User login
Interstitial Granulomatous Dermatitis and Palisaded Neutrophilic Granulomatous Dermatitis
To the Editor:
Palisaded neutrophilic granulomatous dermatitis (PNGD) is a rare disorder that often is associated with systemic disease. It has been shown to manifest in the presence of systemic lupus erythematosus; rheumatoid arthritis; Wegener granulomatosis; and other diseases, mainly autoimmune conditions. Interstitial granulomatous dermatitis (IGD) associated with arthritis was first described by Ackerman et al1 in 1993. In 1994, IGD was placed among the spectrum of PNGD by Chu et al.2 The disease entities included in the spectrum of PNGD of the immune complex disease are Churg-Strauss granuloma, cutaneous extravascular necrotizing granuloma, rheumatoid papules, superficial ulcerating rheumatoid necrobiosis, and IGD with arthritis.2 It has been suggested that IGD has a distinct clinical presentation with associated histopathology, while others suggest it still is part of the PNGD spectrum.2,3 We present 2 cases of granulomatous dermatitis and their findings related to IGD and PNGD.
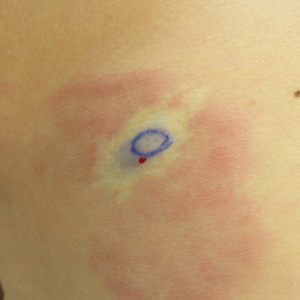
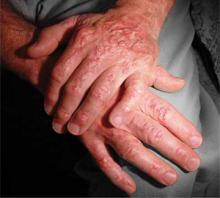
A 58-year-old woman presented with recurrent painful lesions on the trunk, arms, and legs of 2 years’ duration. The lesions spontaneously resolved without scarring or hyperpigmentation but would recur in different areas on the trunk. She was diagnosed with rheumatoid arthritis following a recent autoimmune workup. At presentation, physical examination revealed tender erythematous edematous plaques on the bilateral upper back (Figure 1) and erythematous nodules on the bilateral upper arms. The patient previously had an antinuclear antibody titer of 1:320 with a speckled pattern. A repeat antinuclear antibody titer taken 1 year later was negative. Her rheumatoid factor initially was positive and remained positive upon repeat testing. Punch biopsies were performed for histologic evaluation of the lesions and immunofluorescence. Biopsies examined with hematoxylin and eosin stain revealed perivascular and interstitial mixed (lymphocytic, neutrophilic, eosinophilic) bottom-heavy inflammation with nuclear dust and basophilic degeneration of collagen (Figure 2). Immunofluorescence studies were negative. The patient deferred treatment.
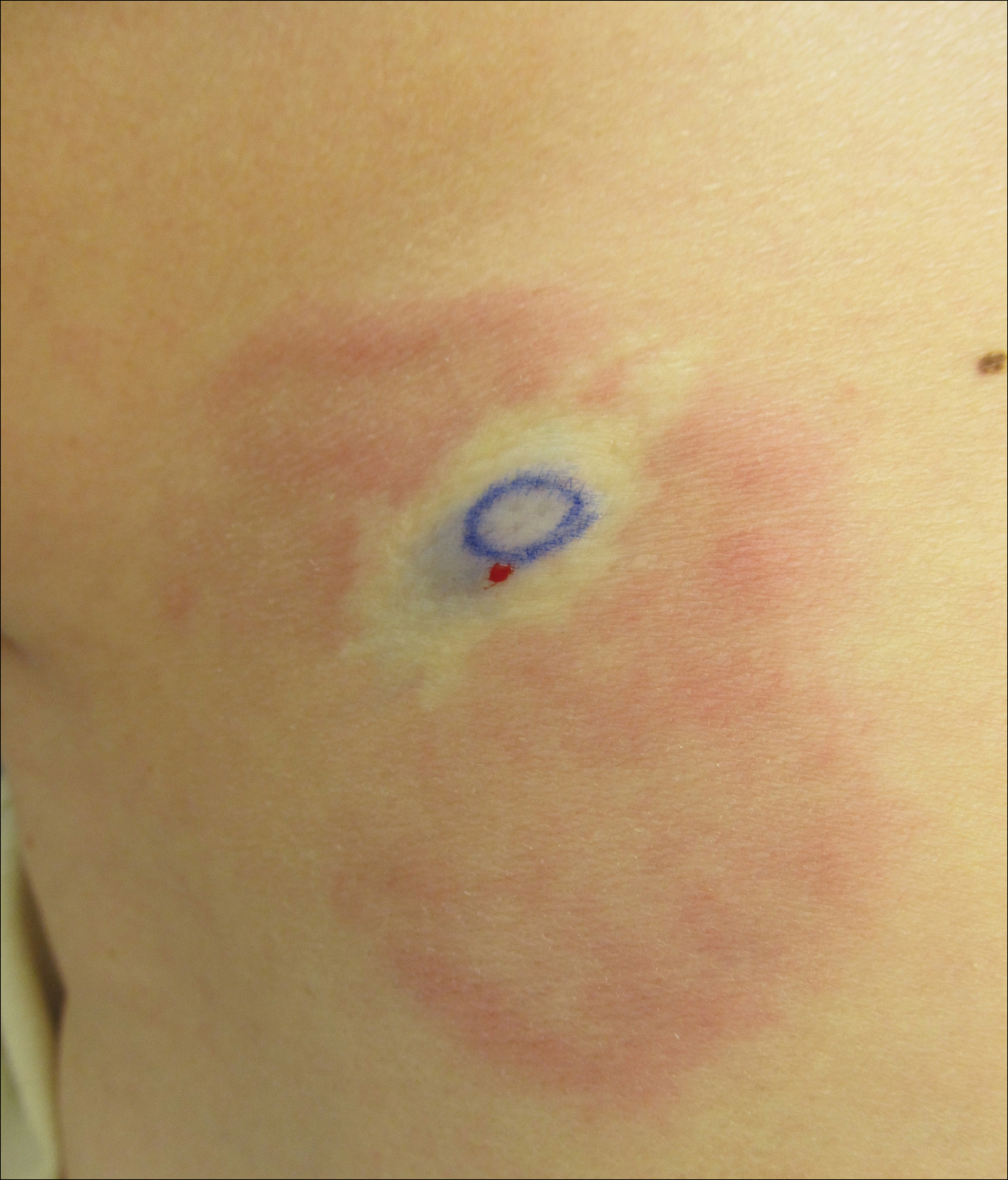
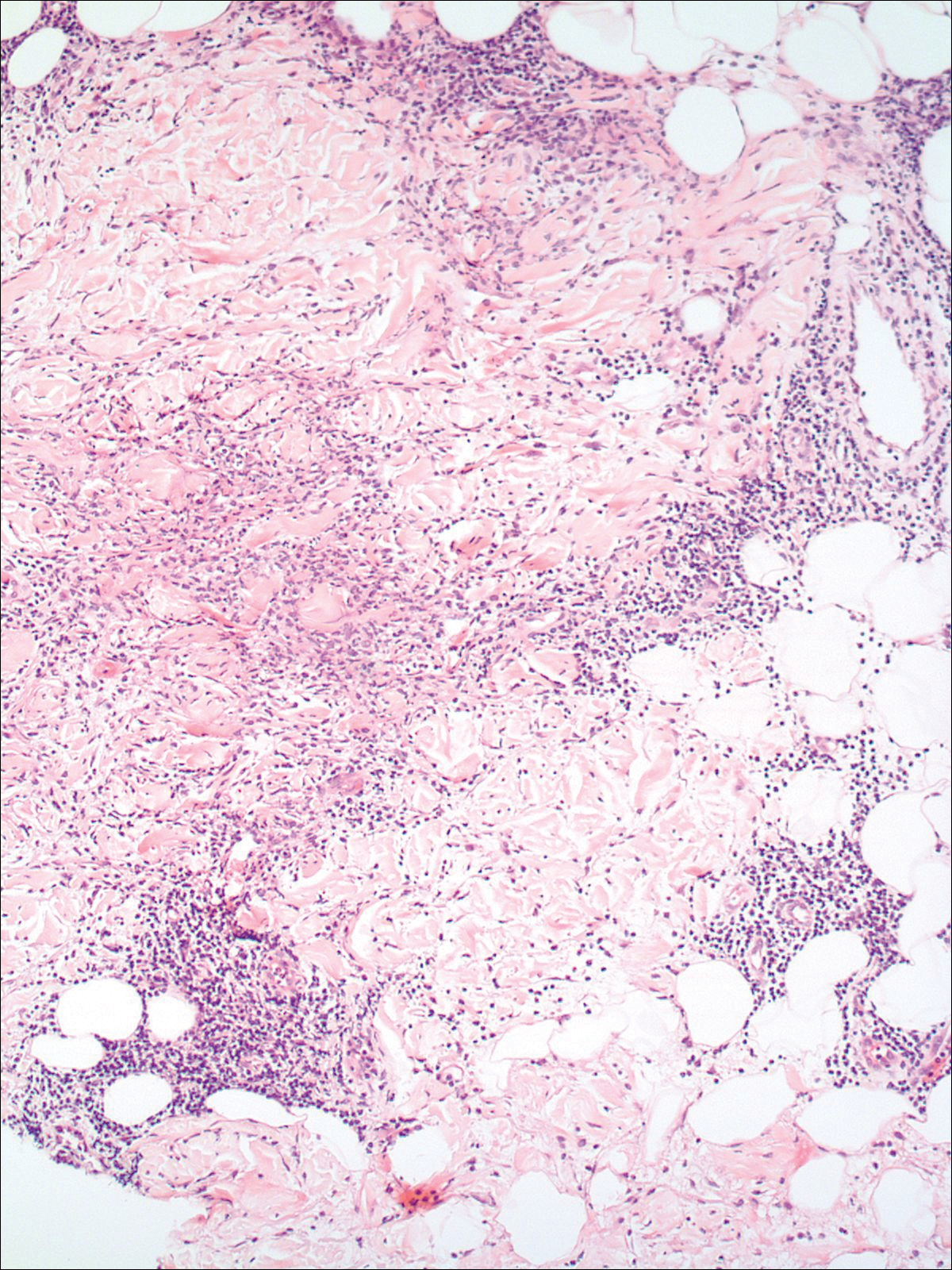
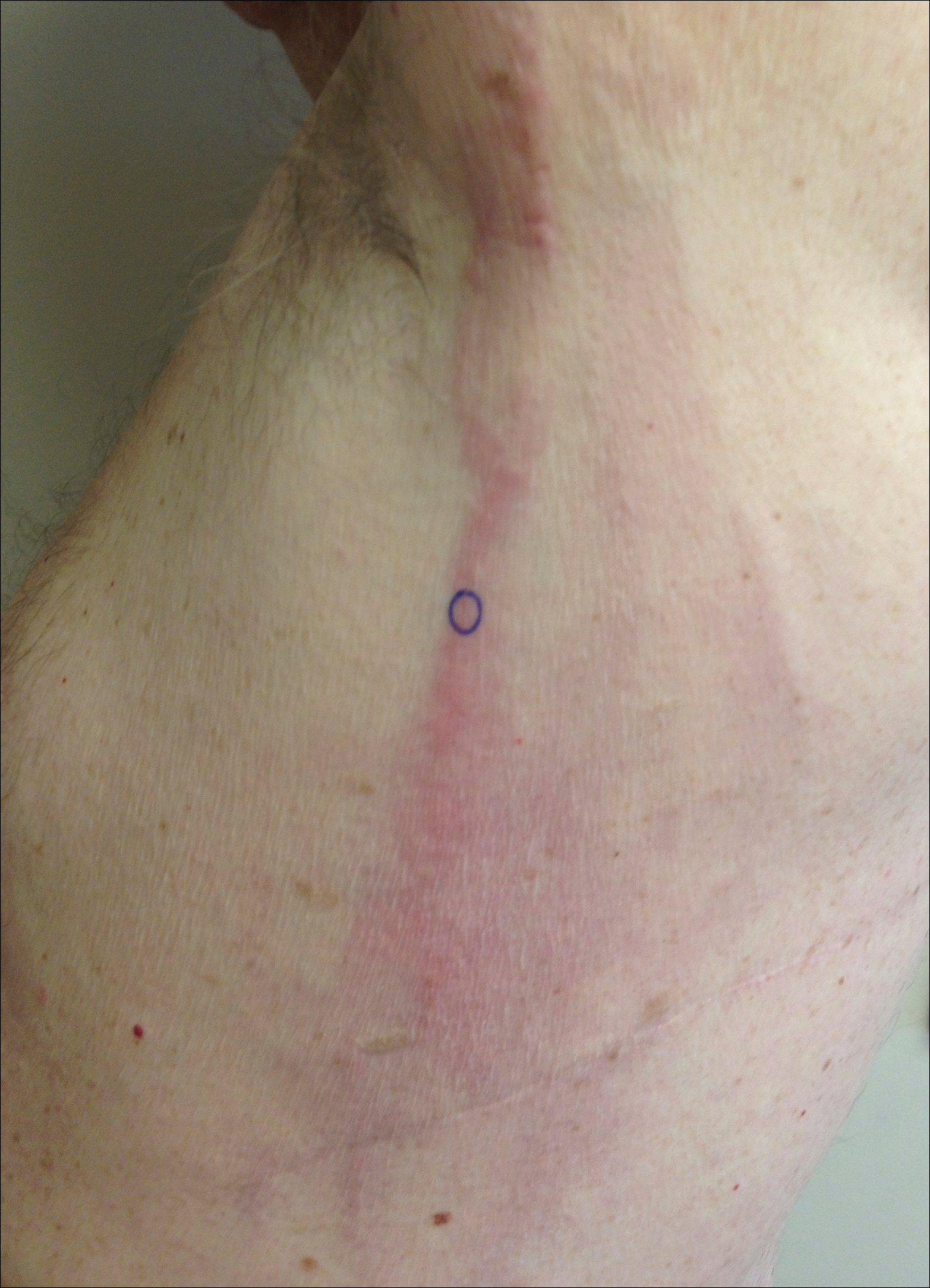
A 74-year-old man presented with a rash on the flank and back with associated pruritus and occasional pain of 2 months’ duration. His primary care physician prescribed a course of cephalexin, but the rash did not improve. Review of systems was positive for intermittent swelling of the hands, feet, and lips, and negative for arthritis. His medical history included 2 episodes of rheumatic fever, one complicated by pneumonia. His medications included finasteride, simvastatin, bisoprolol-hydrochlorothiazide, aspirin, tiotropium, vitamin D, and fish oil. At presentation, physical examination revealed tender violaceous plaques with induration and central clearing distributed on the left side of the back, left side of the flank, and left axilla. The lesion on the axilla measured 30.0×3.5 cm and the lesions on the left side of the back measured 30.0×9.0 cm. The rims of the lesions were elevated and consistent with the rope sign (Figure 3). A punch biopsy of the lesion on the left axilla showed perivascular and interstitial infiltrate of lymphocytes, neutrophils, histiocytes, and eosinophils. There was no evidence of fibrin deposition in the blood vessels. Small areas of necrobiotic collagen surrounded by multinucleated giant cells and lymphocytes were noted (Figure 4). The rash improved spontaneously at the time of suture removal. No treatment was initiated.
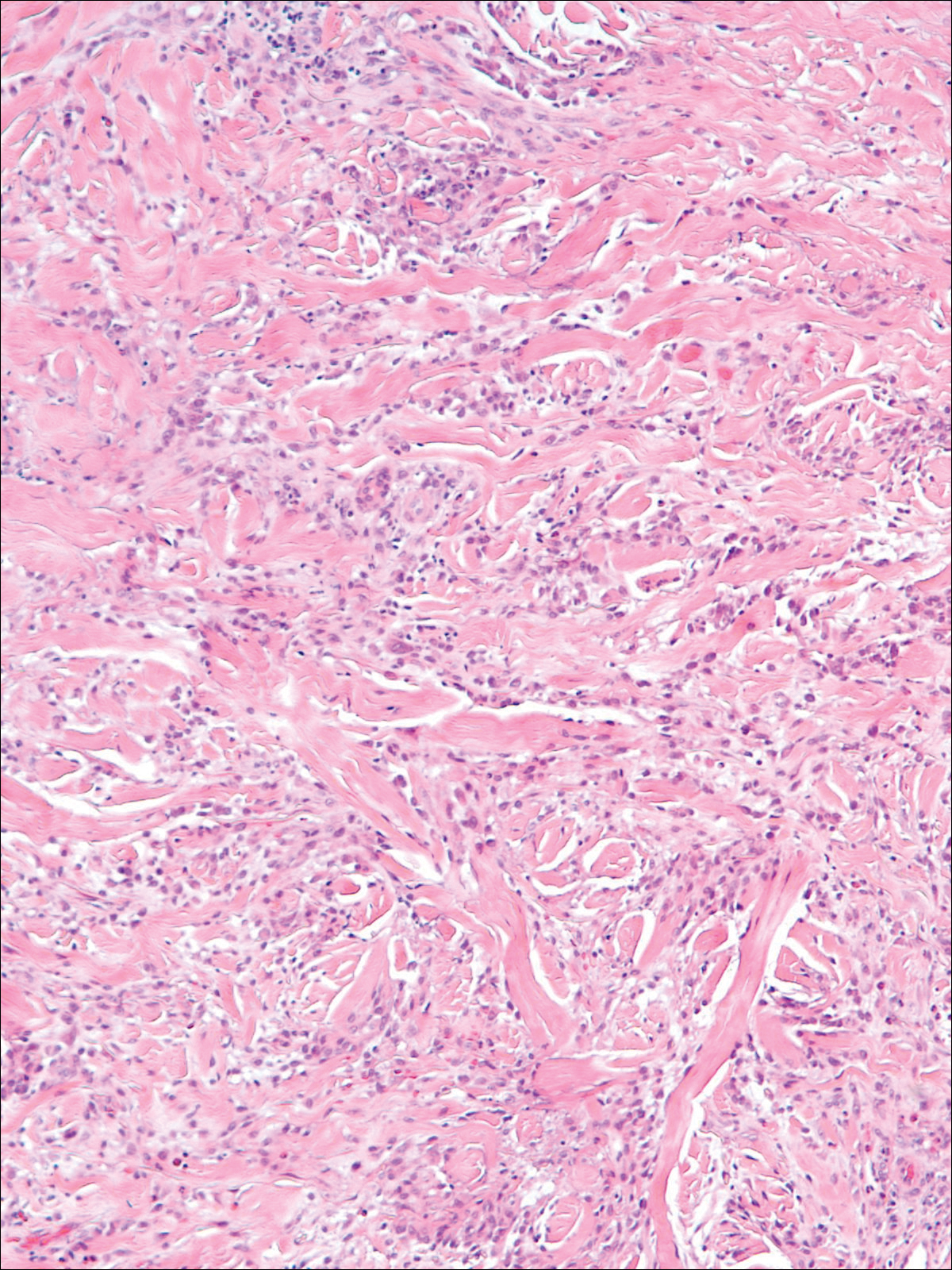
Granulomatous dermatitis in the presence of an autoimmune disorder can present as IGD or PNGD. Both forms of granulomatous dermatitis are rare conditions and considered to be part of the same clinicopathological spectrum. These conditions can be difficult to distinguish clinically but are histologically unique.
Interstitial granulomatous dermatitis and PNGD can have a variable clinical expression. Palisaded neutrophilic granulomatous dermatitis generally presents as flesh-colored to erythematous papules or plaques, most commonly located on the upper arms. The lesions may have a central umbilication with perforation and ulceration.4 Interstitial granulomatous dermatitis most commonly presents as erythematous plaques and papules. The lesions are symmetric and asymptomatic. They most commonly appear on the trunk, axillae, buttocks, thighs, and groin. Subcutaneous linear cords (the rope sign) is a characteristic associated with IGD.3,5 However, the rope sign also has been reported in a patient with PNGD with systemic lupus,6 which further demonstrates the overlapping spectrum of clinical expression seen in these 2 forms of granulomatous dermatitis. Therefore, a diagnosis cannot be made by clinical expression alone; histologic findings are needed for confirmation.
When differentiating IGD and PNGD histologically, it is important to keep in mind that these features exist on a spectrum and depend on the age of the lesion. Deposition of the immune complex around the dermal blood vessel initiates the pathogenesis. Early lesions of PNGD show a neutrophilic infiltrate, focal leukocytoclastic vasculitis, and dense nuclear dust. Developed lesions show zones of basophilic degenerated collagen surrounded by palisades of histiocytes, neutrophils, and nuclear debris.2 The histologic pattern of IGD features smaller areas of palisading histiocytes surrounding foci of degenerated collagen. Neutrophils and eosinophils are seen among the degenerated collagen. There is no evidence of vasculitis and dermal mucin usually is absent.7
Palisaded neutrophilic granulomatous dermatitis has been reported to improve with systemic steroids and dapsone.8 Th
Some authors have disputed the spectrum that Chu et al2 had determined in their study and proposed IGD is a separate entity from the PNGD spectrum. Verneuil et al9 stated that the clinical presentations in Chu et al’s2 study (symmetric papules of the extremities) had not been reported in a patient with IGD. However, in a study of IGD by Peroni et al,3 7 of 12 patients presented with symmetrical papules of the extremities. We believe that the spectrum proposed by Chu et al2 still holds true.
These 2 reports demonstrate the diverse presentation of IGD and PNGD. It is important for dermatologists to keep in mind the PNGD spectrum when a patient presents with granulomatous dermatitis in the presence of an autoimmune disorder.
- Ackerman AB, Guo Y, Vitale P. Clues to diagnosis in dermatopathology. Am Society Clin Pathol. 1993;3:309-312.
- Chu P, Connolly MK, LeBoit PE. The histopathologic spectrum of palisaded neutrophilic and granulomatous dermatitis in patients with collagen vascular disease. Arch Dermatol. 1994;130:1278-1283.
- Peroni A, Colato C, Schena D, et al. Interstitial granulomatous dermatitis: a distinct entity with characteristic histological and clinical pattern. Br J Dermatol. 2012;166:775-783.
- Hantash BM, Chiang D, Kohler S, et al. Palisaded neutrophilic and granulomatous dermatitis associated with limited systemic sclerosis. J Am Acad Dermatol. 2008;58:661-664.
- Garcia-Rabasco A, Esteve-Martinez A, Zaragoza-Ninet V, et al. Interstitial granulomatous dermatitis in a patient with lupus erythematosus. Am J Dermatopathol. 2011;33:871-872.
- Gulati A, Paige D, Yaqoob M, et al. Palisaded neutrophilic granulomatous dermatitis associated with systemic lupus erythematosus presenting with the burning rope sign. J Am Acad Dermatol. 2009;61:711-714.
- Tomasini C, Pippione M. Interstitial granulomatous dermatitis with plaques. J Am Acad Dermatol. 2002;46:892-899.
- Fett N, Kovarik C, Bennett D. Palisaded neutrophilic granulomatous dermatitis without a definable underlying disorder treated with dapsone. J Am Acad Dermatol. 2011;65:E92-E93.
- Verneuil L, Dompmartin A, Comoz F, et al. Interstitial granulomatous dermatitis with cutaneous cords and arthritis: a disorder associated with autoantibodies. J Am Acad Dermatol. 2001;45:286-291.
To the Editor:
Palisaded neutrophilic granulomatous dermatitis (PNGD) is a rare disorder that often is associated with systemic disease. It has been shown to manifest in the presence of systemic lupus erythematosus; rheumatoid arthritis; Wegener granulomatosis; and other diseases, mainly autoimmune conditions. Interstitial granulomatous dermatitis (IGD) associated with arthritis was first described by Ackerman et al1 in 1993. In 1994, IGD was placed among the spectrum of PNGD by Chu et al.2 The disease entities included in the spectrum of PNGD of the immune complex disease are Churg-Strauss granuloma, cutaneous extravascular necrotizing granuloma, rheumatoid papules, superficial ulcerating rheumatoid necrobiosis, and IGD with arthritis.2 It has been suggested that IGD has a distinct clinical presentation with associated histopathology, while others suggest it still is part of the PNGD spectrum.2,3 We present 2 cases of granulomatous dermatitis and their findings related to IGD and PNGD.
