User login
Sudden hypoxia during knee surgery
A 75-year-old man with type 2 diabetes and hypothyroidism underwent bilateral total knee replacement at our hospital.
His functional capacity had been moderately limited by knee pain, but he could easily climb one flight of stairs without symptoms. His medications at that time included levothyroxine (Synthroid) and metformin (Glucophage). He had no known cardiac or pulmonary disease. The preoperative evaluation, including laboratory tests and electrocardiography, was within normal limits.
Spinal anesthesia was used for surgery, and he was given 2 mg of midazolam (Versed) intravenously for sedation. No additional sedation was given. He was given oxygen via nasal cannula at 2 L/min.
All vital signs were stable at the start of the procedure. However, about halfway through, when the thigh tourniquet was released, his oxygen saturation dropped abruptly from 100% to 92%. All other vital signs remained stable, and he was asymptomatic, was oriented to person, time, and place, was conversing freely, and was in no distress. The oxygen flow was increased to 6 L/min, his oxygen saturation improved, and the procedure was then completed as planned.
At the conclusion of the surgery, before the patient was transported to the postanesthesia care unit (PACU) and while his oxygen flow rate was still 6 L/min, his oxygen saturation again dropped to 92%. A simple face mask was placed, and the oxygen flow rate was increased to 10 L/min. His oxygen saturation stayed low, near 90%.
Bleeding during surgery had been nominal. He had received 2 L of lactated Ringer’s solution and 500 mL of hetastarch (Hextend) during surgery. He continued to be asymptomatic in the PACU.
1. What is the most likely cause of oxygen desaturation during bilateral total knee arthroplasty?
- Fat embolism
- Intraoperative pneumonia
- Venous thromboembolism with pulmonary embolism
- Acute myocardial infarction
- Acute pulmonary edema
- Excessive sedation
The differential diagnosis of oxygen desaturation during orthopedic procedures is listed in Table 1.
Fat embolism is the most likely cause, particularly given the greater fatty embolic load that occurs with bilateral total knee arthroplasty than with unilateral total knee arthroplasty.
At what point the maximal showering of fat emboli occurs is not known. Fat may be released into the circulation with pressurization of the medullary canal during surgery or with manipulation of a fracture. The emboli may collect in the leg veins and then be released in a shower when the thigh tourniquet is released. Vasoactive mediators and methylmethacrylate cement released into the circulatory system after tourniquet deflation may also cause vasodilation, hypotension, and increased dead-space ventilation, resulting in hypoxia and a drop in end-tidal CO2.
Pneumonia during surgery is rare without an apparent aspiration event.
Venous thromboembolism is possible but is more likely later in the postoperative period after major orthopedic surgery.
Acute myocardial infarction could present with hypoxia, particularly in a diabetic patient, who may not experience chest pain. However, intraoperative electrocardiographic changes would likely be seen. If myocardial infarction is suspected, postoperative serial electrocardiograms and measuring troponin and cardiac enzyme levels aid in the diagnosis.
Acute pulmonary edema is possible but not as highly suspected, as the patient had no history of congestive heart failure and received an appropriate amount of fluid for this type of surgery.
Excessive sedation could cause hypoventilation and, thus, oxygen desaturatation. However, this patient’s oxygen desaturatation began more than an hour after the midazolam was given. Midazolam is a short-acting benzodiazepine. It is unlikely that the patient would show signs of hypoventilation and oversedation an hour after the drug was given. Our patient also did not show any signs of excessive sedation, as he was awake and conversing during the surgery.
Fat emboli vs fat embolism syndrome
Fat embolism is the presence of fat drops within the systemic and pulmonary microcirculation, with or without clinical sequelae.1 Fat embolism syndrome, on the other hand, is defined as injury to and dysfunction of one or more organs as a result of the embolization of fat, usually within 24 hours of injury or orthopedic surgery.2
Fat embolism syndrome is an unpredictable condition with a varied presentation. Fat droplets are thought to embolize via the venous circulation into the pulmonary arteries, occluding small blood vessels in the lung. However, they also get into the arterial circulation and occlude arteries in the brain, kidney, heart, and liver (more on this phenomenon below).
Fat embolism is reported to originate primarily from fractures of the femur, tibia, and pelvis.2,3 As many as 90% of trauma patients have been shown to have evidence of fat embolism on autopsy.4 However, only a small number of patients develop the classic fat embolism syndrome,2,3,5 Why some develop the syndrome and others do not is still unknown.
Orthopedic procedures associated with fat embolization include knee arthroplasty and hip arthroplasty, particularly if it involves intramedullary manipulation or medullary fixation.6 It has also been reported during spinal procedures in which pedicular screws are used.7 The syndrome occurs in 0.25% to 30% of patients following multiple fractures and in 0.1% to 12% of patients during or following knee or hip arthroplasty.
One study8 showed evidence of fat on transesophageal echocardiography in 88% of patients undergoing medullary reaming of lower-extremity fractures and hip hemiarthroplasty. Blood sampling from the right atrium confirmed that fat was responsible for the echocardiographic abnormalities. The study also showed that the severity of the embolic showering correlated with the severity of hypoxia and the decrease in end-tidal CO2.8
CASE CONTINUED
On arrival at the PACU, our patient’s oxygen saturation was 94% while he was breathing oxygen via a simple face mask at a flow rate of 10 L/min. His heart rate was 60 bpm, blood pressure 110/60 mm Hg, and temperature 37.5°C (96.3°F). Chest sounds were normal on auscultation.
However, 3 hours later, his mental status rapidly deteriorated. He was oriented only to person, and he was drowsy. He had escalating respiratory distress with a rapid respiratory rate and decreasing oxygen saturation. At this point, auscultation of his chest wall revealed bilateral crackles and rales.
He was promptly intubated. Profuse fluid and secretions were noted to be coming from his lungs, filling the endotracheal tube. Arterial blood gas measurement showed a pH of 7.22, Pao2 64 mm Hg, and Paco2 56 mm Hg on 100% fraction of inspired oxygen, with no increased anion gap.
2. Which consequence of fat embolism is most likely at this time in this patient?
- Coexisting sepsis
- Fat embolism syndrome
- Acute cardioembolic stroke
- Anaphylaxis
Fat embolism syndrome should be highly suspected in this patient. As mentioned, it can affect many different organs. It is the most serious condition resulting from fat embolization after surgery or trauma.
Sepsis was unlikely in our patient, since he presented for his surgery in good health and with no preexisting signs or symptoms of infection. Acute cardioembolic stroke could have caused the neurologic signs, but this would not necessarily explain the coexisting hypoxia. An anaphylactic reaction to drugs or surgical cement would most likely present intraoperatively, shortly after exposure occurred, rather than several hours after surgery.
How common is fat embolism syndrome?
The occurrence rate of fat embolism syndrome has been reported to be 0.25% to 30% after multiple fractures and 0.1% to 12% after knee and hip joint surgery, with a mortality rate of 13% to 36%.2,9–14 The rate of occurrence after unilateral total knee joint replacement has been reported to be 1.8% to 5%, and 4% to 12% after bilateral total knee replacement.15–19
The syndrome is relatively more common with traumatic fractures of the lower extremities. However, it has also been reported with liposuction, total parenteral nutrition, bone marrow harvest and transplantation, burns, and acute pancreatitis, to mention a few.10
The broad range of reported incidence rates can be attributed to the fact that many studies were in patients with multiple trauma, whose concomitant injuries may have made it difficult to clearly define the contribution of fat embolism syndrome to the overall rates of morbidity and mortality. Also, different studies used different criteria to define the syndrome.
How does fat embolism syndrome occur?
Two hypotheses for how this syndrome occurs were proposed nearly a century ago.20,21
The “mechanical” theory is that fat emboli are formed as a result of trauma and disruption of adipose tissue and other cells in the bone marrow. Increases in intramedullary pressure force the fat emboli through damaged medullary venous channels in the bone and into the circulation of the lower extremities. This embolization of fat causes an initial mechanical pulmonary obstruction. Mechanical obstruction by fat emboli in the pulmonary system leads to increased pulmonary pressures and an increase in right heart outflow pressure. The right heart becomes strained, leading to a decreased right-sided cardiac output. As a result, the left heart filling pressures diminish and hypotension ensues.20
The “biochemical” theory, on the other hand, is that chylomicrons within the vascular system are modified and their stability is compromised as a result of stress. These traumatized chylomicrons then coalesce to form droplets of fat that accumulate in the pulmonary circulation and produce a mechanical obstruction. This would explain why nontraumatic, nonorthopedic insults can produce this syndrome.
Autopsy studies show that there is little correlation between the presence and quantity of intravascular fat and the severity of clinical symptoms, thus implying that the syndrome is caused by more than just mechanical obstruction. The biochemical theory postulates that fat globules within the circulatory system then cause the release of lipase from the pulmonary alveolar cells, which then hydrolyses the fat into free fatty acids. These free fatty acids cause an inflammatory reaction, complementmediated leukocyte aggregation, chemotoxin release, and subsequently endothelial damage. These vasoactive substances damage type 2 pneumocytes and lead to an increased permeability of the pulmonary capillary beds. Acute respiratory distress syndrome (ARDS) may ensue. Disseminated intravascular coagulation may occur as a result of the formation of microthrombi involving lipids, platelets, and fibrin.21,22
Embolization of fat to the central nervous system can occur as fat globules cross into the systemic circulation via a patent foramen ovale, an atrioventricular shunt, or the pulmonary capillaries. This can then result in cerebral ischemia.23
Although patent foramen ovale may seem the most direct route for cerebral embolization, the neurologic impairment and signs of cerebral emboli in fat embolism syndrome may occur in the absence of patent foramen ovale.24,25 The fat globules may actually go through the lung capillaries, being flexible and forced through by increased pulmonary pressure.
But whether the cause of fat embolism syndrome is occlusion by globules, the release of biochemical mediators, or a combination of both is unknown. Both mechanisms are likely responsible. We can only suspect that the degree of fat load and intrinsic metabolic differences between individuals account for the variation in susceptibility.
FAT EMBOLI AFFECT THE LUNGS, SKIN, AND BRAIN
3. Where on the body is the rash associated with fat embolism syndrome usually seen?
- Face
- Near a site of fracture or surgery
- Chest, axilla, conjunctiva
- Distal extremities
Petechiae are part of the classic presenting triad of fat embolism syndrome, which also includes pulmonary and cerebral dysfunction.
Petechiae usually appear on the 2nd to 4th day after injury.26 They are usually found across the chest, the anterior axillary folds, and the neck, as well as on the oral mucosa and the conjunctiva. The rash is caused by occlusion of dermal capillaries by fat, which increases their fragility.10
Pulmonary changes usually begin with tachypnea, dyspnea, and a drop in oxygen saturation, leading to generalized hypoxia. Respiratory symptoms are present in 100% of cases.2 Respiratory symptoms can acutely develop with the sudden manipulation of a fracture, reaming of bone, or release of a limb tourniquet.27
Body systems affected by fat embolism syndrome are summarized in Table 2.
