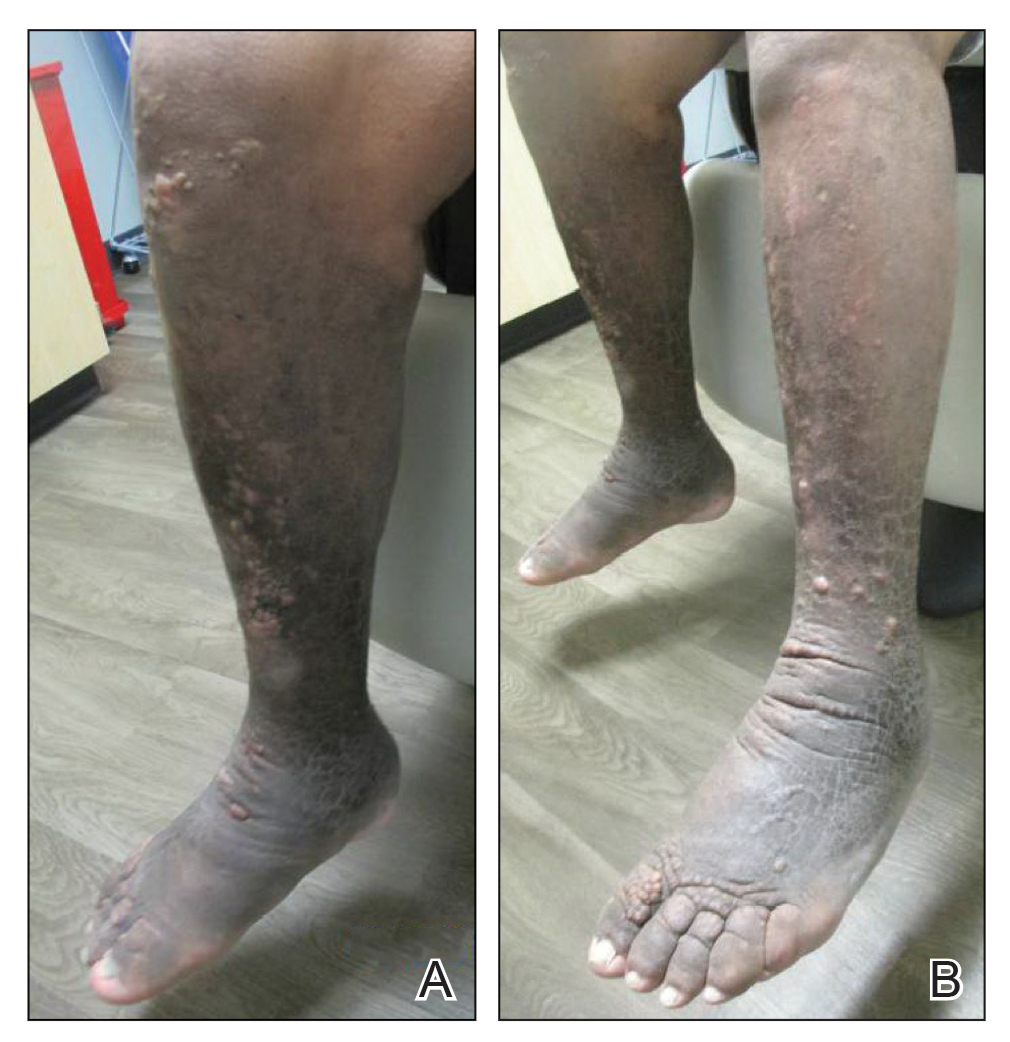
A 59-year-old man presented to dermatology with leonine facies with pronounced glabellar creases and indentations of the earlobes. He had diffuse woody induration, hyperpigmentation, and nonpitting edema of the lower extremities as well as several flesh-colored exophytic nodules scattered throughout the anterior shins and dorsal feet (Figure 1). On the left posterior calf, there was a large, 3-cm, exophytic, firm, flesh-colored nodule. Examination of the hands revealed mild hyperpigmentation of the distal digits, clubbing of the distal phalanges, and cheiroarthropathy.
The patient was diagnosed with Graves disease after experiencing the classic symptoms of hyperthyroidism, including heat intolerance, tremor, palpitations, and anxiety. He received thyroid ablation and subsequently was supplemented with levothyroxine 75 mg daily. Twelve years later, he was diagnosed with Graves ophthalmopathy with ocular proptosis requiring multiple courses of retro-orbital irradiation and surgical procedures for decompression. Approximately 1 year later, he noted increased swelling, firmness, and darkening of the pretibial surfaces. Initially, he was referred to vascular surgery and underwent bilateral saphenous vein ablation. He also was referred to a lymphedema specialist, and workup revealed an unremarkable lymphatic system. Minimal improvement was noted following the saphenous vein ablation, and he subsequently was referred to dermatology for further workup.
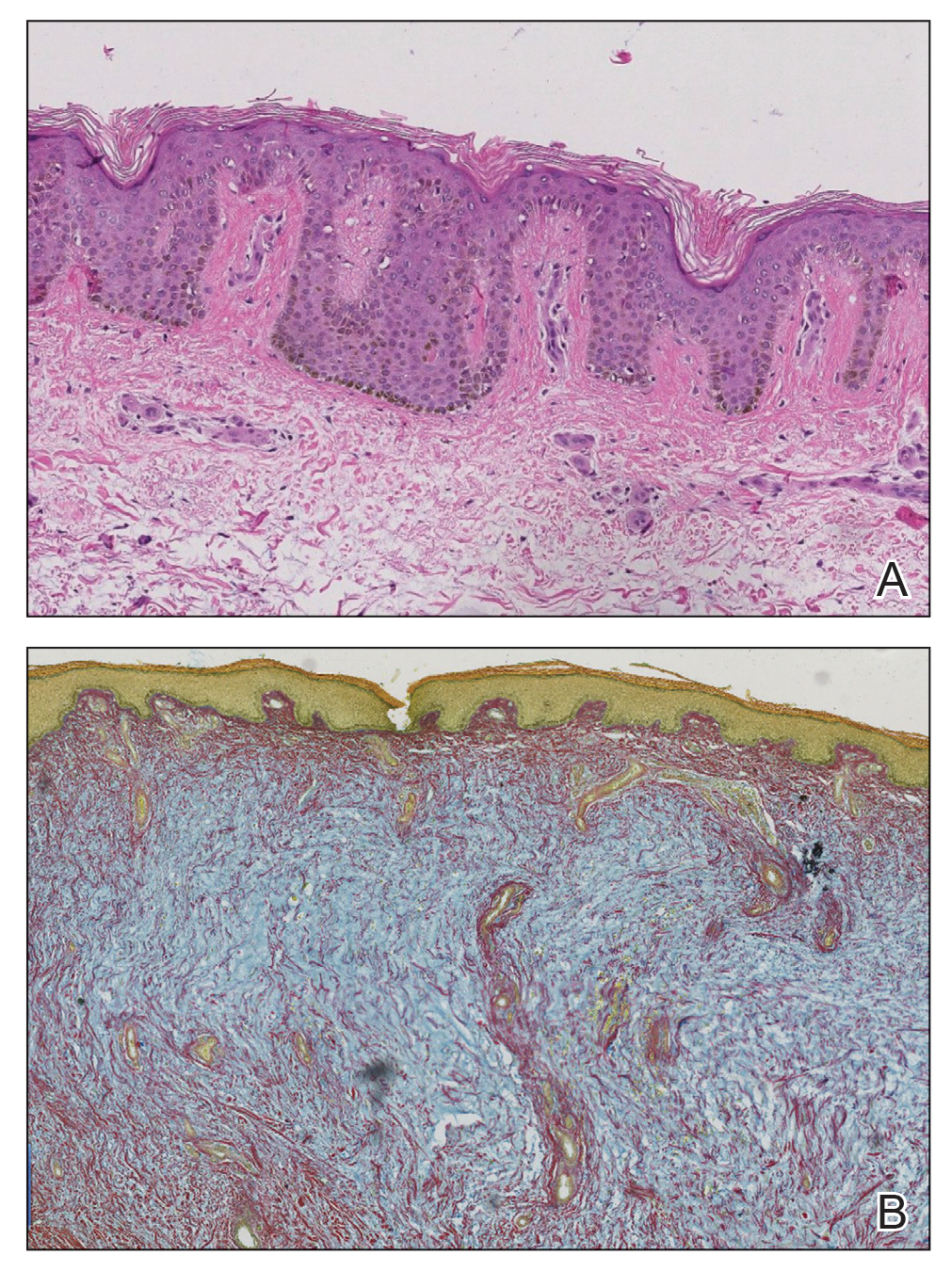
At the current presentation, laboratory analysis revealed a low thyrotropin level (0.03 mIU/L [reference range, 0.4–4.2 mIU/L]), and free thyroxine was within reference range. Radiography of the chest was unremarkable; however, radiography of the hand demonstrated arthrosis of the left fifth proximal interphalangeal joint. Nuclear medicine lymphoscintigraphy and lower extremity ultrasonography were unremarkable. Punch biopsies were performed of the left lateral leg and posterior calf. Hematoxylin and eosin staining demonstrated marked mucin deposition extending to the deep dermis along with deep fibroplasia and was read as consistent with PTM. Colloidal iron highlighted prominent mucin within the dermis (Figure 2).