A 58-year-old woman presented with recurrent painful lesions on the trunk, arms, and legs of 2 years’ duration. The lesions spontaneously resolved without scarring or hyperpigmentation but would recur in different areas on the trunk. She was diagnosed with rheumatoid arthritis following a recent autoimmune workup. At presentation, physical examination revealed tender erythematous edematous plaques on the bilateral upper back (Figure 1) and erythematous nodules on the bilateral upper arms. The patient previously had an antinuclear antibody titer of 1:320 with a speckled pattern. A repeat antinuclear antibody titer taken 1 year later was negative. Her rheumatoid factor initially was positive and remained positive upon repeat testing. Punch biopsies were performed for histologic evaluation of the lesions and immunofluorescence. Biopsies examined with hematoxylin and eosin stain revealed perivascular and interstitial mixed (lymphocytic, neutrophilic, eosinophilic) bottom-heavy inflammation with nuclear dust and basophilic degeneration of collagen (Figure 2). Immunofluorescence studies were negative. The patient deferred treatment.


A 74-year-old man presented with a rash on the flank and back with associated pruritus and occasional pain of 2 months’ duration. His primary care physician prescribed a course of cephalexin, but the rash did not improve. Review of systems was positive for intermittent swelling of the hands, feet, and lips, and negative for arthritis. His medical history included 2 episodes of rheumatic fever, one complicated by pneumonia. His medications included finasteride, simvastatin, bisoprolol-hydrochlorothiazide, aspirin, tiotropium, vitamin D, and fish oil. At presentation, physical examination revealed tender violaceous plaques with induration and central clearing distributed on the left side of the back, left side of the flank, and left axilla. The lesion on the axilla measured 30.0×3.5 cm and the lesions on the left side of the back measured 30.0×9.0 cm. The rims of the lesions were elevated and consistent with the rope sign (Figure 3). A punch biopsy of the lesion on the left axilla showed perivascular and interstitial infiltrate of lymphocytes, neutrophils, histiocytes, and eosinophils. There was no evidence of fibrin deposition in the blood vessels. Small areas of necrobiotic collagen surrounded by multinucleated giant cells and lymphocytes were noted (Figure 4). The rash improved spontaneously at the time of suture removal. No treatment was initiated.


Granulomatous dermatitis in the presence of an autoimmune disorder can present as IGD or PNGD. Both forms of granulomatous dermatitis are rare conditions and considered to be part of the same clinicopathological spectrum. These conditions can be difficult to distinguish clinically but are histologically unique.
Interstitial granulomatous dermatitis and PNGD can have a variable clinical expression. Palisaded neutrophilic granulomatous dermatitis generally presents as flesh-colored to erythematous papules or plaques, most commonly located on the upper arms. The lesions may have a central umbilication with perforation and ulceration.4 Interstitial granulomatous dermatitis most commonly presents as erythematous plaques and papules. The lesions are symmetric and asymptomatic. They most commonly appear on the trunk, axillae, buttocks, thighs, and groin. Subcutaneous linear cords (the rope sign) is a characteristic associated with IGD.3,5 However, the rope sign also has been reported in a patient with PNGD with systemic lupus,6 which further demonstrates the overlapping spectrum of clinical expression seen in these 2 forms of granulomatous dermatitis. Therefore, a diagnosis cannot be made by clinical expression alone; histologic findings are needed for confirmation.
When differentiating IGD and PNGD histologically, it is important to keep in mind that these features exist on a spectrum and depend on the age of the lesion. Deposition of the immune complex around the dermal blood vessel initiates the pathogenesis. Early lesions of PNGD show a neutrophilic infiltrate, focal leukocytoclastic vasculitis, and dense nuclear dust. Developed lesions show zones of basophilic degenerated collagen surrounded by palisades of histiocytes, neutrophils, and nuclear debris.2 The histologic pattern of IGD features smaller areas of palisading histiocytes surrounding foci of degenerated collagen. Neutrophils and eosinophils are seen among the degenerated collagen. There is no evidence of vasculitis and dermal mucin usually is absent.7
Palisaded neutrophilic granulomatous dermatitis has been reported to improve with systemic steroids and dapsone.8 Th
Some authors have disputed the spectrum that Chu et al2 had determined in their study and proposed IGD is a separate entity from the PNGD spectrum. Verneuil et al9 stated that the clinical presentations in Chu et al’s2 study (symmetric papules of the extremities) had not been reported in a patient with IGD. However, in a study of IGD by Peroni et al,3 7 of 12 patients presented with symmetrical papules of the extremities. We believe that the spectrum proposed by Chu et al2 still holds true.
These 2 reports demonstrate the diverse presentation of IGD and PNGD. It is important for dermatologists to keep in mind the PNGD spectrum when a patient presents with granulomatous dermatitis in the presence of an autoimmune disorder.
To the Editor:
Palisaded neutrophilic granulomatous dermatitis (PNGD) is a rare disorder that often is associated with systemic disease. It has been shown to manifest in the presence of systemic lupus erythematosus; rheumatoid arthritis; Wegener granulomatosis; and other diseases, mainly autoimmune conditions. Interstitial granulomatous dermatitis (IGD) associated with arthritis was first described by Ackerman et al1 in 1993. In 1994, IGD was placed among the spectrum of PNGD by Chu et al.2 The disease entities included in the spectrum of PNGD of the immune complex disease are Churg-Strauss granuloma, cutaneous extravascular necrotizing granuloma, rheumatoid papules, superficial ulcerating rheumatoid necrobiosis, and IGD with arthritis.2 It has been suggested that IGD has a distinct clinical presentation with associated histopathology, while others suggest it still is part of the PNGD spectrum.2,3 We present 2 cases of granulomatous dermatitis and their findings related to IGD and PNGD.
A 58-year-old woman presented with recurrent painful lesions on the trunk, arms, and legs of 2 years’ duration. The lesions spontaneously resolved without scarring or hyperpigmentation but would recur in different areas on the trunk. She was diagnosed with rheumatoid arthritis following a recent autoimmune workup. At presentation, physical examination revealed tender erythematous edematous plaques on the bilateral upper back (Figure 1) and erythematous nodules on the bilateral upper arms. The patient previously had an antinuclear antibody titer of 1:320 with a speckled pattern. A repeat antinuclear antibody titer taken 1 year later was negative. Her rheumatoid factor initially was positive and remained positive upon repeat testing. Punch biopsies were performed for histologic evaluation of the lesions and immunofluorescence. Biopsies examined with hematoxylin and eosin stain revealed perivascular and interstitial mixed (lymphocytic, neutrophilic, eosinophilic) bottom-heavy inflammation with nuclear dust and basophilic degeneration of collagen (Figure 2). Immunofluorescence studies were negative. The patient deferred treatment.


A 74-year-old man presented with a rash on the flank and back with associated pruritus and occasional pain of 2 months’ duration. His primary care physician prescribed a course of cephalexin, but the rash did not improve. Review of systems was positive for intermittent swelling of the hands, feet, and lips, and negative for arthritis. His medical history included 2 episodes of rheumatic fever, one complicated by pneumonia. His medications included finasteride, simvastatin, bisoprolol-hydrochlorothiazide, aspirin, tiotropium, vitamin D, and fish oil. At presentation, physical examination revealed tender violaceous plaques with induration and central clearing distributed on the left side of the back, left side of the flank, and left axilla. The lesion on the axilla measured 30.0×3.5 cm and the lesions on the left side of the back measured 30.0×9.0 cm. The rims of the lesions were elevated and consistent with the rope sign (Figure 3). A punch biopsy of the lesion on the left axilla showed perivascular and interstitial infiltrate of lymphocytes, neutrophils, histiocytes, and eosinophils. There was no evidence of fibrin deposition in the blood vessels. Small areas of necrobiotic collagen surrounded by multinucleated giant cells and lymphocytes were noted (Figure 4). The rash improved spontaneously at the time of suture removal. No treatment was initiated.


Granulomatous dermatitis in the presence of an autoimmune disorder can present as IGD or PNGD. Both forms of granulomatous dermatitis are rare conditions and considered to be part of the same clinicopathological spectrum. These conditions can be difficult to distinguish clinically but are histologically unique.
Interstitial granulomatous dermatitis and PNGD can have a variable clinical expression. Palisaded neutrophilic granulomatous dermatitis generally presents as flesh-colored to erythematous papules or plaques, most commonly located on the upper arms. The lesions may have a central umbilication with perforation and ulceration.4 Interstitial granulomatous dermatitis most commonly presents as erythematous plaques and papules. The lesions are symmetric and asymptomatic. They most commonly appear on the trunk, axillae, buttocks, thighs, and groin. Subcutaneous linear cords (the rope sign) is a characteristic associated with IGD.3,5 However, the rope sign also has been reported in a patient with PNGD with systemic lupus,6 which further demonstrates the overlapping spectrum of clinical expression seen in these 2 forms of granulomatous dermatitis. Therefore, a diagnosis cannot be made by clinical expression alone; histologic findings are needed for confirmation.
When differentiating IGD and PNGD histologically, it is important to keep in mind that these features exist on a spectrum and depend on the age of the lesion. Deposition of the immune complex around the dermal blood vessel initiates the pathogenesis. Early lesions of PNGD show a neutrophilic infiltrate, focal leukocytoclastic vasculitis, and dense nuclear dust. Developed lesions show zones of basophilic degenerated collagen surrounded by palisades of histiocytes, neutrophils, and nuclear debris.2 The histologic pattern of IGD features smaller areas of palisading histiocytes surrounding foci of degenerated collagen. Neutrophils and eosinophils are seen among the degenerated collagen. There is no evidence of vasculitis and dermal mucin usually is absent.7
Palisaded neutrophilic granulomatous dermatitis has been reported to improve with systemic steroids and dapsone.8 Th
Some authors have disputed the spectrum that Chu et al2 had determined in their study and proposed IGD is a separate entity from the PNGD spectrum. Verneuil et al9 stated that the clinical presentations in Chu et al’s2 study (symmetric papules of the extremities) had not been reported in a patient with IGD. However, in a study of IGD by Peroni et al,3 7 of 12 patients presented with symmetrical papules of the extremities. We believe that the spectrum proposed by Chu et al2 still holds true.
These 2 reports demonstrate the diverse presentation of IGD and PNGD. It is important for dermatologists to keep in mind the PNGD spectrum when a patient presents with granulomatous dermatitis in the presence of an autoimmune disorder.
- Ackerman AB, Guo Y, Vitale P. Clues to diagnosis in dermatopathology. Am Society Clin Pathol. 1993;3:309-312.
- Chu P, Connolly MK, LeBoit PE. The histopathologic spectrum of palisaded neutrophilic and granulomatous dermatitis in patients with collagen vascular disease. Arch Dermatol. 1994;130:1278-1283.
- Peroni A, Colato C, Schena D, et al. Interstitial granulomatous dermatitis: a distinct entity with characteristic histological and clinical pattern. Br J Dermatol. 2012;166:775-783.
- Hantash BM, Chiang D, Kohler S, et al. Palisaded neutrophilic and granulomatous dermatitis associated with limited systemic sclerosis. J Am Acad Dermatol. 2008;58:661-664.
- Garcia-Rabasco A, Esteve-Martinez A, Zaragoza-Ninet V, et al. Interstitial granulomatous dermatitis in a patient with lupus erythematosus. Am J Dermatopathol. 2011;33:871-872.
- Gulati A, Paige D, Yaqoob M, et al. Palisaded neutrophilic granulomatous dermatitis associated with systemic lupus erythematosus presenting with the burning rope sign. J Am Acad Dermatol. 2009;61:711-714.
- Tomasini C, Pippione M. Interstitial granulomatous dermatitis with plaques. J Am Acad Dermatol. 2002;46:892-899.
- Fett N, Kovarik C, Bennett D. Palisaded neutrophilic granulomatous dermatitis without a definable underlying disorder treated with dapsone. J Am Acad Dermatol. 2011;65:E92-E93.
- Verneuil L, Dompmartin A, Comoz F, et al. Interstitial granulomatous dermatitis with cutaneous cords and arthritis: a disorder associated with autoantibodies. J Am Acad Dermatol. 2001;45:286-291.
- Ackerman AB, Guo Y, Vitale P. Clues to diagnosis in dermatopathology. Am Society Clin Pathol. 1993;3:309-312.
- Chu P, Connolly MK, LeBoit PE. The histopathologic spectrum of palisaded neutrophilic and granulomatous dermatitis in patients with collagen vascular disease. Arch Dermatol. 1994;130:1278-1283.
- Peroni A, Colato C, Schena D, et al. Interstitial granulomatous dermatitis: a distinct entity with characteristic histological and clinical pattern. Br J Dermatol. 2012;166:775-783.
- Hantash BM, Chiang D, Kohler S, et al. Palisaded neutrophilic and granulomatous dermatitis associated with limited systemic sclerosis. J Am Acad Dermatol. 2008;58:661-664.
- Garcia-Rabasco A, Esteve-Martinez A, Zaragoza-Ninet V, et al. Interstitial granulomatous dermatitis in a patient with lupus erythematosus. Am J Dermatopathol. 2011;33:871-872.
- Gulati A, Paige D, Yaqoob M, et al. Palisaded neutrophilic granulomatous dermatitis associated with systemic lupus erythematosus presenting with the burning rope sign. J Am Acad Dermatol. 2009;61:711-714.
- Tomasini C, Pippione M. Interstitial granulomatous dermatitis with plaques. J Am Acad Dermatol. 2002;46:892-899.
- Fett N, Kovarik C, Bennett D. Palisaded neutrophilic granulomatous dermatitis without a definable underlying disorder treated with dapsone. J Am Acad Dermatol. 2011;65:E92-E93.
- Verneuil L, Dompmartin A, Comoz F, et al. Interstitial granulomatous dermatitis with cutaneous cords and arthritis: a disorder associated with autoantibodies. J Am Acad Dermatol. 2001;45:286-291.
Practice Points
- The clinical features of interstitial granulomatous dermatitis and palisaded neutrophilic granulomatous dermatitis exist on a spectrum, and these is considerable overlap between the features of these 2 clinicopathologic entities.
- Interstitial granulomatous dermatitis and palisaded neutrophilic granulomatous dermatitis may respond to systemic steroids or treatment of the underlying systemic disease. Some cases spontaneously resolve.
Managing psoriasis: What’s best for your patient?
• Prescribe high-potency (Class 1) topical steroids for use on thicker, chronic plaques and low-potency (Class 7) steroids for the face, intertriginous areas, and the groin. A
• Recommend folate supplementation (1-5 mg/d) for patients being treated with methotrexate, as it may reduce the drug’s hematologic and gastrointestinal adverse effects without decreasing efficacy. A
• Advise patients scheduled to begin biologic therapy to get standard vaccinations—eg, pneumococcal, influenza, hepatitis A and B—before treatment is initiated and to avoid live and live-attenuated vaccines thereafter. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Psoriasis is a systemic inflammatory disorder altered by environmental and genetic factors that presents as scaly erythematous plaques and affects 2% to 3% of the population.1 Eighty percent have mild-to-moderate cutaneous disease; disabling arthritis occurs in as many as 42% of cases.1,2 The devastating effects on quality of life— including social stigmatization, pain, physical disability, and psychological distress—are comparable to the effects of conditions like cancer and depression.2 There is no definitive cure, and patients are left with a lifetime of waxing and waning symptoms.
Helping to create an individualized treatment plan tailored to disease type and extent, comorbidities, and needs of the patient can directly impact the quality of his or her life.3 For those with localized disease, topical therapy is a suitable first choice. Phototherapy is generally the first-line treatment for patients with extensive psoriasis or disabling symptoms. When phototherapy is not feasible or is ineffective, systemic treatments with conventional oral agents or biologics are indicated.
Discussions about therapeutic options should include expected results and duration of remission, cost, convenience, adverse effects, insurance coverage, and safety concerns. The patient’s preferences should be taken into account in the treatment plan you create.3 A guiding principle: The optimal protocol is the one the patient is motivated to adhere to.
The limits of topical therapy
Topical treatments are safe and effective when used properly, as monotherapy for localized disease and adjunctive therapy for resistant lesions. Monotherapy with topical agents is not recommended for sites that have a significant impact on quality of life, such as the palms and soles, and is not practical for patients with extensive disease (>10% of body surface).4
More potent topicals, such as corticosteroids, are recommended for acute flares; less potent agents with fewer adverse effects, such as calcipotriene, are typically used for maintenance ( TABLE 1 ).5-8 Topical agents can be used either long term or intermittently. Here, too, the most effective treatment is the one the patient will actually apply.5
TABLE 1
A look at nonsteroidal topical treatments5-8
| Class | Examples | Adverse effects | Comments |
|---|---|---|---|
| Vitamin D analogues | Calcipotriol (calcipotriene), calcitriol | Burning, pruritus, edema, peeling, dryness, erythema | Combining with beta-methasone dipropionate increases efficacy |
| Retinoids | Tazarotene, tretinoin | Teratogenic, photosensitivity, irritation | Increased efficacy when combined with NB-UVB (and less UV exposure); increased efficacy, duration of remission, and reduction in steroid-induced atrophy when used with steroids |
| Calcineurin inhibitors | Tacrolimus, pimecrolimus | Burning and pruritus Black box warning for risk of malignancies* | No clinical evidence of increased cancer risk |
| Others | Emollients, salicylic acid, anthralin, coal tar | Severe skin irritation, staining of clothes, odor with anthralin and tar | Salicylic acid works well with steroids and topical immunomodulators, but is not compatible with calcipotriene |
| *Lymphoma seen with oral therapy. UVB, ultraviolet B; NB-UVB, narrow-band ultraviolet B. | |||
Steroid selection is based on site, severity
Corticosteroids are antiproliferative, immunosuppressive, anti-inflammatory, and vasoconstrictive, and divided into 7 classes. Low-potency agents (Class 7) are used on thinner skin like the face, intertriginous areas, and groin; high-potency steroids (Class 1) are reserved for thicker, chronic plaques.9 As a general rule, Class 1 steroids can be safely used for 2 to 4 weeks, with increased risk of both cutaneous effects and systemic absorption if used continuously for longer periods.5 The optimal end point for less potent agents is not known.
Cutaneous adverse effects are more common than systemic ones and include skin atrophy, telangiectasia, striae distensae, acne, folliculitis, and purpura.
Systemic adverse effects include Cushing’s syndrome, osteonecrosis of the femoral head, cataracts, and glaucoma.5 The greatest risk of systemic effects is associated with prolonged use of high-potency corticosteroids over large surfaces or under occlusion with dressings or plastic wrap. Patients should be transitioned to the lowest potency possible to maintain efficacy, use corticosteroids intermittently, or combine them with nonsteroidal agents to avoid unwanted effects.
Topicals aren’t working? Move on to phototherapy
Phototherapy is an option for patients with extensive disease or skin manifestations that are recalcitrant to topicals. It is efficacious, cost-effective, and lacks systemic immunosuppression. Ultraviolet (UV) A and B act on Langerhans cells, cytokines, and adhesion molecules, inhibiting epidermal proliferation and angiogenesis.10
Broadband UVB (BB-UVB) was first used during the 1950s, with crude tar or anthralin, but is rarely used today.
Narrow-band UVB (NB-UVB) (311-313 nm), developed in the 1980s, has largely replaced BB-UVB. In addition to providing more rapid clearing and resolution of psoriasis compared with BB-UVB, NB-UVB may have less phototoxicity.11 Between 20 and 25 NB-UVB treatments, 2 to 3 times a week (in the office or at home) are usually required for significant improvement.
Photocarcinogenesis is a concern but numerous studies, including a review of 3867 patients treated with NB-UVB with a median 5.5-year follow-up, found no significant association with cutaneous malignancies.12 NB-UVB is considered safe during pregnancy and used as first-line therapy for pregnant patients.13
Targeted UVB therapy using a 308-nm excimer laser, another option, selectively targets psoriatic lesions, leaving normal skin untreated. This makes supraerythemogenic doses possible, which increases UVB’s efficacy. Long-term adverse effects and duration of remission have not been clearly established.14
Psoralen and UVA (PUVA), which uses oral or topical psoralens to sensitize the skin to UVA, has a slightly higher efficacy than NB-UVB, but with increased risk of squamous cell carcinoma (SCC) and possibly melanoma.15 Clearing can occur within 24 treatments, with remissions lasting 3 to 6 months;16 monthly maintenance has not been found to lengthen remission.13
Common adverse effects include erythema that peaks 48 to 96 hours after a treatment, pruritus, xerosis, irregular pigmentation, and gastrointestinal symptoms that can be reduced by decreasing psoralen and/or UVA doses.13 High cumulative doses of oral PUVA (>200 treatments) is associated with a dose-related increased risk of nonmelanoma skin cancer, particularly SCC, in the Caucasian population. This increased risk has not been demonstrated in patients treated with PUVA bath therapy, which is more common in Scandinavian countries.17 There is no consensus regarding the risk of melanoma.15
A careful risk-benefit analysis is needed before initiating phototherapy in patients who take photosensitizing drugs, are immunosuppressed, or have a photosensitivity disorder or a history of melanoma, atypical nevi, multiple melanoma risk factors, or multiple nonmelanoma skin cancers.13 Regardless of the type of UV therapy administered, eye protection with goggles is required to decrease the risk of UV-related cataract formation, and genital shielding is needed to prevent increased risk of tumors.13 Photoaging is a long-term effect.
CASE When questioned further about the challenges that Tom has had with controlling his symptoms, he admitted to being noncompliant. As a busy executive, he said he didn’t have time to use the topical corticosteroids regularly. Phototherapy could alleviate his cutaneous symptoms, but would not address his symptoms that were consistent with psoriatic arthritis.
Pairing therapeutic modalities decreases exposure
Combining therapeutic modalities like emollients and topical or oral retinoids with NB-UVB improves efficacy while reducing the number of treatment sessions and cumulative UVB dosage. If calcipotriene is used, it should be applied after phototherapy because it is degraded upon UVB exposure.18 Acitretin should be started 2 weeks prior to initiation of phototherapy, and its use accompanied by a 25% reduction in initial UV dosage.13
PUVA may also be combined with topical calcipotriene or retinoids.19 In both cases, the addition of the other agent typically decreases the duration of phototherapy, improves the clinical response, and reduces the risk of cancer.13,20
Extensive disease? Consider a traditional systemic agent
Traditional systemic therapy is used to treat extensive disease ( FIGURE ), psoriasis refractory to topical agents, and debilitating disease on the palms, soles, or scalp. Biologics are a recent alternative, but traditional systemics have been utilized longer and have a more longstanding adverse effect and safety profile, are administered orally, and are much less expensive than biologics. Monitoring patients on systemic therapy is necessary ( TABLE 2 ).21-24
Methotrexate (MTX), a competitive inhibitor of dihydrofolate reductase, is the most commonly prescribed traditional systemic psoriasis treatment.21 It is administered in a single weekly dose via tablet, parenteral solution, or intramuscular (IM) or subcutaneous (SC) injection.25 A test dose (2.5 or 5 mg) is given initially and complete blood cell count is monitored within one week to evaluate for potential bone marrow toxicity. If none is observed, the dose may be increased to control the disease while minimizing adverse effects.21
Common adverse effects of MTX, such as nausea, vomiting, stomatitis, and fatigue, may be minimized by IM or SC administration, splitting the dose, or providing folate supplementation.21-24 Given in doses of 1 to 5 mg/d, folate may reduce adverse hematologic, gastrointestinal, and hepatic effects without decreasing efficacy.22
The major severe toxicities are myelosuppression, hepatotoxicity, and pulmonary fibrosis.24 MTX-induced hepatotoxicity is similar to nonalcoholic fatty liver disease (NAFLD) and is thought to exacerbate preexisting NAFLD, which is common in patients with metabolic syndrome. A liver biopsy or serum assays for liver fibrosis (amino-terminal peptide of pro-collagen III) may be warranted during therapy.24
MTX is an abortifacient and teratogen, so contraception during treatment and for up to 3 months thereafter is mandatory for women of childbearing age.26 Men should be advised that MTX decreases sperm count. (For more on methotrexate, see: “When a fetus survives methotrexate exposure,” at http://www.jfponline.com/Pages.asp?AID=10299).