4. How many hours after injury does fat embolism syndrome typically manifest?
- 1 to 2 hours
- 6 to 12 hours
- 12 to 20 hours
- 24 to 48 hours
- 72 to 84 hours
Most patients develop signs and symptoms 24 to 48 hours after injury. Patients presenting earlier than 12 hours usually have a more fulminant course.29
The time between fat embolization and the development of fat embolism syndrome is thought to be related to the time required for the metabolic conversion of fat to free fatty acids.30 We suspect that the early desaturation seen in our patient was the result of a heavy showering of fat intraoperatively. However, this could only be concluded after we had ruled out other causes of acute hypoxia and hypotension.
Fat embolism syndrome is a diagnosis of exclusion and is based on clinical criteria. No specific sign, symptom, or test is pathognomonic. It may often be confused with other conditions such as systemic inflammatory response syndrome or sepsis. However, the triad of respiratory and neurologic symptoms and petechiae coupled with the clinical picture of recent trauma or orthopedic surgery almost assures the diagnosis.
Fat embolism syndrome can range from subclinical to fulminating, with the more fulminating course attributable to a huge load of fat emboli, which leads to acute cor pulmonale.
Regardless of the criteria used, one must have a high index of suspicion for fat embolization syndrome in patients undergoing orthopedic procedures, particularly hip and knee surgery, and in patients with fractures, especially fractures of the femur, tibia, or pelvis and multiple, concomitant fractures.
CASE CONTINUED
Our patient was given furosemide (Lasix) empirically for diuresis and to improve oxygenation. However, his oxygen saturation remained low.
Chest radiography 4 hours after surgery showed bilateral pulmonary infiltrates. Serial electrocardiography showed no acute changes. Levels of cardiac enzymes and troponins were normal. Transthoracic echocardiography showed no left ventricular dysfunction, a normal right ventricle, and no evidence of valvular lesions. Urine and blood fat stains were negative, but the sputum stain was positive for copious extracellular fat. The patient became comatose 5 hours postoperatively. Computed tomography of the brain was normal. He was transferred to the surgical intensive care unit.
The clinical course was marked by hemodynamic instability requiring norepinephrine (Levophed) and vasopressin (Pitressin) for hypotension. Right ventricular filling pressures via central venous pressure monitoring showed no evidence of hypovolemia. The hemoglobin concentration and the hematocrit were stable, with no evidence of acute or ongoing bleeding. Blood, urine, and sputum cultures remained negative. Acute myocardial infarction was ruled out by serial electrocardiography, cardiac enzyme testing, and troponin testing.
Magnetic resonance imaging (MRI) of the brain on postoperative day 2 showed foci of acute ischemia suggestive of embolic phenomena consistent with fat embolism syndrome (Figure 1). Transthoracic echocardiography was repeated but again showed no evidence of a patent foramen ovale. Electroencephalography on postoperative day 4 showed severe, diffuse encephalopathy. There was no petechial skin rash. Other laboratory studies showed progressive thrombocytopenia with a platelet count of 53 × 199/L on postoperative day 3.
TESTS THAT AID THE CLINICAL DIAGNOSIS
Although no single laboratory test is pathognomonic for fat embolism syndrome, several tests may help raise suspicion of it, especially in the setting of fracture or an orthopedic surgical procedure.
Arterial blood gases must be measured. A Pao2 of less than 60 mm Hg with no other obvious lung pathology in an orthopedic surgery patient is highly suspicious.12 An alveolar-arterial gradient of greater than 100 mm Hg may further increase suspicion.
Tests for fat. The blood and urine may be examined for fat, although positive findings are not specific for fat embolism syndrome.33 Fat in the urine indicates the occurrence of massive fat embolism, but this is not always accompanied by the syndrome.34 Gurd and Wilson13 found fat globules larger than 8 μm circulating in the serum in all documented cases. They stated that, even though the relationship of large fat globules to the pathogenesis of the clinical picture remains obscure, the demonstration of their presence can be helpful in the diagnosis.13
Also, samples obtained with bronchoalveolar lavage may be examined for fat. The macrophages may be stained for fat using the oil red O stain. Again, this is a nonspecific marker, as fat-stained macrophages are seen in trauma patients,35 but the finding has a very high negative predictive value.36 Anemia, thrombocytopenia, hypofibrinogenemia, an elevated lipase level, and a high erythrocyte sedimentation rate may be found in fat embolism syndrome.13
Chest radiography may show bilateral infiltrates, as in ARDS, but this is not diagnostic for fat embolism syndrome.
Electrocardiography may show changes in ST and T waves and signs of right heart strain.
Transesophageal echocardiography may show increased right heart and pulmonary artery pressures.
Computed tomography is often negative,37,38 but T2-weighted MRI is useful in the diagnosis of cerebral fat embolism syndrome, as it can show intracerebral microinfarcts as early as 4 hours after the onset of neurologic symptoms, and these findings correlate well with the clinical severity of brain injury.
Diffusion-weighted MRI may enhance the sensitivity and specificity of the neuroradiologic diagnosis. Diffusion-weighted MRI typically shows multiple nonconfluent areas of high-intensity signals or bright spots on a dark background, known as a “starfield pattern.” This pattern has been suggested to be pathognomonic of acute cerebral microinfarction. The abnormalities presumably reflect foci of cytotoxic edema that develops immediately, unlike vasogenic edema, seen in T2-weighted images, which may take up to several days to develop. Although these images are not necessarily specific for fat emboli, they are useful in helping make the diagnosis. Thus, diffusionweighted MRI should be done if fat embolism syndrome is suspected.38,39
CASE CONCLUDED
The patient’s course in the intensive care unit was further complicated by gastrointestinal bleeding and renal failure. His neurologic status did not improve. Repeated MRI of the brain showed evolving bilateral watershed infarction throughout the cortices. The neurologic consult service diagnosed the patient as having severe encephalopathy with a very poor prognosis. The decision was made to withdraw care. He was placed under palliative care and died on postoperative day 22.
DRUG TREATMENT OF FAT EMBOLISM SYNDROME
5. Which of the following drugs has been proven to be effective in treating fat embolism syndrome?
- Intravenous ethanol
- Steroids
- Heparin
- Dextran
- Aspirin
- None of the above
None of the above has been proven to be effective in treating this disorder. The management is largely supportive. Thus, prevention, early diagnosis, and symptom management are vital.
Pulmonary and hemodynamic support are the cornerstones of successful treatment. Aggressive respiratory support is often needed. Management of acute lung injury and ARDS focuses on achieving acceptable gas exchange while preventing ventilator-associated lung injury. Intravascular volume must be supported. Inotropes and pulmonary vasodilators may be required to maintain hemodynamics. Exacerbation of central nervous system ischemia from hypotension or hypoxia should be avoided.
If the thrombocytopenia leads to clinical bleeding, platelet transfusions may be warranted.
Supportive care should include prophylaxis of deep venous thrombosis and of gastrointestinal bleeding, and maintenance of nutrition.40 Patients who receive supportive care generally have a favorable outcome, with a mortality rate of less than 10%.28
Drug studies have been inconclusive
Drugs suggested in the treatment of fat embolism syndrome include heparin, aspirin, dextran, hypertonic glucose, and alcohol, but the results have been inconclusive.3,11,23,40–43
Heparin stimulates lipase activity, consequently decreasing the concentration of circulating fat globules. However, the increase in levels of free fatty acids may actually worsen the clinical picture. For this reason, and because of anticoagulation concerns and evidence of increased mortality rates, heparin is now contraindicated in the treatment of fat embolism syndrome.2,41,43
Alcohol. Patients with a higher blood alcohol level at the time of injury have been reported to have a lower incidence of fat embolism syndrome. Alcohol inhibits lipase, suppressing the rise of free fatty acids. In experimental studies, the incidence of fat embolism syndrome was lower when the blood alcohol level was maintained at 20 mg/dL. However, no prospective randomized trial has been done to determine the clinical efficacy of ethanol as a treatment for this condition.5,42
Dextran has been advocated, owing to its ability to improve small-vessel perfusion, but bleeding risk and acute renal failure associated with this drug have limited its use.5
N-acetylcysteine has been shown to attenuate fat-induced lung injury in a study of rats with induced fat embolism syndrome.44
Corticosteroid treatment for this condition is controversial. Studies in patients with femoral and tibial fractures show that steroids reduce the incidence of fat embolism syndrome when given prophylactically, and those treated with steroids had a higher Pao2 than controls. Doses of methylprednisolone in these studies ranged between 9 mg/kg to 90 mg/kg. A drawback of these studies is their small number of patients.12,32,45,46
A meta-analysis47 of randomized trials of corticosteroids to prevent fat embolism syndrome in patients with long-bone fractures identified 104 such studies. Only 7 of the 104 were considered adequate. In 389 patients with long-bone fractures, prophylactic corticosteroids reduced the risk of fat embolism syndrome by 78% (95% confidence interval 43%–92%) and corticosteroids also significantly reduced the risk of hypoxia with no difference in rates of infection or death. However, the overall quality of the trials was poor, and the authors of the meta-analysis concluded that more study is needed before corticosteroids could be formally recommended.47
There is no evidence that steroids improve the overall clinical course of already established fat embolism syndrome.12,32,45 The dosing and optimal timing of administration have also not been established. High doses pose a risk of septic complications, which may be devastating for the posttrauma or postoperative patient.
- Akhtar S. Fat embolism. Anesthesiol Clin 2009; 27:533–550.
- Filomeno LT, Carelli CR, Silva NC, Filho TE, Amatuzzi MM. Fat embolism: a review for current orthopaedics practice. Acta Ortop Bras 2005; 13:196–208.
- ten Duis HJ. The fat embolism syndrome. Injury 1997; 28:77–85.
- Peltier LF. Fat embolism. A current concept. Clin Orthop Relat Res 1969; 66:241–253.
- Gossling HR, Pellegrini VD. Fat embolism syndrome: a review of the pathophysiology and physiological basis of treatment. Clin Orthop Relat Res 1982; 165:68–82.
- Papagelopoulos PJ, Apostolou CD, Karachalios TS, Themistocleous GS, Giannakopoulos CK, Ioannidis TT. Pulmonary fat embolism after total hip and total knee arthroplasty. Orthopedics 2003; 26:523–527.
- Takahashi S, Kitagawa H, Ishii T. Intraoperative pulmonary embolism during spinal instrumentation surgery. A prospective study using transoesophageal echocardiography. J Bone Joint Surg Br 2003; 85:90–94.
- Christie J, Robinson CM, Pell AC, McBirnie J, Burnett R. Transcardiac echocardiography during invasive intramedullary procedures. J Bone Joint Surg Br 1995; 77:450–455.
- Robert JH, Hoffmeyer P, Broquet PE, Cerutti P, Vasey H. Fat embolism syndrome. Orthop Rev 1993; 22:567–571.
- Mellor A, Soni N. Fat embolism. Anaesthesia 2001; 56:145–154.
- Taviloglu K, Yanar H. Fat embolism syndrome. Surg Today 2007; 37:5–8.
- Lindeque BG, Schoeman HS, Dommisse GF, Boeyens MC, Vlok AL. Fat embolism and the fat embolism syndrome. A double-blind therapeutic study. J Bone Joint Surg Br 1987; 69:128–131.