The patient’s medical history, physical examination, laboratory analysis, imaging, and biopsies were considered, and a diagnosis of elephantiasic PTM was made. Minimal improvement was noted with initial therapeutic interventions including compression therapy and application of super high–potency topical corticosteroids. After further evaluation in our multidisciplinary rheumatology-dermatology clinic, the decision was made to initiate rituximab infusions.
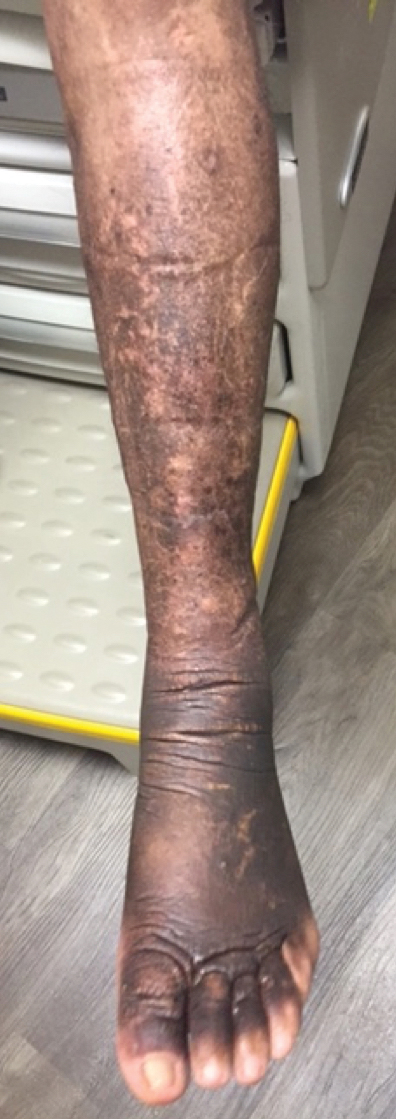
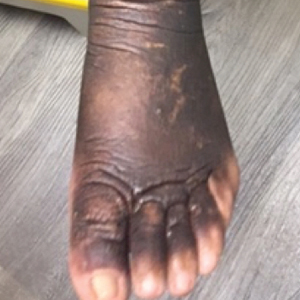
Two months after 1 course of rituximab consisting of two 1000-mg infusions separated by 2 weeks, the patient showed substantial clinical improvement. There was striking improvement of the pretibial surfaces with resolution of the exophytic nodules and improvement of the induration (Figure 3). In addition, there was decreased induration of the glabella and earlobes and decreased fullness of the digital pulp on the hands. The patient also reported subjective improvements in mobility.
Our patient demonstrated all 3 aspects of the Diamond triad: PTM, exophthalmos, and acropachy. Patients present with all 3 features in less than 1% of reported cases of Graves disease.3 Although all 3 features are seen together infrequently, thyroid dermopathy and acropachy often are markers of severe Graves ophthalmopathy. In a study of 114 patients with Graves ophthalmopathy, patients who also had dermopathy and acropachy were more likely to have optic neuropathy or require orbital decompression.4
After overcoming the diagnostic dilemma that the elephantiasic presentation of PTM can present, therapeutic management remains a challenge. Heyes et al5 documented the successful treatment of highly recalcitrant elephantiasic PTM with rituximab and plasmapheresis therapy. In this case, a 44-year-old woman with an 11-year history of Graves disease and elephantiasic PTM received 29 rituximab infusions and 241 plasmapheresis treatments over the course of 3.5 years. Her elephantiasic PTM clinically resolved, and she was able to resume daily activities and wear normal shoes after being nonambulatory for years.5
Rituximab is a monoclonal antibody against CD20, a protein found primarily on the surface of B-cell lymphocytes. Although rituximab initially was approved by the US Food and Drug administration for the treatment of malignant lymphoma, it has had an increasing role in the treatment of autoimmune disorders such as rheumatoid arthritis. Rituximab is postulated to target B lymphocytes and halt their progression to plasma cells. By limiting the population of long-lasting, antibody-producing plasma cells and decreasing the autoantibodies that cause many of the symptoms in Graves disease, rituximab may be an effective therapy to consider in the treatment of elephantiasic PTM.6
Although the exact mechanism is poorly understood, PTM likely is a sequela of hyperthyroidism because of the expression of thyroid-stimulating hormone receptor proteins found on normal dermal fibroblasts. Thyroid-stimulating hormone receptor autoantibodies are thought to stimulate these fibroblasts to produce glycosaminoglycans. Histopathologically, accumulation of glycosaminoglycans deposited in the reticular dermis with high concentrations of hyaluronic acid is observed in PTM.7
Treatment of elephantiasic PTM remains a therapeutic challenge. Given the rarity of the disease process and limited information on effective therapeutic modalities, rituximab should be viewed as a viable treatment option in the management of recalcitrant elephantiasic PTM.
- Schwartz KM, Fatourechi V, Ahmed DDF, et al. Dermopathy of Graves’ disease (pretibial myxedema): long-term outcome. J Clin Endocrinol Metab. 2002;87:438-446.
- Kakati S, Doley B, Pal S, et al. Elephantiasis nostras verrucosa: a rare thyroid dermopathy in Graves’ disease. J Assoc Physicians India. 2005;53:571-572.
- Anderson CK, Miller OF 3rd. Triad of exophthalmos, pretibial myxedema, and acropachy in a patient with Graves’ disease. J Am Acad Dermatol. 2003;48:970-972.
- Fatourechi V, Bartley GB, Eghbali-Fatourechi GZ, et al. Graves’ dermopathy and acropachy are markers of severe Graves’ ophthalmopathy. Thyroid. 2003;13:1141-1144.
- Heyes C, Nolan R, Leahy M, et al. Treatment‐resistant elephantiasic thyroid dermopathy responding to rituximab and plasmapheresis. Australas J Dermatol. 2012;53:E1-E4.
- Salvi M, Vannucchi G, Campi I, et al. Treatment of Graves’ disease and associated ophthalmopathy with the anti-CD20 monoclonal antibody rituximab: an open study. Eur J Endocrinol. 2007;156:33-40.
- Heufelder AE, Dutton CM, Sarkar G, et al. Detection of TSH receptor RNA in cultured fibroblasts from patients with Graves’ ophthalmopathy and pretibial dermopathy. Thyroid. 1993;3:297-300.
To the Editor:
Pretibial myxedema (PTM) is bilateral, nonpitting, scaly thickening and induration of the skin that most commonly occurs on the anterior aspects of the legs and feet. Pretibial myxedema occurs in approximately 0.5% to 4.3% of patients with hyperthyroidism.1 Thyroid dermopathy often is thought of as the classic nonpitting PTM with skin induration and color change. However, rarer forms of PTM, including plaque, nodular, and elephantiasic, also are important to note.2
Elephantiasic PTM is extremely rare, occurring in less than 1% of patients with PTM.2 Elephantiasic PTM is characterized by the persistent swelling of 1 or both legs; thickening of the skin overlying the dorsum of the feet, ankles, and toes; and verrucous irregular plaques that often are fleshy and flattened. The clinical differential diagnosis of elephantiasic PTM includes elephantiasis nostra verrucosa, a late-stage complication of chronic lymphedema that can be related to a variety of infectious or noninfectious obstructive processes. Few effective therapeutic modalities exist in the treatment of elephantiasic PTM. We present a case of elephantiasic PTM.
A 59-year-old man presented to dermatology with leonine facies with pronounced glabellar creases and indentations of the earlobes. He had diffuse woody induration, hyperpigmentation, and nonpitting edema of the lower extremities as well as several flesh-colored exophytic nodules scattered throughout the anterior shins and dorsal feet (Figure 1). On the left posterior calf, there was a large, 3-cm, exophytic, firm, flesh-colored nodule. Examination of the hands revealed mild hyperpigmentation of the distal digits, clubbing of the distal phalanges, and cheiroarthropathy.