Cyclosporine (CSA), an oral calcineurin inhibitor, is a potent immunosuppressant that rapidly clears psoriasis.27 Because duration of use correlates with permanent nephrotoxicity, hypertension, and potential increased risk of SCC and lymphoma, intermittent 12-week courses are recommended. Calcium channel blockers are the preferred treatment for CSA-induced hypertension because of their effect on smooth muscle vasodilation.21
Oral retinoids. Acitretin modulates epidermal proliferation and is anti-inflammatory. Because it lacks immunosuppression, acitretin is generally considered the treatment of choice in HIV patients with severe psoriasis.28 Acitretin is teratogenic and contraindicated in women who plan to become pregnant or who are unwilling to use adequate contraception for 3 years after discontinuing the drug.21
CASE Given the significant percentage of body surface area involved and symptoms consistent with psoriatic arthritis, Tom required an aggressive therapeutic regimen. His history of nonalcoholic fatty liver and social drinking precluded the use of methotrexate. A biologic therapy was the next therapeutic choice that could relieve both his cutaneous and joint symptoms.
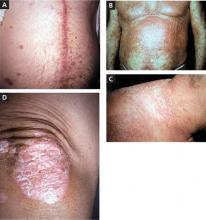
FIGURE
4 psoriasis patients, 4 different presentations
A. The linear erythematous, scaly plaque along this patient’s cardiac bypass scar demonstrates koebnerization of plaque psoriasis.
B. Diffuse erythematous scaly papules coalesce into plaques on this patient’s anterior chest, arms, abdomen, and periumbilicus.
C. The pinpoint pustules on an erythematous base on this patient’s lateral neck, shoulders, and upper back are characteristic of pustular psoriasis.
D. This patient has erythematous plaques with overlying silvery scale on the elbow—a classic
TABLE 2
Traditional systemic therapy21-24
| Methotrexate (MTX) | Cyclosporine (CSA)* | Acitretin | |
|---|---|---|---|
| Dosing | ≤30 mg in one weekly dose (PO, IM, or SC) | 2.5-5.0 mg/kg/d in 2 divided doses for 12 wk, then 12-wk nontreatment period Dose decreased (by 0.5-1.0 mg/kg) with disease clearance or when hypertension or nephrotoxicity are detected | 10-50 mg/d given as a single dose Lower doses (25 mg/d) used to minimize adverse effects and in combination regimens When added to UV, light dose should be reduced 30%-50% |
| Contraindications |
|
|
|
| Baseline monitoring† |
|
|
|
| BP, blood pressure; BUN, blood urea nitrogen; CBC, complete blood cell count; CR, creatinine; H&P, history and physical; IM, intramuscular; LFTs, liver function tests; PO, by mouth; PPD, purified protein derivative; PUVA, psoralen and ultraviolet A; SC, subcutaneous; UV, ultraviolet; UVB, ultraviolet B. *Avoid live vaccinations; caution required with major infection and poorly controlled diabetes. †Ongoing monitoring for MTX: BUN, CBC, CR, LFTs; possible liver biopsy (for high-risk patients or cumulative dose >3.5-4 g); CSA: BP, BUN, CBC, CR, LFTs; lipd profile; magnesium, uric acid, potassium tests; pregnancy testing; Acitretin: CBC, LFTs, lipid profile, renal function test, pregnancy testing. | |||
Biologics require lab work and a detailed medication list
Before beginning biologic therapy for a patient, the National Psoriasis Foundation29 recommends obtaining a complete history, physical, medication list, future plans (ie, pregnancy or travel to locations requiring vaccinations), and baseline labs to identify possible risk factors and/or contraindications. Periodic evaluation to monitor development of new symptoms, including infection and malignancy ( TABLE 3 ),24,30-33 is needed, as well.
TABLE 3
Is your patient a candidate for biologics?24,30-33
| Agent (Drug class) | Alefacept (T-cell inhibitor) | Adalimumab (TNF-inhibitor) | Etanercept (TNF-inhibitor) | Infliximab (TNF-inhibitor) | Ustekinumab (IL-12/23 inhibitor) |
|---|---|---|---|---|---|
| Dosing | 15 mg IM/wk for 12 wk, then 12-wk nontreatment period | 80 mg SC the first wk, 40 mg the 2nd wk, followed by 40 mg every other wk | 50 mg SC twice/wk for 3 mo, then 50 mg/wk | 5 mg/kg IV infusion to start, repeat at 2 and 6 wk, then q6-8 wk | 45 mg SC (for patients <100 kg); 90 mg (for patients >100 kg) to start, repeat at 4 wk, followed by q12 wk for maintenance |
| Contra-indications | HIV |
|
|
| Active TB |
| Baseline monitoring | CD4 count |
|
|
| PPD |
| Ongoing monitoring | Biweekly CD4 count; hold dose for counts <250 |
|
|
| Consider a yearly PPD |
| CBC, complete blood cell count; CHF, congestive heart failure; HIV, human immunodeficiency virus; H&P, history and physical; IL-12/23, interleukin-12, interleukin-23; LFTs, liver function test; MS, multiple sclerosis; NYHA, New York Heart Association; PPD, purified protein derivative; TB, tuberculosis; TNF, tumor necrosis factor. *For this patient population, adalimumab and etanercept have a (theoretical) risk. | |||||
Biologic therapy is contraindicated in patients with active serious infection. If patients develop infections requiring antibiotics while being treated, holding the biologic until infection resolution is advised.34 Standard vaccinations (eg, pneumococcal, hepatitis A and B, influenza, diphtheria, tetanus) are recommended before initiation of immunosuppressive therapy. After therapy starts, patients should avoid live and live-attenuated vaccines (varicella, mumps, measles, and rubella, oral typhoid, yellow fever, herpes zoster, intranasal influenza).35
Currently, none of the biologics are indicated for use in children or adolescents with psoriasis, despite epidemiologic data suggesting that one-third of adults with psoriasis developed it during childhood, in a form severe enough to warrant the use of systemic medications.34 The FDA is currently reviewing the possibility of indicating etanercept for pediatric psoriasis patients. All biologics are category B for pregnancy as there is no evidence that they negatively affect pregnancy.24
T-cell inhibitor. Alefacept binds CD2 on memory-effector T lymphocytes, inhibiting activation. Weekly intramuscular injections of alefacept for 12 weeks can clear lesions with long remissions.30
TNF-inhibitors. The TNF-inhibitors have been available for more than 10 years, predominantly for inflammatory bowel disease (IBD) and rheumatoid arthritis (RA), and more than 1.5 million patients have used adalimumab, etanercept, and infliximab for these disorders. Safety data, especially long term, are mostly derived from patients with IBD or RA, who have often combined TNF-inhibitors with additional immunosuppressive therapies. Thus, for psoriasis patients, who typically use biologics as monotherapy, the risk may be overestimated.24
TNF-inhibitors increase the risk for infection, most commonly of the upper respiratory tract, and, rarely, have been associated with opportunistic infections. Numerous cases of TB reactivation and an increased incidence of disseminated cases have been associated with TNF-inhibitors, so screening is recommended.24
The impact of TNF inhibition on congestive heart failure (CHF) is not well understood. Studies have variously shown that TNF-inhibitors have no effect on CHF morbidity or mortality, increase CHF mortality, or improve left ventricular function. TNF-inhibitors should be avoided in patients with severe CHF (New York Heart Association class III or IV). In milder CHF patients with worsening of symptoms, treatment should be discontinued.36
The increased risk of malignancy, especially lymphoma, is a concern, as there have been numerous case reports of lymphoma occurring with TNF-inhibitors. Psoriasis patients in general have an increased risk of lymphoma that confounds data interpretation.31 A number of case reports and a large observational study have shown patients receiving TNF-inhibitors may be at a greater risk for developing melanoma and nonmelanoma skin cancer.32
Ustekinumab, an interleukin 12/23 inhibitor, is a human monoclonal antibody that is absorbed and eliminated slowly, making dosing injections every 12 weeks convenient with efficacy maintained for at least one year.33 Because of its relative novelty, few studies are published regarding long-term safety. A recent head-to-head trial compared the efficacy and safety of ustekinumab with etanercept and found superior efficacy with ustekinumab, with comparable adverse events.37,38 Similar concerns exist with ustekinumab as with TNF-inhibitors, including infection, malignancy, CHF, and TB.33
CASE Tom denied having a personal family history of multiple sclerosis, or any demyelinating disorder. Nor did he have a history of cancer, tuberculosis exposure, CHF, or hepatitis. A purified protein derivative (PPD) was negative, as was his hepatitis panel, and his complete blood count with differential and metabolic panel were within normal limits.
Tom was started on the TNF-inhibitor adalimumab, after undergoing patient education and training and receiving instructions to stop the medication if he developed a major illness or infection. He received a loading dose of 80 mg SC, followed by 40 mg every other week. He tolerated the treatment well and 70% of his cutaneous symptoms cleared after 12 weeks of therapy; his joint pain also was reduced.
Tom is followed regularly in the clinic, with labs every 4 to 6 months. He is maintained on the injections and happy with the results. At each visit, weight loss and decreased beer intake are encouraged, both of which have been shown to reduce psoriasis severity. Although the beta-blockers and ACE inhibitors he takes are known to exacerbate psoriasis, the medications are necessary to treat Tom’s coronary artery disease.
CORRESPONDENCE Elizabeth Uhlenhake, MD, 18100 Oakwood Boulevard, Suite 300, Dearborn, MI 48124; eulenha@neomed.edu
1. Gelfand JM, Weinstein R, Porter SB, et al. Prevalence and treatment of psoriasis in the United Kingdom: a population-based study. Arch Dermatol. 2005;141:1537-1541.
2. Fortune DG, Richards HL, Griffiths CE. Psychologic factors in psoriasis: consequences, mechanisms, and interventions. Dermatol Clin. 2005;23:681-694.
3. Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332.
4. Pettey AA, Balkrishnan R, Rapp SR, et al. Patients with palmoplantar psoriasis have more physical disability and discomfort than patients with other forms of psoriasis: implications for clinical practice. J Am Acad Dermatol. 2003;49:271-275.
5. Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 3. Guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
6. Kaufmann R, Bibby AJ, Bissonnette R, et al. A new calcipotriol/betamethasone dipropionate formulation (Daivobet) is an effective once-daily treatment for psoriasis vulgaris. Dermatology. 2002;205:389-393.
7. Koo JY, Martin D. Investigator-masked comparison of tazarotene gel q.d. plus mometasone furoate cream q.d. vs. mometasone furoate cream b.i.d. in the treatment of plaque psoriasis. Int J Dermatol. 2001;40:210-212.
8. Berger TG, Duvic M, Van Voorhees AS, et al. The use of topical calcineurin inhibitors in dermatology: safety concerns. Report of the American Academy of Dermatology Association Task Force. J Am Acad Dermatol. 2006;54:818-823.
9. Cornell RC, Stoughton RB. Correlation of the vasoconstriction assay and clinical activity in psoriasis. Arch Dermatol. 1985;121:63-67.
10. Lui H. Phototherapy of psoriasis: update with practical pearls. J Cutan Med Surg. 2002;6(suppl):17-21.
11. Walters IB, Burack LH, Coven TR, et al. Suberythemogenic narrow-band UVB is markedly more effective than conventional UVB in treatment of psoriasis vulgaris. J Am Acad Dermatol. 1999;40:893-900.
12. Stern RS, Laird N. The carcinogenic risk of treatments for severe psoriasis. Photochemotherapy follow-up study. Cancer. 1994;73:2759-2764.
13. Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 5. Guidelines of care for the treatment of psoriasis with phototherapy and photochemotherapy. J Am Acad Dermatol. 2010;62:114-135.
14. Trehan M, Taylor CR. High-dose 308-nm excimer laser for the treatment of psoriasis. J Am Acad Dermatol. 2002;46:732-737.
15. Morison WL, Baughman RD, Day RM, et al. Consensus workshop on the toxic effects of long-term PUVA therapy. Arch Dermatol. 1998;134:595-598.
16. Spuls PI, Witkamp L, Bossuyt PM, et al. A systematic review of five systemic treatments for severe psoriasis. Br J Dermatol. 1997;137:943-949.
17. Murase JE, Lee EE, Koo J. Effect of ethnicity on the risk of developing nonmelanoma skin cancer following long-term PUVA therapy. Int J Dermatol. 2005;44:1016-1021.
18. Lowe NJ, Prystowsky JH, Bourget T, et al. Acitretin plus UVB therapy for psoriasis. Comparisons with placebo plus UVB and acitretin alone. J Am Acad Dermatol. 1991;24:591-594.
19. Torras H, Aliaga A, Lopez-Estebaranz JL, et al. A combination therapy of calcipotriol cream and PUVA reduces the UVA dose and improves the response of psoriasis vulgaris. J Dermatolog Treat. 2004;15:98-103.
20. Tanew A, Guggenbichler A, Honigsmann H, et al. Photochemotherapy for severe psoriasis without or in combination with acitretin: a randomized, double-blind comparison study. J Am Acad Dermatol. 1991;25:682-684.
21. Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 4. Guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol. 2009;61:451-485.
22. Strober BE, Menon K. Folate supplementation during methotrexate therapy for patients with psoriasis. J Am Acad Dermatol. 2005;53:652-659.
23. Taler SJ, Textor SC, Canzanello VJ, et al. Cyclosporine-induced hypertension: incidence, pathogenesis and management. Drug Saf. 1999;20:437-449.
24. Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850.
25. Heydendael VM, Spuls PI, Opmeer BC, et al. Methotrexate versus cyclosporine in moderate-to-severe chronic plaque psoriasis. N Engl J Med. 2003;349:658-665.
26. Lloyd ME, Carr M, McElhatton P, et al. The effects of methotrexate on pregnancy, fertility and lactation. QJM. 1999;92:551-563.
27. Ellis CN, Fradin MS, Messana JM, et al. Cyclosporine for plaque-type psoriasis. Results of a multidose, double-blind trial. N Engl J Med. 1991;324:277-284.
28. Buccheri L, Katchen BR, Karter AJ, et al. Acitretin therapy is effective for psoriasis associated with human immunodeficiency virus infection. Arch Dermatol. 1997;133:711-715.
29. Lebwohl M, Bagel J, Gelfand JM, et al. From the Medical Board of the National Psoriasis Foundation: monitoring and vaccinations in patients treated with biologics for psoriasis. J Am Acad Dermatol. 2008;58:94-105.
30. Gordon KB, Vaishnaw AK, O’Gorman J, et al. Treatment of psoriasis with alefacept: correlation of clinical improvement with reductions of memory T-cell counts. Arch Dermatol. 2003;139:1563-1570.
31. Brown SL, Greene MH, Gershon SK, et al. Tumor necrosis factor antagonist therapy and lymphoma development: twenty-six cases reported to the Food and Drug Administration. Arthritis Rheum. 2002;46:3151-3158.
32. Fulchiero GJ, Jr, Salvaggio H, Drabick JJ, et al. Eruptive latent metastatic melanomas after initiation of antitumor necrosis factor therapies. J Am Acad Dermatol. 2007;56(suppl):S65-S67.
33. Leonardi CL, Kimball AB, Papp KA, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet. 2008;371:1665-1674.
34. US Food and Drug Administration. FDA approves new drug to treat psoriasis. Sept. 25, 2009. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm183851.htm. Accessed June 15, 2012.
35. Duchini A, Goss JA, Karpen S, et al. Vaccinations for adult solid-organ transplant recipients: current recommendations and protocols. Clin Microbiol Rev. 2003;16:357-364.
36. Mann DL, McMurray JJ, Packer M, et al. Targeted anticytokine therapy in patients with chronic heart failure: results of the Randomized Etanercept Worldwide Evaluation (RENEWAL). Circulation. 2004;109:1594-1602.
37. Griffiths CE, Strober BE, van de Kerkhof P, et al. Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N Engl J Med. 2010;362:118-128.
38. Papp KA, Langley RG, Lebwohl M, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet. 2008;371:1675-1684.
• Prescribe high-potency (Class 1) topical steroids for use on thicker, chronic plaques and low-potency (Class 7) steroids for the face, intertriginous areas, and the groin. A
• Recommend folate supplementation (1-5 mg/d) for patients being treated with methotrexate, as it may reduce the drug’s hematologic and gastrointestinal adverse effects without decreasing efficacy. A
• Advise patients scheduled to begin biologic therapy to get standard vaccinations—eg, pneumococcal, influenza, hepatitis A and B—before treatment is initiated and to avoid live and live-attenuated vaccines thereafter. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Psoriasis is a systemic inflammatory disorder altered by environmental and genetic factors that presents as scaly erythematous plaques and affects 2% to 3% of the population.1 Eighty percent have mild-to-moderate cutaneous disease; disabling arthritis occurs in as many as 42% of cases.1,2 The devastating effects on quality of life— including social stigmatization, pain, physical disability, and psychological distress—are comparable to the effects of conditions like cancer and depression.2 There is no definitive cure, and patients are left with a lifetime of waxing and waning symptoms.
Helping to create an individualized treatment plan tailored to disease type and extent, comorbidities, and needs of the patient can directly impact the quality of his or her life.3 For those with localized disease, topical therapy is a suitable first choice. Phototherapy is generally the first-line treatment for patients with extensive psoriasis or disabling symptoms. When phototherapy is not feasible or is ineffective, systemic treatments with conventional oral agents or biologics are indicated.
Discussions about therapeutic options should include expected results and duration of remission, cost, convenience, adverse effects, insurance coverage, and safety concerns. The patient’s preferences should be taken into account in the treatment plan you create.3 A guiding principle: The optimal protocol is the one the patient is motivated to adhere to.
The limits of topical therapy
Topical treatments are safe and effective when used properly, as monotherapy for localized disease and adjunctive therapy for resistant lesions. Monotherapy with topical agents is not recommended for sites that have a significant impact on quality of life, such as the palms and soles, and is not practical for patients with extensive disease (>10% of body surface).4
More potent topicals, such as corticosteroids, are recommended for acute flares; less potent agents with fewer adverse effects, such as calcipotriene, are typically used for maintenance ( TABLE 1 ).5-8 Topical agents can be used either long term or intermittently. Here, too, the most effective treatment is the one the patient will actually apply.5
TABLE 1
A look at nonsteroidal topical treatments5-8
| Class | Examples | Adverse effects | Comments |
|---|---|---|---|
| Vitamin D analogues | Calcipotriol (calcipotriene), calcitriol | Burning, pruritus, edema, peeling, dryness, erythema | Combining with beta-methasone dipropionate increases efficacy |
| Retinoids | Tazarotene, tretinoin | Teratogenic, photosensitivity, irritation | Increased efficacy when combined with NB-UVB (and less UV exposure); increased efficacy, duration of remission, and reduction in steroid-induced atrophy when used with steroids |
| Calcineurin inhibitors | Tacrolimus, pimecrolimus | Burning and pruritus Black box warning for risk of malignancies* | No clinical evidence of increased cancer risk |
| Others | Emollients, salicylic acid, anthralin, coal tar | Severe skin irritation, staining of clothes, odor with anthralin and tar | Salicylic acid works well with steroids and topical immunomodulators, but is not compatible with calcipotriene |
| *Lymphoma seen with oral therapy. UVB, ultraviolet B; NB-UVB, narrow-band ultraviolet B. | |||
Steroid selection is based on site, severity
Corticosteroids are antiproliferative, immunosuppressive, anti-inflammatory, and vasoconstrictive, and divided into 7 classes. Low-potency agents (Class 7) are used on thinner skin like the face, intertriginous areas, and groin; high-potency steroids (Class 1) are reserved for thicker, chronic plaques.9 As a general rule, Class 1 steroids can be safely used for 2 to 4 weeks, with increased risk of both cutaneous effects and systemic absorption if used continuously for longer periods.5 The optimal end point for less potent agents is not known.
Cutaneous adverse effects are more common than systemic ones and include skin atrophy, telangiectasia, striae distensae, acne, folliculitis, and purpura.
Systemic adverse effects include Cushing’s syndrome, osteonecrosis of the femoral head, cataracts, and glaucoma.5 The greatest risk of systemic effects is associated with prolonged use of high-potency corticosteroids over large surfaces or under occlusion with dressings or plastic wrap. Patients should be transitioned to the lowest potency possible to maintain efficacy, use corticosteroids intermittently, or combine them with nonsteroidal agents to avoid unwanted effects.
Topicals aren’t working? Move on to phototherapy
Phototherapy is an option for patients with extensive disease or skin manifestations that are recalcitrant to topicals. It is efficacious, cost-effective, and lacks systemic immunosuppression. Ultraviolet (UV) A and B act on Langerhans cells, cytokines, and adhesion molecules, inhibiting epidermal proliferation and angiogenesis.10
Broadband UVB (BB-UVB) was first used during the 1950s, with crude tar or anthralin, but is rarely used today.
Narrow-band UVB (NB-UVB) (311-313 nm), developed in the 1980s, has largely replaced BB-UVB. In addition to providing more rapid clearing and resolution of psoriasis compared with BB-UVB, NB-UVB may have less phototoxicity.11 Between 20 and 25 NB-UVB treatments, 2 to 3 times a week (in the office or at home) are usually required for significant improvement.
Photocarcinogenesis is a concern but numerous studies, including a review of 3867 patients treated with NB-UVB with a median 5.5-year follow-up, found no significant association with cutaneous malignancies.12 NB-UVB is considered safe during pregnancy and used as first-line therapy for pregnant patients.13
Targeted UVB therapy using a 308-nm excimer laser, another option, selectively targets psoriatic lesions, leaving normal skin untreated. This makes supraerythemogenic doses possible, which increases UVB’s efficacy. Long-term adverse effects and duration of remission have not been clearly established.14
Psoralen and UVA (PUVA), which uses oral or topical psoralens to sensitize the skin to UVA, has a slightly higher efficacy than NB-UVB, but with increased risk of squamous cell carcinoma (SCC) and possibly melanoma.15 Clearing can occur within 24 treatments, with remissions lasting 3 to 6 months;16 monthly maintenance has not been found to lengthen remission.13
Common adverse effects include erythema that peaks 48 to 96 hours after a treatment, pruritus, xerosis, irregular pigmentation, and gastrointestinal symptoms that can be reduced by decreasing psoralen and/or UVA doses.13 High cumulative doses of oral PUVA (>200 treatments) is associated with a dose-related increased risk of nonmelanoma skin cancer, particularly SCC, in the Caucasian population. This increased risk has not been demonstrated in patients treated with PUVA bath therapy, which is more common in Scandinavian countries.17 There is no consensus regarding the risk of melanoma.15
A careful risk-benefit analysis is needed before initiating phototherapy in patients who take photosensitizing drugs, are immunosuppressed, or have a photosensitivity disorder or a history of melanoma, atypical nevi, multiple melanoma risk factors, or multiple nonmelanoma skin cancers.13 Regardless of the type of UV therapy administered, eye protection with goggles is required to decrease the risk of UV-related cataract formation, and genital shielding is needed to prevent increased risk of tumors.13 Photoaging is a long-term effect.
CASE When questioned further about the challenges that Tom has had with controlling his symptoms, he admitted to being noncompliant. As a busy executive, he said he didn’t have time to use the topical corticosteroids regularly. Phototherapy could alleviate his cutaneous symptoms, but would not address his symptoms that were consistent with psoriatic arthritis.