- Gurd AR, Wilson RI. The fat embolism syndrome. J Bone Joint Surg Br 1974; 56B:408–416.
- Ganong RB. Fat emboli syndrome in isolated fractures of the tibia and femur. Clin Orthop Relat Res 1993; 291:208–214.
- Djelouah I, Lefèvre G, Ozier Y, Rosencher N, Tallet F. Fat embolism in orthopedic surgery: role of bone marrow fatty acid. Anesth Analg 1997; 85:441–443.
- Barre J, Lepouse C, Segal P. Embolism and intramedullary femoral surgery. Rev Chir Orthop Reparatrice Appar Mot 1997; 83:9–21.
- Kim YH. Incidence of fat embolism syndrome after cemented or cementless bilateral simultaneous and unilateral total knee arthroplasty. J Arthroplasty 2001; 16:730–739.
- Dorr LD, Merkel C, Mellman MF, Klein I. Fat emboli in bilateral total knee arthroplasty. Predictive factors for neurologic manifestations. Clin Orthop Relat Res 1989; 248:112–118.
- Jankiewicz JJ, Sculco TP, Ranawat CS, Behr C, Tarrentino S. Onestage versus 2-stage bilateral total knee arthroplasty. Clin Orthop Relat Res 1994; 309:94–101.
- Gauss H. The pathology of fat embolism. Arch Surg 1924; 9:593–605.
- Lehman EP, Moore RM. Fat embolism, including experimental production without trauma. Arch Surg 1927; 14:621–662.
- Johnson MJ, Lucas GL. Fat embolism syndrome. Orthopedics 1996; 19:41–48.
- Benson KT. Diagnosis and treatment of fat embolism syndrome. Anesthesiology Rev 1993; 20:165–170.
- Colonna DM, Kilgus D, Brown W, Challa V, Stump DA, Moody DM. Acute brain fat embolization occurring after total hip arthroplasty in the absence of a patent foramen ovale. Anesthesiology 2002; 96:1027–1029.
- Byrick RJ, Mullen JB, Mazer CD, Guest CB. Transpulmonary systemic fat embolism. Studies in mongrel dogs after cemented arthroplasty. Am J Respir Crit Care Med 1994; 150:1416–1422.
- Benestad G. Drei Fälle von Fettembolie mit punktförmigen Blutungen in der Haut. Deutsche Ztschr f Chir 1911; 112:192.
- Hagley SR. The fulminant fat embolism syndrome. Anaesth Intensive Care 1983; 11:167–170.
- Fulde GW, Harrison P. Fat embolism—a review. Arch Emerg Med 1991; 8:233–239.
- Bulger EM, Smith DG, Maier RV, Jurkovich GJ. Fat embolism syndrome. A 10-year review. Arch Surg 1997; 132:435–439.
- King EG, Wagner WW, Ashbaugh DG, Latham LP, Halsey DR. Alterations in pulmonary microanatomy after fat embolism. In vivo observations via thoracic window of the oleic acid-embolized canine lung. Chest 1971; 59:524–530.
- Talbot M, Schemitsch EH. Fat embolism syndrome: history, definition, epidemiology. Injury 2006; 37(suppl 4):S3–S7.
- Schonfeld SA, Ploysongsang Y, DiLisio R, et al. Fat embolism prophylaxis with corticosteroids. A prospective study in high-risk patients. Ann Intern Med 1983; 99:438–443.
- Tetzlaff J, Massoli K. Fat embolism. In:Tetzlaff J, editor. Clinical Orthopedic Anesthesia. Boston, MA: Butterworth-Heinemann; 1995:341–349.
- Capan LM, Miller SM, Patel KP. Fat embolism. Anesthesiol Clin North Am 1993; 11:25–54.
- Reider E, Sherman Y, Weiss Y, Liebergall M, Pizov R. Alveolar macrophages fat stain in early diagnosis of fat embolism syndrome. Isr J Med Sci 1997; 33:654–658.
- Aoki N, Soma K, Shindo M, Kurosawa T, Ohwada T. Evaluation of potential fat emboli during placement of intramedullary nails after orthopedic fractures. Chest 1998; 113:178–181.
- Stoeger A, Daniaux M, Felber S, Stockhammer G, Aichner F, zur Nedden D. MRI findings in cerebral fat embolism. Eur Radiol 1998; 8:1590–1593.
- Takahashi M, Suzuki R, Osakabe Y, et al. Magnetic resonance imaging findings in cerebral fat embolism: correlation with clinical manifestations. J Trauma 1999; 46:324–327.
- Parizel PM, Demey HE, Veeckmans G, et al. Early diagnosis of cerebral fat embolism syndrome by diffusion-weighted MRI (starfield pattern). Stroke 2001; 32:2942–2944.
- Habashi NM, Andrews PL, Scalea TM. Therapeutic aspects of fat embolism syndrome. Injury 2006; 37(suppl 4):S68–S73.
- Sage RH, Tudor RW. Treatment of fat embolism with heparin. Br Med J 1958; 1:1160–1161.
- Myers R, Taljaard JJ. Blood alcohol and fat embolism syndrome. J Bone Joint Surg Am 1977; 59:878–880.
- Denman EE, Cairnes CS, Holmes CM. Case of severe fat embolism treated by intermittent positive-pressure respiration. Br Med J 1964; 2:101–102.
- Liu DD, Kao SJ, Chen HI. N-Acetylcysteine attenuates acute lung injury induced by fat embolism. Crit Care Med 2008; 36:565–571.
- Kallenbach J, Lewis M, Zaltzman M, Feldman C, Orford A, Zwi S. ‘Low-dose’ corticosteroid prophylaxis against fat embolism. J Trauma 1987; 27:1173–1176.
- Stoltenberg JJ, Gustilo RB. The use of methylprednisolone and hypertonic glucose in the prophylaxis of fat embolism syndrome. Clin Orthop Relat Res 1979; 143:211–221.
- Bederman SS, Bhandari M, McKee MD, Schemitsch EH. Do corticosteroids reduce the risk of fat embolism syndrome in patients with long-bone fractures? A meta-analysis. Can J Surg 2009; 52:386–393.
A 75-year-old man with type 2 diabetes and hypothyroidism underwent bilateral total knee replacement at our hospital.
His functional capacity had been moderately limited by knee pain, but he could easily climb one flight of stairs without symptoms. His medications at that time included levothyroxine (Synthroid) and metformin (Glucophage). He had no known cardiac or pulmonary disease. The preoperative evaluation, including laboratory tests and electrocardiography, was within normal limits.
Spinal anesthesia was used for surgery, and he was given 2 mg of midazolam (Versed) intravenously for sedation. No additional sedation was given. He was given oxygen via nasal cannula at 2 L/min.
All vital signs were stable at the start of the procedure. However, about halfway through, when the thigh tourniquet was released, his oxygen saturation dropped abruptly from 100% to 92%. All other vital signs remained stable, and he was asymptomatic, was oriented to person, time, and place, was conversing freely, and was in no distress. The oxygen flow was increased to 6 L/min, his oxygen saturation improved, and the procedure was then completed as planned.
At the conclusion of the surgery, before the patient was transported to the postanesthesia care unit (PACU) and while his oxygen flow rate was still 6 L/min, his oxygen saturation again dropped to 92%. A simple face mask was placed, and the oxygen flow rate was increased to 10 L/min. His oxygen saturation stayed low, near 90%.
Bleeding during surgery had been nominal. He had received 2 L of lactated Ringer’s solution and 500 mL of hetastarch (Hextend) during surgery. He continued to be asymptomatic in the PACU.
1. What is the most likely cause of oxygen desaturation during bilateral total knee arthroplasty?
- Fat embolism
- Intraoperative pneumonia
- Venous thromboembolism with pulmonary embolism
- Acute myocardial infarction
- Acute pulmonary edema
- Excessive sedation
The differential diagnosis of oxygen desaturation during orthopedic procedures is listed in Table 1.
Fat embolism is the most likely cause, particularly given the greater fatty embolic load that occurs with bilateral total knee arthroplasty than with unilateral total knee arthroplasty.
At what point the maximal showering of fat emboli occurs is not known. Fat may be released into the circulation with pressurization of the medullary canal during surgery or with manipulation of a fracture. The emboli may collect in the leg veins and then be released in a shower when the thigh tourniquet is released. Vasoactive mediators and methylmethacrylate cement released into the circulatory system after tourniquet deflation may also cause vasodilation, hypotension, and increased dead-space ventilation, resulting in hypoxia and a drop in end-tidal CO2.
Pneumonia during surgery is rare without an apparent aspiration event.
Venous thromboembolism is possible but is more likely later in the postoperative period after major orthopedic surgery.
Acute myocardial infarction could present with hypoxia, particularly in a diabetic patient, who may not experience chest pain. However, intraoperative electrocardiographic changes would likely be seen. If myocardial infarction is suspected, postoperative serial electrocardiograms and measuring troponin and cardiac enzyme levels aid in the diagnosis.
Acute pulmonary edema is possible but not as highly suspected, as the patient had no history of congestive heart failure and received an appropriate amount of fluid for this type of surgery.
Excessive sedation could cause hypoventilation and, thus, oxygen desaturatation. However, this patient’s oxygen desaturatation began more than an hour after the midazolam was given. Midazolam is a short-acting benzodiazepine. It is unlikely that the patient would show signs of hypoventilation and oversedation an hour after the drug was given. Our patient also did not show any signs of excessive sedation, as he was awake and conversing during the surgery.
Fat emboli vs fat embolism syndrome
Fat embolism is the presence of fat drops within the systemic and pulmonary microcirculation, with or without clinical sequelae.1 Fat embolism syndrome, on the other hand, is defined as injury to and dysfunction of one or more organs as a result of the embolization of fat, usually within 24 hours of injury or orthopedic surgery.2
Fat embolism syndrome is an unpredictable condition with a varied presentation. Fat droplets are thought to embolize via the venous circulation into the pulmonary arteries, occluding small blood vessels in the lung. However, they also get into the arterial circulation and occlude arteries in the brain, kidney, heart, and liver (more on this phenomenon below).
Fat embolism is reported to originate primarily from fractures of the femur, tibia, and pelvis.2,3 As many as 90% of trauma patients have been shown to have evidence of fat embolism on autopsy.4 However, only a small number of patients develop the classic fat embolism syndrome,2,3,5 Why some develop the syndrome and others do not is still unknown.
Orthopedic procedures associated with fat embolization include knee arthroplasty and hip arthroplasty, particularly if it involves intramedullary manipulation or medullary fixation.6 It has also been reported during spinal procedures in which pedicular screws are used.7 The syndrome occurs in 0.25% to 30% of patients following multiple fractures and in 0.1% to 12% of patients during or following knee or hip arthroplasty.