The patient was diagnosed with Graves disease after experiencing the classic symptoms of hyperthyroidism, including heat intolerance, tremor, palpitations, and anxiety. He received thyroid ablation and subsequently was supplemented with levothyroxine 75 mg daily. Twelve years later, he was diagnosed with Graves ophthalmopathy with ocular proptosis requiring multiple courses of retro-orbital irradiation and surgical procedures for decompression. Approximately 1 year later, he noted increased swelling, firmness, and darkening of the pretibial surfaces. Initially, he was referred to vascular surgery and underwent bilateral saphenous vein ablation. He also was referred to a lymphedema specialist, and workup revealed an unremarkable lymphatic system. Minimal improvement was noted following the saphenous vein ablation, and he subsequently was referred to dermatology for further workup.
At the current presentation, laboratory analysis revealed a low thyrotropin level (0.03 mIU/L [reference range, 0.4–4.2 mIU/L]), and free thyroxine was within reference range. Radiography of the chest was unremarkable; however, radiography of the hand demonstrated arthrosis of the left fifth proximal interphalangeal joint. Nuclear medicine lymphoscintigraphy and lower extremity ultrasonography were unremarkable. Punch biopsies were performed of the left lateral leg and posterior calf. Hematoxylin and eosin staining demonstrated marked mucin deposition extending to the deep dermis along with deep fibroplasia and was read as consistent with PTM. Colloidal iron highlighted prominent mucin within the dermis (Figure 2).
The patient’s medical history, physical examination, laboratory analysis, imaging, and biopsies were considered, and a diagnosis of elephantiasic PTM was made. Minimal improvement was noted with initial therapeutic interventions including compression therapy and application of super high–potency topical corticosteroids. After further evaluation in our multidisciplinary rheumatology-dermatology clinic, the decision was made to initiate rituximab infusions.
Two months after 1 course of rituximab consisting of two 1000-mg infusions separated by 2 weeks, the patient showed substantial clinical improvement. There was striking improvement of the pretibial surfaces with resolution of the exophytic nodules and improvement of the induration (Figure 3). In addition, there was decreased induration of the glabella and earlobes and decreased fullness of the digital pulp on the hands. The patient also reported subjective improvements in mobility.
Our patient demonstrated all 3 aspects of the Diamond triad: PTM, exophthalmos, and acropachy. Patients present with all 3 features in less than 1% of reported cases of Graves disease.3 Although all 3 features are seen together infrequently, thyroid dermopathy and acropachy often are markers of severe Graves ophthalmopathy. In a study of 114 patients with Graves ophthalmopathy, patients who also had dermopathy and acropachy were more likely to have optic neuropathy or require orbital decompression.4
After overcoming the diagnostic dilemma that the elephantiasic presentation of PTM can present, therapeutic management remains a challenge. Heyes et al5 documented the successful treatment of highly recalcitrant elephantiasic PTM with rituximab and plasmapheresis therapy. In this case, a 44-year-old woman with an 11-year history of Graves disease and elephantiasic PTM received 29 rituximab infusions and 241 plasmapheresis treatments over the course of 3.5 years. Her elephantiasic PTM clinically resolved, and she was able to resume daily activities and wear normal shoes after being nonambulatory for years.5
Rituximab is a monoclonal antibody against CD20, a protein found primarily on the surface of B-cell lymphocytes. Although rituximab initially was approved by the US Food and Drug administration for the treatment of malignant lymphoma, it has had an increasing role in the treatment of autoimmune disorders such as rheumatoid arthritis. Rituximab is postulated to target B lymphocytes and halt their progression to plasma cells. By limiting the population of long-lasting, antibody-producing plasma cells and decreasing the autoantibodies that cause many of the symptoms in Graves disease, rituximab may be an effective therapy to consider in the treatment of elephantiasic PTM.6
Although the exact mechanism is poorly understood, PTM likely is a sequela of hyperthyroidism because of the expression of thyroid-stimulating hormone receptor proteins found on normal dermal fibroblasts. Thyroid-stimulating hormone receptor autoantibodies are thought to stimulate these fibroblasts to produce glycosaminoglycans. Histopathologically, accumulation of glycosaminoglycans deposited in the reticular dermis with high concentrations of hyaluronic acid is observed in PTM.7
Treatment of elephantiasic PTM remains a therapeutic challenge. Given the rarity of the disease process and limited information on effective therapeutic modalities, rituximab should be viewed as a viable treatment option in the management of recalcitrant elephantiasic PTM.
To the Editor:
Pretibial myxedema (PTM) is bilateral, nonpitting, scaly thickening and induration of the skin that most commonly occurs on the anterior aspects of the legs and feet. Pretibial myxedema occurs in approximately 0.5% to 4.3% of patients with hyperthyroidism.1 Thyroid dermopathy often is thought of as the classic nonpitting PTM with skin induration and color change. However, rarer forms of PTM, including plaque, nodular, and elephantiasic, also are important to note.2
Elephantiasic PTM is extremely rare, occurring in less than 1% of patients with PTM.2 Elephantiasic PTM is characterized by the persistent swelling of 1 or both legs; thickening of the skin overlying the dorsum of the feet, ankles, and toes; and verrucous irregular plaques that often are fleshy and flattened. The clinical differential diagnosis of elephantiasic PTM includes elephantiasis nostra verrucosa, a late-stage complication of chronic lymphedema that can be related to a variety of infectious or noninfectious obstructive processes. Few effective therapeutic modalities exist in the treatment of elephantiasic PTM. We present a case of elephantiasic PTM.
A 59-year-old man presented to dermatology with leonine facies with pronounced glabellar creases and indentations of the earlobes. He had diffuse woody induration, hyperpigmentation, and nonpitting edema of the lower extremities as well as several flesh-colored exophytic nodules scattered throughout the anterior shins and dorsal feet (Figure 1). On the left posterior calf, there was a large, 3-cm, exophytic, firm, flesh-colored nodule. Examination of the hands revealed mild hyperpigmentation of the distal digits, clubbing of the distal phalanges, and cheiroarthropathy.
The patient was diagnosed with Graves disease after experiencing the classic symptoms of hyperthyroidism, including heat intolerance, tremor, palpitations, and anxiety. He received thyroid ablation and subsequently was supplemented with levothyroxine 75 mg daily. Twelve years later, he was diagnosed with Graves ophthalmopathy with ocular proptosis requiring multiple courses of retro-orbital irradiation and surgical procedures for decompression. Approximately 1 year later, he noted increased swelling, firmness, and darkening of the pretibial surfaces. Initially, he was referred to vascular surgery and underwent bilateral saphenous vein ablation. He also was referred to a lymphedema specialist, and workup revealed an unremarkable lymphatic system. Minimal improvement was noted following the saphenous vein ablation, and he subsequently was referred to dermatology for further workup.
At the current presentation, laboratory analysis revealed a low thyrotropin level (0.03 mIU/L [reference range, 0.4–4.2 mIU/L]), and free thyroxine was within reference range. Radiography of the chest was unremarkable; however, radiography of the hand demonstrated arthrosis of the left fifth proximal interphalangeal joint. Nuclear medicine lymphoscintigraphy and lower extremity ultrasonography were unremarkable. Punch biopsies were performed of the left lateral leg and posterior calf. Hematoxylin and eosin staining demonstrated marked mucin deposition extending to the deep dermis along with deep fibroplasia and was read as consistent with PTM. Colloidal iron highlighted prominent mucin within the dermis (Figure 2).
The patient’s medical history, physical examination, laboratory analysis, imaging, and biopsies were considered, and a diagnosis of elephantiasic PTM was made. Minimal improvement was noted with initial therapeutic interventions including compression therapy and application of super high–potency topical corticosteroids. After further evaluation in our multidisciplinary rheumatology-dermatology clinic, the decision was made to initiate rituximab infusions.
Two months after 1 course of rituximab consisting of two 1000-mg infusions separated by 2 weeks, the patient showed substantial clinical improvement. There was striking improvement of the pretibial surfaces with resolution of the exophytic nodules and improvement of the induration (Figure 3). In addition, there was decreased induration of the glabella and earlobes and decreased fullness of the digital pulp on the hands. The patient also reported subjective improvements in mobility.