Pairing therapeutic modalities decreases exposure
Combining therapeutic modalities like emollients and topical or oral retinoids with NB-UVB improves efficacy while reducing the number of treatment sessions and cumulative UVB dosage. If calcipotriene is used, it should be applied after phototherapy because it is degraded upon UVB exposure.18 Acitretin should be started 2 weeks prior to initiation of phototherapy, and its use accompanied by a 25% reduction in initial UV dosage.13
PUVA may also be combined with topical calcipotriene or retinoids.19 In both cases, the addition of the other agent typically decreases the duration of phototherapy, improves the clinical response, and reduces the risk of cancer.13,20
Extensive disease? Consider a traditional systemic agent
Traditional systemic therapy is used to treat extensive disease ( FIGURE ), psoriasis refractory to topical agents, and debilitating disease on the palms, soles, or scalp. Biologics are a recent alternative, but traditional systemics have been utilized longer and have a more longstanding adverse effect and safety profile, are administered orally, and are much less expensive than biologics. Monitoring patients on systemic therapy is necessary ( TABLE 2 ).21-24
Methotrexate (MTX), a competitive inhibitor of dihydrofolate reductase, is the most commonly prescribed traditional systemic psoriasis treatment.21 It is administered in a single weekly dose via tablet, parenteral solution, or intramuscular (IM) or subcutaneous (SC) injection.25 A test dose (2.5 or 5 mg) is given initially and complete blood cell count is monitored within one week to evaluate for potential bone marrow toxicity. If none is observed, the dose may be increased to control the disease while minimizing adverse effects.21
Common adverse effects of MTX, such as nausea, vomiting, stomatitis, and fatigue, may be minimized by IM or SC administration, splitting the dose, or providing folate supplementation.21-24 Given in doses of 1 to 5 mg/d, folate may reduce adverse hematologic, gastrointestinal, and hepatic effects without decreasing efficacy.22
The major severe toxicities are myelosuppression, hepatotoxicity, and pulmonary fibrosis.24 MTX-induced hepatotoxicity is similar to nonalcoholic fatty liver disease (NAFLD) and is thought to exacerbate preexisting NAFLD, which is common in patients with metabolic syndrome. A liver biopsy or serum assays for liver fibrosis (amino-terminal peptide of pro-collagen III) may be warranted during therapy.24
MTX is an abortifacient and teratogen, so contraception during treatment and for up to 3 months thereafter is mandatory for women of childbearing age.26 Men should be advised that MTX decreases sperm count. (For more on methotrexate, see: “When a fetus survives methotrexate exposure,” at http://www.jfponline.com/Pages.asp?AID=10299).
Cyclosporine (CSA), an oral calcineurin inhibitor, is a potent immunosuppressant that rapidly clears psoriasis.27 Because duration of use correlates with permanent nephrotoxicity, hypertension, and potential increased risk of SCC and lymphoma, intermittent 12-week courses are recommended. Calcium channel blockers are the preferred treatment for CSA-induced hypertension because of their effect on smooth muscle vasodilation.21
Oral retinoids. Acitretin modulates epidermal proliferation and is anti-inflammatory. Because it lacks immunosuppression, acitretin is generally considered the treatment of choice in HIV patients with severe psoriasis.28 Acitretin is teratogenic and contraindicated in women who plan to become pregnant or who are unwilling to use adequate contraception for 3 years after discontinuing the drug.21
CASE Given the significant percentage of body surface area involved and symptoms consistent with psoriatic arthritis, Tom required an aggressive therapeutic regimen. His history of nonalcoholic fatty liver and social drinking precluded the use of methotrexate. A biologic therapy was the next therapeutic choice that could relieve both his cutaneous and joint symptoms.
FIGURE
4 psoriasis patients, 4 different presentations
A. The linear erythematous, scaly plaque along this patient’s cardiac bypass scar demonstrates koebnerization of plaque psoriasis.
B. Diffuse erythematous scaly papules coalesce into plaques on this patient’s anterior chest, arms, abdomen, and periumbilicus.
C. The pinpoint pustules on an erythematous base on this patient’s lateral neck, shoulders, and upper back are characteristic of pustular psoriasis.
D. This patient has erythematous plaques with overlying silvery scale on the elbow—a classic
TABLE 2
Traditional systemic therapy21-24
| Methotrexate (MTX) | Cyclosporine (CSA)* | Acitretin | |
|---|---|---|---|
| Dosing | ≤30 mg in one weekly dose (PO, IM, or SC) | 2.5-5.0 mg/kg/d in 2 divided doses for 12 wk, then 12-wk nontreatment period Dose decreased (by 0.5-1.0 mg/kg) with disease clearance or when hypertension or nephrotoxicity are detected | 10-50 mg/d given as a single dose Lower doses (25 mg/d) used to minimize adverse effects and in combination regimens When added to UV, light dose should be reduced 30%-50% |
| Contraindications |
|
|
|
| Baseline monitoring† |
|
|
|
| BP, blood pressure; BUN, blood urea nitrogen; CBC, complete blood cell count; CR, creatinine; H&P, history and physical; IM, intramuscular; LFTs, liver function tests; PO, by mouth; PPD, purified protein derivative; PUVA, psoralen and ultraviolet A; SC, subcutaneous; UV, ultraviolet; UVB, ultraviolet B. *Avoid live vaccinations; caution required with major infection and poorly controlled diabetes. †Ongoing monitoring for MTX: BUN, CBC, CR, LFTs; possible liver biopsy (for high-risk patients or cumulative dose >3.5-4 g); CSA: BP, BUN, CBC, CR, LFTs; lipd profile; magnesium, uric acid, potassium tests; pregnancy testing; Acitretin: CBC, LFTs, lipid profile, renal function test, pregnancy testing. | |||
Biologics require lab work and a detailed medication list
Before beginning biologic therapy for a patient, the National Psoriasis Foundation29 recommends obtaining a complete history, physical, medication list, future plans (ie, pregnancy or travel to locations requiring vaccinations), and baseline labs to identify possible risk factors and/or contraindications. Periodic evaluation to monitor development of new symptoms, including infection and malignancy ( TABLE 3 ),24,30-33 is needed, as well.
TABLE 3
Is your patient a candidate for biologics?24,30-33
| Agent (Drug class) | Alefacept (T-cell inhibitor) | Adalimumab (TNF-inhibitor) | Etanercept (TNF-inhibitor) | Infliximab (TNF-inhibitor) | Ustekinumab (IL-12/23 inhibitor) |
|---|---|---|---|---|---|
| Dosing | 15 mg IM/wk for 12 wk, then 12-wk nontreatment period | 80 mg SC the first wk, 40 mg the 2nd wk, followed by 40 mg every other wk | 50 mg SC twice/wk for 3 mo, then 50 mg/wk | 5 mg/kg IV infusion to start, repeat at 2 and 6 wk, then q6-8 wk | 45 mg SC (for patients <100 kg); 90 mg (for patients >100 kg) to start, repeat at 4 wk, followed by q12 wk for maintenance |
| Contra-indications | HIV |
|
|
| Active TB |
| Baseline monitoring | CD4 count |
|
|
| PPD |
| Ongoing monitoring | Biweekly CD4 count; hold dose for counts <250 |
|
|
| Consider a yearly PPD |
| CBC, complete blood cell count; CHF, congestive heart failure; HIV, human immunodeficiency virus; H&P, history and physical; IL-12/23, interleukin-12, interleukin-23; LFTs, liver function test; MS, multiple sclerosis; NYHA, New York Heart Association; PPD, purified protein derivative; TB, tuberculosis; TNF, tumor necrosis factor. *For this patient population, adalimumab and etanercept have a (theoretical) risk. | |||||
Biologic therapy is contraindicated in patients with active serious infection. If patients develop infections requiring antibiotics while being treated, holding the biologic until infection resolution is advised.34 Standard vaccinations (eg, pneumococcal, hepatitis A and B, influenza, diphtheria, tetanus) are recommended before initiation of immunosuppressive therapy. After therapy starts, patients should avoid live and live-attenuated vaccines (varicella, mumps, measles, and rubella, oral typhoid, yellow fever, herpes zoster, intranasal influenza).35
Currently, none of the biologics are indicated for use in children or adolescents with psoriasis, despite epidemiologic data suggesting that one-third of adults with psoriasis developed it during childhood, in a form severe enough to warrant the use of systemic medications.34 The FDA is currently reviewing the possibility of indicating etanercept for pediatric psoriasis patients. All biologics are category B for pregnancy as there is no evidence that they negatively affect pregnancy.24
T-cell inhibitor. Alefacept binds CD2 on memory-effector T lymphocytes, inhibiting activation. Weekly intramuscular injections of alefacept for 12 weeks can clear lesions with long remissions.30
TNF-inhibitors. The TNF-inhibitors have been available for more than 10 years, predominantly for inflammatory bowel disease (IBD) and rheumatoid arthritis (RA), and more than 1.5 million patients have used adalimumab, etanercept, and infliximab for these disorders. Safety data, especially long term, are mostly derived from patients with IBD or RA, who have often combined TNF-inhibitors with additional immunosuppressive therapies. Thus, for psoriasis patients, who typically use biologics as monotherapy, the risk may be overestimated.24
TNF-inhibitors increase the risk for infection, most commonly of the upper respiratory tract, and, rarely, have been associated with opportunistic infections. Numerous cases of TB reactivation and an increased incidence of disseminated cases have been associated with TNF-inhibitors, so screening is recommended.24
The impact of TNF inhibition on congestive heart failure (CHF) is not well understood. Studies have variously shown that TNF-inhibitors have no effect on CHF morbidity or mortality, increase CHF mortality, or improve left ventricular function. TNF-inhibitors should be avoided in patients with severe CHF (New York Heart Association class III or IV). In milder CHF patients with worsening of symptoms, treatment should be discontinued.36
The increased risk of malignancy, especially lymphoma, is a concern, as there have been numerous case reports of lymphoma occurring with TNF-inhibitors. Psoriasis patients in general have an increased risk of lymphoma that confounds data interpretation.31 A number of case reports and a large observational study have shown patients receiving TNF-inhibitors may be at a greater risk for developing melanoma and nonmelanoma skin cancer.32
Ustekinumab, an interleukin 12/23 inhibitor, is a human monoclonal antibody that is absorbed and eliminated slowly, making dosing injections every 12 weeks convenient with efficacy maintained for at least one year.33 Because of its relative novelty, few studies are published regarding long-term safety. A recent head-to-head trial compared the efficacy and safety of ustekinumab with etanercept and found superior efficacy with ustekinumab, with comparable adverse events.37,38 Similar concerns exist with ustekinumab as with TNF-inhibitors, including infection, malignancy, CHF, and TB.33
CASE Tom denied having a personal family history of multiple sclerosis, or any demyelinating disorder. Nor did he have a history of cancer, tuberculosis exposure, CHF, or hepatitis. A purified protein derivative (PPD) was negative, as was his hepatitis panel, and his complete blood count with differential and metabolic panel were within normal limits.
Tom was started on the TNF-inhibitor adalimumab, after undergoing patient education and training and receiving instructions to stop the medication if he developed a major illness or infection. He received a loading dose of 80 mg SC, followed by 40 mg every other week. He tolerated the treatment well and 70% of his cutaneous symptoms cleared after 12 weeks of therapy; his joint pain also was reduced.
Tom is followed regularly in the clinic, with labs every 4 to 6 months. He is maintained on the injections and happy with the results. At each visit, weight loss and decreased beer intake are encouraged, both of which have been shown to reduce psoriasis severity. Although the beta-blockers and ACE inhibitors he takes are known to exacerbate psoriasis, the medications are necessary to treat Tom’s coronary artery disease.
CORRESPONDENCE Elizabeth Uhlenhake, MD, 18100 Oakwood Boulevard, Suite 300, Dearborn, MI 48124; eulenha@neomed.edu
• Prescribe high-potency (Class 1) topical steroids for use on thicker, chronic plaques and low-potency (Class 7) steroids for the face, intertriginous areas, and the groin. A
• Recommend folate supplementation (1-5 mg/d) for patients being treated with methotrexate, as it may reduce the drug’s hematologic and gastrointestinal adverse effects without decreasing efficacy. A
• Advise patients scheduled to begin biologic therapy to get standard vaccinations—eg, pneumococcal, influenza, hepatitis A and B—before treatment is initiated and to avoid live and live-attenuated vaccines thereafter. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Psoriasis is a systemic inflammatory disorder altered by environmental and genetic factors that presents as scaly erythematous plaques and affects 2% to 3% of the population.1 Eighty percent have mild-to-moderate cutaneous disease; disabling arthritis occurs in as many as 42% of cases.1,2 The devastating effects on quality of life— including social stigmatization, pain, physical disability, and psychological distress—are comparable to the effects of conditions like cancer and depression.2 There is no definitive cure, and patients are left with a lifetime of waxing and waning symptoms.
Helping to create an individualized treatment plan tailored to disease type and extent, comorbidities, and needs of the patient can directly impact the quality of his or her life.3 For those with localized disease, topical therapy is a suitable first choice. Phototherapy is generally the first-line treatment for patients with extensive psoriasis or disabling symptoms. When phototherapy is not feasible or is ineffective, systemic treatments with conventional oral agents or biologics are indicated.
Discussions about therapeutic options should include expected results and duration of remission, cost, convenience, adverse effects, insurance coverage, and safety concerns. The patient’s preferences should be taken into account in the treatment plan you create.3 A guiding principle: The optimal protocol is the one the patient is motivated to adhere to.
The limits of topical therapy
Topical treatments are safe and effective when used properly, as monotherapy for localized disease and adjunctive therapy for resistant lesions. Monotherapy with topical agents is not recommended for sites that have a significant impact on quality of life, such as the palms and soles, and is not practical for patients with extensive disease (>10% of body surface).4
More potent topicals, such as corticosteroids, are recommended for acute flares; less potent agents with fewer adverse effects, such as calcipotriene, are typically used for maintenance ( TABLE 1 ).5-8 Topical agents can be used either long term or intermittently. Here, too, the most effective treatment is the one the patient will actually apply.5
TABLE 1
A look at nonsteroidal topical treatments5-8
| Class | Examples | Adverse effects | Comments |
|---|---|---|---|
| Vitamin D analogues | Calcipotriol (calcipotriene), calcitriol | Burning, pruritus, edema, peeling, dryness, erythema | Combining with beta-methasone dipropionate increases efficacy |
| Retinoids | Tazarotene, tretinoin | Teratogenic, photosensitivity, irritation | Increased efficacy when combined with NB-UVB (and less UV exposure); increased efficacy, duration of remission, and reduction in steroid-induced atrophy when used with steroids |
| Calcineurin inhibitors | Tacrolimus, pimecrolimus | Burning and pruritus Black box warning for risk of malignancies* | No clinical evidence of increased cancer risk |
| Others | Emollients, salicylic acid, anthralin, coal tar | Severe skin irritation, staining of clothes, odor with anthralin and tar | Salicylic acid works well with steroids and topical immunomodulators, but is not compatible with calcipotriene |
| *Lymphoma seen with oral therapy. UVB, ultraviolet B; NB-UVB, narrow-band ultraviolet B. | |||
Steroid selection is based on site, severity
Corticosteroids are antiproliferative, immunosuppressive, anti-inflammatory, and vasoconstrictive, and divided into 7 classes. Low-potency agents (Class 7) are used on thinner skin like the face, intertriginous areas, and groin; high-potency steroids (Class 1) are reserved for thicker, chronic plaques.9 As a general rule, Class 1 steroids can be safely used for 2 to 4 weeks, with increased risk of both cutaneous effects and systemic absorption if used continuously for longer periods.5 The optimal end point for less potent agents is not known.
Cutaneous adverse effects are more common than systemic ones and include skin atrophy, telangiectasia, striae distensae, acne, folliculitis, and purpura.
Systemic adverse effects include Cushing’s syndrome, osteonecrosis of the femoral head, cataracts, and glaucoma.5 The greatest risk of systemic effects is associated with prolonged use of high-potency corticosteroids over large surfaces or under occlusion with dressings or plastic wrap. Patients should be transitioned to the lowest potency possible to maintain efficacy, use corticosteroids intermittently, or combine them with nonsteroidal agents to avoid unwanted effects.
Topicals aren’t working? Move on to phototherapy
Phototherapy is an option for patients with extensive disease or skin manifestations that are recalcitrant to topicals. It is efficacious, cost-effective, and lacks systemic immunosuppression. Ultraviolet (UV) A and B act on Langerhans cells, cytokines, and adhesion molecules, inhibiting epidermal proliferation and angiogenesis.10
Broadband UVB (BB-UVB) was first used during the 1950s, with crude tar or anthralin, but is rarely used today.
Narrow-band UVB (NB-UVB) (311-313 nm), developed in the 1980s, has largely replaced BB-UVB. In addition to providing more rapid clearing and resolution of psoriasis compared with BB-UVB, NB-UVB may have less phototoxicity.11 Between 20 and 25 NB-UVB treatments, 2 to 3 times a week (in the office or at home) are usually required for significant improvement.
Photocarcinogenesis is a concern but numerous studies, including a review of 3867 patients treated with NB-UVB with a median 5.5-year follow-up, found no significant association with cutaneous malignancies.12 NB-UVB is considered safe during pregnancy and used as first-line therapy for pregnant patients.13
Targeted UVB therapy using a 308-nm excimer laser, another option, selectively targets psoriatic lesions, leaving normal skin untreated. This makes supraerythemogenic doses possible, which increases UVB’s efficacy. Long-term adverse effects and duration of remission have not been clearly established.14
Psoralen and UVA (PUVA), which uses oral or topical psoralens to sensitize the skin to UVA, has a slightly higher efficacy than NB-UVB, but with increased risk of squamous cell carcinoma (SCC) and possibly melanoma.15 Clearing can occur within 24 treatments, with remissions lasting 3 to 6 months;16 monthly maintenance has not been found to lengthen remission.13
Common adverse effects include erythema that peaks 48 to 96 hours after a treatment, pruritus, xerosis, irregular pigmentation, and gastrointestinal symptoms that can be reduced by decreasing psoralen and/or UVA doses.13 High cumulative doses of oral PUVA (>200 treatments) is associated with a dose-related increased risk of nonmelanoma skin cancer, particularly SCC, in the Caucasian population. This increased risk has not been demonstrated in patients treated with PUVA bath therapy, which is more common in Scandinavian countries.17 There is no consensus regarding the risk of melanoma.15
A careful risk-benefit analysis is needed before initiating phototherapy in patients who take photosensitizing drugs, are immunosuppressed, or have a photosensitivity disorder or a history of melanoma, atypical nevi, multiple melanoma risk factors, or multiple nonmelanoma skin cancers.13 Regardless of the type of UV therapy administered, eye protection with goggles is required to decrease the risk of UV-related cataract formation, and genital shielding is needed to prevent increased risk of tumors.13 Photoaging is a long-term effect.
CASE When questioned further about the challenges that Tom has had with controlling his symptoms, he admitted to being noncompliant. As a busy executive, he said he didn’t have time to use the topical corticosteroids regularly. Phototherapy could alleviate his cutaneous symptoms, but would not address his symptoms that were consistent with psoriatic arthritis.
Pairing therapeutic modalities decreases exposure
Combining therapeutic modalities like emollients and topical or oral retinoids with NB-UVB improves efficacy while reducing the number of treatment sessions and cumulative UVB dosage. If calcipotriene is used, it should be applied after phototherapy because it is degraded upon UVB exposure.18 Acitretin should be started 2 weeks prior to initiation of phototherapy, and its use accompanied by a 25% reduction in initial UV dosage.13
PUVA may also be combined with topical calcipotriene or retinoids.19 In both cases, the addition of the other agent typically decreases the duration of phototherapy, improves the clinical response, and reduces the risk of cancer.13,20
Extensive disease? Consider a traditional systemic agent
Traditional systemic therapy is used to treat extensive disease ( FIGURE ), psoriasis refractory to topical agents, and debilitating disease on the palms, soles, or scalp. Biologics are a recent alternative, but traditional systemics have been utilized longer and have a more longstanding adverse effect and safety profile, are administered orally, and are much less expensive than biologics. Monitoring patients on systemic therapy is necessary ( TABLE 2 ).21-24
Methotrexate (MTX), a competitive inhibitor of dihydrofolate reductase, is the most commonly prescribed traditional systemic psoriasis treatment.21 It is administered in a single weekly dose via tablet, parenteral solution, or intramuscular (IM) or subcutaneous (SC) injection.25 A test dose (2.5 or 5 mg) is given initially and complete blood cell count is monitored within one week to evaluate for potential bone marrow toxicity. If none is observed, the dose may be increased to control the disease while minimizing adverse effects.21
Common adverse effects of MTX, such as nausea, vomiting, stomatitis, and fatigue, may be minimized by IM or SC administration, splitting the dose, or providing folate supplementation.21-24 Given in doses of 1 to 5 mg/d, folate may reduce adverse hematologic, gastrointestinal, and hepatic effects without decreasing efficacy.22
The major severe toxicities are myelosuppression, hepatotoxicity, and pulmonary fibrosis.24 MTX-induced hepatotoxicity is similar to nonalcoholic fatty liver disease (NAFLD) and is thought to exacerbate preexisting NAFLD, which is common in patients with metabolic syndrome. A liver biopsy or serum assays for liver fibrosis (amino-terminal peptide of pro-collagen III) may be warranted during therapy.24
MTX is an abortifacient and teratogen, so contraception during treatment and for up to 3 months thereafter is mandatory for women of childbearing age.26 Men should be advised that MTX decreases sperm count. (For more on methotrexate, see: “When a fetus survives methotrexate exposure,” at http://www.jfponline.com/Pages.asp?AID=10299).
Cyclosporine (CSA), an oral calcineurin inhibitor, is a potent immunosuppressant that rapidly clears psoriasis.27 Because duration of use correlates with permanent nephrotoxicity, hypertension, and potential increased risk of SCC and lymphoma, intermittent 12-week courses are recommended. Calcium channel blockers are the preferred treatment for CSA-induced hypertension because of their effect on smooth muscle vasodilation.21
Oral retinoids. Acitretin modulates epidermal proliferation and is anti-inflammatory. Because it lacks immunosuppression, acitretin is generally considered the treatment of choice in HIV patients with severe psoriasis.28 Acitretin is teratogenic and contraindicated in women who plan to become pregnant or who are unwilling to use adequate contraception for 3 years after discontinuing the drug.21
CASE Given the significant percentage of body surface area involved and symptoms consistent with psoriatic arthritis, Tom required an aggressive therapeutic regimen. His history of nonalcoholic fatty liver and social drinking precluded the use of methotrexate. A biologic therapy was the next therapeutic choice that could relieve both his cutaneous and joint symptoms.
FIGURE
4 psoriasis patients, 4 different presentations
A. The linear erythematous, scaly plaque along this patient’s cardiac bypass scar demonstrates koebnerization of plaque psoriasis.
B. Diffuse erythematous scaly papules coalesce into plaques on this patient’s anterior chest, arms, abdomen, and periumbilicus.
C. The pinpoint pustules on an erythematous base on this patient’s lateral neck, shoulders, and upper back are characteristic of pustular psoriasis.
D. This patient has erythematous plaques with overlying silvery scale on the elbow—a classic
TABLE 2
Traditional systemic therapy21-24
| Methotrexate (MTX) | Cyclosporine (CSA)* | Acitretin | |
|---|---|---|---|
| Dosing | ≤30 mg in one weekly dose (PO, IM, or SC) | 2.5-5.0 mg/kg/d in 2 divided doses for 12 wk, then 12-wk nontreatment period Dose decreased (by 0.5-1.0 mg/kg) with disease clearance or when hypertension or nephrotoxicity are detected | 10-50 mg/d given as a single dose Lower doses (25 mg/d) used to minimize adverse effects and in combination regimens When added to UV, light dose should be reduced 30%-50% |
| Contraindications |
|
|
|
| Baseline monitoring† |
|
|
|
| BP, blood pressure; BUN, blood urea nitrogen; CBC, complete blood cell count; CR, creatinine; H&P, history and physical; IM, intramuscular; LFTs, liver function tests; PO, by mouth; PPD, purified protein derivative; PUVA, psoralen and ultraviolet A; SC, subcutaneous; UV, ultraviolet; UVB, ultraviolet B. *Avoid live vaccinations; caution required with major infection and poorly controlled diabetes. †Ongoing monitoring for MTX: BUN, CBC, CR, LFTs; possible liver biopsy (for high-risk patients or cumulative dose >3.5-4 g); CSA: BP, BUN, CBC, CR, LFTs; lipd profile; magnesium, uric acid, potassium tests; pregnancy testing; Acitretin: CBC, LFTs, lipid profile, renal function test, pregnancy testing. | |||
Biologics require lab work and a detailed medication list
Before beginning biologic therapy for a patient, the National Psoriasis Foundation29 recommends obtaining a complete history, physical, medication list, future plans (ie, pregnancy or travel to locations requiring vaccinations), and baseline labs to identify possible risk factors and/or contraindications. Periodic evaluation to monitor development of new symptoms, including infection and malignancy ( TABLE 3 ),24,30-33 is needed, as well.