One study8 showed evidence of fat on transesophageal echocardiography in 88% of patients undergoing medullary reaming of lower-extremity fractures and hip hemiarthroplasty. Blood sampling from the right atrium confirmed that fat was responsible for the echocardiographic abnormalities. The study also showed that the severity of the embolic showering correlated with the severity of hypoxia and the decrease in end-tidal CO2.8
CASE CONTINUED
On arrival at the PACU, our patient’s oxygen saturation was 94% while he was breathing oxygen via a simple face mask at a flow rate of 10 L/min. His heart rate was 60 bpm, blood pressure 110/60 mm Hg, and temperature 37.5°C (96.3°F). Chest sounds were normal on auscultation.
However, 3 hours later, his mental status rapidly deteriorated. He was oriented only to person, and he was drowsy. He had escalating respiratory distress with a rapid respiratory rate and decreasing oxygen saturation. At this point, auscultation of his chest wall revealed bilateral crackles and rales.
He was promptly intubated. Profuse fluid and secretions were noted to be coming from his lungs, filling the endotracheal tube. Arterial blood gas measurement showed a pH of 7.22, Pao2 64 mm Hg, and Paco2 56 mm Hg on 100% fraction of inspired oxygen, with no increased anion gap.
2. Which consequence of fat embolism is most likely at this time in this patient?
- Coexisting sepsis
- Fat embolism syndrome
- Acute cardioembolic stroke
- Anaphylaxis
Fat embolism syndrome should be highly suspected in this patient. As mentioned, it can affect many different organs. It is the most serious condition resulting from fat embolization after surgery or trauma.
Sepsis was unlikely in our patient, since he presented for his surgery in good health and with no preexisting signs or symptoms of infection. Acute cardioembolic stroke could have caused the neurologic signs, but this would not necessarily explain the coexisting hypoxia. An anaphylactic reaction to drugs or surgical cement would most likely present intraoperatively, shortly after exposure occurred, rather than several hours after surgery.
How common is fat embolism syndrome?
The occurrence rate of fat embolism syndrome has been reported to be 0.25% to 30% after multiple fractures and 0.1% to 12% after knee and hip joint surgery, with a mortality rate of 13% to 36%.2,9–14 The rate of occurrence after unilateral total knee joint replacement has been reported to be 1.8% to 5%, and 4% to 12% after bilateral total knee replacement.15–19
The syndrome is relatively more common with traumatic fractures of the lower extremities. However, it has also been reported with liposuction, total parenteral nutrition, bone marrow harvest and transplantation, burns, and acute pancreatitis, to mention a few.10
The broad range of reported incidence rates can be attributed to the fact that many studies were in patients with multiple trauma, whose concomitant injuries may have made it difficult to clearly define the contribution of fat embolism syndrome to the overall rates of morbidity and mortality. Also, different studies used different criteria to define the syndrome.
How does fat embolism syndrome occur?
Two hypotheses for how this syndrome occurs were proposed nearly a century ago.20,21
The “mechanical” theory is that fat emboli are formed as a result of trauma and disruption of adipose tissue and other cells in the bone marrow. Increases in intramedullary pressure force the fat emboli through damaged medullary venous channels in the bone and into the circulation of the lower extremities. This embolization of fat causes an initial mechanical pulmonary obstruction. Mechanical obstruction by fat emboli in the pulmonary system leads to increased pulmonary pressures and an increase in right heart outflow pressure. The right heart becomes strained, leading to a decreased right-sided cardiac output. As a result, the left heart filling pressures diminish and hypotension ensues.20
The “biochemical” theory, on the other hand, is that chylomicrons within the vascular system are modified and their stability is compromised as a result of stress. These traumatized chylomicrons then coalesce to form droplets of fat that accumulate in the pulmonary circulation and produce a mechanical obstruction. This would explain why nontraumatic, nonorthopedic insults can produce this syndrome.
Autopsy studies show that there is little correlation between the presence and quantity of intravascular fat and the severity of clinical symptoms, thus implying that the syndrome is caused by more than just mechanical obstruction. The biochemical theory postulates that fat globules within the circulatory system then cause the release of lipase from the pulmonary alveolar cells, which then hydrolyses the fat into free fatty acids. These free fatty acids cause an inflammatory reaction, complementmediated leukocyte aggregation, chemotoxin release, and subsequently endothelial damage. These vasoactive substances damage type 2 pneumocytes and lead to an increased permeability of the pulmonary capillary beds. Acute respiratory distress syndrome (ARDS) may ensue. Disseminated intravascular coagulation may occur as a result of the formation of microthrombi involving lipids, platelets, and fibrin.21,22
Embolization of fat to the central nervous system can occur as fat globules cross into the systemic circulation via a patent foramen ovale, an atrioventricular shunt, or the pulmonary capillaries. This can then result in cerebral ischemia.23
Although patent foramen ovale may seem the most direct route for cerebral embolization, the neurologic impairment and signs of cerebral emboli in fat embolism syndrome may occur in the absence of patent foramen ovale.24,25 The fat globules may actually go through the lung capillaries, being flexible and forced through by increased pulmonary pressure.
But whether the cause of fat embolism syndrome is occlusion by globules, the release of biochemical mediators, or a combination of both is unknown. Both mechanisms are likely responsible. We can only suspect that the degree of fat load and intrinsic metabolic differences between individuals account for the variation in susceptibility.
FAT EMBOLI AFFECT THE LUNGS, SKIN, AND BRAIN
3. Where on the body is the rash associated with fat embolism syndrome usually seen?
- Face
- Near a site of fracture or surgery
- Chest, axilla, conjunctiva
- Distal extremities
Petechiae are part of the classic presenting triad of fat embolism syndrome, which also includes pulmonary and cerebral dysfunction.
Petechiae usually appear on the 2nd to 4th day after injury.26 They are usually found across the chest, the anterior axillary folds, and the neck, as well as on the oral mucosa and the conjunctiva. The rash is caused by occlusion of dermal capillaries by fat, which increases their fragility.10
Pulmonary changes usually begin with tachypnea, dyspnea, and a drop in oxygen saturation, leading to generalized hypoxia. Respiratory symptoms are present in 100% of cases.2 Respiratory symptoms can acutely develop with the sudden manipulation of a fracture, reaming of bone, or release of a limb tourniquet.27
Body systems affected by fat embolism syndrome are summarized in Table 2.
4. How many hours after injury does fat embolism syndrome typically manifest?
- 1 to 2 hours
- 6 to 12 hours
- 12 to 20 hours
- 24 to 48 hours
- 72 to 84 hours
Most patients develop signs and symptoms 24 to 48 hours after injury. Patients presenting earlier than 12 hours usually have a more fulminant course.29
The time between fat embolization and the development of fat embolism syndrome is thought to be related to the time required for the metabolic conversion of fat to free fatty acids.30 We suspect that the early desaturation seen in our patient was the result of a heavy showering of fat intraoperatively. However, this could only be concluded after we had ruled out other causes of acute hypoxia and hypotension.
Fat embolism syndrome is a diagnosis of exclusion and is based on clinical criteria. No specific sign, symptom, or test is pathognomonic. It may often be confused with other conditions such as systemic inflammatory response syndrome or sepsis. However, the triad of respiratory and neurologic symptoms and petechiae coupled with the clinical picture of recent trauma or orthopedic surgery almost assures the diagnosis.
Fat embolism syndrome can range from subclinical to fulminating, with the more fulminating course attributable to a huge load of fat emboli, which leads to acute cor pulmonale.
Regardless of the criteria used, one must have a high index of suspicion for fat embolization syndrome in patients undergoing orthopedic procedures, particularly hip and knee surgery, and in patients with fractures, especially fractures of the femur, tibia, or pelvis and multiple, concomitant fractures.
CASE CONTINUED
Our patient was given furosemide (Lasix) empirically for diuresis and to improve oxygenation. However, his oxygen saturation remained low.
Chest radiography 4 hours after surgery showed bilateral pulmonary infiltrates. Serial electrocardiography showed no acute changes. Levels of cardiac enzymes and troponins were normal. Transthoracic echocardiography showed no left ventricular dysfunction, a normal right ventricle, and no evidence of valvular lesions. Urine and blood fat stains were negative, but the sputum stain was positive for copious extracellular fat. The patient became comatose 5 hours postoperatively. Computed tomography of the brain was normal. He was transferred to the surgical intensive care unit.
The clinical course was marked by hemodynamic instability requiring norepinephrine (Levophed) and vasopressin (Pitressin) for hypotension. Right ventricular filling pressures via central venous pressure monitoring showed no evidence of hypovolemia. The hemoglobin concentration and the hematocrit were stable, with no evidence of acute or ongoing bleeding. Blood, urine, and sputum cultures remained negative. Acute myocardial infarction was ruled out by serial electrocardiography, cardiac enzyme testing, and troponin testing.
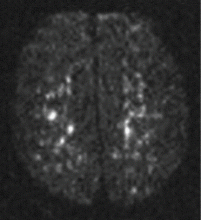
Magnetic resonance imaging (MRI) of the brain on postoperative day 2 showed foci of acute ischemia suggestive of embolic phenomena consistent with fat embolism syndrome (Figure 1). Transthoracic echocardiography was repeated but again showed no evidence of a patent foramen ovale. Electroencephalography on postoperative day 4 showed severe, diffuse encephalopathy. There was no petechial skin rash. Other laboratory studies showed progressive thrombocytopenia with a platelet count of 53 × 199/L on postoperative day 3.
TESTS THAT AID THE CLINICAL DIAGNOSIS
Although no single laboratory test is pathognomonic for fat embolism syndrome, several tests may help raise suspicion of it, especially in the setting of fracture or an orthopedic surgical procedure.
Arterial blood gases must be measured. A Pao2 of less than 60 mm Hg with no other obvious lung pathology in an orthopedic surgery patient is highly suspicious.12 An alveolar-arterial gradient of greater than 100 mm Hg may further increase suspicion.
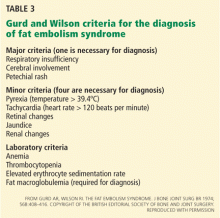
Tests for fat. The blood and urine may be examined for fat, although positive findings are not specific for fat embolism syndrome.33 Fat in the urine indicates the occurrence of massive fat embolism, but this is not always accompanied by the syndrome.34 Gurd and Wilson13 found fat globules larger than 8 μm circulating in the serum in all documented cases. They stated that, even though the relationship of large fat globules to the pathogenesis of the clinical picture remains obscure, the demonstration of their presence can be helpful in the diagnosis.13
Also, samples obtained with bronchoalveolar lavage may be examined for fat. The macrophages may be stained for fat using the oil red O stain. Again, this is a nonspecific marker, as fat-stained macrophages are seen in trauma patients,35 but the finding has a very high negative predictive value.36 Anemia, thrombocytopenia, hypofibrinogenemia, an elevated lipase level, and a high erythrocyte sedimentation rate may be found in fat embolism syndrome.13
Chest radiography may show bilateral infiltrates, as in ARDS, but this is not diagnostic for fat embolism syndrome.
Electrocardiography may show changes in ST and T waves and signs of right heart strain.
Transesophageal echocardiography may show increased right heart and pulmonary artery pressures.