Our patient demonstrated all 3 aspects of the Diamond triad: PTM, exophthalmos, and acropachy. Patients present with all 3 features in less than 1% of reported cases of Graves disease.3 Although all 3 features are seen together infrequently, thyroid dermopathy and acropachy often are markers of severe Graves ophthalmopathy. In a study of 114 patients with Graves ophthalmopathy, patients who also had dermopathy and acropachy were more likely to have optic neuropathy or require orbital decompression.4
After overcoming the diagnostic dilemma that the elephantiasic presentation of PTM can present, therapeutic management remains a challenge. Heyes et al5 documented the successful treatment of highly recalcitrant elephantiasic PTM with rituximab and plasmapheresis therapy. In this case, a 44-year-old woman with an 11-year history of Graves disease and elephantiasic PTM received 29 rituximab infusions and 241 plasmapheresis treatments over the course of 3.5 years. Her elephantiasic PTM clinically resolved, and she was able to resume daily activities and wear normal shoes after being nonambulatory for years.5
Rituximab is a monoclonal antibody against CD20, a protein found primarily on the surface of B-cell lymphocytes. Although rituximab initially was approved by the US Food and Drug administration for the treatment of malignant lymphoma, it has had an increasing role in the treatment of autoimmune disorders such as rheumatoid arthritis. Rituximab is postulated to target B lymphocytes and halt their progression to plasma cells. By limiting the population of long-lasting, antibody-producing plasma cells and decreasing the autoantibodies that cause many of the symptoms in Graves disease, rituximab may be an effective therapy to consider in the treatment of elephantiasic PTM.6
Although the exact mechanism is poorly understood, PTM likely is a sequela of hyperthyroidism because of the expression of thyroid-stimulating hormone receptor proteins found on normal dermal fibroblasts. Thyroid-stimulating hormone receptor autoantibodies are thought to stimulate these fibroblasts to produce glycosaminoglycans. Histopathologically, accumulation of glycosaminoglycans deposited in the reticular dermis with high concentrations of hyaluronic acid is observed in PTM.7
Treatment of elephantiasic PTM remains a therapeutic challenge. Given the rarity of the disease process and limited information on effective therapeutic modalities, rituximab should be viewed as a viable treatment option in the management of recalcitrant elephantiasic PTM.
- Schwartz KM, Fatourechi V, Ahmed DDF, et al. Dermopathy of Graves’ disease (pretibial myxedema): long-term outcome. J Clin Endocrinol Metab. 2002;87:438-446.
- Kakati S, Doley B, Pal S, et al. Elephantiasis nostras verrucosa: a rare thyroid dermopathy in Graves’ disease. J Assoc Physicians India. 2005;53:571-572.
- Anderson CK, Miller OF 3rd. Triad of exophthalmos, pretibial myxedema, and acropachy in a patient with Graves’ disease. J Am Acad Dermatol. 2003;48:970-972.
- Fatourechi V, Bartley GB, Eghbali-Fatourechi GZ, et al. Graves’ dermopathy and acropachy are markers of severe Graves’ ophthalmopathy. Thyroid. 2003;13:1141-1144.
- Heyes C, Nolan R, Leahy M, et al. Treatment‐resistant elephantiasic thyroid dermopathy responding to rituximab and plasmapheresis. Australas J Dermatol. 2012;53:E1-E4.
- Salvi M, Vannucchi G, Campi I, et al. Treatment of Graves’ disease and associated ophthalmopathy with the anti-CD20 monoclonal antibody rituximab: an open study. Eur J Endocrinol. 2007;156:33-40.
- Heufelder AE, Dutton CM, Sarkar G, et al. Detection of TSH receptor RNA in cultured fibroblasts from patients with Graves’ ophthalmopathy and pretibial dermopathy. Thyroid. 1993;3:297-300.
- Schwartz KM, Fatourechi V, Ahmed DDF, et al. Dermopathy of Graves’ disease (pretibial myxedema): long-term outcome. J Clin Endocrinol Metab. 2002;87:438-446.
- Kakati S, Doley B, Pal S, et al. Elephantiasis nostras verrucosa: a rare thyroid dermopathy in Graves’ disease. J Assoc Physicians India. 2005;53:571-572.
- Anderson CK, Miller OF 3rd. Triad of exophthalmos, pretibial myxedema, and acropachy in a patient with Graves’ disease. J Am Acad Dermatol. 2003;48:970-972.
- Fatourechi V, Bartley GB, Eghbali-Fatourechi GZ, et al. Graves’ dermopathy and acropachy are markers of severe Graves’ ophthalmopathy. Thyroid. 2003;13:1141-1144.
- Heyes C, Nolan R, Leahy M, et al. Treatment‐resistant elephantiasic thyroid dermopathy responding to rituximab and plasmapheresis. Australas J Dermatol. 2012;53:E1-E4.
- Salvi M, Vannucchi G, Campi I, et al. Treatment of Graves’ disease and associated ophthalmopathy with the anti-CD20 monoclonal antibody rituximab: an open study. Eur J Endocrinol. 2007;156:33-40.
- Heufelder AE, Dutton CM, Sarkar G, et al. Detection of TSH receptor RNA in cultured fibroblasts from patients with Graves’ ophthalmopathy and pretibial dermopathy. Thyroid. 1993;3:297-300.
Practice Points
- Pretibial myxedema (PTM) is bilateral, nonpitting, scaly thickening and induration of the skin that most commonly occurs on the anterior aspects of the legs and feet.
- Although many therapeutic modalities have been described for the management of the elephantiasis variant of PTM, few treatments have shown notable efficacy.
- Rituximab may be an effective therapy to consider in the treatment of elephantiasic PTM.
Management of Refractory Pain From Hereditary Cutaneous Leiomyomas With Nifedipine and Gabapentin
To the Editor:
Leiomyomas are benign smooth muscle tumors. There are 3 types of cutaneous leiomyomas: (1) piloleiomyomas, arising from the arrector pili muscles; (2) angioleiomyomas, arising from the muscles surrounding dermal blood vessels; and (3) leiomyomas of the external genitalia, arising from the dartoic, vulvar, or mammary smooth muscles.1 There is no gender predilection for cutaneous leiomyomas, and lesions present on average at approximately 40 to 45 years of age.2
Piloleiomyomas are the most common type of cutaneous leiomyomas and typically present as red-brown papules and nodules on the trunk, arms, and legs.3 Piloleiomyomas often are associated with spontaneous or induced pain (eg, with cold exposure). The pain associated with piloleiomyomas can be severely debilitating to patients and may have a considerable impact on their quality of life.
A 40-year-old woman presented to our clinic with numerous widespread, painful, red-brown papules and nodules on the head, neck, chest, abdomen, back, arms, and legs of 6 years’ duration that were increasing in number (Figure 1). She had a history of uterine leiomyomas and type 2 renal papillary carcinoma following a left nephrectomy at 38 years of age. The patient’s mother had a history of similar skin lesions as well as uterine cancer. Multiple excisional biopsies were performed, all of which showed piloleiomyomas on histopathology (Figure 2). The pain associated with the patient’s extensive cutaneous leiomyomas considerably impaired her quality of life. Although she experienced pain in all affected areas of the body, the pain was the worst in the upper arms. She reported having requested a nerve ablation procedure from an outside pain management clinic, which was denied for unknown reasons.
Two years prior to the current presentation, the patient had been treated by a pain management specialist with gabapentin 300 mg twice daily as needed for pain associated with leiomyomas. The patient followed this regimen approximately 3 times weekly for the preceding 1 to 2 years with reduction in her pain symptoms; however, the painful episodes became more frequent and severe over time. The patient reported being unable to further increase the gabapentin dosing frequency because it made her too drowsy and impacted her ability to work a job that required heavy lifting. Thus, the patient requested additional therapy and was subsequently treated at our clinic with numerous excisional biopsies of the most painful lesions during the 2 years prior to her current presentation.
When the patient re-presented to our clinic, she requested additional lesion excisions given that she had experienced some pain relief from this treatment modality in the past; however, these prior excisions only resulted in local pain relief limited to the site of the excision. Because of the extent of the lesions and the patient’s inability to tolerate pain from the lidocaine injections, we did not feel multiple excisions were a practical treatment option. The patient subsequently was offered a trial of cryotherapy for symptom relief based on a reported case in which this modality was successfully used.4 After discussing the risks and benefits associated with this treatment, cryotherapy was attempted on a few of the leiomyomas on the patient’s right shoulder; however, she experienced severe pain during cryotherapy treatment, and the procedure had to be aborted.