TABLE 3
Is your patient a candidate for biologics?24,30-33
| Agent (Drug class) | Alefacept (T-cell inhibitor) | Adalimumab (TNF-inhibitor) | Etanercept (TNF-inhibitor) | Infliximab (TNF-inhibitor) | Ustekinumab (IL-12/23 inhibitor) |
|---|---|---|---|---|---|
| Dosing | 15 mg IM/wk for 12 wk, then 12-wk nontreatment period | 80 mg SC the first wk, 40 mg the 2nd wk, followed by 40 mg every other wk | 50 mg SC twice/wk for 3 mo, then 50 mg/wk | 5 mg/kg IV infusion to start, repeat at 2 and 6 wk, then q6-8 wk | 45 mg SC (for patients <100 kg); 90 mg (for patients >100 kg) to start, repeat at 4 wk, followed by q12 wk for maintenance |
| Contra-indications | HIV |
|
|
| Active TB |
| Baseline monitoring | CD4 count |
|
|
| PPD |
| Ongoing monitoring | Biweekly CD4 count; hold dose for counts <250 |
|
|
| Consider a yearly PPD |
| CBC, complete blood cell count; CHF, congestive heart failure; HIV, human immunodeficiency virus; H&P, history and physical; IL-12/23, interleukin-12, interleukin-23; LFTs, liver function test; MS, multiple sclerosis; NYHA, New York Heart Association; PPD, purified protein derivative; TB, tuberculosis; TNF, tumor necrosis factor. *For this patient population, adalimumab and etanercept have a (theoretical) risk. | |||||
Biologic therapy is contraindicated in patients with active serious infection. If patients develop infections requiring antibiotics while being treated, holding the biologic until infection resolution is advised.34 Standard vaccinations (eg, pneumococcal, hepatitis A and B, influenza, diphtheria, tetanus) are recommended before initiation of immunosuppressive therapy. After therapy starts, patients should avoid live and live-attenuated vaccines (varicella, mumps, measles, and rubella, oral typhoid, yellow fever, herpes zoster, intranasal influenza).35
Currently, none of the biologics are indicated for use in children or adolescents with psoriasis, despite epidemiologic data suggesting that one-third of adults with psoriasis developed it during childhood, in a form severe enough to warrant the use of systemic medications.34 The FDA is currently reviewing the possibility of indicating etanercept for pediatric psoriasis patients. All biologics are category B for pregnancy as there is no evidence that they negatively affect pregnancy.24
T-cell inhibitor. Alefacept binds CD2 on memory-effector T lymphocytes, inhibiting activation. Weekly intramuscular injections of alefacept for 12 weeks can clear lesions with long remissions.30
TNF-inhibitors. The TNF-inhibitors have been available for more than 10 years, predominantly for inflammatory bowel disease (IBD) and rheumatoid arthritis (RA), and more than 1.5 million patients have used adalimumab, etanercept, and infliximab for these disorders. Safety data, especially long term, are mostly derived from patients with IBD or RA, who have often combined TNF-inhibitors with additional immunosuppressive therapies. Thus, for psoriasis patients, who typically use biologics as monotherapy, the risk may be overestimated.24
TNF-inhibitors increase the risk for infection, most commonly of the upper respiratory tract, and, rarely, have been associated with opportunistic infections. Numerous cases of TB reactivation and an increased incidence of disseminated cases have been associated with TNF-inhibitors, so screening is recommended.24
The impact of TNF inhibition on congestive heart failure (CHF) is not well understood. Studies have variously shown that TNF-inhibitors have no effect on CHF morbidity or mortality, increase CHF mortality, or improve left ventricular function. TNF-inhibitors should be avoided in patients with severe CHF (New York Heart Association class III or IV). In milder CHF patients with worsening of symptoms, treatment should be discontinued.36
The increased risk of malignancy, especially lymphoma, is a concern, as there have been numerous case reports of lymphoma occurring with TNF-inhibitors. Psoriasis patients in general have an increased risk of lymphoma that confounds data interpretation.31 A number of case reports and a large observational study have shown patients receiving TNF-inhibitors may be at a greater risk for developing melanoma and nonmelanoma skin cancer.32
Ustekinumab, an interleukin 12/23 inhibitor, is a human monoclonal antibody that is absorbed and eliminated slowly, making dosing injections every 12 weeks convenient with efficacy maintained for at least one year.33 Because of its relative novelty, few studies are published regarding long-term safety. A recent head-to-head trial compared the efficacy and safety of ustekinumab with etanercept and found superior efficacy with ustekinumab, with comparable adverse events.37,38 Similar concerns exist with ustekinumab as with TNF-inhibitors, including infection, malignancy, CHF, and TB.33
CASE Tom denied having a personal family history of multiple sclerosis, or any demyelinating disorder. Nor did he have a history of cancer, tuberculosis exposure, CHF, or hepatitis. A purified protein derivative (PPD) was negative, as was his hepatitis panel, and his complete blood count with differential and metabolic panel were within normal limits.
Tom was started on the TNF-inhibitor adalimumab, after undergoing patient education and training and receiving instructions to stop the medication if he developed a major illness or infection. He received a loading dose of 80 mg SC, followed by 40 mg every other week. He tolerated the treatment well and 70% of his cutaneous symptoms cleared after 12 weeks of therapy; his joint pain also was reduced.
Tom is followed regularly in the clinic, with labs every 4 to 6 months. He is maintained on the injections and happy with the results. At each visit, weight loss and decreased beer intake are encouraged, both of which have been shown to reduce psoriasis severity. Although the beta-blockers and ACE inhibitors he takes are known to exacerbate psoriasis, the medications are necessary to treat Tom’s coronary artery disease.
CORRESPONDENCE Elizabeth Uhlenhake, MD, 18100 Oakwood Boulevard, Suite 300, Dearborn, MI 48124; eulenha@neomed.edu
1. Gelfand JM, Weinstein R, Porter SB, et al. Prevalence and treatment of psoriasis in the United Kingdom: a population-based study. Arch Dermatol. 2005;141:1537-1541.
2. Fortune DG, Richards HL, Griffiths CE. Psychologic factors in psoriasis: consequences, mechanisms, and interventions. Dermatol Clin. 2005;23:681-694.
3. Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332.
4. Pettey AA, Balkrishnan R, Rapp SR, et al. Patients with palmoplantar psoriasis have more physical disability and discomfort than patients with other forms of psoriasis: implications for clinical practice. J Am Acad Dermatol. 2003;49:271-275.
5. Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 3. Guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
6. Kaufmann R, Bibby AJ, Bissonnette R, et al. A new calcipotriol/betamethasone dipropionate formulation (Daivobet) is an effective once-daily treatment for psoriasis vulgaris. Dermatology. 2002;205:389-393.
7. Koo JY, Martin D. Investigator-masked comparison of tazarotene gel q.d. plus mometasone furoate cream q.d. vs. mometasone furoate cream b.i.d. in the treatment of plaque psoriasis. Int J Dermatol. 2001;40:210-212.
8. Berger TG, Duvic M, Van Voorhees AS, et al. The use of topical calcineurin inhibitors in dermatology: safety concerns. Report of the American Academy of Dermatology Association Task Force. J Am Acad Dermatol. 2006;54:818-823.
9. Cornell RC, Stoughton RB. Correlation of the vasoconstriction assay and clinical activity in psoriasis. Arch Dermatol. 1985;121:63-67.
10. Lui H. Phototherapy of psoriasis: update with practical pearls. J Cutan Med Surg. 2002;6(suppl):17-21.
11. Walters IB, Burack LH, Coven TR, et al. Suberythemogenic narrow-band UVB is markedly more effective than conventional UVB in treatment of psoriasis vulgaris. J Am Acad Dermatol. 1999;40:893-900.
12. Stern RS, Laird N. The carcinogenic risk of treatments for severe psoriasis. Photochemotherapy follow-up study. Cancer. 1994;73:2759-2764.
13. Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 5. Guidelines of care for the treatment of psoriasis with phototherapy and photochemotherapy. J Am Acad Dermatol. 2010;62:114-135.
14. Trehan M, Taylor CR. High-dose 308-nm excimer laser for the treatment of psoriasis. J Am Acad Dermatol. 2002;46:732-737.
15. Morison WL, Baughman RD, Day RM, et al. Consensus workshop on the toxic effects of long-term PUVA therapy. Arch Dermatol. 1998;134:595-598.
16. Spuls PI, Witkamp L, Bossuyt PM, et al. A systematic review of five systemic treatments for severe psoriasis. Br J Dermatol. 1997;137:943-949.
17. Murase JE, Lee EE, Koo J. Effect of ethnicity on the risk of developing nonmelanoma skin cancer following long-term PUVA therapy. Int J Dermatol. 2005;44:1016-1021.
18. Lowe NJ, Prystowsky JH, Bourget T, et al. Acitretin plus UVB therapy for psoriasis. Comparisons with placebo plus UVB and acitretin alone. J Am Acad Dermatol. 1991;24:591-594.
19. Torras H, Aliaga A, Lopez-Estebaranz JL, et al. A combination therapy of calcipotriol cream and PUVA reduces the UVA dose and improves the response of psoriasis vulgaris. J Dermatolog Treat. 2004;15:98-103.
20. Tanew A, Guggenbichler A, Honigsmann H, et al. Photochemotherapy for severe psoriasis without or in combination with acitretin: a randomized, double-blind comparison study. J Am Acad Dermatol. 1991;25:682-684.
21. Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 4. Guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol. 2009;61:451-485.
22. Strober BE, Menon K. Folate supplementation during methotrexate therapy for patients with psoriasis. J Am Acad Dermatol. 2005;53:652-659.
23. Taler SJ, Textor SC, Canzanello VJ, et al. Cyclosporine-induced hypertension: incidence, pathogenesis and management. Drug Saf. 1999;20:437-449.
24. Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850.
25. Heydendael VM, Spuls PI, Opmeer BC, et al. Methotrexate versus cyclosporine in moderate-to-severe chronic plaque psoriasis. N Engl J Med. 2003;349:658-665.
26. Lloyd ME, Carr M, McElhatton P, et al. The effects of methotrexate on pregnancy, fertility and lactation. QJM. 1999;92:551-563.
27. Ellis CN, Fradin MS, Messana JM, et al. Cyclosporine for plaque-type psoriasis. Results of a multidose, double-blind trial. N Engl J Med. 1991;324:277-284.
28. Buccheri L, Katchen BR, Karter AJ, et al. Acitretin therapy is effective for psoriasis associated with human immunodeficiency virus infection. Arch Dermatol. 1997;133:711-715.
29. Lebwohl M, Bagel J, Gelfand JM, et al. From the Medical Board of the National Psoriasis Foundation: monitoring and vaccinations in patients treated with biologics for psoriasis. J Am Acad Dermatol. 2008;58:94-105.
30. Gordon KB, Vaishnaw AK, O’Gorman J, et al. Treatment of psoriasis with alefacept: correlation of clinical improvement with reductions of memory T-cell counts. Arch Dermatol. 2003;139:1563-1570.
31. Brown SL, Greene MH, Gershon SK, et al. Tumor necrosis factor antagonist therapy and lymphoma development: twenty-six cases reported to the Food and Drug Administration. Arthritis Rheum. 2002;46:3151-3158.
32. Fulchiero GJ, Jr, Salvaggio H, Drabick JJ, et al. Eruptive latent metastatic melanomas after initiation of antitumor necrosis factor therapies. J Am Acad Dermatol. 2007;56(suppl):S65-S67.
33. Leonardi CL, Kimball AB, Papp KA, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet. 2008;371:1665-1674.
34. US Food and Drug Administration. FDA approves new drug to treat psoriasis. Sept. 25, 2009. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm183851.htm. Accessed June 15, 2012.
35. Duchini A, Goss JA, Karpen S, et al. Vaccinations for adult solid-organ transplant recipients: current recommendations and protocols. Clin Microbiol Rev. 2003;16:357-364.
36. Mann DL, McMurray JJ, Packer M, et al. Targeted anticytokine therapy in patients with chronic heart failure: results of the Randomized Etanercept Worldwide Evaluation (RENEWAL). Circulation. 2004;109:1594-1602.
37. Griffiths CE, Strober BE, van de Kerkhof P, et al. Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N Engl J Med. 2010;362:118-128.
38. Papp KA, Langley RG, Lebwohl M, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet. 2008;371:1675-1684.
1. Gelfand JM, Weinstein R, Porter SB, et al. Prevalence and treatment of psoriasis in the United Kingdom: a population-based study. Arch Dermatol. 2005;141:1537-1541.
2. Fortune DG, Richards HL, Griffiths CE. Psychologic factors in psoriasis: consequences, mechanisms, and interventions. Dermatol Clin. 2005;23:681-694.
3. Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332.
4. Pettey AA, Balkrishnan R, Rapp SR, et al. Patients with palmoplantar psoriasis have more physical disability and discomfort than patients with other forms of psoriasis: implications for clinical practice. J Am Acad Dermatol. 2003;49:271-275.
5. Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 3. Guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
6. Kaufmann R, Bibby AJ, Bissonnette R, et al. A new calcipotriol/betamethasone dipropionate formulation (Daivobet) is an effective once-daily treatment for psoriasis vulgaris. Dermatology. 2002;205:389-393.
7. Koo JY, Martin D. Investigator-masked comparison of tazarotene gel q.d. plus mometasone furoate cream q.d. vs. mometasone furoate cream b.i.d. in the treatment of plaque psoriasis. Int J Dermatol. 2001;40:210-212.
8. Berger TG, Duvic M, Van Voorhees AS, et al. The use of topical calcineurin inhibitors in dermatology: safety concerns. Report of the American Academy of Dermatology Association Task Force. J Am Acad Dermatol. 2006;54:818-823.
9. Cornell RC, Stoughton RB. Correlation of the vasoconstriction assay and clinical activity in psoriasis. Arch Dermatol. 1985;121:63-67.
10. Lui H. Phototherapy of psoriasis: update with practical pearls. J Cutan Med Surg. 2002;6(suppl):17-21.
11. Walters IB, Burack LH, Coven TR, et al. Suberythemogenic narrow-band UVB is markedly more effective than conventional UVB in treatment of psoriasis vulgaris. J Am Acad Dermatol. 1999;40:893-900.
12. Stern RS, Laird N. The carcinogenic risk of treatments for severe psoriasis. Photochemotherapy follow-up study. Cancer. 1994;73:2759-2764.
13. Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 5. Guidelines of care for the treatment of psoriasis with phototherapy and photochemotherapy. J Am Acad Dermatol. 2010;62:114-135.
14. Trehan M, Taylor CR. High-dose 308-nm excimer laser for the treatment of psoriasis. J Am Acad Dermatol. 2002;46:732-737.
15. Morison WL, Baughman RD, Day RM, et al. Consensus workshop on the toxic effects of long-term PUVA therapy. Arch Dermatol. 1998;134:595-598.
16. Spuls PI, Witkamp L, Bossuyt PM, et al. A systematic review of five systemic treatments for severe psoriasis. Br J Dermatol. 1997;137:943-949.
17. Murase JE, Lee EE, Koo J. Effect of ethnicity on the risk of developing nonmelanoma skin cancer following long-term PUVA therapy. Int J Dermatol. 2005;44:1016-1021.
18. Lowe NJ, Prystowsky JH, Bourget T, et al. Acitretin plus UVB therapy for psoriasis. Comparisons with placebo plus UVB and acitretin alone. J Am Acad Dermatol. 1991;24:591-594.
19. Torras H, Aliaga A, Lopez-Estebaranz JL, et al. A combination therapy of calcipotriol cream and PUVA reduces the UVA dose and improves the response of psoriasis vulgaris. J Dermatolog Treat. 2004;15:98-103.
20. Tanew A, Guggenbichler A, Honigsmann H, et al. Photochemotherapy for severe psoriasis without or in combination with acitretin: a randomized, double-blind comparison study. J Am Acad Dermatol. 1991;25:682-684.
21. Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 4. Guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol. 2009;61:451-485.
22. Strober BE, Menon K. Folate supplementation during methotrexate therapy for patients with psoriasis. J Am Acad Dermatol. 2005;53:652-659.
23. Taler SJ, Textor SC, Canzanello VJ, et al. Cyclosporine-induced hypertension: incidence, pathogenesis and management. Drug Saf. 1999;20:437-449.
24. Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850.
25. Heydendael VM, Spuls PI, Opmeer BC, et al. Methotrexate versus cyclosporine in moderate-to-severe chronic plaque psoriasis. N Engl J Med. 2003;349:658-665.
26. Lloyd ME, Carr M, McElhatton P, et al. The effects of methotrexate on pregnancy, fertility and lactation. QJM. 1999;92:551-563.
27. Ellis CN, Fradin MS, Messana JM, et al. Cyclosporine for plaque-type psoriasis. Results of a multidose, double-blind trial. N Engl J Med. 1991;324:277-284.
28. Buccheri L, Katchen BR, Karter AJ, et al. Acitretin therapy is effective for psoriasis associated with human immunodeficiency virus infection. Arch Dermatol. 1997;133:711-715.
29. Lebwohl M, Bagel J, Gelfand JM, et al. From the Medical Board of the National Psoriasis Foundation: monitoring and vaccinations in patients treated with biologics for psoriasis. J Am Acad Dermatol. 2008;58:94-105.
30. Gordon KB, Vaishnaw AK, O’Gorman J, et al. Treatment of psoriasis with alefacept: correlation of clinical improvement with reductions of memory T-cell counts. Arch Dermatol. 2003;139:1563-1570.
31. Brown SL, Greene MH, Gershon SK, et al. Tumor necrosis factor antagonist therapy and lymphoma development: twenty-six cases reported to the Food and Drug Administration. Arthritis Rheum. 2002;46:3151-3158.
32. Fulchiero GJ, Jr, Salvaggio H, Drabick JJ, et al. Eruptive latent metastatic melanomas after initiation of antitumor necrosis factor therapies. J Am Acad Dermatol. 2007;56(suppl):S65-S67.
33. Leonardi CL, Kimball AB, Papp KA, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet. 2008;371:1665-1674.
34. US Food and Drug Administration. FDA approves new drug to treat psoriasis. Sept. 25, 2009. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm183851.htm. Accessed June 15, 2012.
35. Duchini A, Goss JA, Karpen S, et al. Vaccinations for adult solid-organ transplant recipients: current recommendations and protocols. Clin Microbiol Rev. 2003;16:357-364.
36. Mann DL, McMurray JJ, Packer M, et al. Targeted anticytokine therapy in patients with chronic heart failure: results of the Randomized Etanercept Worldwide Evaluation (RENEWAL). Circulation. 2004;109:1594-1602.
37. Griffiths CE, Strober BE, van de Kerkhof P, et al. Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N Engl J Med. 2010;362:118-128.
38. Papp KA, Langley RG, Lebwohl M, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet. 2008;371:1675-1684.
Managing psoriasis: What’s best for your patient?
• Prescribe high-potency (Class 1) topical steroids for use on thicker, chronic plaques and low-potency (Class 7) steroids for the face, intertriginous areas, and the groin. A
• Recommend folate supplementation (1-5 mg/d) for patients being treated with methotrexate, as it may reduce the drug’s hematologic and gastrointestinal adverse effects without decreasing efficacy. A
• Advise patients scheduled to begin biologic therapy to get standard vaccinations—eg, pneumococcal, influenza, hepatitis A and B—before treatment is initiated and to avoid live and live-attenuated vaccines thereafter. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Psoriasis is a systemic inflammatory disorder altered by environmental and genetic factors that presents as scaly erythematous plaques and affects 2% to 3% of the population.1 Eighty percent have mild-to-moderate cutaneous disease; disabling arthritis occurs in as many as 42% of cases.1,2 The devastating effects on quality of life— including social stigmatization, pain, physical disability, and psychological distress—are comparable to the effects of conditions like cancer and depression.2 There is no definitive cure, and patients are left with a lifetime of waxing and waning symptoms.
Helping to create an individualized treatment plan tailored to disease type and extent, comorbidities, and needs of the patient can directly impact the quality of his or her life.3 For those with localized disease, topical therapy is a suitable first choice. Phototherapy is generally the first-line treatment for patients with extensive psoriasis or disabling symptoms. When phototherapy is not feasible or is ineffective, systemic treatments with conventional oral agents or biologics are indicated.
Discussions about therapeutic options should include expected results and duration of remission, cost, convenience, adverse effects, insurance coverage, and safety concerns. The patient’s preferences should be taken into account in the treatment plan you create.3 A guiding principle: The optimal protocol is the one the patient is motivated to adhere to.
The limits of topical therapy
Topical treatments are safe and effective when used properly, as monotherapy for localized disease and adjunctive therapy for resistant lesions. Monotherapy with topical agents is not recommended for sites that have a significant impact on quality of life, such as the palms and soles, and is not practical for patients with extensive disease (>10% of body surface).4
More potent topicals, such as corticosteroids, are recommended for acute flares; less potent agents with fewer adverse effects, such as calcipotriene, are typically used for maintenance ( TABLE 1 ).5-8 Topical agents can be used either long term or intermittently. Here, too, the most effective treatment is the one the patient will actually apply.5
TABLE 1
A look at nonsteroidal topical treatments5-8
| Class | Examples | Adverse effects | Comments |
|---|---|---|---|
| Vitamin D analogues | Calcipotriol (calcipotriene), calcitriol | Burning, pruritus, edema, peeling, dryness, erythema | Combining with beta-methasone dipropionate increases efficacy |
| Retinoids | Tazarotene, tretinoin | Teratogenic, photosensitivity, irritation | Increased efficacy when combined with NB-UVB (and less UV exposure); increased efficacy, duration of remission, and reduction in steroid-induced atrophy when used with steroids |
| Calcineurin inhibitors | Tacrolimus, pimecrolimus | Burning and pruritus Black box warning for risk of malignancies* | No clinical evidence of increased cancer risk |
| Others | Emollients, salicylic acid, anthralin, coal tar | Severe skin irritation, staining of clothes, odor with anthralin and tar | Salicylic acid works well with steroids and topical immunomodulators, but is not compatible with calcipotriene |
| *Lymphoma seen with oral therapy. UVB, ultraviolet B; NB-UVB, narrow-band ultraviolet B. | |||
Steroid selection is based on site, severity
Corticosteroids are antiproliferative, immunosuppressive, anti-inflammatory, and vasoconstrictive, and divided into 7 classes. Low-potency agents (Class 7) are used on thinner skin like the face, intertriginous areas, and groin; high-potency steroids (Class 1) are reserved for thicker, chronic plaques.9 As a general rule, Class 1 steroids can be safely used for 2 to 4 weeks, with increased risk of both cutaneous effects and systemic absorption if used continuously for longer periods.5 The optimal end point for less potent agents is not known.
Cutaneous adverse effects are more common than systemic ones and include skin atrophy, telangiectasia, striae distensae, acne, folliculitis, and purpura.
Systemic adverse effects include Cushing’s syndrome, osteonecrosis of the femoral head, cataracts, and glaucoma.5 The greatest risk of systemic effects is associated with prolonged use of high-potency corticosteroids over large surfaces or under occlusion with dressings or plastic wrap. Patients should be transitioned to the lowest potency possible to maintain efficacy, use corticosteroids intermittently, or combine them with nonsteroidal agents to avoid unwanted effects.
Topicals aren’t working? Move on to phototherapy
Phototherapy is an option for patients with extensive disease or skin manifestations that are recalcitrant to topicals. It is efficacious, cost-effective, and lacks systemic immunosuppression. Ultraviolet (UV) A and B act on Langerhans cells, cytokines, and adhesion molecules, inhibiting epidermal proliferation and angiogenesis.10
Broadband UVB (BB-UVB) was first used during the 1950s, with crude tar or anthralin, but is rarely used today.