Computed tomography is often negative,37,38 but T2-weighted MRI is useful in the diagnosis of cerebral fat embolism syndrome, as it can show intracerebral microinfarcts as early as 4 hours after the onset of neurologic symptoms, and these findings correlate well with the clinical severity of brain injury.
Diffusion-weighted MRI may enhance the sensitivity and specificity of the neuroradiologic diagnosis. Diffusion-weighted MRI typically shows multiple nonconfluent areas of high-intensity signals or bright spots on a dark background, known as a “starfield pattern.” This pattern has been suggested to be pathognomonic of acute cerebral microinfarction. The abnormalities presumably reflect foci of cytotoxic edema that develops immediately, unlike vasogenic edema, seen in T2-weighted images, which may take up to several days to develop. Although these images are not necessarily specific for fat emboli, they are useful in helping make the diagnosis. Thus, diffusionweighted MRI should be done if fat embolism syndrome is suspected.38,39
CASE CONCLUDED
The patient’s course in the intensive care unit was further complicated by gastrointestinal bleeding and renal failure. His neurologic status did not improve. Repeated MRI of the brain showed evolving bilateral watershed infarction throughout the cortices. The neurologic consult service diagnosed the patient as having severe encephalopathy with a very poor prognosis. The decision was made to withdraw care. He was placed under palliative care and died on postoperative day 22.
DRUG TREATMENT OF FAT EMBOLISM SYNDROME
5. Which of the following drugs has been proven to be effective in treating fat embolism syndrome?
- Intravenous ethanol
- Steroids
- Heparin
- Dextran
- Aspirin
- None of the above
None of the above has been proven to be effective in treating this disorder. The management is largely supportive. Thus, prevention, early diagnosis, and symptom management are vital.
Pulmonary and hemodynamic support are the cornerstones of successful treatment. Aggressive respiratory support is often needed. Management of acute lung injury and ARDS focuses on achieving acceptable gas exchange while preventing ventilator-associated lung injury. Intravascular volume must be supported. Inotropes and pulmonary vasodilators may be required to maintain hemodynamics. Exacerbation of central nervous system ischemia from hypotension or hypoxia should be avoided.
If the thrombocytopenia leads to clinical bleeding, platelet transfusions may be warranted.
Supportive care should include prophylaxis of deep venous thrombosis and of gastrointestinal bleeding, and maintenance of nutrition.40 Patients who receive supportive care generally have a favorable outcome, with a mortality rate of less than 10%.28
Drug studies have been inconclusive
Drugs suggested in the treatment of fat embolism syndrome include heparin, aspirin, dextran, hypertonic glucose, and alcohol, but the results have been inconclusive.3,11,23,40–43
Heparin stimulates lipase activity, consequently decreasing the concentration of circulating fat globules. However, the increase in levels of free fatty acids may actually worsen the clinical picture. For this reason, and because of anticoagulation concerns and evidence of increased mortality rates, heparin is now contraindicated in the treatment of fat embolism syndrome.2,41,43
Alcohol. Patients with a higher blood alcohol level at the time of injury have been reported to have a lower incidence of fat embolism syndrome. Alcohol inhibits lipase, suppressing the rise of free fatty acids. In experimental studies, the incidence of fat embolism syndrome was lower when the blood alcohol level was maintained at 20 mg/dL. However, no prospective randomized trial has been done to determine the clinical efficacy of ethanol as a treatment for this condition.5,42
Dextran has been advocated, owing to its ability to improve small-vessel perfusion, but bleeding risk and acute renal failure associated with this drug have limited its use.5
N-acetylcysteine has been shown to attenuate fat-induced lung injury in a study of rats with induced fat embolism syndrome.44
Corticosteroid treatment for this condition is controversial. Studies in patients with femoral and tibial fractures show that steroids reduce the incidence of fat embolism syndrome when given prophylactically, and those treated with steroids had a higher Pao2 than controls. Doses of methylprednisolone in these studies ranged between 9 mg/kg to 90 mg/kg. A drawback of these studies is their small number of patients.12,32,45,46
A meta-analysis47 of randomized trials of corticosteroids to prevent fat embolism syndrome in patients with long-bone fractures identified 104 such studies. Only 7 of the 104 were considered adequate. In 389 patients with long-bone fractures, prophylactic corticosteroids reduced the risk of fat embolism syndrome by 78% (95% confidence interval 43%–92%) and corticosteroids also significantly reduced the risk of hypoxia with no difference in rates of infection or death. However, the overall quality of the trials was poor, and the authors of the meta-analysis concluded that more study is needed before corticosteroids could be formally recommended.47
There is no evidence that steroids improve the overall clinical course of already established fat embolism syndrome.12,32,45 The dosing and optimal timing of administration have also not been established. High doses pose a risk of septic complications, which may be devastating for the posttrauma or postoperative patient.
A 75-year-old man with type 2 diabetes and hypothyroidism underwent bilateral total knee replacement at our hospital.
His functional capacity had been moderately limited by knee pain, but he could easily climb one flight of stairs without symptoms. His medications at that time included levothyroxine (Synthroid) and metformin (Glucophage). He had no known cardiac or pulmonary disease. The preoperative evaluation, including laboratory tests and electrocardiography, was within normal limits.
Spinal anesthesia was used for surgery, and he was given 2 mg of midazolam (Versed) intravenously for sedation. No additional sedation was given. He was given oxygen via nasal cannula at 2 L/min.
All vital signs were stable at the start of the procedure. However, about halfway through, when the thigh tourniquet was released, his oxygen saturation dropped abruptly from 100% to 92%. All other vital signs remained stable, and he was asymptomatic, was oriented to person, time, and place, was conversing freely, and was in no distress. The oxygen flow was increased to 6 L/min, his oxygen saturation improved, and the procedure was then completed as planned.
At the conclusion of the surgery, before the patient was transported to the postanesthesia care unit (PACU) and while his oxygen flow rate was still 6 L/min, his oxygen saturation again dropped to 92%. A simple face mask was placed, and the oxygen flow rate was increased to 10 L/min. His oxygen saturation stayed low, near 90%.
Bleeding during surgery had been nominal. He had received 2 L of lactated Ringer’s solution and 500 mL of hetastarch (Hextend) during surgery. He continued to be asymptomatic in the PACU.
1. What is the most likely cause of oxygen desaturation during bilateral total knee arthroplasty?
- Fat embolism
- Intraoperative pneumonia
- Venous thromboembolism with pulmonary embolism
- Acute myocardial infarction
- Acute pulmonary edema
- Excessive sedation
The differential diagnosis of oxygen desaturation during orthopedic procedures is listed in Table 1.
Fat embolism is the most likely cause, particularly given the greater fatty embolic load that occurs with bilateral total knee arthroplasty than with unilateral total knee arthroplasty.
At what point the maximal showering of fat emboli occurs is not known. Fat may be released into the circulation with pressurization of the medullary canal during surgery or with manipulation of a fracture. The emboli may collect in the leg veins and then be released in a shower when the thigh tourniquet is released. Vasoactive mediators and methylmethacrylate cement released into the circulatory system after tourniquet deflation may also cause vasodilation, hypotension, and increased dead-space ventilation, resulting in hypoxia and a drop in end-tidal CO2.
Pneumonia during surgery is rare without an apparent aspiration event.
Venous thromboembolism is possible but is more likely later in the postoperative period after major orthopedic surgery.
Acute myocardial infarction could present with hypoxia, particularly in a diabetic patient, who may not experience chest pain. However, intraoperative electrocardiographic changes would likely be seen. If myocardial infarction is suspected, postoperative serial electrocardiograms and measuring troponin and cardiac enzyme levels aid in the diagnosis.
Acute pulmonary edema is possible but not as highly suspected, as the patient had no history of congestive heart failure and received an appropriate amount of fluid for this type of surgery.
Excessive sedation could cause hypoventilation and, thus, oxygen desaturatation. However, this patient’s oxygen desaturatation began more than an hour after the midazolam was given. Midazolam is a short-acting benzodiazepine. It is unlikely that the patient would show signs of hypoventilation and oversedation an hour after the drug was given. Our patient also did not show any signs of excessive sedation, as he was awake and conversing during the surgery.
Fat emboli vs fat embolism syndrome
Fat embolism is the presence of fat drops within the systemic and pulmonary microcirculation, with or without clinical sequelae.1 Fat embolism syndrome, on the other hand, is defined as injury to and dysfunction of one or more organs as a result of the embolization of fat, usually within 24 hours of injury or orthopedic surgery.2
Fat embolism syndrome is an unpredictable condition with a varied presentation. Fat droplets are thought to embolize via the venous circulation into the pulmonary arteries, occluding small blood vessels in the lung. However, they also get into the arterial circulation and occlude arteries in the brain, kidney, heart, and liver (more on this phenomenon below).
Fat embolism is reported to originate primarily from fractures of the femur, tibia, and pelvis.2,3 As many as 90% of trauma patients have been shown to have evidence of fat embolism on autopsy.4 However, only a small number of patients develop the classic fat embolism syndrome,2,3,5 Why some develop the syndrome and others do not is still unknown.
Orthopedic procedures associated with fat embolization include knee arthroplasty and hip arthroplasty, particularly if it involves intramedullary manipulation or medullary fixation.6 It has also been reported during spinal procedures in which pedicular screws are used.7 The syndrome occurs in 0.25% to 30% of patients following multiple fractures and in 0.1% to 12% of patients during or following knee or hip arthroplasty.
One study8 showed evidence of fat on transesophageal echocardiography in 88% of patients undergoing medullary reaming of lower-extremity fractures and hip hemiarthroplasty. Blood sampling from the right atrium confirmed that fat was responsible for the echocardiographic abnormalities. The study also showed that the severity of the embolic showering correlated with the severity of hypoxia and the decrease in end-tidal CO2.8
CASE CONTINUED
On arrival at the PACU, our patient’s oxygen saturation was 94% while he was breathing oxygen via a simple face mask at a flow rate of 10 L/min. His heart rate was 60 bpm, blood pressure 110/60 mm Hg, and temperature 37.5°C (96.3°F). Chest sounds were normal on auscultation.
However, 3 hours later, his mental status rapidly deteriorated. He was oriented only to person, and he was drowsy. He had escalating respiratory distress with a rapid respiratory rate and decreasing oxygen saturation. At this point, auscultation of his chest wall revealed bilateral crackles and rales.
He was promptly intubated. Profuse fluid and secretions were noted to be coming from his lungs, filling the endotracheal tube. Arterial blood gas measurement showed a pH of 7.22, Pao2 64 mm Hg, and Paco2 56 mm Hg on 100% fraction of inspired oxygen, with no increased anion gap.
2. Which consequence of fat embolism is most likely at this time in this patient?
- Coexisting sepsis
- Fat embolism syndrome
- Acute cardioembolic stroke
- Anaphylaxis
Fat embolism syndrome should be highly suspected in this patient. As mentioned, it can affect many different organs. It is the most serious condition resulting from fat embolization after surgery or trauma.
Sepsis was unlikely in our patient, since he presented for his surgery in good health and with no preexisting signs or symptoms of infection. Acute cardioembolic stroke could have caused the neurologic signs, but this would not necessarily explain the coexisting hypoxia. An anaphylactic reaction to drugs or surgical cement would most likely present intraoperatively, shortly after exposure occurred, rather than several hours after surgery.