We then increased the patient’s gabapentin regimen to 300 mg in the morning and 600 mg in the evening, as tolerated. The patient reported that she was better able to tolerate the sedating side effects of the increased dose of gabapentin because she had stopped working due to her severe pain episodes. We also added oral nifedipine 10 mg 3 times daily, as needed. Within 30 minutes of starting this treatment regimen, the pain associated with the lesions remarkably improved (10/10 severity before starting treatment vs 3/10 after starting treatment). Her pain levels remained stable (3/10 severity) during 3 weeks of treatment with this combination regimen, but unfortunately she developed headaches and malaise, which she associated with the nifedipine at the 3 times daily dose.
The patient was able to better tolerate the nifedipine after reducing the dose to once daily on an as-needed basis. On average, the patient took nifedipine once every 3 days; however, she reported that she had to periodically increase the frequency of the nifedipine to once daily for up to 2 weeks at a time for periods of more frequent pain flares. The patient reported a consistent pattern of the breakthrough symptoms rapidly improving with each dose of nifedipine, though she did feel that taking consistent gabapentin enhanced baseline symptom control. The patient also noticed on a few occasions when she did not have access to her nifedipine that her pain would flare to 10/10 severity and would decrease to 4/10 severity 30 minutes after restarting nifedipine at 10 mg once daily. She experienced breakthrough pain due to exacerbating factors including her menstrual cycle; exposure to the sun and cold temperatures or water; excessive physical activity; and mild trauma. Due to exacerbations from sun exposure, the patient often wore long-sleeved shirts, which helped reduce the severity of the pain episodes while she was outdoors.
The exact mechanism for the pain associated with cutaneous leiomyomas is unknown but is thought to be due to infringement of the lesion on the surrounding cutaneous nerves. In addition, norepinephrine activates alpha receptors on the smooth muscle to contract through an influx of ions such as calcium. When smooth muscle contracts, the compression of nerves likely is worsened.
There are a limited number of case reports in the literature that have demonstrated successful treatment of the pain associated with cutaneous leiomyomas. Previously reported treatment modalities have included phenoxybenzamine, an alpha-blocking agent that may reduce pain through its antiadrenergic effects2; nitroglycerin, a venous and arterial dilator that may reduce pain by decreasing muscle oxygen requirements2; gabapentin, an antiepileptic and analgesic medication with structural similarity to the gamma-aminobutyric acid neurotransmitter for which the exact mechanism of action is unknown3; botulinum toxin, a neuromuscular blocker that prevents the release of presynaptic acetylcholine and may decrease neuropathic pain by reducing hyperactive nerves5,6; hyoscine butylbromide and topical hyoscine hydrobromide, both antispasmodics that may reduce pain through their anticholinergic effects, which relax smooth muscle7,8; and the CO2 laser, a treatment that has been utilized for its resurfacing, excisional, and ablative properties.9,10
Calcium channel blockers such as amlodipine, verapamil, and nifedipine also have been used to treat the pain associated with piloleiomyomas.11 Calcium ion channel antagonists inhibit the influx of calcium ions across the cell membrane; therefore, nifedipine and other calcium channel blockers may prevent the smooth muscle contraction that is hypothesized to cause pain in patients with cutaneous leiomyomas.12
Mean plasma concentration of nifedipine has been shown to reach maximum values of 160 +/− 49 µg/L after 30 to 60 minutes following oral administration of 10 mg of nifedipine.13 After 8 hours, the mean concentration drops to 3.4 +/− 1.2 µg/L. The clinical response in our patient appeared consistent with the reported pharmacokinetics of the drug, as she was able to consistently obtain considerable reduction in her pain symptoms within 30 minutes of starting nifedipine, coinciding with the period of time it takes for the nifedipine to reach maximum plasma concentrations.13
Interestingly, our patient had worsening pain episodes associated with sun exposure, which typically is not reported as one of the usual triggers for cutaneous leiomyomas. We are not aware of any described mechanisms that would explain this phenomenon.
Importantly, any patient presenting with multiple cutaneous and uterine (if female) leiomyomas should be screened for hereditary leiomyomatosis and renal cell carcinoma syndrome (HLRCC), an autosomal-dominant disorder linked to a mutation in the fumarate hydratase tumor suppressor gene. Clinically, HLRCC patients typically present with multiple cutaneous leiomyomas, uterine leiomyomas, and renal cell cancer (most often type 2 papillary renal cell carcinoma).14 Hereditary leiomyomatosis and renal cell carcinoma syndrome (also known as multiple cutaneous and uterine leiomyomatosis syndrome) previously was thought to be a separate disease entity from Reed syndrome; however, after the same mutation in the fumarate hydratase tumor suppressor gene was found to be responsible for both Reed syndrome and HLRCC, they are now thought to be the same disease process.15
Diagnosis of HLRCC is likely when the patient meets the major criterion of multiple cutaneous piloleiomyomas confirmed histopathologically. Clinical diagnosis of HLRCC is suspected if 2 or more of the following minor criteria are present: type 2 papillary renal cell carcinoma before 40 years of age; onset of severely symptomatic (requiring surgery) uterine fibroids before 40 years of age in females; and first-degree family member who meets 1 or more of these criteria.15 At the time of presentation, the patient met clinical criteria for HLRCC, including multiple cutaneous leiomyomas (major criterion) and type 2 papillary renal cell carcinoma before 40 years of age (minor criterion). The patient also had a history of uterine leiomyomas, but these lesions did not fulfill the criterion of being severely symptomatic requiring surgery. Furthermore, the patient’s mother had similar cutaneous leiomyomas and a history of uterine cancer, which fulfilled additional minor criterion, consistent with an autosomal-dominant inheritance pattern (with variable penetrance) seen in HLRCC. An important issue for counseling and monitoring patients is that premenopausal women with HLRCC are at an increased risk of developing uterine leiomyosarcoma.15 Our patient followed up with an oncologist for tumor surveillance and subsequently underwent genetic testing, which revealed a mutation in the fumarate hydratase gene.
Treatment of painful cutaneous leiomyomas, particularly in patients with HLRCC, remains a therapeutic challenge. Although surgical and/or destructive treatments can provide pain relief for patients who have a limited number of lesions, these options are impracticable when a patient has numerous widespread leiomyomas; therefore, systemic therapies may be more beneficial. Clinicians should be aware of nifedipine, which may be used in combination with gabapentin as a viable treatment option in the management of acute and breakthrough pain associated with cutaneous leiomyomas.
Acknowledgment
The a
- Holst VA, Junkins-Hopkins JM, Elenitsas R. Cutaneous smooth muscle neoplasms: clinical features, histologic findings, and treatment options. J Am Acad Dermatol. 2002;46:477-494.
- Raj S, Calonje E, Kraus M, et al. Cutaneous pilar leiomyoma: clinicopathologic analysis of 53 lesions in 45 patients. Am J Dermatopathol. 1997;19:2-9.
- Alam M, Rabinowitz AD, Engler DE. Gabapentin treatment of multiple piloleiomyoma-related pain. J Am Acad Dermatol. 2002;46:S27-S29.
- Basendwh MA, Fatani M, Baltow B. Reed’s syndrome: a case of multiple cutaneous leiomyomas treated with liquid nitrogen cryotherapy. Case Rep Dermatol. 2016;8:65-70.
- Sifaki MK, Krueger-Krasagakis S, Koutsopoulos A, et al. Botulinum toxin type A–treatment of a patient with multiple cutaneous piloleiomyomas. Dermatology. 2008;218:44-47.
- Onder M, Adıs¸en E. A new indication of botulinum toxin: leiomyoma-related pain. J Am Acad Dermatol. 2009;60:325-328.
- Kaliyadan F, Manoj J, Dharmaratnam AD. Multiple cutaneous leiomyomas: pain relief with pulsed hysocine butyl bromide. Indian J Dermatol. 2009;54:72.
- Archer CB, Whittaker S, Greaves MW. Pharmacological modulation of cold‐induced pain in cutaneous leiomyomata. Br J Dermatol. 1988;118:255-260.
- Christenson LJ, Smith K, Arpey CJ. Treatment of multiple cutaneous leiomyomas with CO2 laser ablation. Dermatol Surg. 2000;26:319-322.
- Michajłowski I, Błaz˙ewicz I, Karpinsky G, et al. Successful treatment of multiple cutaneous leiomyomas with carbon dioxide laser ablation. Postepy Dermatol Alergol. 2015;32:480-482.
- Archer CB, Greaves MW. Assessment of treatment for painful cutaneous leiomyomas. J Am Acad Dermatol. 1987;17:141-142.