Narrow-band UVB (NB-UVB) (311-313 nm), developed in the 1980s, has largely replaced BB-UVB. In addition to providing more rapid clearing and resolution of psoriasis compared with BB-UVB, NB-UVB may have less phototoxicity.11 Between 20 and 25 NB-UVB treatments, 2 to 3 times a week (in the office or at home) are usually required for significant improvement.
Photocarcinogenesis is a concern but numerous studies, including a review of 3867 patients treated with NB-UVB with a median 5.5-year follow-up, found no significant association with cutaneous malignancies.12 NB-UVB is considered safe during pregnancy and used as first-line therapy for pregnant patients.13
Targeted UVB therapy using a 308-nm excimer laser, another option, selectively targets psoriatic lesions, leaving normal skin untreated. This makes supraerythemogenic doses possible, which increases UVB’s efficacy. Long-term adverse effects and duration of remission have not been clearly established.14
Psoralen and UVA (PUVA), which uses oral or topical psoralens to sensitize the skin to UVA, has a slightly higher efficacy than NB-UVB, but with increased risk of squamous cell carcinoma (SCC) and possibly melanoma.15 Clearing can occur within 24 treatments, with remissions lasting 3 to 6 months;16 monthly maintenance has not been found to lengthen remission.13
Common adverse effects include erythema that peaks 48 to 96 hours after a treatment, pruritus, xerosis, irregular pigmentation, and gastrointestinal symptoms that can be reduced by decreasing psoralen and/or UVA doses.13 High cumulative doses of oral PUVA (>200 treatments) is associated with a dose-related increased risk of nonmelanoma skin cancer, particularly SCC, in the Caucasian population. This increased risk has not been demonstrated in patients treated with PUVA bath therapy, which is more common in Scandinavian countries.17 There is no consensus regarding the risk of melanoma.15
A careful risk-benefit analysis is needed before initiating phototherapy in patients who take photosensitizing drugs, are immunosuppressed, or have a photosensitivity disorder or a history of melanoma, atypical nevi, multiple melanoma risk factors, or multiple nonmelanoma skin cancers.13 Regardless of the type of UV therapy administered, eye protection with goggles is required to decrease the risk of UV-related cataract formation, and genital shielding is needed to prevent increased risk of tumors.13 Photoaging is a long-term effect.
CASE When questioned further about the challenges that Tom has had with controlling his symptoms, he admitted to being noncompliant. As a busy executive, he said he didn’t have time to use the topical corticosteroids regularly. Phototherapy could alleviate his cutaneous symptoms, but would not address his symptoms that were consistent with psoriatic arthritis.
Pairing therapeutic modalities decreases exposure
Combining therapeutic modalities like emollients and topical or oral retinoids with NB-UVB improves efficacy while reducing the number of treatment sessions and cumulative UVB dosage. If calcipotriene is used, it should be applied after phototherapy because it is degraded upon UVB exposure.18 Acitretin should be started 2 weeks prior to initiation of phototherapy, and its use accompanied by a 25% reduction in initial UV dosage.13
PUVA may also be combined with topical calcipotriene or retinoids.19 In both cases, the addition of the other agent typically decreases the duration of phototherapy, improves the clinical response, and reduces the risk of cancer.13,20
Extensive disease? Consider a traditional systemic agent
Traditional systemic therapy is used to treat extensive disease ( FIGURE ), psoriasis refractory to topical agents, and debilitating disease on the palms, soles, or scalp. Biologics are a recent alternative, but traditional systemics have been utilized longer and have a more longstanding adverse effect and safety profile, are administered orally, and are much less expensive than biologics. Monitoring patients on systemic therapy is necessary ( TABLE 2 ).21-24
Methotrexate (MTX), a competitive inhibitor of dihydrofolate reductase, is the most commonly prescribed traditional systemic psoriasis treatment.21 It is administered in a single weekly dose via tablet, parenteral solution, or intramuscular (IM) or subcutaneous (SC) injection.25 A test dose (2.5 or 5 mg) is given initially and complete blood cell count is monitored within one week to evaluate for potential bone marrow toxicity. If none is observed, the dose may be increased to control the disease while minimizing adverse effects.21
Common adverse effects of MTX, such as nausea, vomiting, stomatitis, and fatigue, may be minimized by IM or SC administration, splitting the dose, or providing folate supplementation.21-24 Given in doses of 1 to 5 mg/d, folate may reduce adverse hematologic, gastrointestinal, and hepatic effects without decreasing efficacy.22
The major severe toxicities are myelosuppression, hepatotoxicity, and pulmonary fibrosis.24 MTX-induced hepatotoxicity is similar to nonalcoholic fatty liver disease (NAFLD) and is thought to exacerbate preexisting NAFLD, which is common in patients with metabolic syndrome. A liver biopsy or serum assays for liver fibrosis (amino-terminal peptide of pro-collagen III) may be warranted during therapy.24
MTX is an abortifacient and teratogen, so contraception during treatment and for up to 3 months thereafter is mandatory for women of childbearing age.26 Men should be advised that MTX decreases sperm count. (For more on methotrexate, see: “When a fetus survives methotrexate exposure,” at http://www.jfponline.com/Pages.asp?AID=10299).
Cyclosporine (CSA), an oral calcineurin inhibitor, is a potent immunosuppressant that rapidly clears psoriasis.27 Because duration of use correlates with permanent nephrotoxicity, hypertension, and potential increased risk of SCC and lymphoma, intermittent 12-week courses are recommended. Calcium channel blockers are the preferred treatment for CSA-induced hypertension because of their effect on smooth muscle vasodilation.21
Oral retinoids. Acitretin modulates epidermal proliferation and is anti-inflammatory. Because it lacks immunosuppression, acitretin is generally considered the treatment of choice in HIV patients with severe psoriasis.28 Acitretin is teratogenic and contraindicated in women who plan to become pregnant or who are unwilling to use adequate contraception for 3 years after discontinuing the drug.21
CASE Given the significant percentage of body surface area involved and symptoms consistent with psoriatic arthritis, Tom required an aggressive therapeutic regimen. His history of nonalcoholic fatty liver and social drinking precluded the use of methotrexate. A biologic therapy was the next therapeutic choice that could relieve both his cutaneous and joint symptoms.
FIGURE
4 psoriasis patients, 4 different presentations
A. The linear erythematous, scaly plaque along this patient’s cardiac bypass scar demonstrates koebnerization of plaque psoriasis.
B. Diffuse erythematous scaly papules coalesce into plaques on this patient’s anterior chest, arms, abdomen, and periumbilicus.
C. The pinpoint pustules on an erythematous base on this patient’s lateral neck, shoulders, and upper back are characteristic of pustular psoriasis.
D. This patient has erythematous plaques with overlying silvery scale on the elbow—a classic
TABLE 2
Traditional systemic therapy21-24
| Methotrexate (MTX) | Cyclosporine (CSA)* | Acitretin | |
|---|---|---|---|
| Dosing | ≤30 mg in one weekly dose (PO, IM, or SC) | 2.5-5.0 mg/kg/d in 2 divided doses for 12 wk, then 12-wk nontreatment period Dose decreased (by 0.5-1.0 mg/kg) with disease clearance or when hypertension or nephrotoxicity are detected | 10-50 mg/d given as a single dose Lower doses (25 mg/d) used to minimize adverse effects and in combination regimens When added to UV, light dose should be reduced 30%-50% |
| Contraindications |
|
|
|
| Baseline monitoring† |
|
|
|
| BP, blood pressure; BUN, blood urea nitrogen; CBC, complete blood cell count; CR, creatinine; H&P, history and physical; IM, intramuscular; LFTs, liver function tests; PO, by mouth; PPD, purified protein derivative; PUVA, psoralen and ultraviolet A; SC, subcutaneous; UV, ultraviolet; UVB, ultraviolet B. *Avoid live vaccinations; caution required with major infection and poorly controlled diabetes. †Ongoing monitoring for MTX: BUN, CBC, CR, LFTs; possible liver biopsy (for high-risk patients or cumulative dose >3.5-4 g); CSA: BP, BUN, CBC, CR, LFTs; lipd profile; magnesium, uric acid, potassium tests; pregnancy testing; Acitretin: CBC, LFTs, lipid profile, renal function test, pregnancy testing. | |||
Biologics require lab work and a detailed medication list
Before beginning biologic therapy for a patient, the National Psoriasis Foundation29 recommends obtaining a complete history, physical, medication list, future plans (ie, pregnancy or travel to locations requiring vaccinations), and baseline labs to identify possible risk factors and/or contraindications. Periodic evaluation to monitor development of new symptoms, including infection and malignancy ( TABLE 3 ),24,30-33 is needed, as well.
TABLE 3
Is your patient a candidate for biologics?24,30-33
| Agent (Drug class) | Alefacept (T-cell inhibitor) | Adalimumab (TNF-inhibitor) | Etanercept (TNF-inhibitor) | Infliximab (TNF-inhibitor) | Ustekinumab (IL-12/23 inhibitor) |
|---|---|---|---|---|---|
| Dosing | 15 mg IM/wk for 12 wk, then 12-wk nontreatment period | 80 mg SC the first wk, 40 mg the 2nd wk, followed by 40 mg every other wk | 50 mg SC twice/wk for 3 mo, then 50 mg/wk | 5 mg/kg IV infusion to start, repeat at 2 and 6 wk, then q6-8 wk | 45 mg SC (for patients <100 kg); 90 mg (for patients >100 kg) to start, repeat at 4 wk, followed by q12 wk for maintenance |
| Contra-indications | HIV |
|
|
| Active TB |
| Baseline monitoring | CD4 count |
|
|
| PPD |
| Ongoing monitoring | Biweekly CD4 count; hold dose for counts <250 |
|
|
| Consider a yearly PPD |
| CBC, complete blood cell count; CHF, congestive heart failure; HIV, human immunodeficiency virus; H&P, history and physical; IL-12/23, interleukin-12, interleukin-23; LFTs, liver function test; MS, multiple sclerosis; NYHA, New York Heart Association; PPD, purified protein derivative; TB, tuberculosis; TNF, tumor necrosis factor. *For this patient population, adalimumab and etanercept have a (theoretical) risk. | |||||
Biologic therapy is contraindicated in patients with active serious infection. If patients develop infections requiring antibiotics while being treated, holding the biologic until infection resolution is advised.34 Standard vaccinations (eg, pneumococcal, hepatitis A and B, influenza, diphtheria, tetanus) are recommended before initiation of immunosuppressive therapy. After therapy starts, patients should avoid live and live-attenuated vaccines (varicella, mumps, measles, and rubella, oral typhoid, yellow fever, herpes zoster, intranasal influenza).35
Currently, none of the biologics are indicated for use in children or adolescents with psoriasis, despite epidemiologic data suggesting that one-third of adults with psoriasis developed it during childhood, in a form severe enough to warrant the use of systemic medications.34 The FDA is currently reviewing the possibility of indicating etanercept for pediatric psoriasis patients. All biologics are category B for pregnancy as there is no evidence that they negatively affect pregnancy.24
T-cell inhibitor. Alefacept binds CD2 on memory-effector T lymphocytes, inhibiting activation. Weekly intramuscular injections of alefacept for 12 weeks can clear lesions with long remissions.30
TNF-inhibitors. The TNF-inhibitors have been available for more than 10 years, predominantly for inflammatory bowel disease (IBD) and rheumatoid arthritis (RA), and more than 1.5 million patients have used adalimumab, etanercept, and infliximab for these disorders. Safety data, especially long term, are mostly derived from patients with IBD or RA, who have often combined TNF-inhibitors with additional immunosuppressive therapies. Thus, for psoriasis patients, who typically use biologics as monotherapy, the risk may be overestimated.24
TNF-inhibitors increase the risk for infection, most commonly of the upper respiratory tract, and, rarely, have been associated with opportunistic infections. Numerous cases of TB reactivation and an increased incidence of disseminated cases have been associated with TNF-inhibitors, so screening is recommended.24
The impact of TNF inhibition on congestive heart failure (CHF) is not well understood. Studies have variously shown that TNF-inhibitors have no effect on CHF morbidity or mortality, increase CHF mortality, or improve left ventricular function. TNF-inhibitors should be avoided in patients with severe CHF (New York Heart Association class III or IV). In milder CHF patients with worsening of symptoms, treatment should be discontinued.36
The increased risk of malignancy, especially lymphoma, is a concern, as there have been numerous case reports of lymphoma occurring with TNF-inhibitors. Psoriasis patients in general have an increased risk of lymphoma that confounds data interpretation.31 A number of case reports and a large observational study have shown patients receiving TNF-inhibitors may be at a greater risk for developing melanoma and nonmelanoma skin cancer.32
Ustekinumab, an interleukin 12/23 inhibitor, is a human monoclonal antibody that is absorbed and eliminated slowly, making dosing injections every 12 weeks convenient with efficacy maintained for at least one year.33 Because of its relative novelty, few studies are published regarding long-term safety. A recent head-to-head trial compared the efficacy and safety of ustekinumab with etanercept and found superior efficacy with ustekinumab, with comparable adverse events.37,38 Similar concerns exist with ustekinumab as with TNF-inhibitors, including infection, malignancy, CHF, and TB.33
CASE Tom denied having a personal family history of multiple sclerosis, or any demyelinating disorder. Nor did he have a history of cancer, tuberculosis exposure, CHF, or hepatitis. A purified protein derivative (PPD) was negative, as was his hepatitis panel, and his complete blood count with differential and metabolic panel were within normal limits.
Tom was started on the TNF-inhibitor adalimumab, after undergoing patient education and training and receiving instructions to stop the medication if he developed a major illness or infection. He received a loading dose of 80 mg SC, followed by 40 mg every other week. He tolerated the treatment well and 70% of his cutaneous symptoms cleared after 12 weeks of therapy; his joint pain also was reduced.
Tom is followed regularly in the clinic, with labs every 4 to 6 months. He is maintained on the injections and happy with the results. At each visit, weight loss and decreased beer intake are encouraged, both of which have been shown to reduce psoriasis severity. Although the beta-blockers and ACE inhibitors he takes are known to exacerbate psoriasis, the medications are necessary to treat Tom’s coronary artery disease.
CORRESPONDENCE Elizabeth Uhlenhake, MD, 18100 Oakwood Boulevard, Suite 300, Dearborn, MI 48124; eulenha@neomed.edu
1. Gelfand JM, Weinstein R, Porter SB, et al. Prevalence and treatment of psoriasis in the United Kingdom: a population-based study. Arch Dermatol. 2005;141:1537-1541.
2. Fortune DG, Richards HL, Griffiths CE. Psychologic factors in psoriasis: consequences, mechanisms, and interventions. Dermatol Clin. 2005;23:681-694.
3. Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332.
4. Pettey AA, Balkrishnan R, Rapp SR, et al. Patients with palmoplantar psoriasis have more physical disability and discomfort than patients with other forms of psoriasis: implications for clinical practice. J Am Acad Dermatol. 2003;49:271-275.
5. Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 3. Guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
6. Kaufmann R, Bibby AJ, Bissonnette R, et al. A new calcipotriol/betamethasone dipropionate formulation (Daivobet) is an effective once-daily treatment for psoriasis vulgaris. Dermatology. 2002;205:389-393.
7. Koo JY, Martin D. Investigator-masked comparison of tazarotene gel q.d. plus mometasone furoate cream q.d. vs. mometasone furoate cream b.i.d. in the treatment of plaque psoriasis. Int J Dermatol. 2001;40:210-212.
8. Berger TG, Duvic M, Van Voorhees AS, et al. The use of topical calcineurin inhibitors in dermatology: safety concerns. Report of the American Academy of Dermatology Association Task Force. J Am Acad Dermatol. 2006;54:818-823.
9. Cornell RC, Stoughton RB. Correlation of the vasoconstriction assay and clinical activity in psoriasis. Arch Dermatol. 1985;121:63-67.
10. Lui H. Phototherapy of psoriasis: update with practical pearls. J Cutan Med Surg. 2002;6(suppl):17-21.
11. Walters IB, Burack LH, Coven TR, et al. Suberythemogenic narrow-band UVB is markedly more effective than conventional UVB in treatment of psoriasis vulgaris. J Am Acad Dermatol. 1999;40:893-900.
12. Stern RS, Laird N. The carcinogenic risk of treatments for severe psoriasis. Photochemotherapy follow-up study. Cancer. 1994;73:2759-2764.
13. Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 5. Guidelines of care for the treatment of psoriasis with phototherapy and photochemotherapy. J Am Acad Dermatol. 2010;62:114-135.
14. Trehan M, Taylor CR. High-dose 308-nm excimer laser for the treatment of psoriasis. J Am Acad Dermatol. 2002;46:732-737.
15. Morison WL, Baughman RD, Day RM, et al. Consensus workshop on the toxic effects of long-term PUVA therapy. Arch Dermatol. 1998;134:595-598.
16. Spuls PI, Witkamp L, Bossuyt PM, et al. A systematic review of five systemic treatments for severe psoriasis. Br J Dermatol. 1997;137:943-949.
17. Murase JE, Lee EE, Koo J. Effect of ethnicity on the risk of developing nonmelanoma skin cancer following long-term PUVA therapy. Int J Dermatol. 2005;44:1016-1021.
18. Lowe NJ, Prystowsky JH, Bourget T, et al. Acitretin plus UVB therapy for psoriasis. Comparisons with placebo plus UVB and acitretin alone. J Am Acad Dermatol. 1991;24:591-594.
19. Torras H, Aliaga A, Lopez-Estebaranz JL, et al. A combination therapy of calcipotriol cream and PUVA reduces the UVA dose and improves the response of psoriasis vulgaris. J Dermatolog Treat. 2004;15:98-103.
20. Tanew A, Guggenbichler A, Honigsmann H, et al. Photochemotherapy for severe psoriasis without or in combination with acitretin: a randomized, double-blind comparison study. J Am Acad Dermatol. 1991;25:682-684.
21. Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 4. Guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol. 2009;61:451-485.
22. Strober BE, Menon K. Folate supplementation during methotrexate therapy for patients with psoriasis. J Am Acad Dermatol. 2005;53:652-659.
23. Taler SJ, Textor SC, Canzanello VJ, et al. Cyclosporine-induced hypertension: incidence, pathogenesis and management. Drug Saf. 1999;20:437-449.
24. Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850.
25. Heydendael VM, Spuls PI, Opmeer BC, et al. Methotrexate versus cyclosporine in moderate-to-severe chronic plaque psoriasis. N Engl J Med. 2003;349:658-665.
26. Lloyd ME, Carr M, McElhatton P, et al. The effects of methotrexate on pregnancy, fertility and lactation. QJM. 1999;92:551-563.
27. Ellis CN, Fradin MS, Messana JM, et al. Cyclosporine for plaque-type psoriasis. Results of a multidose, double-blind trial. N Engl J Med. 1991;324:277-284.
28. Buccheri L, Katchen BR, Karter AJ, et al. Acitretin therapy is effective for psoriasis associated with human immunodeficiency virus infection. Arch Dermatol. 1997;133:711-715.
29. Lebwohl M, Bagel J, Gelfand JM, et al. From the Medical Board of the National Psoriasis Foundation: monitoring and vaccinations in patients treated with biologics for psoriasis. J Am Acad Dermatol. 2008;58:94-105.
30. Gordon KB, Vaishnaw AK, O’Gorman J, et al. Treatment of psoriasis with alefacept: correlation of clinical improvement with reductions of memory T-cell counts. Arch Dermatol. 2003;139:1563-1570.
31. Brown SL, Greene MH, Gershon SK, et al. Tumor necrosis factor antagonist therapy and lymphoma development: twenty-six cases reported to the Food and Drug Administration. Arthritis Rheum. 2002;46:3151-3158.
32. Fulchiero GJ, Jr, Salvaggio H, Drabick JJ, et al. Eruptive latent metastatic melanomas after initiation of antitumor necrosis factor therapies. J Am Acad Dermatol. 2007;56(suppl):S65-S67.
33. Leonardi CL, Kimball AB, Papp KA, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet. 2008;371:1665-1674.
34. US Food and Drug Administration. FDA approves new drug to treat psoriasis. Sept. 25, 2009. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm183851.htm. Accessed June 15, 2012.
35. Duchini A, Goss JA, Karpen S, et al. Vaccinations for adult solid-organ transplant recipients: current recommendations and protocols. Clin Microbiol Rev. 2003;16:357-364.
36. Mann DL, McMurray JJ, Packer M, et al. Targeted anticytokine therapy in patients with chronic heart failure: results of the Randomized Etanercept Worldwide Evaluation (RENEWAL). Circulation. 2004;109:1594-1602.
37. Griffiths CE, Strober BE, van de Kerkhof P, et al. Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N Engl J Med. 2010;362:118-128.
38. Papp KA, Langley RG, Lebwohl M, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet. 2008;371:1675-1684.
• Prescribe high-potency (Class 1) topical steroids for use on thicker, chronic plaques and low-potency (Class 7) steroids for the face, intertriginous areas, and the groin. A
• Recommend folate supplementation (1-5 mg/d) for patients being treated with methotrexate, as it may reduce the drug’s hematologic and gastrointestinal adverse effects without decreasing efficacy. A
• Advise patients scheduled to begin biologic therapy to get standard vaccinations—eg, pneumococcal, influenza, hepatitis A and B—before treatment is initiated and to avoid live and live-attenuated vaccines thereafter. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Psoriasis is a systemic inflammatory disorder altered by environmental and genetic factors that presents as scaly erythematous plaques and affects 2% to 3% of the population.1 Eighty percent have mild-to-moderate cutaneous disease; disabling arthritis occurs in as many as 42% of cases.1,2 The devastating effects on quality of life— including social stigmatization, pain, physical disability, and psychological distress—are comparable to the effects of conditions like cancer and depression.2 There is no definitive cure, and patients are left with a lifetime of waxing and waning symptoms.
Helping to create an individualized treatment plan tailored to disease type and extent, comorbidities, and needs of the patient can directly impact the quality of his or her life.3 For those with localized disease, topical therapy is a suitable first choice. Phototherapy is generally the first-line treatment for patients with extensive psoriasis or disabling symptoms. When phototherapy is not feasible or is ineffective, systemic treatments with conventional oral agents or biologics are indicated.
Discussions about therapeutic options should include expected results and duration of remission, cost, convenience, adverse effects, insurance coverage, and safety concerns. The patient’s preferences should be taken into account in the treatment plan you create.3 A guiding principle: The optimal protocol is the one the patient is motivated to adhere to.
The limits of topical therapy
Topical treatments are safe and effective when used properly, as monotherapy for localized disease and adjunctive therapy for resistant lesions. Monotherapy with topical agents is not recommended for sites that have a significant impact on quality of life, such as the palms and soles, and is not practical for patients with extensive disease (>10% of body surface).4
More potent topicals, such as corticosteroids, are recommended for acute flares; less potent agents with fewer adverse effects, such as calcipotriene, are typically used for maintenance ( TABLE 1 ).5-8 Topical agents can be used either long term or intermittently. Here, too, the most effective treatment is the one the patient will actually apply.5
TABLE 1
A look at nonsteroidal topical treatments5-8
| Class | Examples | Adverse effects | Comments |
|---|---|---|---|
| Vitamin D analogues | Calcipotriol (calcipotriene), calcitriol | Burning, pruritus, edema, peeling, dryness, erythema | Combining with beta-methasone dipropionate increases efficacy |
| Retinoids | Tazarotene, tretinoin | Teratogenic, photosensitivity, irritation | Increased efficacy when combined with NB-UVB (and less UV exposure); increased efficacy, duration of remission, and reduction in steroid-induced atrophy when used with steroids |
| Calcineurin inhibitors | Tacrolimus, pimecrolimus | Burning and pruritus Black box warning for risk of malignancies* | No clinical evidence of increased cancer risk |
| Others | Emollients, salicylic acid, anthralin, coal tar | Severe skin irritation, staining of clothes, odor with anthralin and tar | Salicylic acid works well with steroids and topical immunomodulators, but is not compatible with calcipotriene |
| *Lymphoma seen with oral therapy. UVB, ultraviolet B; NB-UVB, narrow-band ultraviolet B. | |||
Steroid selection is based on site, severity
Corticosteroids are antiproliferative, immunosuppressive, anti-inflammatory, and vasoconstrictive, and divided into 7 classes. Low-potency agents (Class 7) are used on thinner skin like the face, intertriginous areas, and groin; high-potency steroids (Class 1) are reserved for thicker, chronic plaques.9 As a general rule, Class 1 steroids can be safely used for 2 to 4 weeks, with increased risk of both cutaneous effects and systemic absorption if used continuously for longer periods.5 The optimal end point for less potent agents is not known.