How common is fat embolism syndrome?
The occurrence rate of fat embolism syndrome has been reported to be 0.25% to 30% after multiple fractures and 0.1% to 12% after knee and hip joint surgery, with a mortality rate of 13% to 36%.2,9–14 The rate of occurrence after unilateral total knee joint replacement has been reported to be 1.8% to 5%, and 4% to 12% after bilateral total knee replacement.15–19
The syndrome is relatively more common with traumatic fractures of the lower extremities. However, it has also been reported with liposuction, total parenteral nutrition, bone marrow harvest and transplantation, burns, and acute pancreatitis, to mention a few.10
The broad range of reported incidence rates can be attributed to the fact that many studies were in patients with multiple trauma, whose concomitant injuries may have made it difficult to clearly define the contribution of fat embolism syndrome to the overall rates of morbidity and mortality. Also, different studies used different criteria to define the syndrome.
How does fat embolism syndrome occur?
Two hypotheses for how this syndrome occurs were proposed nearly a century ago.20,21
The “mechanical” theory is that fat emboli are formed as a result of trauma and disruption of adipose tissue and other cells in the bone marrow. Increases in intramedullary pressure force the fat emboli through damaged medullary venous channels in the bone and into the circulation of the lower extremities. This embolization of fat causes an initial mechanical pulmonary obstruction. Mechanical obstruction by fat emboli in the pulmonary system leads to increased pulmonary pressures and an increase in right heart outflow pressure. The right heart becomes strained, leading to a decreased right-sided cardiac output. As a result, the left heart filling pressures diminish and hypotension ensues.20
The “biochemical” theory, on the other hand, is that chylomicrons within the vascular system are modified and their stability is compromised as a result of stress. These traumatized chylomicrons then coalesce to form droplets of fat that accumulate in the pulmonary circulation and produce a mechanical obstruction. This would explain why nontraumatic, nonorthopedic insults can produce this syndrome.
Autopsy studies show that there is little correlation between the presence and quantity of intravascular fat and the severity of clinical symptoms, thus implying that the syndrome is caused by more than just mechanical obstruction. The biochemical theory postulates that fat globules within the circulatory system then cause the release of lipase from the pulmonary alveolar cells, which then hydrolyses the fat into free fatty acids. These free fatty acids cause an inflammatory reaction, complementmediated leukocyte aggregation, chemotoxin release, and subsequently endothelial damage. These vasoactive substances damage type 2 pneumocytes and lead to an increased permeability of the pulmonary capillary beds. Acute respiratory distress syndrome (ARDS) may ensue. Disseminated intravascular coagulation may occur as a result of the formation of microthrombi involving lipids, platelets, and fibrin.21,22
Embolization of fat to the central nervous system can occur as fat globules cross into the systemic circulation via a patent foramen ovale, an atrioventricular shunt, or the pulmonary capillaries. This can then result in cerebral ischemia.23
Although patent foramen ovale may seem the most direct route for cerebral embolization, the neurologic impairment and signs of cerebral emboli in fat embolism syndrome may occur in the absence of patent foramen ovale.24,25 The fat globules may actually go through the lung capillaries, being flexible and forced through by increased pulmonary pressure.
But whether the cause of fat embolism syndrome is occlusion by globules, the release of biochemical mediators, or a combination of both is unknown. Both mechanisms are likely responsible. We can only suspect that the degree of fat load and intrinsic metabolic differences between individuals account for the variation in susceptibility.
FAT EMBOLI AFFECT THE LUNGS, SKIN, AND BRAIN
3. Where on the body is the rash associated with fat embolism syndrome usually seen?
- Face
- Near a site of fracture or surgery
- Chest, axilla, conjunctiva
- Distal extremities
Petechiae are part of the classic presenting triad of fat embolism syndrome, which also includes pulmonary and cerebral dysfunction.
Petechiae usually appear on the 2nd to 4th day after injury.26 They are usually found across the chest, the anterior axillary folds, and the neck, as well as on the oral mucosa and the conjunctiva. The rash is caused by occlusion of dermal capillaries by fat, which increases their fragility.10
Pulmonary changes usually begin with tachypnea, dyspnea, and a drop in oxygen saturation, leading to generalized hypoxia. Respiratory symptoms are present in 100% of cases.2 Respiratory symptoms can acutely develop with the sudden manipulation of a fracture, reaming of bone, or release of a limb tourniquet.27
Body systems affected by fat embolism syndrome are summarized in Table 2.
4. How many hours after injury does fat embolism syndrome typically manifest?
- 1 to 2 hours
- 6 to 12 hours
- 12 to 20 hours
- 24 to 48 hours
- 72 to 84 hours
Most patients develop signs and symptoms 24 to 48 hours after injury. Patients presenting earlier than 12 hours usually have a more fulminant course.29
The time between fat embolization and the development of fat embolism syndrome is thought to be related to the time required for the metabolic conversion of fat to free fatty acids.30 We suspect that the early desaturation seen in our patient was the result of a heavy showering of fat intraoperatively. However, this could only be concluded after we had ruled out other causes of acute hypoxia and hypotension.
Fat embolism syndrome is a diagnosis of exclusion and is based on clinical criteria. No specific sign, symptom, or test is pathognomonic. It may often be confused with other conditions such as systemic inflammatory response syndrome or sepsis. However, the triad of respiratory and neurologic symptoms and petechiae coupled with the clinical picture of recent trauma or orthopedic surgery almost assures the diagnosis.
Fat embolism syndrome can range from subclinical to fulminating, with the more fulminating course attributable to a huge load of fat emboli, which leads to acute cor pulmonale.
Regardless of the criteria used, one must have a high index of suspicion for fat embolization syndrome in patients undergoing orthopedic procedures, particularly hip and knee surgery, and in patients with fractures, especially fractures of the femur, tibia, or pelvis and multiple, concomitant fractures.
CASE CONTINUED
Our patient was given furosemide (Lasix) empirically for diuresis and to improve oxygenation. However, his oxygen saturation remained low.
Chest radiography 4 hours after surgery showed bilateral pulmonary infiltrates. Serial electrocardiography showed no acute changes. Levels of cardiac enzymes and troponins were normal. Transthoracic echocardiography showed no left ventricular dysfunction, a normal right ventricle, and no evidence of valvular lesions. Urine and blood fat stains were negative, but the sputum stain was positive for copious extracellular fat. The patient became comatose 5 hours postoperatively. Computed tomography of the brain was normal. He was transferred to the surgical intensive care unit.
The clinical course was marked by hemodynamic instability requiring norepinephrine (Levophed) and vasopressin (Pitressin) for hypotension. Right ventricular filling pressures via central venous pressure monitoring showed no evidence of hypovolemia. The hemoglobin concentration and the hematocrit were stable, with no evidence of acute or ongoing bleeding. Blood, urine, and sputum cultures remained negative. Acute myocardial infarction was ruled out by serial electrocardiography, cardiac enzyme testing, and troponin testing.
Magnetic resonance imaging (MRI) of the brain on postoperative day 2 showed foci of acute ischemia suggestive of embolic phenomena consistent with fat embolism syndrome (Figure 1). Transthoracic echocardiography was repeated but again showed no evidence of a patent foramen ovale. Electroencephalography on postoperative day 4 showed severe, diffuse encephalopathy. There was no petechial skin rash. Other laboratory studies showed progressive thrombocytopenia with a platelet count of 53 × 199/L on postoperative day 3.
TESTS THAT AID THE CLINICAL DIAGNOSIS
Although no single laboratory test is pathognomonic for fat embolism syndrome, several tests may help raise suspicion of it, especially in the setting of fracture or an orthopedic surgical procedure.
Arterial blood gases must be measured. A Pao2 of less than 60 mm Hg with no other obvious lung pathology in an orthopedic surgery patient is highly suspicious.12 An alveolar-arterial gradient of greater than 100 mm Hg may further increase suspicion.
Tests for fat. The blood and urine may be examined for fat, although positive findings are not specific for fat embolism syndrome.33 Fat in the urine indicates the occurrence of massive fat embolism, but this is not always accompanied by the syndrome.34 Gurd and Wilson13 found fat globules larger than 8 μm circulating in the serum in all documented cases. They stated that, even though the relationship of large fat globules to the pathogenesis of the clinical picture remains obscure, the demonstration of their presence can be helpful in the diagnosis.13
Also, samples obtained with bronchoalveolar lavage may be examined for fat. The macrophages may be stained for fat using the oil red O stain. Again, this is a nonspecific marker, as fat-stained macrophages are seen in trauma patients,35 but the finding has a very high negative predictive value.36 Anemia, thrombocytopenia, hypofibrinogenemia, an elevated lipase level, and a high erythrocyte sedimentation rate may be found in fat embolism syndrome.13
Chest radiography may show bilateral infiltrates, as in ARDS, but this is not diagnostic for fat embolism syndrome.
Electrocardiography may show changes in ST and T waves and signs of right heart strain.
Transesophageal echocardiography may show increased right heart and pulmonary artery pressures.
Computed tomography is often negative,37,38 but T2-weighted MRI is useful in the diagnosis of cerebral fat embolism syndrome, as it can show intracerebral microinfarcts as early as 4 hours after the onset of neurologic symptoms, and these findings correlate well with the clinical severity of brain injury.
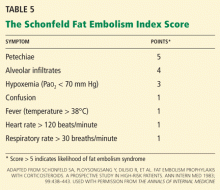
Diffusion-weighted MRI may enhance the sensitivity and specificity of the neuroradiologic diagnosis. Diffusion-weighted MRI typically shows multiple nonconfluent areas of high-intensity signals or bright spots on a dark background, known as a “starfield pattern.” This pattern has been suggested to be pathognomonic of acute cerebral microinfarction. The abnormalities presumably reflect foci of cytotoxic edema that develops immediately, unlike vasogenic edema, seen in T2-weighted images, which may take up to several days to develop. Although these images are not necessarily specific for fat emboli, they are useful in helping make the diagnosis. Thus, diffusionweighted MRI should be done if fat embolism syndrome is suspected.38,39
CASE CONCLUDED
The patient’s course in the intensive care unit was further complicated by gastrointestinal bleeding and renal failure. His neurologic status did not improve. Repeated MRI of the brain showed evolving bilateral watershed infarction throughout the cortices. The neurologic consult service diagnosed the patient as having severe encephalopathy with a very poor prognosis. The decision was made to withdraw care. He was placed under palliative care and died on postoperative day 22.
DRUG TREATMENT OF FAT EMBOLISM SYNDROME
5. Which of the following drugs has been proven to be effective in treating fat embolism syndrome?
- Intravenous ethanol
- Steroids
- Heparin
- Dextran
- Aspirin
- None of the above
None of the above has been proven to be effective in treating this disorder. The management is largely supportive. Thus, prevention, early diagnosis, and symptom management are vital.