- Thompson JA. Therapy for painful cutaneous leiomyomas. J Am Acad Dermatol. 1985;13:865-867.
- Raemsch KD, Sommer J. Pharmacokinetics and metabolism of nifedipine. Hypertension. 1983;5(4 pt 2):II18-II24.
- Toro JR, Nickerson ML, Wei MH, et al. Mutations in the fumarate hydratase gene cause hereditary leiomyomatosis and renal cell cancer in families in North America. Am J Hum Genet. 2003;73:95-106.
- Smit DL, Mensenkamp AR, Badeloe S, et al. Hereditary leiomyomatosis and renal cell cancer in families referred for fumarate hydratase germline mutation analysis. Clin Genet. 2011;79:49-59.
To the Editor:
Leiomyomas are benign smooth muscle tumors. There are 3 types of cutaneous leiomyomas: (1) piloleiomyomas, arising from the arrector pili muscles; (2) angioleiomyomas, arising from the muscles surrounding dermal blood vessels; and (3) leiomyomas of the external genitalia, arising from the dartoic, vulvar, or mammary smooth muscles.1 There is no gender predilection for cutaneous leiomyomas, and lesions present on average at approximately 40 to 45 years of age.2
Piloleiomyomas are the most common type of cutaneous leiomyomas and typically present as red-brown papules and nodules on the trunk, arms, and legs.3 Piloleiomyomas often are associated with spontaneous or induced pain (eg, with cold exposure). The pain associated with piloleiomyomas can be severely debilitating to patients and may have a considerable impact on their quality of life.
A 40-year-old woman presented to our clinic with numerous widespread, painful, red-brown papules and nodules on the head, neck, chest, abdomen, back, arms, and legs of 6 years’ duration that were increasing in number (Figure 1). She had a history of uterine leiomyomas and type 2 renal papillary carcinoma following a left nephrectomy at 38 years of age. The patient’s mother had a history of similar skin lesions as well as uterine cancer. Multiple excisional biopsies were performed, all of which showed piloleiomyomas on histopathology (Figure 2). The pain associated with the patient’s extensive cutaneous leiomyomas considerably impaired her quality of life. Although she experienced pain in all affected areas of the body, the pain was the worst in the upper arms. She reported having requested a nerve ablation procedure from an outside pain management clinic, which was denied for unknown reasons.
Two years prior to the current presentation, the patient had been treated by a pain management specialist with gabapentin 300 mg twice daily as needed for pain associated with leiomyomas. The patient followed this regimen approximately 3 times weekly for the preceding 1 to 2 years with reduction in her pain symptoms; however, the painful episodes became more frequent and severe over time. The patient reported being unable to further increase the gabapentin dosing frequency because it made her too drowsy and impacted her ability to work a job that required heavy lifting. Thus, the patient requested additional therapy and was subsequently treated at our clinic with numerous excisional biopsies of the most painful lesions during the 2 years prior to her current presentation.
When the patient re-presented to our clinic, she requested additional lesion excisions given that she had experienced some pain relief from this treatment modality in the past; however, these prior excisions only resulted in local pain relief limited to the site of the excision. Because of the extent of the lesions and the patient’s inability to tolerate pain from the lidocaine injections, we did not feel multiple excisions were a practical treatment option. The patient subsequently was offered a trial of cryotherapy for symptom relief based on a reported case in which this modality was successfully used.4 After discussing the risks and benefits associated with this treatment, cryotherapy was attempted on a few of the leiomyomas on the patient’s right shoulder; however, she experienced severe pain during cryotherapy treatment, and the procedure had to be aborted.
We then increased the patient’s gabapentin regimen to 300 mg in the morning and 600 mg in the evening, as tolerated. The patient reported that she was better able to tolerate the sedating side effects of the increased dose of gabapentin because she had stopped working due to her severe pain episodes. We also added oral nifedipine 10 mg 3 times daily, as needed. Within 30 minutes of starting this treatment regimen, the pain associated with the lesions remarkably improved (10/10 severity before starting treatment vs 3/10 after starting treatment). Her pain levels remained stable (3/10 severity) during 3 weeks of treatment with this combination regimen, but unfortunately she developed headaches and malaise, which she associated with the nifedipine at the 3 times daily dose.
The patient was able to better tolerate the nifedipine after reducing the dose to once daily on an as-needed basis. On average, the patient took nifedipine once every 3 days; however, she reported that she had to periodically increase the frequency of the nifedipine to once daily for up to 2 weeks at a time for periods of more frequent pain flares. The patient reported a consistent pattern of the breakthrough symptoms rapidly improving with each dose of nifedipine, though she did feel that taking consistent gabapentin enhanced baseline symptom control. The patient also noticed on a few occasions when she did not have access to her nifedipine that her pain would flare to 10/10 severity and would decrease to 4/10 severity 30 minutes after restarting nifedipine at 10 mg once daily. She experienced breakthrough pain due to exacerbating factors including her menstrual cycle; exposure to the sun and cold temperatures or water; excessive physical activity; and mild trauma. Due to exacerbations from sun exposure, the patient often wore long-sleeved shirts, which helped reduce the severity of the pain episodes while she was outdoors.
The exact mechanism for the pain associated with cutaneous leiomyomas is unknown but is thought to be due to infringement of the lesion on the surrounding cutaneous nerves. In addition, norepinephrine activates alpha receptors on the smooth muscle to contract through an influx of ions such as calcium. When smooth muscle contracts, the compression of nerves likely is worsened.
There are a limited number of case reports in the literature that have demonstrated successful treatment of the pain associated with cutaneous leiomyomas. Previously reported treatment modalities have included phenoxybenzamine, an alpha-blocking agent that may reduce pain through its antiadrenergic effects2; nitroglycerin, a venous and arterial dilator that may reduce pain by decreasing muscle oxygen requirements2; gabapentin, an antiepileptic and analgesic medication with structural similarity to the gamma-aminobutyric acid neurotransmitter for which the exact mechanism of action is unknown3; botulinum toxin, a neuromuscular blocker that prevents the release of presynaptic acetylcholine and may decrease neuropathic pain by reducing hyperactive nerves5,6; hyoscine butylbromide and topical hyoscine hydrobromide, both antispasmodics that may reduce pain through their anticholinergic effects, which relax smooth muscle7,8; and the CO2 laser, a treatment that has been utilized for its resurfacing, excisional, and ablative properties.9,10
Calcium channel blockers such as amlodipine, verapamil, and nifedipine also have been used to treat the pain associated with piloleiomyomas.11 Calcium ion channel antagonists inhibit the influx of calcium ions across the cell membrane; therefore, nifedipine and other calcium channel blockers may prevent the smooth muscle contraction that is hypothesized to cause pain in patients with cutaneous leiomyomas.12
Mean plasma concentration of nifedipine has been shown to reach maximum values of 160 +/− 49 µg/L after 30 to 60 minutes following oral administration of 10 mg of nifedipine.13 After 8 hours, the mean concentration drops to 3.4 +/− 1.2 µg/L. The clinical response in our patient appeared consistent with the reported pharmacokinetics of the drug, as she was able to consistently obtain considerable reduction in her pain symptoms within 30 minutes of starting nifedipine, coinciding with the period of time it takes for the nifedipine to reach maximum plasma concentrations.13
Interestingly, our patient had worsening pain episodes associated with sun exposure, which typically is not reported as one of the usual triggers for cutaneous leiomyomas. We are not aware of any described mechanisms that would explain this phenomenon.