Cutaneous adverse effects are more common than systemic ones and include skin atrophy, telangiectasia, striae distensae, acne, folliculitis, and purpura.
Systemic adverse effects include Cushing’s syndrome, osteonecrosis of the femoral head, cataracts, and glaucoma.5 The greatest risk of systemic effects is associated with prolonged use of high-potency corticosteroids over large surfaces or under occlusion with dressings or plastic wrap. Patients should be transitioned to the lowest potency possible to maintain efficacy, use corticosteroids intermittently, or combine them with nonsteroidal agents to avoid unwanted effects.
Topicals aren’t working? Move on to phototherapy
Phototherapy is an option for patients with extensive disease or skin manifestations that are recalcitrant to topicals. It is efficacious, cost-effective, and lacks systemic immunosuppression. Ultraviolet (UV) A and B act on Langerhans cells, cytokines, and adhesion molecules, inhibiting epidermal proliferation and angiogenesis.10
Broadband UVB (BB-UVB) was first used during the 1950s, with crude tar or anthralin, but is rarely used today.
Narrow-band UVB (NB-UVB) (311-313 nm), developed in the 1980s, has largely replaced BB-UVB. In addition to providing more rapid clearing and resolution of psoriasis compared with BB-UVB, NB-UVB may have less phototoxicity.11 Between 20 and 25 NB-UVB treatments, 2 to 3 times a week (in the office or at home) are usually required for significant improvement.
Photocarcinogenesis is a concern but numerous studies, including a review of 3867 patients treated with NB-UVB with a median 5.5-year follow-up, found no significant association with cutaneous malignancies.12 NB-UVB is considered safe during pregnancy and used as first-line therapy for pregnant patients.13
Targeted UVB therapy using a 308-nm excimer laser, another option, selectively targets psoriatic lesions, leaving normal skin untreated. This makes supraerythemogenic doses possible, which increases UVB’s efficacy. Long-term adverse effects and duration of remission have not been clearly established.14
Psoralen and UVA (PUVA), which uses oral or topical psoralens to sensitize the skin to UVA, has a slightly higher efficacy than NB-UVB, but with increased risk of squamous cell carcinoma (SCC) and possibly melanoma.15 Clearing can occur within 24 treatments, with remissions lasting 3 to 6 months;16 monthly maintenance has not been found to lengthen remission.13
Common adverse effects include erythema that peaks 48 to 96 hours after a treatment, pruritus, xerosis, irregular pigmentation, and gastrointestinal symptoms that can be reduced by decreasing psoralen and/or UVA doses.13 High cumulative doses of oral PUVA (>200 treatments) is associated with a dose-related increased risk of nonmelanoma skin cancer, particularly SCC, in the Caucasian population. This increased risk has not been demonstrated in patients treated with PUVA bath therapy, which is more common in Scandinavian countries.17 There is no consensus regarding the risk of melanoma.15
A careful risk-benefit analysis is needed before initiating phototherapy in patients who take photosensitizing drugs, are immunosuppressed, or have a photosensitivity disorder or a history of melanoma, atypical nevi, multiple melanoma risk factors, or multiple nonmelanoma skin cancers.13 Regardless of the type of UV therapy administered, eye protection with goggles is required to decrease the risk of UV-related cataract formation, and genital shielding is needed to prevent increased risk of tumors.13 Photoaging is a long-term effect.
CASE When questioned further about the challenges that Tom has had with controlling his symptoms, he admitted to being noncompliant. As a busy executive, he said he didn’t have time to use the topical corticosteroids regularly. Phototherapy could alleviate his cutaneous symptoms, but would not address his symptoms that were consistent with psoriatic arthritis.
Pairing therapeutic modalities decreases exposure
Combining therapeutic modalities like emollients and topical or oral retinoids with NB-UVB improves efficacy while reducing the number of treatment sessions and cumulative UVB dosage. If calcipotriene is used, it should be applied after phototherapy because it is degraded upon UVB exposure.18 Acitretin should be started 2 weeks prior to initiation of phototherapy, and its use accompanied by a 25% reduction in initial UV dosage.13
PUVA may also be combined with topical calcipotriene or retinoids.19 In both cases, the addition of the other agent typically decreases the duration of phototherapy, improves the clinical response, and reduces the risk of cancer.13,20
Extensive disease? Consider a traditional systemic agent
Traditional systemic therapy is used to treat extensive disease ( FIGURE ), psoriasis refractory to topical agents, and debilitating disease on the palms, soles, or scalp. Biologics are a recent alternative, but traditional systemics have been utilized longer and have a more longstanding adverse effect and safety profile, are administered orally, and are much less expensive than biologics. Monitoring patients on systemic therapy is necessary ( TABLE 2 ).21-24
Methotrexate (MTX), a competitive inhibitor of dihydrofolate reductase, is the most commonly prescribed traditional systemic psoriasis treatment.21 It is administered in a single weekly dose via tablet, parenteral solution, or intramuscular (IM) or subcutaneous (SC) injection.25 A test dose (2.5 or 5 mg) is given initially and complete blood cell count is monitored within one week to evaluate for potential bone marrow toxicity. If none is observed, the dose may be increased to control the disease while minimizing adverse effects.21
Common adverse effects of MTX, such as nausea, vomiting, stomatitis, and fatigue, may be minimized by IM or SC administration, splitting the dose, or providing folate supplementation.21-24 Given in doses of 1 to 5 mg/d, folate may reduce adverse hematologic, gastrointestinal, and hepatic effects without decreasing efficacy.22
The major severe toxicities are myelosuppression, hepatotoxicity, and pulmonary fibrosis.24 MTX-induced hepatotoxicity is similar to nonalcoholic fatty liver disease (NAFLD) and is thought to exacerbate preexisting NAFLD, which is common in patients with metabolic syndrome. A liver biopsy or serum assays for liver fibrosis (amino-terminal peptide of pro-collagen III) may be warranted during therapy.24
MTX is an abortifacient and teratogen, so contraception during treatment and for up to 3 months thereafter is mandatory for women of childbearing age.26 Men should be advised that MTX decreases sperm count. (For more on methotrexate, see: “When a fetus survives methotrexate exposure,” at http://www.jfponline.com/Pages.asp?AID=10299).
Cyclosporine (CSA), an oral calcineurin inhibitor, is a potent immunosuppressant that rapidly clears psoriasis.27 Because duration of use correlates with permanent nephrotoxicity, hypertension, and potential increased risk of SCC and lymphoma, intermittent 12-week courses are recommended. Calcium channel blockers are the preferred treatment for CSA-induced hypertension because of their effect on smooth muscle vasodilation.21
Oral retinoids. Acitretin modulates epidermal proliferation and is anti-inflammatory. Because it lacks immunosuppression, acitretin is generally considered the treatment of choice in HIV patients with severe psoriasis.28 Acitretin is teratogenic and contraindicated in women who plan to become pregnant or who are unwilling to use adequate contraception for 3 years after discontinuing the drug.21
CASE Given the significant percentage of body surface area involved and symptoms consistent with psoriatic arthritis, Tom required an aggressive therapeutic regimen. His history of nonalcoholic fatty liver and social drinking precluded the use of methotrexate. A biologic therapy was the next therapeutic choice that could relieve both his cutaneous and joint symptoms.
FIGURE
4 psoriasis patients, 4 different presentations
A. The linear erythematous, scaly plaque along this patient’s cardiac bypass scar demonstrates koebnerization of plaque psoriasis.
B. Diffuse erythematous scaly papules coalesce into plaques on this patient’s anterior chest, arms, abdomen, and periumbilicus.
C. The pinpoint pustules on an erythematous base on this patient’s lateral neck, shoulders, and upper back are characteristic of pustular psoriasis.
D. This patient has erythematous plaques with overlying silvery scale on the elbow—a classic
TABLE 2
Traditional systemic therapy21-24
| Methotrexate (MTX) | Cyclosporine (CSA)* | Acitretin | |
|---|---|---|---|
| Dosing | ≤30 mg in one weekly dose (PO, IM, or SC) | 2.5-5.0 mg/kg/d in 2 divided doses for 12 wk, then 12-wk nontreatment period Dose decreased (by 0.5-1.0 mg/kg) with disease clearance or when hypertension or nephrotoxicity are detected | 10-50 mg/d given as a single dose Lower doses (25 mg/d) used to minimize adverse effects and in combination regimens When added to UV, light dose should be reduced 30%-50% |
| Contraindications |
|
|
|
| Baseline monitoring† |
|
|
|
| BP, blood pressure; BUN, blood urea nitrogen; CBC, complete blood cell count; CR, creatinine; H&P, history and physical; IM, intramuscular; LFTs, liver function tests; PO, by mouth; PPD, purified protein derivative; PUVA, psoralen and ultraviolet A; SC, subcutaneous; UV, ultraviolet; UVB, ultraviolet B. *Avoid live vaccinations; caution required with major infection and poorly controlled diabetes. †Ongoing monitoring for MTX: BUN, CBC, CR, LFTs; possible liver biopsy (for high-risk patients or cumulative dose >3.5-4 g); CSA: BP, BUN, CBC, CR, LFTs; lipd profile; magnesium, uric acid, potassium tests; pregnancy testing; Acitretin: CBC, LFTs, lipid profile, renal function test, pregnancy testing. | |||
Biologics require lab work and a detailed medication list
Before beginning biologic therapy for a patient, the National Psoriasis Foundation29 recommends obtaining a complete history, physical, medication list, future plans (ie, pregnancy or travel to locations requiring vaccinations), and baseline labs to identify possible risk factors and/or contraindications. Periodic evaluation to monitor development of new symptoms, including infection and malignancy ( TABLE 3 ),24,30-33 is needed, as well.
TABLE 3
Is your patient a candidate for biologics?24,30-33
| Agent (Drug class) | Alefacept (T-cell inhibitor) | Adalimumab (TNF-inhibitor) | Etanercept (TNF-inhibitor) | Infliximab (TNF-inhibitor) | Ustekinumab (IL-12/23 inhibitor) |
|---|---|---|---|---|---|
| Dosing | 15 mg IM/wk for 12 wk, then 12-wk nontreatment period | 80 mg SC the first wk, 40 mg the 2nd wk, followed by 40 mg every other wk | 50 mg SC twice/wk for 3 mo, then 50 mg/wk | 5 mg/kg IV infusion to start, repeat at 2 and 6 wk, then q6-8 wk | 45 mg SC (for patients <100 kg); 90 mg (for patients >100 kg) to start, repeat at 4 wk, followed by q12 wk for maintenance |
| Contra-indications | HIV |
|
|
| Active TB |
| Baseline monitoring | CD4 count |
|
|
| PPD |
| Ongoing monitoring | Biweekly CD4 count; hold dose for counts <250 |
|
|
| Consider a yearly PPD |
| CBC, complete blood cell count; CHF, congestive heart failure; HIV, human immunodeficiency virus; H&P, history and physical; IL-12/23, interleukin-12, interleukin-23; LFTs, liver function test; MS, multiple sclerosis; NYHA, New York Heart Association; PPD, purified protein derivative; TB, tuberculosis; TNF, tumor necrosis factor. *For this patient population, adalimumab and etanercept have a (theoretical) risk. | |||||
Biologic therapy is contraindicated in patients with active serious infection. If patients develop infections requiring antibiotics while being treated, holding the biologic until infection resolution is advised.34 Standard vaccinations (eg, pneumococcal, hepatitis A and B, influenza, diphtheria, tetanus) are recommended before initiation of immunosuppressive therapy. After therapy starts, patients should avoid live and live-attenuated vaccines (varicella, mumps, measles, and rubella, oral typhoid, yellow fever, herpes zoster, intranasal influenza).35
Currently, none of the biologics are indicated for use in children or adolescents with psoriasis, despite epidemiologic data suggesting that one-third of adults with psoriasis developed it during childhood, in a form severe enough to warrant the use of systemic medications.34 The FDA is currently reviewing the possibility of indicating etanercept for pediatric psoriasis patients. All biologics are category B for pregnancy as there is no evidence that they negatively affect pregnancy.24
T-cell inhibitor. Alefacept binds CD2 on memory-effector T lymphocytes, inhibiting activation. Weekly intramuscular injections of alefacept for 12 weeks can clear lesions with long remissions.30
TNF-inhibitors. The TNF-inhibitors have been available for more than 10 years, predominantly for inflammatory bowel disease (IBD) and rheumatoid arthritis (RA), and more than 1.5 million patients have used adalimumab, etanercept, and infliximab for these disorders. Safety data, especially long term, are mostly derived from patients with IBD or RA, who have often combined TNF-inhibitors with additional immunosuppressive therapies. Thus, for psoriasis patients, who typically use biologics as monotherapy, the risk may be overestimated.24
TNF-inhibitors increase the risk for infection, most commonly of the upper respiratory tract, and, rarely, have been associated with opportunistic infections. Numerous cases of TB reactivation and an increased incidence of disseminated cases have been associated with TNF-inhibitors, so screening is recommended.24
The impact of TNF inhibition on congestive heart failure (CHF) is not well understood. Studies have variously shown that TNF-inhibitors have no effect on CHF morbidity or mortality, increase CHF mortality, or improve left ventricular function. TNF-inhibitors should be avoided in patients with severe CHF (New York Heart Association class III or IV). In milder CHF patients with worsening of symptoms, treatment should be discontinued.36
The increased risk of malignancy, especially lymphoma, is a concern, as there have been numerous case reports of lymphoma occurring with TNF-inhibitors. Psoriasis patients in general have an increased risk of lymphoma that confounds data interpretation.31 A number of case reports and a large observational study have shown patients receiving TNF-inhibitors may be at a greater risk for developing melanoma and nonmelanoma skin cancer.32
Ustekinumab, an interleukin 12/23 inhibitor, is a human monoclonal antibody that is absorbed and eliminated slowly, making dosing injections every 12 weeks convenient with efficacy maintained for at least one year.33 Because of its relative novelty, few studies are published regarding long-term safety. A recent head-to-head trial compared the efficacy and safety of ustekinumab with etanercept and found superior efficacy with ustekinumab, with comparable adverse events.37,38 Similar concerns exist with ustekinumab as with TNF-inhibitors, including infection, malignancy, CHF, and TB.33
CASE Tom denied having a personal family history of multiple sclerosis, or any demyelinating disorder. Nor did he have a history of cancer, tuberculosis exposure, CHF, or hepatitis. A purified protein derivative (PPD) was negative, as was his hepatitis panel, and his complete blood count with differential and metabolic panel were within normal limits.
Tom was started on the TNF-inhibitor adalimumab, after undergoing patient education and training and receiving instructions to stop the medication if he developed a major illness or infection. He received a loading dose of 80 mg SC, followed by 40 mg every other week. He tolerated the treatment well and 70% of his cutaneous symptoms cleared after 12 weeks of therapy; his joint pain also was reduced.
Tom is followed regularly in the clinic, with labs every 4 to 6 months. He is maintained on the injections and happy with the results. At each visit, weight loss and decreased beer intake are encouraged, both of which have been shown to reduce psoriasis severity. Although the beta-blockers and ACE inhibitors he takes are known to exacerbate psoriasis, the medications are necessary to treat Tom’s coronary artery disease.
CORRESPONDENCE Elizabeth Uhlenhake, MD, 18100 Oakwood Boulevard, Suite 300, Dearborn, MI 48124; eulenha@neomed.edu
• Prescribe high-potency (Class 1) topical steroids for use on thicker, chronic plaques and low-potency (Class 7) steroids for the face, intertriginous areas, and the groin. A
• Recommend folate supplementation (1-5 mg/d) for patients being treated with methotrexate, as it may reduce the drug’s hematologic and gastrointestinal adverse effects without decreasing efficacy. A
• Advise patients scheduled to begin biologic therapy to get standard vaccinations—eg, pneumococcal, influenza, hepatitis A and B—before treatment is initiated and to avoid live and live-attenuated vaccines thereafter. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Psoriasis is a systemic inflammatory disorder altered by environmental and genetic factors that presents as scaly erythematous plaques and affects 2% to 3% of the population.1 Eighty percent have mild-to-moderate cutaneous disease; disabling arthritis occurs in as many as 42% of cases.1,2 The devastating effects on quality of life— including social stigmatization, pain, physical disability, and psychological distress—are comparable to the effects of conditions like cancer and depression.2 There is no definitive cure, and patients are left with a lifetime of waxing and waning symptoms.
Helping to create an individualized treatment plan tailored to disease type and extent, comorbidities, and needs of the patient can directly impact the quality of his or her life.3 For those with localized disease, topical therapy is a suitable first choice. Phototherapy is generally the first-line treatment for patients with extensive psoriasis or disabling symptoms. When phototherapy is not feasible or is ineffective, systemic treatments with conventional oral agents or biologics are indicated.
Discussions about therapeutic options should include expected results and duration of remission, cost, convenience, adverse effects, insurance coverage, and safety concerns. The patient’s preferences should be taken into account in the treatment plan you create.3 A guiding principle: The optimal protocol is the one the patient is motivated to adhere to.
The limits of topical therapy
Topical treatments are safe and effective when used properly, as monotherapy for localized disease and adjunctive therapy for resistant lesions. Monotherapy with topical agents is not recommended for sites that have a significant impact on quality of life, such as the palms and soles, and is not practical for patients with extensive disease (>10% of body surface).4
More potent topicals, such as corticosteroids, are recommended for acute flares; less potent agents with fewer adverse effects, such as calcipotriene, are typically used for maintenance ( TABLE 1 ).5-8 Topical agents can be used either long term or intermittently. Here, too, the most effective treatment is the one the patient will actually apply.5
TABLE 1
A look at nonsteroidal topical treatments5-8
| Class | Examples | Adverse effects | Comments |
|---|---|---|---|
| Vitamin D analogues | Calcipotriol (calcipotriene), calcitriol | Burning, pruritus, edema, peeling, dryness, erythema | Combining with beta-methasone dipropionate increases efficacy |
| Retinoids | Tazarotene, tretinoin | Teratogenic, photosensitivity, irritation | Increased efficacy when combined with NB-UVB (and less UV exposure); increased efficacy, duration of remission, and reduction in steroid-induced atrophy when used with steroids |
| Calcineurin inhibitors | Tacrolimus, pimecrolimus | Burning and pruritus Black box warning for risk of malignancies* | No clinical evidence of increased cancer risk |
| Others | Emollients, salicylic acid, anthralin, coal tar | Severe skin irritation, staining of clothes, odor with anthralin and tar | Salicylic acid works well with steroids and topical immunomodulators, but is not compatible with calcipotriene |
| *Lymphoma seen with oral therapy. UVB, ultraviolet B; NB-UVB, narrow-band ultraviolet B. | |||
Steroid selection is based on site, severity
Corticosteroids are antiproliferative, immunosuppressive, anti-inflammatory, and vasoconstrictive, and divided into 7 classes. Low-potency agents (Class 7) are used on thinner skin like the face, intertriginous areas, and groin; high-potency steroids (Class 1) are reserved for thicker, chronic plaques.9 As a general rule, Class 1 steroids can be safely used for 2 to 4 weeks, with increased risk of both cutaneous effects and systemic absorption if used continuously for longer periods.5 The optimal end point for less potent agents is not known.
Cutaneous adverse effects are more common than systemic ones and include skin atrophy, telangiectasia, striae distensae, acne, folliculitis, and purpura.
Systemic adverse effects include Cushing’s syndrome, osteonecrosis of the femoral head, cataracts, and glaucoma.5 The greatest risk of systemic effects is associated with prolonged use of high-potency corticosteroids over large surfaces or under occlusion with dressings or plastic wrap. Patients should be transitioned to the lowest potency possible to maintain efficacy, use corticosteroids intermittently, or combine them with nonsteroidal agents to avoid unwanted effects.
Topicals aren’t working? Move on to phototherapy
Phototherapy is an option for patients with extensive disease or skin manifestations that are recalcitrant to topicals. It is efficacious, cost-effective, and lacks systemic immunosuppression. Ultraviolet (UV) A and B act on Langerhans cells, cytokines, and adhesion molecules, inhibiting epidermal proliferation and angiogenesis.10
Broadband UVB (BB-UVB) was first used during the 1950s, with crude tar or anthralin, but is rarely used today.
Narrow-band UVB (NB-UVB) (311-313 nm), developed in the 1980s, has largely replaced BB-UVB. In addition to providing more rapid clearing and resolution of psoriasis compared with BB-UVB, NB-UVB may have less phototoxicity.11 Between 20 and 25 NB-UVB treatments, 2 to 3 times a week (in the office or at home) are usually required for significant improvement.
Photocarcinogenesis is a concern but numerous studies, including a review of 3867 patients treated with NB-UVB with a median 5.5-year follow-up, found no significant association with cutaneous malignancies.12 NB-UVB is considered safe during pregnancy and used as first-line therapy for pregnant patients.13
Targeted UVB therapy using a 308-nm excimer laser, another option, selectively targets psoriatic lesions, leaving normal skin untreated. This makes supraerythemogenic doses possible, which increases UVB’s efficacy. Long-term adverse effects and duration of remission have not been clearly established.14
Psoralen and UVA (PUVA), which uses oral or topical psoralens to sensitize the skin to UVA, has a slightly higher efficacy than NB-UVB, but with increased risk of squamous cell carcinoma (SCC) and possibly melanoma.15 Clearing can occur within 24 treatments, with remissions lasting 3 to 6 months;16 monthly maintenance has not been found to lengthen remission.13
Common adverse effects include erythema that peaks 48 to 96 hours after a treatment, pruritus, xerosis, irregular pigmentation, and gastrointestinal symptoms that can be reduced by decreasing psoralen and/or UVA doses.13 High cumulative doses of oral PUVA (>200 treatments) is associated with a dose-related increased risk of nonmelanoma skin cancer, particularly SCC, in the Caucasian population. This increased risk has not been demonstrated in patients treated with PUVA bath therapy, which is more common in Scandinavian countries.17 There is no consensus regarding the risk of melanoma.15
A careful risk-benefit analysis is needed before initiating phototherapy in patients who take photosensitizing drugs, are immunosuppressed, or have a photosensitivity disorder or a history of melanoma, atypical nevi, multiple melanoma risk factors, or multiple nonmelanoma skin cancers.13 Regardless of the type of UV therapy administered, eye protection with goggles is required to decrease the risk of UV-related cataract formation, and genital shielding is needed to prevent increased risk of tumors.13 Photoaging is a long-term effect.
CASE When questioned further about the challenges that Tom has had with controlling his symptoms, he admitted to being noncompliant. As a busy executive, he said he didn’t have time to use the topical corticosteroids regularly. Phototherapy could alleviate his cutaneous symptoms, but would not address his symptoms that were consistent with psoriatic arthritis.