Pulmonary and hemodynamic support are the cornerstones of successful treatment. Aggressive respiratory support is often needed. Management of acute lung injury and ARDS focuses on achieving acceptable gas exchange while preventing ventilator-associated lung injury. Intravascular volume must be supported. Inotropes and pulmonary vasodilators may be required to maintain hemodynamics. Exacerbation of central nervous system ischemia from hypotension or hypoxia should be avoided.
If the thrombocytopenia leads to clinical bleeding, platelet transfusions may be warranted.
Supportive care should include prophylaxis of deep venous thrombosis and of gastrointestinal bleeding, and maintenance of nutrition.40 Patients who receive supportive care generally have a favorable outcome, with a mortality rate of less than 10%.28
Drug studies have been inconclusive
Drugs suggested in the treatment of fat embolism syndrome include heparin, aspirin, dextran, hypertonic glucose, and alcohol, but the results have been inconclusive.3,11,23,40–43
Heparin stimulates lipase activity, consequently decreasing the concentration of circulating fat globules. However, the increase in levels of free fatty acids may actually worsen the clinical picture. For this reason, and because of anticoagulation concerns and evidence of increased mortality rates, heparin is now contraindicated in the treatment of fat embolism syndrome.2,41,43
Alcohol. Patients with a higher blood alcohol level at the time of injury have been reported to have a lower incidence of fat embolism syndrome. Alcohol inhibits lipase, suppressing the rise of free fatty acids. In experimental studies, the incidence of fat embolism syndrome was lower when the blood alcohol level was maintained at 20 mg/dL. However, no prospective randomized trial has been done to determine the clinical efficacy of ethanol as a treatment for this condition.5,42
Dextran has been advocated, owing to its ability to improve small-vessel perfusion, but bleeding risk and acute renal failure associated with this drug have limited its use.5
N-acetylcysteine has been shown to attenuate fat-induced lung injury in a study of rats with induced fat embolism syndrome.44
Corticosteroid treatment for this condition is controversial. Studies in patients with femoral and tibial fractures show that steroids reduce the incidence of fat embolism syndrome when given prophylactically, and those treated with steroids had a higher Pao2 than controls. Doses of methylprednisolone in these studies ranged between 9 mg/kg to 90 mg/kg. A drawback of these studies is their small number of patients.12,32,45,46
A meta-analysis47 of randomized trials of corticosteroids to prevent fat embolism syndrome in patients with long-bone fractures identified 104 such studies. Only 7 of the 104 were considered adequate. In 389 patients with long-bone fractures, prophylactic corticosteroids reduced the risk of fat embolism syndrome by 78% (95% confidence interval 43%–92%) and corticosteroids also significantly reduced the risk of hypoxia with no difference in rates of infection or death. However, the overall quality of the trials was poor, and the authors of the meta-analysis concluded that more study is needed before corticosteroids could be formally recommended.47
There is no evidence that steroids improve the overall clinical course of already established fat embolism syndrome.12,32,45 The dosing and optimal timing of administration have also not been established. High doses pose a risk of septic complications, which may be devastating for the posttrauma or postoperative patient.
- Akhtar S. Fat embolism. Anesthesiol Clin 2009; 27:533–550.
- Filomeno LT, Carelli CR, Silva NC, Filho TE, Amatuzzi MM. Fat embolism: a review for current orthopaedics practice. Acta Ortop Bras 2005; 13:196–208.
- ten Duis HJ. The fat embolism syndrome. Injury 1997; 28:77–85.
- Peltier LF. Fat embolism. A current concept. Clin Orthop Relat Res 1969; 66:241–253.
- Gossling HR, Pellegrini VD. Fat embolism syndrome: a review of the pathophysiology and physiological basis of treatment. Clin Orthop Relat Res 1982; 165:68–82.
- Papagelopoulos PJ, Apostolou CD, Karachalios TS, Themistocleous GS, Giannakopoulos CK, Ioannidis TT. Pulmonary fat embolism after total hip and total knee arthroplasty. Orthopedics 2003; 26:523–527.
- Takahashi S, Kitagawa H, Ishii T. Intraoperative pulmonary embolism during spinal instrumentation surgery. A prospective study using transoesophageal echocardiography. J Bone Joint Surg Br 2003; 85:90–94.
- Christie J, Robinson CM, Pell AC, McBirnie J, Burnett R. Transcardiac echocardiography during invasive intramedullary procedures. J Bone Joint Surg Br 1995; 77:450–455.
- Robert JH, Hoffmeyer P, Broquet PE, Cerutti P, Vasey H. Fat embolism syndrome. Orthop Rev 1993; 22:567–571.
- Mellor A, Soni N. Fat embolism. Anaesthesia 2001; 56:145–154.
- Taviloglu K, Yanar H. Fat embolism syndrome. Surg Today 2007; 37:5–8.
- Lindeque BG, Schoeman HS, Dommisse GF, Boeyens MC, Vlok AL. Fat embolism and the fat embolism syndrome. A double-blind therapeutic study. J Bone Joint Surg Br 1987; 69:128–131.
- Gurd AR, Wilson RI. The fat embolism syndrome. J Bone Joint Surg Br 1974; 56B:408–416.
- Ganong RB. Fat emboli syndrome in isolated fractures of the tibia and femur. Clin Orthop Relat Res 1993; 291:208–214.
- Djelouah I, Lefèvre G, Ozier Y, Rosencher N, Tallet F. Fat embolism in orthopedic surgery: role of bone marrow fatty acid. Anesth Analg 1997; 85:441–443.
- Barre J, Lepouse C, Segal P. Embolism and intramedullary femoral surgery. Rev Chir Orthop Reparatrice Appar Mot 1997; 83:9–21.
- Kim YH. Incidence of fat embolism syndrome after cemented or cementless bilateral simultaneous and unilateral total knee arthroplasty. J Arthroplasty 2001; 16:730–739.
- Dorr LD, Merkel C, Mellman MF, Klein I. Fat emboli in bilateral total knee arthroplasty. Predictive factors for neurologic manifestations. Clin Orthop Relat Res 1989; 248:112–118.
- Jankiewicz JJ, Sculco TP, Ranawat CS, Behr C, Tarrentino S. Onestage versus 2-stage bilateral total knee arthroplasty. Clin Orthop Relat Res 1994; 309:94–101.
- Gauss H. The pathology of fat embolism. Arch Surg 1924; 9:593–605.
- Lehman EP, Moore RM. Fat embolism, including experimental production without trauma. Arch Surg 1927; 14:621–662.
- Johnson MJ, Lucas GL. Fat embolism syndrome. Orthopedics 1996; 19:41–48.
- Benson KT. Diagnosis and treatment of fat embolism syndrome. Anesthesiology Rev 1993; 20:165–170.
- Colonna DM, Kilgus D, Brown W, Challa V, Stump DA, Moody DM. Acute brain fat embolization occurring after total hip arthroplasty in the absence of a patent foramen ovale. Anesthesiology 2002; 96:1027–1029.
- Byrick RJ, Mullen JB, Mazer CD, Guest CB. Transpulmonary systemic fat embolism. Studies in mongrel dogs after cemented arthroplasty. Am J Respir Crit Care Med 1994; 150:1416–1422.
- Benestad G. Drei Fälle von Fettembolie mit punktförmigen Blutungen in der Haut. Deutsche Ztschr f Chir 1911; 112:192.
- Hagley SR. The fulminant fat embolism syndrome. Anaesth Intensive Care 1983; 11:167–170.
- Fulde GW, Harrison P. Fat embolism—a review. Arch Emerg Med 1991; 8:233–239.
- Bulger EM, Smith DG, Maier RV, Jurkovich GJ. Fat embolism syndrome. A 10-year review. Arch Surg 1997; 132:435–439.
- King EG, Wagner WW, Ashbaugh DG, Latham LP, Halsey DR. Alterations in pulmonary microanatomy after fat embolism. In vivo observations via thoracic window of the oleic acid-embolized canine lung. Chest 1971; 59:524–530.
- Talbot M, Schemitsch EH. Fat embolism syndrome: history, definition, epidemiology. Injury 2006; 37(suppl 4):S3–S7.
- Schonfeld SA, Ploysongsang Y, DiLisio R, et al. Fat embolism prophylaxis with corticosteroids. A prospective study in high-risk patients. Ann Intern Med 1983; 99:438–443.
- Tetzlaff J, Massoli K. Fat embolism. In:Tetzlaff J, editor. Clinical Orthopedic Anesthesia. Boston, MA: Butterworth-Heinemann; 1995:341–349.
- Capan LM, Miller SM, Patel KP. Fat embolism. Anesthesiol Clin North Am 1993; 11:25–54.
- Reider E, Sherman Y, Weiss Y, Liebergall M, Pizov R. Alveolar macrophages fat stain in early diagnosis of fat embolism syndrome. Isr J Med Sci 1997; 33:654–658.
- Aoki N, Soma K, Shindo M, Kurosawa T, Ohwada T. Evaluation of potential fat emboli during placement of intramedullary nails after orthopedic fractures. Chest 1998; 113:178–181.
- Stoeger A, Daniaux M, Felber S, Stockhammer G, Aichner F, zur Nedden D. MRI findings in cerebral fat embolism. Eur Radiol 1998; 8:1590–1593.
- Takahashi M, Suzuki R, Osakabe Y, et al. Magnetic resonance imaging findings in cerebral fat embolism: correlation with clinical manifestations. J Trauma 1999; 46:324–327.
- Parizel PM, Demey HE, Veeckmans G, et al. Early diagnosis of cerebral fat embolism syndrome by diffusion-weighted MRI (starfield pattern). Stroke 2001; 32:2942–2944.
- Habashi NM, Andrews PL, Scalea TM. Therapeutic aspects of fat embolism syndrome. Injury 2006; 37(suppl 4):S68–S73.
- Sage RH, Tudor RW. Treatment of fat embolism with heparin. Br Med J 1958; 1:1160–1161.
- Myers R, Taljaard JJ. Blood alcohol and fat embolism syndrome. J Bone Joint Surg Am 1977; 59:878–880.
- Denman EE, Cairnes CS, Holmes CM. Case of severe fat embolism treated by intermittent positive-pressure respiration. Br Med J 1964; 2:101–102.
- Liu DD, Kao SJ, Chen HI. N-Acetylcysteine attenuates acute lung injury induced by fat embolism. Crit Care Med 2008; 36:565–571.
- Kallenbach J, Lewis M, Zaltzman M, Feldman C, Orford A, Zwi S. ‘Low-dose’ corticosteroid prophylaxis against fat embolism. J Trauma 1987; 27:1173–1176.
- Stoltenberg JJ, Gustilo RB. The use of methylprednisolone and hypertonic glucose in the prophylaxis of fat embolism syndrome. Clin Orthop Relat Res 1979; 143:211–221.
- Bederman SS, Bhandari M, McKee MD, Schemitsch EH. Do corticosteroids reduce the risk of fat embolism syndrome in patients with long-bone fractures? A meta-analysis. Can J Surg 2009; 52:386–393.
- Akhtar S. Fat embolism. Anesthesiol Clin 2009; 27:533–550.
- Filomeno LT, Carelli CR, Silva NC, Filho TE, Amatuzzi MM. Fat embolism: a review for current orthopaedics practice. Acta Ortop Bras 2005; 13:196–208.