Importantly, any patient presenting with multiple cutaneous and uterine (if female) leiomyomas should be screened for hereditary leiomyomatosis and renal cell carcinoma syndrome (HLRCC), an autosomal-dominant disorder linked to a mutation in the fumarate hydratase tumor suppressor gene. Clinically, HLRCC patients typically present with multiple cutaneous leiomyomas, uterine leiomyomas, and renal cell cancer (most often type 2 papillary renal cell carcinoma).14 Hereditary leiomyomatosis and renal cell carcinoma syndrome (also known as multiple cutaneous and uterine leiomyomatosis syndrome) previously was thought to be a separate disease entity from Reed syndrome; however, after the same mutation in the fumarate hydratase tumor suppressor gene was found to be responsible for both Reed syndrome and HLRCC, they are now thought to be the same disease process.15
Diagnosis of HLRCC is likely when the patient meets the major criterion of multiple cutaneous piloleiomyomas confirmed histopathologically. Clinical diagnosis of HLRCC is suspected if 2 or more of the following minor criteria are present: type 2 papillary renal cell carcinoma before 40 years of age; onset of severely symptomatic (requiring surgery) uterine fibroids before 40 years of age in females; and first-degree family member who meets 1 or more of these criteria.15 At the time of presentation, the patient met clinical criteria for HLRCC, including multiple cutaneous leiomyomas (major criterion) and type 2 papillary renal cell carcinoma before 40 years of age (minor criterion). The patient also had a history of uterine leiomyomas, but these lesions did not fulfill the criterion of being severely symptomatic requiring surgery. Furthermore, the patient’s mother had similar cutaneous leiomyomas and a history of uterine cancer, which fulfilled additional minor criterion, consistent with an autosomal-dominant inheritance pattern (with variable penetrance) seen in HLRCC. An important issue for counseling and monitoring patients is that premenopausal women with HLRCC are at an increased risk of developing uterine leiomyosarcoma.15 Our patient followed up with an oncologist for tumor surveillance and subsequently underwent genetic testing, which revealed a mutation in the fumarate hydratase gene.
Treatment of painful cutaneous leiomyomas, particularly in patients with HLRCC, remains a therapeutic challenge. Although surgical and/or destructive treatments can provide pain relief for patients who have a limited number of lesions, these options are impracticable when a patient has numerous widespread leiomyomas; therefore, systemic therapies may be more beneficial. Clinicians should be aware of nifedipine, which may be used in combination with gabapentin as a viable treatment option in the management of acute and breakthrough pain associated with cutaneous leiomyomas.
Acknowledgment
The a
To the Editor:
Leiomyomas are benign smooth muscle tumors. There are 3 types of cutaneous leiomyomas: (1) piloleiomyomas, arising from the arrector pili muscles; (2) angioleiomyomas, arising from the muscles surrounding dermal blood vessels; and (3) leiomyomas of the external genitalia, arising from the dartoic, vulvar, or mammary smooth muscles.1 There is no gender predilection for cutaneous leiomyomas, and lesions present on average at approximately 40 to 45 years of age.2
Piloleiomyomas are the most common type of cutaneous leiomyomas and typically present as red-brown papules and nodules on the trunk, arms, and legs.3 Piloleiomyomas often are associated with spontaneous or induced pain (eg, with cold exposure). The pain associated with piloleiomyomas can be severely debilitating to patients and may have a considerable impact on their quality of life.
A 40-year-old woman presented to our clinic with numerous widespread, painful, red-brown papules and nodules on the head, neck, chest, abdomen, back, arms, and legs of 6 years’ duration that were increasing in number (Figure 1). She had a history of uterine leiomyomas and type 2 renal papillary carcinoma following a left nephrectomy at 38 years of age. The patient’s mother had a history of similar skin lesions as well as uterine cancer. Multiple excisional biopsies were performed, all of which showed piloleiomyomas on histopathology (Figure 2). The pain associated with the patient’s extensive cutaneous leiomyomas considerably impaired her quality of life. Although she experienced pain in all affected areas of the body, the pain was the worst in the upper arms. She reported having requested a nerve ablation procedure from an outside pain management clinic, which was denied for unknown reasons.
Two years prior to the current presentation, the patient had been treated by a pain management specialist with gabapentin 300 mg twice daily as needed for pain associated with leiomyomas. The patient followed this regimen approximately 3 times weekly for the preceding 1 to 2 years with reduction in her pain symptoms; however, the painful episodes became more frequent and severe over time. The patient reported being unable to further increase the gabapentin dosing frequency because it made her too drowsy and impacted her ability to work a job that required heavy lifting. Thus, the patient requested additional therapy and was subsequently treated at our clinic with numerous excisional biopsies of the most painful lesions during the 2 years prior to her current presentation.
When the patient re-presented to our clinic, she requested additional lesion excisions given that she had experienced some pain relief from this treatment modality in the past; however, these prior excisions only resulted in local pain relief limited to the site of the excision. Because of the extent of the lesions and the patient’s inability to tolerate pain from the lidocaine injections, we did not feel multiple excisions were a practical treatment option. The patient subsequently was offered a trial of cryotherapy for symptom relief based on a reported case in which this modality was successfully used.4 After discussing the risks and benefits associated with this treatment, cryotherapy was attempted on a few of the leiomyomas on the patient’s right shoulder; however, she experienced severe pain during cryotherapy treatment, and the procedure had to be aborted.
We then increased the patient’s gabapentin regimen to 300 mg in the morning and 600 mg in the evening, as tolerated. The patient reported that she was better able to tolerate the sedating side effects of the increased dose of gabapentin because she had stopped working due to her severe pain episodes. We also added oral nifedipine 10 mg 3 times daily, as needed. Within 30 minutes of starting this treatment regimen, the pain associated with the lesions remarkably improved (10/10 severity before starting treatment vs 3/10 after starting treatment). Her pain levels remained stable (3/10 severity) during 3 weeks of treatment with this combination regimen, but unfortunately she developed headaches and malaise, which she associated with the nifedipine at the 3 times daily dose.
The patient was able to better tolerate the nifedipine after reducing the dose to once daily on an as-needed basis. On average, the patient took nifedipine once every 3 days; however, she reported that she had to periodically increase the frequency of the nifedipine to once daily for up to 2 weeks at a time for periods of more frequent pain flares. The patient reported a consistent pattern of the breakthrough symptoms rapidly improving with each dose of nifedipine, though she did feel that taking consistent gabapentin enhanced baseline symptom control. The patient also noticed on a few occasions when she did not have access to her nifedipine that her pain would flare to 10/10 severity and would decrease to 4/10 severity 30 minutes after restarting nifedipine at 10 mg once daily. She experienced breakthrough pain due to exacerbating factors including her menstrual cycle; exposure to the sun and cold temperatures or water; excessive physical activity; and mild trauma. Due to exacerbations from sun exposure, the patient often wore long-sleeved shirts, which helped reduce the severity of the pain episodes while she was outdoors.
The exact mechanism for the pain associated with cutaneous leiomyomas is unknown but is thought to be due to infringement of the lesion on the surrounding cutaneous nerves. In addition, norepinephrine activates alpha receptors on the smooth muscle to contract through an influx of ions such as calcium. When smooth muscle contracts, the compression of nerves likely is worsened.
There are a limited number of case reports in the literature that have demonstrated successful treatment of the pain associated with cutaneous leiomyomas. Previously reported treatment modalities have included phenoxybenzamine, an alpha-blocking agent that may reduce pain through its antiadrenergic effects2; nitroglycerin, a venous and arterial dilator that may reduce pain by decreasing muscle oxygen requirements2; gabapentin, an antiepileptic and analgesic medication with structural similarity to the gamma-aminobutyric acid neurotransmitter for which the exact mechanism of action is unknown3; botulinum toxin, a neuromuscular blocker that prevents the release of presynaptic acetylcholine and may decrease neuropathic pain by reducing hyperactive nerves5,6; hyoscine butylbromide and topical hyoscine hydrobromide, both antispasmodics that may reduce pain through their anticholinergic effects, which relax smooth muscle7,8; and the CO2 laser, a treatment that has been utilized for its resurfacing, excisional, and ablative properties.9,10
Calcium channel blockers such as amlodipine, verapamil, and nifedipine also have been used to treat the pain associated with piloleiomyomas.11 Calcium ion channel antagonists inhibit the influx of calcium ions across the cell membrane; therefore, nifedipine and other calcium channel blockers may prevent the smooth muscle contraction that is hypothesized to cause pain in patients with cutaneous leiomyomas.12
Mean plasma concentration of nifedipine has been shown to reach maximum values of 160 +/− 49 µg/L after 30 to 60 minutes following oral administration of 10 mg of nifedipine.13 After 8 hours, the mean concentration drops to 3.4 +/− 1.2 µg/L. The clinical response in our patient appeared consistent with the reported pharmacokinetics of the drug, as she was able to consistently obtain considerable reduction in her pain symptoms within 30 minutes of starting nifedipine, coinciding with the period of time it takes for the nifedipine to reach maximum plasma concentrations.13
Interestingly, our patient had worsening pain episodes associated with sun exposure, which typically is not reported as one of the usual triggers for cutaneous leiomyomas. We are not aware of any described mechanisms that would explain this phenomenon.