Pairing therapeutic modalities decreases exposure
Combining therapeutic modalities like emollients and topical or oral retinoids with NB-UVB improves efficacy while reducing the number of treatment sessions and cumulative UVB dosage. If calcipotriene is used, it should be applied after phototherapy because it is degraded upon UVB exposure.18 Acitretin should be started 2 weeks prior to initiation of phototherapy, and its use accompanied by a 25% reduction in initial UV dosage.13
PUVA may also be combined with topical calcipotriene or retinoids.19 In both cases, the addition of the other agent typically decreases the duration of phototherapy, improves the clinical response, and reduces the risk of cancer.13,20
Extensive disease? Consider a traditional systemic agent
Traditional systemic therapy is used to treat extensive disease ( FIGURE ), psoriasis refractory to topical agents, and debilitating disease on the palms, soles, or scalp. Biologics are a recent alternative, but traditional systemics have been utilized longer and have a more longstanding adverse effect and safety profile, are administered orally, and are much less expensive than biologics. Monitoring patients on systemic therapy is necessary ( TABLE 2 ).21-24
Methotrexate (MTX), a competitive inhibitor of dihydrofolate reductase, is the most commonly prescribed traditional systemic psoriasis treatment.21 It is administered in a single weekly dose via tablet, parenteral solution, or intramuscular (IM) or subcutaneous (SC) injection.25 A test dose (2.5 or 5 mg) is given initially and complete blood cell count is monitored within one week to evaluate for potential bone marrow toxicity. If none is observed, the dose may be increased to control the disease while minimizing adverse effects.21
Common adverse effects of MTX, such as nausea, vomiting, stomatitis, and fatigue, may be minimized by IM or SC administration, splitting the dose, or providing folate supplementation.21-24 Given in doses of 1 to 5 mg/d, folate may reduce adverse hematologic, gastrointestinal, and hepatic effects without decreasing efficacy.22
The major severe toxicities are myelosuppression, hepatotoxicity, and pulmonary fibrosis.24 MTX-induced hepatotoxicity is similar to nonalcoholic fatty liver disease (NAFLD) and is thought to exacerbate preexisting NAFLD, which is common in patients with metabolic syndrome. A liver biopsy or serum assays for liver fibrosis (amino-terminal peptide of pro-collagen III) may be warranted during therapy.24
MTX is an abortifacient and teratogen, so contraception during treatment and for up to 3 months thereafter is mandatory for women of childbearing age.26 Men should be advised that MTX decreases sperm count. (For more on methotrexate, see: “When a fetus survives methotrexate exposure,” at http://www.jfponline.com/Pages.asp?AID=10299).
Cyclosporine (CSA), an oral calcineurin inhibitor, is a potent immunosuppressant that rapidly clears psoriasis.27 Because duration of use correlates with permanent nephrotoxicity, hypertension, and potential increased risk of SCC and lymphoma, intermittent 12-week courses are recommended. Calcium channel blockers are the preferred treatment for CSA-induced hypertension because of their effect on smooth muscle vasodilation.21
Oral retinoids. Acitretin modulates epidermal proliferation and is anti-inflammatory. Because it lacks immunosuppression, acitretin is generally considered the treatment of choice in HIV patients with severe psoriasis.28 Acitretin is teratogenic and contraindicated in women who plan to become pregnant or who are unwilling to use adequate contraception for 3 years after discontinuing the drug.21
CASE Given the significant percentage of body surface area involved and symptoms consistent with psoriatic arthritis, Tom required an aggressive therapeutic regimen. His history of nonalcoholic fatty liver and social drinking precluded the use of methotrexate. A biologic therapy was the next therapeutic choice that could relieve both his cutaneous and joint symptoms.
FIGURE
4 psoriasis patients, 4 different presentations
A. The linear erythematous, scaly plaque along this patient’s cardiac bypass scar demonstrates koebnerization of plaque psoriasis.
B. Diffuse erythematous scaly papules coalesce into plaques on this patient’s anterior chest, arms, abdomen, and periumbilicus.
C. The pinpoint pustules on an erythematous base on this patient’s lateral neck, shoulders, and upper back are characteristic of pustular psoriasis.
D. This patient has erythematous plaques with overlying silvery scale on the elbow—a classic
TABLE 2
Traditional systemic therapy21-24
| Methotrexate (MTX) | Cyclosporine (CSA)* | Acitretin | |
|---|---|---|---|
| Dosing | ≤30 mg in one weekly dose (PO, IM, or SC) | 2.5-5.0 mg/kg/d in 2 divided doses for 12 wk, then 12-wk nontreatment period Dose decreased (by 0.5-1.0 mg/kg) with disease clearance or when hypertension or nephrotoxicity are detected | 10-50 mg/d given as a single dose Lower doses (25 mg/d) used to minimize adverse effects and in combination regimens When added to UV, light dose should be reduced 30%-50% |
| Contraindications |
|
|
|
| Baseline monitoring† |
|
|
|
| BP, blood pressure; BUN, blood urea nitrogen; CBC, complete blood cell count; CR, creatinine; H&P, history and physical; IM, intramuscular; LFTs, liver function tests; PO, by mouth; PPD, purified protein derivative; PUVA, psoralen and ultraviolet A; SC, subcutaneous; UV, ultraviolet; UVB, ultraviolet B. *Avoid live vaccinations; caution required with major infection and poorly controlled diabetes. †Ongoing monitoring for MTX: BUN, CBC, CR, LFTs; possible liver biopsy (for high-risk patients or cumulative dose >3.5-4 g); CSA: BP, BUN, CBC, CR, LFTs; lipd profile; magnesium, uric acid, potassium tests; pregnancy testing; Acitretin: CBC, LFTs, lipid profile, renal function test, pregnancy testing. | |||
Biologics require lab work and a detailed medication list
Before beginning biologic therapy for a patient, the National Psoriasis Foundation29 recommends obtaining a complete history, physical, medication list, future plans (ie, pregnancy or travel to locations requiring vaccinations), and baseline labs to identify possible risk factors and/or contraindications. Periodic evaluation to monitor development of new symptoms, including infection and malignancy ( TABLE 3 ),24,30-33 is needed, as well.
TABLE 3
Is your patient a candidate for biologics?24,30-33
| Agent (Drug class) | Alefacept (T-cell inhibitor) | Adalimumab (TNF-inhibitor) | Etanercept (TNF-inhibitor) | Infliximab (TNF-inhibitor) | Ustekinumab (IL-12/23 inhibitor) |
|---|---|---|---|---|---|
| Dosing | 15 mg IM/wk for 12 wk, then 12-wk nontreatment period | 80 mg SC the first wk, 40 mg the 2nd wk, followed by 40 mg every other wk | 50 mg SC twice/wk for 3 mo, then 50 mg/wk | 5 mg/kg IV infusion to start, repeat at 2 and 6 wk, then q6-8 wk | 45 mg SC (for patients <100 kg); 90 mg (for patients >100 kg) to start, repeat at 4 wk, followed by q12 wk for maintenance |
| Contra-indications | HIV |
|
|
| Active TB |
| Baseline monitoring | CD4 count |
|
|
| PPD |
| Ongoing monitoring | Biweekly CD4 count; hold dose for counts <250 |
|
|
| Consider a yearly PPD |
| CBC, complete blood cell count; CHF, congestive heart failure; HIV, human immunodeficiency virus; H&P, history and physical; IL-12/23, interleukin-12, interleukin-23; LFTs, liver function test; MS, multiple sclerosis; NYHA, New York Heart Association; PPD, purified protein derivative; TB, tuberculosis; TNF, tumor necrosis factor. *For this patient population, adalimumab and etanercept have a (theoretical) risk. | |||||
Biologic therapy is contraindicated in patients with active serious infection. If patients develop infections requiring antibiotics while being treated, holding the biologic until infection resolution is advised.34 Standard vaccinations (eg, pneumococcal, hepatitis A and B, influenza, diphtheria, tetanus) are recommended before initiation of immunosuppressive therapy. After therapy starts, patients should avoid live and live-attenuated vaccines (varicella, mumps, measles, and rubella, oral typhoid, yellow fever, herpes zoster, intranasal influenza).35
Currently, none of the biologics are indicated for use in children or adolescents with psoriasis, despite epidemiologic data suggesting that one-third of adults with psoriasis developed it during childhood, in a form severe enough to warrant the use of systemic medications.34 The FDA is currently reviewing the possibility of indicating etanercept for pediatric psoriasis patients. All biologics are category B for pregnancy as there is no evidence that they negatively affect pregnancy.24
T-cell inhibitor. Alefacept binds CD2 on memory-effector T lymphocytes, inhibiting activation. Weekly intramuscular injections of alefacept for 12 weeks can clear lesions with long remissions.30
TNF-inhibitors. The TNF-inhibitors have been available for more than 10 years, predominantly for inflammatory bowel disease (IBD) and rheumatoid arthritis (RA), and more than 1.5 million patients have used adalimumab, etanercept, and infliximab for these disorders. Safety data, especially long term, are mostly derived from patients with IBD or RA, who have often combined TNF-inhibitors with additional immunosuppressive therapies. Thus, for psoriasis patients, who typically use biologics as monotherapy, the risk may be overestimated.24
TNF-inhibitors increase the risk for infection, most commonly of the upper respiratory tract, and, rarely, have been associated with opportunistic infections. Numerous cases of TB reactivation and an increased incidence of disseminated cases have been associated with TNF-inhibitors, so screening is recommended.24
The impact of TNF inhibition on congestive heart failure (CHF) is not well understood. Studies have variously shown that TNF-inhibitors have no effect on CHF morbidity or mortality, increase CHF mortality, or improve left ventricular function. TNF-inhibitors should be avoided in patients with severe CHF (New York Heart Association class III or IV). In milder CHF patients with worsening of symptoms, treatment should be discontinued.36
The increased risk of malignancy, especially lymphoma, is a concern, as there have been numerous case reports of lymphoma occurring with TNF-inhibitors. Psoriasis patients in general have an increased risk of lymphoma that confounds data interpretation.31 A number of case reports and a large observational study have shown patients receiving TNF-inhibitors may be at a greater risk for developing melanoma and nonmelanoma skin cancer.32
Ustekinumab, an interleukin 12/23 inhibitor, is a human monoclonal antibody that is absorbed and eliminated slowly, making dosing injections every 12 weeks convenient with efficacy maintained for at least one year.33 Because of its relative novelty, few studies are published regarding long-term safety. A recent head-to-head trial compared the efficacy and safety of ustekinumab with etanercept and found superior efficacy with ustekinumab, with comparable adverse events.37,38 Similar concerns exist with ustekinumab as with TNF-inhibitors, including infection, malignancy, CHF, and TB.33
CASE Tom denied having a personal family history of multiple sclerosis, or any demyelinating disorder. Nor did he have a history of cancer, tuberculosis exposure, CHF, or hepatitis. A purified protein derivative (PPD) was negative, as was his hepatitis panel, and his complete blood count with differential and metabolic panel were within normal limits.
Tom was started on the TNF-inhibitor adalimumab, after undergoing patient education and training and receiving instructions to stop the medication if he developed a major illness or infection. He received a loading dose of 80 mg SC, followed by 40 mg every other week. He tolerated the treatment well and 70% of his cutaneous symptoms cleared after 12 weeks of therapy; his joint pain also was reduced.
Tom is followed regularly in the clinic, with labs every 4 to 6 months. He is maintained on the injections and happy with the results. At each visit, weight loss and decreased beer intake are encouraged, both of which have been shown to reduce psoriasis severity. Although the beta-blockers and ACE inhibitors he takes are known to exacerbate psoriasis, the medications are necessary to treat Tom’s coronary artery disease.
CORRESPONDENCE Elizabeth Uhlenhake, MD, 18100 Oakwood Boulevard, Suite 300, Dearborn, MI 48124; eulenha@neomed.edu
1. Gelfand JM, Weinstein R, Porter SB, et al. Prevalence and treatment of psoriasis in the United Kingdom: a population-based study. Arch Dermatol. 2005;141:1537-1541.
2. Fortune DG, Richards HL, Griffiths CE. Psychologic factors in psoriasis: consequences, mechanisms, and interventions. Dermatol Clin. 2005;23:681-694.
3. Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332.
4. Pettey AA, Balkrishnan R, Rapp SR, et al. Patients with palmoplantar psoriasis have more physical disability and discomfort than patients with other forms of psoriasis: implications for clinical practice. J Am Acad Dermatol. 2003;49:271-275.
5. Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 3. Guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
6. Kaufmann R, Bibby AJ, Bissonnette R, et al. A new calcipotriol/betamethasone dipropionate formulation (Daivobet) is an effective once-daily treatment for psoriasis vulgaris. Dermatology. 2002;205:389-393.
7. Koo JY, Martin D. Investigator-masked comparison of tazarotene gel q.d. plus mometasone furoate cream q.d. vs. mometasone furoate cream b.i.d. in the treatment of plaque psoriasis. Int J Dermatol. 2001;40:210-212.
8. Berger TG, Duvic M, Van Voorhees AS, et al. The use of topical calcineurin inhibitors in dermatology: safety concerns. Report of the American Academy of Dermatology Association Task Force. J Am Acad Dermatol. 2006;54:818-823.
9. Cornell RC, Stoughton RB. Correlation of the vasoconstriction assay and clinical activity in psoriasis. Arch Dermatol. 1985;121:63-67.
10. Lui H. Phototherapy of psoriasis: update with practical pearls. J Cutan Med Surg. 2002;6(suppl):17-21.
11. Walters IB, Burack LH, Coven TR, et al. Suberythemogenic narrow-band UVB is markedly more effective than conventional UVB in treatment of psoriasis vulgaris. J Am Acad Dermatol. 1999;40:893-900.
12. Stern RS, Laird N. The carcinogenic risk of treatments for severe psoriasis. Photochemotherapy follow-up study. Cancer. 1994;73:2759-2764.
13. Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 5. Guidelines of care for the treatment of psoriasis with phototherapy and photochemotherapy. J Am Acad Dermatol. 2010;62:114-135.
14. Trehan M, Taylor CR. High-dose 308-nm excimer laser for the treatment of psoriasis. J Am Acad Dermatol. 2002;46:732-737.
15. Morison WL, Baughman RD, Day RM, et al. Consensus workshop on the toxic effects of long-term PUVA therapy. Arch Dermatol. 1998;134:595-598.
16. Spuls PI, Witkamp L, Bossuyt PM, et al. A systematic review of five systemic treatments for severe psoriasis. Br J Dermatol. 1997;137:943-949.
17. Murase JE, Lee EE, Koo J. Effect of ethnicity on the risk of developing nonmelanoma skin cancer following long-term PUVA therapy. Int J Dermatol. 2005;44:1016-1021.
18. Lowe NJ, Prystowsky JH, Bourget T, et al. Acitretin plus UVB therapy for psoriasis. Comparisons with placebo plus UVB and acitretin alone. J Am Acad Dermatol. 1991;24:591-594.
19. Torras H, Aliaga A, Lopez-Estebaranz JL, et al. A combination therapy of calcipotriol cream and PUVA reduces the UVA dose and improves the response of psoriasis vulgaris. J Dermatolog Treat. 2004;15:98-103.
20. Tanew A, Guggenbichler A, Honigsmann H, et al. Photochemotherapy for severe psoriasis without or in combination with acitretin: a randomized, double-blind comparison study. J Am Acad Dermatol. 1991;25:682-684.
21. Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 4. Guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol. 2009;61:451-485.
22. Strober BE, Menon K. Folate supplementation during methotrexate therapy for patients with psoriasis. J Am Acad Dermatol. 2005;53:652-659.
23. Taler SJ, Textor SC, Canzanello VJ, et al. Cyclosporine-induced hypertension: incidence, pathogenesis and management. Drug Saf. 1999;20:437-449.
24. Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850.
25. Heydendael VM, Spuls PI, Opmeer BC, et al. Methotrexate versus cyclosporine in moderate-to-severe chronic plaque psoriasis. N Engl J Med. 2003;349:658-665.
26. Lloyd ME, Carr M, McElhatton P, et al. The effects of methotrexate on pregnancy, fertility and lactation. QJM. 1999;92:551-563.
27. Ellis CN, Fradin MS, Messana JM, et al. Cyclosporine for plaque-type psoriasis. Results of a multidose, double-blind trial. N Engl J Med. 1991;324:277-284.
28. Buccheri L, Katchen BR, Karter AJ, et al. Acitretin therapy is effective for psoriasis associated with human immunodeficiency virus infection. Arch Dermatol. 1997;133:711-715.
29. Lebwohl M, Bagel J, Gelfand JM, et al. From the Medical Board of the National Psoriasis Foundation: monitoring and vaccinations in patients treated with biologics for psoriasis. J Am Acad Dermatol. 2008;58:94-105.
30. Gordon KB, Vaishnaw AK, O’Gorman J, et al. Treatment of psoriasis with alefacept: correlation of clinical improvement with reductions of memory T-cell counts. Arch Dermatol. 2003;139:1563-1570.
31. Brown SL, Greene MH, Gershon SK, et al. Tumor necrosis factor antagonist therapy and lymphoma development: twenty-six cases reported to the Food and Drug Administration. Arthritis Rheum. 2002;46:3151-3158.
32. Fulchiero GJ, Jr, Salvaggio H, Drabick JJ, et al. Eruptive latent metastatic melanomas after initiation of antitumor necrosis factor therapies. J Am Acad Dermatol. 2007;56(suppl):S65-S67.
33. Leonardi CL, Kimball AB, Papp KA, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet. 2008;371:1665-1674.
34. US Food and Drug Administration. FDA approves new drug to treat psoriasis. Sept. 25, 2009. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm183851.htm. Accessed June 15, 2012.
35. Duchini A, Goss JA, Karpen S, et al. Vaccinations for adult solid-organ transplant recipients: current recommendations and protocols. Clin Microbiol Rev. 2003;16:357-364.
36. Mann DL, McMurray JJ, Packer M, et al. Targeted anticytokine therapy in patients with chronic heart failure: results of the Randomized Etanercept Worldwide Evaluation (RENEWAL). Circulation. 2004;109:1594-1602.
37. Griffiths CE, Strober BE, van de Kerkhof P, et al. Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N Engl J Med. 2010;362:118-128.
38. Papp KA, Langley RG, Lebwohl M, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet. 2008;371:1675-1684.
1. Gelfand JM, Weinstein R, Porter SB, et al. Prevalence and treatment of psoriasis in the United Kingdom: a population-based study. Arch Dermatol. 2005;141:1537-1541.
2. Fortune DG, Richards HL, Griffiths CE. Psychologic factors in psoriasis: consequences, mechanisms, and interventions. Dermatol Clin. 2005;23:681-694.
3. Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332.
4. Pettey AA, Balkrishnan R, Rapp SR, et al. Patients with palmoplantar psoriasis have more physical disability and discomfort than patients with other forms of psoriasis: implications for clinical practice. J Am Acad Dermatol. 2003;49:271-275.
5. Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 3. Guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
6. Kaufmann R, Bibby AJ, Bissonnette R, et al. A new calcipotriol/betamethasone dipropionate formulation (Daivobet) is an effective once-daily treatment for psoriasis vulgaris. Dermatology. 2002;205:389-393.
7. Koo JY, Martin D. Investigator-masked comparison of tazarotene gel q.d. plus mometasone furoate cream q.d. vs. mometasone furoate cream b.i.d. in the treatment of plaque psoriasis. Int J Dermatol. 2001;40:210-212.
8. Berger TG, Duvic M, Van Voorhees AS, et al. The use of topical calcineurin inhibitors in dermatology: safety concerns. Report of the American Academy of Dermatology Association Task Force. J Am Acad Dermatol. 2006;54:818-823.
9. Cornell RC, Stoughton RB. Correlation of the vasoconstriction assay and clinical activity in psoriasis. Arch Dermatol. 1985;121:63-67.
10. Lui H. Phototherapy of psoriasis: update with practical pearls. J Cutan Med Surg. 2002;6(suppl):17-21.
11. Walters IB, Burack LH, Coven TR, et al. Suberythemogenic narrow-band UVB is markedly more effective than conventional UVB in treatment of psoriasis vulgaris. J Am Acad Dermatol. 1999;40:893-900.
12. Stern RS, Laird N. The carcinogenic risk of treatments for severe psoriasis. Photochemotherapy follow-up study. Cancer. 1994;73:2759-2764.
13. Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 5. Guidelines of care for the treatment of psoriasis with phototherapy and photochemotherapy. J Am Acad Dermatol. 2010;62:114-135.
14. Trehan M, Taylor CR. High-dose 308-nm excimer laser for the treatment of psoriasis. J Am Acad Dermatol. 2002;46:732-737.
15. Morison WL, Baughman RD, Day RM, et al. Consensus workshop on the toxic effects of long-term PUVA therapy. Arch Dermatol. 1998;134:595-598.
16. Spuls PI, Witkamp L, Bossuyt PM, et al. A systematic review of five systemic treatments for severe psoriasis. Br J Dermatol. 1997;137:943-949.
17. Murase JE, Lee EE, Koo J. Effect of ethnicity on the risk of developing nonmelanoma skin cancer following long-term PUVA therapy. Int J Dermatol. 2005;44:1016-1021.
18. Lowe NJ, Prystowsky JH, Bourget T, et al. Acitretin plus UVB therapy for psoriasis. Comparisons with placebo plus UVB and acitretin alone. J Am Acad Dermatol. 1991;24:591-594.
19. Torras H, Aliaga A, Lopez-Estebaranz JL, et al. A combination therapy of calcipotriol cream and PUVA reduces the UVA dose and improves the response of psoriasis vulgaris. J Dermatolog Treat. 2004;15:98-103.
20. Tanew A, Guggenbichler A, Honigsmann H, et al. Photochemotherapy for severe psoriasis without or in combination with acitretin: a randomized, double-blind comparison study. J Am Acad Dermatol. 1991;25:682-684.
21. Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 4. Guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol. 2009;61:451-485.
22. Strober BE, Menon K. Folate supplementation during methotrexate therapy for patients with psoriasis. J Am Acad Dermatol. 2005;53:652-659.
23. Taler SJ, Textor SC, Canzanello VJ, et al. Cyclosporine-induced hypertension: incidence, pathogenesis and management. Drug Saf. 1999;20:437-449.
24. Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850.
25. Heydendael VM, Spuls PI, Opmeer BC, et al. Methotrexate versus cyclosporine in moderate-to-severe chronic plaque psoriasis. N Engl J Med. 2003;349:658-665.
26. Lloyd ME, Carr M, McElhatton P, et al. The effects of methotrexate on pregnancy, fertility and lactation. QJM. 1999;92:551-563.
27. Ellis CN, Fradin MS, Messana JM, et al. Cyclosporine for plaque-type psoriasis. Results of a multidose, double-blind trial. N Engl J Med. 1991;324:277-284.
28. Buccheri L, Katchen BR, Karter AJ, et al. Acitretin therapy is effective for psoriasis associated with human immunodeficiency virus infection. Arch Dermatol. 1997;133:711-715.
29. Lebwohl M, Bagel J, Gelfand JM, et al. From the Medical Board of the National Psoriasis Foundation: monitoring and vaccinations in patients treated with biologics for psoriasis. J Am Acad Dermatol. 2008;58:94-105.
30. Gordon KB, Vaishnaw AK, O’Gorman J, et al. Treatment of psoriasis with alefacept: correlation of clinical improvement with reductions of memory T-cell counts. Arch Dermatol. 2003;139:1563-1570.
31. Brown SL, Greene MH, Gershon SK, et al. Tumor necrosis factor antagonist therapy and lymphoma development: twenty-six cases reported to the Food and Drug Administration. Arthritis Rheum. 2002;46:3151-3158.
32. Fulchiero GJ, Jr, Salvaggio H, Drabick JJ, et al. Eruptive latent metastatic melanomas after initiation of antitumor necrosis factor therapies. J Am Acad Dermatol. 2007;56(suppl):S65-S67.
33. Leonardi CL, Kimball AB, Papp KA, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet. 2008;371:1665-1674.
34. US Food and Drug Administration. FDA approves new drug to treat psoriasis. Sept. 25, 2009. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm183851.htm. Accessed June 15, 2012.
35. Duchini A, Goss JA, Karpen S, et al. Vaccinations for adult solid-organ transplant recipients: current recommendations and protocols. Clin Microbiol Rev. 2003;16:357-364.
36. Mann DL, McMurray JJ, Packer M, et al. Targeted anticytokine therapy in patients with chronic heart failure: results of the Randomized Etanercept Worldwide Evaluation (RENEWAL). Circulation. 2004;109:1594-1602.
37. Griffiths CE, Strober BE, van de Kerkhof P, et al. Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N Engl J Med. 2010;362:118-128.
38. Papp KA, Langley RG, Lebwohl M, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet. 2008;371:1675-1684.