- ten Duis HJ. The fat embolism syndrome. Injury 1997; 28:77–85.
- Peltier LF. Fat embolism. A current concept. Clin Orthop Relat Res 1969; 66:241–253.
- Gossling HR, Pellegrini VD. Fat embolism syndrome: a review of the pathophysiology and physiological basis of treatment. Clin Orthop Relat Res 1982; 165:68–82.
- Papagelopoulos PJ, Apostolou CD, Karachalios TS, Themistocleous GS, Giannakopoulos CK, Ioannidis TT. Pulmonary fat embolism after total hip and total knee arthroplasty. Orthopedics 2003; 26:523–527.
- Takahashi S, Kitagawa H, Ishii T. Intraoperative pulmonary embolism during spinal instrumentation surgery. A prospective study using transoesophageal echocardiography. J Bone Joint Surg Br 2003; 85:90–94.
- Christie J, Robinson CM, Pell AC, McBirnie J, Burnett R. Transcardiac echocardiography during invasive intramedullary procedures. J Bone Joint Surg Br 1995; 77:450–455.
- Robert JH, Hoffmeyer P, Broquet PE, Cerutti P, Vasey H. Fat embolism syndrome. Orthop Rev 1993; 22:567–571.
- Mellor A, Soni N. Fat embolism. Anaesthesia 2001; 56:145–154.
- Taviloglu K, Yanar H. Fat embolism syndrome. Surg Today 2007; 37:5–8.
- Lindeque BG, Schoeman HS, Dommisse GF, Boeyens MC, Vlok AL. Fat embolism and the fat embolism syndrome. A double-blind therapeutic study. J Bone Joint Surg Br 1987; 69:128–131.
- Gurd AR, Wilson RI. The fat embolism syndrome. J Bone Joint Surg Br 1974; 56B:408–416.
- Ganong RB. Fat emboli syndrome in isolated fractures of the tibia and femur. Clin Orthop Relat Res 1993; 291:208–214.
- Djelouah I, Lefèvre G, Ozier Y, Rosencher N, Tallet F. Fat embolism in orthopedic surgery: role of bone marrow fatty acid. Anesth Analg 1997; 85:441–443.
- Barre J, Lepouse C, Segal P. Embolism and intramedullary femoral surgery. Rev Chir Orthop Reparatrice Appar Mot 1997; 83:9–21.
- Kim YH. Incidence of fat embolism syndrome after cemented or cementless bilateral simultaneous and unilateral total knee arthroplasty. J Arthroplasty 2001; 16:730–739.
- Dorr LD, Merkel C, Mellman MF, Klein I. Fat emboli in bilateral total knee arthroplasty. Predictive factors for neurologic manifestations. Clin Orthop Relat Res 1989; 248:112–118.
- Jankiewicz JJ, Sculco TP, Ranawat CS, Behr C, Tarrentino S. Onestage versus 2-stage bilateral total knee arthroplasty. Clin Orthop Relat Res 1994; 309:94–101.
- Gauss H. The pathology of fat embolism. Arch Surg 1924; 9:593–605.
- Lehman EP, Moore RM. Fat embolism, including experimental production without trauma. Arch Surg 1927; 14:621–662.
- Johnson MJ, Lucas GL. Fat embolism syndrome. Orthopedics 1996; 19:41–48.
- Benson KT. Diagnosis and treatment of fat embolism syndrome. Anesthesiology Rev 1993; 20:165–170.
- Colonna DM, Kilgus D, Brown W, Challa V, Stump DA, Moody DM. Acute brain fat embolization occurring after total hip arthroplasty in the absence of a patent foramen ovale. Anesthesiology 2002; 96:1027–1029.
- Byrick RJ, Mullen JB, Mazer CD, Guest CB. Transpulmonary systemic fat embolism. Studies in mongrel dogs after cemented arthroplasty. Am J Respir Crit Care Med 1994; 150:1416–1422.
- Benestad G. Drei Fälle von Fettembolie mit punktförmigen Blutungen in der Haut. Deutsche Ztschr f Chir 1911; 112:192.
- Hagley SR. The fulminant fat embolism syndrome. Anaesth Intensive Care 1983; 11:167–170.
- Fulde GW, Harrison P. Fat embolism—a review. Arch Emerg Med 1991; 8:233–239.
- Bulger EM, Smith DG, Maier RV, Jurkovich GJ. Fat embolism syndrome. A 10-year review. Arch Surg 1997; 132:435–439.
- King EG, Wagner WW, Ashbaugh DG, Latham LP, Halsey DR. Alterations in pulmonary microanatomy after fat embolism. In vivo observations via thoracic window of the oleic acid-embolized canine lung. Chest 1971; 59:524–530.
- Talbot M, Schemitsch EH. Fat embolism syndrome: history, definition, epidemiology. Injury 2006; 37(suppl 4):S3–S7.
- Schonfeld SA, Ploysongsang Y, DiLisio R, et al. Fat embolism prophylaxis with corticosteroids. A prospective study in high-risk patients. Ann Intern Med 1983; 99:438–443.
- Tetzlaff J, Massoli K. Fat embolism. In:Tetzlaff J, editor. Clinical Orthopedic Anesthesia. Boston, MA: Butterworth-Heinemann; 1995:341–349.
- Capan LM, Miller SM, Patel KP. Fat embolism. Anesthesiol Clin North Am 1993; 11:25–54.
- Reider E, Sherman Y, Weiss Y, Liebergall M, Pizov R. Alveolar macrophages fat stain in early diagnosis of fat embolism syndrome. Isr J Med Sci 1997; 33:654–658.
- Aoki N, Soma K, Shindo M, Kurosawa T, Ohwada T. Evaluation of potential fat emboli during placement of intramedullary nails after orthopedic fractures. Chest 1998; 113:178–181.
- Stoeger A, Daniaux M, Felber S, Stockhammer G, Aichner F, zur Nedden D. MRI findings in cerebral fat embolism. Eur Radiol 1998; 8:1590–1593.
- Takahashi M, Suzuki R, Osakabe Y, et al. Magnetic resonance imaging findings in cerebral fat embolism: correlation with clinical manifestations. J Trauma 1999; 46:324–327.
- Parizel PM, Demey HE, Veeckmans G, et al. Early diagnosis of cerebral fat embolism syndrome by diffusion-weighted MRI (starfield pattern). Stroke 2001; 32:2942–2944.
- Habashi NM, Andrews PL, Scalea TM. Therapeutic aspects of fat embolism syndrome. Injury 2006; 37(suppl 4):S68–S73.
- Sage RH, Tudor RW. Treatment of fat embolism with heparin. Br Med J 1958; 1:1160–1161.
- Myers R, Taljaard JJ. Blood alcohol and fat embolism syndrome. J Bone Joint Surg Am 1977; 59:878–880.
- Denman EE, Cairnes CS, Holmes CM. Case of severe fat embolism treated by intermittent positive-pressure respiration. Br Med J 1964; 2:101–102.
- Liu DD, Kao SJ, Chen HI. N-Acetylcysteine attenuates acute lung injury induced by fat embolism. Crit Care Med 2008; 36:565–571.
- Kallenbach J, Lewis M, Zaltzman M, Feldman C, Orford A, Zwi S. ‘Low-dose’ corticosteroid prophylaxis against fat embolism. J Trauma 1987; 27:1173–1176.
- Stoltenberg JJ, Gustilo RB. The use of methylprednisolone and hypertonic glucose in the prophylaxis of fat embolism syndrome. Clin Orthop Relat Res 1979; 143:211–221.
- Bederman SS, Bhandari M, McKee MD, Schemitsch EH. Do corticosteroids reduce the risk of fat embolism syndrome in patients with long-bone fractures? A meta-analysis. Can J Surg 2009; 52:386–393.
Roth Spots—More than Meets the Eye
A 50‐year‐old female patient with a past medical history of Sjogren's syndrome and polymyositis presented with fever, rash, swelling, and pain in her extremities. Skin biopsy confirmed vasculitis. She was treated with steroids and azathioprine. However, she developed sudden‐onset central visual blurring in her right eye on the fifth day of hospitalization. Fundoscopic exam showed multiple central white‐centered retinal hemorrhages (Roth spots, Figures 1, 2) and vascular sheathing, consistent with retinal vasculitis. Blood cultures were negative. Transthoracic and transesophageal echocardiograms were normal. She was treated with high‐dose intravenous steroids and cyclophosphamide, with visual improvement and a marked reduction in the number of Roth spots.
Roth spots 1 are nonspecific intraretinal hemorrhagic lesions with a white center due to fibrin deposition. Although historically associated with infective endocarditis, they can also occur in other systemic diseases such as connective tissue disorders, vasculitis, leukemia, diabetes, hypertension, anemia, trauma, as well as disseminated bacterial and fungal infections.
- , , .White centered hemorrhages: their significance.Ophthalmology.1980;87:66–69.
A 50‐year‐old female patient with a past medical history of Sjogren's syndrome and polymyositis presented with fever, rash, swelling, and pain in her extremities. Skin biopsy confirmed vasculitis. She was treated with steroids and azathioprine. However, she developed sudden‐onset central visual blurring in her right eye on the fifth day of hospitalization. Fundoscopic exam showed multiple central white‐centered retinal hemorrhages (Roth spots, Figures 1, 2) and vascular sheathing, consistent with retinal vasculitis. Blood cultures were negative. Transthoracic and transesophageal echocardiograms were normal. She was treated with high‐dose intravenous steroids and cyclophosphamide, with visual improvement and a marked reduction in the number of Roth spots.


Roth spots 1 are nonspecific intraretinal hemorrhagic lesions with a white center due to fibrin deposition. Although historically associated with infective endocarditis, they can also occur in other systemic diseases such as connective tissue disorders, vasculitis, leukemia, diabetes, hypertension, anemia, trauma, as well as disseminated bacterial and fungal infections.
A 50‐year‐old female patient with a past medical history of Sjogren's syndrome and polymyositis presented with fever, rash, swelling, and pain in her extremities. Skin biopsy confirmed vasculitis. She was treated with steroids and azathioprine. However, she developed sudden‐onset central visual blurring in her right eye on the fifth day of hospitalization. Fundoscopic exam showed multiple central white‐centered retinal hemorrhages (Roth spots, Figures 1, 2) and vascular sheathing, consistent with retinal vasculitis. Blood cultures were negative. Transthoracic and transesophageal echocardiograms were normal. She was treated with high‐dose intravenous steroids and cyclophosphamide, with visual improvement and a marked reduction in the number of Roth spots.


Roth spots 1 are nonspecific intraretinal hemorrhagic lesions with a white center due to fibrin deposition. Although historically associated with infective endocarditis, they can also occur in other systemic diseases such as connective tissue disorders, vasculitis, leukemia, diabetes, hypertension, anemia, trauma, as well as disseminated bacterial and fungal infections.
- , , .White centered hemorrhages: their significance.Ophthalmology.1980;87:66–69.
- , , .White centered hemorrhages: their significance.Ophthalmology.1980;87:66–69.