Importantly, any patient presenting with multiple cutaneous and uterine (if female) leiomyomas should be screened for hereditary leiomyomatosis and renal cell carcinoma syndrome (HLRCC), an autosomal-dominant disorder linked to a mutation in the fumarate hydratase tumor suppressor gene. Clinically, HLRCC patients typically present with multiple cutaneous leiomyomas, uterine leiomyomas, and renal cell cancer (most often type 2 papillary renal cell carcinoma).14 Hereditary leiomyomatosis and renal cell carcinoma syndrome (also known as multiple cutaneous and uterine leiomyomatosis syndrome) previously was thought to be a separate disease entity from Reed syndrome; however, after the same mutation in the fumarate hydratase tumor suppressor gene was found to be responsible for both Reed syndrome and HLRCC, they are now thought to be the same disease process.15
Diagnosis of HLRCC is likely when the patient meets the major criterion of multiple cutaneous piloleiomyomas confirmed histopathologically. Clinical diagnosis of HLRCC is suspected if 2 or more of the following minor criteria are present: type 2 papillary renal cell carcinoma before 40 years of age; onset of severely symptomatic (requiring surgery) uterine fibroids before 40 years of age in females; and first-degree family member who meets 1 or more of these criteria.15 At the time of presentation, the patient met clinical criteria for HLRCC, including multiple cutaneous leiomyomas (major criterion) and type 2 papillary renal cell carcinoma before 40 years of age (minor criterion). The patient also had a history of uterine leiomyomas, but these lesions did not fulfill the criterion of being severely symptomatic requiring surgery. Furthermore, the patient’s mother had similar cutaneous leiomyomas and a history of uterine cancer, which fulfilled additional minor criterion, consistent with an autosomal-dominant inheritance pattern (with variable penetrance) seen in HLRCC. An important issue for counseling and monitoring patients is that premenopausal women with HLRCC are at an increased risk of developing uterine leiomyosarcoma.15 Our patient followed up with an oncologist for tumor surveillance and subsequently underwent genetic testing, which revealed a mutation in the fumarate hydratase gene.
Treatment of painful cutaneous leiomyomas, particularly in patients with HLRCC, remains a therapeutic challenge. Although surgical and/or destructive treatments can provide pain relief for patients who have a limited number of lesions, these options are impracticable when a patient has numerous widespread leiomyomas; therefore, systemic therapies may be more beneficial. Clinicians should be aware of nifedipine, which may be used in combination with gabapentin as a viable treatment option in the management of acute and breakthrough pain associated with cutaneous leiomyomas.
Acknowledgment
The a
- Holst VA, Junkins-Hopkins JM, Elenitsas R. Cutaneous smooth muscle neoplasms: clinical features, histologic findings, and treatment options. J Am Acad Dermatol. 2002;46:477-494.
- Raj S, Calonje E, Kraus M, et al. Cutaneous pilar leiomyoma: clinicopathologic analysis of 53 lesions in 45 patients. Am J Dermatopathol. 1997;19:2-9.
- Alam M, Rabinowitz AD, Engler DE. Gabapentin treatment of multiple piloleiomyoma-related pain. J Am Acad Dermatol. 2002;46:S27-S29.
- Basendwh MA, Fatani M, Baltow B. Reed’s syndrome: a case of multiple cutaneous leiomyomas treated with liquid nitrogen cryotherapy. Case Rep Dermatol. 2016;8:65-70.
- Sifaki MK, Krueger-Krasagakis S, Koutsopoulos A, et al. Botulinum toxin type A–treatment of a patient with multiple cutaneous piloleiomyomas. Dermatology. 2008;218:44-47.
- Onder M, Adıs¸en E. A new indication of botulinum toxin: leiomyoma-related pain. J Am Acad Dermatol. 2009;60:325-328.
- Kaliyadan F, Manoj J, Dharmaratnam AD. Multiple cutaneous leiomyomas: pain relief with pulsed hysocine butyl bromide. Indian J Dermatol. 2009;54:72.
- Archer CB, Whittaker S, Greaves MW. Pharmacological modulation of cold‐induced pain in cutaneous leiomyomata. Br J Dermatol. 1988;118:255-260.
- Christenson LJ, Smith K, Arpey CJ. Treatment of multiple cutaneous leiomyomas with CO2 laser ablation. Dermatol Surg. 2000;26:319-322.
- Michajłowski I, Błaz˙ewicz I, Karpinsky G, et al. Successful treatment of multiple cutaneous leiomyomas with carbon dioxide laser ablation. Postepy Dermatol Alergol. 2015;32:480-482.
- Archer CB, Greaves MW. Assessment of treatment for painful cutaneous leiomyomas. J Am Acad Dermatol. 1987;17:141-142.
- Thompson JA. Therapy for painful cutaneous leiomyomas. J Am Acad Dermatol. 1985;13:865-867.
- Raemsch KD, Sommer J. Pharmacokinetics and metabolism of nifedipine. Hypertension. 1983;5(4 pt 2):II18-II24.
- Toro JR, Nickerson ML, Wei MH, et al. Mutations in the fumarate hydratase gene cause hereditary leiomyomatosis and renal cell cancer in families in North America. Am J Hum Genet. 2003;73:95-106.
- Smit DL, Mensenkamp AR, Badeloe S, et al. Hereditary leiomyomatosis and renal cell cancer in families referred for fumarate hydratase germline mutation analysis. Clin Genet. 2011;79:49-59.
- Holst VA, Junkins-Hopkins JM, Elenitsas R. Cutaneous smooth muscle neoplasms: clinical features, histologic findings, and treatment options. J Am Acad Dermatol. 2002;46:477-494.
- Raj S, Calonje E, Kraus M, et al. Cutaneous pilar leiomyoma: clinicopathologic analysis of 53 lesions in 45 patients. Am J Dermatopathol. 1997;19:2-9.
- Alam M, Rabinowitz AD, Engler DE. Gabapentin treatment of multiple piloleiomyoma-related pain. J Am Acad Dermatol. 2002;46:S27-S29.
- Basendwh MA, Fatani M, Baltow B. Reed’s syndrome: a case of multiple cutaneous leiomyomas treated with liquid nitrogen cryotherapy. Case Rep Dermatol. 2016;8:65-70.
- Sifaki MK, Krueger-Krasagakis S, Koutsopoulos A, et al. Botulinum toxin type A–treatment of a patient with multiple cutaneous piloleiomyomas. Dermatology. 2008;218:44-47.
- Onder M, Adıs¸en E. A new indication of botulinum toxin: leiomyoma-related pain. J Am Acad Dermatol. 2009;60:325-328.
- Kaliyadan F, Manoj J, Dharmaratnam AD. Multiple cutaneous leiomyomas: pain relief with pulsed hysocine butyl bromide. Indian J Dermatol. 2009;54:72.
- Archer CB, Whittaker S, Greaves MW. Pharmacological modulation of cold‐induced pain in cutaneous leiomyomata. Br J Dermatol. 1988;118:255-260.
- Christenson LJ, Smith K, Arpey CJ. Treatment of multiple cutaneous leiomyomas with CO2 laser ablation. Dermatol Surg. 2000;26:319-322.
- Michajłowski I, Błaz˙ewicz I, Karpinsky G, et al. Successful treatment of multiple cutaneous leiomyomas with carbon dioxide laser ablation. Postepy Dermatol Alergol. 2015;32:480-482.
- Archer CB, Greaves MW. Assessment of treatment for painful cutaneous leiomyomas. J Am Acad Dermatol. 1987;17:141-142.
- Thompson JA. Therapy for painful cutaneous leiomyomas. J Am Acad Dermatol. 1985;13:865-867.
- Raemsch KD, Sommer J. Pharmacokinetics and metabolism of nifedipine. Hypertension. 1983;5(4 pt 2):II18-II24.
- Toro JR, Nickerson ML, Wei MH, et al. Mutations in the fumarate hydratase gene cause hereditary leiomyomatosis and renal cell cancer in families in North America. Am J Hum Genet. 2003;73:95-106.
- Smit DL, Mensenkamp AR, Badeloe S, et al. Hereditary leiomyomatosis and renal cell cancer in families referred for fumarate hydratase germline mutation analysis. Clin Genet. 2011;79:49-59.
Practice Points
- Cutaneous leiomyomas (piloleiomyomas) are benign smooth muscle tumors derived from the arrector pili muscle.
- Patients presenting with multiple cutaneous leiomyomas should be evaluated for hereditary leiomyomatosis and renal cell carcinoma syndrome, an autosomal-dominant disorder, which also predisposes to the development of symptomatic uterine fibroids and uterine leiomyosarcoma.
- Cutaneous leiomyomas may be a source of considerable pain, which may respond to treatment with nifedipine in combination with gabapentin.